User login
Salvage chemo for NSCLC more effective after PD-1/PD-L1 inhibitors
GENEVA – Patients with stage IV non–small cell lung cancer (NSCLC) who have disease progression following treatment with an immune checkpoint inhibitor have a 30% better chance of achieving at least a partial response with salvage chemotherapy compared with patients who had received prior chemotherapy but not immunotherapy, according to Swiss and U.S. investigators.
In a study of 82 patients with advanced NSCLC, 18 of 67 patients (27%) who had progressed on an inhibitor of programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) had a partial response to combination chemotherapy, compared with just 1 of 15 (7%) of patients who had not received a checkpoint inhibitor, reported Sacha Rothschild, MD, PhD, of University Hospital Basel, Switzerland, and colleagues.
“The odds of achieving a partial response to salvage chemotherapy were significantly higher in patients with prior exposure to PD-1/PD-L1 inhibitors. This observed difference, however, warrants confirmation in larger cohorts. Ongoing investigations include the duration of response as well as evaluation of toxicity,” the researchers wrote in a scientific poster presented at the European Lung Cancer Conference.
Immune checkpoint inhibitors have been shown to be active in patients with late-stage NSCLC who have experienced disease progression following chemotherapy. To see whether salvage chemotherapy could offer any additional benefit to patients who had disease progression on immunotherapy, the investigators conducted a retrospective case-control study. They reviewed 355 patient records, and identified 82 patients – 46 men and 36 women – who met the study criteria.
Of this group, 67 patients had received a PD-1/PD-L1 checkpoint inhibitor, either nivolumab (Opdivo, 56 patients), pembrolizumab (Keytruda, 7), or atezolizumab (Tecentriq, 4). These patients were designated as cases. The remaining 15 patients, who had received prior chemotherapy or chemoradiotherapy only, were designated as controls.
Of all 82 patients, 63 (77%) had adenocarcinomas, 18 (22%) had squamous cell carcinomas, and 1 (1%) had a large-cell carcinoma.
Cases had a mean of 2.37 chemotherapy regimens prior to salvage chemotherapy, and controls had a mean of 1.93. Drugs used in the salvage regimens were docetaxel in 62% of patients, pemetrexed in 20%, gemcitabine in 12%, and paclitaxel in 6%.
The odds ratio for achieving a partial response was 0.30 (95% confidence interval, 0.18-0.50, P less than .0001).
In a multiple logistic regression model, neither age, sex, number of prior chemotherapy regimens, tumor histology, smoking status, nor type of salvage chemotherapy regimen were significantly associated with the likelihood of achieving a partial response.
In a poster discussion session, Simon Ekman, MD, of Karolinska Institute in Stockholm, Sweden, the invited discussant, said that the data from the study are convincing, and raise the question of what to do next.
“The question is how do we combine immunotherapy with chemotherapy? Should we use the immune therapy first, like in this trial, or vice versa, or should we combine concurrently” he said.
The strategy of chemotherapy first is supported by research showing that neoantigens crucial for the immune response are released during chemotherapy and radiotherapy, enabling immunotherapeutic agents to work better, he said.
The study was internally funded. Dr. Rothschild and Dr. Ekman reported no conflicts relevant to the research.
GENEVA – Patients with stage IV non–small cell lung cancer (NSCLC) who have disease progression following treatment with an immune checkpoint inhibitor have a 30% better chance of achieving at least a partial response with salvage chemotherapy compared with patients who had received prior chemotherapy but not immunotherapy, according to Swiss and U.S. investigators.
In a study of 82 patients with advanced NSCLC, 18 of 67 patients (27%) who had progressed on an inhibitor of programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) had a partial response to combination chemotherapy, compared with just 1 of 15 (7%) of patients who had not received a checkpoint inhibitor, reported Sacha Rothschild, MD, PhD, of University Hospital Basel, Switzerland, and colleagues.
“The odds of achieving a partial response to salvage chemotherapy were significantly higher in patients with prior exposure to PD-1/PD-L1 inhibitors. This observed difference, however, warrants confirmation in larger cohorts. Ongoing investigations include the duration of response as well as evaluation of toxicity,” the researchers wrote in a scientific poster presented at the European Lung Cancer Conference.
Immune checkpoint inhibitors have been shown to be active in patients with late-stage NSCLC who have experienced disease progression following chemotherapy. To see whether salvage chemotherapy could offer any additional benefit to patients who had disease progression on immunotherapy, the investigators conducted a retrospective case-control study. They reviewed 355 patient records, and identified 82 patients – 46 men and 36 women – who met the study criteria.
Of this group, 67 patients had received a PD-1/PD-L1 checkpoint inhibitor, either nivolumab (Opdivo, 56 patients), pembrolizumab (Keytruda, 7), or atezolizumab (Tecentriq, 4). These patients were designated as cases. The remaining 15 patients, who had received prior chemotherapy or chemoradiotherapy only, were designated as controls.
Of all 82 patients, 63 (77%) had adenocarcinomas, 18 (22%) had squamous cell carcinomas, and 1 (1%) had a large-cell carcinoma.
Cases had a mean of 2.37 chemotherapy regimens prior to salvage chemotherapy, and controls had a mean of 1.93. Drugs used in the salvage regimens were docetaxel in 62% of patients, pemetrexed in 20%, gemcitabine in 12%, and paclitaxel in 6%.
The odds ratio for achieving a partial response was 0.30 (95% confidence interval, 0.18-0.50, P less than .0001).
In a multiple logistic regression model, neither age, sex, number of prior chemotherapy regimens, tumor histology, smoking status, nor type of salvage chemotherapy regimen were significantly associated with the likelihood of achieving a partial response.
In a poster discussion session, Simon Ekman, MD, of Karolinska Institute in Stockholm, Sweden, the invited discussant, said that the data from the study are convincing, and raise the question of what to do next.
“The question is how do we combine immunotherapy with chemotherapy? Should we use the immune therapy first, like in this trial, or vice versa, or should we combine concurrently” he said.
The strategy of chemotherapy first is supported by research showing that neoantigens crucial for the immune response are released during chemotherapy and radiotherapy, enabling immunotherapeutic agents to work better, he said.
The study was internally funded. Dr. Rothschild and Dr. Ekman reported no conflicts relevant to the research.
GENEVA – Patients with stage IV non–small cell lung cancer (NSCLC) who have disease progression following treatment with an immune checkpoint inhibitor have a 30% better chance of achieving at least a partial response with salvage chemotherapy compared with patients who had received prior chemotherapy but not immunotherapy, according to Swiss and U.S. investigators.
In a study of 82 patients with advanced NSCLC, 18 of 67 patients (27%) who had progressed on an inhibitor of programmed death-1 (PD-1) or programmed death ligand-1 (PD-L1) had a partial response to combination chemotherapy, compared with just 1 of 15 (7%) of patients who had not received a checkpoint inhibitor, reported Sacha Rothschild, MD, PhD, of University Hospital Basel, Switzerland, and colleagues.
“The odds of achieving a partial response to salvage chemotherapy were significantly higher in patients with prior exposure to PD-1/PD-L1 inhibitors. This observed difference, however, warrants confirmation in larger cohorts. Ongoing investigations include the duration of response as well as evaluation of toxicity,” the researchers wrote in a scientific poster presented at the European Lung Cancer Conference.
Immune checkpoint inhibitors have been shown to be active in patients with late-stage NSCLC who have experienced disease progression following chemotherapy. To see whether salvage chemotherapy could offer any additional benefit to patients who had disease progression on immunotherapy, the investigators conducted a retrospective case-control study. They reviewed 355 patient records, and identified 82 patients – 46 men and 36 women – who met the study criteria.
Of this group, 67 patients had received a PD-1/PD-L1 checkpoint inhibitor, either nivolumab (Opdivo, 56 patients), pembrolizumab (Keytruda, 7), or atezolizumab (Tecentriq, 4). These patients were designated as cases. The remaining 15 patients, who had received prior chemotherapy or chemoradiotherapy only, were designated as controls.
Of all 82 patients, 63 (77%) had adenocarcinomas, 18 (22%) had squamous cell carcinomas, and 1 (1%) had a large-cell carcinoma.
Cases had a mean of 2.37 chemotherapy regimens prior to salvage chemotherapy, and controls had a mean of 1.93. Drugs used in the salvage regimens were docetaxel in 62% of patients, pemetrexed in 20%, gemcitabine in 12%, and paclitaxel in 6%.
The odds ratio for achieving a partial response was 0.30 (95% confidence interval, 0.18-0.50, P less than .0001).
In a multiple logistic regression model, neither age, sex, number of prior chemotherapy regimens, tumor histology, smoking status, nor type of salvage chemotherapy regimen were significantly associated with the likelihood of achieving a partial response.
In a poster discussion session, Simon Ekman, MD, of Karolinska Institute in Stockholm, Sweden, the invited discussant, said that the data from the study are convincing, and raise the question of what to do next.
“The question is how do we combine immunotherapy with chemotherapy? Should we use the immune therapy first, like in this trial, or vice versa, or should we combine concurrently” he said.
The strategy of chemotherapy first is supported by research showing that neoantigens crucial for the immune response are released during chemotherapy and radiotherapy, enabling immunotherapeutic agents to work better, he said.
The study was internally funded. Dr. Rothschild and Dr. Ekman reported no conflicts relevant to the research.
FROM ELCC
Key clinical point: Salvage chemotherapy was more effective among patients who had disease progression following immunotherapy in the second line than among patients who received only chemotherapy.
Major finding: Among patients with disease progression while on a checkpoint inhibitor, 18 of 67 had a partial response to salvage chemotherapy, compared with just 1 of 15 controls.
Data source: Retrospective case-control study of 82 patients with advanced NSCLC.
Disclosures: The study was internally funded. Dr. Rothschild and Dr. Ekman reported no conflicts relevant to the research.
Atezolizumab improves OS in NSCLC with brain metastases
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
Key clinical point: The PD-L1 inhibitor atezolizumab improved overall survival, vs. docetaxel, in patients with non–small cell lung cancer and brain metastases.
Major finding: The hazard ratio for overall survival was 0.54 for patients assigned to atezolizumab, vs. docetaxel, in the OAK trial.
Data source: Pooled safety analysis of 1452 patients and efficacy analysis of 850 patients with NSCLC with or without brain metastases.
Disclosures: The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
Second generation ALK inhibitors show good response in second-line NSCLC
GENEVA – Second-generation inhibitors of the anaplastic lymphoma kinase (ALK) are associated with high response rates in patients with non–small cell lung cancer (NSCLC) who have disease progression after treatment with the first-generation ALK inhibitor crizotinib (Xalkori), investigators reported.
Of 117 patients with ALK-positive NSCLC that progressed while on treatment with the ALK-targeting tyrosine-kinase inhibitor (TKI) crizotinib, both ceritinib (Zykadia) and alectinib (Alecensa) were associated with high combined partial and complete response rates, reported Christian Britschgi, MD, PhD, of University Hospital Zürich, and his colleagues.
“We can confirm the high rate of response of ceritinib and alectinib as second TKIs,” Dr. Britschgi said in an interview.
“With ceritinib or alectinib in the first line, however, it’s hard to draw any conclusions because there are very few patients treated with these drugs in the first line who were included in the trials,” he added.
ALK rearrangements occur in 3%-5% of lung adenocarcinomas, and these mutations are potential targets for TKIs directed against ALK.
The investigators analyzed the clinical characteristics of response to, and disease progression on, available ALK-TKIs. They also performed next-generation sequencing on a small group of tumor samples to investigate mechanisms of resistance and to look for new mutations that could be targetable.
They collected data on patients with ALK-positive NSCLC who were receiving palliative care and looked at clinical data and biopsy samples collected before treatment, and where available, post–ALK-TKI therapy.
They collected clinical data on 139 patients, 117 of whom had undergone first-line treatment with chemotherapy (74%), ALK-TKIs (25%), or other therapies (1%). Of this group, 92 received second-line therapy – chemotherapy (17%), ALK-TKIs (74%), or immunotherapies/other (9%).
Third-line treatments were given to 55 patients, 20% of whom received chemotherapy, 75% of whom received ALK-TKIs, and 5% of whom received other therapies.
Twenty-four patients survived long enough to receive fourth-line treatments, which included chemotherapy in 8 patients (33%), ALK-TKIs in 15 (63%), or other therapies in 1 patient.
In all, 102 patients received crizotinib, all but one in the first line and the remaining patient in the second line. The overall response rate was 58.8% (59.4% for the 101 first-line patients). Median progression-free survival (PFS) in these patients was 11.7 months.
Ceritinib was given to 2 patients in the first line, 7 in the second line, and 28 in the third line. The overall response rate was 48.6% (50% in the two patients who received it in the first line, and 50% in patients who received it after crizotinib). The median PFS was 6.5 months.
For the 26 patients who received alectinib – 3 in the first line and 23 after crizotinib – the overall response rate was 100% when the drug was used in the first line, and 60.9% when used in the second and subsequent lines. Median PFS was 12.4 months.
Only a few patients to date have had next-generation sequencing of tumors performed, Dr. Britschgi said. One patient who developed resistance while on alectinib was found to have a point mutation (ALK p.lle117Ser) that was not found in a pretreatment biopsy sample, indicating that it had emerged during therapy. This patient was then started on and had a response to lorlatinib.
A second patient who had received several lines of therapy including chemotherapy and TKIs and was enrolled in the expanded access program for lorlatinib developed hepatic progression while on that drug. A biopsy showed that the tumor carried epidermal growth-factor receptor variant 3, which is known to be highly mutagenic and is associated with glioblastoma. The patient was then put on nivolumab (Opdivo) and had a good response, but had further progression, and was then put on alectinib and again had a good response that is currently ongoing, Dr. Britschgi said.
The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer and Roche.
GENEVA – Second-generation inhibitors of the anaplastic lymphoma kinase (ALK) are associated with high response rates in patients with non–small cell lung cancer (NSCLC) who have disease progression after treatment with the first-generation ALK inhibitor crizotinib (Xalkori), investigators reported.
Of 117 patients with ALK-positive NSCLC that progressed while on treatment with the ALK-targeting tyrosine-kinase inhibitor (TKI) crizotinib, both ceritinib (Zykadia) and alectinib (Alecensa) were associated with high combined partial and complete response rates, reported Christian Britschgi, MD, PhD, of University Hospital Zürich, and his colleagues.
“We can confirm the high rate of response of ceritinib and alectinib as second TKIs,” Dr. Britschgi said in an interview.
“With ceritinib or alectinib in the first line, however, it’s hard to draw any conclusions because there are very few patients treated with these drugs in the first line who were included in the trials,” he added.
ALK rearrangements occur in 3%-5% of lung adenocarcinomas, and these mutations are potential targets for TKIs directed against ALK.
The investigators analyzed the clinical characteristics of response to, and disease progression on, available ALK-TKIs. They also performed next-generation sequencing on a small group of tumor samples to investigate mechanisms of resistance and to look for new mutations that could be targetable.
They collected data on patients with ALK-positive NSCLC who were receiving palliative care and looked at clinical data and biopsy samples collected before treatment, and where available, post–ALK-TKI therapy.
They collected clinical data on 139 patients, 117 of whom had undergone first-line treatment with chemotherapy (74%), ALK-TKIs (25%), or other therapies (1%). Of this group, 92 received second-line therapy – chemotherapy (17%), ALK-TKIs (74%), or immunotherapies/other (9%).
Third-line treatments were given to 55 patients, 20% of whom received chemotherapy, 75% of whom received ALK-TKIs, and 5% of whom received other therapies.
Twenty-four patients survived long enough to receive fourth-line treatments, which included chemotherapy in 8 patients (33%), ALK-TKIs in 15 (63%), or other therapies in 1 patient.
In all, 102 patients received crizotinib, all but one in the first line and the remaining patient in the second line. The overall response rate was 58.8% (59.4% for the 101 first-line patients). Median progression-free survival (PFS) in these patients was 11.7 months.
Ceritinib was given to 2 patients in the first line, 7 in the second line, and 28 in the third line. The overall response rate was 48.6% (50% in the two patients who received it in the first line, and 50% in patients who received it after crizotinib). The median PFS was 6.5 months.
For the 26 patients who received alectinib – 3 in the first line and 23 after crizotinib – the overall response rate was 100% when the drug was used in the first line, and 60.9% when used in the second and subsequent lines. Median PFS was 12.4 months.
Only a few patients to date have had next-generation sequencing of tumors performed, Dr. Britschgi said. One patient who developed resistance while on alectinib was found to have a point mutation (ALK p.lle117Ser) that was not found in a pretreatment biopsy sample, indicating that it had emerged during therapy. This patient was then started on and had a response to lorlatinib.
A second patient who had received several lines of therapy including chemotherapy and TKIs and was enrolled in the expanded access program for lorlatinib developed hepatic progression while on that drug. A biopsy showed that the tumor carried epidermal growth-factor receptor variant 3, which is known to be highly mutagenic and is associated with glioblastoma. The patient was then put on nivolumab (Opdivo) and had a good response, but had further progression, and was then put on alectinib and again had a good response that is currently ongoing, Dr. Britschgi said.
The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer and Roche.
GENEVA – Second-generation inhibitors of the anaplastic lymphoma kinase (ALK) are associated with high response rates in patients with non–small cell lung cancer (NSCLC) who have disease progression after treatment with the first-generation ALK inhibitor crizotinib (Xalkori), investigators reported.
Of 117 patients with ALK-positive NSCLC that progressed while on treatment with the ALK-targeting tyrosine-kinase inhibitor (TKI) crizotinib, both ceritinib (Zykadia) and alectinib (Alecensa) were associated with high combined partial and complete response rates, reported Christian Britschgi, MD, PhD, of University Hospital Zürich, and his colleagues.
“We can confirm the high rate of response of ceritinib and alectinib as second TKIs,” Dr. Britschgi said in an interview.
“With ceritinib or alectinib in the first line, however, it’s hard to draw any conclusions because there are very few patients treated with these drugs in the first line who were included in the trials,” he added.
ALK rearrangements occur in 3%-5% of lung adenocarcinomas, and these mutations are potential targets for TKIs directed against ALK.
The investigators analyzed the clinical characteristics of response to, and disease progression on, available ALK-TKIs. They also performed next-generation sequencing on a small group of tumor samples to investigate mechanisms of resistance and to look for new mutations that could be targetable.
They collected data on patients with ALK-positive NSCLC who were receiving palliative care and looked at clinical data and biopsy samples collected before treatment, and where available, post–ALK-TKI therapy.
They collected clinical data on 139 patients, 117 of whom had undergone first-line treatment with chemotherapy (74%), ALK-TKIs (25%), or other therapies (1%). Of this group, 92 received second-line therapy – chemotherapy (17%), ALK-TKIs (74%), or immunotherapies/other (9%).
Third-line treatments were given to 55 patients, 20% of whom received chemotherapy, 75% of whom received ALK-TKIs, and 5% of whom received other therapies.
Twenty-four patients survived long enough to receive fourth-line treatments, which included chemotherapy in 8 patients (33%), ALK-TKIs in 15 (63%), or other therapies in 1 patient.
In all, 102 patients received crizotinib, all but one in the first line and the remaining patient in the second line. The overall response rate was 58.8% (59.4% for the 101 first-line patients). Median progression-free survival (PFS) in these patients was 11.7 months.
Ceritinib was given to 2 patients in the first line, 7 in the second line, and 28 in the third line. The overall response rate was 48.6% (50% in the two patients who received it in the first line, and 50% in patients who received it after crizotinib). The median PFS was 6.5 months.
For the 26 patients who received alectinib – 3 in the first line and 23 after crizotinib – the overall response rate was 100% when the drug was used in the first line, and 60.9% when used in the second and subsequent lines. Median PFS was 12.4 months.
Only a few patients to date have had next-generation sequencing of tumors performed, Dr. Britschgi said. One patient who developed resistance while on alectinib was found to have a point mutation (ALK p.lle117Ser) that was not found in a pretreatment biopsy sample, indicating that it had emerged during therapy. This patient was then started on and had a response to lorlatinib.
A second patient who had received several lines of therapy including chemotherapy and TKIs and was enrolled in the expanded access program for lorlatinib developed hepatic progression while on that drug. A biopsy showed that the tumor carried epidermal growth-factor receptor variant 3, which is known to be highly mutagenic and is associated with glioblastoma. The patient was then put on nivolumab (Opdivo) and had a good response, but had further progression, and was then put on alectinib and again had a good response that is currently ongoing, Dr. Britschgi said.
The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer and Roche.
FROM ELCC
Key clinical point:
Major finding: Overall response rates after crizotinib were 50% for ceritinib and 60.9% for alectinib.
Data source: A retrospective review of data on 117 patients with ALK-positive NSCLC in Swiss and Italian hospitals.
Disclosures: The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer, and Roche.
Lung cancer metastatic sites differ by histology, tumor factors
GENEVA – A review of data on more than 75,000 patients with lung cancer has revealed distinct patterns of metastasis according to subtype, a finding that could help in surveillance, treatment planning, and prophylaxis, an investigator contends.
Patients with small cell lung cancer (SCLC) had significantly higher rates of liver metastases than patients with non–small cell lung cancer (NSCLC), while patients with NSCLC had significantly higher rates of metastases to bone, reported Mohamed Hendawi, MD, a visiting scholar at the Ohio State University Medical Center in Columbus.
“Predictors for liver metastasis were small cell and adenocarcinoma histology, lower and upper lobe locations, and high grade tumors. Predictors for metastasis to brain were advanced age at diagnosis, adenocarcinoma and small-cell histology, lower lobe [and] main bronchus locations, and high grade tumors,” he wrote in a scientific poster presented at the European Lung Cancer Conference.
Dr. Hendawi drew records on all patients with metastatic lung cancer included in the 2010-2013 Surveillance, Epidemiology, and End Results database. He used univariate and multivariate logistic regression models to evaluate predictors of metastasis.
The data set included a total of 76,254 patients with metastatic lung cancer, of which 17% were SCLC and 83% were NSCLC tumors. In 54% of patients, the primary tumor was in the right lung; in 38%, it was in the left lung; and, in 8% of patients, the primary tumor was bilateral.
The rates of metastases to bone were high in both major lung cancer types but, as noted before, were significantly higher in patients with NSCLC: 37% compared with 34% for patients with SCLC (P less than .001).
In contrast, the incidence of liver metastases in SCLC was more than double that of NSCLC: 46% vs. 20%, respectively (P less than .001). There were slightly, but significantly, fewer cases of brain metastases at the time of diagnosis among patients with SCLC: 25% vs. 26% (P = .003).
Histologic subtypes significantly associated with both brain and liver metastases were, in descending order, adenocarcinomas, small cell, and squamous cell cancers.
Although carcinoid lung cancers accounted for only 2.1% of all tumors, they were associated with a high rate of metastasis to brain at diagnosis (44.8%).
As noted, independent risk factors for liver metastasis were small cell and adenocarcinoma histologies (P less than .001), tumors in the upper lobe (P = .028), and high-grade tumor (P less than .001).
Independent predictors for brain metastases were advanced age at diagnosis (P less than .001), adenocarcinoma and small-cell histologies (P less than .001), lower lobe or main bronchus locations (P = .004), and higher-grade tumors (P less than .001).
In a poster discussion session, Paolo Boffetta, MD, MPH, from the Icahn School of Medicine at Mount Sinai in New York City, the invited discussant, commented that, while he thought that the data were interesting, “the main issue I had with this poster is that it’s limited to patients with metastasis, so we cannot really evaluate the risk of metastasis according to the different histological types and the absolute risk of developing metastases in one or the other organ but only the relative risk of developing metastasis in one organ versus the other having one or the other histology.”
“So, we really don’t know whether the risk is increased in one group or decreased in the other one that generates these differences,” he said.
The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
GENEVA – A review of data on more than 75,000 patients with lung cancer has revealed distinct patterns of metastasis according to subtype, a finding that could help in surveillance, treatment planning, and prophylaxis, an investigator contends.
Patients with small cell lung cancer (SCLC) had significantly higher rates of liver metastases than patients with non–small cell lung cancer (NSCLC), while patients with NSCLC had significantly higher rates of metastases to bone, reported Mohamed Hendawi, MD, a visiting scholar at the Ohio State University Medical Center in Columbus.
“Predictors for liver metastasis were small cell and adenocarcinoma histology, lower and upper lobe locations, and high grade tumors. Predictors for metastasis to brain were advanced age at diagnosis, adenocarcinoma and small-cell histology, lower lobe [and] main bronchus locations, and high grade tumors,” he wrote in a scientific poster presented at the European Lung Cancer Conference.
Dr. Hendawi drew records on all patients with metastatic lung cancer included in the 2010-2013 Surveillance, Epidemiology, and End Results database. He used univariate and multivariate logistic regression models to evaluate predictors of metastasis.
The data set included a total of 76,254 patients with metastatic lung cancer, of which 17% were SCLC and 83% were NSCLC tumors. In 54% of patients, the primary tumor was in the right lung; in 38%, it was in the left lung; and, in 8% of patients, the primary tumor was bilateral.
The rates of metastases to bone were high in both major lung cancer types but, as noted before, were significantly higher in patients with NSCLC: 37% compared with 34% for patients with SCLC (P less than .001).
In contrast, the incidence of liver metastases in SCLC was more than double that of NSCLC: 46% vs. 20%, respectively (P less than .001). There were slightly, but significantly, fewer cases of brain metastases at the time of diagnosis among patients with SCLC: 25% vs. 26% (P = .003).
Histologic subtypes significantly associated with both brain and liver metastases were, in descending order, adenocarcinomas, small cell, and squamous cell cancers.
Although carcinoid lung cancers accounted for only 2.1% of all tumors, they were associated with a high rate of metastasis to brain at diagnosis (44.8%).
As noted, independent risk factors for liver metastasis were small cell and adenocarcinoma histologies (P less than .001), tumors in the upper lobe (P = .028), and high-grade tumor (P less than .001).
Independent predictors for brain metastases were advanced age at diagnosis (P less than .001), adenocarcinoma and small-cell histologies (P less than .001), lower lobe or main bronchus locations (P = .004), and higher-grade tumors (P less than .001).
In a poster discussion session, Paolo Boffetta, MD, MPH, from the Icahn School of Medicine at Mount Sinai in New York City, the invited discussant, commented that, while he thought that the data were interesting, “the main issue I had with this poster is that it’s limited to patients with metastasis, so we cannot really evaluate the risk of metastasis according to the different histological types and the absolute risk of developing metastases in one or the other organ but only the relative risk of developing metastasis in one organ versus the other having one or the other histology.”
“So, we really don’t know whether the risk is increased in one group or decreased in the other one that generates these differences,” he said.
The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
GENEVA – A review of data on more than 75,000 patients with lung cancer has revealed distinct patterns of metastasis according to subtype, a finding that could help in surveillance, treatment planning, and prophylaxis, an investigator contends.
Patients with small cell lung cancer (SCLC) had significantly higher rates of liver metastases than patients with non–small cell lung cancer (NSCLC), while patients with NSCLC had significantly higher rates of metastases to bone, reported Mohamed Hendawi, MD, a visiting scholar at the Ohio State University Medical Center in Columbus.
“Predictors for liver metastasis were small cell and adenocarcinoma histology, lower and upper lobe locations, and high grade tumors. Predictors for metastasis to brain were advanced age at diagnosis, adenocarcinoma and small-cell histology, lower lobe [and] main bronchus locations, and high grade tumors,” he wrote in a scientific poster presented at the European Lung Cancer Conference.
Dr. Hendawi drew records on all patients with metastatic lung cancer included in the 2010-2013 Surveillance, Epidemiology, and End Results database. He used univariate and multivariate logistic regression models to evaluate predictors of metastasis.
The data set included a total of 76,254 patients with metastatic lung cancer, of which 17% were SCLC and 83% were NSCLC tumors. In 54% of patients, the primary tumor was in the right lung; in 38%, it was in the left lung; and, in 8% of patients, the primary tumor was bilateral.
The rates of metastases to bone were high in both major lung cancer types but, as noted before, were significantly higher in patients with NSCLC: 37% compared with 34% for patients with SCLC (P less than .001).
In contrast, the incidence of liver metastases in SCLC was more than double that of NSCLC: 46% vs. 20%, respectively (P less than .001). There were slightly, but significantly, fewer cases of brain metastases at the time of diagnosis among patients with SCLC: 25% vs. 26% (P = .003).
Histologic subtypes significantly associated with both brain and liver metastases were, in descending order, adenocarcinomas, small cell, and squamous cell cancers.
Although carcinoid lung cancers accounted for only 2.1% of all tumors, they were associated with a high rate of metastasis to brain at diagnosis (44.8%).
As noted, independent risk factors for liver metastasis were small cell and adenocarcinoma histologies (P less than .001), tumors in the upper lobe (P = .028), and high-grade tumor (P less than .001).
Independent predictors for brain metastases were advanced age at diagnosis (P less than .001), adenocarcinoma and small-cell histologies (P less than .001), lower lobe or main bronchus locations (P = .004), and higher-grade tumors (P less than .001).
In a poster discussion session, Paolo Boffetta, MD, MPH, from the Icahn School of Medicine at Mount Sinai in New York City, the invited discussant, commented that, while he thought that the data were interesting, “the main issue I had with this poster is that it’s limited to patients with metastasis, so we cannot really evaluate the risk of metastasis according to the different histological types and the absolute risk of developing metastases in one or the other organ but only the relative risk of developing metastasis in one organ versus the other having one or the other histology.”
“So, we really don’t know whether the risk is increased in one group or decreased in the other one that generates these differences,” he said.
The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
FROM ELCC
Key clinical point: Lung cancers tend to metastasize to different organs based on histology and other clinical factors.
Major finding: The incidence of metastasis to bone was higher in patients with NSCLC than SCLC, while liver metastases were more than twice as high among patients with SCLC.
Data source: Review of Surveillance, Epidemiology, and End Results data on 76,254 patients with metastatic lung cancer.
Disclosures: The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
G-CSF safe, but antibiotics are more concerning in SCLC
GENEVA – In patients with limited stage–small cell lung cancer (LS-SCLC) treated with concurrent chemotherapy and radiation, the use of antibiotics to prevent febrile neutropenia was associated with worse outcomes, but granulocyte-colony stimulating factor (G-CSF) prescribed for the same purposes appeared to be safe, reported investigators.
In a subanalysis of data on patients with early SCLC enrolled in the phase III CONVERT trial comparing chemotherapy with concurrent once-daily vs. twice-daily radiation, the use of antibiotic prophylaxis of neutropenia was associated with worse overall survival (OS) and progression-free survival, (PFS) compared with no antibiotics, reported Fabio Gomes, MD, from the Christie NHS Foundation Trust Hospital in Manchester, England.
The use of G-CSF was, however, associated with higher rates of grade 3 or 4 thrombocytopenia and anemia, requiring supportive measures, he acknowledged.
The role of G-CSF with concurrent thoracic radiotherapy is controversial because of safety concerns, but data are scarce, Dr. Gomes said. He noted that the American Society of Clinical Oncology guidelines on the use of white blood cell growth factors recommend against their routine use.
However, some of those concerns arose in the mid-1990s when granulocyt macrophage–stimulating colony factor (GM-CSF) was used, rather than G-CSF, which acts on only a single blood lineage, namely neutrophils. Additionally, modern radiology techniques are more precise than they were 20 years ago, reducing the risk of toxicity, he noted.
In the CONVERT trial, 547 patients with LS-SCLC were randomly assigned to receive four to six cycles of cisplatin and etoposide chemotherapy concurrently with either once daily thoracic radiation for a total dose of 66 Gy divided into 33 fractions delivered over 45 days or to twice-daily radiation at a total dose of 45 Gy divided into 30 fractions delivered over 19 days.
There was no difference between the groups in the primary endpoint of overall survival.
In the subanalysis reported here, Gomes et al. looked at the use of G-CSF, delivered at the investigator’s discretion, in 487 patients. Approximately 40% of patients in the subanalysis received G-CSF during at least one treatment cycle.
Prophylactic antibiotics were recommended by the investigators for use in association with at least one chemotherapy cycle, and 49% of patients in the subanalysis received them during at least one cycle.
Hematological toxicities included grade 3 or 4 thrombocytopenia occurring in 29.9% of patients who received G-CSF, vs. 13.3% of those who did not (P less than .001). The rates were similar between the once-daily and twice-daily radiation groups.
Grade 3 or 4 anemia occurred in 16.9% of patients who received G-CSF, vs. 10.7% of those who didn’t (P = .027). The difference was significant only among patients in the twice-daily radiation arm (20.9% vs. 8.3%, respectively; P = .004).
Patients in the twice-daily radiation arms who received G-CSF also required more platelet transfusion, compared with the once-daily arm (P less than .001), and, in both arms, G-CSF was associated with more red-cell transfusions (P = .007 for once-daily and .001 for twice daily).
G-CSF was not associated with either pneumonitis or esophagitis, and there were no differences in treatment-related deaths with either G-CSF or antibiotics.
Median OS by G-CSF use was 29 months with and 27 months without, a difference that was not significant (P = .08). Median PFS also did not differ by G-CSF use or nonuse.
When it came to antibiotic prophylaxis, however, both median OS and PFS were significant worse with antibiotic use (OS, 24 months with vs. 33 months without; P = .016; PFS, P = .03).
“We are very reassured that there are no significant additional toxicities [with G-CSF] from radiation in the acute setting,” commented Sanjay Popat, FRCP, PhD, from the Royal Marsden Hospital in London, the invited discussant.
“However, we have no data as yet on the impact of G-CSF usage on febrile neutropenia, which is of course the fundamental that we’re aiming to improve in the hope that this will contribute to [lowering] costs,” he added.
The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
GENEVA – In patients with limited stage–small cell lung cancer (LS-SCLC) treated with concurrent chemotherapy and radiation, the use of antibiotics to prevent febrile neutropenia was associated with worse outcomes, but granulocyte-colony stimulating factor (G-CSF) prescribed for the same purposes appeared to be safe, reported investigators.
In a subanalysis of data on patients with early SCLC enrolled in the phase III CONVERT trial comparing chemotherapy with concurrent once-daily vs. twice-daily radiation, the use of antibiotic prophylaxis of neutropenia was associated with worse overall survival (OS) and progression-free survival, (PFS) compared with no antibiotics, reported Fabio Gomes, MD, from the Christie NHS Foundation Trust Hospital in Manchester, England.
The use of G-CSF was, however, associated with higher rates of grade 3 or 4 thrombocytopenia and anemia, requiring supportive measures, he acknowledged.
The role of G-CSF with concurrent thoracic radiotherapy is controversial because of safety concerns, but data are scarce, Dr. Gomes said. He noted that the American Society of Clinical Oncology guidelines on the use of white blood cell growth factors recommend against their routine use.
However, some of those concerns arose in the mid-1990s when granulocyt macrophage–stimulating colony factor (GM-CSF) was used, rather than G-CSF, which acts on only a single blood lineage, namely neutrophils. Additionally, modern radiology techniques are more precise than they were 20 years ago, reducing the risk of toxicity, he noted.
In the CONVERT trial, 547 patients with LS-SCLC were randomly assigned to receive four to six cycles of cisplatin and etoposide chemotherapy concurrently with either once daily thoracic radiation for a total dose of 66 Gy divided into 33 fractions delivered over 45 days or to twice-daily radiation at a total dose of 45 Gy divided into 30 fractions delivered over 19 days.
There was no difference between the groups in the primary endpoint of overall survival.
In the subanalysis reported here, Gomes et al. looked at the use of G-CSF, delivered at the investigator’s discretion, in 487 patients. Approximately 40% of patients in the subanalysis received G-CSF during at least one treatment cycle.
Prophylactic antibiotics were recommended by the investigators for use in association with at least one chemotherapy cycle, and 49% of patients in the subanalysis received them during at least one cycle.
Hematological toxicities included grade 3 or 4 thrombocytopenia occurring in 29.9% of patients who received G-CSF, vs. 13.3% of those who did not (P less than .001). The rates were similar between the once-daily and twice-daily radiation groups.
Grade 3 or 4 anemia occurred in 16.9% of patients who received G-CSF, vs. 10.7% of those who didn’t (P = .027). The difference was significant only among patients in the twice-daily radiation arm (20.9% vs. 8.3%, respectively; P = .004).
Patients in the twice-daily radiation arms who received G-CSF also required more platelet transfusion, compared with the once-daily arm (P less than .001), and, in both arms, G-CSF was associated with more red-cell transfusions (P = .007 for once-daily and .001 for twice daily).
G-CSF was not associated with either pneumonitis or esophagitis, and there were no differences in treatment-related deaths with either G-CSF or antibiotics.
Median OS by G-CSF use was 29 months with and 27 months without, a difference that was not significant (P = .08). Median PFS also did not differ by G-CSF use or nonuse.
When it came to antibiotic prophylaxis, however, both median OS and PFS were significant worse with antibiotic use (OS, 24 months with vs. 33 months without; P = .016; PFS, P = .03).
“We are very reassured that there are no significant additional toxicities [with G-CSF] from radiation in the acute setting,” commented Sanjay Popat, FRCP, PhD, from the Royal Marsden Hospital in London, the invited discussant.
“However, we have no data as yet on the impact of G-CSF usage on febrile neutropenia, which is of course the fundamental that we’re aiming to improve in the hope that this will contribute to [lowering] costs,” he added.
The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
GENEVA – In patients with limited stage–small cell lung cancer (LS-SCLC) treated with concurrent chemotherapy and radiation, the use of antibiotics to prevent febrile neutropenia was associated with worse outcomes, but granulocyte-colony stimulating factor (G-CSF) prescribed for the same purposes appeared to be safe, reported investigators.
In a subanalysis of data on patients with early SCLC enrolled in the phase III CONVERT trial comparing chemotherapy with concurrent once-daily vs. twice-daily radiation, the use of antibiotic prophylaxis of neutropenia was associated with worse overall survival (OS) and progression-free survival, (PFS) compared with no antibiotics, reported Fabio Gomes, MD, from the Christie NHS Foundation Trust Hospital in Manchester, England.
The use of G-CSF was, however, associated with higher rates of grade 3 or 4 thrombocytopenia and anemia, requiring supportive measures, he acknowledged.
The role of G-CSF with concurrent thoracic radiotherapy is controversial because of safety concerns, but data are scarce, Dr. Gomes said. He noted that the American Society of Clinical Oncology guidelines on the use of white blood cell growth factors recommend against their routine use.
However, some of those concerns arose in the mid-1990s when granulocyt macrophage–stimulating colony factor (GM-CSF) was used, rather than G-CSF, which acts on only a single blood lineage, namely neutrophils. Additionally, modern radiology techniques are more precise than they were 20 years ago, reducing the risk of toxicity, he noted.
In the CONVERT trial, 547 patients with LS-SCLC were randomly assigned to receive four to six cycles of cisplatin and etoposide chemotherapy concurrently with either once daily thoracic radiation for a total dose of 66 Gy divided into 33 fractions delivered over 45 days or to twice-daily radiation at a total dose of 45 Gy divided into 30 fractions delivered over 19 days.
There was no difference between the groups in the primary endpoint of overall survival.
In the subanalysis reported here, Gomes et al. looked at the use of G-CSF, delivered at the investigator’s discretion, in 487 patients. Approximately 40% of patients in the subanalysis received G-CSF during at least one treatment cycle.
Prophylactic antibiotics were recommended by the investigators for use in association with at least one chemotherapy cycle, and 49% of patients in the subanalysis received them during at least one cycle.
Hematological toxicities included grade 3 or 4 thrombocytopenia occurring in 29.9% of patients who received G-CSF, vs. 13.3% of those who did not (P less than .001). The rates were similar between the once-daily and twice-daily radiation groups.
Grade 3 or 4 anemia occurred in 16.9% of patients who received G-CSF, vs. 10.7% of those who didn’t (P = .027). The difference was significant only among patients in the twice-daily radiation arm (20.9% vs. 8.3%, respectively; P = .004).
Patients in the twice-daily radiation arms who received G-CSF also required more platelet transfusion, compared with the once-daily arm (P less than .001), and, in both arms, G-CSF was associated with more red-cell transfusions (P = .007 for once-daily and .001 for twice daily).
G-CSF was not associated with either pneumonitis or esophagitis, and there were no differences in treatment-related deaths with either G-CSF or antibiotics.
Median OS by G-CSF use was 29 months with and 27 months without, a difference that was not significant (P = .08). Median PFS also did not differ by G-CSF use or nonuse.
When it came to antibiotic prophylaxis, however, both median OS and PFS were significant worse with antibiotic use (OS, 24 months with vs. 33 months without; P = .016; PFS, P = .03).
“We are very reassured that there are no significant additional toxicities [with G-CSF] from radiation in the acute setting,” commented Sanjay Popat, FRCP, PhD, from the Royal Marsden Hospital in London, the invited discussant.
“However, we have no data as yet on the impact of G-CSF usage on febrile neutropenia, which is of course the fundamental that we’re aiming to improve in the hope that this will contribute to [lowering] costs,” he added.
The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
FROM ELCC
Key clinical point: Febrile neutropenia prophylaxis with G-CSF was safe, but prophylactic antibiotics were associated with worse overall survival in patients with limited stage–small cell lung cancer.
Major finding: Both median overall and progression-free survival were lower among patients who received prophylactic antibiotics. There were no differences in survival by G-CSF use.
Data source: Subanalysis of data on 487 patients in the phase III CONVERT trial comparing once-daily and twice daily radiation concurrent with chemotherapy in LS-SCLC.
Disclosures: The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
Chemo sequencing in elderly adults with NSCLC linked with survival
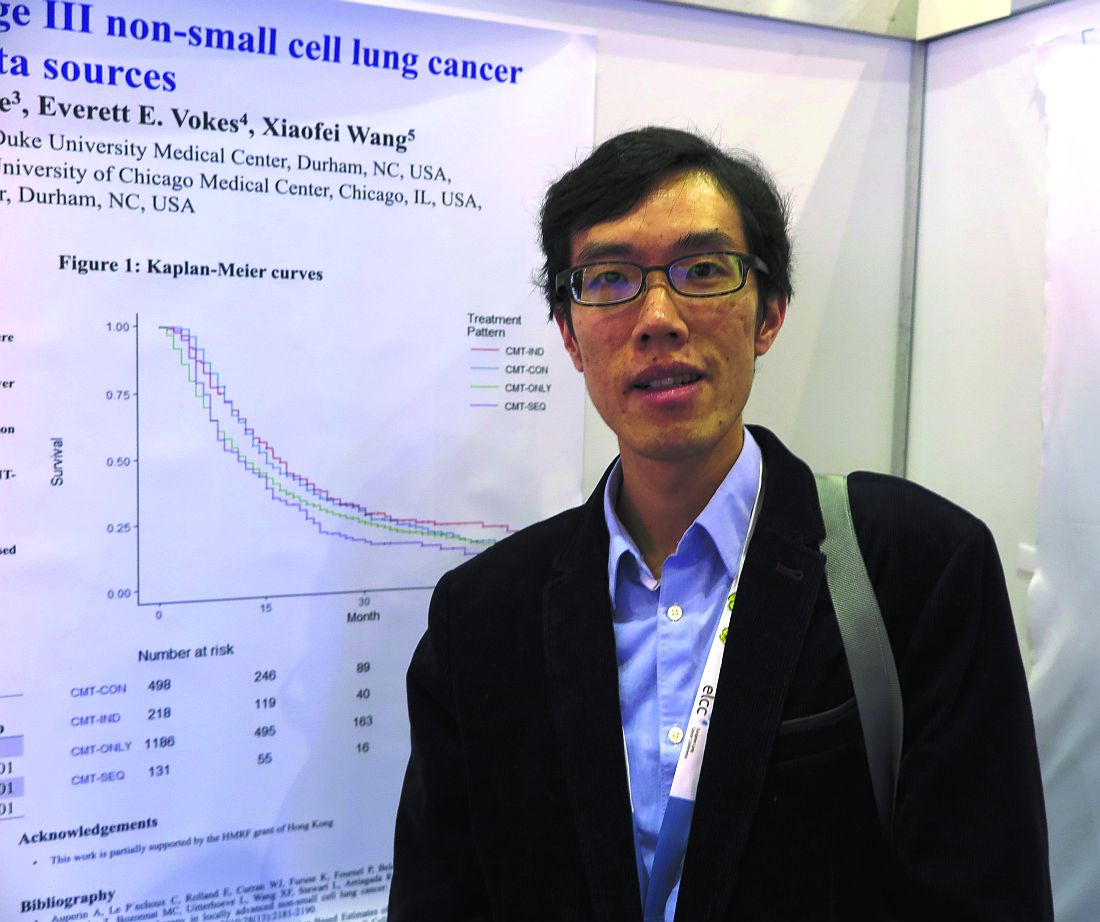
GENEVA – Among older adults with stage III non–small cell lung cancer (NSCLC), the sequencing of chemotherapy and radiation has a significant effect on overall survival, a team of investigators from Hong Kong and the United States reported.
Among 2,033 adults who were 65 years or older with locally advanced NSCLC and treated with one of four combined-modality therapy (CMT) schedules, both chemotherapy induction followed by concurrent therapy (CMT-IND) and concurrent therapy followed by consolidation chemoradiation (CMT-CON) were associated with an approximately 30% improvement in survival, compared with either sequential chemotherapy followed by radiation (CMT-SEQ) or concurrent therapy only (CMT-ONLY), reported Hei Man Herbert Pang, MD, of the University of Hong Kong and his colleagues.
The investigators used retrospective data from U.S. and Chinese sources to compare the relative survival benefits with various combined modality therapies. These included a Surveillance, Epidemiology, and End Results–Medicare cohort of patients 65 years and older with stage IIIA or IIIB NSCLC treated with CMT from 2006 through 2010 and a cohort of patients with the same age and NSCLC treated at Queen Mary Hospital in Hong Kong from 2007 through 2016.
They assessed neutropenia using inpatient claims data for episodes occurring within 130 days of the first chemotherapy cycle.
In an unadjusted analysis, they found that median overall survival, in descending order, was 16.1 months for CMT-SEQ,15.0 months for CMT-ONLY, 12.0 months for CMT-IND, and 11.0 months for CMT-CON.
When they controlled for variables, however, a different picture began to emerge.
For example, patients who were treated with CMT-SEQ had lower Charlson Comorbidity Index scores and, thus, were comparatively healthier than patients treated with other combined modalities.
Hospitalizations for neutropenia were most common with CMT-CON, occurring in 13.3% of patients, compared with 9.8% of patients treated with CMT-ONLY, 9.2% with CMT-IND, and 2.3% with CMT-SEQ.
In multivariable models controlling for sex, race, ethnicity, histology, and Charlson score, CMT-CON and CMT-IND were associated with significantly better overall, compared with CMT-SEQ, with respective hazard ratios for death of 0.68 (P less than .001) and 0.67 (P = .001). In this model, CMT-ONLY was not associated with significantly better survival.
In a propensity score model adjusted for the same factors, the respective HRs for CMT-CON, CMT-IND, and CMT-ONLY vs. CMT-SEQ were 0.69, 0.70, and 0.86 (P less than .001 for all three comparisons).
“The findings on efficacy and toxicity are quite consistent with previously reported studies based on clinical trials or observational databases,” the investigators said.
The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
GENEVA – Among older adults with stage III non–small cell lung cancer (NSCLC), the sequencing of chemotherapy and radiation has a significant effect on overall survival, a team of investigators from Hong Kong and the United States reported.
Among 2,033 adults who were 65 years or older with locally advanced NSCLC and treated with one of four combined-modality therapy (CMT) schedules, both chemotherapy induction followed by concurrent therapy (CMT-IND) and concurrent therapy followed by consolidation chemoradiation (CMT-CON) were associated with an approximately 30% improvement in survival, compared with either sequential chemotherapy followed by radiation (CMT-SEQ) or concurrent therapy only (CMT-ONLY), reported Hei Man Herbert Pang, MD, of the University of Hong Kong and his colleagues.
The investigators used retrospective data from U.S. and Chinese sources to compare the relative survival benefits with various combined modality therapies. These included a Surveillance, Epidemiology, and End Results–Medicare cohort of patients 65 years and older with stage IIIA or IIIB NSCLC treated with CMT from 2006 through 2010 and a cohort of patients with the same age and NSCLC treated at Queen Mary Hospital in Hong Kong from 2007 through 2016.
They assessed neutropenia using inpatient claims data for episodes occurring within 130 days of the first chemotherapy cycle.
In an unadjusted analysis, they found that median overall survival, in descending order, was 16.1 months for CMT-SEQ,15.0 months for CMT-ONLY, 12.0 months for CMT-IND, and 11.0 months for CMT-CON.
When they controlled for variables, however, a different picture began to emerge.
For example, patients who were treated with CMT-SEQ had lower Charlson Comorbidity Index scores and, thus, were comparatively healthier than patients treated with other combined modalities.
Hospitalizations for neutropenia were most common with CMT-CON, occurring in 13.3% of patients, compared with 9.8% of patients treated with CMT-ONLY, 9.2% with CMT-IND, and 2.3% with CMT-SEQ.
In multivariable models controlling for sex, race, ethnicity, histology, and Charlson score, CMT-CON and CMT-IND were associated with significantly better overall, compared with CMT-SEQ, with respective hazard ratios for death of 0.68 (P less than .001) and 0.67 (P = .001). In this model, CMT-ONLY was not associated with significantly better survival.
In a propensity score model adjusted for the same factors, the respective HRs for CMT-CON, CMT-IND, and CMT-ONLY vs. CMT-SEQ were 0.69, 0.70, and 0.86 (P less than .001 for all three comparisons).
“The findings on efficacy and toxicity are quite consistent with previously reported studies based on clinical trials or observational databases,” the investigators said.
The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
GENEVA – Among older adults with stage III non–small cell lung cancer (NSCLC), the sequencing of chemotherapy and radiation has a significant effect on overall survival, a team of investigators from Hong Kong and the United States reported.
Among 2,033 adults who were 65 years or older with locally advanced NSCLC and treated with one of four combined-modality therapy (CMT) schedules, both chemotherapy induction followed by concurrent therapy (CMT-IND) and concurrent therapy followed by consolidation chemoradiation (CMT-CON) were associated with an approximately 30% improvement in survival, compared with either sequential chemotherapy followed by radiation (CMT-SEQ) or concurrent therapy only (CMT-ONLY), reported Hei Man Herbert Pang, MD, of the University of Hong Kong and his colleagues.
The investigators used retrospective data from U.S. and Chinese sources to compare the relative survival benefits with various combined modality therapies. These included a Surveillance, Epidemiology, and End Results–Medicare cohort of patients 65 years and older with stage IIIA or IIIB NSCLC treated with CMT from 2006 through 2010 and a cohort of patients with the same age and NSCLC treated at Queen Mary Hospital in Hong Kong from 2007 through 2016.
They assessed neutropenia using inpatient claims data for episodes occurring within 130 days of the first chemotherapy cycle.
In an unadjusted analysis, they found that median overall survival, in descending order, was 16.1 months for CMT-SEQ,15.0 months for CMT-ONLY, 12.0 months for CMT-IND, and 11.0 months for CMT-CON.
When they controlled for variables, however, a different picture began to emerge.
For example, patients who were treated with CMT-SEQ had lower Charlson Comorbidity Index scores and, thus, were comparatively healthier than patients treated with other combined modalities.
Hospitalizations for neutropenia were most common with CMT-CON, occurring in 13.3% of patients, compared with 9.8% of patients treated with CMT-ONLY, 9.2% with CMT-IND, and 2.3% with CMT-SEQ.
In multivariable models controlling for sex, race, ethnicity, histology, and Charlson score, CMT-CON and CMT-IND were associated with significantly better overall, compared with CMT-SEQ, with respective hazard ratios for death of 0.68 (P less than .001) and 0.67 (P = .001). In this model, CMT-ONLY was not associated with significantly better survival.
In a propensity score model adjusted for the same factors, the respective HRs for CMT-CON, CMT-IND, and CMT-ONLY vs. CMT-SEQ were 0.69, 0.70, and 0.86 (P less than .001 for all three comparisons).
“The findings on efficacy and toxicity are quite consistent with previously reported studies based on clinical trials or observational databases,” the investigators said.
The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
FROM ELCC
Key clinical point: Some combined modality therapy options for older adults with NSCLC are associated with better overall survival.
Major finding: CMT-IND and CMT-CON were associated with a 30% improvement in overall survival, compared with CMT-SEQ.
Data source: Retrospective review of data on 2,033 adults 65 years and older with NSCLC in the United States and Hong Kong.
Disclosures: The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
Sex-based differences in lung cancer screening intervals suggested
GENEVA – A lung-cancer screening CT interval of once-yearly for men and once every 3 years for women appears to be the optimum schedule for detecting most early-stage lung cancers while minimizing radiation exposure, results of a retrospective study suggest.
Among 96 patients (85 men and 11 women) with lung cancers detected on follow-up screening CT, the mean interval time between initial CT and diagnostic CT was significantly longer among women than among men, at 5.6 vs. 3.6 years (P = .02), reported Mi-Young Kim, MD, a radiologist at Asan Medical Center in Seoul, South Korea.
Men tended to have a higher stage at diagnosis, however. Stage I cancers were diagnosed in 82% of women, but only 49% of men. Tumor size was also larger among men at presentation at a mean of 29.5 mm vs. 15.5 mm, Dr. Kim and her colleagues found.
Current lung cancer screening guidelines vary somewhat, but most recommend annual screening for people aged 55-80 years who have a 30 pack-year or greater smoking history and are current smokers or have quit within the last 15 years.
Prior studies to see whether longer screening intervals were safe have yielded mixed results, possibly because of differences in clinical and radiologic presentation between men and women, Dr. Kim said.
To explore sex differences in lung cancer at the time of diagnosis, she and her colleagues retrospectively reviewed records for 46,766 patients who underwent screening at their center from January 2000 through February 2016, during which time, 282 patients were diagnosed with lung cancer. Of this group, 186 were diagnosed from the initial screening CT scan, and 96 – the cohort included in the study – were diagnosed from subsequent scans.
The authors found that the majority of men (72%) had solid nodules as the primary pathology. In contrast, ground-glass opacities were the most common nodular finding among women, occurring in 45% of the cases. The most common histology among men was adenocarcinoma (42%), followed by squamous-cell carcinoma (35%), small cell lung cancer (18%), and others (5%). All women presented with adenocarcinoma histology.
“Because ground-glass opacity nodule is the most common feature of lung cancer in women, and all cases are adenocarcinoma, the growth rate of cancers might be low,” Dr. Kim said in a statement.
Only 2 of the 11 women in the study were smokers, compared with 74 of the 85 men.
Looking at the operability of lung cancer according to screening intervals, the investigators found that 100% of tumors detected at 1 year in men were operable, compared with 94% of those detected at 2 years, and 55% for those detected at the 3-year interval. In contrast, among women, there were no tumors detected at 1 year, one operable tumor and no inoperable tumors at 2 years, and two operable and no inoperable tumors at 3 years. Beyond 3 years, however, the rate of inoperable tumors at the time of diagnosis was 32% in men and 25% in women.
“We included all patients screened for lung cancer in a 17-year period, but the number of women patients was low and further studies are needed to confirm the sex differences we found,” Dr. Kim acknowledged.
“Only when this is adjusted for – when we compare smokers of the same pack-year history – then we can look at whether women and men do have different rates of appearance of nodules,” he said.
“To me, this is a very interesting suggestion that indeed the biology of the growth of these nodules may be different in men and women, but only if we are sure that the main environmental factor is taken care of.”
The study funding source was not disclosed.
Dr. Kim and Dr. Boffetta reported no relevant disclosures.
GENEVA – A lung-cancer screening CT interval of once-yearly for men and once every 3 years for women appears to be the optimum schedule for detecting most early-stage lung cancers while minimizing radiation exposure, results of a retrospective study suggest.
Among 96 patients (85 men and 11 women) with lung cancers detected on follow-up screening CT, the mean interval time between initial CT and diagnostic CT was significantly longer among women than among men, at 5.6 vs. 3.6 years (P = .02), reported Mi-Young Kim, MD, a radiologist at Asan Medical Center in Seoul, South Korea.
Men tended to have a higher stage at diagnosis, however. Stage I cancers were diagnosed in 82% of women, but only 49% of men. Tumor size was also larger among men at presentation at a mean of 29.5 mm vs. 15.5 mm, Dr. Kim and her colleagues found.
Current lung cancer screening guidelines vary somewhat, but most recommend annual screening for people aged 55-80 years who have a 30 pack-year or greater smoking history and are current smokers or have quit within the last 15 years.
Prior studies to see whether longer screening intervals were safe have yielded mixed results, possibly because of differences in clinical and radiologic presentation between men and women, Dr. Kim said.
To explore sex differences in lung cancer at the time of diagnosis, she and her colleagues retrospectively reviewed records for 46,766 patients who underwent screening at their center from January 2000 through February 2016, during which time, 282 patients were diagnosed with lung cancer. Of this group, 186 were diagnosed from the initial screening CT scan, and 96 – the cohort included in the study – were diagnosed from subsequent scans.
The authors found that the majority of men (72%) had solid nodules as the primary pathology. In contrast, ground-glass opacities were the most common nodular finding among women, occurring in 45% of the cases. The most common histology among men was adenocarcinoma (42%), followed by squamous-cell carcinoma (35%), small cell lung cancer (18%), and others (5%). All women presented with adenocarcinoma histology.
“Because ground-glass opacity nodule is the most common feature of lung cancer in women, and all cases are adenocarcinoma, the growth rate of cancers might be low,” Dr. Kim said in a statement.
Only 2 of the 11 women in the study were smokers, compared with 74 of the 85 men.
Looking at the operability of lung cancer according to screening intervals, the investigators found that 100% of tumors detected at 1 year in men were operable, compared with 94% of those detected at 2 years, and 55% for those detected at the 3-year interval. In contrast, among women, there were no tumors detected at 1 year, one operable tumor and no inoperable tumors at 2 years, and two operable and no inoperable tumors at 3 years. Beyond 3 years, however, the rate of inoperable tumors at the time of diagnosis was 32% in men and 25% in women.
“We included all patients screened for lung cancer in a 17-year period, but the number of women patients was low and further studies are needed to confirm the sex differences we found,” Dr. Kim acknowledged.
“Only when this is adjusted for – when we compare smokers of the same pack-year history – then we can look at whether women and men do have different rates of appearance of nodules,” he said.
“To me, this is a very interesting suggestion that indeed the biology of the growth of these nodules may be different in men and women, but only if we are sure that the main environmental factor is taken care of.”
The study funding source was not disclosed.
Dr. Kim and Dr. Boffetta reported no relevant disclosures.
GENEVA – A lung-cancer screening CT interval of once-yearly for men and once every 3 years for women appears to be the optimum schedule for detecting most early-stage lung cancers while minimizing radiation exposure, results of a retrospective study suggest.
Among 96 patients (85 men and 11 women) with lung cancers detected on follow-up screening CT, the mean interval time between initial CT and diagnostic CT was significantly longer among women than among men, at 5.6 vs. 3.6 years (P = .02), reported Mi-Young Kim, MD, a radiologist at Asan Medical Center in Seoul, South Korea.
Men tended to have a higher stage at diagnosis, however. Stage I cancers were diagnosed in 82% of women, but only 49% of men. Tumor size was also larger among men at presentation at a mean of 29.5 mm vs. 15.5 mm, Dr. Kim and her colleagues found.
Current lung cancer screening guidelines vary somewhat, but most recommend annual screening for people aged 55-80 years who have a 30 pack-year or greater smoking history and are current smokers or have quit within the last 15 years.
Prior studies to see whether longer screening intervals were safe have yielded mixed results, possibly because of differences in clinical and radiologic presentation between men and women, Dr. Kim said.
To explore sex differences in lung cancer at the time of diagnosis, she and her colleagues retrospectively reviewed records for 46,766 patients who underwent screening at their center from January 2000 through February 2016, during which time, 282 patients were diagnosed with lung cancer. Of this group, 186 were diagnosed from the initial screening CT scan, and 96 – the cohort included in the study – were diagnosed from subsequent scans.
The authors found that the majority of men (72%) had solid nodules as the primary pathology. In contrast, ground-glass opacities were the most common nodular finding among women, occurring in 45% of the cases. The most common histology among men was adenocarcinoma (42%), followed by squamous-cell carcinoma (35%), small cell lung cancer (18%), and others (5%). All women presented with adenocarcinoma histology.
“Because ground-glass opacity nodule is the most common feature of lung cancer in women, and all cases are adenocarcinoma, the growth rate of cancers might be low,” Dr. Kim said in a statement.
Only 2 of the 11 women in the study were smokers, compared with 74 of the 85 men.
Looking at the operability of lung cancer according to screening intervals, the investigators found that 100% of tumors detected at 1 year in men were operable, compared with 94% of those detected at 2 years, and 55% for those detected at the 3-year interval. In contrast, among women, there were no tumors detected at 1 year, one operable tumor and no inoperable tumors at 2 years, and two operable and no inoperable tumors at 3 years. Beyond 3 years, however, the rate of inoperable tumors at the time of diagnosis was 32% in men and 25% in women.
“We included all patients screened for lung cancer in a 17-year period, but the number of women patients was low and further studies are needed to confirm the sex differences we found,” Dr. Kim acknowledged.
“Only when this is adjusted for – when we compare smokers of the same pack-year history – then we can look at whether women and men do have different rates of appearance of nodules,” he said.
“To me, this is a very interesting suggestion that indeed the biology of the growth of these nodules may be different in men and women, but only if we are sure that the main environmental factor is taken care of.”
The study funding source was not disclosed.
Dr. Kim and Dr. Boffetta reported no relevant disclosures.
FROM ELCC
Key clinical point:
Major finding: A repeated CT scan for lung cancer every year in men and every 3 years in women picked up most new, operable lesions.
Data source: Retrospective review of data on 96 patients with lung cancer diagnosed at a second or subsequent CT screening.
Disclosures: The study funding source was not disclosed. Dr. Kim and Dr. Boffetta reported no relevant disclosures.
Flu shots may spark immune adverse events in PD-1 blockade for NSCLC
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
AT ELCC
Key clinical point:
Major finding: Of 23 patients who were vaccinated, 12 developed IrAEs, including 6 grade 1/2 and 6 grade 3/4 events.
Data source: Prospective study of 23 patients with NSCLC and 23 healthy controls.
Disclosures: The study was supported by institutional funding. The investigators reported no relevant conflicts of interest.