User login
Navigating Ethical and Clinical Considerations Relating to Percutaneous Gastrostomy (PEG) Tubes
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
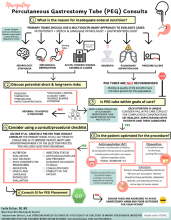
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Cases
Consults for percutaneous gastrostomy (PEG) tube placement for a patient ...
- With dysphagia after stroke: A 70-year-old female with a history of hypertension presented to the hospital with altered mental status and left-sided weakness. She was previously active and independently living. MRI of the brain revealed a right basal ganglia infarct. As a result, she developed dysphagia. She was evaluated by speech and language pathology and underwent a modified barium swallow. Given concerns for aspiration, the recommendation was made for gastroenterology (GI) consultation to place PEG tube for nutrition and medication administration.
- With advanced dementia: An 85-year-old male with an extensive medical history including advanced dementia was admitted from his nursing home for decreased oral intake. His baseline mental status is awake and alert, but he is nonverbal and does not follow commands. Upon 72-hour calorie count, the nutrition consultants determined that he cannot independently meet his nutrition goals. His family wants “everything done” and are asking about a “feeding tube.” The primary team has now consulted GI for PEG tube placement.
- Who is being discharged to a long-term care facility: A 45-year-old male was admitted to the ICU after a heroin overdose. CPR was initiated in the field and return of spontaneous circulation was obtained after 25 minutes. The patient has minimal brainstem reflexes. He is ventilator dependent. He has no family, and now is status-post tracheostomy placement by two-physician consent. The patient is ready for discharge to a long-term care facility that will not accept patients with nasogastric tubes. GI is consulted for PEG tube placement.
Discussion
Gastroenterologists are often consulted for PEG tube placement. However, This is rooted in the fact that, as one expert wrote, “feeding, unlike any other medical treatment, has a moral and emotional significance derived from culture.”1 Understanding the evidence, ethical considerations, and team dynamic behind PEG tube placement is critical for every gastroenterologist. Herein we review these topics and offer guidelines for having patient-centered conversations involving these fundamental concepts.
First, the gastroenterologist should understand the evidence to debunk myths and clarify truths surrounding PEG tube placement. While PEG tubes may help patients with amyotrophic lateral sclerosis stabilize their weight and can even be prophylactically placed in select patients with head and neck cancer,2,3 they are not always appropriate in patients in early recovery from stroke and have not been shown to improve outcomes in patients with advanced dementia. At least 50% of stroke-related dysphagia resolves within 1-2 weeks, and so the American Heart Association Stroke Council recommends continuing nasogastric tube feeding for 2-3 weeks in patients such as the one presented in case 1 before considering PEG tube placement.4
In situations of advanced dementia such as in case,2 several studies demonstrate that PEG tubes do not reduce or prevent aspiration pneumonia, prevent consequences of malnutrition, prolong life, reduce pressure ulcers, reduce urinary of gastrointestinal tract infections, lead to functional improvement, mitigate decline, or even improve comfort or quality of life for patients or their caregivers.5-7 Despite this evidence, as demonstrated in case,3 it is true that many American skilled nursing facilities will not accept a patient without a PEG if enteral feeding is needed. This restriction may vary by state: One study found that skilled nursing facilities in New York City are much less likely to accept patients with nasogastric feeding tubes than randomly selected skilled nursing facilities throughout the country.6 Nonetheless, gastroenterologists should look to the literature to understand the outcomes of populations of patients after PEG tube placement and use that data to guide decision-making.
Secondly, the five ethical principles that inform all medical decision making – autonomy, beneficence, nonmaleficence, justice, and futility – should also inform the gastroenterologist’s rationale in offering PEG placement.8
Autonomy implies that the medical team has determined who is able to make the decision regarding PEG tube placement for the patient. Beneficence connects the patient’s medical diagnosis and technical parameters of PEG tube placement with his or her goals of care. Nonmaleficence ensures the decision-making party understands the benefits and risks of the procedure, including anticipatory guidance on possible PEG tube management, complications, risks, and need for replacement. Justice incorporates the context of the patient’s life, including family dynamics, religious, cultural, and financial factors. Futility connects the patient’s prognosis with practical aspects of having a PEG tube.
The complexity of PEG placement lies in the fact that these ethical principles are often at odds with each other. For example, case 2 highlights the conflicting principles of autonomy and futility for elderly dementia patients: While PEG tube placements do not improve comfort or quality of life in advanced dementia (futility), the family representing the patient has stated they want everything done for his care, including PEG tube placement (autonomy). Navigating these ethical principles can be difficult, but having a framework to organize the different factors offers sound guidance for the gastroenterologist.
Finally, the gastroenterologist should recognize the roles of the multidisciplinary team members, including the patient and their representatives, regarding PEG tube placement consults. While gastroenterologists can be viewed as the technicians consulted to simply “place the tube,” they must seek to understand the members of the team representing the patient to be stewards of their skill set. Consulting team physicians carry great responsibility in organizing the medical and psychosocial aspects of each patient’s care, and their proper goals to relieve suffering and prevent death may color their judgment regarding who they believe is a candidate for a PEG tube. Nutritionists, speech therapists, and case managers can help provide objective data on the practicality and feasibility of a PEG tube in their patients. The healthcare system may influence the decision to consult heavily, as seen in the rules of the long-term care facility in case.3 While it is the job of the multidisciplinary medical team to explain the evidence and ethical considerations of PEG tube placement in a patient-centered manner, ultimately the decision belongs to the patient and their family or representatives.
The moral burden of not pursuing PEG placement may supersede the medical advice in many situations. There is an emotionally taxing perception that withholding nutrition via PEG is “starving the patient,” despite literature showing many terminally ill patients do not experience thirst or hunger, and those who do have alleviation of these symptoms with small amounts of food or liquid, not with PEG placement.5 As every patient is unique, PEG tube consultation guidelines created with input from all stakeholders have been utilized to ensure that patients are medically optimized for PEG tube placement and that evidence and ethics-based considerations are evaluated by the multidisciplinary team. An example of such a guideline is shown in Figure 1.
If the gastroenterologist encounters more contentious consultations, there are ways to build consensus to both alleviate patient and family suffering as well as elevate the discussions between teams.
First, identify the type of consult that is repeatedly bringing differing viewpoints and differing ethical principles into play. Second, get representatives from teams together in a neutral environment to understand stakeholders needs. New data suggest, in stroke cases like case 1, there may be dramatic benefit in long-term ability to recover if patients can get early intensive rehabilitation.9 This intense daily rehabilitation is not available within the hospital setting at many locations, and facilitation of discharge may be requested earlier than usually advised tube placement. Third, build a common language for requests and responses between teams. For instance, neurologists can identify and document which patients have less likelihood of early spontaneous recovery, and this can allow gastroenterologists to understand that those patients with little potential for early swallowing recovery can safely be targeted for PEG earlier during the hospital course. Other patients described as having a potential for spontaneous improvement should be given time to recover before an intervention is considered.10 Having a common understanding of goals and a better-informed decision pathway helps each team member feel fulfilled and rewarded, which will ultimately help reduce compassion fatigue and moral burden on providers.
In conclusion, PEG tube placement can be a challenging consultation for gastroenterologists because of the clinical, social, and ethical ramifications at stake for the patient. Even when PEG tube placement is technically feasible, the gastroenterologist should feel empowered to address the evidence-based outcomes of PEG tube placement, discuss the ethical principles of the decision-making process, and communicate with a multidisciplinary team using guidelines as set forth by this paper to best serve the patient.
Dr. Seltzer is based in the Department of Internal Medicine, Mount Sinai Morningside-West, New York City. Dr. Pusateri is based in the Division of Gastroenterology, Hepatology and Nutrition, Ohio State University Wexner Medical Center, Columbus. Dr. Nguyen is based in the Division of Gastroenterology and Center for Esophageal Diseases, Baylor Scott & White Health, Dallas, Texas. Dr. Stein is based in the Division of Gastroenterology, Robert Wood Johnson University Hospital, Rutgers University, New Brunswick, New Jersey. All authors contributed equally to this manuscript, and have no disclosures related to this article.
References
1. Mackie S. Gastroenterol Nurs. 2001 May-Jun;24(3):138-42.
2. Miller RG et al. Neurology. 2009 Oct. doi: 10.1212/WNL.0b013e3181bc0141.
3. Colevas AD et al. J Natl Compr Canc Netw. 2018 May. doi: 10.6004/jnccn.2018.0026.
4. Holloway RG et al. Stroke. 2014 Jun. doi: 10.1161/STR.0000000000000015.
5. Finucane TE et al. JAMA. 1999 Oct. doi: 10.1001/jama.282.14.1365.
6. Burgermaster M et al. Nutr Clin Pract. 2016 Jun. doi: 10.1177/0884533616629636.
7. American Geriatrics Society Ethics C, Clinical P, Models of Care C. J Am Geriatr Soc. 2014 Aug. doi: 10.1111/jgs.12924.
8. Beauchamp TL. Principlism in Bioethics. In: Serna P, eds. Bioethical Decision Making and Argumentation. International Library of Ethics, Law, and the New Medicine, vol 70. Springer; Cham. 2016 Sept:1-16. doi: 10.1007/978-3-319-43419-3_1.
9. Powers WJ et al. Stroke. 2019 Oct. doi: 10.1161/STR.0000000000000211.
10. Galovic M et al. JAMA Neurol. 2019 May. doi: 10.1001/jamaneurol.2018.4858.
Delivery of Care: The Ethical Imperative in Healthcare
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
The ethical imperative in healthcare necessitates equitable delivery of care to all individuals, regardless of their socio-economic status or insurance coverage. This principle is rooted in the concept of justice and is crucial to achieving health equity.
As gastroenterologists, despite our various practice settings, we have seen the harmful effects of economic and social disparities on health outcomes. We must therefore ensure that we acknowledge the existence of these disparities, and then begin to provide a framework that allows us to ethically and successfully navigate these complexities for our patients and our affiliated structures.
The following cases illustrate the complexities and ethical dilemmas that gastroenterology and hepatology healthcare professionals encounter in delivering care within the traditional healthcare system.
- Case 1: A 44-year-old male presents to the hospital with intermittent rectal bleeding every few weeks without associated abdominal pain or weight loss and not associated with straining. He has bowel movements every 2-3 days. There is no family history of underlying gastrointestinal disease or associated neoplasm. He is accompanied at the time of the interview by his coworker who offered to drive him to the hospital as he is having personal car trouble. Physical examination reveals normal hemodynamics, abdomen is benign, a digital rectal exam reveals small internal hemorrhoids without pain. Hemoglobin is 10, MCV 85. There is scant blood on the glove. He is uninsured. A GI consult is placed to determine the disposition of the patient. The resident on service suggests outpatient follow-up given low risk of clinical deterioration.
- Case 2: A 28-year-old woman postpartum 6 weeks presents in the office with a history of ulcerative colitis which was diagnosed 2 years prior. She was initially placed on steroid therapy. She underwent a colonoscopy at the time of her diagnosis and was following with a gastroenterologist at which time she was found to have moderate left-sided disease with a modified Mayo score of 9. She complains of urgency and rectal bleeding. She saw a gastroenterologist during her pregnancy and was placed on oral mesalamine, which she remains on at the time of evaluation. Once her physical examination is completed and laboratory values are reviewed, you begin to discuss advanced therapies including biologics as she has failed conventional therapies.
- Case 3: You receive a phone call from an outside hospital about a potential transfer for a 46-year-old male who is an immigrant of unknown citizenship status with fulminant liver failure. He meets all criteria including encephalopathy and coagulopathy. He drinks only socially. His secondary liver workup for extensive disease including ceruloplasmin remains pending. Viral hepatology serologies and autoimmune serologies are negative.
Challenges to the Delivery of Equitable Care
These cases underscore the challenges of delivering equitable care within a system that often fails to address the social determinants of health (SDOH). The disparity in the evaluation and treatment of patients based on insurance status not only affects patient outcomes, but also emphasizes the ethical dilemma of balancing cost with population health management.
The introduction of measures SDOH-1 and SDOH-2 by the Centers for Medicare & Medicaid Services in the 2023 IPPS Final Rule is a step towards requiring hospitals to systematically collect patient-level SDOH data, aiming to establish meaningful collaborations between healthcare providers and community-based organizations for whole-person care.1 The primary goal is to allow ecosystems to collect patient-level social risk factors followed by the creation of meaningful collaboration between healthcare providers and the community-based organizations.
The office settings may or may not implement the SDOH and the current electronic medical record systems. However, from a social history standpoint and certainly from a decision standpoint, the impact of SDOH is realized in all settings.
Interplay of SDOH and Ethical Considerations
The recognition of social determinants of health is crucial for ethical healthcare delivery. In the first case, considering the patient’s identified social determinants of health — including lack of insurance and transportation, combined with the rising incidence of colorectal cancer in individuals under 55 — an argument could be made for admitting the patient under observation for inpatient colonoscopy.
Data have shown disparities in treatment and referrals in emergency care setting for Black patients with rectal bleeding.2 It is imperative that we recognize these existing disparities in diagnosis and outcomes, along with determining SDOH to appropriately come to a final disposition. This approach aligns with the principle of justice and the imperative to deliver equitable care.
In the third case study, we have a patient facing the life-or-death situation of fulminant liver failure. He requires an expeditious decision to be made about transfer candidacy for liver transplant evaluation by the hepatology team.
Impact of Insurance Status on Healthcare Access
Insurance status significantly influences access to healthcare and disparities in treatment outcomes. As seen in case 2 and case 3, our therapies often hinge upon access.
In the inflammatory bowel disease (IBD) case, the therapy that we will choose for our IBD patient may be more influenced by access than efficacy. In a national sample of children with Crohn’s disease, publicly insured children were more likely to receive a biologic within 18 months of diagnosis compared to children with private insurance.3 This would suggest that those with private insurance perhaps experience increased barriers.
In the IBD case that we presented here, we do have a publicly insured woman who will face a potential loss of her Medicaid coverage. Our therapeutic decision will therefore not just rely on risk stratification and individualized approach, but rather the programs that are put in place by our pharmaceutical partners to support a future self-pay patient. This may or may not be favorable to her outcome. This discrepancy points to systemic inequalities in healthcare access and the need for policies that ensure equitable treatment for all, regardless of insurance status.
Conclusion
The delivery of care in healthcare is an ethical imperative that demands equity and justice. The cases discussed above illustrate the complex interplay between socioeconomic factors, insurance status, and the ethical challenges in providing equitable care.
Systematic efforts to address social determinants of health, as mandated by recent CMS measures, along with a commitment to ethical principles, are essential steps toward reducing disparities and ensuring that all individuals receive the care they need. As healthcare expenditures continue to rise, particularly in areas like gastrointestinal health, addressing these ethical and systemic challenges becomes even more critical for the sustainability of the healthcare system and the well-being of the population it serves.
Gastrointestinal healthcare expenditures totaled $119.6 billion in 2018. Annually there were more than 36.8 million ambulatory visits for GI symptoms and 43.4 million ambulatory visits with primary GI diagnosis.4 The use of higher-acuity settings and lack of continuity of care, and the under-recognition and lack of longitudinal framework to follow those families at risk continue to compromise our healthcare system.
Dr. McCutchen is a gastroenterologist at United Digestive, Atlanta, Georgia. She is vice chair of the AGA Research Foundation. Dr. Boules is vice president of global medical and scientific affairs at Ironwood Pharmaceuticals, Cleveland, Ohio.
References
1. www.govinfo.gov/content/pkg/FR-2022-08-10/pdf/2022-16472.pdf.
2. Shields HM et al. Disparities in evaluation of patients with rectal bleeding 40 years and older. Clin Gastroenterol Hepatol. 2014 Apr. doi: 10.1016/j.cgh.2013.07.008.
3. Quiros JA et al. Insurance type influences access to biologics and healthcare utilization in pediatric Crohn’s disease. Crohns Colitis 360. 2021 Aug. doi: 10.1093/crocol/otab057.
4. Peery AF et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022 Feb. doi: 10.1053/j.gastro.2021.10.017.
A case for when, how, and why to evaluate capacity
Case
Ms. F. is a 68-year-old woman who presented to the hospital with sepsis, developed delirium, and stopped eating. Her clinicians recommended a PEG tube. Although she was inconsistently oriented to self, time, and place, she reiterated the same decision across multiple discussions: She did not want the PEG tube. Her replies to what would happen if she didn’t have the procedure and continued not to eat were consistent, too: “I’ll wither away.”
Ms. F. had impaired cognition. Do these impairments mean her clinicians should over-rule her choice? What evidence indicates whether she lacks decision-making capacity? This case of a patient refusing a potentially life-saving procedure amplifies the importance of asking these questions and integrating capacity assessments into clinical care. In this article, we will describe what capacity is, when and how to assess it, and the alternatives when a patient does not have capacity.
The ethical background
Before starting a medical treatment or procedure, a physician must obtain the patient’s informed consent. This is a core ethic of medicine. Informed consent describes the voluntary decision made by a competent patient following the disclosure of necessary information. Informed consent is key to achieving a balance between promoting patient self-determination and protecting vulnerable patients from harm. In most clinical encounters, informed consent unfolds effortlessly. However, in the care of patients who are acutely ill, particularly those in hospitals, fulfilling the ethic can be challenging.
It is important to have skills to recognize and address these challenges. One of the most common challenges to practicing the ethic of informed consent is the impact of illness on a person’s decision-making capacity. A patient who retains capacity ought to make his or her decisions and does not need someone else (a friend or a family member) to help with the decision.
Incapacity is unfortunately common among the acutely ill medical inpatient population, which typically skews older with more comorbidities.1 Impairments frequently are overlooked for a variety of reasons,2-6 including that many hospitalized patients do not challenge their doctors’ decisions. Doctors may be reluctant to assess capacity because the assessment may medically, legally, or ethically complicate the patient’s care.
Two common terms describe the outcome of an assessment of a patient’s decision-making abilities: competency and capacity. Competency describes a legal principle. It is granted or withdrawn by judicial review. The consequences of a judge rescinding competency are severe: A patient would need a guardian to make choices on his or her behalf.
Capacity, on the other hand, is a clinical concept. A physician assesses whether the patient can make a specific decision in a specific context. The difference between the two terms – competency and capacity – delineates what are the consequences of the assessment and which authority, a judge or a physician, has the right to withdraw a person’s decision-making authority.
The judge offers a global assessment that can lead to a guardianship. The physician’s decision is temporal and situational. Patients can lack capacity when they are ill and recover it when they are healed. Capacity is specific to each medical decision that the patient makes and so a person can lack capacity to make some decisions but not others.
Ethical framework to make assessment
Capacity is described by four decisional abilities: 1) communicate a choice, 2) understand relevant information, 3) appreciation, and 4) reasoning.7
Communication of a choice may be verbal or nonverbal, but the patient must be able to indicate the treatment choice clearly and consistently. Understanding describes knowing essential information a physician has conveyed. This is assessed by having the patients say back what they were told, such as: “Can you tell me in your own words what is a PEG tube?”
The components of appreciation are: the diagnosis or disorder and the benefits and risks of the proposed intervention as it relates to the diagnosis or disorder. Patients who appreciate their disorder have insight into their condition: “I’m not eating because I have an infection.” This can be assessed with a question such as: “Can you tell me in your own words what are the risks or downsides to you?” This prompt assesses the patient’s appreciation of risk. Reframing the question to ask, “Can you tell me about the upsides of this intervention?” will assess the patient’s appreciation of benefit.
Reasoning assesses the thought process and rationale for a person’s decision. It has two components – comparative and consequential reasoning. The first compares the different choices presented about the proposed intervention: “How does having a PEG tube compare to not having it?” The second asks about the consequences of each choice: “What might happen to a person who has the PEG tube?”
The capacity assessment evaluates a patient's performance on these decision-making abilities. This informs the clinician’s judgment of whether the patient has the capacity to make a decision. A patient who has capacity makes the choice, regardless of the physician’s preference or recommendation.
The physician’s duty is to decide which decision-making abilities to assess. Choice and understanding are essential. In riskier or more consequential decisions, a physician may raise the rigor of the assessment to include appreciation and reasoning.8 It is common practice for physicians to raise the standard for when to evaluate and how extensive their evaluation is when the decision is life-altering, as with a PEG tube versus a more routine, non–life-altering decision such as drawing blood for a routine wellness visit.
A simple scoring rubric determines the patient’s ability to answer each question along a range from adequate = 2, marginal = 1, to inadequate = 0. The extremes or adequate or inadequate are straightforward. Judgment is needed when performance is marginal. In the case of repeated marginal answers, a physician must strongly consider whether the patient lacks capacity to make the decision in question.9
Who receives a capacity assessment and when?
A good doctor is a good teacher. A doctor should therefore check that patients understand what is happening with their health. Assessing understanding is simply good medicine; for example, a good teacher ought to be asking an unimpaired patient without impaired cognition, “Can you say back to me the key points of what I explained?” With this approach, every patient is effectively “screened” for a capacity impairment.
Certain patients ought to trigger a more thorough examination of decisional abilities. Across multiple articles, the strongest factors associated with incapacity are older age and diminished cognitive function (often detected by MMSE scores below the low 20s).1,7,10 Other factors that may amplify these deficits and thus should raise clinician concern would be patients with brain diseases such as Alzheimer’s or Parkinson’s, persons with lower education levels, or those who already have someone who helps them make decisions. To be sure, many older adults, even those with cognitive impairments, retain capacity, but extra protection should be in place to ensure their well-being.
Consequences of incapacity
If a careful assessment shows a patient has sound decision-making abilities, the patient is free to make the choice. On the other hand, a person does not have the capacity to make the decision at hand if he or she cannot communicate a choice or understand relevant information. Whether appreciation or reasoning ought to be assessed depends on the complexity and the significance of the decision. An assessment of decisional ability is not the end of the decision-making process. The goal is to maximize the patient’s autonomy.
Capacity can change over time. Factors that may inhibit capacity, such as medications, time of day, and even illness acuity, need to be accounted for and, if possible, addressed. The decision ought to be delayed, if possible, to a time when the patient has better chances of having capacity. If it is unlikely that patients’ status will change in the time frame needed to make the choice and they are found to not have capacity, then the decision making can be aided by advance directives or substitute decision makers such as family members or legal guardians.
Revisiting the case
Ms. F., who was delirious, retained notable decisional abilities. She understood the procedure of receiving the PEG tube and how the risk of continuing to not eat and not receive the PEG would result in dying by starvation. She appreciated her own diagnosis and how the proposed intervention could alter her condition. She appreciated how not having a PEG would lead to her death. Her choice to refuse the procedure was consistent. Ms. F. showed she retained capacity to make this decision. It was the physician’s duty to respect her autonomy and so to respect her refusal of the PEG.
Dr. Ney is a physician resident, department of psychiatry and human behavior, Thomas Jefferson University Hospital, Philadelphia. He has no conflicts to disclose. Dr. Karlawish is a professor in the departments of medicine, medical ethics and health policy, and neurology, University of Pennsylvania, Philadelphia. He is a site investigator for clinical trials sponsored by Biogen, Eisai, and Lilly.
References
1. Raymont V et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364(9443):1421-7. doi: 10.1016/S0140-6736(04)17224-3.
2. Hanson M and Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60(1):69-70. doi: 10.1503/cjs.004816.
3. Dahlberg J et al. Lack of informed consent for surgical procedures by elderly patients with inability to consent: A retrospective chart review from an academic medical center in Norway. Patient Saf Surg. 2019;13:24. doi: 10.1186/s13037-019-0205-5.
4. Sessums LL et al. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-7. doi: 10.1001/jama.2011.1023.
5. Terranova C et al. Ethical and medicolegal implications of capacity of patients in geriatric surgery. Med Sci Law. 2013;53(3):166-71. doi: 10.1177/0025802412473963.
6. John S et al. Assessing patients decision-making capacity in the hospital setting: A literature review. Aust J Rural Health. 2020;28(2):141-8. doi: 10.1111/ajr.12592.
7. Kim SYH et al. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47(4):325-9. doi: 10.1176/appi.psy.47.4.325.
8. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-40. doi: 10.1056/NEJMcp074045.
9. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16(1):91-8. doi: 10.1159/000109763.
10. Christensen K et al. Decision-making capacity for informed consent in the older population. Bull Am Acad Psychiatry Law. 1995;23(3):353-65.
Case
Ms. F. is a 68-year-old woman who presented to the hospital with sepsis, developed delirium, and stopped eating. Her clinicians recommended a PEG tube. Although she was inconsistently oriented to self, time, and place, she reiterated the same decision across multiple discussions: She did not want the PEG tube. Her replies to what would happen if she didn’t have the procedure and continued not to eat were consistent, too: “I’ll wither away.”
Ms. F. had impaired cognition. Do these impairments mean her clinicians should over-rule her choice? What evidence indicates whether she lacks decision-making capacity? This case of a patient refusing a potentially life-saving procedure amplifies the importance of asking these questions and integrating capacity assessments into clinical care. In this article, we will describe what capacity is, when and how to assess it, and the alternatives when a patient does not have capacity.
The ethical background
Before starting a medical treatment or procedure, a physician must obtain the patient’s informed consent. This is a core ethic of medicine. Informed consent describes the voluntary decision made by a competent patient following the disclosure of necessary information. Informed consent is key to achieving a balance between promoting patient self-determination and protecting vulnerable patients from harm. In most clinical encounters, informed consent unfolds effortlessly. However, in the care of patients who are acutely ill, particularly those in hospitals, fulfilling the ethic can be challenging.
It is important to have skills to recognize and address these challenges. One of the most common challenges to practicing the ethic of informed consent is the impact of illness on a person’s decision-making capacity. A patient who retains capacity ought to make his or her decisions and does not need someone else (a friend or a family member) to help with the decision.
Incapacity is unfortunately common among the acutely ill medical inpatient population, which typically skews older with more comorbidities.1 Impairments frequently are overlooked for a variety of reasons,2-6 including that many hospitalized patients do not challenge their doctors’ decisions. Doctors may be reluctant to assess capacity because the assessment may medically, legally, or ethically complicate the patient’s care.
Two common terms describe the outcome of an assessment of a patient’s decision-making abilities: competency and capacity. Competency describes a legal principle. It is granted or withdrawn by judicial review. The consequences of a judge rescinding competency are severe: A patient would need a guardian to make choices on his or her behalf.
Capacity, on the other hand, is a clinical concept. A physician assesses whether the patient can make a specific decision in a specific context. The difference between the two terms – competency and capacity – delineates what are the consequences of the assessment and which authority, a judge or a physician, has the right to withdraw a person’s decision-making authority.
The judge offers a global assessment that can lead to a guardianship. The physician’s decision is temporal and situational. Patients can lack capacity when they are ill and recover it when they are healed. Capacity is specific to each medical decision that the patient makes and so a person can lack capacity to make some decisions but not others.
Ethical framework to make assessment
Capacity is described by four decisional abilities: 1) communicate a choice, 2) understand relevant information, 3) appreciation, and 4) reasoning.7
Communication of a choice may be verbal or nonverbal, but the patient must be able to indicate the treatment choice clearly and consistently. Understanding describes knowing essential information a physician has conveyed. This is assessed by having the patients say back what they were told, such as: “Can you tell me in your own words what is a PEG tube?”
The components of appreciation are: the diagnosis or disorder and the benefits and risks of the proposed intervention as it relates to the diagnosis or disorder. Patients who appreciate their disorder have insight into their condition: “I’m not eating because I have an infection.” This can be assessed with a question such as: “Can you tell me in your own words what are the risks or downsides to you?” This prompt assesses the patient’s appreciation of risk. Reframing the question to ask, “Can you tell me about the upsides of this intervention?” will assess the patient’s appreciation of benefit.
Reasoning assesses the thought process and rationale for a person’s decision. It has two components – comparative and consequential reasoning. The first compares the different choices presented about the proposed intervention: “How does having a PEG tube compare to not having it?” The second asks about the consequences of each choice: “What might happen to a person who has the PEG tube?”
The capacity assessment evaluates a patient's performance on these decision-making abilities. This informs the clinician’s judgment of whether the patient has the capacity to make a decision. A patient who has capacity makes the choice, regardless of the physician’s preference or recommendation.
The physician’s duty is to decide which decision-making abilities to assess. Choice and understanding are essential. In riskier or more consequential decisions, a physician may raise the rigor of the assessment to include appreciation and reasoning.8 It is common practice for physicians to raise the standard for when to evaluate and how extensive their evaluation is when the decision is life-altering, as with a PEG tube versus a more routine, non–life-altering decision such as drawing blood for a routine wellness visit.
A simple scoring rubric determines the patient’s ability to answer each question along a range from adequate = 2, marginal = 1, to inadequate = 0. The extremes or adequate or inadequate are straightforward. Judgment is needed when performance is marginal. In the case of repeated marginal answers, a physician must strongly consider whether the patient lacks capacity to make the decision in question.9
Who receives a capacity assessment and when?
A good doctor is a good teacher. A doctor should therefore check that patients understand what is happening with their health. Assessing understanding is simply good medicine; for example, a good teacher ought to be asking an unimpaired patient without impaired cognition, “Can you say back to me the key points of what I explained?” With this approach, every patient is effectively “screened” for a capacity impairment.
Certain patients ought to trigger a more thorough examination of decisional abilities. Across multiple articles, the strongest factors associated with incapacity are older age and diminished cognitive function (often detected by MMSE scores below the low 20s).1,7,10 Other factors that may amplify these deficits and thus should raise clinician concern would be patients with brain diseases such as Alzheimer’s or Parkinson’s, persons with lower education levels, or those who already have someone who helps them make decisions. To be sure, many older adults, even those with cognitive impairments, retain capacity, but extra protection should be in place to ensure their well-being.
Consequences of incapacity
If a careful assessment shows a patient has sound decision-making abilities, the patient is free to make the choice. On the other hand, a person does not have the capacity to make the decision at hand if he or she cannot communicate a choice or understand relevant information. Whether appreciation or reasoning ought to be assessed depends on the complexity and the significance of the decision. An assessment of decisional ability is not the end of the decision-making process. The goal is to maximize the patient’s autonomy.
Capacity can change over time. Factors that may inhibit capacity, such as medications, time of day, and even illness acuity, need to be accounted for and, if possible, addressed. The decision ought to be delayed, if possible, to a time when the patient has better chances of having capacity. If it is unlikely that patients’ status will change in the time frame needed to make the choice and they are found to not have capacity, then the decision making can be aided by advance directives or substitute decision makers such as family members or legal guardians.
Revisiting the case
Ms. F., who was delirious, retained notable decisional abilities. She understood the procedure of receiving the PEG tube and how the risk of continuing to not eat and not receive the PEG would result in dying by starvation. She appreciated her own diagnosis and how the proposed intervention could alter her condition. She appreciated how not having a PEG would lead to her death. Her choice to refuse the procedure was consistent. Ms. F. showed she retained capacity to make this decision. It was the physician’s duty to respect her autonomy and so to respect her refusal of the PEG.
Dr. Ney is a physician resident, department of psychiatry and human behavior, Thomas Jefferson University Hospital, Philadelphia. He has no conflicts to disclose. Dr. Karlawish is a professor in the departments of medicine, medical ethics and health policy, and neurology, University of Pennsylvania, Philadelphia. He is a site investigator for clinical trials sponsored by Biogen, Eisai, and Lilly.
References
1. Raymont V et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364(9443):1421-7. doi: 10.1016/S0140-6736(04)17224-3.
2. Hanson M and Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60(1):69-70. doi: 10.1503/cjs.004816.
3. Dahlberg J et al. Lack of informed consent for surgical procedures by elderly patients with inability to consent: A retrospective chart review from an academic medical center in Norway. Patient Saf Surg. 2019;13:24. doi: 10.1186/s13037-019-0205-5.
4. Sessums LL et al. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-7. doi: 10.1001/jama.2011.1023.
5. Terranova C et al. Ethical and medicolegal implications of capacity of patients in geriatric surgery. Med Sci Law. 2013;53(3):166-71. doi: 10.1177/0025802412473963.
6. John S et al. Assessing patients decision-making capacity in the hospital setting: A literature review. Aust J Rural Health. 2020;28(2):141-8. doi: 10.1111/ajr.12592.
7. Kim SYH et al. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47(4):325-9. doi: 10.1176/appi.psy.47.4.325.
8. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-40. doi: 10.1056/NEJMcp074045.
9. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16(1):91-8. doi: 10.1159/000109763.
10. Christensen K et al. Decision-making capacity for informed consent in the older population. Bull Am Acad Psychiatry Law. 1995;23(3):353-65.
Case
Ms. F. is a 68-year-old woman who presented to the hospital with sepsis, developed delirium, and stopped eating. Her clinicians recommended a PEG tube. Although she was inconsistently oriented to self, time, and place, she reiterated the same decision across multiple discussions: She did not want the PEG tube. Her replies to what would happen if she didn’t have the procedure and continued not to eat were consistent, too: “I’ll wither away.”
Ms. F. had impaired cognition. Do these impairments mean her clinicians should over-rule her choice? What evidence indicates whether she lacks decision-making capacity? This case of a patient refusing a potentially life-saving procedure amplifies the importance of asking these questions and integrating capacity assessments into clinical care. In this article, we will describe what capacity is, when and how to assess it, and the alternatives when a patient does not have capacity.
The ethical background
Before starting a medical treatment or procedure, a physician must obtain the patient’s informed consent. This is a core ethic of medicine. Informed consent describes the voluntary decision made by a competent patient following the disclosure of necessary information. Informed consent is key to achieving a balance between promoting patient self-determination and protecting vulnerable patients from harm. In most clinical encounters, informed consent unfolds effortlessly. However, in the care of patients who are acutely ill, particularly those in hospitals, fulfilling the ethic can be challenging.
It is important to have skills to recognize and address these challenges. One of the most common challenges to practicing the ethic of informed consent is the impact of illness on a person’s decision-making capacity. A patient who retains capacity ought to make his or her decisions and does not need someone else (a friend or a family member) to help with the decision.
Incapacity is unfortunately common among the acutely ill medical inpatient population, which typically skews older with more comorbidities.1 Impairments frequently are overlooked for a variety of reasons,2-6 including that many hospitalized patients do not challenge their doctors’ decisions. Doctors may be reluctant to assess capacity because the assessment may medically, legally, or ethically complicate the patient’s care.
Two common terms describe the outcome of an assessment of a patient’s decision-making abilities: competency and capacity. Competency describes a legal principle. It is granted or withdrawn by judicial review. The consequences of a judge rescinding competency are severe: A patient would need a guardian to make choices on his or her behalf.
Capacity, on the other hand, is a clinical concept. A physician assesses whether the patient can make a specific decision in a specific context. The difference between the two terms – competency and capacity – delineates what are the consequences of the assessment and which authority, a judge or a physician, has the right to withdraw a person’s decision-making authority.
The judge offers a global assessment that can lead to a guardianship. The physician’s decision is temporal and situational. Patients can lack capacity when they are ill and recover it when they are healed. Capacity is specific to each medical decision that the patient makes and so a person can lack capacity to make some decisions but not others.
Ethical framework to make assessment
Capacity is described by four decisional abilities: 1) communicate a choice, 2) understand relevant information, 3) appreciation, and 4) reasoning.7
Communication of a choice may be verbal or nonverbal, but the patient must be able to indicate the treatment choice clearly and consistently. Understanding describes knowing essential information a physician has conveyed. This is assessed by having the patients say back what they were told, such as: “Can you tell me in your own words what is a PEG tube?”
The components of appreciation are: the diagnosis or disorder and the benefits and risks of the proposed intervention as it relates to the diagnosis or disorder. Patients who appreciate their disorder have insight into their condition: “I’m not eating because I have an infection.” This can be assessed with a question such as: “Can you tell me in your own words what are the risks or downsides to you?” This prompt assesses the patient’s appreciation of risk. Reframing the question to ask, “Can you tell me about the upsides of this intervention?” will assess the patient’s appreciation of benefit.
Reasoning assesses the thought process and rationale for a person’s decision. It has two components – comparative and consequential reasoning. The first compares the different choices presented about the proposed intervention: “How does having a PEG tube compare to not having it?” The second asks about the consequences of each choice: “What might happen to a person who has the PEG tube?”
The capacity assessment evaluates a patient's performance on these decision-making abilities. This informs the clinician’s judgment of whether the patient has the capacity to make a decision. A patient who has capacity makes the choice, regardless of the physician’s preference or recommendation.
The physician’s duty is to decide which decision-making abilities to assess. Choice and understanding are essential. In riskier or more consequential decisions, a physician may raise the rigor of the assessment to include appreciation and reasoning.8 It is common practice for physicians to raise the standard for when to evaluate and how extensive their evaluation is when the decision is life-altering, as with a PEG tube versus a more routine, non–life-altering decision such as drawing blood for a routine wellness visit.
A simple scoring rubric determines the patient’s ability to answer each question along a range from adequate = 2, marginal = 1, to inadequate = 0. The extremes or adequate or inadequate are straightforward. Judgment is needed when performance is marginal. In the case of repeated marginal answers, a physician must strongly consider whether the patient lacks capacity to make the decision in question.9
Who receives a capacity assessment and when?
A good doctor is a good teacher. A doctor should therefore check that patients understand what is happening with their health. Assessing understanding is simply good medicine; for example, a good teacher ought to be asking an unimpaired patient without impaired cognition, “Can you say back to me the key points of what I explained?” With this approach, every patient is effectively “screened” for a capacity impairment.
Certain patients ought to trigger a more thorough examination of decisional abilities. Across multiple articles, the strongest factors associated with incapacity are older age and diminished cognitive function (often detected by MMSE scores below the low 20s).1,7,10 Other factors that may amplify these deficits and thus should raise clinician concern would be patients with brain diseases such as Alzheimer’s or Parkinson’s, persons with lower education levels, or those who already have someone who helps them make decisions. To be sure, many older adults, even those with cognitive impairments, retain capacity, but extra protection should be in place to ensure their well-being.
Consequences of incapacity
If a careful assessment shows a patient has sound decision-making abilities, the patient is free to make the choice. On the other hand, a person does not have the capacity to make the decision at hand if he or she cannot communicate a choice or understand relevant information. Whether appreciation or reasoning ought to be assessed depends on the complexity and the significance of the decision. An assessment of decisional ability is not the end of the decision-making process. The goal is to maximize the patient’s autonomy.
Capacity can change over time. Factors that may inhibit capacity, such as medications, time of day, and even illness acuity, need to be accounted for and, if possible, addressed. The decision ought to be delayed, if possible, to a time when the patient has better chances of having capacity. If it is unlikely that patients’ status will change in the time frame needed to make the choice and they are found to not have capacity, then the decision making can be aided by advance directives or substitute decision makers such as family members or legal guardians.
Revisiting the case
Ms. F., who was delirious, retained notable decisional abilities. She understood the procedure of receiving the PEG tube and how the risk of continuing to not eat and not receive the PEG would result in dying by starvation. She appreciated her own diagnosis and how the proposed intervention could alter her condition. She appreciated how not having a PEG would lead to her death. Her choice to refuse the procedure was consistent. Ms. F. showed she retained capacity to make this decision. It was the physician’s duty to respect her autonomy and so to respect her refusal of the PEG.
Dr. Ney is a physician resident, department of psychiatry and human behavior, Thomas Jefferson University Hospital, Philadelphia. He has no conflicts to disclose. Dr. Karlawish is a professor in the departments of medicine, medical ethics and health policy, and neurology, University of Pennsylvania, Philadelphia. He is a site investigator for clinical trials sponsored by Biogen, Eisai, and Lilly.
References
1. Raymont V et al. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364(9443):1421-7. doi: 10.1016/S0140-6736(04)17224-3.
2. Hanson M and Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60(1):69-70. doi: 10.1503/cjs.004816.
3. Dahlberg J et al. Lack of informed consent for surgical procedures by elderly patients with inability to consent: A retrospective chart review from an academic medical center in Norway. Patient Saf Surg. 2019;13:24. doi: 10.1186/s13037-019-0205-5.
4. Sessums LL et al. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-7. doi: 10.1001/jama.2011.1023.
5. Terranova C et al. Ethical and medicolegal implications of capacity of patients in geriatric surgery. Med Sci Law. 2013;53(3):166-71. doi: 10.1177/0025802412473963.
6. John S et al. Assessing patients decision-making capacity in the hospital setting: A literature review. Aust J Rural Health. 2020;28(2):141-8. doi: 10.1111/ajr.12592.
7. Kim SYH et al. Do clinicians follow a risk-sensitive model of capacity-determination? An experimental video survey. Psychosomatics. 2006;47(4):325-9. doi: 10.1176/appi.psy.47.4.325.
8. Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-40. doi: 10.1056/NEJMcp074045.
9. Karlawish J. Measuring decision-making capacity in cognitively impaired individuals. Neurosignals. 2008;16(1):91-8. doi: 10.1159/000109763.
10. Christensen K et al. Decision-making capacity for informed consent in the older population. Bull Am Acad Psychiatry Law. 1995;23(3):353-65.
Repeat endoscopy for deliberate foreign body ingestions
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
The central role of informed consent in novel procedures
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Mrs. Jones is a 44-year-old woman who has struggled with her weight. She has a body mass index (BMI) of 35 kg/m2 and hypertension requiring daily medication. She has tried various diets over the years and has never been able to exercise consistently. She desperately wants to lose weight to improve her confidence and to avoid developing diabetes and dialysis that her parents required. She has considered weight loss surgery but is afraid after her best friend died following uterine fibroid surgery. She saw a billboard that advertised a new weight loss procedure without surgery. She looked up the procedure, found Dr. Indo on the university medical center’s website, and booked an appointment. Dr. Indo talked about performing an incisionless procedure done with an endoscope through her mouth. It would make her stomach into a tube to reduce the amount of food she could eat as well as prevent some absorption of food in her intestines. When Mrs. Jones asked how many of these the doctor had performed, Dr. Indo remarked she personally had done “several” in the past few years including training. Dr. Indo reassured Mrs. Jones that the procedure has been performed hundreds of times around the country and has been shown to be safe. Dr. Indo also explained that studies were still ongoing, including possibly at the university medical center, but that she had never personally seen any serious complications or death, and only one patient she knew of converted to a traditional bariatric surgery.
Obesity is a large international public health problem, with the World Health Organization estimating that there are 600 million obese adults worldwide.1 Bariatric surgery has been an effective way to improve complications related to obesity and quality of life. Endoscopic approaches to bariatric surgery have appeared since at least the late 1980s and, similarly to their traditional surgical counterparts, work in two main categories: restrictive or malabsorptive.1 Restrictive endoscopic bariatric therapies (EBTs) include intragastric balloons (IGB) that are filled with saline or gas to decrease intragastric luminal size, endoscopic sleeve gastroplasty that makes full-thickness plications of the gastric wall to tubularize the stomach like a sleeve gastrectomy, and AspireAssist where patients use a percutaneous gastrostomy to remove part of an ingested meal.1 Malabsorptive procedures include bypass sleeves that use a stentlike device to bypass absorption of food in the duodenum and proximal jejunum, the incisionless magnetic anastomosis system (IMAS) that creates a gastrojejunal bypass for diverting absorption, and duodenal mucosal resurfacing (DMR) that ablates the duodenal mucosa.1,2
The benefits of EBTs over traditional bariatric surgery are that they have a lower risk profile, there is limited anatomic alternation, and they are potentially reversible.1 Although no formal guidelines exist in the United States for the use of EBTs, the American Society for Gastrointestinal Endoscopy (ASGE) preliminary recommendations describe EBTs as applicable for patients who have failed lifestyle interventions and have BMIs between 30 and 45.1 While some of these techniques were first described in the 1980s, many individual companies and devices still do not have Food and Drug Administration approval and some have even had approval withdrawn. While traditional bariatric surgery may have complication rates up to 17%, EBTs are not without complications.1 Endoscopic barriers can migrate and occlude, cause pancreatitis, cause liver abscesses from biliary occlusion, and more severely cause GI bleeding and perforations.1 Many EBTs are also temporary treatments with IGBs and barrier bypasses placed only for 6-12 months.1 While there have been some studies looking at individual outcomes of the various EBTs, large prospective research trials looking at safety and efficacy, especially when comparing EBT to traditional bariatric surgery or in combination, are lacking.
Continued innovation in medicine and technology is critical to improving patient care. New innovations in medicine have allowed us to treat more disease, save lives, reduce complications, and better care for patients. But what exactly is innovation and when does it become research? The landmark Belmont Report in 1979 distinguishes research from innovative therapy, calling research “an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge.”3 Patients in research thus bear the risks while others stand to benefit. The report affirms then that routine medical practice involves interventions designed specifically to benefit the individual patient. The European Association for Endoscopic Surgery defines innovations as any “significant modification of a standard technique, a new application of or new indication for an established technique, or an alternative combination of an established technique with another therapeutic modality.”4 As such, innovations should eventually be formally studied with institutional review board (IRB) approval and protocols to establish safety and efficacy. Another complicating factor is that there is no FDA approval for surgical and procedural techniques as there is for medications and certain devices. Therefore, no robust regulatory mechanisms exist to ensure patient safety and benefit. Further complicating matters is that innovative procedures often start as modifications of techniques and are often done regularly to fit specific situations – for example, an additional stitch in a different location or in a different orientation to what is done in the standard fashion. However, true innovations should be distinguished from these modifications. Perhaps then another way to think about the two is to splinter them into three types of activity: research, routine accepted practice, and innovative medicine.5
Given this potential for blurred lines about novel approaches to medical conditions, how do we communicate this to patients? This is where the role of informed consent becomes essential. Informed consent is key to respecting patients’ autonomy – a central tenet of medical ethics. For patients to make autonomous choices they need basic facts to make informed decisions.6 These facts must be unbiased and free from conflicts, and they must not only be truthful but also be comprehensive and free from omission. It is in this informed consent process that we must explain that a technique or procedure is new, outline the risks and benefits, and share our actual experiences with said procedure especially if it is limited.7 We must also be aware of how certain biases and conflicts can affect our decisions to adapt and recommend innovative therapies. We may have incentives to offer innovative therapies to be on the “cutting edge” and attract patients. We may have explicit financial gain if working directly with device manufacturers or reimbursed by our institutions per procedure. Conflicts of interest are not only financial, but they can also be the prospects of promotion or career advancement.3 Institutions as well are incentivized to advertise the “latest” to bolster their prestige and reputations. Ultimately, we should act to the highest levels of professionalism, and ethics, by ignoring benefit to ourselves as physicians and always focusing on the benefits for our patients.7
What about when patients ask for specific innovative procedures as Mrs. Jones did above? What is our responsibility then? In situations where patients specifically push for a new procedure, it remains our duty to inform patients about the novelty of the procedure and the limited study of its safety and efficacy. When speaking about the “experience” with a novel procedure, it is tempting to speak globally and broadly. For example, Dr. Indo spoke about the procedure being done hundreds of times across the country and being safe in this context. It is our duty to be transparent, disclose our own experiences, and consider our own skills when recommending a novel procedure.7 It should be noted that patients are a vulnerable population and many times at the mercy of our recommendations. We’ve often heard patients say “Whatever you say doc; You’re the doctor;” or “I’ll do what you think is best” when presented with treatment options. This is an incredible amount of power, and we must protect this trust patients place in us by clearly acknowledging the uncertainties of new procedures and placing their benefit over our own potential gain.
Dr. Williams is a general surgery resident at the University of Chicago and a fellow at the MacLean Center for clinical medical ethics. Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, vice chairman for ethics, professional development, and wellness, and chief of endocrine surgery, department of surgery, and the associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago. The authors have no conflicts to disclose.
References
1. Goyal H et al. Ther Adv Gastrointest Endosc. 2021;14. doi: 10.1177/2631774520984627.
2. Machytka E et al. Gastrointestinal Endosc. 2017;86(5):904-12. doi: 10.1016/j.gie.2017.07.009.
3. Eastwood GL. J Gastroenterol Hepatol (Australia). 2015;30(S1):8-11. doi: 10.1111/jgh.12755.
4. Neugebauer EAM et al. Surg Endosc. 2010;24(7):1594-1615. doi: 10.1007/s00464-009-0818-3.
5. Eaton, ML and Kennedy, DL. Innovation in Medical Technology: Ethical Issues and Challenges. Baltimore: Johns Hopkins University Press, 2007.
6. Angelos P. Ann Thorac Surg. 2019;108(6):1611-2. doi: 10.1016/j.athoracsur.2019.08.010.
7. Angelos P. Virtual Mentor. 2011;13(1):6-9. doi: 10.1001/virtualmentor.2011.13.1.ccas1-1101.
Sharing notes with our patients: Ethical considerations
Even a decade ago, the idea of providers sharing clinical notes with patients was almost unfathomable to most in medicine. We have since seen a sea change regarding the need for transparency in health care, leading to dramatic legislative and policy shifts in recent years.
On April 5, 2021, the federal program rule on Interoperability, Information Blocking, and ONC Health IT Certification took effect, which implemented a part of the bipartisan 21st Century Cures Act of 2016 requiring most of a patient’s electronic health information (EHI) be made easily accessible free of charge and “without delay.”1
Included in this defined set of EHI, known as the United States Core Data for Interoperability, are eight types of clinical notes that must be shared with patients, including: progress notes, history and physical notes, consultation notes, discharge summary notes, procedure notes, laboratory report narratives, imaging narratives, and pathology report narratives. Many clinicians viewed this federally mandated transition to note sharing with patients with concern, fearing increased documentation burdens, needless patient anxiety, and inevitable deluge of follow-up questions and requests for chart corrections.
In reality, the Health Insurance Portability and Accountability Act (HIPAA) granted virtually all patients the right to review a paper copy of their medical records, including all clinical notes, way back in 1996. Practically speaking, though, the multiple steps required to formally make these requests kept most patients from regularly accessing their health information.
The 21st Century Cures Act streamlines and modernizes this process by requiring electronic access. Certain note types, including psychotherapy notes, are exempt from this requirement. As has always been true since HIPAA was enacted, exceptions may be used for circumstances in which a clinician holds a reasonable belief that blocking information is necessary to prevent harm to a patient or another person or to protect an individual’s privacy. By continuing to allow for these exceptions, clinicians maintain the autonomy to block sharing of notes in the rare, complex situations in which doing so may truly be harmful.
And while the legal requirement to share most clinical notes is new, there is already a wealth of evidence from the earliest adopters (part of the OpenNotes movement) affirming the significant benefits from this practice – for patients and providers – with few negative effects on workflows or documentation patterns.2 Findings published as early as 2012, and regularly since then, among OpenNotes adopters from a diverse set of health care institutions have shown access to notes improves patient engagement, activation, and communication, as well as patient and clinician satisfaction.3
Still, providers may argue, shouldn’t clinical notes be a space where providers are free to articulate uncertainties, work through clinical reasoning, and share subtle observations about a patient’s presentation and findings with colleagues without having to worry about alarming patients who may lack the background to understand medical nuances?
It’s a fine balance in certain situations since we want to document our objective clinical assessments and prognoses without needlessly upsetting our patients, especially when considering a potentially life-changing diagnosis. How do we continue offering hope to our patients while still respecting their autonomy and sharing their health information with them? There is no uniform approach or standard playbook to follow since each patient and clinical circumstance is unique.
Fundamentally, sharing clinical notes is about granting access to one’s own health information, promoting patient activation and engagement, and making health care more patient centered. As a clinician, it’s important to frame the conversations we have with our patients so they are not surprised or caught off guard by what we have written in our notes. If you had a difficult or contentious conversation, document it objectively and without bias. If you are discussing obesity, substance abuse, or mental health, do so respectfully, supportively, and without judgment. If one of the reasons you are doing a CT scan is to rule out pancreatic cancer, it’s hard to argue that the patient does not deserve to know that beforehand.
The OpenNotes experience to date has consistently shown that patients benefit from direct discussions and transparency, which can even motivate difficult behavior changes.4 As clinicians, we may have to make minor changes in how we document, such as using less medical jargon and fewer abbreviations, but based on data from the longest tenured participants in OpenNotes, these adjustments do not add to documentation burdens.5 An activated patient who is reading their notes is an engaged patient, one who will often collaborate more in their own care, offer additional insights, and feel more empowered to take responsibility for their own health.6
When surveyed, patients report that access to their clinical notes helps them feel more in control of their health by understanding their medical conditions better, which makes them feel more prepared for their visits.4 Studies have shown that patients forget between 40%-80% of the information communicated during a visit, making clinical notes a valuable reminder and reference. Over 75% of patients in one study reported that reading notes helped them better understand the meaning of results and the rationale for referrals and tests, which led to greater follow-through with their treatment plans and follow-up appointments.3 A remarkable 99% of patients in the same study reported feeling the same or better about their physician after reading their notes.
Sharing notes with patients also makes care safer and more equitable. A written record of a visit serves as an important source of information about why a medicine is prescribed, a reminder about additions or changes to a regimen, and potential adverse effects of medications. In the first OpenNotes study, which had more than 100 primary care physicians and 20,000 patients, 60%-78% of patients with access to their notes reported improved medication adherence.2 A later study reported similar benefits, particularly among patients who identify as racial or ethnic minorities, non-native English speakers, and those with a high school education or less. These findings may reflect increased trust that comes with a more collaborative relationship between providers and patients. Patients who can read their notes also show a willingness to review their medication lists and report discrepancies and errors, making their care safer still.7
Conclusion
The move to widespread shared notes, though prompted by a federal mandate, is a critical step forward in patient activation, engagement, and satisfaction. Importantly, there is a large body of evidence showing multiple benefits, including better communication and safer and more equitable care at sites that have already been sharing notes for over a decade. When surveyed, both patients and providers who have been participating in shared notes believe the practice should continue.
In April 2021, we began a massive natural experiment in the U.S. with ubiquitous sharing of clinical notes, one that will help us learn more about how best to make our patients’ health information accessible, meaningful, and most meaningful in improving their overall health and well-being. Sharing notes with our patients is at once relatively easy to implement but complex in its implications and represents a significant paradigm shift in medicine toward a safer, more patient-centered approach. The evidence to date has shown that embracing shared notes promotes greater patient activation and engagement, and with it a more transparent and collaborative relationship between providers and patients that could lead to transformative benefits to the quality of the care we can achieve together.
Dr. Shah is an associate professor of medicine and pediatrics and associate chief medical information officer at University of Chicago Medicine. He has no disclosures
References
1. 21st Century Cures Act, HR 34, 114th Congress (2015). Accessed 2021 Sep 23. https://www.congress.gov/bill/114th-congress/house-bill/34.
2. Delbanco T et al. Ann Intern Med. 2012 Oct;157(7):461-70.
3. Bell S et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
4. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
5. DesRoches C et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Blease C et al. J Med Ethics. 2021 May. doi: 10.1136/medethics-2021-107275.
7. DesRoches C et al. Ann Intern Med. 2019 Jul 2;171(1):69-71.
Even a decade ago, the idea of providers sharing clinical notes with patients was almost unfathomable to most in medicine. We have since seen a sea change regarding the need for transparency in health care, leading to dramatic legislative and policy shifts in recent years.
On April 5, 2021, the federal program rule on Interoperability, Information Blocking, and ONC Health IT Certification took effect, which implemented a part of the bipartisan 21st Century Cures Act of 2016 requiring most of a patient’s electronic health information (EHI) be made easily accessible free of charge and “without delay.”1
Included in this defined set of EHI, known as the United States Core Data for Interoperability, are eight types of clinical notes that must be shared with patients, including: progress notes, history and physical notes, consultation notes, discharge summary notes, procedure notes, laboratory report narratives, imaging narratives, and pathology report narratives. Many clinicians viewed this federally mandated transition to note sharing with patients with concern, fearing increased documentation burdens, needless patient anxiety, and inevitable deluge of follow-up questions and requests for chart corrections.
In reality, the Health Insurance Portability and Accountability Act (HIPAA) granted virtually all patients the right to review a paper copy of their medical records, including all clinical notes, way back in 1996. Practically speaking, though, the multiple steps required to formally make these requests kept most patients from regularly accessing their health information.
The 21st Century Cures Act streamlines and modernizes this process by requiring electronic access. Certain note types, including psychotherapy notes, are exempt from this requirement. As has always been true since HIPAA was enacted, exceptions may be used for circumstances in which a clinician holds a reasonable belief that blocking information is necessary to prevent harm to a patient or another person or to protect an individual’s privacy. By continuing to allow for these exceptions, clinicians maintain the autonomy to block sharing of notes in the rare, complex situations in which doing so may truly be harmful.
And while the legal requirement to share most clinical notes is new, there is already a wealth of evidence from the earliest adopters (part of the OpenNotes movement) affirming the significant benefits from this practice – for patients and providers – with few negative effects on workflows or documentation patterns.2 Findings published as early as 2012, and regularly since then, among OpenNotes adopters from a diverse set of health care institutions have shown access to notes improves patient engagement, activation, and communication, as well as patient and clinician satisfaction.3
Still, providers may argue, shouldn’t clinical notes be a space where providers are free to articulate uncertainties, work through clinical reasoning, and share subtle observations about a patient’s presentation and findings with colleagues without having to worry about alarming patients who may lack the background to understand medical nuances?
It’s a fine balance in certain situations since we want to document our objective clinical assessments and prognoses without needlessly upsetting our patients, especially when considering a potentially life-changing diagnosis. How do we continue offering hope to our patients while still respecting their autonomy and sharing their health information with them? There is no uniform approach or standard playbook to follow since each patient and clinical circumstance is unique.
Fundamentally, sharing clinical notes is about granting access to one’s own health information, promoting patient activation and engagement, and making health care more patient centered. As a clinician, it’s important to frame the conversations we have with our patients so they are not surprised or caught off guard by what we have written in our notes. If you had a difficult or contentious conversation, document it objectively and without bias. If you are discussing obesity, substance abuse, or mental health, do so respectfully, supportively, and without judgment. If one of the reasons you are doing a CT scan is to rule out pancreatic cancer, it’s hard to argue that the patient does not deserve to know that beforehand.
The OpenNotes experience to date has consistently shown that patients benefit from direct discussions and transparency, which can even motivate difficult behavior changes.4 As clinicians, we may have to make minor changes in how we document, such as using less medical jargon and fewer abbreviations, but based on data from the longest tenured participants in OpenNotes, these adjustments do not add to documentation burdens.5 An activated patient who is reading their notes is an engaged patient, one who will often collaborate more in their own care, offer additional insights, and feel more empowered to take responsibility for their own health.6
When surveyed, patients report that access to their clinical notes helps them feel more in control of their health by understanding their medical conditions better, which makes them feel more prepared for their visits.4 Studies have shown that patients forget between 40%-80% of the information communicated during a visit, making clinical notes a valuable reminder and reference. Over 75% of patients in one study reported that reading notes helped them better understand the meaning of results and the rationale for referrals and tests, which led to greater follow-through with their treatment plans and follow-up appointments.3 A remarkable 99% of patients in the same study reported feeling the same or better about their physician after reading their notes.
Sharing notes with patients also makes care safer and more equitable. A written record of a visit serves as an important source of information about why a medicine is prescribed, a reminder about additions or changes to a regimen, and potential adverse effects of medications. In the first OpenNotes study, which had more than 100 primary care physicians and 20,000 patients, 60%-78% of patients with access to their notes reported improved medication adherence.2 A later study reported similar benefits, particularly among patients who identify as racial or ethnic minorities, non-native English speakers, and those with a high school education or less. These findings may reflect increased trust that comes with a more collaborative relationship between providers and patients. Patients who can read their notes also show a willingness to review their medication lists and report discrepancies and errors, making their care safer still.7
Conclusion
The move to widespread shared notes, though prompted by a federal mandate, is a critical step forward in patient activation, engagement, and satisfaction. Importantly, there is a large body of evidence showing multiple benefits, including better communication and safer and more equitable care at sites that have already been sharing notes for over a decade. When surveyed, both patients and providers who have been participating in shared notes believe the practice should continue.
In April 2021, we began a massive natural experiment in the U.S. with ubiquitous sharing of clinical notes, one that will help us learn more about how best to make our patients’ health information accessible, meaningful, and most meaningful in improving their overall health and well-being. Sharing notes with our patients is at once relatively easy to implement but complex in its implications and represents a significant paradigm shift in medicine toward a safer, more patient-centered approach. The evidence to date has shown that embracing shared notes promotes greater patient activation and engagement, and with it a more transparent and collaborative relationship between providers and patients that could lead to transformative benefits to the quality of the care we can achieve together.
Dr. Shah is an associate professor of medicine and pediatrics and associate chief medical information officer at University of Chicago Medicine. He has no disclosures
References
1. 21st Century Cures Act, HR 34, 114th Congress (2015). Accessed 2021 Sep 23. https://www.congress.gov/bill/114th-congress/house-bill/34.
2. Delbanco T et al. Ann Intern Med. 2012 Oct;157(7):461-70.
3. Bell S et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
4. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
5. DesRoches C et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Blease C et al. J Med Ethics. 2021 May. doi: 10.1136/medethics-2021-107275.
7. DesRoches C et al. Ann Intern Med. 2019 Jul 2;171(1):69-71.
Even a decade ago, the idea of providers sharing clinical notes with patients was almost unfathomable to most in medicine. We have since seen a sea change regarding the need for transparency in health care, leading to dramatic legislative and policy shifts in recent years.
On April 5, 2021, the federal program rule on Interoperability, Information Blocking, and ONC Health IT Certification took effect, which implemented a part of the bipartisan 21st Century Cures Act of 2016 requiring most of a patient’s electronic health information (EHI) be made easily accessible free of charge and “without delay.”1
Included in this defined set of EHI, known as the United States Core Data for Interoperability, are eight types of clinical notes that must be shared with patients, including: progress notes, history and physical notes, consultation notes, discharge summary notes, procedure notes, laboratory report narratives, imaging narratives, and pathology report narratives. Many clinicians viewed this federally mandated transition to note sharing with patients with concern, fearing increased documentation burdens, needless patient anxiety, and inevitable deluge of follow-up questions and requests for chart corrections.
In reality, the Health Insurance Portability and Accountability Act (HIPAA) granted virtually all patients the right to review a paper copy of their medical records, including all clinical notes, way back in 1996. Practically speaking, though, the multiple steps required to formally make these requests kept most patients from regularly accessing their health information.
The 21st Century Cures Act streamlines and modernizes this process by requiring electronic access. Certain note types, including psychotherapy notes, are exempt from this requirement. As has always been true since HIPAA was enacted, exceptions may be used for circumstances in which a clinician holds a reasonable belief that blocking information is necessary to prevent harm to a patient or another person or to protect an individual’s privacy. By continuing to allow for these exceptions, clinicians maintain the autonomy to block sharing of notes in the rare, complex situations in which doing so may truly be harmful.
And while the legal requirement to share most clinical notes is new, there is already a wealth of evidence from the earliest adopters (part of the OpenNotes movement) affirming the significant benefits from this practice – for patients and providers – with few negative effects on workflows or documentation patterns.2 Findings published as early as 2012, and regularly since then, among OpenNotes adopters from a diverse set of health care institutions have shown access to notes improves patient engagement, activation, and communication, as well as patient and clinician satisfaction.3
Still, providers may argue, shouldn’t clinical notes be a space where providers are free to articulate uncertainties, work through clinical reasoning, and share subtle observations about a patient’s presentation and findings with colleagues without having to worry about alarming patients who may lack the background to understand medical nuances?
It’s a fine balance in certain situations since we want to document our objective clinical assessments and prognoses without needlessly upsetting our patients, especially when considering a potentially life-changing diagnosis. How do we continue offering hope to our patients while still respecting their autonomy and sharing their health information with them? There is no uniform approach or standard playbook to follow since each patient and clinical circumstance is unique.
Fundamentally, sharing clinical notes is about granting access to one’s own health information, promoting patient activation and engagement, and making health care more patient centered. As a clinician, it’s important to frame the conversations we have with our patients so they are not surprised or caught off guard by what we have written in our notes. If you had a difficult or contentious conversation, document it objectively and without bias. If you are discussing obesity, substance abuse, or mental health, do so respectfully, supportively, and without judgment. If one of the reasons you are doing a CT scan is to rule out pancreatic cancer, it’s hard to argue that the patient does not deserve to know that beforehand.
The OpenNotes experience to date has consistently shown that patients benefit from direct discussions and transparency, which can even motivate difficult behavior changes.4 As clinicians, we may have to make minor changes in how we document, such as using less medical jargon and fewer abbreviations, but based on data from the longest tenured participants in OpenNotes, these adjustments do not add to documentation burdens.5 An activated patient who is reading their notes is an engaged patient, one who will often collaborate more in their own care, offer additional insights, and feel more empowered to take responsibility for their own health.6
When surveyed, patients report that access to their clinical notes helps them feel more in control of their health by understanding their medical conditions better, which makes them feel more prepared for their visits.4 Studies have shown that patients forget between 40%-80% of the information communicated during a visit, making clinical notes a valuable reminder and reference. Over 75% of patients in one study reported that reading notes helped them better understand the meaning of results and the rationale for referrals and tests, which led to greater follow-through with their treatment plans and follow-up appointments.3 A remarkable 99% of patients in the same study reported feeling the same or better about their physician after reading their notes.
Sharing notes with patients also makes care safer and more equitable. A written record of a visit serves as an important source of information about why a medicine is prescribed, a reminder about additions or changes to a regimen, and potential adverse effects of medications. In the first OpenNotes study, which had more than 100 primary care physicians and 20,000 patients, 60%-78% of patients with access to their notes reported improved medication adherence.2 A later study reported similar benefits, particularly among patients who identify as racial or ethnic minorities, non-native English speakers, and those with a high school education or less. These findings may reflect increased trust that comes with a more collaborative relationship between providers and patients. Patients who can read their notes also show a willingness to review their medication lists and report discrepancies and errors, making their care safer still.7
Conclusion
The move to widespread shared notes, though prompted by a federal mandate, is a critical step forward in patient activation, engagement, and satisfaction. Importantly, there is a large body of evidence showing multiple benefits, including better communication and safer and more equitable care at sites that have already been sharing notes for over a decade. When surveyed, both patients and providers who have been participating in shared notes believe the practice should continue.
In April 2021, we began a massive natural experiment in the U.S. with ubiquitous sharing of clinical notes, one that will help us learn more about how best to make our patients’ health information accessible, meaningful, and most meaningful in improving their overall health and well-being. Sharing notes with our patients is at once relatively easy to implement but complex in its implications and represents a significant paradigm shift in medicine toward a safer, more patient-centered approach. The evidence to date has shown that embracing shared notes promotes greater patient activation and engagement, and with it a more transparent and collaborative relationship between providers and patients that could lead to transformative benefits to the quality of the care we can achieve together.
Dr. Shah is an associate professor of medicine and pediatrics and associate chief medical information officer at University of Chicago Medicine. He has no disclosures
References
1. 21st Century Cures Act, HR 34, 114th Congress (2015). Accessed 2021 Sep 23. https://www.congress.gov/bill/114th-congress/house-bill/34.
2. Delbanco T et al. Ann Intern Med. 2012 Oct;157(7):461-70.
3. Bell S et al. BMJ Qual Saf. 2017 Apr;26(4):262-70.
4. Walker J et al. J Med Internet Res. 2019 May. doi: 10.2196/13876.
5. DesRoches C et al. JAMA Netw Open. 2020 Mar. doi: 10.1001/jamanetworkopen.2020.1753.
6. Blease C et al. J Med Ethics. 2021 May. doi: 10.1136/medethics-2021-107275.
7. DesRoches C et al. Ann Intern Med. 2019 Jul 2;171(1):69-71.
Coerced invasive procedures: Policy overriding indication in gastrostomy tube placement
Clinical scenario
An 83-year-old man is admitted with a hemiplegic cerebrovascular accident. He is found to have dysphagia, and a nasogastric feeding tube is placed. Over the next several days, his strength begins to recover, and he tolerates his tube feeding well. Discharge to a skilled nursing facility (SNF) for subacute rehabilitation is planned. His swallowing is showing signs of recovery; it has not recovered adequately but is expected to continue to improve such that he is predicted to be independent of tube feeding within 7-14 days. None of the facilities in the region are willing to admit a patient with a nasal feeding tube, despite the anticipated short duration. The patient is medically ready for discharge but is refusing the feeding gastrostomy. “Why would I want a hole in my stomach, if I’m only going to need it for 1-2 weeks and this tube in my nose is working fine and is comfortable?” he pleads with tears in his eyes.
Feeding dysphagic patients after stroke
Dysphagia, potentially leading to aspiration and/or pneumonia, is a common sequela of stroke – up to half of hospitalized patients are affected.1 When oral intake is contraindicated, patients are often fed by nasogastric tube (NGT) or by surgically or endoscopically placed gastrostomy tube (GT). Without good justification based on outcomes, NGTs are traditionally used when the need for feeding is thought to be short term (<4 weeks) and GTs are used for long term (>4 weeks). However, in 2005, a large multicenter randomized control trial found that the majority of stroke patients with dysphagia that would resolve had resolution within 2-3 weeks. Moreover, outcomes were equivalent or better for patients fed with an NGT versus GT.
The authors concluded by recommending feeding via NGT for 2-3 weeks, after which conversion to GT can be considered if dysphagia persists.1 Notably, the recommendation allows consideration, and no evidence-based guideline requires or recommends GT be placed based on duration of tube feed dependence. Currently, while nutrition and neurology authorities have adopted these recommendations,2,3 many authors have noted poor adherence to this guideline, and many find that the median period between stroke and GT placement is 7 days rather than the recommended minimum of 14.4,5,6 While ignorance can partially explain the lack of widespread compliance,6 the policies of posthospital facilities are another culprit. Increasingly, and for a variety of reasons unsupported by the literature, SNFs refuse NGT and require GT.4,7,8,9
Ethical considerations
The four principles of medical ethics – autonomy, beneficence, nonmaleficence, and justice – can guide clinicians, patients, and family members in decision-making. In our case, by withholding needed and desired treatment (discharge to and treatment by a rehabilitation facility) the patient is being coerced to undergo a procedure he does not want, and clinicians participate in denying him autonomy. Further, given that the evidence, national guidelines, and in fact federal regulations indicate that his preferences are congruent with best practices, pressuring him to accept gastrostomy placement runs afoul of the principles of beneficence and nonmaleficence. Though the mechanism is unclear, early gastrostomy (<14-21 days) is associated with increased risk of death, worse functional outcomes, and a lower rate of return to oral feeding, as well as a significant procedure-specific complication rate.1,10 By insisting on gastrostomy, we neither act in this patient’s best interests nor “do no harm.”
However, the medical system is complex. The clinician at the bedside can evaluate this scenario, review the national guidelines, discuss the procedure and risks with the patient and family, and conclude that the patient should be discharged with a nasal feeding tube. Nevertheless, if no facility is willing to accept him without a gastrostomy, our decision-making model – previously limited to our patient’s best interests alone – is forced to change. Despite our misgivings, we often conclude that the harm done by an early gastrostomy is outweighed by the harm of remaining unnecessarily in the acute hospital setting. We further worry about other patients lingering in the emergency department for lack of an inpatient bed and the possible – though unknowable – harm done to them.
Looking forward
It is an unfortunate fact that medical decision-making must often include factors unrelated to the patient’s best interests, with financial considerations and structural barriers frequently driving deviation from ideal care. Providers and patients navigate these decisions to their best abilities, making compromises when forced. However, with education and professional activism, providers can advocate for the elimination of barriers to providing medically sound and ethically appropriate care. In our experience, delay of gastrostomy placement, until discharge is imminent and planning for postdischarge care is initiated, has resulted in a decrease by half the fraction of patients with tracheostomies who had gastrostomies placed prior to discharge.11 With aggressive outreach and education, we now have nursing homes willing to accept patients with NGTs.
Criteria for admission to discharge facilities can drive medical decision-making that is unethical and unsupported by evidence. Continued efforts to eliminate barriers to appropriate and ethical care have been successful and are encouraged.
Dr. Cowan is administrative chief resident in the department of surgery at Columbia University Irving Medical Center, New York. Dr. Seres is professor of medicine in the Institute of Human Nutrition and associate clinical ethicist at Columbia University Irving Medical Center. The authors have no conflicts of interest to disclose.
References
1. Dennis MS et al. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72.
2. Powers W. et al. Stroke. 2018 Mar;49(3):e46-e110.
3. Burgos R et al. Clin Nutr. 2018 Feb;37(1):354-96.
4. Wilmskoetter J et al. J Stroke Cerebrovasc Dis. 2016 Nov;25(11):2694-700.
5. George BP et al. Stroke. 2017 Feb;48(2):420-7.
6. Fessler TA. et al. Surg Endosc. 2019 Dec;33(12):4089-97.
7. Burgermaster M et al. Nutr Clin Pract. 2016 Jun;31(3):342-8.
8. Moran C and O’Mahoney S. Curr Opin Gastroenterol. 2015 Mar;31(2):137-42.
9. Gomes CA et al. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008096.
10. Joundi RA et al. Neurology. 2018 Feb 13;90(7):e544-52.
11. Bothra A et al. J Parenter Enteral Nutr. 2018 Feb;42(2):491.
Clinical scenario
An 83-year-old man is admitted with a hemiplegic cerebrovascular accident. He is found to have dysphagia, and a nasogastric feeding tube is placed. Over the next several days, his strength begins to recover, and he tolerates his tube feeding well. Discharge to a skilled nursing facility (SNF) for subacute rehabilitation is planned. His swallowing is showing signs of recovery; it has not recovered adequately but is expected to continue to improve such that he is predicted to be independent of tube feeding within 7-14 days. None of the facilities in the region are willing to admit a patient with a nasal feeding tube, despite the anticipated short duration. The patient is medically ready for discharge but is refusing the feeding gastrostomy. “Why would I want a hole in my stomach, if I’m only going to need it for 1-2 weeks and this tube in my nose is working fine and is comfortable?” he pleads with tears in his eyes.
Feeding dysphagic patients after stroke
Dysphagia, potentially leading to aspiration and/or pneumonia, is a common sequela of stroke – up to half of hospitalized patients are affected.1 When oral intake is contraindicated, patients are often fed by nasogastric tube (NGT) or by surgically or endoscopically placed gastrostomy tube (GT). Without good justification based on outcomes, NGTs are traditionally used when the need for feeding is thought to be short term (<4 weeks) and GTs are used for long term (>4 weeks). However, in 2005, a large multicenter randomized control trial found that the majority of stroke patients with dysphagia that would resolve had resolution within 2-3 weeks. Moreover, outcomes were equivalent or better for patients fed with an NGT versus GT.
The authors concluded by recommending feeding via NGT for 2-3 weeks, after which conversion to GT can be considered if dysphagia persists.1 Notably, the recommendation allows consideration, and no evidence-based guideline requires or recommends GT be placed based on duration of tube feed dependence. Currently, while nutrition and neurology authorities have adopted these recommendations,2,3 many authors have noted poor adherence to this guideline, and many find that the median period between stroke and GT placement is 7 days rather than the recommended minimum of 14.4,5,6 While ignorance can partially explain the lack of widespread compliance,6 the policies of posthospital facilities are another culprit. Increasingly, and for a variety of reasons unsupported by the literature, SNFs refuse NGT and require GT.4,7,8,9
Ethical considerations
The four principles of medical ethics – autonomy, beneficence, nonmaleficence, and justice – can guide clinicians, patients, and family members in decision-making. In our case, by withholding needed and desired treatment (discharge to and treatment by a rehabilitation facility) the patient is being coerced to undergo a procedure he does not want, and clinicians participate in denying him autonomy. Further, given that the evidence, national guidelines, and in fact federal regulations indicate that his preferences are congruent with best practices, pressuring him to accept gastrostomy placement runs afoul of the principles of beneficence and nonmaleficence. Though the mechanism is unclear, early gastrostomy (<14-21 days) is associated with increased risk of death, worse functional outcomes, and a lower rate of return to oral feeding, as well as a significant procedure-specific complication rate.1,10 By insisting on gastrostomy, we neither act in this patient’s best interests nor “do no harm.”
However, the medical system is complex. The clinician at the bedside can evaluate this scenario, review the national guidelines, discuss the procedure and risks with the patient and family, and conclude that the patient should be discharged with a nasal feeding tube. Nevertheless, if no facility is willing to accept him without a gastrostomy, our decision-making model – previously limited to our patient’s best interests alone – is forced to change. Despite our misgivings, we often conclude that the harm done by an early gastrostomy is outweighed by the harm of remaining unnecessarily in the acute hospital setting. We further worry about other patients lingering in the emergency department for lack of an inpatient bed and the possible – though unknowable – harm done to them.
Looking forward
It is an unfortunate fact that medical decision-making must often include factors unrelated to the patient’s best interests, with financial considerations and structural barriers frequently driving deviation from ideal care. Providers and patients navigate these decisions to their best abilities, making compromises when forced. However, with education and professional activism, providers can advocate for the elimination of barriers to providing medically sound and ethically appropriate care. In our experience, delay of gastrostomy placement, until discharge is imminent and planning for postdischarge care is initiated, has resulted in a decrease by half the fraction of patients with tracheostomies who had gastrostomies placed prior to discharge.11 With aggressive outreach and education, we now have nursing homes willing to accept patients with NGTs.
Criteria for admission to discharge facilities can drive medical decision-making that is unethical and unsupported by evidence. Continued efforts to eliminate barriers to appropriate and ethical care have been successful and are encouraged.
Dr. Cowan is administrative chief resident in the department of surgery at Columbia University Irving Medical Center, New York. Dr. Seres is professor of medicine in the Institute of Human Nutrition and associate clinical ethicist at Columbia University Irving Medical Center. The authors have no conflicts of interest to disclose.
References
1. Dennis MS et al. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72.
2. Powers W. et al. Stroke. 2018 Mar;49(3):e46-e110.
3. Burgos R et al. Clin Nutr. 2018 Feb;37(1):354-96.
4. Wilmskoetter J et al. J Stroke Cerebrovasc Dis. 2016 Nov;25(11):2694-700.
5. George BP et al. Stroke. 2017 Feb;48(2):420-7.
6. Fessler TA. et al. Surg Endosc. 2019 Dec;33(12):4089-97.
7. Burgermaster M et al. Nutr Clin Pract. 2016 Jun;31(3):342-8.
8. Moran C and O’Mahoney S. Curr Opin Gastroenterol. 2015 Mar;31(2):137-42.
9. Gomes CA et al. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008096.
10. Joundi RA et al. Neurology. 2018 Feb 13;90(7):e544-52.
11. Bothra A et al. J Parenter Enteral Nutr. 2018 Feb;42(2):491.
Clinical scenario
An 83-year-old man is admitted with a hemiplegic cerebrovascular accident. He is found to have dysphagia, and a nasogastric feeding tube is placed. Over the next several days, his strength begins to recover, and he tolerates his tube feeding well. Discharge to a skilled nursing facility (SNF) for subacute rehabilitation is planned. His swallowing is showing signs of recovery; it has not recovered adequately but is expected to continue to improve such that he is predicted to be independent of tube feeding within 7-14 days. None of the facilities in the region are willing to admit a patient with a nasal feeding tube, despite the anticipated short duration. The patient is medically ready for discharge but is refusing the feeding gastrostomy. “Why would I want a hole in my stomach, if I’m only going to need it for 1-2 weeks and this tube in my nose is working fine and is comfortable?” he pleads with tears in his eyes.
Feeding dysphagic patients after stroke
Dysphagia, potentially leading to aspiration and/or pneumonia, is a common sequela of stroke – up to half of hospitalized patients are affected.1 When oral intake is contraindicated, patients are often fed by nasogastric tube (NGT) or by surgically or endoscopically placed gastrostomy tube (GT). Without good justification based on outcomes, NGTs are traditionally used when the need for feeding is thought to be short term (<4 weeks) and GTs are used for long term (>4 weeks). However, in 2005, a large multicenter randomized control trial found that the majority of stroke patients with dysphagia that would resolve had resolution within 2-3 weeks. Moreover, outcomes were equivalent or better for patients fed with an NGT versus GT.
The authors concluded by recommending feeding via NGT for 2-3 weeks, after which conversion to GT can be considered if dysphagia persists.1 Notably, the recommendation allows consideration, and no evidence-based guideline requires or recommends GT be placed based on duration of tube feed dependence. Currently, while nutrition and neurology authorities have adopted these recommendations,2,3 many authors have noted poor adherence to this guideline, and many find that the median period between stroke and GT placement is 7 days rather than the recommended minimum of 14.4,5,6 While ignorance can partially explain the lack of widespread compliance,6 the policies of posthospital facilities are another culprit. Increasingly, and for a variety of reasons unsupported by the literature, SNFs refuse NGT and require GT.4,7,8,9
Ethical considerations
The four principles of medical ethics – autonomy, beneficence, nonmaleficence, and justice – can guide clinicians, patients, and family members in decision-making. In our case, by withholding needed and desired treatment (discharge to and treatment by a rehabilitation facility) the patient is being coerced to undergo a procedure he does not want, and clinicians participate in denying him autonomy. Further, given that the evidence, national guidelines, and in fact federal regulations indicate that his preferences are congruent with best practices, pressuring him to accept gastrostomy placement runs afoul of the principles of beneficence and nonmaleficence. Though the mechanism is unclear, early gastrostomy (<14-21 days) is associated with increased risk of death, worse functional outcomes, and a lower rate of return to oral feeding, as well as a significant procedure-specific complication rate.1,10 By insisting on gastrostomy, we neither act in this patient’s best interests nor “do no harm.”
However, the medical system is complex. The clinician at the bedside can evaluate this scenario, review the national guidelines, discuss the procedure and risks with the patient and family, and conclude that the patient should be discharged with a nasal feeding tube. Nevertheless, if no facility is willing to accept him without a gastrostomy, our decision-making model – previously limited to our patient’s best interests alone – is forced to change. Despite our misgivings, we often conclude that the harm done by an early gastrostomy is outweighed by the harm of remaining unnecessarily in the acute hospital setting. We further worry about other patients lingering in the emergency department for lack of an inpatient bed and the possible – though unknowable – harm done to them.
Looking forward
It is an unfortunate fact that medical decision-making must often include factors unrelated to the patient’s best interests, with financial considerations and structural barriers frequently driving deviation from ideal care. Providers and patients navigate these decisions to their best abilities, making compromises when forced. However, with education and professional activism, providers can advocate for the elimination of barriers to providing medically sound and ethically appropriate care. In our experience, delay of gastrostomy placement, until discharge is imminent and planning for postdischarge care is initiated, has resulted in a decrease by half the fraction of patients with tracheostomies who had gastrostomies placed prior to discharge.11 With aggressive outreach and education, we now have nursing homes willing to accept patients with NGTs.
Criteria for admission to discharge facilities can drive medical decision-making that is unethical and unsupported by evidence. Continued efforts to eliminate barriers to appropriate and ethical care have been successful and are encouraged.
Dr. Cowan is administrative chief resident in the department of surgery at Columbia University Irving Medical Center, New York. Dr. Seres is professor of medicine in the Institute of Human Nutrition and associate clinical ethicist at Columbia University Irving Medical Center. The authors have no conflicts of interest to disclose.
References
1. Dennis MS et al. Lancet. 2005 Feb 26-Mar 4;365(9461):764-72.
2. Powers W. et al. Stroke. 2018 Mar;49(3):e46-e110.
3. Burgos R et al. Clin Nutr. 2018 Feb;37(1):354-96.
4. Wilmskoetter J et al. J Stroke Cerebrovasc Dis. 2016 Nov;25(11):2694-700.
5. George BP et al. Stroke. 2017 Feb;48(2):420-7.
6. Fessler TA. et al. Surg Endosc. 2019 Dec;33(12):4089-97.
7. Burgermaster M et al. Nutr Clin Pract. 2016 Jun;31(3):342-8.
8. Moran C and O’Mahoney S. Curr Opin Gastroenterol. 2015 Mar;31(2):137-42.
9. Gomes CA et al. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008096.
10. Joundi RA et al. Neurology. 2018 Feb 13;90(7):e544-52.
11. Bothra A et al. J Parenter Enteral Nutr. 2018 Feb;42(2):491.
Mindful mentoring
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Case of the inappropriate endoscopy referral
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A 53-year-old woman was referred for surveillance colonoscopy. She is a current smoker with a history of chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, and two diminutive hyperplastic polyps found on average-risk screening colonoscopy 3 years previously. Her prep at the time was excellent and she was advised to return in 10 years for follow-up. She has taken the day off work, arranged for a driver, is prepped, and is on your schedule for a colonoscopy for a “history of polyps.” Is this an appropriate referral and how should you handle it?
Most of us have had questionable referrals on our endoscopy schedules. While judgments can vary among providers about when a patient should undergo a procedure or what intervention is most needed, some direct-access referrals for endoscopy are considered inappropriate by most standards. In examining referrals for colonoscopy, studies have shown that as many as 23% of screening colonoscopies among Medicare beneficiaries and 14.2% of Veterans Affairs patients in a large colorectal cancer screening study are inappropriate.1,2 A prospective multicenter study found 29% of colonoscopies to be inappropriate, and surveillance studies were confirmed as the most frequent source of inappropriate procedures.3,4 Endoscopies are performed so frequently, effectively, and safely that they can be readily scheduled by gastroenterologists and nongastroenterologists alike. Open access has facilitated and expedited needed procedures, providing benefit to patient and provider and freeing clinic visit time for more complex consults. But while endoscopy is very safe, it is not without risk or cost. What should be the response when a patient in the endoscopy unit appears to be inappropriately referred?
The first step is to determine what is inappropriate. There are several situations when a procedure might be considered inappropriate, particularly when we try to apply ethical principles.
1. The performance of the procedure is contrary to society guidelines. The American Gastroenterological Association, American Society for Gastrointestinal Endoscopy, and American College of Gastroenterology publish clinical guidelines. These documents are drafted after rigorous research and literature review, and the strength of the recommendations is confirmed by incorporation of GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology. Such guidelines allow gastroenterologists across the country to practice confidently in a manner consistent with the current available data and the standards of care for the GI community. A patient who is referred for a procedure for an indication that does not adhere to – or contradicts – guidelines, may be at risk for substandard care and possibly at risk for harm. It is the physician’s ethical responsibility to provide the most “good” and the least harm for patients, consistent with the ethical principle of beneficence.
Guidelines, however, are not mandates, and an argument may be made that in order to provide the best care, alternatives may be offered to a patient. Some circumstances require clinical judgments based on unique patient characteristics and the need for individualized care. As a rule, however, the goal of guidelines is to assist doctors in providing the best care.
2. The procedure is not the correct test for the clinical question. While endoscopy can address many clinical queries, endoscopy is not always the right procedure for a specific medical question. A patient referred for an esophagogastroduodenoscopy (EGD) to rule out gastroparesis is being subjected to the wrong test to answer the clinical question. Some information may be obtained from an EGD (e.g., retained food may suggest dysmotility or the patient could have gastric outlet obstruction) but this is not the recommended initial management step. Is it reasonable to proceed with a test that cannot answer the question asked? Continuing with the endoscopy would not enhance beneficence and might be a futile service for the patient. Is this doing the best for the patient?
3. The risks of the procedure outweigh the benefits. Some procedures may be consistent with guidelines and able to answer the questions asked, but may present more risk than benefit. Should an elderly patient with multiple significant comorbidities and a likely limited life span undergo a follow-up colonoscopy even at an appropriate interval? The principle of nonmaleficence is the clear standard here.
4. The intent for doing the procedure has questionable merit. Some patients may request an EGD at the time of the screening colonoscopy just to “check,” regardless of symptoms or risk category. A patient has a right to make her/his own decisions but patient autonomy should not be an excuse for a nonindicated procedure.
In the case of the 53-year-old woman referred for surveillance colonoscopy, the physician needs to consider whether performing the test is inappropriate for any of the above reasons. First and foremost, is it doing the most good for the patient?
On the one hand, performing an inappropriately referred procedure contradicts guidelines and may present undue risk of complication from anesthesia or endoscopy. Would the physician be ethically compromised in this situation, or even legally liable should a complication arise during a procedure done for a questionable indication?
On the other hand, canceling such a procedure creates multiple dilemmas. The autonomy and the convenience of the patient need to be respected. The patient who has followed all the instructions, is prepped, has taken off work, arranged for transportation, and wants to have the procedure done may have difficulty accepting a cancellation. Colonoscopy is a safe test. Is it the right thing to cancel her procedure because of an imprudent referral? Would this undermine the patient’s confidence in her referring provider? Physicians may face other pressures to proceed, such as practice or institutional restraints that discourage same-day cancellations. Maintenance of robust financial practices, stable referral sources, and excellent patient satisfaction measures are critical to running an efficient endoscopy unit and maximizing patient service and care.
Is there a sensible way to address the dilemma? One approach is simply to move ahead with the procedure if the physician feels that the benefits outweigh the medical and ethical risks. Besides patient convenience, other “benefits” could be relevant: clinical value from an unexpected finding, affirmation of the patient’s invested time and effort, and avoidance of the apparent undermining of the authority of a referring colleague. Finally, maintaining productive and efficient practices or institutions ultimately allows for better patient care. The physician can explain the enhanced risks, present the alternatives, and – perhaps in less time than the ethical deliberations might take – complete the procedure and have the patient resting comfortably in the recovery unit.
An alternative approach is to cancel the procedure if the physician feels that the indication is not legitimate, or that the risks to the patient and the physician are significant. Explaining the cancellation can be difficult but may be the right decision if ethical principles of beneficence are upheld. It is understood that procedures consume health care resources and can present an undue expense to society if done for improper reasons. Unnecessary procedures clutter schedules for patients who truly need an endoscopy.
Neither approach is completely satisfying, although moving forward with a likely very safe procedure is often the easiest step and probably what many physicians do in this setting.
Is there a better way to approach this problem? Preventing the ethical dilemma is the ideal scenario, although not always feasible. Here are some suggestions to consider.
Reviewing referrals prior to the procedure day allows endoscopists to contact and cancel patients if needed, before the prep and travel begin. This addresses the convenience aspects but not the issue regarding the underlying indication.
The most important step toward avoiding inappropriate referrals is better education for referring providers. Even gastroenterologists, let alone primary care physicians, may struggle to stay current on changing clinical GI guidelines. Colorectal cancer screening, for example, is an area that gives gastroenterologists an opportunity to communicate with and educate colleagues about appropriate management. Keeping our referral base up to date about guidelines and prep and safety recommendations will likely reduce the number of inappropriate colonoscopy referrals and provide many of the benefits described above.
Providing the best care for patients by adhering to medical ethical principles is the goal of our work as physicians. Implementing this goal may demand tough decisions.
Dr. Fisher is professor of clinical medicine and director of small-bowel imaging, division of gastroenterology, University of Pennsylvania, Philadelphia.
References
1. Sheffield KM et al. JAMA Intern Med. 2013 Apr 8;173(7):542-50.
2. Powell AA et al. J Gen Intern Med. 2015 Jun;30(6):732-41.
3. Petruzziello L et al. J Clin Gastroenterol. 2012;46(7):590-4.
4. Kapila N et al. Dig Dis Sci. 2019;64(10):2798-805.
A practical approach to utilizing cannabis as adjuvant therapy in inflammatory bowel disease
Case 1
A 30 year-old female with longstanding ulcerative colitis who has a history of medically refractory steroid-dependent disease and was able to achieve remission with vedolizumab for the last 5 years. Most recent objective assessment showed histologic remission. She has been using daily cannabis medicinally for the last year (high CBD:THC [cannabidiol:delta-9-tetracannabidol] concentration). She notes that she has felt better in the last year since introducing cannabis (improved stool frequency/formation, sleep quality). She inquires about discontinuing her biologic therapy in the hope of using cannabis alone to maintain remission.

Figure 1.
Case 2
A 22-year-old male with ileocolonic inflammatory Crohn’s disease escalated to adalimumab requiring an intensification of therapy to weekly dosing to normalize C-reactive protein (CRP). A recent colonoscopy showed endoscopic improvement (colonic normalization and rare aphthae in ileum). He notes clear clinical improvement, but he continues to experience diarrhea and abdominal cramping (no relationship to meals). Declines addition of immunomodulator (nervous about returning to college during the COVID-19 pandemic). He wonders whether cannabis could be effective in controlling his symptoms as he has had improvement in symptoms during his sporadic recreational cannabis exposure.
Discussion
These cases outline the challenges that providers face when managing patients with inflammatory bowel disease (IBD) when a patient would like to either substitute or incorporate cannabis into their treatment plan. Studies have shown a high prevalence of cannabis use among patients with IBD. With the restrictions surrounding the use of cannabis – either medically or recreationally – being liberalized in many states, these conversations are likely to become more frequent in your practice. However, one of the first challenges that providers face surrounding cannabis is that many patients who use cannabis do not disclose use to their health care team for fear of being judged negatively. In addition, many providers do not routinely ask about cannabis use during office visits. This might be directly related to being unprepared to have a knowledge-based discussion on the risks and benefits of cannabis use in IBD, with the same confidence present during discussion of biologic therapies.
For background, Cannabis sativa (cannabis) is composed of hundreds of phytocannabinoids, the two most common are THC and CBD. These cannabinoids act at the endocannabinoid receptors, which are expressed in the central and peripheral nervous systems and immune cells/tissues, and help explain the clinical changes experienced by cannabis users. Both THC and CBD have been studied in varying doses and routes of administration in patients with IBD, making it challenging to translate into real-world recommendations for patients. Some of the most common reported benefits of cannabis use (particularly in an IBD population) are improvement in pain, diarrhea, nausea, and joint pain. Some studies have shown overall improvement in quality of life (Figure 1).
Some common questions that arise surrounding cannabis use in IBD patients include:
1. Is it possible to stop traditional medical therapy and replace it with cannabis therapy?
No studies have directly addressed this exact question. The small studies, both randomized controlled trials and retrospective ones, have studied the effects of cannabis as adjuvant therapy only. None of the data available to date suggest that cannabis has any anti-inflammatory properties with absence of improvement in biomarkers or endoscopic measures of inflammation. In effect, any attempt to discontinue standard therapy with substitution of cannabis-based therapy should be seen as no different than simply discontinuing standard therapy. There exists the argument that – among those with moderate to severe disease – cannabis might suppress the investigation of mild symptoms which may herald a flare of disease, thus lulling the patient into a state of false stability. We do not advocate the substitution of cannabis products in place of standard medical therapy.
2. Is there a role for cannabis as adjuvant therapy in patients with IBD?
Studies to date have included only symptomatic patients with objective evidence of inflammation and assessed clinical, biochemical, or endoscopic endpoints. In Crohn’s disease, two studies showed no improvement in clinical remission rates but showed improvement in clinical response; a third study showed both improvement in clinical remission/response as well as improved quality of life. No study showed a change in disease markers of activity including CRP, fecal calprotectin, or endoscopic scoring. In one study, all patients relapsed shortly after cannabis discontinuation suggesting that, while there was benefit in symptom control, there was no improvement of the underlying chronic inflammation.
In patients with ulcerative colitis, there were two studies. One study showed no improvement and high rates of intolerance in the treatment group, while the other study reported improved disease activity but no objective improvement. The variation in results between disease states and between studies might be because of cannabis formulations. In patients with persistent symptoms despite current medical therapy, there might be a role in those patients for adjuvant therapy for improvement symptom control but not disease control. Optimization of medical therapy would still be indicated.
3. What dose and formulation of cannabis should I recommend to a patient as adjuvant therapy?
This is an excellent question and one that unfortunately we do not have the answer to. As mentioned previously, the studies have looked at varying formulations (THC alone, CBD:THC with varying percentages of THC, CBD alone) and varying routes of administration (sublingual, oral, inhalation). The IBD studies looking at CBD-alone formulations lacked clinical efficacy. In states where cannabis products have been accessible to IBD patients, no data on the product type (THC:CBD), method of administration, or prescriber preferences have been published.
4. What risks should I advise my patients about with cannabis use?
The challenge is that we don’t have large population-based studies in IBD looking at long-term risks of cannabis use. However, in the small RCT studies there were minimal reported side effects and no major adverse events over 8-10 weeks. Larger IBD population-based studies have shown that cannabis users were more likely to discontinue traditional medical therapy, and there is an increased risk for surgery in patients with Crohn’s disease. Larger studies in non-IBD patients have shown risk for addiction to other substances, diminished life achievement, increased motor vehicle accidents, chronic bronchitis, psychiatric disturbances and cannabis dependence, and cannabis hyperemesis syndrome (with an uncanny presentation resembling Crohn’s disease flare with partial small bowel obstruction). Patients should also be advised about legal implications of use (given its continued classification as a federal schedule 1 drug), possible drug interactions, and special considerations in pediatric patients (increased risk of addiction), elderly patients (increased risk of neuropsychological effects), and during pregnancy (with national obstetric society guidelines warning against use because of fetal exposure and increased risk of stillbirth).
5. What are the legal implications for providers? Patients?
As of July 2020, cannabis is available for recreational use in 12 states, for medicinal use in 28 states, and illegal in 11 states. So the answer really depends on what state the patient lives in. As a provider who might certify patients (in some medicinal states) or recommend cannabis to patients, you should consider legal and licensing implications. Again, this might vary state to state, and you should also take into account federal status. Providers acting in compliance with state laws are unlikely to have federal consequences. However, remember that malpractice insurance only covers FDA-approved medical therapies. Patients should be advised to consider the potential (although highly unlikely) to face federal prosecution and implications of use for employment, school, camp, or travel, and driving restrictions.
Take home points
- Inquire about cannabis to start the conversation.
- Know your state’s legalization status surrounding cannabis.
- Patients with IBD report improvement in symptoms and quality of life with adjuvant cannabis use; however, there is no change in disease activity.
- Encourage your patients to continue and optimize their maintenance therapy.
- Educate your patients about the legal considerations and known risks.
In conclusion, the use of cannabis in IBD patients has increased in recent years. It is important to be able to discuss the risks and benefits of use with your IBD patients. Focus on the lack of data showing that cannabis improves disease activity, and has shown benefit only in improving IBD-associated symptoms. In some patients there might be a role for adjuvant cannabis therapy to improve overall symptom control and quality of life.
Dr. Kinnucan is an assistant professor of medicine, division of gastroenterology, Michigan Medicine, University of Michigan, Ann Arbor; Dr. Swaminath is an associate professor of medicine, division of gastroenterology, Lenox Hill Hospital, Northwell Health, New York.
Case 1
A 30 year-old female with longstanding ulcerative colitis who has a history of medically refractory steroid-dependent disease and was able to achieve remission with vedolizumab for the last 5 years. Most recent objective assessment showed histologic remission. She has been using daily cannabis medicinally for the last year (high CBD:THC [cannabidiol:delta-9-tetracannabidol] concentration). She notes that she has felt better in the last year since introducing cannabis (improved stool frequency/formation, sleep quality). She inquires about discontinuing her biologic therapy in the hope of using cannabis alone to maintain remission.

Figure 1.
Case 2
A 22-year-old male with ileocolonic inflammatory Crohn’s disease escalated to adalimumab requiring an intensification of therapy to weekly dosing to normalize C-reactive protein (CRP). A recent colonoscopy showed endoscopic improvement (colonic normalization and rare aphthae in ileum). He notes clear clinical improvement, but he continues to experience diarrhea and abdominal cramping (no relationship to meals). Declines addition of immunomodulator (nervous about returning to college during the COVID-19 pandemic). He wonders whether cannabis could be effective in controlling his symptoms as he has had improvement in symptoms during his sporadic recreational cannabis exposure.
Discussion
These cases outline the challenges that providers face when managing patients with inflammatory bowel disease (IBD) when a patient would like to either substitute or incorporate cannabis into their treatment plan. Studies have shown a high prevalence of cannabis use among patients with IBD. With the restrictions surrounding the use of cannabis – either medically or recreationally – being liberalized in many states, these conversations are likely to become more frequent in your practice. However, one of the first challenges that providers face surrounding cannabis is that many patients who use cannabis do not disclose use to their health care team for fear of being judged negatively. In addition, many providers do not routinely ask about cannabis use during office visits. This might be directly related to being unprepared to have a knowledge-based discussion on the risks and benefits of cannabis use in IBD, with the same confidence present during discussion of biologic therapies.
For background, Cannabis sativa (cannabis) is composed of hundreds of phytocannabinoids, the two most common are THC and CBD. These cannabinoids act at the endocannabinoid receptors, which are expressed in the central and peripheral nervous systems and immune cells/tissues, and help explain the clinical changes experienced by cannabis users. Both THC and CBD have been studied in varying doses and routes of administration in patients with IBD, making it challenging to translate into real-world recommendations for patients. Some of the most common reported benefits of cannabis use (particularly in an IBD population) are improvement in pain, diarrhea, nausea, and joint pain. Some studies have shown overall improvement in quality of life (Figure 1).
Some common questions that arise surrounding cannabis use in IBD patients include:
1. Is it possible to stop traditional medical therapy and replace it with cannabis therapy?
No studies have directly addressed this exact question. The small studies, both randomized controlled trials and retrospective ones, have studied the effects of cannabis as adjuvant therapy only. None of the data available to date suggest that cannabis has any anti-inflammatory properties with absence of improvement in biomarkers or endoscopic measures of inflammation. In effect, any attempt to discontinue standard therapy with substitution of cannabis-based therapy should be seen as no different than simply discontinuing standard therapy. There exists the argument that – among those with moderate to severe disease – cannabis might suppress the investigation of mild symptoms which may herald a flare of disease, thus lulling the patient into a state of false stability. We do not advocate the substitution of cannabis products in place of standard medical therapy.
2. Is there a role for cannabis as adjuvant therapy in patients with IBD?
Studies to date have included only symptomatic patients with objective evidence of inflammation and assessed clinical, biochemical, or endoscopic endpoints. In Crohn’s disease, two studies showed no improvement in clinical remission rates but showed improvement in clinical response; a third study showed both improvement in clinical remission/response as well as improved quality of life. No study showed a change in disease markers of activity including CRP, fecal calprotectin, or endoscopic scoring. In one study, all patients relapsed shortly after cannabis discontinuation suggesting that, while there was benefit in symptom control, there was no improvement of the underlying chronic inflammation.
In patients with ulcerative colitis, there were two studies. One study showed no improvement and high rates of intolerance in the treatment group, while the other study reported improved disease activity but no objective improvement. The variation in results between disease states and between studies might be because of cannabis formulations. In patients with persistent symptoms despite current medical therapy, there might be a role in those patients for adjuvant therapy for improvement symptom control but not disease control. Optimization of medical therapy would still be indicated.
3. What dose and formulation of cannabis should I recommend to a patient as adjuvant therapy?
This is an excellent question and one that unfortunately we do not have the answer to. As mentioned previously, the studies have looked at varying formulations (THC alone, CBD:THC with varying percentages of THC, CBD alone) and varying routes of administration (sublingual, oral, inhalation). The IBD studies looking at CBD-alone formulations lacked clinical efficacy. In states where cannabis products have been accessible to IBD patients, no data on the product type (THC:CBD), method of administration, or prescriber preferences have been published.
4. What risks should I advise my patients about with cannabis use?
The challenge is that we don’t have large population-based studies in IBD looking at long-term risks of cannabis use. However, in the small RCT studies there were minimal reported side effects and no major adverse events over 8-10 weeks. Larger IBD population-based studies have shown that cannabis users were more likely to discontinue traditional medical therapy, and there is an increased risk for surgery in patients with Crohn’s disease. Larger studies in non-IBD patients have shown risk for addiction to other substances, diminished life achievement, increased motor vehicle accidents, chronic bronchitis, psychiatric disturbances and cannabis dependence, and cannabis hyperemesis syndrome (with an uncanny presentation resembling Crohn’s disease flare with partial small bowel obstruction). Patients should also be advised about legal implications of use (given its continued classification as a federal schedule 1 drug), possible drug interactions, and special considerations in pediatric patients (increased risk of addiction), elderly patients (increased risk of neuropsychological effects), and during pregnancy (with national obstetric society guidelines warning against use because of fetal exposure and increased risk of stillbirth).
5. What are the legal implications for providers? Patients?
As of July 2020, cannabis is available for recreational use in 12 states, for medicinal use in 28 states, and illegal in 11 states. So the answer really depends on what state the patient lives in. As a provider who might certify patients (in some medicinal states) or recommend cannabis to patients, you should consider legal and licensing implications. Again, this might vary state to state, and you should also take into account federal status. Providers acting in compliance with state laws are unlikely to have federal consequences. However, remember that malpractice insurance only covers FDA-approved medical therapies. Patients should be advised to consider the potential (although highly unlikely) to face federal prosecution and implications of use for employment, school, camp, or travel, and driving restrictions.
Take home points
- Inquire about cannabis to start the conversation.
- Know your state’s legalization status surrounding cannabis.
- Patients with IBD report improvement in symptoms and quality of life with adjuvant cannabis use; however, there is no change in disease activity.
- Encourage your patients to continue and optimize their maintenance therapy.
- Educate your patients about the legal considerations and known risks.
In conclusion, the use of cannabis in IBD patients has increased in recent years. It is important to be able to discuss the risks and benefits of use with your IBD patients. Focus on the lack of data showing that cannabis improves disease activity, and has shown benefit only in improving IBD-associated symptoms. In some patients there might be a role for adjuvant cannabis therapy to improve overall symptom control and quality of life.
Dr. Kinnucan is an assistant professor of medicine, division of gastroenterology, Michigan Medicine, University of Michigan, Ann Arbor; Dr. Swaminath is an associate professor of medicine, division of gastroenterology, Lenox Hill Hospital, Northwell Health, New York.
Case 1
A 30 year-old female with longstanding ulcerative colitis who has a history of medically refractory steroid-dependent disease and was able to achieve remission with vedolizumab for the last 5 years. Most recent objective assessment showed histologic remission. She has been using daily cannabis medicinally for the last year (high CBD:THC [cannabidiol:delta-9-tetracannabidol] concentration). She notes that she has felt better in the last year since introducing cannabis (improved stool frequency/formation, sleep quality). She inquires about discontinuing her biologic therapy in the hope of using cannabis alone to maintain remission.

Figure 1.
Case 2
A 22-year-old male with ileocolonic inflammatory Crohn’s disease escalated to adalimumab requiring an intensification of therapy to weekly dosing to normalize C-reactive protein (CRP). A recent colonoscopy showed endoscopic improvement (colonic normalization and rare aphthae in ileum). He notes clear clinical improvement, but he continues to experience diarrhea and abdominal cramping (no relationship to meals). Declines addition of immunomodulator (nervous about returning to college during the COVID-19 pandemic). He wonders whether cannabis could be effective in controlling his symptoms as he has had improvement in symptoms during his sporadic recreational cannabis exposure.
Discussion
These cases outline the challenges that providers face when managing patients with inflammatory bowel disease (IBD) when a patient would like to either substitute or incorporate cannabis into their treatment plan. Studies have shown a high prevalence of cannabis use among patients with IBD. With the restrictions surrounding the use of cannabis – either medically or recreationally – being liberalized in many states, these conversations are likely to become more frequent in your practice. However, one of the first challenges that providers face surrounding cannabis is that many patients who use cannabis do not disclose use to their health care team for fear of being judged negatively. In addition, many providers do not routinely ask about cannabis use during office visits. This might be directly related to being unprepared to have a knowledge-based discussion on the risks and benefits of cannabis use in IBD, with the same confidence present during discussion of biologic therapies.
For background, Cannabis sativa (cannabis) is composed of hundreds of phytocannabinoids, the two most common are THC and CBD. These cannabinoids act at the endocannabinoid receptors, which are expressed in the central and peripheral nervous systems and immune cells/tissues, and help explain the clinical changes experienced by cannabis users. Both THC and CBD have been studied in varying doses and routes of administration in patients with IBD, making it challenging to translate into real-world recommendations for patients. Some of the most common reported benefits of cannabis use (particularly in an IBD population) are improvement in pain, diarrhea, nausea, and joint pain. Some studies have shown overall improvement in quality of life (Figure 1).
Some common questions that arise surrounding cannabis use in IBD patients include:
1. Is it possible to stop traditional medical therapy and replace it with cannabis therapy?
No studies have directly addressed this exact question. The small studies, both randomized controlled trials and retrospective ones, have studied the effects of cannabis as adjuvant therapy only. None of the data available to date suggest that cannabis has any anti-inflammatory properties with absence of improvement in biomarkers or endoscopic measures of inflammation. In effect, any attempt to discontinue standard therapy with substitution of cannabis-based therapy should be seen as no different than simply discontinuing standard therapy. There exists the argument that – among those with moderate to severe disease – cannabis might suppress the investigation of mild symptoms which may herald a flare of disease, thus lulling the patient into a state of false stability. We do not advocate the substitution of cannabis products in place of standard medical therapy.
2. Is there a role for cannabis as adjuvant therapy in patients with IBD?
Studies to date have included only symptomatic patients with objective evidence of inflammation and assessed clinical, biochemical, or endoscopic endpoints. In Crohn’s disease, two studies showed no improvement in clinical remission rates but showed improvement in clinical response; a third study showed both improvement in clinical remission/response as well as improved quality of life. No study showed a change in disease markers of activity including CRP, fecal calprotectin, or endoscopic scoring. In one study, all patients relapsed shortly after cannabis discontinuation suggesting that, while there was benefit in symptom control, there was no improvement of the underlying chronic inflammation.
In patients with ulcerative colitis, there were two studies. One study showed no improvement and high rates of intolerance in the treatment group, while the other study reported improved disease activity but no objective improvement. The variation in results between disease states and between studies might be because of cannabis formulations. In patients with persistent symptoms despite current medical therapy, there might be a role in those patients for adjuvant therapy for improvement symptom control but not disease control. Optimization of medical therapy would still be indicated.
3. What dose and formulation of cannabis should I recommend to a patient as adjuvant therapy?
This is an excellent question and one that unfortunately we do not have the answer to. As mentioned previously, the studies have looked at varying formulations (THC alone, CBD:THC with varying percentages of THC, CBD alone) and varying routes of administration (sublingual, oral, inhalation). The IBD studies looking at CBD-alone formulations lacked clinical efficacy. In states where cannabis products have been accessible to IBD patients, no data on the product type (THC:CBD), method of administration, or prescriber preferences have been published.
4. What risks should I advise my patients about with cannabis use?
The challenge is that we don’t have large population-based studies in IBD looking at long-term risks of cannabis use. However, in the small RCT studies there were minimal reported side effects and no major adverse events over 8-10 weeks. Larger IBD population-based studies have shown that cannabis users were more likely to discontinue traditional medical therapy, and there is an increased risk for surgery in patients with Crohn’s disease. Larger studies in non-IBD patients have shown risk for addiction to other substances, diminished life achievement, increased motor vehicle accidents, chronic bronchitis, psychiatric disturbances and cannabis dependence, and cannabis hyperemesis syndrome (with an uncanny presentation resembling Crohn’s disease flare with partial small bowel obstruction). Patients should also be advised about legal implications of use (given its continued classification as a federal schedule 1 drug), possible drug interactions, and special considerations in pediatric patients (increased risk of addiction), elderly patients (increased risk of neuropsychological effects), and during pregnancy (with national obstetric society guidelines warning against use because of fetal exposure and increased risk of stillbirth).
5. What are the legal implications for providers? Patients?
As of July 2020, cannabis is available for recreational use in 12 states, for medicinal use in 28 states, and illegal in 11 states. So the answer really depends on what state the patient lives in. As a provider who might certify patients (in some medicinal states) or recommend cannabis to patients, you should consider legal and licensing implications. Again, this might vary state to state, and you should also take into account federal status. Providers acting in compliance with state laws are unlikely to have federal consequences. However, remember that malpractice insurance only covers FDA-approved medical therapies. Patients should be advised to consider the potential (although highly unlikely) to face federal prosecution and implications of use for employment, school, camp, or travel, and driving restrictions.
Take home points
- Inquire about cannabis to start the conversation.
- Know your state’s legalization status surrounding cannabis.
- Patients with IBD report improvement in symptoms and quality of life with adjuvant cannabis use; however, there is no change in disease activity.
- Encourage your patients to continue and optimize their maintenance therapy.
- Educate your patients about the legal considerations and known risks.
In conclusion, the use of cannabis in IBD patients has increased in recent years. It is important to be able to discuss the risks and benefits of use with your IBD patients. Focus on the lack of data showing that cannabis improves disease activity, and has shown benefit only in improving IBD-associated symptoms. In some patients there might be a role for adjuvant cannabis therapy to improve overall symptom control and quality of life.
Dr. Kinnucan is an assistant professor of medicine, division of gastroenterology, Michigan Medicine, University of Michigan, Ann Arbor; Dr. Swaminath is an associate professor of medicine, division of gastroenterology, Lenox Hill Hospital, Northwell Health, New York.