User login
Discussing breast cancer and hormone replacement therapy with women
Erratum (2002;70:185-192)
How to control migraines in patients with psychiatric disorders
Many of the 28 million people who suffer from migraine headaches each year1 need psychiatric care in addition to headache relief. Migraine headaches often coexist with depression,2 anxiety/panic disorders,2,3 bipolar disorder,4 and phobias,5 as well as with stroke6 and epilepsy.7 A study of 995 young adults found that anxiety disorders, phobias, major depression, panic disorder, and obsessive-compulsive disorder were two to five times more prevalent among migraine sufferers than among a control group (Table 1 ).2
Migraine sufferers know that at any time an attack could hamper their ability to work, care for their families, or engage in social activities. A nationwide study of migraineurs found that attacks often impaired their relationships with family and friends.8
Psychiatrists should screen patients for a history of migraine or other headaches and carefully consider the relationship between migraines and psychiatric disorders when prescribing treatment. In this article, we outline acute and preventive headache treatments and present two cases to help you treat these patients appropriately.
Table 1
PSYCHIATRIC COMORBIDITIES IN PATIENTS WITH VS. WITHOUT MIGRAINES*
| Migraineurs (%) (n = 128) | Controls (%) (n = 879) | |
|---|---|---|
| Any anxiety | 54 | 27 |
| Generalized anxiety disorder | 10 | 2 |
| Phobia | 40 | 21 |
| Major depression | 35 | 10 |
| Panic disorder | 11 | 2 |
| Obsessive-compulsive disorder | 9 | 2 |
| * Prevalence Source: Breslau N, Davis GC. Cephalalgia 1992;12(2):85-90. | ||
Table 2
THREE TYPES OF PRIMARY HEADACHE: DIAGNOSTIC CRITERIA
| Headache type | Age of onset (years) | Location | Duration | Frequency/timing | Severity | Quality | Features |
|---|---|---|---|---|---|---|---|
| Migraine | 10 to 40 | Hemicranial | 4 to 72 hr | Variable | Moderate to severe | Throbbing, steady ache | Nausea; vomiting; photo/phono/osmophobia; neurologic deficits; aura |
| Tension-type | 20 to 50 | Bilateral/generalized | 30 min to 7 days+ | Variable | Dull ache, may wax and wane | Tight, band-like pressure | Generally none |
| Cluster | 15 to 40 | Unilateral, periorbital or retro-orbital | 15 to 180 min | 1 to 8 times per day or night | Excruciating | Boring, piercing | Ipsilateral, conjunctival injection, nasal congestion, rhinorrhea, miosis, facial seating |
| Source: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96. | |||||||
Headache definitions and diagnosis
Primary or secondary. Under the International Headache Society’s (IHS) 1988 headache classification and diagnostic criteria,9 headaches are primary or secondary:
- Primary headaches are benign recurrent headaches that commonly present in practice.
- Secondary headaches occur much less frequently and are caused by underlying pathology.
The possibility of secondary headache should be ruled out before a primary headache can be diagnosed. The following headache features should cause concern:
- Severe headache with abrupt onset
- Subacute or progressive headache over days or months
- Headache, nausea, vomiting, and fever not explained by systemic illness
- New-onset headache late in life
- Headache with neurologic signs or symptoms such as confusion, decreased level of consciousness, meningismus, or papilledema
- Headache following head trauma
- Patient history of sickle cell disease, malignancy, or HIV.
Headache types. The three major types of primary headache are migraine, tension-type, and cluster (Table 2 ). Tension-type is the most common, is often mild, and is either self-treated with over-the-counter medications or ignored. Migraine is the most troublesome headache in everyday practice. Cluster is the most severe and fortunately is rare.
Migraine with aura and migraine without aura are separate diagnoses. IHS criteria for diagnosing migraines without aura are listed in Table 3. According to the IHS, migraine with aura (or “classic migraine”) fulfills all the criteria for migraine without aura, with fully reversible neurologic symptoms indicating focal cerebral cortical and/or brain stem dysfunction.
Auras. About 15% of migraineurs experience auras. Symptoms develop within 5 to 20 minutes, usually last less than 1 hour, and fade before the headache’s onset. Gradual onset and history of previous attacks helps to distinguish aura from transient ischemic attacks. Auras may manifest as visual, sensory, motor, or brain-stem symptoms, or as combinations of these:
- Visual auras are most common, presenting as localized visual loss (scotoma), with flashing lights (scintillation) at margins or jagged edges (fortification).
- Sensory auras present as facial or limb paresthesias.
- Motor auras manifest as weakness or lack of coordination.
- Brain stem auras manifest as vertigo or double vision.
Migraine aura is considered part of the headache’s prodrome, which may occur days or hours before the headache’s onset. The aura may bring about:
- an altered mental state (e.g., depression, hyperactivity, euphoria, difficulty concentrating, dysphasia)
- neurologic symptoms (e.g., photophobia, phonophobia, hyperosmia, yawning)
- general bodily discomforts (e.g., anorexia, food craving, diarrhea, thirst, urination, fluid retention, cold feeling).
Despite their sometimes severe effects, migraines often remain undiagnosed.10 Migraine should be suspected in patients with recurrent moderate to severe disabling headaches (Box).11-15
Case 1: “Bad, sick headaches”
Ms. A, 23, a single parent with a 2-year-old child, has had trouble staying employed because of repeated illnesses. She made 17 visits to her primary care physician within 26 months. While her main complaint was headache, she also complained of other aches and pains, a lack of energy, and insomnia. Numerous examinations revealed no physical abnormalities.
She reported having “bad, sick headaches” that sometimes lasted 2 to 3 days. Bed rest helped but this was not always possible. The headache was throbbing and usually one-sided. She had no aura, and ibuprofen gave partial relief. She noted that her mother gets similar headaches.
Table 3
DIAGNOSTIC REQUIREMENTS FOR MIGRAINE WITHOUT AURA
Mandatory
|
At least 2 of the following:
|
| During headache At least one of the following:
|
| Additional features Migraine prodrome—A range of general, neurologic, and mental changes may occur hours or days before the headache’s onset General—Anorexia, food craving, diarrhea or constipation, thirst, urination, fluid retention, cold feeling Mental—Depression, hyperactivity, euphoria, difficulty concentrating, dysphasia Neurologic—Photophobia, phonophobia, hyperosmia, yawning |
| SOURCE: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96. |
Ms. A was diagnosed with migraine without aura, and she was treated with sumatriptan, 100 mg (1 or 2 doses, as needed). Her headaches responded well to this treatment, but the frequency of attacks remained unchanged. She requested a change in her medicine.
The underlying mechanisms of migraine headaches are not completely understood.
Vascular causes. A recently described neurovascular mechanism11 suggests that perivascular neurogenic inflammation involving meningeal vessels causes migraine. The triptan drugs have been found to reverse this process and relieve the headache.12
Positron emission tomography has demonstrated increased blood flow during acute migraine in midline brain stem structures. This suggests the presence of a central migraine generator in that location.13
Heredity. A rare form of migraine, familial hemiplegic migraine, is associated with a genetic abnormality on chromosome 19.14
Nitric oxide. Nitroglycerine-induced migraine headache, caused by the release of nitric oxide in cerebral vessels, can be reversed by nitrous oxide synthase inhibitors, thus opening up intriguing possibilities of new therapeutic agents and increased understanding of underlying migraine mechanisms.15
Treating migraines
Acute treatment. Migraineurs whose attacks are infrequent and mild may find OTC analgesics or NSAIDs adequate. Most patients, however, require specific migraine treatment, usually with triptans. Acute oral treatment options include sumatriptan, 50 to 100 mg; rizatriptan, 10 mg; zolmitriptan, 2.5 to 5 mg; and eletriptan, 40 mg.
In case of vomiting or nausea, options include sumatriptan, 20 mg nasal spray or 6 mg SC; rizatriptan, 10 mg on a dissolving wafer; or dihydroergotamine, 2 mg nasal spray or 1 mg IM or SC. For severe nausea or vomiting, an anti-nauseant (e.g., prochlorperazine suppositories, 25 mg) may be of value.
Preventive treatment. Preventive treatment may be warranted, depending on attack frequency, severity, and the extent of disability caused. One prolonged, severe attack per month that responds poorly to acute treatment may indicate the need for preventive treatment. A range of preventative treatments is available (Table 4).
In Ms. A’s case, oral sumatriptan lessened the severity of the migraine attacks, and the addition of nortriptyline, 50 mg/d, reduced frequency by about 50%. She felt more energetic overall and was sleeping better.
Treating the psychiatric comorbidity
Behavioral therapy is used as an adjunct to pharmacologic headache treatment. This approach is usually considered after a poor or adverse response to treatment, or when pharmacologic treatment is contraindicated (e.g., during pregnancy).
Relaxation training, biofeedback, and cognitive-behavioral stress management are the most commonly used forms of behavioral therapy. Thirty-five to 55% improvement in migraine has been reported following such treatments.16
Cognitive-behavioral intervention has been shown to be effective in depression17 and anxiety disorders.18 When either psychiatric problem is comorbid with migraine, cognitive therapy can improve both the migraine and the psychiatric comorbidity.
Pharmacologic therapy. Depression is commonly associated with migraine and may be caused by living with chronic disabling headaches over time. In such cases, the depression will improve as the migraine responds to treatment. However, in cases where comorbid depression or anxiety trigger or exacerbate acute migraine attacks, neither the migraine nor the psychiatric problem responds until the underlying psychopathology is treated. In such cases, simultaneous psychiatric and migraine pharmacologic treatment is required.
We recommend that you treat the psychiatric comorbidity as it would be treated without a co-existing migraine. Be advised, however, that monoamine oxidase inhibitors are contraindicated in depression during the 2 weeks before treating the comorbid migraine with a triptan. If the patient does not respond or if there is concern regarding possible underlying pathology, consult with a clinician who specializes in headache treatment.
Precipitating and aggravating factors
Headache triggers. Helping patients to recognize headache triggers and aggravating factors is an important element in treating and preventing migraines. Identifying these factors in the patient history can help you establish a diagnosis and implement steps to avoid or reduce attack severity.
Table 4
TREATMENT OPTIONS FOR PREVENTING MIGRAINE ATTACKS
| Drug | Efficacy* | Side effects* | Indications | Contraindications |
|---|---|---|---|---|
| β blockers | 4+ | 2+ | Hypertension | Depression, asthma, diabetes, hypotension, congestive heart failure, peripheral vascular disease |
| Ca channel blockers | 2+ | 1+ | Hypertension, angina, asthma, migraine aura | Constipation, hypotension |
| Tricyclic antidepressants | 4+ | 2+ | Depression, anxiety disorders, insomnia | Heart block, urinary retention, mania |
| Selective serotonin reuptake inhibitors | 2+ | 1+ | Depression, obsessive-compulsive disorder | Mania |
| Monoamine oxidase inhibitors | 4+ | 4+ | Depression | Dietary restrictions |
| Divalproex/valproate | 4+ | 2+ | Epilepsy, anxiety disorders, mania | Liver disease, bleeding disorders, hair loss |
| Naproxen | 2+ | 2+ | Arthritis, other pain disorders | Gastritis, peptic ulcer |
| * Ratings on a scale from 1+ (lowest) to 4+ (highest). Table partially derived from data in: Silberstein SD, Lipton RB, Goadsby PJ, Smith, R (eds). Headache in primary care. Oxford, UK: Isis Medical Media, 1999. | ||||
Common migraine headache triggers include menstruation, stress, relaxation after stress, fatigue, too much or too little sleep, skipping meals, weather changes, high humidity, glare and flickering lights, loud or high-pitched noises, smoke or dust, strong perfumes or cooking aromas. Food triggers cause 10% of migraine cases. Chocolate, strong cheeses, red wine, beer, citrus fruits, and foods with monosodium glutamate and nitrate preservatives are common food triggers.
Tension-type headaches are triggered by stress or the end of a stress-filled day. Triggers for cluster-type headaches include alcohol, smoking during the cluster phase, and lying down during an attack.
Case 2: Flying the unfriendly skies
Ms. B, 38, is a mother of three who works as a flight attendant. She is separated from her husband and had filed for divorce because of repeated spousal abuse. She has visited her primary care physician multiple times for migraine, sinus problems, backache, and coccygodynia. Orthopedic and rectal examinations revealed no abnormalities.
Her headaches met the IHS diagnostic criteria for migraine with aura, and these responded well to zolmitriptan, 5 mg. The headaches usually occurred during days off from work, but her sinus problems also led to headaches and nasal stuffiness when she flew. Her headaches eventually occurred almost daily.
Her supervisor was unsympathetic. An otolaryngologist had prescribed decongestants and a course of desensitization, both of which brought only transient relief.
A counselor at work recommended that Ms. B go on sick leave and accept a transfer to a non-flying job. The patient was tearful and felt overwhelmed by her problems. She felt that life was no longer worth living. She agreed to see a psychiatrist, who diagnosed depression and anxiety disorder. The psychiatrist prescribed citalopram, 20 mg/d, and agreed to see her regularly to monitor progress.
Discussion. As a migraineur, Ms. B was at increased risk for depression and anxiety disorders.19 Migraine with aura is associated with an increased lifetime prevalence of suicidal ideation and suicide attempts.20
The exact mechanisms by which migraine and depression are related are unknown. Each disorder increases the risk for developing the other. The specificity of this relationship is strengthened by the fact that depression is not associated with a greater risk of severe nonmigrainous headache, even though a severe nonmigrainous headache may cause depression.21
The patient in case 1 responded well when an antidepressant was added to her treatment. In her case, the diagnosis of a depressive disorder remained an open question. Migraine attacks are known to be associated with mood change, lethargy, and cognitive changes. The picture may be further confounded because migraine without depression responds well to prophylaxis with antidepressants.
The patient in case 2, however, presented with a complex of interrelated headache and psychiatric problems of potentially dangerous proportions. Psychiatric problems in migraineurs may be deep-seated, and these patients may require urgent, specialized attention to avoid further serious disability and a possible tragic outcome.
Related resources
- Silberstein SD, Lipton RB, Goadsby PJ, Smith, R, eds. Headache in primary care. Oxford, UK: Isis Medical Media, 1999.
- Silberstein SD, Lipton RB, Dalessio DJ. Wolff’s headache and other head pain (7th ed). New York: Oxford University Press, 2001.
- Davidoff RA. Migraine. Manifestations, pathogenesis and management (2nd ed). New York: Oxford University Press, 2002.
- International Headache Society. http://www.i-h-s.org
Drug brand names
- Citalopram • Celexa
- Dihydroergotamine • Migranal
- Eletriptan • Relpax
- Rizatriptan • Maxalt
- Sumatriptan • Imitrex
- Valproate sodium • Depakote
- Zolmitriptan • Zomig
Disclosure
Dr. Smith reports that he serves as a consultant to and is on the speakers’ bureau of AstraZeneca Pharmaceuticals.
Dr. Hasse reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lipton RB, Stewart WF, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41(7):646-57.
2. Breslau N, Davis GC. Migraine, major depression and panic disorder: A prospective epidemiologic study of young adults. Cephalalgia 1992;12(2):85-90.
3. Stewart WF, Linet MS, Celentano DD. Migraine headaches and panic attacks. Psychosom Med 1989;51(5):559-69.
4. Mersky H, Peatfield RC. Headache in the psychiatrically ill. In: Olesen J, Tfelt-Hensen P, Welch KMA (eds). The headaches (2nd ed). Baltimore: Lippincott, Williams and Wilkins, 1999;962-3.
5. Davidoff RA. Comorbidity. In: Davidoff RA (ed). Migraine manifestations, pathogenesis, and management (2nd ed). Oxford, UK: Oxford University Press, 2002;21-2.
6. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. BMJ 1999;318(7175):13-8.
7. Migraine-epilepsy relationships: epidemiological and genetic aspects. In: Andermann FA, Lugaresi E (eds). Migraine and epilepsy. Boston: Butterworths, 1987.
8. Smith R. Impact of migraine on the family. Headache 1998;38(6):423-6.
9. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96.
10. Smith R, Hasse LA, Ritchey PN, et al. Extent of migraine and migrainous headache in headache NOS patients in family practice. Cephalalgia 2001;21:291-2.
11. Olesen J, Friberg L, et al. Basic mechanisms in vascular headache. Neurol Clin 1990;8:801-15.
12. Sumatriptan—an oral dose-defining study. The Oral Sumatriptan Dose-Defining Study Group. Eur Neurol 1991;31(5):300-5.
13. Diener HC. Positron emission tomography studies in headache. Headache 1997;37(10):622-5.
14. Joutel A, Bousser MG, V Biousse, et al. A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet 1993;5(1):40-5.
15. Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab 1994;14(2):175-92.
16. Holroyd KA, Penzien DB, Lipchik GL. Efficacy of Behavioral Treatments. In: Silberstein SD, Lipton RB, Dalessio DJ (eds). Wolf’s headache and other head pain (7th ed). New York: Oxford University Press, 2001;563-6.
17. Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: A comprehensive review of controlled outcome research. Psychol Bull 1990;108:30-49.
18. Gould RA, Otto MW, et al. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder. Behav Ther 1997;28:285-305.
19. Breslau N, Andreski P. Migraine, personality and psychiatric comorbidity. Headache 1995;35(7):382-6.
20. Breslau N. Migraine, suicidal ideation, and suicide attempts. Neurology 1992;42(2):392-5.
21. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54(2):308-13.
Many of the 28 million people who suffer from migraine headaches each year1 need psychiatric care in addition to headache relief. Migraine headaches often coexist with depression,2 anxiety/panic disorders,2,3 bipolar disorder,4 and phobias,5 as well as with stroke6 and epilepsy.7 A study of 995 young adults found that anxiety disorders, phobias, major depression, panic disorder, and obsessive-compulsive disorder were two to five times more prevalent among migraine sufferers than among a control group (Table 1 ).2
Migraine sufferers know that at any time an attack could hamper their ability to work, care for their families, or engage in social activities. A nationwide study of migraineurs found that attacks often impaired their relationships with family and friends.8
Psychiatrists should screen patients for a history of migraine or other headaches and carefully consider the relationship between migraines and psychiatric disorders when prescribing treatment. In this article, we outline acute and preventive headache treatments and present two cases to help you treat these patients appropriately.
Table 1
PSYCHIATRIC COMORBIDITIES IN PATIENTS WITH VS. WITHOUT MIGRAINES*
| Migraineurs (%) (n = 128) | Controls (%) (n = 879) | |
|---|---|---|
| Any anxiety | 54 | 27 |
| Generalized anxiety disorder | 10 | 2 |
| Phobia | 40 | 21 |
| Major depression | 35 | 10 |
| Panic disorder | 11 | 2 |
| Obsessive-compulsive disorder | 9 | 2 |
| * Prevalence Source: Breslau N, Davis GC. Cephalalgia 1992;12(2):85-90. | ||
Table 2
THREE TYPES OF PRIMARY HEADACHE: DIAGNOSTIC CRITERIA
| Headache type | Age of onset (years) | Location | Duration | Frequency/timing | Severity | Quality | Features |
|---|---|---|---|---|---|---|---|
| Migraine | 10 to 40 | Hemicranial | 4 to 72 hr | Variable | Moderate to severe | Throbbing, steady ache | Nausea; vomiting; photo/phono/osmophobia; neurologic deficits; aura |
| Tension-type | 20 to 50 | Bilateral/generalized | 30 min to 7 days+ | Variable | Dull ache, may wax and wane | Tight, band-like pressure | Generally none |
| Cluster | 15 to 40 | Unilateral, periorbital or retro-orbital | 15 to 180 min | 1 to 8 times per day or night | Excruciating | Boring, piercing | Ipsilateral, conjunctival injection, nasal congestion, rhinorrhea, miosis, facial seating |
| Source: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96. | |||||||
Headache definitions and diagnosis
Primary or secondary. Under the International Headache Society’s (IHS) 1988 headache classification and diagnostic criteria,9 headaches are primary or secondary:
- Primary headaches are benign recurrent headaches that commonly present in practice.
- Secondary headaches occur much less frequently and are caused by underlying pathology.
The possibility of secondary headache should be ruled out before a primary headache can be diagnosed. The following headache features should cause concern:
- Severe headache with abrupt onset
- Subacute or progressive headache over days or months
- Headache, nausea, vomiting, and fever not explained by systemic illness
- New-onset headache late in life
- Headache with neurologic signs or symptoms such as confusion, decreased level of consciousness, meningismus, or papilledema
- Headache following head trauma
- Patient history of sickle cell disease, malignancy, or HIV.
Headache types. The three major types of primary headache are migraine, tension-type, and cluster (Table 2 ). Tension-type is the most common, is often mild, and is either self-treated with over-the-counter medications or ignored. Migraine is the most troublesome headache in everyday practice. Cluster is the most severe and fortunately is rare.
Migraine with aura and migraine without aura are separate diagnoses. IHS criteria for diagnosing migraines without aura are listed in Table 3. According to the IHS, migraine with aura (or “classic migraine”) fulfills all the criteria for migraine without aura, with fully reversible neurologic symptoms indicating focal cerebral cortical and/or brain stem dysfunction.
Auras. About 15% of migraineurs experience auras. Symptoms develop within 5 to 20 minutes, usually last less than 1 hour, and fade before the headache’s onset. Gradual onset and history of previous attacks helps to distinguish aura from transient ischemic attacks. Auras may manifest as visual, sensory, motor, or brain-stem symptoms, or as combinations of these:
- Visual auras are most common, presenting as localized visual loss (scotoma), with flashing lights (scintillation) at margins or jagged edges (fortification).
- Sensory auras present as facial or limb paresthesias.
- Motor auras manifest as weakness or lack of coordination.
- Brain stem auras manifest as vertigo or double vision.
Migraine aura is considered part of the headache’s prodrome, which may occur days or hours before the headache’s onset. The aura may bring about:
- an altered mental state (e.g., depression, hyperactivity, euphoria, difficulty concentrating, dysphasia)
- neurologic symptoms (e.g., photophobia, phonophobia, hyperosmia, yawning)
- general bodily discomforts (e.g., anorexia, food craving, diarrhea, thirst, urination, fluid retention, cold feeling).
Despite their sometimes severe effects, migraines often remain undiagnosed.10 Migraine should be suspected in patients with recurrent moderate to severe disabling headaches (Box).11-15
Case 1: “Bad, sick headaches”
Ms. A, 23, a single parent with a 2-year-old child, has had trouble staying employed because of repeated illnesses. She made 17 visits to her primary care physician within 26 months. While her main complaint was headache, she also complained of other aches and pains, a lack of energy, and insomnia. Numerous examinations revealed no physical abnormalities.
She reported having “bad, sick headaches” that sometimes lasted 2 to 3 days. Bed rest helped but this was not always possible. The headache was throbbing and usually one-sided. She had no aura, and ibuprofen gave partial relief. She noted that her mother gets similar headaches.
Table 3
DIAGNOSTIC REQUIREMENTS FOR MIGRAINE WITHOUT AURA
Mandatory
|
At least 2 of the following:
|
| During headache At least one of the following:
|
| Additional features Migraine prodrome—A range of general, neurologic, and mental changes may occur hours or days before the headache’s onset General—Anorexia, food craving, diarrhea or constipation, thirst, urination, fluid retention, cold feeling Mental—Depression, hyperactivity, euphoria, difficulty concentrating, dysphasia Neurologic—Photophobia, phonophobia, hyperosmia, yawning |
| SOURCE: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96. |
Ms. A was diagnosed with migraine without aura, and she was treated with sumatriptan, 100 mg (1 or 2 doses, as needed). Her headaches responded well to this treatment, but the frequency of attacks remained unchanged. She requested a change in her medicine.
The underlying mechanisms of migraine headaches are not completely understood.
Vascular causes. A recently described neurovascular mechanism11 suggests that perivascular neurogenic inflammation involving meningeal vessels causes migraine. The triptan drugs have been found to reverse this process and relieve the headache.12
Positron emission tomography has demonstrated increased blood flow during acute migraine in midline brain stem structures. This suggests the presence of a central migraine generator in that location.13
Heredity. A rare form of migraine, familial hemiplegic migraine, is associated with a genetic abnormality on chromosome 19.14
Nitric oxide. Nitroglycerine-induced migraine headache, caused by the release of nitric oxide in cerebral vessels, can be reversed by nitrous oxide synthase inhibitors, thus opening up intriguing possibilities of new therapeutic agents and increased understanding of underlying migraine mechanisms.15
Treating migraines
Acute treatment. Migraineurs whose attacks are infrequent and mild may find OTC analgesics or NSAIDs adequate. Most patients, however, require specific migraine treatment, usually with triptans. Acute oral treatment options include sumatriptan, 50 to 100 mg; rizatriptan, 10 mg; zolmitriptan, 2.5 to 5 mg; and eletriptan, 40 mg.
In case of vomiting or nausea, options include sumatriptan, 20 mg nasal spray or 6 mg SC; rizatriptan, 10 mg on a dissolving wafer; or dihydroergotamine, 2 mg nasal spray or 1 mg IM or SC. For severe nausea or vomiting, an anti-nauseant (e.g., prochlorperazine suppositories, 25 mg) may be of value.
Preventive treatment. Preventive treatment may be warranted, depending on attack frequency, severity, and the extent of disability caused. One prolonged, severe attack per month that responds poorly to acute treatment may indicate the need for preventive treatment. A range of preventative treatments is available (Table 4).
In Ms. A’s case, oral sumatriptan lessened the severity of the migraine attacks, and the addition of nortriptyline, 50 mg/d, reduced frequency by about 50%. She felt more energetic overall and was sleeping better.
Treating the psychiatric comorbidity
Behavioral therapy is used as an adjunct to pharmacologic headache treatment. This approach is usually considered after a poor or adverse response to treatment, or when pharmacologic treatment is contraindicated (e.g., during pregnancy).
Relaxation training, biofeedback, and cognitive-behavioral stress management are the most commonly used forms of behavioral therapy. Thirty-five to 55% improvement in migraine has been reported following such treatments.16
Cognitive-behavioral intervention has been shown to be effective in depression17 and anxiety disorders.18 When either psychiatric problem is comorbid with migraine, cognitive therapy can improve both the migraine and the psychiatric comorbidity.
Pharmacologic therapy. Depression is commonly associated with migraine and may be caused by living with chronic disabling headaches over time. In such cases, the depression will improve as the migraine responds to treatment. However, in cases where comorbid depression or anxiety trigger or exacerbate acute migraine attacks, neither the migraine nor the psychiatric problem responds until the underlying psychopathology is treated. In such cases, simultaneous psychiatric and migraine pharmacologic treatment is required.
We recommend that you treat the psychiatric comorbidity as it would be treated without a co-existing migraine. Be advised, however, that monoamine oxidase inhibitors are contraindicated in depression during the 2 weeks before treating the comorbid migraine with a triptan. If the patient does not respond or if there is concern regarding possible underlying pathology, consult with a clinician who specializes in headache treatment.
Precipitating and aggravating factors
Headache triggers. Helping patients to recognize headache triggers and aggravating factors is an important element in treating and preventing migraines. Identifying these factors in the patient history can help you establish a diagnosis and implement steps to avoid or reduce attack severity.
Table 4
TREATMENT OPTIONS FOR PREVENTING MIGRAINE ATTACKS
| Drug | Efficacy* | Side effects* | Indications | Contraindications |
|---|---|---|---|---|
| β blockers | 4+ | 2+ | Hypertension | Depression, asthma, diabetes, hypotension, congestive heart failure, peripheral vascular disease |
| Ca channel blockers | 2+ | 1+ | Hypertension, angina, asthma, migraine aura | Constipation, hypotension |
| Tricyclic antidepressants | 4+ | 2+ | Depression, anxiety disorders, insomnia | Heart block, urinary retention, mania |
| Selective serotonin reuptake inhibitors | 2+ | 1+ | Depression, obsessive-compulsive disorder | Mania |
| Monoamine oxidase inhibitors | 4+ | 4+ | Depression | Dietary restrictions |
| Divalproex/valproate | 4+ | 2+ | Epilepsy, anxiety disorders, mania | Liver disease, bleeding disorders, hair loss |
| Naproxen | 2+ | 2+ | Arthritis, other pain disorders | Gastritis, peptic ulcer |
| * Ratings on a scale from 1+ (lowest) to 4+ (highest). Table partially derived from data in: Silberstein SD, Lipton RB, Goadsby PJ, Smith, R (eds). Headache in primary care. Oxford, UK: Isis Medical Media, 1999. | ||||
Common migraine headache triggers include menstruation, stress, relaxation after stress, fatigue, too much or too little sleep, skipping meals, weather changes, high humidity, glare and flickering lights, loud or high-pitched noises, smoke or dust, strong perfumes or cooking aromas. Food triggers cause 10% of migraine cases. Chocolate, strong cheeses, red wine, beer, citrus fruits, and foods with monosodium glutamate and nitrate preservatives are common food triggers.
Tension-type headaches are triggered by stress or the end of a stress-filled day. Triggers for cluster-type headaches include alcohol, smoking during the cluster phase, and lying down during an attack.
Case 2: Flying the unfriendly skies
Ms. B, 38, is a mother of three who works as a flight attendant. She is separated from her husband and had filed for divorce because of repeated spousal abuse. She has visited her primary care physician multiple times for migraine, sinus problems, backache, and coccygodynia. Orthopedic and rectal examinations revealed no abnormalities.
Her headaches met the IHS diagnostic criteria for migraine with aura, and these responded well to zolmitriptan, 5 mg. The headaches usually occurred during days off from work, but her sinus problems also led to headaches and nasal stuffiness when she flew. Her headaches eventually occurred almost daily.
Her supervisor was unsympathetic. An otolaryngologist had prescribed decongestants and a course of desensitization, both of which brought only transient relief.
A counselor at work recommended that Ms. B go on sick leave and accept a transfer to a non-flying job. The patient was tearful and felt overwhelmed by her problems. She felt that life was no longer worth living. She agreed to see a psychiatrist, who diagnosed depression and anxiety disorder. The psychiatrist prescribed citalopram, 20 mg/d, and agreed to see her regularly to monitor progress.
Discussion. As a migraineur, Ms. B was at increased risk for depression and anxiety disorders.19 Migraine with aura is associated with an increased lifetime prevalence of suicidal ideation and suicide attempts.20
The exact mechanisms by which migraine and depression are related are unknown. Each disorder increases the risk for developing the other. The specificity of this relationship is strengthened by the fact that depression is not associated with a greater risk of severe nonmigrainous headache, even though a severe nonmigrainous headache may cause depression.21
The patient in case 1 responded well when an antidepressant was added to her treatment. In her case, the diagnosis of a depressive disorder remained an open question. Migraine attacks are known to be associated with mood change, lethargy, and cognitive changes. The picture may be further confounded because migraine without depression responds well to prophylaxis with antidepressants.
The patient in case 2, however, presented with a complex of interrelated headache and psychiatric problems of potentially dangerous proportions. Psychiatric problems in migraineurs may be deep-seated, and these patients may require urgent, specialized attention to avoid further serious disability and a possible tragic outcome.
Related resources
- Silberstein SD, Lipton RB, Goadsby PJ, Smith, R, eds. Headache in primary care. Oxford, UK: Isis Medical Media, 1999.
- Silberstein SD, Lipton RB, Dalessio DJ. Wolff’s headache and other head pain (7th ed). New York: Oxford University Press, 2001.
- Davidoff RA. Migraine. Manifestations, pathogenesis and management (2nd ed). New York: Oxford University Press, 2002.
- International Headache Society. http://www.i-h-s.org
Drug brand names
- Citalopram • Celexa
- Dihydroergotamine • Migranal
- Eletriptan • Relpax
- Rizatriptan • Maxalt
- Sumatriptan • Imitrex
- Valproate sodium • Depakote
- Zolmitriptan • Zomig
Disclosure
Dr. Smith reports that he serves as a consultant to and is on the speakers’ bureau of AstraZeneca Pharmaceuticals.
Dr. Hasse reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many of the 28 million people who suffer from migraine headaches each year1 need psychiatric care in addition to headache relief. Migraine headaches often coexist with depression,2 anxiety/panic disorders,2,3 bipolar disorder,4 and phobias,5 as well as with stroke6 and epilepsy.7 A study of 995 young adults found that anxiety disorders, phobias, major depression, panic disorder, and obsessive-compulsive disorder were two to five times more prevalent among migraine sufferers than among a control group (Table 1 ).2
Migraine sufferers know that at any time an attack could hamper their ability to work, care for their families, or engage in social activities. A nationwide study of migraineurs found that attacks often impaired their relationships with family and friends.8
Psychiatrists should screen patients for a history of migraine or other headaches and carefully consider the relationship between migraines and psychiatric disorders when prescribing treatment. In this article, we outline acute and preventive headache treatments and present two cases to help you treat these patients appropriately.
Table 1
PSYCHIATRIC COMORBIDITIES IN PATIENTS WITH VS. WITHOUT MIGRAINES*
| Migraineurs (%) (n = 128) | Controls (%) (n = 879) | |
|---|---|---|
| Any anxiety | 54 | 27 |
| Generalized anxiety disorder | 10 | 2 |
| Phobia | 40 | 21 |
| Major depression | 35 | 10 |
| Panic disorder | 11 | 2 |
| Obsessive-compulsive disorder | 9 | 2 |
| * Prevalence Source: Breslau N, Davis GC. Cephalalgia 1992;12(2):85-90. | ||
Table 2
THREE TYPES OF PRIMARY HEADACHE: DIAGNOSTIC CRITERIA
| Headache type | Age of onset (years) | Location | Duration | Frequency/timing | Severity | Quality | Features |
|---|---|---|---|---|---|---|---|
| Migraine | 10 to 40 | Hemicranial | 4 to 72 hr | Variable | Moderate to severe | Throbbing, steady ache | Nausea; vomiting; photo/phono/osmophobia; neurologic deficits; aura |
| Tension-type | 20 to 50 | Bilateral/generalized | 30 min to 7 days+ | Variable | Dull ache, may wax and wane | Tight, band-like pressure | Generally none |
| Cluster | 15 to 40 | Unilateral, periorbital or retro-orbital | 15 to 180 min | 1 to 8 times per day or night | Excruciating | Boring, piercing | Ipsilateral, conjunctival injection, nasal congestion, rhinorrhea, miosis, facial seating |
| Source: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96. | |||||||
Headache definitions and diagnosis
Primary or secondary. Under the International Headache Society’s (IHS) 1988 headache classification and diagnostic criteria,9 headaches are primary or secondary:
- Primary headaches are benign recurrent headaches that commonly present in practice.
- Secondary headaches occur much less frequently and are caused by underlying pathology.
The possibility of secondary headache should be ruled out before a primary headache can be diagnosed. The following headache features should cause concern:
- Severe headache with abrupt onset
- Subacute or progressive headache over days or months
- Headache, nausea, vomiting, and fever not explained by systemic illness
- New-onset headache late in life
- Headache with neurologic signs or symptoms such as confusion, decreased level of consciousness, meningismus, or papilledema
- Headache following head trauma
- Patient history of sickle cell disease, malignancy, or HIV.
Headache types. The three major types of primary headache are migraine, tension-type, and cluster (Table 2 ). Tension-type is the most common, is often mild, and is either self-treated with over-the-counter medications or ignored. Migraine is the most troublesome headache in everyday practice. Cluster is the most severe and fortunately is rare.
Migraine with aura and migraine without aura are separate diagnoses. IHS criteria for diagnosing migraines without aura are listed in Table 3. According to the IHS, migraine with aura (or “classic migraine”) fulfills all the criteria for migraine without aura, with fully reversible neurologic symptoms indicating focal cerebral cortical and/or brain stem dysfunction.
Auras. About 15% of migraineurs experience auras. Symptoms develop within 5 to 20 minutes, usually last less than 1 hour, and fade before the headache’s onset. Gradual onset and history of previous attacks helps to distinguish aura from transient ischemic attacks. Auras may manifest as visual, sensory, motor, or brain-stem symptoms, or as combinations of these:
- Visual auras are most common, presenting as localized visual loss (scotoma), with flashing lights (scintillation) at margins or jagged edges (fortification).
- Sensory auras present as facial or limb paresthesias.
- Motor auras manifest as weakness or lack of coordination.
- Brain stem auras manifest as vertigo or double vision.
Migraine aura is considered part of the headache’s prodrome, which may occur days or hours before the headache’s onset. The aura may bring about:
- an altered mental state (e.g., depression, hyperactivity, euphoria, difficulty concentrating, dysphasia)
- neurologic symptoms (e.g., photophobia, phonophobia, hyperosmia, yawning)
- general bodily discomforts (e.g., anorexia, food craving, diarrhea, thirst, urination, fluid retention, cold feeling).
Despite their sometimes severe effects, migraines often remain undiagnosed.10 Migraine should be suspected in patients with recurrent moderate to severe disabling headaches (Box).11-15
Case 1: “Bad, sick headaches”
Ms. A, 23, a single parent with a 2-year-old child, has had trouble staying employed because of repeated illnesses. She made 17 visits to her primary care physician within 26 months. While her main complaint was headache, she also complained of other aches and pains, a lack of energy, and insomnia. Numerous examinations revealed no physical abnormalities.
She reported having “bad, sick headaches” that sometimes lasted 2 to 3 days. Bed rest helped but this was not always possible. The headache was throbbing and usually one-sided. She had no aura, and ibuprofen gave partial relief. She noted that her mother gets similar headaches.
Table 3
DIAGNOSTIC REQUIREMENTS FOR MIGRAINE WITHOUT AURA
Mandatory
|
At least 2 of the following:
|
| During headache At least one of the following:
|
| Additional features Migraine prodrome—A range of general, neurologic, and mental changes may occur hours or days before the headache’s onset General—Anorexia, food craving, diarrhea or constipation, thirst, urination, fluid retention, cold feeling Mental—Depression, hyperactivity, euphoria, difficulty concentrating, dysphasia Neurologic—Photophobia, phonophobia, hyperosmia, yawning |
| SOURCE: Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96. |
Ms. A was diagnosed with migraine without aura, and she was treated with sumatriptan, 100 mg (1 or 2 doses, as needed). Her headaches responded well to this treatment, but the frequency of attacks remained unchanged. She requested a change in her medicine.
The underlying mechanisms of migraine headaches are not completely understood.
Vascular causes. A recently described neurovascular mechanism11 suggests that perivascular neurogenic inflammation involving meningeal vessels causes migraine. The triptan drugs have been found to reverse this process and relieve the headache.12
Positron emission tomography has demonstrated increased blood flow during acute migraine in midline brain stem structures. This suggests the presence of a central migraine generator in that location.13
Heredity. A rare form of migraine, familial hemiplegic migraine, is associated with a genetic abnormality on chromosome 19.14
Nitric oxide. Nitroglycerine-induced migraine headache, caused by the release of nitric oxide in cerebral vessels, can be reversed by nitrous oxide synthase inhibitors, thus opening up intriguing possibilities of new therapeutic agents and increased understanding of underlying migraine mechanisms.15
Treating migraines
Acute treatment. Migraineurs whose attacks are infrequent and mild may find OTC analgesics or NSAIDs adequate. Most patients, however, require specific migraine treatment, usually with triptans. Acute oral treatment options include sumatriptan, 50 to 100 mg; rizatriptan, 10 mg; zolmitriptan, 2.5 to 5 mg; and eletriptan, 40 mg.
In case of vomiting or nausea, options include sumatriptan, 20 mg nasal spray or 6 mg SC; rizatriptan, 10 mg on a dissolving wafer; or dihydroergotamine, 2 mg nasal spray or 1 mg IM or SC. For severe nausea or vomiting, an anti-nauseant (e.g., prochlorperazine suppositories, 25 mg) may be of value.
Preventive treatment. Preventive treatment may be warranted, depending on attack frequency, severity, and the extent of disability caused. One prolonged, severe attack per month that responds poorly to acute treatment may indicate the need for preventive treatment. A range of preventative treatments is available (Table 4).
In Ms. A’s case, oral sumatriptan lessened the severity of the migraine attacks, and the addition of nortriptyline, 50 mg/d, reduced frequency by about 50%. She felt more energetic overall and was sleeping better.
Treating the psychiatric comorbidity
Behavioral therapy is used as an adjunct to pharmacologic headache treatment. This approach is usually considered after a poor or adverse response to treatment, or when pharmacologic treatment is contraindicated (e.g., during pregnancy).
Relaxation training, biofeedback, and cognitive-behavioral stress management are the most commonly used forms of behavioral therapy. Thirty-five to 55% improvement in migraine has been reported following such treatments.16
Cognitive-behavioral intervention has been shown to be effective in depression17 and anxiety disorders.18 When either psychiatric problem is comorbid with migraine, cognitive therapy can improve both the migraine and the psychiatric comorbidity.
Pharmacologic therapy. Depression is commonly associated with migraine and may be caused by living with chronic disabling headaches over time. In such cases, the depression will improve as the migraine responds to treatment. However, in cases where comorbid depression or anxiety trigger or exacerbate acute migraine attacks, neither the migraine nor the psychiatric problem responds until the underlying psychopathology is treated. In such cases, simultaneous psychiatric and migraine pharmacologic treatment is required.
We recommend that you treat the psychiatric comorbidity as it would be treated without a co-existing migraine. Be advised, however, that monoamine oxidase inhibitors are contraindicated in depression during the 2 weeks before treating the comorbid migraine with a triptan. If the patient does not respond or if there is concern regarding possible underlying pathology, consult with a clinician who specializes in headache treatment.
Precipitating and aggravating factors
Headache triggers. Helping patients to recognize headache triggers and aggravating factors is an important element in treating and preventing migraines. Identifying these factors in the patient history can help you establish a diagnosis and implement steps to avoid or reduce attack severity.
Table 4
TREATMENT OPTIONS FOR PREVENTING MIGRAINE ATTACKS
| Drug | Efficacy* | Side effects* | Indications | Contraindications |
|---|---|---|---|---|
| β blockers | 4+ | 2+ | Hypertension | Depression, asthma, diabetes, hypotension, congestive heart failure, peripheral vascular disease |
| Ca channel blockers | 2+ | 1+ | Hypertension, angina, asthma, migraine aura | Constipation, hypotension |
| Tricyclic antidepressants | 4+ | 2+ | Depression, anxiety disorders, insomnia | Heart block, urinary retention, mania |
| Selective serotonin reuptake inhibitors | 2+ | 1+ | Depression, obsessive-compulsive disorder | Mania |
| Monoamine oxidase inhibitors | 4+ | 4+ | Depression | Dietary restrictions |
| Divalproex/valproate | 4+ | 2+ | Epilepsy, anxiety disorders, mania | Liver disease, bleeding disorders, hair loss |
| Naproxen | 2+ | 2+ | Arthritis, other pain disorders | Gastritis, peptic ulcer |
| * Ratings on a scale from 1+ (lowest) to 4+ (highest). Table partially derived from data in: Silberstein SD, Lipton RB, Goadsby PJ, Smith, R (eds). Headache in primary care. Oxford, UK: Isis Medical Media, 1999. | ||||
Common migraine headache triggers include menstruation, stress, relaxation after stress, fatigue, too much or too little sleep, skipping meals, weather changes, high humidity, glare and flickering lights, loud or high-pitched noises, smoke or dust, strong perfumes or cooking aromas. Food triggers cause 10% of migraine cases. Chocolate, strong cheeses, red wine, beer, citrus fruits, and foods with monosodium glutamate and nitrate preservatives are common food triggers.
Tension-type headaches are triggered by stress or the end of a stress-filled day. Triggers for cluster-type headaches include alcohol, smoking during the cluster phase, and lying down during an attack.
Case 2: Flying the unfriendly skies
Ms. B, 38, is a mother of three who works as a flight attendant. She is separated from her husband and had filed for divorce because of repeated spousal abuse. She has visited her primary care physician multiple times for migraine, sinus problems, backache, and coccygodynia. Orthopedic and rectal examinations revealed no abnormalities.
Her headaches met the IHS diagnostic criteria for migraine with aura, and these responded well to zolmitriptan, 5 mg. The headaches usually occurred during days off from work, but her sinus problems also led to headaches and nasal stuffiness when she flew. Her headaches eventually occurred almost daily.
Her supervisor was unsympathetic. An otolaryngologist had prescribed decongestants and a course of desensitization, both of which brought only transient relief.
A counselor at work recommended that Ms. B go on sick leave and accept a transfer to a non-flying job. The patient was tearful and felt overwhelmed by her problems. She felt that life was no longer worth living. She agreed to see a psychiatrist, who diagnosed depression and anxiety disorder. The psychiatrist prescribed citalopram, 20 mg/d, and agreed to see her regularly to monitor progress.
Discussion. As a migraineur, Ms. B was at increased risk for depression and anxiety disorders.19 Migraine with aura is associated with an increased lifetime prevalence of suicidal ideation and suicide attempts.20
The exact mechanisms by which migraine and depression are related are unknown. Each disorder increases the risk for developing the other. The specificity of this relationship is strengthened by the fact that depression is not associated with a greater risk of severe nonmigrainous headache, even though a severe nonmigrainous headache may cause depression.21
The patient in case 1 responded well when an antidepressant was added to her treatment. In her case, the diagnosis of a depressive disorder remained an open question. Migraine attacks are known to be associated with mood change, lethargy, and cognitive changes. The picture may be further confounded because migraine without depression responds well to prophylaxis with antidepressants.
The patient in case 2, however, presented with a complex of interrelated headache and psychiatric problems of potentially dangerous proportions. Psychiatric problems in migraineurs may be deep-seated, and these patients may require urgent, specialized attention to avoid further serious disability and a possible tragic outcome.
Related resources
- Silberstein SD, Lipton RB, Goadsby PJ, Smith, R, eds. Headache in primary care. Oxford, UK: Isis Medical Media, 1999.
- Silberstein SD, Lipton RB, Dalessio DJ. Wolff’s headache and other head pain (7th ed). New York: Oxford University Press, 2001.
- Davidoff RA. Migraine. Manifestations, pathogenesis and management (2nd ed). New York: Oxford University Press, 2002.
- International Headache Society. http://www.i-h-s.org
Drug brand names
- Citalopram • Celexa
- Dihydroergotamine • Migranal
- Eletriptan • Relpax
- Rizatriptan • Maxalt
- Sumatriptan • Imitrex
- Valproate sodium • Depakote
- Zolmitriptan • Zomig
Disclosure
Dr. Smith reports that he serves as a consultant to and is on the speakers’ bureau of AstraZeneca Pharmaceuticals.
Dr. Hasse reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lipton RB, Stewart WF, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41(7):646-57.
2. Breslau N, Davis GC. Migraine, major depression and panic disorder: A prospective epidemiologic study of young adults. Cephalalgia 1992;12(2):85-90.
3. Stewart WF, Linet MS, Celentano DD. Migraine headaches and panic attacks. Psychosom Med 1989;51(5):559-69.
4. Mersky H, Peatfield RC. Headache in the psychiatrically ill. In: Olesen J, Tfelt-Hensen P, Welch KMA (eds). The headaches (2nd ed). Baltimore: Lippincott, Williams and Wilkins, 1999;962-3.
5. Davidoff RA. Comorbidity. In: Davidoff RA (ed). Migraine manifestations, pathogenesis, and management (2nd ed). Oxford, UK: Oxford University Press, 2002;21-2.
6. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. BMJ 1999;318(7175):13-8.
7. Migraine-epilepsy relationships: epidemiological and genetic aspects. In: Andermann FA, Lugaresi E (eds). Migraine and epilepsy. Boston: Butterworths, 1987.
8. Smith R. Impact of migraine on the family. Headache 1998;38(6):423-6.
9. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96.
10. Smith R, Hasse LA, Ritchey PN, et al. Extent of migraine and migrainous headache in headache NOS patients in family practice. Cephalalgia 2001;21:291-2.
11. Olesen J, Friberg L, et al. Basic mechanisms in vascular headache. Neurol Clin 1990;8:801-15.
12. Sumatriptan—an oral dose-defining study. The Oral Sumatriptan Dose-Defining Study Group. Eur Neurol 1991;31(5):300-5.
13. Diener HC. Positron emission tomography studies in headache. Headache 1997;37(10):622-5.
14. Joutel A, Bousser MG, V Biousse, et al. A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet 1993;5(1):40-5.
15. Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab 1994;14(2):175-92.
16. Holroyd KA, Penzien DB, Lipchik GL. Efficacy of Behavioral Treatments. In: Silberstein SD, Lipton RB, Dalessio DJ (eds). Wolf’s headache and other head pain (7th ed). New York: Oxford University Press, 2001;563-6.
17. Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: A comprehensive review of controlled outcome research. Psychol Bull 1990;108:30-49.
18. Gould RA, Otto MW, et al. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder. Behav Ther 1997;28:285-305.
19. Breslau N, Andreski P. Migraine, personality and psychiatric comorbidity. Headache 1995;35(7):382-6.
20. Breslau N. Migraine, suicidal ideation, and suicide attempts. Neurology 1992;42(2):392-5.
21. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54(2):308-13.
1. Lipton RB, Stewart WF, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41(7):646-57.
2. Breslau N, Davis GC. Migraine, major depression and panic disorder: A prospective epidemiologic study of young adults. Cephalalgia 1992;12(2):85-90.
3. Stewart WF, Linet MS, Celentano DD. Migraine headaches and panic attacks. Psychosom Med 1989;51(5):559-69.
4. Mersky H, Peatfield RC. Headache in the psychiatrically ill. In: Olesen J, Tfelt-Hensen P, Welch KMA (eds). The headaches (2nd ed). Baltimore: Lippincott, Williams and Wilkins, 1999;962-3.
5. Davidoff RA. Comorbidity. In: Davidoff RA (ed). Migraine manifestations, pathogenesis, and management (2nd ed). Oxford, UK: Oxford University Press, 2002;21-2.
6. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. BMJ 1999;318(7175):13-8.
7. Migraine-epilepsy relationships: epidemiological and genetic aspects. In: Andermann FA, Lugaresi E (eds). Migraine and epilepsy. Boston: Butterworths, 1987.
8. Smith R. Impact of migraine on the family. Headache 1998;38(6):423-6.
9. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 1988;8(suppl 7):1-96.
10. Smith R, Hasse LA, Ritchey PN, et al. Extent of migraine and migrainous headache in headache NOS patients in family practice. Cephalalgia 2001;21:291-2.
11. Olesen J, Friberg L, et al. Basic mechanisms in vascular headache. Neurol Clin 1990;8:801-15.
12. Sumatriptan—an oral dose-defining study. The Oral Sumatriptan Dose-Defining Study Group. Eur Neurol 1991;31(5):300-5.
13. Diener HC. Positron emission tomography studies in headache. Headache 1997;37(10):622-5.
14. Joutel A, Bousser MG, V Biousse, et al. A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet 1993;5(1):40-5.
15. Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab 1994;14(2):175-92.
16. Holroyd KA, Penzien DB, Lipchik GL. Efficacy of Behavioral Treatments. In: Silberstein SD, Lipton RB, Dalessio DJ (eds). Wolf’s headache and other head pain (7th ed). New York: Oxford University Press, 2001;563-6.
17. Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: A comprehensive review of controlled outcome research. Psychol Bull 1990;108:30-49.
18. Gould RA, Otto MW, et al. Cognitive behavioral and pharmacological treatment of generalized anxiety disorder. Behav Ther 1997;28:285-305.
19. Breslau N, Andreski P. Migraine, personality and psychiatric comorbidity. Headache 1995;35(7):382-6.
20. Breslau N. Migraine, suicidal ideation, and suicide attempts. Neurology 1992;42(2):392-5.
21. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54(2):308-13.
A showdown with severe social phobia
History: Living in fear
Mr. I, 41, presents for an initial psychiatric evaluation. He saw a psychologist 8 years ago for a “mild depression,” which he described as a lack of motivation and difficulty concentrating. His mood has been chronically “flat” for the last 10 years. He complains of poor energy and decreased sleep because of irregular work hours, and admits to using over-the-counter caffeine pills to help him function.
The patient denies suicidal ideations, symptoms of guilt, psychotic symptoms, or crying spells, but has a history of alcoholism and cocaine abuse. (He has been sober for 5 years.) Significant recent stressors include a recent breakup with his girlfriend, which he adds “really hasn’t bothered me at all.”
Mr. I has been increasingly avoiding social situations. Though he denies having panic attacks, interaction with other people triggers shortness of breath and chest tight-ness, especially when speaking in public to strangers.
The fear of what others might think of him is dominating Mr. I’s life. For example, he would like to console a housemate whose mother died, but because he is afraid of how the friend will react, Mr. I has not approached him. He adds that he goes out of his way to avoid contact with his co-workers, working irregular hours and eating his lunch in his car rather than the office lounge—even in inclement weather.
Mr. I does attend Alcoholic Anonymous meetings, but often sits toward the back. He had led some meetings, but refused to even look up from the podium while doing so. His anxiety worsened, his heart rate increased, and his palms sweated while leading the group. He began attending different AA meetings so that others would not recognize him and volunteer him to lead.
He adds that he feels comfortable meeting and dating women, since these exchanges are “scripted.” As he gets to know his partner better, however, Mr. I becomes more self-conscious.
Which of Mr. I’s symptoms would you address first: the depressive or the phobic?
Drs. Yu’s, Gordon’s, and Maguire’s observations
Based on Mr. I’s presentation, one might at first diagnose major depressive disorder, but chronic avoidance patterns differentiate his illness from an endogenous depression. Mr. I was diagnosed as having social phobia, a disorder that has been gaining attention among researchers.
A phobia is defined as an irrational fear that produces conscious avoidance of the feared subject, activity, or situation. The presence or anticipation of the phobic entity elicits severe distress, though the affected person usually recognizes that the reaction is excessive. DSM-IV defines social phobia as a strong, persisting fear of potentially embarrassing situations (Box).1
Two peaks of onset have been described: one occurring before age 5, and the other between ages 11 and 17.2 The mean age of onset has been reported to be 15.2
- A marked and persistent fear of one or more social or performance situations in which the person is exposed to unfamiliar people or to possible scrutiny by others. The individual fears that he or she will act in a way (or show anxiety symptoms) that will be humiliating or embarrassing.
- Exposure to the feared social situation almost invariably provokes anxiety, which may take the form of a situational or predisposed panic attack.
- The person recognizes that the fear is excessive or unreasonable.
- The feared social or performance situations are avoided, or else endured with intense anxiety or distress.
- The avoidance, anxious anticipation, or distress in the feared social or performance situation(s) interferes significantly with the person’s normal routine, occupational or academic functioning, or social activities or relationships with others, or there is marked distress about having the phobia.
- In individuals younger than 18, the duration is at least 6 months.
- The fear or avoidance is not caused directly by a substance (e.g., a drug of abuse or medication) or general medical condition, and is not better accounted for by another mental disorder (e.g., panic disorder with or without agoraphobia, separation anxiety disorder, body dysmorphic disorder, a pervasive developmental disorder, or schizoid personality disorder).
- If a general medical condition or other mental disorder is present, the fear in criterion A is unrelated to it (e.g., the patient does not fear stuttering, trembling in Parkinson’s disease, or exhibiting abnormal eating behavior in anorexia nervosa or bulimia nervosa). Specify if:
SOURCE: DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000.
DSM-IV describes two types of social phobia: generalized social phobia and performance phobia. Normal fear and shyness should be differentiated from social phobia. Medical conditions—including CNS tumors and cerebrovascular diseases—and drugs typically bring about neurologic and mental status symptoms that can confound the diagnosis.
Symptoms of other anxiety disorders, including panic disorder and agoraphobia, may mimic social phobia. Fear in social phobia is not present outside of, or in anticipation of, the feared situation. Social phobia also can be misdiagnosed as an avoidant personality, schizoid personality, or major depressive disorder.1
At this point, one should consider diagnosing Mr. I with social phobia, as evinced by his excessive avoidance of social situations. Mr. I also recognizes that his avoidance is excessive and causes difficulty in his daily functioning.
Behavioral inhibition in childhood is suggested to be more common in children with parents who had panic disorder. Generalized fear may manifest later as excessive shyness. Several studies have linked childhood behavior inhibition to social phobia.5-7’
First treatment: Pharmacotherapy and psychotherapy
Mr. I is interested in trying an antidepressant, but noted that about 6 years ago a brief course of a selective serotonin reuptake inhibitor led to difficulty sleeping and decreased libido and orgasm. He agrees to take bupropion SR, 100 mg/d, titrated after 2 weeks to 100 mg bid.
He also begins cognitive-behavioral and supportive therapy, during which he reveals that his pattern of avoidance took root in grade school, where he was often a quiet sidekick to the popular kids. During therapy, he describes visitations with his daughters, both of whom live with his ex-wife, as extremely difficult.
“I really don’t know what to say,” Mr. I says. “We often stare at each other during dessert, and I want to get it over with and go home.”
After 1 month, his bupropion SR is increased to 150 mg bid. One month later, his feelings of depression are under control. He sleeps well, no longer feels fatigued, and can concentrate. Still he isolates himself, fearing others’ disapproval. He has become more resistant to psychotherapy. “I know my patterns. I know what I do, but I can’t change it,” he says.
How would you treat Mr. I’s persistent social phobia? Would you switch his medications or augment existing ones? Does psychotherapy still have a role in treatment?
Drs. Yu’s, Gordon’s, and Maguire’s observations
Pharmacologic treatment of social phobia has spawned several neurochemical hypotheses. As beta-adrenergic antagonists have been proven efficacious in treating performance phobia, an adrenergic hypothesis suggests patients with performance phobia release more norepinephrine and epinephrine or are more sensitive to normal levels of these neuro-transmitters.8
The success of monoamine oxidase inhibitors in generalized social phobia suggests that dopamine plays a role in treating this form of the disorder. Central dopamine activity has been associated with positive emotions or extraversion.9
SSRIs have also demonstrated efficacy against generalized social phobia.10 Researchers have associated higher serotonin levels with increased social dominance11 and suggest that abnormal dopamine and serotonin levels contribute to the disorder’s pathogenesis.
Current treatments include psychotherapy and pharmacotherapy, and studies suggest that a combination of the two may be more efficacious than either alone.8 Venlafaxine, phenelzine, buspirone, benzodiazepines, and SSRIs have all demonstrated effectiveness and tolerability in generalized social phobia. Beta-adrenergic receptor antagonists (e.g., atenolol, propranolol) are commonly administered to treat performance phobia shortly before exposure to the phobic stimulus.
An adequate time frame for psychopharmacologic treatment of social phobia has not been defined. In depression therapy, medications should be maintained for at least 4 to 5 weeks before considering the regimen unsuccessful.1
While Mr. I’s depression responded well to bupropion SR, a medication whose mechanism involves dopamine and norepinephrine reuptake, use of a medication that augments his serotonin may further improve his condition.
Cognitive and behavioral therapies also are indicated for both generalized social phobia and performance phobia. These should be considered along with medication therapy for Mr. I to treat his social phobia and prevent a relapse.
Despite the available evidence, however, the course and prognosis of social phobia are not clear. Data are still forthcoming on this recently recognized disorder.
Further treatment: Another neurotransmitter
Again cautious of potential adverse sexual effects, Mr. I agrees to try mirtazapine, 30 mg at bedtime, in addition to bupropion SR. He initially complains of sedation and lowered energy, but is willing to continue the mirtazapine, hoping that it will help his social phobia.
Two months after starting mirtazapine, Mr. I is still fearful at work and home, and his relationship with his order. Generalized fear may manifest later as excessive shyness. Several studies have linked childhood behavior inhibition to social phobia.5-7’ daughters has not improved. After another month, he reports that his sedation has resolved, but complains of increased fatigue and difficulty concentrating. He suspects that the bupropion SR has stopped working.
After another month, Mr. I self-discontinues the mirtazapine. Though he tries to participate in social situations, his anxiety has worsened. He goes to a country music club once a week but is afraid to ask anyone to dance.
Should you focus on Mr. I’s depression rather than his social phobia? If so, how do you change his treatment?
Drs. Yu’s, Gordon’s, and Maguire’s observations
The severity of Mr. I’s social phobia may be causing his depression. Both trials of bupropion SR and mirtazapine have been adequate for his depression but have not alleviated his social phobia.
Medications that affect gamma-aminobutyric acid (GABA) levels, specifically benzodiazepines, have not been tried. Benzodiazepines provide rapid relief with little risk in short-term treatment, but dependence/withdrawal risks increase greatly when given more than 4 to 6 months.8 Because of Mr. I’s chronic anxiety in social phobia and his history of alcoholism, benzodiazepines are not recommended.
The novel compound tiagabine has been shown to increase GABA in the synaptic cleft.12 GABA increases chloride conduction through its ligand-gated channels, creating a potential antianxiety effect similar to that produced by benzodiazepines.8,13,14 GAT-1, the predominant transporter, removes excess GABA.
Just as SSRIs inhibit serotonin reuptake and allow the neurotransmitter to act on its receptors to alleviate depression and anxiety, so does tiagabine inhibit GAT-1. Theoretically, tiagabine may relieve anxiety by increasing synaptic concentrations of GABA.
Tiagabine also has been shown to be well-tolerated without a known abuse or dependence potential.12 Possible adverse effects include impaired concentration, somnolence, fatigue, nausea, and dizziness. To avoid adverse effects, slow titration (about 4 mg per week) is recommended.
Changing treatment: Looking up
Tiagabine, 4 mg at bedtime, is added to help with Mr. I’s anxiety; he is instructed to increase the dosage by 4 mg every 5 days in divided doses. He began to sense improvement during the second week, at 4 mg bid, and 2 weeks later his anxiety has been greatly reduced. He can now sit quietly with his co-workers during coffee breaks and has begun training a co-worker, which he never dared to attempt before. At this point, he was tolerating tiagabine at 8 mg bid.
One month later, tiagabine is increased to 16 mg bid. Mr. I has noticed mild dizziness with each dosage increase, but each time it subsided within a day. He has been maintained on 16 mg bid.
Saying that his anxiety is now well-controlled, Mr. I enjoys at least one dance each week at the country music club he frequents. One week later, he led an Alcoholics Anonymous meeting—while looking up to his audience for the first time. He continues these activities and his therapy sessions, which are geared toward developing stronger skills to minimize his anxiety. He is considering lowering his medication dosages (though he is wary of a possible relapse) and furthering his therapy.
Related resources
- Anxiety Disorders Association of America www.adaa.org
- National Institute of Mental Health: Phobias from NLM’s MEDLINEplus http://www.nlm.nih.gov/medlineplus/phobias.html
Author affiliations
Dr. Yu is a fellow in child and adolescent psychiatry, Dr. Gordon is a resident physician; and Dr. Maguire is assistant dean for continuing medical education, director of resident training, and associate clinical professor, department of psychiatry, University of California, Irvine.
Drug brand names
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Mirtazapine • Remeron
- Phenelzine • Nardil
- Tiagabine • Gabitril
- Venlafaxine • Effexor
Disclosure
Dr. Yu reports that he serves on the speaker’s bureau of Cephalon Inc., Novartis Pharmaceuticals Corp., and Pfizer Inc., and receives research/grant support from and serves as a consultant to Eli Lilly and Co.
Dr. Gordon reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Dr. Maguire reports that he receives research/grant support from, serves as a consultant to, and is on the speaker’s bureau of Eli Lilly and Co., is on the speaker’s bureau of Pfizer Inc., and receives research/grant support from Forest Laboratories and GlaxoSmithKline.
1. Sadock BJ, Sadock VA. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry (7th ed). Philadelphia: Lippincott Williams & Wilkins, 2000.
2. Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia: comorbidity and morbidity in an epidemiological sample. Arch Gen Psychiatry 1992;49:282-8.
3. Wacker HR, Mhllejans R, Klein KH, Battegay R. Identification of cases of anxiety disorders and affective disorders in the community according to ICD-10 and DSM-III-R using the composite international diagnostic interview (CIDI). Int J Methods Psychiatr Res 1992;2:91-100.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed), text revision. Washington, DC: American Psychiatric Association, 2000.
5. Van Ameringen M, Mancini C, Oakman JM. The relationship of behavioral inhibition and shyness to anxiety disorder. J Nerv Ment Dis 1998;186:425-31.
6. Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry 1999;38:1008-15.
7. Turner SM, Beidel DC, Townsley RM. Social phobia: relationship to shyness. Behav Res Ther 1990;28:497-505.
8. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical application (2nd ed). Cambridge University Press, Cambridge, 2000.
9. Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist induced dopamine activity to positive emotionality. J Pers Soc Psychol 1994;67:485-98.
10. Van Ameringen M, Mancini C, Oakman JM, Farvolden P. Selective serotonin reuptake inhibitors in the treatment of social phobia: the emerging gold standard. CNS Drugs 1999;11:307-15.
11. Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 1998;155:373-9.
12. Physician’s Desk Reference (55th ed). Montvale, NJ: Medical Economics, 2001.
13. Bowery NG. Pharmacology of mammalian GABAB receptors. In: Enna SJ, Bowery NG (eds). The GABA receptors (2nd ed). Totowa, NJ: Humana Press, 1997;209-36.
14. Johnston GAR. Molecular biology, pharmacology, and physiology of GABAC receptors. In: Enna SJ, Bowery NG (eds). The GABA receptors (2nd ed). Totowa, NJ: Humana Press, 1997;297-323.
History: Living in fear
Mr. I, 41, presents for an initial psychiatric evaluation. He saw a psychologist 8 years ago for a “mild depression,” which he described as a lack of motivation and difficulty concentrating. His mood has been chronically “flat” for the last 10 years. He complains of poor energy and decreased sleep because of irregular work hours, and admits to using over-the-counter caffeine pills to help him function.
The patient denies suicidal ideations, symptoms of guilt, psychotic symptoms, or crying spells, but has a history of alcoholism and cocaine abuse. (He has been sober for 5 years.) Significant recent stressors include a recent breakup with his girlfriend, which he adds “really hasn’t bothered me at all.”
Mr. I has been increasingly avoiding social situations. Though he denies having panic attacks, interaction with other people triggers shortness of breath and chest tight-ness, especially when speaking in public to strangers.
The fear of what others might think of him is dominating Mr. I’s life. For example, he would like to console a housemate whose mother died, but because he is afraid of how the friend will react, Mr. I has not approached him. He adds that he goes out of his way to avoid contact with his co-workers, working irregular hours and eating his lunch in his car rather than the office lounge—even in inclement weather.
Mr. I does attend Alcoholic Anonymous meetings, but often sits toward the back. He had led some meetings, but refused to even look up from the podium while doing so. His anxiety worsened, his heart rate increased, and his palms sweated while leading the group. He began attending different AA meetings so that others would not recognize him and volunteer him to lead.
He adds that he feels comfortable meeting and dating women, since these exchanges are “scripted.” As he gets to know his partner better, however, Mr. I becomes more self-conscious.
Which of Mr. I’s symptoms would you address first: the depressive or the phobic?
Drs. Yu’s, Gordon’s, and Maguire’s observations
Based on Mr. I’s presentation, one might at first diagnose major depressive disorder, but chronic avoidance patterns differentiate his illness from an endogenous depression. Mr. I was diagnosed as having social phobia, a disorder that has been gaining attention among researchers.
A phobia is defined as an irrational fear that produces conscious avoidance of the feared subject, activity, or situation. The presence or anticipation of the phobic entity elicits severe distress, though the affected person usually recognizes that the reaction is excessive. DSM-IV defines social phobia as a strong, persisting fear of potentially embarrassing situations (Box).1
Two peaks of onset have been described: one occurring before age 5, and the other between ages 11 and 17.2 The mean age of onset has been reported to be 15.2
- A marked and persistent fear of one or more social or performance situations in which the person is exposed to unfamiliar people or to possible scrutiny by others. The individual fears that he or she will act in a way (or show anxiety symptoms) that will be humiliating or embarrassing.
- Exposure to the feared social situation almost invariably provokes anxiety, which may take the form of a situational or predisposed panic attack.
- The person recognizes that the fear is excessive or unreasonable.
- The feared social or performance situations are avoided, or else endured with intense anxiety or distress.
- The avoidance, anxious anticipation, or distress in the feared social or performance situation(s) interferes significantly with the person’s normal routine, occupational or academic functioning, or social activities or relationships with others, or there is marked distress about having the phobia.
- In individuals younger than 18, the duration is at least 6 months.
- The fear or avoidance is not caused directly by a substance (e.g., a drug of abuse or medication) or general medical condition, and is not better accounted for by another mental disorder (e.g., panic disorder with or without agoraphobia, separation anxiety disorder, body dysmorphic disorder, a pervasive developmental disorder, or schizoid personality disorder).
- If a general medical condition or other mental disorder is present, the fear in criterion A is unrelated to it (e.g., the patient does not fear stuttering, trembling in Parkinson’s disease, or exhibiting abnormal eating behavior in anorexia nervosa or bulimia nervosa). Specify if:
SOURCE: DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000.
DSM-IV describes two types of social phobia: generalized social phobia and performance phobia. Normal fear and shyness should be differentiated from social phobia. Medical conditions—including CNS tumors and cerebrovascular diseases—and drugs typically bring about neurologic and mental status symptoms that can confound the diagnosis.
Symptoms of other anxiety disorders, including panic disorder and agoraphobia, may mimic social phobia. Fear in social phobia is not present outside of, or in anticipation of, the feared situation. Social phobia also can be misdiagnosed as an avoidant personality, schizoid personality, or major depressive disorder.1
At this point, one should consider diagnosing Mr. I with social phobia, as evinced by his excessive avoidance of social situations. Mr. I also recognizes that his avoidance is excessive and causes difficulty in his daily functioning.
Behavioral inhibition in childhood is suggested to be more common in children with parents who had panic disorder. Generalized fear may manifest later as excessive shyness. Several studies have linked childhood behavior inhibition to social phobia.5-7’
First treatment: Pharmacotherapy and psychotherapy
Mr. I is interested in trying an antidepressant, but noted that about 6 years ago a brief course of a selective serotonin reuptake inhibitor led to difficulty sleeping and decreased libido and orgasm. He agrees to take bupropion SR, 100 mg/d, titrated after 2 weeks to 100 mg bid.
He also begins cognitive-behavioral and supportive therapy, during which he reveals that his pattern of avoidance took root in grade school, where he was often a quiet sidekick to the popular kids. During therapy, he describes visitations with his daughters, both of whom live with his ex-wife, as extremely difficult.
“I really don’t know what to say,” Mr. I says. “We often stare at each other during dessert, and I want to get it over with and go home.”
After 1 month, his bupropion SR is increased to 150 mg bid. One month later, his feelings of depression are under control. He sleeps well, no longer feels fatigued, and can concentrate. Still he isolates himself, fearing others’ disapproval. He has become more resistant to psychotherapy. “I know my patterns. I know what I do, but I can’t change it,” he says.
How would you treat Mr. I’s persistent social phobia? Would you switch his medications or augment existing ones? Does psychotherapy still have a role in treatment?
Drs. Yu’s, Gordon’s, and Maguire’s observations
Pharmacologic treatment of social phobia has spawned several neurochemical hypotheses. As beta-adrenergic antagonists have been proven efficacious in treating performance phobia, an adrenergic hypothesis suggests patients with performance phobia release more norepinephrine and epinephrine or are more sensitive to normal levels of these neuro-transmitters.8
The success of monoamine oxidase inhibitors in generalized social phobia suggests that dopamine plays a role in treating this form of the disorder. Central dopamine activity has been associated with positive emotions or extraversion.9
SSRIs have also demonstrated efficacy against generalized social phobia.10 Researchers have associated higher serotonin levels with increased social dominance11 and suggest that abnormal dopamine and serotonin levels contribute to the disorder’s pathogenesis.
Current treatments include psychotherapy and pharmacotherapy, and studies suggest that a combination of the two may be more efficacious than either alone.8 Venlafaxine, phenelzine, buspirone, benzodiazepines, and SSRIs have all demonstrated effectiveness and tolerability in generalized social phobia. Beta-adrenergic receptor antagonists (e.g., atenolol, propranolol) are commonly administered to treat performance phobia shortly before exposure to the phobic stimulus.
An adequate time frame for psychopharmacologic treatment of social phobia has not been defined. In depression therapy, medications should be maintained for at least 4 to 5 weeks before considering the regimen unsuccessful.1
While Mr. I’s depression responded well to bupropion SR, a medication whose mechanism involves dopamine and norepinephrine reuptake, use of a medication that augments his serotonin may further improve his condition.
Cognitive and behavioral therapies also are indicated for both generalized social phobia and performance phobia. These should be considered along with medication therapy for Mr. I to treat his social phobia and prevent a relapse.
Despite the available evidence, however, the course and prognosis of social phobia are not clear. Data are still forthcoming on this recently recognized disorder.
Further treatment: Another neurotransmitter
Again cautious of potential adverse sexual effects, Mr. I agrees to try mirtazapine, 30 mg at bedtime, in addition to bupropion SR. He initially complains of sedation and lowered energy, but is willing to continue the mirtazapine, hoping that it will help his social phobia.
Two months after starting mirtazapine, Mr. I is still fearful at work and home, and his relationship with his order. Generalized fear may manifest later as excessive shyness. Several studies have linked childhood behavior inhibition to social phobia.5-7’ daughters has not improved. After another month, he reports that his sedation has resolved, but complains of increased fatigue and difficulty concentrating. He suspects that the bupropion SR has stopped working.
After another month, Mr. I self-discontinues the mirtazapine. Though he tries to participate in social situations, his anxiety has worsened. He goes to a country music club once a week but is afraid to ask anyone to dance.
Should you focus on Mr. I’s depression rather than his social phobia? If so, how do you change his treatment?
Drs. Yu’s, Gordon’s, and Maguire’s observations
The severity of Mr. I’s social phobia may be causing his depression. Both trials of bupropion SR and mirtazapine have been adequate for his depression but have not alleviated his social phobia.
Medications that affect gamma-aminobutyric acid (GABA) levels, specifically benzodiazepines, have not been tried. Benzodiazepines provide rapid relief with little risk in short-term treatment, but dependence/withdrawal risks increase greatly when given more than 4 to 6 months.8 Because of Mr. I’s chronic anxiety in social phobia and his history of alcoholism, benzodiazepines are not recommended.
The novel compound tiagabine has been shown to increase GABA in the synaptic cleft.12 GABA increases chloride conduction through its ligand-gated channels, creating a potential antianxiety effect similar to that produced by benzodiazepines.8,13,14 GAT-1, the predominant transporter, removes excess GABA.
Just as SSRIs inhibit serotonin reuptake and allow the neurotransmitter to act on its receptors to alleviate depression and anxiety, so does tiagabine inhibit GAT-1. Theoretically, tiagabine may relieve anxiety by increasing synaptic concentrations of GABA.
Tiagabine also has been shown to be well-tolerated without a known abuse or dependence potential.12 Possible adverse effects include impaired concentration, somnolence, fatigue, nausea, and dizziness. To avoid adverse effects, slow titration (about 4 mg per week) is recommended.
Changing treatment: Looking up
Tiagabine, 4 mg at bedtime, is added to help with Mr. I’s anxiety; he is instructed to increase the dosage by 4 mg every 5 days in divided doses. He began to sense improvement during the second week, at 4 mg bid, and 2 weeks later his anxiety has been greatly reduced. He can now sit quietly with his co-workers during coffee breaks and has begun training a co-worker, which he never dared to attempt before. At this point, he was tolerating tiagabine at 8 mg bid.
One month later, tiagabine is increased to 16 mg bid. Mr. I has noticed mild dizziness with each dosage increase, but each time it subsided within a day. He has been maintained on 16 mg bid.
Saying that his anxiety is now well-controlled, Mr. I enjoys at least one dance each week at the country music club he frequents. One week later, he led an Alcoholics Anonymous meeting—while looking up to his audience for the first time. He continues these activities and his therapy sessions, which are geared toward developing stronger skills to minimize his anxiety. He is considering lowering his medication dosages (though he is wary of a possible relapse) and furthering his therapy.
Related resources
- Anxiety Disorders Association of America www.adaa.org
- National Institute of Mental Health: Phobias from NLM’s MEDLINEplus http://www.nlm.nih.gov/medlineplus/phobias.html
Author affiliations
Dr. Yu is a fellow in child and adolescent psychiatry, Dr. Gordon is a resident physician; and Dr. Maguire is assistant dean for continuing medical education, director of resident training, and associate clinical professor, department of psychiatry, University of California, Irvine.
Drug brand names
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Mirtazapine • Remeron
- Phenelzine • Nardil
- Tiagabine • Gabitril
- Venlafaxine • Effexor
Disclosure
Dr. Yu reports that he serves on the speaker’s bureau of Cephalon Inc., Novartis Pharmaceuticals Corp., and Pfizer Inc., and receives research/grant support from and serves as a consultant to Eli Lilly and Co.
Dr. Gordon reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Dr. Maguire reports that he receives research/grant support from, serves as a consultant to, and is on the speaker’s bureau of Eli Lilly and Co., is on the speaker’s bureau of Pfizer Inc., and receives research/grant support from Forest Laboratories and GlaxoSmithKline.
History: Living in fear
Mr. I, 41, presents for an initial psychiatric evaluation. He saw a psychologist 8 years ago for a “mild depression,” which he described as a lack of motivation and difficulty concentrating. His mood has been chronically “flat” for the last 10 years. He complains of poor energy and decreased sleep because of irregular work hours, and admits to using over-the-counter caffeine pills to help him function.
The patient denies suicidal ideations, symptoms of guilt, psychotic symptoms, or crying spells, but has a history of alcoholism and cocaine abuse. (He has been sober for 5 years.) Significant recent stressors include a recent breakup with his girlfriend, which he adds “really hasn’t bothered me at all.”
Mr. I has been increasingly avoiding social situations. Though he denies having panic attacks, interaction with other people triggers shortness of breath and chest tight-ness, especially when speaking in public to strangers.
The fear of what others might think of him is dominating Mr. I’s life. For example, he would like to console a housemate whose mother died, but because he is afraid of how the friend will react, Mr. I has not approached him. He adds that he goes out of his way to avoid contact with his co-workers, working irregular hours and eating his lunch in his car rather than the office lounge—even in inclement weather.
Mr. I does attend Alcoholic Anonymous meetings, but often sits toward the back. He had led some meetings, but refused to even look up from the podium while doing so. His anxiety worsened, his heart rate increased, and his palms sweated while leading the group. He began attending different AA meetings so that others would not recognize him and volunteer him to lead.
He adds that he feels comfortable meeting and dating women, since these exchanges are “scripted.” As he gets to know his partner better, however, Mr. I becomes more self-conscious.
Which of Mr. I’s symptoms would you address first: the depressive or the phobic?
Drs. Yu’s, Gordon’s, and Maguire’s observations
Based on Mr. I’s presentation, one might at first diagnose major depressive disorder, but chronic avoidance patterns differentiate his illness from an endogenous depression. Mr. I was diagnosed as having social phobia, a disorder that has been gaining attention among researchers.
A phobia is defined as an irrational fear that produces conscious avoidance of the feared subject, activity, or situation. The presence or anticipation of the phobic entity elicits severe distress, though the affected person usually recognizes that the reaction is excessive. DSM-IV defines social phobia as a strong, persisting fear of potentially embarrassing situations (Box).1
Two peaks of onset have been described: one occurring before age 5, and the other between ages 11 and 17.2 The mean age of onset has been reported to be 15.2
- A marked and persistent fear of one or more social or performance situations in which the person is exposed to unfamiliar people or to possible scrutiny by others. The individual fears that he or she will act in a way (or show anxiety symptoms) that will be humiliating or embarrassing.
- Exposure to the feared social situation almost invariably provokes anxiety, which may take the form of a situational or predisposed panic attack.
- The person recognizes that the fear is excessive or unreasonable.
- The feared social or performance situations are avoided, or else endured with intense anxiety or distress.
- The avoidance, anxious anticipation, or distress in the feared social or performance situation(s) interferes significantly with the person’s normal routine, occupational or academic functioning, or social activities or relationships with others, or there is marked distress about having the phobia.
- In individuals younger than 18, the duration is at least 6 months.
- The fear or avoidance is not caused directly by a substance (e.g., a drug of abuse or medication) or general medical condition, and is not better accounted for by another mental disorder (e.g., panic disorder with or without agoraphobia, separation anxiety disorder, body dysmorphic disorder, a pervasive developmental disorder, or schizoid personality disorder).
- If a general medical condition or other mental disorder is present, the fear in criterion A is unrelated to it (e.g., the patient does not fear stuttering, trembling in Parkinson’s disease, or exhibiting abnormal eating behavior in anorexia nervosa or bulimia nervosa). Specify if:
SOURCE: DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000.
DSM-IV describes two types of social phobia: generalized social phobia and performance phobia. Normal fear and shyness should be differentiated from social phobia. Medical conditions—including CNS tumors and cerebrovascular diseases—and drugs typically bring about neurologic and mental status symptoms that can confound the diagnosis.
Symptoms of other anxiety disorders, including panic disorder and agoraphobia, may mimic social phobia. Fear in social phobia is not present outside of, or in anticipation of, the feared situation. Social phobia also can be misdiagnosed as an avoidant personality, schizoid personality, or major depressive disorder.1
At this point, one should consider diagnosing Mr. I with social phobia, as evinced by his excessive avoidance of social situations. Mr. I also recognizes that his avoidance is excessive and causes difficulty in his daily functioning.
Behavioral inhibition in childhood is suggested to be more common in children with parents who had panic disorder. Generalized fear may manifest later as excessive shyness. Several studies have linked childhood behavior inhibition to social phobia.5-7’
First treatment: Pharmacotherapy and psychotherapy
Mr. I is interested in trying an antidepressant, but noted that about 6 years ago a brief course of a selective serotonin reuptake inhibitor led to difficulty sleeping and decreased libido and orgasm. He agrees to take bupropion SR, 100 mg/d, titrated after 2 weeks to 100 mg bid.
He also begins cognitive-behavioral and supportive therapy, during which he reveals that his pattern of avoidance took root in grade school, where he was often a quiet sidekick to the popular kids. During therapy, he describes visitations with his daughters, both of whom live with his ex-wife, as extremely difficult.
“I really don’t know what to say,” Mr. I says. “We often stare at each other during dessert, and I want to get it over with and go home.”
After 1 month, his bupropion SR is increased to 150 mg bid. One month later, his feelings of depression are under control. He sleeps well, no longer feels fatigued, and can concentrate. Still he isolates himself, fearing others’ disapproval. He has become more resistant to psychotherapy. “I know my patterns. I know what I do, but I can’t change it,” he says.
How would you treat Mr. I’s persistent social phobia? Would you switch his medications or augment existing ones? Does psychotherapy still have a role in treatment?
Drs. Yu’s, Gordon’s, and Maguire’s observations
Pharmacologic treatment of social phobia has spawned several neurochemical hypotheses. As beta-adrenergic antagonists have been proven efficacious in treating performance phobia, an adrenergic hypothesis suggests patients with performance phobia release more norepinephrine and epinephrine or are more sensitive to normal levels of these neuro-transmitters.8
The success of monoamine oxidase inhibitors in generalized social phobia suggests that dopamine plays a role in treating this form of the disorder. Central dopamine activity has been associated with positive emotions or extraversion.9
SSRIs have also demonstrated efficacy against generalized social phobia.10 Researchers have associated higher serotonin levels with increased social dominance11 and suggest that abnormal dopamine and serotonin levels contribute to the disorder’s pathogenesis.
Current treatments include psychotherapy and pharmacotherapy, and studies suggest that a combination of the two may be more efficacious than either alone.8 Venlafaxine, phenelzine, buspirone, benzodiazepines, and SSRIs have all demonstrated effectiveness and tolerability in generalized social phobia. Beta-adrenergic receptor antagonists (e.g., atenolol, propranolol) are commonly administered to treat performance phobia shortly before exposure to the phobic stimulus.
An adequate time frame for psychopharmacologic treatment of social phobia has not been defined. In depression therapy, medications should be maintained for at least 4 to 5 weeks before considering the regimen unsuccessful.1
While Mr. I’s depression responded well to bupropion SR, a medication whose mechanism involves dopamine and norepinephrine reuptake, use of a medication that augments his serotonin may further improve his condition.
Cognitive and behavioral therapies also are indicated for both generalized social phobia and performance phobia. These should be considered along with medication therapy for Mr. I to treat his social phobia and prevent a relapse.
Despite the available evidence, however, the course and prognosis of social phobia are not clear. Data are still forthcoming on this recently recognized disorder.
Further treatment: Another neurotransmitter
Again cautious of potential adverse sexual effects, Mr. I agrees to try mirtazapine, 30 mg at bedtime, in addition to bupropion SR. He initially complains of sedation and lowered energy, but is willing to continue the mirtazapine, hoping that it will help his social phobia.
Two months after starting mirtazapine, Mr. I is still fearful at work and home, and his relationship with his order. Generalized fear may manifest later as excessive shyness. Several studies have linked childhood behavior inhibition to social phobia.5-7’ daughters has not improved. After another month, he reports that his sedation has resolved, but complains of increased fatigue and difficulty concentrating. He suspects that the bupropion SR has stopped working.
After another month, Mr. I self-discontinues the mirtazapine. Though he tries to participate in social situations, his anxiety has worsened. He goes to a country music club once a week but is afraid to ask anyone to dance.
Should you focus on Mr. I’s depression rather than his social phobia? If so, how do you change his treatment?
Drs. Yu’s, Gordon’s, and Maguire’s observations
The severity of Mr. I’s social phobia may be causing his depression. Both trials of bupropion SR and mirtazapine have been adequate for his depression but have not alleviated his social phobia.
Medications that affect gamma-aminobutyric acid (GABA) levels, specifically benzodiazepines, have not been tried. Benzodiazepines provide rapid relief with little risk in short-term treatment, but dependence/withdrawal risks increase greatly when given more than 4 to 6 months.8 Because of Mr. I’s chronic anxiety in social phobia and his history of alcoholism, benzodiazepines are not recommended.
The novel compound tiagabine has been shown to increase GABA in the synaptic cleft.12 GABA increases chloride conduction through its ligand-gated channels, creating a potential antianxiety effect similar to that produced by benzodiazepines.8,13,14 GAT-1, the predominant transporter, removes excess GABA.
Just as SSRIs inhibit serotonin reuptake and allow the neurotransmitter to act on its receptors to alleviate depression and anxiety, so does tiagabine inhibit GAT-1. Theoretically, tiagabine may relieve anxiety by increasing synaptic concentrations of GABA.
Tiagabine also has been shown to be well-tolerated without a known abuse or dependence potential.12 Possible adverse effects include impaired concentration, somnolence, fatigue, nausea, and dizziness. To avoid adverse effects, slow titration (about 4 mg per week) is recommended.
Changing treatment: Looking up
Tiagabine, 4 mg at bedtime, is added to help with Mr. I’s anxiety; he is instructed to increase the dosage by 4 mg every 5 days in divided doses. He began to sense improvement during the second week, at 4 mg bid, and 2 weeks later his anxiety has been greatly reduced. He can now sit quietly with his co-workers during coffee breaks and has begun training a co-worker, which he never dared to attempt before. At this point, he was tolerating tiagabine at 8 mg bid.
One month later, tiagabine is increased to 16 mg bid. Mr. I has noticed mild dizziness with each dosage increase, but each time it subsided within a day. He has been maintained on 16 mg bid.
Saying that his anxiety is now well-controlled, Mr. I enjoys at least one dance each week at the country music club he frequents. One week later, he led an Alcoholics Anonymous meeting—while looking up to his audience for the first time. He continues these activities and his therapy sessions, which are geared toward developing stronger skills to minimize his anxiety. He is considering lowering his medication dosages (though he is wary of a possible relapse) and furthering his therapy.
Related resources
- Anxiety Disorders Association of America www.adaa.org
- National Institute of Mental Health: Phobias from NLM’s MEDLINEplus http://www.nlm.nih.gov/medlineplus/phobias.html
Author affiliations
Dr. Yu is a fellow in child and adolescent psychiatry, Dr. Gordon is a resident physician; and Dr. Maguire is assistant dean for continuing medical education, director of resident training, and associate clinical professor, department of psychiatry, University of California, Irvine.
Drug brand names
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Mirtazapine • Remeron
- Phenelzine • Nardil
- Tiagabine • Gabitril
- Venlafaxine • Effexor
Disclosure
Dr. Yu reports that he serves on the speaker’s bureau of Cephalon Inc., Novartis Pharmaceuticals Corp., and Pfizer Inc., and receives research/grant support from and serves as a consultant to Eli Lilly and Co.
Dr. Gordon reports no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Dr. Maguire reports that he receives research/grant support from, serves as a consultant to, and is on the speaker’s bureau of Eli Lilly and Co., is on the speaker’s bureau of Pfizer Inc., and receives research/grant support from Forest Laboratories and GlaxoSmithKline.
1. Sadock BJ, Sadock VA. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry (7th ed). Philadelphia: Lippincott Williams & Wilkins, 2000.
2. Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia: comorbidity and morbidity in an epidemiological sample. Arch Gen Psychiatry 1992;49:282-8.
3. Wacker HR, Mhllejans R, Klein KH, Battegay R. Identification of cases of anxiety disorders and affective disorders in the community according to ICD-10 and DSM-III-R using the composite international diagnostic interview (CIDI). Int J Methods Psychiatr Res 1992;2:91-100.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed), text revision. Washington, DC: American Psychiatric Association, 2000.
5. Van Ameringen M, Mancini C, Oakman JM. The relationship of behavioral inhibition and shyness to anxiety disorder. J Nerv Ment Dis 1998;186:425-31.
6. Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry 1999;38:1008-15.
7. Turner SM, Beidel DC, Townsley RM. Social phobia: relationship to shyness. Behav Res Ther 1990;28:497-505.
8. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical application (2nd ed). Cambridge University Press, Cambridge, 2000.
9. Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist induced dopamine activity to positive emotionality. J Pers Soc Psychol 1994;67:485-98.
10. Van Ameringen M, Mancini C, Oakman JM, Farvolden P. Selective serotonin reuptake inhibitors in the treatment of social phobia: the emerging gold standard. CNS Drugs 1999;11:307-15.
11. Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 1998;155:373-9.
12. Physician’s Desk Reference (55th ed). Montvale, NJ: Medical Economics, 2001.
13. Bowery NG. Pharmacology of mammalian GABAB receptors. In: Enna SJ, Bowery NG (eds). The GABA receptors (2nd ed). Totowa, NJ: Humana Press, 1997;209-36.
14. Johnston GAR. Molecular biology, pharmacology, and physiology of GABAC receptors. In: Enna SJ, Bowery NG (eds). The GABA receptors (2nd ed). Totowa, NJ: Humana Press, 1997;297-323.
1. Sadock BJ, Sadock VA. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry (7th ed). Philadelphia: Lippincott Williams & Wilkins, 2000.
2. Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia: comorbidity and morbidity in an epidemiological sample. Arch Gen Psychiatry 1992;49:282-8.
3. Wacker HR, Mhllejans R, Klein KH, Battegay R. Identification of cases of anxiety disorders and affective disorders in the community according to ICD-10 and DSM-III-R using the composite international diagnostic interview (CIDI). Int J Methods Psychiatr Res 1992;2:91-100.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed), text revision. Washington, DC: American Psychiatric Association, 2000.
5. Van Ameringen M, Mancini C, Oakman JM. The relationship of behavioral inhibition and shyness to anxiety disorder. J Nerv Ment Dis 1998;186:425-31.
6. Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry 1999;38:1008-15.
7. Turner SM, Beidel DC, Townsley RM. Social phobia: relationship to shyness. Behav Res Ther 1990;28:497-505.
8. Stahl SM. Essential psychopharmacology: neuroscientific basis and practical application (2nd ed). Cambridge University Press, Cambridge, 2000.
9. Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist induced dopamine activity to positive emotionality. J Pers Soc Psychol 1994;67:485-98.
10. Van Ameringen M, Mancini C, Oakman JM, Farvolden P. Selective serotonin reuptake inhibitors in the treatment of social phobia: the emerging gold standard. CNS Drugs 1999;11:307-15.
11. Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry 1998;155:373-9.
12. Physician’s Desk Reference (55th ed). Montvale, NJ: Medical Economics, 2001.
13. Bowery NG. Pharmacology of mammalian GABAB receptors. In: Enna SJ, Bowery NG (eds). The GABA receptors (2nd ed). Totowa, NJ: Humana Press, 1997;209-36.
14. Johnston GAR. Molecular biology, pharmacology, and physiology of GABAC receptors. In: Enna SJ, Bowery NG (eds). The GABA receptors (2nd ed). Totowa, NJ: Humana Press, 1997;297-323.
Borderline personality disorder: The lability of psychiatric diagnosis
Not everyone agrees that borderline personality disorder (BPD) should be a diagnostic category. BPD became “official” with DSM-III in 1980, although the term had been used for 40 years to describe various patient groups. Being listed in DSM-III legitimized BPD, which was thought to represent a specific—though not necessarily distinct—diagnostic category.
The history of the BPD diagnosis and opinions as to its usefulness can be viewed as a microcosm of psychiatric diagnosis in general. Before DSM-III, diagnoses were broadly defined and did not contain specific inclusion or exclusion criteria.1 For the 5 to 10 years prior to DSM-III, however, two assumptions developed:
- distinct diagnostic categories did, in fact, exist
- by rigorously defining and studying those categories we could develop more specific and effective treatments for our patients.2
The specificity and exclusivity that we assumed we could achieve by categorical diagnoses, however, remain a distant wish. Comorbidity appears more common in psychiatry than was originally thought and confounds both treatment and outcome.3 Also, many patients appear treatment-resistant, despite fitting neatly into diagnostic categories.4
Miss A, age 35, presents to the emergency room with a long history of intermittent depression and self-mutilation. She has never been hospitalized nor on psychotropic medication but has been in and out of psychotherapy for years. She has had intermittent depressive episodes for many years, though the episodes often lasted 2 to 3 weeks and appeared to correct themselves spontaneously.
Agitated and afraid. She is extremely agitated when she arrives at the emergency department. She has hardly slept or eaten but insists she is not hungry. She reports that she cannot concentrate or do her work as an accountant. She says she is hearing voices, knows they are in her head, but nonetheless is terrified that something horrible is about to happen—though she cannot say what it might be.
Voice ‘calling my name.’ When the psychiatric resident inquires further, Miss A says a male voice is calling her name and mumbling some short phrase she cannot understand. She says she has heard the voice the last few days, perhaps for 10 to 15 minutes every few hours, particularly when she ruminates about how she messed up a relationship with her now ex-boyfriend. The breakup occurred 1 week ago.
Feeling detached. She claims she has never heard voices before but describes periods when she has felt detached and unreal. Often these were short-term dissociative episodes that occurred in the wake of what she perceived as a personal failure or a distressful interpersonal encounter (often with a man). Relationships frequently were very difficult for her, and she felt she could easily go from infatuation to detesting someone.
Diagnosis? Talking appears to calm her down. After being in the emergency room for 2 hours, she says she no longer hears the voice. The resident tells the attending psychiatrist he believes the patient is in a major depressive episode, perhaps a psychotic depression, and proposes starting antidepressant treatment. The attending argues that the patient appears to have borderline personality disorder and suggests that she be sent home without medication and given an appointment to the outpatient clinic within the next few days.
As psychiatry considers DSM-V, questions linger as to whether BPD (and personality disorder in general) should remain as a categorical diagnosis or if dimensional measures may be more appropriate. Dimensions imply that no one ever fits into a given box because no specific box exists. Rather, patients are described as being closer to or more distant from a prototypic model of the diagnosis. In personality disorders, the dimensions most often mentioned are cognition, impulsivity, emotional lability, environmental hyperreactivity, and anxiety. The case report (above) illustrates the interplay of these dimensions in a typical patient with presumed BPD.
What’s in a name?
The symptom complex or syndrome that bears the name borderline personality disorder has probably existed for as long as people have thought about patients in psychopathologic terms.5 Before 1980, the term “borderline” applied primarily to two separate but overlapping concepts:
- Patients thought to reside on the “border” with psychosis, such as the patient in our case example. They seemed to have an underlying psychotic disorder, but the psychosis—if it surfaced—appeared briefly, was not exceptionally deep or firmly held, and was not regularly evident or immediately accessible to the clinician.
- Patients who appeared to occupy the space between neurosis and psychosis. This concept evolved into the idea of a character or personality disorder distinguished primarily by unstable interpersonal relationships, a confused or inconsistent sense of identity, and emotional instability.
How DSM is changing. Comparing the disorders listed in DSM-IV (1994)6 versus DSM-II (1968)1 suggests that psychiatry has become enamored of the naming process. For example, DSM-II lists anxiety neurosis (300.0), phobic neurosis (300.2), and obsessive-compulsive neurosis (300.3), whereas DSM-IV lists 11 different categories of anxiety disorders.
But beyond naming, subsequent DSMs have differed even more dramatically from DSM-II. We have seen a shift from describing a diagnostic category with a simple explanatory paragraph to lists of specific inclusion and exclusion criteria. These more-specific lists imply that they define categories closer to some reality or authenticity than did previous definitions.
Before DSM-III, the borderline concept was conceived in broad object relational and psychodynamic terms. In contrast, DSM-III produced a definitive set of criteria and required that a subset be met before the diagnosis could be made.7 An example of this criteria-based model is shown in Box 1, which lists the DSM-IV-TR criteria for BPD.
Some psychiatrists objected that BPD was solely a psychoanalytic construct and too theoretical for inclusion in DSM-III. Others argued that if BPD were not defined, it would be difficult to study the clinical usefulness of that definition or any other. Nonetheless, many have argued that BPD does not exist, though to what category BPD patients should belong has changed over the years:
- Is BPD nothing more than a milder or unusual presentation of an affective disorder8 or actually bipolar II disorder?9
- Is it a presentation of posttraumatic stress disorder (PTSD) called “complex PTSD,”10-11 or an adult presentation of attention-deficit/ hyperactivity or other brain disorder?12
- Is it a stigmatizing diagnosis that we apply to patients whom we do not like?13
In truth, the diagnosis of BPD reflects a particular clinical presentation no more or less accurately than many of the well-accepted axis I disorders. Despite recent advances in the neurosciences, the dilemma we face as psychiatrists is that we make a diagnosis based upon what we see in the clinical setting (i.e., a phenotype). Yet in labeling what we believe is a specific psychiatric disorder, we make assumptions—for better or for worse, consciously or unconsciously—about pathophysiology and indirectly about genotype.
A pervasive pattern of instability of interpersonal relationships, self-image, and affects and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by five (or more) of the following:
- Frantic efforts to avoid real or imagined abandonment
- A pattern of unstable and intense interpersonal relationships characterized by alternating between extremes of idealization and devaluation
- Identity disturbance: markedly and persistent unstable self-image or sense of self
- Impulsivity in at least two areas that are potentially self-damaging (spending, sex, substance abuse, binge eating, reckless driving)
- Recurrent suicidal behavior, gestures, or threats; self-mutilating behavior
- Affective instability due to a marked reactivity of mood
- Chronic feelings of emptiness
- Inappropriate, intense anger or difficulty controlling anger
- Transient, stress-related paranoid ideation or severe dissociative symptoms
Source: DSM-IV6
Defining the borderline personality
Stern first used the term “borderline” in 1938 to describe patients who appeared to occupy the border between neurosis and psychosis.14 In 1942, Deutsch described the “as if” personality in patients who seemed chameleon-like. They could adapt or play the role demanded of them in specific situations, yet elsewhere—as in the analyst’s office—they had little sense of themselves and were thought to be internally disorganized and probably psychotic.15
Border to psychosis. The idea that borderline-type patients were psychotic continued in Hoch and Polatin’s description of the “pseudoneurotic schizophrenic,”16 a patient who appeared severely neurotic but was thought to employ many defenses and interpersonal styles to ward off a fundamental inner psychosis. Knight used the label “borderline states”17 to describe severely ill patients who were not frankly psychotic but fell within the realm of psychosis without qualifying for a diagnosis of schizophrenia. Knight was the first person to use the term “borderline” as a diagnostic entity, though simultaneously he argued against its use as a label because the term lacked precision.
Psychotic character. About the same time, Schmideberg characterized a group of patients whose emotional lability or affective reactivity seemed to be a consistent aspect of their clinical presentation. She believed this appearance of “stable instability”18 represented the patient’s characterologic adaptation to the world.
Frosch coined the term “psychotic character”19 that aptly captured both the characterologic and the border-to-psychosis aspects of these patients’ clinical picture. According to Frosch, these patients appeared to regress readily into psychotic thinking, yet they did not lose their ability to test reality.
Affective and emotional instability. Thus until the 1960s, the term borderline was applied primarily to patients who appeared to occupy the border between neurosis and psychosis but were thought to be closer to psychotic than neurotic. And this sitting close to the edge of psychosis appeared to be a stable condition.
Most of the attention up until this point had been paid to how these patients thought—with little attention to their affective lability or emotional instability, save for Schmideberg’s comments. In the 1960s, however, the term borderline was applied somewhat differently—not completely divorced from previous concepts but with greater emphasis on borderline as a stable but psychopathologic functioning of the personality that included affective and emotional instability and an impaired sense of self.
- Intense affect, usually depressive or hostile
- History of impulsive, often self-destructive behavior
- Social adaptiveness that may mask a disturbed identity
- Brief psychotic episodes, often paranoid and evident in unstructured situations
- “Loose thinking” or primitive answers on unstructured psychological tests
- Relationships vacillate between transient superficiality and intense dependency
Impaired personality organization. In 1967, Kernberg published a seminal article in the history of BPD diagnosis. Although he did not discuss the diagnosis of BPD, Kernberg did develop a concept concerning a specific organization of the personality based upon impaired object relations. This impaired organization could apply across several personality disorders. The construct, named borderline personality organization (BPO),20 was defined by:
- an impaired sense of identity and lack of integration of one’s own identity
- use of primitive defenses, including splitting, rage, and regression
- ability to test reality.
Kernberg’s theory is too complex to summarize here, but he—along with Roy Grinker—is responsible for placing BPD on the diagnostic map. He was the first to describe BPO (and by extension BPD) in terms of a personality disorder.
Grinker’s ‘core’ group. Almost simultaneously (in 1968), Grinker published a careful study of 50 hospitalized patients. His work on the “borderline syndrome”21 revealed four subgroups to which the label of borderline had been applied:
- those occupying the border with psychosis
- those occupying the border with neurosis
- those similar to Deutsch’s “as if” group
- the “core” borderline group.
The core group—with its symptoms of anger and loneliness, a nonintegrated sense of self, and labile and oscillating interpersonal relationships— defined patients closest to our current definition.
Six criteria for BPD. In 1975, Gunderson and Singer published an article that greatly influenced our definition of BPD. They reviewed major descriptive accounts of BPD or BPD-like syndromes22 and proposed six diagnostic criteria (Box 2), though they did not identify a specific number or subset of the criteria as needing to be met for the diagnosis. (It is important to note that the term BPD did not become official for 5 more years.)
DSM-IV’s definition of BPD retains four of Gunderson and Singer‘s criteria among the nine it lists (five being necessary for a diagnosis of BPD). Missing are:
- social adaptiveness—though DSM-IV does say that social adaptiveness may be superficial (as in the “as if” personality) and may hide a disturbed identity6
- and the criterion relating to psychological test performance (this omission reflects a movement since 1980 away from listing “psychological” or psychodynamic criteria in the DSM).
DSM-III. BPD was included in DSM-III7 following an important study that tried to determine whether the term “borderline” refers to patients at the border of psychosis or to a stable group with mood instability and affective lability as part of a personality disorder. Spitzer et al23 asked 808 clinicians to describe patients they would label as borderline and to use 22 items gleaned from the literature to score two of their own patients:
- one patient who the clinician felt truly had borderline personality, borderline personality organization, or borderline schizophrenia
- and a control patient who was not diagnosed as psychotic and did not fall into any borderline category.
- The concept of abandonment, introduced in DSM-III-R, replaced the concept of aloneness in DSM-III.
- In DSM-III and DSM-III-R, a patient needed to meet 5 of 8 criteria for a diagnosis of BPD.
- DSM-IV introduced the ninth criterion, “transient, stress-related paranoid ideation or severe dissociative symptoms.” Since then, a patient has needed to meet 5 of 9 criteria for a diagnosis of BPD.
Their responses showed that BPD and schizotypal personality disorder (SPD) were separate, independent (though not mutually exclusive) disorders. Spitzer et al preserved the “schizotypal” label in DSM-III to describe the personality disorder that closely matched the border-to-psychosis subset. The other criteria set, which they labeled the “unstable personality item set,” was renamed “borderline” in DSM-III to describe the personality disorder that closely matched the emotional lability subset.
From one DSM edition to another, the concept of brief transient psychotic episodes has been included in and excluded from the diagnosis of borderline personality disorder (BPD).
In DSM-III. Because of work by Spitzer et al, these “experiences” were placed within schizotypal personality disorder (SPD) in DSM-III in 1980, though historically they had always been within the borderline concept and were one of Gunderson and Singer’s six criteria for diagnosing BPD (Box 2).22
Out of DSM-III-R. Research in the late 1980s suggested that when patients with BPD were depressed, they had a greater tendency to have psychotic–like episodes.24 Evidence indicated that attributing these psychotic and dissociative phenomena to SPD, rather than—perhaps more appropriately—to BPD, was one of the main reasons for the overlap between the definitions of BPD and SPD.25 Therefore, in DSM-III-R, the transient psychotic/dissociative criterion was removed from the SPD criteria set.
Back in DSM-IV. The criterion “transient stress-related paranoid ideation or severe dissociative symptoms” was placed into the BPD criteria set in DSM-IV. In DSM-IV, these symptoms were further characterized as usually not of “sufficient severity or duration to warrant an additional diagnosis.”
What is “sufficient” duration? The psychotic episodes of BPD last for minutes to hours and often appear when the patient imagines being (or actually is) abandoned by others. Not all agree that the criteria for BPD are met if these episodes last longer (e.g.,a day or two). In that case, they may exceed the transient time frame. More research is needed to better understand the quality and duration of these psychotic-like phenomena.
Not everyone agrees with renaming the unstable set “borderline” because the word:
- has always been ambiguous
- does not connote or denote any specific criteria or characteristic of patients who bear the label
- brands the patient as untreatable, defiant, or just “bad.”
Post-DSM-III: Where are we now?
From DSM-III evolved the hope that psychiatry could describe valid, well-defined diagnostic categories. Lost in the DSM-III enthusiasm was the fact that the categories were based upon theoretic constructs—theories no more or less valid than other theories that had preceded them. Because some of these categories were based upon empiric data— such as the Spitzer et al study—these diagnoses were perceived as more valid and more related to pathophysiology and perhaps genotype than prior constructs and definitions.
In the 1980s and early 1990s, a proliferation of studies attempted to examine the validity and reliability of DSM-III definitions, and BPD became the most studied of the personality disorders. The BPD concept took hold, even though several studies did not support it and despite refinements in subsequent DSM editions (Box 3).
One refinement in the BPD construct applied to transient psychotic or psychotic-like experiences, including dissociative phenomena. Yet questions remain about the duration of these transient episodes (Box 4).
Categorical versus dimensional
The categorical concept of BPD is facing new scrutiny,26,27 as recent studies have implied that biological disturbances may be spread across a number of personality disorders.28 If biological findings are found to be more closely allied with genotypic variations (alleles),29 then perhaps a dimensional classification system is needed for BPD and personality disorders in general.
On the other hand, categories provide a well-defined population that we can study and try to delineate from other populations, whereas dimensions—while perhaps closer to the reality of clinical presentation—may allow too much variability for research to proceed without confounding restraints.
BPD will continue to evolve, as will all psychiatric diagnostic categories, but the need to modify its definition does not negate its usefulness and clinical applicability. Most of our patients do not read the DSM before coming to us. They present with symptom complexes and problems that demand that we listen to what they say and understand who they are while we also try to fit them—as best we can—into categories or dimensions30 that help us choose the most appropriate interventions.
Related resources
- BPD Sanctuary (borderline personality disorder education, communities, support, books, and resources) http://www.mhsanctuary.com/borderline/ Borderline Central (resources for people who care about someone with borderline personality disorder) http://www.bpdcentral.com/
- Gunderson JG. Borderline personality disorder: a clinical guide. Washington, DC: American Psychiatric Publishing, Inc., 200l.
- Paris J (ed). Borderline personality disorder. Psych Clin N Am 2000;23:1 (entire volume devoted to BPD).
- Silk KR (ed). Biological and neurobehavioral studies of borderline personality disorder. Washington, DC: American Psychiatric Press, 1994.
1. Diagnostic and statistical manual of mental disorders (2nd ed). Washington, DC: American Psychiatric Association, 1968.
2. Feighner JP, Robins E, Guze SB, et al. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972;26:57-63.
3. Merikangas KR, Angst J, Eaton W, et al. Comorbidity and boundaries of affective disorders with anxiety disorders and substance misuse: results of an international task force. Br J Psychiatry 1996;30(suppl):58-67.
4. Nierenberg AA. DeCecco LM.Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 16):5-9.
5. Stone MH (ed). Essential papers on borderline disorders: one hundred years at the border. New York: New York University Press, 1986.
6. Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC: American Psychiatric Association, 1994.
7. Diagnostic and statistical manual of mental disorders (3rd ed). Washington, DC: American Psychiatric Association, 1980.
8. Akiskal HS. Subaffective disorders: dysthymic, cyclothymic and bipolar II disorders in the “borderline” realm. Psychiatric Clin N Am 1981;4:25-46.
9. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatric Res 2001;35:307-12.
10. Herman JL, van der Kolk BA. Traumatic antecedents of borderline personality disorder. In: van der Kolk, BA. Psychological trauma. Washington, DC: American Psychiatric Press, 1987;111-26.
11. Silk KR, Lee S, Hill EM, Lohr NE. Borderline symptoms and severity of sexual abuse. Am J Psychiatry 1995;152:1059-64.
12. Streeter CC, Van Reekum R, Shorr RI, Bachman DL. Prior head injury in male veterans with borderline personality disorder. J Nerv Ment Dis 1995;183:577-81.
13. Maltsberger JT. Countertransference in borderline conditions: some further notes. Int J Psychoanal Psychother 1982-83;9:125-34.
14. Stern A. Psychoanalytic investigation and therapy in the borderline group of neuroses. Psychoanal Q 1938;7:467-89.
15. Deutsch H. Some forms of emotional disturbance and their relationship to schizophrenia. Psychoanal Q 1942;11:301-21.
16. Hoch P, Polatin P. Pseudoneurotic forms of schizophrenia. Psychiatric Q 1949;23:248-76.
17. Knight R. Borderline states. Bull Menn Clin 1953;17:1-12.
18. Schmideberg M. The treatment of psychopaths and borderline patients. Am J Psychotherapy 1947;1:45-70.
19. Frosch J. The psychotic character: clinical psychiatric considerations. Psychiatric Q 1964;38:81-96.
20. Kernberg O. Borderline personality organization. J Am Psychoanal Assoc 1967;15:641-85.
21. Grinker RR, Werble B, Drye R. The borderline syndrome: a behavioral study of ego functions. New York: Basic Books, 1968.
22. Gunderson JG, Singer MT. Defining borderline patients: an overview. Am J Psychiatry 1975;132:1-10.
23. Spitzer RL, Endicott J, Gibbon M. Crossing the border into borderline personality and borderline schizophrenia: the development of criteria. Arch Gen Psychiatry 1979;36:17-24.
24. Silk KR, Lohr NE, Westen D, Goodrich S. Psychosis in borderline patients with depression. J Personality Disord 1989;3:92-100.
25. Silk KR, Westen D, Lohr NE, et al. DSM-III and DSM-III-R schizotypal symptoms in borderline personality disorder. Comprehen Psychiatry 1990;31:103-10.
26. Livesley WJ, Jang KL, Vernon PA. Phenotypic and genetic structure of traits delineating personality disorder. Arch Gen Psychiatry 1998;55:941-8.
27. McCrae RR, Yang J, Costa PT, Jr, et al. Personality profiles and the prediction of categorical personality disorders. J Personality 2001;69:155-74.
28. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry 1991;148:1647-58.
29. New AS, Gelernter J, Goodman M, et al. Suicide, impulsive aggression, and HTR1B genotype. Biolog Psychiatry 2001;50:62-5.
30. Oldham JM, Skodol AE. Charting the future of axis II. J Personality Disord 2000;14:17-29.
Not everyone agrees that borderline personality disorder (BPD) should be a diagnostic category. BPD became “official” with DSM-III in 1980, although the term had been used for 40 years to describe various patient groups. Being listed in DSM-III legitimized BPD, which was thought to represent a specific—though not necessarily distinct—diagnostic category.
The history of the BPD diagnosis and opinions as to its usefulness can be viewed as a microcosm of psychiatric diagnosis in general. Before DSM-III, diagnoses were broadly defined and did not contain specific inclusion or exclusion criteria.1 For the 5 to 10 years prior to DSM-III, however, two assumptions developed:
- distinct diagnostic categories did, in fact, exist
- by rigorously defining and studying those categories we could develop more specific and effective treatments for our patients.2
The specificity and exclusivity that we assumed we could achieve by categorical diagnoses, however, remain a distant wish. Comorbidity appears more common in psychiatry than was originally thought and confounds both treatment and outcome.3 Also, many patients appear treatment-resistant, despite fitting neatly into diagnostic categories.4
Miss A, age 35, presents to the emergency room with a long history of intermittent depression and self-mutilation. She has never been hospitalized nor on psychotropic medication but has been in and out of psychotherapy for years. She has had intermittent depressive episodes for many years, though the episodes often lasted 2 to 3 weeks and appeared to correct themselves spontaneously.
Agitated and afraid. She is extremely agitated when she arrives at the emergency department. She has hardly slept or eaten but insists she is not hungry. She reports that she cannot concentrate or do her work as an accountant. She says she is hearing voices, knows they are in her head, but nonetheless is terrified that something horrible is about to happen—though she cannot say what it might be.
Voice ‘calling my name.’ When the psychiatric resident inquires further, Miss A says a male voice is calling her name and mumbling some short phrase she cannot understand. She says she has heard the voice the last few days, perhaps for 10 to 15 minutes every few hours, particularly when she ruminates about how she messed up a relationship with her now ex-boyfriend. The breakup occurred 1 week ago.
Feeling detached. She claims she has never heard voices before but describes periods when she has felt detached and unreal. Often these were short-term dissociative episodes that occurred in the wake of what she perceived as a personal failure or a distressful interpersonal encounter (often with a man). Relationships frequently were very difficult for her, and she felt she could easily go from infatuation to detesting someone.
Diagnosis? Talking appears to calm her down. After being in the emergency room for 2 hours, she says she no longer hears the voice. The resident tells the attending psychiatrist he believes the patient is in a major depressive episode, perhaps a psychotic depression, and proposes starting antidepressant treatment. The attending argues that the patient appears to have borderline personality disorder and suggests that she be sent home without medication and given an appointment to the outpatient clinic within the next few days.
As psychiatry considers DSM-V, questions linger as to whether BPD (and personality disorder in general) should remain as a categorical diagnosis or if dimensional measures may be more appropriate. Dimensions imply that no one ever fits into a given box because no specific box exists. Rather, patients are described as being closer to or more distant from a prototypic model of the diagnosis. In personality disorders, the dimensions most often mentioned are cognition, impulsivity, emotional lability, environmental hyperreactivity, and anxiety. The case report (above) illustrates the interplay of these dimensions in a typical patient with presumed BPD.
What’s in a name?
The symptom complex or syndrome that bears the name borderline personality disorder has probably existed for as long as people have thought about patients in psychopathologic terms.5 Before 1980, the term “borderline” applied primarily to two separate but overlapping concepts:
- Patients thought to reside on the “border” with psychosis, such as the patient in our case example. They seemed to have an underlying psychotic disorder, but the psychosis—if it surfaced—appeared briefly, was not exceptionally deep or firmly held, and was not regularly evident or immediately accessible to the clinician.
- Patients who appeared to occupy the space between neurosis and psychosis. This concept evolved into the idea of a character or personality disorder distinguished primarily by unstable interpersonal relationships, a confused or inconsistent sense of identity, and emotional instability.
How DSM is changing. Comparing the disorders listed in DSM-IV (1994)6 versus DSM-II (1968)1 suggests that psychiatry has become enamored of the naming process. For example, DSM-II lists anxiety neurosis (300.0), phobic neurosis (300.2), and obsessive-compulsive neurosis (300.3), whereas DSM-IV lists 11 different categories of anxiety disorders.
But beyond naming, subsequent DSMs have differed even more dramatically from DSM-II. We have seen a shift from describing a diagnostic category with a simple explanatory paragraph to lists of specific inclusion and exclusion criteria. These more-specific lists imply that they define categories closer to some reality or authenticity than did previous definitions.
Before DSM-III, the borderline concept was conceived in broad object relational and psychodynamic terms. In contrast, DSM-III produced a definitive set of criteria and required that a subset be met before the diagnosis could be made.7 An example of this criteria-based model is shown in Box 1, which lists the DSM-IV-TR criteria for BPD.
Some psychiatrists objected that BPD was solely a psychoanalytic construct and too theoretical for inclusion in DSM-III. Others argued that if BPD were not defined, it would be difficult to study the clinical usefulness of that definition or any other. Nonetheless, many have argued that BPD does not exist, though to what category BPD patients should belong has changed over the years:
- Is BPD nothing more than a milder or unusual presentation of an affective disorder8 or actually bipolar II disorder?9
- Is it a presentation of posttraumatic stress disorder (PTSD) called “complex PTSD,”10-11 or an adult presentation of attention-deficit/ hyperactivity or other brain disorder?12
- Is it a stigmatizing diagnosis that we apply to patients whom we do not like?13
In truth, the diagnosis of BPD reflects a particular clinical presentation no more or less accurately than many of the well-accepted axis I disorders. Despite recent advances in the neurosciences, the dilemma we face as psychiatrists is that we make a diagnosis based upon what we see in the clinical setting (i.e., a phenotype). Yet in labeling what we believe is a specific psychiatric disorder, we make assumptions—for better or for worse, consciously or unconsciously—about pathophysiology and indirectly about genotype.
A pervasive pattern of instability of interpersonal relationships, self-image, and affects and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by five (or more) of the following:
- Frantic efforts to avoid real or imagined abandonment
- A pattern of unstable and intense interpersonal relationships characterized by alternating between extremes of idealization and devaluation
- Identity disturbance: markedly and persistent unstable self-image or sense of self
- Impulsivity in at least two areas that are potentially self-damaging (spending, sex, substance abuse, binge eating, reckless driving)
- Recurrent suicidal behavior, gestures, or threats; self-mutilating behavior
- Affective instability due to a marked reactivity of mood
- Chronic feelings of emptiness
- Inappropriate, intense anger or difficulty controlling anger
- Transient, stress-related paranoid ideation or severe dissociative symptoms
Source: DSM-IV6
Defining the borderline personality
Stern first used the term “borderline” in 1938 to describe patients who appeared to occupy the border between neurosis and psychosis.14 In 1942, Deutsch described the “as if” personality in patients who seemed chameleon-like. They could adapt or play the role demanded of them in specific situations, yet elsewhere—as in the analyst’s office—they had little sense of themselves and were thought to be internally disorganized and probably psychotic.15
Border to psychosis. The idea that borderline-type patients were psychotic continued in Hoch and Polatin’s description of the “pseudoneurotic schizophrenic,”16 a patient who appeared severely neurotic but was thought to employ many defenses and interpersonal styles to ward off a fundamental inner psychosis. Knight used the label “borderline states”17 to describe severely ill patients who were not frankly psychotic but fell within the realm of psychosis without qualifying for a diagnosis of schizophrenia. Knight was the first person to use the term “borderline” as a diagnostic entity, though simultaneously he argued against its use as a label because the term lacked precision.
Psychotic character. About the same time, Schmideberg characterized a group of patients whose emotional lability or affective reactivity seemed to be a consistent aspect of their clinical presentation. She believed this appearance of “stable instability”18 represented the patient’s characterologic adaptation to the world.
Frosch coined the term “psychotic character”19 that aptly captured both the characterologic and the border-to-psychosis aspects of these patients’ clinical picture. According to Frosch, these patients appeared to regress readily into psychotic thinking, yet they did not lose their ability to test reality.
Affective and emotional instability. Thus until the 1960s, the term borderline was applied primarily to patients who appeared to occupy the border between neurosis and psychosis but were thought to be closer to psychotic than neurotic. And this sitting close to the edge of psychosis appeared to be a stable condition.
Most of the attention up until this point had been paid to how these patients thought—with little attention to their affective lability or emotional instability, save for Schmideberg’s comments. In the 1960s, however, the term borderline was applied somewhat differently—not completely divorced from previous concepts but with greater emphasis on borderline as a stable but psychopathologic functioning of the personality that included affective and emotional instability and an impaired sense of self.
- Intense affect, usually depressive or hostile
- History of impulsive, often self-destructive behavior
- Social adaptiveness that may mask a disturbed identity
- Brief psychotic episodes, often paranoid and evident in unstructured situations
- “Loose thinking” or primitive answers on unstructured psychological tests
- Relationships vacillate between transient superficiality and intense dependency
Impaired personality organization. In 1967, Kernberg published a seminal article in the history of BPD diagnosis. Although he did not discuss the diagnosis of BPD, Kernberg did develop a concept concerning a specific organization of the personality based upon impaired object relations. This impaired organization could apply across several personality disorders. The construct, named borderline personality organization (BPO),20 was defined by:
- an impaired sense of identity and lack of integration of one’s own identity
- use of primitive defenses, including splitting, rage, and regression
- ability to test reality.
Kernberg’s theory is too complex to summarize here, but he—along with Roy Grinker—is responsible for placing BPD on the diagnostic map. He was the first to describe BPO (and by extension BPD) in terms of a personality disorder.
Grinker’s ‘core’ group. Almost simultaneously (in 1968), Grinker published a careful study of 50 hospitalized patients. His work on the “borderline syndrome”21 revealed four subgroups to which the label of borderline had been applied:
- those occupying the border with psychosis
- those occupying the border with neurosis
- those similar to Deutsch’s “as if” group
- the “core” borderline group.
The core group—with its symptoms of anger and loneliness, a nonintegrated sense of self, and labile and oscillating interpersonal relationships— defined patients closest to our current definition.
Six criteria for BPD. In 1975, Gunderson and Singer published an article that greatly influenced our definition of BPD. They reviewed major descriptive accounts of BPD or BPD-like syndromes22 and proposed six diagnostic criteria (Box 2), though they did not identify a specific number or subset of the criteria as needing to be met for the diagnosis. (It is important to note that the term BPD did not become official for 5 more years.)
DSM-IV’s definition of BPD retains four of Gunderson and Singer‘s criteria among the nine it lists (five being necessary for a diagnosis of BPD). Missing are:
- social adaptiveness—though DSM-IV does say that social adaptiveness may be superficial (as in the “as if” personality) and may hide a disturbed identity6
- and the criterion relating to psychological test performance (this omission reflects a movement since 1980 away from listing “psychological” or psychodynamic criteria in the DSM).
DSM-III. BPD was included in DSM-III7 following an important study that tried to determine whether the term “borderline” refers to patients at the border of psychosis or to a stable group with mood instability and affective lability as part of a personality disorder. Spitzer et al23 asked 808 clinicians to describe patients they would label as borderline and to use 22 items gleaned from the literature to score two of their own patients:
- one patient who the clinician felt truly had borderline personality, borderline personality organization, or borderline schizophrenia
- and a control patient who was not diagnosed as psychotic and did not fall into any borderline category.
- The concept of abandonment, introduced in DSM-III-R, replaced the concept of aloneness in DSM-III.
- In DSM-III and DSM-III-R, a patient needed to meet 5 of 8 criteria for a diagnosis of BPD.
- DSM-IV introduced the ninth criterion, “transient, stress-related paranoid ideation or severe dissociative symptoms.” Since then, a patient has needed to meet 5 of 9 criteria for a diagnosis of BPD.
Their responses showed that BPD and schizotypal personality disorder (SPD) were separate, independent (though not mutually exclusive) disorders. Spitzer et al preserved the “schizotypal” label in DSM-III to describe the personality disorder that closely matched the border-to-psychosis subset. The other criteria set, which they labeled the “unstable personality item set,” was renamed “borderline” in DSM-III to describe the personality disorder that closely matched the emotional lability subset.
From one DSM edition to another, the concept of brief transient psychotic episodes has been included in and excluded from the diagnosis of borderline personality disorder (BPD).
In DSM-III. Because of work by Spitzer et al, these “experiences” were placed within schizotypal personality disorder (SPD) in DSM-III in 1980, though historically they had always been within the borderline concept and were one of Gunderson and Singer’s six criteria for diagnosing BPD (Box 2).22
Out of DSM-III-R. Research in the late 1980s suggested that when patients with BPD were depressed, they had a greater tendency to have psychotic–like episodes.24 Evidence indicated that attributing these psychotic and dissociative phenomena to SPD, rather than—perhaps more appropriately—to BPD, was one of the main reasons for the overlap between the definitions of BPD and SPD.25 Therefore, in DSM-III-R, the transient psychotic/dissociative criterion was removed from the SPD criteria set.
Back in DSM-IV. The criterion “transient stress-related paranoid ideation or severe dissociative symptoms” was placed into the BPD criteria set in DSM-IV. In DSM-IV, these symptoms were further characterized as usually not of “sufficient severity or duration to warrant an additional diagnosis.”
What is “sufficient” duration? The psychotic episodes of BPD last for minutes to hours and often appear when the patient imagines being (or actually is) abandoned by others. Not all agree that the criteria for BPD are met if these episodes last longer (e.g.,a day or two). In that case, they may exceed the transient time frame. More research is needed to better understand the quality and duration of these psychotic-like phenomena.
Not everyone agrees with renaming the unstable set “borderline” because the word:
- has always been ambiguous
- does not connote or denote any specific criteria or characteristic of patients who bear the label
- brands the patient as untreatable, defiant, or just “bad.”
Post-DSM-III: Where are we now?
From DSM-III evolved the hope that psychiatry could describe valid, well-defined diagnostic categories. Lost in the DSM-III enthusiasm was the fact that the categories were based upon theoretic constructs—theories no more or less valid than other theories that had preceded them. Because some of these categories were based upon empiric data— such as the Spitzer et al study—these diagnoses were perceived as more valid and more related to pathophysiology and perhaps genotype than prior constructs and definitions.
In the 1980s and early 1990s, a proliferation of studies attempted to examine the validity and reliability of DSM-III definitions, and BPD became the most studied of the personality disorders. The BPD concept took hold, even though several studies did not support it and despite refinements in subsequent DSM editions (Box 3).
One refinement in the BPD construct applied to transient psychotic or psychotic-like experiences, including dissociative phenomena. Yet questions remain about the duration of these transient episodes (Box 4).
Categorical versus dimensional
The categorical concept of BPD is facing new scrutiny,26,27 as recent studies have implied that biological disturbances may be spread across a number of personality disorders.28 If biological findings are found to be more closely allied with genotypic variations (alleles),29 then perhaps a dimensional classification system is needed for BPD and personality disorders in general.
On the other hand, categories provide a well-defined population that we can study and try to delineate from other populations, whereas dimensions—while perhaps closer to the reality of clinical presentation—may allow too much variability for research to proceed without confounding restraints.
BPD will continue to evolve, as will all psychiatric diagnostic categories, but the need to modify its definition does not negate its usefulness and clinical applicability. Most of our patients do not read the DSM before coming to us. They present with symptom complexes and problems that demand that we listen to what they say and understand who they are while we also try to fit them—as best we can—into categories or dimensions30 that help us choose the most appropriate interventions.
Related resources
- BPD Sanctuary (borderline personality disorder education, communities, support, books, and resources) http://www.mhsanctuary.com/borderline/ Borderline Central (resources for people who care about someone with borderline personality disorder) http://www.bpdcentral.com/
- Gunderson JG. Borderline personality disorder: a clinical guide. Washington, DC: American Psychiatric Publishing, Inc., 200l.
- Paris J (ed). Borderline personality disorder. Psych Clin N Am 2000;23:1 (entire volume devoted to BPD).
- Silk KR (ed). Biological and neurobehavioral studies of borderline personality disorder. Washington, DC: American Psychiatric Press, 1994.
Not everyone agrees that borderline personality disorder (BPD) should be a diagnostic category. BPD became “official” with DSM-III in 1980, although the term had been used for 40 years to describe various patient groups. Being listed in DSM-III legitimized BPD, which was thought to represent a specific—though not necessarily distinct—diagnostic category.
The history of the BPD diagnosis and opinions as to its usefulness can be viewed as a microcosm of psychiatric diagnosis in general. Before DSM-III, diagnoses were broadly defined and did not contain specific inclusion or exclusion criteria.1 For the 5 to 10 years prior to DSM-III, however, two assumptions developed:
- distinct diagnostic categories did, in fact, exist
- by rigorously defining and studying those categories we could develop more specific and effective treatments for our patients.2
The specificity and exclusivity that we assumed we could achieve by categorical diagnoses, however, remain a distant wish. Comorbidity appears more common in psychiatry than was originally thought and confounds both treatment and outcome.3 Also, many patients appear treatment-resistant, despite fitting neatly into diagnostic categories.4
Miss A, age 35, presents to the emergency room with a long history of intermittent depression and self-mutilation. She has never been hospitalized nor on psychotropic medication but has been in and out of psychotherapy for years. She has had intermittent depressive episodes for many years, though the episodes often lasted 2 to 3 weeks and appeared to correct themselves spontaneously.
Agitated and afraid. She is extremely agitated when she arrives at the emergency department. She has hardly slept or eaten but insists she is not hungry. She reports that she cannot concentrate or do her work as an accountant. She says she is hearing voices, knows they are in her head, but nonetheless is terrified that something horrible is about to happen—though she cannot say what it might be.
Voice ‘calling my name.’ When the psychiatric resident inquires further, Miss A says a male voice is calling her name and mumbling some short phrase she cannot understand. She says she has heard the voice the last few days, perhaps for 10 to 15 minutes every few hours, particularly when she ruminates about how she messed up a relationship with her now ex-boyfriend. The breakup occurred 1 week ago.
Feeling detached. She claims she has never heard voices before but describes periods when she has felt detached and unreal. Often these were short-term dissociative episodes that occurred in the wake of what she perceived as a personal failure or a distressful interpersonal encounter (often with a man). Relationships frequently were very difficult for her, and she felt she could easily go from infatuation to detesting someone.
Diagnosis? Talking appears to calm her down. After being in the emergency room for 2 hours, she says she no longer hears the voice. The resident tells the attending psychiatrist he believes the patient is in a major depressive episode, perhaps a psychotic depression, and proposes starting antidepressant treatment. The attending argues that the patient appears to have borderline personality disorder and suggests that she be sent home without medication and given an appointment to the outpatient clinic within the next few days.
As psychiatry considers DSM-V, questions linger as to whether BPD (and personality disorder in general) should remain as a categorical diagnosis or if dimensional measures may be more appropriate. Dimensions imply that no one ever fits into a given box because no specific box exists. Rather, patients are described as being closer to or more distant from a prototypic model of the diagnosis. In personality disorders, the dimensions most often mentioned are cognition, impulsivity, emotional lability, environmental hyperreactivity, and anxiety. The case report (above) illustrates the interplay of these dimensions in a typical patient with presumed BPD.
What’s in a name?
The symptom complex or syndrome that bears the name borderline personality disorder has probably existed for as long as people have thought about patients in psychopathologic terms.5 Before 1980, the term “borderline” applied primarily to two separate but overlapping concepts:
- Patients thought to reside on the “border” with psychosis, such as the patient in our case example. They seemed to have an underlying psychotic disorder, but the psychosis—if it surfaced—appeared briefly, was not exceptionally deep or firmly held, and was not regularly evident or immediately accessible to the clinician.
- Patients who appeared to occupy the space between neurosis and psychosis. This concept evolved into the idea of a character or personality disorder distinguished primarily by unstable interpersonal relationships, a confused or inconsistent sense of identity, and emotional instability.
How DSM is changing. Comparing the disorders listed in DSM-IV (1994)6 versus DSM-II (1968)1 suggests that psychiatry has become enamored of the naming process. For example, DSM-II lists anxiety neurosis (300.0), phobic neurosis (300.2), and obsessive-compulsive neurosis (300.3), whereas DSM-IV lists 11 different categories of anxiety disorders.
But beyond naming, subsequent DSMs have differed even more dramatically from DSM-II. We have seen a shift from describing a diagnostic category with a simple explanatory paragraph to lists of specific inclusion and exclusion criteria. These more-specific lists imply that they define categories closer to some reality or authenticity than did previous definitions.
Before DSM-III, the borderline concept was conceived in broad object relational and psychodynamic terms. In contrast, DSM-III produced a definitive set of criteria and required that a subset be met before the diagnosis could be made.7 An example of this criteria-based model is shown in Box 1, which lists the DSM-IV-TR criteria for BPD.
Some psychiatrists objected that BPD was solely a psychoanalytic construct and too theoretical for inclusion in DSM-III. Others argued that if BPD were not defined, it would be difficult to study the clinical usefulness of that definition or any other. Nonetheless, many have argued that BPD does not exist, though to what category BPD patients should belong has changed over the years:
- Is BPD nothing more than a milder or unusual presentation of an affective disorder8 or actually bipolar II disorder?9
- Is it a presentation of posttraumatic stress disorder (PTSD) called “complex PTSD,”10-11 or an adult presentation of attention-deficit/ hyperactivity or other brain disorder?12
- Is it a stigmatizing diagnosis that we apply to patients whom we do not like?13
In truth, the diagnosis of BPD reflects a particular clinical presentation no more or less accurately than many of the well-accepted axis I disorders. Despite recent advances in the neurosciences, the dilemma we face as psychiatrists is that we make a diagnosis based upon what we see in the clinical setting (i.e., a phenotype). Yet in labeling what we believe is a specific psychiatric disorder, we make assumptions—for better or for worse, consciously or unconsciously—about pathophysiology and indirectly about genotype.
A pervasive pattern of instability of interpersonal relationships, self-image, and affects and marked impulsivity beginning by early adulthood and present in a variety of contexts, as indicated by five (or more) of the following:
- Frantic efforts to avoid real or imagined abandonment
- A pattern of unstable and intense interpersonal relationships characterized by alternating between extremes of idealization and devaluation
- Identity disturbance: markedly and persistent unstable self-image or sense of self
- Impulsivity in at least two areas that are potentially self-damaging (spending, sex, substance abuse, binge eating, reckless driving)
- Recurrent suicidal behavior, gestures, or threats; self-mutilating behavior
- Affective instability due to a marked reactivity of mood
- Chronic feelings of emptiness
- Inappropriate, intense anger or difficulty controlling anger
- Transient, stress-related paranoid ideation or severe dissociative symptoms
Source: DSM-IV6
Defining the borderline personality
Stern first used the term “borderline” in 1938 to describe patients who appeared to occupy the border between neurosis and psychosis.14 In 1942, Deutsch described the “as if” personality in patients who seemed chameleon-like. They could adapt or play the role demanded of them in specific situations, yet elsewhere—as in the analyst’s office—they had little sense of themselves and were thought to be internally disorganized and probably psychotic.15
Border to psychosis. The idea that borderline-type patients were psychotic continued in Hoch and Polatin’s description of the “pseudoneurotic schizophrenic,”16 a patient who appeared severely neurotic but was thought to employ many defenses and interpersonal styles to ward off a fundamental inner psychosis. Knight used the label “borderline states”17 to describe severely ill patients who were not frankly psychotic but fell within the realm of psychosis without qualifying for a diagnosis of schizophrenia. Knight was the first person to use the term “borderline” as a diagnostic entity, though simultaneously he argued against its use as a label because the term lacked precision.
Psychotic character. About the same time, Schmideberg characterized a group of patients whose emotional lability or affective reactivity seemed to be a consistent aspect of their clinical presentation. She believed this appearance of “stable instability”18 represented the patient’s characterologic adaptation to the world.
Frosch coined the term “psychotic character”19 that aptly captured both the characterologic and the border-to-psychosis aspects of these patients’ clinical picture. According to Frosch, these patients appeared to regress readily into psychotic thinking, yet they did not lose their ability to test reality.
Affective and emotional instability. Thus until the 1960s, the term borderline was applied primarily to patients who appeared to occupy the border between neurosis and psychosis but were thought to be closer to psychotic than neurotic. And this sitting close to the edge of psychosis appeared to be a stable condition.
Most of the attention up until this point had been paid to how these patients thought—with little attention to their affective lability or emotional instability, save for Schmideberg’s comments. In the 1960s, however, the term borderline was applied somewhat differently—not completely divorced from previous concepts but with greater emphasis on borderline as a stable but psychopathologic functioning of the personality that included affective and emotional instability and an impaired sense of self.
- Intense affect, usually depressive or hostile
- History of impulsive, often self-destructive behavior
- Social adaptiveness that may mask a disturbed identity
- Brief psychotic episodes, often paranoid and evident in unstructured situations
- “Loose thinking” or primitive answers on unstructured psychological tests
- Relationships vacillate between transient superficiality and intense dependency
Impaired personality organization. In 1967, Kernberg published a seminal article in the history of BPD diagnosis. Although he did not discuss the diagnosis of BPD, Kernberg did develop a concept concerning a specific organization of the personality based upon impaired object relations. This impaired organization could apply across several personality disorders. The construct, named borderline personality organization (BPO),20 was defined by:
- an impaired sense of identity and lack of integration of one’s own identity
- use of primitive defenses, including splitting, rage, and regression
- ability to test reality.
Kernberg’s theory is too complex to summarize here, but he—along with Roy Grinker—is responsible for placing BPD on the diagnostic map. He was the first to describe BPO (and by extension BPD) in terms of a personality disorder.
Grinker’s ‘core’ group. Almost simultaneously (in 1968), Grinker published a careful study of 50 hospitalized patients. His work on the “borderline syndrome”21 revealed four subgroups to which the label of borderline had been applied:
- those occupying the border with psychosis
- those occupying the border with neurosis
- those similar to Deutsch’s “as if” group
- the “core” borderline group.
The core group—with its symptoms of anger and loneliness, a nonintegrated sense of self, and labile and oscillating interpersonal relationships— defined patients closest to our current definition.
Six criteria for BPD. In 1975, Gunderson and Singer published an article that greatly influenced our definition of BPD. They reviewed major descriptive accounts of BPD or BPD-like syndromes22 and proposed six diagnostic criteria (Box 2), though they did not identify a specific number or subset of the criteria as needing to be met for the diagnosis. (It is important to note that the term BPD did not become official for 5 more years.)
DSM-IV’s definition of BPD retains four of Gunderson and Singer‘s criteria among the nine it lists (five being necessary for a diagnosis of BPD). Missing are:
- social adaptiveness—though DSM-IV does say that social adaptiveness may be superficial (as in the “as if” personality) and may hide a disturbed identity6
- and the criterion relating to psychological test performance (this omission reflects a movement since 1980 away from listing “psychological” or psychodynamic criteria in the DSM).
DSM-III. BPD was included in DSM-III7 following an important study that tried to determine whether the term “borderline” refers to patients at the border of psychosis or to a stable group with mood instability and affective lability as part of a personality disorder. Spitzer et al23 asked 808 clinicians to describe patients they would label as borderline and to use 22 items gleaned from the literature to score two of their own patients:
- one patient who the clinician felt truly had borderline personality, borderline personality organization, or borderline schizophrenia
- and a control patient who was not diagnosed as psychotic and did not fall into any borderline category.
- The concept of abandonment, introduced in DSM-III-R, replaced the concept of aloneness in DSM-III.
- In DSM-III and DSM-III-R, a patient needed to meet 5 of 8 criteria for a diagnosis of BPD.
- DSM-IV introduced the ninth criterion, “transient, stress-related paranoid ideation or severe dissociative symptoms.” Since then, a patient has needed to meet 5 of 9 criteria for a diagnosis of BPD.
Their responses showed that BPD and schizotypal personality disorder (SPD) were separate, independent (though not mutually exclusive) disorders. Spitzer et al preserved the “schizotypal” label in DSM-III to describe the personality disorder that closely matched the border-to-psychosis subset. The other criteria set, which they labeled the “unstable personality item set,” was renamed “borderline” in DSM-III to describe the personality disorder that closely matched the emotional lability subset.
From one DSM edition to another, the concept of brief transient psychotic episodes has been included in and excluded from the diagnosis of borderline personality disorder (BPD).
In DSM-III. Because of work by Spitzer et al, these “experiences” were placed within schizotypal personality disorder (SPD) in DSM-III in 1980, though historically they had always been within the borderline concept and were one of Gunderson and Singer’s six criteria for diagnosing BPD (Box 2).22
Out of DSM-III-R. Research in the late 1980s suggested that when patients with BPD were depressed, they had a greater tendency to have psychotic–like episodes.24 Evidence indicated that attributing these psychotic and dissociative phenomena to SPD, rather than—perhaps more appropriately—to BPD, was one of the main reasons for the overlap between the definitions of BPD and SPD.25 Therefore, in DSM-III-R, the transient psychotic/dissociative criterion was removed from the SPD criteria set.
Back in DSM-IV. The criterion “transient stress-related paranoid ideation or severe dissociative symptoms” was placed into the BPD criteria set in DSM-IV. In DSM-IV, these symptoms were further characterized as usually not of “sufficient severity or duration to warrant an additional diagnosis.”
What is “sufficient” duration? The psychotic episodes of BPD last for minutes to hours and often appear when the patient imagines being (or actually is) abandoned by others. Not all agree that the criteria for BPD are met if these episodes last longer (e.g.,a day or two). In that case, they may exceed the transient time frame. More research is needed to better understand the quality and duration of these psychotic-like phenomena.
Not everyone agrees with renaming the unstable set “borderline” because the word:
- has always been ambiguous
- does not connote or denote any specific criteria or characteristic of patients who bear the label
- brands the patient as untreatable, defiant, or just “bad.”
Post-DSM-III: Where are we now?
From DSM-III evolved the hope that psychiatry could describe valid, well-defined diagnostic categories. Lost in the DSM-III enthusiasm was the fact that the categories were based upon theoretic constructs—theories no more or less valid than other theories that had preceded them. Because some of these categories were based upon empiric data— such as the Spitzer et al study—these diagnoses were perceived as more valid and more related to pathophysiology and perhaps genotype than prior constructs and definitions.
In the 1980s and early 1990s, a proliferation of studies attempted to examine the validity and reliability of DSM-III definitions, and BPD became the most studied of the personality disorders. The BPD concept took hold, even though several studies did not support it and despite refinements in subsequent DSM editions (Box 3).
One refinement in the BPD construct applied to transient psychotic or psychotic-like experiences, including dissociative phenomena. Yet questions remain about the duration of these transient episodes (Box 4).
Categorical versus dimensional
The categorical concept of BPD is facing new scrutiny,26,27 as recent studies have implied that biological disturbances may be spread across a number of personality disorders.28 If biological findings are found to be more closely allied with genotypic variations (alleles),29 then perhaps a dimensional classification system is needed for BPD and personality disorders in general.
On the other hand, categories provide a well-defined population that we can study and try to delineate from other populations, whereas dimensions—while perhaps closer to the reality of clinical presentation—may allow too much variability for research to proceed without confounding restraints.
BPD will continue to evolve, as will all psychiatric diagnostic categories, but the need to modify its definition does not negate its usefulness and clinical applicability. Most of our patients do not read the DSM before coming to us. They present with symptom complexes and problems that demand that we listen to what they say and understand who they are while we also try to fit them—as best we can—into categories or dimensions30 that help us choose the most appropriate interventions.
Related resources
- BPD Sanctuary (borderline personality disorder education, communities, support, books, and resources) http://www.mhsanctuary.com/borderline/ Borderline Central (resources for people who care about someone with borderline personality disorder) http://www.bpdcentral.com/
- Gunderson JG. Borderline personality disorder: a clinical guide. Washington, DC: American Psychiatric Publishing, Inc., 200l.
- Paris J (ed). Borderline personality disorder. Psych Clin N Am 2000;23:1 (entire volume devoted to BPD).
- Silk KR (ed). Biological and neurobehavioral studies of borderline personality disorder. Washington, DC: American Psychiatric Press, 1994.
1. Diagnostic and statistical manual of mental disorders (2nd ed). Washington, DC: American Psychiatric Association, 1968.
2. Feighner JP, Robins E, Guze SB, et al. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972;26:57-63.
3. Merikangas KR, Angst J, Eaton W, et al. Comorbidity and boundaries of affective disorders with anxiety disorders and substance misuse: results of an international task force. Br J Psychiatry 1996;30(suppl):58-67.
4. Nierenberg AA. DeCecco LM.Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 16):5-9.
5. Stone MH (ed). Essential papers on borderline disorders: one hundred years at the border. New York: New York University Press, 1986.
6. Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC: American Psychiatric Association, 1994.
7. Diagnostic and statistical manual of mental disorders (3rd ed). Washington, DC: American Psychiatric Association, 1980.
8. Akiskal HS. Subaffective disorders: dysthymic, cyclothymic and bipolar II disorders in the “borderline” realm. Psychiatric Clin N Am 1981;4:25-46.
9. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatric Res 2001;35:307-12.
10. Herman JL, van der Kolk BA. Traumatic antecedents of borderline personality disorder. In: van der Kolk, BA. Psychological trauma. Washington, DC: American Psychiatric Press, 1987;111-26.
11. Silk KR, Lee S, Hill EM, Lohr NE. Borderline symptoms and severity of sexual abuse. Am J Psychiatry 1995;152:1059-64.
12. Streeter CC, Van Reekum R, Shorr RI, Bachman DL. Prior head injury in male veterans with borderline personality disorder. J Nerv Ment Dis 1995;183:577-81.
13. Maltsberger JT. Countertransference in borderline conditions: some further notes. Int J Psychoanal Psychother 1982-83;9:125-34.
14. Stern A. Psychoanalytic investigation and therapy in the borderline group of neuroses. Psychoanal Q 1938;7:467-89.
15. Deutsch H. Some forms of emotional disturbance and their relationship to schizophrenia. Psychoanal Q 1942;11:301-21.
16. Hoch P, Polatin P. Pseudoneurotic forms of schizophrenia. Psychiatric Q 1949;23:248-76.
17. Knight R. Borderline states. Bull Menn Clin 1953;17:1-12.
18. Schmideberg M. The treatment of psychopaths and borderline patients. Am J Psychotherapy 1947;1:45-70.
19. Frosch J. The psychotic character: clinical psychiatric considerations. Psychiatric Q 1964;38:81-96.
20. Kernberg O. Borderline personality organization. J Am Psychoanal Assoc 1967;15:641-85.
21. Grinker RR, Werble B, Drye R. The borderline syndrome: a behavioral study of ego functions. New York: Basic Books, 1968.
22. Gunderson JG, Singer MT. Defining borderline patients: an overview. Am J Psychiatry 1975;132:1-10.
23. Spitzer RL, Endicott J, Gibbon M. Crossing the border into borderline personality and borderline schizophrenia: the development of criteria. Arch Gen Psychiatry 1979;36:17-24.
24. Silk KR, Lohr NE, Westen D, Goodrich S. Psychosis in borderline patients with depression. J Personality Disord 1989;3:92-100.
25. Silk KR, Westen D, Lohr NE, et al. DSM-III and DSM-III-R schizotypal symptoms in borderline personality disorder. Comprehen Psychiatry 1990;31:103-10.
26. Livesley WJ, Jang KL, Vernon PA. Phenotypic and genetic structure of traits delineating personality disorder. Arch Gen Psychiatry 1998;55:941-8.
27. McCrae RR, Yang J, Costa PT, Jr, et al. Personality profiles and the prediction of categorical personality disorders. J Personality 2001;69:155-74.
28. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry 1991;148:1647-58.
29. New AS, Gelernter J, Goodman M, et al. Suicide, impulsive aggression, and HTR1B genotype. Biolog Psychiatry 2001;50:62-5.
30. Oldham JM, Skodol AE. Charting the future of axis II. J Personality Disord 2000;14:17-29.
1. Diagnostic and statistical manual of mental disorders (2nd ed). Washington, DC: American Psychiatric Association, 1968.
2. Feighner JP, Robins E, Guze SB, et al. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972;26:57-63.
3. Merikangas KR, Angst J, Eaton W, et al. Comorbidity and boundaries of affective disorders with anxiety disorders and substance misuse: results of an international task force. Br J Psychiatry 1996;30(suppl):58-67.
4. Nierenberg AA. DeCecco LM.Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry 2001;62(suppl 16):5-9.
5. Stone MH (ed). Essential papers on borderline disorders: one hundred years at the border. New York: New York University Press, 1986.
6. Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC: American Psychiatric Association, 1994.
7. Diagnostic and statistical manual of mental disorders (3rd ed). Washington, DC: American Psychiatric Association, 1980.
8. Akiskal HS. Subaffective disorders: dysthymic, cyclothymic and bipolar II disorders in the “borderline” realm. Psychiatric Clin N Am 1981;4:25-46.
9. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatric Res 2001;35:307-12.
10. Herman JL, van der Kolk BA. Traumatic antecedents of borderline personality disorder. In: van der Kolk, BA. Psychological trauma. Washington, DC: American Psychiatric Press, 1987;111-26.
11. Silk KR, Lee S, Hill EM, Lohr NE. Borderline symptoms and severity of sexual abuse. Am J Psychiatry 1995;152:1059-64.
12. Streeter CC, Van Reekum R, Shorr RI, Bachman DL. Prior head injury in male veterans with borderline personality disorder. J Nerv Ment Dis 1995;183:577-81.
13. Maltsberger JT. Countertransference in borderline conditions: some further notes. Int J Psychoanal Psychother 1982-83;9:125-34.
14. Stern A. Psychoanalytic investigation and therapy in the borderline group of neuroses. Psychoanal Q 1938;7:467-89.
15. Deutsch H. Some forms of emotional disturbance and their relationship to schizophrenia. Psychoanal Q 1942;11:301-21.
16. Hoch P, Polatin P. Pseudoneurotic forms of schizophrenia. Psychiatric Q 1949;23:248-76.
17. Knight R. Borderline states. Bull Menn Clin 1953;17:1-12.
18. Schmideberg M. The treatment of psychopaths and borderline patients. Am J Psychotherapy 1947;1:45-70.
19. Frosch J. The psychotic character: clinical psychiatric considerations. Psychiatric Q 1964;38:81-96.
20. Kernberg O. Borderline personality organization. J Am Psychoanal Assoc 1967;15:641-85.
21. Grinker RR, Werble B, Drye R. The borderline syndrome: a behavioral study of ego functions. New York: Basic Books, 1968.
22. Gunderson JG, Singer MT. Defining borderline patients: an overview. Am J Psychiatry 1975;132:1-10.
23. Spitzer RL, Endicott J, Gibbon M. Crossing the border into borderline personality and borderline schizophrenia: the development of criteria. Arch Gen Psychiatry 1979;36:17-24.
24. Silk KR, Lohr NE, Westen D, Goodrich S. Psychosis in borderline patients with depression. J Personality Disord 1989;3:92-100.
25. Silk KR, Westen D, Lohr NE, et al. DSM-III and DSM-III-R schizotypal symptoms in borderline personality disorder. Comprehen Psychiatry 1990;31:103-10.
26. Livesley WJ, Jang KL, Vernon PA. Phenotypic and genetic structure of traits delineating personality disorder. Arch Gen Psychiatry 1998;55:941-8.
27. McCrae RR, Yang J, Costa PT, Jr, et al. Personality profiles and the prediction of categorical personality disorders. J Personality 2001;69:155-74.
28. Siever LJ, Davis KL. A psychobiological perspective on the personality disorders. Am J Psychiatry 1991;148:1647-58.
29. New AS, Gelernter J, Goodman M, et al. Suicide, impulsive aggression, and HTR1B genotype. Biolog Psychiatry 2001;50:62-5.
30. Oldham JM, Skodol AE. Charting the future of axis II. J Personality Disord 2000;14:17-29.
ADHD and substance abuse: 4 therapeutic options for patients with addictions
Should you prescribe a stimulant to treat attention and hyperactivity problems in teenagers and adults with a history of substance abuse? Evidence suggests that using a stimulant to treat attention-deficit/hyperactivity disorder (ADHD) may place such patients at risk for stimulant abuse or for relapse into abuse of other substances. But a stimulant may be the only option for patients whose ADHD symptoms do not respond to alternate medications, such as antidepressants.
Growing numbers of adults are being treated for ADHD. Because substance abuse problems are common in adults with ADHD (Box 1 ),1-3 prescribing an antidepressant instead of a stimulant in some cases may be prudent. Consider the following factors when choosing ADHD therapy for patients with a history of substance abuse.
Prevalence of stimulant use and abuse
In the United States, more than 95% of medications prescribed for children and adults with ADHD are stimulants—usually methylphenidate.4 Stimulant use has increased as more children and adults are diagnosed with ADHD. Methylphenidate prescriptions increased five-fold from 1990 to 1995.5 Visits to psychiatrists and physicians that included stimulant prescriptions grew from 570,000 to 2.86 million from 1985 to 1994, with most of that increase occurring during visits to primary care and other physicians.6
When used as prescribed, methylphenidate is safe and effective for treating most children and adults with ADHD. Methylphenidate’s pharmacologic properties, however, are similar to those of amphetamines and cocaine (Box 2, Figure 1),7,8 which is why methylphenidate is a schedule-II controlled substance.
Published data. Fifteen reports of methylphenidate abuse were published in the medical literature between 1960 and 1999,7 but little is known about the prevalence of stimulant abuse among patients with ADHD. Banov and colleagues recently published what may be the only data available, when they reported that 3 of 37 (8%) patients abused the stimulants they were prescribed for ADHD.9 The three patients who abused stimulants had histories of drug and alcohol abuse at study entry. In all three cases, stimulant abuse did not develop immediately but became apparent within 6 months after the study began.
In a study of 651 students ages 11 to 18 in Wisconsin and Minnesota, more than one-third of those taking stimulants reported being asked to sell or trade their medications. More than one-half of those not taking ADHD medications said they knew someone who sold or gave away his or her medication.10
Stimulant theft, recreational use. Methylphenidate has been identified as the third most abused prescribed substance in the United States.11 It was the 10th most frequently stolen controlled drug from pharmacies between 1990 and 1995, and 700,000 dosage units were reported stolen in 1996 and 1997.12
As many as 50% of adults with ADHD have substance abuse problems (including alcohol, cocaine, and marijuana), and as many as 30% have antisocial personality disorder (with increased potential for drug-seeking behaviors).1 Compared with the general population, persons with ADHD have an earlier onset of substance abuse that is less responsive to treatment and more likely to progress from alcohol to other drugs.2
The elevated risk of substance abuse in ADHD may be related to a subtle lack of response to normal positive and negative reinforcements. Hunt has outlined four neurobehavioral deficits that define ADHD.3 Besides inattention, hyperarousal, and impulsiveness, he proposes that persons with ADHD have a reward system deficit. They may gravitate toward substance abuse because drugs, alcohol, and nicotine provide stronger rewards than life’s more subtle social interactions.
The popular media have reported recreational use of methylphenidate—with street names such as “R-Ball” and “Vitamin R”—among teens and college students.13 Illegal stimulants are perceived to be easily accessible on college campuses, but no data have been reported.
The use of stimulant medication for ADHD patients with substance abuse problems remains controversial. For such patients, this author reserves stimulant medication for those:
- whose ADHD symptoms have not responded adequately to alternate treatments
- who have been reliable with prescription medications
- and whose functional level is seriously impaired by their ADHD.
Antidepressants vs. stimulants
Although few well-designed controlled studies have been published, four antidepressants appear to be reasonably equivalent in effectiveness for adults with ADHD and do not carry potential for stimulant abuse.14
Desipraime, bupropion, venlafaxine, and the experimental drug atomoxetine (Table 1) all increase norepinephrine at the synapse by inhibiting presynaptic reuptake. Though dopamine has traditionally been considered the neurotransmitter of choice for ADHD treatment, norepinephrine may be equally potent.
Impulse control center. Several lines of research have recently established a connection between the prefrontal cortex, norepinephrine, and ADHD.15 This evidence suggests that the prefrontal cortex plays a major role in inhibiting impulses and responses to distractions:
Figure 1
PET scans of the brain using carbon 11 (11C)-labeled cocaine and methylphenidate HCl show similar distributions in the striatium when the drugs are administered intravenously.
Source: Reproduced with permission from Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
Stimulants are classified as schedule-II drugs because they produce powerful reinforcing effects by increasing synaptic dopamine.7 Positron-emission tomography (PET) scans using carbon labeling have shown similar distributions of methylphenidate and cocaine in the brain (Figure 1).8 When administered intravenously, both drugs occupy the same receptors in the striatum and produce a “high” that parallels rapid neuronal uptake. Methylphenidate and cocaine similarly increase stimulation of the postsynaptic neuron by blocking the dopamine reuptake pump (dopamine transporter).
How a substance gets to the brain’s euphoric receptors greatly affects its addictive properties. Delivery systems with rapid onset—smoking, “snorting,” or IV injection—have much greater ability to produce a “high” than do oral or transdermal routes. The greater the “high,” the greater the potential for abuse.
Because methylphenidate is prescribed for oral use, the potential for abuse is minimal. However, we need to be extremely cautious when giving methylphenidate or similar stimulant medications to patients who have shown they are unable to control their abuse of other substances.
Stimulants can also re-ignite a dormant substance abuse problem. Though little has been written about this in the medical literature, Elizabeth Wurtzel, author of the controversial Prozac Nation, chronicles the resumption of her cocaine abuse in More, Now, Again: A Memoir of Addiction. She contends that after her doctor added methylphenidate to augment treatment of partially remitting depression, she began abusing it and eventually was using 40 tablets per day before slipping back into cocaine dependence.
- Patients with prefrontal cortex deficits can have problems with inattention and poor impulse control.
- Patients with ADHD have frontal lobe impairments, as neuropsychological testing and imaging studies have shown.
- Norepinephrine neurons, with cell bodies in the locus coeruleus, have projections that terminate in the prefrontal cortex.
- Agents with norepinephrine activity, but without mood-altering properties (e.g., clonidine), have been shown to improve ADHD symptoms.
Table 1
ADHD IN ADULTS: ANTIDEPRESSANT DOSAGES AND SIDE EFFECTS
| Medication | Class | Effective dosage | Side effects |
|---|---|---|---|
| Desipramine | Tricyclic | 100 to 200 mg/d | Sedation, weight gain, dry mouth, constipation, orthostatic hypotension, prolonged cardiac conduction time; may be lethal in overdose |
| Bupropion SR | Norepinephrine and dopamine reuptake inhibitor | 150 mg bid to 200 mg bid | Headaches, insomnia, agitation, increased risk of seizures |
| Venlafaxine XR | Serotonin and norepinephrine reuptake inhibitor | 75 to 225 mg/d | Nausea, sexual side effects, agitation, increased blood pressure at higher dosages |
| Atomoxetine* | Norepinephrine reuptake inhibitor | To be determined | To be determined |
| * Investigational agent; not FDA-approved | |||
Additional evidence suggests that the prefrontal cortex has projections back to the locus coeruleus, which may explain the relationship between the two areas. It may be that the brain’s higher-functioning areas, such as the prefrontal cortex, provide intelligent screening of impulses from the brain’s older areas, such as the locus coeruleus. Therefore, increased prefrontal cortex activity may modulate some impulses that ADHD patients cannot control otherwise.

No ‘high’ with antidepressants. Patients with ADHD who have experienced the powerful effects of street drugs such as cocaine, methamphetamine, or even alcohol may report that antidepressants do not provide the effect they desire. It is difficult to know if these patients are reporting a lack of benefit or simply the absence of a euphoric “high” they are used to experiencing with substances of abuse. The newer antidepressants do not activate the brain’s euphoric receptors to an appreciable degree.
Patients who take stimulants as prescribed also do not report a “high” but can detect the medication’s presence and absence. Most do not crave this feeling, but substance abusers tend to like it. A patient recently told me he didn’t think stimulants improved his ADHD, but said, “I just liked the way they made me feel.”
Desipramine
The tricyclic antidepressant desipramine is a potent norepinephrine reuptake inhibitor that is effective in treating ADHD in children and adults. In a double-blind, placebo-controlled study of adults with ADHD, subjects receiving desipramine showed robust improvement in symptom scores on the ADHD Rating Scale,16 compared with those receiving the placebo (Figure 2).17
During the 6-week trial, 41 adults with ADHD received desipramine, 200 mg/d, or a placebo. Those receiving desipramine showed significant improvement in 12 of 14 ADHD symptoms and less hyperactivity, impulsivity, and inattentiveness, whereas those receiving the placebo showed no improvement. According to the study criteria, 68% of those who received desipramine and none who received the placebo were considered positive responders.
Though no head-to-head studies have compared desipramine with methylphenidate, the same researchers conducted a similar placebo-controlled study with methylphenidate. The 6-week symptom score on the ADHD Rating Scale was 12.5 for methylphenidate, compared with a score of 12 for desipramine.18
Recommendation. Desipramine may be the most effective of the antidepressant treatments for patients with ADHD. Because of its side effects, however, it is not this author’s first choice and is usually reserved for patients whose symptoms fail to respond to other antidepressants. Desipramine can cause sedation, dry mouth, and constipation, which are related to blockade of adrenergic, histamine, and muscarinic cholinergic receptors. It also can be lethal in overdose.
Some substance abusers lose confidence in a medication that they cannot feel working. The side effects of desipramine, which can be intolerable for some patients, can reassure others with a history of substance abuse that they are being medicated.
Bupropion
Bupropion is a unique antidepressant that inhibits the presynaptic reuptake of dopamine and norepinephrine. Open-label studies demonstrate good responses to bupropion by adults with ADHD. One placebo-controlled, double-blind study found improved ADHD symptoms in 76% of patients receiving bupropion SR, compared with 37% of those receiving a placebo; the difference was statistically significant.19
Figure 2 IMPROVED ADHD SYMPTOMS WITH DESIPRAMINE
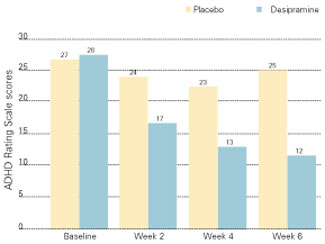
Adults with ADHD who received desipramine, 200 mg/d, in a double-blind trial showed significantly less hyperactivity, impulsivity, and inattentiveness after 6 weeks of therapy than a control group that received a placebo.
Source: Adapted with permission from Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.Another study of adults with ADHD compared bupropion SR to methylphenidate and a placebo.20 Using a primary outcome of the Clinical Global Impression (CGI) scale, response rates were 64% for bupropion SR, 50% for methylphenidate, and 27% for the placebo. The difference in response rates between the two agents was not statistically significant (p = 0.14).
Recommendation. The risk of seizures with bupropion is about 1 in 1,000. Therefore, bupropion should not be given to patients with a seizure disorder or to those with conditions that alter the seizure threshold (e.g., eating disorders, recent head trauma, or benzodiazepine withdrawal).21
This author uses bupropion as first-line treatment for appropriate patients with ADHD and a substance abuse history. Bupropion’s mild benefit with smoking cessation may provide some crossover effect for other substances of abuse. The low incidence of sexual side effects is another benefit. Drawbacks include twice-daily dosing and lack of a robust effect on attention and concentration.
Venlafaxine
Venlafaxine is a potent inhibitor of serotonin reuptake, a moderate inhibitor of norepinephrine, and a mild inhibitor of dopamine. Venlafaxine has displayed response rates similar to those of desipramine and bupropion in open-label studies in adults with ADHD,14 but no placebo-controlled studies exist.
As noted above, these antidepressants are believed to improve ADHD symptoms by making norepinephrine more available at the synapse. Hypothetically, then, one would need to administer venlafaxine at dosages that adequately inhibit the norepinephrine reuptake receptor. Venlafaxine XR, 150 mg/d, provides significant norepinephrine activity, according to several lines of evidence.
Recently, Upadhyaya et al reported the use of venlafaxine in one of the few treatment studies of patients with ADHD and comorbid alcohol/cocaine abuse. In an open-label trial, 10 subjects received venlafaxine, up to 300 mg/d, along with psychotherapy and attendance at Alcoholics Anonymous meetings. The nine who completed 4 weeks of treatment showed significantly improved ADHD symptoms and decreased alcohol craving.22
Recommendation. Venlafaxine is this author’s second choice for patients with ADHD and substance abuse problems. Sexual side effects that some patients experience with venlafaxine can limit its use. Some clinicians are concerned about increases in blood pressure associated with venlafaxine, although significant changes do not seem to occur at dosages below 300 mg/d.23
Atomoxetine
Atomoxetine is an investigational antidepressant in phase-III trials as a treatment for ADHD. Evidence shows atomoxetine to be a potent inhibitor of the presynaptic norepinephrine transporter, with minimal affinity for other neurotransmitter receptors. Initial studies suggest that atomoxetine is effective for adults and children with ADHD:
- In a small, double-blind, placebo-controlled, crossover trial, 11 of 20 adults showed improvement in ADHD symptoms within 3 weeks of starting atomoxetine.24
- In 297 children and adolescents, atomoxetine at dosages averaging approximately 1.2 mg/kg/d was more effective than a placebo in reducing ADHD symptoms and improving social and family functioning. Treatment was well tolerated and without significant side effects.25
- In a randomized open-label trial, 228 children received atomoxetine or methylphenidate. Both treatments significantly reduced inattention and hyperactive/impulsive symptoms.26
Related resources
- Arnsten AF. Genetics of childhood disorders (XVIII). ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201-3.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention-deficit/hyperactivity disorder. Am J Psychiatry. 1993;150:885-90.
- Research Report: Prescription Drugs—abuse and addiction. National Institute on Drug Abuse, Substance Abuse and Mental Health Services Administration.
Drug brand names
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Ritalin, Concerta
- Venlafaxine • Effexor
Disclosure
Dr. Higgins reports that he is on the speakers’ bureaus for Wyeth-Ayerst Pharmaceuticals and Eli Lilly and Co.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76.
2. Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann NY Acad Sci 2001;931:251-70.
3. Hunt RD. Nosology, neurobiology, and clinical patterns of ADHD in adults. Psychiatric Ann 1997;27:572-81.
4. Taylor MA. Evaluation and management of attention-deficit hyperactivity disorder. Am Fam Phys 1997;55:887-904.
5. Diller LH. The run on Ritalin. Attention deficit disorder and stimulant treatment in the 1990s. Hasting Center Report 1996;26:12-18.
6. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotrophic medications: primary care, psychiatry, and other medical specialties. JAMA 1998;279:526-31.
7. Mortan WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. J Clin Psychiatry (Primary Care) 2000;2:159-64.
8. Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
9. Banov MD, Palmer T, Brody E. Antidepressants are as effective as stimulants in the long-term treatment of ADHD in Adults. Primary Psychiatry 2001;8:54-7.
10. Moline S, Frankenberger W. Use of stimulant medication for treatment of attention-deficit/hyperactivity disorder. A survey of middle and high school students attitudes. Psychology in the Schools 2001;38:569-84.
11. Prescription drugs: abuse and addiction. National Institute on Drug Abuse Research Report Series. NIH publication number 01-4881, July 2001.
12. Mann A. Illicit methylphenidate trade appears widespread. Clinical Psychiatry News June 2000;28(6):5-
13. ABCNews.com. http://more/abcnews.go.com/sections/living/DailyNews/ritalin0505.html
14. Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999;48:15-20.
15. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry 1996;53:448-55.
16. DuPaul G. The ADHD Rating Scale: normative data, reliability, and validity. Worcester, MA: University of Massachusetts Medical School, 1990.
17. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
18. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
19. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
20. Kuperman S, Perry PJ, Gaffney GR, et al. Bupropion SR vs. methylphenidate vs. placebo for attention-deficit/hyperactivity disorder in adults. Ann Clin Psychiatry 2001;13:129-34.
21. Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of death, seizures, serum sickness. Can Med J 2002;166:68.-
22. Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol 2001;21:116-8.
23. Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 1998;59:502-8.
24. Spencer T, Biederman J, Wilens T, Prince J, Hatch M. Effectiveness and tolerability of tomoxetine in adults with attention-deficit/hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
25. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108:E83.-
26. Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in ADHD children: a randomized, open-label trial (presentation). Honolulu: American Academy of Child and Adolescent Psychiatry, October, 2001.
Should you prescribe a stimulant to treat attention and hyperactivity problems in teenagers and adults with a history of substance abuse? Evidence suggests that using a stimulant to treat attention-deficit/hyperactivity disorder (ADHD) may place such patients at risk for stimulant abuse or for relapse into abuse of other substances. But a stimulant may be the only option for patients whose ADHD symptoms do not respond to alternate medications, such as antidepressants.
Growing numbers of adults are being treated for ADHD. Because substance abuse problems are common in adults with ADHD (Box 1 ),1-3 prescribing an antidepressant instead of a stimulant in some cases may be prudent. Consider the following factors when choosing ADHD therapy for patients with a history of substance abuse.
Prevalence of stimulant use and abuse
In the United States, more than 95% of medications prescribed for children and adults with ADHD are stimulants—usually methylphenidate.4 Stimulant use has increased as more children and adults are diagnosed with ADHD. Methylphenidate prescriptions increased five-fold from 1990 to 1995.5 Visits to psychiatrists and physicians that included stimulant prescriptions grew from 570,000 to 2.86 million from 1985 to 1994, with most of that increase occurring during visits to primary care and other physicians.6
When used as prescribed, methylphenidate is safe and effective for treating most children and adults with ADHD. Methylphenidate’s pharmacologic properties, however, are similar to those of amphetamines and cocaine (Box 2, Figure 1),7,8 which is why methylphenidate is a schedule-II controlled substance.
Published data. Fifteen reports of methylphenidate abuse were published in the medical literature between 1960 and 1999,7 but little is known about the prevalence of stimulant abuse among patients with ADHD. Banov and colleagues recently published what may be the only data available, when they reported that 3 of 37 (8%) patients abused the stimulants they were prescribed for ADHD.9 The three patients who abused stimulants had histories of drug and alcohol abuse at study entry. In all three cases, stimulant abuse did not develop immediately but became apparent within 6 months after the study began.
In a study of 651 students ages 11 to 18 in Wisconsin and Minnesota, more than one-third of those taking stimulants reported being asked to sell or trade their medications. More than one-half of those not taking ADHD medications said they knew someone who sold or gave away his or her medication.10
Stimulant theft, recreational use. Methylphenidate has been identified as the third most abused prescribed substance in the United States.11 It was the 10th most frequently stolen controlled drug from pharmacies between 1990 and 1995, and 700,000 dosage units were reported stolen in 1996 and 1997.12
As many as 50% of adults with ADHD have substance abuse problems (including alcohol, cocaine, and marijuana), and as many as 30% have antisocial personality disorder (with increased potential for drug-seeking behaviors).1 Compared with the general population, persons with ADHD have an earlier onset of substance abuse that is less responsive to treatment and more likely to progress from alcohol to other drugs.2
The elevated risk of substance abuse in ADHD may be related to a subtle lack of response to normal positive and negative reinforcements. Hunt has outlined four neurobehavioral deficits that define ADHD.3 Besides inattention, hyperarousal, and impulsiveness, he proposes that persons with ADHD have a reward system deficit. They may gravitate toward substance abuse because drugs, alcohol, and nicotine provide stronger rewards than life’s more subtle social interactions.
The popular media have reported recreational use of methylphenidate—with street names such as “R-Ball” and “Vitamin R”—among teens and college students.13 Illegal stimulants are perceived to be easily accessible on college campuses, but no data have been reported.
The use of stimulant medication for ADHD patients with substance abuse problems remains controversial. For such patients, this author reserves stimulant medication for those:
- whose ADHD symptoms have not responded adequately to alternate treatments
- who have been reliable with prescription medications
- and whose functional level is seriously impaired by their ADHD.
Antidepressants vs. stimulants
Although few well-designed controlled studies have been published, four antidepressants appear to be reasonably equivalent in effectiveness for adults with ADHD and do not carry potential for stimulant abuse.14
Desipraime, bupropion, venlafaxine, and the experimental drug atomoxetine (Table 1) all increase norepinephrine at the synapse by inhibiting presynaptic reuptake. Though dopamine has traditionally been considered the neurotransmitter of choice for ADHD treatment, norepinephrine may be equally potent.
Impulse control center. Several lines of research have recently established a connection between the prefrontal cortex, norepinephrine, and ADHD.15 This evidence suggests that the prefrontal cortex plays a major role in inhibiting impulses and responses to distractions:
Figure 1

PET scans of the brain using carbon 11 (11C)-labeled cocaine and methylphenidate HCl show similar distributions in the striatium when the drugs are administered intravenously.
Source: Reproduced with permission from Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
Stimulants are classified as schedule-II drugs because they produce powerful reinforcing effects by increasing synaptic dopamine.7 Positron-emission tomography (PET) scans using carbon labeling have shown similar distributions of methylphenidate and cocaine in the brain (Figure 1).8 When administered intravenously, both drugs occupy the same receptors in the striatum and produce a “high” that parallels rapid neuronal uptake. Methylphenidate and cocaine similarly increase stimulation of the postsynaptic neuron by blocking the dopamine reuptake pump (dopamine transporter).
How a substance gets to the brain’s euphoric receptors greatly affects its addictive properties. Delivery systems with rapid onset—smoking, “snorting,” or IV injection—have much greater ability to produce a “high” than do oral or transdermal routes. The greater the “high,” the greater the potential for abuse.
Because methylphenidate is prescribed for oral use, the potential for abuse is minimal. However, we need to be extremely cautious when giving methylphenidate or similar stimulant medications to patients who have shown they are unable to control their abuse of other substances.
Stimulants can also re-ignite a dormant substance abuse problem. Though little has been written about this in the medical literature, Elizabeth Wurtzel, author of the controversial Prozac Nation, chronicles the resumption of her cocaine abuse in More, Now, Again: A Memoir of Addiction. She contends that after her doctor added methylphenidate to augment treatment of partially remitting depression, she began abusing it and eventually was using 40 tablets per day before slipping back into cocaine dependence.
- Patients with prefrontal cortex deficits can have problems with inattention and poor impulse control.
- Patients with ADHD have frontal lobe impairments, as neuropsychological testing and imaging studies have shown.
- Norepinephrine neurons, with cell bodies in the locus coeruleus, have projections that terminate in the prefrontal cortex.
- Agents with norepinephrine activity, but without mood-altering properties (e.g., clonidine), have been shown to improve ADHD symptoms.
Table 1
ADHD IN ADULTS: ANTIDEPRESSANT DOSAGES AND SIDE EFFECTS
| Medication | Class | Effective dosage | Side effects |
|---|---|---|---|
| Desipramine | Tricyclic | 100 to 200 mg/d | Sedation, weight gain, dry mouth, constipation, orthostatic hypotension, prolonged cardiac conduction time; may be lethal in overdose |
| Bupropion SR | Norepinephrine and dopamine reuptake inhibitor | 150 mg bid to 200 mg bid | Headaches, insomnia, agitation, increased risk of seizures |
| Venlafaxine XR | Serotonin and norepinephrine reuptake inhibitor | 75 to 225 mg/d | Nausea, sexual side effects, agitation, increased blood pressure at higher dosages |
| Atomoxetine* | Norepinephrine reuptake inhibitor | To be determined | To be determined |
| * Investigational agent; not FDA-approved | |||
Additional evidence suggests that the prefrontal cortex has projections back to the locus coeruleus, which may explain the relationship between the two areas. It may be that the brain’s higher-functioning areas, such as the prefrontal cortex, provide intelligent screening of impulses from the brain’s older areas, such as the locus coeruleus. Therefore, increased prefrontal cortex activity may modulate some impulses that ADHD patients cannot control otherwise.

No ‘high’ with antidepressants. Patients with ADHD who have experienced the powerful effects of street drugs such as cocaine, methamphetamine, or even alcohol may report that antidepressants do not provide the effect they desire. It is difficult to know if these patients are reporting a lack of benefit or simply the absence of a euphoric “high” they are used to experiencing with substances of abuse. The newer antidepressants do not activate the brain’s euphoric receptors to an appreciable degree.
Patients who take stimulants as prescribed also do not report a “high” but can detect the medication’s presence and absence. Most do not crave this feeling, but substance abusers tend to like it. A patient recently told me he didn’t think stimulants improved his ADHD, but said, “I just liked the way they made me feel.”
Desipramine
The tricyclic antidepressant desipramine is a potent norepinephrine reuptake inhibitor that is effective in treating ADHD in children and adults. In a double-blind, placebo-controlled study of adults with ADHD, subjects receiving desipramine showed robust improvement in symptom scores on the ADHD Rating Scale,16 compared with those receiving the placebo (Figure 2).17
During the 6-week trial, 41 adults with ADHD received desipramine, 200 mg/d, or a placebo. Those receiving desipramine showed significant improvement in 12 of 14 ADHD symptoms and less hyperactivity, impulsivity, and inattentiveness, whereas those receiving the placebo showed no improvement. According to the study criteria, 68% of those who received desipramine and none who received the placebo were considered positive responders.
Though no head-to-head studies have compared desipramine with methylphenidate, the same researchers conducted a similar placebo-controlled study with methylphenidate. The 6-week symptom score on the ADHD Rating Scale was 12.5 for methylphenidate, compared with a score of 12 for desipramine.18
Recommendation. Desipramine may be the most effective of the antidepressant treatments for patients with ADHD. Because of its side effects, however, it is not this author’s first choice and is usually reserved for patients whose symptoms fail to respond to other antidepressants. Desipramine can cause sedation, dry mouth, and constipation, which are related to blockade of adrenergic, histamine, and muscarinic cholinergic receptors. It also can be lethal in overdose.
Some substance abusers lose confidence in a medication that they cannot feel working. The side effects of desipramine, which can be intolerable for some patients, can reassure others with a history of substance abuse that they are being medicated.
Bupropion
Bupropion is a unique antidepressant that inhibits the presynaptic reuptake of dopamine and norepinephrine. Open-label studies demonstrate good responses to bupropion by adults with ADHD. One placebo-controlled, double-blind study found improved ADHD symptoms in 76% of patients receiving bupropion SR, compared with 37% of those receiving a placebo; the difference was statistically significant.19
Figure 2 IMPROVED ADHD SYMPTOMS WITH DESIPRAMINE

Adults with ADHD who received desipramine, 200 mg/d, in a double-blind trial showed significantly less hyperactivity, impulsivity, and inattentiveness after 6 weeks of therapy than a control group that received a placebo.
Source: Adapted with permission from Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.Another study of adults with ADHD compared bupropion SR to methylphenidate and a placebo.20 Using a primary outcome of the Clinical Global Impression (CGI) scale, response rates were 64% for bupropion SR, 50% for methylphenidate, and 27% for the placebo. The difference in response rates between the two agents was not statistically significant (p = 0.14).
Recommendation. The risk of seizures with bupropion is about 1 in 1,000. Therefore, bupropion should not be given to patients with a seizure disorder or to those with conditions that alter the seizure threshold (e.g., eating disorders, recent head trauma, or benzodiazepine withdrawal).21
This author uses bupropion as first-line treatment for appropriate patients with ADHD and a substance abuse history. Bupropion’s mild benefit with smoking cessation may provide some crossover effect for other substances of abuse. The low incidence of sexual side effects is another benefit. Drawbacks include twice-daily dosing and lack of a robust effect on attention and concentration.
Venlafaxine
Venlafaxine is a potent inhibitor of serotonin reuptake, a moderate inhibitor of norepinephrine, and a mild inhibitor of dopamine. Venlafaxine has displayed response rates similar to those of desipramine and bupropion in open-label studies in adults with ADHD,14 but no placebo-controlled studies exist.
As noted above, these antidepressants are believed to improve ADHD symptoms by making norepinephrine more available at the synapse. Hypothetically, then, one would need to administer venlafaxine at dosages that adequately inhibit the norepinephrine reuptake receptor. Venlafaxine XR, 150 mg/d, provides significant norepinephrine activity, according to several lines of evidence.
Recently, Upadhyaya et al reported the use of venlafaxine in one of the few treatment studies of patients with ADHD and comorbid alcohol/cocaine abuse. In an open-label trial, 10 subjects received venlafaxine, up to 300 mg/d, along with psychotherapy and attendance at Alcoholics Anonymous meetings. The nine who completed 4 weeks of treatment showed significantly improved ADHD symptoms and decreased alcohol craving.22
Recommendation. Venlafaxine is this author’s second choice for patients with ADHD and substance abuse problems. Sexual side effects that some patients experience with venlafaxine can limit its use. Some clinicians are concerned about increases in blood pressure associated with venlafaxine, although significant changes do not seem to occur at dosages below 300 mg/d.23
Atomoxetine
Atomoxetine is an investigational antidepressant in phase-III trials as a treatment for ADHD. Evidence shows atomoxetine to be a potent inhibitor of the presynaptic norepinephrine transporter, with minimal affinity for other neurotransmitter receptors. Initial studies suggest that atomoxetine is effective for adults and children with ADHD:
- In a small, double-blind, placebo-controlled, crossover trial, 11 of 20 adults showed improvement in ADHD symptoms within 3 weeks of starting atomoxetine.24
- In 297 children and adolescents, atomoxetine at dosages averaging approximately 1.2 mg/kg/d was more effective than a placebo in reducing ADHD symptoms and improving social and family functioning. Treatment was well tolerated and without significant side effects.25
- In a randomized open-label trial, 228 children received atomoxetine or methylphenidate. Both treatments significantly reduced inattention and hyperactive/impulsive symptoms.26
Related resources
- Arnsten AF. Genetics of childhood disorders (XVIII). ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201-3.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention-deficit/hyperactivity disorder. Am J Psychiatry. 1993;150:885-90.
- Research Report: Prescription Drugs—abuse and addiction. National Institute on Drug Abuse, Substance Abuse and Mental Health Services Administration.
Drug brand names
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Ritalin, Concerta
- Venlafaxine • Effexor
Disclosure
Dr. Higgins reports that he is on the speakers’ bureaus for Wyeth-Ayerst Pharmaceuticals and Eli Lilly and Co.
Should you prescribe a stimulant to treat attention and hyperactivity problems in teenagers and adults with a history of substance abuse? Evidence suggests that using a stimulant to treat attention-deficit/hyperactivity disorder (ADHD) may place such patients at risk for stimulant abuse or for relapse into abuse of other substances. But a stimulant may be the only option for patients whose ADHD symptoms do not respond to alternate medications, such as antidepressants.
Growing numbers of adults are being treated for ADHD. Because substance abuse problems are common in adults with ADHD (Box 1 ),1-3 prescribing an antidepressant instead of a stimulant in some cases may be prudent. Consider the following factors when choosing ADHD therapy for patients with a history of substance abuse.
Prevalence of stimulant use and abuse
In the United States, more than 95% of medications prescribed for children and adults with ADHD are stimulants—usually methylphenidate.4 Stimulant use has increased as more children and adults are diagnosed with ADHD. Methylphenidate prescriptions increased five-fold from 1990 to 1995.5 Visits to psychiatrists and physicians that included stimulant prescriptions grew from 570,000 to 2.86 million from 1985 to 1994, with most of that increase occurring during visits to primary care and other physicians.6
When used as prescribed, methylphenidate is safe and effective for treating most children and adults with ADHD. Methylphenidate’s pharmacologic properties, however, are similar to those of amphetamines and cocaine (Box 2, Figure 1),7,8 which is why methylphenidate is a schedule-II controlled substance.
Published data. Fifteen reports of methylphenidate abuse were published in the medical literature between 1960 and 1999,7 but little is known about the prevalence of stimulant abuse among patients with ADHD. Banov and colleagues recently published what may be the only data available, when they reported that 3 of 37 (8%) patients abused the stimulants they were prescribed for ADHD.9 The three patients who abused stimulants had histories of drug and alcohol abuse at study entry. In all three cases, stimulant abuse did not develop immediately but became apparent within 6 months after the study began.
In a study of 651 students ages 11 to 18 in Wisconsin and Minnesota, more than one-third of those taking stimulants reported being asked to sell or trade their medications. More than one-half of those not taking ADHD medications said they knew someone who sold or gave away his or her medication.10
Stimulant theft, recreational use. Methylphenidate has been identified as the third most abused prescribed substance in the United States.11 It was the 10th most frequently stolen controlled drug from pharmacies between 1990 and 1995, and 700,000 dosage units were reported stolen in 1996 and 1997.12
As many as 50% of adults with ADHD have substance abuse problems (including alcohol, cocaine, and marijuana), and as many as 30% have antisocial personality disorder (with increased potential for drug-seeking behaviors).1 Compared with the general population, persons with ADHD have an earlier onset of substance abuse that is less responsive to treatment and more likely to progress from alcohol to other drugs.2
The elevated risk of substance abuse in ADHD may be related to a subtle lack of response to normal positive and negative reinforcements. Hunt has outlined four neurobehavioral deficits that define ADHD.3 Besides inattention, hyperarousal, and impulsiveness, he proposes that persons with ADHD have a reward system deficit. They may gravitate toward substance abuse because drugs, alcohol, and nicotine provide stronger rewards than life’s more subtle social interactions.
The popular media have reported recreational use of methylphenidate—with street names such as “R-Ball” and “Vitamin R”—among teens and college students.13 Illegal stimulants are perceived to be easily accessible on college campuses, but no data have been reported.
The use of stimulant medication for ADHD patients with substance abuse problems remains controversial. For such patients, this author reserves stimulant medication for those:
- whose ADHD symptoms have not responded adequately to alternate treatments
- who have been reliable with prescription medications
- and whose functional level is seriously impaired by their ADHD.
Antidepressants vs. stimulants
Although few well-designed controlled studies have been published, four antidepressants appear to be reasonably equivalent in effectiveness for adults with ADHD and do not carry potential for stimulant abuse.14
Desipraime, bupropion, venlafaxine, and the experimental drug atomoxetine (Table 1) all increase norepinephrine at the synapse by inhibiting presynaptic reuptake. Though dopamine has traditionally been considered the neurotransmitter of choice for ADHD treatment, norepinephrine may be equally potent.
Impulse control center. Several lines of research have recently established a connection between the prefrontal cortex, norepinephrine, and ADHD.15 This evidence suggests that the prefrontal cortex plays a major role in inhibiting impulses and responses to distractions:
Figure 1

PET scans of the brain using carbon 11 (11C)-labeled cocaine and methylphenidate HCl show similar distributions in the striatium when the drugs are administered intravenously.
Source: Reproduced with permission from Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
Stimulants are classified as schedule-II drugs because they produce powerful reinforcing effects by increasing synaptic dopamine.7 Positron-emission tomography (PET) scans using carbon labeling have shown similar distributions of methylphenidate and cocaine in the brain (Figure 1).8 When administered intravenously, both drugs occupy the same receptors in the striatum and produce a “high” that parallels rapid neuronal uptake. Methylphenidate and cocaine similarly increase stimulation of the postsynaptic neuron by blocking the dopamine reuptake pump (dopamine transporter).
How a substance gets to the brain’s euphoric receptors greatly affects its addictive properties. Delivery systems with rapid onset—smoking, “snorting,” or IV injection—have much greater ability to produce a “high” than do oral or transdermal routes. The greater the “high,” the greater the potential for abuse.
Because methylphenidate is prescribed for oral use, the potential for abuse is minimal. However, we need to be extremely cautious when giving methylphenidate or similar stimulant medications to patients who have shown they are unable to control their abuse of other substances.
Stimulants can also re-ignite a dormant substance abuse problem. Though little has been written about this in the medical literature, Elizabeth Wurtzel, author of the controversial Prozac Nation, chronicles the resumption of her cocaine abuse in More, Now, Again: A Memoir of Addiction. She contends that after her doctor added methylphenidate to augment treatment of partially remitting depression, she began abusing it and eventually was using 40 tablets per day before slipping back into cocaine dependence.
- Patients with prefrontal cortex deficits can have problems with inattention and poor impulse control.
- Patients with ADHD have frontal lobe impairments, as neuropsychological testing and imaging studies have shown.
- Norepinephrine neurons, with cell bodies in the locus coeruleus, have projections that terminate in the prefrontal cortex.
- Agents with norepinephrine activity, but without mood-altering properties (e.g., clonidine), have been shown to improve ADHD symptoms.
Table 1
ADHD IN ADULTS: ANTIDEPRESSANT DOSAGES AND SIDE EFFECTS
| Medication | Class | Effective dosage | Side effects |
|---|---|---|---|
| Desipramine | Tricyclic | 100 to 200 mg/d | Sedation, weight gain, dry mouth, constipation, orthostatic hypotension, prolonged cardiac conduction time; may be lethal in overdose |
| Bupropion SR | Norepinephrine and dopamine reuptake inhibitor | 150 mg bid to 200 mg bid | Headaches, insomnia, agitation, increased risk of seizures |
| Venlafaxine XR | Serotonin and norepinephrine reuptake inhibitor | 75 to 225 mg/d | Nausea, sexual side effects, agitation, increased blood pressure at higher dosages |
| Atomoxetine* | Norepinephrine reuptake inhibitor | To be determined | To be determined |
| * Investigational agent; not FDA-approved | |||
Additional evidence suggests that the prefrontal cortex has projections back to the locus coeruleus, which may explain the relationship between the two areas. It may be that the brain’s higher-functioning areas, such as the prefrontal cortex, provide intelligent screening of impulses from the brain’s older areas, such as the locus coeruleus. Therefore, increased prefrontal cortex activity may modulate some impulses that ADHD patients cannot control otherwise.

No ‘high’ with antidepressants. Patients with ADHD who have experienced the powerful effects of street drugs such as cocaine, methamphetamine, or even alcohol may report that antidepressants do not provide the effect they desire. It is difficult to know if these patients are reporting a lack of benefit or simply the absence of a euphoric “high” they are used to experiencing with substances of abuse. The newer antidepressants do not activate the brain’s euphoric receptors to an appreciable degree.
Patients who take stimulants as prescribed also do not report a “high” but can detect the medication’s presence and absence. Most do not crave this feeling, but substance abusers tend to like it. A patient recently told me he didn’t think stimulants improved his ADHD, but said, “I just liked the way they made me feel.”
Desipramine
The tricyclic antidepressant desipramine is a potent norepinephrine reuptake inhibitor that is effective in treating ADHD in children and adults. In a double-blind, placebo-controlled study of adults with ADHD, subjects receiving desipramine showed robust improvement in symptom scores on the ADHD Rating Scale,16 compared with those receiving the placebo (Figure 2).17
During the 6-week trial, 41 adults with ADHD received desipramine, 200 mg/d, or a placebo. Those receiving desipramine showed significant improvement in 12 of 14 ADHD symptoms and less hyperactivity, impulsivity, and inattentiveness, whereas those receiving the placebo showed no improvement. According to the study criteria, 68% of those who received desipramine and none who received the placebo were considered positive responders.
Though no head-to-head studies have compared desipramine with methylphenidate, the same researchers conducted a similar placebo-controlled study with methylphenidate. The 6-week symptom score on the ADHD Rating Scale was 12.5 for methylphenidate, compared with a score of 12 for desipramine.18
Recommendation. Desipramine may be the most effective of the antidepressant treatments for patients with ADHD. Because of its side effects, however, it is not this author’s first choice and is usually reserved for patients whose symptoms fail to respond to other antidepressants. Desipramine can cause sedation, dry mouth, and constipation, which are related to blockade of adrenergic, histamine, and muscarinic cholinergic receptors. It also can be lethal in overdose.
Some substance abusers lose confidence in a medication that they cannot feel working. The side effects of desipramine, which can be intolerable for some patients, can reassure others with a history of substance abuse that they are being medicated.
Bupropion
Bupropion is a unique antidepressant that inhibits the presynaptic reuptake of dopamine and norepinephrine. Open-label studies demonstrate good responses to bupropion by adults with ADHD. One placebo-controlled, double-blind study found improved ADHD symptoms in 76% of patients receiving bupropion SR, compared with 37% of those receiving a placebo; the difference was statistically significant.19
Figure 2 IMPROVED ADHD SYMPTOMS WITH DESIPRAMINE

Adults with ADHD who received desipramine, 200 mg/d, in a double-blind trial showed significantly less hyperactivity, impulsivity, and inattentiveness after 6 weeks of therapy than a control group that received a placebo.
Source: Adapted with permission from Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.Another study of adults with ADHD compared bupropion SR to methylphenidate and a placebo.20 Using a primary outcome of the Clinical Global Impression (CGI) scale, response rates were 64% for bupropion SR, 50% for methylphenidate, and 27% for the placebo. The difference in response rates between the two agents was not statistically significant (p = 0.14).
Recommendation. The risk of seizures with bupropion is about 1 in 1,000. Therefore, bupropion should not be given to patients with a seizure disorder or to those with conditions that alter the seizure threshold (e.g., eating disorders, recent head trauma, or benzodiazepine withdrawal).21
This author uses bupropion as first-line treatment for appropriate patients with ADHD and a substance abuse history. Bupropion’s mild benefit with smoking cessation may provide some crossover effect for other substances of abuse. The low incidence of sexual side effects is another benefit. Drawbacks include twice-daily dosing and lack of a robust effect on attention and concentration.
Venlafaxine
Venlafaxine is a potent inhibitor of serotonin reuptake, a moderate inhibitor of norepinephrine, and a mild inhibitor of dopamine. Venlafaxine has displayed response rates similar to those of desipramine and bupropion in open-label studies in adults with ADHD,14 but no placebo-controlled studies exist.
As noted above, these antidepressants are believed to improve ADHD symptoms by making norepinephrine more available at the synapse. Hypothetically, then, one would need to administer venlafaxine at dosages that adequately inhibit the norepinephrine reuptake receptor. Venlafaxine XR, 150 mg/d, provides significant norepinephrine activity, according to several lines of evidence.
Recently, Upadhyaya et al reported the use of venlafaxine in one of the few treatment studies of patients with ADHD and comorbid alcohol/cocaine abuse. In an open-label trial, 10 subjects received venlafaxine, up to 300 mg/d, along with psychotherapy and attendance at Alcoholics Anonymous meetings. The nine who completed 4 weeks of treatment showed significantly improved ADHD symptoms and decreased alcohol craving.22
Recommendation. Venlafaxine is this author’s second choice for patients with ADHD and substance abuse problems. Sexual side effects that some patients experience with venlafaxine can limit its use. Some clinicians are concerned about increases in blood pressure associated with venlafaxine, although significant changes do not seem to occur at dosages below 300 mg/d.23
Atomoxetine
Atomoxetine is an investigational antidepressant in phase-III trials as a treatment for ADHD. Evidence shows atomoxetine to be a potent inhibitor of the presynaptic norepinephrine transporter, with minimal affinity for other neurotransmitter receptors. Initial studies suggest that atomoxetine is effective for adults and children with ADHD:
- In a small, double-blind, placebo-controlled, crossover trial, 11 of 20 adults showed improvement in ADHD symptoms within 3 weeks of starting atomoxetine.24
- In 297 children and adolescents, atomoxetine at dosages averaging approximately 1.2 mg/kg/d was more effective than a placebo in reducing ADHD symptoms and improving social and family functioning. Treatment was well tolerated and without significant side effects.25
- In a randomized open-label trial, 228 children received atomoxetine or methylphenidate. Both treatments significantly reduced inattention and hyperactive/impulsive symptoms.26
Related resources
- Arnsten AF. Genetics of childhood disorders (XVIII). ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201-3.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention-deficit/hyperactivity disorder. Am J Psychiatry. 1993;150:885-90.
- Research Report: Prescription Drugs—abuse and addiction. National Institute on Drug Abuse, Substance Abuse and Mental Health Services Administration.
Drug brand names
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Methylphenidate • Ritalin, Concerta
- Venlafaxine • Effexor
Disclosure
Dr. Higgins reports that he is on the speakers’ bureaus for Wyeth-Ayerst Pharmaceuticals and Eli Lilly and Co.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76.
2. Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann NY Acad Sci 2001;931:251-70.
3. Hunt RD. Nosology, neurobiology, and clinical patterns of ADHD in adults. Psychiatric Ann 1997;27:572-81.
4. Taylor MA. Evaluation and management of attention-deficit hyperactivity disorder. Am Fam Phys 1997;55:887-904.
5. Diller LH. The run on Ritalin. Attention deficit disorder and stimulant treatment in the 1990s. Hasting Center Report 1996;26:12-18.
6. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotrophic medications: primary care, psychiatry, and other medical specialties. JAMA 1998;279:526-31.
7. Mortan WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. J Clin Psychiatry (Primary Care) 2000;2:159-64.
8. Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
9. Banov MD, Palmer T, Brody E. Antidepressants are as effective as stimulants in the long-term treatment of ADHD in Adults. Primary Psychiatry 2001;8:54-7.
10. Moline S, Frankenberger W. Use of stimulant medication for treatment of attention-deficit/hyperactivity disorder. A survey of middle and high school students attitudes. Psychology in the Schools 2001;38:569-84.
11. Prescription drugs: abuse and addiction. National Institute on Drug Abuse Research Report Series. NIH publication number 01-4881, July 2001.
12. Mann A. Illicit methylphenidate trade appears widespread. Clinical Psychiatry News June 2000;28(6):5-
13. ABCNews.com. http://more/abcnews.go.com/sections/living/DailyNews/ritalin0505.html
14. Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999;48:15-20.
15. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry 1996;53:448-55.
16. DuPaul G. The ADHD Rating Scale: normative data, reliability, and validity. Worcester, MA: University of Massachusetts Medical School, 1990.
17. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
18. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
19. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
20. Kuperman S, Perry PJ, Gaffney GR, et al. Bupropion SR vs. methylphenidate vs. placebo for attention-deficit/hyperactivity disorder in adults. Ann Clin Psychiatry 2001;13:129-34.
21. Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of death, seizures, serum sickness. Can Med J 2002;166:68.-
22. Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol 2001;21:116-8.
23. Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 1998;59:502-8.
24. Spencer T, Biederman J, Wilens T, Prince J, Hatch M. Effectiveness and tolerability of tomoxetine in adults with attention-deficit/hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
25. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108:E83.-
26. Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in ADHD children: a randomized, open-label trial (presentation). Honolulu: American Academy of Child and Adolescent Psychiatry, October, 2001.
1. Mannuzza S, Klein RG, Bessler A, et al. Adult outcome of hyperactive boys. Educational achievement, occupational rank, and psychiatric status. Arch Gen Psychiatry 1993;50:565-76.
2. Sullivan MA, Rudnik-Levin F. Attention deficit/hyperactivity disorder and substance abuse. Diagnostic and therapeutic considerations. Ann NY Acad Sci 2001;931:251-70.
3. Hunt RD. Nosology, neurobiology, and clinical patterns of ADHD in adults. Psychiatric Ann 1997;27:572-81.
4. Taylor MA. Evaluation and management of attention-deficit hyperactivity disorder. Am Fam Phys 1997;55:887-904.
5. Diller LH. The run on Ritalin. Attention deficit disorder and stimulant treatment in the 1990s. Hasting Center Report 1996;26:12-18.
6. Pincus HA, Tanielian TL, Marcus SC, et al. Prescribing trends in psychotrophic medications: primary care, psychiatry, and other medical specialties. JAMA 1998;279:526-31.
7. Mortan WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. J Clin Psychiatry (Primary Care) 2000;2:159-64.
8. Volkow ND, Ding YS, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 1995;52:456-63.
9. Banov MD, Palmer T, Brody E. Antidepressants are as effective as stimulants in the long-term treatment of ADHD in Adults. Primary Psychiatry 2001;8:54-7.
10. Moline S, Frankenberger W. Use of stimulant medication for treatment of attention-deficit/hyperactivity disorder. A survey of middle and high school students attitudes. Psychology in the Schools 2001;38:569-84.
11. Prescription drugs: abuse and addiction. National Institute on Drug Abuse Research Report Series. NIH publication number 01-4881, July 2001.
12. Mann A. Illicit methylphenidate trade appears widespread. Clinical Psychiatry News June 2000;28(6):5-
13. ABCNews.com. http://more/abcnews.go.com/sections/living/DailyNews/ritalin0505.html
14. Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999;48:15-20.
15. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry 1996;53:448-55.
16. DuPaul G. The ADHD Rating Scale: normative data, reliability, and validity. Worcester, MA: University of Massachusetts Medical School, 1990.
17. Wilens TE, Biederman J, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention-deficit/hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
18. Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K. A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1995;52:434-43.
19. Wilens TE, Spencer TJ, Biederman J, et al. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
20. Kuperman S, Perry PJ, Gaffney GR, et al. Bupropion SR vs. methylphenidate vs. placebo for attention-deficit/hyperactivity disorder in adults. Ann Clin Psychiatry 2001;13:129-34.
21. Wooltorton E. Bupropion (Zyban, Wellbutrin SR): reports of death, seizures, serum sickness. Can Med J 2002;166:68.-
22. Upadhyaya HP, Brady KT, Sethuraman G, et al. Venlafaxine treatment of patients with comorbid alcohol/cocaine abuse and attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychopharmacol 2001;21:116-8.
23. Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry 1998;59:502-8.
24. Spencer T, Biederman J, Wilens T, Prince J, Hatch M. Effectiveness and tolerability of tomoxetine in adults with attention-deficit/hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
25. Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108:E83.-
26. Kratochvil C, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in ADHD children: a randomized, open-label trial (presentation). Honolulu: American Academy of Child and Adolescent Psychiatry, October, 2001.
Vitamin E may worsen acute respiratory tract infections in the elderly
ABSTRACT
BACKGROUND: The geriatric population has a potentially increased risk of infectious diseases and related sequelae because of decreasing immunocompetency. Currently available trials assessing efficacy of multivitamins and minerals are limited and contradictory. In this study the authors compared whether daily supplementation with a multivitamin containing minerals, with or without vitamin E, or vitamin E alone, affected the incidence and severity of acute respiratory tract infections in elderly individuals.
POPULATION STUDIED: The study population included 652 noninstitutionalized men and women living in the Netherlands who were at least 60 years of age. Patients were excluded if they were taking immunosuppressive agents, anticoagulants that could interfere with vitamin K metabolism, or dietary supplements within the preceding 2 months. Additional exclusion criteria included a history of cancer, liver disease, or fat malabsorption within the preceding 5 years.
STUDY DESIGN AND VALIDITY: Allocation assignment was concealed in this randomized, placebo-controlled trial. Participants took either 2 capsules daily of a multivitamin and mineral complex; vitamin E (200 mg/dL α-tocopheryl acetate); multivitamin-mineral complex plus vitamin E; or placebo for a maximum of 15 months. Doses of multivitamins were at recommended daily allowance (RDA) levels and doses of minerals were 25% to 50% of RDA levels. Patients recorded signs and acute symptoms of respiratory tract infections using a diary. A study nurse confirmed possible respiratory tract infections based on predetermined definitions. Data analysis was performed on an intention-to-treat and per-protocol basis.
OUTCOMES MEASURED: The primary outcomes measured were incidence and severity of acute respiratory tract infections. Microbiology and serology testing for 9 common respiratory pathogens was preformed for a random subsample of symptomatic patients. Baseline and post-study plasma levels of α-tocopherol, ascorbic acid, retinol, and carotenoids were also measured.
RESULTS: After a median study duration of 441 days, 1024 episodes of acute respiratory tract infections were reported by 68% of the participants. Of the 74.4% of these reported to the study nurse, 99.2% were confirmed as respiratory tract infections. Of the 107 symptomatic episodes randomly selected for microbiological testing, 58% had a confirmed pathogen, the most common being rhinovirus (54%). When treatment groups were evaluated individually compared with placebo, results were similar for all aspects of incidence and severity of acute respiratory tract infection, except that more patients in the multivitamin-mineral treatment group experienced a significant reduction in activity restriction (34.8% vs 48.5%; P = .04; number needed to treat = 8). Participants taking either multivitamin-mineral or vitamin E supplementation did not have a decreased incidence rate ratio of acute respiratory tract infections, 0.95 (95% CI, 0.75–1.15) and 1.12 (95% CI, 0.88–1.25), respectively. Multivitamin-mineral supplementation had no effect on severity of infection, whereas vitamin E supplementation was associated with illnesses of significantly greater severity: median illness duration was 19 versus 14 days (P = .02); median number of symptoms was 6 versus 4 (P = .03); fever occurrence in 37% versus 25% (P = .009); and restriction of activity in 52% versus 41% (P = .02).
For the elderly living in noninstitutionalized settings, supplementation with multivitamins at RDA levels with minerals at 25% to 50% RDA levels exhibited no effect on incidence or severity of acute respiratory tract infections. However, vitamin E 200 mg daily adversely affected the severity, but not the incidence, of acute respiratory tract infections. Before altering current vitamin E prescribing patterns or counseling patients to discontinue use, these findings should be confirmed.
ABSTRACT
BACKGROUND: The geriatric population has a potentially increased risk of infectious diseases and related sequelae because of decreasing immunocompetency. Currently available trials assessing efficacy of multivitamins and minerals are limited and contradictory. In this study the authors compared whether daily supplementation with a multivitamin containing minerals, with or without vitamin E, or vitamin E alone, affected the incidence and severity of acute respiratory tract infections in elderly individuals.
POPULATION STUDIED: The study population included 652 noninstitutionalized men and women living in the Netherlands who were at least 60 years of age. Patients were excluded if they were taking immunosuppressive agents, anticoagulants that could interfere with vitamin K metabolism, or dietary supplements within the preceding 2 months. Additional exclusion criteria included a history of cancer, liver disease, or fat malabsorption within the preceding 5 years.
STUDY DESIGN AND VALIDITY: Allocation assignment was concealed in this randomized, placebo-controlled trial. Participants took either 2 capsules daily of a multivitamin and mineral complex; vitamin E (200 mg/dL α-tocopheryl acetate); multivitamin-mineral complex plus vitamin E; or placebo for a maximum of 15 months. Doses of multivitamins were at recommended daily allowance (RDA) levels and doses of minerals were 25% to 50% of RDA levels. Patients recorded signs and acute symptoms of respiratory tract infections using a diary. A study nurse confirmed possible respiratory tract infections based on predetermined definitions. Data analysis was performed on an intention-to-treat and per-protocol basis.
OUTCOMES MEASURED: The primary outcomes measured were incidence and severity of acute respiratory tract infections. Microbiology and serology testing for 9 common respiratory pathogens was preformed for a random subsample of symptomatic patients. Baseline and post-study plasma levels of α-tocopherol, ascorbic acid, retinol, and carotenoids were also measured.
RESULTS: After a median study duration of 441 days, 1024 episodes of acute respiratory tract infections were reported by 68% of the participants. Of the 74.4% of these reported to the study nurse, 99.2% were confirmed as respiratory tract infections. Of the 107 symptomatic episodes randomly selected for microbiological testing, 58% had a confirmed pathogen, the most common being rhinovirus (54%). When treatment groups were evaluated individually compared with placebo, results were similar for all aspects of incidence and severity of acute respiratory tract infection, except that more patients in the multivitamin-mineral treatment group experienced a significant reduction in activity restriction (34.8% vs 48.5%; P = .04; number needed to treat = 8). Participants taking either multivitamin-mineral or vitamin E supplementation did not have a decreased incidence rate ratio of acute respiratory tract infections, 0.95 (95% CI, 0.75–1.15) and 1.12 (95% CI, 0.88–1.25), respectively. Multivitamin-mineral supplementation had no effect on severity of infection, whereas vitamin E supplementation was associated with illnesses of significantly greater severity: median illness duration was 19 versus 14 days (P = .02); median number of symptoms was 6 versus 4 (P = .03); fever occurrence in 37% versus 25% (P = .009); and restriction of activity in 52% versus 41% (P = .02).
For the elderly living in noninstitutionalized settings, supplementation with multivitamins at RDA levels with minerals at 25% to 50% RDA levels exhibited no effect on incidence or severity of acute respiratory tract infections. However, vitamin E 200 mg daily adversely affected the severity, but not the incidence, of acute respiratory tract infections. Before altering current vitamin E prescribing patterns or counseling patients to discontinue use, these findings should be confirmed.
ABSTRACT
BACKGROUND: The geriatric population has a potentially increased risk of infectious diseases and related sequelae because of decreasing immunocompetency. Currently available trials assessing efficacy of multivitamins and minerals are limited and contradictory. In this study the authors compared whether daily supplementation with a multivitamin containing minerals, with or without vitamin E, or vitamin E alone, affected the incidence and severity of acute respiratory tract infections in elderly individuals.
POPULATION STUDIED: The study population included 652 noninstitutionalized men and women living in the Netherlands who were at least 60 years of age. Patients were excluded if they were taking immunosuppressive agents, anticoagulants that could interfere with vitamin K metabolism, or dietary supplements within the preceding 2 months. Additional exclusion criteria included a history of cancer, liver disease, or fat malabsorption within the preceding 5 years.
STUDY DESIGN AND VALIDITY: Allocation assignment was concealed in this randomized, placebo-controlled trial. Participants took either 2 capsules daily of a multivitamin and mineral complex; vitamin E (200 mg/dL α-tocopheryl acetate); multivitamin-mineral complex plus vitamin E; or placebo for a maximum of 15 months. Doses of multivitamins were at recommended daily allowance (RDA) levels and doses of minerals were 25% to 50% of RDA levels. Patients recorded signs and acute symptoms of respiratory tract infections using a diary. A study nurse confirmed possible respiratory tract infections based on predetermined definitions. Data analysis was performed on an intention-to-treat and per-protocol basis.
OUTCOMES MEASURED: The primary outcomes measured were incidence and severity of acute respiratory tract infections. Microbiology and serology testing for 9 common respiratory pathogens was preformed for a random subsample of symptomatic patients. Baseline and post-study plasma levels of α-tocopherol, ascorbic acid, retinol, and carotenoids were also measured.
RESULTS: After a median study duration of 441 days, 1024 episodes of acute respiratory tract infections were reported by 68% of the participants. Of the 74.4% of these reported to the study nurse, 99.2% were confirmed as respiratory tract infections. Of the 107 symptomatic episodes randomly selected for microbiological testing, 58% had a confirmed pathogen, the most common being rhinovirus (54%). When treatment groups were evaluated individually compared with placebo, results were similar for all aspects of incidence and severity of acute respiratory tract infection, except that more patients in the multivitamin-mineral treatment group experienced a significant reduction in activity restriction (34.8% vs 48.5%; P = .04; number needed to treat = 8). Participants taking either multivitamin-mineral or vitamin E supplementation did not have a decreased incidence rate ratio of acute respiratory tract infections, 0.95 (95% CI, 0.75–1.15) and 1.12 (95% CI, 0.88–1.25), respectively. Multivitamin-mineral supplementation had no effect on severity of infection, whereas vitamin E supplementation was associated with illnesses of significantly greater severity: median illness duration was 19 versus 14 days (P = .02); median number of symptoms was 6 versus 4 (P = .03); fever occurrence in 37% versus 25% (P = .009); and restriction of activity in 52% versus 41% (P = .02).
For the elderly living in noninstitutionalized settings, supplementation with multivitamins at RDA levels with minerals at 25% to 50% RDA levels exhibited no effect on incidence or severity of acute respiratory tract infections. However, vitamin E 200 mg daily adversely affected the severity, but not the incidence, of acute respiratory tract infections. Before altering current vitamin E prescribing patterns or counseling patients to discontinue use, these findings should be confirmed.
Ginkgo is not a smart pill
ABSTRACT
BACKGROUND: Extracts of ginkgo have shown some promise in enhancing memory in demented elderly individuals.1 These authors set out to test the veracity of one manufacturer’s claim that ginkgo biloba “enhances mental focus and improves memory and concentration” in nondemented older adults.
POPULATION STUDIED: The authors solicited community-dwelling, functionally independent individuals older than 60 years who volunteered to participate in a study to improve memory. They excluded individuals with recent strokes, head injuries, or other “life-threatening illnesses,” mental illness or retardation, and persons who lacked someone to provide an evaluation of their memory and concentration. Participants were excluded if they scored less than 27 out of a possible 30 points on the Mini-Mental Status Examination at baseline. Highly educated or intelligent participants with mild dementia may have been included.
STUDY DESIGN AND VALIDITY: In this randomized controlled trial, participants received 40 mg of ginkgo biloba 3 times daily (the dose recommended by the manufacturer) or identical-appearing placebo for 6 weeks. This duration is 2 weeks longer than the manufacturer’s indicated onset of action. The number of subjects (230) was sufficient to detect a large effect. The researchers involved in dispensing study drugs and evaluating outcomes were effectively blinded, as were patients. Allocation to the control treatment groups may not have been concealed from the enrolling investigators. All persons screened for participation were accounted for, and the few dropouts were evenly balanced between the treatment and placebo groups. Analysis was by intention to treat.
OUTCOMES MEASURED: The authors measured performance on a wide variety of age-normed tests of memory, intelligence, and word-finding, such as the Wechsler Adult Intelligence Scale, well chosen to allow measurements to increase dramatically from baseline (ie, avoiding the ceiling effect that would be present if only tests for dementia were used). No quality of life measures were included. Companions were asked whether the subjects’ memory improved.
RESULTS: No statistically significant differences were noted between placebo and control groups on any of the scales measured. No significant changes from baseline to posttreatment and no adverse effects were found in either of the groups.
Ginkgo, in standard doses for 6 weeks, was ineffective in improving memory, intelligence, and concentration in older patients without dementia. Because of the lack of regulation among herbal supplements, ginkgo products by other manufacturers might be effective. Nevertheless, if you do not currently recommend ginkgo supplements to older patients who are worried about memory loss, do not start now.
ABSTRACT
BACKGROUND: Extracts of ginkgo have shown some promise in enhancing memory in demented elderly individuals.1 These authors set out to test the veracity of one manufacturer’s claim that ginkgo biloba “enhances mental focus and improves memory and concentration” in nondemented older adults.
POPULATION STUDIED: The authors solicited community-dwelling, functionally independent individuals older than 60 years who volunteered to participate in a study to improve memory. They excluded individuals with recent strokes, head injuries, or other “life-threatening illnesses,” mental illness or retardation, and persons who lacked someone to provide an evaluation of their memory and concentration. Participants were excluded if they scored less than 27 out of a possible 30 points on the Mini-Mental Status Examination at baseline. Highly educated or intelligent participants with mild dementia may have been included.
STUDY DESIGN AND VALIDITY: In this randomized controlled trial, participants received 40 mg of ginkgo biloba 3 times daily (the dose recommended by the manufacturer) or identical-appearing placebo for 6 weeks. This duration is 2 weeks longer than the manufacturer’s indicated onset of action. The number of subjects (230) was sufficient to detect a large effect. The researchers involved in dispensing study drugs and evaluating outcomes were effectively blinded, as were patients. Allocation to the control treatment groups may not have been concealed from the enrolling investigators. All persons screened for participation were accounted for, and the few dropouts were evenly balanced between the treatment and placebo groups. Analysis was by intention to treat.
OUTCOMES MEASURED: The authors measured performance on a wide variety of age-normed tests of memory, intelligence, and word-finding, such as the Wechsler Adult Intelligence Scale, well chosen to allow measurements to increase dramatically from baseline (ie, avoiding the ceiling effect that would be present if only tests for dementia were used). No quality of life measures were included. Companions were asked whether the subjects’ memory improved.
RESULTS: No statistically significant differences were noted between placebo and control groups on any of the scales measured. No significant changes from baseline to posttreatment and no adverse effects were found in either of the groups.
Ginkgo, in standard doses for 6 weeks, was ineffective in improving memory, intelligence, and concentration in older patients without dementia. Because of the lack of regulation among herbal supplements, ginkgo products by other manufacturers might be effective. Nevertheless, if you do not currently recommend ginkgo supplements to older patients who are worried about memory loss, do not start now.
ABSTRACT
BACKGROUND: Extracts of ginkgo have shown some promise in enhancing memory in demented elderly individuals.1 These authors set out to test the veracity of one manufacturer’s claim that ginkgo biloba “enhances mental focus and improves memory and concentration” in nondemented older adults.
POPULATION STUDIED: The authors solicited community-dwelling, functionally independent individuals older than 60 years who volunteered to participate in a study to improve memory. They excluded individuals with recent strokes, head injuries, or other “life-threatening illnesses,” mental illness or retardation, and persons who lacked someone to provide an evaluation of their memory and concentration. Participants were excluded if they scored less than 27 out of a possible 30 points on the Mini-Mental Status Examination at baseline. Highly educated or intelligent participants with mild dementia may have been included.
STUDY DESIGN AND VALIDITY: In this randomized controlled trial, participants received 40 mg of ginkgo biloba 3 times daily (the dose recommended by the manufacturer) or identical-appearing placebo for 6 weeks. This duration is 2 weeks longer than the manufacturer’s indicated onset of action. The number of subjects (230) was sufficient to detect a large effect. The researchers involved in dispensing study drugs and evaluating outcomes were effectively blinded, as were patients. Allocation to the control treatment groups may not have been concealed from the enrolling investigators. All persons screened for participation were accounted for, and the few dropouts were evenly balanced between the treatment and placebo groups. Analysis was by intention to treat.
OUTCOMES MEASURED: The authors measured performance on a wide variety of age-normed tests of memory, intelligence, and word-finding, such as the Wechsler Adult Intelligence Scale, well chosen to allow measurements to increase dramatically from baseline (ie, avoiding the ceiling effect that would be present if only tests for dementia were used). No quality of life measures were included. Companions were asked whether the subjects’ memory improved.
RESULTS: No statistically significant differences were noted between placebo and control groups on any of the scales measured. No significant changes from baseline to posttreatment and no adverse effects were found in either of the groups.
Ginkgo, in standard doses for 6 weeks, was ineffective in improving memory, intelligence, and concentration in older patients without dementia. Because of the lack of regulation among herbal supplements, ginkgo products by other manufacturers might be effective. Nevertheless, if you do not currently recommend ginkgo supplements to older patients who are worried about memory loss, do not start now.
Sexual addiction: disorder vs. personality
The two articles in your July issue on sexual addiction address two different subjects.
Steven L. Mahorney, MD, writes about a problem that troubles patients and for which they seek help. These people are troubled by how their sexual activities disturb their lives. Since we physicians may be able to help these troubled persons, we call it a “disorder.”
Neal W. Dunsieth, MD, writes about people whose sexual acts disturb others and thus create a problem for society. Society tries to cope with these disturbers of our peace in several ways, one of which is to lock them up in hospitals and in jails. If we confine these offenders and try to treat them, we are more or less obligated to give them a medical diagnosis. But it should not bear the same name as the disorder that applies to the people Dr. Mahorney writes about.
The distinction is similar to the difference between “obsessive-compulsive disorder” and “obsessive-compulsive personality.” The person with the “disorder” is troubled by it, but the one with the “personality” sees himself as orderly, neat, and thinks that “if a task is worth doing, it is worth doing correctly.” Others may find that person to be a problem.
Unfortunately, some lawyers think that a client with a medical diagnosis should not be held responsible for his or her behavior, which may be seen as part of the diagnosis. This type of thinking would dictate that a person with a smoking addiction is not responsible for quitting smoking.
Yehuda Sherman, MD
Lafayette, CA
Dr. Dunsieth responds
Although I agree there may be an analogy between degrees of problem sexual behavior and obsessive-compulsive disorder versus obsessive-compulsive personality, I view the distinction somewhat differently.
We probably have a “neurotic/organic” split that sorts self-identified patients from felons. This would follow somewhat logically because the felons have lost control of their impulses to the point that their infractions are severe and harmful to society. Therefore, their underlying problems probably are more severe. This may have implications for treatment.
Overall, though, there is too much heterogeneity among individuals with problem sexual behavior to make many generalizations. I will hold to only one generalization with these patients as a whole: Defensiveness surrounding problem sexual behavior runs higher among individuals than it does for virtually any other problem in psychiatry. When treating sexual behavior, the clinician must employ a high degree of structure and a somewhat skeptical ear.
Neal W. Dunsieth, MD
University of Cincinnat
College of Medicine
Dr. Mahorney responds
I appreciate Dr. Sherman’s letter. I had the same reaction to the juxtaposition of my article with that of Dr. Dunsieth.
The combination of DSM labeling, pharmaceutical marketing, and managed care has left many of us feeling that the best a psychiatrist can hope for is to sign the prescriptions and orders of a multidisciplinary team in a prison or jail, give the prisoners structured interviews, and pass the statistical analysis of those interviews off as “research.” The “research” can be published and the authors declared “experts” whose testimony can then be peddled to lawyers and their clients. I didn’t get the idea from Dr. Hillard’s first editorial, justifying the creation of a new journal, that that was the only vision out there. It certainly isn’t what my article was about.
I still think that as doctors, we should try to find ways to help our patients—before they go to jail.
I also want to disavow the automatic connection some have seen in my article between the usefulness of the sexual addiction paradigm and 12-step programs as treatment modalities. An exciting debate on this subject is emerging in clinical psychology circles. Psychiatrists should be part of that debate, and we can be if we could just tear ourselves away from our DSM bean counting.
Steven L. Mahorney, MD
University of North Carolina
School of Medicine, Chapel Hill
The two articles in your July issue on sexual addiction address two different subjects.
Steven L. Mahorney, MD, writes about a problem that troubles patients and for which they seek help. These people are troubled by how their sexual activities disturb their lives. Since we physicians may be able to help these troubled persons, we call it a “disorder.”
Neal W. Dunsieth, MD, writes about people whose sexual acts disturb others and thus create a problem for society. Society tries to cope with these disturbers of our peace in several ways, one of which is to lock them up in hospitals and in jails. If we confine these offenders and try to treat them, we are more or less obligated to give them a medical diagnosis. But it should not bear the same name as the disorder that applies to the people Dr. Mahorney writes about.
The distinction is similar to the difference between “obsessive-compulsive disorder” and “obsessive-compulsive personality.” The person with the “disorder” is troubled by it, but the one with the “personality” sees himself as orderly, neat, and thinks that “if a task is worth doing, it is worth doing correctly.” Others may find that person to be a problem.
Unfortunately, some lawyers think that a client with a medical diagnosis should not be held responsible for his or her behavior, which may be seen as part of the diagnosis. This type of thinking would dictate that a person with a smoking addiction is not responsible for quitting smoking.
Yehuda Sherman, MD
Lafayette, CA
Dr. Dunsieth responds
Although I agree there may be an analogy between degrees of problem sexual behavior and obsessive-compulsive disorder versus obsessive-compulsive personality, I view the distinction somewhat differently.
We probably have a “neurotic/organic” split that sorts self-identified patients from felons. This would follow somewhat logically because the felons have lost control of their impulses to the point that their infractions are severe and harmful to society. Therefore, their underlying problems probably are more severe. This may have implications for treatment.
Overall, though, there is too much heterogeneity among individuals with problem sexual behavior to make many generalizations. I will hold to only one generalization with these patients as a whole: Defensiveness surrounding problem sexual behavior runs higher among individuals than it does for virtually any other problem in psychiatry. When treating sexual behavior, the clinician must employ a high degree of structure and a somewhat skeptical ear.
Neal W. Dunsieth, MD
University of Cincinnat
College of Medicine
Dr. Mahorney responds
I appreciate Dr. Sherman’s letter. I had the same reaction to the juxtaposition of my article with that of Dr. Dunsieth.
The combination of DSM labeling, pharmaceutical marketing, and managed care has left many of us feeling that the best a psychiatrist can hope for is to sign the prescriptions and orders of a multidisciplinary team in a prison or jail, give the prisoners structured interviews, and pass the statistical analysis of those interviews off as “research.” The “research” can be published and the authors declared “experts” whose testimony can then be peddled to lawyers and their clients. I didn’t get the idea from Dr. Hillard’s first editorial, justifying the creation of a new journal, that that was the only vision out there. It certainly isn’t what my article was about.
I still think that as doctors, we should try to find ways to help our patients—before they go to jail.
I also want to disavow the automatic connection some have seen in my article between the usefulness of the sexual addiction paradigm and 12-step programs as treatment modalities. An exciting debate on this subject is emerging in clinical psychology circles. Psychiatrists should be part of that debate, and we can be if we could just tear ourselves away from our DSM bean counting.
Steven L. Mahorney, MD
University of North Carolina
School of Medicine, Chapel Hill
The two articles in your July issue on sexual addiction address two different subjects.
Steven L. Mahorney, MD, writes about a problem that troubles patients and for which they seek help. These people are troubled by how their sexual activities disturb their lives. Since we physicians may be able to help these troubled persons, we call it a “disorder.”
Neal W. Dunsieth, MD, writes about people whose sexual acts disturb others and thus create a problem for society. Society tries to cope with these disturbers of our peace in several ways, one of which is to lock them up in hospitals and in jails. If we confine these offenders and try to treat them, we are more or less obligated to give them a medical diagnosis. But it should not bear the same name as the disorder that applies to the people Dr. Mahorney writes about.
The distinction is similar to the difference between “obsessive-compulsive disorder” and “obsessive-compulsive personality.” The person with the “disorder” is troubled by it, but the one with the “personality” sees himself as orderly, neat, and thinks that “if a task is worth doing, it is worth doing correctly.” Others may find that person to be a problem.
Unfortunately, some lawyers think that a client with a medical diagnosis should not be held responsible for his or her behavior, which may be seen as part of the diagnosis. This type of thinking would dictate that a person with a smoking addiction is not responsible for quitting smoking.
Yehuda Sherman, MD
Lafayette, CA
Dr. Dunsieth responds
Although I agree there may be an analogy between degrees of problem sexual behavior and obsessive-compulsive disorder versus obsessive-compulsive personality, I view the distinction somewhat differently.
We probably have a “neurotic/organic” split that sorts self-identified patients from felons. This would follow somewhat logically because the felons have lost control of their impulses to the point that their infractions are severe and harmful to society. Therefore, their underlying problems probably are more severe. This may have implications for treatment.
Overall, though, there is too much heterogeneity among individuals with problem sexual behavior to make many generalizations. I will hold to only one generalization with these patients as a whole: Defensiveness surrounding problem sexual behavior runs higher among individuals than it does for virtually any other problem in psychiatry. When treating sexual behavior, the clinician must employ a high degree of structure and a somewhat skeptical ear.
Neal W. Dunsieth, MD
University of Cincinnat
College of Medicine
Dr. Mahorney responds
I appreciate Dr. Sherman’s letter. I had the same reaction to the juxtaposition of my article with that of Dr. Dunsieth.
The combination of DSM labeling, pharmaceutical marketing, and managed care has left many of us feeling that the best a psychiatrist can hope for is to sign the prescriptions and orders of a multidisciplinary team in a prison or jail, give the prisoners structured interviews, and pass the statistical analysis of those interviews off as “research.” The “research” can be published and the authors declared “experts” whose testimony can then be peddled to lawyers and their clients. I didn’t get the idea from Dr. Hillard’s first editorial, justifying the creation of a new journal, that that was the only vision out there. It certainly isn’t what my article was about.
I still think that as doctors, we should try to find ways to help our patients—before they go to jail.
I also want to disavow the automatic connection some have seen in my article between the usefulness of the sexual addiction paradigm and 12-step programs as treatment modalities. An exciting debate on this subject is emerging in clinical psychology circles. Psychiatrists should be part of that debate, and we can be if we could just tear ourselves away from our DSM bean counting.
Steven L. Mahorney, MD
University of North Carolina
School of Medicine, Chapel Hill
Sexual addiction: disorder vs. personality
The two articles in your July issue on sexual addiction address two different subjects.
Steven L. Mahorney, MD, writes about a problem that troubles patients and for which they seek help. These people are troubled by how their sexual activities disturb their lives. Since we physicians may be able to help these troubled persons, we call it a “disorder.”
Neal W. Dunsieth, MD, writes about people whose sexual acts disturb others and thus create a problem for society. Society tries to cope with these disturbers of our peace in several ways, one of which is to lock them up in hospitals and in jails. If we confine these offenders and try to treat them, we are more or less obligated to give them a medical diagnosis. But it should not bear the same name as the disorder that applies to the people Dr. Mahorney writes about.
The distinction is similar to the difference between “obsessive-compulsive disorder” and “obsessive-compulsive personality.” The person with the “disorder” is troubled by it, but the one with the “personality” sees himself as orderly, neat, and thinks that “if a task is worth doing, it is worth doing correctly.” Others may find that person to be a problem.
Unfortunately, some lawyers think that a client with a medical diagnosis should not be held responsible for his or her behavior, which may be seen as part of the diagnosis. This type of thinking would dictate that a person with a smoking addiction is not responsible for quitting smoking.
Yehuda Sherman, MD
Lafayette, CA
Dr. Dunsieth responds
Although I agree there may be an analogy between degrees of problem sexual behavior and obsessive-compulsive disorder versus obsessive-compulsive personality, I view the distinction somewhat differently.
We probably have a “neurotic/organic” split that sorts self-identified patients from felons. This would follow somewhat logically because the felons have lost control of their impulses to the point that their infractions are severe and harmful to society. Therefore, their underlying problems probably are more severe. This may have implications for treatment.
Overall, though, there is too much heterogeneity among individuals with problem sexual behavior to make many generalizations. I will hold to only one generalization with these patients as a whole: Defensiveness surrounding problem sexual behavior runs higher among individuals than it does for virtually any other problem in psychiatry. When treating sexual behavior, the clinician must employ a high degree of structure and a somewhat skeptical ear.
Neal W. Dunsieth, MD
University of Cincinnat
College of Medicine
Dr. Mahorney responds
I appreciate Dr. Sherman’s letter. I had the same reaction to the juxtaposition of my article with that of Dr. Dunsieth.
The combination of DSM labeling, pharmaceutical marketing, and managed care has left many of us feeling that the best a psychiatrist can hope for is to sign the prescriptions and orders of a multidisciplinary team in a prison or jail, give the prisoners structured interviews, and pass the statistical analysis of those interviews off as “research.” The “research” can be published and the authors declared “experts” whose testimony can then be peddled to lawyers and their clients. I didn’t get the idea from Dr. Hillard’s first editorial, justifying the creation of a new journal, that that was the only vision out there. It certainly isn’t what my article was about.
I still think that as doctors, we should try to find ways to help our patients—before they go to jail.
I also want to disavow the automatic connection some have seen in my article between the usefulness of the sexual addiction paradigm and 12-step programs as treatment modalities. An exciting debate on this subject is emerging in clinical psychology circles. Psychiatrists should be part of that debate, and we can be if we could just tear ourselves away from our DSM bean counting.
Steven L. Mahorney, MD
University of North Carolina
School of Medicine, Chapel Hill
The two articles in your July issue on sexual addiction address two different subjects.
Steven L. Mahorney, MD, writes about a problem that troubles patients and for which they seek help. These people are troubled by how their sexual activities disturb their lives. Since we physicians may be able to help these troubled persons, we call it a “disorder.”
Neal W. Dunsieth, MD, writes about people whose sexual acts disturb others and thus create a problem for society. Society tries to cope with these disturbers of our peace in several ways, one of which is to lock them up in hospitals and in jails. If we confine these offenders and try to treat them, we are more or less obligated to give them a medical diagnosis. But it should not bear the same name as the disorder that applies to the people Dr. Mahorney writes about.
The distinction is similar to the difference between “obsessive-compulsive disorder” and “obsessive-compulsive personality.” The person with the “disorder” is troubled by it, but the one with the “personality” sees himself as orderly, neat, and thinks that “if a task is worth doing, it is worth doing correctly.” Others may find that person to be a problem.
Unfortunately, some lawyers think that a client with a medical diagnosis should not be held responsible for his or her behavior, which may be seen as part of the diagnosis. This type of thinking would dictate that a person with a smoking addiction is not responsible for quitting smoking.
Yehuda Sherman, MD
Lafayette, CA
Dr. Dunsieth responds
Although I agree there may be an analogy between degrees of problem sexual behavior and obsessive-compulsive disorder versus obsessive-compulsive personality, I view the distinction somewhat differently.
We probably have a “neurotic/organic” split that sorts self-identified patients from felons. This would follow somewhat logically because the felons have lost control of their impulses to the point that their infractions are severe and harmful to society. Therefore, their underlying problems probably are more severe. This may have implications for treatment.
Overall, though, there is too much heterogeneity among individuals with problem sexual behavior to make many generalizations. I will hold to only one generalization with these patients as a whole: Defensiveness surrounding problem sexual behavior runs higher among individuals than it does for virtually any other problem in psychiatry. When treating sexual behavior, the clinician must employ a high degree of structure and a somewhat skeptical ear.
Neal W. Dunsieth, MD
University of Cincinnat
College of Medicine
Dr. Mahorney responds
I appreciate Dr. Sherman’s letter. I had the same reaction to the juxtaposition of my article with that of Dr. Dunsieth.
The combination of DSM labeling, pharmaceutical marketing, and managed care has left many of us feeling that the best a psychiatrist can hope for is to sign the prescriptions and orders of a multidisciplinary team in a prison or jail, give the prisoners structured interviews, and pass the statistical analysis of those interviews off as “research.” The “research” can be published and the authors declared “experts” whose testimony can then be peddled to lawyers and their clients. I didn’t get the idea from Dr. Hillard’s first editorial, justifying the creation of a new journal, that that was the only vision out there. It certainly isn’t what my article was about.
I still think that as doctors, we should try to find ways to help our patients—before they go to jail.
I also want to disavow the automatic connection some have seen in my article between the usefulness of the sexual addiction paradigm and 12-step programs as treatment modalities. An exciting debate on this subject is emerging in clinical psychology circles. Psychiatrists should be part of that debate, and we can be if we could just tear ourselves away from our DSM bean counting.
Steven L. Mahorney, MD
University of North Carolina
School of Medicine, Chapel Hill
The two articles in your July issue on sexual addiction address two different subjects.
Steven L. Mahorney, MD, writes about a problem that troubles patients and for which they seek help. These people are troubled by how their sexual activities disturb their lives. Since we physicians may be able to help these troubled persons, we call it a “disorder.”
Neal W. Dunsieth, MD, writes about people whose sexual acts disturb others and thus create a problem for society. Society tries to cope with these disturbers of our peace in several ways, one of which is to lock them up in hospitals and in jails. If we confine these offenders and try to treat them, we are more or less obligated to give them a medical diagnosis. But it should not bear the same name as the disorder that applies to the people Dr. Mahorney writes about.
The distinction is similar to the difference between “obsessive-compulsive disorder” and “obsessive-compulsive personality.” The person with the “disorder” is troubled by it, but the one with the “personality” sees himself as orderly, neat, and thinks that “if a task is worth doing, it is worth doing correctly.” Others may find that person to be a problem.
Unfortunately, some lawyers think that a client with a medical diagnosis should not be held responsible for his or her behavior, which may be seen as part of the diagnosis. This type of thinking would dictate that a person with a smoking addiction is not responsible for quitting smoking.
Yehuda Sherman, MD
Lafayette, CA
Dr. Dunsieth responds
Although I agree there may be an analogy between degrees of problem sexual behavior and obsessive-compulsive disorder versus obsessive-compulsive personality, I view the distinction somewhat differently.
We probably have a “neurotic/organic” split that sorts self-identified patients from felons. This would follow somewhat logically because the felons have lost control of their impulses to the point that their infractions are severe and harmful to society. Therefore, their underlying problems probably are more severe. This may have implications for treatment.
Overall, though, there is too much heterogeneity among individuals with problem sexual behavior to make many generalizations. I will hold to only one generalization with these patients as a whole: Defensiveness surrounding problem sexual behavior runs higher among individuals than it does for virtually any other problem in psychiatry. When treating sexual behavior, the clinician must employ a high degree of structure and a somewhat skeptical ear.
Neal W. Dunsieth, MD
University of Cincinnat
College of Medicine
Dr. Mahorney responds
I appreciate Dr. Sherman’s letter. I had the same reaction to the juxtaposition of my article with that of Dr. Dunsieth.
The combination of DSM labeling, pharmaceutical marketing, and managed care has left many of us feeling that the best a psychiatrist can hope for is to sign the prescriptions and orders of a multidisciplinary team in a prison or jail, give the prisoners structured interviews, and pass the statistical analysis of those interviews off as “research.” The “research” can be published and the authors declared “experts” whose testimony can then be peddled to lawyers and their clients. I didn’t get the idea from Dr. Hillard’s first editorial, justifying the creation of a new journal, that that was the only vision out there. It certainly isn’t what my article was about.
I still think that as doctors, we should try to find ways to help our patients—before they go to jail.
I also want to disavow the automatic connection some have seen in my article between the usefulness of the sexual addiction paradigm and 12-step programs as treatment modalities. An exciting debate on this subject is emerging in clinical psychology circles. Psychiatrists should be part of that debate, and we can be if we could just tear ourselves away from our DSM bean counting.
Steven L. Mahorney, MD
University of North Carolina
School of Medicine, Chapel Hill