User login
Adverse childhood experiences link to worse SLE
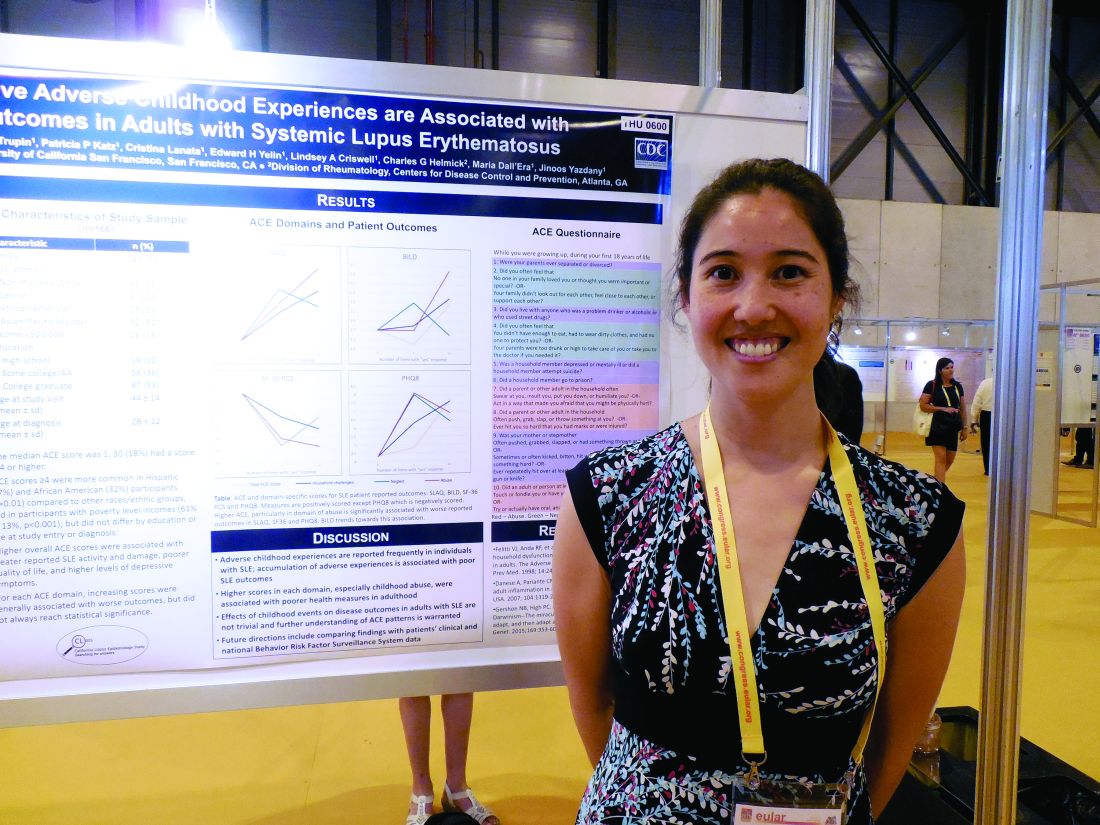
MADRID – Adult patients with systemic lupus erythematosus (SLE) who had several adverse experiences as children generally had a worse disease state than did similar adult lupus patients with no adverse childhood experiences in a study of 166 California lupus patients.
Higher numbers of self-reported episodes of household challenges, neglect, and especially childhood abuse were associated with worse SLE outcomes, Kimberly DeQuattro, MD, said while presenting a poster at the European Congress of Rheumatology.
It remains unclear “why there is an association between childhood trauma and outcomes of chronic disease, especially autoimmune disease,” she said in an interview. “We want to know, What does it mean [clinically] to have been abused or neglected, and is it irreversible?” She called for further understanding of childhood adverse experiences and their consequences. These patients might benefit from referrals to psychologists or social workers, Dr. DeQuattro suggested.
The data for her analysis came from 166 adult SLE patients who participated in the California Lupus Epidemiology Study and completed several disease activity surveys, as well as the Adverse Childhood Experience survey, which measures episodes of abuse, neglect, and household challenges. The patients averaged 44 years old and had been diagnosed with SLE for an average of about 16 years. More than 60% of the 166 participants reported at least one episode on this survey. “One event does not make a big difference,” Dr. DeQuattro said, but that wasn’t the case for the SLE patients who reported having four of more of these childhood events.
Patients with a higher number of adverse childhood experiences had worse disease states, as measured by the Systemic Lupus Activity Questionnaire; the Brief Index of Lupus Damage; the 36-Item Short Form Health Survey, a measure of quality of life; and the Patient Health Questionnaire 8, a measure of depression, the researchers said in their report. For example, the average score on the Systemic Lupus Activity Questionnaire was 6.0 in 66 patients with no adverse childhood experiences and 11.8 in the 30 patients who reported having four or more adverse childhood experiences.
Dr. DeQuattro had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Adult patients with systemic lupus erythematosus (SLE) who had several adverse experiences as children generally had a worse disease state than did similar adult lupus patients with no adverse childhood experiences in a study of 166 California lupus patients.
Higher numbers of self-reported episodes of household challenges, neglect, and especially childhood abuse were associated with worse SLE outcomes, Kimberly DeQuattro, MD, said while presenting a poster at the European Congress of Rheumatology.
It remains unclear “why there is an association between childhood trauma and outcomes of chronic disease, especially autoimmune disease,” she said in an interview. “We want to know, What does it mean [clinically] to have been abused or neglected, and is it irreversible?” She called for further understanding of childhood adverse experiences and their consequences. These patients might benefit from referrals to psychologists or social workers, Dr. DeQuattro suggested.
The data for her analysis came from 166 adult SLE patients who participated in the California Lupus Epidemiology Study and completed several disease activity surveys, as well as the Adverse Childhood Experience survey, which measures episodes of abuse, neglect, and household challenges. The patients averaged 44 years old and had been diagnosed with SLE for an average of about 16 years. More than 60% of the 166 participants reported at least one episode on this survey. “One event does not make a big difference,” Dr. DeQuattro said, but that wasn’t the case for the SLE patients who reported having four of more of these childhood events.
Patients with a higher number of adverse childhood experiences had worse disease states, as measured by the Systemic Lupus Activity Questionnaire; the Brief Index of Lupus Damage; the 36-Item Short Form Health Survey, a measure of quality of life; and the Patient Health Questionnaire 8, a measure of depression, the researchers said in their report. For example, the average score on the Systemic Lupus Activity Questionnaire was 6.0 in 66 patients with no adverse childhood experiences and 11.8 in the 30 patients who reported having four or more adverse childhood experiences.
Dr. DeQuattro had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Adult patients with systemic lupus erythematosus (SLE) who had several adverse experiences as children generally had a worse disease state than did similar adult lupus patients with no adverse childhood experiences in a study of 166 California lupus patients.
Higher numbers of self-reported episodes of household challenges, neglect, and especially childhood abuse were associated with worse SLE outcomes, Kimberly DeQuattro, MD, said while presenting a poster at the European Congress of Rheumatology.
It remains unclear “why there is an association between childhood trauma and outcomes of chronic disease, especially autoimmune disease,” she said in an interview. “We want to know, What does it mean [clinically] to have been abused or neglected, and is it irreversible?” She called for further understanding of childhood adverse experiences and their consequences. These patients might benefit from referrals to psychologists or social workers, Dr. DeQuattro suggested.
The data for her analysis came from 166 adult SLE patients who participated in the California Lupus Epidemiology Study and completed several disease activity surveys, as well as the Adverse Childhood Experience survey, which measures episodes of abuse, neglect, and household challenges. The patients averaged 44 years old and had been diagnosed with SLE for an average of about 16 years. More than 60% of the 166 participants reported at least one episode on this survey. “One event does not make a big difference,” Dr. DeQuattro said, but that wasn’t the case for the SLE patients who reported having four of more of these childhood events.
Patients with a higher number of adverse childhood experiences had worse disease states, as measured by the Systemic Lupus Activity Questionnaire; the Brief Index of Lupus Damage; the 36-Item Short Form Health Survey, a measure of quality of life; and the Patient Health Questionnaire 8, a measure of depression, the researchers said in their report. For example, the average score on the Systemic Lupus Activity Questionnaire was 6.0 in 66 patients with no adverse childhood experiences and 11.8 in the 30 patients who reported having four or more adverse childhood experiences.
Dr. DeQuattro had no relevant financial disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: The Systemic Lupus Activity Questionnaire score averaged 6.0 with no adverse childhood experiences and 11.8 with four or more experiences.
Data source: Survey results from 166 adult lupus patients enrolled in the California Lupus Epidemiology Study.
Disclosures: Dr. DeQuattro had no relevant financial disclosures.
Three-drug combo keeps early RA at bay long term
MADRID – A triple combination of methotrexate, hydroxychloroquine, and triamcinolone used to induce remission in patients with early rheumatoid arthritis (RA) was associated with higher long-term remission rates than methotrexate used alone in a study presented at the European Congress of Rheumatology.
The percentages of patients in remission were a respective 88.2% versus 72.1% at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
“Combination treatment results in a higher remission rate in the first 2 years of treatment and a similar remission rate in the third year,” said Tammo Brunekreef, a medical student at Ziekenhuisgroep Twente in Almelo, the Netherlands, who presented the findings.
“There is no consensus on what the initial treatment should look like, however,” he said. For instance, should remission be induced with methotrexate alone? Or should methotrexate be used in combination with other synthetic disease-modifying antirheumatic drugs (DMARDs)? Or is methotrexate best combined with steroids?
“The early treatment [of RA] is very important because a shorter time to remission is related to sustainability of remission,” Mr. Brunekreef said. “Combination therapy has also been compared in previous studies with methotrexate monotherapy and had been shown to be more effective at 3 and 6 months, although not at 12 months.”
Data on the longer-term follow-up in routine care is lacking, so Mr. Brunekreef and Dutch rheumatologist Hein J. Bernelot Moens, MD, created two historical cohorts of patients with early RA. One cohort of 296 patients had a disease onset between 2006 and 2011 and had received methotrexate monotherapy initiated at 15-20 mg/week. The other cohort of 157 patients had a disease onset between 2012 and 2014 and had been given a combination of oral methotrexate, started at 20 mg/week; oral hydroxychloroquine, started at 200 mg twice daily; and a single 80-120 mg intramuscular injection of triamcinolone that could be repeated after 4 weeks, if necessary.
The mean age of the recruited patients in the monotherapy and combination cohorts was a respective 59.5 and 58.9 years, and 60.5% and 65% were female. More patients in the combination than monotherapy group were rheumatoid factor positive (72.3% vs. 62.2%), with 72.1% and 64.5% being positive for anticitrullinated protein antibodies.
“A number of patients were lost to follow-up due to death (3.7% vs. 1.9%) [or] drug-free remission (1.7% vs. 0.6%) or did not start methotrexate or for other reasons (2.4% vs. 0.6%), but this was not significantly different,” Mr. Brunekreef reported. This left 273 and 124 in each cohort, respectively, who completed 3 years’ of follow-up.
The same percentage of patients in the methotrexate and combination cohorts started a biologic DMARD in the first year (10.8%). A biologic DMARD was recommended if remission was not achieved within 6 months or if there was sustained disease activity after 6 months.
In the second year, however, 6.6% more patients in the methotrexate cohort started a biologic (9.8% vs. 3.2%). Conversely, 1.3% more patients started a biologic in the third year in the combination arm (4% vs. 2.7%). Overall, the receipt of biologics by 21% of monotherapy patients and 14% of combination therapy patients did not differ significantly.
The mean time to the start of the biologic DMARD was similar, however, at around 11-12 months.
Mr. Brunekreef answered that the DAS28-ESR had been used up to 2015 and then the DAS-CRP from 2016 onward, although the latter was only for patients in year 3, a small number of patients. “We’re looking into a way to translate those data to make them comparable,” Brunekreef said.
“That’s a real problem,” Dr. Fleischmann said, as the DAS28-ESR and DAS28-CRP are not interchangeable. He proposed that these data needed to be looked at using another measure, perhaps the Clinical Disease Activity Index.
Further, Dr. Fleischmann observed that the methotrexate monotherapy data were “absolutely incredible, compared to what we’ve seen in randomized, controlled trials, with 40%-50% of patients in remission.” In clinical trials, about 15% on methotrexate alone achieve an American College of Rheumatology 20 Response Criteria.
“These are very, very strong data, but now I wonder whether or not it’s because of that switch” in DAS28 scoring, Dr. Fleischmann said.
Mr. Brunekreef had no disclosures to report. Dr. Fleischmann has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
MADRID – A triple combination of methotrexate, hydroxychloroquine, and triamcinolone used to induce remission in patients with early rheumatoid arthritis (RA) was associated with higher long-term remission rates than methotrexate used alone in a study presented at the European Congress of Rheumatology.
The percentages of patients in remission were a respective 88.2% versus 72.1% at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
“Combination treatment results in a higher remission rate in the first 2 years of treatment and a similar remission rate in the third year,” said Tammo Brunekreef, a medical student at Ziekenhuisgroep Twente in Almelo, the Netherlands, who presented the findings.
“There is no consensus on what the initial treatment should look like, however,” he said. For instance, should remission be induced with methotrexate alone? Or should methotrexate be used in combination with other synthetic disease-modifying antirheumatic drugs (DMARDs)? Or is methotrexate best combined with steroids?
“The early treatment [of RA] is very important because a shorter time to remission is related to sustainability of remission,” Mr. Brunekreef said. “Combination therapy has also been compared in previous studies with methotrexate monotherapy and had been shown to be more effective at 3 and 6 months, although not at 12 months.”
Data on the longer-term follow-up in routine care is lacking, so Mr. Brunekreef and Dutch rheumatologist Hein J. Bernelot Moens, MD, created two historical cohorts of patients with early RA. One cohort of 296 patients had a disease onset between 2006 and 2011 and had received methotrexate monotherapy initiated at 15-20 mg/week. The other cohort of 157 patients had a disease onset between 2012 and 2014 and had been given a combination of oral methotrexate, started at 20 mg/week; oral hydroxychloroquine, started at 200 mg twice daily; and a single 80-120 mg intramuscular injection of triamcinolone that could be repeated after 4 weeks, if necessary.
The mean age of the recruited patients in the monotherapy and combination cohorts was a respective 59.5 and 58.9 years, and 60.5% and 65% were female. More patients in the combination than monotherapy group were rheumatoid factor positive (72.3% vs. 62.2%), with 72.1% and 64.5% being positive for anticitrullinated protein antibodies.
“A number of patients were lost to follow-up due to death (3.7% vs. 1.9%) [or] drug-free remission (1.7% vs. 0.6%) or did not start methotrexate or for other reasons (2.4% vs. 0.6%), but this was not significantly different,” Mr. Brunekreef reported. This left 273 and 124 in each cohort, respectively, who completed 3 years’ of follow-up.
The same percentage of patients in the methotrexate and combination cohorts started a biologic DMARD in the first year (10.8%). A biologic DMARD was recommended if remission was not achieved within 6 months or if there was sustained disease activity after 6 months.
In the second year, however, 6.6% more patients in the methotrexate cohort started a biologic (9.8% vs. 3.2%). Conversely, 1.3% more patients started a biologic in the third year in the combination arm (4% vs. 2.7%). Overall, the receipt of biologics by 21% of monotherapy patients and 14% of combination therapy patients did not differ significantly.
The mean time to the start of the biologic DMARD was similar, however, at around 11-12 months.
Mr. Brunekreef answered that the DAS28-ESR had been used up to 2015 and then the DAS-CRP from 2016 onward, although the latter was only for patients in year 3, a small number of patients. “We’re looking into a way to translate those data to make them comparable,” Brunekreef said.
“That’s a real problem,” Dr. Fleischmann said, as the DAS28-ESR and DAS28-CRP are not interchangeable. He proposed that these data needed to be looked at using another measure, perhaps the Clinical Disease Activity Index.
Further, Dr. Fleischmann observed that the methotrexate monotherapy data were “absolutely incredible, compared to what we’ve seen in randomized, controlled trials, with 40%-50% of patients in remission.” In clinical trials, about 15% on methotrexate alone achieve an American College of Rheumatology 20 Response Criteria.
“These are very, very strong data, but now I wonder whether or not it’s because of that switch” in DAS28 scoring, Dr. Fleischmann said.
Mr. Brunekreef had no disclosures to report. Dr. Fleischmann has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
MADRID – A triple combination of methotrexate, hydroxychloroquine, and triamcinolone used to induce remission in patients with early rheumatoid arthritis (RA) was associated with higher long-term remission rates than methotrexate used alone in a study presented at the European Congress of Rheumatology.
The percentages of patients in remission were a respective 88.2% versus 72.1% at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
“Combination treatment results in a higher remission rate in the first 2 years of treatment and a similar remission rate in the third year,” said Tammo Brunekreef, a medical student at Ziekenhuisgroep Twente in Almelo, the Netherlands, who presented the findings.
“There is no consensus on what the initial treatment should look like, however,” he said. For instance, should remission be induced with methotrexate alone? Or should methotrexate be used in combination with other synthetic disease-modifying antirheumatic drugs (DMARDs)? Or is methotrexate best combined with steroids?
“The early treatment [of RA] is very important because a shorter time to remission is related to sustainability of remission,” Mr. Brunekreef said. “Combination therapy has also been compared in previous studies with methotrexate monotherapy and had been shown to be more effective at 3 and 6 months, although not at 12 months.”
Data on the longer-term follow-up in routine care is lacking, so Mr. Brunekreef and Dutch rheumatologist Hein J. Bernelot Moens, MD, created two historical cohorts of patients with early RA. One cohort of 296 patients had a disease onset between 2006 and 2011 and had received methotrexate monotherapy initiated at 15-20 mg/week. The other cohort of 157 patients had a disease onset between 2012 and 2014 and had been given a combination of oral methotrexate, started at 20 mg/week; oral hydroxychloroquine, started at 200 mg twice daily; and a single 80-120 mg intramuscular injection of triamcinolone that could be repeated after 4 weeks, if necessary.
The mean age of the recruited patients in the monotherapy and combination cohorts was a respective 59.5 and 58.9 years, and 60.5% and 65% were female. More patients in the combination than monotherapy group were rheumatoid factor positive (72.3% vs. 62.2%), with 72.1% and 64.5% being positive for anticitrullinated protein antibodies.
“A number of patients were lost to follow-up due to death (3.7% vs. 1.9%) [or] drug-free remission (1.7% vs. 0.6%) or did not start methotrexate or for other reasons (2.4% vs. 0.6%), but this was not significantly different,” Mr. Brunekreef reported. This left 273 and 124 in each cohort, respectively, who completed 3 years’ of follow-up.
The same percentage of patients in the methotrexate and combination cohorts started a biologic DMARD in the first year (10.8%). A biologic DMARD was recommended if remission was not achieved within 6 months or if there was sustained disease activity after 6 months.
In the second year, however, 6.6% more patients in the methotrexate cohort started a biologic (9.8% vs. 3.2%). Conversely, 1.3% more patients started a biologic in the third year in the combination arm (4% vs. 2.7%). Overall, the receipt of biologics by 21% of monotherapy patients and 14% of combination therapy patients did not differ significantly.
The mean time to the start of the biologic DMARD was similar, however, at around 11-12 months.
Mr. Brunekreef answered that the DAS28-ESR had been used up to 2015 and then the DAS-CRP from 2016 onward, although the latter was only for patients in year 3, a small number of patients. “We’re looking into a way to translate those data to make them comparable,” Brunekreef said.
“That’s a real problem,” Dr. Fleischmann said, as the DAS28-ESR and DAS28-CRP are not interchangeable. He proposed that these data needed to be looked at using another measure, perhaps the Clinical Disease Activity Index.
Further, Dr. Fleischmann observed that the methotrexate monotherapy data were “absolutely incredible, compared to what we’ve seen in randomized, controlled trials, with 40%-50% of patients in remission.” In clinical trials, about 15% on methotrexate alone achieve an American College of Rheumatology 20 Response Criteria.
“These are very, very strong data, but now I wonder whether or not it’s because of that switch” in DAS28 scoring, Dr. Fleischmann said.
Mr. Brunekreef had no disclosures to report. Dr. Fleischmann has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: A DAS28 less than 2.6 was achieved in 88.2% who received a methotrexate-based triple combination versus 72.1% with methotrexate monotherapy at 1 year, 86.6% versus 81.9% at 2 years, and 88.0% versus 85.8% at 3 years.
Data source: Two historical cohorts of early arthritis patients treated with methotrexate alone (n = 296) or a combination of methotrexate, hydroxychloroquine, and triamcinolone (n = 157) in routine care.
Disclosures: The study presenter had no disclosures to report. An independent commentator has received research grants, research support, and consultancy fees from Pfizer and from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Sanofi Genzyme, and UCB.
Sustained remission on biologics bodes well for children with JIA
MADRID – Many children with juvenile idiopathic arthritis (JIA) who do well on biologics for a prolonged period will stay in remission after the medication is withdrawn, some new real-world data suggest.
A database review found that 70% of those in remission for at least 1.5 years remained in remission after stopping their biologic agent. Patients taking tocilizumab had the best outcomes, with 8 months of sustained, drug-free remission and only a 12% rate of disease flare, Ekaterina Alexeeva, MD, reported at the European Congress of Rheumatology.
“Prolonged therapy with biologic agents may cause adverse events which lead to the necessity of discontinuation of therapy in patients once complete disease quiescence has been achieved,” she said. While long-term drug studies do offer some glimpse into the stability of remission after drug discontinuation, these data don’t often reflect real-world experience.
“Clinical trials are made up of highly selected participants in contorted conditions with limited duration. Real-word data, collected under real-life practical circumstances, provide additional characteristics of patient populations, information on the effectiveness and safety of treatment over time, and the outcomes we can achieve under real-world conditions,” Dr. Alexeeva said.
She plumbed a national JIA database to find 83 patients who had achieved longstanding clinical remission on a biologic therapy, then either rapidly discontinued treatment (61) or went through a tapering protocol (22), according to their doctors’ decision. These children were a mean of 11 years old, with mean disease duration of 2 years before the initiation of a biologic treatment. Systemic JIA was present in 40%; 22% had oligoarthritis, and 38% had polyarthritis.
All of the patients with systemic JIA were taking tocilizumab, although only 25% took it as monotherapy. Other medications being used were methotrexate (42%), cyclosporine (15%), glucocorticoids (15%), and leflunomide (3%).
For those with oligo- and polyarthritis, etanercept was the most commonly employed biologic (70%), followed by adalimumab (30%). Most (68%) were on monotherapy with their agent; however, 18% of those taking etanercept and 14% of those taking adalimumab were also taking methotrexate.
Before discontinuing their medication, the systemic JIA patients taking tocilizumab had a mean 43 months of inactive disease and a mean 37 months of remission. Among those taking adalimumab, the mean period of inactive disease was 48 months and the mean remission was 40 months. Among those taking etanercept, the mean period of inactive disease was 40 months and the mean remission was 34 months.
After discontinuing the biologic, the mean overall remission length was 6 months for all patients. However, this varied considerably with diagnosis and medication, Dr. Alexeeva noted. For systemic JIA patients taking tocilizumab, remission ranged from a minimum of 1 month to a maximum of 48 months. For those taking adalimumab, remission ranged from 4 to 38 months. Remission ranged from 1 to 20 months among those taking etanercept.
Disease flare occurred in 12% of those taking tocilizumab, at a mean of 8 months after discontinuation; 31% of those taking etanercept at a mean of 5.5 months; and 60% of those taking adalimumab at a mean of 4 months. Time to flare was longest among those taking tocilizumab (6-18 months), followed by etanercept (1.5-12 months) and adalimumab (1-13 months).
Dr. Alexeeva disclosed research funding and support from numerous pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
MADRID – Many children with juvenile idiopathic arthritis (JIA) who do well on biologics for a prolonged period will stay in remission after the medication is withdrawn, some new real-world data suggest.
A database review found that 70% of those in remission for at least 1.5 years remained in remission after stopping their biologic agent. Patients taking tocilizumab had the best outcomes, with 8 months of sustained, drug-free remission and only a 12% rate of disease flare, Ekaterina Alexeeva, MD, reported at the European Congress of Rheumatology.
“Prolonged therapy with biologic agents may cause adverse events which lead to the necessity of discontinuation of therapy in patients once complete disease quiescence has been achieved,” she said. While long-term drug studies do offer some glimpse into the stability of remission after drug discontinuation, these data don’t often reflect real-world experience.
“Clinical trials are made up of highly selected participants in contorted conditions with limited duration. Real-word data, collected under real-life practical circumstances, provide additional characteristics of patient populations, information on the effectiveness and safety of treatment over time, and the outcomes we can achieve under real-world conditions,” Dr. Alexeeva said.
She plumbed a national JIA database to find 83 patients who had achieved longstanding clinical remission on a biologic therapy, then either rapidly discontinued treatment (61) or went through a tapering protocol (22), according to their doctors’ decision. These children were a mean of 11 years old, with mean disease duration of 2 years before the initiation of a biologic treatment. Systemic JIA was present in 40%; 22% had oligoarthritis, and 38% had polyarthritis.
All of the patients with systemic JIA were taking tocilizumab, although only 25% took it as monotherapy. Other medications being used were methotrexate (42%), cyclosporine (15%), glucocorticoids (15%), and leflunomide (3%).
For those with oligo- and polyarthritis, etanercept was the most commonly employed biologic (70%), followed by adalimumab (30%). Most (68%) were on monotherapy with their agent; however, 18% of those taking etanercept and 14% of those taking adalimumab were also taking methotrexate.
Before discontinuing their medication, the systemic JIA patients taking tocilizumab had a mean 43 months of inactive disease and a mean 37 months of remission. Among those taking adalimumab, the mean period of inactive disease was 48 months and the mean remission was 40 months. Among those taking etanercept, the mean period of inactive disease was 40 months and the mean remission was 34 months.
After discontinuing the biologic, the mean overall remission length was 6 months for all patients. However, this varied considerably with diagnosis and medication, Dr. Alexeeva noted. For systemic JIA patients taking tocilizumab, remission ranged from a minimum of 1 month to a maximum of 48 months. For those taking adalimumab, remission ranged from 4 to 38 months. Remission ranged from 1 to 20 months among those taking etanercept.
Disease flare occurred in 12% of those taking tocilizumab, at a mean of 8 months after discontinuation; 31% of those taking etanercept at a mean of 5.5 months; and 60% of those taking adalimumab at a mean of 4 months. Time to flare was longest among those taking tocilizumab (6-18 months), followed by etanercept (1.5-12 months) and adalimumab (1-13 months).
Dr. Alexeeva disclosed research funding and support from numerous pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
MADRID – Many children with juvenile idiopathic arthritis (JIA) who do well on biologics for a prolonged period will stay in remission after the medication is withdrawn, some new real-world data suggest.
A database review found that 70% of those in remission for at least 1.5 years remained in remission after stopping their biologic agent. Patients taking tocilizumab had the best outcomes, with 8 months of sustained, drug-free remission and only a 12% rate of disease flare, Ekaterina Alexeeva, MD, reported at the European Congress of Rheumatology.
“Prolonged therapy with biologic agents may cause adverse events which lead to the necessity of discontinuation of therapy in patients once complete disease quiescence has been achieved,” she said. While long-term drug studies do offer some glimpse into the stability of remission after drug discontinuation, these data don’t often reflect real-world experience.
“Clinical trials are made up of highly selected participants in contorted conditions with limited duration. Real-word data, collected under real-life practical circumstances, provide additional characteristics of patient populations, information on the effectiveness and safety of treatment over time, and the outcomes we can achieve under real-world conditions,” Dr. Alexeeva said.
She plumbed a national JIA database to find 83 patients who had achieved longstanding clinical remission on a biologic therapy, then either rapidly discontinued treatment (61) or went through a tapering protocol (22), according to their doctors’ decision. These children were a mean of 11 years old, with mean disease duration of 2 years before the initiation of a biologic treatment. Systemic JIA was present in 40%; 22% had oligoarthritis, and 38% had polyarthritis.
All of the patients with systemic JIA were taking tocilizumab, although only 25% took it as monotherapy. Other medications being used were methotrexate (42%), cyclosporine (15%), glucocorticoids (15%), and leflunomide (3%).
For those with oligo- and polyarthritis, etanercept was the most commonly employed biologic (70%), followed by adalimumab (30%). Most (68%) were on monotherapy with their agent; however, 18% of those taking etanercept and 14% of those taking adalimumab were also taking methotrexate.
Before discontinuing their medication, the systemic JIA patients taking tocilizumab had a mean 43 months of inactive disease and a mean 37 months of remission. Among those taking adalimumab, the mean period of inactive disease was 48 months and the mean remission was 40 months. Among those taking etanercept, the mean period of inactive disease was 40 months and the mean remission was 34 months.
After discontinuing the biologic, the mean overall remission length was 6 months for all patients. However, this varied considerably with diagnosis and medication, Dr. Alexeeva noted. For systemic JIA patients taking tocilizumab, remission ranged from a minimum of 1 month to a maximum of 48 months. For those taking adalimumab, remission ranged from 4 to 38 months. Remission ranged from 1 to 20 months among those taking etanercept.
Disease flare occurred in 12% of those taking tocilizumab, at a mean of 8 months after discontinuation; 31% of those taking etanercept at a mean of 5.5 months; and 60% of those taking adalimumab at a mean of 4 months. Time to flare was longest among those taking tocilizumab (6-18 months), followed by etanercept (1.5-12 months) and adalimumab (1-13 months).
Dr. Alexeeva disclosed research funding and support from numerous pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Overall, 70% of those who were in remission for at least 1.5 years remained in remission after stopping their biologic.
Data source: A database review comprising 83 children.
Disclosures: Dr. Alexeeva disclosed research and grant support from numerous pharmaceutical companies.
Allopurinol and ventricular arrhythmias: Is there a link?
MADRID – Newly begun allopurinol treatment of elderly patients was linked with a significantly reduced incidence of ventricular arrhythmias in a review of more than 28,000 Medicare beneficiaries.
The antiarrhythmic effect appeared to strengthen with more prolonged allopurinol treatment, reaching a 28% reduction in ventricular arrhythmia incidence among patients on allopurinol for more than 2 years, compared with patients who never received the uric acid reducer, Jasvinder A. Singh, MD, reported in a poster at the European Congress of Rheumatology.
He and his associates used data collected from more than 3 million U.S. Medicare beneficiaries during 2006-2012 from a random Medicare 5% sample. This group included 28,755 patients who began a prescription for allopurinol after not having filled a prescription for the drug during the prior 365 days. The patients averaged 77 years of age.
The outcome of interest was a new onset ventricular arrhythmia, defined as an arrhythmia episode in a patient with no prior record of arrhythmia during the previous 365 days. Arrhythmia occurred in 2,538 of the patients on new allopurinol treatment (9%).
In a multivariate analysis that compared new allopurinol users with patients without allopurinol exposure and controlled for several demographic and clinical features, allopurinol use was linked with a statistically significant 18% reduced rate of incident ventricular arrhythmias in those who recieved it, compared with similar patients who did not receive allopurinol, Dr. Singh and his associates reported.
An additional analysis looked at the relative risk reduction associated with various lengths of allopurinol use. Compared with patients not on allopurinol, those taking it for more than 2 years had a significant 28% reduced rate of arrhythmias; those on it for 6 months to 2 years had a significant 24% reduction in incident arrhythmias; and those on it for 1-180 days had no significant change in their arrhythmia incidence.
Other individual factors that significantly correlated with increased ventricular arrhythmias included older age, male sex, African American race, presence of comorbidities, and treatment with a beta-blockers. Like allopurinol, treatment with a statin linked with a significantly reduced arrhythmia incidence.
In a second, related report at the meeting, Dr. Singh and his associates used a very similar data set to see if a link existed between new-onset allopurinol treatment and a reduced incidence of peripheral artery disease. This analysis showed that allopurinol use linked with a statistically significant 12% reduction in new onset peripheral artery disease. They again found that the clinical impact of allopurinol treatment grew larger as the duration of allopurinol treatment increased.
Dr. Singh has financial ties to Allergan, Bioiberica, Crealta, Iroko, Merz, Regeneron, Savient, and Takeda.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Newly begun allopurinol treatment of elderly patients was linked with a significantly reduced incidence of ventricular arrhythmias in a review of more than 28,000 Medicare beneficiaries.
The antiarrhythmic effect appeared to strengthen with more prolonged allopurinol treatment, reaching a 28% reduction in ventricular arrhythmia incidence among patients on allopurinol for more than 2 years, compared with patients who never received the uric acid reducer, Jasvinder A. Singh, MD, reported in a poster at the European Congress of Rheumatology.
He and his associates used data collected from more than 3 million U.S. Medicare beneficiaries during 2006-2012 from a random Medicare 5% sample. This group included 28,755 patients who began a prescription for allopurinol after not having filled a prescription for the drug during the prior 365 days. The patients averaged 77 years of age.
The outcome of interest was a new onset ventricular arrhythmia, defined as an arrhythmia episode in a patient with no prior record of arrhythmia during the previous 365 days. Arrhythmia occurred in 2,538 of the patients on new allopurinol treatment (9%).
In a multivariate analysis that compared new allopurinol users with patients without allopurinol exposure and controlled for several demographic and clinical features, allopurinol use was linked with a statistically significant 18% reduced rate of incident ventricular arrhythmias in those who recieved it, compared with similar patients who did not receive allopurinol, Dr. Singh and his associates reported.
An additional analysis looked at the relative risk reduction associated with various lengths of allopurinol use. Compared with patients not on allopurinol, those taking it for more than 2 years had a significant 28% reduced rate of arrhythmias; those on it for 6 months to 2 years had a significant 24% reduction in incident arrhythmias; and those on it for 1-180 days had no significant change in their arrhythmia incidence.
Other individual factors that significantly correlated with increased ventricular arrhythmias included older age, male sex, African American race, presence of comorbidities, and treatment with a beta-blockers. Like allopurinol, treatment with a statin linked with a significantly reduced arrhythmia incidence.
In a second, related report at the meeting, Dr. Singh and his associates used a very similar data set to see if a link existed between new-onset allopurinol treatment and a reduced incidence of peripheral artery disease. This analysis showed that allopurinol use linked with a statistically significant 12% reduction in new onset peripheral artery disease. They again found that the clinical impact of allopurinol treatment grew larger as the duration of allopurinol treatment increased.
Dr. Singh has financial ties to Allergan, Bioiberica, Crealta, Iroko, Merz, Regeneron, Savient, and Takeda.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Newly begun allopurinol treatment of elderly patients was linked with a significantly reduced incidence of ventricular arrhythmias in a review of more than 28,000 Medicare beneficiaries.
The antiarrhythmic effect appeared to strengthen with more prolonged allopurinol treatment, reaching a 28% reduction in ventricular arrhythmia incidence among patients on allopurinol for more than 2 years, compared with patients who never received the uric acid reducer, Jasvinder A. Singh, MD, reported in a poster at the European Congress of Rheumatology.
He and his associates used data collected from more than 3 million U.S. Medicare beneficiaries during 2006-2012 from a random Medicare 5% sample. This group included 28,755 patients who began a prescription for allopurinol after not having filled a prescription for the drug during the prior 365 days. The patients averaged 77 years of age.
The outcome of interest was a new onset ventricular arrhythmia, defined as an arrhythmia episode in a patient with no prior record of arrhythmia during the previous 365 days. Arrhythmia occurred in 2,538 of the patients on new allopurinol treatment (9%).
In a multivariate analysis that compared new allopurinol users with patients without allopurinol exposure and controlled for several demographic and clinical features, allopurinol use was linked with a statistically significant 18% reduced rate of incident ventricular arrhythmias in those who recieved it, compared with similar patients who did not receive allopurinol, Dr. Singh and his associates reported.
An additional analysis looked at the relative risk reduction associated with various lengths of allopurinol use. Compared with patients not on allopurinol, those taking it for more than 2 years had a significant 28% reduced rate of arrhythmias; those on it for 6 months to 2 years had a significant 24% reduction in incident arrhythmias; and those on it for 1-180 days had no significant change in their arrhythmia incidence.
Other individual factors that significantly correlated with increased ventricular arrhythmias included older age, male sex, African American race, presence of comorbidities, and treatment with a beta-blockers. Like allopurinol, treatment with a statin linked with a significantly reduced arrhythmia incidence.
In a second, related report at the meeting, Dr. Singh and his associates used a very similar data set to see if a link existed between new-onset allopurinol treatment and a reduced incidence of peripheral artery disease. This analysis showed that allopurinol use linked with a statistically significant 12% reduction in new onset peripheral artery disease. They again found that the clinical impact of allopurinol treatment grew larger as the duration of allopurinol treatment increased.
Dr. Singh has financial ties to Allergan, Bioiberica, Crealta, Iroko, Merz, Regeneron, Savient, and Takeda.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Patients on allopurinol for more than 2 years had a 28% reduced rate of ventricular arrhythmias, compared with those not on allopurinol treatment.
Data source: A 5% random sample of Medicare beneficiaries during 2006-2012.
Disclosures: Dr. Singh has been a consultant to Allergan, Bioiberica, Crealta, Iroko, Merz, Regeneron, Savient, and Takeda and has received research support from Savient and Takeda.
VIDEO: Cardiovascular events in rheumatoid arthritis have decreased over decades
MADRID – Recent improvements in the management of rheumatoid arthritis may have had a positive impact on common cardiovascular comorbidities, according to the results of a systematic review and meta-analysis.
Risk ratios (RR) for several CV events in rheumatoid arthritis (RA) patients were found to be lower for data published after 2000 and up to March 2016 when compared with data published up until 2000. Indeed, comparing these two time periods, French researchers found that the RR for myocardial infarction (MI) were a respective 1.32 and 1.18, for heart failure a respective 1.25 and 1.17, and for CV mortality a respective 1.21 and 1.07.
“Systemic inflammation is the cornerstone of both rheumatoid arthritis and atherosclerosis,” Cécile Gaujoux-Viala, MD, PhD, professor of rheumatology at Montpellier University, Nîmes, France, and chief of the rheumatology service at Nîmes University Hospital, said during a press briefing at the European Congress of Rheumatology.
“Over the past 15 years, new treatment strategies such as ‘tight control,’ ‘treat-to-target,’ methotrexate optimization, and use of biologic DMARDs [disease-modifying antirheumatic drugs] have led to better control of this inflammation,” Dr. Gaujoux-Viala added.
The aim of the meta-analysis was to look at the overall risk for CV events in RA patients versus the general population, she said, as well as to see if there had been any temporal shift by analyzing data obtained within two time periods – before 2000 and after 2000.
A systematic literature review was performed using the PubMed and Cochrane Library databases to search for observational studies that provided data about the occurrence of CV events in RA patients and controls. Of 5,714 papers that included reports of stroke, MI, heart failure, or CV death, 28 had the necessary data that could be used for the meta-analysis. Overall, the 28 studies included 227,871 RA patients, with a mean age of 55 years.
Results showed that RA patients had a 17% increased risk for stroke versus controls overall (P = .002), with a RR of 1.17. The RRs were 1.12 before 2000 and 1.23 after 2000, making stroke the only CV event that did not appear to show a downward trend.
Compared with the general population, RA patients had a 24% excess risk of MI, a 22% excess risk of heart failure, and a 18% excess risk of dying from a CV event (all P less than .00001).
These data provide “confirmation of an increased CV risk in RA patients compared to the general population,” said Dr. Gaujoux-Viala, who also discussed the study and its implications in a video interview.
Commenting on the study, Philip J. Mease, MD, of the University of Washington, Seattle, wondered where the studies used in the meta-analysis had been performed because of the potential impact that reduced access to CV medications or prevention strategies in certain countries could have on the results. However, the investigators did not determine where each of the studies used in the review took place.
Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Recent improvements in the management of rheumatoid arthritis may have had a positive impact on common cardiovascular comorbidities, according to the results of a systematic review and meta-analysis.
Risk ratios (RR) for several CV events in rheumatoid arthritis (RA) patients were found to be lower for data published after 2000 and up to March 2016 when compared with data published up until 2000. Indeed, comparing these two time periods, French researchers found that the RR for myocardial infarction (MI) were a respective 1.32 and 1.18, for heart failure a respective 1.25 and 1.17, and for CV mortality a respective 1.21 and 1.07.
“Systemic inflammation is the cornerstone of both rheumatoid arthritis and atherosclerosis,” Cécile Gaujoux-Viala, MD, PhD, professor of rheumatology at Montpellier University, Nîmes, France, and chief of the rheumatology service at Nîmes University Hospital, said during a press briefing at the European Congress of Rheumatology.
“Over the past 15 years, new treatment strategies such as ‘tight control,’ ‘treat-to-target,’ methotrexate optimization, and use of biologic DMARDs [disease-modifying antirheumatic drugs] have led to better control of this inflammation,” Dr. Gaujoux-Viala added.
The aim of the meta-analysis was to look at the overall risk for CV events in RA patients versus the general population, she said, as well as to see if there had been any temporal shift by analyzing data obtained within two time periods – before 2000 and after 2000.
A systematic literature review was performed using the PubMed and Cochrane Library databases to search for observational studies that provided data about the occurrence of CV events in RA patients and controls. Of 5,714 papers that included reports of stroke, MI, heart failure, or CV death, 28 had the necessary data that could be used for the meta-analysis. Overall, the 28 studies included 227,871 RA patients, with a mean age of 55 years.
Results showed that RA patients had a 17% increased risk for stroke versus controls overall (P = .002), with a RR of 1.17. The RRs were 1.12 before 2000 and 1.23 after 2000, making stroke the only CV event that did not appear to show a downward trend.
Compared with the general population, RA patients had a 24% excess risk of MI, a 22% excess risk of heart failure, and a 18% excess risk of dying from a CV event (all P less than .00001).
These data provide “confirmation of an increased CV risk in RA patients compared to the general population,” said Dr. Gaujoux-Viala, who also discussed the study and its implications in a video interview.
Commenting on the study, Philip J. Mease, MD, of the University of Washington, Seattle, wondered where the studies used in the meta-analysis had been performed because of the potential impact that reduced access to CV medications or prevention strategies in certain countries could have on the results. However, the investigators did not determine where each of the studies used in the review took place.
Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MADRID – Recent improvements in the management of rheumatoid arthritis may have had a positive impact on common cardiovascular comorbidities, according to the results of a systematic review and meta-analysis.
Risk ratios (RR) for several CV events in rheumatoid arthritis (RA) patients were found to be lower for data published after 2000 and up to March 2016 when compared with data published up until 2000. Indeed, comparing these two time periods, French researchers found that the RR for myocardial infarction (MI) were a respective 1.32 and 1.18, for heart failure a respective 1.25 and 1.17, and for CV mortality a respective 1.21 and 1.07.
“Systemic inflammation is the cornerstone of both rheumatoid arthritis and atherosclerosis,” Cécile Gaujoux-Viala, MD, PhD, professor of rheumatology at Montpellier University, Nîmes, France, and chief of the rheumatology service at Nîmes University Hospital, said during a press briefing at the European Congress of Rheumatology.
“Over the past 15 years, new treatment strategies such as ‘tight control,’ ‘treat-to-target,’ methotrexate optimization, and use of biologic DMARDs [disease-modifying antirheumatic drugs] have led to better control of this inflammation,” Dr. Gaujoux-Viala added.
The aim of the meta-analysis was to look at the overall risk for CV events in RA patients versus the general population, she said, as well as to see if there had been any temporal shift by analyzing data obtained within two time periods – before 2000 and after 2000.
A systematic literature review was performed using the PubMed and Cochrane Library databases to search for observational studies that provided data about the occurrence of CV events in RA patients and controls. Of 5,714 papers that included reports of stroke, MI, heart failure, or CV death, 28 had the necessary data that could be used for the meta-analysis. Overall, the 28 studies included 227,871 RA patients, with a mean age of 55 years.
Results showed that RA patients had a 17% increased risk for stroke versus controls overall (P = .002), with a RR of 1.17. The RRs were 1.12 before 2000 and 1.23 after 2000, making stroke the only CV event that did not appear to show a downward trend.
Compared with the general population, RA patients had a 24% excess risk of MI, a 22% excess risk of heart failure, and a 18% excess risk of dying from a CV event (all P less than .00001).
These data provide “confirmation of an increased CV risk in RA patients compared to the general population,” said Dr. Gaujoux-Viala, who also discussed the study and its implications in a video interview.
Commenting on the study, Philip J. Mease, MD, of the University of Washington, Seattle, wondered where the studies used in the meta-analysis had been performed because of the potential impact that reduced access to CV medications or prevention strategies in certain countries could have on the results. However, the investigators did not determine where each of the studies used in the review took place.
Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Risk ratios for myocardial infarction, heart failure, and CV mortality were lower between the period of 2000-2016 than for the period up to 2000.
Data source: Meta-analysis of 28 studies published up to March 2016 that provided data on CV event rates in RA patients and the general population.
Disclosures: Dr. Gaujoux-Viala had no relevant conflicts of interest to disclose.
Tool indicates fracture risk after HSCT
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
AT THE EULAR 2017 CONGRESS
Key clinical point: The Fracture Risk Assessment Tool (FRAX) helped in predicting osteoporotic fracture risk after hematopoietic stem cell transplantation (HSCT).
Major finding: The area under the receiver operating characteristic curve was 0.66, indicating modest predictive ability,10 years after HSCT.
Data source: A retrospective cohort study of 5,170 adult patients who received HSCT at the University of Texas MD Anderson Cancer Center between 2001 and 2010.
Disclosures: Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
Prior mycobacterial infection linked to Sjögren’s syndrome
MADRID – in a large population-based study reported at the European Congress of Rheumatology.
Study investigator Hsin-Hua Chen, MD, and his colleagues at the Taichung (Taiwan) Veterans Hospital found that the adjusted odds ratio for having Sjögren’s syndrome after nontuberculous mycobacteria infection (NTM) was 11.24, with a 95% confidence interval of 2.37-53.24.
The risk for having Sjögren’s syndrome was found to be highest in those aged 40-65 years, versus those older than 65 (aOR, 39.24; P = .09) and in those with no prior history of bronchiectasis (aOR, 37.98; P = .09). Although, in both analyses, the 95% CIs were very wide (3.97-387.75 and 3.83-376.92, respectively), and the P values were not significant.
Dr. Chen and his colleagues decided to look at the association between tuberculous or nontuberculous mycobacteria with Sjögren’s syndrome for several reasons. First, mycobacterial infections have been linked to the development of autoimmunity. Second, there has been an increased incidence of tuberculosis reported in patients with Sjögren’s syndrome. Third, both Sjögren’s and infection with NTM occurred predominantly in middle-aged women, suggesting a shared potential mechanism.
To investigate a possible association, a matched case-control study was conducted, with data obtained from the Taiwan National Health Insurance Database. There were 5,751 new cases of Sjögren’s syndrome that were identified and validated by at least two qualified rheumatologists and matched to 86,265 controls from the general population according to age, gender, and year of diagnosis. Patients with rheumatoid arthritis and systemic lupus erythematosus were excluded. International Classification of Disease codes were used to identify individuals who had prior TB or NTM infections.
The mean age of patients in both groups was 55 years, and approximately 87% of participants in both groups were female. There was a significant difference in baseline Charlson Comorbidity Index scores between cases and controls (0.5 vs. 0.4; P less than .001), and more cases than controls had bronchiectasis (4.1% vs. 1.3%; P less than .001). Results were adjusted accordingly.
While there was an association between NTM infection and Sjögren’s syndrome, there was no association with tuberculous mycobacteria infection.
Of course, it is not clear if infection with NTM actually causes the condition, and reverse causality cannot be ruled out, Dr. Chen said, so further mechanistic studies would be needed to investigate NTM’s possible role in the development of Sjögren’s syndrome.
Dr. Chen and coauthors had nothing to disclose.
MADRID – in a large population-based study reported at the European Congress of Rheumatology.
Study investigator Hsin-Hua Chen, MD, and his colleagues at the Taichung (Taiwan) Veterans Hospital found that the adjusted odds ratio for having Sjögren’s syndrome after nontuberculous mycobacteria infection (NTM) was 11.24, with a 95% confidence interval of 2.37-53.24.
The risk for having Sjögren’s syndrome was found to be highest in those aged 40-65 years, versus those older than 65 (aOR, 39.24; P = .09) and in those with no prior history of bronchiectasis (aOR, 37.98; P = .09). Although, in both analyses, the 95% CIs were very wide (3.97-387.75 and 3.83-376.92, respectively), and the P values were not significant.
Dr. Chen and his colleagues decided to look at the association between tuberculous or nontuberculous mycobacteria with Sjögren’s syndrome for several reasons. First, mycobacterial infections have been linked to the development of autoimmunity. Second, there has been an increased incidence of tuberculosis reported in patients with Sjögren’s syndrome. Third, both Sjögren’s and infection with NTM occurred predominantly in middle-aged women, suggesting a shared potential mechanism.
To investigate a possible association, a matched case-control study was conducted, with data obtained from the Taiwan National Health Insurance Database. There were 5,751 new cases of Sjögren’s syndrome that were identified and validated by at least two qualified rheumatologists and matched to 86,265 controls from the general population according to age, gender, and year of diagnosis. Patients with rheumatoid arthritis and systemic lupus erythematosus were excluded. International Classification of Disease codes were used to identify individuals who had prior TB or NTM infections.
The mean age of patients in both groups was 55 years, and approximately 87% of participants in both groups were female. There was a significant difference in baseline Charlson Comorbidity Index scores between cases and controls (0.5 vs. 0.4; P less than .001), and more cases than controls had bronchiectasis (4.1% vs. 1.3%; P less than .001). Results were adjusted accordingly.
While there was an association between NTM infection and Sjögren’s syndrome, there was no association with tuberculous mycobacteria infection.
Of course, it is not clear if infection with NTM actually causes the condition, and reverse causality cannot be ruled out, Dr. Chen said, so further mechanistic studies would be needed to investigate NTM’s possible role in the development of Sjögren’s syndrome.
Dr. Chen and coauthors had nothing to disclose.
MADRID – in a large population-based study reported at the European Congress of Rheumatology.
Study investigator Hsin-Hua Chen, MD, and his colleagues at the Taichung (Taiwan) Veterans Hospital found that the adjusted odds ratio for having Sjögren’s syndrome after nontuberculous mycobacteria infection (NTM) was 11.24, with a 95% confidence interval of 2.37-53.24.
The risk for having Sjögren’s syndrome was found to be highest in those aged 40-65 years, versus those older than 65 (aOR, 39.24; P = .09) and in those with no prior history of bronchiectasis (aOR, 37.98; P = .09). Although, in both analyses, the 95% CIs were very wide (3.97-387.75 and 3.83-376.92, respectively), and the P values were not significant.
Dr. Chen and his colleagues decided to look at the association between tuberculous or nontuberculous mycobacteria with Sjögren’s syndrome for several reasons. First, mycobacterial infections have been linked to the development of autoimmunity. Second, there has been an increased incidence of tuberculosis reported in patients with Sjögren’s syndrome. Third, both Sjögren’s and infection with NTM occurred predominantly in middle-aged women, suggesting a shared potential mechanism.
To investigate a possible association, a matched case-control study was conducted, with data obtained from the Taiwan National Health Insurance Database. There were 5,751 new cases of Sjögren’s syndrome that were identified and validated by at least two qualified rheumatologists and matched to 86,265 controls from the general population according to age, gender, and year of diagnosis. Patients with rheumatoid arthritis and systemic lupus erythematosus were excluded. International Classification of Disease codes were used to identify individuals who had prior TB or NTM infections.
The mean age of patients in both groups was 55 years, and approximately 87% of participants in both groups were female. There was a significant difference in baseline Charlson Comorbidity Index scores between cases and controls (0.5 vs. 0.4; P less than .001), and more cases than controls had bronchiectasis (4.1% vs. 1.3%; P less than .001). Results were adjusted accordingly.
While there was an association between NTM infection and Sjögren’s syndrome, there was no association with tuberculous mycobacteria infection.
Of course, it is not clear if infection with NTM actually causes the condition, and reverse causality cannot be ruled out, Dr. Chen said, so further mechanistic studies would be needed to investigate NTM’s possible role in the development of Sjögren’s syndrome.
Dr. Chen and coauthors had nothing to disclose.
AT THE EULAR 2017 CONGRESS
Key clinical point: Screening for Sjögren’s syndrome might be needed among people infected with nontuberculous mycobacteria if these data are confirmed.
Major finding: A strong association between prior infection with NTM and the development of Sjögren’s syndrome was found.
Data source: A retrospective, population-based study involving 5,751 newly diagnosed cases of Sjögren’s syndrome and 86,265 control subjects.
Disclosures: The author had no disclosures.
Comorbidities in psoriatic arthritis flag worse prognosis
MADRID – Comorbidities are relatively common in psoriatic arthritis patients, and they are more prevalent in patients with a worse disease course while on initial treatment with a tumor necrosis factor inhibitor, based on data from more than 1,700 Danish patients.
The presence of comorbidities in psoriatic arthritis (PsA) patients on initial tumor necrosis factor inhibitor (TNFi) treatment “was associated with higher disease activity, shorter adherence to the first TNFi, and reduced clinical response,” Lars Erik Kristensen, MD, said at the European Congress of Rheumatology.
To better understand the possible impact of comorbidities on PsA, he and his associates reviewed 1,750 Danish patients with PsA enrolled in a national registry at the time they began treatment with a TNFi. At the time they started treatment, 1,066 (61%) had no comorbidities, 493 (28%) had one comorbidity, and 191 (11%) had two or more comorbidities.
A comparison of the subgroups with no comorbidities and those with two or more showed several important and statistically significant differences in their baseline characteristics. Patients with at least two comorbidities had longer disease duration, and they had more active disease as measured by parameters including the Disease Activity Score 28 and the Health Assessment Questionnaire. Patients with two or more comorbidities also were older and had a higher average body mass index.
Further analyses showed that patients with two or more comorbidities were 72% more like to discontinue their TNFi treatment, compared with patients with no comorbidities – a statistically significant difference, Dr. Kristensen reported.
After 6 months of TNFi treatment, patients with two or more comorbidities had lower rates of achieving the American College of Rheumatology 20%, 50%, or 70% improvement criteria compared with patients with no comorbidities. For example, an ACR20 response occurred in 40% of patients with no comorbidities and in 31% of patients with two or more comorbidities after 6 months in an adjusted analysis.
Dr. Kristensen has been a consultant to or a speaker for several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Comorbidities are relatively common in psoriatic arthritis patients, and they are more prevalent in patients with a worse disease course while on initial treatment with a tumor necrosis factor inhibitor, based on data from more than 1,700 Danish patients.
The presence of comorbidities in psoriatic arthritis (PsA) patients on initial tumor necrosis factor inhibitor (TNFi) treatment “was associated with higher disease activity, shorter adherence to the first TNFi, and reduced clinical response,” Lars Erik Kristensen, MD, said at the European Congress of Rheumatology.
To better understand the possible impact of comorbidities on PsA, he and his associates reviewed 1,750 Danish patients with PsA enrolled in a national registry at the time they began treatment with a TNFi. At the time they started treatment, 1,066 (61%) had no comorbidities, 493 (28%) had one comorbidity, and 191 (11%) had two or more comorbidities.
A comparison of the subgroups with no comorbidities and those with two or more showed several important and statistically significant differences in their baseline characteristics. Patients with at least two comorbidities had longer disease duration, and they had more active disease as measured by parameters including the Disease Activity Score 28 and the Health Assessment Questionnaire. Patients with two or more comorbidities also were older and had a higher average body mass index.
Further analyses showed that patients with two or more comorbidities were 72% more like to discontinue their TNFi treatment, compared with patients with no comorbidities – a statistically significant difference, Dr. Kristensen reported.
After 6 months of TNFi treatment, patients with two or more comorbidities had lower rates of achieving the American College of Rheumatology 20%, 50%, or 70% improvement criteria compared with patients with no comorbidities. For example, an ACR20 response occurred in 40% of patients with no comorbidities and in 31% of patients with two or more comorbidities after 6 months in an adjusted analysis.
Dr. Kristensen has been a consultant to or a speaker for several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – Comorbidities are relatively common in psoriatic arthritis patients, and they are more prevalent in patients with a worse disease course while on initial treatment with a tumor necrosis factor inhibitor, based on data from more than 1,700 Danish patients.
The presence of comorbidities in psoriatic arthritis (PsA) patients on initial tumor necrosis factor inhibitor (TNFi) treatment “was associated with higher disease activity, shorter adherence to the first TNFi, and reduced clinical response,” Lars Erik Kristensen, MD, said at the European Congress of Rheumatology.
To better understand the possible impact of comorbidities on PsA, he and his associates reviewed 1,750 Danish patients with PsA enrolled in a national registry at the time they began treatment with a TNFi. At the time they started treatment, 1,066 (61%) had no comorbidities, 493 (28%) had one comorbidity, and 191 (11%) had two or more comorbidities.
A comparison of the subgroups with no comorbidities and those with two or more showed several important and statistically significant differences in their baseline characteristics. Patients with at least two comorbidities had longer disease duration, and they had more active disease as measured by parameters including the Disease Activity Score 28 and the Health Assessment Questionnaire. Patients with two or more comorbidities also were older and had a higher average body mass index.
Further analyses showed that patients with two or more comorbidities were 72% more like to discontinue their TNFi treatment, compared with patients with no comorbidities – a statistically significant difference, Dr. Kristensen reported.
After 6 months of TNFi treatment, patients with two or more comorbidities had lower rates of achieving the American College of Rheumatology 20%, 50%, or 70% improvement criteria compared with patients with no comorbidities. For example, an ACR20 response occurred in 40% of patients with no comorbidities and in 31% of patients with two or more comorbidities after 6 months in an adjusted analysis.
Dr. Kristensen has been a consultant to or a speaker for several drug companies.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point: , compared with patients with no comorbidities.
Major finding: An ACR20 response occurred in 40% of patients with no comorbidities but only 31% of those with two or more comorbidities.
Data source: Review of national registry data for 1,750 Danish psoriatic arthritis patients.
Disclosures: Dr. Kristensen has been a consultant to or a speaker for several drug companies.
TNFi treatment halves ankylosing spondylitis progression
MADRID – At least 2 years of tumor necrosis factor–inhibitor treatment of patients with ankylosing spondylitis nearly halved the rate of spinal radiographic progression in a study involving 432 Swiss patients.
In addition, patients on a tumor necrosis factor inhibitor (TNFi) who achieved low disease activity, reflected in an Ankylosing Spondylitis (AS) Disease Activity Score of 1.3 or less, showed virtually no spinal radiographic progression during a 2-year follow-up, Adrian Ciurea, MD, reported at the European Congress of Rheumatology.
He cautioned, however, that the evidence only shows correlation and can’t prove a causal relationship between TNFi treatment and slowed spinal radiographic progression because of potential residual confounding.
Dr. Ciurea and his associates analyzed records for AS patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases cohort who underwent at least two spinal radiographs separated by a 2-year gap. They assessed the radiographs using the modified Stoke AS Spinal Score (mSASSS), and they defined progression as a gain of at least two units on the mSASSS during a 2-year period between radiographs.
The 432 AS patients in the study averaged 40 years old, two-thirds were men, and they had AS symptoms for an average of nearly 14 years. Their average AS Disease Activity Score (ASDAS) at entry was 2.8.
A multivariate analysis that controlled for several variables, including sex, smoking history, baseline mSASSS, and exercise, identified three parameters that had significant correlations with radiographic progression: Men had more than double the rate of progression, compared with women; higher baseline mSASSS was linked with a higher rate of progression; and a greater-than-2-year history of treatment with a TNFi was linked with a 48% reduced rate of progression, reported Dr. Ciurea, a rheumatologist at the Zürich University Hospital.
The duration of treatment also mattered. Patients who received at least 4 years of TNFi treatment had a statistically significant 68% reduced rate of radiographic spinal progression. In contrast, patients who received a TNFi for fewer than 4 years but more than 2 years had a 42% lower rate of progression that was of borderline statistical significance. TNFi treatment that started during the 2 years immediately preceding the radiograph failed to show a significant link with reduced progression.
Further analysis also showed a tight correlation between patients’ disease activity while on TNFi treatment and radiographic progression. Patients who maintained an average ASDAS of 2.1 or less during the 2 years prior to radiographic assessment showed an average mSASSS gain of 0.31 units over that 2-year period, compared with an average 1.45-unit mSASSS gain among patients whose average ASDAS remained above 2.1, a statistically significant difference between these two groups. Patients with even more inactive disease on TNFi treatment – those who maintained an average ASDAS of 1.3 or less – had an average 0.01-unit rise in their mSASSS after 2 years of treatment, compared with an average 0.52-unit mSASSS rise after 2 years in patients with an ASDAS of more than 1.3 but less than 2.1, he said.
The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – At least 2 years of tumor necrosis factor–inhibitor treatment of patients with ankylosing spondylitis nearly halved the rate of spinal radiographic progression in a study involving 432 Swiss patients.
In addition, patients on a tumor necrosis factor inhibitor (TNFi) who achieved low disease activity, reflected in an Ankylosing Spondylitis (AS) Disease Activity Score of 1.3 or less, showed virtually no spinal radiographic progression during a 2-year follow-up, Adrian Ciurea, MD, reported at the European Congress of Rheumatology.
He cautioned, however, that the evidence only shows correlation and can’t prove a causal relationship between TNFi treatment and slowed spinal radiographic progression because of potential residual confounding.
Dr. Ciurea and his associates analyzed records for AS patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases cohort who underwent at least two spinal radiographs separated by a 2-year gap. They assessed the radiographs using the modified Stoke AS Spinal Score (mSASSS), and they defined progression as a gain of at least two units on the mSASSS during a 2-year period between radiographs.
The 432 AS patients in the study averaged 40 years old, two-thirds were men, and they had AS symptoms for an average of nearly 14 years. Their average AS Disease Activity Score (ASDAS) at entry was 2.8.
A multivariate analysis that controlled for several variables, including sex, smoking history, baseline mSASSS, and exercise, identified three parameters that had significant correlations with radiographic progression: Men had more than double the rate of progression, compared with women; higher baseline mSASSS was linked with a higher rate of progression; and a greater-than-2-year history of treatment with a TNFi was linked with a 48% reduced rate of progression, reported Dr. Ciurea, a rheumatologist at the Zürich University Hospital.
The duration of treatment also mattered. Patients who received at least 4 years of TNFi treatment had a statistically significant 68% reduced rate of radiographic spinal progression. In contrast, patients who received a TNFi for fewer than 4 years but more than 2 years had a 42% lower rate of progression that was of borderline statistical significance. TNFi treatment that started during the 2 years immediately preceding the radiograph failed to show a significant link with reduced progression.
Further analysis also showed a tight correlation between patients’ disease activity while on TNFi treatment and radiographic progression. Patients who maintained an average ASDAS of 2.1 or less during the 2 years prior to radiographic assessment showed an average mSASSS gain of 0.31 units over that 2-year period, compared with an average 1.45-unit mSASSS gain among patients whose average ASDAS remained above 2.1, a statistically significant difference between these two groups. Patients with even more inactive disease on TNFi treatment – those who maintained an average ASDAS of 1.3 or less – had an average 0.01-unit rise in their mSASSS after 2 years of treatment, compared with an average 0.52-unit mSASSS rise after 2 years in patients with an ASDAS of more than 1.3 but less than 2.1, he said.
The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
MADRID – At least 2 years of tumor necrosis factor–inhibitor treatment of patients with ankylosing spondylitis nearly halved the rate of spinal radiographic progression in a study involving 432 Swiss patients.
In addition, patients on a tumor necrosis factor inhibitor (TNFi) who achieved low disease activity, reflected in an Ankylosing Spondylitis (AS) Disease Activity Score of 1.3 or less, showed virtually no spinal radiographic progression during a 2-year follow-up, Adrian Ciurea, MD, reported at the European Congress of Rheumatology.
He cautioned, however, that the evidence only shows correlation and can’t prove a causal relationship between TNFi treatment and slowed spinal radiographic progression because of potential residual confounding.
Dr. Ciurea and his associates analyzed records for AS patients enrolled in the Swiss Clinical Quality Management in Rheumatic Diseases cohort who underwent at least two spinal radiographs separated by a 2-year gap. They assessed the radiographs using the modified Stoke AS Spinal Score (mSASSS), and they defined progression as a gain of at least two units on the mSASSS during a 2-year period between radiographs.
The 432 AS patients in the study averaged 40 years old, two-thirds were men, and they had AS symptoms for an average of nearly 14 years. Their average AS Disease Activity Score (ASDAS) at entry was 2.8.
A multivariate analysis that controlled for several variables, including sex, smoking history, baseline mSASSS, and exercise, identified three parameters that had significant correlations with radiographic progression: Men had more than double the rate of progression, compared with women; higher baseline mSASSS was linked with a higher rate of progression; and a greater-than-2-year history of treatment with a TNFi was linked with a 48% reduced rate of progression, reported Dr. Ciurea, a rheumatologist at the Zürich University Hospital.
The duration of treatment also mattered. Patients who received at least 4 years of TNFi treatment had a statistically significant 68% reduced rate of radiographic spinal progression. In contrast, patients who received a TNFi for fewer than 4 years but more than 2 years had a 42% lower rate of progression that was of borderline statistical significance. TNFi treatment that started during the 2 years immediately preceding the radiograph failed to show a significant link with reduced progression.
Further analysis also showed a tight correlation between patients’ disease activity while on TNFi treatment and radiographic progression. Patients who maintained an average ASDAS of 2.1 or less during the 2 years prior to radiographic assessment showed an average mSASSS gain of 0.31 units over that 2-year period, compared with an average 1.45-unit mSASSS gain among patients whose average ASDAS remained above 2.1, a statistically significant difference between these two groups. Patients with even more inactive disease on TNFi treatment – those who maintained an average ASDAS of 1.3 or less – had an average 0.01-unit rise in their mSASSS after 2 years of treatment, compared with an average 0.52-unit mSASSS rise after 2 years in patients with an ASDAS of more than 1.3 but less than 2.1, he said.
The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Prolonged TNFi treatment was linked with a 48% lower rate of spinal radiographic progression, compared with shorter treatment.
Data source: Review of 432 patients in the Swiss Clinical Quality Management in Rheumatic Diseases cohort.
Disclosures: The cohort study received partial support from Merck Sharpe & Dohme. Dr. Ciurea has been a consultant to or speaker for Abbvie, Celgene, Eli Lilly, Janssen-Cilag, Merck Sharp & Dohme, Novartis, Pfizer, and UCB.
Meta-analysis: Bisphosphonates mitigate glucocorticoid-induced bone loss
MADRID – Bisphosphonates mitigate the damaging effects of glucocorticoid on bone, boosting bone mineral density and reducing the risk of fracture by up to 33%, compared with placebo, according to a systematic review and meta-analysis.
The review of 11 randomized, controlled trials found that bisphosphonates consistently improved bone outcomes among patients taking prednisone, Anas Makhzoum, MD, said at the European Congress of Rheumatology.
The primary outcomes of these trials were mean percentage change in bone mineral density at lumbar spine and femoral neck, and fracture incidence.
The drugs examined were ibandronate, alendronate, risedronate, etidronate, and clodronate. The mean duration of these trials was 71 weeks. Patients took a mean steroid dose of 15 mg.
Dr. Makhzoum, a resident at Queen’s University, Kingston, Ont., pooled nine of these trials for the outcome of mean percentage change in lumbar spine bone mineral density. The pooled mean percentage change of lumbar spine consistently favored bisphosphonates, compared with placebo, with a mean, statistically significant difference of approximately 4%.
Six studies were pooled for the outcome of mean percentage change in femoral neck bone mineral density. The pooled mean percentage change consistently favored bisphosphonates, with a mean, statistically significant difference of 2.95% relative to placebo.
Seven studies were pooled for outcome of incident fracture and the results consistently favored bisphosphonates, with a mean, statistically significant 33% decrease in the risk of a new fracture, compared with the placebo group (relative risk, 0.66).
“Bisphosphonates remain the standard of care for prevention and treatment of bone loss in patients on chronic steroids treatment,” Dr. Makhzoum noted.
He had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
MADRID – Bisphosphonates mitigate the damaging effects of glucocorticoid on bone, boosting bone mineral density and reducing the risk of fracture by up to 33%, compared with placebo, according to a systematic review and meta-analysis.
The review of 11 randomized, controlled trials found that bisphosphonates consistently improved bone outcomes among patients taking prednisone, Anas Makhzoum, MD, said at the European Congress of Rheumatology.
The primary outcomes of these trials were mean percentage change in bone mineral density at lumbar spine and femoral neck, and fracture incidence.
The drugs examined were ibandronate, alendronate, risedronate, etidronate, and clodronate. The mean duration of these trials was 71 weeks. Patients took a mean steroid dose of 15 mg.
Dr. Makhzoum, a resident at Queen’s University, Kingston, Ont., pooled nine of these trials for the outcome of mean percentage change in lumbar spine bone mineral density. The pooled mean percentage change of lumbar spine consistently favored bisphosphonates, compared with placebo, with a mean, statistically significant difference of approximately 4%.
Six studies were pooled for the outcome of mean percentage change in femoral neck bone mineral density. The pooled mean percentage change consistently favored bisphosphonates, with a mean, statistically significant difference of 2.95% relative to placebo.
Seven studies were pooled for outcome of incident fracture and the results consistently favored bisphosphonates, with a mean, statistically significant 33% decrease in the risk of a new fracture, compared with the placebo group (relative risk, 0.66).
“Bisphosphonates remain the standard of care for prevention and treatment of bone loss in patients on chronic steroids treatment,” Dr. Makhzoum noted.
He had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
MADRID – Bisphosphonates mitigate the damaging effects of glucocorticoid on bone, boosting bone mineral density and reducing the risk of fracture by up to 33%, compared with placebo, according to a systematic review and meta-analysis.
The review of 11 randomized, controlled trials found that bisphosphonates consistently improved bone outcomes among patients taking prednisone, Anas Makhzoum, MD, said at the European Congress of Rheumatology.
The primary outcomes of these trials were mean percentage change in bone mineral density at lumbar spine and femoral neck, and fracture incidence.
The drugs examined were ibandronate, alendronate, risedronate, etidronate, and clodronate. The mean duration of these trials was 71 weeks. Patients took a mean steroid dose of 15 mg.
Dr. Makhzoum, a resident at Queen’s University, Kingston, Ont., pooled nine of these trials for the outcome of mean percentage change in lumbar spine bone mineral density. The pooled mean percentage change of lumbar spine consistently favored bisphosphonates, compared with placebo, with a mean, statistically significant difference of approximately 4%.
Six studies were pooled for the outcome of mean percentage change in femoral neck bone mineral density. The pooled mean percentage change consistently favored bisphosphonates, with a mean, statistically significant difference of 2.95% relative to placebo.
Seven studies were pooled for outcome of incident fracture and the results consistently favored bisphosphonates, with a mean, statistically significant 33% decrease in the risk of a new fracture, compared with the placebo group (relative risk, 0.66).
“Bisphosphonates remain the standard of care for prevention and treatment of bone loss in patients on chronic steroids treatment,” Dr. Makhzoum noted.
He had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Relative to placebo, bisphosphonates reduced the risk of fracture by up to 33%.
Data source: A meta-analysis comprising 11 randomized, placebo-controlled trials with more than 2,000 patients.
Disclosures: Dr. Makhzoum had no financial disclosures.