User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Part of Taking a Good (Human) Patient History Includes Asking About Pet Vaccinations
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In my job, I spend 99% of my time thinking about ethical issues that arise in the care of human beings. That is the focus of our medical school, and that’s what we do.
However,
Recently, there has been a great increase in the number of pet owners who are saying, “I’m not going to vaccinate my pets.” As horrible as this sounds, what’s happening is vaccine hesitancy about vaccines used in humans is extending through some people to their pets.
The number of people who say they don’t trust things like rabies vaccine to be effective or safe for their pet animals is 40%, at least in surveys, and the American Veterinary Medical Association reports that 15%-18% of pet owners are not, in fact, vaccinating their pets against rabies.
Rabies, as I hope everybody knows, is one horrible disease. Even the treatment of it, should you get bitten by a rabid animal, is no fun, expensive, and hopefully something that can be administered quickly. It’s not always the case. Worldwide, at least 70,000 people die from rabies every year.
Obviously, there are many countries that are so terrified of rabies, they won’t let you bring pets in without quarantining them, say, England, for at least 6 months to a year, I believe, because they don’t want rabies getting into their country. They’re very strict about the movement of pets.
It is inexcusable for people, first, not to give their pets vaccines that prevent them getting distemper, parvovirus, or many other diseases that harm the pet. It’s also inexcusable to shorten your pet’s life or ask your patients to care for pets who get sick from many of these diseases that are vaccine preventable.
Worst of all, it’s inexcusable for any pet owner not to give a rabies vaccine to their pets. Were it up to me, I’d say you have to license your pet, and as part of that, you must mandate rabies vaccines for your dogs, cats, and other pets.
We know what happens when people encounter wild animals like raccoons and rabbits. It is not a good situation. Your pets can easily encounter a rabid animal and then put themselves in a position where they can harm their human owners.
We have an efficacious, safe treatment. If you’re dealing with someone, it might make sense to ask them, “Do you own a pet? Are you vaccinating?” It may not be something you’d ever thought about, but what we don’t need is rabies back in a bigger way in the United States than it’s been in the past.
I think, as a matter of prudence and public health, maybe firing up that question, “Got a pet in the house and are you vaccinating,” could be part of taking a good history.
Dr. Caplan is director of the division of medical ethics at New York University Langone Medical Center, New York City. He disclosed conflicts of interest with Johnson & Johnson and Medscape.
A version of this article first appeared on Medscape.com.
From Scrubs to Social Media: How Some Med Students Become Influencers
A medical student’s life is an endless cycle of classes, exams, clinical rotations, and residency preparation. On TikTok and Instagram, among other sites, they share medical school experiences and lessons learned in the classroom and advocate for causes such as increased diversity and gender rights in the medical field.
This news organization caught up with a few social media influencers with a large online following to learn how medical students can effectively use social media to build a professional brand and network. Most of the students interviewed said that their social media platforms offered an opportunity to educate others about significant medical developments, feel part of a community with a like-minded audience, and network with doctors who may lead them to a future residency or career path.
Many med students said that they built their large audiences by creating a platform for people of their ethnic background, nationality, race, gender, or simply what others weren’t already talking about. They said they saw a niche in social media that was missing or others hadn’t tackled in the same way.
When Joel Bervell began med school in 2020, he questioned some of the lessons he learned about how race is used in medical practice, which didn’t make sense to him. So, he began his own research. He had about 2000 followers on Instagram at the time.
Mr. Bervell read a new study about pulse oximeters and how they often produce misleading readings on patients with dark skin.
He wondered why he hadn’t learned this in medical school, so he posted it on TikTok. Within 24 hours, about 500,000 people viewed it. Most of the comments were from doctors, nurses, and physician assistants who said they weren’t aware of the disparity.
While his initial posts detailed his journey to medical school and a day-in-the-life of a medical student, he transitioned to posts primarily about race, health equity, and what he perceives as racial bias in medicine.
Now, the fourth-year Ghanaian-American student at the Elson S. Floyd College of Medicine at Washington State University Spokane has close to 1.2 million followers on Instagram and TikTok combined. He frequently visits the White House to advise on social media’s influence on healthcare and has appeared on the Kelly Clarkson Show, Good Morning America, CNN, and ABC, among others.
He said he also uses social media to translate complex medical information for a general audience, many of whom access health information online so they can manage their own healthcare. He sees his social media work as an extension of his medical education, allowing him to delve deeper into subjects and report on them as if he were publishing research in a medical journal.
“When I came to medical school, yes, I wanted to be a doctor. But I also wanted to impact people.” Social media allows him to educate many more people than individual patients, the 29-year-old told this news organization.
Inspiring Minorities
Tabhata Paulet, 27, started her TikTok presence as a premed student in 2021. She aimed to provide free resources to help low-income, first-generation Latinx students like herself study for standardized exams.
“I always looked online for guidance and resources, and the medical influencers did not share a similar background. So, I shared my story and what I had to do as a first-generation and first person in my family to become a physician. I did not have access to the same resources as my peers,” said Ms. Paulet, who was born in Peru and came to New Jersey as a child.
Students who are Hispanic, Latinx, or of Spanish origin made up 6.8% of total medical school enrollment in 2023-2024, up slightly from 6.7% in 2022-2023, according to the Association of American Medical Colleges (AAMC).
Ms. Paulet’s online presence grew when she began documenting her experiences as a first-year medical student, bridging the language barrier for Spanish-speaking patients so they could understand their diagnosis and treatment. She often posts about health disparity and barriers to care for underserved communities.
Most of her nearly 22,000 followers are Hispanic, said the now fourth-year student at Rutgers New Jersey Medical School in Newark, New Jersey. “I talk a lot about my interesting Spanish-speaking patients ... and how sometimes speaking their native language truly makes a difference in their care.”
She believes that she serves an important role in social media. “It can be very inspirational for those who come after you [in med school] to see someone from a similar culture and upbringing.”
Creating a Community
It was during a therapy session 4 years ago that Jeremy “JP” Scott decided to share Instagram posts about his experiences as a nontraditional medical student. The 37-year-old was studying at Ross University School of Medicine in Barbados and was feeling lonely as an international medical student training to be a doctor as a second career.
Before starting med school, Mr. Scott was an adjunct professor and lab supervisor at the University of Hartford Biology Department, West Hartford, Connecticut, and then a research assistant and lab manager at the Wistar Institute in Philadelphia.
Although he wanted to follow his mother’s path to becoming a doctor, it was more difficult than he envisioned, said the fourth-year student who completed clinical rotations in the United States and is now applying for residencies.
“I talked about how medical school is not what it appears to be ... There are a lot of challenges we are going through,” especially as people of color, he said.
Mr. Scott believes social media helps people feel included and less alone. He said many of his followers are med students and physicians.
His posts often focus on LGBTQIA+ pride and being a minority as a Black man in medicine.
“The pandemic spurred a lot of us. We had a racial reckoning in our country at the time. It inspired us to talk as Black creators and Black medical students.”
Black or African American medical students made up 8.5% of total med school enrollment in 2023-2024, a slight increase from 2022 to 2023, according to AAMC figures. Black men represented 7% of total enrollment in 2023-2024, while Black women represented 9.8%.
After only a handful of online posts in which Mr. Scott candidly discussed his mental health struggles and relationships, he attracted the attention of several medical apparel companies, including the popular FIGS scrubs. He’s now an ambassador for the company, which supports him and his content.
“My association with FIGS has helped attract a wider online audience, increasing my presence.” Today, he has 14,000 Instagram followers. “It opened up so many opportunities,” Mr. Scott said. One example is working with the national LGBTQIA+ community.
“The goal was never to be a social media influencer, to gain sponsorships or photo opportunities,” he said.
“My job, first, is as a medical student. Everything else is second. I am not trying to be a professional social media personality. I’m trying to be an actual physician.” He also tries to separate JP “social media” from Jeremy, the medical student.
“On Instagram, anyone can pull it up and see what you’re doing. The last thing I want is for them to think that I’m not serious about what I’m doing, that I’m not here to learn and become a doctor.”
Benefits and Drawbacks
Ms. Paulet said her social media following helped her connect with leaders in the Latinx medical community, including an obstetrics anesthesiologist, her intended specialty. “I don’t think I’d be able to do that without a social media platform.”
Her online activity also propelled her from regional to national leadership in the Latino Medical Student Association (LMSA). She now also runs their Instagram page, which has 14,000 followers.
Mr. Bervell believes social media is a great way to network. He’s connected with people he wouldn’t have met otherwise, including physicians. “I think it will help me get into a residency,” he said. “It allows people to know who you are ... They will be able to tell in a few videos the type of doctor I want to be.”
On the other hand, Mr. Bervell is aware of the negative impacts of social media on mental health. “You can get lost in social media.” For that reason, he often tries to disconnect. “I can go days without my phone.”
Posting on social media can be time-consuming, Mr. Bervell admitted. He said he spent about 2 hours a day researching, editing, and posting on TikTok when he first started building his following. Now, he spends about 2-3 hours a week creating videos. “I don’t post every day anymore. I don’t have the time.”
When she started building her TikTok presence, Ms. Paulet said she devoted 15 hours a week to the endeavor, but now she spends 10-12 hours a week posting online, including on LMSA’s Instagram page. “Whenever you are done with an exam or have a study break, this is something fun to do.” She also says you never know who you’re going to inspire when you put yourself out there.
“Talk about your journey, rotations, or your experience in your first or second year of medical school. Talk about milestones like board exams.”
Word to the Wise
Some students may be concerned that their posts might affect a potential residency program. But the medical students interviewed say they want to find programs that align with their values and accept them for who they are.
Mr. Scott said he’s not worried about someone not liking him because of who he is. “I am Black and openly gay. If it’s a problem, I don’t need to work with you or your institution.”
Mr. Bervell stressed that medical students should stay professional online. “I reach 5-10 million people a month, and I have to think: Would I want them to see this? You have to know at all times that someone is watching. I’m very careful about how I post. I script out every video.”
Mr. Scott agreed. He advises those interested in becoming medical influencers to know what they can’t post online. For example, to ensure safety and privacy, Mr. Scott doesn’t take photos in the hospital, show his medical badge, or post patient information. “You want to be respectful of your future medical profession,” he said.
“If it’s something my mother would be ashamed of, I don’t need to post about it.”
A version of this article first appeared on Medscape.com.
A medical student’s life is an endless cycle of classes, exams, clinical rotations, and residency preparation. On TikTok and Instagram, among other sites, they share medical school experiences and lessons learned in the classroom and advocate for causes such as increased diversity and gender rights in the medical field.
This news organization caught up with a few social media influencers with a large online following to learn how medical students can effectively use social media to build a professional brand and network. Most of the students interviewed said that their social media platforms offered an opportunity to educate others about significant medical developments, feel part of a community with a like-minded audience, and network with doctors who may lead them to a future residency or career path.
Many med students said that they built their large audiences by creating a platform for people of their ethnic background, nationality, race, gender, or simply what others weren’t already talking about. They said they saw a niche in social media that was missing or others hadn’t tackled in the same way.
When Joel Bervell began med school in 2020, he questioned some of the lessons he learned about how race is used in medical practice, which didn’t make sense to him. So, he began his own research. He had about 2000 followers on Instagram at the time.
Mr. Bervell read a new study about pulse oximeters and how they often produce misleading readings on patients with dark skin.
He wondered why he hadn’t learned this in medical school, so he posted it on TikTok. Within 24 hours, about 500,000 people viewed it. Most of the comments were from doctors, nurses, and physician assistants who said they weren’t aware of the disparity.
While his initial posts detailed his journey to medical school and a day-in-the-life of a medical student, he transitioned to posts primarily about race, health equity, and what he perceives as racial bias in medicine.
Now, the fourth-year Ghanaian-American student at the Elson S. Floyd College of Medicine at Washington State University Spokane has close to 1.2 million followers on Instagram and TikTok combined. He frequently visits the White House to advise on social media’s influence on healthcare and has appeared on the Kelly Clarkson Show, Good Morning America, CNN, and ABC, among others.
He said he also uses social media to translate complex medical information for a general audience, many of whom access health information online so they can manage their own healthcare. He sees his social media work as an extension of his medical education, allowing him to delve deeper into subjects and report on them as if he were publishing research in a medical journal.
“When I came to medical school, yes, I wanted to be a doctor. But I also wanted to impact people.” Social media allows him to educate many more people than individual patients, the 29-year-old told this news organization.
Inspiring Minorities
Tabhata Paulet, 27, started her TikTok presence as a premed student in 2021. She aimed to provide free resources to help low-income, first-generation Latinx students like herself study for standardized exams.
“I always looked online for guidance and resources, and the medical influencers did not share a similar background. So, I shared my story and what I had to do as a first-generation and first person in my family to become a physician. I did not have access to the same resources as my peers,” said Ms. Paulet, who was born in Peru and came to New Jersey as a child.
Students who are Hispanic, Latinx, or of Spanish origin made up 6.8% of total medical school enrollment in 2023-2024, up slightly from 6.7% in 2022-2023, according to the Association of American Medical Colleges (AAMC).
Ms. Paulet’s online presence grew when she began documenting her experiences as a first-year medical student, bridging the language barrier for Spanish-speaking patients so they could understand their diagnosis and treatment. She often posts about health disparity and barriers to care for underserved communities.
Most of her nearly 22,000 followers are Hispanic, said the now fourth-year student at Rutgers New Jersey Medical School in Newark, New Jersey. “I talk a lot about my interesting Spanish-speaking patients ... and how sometimes speaking their native language truly makes a difference in their care.”
She believes that she serves an important role in social media. “It can be very inspirational for those who come after you [in med school] to see someone from a similar culture and upbringing.”
Creating a Community
It was during a therapy session 4 years ago that Jeremy “JP” Scott decided to share Instagram posts about his experiences as a nontraditional medical student. The 37-year-old was studying at Ross University School of Medicine in Barbados and was feeling lonely as an international medical student training to be a doctor as a second career.
Before starting med school, Mr. Scott was an adjunct professor and lab supervisor at the University of Hartford Biology Department, West Hartford, Connecticut, and then a research assistant and lab manager at the Wistar Institute in Philadelphia.
Although he wanted to follow his mother’s path to becoming a doctor, it was more difficult than he envisioned, said the fourth-year student who completed clinical rotations in the United States and is now applying for residencies.
“I talked about how medical school is not what it appears to be ... There are a lot of challenges we are going through,” especially as people of color, he said.
Mr. Scott believes social media helps people feel included and less alone. He said many of his followers are med students and physicians.
His posts often focus on LGBTQIA+ pride and being a minority as a Black man in medicine.
“The pandemic spurred a lot of us. We had a racial reckoning in our country at the time. It inspired us to talk as Black creators and Black medical students.”
Black or African American medical students made up 8.5% of total med school enrollment in 2023-2024, a slight increase from 2022 to 2023, according to AAMC figures. Black men represented 7% of total enrollment in 2023-2024, while Black women represented 9.8%.
After only a handful of online posts in which Mr. Scott candidly discussed his mental health struggles and relationships, he attracted the attention of several medical apparel companies, including the popular FIGS scrubs. He’s now an ambassador for the company, which supports him and his content.
“My association with FIGS has helped attract a wider online audience, increasing my presence.” Today, he has 14,000 Instagram followers. “It opened up so many opportunities,” Mr. Scott said. One example is working with the national LGBTQIA+ community.
“The goal was never to be a social media influencer, to gain sponsorships or photo opportunities,” he said.
“My job, first, is as a medical student. Everything else is second. I am not trying to be a professional social media personality. I’m trying to be an actual physician.” He also tries to separate JP “social media” from Jeremy, the medical student.
“On Instagram, anyone can pull it up and see what you’re doing. The last thing I want is for them to think that I’m not serious about what I’m doing, that I’m not here to learn and become a doctor.”
Benefits and Drawbacks
Ms. Paulet said her social media following helped her connect with leaders in the Latinx medical community, including an obstetrics anesthesiologist, her intended specialty. “I don’t think I’d be able to do that without a social media platform.”
Her online activity also propelled her from regional to national leadership in the Latino Medical Student Association (LMSA). She now also runs their Instagram page, which has 14,000 followers.
Mr. Bervell believes social media is a great way to network. He’s connected with people he wouldn’t have met otherwise, including physicians. “I think it will help me get into a residency,” he said. “It allows people to know who you are ... They will be able to tell in a few videos the type of doctor I want to be.”
On the other hand, Mr. Bervell is aware of the negative impacts of social media on mental health. “You can get lost in social media.” For that reason, he often tries to disconnect. “I can go days without my phone.”
Posting on social media can be time-consuming, Mr. Bervell admitted. He said he spent about 2 hours a day researching, editing, and posting on TikTok when he first started building his following. Now, he spends about 2-3 hours a week creating videos. “I don’t post every day anymore. I don’t have the time.”
When she started building her TikTok presence, Ms. Paulet said she devoted 15 hours a week to the endeavor, but now she spends 10-12 hours a week posting online, including on LMSA’s Instagram page. “Whenever you are done with an exam or have a study break, this is something fun to do.” She also says you never know who you’re going to inspire when you put yourself out there.
“Talk about your journey, rotations, or your experience in your first or second year of medical school. Talk about milestones like board exams.”
Word to the Wise
Some students may be concerned that their posts might affect a potential residency program. But the medical students interviewed say they want to find programs that align with their values and accept them for who they are.
Mr. Scott said he’s not worried about someone not liking him because of who he is. “I am Black and openly gay. If it’s a problem, I don’t need to work with you or your institution.”
Mr. Bervell stressed that medical students should stay professional online. “I reach 5-10 million people a month, and I have to think: Would I want them to see this? You have to know at all times that someone is watching. I’m very careful about how I post. I script out every video.”
Mr. Scott agreed. He advises those interested in becoming medical influencers to know what they can’t post online. For example, to ensure safety and privacy, Mr. Scott doesn’t take photos in the hospital, show his medical badge, or post patient information. “You want to be respectful of your future medical profession,” he said.
“If it’s something my mother would be ashamed of, I don’t need to post about it.”
A version of this article first appeared on Medscape.com.
A medical student’s life is an endless cycle of classes, exams, clinical rotations, and residency preparation. On TikTok and Instagram, among other sites, they share medical school experiences and lessons learned in the classroom and advocate for causes such as increased diversity and gender rights in the medical field.
This news organization caught up with a few social media influencers with a large online following to learn how medical students can effectively use social media to build a professional brand and network. Most of the students interviewed said that their social media platforms offered an opportunity to educate others about significant medical developments, feel part of a community with a like-minded audience, and network with doctors who may lead them to a future residency or career path.
Many med students said that they built their large audiences by creating a platform for people of their ethnic background, nationality, race, gender, or simply what others weren’t already talking about. They said they saw a niche in social media that was missing or others hadn’t tackled in the same way.
When Joel Bervell began med school in 2020, he questioned some of the lessons he learned about how race is used in medical practice, which didn’t make sense to him. So, he began his own research. He had about 2000 followers on Instagram at the time.
Mr. Bervell read a new study about pulse oximeters and how they often produce misleading readings on patients with dark skin.
He wondered why he hadn’t learned this in medical school, so he posted it on TikTok. Within 24 hours, about 500,000 people viewed it. Most of the comments were from doctors, nurses, and physician assistants who said they weren’t aware of the disparity.
While his initial posts detailed his journey to medical school and a day-in-the-life of a medical student, he transitioned to posts primarily about race, health equity, and what he perceives as racial bias in medicine.
Now, the fourth-year Ghanaian-American student at the Elson S. Floyd College of Medicine at Washington State University Spokane has close to 1.2 million followers on Instagram and TikTok combined. He frequently visits the White House to advise on social media’s influence on healthcare and has appeared on the Kelly Clarkson Show, Good Morning America, CNN, and ABC, among others.
He said he also uses social media to translate complex medical information for a general audience, many of whom access health information online so they can manage their own healthcare. He sees his social media work as an extension of his medical education, allowing him to delve deeper into subjects and report on them as if he were publishing research in a medical journal.
“When I came to medical school, yes, I wanted to be a doctor. But I also wanted to impact people.” Social media allows him to educate many more people than individual patients, the 29-year-old told this news organization.
Inspiring Minorities
Tabhata Paulet, 27, started her TikTok presence as a premed student in 2021. She aimed to provide free resources to help low-income, first-generation Latinx students like herself study for standardized exams.
“I always looked online for guidance and resources, and the medical influencers did not share a similar background. So, I shared my story and what I had to do as a first-generation and first person in my family to become a physician. I did not have access to the same resources as my peers,” said Ms. Paulet, who was born in Peru and came to New Jersey as a child.
Students who are Hispanic, Latinx, or of Spanish origin made up 6.8% of total medical school enrollment in 2023-2024, up slightly from 6.7% in 2022-2023, according to the Association of American Medical Colleges (AAMC).
Ms. Paulet’s online presence grew when she began documenting her experiences as a first-year medical student, bridging the language barrier for Spanish-speaking patients so they could understand their diagnosis and treatment. She often posts about health disparity and barriers to care for underserved communities.
Most of her nearly 22,000 followers are Hispanic, said the now fourth-year student at Rutgers New Jersey Medical School in Newark, New Jersey. “I talk a lot about my interesting Spanish-speaking patients ... and how sometimes speaking their native language truly makes a difference in their care.”
She believes that she serves an important role in social media. “It can be very inspirational for those who come after you [in med school] to see someone from a similar culture and upbringing.”
Creating a Community
It was during a therapy session 4 years ago that Jeremy “JP” Scott decided to share Instagram posts about his experiences as a nontraditional medical student. The 37-year-old was studying at Ross University School of Medicine in Barbados and was feeling lonely as an international medical student training to be a doctor as a second career.
Before starting med school, Mr. Scott was an adjunct professor and lab supervisor at the University of Hartford Biology Department, West Hartford, Connecticut, and then a research assistant and lab manager at the Wistar Institute in Philadelphia.
Although he wanted to follow his mother’s path to becoming a doctor, it was more difficult than he envisioned, said the fourth-year student who completed clinical rotations in the United States and is now applying for residencies.
“I talked about how medical school is not what it appears to be ... There are a lot of challenges we are going through,” especially as people of color, he said.
Mr. Scott believes social media helps people feel included and less alone. He said many of his followers are med students and physicians.
His posts often focus on LGBTQIA+ pride and being a minority as a Black man in medicine.
“The pandemic spurred a lot of us. We had a racial reckoning in our country at the time. It inspired us to talk as Black creators and Black medical students.”
Black or African American medical students made up 8.5% of total med school enrollment in 2023-2024, a slight increase from 2022 to 2023, according to AAMC figures. Black men represented 7% of total enrollment in 2023-2024, while Black women represented 9.8%.
After only a handful of online posts in which Mr. Scott candidly discussed his mental health struggles and relationships, he attracted the attention of several medical apparel companies, including the popular FIGS scrubs. He’s now an ambassador for the company, which supports him and his content.
“My association with FIGS has helped attract a wider online audience, increasing my presence.” Today, he has 14,000 Instagram followers. “It opened up so many opportunities,” Mr. Scott said. One example is working with the national LGBTQIA+ community.
“The goal was never to be a social media influencer, to gain sponsorships or photo opportunities,” he said.
“My job, first, is as a medical student. Everything else is second. I am not trying to be a professional social media personality. I’m trying to be an actual physician.” He also tries to separate JP “social media” from Jeremy, the medical student.
“On Instagram, anyone can pull it up and see what you’re doing. The last thing I want is for them to think that I’m not serious about what I’m doing, that I’m not here to learn and become a doctor.”
Benefits and Drawbacks
Ms. Paulet said her social media following helped her connect with leaders in the Latinx medical community, including an obstetrics anesthesiologist, her intended specialty. “I don’t think I’d be able to do that without a social media platform.”
Her online activity also propelled her from regional to national leadership in the Latino Medical Student Association (LMSA). She now also runs their Instagram page, which has 14,000 followers.
Mr. Bervell believes social media is a great way to network. He’s connected with people he wouldn’t have met otherwise, including physicians. “I think it will help me get into a residency,” he said. “It allows people to know who you are ... They will be able to tell in a few videos the type of doctor I want to be.”
On the other hand, Mr. Bervell is aware of the negative impacts of social media on mental health. “You can get lost in social media.” For that reason, he often tries to disconnect. “I can go days without my phone.”
Posting on social media can be time-consuming, Mr. Bervell admitted. He said he spent about 2 hours a day researching, editing, and posting on TikTok when he first started building his following. Now, he spends about 2-3 hours a week creating videos. “I don’t post every day anymore. I don’t have the time.”
When she started building her TikTok presence, Ms. Paulet said she devoted 15 hours a week to the endeavor, but now she spends 10-12 hours a week posting online, including on LMSA’s Instagram page. “Whenever you are done with an exam or have a study break, this is something fun to do.” She also says you never know who you’re going to inspire when you put yourself out there.
“Talk about your journey, rotations, or your experience in your first or second year of medical school. Talk about milestones like board exams.”
Word to the Wise
Some students may be concerned that their posts might affect a potential residency program. But the medical students interviewed say they want to find programs that align with their values and accept them for who they are.
Mr. Scott said he’s not worried about someone not liking him because of who he is. “I am Black and openly gay. If it’s a problem, I don’t need to work with you or your institution.”
Mr. Bervell stressed that medical students should stay professional online. “I reach 5-10 million people a month, and I have to think: Would I want them to see this? You have to know at all times that someone is watching. I’m very careful about how I post. I script out every video.”
Mr. Scott agreed. He advises those interested in becoming medical influencers to know what they can’t post online. For example, to ensure safety and privacy, Mr. Scott doesn’t take photos in the hospital, show his medical badge, or post patient information. “You want to be respectful of your future medical profession,” he said.
“If it’s something my mother would be ashamed of, I don’t need to post about it.”
A version of this article first appeared on Medscape.com.
COVID-19 Booster Vaccine Shortens Menstrual Cycles in Teens
TOPLINE:
The vaccine did not appear to be associated with shifts in menstrual flow, pain, or other symptoms.
METHODOLOGY:
- Reports of menstrual cycle changes following the COVID-19 vaccination began to emerge in early 2021, raising concerns about the impact of the vaccine on menstrual health.
- Researchers conducted a prospective study including 65 adolescent girls (mean age, 17.3 years), of whom 47 had received an initial series of COVID-19 vaccination at least 6 months prior to receiving a booster dose (booster group), and 18 had not received the booster vaccine (control group), two of whom had never received any COVID-19 vaccine, four who had received an initial vaccine but not a booster, and 12 who had received an initial vaccine and booster but more than 6 months prior to the study.
- Menstrual cycle length was measured for three cycles prior to and four cycles after vaccination in the booster group and for seven cycles in the control group.
- Menstrual flow, pain, and stress were measured at baseline and monthly for 3 months post vaccination.
TAKEAWAY:
- Participants in the booster group experienced shorter cycles by an average of 5.35 days after receiving the COVID-19 booster vaccine (P = .03), particularly during the second cycle. In contrast, those in the control group did not experience any changes in the menstrual cycle length.
- Receiving the booster dose in the follicular phase was associated with significantly shorter menstrual cycles, compared with pre-booster cycles (P = .0157).
- Menstrual flow, pain, and other symptoms remained unaffected after the COVID-19 booster vaccination.
- Higher stress levels at baseline were also associated with a shorter length of the menstrual cycle (P = .03) in both groups, regardless of the booster vaccination status.
IN PRACTICE:
“These data are potentially important for counseling parents regarding potential vaccine refusal in the future for their teen daughters,” the authors wrote.
SOURCE:
This study was led by Laura A. Payne, PhD, from McLean Hospital in Boston, and was published online in the Journal of Adolescent Health.
LIMITATIONS:
The sample size for the booster and control groups was relatively small and homogeneous. The study did not include the height, weight, birth control use, or other chronic conditions of the participants, which may have influenced the functioning of the menstrual cycle. The control group included a majority of teens who had previously received a vaccine and even a booster, which could have affected results.
DISCLOSURES:
This study was supported by grants from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Some authors received consulting fees, travel reimbursements, honoraria, research funding, and royalties from Bayer Healthcare, Mahana Therapeutics, Gates, and Merck, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The vaccine did not appear to be associated with shifts in menstrual flow, pain, or other symptoms.
METHODOLOGY:
- Reports of menstrual cycle changes following the COVID-19 vaccination began to emerge in early 2021, raising concerns about the impact of the vaccine on menstrual health.
- Researchers conducted a prospective study including 65 adolescent girls (mean age, 17.3 years), of whom 47 had received an initial series of COVID-19 vaccination at least 6 months prior to receiving a booster dose (booster group), and 18 had not received the booster vaccine (control group), two of whom had never received any COVID-19 vaccine, four who had received an initial vaccine but not a booster, and 12 who had received an initial vaccine and booster but more than 6 months prior to the study.
- Menstrual cycle length was measured for three cycles prior to and four cycles after vaccination in the booster group and for seven cycles in the control group.
- Menstrual flow, pain, and stress were measured at baseline and monthly for 3 months post vaccination.
TAKEAWAY:
- Participants in the booster group experienced shorter cycles by an average of 5.35 days after receiving the COVID-19 booster vaccine (P = .03), particularly during the second cycle. In contrast, those in the control group did not experience any changes in the menstrual cycle length.
- Receiving the booster dose in the follicular phase was associated with significantly shorter menstrual cycles, compared with pre-booster cycles (P = .0157).
- Menstrual flow, pain, and other symptoms remained unaffected after the COVID-19 booster vaccination.
- Higher stress levels at baseline were also associated with a shorter length of the menstrual cycle (P = .03) in both groups, regardless of the booster vaccination status.
IN PRACTICE:
“These data are potentially important for counseling parents regarding potential vaccine refusal in the future for their teen daughters,” the authors wrote.
SOURCE:
This study was led by Laura A. Payne, PhD, from McLean Hospital in Boston, and was published online in the Journal of Adolescent Health.
LIMITATIONS:
The sample size for the booster and control groups was relatively small and homogeneous. The study did not include the height, weight, birth control use, or other chronic conditions of the participants, which may have influenced the functioning of the menstrual cycle. The control group included a majority of teens who had previously received a vaccine and even a booster, which could have affected results.
DISCLOSURES:
This study was supported by grants from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Some authors received consulting fees, travel reimbursements, honoraria, research funding, and royalties from Bayer Healthcare, Mahana Therapeutics, Gates, and Merck, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
The vaccine did not appear to be associated with shifts in menstrual flow, pain, or other symptoms.
METHODOLOGY:
- Reports of menstrual cycle changes following the COVID-19 vaccination began to emerge in early 2021, raising concerns about the impact of the vaccine on menstrual health.
- Researchers conducted a prospective study including 65 adolescent girls (mean age, 17.3 years), of whom 47 had received an initial series of COVID-19 vaccination at least 6 months prior to receiving a booster dose (booster group), and 18 had not received the booster vaccine (control group), two of whom had never received any COVID-19 vaccine, four who had received an initial vaccine but not a booster, and 12 who had received an initial vaccine and booster but more than 6 months prior to the study.
- Menstrual cycle length was measured for three cycles prior to and four cycles after vaccination in the booster group and for seven cycles in the control group.
- Menstrual flow, pain, and stress were measured at baseline and monthly for 3 months post vaccination.
TAKEAWAY:
- Participants in the booster group experienced shorter cycles by an average of 5.35 days after receiving the COVID-19 booster vaccine (P = .03), particularly during the second cycle. In contrast, those in the control group did not experience any changes in the menstrual cycle length.
- Receiving the booster dose in the follicular phase was associated with significantly shorter menstrual cycles, compared with pre-booster cycles (P = .0157).
- Menstrual flow, pain, and other symptoms remained unaffected after the COVID-19 booster vaccination.
- Higher stress levels at baseline were also associated with a shorter length of the menstrual cycle (P = .03) in both groups, regardless of the booster vaccination status.
IN PRACTICE:
“These data are potentially important for counseling parents regarding potential vaccine refusal in the future for their teen daughters,” the authors wrote.
SOURCE:
This study was led by Laura A. Payne, PhD, from McLean Hospital in Boston, and was published online in the Journal of Adolescent Health.
LIMITATIONS:
The sample size for the booster and control groups was relatively small and homogeneous. The study did not include the height, weight, birth control use, or other chronic conditions of the participants, which may have influenced the functioning of the menstrual cycle. The control group included a majority of teens who had previously received a vaccine and even a booster, which could have affected results.
DISCLOSURES:
This study was supported by grants from the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Some authors received consulting fees, travel reimbursements, honoraria, research funding, and royalties from Bayer Healthcare, Mahana Therapeutics, Gates, and Merck, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The Battle Against Recurrent UTIs in Welsh Women
TOPLINE:
The prevalence of recurrent urinary tract infections (rUTIs) and the use of antibiotics for prevention are substantial among women in Wales, particularly among those over the age of 57 years. A high level of resistance to two recommended antibiotics was observed, suggesting that more frequent urine cultures could better guide antibiotic selection for treatment and prophylaxis.
METHODOLOGY:
- The researchers conducted a retrospective cross-sectional study using a large databank of patients in Wales to describe the characteristics and urine profiles of women with rUTIs between 2010 and 2022.
- They created two cohorts: One with 92,213 women (median age, 60 years) who experienced rUTIs, defined as two or more acute episodes within 6 months or three or more acute episodes within 12 months.
- Another cohort comprised of 26,862 women (median age, 71 years) were prescribed prophylactic antibiotics, which was defined as receiving three or more consecutive prescriptions of the same UTI-specific antibiotic (trimethoprim, nitrofurantoin, or cefalexin), with intervals of 21-56 days between prescriptions.
- Urine culture results in the 12 months before a rUTI diagnosis and 18 months before prophylactic antibiotic initiation and all urine culture results within 7 days of an acute UTI were analyzed to assess antibiotic resistance patterns.
TAKEAWAY:
- Overall, 6% of women had rUTIs, 1.7% of which were prescribed prophylactic antibiotics with proportions increasing sharply after age 57.
- Nearly half of the women (49%) who were prescribed a prophylactic antibiotic qualified as having rUTIs in the 18 months before initiation.
- This study showed that 80.8% of women with rUTIs had a urine culture result documented in the 12 months preceding the diagnosis.
- More than half (64%) of the women taking prophylactic antibiotics had a urine culture result documented before starting treatment, and 18% of those prescribed trimethoprim had resistance to the antibiotic.
IN PRACTICE:
“More frequent urine cultures in the workup of rUTI diagnosis and prophylactic antibiotic initiation could better inform antibiotic choice,” the authors wrote.
SOURCE:
The study was led by Leigh Sanyaolu, BSc (Hons), MRCS, MRCGP, PGDip, a general practitioner from the Division of Population Medicine and PRIME Centre Wales at Cardiff University in Cardiff, and was published online in the British Journal of General Practice.
LIMITATIONS:
The study’s reliance on electronic health records may have led to coding errors and missing data. The diagnosis of UTIs may have been difficult in older women with increased frailty as they can have fewer specific symptoms and asymptomatic bacteriuria, which can be misdiagnosed as a UTI.
DISCLOSURES:
This work was supported by Health and Care Research Wales. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of recurrent urinary tract infections (rUTIs) and the use of antibiotics for prevention are substantial among women in Wales, particularly among those over the age of 57 years. A high level of resistance to two recommended antibiotics was observed, suggesting that more frequent urine cultures could better guide antibiotic selection for treatment and prophylaxis.
METHODOLOGY:
- The researchers conducted a retrospective cross-sectional study using a large databank of patients in Wales to describe the characteristics and urine profiles of women with rUTIs between 2010 and 2022.
- They created two cohorts: One with 92,213 women (median age, 60 years) who experienced rUTIs, defined as two or more acute episodes within 6 months or three or more acute episodes within 12 months.
- Another cohort comprised of 26,862 women (median age, 71 years) were prescribed prophylactic antibiotics, which was defined as receiving three or more consecutive prescriptions of the same UTI-specific antibiotic (trimethoprim, nitrofurantoin, or cefalexin), with intervals of 21-56 days between prescriptions.
- Urine culture results in the 12 months before a rUTI diagnosis and 18 months before prophylactic antibiotic initiation and all urine culture results within 7 days of an acute UTI were analyzed to assess antibiotic resistance patterns.
TAKEAWAY:
- Overall, 6% of women had rUTIs, 1.7% of which were prescribed prophylactic antibiotics with proportions increasing sharply after age 57.
- Nearly half of the women (49%) who were prescribed a prophylactic antibiotic qualified as having rUTIs in the 18 months before initiation.
- This study showed that 80.8% of women with rUTIs had a urine culture result documented in the 12 months preceding the diagnosis.
- More than half (64%) of the women taking prophylactic antibiotics had a urine culture result documented before starting treatment, and 18% of those prescribed trimethoprim had resistance to the antibiotic.
IN PRACTICE:
“More frequent urine cultures in the workup of rUTI diagnosis and prophylactic antibiotic initiation could better inform antibiotic choice,” the authors wrote.
SOURCE:
The study was led by Leigh Sanyaolu, BSc (Hons), MRCS, MRCGP, PGDip, a general practitioner from the Division of Population Medicine and PRIME Centre Wales at Cardiff University in Cardiff, and was published online in the British Journal of General Practice.
LIMITATIONS:
The study’s reliance on electronic health records may have led to coding errors and missing data. The diagnosis of UTIs may have been difficult in older women with increased frailty as they can have fewer specific symptoms and asymptomatic bacteriuria, which can be misdiagnosed as a UTI.
DISCLOSURES:
This work was supported by Health and Care Research Wales. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of recurrent urinary tract infections (rUTIs) and the use of antibiotics for prevention are substantial among women in Wales, particularly among those over the age of 57 years. A high level of resistance to two recommended antibiotics was observed, suggesting that more frequent urine cultures could better guide antibiotic selection for treatment and prophylaxis.
METHODOLOGY:
- The researchers conducted a retrospective cross-sectional study using a large databank of patients in Wales to describe the characteristics and urine profiles of women with rUTIs between 2010 and 2022.
- They created two cohorts: One with 92,213 women (median age, 60 years) who experienced rUTIs, defined as two or more acute episodes within 6 months or three or more acute episodes within 12 months.
- Another cohort comprised of 26,862 women (median age, 71 years) were prescribed prophylactic antibiotics, which was defined as receiving three or more consecutive prescriptions of the same UTI-specific antibiotic (trimethoprim, nitrofurantoin, or cefalexin), with intervals of 21-56 days between prescriptions.
- Urine culture results in the 12 months before a rUTI diagnosis and 18 months before prophylactic antibiotic initiation and all urine culture results within 7 days of an acute UTI were analyzed to assess antibiotic resistance patterns.
TAKEAWAY:
- Overall, 6% of women had rUTIs, 1.7% of which were prescribed prophylactic antibiotics with proportions increasing sharply after age 57.
- Nearly half of the women (49%) who were prescribed a prophylactic antibiotic qualified as having rUTIs in the 18 months before initiation.
- This study showed that 80.8% of women with rUTIs had a urine culture result documented in the 12 months preceding the diagnosis.
- More than half (64%) of the women taking prophylactic antibiotics had a urine culture result documented before starting treatment, and 18% of those prescribed trimethoprim had resistance to the antibiotic.
IN PRACTICE:
“More frequent urine cultures in the workup of rUTI diagnosis and prophylactic antibiotic initiation could better inform antibiotic choice,” the authors wrote.
SOURCE:
The study was led by Leigh Sanyaolu, BSc (Hons), MRCS, MRCGP, PGDip, a general practitioner from the Division of Population Medicine and PRIME Centre Wales at Cardiff University in Cardiff, and was published online in the British Journal of General Practice.
LIMITATIONS:
The study’s reliance on electronic health records may have led to coding errors and missing data. The diagnosis of UTIs may have been difficult in older women with increased frailty as they can have fewer specific symptoms and asymptomatic bacteriuria, which can be misdiagnosed as a UTI.
DISCLOSURES:
This work was supported by Health and Care Research Wales. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
The New Formula for Stronger, Longer-Lasting Vaccines
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
Vaccines work pretty well. But with a little help, they could work better.
Stanford researchers have developed a new vaccine helper that combines two kinds of adjuvants, ingredients that improve a vaccine’s efficacy, in a novel, customizable system.
In lab tests, the experimental additive improved the effectiveness of COVID-19 and HIV vaccine candidates, though it could be adapted to stimulate immune responses to a variety of pathogens, the researchers said. It could also be used one day to fine-tune vaccines for vulnerable groups like young children, older adults, and those with compromised immune systems.
“Current vaccines are not perfect,” said lead study author Ben Ou, a PhD candidate and researcher in the lab of Eric Appel, PhD, an associate professor of materials science and engineering, at Stanford University in California. “Many fail to generate long-lasting immunity or immunity against closely related strains [such as] flu or COVID vaccines. One way to improve them is to design more potent vaccine adjuvants.”
The study marks an advance in an area of growing scientific interest: Combining different adjuvants to enhance the immune-stimulating effect.
The Stanford scientists developed sphere-shaped nanoparticles, like tiny round cages, made of saponins, immune-stimulating molecules common in adjuvant development. To these nanoparticles, they attached Toll-like receptor (TLR) agonists, molecules that have become a focus in vaccine research because they stimulate a variety of immune responses.
Dr. Ou and the team tested the new adjuvant platform in COVID and HIV vaccines, comparing it to vaccines containing alum, a widely used adjuvant. (Alum is not used in COVID vaccines available in the United States.)
The nanoparticle-adjuvanted vaccines triggered stronger, longer-lasting effects.
Notably, the combination of the new adjuvant system with a SARS-CoV-2 virus vaccine was effective in mice against the original SARS-CoV-2 virus and against Delta, Omicron, and other variants that emerged in the months and years after the initial outbreak.
“Since our nanoparticle adjuvant platform is more potent than traditional/clinical vaccine adjuvants,” Dr. Ou said, “we expected mice to produce broadly neutralizing antibodies and better breadth responses.”
100 Years of Adjuvants
The first vaccine adjuvants were aluminum salts mixed into shots against pertussis, diphtheria, and tetanus in the 1920s. Today, alum is still used in many vaccines, including shots for diphtheria, tetanus, and pertussis; hepatitis A and B; human papillomavirus; and pneumococcal disease.
But since the 1990s, new adjuvants have come on the scene. Saponin-based compounds, harvested from the soapbark tree, are used in the Novavax COVID-19 Vaccine, Adjuvanted; a synthetic DNA adjuvant in the Heplisav-B vaccine against hepatitis B; and oil in water adjuvants using squalene in the Fluad and Fluad Quadrivalent influenza vaccines. Other vaccines, including those for chickenpox, cholera, measles, mumps, rubella, and mRNA-based COVID vaccines from Pfizer-BioNTech and Moderna, don’t contain adjuvants.
TLR agonists have recently become research hotspots in vaccine science.
“TLR agonists activate the innate immune system, putting it on a heightened alert state that can result in a higher antibody production and longer-lasting protection,” said David Burkhart, PhD, a research professor in biomedical and pharmaceutical sciences at the University of Montana in Missoula. He is also the chief operating officer of Inimmune, a biotech company developing vaccines and immunotherapies.
Dr. Burkhart studies TLR agonists in vaccines and other applications. “Different combinations activate different parts of the immune system,” he said. “TLR4 might activate the army, while TLR7 might activate the air force. You might need both in one vaccine.”
TLR agonists have also shown promise against Alzheimer’s disease, allergies, cancer, and even addiction. In immune’s experimental immunotherapy using TLR agonists for advanced solid tumors has just entered human trials, and the company is looking at a TLR agonist therapy for allergic rhinitis.
Combining Forces
In the new study, researchers tested five different combinations of TLR agonists hooked to the saponin nanoparticle framework. Each elicited a slightly different response from the immune cells.
“Our immune systems generate different downstream immune responses based on which TLRs are activated,” Dr. Ou said.
Ultimately, the advance could spur the development of vaccines tuned for stronger immune protection.
“We need different immune responses to fight different types of pathogens,” Dr. Ou said. “Depending on what specific virus/disease the vaccine is formulated for, activation of one specific TLR may confer better protection than another TLR.”
According to Dr. Burkhart, combining a saponin with a TLR agonist has found success before.
Biopharma company GSK (formerly GlaxoSmithKline) used the combination in its AS01 adjuvant, in the vaccine Shingrix against herpes zoster. The live-attenuated yellow fever vaccine, given to more than 600 million people around the world and considered one of the most powerful vaccines ever developed, uses several TLR agonists.
The Stanford paper, Dr. Burkhart said, “is a nice demonstration of the enhanced efficacy [that] adjuvants can provide to vaccines by exploiting the synergy different adjuvants and TLR agonists can provide when used in combination.”
Tailoring Vaccines
The customizable aspect of TLR agonists is important too, Dr. Burkhart said.
“The human immune system changes dramatically from birth to childhood into adulthood into older maturity,” he said. “It’s not a one-size-fits-all. Vaccines need to be tailored to these populations for maximum effectiveness and safety. TLRAs [TLR agonists] are a highly valuable tool in the vaccine toolbox. I think it’s inevitable we’ll have more in the future.”
That’s what the Stanford researchers hope for.
They noted in the study that the nanoparticle platform could easily be used to test different TLR agonist adjuvant combinations in vaccines.
But human studies are still a ways off. Tests in larger animals would likely come next, Dr. Ou said.
“We now have a single nanoparticle adjuvant platform with formulations containing different TLRs,” Dr. Ou said. “Scientists can pick which specific formulation is the most suitable for their needs.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
The Next Frontier of Antibiotic Discovery: Inside Your Gut
Scientists at Stanford University and the University of Pennsylvania have discovered a new antibiotic candidate in a surprising place: the human gut.
In mice, the antibiotic — a peptide known as prevotellin-2 — showed antimicrobial potency on par with polymyxin B, an antibiotic medication used to treat multidrug-resistant infections. Meanwhile, the peptide mainly left commensal, or beneficial, bacteria alone. The study, published in Cell, also identified several other potent antibiotic peptides with the potential to combat antimicrobial-resistant infections.
The research is part of a larger quest to find new antibiotics that can fight drug-resistant infections, a critical public health threat with more than 2.8 million cases and 35,000 deaths annually in the United States. That quest is urgent, said study author César de la Fuente, PhD, professor of bioengineering at the University of Pennsylvania, Philadelphia.
“The main pillars that have enabled us to almost double our lifespan in the last 100 years or so have been antibiotics, vaccines, and clean water,” said Dr. de la Fuente. “Imagine taking out one of those. I think it would be pretty dramatic.” (Dr. De la Fuente’s lab has become known for finding antibiotic candidates in unusual places, like ancient genetic information of Neanderthals and woolly mammoths.)
The first widely used antibiotic, penicillin, was discovered in 1928, when a physician studying Staphylococcus bacteria returned to his lab after summer break to find mold growing in one of his petri dishes. But many other antibiotics — like streptomycin, tetracycline, and erythromycin — were discovered from soil bacteria, which produce variations of these substances to compete with other microorganisms.
By looking in the gut microbiome, the researchers hoped to identify peptides that the trillions of microbes use against each other in the fight for limited resources — ideally, peptides that wouldn’t broadly kill off the entire microbiome.
Kill the Bad, Spare the Good
Many traditional antibiotics are small molecules. This means they can wipe out the good bacteria in your body, and because each targets a specific bacterial function, bad bacteria can become resistant to them.
Peptide antibiotics, on the other hand, don’t diffuse into the whole body. If taken orally, they stay in the gut; if taken intravenously, they generally stay in the blood. And because of how they kill bacteria, targeting the membrane, they’re also less prone to bacterial resistance.
The microbiome is like a big reservoir of pathogens, said Ami Bhatt, MD, PhD, hematologist at Stanford University in California and one of the study’s authors. Because many antibiotics kill healthy gut bacteria, “what you have left over,” Dr. Bhatt said, “is this big open niche that gets filled up with multidrug-resistant organisms like E coli [Escherichia coli] or vancomycin-resistant Enterococcus.”
Dr. Bhatt has seen cancer patients undergo successful treatment only to die of a multidrug-resistant infection, because current antibiotics fail against those pathogens. “That’s like winning the battle to lose the war.”
By investigating the microbiome, “we wanted to see if we could identify antimicrobial peptides that might spare key members of our regular microbiome, so that we wouldn’t totally disrupt the microbiome the way we do when we use broad-spectrum, small molecule–based antibiotics,” Dr. Bhatt said.
The researchers used artificial intelligence to sift through 400,000 proteins to predict, based on known antibiotics, which peptide sequences might have antimicrobial properties. From the results, they chose 78 peptides to synthesize and test.
“The application of computational approaches combined with experimental validation is very powerful and exciting,” said Jennifer Geddes-McAlister, PhD, professor of cell biology at the University of Guelph in Ontario, Canada, who was not involved in the study. “The study is robust in its approach to microbiome sampling.”
The Long Journey from Lab to Clinic
More than half of the peptides the team tested effectively inhibited the growth of harmful bacteria, and prevotellin-2 (derived from the bacteria Prevotella copri)stood out as the most powerful.
“The study validates experimental data from the lab using animal models, which moves discoveries closer to the clinic,” said Dr. Geddes-McAlister. “Further testing with clinical trials is needed, but the potential for clinical application is promising.”
Unfortunately, that’s not likely to happen anytime soon, said Dr. de la Fuente. “There is not enough economic incentive” for companies to develop new antibiotics. Ten years is his most hopeful guess for when we might see prevotellin-2, or a similar antibiotic, complete clinical trials.
A version of this article first appeared on Medscape.com.
Scientists at Stanford University and the University of Pennsylvania have discovered a new antibiotic candidate in a surprising place: the human gut.
In mice, the antibiotic — a peptide known as prevotellin-2 — showed antimicrobial potency on par with polymyxin B, an antibiotic medication used to treat multidrug-resistant infections. Meanwhile, the peptide mainly left commensal, or beneficial, bacteria alone. The study, published in Cell, also identified several other potent antibiotic peptides with the potential to combat antimicrobial-resistant infections.
The research is part of a larger quest to find new antibiotics that can fight drug-resistant infections, a critical public health threat with more than 2.8 million cases and 35,000 deaths annually in the United States. That quest is urgent, said study author César de la Fuente, PhD, professor of bioengineering at the University of Pennsylvania, Philadelphia.
“The main pillars that have enabled us to almost double our lifespan in the last 100 years or so have been antibiotics, vaccines, and clean water,” said Dr. de la Fuente. “Imagine taking out one of those. I think it would be pretty dramatic.” (Dr. De la Fuente’s lab has become known for finding antibiotic candidates in unusual places, like ancient genetic information of Neanderthals and woolly mammoths.)
The first widely used antibiotic, penicillin, was discovered in 1928, when a physician studying Staphylococcus bacteria returned to his lab after summer break to find mold growing in one of his petri dishes. But many other antibiotics — like streptomycin, tetracycline, and erythromycin — were discovered from soil bacteria, which produce variations of these substances to compete with other microorganisms.
By looking in the gut microbiome, the researchers hoped to identify peptides that the trillions of microbes use against each other in the fight for limited resources — ideally, peptides that wouldn’t broadly kill off the entire microbiome.
Kill the Bad, Spare the Good
Many traditional antibiotics are small molecules. This means they can wipe out the good bacteria in your body, and because each targets a specific bacterial function, bad bacteria can become resistant to them.
Peptide antibiotics, on the other hand, don’t diffuse into the whole body. If taken orally, they stay in the gut; if taken intravenously, they generally stay in the blood. And because of how they kill bacteria, targeting the membrane, they’re also less prone to bacterial resistance.
The microbiome is like a big reservoir of pathogens, said Ami Bhatt, MD, PhD, hematologist at Stanford University in California and one of the study’s authors. Because many antibiotics kill healthy gut bacteria, “what you have left over,” Dr. Bhatt said, “is this big open niche that gets filled up with multidrug-resistant organisms like E coli [Escherichia coli] or vancomycin-resistant Enterococcus.”
Dr. Bhatt has seen cancer patients undergo successful treatment only to die of a multidrug-resistant infection, because current antibiotics fail against those pathogens. “That’s like winning the battle to lose the war.”
By investigating the microbiome, “we wanted to see if we could identify antimicrobial peptides that might spare key members of our regular microbiome, so that we wouldn’t totally disrupt the microbiome the way we do when we use broad-spectrum, small molecule–based antibiotics,” Dr. Bhatt said.
The researchers used artificial intelligence to sift through 400,000 proteins to predict, based on known antibiotics, which peptide sequences might have antimicrobial properties. From the results, they chose 78 peptides to synthesize and test.
“The application of computational approaches combined with experimental validation is very powerful and exciting,” said Jennifer Geddes-McAlister, PhD, professor of cell biology at the University of Guelph in Ontario, Canada, who was not involved in the study. “The study is robust in its approach to microbiome sampling.”
The Long Journey from Lab to Clinic
More than half of the peptides the team tested effectively inhibited the growth of harmful bacteria, and prevotellin-2 (derived from the bacteria Prevotella copri)stood out as the most powerful.
“The study validates experimental data from the lab using animal models, which moves discoveries closer to the clinic,” said Dr. Geddes-McAlister. “Further testing with clinical trials is needed, but the potential for clinical application is promising.”
Unfortunately, that’s not likely to happen anytime soon, said Dr. de la Fuente. “There is not enough economic incentive” for companies to develop new antibiotics. Ten years is his most hopeful guess for when we might see prevotellin-2, or a similar antibiotic, complete clinical trials.
A version of this article first appeared on Medscape.com.
Scientists at Stanford University and the University of Pennsylvania have discovered a new antibiotic candidate in a surprising place: the human gut.
In mice, the antibiotic — a peptide known as prevotellin-2 — showed antimicrobial potency on par with polymyxin B, an antibiotic medication used to treat multidrug-resistant infections. Meanwhile, the peptide mainly left commensal, or beneficial, bacteria alone. The study, published in Cell, also identified several other potent antibiotic peptides with the potential to combat antimicrobial-resistant infections.
The research is part of a larger quest to find new antibiotics that can fight drug-resistant infections, a critical public health threat with more than 2.8 million cases and 35,000 deaths annually in the United States. That quest is urgent, said study author César de la Fuente, PhD, professor of bioengineering at the University of Pennsylvania, Philadelphia.
“The main pillars that have enabled us to almost double our lifespan in the last 100 years or so have been antibiotics, vaccines, and clean water,” said Dr. de la Fuente. “Imagine taking out one of those. I think it would be pretty dramatic.” (Dr. De la Fuente’s lab has become known for finding antibiotic candidates in unusual places, like ancient genetic information of Neanderthals and woolly mammoths.)
The first widely used antibiotic, penicillin, was discovered in 1928, when a physician studying Staphylococcus bacteria returned to his lab after summer break to find mold growing in one of his petri dishes. But many other antibiotics — like streptomycin, tetracycline, and erythromycin — were discovered from soil bacteria, which produce variations of these substances to compete with other microorganisms.
By looking in the gut microbiome, the researchers hoped to identify peptides that the trillions of microbes use against each other in the fight for limited resources — ideally, peptides that wouldn’t broadly kill off the entire microbiome.
Kill the Bad, Spare the Good
Many traditional antibiotics are small molecules. This means they can wipe out the good bacteria in your body, and because each targets a specific bacterial function, bad bacteria can become resistant to them.
Peptide antibiotics, on the other hand, don’t diffuse into the whole body. If taken orally, they stay in the gut; if taken intravenously, they generally stay in the blood. And because of how they kill bacteria, targeting the membrane, they’re also less prone to bacterial resistance.
The microbiome is like a big reservoir of pathogens, said Ami Bhatt, MD, PhD, hematologist at Stanford University in California and one of the study’s authors. Because many antibiotics kill healthy gut bacteria, “what you have left over,” Dr. Bhatt said, “is this big open niche that gets filled up with multidrug-resistant organisms like E coli [Escherichia coli] or vancomycin-resistant Enterococcus.”
Dr. Bhatt has seen cancer patients undergo successful treatment only to die of a multidrug-resistant infection, because current antibiotics fail against those pathogens. “That’s like winning the battle to lose the war.”
By investigating the microbiome, “we wanted to see if we could identify antimicrobial peptides that might spare key members of our regular microbiome, so that we wouldn’t totally disrupt the microbiome the way we do when we use broad-spectrum, small molecule–based antibiotics,” Dr. Bhatt said.
The researchers used artificial intelligence to sift through 400,000 proteins to predict, based on known antibiotics, which peptide sequences might have antimicrobial properties. From the results, they chose 78 peptides to synthesize and test.
“The application of computational approaches combined with experimental validation is very powerful and exciting,” said Jennifer Geddes-McAlister, PhD, professor of cell biology at the University of Guelph in Ontario, Canada, who was not involved in the study. “The study is robust in its approach to microbiome sampling.”
The Long Journey from Lab to Clinic
More than half of the peptides the team tested effectively inhibited the growth of harmful bacteria, and prevotellin-2 (derived from the bacteria Prevotella copri)stood out as the most powerful.
“The study validates experimental data from the lab using animal models, which moves discoveries closer to the clinic,” said Dr. Geddes-McAlister. “Further testing with clinical trials is needed, but the potential for clinical application is promising.”
Unfortunately, that’s not likely to happen anytime soon, said Dr. de la Fuente. “There is not enough economic incentive” for companies to develop new antibiotics. Ten years is his most hopeful guess for when we might see prevotellin-2, or a similar antibiotic, complete clinical trials.
A version of this article first appeared on Medscape.com.
FROM CELL
Physicians Lament Over Reliance on Relative Value Units: Survey
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
PrEP Prescription Pickups Vary With Prescriber Specialty
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
FROM JAMA INTERNAL MEDICINE
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6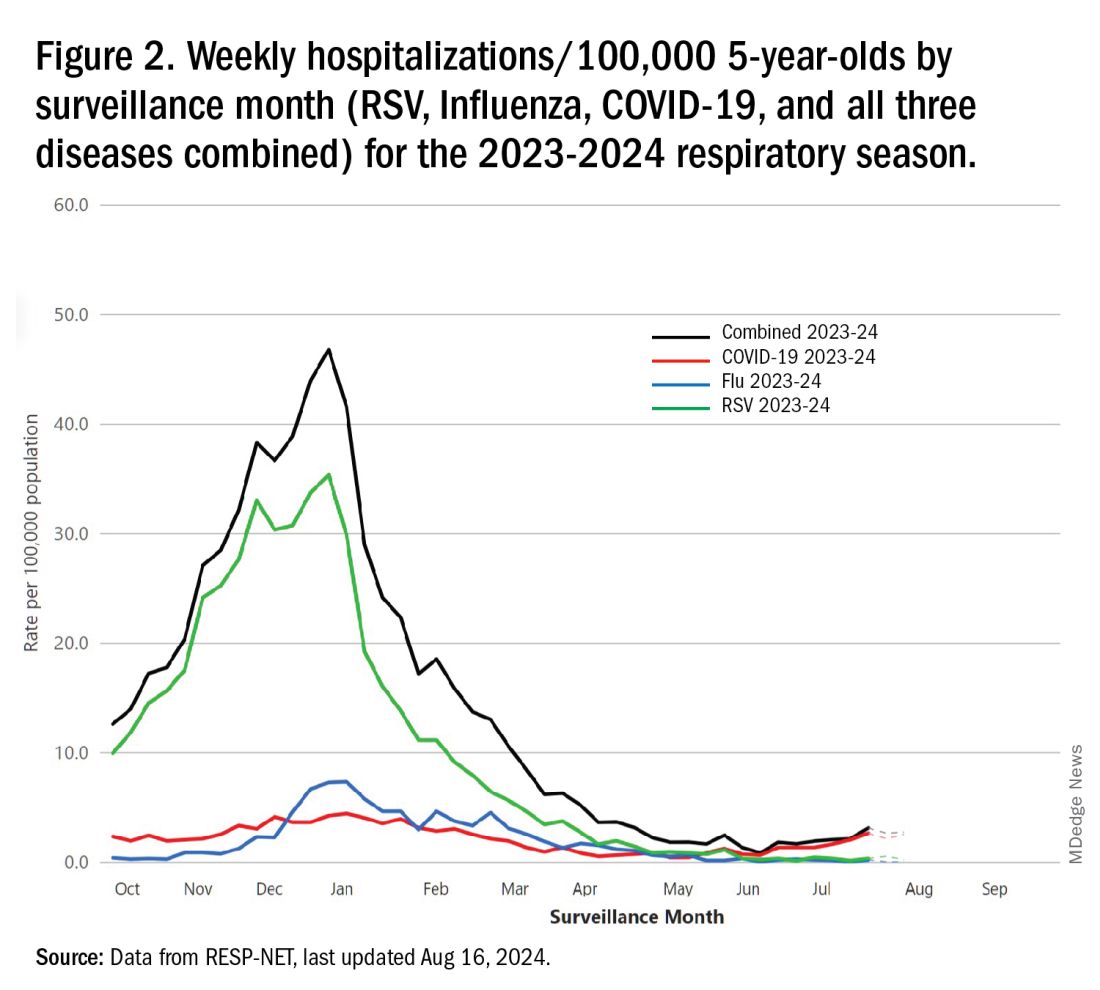
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 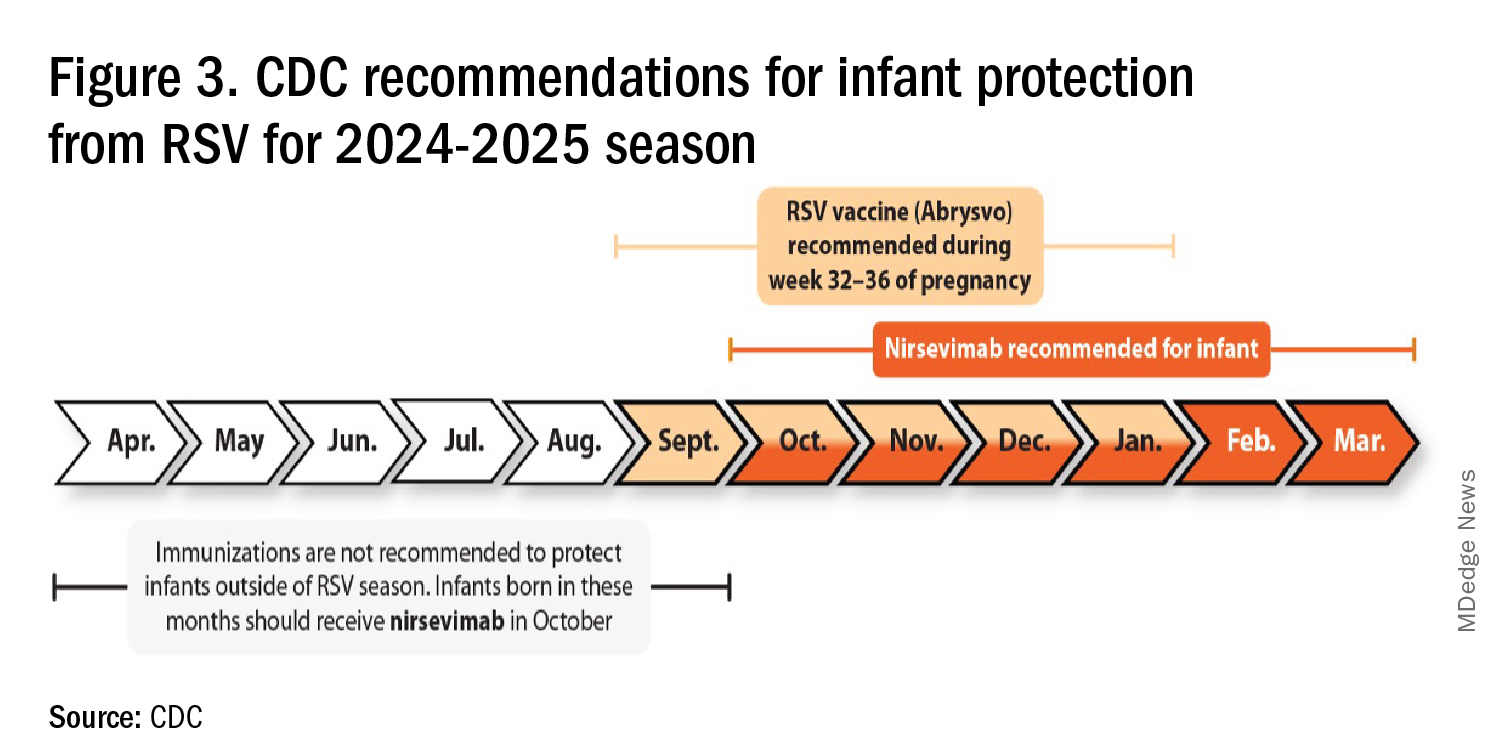
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.