User login
Minority-serving hospitals had similar survival after liver cancer surgery
Overall survival after liver cancer surgery was similar regardless of whether patients were treated at minority-serving hospitals or at hospitals with proportionally fewer African American or Hispanic patients, investigators have found.
“[T]reatment of racial minorities is largely restricted to a subset of hospitals, often referred to as minority-serving hospitals. We sought to examine whether racial and ethnic minorities with hepatocellular carcinoma receive their surgical care at minority-serving hospitals, and whether treatment at minority-serving hospitals is associated with differences in overall survival,” explained Winta T. Mehtsun, MD, MPH, of Dana-Farber Cancer Institute in Boston and associates in an abstract released as part of the annual Digestive Disease Week.®
Hepatocellular carcinoma continues to have a low 5-year survival rate and exhibits marked racial and ethnic disparities in diagnosis, treatment, and outcomes. In a recent study of Surveillance Epidemiology and End Results (SEER) data, African American patients with hepatocellular carcinoma were significantly younger at diagnosis, were more likely to have metastatic disease, and were less likely to receive surgical treatment compared with whites (Am J Prevent Med 2018;55:S40-48). Among patients with early-stage liver cancer, Hispanic and African American patients are less likely to receive curative therapy and die sooner, on average, than do other patients (Clin Gastroenterol Hepatol. 2019;17:551-9).
Minority-serving hospitals also have improved significantly less over time on measures of critical care, length of stay, and mortality, but whether these issues extend to hepatocellular carcinoma remains unclear. Therefore, Dr. Mehtsun and her associates studied all 2,609 patients in the National Cancer Database who received surgical resection (not transplantation or local therapy) for nonmetastatic hepatocellular carcinoma between 2004 and 2014. They compared survival at minority-serving hospitals – those in the top 10% based on the proportion of patients who were African American or Hispanic – with survival at other hospitals.
“There was no association between minority-serving hospital and overall survival,” the researchers reported (multivariable hazard ratio for death, 0.89; 95% confidence interval, 0.72-1.11). In contrast, survival was significantly shorter among patients with more advanced disease (HR, 2.5; 95% CI, 2.1-2.8), patients who were treated at a community cancer program (HR, 1.7; 95% CI, 1.3-2.4), and patients whose Charlson Comorbidity Index was greater than 2 (HR, 1.2; 95% CI, 1.1-1.4).
Stage at diagnosis, comorbidities, and sex were not significantly related to hospital type, the investigators noted. A total of 298 patients (11%) were treated at minority-serving hospitals. Patients treated at minority-serving hospitals were significantly more likely to be uninsured (11% vs. 4% at other hospitals) and significantly less likely to be treated at an academic center (55% vs. 69%; both P less than .001).
Dr. Mehtsun reported having no relevant conflicts of interest.
SOURCE: Mehtsun WT et al. DDW 2020, Abstract Tu2043.
Overall survival after liver cancer surgery was similar regardless of whether patients were treated at minority-serving hospitals or at hospitals with proportionally fewer African American or Hispanic patients, investigators have found.
“[T]reatment of racial minorities is largely restricted to a subset of hospitals, often referred to as minority-serving hospitals. We sought to examine whether racial and ethnic minorities with hepatocellular carcinoma receive their surgical care at minority-serving hospitals, and whether treatment at minority-serving hospitals is associated with differences in overall survival,” explained Winta T. Mehtsun, MD, MPH, of Dana-Farber Cancer Institute in Boston and associates in an abstract released as part of the annual Digestive Disease Week.®
Hepatocellular carcinoma continues to have a low 5-year survival rate and exhibits marked racial and ethnic disparities in diagnosis, treatment, and outcomes. In a recent study of Surveillance Epidemiology and End Results (SEER) data, African American patients with hepatocellular carcinoma were significantly younger at diagnosis, were more likely to have metastatic disease, and were less likely to receive surgical treatment compared with whites (Am J Prevent Med 2018;55:S40-48). Among patients with early-stage liver cancer, Hispanic and African American patients are less likely to receive curative therapy and die sooner, on average, than do other patients (Clin Gastroenterol Hepatol. 2019;17:551-9).
Minority-serving hospitals also have improved significantly less over time on measures of critical care, length of stay, and mortality, but whether these issues extend to hepatocellular carcinoma remains unclear. Therefore, Dr. Mehtsun and her associates studied all 2,609 patients in the National Cancer Database who received surgical resection (not transplantation or local therapy) for nonmetastatic hepatocellular carcinoma between 2004 and 2014. They compared survival at minority-serving hospitals – those in the top 10% based on the proportion of patients who were African American or Hispanic – with survival at other hospitals.
“There was no association between minority-serving hospital and overall survival,” the researchers reported (multivariable hazard ratio for death, 0.89; 95% confidence interval, 0.72-1.11). In contrast, survival was significantly shorter among patients with more advanced disease (HR, 2.5; 95% CI, 2.1-2.8), patients who were treated at a community cancer program (HR, 1.7; 95% CI, 1.3-2.4), and patients whose Charlson Comorbidity Index was greater than 2 (HR, 1.2; 95% CI, 1.1-1.4).
Stage at diagnosis, comorbidities, and sex were not significantly related to hospital type, the investigators noted. A total of 298 patients (11%) were treated at minority-serving hospitals. Patients treated at minority-serving hospitals were significantly more likely to be uninsured (11% vs. 4% at other hospitals) and significantly less likely to be treated at an academic center (55% vs. 69%; both P less than .001).
Dr. Mehtsun reported having no relevant conflicts of interest.
SOURCE: Mehtsun WT et al. DDW 2020, Abstract Tu2043.
Overall survival after liver cancer surgery was similar regardless of whether patients were treated at minority-serving hospitals or at hospitals with proportionally fewer African American or Hispanic patients, investigators have found.
“[T]reatment of racial minorities is largely restricted to a subset of hospitals, often referred to as minority-serving hospitals. We sought to examine whether racial and ethnic minorities with hepatocellular carcinoma receive their surgical care at minority-serving hospitals, and whether treatment at minority-serving hospitals is associated with differences in overall survival,” explained Winta T. Mehtsun, MD, MPH, of Dana-Farber Cancer Institute in Boston and associates in an abstract released as part of the annual Digestive Disease Week.®
Hepatocellular carcinoma continues to have a low 5-year survival rate and exhibits marked racial and ethnic disparities in diagnosis, treatment, and outcomes. In a recent study of Surveillance Epidemiology and End Results (SEER) data, African American patients with hepatocellular carcinoma were significantly younger at diagnosis, were more likely to have metastatic disease, and were less likely to receive surgical treatment compared with whites (Am J Prevent Med 2018;55:S40-48). Among patients with early-stage liver cancer, Hispanic and African American patients are less likely to receive curative therapy and die sooner, on average, than do other patients (Clin Gastroenterol Hepatol. 2019;17:551-9).
Minority-serving hospitals also have improved significantly less over time on measures of critical care, length of stay, and mortality, but whether these issues extend to hepatocellular carcinoma remains unclear. Therefore, Dr. Mehtsun and her associates studied all 2,609 patients in the National Cancer Database who received surgical resection (not transplantation or local therapy) for nonmetastatic hepatocellular carcinoma between 2004 and 2014. They compared survival at minority-serving hospitals – those in the top 10% based on the proportion of patients who were African American or Hispanic – with survival at other hospitals.
“There was no association between minority-serving hospital and overall survival,” the researchers reported (multivariable hazard ratio for death, 0.89; 95% confidence interval, 0.72-1.11). In contrast, survival was significantly shorter among patients with more advanced disease (HR, 2.5; 95% CI, 2.1-2.8), patients who were treated at a community cancer program (HR, 1.7; 95% CI, 1.3-2.4), and patients whose Charlson Comorbidity Index was greater than 2 (HR, 1.2; 95% CI, 1.1-1.4).
Stage at diagnosis, comorbidities, and sex were not significantly related to hospital type, the investigators noted. A total of 298 patients (11%) were treated at minority-serving hospitals. Patients treated at minority-serving hospitals were significantly more likely to be uninsured (11% vs. 4% at other hospitals) and significantly less likely to be treated at an academic center (55% vs. 69%; both P less than .001).
Dr. Mehtsun reported having no relevant conflicts of interest.
SOURCE: Mehtsun WT et al. DDW 2020, Abstract Tu2043.
FROM DDW 2020
Large study finds no link between gluten, IBD risk
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
Among women without celiac disease, dietary gluten intake was not associated with the risk of developing either Crohn’s disease or ulcerative colitis, investigators reported.
The findings spanned subgroups stratified by age, body mass index, smoking status, and whether individuals primarily consumed refined or whole grains, said Emily Walsh Lopes, MD, gastroenterology clinical and research fellow at Massachusetts General Hospital in Boston. She and associates reported the combined analysis of the prospective Nurses’ Health Study and Nurses’ Health Study II in an abstract released as part of the annual Digestive Disease Week.®
“Avoidance of dietary gluten is common, and many patients attribute gastrointestinal symptoms to gluten intake,” Dr. Lopes said in an interview. “Though our findings warrant further study, the results suggest to patients and providers that eating gluten does not increase a person’s chance of getting diagnosed with inflammatory bowel disease.”
Prior studies have found that many individuals with inflammatory bowel disease avoid gluten and report subsequent improvements in gastrointestinal symptoms, even if they do not have celiac disease. However, it remains unclear whether dietary gluten is a risk factor for new-onset inflammatory bowel disease.
To address this question, Dr. Lopes and associates analyzed data collected from 165,327 women who took part in the Nurses’ Health Study (1986 to 2016) or the Nurses’ Health Study II (1991 through 2017). None of the women had a preexisting diagnosis of celiac disease or inflammatory bowel disease. Dietary gluten intake was estimated based on food frequency questionnaires completed by the women at baseline and every 4 years. The researchers also reviewed medical records to confirm self-reported cases of new-onset ulcerative colitis and Crohn’s disease.
Over 4.02 million person-years of follow-up, 277 women developed Crohn’s disease and 359 developed ulcerative colitis. Gluten intake was not associated with the risk of either type of inflammatory bowel disease, even after the researchers controlled for multiple demographic and clinical risk factors.
After submitting their abstract, Dr. Lopes and coinvestigators expanded the dataset to include a large cohort of men from the prospective Health Professionals Follow-up Study. The final pooled cohort included more than 208,000 women and men followed for more than 20 years. Through the end of follow-up, the researchers documented 337 cases of Crohn’s disease and 446 cases of ulcerative colitis. “Inclusion of the male cohort in the pooled analysis did not materially change our estimates,” Dr. Lopes told MDedge. “That is, no association was seen between gluten intake and risk of either Crohn’s disease or ulcerative colitis in the final cohort.”
She noted that the findings cannot be extrapolated to individuals who are already diagnosed with inflammatory bowel disease. “It is possible that different mechanisms exist to explain how gluten intake impacts those already diagnosed with IBD, and this topic warrants further study,” she said. Also, because the three cohort studies were observational, they are subject to bias. “While we tried to account for this in our analyses, residual bias may still exist.”
Dr. Lopes reported having no conflicts of interest.
SOURCE: Walsh Lopes E et al. DDW 2020, abstract 847.
FROM DDW 2020
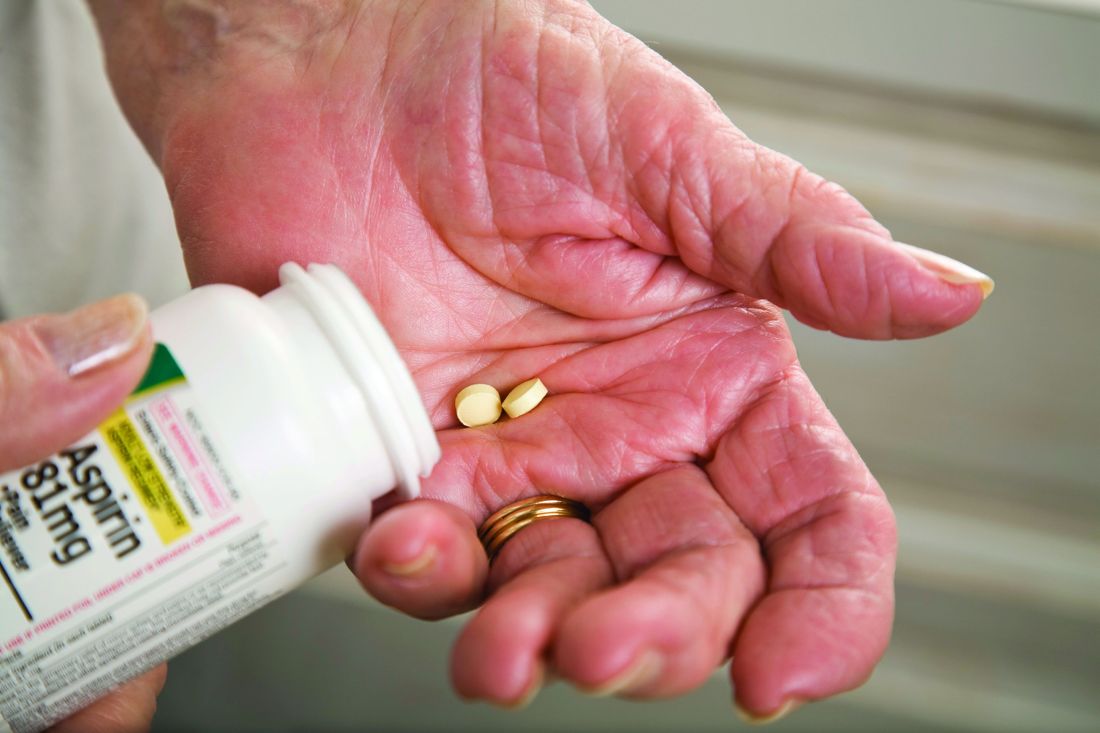
Major GI bleeding risk calculated for primary prevention aspirin in elderly
in a new analysis from the large randomized ASPREE trial released as part of the annual Digestive Disease Week.
The analysis identified several independent risk factors for major GI bleeding – advanced age, hypertension, obesity, smoking, and chronic kidney disease – according to Suzanne E. Mahady, MBBS, PhD, a gastroenterologist and clinical epidemiologist at Monash University in Melbourne.
“To date, there [are] no comparable data assessing aspirin-related bleeding in older healthy people from a large randomized, controlled trial. Previous data [have] been observational, with variable definitions of significant bleeding, and retrospective. We derived a standard definition for bleeding, used physicians to adjudicate bleeding endpoints, and followed older people for 5 years,” she explained in an interview.
“Our data on bleeding [are] novel,” Dr. Mahady added. “It will help clinicians assess who is most at risk of bleeding with aspirin and target modifiable bleeding risk factors where possible.”
ASPREE was a double-blind trial including 19,114 apparently healthy Australian and American adults age 70 or older, or age 65-plus for blacks and Hispanics in the United States. Participants were randomized to 100 mg/day of enteric-coated aspirin or placebo. At a median 4.7 years of follow-up, there was no between-group difference in major adverse cardiovascular events, a lack of benefit accompanied by a 38% greater risk of major hemorrhage risk and a statistically significant 14% increase in all-cause mortality in the aspirin group. (N Engl J Med. 2018 Oct 18;379[16]:1509-18). The chief contributor to the excess mortality in the aspirin group was their 31% greater risk of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
The new analysis of severe GI bleeding documented an absolute 5-year risk of 0.2% for 70-year-olds and 0.4% in 80-year-olds on aspirin. In 80-year-olds with additional GI bleeding risk factors as identified in the study, the rate reached up to 5.5%. The risk of major upper GI bleeding events was 87% greater in the aspirin group, compared with placebo-treated controls, and the risk of serious lower GI bleeding was increased 36%.
ASPREE coinvestigator Andrew T. Chan, MD, said that the bleeding data should prove useful in future efforts to appropriately weight the risks and benefits of low-dose aspirin treatment.
“We need to better understand how to incorporate bleeding risk in clinical decision making about how to use aspirin among older adults because aspirin has many potential benefits, including prevention of colorectal cancer,” said Dr. Chan, a gastroenterologist and professor of medicine at Harvard Medical School and director for cancer epidemiology at Massachusetts General Hospital, both in Boston.
However, ASPREE has soured cardiologists on the decades-long practice of recommending aspirin for primary prevention of cardiovascular disease in older individuals. In response to the publication of primary outcomes in ASPREE, which was closely bracketed by publication of the largely negative results of the randomized ARRIVE and ASCEND trials in a collective 47,000-plus randomized patients, the American College of Cardiology/American Heart Association clipped aspirin’s role for primary prevention of atherosclerotic cardiovascular disease. The current recommendation is that low-dose aspirin should not be administered on a routine basis for primary cardiovascular prevention in people above age 70, nor in adults at any age at increased bleeding risk, although the practice “might be considered” for primary prevention in select higher atherosclerotic cardiovascular disease–risk 40- to 70-year-olds, provided they are not at increased bleeding risk (J Am Coll Cardiol. 2019 Sep. doi: 10.1016/j.jacc.2019.03.010).
Dr. Mahady reported having no financial conflicts of interest. Dr. Chan serves as a consultant to Bayer Pharma, Janssen, and Pfizer.
in a new analysis from the large randomized ASPREE trial released as part of the annual Digestive Disease Week.
The analysis identified several independent risk factors for major GI bleeding – advanced age, hypertension, obesity, smoking, and chronic kidney disease – according to Suzanne E. Mahady, MBBS, PhD, a gastroenterologist and clinical epidemiologist at Monash University in Melbourne.
“To date, there [are] no comparable data assessing aspirin-related bleeding in older healthy people from a large randomized, controlled trial. Previous data [have] been observational, with variable definitions of significant bleeding, and retrospective. We derived a standard definition for bleeding, used physicians to adjudicate bleeding endpoints, and followed older people for 5 years,” she explained in an interview.
“Our data on bleeding [are] novel,” Dr. Mahady added. “It will help clinicians assess who is most at risk of bleeding with aspirin and target modifiable bleeding risk factors where possible.”
ASPREE was a double-blind trial including 19,114 apparently healthy Australian and American adults age 70 or older, or age 65-plus for blacks and Hispanics in the United States. Participants were randomized to 100 mg/day of enteric-coated aspirin or placebo. At a median 4.7 years of follow-up, there was no between-group difference in major adverse cardiovascular events, a lack of benefit accompanied by a 38% greater risk of major hemorrhage risk and a statistically significant 14% increase in all-cause mortality in the aspirin group. (N Engl J Med. 2018 Oct 18;379[16]:1509-18). The chief contributor to the excess mortality in the aspirin group was their 31% greater risk of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
The new analysis of severe GI bleeding documented an absolute 5-year risk of 0.2% for 70-year-olds and 0.4% in 80-year-olds on aspirin. In 80-year-olds with additional GI bleeding risk factors as identified in the study, the rate reached up to 5.5%. The risk of major upper GI bleeding events was 87% greater in the aspirin group, compared with placebo-treated controls, and the risk of serious lower GI bleeding was increased 36%.
ASPREE coinvestigator Andrew T. Chan, MD, said that the bleeding data should prove useful in future efforts to appropriately weight the risks and benefits of low-dose aspirin treatment.
“We need to better understand how to incorporate bleeding risk in clinical decision making about how to use aspirin among older adults because aspirin has many potential benefits, including prevention of colorectal cancer,” said Dr. Chan, a gastroenterologist and professor of medicine at Harvard Medical School and director for cancer epidemiology at Massachusetts General Hospital, both in Boston.
However, ASPREE has soured cardiologists on the decades-long practice of recommending aspirin for primary prevention of cardiovascular disease in older individuals. In response to the publication of primary outcomes in ASPREE, which was closely bracketed by publication of the largely negative results of the randomized ARRIVE and ASCEND trials in a collective 47,000-plus randomized patients, the American College of Cardiology/American Heart Association clipped aspirin’s role for primary prevention of atherosclerotic cardiovascular disease. The current recommendation is that low-dose aspirin should not be administered on a routine basis for primary cardiovascular prevention in people above age 70, nor in adults at any age at increased bleeding risk, although the practice “might be considered” for primary prevention in select higher atherosclerotic cardiovascular disease–risk 40- to 70-year-olds, provided they are not at increased bleeding risk (J Am Coll Cardiol. 2019 Sep. doi: 10.1016/j.jacc.2019.03.010).
Dr. Mahady reported having no financial conflicts of interest. Dr. Chan serves as a consultant to Bayer Pharma, Janssen, and Pfizer.
in a new analysis from the large randomized ASPREE trial released as part of the annual Digestive Disease Week.
The analysis identified several independent risk factors for major GI bleeding – advanced age, hypertension, obesity, smoking, and chronic kidney disease – according to Suzanne E. Mahady, MBBS, PhD, a gastroenterologist and clinical epidemiologist at Monash University in Melbourne.
“To date, there [are] no comparable data assessing aspirin-related bleeding in older healthy people from a large randomized, controlled trial. Previous data [have] been observational, with variable definitions of significant bleeding, and retrospective. We derived a standard definition for bleeding, used physicians to adjudicate bleeding endpoints, and followed older people for 5 years,” she explained in an interview.
“Our data on bleeding [are] novel,” Dr. Mahady added. “It will help clinicians assess who is most at risk of bleeding with aspirin and target modifiable bleeding risk factors where possible.”
ASPREE was a double-blind trial including 19,114 apparently healthy Australian and American adults age 70 or older, or age 65-plus for blacks and Hispanics in the United States. Participants were randomized to 100 mg/day of enteric-coated aspirin or placebo. At a median 4.7 years of follow-up, there was no between-group difference in major adverse cardiovascular events, a lack of benefit accompanied by a 38% greater risk of major hemorrhage risk and a statistically significant 14% increase in all-cause mortality in the aspirin group. (N Engl J Med. 2018 Oct 18;379[16]:1509-18). The chief contributor to the excess mortality in the aspirin group was their 31% greater risk of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
The new analysis of severe GI bleeding documented an absolute 5-year risk of 0.2% for 70-year-olds and 0.4% in 80-year-olds on aspirin. In 80-year-olds with additional GI bleeding risk factors as identified in the study, the rate reached up to 5.5%. The risk of major upper GI bleeding events was 87% greater in the aspirin group, compared with placebo-treated controls, and the risk of serious lower GI bleeding was increased 36%.
ASPREE coinvestigator Andrew T. Chan, MD, said that the bleeding data should prove useful in future efforts to appropriately weight the risks and benefits of low-dose aspirin treatment.
“We need to better understand how to incorporate bleeding risk in clinical decision making about how to use aspirin among older adults because aspirin has many potential benefits, including prevention of colorectal cancer,” said Dr. Chan, a gastroenterologist and professor of medicine at Harvard Medical School and director for cancer epidemiology at Massachusetts General Hospital, both in Boston.
However, ASPREE has soured cardiologists on the decades-long practice of recommending aspirin for primary prevention of cardiovascular disease in older individuals. In response to the publication of primary outcomes in ASPREE, which was closely bracketed by publication of the largely negative results of the randomized ARRIVE and ASCEND trials in a collective 47,000-plus randomized patients, the American College of Cardiology/American Heart Association clipped aspirin’s role for primary prevention of atherosclerotic cardiovascular disease. The current recommendation is that low-dose aspirin should not be administered on a routine basis for primary cardiovascular prevention in people above age 70, nor in adults at any age at increased bleeding risk, although the practice “might be considered” for primary prevention in select higher atherosclerotic cardiovascular disease–risk 40- to 70-year-olds, provided they are not at increased bleeding risk (J Am Coll Cardiol. 2019 Sep. doi: 10.1016/j.jacc.2019.03.010).
Dr. Mahady reported having no financial conflicts of interest. Dr. Chan serves as a consultant to Bayer Pharma, Janssen, and Pfizer.
FROM DDW 2020
Bariatric surgery in advanced heart failure wins transplant eligibility
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
Bariatric surgery is a safe and effective means for obese patients with advanced heart failure supported by a left ventricular assist device to qualify for heart transplantation, Praneet Wander, MD, reported in an abstract released as part of the annual Digestive Disease Week®.
She presented a systematic review and meta-analysis of nine retrospective or cross-sectional cohort studies totaling
Of the 86 patients, 50 (58%) were able to drop their BMI below 35, a requirement for inclusion on the heart transplant waiting list, noted Dr. Wander, a gastroenterology fellow at Hofstra University, Hempstead, N.Y., and North Shore LIJ Hospital in Manhasset, N.Y.
“A lot of bariatric surgeons don’t feel comfortable operating on patients who have a low ejection fraction,” she explained in an interview. “This study should encourage bariatric surgeons to do procedures even in patients with advanced heart failure so they can meet the BMI requirement for heart transplantation.”
Even if patients don’t actually undergo heart transplantation because of the perpetual donor organ shortage or inability to meet non–BMI-related eligibility criteria, they gain other major benefits from bariatric surgery: Their blood pressure goes down, their diabetes improves, and they become better able to engage in physical activity, she added.
Of the 86 patients in the meta-analysis, 84 underwent laparoscopic sleeve gastrectomy. That’s the preferred bariatric operation in patients with advanced heart failure at the Mayo Clinic as well, according to Andres J. Acosta, MD, PhD, a gastroenterologist at the medical center in Rochester, Minn.
There’s less weight loss achieved than with an open Roux-en-Y gastric bypass, but it’s a simpler operation in these high-risk patients, who typically have multiple comorbid conditions, he explained.
He predicted that Dr. Wander’s study will indeed influence bariatric surgeons at tertiary medical centers around the country to become more willing to consider weight-loss surgery in patients with advanced heart failure, while those in community practice will likely continue to be most comfortable operating on more stable patients with minimal comorbidities aside from their obesity.
“Data such as [these] will be reassuring to bariatric surgery programs such as ours, where we’re able to say: ‘Yes, there are risks, but these patients will benefit in the long term if we assume those risks,’ ” Dr. Acosta said.
He’s confident that, in the near future, the preferred form of bariatric surgery in patients with advanced heart failure will be a minimally invasive procedure performed endoscopically by gastroenterologists. He and his Mayo Clinic colleagues have already established a track record of success with endoscopic sleeve gastrectomy in patients with advanced kidney, liver, or lung disease in order to make them eligible for transplantation, as well as for the ancillary benefits provided by massive weight loss.
“There’s a little less weight loss than with laparoscopic sleeve gastrectomy, but it’s a significantly less risky operation. Shorter operative time, shorter hospital length of stay, less risk of infections and leaks,” he said in an interview. “We haven’t done it yet in heart disease, but I think based on this study this should be the next step at Mayo.”
Radha Gopalan, MD, director of heart failure and transplantation at Banner–University Medical Center in Phoenix, pronounced Dr. Wander’s meta-analysis “a positive study that’s very supportive of what we’re doing at our center.
“At a busy heart transplant center like ours, we are comfortable managing these patients, so the bariatric surgeons are reassured that the heart failure team is behind them. The risk of the procedure is mitigated by the availability of the multidisciplinary team to get the patient with obesity and heart failure through the surgery,” he explained.
Dr. Gopalan heads a novel bariatric heart failure program at Banner. While Dr. Wander’s meta-analysis focused on bariatric surgery in heart failure patients on LVAD circulatory support, Dr. Gopalan and colleagues are moving the intervention upstream. Roughly roughly 80% of patients in his bariatric heart failure program who meet criteria for LVAD implantation are now offered bariatric surgery before an LVAD is put in.
“I am moving away from putting the LVAD in first and then doing bariatric surgery. We have gotten comfortable taking these patients for bariatric surgery with inotropic support before going to the LVAD, which has the potential to even eliminate the requirement for an LVAD. Some patients get so much better that they become transplant ineligible,” Dr. Gopalan said.
Dr. Wander reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Wander P. DDW 2020 Abstract, #Mo2010.
FROM DDW 2020
Blood test detects colon cancer in single-center study
Blood assay studied for colorectal cancer screening.
A blood test detected 11 of 11 cases of colorectal cancer in a study involving 354 patients, and also spotted a majority of cases – 40 out of 53 – in which participants had advanced adenomas, an investigator said.
Results from a single-center study of CellMax Life’s FirstSight blood test were released as a poster as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
For a study conducted at one site, the Veterans Affairs Palo Alto (Calif.) Healthcare System, Shai Friedland, MD, and colleagues recruited 354 patients between ages 45 and 80 who were scheduled for elective colonoscopy. The researchers excluded people with a personal history of cancer or inflammatory bowel disease. They used CellMax’s FirstSight test on blood samples from the study participants.
The FirstSight test result was positive for colorectal cancer in all 11 patients in the study who were found by colonoscopy to have this condition, said Dr. Friedland, who is a professor of medicine at Stanford (Calif.) University and chief of gastroenterology at the VA Palo Alto Healthcare System. Thus, the test showed a sensitivity of 100% in this instance.
Among the 53 study participants found by colonoscopy to have advanced adenoma, 40 were positive on FirstSight; thus, so the test has a sensitivity of 75.5% for this result.
Among 79 patients who had negative colonoscopy results, meaning they were judged free of cancer or polyps, the test showed 8 as having signs of disease or growths.
“If you had a large adenoma that was removed years ago and now you have a negative colonoscopy, your score might still be high,” Dr. Friedland said in a recorded presentation for DDW. “In other words, the changes that are detectable in your blood might persist even after the polypectomy.”
He said there are plans to soon start a large-scale multicenter study of the CellMax assay.
“The blood test has the potential to fill an unmet need by giving patients a highly sensitive convenient option for colorectal cancer screening,” he said.
CellMax already is seeking to position its test as a more convenient alternative to either colonoscopy or the Cologuard screening test. Many patients put off cancer screening because of the need to take time off from work and the invasive nature of colonoscopy. Exact Sciences has used direct-to-consumer advertising to promote its Cologuard home-based test as a more convenient alternative to colonoscopy, but its product requires patients to collect their own stool samples and mail them to a lab, a process many people find off-putting.
Public health advocates, including the U.S. Preventive Services Task Force (USPSTF), have for years been pressing for wider screening of American adults for colon cancer. USPSTF is in the midst of updating its recommendations on colon cancer. In announcing its latest update of these recommendations in 2016, USPSTF said “the best screening test is the one that gets done” (JAMA. 2016;315[23]:2564-75).
USPSTF pressed for maximizing the total proportion of the eligible population, a point Dr. Friedland echoed in a CellMax press release.
“For colon cancer screening to be most effective, it is essential to detect precancerous polyps and then perform a colonoscopy to remove the polyps,” said Dr. Friedland in the CellMax press release. “Giving patients the option of getting a blood test for screening would undoubtedly increase compliance and thereby reduce mortality from colorectal cancer.”
In the DDW presentation, Dr. Friedland and colleagues also said the CellMax test showed greater sensitivity (100%) for colorectal cancer and advanced precancerous lesions (75.5%) than did Cologuard (92.3% for colorectal cancer and 42.4% for advanced precancerous lesions).
Cara Connelly, Director of Public Relations and Corporate Communications for Exact Sciences said that the company “is dedicated to getting more people screened for colorectal cancer and applaud the researchers for their efforts. We look forward to hearing more about the performance of this test in a prospective multisite study with nonsymptomatic patients.”
Naresh T. Gunaratnam, MD, a gastroenterologist and research director at Huron Gastro in Ypsilanti, Mich., said he is concerned that aggressive promotion of alternative tests may obscure the benefits of colonoscopy. Dr. Gunaratnam, a 2019 winner of the American Gastroenterological Association (AGA) Distinguished Clinician Award, has been a public critic of the marketing of colon cancer tests, which emphasize the convenience of these products. When asked by MDedge to comment on the CellMax-funded study, Dr. Gunaratnam said alternative tests do have a place for the care of patients who cannot or will not have a colonoscopy.
“But if you convince a patient who would be willing to have a colonoscopy not to, that’s a disservice,” he said.
“If you want the best test, the one that is best at finding cancers and finding polyps and the only one that can remove the polyp, that’s colonoscopy,” Dr. Gunaratnam added. “One day there may be a pill you can swallow that blows up the polyps, but we’re not there yet. We have to mechanically remove them.”
SOURCE: Friedland S et al. DDW 2020, eposter 575.
Blood assay studied for colorectal cancer screening.
Blood assay studied for colorectal cancer screening.
A blood test detected 11 of 11 cases of colorectal cancer in a study involving 354 patients, and also spotted a majority of cases – 40 out of 53 – in which participants had advanced adenomas, an investigator said.
Results from a single-center study of CellMax Life’s FirstSight blood test were released as a poster as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
For a study conducted at one site, the Veterans Affairs Palo Alto (Calif.) Healthcare System, Shai Friedland, MD, and colleagues recruited 354 patients between ages 45 and 80 who were scheduled for elective colonoscopy. The researchers excluded people with a personal history of cancer or inflammatory bowel disease. They used CellMax’s FirstSight test on blood samples from the study participants.
The FirstSight test result was positive for colorectal cancer in all 11 patients in the study who were found by colonoscopy to have this condition, said Dr. Friedland, who is a professor of medicine at Stanford (Calif.) University and chief of gastroenterology at the VA Palo Alto Healthcare System. Thus, the test showed a sensitivity of 100% in this instance.
Among the 53 study participants found by colonoscopy to have advanced adenoma, 40 were positive on FirstSight; thus, so the test has a sensitivity of 75.5% for this result.
Among 79 patients who had negative colonoscopy results, meaning they were judged free of cancer or polyps, the test showed 8 as having signs of disease or growths.
“If you had a large adenoma that was removed years ago and now you have a negative colonoscopy, your score might still be high,” Dr. Friedland said in a recorded presentation for DDW. “In other words, the changes that are detectable in your blood might persist even after the polypectomy.”
He said there are plans to soon start a large-scale multicenter study of the CellMax assay.
“The blood test has the potential to fill an unmet need by giving patients a highly sensitive convenient option for colorectal cancer screening,” he said.
CellMax already is seeking to position its test as a more convenient alternative to either colonoscopy or the Cologuard screening test. Many patients put off cancer screening because of the need to take time off from work and the invasive nature of colonoscopy. Exact Sciences has used direct-to-consumer advertising to promote its Cologuard home-based test as a more convenient alternative to colonoscopy, but its product requires patients to collect their own stool samples and mail them to a lab, a process many people find off-putting.
Public health advocates, including the U.S. Preventive Services Task Force (USPSTF), have for years been pressing for wider screening of American adults for colon cancer. USPSTF is in the midst of updating its recommendations on colon cancer. In announcing its latest update of these recommendations in 2016, USPSTF said “the best screening test is the one that gets done” (JAMA. 2016;315[23]:2564-75).
USPSTF pressed for maximizing the total proportion of the eligible population, a point Dr. Friedland echoed in a CellMax press release.
“For colon cancer screening to be most effective, it is essential to detect precancerous polyps and then perform a colonoscopy to remove the polyps,” said Dr. Friedland in the CellMax press release. “Giving patients the option of getting a blood test for screening would undoubtedly increase compliance and thereby reduce mortality from colorectal cancer.”
In the DDW presentation, Dr. Friedland and colleagues also said the CellMax test showed greater sensitivity (100%) for colorectal cancer and advanced precancerous lesions (75.5%) than did Cologuard (92.3% for colorectal cancer and 42.4% for advanced precancerous lesions).
Cara Connelly, Director of Public Relations and Corporate Communications for Exact Sciences said that the company “is dedicated to getting more people screened for colorectal cancer and applaud the researchers for their efforts. We look forward to hearing more about the performance of this test in a prospective multisite study with nonsymptomatic patients.”
Naresh T. Gunaratnam, MD, a gastroenterologist and research director at Huron Gastro in Ypsilanti, Mich., said he is concerned that aggressive promotion of alternative tests may obscure the benefits of colonoscopy. Dr. Gunaratnam, a 2019 winner of the American Gastroenterological Association (AGA) Distinguished Clinician Award, has been a public critic of the marketing of colon cancer tests, which emphasize the convenience of these products. When asked by MDedge to comment on the CellMax-funded study, Dr. Gunaratnam said alternative tests do have a place for the care of patients who cannot or will not have a colonoscopy.
“But if you convince a patient who would be willing to have a colonoscopy not to, that’s a disservice,” he said.
“If you want the best test, the one that is best at finding cancers and finding polyps and the only one that can remove the polyp, that’s colonoscopy,” Dr. Gunaratnam added. “One day there may be a pill you can swallow that blows up the polyps, but we’re not there yet. We have to mechanically remove them.”
SOURCE: Friedland S et al. DDW 2020, eposter 575.
A blood test detected 11 of 11 cases of colorectal cancer in a study involving 354 patients, and also spotted a majority of cases – 40 out of 53 – in which participants had advanced adenomas, an investigator said.
Results from a single-center study of CellMax Life’s FirstSight blood test were released as a poster as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
For a study conducted at one site, the Veterans Affairs Palo Alto (Calif.) Healthcare System, Shai Friedland, MD, and colleagues recruited 354 patients between ages 45 and 80 who were scheduled for elective colonoscopy. The researchers excluded people with a personal history of cancer or inflammatory bowel disease. They used CellMax’s FirstSight test on blood samples from the study participants.
The FirstSight test result was positive for colorectal cancer in all 11 patients in the study who were found by colonoscopy to have this condition, said Dr. Friedland, who is a professor of medicine at Stanford (Calif.) University and chief of gastroenterology at the VA Palo Alto Healthcare System. Thus, the test showed a sensitivity of 100% in this instance.
Among the 53 study participants found by colonoscopy to have advanced adenoma, 40 were positive on FirstSight; thus, so the test has a sensitivity of 75.5% for this result.
Among 79 patients who had negative colonoscopy results, meaning they were judged free of cancer or polyps, the test showed 8 as having signs of disease or growths.
“If you had a large adenoma that was removed years ago and now you have a negative colonoscopy, your score might still be high,” Dr. Friedland said in a recorded presentation for DDW. “In other words, the changes that are detectable in your blood might persist even after the polypectomy.”
He said there are plans to soon start a large-scale multicenter study of the CellMax assay.
“The blood test has the potential to fill an unmet need by giving patients a highly sensitive convenient option for colorectal cancer screening,” he said.
CellMax already is seeking to position its test as a more convenient alternative to either colonoscopy or the Cologuard screening test. Many patients put off cancer screening because of the need to take time off from work and the invasive nature of colonoscopy. Exact Sciences has used direct-to-consumer advertising to promote its Cologuard home-based test as a more convenient alternative to colonoscopy, but its product requires patients to collect their own stool samples and mail them to a lab, a process many people find off-putting.
Public health advocates, including the U.S. Preventive Services Task Force (USPSTF), have for years been pressing for wider screening of American adults for colon cancer. USPSTF is in the midst of updating its recommendations on colon cancer. In announcing its latest update of these recommendations in 2016, USPSTF said “the best screening test is the one that gets done” (JAMA. 2016;315[23]:2564-75).
USPSTF pressed for maximizing the total proportion of the eligible population, a point Dr. Friedland echoed in a CellMax press release.
“For colon cancer screening to be most effective, it is essential to detect precancerous polyps and then perform a colonoscopy to remove the polyps,” said Dr. Friedland in the CellMax press release. “Giving patients the option of getting a blood test for screening would undoubtedly increase compliance and thereby reduce mortality from colorectal cancer.”
In the DDW presentation, Dr. Friedland and colleagues also said the CellMax test showed greater sensitivity (100%) for colorectal cancer and advanced precancerous lesions (75.5%) than did Cologuard (92.3% for colorectal cancer and 42.4% for advanced precancerous lesions).
Cara Connelly, Director of Public Relations and Corporate Communications for Exact Sciences said that the company “is dedicated to getting more people screened for colorectal cancer and applaud the researchers for their efforts. We look forward to hearing more about the performance of this test in a prospective multisite study with nonsymptomatic patients.”
Naresh T. Gunaratnam, MD, a gastroenterologist and research director at Huron Gastro in Ypsilanti, Mich., said he is concerned that aggressive promotion of alternative tests may obscure the benefits of colonoscopy. Dr. Gunaratnam, a 2019 winner of the American Gastroenterological Association (AGA) Distinguished Clinician Award, has been a public critic of the marketing of colon cancer tests, which emphasize the convenience of these products. When asked by MDedge to comment on the CellMax-funded study, Dr. Gunaratnam said alternative tests do have a place for the care of patients who cannot or will not have a colonoscopy.
“But if you convince a patient who would be willing to have a colonoscopy not to, that’s a disservice,” he said.
“If you want the best test, the one that is best at finding cancers and finding polyps and the only one that can remove the polyp, that’s colonoscopy,” Dr. Gunaratnam added. “One day there may be a pill you can swallow that blows up the polyps, but we’re not there yet. We have to mechanically remove them.”
SOURCE: Friedland S et al. DDW 2020, eposter 575.
FROM DDW 2020
Sleeve gastrectomy, antiobesity drugs underutilized
Despite an increasing rate of obesity in the United States, sleeve gastrectomy and postoperative antiobesity pharmacotherapy remain significantly underutilized, according to investigators.
A retrospective study involving almost 3 million adults with obesity found that only 0.94% had undergone sleeve gastrectomy, with 5.6% of those receiving weight-loss drugs after discharge, reported lead author Raj Shah, MD, of University Hospitals Cleveland Medical Center, and colleagues.
“While obesity has increased exponentially in the past decade, the trends of bariatric procedures and postoperative pharmacotherapy in this timeline is not well established,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
According to coauthor Abbinaya Elangovan, MD, of MetroHealth Medical Center, Cleveland, existing data suggest a practice gap.
“We know from published studies that antiobesity measures – both surgical and pharmacotherapeutic – do not match the rates of obesity,” Dr. Elangovan said. “We wanted to see how many of the morbidly obese [patients] who get bariatric surgery get started on antiobesity pharmacotherapy. We selected sleeve gastrectomy, as that is the most common bariatric procedure performed in the United States in recent times.”
The investigators began by retrospectively screening 2,717,000 individuals with a body mass index (in kg/m2) of at least 40 who entered the IBM Explorys database from 2010 to 2019. Out of this group, 25,540 individuals (0.94%) had undergone sleeve gastrectomy. Annual rates of the procedure increased from 0.06% in 2010 to 0.4% in 2019 (P < .0001).
Of the 25,540 patients who underwent sleeve gastrectomy, 1,440 (5.6%) were prescribed antiobesity medication after surgery, with about half (47%) of these prescriptions written within a year. The most common medication was phentermine (66%), followed by bupropion/naltrexone (16%) and phentermine/topiramate (14.4%).
Dr. Elangovan said that the rates of surgery and antiobesity pharmacotherapy found in the study were “sparse” compared with rates of obesity.
“[Future studies need] to find the barriers to antiobesity pharmacotherapy,” Dr. Elangovan said. “We know from some of the published studies that there are differences in provider perceptions, as well as patient populations who get the therapy.”
The present analysis showed that women, African Americans, and patients with commercial insurance were significantly more likely to receive postoperative weight-loss medications than other patient subgroups.
“I think insurance could be a potential concern,” Dr. Elangovan said. “This has been shown previously in the literature.” She also suggested that women may be accessing obesity-related health care more often than men.
Discussing steps to improve interventions for patients with obesity, Dr. Elangovan emphasized the amount of data supporting antiobesity pharmacotherapy.
“We know from studies published so far that combining pharmacotherapy with behavioral modifications has a greater percentage of success, compared to behavioral modifications by themselves,” Dr. Elangovan said.
According to Dr. Elangovan, primary care providers play a key role in connecting obese patients with the treatments they need, requiring familiarity with existing guidelines.
“It helps if practicing clinicians, especially primary care providers, are familiar with bariatric surgery criteria and institution policies,” Dr. Elangovan said. “It has been shown in some studies that limited experience in prescribing and concern for adverse reactions could affect the prescription of antiobesity pharmacotherapy. Targeted interventions such as educational programs may increase the appropriate usage of medications.”
Dr. Smith disclosed a relationship with US Endoscopy.
SOURCE: Shah R et al. DDW 2020, Abstract 791.
Despite an increasing rate of obesity in the United States, sleeve gastrectomy and postoperative antiobesity pharmacotherapy remain significantly underutilized, according to investigators.
A retrospective study involving almost 3 million adults with obesity found that only 0.94% had undergone sleeve gastrectomy, with 5.6% of those receiving weight-loss drugs after discharge, reported lead author Raj Shah, MD, of University Hospitals Cleveland Medical Center, and colleagues.
“While obesity has increased exponentially in the past decade, the trends of bariatric procedures and postoperative pharmacotherapy in this timeline is not well established,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
According to coauthor Abbinaya Elangovan, MD, of MetroHealth Medical Center, Cleveland, existing data suggest a practice gap.
“We know from published studies that antiobesity measures – both surgical and pharmacotherapeutic – do not match the rates of obesity,” Dr. Elangovan said. “We wanted to see how many of the morbidly obese [patients] who get bariatric surgery get started on antiobesity pharmacotherapy. We selected sleeve gastrectomy, as that is the most common bariatric procedure performed in the United States in recent times.”
The investigators began by retrospectively screening 2,717,000 individuals with a body mass index (in kg/m2) of at least 40 who entered the IBM Explorys database from 2010 to 2019. Out of this group, 25,540 individuals (0.94%) had undergone sleeve gastrectomy. Annual rates of the procedure increased from 0.06% in 2010 to 0.4% in 2019 (P < .0001).
Of the 25,540 patients who underwent sleeve gastrectomy, 1,440 (5.6%) were prescribed antiobesity medication after surgery, with about half (47%) of these prescriptions written within a year. The most common medication was phentermine (66%), followed by bupropion/naltrexone (16%) and phentermine/topiramate (14.4%).
Dr. Elangovan said that the rates of surgery and antiobesity pharmacotherapy found in the study were “sparse” compared with rates of obesity.
“[Future studies need] to find the barriers to antiobesity pharmacotherapy,” Dr. Elangovan said. “We know from some of the published studies that there are differences in provider perceptions, as well as patient populations who get the therapy.”
The present analysis showed that women, African Americans, and patients with commercial insurance were significantly more likely to receive postoperative weight-loss medications than other patient subgroups.
“I think insurance could be a potential concern,” Dr. Elangovan said. “This has been shown previously in the literature.” She also suggested that women may be accessing obesity-related health care more often than men.
Discussing steps to improve interventions for patients with obesity, Dr. Elangovan emphasized the amount of data supporting antiobesity pharmacotherapy.
“We know from studies published so far that combining pharmacotherapy with behavioral modifications has a greater percentage of success, compared to behavioral modifications by themselves,” Dr. Elangovan said.
According to Dr. Elangovan, primary care providers play a key role in connecting obese patients with the treatments they need, requiring familiarity with existing guidelines.
“It helps if practicing clinicians, especially primary care providers, are familiar with bariatric surgery criteria and institution policies,” Dr. Elangovan said. “It has been shown in some studies that limited experience in prescribing and concern for adverse reactions could affect the prescription of antiobesity pharmacotherapy. Targeted interventions such as educational programs may increase the appropriate usage of medications.”
Dr. Smith disclosed a relationship with US Endoscopy.
SOURCE: Shah R et al. DDW 2020, Abstract 791.
Despite an increasing rate of obesity in the United States, sleeve gastrectomy and postoperative antiobesity pharmacotherapy remain significantly underutilized, according to investigators.
A retrospective study involving almost 3 million adults with obesity found that only 0.94% had undergone sleeve gastrectomy, with 5.6% of those receiving weight-loss drugs after discharge, reported lead author Raj Shah, MD, of University Hospitals Cleveland Medical Center, and colleagues.
“While obesity has increased exponentially in the past decade, the trends of bariatric procedures and postoperative pharmacotherapy in this timeline is not well established,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
According to coauthor Abbinaya Elangovan, MD, of MetroHealth Medical Center, Cleveland, existing data suggest a practice gap.
“We know from published studies that antiobesity measures – both surgical and pharmacotherapeutic – do not match the rates of obesity,” Dr. Elangovan said. “We wanted to see how many of the morbidly obese [patients] who get bariatric surgery get started on antiobesity pharmacotherapy. We selected sleeve gastrectomy, as that is the most common bariatric procedure performed in the United States in recent times.”
The investigators began by retrospectively screening 2,717,000 individuals with a body mass index (in kg/m2) of at least 40 who entered the IBM Explorys database from 2010 to 2019. Out of this group, 25,540 individuals (0.94%) had undergone sleeve gastrectomy. Annual rates of the procedure increased from 0.06% in 2010 to 0.4% in 2019 (P < .0001).
Of the 25,540 patients who underwent sleeve gastrectomy, 1,440 (5.6%) were prescribed antiobesity medication after surgery, with about half (47%) of these prescriptions written within a year. The most common medication was phentermine (66%), followed by bupropion/naltrexone (16%) and phentermine/topiramate (14.4%).
Dr. Elangovan said that the rates of surgery and antiobesity pharmacotherapy found in the study were “sparse” compared with rates of obesity.
“[Future studies need] to find the barriers to antiobesity pharmacotherapy,” Dr. Elangovan said. “We know from some of the published studies that there are differences in provider perceptions, as well as patient populations who get the therapy.”
The present analysis showed that women, African Americans, and patients with commercial insurance were significantly more likely to receive postoperative weight-loss medications than other patient subgroups.
“I think insurance could be a potential concern,” Dr. Elangovan said. “This has been shown previously in the literature.” She also suggested that women may be accessing obesity-related health care more often than men.
Discussing steps to improve interventions for patients with obesity, Dr. Elangovan emphasized the amount of data supporting antiobesity pharmacotherapy.
“We know from studies published so far that combining pharmacotherapy with behavioral modifications has a greater percentage of success, compared to behavioral modifications by themselves,” Dr. Elangovan said.
According to Dr. Elangovan, primary care providers play a key role in connecting obese patients with the treatments they need, requiring familiarity with existing guidelines.
“It helps if practicing clinicians, especially primary care providers, are familiar with bariatric surgery criteria and institution policies,” Dr. Elangovan said. “It has been shown in some studies that limited experience in prescribing and concern for adverse reactions could affect the prescription of antiobesity pharmacotherapy. Targeted interventions such as educational programs may increase the appropriate usage of medications.”
Dr. Smith disclosed a relationship with US Endoscopy.
SOURCE: Shah R et al. DDW 2020, Abstract 791.
FROM DDW 2020
Parental injury, illness linked to increased pediatric GI visits, prescriptions
In a self-controlled case series using records from the Military Health System Data Repository, pediatric visits for disorders linked to gut-brain interactions were found to have increased 9% (incidence rate ratio, 1.09; 95% CI, 1.07-1.10) following a parent’s illness or injury, reported lead author Patrick Short, MD, of the Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview. The Military Health System Data Repository receives records from the Department of Defense’s global network of more than 260 medical facilities as well as outside health care organizations where military families are seen.
A secondary analysis done for this study found children of brain injured parents had 4% more postinjury visits for abdominal pain and 23% increased odds of antispasmodic prescription, compared with children whose parents had other physical injuries, Dr. Short said. He presented his research in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19. The study focused on children aged 3-16 years with a parent who served in the military and was ill or injured between 2004 and 2014. Excluded from this research were records for children with diagnosed systemic or organic gastrointestinal disease, such as celiac disease.
The study used ICD-9 codes to identify outpatient visits for irritable bowel syndrome, abdominal pain, constipation, and fecal incontinence in the 2 years before and after parental injury or diagnosis of illness. Outpatient pharmacy records showed which of the children studied took laxatives and antispasmodics.
Parental injury or illness was defined by the placement of the children’s mothers and fathers on the injured, ill, or wounded file in the data repository. The data file generally covers people with conditions that severely limit their ability to do their usual jobs. These include traumatic brain injury, PTSD, amputation, shrapnel injury, and illnesses such as cancer.
There was a 7% increase in visits for constipation but fecal incontinence did not significantly change following parental illness or injury, Dr. Short said. But the odds of being prescribed an antispasmodic increased 23% following parents’ injuries and serious illnesses, while the odds for laxative prescription decreased by 5%.
The study highlights the potential physical impact of stress on children when families experience a crisis, Dr. Short said in an interview. Children may feel anxious about their parent’s health, while at the same time experiencing unavoidable disruption in family life because of an injury or illness.
“It impacts the day-to-day regimens and routines and decreases the family support,” Dr. Short said. “As humans we are limited in what we have to offer. When we are trying to take care of things on our own, it limits what we can give to people around us.”
The findings of this study should serve to remind physicians to alert parents that their children could experience worsening of GI conditions because of the stress of an ill or injured parent. They then can focus on securing help ahead of the time for the child, such as therapy, he said.
The next step in advancing on the research he prepared for DDW could be testing through prospective studies how well preventive measures such as family counseling work, Dr. Short said.
Dr. Short’s research adds to the growing body of evidence about the brain-gut connection, said Kara Gross Margolis, MD, a spokesperson for the American Gastroenterological Association. An associate professor of pediatrics at Columbia University Medical Center, New York, Dr. Margolis has published research on the brain-gut axis. Her lab focuses on the effects of neurotransmitters and inflammation on enteric nervous system development and function.
Physicians should take a broad view when treating children for functional GI illnesses. Behavioral therapy and antidepressants, for example, have been shown to help children with conditions such as irritable bowel syndrome and other functional gastrointestinal diseases, said Dr. Margolis.
“In a number of these cases, we not only have to treat the gut. We have to treat the brain as well,” Dr. Margolis said.
“When mental health issues are involved that impact the parents of these kids, You have to look at a family as an entire unit,” she added. “You not only treat the child for those symptoms, but you really have to look at how their parents can also be cared for so that their impact on their children will be positive as well.”
Research in the vein explored by Dr. Short will be important to remember as society works through the legacy of the COVID-19 pandemic, Dr. Margolis said. “We have huge numbers of families undergoing tremendous stress due to loss of jobs, health care, medical issues, and parental injury potentially from coronavirus.”
No outside funding was reported, and the study was covered through Uniformed Services University budget.
SOURCE: Short P et al. DDW 2020, Abstract 815.
In a self-controlled case series using records from the Military Health System Data Repository, pediatric visits for disorders linked to gut-brain interactions were found to have increased 9% (incidence rate ratio, 1.09; 95% CI, 1.07-1.10) following a parent’s illness or injury, reported lead author Patrick Short, MD, of the Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview. The Military Health System Data Repository receives records from the Department of Defense’s global network of more than 260 medical facilities as well as outside health care organizations where military families are seen.
A secondary analysis done for this study found children of brain injured parents had 4% more postinjury visits for abdominal pain and 23% increased odds of antispasmodic prescription, compared with children whose parents had other physical injuries, Dr. Short said. He presented his research in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19. The study focused on children aged 3-16 years with a parent who served in the military and was ill or injured between 2004 and 2014. Excluded from this research were records for children with diagnosed systemic or organic gastrointestinal disease, such as celiac disease.
The study used ICD-9 codes to identify outpatient visits for irritable bowel syndrome, abdominal pain, constipation, and fecal incontinence in the 2 years before and after parental injury or diagnosis of illness. Outpatient pharmacy records showed which of the children studied took laxatives and antispasmodics.
Parental injury or illness was defined by the placement of the children’s mothers and fathers on the injured, ill, or wounded file in the data repository. The data file generally covers people with conditions that severely limit their ability to do their usual jobs. These include traumatic brain injury, PTSD, amputation, shrapnel injury, and illnesses such as cancer.
There was a 7% increase in visits for constipation but fecal incontinence did not significantly change following parental illness or injury, Dr. Short said. But the odds of being prescribed an antispasmodic increased 23% following parents’ injuries and serious illnesses, while the odds for laxative prescription decreased by 5%.
The study highlights the potential physical impact of stress on children when families experience a crisis, Dr. Short said in an interview. Children may feel anxious about their parent’s health, while at the same time experiencing unavoidable disruption in family life because of an injury or illness.
“It impacts the day-to-day regimens and routines and decreases the family support,” Dr. Short said. “As humans we are limited in what we have to offer. When we are trying to take care of things on our own, it limits what we can give to people around us.”
The findings of this study should serve to remind physicians to alert parents that their children could experience worsening of GI conditions because of the stress of an ill or injured parent. They then can focus on securing help ahead of the time for the child, such as therapy, he said.
The next step in advancing on the research he prepared for DDW could be testing through prospective studies how well preventive measures such as family counseling work, Dr. Short said.
Dr. Short’s research adds to the growing body of evidence about the brain-gut connection, said Kara Gross Margolis, MD, a spokesperson for the American Gastroenterological Association. An associate professor of pediatrics at Columbia University Medical Center, New York, Dr. Margolis has published research on the brain-gut axis. Her lab focuses on the effects of neurotransmitters and inflammation on enteric nervous system development and function.
Physicians should take a broad view when treating children for functional GI illnesses. Behavioral therapy and antidepressants, for example, have been shown to help children with conditions such as irritable bowel syndrome and other functional gastrointestinal diseases, said Dr. Margolis.
“In a number of these cases, we not only have to treat the gut. We have to treat the brain as well,” Dr. Margolis said.
“When mental health issues are involved that impact the parents of these kids, You have to look at a family as an entire unit,” she added. “You not only treat the child for those symptoms, but you really have to look at how their parents can also be cared for so that their impact on their children will be positive as well.”
Research in the vein explored by Dr. Short will be important to remember as society works through the legacy of the COVID-19 pandemic, Dr. Margolis said. “We have huge numbers of families undergoing tremendous stress due to loss of jobs, health care, medical issues, and parental injury potentially from coronavirus.”
No outside funding was reported, and the study was covered through Uniformed Services University budget.
SOURCE: Short P et al. DDW 2020, Abstract 815.
In a self-controlled case series using records from the Military Health System Data Repository, pediatric visits for disorders linked to gut-brain interactions were found to have increased 9% (incidence rate ratio, 1.09; 95% CI, 1.07-1.10) following a parent’s illness or injury, reported lead author Patrick Short, MD, of the Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview. The Military Health System Data Repository receives records from the Department of Defense’s global network of more than 260 medical facilities as well as outside health care organizations where military families are seen.
A secondary analysis done for this study found children of brain injured parents had 4% more postinjury visits for abdominal pain and 23% increased odds of antispasmodic prescription, compared with children whose parents had other physical injuries, Dr. Short said. He presented his research in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19. The study focused on children aged 3-16 years with a parent who served in the military and was ill or injured between 2004 and 2014. Excluded from this research were records for children with diagnosed systemic or organic gastrointestinal disease, such as celiac disease.
The study used ICD-9 codes to identify outpatient visits for irritable bowel syndrome, abdominal pain, constipation, and fecal incontinence in the 2 years before and after parental injury or diagnosis of illness. Outpatient pharmacy records showed which of the children studied took laxatives and antispasmodics.
Parental injury or illness was defined by the placement of the children’s mothers and fathers on the injured, ill, or wounded file in the data repository. The data file generally covers people with conditions that severely limit their ability to do their usual jobs. These include traumatic brain injury, PTSD, amputation, shrapnel injury, and illnesses such as cancer.
There was a 7% increase in visits for constipation but fecal incontinence did not significantly change following parental illness or injury, Dr. Short said. But the odds of being prescribed an antispasmodic increased 23% following parents’ injuries and serious illnesses, while the odds for laxative prescription decreased by 5%.
The study highlights the potential physical impact of stress on children when families experience a crisis, Dr. Short said in an interview. Children may feel anxious about their parent’s health, while at the same time experiencing unavoidable disruption in family life because of an injury or illness.
“It impacts the day-to-day regimens and routines and decreases the family support,” Dr. Short said. “As humans we are limited in what we have to offer. When we are trying to take care of things on our own, it limits what we can give to people around us.”
The findings of this study should serve to remind physicians to alert parents that their children could experience worsening of GI conditions because of the stress of an ill or injured parent. They then can focus on securing help ahead of the time for the child, such as therapy, he said.
The next step in advancing on the research he prepared for DDW could be testing through prospective studies how well preventive measures such as family counseling work, Dr. Short said.
Dr. Short’s research adds to the growing body of evidence about the brain-gut connection, said Kara Gross Margolis, MD, a spokesperson for the American Gastroenterological Association. An associate professor of pediatrics at Columbia University Medical Center, New York, Dr. Margolis has published research on the brain-gut axis. Her lab focuses on the effects of neurotransmitters and inflammation on enteric nervous system development and function.
Physicians should take a broad view when treating children for functional GI illnesses. Behavioral therapy and antidepressants, for example, have been shown to help children with conditions such as irritable bowel syndrome and other functional gastrointestinal diseases, said Dr. Margolis.
“In a number of these cases, we not only have to treat the gut. We have to treat the brain as well,” Dr. Margolis said.
“When mental health issues are involved that impact the parents of these kids, You have to look at a family as an entire unit,” she added. “You not only treat the child for those symptoms, but you really have to look at how their parents can also be cared for so that their impact on their children will be positive as well.”
Research in the vein explored by Dr. Short will be important to remember as society works through the legacy of the COVID-19 pandemic, Dr. Margolis said. “We have huge numbers of families undergoing tremendous stress due to loss of jobs, health care, medical issues, and parental injury potentially from coronavirus.”
No outside funding was reported, and the study was covered through Uniformed Services University budget.
SOURCE: Short P et al. DDW 2020, Abstract 815.
FROM DDW 2020
Postcolonoscopy antibiotics linked with IBS
Antibiotic exposure within 14 days after screening colonoscopy may increase risk of irritable bowel syndrome (IBS), based on a retrospective analysis of more than 400,000 individuals.
Antibiotic use in the 2 weeks leading up to colonoscopy also trended toward an association with IBS, reported lead author Ravy Vajravelu, MD, of University of Pennsylvania, Philadelphia, and colleagues.
“Laboratory studies in mice have demonstrated that colon cleansing in conjunction with systemic antibiotic use can cause persistent intestinal dysbiosis,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19. “Because perturbation of the gut microbiome is thought to be a trigger for the development of IBS, we sought to assess whether humans who undergo bowel cleanse for colonoscopy in conjunction with antibiotic exposure develop new-onset IBS or IBS-related symptoms.”
According to Dr. Vajravelu, previous human studies have shown that bowel cleansing or antibiotics can alter the baseline gut microbiome, but no previous human research explored the impact of both triggers at once.
The present study involved individuals 50 years or older from the OptumInsight Clinformatics database who underwent screening colonoscopy between 2000 and 2016. Those with preexisting gastrointestinal conditions or symptoms within 180 days leading up to colonoscopy were excluded, leaving 402,259 individuals in the final cohort. From this group, individuals were identified who had exposure to antibiotics within 14 days before and/or after colonoscopy.
The primary outcome was a diagnosis of IBS in the 180 days following the antibiotic exposure window. Secondary outcomes included newly diagnosed diarrhea, change in bowel habits, and abdominal pain. A variety of covariates were tested through multivariable logistical regression, including gastrointestinal infections, medical comorbidities, and demographic factors, with only sex and age remaining in the final model.
Across the cohort, 2% of patients received antibiotics either before or after colonoscopy, while 1% had exposure both before and after. A total of 1,002 individuals (0.2%) were diagnosed with IBS within a median time frame of 112 days.
Multivariate analysis revealed that individuals exposed to antibiotics in the 14 days following colonoscopy had a 77% increased risk of developing IBS (adjusted odds ratio, 1.77; 95% confidence interval, 1.31-2.39). To a lesser degree, and not quite achieving statistical significance, trends toward an association were found for antibiotic exposure before colonoscopy (aOR, 1.38; 95% CI, 0.99-1.92), and for antibiotic exposure both before and after colonoscopy (aOR, 1.41; 95% CI, 0.97-2.04).
Dr. Vajravelu said that these preliminary findings are currently undergoing further analysis.
“In particular, we are interested in determining whether antibiotics that target gram-negative bacteria, which are abundant in the gut, have a greater association with subsequent IBS,” Dr. Vajravelu said.
In addition, they are taking steps to eliminate other confounding factors.
“The main objective of these new analyses is to ensure that the association between bowel cleanse and antibiotics with subsequent IBS is not related to the reasons antibiotics were prescribed initially,” Dr. Vajravelu said. “For example, someone experiencing diarrhea could receive a trial of empiric antibiotics and then receive a colonoscopy when the diarrhea does not resolve. In [the present analysis], we avoided including individuals like this by including only those who underwent screening colonoscopy, and therefore did not have any prior documented GI symptoms. In our [ongoing] analyses, we are including additional restrictions to strengthen the findings.”
If the findings do hold, Dr. Vajravelu suggested that they may have clinical implications.
“[I]t may be important to review whether patients scheduled for colonoscopy have received recent antibiotics and warn them to avoid antibiotics after colonoscopy, if possible,” Dr. Vajravelu said. “Additionally, for gastroenterologists, these data may underscore the importance of adhering to preprocedural antibiotic prophylaxis guidelines put forth by GI societies.”The investigators disclosed relationships with Merck, Pfizer, Gilead, and others.
SOURCE: Vajravelu R et al. DDW 2020. Abstract 404.
Antibiotic exposure within 14 days after screening colonoscopy may increase risk of irritable bowel syndrome (IBS), based on a retrospective analysis of more than 400,000 individuals.
Antibiotic use in the 2 weeks leading up to colonoscopy also trended toward an association with IBS, reported lead author Ravy Vajravelu, MD, of University of Pennsylvania, Philadelphia, and colleagues.
“Laboratory studies in mice have demonstrated that colon cleansing in conjunction with systemic antibiotic use can cause persistent intestinal dysbiosis,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19. “Because perturbation of the gut microbiome is thought to be a trigger for the development of IBS, we sought to assess whether humans who undergo bowel cleanse for colonoscopy in conjunction with antibiotic exposure develop new-onset IBS or IBS-related symptoms.”
According to Dr. Vajravelu, previous human studies have shown that bowel cleansing or antibiotics can alter the baseline gut microbiome, but no previous human research explored the impact of both triggers at once.
The present study involved individuals 50 years or older from the OptumInsight Clinformatics database who underwent screening colonoscopy between 2000 and 2016. Those with preexisting gastrointestinal conditions or symptoms within 180 days leading up to colonoscopy were excluded, leaving 402,259 individuals in the final cohort. From this group, individuals were identified who had exposure to antibiotics within 14 days before and/or after colonoscopy.
The primary outcome was a diagnosis of IBS in the 180 days following the antibiotic exposure window. Secondary outcomes included newly diagnosed diarrhea, change in bowel habits, and abdominal pain. A variety of covariates were tested through multivariable logistical regression, including gastrointestinal infections, medical comorbidities, and demographic factors, with only sex and age remaining in the final model.
Across the cohort, 2% of patients received antibiotics either before or after colonoscopy, while 1% had exposure both before and after. A total of 1,002 individuals (0.2%) were diagnosed with IBS within a median time frame of 112 days.
Multivariate analysis revealed that individuals exposed to antibiotics in the 14 days following colonoscopy had a 77% increased risk of developing IBS (adjusted odds ratio, 1.77; 95% confidence interval, 1.31-2.39). To a lesser degree, and not quite achieving statistical significance, trends toward an association were found for antibiotic exposure before colonoscopy (aOR, 1.38; 95% CI, 0.99-1.92), and for antibiotic exposure both before and after colonoscopy (aOR, 1.41; 95% CI, 0.97-2.04).
Dr. Vajravelu said that these preliminary findings are currently undergoing further analysis.
“In particular, we are interested in determining whether antibiotics that target gram-negative bacteria, which are abundant in the gut, have a greater association with subsequent IBS,” Dr. Vajravelu said.
In addition, they are taking steps to eliminate other confounding factors.
“The main objective of these new analyses is to ensure that the association between bowel cleanse and antibiotics with subsequent IBS is not related to the reasons antibiotics were prescribed initially,” Dr. Vajravelu said. “For example, someone experiencing diarrhea could receive a trial of empiric antibiotics and then receive a colonoscopy when the diarrhea does not resolve. In [the present analysis], we avoided including individuals like this by including only those who underwent screening colonoscopy, and therefore did not have any prior documented GI symptoms. In our [ongoing] analyses, we are including additional restrictions to strengthen the findings.”
If the findings do hold, Dr. Vajravelu suggested that they may have clinical implications.
“[I]t may be important to review whether patients scheduled for colonoscopy have received recent antibiotics and warn them to avoid antibiotics after colonoscopy, if possible,” Dr. Vajravelu said. “Additionally, for gastroenterologists, these data may underscore the importance of adhering to preprocedural antibiotic prophylaxis guidelines put forth by GI societies.”The investigators disclosed relationships with Merck, Pfizer, Gilead, and others.
SOURCE: Vajravelu R et al. DDW 2020. Abstract 404.
Antibiotic exposure within 14 days after screening colonoscopy may increase risk of irritable bowel syndrome (IBS), based on a retrospective analysis of more than 400,000 individuals.
Antibiotic use in the 2 weeks leading up to colonoscopy also trended toward an association with IBS, reported lead author Ravy Vajravelu, MD, of University of Pennsylvania, Philadelphia, and colleagues.
“Laboratory studies in mice have demonstrated that colon cleansing in conjunction with systemic antibiotic use can cause persistent intestinal dysbiosis,” the investigators wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19. “Because perturbation of the gut microbiome is thought to be a trigger for the development of IBS, we sought to assess whether humans who undergo bowel cleanse for colonoscopy in conjunction with antibiotic exposure develop new-onset IBS or IBS-related symptoms.”
According to Dr. Vajravelu, previous human studies have shown that bowel cleansing or antibiotics can alter the baseline gut microbiome, but no previous human research explored the impact of both triggers at once.
The present study involved individuals 50 years or older from the OptumInsight Clinformatics database who underwent screening colonoscopy between 2000 and 2016. Those with preexisting gastrointestinal conditions or symptoms within 180 days leading up to colonoscopy were excluded, leaving 402,259 individuals in the final cohort. From this group, individuals were identified who had exposure to antibiotics within 14 days before and/or after colonoscopy.
The primary outcome was a diagnosis of IBS in the 180 days following the antibiotic exposure window. Secondary outcomes included newly diagnosed diarrhea, change in bowel habits, and abdominal pain. A variety of covariates were tested through multivariable logistical regression, including gastrointestinal infections, medical comorbidities, and demographic factors, with only sex and age remaining in the final model.
Across the cohort, 2% of patients received antibiotics either before or after colonoscopy, while 1% had exposure both before and after. A total of 1,002 individuals (0.2%) were diagnosed with IBS within a median time frame of 112 days.
Multivariate analysis revealed that individuals exposed to antibiotics in the 14 days following colonoscopy had a 77% increased risk of developing IBS (adjusted odds ratio, 1.77; 95% confidence interval, 1.31-2.39). To a lesser degree, and not quite achieving statistical significance, trends toward an association were found for antibiotic exposure before colonoscopy (aOR, 1.38; 95% CI, 0.99-1.92), and for antibiotic exposure both before and after colonoscopy (aOR, 1.41; 95% CI, 0.97-2.04).
Dr. Vajravelu said that these preliminary findings are currently undergoing further analysis.
“In particular, we are interested in determining whether antibiotics that target gram-negative bacteria, which are abundant in the gut, have a greater association with subsequent IBS,” Dr. Vajravelu said.
In addition, they are taking steps to eliminate other confounding factors.
“The main objective of these new analyses is to ensure that the association between bowel cleanse and antibiotics with subsequent IBS is not related to the reasons antibiotics were prescribed initially,” Dr. Vajravelu said. “For example, someone experiencing diarrhea could receive a trial of empiric antibiotics and then receive a colonoscopy when the diarrhea does not resolve. In [the present analysis], we avoided including individuals like this by including only those who underwent screening colonoscopy, and therefore did not have any prior documented GI symptoms. In our [ongoing] analyses, we are including additional restrictions to strengthen the findings.”
If the findings do hold, Dr. Vajravelu suggested that they may have clinical implications.
“[I]t may be important to review whether patients scheduled for colonoscopy have received recent antibiotics and warn them to avoid antibiotics after colonoscopy, if possible,” Dr. Vajravelu said. “Additionally, for gastroenterologists, these data may underscore the importance of adhering to preprocedural antibiotic prophylaxis guidelines put forth by GI societies.”The investigators disclosed relationships with Merck, Pfizer, Gilead, and others.
SOURCE: Vajravelu R et al. DDW 2020. Abstract 404.
FROM DDW 2020
Too much or too little sleep spikes constipation
Individuals who sleep more or less than average report significantly more constipation, compared with normal sleepers, based on data from 14,590 adults.
“Normal sleep duration is thought to be essential for healthy bowel function; however, the effect of either limited or excessive sleep duration on bowel patterns is poorly understood,” Adeyinka Adejumo, MD, of North Shore Medical Center, Salem, Mass., and colleagues wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
To examine the association between sleep duration and bowel function, the researchers identified 14,590 adults aged 20 years and older who completed questionnaires on sleep and bowel health as part of the National Health and Nutrition Examination Survey (NHANES) during 2005-2010.
Sleep was divided into three categories based on standards from the National Sleep Foundation: short (less than 7 hours), normal (7-8 hours) and long (more than 8 hours).
Overall, constipation rates were significantly lower among normal sleepers (8.3%) compared with both short and long sleepers (11.0% and 12.5%, respectively; P < .0001 for both).
Bowel function was defined as normal, constipation, or diarrhea based on stool form and bowel movements per week. After controlling for demographic, lifestyle, and dietary factors, long sleepers and short sleepers were 61% and 38% more likely, respectively, to report constipation, compared with normal sleepers.
However, sleep duration was not related to diarrhea, the researchers noted. In addition, “A sensitivity analysis revealed that sleep duration did not mediate the relationship between comorbid factors (such as overall health, poverty index, obesity, and body mass index) and constipation,” they wrote.
The results suggest that decreased sleep is associated with constipation among adults in the United States, the researchers said. However, “further studies are needed to evaluate the physiologic mechanisms driving the impact of sleep duration on bowel function to determine whether sleep disorders or their underlying causes affect constipation,” they concluded.
“This study was necessary because up to 50% of Americans suffer from sleep disorders, out of which abnormal sleep duration is one of the most common and underdiagnosed, and associated with other diseases such as hypertension and diabetes,” Dr. Adejumo said in an interview. “However, disorders of bowel function (constipation and diarrhea), which affect almost 10%-15% of the population and result in significant health care burden, such as higher cost, hospital visits, abdominal discomfort, have not been studied among individuals with suboptimal sleep duration.”
Dr. Adejumo said he and his colleagues were surprised by their findings. “Although, based on our hypothesis, we thought that sleeping too long may be associated with constipation, we were shocked to note similar results among people who also sleep for short durations,” he noted.
“Previous studies had suggested that bowel contraction slows down considerably during sleep. It, therefore, will make sense that sleeping for too long may result in suppressed bowel motility and decreased bowel movement,” he said. “However, our results showed similar findings among short sleepers. We do not know the exact mechanism of these results. It may be that short sleep resulted in inadequate bowel rest, bowel muscle fatigue, and, subsequently, decreased bowel movement,” said Dr. Adejumo. “Or it may also be that brain-gut signaling pathways are disrupted among short sleepers, as is seen among IBS patients after a poor night sleep, resulting in higher constipation,” he added.
Clinicians should be aware of the impact of both short and long sleep on constipation, said Dr. Adejumo. “Individuals who are unable to have adequate periods of sleep due to other diseases, including insomnia, disrupted job schedules, or conditions with too long sleep, such as narcolepsy, may all additionally suffer from constipation. Such patients may need regular evaluation and treatment for constipation to improve their discomfort,” he said.
“To confirm our findings, other clinical studies with more granular data on sleep and constipation, are needed, as well as translational research to uncover the potential mechanisms of these findings,” he emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Adejumo A et al. DDW 2020. Abstract Sa1711.
Individuals who sleep more or less than average report significantly more constipation, compared with normal sleepers, based on data from 14,590 adults.
“Normal sleep duration is thought to be essential for healthy bowel function; however, the effect of either limited or excessive sleep duration on bowel patterns is poorly understood,” Adeyinka Adejumo, MD, of North Shore Medical Center, Salem, Mass., and colleagues wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
To examine the association between sleep duration and bowel function, the researchers identified 14,590 adults aged 20 years and older who completed questionnaires on sleep and bowel health as part of the National Health and Nutrition Examination Survey (NHANES) during 2005-2010.
Sleep was divided into three categories based on standards from the National Sleep Foundation: short (less than 7 hours), normal (7-8 hours) and long (more than 8 hours).
Overall, constipation rates were significantly lower among normal sleepers (8.3%) compared with both short and long sleepers (11.0% and 12.5%, respectively; P < .0001 for both).
Bowel function was defined as normal, constipation, or diarrhea based on stool form and bowel movements per week. After controlling for demographic, lifestyle, and dietary factors, long sleepers and short sleepers were 61% and 38% more likely, respectively, to report constipation, compared with normal sleepers.
However, sleep duration was not related to diarrhea, the researchers noted. In addition, “A sensitivity analysis revealed that sleep duration did not mediate the relationship between comorbid factors (such as overall health, poverty index, obesity, and body mass index) and constipation,” they wrote.
The results suggest that decreased sleep is associated with constipation among adults in the United States, the researchers said. However, “further studies are needed to evaluate the physiologic mechanisms driving the impact of sleep duration on bowel function to determine whether sleep disorders or their underlying causes affect constipation,” they concluded.
“This study was necessary because up to 50% of Americans suffer from sleep disorders, out of which abnormal sleep duration is one of the most common and underdiagnosed, and associated with other diseases such as hypertension and diabetes,” Dr. Adejumo said in an interview. “However, disorders of bowel function (constipation and diarrhea), which affect almost 10%-15% of the population and result in significant health care burden, such as higher cost, hospital visits, abdominal discomfort, have not been studied among individuals with suboptimal sleep duration.”
Dr. Adejumo said he and his colleagues were surprised by their findings. “Although, based on our hypothesis, we thought that sleeping too long may be associated with constipation, we were shocked to note similar results among people who also sleep for short durations,” he noted.
“Previous studies had suggested that bowel contraction slows down considerably during sleep. It, therefore, will make sense that sleeping for too long may result in suppressed bowel motility and decreased bowel movement,” he said. “However, our results showed similar findings among short sleepers. We do not know the exact mechanism of these results. It may be that short sleep resulted in inadequate bowel rest, bowel muscle fatigue, and, subsequently, decreased bowel movement,” said Dr. Adejumo. “Or it may also be that brain-gut signaling pathways are disrupted among short sleepers, as is seen among IBS patients after a poor night sleep, resulting in higher constipation,” he added.
Clinicians should be aware of the impact of both short and long sleep on constipation, said Dr. Adejumo. “Individuals who are unable to have adequate periods of sleep due to other diseases, including insomnia, disrupted job schedules, or conditions with too long sleep, such as narcolepsy, may all additionally suffer from constipation. Such patients may need regular evaluation and treatment for constipation to improve their discomfort,” he said.
“To confirm our findings, other clinical studies with more granular data on sleep and constipation, are needed, as well as translational research to uncover the potential mechanisms of these findings,” he emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Adejumo A et al. DDW 2020. Abstract Sa1711.
Individuals who sleep more or less than average report significantly more constipation, compared with normal sleepers, based on data from 14,590 adults.
“Normal sleep duration is thought to be essential for healthy bowel function; however, the effect of either limited or excessive sleep duration on bowel patterns is poorly understood,” Adeyinka Adejumo, MD, of North Shore Medical Center, Salem, Mass., and colleagues wrote in an abstract released as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
To examine the association between sleep duration and bowel function, the researchers identified 14,590 adults aged 20 years and older who completed questionnaires on sleep and bowel health as part of the National Health and Nutrition Examination Survey (NHANES) during 2005-2010.
Sleep was divided into three categories based on standards from the National Sleep Foundation: short (less than 7 hours), normal (7-8 hours) and long (more than 8 hours).
Overall, constipation rates were significantly lower among normal sleepers (8.3%) compared with both short and long sleepers (11.0% and 12.5%, respectively; P < .0001 for both).
Bowel function was defined as normal, constipation, or diarrhea based on stool form and bowel movements per week. After controlling for demographic, lifestyle, and dietary factors, long sleepers and short sleepers were 61% and 38% more likely, respectively, to report constipation, compared with normal sleepers.
However, sleep duration was not related to diarrhea, the researchers noted. In addition, “A sensitivity analysis revealed that sleep duration did not mediate the relationship between comorbid factors (such as overall health, poverty index, obesity, and body mass index) and constipation,” they wrote.
The results suggest that decreased sleep is associated with constipation among adults in the United States, the researchers said. However, “further studies are needed to evaluate the physiologic mechanisms driving the impact of sleep duration on bowel function to determine whether sleep disorders or their underlying causes affect constipation,” they concluded.
“This study was necessary because up to 50% of Americans suffer from sleep disorders, out of which abnormal sleep duration is one of the most common and underdiagnosed, and associated with other diseases such as hypertension and diabetes,” Dr. Adejumo said in an interview. “However, disorders of bowel function (constipation and diarrhea), which affect almost 10%-15% of the population and result in significant health care burden, such as higher cost, hospital visits, abdominal discomfort, have not been studied among individuals with suboptimal sleep duration.”
Dr. Adejumo said he and his colleagues were surprised by their findings. “Although, based on our hypothesis, we thought that sleeping too long may be associated with constipation, we were shocked to note similar results among people who also sleep for short durations,” he noted.
“Previous studies had suggested that bowel contraction slows down considerably during sleep. It, therefore, will make sense that sleeping for too long may result in suppressed bowel motility and decreased bowel movement,” he said. “However, our results showed similar findings among short sleepers. We do not know the exact mechanism of these results. It may be that short sleep resulted in inadequate bowel rest, bowel muscle fatigue, and, subsequently, decreased bowel movement,” said Dr. Adejumo. “Or it may also be that brain-gut signaling pathways are disrupted among short sleepers, as is seen among IBS patients after a poor night sleep, resulting in higher constipation,” he added.
Clinicians should be aware of the impact of both short and long sleep on constipation, said Dr. Adejumo. “Individuals who are unable to have adequate periods of sleep due to other diseases, including insomnia, disrupted job schedules, or conditions with too long sleep, such as narcolepsy, may all additionally suffer from constipation. Such patients may need regular evaluation and treatment for constipation to improve their discomfort,” he said.
“To confirm our findings, other clinical studies with more granular data on sleep and constipation, are needed, as well as translational research to uncover the potential mechanisms of these findings,” he emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Adejumo A et al. DDW 2020. Abstract Sa1711.
FROM DDW 2020
Key clinical point: Both short sleepers and long sleepers reported significantly more constipation compared with normal sleepers.
Major finding: Overall constipation rates were 8.3% in normal sleepers, compared with 11.0% for short sleepers and 12.5% for long sleepers.
Study details: The data come from 14,590 adults who participated in NHANES between 2005 and 2010.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Adejumo A et al. DDW 2020. Abstract Sa1711.
Postpropofol driving poses low risk to endoscopy patients
Adults given propofol as part of an elective outpatient endoscopy procedure showed similar driving skills after postsedation recovery and prior to the procedure, based on simulation data from an open-label study of 41 patients.
Although current guidelines recommend that patients refrain from driving for 24 hours after propofol sedation and be accompanied by a responsible adult, data on the driving skills of patients after postsedation recovery are limited, Pooja Lal, MD, of the Cleveland Clinic Foundation and colleagues wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“We assessed psychomotor recovery using a driving simulator which mimics real-life driving in outpatients undergoing gastrointestinal endoscopy with propofol,” they wrote.
The researchers enrolled 41 outpatients who were given propofol for various elective procedures at an endoscopy unit of a single center. Patients’ driving skills were tested at baseline and after postsedation recovery using a driving simulator. Postsedation recovery was defined as Aldrete score of 9 in the recovery room. Patients were excluded from the study if they demonstrated altered mental status of any type including dementia, delirium, and hepatic encephalopathy; if they were legally blind; or were currently inpatients.
Overall, driving skills were not significantly different between preprocedure and postsedation recovery on measures of number of times over the speed limit (3.2 vs. 3.4), number of times drivers went off the road (0.37 vs. 0.54), and total pedal reaction time (6.1 seconds vs. 7.6 seconds).
“The two variables including gas pedal reaction time and the total number of collisions did not follow normal distribution,” with medians of 0.70 for gas pedal reaction time in both groups and medians of 0 collisions for both groups, the investigators noted.
The study findings were limited by the small sample size and open-label design. However, the results suggest that driving skills were similar for patients at baseline and after achieving a postsedation Aldrete score of 9, the researchers wrote. Based on these findings, “current recommendations that patients should refrain from driving and unescorted use of public transport for 24 hours after sedation may need to be reconsidered in patients who receive propofol sedation.”
“With this study, we are aiming to identify the correct patient population who could potentially drive themselves home after their procedure rather than having to arrange for transportation,” Dr. Lal said in an interview.
“The significant cost associated with the daylong interruption of the activities of daily living, having a family member accompany them for their procedures, or not being able to use public transport unescorted may deter some patients from complying with colonoscopy screenings and other important endoscopic procedures,” she explained.
“There are patients who arrive for their procedure without any accompanying family members and we have to admit them to an observation unit overnight or reschedule their procedures. The expense and inconvenience associated with the extended recovery potentially could be an impediment to many important screening exams,” she noted. “In the setting of increased awareness regarding the need for these screening exams, this study is important to highlight these barriers and propose a solution for them.”
The potential costs of lost salaries is extremely high, she said. “If we assume that the 24-hour recovery period necessitates taking a day off from work, the value of lost salary per patient would be $183.68 as per the 2019 national hourly average wage. With an estimated 19 million colonoscopies performed annually in the United States, the aggregate cost of lost wages with the 24-hour guideline would be $3.5 billion. This amount does not account for the lost wages of the accompanying family member.”
Dr. Lal said she and her colleagues were not surprised by the findings. “The endoscopists in our endoscopy unit have noted that patients recover much more rapidly after sedation with propofol compared with other sedative agents. This observation led to the hypothesis that those patients receiving propofol should have a speedy psychomotor recovery and should be able to drive the same day. Our findings are in accordance with our observations.” However, “larger and preferably multicenter studies are needed to validate these findings and add to our knowledge about the postsedation psychomotor recovery,” said Dr. Lal. She added that the study is ongoing and the researchers have collected data from a total of 63 patients, which increases the power of the results.
The researchers had no financial conflicts to disclose.
*This story was updated on 5/8/2020.
SOURCE: Lal P et al. DDW 2020, Abstract 295.
Adults given propofol as part of an elective outpatient endoscopy procedure showed similar driving skills after postsedation recovery and prior to the procedure, based on simulation data from an open-label study of 41 patients.
Although current guidelines recommend that patients refrain from driving for 24 hours after propofol sedation and be accompanied by a responsible adult, data on the driving skills of patients after postsedation recovery are limited, Pooja Lal, MD, of the Cleveland Clinic Foundation and colleagues wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“We assessed psychomotor recovery using a driving simulator which mimics real-life driving in outpatients undergoing gastrointestinal endoscopy with propofol,” they wrote.
The researchers enrolled 41 outpatients who were given propofol for various elective procedures at an endoscopy unit of a single center. Patients’ driving skills were tested at baseline and after postsedation recovery using a driving simulator. Postsedation recovery was defined as Aldrete score of 9 in the recovery room. Patients were excluded from the study if they demonstrated altered mental status of any type including dementia, delirium, and hepatic encephalopathy; if they were legally blind; or were currently inpatients.
Overall, driving skills were not significantly different between preprocedure and postsedation recovery on measures of number of times over the speed limit (3.2 vs. 3.4), number of times drivers went off the road (0.37 vs. 0.54), and total pedal reaction time (6.1 seconds vs. 7.6 seconds).
“The two variables including gas pedal reaction time and the total number of collisions did not follow normal distribution,” with medians of 0.70 for gas pedal reaction time in both groups and medians of 0 collisions for both groups, the investigators noted.
The study findings were limited by the small sample size and open-label design. However, the results suggest that driving skills were similar for patients at baseline and after achieving a postsedation Aldrete score of 9, the researchers wrote. Based on these findings, “current recommendations that patients should refrain from driving and unescorted use of public transport for 24 hours after sedation may need to be reconsidered in patients who receive propofol sedation.”
“With this study, we are aiming to identify the correct patient population who could potentially drive themselves home after their procedure rather than having to arrange for transportation,” Dr. Lal said in an interview.
“The significant cost associated with the daylong interruption of the activities of daily living, having a family member accompany them for their procedures, or not being able to use public transport unescorted may deter some patients from complying with colonoscopy screenings and other important endoscopic procedures,” she explained.
“There are patients who arrive for their procedure without any accompanying family members and we have to admit them to an observation unit overnight or reschedule their procedures. The expense and inconvenience associated with the extended recovery potentially could be an impediment to many important screening exams,” she noted. “In the setting of increased awareness regarding the need for these screening exams, this study is important to highlight these barriers and propose a solution for them.”
The potential costs of lost salaries is extremely high, she said. “If we assume that the 24-hour recovery period necessitates taking a day off from work, the value of lost salary per patient would be $183.68 as per the 2019 national hourly average wage. With an estimated 19 million colonoscopies performed annually in the United States, the aggregate cost of lost wages with the 24-hour guideline would be $3.5 billion. This amount does not account for the lost wages of the accompanying family member.”
Dr. Lal said she and her colleagues were not surprised by the findings. “The endoscopists in our endoscopy unit have noted that patients recover much more rapidly after sedation with propofol compared with other sedative agents. This observation led to the hypothesis that those patients receiving propofol should have a speedy psychomotor recovery and should be able to drive the same day. Our findings are in accordance with our observations.” However, “larger and preferably multicenter studies are needed to validate these findings and add to our knowledge about the postsedation psychomotor recovery,” said Dr. Lal. She added that the study is ongoing and the researchers have collected data from a total of 63 patients, which increases the power of the results.
The researchers had no financial conflicts to disclose.
*This story was updated on 5/8/2020.
SOURCE: Lal P et al. DDW 2020, Abstract 295.
Adults given propofol as part of an elective outpatient endoscopy procedure showed similar driving skills after postsedation recovery and prior to the procedure, based on simulation data from an open-label study of 41 patients.
Although current guidelines recommend that patients refrain from driving for 24 hours after propofol sedation and be accompanied by a responsible adult, data on the driving skills of patients after postsedation recovery are limited, Pooja Lal, MD, of the Cleveland Clinic Foundation and colleagues wrote in an abstract released as part of the annual Digestive Disease Week, which was canceled because of COVID-19.
“We assessed psychomotor recovery using a driving simulator which mimics real-life driving in outpatients undergoing gastrointestinal endoscopy with propofol,” they wrote.
The researchers enrolled 41 outpatients who were given propofol for various elective procedures at an endoscopy unit of a single center. Patients’ driving skills were tested at baseline and after postsedation recovery using a driving simulator. Postsedation recovery was defined as Aldrete score of 9 in the recovery room. Patients were excluded from the study if they demonstrated altered mental status of any type including dementia, delirium, and hepatic encephalopathy; if they were legally blind; or were currently inpatients.
Overall, driving skills were not significantly different between preprocedure and postsedation recovery on measures of number of times over the speed limit (3.2 vs. 3.4), number of times drivers went off the road (0.37 vs. 0.54), and total pedal reaction time (6.1 seconds vs. 7.6 seconds).
“The two variables including gas pedal reaction time and the total number of collisions did not follow normal distribution,” with medians of 0.70 for gas pedal reaction time in both groups and medians of 0 collisions for both groups, the investigators noted.
The study findings were limited by the small sample size and open-label design. However, the results suggest that driving skills were similar for patients at baseline and after achieving a postsedation Aldrete score of 9, the researchers wrote. Based on these findings, “current recommendations that patients should refrain from driving and unescorted use of public transport for 24 hours after sedation may need to be reconsidered in patients who receive propofol sedation.”
“With this study, we are aiming to identify the correct patient population who could potentially drive themselves home after their procedure rather than having to arrange for transportation,” Dr. Lal said in an interview.
“The significant cost associated with the daylong interruption of the activities of daily living, having a family member accompany them for their procedures, or not being able to use public transport unescorted may deter some patients from complying with colonoscopy screenings and other important endoscopic procedures,” she explained.
“There are patients who arrive for their procedure without any accompanying family members and we have to admit them to an observation unit overnight or reschedule their procedures. The expense and inconvenience associated with the extended recovery potentially could be an impediment to many important screening exams,” she noted. “In the setting of increased awareness regarding the need for these screening exams, this study is important to highlight these barriers and propose a solution for them.”
The potential costs of lost salaries is extremely high, she said. “If we assume that the 24-hour recovery period necessitates taking a day off from work, the value of lost salary per patient would be $183.68 as per the 2019 national hourly average wage. With an estimated 19 million colonoscopies performed annually in the United States, the aggregate cost of lost wages with the 24-hour guideline would be $3.5 billion. This amount does not account for the lost wages of the accompanying family member.”
Dr. Lal said she and her colleagues were not surprised by the findings. “The endoscopists in our endoscopy unit have noted that patients recover much more rapidly after sedation with propofol compared with other sedative agents. This observation led to the hypothesis that those patients receiving propofol should have a speedy psychomotor recovery and should be able to drive the same day. Our findings are in accordance with our observations.” However, “larger and preferably multicenter studies are needed to validate these findings and add to our knowledge about the postsedation psychomotor recovery,” said Dr. Lal. She added that the study is ongoing and the researchers have collected data from a total of 63 patients, which increases the power of the results.
The researchers had no financial conflicts to disclose.
*This story was updated on 5/8/2020.
SOURCE: Lal P et al. DDW 2020, Abstract 295.
FROM DDW 2020
Key clinical point: Patients who received propofol prior to endoscopic procedures showed no significant difference in driving ability from before the procedure to postsedation recovery.
Major finding: A simulated measure of driving ability showed similar competence before and after endoscopic procedures (number of times over the speed limit, 3.2 and 3.4, respectively).
Study details: The data come from a prospective, open-label study of 41 adults who underwent endoscopic procedures at a single center.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Lal P et al. DDW 2020, Abstract 295.