User login
Developing vaccines against enterovirus-A71 called a priority
MADRID – Is there a need for an enterovirus-A71 vaccine?
This is a new question for North American and European physicians, but not so new in Asia.
“China says yes, with more than 15 million cases of hand, foot, and mouth disease resulting in 3,500 deaths since surveillance started in 2009,” Heli Harvala, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
Seroconversion rates 28 days after the second dose of these vaccines, both directed specifically against viral subgenotype C-4, are 92%-100%. Vaccine efficacy is 91%-97%, according to Dr. Harvala, a consultant medical virologist at University College London.
It remains a mystery why major outbreaks of severe EV-A71 disease have mostly occurred in Asia, with the notable exception of a Spanish outbreak of EV-A71 encephalitis in 2016. The possibility of much wider spread is concerning.
The Chinese monovalent EV-A71 vaccines, however, are seen as a stopgap. For one thing, recent evidence suggests that it’s probably not the specific EV-A71 C-4 viral subgenotype that accounts for all severe disease.
“I think we have to aim for a multivalent vaccine,” Dr. Harvala said.
Now in clinical trials, investigational bivalent vaccines are directed against other EV-A71 subgenotypes in addition to C-4, and also against another enterovirus, coxsackievirus serotype A16, the most common cause of classic hand, foot, and mouth disease in the United States. But that’s probably not enough, according to Dr. Harvala. She noted that coxsackievirus A6, which was first identified more than 50 years ago, abruptly became the main cause of mild hand, foot, and mouth disease in China in 2013 and again in 2015. Moreover, its role in severe cases is growing, and there have been important outbreaks in the United States in recent years. These severe cases come in three main presentations, resembling either erythema multiforme, chicken pox, or eczema herpeticum.
Dr. Harvala added that a next-generation vaccine probably also should offer protection against enterovirus-D68. In 2014, there were 1,153 laboratory-confirmed EV-D68 infections and 14 deaths in the United States and Canada. This infection poses a diagnostic challenge: while the virus is readily detectable on throat swabs, it’s only rarely present in stool or cerebrospinal fluid samples.
“It’s important to keep in mind that this infection is still underdiagnosed. We are not really looking for it,” she said.
No specific treatment for enterovirus infections is available. Three capsid-binding antiviral agents now are in clinical trials: pleconaril, vapendavir, and pocapavir. In addition, translational studies have demonstrated that the SSRI fluoxetine inhibits enterovirus replication, but there have been no clinical trials as yet.
Although development of antivirals effective against enterovirus is an active area of research, Dr. Harvala thinks drug resistance will be an issue, underscoring the importance of vaccine development.
She reported having no financial conflicts of interest regarding her presentation.
MADRID – Is there a need for an enterovirus-A71 vaccine?
This is a new question for North American and European physicians, but not so new in Asia.
“China says yes, with more than 15 million cases of hand, foot, and mouth disease resulting in 3,500 deaths since surveillance started in 2009,” Heli Harvala, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
Seroconversion rates 28 days after the second dose of these vaccines, both directed specifically against viral subgenotype C-4, are 92%-100%. Vaccine efficacy is 91%-97%, according to Dr. Harvala, a consultant medical virologist at University College London.
It remains a mystery why major outbreaks of severe EV-A71 disease have mostly occurred in Asia, with the notable exception of a Spanish outbreak of EV-A71 encephalitis in 2016. The possibility of much wider spread is concerning.
The Chinese monovalent EV-A71 vaccines, however, are seen as a stopgap. For one thing, recent evidence suggests that it’s probably not the specific EV-A71 C-4 viral subgenotype that accounts for all severe disease.
“I think we have to aim for a multivalent vaccine,” Dr. Harvala said.
Now in clinical trials, investigational bivalent vaccines are directed against other EV-A71 subgenotypes in addition to C-4, and also against another enterovirus, coxsackievirus serotype A16, the most common cause of classic hand, foot, and mouth disease in the United States. But that’s probably not enough, according to Dr. Harvala. She noted that coxsackievirus A6, which was first identified more than 50 years ago, abruptly became the main cause of mild hand, foot, and mouth disease in China in 2013 and again in 2015. Moreover, its role in severe cases is growing, and there have been important outbreaks in the United States in recent years. These severe cases come in three main presentations, resembling either erythema multiforme, chicken pox, or eczema herpeticum.
Dr. Harvala added that a next-generation vaccine probably also should offer protection against enterovirus-D68. In 2014, there were 1,153 laboratory-confirmed EV-D68 infections and 14 deaths in the United States and Canada. This infection poses a diagnostic challenge: while the virus is readily detectable on throat swabs, it’s only rarely present in stool or cerebrospinal fluid samples.
“It’s important to keep in mind that this infection is still underdiagnosed. We are not really looking for it,” she said.
No specific treatment for enterovirus infections is available. Three capsid-binding antiviral agents now are in clinical trials: pleconaril, vapendavir, and pocapavir. In addition, translational studies have demonstrated that the SSRI fluoxetine inhibits enterovirus replication, but there have been no clinical trials as yet.
Although development of antivirals effective against enterovirus is an active area of research, Dr. Harvala thinks drug resistance will be an issue, underscoring the importance of vaccine development.
She reported having no financial conflicts of interest regarding her presentation.
MADRID – Is there a need for an enterovirus-A71 vaccine?
This is a new question for North American and European physicians, but not so new in Asia.
“China says yes, with more than 15 million cases of hand, foot, and mouth disease resulting in 3,500 deaths since surveillance started in 2009,” Heli Harvala, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
Seroconversion rates 28 days after the second dose of these vaccines, both directed specifically against viral subgenotype C-4, are 92%-100%. Vaccine efficacy is 91%-97%, according to Dr. Harvala, a consultant medical virologist at University College London.
It remains a mystery why major outbreaks of severe EV-A71 disease have mostly occurred in Asia, with the notable exception of a Spanish outbreak of EV-A71 encephalitis in 2016. The possibility of much wider spread is concerning.
The Chinese monovalent EV-A71 vaccines, however, are seen as a stopgap. For one thing, recent evidence suggests that it’s probably not the specific EV-A71 C-4 viral subgenotype that accounts for all severe disease.
“I think we have to aim for a multivalent vaccine,” Dr. Harvala said.
Now in clinical trials, investigational bivalent vaccines are directed against other EV-A71 subgenotypes in addition to C-4, and also against another enterovirus, coxsackievirus serotype A16, the most common cause of classic hand, foot, and mouth disease in the United States. But that’s probably not enough, according to Dr. Harvala. She noted that coxsackievirus A6, which was first identified more than 50 years ago, abruptly became the main cause of mild hand, foot, and mouth disease in China in 2013 and again in 2015. Moreover, its role in severe cases is growing, and there have been important outbreaks in the United States in recent years. These severe cases come in three main presentations, resembling either erythema multiforme, chicken pox, or eczema herpeticum.
Dr. Harvala added that a next-generation vaccine probably also should offer protection against enterovirus-D68. In 2014, there were 1,153 laboratory-confirmed EV-D68 infections and 14 deaths in the United States and Canada. This infection poses a diagnostic challenge: while the virus is readily detectable on throat swabs, it’s only rarely present in stool or cerebrospinal fluid samples.
“It’s important to keep in mind that this infection is still underdiagnosed. We are not really looking for it,” she said.
No specific treatment for enterovirus infections is available. Three capsid-binding antiviral agents now are in clinical trials: pleconaril, vapendavir, and pocapavir. In addition, translational studies have demonstrated that the SSRI fluoxetine inhibits enterovirus replication, but there have been no clinical trials as yet.
Although development of antivirals effective against enterovirus is an active area of research, Dr. Harvala thinks drug resistance will be an issue, underscoring the importance of vaccine development.
She reported having no financial conflicts of interest regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2017
Acute lobar nephronia often has misleading presentation
MADRID – Acute lobar nephronia needs to be considered in children with high fever, abdominal pain, and markedly elevated acute-phase reactants, even if their urinalysis and ultrasound results are negative, Paula Sanchez-Marcos, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a retrospective study of 18 episodes of acute lobar nephronia (ALN) in 16 children seen at the hospital, a tertiary referral center. Six of the children had vesicoureteral reflux or another underlying uropathy. Mean age at diagnosis was 79 months, with a range of 5 to 180 months.
All patients had a fever greater than 38.5° C when they presented with a mean 6-day history of illness. Of the 16 children, 14 had abdominal pain. The mean C-reactive protein level was 197 mg/L, with a WBC count of 21,962 cells/mcL and a neutrophil count of 17,372 cells/mcL.
Urine dipstick was negative in five episodes. However, urine culture was eventually productive in 10 episodes, with Escherichia coli the most commonly isolated microorganism, found in five of these cases.
All patients underwent ultrasound imaging a mean of 1.7 days into their hospital admission, although it established the diagnosis of ALN in only two episodes. Additional imaging with CT had a 91% sensitivity, showing positive results in 10 of 11 cases, while MRI had 100% sensitivity.
Patients received IV antibiotics for a median of 14 days before switching to sequential oral antibiotics for a median of 8.7 days.
Three patients developed renal abscesses, with percutaneous drainage required in two instances. Unilateral renal scarring occurred in 7 of 16 patients.
Dr. Sanchez-Marcos recommended technetium-99m dimercaptosuccinic acid renal scintigraphy as a tool to confirm improvement in response to antimicrobial therapy.
She reported having no financial conflicts regarding her presentation.
MADRID – Acute lobar nephronia needs to be considered in children with high fever, abdominal pain, and markedly elevated acute-phase reactants, even if their urinalysis and ultrasound results are negative, Paula Sanchez-Marcos, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a retrospective study of 18 episodes of acute lobar nephronia (ALN) in 16 children seen at the hospital, a tertiary referral center. Six of the children had vesicoureteral reflux or another underlying uropathy. Mean age at diagnosis was 79 months, with a range of 5 to 180 months.
All patients had a fever greater than 38.5° C when they presented with a mean 6-day history of illness. Of the 16 children, 14 had abdominal pain. The mean C-reactive protein level was 197 mg/L, with a WBC count of 21,962 cells/mcL and a neutrophil count of 17,372 cells/mcL.
Urine dipstick was negative in five episodes. However, urine culture was eventually productive in 10 episodes, with Escherichia coli the most commonly isolated microorganism, found in five of these cases.
All patients underwent ultrasound imaging a mean of 1.7 days into their hospital admission, although it established the diagnosis of ALN in only two episodes. Additional imaging with CT had a 91% sensitivity, showing positive results in 10 of 11 cases, while MRI had 100% sensitivity.
Patients received IV antibiotics for a median of 14 days before switching to sequential oral antibiotics for a median of 8.7 days.
Three patients developed renal abscesses, with percutaneous drainage required in two instances. Unilateral renal scarring occurred in 7 of 16 patients.
Dr. Sanchez-Marcos recommended technetium-99m dimercaptosuccinic acid renal scintigraphy as a tool to confirm improvement in response to antimicrobial therapy.
She reported having no financial conflicts regarding her presentation.
MADRID – Acute lobar nephronia needs to be considered in children with high fever, abdominal pain, and markedly elevated acute-phase reactants, even if their urinalysis and ultrasound results are negative, Paula Sanchez-Marcos, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a retrospective study of 18 episodes of acute lobar nephronia (ALN) in 16 children seen at the hospital, a tertiary referral center. Six of the children had vesicoureteral reflux or another underlying uropathy. Mean age at diagnosis was 79 months, with a range of 5 to 180 months.
All patients had a fever greater than 38.5° C when they presented with a mean 6-day history of illness. Of the 16 children, 14 had abdominal pain. The mean C-reactive protein level was 197 mg/L, with a WBC count of 21,962 cells/mcL and a neutrophil count of 17,372 cells/mcL.
Urine dipstick was negative in five episodes. However, urine culture was eventually productive in 10 episodes, with Escherichia coli the most commonly isolated microorganism, found in five of these cases.
All patients underwent ultrasound imaging a mean of 1.7 days into their hospital admission, although it established the diagnosis of ALN in only two episodes. Additional imaging with CT had a 91% sensitivity, showing positive results in 10 of 11 cases, while MRI had 100% sensitivity.
Patients received IV antibiotics for a median of 14 days before switching to sequential oral antibiotics for a median of 8.7 days.
Three patients developed renal abscesses, with percutaneous drainage required in two instances. Unilateral renal scarring occurred in 7 of 16 patients.
Dr. Sanchez-Marcos recommended technetium-99m dimercaptosuccinic acid renal scintigraphy as a tool to confirm improvement in response to antimicrobial therapy.
She reported having no financial conflicts regarding her presentation.
AT ESPID 2017
Key clinical point:
Major finding: Urine dipstick results were negative in 5 instances, and ultrasound was negative in 16 cases.
Data source: This was a single-center, retrospective, descriptive study of 18 episodes of acute lobar nephronia in 16 children.
Disclosures: Dr. Sanchez-Marcos reported having no financial conflicts of interest.
New acellular pertussis vaccine may solve waning immunogenicity problem
MADRID – A novel, monovalent, acellular pertussis vaccine containing a recombinant, genetically inactivated pertussis toxin displayed markedly greater sustained immunogenicity than the widely used Sanofi Pasteur Tdap, known as Adacel, which is used as a booster vaccination of adolescents and young adults, in a pivotal phase 3, randomized trial, Simonetta Viviani, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Our interpretation of these results is that they open up a new way to approach pertussis vaccination,” declared Dr. Viviani, director of clinical development at BioNet-Asia, a Bangkok-based biotech vaccine company.
The impetus for developing new acellular pertussis vaccines is the documented resurgence of pertussis.
“One suggested approach has been to replace chemically inactivated PT with a genetically inactivated PT. The rationale for that is the epitopes of the PT are conserved in the genetically modified PT toxin, as opposed to being destroyed in the chemical inactivation process,” Dr. Viviani explained.
The significant phase 3 trial included 450 Thai 12- to 17-year-olds who were randomized to a single 0.5-mL dose of Pertagen, Boostagen, or Adacel. Both Pertagen and Boostagen contain 5 mcg of the genetically inactivated PT and 5 mcg of filamentous hemagglutinin.
The seroconversion rate, defined as the proportion of subjects who reached at least a fourfold increase in titers of PT and filamentous-hemagglutinin antibodies over baseline, was far superior at both 28 days and 1 year in subjects who got Pertagen or Boostagen, compared with those who received Adacel.
Session chair Ulrich Heininger, MD, declared, “This is really, really exciting.”
It now will be very important that the monovalent Pertagen vaccine be formally studied in pregnant women, he observed.
“Since we’d like to immunize women in every pregnancy and they don’t necessarily need the Td component of Tdap every time, a monovalent vaccine might open a new path for acceptance,” commented Dr. Heininger, professor of pediatric infectious diseases at University Children’s Hospital in Basel, Switz.
Dr. Viviani said that a study in pregnant women is now in the early planning stages.
The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
MADRID – A novel, monovalent, acellular pertussis vaccine containing a recombinant, genetically inactivated pertussis toxin displayed markedly greater sustained immunogenicity than the widely used Sanofi Pasteur Tdap, known as Adacel, which is used as a booster vaccination of adolescents and young adults, in a pivotal phase 3, randomized trial, Simonetta Viviani, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Our interpretation of these results is that they open up a new way to approach pertussis vaccination,” declared Dr. Viviani, director of clinical development at BioNet-Asia, a Bangkok-based biotech vaccine company.
The impetus for developing new acellular pertussis vaccines is the documented resurgence of pertussis.
“One suggested approach has been to replace chemically inactivated PT with a genetically inactivated PT. The rationale for that is the epitopes of the PT are conserved in the genetically modified PT toxin, as opposed to being destroyed in the chemical inactivation process,” Dr. Viviani explained.
The significant phase 3 trial included 450 Thai 12- to 17-year-olds who were randomized to a single 0.5-mL dose of Pertagen, Boostagen, or Adacel. Both Pertagen and Boostagen contain 5 mcg of the genetically inactivated PT and 5 mcg of filamentous hemagglutinin.
The seroconversion rate, defined as the proportion of subjects who reached at least a fourfold increase in titers of PT and filamentous-hemagglutinin antibodies over baseline, was far superior at both 28 days and 1 year in subjects who got Pertagen or Boostagen, compared with those who received Adacel.
Session chair Ulrich Heininger, MD, declared, “This is really, really exciting.”
It now will be very important that the monovalent Pertagen vaccine be formally studied in pregnant women, he observed.
“Since we’d like to immunize women in every pregnancy and they don’t necessarily need the Td component of Tdap every time, a monovalent vaccine might open a new path for acceptance,” commented Dr. Heininger, professor of pediatric infectious diseases at University Children’s Hospital in Basel, Switz.
Dr. Viviani said that a study in pregnant women is now in the early planning stages.
The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
MADRID – A novel, monovalent, acellular pertussis vaccine containing a recombinant, genetically inactivated pertussis toxin displayed markedly greater sustained immunogenicity than the widely used Sanofi Pasteur Tdap, known as Adacel, which is used as a booster vaccination of adolescents and young adults, in a pivotal phase 3, randomized trial, Simonetta Viviani, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Our interpretation of these results is that they open up a new way to approach pertussis vaccination,” declared Dr. Viviani, director of clinical development at BioNet-Asia, a Bangkok-based biotech vaccine company.
The impetus for developing new acellular pertussis vaccines is the documented resurgence of pertussis.
“One suggested approach has been to replace chemically inactivated PT with a genetically inactivated PT. The rationale for that is the epitopes of the PT are conserved in the genetically modified PT toxin, as opposed to being destroyed in the chemical inactivation process,” Dr. Viviani explained.
The significant phase 3 trial included 450 Thai 12- to 17-year-olds who were randomized to a single 0.5-mL dose of Pertagen, Boostagen, or Adacel. Both Pertagen and Boostagen contain 5 mcg of the genetically inactivated PT and 5 mcg of filamentous hemagglutinin.
The seroconversion rate, defined as the proportion of subjects who reached at least a fourfold increase in titers of PT and filamentous-hemagglutinin antibodies over baseline, was far superior at both 28 days and 1 year in subjects who got Pertagen or Boostagen, compared with those who received Adacel.
Session chair Ulrich Heininger, MD, declared, “This is really, really exciting.”
It now will be very important that the monovalent Pertagen vaccine be formally studied in pregnant women, he observed.
“Since we’d like to immunize women in every pregnancy and they don’t necessarily need the Td component of Tdap every time, a monovalent vaccine might open a new path for acceptance,” commented Dr. Heininger, professor of pediatric infectious diseases at University Children’s Hospital in Basel, Switz.
Dr. Viviani said that a study in pregnant women is now in the early planning stages.
The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
AT ESPID 2017
Key clinical point:
Major finding: One year after teens received a single dose of a novel acellular pertussis vaccine, they had a geometric mean titer of PT neutralizing antibody of 77 IU/mL, compared with just 12 IU/mL in adolescents who received a conventional Tdap vaccine.
Data source: This randomized, triple-arm, pivotal phase 3 clinical trial included 450 Thai 12- to 17-year-olds followed for 1 year after receiving a single dose of a novel monovalent pertussis vaccine or a novel Tdap vaccine, both of which contain genetically inactivated pertussis toxin, or, instead of those, a widely utilized conventional Tdap vaccine.
Disclosures: The study was sponsored by BioNet-Asia and Mahidol University. Dr. Viviani is a BioNet employee.
Inactivated quadrivalent influenza vaccine safe, effective in 6- to 35-month-olds
MADRID – An intramuscular inactivated quadrivalent influenza vaccine reduced the risk of laboratory-confirmed influenza by up to 69% in previously unvaccinated children aged 6-35 months in a large randomized trial, Stephanie Pepin, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The quadrivalent influenza vaccine (QIV) is licensed by the Food and Drug Administration as Fluzone Quadrivalent for use in patients as young as 6 months of age. It contains two A- and two B-lineage influenza strains in order to address the common problem of mismatches between circulating influenza B and the single B-lineage strain included in trivalent vaccines.
The incidence of any laboratory confirmed strain of influenza illness during the period from 14 days post-vaccination to the end of flu season was 4.72% in the QIV group, compared with 9.84% in controls who received placebo, which translated to 52% efficacy. The incidence of influenza from vaccine-similar strains as determined by the Sanger sequencing method was 1.01% in children randomized to QIV, compared with 3.28% with placebo, for a 69% efficacy rate.
The QIV had a safety profile in this young population that was similar to the older licensed trivalent vaccine. The most frequently reported adverse reactions to the QIV were injection site pain, irritability, loss of appetite, abnormal crying, and malaise, each reported in 19%-25% of children after the first injection and in 14%-18% after the second. These were typically mild grade 1 or 2 reactions, which arose in the first 3 days after vaccination and resolved spontaneously 1-3 days later.
The trial was sponsored by Sanofi Pasteur and presented by a company employee.
MADRID – An intramuscular inactivated quadrivalent influenza vaccine reduced the risk of laboratory-confirmed influenza by up to 69% in previously unvaccinated children aged 6-35 months in a large randomized trial, Stephanie Pepin, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The quadrivalent influenza vaccine (QIV) is licensed by the Food and Drug Administration as Fluzone Quadrivalent for use in patients as young as 6 months of age. It contains two A- and two B-lineage influenza strains in order to address the common problem of mismatches between circulating influenza B and the single B-lineage strain included in trivalent vaccines.
The incidence of any laboratory confirmed strain of influenza illness during the period from 14 days post-vaccination to the end of flu season was 4.72% in the QIV group, compared with 9.84% in controls who received placebo, which translated to 52% efficacy. The incidence of influenza from vaccine-similar strains as determined by the Sanger sequencing method was 1.01% in children randomized to QIV, compared with 3.28% with placebo, for a 69% efficacy rate.
The QIV had a safety profile in this young population that was similar to the older licensed trivalent vaccine. The most frequently reported adverse reactions to the QIV were injection site pain, irritability, loss of appetite, abnormal crying, and malaise, each reported in 19%-25% of children after the first injection and in 14%-18% after the second. These were typically mild grade 1 or 2 reactions, which arose in the first 3 days after vaccination and resolved spontaneously 1-3 days later.
The trial was sponsored by Sanofi Pasteur and presented by a company employee.
MADRID – An intramuscular inactivated quadrivalent influenza vaccine reduced the risk of laboratory-confirmed influenza by up to 69% in previously unvaccinated children aged 6-35 months in a large randomized trial, Stephanie Pepin, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
The quadrivalent influenza vaccine (QIV) is licensed by the Food and Drug Administration as Fluzone Quadrivalent for use in patients as young as 6 months of age. It contains two A- and two B-lineage influenza strains in order to address the common problem of mismatches between circulating influenza B and the single B-lineage strain included in trivalent vaccines.
The incidence of any laboratory confirmed strain of influenza illness during the period from 14 days post-vaccination to the end of flu season was 4.72% in the QIV group, compared with 9.84% in controls who received placebo, which translated to 52% efficacy. The incidence of influenza from vaccine-similar strains as determined by the Sanger sequencing method was 1.01% in children randomized to QIV, compared with 3.28% with placebo, for a 69% efficacy rate.
The QIV had a safety profile in this young population that was similar to the older licensed trivalent vaccine. The most frequently reported adverse reactions to the QIV were injection site pain, irritability, loss of appetite, abnormal crying, and malaise, each reported in 19%-25% of children after the first injection and in 14%-18% after the second. These were typically mild grade 1 or 2 reactions, which arose in the first 3 days after vaccination and resolved spontaneously 1-3 days later.
The trial was sponsored by Sanofi Pasteur and presented by a company employee.
AT ESPID 2017
Key clinical point:
Major finding: The intramuscular inactivated influenza vaccine, Fluzone Quadrivalent, reduced the risk of laboratory-confirmed influenza by up to 69% in 6- to 35-month-olds.
Data source: This randomized, multinational, placebo-controlled trial included 5,806 healthy 6- to 35-month-old children.
Disclosures: The study was sponsored by Sanofi Pasteur and presented by a company employee.
Pertussis resurgence is real, but possible solutions exist
MADRID – The explanation for the ongoing resurgence in pertussis in adolescents and adults in the United States and other developed countries lies largely in the waning effectiveness of current acellular pertussis vaccines as early as 2-3 years post boosters, according to Stanley A. Plotkin, MD, chair of the steering committee for the Global Pertussis Initiative.
“The problem seems to lie in the lack of persistence of immunity after vaccination using the acellular pertussis vaccines. To say that this is not controversial would clearly be wrong, but that is my view,” he declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
It’s a view supported by persuasive evidence, added Dr. Plotkin, emeritus professor of pediatrics at the University of Pennsylvania, Philadelphia.
In the United States, investigators at Northern California Kaiser Permanente have shown that the effectiveness of acellular pertussis in the Tdap vaccine wanes rapidly in adolescents. Indeed, it plunged from 69% effectiveness in the first year after vaccination to less than 9% by year 4 (Pediatrics. 2016 Mar;137[3]:e20153326).
In contrast, whole-cell pertussis vaccines provide roughly 6-10 years of protection against infection, and native infection provides persistent protection against reinfection for 7-20 years, Dr. Plotkin noted.
He was senior coauthor of a recent study that addresses why acellular pertussis vaccine immunity wanes so quickly. He and his coinvestigators demonstrated that while whole-cell pertussis vaccines promote vigorous Th1 and Th17 responses, which discourage pharyngeal colonization, acellular pertussis vaccines orient the immune system toward a less salutary Th1/Th2 response (Cold Spring Harb Perspect Biol. 2017 Mar 13. doi: 10.1101/cshperspect.a029454).
In addition, other investigators have shown that repeated booster doses of acellular pertussis vaccine generate higher levels of antigen-specific IgG4, which doesn’t bind complement and results in impaired phagocytosis and a suboptimal inflammatory response. In contrast, priming of the immune system via administration of a whole-cell pertussis vaccine at birth followed by acellular pertussis boosters results in improved phagocytosis and complement-mediated microbial killing via preferential induction of IgG1(Cold Spring Harb Perspect Biol. 2017 Mar 13. doi: 10.1101/cshperspect.a029553).
Possible solutions to the pertussis problem
Infants don’t need a new vaccine; that’s not where the vaccine failures are occurring. “Again, I stress that the problem so far has not been in infants, it has been in adolescents and adults,” he said.
A new vaccine is a daunting prospect. Given the huge investment vaccine manufacturers made in the 1990s to bring the current acellular vaccines to the market, they are hardly eager to launch development programs for new pertussis vaccines. They have other vaccine development priorities.
Moreover, the regulatory challenges are huge unless the Food and Drug Administration and other licensing authorities are willing to forgo the large, long, and expensive clinical trials that have traditionally been required. In lieu of such efficacy studies, they would need to consider studies demonstrating better immunogenicity based upon antibody titers, or animal studies.
“The possibility of a human challenge study in adults is an idea I like; I’m not sure about the FDA,” the pediatrician said.
Until a new or improved vaccine becomes available, the most important strategy to control the resurgence of pertussis is acellular vaccination of pregnant women in their third trimester to provide passive protection to the newborn via transplacental antibody. That practice is already recommended in the United States and many other countries. And while it reduces the risk of pertussis in early infancy – the most serious form of the disease – that strategy won’t have any real impact on the adult burden of disease, which Dr. Plotkin estimated at more than 600,000 cases annually.
Cocooning – a strategy of vaccinating all of a newborn’s family contacts – has been promoted in guidelines but has proved difficult to implement. “I think cocooning strategies by and large have been a failure,” he declared.
More frequent boosters of current acellular pertussis vaccines would presumably increase effectiveness, but that would be costly and tough to put in place on a public health scale.
A return to using conventional whole-cell pertussis vaccines would be a tough sell to the public and is probably flat out unacceptable. Developing a less reactogenic whole-cell vaccine might be a work-around, but it hasn’t been done yet.
The easiest way to improve acellular pertussis vaccine for adolescents and adults is to improve the pertussis toxin antigen component. Increasing the dose of pertussis toxin could generate more and longer-lasting antibodies to it. An even more exciting possibility is based upon evidence more than a decade old that genetic inactivation of pertussis toxin results in antibody levels far higher and presumably more bactericidal than the formalin-inactivated pertussis toxin included in current vaccines, according to Dr. Plotkin.
Adding stronger adjuvants to a Tdap vaccine for adolescents is another appealing strategy. There are plenty to choose from, including some that would presumably have an easier pathway to regulatory approval because they are already contained in licensed vaccines. This beefed-up adjuvant strategy, like the notion of changing the antigens in acellular pertussis vaccines to those from currently circulating strains, is feasible albeit more difficult than simply improving the pertussis toxin component of existing vaccines, he said.
The Global Pertussis Initiative is sponsored by Sanofi Pasteur. Dr. Plotkin reported serving as a consultant to that vaccine manufacturer and numerous others but declared he had no financial conflicts regarding his presentation.
MADRID – The explanation for the ongoing resurgence in pertussis in adolescents and adults in the United States and other developed countries lies largely in the waning effectiveness of current acellular pertussis vaccines as early as 2-3 years post boosters, according to Stanley A. Plotkin, MD, chair of the steering committee for the Global Pertussis Initiative.
“The problem seems to lie in the lack of persistence of immunity after vaccination using the acellular pertussis vaccines. To say that this is not controversial would clearly be wrong, but that is my view,” he declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
It’s a view supported by persuasive evidence, added Dr. Plotkin, emeritus professor of pediatrics at the University of Pennsylvania, Philadelphia.
In the United States, investigators at Northern California Kaiser Permanente have shown that the effectiveness of acellular pertussis in the Tdap vaccine wanes rapidly in adolescents. Indeed, it plunged from 69% effectiveness in the first year after vaccination to less than 9% by year 4 (Pediatrics. 2016 Mar;137[3]:e20153326).
In contrast, whole-cell pertussis vaccines provide roughly 6-10 years of protection against infection, and native infection provides persistent protection against reinfection for 7-20 years, Dr. Plotkin noted.
He was senior coauthor of a recent study that addresses why acellular pertussis vaccine immunity wanes so quickly. He and his coinvestigators demonstrated that while whole-cell pertussis vaccines promote vigorous Th1 and Th17 responses, which discourage pharyngeal colonization, acellular pertussis vaccines orient the immune system toward a less salutary Th1/Th2 response (Cold Spring Harb Perspect Biol. 2017 Mar 13. doi: 10.1101/cshperspect.a029454).
In addition, other investigators have shown that repeated booster doses of acellular pertussis vaccine generate higher levels of antigen-specific IgG4, which doesn’t bind complement and results in impaired phagocytosis and a suboptimal inflammatory response. In contrast, priming of the immune system via administration of a whole-cell pertussis vaccine at birth followed by acellular pertussis boosters results in improved phagocytosis and complement-mediated microbial killing via preferential induction of IgG1(Cold Spring Harb Perspect Biol. 2017 Mar 13. doi: 10.1101/cshperspect.a029553).
Possible solutions to the pertussis problem
Infants don’t need a new vaccine; that’s not where the vaccine failures are occurring. “Again, I stress that the problem so far has not been in infants, it has been in adolescents and adults,” he said.
A new vaccine is a daunting prospect. Given the huge investment vaccine manufacturers made in the 1990s to bring the current acellular vaccines to the market, they are hardly eager to launch development programs for new pertussis vaccines. They have other vaccine development priorities.
Moreover, the regulatory challenges are huge unless the Food and Drug Administration and other licensing authorities are willing to forgo the large, long, and expensive clinical trials that have traditionally been required. In lieu of such efficacy studies, they would need to consider studies demonstrating better immunogenicity based upon antibody titers, or animal studies.
“The possibility of a human challenge study in adults is an idea I like; I’m not sure about the FDA,” the pediatrician said.
Until a new or improved vaccine becomes available, the most important strategy to control the resurgence of pertussis is acellular vaccination of pregnant women in their third trimester to provide passive protection to the newborn via transplacental antibody. That practice is already recommended in the United States and many other countries. And while it reduces the risk of pertussis in early infancy – the most serious form of the disease – that strategy won’t have any real impact on the adult burden of disease, which Dr. Plotkin estimated at more than 600,000 cases annually.
Cocooning – a strategy of vaccinating all of a newborn’s family contacts – has been promoted in guidelines but has proved difficult to implement. “I think cocooning strategies by and large have been a failure,” he declared.
More frequent boosters of current acellular pertussis vaccines would presumably increase effectiveness, but that would be costly and tough to put in place on a public health scale.
A return to using conventional whole-cell pertussis vaccines would be a tough sell to the public and is probably flat out unacceptable. Developing a less reactogenic whole-cell vaccine might be a work-around, but it hasn’t been done yet.
The easiest way to improve acellular pertussis vaccine for adolescents and adults is to improve the pertussis toxin antigen component. Increasing the dose of pertussis toxin could generate more and longer-lasting antibodies to it. An even more exciting possibility is based upon evidence more than a decade old that genetic inactivation of pertussis toxin results in antibody levels far higher and presumably more bactericidal than the formalin-inactivated pertussis toxin included in current vaccines, according to Dr. Plotkin.
Adding stronger adjuvants to a Tdap vaccine for adolescents is another appealing strategy. There are plenty to choose from, including some that would presumably have an easier pathway to regulatory approval because they are already contained in licensed vaccines. This beefed-up adjuvant strategy, like the notion of changing the antigens in acellular pertussis vaccines to those from currently circulating strains, is feasible albeit more difficult than simply improving the pertussis toxin component of existing vaccines, he said.
The Global Pertussis Initiative is sponsored by Sanofi Pasteur. Dr. Plotkin reported serving as a consultant to that vaccine manufacturer and numerous others but declared he had no financial conflicts regarding his presentation.
MADRID – The explanation for the ongoing resurgence in pertussis in adolescents and adults in the United States and other developed countries lies largely in the waning effectiveness of current acellular pertussis vaccines as early as 2-3 years post boosters, according to Stanley A. Plotkin, MD, chair of the steering committee for the Global Pertussis Initiative.
“The problem seems to lie in the lack of persistence of immunity after vaccination using the acellular pertussis vaccines. To say that this is not controversial would clearly be wrong, but that is my view,” he declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
It’s a view supported by persuasive evidence, added Dr. Plotkin, emeritus professor of pediatrics at the University of Pennsylvania, Philadelphia.
In the United States, investigators at Northern California Kaiser Permanente have shown that the effectiveness of acellular pertussis in the Tdap vaccine wanes rapidly in adolescents. Indeed, it plunged from 69% effectiveness in the first year after vaccination to less than 9% by year 4 (Pediatrics. 2016 Mar;137[3]:e20153326).
In contrast, whole-cell pertussis vaccines provide roughly 6-10 years of protection against infection, and native infection provides persistent protection against reinfection for 7-20 years, Dr. Plotkin noted.
He was senior coauthor of a recent study that addresses why acellular pertussis vaccine immunity wanes so quickly. He and his coinvestigators demonstrated that while whole-cell pertussis vaccines promote vigorous Th1 and Th17 responses, which discourage pharyngeal colonization, acellular pertussis vaccines orient the immune system toward a less salutary Th1/Th2 response (Cold Spring Harb Perspect Biol. 2017 Mar 13. doi: 10.1101/cshperspect.a029454).
In addition, other investigators have shown that repeated booster doses of acellular pertussis vaccine generate higher levels of antigen-specific IgG4, which doesn’t bind complement and results in impaired phagocytosis and a suboptimal inflammatory response. In contrast, priming of the immune system via administration of a whole-cell pertussis vaccine at birth followed by acellular pertussis boosters results in improved phagocytosis and complement-mediated microbial killing via preferential induction of IgG1(Cold Spring Harb Perspect Biol. 2017 Mar 13. doi: 10.1101/cshperspect.a029553).
Possible solutions to the pertussis problem
Infants don’t need a new vaccine; that’s not where the vaccine failures are occurring. “Again, I stress that the problem so far has not been in infants, it has been in adolescents and adults,” he said.
A new vaccine is a daunting prospect. Given the huge investment vaccine manufacturers made in the 1990s to bring the current acellular vaccines to the market, they are hardly eager to launch development programs for new pertussis vaccines. They have other vaccine development priorities.
Moreover, the regulatory challenges are huge unless the Food and Drug Administration and other licensing authorities are willing to forgo the large, long, and expensive clinical trials that have traditionally been required. In lieu of such efficacy studies, they would need to consider studies demonstrating better immunogenicity based upon antibody titers, or animal studies.
“The possibility of a human challenge study in adults is an idea I like; I’m not sure about the FDA,” the pediatrician said.
Until a new or improved vaccine becomes available, the most important strategy to control the resurgence of pertussis is acellular vaccination of pregnant women in their third trimester to provide passive protection to the newborn via transplacental antibody. That practice is already recommended in the United States and many other countries. And while it reduces the risk of pertussis in early infancy – the most serious form of the disease – that strategy won’t have any real impact on the adult burden of disease, which Dr. Plotkin estimated at more than 600,000 cases annually.
Cocooning – a strategy of vaccinating all of a newborn’s family contacts – has been promoted in guidelines but has proved difficult to implement. “I think cocooning strategies by and large have been a failure,” he declared.
More frequent boosters of current acellular pertussis vaccines would presumably increase effectiveness, but that would be costly and tough to put in place on a public health scale.
A return to using conventional whole-cell pertussis vaccines would be a tough sell to the public and is probably flat out unacceptable. Developing a less reactogenic whole-cell vaccine might be a work-around, but it hasn’t been done yet.
The easiest way to improve acellular pertussis vaccine for adolescents and adults is to improve the pertussis toxin antigen component. Increasing the dose of pertussis toxin could generate more and longer-lasting antibodies to it. An even more exciting possibility is based upon evidence more than a decade old that genetic inactivation of pertussis toxin results in antibody levels far higher and presumably more bactericidal than the formalin-inactivated pertussis toxin included in current vaccines, according to Dr. Plotkin.
Adding stronger adjuvants to a Tdap vaccine for adolescents is another appealing strategy. There are plenty to choose from, including some that would presumably have an easier pathway to regulatory approval because they are already contained in licensed vaccines. This beefed-up adjuvant strategy, like the notion of changing the antigens in acellular pertussis vaccines to those from currently circulating strains, is feasible albeit more difficult than simply improving the pertussis toxin component of existing vaccines, he said.
The Global Pertussis Initiative is sponsored by Sanofi Pasteur. Dr. Plotkin reported serving as a consultant to that vaccine manufacturer and numerous others but declared he had no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ESPID 2017
Oral antibiotics successfully treat community-acquired pneumonia with empyema
MADRID – Outpatient oral antibiotics were more successful than outpatient parenteral antibiotic therapy at treating children with community-acquired pneumonia complicated by empyema, in a study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
Thirty-five percent of the patients were culture positive, a typically low rate that makes treatment of this disease particularly challenging, Lauren Kushner, a medical student at the University of California, Irvine, and one of the study’s authors, said at the meeting.
The treatment success rates, which were defined as improvement with no change in treatment, were 93% for the patients taking oral antibiotics and 58% in the patients on outpatient parenteral antibiotic therapy.
This retrospective observational study included 149 patients under age 18 years hospitalized for community-acquired pneumonia complicated by empyema, at Children’s Hospital of Orange County, Calif. Only 12 of the patients were treated with parenteral antibiotic therapy and none of the study participants had comorbid chronic medical conditions. As in other studies, Streptococcus pneumoniae was the most commonly identified pathogen.
Laboratory markers of inflammation are useful in guiding oral antibiotic therapy for children with CAP complicated by empyema, reported Ms. Kushner.
“A rapid drop in C-reactive protein [CRP] in combination with a decrease in white blood cell count [WBC] can be used acutely in the hospitalization phase to tell you the patient is improving on the selected antibiotic and also to help dictate when the patient might be able to go home, whereas improvement in the erythrocyte sedimentation rate [ESR] does not happen until much later in the course of treatment but can be used to tell you when a patient has been adequately treated,” said Ms. Kushner.
One hundred thirty-seven patients were discharged on oral antibiotic therapy, as is strongly recommended in Infectious Diseases Society of America guidelines for postdischarge treatment of complicated pneumonia, even though there are no randomized clinical trials demonstrating it to be superior or even noninferior to outpatient parenteral antibiotics. An aminopenicillin was the most frequently prescribed type of oral antibiotic, while ceftriaxone was the top choice for outpatient parenteral therapy.
The average total duration of antibiotic therapy, inpatient plus outpatient, was similar in the two groups: 30.4 days in the oral antibiotic group and 33.2 days in children on outpatient IV therapy.
The transition to oral therapy occurred a median of 6 days after admission. At that point, CRP levels had dropped sharply by a mean of 204 mg/L from a baseline of more than 250 mg/L at admission. In the same time frame, mean WBC dropped by 6,400 cells/mcL from close to 20,000/mcL at admission. Thus, sharp declines in these two inflammatory markers while a patient is still in the hospital provide reassurance that antibiotic therapy is on the right track. Their rate of decline slowed considerably after the switch to oral therapy: for example, mean CRP decreased by only another 44 mg/L from switch to discharge, and by a further 19 mg/L from discharge to end of treatment.
In contrast, the mean ESR remained elevated at a level approaching 100 mm/hour with little fluctuation from admission through discharge. Weekly monitoring of ESR post discharge showed that this inflammatory marker improved only late in the course of oral therapy. A drop to less than 30 mm/hour indicates the infection has resolved, Ms. Kushner said.
She noted that in contrast to her study findings, a recent multicenter, 2,123-patient study by the Pediatric Research in Inpatient Settings Network found that treatment failure rates didn’t differ significantly between the two treatment strategies (Pediatrics. 2016 Dec;138[6]. pii: e20161692). Similarly, a retrospective study of 391 children with empyema admitted to Primary Children’s Hospital in Salt Lake City found closely similar rates of treatment failure and other complications regardless of whether the patients were placed on outpatient oral or parenteral antibiotic therapy (Hosp Pediatr. 2015 Dec;5[12]:605-12).
Ms. Kushner reported having no financial conflicts of interest regarding the study.
MADRID – Outpatient oral antibiotics were more successful than outpatient parenteral antibiotic therapy at treating children with community-acquired pneumonia complicated by empyema, in a study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
Thirty-five percent of the patients were culture positive, a typically low rate that makes treatment of this disease particularly challenging, Lauren Kushner, a medical student at the University of California, Irvine, and one of the study’s authors, said at the meeting.
The treatment success rates, which were defined as improvement with no change in treatment, were 93% for the patients taking oral antibiotics and 58% in the patients on outpatient parenteral antibiotic therapy.
This retrospective observational study included 149 patients under age 18 years hospitalized for community-acquired pneumonia complicated by empyema, at Children’s Hospital of Orange County, Calif. Only 12 of the patients were treated with parenteral antibiotic therapy and none of the study participants had comorbid chronic medical conditions. As in other studies, Streptococcus pneumoniae was the most commonly identified pathogen.
Laboratory markers of inflammation are useful in guiding oral antibiotic therapy for children with CAP complicated by empyema, reported Ms. Kushner.
“A rapid drop in C-reactive protein [CRP] in combination with a decrease in white blood cell count [WBC] can be used acutely in the hospitalization phase to tell you the patient is improving on the selected antibiotic and also to help dictate when the patient might be able to go home, whereas improvement in the erythrocyte sedimentation rate [ESR] does not happen until much later in the course of treatment but can be used to tell you when a patient has been adequately treated,” said Ms. Kushner.
One hundred thirty-seven patients were discharged on oral antibiotic therapy, as is strongly recommended in Infectious Diseases Society of America guidelines for postdischarge treatment of complicated pneumonia, even though there are no randomized clinical trials demonstrating it to be superior or even noninferior to outpatient parenteral antibiotics. An aminopenicillin was the most frequently prescribed type of oral antibiotic, while ceftriaxone was the top choice for outpatient parenteral therapy.
The average total duration of antibiotic therapy, inpatient plus outpatient, was similar in the two groups: 30.4 days in the oral antibiotic group and 33.2 days in children on outpatient IV therapy.
The transition to oral therapy occurred a median of 6 days after admission. At that point, CRP levels had dropped sharply by a mean of 204 mg/L from a baseline of more than 250 mg/L at admission. In the same time frame, mean WBC dropped by 6,400 cells/mcL from close to 20,000/mcL at admission. Thus, sharp declines in these two inflammatory markers while a patient is still in the hospital provide reassurance that antibiotic therapy is on the right track. Their rate of decline slowed considerably after the switch to oral therapy: for example, mean CRP decreased by only another 44 mg/L from switch to discharge, and by a further 19 mg/L from discharge to end of treatment.
In contrast, the mean ESR remained elevated at a level approaching 100 mm/hour with little fluctuation from admission through discharge. Weekly monitoring of ESR post discharge showed that this inflammatory marker improved only late in the course of oral therapy. A drop to less than 30 mm/hour indicates the infection has resolved, Ms. Kushner said.
She noted that in contrast to her study findings, a recent multicenter, 2,123-patient study by the Pediatric Research in Inpatient Settings Network found that treatment failure rates didn’t differ significantly between the two treatment strategies (Pediatrics. 2016 Dec;138[6]. pii: e20161692). Similarly, a retrospective study of 391 children with empyema admitted to Primary Children’s Hospital in Salt Lake City found closely similar rates of treatment failure and other complications regardless of whether the patients were placed on outpatient oral or parenteral antibiotic therapy (Hosp Pediatr. 2015 Dec;5[12]:605-12).
Ms. Kushner reported having no financial conflicts of interest regarding the study.
MADRID – Outpatient oral antibiotics were more successful than outpatient parenteral antibiotic therapy at treating children with community-acquired pneumonia complicated by empyema, in a study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
Thirty-five percent of the patients were culture positive, a typically low rate that makes treatment of this disease particularly challenging, Lauren Kushner, a medical student at the University of California, Irvine, and one of the study’s authors, said at the meeting.
The treatment success rates, which were defined as improvement with no change in treatment, were 93% for the patients taking oral antibiotics and 58% in the patients on outpatient parenteral antibiotic therapy.
This retrospective observational study included 149 patients under age 18 years hospitalized for community-acquired pneumonia complicated by empyema, at Children’s Hospital of Orange County, Calif. Only 12 of the patients were treated with parenteral antibiotic therapy and none of the study participants had comorbid chronic medical conditions. As in other studies, Streptococcus pneumoniae was the most commonly identified pathogen.
Laboratory markers of inflammation are useful in guiding oral antibiotic therapy for children with CAP complicated by empyema, reported Ms. Kushner.
“A rapid drop in C-reactive protein [CRP] in combination with a decrease in white blood cell count [WBC] can be used acutely in the hospitalization phase to tell you the patient is improving on the selected antibiotic and also to help dictate when the patient might be able to go home, whereas improvement in the erythrocyte sedimentation rate [ESR] does not happen until much later in the course of treatment but can be used to tell you when a patient has been adequately treated,” said Ms. Kushner.
One hundred thirty-seven patients were discharged on oral antibiotic therapy, as is strongly recommended in Infectious Diseases Society of America guidelines for postdischarge treatment of complicated pneumonia, even though there are no randomized clinical trials demonstrating it to be superior or even noninferior to outpatient parenteral antibiotics. An aminopenicillin was the most frequently prescribed type of oral antibiotic, while ceftriaxone was the top choice for outpatient parenteral therapy.
The average total duration of antibiotic therapy, inpatient plus outpatient, was similar in the two groups: 30.4 days in the oral antibiotic group and 33.2 days in children on outpatient IV therapy.
The transition to oral therapy occurred a median of 6 days after admission. At that point, CRP levels had dropped sharply by a mean of 204 mg/L from a baseline of more than 250 mg/L at admission. In the same time frame, mean WBC dropped by 6,400 cells/mcL from close to 20,000/mcL at admission. Thus, sharp declines in these two inflammatory markers while a patient is still in the hospital provide reassurance that antibiotic therapy is on the right track. Their rate of decline slowed considerably after the switch to oral therapy: for example, mean CRP decreased by only another 44 mg/L from switch to discharge, and by a further 19 mg/L from discharge to end of treatment.
In contrast, the mean ESR remained elevated at a level approaching 100 mm/hour with little fluctuation from admission through discharge. Weekly monitoring of ESR post discharge showed that this inflammatory marker improved only late in the course of oral therapy. A drop to less than 30 mm/hour indicates the infection has resolved, Ms. Kushner said.
She noted that in contrast to her study findings, a recent multicenter, 2,123-patient study by the Pediatric Research in Inpatient Settings Network found that treatment failure rates didn’t differ significantly between the two treatment strategies (Pediatrics. 2016 Dec;138[6]. pii: e20161692). Similarly, a retrospective study of 391 children with empyema admitted to Primary Children’s Hospital in Salt Lake City found closely similar rates of treatment failure and other complications regardless of whether the patients were placed on outpatient oral or parenteral antibiotic therapy (Hosp Pediatr. 2015 Dec;5[12]:605-12).
Ms. Kushner reported having no financial conflicts of interest regarding the study.
AT ESPID 2017
Key clinical point:
Major finding: The treatment success rate with outpatient oral antibiotic therapy for pediatric community-acquired pneumonia with empyema was 93% in a retrospective study, significantly better than the 58% rate in a much smaller group of patients discharged with outpatient parenteral antibiotic therapy.
Data source: This retrospective single-center study included 149 patients under age 18 years hospitalized for community-acquired pneumonia complicated by empyema and later sent home on either oral or parenteral antibiotic therapy.
Disclosures: The study presenter reported having no financial conflicts of interest.
Lessons emerge from Europe’s first enterovirus-related brain stem encephalitis outbreak
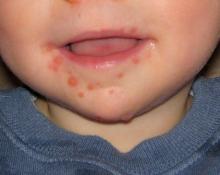
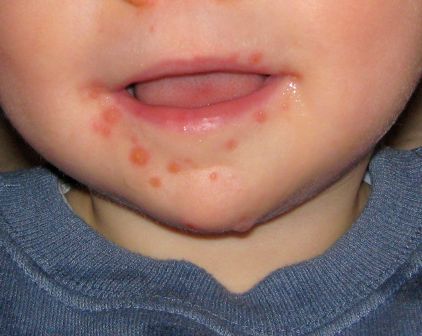
MADRID – Ninety-two percent of Spanish children sickened during the first-ever outbreak of enterovirus-associated brain stem encephalitis in western Europe survived with no long-term sequelae, Nuria Worner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We think that aggressive treatments should be restricted to those patients with important neurologic involvement,” declared Dr. Worner of Vall d’Hebron University Hospital in Barcelona. “We can say that no patients with milder involvement and without warning signs during the first 24 hours after onset of neurologic involvement went on to develop fulminant symptoms.”
Notable outbreaks of enterovirus A71 (EV-A71)-associated brain stem encephalitis occurred in Southeast Asia, Australia, and China in the late 1990s.
Dr. Worner reported on 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016. Their median age was 25 months, 57% were male, and a median of 2 days of symptoms of mild viral illness transpired before neurologic symptoms arose. Prior to presenting to a hospital, 21% of the children had been diagnosed with hand-foot-and-mouth disease, and 13% with herpangina.
Initial preadmission symptoms included fever in 94% of cases, sleepiness in 86%, ataxia in 75%, tremor in 47%, myoclonus in 40%, and a rash in 26%.
Fifty-five percent of the children had EV RNA isolated from both throat and feces, 26% from the throat only, and 19% only from their feces. Eighty-seven percent of serotyped EV were EV-A71.
Ninety percent of children underwent lumbar puncture. Particularly noteworthy was the finding that EV was detected in the cerebrospinal fluid of a mere 3% of patients, although pleocytosis was present in 84%.
Brain MRI showed brain stem encephalitis along with myelitis in 50% of patients, brain stem myelitis without encephalitis in 29%, myelitis elsewhere in 2%, and normal findings in 19%.
Ground zero for the outbreak was Barcelona and the surrounding region of Catalonia; indeed, 130 of the 196 (66%) affected children came from there. The Catalan health department and pediatric infectious disease specialists quickly created standardized case severity definitions and treatment recommendations; they distributed them nationally.
Mild EV-A71–associated brain stem disease was defined as two or more of the following: tremor, myoclonus, mild ataxia, and/or significant drowsiness. The recommendation in these mild cases was for no treatment other than supportive care and careful in-hospital monitoring.
Patients with moderate involvement had to meet the definition for mild disease plus more pronounced ataxia or bulbar motor neuron involvement marked by slurred speech, drooling, dysphagia, apnea, abolition of the gag reflex, and/or an abnormal respiratory pattern. Moderately affected patients received two doses of intravenous immunoglobulin (IVIG), each dosed at 1 g/kg per 24 hours. Admission to the pediatric ICU was individualized for patients with moderate EV-A71–associated brain stem encephalitis.
Severe disease was categorized as bulbar motor neuron involvement plus neurogenic cardiorespiratory failure. Those patients were uniformly admitted to a pediatric ICU and given the two doses of IVIG. The need for systemic steroids was determined on an individual basis.
Forty percent of patients received IVIG and systemic steroids, 24% received IVIG only, 2% systemic steroids only, and 34% received no treatment other than supportive care.
Twenty-six percent of children were admitted to a pediatric ICU for a median stay of 3.5 days. Nine percent of children were placed on mechanical ventilation.
As the disease evolved, the most frequent neurologic complications included slurred speech in 15% of children, abnormal breathing pattern in 11%, seizures in 10%, acute flaccid paralysis in 9%, and cardiorespiratory failure with pulmonary edema in 9%, all occurring within the first hours after hospital admission.
The median hospital length of stay for the full study population was 6 days. The survival rate was 99.5%, with the sole death being due to cardiorespiratory failure.
With 1-6 months of follow-up since the acute episode of EV-A71–associated brain stem encephalitis, the long-term sequelae included two cases of limb paresis and two cases of paresis of a cranial nerve, one child with residual seizures, and one with hypoxic-ischemic encephalopathy.
Asked why the fatality rate in the Spanish outbreak was so much lower than in the earlier Australasian outbreaks, Dr. Worner cited Catalan physicians’ quick recognition of what was underway – and, more importantly, a difference in the EV-A71 viral subgenotype. Most of the most severe cases in Asia and Australia involved the C-4 subgenotype, while in Spain, the predominant subgenotype involved in the outbreak was C-1.
As for the curious finding that EV was detectable in the cerebrospinal fluid of a mere 3% of the Spanish children, she said the explanation is unknown. The two main possibilities are that the CNS symptoms were due to a parenchymal brain infection rather than to EV-A71 infection of meningeal tissue. Alternatively, the CNS involvement may have been a manifestation of an immunologic response to the infection, rather than being due to the virus itself.
Dr. Worner reported having no financial conflicts of interest.
MADRID – Ninety-two percent of Spanish children sickened during the first-ever outbreak of enterovirus-associated brain stem encephalitis in western Europe survived with no long-term sequelae, Nuria Worner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We think that aggressive treatments should be restricted to those patients with important neurologic involvement,” declared Dr. Worner of Vall d’Hebron University Hospital in Barcelona. “We can say that no patients with milder involvement and without warning signs during the first 24 hours after onset of neurologic involvement went on to develop fulminant symptoms.”
Notable outbreaks of enterovirus A71 (EV-A71)-associated brain stem encephalitis occurred in Southeast Asia, Australia, and China in the late 1990s.
Dr. Worner reported on 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016. Their median age was 25 months, 57% were male, and a median of 2 days of symptoms of mild viral illness transpired before neurologic symptoms arose. Prior to presenting to a hospital, 21% of the children had been diagnosed with hand-foot-and-mouth disease, and 13% with herpangina.
Initial preadmission symptoms included fever in 94% of cases, sleepiness in 86%, ataxia in 75%, tremor in 47%, myoclonus in 40%, and a rash in 26%.
Fifty-five percent of the children had EV RNA isolated from both throat and feces, 26% from the throat only, and 19% only from their feces. Eighty-seven percent of serotyped EV were EV-A71.
Ninety percent of children underwent lumbar puncture. Particularly noteworthy was the finding that EV was detected in the cerebrospinal fluid of a mere 3% of patients, although pleocytosis was present in 84%.
Brain MRI showed brain stem encephalitis along with myelitis in 50% of patients, brain stem myelitis without encephalitis in 29%, myelitis elsewhere in 2%, and normal findings in 19%.
Ground zero for the outbreak was Barcelona and the surrounding region of Catalonia; indeed, 130 of the 196 (66%) affected children came from there. The Catalan health department and pediatric infectious disease specialists quickly created standardized case severity definitions and treatment recommendations; they distributed them nationally.
Mild EV-A71–associated brain stem disease was defined as two or more of the following: tremor, myoclonus, mild ataxia, and/or significant drowsiness. The recommendation in these mild cases was for no treatment other than supportive care and careful in-hospital monitoring.
Patients with moderate involvement had to meet the definition for mild disease plus more pronounced ataxia or bulbar motor neuron involvement marked by slurred speech, drooling, dysphagia, apnea, abolition of the gag reflex, and/or an abnormal respiratory pattern. Moderately affected patients received two doses of intravenous immunoglobulin (IVIG), each dosed at 1 g/kg per 24 hours. Admission to the pediatric ICU was individualized for patients with moderate EV-A71–associated brain stem encephalitis.
Severe disease was categorized as bulbar motor neuron involvement plus neurogenic cardiorespiratory failure. Those patients were uniformly admitted to a pediatric ICU and given the two doses of IVIG. The need for systemic steroids was determined on an individual basis.
Forty percent of patients received IVIG and systemic steroids, 24% received IVIG only, 2% systemic steroids only, and 34% received no treatment other than supportive care.
Twenty-six percent of children were admitted to a pediatric ICU for a median stay of 3.5 days. Nine percent of children were placed on mechanical ventilation.
As the disease evolved, the most frequent neurologic complications included slurred speech in 15% of children, abnormal breathing pattern in 11%, seizures in 10%, acute flaccid paralysis in 9%, and cardiorespiratory failure with pulmonary edema in 9%, all occurring within the first hours after hospital admission.
The median hospital length of stay for the full study population was 6 days. The survival rate was 99.5%, with the sole death being due to cardiorespiratory failure.
With 1-6 months of follow-up since the acute episode of EV-A71–associated brain stem encephalitis, the long-term sequelae included two cases of limb paresis and two cases of paresis of a cranial nerve, one child with residual seizures, and one with hypoxic-ischemic encephalopathy.
Asked why the fatality rate in the Spanish outbreak was so much lower than in the earlier Australasian outbreaks, Dr. Worner cited Catalan physicians’ quick recognition of what was underway – and, more importantly, a difference in the EV-A71 viral subgenotype. Most of the most severe cases in Asia and Australia involved the C-4 subgenotype, while in Spain, the predominant subgenotype involved in the outbreak was C-1.
As for the curious finding that EV was detectable in the cerebrospinal fluid of a mere 3% of the Spanish children, she said the explanation is unknown. The two main possibilities are that the CNS symptoms were due to a parenchymal brain infection rather than to EV-A71 infection of meningeal tissue. Alternatively, the CNS involvement may have been a manifestation of an immunologic response to the infection, rather than being due to the virus itself.
Dr. Worner reported having no financial conflicts of interest.
MADRID – Ninety-two percent of Spanish children sickened during the first-ever outbreak of enterovirus-associated brain stem encephalitis in western Europe survived with no long-term sequelae, Nuria Worner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We think that aggressive treatments should be restricted to those patients with important neurologic involvement,” declared Dr. Worner of Vall d’Hebron University Hospital in Barcelona. “We can say that no patients with milder involvement and without warning signs during the first 24 hours after onset of neurologic involvement went on to develop fulminant symptoms.”
Notable outbreaks of enterovirus A71 (EV-A71)-associated brain stem encephalitis occurred in Southeast Asia, Australia, and China in the late 1990s.
Dr. Worner reported on 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016. Their median age was 25 months, 57% were male, and a median of 2 days of symptoms of mild viral illness transpired before neurologic symptoms arose. Prior to presenting to a hospital, 21% of the children had been diagnosed with hand-foot-and-mouth disease, and 13% with herpangina.
Initial preadmission symptoms included fever in 94% of cases, sleepiness in 86%, ataxia in 75%, tremor in 47%, myoclonus in 40%, and a rash in 26%.
Fifty-five percent of the children had EV RNA isolated from both throat and feces, 26% from the throat only, and 19% only from their feces. Eighty-seven percent of serotyped EV were EV-A71.
Ninety percent of children underwent lumbar puncture. Particularly noteworthy was the finding that EV was detected in the cerebrospinal fluid of a mere 3% of patients, although pleocytosis was present in 84%.
Brain MRI showed brain stem encephalitis along with myelitis in 50% of patients, brain stem myelitis without encephalitis in 29%, myelitis elsewhere in 2%, and normal findings in 19%.
Ground zero for the outbreak was Barcelona and the surrounding region of Catalonia; indeed, 130 of the 196 (66%) affected children came from there. The Catalan health department and pediatric infectious disease specialists quickly created standardized case severity definitions and treatment recommendations; they distributed them nationally.
Mild EV-A71–associated brain stem disease was defined as two or more of the following: tremor, myoclonus, mild ataxia, and/or significant drowsiness. The recommendation in these mild cases was for no treatment other than supportive care and careful in-hospital monitoring.
Patients with moderate involvement had to meet the definition for mild disease plus more pronounced ataxia or bulbar motor neuron involvement marked by slurred speech, drooling, dysphagia, apnea, abolition of the gag reflex, and/or an abnormal respiratory pattern. Moderately affected patients received two doses of intravenous immunoglobulin (IVIG), each dosed at 1 g/kg per 24 hours. Admission to the pediatric ICU was individualized for patients with moderate EV-A71–associated brain stem encephalitis.
Severe disease was categorized as bulbar motor neuron involvement plus neurogenic cardiorespiratory failure. Those patients were uniformly admitted to a pediatric ICU and given the two doses of IVIG. The need for systemic steroids was determined on an individual basis.
Forty percent of patients received IVIG and systemic steroids, 24% received IVIG only, 2% systemic steroids only, and 34% received no treatment other than supportive care.
Twenty-six percent of children were admitted to a pediatric ICU for a median stay of 3.5 days. Nine percent of children were placed on mechanical ventilation.
As the disease evolved, the most frequent neurologic complications included slurred speech in 15% of children, abnormal breathing pattern in 11%, seizures in 10%, acute flaccid paralysis in 9%, and cardiorespiratory failure with pulmonary edema in 9%, all occurring within the first hours after hospital admission.
The median hospital length of stay for the full study population was 6 days. The survival rate was 99.5%, with the sole death being due to cardiorespiratory failure.
With 1-6 months of follow-up since the acute episode of EV-A71–associated brain stem encephalitis, the long-term sequelae included two cases of limb paresis and two cases of paresis of a cranial nerve, one child with residual seizures, and one with hypoxic-ischemic encephalopathy.
Asked why the fatality rate in the Spanish outbreak was so much lower than in the earlier Australasian outbreaks, Dr. Worner cited Catalan physicians’ quick recognition of what was underway – and, more importantly, a difference in the EV-A71 viral subgenotype. Most of the most severe cases in Asia and Australia involved the C-4 subgenotype, while in Spain, the predominant subgenotype involved in the outbreak was C-1.
As for the curious finding that EV was detectable in the cerebrospinal fluid of a mere 3% of the Spanish children, she said the explanation is unknown. The two main possibilities are that the CNS symptoms were due to a parenchymal brain infection rather than to EV-A71 infection of meningeal tissue. Alternatively, the CNS involvement may have been a manifestation of an immunologic response to the infection, rather than being due to the virus itself.
Dr. Worner reported having no financial conflicts of interest.
AT ESPID 2017
Key clinical point:
Major finding: Ninety-two percent of Spanish children involved in an outbreak of enterovirus-associated brain stem encephalitis survived with no long-term sequelae.
Data source: A retrospective review of 196 children treated for laboratory-confirmed EV-A71–associated brain stem encephalitis at 16 Spanish hospitals in April-December 2016 during the first-ever outbreak of this condition in western Europe.
Disclosures: Dr. Worner reported having no financial conflicts of interest.
Meningitis before age 3 months: Consider enterovirus and parechovirus
MADRID – Ninety-five percent of cases of enterovirus and parechovirus meningitis in infants younger than 90 days old in the United Kingdom and Ireland were diagnosed through lumbar puncture and identification of the virus in the CSF by PCR, Seilesh Kadambari, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We recommend that routine testing of CSF for enterovirus and parechovirus in febrile infants should be promoted, even in the absence of pleocytosis,” said Dr. Kadambari of John Radcliffe Hospital in Oxford, England.
This active enhanced prospective surveillance study was undertaken because an earlier retrospective study concluded that the rate of viral meningitis across all pediatric and adult age groups had increased rapidly during a recent 10-year period in the United Kingdom and Ireland. Infants younger than 3 months of age were especially hard hit by enterovirus, which accounted in the earlier study for 92% of all viral meningitis cases in that age group. Dr. Kadambari and his coinvestigators decided to take a closer prospective look at the sub-3-month age group because it’s such an important time neurodevelopmentally.
During the 13-month period of June 2014 to June 2015, 710 patients younger than 90 days old were hospitalized for enterovirus or parechovirus meningitis across the United Kingdom and Republic of Ireland. Ninety-five percent were due to enterovirus. Only 6% of affected infants were born prematurely.
“One of the take-home messages for me from the study was that 12% of enterovirus cases and 23% of parechovirus meningitis cases required admission to an ICU setting,” the pediatrician observed.
Among the infants admitted to a pediatric ICU, half of those with enterovirus meningitis and all those with parechovirus meningitis required intubation and mechanical ventilation. One-fifth of the enterovirus meningitis patients in pediatric ICUs required inotropic support for cardiovascular stabilization, as did all young infants with parechovirus meningitis.
Among the 710 patients, the three most common clinical presenting features were fever, irritability, and reduced feeding, present in 85%, 66%, and 54%, respectively.
Upon physical examination, two noteworthy common findings were signs of shock, present in 27% of infants with enterovirus meningitis and 43% with parechovirus meningitis, and respiratory distress, seen in 12% and 26%, respectively.
None of the 710 infants had a secondary bacterial infection. “That has implications for antimicrobial stewardship programs,” according to Dr. Kadambari.
The majority of infants had a normal CSF WBC count and a normal-range C-reactive protein level.
“Raised inflammatory markers were not a common feature in our cohort,” he noted.
Two infants died: one of a massive pulmonary hemorrhage and the other as a result of septic shock. Of the remaining 708 patients, however, 699 (98.7%) were discharged without significant neurologic impairment. Seven infants with enterovirus meningitis and two with parechovirus meningitis were discharged with severe delay, including cases of fine motor and gross motor delay, visual abnormalities, and a single case of severe cardiac dysfunction requiring discharge on an ACE inhibitor.
This surveillance study was a collaboration between St. George’s University of London and Public Health England. Dr. Kadambari reported having no financial conflicts.
Future studies need to examine long-term neurodevelopmental outcomes in affected young infants out to age 24 months, he said.
MADRID – Ninety-five percent of cases of enterovirus and parechovirus meningitis in infants younger than 90 days old in the United Kingdom and Ireland were diagnosed through lumbar puncture and identification of the virus in the CSF by PCR, Seilesh Kadambari, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We recommend that routine testing of CSF for enterovirus and parechovirus in febrile infants should be promoted, even in the absence of pleocytosis,” said Dr. Kadambari of John Radcliffe Hospital in Oxford, England.
This active enhanced prospective surveillance study was undertaken because an earlier retrospective study concluded that the rate of viral meningitis across all pediatric and adult age groups had increased rapidly during a recent 10-year period in the United Kingdom and Ireland. Infants younger than 3 months of age were especially hard hit by enterovirus, which accounted in the earlier study for 92% of all viral meningitis cases in that age group. Dr. Kadambari and his coinvestigators decided to take a closer prospective look at the sub-3-month age group because it’s such an important time neurodevelopmentally.
During the 13-month period of June 2014 to June 2015, 710 patients younger than 90 days old were hospitalized for enterovirus or parechovirus meningitis across the United Kingdom and Republic of Ireland. Ninety-five percent were due to enterovirus. Only 6% of affected infants were born prematurely.
“One of the take-home messages for me from the study was that 12% of enterovirus cases and 23% of parechovirus meningitis cases required admission to an ICU setting,” the pediatrician observed.
Among the infants admitted to a pediatric ICU, half of those with enterovirus meningitis and all those with parechovirus meningitis required intubation and mechanical ventilation. One-fifth of the enterovirus meningitis patients in pediatric ICUs required inotropic support for cardiovascular stabilization, as did all young infants with parechovirus meningitis.
Among the 710 patients, the three most common clinical presenting features were fever, irritability, and reduced feeding, present in 85%, 66%, and 54%, respectively.
Upon physical examination, two noteworthy common findings were signs of shock, present in 27% of infants with enterovirus meningitis and 43% with parechovirus meningitis, and respiratory distress, seen in 12% and 26%, respectively.
None of the 710 infants had a secondary bacterial infection. “That has implications for antimicrobial stewardship programs,” according to Dr. Kadambari.
The majority of infants had a normal CSF WBC count and a normal-range C-reactive protein level.
“Raised inflammatory markers were not a common feature in our cohort,” he noted.
Two infants died: one of a massive pulmonary hemorrhage and the other as a result of septic shock. Of the remaining 708 patients, however, 699 (98.7%) were discharged without significant neurologic impairment. Seven infants with enterovirus meningitis and two with parechovirus meningitis were discharged with severe delay, including cases of fine motor and gross motor delay, visual abnormalities, and a single case of severe cardiac dysfunction requiring discharge on an ACE inhibitor.
This surveillance study was a collaboration between St. George’s University of London and Public Health England. Dr. Kadambari reported having no financial conflicts.
Future studies need to examine long-term neurodevelopmental outcomes in affected young infants out to age 24 months, he said.
MADRID – Ninety-five percent of cases of enterovirus and parechovirus meningitis in infants younger than 90 days old in the United Kingdom and Ireland were diagnosed through lumbar puncture and identification of the virus in the CSF by PCR, Seilesh Kadambari, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We recommend that routine testing of CSF for enterovirus and parechovirus in febrile infants should be promoted, even in the absence of pleocytosis,” said Dr. Kadambari of John Radcliffe Hospital in Oxford, England.
This active enhanced prospective surveillance study was undertaken because an earlier retrospective study concluded that the rate of viral meningitis across all pediatric and adult age groups had increased rapidly during a recent 10-year period in the United Kingdom and Ireland. Infants younger than 3 months of age were especially hard hit by enterovirus, which accounted in the earlier study for 92% of all viral meningitis cases in that age group. Dr. Kadambari and his coinvestigators decided to take a closer prospective look at the sub-3-month age group because it’s such an important time neurodevelopmentally.
During the 13-month period of June 2014 to June 2015, 710 patients younger than 90 days old were hospitalized for enterovirus or parechovirus meningitis across the United Kingdom and Republic of Ireland. Ninety-five percent were due to enterovirus. Only 6% of affected infants were born prematurely.
“One of the take-home messages for me from the study was that 12% of enterovirus cases and 23% of parechovirus meningitis cases required admission to an ICU setting,” the pediatrician observed.
Among the infants admitted to a pediatric ICU, half of those with enterovirus meningitis and all those with parechovirus meningitis required intubation and mechanical ventilation. One-fifth of the enterovirus meningitis patients in pediatric ICUs required inotropic support for cardiovascular stabilization, as did all young infants with parechovirus meningitis.
Among the 710 patients, the three most common clinical presenting features were fever, irritability, and reduced feeding, present in 85%, 66%, and 54%, respectively.
Upon physical examination, two noteworthy common findings were signs of shock, present in 27% of infants with enterovirus meningitis and 43% with parechovirus meningitis, and respiratory distress, seen in 12% and 26%, respectively.
None of the 710 infants had a secondary bacterial infection. “That has implications for antimicrobial stewardship programs,” according to Dr. Kadambari.
The majority of infants had a normal CSF WBC count and a normal-range C-reactive protein level.
“Raised inflammatory markers were not a common feature in our cohort,” he noted.
Two infants died: one of a massive pulmonary hemorrhage and the other as a result of septic shock. Of the remaining 708 patients, however, 699 (98.7%) were discharged without significant neurologic impairment. Seven infants with enterovirus meningitis and two with parechovirus meningitis were discharged with severe delay, including cases of fine motor and gross motor delay, visual abnormalities, and a single case of severe cardiac dysfunction requiring discharge on an ACE inhibitor.
This surveillance study was a collaboration between St. George’s University of London and Public Health England. Dr. Kadambari reported having no financial conflicts.
Future studies need to examine long-term neurodevelopmental outcomes in affected young infants out to age 24 months, he said.
AT ESPID 2017
Key clinical point:
Major finding: More than 98% of infants in the United Kingdom and Ireland younger than 90 days old with enterovirus or parechovirus meningitis were discharged without significant neurologic impairment.
Data source: A prospective active enhanced surveillance study including all 710 infants younger than 90 days old hospitalized for enterovirus or parechovirus meningitis in the United Kingdom and Ireland in a 13-month period.
Disclosures: The study was jointly sponsored by Public Health England and St. George’s University of London. The presenter reported having no financial conflicts.
Start ART in first 3 months in infants with perinatal HIV
MADRID – Infants with perinatal HIV infection are significantly more likely to achieve viral suppression by age 12 months if they start antiretroviral therapy (ART) before age 3 months than if physicians wait until 3-6 months of age, Paolo Palma, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a study of the factors associated with time to virologic sup
The purpose of this study was to identify the key factors involved in attaining early virologic control of perinatally acquired HIV. This information is necessary to lay the groundwork for planned future investigations of immunotherapeutic strategies designed to achieve sustained ART-free remission. Such strategies are most likely to be successful in very young children who have not yet built up a massive viral load, explained Dr. Palma of Bambino Gesù Children’s Hospital in Rome.
“A major obstacle to curing HIV infection is persistence of virus as integrated proviral DNA in long-lived cells even after many years on ART. ART-free HIV remission is more likely to occur if viral suppression is achieved very early in infection,” he said.
The median age of the 420 subjects at the time ART was initiated was 2.9 months. Their CD4 cell percentage was 34%, with a median CD4 cell count of 1,780 and an average viral load at baseline of 316,228 copies/mL.
At 12 months of age, 84% of patients had achieved viral suppression. In multivariate analyses adjusted for initial ART regimen and geographic location, three factors were associated with this outcome: younger age at ART onset, a lower baseline viral load, and a higher per
Indeed, for each 1-month increase in age at onset of ART, the likelihood of virologic response at age 12 months decreased by 16%. Similarly, the rate of virologic response at 1 year of age decreased by 15% for each 10 copies/mL increase in viral load at the start of ART. In contrast, the likelihood of virologic suppression at age 12 months increased by 10% for each 10% increase in CD4 cell percentage at the start of treatment.
Among the variables that proved unrelated to virologic suppression at 1 year of age were gender, AIDS status, feeding style (breastfed versus bottle-fed), and ethnicity.
Dr. Palma reported having no relevant financial disclosures.
MADRID – Infants with perinatal HIV infection are significantly more likely to achieve viral suppression by age 12 months if they start antiretroviral therapy (ART) before age 3 months than if physicians wait until 3-6 months of age, Paolo Palma, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a study of the factors associated with time to virologic sup
The purpose of this study was to identify the key factors involved in attaining early virologic control of perinatally acquired HIV. This information is necessary to lay the groundwork for planned future investigations of immunotherapeutic strategies designed to achieve sustained ART-free remission. Such strategies are most likely to be successful in very young children who have not yet built up a massive viral load, explained Dr. Palma of Bambino Gesù Children’s Hospital in Rome.
“A major obstacle to curing HIV infection is persistence of virus as integrated proviral DNA in long-lived cells even after many years on ART. ART-free HIV remission is more likely to occur if viral suppression is achieved very early in infection,” he said.
The median age of the 420 subjects at the time ART was initiated was 2.9 months. Their CD4 cell percentage was 34%, with a median CD4 cell count of 1,780 and an average viral load at baseline of 316,228 copies/mL.
At 12 months of age, 84% of patients had achieved viral suppression. In multivariate analyses adjusted for initial ART regimen and geographic location, three factors were associated with this outcome: younger age at ART onset, a lower baseline viral load, and a higher per
Indeed, for each 1-month increase in age at onset of ART, the likelihood of virologic response at age 12 months decreased by 16%. Similarly, the rate of virologic response at 1 year of age decreased by 15% for each 10 copies/mL increase in viral load at the start of ART. In contrast, the likelihood of virologic suppression at age 12 months increased by 10% for each 10% increase in CD4 cell percentage at the start of treatment.
Among the variables that proved unrelated to virologic suppression at 1 year of age were gender, AIDS status, feeding style (breastfed versus bottle-fed), and ethnicity.
Dr. Palma reported having no relevant financial disclosures.
MADRID – Infants with perinatal HIV infection are significantly more likely to achieve viral suppression by age 12 months if they start antiretroviral therapy (ART) before age 3 months than if physicians wait until 3-6 months of age, Paolo Palma, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a study of the factors associated with time to virologic sup
The purpose of this study was to identify the key factors involved in attaining early virologic control of perinatally acquired HIV. This information is necessary to lay the groundwork for planned future investigations of immunotherapeutic strategies designed to achieve sustained ART-free remission. Such strategies are most likely to be successful in very young children who have not yet built up a massive viral load, explained Dr. Palma of Bambino Gesù Children’s Hospital in Rome.
“A major obstacle to curing HIV infection is persistence of virus as integrated proviral DNA in long-lived cells even after many years on ART. ART-free HIV remission is more likely to occur if viral suppression is achieved very early in infection,” he said.
The median age of the 420 subjects at the time ART was initiated was 2.9 months. Their CD4 cell percentage was 34%, with a median CD4 cell count of 1,780 and an average viral load at baseline of 316,228 copies/mL.
At 12 months of age, 84% of patients had achieved viral suppression. In multivariate analyses adjusted for initial ART regimen and geographic location, three factors were associated with this outcome: younger age at ART onset, a lower baseline viral load, and a higher per
Indeed, for each 1-month increase in age at onset of ART, the likelihood of virologic response at age 12 months decreased by 16%. Similarly, the rate of virologic response at 1 year of age decreased by 15% for each 10 copies/mL increase in viral load at the start of ART. In contrast, the likelihood of virologic suppression at age 12 months increased by 10% for each 10% increase in CD4 cell percentage at the start of treatment.
Among the variables that proved unrelated to virologic suppression at 1 year of age were gender, AIDS status, feeding style (breastfed versus bottle-fed), and ethnicity.
Dr. Palma reported having no relevant financial disclosures.
AT ESPID 2017
Key clinical point:
Major finding: With each 1-month delay in starting antiretroviral therapy, the likelihood of attaining viral suppression by age 12 months drops by 16%.
Data source: This observational study included 420 infants with perinatally acquired HIV infection who began antiretroviral therapy prior to age 6 months.
Disclosures: Dr. Palma reported having no relevant financial disclosures.
English infant quadravalent group B meningococcal vaccine pays off
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
MADRID – Cases of invasive meningococcal disease in English 1 year olds dropped fourfold in the first year after the multicomponent group B meningococcal (MenB) vaccine (4CMenB, Bexsero) was added to the national infant immunization list, Shamez Ladhani, MD, Ph.D, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In 1 year olds, we have seen a significant decrease in the number of cases, compared with the number of cases predicted. I suspect the vaccine effectiveness is close to 94% against meningococcal group B,” said Dr. Ladhani of Public Health England, London.
Meningococcal meningitis and septicemia remain the leading cause of death for children under 5 in the UK. Public awareness of this threat is high, so 4CMenB has been well accepted. Of eligible infants, 96% have received the first dose and 89% the second. The first cohort became available for the 12-month booster dose in May 2016.
Between May and December of 2016, the Public Health England intensive surveillance program confirmed six cases of invasive meningococcal disease. During the same time frame in 2015, there were 24 cases, whereas, during the 4 years prior to introduction of 4CMenB, the average was 18 cases per year.
Three of the six 1-year-olds had meningitis, and three had septicemia. One was admitted to an ICU. None died. None had comorbid conditions placing them at increased risk. Five of the six children with invasive meningococcal disease had received two doses of the vaccine, and one child became ill 3 months after receiving the third dose.
The vaccine is licensed to be given as a four-dose series: three primary doses plus one booster dose. UK health officials deemed that excessive and not cost effective. Based on data from the vaccine trials, they determined that three doses are sufficient. This cut the cost of the national program by 25% while maintaining protection (Lancet. 2016 Dec 3;388(10061):2775-82).
Pediatricians from other countries were extremely curious about this innovative immunization program. What about vaccine side effects in infants? they asked.
Dr. Ladhani replied that studies in Australia, Northern Ireland, and Scotland have all shown a small uptick in mild fever and irritability in the first 3 days after the first dose of 4CMenB. It’s less of an issue with the second dose.
“One dose is not protective. You need two. Parents understand that, while these side effects are annoying, the risks associated with meningitis are far greater. They are very, very accepting of the vaccine,” according to Dr. Ladhani.
What about using 4CMenB in teenagers? physicians asked.
Dr. Ladhani predicted that the infant immunization program will have zero effect in adolescents. Teens are the main carriers of meningococcal bacteria. So, in theory, vaccinating them could not only protect the adolescents themselves but could benefit the whole population through herd immunity.
“The problem is we don’t have solid evidence that Bexsero protects against carriage. A massive carriage study is underway in Australia. It should provide data in 2 or 3 years. If we do see a carriage effect, then vaccinating teenagers becomes a very attractive option because you protect them and others around them,” he explained.
Dr. Ladhani reported having no financial conflicts regarding his study, funded by Public Health England.
AT ESPID 2017
Key clinical point:
Major finding: The number of confirmed cases of invasive meningococcal disease in English 1-year-olds dropped to six the year after introduction of the 4CMenB vaccine in the infant immunization schedule, down from 24 cases the previous year.
Data source: An enhanced surveillance study utilizing a combination of laboratory, public health, and clinical reporting to track and confirm all cases of invasive meningococcal disease in English 1-year-olds.
Disclosures: The study was funded by Public Health England. The presenter, who is employed by the agency, reported having no financial conflicts.