User login
Short Interval Repeat Colonoscopy After Inadequate Bowel Preparation Is Low Among Veterans
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
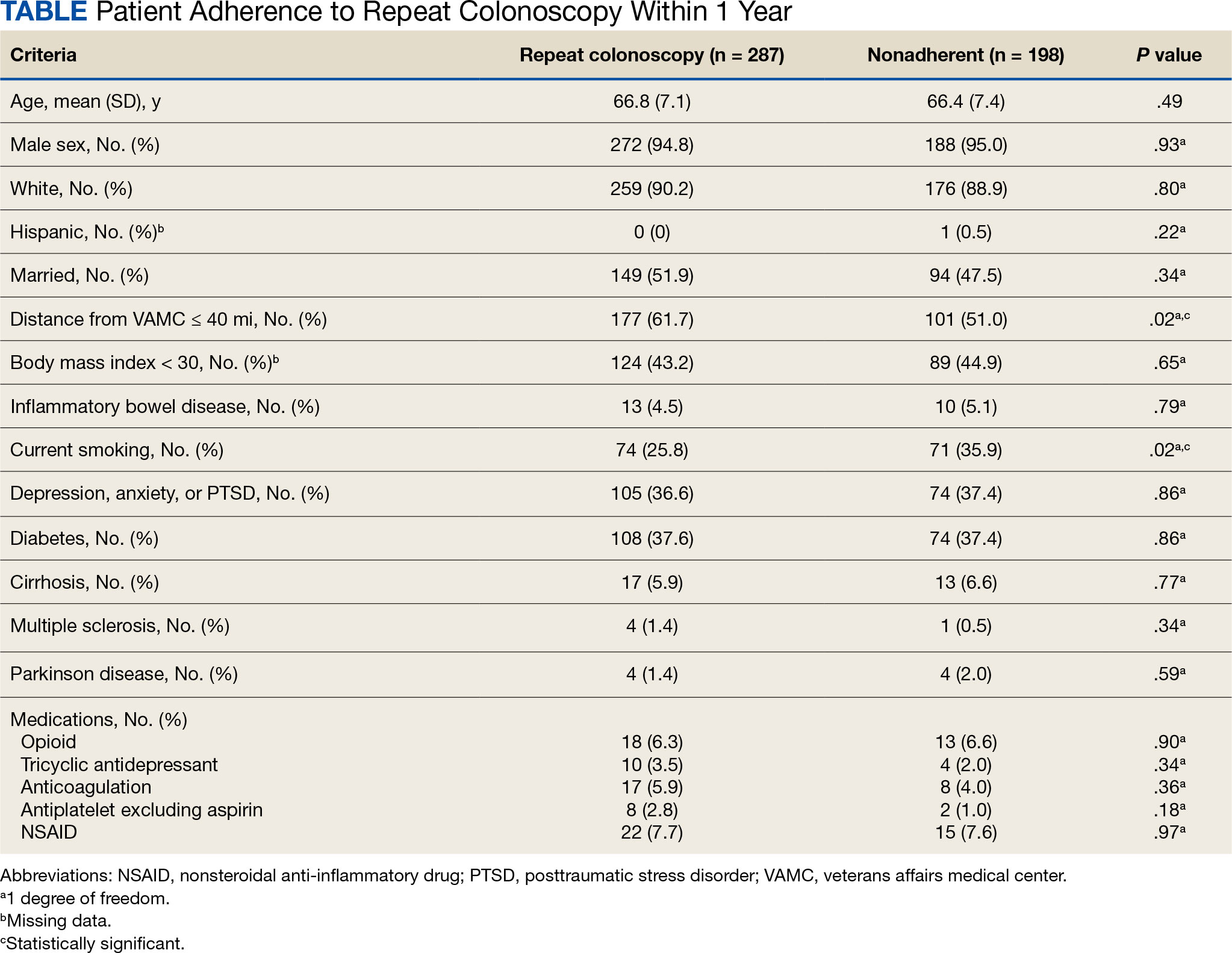
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
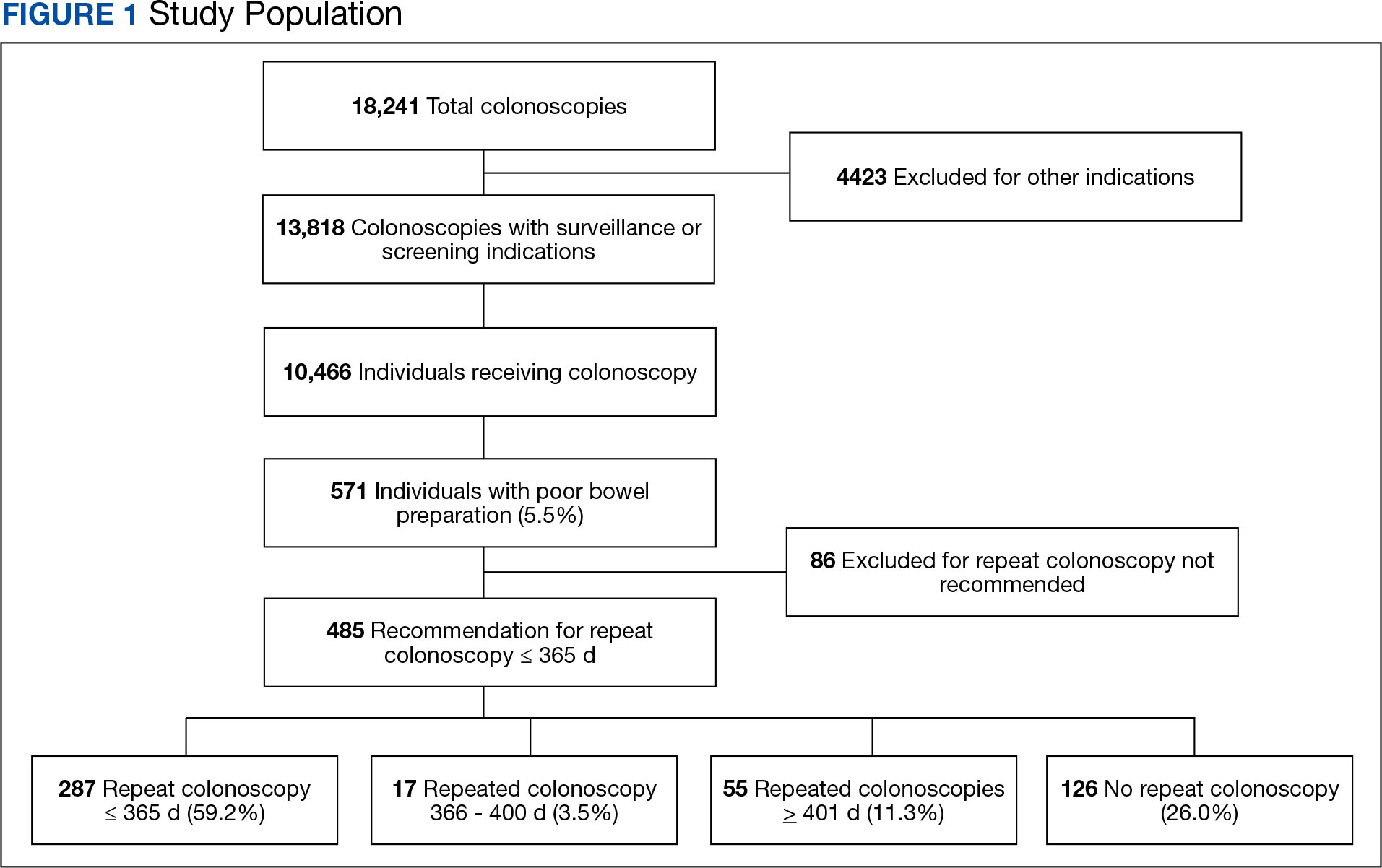
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.
Adherence to Recommended Interval Colonoscopy
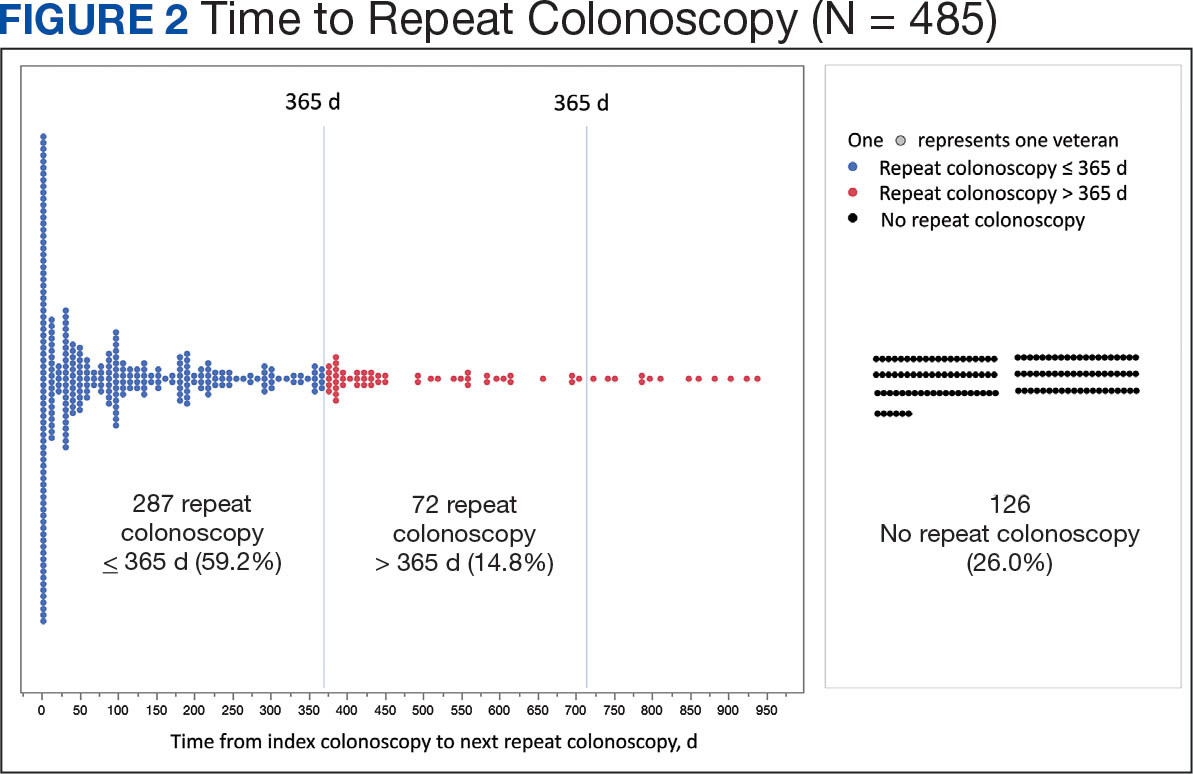
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).
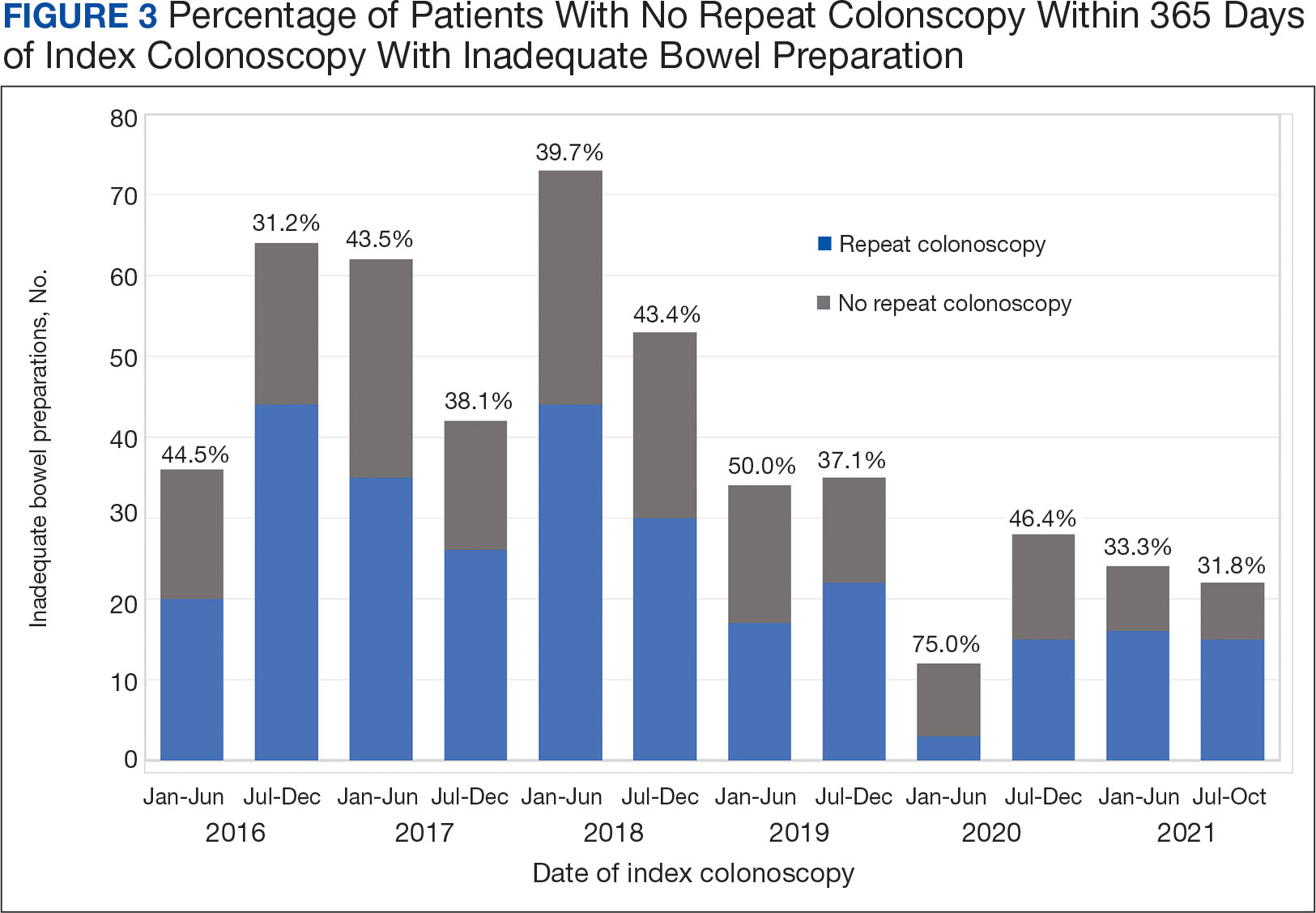
To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).
Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.


Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).

To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).

Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
Colorectal cancer (CRC) is the third-most diagnosed cancer after breast and lung cancer, and is the second leading cause of global cancer related deaths.1 In 2023 in the United States, > 150,000 individuals were diagnosed with CRC and 52,000 died.2
Colonoscopy is an effective CRC screening method and the lone method recommended for polyp surveillance. Inadequate bowel preparation (IBP) has been estimated to occur in about 6% to 26% of colonoscopies. 3,4 The prevalence varies based on a variety of comorbidities, including immobility, diabetes mellitus, neurologic disorders, and use of opioids, with more occurrences of IBP noted in older adult, non-English speaking, and male individuals.4-6
The quality of bowel preparation is integral to the effectiveness of screening and surveillance colonoscopies. IBP has been associated with missed adenomas and significantly lower adenoma detection rates.7-9 In particular, IBP is independently associated with an increased risk of CRC in the future.3 Accordingly, the US Multisociety Task Force recommends repeat colonoscopies for individuals with IBP within 1 year.10 Ensuring that these individuals receive repeat colonoscopies is an essential part of CRC prevention. The benefit of repeat colonoscopy after IBP is highlighted by a retrospective analysis from Fung and colleagues that showed 81% of repeat colonoscopies had adequate bowel preparation, with higher numbers of adenomas detected on repeat compared to initial colonoscopies.11
Given the impact of bowel preparation quality on the diagnostic capability of the colonoscopy, adherence to guidelines for repeat colonoscopies in cases of IBP is paramount for effective CRC prevention. This study aims to measure the frequency of repeat colonoscopy after IBP and the factors associated with adherence to recommendations.
METHODS
Individuals who underwent colonoscopy at the Minneapolis Veterans Affairs Medical Center (MVAMC) from January 1, 2016, to October 19, 2021, were identified to allow for 400 days of follow-up from the index colonoscopy to the data collection date. During the COVID-19 pandemic, the colonoscopy procedure capacity was reduced by 50% from June 1, 2020, to December 1, 2020, delaying nonurgent procedures, including screening and surveillance colonoscopies.
Individuals who underwent colonoscopy for CRC screening or polyp surveillance, or following a positive fecal immunohistochemistry test (FIT) or virtual computed tomography colonoscopy were included. Patients with colonoscopy indications for iron deficiency anemia, gastrointestinal bleeding, disease activity assessment of inflammatory bowel disease, abdominal pain, or changes in bowel movement pattern were excluded. IBP was defined as recording a Boston Bowel Preparation Scale (BBPS) score of < 6, or < 2 in any segment, or described as poor or inadequate using the Aronchick scale.
Age, sex, race, marital status, distance to MVAMC, smoking status, comorbidities, and concurrent medication use, including antiplatelet, anticoagulation, and prescription opiates at the time of index colonoscopy were obtained from the Veterans Health Administration (VHA) Corporate Data Warehouse (CDW) using structured query language processing of colonoscopy procedure notes to extract preparation scores and other procedure information. The CDW contains extracts from VHA clinical and administrative systems that contain complete clinical data from October 1999.12 Current smoking status was defined as any smoking activity at the time the questionnaire was administered during a routine clinic visit within 400 days from the index colonoscopy.
Only individuals who were recommended to have repeat colonoscopy within 1 year were included. The intervals of 365 days and 400 days (1 year + about 1 additional month) were used in the event that the individual had a delay in scheduling their 1-year repeat colonoscopy. For individuals who did not undergo a colonoscopy at MVAMC within 400 days, a manual chart review of all available records was performed to determine whether a colonoscopy was performed at a non-VA facility.
Patients received written instructions for bowel preparation 2 weeks prior to the procedure. The preparation included magnesium citrate and a split dose of 4 liters of polyethylene glycol. Patients were also advised to start a low-fiber diet 3 days prior to the procedure and a clear liquid diet the day before the procedure. Patients with a history of IBP or those undergoing procedures with anesthesia received an additional 2 liters for a total of 6 liters of polyethylene glycol.
Statistical analysis
Baseline characteristics were reported as mean (SD) or median and IQR for continuous variables and percentage for categorical variables. Individuals who returned for colonoscopy within 400 days were compared to those who did not identify factors associated with adherence to recommendations. The data on individuals who returned for colonoscopy within 400 days were also analyzed for additional minor delays in the timing of the repeat colonoscopy. Continuous data were compared using Mann-Whitney U tests. Categorical data were compared using X2 or Fisher exact tests. Missing data were imputed from the analyses. All analyses were performed using SAS JMP Pro version 16. P < .05 was considered statistically significant.
RESULTS
There were 18,241 total colonoscopies performed between January 1, 2016, to October 19, 2021, and 13,818 colonoscopies had indications for screening for colon cancer, positive FIT, virtual colonoscopy, or surveillance. Of the 10,466 unique patients there were 5369 patients for polyp surveillance, 4054 patients for CRC screening, and 1043 patients for positive FIT or virtual colonoscopy. Of these, 571 individuals (5.5%) had IBP. Repeat colonoscopy within 1 year was recommended for 485 individuals (84.9%) who were included in this study (153 CRC screenings and 46 positive FITs) but not for 86 individuals (15.1%) (Figure 1). Among included patients, the mean (SD) age was 66.6 (7.2) years, and the majority were male (460 [94.8%]) and White (435 [89.7%]) (Table). Two hundred and forty-three (50.1%) were married.


Adherence to Recommended Interval Colonoscopy
Of the 485 patients with IBP who were recommended for follow-up colonoscopy, 287 (59.2%) had a colonoscopy within 1 year, and 198 (40.8%) did not; 17 patients (13.5%) had repeat colonoscopy within 366 to 400 days. Five (1.0%) individuals had a repeat colonoscopy the next day, and 77 (15.9%) had a repeat colonoscopy within 7 days. One hundred and twentysix (26.0%) individuals underwent no repeat colonoscopy during the study period (Figure 2).

To account for the COVID-19 pandemic, the adherence rate of repeat colonoscopy within 1 year prepandemic (January 1, 2016, to December 1, 2018) was calculated along with the adherence rate postpandemic (January 1, 2019 to the end of the study). The rates were similar: 199 of 330 (60.3%) individuals prepandemic vs 88 of 155 (56.8%) individuals postpandemic (Figure 3).

Significant Associations
Age, sex, and race were not associated with adherence to repeat colonoscopy within 1 year. Individuals living ≤ 40 miles from the endoscopy center were more likely to undergo a repeat colonoscopy within 1 year compared with those who lived > 40 miles away (61.7% vs 51.0%, P = .02). Current smoking status was associated with a lower rate of repeat colonoscopy within 1 year (25.8% vs 35.9%; P = .02). There were no differences with respect to inflammatory bowel disease diagnosis, mental health diagnosis, diabetes mellitus, cirrhosis, or medications used, including opioids, anticoagulation, and antiplatelet therapy.
Outcomes
Among individuals who had a repeat colonoscopy the day after the index colonoscopy, 53 of 56 individuals (94.6%) had adequate bowel preparation. Among individuals who had a repeat colonoscopy within 7 days, 70 of 77 (90.9%) had adequate bowel preparation. Of 287 individuals with a repeat colonoscopy within 1 year, 251 (87.5%) had adequate bowel preparation on the repeat colonoscopy. By 400 days after the index colonoscopy, 268 of 304 individuals (88.2%) had adequate bowel preparation.
In this study conducted at a large VA medical center, we found that 5.6% of individuals undergoing colonoscopies had IBP, a rate comparable to prior studies (6% to 26%).3,4 Only 59.2% of individuals underwent repeat colonoscopies within 1 year, as recommended after an index colonoscopy with IBP. Smoking and living longer distances (> 40 miles) from the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation.
Current guidelines recommend repeat colonoscopy for individuals with IBP within 1 year.10 In cases of IBP, the advanced adenoma miss rate is 36% upon repeat colonoscopy within 1 year.13 Despite the importance of a follow-up colonoscopy, clinician adherence with this recommendation remains low.10,14,15 However, in this study cohort, 485 of 571 individuals with IBP (84.9%) received recommendations for a repeat colonoscopy within 1 year. In the US, only 31.9% of 260,314 colonoscopies with IBP included recommendations for a follow-up colonoscopy within 1 year.14 This could be related to variations in endoscopist practice as well as patient risk factors for developing polyps, including family history of cancer and personal history of prior polyps. The findings of multiple polyps, high-risk adenomas, and cancer on the index colonoscopy also influences the endoscopist for repeat colonoscopy within 1 year.14
The timing for repeat colonoscopies within 1 year will be determined by the patients, clinicians, and available scheduling. In this study, the earlier repeat colonoscopies, especially those occurring the day after the index colonoscopy, had the highest success rate of adequate bowel preparation. In a prior study, repeating colonoscopies within the same day or the next day was also found to have a higher rate of adequate bowel preparation than repeat colonoscopies within 1 year (88.9% vs 83.5%).16
Ensuring the return of individuals with IBP for repeat colonoscopy is a challenging task. We identified that individuals who live further away from MVAMC and current smokers had a decreased probability of returning for a repeat colonoscopy. Toro and colleagues found a 68.7% return rate for a repeat colonoscopy within 1 year with individuals age ≥ 60 years, and patients who were White were less likely to proceed with a repeat colonoscopy within 1 year.17 The study did not provide data regarding smoking status or distance to the endoscopy center.17 In a prior study of veterans, the dual diagnosis of psychiatric disorders and substance abuse was associated with missed and canceled colonoscopy appointments.18 The distance to the endoscopy center has also been previously identified as a barrier to a colonoscopy following an abnormal FIT.19 Although not identified in this study due to the homogenous demographic profile, social determinants of health such as socioeconomic status, education, and insurance coverage are known barriers to cancer screening but were not evaluated in this study.20
Based on the identified risk factors, we have created a model for utilizing those risk factors to identify individuals at higher risk for noncompliance (ie, those who live further away from the endoscopy center or currently smoke). These individuals are proactively offered to use an intraprocedural bowel cleansing device to achieve adequate bowel preparation or priority rescheduling for a next-day colonoscopy.
Limitations
This study was a single-center study of the veteran population, which is predominantly White and male, thus limiting generalizability. The study is also limited by minimal available data on adenoma detection and colon cancer incidence on subsequent colonoscopies.
CONCLUSIONS
The rate of IBP was 5.5% in individuals undergoing colonoscopy for colon cancer screening, surveillance, positive FIT, or computed tomography colonography. Only 59.2% of those with IBP underwent the recommended repeat colonoscopy within 1 year. Smoking and distance to the endoscopy center were associated with a decreased adherence to the repeat colonoscopy recommendation. Additional efforts are needed to ensure that individuals with IBP return for timely repeat colonoscopy.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322/caac.21772
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823- 834. doi:10.1016/S1470-2045(17)30187-0
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378- 384. doi:10.1016/s0016-5107(04)02776-2
- Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(8):819-826. doi:10.1097/MEG.0000000000001175
- ASGE Standards of Practice Committee, Saltzman JR, Cash BD, et al. Bowel preparation before colonoscopy. Gastrointest Endosc. 2015;81(4):781-794. doi:10.1016/j.gie.2014.09.048
- Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150(2):396- e15. doi:10.1053/j.gastro.2015.09.041
- Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11(6):e0154149. Published 2016 Jun 3. doi:10.1371/journal.pone.0154149
- Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-1203. doi:10.1016/j.gie.2012.01.005
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. doi:10.1053/j.gastro.2012.06.001
- Fung P, Syed A, Cole R, Farah K. Poor bowel prep: are you really going to come back within a year? Abstract presented at American Gastroenterological Association DDW 2021, May 21-23, 2021. doi:10.1016/S0016-5085(21)01204-X
- US Department of Veterans Affairs, VA Health Systems Research. Corporate data warehouse (CDW). Updated January 11, 2023. Accessed August 6, 2024. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm
- Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73(6):1207-1214. doi:10.1016/j.gie.2011.01.051
- Calderwood AH, Holub JL, Greenwald DA. Recommendations for follow-up interval after colonoscopy with inadequate bowel preparation in a national colonoscopy quality registry. Gastrointest Endosc. 2022;95(2):360-367. e2. doi:10.1016/j.gie.2021.09.027
- Latorre M, Roy A, Spyrou E, Garcia-Carrasquillo R, Rosenberg R, Lebwohl B. Adherence to guidelines after poor colonoscopy preparation: experience from a patient navigator program. Gastroenterology. 2016;151(1):P196. doi:10.1053/j.gastro.2016.05.027
- Bouquet E, Tomal J, Choksi Y. Next-day screening colonoscopy following inadequate bowel preparation may improve quality of preparation and adenoma detection in a veteran population. Am J Gastroenterol. 2020;115:S259. doi:10.14309/ajg.0000000000000853
- Toro B, Dawkins G, Friedenberg FK, Ehrlich AC. Risk factors for failure to return after a poor preparation colonoscopy: experience in a safety-net hospital, 255. Abstract presented at ACG October 2016. https://journals.lww.com/ajg/fulltext/2016/10001/risk_factors_for_failure_to_return_after_a_poor.255.aspx
- Partin MR, Gravely A, Gellad ZF, et al. Factors Associated With Missed and Cancelled Colonoscopy Appointments at Veterans Health Administration Facilities. Clin Gastroenterol Hepatol. 2016;14(2):259-267. doi:10.1016/j.cgh.2015.07.051
- Idos GE, Bonner JD, Haghighat S, et al. Bridging the Gap: Patient Navigation Increases Colonoscopy Follow-up After Abnormal FIT. Clin Transl Gastroenterol. 2021;12(2):e00307. doi:10.14309/ctg.0000000000000307
- Islami F, Baeker Bispo J, Lee H, et al. American Cancer Society’s report on the status of cancer disparities in the United States, 2023. CA Cancer J Clin. 2024;74(2):136- 166. doi:10.3322/caac.21812
Establishing a Just Culture: Implications for the Veterans Health Administration Journey to High Reliability
Medical errors are a persistent problem and leading cause of preventable death in the United States. There is considerable momentum behind the idea that implementation of a just culture is foundational to detecting and learning from errors in pursuit of zero patient harm.1-6 Just culture is a framework that fosters an environment of trust within health care organizations, aiming to achieve fair outcomes for those involved in incidents or near misses. It emphasizes openness, accountability, and learning, prioritizing the repair of harm and systemic improvement over assigning blame.7
A just culture mindset reflects a significant shift in thinking that moves from the tendency to blame and punish others toward a focus on organizational learning and continued process improvement.8,9 This systemic shift in fundamental thinking transforms how leaders approach staff errors and how they are addressed.10 In essence, just culture reflects an ethos centered on openness, a deep appreciation of human fallibility, and shared accountability at both the individual and organizational levels.
Organizational learning and innovation are stifled in the absence of a just culture, and there is a tendency for employees to avoid disclosing their own errors as well as those of their colleagues.11 The transformation to a just culture is often slowed or disrupted by personal, systemic, and cultural barriers.12 It is imperative that all executive, service line, and frontline managers recognize and execute their distinct responsibilities while adjudicating the appropriate course of action in the aftermath of adverse events or near misses. This requires a nuanced understanding of the factors that contribute to errors at the individual and organizational levels to ensure an appropriate response.
The Veterans Health Administration (VHA) is orchestrating an enterprise transformation to develop into a high reliability organization (HRO). This began with a single-site test in 2016, which demonstrated successful results in patient safety culture, patient safety event reporting, and patient safety outcomes.13 In 2019, the VHA formally launched its enterprise-wide HRO journey in 18 hospital facilities, followed by successive waves of 67 and 54 facilities in 2021 and 2022, respectively. The VHA journey to transform into an HRO aligns with 3 pillars, 5 principles, and 7 values. The VHA has emphasized the importance of just culture as a foundational element of the HRO framework, specifically under the pillar of leadership. To promote leadership engagement, the VHA has employed an array of approaches that include education, leader coaching, and change management strategies. Given the diversity among VHA facilities, each with local cultures and histories, some sites have more readily implemented a just culture than others.14 A deeper exploration into potential obstacles, particularly concerning leadership engagement, could be instrumental for formulating strategies that further establish a just culture across the VHA.15
There is a paucity of empirical research regarding factors that facilitate and/or impede the implementation of a just culture in health care settings.16,17 Likert scale surveys, such as the Patient Safety Culture Module for the VHA All Employee Survey and its predecessor, the Patient Safety Culture Survey, have been used to assess culture and climate.18 However, qualitative evaluations directly assessing the lived experiences of those trying to implement a just culture provide additional depth and context that can help identify specific factors that support or impede becoming an HRO. The purpose of this study was to increase understanding of factors that influence the establishment and sustainment of a just culture and to identify specific methods for improving the implementation of just culture principles and practices aligned with HRO.
METHODS
This qualitative study explored facilitators and barriers to establishing and sustaining a just culture as experienced across a subset of VHA facilities by HRO leads or staff assigned with the primary responsibilities of supporting facility-level HRO transformation. HRO leads are assigned responsibility for supporting executive leadership in planning, coordinating, implementing, and monitoring activities to ensure effective high reliability efforts, including focused efforts to establish a robust patient safety program, a culture of safety, and a culture of continuous process improvement.
Virtual focus group discussions held via Microsoft Teams generated in-depth, diverse perspectives from participants across 16 VHA facilities. Qualitative research and evaluation methods provide an enhanced depth of understanding and allow the emergence of detailed data.19 A qualitative grounded theory approach elicits complex, multifaceted phenomena that cannot be appreciated solely by numeric data.20 Grounded theory was selected to limit preconceived notions and provide a more systematic analysis, including open, axial, and thematic coding. Such methods afford opportunities to adapt to unplanned follow-up questions and thus provide a flexible approach to generate new ideas and concepts.21 Additionally, qualitative methods help overcome the tendencies of respondents to agree rather than disagree when presented with Likert-style scales, which tend to skew responses toward the positive.22
Participants must have been assigned as an HRO lead for ≥ 6 months at the same facility. Potential participants were identified through purposive sampling, considering their leadership roles in HRO and experience with just culture implementation, the size and complexity of their facility, and geographic distribution. Invitations explaining the study and encouraging voluntary participation to participate were emailed. Of 37 HRO leads invited to participate in the study, 16 agreed to participate and attended 1 of 3 hour-long focus group sessions. One session was rescheduled due to limited attendance. Participants represented a mix of VHA sites in terms of geography, facility size, and complexity.
Focus Group Procedures
Demographic data were collected prior to sessions via an online form to better understand the participant population, including facility complexity level, length of time in HRO lead role, clinical background, and facility level just culture training. Each session was led by an experienced focus group facilitator (CV) who was not directly involved with the overall HRO implementation to establish a neutral perspective. Each session was attended by 4 to 7 participants and 2 observers who took notes. The sessions were recorded and included automated transcriptions, which were edited for accuracy.
Focus group sessions began with a brief introduction and an opportunity for participants to ask questions. Participants were then asked a series of open-ended questions to elicit responses regardingfacilitators, barriers, and leadership support needed for implementing just culture. The questions were part of a facilitator guide that included an introductory script and discussion prompts to ensure consistency across focus groups.
Facilitators were defined as factors that increase the likelihood of establishing or sustaining a just culture. Barriers were defined as factors that decrease or inhibit the likelihood of establishing or sustaining just culture. The focus group facilitator encouraged all participants to share their views and provided clarification if needed, as well as prompts and examples where appropriate, but primarily sought to elicit responses to the questions.
Institutional review board review and approval were not required for this quality improvement initiative. The project adhered to ethical standards of research, including asking participants for verbal consent and preserving their confidentiality. Participation was voluntary, and prior to the focus group sessions, participants were provided information explaining the study’s purpose, procedures, and their rights. Participant identities were kept confidential, and all data were anonymized during the analysis phase. Pseudonyms or identifiers were used during data transcription to protect participant identity. All data, including recordings and transcriptions, were stored on password-protected devices accessible only to the research team. Any identifiable information was removed during data analysis to ensure confidentiality.
Analysis
Focus group recordings were transcribed verbatim, capturing all verbal interactions and nonverbal cues that may contribute to understanding the participants' perspectives. Thematic analysis was used to analyze the qualitative data from the focus group discussions.23 The transcribed data were organized, coded, and analyzed using ATLAS.ti 23 qualitative data software to identify key themes and patterns.
Results
The themes identified include the 5 facilitators, barriers, and recommendations most frequently mentioned by HRO leads across focus group sessions. The nature of each theme is described, along with commonly mentioned examples and direct quotes from participants that illustrate our understanding of their perspectives.
Facilitators
Training and coaching (26 responses). The availability of training around the Just Culture Decision Support Tool (DST) was cited as a practical aid in guiding leaders through complex just culture decisions to ensure consistency and fairness. Additionally, an executive leadership team that served as champions for just culture principles played a vital role in promoting and sustaining the approach: “Training them on the roll-out of the decision support tool with supervisors at all levels, and education for just culture and making it part of our safety forum has helped for the last 4 months.” “Having some regular training and share-out cadences embedded within the schedule as well as dynamic directors and well-trained executive leadership team (ELT) for support has been a facilitator.”
Increased transparency (16 responses). Participants consistently highlighted the importance of leadership transparency as a key facilitator for implementing just culture. Open and honest communication from top-level executives fostered an environment of trust and accountability. Approachable and physically present leadership was seen as essential for creating a culture where employees felt comfortable reporting errors and concerns without fear of retaliation: “They’re surprisingly honest with themselves about what we can do, what we cannot do, and they set the expectations exactly at that.”
Approachable leadership (15 responses). Participants frequently mentioned the importance of having dynamic leadership spearheading the implementation of just culture and leading by example. Having a leadership team that accepts accountability and reinforces consistency in the manner that near misses or mistakes are addressed is paramount to promoting the principles of just culture and increasing psychological safety: “We do have very approachable leadership, which I know is hard if you’re trying to implement that nationwide, it’s hard to implement approachability. But I do think that people raise their concerns, and they’ll stop them in the hallway and ask them questions. So, in terms of comfort level with the executive leadership, I do think that’s high, which would promote psychological safety.”
Feedback loops and follow through (13 responses). Participants emphasized the importance of taking concrete actions to address concerns and improve processes. Regular check-ins with supervisors to discuss matters related to just culture provided a structured opportunity for addressing issues and reinforcing the importance of the approach: “One thing that we’ve really focused on is not only identifying mistakes, but [taking] ownership. We continue to track it until … it’s completed and then a process of how to communicate that back and really using closed loop communication with the staff and letting them know.”
Forums and town halls (10 responses). These platforms created feedback loops that were seen as invaluable tools for sharing near misses, celebrating successes, and promoting open dialogue. Forums and town halls cultivated a culture of continuous improvement and trust: “We’ll celebrate catches, a safety story is inside that catch. So, if we celebrate the change, people feel safer to speak up.” “Truthfully, we’ve had a great relationship since establishing our safety forums and just value open lines of communication.”
Barriers
Inadequate training (30 responses). Insufficient engagement during training—limited bandwidth and availability to attend and actively participate in training—was perceived as detrimental to creating awareness and buy-in from staff, supervisors, and leadership, thereby hindering successful integration of just culture principles. Participants also identified too many conflicting priorities from VHA leadership, which contributes to training and information fatigue among staff and supervisors. “Our biggest barrier is just so many different competing priorities going on. We have so much that we’re asking people to do.” “One hundred percent training is feeling more like a ticked box than actually yielding results, I have a very hard time getting staff engaged.”
Inconsistency between executive leaders and middle managers (28 responses). A lack of consistency in the commitment to and enactment of just culture principles among leaders poses a challenge. Participants gave several examples of inconsistencies in messaging and reinforcement of just culture principles, leading to confusion among staff and hindering adoption. Likewise, the absence of standardized procedures for implementing just culture created variability: “The director coming in and trying to change things, it put a lot of resistance, we struggle with getting the other ELT members on board … some of the messages that come out at times can feel more punitive.”
Middle management resistance (22 responses). In some instances, participants reported middle managers exhibiting attitudes and behaviors that undermined the application of just culture principles and effectiveness. Such attitudes and behaviors were attributed to a lack of adequate training, coaching, and awareness. Other perceived contributions included fear of failure and a desire to distance oneself from staff who have made mistakes: “As soon as someone makes an error, they go straight to suspend them, and that’s the disconnect right there.” “There’s almost a level of working in the opposite direction in some of the mid-management.”
Cultural misalignment (18 responses). The existing culture of distancing oneself from mistakes presented a significant barrier to the adoption of just culture because it clashed with the principles of open reporting and accountability. Staff underreported errors or framed them in a way that minimized personal responsibility, thereby making it more essential to put in the necessary and difficult work to learn from mistakes: “One, you’re going to get in trouble. There’s going to be more work added to you or something of that nature."
Lack of accountability for opposition(17 responses). Participants noted a clear lack of accountability for those who opposed or showed resistance to just culture, which allowed resistance to persist without consequences. In many instances, leaders were described as having overlooked repeated instances of unjust attitudes and behaviors (eg, inappropriate blame or punishment), which allowed those practices to continue. “Executive leadership is standing on the hill and saying we’re a just culture and we do everything correctly, and staff has the expectation that they’re going to be treated with just culture and then the middle management is setting that on fire, then we show them that that’s not just culture, and they continue to have those poor behaviors, but there’s a lack of accountability.”
Limited bandwidth and lack of coordination (14 responses). HRO leads often faced role-specific constraints in having adequate time and authority to coordinate efforts to implement or sustain just culture. This includes challenges with coordination across organizational levels (eg, between the hospital and regional Veterans Integrated Service Network [VISN] management levels) and across departments within the hospital (eg, between human resources and service lines or units). “Our VISN human resources is completely detached. They’ll not cooperate with these efforts, which is hard.” “There’s not enough bandwidth to actually support, I’m just 1 person.” “[There’s] all these mandated trainings of 8 hours when we’re already fatigued, short-staffed, taking 3 other HRO classes.”
Recommendations
Training improvements (24 responses). HRO leads recommended that comprehensive training programs be developed and implemented for staff, supervisors, and leadership to increase awareness and understanding of just culture principles. These training initiatives should focus on fostering a shared understanding of the core tenets of just culture, the importance of error reporting, and the processes involved in fair and consistent decision making (eg, training simulations on use of the Just Culture DST). “We’ve really never had any formal training on the decision support tool. I hope that what’s coming out for next year. We’ll have some more formal training for the tool because I think it would be great to really have our leadership and our supervisors and our managers use that tool.” “We can give a more directed and intentional training to leadership on the 4 foundational practices and what it means to implement those and what it means to utilize that behavioral component of HRO.”
Clear and consistent procedures toincrease accountability (22 responses). To promote a culture of accountability and consistency in the application of just culture principles, organizations should establish clear mechanisms for reporting, investigating, and addressing incidents. Standardized procedures and DSTs can aid in ensuring that responses to errors are equitable and align with just culture principles: “I recommend accountability; if it’s clearly evidenced that you’re not toeing the just culture line, then we need to be able to do something about it and not just finger wag.” “[We need to have] a templated way to approach just culture implementation. The decision support tool is great, I absolutely love having the resources and being able to find a lot of clinical examples and discussion tools like that. But when it comes down to it, not having that kind of official thing to fall back on it can be a little bit rough.”
Additional coaching and consultationsupport (15 responses). To support supervisors in effectively implementing just culture within their teams, participants recommended that organizations provide ongoing coaching and mentorship opportunities. Additionally, third-party consultants with expertise in just culture were described as offering valuable guidance, particularly in cases where internal staff resources or HRO lead bandwidth may be limited. “There are so many consulting agencies with HRO that have been contracted to do different projects, but maybe that can help with an educational program.” “I want to see my executive leadership coach the supervisors up right and then allow them to do one-on-ones and facilitate and empower the frontline staff, and it’s just a good way of transparency and communication.”
Improved leadership sponsorship (15 responses). Participants noted that leadership buy-in is crucial for the successful implementation of just culture. Facilities should actively engage and educate leadership teams on the benefits of just culture and how it aligns with broader patient safety and organizational goals. Leaders should be visible and active champions of its principles, supporting change in their daily engagements with staff. “ELT support is absolutely necessary. Why? Because they will make it important to those in their service lines. They will make it important to those supervisors and managers. If it’s not important to that ELT member, then it’s not going to be important to that manager or that supervisor.”
Improved collaboration with patient safety and human resources (6 responses). Collaborative efforts with patient safety and human resources departments were seen as instrumental in supporting just culture, emphasizing its importance, and effectively addressing issues. Coordinating with these departments specifically contributes to consistent reinforcement and expands the bandwidth of HRO leads. These departments play integral roles in supporting just culture through effective policies, procedures, and communication. “I think it would be really helpful to have common language between what human resources teaches and what is in our decision support tool.”
DISCUSSION
This study sought to collect and synthesize the experiences of leaders across a large, integrated health care system in establishing and sustaining a just culture as part of an enterprise journey to become an HRO.24 The VHA has provided enterprise-wide support (eg, training, leader coaching, and communications) for the implementation of HRO principles and practices with the goal of creating a culture of safety, which includes just culture elements. This support includes enterprise program offices, VISNs, and hospital facilities, though notably, there is variability in how HRO is implemented at the local level. The facilitators, barriers, and recommendations presented in this article are representative of the designated HRO leads at VHA hospital facilities who have direct experience with implementing and sustaining just culture. The themes presented offer specific opportunities for intervention and actionable strategies to enhance just culture initiatives, foster psychological safety and accountability, and ultimately improve the quality of care and patient outcomes.3,25
Frequently identified facilitators such as providing training and coaching, having leaders who are available and approachable, demonstrating follow-through to address identified issues, and creating venues where errors and successes can be openly discussed.26 These facilitators are aligned with enterprise HRO support strategies orchestrated by the VHA at the enterprise VISN and facility levels to support a culture of safety, continuous process improvement, and leadership commitment.
Frequently identified barriers included inadequate training, inconsistent application of just culture by middle managers vs senior leaders, a lack of accountability or corrective action when unjust corrective actions took place, time and resource constraints, and inadequate coordination across departments (eg, operational departments and human resources) and organizational levels. These factors were identified through focus groups with a limited set of HRO leads. They may reflect challenges to changing culture that may be deeply engrained in individual histories, organizational norms, and systemic practices. Improving upon these just culture initiatives requires multifaceted approaches and working through resistance to change.
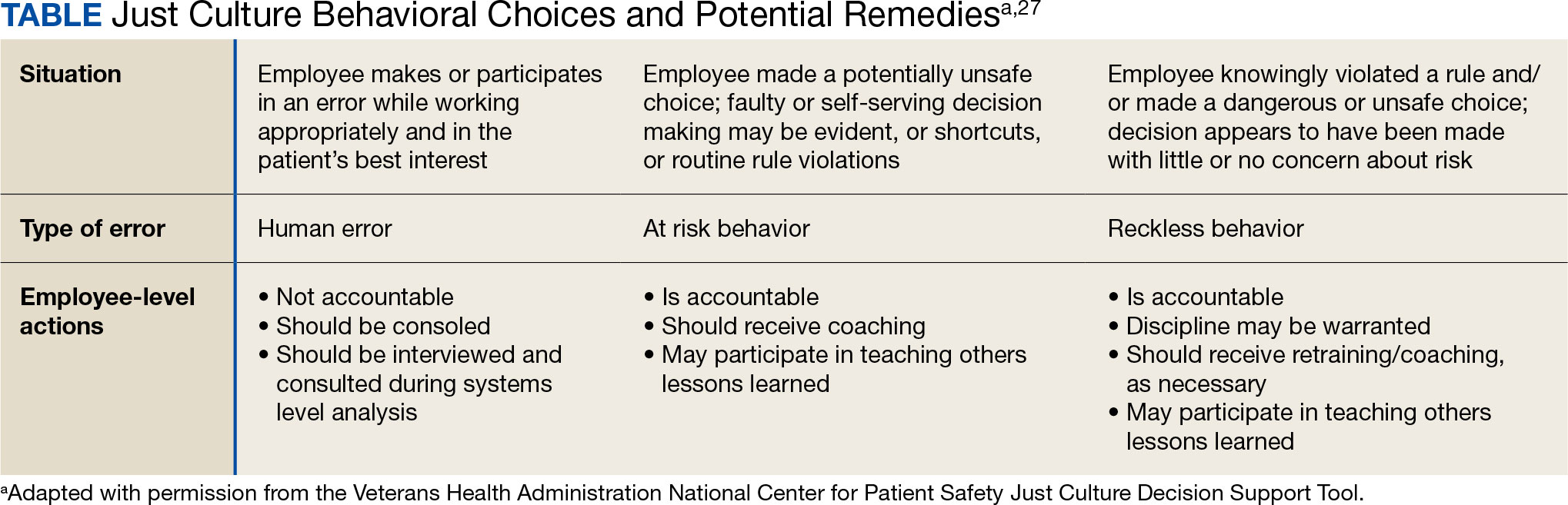
VHA HRO leads identified several actionable recommendations that may be used in pursuit of a just culture. First, improvements in training involving how to apply just culture principles and, specifically, the use of the Just Culture DST were identified as an opportunity for improvement. The VHA National Center for Patient Safety developed the DST as an aid for leaders to effectively address errors in line with just culture principles, balancing individual and system accountability.27 The DST specifically addresses human error as well as risky and reckless behavior, and it clarifies the delineation between individual and organizational accountability (Table).3
Scenario-based interactive training and simulations may prove especially useful for middle managers and frontline supervisors who are closest to errors. Clear and repeatable procedures for determining courses of action for accountability in response are needed, and support for their application must be coordinated across multiple departments (eg, patient safety and human resources) to ensure consistency and fairness. Coaching and consultation are also viewed as beneficial in supporting applications. Coaching is provided to senior leaders across most facilities, but the availability of specific, role-based coaching and training is more limited for middle managers and frontline supervisors who may benefit most from hands-on support.
Lastly, sponsorship from leaders was viewed as critical to success, but follow through to ensure support flows down from the executive suite to the frontline is variable across facilities and requires consistent effort over time. This is especially challenging given the frequent turnover in leadership roles evident in the VHA and other health care systems.
Limitations
This study employed qualitative methods and sampled a relatively small subset of experienced leaders with specific roles in implementing HRO in the VHA. Thus, it should not be considered representative of the perspectives of all leaders within the VHA or other health care systems. Future studies should assess facilitators and barriers beyond the facility level, including a focus incorporating both the VISN and VHA. More broadly, qualitative methods such as those employed in this study offer great depth and nuance but have limited ability to identify system-wide trends and differences. As such, it may be beneficial to specifically look at sites that are high- or low-performing on measures of patient safety culture to identify differences that may inform implementation strategies based on organizational maturity and readiness for change.
Conclusions
Successful implementation of these recommendations will require ongoing commitment, collaboration, and a sustained effort from all stakeholders involved at multiple levels of the health care system. Monitoring and evaluating progress should be conducted regularly to ensure that recommendations lead to improvements in implementing just culture principles. This quality improvement study adds to the knowledge base on factors that impact the just culture and broader efforts to realize HRO principles and practices in health care systems. The approach of this study may serve as a model for identifying opportunities to improve HRO implementation within the VHA and other settings, especially when paired with ongoing quantitative evaluation of organizational safety culture, just culture behaviors, and patient outcomes.
- Aljabari S, Kadhim Z. Common barriers to reporting medical errors. ScientificWorldJournal. 2021;2021:6494889. doi:10.1155/2021/6494889
- Arnal-Velasco D, Heras-Hernando V. Learning from errors and resilience. Curr Opin Anaesthesiol. 2023;36(3):376-381. doi:10.1097/ACO.0000000000001257
- Murray JS, Clifford J, Larson S, Lee JK, Sculli GL. Implementing just culture to improve patient safety. Mil Med. doi:10.1093/milmed/usac115
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- van Baarle E, Hartman L, Rooijakkers S, et al. Fostering a just culture in healthcare organizations: experiences in practice. BMC Health Serv Res. 2022;22(1):1035. doi:10.1186/s12913-022-08418-z
- Weenink JW, Wallenburg I, Hartman L, et al. Role of the regulator in enabling a just culture: a qualitative study in mental health and hospital care. BMJ Open. 2022;12(7):e061321. doi:10.1136/bmjopen-2022-061321
- White RM, Delacroix R. Second victim phenomenon: is ‘just culture’ a reality? An integrative review. Appl Nurs Res. 2020;56:151319. doi:10.1016/j.apnr.2020.151319
- Cribb A, O’Hara JK, Waring J. Improving responses to safety incidents: we need to talk about justice. BMJ Qual Saf. 2022;31(4):327-330. doi:10.1136/bmjqs-2021-014333
- Rocco C, Rodríguez AM, Noya B. Elimination of punitive outcomes and criminalization of medical errors. Curr Opin Anaesthesiol. 2022;35(6):728-732. doi:10.1097/ACO.0000000000001197
- Dekker S, Rafferty J, Oates A. Restorative Just Culture in Practice: Implementation and Evaluation. Routledge; 2022.
- Brattebø G, Flaatten HK. Errors in medicine: punishment versus learning medical adverse events revisited - expanding the frame. Curr Opin Anaesthesiol. 2023;36(2):240-245. doi:10.1097/ACO.0000000000001235
- Shabel W, Dennis JL. Missouri’s just culture collaborative. J Healthc Risk Manag. 2012;32(2):38-43. doi:10.1002/jhrm.21093
- Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
- Martin G, Chew S, McCarthy I, Dawson J, Dixon-Woods M. Encouraging openness in health care: policy and practice implications of a mixed-methods study in the English National Health Service. J Health Serv Res Policy. 2023;28(1):14-24. doi:10.1177/13558196221109053
- Siewert B, Brook OR, Swedeen S, Eisenberg RL, Hochman M. Overcoming human barriers to safety event reporting in radiology. Radiographics. 2019;39(1):251-263. doi:10.1148/rg.2019180135
- Barkell NP, Snyder SS. Just culture in healthcare: an integrative review. Nurs Forum. 2021;56(1):103-111. doi:10.1111/nuf.12525
- Murray JS, Lee J, Larson S, Range A, Scott D, Clifford J. Requirements for implementing a ‘just culture’ within healthcare organisations: an integrative review. BMJ Open Qual. 2023;12(2)e002237. doi:10.1136/bmjoq-2022-002237
- Mohr DC, Chen C, Sullivan J, Gunnar W, Damschroder L. Development and validation of the Veterans Health Administration patient safety culture survey. J Patient Saf. 2022;18(6):539-545. doi:10.1097/PTS.0000000000001027
- Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. 4th ed. SAGE Publications, Inc.; 2014.
- Patton MQ. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. 4th ed. SAGE Publications, Inc.; 2015.
- Maxwell JA. Qualitative Research Design: An Interactive Approach. 3rd ed. SAGE Publications, Inc.; 2013.
- Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47(4):2025-2047. doi:10.1007/s11135-011-9640-9
- Braun V, Clarke V. Thematic Analysis: A Practical Guide. SAGE Publications, Inc; 2021.
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68(3):151-157. doi:10.1097/JDM-D-23-00056
- Dietl JE, Derksen C, Keller FM, Lippke S. Interdisciplinary and interprofessional communication intervention: how psychological safety fosters communication and increases patient safety. Front Psychol. 2023;14:1164288. doi:10.3389/fpsyg.2023.1164288
- Eng DM, Schweikart SJ. Why accountability sharing in health care organizational cultures means patients are probably safer. AMA J Ethics. 2020;22(9):E779-E783. doi:10.1001/amajethics.2020.779
- Veterans Health Administration National Center for Patient Safety. Just Culture Decision Support Tool. Revised May 2021. Accessed August 5, 2024.https://www.patientsafety.va.gov/docs/Just-Culture-Decision-Support-Tool-2022.pdf
Medical errors are a persistent problem and leading cause of preventable death in the United States. There is considerable momentum behind the idea that implementation of a just culture is foundational to detecting and learning from errors in pursuit of zero patient harm.1-6 Just culture is a framework that fosters an environment of trust within health care organizations, aiming to achieve fair outcomes for those involved in incidents or near misses. It emphasizes openness, accountability, and learning, prioritizing the repair of harm and systemic improvement over assigning blame.7
A just culture mindset reflects a significant shift in thinking that moves from the tendency to blame and punish others toward a focus on organizational learning and continued process improvement.8,9 This systemic shift in fundamental thinking transforms how leaders approach staff errors and how they are addressed.10 In essence, just culture reflects an ethos centered on openness, a deep appreciation of human fallibility, and shared accountability at both the individual and organizational levels.
Organizational learning and innovation are stifled in the absence of a just culture, and there is a tendency for employees to avoid disclosing their own errors as well as those of their colleagues.11 The transformation to a just culture is often slowed or disrupted by personal, systemic, and cultural barriers.12 It is imperative that all executive, service line, and frontline managers recognize and execute their distinct responsibilities while adjudicating the appropriate course of action in the aftermath of adverse events or near misses. This requires a nuanced understanding of the factors that contribute to errors at the individual and organizational levels to ensure an appropriate response.
The Veterans Health Administration (VHA) is orchestrating an enterprise transformation to develop into a high reliability organization (HRO). This began with a single-site test in 2016, which demonstrated successful results in patient safety culture, patient safety event reporting, and patient safety outcomes.13 In 2019, the VHA formally launched its enterprise-wide HRO journey in 18 hospital facilities, followed by successive waves of 67 and 54 facilities in 2021 and 2022, respectively. The VHA journey to transform into an HRO aligns with 3 pillars, 5 principles, and 7 values. The VHA has emphasized the importance of just culture as a foundational element of the HRO framework, specifically under the pillar of leadership. To promote leadership engagement, the VHA has employed an array of approaches that include education, leader coaching, and change management strategies. Given the diversity among VHA facilities, each with local cultures and histories, some sites have more readily implemented a just culture than others.14 A deeper exploration into potential obstacles, particularly concerning leadership engagement, could be instrumental for formulating strategies that further establish a just culture across the VHA.15
There is a paucity of empirical research regarding factors that facilitate and/or impede the implementation of a just culture in health care settings.16,17 Likert scale surveys, such as the Patient Safety Culture Module for the VHA All Employee Survey and its predecessor, the Patient Safety Culture Survey, have been used to assess culture and climate.18 However, qualitative evaluations directly assessing the lived experiences of those trying to implement a just culture provide additional depth and context that can help identify specific factors that support or impede becoming an HRO. The purpose of this study was to increase understanding of factors that influence the establishment and sustainment of a just culture and to identify specific methods for improving the implementation of just culture principles and practices aligned with HRO.
METHODS
This qualitative study explored facilitators and barriers to establishing and sustaining a just culture as experienced across a subset of VHA facilities by HRO leads or staff assigned with the primary responsibilities of supporting facility-level HRO transformation. HRO leads are assigned responsibility for supporting executive leadership in planning, coordinating, implementing, and monitoring activities to ensure effective high reliability efforts, including focused efforts to establish a robust patient safety program, a culture of safety, and a culture of continuous process improvement.
Virtual focus group discussions held via Microsoft Teams generated in-depth, diverse perspectives from participants across 16 VHA facilities. Qualitative research and evaluation methods provide an enhanced depth of understanding and allow the emergence of detailed data.19 A qualitative grounded theory approach elicits complex, multifaceted phenomena that cannot be appreciated solely by numeric data.20 Grounded theory was selected to limit preconceived notions and provide a more systematic analysis, including open, axial, and thematic coding. Such methods afford opportunities to adapt to unplanned follow-up questions and thus provide a flexible approach to generate new ideas and concepts.21 Additionally, qualitative methods help overcome the tendencies of respondents to agree rather than disagree when presented with Likert-style scales, which tend to skew responses toward the positive.22
Participants must have been assigned as an HRO lead for ≥ 6 months at the same facility. Potential participants were identified through purposive sampling, considering their leadership roles in HRO and experience with just culture implementation, the size and complexity of their facility, and geographic distribution. Invitations explaining the study and encouraging voluntary participation to participate were emailed. Of 37 HRO leads invited to participate in the study, 16 agreed to participate and attended 1 of 3 hour-long focus group sessions. One session was rescheduled due to limited attendance. Participants represented a mix of VHA sites in terms of geography, facility size, and complexity.
Focus Group Procedures
Demographic data were collected prior to sessions via an online form to better understand the participant population, including facility complexity level, length of time in HRO lead role, clinical background, and facility level just culture training. Each session was led by an experienced focus group facilitator (CV) who was not directly involved with the overall HRO implementation to establish a neutral perspective. Each session was attended by 4 to 7 participants and 2 observers who took notes. The sessions were recorded and included automated transcriptions, which were edited for accuracy.
Focus group sessions began with a brief introduction and an opportunity for participants to ask questions. Participants were then asked a series of open-ended questions to elicit responses regardingfacilitators, barriers, and leadership support needed for implementing just culture. The questions were part of a facilitator guide that included an introductory script and discussion prompts to ensure consistency across focus groups.
Facilitators were defined as factors that increase the likelihood of establishing or sustaining a just culture. Barriers were defined as factors that decrease or inhibit the likelihood of establishing or sustaining just culture. The focus group facilitator encouraged all participants to share their views and provided clarification if needed, as well as prompts and examples where appropriate, but primarily sought to elicit responses to the questions.
Institutional review board review and approval were not required for this quality improvement initiative. The project adhered to ethical standards of research, including asking participants for verbal consent and preserving their confidentiality. Participation was voluntary, and prior to the focus group sessions, participants were provided information explaining the study’s purpose, procedures, and their rights. Participant identities were kept confidential, and all data were anonymized during the analysis phase. Pseudonyms or identifiers were used during data transcription to protect participant identity. All data, including recordings and transcriptions, were stored on password-protected devices accessible only to the research team. Any identifiable information was removed during data analysis to ensure confidentiality.
Analysis
Focus group recordings were transcribed verbatim, capturing all verbal interactions and nonverbal cues that may contribute to understanding the participants' perspectives. Thematic analysis was used to analyze the qualitative data from the focus group discussions.23 The transcribed data were organized, coded, and analyzed using ATLAS.ti 23 qualitative data software to identify key themes and patterns.
Results
The themes identified include the 5 facilitators, barriers, and recommendations most frequently mentioned by HRO leads across focus group sessions. The nature of each theme is described, along with commonly mentioned examples and direct quotes from participants that illustrate our understanding of their perspectives.
Facilitators
Training and coaching (26 responses). The availability of training around the Just Culture Decision Support Tool (DST) was cited as a practical aid in guiding leaders through complex just culture decisions to ensure consistency and fairness. Additionally, an executive leadership team that served as champions for just culture principles played a vital role in promoting and sustaining the approach: “Training them on the roll-out of the decision support tool with supervisors at all levels, and education for just culture and making it part of our safety forum has helped for the last 4 months.” “Having some regular training and share-out cadences embedded within the schedule as well as dynamic directors and well-trained executive leadership team (ELT) for support has been a facilitator.”
Increased transparency (16 responses). Participants consistently highlighted the importance of leadership transparency as a key facilitator for implementing just culture. Open and honest communication from top-level executives fostered an environment of trust and accountability. Approachable and physically present leadership was seen as essential for creating a culture where employees felt comfortable reporting errors and concerns without fear of retaliation: “They’re surprisingly honest with themselves about what we can do, what we cannot do, and they set the expectations exactly at that.”
Approachable leadership (15 responses). Participants frequently mentioned the importance of having dynamic leadership spearheading the implementation of just culture and leading by example. Having a leadership team that accepts accountability and reinforces consistency in the manner that near misses or mistakes are addressed is paramount to promoting the principles of just culture and increasing psychological safety: “We do have very approachable leadership, which I know is hard if you’re trying to implement that nationwide, it’s hard to implement approachability. But I do think that people raise their concerns, and they’ll stop them in the hallway and ask them questions. So, in terms of comfort level with the executive leadership, I do think that’s high, which would promote psychological safety.”
Feedback loops and follow through (13 responses). Participants emphasized the importance of taking concrete actions to address concerns and improve processes. Regular check-ins with supervisors to discuss matters related to just culture provided a structured opportunity for addressing issues and reinforcing the importance of the approach: “One thing that we’ve really focused on is not only identifying mistakes, but [taking] ownership. We continue to track it until … it’s completed and then a process of how to communicate that back and really using closed loop communication with the staff and letting them know.”
Forums and town halls (10 responses). These platforms created feedback loops that were seen as invaluable tools for sharing near misses, celebrating successes, and promoting open dialogue. Forums and town halls cultivated a culture of continuous improvement and trust: “We’ll celebrate catches, a safety story is inside that catch. So, if we celebrate the change, people feel safer to speak up.” “Truthfully, we’ve had a great relationship since establishing our safety forums and just value open lines of communication.”
Barriers
Inadequate training (30 responses). Insufficient engagement during training—limited bandwidth and availability to attend and actively participate in training—was perceived as detrimental to creating awareness and buy-in from staff, supervisors, and leadership, thereby hindering successful integration of just culture principles. Participants also identified too many conflicting priorities from VHA leadership, which contributes to training and information fatigue among staff and supervisors. “Our biggest barrier is just so many different competing priorities going on. We have so much that we’re asking people to do.” “One hundred percent training is feeling more like a ticked box than actually yielding results, I have a very hard time getting staff engaged.”
Inconsistency between executive leaders and middle managers (28 responses). A lack of consistency in the commitment to and enactment of just culture principles among leaders poses a challenge. Participants gave several examples of inconsistencies in messaging and reinforcement of just culture principles, leading to confusion among staff and hindering adoption. Likewise, the absence of standardized procedures for implementing just culture created variability: “The director coming in and trying to change things, it put a lot of resistance, we struggle with getting the other ELT members on board … some of the messages that come out at times can feel more punitive.”
Middle management resistance (22 responses). In some instances, participants reported middle managers exhibiting attitudes and behaviors that undermined the application of just culture principles and effectiveness. Such attitudes and behaviors were attributed to a lack of adequate training, coaching, and awareness. Other perceived contributions included fear of failure and a desire to distance oneself from staff who have made mistakes: “As soon as someone makes an error, they go straight to suspend them, and that’s the disconnect right there.” “There’s almost a level of working in the opposite direction in some of the mid-management.”
Cultural misalignment (18 responses). The existing culture of distancing oneself from mistakes presented a significant barrier to the adoption of just culture because it clashed with the principles of open reporting and accountability. Staff underreported errors or framed them in a way that minimized personal responsibility, thereby making it more essential to put in the necessary and difficult work to learn from mistakes: “One, you’re going to get in trouble. There’s going to be more work added to you or something of that nature."
Lack of accountability for opposition(17 responses). Participants noted a clear lack of accountability for those who opposed or showed resistance to just culture, which allowed resistance to persist without consequences. In many instances, leaders were described as having overlooked repeated instances of unjust attitudes and behaviors (eg, inappropriate blame or punishment), which allowed those practices to continue. “Executive leadership is standing on the hill and saying we’re a just culture and we do everything correctly, and staff has the expectation that they’re going to be treated with just culture and then the middle management is setting that on fire, then we show them that that’s not just culture, and they continue to have those poor behaviors, but there’s a lack of accountability.”
Limited bandwidth and lack of coordination (14 responses). HRO leads often faced role-specific constraints in having adequate time and authority to coordinate efforts to implement or sustain just culture. This includes challenges with coordination across organizational levels (eg, between the hospital and regional Veterans Integrated Service Network [VISN] management levels) and across departments within the hospital (eg, between human resources and service lines or units). “Our VISN human resources is completely detached. They’ll not cooperate with these efforts, which is hard.” “There’s not enough bandwidth to actually support, I’m just 1 person.” “[There’s] all these mandated trainings of 8 hours when we’re already fatigued, short-staffed, taking 3 other HRO classes.”
Recommendations
Training improvements (24 responses). HRO leads recommended that comprehensive training programs be developed and implemented for staff, supervisors, and leadership to increase awareness and understanding of just culture principles. These training initiatives should focus on fostering a shared understanding of the core tenets of just culture, the importance of error reporting, and the processes involved in fair and consistent decision making (eg, training simulations on use of the Just Culture DST). “We’ve really never had any formal training on the decision support tool. I hope that what’s coming out for next year. We’ll have some more formal training for the tool because I think it would be great to really have our leadership and our supervisors and our managers use that tool.” “We can give a more directed and intentional training to leadership on the 4 foundational practices and what it means to implement those and what it means to utilize that behavioral component of HRO.”
Clear and consistent procedures toincrease accountability (22 responses). To promote a culture of accountability and consistency in the application of just culture principles, organizations should establish clear mechanisms for reporting, investigating, and addressing incidents. Standardized procedures and DSTs can aid in ensuring that responses to errors are equitable and align with just culture principles: “I recommend accountability; if it’s clearly evidenced that you’re not toeing the just culture line, then we need to be able to do something about it and not just finger wag.” “[We need to have] a templated way to approach just culture implementation. The decision support tool is great, I absolutely love having the resources and being able to find a lot of clinical examples and discussion tools like that. But when it comes down to it, not having that kind of official thing to fall back on it can be a little bit rough.”
Additional coaching and consultationsupport (15 responses). To support supervisors in effectively implementing just culture within their teams, participants recommended that organizations provide ongoing coaching and mentorship opportunities. Additionally, third-party consultants with expertise in just culture were described as offering valuable guidance, particularly in cases where internal staff resources or HRO lead bandwidth may be limited. “There are so many consulting agencies with HRO that have been contracted to do different projects, but maybe that can help with an educational program.” “I want to see my executive leadership coach the supervisors up right and then allow them to do one-on-ones and facilitate and empower the frontline staff, and it’s just a good way of transparency and communication.”
Improved leadership sponsorship (15 responses). Participants noted that leadership buy-in is crucial for the successful implementation of just culture. Facilities should actively engage and educate leadership teams on the benefits of just culture and how it aligns with broader patient safety and organizational goals. Leaders should be visible and active champions of its principles, supporting change in their daily engagements with staff. “ELT support is absolutely necessary. Why? Because they will make it important to those in their service lines. They will make it important to those supervisors and managers. If it’s not important to that ELT member, then it’s not going to be important to that manager or that supervisor.”
Improved collaboration with patient safety and human resources (6 responses). Collaborative efforts with patient safety and human resources departments were seen as instrumental in supporting just culture, emphasizing its importance, and effectively addressing issues. Coordinating with these departments specifically contributes to consistent reinforcement and expands the bandwidth of HRO leads. These departments play integral roles in supporting just culture through effective policies, procedures, and communication. “I think it would be really helpful to have common language between what human resources teaches and what is in our decision support tool.”
DISCUSSION
This study sought to collect and synthesize the experiences of leaders across a large, integrated health care system in establishing and sustaining a just culture as part of an enterprise journey to become an HRO.24 The VHA has provided enterprise-wide support (eg, training, leader coaching, and communications) for the implementation of HRO principles and practices with the goal of creating a culture of safety, which includes just culture elements. This support includes enterprise program offices, VISNs, and hospital facilities, though notably, there is variability in how HRO is implemented at the local level. The facilitators, barriers, and recommendations presented in this article are representative of the designated HRO leads at VHA hospital facilities who have direct experience with implementing and sustaining just culture. The themes presented offer specific opportunities for intervention and actionable strategies to enhance just culture initiatives, foster psychological safety and accountability, and ultimately improve the quality of care and patient outcomes.3,25
Frequently identified facilitators such as providing training and coaching, having leaders who are available and approachable, demonstrating follow-through to address identified issues, and creating venues where errors and successes can be openly discussed.26 These facilitators are aligned with enterprise HRO support strategies orchestrated by the VHA at the enterprise VISN and facility levels to support a culture of safety, continuous process improvement, and leadership commitment.
Frequently identified barriers included inadequate training, inconsistent application of just culture by middle managers vs senior leaders, a lack of accountability or corrective action when unjust corrective actions took place, time and resource constraints, and inadequate coordination across departments (eg, operational departments and human resources) and organizational levels. These factors were identified through focus groups with a limited set of HRO leads. They may reflect challenges to changing culture that may be deeply engrained in individual histories, organizational norms, and systemic practices. Improving upon these just culture initiatives requires multifaceted approaches and working through resistance to change.
VHA HRO leads identified several actionable recommendations that may be used in pursuit of a just culture. First, improvements in training involving how to apply just culture principles and, specifically, the use of the Just Culture DST were identified as an opportunity for improvement. The VHA National Center for Patient Safety developed the DST as an aid for leaders to effectively address errors in line with just culture principles, balancing individual and system accountability.27 The DST specifically addresses human error as well as risky and reckless behavior, and it clarifies the delineation between individual and organizational accountability (Table).3

Scenario-based interactive training and simulations may prove especially useful for middle managers and frontline supervisors who are closest to errors. Clear and repeatable procedures for determining courses of action for accountability in response are needed, and support for their application must be coordinated across multiple departments (eg, patient safety and human resources) to ensure consistency and fairness. Coaching and consultation are also viewed as beneficial in supporting applications. Coaching is provided to senior leaders across most facilities, but the availability of specific, role-based coaching and training is more limited for middle managers and frontline supervisors who may benefit most from hands-on support.
Lastly, sponsorship from leaders was viewed as critical to success, but follow through to ensure support flows down from the executive suite to the frontline is variable across facilities and requires consistent effort over time. This is especially challenging given the frequent turnover in leadership roles evident in the VHA and other health care systems.
Limitations
This study employed qualitative methods and sampled a relatively small subset of experienced leaders with specific roles in implementing HRO in the VHA. Thus, it should not be considered representative of the perspectives of all leaders within the VHA or other health care systems. Future studies should assess facilitators and barriers beyond the facility level, including a focus incorporating both the VISN and VHA. More broadly, qualitative methods such as those employed in this study offer great depth and nuance but have limited ability to identify system-wide trends and differences. As such, it may be beneficial to specifically look at sites that are high- or low-performing on measures of patient safety culture to identify differences that may inform implementation strategies based on organizational maturity and readiness for change.
Conclusions
Successful implementation of these recommendations will require ongoing commitment, collaboration, and a sustained effort from all stakeholders involved at multiple levels of the health care system. Monitoring and evaluating progress should be conducted regularly to ensure that recommendations lead to improvements in implementing just culture principles. This quality improvement study adds to the knowledge base on factors that impact the just culture and broader efforts to realize HRO principles and practices in health care systems. The approach of this study may serve as a model for identifying opportunities to improve HRO implementation within the VHA and other settings, especially when paired with ongoing quantitative evaluation of organizational safety culture, just culture behaviors, and patient outcomes.
Medical errors are a persistent problem and leading cause of preventable death in the United States. There is considerable momentum behind the idea that implementation of a just culture is foundational to detecting and learning from errors in pursuit of zero patient harm.1-6 Just culture is a framework that fosters an environment of trust within health care organizations, aiming to achieve fair outcomes for those involved in incidents or near misses. It emphasizes openness, accountability, and learning, prioritizing the repair of harm and systemic improvement over assigning blame.7
A just culture mindset reflects a significant shift in thinking that moves from the tendency to blame and punish others toward a focus on organizational learning and continued process improvement.8,9 This systemic shift in fundamental thinking transforms how leaders approach staff errors and how they are addressed.10 In essence, just culture reflects an ethos centered on openness, a deep appreciation of human fallibility, and shared accountability at both the individual and organizational levels.
Organizational learning and innovation are stifled in the absence of a just culture, and there is a tendency for employees to avoid disclosing their own errors as well as those of their colleagues.11 The transformation to a just culture is often slowed or disrupted by personal, systemic, and cultural barriers.12 It is imperative that all executive, service line, and frontline managers recognize and execute their distinct responsibilities while adjudicating the appropriate course of action in the aftermath of adverse events or near misses. This requires a nuanced understanding of the factors that contribute to errors at the individual and organizational levels to ensure an appropriate response.
The Veterans Health Administration (VHA) is orchestrating an enterprise transformation to develop into a high reliability organization (HRO). This began with a single-site test in 2016, which demonstrated successful results in patient safety culture, patient safety event reporting, and patient safety outcomes.13 In 2019, the VHA formally launched its enterprise-wide HRO journey in 18 hospital facilities, followed by successive waves of 67 and 54 facilities in 2021 and 2022, respectively. The VHA journey to transform into an HRO aligns with 3 pillars, 5 principles, and 7 values. The VHA has emphasized the importance of just culture as a foundational element of the HRO framework, specifically under the pillar of leadership. To promote leadership engagement, the VHA has employed an array of approaches that include education, leader coaching, and change management strategies. Given the diversity among VHA facilities, each with local cultures and histories, some sites have more readily implemented a just culture than others.14 A deeper exploration into potential obstacles, particularly concerning leadership engagement, could be instrumental for formulating strategies that further establish a just culture across the VHA.15
There is a paucity of empirical research regarding factors that facilitate and/or impede the implementation of a just culture in health care settings.16,17 Likert scale surveys, such as the Patient Safety Culture Module for the VHA All Employee Survey and its predecessor, the Patient Safety Culture Survey, have been used to assess culture and climate.18 However, qualitative evaluations directly assessing the lived experiences of those trying to implement a just culture provide additional depth and context that can help identify specific factors that support or impede becoming an HRO. The purpose of this study was to increase understanding of factors that influence the establishment and sustainment of a just culture and to identify specific methods for improving the implementation of just culture principles and practices aligned with HRO.
METHODS
This qualitative study explored facilitators and barriers to establishing and sustaining a just culture as experienced across a subset of VHA facilities by HRO leads or staff assigned with the primary responsibilities of supporting facility-level HRO transformation. HRO leads are assigned responsibility for supporting executive leadership in planning, coordinating, implementing, and monitoring activities to ensure effective high reliability efforts, including focused efforts to establish a robust patient safety program, a culture of safety, and a culture of continuous process improvement.
Virtual focus group discussions held via Microsoft Teams generated in-depth, diverse perspectives from participants across 16 VHA facilities. Qualitative research and evaluation methods provide an enhanced depth of understanding and allow the emergence of detailed data.19 A qualitative grounded theory approach elicits complex, multifaceted phenomena that cannot be appreciated solely by numeric data.20 Grounded theory was selected to limit preconceived notions and provide a more systematic analysis, including open, axial, and thematic coding. Such methods afford opportunities to adapt to unplanned follow-up questions and thus provide a flexible approach to generate new ideas and concepts.21 Additionally, qualitative methods help overcome the tendencies of respondents to agree rather than disagree when presented with Likert-style scales, which tend to skew responses toward the positive.22
Participants must have been assigned as an HRO lead for ≥ 6 months at the same facility. Potential participants were identified through purposive sampling, considering their leadership roles in HRO and experience with just culture implementation, the size and complexity of their facility, and geographic distribution. Invitations explaining the study and encouraging voluntary participation to participate were emailed. Of 37 HRO leads invited to participate in the study, 16 agreed to participate and attended 1 of 3 hour-long focus group sessions. One session was rescheduled due to limited attendance. Participants represented a mix of VHA sites in terms of geography, facility size, and complexity.
Focus Group Procedures
Demographic data were collected prior to sessions via an online form to better understand the participant population, including facility complexity level, length of time in HRO lead role, clinical background, and facility level just culture training. Each session was led by an experienced focus group facilitator (CV) who was not directly involved with the overall HRO implementation to establish a neutral perspective. Each session was attended by 4 to 7 participants and 2 observers who took notes. The sessions were recorded and included automated transcriptions, which were edited for accuracy.
Focus group sessions began with a brief introduction and an opportunity for participants to ask questions. Participants were then asked a series of open-ended questions to elicit responses regardingfacilitators, barriers, and leadership support needed for implementing just culture. The questions were part of a facilitator guide that included an introductory script and discussion prompts to ensure consistency across focus groups.
Facilitators were defined as factors that increase the likelihood of establishing or sustaining a just culture. Barriers were defined as factors that decrease or inhibit the likelihood of establishing or sustaining just culture. The focus group facilitator encouraged all participants to share their views and provided clarification if needed, as well as prompts and examples where appropriate, but primarily sought to elicit responses to the questions.
Institutional review board review and approval were not required for this quality improvement initiative. The project adhered to ethical standards of research, including asking participants for verbal consent and preserving their confidentiality. Participation was voluntary, and prior to the focus group sessions, participants were provided information explaining the study’s purpose, procedures, and their rights. Participant identities were kept confidential, and all data were anonymized during the analysis phase. Pseudonyms or identifiers were used during data transcription to protect participant identity. All data, including recordings and transcriptions, were stored on password-protected devices accessible only to the research team. Any identifiable information was removed during data analysis to ensure confidentiality.
Analysis
Focus group recordings were transcribed verbatim, capturing all verbal interactions and nonverbal cues that may contribute to understanding the participants' perspectives. Thematic analysis was used to analyze the qualitative data from the focus group discussions.23 The transcribed data were organized, coded, and analyzed using ATLAS.ti 23 qualitative data software to identify key themes and patterns.
Results
The themes identified include the 5 facilitators, barriers, and recommendations most frequently mentioned by HRO leads across focus group sessions. The nature of each theme is described, along with commonly mentioned examples and direct quotes from participants that illustrate our understanding of their perspectives.
Facilitators
Training and coaching (26 responses). The availability of training around the Just Culture Decision Support Tool (DST) was cited as a practical aid in guiding leaders through complex just culture decisions to ensure consistency and fairness. Additionally, an executive leadership team that served as champions for just culture principles played a vital role in promoting and sustaining the approach: “Training them on the roll-out of the decision support tool with supervisors at all levels, and education for just culture and making it part of our safety forum has helped for the last 4 months.” “Having some regular training and share-out cadences embedded within the schedule as well as dynamic directors and well-trained executive leadership team (ELT) for support has been a facilitator.”
Increased transparency (16 responses). Participants consistently highlighted the importance of leadership transparency as a key facilitator for implementing just culture. Open and honest communication from top-level executives fostered an environment of trust and accountability. Approachable and physically present leadership was seen as essential for creating a culture where employees felt comfortable reporting errors and concerns without fear of retaliation: “They’re surprisingly honest with themselves about what we can do, what we cannot do, and they set the expectations exactly at that.”
Approachable leadership (15 responses). Participants frequently mentioned the importance of having dynamic leadership spearheading the implementation of just culture and leading by example. Having a leadership team that accepts accountability and reinforces consistency in the manner that near misses or mistakes are addressed is paramount to promoting the principles of just culture and increasing psychological safety: “We do have very approachable leadership, which I know is hard if you’re trying to implement that nationwide, it’s hard to implement approachability. But I do think that people raise their concerns, and they’ll stop them in the hallway and ask them questions. So, in terms of comfort level with the executive leadership, I do think that’s high, which would promote psychological safety.”
Feedback loops and follow through (13 responses). Participants emphasized the importance of taking concrete actions to address concerns and improve processes. Regular check-ins with supervisors to discuss matters related to just culture provided a structured opportunity for addressing issues and reinforcing the importance of the approach: “One thing that we’ve really focused on is not only identifying mistakes, but [taking] ownership. We continue to track it until … it’s completed and then a process of how to communicate that back and really using closed loop communication with the staff and letting them know.”
Forums and town halls (10 responses). These platforms created feedback loops that were seen as invaluable tools for sharing near misses, celebrating successes, and promoting open dialogue. Forums and town halls cultivated a culture of continuous improvement and trust: “We’ll celebrate catches, a safety story is inside that catch. So, if we celebrate the change, people feel safer to speak up.” “Truthfully, we’ve had a great relationship since establishing our safety forums and just value open lines of communication.”
Barriers
Inadequate training (30 responses). Insufficient engagement during training—limited bandwidth and availability to attend and actively participate in training—was perceived as detrimental to creating awareness and buy-in from staff, supervisors, and leadership, thereby hindering successful integration of just culture principles. Participants also identified too many conflicting priorities from VHA leadership, which contributes to training and information fatigue among staff and supervisors. “Our biggest barrier is just so many different competing priorities going on. We have so much that we’re asking people to do.” “One hundred percent training is feeling more like a ticked box than actually yielding results, I have a very hard time getting staff engaged.”
Inconsistency between executive leaders and middle managers (28 responses). A lack of consistency in the commitment to and enactment of just culture principles among leaders poses a challenge. Participants gave several examples of inconsistencies in messaging and reinforcement of just culture principles, leading to confusion among staff and hindering adoption. Likewise, the absence of standardized procedures for implementing just culture created variability: “The director coming in and trying to change things, it put a lot of resistance, we struggle with getting the other ELT members on board … some of the messages that come out at times can feel more punitive.”
Middle management resistance (22 responses). In some instances, participants reported middle managers exhibiting attitudes and behaviors that undermined the application of just culture principles and effectiveness. Such attitudes and behaviors were attributed to a lack of adequate training, coaching, and awareness. Other perceived contributions included fear of failure and a desire to distance oneself from staff who have made mistakes: “As soon as someone makes an error, they go straight to suspend them, and that’s the disconnect right there.” “There’s almost a level of working in the opposite direction in some of the mid-management.”
Cultural misalignment (18 responses). The existing culture of distancing oneself from mistakes presented a significant barrier to the adoption of just culture because it clashed with the principles of open reporting and accountability. Staff underreported errors or framed them in a way that minimized personal responsibility, thereby making it more essential to put in the necessary and difficult work to learn from mistakes: “One, you’re going to get in trouble. There’s going to be more work added to you or something of that nature."
Lack of accountability for opposition(17 responses). Participants noted a clear lack of accountability for those who opposed or showed resistance to just culture, which allowed resistance to persist without consequences. In many instances, leaders were described as having overlooked repeated instances of unjust attitudes and behaviors (eg, inappropriate blame or punishment), which allowed those practices to continue. “Executive leadership is standing on the hill and saying we’re a just culture and we do everything correctly, and staff has the expectation that they’re going to be treated with just culture and then the middle management is setting that on fire, then we show them that that’s not just culture, and they continue to have those poor behaviors, but there’s a lack of accountability.”
Limited bandwidth and lack of coordination (14 responses). HRO leads often faced role-specific constraints in having adequate time and authority to coordinate efforts to implement or sustain just culture. This includes challenges with coordination across organizational levels (eg, between the hospital and regional Veterans Integrated Service Network [VISN] management levels) and across departments within the hospital (eg, between human resources and service lines or units). “Our VISN human resources is completely detached. They’ll not cooperate with these efforts, which is hard.” “There’s not enough bandwidth to actually support, I’m just 1 person.” “[There’s] all these mandated trainings of 8 hours when we’re already fatigued, short-staffed, taking 3 other HRO classes.”
Recommendations
Training improvements (24 responses). HRO leads recommended that comprehensive training programs be developed and implemented for staff, supervisors, and leadership to increase awareness and understanding of just culture principles. These training initiatives should focus on fostering a shared understanding of the core tenets of just culture, the importance of error reporting, and the processes involved in fair and consistent decision making (eg, training simulations on use of the Just Culture DST). “We’ve really never had any formal training on the decision support tool. I hope that what’s coming out for next year. We’ll have some more formal training for the tool because I think it would be great to really have our leadership and our supervisors and our managers use that tool.” “We can give a more directed and intentional training to leadership on the 4 foundational practices and what it means to implement those and what it means to utilize that behavioral component of HRO.”
Clear and consistent procedures toincrease accountability (22 responses). To promote a culture of accountability and consistency in the application of just culture principles, organizations should establish clear mechanisms for reporting, investigating, and addressing incidents. Standardized procedures and DSTs can aid in ensuring that responses to errors are equitable and align with just culture principles: “I recommend accountability; if it’s clearly evidenced that you’re not toeing the just culture line, then we need to be able to do something about it and not just finger wag.” “[We need to have] a templated way to approach just culture implementation. The decision support tool is great, I absolutely love having the resources and being able to find a lot of clinical examples and discussion tools like that. But when it comes down to it, not having that kind of official thing to fall back on it can be a little bit rough.”
Additional coaching and consultationsupport (15 responses). To support supervisors in effectively implementing just culture within their teams, participants recommended that organizations provide ongoing coaching and mentorship opportunities. Additionally, third-party consultants with expertise in just culture were described as offering valuable guidance, particularly in cases where internal staff resources or HRO lead bandwidth may be limited. “There are so many consulting agencies with HRO that have been contracted to do different projects, but maybe that can help with an educational program.” “I want to see my executive leadership coach the supervisors up right and then allow them to do one-on-ones and facilitate and empower the frontline staff, and it’s just a good way of transparency and communication.”
Improved leadership sponsorship (15 responses). Participants noted that leadership buy-in is crucial for the successful implementation of just culture. Facilities should actively engage and educate leadership teams on the benefits of just culture and how it aligns with broader patient safety and organizational goals. Leaders should be visible and active champions of its principles, supporting change in their daily engagements with staff. “ELT support is absolutely necessary. Why? Because they will make it important to those in their service lines. They will make it important to those supervisors and managers. If it’s not important to that ELT member, then it’s not going to be important to that manager or that supervisor.”
Improved collaboration with patient safety and human resources (6 responses). Collaborative efforts with patient safety and human resources departments were seen as instrumental in supporting just culture, emphasizing its importance, and effectively addressing issues. Coordinating with these departments specifically contributes to consistent reinforcement and expands the bandwidth of HRO leads. These departments play integral roles in supporting just culture through effective policies, procedures, and communication. “I think it would be really helpful to have common language between what human resources teaches and what is in our decision support tool.”
DISCUSSION
This study sought to collect and synthesize the experiences of leaders across a large, integrated health care system in establishing and sustaining a just culture as part of an enterprise journey to become an HRO.24 The VHA has provided enterprise-wide support (eg, training, leader coaching, and communications) for the implementation of HRO principles and practices with the goal of creating a culture of safety, which includes just culture elements. This support includes enterprise program offices, VISNs, and hospital facilities, though notably, there is variability in how HRO is implemented at the local level. The facilitators, barriers, and recommendations presented in this article are representative of the designated HRO leads at VHA hospital facilities who have direct experience with implementing and sustaining just culture. The themes presented offer specific opportunities for intervention and actionable strategies to enhance just culture initiatives, foster psychological safety and accountability, and ultimately improve the quality of care and patient outcomes.3,25
Frequently identified facilitators such as providing training and coaching, having leaders who are available and approachable, demonstrating follow-through to address identified issues, and creating venues where errors and successes can be openly discussed.26 These facilitators are aligned with enterprise HRO support strategies orchestrated by the VHA at the enterprise VISN and facility levels to support a culture of safety, continuous process improvement, and leadership commitment.
Frequently identified barriers included inadequate training, inconsistent application of just culture by middle managers vs senior leaders, a lack of accountability or corrective action when unjust corrective actions took place, time and resource constraints, and inadequate coordination across departments (eg, operational departments and human resources) and organizational levels. These factors were identified through focus groups with a limited set of HRO leads. They may reflect challenges to changing culture that may be deeply engrained in individual histories, organizational norms, and systemic practices. Improving upon these just culture initiatives requires multifaceted approaches and working through resistance to change.
VHA HRO leads identified several actionable recommendations that may be used in pursuit of a just culture. First, improvements in training involving how to apply just culture principles and, specifically, the use of the Just Culture DST were identified as an opportunity for improvement. The VHA National Center for Patient Safety developed the DST as an aid for leaders to effectively address errors in line with just culture principles, balancing individual and system accountability.27 The DST specifically addresses human error as well as risky and reckless behavior, and it clarifies the delineation between individual and organizational accountability (Table).3

Scenario-based interactive training and simulations may prove especially useful for middle managers and frontline supervisors who are closest to errors. Clear and repeatable procedures for determining courses of action for accountability in response are needed, and support for their application must be coordinated across multiple departments (eg, patient safety and human resources) to ensure consistency and fairness. Coaching and consultation are also viewed as beneficial in supporting applications. Coaching is provided to senior leaders across most facilities, but the availability of specific, role-based coaching and training is more limited for middle managers and frontline supervisors who may benefit most from hands-on support.
Lastly, sponsorship from leaders was viewed as critical to success, but follow through to ensure support flows down from the executive suite to the frontline is variable across facilities and requires consistent effort over time. This is especially challenging given the frequent turnover in leadership roles evident in the VHA and other health care systems.
Limitations
This study employed qualitative methods and sampled a relatively small subset of experienced leaders with specific roles in implementing HRO in the VHA. Thus, it should not be considered representative of the perspectives of all leaders within the VHA or other health care systems. Future studies should assess facilitators and barriers beyond the facility level, including a focus incorporating both the VISN and VHA. More broadly, qualitative methods such as those employed in this study offer great depth and nuance but have limited ability to identify system-wide trends and differences. As such, it may be beneficial to specifically look at sites that are high- or low-performing on measures of patient safety culture to identify differences that may inform implementation strategies based on organizational maturity and readiness for change.
Conclusions
Successful implementation of these recommendations will require ongoing commitment, collaboration, and a sustained effort from all stakeholders involved at multiple levels of the health care system. Monitoring and evaluating progress should be conducted regularly to ensure that recommendations lead to improvements in implementing just culture principles. This quality improvement study adds to the knowledge base on factors that impact the just culture and broader efforts to realize HRO principles and practices in health care systems. The approach of this study may serve as a model for identifying opportunities to improve HRO implementation within the VHA and other settings, especially when paired with ongoing quantitative evaluation of organizational safety culture, just culture behaviors, and patient outcomes.
- Aljabari S, Kadhim Z. Common barriers to reporting medical errors. ScientificWorldJournal. 2021;2021:6494889. doi:10.1155/2021/6494889
- Arnal-Velasco D, Heras-Hernando V. Learning from errors and resilience. Curr Opin Anaesthesiol. 2023;36(3):376-381. doi:10.1097/ACO.0000000000001257
- Murray JS, Clifford J, Larson S, Lee JK, Sculli GL. Implementing just culture to improve patient safety. Mil Med. doi:10.1093/milmed/usac115
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- van Baarle E, Hartman L, Rooijakkers S, et al. Fostering a just culture in healthcare organizations: experiences in practice. BMC Health Serv Res. 2022;22(1):1035. doi:10.1186/s12913-022-08418-z
- Weenink JW, Wallenburg I, Hartman L, et al. Role of the regulator in enabling a just culture: a qualitative study in mental health and hospital care. BMJ Open. 2022;12(7):e061321. doi:10.1136/bmjopen-2022-061321
- White RM, Delacroix R. Second victim phenomenon: is ‘just culture’ a reality? An integrative review. Appl Nurs Res. 2020;56:151319. doi:10.1016/j.apnr.2020.151319
- Cribb A, O’Hara JK, Waring J. Improving responses to safety incidents: we need to talk about justice. BMJ Qual Saf. 2022;31(4):327-330. doi:10.1136/bmjqs-2021-014333
- Rocco C, Rodríguez AM, Noya B. Elimination of punitive outcomes and criminalization of medical errors. Curr Opin Anaesthesiol. 2022;35(6):728-732. doi:10.1097/ACO.0000000000001197
- Dekker S, Rafferty J, Oates A. Restorative Just Culture in Practice: Implementation and Evaluation. Routledge; 2022.
- Brattebø G, Flaatten HK. Errors in medicine: punishment versus learning medical adverse events revisited - expanding the frame. Curr Opin Anaesthesiol. 2023;36(2):240-245. doi:10.1097/ACO.0000000000001235
- Shabel W, Dennis JL. Missouri’s just culture collaborative. J Healthc Risk Manag. 2012;32(2):38-43. doi:10.1002/jhrm.21093
- Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
- Martin G, Chew S, McCarthy I, Dawson J, Dixon-Woods M. Encouraging openness in health care: policy and practice implications of a mixed-methods study in the English National Health Service. J Health Serv Res Policy. 2023;28(1):14-24. doi:10.1177/13558196221109053
- Siewert B, Brook OR, Swedeen S, Eisenberg RL, Hochman M. Overcoming human barriers to safety event reporting in radiology. Radiographics. 2019;39(1):251-263. doi:10.1148/rg.2019180135
- Barkell NP, Snyder SS. Just culture in healthcare: an integrative review. Nurs Forum. 2021;56(1):103-111. doi:10.1111/nuf.12525
- Murray JS, Lee J, Larson S, Range A, Scott D, Clifford J. Requirements for implementing a ‘just culture’ within healthcare organisations: an integrative review. BMJ Open Qual. 2023;12(2)e002237. doi:10.1136/bmjoq-2022-002237
- Mohr DC, Chen C, Sullivan J, Gunnar W, Damschroder L. Development and validation of the Veterans Health Administration patient safety culture survey. J Patient Saf. 2022;18(6):539-545. doi:10.1097/PTS.0000000000001027
- Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. 4th ed. SAGE Publications, Inc.; 2014.
- Patton MQ. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. 4th ed. SAGE Publications, Inc.; 2015.
- Maxwell JA. Qualitative Research Design: An Interactive Approach. 3rd ed. SAGE Publications, Inc.; 2013.
- Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47(4):2025-2047. doi:10.1007/s11135-011-9640-9
- Braun V, Clarke V. Thematic Analysis: A Practical Guide. SAGE Publications, Inc; 2021.
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68(3):151-157. doi:10.1097/JDM-D-23-00056
- Dietl JE, Derksen C, Keller FM, Lippke S. Interdisciplinary and interprofessional communication intervention: how psychological safety fosters communication and increases patient safety. Front Psychol. 2023;14:1164288. doi:10.3389/fpsyg.2023.1164288
- Eng DM, Schweikart SJ. Why accountability sharing in health care organizational cultures means patients are probably safer. AMA J Ethics. 2020;22(9):E779-E783. doi:10.1001/amajethics.2020.779
- Veterans Health Administration National Center for Patient Safety. Just Culture Decision Support Tool. Revised May 2021. Accessed August 5, 2024.https://www.patientsafety.va.gov/docs/Just-Culture-Decision-Support-Tool-2022.pdf
- Aljabari S, Kadhim Z. Common barriers to reporting medical errors. ScientificWorldJournal. 2021;2021:6494889. doi:10.1155/2021/6494889
- Arnal-Velasco D, Heras-Hernando V. Learning from errors and resilience. Curr Opin Anaesthesiol. 2023;36(3):376-381. doi:10.1097/ACO.0000000000001257
- Murray JS, Clifford J, Larson S, Lee JK, Sculli GL. Implementing just culture to improve patient safety. Mil Med. doi:10.1093/milmed/usac115
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187(7-8):808-810. doi:10.1093/milmed/usac041
- van Baarle E, Hartman L, Rooijakkers S, et al. Fostering a just culture in healthcare organizations: experiences in practice. BMC Health Serv Res. 2022;22(1):1035. doi:10.1186/s12913-022-08418-z
- Weenink JW, Wallenburg I, Hartman L, et al. Role of the regulator in enabling a just culture: a qualitative study in mental health and hospital care. BMJ Open. 2022;12(7):e061321. doi:10.1136/bmjopen-2022-061321
- White RM, Delacroix R. Second victim phenomenon: is ‘just culture’ a reality? An integrative review. Appl Nurs Res. 2020;56:151319. doi:10.1016/j.apnr.2020.151319
- Cribb A, O’Hara JK, Waring J. Improving responses to safety incidents: we need to talk about justice. BMJ Qual Saf. 2022;31(4):327-330. doi:10.1136/bmjqs-2021-014333
- Rocco C, Rodríguez AM, Noya B. Elimination of punitive outcomes and criminalization of medical errors. Curr Opin Anaesthesiol. 2022;35(6):728-732. doi:10.1097/ACO.0000000000001197
- Dekker S, Rafferty J, Oates A. Restorative Just Culture in Practice: Implementation and Evaluation. Routledge; 2022.
- Brattebø G, Flaatten HK. Errors in medicine: punishment versus learning medical adverse events revisited - expanding the frame. Curr Opin Anaesthesiol. 2023;36(2):240-245. doi:10.1097/ACO.0000000000001235
- Shabel W, Dennis JL. Missouri’s just culture collaborative. J Healthc Risk Manag. 2012;32(2):38-43. doi:10.1002/jhrm.21093
- Sculli GL, Pendley-Louis R, Neily J, et al. A high-reliability organization framework for health care: a multiyear implementation strategy and associated outcomes. J Patient Saf. 2022;18(1):64-70. doi:10.1097/PTS.0000000000000788
- Martin G, Chew S, McCarthy I, Dawson J, Dixon-Woods M. Encouraging openness in health care: policy and practice implications of a mixed-methods study in the English National Health Service. J Health Serv Res Policy. 2023;28(1):14-24. doi:10.1177/13558196221109053
- Siewert B, Brook OR, Swedeen S, Eisenberg RL, Hochman M. Overcoming human barriers to safety event reporting in radiology. Radiographics. 2019;39(1):251-263. doi:10.1148/rg.2019180135
- Barkell NP, Snyder SS. Just culture in healthcare: an integrative review. Nurs Forum. 2021;56(1):103-111. doi:10.1111/nuf.12525
- Murray JS, Lee J, Larson S, Range A, Scott D, Clifford J. Requirements for implementing a ‘just culture’ within healthcare organisations: an integrative review. BMJ Open Qual. 2023;12(2)e002237. doi:10.1136/bmjoq-2022-002237
- Mohr DC, Chen C, Sullivan J, Gunnar W, Damschroder L. Development and validation of the Veterans Health Administration patient safety culture survey. J Patient Saf. 2022;18(6):539-545. doi:10.1097/PTS.0000000000001027
- Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. 4th ed. SAGE Publications, Inc.; 2014.
- Patton MQ. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. 4th ed. SAGE Publications, Inc.; 2015.
- Maxwell JA. Qualitative Research Design: An Interactive Approach. 3rd ed. SAGE Publications, Inc.; 2013.
- Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47(4):2025-2047. doi:10.1007/s11135-011-9640-9
- Braun V, Clarke V. Thematic Analysis: A Practical Guide. SAGE Publications, Inc; 2021.
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68(3):151-157. doi:10.1097/JDM-D-23-00056
- Dietl JE, Derksen C, Keller FM, Lippke S. Interdisciplinary and interprofessional communication intervention: how psychological safety fosters communication and increases patient safety. Front Psychol. 2023;14:1164288. doi:10.3389/fpsyg.2023.1164288
- Eng DM, Schweikart SJ. Why accountability sharing in health care organizational cultures means patients are probably safer. AMA J Ethics. 2020;22(9):E779-E783. doi:10.1001/amajethics.2020.779
- Veterans Health Administration National Center for Patient Safety. Just Culture Decision Support Tool. Revised May 2021. Accessed August 5, 2024.https://www.patientsafety.va.gov/docs/Just-Culture-Decision-Support-Tool-2022.pdf
VA Cancer Clinical Trials as a Strategy for Increasing Accrual of Racial and Ethnic Underrepresented Groups
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
Background
Cancer clinical trials (CCTs) are central to improving cancer care. However, generalizability of findings from CCTs is difficult due to the lack of diversity in most United States CCTs. Clinical trial accrual of underrepresented groups, is low throughout the United States and is approximately 4-5% in most CCTs. Reasons for low accrual in this population are multifactorial. Despite numerous factors related to accruing racial and ethnic underrepresented groups, many institutions have sought to address these barriers. We conducted a scoping review to identify evidence-based approaches to increase participation in cancer treatment clinical trials.
Methods
We reviewed the Salisbury VA Medical Center Oncology clinical trial database from October 2019 to June 2024. The participants in these clinical trials required consent. These clinical trials included treatment interventional as well as non-treatment interventional. Fifteen studies were included and over 260 Veterans participated.
Results
Key themes emerged that included a focus on patient education, cultural competency, and building capacity in the clinics to care for the Veteran population at three separate sites in the Salisbury VA system. The Black Veteran accrual rate of 29% was achieved. This accrual rate is representative of our VA catchment population of 33% for Black Veterans, and is five times the national average.
Conclusions
The research team’s success in enrolling Black Veterans in clinical trials is attributed to several factors. The demographic composition of Veterans served by the Salisbury, Charlotte, and Kernersville VA provided a diverse population that included a 33% Black group. The type of clinical trials focused on patients who were most impacted by the disease. The VA did afford less barriers to access to health care.
CDK7 Inhibition in Patient-Derived Organoid Modeling of Biliary Tract Cancers
Background
Biliary tract cancers (BTC) represent an important rare cancer type in Veterans. The heterogeneity of BTC has revealed distinct molecular subtypes, however a majority of patients remain without precision-based targeted therapeutics. Epigenomic remodeling has been considered as a shared mechanism of therapeutic resistance. Cyclin dependant kinase 7 (CDK7) is an emerging therapeutic target that functions by phosphorylation of RNA polymerase II and cell cycle progression. Here, we investigate CDK7 inhibition using small molecule inhibition (SY-5609) across a panel of BTC organoid models.
Methods
PCOs were expanded from patient-derived tissues and shared models provided from the NCI. Organoid response was tracked from growth using Z-stacked high content imaging (Cytation5) to track individual organoid growth and established viability markers of Caspase-3/7 (C3/7) and ToPro3, for induced apoptosis and necrosis for phenotypic screening. Treatment groups included media control, positive control (cycloheximide) 200uM continuous, gemcitabine (gem) 10uM 24h, cisplatin (cis) 5uM 48h, combination gem+cis, and SY-5609 10nM 144h. Glass’s delta was used to standardize effect size relative to media control.
Results
Patient-derived cancer organoids were generated across four unique models including pathogenic (A-B) IDH1 p.R132G, (C) FGFR2-HPGDS fusion and (D) non-targetable molecular profile (CCNE1 amplified, BRCA1 splice variant). In the non-targeted model, CDK7 inhibition achieved growth arrest +2.0% (SY-5607) v. +43.0% (media control) with effect size >1.1. This response was similar to standard of care gem+cis with growth of +1.5% and augmented using the combination of gem+SY-5609 -3.1% with effect size of >1.3. When treated with CDK7 inhibition, persistent growth was seen across models of IDH1 mutant and FGFR2-HPGDS2 fusion cancers. High content imaging revealed subclonal populations with failed induction of apoptosis and necrosis at 144h, suggestive of the critical need to address intrinsic resistant populations to both SOC chemotherapy and novel targeted strategies.
Conclusions
Across a diversity of BTC cancer models, CDK7 inhibition was found to achieve growth arrest in a CCNE1 amplified cancer model. High content imaging of organoids can identify subclonal resistant populations as a critical unmet need in future therapeutic development. Ongoing work is adapting these techniques to multiple small molecule inhibitors that target transcription including EZH1/2 and CDK9.
Background
Biliary tract cancers (BTC) represent an important rare cancer type in Veterans. The heterogeneity of BTC has revealed distinct molecular subtypes, however a majority of patients remain without precision-based targeted therapeutics. Epigenomic remodeling has been considered as a shared mechanism of therapeutic resistance. Cyclin dependant kinase 7 (CDK7) is an emerging therapeutic target that functions by phosphorylation of RNA polymerase II and cell cycle progression. Here, we investigate CDK7 inhibition using small molecule inhibition (SY-5609) across a panel of BTC organoid models.
Methods
PCOs were expanded from patient-derived tissues and shared models provided from the NCI. Organoid response was tracked from growth using Z-stacked high content imaging (Cytation5) to track individual organoid growth and established viability markers of Caspase-3/7 (C3/7) and ToPro3, for induced apoptosis and necrosis for phenotypic screening. Treatment groups included media control, positive control (cycloheximide) 200uM continuous, gemcitabine (gem) 10uM 24h, cisplatin (cis) 5uM 48h, combination gem+cis, and SY-5609 10nM 144h. Glass’s delta was used to standardize effect size relative to media control.
Results
Patient-derived cancer organoids were generated across four unique models including pathogenic (A-B) IDH1 p.R132G, (C) FGFR2-HPGDS fusion and (D) non-targetable molecular profile (CCNE1 amplified, BRCA1 splice variant). In the non-targeted model, CDK7 inhibition achieved growth arrest +2.0% (SY-5607) v. +43.0% (media control) with effect size >1.1. This response was similar to standard of care gem+cis with growth of +1.5% and augmented using the combination of gem+SY-5609 -3.1% with effect size of >1.3. When treated with CDK7 inhibition, persistent growth was seen across models of IDH1 mutant and FGFR2-HPGDS2 fusion cancers. High content imaging revealed subclonal populations with failed induction of apoptosis and necrosis at 144h, suggestive of the critical need to address intrinsic resistant populations to both SOC chemotherapy and novel targeted strategies.
Conclusions
Across a diversity of BTC cancer models, CDK7 inhibition was found to achieve growth arrest in a CCNE1 amplified cancer model. High content imaging of organoids can identify subclonal resistant populations as a critical unmet need in future therapeutic development. Ongoing work is adapting these techniques to multiple small molecule inhibitors that target transcription including EZH1/2 and CDK9.
Background
Biliary tract cancers (BTC) represent an important rare cancer type in Veterans. The heterogeneity of BTC has revealed distinct molecular subtypes, however a majority of patients remain without precision-based targeted therapeutics. Epigenomic remodeling has been considered as a shared mechanism of therapeutic resistance. Cyclin dependant kinase 7 (CDK7) is an emerging therapeutic target that functions by phosphorylation of RNA polymerase II and cell cycle progression. Here, we investigate CDK7 inhibition using small molecule inhibition (SY-5609) across a panel of BTC organoid models.
Methods
PCOs were expanded from patient-derived tissues and shared models provided from the NCI. Organoid response was tracked from growth using Z-stacked high content imaging (Cytation5) to track individual organoid growth and established viability markers of Caspase-3/7 (C3/7) and ToPro3, for induced apoptosis and necrosis for phenotypic screening. Treatment groups included media control, positive control (cycloheximide) 200uM continuous, gemcitabine (gem) 10uM 24h, cisplatin (cis) 5uM 48h, combination gem+cis, and SY-5609 10nM 144h. Glass’s delta was used to standardize effect size relative to media control.
Results
Patient-derived cancer organoids were generated across four unique models including pathogenic (A-B) IDH1 p.R132G, (C) FGFR2-HPGDS fusion and (D) non-targetable molecular profile (CCNE1 amplified, BRCA1 splice variant). In the non-targeted model, CDK7 inhibition achieved growth arrest +2.0% (SY-5607) v. +43.0% (media control) with effect size >1.1. This response was similar to standard of care gem+cis with growth of +1.5% and augmented using the combination of gem+SY-5609 -3.1% with effect size of >1.3. When treated with CDK7 inhibition, persistent growth was seen across models of IDH1 mutant and FGFR2-HPGDS2 fusion cancers. High content imaging revealed subclonal populations with failed induction of apoptosis and necrosis at 144h, suggestive of the critical need to address intrinsic resistant populations to both SOC chemotherapy and novel targeted strategies.
Conclusions
Across a diversity of BTC cancer models, CDK7 inhibition was found to achieve growth arrest in a CCNE1 amplified cancer model. High content imaging of organoids can identify subclonal resistant populations as a critical unmet need in future therapeutic development. Ongoing work is adapting these techniques to multiple small molecule inhibitors that target transcription including EZH1/2 and CDK9.
Carboplatin as a Radiosensitizing Agent in Locally Advanced Head and Neck Cancer: Friendly to an Older Veteran Population
Background
The standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is combination chemoradiotherapy. Platinum-based chemotherapy is used for radiosensitization and significantly improves locoregional control and survival. Cisplatin is the standard of care; however, many patients are cisplatin-ineligible due to underlying comorbidities. Carboplatin is an alternative chemotherapy in these patients, but efficacy data are lacking. Purpose: To evaluate the efficacy and tolerability of weekly carboplatin concurrent with radiation in veterans with locally advanced HNSCC.
Methods
Our tumor registry was used to identify patients who received platinum-based chemoradiotherapy for stage III-IVB HNSCC at a single center between 2007 to 2017. Patients who received carboplatin were identified. Data including dosing, toxicities, and disease response was collected and analyzed.
Results
A total of 26 patients who received weekly carboplatin were analyzed. All patients were male with an average age of 65. A usual dose of carboplatin AUC 2 was utilized. The average cumulative dose for weekly carboplatin was AUC 12, with most patients (65%) receiving 6 doses or more. The mean number of weekly carboplatin doses held was 0.3. 7 patients (27%) had at least one dose held. 21 (81%) patients showed treatment benefit: 19 (73%) had complete response and 2 (8%) had partial response on first scan following treatment. The four most common toxicities were mucositis (69%), nausea/vomiting (23%), oral thrush (19%), and dermatologic toxicities (19%). The most common toxicities causing dose interruption were fatigue (12%), neutropenia (8%), and thrombocytopenia (8%). Grade 3/4 mucositis was experienced in 6 patients (23%). Other grade 3/4 toxicities included neutropenia (8%), anemia (8%), thrombocytopenia (1%), nephrotoxicity (1%) and nausea (1%).
Conclusions
Carboplatin was both efficacious and well tolerated in our older veteran population. These findings add to the limited body of evidence examining weekly carboplatin in patients with advanced head and neck cancer. While cisplatin remains standard of care, carboplatin may be a reasonable alternative as evidenced in a real-world veteran population.
Background
The standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is combination chemoradiotherapy. Platinum-based chemotherapy is used for radiosensitization and significantly improves locoregional control and survival. Cisplatin is the standard of care; however, many patients are cisplatin-ineligible due to underlying comorbidities. Carboplatin is an alternative chemotherapy in these patients, but efficacy data are lacking. Purpose: To evaluate the efficacy and tolerability of weekly carboplatin concurrent with radiation in veterans with locally advanced HNSCC.
Methods
Our tumor registry was used to identify patients who received platinum-based chemoradiotherapy for stage III-IVB HNSCC at a single center between 2007 to 2017. Patients who received carboplatin were identified. Data including dosing, toxicities, and disease response was collected and analyzed.
Results
A total of 26 patients who received weekly carboplatin were analyzed. All patients were male with an average age of 65. A usual dose of carboplatin AUC 2 was utilized. The average cumulative dose for weekly carboplatin was AUC 12, with most patients (65%) receiving 6 doses or more. The mean number of weekly carboplatin doses held was 0.3. 7 patients (27%) had at least one dose held. 21 (81%) patients showed treatment benefit: 19 (73%) had complete response and 2 (8%) had partial response on first scan following treatment. The four most common toxicities were mucositis (69%), nausea/vomiting (23%), oral thrush (19%), and dermatologic toxicities (19%). The most common toxicities causing dose interruption were fatigue (12%), neutropenia (8%), and thrombocytopenia (8%). Grade 3/4 mucositis was experienced in 6 patients (23%). Other grade 3/4 toxicities included neutropenia (8%), anemia (8%), thrombocytopenia (1%), nephrotoxicity (1%) and nausea (1%).
Conclusions
Carboplatin was both efficacious and well tolerated in our older veteran population. These findings add to the limited body of evidence examining weekly carboplatin in patients with advanced head and neck cancer. While cisplatin remains standard of care, carboplatin may be a reasonable alternative as evidenced in a real-world veteran population.
Background
The standard of care for locally advanced head and neck squamous cell carcinoma (HNSCC) is combination chemoradiotherapy. Platinum-based chemotherapy is used for radiosensitization and significantly improves locoregional control and survival. Cisplatin is the standard of care; however, many patients are cisplatin-ineligible due to underlying comorbidities. Carboplatin is an alternative chemotherapy in these patients, but efficacy data are lacking. Purpose: To evaluate the efficacy and tolerability of weekly carboplatin concurrent with radiation in veterans with locally advanced HNSCC.
Methods
Our tumor registry was used to identify patients who received platinum-based chemoradiotherapy for stage III-IVB HNSCC at a single center between 2007 to 2017. Patients who received carboplatin were identified. Data including dosing, toxicities, and disease response was collected and analyzed.
Results
A total of 26 patients who received weekly carboplatin were analyzed. All patients were male with an average age of 65. A usual dose of carboplatin AUC 2 was utilized. The average cumulative dose for weekly carboplatin was AUC 12, with most patients (65%) receiving 6 doses or more. The mean number of weekly carboplatin doses held was 0.3. 7 patients (27%) had at least one dose held. 21 (81%) patients showed treatment benefit: 19 (73%) had complete response and 2 (8%) had partial response on first scan following treatment. The four most common toxicities were mucositis (69%), nausea/vomiting (23%), oral thrush (19%), and dermatologic toxicities (19%). The most common toxicities causing dose interruption were fatigue (12%), neutropenia (8%), and thrombocytopenia (8%). Grade 3/4 mucositis was experienced in 6 patients (23%). Other grade 3/4 toxicities included neutropenia (8%), anemia (8%), thrombocytopenia (1%), nephrotoxicity (1%) and nausea (1%).
Conclusions
Carboplatin was both efficacious and well tolerated in our older veteran population. These findings add to the limited body of evidence examining weekly carboplatin in patients with advanced head and neck cancer. While cisplatin remains standard of care, carboplatin may be a reasonable alternative as evidenced in a real-world veteran population.
Variation in Cardiovascular Risk Assessment Status in Patients Receiving Oral Anti-Cancer Therapies: A Focus on Equity throughout VISN (Veteran Integrated Service Network) 12
Background
Oral anti-cancer therapies have quickly moved to the forefront of cancer treatment for several oncologic disease states. While these treatments have led to improvements in prognosis and ease of administration, many of these agents carry the risk of serious short- and long-term toxicities affecting the cardiovascular system. This prompted the Journal of the American Heart Association (JAHA) to release special guidance focused on cardiovascular monitoring strategies for anti-cancer agents. The primary objective of this retrospective review was to evaluate compliance with cardiovascular monitoring based on JAHA cardio-oncologic guidelines. The secondary objective was to assess disparities in cardiovascular monitoring based on markers of equity such as race/ ethnicity, rurality, socioeconomic status and gender.
Methods
Patients who initiated pazopanib, cabozantinib, lenvatinib, axitinib, regorafenib, nilotinib, ibrutinib, sorafenib, sunitinib, ponatinib or everolimus between January 1, 2019 and December 31, 2022 at a VHA VISN 12 site with oncology services were followed forward until treatment discontinuation or 12 months of therapy had been completed. Data was acquired utilizing the VA Informatics and Computing Infrastructure (VINCI) and the Corporate Data Warehouse (CDW). The following cardiovascular monitoring markers were recorded at baseline and months 3, 6, 9 and 12 after initiation anti-cancer therapy: blood pressure, blood glucose, cholesterol, ECG and echocardiogram. Descriptive statistics were used to examine all continuous variables, while frequencies were used to examine categorical variables. Univariate statistics were performed on all items respectively.
Results
A total of 219 patients were identified initiating pre-specified oral anti-cancer therapies during the study time period. Of these, a total of n=145 met study inclusion criteria. 97% were male (n=141), 80% (n=116) had a racial background of white, 36% (n=52) live in rural or highly rural locations and 23% (n=34) lived in a high poverty area. Based on the primary endpoint, the mean compliance with recommended cardiovascular monitoring was 44.95% [IQR 12]. There was no statistically significant difference in cardiovascular monitoring based on equity.
Conclusions
Overall uptake of cardiovascular monitoring markers recommended by JAHA guidance is low. We plan to evaluate methods to increase these measures, utilizing clinical pharmacy provider support throughout VISN 12.
Background
Oral anti-cancer therapies have quickly moved to the forefront of cancer treatment for several oncologic disease states. While these treatments have led to improvements in prognosis and ease of administration, many of these agents carry the risk of serious short- and long-term toxicities affecting the cardiovascular system. This prompted the Journal of the American Heart Association (JAHA) to release special guidance focused on cardiovascular monitoring strategies for anti-cancer agents. The primary objective of this retrospective review was to evaluate compliance with cardiovascular monitoring based on JAHA cardio-oncologic guidelines. The secondary objective was to assess disparities in cardiovascular monitoring based on markers of equity such as race/ ethnicity, rurality, socioeconomic status and gender.
Methods
Patients who initiated pazopanib, cabozantinib, lenvatinib, axitinib, regorafenib, nilotinib, ibrutinib, sorafenib, sunitinib, ponatinib or everolimus between January 1, 2019 and December 31, 2022 at a VHA VISN 12 site with oncology services were followed forward until treatment discontinuation or 12 months of therapy had been completed. Data was acquired utilizing the VA Informatics and Computing Infrastructure (VINCI) and the Corporate Data Warehouse (CDW). The following cardiovascular monitoring markers were recorded at baseline and months 3, 6, 9 and 12 after initiation anti-cancer therapy: blood pressure, blood glucose, cholesterol, ECG and echocardiogram. Descriptive statistics were used to examine all continuous variables, while frequencies were used to examine categorical variables. Univariate statistics were performed on all items respectively.
Results
A total of 219 patients were identified initiating pre-specified oral anti-cancer therapies during the study time period. Of these, a total of n=145 met study inclusion criteria. 97% were male (n=141), 80% (n=116) had a racial background of white, 36% (n=52) live in rural or highly rural locations and 23% (n=34) lived in a high poverty area. Based on the primary endpoint, the mean compliance with recommended cardiovascular monitoring was 44.95% [IQR 12]. There was no statistically significant difference in cardiovascular monitoring based on equity.
Conclusions
Overall uptake of cardiovascular monitoring markers recommended by JAHA guidance is low. We plan to evaluate methods to increase these measures, utilizing clinical pharmacy provider support throughout VISN 12.
Background
Oral anti-cancer therapies have quickly moved to the forefront of cancer treatment for several oncologic disease states. While these treatments have led to improvements in prognosis and ease of administration, many of these agents carry the risk of serious short- and long-term toxicities affecting the cardiovascular system. This prompted the Journal of the American Heart Association (JAHA) to release special guidance focused on cardiovascular monitoring strategies for anti-cancer agents. The primary objective of this retrospective review was to evaluate compliance with cardiovascular monitoring based on JAHA cardio-oncologic guidelines. The secondary objective was to assess disparities in cardiovascular monitoring based on markers of equity such as race/ ethnicity, rurality, socioeconomic status and gender.
Methods
Patients who initiated pazopanib, cabozantinib, lenvatinib, axitinib, regorafenib, nilotinib, ibrutinib, sorafenib, sunitinib, ponatinib or everolimus between January 1, 2019 and December 31, 2022 at a VHA VISN 12 site with oncology services were followed forward until treatment discontinuation or 12 months of therapy had been completed. Data was acquired utilizing the VA Informatics and Computing Infrastructure (VINCI) and the Corporate Data Warehouse (CDW). The following cardiovascular monitoring markers were recorded at baseline and months 3, 6, 9 and 12 after initiation anti-cancer therapy: blood pressure, blood glucose, cholesterol, ECG and echocardiogram. Descriptive statistics were used to examine all continuous variables, while frequencies were used to examine categorical variables. Univariate statistics were performed on all items respectively.
Results
A total of 219 patients were identified initiating pre-specified oral anti-cancer therapies during the study time period. Of these, a total of n=145 met study inclusion criteria. 97% were male (n=141), 80% (n=116) had a racial background of white, 36% (n=52) live in rural or highly rural locations and 23% (n=34) lived in a high poverty area. Based on the primary endpoint, the mean compliance with recommended cardiovascular monitoring was 44.95% [IQR 12]. There was no statistically significant difference in cardiovascular monitoring based on equity.
Conclusions
Overall uptake of cardiovascular monitoring markers recommended by JAHA guidance is low. We plan to evaluate methods to increase these measures, utilizing clinical pharmacy provider support throughout VISN 12.
Developing a Cancer Rehabilitation Program—Improving Access to Ancillary Services to Mitigate the Impact of Cancer and its Treatments for Veterans Diagnosed With Cancer
Background
Approximately 56,000 Veterans are diagnosed with cancer every year in the VA system. Up to 90% of survivors have at least one impairment that decreases their quality of life, but only 2-9% are receiving cancer rehabilitation. Current research in cancer care demonstrates the importance of prospective surveillance, rehabilitation, and a multidisciplinary (MultiD) approach to cancer survivorship. Multi-D treatments help mitigate the effects of cancer and its treatments as the veterans proceed through care, improve outcomes, and streamline the process to meet all rehabilitation needs for those affected by cancer. Prior to the development of this program all services except navigation were available. Those diagnosed with cancer were not receiving prehabilitation and consults to ancillary services did not occur until after active cancer treatment was completed. CCRP united existing Multi-D programs to better serve the needs of veterans with cancer. Development of the CCRP CPRS Consult menu has allowed for improved access for both providers and veterans.
Methods
Identified the need for ancillary services during cancer survivorship, regardless of Veterans treatment location within or outside the VA system. Initiated tracking via CCR consults, developed a CCRP guidebook to identify all services available and how to access them as well as the CCCRP consult menu to create easier access for providers and veterans. Tracking via Multi-D departments that allow for tracking in CPRS via CCRP Consult.
Results
Prior to FY23 no cancer rehab consults existed. Consults received since program implementation: Navigation: 144, Physical Therapy: 102, Occupational Therapy: 7, Speech: 15. All other Multi-D did not track CCRP-specific consults. Other tools for data analysis are utilized in other departments in which gaps in coordination of care have been caught/resolved, and advocacy has increased.
Conclusions
Comprehensive cancer care from diagnosis throughout survivorship improves quality of life. A Multi-D comprehensive Cancer rehabilitation provides an opportunity to streamline care via a CPRS Menu. Other VA medical centers can develop a Multi-D cancer rehabilitation program to coordinate treatments from diagnosis through survivorship. This is an opportunity to make the VA the forefront of oncology care – by providing all services within one system.
Background
Approximately 56,000 Veterans are diagnosed with cancer every year in the VA system. Up to 90% of survivors have at least one impairment that decreases their quality of life, but only 2-9% are receiving cancer rehabilitation. Current research in cancer care demonstrates the importance of prospective surveillance, rehabilitation, and a multidisciplinary (MultiD) approach to cancer survivorship. Multi-D treatments help mitigate the effects of cancer and its treatments as the veterans proceed through care, improve outcomes, and streamline the process to meet all rehabilitation needs for those affected by cancer. Prior to the development of this program all services except navigation were available. Those diagnosed with cancer were not receiving prehabilitation and consults to ancillary services did not occur until after active cancer treatment was completed. CCRP united existing Multi-D programs to better serve the needs of veterans with cancer. Development of the CCRP CPRS Consult menu has allowed for improved access for both providers and veterans.
Methods
Identified the need for ancillary services during cancer survivorship, regardless of Veterans treatment location within or outside the VA system. Initiated tracking via CCR consults, developed a CCRP guidebook to identify all services available and how to access them as well as the CCCRP consult menu to create easier access for providers and veterans. Tracking via Multi-D departments that allow for tracking in CPRS via CCRP Consult.
Results
Prior to FY23 no cancer rehab consults existed. Consults received since program implementation: Navigation: 144, Physical Therapy: 102, Occupational Therapy: 7, Speech: 15. All other Multi-D did not track CCRP-specific consults. Other tools for data analysis are utilized in other departments in which gaps in coordination of care have been caught/resolved, and advocacy has increased.
Conclusions
Comprehensive cancer care from diagnosis throughout survivorship improves quality of life. A Multi-D comprehensive Cancer rehabilitation provides an opportunity to streamline care via a CPRS Menu. Other VA medical centers can develop a Multi-D cancer rehabilitation program to coordinate treatments from diagnosis through survivorship. This is an opportunity to make the VA the forefront of oncology care – by providing all services within one system.
Background
Approximately 56,000 Veterans are diagnosed with cancer every year in the VA system. Up to 90% of survivors have at least one impairment that decreases their quality of life, but only 2-9% are receiving cancer rehabilitation. Current research in cancer care demonstrates the importance of prospective surveillance, rehabilitation, and a multidisciplinary (MultiD) approach to cancer survivorship. Multi-D treatments help mitigate the effects of cancer and its treatments as the veterans proceed through care, improve outcomes, and streamline the process to meet all rehabilitation needs for those affected by cancer. Prior to the development of this program all services except navigation were available. Those diagnosed with cancer were not receiving prehabilitation and consults to ancillary services did not occur until after active cancer treatment was completed. CCRP united existing Multi-D programs to better serve the needs of veterans with cancer. Development of the CCRP CPRS Consult menu has allowed for improved access for both providers and veterans.
Methods
Identified the need for ancillary services during cancer survivorship, regardless of Veterans treatment location within or outside the VA system. Initiated tracking via CCR consults, developed a CCRP guidebook to identify all services available and how to access them as well as the CCCRP consult menu to create easier access for providers and veterans. Tracking via Multi-D departments that allow for tracking in CPRS via CCRP Consult.
Results
Prior to FY23 no cancer rehab consults existed. Consults received since program implementation: Navigation: 144, Physical Therapy: 102, Occupational Therapy: 7, Speech: 15. All other Multi-D did not track CCRP-specific consults. Other tools for data analysis are utilized in other departments in which gaps in coordination of care have been caught/resolved, and advocacy has increased.
Conclusions
Comprehensive cancer care from diagnosis throughout survivorship improves quality of life. A Multi-D comprehensive Cancer rehabilitation provides an opportunity to streamline care via a CPRS Menu. Other VA medical centers can develop a Multi-D cancer rehabilitation program to coordinate treatments from diagnosis through survivorship. This is an opportunity to make the VA the forefront of oncology care – by providing all services within one system.
Optimization of Hematology/ Oncology E-Consult Ordering Process
Background
Multiple responses or repeat e-consults were observed by Hematology/Oncology Department. Root cause analysis uncovered that 60% of e-consults ordered required multiple responses or repeat econsults for the same clinical situation, often due to the need for additional lab testing before the e-consult question could be addressed. Hematology/Oncology econsult ordering process did not have an order design menu to provide guidance on appropriate questions, simplified ordering of relevant tests, or ways to identify patients that were either already established in the Hem/Onc clinic or patients that would be better managed with a more urgent or in-person consultation. This quality improvement project was created to improve the appropriateness and efficiency of hematology/oncology e-consult ordering process.
Methods
Using Plan-Do-Study-Act (PDSA) quality improvement methodology, a project team lead by Hematology/Oncology, Clinical Informatics, Clinical Application Coordinator and the Systems Redesign Coordinator, rebuilt menus to navigate referring providers to the appropriate e-consults. This would improve the process flow and enhance clear communication. The primary process improvement goals were 1) to decrease the number of e-consults that were better suited for inperson evaluation; 2) decrease the number of Hem/Onc e-consults that lack adequate clinical lab information and 3) decrease the number of e-consults for patients that are already established with a Hematology/Oncology provider.
Results
Baseline sample data (7-1-23-11-30-22)-revealed only 60% of e-consults placed were deemed appropriate. 13% required certain minimum lab testing, 11% were already established patients and 11% were better managed through in-person consultation. After the first PDSA cycle, from 9/21/23-3/29/24, 72% of econsults were deemed appropriate (114/158), a 12% improvement.
Conclusions
The success of the project supports the use of existing VA hospital-based program resources such as clinical informatics and utilizing frontline physician input. This input was critical to the redesigned ordering process. Ultimately, our process improvement efforts helped facilitate communication and information flow which improved our ability to better coordinate our Veteran’s care.
Background
Multiple responses or repeat e-consults were observed by Hematology/Oncology Department. Root cause analysis uncovered that 60% of e-consults ordered required multiple responses or repeat econsults for the same clinical situation, often due to the need for additional lab testing before the e-consult question could be addressed. Hematology/Oncology econsult ordering process did not have an order design menu to provide guidance on appropriate questions, simplified ordering of relevant tests, or ways to identify patients that were either already established in the Hem/Onc clinic or patients that would be better managed with a more urgent or in-person consultation. This quality improvement project was created to improve the appropriateness and efficiency of hematology/oncology e-consult ordering process.
Methods
Using Plan-Do-Study-Act (PDSA) quality improvement methodology, a project team lead by Hematology/Oncology, Clinical Informatics, Clinical Application Coordinator and the Systems Redesign Coordinator, rebuilt menus to navigate referring providers to the appropriate e-consults. This would improve the process flow and enhance clear communication. The primary process improvement goals were 1) to decrease the number of e-consults that were better suited for inperson evaluation; 2) decrease the number of Hem/Onc e-consults that lack adequate clinical lab information and 3) decrease the number of e-consults for patients that are already established with a Hematology/Oncology provider.
Results
Baseline sample data (7-1-23-11-30-22)-revealed only 60% of e-consults placed were deemed appropriate. 13% required certain minimum lab testing, 11% were already established patients and 11% were better managed through in-person consultation. After the first PDSA cycle, from 9/21/23-3/29/24, 72% of econsults were deemed appropriate (114/158), a 12% improvement.
Conclusions
The success of the project supports the use of existing VA hospital-based program resources such as clinical informatics and utilizing frontline physician input. This input was critical to the redesigned ordering process. Ultimately, our process improvement efforts helped facilitate communication and information flow which improved our ability to better coordinate our Veteran’s care.
Background
Multiple responses or repeat e-consults were observed by Hematology/Oncology Department. Root cause analysis uncovered that 60% of e-consults ordered required multiple responses or repeat econsults for the same clinical situation, often due to the need for additional lab testing before the e-consult question could be addressed. Hematology/Oncology econsult ordering process did not have an order design menu to provide guidance on appropriate questions, simplified ordering of relevant tests, or ways to identify patients that were either already established in the Hem/Onc clinic or patients that would be better managed with a more urgent or in-person consultation. This quality improvement project was created to improve the appropriateness and efficiency of hematology/oncology e-consult ordering process.
Methods
Using Plan-Do-Study-Act (PDSA) quality improvement methodology, a project team lead by Hematology/Oncology, Clinical Informatics, Clinical Application Coordinator and the Systems Redesign Coordinator, rebuilt menus to navigate referring providers to the appropriate e-consults. This would improve the process flow and enhance clear communication. The primary process improvement goals were 1) to decrease the number of e-consults that were better suited for inperson evaluation; 2) decrease the number of Hem/Onc e-consults that lack adequate clinical lab information and 3) decrease the number of e-consults for patients that are already established with a Hematology/Oncology provider.
Results
Baseline sample data (7-1-23-11-30-22)-revealed only 60% of e-consults placed were deemed appropriate. 13% required certain minimum lab testing, 11% were already established patients and 11% were better managed through in-person consultation. After the first PDSA cycle, from 9/21/23-3/29/24, 72% of econsults were deemed appropriate (114/158), a 12% improvement.
Conclusions
The success of the project supports the use of existing VA hospital-based program resources such as clinical informatics and utilizing frontline physician input. This input was critical to the redesigned ordering process. Ultimately, our process improvement efforts helped facilitate communication and information flow which improved our ability to better coordinate our Veteran’s care.
Melasma Risk Factors: A Matched Cohort Study Using Data From the All of Us Research Program
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.
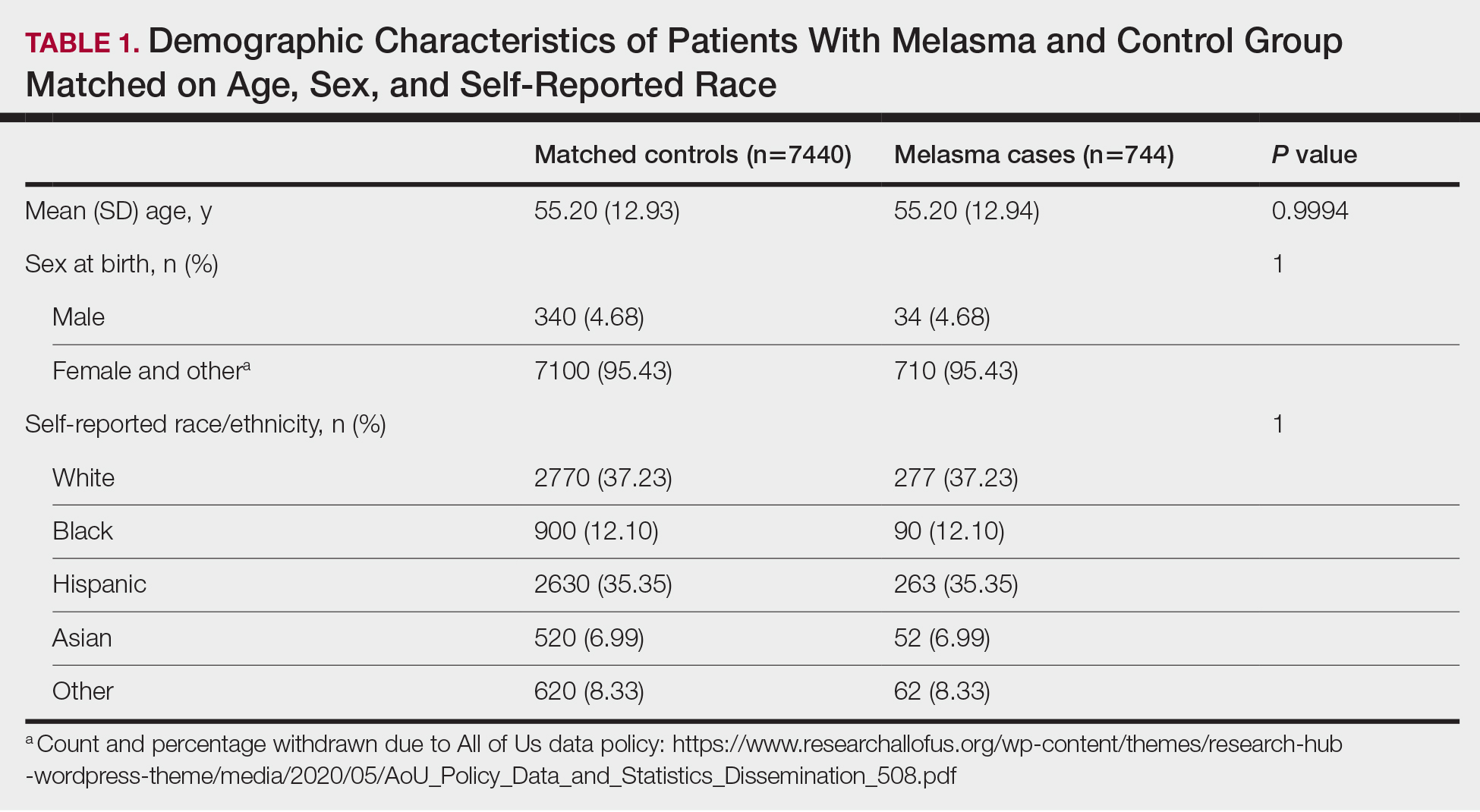
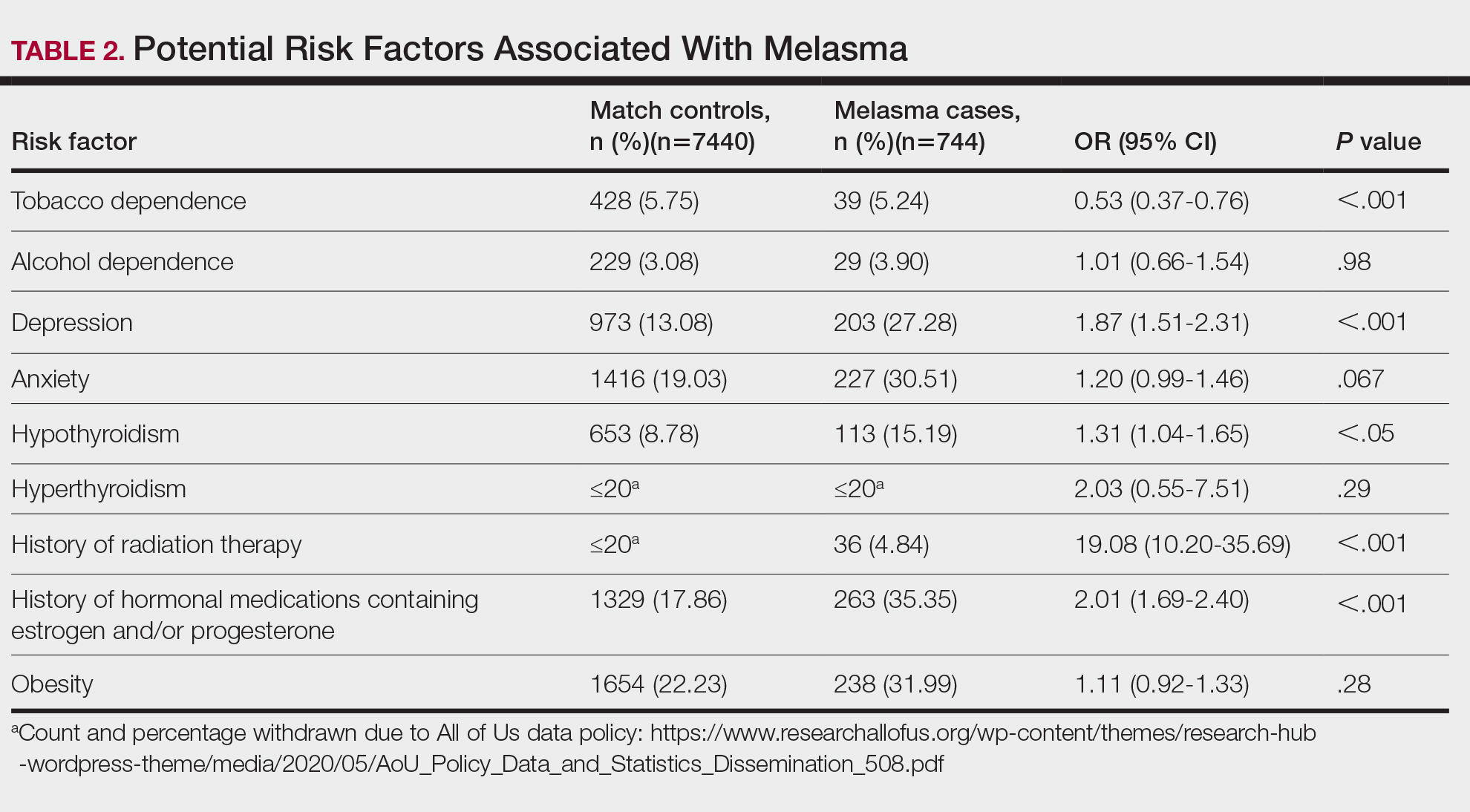
Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.


Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.


Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
Practice Points
- Treatment options for melasma are limited due to its poorly understood pathogenesis.
- Depression and hypothyroidism and/or history of exposure to radiation and hormonal therapies may increase melasma risk.
- We recommend that patients with melasma be screened for depression and thyroid dysfunction. Patients undergoing radiation therapy or starting estrogen and/ or progesterone therapy should be counseled on the increased risk for melasma.
“It Takes a Village”: Benefits and Challenges of Navigating Cancer Care with the Pacific Community and the Veterans Health Administration
Background
The Palliative Care in Hawaii/Pacific Island Communities for Veterans (PaCiHPIC Veterans) study is a VA-funded research study that explores social determinants of health, cultural values, and cancer disparities impacting Native Hawaiian/Pacific Islander/US-affiliated Pacific Island resident (NHPI/USAPI) Veterans.Cancer prevalence and mortality are increasing among NHPI/ USAPI Veterans which can be partly attributed to nuclear fallout from U.S. military activities in the region. This population faces geographic, financial, and logistical barriers to cancer care. There is an imminent need to understand and address access to cancer care and palliative care to reduce disparities within this population.
Methods
We interviewed 15 clinicians including physicians, nurses, nurse practitioners, social workers, and clinical psychologists specializing in primary care, palliative care, and oncology, self-identifying as White, Asian American, NHPI, and Multiracial. Interviews were transcribed verbatim and de-identified. Using inductive and deductive strategies, we iteratively collapsed content into codes formulating a codebook. Thematic analyses were performed using dual-coder review in Atlas.ti v23. Themes were mapped to the socioecological model.
Results
Clinicians described how NHPI/USAPI Veterans receive healthcare and instrumental support at individual, community, and systems levels, including from family caregivers, “high-talking chiefs,” traditional healers (“suruhanu”), community health clinics, and the VHA. Clinicians identified challenges and opportunities for care coordination: (1) financial and logistical barriers to involve family and decision-makers; (2) clinician understanding of cultural values and influence on medical decision-making; (3) care fragmentation resulting from transitions between community care and VHA; and (4) collaboration with key individuals in Pacific social hierarchies.
Conclusions
Cancer navigation and care coordination gaps create challenges for clinicians and NHPI/USAPI Veterans managing cancer in the Pacific Islands. Better understanding of these systems of care and associated gaps can inform the development of an intervention to improve cancer care delivery to this population. NHPI/ USAPI Veterans may experience care fragmentation due to care transitions between community care and the VHA. At the same time, these sources also create multiple layers of support for Veterans. Interventions to address these challenges can leverage the strengths of Pacific communities, while striving to better integrate care between community healthcare providers and VHA.
Background
The Palliative Care in Hawaii/Pacific Island Communities for Veterans (PaCiHPIC Veterans) study is a VA-funded research study that explores social determinants of health, cultural values, and cancer disparities impacting Native Hawaiian/Pacific Islander/US-affiliated Pacific Island resident (NHPI/USAPI) Veterans.Cancer prevalence and mortality are increasing among NHPI/ USAPI Veterans which can be partly attributed to nuclear fallout from U.S. military activities in the region. This population faces geographic, financial, and logistical barriers to cancer care. There is an imminent need to understand and address access to cancer care and palliative care to reduce disparities within this population.
Methods
We interviewed 15 clinicians including physicians, nurses, nurse practitioners, social workers, and clinical psychologists specializing in primary care, palliative care, and oncology, self-identifying as White, Asian American, NHPI, and Multiracial. Interviews were transcribed verbatim and de-identified. Using inductive and deductive strategies, we iteratively collapsed content into codes formulating a codebook. Thematic analyses were performed using dual-coder review in Atlas.ti v23. Themes were mapped to the socioecological model.
Results
Clinicians described how NHPI/USAPI Veterans receive healthcare and instrumental support at individual, community, and systems levels, including from family caregivers, “high-talking chiefs,” traditional healers (“suruhanu”), community health clinics, and the VHA. Clinicians identified challenges and opportunities for care coordination: (1) financial and logistical barriers to involve family and decision-makers; (2) clinician understanding of cultural values and influence on medical decision-making; (3) care fragmentation resulting from transitions between community care and VHA; and (4) collaboration with key individuals in Pacific social hierarchies.
Conclusions
Cancer navigation and care coordination gaps create challenges for clinicians and NHPI/USAPI Veterans managing cancer in the Pacific Islands. Better understanding of these systems of care and associated gaps can inform the development of an intervention to improve cancer care delivery to this population. NHPI/ USAPI Veterans may experience care fragmentation due to care transitions between community care and the VHA. At the same time, these sources also create multiple layers of support for Veterans. Interventions to address these challenges can leverage the strengths of Pacific communities, while striving to better integrate care between community healthcare providers and VHA.
Background
The Palliative Care in Hawaii/Pacific Island Communities for Veterans (PaCiHPIC Veterans) study is a VA-funded research study that explores social determinants of health, cultural values, and cancer disparities impacting Native Hawaiian/Pacific Islander/US-affiliated Pacific Island resident (NHPI/USAPI) Veterans.Cancer prevalence and mortality are increasing among NHPI/ USAPI Veterans which can be partly attributed to nuclear fallout from U.S. military activities in the region. This population faces geographic, financial, and logistical barriers to cancer care. There is an imminent need to understand and address access to cancer care and palliative care to reduce disparities within this population.
Methods
We interviewed 15 clinicians including physicians, nurses, nurse practitioners, social workers, and clinical psychologists specializing in primary care, palliative care, and oncology, self-identifying as White, Asian American, NHPI, and Multiracial. Interviews were transcribed verbatim and de-identified. Using inductive and deductive strategies, we iteratively collapsed content into codes formulating a codebook. Thematic analyses were performed using dual-coder review in Atlas.ti v23. Themes were mapped to the socioecological model.
Results
Clinicians described how NHPI/USAPI Veterans receive healthcare and instrumental support at individual, community, and systems levels, including from family caregivers, “high-talking chiefs,” traditional healers (“suruhanu”), community health clinics, and the VHA. Clinicians identified challenges and opportunities for care coordination: (1) financial and logistical barriers to involve family and decision-makers; (2) clinician understanding of cultural values and influence on medical decision-making; (3) care fragmentation resulting from transitions between community care and VHA; and (4) collaboration with key individuals in Pacific social hierarchies.
Conclusions
Cancer navigation and care coordination gaps create challenges for clinicians and NHPI/USAPI Veterans managing cancer in the Pacific Islands. Better understanding of these systems of care and associated gaps can inform the development of an intervention to improve cancer care delivery to this population. NHPI/ USAPI Veterans may experience care fragmentation due to care transitions between community care and the VHA. At the same time, these sources also create multiple layers of support for Veterans. Interventions to address these challenges can leverage the strengths of Pacific communities, while striving to better integrate care between community healthcare providers and VHA.