User login
Is there a relationship between hypertension and cognitive function in older adults?
Vasectomy not a risk factor for prostate cancer
ABSTRACT
BACKGROUND: Several case-control and cohort studies since the early 1990s have shown conflicting results on a possible association between vasectomy and prostate cancer risk. A recent systematic review failed to show a causal association and suggested several possible mechanisms for inconclusive results. This study addressed some of these limitations.
POPULATION STUDIED: The study included 923 men in New Zealand between the ages of 40 and 74 years with newly diagnosed prostate cancer (cases). All men were on the general electoral roll and had a history of marriage. The control group was randomly selected from the general electoral roll (n = 1224), and frequency matching to cases was performed in 5-year age groups. The mean age for cases and controls was 66.3 and 65.1 years, respectively. All cases and controls had telephone numbers for data collection purposes. Because nearly all study subjects were of European descent (97%), the results may not apply to other ethnic groups.
STUDY DESIGN AND VALIDITY: This national, population-based, case-control study was performed on all newly diagnosed cases of prostate cancer during a specified time (April 1, 1996, to December 31, 1998). Controls were randomly selected from the general electoral roll in which about 95% of adults are listed. Of potential cases and controls, only 12% and 20%, respectively, could not be contacted due to death, doctor or subject refusal, severe illness, inability to trace, or language difficulties.
OUTCOMES MEASURED: The primary outcome measured was the relative risk (RR) of prostate cancer for men who had vasectomies compared with that for men who had not undergone the procedure.
RESULTS: No association between prostate cancer and vasectomy was found (RR = 0.92; 95% confidence interval [CI], 0.75–1.14). Even after 25 years since vasectomy, no association was found (RR = 0.92; 95% CI, 0.68–1.23). Adjustments were made for social class, geographic region, religious affiliation, and family history of prostate cancer without any effect on the risk.
This study found that having a vasectomy does not increase a man’s risk of developing prostate cancer, even after 25 or more years of follow-up. Because a previous systematic review also showed no conclusive evidence for an increased risk of prostate cancer after vasectomy, practitioners can confidently advise patients requesting vasectomies of the safety advantages compared with other methods of sterilization.
ABSTRACT
BACKGROUND: Several case-control and cohort studies since the early 1990s have shown conflicting results on a possible association between vasectomy and prostate cancer risk. A recent systematic review failed to show a causal association and suggested several possible mechanisms for inconclusive results. This study addressed some of these limitations.
POPULATION STUDIED: The study included 923 men in New Zealand between the ages of 40 and 74 years with newly diagnosed prostate cancer (cases). All men were on the general electoral roll and had a history of marriage. The control group was randomly selected from the general electoral roll (n = 1224), and frequency matching to cases was performed in 5-year age groups. The mean age for cases and controls was 66.3 and 65.1 years, respectively. All cases and controls had telephone numbers for data collection purposes. Because nearly all study subjects were of European descent (97%), the results may not apply to other ethnic groups.
STUDY DESIGN AND VALIDITY: This national, population-based, case-control study was performed on all newly diagnosed cases of prostate cancer during a specified time (April 1, 1996, to December 31, 1998). Controls were randomly selected from the general electoral roll in which about 95% of adults are listed. Of potential cases and controls, only 12% and 20%, respectively, could not be contacted due to death, doctor or subject refusal, severe illness, inability to trace, or language difficulties.
OUTCOMES MEASURED: The primary outcome measured was the relative risk (RR) of prostate cancer for men who had vasectomies compared with that for men who had not undergone the procedure.
RESULTS: No association between prostate cancer and vasectomy was found (RR = 0.92; 95% confidence interval [CI], 0.75–1.14). Even after 25 years since vasectomy, no association was found (RR = 0.92; 95% CI, 0.68–1.23). Adjustments were made for social class, geographic region, religious affiliation, and family history of prostate cancer without any effect on the risk.
This study found that having a vasectomy does not increase a man’s risk of developing prostate cancer, even after 25 or more years of follow-up. Because a previous systematic review also showed no conclusive evidence for an increased risk of prostate cancer after vasectomy, practitioners can confidently advise patients requesting vasectomies of the safety advantages compared with other methods of sterilization.
ABSTRACT
BACKGROUND: Several case-control and cohort studies since the early 1990s have shown conflicting results on a possible association between vasectomy and prostate cancer risk. A recent systematic review failed to show a causal association and suggested several possible mechanisms for inconclusive results. This study addressed some of these limitations.
POPULATION STUDIED: The study included 923 men in New Zealand between the ages of 40 and 74 years with newly diagnosed prostate cancer (cases). All men were on the general electoral roll and had a history of marriage. The control group was randomly selected from the general electoral roll (n = 1224), and frequency matching to cases was performed in 5-year age groups. The mean age for cases and controls was 66.3 and 65.1 years, respectively. All cases and controls had telephone numbers for data collection purposes. Because nearly all study subjects were of European descent (97%), the results may not apply to other ethnic groups.
STUDY DESIGN AND VALIDITY: This national, population-based, case-control study was performed on all newly diagnosed cases of prostate cancer during a specified time (April 1, 1996, to December 31, 1998). Controls were randomly selected from the general electoral roll in which about 95% of adults are listed. Of potential cases and controls, only 12% and 20%, respectively, could not be contacted due to death, doctor or subject refusal, severe illness, inability to trace, or language difficulties.
OUTCOMES MEASURED: The primary outcome measured was the relative risk (RR) of prostate cancer for men who had vasectomies compared with that for men who had not undergone the procedure.
RESULTS: No association between prostate cancer and vasectomy was found (RR = 0.92; 95% confidence interval [CI], 0.75–1.14). Even after 25 years since vasectomy, no association was found (RR = 0.92; 95% CI, 0.68–1.23). Adjustments were made for social class, geographic region, religious affiliation, and family history of prostate cancer without any effect on the risk.
This study found that having a vasectomy does not increase a man’s risk of developing prostate cancer, even after 25 or more years of follow-up. Because a previous systematic review also showed no conclusive evidence for an increased risk of prostate cancer after vasectomy, practitioners can confidently advise patients requesting vasectomies of the safety advantages compared with other methods of sterilization.
Inhaled fluticasone superior to montelukast in persistent asthma
ABSTRACT
BACKGROUND: Asthma management guidelines recommend patients with persistent asthma use asthma controller therapy in addition to as-needed short-acting beta-agonist therapy to improve symptom control, maintain pulmonary function, and decrease exacerbations. This study compared 2 asthma controllers, inhaled fluticasone and oral montelukast, with respect to clinical efficacy, patient preference, asthma-specific quality of life, and safety.
POPULATION STUDIED: The patients in this study were men and women aged 15 years and older with asthma recruited from multiple centers across the United States. Nonsmoking patients were included with a forced expiratory volume in 1 second (FEV 1 ) of 50% to 80% of predicted that reversed by at least 15% with bronchodilator use. Patients were then eligible for randomization if, after an 8- to 14-day run-in period, their FEV 1 remained within 15% of initial values, they used albuterol at least 6 of the last 7 days, and they had asthma symptom scores of 2 (on a 0 to 5 scale) for at least 4 of the last 7 days.
STUDY DESIGN AND VALIDITY: This study was a double-blinded, randomized trial sponsored by the makers of fluticasone. Patients meeting initial inclusion criteria underwent an 8- to 14-day run-in period in which only short-acting beta-agonist use was allowed. Patients were then randomized to 1 of 2 treatment groups if they met the secondary inclusion criteria. Personal communication with the lead author confirmed that allocation assignment was concealed. Patients received either fluticasone 88 μg twice daily via metered dose inhaler (MDI) and montelukast placebo, or montelukast 10 mg daily with a placebo MDI. Patients kept daily records and had clinical evaluations at regular intervals for 24 weeks. Seventy-six percent of the patients completed the study.
OUTCOMES MEASURED: The primary outcome was percent change in FEV 1 . Other outcomes included peak flow rate, symptom-free days, daily albuterol use, asthma symptom scores, asthma quality-of-life scores, and patient-rated satisfaction with treatment. Safety was also assessed by reports of clinical adverse events and number of asthma exacerbations.
RESULTS: Using an intent-to-treat analysis, the fluticasone group had a significantly greater sustained change in FEV 1 (22% vs 14%; P < .001). Significant differences were noted after just 2 weeks of treatment. Significant differences favoring fluticasone were also found in all secondary outcomes including the patient-oriented outcomes of change in asthma symptom scores (–0.91 vs –0.57; P < .001), asthma quality-of-life scores (1.3 vs 1.0; P = .004), and patient-rated satisfaction with treatment (83% of fluticasone patients satisfied vs 66% of montelukast patients satisfied; P < .001). No differences were noted in overall incidence of adverse events between treatment groups, but significantly more fluticasone-treated patients reported hoarseness (9 vs 0; P = .002) and oral pharyngeal candidiasis (8 vs 0; P = .008). The incidence of asthma exacerbations was similar (19 fluticasone-treated patients vs 21 montelukast-treated patients).
This study confirms earlier studies indicating that inhaled steroids should be first-line treatment for moderate-to-severe persistent asthma. When compared with montelukast, inhaled fluticasone showed greater improvements in clinical measures of asthma, as well as patient-oriented measures such as symptom scores, quality-of-life scores, and patientrated satisfaction. However, moderate-to-severe persistent asthma appears to require more therapeutic measures than just low-dose fluticasone. Despite treatment, patients still used albuterol on more than half of the days, only one third of days were symptom-free, and symptom scores improved by less than 1 point on a 6-point scale.
ABSTRACT
BACKGROUND: Asthma management guidelines recommend patients with persistent asthma use asthma controller therapy in addition to as-needed short-acting beta-agonist therapy to improve symptom control, maintain pulmonary function, and decrease exacerbations. This study compared 2 asthma controllers, inhaled fluticasone and oral montelukast, with respect to clinical efficacy, patient preference, asthma-specific quality of life, and safety.
POPULATION STUDIED: The patients in this study were men and women aged 15 years and older with asthma recruited from multiple centers across the United States. Nonsmoking patients were included with a forced expiratory volume in 1 second (FEV 1 ) of 50% to 80% of predicted that reversed by at least 15% with bronchodilator use. Patients were then eligible for randomization if, after an 8- to 14-day run-in period, their FEV 1 remained within 15% of initial values, they used albuterol at least 6 of the last 7 days, and they had asthma symptom scores of 2 (on a 0 to 5 scale) for at least 4 of the last 7 days.
STUDY DESIGN AND VALIDITY: This study was a double-blinded, randomized trial sponsored by the makers of fluticasone. Patients meeting initial inclusion criteria underwent an 8- to 14-day run-in period in which only short-acting beta-agonist use was allowed. Patients were then randomized to 1 of 2 treatment groups if they met the secondary inclusion criteria. Personal communication with the lead author confirmed that allocation assignment was concealed. Patients received either fluticasone 88 μg twice daily via metered dose inhaler (MDI) and montelukast placebo, or montelukast 10 mg daily with a placebo MDI. Patients kept daily records and had clinical evaluations at regular intervals for 24 weeks. Seventy-six percent of the patients completed the study.
OUTCOMES MEASURED: The primary outcome was percent change in FEV 1 . Other outcomes included peak flow rate, symptom-free days, daily albuterol use, asthma symptom scores, asthma quality-of-life scores, and patient-rated satisfaction with treatment. Safety was also assessed by reports of clinical adverse events and number of asthma exacerbations.
RESULTS: Using an intent-to-treat analysis, the fluticasone group had a significantly greater sustained change in FEV 1 (22% vs 14%; P < .001). Significant differences were noted after just 2 weeks of treatment. Significant differences favoring fluticasone were also found in all secondary outcomes including the patient-oriented outcomes of change in asthma symptom scores (–0.91 vs –0.57; P < .001), asthma quality-of-life scores (1.3 vs 1.0; P = .004), and patient-rated satisfaction with treatment (83% of fluticasone patients satisfied vs 66% of montelukast patients satisfied; P < .001). No differences were noted in overall incidence of adverse events between treatment groups, but significantly more fluticasone-treated patients reported hoarseness (9 vs 0; P = .002) and oral pharyngeal candidiasis (8 vs 0; P = .008). The incidence of asthma exacerbations was similar (19 fluticasone-treated patients vs 21 montelukast-treated patients).
This study confirms earlier studies indicating that inhaled steroids should be first-line treatment for moderate-to-severe persistent asthma. When compared with montelukast, inhaled fluticasone showed greater improvements in clinical measures of asthma, as well as patient-oriented measures such as symptom scores, quality-of-life scores, and patientrated satisfaction. However, moderate-to-severe persistent asthma appears to require more therapeutic measures than just low-dose fluticasone. Despite treatment, patients still used albuterol on more than half of the days, only one third of days were symptom-free, and symptom scores improved by less than 1 point on a 6-point scale.
ABSTRACT
BACKGROUND: Asthma management guidelines recommend patients with persistent asthma use asthma controller therapy in addition to as-needed short-acting beta-agonist therapy to improve symptom control, maintain pulmonary function, and decrease exacerbations. This study compared 2 asthma controllers, inhaled fluticasone and oral montelukast, with respect to clinical efficacy, patient preference, asthma-specific quality of life, and safety.
POPULATION STUDIED: The patients in this study were men and women aged 15 years and older with asthma recruited from multiple centers across the United States. Nonsmoking patients were included with a forced expiratory volume in 1 second (FEV 1 ) of 50% to 80% of predicted that reversed by at least 15% with bronchodilator use. Patients were then eligible for randomization if, after an 8- to 14-day run-in period, their FEV 1 remained within 15% of initial values, they used albuterol at least 6 of the last 7 days, and they had asthma symptom scores of 2 (on a 0 to 5 scale) for at least 4 of the last 7 days.
STUDY DESIGN AND VALIDITY: This study was a double-blinded, randomized trial sponsored by the makers of fluticasone. Patients meeting initial inclusion criteria underwent an 8- to 14-day run-in period in which only short-acting beta-agonist use was allowed. Patients were then randomized to 1 of 2 treatment groups if they met the secondary inclusion criteria. Personal communication with the lead author confirmed that allocation assignment was concealed. Patients received either fluticasone 88 μg twice daily via metered dose inhaler (MDI) and montelukast placebo, or montelukast 10 mg daily with a placebo MDI. Patients kept daily records and had clinical evaluations at regular intervals for 24 weeks. Seventy-six percent of the patients completed the study.
OUTCOMES MEASURED: The primary outcome was percent change in FEV 1 . Other outcomes included peak flow rate, symptom-free days, daily albuterol use, asthma symptom scores, asthma quality-of-life scores, and patient-rated satisfaction with treatment. Safety was also assessed by reports of clinical adverse events and number of asthma exacerbations.
RESULTS: Using an intent-to-treat analysis, the fluticasone group had a significantly greater sustained change in FEV 1 (22% vs 14%; P < .001). Significant differences were noted after just 2 weeks of treatment. Significant differences favoring fluticasone were also found in all secondary outcomes including the patient-oriented outcomes of change in asthma symptom scores (–0.91 vs –0.57; P < .001), asthma quality-of-life scores (1.3 vs 1.0; P = .004), and patient-rated satisfaction with treatment (83% of fluticasone patients satisfied vs 66% of montelukast patients satisfied; P < .001). No differences were noted in overall incidence of adverse events between treatment groups, but significantly more fluticasone-treated patients reported hoarseness (9 vs 0; P = .002) and oral pharyngeal candidiasis (8 vs 0; P = .008). The incidence of asthma exacerbations was similar (19 fluticasone-treated patients vs 21 montelukast-treated patients).
This study confirms earlier studies indicating that inhaled steroids should be first-line treatment for moderate-to-severe persistent asthma. When compared with montelukast, inhaled fluticasone showed greater improvements in clinical measures of asthma, as well as patient-oriented measures such as symptom scores, quality-of-life scores, and patientrated satisfaction. However, moderate-to-severe persistent asthma appears to require more therapeutic measures than just low-dose fluticasone. Despite treatment, patients still used albuterol on more than half of the days, only one third of days were symptom-free, and symptom scores improved by less than 1 point on a 6-point scale.
Is there a role for theophylline in treating patients with asthma?
With adults, oral theophylline may help lower the dosage of inhaled steroids needed to control chronic asthma. It offers no benefit for acute asthma exacerbations. For children, intravenous aminophylline may improve the clinical course of severe asthma attacks. Side effects and toxicity limit use of these medications in most settings. (Grade of recommendation: A, based on systematic reviews and randomized control trials [RCTs]).
Evidence summary
Several systematic reviews help clarify theophylline’s role in asthma management. When compared with placebo in the management of acute exacerbations, theophylline confers no added benefit to beta-agonist therapy (with or without steroids) in improving pulmonary function or reducing hospitalization rates. Side effects occurred more often in the theophylline group: palpitations/arrhythmias (OR = 2.9; 95% CI: 1.5 to 5.7) and vomiting (OR = 4.2; 95% CI: 2.4 to 7.4).1 For moderately severe asthma in patients already receiving inhaled corticosteroids (ICS), theophylline as maintenance therapy equaled long-acting beta-2-agonists in increasing FEV 1 and PEFR, but was less effective in controlling night time symptoms. Use of long-acting beta-agonists resulted in fewer side effects (RR = 0.38; 95%CI: 0.25-0.57).2 When added to low-dose ICS for maintenance, theophylline was as effective as high-dose ICS alone in improving FEV 1 , decreasing day and night symptoms, and reducing the need for rescue medications and the incidence of attacks. This suggests theophylline has utility as a steroid sparing agent.3
Intravenous aminophylline does appear to be clinically beneficial for children with severe exacerbations, defined as an FEV 1 of 35%-40% of predicted value. Critically ill children receiving aminophylline in addition to usual care exhibited an improved FEV 1 at 24 hours (mean difference = 8.4%; 95% CI: 0.82 to 15.92) and reduced symptom scores at 6 hours.4 The largest RCT of aminophylline in children demonstrated a reduced intubation rate (NNT = 14 CI: 7.8-77).5 Children receiving aminophylline experienced more vomiting (RR = 3.69; 95%CI: 2.15-6.33). Treatment with aminophylline did not reduce length of hospital stay or the number of rescue nebulizers needed (Table).4
TABLE
Theophylline use in asthma
| Adults | Children | |
|---|---|---|
| Acute Treatment | No added benefit to corticosteroids and beta-agonist therapy; increased GI and cardiac side effects. | 24 hours of IV aminophylline improves symptom scores without reducing LOS or nebulizer requirements; may reduce intubation |
| Maintenance Therapy | ||
| Mild | No clinical benefit | Not recommended |
| Moderate | Performs worse than long-acting beta-agonists and has more side effects; may limit the need for high-dose ICS if not using long beta agonists. | No advantage over long-acting beta agonists when added to ICS. More side effects |
| Severe | Same for moderate; does not limit the need for oral corticosteroids in this setting. | Same as moderate |
| LOS = length of stay; ICS = inhaled corticosteroids. | ||
Recommendations from others
Three evidence-supported guidelines concur that theophylline has a limited role as maintenance therapy for moderate-to-severe persistent asthma when symptom control with ICS alone is not adequate. Much stronger evidence supports the use of long-acting beta-2-agonists or leukotriene modifiers in this setting.6-8 The guidelines do not recommend using theophylline to treat acute asthma exacerbations; nor do they address using theophylline in children.
Read a Clinical Commentary by M. Lee Chambliss, MD, MSPH, at www.fpin.org.
1. Wilson AJ, Gibson, PG, Coughlan J. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
2. Parameswaran K, Belda J, Rowe BH. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
3. Evans DJ, Taylor DA, Zetterstrom O, et al. N Engl J Med 1997;337:1412-8.
4. Mitra A, Bassler D, Ducharme FM. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Yung M, South M. Arch Dis Child 1998;79:405-410.
6. Management of Chronic Asthma. Evidence Report/Technology Assessment. Number 44. AHQR Publication Number 01-E043, September 2001.
7. Global Initiative for Asthma, National Heart, Lung and Blood Institute, (U.S.)/World Health Organization. 1995 Jan (revised 1998).
8. Expert Panel Report 2:Guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program/National Heart, Lung and Blood Institute (U.S.). 1997 Jul, (reprinted 1998 Apr, 1999 Mar).
With adults, oral theophylline may help lower the dosage of inhaled steroids needed to control chronic asthma. It offers no benefit for acute asthma exacerbations. For children, intravenous aminophylline may improve the clinical course of severe asthma attacks. Side effects and toxicity limit use of these medications in most settings. (Grade of recommendation: A, based on systematic reviews and randomized control trials [RCTs]).
Evidence summary
Several systematic reviews help clarify theophylline’s role in asthma management. When compared with placebo in the management of acute exacerbations, theophylline confers no added benefit to beta-agonist therapy (with or without steroids) in improving pulmonary function or reducing hospitalization rates. Side effects occurred more often in the theophylline group: palpitations/arrhythmias (OR = 2.9; 95% CI: 1.5 to 5.7) and vomiting (OR = 4.2; 95% CI: 2.4 to 7.4).1 For moderately severe asthma in patients already receiving inhaled corticosteroids (ICS), theophylline as maintenance therapy equaled long-acting beta-2-agonists in increasing FEV 1 and PEFR, but was less effective in controlling night time symptoms. Use of long-acting beta-agonists resulted in fewer side effects (RR = 0.38; 95%CI: 0.25-0.57).2 When added to low-dose ICS for maintenance, theophylline was as effective as high-dose ICS alone in improving FEV 1 , decreasing day and night symptoms, and reducing the need for rescue medications and the incidence of attacks. This suggests theophylline has utility as a steroid sparing agent.3
Intravenous aminophylline does appear to be clinically beneficial for children with severe exacerbations, defined as an FEV 1 of 35%-40% of predicted value. Critically ill children receiving aminophylline in addition to usual care exhibited an improved FEV 1 at 24 hours (mean difference = 8.4%; 95% CI: 0.82 to 15.92) and reduced symptom scores at 6 hours.4 The largest RCT of aminophylline in children demonstrated a reduced intubation rate (NNT = 14 CI: 7.8-77).5 Children receiving aminophylline experienced more vomiting (RR = 3.69; 95%CI: 2.15-6.33). Treatment with aminophylline did not reduce length of hospital stay or the number of rescue nebulizers needed (Table).4
TABLE
Theophylline use in asthma
| Adults | Children | |
|---|---|---|
| Acute Treatment | No added benefit to corticosteroids and beta-agonist therapy; increased GI and cardiac side effects. | 24 hours of IV aminophylline improves symptom scores without reducing LOS or nebulizer requirements; may reduce intubation |
| Maintenance Therapy | ||
| Mild | No clinical benefit | Not recommended |
| Moderate | Performs worse than long-acting beta-agonists and has more side effects; may limit the need for high-dose ICS if not using long beta agonists. | No advantage over long-acting beta agonists when added to ICS. More side effects |
| Severe | Same for moderate; does not limit the need for oral corticosteroids in this setting. | Same as moderate |
| LOS = length of stay; ICS = inhaled corticosteroids. | ||
Recommendations from others
Three evidence-supported guidelines concur that theophylline has a limited role as maintenance therapy for moderate-to-severe persistent asthma when symptom control with ICS alone is not adequate. Much stronger evidence supports the use of long-acting beta-2-agonists or leukotriene modifiers in this setting.6-8 The guidelines do not recommend using theophylline to treat acute asthma exacerbations; nor do they address using theophylline in children.
Read a Clinical Commentary by M. Lee Chambliss, MD, MSPH, at www.fpin.org.
With adults, oral theophylline may help lower the dosage of inhaled steroids needed to control chronic asthma. It offers no benefit for acute asthma exacerbations. For children, intravenous aminophylline may improve the clinical course of severe asthma attacks. Side effects and toxicity limit use of these medications in most settings. (Grade of recommendation: A, based on systematic reviews and randomized control trials [RCTs]).
Evidence summary
Several systematic reviews help clarify theophylline’s role in asthma management. When compared with placebo in the management of acute exacerbations, theophylline confers no added benefit to beta-agonist therapy (with or without steroids) in improving pulmonary function or reducing hospitalization rates. Side effects occurred more often in the theophylline group: palpitations/arrhythmias (OR = 2.9; 95% CI: 1.5 to 5.7) and vomiting (OR = 4.2; 95% CI: 2.4 to 7.4).1 For moderately severe asthma in patients already receiving inhaled corticosteroids (ICS), theophylline as maintenance therapy equaled long-acting beta-2-agonists in increasing FEV 1 and PEFR, but was less effective in controlling night time symptoms. Use of long-acting beta-agonists resulted in fewer side effects (RR = 0.38; 95%CI: 0.25-0.57).2 When added to low-dose ICS for maintenance, theophylline was as effective as high-dose ICS alone in improving FEV 1 , decreasing day and night symptoms, and reducing the need for rescue medications and the incidence of attacks. This suggests theophylline has utility as a steroid sparing agent.3
Intravenous aminophylline does appear to be clinically beneficial for children with severe exacerbations, defined as an FEV 1 of 35%-40% of predicted value. Critically ill children receiving aminophylline in addition to usual care exhibited an improved FEV 1 at 24 hours (mean difference = 8.4%; 95% CI: 0.82 to 15.92) and reduced symptom scores at 6 hours.4 The largest RCT of aminophylline in children demonstrated a reduced intubation rate (NNT = 14 CI: 7.8-77).5 Children receiving aminophylline experienced more vomiting (RR = 3.69; 95%CI: 2.15-6.33). Treatment with aminophylline did not reduce length of hospital stay or the number of rescue nebulizers needed (Table).4
TABLE
Theophylline use in asthma
| Adults | Children | |
|---|---|---|
| Acute Treatment | No added benefit to corticosteroids and beta-agonist therapy; increased GI and cardiac side effects. | 24 hours of IV aminophylline improves symptom scores without reducing LOS or nebulizer requirements; may reduce intubation |
| Maintenance Therapy | ||
| Mild | No clinical benefit | Not recommended |
| Moderate | Performs worse than long-acting beta-agonists and has more side effects; may limit the need for high-dose ICS if not using long beta agonists. | No advantage over long-acting beta agonists when added to ICS. More side effects |
| Severe | Same for moderate; does not limit the need for oral corticosteroids in this setting. | Same as moderate |
| LOS = length of stay; ICS = inhaled corticosteroids. | ||
Recommendations from others
Three evidence-supported guidelines concur that theophylline has a limited role as maintenance therapy for moderate-to-severe persistent asthma when symptom control with ICS alone is not adequate. Much stronger evidence supports the use of long-acting beta-2-agonists or leukotriene modifiers in this setting.6-8 The guidelines do not recommend using theophylline to treat acute asthma exacerbations; nor do they address using theophylline in children.
Read a Clinical Commentary by M. Lee Chambliss, MD, MSPH, at www.fpin.org.
1. Wilson AJ, Gibson, PG, Coughlan J. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
2. Parameswaran K, Belda J, Rowe BH. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
3. Evans DJ, Taylor DA, Zetterstrom O, et al. N Engl J Med 1997;337:1412-8.
4. Mitra A, Bassler D, Ducharme FM. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Yung M, South M. Arch Dis Child 1998;79:405-410.
6. Management of Chronic Asthma. Evidence Report/Technology Assessment. Number 44. AHQR Publication Number 01-E043, September 2001.
7. Global Initiative for Asthma, National Heart, Lung and Blood Institute, (U.S.)/World Health Organization. 1995 Jan (revised 1998).
8. Expert Panel Report 2:Guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program/National Heart, Lung and Blood Institute (U.S.). 1997 Jul, (reprinted 1998 Apr, 1999 Mar).
1. Wilson AJ, Gibson, PG, Coughlan J. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
2. Parameswaran K, Belda J, Rowe BH. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
3. Evans DJ, Taylor DA, Zetterstrom O, et al. N Engl J Med 1997;337:1412-8.
4. Mitra A, Bassler D, Ducharme FM. The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Yung M, South M. Arch Dis Child 1998;79:405-410.
6. Management of Chronic Asthma. Evidence Report/Technology Assessment. Number 44. AHQR Publication Number 01-E043, September 2001.
7. Global Initiative for Asthma, National Heart, Lung and Blood Institute, (U.S.)/World Health Organization. 1995 Jan (revised 1998).
8. Expert Panel Report 2:Guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program/National Heart, Lung and Blood Institute (U.S.). 1997 Jul, (reprinted 1998 Apr, 1999 Mar).
Evidence-based answers from the Family Physicians Inquiries Network
Endometriosis: What it is and how it is treated
A 62-year-old man with hypotension and an abnormal chest radiograph
Asymptomatic hyperuricemia: To treat or not to treat
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
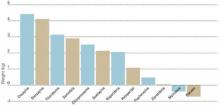
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
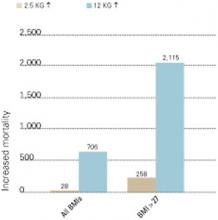
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
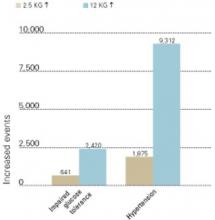
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Kleptomania: Emerging therapies target mood, impulsive behavior
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.