User login
European Society of Cardiology (ESC): Annual Congress
ESC: New support for aspirin’s anticancer effect
LONDON – The new U.S. Preventive Services Task Force draft recommendation for daily low-dose aspirin to reduce the risk of colorectal cancer has been shored up by three large new positive case-control studies presented at the annual congress of the European Society of Cardiology.
The studies weren’t considered by the USPSTF in its deliberations. They’re too new. But the three studies, each with a different methodologic design, collectively included more than 2 million subjects. And each study showed roughly a 30% reduction in the risk of colorectal cancer in daily aspirin users.
Dr. Luis A. Garcia Rodriguez presented the three studies, each one an analysis based on data extracted from the Health Improvement Network U.K. primary care database.
The first study included 170,336 new users of low-dose aspirin for primary or secondary prevention of cardiovascular disease and an equal number of individually matched non–aspirin-user controls. This study was designed to simulate enrollment into a placebo-controlled clinical trial of aspirin. During a median 5.1 years of follow-up and after adjustment for numerous potential confounders including smoking, body mass index, and use of nonsteroidal anti-inflammatory drugs, current users of aspirin were found to have a 34% relative risk reduction for colorectal cancer, reported Dr. Garcia Rodriguez, director of the Spanish Center for Pharmacoepidemiologic Research in Madrid.
The second study compared 171,527 new users of low-dose aspirin with 149,597 new users of acetaminophen. Acetaminophen users were selected as a control group to minimize any healthy-user bias arising from differences between aspirin users and nonusers in the first study. In study two, over a median 5.8 years of follow-up, the aspirin users had an adjusted 29% relative risk reduction for colorectal cancer.
The third study used a nested case-control methodology. Dr. Garcia Rodriguez and his associates began with a pool of 1,935,801 patients naive to daily low-dose aspirin. They then identified the individuals within that cohort who were diagnosed with colorectal cancer during 7.5 years of follow-up, matched them to 20,000 controls by age and gender, and determined who had initiated daily low-dose aspirin for cardiovascular prevention during the follow-up period. The daily aspirin users proved to be at an adjusted 31% lower risk of colorectal cancer than nonusers.
The fact that a consistent protective effect against colorectal cancer was seen in all three studies argues against the findings being explainable on the basis of selection bias, Dr. Garcia Rodriguez asserted.
In all three studies, the protective effect against colorectal cancer was greater in patients on low-dose aspirin for secondary rather than primary cardiovascular prevention. Patients taking aspirin for secondary prevention in studies one, two, and three had relative risk reductions in colorectal cancer of 40%, 38%, and 39%, respectively, compared with non–aspirin-using controls. Among individuals on aspirin for primary cardiovascular prevention, the relative risk reductions for colorectal cancer were 29%, 22%, and 25% across the three studies.
The studies were funded by Bayer Pharma. Dr. Garcia Rodriguez has served as an advisory board member for the company.
This article was updated 10/14/2015.
LONDON – The new U.S. Preventive Services Task Force draft recommendation for daily low-dose aspirin to reduce the risk of colorectal cancer has been shored up by three large new positive case-control studies presented at the annual congress of the European Society of Cardiology.
The studies weren’t considered by the USPSTF in its deliberations. They’re too new. But the three studies, each with a different methodologic design, collectively included more than 2 million subjects. And each study showed roughly a 30% reduction in the risk of colorectal cancer in daily aspirin users.
Dr. Luis A. Garcia Rodriguez presented the three studies, each one an analysis based on data extracted from the Health Improvement Network U.K. primary care database.
The first study included 170,336 new users of low-dose aspirin for primary or secondary prevention of cardiovascular disease and an equal number of individually matched non–aspirin-user controls. This study was designed to simulate enrollment into a placebo-controlled clinical trial of aspirin. During a median 5.1 years of follow-up and after adjustment for numerous potential confounders including smoking, body mass index, and use of nonsteroidal anti-inflammatory drugs, current users of aspirin were found to have a 34% relative risk reduction for colorectal cancer, reported Dr. Garcia Rodriguez, director of the Spanish Center for Pharmacoepidemiologic Research in Madrid.
The second study compared 171,527 new users of low-dose aspirin with 149,597 new users of acetaminophen. Acetaminophen users were selected as a control group to minimize any healthy-user bias arising from differences between aspirin users and nonusers in the first study. In study two, over a median 5.8 years of follow-up, the aspirin users had an adjusted 29% relative risk reduction for colorectal cancer.
The third study used a nested case-control methodology. Dr. Garcia Rodriguez and his associates began with a pool of 1,935,801 patients naive to daily low-dose aspirin. They then identified the individuals within that cohort who were diagnosed with colorectal cancer during 7.5 years of follow-up, matched them to 20,000 controls by age and gender, and determined who had initiated daily low-dose aspirin for cardiovascular prevention during the follow-up period. The daily aspirin users proved to be at an adjusted 31% lower risk of colorectal cancer than nonusers.
The fact that a consistent protective effect against colorectal cancer was seen in all three studies argues against the findings being explainable on the basis of selection bias, Dr. Garcia Rodriguez asserted.
In all three studies, the protective effect against colorectal cancer was greater in patients on low-dose aspirin for secondary rather than primary cardiovascular prevention. Patients taking aspirin for secondary prevention in studies one, two, and three had relative risk reductions in colorectal cancer of 40%, 38%, and 39%, respectively, compared with non–aspirin-using controls. Among individuals on aspirin for primary cardiovascular prevention, the relative risk reductions for colorectal cancer were 29%, 22%, and 25% across the three studies.
The studies were funded by Bayer Pharma. Dr. Garcia Rodriguez has served as an advisory board member for the company.
This article was updated 10/14/2015.
LONDON – The new U.S. Preventive Services Task Force draft recommendation for daily low-dose aspirin to reduce the risk of colorectal cancer has been shored up by three large new positive case-control studies presented at the annual congress of the European Society of Cardiology.
The studies weren’t considered by the USPSTF in its deliberations. They’re too new. But the three studies, each with a different methodologic design, collectively included more than 2 million subjects. And each study showed roughly a 30% reduction in the risk of colorectal cancer in daily aspirin users.
Dr. Luis A. Garcia Rodriguez presented the three studies, each one an analysis based on data extracted from the Health Improvement Network U.K. primary care database.
The first study included 170,336 new users of low-dose aspirin for primary or secondary prevention of cardiovascular disease and an equal number of individually matched non–aspirin-user controls. This study was designed to simulate enrollment into a placebo-controlled clinical trial of aspirin. During a median 5.1 years of follow-up and after adjustment for numerous potential confounders including smoking, body mass index, and use of nonsteroidal anti-inflammatory drugs, current users of aspirin were found to have a 34% relative risk reduction for colorectal cancer, reported Dr. Garcia Rodriguez, director of the Spanish Center for Pharmacoepidemiologic Research in Madrid.
The second study compared 171,527 new users of low-dose aspirin with 149,597 new users of acetaminophen. Acetaminophen users were selected as a control group to minimize any healthy-user bias arising from differences between aspirin users and nonusers in the first study. In study two, over a median 5.8 years of follow-up, the aspirin users had an adjusted 29% relative risk reduction for colorectal cancer.
The third study used a nested case-control methodology. Dr. Garcia Rodriguez and his associates began with a pool of 1,935,801 patients naive to daily low-dose aspirin. They then identified the individuals within that cohort who were diagnosed with colorectal cancer during 7.5 years of follow-up, matched them to 20,000 controls by age and gender, and determined who had initiated daily low-dose aspirin for cardiovascular prevention during the follow-up period. The daily aspirin users proved to be at an adjusted 31% lower risk of colorectal cancer than nonusers.
The fact that a consistent protective effect against colorectal cancer was seen in all three studies argues against the findings being explainable on the basis of selection bias, Dr. Garcia Rodriguez asserted.
In all three studies, the protective effect against colorectal cancer was greater in patients on low-dose aspirin for secondary rather than primary cardiovascular prevention. Patients taking aspirin for secondary prevention in studies one, two, and three had relative risk reductions in colorectal cancer of 40%, 38%, and 39%, respectively, compared with non–aspirin-using controls. Among individuals on aspirin for primary cardiovascular prevention, the relative risk reductions for colorectal cancer were 29%, 22%, and 25% across the three studies.
The studies were funded by Bayer Pharma. Dr. Garcia Rodriguez has served as an advisory board member for the company.
This article was updated 10/14/2015.
AT THE ESC CONGRESS 2015
Key clinical point: Each of three new case-control studies concluded that daily low-dose aspirin for cardiovascular prevention reduced the risk of developing colorectal cancer by about 30%.
Major finding: Relative risk reductions for colorectal cancer among aspirin users during median follow-up periods of 5.1-7.5 years in the three studies were 34%, 29%, and 31%.
Data source: The three studies, which included well over 2 million patients, were based on data extracted from a large U.K. primary care network database.
Disclosures: The studies were funded by Bayer Pharma. Dr. Rodriguez has served as an advisory board member for the company.
ESC: Obstructive sleep apnea often complicates heart failure
LONDON – The majority of patients with severe heart failure had sleep-disordered breathing and, in most affected patients, this manifested as obstructive sleep apnea, in an analysis of more than 1,000 German heart failure patients enrolled in a multicenter registry.
“The vast majority of heart failure patients with sleep-disordered breathing [SDB] have obstructive sleep apnea, which differs from previous results,” said Dr. Olaf Oldenburg at the annual congress of the European Society of Cardiology. Possible reasons why this German registry had different findings, compared with prior reports, were its inclusion of heart failure patients with milder symptoms, inclusion of patients with preserved ejection fraction, and inclusion of more women, suggested Dr. Oldenburg, director of the sleep laboratory at the Heart and Diabetes Center of Ruhr University of Bochum in Bad Oeynhausen, Germany.
His finding that nearly two-thirds of the heart failure patients with SDB in this registry had obstructive sleep apnea and that one-third had moderate or severe obstructive sleep apnea was notable because this remains a form of sleep-disordered breathing that can be treated, he said. “There is still enough evidence to treat obstructive sleep apnea” in heart failure patients when it has a moderate or severe presentation, which is defined as causing 15 or more apnea-hypopnea events/hour during sleep. “Obstructive sleep apnea is definitely not a compensatory mechanism in heart failure,” Dr. Oldenburg said.
He highlighted the ongoing need to treat more severe obstructive sleep apnea in heart failure patients because this form of SDB sharply contrasts with the results of the SERVE-HF trial, also reported at the congress, which showed that in patients with advanced heart failure and central sleep apnea nocturnal treatment with adaptive servo ventilation failed to provide benefit and also appeared to boost patient mortality (N Engl J Med. 2015 Sep 17;373[12]:1095-105).
Following that report “we need to think about which heart failure patients to treat” with nocturnal ventilation, and differentiate between heart failure patients with obstructive sleep apnea and those with central sleep apnea, Dr. Oldenburg said.
The data he reported came from the SchlaHF-XT (Sleep Disordered Breathing in Heart Failure) registry, which enrolled patients with heart failure and reduced or preserved ejection fraction and any New York Heart Association functional class treated either at German hospitals or in physician offices. He reported data for 1,186 fully assessed and classified patients, who averaged 68 years old and two-thirds of whom were men. Slightly more than half had heart failure with reduced ejection fraction, and about half had New York Heart Association class II heart failure, a quarter had class III heart failure, with the remaining patients divided roughly equally between class I and IV.
Screening for SDB showed that 24% had no SDB, 37% had mild SBD, 21% had moderate SDB, and 19% had severe SDB (percentages total 101% because of rounding). Among those with SDB, 64% had obstructive sleep apnea, 22% had central sleep apnea, and the remaining 14% had either a mixed form of sleep apnea or were not classifiable.
The analysis also showed that moderate and severe SDB, the forms that require treatment, occurred more often among patients with heart failure and reduced ejection fraction, 43%, compared with patients with heart failure and preserved ejection fraction, who had a 36% prevalence of SDB requiring treatment. Moderate or severe central sleep apnea occurred in 15% of patients with reduced ejection fraction and in 9% of patients with preserved ejection fraction.
A second report at the congress by Dr. Oldenburg showed that the duration of time when a patient’s oxygen saturation fell below 90% was a better gauge of the severity of SDB than was the traditional measure of the apnea-hypopnea index (AHI), the average number of apnea-hypopnea episodes a patient has during an hour of sleep. For this analysis, he used data collected on 963 patients with chronic, stable heart failure with reduced ejection fraction who underwent a comprehensive sleep study with pulse oximetry measurements during 2002-2013.
The results showed that while the measured AHI significantly linked with the 5-year mortality rate of these patients, the relationship became statistically insignificant after researchers adjusted for age, sex, body mass index, heart failure severity, ejection fraction, medications, and other clinical variables.
In contrast, the average time a patient spent with an oxygen saturation level below 90% overnight strong linked with 5-year mortality even after adjusting for all these covariables. The analysis showed that each hour of sleep a heart failure patient spent with an oxygen saturation level below 90% linked with a relative 16% reduction in 5-year survival. Patients in the quartile with the greatest amount of time spent with a oxygen saturation level below 90% had a 50% 5-year mortality rate, while those in the quartile with the least amount of time spent with severely depressed oxygen saturation had a 30% 5-year mortality rate.
Based on this finding “you need to look at the effect of SDB and not just the apnea-hypopnea index” when assessing SDB in patients with heart failure, Dr. Oldenburg said.
On Twitter @mitchelzoler
LONDON – The majority of patients with severe heart failure had sleep-disordered breathing and, in most affected patients, this manifested as obstructive sleep apnea, in an analysis of more than 1,000 German heart failure patients enrolled in a multicenter registry.
“The vast majority of heart failure patients with sleep-disordered breathing [SDB] have obstructive sleep apnea, which differs from previous results,” said Dr. Olaf Oldenburg at the annual congress of the European Society of Cardiology. Possible reasons why this German registry had different findings, compared with prior reports, were its inclusion of heart failure patients with milder symptoms, inclusion of patients with preserved ejection fraction, and inclusion of more women, suggested Dr. Oldenburg, director of the sleep laboratory at the Heart and Diabetes Center of Ruhr University of Bochum in Bad Oeynhausen, Germany.
His finding that nearly two-thirds of the heart failure patients with SDB in this registry had obstructive sleep apnea and that one-third had moderate or severe obstructive sleep apnea was notable because this remains a form of sleep-disordered breathing that can be treated, he said. “There is still enough evidence to treat obstructive sleep apnea” in heart failure patients when it has a moderate or severe presentation, which is defined as causing 15 or more apnea-hypopnea events/hour during sleep. “Obstructive sleep apnea is definitely not a compensatory mechanism in heart failure,” Dr. Oldenburg said.
He highlighted the ongoing need to treat more severe obstructive sleep apnea in heart failure patients because this form of SDB sharply contrasts with the results of the SERVE-HF trial, also reported at the congress, which showed that in patients with advanced heart failure and central sleep apnea nocturnal treatment with adaptive servo ventilation failed to provide benefit and also appeared to boost patient mortality (N Engl J Med. 2015 Sep 17;373[12]:1095-105).
Following that report “we need to think about which heart failure patients to treat” with nocturnal ventilation, and differentiate between heart failure patients with obstructive sleep apnea and those with central sleep apnea, Dr. Oldenburg said.
The data he reported came from the SchlaHF-XT (Sleep Disordered Breathing in Heart Failure) registry, which enrolled patients with heart failure and reduced or preserved ejection fraction and any New York Heart Association functional class treated either at German hospitals or in physician offices. He reported data for 1,186 fully assessed and classified patients, who averaged 68 years old and two-thirds of whom were men. Slightly more than half had heart failure with reduced ejection fraction, and about half had New York Heart Association class II heart failure, a quarter had class III heart failure, with the remaining patients divided roughly equally between class I and IV.
Screening for SDB showed that 24% had no SDB, 37% had mild SBD, 21% had moderate SDB, and 19% had severe SDB (percentages total 101% because of rounding). Among those with SDB, 64% had obstructive sleep apnea, 22% had central sleep apnea, and the remaining 14% had either a mixed form of sleep apnea or were not classifiable.
The analysis also showed that moderate and severe SDB, the forms that require treatment, occurred more often among patients with heart failure and reduced ejection fraction, 43%, compared with patients with heart failure and preserved ejection fraction, who had a 36% prevalence of SDB requiring treatment. Moderate or severe central sleep apnea occurred in 15% of patients with reduced ejection fraction and in 9% of patients with preserved ejection fraction.
A second report at the congress by Dr. Oldenburg showed that the duration of time when a patient’s oxygen saturation fell below 90% was a better gauge of the severity of SDB than was the traditional measure of the apnea-hypopnea index (AHI), the average number of apnea-hypopnea episodes a patient has during an hour of sleep. For this analysis, he used data collected on 963 patients with chronic, stable heart failure with reduced ejection fraction who underwent a comprehensive sleep study with pulse oximetry measurements during 2002-2013.
The results showed that while the measured AHI significantly linked with the 5-year mortality rate of these patients, the relationship became statistically insignificant after researchers adjusted for age, sex, body mass index, heart failure severity, ejection fraction, medications, and other clinical variables.
In contrast, the average time a patient spent with an oxygen saturation level below 90% overnight strong linked with 5-year mortality even after adjusting for all these covariables. The analysis showed that each hour of sleep a heart failure patient spent with an oxygen saturation level below 90% linked with a relative 16% reduction in 5-year survival. Patients in the quartile with the greatest amount of time spent with a oxygen saturation level below 90% had a 50% 5-year mortality rate, while those in the quartile with the least amount of time spent with severely depressed oxygen saturation had a 30% 5-year mortality rate.
Based on this finding “you need to look at the effect of SDB and not just the apnea-hypopnea index” when assessing SDB in patients with heart failure, Dr. Oldenburg said.
On Twitter @mitchelzoler
LONDON – The majority of patients with severe heart failure had sleep-disordered breathing and, in most affected patients, this manifested as obstructive sleep apnea, in an analysis of more than 1,000 German heart failure patients enrolled in a multicenter registry.
“The vast majority of heart failure patients with sleep-disordered breathing [SDB] have obstructive sleep apnea, which differs from previous results,” said Dr. Olaf Oldenburg at the annual congress of the European Society of Cardiology. Possible reasons why this German registry had different findings, compared with prior reports, were its inclusion of heart failure patients with milder symptoms, inclusion of patients with preserved ejection fraction, and inclusion of more women, suggested Dr. Oldenburg, director of the sleep laboratory at the Heart and Diabetes Center of Ruhr University of Bochum in Bad Oeynhausen, Germany.
His finding that nearly two-thirds of the heart failure patients with SDB in this registry had obstructive sleep apnea and that one-third had moderate or severe obstructive sleep apnea was notable because this remains a form of sleep-disordered breathing that can be treated, he said. “There is still enough evidence to treat obstructive sleep apnea” in heart failure patients when it has a moderate or severe presentation, which is defined as causing 15 or more apnea-hypopnea events/hour during sleep. “Obstructive sleep apnea is definitely not a compensatory mechanism in heart failure,” Dr. Oldenburg said.
He highlighted the ongoing need to treat more severe obstructive sleep apnea in heart failure patients because this form of SDB sharply contrasts with the results of the SERVE-HF trial, also reported at the congress, which showed that in patients with advanced heart failure and central sleep apnea nocturnal treatment with adaptive servo ventilation failed to provide benefit and also appeared to boost patient mortality (N Engl J Med. 2015 Sep 17;373[12]:1095-105).
Following that report “we need to think about which heart failure patients to treat” with nocturnal ventilation, and differentiate between heart failure patients with obstructive sleep apnea and those with central sleep apnea, Dr. Oldenburg said.
The data he reported came from the SchlaHF-XT (Sleep Disordered Breathing in Heart Failure) registry, which enrolled patients with heart failure and reduced or preserved ejection fraction and any New York Heart Association functional class treated either at German hospitals or in physician offices. He reported data for 1,186 fully assessed and classified patients, who averaged 68 years old and two-thirds of whom were men. Slightly more than half had heart failure with reduced ejection fraction, and about half had New York Heart Association class II heart failure, a quarter had class III heart failure, with the remaining patients divided roughly equally between class I and IV.
Screening for SDB showed that 24% had no SDB, 37% had mild SBD, 21% had moderate SDB, and 19% had severe SDB (percentages total 101% because of rounding). Among those with SDB, 64% had obstructive sleep apnea, 22% had central sleep apnea, and the remaining 14% had either a mixed form of sleep apnea or were not classifiable.
The analysis also showed that moderate and severe SDB, the forms that require treatment, occurred more often among patients with heart failure and reduced ejection fraction, 43%, compared with patients with heart failure and preserved ejection fraction, who had a 36% prevalence of SDB requiring treatment. Moderate or severe central sleep apnea occurred in 15% of patients with reduced ejection fraction and in 9% of patients with preserved ejection fraction.
A second report at the congress by Dr. Oldenburg showed that the duration of time when a patient’s oxygen saturation fell below 90% was a better gauge of the severity of SDB than was the traditional measure of the apnea-hypopnea index (AHI), the average number of apnea-hypopnea episodes a patient has during an hour of sleep. For this analysis, he used data collected on 963 patients with chronic, stable heart failure with reduced ejection fraction who underwent a comprehensive sleep study with pulse oximetry measurements during 2002-2013.
The results showed that while the measured AHI significantly linked with the 5-year mortality rate of these patients, the relationship became statistically insignificant after researchers adjusted for age, sex, body mass index, heart failure severity, ejection fraction, medications, and other clinical variables.
In contrast, the average time a patient spent with an oxygen saturation level below 90% overnight strong linked with 5-year mortality even after adjusting for all these covariables. The analysis showed that each hour of sleep a heart failure patient spent with an oxygen saturation level below 90% linked with a relative 16% reduction in 5-year survival. Patients in the quartile with the greatest amount of time spent with a oxygen saturation level below 90% had a 50% 5-year mortality rate, while those in the quartile with the least amount of time spent with severely depressed oxygen saturation had a 30% 5-year mortality rate.
Based on this finding “you need to look at the effect of SDB and not just the apnea-hypopnea index” when assessing SDB in patients with heart failure, Dr. Oldenburg said.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2015
Key clinical point: Significant sleep disordered breathing is common among heart failure patients, most often manifesting as obstructive sleep apnea.
Major finding: In a real-world population of heart failure patients 64% of those with sleep disordered breathing had obstructive sleep apnea.
Data source: The SchlaHF-XT registry with 1,1816 heart failure patients enrolled at several German hospitals and private practices.
Disclosures: The SchlaHF-XT registry was sponsored by ResMed. Dr. Oldenburg said that he has received research support from several companies.
ESC: Registry confirms liver dysfunction’s heart-failure importance
LONDON – Hepatic dysfunction, which is beginning to be appreciated as an important complication of patients with acute heart failure, boosted the relative rate of death in patients with acute heart failure by 57% in an analysis of more than 5,000 patients followed for a year in a registry maintained by the European Society of Cardiology (ESC).
The registry data also showed that at entry into the registry the acute heart failure patients had an 8% prevalence of hepatic dysfunction.
These observations on the prevalence and impact of liver dysfunction in acute heart failure patients highlight a new recognition that acute heart failure can trigger morbidity and mortality through its hepatic effects in a manner similar to the better-appreciated effect that acute heart failure has on renal and pulmonary function, Dr. Alexandre Mebazaa said at the annual congress of the European Society of Cardiology.
“Everybody knows about the kidney, but not many know about the liver,” said Dr. Mebazaa in an interview. Recent studies on mechanisms of organ damage during acute heart failure episodes indicates that the liver, kidneys, and lungs are all damaged by fluid congestion in these organs, he said. “Fluid overload leads to organ dysfunction,” and this complication seems relatively resistant to the diuretic treatment that acute heart failure patients typically receive when they are hospitalized for decompensation episodes. “New approaches are needed to remove fluid from the organs. Diuretics remove fluid from the blood, but not from the organs,” said Dr. Mebazaa, a professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
The data Dr. Mebazaa reported at the congress were the first 1-year follow-up data from the ESC’s Heart Failure Long-Term Registry, begun in 2012 and involving centers from more than 30 countries in Europe as well as in North Africa and the Middle East. The panel of ESC members who oversee this registry “selected centers dedicated to the database,” he said.
The registry followed 5,039 patients with acute heart failure for at least 1 year, as well as 7,401 patients diagnosed with chronic heart failure. Another striking, though not surprising finding of the 1-year follow-up data was the disparity in mortality and hospitalization rates between these two subgroups.
After 1 year, the mortality rate was 24% among the acute heart failure patients, compared with 6% among the chronic heart failure patients. Dr. Mebazaa called the acute heart failure mortality rate “terrible.”
Hospitalization rates in the two subgroups over the 1-year follow-up ran to 19% among the acute heart failure patients ands 10% for chronic heart failure patients. The combined rate of death or hospitalization over 1 year was 36% in the acute heart failure patients and 15% in those with chronic heart failure.
These data “show that we really need new treatments for acute heart failure to reduce rehospitalizations and deaths,” Dr. Mebazaa said.
A multivariate adjusted analysis identified several baseline factors that significantly linked with mortality among the acute heart-failure patients during the 1-year follow-up. In addition to hepatic dysfunction linking with a 57% increased rate of death other factors included age, which linked with a 24% increased rate for every 5 years of older age; New York Heart Association class III or IV severity, which linked with 50% higher mortality; renal dysfunction, which linked with a 52% higher mortality rate; and aortic stenosis, linked with a 54% increased mortality rate.
The finding that hepatic dysfunction was an independent mortality risk in acute heart failure confirmed a finding that Dr. Mebazaa and his associates reported in 2013 in a post hoc analysis of 1,134 patients with acute heart failure who had been enrolled in a drug-intervention trial (Eur Heart J. 2013 Sep 15;34:742-9). The registry results confirm for the first time that finding in an independent and much larger group of patients, Dr. Mebazaa said.
On Twitter @mitchelzoler
LONDON – Hepatic dysfunction, which is beginning to be appreciated as an important complication of patients with acute heart failure, boosted the relative rate of death in patients with acute heart failure by 57% in an analysis of more than 5,000 patients followed for a year in a registry maintained by the European Society of Cardiology (ESC).
The registry data also showed that at entry into the registry the acute heart failure patients had an 8% prevalence of hepatic dysfunction.
These observations on the prevalence and impact of liver dysfunction in acute heart failure patients highlight a new recognition that acute heart failure can trigger morbidity and mortality through its hepatic effects in a manner similar to the better-appreciated effect that acute heart failure has on renal and pulmonary function, Dr. Alexandre Mebazaa said at the annual congress of the European Society of Cardiology.
“Everybody knows about the kidney, but not many know about the liver,” said Dr. Mebazaa in an interview. Recent studies on mechanisms of organ damage during acute heart failure episodes indicates that the liver, kidneys, and lungs are all damaged by fluid congestion in these organs, he said. “Fluid overload leads to organ dysfunction,” and this complication seems relatively resistant to the diuretic treatment that acute heart failure patients typically receive when they are hospitalized for decompensation episodes. “New approaches are needed to remove fluid from the organs. Diuretics remove fluid from the blood, but not from the organs,” said Dr. Mebazaa, a professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
The data Dr. Mebazaa reported at the congress were the first 1-year follow-up data from the ESC’s Heart Failure Long-Term Registry, begun in 2012 and involving centers from more than 30 countries in Europe as well as in North Africa and the Middle East. The panel of ESC members who oversee this registry “selected centers dedicated to the database,” he said.
The registry followed 5,039 patients with acute heart failure for at least 1 year, as well as 7,401 patients diagnosed with chronic heart failure. Another striking, though not surprising finding of the 1-year follow-up data was the disparity in mortality and hospitalization rates between these two subgroups.
After 1 year, the mortality rate was 24% among the acute heart failure patients, compared with 6% among the chronic heart failure patients. Dr. Mebazaa called the acute heart failure mortality rate “terrible.”
Hospitalization rates in the two subgroups over the 1-year follow-up ran to 19% among the acute heart failure patients ands 10% for chronic heart failure patients. The combined rate of death or hospitalization over 1 year was 36% in the acute heart failure patients and 15% in those with chronic heart failure.
These data “show that we really need new treatments for acute heart failure to reduce rehospitalizations and deaths,” Dr. Mebazaa said.
A multivariate adjusted analysis identified several baseline factors that significantly linked with mortality among the acute heart-failure patients during the 1-year follow-up. In addition to hepatic dysfunction linking with a 57% increased rate of death other factors included age, which linked with a 24% increased rate for every 5 years of older age; New York Heart Association class III or IV severity, which linked with 50% higher mortality; renal dysfunction, which linked with a 52% higher mortality rate; and aortic stenosis, linked with a 54% increased mortality rate.
The finding that hepatic dysfunction was an independent mortality risk in acute heart failure confirmed a finding that Dr. Mebazaa and his associates reported in 2013 in a post hoc analysis of 1,134 patients with acute heart failure who had been enrolled in a drug-intervention trial (Eur Heart J. 2013 Sep 15;34:742-9). The registry results confirm for the first time that finding in an independent and much larger group of patients, Dr. Mebazaa said.
On Twitter @mitchelzoler
LONDON – Hepatic dysfunction, which is beginning to be appreciated as an important complication of patients with acute heart failure, boosted the relative rate of death in patients with acute heart failure by 57% in an analysis of more than 5,000 patients followed for a year in a registry maintained by the European Society of Cardiology (ESC).
The registry data also showed that at entry into the registry the acute heart failure patients had an 8% prevalence of hepatic dysfunction.
These observations on the prevalence and impact of liver dysfunction in acute heart failure patients highlight a new recognition that acute heart failure can trigger morbidity and mortality through its hepatic effects in a manner similar to the better-appreciated effect that acute heart failure has on renal and pulmonary function, Dr. Alexandre Mebazaa said at the annual congress of the European Society of Cardiology.
“Everybody knows about the kidney, but not many know about the liver,” said Dr. Mebazaa in an interview. Recent studies on mechanisms of organ damage during acute heart failure episodes indicates that the liver, kidneys, and lungs are all damaged by fluid congestion in these organs, he said. “Fluid overload leads to organ dysfunction,” and this complication seems relatively resistant to the diuretic treatment that acute heart failure patients typically receive when they are hospitalized for decompensation episodes. “New approaches are needed to remove fluid from the organs. Diuretics remove fluid from the blood, but not from the organs,” said Dr. Mebazaa, a professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
The data Dr. Mebazaa reported at the congress were the first 1-year follow-up data from the ESC’s Heart Failure Long-Term Registry, begun in 2012 and involving centers from more than 30 countries in Europe as well as in North Africa and the Middle East. The panel of ESC members who oversee this registry “selected centers dedicated to the database,” he said.
The registry followed 5,039 patients with acute heart failure for at least 1 year, as well as 7,401 patients diagnosed with chronic heart failure. Another striking, though not surprising finding of the 1-year follow-up data was the disparity in mortality and hospitalization rates between these two subgroups.
After 1 year, the mortality rate was 24% among the acute heart failure patients, compared with 6% among the chronic heart failure patients. Dr. Mebazaa called the acute heart failure mortality rate “terrible.”
Hospitalization rates in the two subgroups over the 1-year follow-up ran to 19% among the acute heart failure patients ands 10% for chronic heart failure patients. The combined rate of death or hospitalization over 1 year was 36% in the acute heart failure patients and 15% in those with chronic heart failure.
These data “show that we really need new treatments for acute heart failure to reduce rehospitalizations and deaths,” Dr. Mebazaa said.
A multivariate adjusted analysis identified several baseline factors that significantly linked with mortality among the acute heart-failure patients during the 1-year follow-up. In addition to hepatic dysfunction linking with a 57% increased rate of death other factors included age, which linked with a 24% increased rate for every 5 years of older age; New York Heart Association class III or IV severity, which linked with 50% higher mortality; renal dysfunction, which linked with a 52% higher mortality rate; and aortic stenosis, linked with a 54% increased mortality rate.
The finding that hepatic dysfunction was an independent mortality risk in acute heart failure confirmed a finding that Dr. Mebazaa and his associates reported in 2013 in a post hoc analysis of 1,134 patients with acute heart failure who had been enrolled in a drug-intervention trial (Eur Heart J. 2013 Sep 15;34:742-9). The registry results confirm for the first time that finding in an independent and much larger group of patients, Dr. Mebazaa said.
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2015
Key clinical point: Liver dysfunction was a statistically significant, independent predictor of 1-year mortality in acute heart-failure patients.
Major finding: Acute heart-failure patients had a 57% relative increased rate of 1-year death, compared with patients without liver dysfunction.
Data source: One-year follow-up of 5,039 patients with acute heart failure enrolled in a multinational registry maintained by the European Society of Cardiology.
Disclosures: Dr. Mebazaa has received speaking honoraria and consulting fees from 11 drug or diagnostic companies.
ESC: Alirocumab ‘cures’ heterozygous familial hypercholesterolemia
LONDON – The largest-ever treatment study of patients with heterozygous familial hypercholesterolemia shows that the PCSK9 inhibitor alirocumab achieves “truly astounding” reductions in LDL cholesterol and atherogenic lipid particles through 78 weeks of follow-up, Dr. John J.P. Kastelein said at the annual congress of the European Society of Cardiology.
“These findings show reductions in LDL levels in these individuals that were completely impossible until now. Basically, this therapy in conjunction with statins and ezetimibe cures this genetic disease because LDL levels in these individuals are now lower than in the general population, which is, of course, something that we’ve never seen before,” said Dr. Kastelein, chairman of the department of vascular medicine at the University of Amsterdam.
Heterozygous familial hypercholesterolemia (HeFH) is the most common autosomal dominant genetic disorder, with an estimated prevalence worldwide of 1 in 200 in the general population. The associated highly elevated LDL levels bring a markedly increased risk of premature cardiovascular events. Only about 20% of patients with HeFH are able to achieve an LDL of 100 mg/dL or less despite maximum-tolerated doses of statins plus ezetimibe (Vytorin).
Dr. Kastelein presented the 78-week lipid-lowering results from four phase III randomized, double-blind clinical trials involving 1,257 HeFH patients unable to attain LDL goals despite maximum-tolerated statin therapy, typically supplemented with ezetimibe.
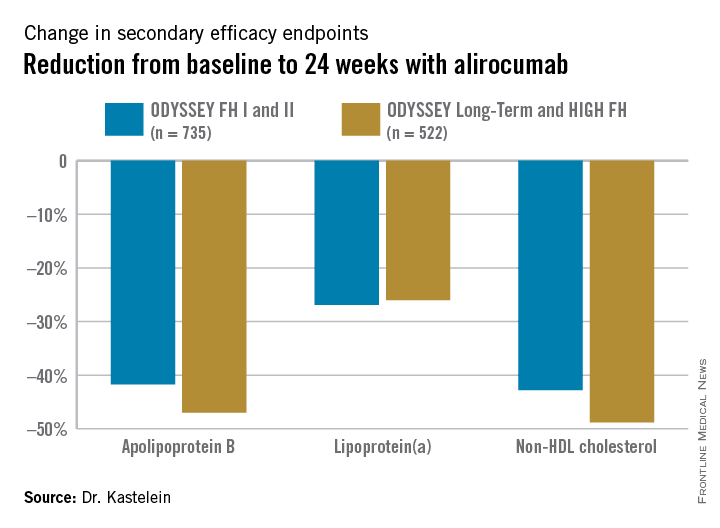
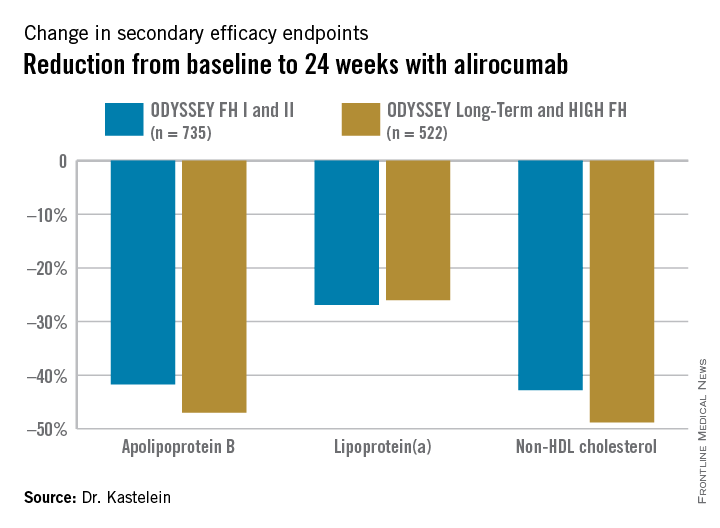
Participants were randomly assigned 2:1 to biweekly subcutaneous injections of alirocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) or placebo on top of background maximum tolerated statin therapy, usually accompanied by ezetimibe. The ODYSSEY FH I and FH II studies included 735 patients, with the alirocumab group starting on the PCSK9 inhibitor at 75 mg every 2 weeks, with the dose being doubled to 150 mg at 24 weeks in patients who hadn’t achieved an LDL level of 70 mg/dL or less at that point. The 522 HeFH patients in the ODYSSEY Long-Term study and the HIGH FH trial had higher baseline LDL levels and greater cardiovascular risk, and they therefore were on alirocumab at 150 mg every 2 weeks or placebo from the outset.
Fifty percent of alirocumab-treated patients in the FH I and II studies and 63% in the other two trials achieved an LDL level below 70 mg/dL at week 24, as did 0.6% and 1% of control patients, respectively. Dr. Kastelein characterized that as a jaw-dropping result.
“I have seen very few HeFH patients in my clinic – which is one of the largest in the world in terms of numbers of patients – who reached the goal of an LDL below 70 mg/dL,” he commented.
The outcomes in terms of secondary lipid endpoints provided an added bonus.
Absolutely no tachyphylaxis or return toward baseline LDL occurred over the course of 78 weeks of treatment, Dr. Kastelein continued.
The safety performance of alirocumab was highly reassuring: Treatment-emergent adverse events occurred in 3.9% of 837 alirocumab-treated patients and 3.6% on placebo. Overall, there was no significant difference between the two groups in rates of headache, musculoskeletal or connective tissue disorders, infections, injection-site reactions, adjudicated cardiovascular events, allergic reactions, neurocognitive disorders, elevated liver enzymes, or any other safety endpoint.
Both alirocumab (Praluent) and evolocumab (Repatha), another PCSK9 inhibitor, have been approved for the treatment of familial hypercholesterolemia by the U.S. Food and Drug Administration and European regulators.
The next round of PCSK9 inhibitor studies, now ongoing, involves enrolling 70,000 subjects at increased cardiovascular risk in order to assess whether the level of LDL lowering achieved with these novel agents translates into a reduction in cardiovascular events.
Session cochair Dr. Barbara Casadei, professor of cardiovascular medicine at the University of Oxford (England), posed a question: Statin therapy has been shown to increase the risk of new-onset type 2 diabetes – how about the PCSK9 inhibitors?
Dr. Kastelein replied that he recently published a study of more than 25,000 Dutch individuals with familial hypercholesterolemia and showed that their prevalence of type 2 diabetes was significantly lower than in their unaffected relatives (JAMA 2015 Mar 10;313[10]:1029-36).
“One of my theories is that the PCSK9 inhibitors reduce the number of LDL receptors on beta cells in the pancreas, so there’s less cholesterol influx, and since cholesterol is toxic to beta cells, the beta cells of PCSK9-inhibitor-treated patients live longer and therefore these individuals will have less type 2 diabetes. Statins do exactly the opposite: They up-regulate LDL receptors, so there will be more cholesterol coming into the beta cell and the theory is that this will result in an increase in type 2 diabetes,” he explained.
The HeFH studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
LONDON – The largest-ever treatment study of patients with heterozygous familial hypercholesterolemia shows that the PCSK9 inhibitor alirocumab achieves “truly astounding” reductions in LDL cholesterol and atherogenic lipid particles through 78 weeks of follow-up, Dr. John J.P. Kastelein said at the annual congress of the European Society of Cardiology.
“These findings show reductions in LDL levels in these individuals that were completely impossible until now. Basically, this therapy in conjunction with statins and ezetimibe cures this genetic disease because LDL levels in these individuals are now lower than in the general population, which is, of course, something that we’ve never seen before,” said Dr. Kastelein, chairman of the department of vascular medicine at the University of Amsterdam.

Heterozygous familial hypercholesterolemia (HeFH) is the most common autosomal dominant genetic disorder, with an estimated prevalence worldwide of 1 in 200 in the general population. The associated highly elevated LDL levels bring a markedly increased risk of premature cardiovascular events. Only about 20% of patients with HeFH are able to achieve an LDL of 100 mg/dL or less despite maximum-tolerated doses of statins plus ezetimibe (Vytorin).
Dr. Kastelein presented the 78-week lipid-lowering results from four phase III randomized, double-blind clinical trials involving 1,257 HeFH patients unable to attain LDL goals despite maximum-tolerated statin therapy, typically supplemented with ezetimibe.
Participants were randomly assigned 2:1 to biweekly subcutaneous injections of alirocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) or placebo on top of background maximum tolerated statin therapy, usually accompanied by ezetimibe. The ODYSSEY FH I and FH II studies included 735 patients, with the alirocumab group starting on the PCSK9 inhibitor at 75 mg every 2 weeks, with the dose being doubled to 150 mg at 24 weeks in patients who hadn’t achieved an LDL level of 70 mg/dL or less at that point. The 522 HeFH patients in the ODYSSEY Long-Term study and the HIGH FH trial had higher baseline LDL levels and greater cardiovascular risk, and they therefore were on alirocumab at 150 mg every 2 weeks or placebo from the outset.
Fifty percent of alirocumab-treated patients in the FH I and II studies and 63% in the other two trials achieved an LDL level below 70 mg/dL at week 24, as did 0.6% and 1% of control patients, respectively. Dr. Kastelein characterized that as a jaw-dropping result.
“I have seen very few HeFH patients in my clinic – which is one of the largest in the world in terms of numbers of patients – who reached the goal of an LDL below 70 mg/dL,” he commented.
The outcomes in terms of secondary lipid endpoints provided an added bonus.
Absolutely no tachyphylaxis or return toward baseline LDL occurred over the course of 78 weeks of treatment, Dr. Kastelein continued.
The safety performance of alirocumab was highly reassuring: Treatment-emergent adverse events occurred in 3.9% of 837 alirocumab-treated patients and 3.6% on placebo. Overall, there was no significant difference between the two groups in rates of headache, musculoskeletal or connective tissue disorders, infections, injection-site reactions, adjudicated cardiovascular events, allergic reactions, neurocognitive disorders, elevated liver enzymes, or any other safety endpoint.
Both alirocumab (Praluent) and evolocumab (Repatha), another PCSK9 inhibitor, have been approved for the treatment of familial hypercholesterolemia by the U.S. Food and Drug Administration and European regulators.
The next round of PCSK9 inhibitor studies, now ongoing, involves enrolling 70,000 subjects at increased cardiovascular risk in order to assess whether the level of LDL lowering achieved with these novel agents translates into a reduction in cardiovascular events.
Session cochair Dr. Barbara Casadei, professor of cardiovascular medicine at the University of Oxford (England), posed a question: Statin therapy has been shown to increase the risk of new-onset type 2 diabetes – how about the PCSK9 inhibitors?
Dr. Kastelein replied that he recently published a study of more than 25,000 Dutch individuals with familial hypercholesterolemia and showed that their prevalence of type 2 diabetes was significantly lower than in their unaffected relatives (JAMA 2015 Mar 10;313[10]:1029-36).
“One of my theories is that the PCSK9 inhibitors reduce the number of LDL receptors on beta cells in the pancreas, so there’s less cholesterol influx, and since cholesterol is toxic to beta cells, the beta cells of PCSK9-inhibitor-treated patients live longer and therefore these individuals will have less type 2 diabetes. Statins do exactly the opposite: They up-regulate LDL receptors, so there will be more cholesterol coming into the beta cell and the theory is that this will result in an increase in type 2 diabetes,” he explained.
The HeFH studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
LONDON – The largest-ever treatment study of patients with heterozygous familial hypercholesterolemia shows that the PCSK9 inhibitor alirocumab achieves “truly astounding” reductions in LDL cholesterol and atherogenic lipid particles through 78 weeks of follow-up, Dr. John J.P. Kastelein said at the annual congress of the European Society of Cardiology.
“These findings show reductions in LDL levels in these individuals that were completely impossible until now. Basically, this therapy in conjunction with statins and ezetimibe cures this genetic disease because LDL levels in these individuals are now lower than in the general population, which is, of course, something that we’ve never seen before,” said Dr. Kastelein, chairman of the department of vascular medicine at the University of Amsterdam.

Heterozygous familial hypercholesterolemia (HeFH) is the most common autosomal dominant genetic disorder, with an estimated prevalence worldwide of 1 in 200 in the general population. The associated highly elevated LDL levels bring a markedly increased risk of premature cardiovascular events. Only about 20% of patients with HeFH are able to achieve an LDL of 100 mg/dL or less despite maximum-tolerated doses of statins plus ezetimibe (Vytorin).
Dr. Kastelein presented the 78-week lipid-lowering results from four phase III randomized, double-blind clinical trials involving 1,257 HeFH patients unable to attain LDL goals despite maximum-tolerated statin therapy, typically supplemented with ezetimibe.
Participants were randomly assigned 2:1 to biweekly subcutaneous injections of alirocumab, a monoclonal antibody that inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) or placebo on top of background maximum tolerated statin therapy, usually accompanied by ezetimibe. The ODYSSEY FH I and FH II studies included 735 patients, with the alirocumab group starting on the PCSK9 inhibitor at 75 mg every 2 weeks, with the dose being doubled to 150 mg at 24 weeks in patients who hadn’t achieved an LDL level of 70 mg/dL or less at that point. The 522 HeFH patients in the ODYSSEY Long-Term study and the HIGH FH trial had higher baseline LDL levels and greater cardiovascular risk, and they therefore were on alirocumab at 150 mg every 2 weeks or placebo from the outset.
Fifty percent of alirocumab-treated patients in the FH I and II studies and 63% in the other two trials achieved an LDL level below 70 mg/dL at week 24, as did 0.6% and 1% of control patients, respectively. Dr. Kastelein characterized that as a jaw-dropping result.
“I have seen very few HeFH patients in my clinic – which is one of the largest in the world in terms of numbers of patients – who reached the goal of an LDL below 70 mg/dL,” he commented.
The outcomes in terms of secondary lipid endpoints provided an added bonus.
Absolutely no tachyphylaxis or return toward baseline LDL occurred over the course of 78 weeks of treatment, Dr. Kastelein continued.
The safety performance of alirocumab was highly reassuring: Treatment-emergent adverse events occurred in 3.9% of 837 alirocumab-treated patients and 3.6% on placebo. Overall, there was no significant difference between the two groups in rates of headache, musculoskeletal or connective tissue disorders, infections, injection-site reactions, adjudicated cardiovascular events, allergic reactions, neurocognitive disorders, elevated liver enzymes, or any other safety endpoint.
Both alirocumab (Praluent) and evolocumab (Repatha), another PCSK9 inhibitor, have been approved for the treatment of familial hypercholesterolemia by the U.S. Food and Drug Administration and European regulators.
The next round of PCSK9 inhibitor studies, now ongoing, involves enrolling 70,000 subjects at increased cardiovascular risk in order to assess whether the level of LDL lowering achieved with these novel agents translates into a reduction in cardiovascular events.
Session cochair Dr. Barbara Casadei, professor of cardiovascular medicine at the University of Oxford (England), posed a question: Statin therapy has been shown to increase the risk of new-onset type 2 diabetes – how about the PCSK9 inhibitors?
Dr. Kastelein replied that he recently published a study of more than 25,000 Dutch individuals with familial hypercholesterolemia and showed that their prevalence of type 2 diabetes was significantly lower than in their unaffected relatives (JAMA 2015 Mar 10;313[10]:1029-36).
“One of my theories is that the PCSK9 inhibitors reduce the number of LDL receptors on beta cells in the pancreas, so there’s less cholesterol influx, and since cholesterol is toxic to beta cells, the beta cells of PCSK9-inhibitor-treated patients live longer and therefore these individuals will have less type 2 diabetes. Statins do exactly the opposite: They up-regulate LDL receptors, so there will be more cholesterol coming into the beta cell and the theory is that this will result in an increase in type 2 diabetes,” he explained.
The HeFH studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
AT THE ESC CONGRESS 2015
Key clinical point: The PCSK9 inhibitor alirocumab lowers LDL cholesterol to unprecedented levels patients with heterozygous familial hypercholesterolemia.
Major finding: Of patients with heterozygous familial hypercholesterolemia with unacceptably high LDL levels despite intensive conventional lipid-lowering agents, 56% achieved an LDL cholesterol below 70 mg/dL after 24 weeks of alirocumab, compared with 1% of placebo-treated controls.
Data source: This analysis included 1,257 individuals with heterozygous familial hypercholesterolemia in four phase III, randomized, double-blind clinical trials who were randomized 2-to-1 to alirocumab or placebo plus background maximally tolerated conventional lipid-lowering medications and followed prospectively for 78 weeks.
Disclosures: The studies were supported by Sanofi and Regeneron Pharmaceuticals. Dr. Kastelein reported having received consulting fees from those pharmaceutical companies and nearly two dozen others.
ESC: Noncardiac surgery in HCM patients warrants special attention
LONDON – Hypertrophic cardiomyopathy patients undergoing noncardiac surgery posted significantly worse 30-day composite outcomes than did closely matched controls undergoing the same sorts of surgical procedures.
“Our recommendation is that when hypertrophic cardiomyopathy patients need noncardiac surgery they should be evaluated and treated at an experienced center,” Dr. Milind Y. Desai concluded at the annual congress of the European Society of Cardiology.
There is a dearth of data on outcomes of noncardiac surgery in patients with hypertrophic cardiomyopathy (HCM). This was the impetus for Dr. Desai and his coinvestigators at the Cleveland Clinic to conduct a case-control study involving 92 consecutive adults with HCM undergoing intermediate- or high-cardiovascular-risk noncardiac surgery and 184 controls matched for age, gender, and type of surgery. Enrollment was restricted to HCM patients who hadn’t previously undergone septal myectomy or alcohol ablation.
The primary outcome was the 30-day composite of postoperative death, MI, stroke, or heart failure. The incidence was 10% in the HCM group, significantly greater than the 3% rate in controls. Moreover, 4% of HCM patients developed postoperative atrial fibrillation, compared with none of the controls. Three deaths occurred among the 92 HCM patients, an incidence twice that in the control group.
The special challenge of noncardiac surgery in HCM patients is that their heart condition is characterized by systolic anterior motion of the mitral valve, dynamic left ventricular outflow tract obstruction, diastolic dysfunction, and mitral regurgitation. The rapid blood pressure and fluid shifts that occur during noncardiac surgery require special attention in such patients, Dr. Desai observed.
The HCM patients in this series received such attention, he added. They were more likely than controls to be on beta-blocker therapy at surgery, they received lower doses of ephedrine intraoperatively so as to avoid aggravating outflow tract obstruction, and they spent half as much time as controls with a systolic blood pressure below 90 mm Hg or a heart rate greater than 100 bpm.
“Care was taken to make sure these patients did not decompensate,” he noted.
Dr. Desai reported no financial conflicts regarding this study, conducted free of commercial support.
LONDON – Hypertrophic cardiomyopathy patients undergoing noncardiac surgery posted significantly worse 30-day composite outcomes than did closely matched controls undergoing the same sorts of surgical procedures.
“Our recommendation is that when hypertrophic cardiomyopathy patients need noncardiac surgery they should be evaluated and treated at an experienced center,” Dr. Milind Y. Desai concluded at the annual congress of the European Society of Cardiology.
There is a dearth of data on outcomes of noncardiac surgery in patients with hypertrophic cardiomyopathy (HCM). This was the impetus for Dr. Desai and his coinvestigators at the Cleveland Clinic to conduct a case-control study involving 92 consecutive adults with HCM undergoing intermediate- or high-cardiovascular-risk noncardiac surgery and 184 controls matched for age, gender, and type of surgery. Enrollment was restricted to HCM patients who hadn’t previously undergone septal myectomy or alcohol ablation.
The primary outcome was the 30-day composite of postoperative death, MI, stroke, or heart failure. The incidence was 10% in the HCM group, significantly greater than the 3% rate in controls. Moreover, 4% of HCM patients developed postoperative atrial fibrillation, compared with none of the controls. Three deaths occurred among the 92 HCM patients, an incidence twice that in the control group.
The special challenge of noncardiac surgery in HCM patients is that their heart condition is characterized by systolic anterior motion of the mitral valve, dynamic left ventricular outflow tract obstruction, diastolic dysfunction, and mitral regurgitation. The rapid blood pressure and fluid shifts that occur during noncardiac surgery require special attention in such patients, Dr. Desai observed.
The HCM patients in this series received such attention, he added. They were more likely than controls to be on beta-blocker therapy at surgery, they received lower doses of ephedrine intraoperatively so as to avoid aggravating outflow tract obstruction, and they spent half as much time as controls with a systolic blood pressure below 90 mm Hg or a heart rate greater than 100 bpm.
“Care was taken to make sure these patients did not decompensate,” he noted.
Dr. Desai reported no financial conflicts regarding this study, conducted free of commercial support.
LONDON – Hypertrophic cardiomyopathy patients undergoing noncardiac surgery posted significantly worse 30-day composite outcomes than did closely matched controls undergoing the same sorts of surgical procedures.
“Our recommendation is that when hypertrophic cardiomyopathy patients need noncardiac surgery they should be evaluated and treated at an experienced center,” Dr. Milind Y. Desai concluded at the annual congress of the European Society of Cardiology.
There is a dearth of data on outcomes of noncardiac surgery in patients with hypertrophic cardiomyopathy (HCM). This was the impetus for Dr. Desai and his coinvestigators at the Cleveland Clinic to conduct a case-control study involving 92 consecutive adults with HCM undergoing intermediate- or high-cardiovascular-risk noncardiac surgery and 184 controls matched for age, gender, and type of surgery. Enrollment was restricted to HCM patients who hadn’t previously undergone septal myectomy or alcohol ablation.
The primary outcome was the 30-day composite of postoperative death, MI, stroke, or heart failure. The incidence was 10% in the HCM group, significantly greater than the 3% rate in controls. Moreover, 4% of HCM patients developed postoperative atrial fibrillation, compared with none of the controls. Three deaths occurred among the 92 HCM patients, an incidence twice that in the control group.
The special challenge of noncardiac surgery in HCM patients is that their heart condition is characterized by systolic anterior motion of the mitral valve, dynamic left ventricular outflow tract obstruction, diastolic dysfunction, and mitral regurgitation. The rapid blood pressure and fluid shifts that occur during noncardiac surgery require special attention in such patients, Dr. Desai observed.
The HCM patients in this series received such attention, he added. They were more likely than controls to be on beta-blocker therapy at surgery, they received lower doses of ephedrine intraoperatively so as to avoid aggravating outflow tract obstruction, and they spent half as much time as controls with a systolic blood pressure below 90 mm Hg or a heart rate greater than 100 bpm.
“Care was taken to make sure these patients did not decompensate,” he noted.
Dr. Desai reported no financial conflicts regarding this study, conducted free of commercial support.
AT THE ESC CONGRESS 2015
Key clinical point: Hypertrophic cardiomyopathy patients undergoing noncardiac surgery have significantly worse outcomes than do matched controls undergoing similar operations.
Major finding: The 30-day composite endpoint of death, MI, stroke, or heart failure occurred in 10% of hypertrophic cardiomyopathy patients who underwent noncardiac surgery, compared with 3% of matched controls.
Data source: A case-control study comparing 30-day outcomes in 92 consecutive hypertrophic cardiomyopathy patients undergoing intermediate- or high-cardiovascular-risk noncardiac surgery and 184 matched controls.
Disclosures: This study was conducted free of commercial support, and the presenter reported having no financial conflicts.
ESC: Nonobstructive CAD spells below-average mortality
LONDON – Patients with stable angina pectoris who are found to have no obstructive coronary artery disease upon diagnostic coronary angiography actually have significantly lower rates of all-cause mortality and acute MI over the next 7 years than the age- and gender-matched general population, according to a large Danish registry study.
“This is a novel finding that’s never been reported before,” Dr. Kris K. Olesen noted in presenting the results at the annual congress of the European Society of Cardiology.
His retrospective, population-based cohort study of patients in the Western Denmark Heart Registry who were referred for diagnostic coronary angiography during 2003-2012 included nearly 40,000 individuals with stable angina pectoris who were found on elective coronary angiography to have no obstructive CAD, meaning no lesions involving 50% or greater luminal narrowing. They were compared to the age- and gender-matched general western Danish population without a history of ischemic heart disease.
The upshot: Individuals without angiographic obstructive CAD had an absolute 1.61% lower rate of all-cause mortality, compared with the general western Danish population, during a median 3.9 and maximum 7 years of follow-up. They also had an absolute 0.41% lower risk of acute MI. After adjustment for comorbid conditions, this translated to a 39% relative risk reduction in all-cause mortality and a 41% reduction in MI risk, with both results being statistically significant with P values of less than 0.0001, reported Dr. Olesen of Aahus (Denmark) University.
One possible explanation for these surprising results, he said, is that patients with stable angina who elect to undergo diagnostic angiography are more health-aware than the general population and take better care of themselves. Another factor is that the general population with no history of ischemic heart disease probably includes individuals with significant CAD and chest pain who have never sought medical attention; they would drive up rates of MI and all-cause mortality in the comparison group.
Discussant Dr. Elmir Omerovic of the University of Goteborg (Sweden) offered up another possibility: Patients without obstructive CAD avoid percutaneous coronary intervention and are thereby spared the risks associated with an invasive procedure, including the hazard of serious bleeding events due to dual antiplatelet therapy.
Dr. Olesen reported having no financial conflicts with regard to this study, conducted with institutional funds.
LONDON – Patients with stable angina pectoris who are found to have no obstructive coronary artery disease upon diagnostic coronary angiography actually have significantly lower rates of all-cause mortality and acute MI over the next 7 years than the age- and gender-matched general population, according to a large Danish registry study.
“This is a novel finding that’s never been reported before,” Dr. Kris K. Olesen noted in presenting the results at the annual congress of the European Society of Cardiology.
His retrospective, population-based cohort study of patients in the Western Denmark Heart Registry who were referred for diagnostic coronary angiography during 2003-2012 included nearly 40,000 individuals with stable angina pectoris who were found on elective coronary angiography to have no obstructive CAD, meaning no lesions involving 50% or greater luminal narrowing. They were compared to the age- and gender-matched general western Danish population without a history of ischemic heart disease.
The upshot: Individuals without angiographic obstructive CAD had an absolute 1.61% lower rate of all-cause mortality, compared with the general western Danish population, during a median 3.9 and maximum 7 years of follow-up. They also had an absolute 0.41% lower risk of acute MI. After adjustment for comorbid conditions, this translated to a 39% relative risk reduction in all-cause mortality and a 41% reduction in MI risk, with both results being statistically significant with P values of less than 0.0001, reported Dr. Olesen of Aahus (Denmark) University.
One possible explanation for these surprising results, he said, is that patients with stable angina who elect to undergo diagnostic angiography are more health-aware than the general population and take better care of themselves. Another factor is that the general population with no history of ischemic heart disease probably includes individuals with significant CAD and chest pain who have never sought medical attention; they would drive up rates of MI and all-cause mortality in the comparison group.
Discussant Dr. Elmir Omerovic of the University of Goteborg (Sweden) offered up another possibility: Patients without obstructive CAD avoid percutaneous coronary intervention and are thereby spared the risks associated with an invasive procedure, including the hazard of serious bleeding events due to dual antiplatelet therapy.
Dr. Olesen reported having no financial conflicts with regard to this study, conducted with institutional funds.
LONDON – Patients with stable angina pectoris who are found to have no obstructive coronary artery disease upon diagnostic coronary angiography actually have significantly lower rates of all-cause mortality and acute MI over the next 7 years than the age- and gender-matched general population, according to a large Danish registry study.
“This is a novel finding that’s never been reported before,” Dr. Kris K. Olesen noted in presenting the results at the annual congress of the European Society of Cardiology.
His retrospective, population-based cohort study of patients in the Western Denmark Heart Registry who were referred for diagnostic coronary angiography during 2003-2012 included nearly 40,000 individuals with stable angina pectoris who were found on elective coronary angiography to have no obstructive CAD, meaning no lesions involving 50% or greater luminal narrowing. They were compared to the age- and gender-matched general western Danish population without a history of ischemic heart disease.
The upshot: Individuals without angiographic obstructive CAD had an absolute 1.61% lower rate of all-cause mortality, compared with the general western Danish population, during a median 3.9 and maximum 7 years of follow-up. They also had an absolute 0.41% lower risk of acute MI. After adjustment for comorbid conditions, this translated to a 39% relative risk reduction in all-cause mortality and a 41% reduction in MI risk, with both results being statistically significant with P values of less than 0.0001, reported Dr. Olesen of Aahus (Denmark) University.
One possible explanation for these surprising results, he said, is that patients with stable angina who elect to undergo diagnostic angiography are more health-aware than the general population and take better care of themselves. Another factor is that the general population with no history of ischemic heart disease probably includes individuals with significant CAD and chest pain who have never sought medical attention; they would drive up rates of MI and all-cause mortality in the comparison group.
Discussant Dr. Elmir Omerovic of the University of Goteborg (Sweden) offered up another possibility: Patients without obstructive CAD avoid percutaneous coronary intervention and are thereby spared the risks associated with an invasive procedure, including the hazard of serious bleeding events due to dual antiplatelet therapy.
Dr. Olesen reported having no financial conflicts with regard to this study, conducted with institutional funds.
AT THE ESC CONGRESS 2015
Key clinical point: Rates of acute MI and all-cause mortality in patients with stable angina pectoris but no obstructive CAD are significantly lower than in the general population.
Major finding: Danish adults with stable angina pectoris but no obstructive CAD upon diagnostic coronary angiography had a 41% lower risk of acute MI and a 39% relative risk reduction in all-cause mortality compared with the age- and gender-matched general Danish population.
Data source: This retrospective population-based cohort study included nearly 40,000 Danish adults with stable angina pectoris who upon elective coronary angiography were found to have no obstructive CAD.
Disclosures: The study was supported by institutional funds. The presenter reported having no financial conflicts.
ESC: Further data indicate no excess heart failure risk with lixisenatide or sitagliptin
LONDON – Further data from two large-scale diabetes trials continue to indicate that there is no increased risk for heart failure with either lixisenatide or sitagliptin compared to placebo.
The findings from ELIXA (the evaluation of lixisenatide in acute coronary syndromes) and TECOS (the Trial Evaluating Cardiovascular Outcomes With Sitagliptin), presented at the annual congress of the European Society of Cardiology, provide reassuring evidence of the cardiovascular safety of both drugs, and add to the trials’ results previously presented at the 2015 annual scientific sessions of the American Diabetes Association, according to the key investigators for the separately-run trials.
“ELIXA demonstrates the cardiovascular safety of lixisenatide,” Dr. Eldrin Lewis, associate professor of medicine at Harvard Medical School and of Brigham and Women’s Hospital in Boston. “Additional analyses indicate safety with respect to heart failure events as well as all-cause mortality, and the hazard ratio after heart failure hospitalizations demonstrate that this endpoint is very meaningful in diabetes as it is in other populations” he added. Furthermore, the neutral effects of lixisenatide were seen across a wide spectrum of heart failure risk.
TECOS investigator Dr. Paul Armstrong of the University of Alberta in Canada noted similar safety findings with sitagliptin. “We found no increase in the risk of heart failure or related adverse outcomes after sitagliptin therapy,” he said at a press briefing. “We can safely conclude that sitagliptin can be used in patients with type 2 diabetes without concern for worsening heart failure.”
ELIXA and TECOS were two phase 3, randomized, double blind placebo-controlled trials specifically designed to look at cardiovascular outcomes after treatment with the glucagon-like peptide 1 (GLP-1) receptor agonist lixisenatide (intended trade name Lyxumia) or the dipeptyl peptidase 4 (DPP-4) inhibitor sitagliptin (Januvia). The trials enrolled 6,068 and 14,671 patients, respectively, and were performed in the wake of prior trials that had suggested an increased risk for hospitalization for heart failure in patients treated with other DPP-4 inhibitors including saxagliptin (Onglyza) in the SAVOR-TIMI 53 trial and alogliptin (Nesina) in the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial.
Although powered to show superiority of lixisenatide versus placebo in terms of cardiovascular safety, ELIXA was only able to establish noninferiority (HR, 1.02), but results were below limits for noninferiority set by the United States Food and Drug Administration, Dr. Lewis observed. In the updated analysis of ELIXA there was no difference between treatment with the lixisenatide and placebo for the primary composite cardiovascular endpoint plus heart failure hospitalization (hazard ratio, 0.97, 95% confidence interval 0.85-1.10) after 3 years of follow-up. There were also no differences in the rates of heart failure hospitalization (HR, 0.96, 95% CI 0.75-1.23), the primary endpoint plus heart failure hospitalization and coronary revascularization (HR=1.00, 95% CI 0.90-1.11), or death from any cause (HR, 0.94, 95% CI 0.78-1.13).Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure in ELIXA than in those who did not there was there was no difference between treatment with the GLP-1 agonist or placebo in patients with (9.7% vs. 10.2%, HR, 0.93, 95% CI 0.66-1.30) or without (2.4% vs. 2.5%, HR, 0.97, 95% CI 0.67-1.40) a history of the disease.
The primary findings of TECOS have been published (doi:10.1056/NEJMoa1501352) and showed sitagliptin to be noninferior to placebo for the primary composite cardiovascular endpoint (HR,0.98; 95% CI, 0.88-1.09; P less than .001). Heart failure hospitalization rates were similar (HR, 1.00; 95% CI, 0.83-1.20; P = .98).
In the updated analysis of TECOS, the prespecified secondary endpoint of time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms (HR, 1.0; 95% CI, 0.84-1.20, P = .95), nor were there any differences between the treatments in terms of other prespecified secondary endpoints of hospitalization for heart failure or cardiovascular death (HR, 1.02, 95% CI 0.90-1.14, P =.74), or hospitalization for heart failure or all-cause death (HR, 1.00, 95% CI 0.90-1.11, P = .93).
Dr. Armstrong acknowledged that the findings of TECOS were in contrast to the SAVOR-TIMI 53 and EXAMINE trials but that this could be due to several factors: differences in the patients enrolled, the background care provided, and variation in the acquisition or definition of heart failure events between the trials. It could also be due to intrinsic differences among the DPP-4 inhibitors themselves or “chance.”
Dr. Gabriel Steg of Hôpital Bichat in Paris, France who provided independent comment after the presentation of the ELIXA data questioned the value of continuing to perform large-scale noninferiority studies assessing the cardiovascular safety of novel diabetes medicines. More than 150,000 patients have been studied in such trials to date which have shown noninferiority for cardiovascular outcomes.
“Non-inferiority trials are often mistakenly being interpreted as ‘lack of efficacy’ trials and can among nonspecialists generate skepticism regarding treatment of diabetes or the need to control glycemia,” he said. “My conclusion would be to suggest that it is time to stop wasting resources on noninferiority trials and to work on truly improving diabetes care.”
ELXIA was sponsored by Sanofi. Dr. Lewis disclosed receiving research funding from Sanofi, and other drug companies. TECOS was sponsored by Merck. Dr. Armstrong disclosed receiving research funding, educational grants and consulting fees from Merck as well as other companies. Dr. Steg had received research grants from and has acted as a consultant for Sanofi, Merck, and other companies.
LONDON – Further data from two large-scale diabetes trials continue to indicate that there is no increased risk for heart failure with either lixisenatide or sitagliptin compared to placebo.
The findings from ELIXA (the evaluation of lixisenatide in acute coronary syndromes) and TECOS (the Trial Evaluating Cardiovascular Outcomes With Sitagliptin), presented at the annual congress of the European Society of Cardiology, provide reassuring evidence of the cardiovascular safety of both drugs, and add to the trials’ results previously presented at the 2015 annual scientific sessions of the American Diabetes Association, according to the key investigators for the separately-run trials.
“ELIXA demonstrates the cardiovascular safety of lixisenatide,” Dr. Eldrin Lewis, associate professor of medicine at Harvard Medical School and of Brigham and Women’s Hospital in Boston. “Additional analyses indicate safety with respect to heart failure events as well as all-cause mortality, and the hazard ratio after heart failure hospitalizations demonstrate that this endpoint is very meaningful in diabetes as it is in other populations” he added. Furthermore, the neutral effects of lixisenatide were seen across a wide spectrum of heart failure risk.
TECOS investigator Dr. Paul Armstrong of the University of Alberta in Canada noted similar safety findings with sitagliptin. “We found no increase in the risk of heart failure or related adverse outcomes after sitagliptin therapy,” he said at a press briefing. “We can safely conclude that sitagliptin can be used in patients with type 2 diabetes without concern for worsening heart failure.”
ELIXA and TECOS were two phase 3, randomized, double blind placebo-controlled trials specifically designed to look at cardiovascular outcomes after treatment with the glucagon-like peptide 1 (GLP-1) receptor agonist lixisenatide (intended trade name Lyxumia) or the dipeptyl peptidase 4 (DPP-4) inhibitor sitagliptin (Januvia). The trials enrolled 6,068 and 14,671 patients, respectively, and were performed in the wake of prior trials that had suggested an increased risk for hospitalization for heart failure in patients treated with other DPP-4 inhibitors including saxagliptin (Onglyza) in the SAVOR-TIMI 53 trial and alogliptin (Nesina) in the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial.
Although powered to show superiority of lixisenatide versus placebo in terms of cardiovascular safety, ELIXA was only able to establish noninferiority (HR, 1.02), but results were below limits for noninferiority set by the United States Food and Drug Administration, Dr. Lewis observed. In the updated analysis of ELIXA there was no difference between treatment with the lixisenatide and placebo for the primary composite cardiovascular endpoint plus heart failure hospitalization (hazard ratio, 0.97, 95% confidence interval 0.85-1.10) after 3 years of follow-up. There were also no differences in the rates of heart failure hospitalization (HR, 0.96, 95% CI 0.75-1.23), the primary endpoint plus heart failure hospitalization and coronary revascularization (HR=1.00, 95% CI 0.90-1.11), or death from any cause (HR, 0.94, 95% CI 0.78-1.13).Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure in ELIXA than in those who did not there was there was no difference between treatment with the GLP-1 agonist or placebo in patients with (9.7% vs. 10.2%, HR, 0.93, 95% CI 0.66-1.30) or without (2.4% vs. 2.5%, HR, 0.97, 95% CI 0.67-1.40) a history of the disease.
The primary findings of TECOS have been published (doi:10.1056/NEJMoa1501352) and showed sitagliptin to be noninferior to placebo for the primary composite cardiovascular endpoint (HR,0.98; 95% CI, 0.88-1.09; P less than .001). Heart failure hospitalization rates were similar (HR, 1.00; 95% CI, 0.83-1.20; P = .98).
In the updated analysis of TECOS, the prespecified secondary endpoint of time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms (HR, 1.0; 95% CI, 0.84-1.20, P = .95), nor were there any differences between the treatments in terms of other prespecified secondary endpoints of hospitalization for heart failure or cardiovascular death (HR, 1.02, 95% CI 0.90-1.14, P =.74), or hospitalization for heart failure or all-cause death (HR, 1.00, 95% CI 0.90-1.11, P = .93).
Dr. Armstrong acknowledged that the findings of TECOS were in contrast to the SAVOR-TIMI 53 and EXAMINE trials but that this could be due to several factors: differences in the patients enrolled, the background care provided, and variation in the acquisition or definition of heart failure events between the trials. It could also be due to intrinsic differences among the DPP-4 inhibitors themselves or “chance.”
Dr. Gabriel Steg of Hôpital Bichat in Paris, France who provided independent comment after the presentation of the ELIXA data questioned the value of continuing to perform large-scale noninferiority studies assessing the cardiovascular safety of novel diabetes medicines. More than 150,000 patients have been studied in such trials to date which have shown noninferiority for cardiovascular outcomes.
“Non-inferiority trials are often mistakenly being interpreted as ‘lack of efficacy’ trials and can among nonspecialists generate skepticism regarding treatment of diabetes or the need to control glycemia,” he said. “My conclusion would be to suggest that it is time to stop wasting resources on noninferiority trials and to work on truly improving diabetes care.”
ELXIA was sponsored by Sanofi. Dr. Lewis disclosed receiving research funding from Sanofi, and other drug companies. TECOS was sponsored by Merck. Dr. Armstrong disclosed receiving research funding, educational grants and consulting fees from Merck as well as other companies. Dr. Steg had received research grants from and has acted as a consultant for Sanofi, Merck, and other companies.
LONDON – Further data from two large-scale diabetes trials continue to indicate that there is no increased risk for heart failure with either lixisenatide or sitagliptin compared to placebo.
The findings from ELIXA (the evaluation of lixisenatide in acute coronary syndromes) and TECOS (the Trial Evaluating Cardiovascular Outcomes With Sitagliptin), presented at the annual congress of the European Society of Cardiology, provide reassuring evidence of the cardiovascular safety of both drugs, and add to the trials’ results previously presented at the 2015 annual scientific sessions of the American Diabetes Association, according to the key investigators for the separately-run trials.
“ELIXA demonstrates the cardiovascular safety of lixisenatide,” Dr. Eldrin Lewis, associate professor of medicine at Harvard Medical School and of Brigham and Women’s Hospital in Boston. “Additional analyses indicate safety with respect to heart failure events as well as all-cause mortality, and the hazard ratio after heart failure hospitalizations demonstrate that this endpoint is very meaningful in diabetes as it is in other populations” he added. Furthermore, the neutral effects of lixisenatide were seen across a wide spectrum of heart failure risk.
TECOS investigator Dr. Paul Armstrong of the University of Alberta in Canada noted similar safety findings with sitagliptin. “We found no increase in the risk of heart failure or related adverse outcomes after sitagliptin therapy,” he said at a press briefing. “We can safely conclude that sitagliptin can be used in patients with type 2 diabetes without concern for worsening heart failure.”
ELIXA and TECOS were two phase 3, randomized, double blind placebo-controlled trials specifically designed to look at cardiovascular outcomes after treatment with the glucagon-like peptide 1 (GLP-1) receptor agonist lixisenatide (intended trade name Lyxumia) or the dipeptyl peptidase 4 (DPP-4) inhibitor sitagliptin (Januvia). The trials enrolled 6,068 and 14,671 patients, respectively, and were performed in the wake of prior trials that had suggested an increased risk for hospitalization for heart failure in patients treated with other DPP-4 inhibitors including saxagliptin (Onglyza) in the SAVOR-TIMI 53 trial and alogliptin (Nesina) in the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care) trial.
Although powered to show superiority of lixisenatide versus placebo in terms of cardiovascular safety, ELIXA was only able to establish noninferiority (HR, 1.02), but results were below limits for noninferiority set by the United States Food and Drug Administration, Dr. Lewis observed. In the updated analysis of ELIXA there was no difference between treatment with the lixisenatide and placebo for the primary composite cardiovascular endpoint plus heart failure hospitalization (hazard ratio, 0.97, 95% confidence interval 0.85-1.10) after 3 years of follow-up. There were also no differences in the rates of heart failure hospitalization (HR, 0.96, 95% CI 0.75-1.23), the primary endpoint plus heart failure hospitalization and coronary revascularization (HR=1.00, 95% CI 0.90-1.11), or death from any cause (HR, 0.94, 95% CI 0.78-1.13).Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure in ELIXA than in those who did not there was there was no difference between treatment with the GLP-1 agonist or placebo in patients with (9.7% vs. 10.2%, HR, 0.93, 95% CI 0.66-1.30) or without (2.4% vs. 2.5%, HR, 0.97, 95% CI 0.67-1.40) a history of the disease.
The primary findings of TECOS have been published (doi:10.1056/NEJMoa1501352) and showed sitagliptin to be noninferior to placebo for the primary composite cardiovascular endpoint (HR,0.98; 95% CI, 0.88-1.09; P less than .001). Heart failure hospitalization rates were similar (HR, 1.00; 95% CI, 0.83-1.20; P = .98).
In the updated analysis of TECOS, the prespecified secondary endpoint of time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms (HR, 1.0; 95% CI, 0.84-1.20, P = .95), nor were there any differences between the treatments in terms of other prespecified secondary endpoints of hospitalization for heart failure or cardiovascular death (HR, 1.02, 95% CI 0.90-1.14, P =.74), or hospitalization for heart failure or all-cause death (HR, 1.00, 95% CI 0.90-1.11, P = .93).
Dr. Armstrong acknowledged that the findings of TECOS were in contrast to the SAVOR-TIMI 53 and EXAMINE trials but that this could be due to several factors: differences in the patients enrolled, the background care provided, and variation in the acquisition or definition of heart failure events between the trials. It could also be due to intrinsic differences among the DPP-4 inhibitors themselves or “chance.”
Dr. Gabriel Steg of Hôpital Bichat in Paris, France who provided independent comment after the presentation of the ELIXA data questioned the value of continuing to perform large-scale noninferiority studies assessing the cardiovascular safety of novel diabetes medicines. More than 150,000 patients have been studied in such trials to date which have shown noninferiority for cardiovascular outcomes.
“Non-inferiority trials are often mistakenly being interpreted as ‘lack of efficacy’ trials and can among nonspecialists generate skepticism regarding treatment of diabetes or the need to control glycemia,” he said. “My conclusion would be to suggest that it is time to stop wasting resources on noninferiority trials and to work on truly improving diabetes care.”
ELXIA was sponsored by Sanofi. Dr. Lewis disclosed receiving research funding from Sanofi, and other drug companies. TECOS was sponsored by Merck. Dr. Armstrong disclosed receiving research funding, educational grants and consulting fees from Merck as well as other companies. Dr. Steg had received research grants from and has acted as a consultant for Sanofi, Merck, and other companies.
AT THE ESC CONGRESS 2015
Key clinical point:Patients with type 2 diabetes who are treated with lixisenatide or sitagliptin are at no greater risk for heart failure than if they are given placebo.
Major finding: The rates of heart failure hospitalization versus placebo were comparable for either lixisenatide (HR, 0.96, 95% CI 0.75-1.23) or sitagliptin (HR, 1.00; 95% CI, 0.83-1.20; P = .98).
Data source: ELIXA and TECOS: Two separately run, randomized, double blind, placebo-controlled cardiovascular safety trials of over 20,000 patients with type 2 diabetes mellitus.
Disclosures: ELIXA was sponsored by Sanofi, the maker of lixisenatide. Dr. Lewis disclosed receiving research funding from Sanofi. TECOS was sponsored by Merck, the maker of sitagliptin. Dr. Armstrong disclosed receiving research funding, educational grants and consulting fees from Merck as well as other companies. Dr. Steg had received research grants from Sanofi, and has acted as a consultant for Sanofi, Merck, and other companies.
ESC: Atrial fibrillation accelerates brain atrophy
LONDON – Atrial fibrillation in the elderly general population was independently associated with accelerated losses of brain volume and cognitive function in a major longitudinal study.
These findings from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) have the potential to change the management of atrial fibrillation (AF), Dr. David O. Arnar said at the annual congress of the European Society of Cardiology.
“I think these data potentially suggest it’s better for the brain to remain in sinus rhythm than to pursue rate control in AF. We also have other studies, postablation studies, that show doing an ablation procedure to restore sinus rhythm delays the onset of cognitive dysfunction. So I think we have more and more data that are suggesting AF may be bad for the brain in more ways than just causing cerebral infarcts. That possibility needs to be considered as an endpoint in future studies of treatment strategies,” asserted Dr. Arnar, a cardiologist at Landspítali–The National University Hospital of Iceland, Reykjavik.
The AGES-Reykjavik Study is an ongoing project designed to investigate the genetic and environmental factors that contribute to clinical and subclinical diseases in older-age individuals.
The new data Dr. Arnar presented are an outgrowth of an earlier report from AGES-Reykjavik, which concluded that AF was associated with smaller brain volume and diminished cognitive performance independent of cerebral infarcts. The observed deficits were smallest in subjects with no history of AF, larger in those with paroxysmal AF, and largest of all in participants with persistent/permanent AF (Stroke. 2013 Apr;44[4]:1020-5). However, this was a cross-sectional analysis, which by definition doesn’t permit drawing conclusions regarding cause and effect.
That earlier report was the impetus for the new study featuring a mean of 5.2 years of longitudinal follow-up. The study included 2,472 elderly, nondemented subjects with a mean baseline age of 76 years who underwent brain MRIs and structured cognitive function testing, with repeated assessments roughly a half-decade later.
A total of 121 subjects had ECG-confirmed AF or a history of AF at entry. Another 132 developed new-onset AF during follow-up. Since the participants with prevalent or incident AF had significantly higher levels of cardiovascular risk factors, alcohol consumption, history of cerebral infarcts, and other potential confounders, extensive multivariate statistical adjustments were required in analyzing the data, according to the cardiologist.
During the follow-up period, the AF-free subjects experienced a mean 1.8% reduction in gray matter volume, compared with a 2.7% decrease in individuals with prevalent AF and a 3.88% reduction in those with incident AF. All differences were statistically significant.
Loss of white matter volume over time followed a similar pattern: a mean loss of 5.35% in the no-AF group, compared with a 5.5% drop in those with prevalent AF and a 6.56% decrease in individuals with incident AF.
The volume of white matter lesions rose by 31.6% in the elderly no-AF group, 26.9% in those with prevalent AF, and 43.5% in subjects with new-onset AF during follow-up.
“It surprised us that the changes were most pronounced in those with incident AF rather than prevalent AF,” Dr. Arnar confessed. “How do we explain that? Well, I don’t know, but you wonder if the effect of AF on the brain could be most pronounced initially and then as AF goes on, an adaptation process occurs so that the rate of change in the brain becomes less pronounced as the AF becomes more chronic.”
Turning to the results of cognitive function testing, a composite measure of processing speed declined over time by 10% in the no-AF group, 12.7% with prevalent AF, and 13.9% with incident AF. All differences were statistically significant.
The rate of decline in executive function was 8% in the no-AF subjects, 10.2% with prevalent AF, and 11.8% with incident AF.
Similarly, scores on memory testing dropped by 9.3% in the no-AF group, 9.9% with prevalent AF, and 11.9% with incident AF.
The mechanism by which AF accelerates brain aging is unknown. Dr. Arnar strongly suspects it is multifactorial, with candidate processes including altered autonomic regulation of blood flow, microemboli causing brain atrophy, and most assuredly AF-induced diminution of cerebral blood flow.
He presented cerebral blood flow data obtained via phase contrast MRI on 2,125 study participants. Those with no history of AF averaged a total cerebral blood flow of 540 mL/min. Subjects with a history of AF who were in sinus rhythm at the time of the brain scan averaged 520 mL/min. And subjects in AF when they were scanned averaged less than 480 mL/min.
Dr. Arnar also presented preliminary results from an ongoing brain perfusion imaging study conducted in AF patients before and after direct current cardioversion. Among 17 patients who responded to cardioversion by going into sinus rhythm and staying there for at least 10 weeks until their follow-up MRI, total cerebral blood flow improved by a mean of 70 mL/min from a precardioversion figure of 557 mL/min. Both white and gray matter perfusion improved by a mean of 16%.
In contrast, the 10 patients who remained in AF despite the cardioversion attempt showed no improvement in any of these three endpoints over the 10 weeks.
Audience members wondered whether being on warfarin or beta blocker therapy affected the rate of brain volume loss or cognitive function. Not in the cross-sectional study published in Stroke, Dr. Arnar replied. However, those analyses have yet to be conducted in the new longitudinal study.
The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. Dr. Arnar reported having no financial conflicts.
LONDON – Atrial fibrillation in the elderly general population was independently associated with accelerated losses of brain volume and cognitive function in a major longitudinal study.
These findings from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) have the potential to change the management of atrial fibrillation (AF), Dr. David O. Arnar said at the annual congress of the European Society of Cardiology.
“I think these data potentially suggest it’s better for the brain to remain in sinus rhythm than to pursue rate control in AF. We also have other studies, postablation studies, that show doing an ablation procedure to restore sinus rhythm delays the onset of cognitive dysfunction. So I think we have more and more data that are suggesting AF may be bad for the brain in more ways than just causing cerebral infarcts. That possibility needs to be considered as an endpoint in future studies of treatment strategies,” asserted Dr. Arnar, a cardiologist at Landspítali–The National University Hospital of Iceland, Reykjavik.
The AGES-Reykjavik Study is an ongoing project designed to investigate the genetic and environmental factors that contribute to clinical and subclinical diseases in older-age individuals.
The new data Dr. Arnar presented are an outgrowth of an earlier report from AGES-Reykjavik, which concluded that AF was associated with smaller brain volume and diminished cognitive performance independent of cerebral infarcts. The observed deficits were smallest in subjects with no history of AF, larger in those with paroxysmal AF, and largest of all in participants with persistent/permanent AF (Stroke. 2013 Apr;44[4]:1020-5). However, this was a cross-sectional analysis, which by definition doesn’t permit drawing conclusions regarding cause and effect.
That earlier report was the impetus for the new study featuring a mean of 5.2 years of longitudinal follow-up. The study included 2,472 elderly, nondemented subjects with a mean baseline age of 76 years who underwent brain MRIs and structured cognitive function testing, with repeated assessments roughly a half-decade later.
A total of 121 subjects had ECG-confirmed AF or a history of AF at entry. Another 132 developed new-onset AF during follow-up. Since the participants with prevalent or incident AF had significantly higher levels of cardiovascular risk factors, alcohol consumption, history of cerebral infarcts, and other potential confounders, extensive multivariate statistical adjustments were required in analyzing the data, according to the cardiologist.
During the follow-up period, the AF-free subjects experienced a mean 1.8% reduction in gray matter volume, compared with a 2.7% decrease in individuals with prevalent AF and a 3.88% reduction in those with incident AF. All differences were statistically significant.
Loss of white matter volume over time followed a similar pattern: a mean loss of 5.35% in the no-AF group, compared with a 5.5% drop in those with prevalent AF and a 6.56% decrease in individuals with incident AF.
The volume of white matter lesions rose by 31.6% in the elderly no-AF group, 26.9% in those with prevalent AF, and 43.5% in subjects with new-onset AF during follow-up.
“It surprised us that the changes were most pronounced in those with incident AF rather than prevalent AF,” Dr. Arnar confessed. “How do we explain that? Well, I don’t know, but you wonder if the effect of AF on the brain could be most pronounced initially and then as AF goes on, an adaptation process occurs so that the rate of change in the brain becomes less pronounced as the AF becomes more chronic.”
Turning to the results of cognitive function testing, a composite measure of processing speed declined over time by 10% in the no-AF group, 12.7% with prevalent AF, and 13.9% with incident AF. All differences were statistically significant.
The rate of decline in executive function was 8% in the no-AF subjects, 10.2% with prevalent AF, and 11.8% with incident AF.
Similarly, scores on memory testing dropped by 9.3% in the no-AF group, 9.9% with prevalent AF, and 11.9% with incident AF.
The mechanism by which AF accelerates brain aging is unknown. Dr. Arnar strongly suspects it is multifactorial, with candidate processes including altered autonomic regulation of blood flow, microemboli causing brain atrophy, and most assuredly AF-induced diminution of cerebral blood flow.
He presented cerebral blood flow data obtained via phase contrast MRI on 2,125 study participants. Those with no history of AF averaged a total cerebral blood flow of 540 mL/min. Subjects with a history of AF who were in sinus rhythm at the time of the brain scan averaged 520 mL/min. And subjects in AF when they were scanned averaged less than 480 mL/min.
Dr. Arnar also presented preliminary results from an ongoing brain perfusion imaging study conducted in AF patients before and after direct current cardioversion. Among 17 patients who responded to cardioversion by going into sinus rhythm and staying there for at least 10 weeks until their follow-up MRI, total cerebral blood flow improved by a mean of 70 mL/min from a precardioversion figure of 557 mL/min. Both white and gray matter perfusion improved by a mean of 16%.
In contrast, the 10 patients who remained in AF despite the cardioversion attempt showed no improvement in any of these three endpoints over the 10 weeks.
Audience members wondered whether being on warfarin or beta blocker therapy affected the rate of brain volume loss or cognitive function. Not in the cross-sectional study published in Stroke, Dr. Arnar replied. However, those analyses have yet to be conducted in the new longitudinal study.
The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. Dr. Arnar reported having no financial conflicts.
LONDON – Atrial fibrillation in the elderly general population was independently associated with accelerated losses of brain volume and cognitive function in a major longitudinal study.
These findings from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) have the potential to change the management of atrial fibrillation (AF), Dr. David O. Arnar said at the annual congress of the European Society of Cardiology.
“I think these data potentially suggest it’s better for the brain to remain in sinus rhythm than to pursue rate control in AF. We also have other studies, postablation studies, that show doing an ablation procedure to restore sinus rhythm delays the onset of cognitive dysfunction. So I think we have more and more data that are suggesting AF may be bad for the brain in more ways than just causing cerebral infarcts. That possibility needs to be considered as an endpoint in future studies of treatment strategies,” asserted Dr. Arnar, a cardiologist at Landspítali–The National University Hospital of Iceland, Reykjavik.
The AGES-Reykjavik Study is an ongoing project designed to investigate the genetic and environmental factors that contribute to clinical and subclinical diseases in older-age individuals.
The new data Dr. Arnar presented are an outgrowth of an earlier report from AGES-Reykjavik, which concluded that AF was associated with smaller brain volume and diminished cognitive performance independent of cerebral infarcts. The observed deficits were smallest in subjects with no history of AF, larger in those with paroxysmal AF, and largest of all in participants with persistent/permanent AF (Stroke. 2013 Apr;44[4]:1020-5). However, this was a cross-sectional analysis, which by definition doesn’t permit drawing conclusions regarding cause and effect.
That earlier report was the impetus for the new study featuring a mean of 5.2 years of longitudinal follow-up. The study included 2,472 elderly, nondemented subjects with a mean baseline age of 76 years who underwent brain MRIs and structured cognitive function testing, with repeated assessments roughly a half-decade later.
A total of 121 subjects had ECG-confirmed AF or a history of AF at entry. Another 132 developed new-onset AF during follow-up. Since the participants with prevalent or incident AF had significantly higher levels of cardiovascular risk factors, alcohol consumption, history of cerebral infarcts, and other potential confounders, extensive multivariate statistical adjustments were required in analyzing the data, according to the cardiologist.
During the follow-up period, the AF-free subjects experienced a mean 1.8% reduction in gray matter volume, compared with a 2.7% decrease in individuals with prevalent AF and a 3.88% reduction in those with incident AF. All differences were statistically significant.
Loss of white matter volume over time followed a similar pattern: a mean loss of 5.35% in the no-AF group, compared with a 5.5% drop in those with prevalent AF and a 6.56% decrease in individuals with incident AF.
The volume of white matter lesions rose by 31.6% in the elderly no-AF group, 26.9% in those with prevalent AF, and 43.5% in subjects with new-onset AF during follow-up.
“It surprised us that the changes were most pronounced in those with incident AF rather than prevalent AF,” Dr. Arnar confessed. “How do we explain that? Well, I don’t know, but you wonder if the effect of AF on the brain could be most pronounced initially and then as AF goes on, an adaptation process occurs so that the rate of change in the brain becomes less pronounced as the AF becomes more chronic.”
Turning to the results of cognitive function testing, a composite measure of processing speed declined over time by 10% in the no-AF group, 12.7% with prevalent AF, and 13.9% with incident AF. All differences were statistically significant.
The rate of decline in executive function was 8% in the no-AF subjects, 10.2% with prevalent AF, and 11.8% with incident AF.
Similarly, scores on memory testing dropped by 9.3% in the no-AF group, 9.9% with prevalent AF, and 11.9% with incident AF.
The mechanism by which AF accelerates brain aging is unknown. Dr. Arnar strongly suspects it is multifactorial, with candidate processes including altered autonomic regulation of blood flow, microemboli causing brain atrophy, and most assuredly AF-induced diminution of cerebral blood flow.
He presented cerebral blood flow data obtained via phase contrast MRI on 2,125 study participants. Those with no history of AF averaged a total cerebral blood flow of 540 mL/min. Subjects with a history of AF who were in sinus rhythm at the time of the brain scan averaged 520 mL/min. And subjects in AF when they were scanned averaged less than 480 mL/min.
Dr. Arnar also presented preliminary results from an ongoing brain perfusion imaging study conducted in AF patients before and after direct current cardioversion. Among 17 patients who responded to cardioversion by going into sinus rhythm and staying there for at least 10 weeks until their follow-up MRI, total cerebral blood flow improved by a mean of 70 mL/min from a precardioversion figure of 557 mL/min. Both white and gray matter perfusion improved by a mean of 16%.
In contrast, the 10 patients who remained in AF despite the cardioversion attempt showed no improvement in any of these three endpoints over the 10 weeks.
Audience members wondered whether being on warfarin or beta blocker therapy affected the rate of brain volume loss or cognitive function. Not in the cross-sectional study published in Stroke, Dr. Arnar replied. However, those analyses have yet to be conducted in the new longitudinal study.
The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. Dr. Arnar reported having no financial conflicts.
AT THE ESC CONGRESS 2015
Key clinical point: Atrial fibrillation appears to accelerate brain aging in the elderly independent of cerebral embolism.
Major finding: Both prevalent and incident atrial fibrillation were associated with accelerated loss of gray matter and total brain volume as well as key aspects of cognitive function.
Data source: This population-based study included 2,472 nondemented participants with a mean baseline age of 76 years who were prospectively followed with brain scans and structured cognitive function tests for a mean of 5.2 years.
Disclosures: The AGES-Reykjavik Study is funded by the U.S. National Institutes of Health, Icelandic Heart Association, and the Icelandic Parliament. The presenter reported having no financial conflicts.
ESC: Zero risk of death in pregnant women with severe aortic stenosis
LONDON – The mortality risk for pregnant women with moderate or severe aortic stenosis is close to zero in contemporary practice, according to data from the large, international ROPAC registry.
That being said, more than one-third of women with severe aortic stenosis will require hospitalization for cardiac reasons during their pregnancy, with heart failure the number-one cause for admission, Dr. Stefan Orwat reported at the annual congress of the European Society of Cardiology.
Severe fetal complications are rare. However, preterm birth, small for gestational age, and neonatal Apgar scores below 7 are quite common in pregnancies involving severe aortic stenosis, as defined by a prepregnancy transaortic peak gradient of 64 mm Hg or more, according to Dr. Orwat of University Hospital in Muenster, Germany.
ROPAC (the Registry on Pregnancy and Cardiac disease) is a unique ongoing, global, prospective, observational registry created in order to advance understanding of the risks of pregnancy in the setting of structural heart disease. Prior reports from other sources have often provided conflicting guidance because of relatively small patient sample sizes. That’s been especially true with regard to aortic stenosis (AS), the cardiologist said.
Out of 2,966 pregnancies in women with various forms of structural heart disease in ROPAC, Dr. Orwat focused on the 99 pregnancies in 96 women with moderate or severe AS, 21 of whom had a prosthetic heart valve in place prior to pregnancy; 65 of the women had moderate AS as evidenced by an echocardiographic prepregnancy peak gradient of 36-63 mm Hg.
No maternal deaths occurred during pregnancy or within 1 week afterward. However, 13% of women with moderate AS and 35% with severe AS were hospitalized for cardiac reasons during their pregnancy. Most of the admissions were for new or worsening heart failure, which occurred at a median of 28 weeks’ gestation.
The risk of heart failure hospitalization during pregnancy was quite low – in the 6%-8% range – among women with baseline prepregnancy asymptomatic or symptomatic moderate AS or asymptomatic severe AS. In contrast, the rate shot up to 26% in women with symptomatic and severe AS prior to pregnancy.
There were a couple of hospitalizations for arrhythmias and one for endocarditis. Unlike in some prior reports by other research groups, there were no cases of pulmonary embolism, valve thrombosis, cerebrovascular events, or deep vein thrombosis. The presence of a mechanical valve with its required rigorous anticoagulation wasn’t related to worse outcomes in this series, unlike in some others.
The heart failure was generally manageable medically. Only two patients required intervention. One developed endocarditis, then underwent balloon aortic valvuloplasty and aortic valve replacement with a mechanical valve 4 months into pregnancy, with subsequent vaginal delivery at 38 weeks. The other patient, who was unaware she had AS prior to pregnancy, presented with severe AS and a depressed left ventricular ejection fraction at 20 weeks’ gestation. She responded well to valvuloplasty, had an uneventful vaginal delivery at 38 weeks, then underwent aortic valve replacement.
In a multivariate analysis, only two independent predictors of maternal hospitalization during pregnancy were identified: the presence of symptoms prior to pregnancy, and the prepregnancy hemodynamic severity of AS.
Preterm birth prior to 37 weeks occurred in 16% of pregnancies involving moderate and 36% of those featuring severe AS. An Apgar score below 7 occurred in 5% of cases of moderate and 16% of severe AS. The small for gestational age rate was 3% with moderate AS and ballooned to 21% with severe AS. The only independent predictor of fetal complications was the degree of elevation in peak aortic gradient prior to pregnancy.
Audience questions focused on challenging scenarios. Would you allow a pregnancy to continue, Dr. Orwat was asked, if a woman with AS developed an aortic gradient of 90 mm Hg during the first trimester?
“It depends on whether she was asymptomatic prior to pregnancy. If so, I think it’s a low-risk situation, and you can carry on with the pregnancy,” he replied. Remember, he added, it’s the baseline gradient that’s important. Cardiac output goes up during the first trimester as a matter of course, and so will the gradient.
Dr. Orwat emphasized that symptomatic severe AS is an indication for aortic valve replacement, and he would advise an affected patient to undergo the surgery prior to pregnancy.
Audience member Dr. Fiona Walker rose to advocate ordering a prepregnancy treadmill exercise test in all patients with asymptomatic severe AS in order to learn whether they are likely to remain asymptomatic during the stresses of pregnancy. In a patient with severe AS who remains asymptomatic with exercise, it’s probably safer to go through pregnancy without prior valve replacement than to put in a mechanical valve.
“Patients with mechanical valves in pregnancy are one of the highest-risk groups we look after,” commented Dr. Walker, head of the maternal cardiology program at University College London Hospitals.
But another audience member advocated aortic valve replacement prior to pregnancy in all women with severe AS, asymptomatic or not.
“Nowadays, if you are afraid of a mechanical valve, you can put in a bioprosthetic one, although I know that’s considered controversial. I see that asymptomatic severe aortic stenosis seems quite safe in ROPAC, but I still consider severe aortic stenosis one of the truly dangerous problems in pregnancy. Maybe I’ll change my mind after ROPAC is published, but right now I don’t think we’re going to leave a severe aortic stenosis prior to pregnancy without intervention,” declared Dr. Avraham Shotan, head of the Heart Institute at Hillel Yaffe Medical Center in Hadera, Israel.
Session cochair Dr. Christa Gohlke-Baerwolf of Bad Krozingen (Germany) Hospital mediated the dispute and closed discussion by observing, “Severe aortic stenosis is not just one thing, it’s a continuum, and such patients should be evaluated by a multidisciplinary team very carefully before starting pregnancy.“
ROPAC is sponsored by the ESC. Dr. Orwat reported having no financial conflicts regarding his study.
LONDON – The mortality risk for pregnant women with moderate or severe aortic stenosis is close to zero in contemporary practice, according to data from the large, international ROPAC registry.
That being said, more than one-third of women with severe aortic stenosis will require hospitalization for cardiac reasons during their pregnancy, with heart failure the number-one cause for admission, Dr. Stefan Orwat reported at the annual congress of the European Society of Cardiology.
Severe fetal complications are rare. However, preterm birth, small for gestational age, and neonatal Apgar scores below 7 are quite common in pregnancies involving severe aortic stenosis, as defined by a prepregnancy transaortic peak gradient of 64 mm Hg or more, according to Dr. Orwat of University Hospital in Muenster, Germany.
ROPAC (the Registry on Pregnancy and Cardiac disease) is a unique ongoing, global, prospective, observational registry created in order to advance understanding of the risks of pregnancy in the setting of structural heart disease. Prior reports from other sources have often provided conflicting guidance because of relatively small patient sample sizes. That’s been especially true with regard to aortic stenosis (AS), the cardiologist said.
Out of 2,966 pregnancies in women with various forms of structural heart disease in ROPAC, Dr. Orwat focused on the 99 pregnancies in 96 women with moderate or severe AS, 21 of whom had a prosthetic heart valve in place prior to pregnancy; 65 of the women had moderate AS as evidenced by an echocardiographic prepregnancy peak gradient of 36-63 mm Hg.
No maternal deaths occurred during pregnancy or within 1 week afterward. However, 13% of women with moderate AS and 35% with severe AS were hospitalized for cardiac reasons during their pregnancy. Most of the admissions were for new or worsening heart failure, which occurred at a median of 28 weeks’ gestation.
The risk of heart failure hospitalization during pregnancy was quite low – in the 6%-8% range – among women with baseline prepregnancy asymptomatic or symptomatic moderate AS or asymptomatic severe AS. In contrast, the rate shot up to 26% in women with symptomatic and severe AS prior to pregnancy.
There were a couple of hospitalizations for arrhythmias and one for endocarditis. Unlike in some prior reports by other research groups, there were no cases of pulmonary embolism, valve thrombosis, cerebrovascular events, or deep vein thrombosis. The presence of a mechanical valve with its required rigorous anticoagulation wasn’t related to worse outcomes in this series, unlike in some others.
The heart failure was generally manageable medically. Only two patients required intervention. One developed endocarditis, then underwent balloon aortic valvuloplasty and aortic valve replacement with a mechanical valve 4 months into pregnancy, with subsequent vaginal delivery at 38 weeks. The other patient, who was unaware she had AS prior to pregnancy, presented with severe AS and a depressed left ventricular ejection fraction at 20 weeks’ gestation. She responded well to valvuloplasty, had an uneventful vaginal delivery at 38 weeks, then underwent aortic valve replacement.
In a multivariate analysis, only two independent predictors of maternal hospitalization during pregnancy were identified: the presence of symptoms prior to pregnancy, and the prepregnancy hemodynamic severity of AS.
Preterm birth prior to 37 weeks occurred in 16% of pregnancies involving moderate and 36% of those featuring severe AS. An Apgar score below 7 occurred in 5% of cases of moderate and 16% of severe AS. The small for gestational age rate was 3% with moderate AS and ballooned to 21% with severe AS. The only independent predictor of fetal complications was the degree of elevation in peak aortic gradient prior to pregnancy.
Audience questions focused on challenging scenarios. Would you allow a pregnancy to continue, Dr. Orwat was asked, if a woman with AS developed an aortic gradient of 90 mm Hg during the first trimester?
“It depends on whether she was asymptomatic prior to pregnancy. If so, I think it’s a low-risk situation, and you can carry on with the pregnancy,” he replied. Remember, he added, it’s the baseline gradient that’s important. Cardiac output goes up during the first trimester as a matter of course, and so will the gradient.
Dr. Orwat emphasized that symptomatic severe AS is an indication for aortic valve replacement, and he would advise an affected patient to undergo the surgery prior to pregnancy.
Audience member Dr. Fiona Walker rose to advocate ordering a prepregnancy treadmill exercise test in all patients with asymptomatic severe AS in order to learn whether they are likely to remain asymptomatic during the stresses of pregnancy. In a patient with severe AS who remains asymptomatic with exercise, it’s probably safer to go through pregnancy without prior valve replacement than to put in a mechanical valve.
“Patients with mechanical valves in pregnancy are one of the highest-risk groups we look after,” commented Dr. Walker, head of the maternal cardiology program at University College London Hospitals.
But another audience member advocated aortic valve replacement prior to pregnancy in all women with severe AS, asymptomatic or not.
“Nowadays, if you are afraid of a mechanical valve, you can put in a bioprosthetic one, although I know that’s considered controversial. I see that asymptomatic severe aortic stenosis seems quite safe in ROPAC, but I still consider severe aortic stenosis one of the truly dangerous problems in pregnancy. Maybe I’ll change my mind after ROPAC is published, but right now I don’t think we’re going to leave a severe aortic stenosis prior to pregnancy without intervention,” declared Dr. Avraham Shotan, head of the Heart Institute at Hillel Yaffe Medical Center in Hadera, Israel.
Session cochair Dr. Christa Gohlke-Baerwolf of Bad Krozingen (Germany) Hospital mediated the dispute and closed discussion by observing, “Severe aortic stenosis is not just one thing, it’s a continuum, and such patients should be evaluated by a multidisciplinary team very carefully before starting pregnancy.“
ROPAC is sponsored by the ESC. Dr. Orwat reported having no financial conflicts regarding his study.
LONDON – The mortality risk for pregnant women with moderate or severe aortic stenosis is close to zero in contemporary practice, according to data from the large, international ROPAC registry.
That being said, more than one-third of women with severe aortic stenosis will require hospitalization for cardiac reasons during their pregnancy, with heart failure the number-one cause for admission, Dr. Stefan Orwat reported at the annual congress of the European Society of Cardiology.
Severe fetal complications are rare. However, preterm birth, small for gestational age, and neonatal Apgar scores below 7 are quite common in pregnancies involving severe aortic stenosis, as defined by a prepregnancy transaortic peak gradient of 64 mm Hg or more, according to Dr. Orwat of University Hospital in Muenster, Germany.
ROPAC (the Registry on Pregnancy and Cardiac disease) is a unique ongoing, global, prospective, observational registry created in order to advance understanding of the risks of pregnancy in the setting of structural heart disease. Prior reports from other sources have often provided conflicting guidance because of relatively small patient sample sizes. That’s been especially true with regard to aortic stenosis (AS), the cardiologist said.
Out of 2,966 pregnancies in women with various forms of structural heart disease in ROPAC, Dr. Orwat focused on the 99 pregnancies in 96 women with moderate or severe AS, 21 of whom had a prosthetic heart valve in place prior to pregnancy; 65 of the women had moderate AS as evidenced by an echocardiographic prepregnancy peak gradient of 36-63 mm Hg.
No maternal deaths occurred during pregnancy or within 1 week afterward. However, 13% of women with moderate AS and 35% with severe AS were hospitalized for cardiac reasons during their pregnancy. Most of the admissions were for new or worsening heart failure, which occurred at a median of 28 weeks’ gestation.
The risk of heart failure hospitalization during pregnancy was quite low – in the 6%-8% range – among women with baseline prepregnancy asymptomatic or symptomatic moderate AS or asymptomatic severe AS. In contrast, the rate shot up to 26% in women with symptomatic and severe AS prior to pregnancy.
There were a couple of hospitalizations for arrhythmias and one for endocarditis. Unlike in some prior reports by other research groups, there were no cases of pulmonary embolism, valve thrombosis, cerebrovascular events, or deep vein thrombosis. The presence of a mechanical valve with its required rigorous anticoagulation wasn’t related to worse outcomes in this series, unlike in some others.
The heart failure was generally manageable medically. Only two patients required intervention. One developed endocarditis, then underwent balloon aortic valvuloplasty and aortic valve replacement with a mechanical valve 4 months into pregnancy, with subsequent vaginal delivery at 38 weeks. The other patient, who was unaware she had AS prior to pregnancy, presented with severe AS and a depressed left ventricular ejection fraction at 20 weeks’ gestation. She responded well to valvuloplasty, had an uneventful vaginal delivery at 38 weeks, then underwent aortic valve replacement.
In a multivariate analysis, only two independent predictors of maternal hospitalization during pregnancy were identified: the presence of symptoms prior to pregnancy, and the prepregnancy hemodynamic severity of AS.
Preterm birth prior to 37 weeks occurred in 16% of pregnancies involving moderate and 36% of those featuring severe AS. An Apgar score below 7 occurred in 5% of cases of moderate and 16% of severe AS. The small for gestational age rate was 3% with moderate AS and ballooned to 21% with severe AS. The only independent predictor of fetal complications was the degree of elevation in peak aortic gradient prior to pregnancy.
Audience questions focused on challenging scenarios. Would you allow a pregnancy to continue, Dr. Orwat was asked, if a woman with AS developed an aortic gradient of 90 mm Hg during the first trimester?
“It depends on whether she was asymptomatic prior to pregnancy. If so, I think it’s a low-risk situation, and you can carry on with the pregnancy,” he replied. Remember, he added, it’s the baseline gradient that’s important. Cardiac output goes up during the first trimester as a matter of course, and so will the gradient.
Dr. Orwat emphasized that symptomatic severe AS is an indication for aortic valve replacement, and he would advise an affected patient to undergo the surgery prior to pregnancy.
Audience member Dr. Fiona Walker rose to advocate ordering a prepregnancy treadmill exercise test in all patients with asymptomatic severe AS in order to learn whether they are likely to remain asymptomatic during the stresses of pregnancy. In a patient with severe AS who remains asymptomatic with exercise, it’s probably safer to go through pregnancy without prior valve replacement than to put in a mechanical valve.
“Patients with mechanical valves in pregnancy are one of the highest-risk groups we look after,” commented Dr. Walker, head of the maternal cardiology program at University College London Hospitals.
But another audience member advocated aortic valve replacement prior to pregnancy in all women with severe AS, asymptomatic or not.
“Nowadays, if you are afraid of a mechanical valve, you can put in a bioprosthetic one, although I know that’s considered controversial. I see that asymptomatic severe aortic stenosis seems quite safe in ROPAC, but I still consider severe aortic stenosis one of the truly dangerous problems in pregnancy. Maybe I’ll change my mind after ROPAC is published, but right now I don’t think we’re going to leave a severe aortic stenosis prior to pregnancy without intervention,” declared Dr. Avraham Shotan, head of the Heart Institute at Hillel Yaffe Medical Center in Hadera, Israel.
Session cochair Dr. Christa Gohlke-Baerwolf of Bad Krozingen (Germany) Hospital mediated the dispute and closed discussion by observing, “Severe aortic stenosis is not just one thing, it’s a continuum, and such patients should be evaluated by a multidisciplinary team very carefully before starting pregnancy.“
ROPAC is sponsored by the ESC. Dr. Orwat reported having no financial conflicts regarding his study.
AT THE ESC CONGRESS 20015
Key clinical point: Pregnancy in women with aortic stenosis now carries a near-zero risk of maternal mortality.
Major finding: Maternal mortality was zero in 99 pregnancies in 96 women with moderate or severe aortic stenosis.
Data source: The ROPAC registry is an ongoing, prospective, global, observational registry devoted to women with structural heart disease.
Disclosures: The registry is sponsored by the European Society of Cardiology. The presenter reported having no financial conflicts of interest.
ESC: Most heart failure patients’ rehospitalizations are for other reasons
LONDON – More than half of all patients hospitalized for heart failure were readmitted within 1 year of discharge in a large Italian population-based study.
Results from the ARNO-CORE CardioVascular Observatory database showed that 56.6% of 41,417 patients with heart failure who were discharged alive and prescribed at least one drug for heart failure were readmitted to the hospital at least once in the following year, Dr. Aldo Maggioni said at the annual congress of the European Society of Cardiology.
This averaged out to 2.1 readmissions per person, and importantly, those readmissions were frequently for noncardiovascular reasons, he said. Indeed, 23,826 (49.1%) of rehospitalizations were due to respiratory disease, gastrointestinal disease, cancer, trauma, or other reasons.
“This clearly implies that if we want to impact the total burden of [heart failure], we are not to just consider the reduction in hospitalization for heart failure, but we have to take into account the multiplicity of causes that these patients have,” commented Dr. Maggioni of the National Association of Hospital Cardiologists Research Center in Florence, Italy.
The ARNO-CORE CardioVascular Observatory is a large, retrospective observational study looking at the clinical epidemiology of patients with heart failure. Unlike clinical trial populations and even specialty-run heart failure registries, the ARNO database aims to provide a more representative picture of heart failure and its treatment in a routine practice setting, Dr. Maggioni noted.
Of the 41,413 patients with acute heart failure during 2008-2012 included in the present analysis, just over one-quarter (27%) were admitted to a cardiology unit, with the majority (50%) admitted to internal medicine or general medicine practices, and 14% seen in geriatric departments, 2% in functional recovery or rehabilitation units, 2% in pulmonary units, and the remainder in other practices.
The mean age of patients was 78 years, which was older than that seen in heart failure trials such as the RELAX-AHF study (72 years) and the ESC-HF Long-Term Registry(71 years). There was also a higher percentage of women, compared with the percentage for the trial and the other registry population (51% vs. 38% and 27%, respectively), and there were higher incidences of chronic obstructive pulmonary disease (31% vs. 16% and 20%) and depression (21% in the ARNO population vs. 8% in the ESC-HF Long-Term Registry). A substantial percentage (31%) had diabetes (48% in RELAX-AHF and 39% in the ESC registry), but a lower percentage was found to have coexistent chronic kidney disease (4% vs. 26% in the ESC registry). The reason for the latter difference was perhaps that it was possible to more accurately include only those patients with chronic kidney disease using the administrative coding, Dr. Maggioni said.
In terms of pharmacologic treatments, fewer patients treated in routine practice than in the clinical trial or ESC registry settings received guideline-recommended therapies. Two-thirds (66%) of patients in the ARNO database were taking ACE inhibitors or angiotensin II receptor blockers (ARBs), compared with 72% and 77% of the RELAX-AHF and ESC registry populations. Furthermore only about half (52%) were taking beta-blockers, compared with 89% and 72% of the trial and other ESC registry populations.
Importantly, patients were being treated with suboptimal doses, he said, and adherence rates at 1 year were 58% for ACE inhibitors and ARBs and 62% for beta-blockers.
All-cause mortality at 1 year was 28.7%, which was higher than that seen in clinical studies such as EVEREST (26.1%) and at 6 months in the RELAX-AHF study (9.2%). It was also slightly higher than that seen in the ESC-HF Long-Term Registry (23.6%).
“The yearly cost for a patient with heart failure is high and mainly driven by hospitalizations,” Dr. Maggioni said. The estimated costs per patient per year were $13,295.04, which is higher than that for cardiovascular disease in general ($10,689.59) and lower than for acute coronary syndrome ($16,664.74). Of this cost, roughly 83% is linked to hospitalization and less than 10% to medications and 7% to specialist diagnostic procedures.
“Given the poor prognosis and the high health care costs associated with hospital readmissions, even small improvements in the global heart failure patient care can have a substantial impact on patient quality of life and health care costs,” Dr. Maggioni suggested. He added that better adherence to guideline-recommended medications could also play a vital role.The study was partially supported by Novartis Pharma Italy. Dr. Maggioni disclosed serving on committees of heart failure trials sponsored by Bayer Healthcare, Cardiorentis, and Novartis Pharma Italy.
*This story was updated 9/10/2015.
LONDON – More than half of all patients hospitalized for heart failure were readmitted within 1 year of discharge in a large Italian population-based study.
Results from the ARNO-CORE CardioVascular Observatory database showed that 56.6% of 41,417 patients with heart failure who were discharged alive and prescribed at least one drug for heart failure were readmitted to the hospital at least once in the following year, Dr. Aldo Maggioni said at the annual congress of the European Society of Cardiology.
This averaged out to 2.1 readmissions per person, and importantly, those readmissions were frequently for noncardiovascular reasons, he said. Indeed, 23,826 (49.1%) of rehospitalizations were due to respiratory disease, gastrointestinal disease, cancer, trauma, or other reasons.
“This clearly implies that if we want to impact the total burden of [heart failure], we are not to just consider the reduction in hospitalization for heart failure, but we have to take into account the multiplicity of causes that these patients have,” commented Dr. Maggioni of the National Association of Hospital Cardiologists Research Center in Florence, Italy.
The ARNO-CORE CardioVascular Observatory is a large, retrospective observational study looking at the clinical epidemiology of patients with heart failure. Unlike clinical trial populations and even specialty-run heart failure registries, the ARNO database aims to provide a more representative picture of heart failure and its treatment in a routine practice setting, Dr. Maggioni noted.
Of the 41,413 patients with acute heart failure during 2008-2012 included in the present analysis, just over one-quarter (27%) were admitted to a cardiology unit, with the majority (50%) admitted to internal medicine or general medicine practices, and 14% seen in geriatric departments, 2% in functional recovery or rehabilitation units, 2% in pulmonary units, and the remainder in other practices.
The mean age of patients was 78 years, which was older than that seen in heart failure trials such as the RELAX-AHF study (72 years) and the ESC-HF Long-Term Registry(71 years). There was also a higher percentage of women, compared with the percentage for the trial and the other registry population (51% vs. 38% and 27%, respectively), and there were higher incidences of chronic obstructive pulmonary disease (31% vs. 16% and 20%) and depression (21% in the ARNO population vs. 8% in the ESC-HF Long-Term Registry). A substantial percentage (31%) had diabetes (48% in RELAX-AHF and 39% in the ESC registry), but a lower percentage was found to have coexistent chronic kidney disease (4% vs. 26% in the ESC registry). The reason for the latter difference was perhaps that it was possible to more accurately include only those patients with chronic kidney disease using the administrative coding, Dr. Maggioni said.
In terms of pharmacologic treatments, fewer patients treated in routine practice than in the clinical trial or ESC registry settings received guideline-recommended therapies. Two-thirds (66%) of patients in the ARNO database were taking ACE inhibitors or angiotensin II receptor blockers (ARBs), compared with 72% and 77% of the RELAX-AHF and ESC registry populations. Furthermore only about half (52%) were taking beta-blockers, compared with 89% and 72% of the trial and other ESC registry populations.
Importantly, patients were being treated with suboptimal doses, he said, and adherence rates at 1 year were 58% for ACE inhibitors and ARBs and 62% for beta-blockers.
All-cause mortality at 1 year was 28.7%, which was higher than that seen in clinical studies such as EVEREST (26.1%) and at 6 months in the RELAX-AHF study (9.2%). It was also slightly higher than that seen in the ESC-HF Long-Term Registry (23.6%).
“The yearly cost for a patient with heart failure is high and mainly driven by hospitalizations,” Dr. Maggioni said. The estimated costs per patient per year were $13,295.04, which is higher than that for cardiovascular disease in general ($10,689.59) and lower than for acute coronary syndrome ($16,664.74). Of this cost, roughly 83% is linked to hospitalization and less than 10% to medications and 7% to specialist diagnostic procedures.
“Given the poor prognosis and the high health care costs associated with hospital readmissions, even small improvements in the global heart failure patient care can have a substantial impact on patient quality of life and health care costs,” Dr. Maggioni suggested. He added that better adherence to guideline-recommended medications could also play a vital role.The study was partially supported by Novartis Pharma Italy. Dr. Maggioni disclosed serving on committees of heart failure trials sponsored by Bayer Healthcare, Cardiorentis, and Novartis Pharma Italy.
*This story was updated 9/10/2015.
LONDON – More than half of all patients hospitalized for heart failure were readmitted within 1 year of discharge in a large Italian population-based study.
Results from the ARNO-CORE CardioVascular Observatory database showed that 56.6% of 41,417 patients with heart failure who were discharged alive and prescribed at least one drug for heart failure were readmitted to the hospital at least once in the following year, Dr. Aldo Maggioni said at the annual congress of the European Society of Cardiology.
This averaged out to 2.1 readmissions per person, and importantly, those readmissions were frequently for noncardiovascular reasons, he said. Indeed, 23,826 (49.1%) of rehospitalizations were due to respiratory disease, gastrointestinal disease, cancer, trauma, or other reasons.
“This clearly implies that if we want to impact the total burden of [heart failure], we are not to just consider the reduction in hospitalization for heart failure, but we have to take into account the multiplicity of causes that these patients have,” commented Dr. Maggioni of the National Association of Hospital Cardiologists Research Center in Florence, Italy.
The ARNO-CORE CardioVascular Observatory is a large, retrospective observational study looking at the clinical epidemiology of patients with heart failure. Unlike clinical trial populations and even specialty-run heart failure registries, the ARNO database aims to provide a more representative picture of heart failure and its treatment in a routine practice setting, Dr. Maggioni noted.
Of the 41,413 patients with acute heart failure during 2008-2012 included in the present analysis, just over one-quarter (27%) were admitted to a cardiology unit, with the majority (50%) admitted to internal medicine or general medicine practices, and 14% seen in geriatric departments, 2% in functional recovery or rehabilitation units, 2% in pulmonary units, and the remainder in other practices.
The mean age of patients was 78 years, which was older than that seen in heart failure trials such as the RELAX-AHF study (72 years) and the ESC-HF Long-Term Registry(71 years). There was also a higher percentage of women, compared with the percentage for the trial and the other registry population (51% vs. 38% and 27%, respectively), and there were higher incidences of chronic obstructive pulmonary disease (31% vs. 16% and 20%) and depression (21% in the ARNO population vs. 8% in the ESC-HF Long-Term Registry). A substantial percentage (31%) had diabetes (48% in RELAX-AHF and 39% in the ESC registry), but a lower percentage was found to have coexistent chronic kidney disease (4% vs. 26% in the ESC registry). The reason for the latter difference was perhaps that it was possible to more accurately include only those patients with chronic kidney disease using the administrative coding, Dr. Maggioni said.
In terms of pharmacologic treatments, fewer patients treated in routine practice than in the clinical trial or ESC registry settings received guideline-recommended therapies. Two-thirds (66%) of patients in the ARNO database were taking ACE inhibitors or angiotensin II receptor blockers (ARBs), compared with 72% and 77% of the RELAX-AHF and ESC registry populations. Furthermore only about half (52%) were taking beta-blockers, compared with 89% and 72% of the trial and other ESC registry populations.
Importantly, patients were being treated with suboptimal doses, he said, and adherence rates at 1 year were 58% for ACE inhibitors and ARBs and 62% for beta-blockers.
All-cause mortality at 1 year was 28.7%, which was higher than that seen in clinical studies such as EVEREST (26.1%) and at 6 months in the RELAX-AHF study (9.2%). It was also slightly higher than that seen in the ESC-HF Long-Term Registry (23.6%).
“The yearly cost for a patient with heart failure is high and mainly driven by hospitalizations,” Dr. Maggioni said. The estimated costs per patient per year were $13,295.04, which is higher than that for cardiovascular disease in general ($10,689.59) and lower than for acute coronary syndrome ($16,664.74). Of this cost, roughly 83% is linked to hospitalization and less than 10% to medications and 7% to specialist diagnostic procedures.
“Given the poor prognosis and the high health care costs associated with hospital readmissions, even small improvements in the global heart failure patient care can have a substantial impact on patient quality of life and health care costs,” Dr. Maggioni suggested. He added that better adherence to guideline-recommended medications could also play a vital role.The study was partially supported by Novartis Pharma Italy. Dr. Maggioni disclosed serving on committees of heart failure trials sponsored by Bayer Healthcare, Cardiorentis, and Novartis Pharma Italy.
*This story was updated 9/10/2015.
AT THE ESC CONGRESS 2015
Key clinical point: Hospital readmission in patients with heart failure is often a result of noncardiovascular causes, warranting a multidisciplinary approach.
Major finding: More than half (56.6%) of 41,417 patients with heart failure studied were rehospitalized within 1 year, mostly for noncardiovascular reasons (49.1%).
Data source: The ARNO-CORE CardioVascular Observatory, a large retrospective observational study looking at the clinical epidemiology of patients with heart failure.
Disclosures: The study was partially supported by Novartis Pharma Italy. Dr. Maggioni disclosed serving on committees of heart failure trials sponsored by Bayer Healthcare, Cardiorentis, and Novartis Pharma Italy.