User login
New Guidelines: Start PSA Screening Earlier in Black Men
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, baseline PSA testing in Black men should begin at age 40-45, sooner than current guidelines recommend, and should be followed by regular screening intervals, preferably annually, at least until age 70, a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues wrote. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Taskforce recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” Dr. Oh added.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Dr. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, baseline PSA testing in Black men should begin at age 40-45, sooner than current guidelines recommend, and should be followed by regular screening intervals, preferably annually, at least until age 70, a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues wrote. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Taskforce recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” Dr. Oh added.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Dr. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
Lowering the recommended age for baseline prostate-specific antigen (PSA) would reduce prostate cancer deaths by about 30% in Black men without significantly increasing the rate of overdiagnosis, according to new screening guidelines from the Prostate Cancer Foundation.
Specifically, baseline PSA testing in Black men should begin at age 40-45, sooner than current guidelines recommend, and should be followed by regular screening intervals, preferably annually, at least until age 70, a multidisciplinary panel of experts and patient advocates determined based on a comprehensive literature review.
The panel’s findings were presented in a poster at the ASCO Genitourinary Symposium.
“Black men in the United States are considered a high-risk population for being diagnosed with and dying from prostate cancer,” lead author Isla Garraway, MD, PhD, of the University of California, Los Angeles, and colleagues wrote. Specifically, Black men are about two times more likely to be diagnosed with and die from prostate cancer than White men. But, the authors continued, “few guidelines have outlined specific recommendations for PSA-based prostate cancer screening among Black men.”
The US Preventive Services Taskforce recommendations, which are currently being updated, set the PSA screening start age at 55. The task force recommendations, which dictate insurance coverage in the United States, acknowledged “a potential mortality benefit for African American men when beginning screening before age 55 years” but did not explicitly recommend screening earlier.
Current guidelines from the American Cancer Society call for discussions about screening in average-risk men to begin at age 50-55. The recommendations do specify lowering the age to 45 for those at a high risk for prostate cancer, which includes Black men as well as those with a first-degree relative diagnosed with prostate cancer before age 65. In some cases, screening can begin at age 40 in the highest risk men — those with more than one first-degree relative who had prostate cancer at a young age.
The Prostate Cancer Foundation “wanted to address the confusion around different guideline statements and the lack of clarity around screening recommendations for Black men,” said William K. Oh, MD, of The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, who chaired the panel for the new guidelines. “We thus convened a distinguished panel of experts from diverse backgrounds and expertise to create six guidelines statements to help Black men, their families, and their healthcare providers to consider options for prostate cancer screening based on the best available evidence.”
After reviewing 287, the expert panel developed six new guideline statements, reaching at least 80% consensus among panel members, addressing screening for Black men:
Because Black men are at a high risk for prostate cancer, the benefits of screening generally outweigh the risks.
PSA testing should be considered first line for prostate cancer screening, although some providers may recommend an optional digital rectal exam in addition to the PSA test.
Black men should engage in shared decision-making with their healthcare providers and other trusted sources of information to learn about the pros and cons of screening.
For Black men who elect screening, a baseline PSA test should be done between ages 40 and 45, and annual PSA screening should be strongly considered based on the PSA value and the individual’s health status.
Black men over age 70 who have been undergoing prostate cancer screening should talk with their healthcare provider about whether to continue PSA testing and make an informed decision based on their age, life expectancy, health status, family history, and prior PSA levels.
Black men who are at even higher risk due to a strong family history and/or known carriers of high-risk genetic variants should consider initiating annual PSA screening as early as age 40.
These statements are based on “the best available evidence, which overwhelmingly supports the conclusion that Black men in the US could benefit from a risk-adapted PSA screening,” the investigators concluded, noting that the latest evidence “warrants revisiting current recommendations for early [prostate cancer] detection in Black men from other national guideline groups.”
“We believe that the outcome of these more directed guidelines will be to give clarity to these men,” Dr. Oh added.
This research was funded by the Prostate Cancer Foundation, National Cancer Institute, Veterans Affairs, Jean Perkins Foundation, and Department of Defense. Dr. Garraway reported having no disclosures.
A version of this article appeared on Medscape.com.
FROM ASCO GU 2024
CT Poses Risk for Malignant Hematopathies Among Children
More than a million European children undergo a CT scan each year. Ionizing radiation at moderate (> 100 mGy) to high (> 1 Gy) doses is a recognized risk factor for malignant hematopathies. The risk associated with exposure to low doses (< 100 mGy), typically delivered during a CT scan in children or adolescents, is unknown.
Previous studies assessed the risk for malignant hematopathies related to ionizing radiation from CT scans in young patients. Some showed an increased risk for leukemia with repeated scans, but confounding factors resulted in a lack of statistical power or biases in some cases. The EPI-CT study, coordinated by the International Agency for Research on Cancer, aimed to evaluate the cancer risk among children and adolescents after exposure to low doses of ionizing radiation during CT scans.
A European Cohort
A recent article presents an assessment of observed malignant hematopathies following CT scan. The authors followed a multinational European cohort of 948,174 patients who had a CT scan before age 22 years. Ionizing radiation doses to the bone marrow were evaluated based on the scanned body region, patient characteristics, scan year, and the technical parameters of the machine. The analysis involved 876,771 patients who underwent 1,331,896 scans (an average of 1.52 per patient) and were followed for at least 2 years after the first scan.
In total, 790 malignant hematopathies were diagnosed, including 578 lymphoid hematopathies and 203 myeloid hematopathies and acute leukemias. The average follow-up period was 7.8 years. At the time of diagnosis, 51% of patients were under the age of 20 years, and 88.5% were under the age of 30 years. There was an association between cumulative dose and the observed malignant hematopathy, with an observed rate of 1.96 per 100 mGy (790 cases).
This rate corresponds to a 16% increased rate per scan (for a dose observed per scan of 8 mGy). A higher rate for any type of malignant hematopathy was observed for doses > 10 mGy, with an observed rate of 2.66 for doses > 50 mGy, compared with doses < 5 mGy.
The rate of malignant hematopathy increased with older age at the time of radiation exposure, particularly for lymphoid observations. The rate in the 5- to 9-year age group and the > 10-year age group was, respectively, two times and three to four times higher than that in the < 5-year age group. The rate decreased over time, with the highest observed rate between 2 and 5 years after ionizing radiation exposure and the lowest after 10 years.
CT Scans Must Be Warranted
This study, which involved nearly a million patients, has higher statistical power than previous studies, despite missing or approximate data (including that related to actually delivered doses). An association was shown between cumulative dose to the bone marrow and the risk of developing malignant hematopathy, both lymphoid and myeloid, with an increased risk even at low doses (10-15 mGy).
The results suggest that for every 10,000 children examined today (with a dose per scan of 8 mGy), 1-2 could develop a radiation-related malignant hematopathy in the next 12 years (1.4 cases). This study confirms the higher risk for cancer at low radiation doses and emphasizes the importance of justifying each pediatric CT scan and optimizing delivered doses. It is important to recall that an MRI or ultrasound can sometimes be an adequate substitute for a CT scan.
This article was translated from JIM , which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com .
More than a million European children undergo a CT scan each year. Ionizing radiation at moderate (> 100 mGy) to high (> 1 Gy) doses is a recognized risk factor for malignant hematopathies. The risk associated with exposure to low doses (< 100 mGy), typically delivered during a CT scan in children or adolescents, is unknown.
Previous studies assessed the risk for malignant hematopathies related to ionizing radiation from CT scans in young patients. Some showed an increased risk for leukemia with repeated scans, but confounding factors resulted in a lack of statistical power or biases in some cases. The EPI-CT study, coordinated by the International Agency for Research on Cancer, aimed to evaluate the cancer risk among children and adolescents after exposure to low doses of ionizing radiation during CT scans.
A European Cohort
A recent article presents an assessment of observed malignant hematopathies following CT scan. The authors followed a multinational European cohort of 948,174 patients who had a CT scan before age 22 years. Ionizing radiation doses to the bone marrow were evaluated based on the scanned body region, patient characteristics, scan year, and the technical parameters of the machine. The analysis involved 876,771 patients who underwent 1,331,896 scans (an average of 1.52 per patient) and were followed for at least 2 years after the first scan.
In total, 790 malignant hematopathies were diagnosed, including 578 lymphoid hematopathies and 203 myeloid hematopathies and acute leukemias. The average follow-up period was 7.8 years. At the time of diagnosis, 51% of patients were under the age of 20 years, and 88.5% were under the age of 30 years. There was an association between cumulative dose and the observed malignant hematopathy, with an observed rate of 1.96 per 100 mGy (790 cases).
This rate corresponds to a 16% increased rate per scan (for a dose observed per scan of 8 mGy). A higher rate for any type of malignant hematopathy was observed for doses > 10 mGy, with an observed rate of 2.66 for doses > 50 mGy, compared with doses < 5 mGy.
The rate of malignant hematopathy increased with older age at the time of radiation exposure, particularly for lymphoid observations. The rate in the 5- to 9-year age group and the > 10-year age group was, respectively, two times and three to four times higher than that in the < 5-year age group. The rate decreased over time, with the highest observed rate between 2 and 5 years after ionizing radiation exposure and the lowest after 10 years.
CT Scans Must Be Warranted
This study, which involved nearly a million patients, has higher statistical power than previous studies, despite missing or approximate data (including that related to actually delivered doses). An association was shown between cumulative dose to the bone marrow and the risk of developing malignant hematopathy, both lymphoid and myeloid, with an increased risk even at low doses (10-15 mGy).
The results suggest that for every 10,000 children examined today (with a dose per scan of 8 mGy), 1-2 could develop a radiation-related malignant hematopathy in the next 12 years (1.4 cases). This study confirms the higher risk for cancer at low radiation doses and emphasizes the importance of justifying each pediatric CT scan and optimizing delivered doses. It is important to recall that an MRI or ultrasound can sometimes be an adequate substitute for a CT scan.
This article was translated from JIM , which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com .
More than a million European children undergo a CT scan each year. Ionizing radiation at moderate (> 100 mGy) to high (> 1 Gy) doses is a recognized risk factor for malignant hematopathies. The risk associated with exposure to low doses (< 100 mGy), typically delivered during a CT scan in children or adolescents, is unknown.
Previous studies assessed the risk for malignant hematopathies related to ionizing radiation from CT scans in young patients. Some showed an increased risk for leukemia with repeated scans, but confounding factors resulted in a lack of statistical power or biases in some cases. The EPI-CT study, coordinated by the International Agency for Research on Cancer, aimed to evaluate the cancer risk among children and adolescents after exposure to low doses of ionizing radiation during CT scans.
A European Cohort
A recent article presents an assessment of observed malignant hematopathies following CT scan. The authors followed a multinational European cohort of 948,174 patients who had a CT scan before age 22 years. Ionizing radiation doses to the bone marrow were evaluated based on the scanned body region, patient characteristics, scan year, and the technical parameters of the machine. The analysis involved 876,771 patients who underwent 1,331,896 scans (an average of 1.52 per patient) and were followed for at least 2 years after the first scan.
In total, 790 malignant hematopathies were diagnosed, including 578 lymphoid hematopathies and 203 myeloid hematopathies and acute leukemias. The average follow-up period was 7.8 years. At the time of diagnosis, 51% of patients were under the age of 20 years, and 88.5% were under the age of 30 years. There was an association between cumulative dose and the observed malignant hematopathy, with an observed rate of 1.96 per 100 mGy (790 cases).
This rate corresponds to a 16% increased rate per scan (for a dose observed per scan of 8 mGy). A higher rate for any type of malignant hematopathy was observed for doses > 10 mGy, with an observed rate of 2.66 for doses > 50 mGy, compared with doses < 5 mGy.
The rate of malignant hematopathy increased with older age at the time of radiation exposure, particularly for lymphoid observations. The rate in the 5- to 9-year age group and the > 10-year age group was, respectively, two times and three to four times higher than that in the < 5-year age group. The rate decreased over time, with the highest observed rate between 2 and 5 years after ionizing radiation exposure and the lowest after 10 years.
CT Scans Must Be Warranted
This study, which involved nearly a million patients, has higher statistical power than previous studies, despite missing or approximate data (including that related to actually delivered doses). An association was shown between cumulative dose to the bone marrow and the risk of developing malignant hematopathy, both lymphoid and myeloid, with an increased risk even at low doses (10-15 mGy).
The results suggest that for every 10,000 children examined today (with a dose per scan of 8 mGy), 1-2 could develop a radiation-related malignant hematopathy in the next 12 years (1.4 cases). This study confirms the higher risk for cancer at low radiation doses and emphasizes the importance of justifying each pediatric CT scan and optimizing delivered doses. It is important to recall that an MRI or ultrasound can sometimes be an adequate substitute for a CT scan.
This article was translated from JIM , which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com .
Ibuprofen Fails for Patent Ductus Arteriosus in Preterm Infants
The study population included infants born between 23 weeks 0 days’ and 28 weeks 6 days’ gestation. The researchers randomized 326 extremely preterm infants with patent ductus arteriosus (PDA) at 72 hours or less after birth to ibuprofen at a loading dose of 10 mg/kg followed by two doses of 5 mg/kg at least 24 hours apart, and 327 to placebo.
The PDAs in the infants had a diameter of at least 1.5 mm with pulsatile flow.
Severe dysplasia outcome
The study’s primary outcome was a composite of death or moderate to severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. Overall, a primary outcome occurred in 69.2% of infants who received ibuprofen and 63.5% of those who received a placebo.
Risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was not reduced by early ibuprofen vs. placebo for preterm infants, the researchers concluded. Moderate or severe bronchopulmonary dysplasia occurred in 64.2% of the infants in the ibuprofen group and 59.3% of the placebo group who survived to 36 weeks’ postmenstrual age.
‘Unforeseeable’ serious adverse events
Forty-four deaths occurred in the ibuprofen group and 33 in the placebo group (adjusted risk ratio 1.09). Two “unforeseeable” serious adverse events occurred during the study that were potentially related to ibuprofen.
The lead author was Samir Gupta, MD, of Sidra Medicine, Doha, Qatar. The study was published online in the New England Journal of Medicine.
Study limitations include incomplete data for some patients.
The study was supported by the National Institute for Health Research Health Technology Assessment Programme. The researchers had no financial conflicts to disclose.
The study population included infants born between 23 weeks 0 days’ and 28 weeks 6 days’ gestation. The researchers randomized 326 extremely preterm infants with patent ductus arteriosus (PDA) at 72 hours or less after birth to ibuprofen at a loading dose of 10 mg/kg followed by two doses of 5 mg/kg at least 24 hours apart, and 327 to placebo.
The PDAs in the infants had a diameter of at least 1.5 mm with pulsatile flow.
Severe dysplasia outcome
The study’s primary outcome was a composite of death or moderate to severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. Overall, a primary outcome occurred in 69.2% of infants who received ibuprofen and 63.5% of those who received a placebo.
Risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was not reduced by early ibuprofen vs. placebo for preterm infants, the researchers concluded. Moderate or severe bronchopulmonary dysplasia occurred in 64.2% of the infants in the ibuprofen group and 59.3% of the placebo group who survived to 36 weeks’ postmenstrual age.
‘Unforeseeable’ serious adverse events
Forty-four deaths occurred in the ibuprofen group and 33 in the placebo group (adjusted risk ratio 1.09). Two “unforeseeable” serious adverse events occurred during the study that were potentially related to ibuprofen.
The lead author was Samir Gupta, MD, of Sidra Medicine, Doha, Qatar. The study was published online in the New England Journal of Medicine.
Study limitations include incomplete data for some patients.
The study was supported by the National Institute for Health Research Health Technology Assessment Programme. The researchers had no financial conflicts to disclose.
The study population included infants born between 23 weeks 0 days’ and 28 weeks 6 days’ gestation. The researchers randomized 326 extremely preterm infants with patent ductus arteriosus (PDA) at 72 hours or less after birth to ibuprofen at a loading dose of 10 mg/kg followed by two doses of 5 mg/kg at least 24 hours apart, and 327 to placebo.
The PDAs in the infants had a diameter of at least 1.5 mm with pulsatile flow.
Severe dysplasia outcome
The study’s primary outcome was a composite of death or moderate to severe bronchopulmonary dysplasia at 36 weeks’ postmenstrual age. Overall, a primary outcome occurred in 69.2% of infants who received ibuprofen and 63.5% of those who received a placebo.
Risk of death or bronchopulmonary dysplasia at 36 weeks’ postmenstrual age was not reduced by early ibuprofen vs. placebo for preterm infants, the researchers concluded. Moderate or severe bronchopulmonary dysplasia occurred in 64.2% of the infants in the ibuprofen group and 59.3% of the placebo group who survived to 36 weeks’ postmenstrual age.
‘Unforeseeable’ serious adverse events
Forty-four deaths occurred in the ibuprofen group and 33 in the placebo group (adjusted risk ratio 1.09). Two “unforeseeable” serious adverse events occurred during the study that were potentially related to ibuprofen.
The lead author was Samir Gupta, MD, of Sidra Medicine, Doha, Qatar. The study was published online in the New England Journal of Medicine.
Study limitations include incomplete data for some patients.
The study was supported by the National Institute for Health Research Health Technology Assessment Programme. The researchers had no financial conflicts to disclose.
Stockholm3 Prostate Test Bests PSA for Prostate Cancer Risk in North America
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
The Stockholm3 (A3P Biomedical) multiparametic blood test has shown accuracy in assessing the risk of prostate cancer, exceeding that of the standard prostate-specific antigen (PSA)-based test, in Swedish patients.
“The Stockholm3 outperformed the PSA test overall and in every subcohort, with an impressive reduction of unnecessary biopsies of 40% to 50%, while maintaining relative sensitivity,” first author Scott E. Eggener, MD, said in presenting the findings at the ASCO Genitourinary Cancers Symposium. The test “has attractive characteristics in a diverse cohort, including within various racial and ethnic subgroups,” added Dr. Eggener, professor of surgery and radiology at the University of Chicago.
While the PSA test, the standard-of-care in prostate cancer risk assessment, reduces mortality, the test is known to have a risk for false positive results, leading to unnecessary prostate biopsies, as well as overdiagnosis of low-risk prostate cancers, Dr. Eggener explained in his talk.
Randomized trials do show “fewer men die from prostate cancer with screening [with PSA testing], however, the likelihood of unnecessarily finding out about a cancer, undergoing treatment, and exposure to potential treatment-related side effects is significantly higher,” Dr. Eggener said in a interview.
The Stockholm3 clinical diagnostic prostate cancer test, which has been used in Sweden and Norway since 2017, was validated in a sample of nearly 60,000 men in the STHLM3 study (doi: 10.1016/S1470-2045[15]00361-7), which was published in The Lancet Oncology in December 2015. That study showed significant improvement over PSA alone detection of prostate cancers with a Gleason score of at least 7 (P < .0001), Dr. Eggener explained.
The test combines five plasma protein markers, including total and free PSA, PSP94, GDF-15 and KLK2, along with 101 genetic markers and clinical patient data, including age, previous biopsy results and family history.
Because the Stockholm3 test was validated in a Swedish population cohort, evidence on the accuracy of the test in other racial and ethnic populations is lacking, the authors noted in the abstract.
Study Methods and Results
To further investigate, Dr. Eggener and his colleagues conducted the prospective SEPTA trial, involving 2,129 men with no known prostate cancer but clinical indications for prostate biopsy, who were referred for prostate biopsy at 17 North American sites between 2019 and 2023.
Among the men, 24% were self-identified as African American/Black; 46% were White/Caucasian; 14% were Hispanic/Latina; and 16% were Asian. The men’s median age was 63; their median PSA value was 6.1 ng/mL, according to the abstract.
Of the patients, 16% received magnetic resonance imaging (MRI)-targeted biopsies and 20% had prior benign biopsies, the abstract notes.
Biopsy results showed that clinically significant prostate cancer, defined as International Society of Urological Pathology (ISUP) Gleason Grade group ≥ 2, was detected in 29% of patients, with 14% having ISUP 1 cancer and 57% of cases having been benign, according to the abstract.
Overall detection rates of grade 2 or higher were 37% for African American/Black, 28% for White/Caucasian, 29% for Hispanic/Latino, and 21% Asian.
In terms of sensitivity of the two tests, the Stockholm3 (cut-point of ≥ 15) was noninferior compared with the traditional PSA cut-point of ≥ 4 ng/mL (relative sensitivity 0.95).
Results were consistent across ethnic subgroups: noninferior sensitivity (0.91-0.98) and superior specificity (2.51-4.70), the abstract authors reported.
Compared with the use of the PSA test’s cut-point of ≥ 4 ng/mL, the use of Stockholm3’s cut-point of ≥ 15 or higher would have reduced unnecessary biopsies by 45% overall, including by 46% among Asian and Black/African American patients, by 53% in Hispanic patients and 42% in White patients, according to the abstract.
Overall, “utilization of Stockholm3 improves the net benefit:harm ratio of PSA screening by identifying nearly all men with Gleason Grade 2 or higher, while minimizing the number of men undergoing biopsy who show no cancer or an indolent cancer (Gleason Grade 1),” Dr. Eggener said in an interview.
Stockholm3 Expected to be Available in U.S. This Year
The test, which has been available in Sweden since 2018, is expected to become commercially available in the United States in early 2024. Dr. Eggener noted that “cost of the test hasn’t been finalized, but will be considerably more expensive than PSA, which is very cheap.”
Commenting on the findings, Bradley McGregor, MD, of the Dana Farber Cancer Institute and an ASCO oncology expert, noted that “ultimately, the goal [of prostate screening] is to be able to better decide when a biopsy is going to yield a clinically relevant prostate cancer, [and] this study gives us some insight of the use of the Stockholm3 tool in a more diverse population.
“How the tool will be utilized in the clinic and in guidelines is something that is a work in progress,” he added. “But I think this provides some reassurances that this will have implications beyond just the homogeneous populations in the original studies.”
Dr. McGregor noted that considerations of the issue of cost should be weighed against the potential costs involved in unnecessary biopsies and a host of other costs that can arise with an inaccurate risk assessment.
“If there is a way to avoid those costs and help us have more confidence in the prostate test results and intervene at an earlier stage, I think that’s exciting,” he said.
Dr. Eggener has consulted for A3P Biomedical but had no financial relationship with the company to disclose.
FROM ASCO GU 2024
PCPs Increasingly Chained to EHRs
If you feel like the day doesn’t hold enough hours for you to get your work done, you’re right: (EHRs).
Investigators followed 141 academic PCPs between May 2019 and March 2023 and found they spent considerably more time engaging in EHR tasks during the final year of the study than in the prepandemic period. EHR time increased by over 8% on days with scheduled appointments and almost 20% on days without scheduled appointments.
“Physicians spend an unsustainable amount of time on EHR-based work, and that amount has increased steadily from 2019 to 2023,” Christine Sinsky, MD, vice president of professional satisfaction at the American Medical Association (AMA) and the senior author of the study, told this news organization. “It is imperative for healthcare systems to develop strategies to change the overall EHR workload trajectory to minimize PCPs’ occupational stress, including improved workflows, where the work is more appropriately distributed amongst the team.”
The study was published online on January 22, 2024, in the Annals of Family Medicine.
‘Pajama Time’
Dr. Sinsky said the motivation for conducting the current study was that PCPs have reported an increase in their workload, especially EHR tasks outside of work (“pajama time”) since the onset of the pandemic.
The research followed up on a 2017 analysis from the same group and other findings showing an increase in the time physicians spend in EHR tasks and the number of Inbox messages they receive from patients seeking medical advice increased during the months following the start of the pandemic.
“As a busy practicing PCP with a large panel of patients, my sense was that the workload was increasing even more, which is what our study confirmed,” said Brian G. Arndt, MD, of the Department of Family Medicine and Community Heath at the University of Wisconsin School of Medicine and Public Health, in Madison, Wisconsin, who led the new study.
The researchers analyzed EHR usage of 141 academic PCPs practicing family medicine, internal medicine, and general pediatrics, two thirds (66.7%) of whom were female. They compared the amount of time spent on EHR tasks during four timespans:
- May 2019 to February 2020
- June 2020 to March 2021
- May 2021 to March 2022
- April 2022 to March 2023
Each PCP’s time and Inbox message volume were calculated and then normalized over 8 hours of scheduled clinic appointments.
Increased Time, Increased Burnout
The study found evidence PCPs have reduced their clinical hours in response to their growing digital workload.
“We have a serious shortage of primary care physicians,” Dr. Sinsky said. “When PCPs cut back their clinical [work] as a coping mechanism for an unmanageable workload, this further exacerbates the primary care shortage, reducing access to care for patients.”
The researchers found increases from the first prepandemic period to the final period of their study in average time that PCPs spent at the EHR per 8 hours of scheduled clinic appointments (Table).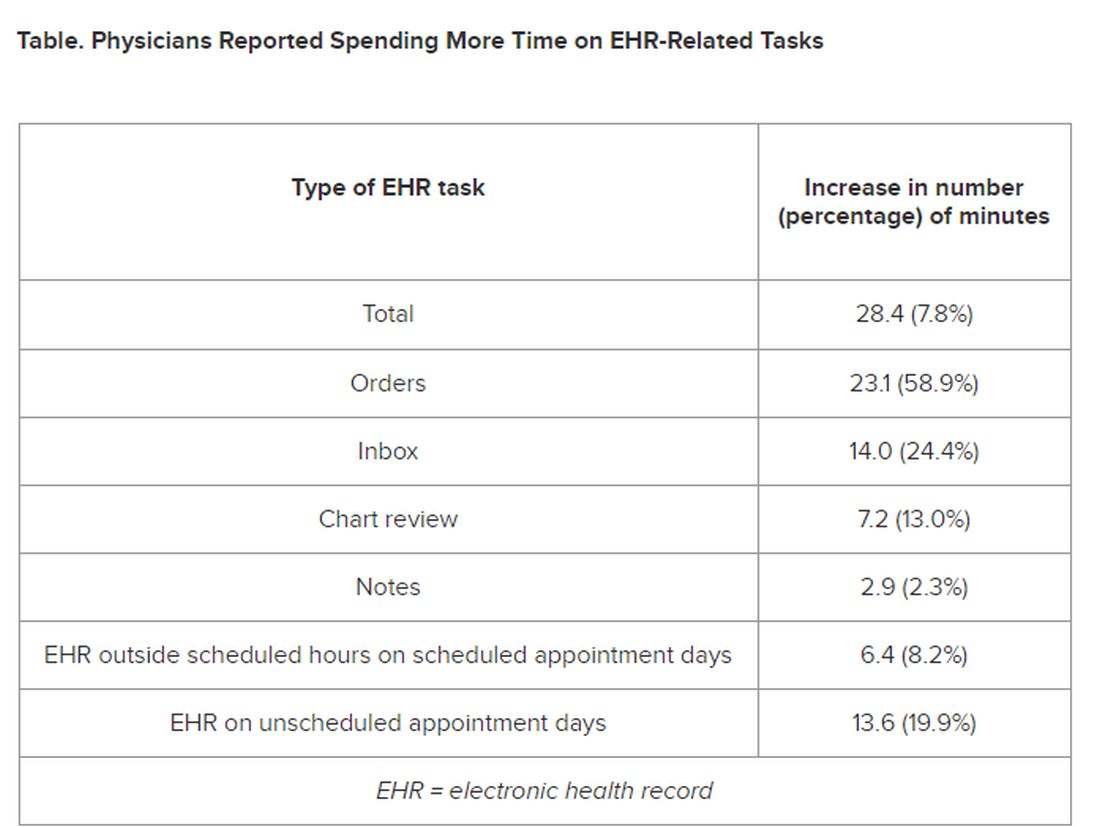
PCPs were inundated with several types of EHR-related responsibilities, including more medical advice requests (+55.5%) and more prescription messages (+19.5%) per 8 hours of scheduled clinic appointments. On the other hand, they had slightly fewer patient calls (−10.5%) and messages concerning test results (−2.7%).
A recent study of 307 PCPs across 31 primary care practices paralleled these findings. It found that physicians spent 36.2 minutes on the EHR per visit (interquartile range, 28.9-45.7 minutes). Included were 6.2 minutes of “pajama time” per visit and 7.8 minutes on the EHR per visit.
The amount of EHR time exceeded the amount of time allotted to a primary care visit (30 minutes). The authors commented that the EHR time burden “and the burnout associated with this burden represent a serious threat to the primary care physician workforce.”
“As more health systems across the country transition from fee-for-service to value-based payment arrangements, they need to balance the time PCPs and their care teams need for face-to-face care — in-person or video visits — with the increasing asynchronous care patients are seeking from us through the portal, for example, MyChart,” Dr. Arndt said.
Sinsky noted that when patients receive care from a PCP, quality is higher and costs are lower. “When access to primary care is further limited by virtue of physicians being overwhelmed by administrative work implemented via the EHR, so that they are reducing their hours, then we can expect negative consequences for patient care and costs of care.”
Tips for Reducing EHR Time
Arndt noted that some “brief investments” of time with patients “lead to high rates of return on decreased MyChart messaging.” For example, he has said to patients: “In the future, there’s no need to respond in MyChart with a ‘Thank you.’” Or “In the future, if you have questions from preappointment labs, no need to send me a separate message in MyChart prior to your visit since they’re typically just a few days out. I look closely at your labs and would always pick up the phone and call you if there was anything more urgent or pressing that needs more immediate action.”
Sinsky recommended two “high-yield opportunities” to reduce EHR-associated workload. The AMA offers a brief Inbox reduction checklist as well as a detailed toolkit to guide physicians and operational leaders in reducing the volume of unnecessary Inbox messages, she said.
Distribution of work among the team also can reduce the time physicians spent on order entry. “It doesn’t take a medical school education to enter orders for flu shots, lipid profiles, mammograms, and other tests, and yet we have primary care physicians around the country spending an hour or more per 8 hours of patient visits on this task,” she said.
‘Growing Mountain’
Sally Baxter, MD, assistant professor of ophthalmology and division chief for Ophthalmology Informatics and Data Sciences at University of California San Diego, said, “Studies like this ... are important for continuing to quantify the burden of EHR work and to evaluate potential interventions to reduce this burden and subsequent burnout.”
Baxter’s health system allows physicians to bill for asynchronous messaging when certain eligibility criteria are met. “This can deter frivolous messaging and also provide some compensation for the work involved,” she said.
“In addition, we’ve recently piloted using AI tools to help draft replies to patient messages in the EHR as another approach to tackling this important issue,” said Baxter, who wasn’t involved with the current study.
Eve Rittenberg, MD, an assistant professor at Harvard Medical School and a PCP at Brigham and Women’s Hospital Fish Center for Women’s Health, in Boston, recommended that healthcare systems “monitor EHR workload across gender, specialty, and other variables to develop equitable support and compensation models.”
Dr. Rittenberg, who wasn’t involved with the current study, said healthcare systems should consider supporting physicians by blocking out time during clinic sessions to manage their EHR work. “Cross-coverage systems are vital so that on their days off, physicians can unplug from the computer and know that their patients’ needs are being met,” she added.
This work was supported in part by the AMA Practice Transformation Initiative: EHR-Use Metrics Research which provided grant funding to several of the authors. Sinsky is employed by the AMA. Dr. Arndt and coauthors disclosed no relevant financial information. Dr. Baxter received nonfinancial support from Optonmed and Topcon for research studies and collaborated with some of the study authors on other research but not this particular study. Dr. Rittenberg received internal funding from the Brigham Care Redesign Incubator and Startup Program, Brigham and Women’s Hospital, for a previous pilot project of inbasket cross-coverage. She had no relevant current disclosures.
A version of this article appeared on Medscape.com.
If you feel like the day doesn’t hold enough hours for you to get your work done, you’re right: (EHRs).
Investigators followed 141 academic PCPs between May 2019 and March 2023 and found they spent considerably more time engaging in EHR tasks during the final year of the study than in the prepandemic period. EHR time increased by over 8% on days with scheduled appointments and almost 20% on days without scheduled appointments.
“Physicians spend an unsustainable amount of time on EHR-based work, and that amount has increased steadily from 2019 to 2023,” Christine Sinsky, MD, vice president of professional satisfaction at the American Medical Association (AMA) and the senior author of the study, told this news organization. “It is imperative for healthcare systems to develop strategies to change the overall EHR workload trajectory to minimize PCPs’ occupational stress, including improved workflows, where the work is more appropriately distributed amongst the team.”
The study was published online on January 22, 2024, in the Annals of Family Medicine.
‘Pajama Time’
Dr. Sinsky said the motivation for conducting the current study was that PCPs have reported an increase in their workload, especially EHR tasks outside of work (“pajama time”) since the onset of the pandemic.
The research followed up on a 2017 analysis from the same group and other findings showing an increase in the time physicians spend in EHR tasks and the number of Inbox messages they receive from patients seeking medical advice increased during the months following the start of the pandemic.
“As a busy practicing PCP with a large panel of patients, my sense was that the workload was increasing even more, which is what our study confirmed,” said Brian G. Arndt, MD, of the Department of Family Medicine and Community Heath at the University of Wisconsin School of Medicine and Public Health, in Madison, Wisconsin, who led the new study.
The researchers analyzed EHR usage of 141 academic PCPs practicing family medicine, internal medicine, and general pediatrics, two thirds (66.7%) of whom were female. They compared the amount of time spent on EHR tasks during four timespans:
- May 2019 to February 2020
- June 2020 to March 2021
- May 2021 to March 2022
- April 2022 to March 2023
Each PCP’s time and Inbox message volume were calculated and then normalized over 8 hours of scheduled clinic appointments.
Increased Time, Increased Burnout
The study found evidence PCPs have reduced their clinical hours in response to their growing digital workload.
“We have a serious shortage of primary care physicians,” Dr. Sinsky said. “When PCPs cut back their clinical [work] as a coping mechanism for an unmanageable workload, this further exacerbates the primary care shortage, reducing access to care for patients.”
The researchers found increases from the first prepandemic period to the final period of their study in average time that PCPs spent at the EHR per 8 hours of scheduled clinic appointments (Table).
PCPs were inundated with several types of EHR-related responsibilities, including more medical advice requests (+55.5%) and more prescription messages (+19.5%) per 8 hours of scheduled clinic appointments. On the other hand, they had slightly fewer patient calls (−10.5%) and messages concerning test results (−2.7%).
A recent study of 307 PCPs across 31 primary care practices paralleled these findings. It found that physicians spent 36.2 minutes on the EHR per visit (interquartile range, 28.9-45.7 minutes). Included were 6.2 minutes of “pajama time” per visit and 7.8 minutes on the EHR per visit.
The amount of EHR time exceeded the amount of time allotted to a primary care visit (30 minutes). The authors commented that the EHR time burden “and the burnout associated with this burden represent a serious threat to the primary care physician workforce.”
“As more health systems across the country transition from fee-for-service to value-based payment arrangements, they need to balance the time PCPs and their care teams need for face-to-face care — in-person or video visits — with the increasing asynchronous care patients are seeking from us through the portal, for example, MyChart,” Dr. Arndt said.
Sinsky noted that when patients receive care from a PCP, quality is higher and costs are lower. “When access to primary care is further limited by virtue of physicians being overwhelmed by administrative work implemented via the EHR, so that they are reducing their hours, then we can expect negative consequences for patient care and costs of care.”
Tips for Reducing EHR Time
Arndt noted that some “brief investments” of time with patients “lead to high rates of return on decreased MyChart messaging.” For example, he has said to patients: “In the future, there’s no need to respond in MyChart with a ‘Thank you.’” Or “In the future, if you have questions from preappointment labs, no need to send me a separate message in MyChart prior to your visit since they’re typically just a few days out. I look closely at your labs and would always pick up the phone and call you if there was anything more urgent or pressing that needs more immediate action.”
Sinsky recommended two “high-yield opportunities” to reduce EHR-associated workload. The AMA offers a brief Inbox reduction checklist as well as a detailed toolkit to guide physicians and operational leaders in reducing the volume of unnecessary Inbox messages, she said.
Distribution of work among the team also can reduce the time physicians spent on order entry. “It doesn’t take a medical school education to enter orders for flu shots, lipid profiles, mammograms, and other tests, and yet we have primary care physicians around the country spending an hour or more per 8 hours of patient visits on this task,” she said.
‘Growing Mountain’
Sally Baxter, MD, assistant professor of ophthalmology and division chief for Ophthalmology Informatics and Data Sciences at University of California San Diego, said, “Studies like this ... are important for continuing to quantify the burden of EHR work and to evaluate potential interventions to reduce this burden and subsequent burnout.”
Baxter’s health system allows physicians to bill for asynchronous messaging when certain eligibility criteria are met. “This can deter frivolous messaging and also provide some compensation for the work involved,” she said.
“In addition, we’ve recently piloted using AI tools to help draft replies to patient messages in the EHR as another approach to tackling this important issue,” said Baxter, who wasn’t involved with the current study.
Eve Rittenberg, MD, an assistant professor at Harvard Medical School and a PCP at Brigham and Women’s Hospital Fish Center for Women’s Health, in Boston, recommended that healthcare systems “monitor EHR workload across gender, specialty, and other variables to develop equitable support and compensation models.”
Dr. Rittenberg, who wasn’t involved with the current study, said healthcare systems should consider supporting physicians by blocking out time during clinic sessions to manage their EHR work. “Cross-coverage systems are vital so that on their days off, physicians can unplug from the computer and know that their patients’ needs are being met,” she added.
This work was supported in part by the AMA Practice Transformation Initiative: EHR-Use Metrics Research which provided grant funding to several of the authors. Sinsky is employed by the AMA. Dr. Arndt and coauthors disclosed no relevant financial information. Dr. Baxter received nonfinancial support from Optonmed and Topcon for research studies and collaborated with some of the study authors on other research but not this particular study. Dr. Rittenberg received internal funding from the Brigham Care Redesign Incubator and Startup Program, Brigham and Women’s Hospital, for a previous pilot project of inbasket cross-coverage. She had no relevant current disclosures.
A version of this article appeared on Medscape.com.
If you feel like the day doesn’t hold enough hours for you to get your work done, you’re right: (EHRs).
Investigators followed 141 academic PCPs between May 2019 and March 2023 and found they spent considerably more time engaging in EHR tasks during the final year of the study than in the prepandemic period. EHR time increased by over 8% on days with scheduled appointments and almost 20% on days without scheduled appointments.
“Physicians spend an unsustainable amount of time on EHR-based work, and that amount has increased steadily from 2019 to 2023,” Christine Sinsky, MD, vice president of professional satisfaction at the American Medical Association (AMA) and the senior author of the study, told this news organization. “It is imperative for healthcare systems to develop strategies to change the overall EHR workload trajectory to minimize PCPs’ occupational stress, including improved workflows, where the work is more appropriately distributed amongst the team.”
The study was published online on January 22, 2024, in the Annals of Family Medicine.
‘Pajama Time’
Dr. Sinsky said the motivation for conducting the current study was that PCPs have reported an increase in their workload, especially EHR tasks outside of work (“pajama time”) since the onset of the pandemic.
The research followed up on a 2017 analysis from the same group and other findings showing an increase in the time physicians spend in EHR tasks and the number of Inbox messages they receive from patients seeking medical advice increased during the months following the start of the pandemic.
“As a busy practicing PCP with a large panel of patients, my sense was that the workload was increasing even more, which is what our study confirmed,” said Brian G. Arndt, MD, of the Department of Family Medicine and Community Heath at the University of Wisconsin School of Medicine and Public Health, in Madison, Wisconsin, who led the new study.
The researchers analyzed EHR usage of 141 academic PCPs practicing family medicine, internal medicine, and general pediatrics, two thirds (66.7%) of whom were female. They compared the amount of time spent on EHR tasks during four timespans:
- May 2019 to February 2020
- June 2020 to March 2021
- May 2021 to March 2022
- April 2022 to March 2023
Each PCP’s time and Inbox message volume were calculated and then normalized over 8 hours of scheduled clinic appointments.
Increased Time, Increased Burnout
The study found evidence PCPs have reduced their clinical hours in response to their growing digital workload.
“We have a serious shortage of primary care physicians,” Dr. Sinsky said. “When PCPs cut back their clinical [work] as a coping mechanism for an unmanageable workload, this further exacerbates the primary care shortage, reducing access to care for patients.”
The researchers found increases from the first prepandemic period to the final period of their study in average time that PCPs spent at the EHR per 8 hours of scheduled clinic appointments (Table).
PCPs were inundated with several types of EHR-related responsibilities, including more medical advice requests (+55.5%) and more prescription messages (+19.5%) per 8 hours of scheduled clinic appointments. On the other hand, they had slightly fewer patient calls (−10.5%) and messages concerning test results (−2.7%).
A recent study of 307 PCPs across 31 primary care practices paralleled these findings. It found that physicians spent 36.2 minutes on the EHR per visit (interquartile range, 28.9-45.7 minutes). Included were 6.2 minutes of “pajama time” per visit and 7.8 minutes on the EHR per visit.
The amount of EHR time exceeded the amount of time allotted to a primary care visit (30 minutes). The authors commented that the EHR time burden “and the burnout associated with this burden represent a serious threat to the primary care physician workforce.”
“As more health systems across the country transition from fee-for-service to value-based payment arrangements, they need to balance the time PCPs and their care teams need for face-to-face care — in-person or video visits — with the increasing asynchronous care patients are seeking from us through the portal, for example, MyChart,” Dr. Arndt said.
Sinsky noted that when patients receive care from a PCP, quality is higher and costs are lower. “When access to primary care is further limited by virtue of physicians being overwhelmed by administrative work implemented via the EHR, so that they are reducing their hours, then we can expect negative consequences for patient care and costs of care.”
Tips for Reducing EHR Time
Arndt noted that some “brief investments” of time with patients “lead to high rates of return on decreased MyChart messaging.” For example, he has said to patients: “In the future, there’s no need to respond in MyChart with a ‘Thank you.’” Or “In the future, if you have questions from preappointment labs, no need to send me a separate message in MyChart prior to your visit since they’re typically just a few days out. I look closely at your labs and would always pick up the phone and call you if there was anything more urgent or pressing that needs more immediate action.”
Sinsky recommended two “high-yield opportunities” to reduce EHR-associated workload. The AMA offers a brief Inbox reduction checklist as well as a detailed toolkit to guide physicians and operational leaders in reducing the volume of unnecessary Inbox messages, she said.
Distribution of work among the team also can reduce the time physicians spent on order entry. “It doesn’t take a medical school education to enter orders for flu shots, lipid profiles, mammograms, and other tests, and yet we have primary care physicians around the country spending an hour or more per 8 hours of patient visits on this task,” she said.
‘Growing Mountain’
Sally Baxter, MD, assistant professor of ophthalmology and division chief for Ophthalmology Informatics and Data Sciences at University of California San Diego, said, “Studies like this ... are important for continuing to quantify the burden of EHR work and to evaluate potential interventions to reduce this burden and subsequent burnout.”
Baxter’s health system allows physicians to bill for asynchronous messaging when certain eligibility criteria are met. “This can deter frivolous messaging and also provide some compensation for the work involved,” she said.
“In addition, we’ve recently piloted using AI tools to help draft replies to patient messages in the EHR as another approach to tackling this important issue,” said Baxter, who wasn’t involved with the current study.
Eve Rittenberg, MD, an assistant professor at Harvard Medical School and a PCP at Brigham and Women’s Hospital Fish Center for Women’s Health, in Boston, recommended that healthcare systems “monitor EHR workload across gender, specialty, and other variables to develop equitable support and compensation models.”
Dr. Rittenberg, who wasn’t involved with the current study, said healthcare systems should consider supporting physicians by blocking out time during clinic sessions to manage their EHR work. “Cross-coverage systems are vital so that on their days off, physicians can unplug from the computer and know that their patients’ needs are being met,” she added.
This work was supported in part by the AMA Practice Transformation Initiative: EHR-Use Metrics Research which provided grant funding to several of the authors. Sinsky is employed by the AMA. Dr. Arndt and coauthors disclosed no relevant financial information. Dr. Baxter received nonfinancial support from Optonmed and Topcon for research studies and collaborated with some of the study authors on other research but not this particular study. Dr. Rittenberg received internal funding from the Brigham Care Redesign Incubator and Startup Program, Brigham and Women’s Hospital, for a previous pilot project of inbasket cross-coverage. She had no relevant current disclosures.
A version of this article appeared on Medscape.com.
Do Your Patients Hate Exercise? Suggest They Do This Instead
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Colchicine May Benefit Patients With Diabetes and Recent MI
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
FDA Expands Dupilumab for EoE to Younger Children
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved dupilumab (Dupixent, Regeneron/Sanofi) for the treatment of eosinophilic esophagitis (EoE) in children aged 1-11 years and weighing ≥ 15 kg. It is the first and only medicine approved to treat these patients.
, as reported by this news organization.
EoE is a chronic inflammatory disorder driven by type 2 inflammation that damages the esophagus and causes difficulty swallowing and eating.
Dupilumab is a monoclonal antibody that acts to inhibit part of the inflammatory pathway.
EoE KIDS Trial
The FDA approval of dupilumab for younger children is based on results from the phase 3 randomized, double-blind, placebo-controlled EoE KIDS trial, which had two parts.
Part A was a 16-week double-blind treatment period that evaluated the safety and efficacy of dupilumab in a tiered weight-based dosing schema.
At 16 weeks, 66% of children who received higher dose dupilumab at tiered dosing regimens based on weight achieved histologic disease remission (six or fewer eosinophils/high power field), which was the primary endpoint, compared with only 3% of children who received placebo.
In addition, a greater decrease in the proportion of days with one or more signs of EoE according to the Pediatric EoE Sign/Symptom Questionnaire caregiver version (PESQ-C) was observed in children treated with dupilumab at 16 weeks compared placebo.
Part B was a 36-week extended active treatment period in which eligible children from Part A in the dupilumab group continued to receive their dose level and those in the placebo group in Part A switched to active treatment.
Histologic remission was sustained at week 52 in 53% of children treated with dupilumab in Parts A and B. Histologic remission was also achieved at week 52 in 53% of children who switched to dupilumab from placebo in Part B.
The safety profile of dupilumab observed through 16 weeks in these children was generally in line to that seen through 24 weeks in persons aged 12 years or older with EoE.
The most common adverse events (≥ 2%) more frequently observed with dupilumab than with placebo were injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections. In EoE KIDS Part B, one case of helminth infection was reported in the dupilumab arm.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
e-Cigarettes Best Nicotine Gum for Smoking Cessation
UPDATE: On March 29, 2024, the authors of this study published in JAMA Internal Medicine issued a formal retraction of their article. "Unfortunately, we have found significant coding errors that are difficult to rectify," the author wrote. "We also discovered discrepancies in the calculation process that cast doubt on the accuracy and reliability of the reported findings." The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
TOPLINE:
and as effective as varenicline in achieving sustained abstinence at 6 months, a randomized trial found. Questions about the long-term safety of e-cigarettes remain, however, according to the researchers.
METHODOLOGY:
- The study included 1068 participants in China who were smoking at least 10 cigarettes per day.
- They were randomly assigned to undergo 12 weeks of treatment with a cartridge-based e-cigarette, varenicline, or nicotine chewing gum.
TAKEAWAY:
- At 6 months, the biochemically validated rate of quitting was 15.7% for those who received e-cigarettes, 14.2% for those who received varenicline, and 8.8% for those who chewed nicotine gum.
- At 6 months, 62.8% of participants in the e-cigarette arm were still using the devices, whereas those in the other study arms had not continued their treatments.
- Adverse reactions with e-cigarettes and nicotine chewing gum included irritation of the throat and mouth, which occurred in 7%-8% of participants.
- In the varenicline group, 8.8% experienced nausea.
- No serious adverse events were reported.
IN PRACTICE:
“A moderate approach would be to recommend approved medications as the first step and, if that fails, then inform the patient of the evidence regarding the use of electronic cigarettes as a possible approach, acknowledging all its caveats,” Dorothy K. Hatsukami, PhD, with the University of Minnesota in Minneapolis, and Judith J. Prochaska, PhD, MPH, with Stanford (California) University, wrote in an invited commentary.
SOURCE:
Zhao Liu, PhD, with the China-Japan Friendship Hospital in Beijing, was the corresponding author for the study. The study was published online on January 29, 2024, in JAMA Internal Medicine.
LIMITATIONS:
The trial had an open-label design, so participants’ expectations about their assigned treatment may have influenced the results.
The study did not include participants older than 45 years, so it is unclear how the results apply to older populations.
More studies are needed to see whether continued use of e-cigarettes is beneficial or harmful, the researchers wrote.
Combining forms of nicotine replacement therapy, such as gum plus a patch, may be more effective than a single form, but the trial did not assess a combined approach, the commentary authors noted. The dose of nicotine gum for some participants may have been suboptimal, they added.
DISCLOSURES:
The study was supported by the Scientific Research Project Fund of China-Japan Friendship Hospital. The researchers had no conflict of interest disclosures. Dr. Prochaska disclosed receiving fees from Achieve Life Sciences, OneLeaf, and attorneys who are involved in litigation against tobacco companies.
A version of this article appeared on Medscape.com.
UPDATE: On March 29, 2024, the authors of this study published in JAMA Internal Medicine issued a formal retraction of their article. "Unfortunately, we have found significant coding errors that are difficult to rectify," the author wrote. "We also discovered discrepancies in the calculation process that cast doubt on the accuracy and reliability of the reported findings." The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
TOPLINE:
and as effective as varenicline in achieving sustained abstinence at 6 months, a randomized trial found. Questions about the long-term safety of e-cigarettes remain, however, according to the researchers.
METHODOLOGY:
- The study included 1068 participants in China who were smoking at least 10 cigarettes per day.
- They were randomly assigned to undergo 12 weeks of treatment with a cartridge-based e-cigarette, varenicline, or nicotine chewing gum.
TAKEAWAY:
- At 6 months, the biochemically validated rate of quitting was 15.7% for those who received e-cigarettes, 14.2% for those who received varenicline, and 8.8% for those who chewed nicotine gum.
- At 6 months, 62.8% of participants in the e-cigarette arm were still using the devices, whereas those in the other study arms had not continued their treatments.
- Adverse reactions with e-cigarettes and nicotine chewing gum included irritation of the throat and mouth, which occurred in 7%-8% of participants.
- In the varenicline group, 8.8% experienced nausea.
- No serious adverse events were reported.
IN PRACTICE:
“A moderate approach would be to recommend approved medications as the first step and, if that fails, then inform the patient of the evidence regarding the use of electronic cigarettes as a possible approach, acknowledging all its caveats,” Dorothy K. Hatsukami, PhD, with the University of Minnesota in Minneapolis, and Judith J. Prochaska, PhD, MPH, with Stanford (California) University, wrote in an invited commentary.
SOURCE:
Zhao Liu, PhD, with the China-Japan Friendship Hospital in Beijing, was the corresponding author for the study. The study was published online on January 29, 2024, in JAMA Internal Medicine.
LIMITATIONS:
The trial had an open-label design, so participants’ expectations about their assigned treatment may have influenced the results.
The study did not include participants older than 45 years, so it is unclear how the results apply to older populations.
More studies are needed to see whether continued use of e-cigarettes is beneficial or harmful, the researchers wrote.
Combining forms of nicotine replacement therapy, such as gum plus a patch, may be more effective than a single form, but the trial did not assess a combined approach, the commentary authors noted. The dose of nicotine gum for some participants may have been suboptimal, they added.
DISCLOSURES:
The study was supported by the Scientific Research Project Fund of China-Japan Friendship Hospital. The researchers had no conflict of interest disclosures. Dr. Prochaska disclosed receiving fees from Achieve Life Sciences, OneLeaf, and attorneys who are involved in litigation against tobacco companies.
A version of this article appeared on Medscape.com.
UPDATE: On March 29, 2024, the authors of this study published in JAMA Internal Medicine issued a formal retraction of their article. "Unfortunately, we have found significant coding errors that are difficult to rectify," the author wrote. "We also discovered discrepancies in the calculation process that cast doubt on the accuracy and reliability of the reported findings." The CHEST Physician® Editorial Board apologizes for any confusion this may have caused.
TOPLINE:
and as effective as varenicline in achieving sustained abstinence at 6 months, a randomized trial found. Questions about the long-term safety of e-cigarettes remain, however, according to the researchers.
METHODOLOGY:
- The study included 1068 participants in China who were smoking at least 10 cigarettes per day.
- They were randomly assigned to undergo 12 weeks of treatment with a cartridge-based e-cigarette, varenicline, or nicotine chewing gum.
TAKEAWAY:
- At 6 months, the biochemically validated rate of quitting was 15.7% for those who received e-cigarettes, 14.2% for those who received varenicline, and 8.8% for those who chewed nicotine gum.
- At 6 months, 62.8% of participants in the e-cigarette arm were still using the devices, whereas those in the other study arms had not continued their treatments.
- Adverse reactions with e-cigarettes and nicotine chewing gum included irritation of the throat and mouth, which occurred in 7%-8% of participants.
- In the varenicline group, 8.8% experienced nausea.
- No serious adverse events were reported.
IN PRACTICE:
“A moderate approach would be to recommend approved medications as the first step and, if that fails, then inform the patient of the evidence regarding the use of electronic cigarettes as a possible approach, acknowledging all its caveats,” Dorothy K. Hatsukami, PhD, with the University of Minnesota in Minneapolis, and Judith J. Prochaska, PhD, MPH, with Stanford (California) University, wrote in an invited commentary.
SOURCE:
Zhao Liu, PhD, with the China-Japan Friendship Hospital in Beijing, was the corresponding author for the study. The study was published online on January 29, 2024, in JAMA Internal Medicine.
LIMITATIONS:
The trial had an open-label design, so participants’ expectations about their assigned treatment may have influenced the results.
The study did not include participants older than 45 years, so it is unclear how the results apply to older populations.
More studies are needed to see whether continued use of e-cigarettes is beneficial or harmful, the researchers wrote.
Combining forms of nicotine replacement therapy, such as gum plus a patch, may be more effective than a single form, but the trial did not assess a combined approach, the commentary authors noted. The dose of nicotine gum for some participants may have been suboptimal, they added.
DISCLOSURES:
The study was supported by the Scientific Research Project Fund of China-Japan Friendship Hospital. The researchers had no conflict of interest disclosures. Dr. Prochaska disclosed receiving fees from Achieve Life Sciences, OneLeaf, and attorneys who are involved in litigation against tobacco companies.
A version of this article appeared on Medscape.com.
HPV Vaccine Shown to Be Highly Effective in Girls Years Later
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.