User login
Oral bacteria linked with pancreatic cancer
Two species of oral bacteria – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – have been linked with an elevated risk of developing pancreatic cancer. This follows previous studies linking poor oral health in general to an increased risk of pancreatic cancer.
“Each [species] is associated with more than 50% higher likelihood to develop pancreatic cancer,” Jiyoung Ahn, Ph.D., of New York University Langone Medical Center, said during a news conference at the annual meeting of the American Association for Cancer Research.
“The findings suggest that carriage of these bacteria is related to subsequent development of pancreatic cancer,” said Dr. Ahn. Given the poor survival rate for pancreatic cancer of 5% at 5 years, identifying risk factors for its development may have implications for screening tools and prevention if the associations are found to be causal.
Employing a prospective, nested case-control study design, Dr. Ahn used samples and data from the Cancer Prevention Study II and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohorts. Both of those studies enrolled healthy people and followed them for several years looking for a variety of outcomes, including cancer. Researchers performed genomic analysis on oral wash samples collected at the beginning of the studies to identify the bacterial species present. Samples from 361 people who eventually developed pancreatic cancer were matched to 371 controls.
After adjustment for covariates of age, race, sex, smoking status, alcohol consumption, body mass index, and diabetes, logistic regression analysis showed that the presence of P. gingivalis in the samples was associated with a 59% increased risk (adjusted odds ratio [OR] = 1.59; 95% confidence interval [CI] 1.15, 2.20) of developing pancreatic cancer, and the presence of A. actinomycetemcomitans was associated with a 119% increased risk (OR = 2.19; 95% CI 1.15, 4.15). The presence of these bacteria remained a risk factor even after the deletion of samples from people who developed pancreatic cancer within the 2 years after collection of their samples to eliminate the possibility that early pancreatic cancers were affecting the populations of bacteria present.
Greater relative abundance of Fusobacteria species was associated with a lower risk (OR per percent increased abundance = 0.92; 95% CI 0.87, 0.98).
“Why this is important is because this is the first evidence suggesting the oral bacteria and pancreatic cancer relationship,” Dr. Ahn said, adding that she foresees eventual clinical applications. “In the long run, knowing these bacteria, we can see who’s more likely to develop pancreatic cancer, and in the more long run, we see that maybe we can control these bacteria ... to prevent pancreatic cancer.”
So far, she said no mechanisms are known to account for the association between bacteria and pancreatic cancer risk although several have been proposed. She said a next step is to collect pancreatic tissue to see if oral bacteria are traveling to the pancreas.
Dr. Ahn noted that a major limitation of the study was that the populations involved in the cohorts from which the oral wash samples were obtained were mainly non-Hispanic whites, so the findings may not be generalizable to other groups.
Dr. Ahn had no disclosures.
Two species of oral bacteria – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – have been linked with an elevated risk of developing pancreatic cancer. This follows previous studies linking poor oral health in general to an increased risk of pancreatic cancer.
“Each [species] is associated with more than 50% higher likelihood to develop pancreatic cancer,” Jiyoung Ahn, Ph.D., of New York University Langone Medical Center, said during a news conference at the annual meeting of the American Association for Cancer Research.
“The findings suggest that carriage of these bacteria is related to subsequent development of pancreatic cancer,” said Dr. Ahn. Given the poor survival rate for pancreatic cancer of 5% at 5 years, identifying risk factors for its development may have implications for screening tools and prevention if the associations are found to be causal.
Employing a prospective, nested case-control study design, Dr. Ahn used samples and data from the Cancer Prevention Study II and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohorts. Both of those studies enrolled healthy people and followed them for several years looking for a variety of outcomes, including cancer. Researchers performed genomic analysis on oral wash samples collected at the beginning of the studies to identify the bacterial species present. Samples from 361 people who eventually developed pancreatic cancer were matched to 371 controls.
After adjustment for covariates of age, race, sex, smoking status, alcohol consumption, body mass index, and diabetes, logistic regression analysis showed that the presence of P. gingivalis in the samples was associated with a 59% increased risk (adjusted odds ratio [OR] = 1.59; 95% confidence interval [CI] 1.15, 2.20) of developing pancreatic cancer, and the presence of A. actinomycetemcomitans was associated with a 119% increased risk (OR = 2.19; 95% CI 1.15, 4.15). The presence of these bacteria remained a risk factor even after the deletion of samples from people who developed pancreatic cancer within the 2 years after collection of their samples to eliminate the possibility that early pancreatic cancers were affecting the populations of bacteria present.
Greater relative abundance of Fusobacteria species was associated with a lower risk (OR per percent increased abundance = 0.92; 95% CI 0.87, 0.98).
“Why this is important is because this is the first evidence suggesting the oral bacteria and pancreatic cancer relationship,” Dr. Ahn said, adding that she foresees eventual clinical applications. “In the long run, knowing these bacteria, we can see who’s more likely to develop pancreatic cancer, and in the more long run, we see that maybe we can control these bacteria ... to prevent pancreatic cancer.”
So far, she said no mechanisms are known to account for the association between bacteria and pancreatic cancer risk although several have been proposed. She said a next step is to collect pancreatic tissue to see if oral bacteria are traveling to the pancreas.
Dr. Ahn noted that a major limitation of the study was that the populations involved in the cohorts from which the oral wash samples were obtained were mainly non-Hispanic whites, so the findings may not be generalizable to other groups.
Dr. Ahn had no disclosures.
Two species of oral bacteria – Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans – have been linked with an elevated risk of developing pancreatic cancer. This follows previous studies linking poor oral health in general to an increased risk of pancreatic cancer.
“Each [species] is associated with more than 50% higher likelihood to develop pancreatic cancer,” Jiyoung Ahn, Ph.D., of New York University Langone Medical Center, said during a news conference at the annual meeting of the American Association for Cancer Research.
“The findings suggest that carriage of these bacteria is related to subsequent development of pancreatic cancer,” said Dr. Ahn. Given the poor survival rate for pancreatic cancer of 5% at 5 years, identifying risk factors for its development may have implications for screening tools and prevention if the associations are found to be causal.
Employing a prospective, nested case-control study design, Dr. Ahn used samples and data from the Cancer Prevention Study II and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial cohorts. Both of those studies enrolled healthy people and followed them for several years looking for a variety of outcomes, including cancer. Researchers performed genomic analysis on oral wash samples collected at the beginning of the studies to identify the bacterial species present. Samples from 361 people who eventually developed pancreatic cancer were matched to 371 controls.
After adjustment for covariates of age, race, sex, smoking status, alcohol consumption, body mass index, and diabetes, logistic regression analysis showed that the presence of P. gingivalis in the samples was associated with a 59% increased risk (adjusted odds ratio [OR] = 1.59; 95% confidence interval [CI] 1.15, 2.20) of developing pancreatic cancer, and the presence of A. actinomycetemcomitans was associated with a 119% increased risk (OR = 2.19; 95% CI 1.15, 4.15). The presence of these bacteria remained a risk factor even after the deletion of samples from people who developed pancreatic cancer within the 2 years after collection of their samples to eliminate the possibility that early pancreatic cancers were affecting the populations of bacteria present.
Greater relative abundance of Fusobacteria species was associated with a lower risk (OR per percent increased abundance = 0.92; 95% CI 0.87, 0.98).
“Why this is important is because this is the first evidence suggesting the oral bacteria and pancreatic cancer relationship,” Dr. Ahn said, adding that she foresees eventual clinical applications. “In the long run, knowing these bacteria, we can see who’s more likely to develop pancreatic cancer, and in the more long run, we see that maybe we can control these bacteria ... to prevent pancreatic cancer.”
So far, she said no mechanisms are known to account for the association between bacteria and pancreatic cancer risk although several have been proposed. She said a next step is to collect pancreatic tissue to see if oral bacteria are traveling to the pancreas.
Dr. Ahn noted that a major limitation of the study was that the populations involved in the cohorts from which the oral wash samples were obtained were mainly non-Hispanic whites, so the findings may not be generalizable to other groups.
Dr. Ahn had no disclosures.
FROM THE AACR ANNUAL MEETING
Key clinical point: Two oral bacteria are associated with increased pancreatic cancer risk.
Major finding: Bacteria are linked to over 50% increased pancreatic cancer risk.
Data source: Prospective nested case-control study of 361 incident pancreatic cancer cases and 371 matched controls.
Disclosures: Dr. Ahn had no disclosures.
Merkel cell carcinoma responds to first-line pembrolizumab
First-line therapy with pembrolizumab is associated with frequent and durable responses in patients with advanced Merkel cell carcinoma (MCC), according to a study presented at the annual meeting of the American Association for Cancer Research.
The objective response rate wad 56% among 25 patients with MCC who had received at least one dose of pembrolizumab in a phase II study, Dr. Paul Nghiem said at a press conference during the meeting.
This rare but aggressive skin cancer has been notoriously hard to treat with typical chemotherapy of platinum compounds and etoposide, with half of tumors progressing by 3 months and 90% progressing by 10 months. MCC is about three times more likely to kill a patient compared with melanoma.
Given that about 40% of the 2,000 cases per year develop advanced disease, pembrolizumab has the potential to be an advance over chemotherapy although at this point no therapy is approved by the Food and Drug Administration for MCC advanced disease. The disease may be caused by ultraviolet light exposure in older individuals or with immune suppression. In about 80% of cases, Merkel cell polyomavirus (MCPyV) is present, but the virus is ubiquitous in the environment, with widespread exposure in the population without ill effects for most people.
Pembrolizumab acts to block the interaction of PD-1 on tumor-specific or MCPyV-specific T cells with its ligand, PD-L1, on tumor cells or antigen-presenting cells, thus allowing the T cells to recognize the tumors and enhance tumor cell killing.
In a phase II multicenter, single-arm, open-label trial, patients with unresectable or metastatic disease who had never before received any systemic therapy received pembrolizumab 2 mg/kg IV every 3 weeks for up to 2 years. Tumor evaluations were done every 9 or 12 weeks with a median follow-up of 7.6 months.
Of the 25 patients who had received at least one dose and had at least one radiological assessment, there was an objective response rate of 56% (14/25), including 4 complete, 10 partial, and 1 unconfirmed partial response. One patient had stable disease, and nine had progressive disease.
“By the first scan at 3 months most patients have found where they were going to go,” said Dr. Nghiem, professor of medicine in dermatology at the University of Washington, Seattle. “Several had failed early, but by the time of the first scan at 3 months there were often very profound and complete responses, and a lot of the partial responses just remain very steady over time.”
With the caveat that this was not a randomized trial, he said the progression-free survival (PFS) among responders was 67% at 6 months and the median PFS was 9 months, compared to a historical median overall survival with chemotherapy of 9.5 months after metastatic diagnosis. Among responders, 86% continue to have excellent disease control more than 6 months after starting therapy.
Objective responses in this small study appeared to be associated with positive viral status of the tumors. Among patients with virus-positive tumors, 62% (10/16) had objective responses vs. 44% (4/9) in whom the tumors were virus negative. “That was not a statistically significant difference … but maybe suggests, like head and neck [cancers], there may be a better story there for virus positive,” Dr. Nghiem said. PD-L1 expression in the tumors did not predict responses.
In response to a question during the news conference about the high response rate compared to responses to therapy of most other solid tumors, Dr. Nghiem pointed out, “Historically, this has been a very immune-associated cancer … If you had CD8 T cells infiltrating into this cancer at any reasonable number, among 300 patients not one died of the disease.” Unleashing the immune system in this trial through PD-1 blockade may therefore help to explain the good outcomes.
Adverse events were managed with corticosteroid treatment and discontinuing the drug. Two patients who had severe drug-related toxicities improved after steroids and stopping the drug. But, nonetheless, they have ongoing antitumor responses several months after stopping pembrolizumab.
The investigators are currently expanding the trial and recruiting more patients.
The study was simultaneously published online in the New England Journal of Medicine (2016 April 19. doi: 10.1056/NEJMoa1603702).
The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
First-line therapy with pembrolizumab is associated with frequent and durable responses in patients with advanced Merkel cell carcinoma (MCC), according to a study presented at the annual meeting of the American Association for Cancer Research.
The objective response rate wad 56% among 25 patients with MCC who had received at least one dose of pembrolizumab in a phase II study, Dr. Paul Nghiem said at a press conference during the meeting.
This rare but aggressive skin cancer has been notoriously hard to treat with typical chemotherapy of platinum compounds and etoposide, with half of tumors progressing by 3 months and 90% progressing by 10 months. MCC is about three times more likely to kill a patient compared with melanoma.
Given that about 40% of the 2,000 cases per year develop advanced disease, pembrolizumab has the potential to be an advance over chemotherapy although at this point no therapy is approved by the Food and Drug Administration for MCC advanced disease. The disease may be caused by ultraviolet light exposure in older individuals or with immune suppression. In about 80% of cases, Merkel cell polyomavirus (MCPyV) is present, but the virus is ubiquitous in the environment, with widespread exposure in the population without ill effects for most people.
Pembrolizumab acts to block the interaction of PD-1 on tumor-specific or MCPyV-specific T cells with its ligand, PD-L1, on tumor cells or antigen-presenting cells, thus allowing the T cells to recognize the tumors and enhance tumor cell killing.
In a phase II multicenter, single-arm, open-label trial, patients with unresectable or metastatic disease who had never before received any systemic therapy received pembrolizumab 2 mg/kg IV every 3 weeks for up to 2 years. Tumor evaluations were done every 9 or 12 weeks with a median follow-up of 7.6 months.
Of the 25 patients who had received at least one dose and had at least one radiological assessment, there was an objective response rate of 56% (14/25), including 4 complete, 10 partial, and 1 unconfirmed partial response. One patient had stable disease, and nine had progressive disease.
“By the first scan at 3 months most patients have found where they were going to go,” said Dr. Nghiem, professor of medicine in dermatology at the University of Washington, Seattle. “Several had failed early, but by the time of the first scan at 3 months there were often very profound and complete responses, and a lot of the partial responses just remain very steady over time.”
With the caveat that this was not a randomized trial, he said the progression-free survival (PFS) among responders was 67% at 6 months and the median PFS was 9 months, compared to a historical median overall survival with chemotherapy of 9.5 months after metastatic diagnosis. Among responders, 86% continue to have excellent disease control more than 6 months after starting therapy.
Objective responses in this small study appeared to be associated with positive viral status of the tumors. Among patients with virus-positive tumors, 62% (10/16) had objective responses vs. 44% (4/9) in whom the tumors were virus negative. “That was not a statistically significant difference … but maybe suggests, like head and neck [cancers], there may be a better story there for virus positive,” Dr. Nghiem said. PD-L1 expression in the tumors did not predict responses.
In response to a question during the news conference about the high response rate compared to responses to therapy of most other solid tumors, Dr. Nghiem pointed out, “Historically, this has been a very immune-associated cancer … If you had CD8 T cells infiltrating into this cancer at any reasonable number, among 300 patients not one died of the disease.” Unleashing the immune system in this trial through PD-1 blockade may therefore help to explain the good outcomes.
Adverse events were managed with corticosteroid treatment and discontinuing the drug. Two patients who had severe drug-related toxicities improved after steroids and stopping the drug. But, nonetheless, they have ongoing antitumor responses several months after stopping pembrolizumab.
The investigators are currently expanding the trial and recruiting more patients.
The study was simultaneously published online in the New England Journal of Medicine (2016 April 19. doi: 10.1056/NEJMoa1603702).
The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
First-line therapy with pembrolizumab is associated with frequent and durable responses in patients with advanced Merkel cell carcinoma (MCC), according to a study presented at the annual meeting of the American Association for Cancer Research.
The objective response rate wad 56% among 25 patients with MCC who had received at least one dose of pembrolizumab in a phase II study, Dr. Paul Nghiem said at a press conference during the meeting.
This rare but aggressive skin cancer has been notoriously hard to treat with typical chemotherapy of platinum compounds and etoposide, with half of tumors progressing by 3 months and 90% progressing by 10 months. MCC is about three times more likely to kill a patient compared with melanoma.
Given that about 40% of the 2,000 cases per year develop advanced disease, pembrolizumab has the potential to be an advance over chemotherapy although at this point no therapy is approved by the Food and Drug Administration for MCC advanced disease. The disease may be caused by ultraviolet light exposure in older individuals or with immune suppression. In about 80% of cases, Merkel cell polyomavirus (MCPyV) is present, but the virus is ubiquitous in the environment, with widespread exposure in the population without ill effects for most people.
Pembrolizumab acts to block the interaction of PD-1 on tumor-specific or MCPyV-specific T cells with its ligand, PD-L1, on tumor cells or antigen-presenting cells, thus allowing the T cells to recognize the tumors and enhance tumor cell killing.
In a phase II multicenter, single-arm, open-label trial, patients with unresectable or metastatic disease who had never before received any systemic therapy received pembrolizumab 2 mg/kg IV every 3 weeks for up to 2 years. Tumor evaluations were done every 9 or 12 weeks with a median follow-up of 7.6 months.
Of the 25 patients who had received at least one dose and had at least one radiological assessment, there was an objective response rate of 56% (14/25), including 4 complete, 10 partial, and 1 unconfirmed partial response. One patient had stable disease, and nine had progressive disease.
“By the first scan at 3 months most patients have found where they were going to go,” said Dr. Nghiem, professor of medicine in dermatology at the University of Washington, Seattle. “Several had failed early, but by the time of the first scan at 3 months there were often very profound and complete responses, and a lot of the partial responses just remain very steady over time.”
With the caveat that this was not a randomized trial, he said the progression-free survival (PFS) among responders was 67% at 6 months and the median PFS was 9 months, compared to a historical median overall survival with chemotherapy of 9.5 months after metastatic diagnosis. Among responders, 86% continue to have excellent disease control more than 6 months after starting therapy.
Objective responses in this small study appeared to be associated with positive viral status of the tumors. Among patients with virus-positive tumors, 62% (10/16) had objective responses vs. 44% (4/9) in whom the tumors were virus negative. “That was not a statistically significant difference … but maybe suggests, like head and neck [cancers], there may be a better story there for virus positive,” Dr. Nghiem said. PD-L1 expression in the tumors did not predict responses.
In response to a question during the news conference about the high response rate compared to responses to therapy of most other solid tumors, Dr. Nghiem pointed out, “Historically, this has been a very immune-associated cancer … If you had CD8 T cells infiltrating into this cancer at any reasonable number, among 300 patients not one died of the disease.” Unleashing the immune system in this trial through PD-1 blockade may therefore help to explain the good outcomes.
Adverse events were managed with corticosteroid treatment and discontinuing the drug. Two patients who had severe drug-related toxicities improved after steroids and stopping the drug. But, nonetheless, they have ongoing antitumor responses several months after stopping pembrolizumab.
The investigators are currently expanding the trial and recruiting more patients.
The study was simultaneously published online in the New England Journal of Medicine (2016 April 19. doi: 10.1056/NEJMoa1603702).
The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
FROM THE AACR ANNUAL MEETING
Key clinical point: Rare Merkel cell carcinoma responds well to immunotherapy with pembrolizumab.
Major finding: The response rate was 56%, all occurring by 3 months.
Data source: Phase II multicenter, single-arm, open-label trial of 25 patients (NCT02267603).
Disclosures: The study was supported in part by Merck. Dr. Nghiem is a consultant to EMD Serono and has grant/research support from Bristol-Myers Squibb.
VP Biden to AACR: Help me help you
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
FROM THE AACR ANNUAL MEETING
Change in T-cell distribution predicts LFS, OS in AML

NEW ORLEANS—A phase 4 study has revealed biomarkers that appear to predict the efficacy of treatment with histamine dihydrochloride (HDC) and interleukin-2 (IL-2) in patients with acute myeloid leukemia (AML).
Researchers found that patients who remained in complete remission after 1 cycle of HDC/IL-2 experienced a significant reduction in effector memory T cells (TEM) and a concomitant increase in effector T cells (Teff) during therapy.
This “TEM to Teff transition” was associated with favorable leukemia-free survival (LFS) and overall survival (OS), especially among patients older than 60.
Frida Ewald Sander, PhD, of University of Gothenburg in Sweden, and her colleagues presented this research at the 2016 AACR Annual Meeting (abstract CT116*).
The study was supported by The Swedish Research Council, The Swedish Cancer Society, The Swedish Society for Medical Research, Meda Pharma, and Immune Pharmaceuticals, which markets HDC as Ceplene®.
The combination of HDC and IL-2 is currently approved in more than 30 countries to prevent relapse in AML patients. In this phase 4 study (Re:MISSION), the researchers set out to assess the immunomodulatory properties of this treatment and correlate potential biomarkers with clinical outcomes.
The study included 84 non-transplanted AML patients (ages 18 to 79) in first complete remission. The patients received HDC (0.5 mg SC BID) and human recombinant IL-2 (1MIU SC BID) in 3-week cycles for 18 months. The patients were followed for at least 2 years from the start of immunotherapy to evaluate survival.
The researchers collected blood from the patients before they began HDC/IL-2 therapy and at the end of the first treatment cycle. From these samples, the team assessed the frequency of CD8+ T cells, including naïve T cells (CD45RA+CCR7+), central memory T cells (CD45RO+CCR7+), TEM cells (CD45RO+CCR7-), and Teff cells (CD45RA+CCR7-).
The researchers found that non-relapsing patients experienced a significant reduction in TEM cells (P=0.001) and a significant increase in Teff cells (P=0.007) during cycle 1. However, this effect was not observed in patients who did relapse.
Further analysis revealed that the reduction in TEM cells was significantly associated with favorable LFS and OS in the entire cohort (P=0.0007 and P=0.005, respectively) and among patients over 60 (P<0.0001 and P=0.002, respectively).
Likewise, the increase in Teff cells was associated with favorable LFS and OS in the entire cohort (P=0.07 and P=0.04, respectively) and among patients over 60 (P=0.004 and P=0.0001, respectively).
The concomitant reduction of TEM cells and induction of Teff cells—the TEM to Teff transition—was associated with superior LFS and OS in the overall cohort (P=0.0002 and P=0.002, respectively) and in the over-60 population (P<0.0001 for LFS and OS).
The researchers said these predictors of outcome remained significant when they adjusted for potential confounders (age, risk group classification, number of induction courses required to achieve complete response, and number of consolidation courses).
Therefore, the team concluded that the altered distribution of cytotoxic T cells during treatment with HDC/IL-2 can prognosticate LFS and OS in AML patients, particularly those over 60.
“We believe that the new data may allow a personalized approach to selection of patients who are most likely to benefit from Ceplene/IL-2 treatment in AML—in particular, the older patient population, who have demonstrated almost 100% survival when positive for the T-cell transition biomarkers,” said Miri Ben-Ami, MD, executive vice president of oncology at Immune Pharmaceuticals.
“In addition, current research is revealing the potential synergy between immune checkpoint inhibitors and Ceplene, which could open the possibility of additional therapeutic indications for this combination.” ![]()
*Information in the abstract differs from that presented at the meeting.

NEW ORLEANS—A phase 4 study has revealed biomarkers that appear to predict the efficacy of treatment with histamine dihydrochloride (HDC) and interleukin-2 (IL-2) in patients with acute myeloid leukemia (AML).
Researchers found that patients who remained in complete remission after 1 cycle of HDC/IL-2 experienced a significant reduction in effector memory T cells (TEM) and a concomitant increase in effector T cells (Teff) during therapy.
This “TEM to Teff transition” was associated with favorable leukemia-free survival (LFS) and overall survival (OS), especially among patients older than 60.
Frida Ewald Sander, PhD, of University of Gothenburg in Sweden, and her colleagues presented this research at the 2016 AACR Annual Meeting (abstract CT116*).
The study was supported by The Swedish Research Council, The Swedish Cancer Society, The Swedish Society for Medical Research, Meda Pharma, and Immune Pharmaceuticals, which markets HDC as Ceplene®.
The combination of HDC and IL-2 is currently approved in more than 30 countries to prevent relapse in AML patients. In this phase 4 study (Re:MISSION), the researchers set out to assess the immunomodulatory properties of this treatment and correlate potential biomarkers with clinical outcomes.
The study included 84 non-transplanted AML patients (ages 18 to 79) in first complete remission. The patients received HDC (0.5 mg SC BID) and human recombinant IL-2 (1MIU SC BID) in 3-week cycles for 18 months. The patients were followed for at least 2 years from the start of immunotherapy to evaluate survival.
The researchers collected blood from the patients before they began HDC/IL-2 therapy and at the end of the first treatment cycle. From these samples, the team assessed the frequency of CD8+ T cells, including naïve T cells (CD45RA+CCR7+), central memory T cells (CD45RO+CCR7+), TEM cells (CD45RO+CCR7-), and Teff cells (CD45RA+CCR7-).
The researchers found that non-relapsing patients experienced a significant reduction in TEM cells (P=0.001) and a significant increase in Teff cells (P=0.007) during cycle 1. However, this effect was not observed in patients who did relapse.
Further analysis revealed that the reduction in TEM cells was significantly associated with favorable LFS and OS in the entire cohort (P=0.0007 and P=0.005, respectively) and among patients over 60 (P<0.0001 and P=0.002, respectively).
Likewise, the increase in Teff cells was associated with favorable LFS and OS in the entire cohort (P=0.07 and P=0.04, respectively) and among patients over 60 (P=0.004 and P=0.0001, respectively).
The concomitant reduction of TEM cells and induction of Teff cells—the TEM to Teff transition—was associated with superior LFS and OS in the overall cohort (P=0.0002 and P=0.002, respectively) and in the over-60 population (P<0.0001 for LFS and OS).
The researchers said these predictors of outcome remained significant when they adjusted for potential confounders (age, risk group classification, number of induction courses required to achieve complete response, and number of consolidation courses).
Therefore, the team concluded that the altered distribution of cytotoxic T cells during treatment with HDC/IL-2 can prognosticate LFS and OS in AML patients, particularly those over 60.
“We believe that the new data may allow a personalized approach to selection of patients who are most likely to benefit from Ceplene/IL-2 treatment in AML—in particular, the older patient population, who have demonstrated almost 100% survival when positive for the T-cell transition biomarkers,” said Miri Ben-Ami, MD, executive vice president of oncology at Immune Pharmaceuticals.
“In addition, current research is revealing the potential synergy between immune checkpoint inhibitors and Ceplene, which could open the possibility of additional therapeutic indications for this combination.” ![]()
*Information in the abstract differs from that presented at the meeting.

NEW ORLEANS—A phase 4 study has revealed biomarkers that appear to predict the efficacy of treatment with histamine dihydrochloride (HDC) and interleukin-2 (IL-2) in patients with acute myeloid leukemia (AML).
Researchers found that patients who remained in complete remission after 1 cycle of HDC/IL-2 experienced a significant reduction in effector memory T cells (TEM) and a concomitant increase in effector T cells (Teff) during therapy.
This “TEM to Teff transition” was associated with favorable leukemia-free survival (LFS) and overall survival (OS), especially among patients older than 60.
Frida Ewald Sander, PhD, of University of Gothenburg in Sweden, and her colleagues presented this research at the 2016 AACR Annual Meeting (abstract CT116*).
The study was supported by The Swedish Research Council, The Swedish Cancer Society, The Swedish Society for Medical Research, Meda Pharma, and Immune Pharmaceuticals, which markets HDC as Ceplene®.
The combination of HDC and IL-2 is currently approved in more than 30 countries to prevent relapse in AML patients. In this phase 4 study (Re:MISSION), the researchers set out to assess the immunomodulatory properties of this treatment and correlate potential biomarkers with clinical outcomes.
The study included 84 non-transplanted AML patients (ages 18 to 79) in first complete remission. The patients received HDC (0.5 mg SC BID) and human recombinant IL-2 (1MIU SC BID) in 3-week cycles for 18 months. The patients were followed for at least 2 years from the start of immunotherapy to evaluate survival.
The researchers collected blood from the patients before they began HDC/IL-2 therapy and at the end of the first treatment cycle. From these samples, the team assessed the frequency of CD8+ T cells, including naïve T cells (CD45RA+CCR7+), central memory T cells (CD45RO+CCR7+), TEM cells (CD45RO+CCR7-), and Teff cells (CD45RA+CCR7-).
The researchers found that non-relapsing patients experienced a significant reduction in TEM cells (P=0.001) and a significant increase in Teff cells (P=0.007) during cycle 1. However, this effect was not observed in patients who did relapse.
Further analysis revealed that the reduction in TEM cells was significantly associated with favorable LFS and OS in the entire cohort (P=0.0007 and P=0.005, respectively) and among patients over 60 (P<0.0001 and P=0.002, respectively).
Likewise, the increase in Teff cells was associated with favorable LFS and OS in the entire cohort (P=0.07 and P=0.04, respectively) and among patients over 60 (P=0.004 and P=0.0001, respectively).
The concomitant reduction of TEM cells and induction of Teff cells—the TEM to Teff transition—was associated with superior LFS and OS in the overall cohort (P=0.0002 and P=0.002, respectively) and in the over-60 population (P<0.0001 for LFS and OS).
The researchers said these predictors of outcome remained significant when they adjusted for potential confounders (age, risk group classification, number of induction courses required to achieve complete response, and number of consolidation courses).
Therefore, the team concluded that the altered distribution of cytotoxic T cells during treatment with HDC/IL-2 can prognosticate LFS and OS in AML patients, particularly those over 60.
“We believe that the new data may allow a personalized approach to selection of patients who are most likely to benefit from Ceplene/IL-2 treatment in AML—in particular, the older patient population, who have demonstrated almost 100% survival when positive for the T-cell transition biomarkers,” said Miri Ben-Ami, MD, executive vice president of oncology at Immune Pharmaceuticals.
“In addition, current research is revealing the potential synergy between immune checkpoint inhibitors and Ceplene, which could open the possibility of additional therapeutic indications for this combination.” ![]()
*Information in the abstract differs from that presented at the meeting.
MammaPrint bests clinical factors in sparing patients from chemotherapy
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
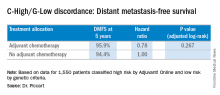
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
FROM THE AACR ANNUAL MEETING
Key clinical point: MammaPrint bests Adjuvant! Online for sparing low-risk patients from chemotherapy.
Major finding: Genotyping results in a 14% reduction in the use of chemotherapy, compared with a clinical strategy.
Data source: Phase III randomized controlled study of 6,693 women with early stage breast cancer.
Disclosures: Entities and funding sources involved in the study include Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
Treatment can produce durable responses in NHL

2016 AACR Annual Meeting
© AACR/Todd Buchanan
NEW ORLEANS—Administering an antibody-radionuclide conjugate after B-cell depletion with rituximab can produce lasting responses in patients with relapsed non-Hodgkin lymphoma (NHL), according to a phase 1/2 study.
The conjugate, 177Lu-DOTA-HH1 (Betalutin), consists of the tumor-specific antibody HH1, which targets the CD37 antigen on the surface of NHL cells, conjugated to the β-emitting isotope lutetium-177 (Lu-177) via the chemical linker DOTA.
In an ongoing phase 1/2 study, Betalutin given after rituximab produced an overall response rate of 63.2%.
The median duration of response has not yet been reached, and 1 patient has maintained a response for more than 36 months.
In addition, the researchers said Betalutin was well tolerated, with a predictable and manageable safety profile. Most adverse events were hematologic, and all have been transient and reversible.
These results were presented at the 2016 AACR Annual Meeting (abstract LB-252*). The study is sponsored by Nordic Nanovector ASA.
Patients and study design
The researchers presented data on 21 patients—19 with follicular lymphoma and 2 with mantle cell lymphoma. All tumors were positive for CD37.
The patients’ median age was 68 (range, 41-78). Sixty-seven percent were male, and they had received 1 to 8 prior treatment regimens.
In this dose-escalation study, patients received Betalutin at 3 different doses, but they were also divided into 2 arms according to predosing with cold HH1 antibody.
In Arm 1 (n=12), patients received rituximab (at 375 mg/m2) on day -28 and -21 to deplete circulating B cells. On day 0, predosing with 50 mg HH1 was given before Betalutin injection. Then, patients received Betalutin at 10 MBq/kg (n=3), 15 MBq/kg (n=6), or 20 MBq/kg (n=3).
In Arm 2 (n=4), patients received rituximab at the same dose and schedule as Arm 1, but Betalutin was administered without HH1 predosing on day 0 at either 10 MBq/kg (n=2) or 15 MBq/kg (n=2).
The first patient treated on this trial received 250 mg/m2 of rituximab on day -7 and day 0 prior to Betalutin administration and was included in the 10 MBq/kg group in Arm 2.
The 15 MBq/kg dose level of Arm 1 has been expanded into the phase 2 portion of the study, as dose-limiting toxicities occurred at the 20 MBq/kg dose. Five patients have been treated in the phase 2 portion.
Safety
Adverse events (AEs) from the phase 2 portion of the study were not reported, as the data are still being collected.
In the phase 1 portion, grade 3/4 AEs were hematologic in nature and included decreases in platelet counts (3 grade 3 and 6 grade 4) and neutrophil counts (5 grade 3 and 4 grade 4).
Serious AEs included decreases in platelet counts (n=2), atrial fibrillation (n=2), epistaxis (n=1), fractured sternum (n=1), decreased neutrophil count (n=1), pharyngitis (n=1), pneumonia (n=1), pulmonary embolism (n=1), and sepsis (n=1).
The pulmonary embolism was deemed unrelated to treatment, but the remaining events were considered possibly or probably related to Betalutin.
The researchers noted that 1 patient experienced pharyngitis, pneumonia, pulmonary embolism, epistaxis, sepsis, and a decrease in lymphocyte count.
All patients’ platelets and neutrophils recovered. Two patients required platelet transfusions—one patient in the 20 MBq/kg cohort of Arm 1 and one patient in the 15 MBq/kg cohort of Arm 2.
Efficacy
Nineteen patients were evaluable for response. The overall response rate was 63.2% (n=12) and included both complete (31.6%, n=6) and partial responses (31.6%, n=6). Progression occurred in 21.1% of patients (n=4), and 15.8% (n=3) had stable disease.
The researchers presented data on 9 patients treated at the recommended 15 MBq/kg dose level with 50 mg HH1 predosing. Five patients were treated in phase 1 and 4 in phase 2. One of these patients was excluded from the analysis due to transformed lymphoma.
Two patients in phase 1 responded—both complete responses—and 3 patients in phase 2 responded—2 complete and 1 partial response.
For the entire study cohort, the median duration of response has not yet been reached. Six responses are ongoing—2 for 3+ months, 1 for 6+ months, 1 for 18+ months, 1 for 24+ months, and 1 for 36+ months. ![]()
*Information in the abstract differs from that presented at the meeting.

2016 AACR Annual Meeting
© AACR/Todd Buchanan
NEW ORLEANS—Administering an antibody-radionuclide conjugate after B-cell depletion with rituximab can produce lasting responses in patients with relapsed non-Hodgkin lymphoma (NHL), according to a phase 1/2 study.
The conjugate, 177Lu-DOTA-HH1 (Betalutin), consists of the tumor-specific antibody HH1, which targets the CD37 antigen on the surface of NHL cells, conjugated to the β-emitting isotope lutetium-177 (Lu-177) via the chemical linker DOTA.
In an ongoing phase 1/2 study, Betalutin given after rituximab produced an overall response rate of 63.2%.
The median duration of response has not yet been reached, and 1 patient has maintained a response for more than 36 months.
In addition, the researchers said Betalutin was well tolerated, with a predictable and manageable safety profile. Most adverse events were hematologic, and all have been transient and reversible.
These results were presented at the 2016 AACR Annual Meeting (abstract LB-252*). The study is sponsored by Nordic Nanovector ASA.
Patients and study design
The researchers presented data on 21 patients—19 with follicular lymphoma and 2 with mantle cell lymphoma. All tumors were positive for CD37.
The patients’ median age was 68 (range, 41-78). Sixty-seven percent were male, and they had received 1 to 8 prior treatment regimens.
In this dose-escalation study, patients received Betalutin at 3 different doses, but they were also divided into 2 arms according to predosing with cold HH1 antibody.
In Arm 1 (n=12), patients received rituximab (at 375 mg/m2) on day -28 and -21 to deplete circulating B cells. On day 0, predosing with 50 mg HH1 was given before Betalutin injection. Then, patients received Betalutin at 10 MBq/kg (n=3), 15 MBq/kg (n=6), or 20 MBq/kg (n=3).
In Arm 2 (n=4), patients received rituximab at the same dose and schedule as Arm 1, but Betalutin was administered without HH1 predosing on day 0 at either 10 MBq/kg (n=2) or 15 MBq/kg (n=2).
The first patient treated on this trial received 250 mg/m2 of rituximab on day -7 and day 0 prior to Betalutin administration and was included in the 10 MBq/kg group in Arm 2.
The 15 MBq/kg dose level of Arm 1 has been expanded into the phase 2 portion of the study, as dose-limiting toxicities occurred at the 20 MBq/kg dose. Five patients have been treated in the phase 2 portion.
Safety
Adverse events (AEs) from the phase 2 portion of the study were not reported, as the data are still being collected.
In the phase 1 portion, grade 3/4 AEs were hematologic in nature and included decreases in platelet counts (3 grade 3 and 6 grade 4) and neutrophil counts (5 grade 3 and 4 grade 4).
Serious AEs included decreases in platelet counts (n=2), atrial fibrillation (n=2), epistaxis (n=1), fractured sternum (n=1), decreased neutrophil count (n=1), pharyngitis (n=1), pneumonia (n=1), pulmonary embolism (n=1), and sepsis (n=1).
The pulmonary embolism was deemed unrelated to treatment, but the remaining events were considered possibly or probably related to Betalutin.
The researchers noted that 1 patient experienced pharyngitis, pneumonia, pulmonary embolism, epistaxis, sepsis, and a decrease in lymphocyte count.
All patients’ platelets and neutrophils recovered. Two patients required platelet transfusions—one patient in the 20 MBq/kg cohort of Arm 1 and one patient in the 15 MBq/kg cohort of Arm 2.
Efficacy
Nineteen patients were evaluable for response. The overall response rate was 63.2% (n=12) and included both complete (31.6%, n=6) and partial responses (31.6%, n=6). Progression occurred in 21.1% of patients (n=4), and 15.8% (n=3) had stable disease.
The researchers presented data on 9 patients treated at the recommended 15 MBq/kg dose level with 50 mg HH1 predosing. Five patients were treated in phase 1 and 4 in phase 2. One of these patients was excluded from the analysis due to transformed lymphoma.
Two patients in phase 1 responded—both complete responses—and 3 patients in phase 2 responded—2 complete and 1 partial response.
For the entire study cohort, the median duration of response has not yet been reached. Six responses are ongoing—2 for 3+ months, 1 for 6+ months, 1 for 18+ months, 1 for 24+ months, and 1 for 36+ months. ![]()
*Information in the abstract differs from that presented at the meeting.

2016 AACR Annual Meeting
© AACR/Todd Buchanan
NEW ORLEANS—Administering an antibody-radionuclide conjugate after B-cell depletion with rituximab can produce lasting responses in patients with relapsed non-Hodgkin lymphoma (NHL), according to a phase 1/2 study.
The conjugate, 177Lu-DOTA-HH1 (Betalutin), consists of the tumor-specific antibody HH1, which targets the CD37 antigen on the surface of NHL cells, conjugated to the β-emitting isotope lutetium-177 (Lu-177) via the chemical linker DOTA.
In an ongoing phase 1/2 study, Betalutin given after rituximab produced an overall response rate of 63.2%.
The median duration of response has not yet been reached, and 1 patient has maintained a response for more than 36 months.
In addition, the researchers said Betalutin was well tolerated, with a predictable and manageable safety profile. Most adverse events were hematologic, and all have been transient and reversible.
These results were presented at the 2016 AACR Annual Meeting (abstract LB-252*). The study is sponsored by Nordic Nanovector ASA.
Patients and study design
The researchers presented data on 21 patients—19 with follicular lymphoma and 2 with mantle cell lymphoma. All tumors were positive for CD37.
The patients’ median age was 68 (range, 41-78). Sixty-seven percent were male, and they had received 1 to 8 prior treatment regimens.
In this dose-escalation study, patients received Betalutin at 3 different doses, but they were also divided into 2 arms according to predosing with cold HH1 antibody.
In Arm 1 (n=12), patients received rituximab (at 375 mg/m2) on day -28 and -21 to deplete circulating B cells. On day 0, predosing with 50 mg HH1 was given before Betalutin injection. Then, patients received Betalutin at 10 MBq/kg (n=3), 15 MBq/kg (n=6), or 20 MBq/kg (n=3).
In Arm 2 (n=4), patients received rituximab at the same dose and schedule as Arm 1, but Betalutin was administered without HH1 predosing on day 0 at either 10 MBq/kg (n=2) or 15 MBq/kg (n=2).
The first patient treated on this trial received 250 mg/m2 of rituximab on day -7 and day 0 prior to Betalutin administration and was included in the 10 MBq/kg group in Arm 2.
The 15 MBq/kg dose level of Arm 1 has been expanded into the phase 2 portion of the study, as dose-limiting toxicities occurred at the 20 MBq/kg dose. Five patients have been treated in the phase 2 portion.
Safety
Adverse events (AEs) from the phase 2 portion of the study were not reported, as the data are still being collected.
In the phase 1 portion, grade 3/4 AEs were hematologic in nature and included decreases in platelet counts (3 grade 3 and 6 grade 4) and neutrophil counts (5 grade 3 and 4 grade 4).
Serious AEs included decreases in platelet counts (n=2), atrial fibrillation (n=2), epistaxis (n=1), fractured sternum (n=1), decreased neutrophil count (n=1), pharyngitis (n=1), pneumonia (n=1), pulmonary embolism (n=1), and sepsis (n=1).
The pulmonary embolism was deemed unrelated to treatment, but the remaining events were considered possibly or probably related to Betalutin.
The researchers noted that 1 patient experienced pharyngitis, pneumonia, pulmonary embolism, epistaxis, sepsis, and a decrease in lymphocyte count.
All patients’ platelets and neutrophils recovered. Two patients required platelet transfusions—one patient in the 20 MBq/kg cohort of Arm 1 and one patient in the 15 MBq/kg cohort of Arm 2.
Efficacy
Nineteen patients were evaluable for response. The overall response rate was 63.2% (n=12) and included both complete (31.6%, n=6) and partial responses (31.6%, n=6). Progression occurred in 21.1% of patients (n=4), and 15.8% (n=3) had stable disease.
The researchers presented data on 9 patients treated at the recommended 15 MBq/kg dose level with 50 mg HH1 predosing. Five patients were treated in phase 1 and 4 in phase 2. One of these patients was excluded from the analysis due to transformed lymphoma.
Two patients in phase 1 responded—both complete responses—and 3 patients in phase 2 responded—2 complete and 1 partial response.
For the entire study cohort, the median duration of response has not yet been reached. Six responses are ongoing—2 for 3+ months, 1 for 6+ months, 1 for 18+ months, 1 for 24+ months, and 1 for 36+ months. ![]()
*Information in the abstract differs from that presented at the meeting.
Drug shows early promise for low-grade lymphoma

follicular lymphoma
NEW ORLEANS—The TLR9 agonist SD-101 has produced encouraging early results in patients with low-grade B-cell lymphoma, according to researchers.
In an ongoing phase 1/2 study, patients received low-dose radiation, followed by an injection of SD-101 into one of their tumors.
This prompted changes in the tumor microenvironment that potentially induced a systemic antitumor response and decreased the volume of both treated and untreated tumors.
In addition, SD-101 was considered well tolerated, with no dose-limiting toxicities or maximum-tolerated dose.
“We are pleased to have already demonstrated a safety profile, pharmacodynamics, and preliminary efficacy in this study,” said Ronald Levy, MD, of Stanford University in California.
Dr Levy and his colleagues presented these results at the 2016 AACR Annual Meeting (abstract CT047). The study is sponsored by Dynavax Technologies Corporation, the company developing SD-101.
The researchers reported data for 13 patients with untreated, low-grade B-cell lymphoma. They had a mean age of 63.2, and 53.8% were male.
Patients had follicular lymphoma (n=9), small lymphocytic lymphoma (n=2), chronic lymphocytic leukemia (n=1), and nodal marginal zone lymphoma (n=1).
At least 2 sites of measurable disease were required for participation in this study. One site was treated with low-dose radiation (2 Gy) and injected with SD-101 on days 1, 8, 15, 22, and 29. Other lesions received no treatment.
In Part 1—the dose-escalation portion of the study—patients received SD-101 at 1 mg (n=3), 2 mg (n=3), 4 mg (n=3), or 8 mg (n=4). The phase 2 expansion portion of the study is ongoing, with enrollment in 2 dose cohorts (1 mg and 8 mg).
“This clinical trial design is unique and takes advantage of the fact that lymphoma patients have easily injectable sites of disease,” Dr Levy said. “The local injections are conveniently added to low-dose radiotherapy, a standard treatment for low-grade lymphoma.”
Safety
All 13 patients experienced at least 1 adverse event (AEs), although nearly all were grade 1 or 2.
The most common treatment-related AEs were local injection-site reactions and flu-like symptoms, including fever, chills, and myalgia (n=11 for all 3). However, the researchers said these AEs were primarily short-lived and controlled by over-the-counter acetaminophen in most cases.
In the 8 mg dosing cohort, 1 patient had grade 3 neutropenia and 2 had grade 3 malaise, all of which were considered treatment-related. In addition, there was a case of grade 3 asymptomatic pulmonary embolism in the 4 mg dose cohort, which was deemed serious but unrelated to treatment.
Efficacy
The researchers observed induction of interferon-responsive genes at all dose levels 24 hours after the second dose of SD-101 was given (Day 9).
In addition, T-cell numbers increased at the treated site by Day 8. The total T cells increased in 7 of 10 evaluable patients by Day 8 (range, >300% to 18%).
CD4+ and CD8+ T cells increased simultaneously in 5 of 7 evaluable patients, regulatory T cells decreased in 8 of 10 evaluable patients by Day 8, and follicular T helper cells decreased in 9 of 9 evaluable patients by Day 8.
Furthermore, treated and untreated tumors decreased in volume across all dose groups.
At Day 90, 12 patients had a reduction of the product of diameters in treated tumors (median -45.3%; range, -87 to +100), and 11 patients had a reduction in untreated tumors (median -8.1%; range, -48 to +45).
In 9 patients, these abscopal effects were sustained for at least 180 to 360 days.
However, 6 patients discontinued from the study due to progression. Three had radiographic progression—1 at Day 92 (in the 4 mg cohort) and 2 at 1 year (1 in the 1 mg cohort and 1 in the 2 mg cohort).
Two patients had clinical progression—1 at Day 197 (4 mg) and 1 at Day 273 (2 mg). And 1 patient discontinued at Day 203 due to a combination of clinical and radiographic progression (8 mg).
The researchers pointed out that there was no evidence of a dose response with SD-101, but the study included a limited number of patients. ![]()

follicular lymphoma
NEW ORLEANS—The TLR9 agonist SD-101 has produced encouraging early results in patients with low-grade B-cell lymphoma, according to researchers.
In an ongoing phase 1/2 study, patients received low-dose radiation, followed by an injection of SD-101 into one of their tumors.
This prompted changes in the tumor microenvironment that potentially induced a systemic antitumor response and decreased the volume of both treated and untreated tumors.
In addition, SD-101 was considered well tolerated, with no dose-limiting toxicities or maximum-tolerated dose.
“We are pleased to have already demonstrated a safety profile, pharmacodynamics, and preliminary efficacy in this study,” said Ronald Levy, MD, of Stanford University in California.
Dr Levy and his colleagues presented these results at the 2016 AACR Annual Meeting (abstract CT047). The study is sponsored by Dynavax Technologies Corporation, the company developing SD-101.
The researchers reported data for 13 patients with untreated, low-grade B-cell lymphoma. They had a mean age of 63.2, and 53.8% were male.
Patients had follicular lymphoma (n=9), small lymphocytic lymphoma (n=2), chronic lymphocytic leukemia (n=1), and nodal marginal zone lymphoma (n=1).
At least 2 sites of measurable disease were required for participation in this study. One site was treated with low-dose radiation (2 Gy) and injected with SD-101 on days 1, 8, 15, 22, and 29. Other lesions received no treatment.
In Part 1—the dose-escalation portion of the study—patients received SD-101 at 1 mg (n=3), 2 mg (n=3), 4 mg (n=3), or 8 mg (n=4). The phase 2 expansion portion of the study is ongoing, with enrollment in 2 dose cohorts (1 mg and 8 mg).
“This clinical trial design is unique and takes advantage of the fact that lymphoma patients have easily injectable sites of disease,” Dr Levy said. “The local injections are conveniently added to low-dose radiotherapy, a standard treatment for low-grade lymphoma.”
Safety
All 13 patients experienced at least 1 adverse event (AEs), although nearly all were grade 1 or 2.
The most common treatment-related AEs were local injection-site reactions and flu-like symptoms, including fever, chills, and myalgia (n=11 for all 3). However, the researchers said these AEs were primarily short-lived and controlled by over-the-counter acetaminophen in most cases.
In the 8 mg dosing cohort, 1 patient had grade 3 neutropenia and 2 had grade 3 malaise, all of which were considered treatment-related. In addition, there was a case of grade 3 asymptomatic pulmonary embolism in the 4 mg dose cohort, which was deemed serious but unrelated to treatment.
Efficacy
The researchers observed induction of interferon-responsive genes at all dose levels 24 hours after the second dose of SD-101 was given (Day 9).
In addition, T-cell numbers increased at the treated site by Day 8. The total T cells increased in 7 of 10 evaluable patients by Day 8 (range, >300% to 18%).
CD4+ and CD8+ T cells increased simultaneously in 5 of 7 evaluable patients, regulatory T cells decreased in 8 of 10 evaluable patients by Day 8, and follicular T helper cells decreased in 9 of 9 evaluable patients by Day 8.
Furthermore, treated and untreated tumors decreased in volume across all dose groups.
At Day 90, 12 patients had a reduction of the product of diameters in treated tumors (median -45.3%; range, -87 to +100), and 11 patients had a reduction in untreated tumors (median -8.1%; range, -48 to +45).
In 9 patients, these abscopal effects were sustained for at least 180 to 360 days.
However, 6 patients discontinued from the study due to progression. Three had radiographic progression—1 at Day 92 (in the 4 mg cohort) and 2 at 1 year (1 in the 1 mg cohort and 1 in the 2 mg cohort).
Two patients had clinical progression—1 at Day 197 (4 mg) and 1 at Day 273 (2 mg). And 1 patient discontinued at Day 203 due to a combination of clinical and radiographic progression (8 mg).
The researchers pointed out that there was no evidence of a dose response with SD-101, but the study included a limited number of patients. ![]()

follicular lymphoma
NEW ORLEANS—The TLR9 agonist SD-101 has produced encouraging early results in patients with low-grade B-cell lymphoma, according to researchers.
In an ongoing phase 1/2 study, patients received low-dose radiation, followed by an injection of SD-101 into one of their tumors.
This prompted changes in the tumor microenvironment that potentially induced a systemic antitumor response and decreased the volume of both treated and untreated tumors.
In addition, SD-101 was considered well tolerated, with no dose-limiting toxicities or maximum-tolerated dose.
“We are pleased to have already demonstrated a safety profile, pharmacodynamics, and preliminary efficacy in this study,” said Ronald Levy, MD, of Stanford University in California.
Dr Levy and his colleagues presented these results at the 2016 AACR Annual Meeting (abstract CT047). The study is sponsored by Dynavax Technologies Corporation, the company developing SD-101.
The researchers reported data for 13 patients with untreated, low-grade B-cell lymphoma. They had a mean age of 63.2, and 53.8% were male.
Patients had follicular lymphoma (n=9), small lymphocytic lymphoma (n=2), chronic lymphocytic leukemia (n=1), and nodal marginal zone lymphoma (n=1).
At least 2 sites of measurable disease were required for participation in this study. One site was treated with low-dose radiation (2 Gy) and injected with SD-101 on days 1, 8, 15, 22, and 29. Other lesions received no treatment.
In Part 1—the dose-escalation portion of the study—patients received SD-101 at 1 mg (n=3), 2 mg (n=3), 4 mg (n=3), or 8 mg (n=4). The phase 2 expansion portion of the study is ongoing, with enrollment in 2 dose cohorts (1 mg and 8 mg).
“This clinical trial design is unique and takes advantage of the fact that lymphoma patients have easily injectable sites of disease,” Dr Levy said. “The local injections are conveniently added to low-dose radiotherapy, a standard treatment for low-grade lymphoma.”
Safety
All 13 patients experienced at least 1 adverse event (AEs), although nearly all were grade 1 or 2.
The most common treatment-related AEs were local injection-site reactions and flu-like symptoms, including fever, chills, and myalgia (n=11 for all 3). However, the researchers said these AEs were primarily short-lived and controlled by over-the-counter acetaminophen in most cases.
In the 8 mg dosing cohort, 1 patient had grade 3 neutropenia and 2 had grade 3 malaise, all of which were considered treatment-related. In addition, there was a case of grade 3 asymptomatic pulmonary embolism in the 4 mg dose cohort, which was deemed serious but unrelated to treatment.
Efficacy
The researchers observed induction of interferon-responsive genes at all dose levels 24 hours after the second dose of SD-101 was given (Day 9).
In addition, T-cell numbers increased at the treated site by Day 8. The total T cells increased in 7 of 10 evaluable patients by Day 8 (range, >300% to 18%).
CD4+ and CD8+ T cells increased simultaneously in 5 of 7 evaluable patients, regulatory T cells decreased in 8 of 10 evaluable patients by Day 8, and follicular T helper cells decreased in 9 of 9 evaluable patients by Day 8.
Furthermore, treated and untreated tumors decreased in volume across all dose groups.
At Day 90, 12 patients had a reduction of the product of diameters in treated tumors (median -45.3%; range, -87 to +100), and 11 patients had a reduction in untreated tumors (median -8.1%; range, -48 to +45).
In 9 patients, these abscopal effects were sustained for at least 180 to 360 days.
However, 6 patients discontinued from the study due to progression. Three had radiographic progression—1 at Day 92 (in the 4 mg cohort) and 2 at 1 year (1 in the 1 mg cohort and 1 in the 2 mg cohort).
Two patients had clinical progression—1 at Day 197 (4 mg) and 1 at Day 273 (2 mg). And 1 patient discontinued at Day 203 due to a combination of clinical and radiographic progression (8 mg).
The researchers pointed out that there was no evidence of a dose response with SD-101, but the study included a limited number of patients. ![]()
Targeting gene rearrangements shows promise in early study
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.
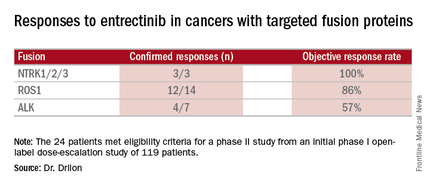
Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.

Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
Entrectinib, an investigational drug that targets several abnormal fusion proteins, showed antitumor activity and was safe in patients with several different types of advanced solid tumors. The patients had never before been exposed to drugs targeting these same genetic alterations.
“Responses can be very rapid and durable … which include colorectal, primary brain tumor, astrocytoma, fibrosarcoma, lung, and mammary analog secretory carcinoma,” Dr. Alexander Drilon of Memorial Sloan Kettering Cancer Center in New York said in a news conference at the annual meeting of the American Association for Cancer Research. “Dramatic intracranial activity … has been demonstrated both in primary brain tumor and also in metastatic.”
NTRK1/2/3, ROS1, and ALK gene–rearranged cancers produce fusion proteins that are ligand independent for their activity and thus constitutively active, driving tumor growth. Entrectinib is a pan-TRK, ROS1, ALK tyrosine kinase inhibitor that targets the abnormal fusion protein products of the genes, is highly potent at low concentrations, and has been designed to cross the blood-brain barrier (BBB). The targeted proteins are present across multiple cancers and are especially prevalent (greater than 80%) among some rare adult and pediatric cancers.

Combined data on 119 patients in two phase I trials established 600 mg orally once daily as the recommended dose to go into phase II trials. Among the 24 patients meeting eligibility criteria for a phase II trial (presence of the targeted gene fusions in their tumors, no prior treatment against these targets, and treatment at or above 600 mg daily), the confirmed response rate was 79% (19/24). Most were partial responses in terms of tumor shrinkage, but two patients had complete responses. Response rates appeared to vary according to the specific fusion protein defect.
All three cases of CNS disease with NTRK-rearranged cancers had intracranial responses, demonstrating that the drug crosses the BBB and is active. In one case, a 46-year-old man with brain metastases heavily pretreated for non–small cell lung cancer with an NTRK1 rearrangement experienced a dramatic response.
“The patient at that point was actually on hospice and was doing extremely poorly on supplemental oxygen,” Dr. Drilon said. “Within a few weeks, the patient had a dramatic clinical response to therapy … At day 26 there was almost a 50% reduction in tumor burden.” At day 317 scans showed he had a complete intracranial response to entrectinib, but he still has visceral disease on therapy past 1 year.
Responses often occurred within the first month of therapy, and many persisted for several months without disease progression, with one patient being followed for more than 2 years with clinical benefit. Nineteen of 24 patients have been on the therapy for more than 6 months, and the therapy appears to be safe and well tolerated.
Commenting on this study and others targeting specific genetic alterations leading to cancer, Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, said, “You’re seeing a series of clinical trials described that aren’t necessarily targeting people with a particular cancer but rather people who have cancers characterized by particular molecular abnormalities.” Not all cancers will have identified molecular abnormalities driving them. “However, I think where you have these drivers, the proper thing to do is not to worry about whether [a drug] works in a given disease but rather whether it works for people with that particular abnormality,” he said.
For the future, the investigators plan a phase II trial called STARTRK-2. It is a multicenter, open-label, global basket study to include any solid tumors with the targeted rearrangements.
Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
FROM THE AACR ANNUAL MEETING
Key clinical point: Entrectinib showed antitumor activity including intracranial responses.
Major finding: Patients with the targeted abnormalities had a 79% response rate.
Data source: Twenty-four patients meeting eligibility criteria for a phase II study from an initial phase I open-label dose-escalation study of 119 patients.
Disclosures: Dr. Drilon disclosed ties with Ignyta, which funded the study, and has received research funding from Foundation Medicine. Dr. Weiner disclosed ties with several pharmaceutical companies.
mAb can reduce CSCs in newly diagnosed MM

Photo by Linda Bartlett
NEW ORLEANS—A small study suggests that treatment with lenalidomide and dexamethasone (len-dex) prompts an increase in cancer stem cells (CSCs) for patients with newly diagnosed multiple myeloma (MM).
However, adding an anti-CD19 monoclonal antibody (mAb) to the regimen can reduce CSCs.
Most patients who received the mAb, MEDI-551, experienced a decrease in CSCs, but the cells rebounded after the patients stopped receiving MEDI-551.
And those patients who did not see a decrease in CSCs progressed. However, some patients are still in response and remain on treatment with len-dex.
The investigators believe these early results suggest prolonged treatment with len-dex and MED-551 may be safe and clinically beneficial for MM patients.
The results were presented at the 2016 AACR Annual Meeting (abstract CT102).
The study included 17 patients with newly diagnosed MM. They had a median age of 65 (range, 34-73). Most had ISS stage I (n=11), 2 had stage II, and 4 had stage III. Seven patients had t(4;14).
“We chose to carry out this clinical trial in newly diagnosed patients because our original data showed that CD19 was almost always expressed by myeloma stem cells in these patients, whereas we don’t know if that is the case in more advanced patients,” said investigator William Matsui, MD, of Johns Hopkins University School of Medicine in Baltimore, Maryland.
The patients received 28-day cycles of len-dex (len at 25 mg PO, days 1-21, and dex at 40 mg PO, weekly). Patients received MEDI-551 (at 4 mg/kg IV) in cycle 3 (day 1, 8) and cycle 4 (day 1). Responding patients continued on len-dex.
The investigators measured MM CSCs by quantifying the growth of MM colonies (CFU-MM) from marrow aspirates at baseline and at the end of cycles 2 and 4.
The team quantified peripheral blood CSCs by flow cytometry (CD19+CD27+ALDH+) at baseline and the end of cycles 2, 4, 5, and 7.
“We wanted to see if these 2 assays gave similar results, and, in this clinical trial, they were almost identical,” said investigator Carol Ann Huff, MD, also of Johns Hopkins.
“Since it is much easier to draw blood than bone marrow from our patients, we think that we can primarily use blood to track multiple myeloma stem cells in the future.”
Response
Two patients did not receive MEDI-551 due to progressive disease and noncompliance. So the investigators assessed responses in 15 patients.
After cycle 2 (len-dex alone), there were 3 very good partial responses (VGPRs), 10 partial responses (PRs), 1 molecular response, and 1 case of stable disease.
After cycle 4 (len-dex plus MEDI-551), there were 6 VGPRs, 8 PRs, and 1 molecular response.
Ten patients who completed treatment with MED-551 remain on len-dex. At the end of cycle 7, there was 1 complete response, 8 VGPRs, and 1 PR.
CFU-MM
When compared to baseline, bone-marrow-derived CFU-MM increased a median of 2.5-fold (range, 0.4-7.4) after cycle 2 but decreased a median of 0.48-fold (range, 0.14-0.85) in 14 patients after cycle 4.
The investigators compared these results to 5 newly diagnosed MM patients who only received standard treatment with len-dex.
In these patients, CFU-MM increased a median of 9.3-fold (range, 4-14) at a median of 4 months (range, 2-4). This is in spite of the fact that all of these patients had a PR or better.
Circulating CSCs
Compared to baseline, circulating MM CSCs increased a median of 1.6-fold (range, 0.4-8.6) in 14 patients after cycle 2 but decreased a median of 0.6-fold (range, 0.01-7.4) in 13 patients after cycle 4.
At the end of cycle 5, MM CSCs had increased in 4 of the 10 patients who were still on len-dex. By the end of cycle 7, MM CSCs had increased in 8 of the patients.
Circulating MM CSCs increased by the end of cycle 4 in 2 patients, and both had progressed by end of cycle 7.
Safety and next steps
The investigators said there were no serious adverse events in this trial, but 2 patients experienced grade 2 infusion reactions after the first MEDI-551 dose.
The team plans to conduct further studies to assess the long-term impact of MED-551 in MM patients and determine how the mAb might work in combination with other treatments, particularly transplant.
“In other studies at Johns Hopkins, we have found that antibody therapies can work much better after a bone marrow transplant, especially allogeneic transplants,” Dr Matsui said.
Funding and drugs for this study were provided by MedImmune Inc., the developers of MEDI-551. Drs Huff and Matsui served as a paid scientific advisory board member and a consultant to MedImmune Inc., respectively. ![]()

Photo by Linda Bartlett
NEW ORLEANS—A small study suggests that treatment with lenalidomide and dexamethasone (len-dex) prompts an increase in cancer stem cells (CSCs) for patients with newly diagnosed multiple myeloma (MM).
However, adding an anti-CD19 monoclonal antibody (mAb) to the regimen can reduce CSCs.
Most patients who received the mAb, MEDI-551, experienced a decrease in CSCs, but the cells rebounded after the patients stopped receiving MEDI-551.
And those patients who did not see a decrease in CSCs progressed. However, some patients are still in response and remain on treatment with len-dex.
The investigators believe these early results suggest prolonged treatment with len-dex and MED-551 may be safe and clinically beneficial for MM patients.
The results were presented at the 2016 AACR Annual Meeting (abstract CT102).
The study included 17 patients with newly diagnosed MM. They had a median age of 65 (range, 34-73). Most had ISS stage I (n=11), 2 had stage II, and 4 had stage III. Seven patients had t(4;14).
“We chose to carry out this clinical trial in newly diagnosed patients because our original data showed that CD19 was almost always expressed by myeloma stem cells in these patients, whereas we don’t know if that is the case in more advanced patients,” said investigator William Matsui, MD, of Johns Hopkins University School of Medicine in Baltimore, Maryland.
The patients received 28-day cycles of len-dex (len at 25 mg PO, days 1-21, and dex at 40 mg PO, weekly). Patients received MEDI-551 (at 4 mg/kg IV) in cycle 3 (day 1, 8) and cycle 4 (day 1). Responding patients continued on len-dex.
The investigators measured MM CSCs by quantifying the growth of MM colonies (CFU-MM) from marrow aspirates at baseline and at the end of cycles 2 and 4.
The team quantified peripheral blood CSCs by flow cytometry (CD19+CD27+ALDH+) at baseline and the end of cycles 2, 4, 5, and 7.
“We wanted to see if these 2 assays gave similar results, and, in this clinical trial, they were almost identical,” said investigator Carol Ann Huff, MD, also of Johns Hopkins.
“Since it is much easier to draw blood than bone marrow from our patients, we think that we can primarily use blood to track multiple myeloma stem cells in the future.”
Response
Two patients did not receive MEDI-551 due to progressive disease and noncompliance. So the investigators assessed responses in 15 patients.
After cycle 2 (len-dex alone), there were 3 very good partial responses (VGPRs), 10 partial responses (PRs), 1 molecular response, and 1 case of stable disease.
After cycle 4 (len-dex plus MEDI-551), there were 6 VGPRs, 8 PRs, and 1 molecular response.
Ten patients who completed treatment with MED-551 remain on len-dex. At the end of cycle 7, there was 1 complete response, 8 VGPRs, and 1 PR.
CFU-MM
When compared to baseline, bone-marrow-derived CFU-MM increased a median of 2.5-fold (range, 0.4-7.4) after cycle 2 but decreased a median of 0.48-fold (range, 0.14-0.85) in 14 patients after cycle 4.
The investigators compared these results to 5 newly diagnosed MM patients who only received standard treatment with len-dex.
In these patients, CFU-MM increased a median of 9.3-fold (range, 4-14) at a median of 4 months (range, 2-4). This is in spite of the fact that all of these patients had a PR or better.
Circulating CSCs
Compared to baseline, circulating MM CSCs increased a median of 1.6-fold (range, 0.4-8.6) in 14 patients after cycle 2 but decreased a median of 0.6-fold (range, 0.01-7.4) in 13 patients after cycle 4.
At the end of cycle 5, MM CSCs had increased in 4 of the 10 patients who were still on len-dex. By the end of cycle 7, MM CSCs had increased in 8 of the patients.
Circulating MM CSCs increased by the end of cycle 4 in 2 patients, and both had progressed by end of cycle 7.
Safety and next steps
The investigators said there were no serious adverse events in this trial, but 2 patients experienced grade 2 infusion reactions after the first MEDI-551 dose.
The team plans to conduct further studies to assess the long-term impact of MED-551 in MM patients and determine how the mAb might work in combination with other treatments, particularly transplant.
“In other studies at Johns Hopkins, we have found that antibody therapies can work much better after a bone marrow transplant, especially allogeneic transplants,” Dr Matsui said.
Funding and drugs for this study were provided by MedImmune Inc., the developers of MEDI-551. Drs Huff and Matsui served as a paid scientific advisory board member and a consultant to MedImmune Inc., respectively. ![]()

Photo by Linda Bartlett
NEW ORLEANS—A small study suggests that treatment with lenalidomide and dexamethasone (len-dex) prompts an increase in cancer stem cells (CSCs) for patients with newly diagnosed multiple myeloma (MM).
However, adding an anti-CD19 monoclonal antibody (mAb) to the regimen can reduce CSCs.
Most patients who received the mAb, MEDI-551, experienced a decrease in CSCs, but the cells rebounded after the patients stopped receiving MEDI-551.
And those patients who did not see a decrease in CSCs progressed. However, some patients are still in response and remain on treatment with len-dex.
The investigators believe these early results suggest prolonged treatment with len-dex and MED-551 may be safe and clinically beneficial for MM patients.
The results were presented at the 2016 AACR Annual Meeting (abstract CT102).
The study included 17 patients with newly diagnosed MM. They had a median age of 65 (range, 34-73). Most had ISS stage I (n=11), 2 had stage II, and 4 had stage III. Seven patients had t(4;14).
“We chose to carry out this clinical trial in newly diagnosed patients because our original data showed that CD19 was almost always expressed by myeloma stem cells in these patients, whereas we don’t know if that is the case in more advanced patients,” said investigator William Matsui, MD, of Johns Hopkins University School of Medicine in Baltimore, Maryland.
The patients received 28-day cycles of len-dex (len at 25 mg PO, days 1-21, and dex at 40 mg PO, weekly). Patients received MEDI-551 (at 4 mg/kg IV) in cycle 3 (day 1, 8) and cycle 4 (day 1). Responding patients continued on len-dex.
The investigators measured MM CSCs by quantifying the growth of MM colonies (CFU-MM) from marrow aspirates at baseline and at the end of cycles 2 and 4.
The team quantified peripheral blood CSCs by flow cytometry (CD19+CD27+ALDH+) at baseline and the end of cycles 2, 4, 5, and 7.
“We wanted to see if these 2 assays gave similar results, and, in this clinical trial, they were almost identical,” said investigator Carol Ann Huff, MD, also of Johns Hopkins.
“Since it is much easier to draw blood than bone marrow from our patients, we think that we can primarily use blood to track multiple myeloma stem cells in the future.”
Response
Two patients did not receive MEDI-551 due to progressive disease and noncompliance. So the investigators assessed responses in 15 patients.
After cycle 2 (len-dex alone), there were 3 very good partial responses (VGPRs), 10 partial responses (PRs), 1 molecular response, and 1 case of stable disease.
After cycle 4 (len-dex plus MEDI-551), there were 6 VGPRs, 8 PRs, and 1 molecular response.
Ten patients who completed treatment with MED-551 remain on len-dex. At the end of cycle 7, there was 1 complete response, 8 VGPRs, and 1 PR.
CFU-MM
When compared to baseline, bone-marrow-derived CFU-MM increased a median of 2.5-fold (range, 0.4-7.4) after cycle 2 but decreased a median of 0.48-fold (range, 0.14-0.85) in 14 patients after cycle 4.
The investigators compared these results to 5 newly diagnosed MM patients who only received standard treatment with len-dex.
In these patients, CFU-MM increased a median of 9.3-fold (range, 4-14) at a median of 4 months (range, 2-4). This is in spite of the fact that all of these patients had a PR or better.
Circulating CSCs
Compared to baseline, circulating MM CSCs increased a median of 1.6-fold (range, 0.4-8.6) in 14 patients after cycle 2 but decreased a median of 0.6-fold (range, 0.01-7.4) in 13 patients after cycle 4.
At the end of cycle 5, MM CSCs had increased in 4 of the 10 patients who were still on len-dex. By the end of cycle 7, MM CSCs had increased in 8 of the patients.
Circulating MM CSCs increased by the end of cycle 4 in 2 patients, and both had progressed by end of cycle 7.
Safety and next steps
The investigators said there were no serious adverse events in this trial, but 2 patients experienced grade 2 infusion reactions after the first MEDI-551 dose.
The team plans to conduct further studies to assess the long-term impact of MED-551 in MM patients and determine how the mAb might work in combination with other treatments, particularly transplant.
“In other studies at Johns Hopkins, we have found that antibody therapies can work much better after a bone marrow transplant, especially allogeneic transplants,” Dr Matsui said.
Funding and drugs for this study were provided by MedImmune Inc., the developers of MEDI-551. Drs Huff and Matsui served as a paid scientific advisory board member and a consultant to MedImmune Inc., respectively. ![]()
Antibody shows activity against ALL, CLL

Photo by Aaron Logan
NEW ORLEANS—Preclinical data suggest IMMU-114, a humanized anti-HLA-DR IgG4 antibody, is active against acute and chronic leukemias.
In a mouse model of chronic lymphocytic leukemia (CLL), IMMU-114 significantly prolonged survival when compared to rituximab.
IMMU-114 also produced a significant survival benefit in a mouse model of acute lymphoblastic leukemia (ALL) that is refractory to doxorubicin.
These results were presented at the 2016 AACR Annual Meeting (abstract 587). The research was carried out by employees of Immunomedics, Inc., the company developing IMMU-114.
The researchers generated a mouse model of human CLL by growing the cell line JVM-3 in SCID mice. The team noted that this model has similar HLA-DR and CD20 expression.
So they compared the efficacy of IMMU-114 and the anti-CD20 antibody rituximab in these mice and found that, at all doses tested, IMMU-114 significantly improved the median survival time (MST).
When both drugs were given at 50 µg, the MST was 42 days with IMMU-114 and 19 days with rituximab (P<0.0001).
When both drugs were given at 100 µg, the MSTs were 54 days and 18 days, respectively (P=0.017). And when both drugs were given at 200 µg, the MSTs were 46 days and 18 days, respectively (P<0.0001).
In control mice that received only saline, the MST was 14 days.
In in vitro experiments with the cell line JVM-3, IMMU-114 and the BTK inhibitor ibrutinib exhibited synergy fighting against the CLL cells. When given with the PI3K inhibitor idelalisib, IMMU-114 produced an additive effect.
In a doxorubicin-refractory mouse model of ALL (MN 60), IMMU-114 provided a significant survival benefit over doxorubicin and saline controls.
The MSTs were 21 days with saline, 23 days with doxorubicin, 39 days with IMMU-114 at 25 µg (P<0.0001), and 42.5 days with IMMU-114 at 50 µg (P<0.0001).
The researchers said IMMU-114 was well tolerated in these experiments, as evidenced by no significant weight loss in the mice. ![]()

Photo by Aaron Logan
NEW ORLEANS—Preclinical data suggest IMMU-114, a humanized anti-HLA-DR IgG4 antibody, is active against acute and chronic leukemias.
In a mouse model of chronic lymphocytic leukemia (CLL), IMMU-114 significantly prolonged survival when compared to rituximab.
IMMU-114 also produced a significant survival benefit in a mouse model of acute lymphoblastic leukemia (ALL) that is refractory to doxorubicin.
These results were presented at the 2016 AACR Annual Meeting (abstract 587). The research was carried out by employees of Immunomedics, Inc., the company developing IMMU-114.
The researchers generated a mouse model of human CLL by growing the cell line JVM-3 in SCID mice. The team noted that this model has similar HLA-DR and CD20 expression.
So they compared the efficacy of IMMU-114 and the anti-CD20 antibody rituximab in these mice and found that, at all doses tested, IMMU-114 significantly improved the median survival time (MST).
When both drugs were given at 50 µg, the MST was 42 days with IMMU-114 and 19 days with rituximab (P<0.0001).
When both drugs were given at 100 µg, the MSTs were 54 days and 18 days, respectively (P=0.017). And when both drugs were given at 200 µg, the MSTs were 46 days and 18 days, respectively (P<0.0001).
In control mice that received only saline, the MST was 14 days.
In in vitro experiments with the cell line JVM-3, IMMU-114 and the BTK inhibitor ibrutinib exhibited synergy fighting against the CLL cells. When given with the PI3K inhibitor idelalisib, IMMU-114 produced an additive effect.
In a doxorubicin-refractory mouse model of ALL (MN 60), IMMU-114 provided a significant survival benefit over doxorubicin and saline controls.
The MSTs were 21 days with saline, 23 days with doxorubicin, 39 days with IMMU-114 at 25 µg (P<0.0001), and 42.5 days with IMMU-114 at 50 µg (P<0.0001).
The researchers said IMMU-114 was well tolerated in these experiments, as evidenced by no significant weight loss in the mice. ![]()

Photo by Aaron Logan
NEW ORLEANS—Preclinical data suggest IMMU-114, a humanized anti-HLA-DR IgG4 antibody, is active against acute and chronic leukemias.
In a mouse model of chronic lymphocytic leukemia (CLL), IMMU-114 significantly prolonged survival when compared to rituximab.
IMMU-114 also produced a significant survival benefit in a mouse model of acute lymphoblastic leukemia (ALL) that is refractory to doxorubicin.
These results were presented at the 2016 AACR Annual Meeting (abstract 587). The research was carried out by employees of Immunomedics, Inc., the company developing IMMU-114.
The researchers generated a mouse model of human CLL by growing the cell line JVM-3 in SCID mice. The team noted that this model has similar HLA-DR and CD20 expression.
So they compared the efficacy of IMMU-114 and the anti-CD20 antibody rituximab in these mice and found that, at all doses tested, IMMU-114 significantly improved the median survival time (MST).
When both drugs were given at 50 µg, the MST was 42 days with IMMU-114 and 19 days with rituximab (P<0.0001).
When both drugs were given at 100 µg, the MSTs were 54 days and 18 days, respectively (P=0.017). And when both drugs were given at 200 µg, the MSTs were 46 days and 18 days, respectively (P<0.0001).
In control mice that received only saline, the MST was 14 days.
In in vitro experiments with the cell line JVM-3, IMMU-114 and the BTK inhibitor ibrutinib exhibited synergy fighting against the CLL cells. When given with the PI3K inhibitor idelalisib, IMMU-114 produced an additive effect.
In a doxorubicin-refractory mouse model of ALL (MN 60), IMMU-114 provided a significant survival benefit over doxorubicin and saline controls.
The MSTs were 21 days with saline, 23 days with doxorubicin, 39 days with IMMU-114 at 25 µg (P<0.0001), and 42.5 days with IMMU-114 at 50 µg (P<0.0001).
The researchers said IMMU-114 was well tolerated in these experiments, as evidenced by no significant weight loss in the mice. ![]()