User login
Caring for Patients at a COVID-19 Field Hospital
During the initial peak of coronavirus disease 2019 (COVID-19) cases, US models suggested hospital bed shortages, hinting at the dire possibility of an overwhelmed healthcare system.1,2 Such projections invoked widespread uncertainty and fear of massive loss of life secondary to an undersupply of treatment resources. This led many state governments to rush into a series of historically unprecedented interventions, including the rapid deployment of field hospitals. US state governments, in partnership with the Army Corps of Engineers, invested more than $660 million to transform convention halls, university campus buildings, and even abandoned industrial warehouses, into overflow hospitals for the care of COVID-19 patients.1 Such a national scale of field hospital construction is truly historic, never before having occurred at this speed and on this scale. The only other time field hospitals were deployed nearly as widely in the United States was during the Civil War.3
FIELD HOSPITALS DURING THE COVID-19 PANDEMIC
The use of COVID-19 field hospital resources has been variable, with patient volumes ranging from 0 at many to more than 1,000 at the Javits Center field hospital in New York City.1 In fact, most field hospitals did not treat any patients because early public health measures, such as stay-at-home orders, helped contain the virus in most states.1 As of this writing, the United States has seen a dramatic surge in COVID-19 transmission and hospitalizations. This has led many states to re-introduce field hospitals into their COVID emergency response.
Our site, the Baltimore Convention Center Field Hospital (BCCFH), is one of few sites that is still operational and, to our knowledge, is the longest-running US COVID-19 field hospital. We have cared for 543 patients since opening and have had no cardiac arrests or on-site deaths. To safely offload lower-acuity COVID-19 patients from Maryland hospitals, we designed admission criteria and care processes to provide medical care on site until patients are ready for discharge. However, we anticipated that some patients would decompensate and need to return to a higher level of care. Here, we share our experience with identifying, assessing, resuscitating, and transporting unstable patients. We believe that this process has allowed us to treat about 80% of our patients in place with successful discharge to outpatient care. We have safely transferred about 20% to a higher level of care, having learned from our early cases to refine and improve our rapid response process.
CASES
Case 1
A 39-year-old man was transferred to the BCCFH on his 9th day of symptoms following a 3-day hospital admission for COVID-19. On BCCFH day 1, he developed an oxygen requirement of 2 L/min and a fever of 39.9 oC. Testing revealed worsening hyponatremia and new proteinuria, and a chest radiograph showed increased bilateral interstitial infiltrates. Cefdinir and fluid restriction were initiated. On BCCFH day 2, the patient developed hypotension (88/55 mm Hg), tachycardia (180 bpm), an oxygen requirement of 3 L/min, and a brief syncopal episode while sitting in bed. The charge physician and nurse were directed to the bedside. They instructed staff to bring a stretcher and intravenous (IV) supplies. Unable to locate these supplies in the triage bay, the staff found them in various locations. An IV line was inserted, and fluids administered, after which vital signs improved. Emergency medical services (EMS), which were on standby outside the field hospital, were alerted via radio; they donned personal protective equipment (PPE) and arrived at the triage bay. They were redirected to patient bedside, whence they transported the patient to the hospital.
Case 2
A 64-year-old man with a history of homelessness, myocardial infarctions, cerebrovascular accident, and paroxysmal atrial fibrillation was transferred to the BCCFH on his 6th day of symptoms after a 2-day hospitalization with COVID-19 respiratory illness. On BCCFH day 1, he had a temperature of 39.3 oC and atypical chest pain. A laboratory workup was unrevealing. On BCCFH day 2, he had asymptomatic hypotension and a heart rate of 60-85 bpm while receiving his usual metoprolol dose. On BCCFH day 3, he reported dizziness and was found to be hypotensive (83/41 mm Hg) and febrile (38.6 oC). The rapid response team (RRT) was called over radio, and they quickly assessed the patient and transported him to the triage bay. EMS, signaled through the RRT radio announcement, arrived at the triage bay and transported the patient to a traditional hospital.
ABOUT THE BCCFH
The BCCFH, which opened in April 2020, is a 252-bed facility that’s spread over a single exhibit hall floor and cares for stable adult COVID-19 patients from any hospital or emergency department in Maryland (Appendix A). The site offers basic laboratory tests, radiography, a limited on-site pharmacy, and spot vital sign monitoring without telemetry. Both EMS and a certified registered nurse anesthetist are on standby in the nonclinical area and must don PPE before entering the patient care area when called. The appendices show the patient beds (Appendix B) and triage area (Appendix C) used for patient evaluation and resuscitation. Unlike conventional hospitals, the BCCFH has limited consultant access, and there are frequent changes in clinical teams. In addition to clinicians, our site has physical therapists, occupational therapists, and social work teams to assist in patient care and discharge planning. As of this writing, we have cared for 543 patients, sent to us from one-third of Maryland’s hospitals. Use during the first wave of COVID was variable, with some hospitals sending us just a few patients. One Baltimore hospital sent us 8% of its COVID-19 patients. Because the patients have an average 5-day stay, the BCCFH has offloaded 2,600 bed-days of care from acute hospitals.
ROLE OF THE RRT IN A FIELD HOSPITAL
COVID-19 field hospitals must be prepared to respond effectively to decompensating patients. In our experience, effective RRTs provide a standard and reproducible approach to patient emergencies. In the conventional hospital setting, these teams consist of clinicians who can be called on by any healthcare worker to quickly assess deteriorating patients and intervene with treatment. The purpose of an RRT is to provide immediate care to a patient before progression to respiratory or cardiac arrest. RRTs proliferated in US hospitals after 2004 when the Institute for Healthcare Improvement in Boston, Massachusetts, recommended such teams for improved quality of care. Though studies report conflicting findings on the impact of RRTs on mortality rates, these studies were performed in traditional hospitals with ample resources, consultants, and clinicians familiar with their patients rather than in resource-limited field hospitals.4-13 Our field hospital has found RRTs, and the principles behind them, useful in the identification and management of decompensating COVID-19 patients.
A FOUR-STEP RAPID RESPONSE FRAMEWORK: CASE CORRELATION
An approach to managing decompensating patients in a COVID-19 field hospital can be considered in four phases: identification, assessment, resuscitation, and transport. Referring to these phases, the first case shows opportunities for improvement in resuscitation and transport. Although decompensation was identified, the patient was not transported to the triage bay for resuscitation, and there was confusion when trying to obtain the proper equipment. Additionally, EMS awaited the patient in the triage bay, while he remained in his cubicle, which delayed transport to an acute care hospital. The second case shows opportunities for improvement in identification and assessment. The patient had signs of impending decompensation that were not immediately recognized and treated. However, once decompensation occurred, the RRT was called and the patient was transported quickly to the triage bay, and then to the hospital via EMS.
In our experience at the BCCFH, identification is a key phase in COVID-19 care at a field hospital. Identification involves recognizing impending deterioration, as well as understanding risk factors for decompensation. For COVID-19 specifically, this requires heightened awareness of patients who are in the 2nd to 3rd week of symptoms. Data from Wuhan, China, suggest that decompensation occurs predictably around symptom day 9.14,15 At the BCCFH, the median symptom duration for patients who decompensated and returned to a hospital was 13 days. In both introductory cases, patients were in the high-risk 2nd week of symptoms when decompensation occurred. Clinicians at the BCCFH now discuss patient symptom day during their handoffs, when rounding, and when making decisions regarding acute care transfer. Our team has also integrated clinical information from our electronic health record to create a dashboard describing those patients requiring acute care transfer to assist in identifying other trends or predictive factors (Appendix D).
LESSONS FROM THE FIELD HOSPITAL: IMPROVING CLINICAL PERFORMANCE
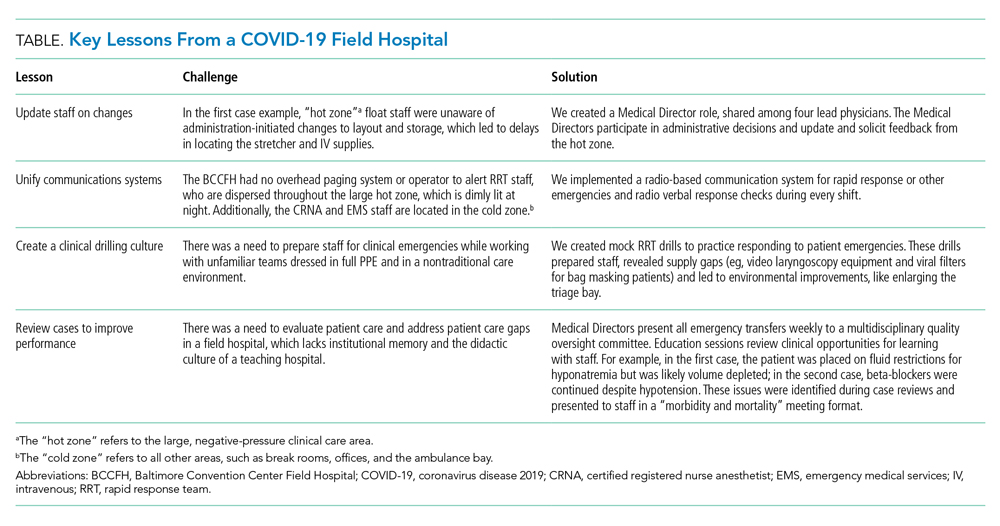
Although RRTs are designed to activate when an individual patient decompensates, they should fit within a larger operational framework for patient safety. Our experience with emergencies at the BCCFH has yielded four opportunities for learning relevant to COVID-19 care in nontraditional settings (Table). These lessons include how to update staff on clinical process changes, unify communication systems, create a clinical drilling culture, and review cases to improve performance. They illustrate the importance of standardizing emergency processes, conducting frequent updates and drills, and ensuring continuous improvement. We found that, while caring for patients with an unpredictable, novel disease in a nontraditional setting and while wearing PPE and working with new colleagues during every shift, the best approach to support patients and staff is to anticipate emergencies rather than relying on individual staff to develop on-the-spot solutions.
CONCLUSION
The COVID-19 era has seen the unprecedented construction and utilization of emergency field hospital facilities. Such facilities can serve to offload some COVID-19 patients from strained healthcare infrastructure and provide essential care to these patients. We share many of the unique physical and logistical considerations specific to a nontraditional site. We optimized our space, our equipment, and our communication system. We learned how to identify, assess, resuscitate, and transport decompensating COVID-19 patients. Ultimately, our field hospital has been well utilized and successful at caring for patients because of its adaptability, accessibility, and safety record. Of the 15% of patients we transferred to a hospital for care, 81% were successfully stabilized and were willing to return to the BCCFH to complete their care. Our design included supportive care such as social work, physical and occupational therapy, and treatment of comorbidities, such as diabetes and substance use disorder. Our model demonstrates an effective nonhospital option for the care of lower-acuity, medically complex COVID-19 patients. If such facilities are used in subsequent COVID-19 outbreaks, we advise structured planning for the care of decompensating patients that takes into account the need for effective communication, drilling, and ongoing process improvement.
1. Rose J. U.S. Field Hospitals Stand Down, Most Without Treating Any COVID-19 Patients. All Things Considered. NPR; May 7, 2020. Accessed July 21, 2020. https://www.npr.org/2020/05/07/851712311/u-s-field-hospitals-stand-down-most-without-treating-any-covid-19-patients
2. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305-1314. https://doi.org/10.1016/s0140-6736(20)30744-3
3. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-142. https://doi.org/10.1080/08998280.2016.11929390
4. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916-21. https://doi.org/10.1097/01.ccm.0000119428.02968.9e
5. Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179(6):283-287.
6. Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236-240.
7. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387-390. https://doi.org/10.1136/bmj.324.7334.387
8. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL; Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. https://doi.org/10.1136/qhc.13.4.251
9. Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A. The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54(9):853-860. https://doi.org/10.1046/j.1365-2044.1999.00996.x
10. Hillman K, Chen J, Cretikos M, et al; MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. https://doi.org/10.1016/s0140-6736(05)66733-5
11. Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257-263. https://doi.org/10.1016/j.resuscitation.2004.01.021
12. Pittard AJ. Out of our reach? assessing the impact of introducing a critical care outreach service. Anaesthesia. 2003;58(9):882-885. https://doi.org/10.1046/j.1365-2044.2003.03331.x
13. Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398-1404. https://doi.org/10.1007/s00134-004-2268-7
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
15. Zhou Y, Li W, Wang D, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177-179. https://doi.org/10.1136/svn-2020-000398
During the initial peak of coronavirus disease 2019 (COVID-19) cases, US models suggested hospital bed shortages, hinting at the dire possibility of an overwhelmed healthcare system.1,2 Such projections invoked widespread uncertainty and fear of massive loss of life secondary to an undersupply of treatment resources. This led many state governments to rush into a series of historically unprecedented interventions, including the rapid deployment of field hospitals. US state governments, in partnership with the Army Corps of Engineers, invested more than $660 million to transform convention halls, university campus buildings, and even abandoned industrial warehouses, into overflow hospitals for the care of COVID-19 patients.1 Such a national scale of field hospital construction is truly historic, never before having occurred at this speed and on this scale. The only other time field hospitals were deployed nearly as widely in the United States was during the Civil War.3
FIELD HOSPITALS DURING THE COVID-19 PANDEMIC
The use of COVID-19 field hospital resources has been variable, with patient volumes ranging from 0 at many to more than 1,000 at the Javits Center field hospital in New York City.1 In fact, most field hospitals did not treat any patients because early public health measures, such as stay-at-home orders, helped contain the virus in most states.1 As of this writing, the United States has seen a dramatic surge in COVID-19 transmission and hospitalizations. This has led many states to re-introduce field hospitals into their COVID emergency response.
Our site, the Baltimore Convention Center Field Hospital (BCCFH), is one of few sites that is still operational and, to our knowledge, is the longest-running US COVID-19 field hospital. We have cared for 543 patients since opening and have had no cardiac arrests or on-site deaths. To safely offload lower-acuity COVID-19 patients from Maryland hospitals, we designed admission criteria and care processes to provide medical care on site until patients are ready for discharge. However, we anticipated that some patients would decompensate and need to return to a higher level of care. Here, we share our experience with identifying, assessing, resuscitating, and transporting unstable patients. We believe that this process has allowed us to treat about 80% of our patients in place with successful discharge to outpatient care. We have safely transferred about 20% to a higher level of care, having learned from our early cases to refine and improve our rapid response process.
CASES
Case 1
A 39-year-old man was transferred to the BCCFH on his 9th day of symptoms following a 3-day hospital admission for COVID-19. On BCCFH day 1, he developed an oxygen requirement of 2 L/min and a fever of 39.9 oC. Testing revealed worsening hyponatremia and new proteinuria, and a chest radiograph showed increased bilateral interstitial infiltrates. Cefdinir and fluid restriction were initiated. On BCCFH day 2, the patient developed hypotension (88/55 mm Hg), tachycardia (180 bpm), an oxygen requirement of 3 L/min, and a brief syncopal episode while sitting in bed. The charge physician and nurse were directed to the bedside. They instructed staff to bring a stretcher and intravenous (IV) supplies. Unable to locate these supplies in the triage bay, the staff found them in various locations. An IV line was inserted, and fluids administered, after which vital signs improved. Emergency medical services (EMS), which were on standby outside the field hospital, were alerted via radio; they donned personal protective equipment (PPE) and arrived at the triage bay. They were redirected to patient bedside, whence they transported the patient to the hospital.
Case 2
A 64-year-old man with a history of homelessness, myocardial infarctions, cerebrovascular accident, and paroxysmal atrial fibrillation was transferred to the BCCFH on his 6th day of symptoms after a 2-day hospitalization with COVID-19 respiratory illness. On BCCFH day 1, he had a temperature of 39.3 oC and atypical chest pain. A laboratory workup was unrevealing. On BCCFH day 2, he had asymptomatic hypotension and a heart rate of 60-85 bpm while receiving his usual metoprolol dose. On BCCFH day 3, he reported dizziness and was found to be hypotensive (83/41 mm Hg) and febrile (38.6 oC). The rapid response team (RRT) was called over radio, and they quickly assessed the patient and transported him to the triage bay. EMS, signaled through the RRT radio announcement, arrived at the triage bay and transported the patient to a traditional hospital.
ABOUT THE BCCFH
The BCCFH, which opened in April 2020, is a 252-bed facility that’s spread over a single exhibit hall floor and cares for stable adult COVID-19 patients from any hospital or emergency department in Maryland (Appendix A). The site offers basic laboratory tests, radiography, a limited on-site pharmacy, and spot vital sign monitoring without telemetry. Both EMS and a certified registered nurse anesthetist are on standby in the nonclinical area and must don PPE before entering the patient care area when called. The appendices show the patient beds (Appendix B) and triage area (Appendix C) used for patient evaluation and resuscitation. Unlike conventional hospitals, the BCCFH has limited consultant access, and there are frequent changes in clinical teams. In addition to clinicians, our site has physical therapists, occupational therapists, and social work teams to assist in patient care and discharge planning. As of this writing, we have cared for 543 patients, sent to us from one-third of Maryland’s hospitals. Use during the first wave of COVID was variable, with some hospitals sending us just a few patients. One Baltimore hospital sent us 8% of its COVID-19 patients. Because the patients have an average 5-day stay, the BCCFH has offloaded 2,600 bed-days of care from acute hospitals.
ROLE OF THE RRT IN A FIELD HOSPITAL
COVID-19 field hospitals must be prepared to respond effectively to decompensating patients. In our experience, effective RRTs provide a standard and reproducible approach to patient emergencies. In the conventional hospital setting, these teams consist of clinicians who can be called on by any healthcare worker to quickly assess deteriorating patients and intervene with treatment. The purpose of an RRT is to provide immediate care to a patient before progression to respiratory or cardiac arrest. RRTs proliferated in US hospitals after 2004 when the Institute for Healthcare Improvement in Boston, Massachusetts, recommended such teams for improved quality of care. Though studies report conflicting findings on the impact of RRTs on mortality rates, these studies were performed in traditional hospitals with ample resources, consultants, and clinicians familiar with their patients rather than in resource-limited field hospitals.4-13 Our field hospital has found RRTs, and the principles behind them, useful in the identification and management of decompensating COVID-19 patients.
A FOUR-STEP RAPID RESPONSE FRAMEWORK: CASE CORRELATION
An approach to managing decompensating patients in a COVID-19 field hospital can be considered in four phases: identification, assessment, resuscitation, and transport. Referring to these phases, the first case shows opportunities for improvement in resuscitation and transport. Although decompensation was identified, the patient was not transported to the triage bay for resuscitation, and there was confusion when trying to obtain the proper equipment. Additionally, EMS awaited the patient in the triage bay, while he remained in his cubicle, which delayed transport to an acute care hospital. The second case shows opportunities for improvement in identification and assessment. The patient had signs of impending decompensation that were not immediately recognized and treated. However, once decompensation occurred, the RRT was called and the patient was transported quickly to the triage bay, and then to the hospital via EMS.
In our experience at the BCCFH, identification is a key phase in COVID-19 care at a field hospital. Identification involves recognizing impending deterioration, as well as understanding risk factors for decompensation. For COVID-19 specifically, this requires heightened awareness of patients who are in the 2nd to 3rd week of symptoms. Data from Wuhan, China, suggest that decompensation occurs predictably around symptom day 9.14,15 At the BCCFH, the median symptom duration for patients who decompensated and returned to a hospital was 13 days. In both introductory cases, patients were in the high-risk 2nd week of symptoms when decompensation occurred. Clinicians at the BCCFH now discuss patient symptom day during their handoffs, when rounding, and when making decisions regarding acute care transfer. Our team has also integrated clinical information from our electronic health record to create a dashboard describing those patients requiring acute care transfer to assist in identifying other trends or predictive factors (Appendix D).
LESSONS FROM THE FIELD HOSPITAL: IMPROVING CLINICAL PERFORMANCE
Although RRTs are designed to activate when an individual patient decompensates, they should fit within a larger operational framework for patient safety. Our experience with emergencies at the BCCFH has yielded four opportunities for learning relevant to COVID-19 care in nontraditional settings (Table). These lessons include how to update staff on clinical process changes, unify communication systems, create a clinical drilling culture, and review cases to improve performance. They illustrate the importance of standardizing emergency processes, conducting frequent updates and drills, and ensuring continuous improvement. We found that, while caring for patients with an unpredictable, novel disease in a nontraditional setting and while wearing PPE and working with new colleagues during every shift, the best approach to support patients and staff is to anticipate emergencies rather than relying on individual staff to develop on-the-spot solutions.

CONCLUSION
The COVID-19 era has seen the unprecedented construction and utilization of emergency field hospital facilities. Such facilities can serve to offload some COVID-19 patients from strained healthcare infrastructure and provide essential care to these patients. We share many of the unique physical and logistical considerations specific to a nontraditional site. We optimized our space, our equipment, and our communication system. We learned how to identify, assess, resuscitate, and transport decompensating COVID-19 patients. Ultimately, our field hospital has been well utilized and successful at caring for patients because of its adaptability, accessibility, and safety record. Of the 15% of patients we transferred to a hospital for care, 81% were successfully stabilized and were willing to return to the BCCFH to complete their care. Our design included supportive care such as social work, physical and occupational therapy, and treatment of comorbidities, such as diabetes and substance use disorder. Our model demonstrates an effective nonhospital option for the care of lower-acuity, medically complex COVID-19 patients. If such facilities are used in subsequent COVID-19 outbreaks, we advise structured planning for the care of decompensating patients that takes into account the need for effective communication, drilling, and ongoing process improvement.
During the initial peak of coronavirus disease 2019 (COVID-19) cases, US models suggested hospital bed shortages, hinting at the dire possibility of an overwhelmed healthcare system.1,2 Such projections invoked widespread uncertainty and fear of massive loss of life secondary to an undersupply of treatment resources. This led many state governments to rush into a series of historically unprecedented interventions, including the rapid deployment of field hospitals. US state governments, in partnership with the Army Corps of Engineers, invested more than $660 million to transform convention halls, university campus buildings, and even abandoned industrial warehouses, into overflow hospitals for the care of COVID-19 patients.1 Such a national scale of field hospital construction is truly historic, never before having occurred at this speed and on this scale. The only other time field hospitals were deployed nearly as widely in the United States was during the Civil War.3
FIELD HOSPITALS DURING THE COVID-19 PANDEMIC
The use of COVID-19 field hospital resources has been variable, with patient volumes ranging from 0 at many to more than 1,000 at the Javits Center field hospital in New York City.1 In fact, most field hospitals did not treat any patients because early public health measures, such as stay-at-home orders, helped contain the virus in most states.1 As of this writing, the United States has seen a dramatic surge in COVID-19 transmission and hospitalizations. This has led many states to re-introduce field hospitals into their COVID emergency response.
Our site, the Baltimore Convention Center Field Hospital (BCCFH), is one of few sites that is still operational and, to our knowledge, is the longest-running US COVID-19 field hospital. We have cared for 543 patients since opening and have had no cardiac arrests or on-site deaths. To safely offload lower-acuity COVID-19 patients from Maryland hospitals, we designed admission criteria and care processes to provide medical care on site until patients are ready for discharge. However, we anticipated that some patients would decompensate and need to return to a higher level of care. Here, we share our experience with identifying, assessing, resuscitating, and transporting unstable patients. We believe that this process has allowed us to treat about 80% of our patients in place with successful discharge to outpatient care. We have safely transferred about 20% to a higher level of care, having learned from our early cases to refine and improve our rapid response process.
CASES
Case 1
A 39-year-old man was transferred to the BCCFH on his 9th day of symptoms following a 3-day hospital admission for COVID-19. On BCCFH day 1, he developed an oxygen requirement of 2 L/min and a fever of 39.9 oC. Testing revealed worsening hyponatremia and new proteinuria, and a chest radiograph showed increased bilateral interstitial infiltrates. Cefdinir and fluid restriction were initiated. On BCCFH day 2, the patient developed hypotension (88/55 mm Hg), tachycardia (180 bpm), an oxygen requirement of 3 L/min, and a brief syncopal episode while sitting in bed. The charge physician and nurse were directed to the bedside. They instructed staff to bring a stretcher and intravenous (IV) supplies. Unable to locate these supplies in the triage bay, the staff found them in various locations. An IV line was inserted, and fluids administered, after which vital signs improved. Emergency medical services (EMS), which were on standby outside the field hospital, were alerted via radio; they donned personal protective equipment (PPE) and arrived at the triage bay. They were redirected to patient bedside, whence they transported the patient to the hospital.
Case 2
A 64-year-old man with a history of homelessness, myocardial infarctions, cerebrovascular accident, and paroxysmal atrial fibrillation was transferred to the BCCFH on his 6th day of symptoms after a 2-day hospitalization with COVID-19 respiratory illness. On BCCFH day 1, he had a temperature of 39.3 oC and atypical chest pain. A laboratory workup was unrevealing. On BCCFH day 2, he had asymptomatic hypotension and a heart rate of 60-85 bpm while receiving his usual metoprolol dose. On BCCFH day 3, he reported dizziness and was found to be hypotensive (83/41 mm Hg) and febrile (38.6 oC). The rapid response team (RRT) was called over radio, and they quickly assessed the patient and transported him to the triage bay. EMS, signaled through the RRT radio announcement, arrived at the triage bay and transported the patient to a traditional hospital.
ABOUT THE BCCFH
The BCCFH, which opened in April 2020, is a 252-bed facility that’s spread over a single exhibit hall floor and cares for stable adult COVID-19 patients from any hospital or emergency department in Maryland (Appendix A). The site offers basic laboratory tests, radiography, a limited on-site pharmacy, and spot vital sign monitoring without telemetry. Both EMS and a certified registered nurse anesthetist are on standby in the nonclinical area and must don PPE before entering the patient care area when called. The appendices show the patient beds (Appendix B) and triage area (Appendix C) used for patient evaluation and resuscitation. Unlike conventional hospitals, the BCCFH has limited consultant access, and there are frequent changes in clinical teams. In addition to clinicians, our site has physical therapists, occupational therapists, and social work teams to assist in patient care and discharge planning. As of this writing, we have cared for 543 patients, sent to us from one-third of Maryland’s hospitals. Use during the first wave of COVID was variable, with some hospitals sending us just a few patients. One Baltimore hospital sent us 8% of its COVID-19 patients. Because the patients have an average 5-day stay, the BCCFH has offloaded 2,600 bed-days of care from acute hospitals.
ROLE OF THE RRT IN A FIELD HOSPITAL
COVID-19 field hospitals must be prepared to respond effectively to decompensating patients. In our experience, effective RRTs provide a standard and reproducible approach to patient emergencies. In the conventional hospital setting, these teams consist of clinicians who can be called on by any healthcare worker to quickly assess deteriorating patients and intervene with treatment. The purpose of an RRT is to provide immediate care to a patient before progression to respiratory or cardiac arrest. RRTs proliferated in US hospitals after 2004 when the Institute for Healthcare Improvement in Boston, Massachusetts, recommended such teams for improved quality of care. Though studies report conflicting findings on the impact of RRTs on mortality rates, these studies were performed in traditional hospitals with ample resources, consultants, and clinicians familiar with their patients rather than in resource-limited field hospitals.4-13 Our field hospital has found RRTs, and the principles behind them, useful in the identification and management of decompensating COVID-19 patients.
A FOUR-STEP RAPID RESPONSE FRAMEWORK: CASE CORRELATION
An approach to managing decompensating patients in a COVID-19 field hospital can be considered in four phases: identification, assessment, resuscitation, and transport. Referring to these phases, the first case shows opportunities for improvement in resuscitation and transport. Although decompensation was identified, the patient was not transported to the triage bay for resuscitation, and there was confusion when trying to obtain the proper equipment. Additionally, EMS awaited the patient in the triage bay, while he remained in his cubicle, which delayed transport to an acute care hospital. The second case shows opportunities for improvement in identification and assessment. The patient had signs of impending decompensation that were not immediately recognized and treated. However, once decompensation occurred, the RRT was called and the patient was transported quickly to the triage bay, and then to the hospital via EMS.
In our experience at the BCCFH, identification is a key phase in COVID-19 care at a field hospital. Identification involves recognizing impending deterioration, as well as understanding risk factors for decompensation. For COVID-19 specifically, this requires heightened awareness of patients who are in the 2nd to 3rd week of symptoms. Data from Wuhan, China, suggest that decompensation occurs predictably around symptom day 9.14,15 At the BCCFH, the median symptom duration for patients who decompensated and returned to a hospital was 13 days. In both introductory cases, patients were in the high-risk 2nd week of symptoms when decompensation occurred. Clinicians at the BCCFH now discuss patient symptom day during their handoffs, when rounding, and when making decisions regarding acute care transfer. Our team has also integrated clinical information from our electronic health record to create a dashboard describing those patients requiring acute care transfer to assist in identifying other trends or predictive factors (Appendix D).
LESSONS FROM THE FIELD HOSPITAL: IMPROVING CLINICAL PERFORMANCE
Although RRTs are designed to activate when an individual patient decompensates, they should fit within a larger operational framework for patient safety. Our experience with emergencies at the BCCFH has yielded four opportunities for learning relevant to COVID-19 care in nontraditional settings (Table). These lessons include how to update staff on clinical process changes, unify communication systems, create a clinical drilling culture, and review cases to improve performance. They illustrate the importance of standardizing emergency processes, conducting frequent updates and drills, and ensuring continuous improvement. We found that, while caring for patients with an unpredictable, novel disease in a nontraditional setting and while wearing PPE and working with new colleagues during every shift, the best approach to support patients and staff is to anticipate emergencies rather than relying on individual staff to develop on-the-spot solutions.

CONCLUSION
The COVID-19 era has seen the unprecedented construction and utilization of emergency field hospital facilities. Such facilities can serve to offload some COVID-19 patients from strained healthcare infrastructure and provide essential care to these patients. We share many of the unique physical and logistical considerations specific to a nontraditional site. We optimized our space, our equipment, and our communication system. We learned how to identify, assess, resuscitate, and transport decompensating COVID-19 patients. Ultimately, our field hospital has been well utilized and successful at caring for patients because of its adaptability, accessibility, and safety record. Of the 15% of patients we transferred to a hospital for care, 81% were successfully stabilized and were willing to return to the BCCFH to complete their care. Our design included supportive care such as social work, physical and occupational therapy, and treatment of comorbidities, such as diabetes and substance use disorder. Our model demonstrates an effective nonhospital option for the care of lower-acuity, medically complex COVID-19 patients. If such facilities are used in subsequent COVID-19 outbreaks, we advise structured planning for the care of decompensating patients that takes into account the need for effective communication, drilling, and ongoing process improvement.
1. Rose J. U.S. Field Hospitals Stand Down, Most Without Treating Any COVID-19 Patients. All Things Considered. NPR; May 7, 2020. Accessed July 21, 2020. https://www.npr.org/2020/05/07/851712311/u-s-field-hospitals-stand-down-most-without-treating-any-covid-19-patients
2. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305-1314. https://doi.org/10.1016/s0140-6736(20)30744-3
3. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-142. https://doi.org/10.1080/08998280.2016.11929390
4. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916-21. https://doi.org/10.1097/01.ccm.0000119428.02968.9e
5. Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179(6):283-287.
6. Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236-240.
7. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387-390. https://doi.org/10.1136/bmj.324.7334.387
8. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL; Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. https://doi.org/10.1136/qhc.13.4.251
9. Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A. The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54(9):853-860. https://doi.org/10.1046/j.1365-2044.1999.00996.x
10. Hillman K, Chen J, Cretikos M, et al; MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. https://doi.org/10.1016/s0140-6736(05)66733-5
11. Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257-263. https://doi.org/10.1016/j.resuscitation.2004.01.021
12. Pittard AJ. Out of our reach? assessing the impact of introducing a critical care outreach service. Anaesthesia. 2003;58(9):882-885. https://doi.org/10.1046/j.1365-2044.2003.03331.x
13. Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398-1404. https://doi.org/10.1007/s00134-004-2268-7
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
15. Zhou Y, Li W, Wang D, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177-179. https://doi.org/10.1136/svn-2020-000398
1. Rose J. U.S. Field Hospitals Stand Down, Most Without Treating Any COVID-19 Patients. All Things Considered. NPR; May 7, 2020. Accessed July 21, 2020. https://www.npr.org/2020/05/07/851712311/u-s-field-hospitals-stand-down-most-without-treating-any-covid-19-patients
2. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305-1314. https://doi.org/10.1016/s0140-6736(20)30744-3
3. Reilly RF. Medical and surgical care during the American Civil War, 1861-1865. Proc (Bayl Univ Med Cent). 2016;29(2):138-142. https://doi.org/10.1080/08998280.2016.11929390
4. Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916-21. https://doi.org/10.1097/01.ccm.0000119428.02968.9e
5. Bellomo R, Goldsmith D, Uchino S, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003;179(6):283-287.
6. Bristow PJ, Hillman KM, Chey T, et al. Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust. 2000;173(5):236-240.
7. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324(7334):387-390. https://doi.org/10.1136/bmj.324.7334.387
8. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL; Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. https://doi.org/10.1136/qhc.13.4.251
9. Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A. The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia. 1999;54(9):853-860. https://doi.org/10.1046/j.1365-2044.1999.00996.x
10. Hillman K, Chen J, Cretikos M, et al; MERIT study investigators. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. https://doi.org/10.1016/s0140-6736(05)66733-5
11. Kenward G, Castle N, Hodgetts T, Shaikh L. Evaluation of a medical emergency team one year after implementation. Resuscitation. 2004;61(3):257-263. https://doi.org/10.1016/j.resuscitation.2004.01.021
12. Pittard AJ. Out of our reach? assessing the impact of introducing a critical care outreach service. Anaesthesia. 2003;58(9):882-885. https://doi.org/10.1046/j.1365-2044.2003.03331.x
13. Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30(7):1398-1404. https://doi.org/10.1007/s00134-004-2268-7
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. https://doi.org/10.1016/s0140-6736(20)30566-3
15. Zhou Y, Li W, Wang D, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. 2020;5(2):177-179. https://doi.org/10.1136/svn-2020-000398
© 2021 Society of Hospital Medicine
Defining a New Normal While Awaiting the Pandemic’s Next Wave
Hospitalists have played a central role in the massive response to the coronavirus disease 2019 (COVID-19) pandemic by creating innovative staffing models, rapidly learning about the disease and teaching others, and working closely with hospital executive leadership to create surge capacity.1 Some hospitals and regions have weathered an initial storm and are now experiencing a slower influx of COVID-19 patients, while others are now seeing a surge, which is expected to persist for the foreseeable future—the marathon has begun.2 We have entered a new COVID-19 reality: disrupted care models, harsh financial consequences,3 and uncertainty about which adaptations should be preserved and for how long. Common operational challenges will define the new normal. In this Perspective, we share strategies to address these challenges, focusing on three emerging themes: realigning staffing to patient volumes, safely managing space limitations, and navigating the financial ramifications of COVID-19 for hospital medicine groups.
BALANCING STAFFING AND PATIENT VOLUME
Hospital medicine groups face uncertainty about future patient volumes and their characteristics. It is unclear when, how, or even whether hospital medicine groups should return to “normal” pre-COVID staffing models. The following principles can guide staffing decisions.
First, maintain nonhospitalist backup pools and define triggers to activate these providers. Despite the impulse to return to prior staffing models, this recovery period provides an opportunity for leaders to create transparent activation protocols and provide additional training to enable seamless backup. In preparation for a surge, our hospital medicine group quickly assembled an emergency staffing pool composed of advanced practice providers, primary care providers, medicine subspecialists, and surgeons who were prepared to temporarily assume unfamiliar roles. Thankfully, we were able to manage our COVID-19 patients without much emergency hospitalist staffing, but for other hospitals with larger community outbreaks, the emergency backup workforce proved invaluable.
Second, use appropriate safeguards and delegate certain aspects of COVID-related care to other healthcare team members. As staff are deployed and redeployed, consider how interprofessional team members can be reintegrated into evaluation and triage protocols. For example, registered nurses can determine appropriate isolation precautions for patients with COVID and patients under investigation.
Third, consider hospital-specific specialty care patterns when planning for COVID-19 redeployment to ensure access to equally critical, nonelective services. For example, Level 1 trauma centers may expect seasonal increases in trauma patient volumes, so consider staffing trauma teams (including surgeons, anesthesiologists, and operating room staff) for their usual roles to prevent critical coverage gaps. Concurrently, hospital medicine consulting and comanagement teams must also be available to support the trauma service. These staffing needs affect who will be available for redeployment for future COVID-related care.
MANAGING THE PHYSICAL LIMITATIONS OF SPACE
As the number of COVID cases increased, numerous hospitals created geographic “hot zones” with defined cold (uncontaminated), warm (transitional), and hot (contaminated) areas by either partitioning off a section of an acute care medical ward or repurposing an entire ward as a COVID-19 unit, and similar zones were made in intensive care units. Hot zones required significant early investments to change infrastructure, including equipping rooms for negative pressurization with HEPA filtration towers and training staff on safety protocols for entering these spaces, performing necessary patient care, and exiting. Ultimately, these investments proved worthwhile and allowed for decreased personal protective equipment (PPE) use, as well as improved efficiency and staff safety. However, as hospitals ramp up non-COVID care, deciding how to best reconfigure or downsize these hot zones has become challenging.
With time to regroup, the newly experienced end users of hot zones—hospitalists, other staff who worked in these spaces, and patients—must be included in discussions with engineers, architects, and administrators regarding future construction. Hot zone plans should specifically address how physical separation of COVID and non-COVID patients will be maintained while providing safe and efficient care. With elective surgeries increasing and non-COVID patients returning to hospitals, leaders must consider the psychological effects that seeing hospital staff doffing PPE and crossing an invisible barrier to a ‘‘cold” area of the floor has on patients and their families. It is important to maintain hot zones in areas that can dynamically flex to accommodate waves of the current and future pandemics, especially because hospitals may be asked to care for patients from overwhelmed distant sites even if the pandemic is locally controlled. We are experimenting with modifications to hospital traffic patterns including “no pass through” zones, one-way hallways, and separate entries and exits to clinical floors for COVID and non-COVID patients. With vigilant adherence to infection prevention guidelines and PPE use, we have not seen hospital-acquired infections with this model of care.
Modifying space and flow patterns also enables clustered care for COVID patients, which allows for the temporary use of modular teams.4 This tactic may be especially useful during surge periods, during which PPE conservation is paramount and isolating cohorts of providers provides an extra layer of safety. In the longer run, however, isolating providers from their peers risks worsening morale and increasing burnout.
NAVIGATING THE FINANCIAL CHALLENGES
The path forward must ensure safety but also allow for a financially sustainable balance of COVID and non-COVID care. To prepare for surges, health systems canceled elective surgeries and other services that generate essential revenue. At both private and public hospitals, systemwide measures have been taken to mitigate these financial losses. These measures have included salary, retirement, and continuing medical education benefit reductions for physicians and senior leadership; limits to physician hiring and recruitment; leaner operations with systemwide expense reductions; and mandatory and voluntary staff furloughs. The frontline hospital staff, including physicians, nurses, technologists, and food and environmental service workers, who have made great sacrifices during this pandemic, may also now be facing significant personal financial consequences.
The following recommendations are offered from the perspective that crisis creates opportunity for hospital medicine leaders grappling with budget shortfalls.
First, maximize budget transparency by explicitly defining the principles and priorities that govern budget decisions, which allows hospitalist group members to understand how the organization determines budget cuts. For example, stating that a key priority is to minimize staff layoffs makes consequent salary reductions more understandable.
Second, solicit hospital medicine group members’ input on these shared challenges and invite their help in identifying and prioritizing potential cost-saving or cost-cutting measures.
Third, highlight hospitalists’ nonfiscal contributions, especially in terms of crisis leadership, to continue engagement with executive leaders.5 This may include a dialogue about the disproportionate influence of work relative value unit production on salary and about how to create compensation systems that can also recognize crisis readiness as an important feature of sustainability and quality care. The next pandemic surge may be weeks or months away, and hospitalists will again need to be leaders in the response.
Fourth, use this crisis to foster fiscal innovation and accelerate participation in value improvement work, such as redesigning pay-for-performance metrics. Financially strapped institutions will value hospitalists who are good financial stewards. For example, leverage hospitalist expertise in progression of care to facilitate timely disposition of COVID patients, thereby minimizing costly extended hospitalizations.
Lastly, hospital medicine groups must match staffing to patient volume to the extent possible. Approximately two-thirds of hospitalist groups entered this crisis already understaffed and partially reliant on moonlighters,6 which allowed some variation of labor expenses to match lower patient volume. During the recovery phase, hospital volumes may either be significantly below or above baseline; many patients are understandably avoiding hospitals due to fear of COVID. However, delayed care may create a different kind of peak demand for services. For hospitalists, uncertainty about expected clinical roles, COVID vs non-COVID patient mix, and patient volume can be stressful. We recommend sustained, frequent communication about census trends and how shifts will be covered to ensure adequate, long-term staffing. Maintaining trust and morale will be equally, if not more, important in the next phase.
CONCLUSION
As we settle into the marathon, hospital medicine leadership must balance competing priorities with increasing finesse. Our hospital medicine group has benefited from continually discussing operational challenges and refining our strategies as we plan for what is ahead. We have highlighted three mission-critical themes and recommend that hospital and hospital medicine group leaders remain mindful of these challenges and potential strategies. Each of our four academic hospitals has considered similar trade-offs and will proceed along slightly different trajectories to meet unique needs. Looking to the future, we anticipate additional challenges requiring greater ongoing attention alongside those already identified. These include mitigating provider burnout, optimizing resident and student education, and maintaining scholarly work as COVID unpredictably waxes and wanes. By accumulating confidence and wisdom about post-COVID hospital medicine group functions, we hope to provide hospitalists with the energy to keep the pace in the next phase of the marathon.
- Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med . 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
- COVIDView - A weekly Surveillance Summary of U.S. COVID-19 Activity. US Centers for Disease Control and Prevention. July 9, 2020. Accessed July 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-10-2020.pdf
- Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. Published online May 4, 2020. https://doi.org/10.1001/jama.2020.6269
- Wang CJ, Bair H, Yeh CC. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med . 2020;15(6):370-371. https://doi.org/10.12788/jhm.3452
- White AA, McIlraith T, Chivu AM, et al. Collaboration, not calculation: a qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(11):662-667. https://doi.org/10.12788/jhm.3249
- 2018 State of Hospital Medicine: 2018 Report Based on 2017 Data . Society of Hospital Medicine; 2018. Accessed July 27, 2020. https://sohm.hospitalmedicine.org/
Hospitalists have played a central role in the massive response to the coronavirus disease 2019 (COVID-19) pandemic by creating innovative staffing models, rapidly learning about the disease and teaching others, and working closely with hospital executive leadership to create surge capacity.1 Some hospitals and regions have weathered an initial storm and are now experiencing a slower influx of COVID-19 patients, while others are now seeing a surge, which is expected to persist for the foreseeable future—the marathon has begun.2 We have entered a new COVID-19 reality: disrupted care models, harsh financial consequences,3 and uncertainty about which adaptations should be preserved and for how long. Common operational challenges will define the new normal. In this Perspective, we share strategies to address these challenges, focusing on three emerging themes: realigning staffing to patient volumes, safely managing space limitations, and navigating the financial ramifications of COVID-19 for hospital medicine groups.
BALANCING STAFFING AND PATIENT VOLUME
Hospital medicine groups face uncertainty about future patient volumes and their characteristics. It is unclear when, how, or even whether hospital medicine groups should return to “normal” pre-COVID staffing models. The following principles can guide staffing decisions.
First, maintain nonhospitalist backup pools and define triggers to activate these providers. Despite the impulse to return to prior staffing models, this recovery period provides an opportunity for leaders to create transparent activation protocols and provide additional training to enable seamless backup. In preparation for a surge, our hospital medicine group quickly assembled an emergency staffing pool composed of advanced practice providers, primary care providers, medicine subspecialists, and surgeons who were prepared to temporarily assume unfamiliar roles. Thankfully, we were able to manage our COVID-19 patients without much emergency hospitalist staffing, but for other hospitals with larger community outbreaks, the emergency backup workforce proved invaluable.
Second, use appropriate safeguards and delegate certain aspects of COVID-related care to other healthcare team members. As staff are deployed and redeployed, consider how interprofessional team members can be reintegrated into evaluation and triage protocols. For example, registered nurses can determine appropriate isolation precautions for patients with COVID and patients under investigation.
Third, consider hospital-specific specialty care patterns when planning for COVID-19 redeployment to ensure access to equally critical, nonelective services. For example, Level 1 trauma centers may expect seasonal increases in trauma patient volumes, so consider staffing trauma teams (including surgeons, anesthesiologists, and operating room staff) for their usual roles to prevent critical coverage gaps. Concurrently, hospital medicine consulting and comanagement teams must also be available to support the trauma service. These staffing needs affect who will be available for redeployment for future COVID-related care.
MANAGING THE PHYSICAL LIMITATIONS OF SPACE
As the number of COVID cases increased, numerous hospitals created geographic “hot zones” with defined cold (uncontaminated), warm (transitional), and hot (contaminated) areas by either partitioning off a section of an acute care medical ward or repurposing an entire ward as a COVID-19 unit, and similar zones were made in intensive care units. Hot zones required significant early investments to change infrastructure, including equipping rooms for negative pressurization with HEPA filtration towers and training staff on safety protocols for entering these spaces, performing necessary patient care, and exiting. Ultimately, these investments proved worthwhile and allowed for decreased personal protective equipment (PPE) use, as well as improved efficiency and staff safety. However, as hospitals ramp up non-COVID care, deciding how to best reconfigure or downsize these hot zones has become challenging.
With time to regroup, the newly experienced end users of hot zones—hospitalists, other staff who worked in these spaces, and patients—must be included in discussions with engineers, architects, and administrators regarding future construction. Hot zone plans should specifically address how physical separation of COVID and non-COVID patients will be maintained while providing safe and efficient care. With elective surgeries increasing and non-COVID patients returning to hospitals, leaders must consider the psychological effects that seeing hospital staff doffing PPE and crossing an invisible barrier to a ‘‘cold” area of the floor has on patients and their families. It is important to maintain hot zones in areas that can dynamically flex to accommodate waves of the current and future pandemics, especially because hospitals may be asked to care for patients from overwhelmed distant sites even if the pandemic is locally controlled. We are experimenting with modifications to hospital traffic patterns including “no pass through” zones, one-way hallways, and separate entries and exits to clinical floors for COVID and non-COVID patients. With vigilant adherence to infection prevention guidelines and PPE use, we have not seen hospital-acquired infections with this model of care.
Modifying space and flow patterns also enables clustered care for COVID patients, which allows for the temporary use of modular teams.4 This tactic may be especially useful during surge periods, during which PPE conservation is paramount and isolating cohorts of providers provides an extra layer of safety. In the longer run, however, isolating providers from their peers risks worsening morale and increasing burnout.
NAVIGATING THE FINANCIAL CHALLENGES
The path forward must ensure safety but also allow for a financially sustainable balance of COVID and non-COVID care. To prepare for surges, health systems canceled elective surgeries and other services that generate essential revenue. At both private and public hospitals, systemwide measures have been taken to mitigate these financial losses. These measures have included salary, retirement, and continuing medical education benefit reductions for physicians and senior leadership; limits to physician hiring and recruitment; leaner operations with systemwide expense reductions; and mandatory and voluntary staff furloughs. The frontline hospital staff, including physicians, nurses, technologists, and food and environmental service workers, who have made great sacrifices during this pandemic, may also now be facing significant personal financial consequences.
The following recommendations are offered from the perspective that crisis creates opportunity for hospital medicine leaders grappling with budget shortfalls.
First, maximize budget transparency by explicitly defining the principles and priorities that govern budget decisions, which allows hospitalist group members to understand how the organization determines budget cuts. For example, stating that a key priority is to minimize staff layoffs makes consequent salary reductions more understandable.
Second, solicit hospital medicine group members’ input on these shared challenges and invite their help in identifying and prioritizing potential cost-saving or cost-cutting measures.
Third, highlight hospitalists’ nonfiscal contributions, especially in terms of crisis leadership, to continue engagement with executive leaders.5 This may include a dialogue about the disproportionate influence of work relative value unit production on salary and about how to create compensation systems that can also recognize crisis readiness as an important feature of sustainability and quality care. The next pandemic surge may be weeks or months away, and hospitalists will again need to be leaders in the response.
Fourth, use this crisis to foster fiscal innovation and accelerate participation in value improvement work, such as redesigning pay-for-performance metrics. Financially strapped institutions will value hospitalists who are good financial stewards. For example, leverage hospitalist expertise in progression of care to facilitate timely disposition of COVID patients, thereby minimizing costly extended hospitalizations.
Lastly, hospital medicine groups must match staffing to patient volume to the extent possible. Approximately two-thirds of hospitalist groups entered this crisis already understaffed and partially reliant on moonlighters,6 which allowed some variation of labor expenses to match lower patient volume. During the recovery phase, hospital volumes may either be significantly below or above baseline; many patients are understandably avoiding hospitals due to fear of COVID. However, delayed care may create a different kind of peak demand for services. For hospitalists, uncertainty about expected clinical roles, COVID vs non-COVID patient mix, and patient volume can be stressful. We recommend sustained, frequent communication about census trends and how shifts will be covered to ensure adequate, long-term staffing. Maintaining trust and morale will be equally, if not more, important in the next phase.
CONCLUSION
As we settle into the marathon, hospital medicine leadership must balance competing priorities with increasing finesse. Our hospital medicine group has benefited from continually discussing operational challenges and refining our strategies as we plan for what is ahead. We have highlighted three mission-critical themes and recommend that hospital and hospital medicine group leaders remain mindful of these challenges and potential strategies. Each of our four academic hospitals has considered similar trade-offs and will proceed along slightly different trajectories to meet unique needs. Looking to the future, we anticipate additional challenges requiring greater ongoing attention alongside those already identified. These include mitigating provider burnout, optimizing resident and student education, and maintaining scholarly work as COVID unpredictably waxes and wanes. By accumulating confidence and wisdom about post-COVID hospital medicine group functions, we hope to provide hospitalists with the energy to keep the pace in the next phase of the marathon.
Hospitalists have played a central role in the massive response to the coronavirus disease 2019 (COVID-19) pandemic by creating innovative staffing models, rapidly learning about the disease and teaching others, and working closely with hospital executive leadership to create surge capacity.1 Some hospitals and regions have weathered an initial storm and are now experiencing a slower influx of COVID-19 patients, while others are now seeing a surge, which is expected to persist for the foreseeable future—the marathon has begun.2 We have entered a new COVID-19 reality: disrupted care models, harsh financial consequences,3 and uncertainty about which adaptations should be preserved and for how long. Common operational challenges will define the new normal. In this Perspective, we share strategies to address these challenges, focusing on three emerging themes: realigning staffing to patient volumes, safely managing space limitations, and navigating the financial ramifications of COVID-19 for hospital medicine groups.
BALANCING STAFFING AND PATIENT VOLUME
Hospital medicine groups face uncertainty about future patient volumes and their characteristics. It is unclear when, how, or even whether hospital medicine groups should return to “normal” pre-COVID staffing models. The following principles can guide staffing decisions.
First, maintain nonhospitalist backup pools and define triggers to activate these providers. Despite the impulse to return to prior staffing models, this recovery period provides an opportunity for leaders to create transparent activation protocols and provide additional training to enable seamless backup. In preparation for a surge, our hospital medicine group quickly assembled an emergency staffing pool composed of advanced practice providers, primary care providers, medicine subspecialists, and surgeons who were prepared to temporarily assume unfamiliar roles. Thankfully, we were able to manage our COVID-19 patients without much emergency hospitalist staffing, but for other hospitals with larger community outbreaks, the emergency backup workforce proved invaluable.
Second, use appropriate safeguards and delegate certain aspects of COVID-related care to other healthcare team members. As staff are deployed and redeployed, consider how interprofessional team members can be reintegrated into evaluation and triage protocols. For example, registered nurses can determine appropriate isolation precautions for patients with COVID and patients under investigation.
Third, consider hospital-specific specialty care patterns when planning for COVID-19 redeployment to ensure access to equally critical, nonelective services. For example, Level 1 trauma centers may expect seasonal increases in trauma patient volumes, so consider staffing trauma teams (including surgeons, anesthesiologists, and operating room staff) for their usual roles to prevent critical coverage gaps. Concurrently, hospital medicine consulting and comanagement teams must also be available to support the trauma service. These staffing needs affect who will be available for redeployment for future COVID-related care.
MANAGING THE PHYSICAL LIMITATIONS OF SPACE
As the number of COVID cases increased, numerous hospitals created geographic “hot zones” with defined cold (uncontaminated), warm (transitional), and hot (contaminated) areas by either partitioning off a section of an acute care medical ward or repurposing an entire ward as a COVID-19 unit, and similar zones were made in intensive care units. Hot zones required significant early investments to change infrastructure, including equipping rooms for negative pressurization with HEPA filtration towers and training staff on safety protocols for entering these spaces, performing necessary patient care, and exiting. Ultimately, these investments proved worthwhile and allowed for decreased personal protective equipment (PPE) use, as well as improved efficiency and staff safety. However, as hospitals ramp up non-COVID care, deciding how to best reconfigure or downsize these hot zones has become challenging.
With time to regroup, the newly experienced end users of hot zones—hospitalists, other staff who worked in these spaces, and patients—must be included in discussions with engineers, architects, and administrators regarding future construction. Hot zone plans should specifically address how physical separation of COVID and non-COVID patients will be maintained while providing safe and efficient care. With elective surgeries increasing and non-COVID patients returning to hospitals, leaders must consider the psychological effects that seeing hospital staff doffing PPE and crossing an invisible barrier to a ‘‘cold” area of the floor has on patients and their families. It is important to maintain hot zones in areas that can dynamically flex to accommodate waves of the current and future pandemics, especially because hospitals may be asked to care for patients from overwhelmed distant sites even if the pandemic is locally controlled. We are experimenting with modifications to hospital traffic patterns including “no pass through” zones, one-way hallways, and separate entries and exits to clinical floors for COVID and non-COVID patients. With vigilant adherence to infection prevention guidelines and PPE use, we have not seen hospital-acquired infections with this model of care.
Modifying space and flow patterns also enables clustered care for COVID patients, which allows for the temporary use of modular teams.4 This tactic may be especially useful during surge periods, during which PPE conservation is paramount and isolating cohorts of providers provides an extra layer of safety. In the longer run, however, isolating providers from their peers risks worsening morale and increasing burnout.
NAVIGATING THE FINANCIAL CHALLENGES
The path forward must ensure safety but also allow for a financially sustainable balance of COVID and non-COVID care. To prepare for surges, health systems canceled elective surgeries and other services that generate essential revenue. At both private and public hospitals, systemwide measures have been taken to mitigate these financial losses. These measures have included salary, retirement, and continuing medical education benefit reductions for physicians and senior leadership; limits to physician hiring and recruitment; leaner operations with systemwide expense reductions; and mandatory and voluntary staff furloughs. The frontline hospital staff, including physicians, nurses, technologists, and food and environmental service workers, who have made great sacrifices during this pandemic, may also now be facing significant personal financial consequences.
The following recommendations are offered from the perspective that crisis creates opportunity for hospital medicine leaders grappling with budget shortfalls.
First, maximize budget transparency by explicitly defining the principles and priorities that govern budget decisions, which allows hospitalist group members to understand how the organization determines budget cuts. For example, stating that a key priority is to minimize staff layoffs makes consequent salary reductions more understandable.
Second, solicit hospital medicine group members’ input on these shared challenges and invite their help in identifying and prioritizing potential cost-saving or cost-cutting measures.
Third, highlight hospitalists’ nonfiscal contributions, especially in terms of crisis leadership, to continue engagement with executive leaders.5 This may include a dialogue about the disproportionate influence of work relative value unit production on salary and about how to create compensation systems that can also recognize crisis readiness as an important feature of sustainability and quality care. The next pandemic surge may be weeks or months away, and hospitalists will again need to be leaders in the response.
Fourth, use this crisis to foster fiscal innovation and accelerate participation in value improvement work, such as redesigning pay-for-performance metrics. Financially strapped institutions will value hospitalists who are good financial stewards. For example, leverage hospitalist expertise in progression of care to facilitate timely disposition of COVID patients, thereby minimizing costly extended hospitalizations.
Lastly, hospital medicine groups must match staffing to patient volume to the extent possible. Approximately two-thirds of hospitalist groups entered this crisis already understaffed and partially reliant on moonlighters,6 which allowed some variation of labor expenses to match lower patient volume. During the recovery phase, hospital volumes may either be significantly below or above baseline; many patients are understandably avoiding hospitals due to fear of COVID. However, delayed care may create a different kind of peak demand for services. For hospitalists, uncertainty about expected clinical roles, COVID vs non-COVID patient mix, and patient volume can be stressful. We recommend sustained, frequent communication about census trends and how shifts will be covered to ensure adequate, long-term staffing. Maintaining trust and morale will be equally, if not more, important in the next phase.
CONCLUSION
As we settle into the marathon, hospital medicine leadership must balance competing priorities with increasing finesse. Our hospital medicine group has benefited from continually discussing operational challenges and refining our strategies as we plan for what is ahead. We have highlighted three mission-critical themes and recommend that hospital and hospital medicine group leaders remain mindful of these challenges and potential strategies. Each of our four academic hospitals has considered similar trade-offs and will proceed along slightly different trajectories to meet unique needs. Looking to the future, we anticipate additional challenges requiring greater ongoing attention alongside those already identified. These include mitigating provider burnout, optimizing resident and student education, and maintaining scholarly work as COVID unpredictably waxes and wanes. By accumulating confidence and wisdom about post-COVID hospital medicine group functions, we hope to provide hospitalists with the energy to keep the pace in the next phase of the marathon.
- Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med . 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
- COVIDView - A weekly Surveillance Summary of U.S. COVID-19 Activity. US Centers for Disease Control and Prevention. July 9, 2020. Accessed July 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-10-2020.pdf
- Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. Published online May 4, 2020. https://doi.org/10.1001/jama.2020.6269
- Wang CJ, Bair H, Yeh CC. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med . 2020;15(6):370-371. https://doi.org/10.12788/jhm.3452
- White AA, McIlraith T, Chivu AM, et al. Collaboration, not calculation: a qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(11):662-667. https://doi.org/10.12788/jhm.3249
- 2018 State of Hospital Medicine: 2018 Report Based on 2017 Data . Society of Hospital Medicine; 2018. Accessed July 27, 2020. https://sohm.hospitalmedicine.org/
- Garg M, Wray CM. Hospital medicine management in the time of COVID-19: preparing for a sprint and a marathon. J Hosp Med . 2020;15(5):305-307. https://doi.org/10.12788/jhm.3427
- COVIDView - A weekly Surveillance Summary of U.S. COVID-19 Activity. US Centers for Disease Control and Prevention. July 9, 2020. Accessed July 13, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-07-10-2020.pdf
- Khullar D, Bond AM, Schpero WL. COVID-19 and the financial health of US hospitals. JAMA. Published online May 4, 2020. https://doi.org/10.1001/jama.2020.6269
- Wang CJ, Bair H, Yeh CC. How to prevent and manage hospital-based infections during coronavirus outbreaks: five lessons from Taiwan. J Hosp Med . 2020;15(6):370-371. https://doi.org/10.12788/jhm.3452
- White AA, McIlraith T, Chivu AM, et al. Collaboration, not calculation: a qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(11):662-667. https://doi.org/10.12788/jhm.3249
- 2018 State of Hospital Medicine: 2018 Report Based on 2017 Data . Society of Hospital Medicine; 2018. Accessed July 27, 2020. https://sohm.hospitalmedicine.org/
© 2021 Society of Hospital Medicine
Email: smookh@u.washington.edu.
The Need for Standardized Metrics to Drive Decision-making During the COVID-19 Pandemic
The rapid onset of the novel coronavirus disease 2019 (COVID-19) pandemic forced the US healthcare system to scramble to prepare for a health crisis with many unknowns. Early on, it was unclear exactly how the virus was transmitted, how many people would fall ill or how ill they would get, what treatments would be most efficacious, and what resources were needed to care for patients.1 Given the short window the healthcare system had to prepare, many initial and important decisions were made quickly and often at a local level, with limited coordination and standardization across localities and organizations. These decisions included what services could be offered, how best to allocate potentially scarce resources (such as personal protective equipment and ventilators), and how much surge capacity to build.2,3 In short, many of the early decisions about the pandemic were understandably varied, and the lack of standardized metrics to help guide decision-making did not help the situation.
CHALLENGES WITH MANAGING THE PANDEMIC WITHOUT STANDARDIZED METRICS
Unfortunately, as the COVID-19 pandemic continues, there has been insufficient movement toward standardizing definitions for many key measures needed to manage the public health response. Even small differences in definitions can have important implications for decision-making.4 For example, public health officials have recommended communities achieve a positivity rate of 5% or lower for 14 straight days before easing virus-related restrictions.5 In Maryland, two different entities are calculating positivity rates for the state using different methodologies and producing different results, which can have significant public health and economic implications for the state. Johns Hopkins University’s Resource Center calculates the positivity rate by comparing the number of people who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to all people who were tested. This method consistently produces a positivity rate for Maryland above the 5% threshold. In contrast, the state of Maryland calculates the positivity rate by comparing the number of positive tests for SARS-CoV-2 to the number of tests conducted, even if the same person had multiple tests (unless the tests are performed the same day at the same location). This method consistently produces a positivity rate for Maryland below the 5% threshold.6
THE POLITICIZATION OF THE DATA
The lack of standardized definitions leads not only to debate and confusion over what steps to take next, but also opens the door to politicization of pandemic data. This is readily apparent when considering mortality due to COVID-19. For example, different states use different definitions for COVID-19 mortality. Alabama defines COVID-19 mortality by only including patients who tested positive for the SARS-CoV-2 virus and the cause of death was attributed to COVID-19. In contrast, Colorado’s COVID-19 mortality definition includes those patients who are believed to have died of COVID-19, but does not require confirmation of SARS-CoV-2 infection by a positive test.7 Further compounding the challenge, some politicians reference the COVID-19 mortality rate as a comparison of those who died from COVID-19 with those who were sick with COVID-19, reflecting the success rate of treating patients with COVID-19, an area in which the United States has done relatively well compared with other countries. This definition of the mortality rate suits a narrative of successful pandemic management.8 However, many public health officials suggest the COVID-19 mortality rate should be defined by comparing the number of deaths from COVID-19 as a percentage of the population, which reflects the percentage of the population dying from the disease. In this regard, the United States has not done as well relative to other countries.9 These different definitions highlight how the United States lacks a standardized way to compare its performance across states and with other countries, even on a straightforward measure like mortality.
CURRENT METRICS THAT NEED STANDARDIZATION
The lack of clarity on, and politicization of, pandemic data demonstrate the need to take stock of what metrics require standardization to help public health officials and health system leaders manage the pandemic response moving forward. The Table provides examples of currently used metrics that would benefit from better standardization to inform decision-making across a broad range of settings, including public health, hospitals, physician clinics, and nursing homes. For example, a commonly referenced metric during the pandemic has been a moving average of the incidence rate of positive COVID-19 cases in a defined geographic area (eg, a state).10,11 This data point is helpful to healthcare delivery organizations for understanding the change in COVID-19 cases in their cities and states, which can inform planning on whether or not to continue elective surgeries or how many beds need to be kept in reserve status for a potential surge of hospitalizations. But there has not been a consensus around whether the reporting of COVID-19 positive tests should reflect the day the test was performed or the day the test results were available. The day the test results were available can be influenced by lengthy or uneven turnaround times for the results (eg, backlogs in labs) and can paint a false picture of trends with the virus.
As another example, knowing the percentage of the population that has tested positive for COVID-19 can help inform both resource planning and reopening decisions. But there has been variation in whether counts of positive COVID-19 tests should only include antigen tests, or antibody tests as well. This exact question played out when the Centers for Disease Control and Prevention (CDC) made decisions that differed from those of many states about whether to include antibody tests in their publicly announced COVID-19 testing numbers,12 perhaps undermining public confidence in the reported data.
MOVING FORWARD WITH STANDARDIZING DEFINITIONS
To capture currently unstandardized metrics with broad applicability, the United States should form a consensus task force to identify and define metrics and, over time, refine them based on current science and public health priorities. The task force would require a mix of individuals with various skill sets, such as expertise in infectious diseases and epidemiology, healthcare operations, statistics, performance measurement, and public health. The US Department of Health and Human Services is likely the appropriate sponsor, with representation from the National Institutes of Health, the CDC, and the Agency for Healthcare Research and Quality, in partnership with national provider and public health group representatives.
Once standardized definitions for metrics have been agreed upon, the metric definitions will need to be made readily available to the public and healthcare organizations. Standardization will permit collection of electronic health records for quick calculation and review, with an output of dashboards for reporting. It would also prevent every public health and healthcare delivery organization from having to define its own metrics, freeing them up to focus on planning. Several metrics already have standard definitions, and those metrics have proven useful for decision-making. For example, there is agreement that the turnaround time for a SARS-CoV-2 test is measured by the difference in time between when the test was performed and when the test results were available. This standard definition allows for performance comparisons across different laboratories within the same service area and comparisons across different regions of the country. Once the metrics are standardized, public health leaders and healthcare organizations can use variation in performance and outcomes to identify leading indicators for planning.
CONCLUSION
Amid the COVID-19 pandemic, the US healthcare system finds itself in a state of managing uncertainty for a prolonged period of time. The unprecedented nature of this crisis means that best practices will not always be clear. Providing access to clearly defined, standardized metrics will be essential to public health officials and healthcare organization leaders’ ability to manage through this pandemic. The risk of not moving in this direction means forcing leaders to make decisions without the best information available. Good data will be essential to guiding the US healthcare system through this extraordinary crisis.
- Weston S, Frieman MB. COVID-19: knowns, unknowns, and questions. mSphere. 2020;5(2):e00203-20. https://doi.org/10.1128/mSphere.00203-20
- Griffin KM, Karas MG, Ivascu NS, Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020;201(11):1337-1344. https://doi.org/10.1164/rccm.202004-1037CP
- De Georgeo MR, De Georgeo JM, Egan TM, et al. Containing SARS-CoV-2 in hospitals facing finite PPE, limited testing, and physical space variability: navigating resource constrained enhanced traffic control bundling. J Microbiol Immunol. 2020;S1684-1182(20)30166-3. https://doi.org/10.1016/j.jmii.2020.07.009
- Fischhoff B. Making decisions in a COVID-19 world. JAMA. 2020;324(2):139-140. https://doi.org/10.1001/jama.2020.10178
- Collins K. Is your state doing enough coronavirus testing? New York Times. October 14, 2020. Updated October 29, 2020. Accessed October 14, 2020. https://www.nytimes.com/interactive/2020/us/coronavirus-testing.html
- Ruiz N. Why is Maryland’s coronavirus positivity rate always lower than what Johns Hopkins says it is — and does it matter? Baltimore Sun. September 10, 2020. Accessed October 14, 2020. https://www.baltimoresun.com/coronavirus/bs-md-maryland-coronavirus-positivity-rate-hopkins-20200817-zoepxdjlxbazdm6kabrjehbemq-story.html
- Brown E, Reinhard B, Thebault R. Which deaths count toward the covid-19 death toll? It depends on the state. Washington Post. April 16, 2020. Accessed July 23, 2020. https://www.washingtonpost.com/investigations/which-deaths-count-toward-the-covid-19-death-toll-it-depends-on-the-state/2020/04/16/bca84ae0-7991-11ea-a130-df573469f094_story.html
- Carlisle M. Here’s what Trump got wrong about America’s COVID-19 death rate. Time. August 4, 2020. Accessed October 14, 2020. https://time.com/5875411/trump-covid-19-death-rate-interview/
- Mortality analyses. Johns Hopkins University & Medicine Coronavirus Resource Center. October 14, 2020. Updated October 29, 2020. Accessed October 14, 2020. https://coronavirus.jhu.edu/data/mortality
- COVID-19 daily case incidence rate maps. Kentucky Cabinet for Health and Family Services. Accessed October 14, 2020. https://chfs.ky.gov/Pages/cv19maps.aspx
- COVID-19 trajectory animations. Pennsylvania Department of Health. Accessed October 14, 2020. https://www.health.pa.gov/topics/disease/coronavirus/Pages/Data-Animations.aspx
- Stolberg SG, Kaplan S, Mervosh S. CDC test counting error leaves epidemiologists ‘really baffled.’ New York Times. May 22, 2020. Updated June 3, 2020. Accessed July 23, 2020. https://www.nytimes.com/2020/05/22/us/politics/coronavirus-tests-cdc.html
The rapid onset of the novel coronavirus disease 2019 (COVID-19) pandemic forced the US healthcare system to scramble to prepare for a health crisis with many unknowns. Early on, it was unclear exactly how the virus was transmitted, how many people would fall ill or how ill they would get, what treatments would be most efficacious, and what resources were needed to care for patients.1 Given the short window the healthcare system had to prepare, many initial and important decisions were made quickly and often at a local level, with limited coordination and standardization across localities and organizations. These decisions included what services could be offered, how best to allocate potentially scarce resources (such as personal protective equipment and ventilators), and how much surge capacity to build.2,3 In short, many of the early decisions about the pandemic were understandably varied, and the lack of standardized metrics to help guide decision-making did not help the situation.
CHALLENGES WITH MANAGING THE PANDEMIC WITHOUT STANDARDIZED METRICS
Unfortunately, as the COVID-19 pandemic continues, there has been insufficient movement toward standardizing definitions for many key measures needed to manage the public health response. Even small differences in definitions can have important implications for decision-making.4 For example, public health officials have recommended communities achieve a positivity rate of 5% or lower for 14 straight days before easing virus-related restrictions.5 In Maryland, two different entities are calculating positivity rates for the state using different methodologies and producing different results, which can have significant public health and economic implications for the state. Johns Hopkins University’s Resource Center calculates the positivity rate by comparing the number of people who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to all people who were tested. This method consistently produces a positivity rate for Maryland above the 5% threshold. In contrast, the state of Maryland calculates the positivity rate by comparing the number of positive tests for SARS-CoV-2 to the number of tests conducted, even if the same person had multiple tests (unless the tests are performed the same day at the same location). This method consistently produces a positivity rate for Maryland below the 5% threshold.6
THE POLITICIZATION OF THE DATA
The lack of standardized definitions leads not only to debate and confusion over what steps to take next, but also opens the door to politicization of pandemic data. This is readily apparent when considering mortality due to COVID-19. For example, different states use different definitions for COVID-19 mortality. Alabama defines COVID-19 mortality by only including patients who tested positive for the SARS-CoV-2 virus and the cause of death was attributed to COVID-19. In contrast, Colorado’s COVID-19 mortality definition includes those patients who are believed to have died of COVID-19, but does not require confirmation of SARS-CoV-2 infection by a positive test.7 Further compounding the challenge, some politicians reference the COVID-19 mortality rate as a comparison of those who died from COVID-19 with those who were sick with COVID-19, reflecting the success rate of treating patients with COVID-19, an area in which the United States has done relatively well compared with other countries. This definition of the mortality rate suits a narrative of successful pandemic management.8 However, many public health officials suggest the COVID-19 mortality rate should be defined by comparing the number of deaths from COVID-19 as a percentage of the population, which reflects the percentage of the population dying from the disease. In this regard, the United States has not done as well relative to other countries.9 These different definitions highlight how the United States lacks a standardized way to compare its performance across states and with other countries, even on a straightforward measure like mortality.
CURRENT METRICS THAT NEED STANDARDIZATION
The lack of clarity on, and politicization of, pandemic data demonstrate the need to take stock of what metrics require standardization to help public health officials and health system leaders manage the pandemic response moving forward. The Table provides examples of currently used metrics that would benefit from better standardization to inform decision-making across a broad range of settings, including public health, hospitals, physician clinics, and nursing homes. For example, a commonly referenced metric during the pandemic has been a moving average of the incidence rate of positive COVID-19 cases in a defined geographic area (eg, a state).10,11 This data point is helpful to healthcare delivery organizations for understanding the change in COVID-19 cases in their cities and states, which can inform planning on whether or not to continue elective surgeries or how many beds need to be kept in reserve status for a potential surge of hospitalizations. But there has not been a consensus around whether the reporting of COVID-19 positive tests should reflect the day the test was performed or the day the test results were available. The day the test results were available can be influenced by lengthy or uneven turnaround times for the results (eg, backlogs in labs) and can paint a false picture of trends with the virus.

As another example, knowing the percentage of the population that has tested positive for COVID-19 can help inform both resource planning and reopening decisions. But there has been variation in whether counts of positive COVID-19 tests should only include antigen tests, or antibody tests as well. This exact question played out when the Centers for Disease Control and Prevention (CDC) made decisions that differed from those of many states about whether to include antibody tests in their publicly announced COVID-19 testing numbers,12 perhaps undermining public confidence in the reported data.
MOVING FORWARD WITH STANDARDIZING DEFINITIONS
To capture currently unstandardized metrics with broad applicability, the United States should form a consensus task force to identify and define metrics and, over time, refine them based on current science and public health priorities. The task force would require a mix of individuals with various skill sets, such as expertise in infectious diseases and epidemiology, healthcare operations, statistics, performance measurement, and public health. The US Department of Health and Human Services is likely the appropriate sponsor, with representation from the National Institutes of Health, the CDC, and the Agency for Healthcare Research and Quality, in partnership with national provider and public health group representatives.
Once standardized definitions for metrics have been agreed upon, the metric definitions will need to be made readily available to the public and healthcare organizations. Standardization will permit collection of electronic health records for quick calculation and review, with an output of dashboards for reporting. It would also prevent every public health and healthcare delivery organization from having to define its own metrics, freeing them up to focus on planning. Several metrics already have standard definitions, and those metrics have proven useful for decision-making. For example, there is agreement that the turnaround time for a SARS-CoV-2 test is measured by the difference in time between when the test was performed and when the test results were available. This standard definition allows for performance comparisons across different laboratories within the same service area and comparisons across different regions of the country. Once the metrics are standardized, public health leaders and healthcare organizations can use variation in performance and outcomes to identify leading indicators for planning.
CONCLUSION
Amid the COVID-19 pandemic, the US healthcare system finds itself in a state of managing uncertainty for a prolonged period of time. The unprecedented nature of this crisis means that best practices will not always be clear. Providing access to clearly defined, standardized metrics will be essential to public health officials and healthcare organization leaders’ ability to manage through this pandemic. The risk of not moving in this direction means forcing leaders to make decisions without the best information available. Good data will be essential to guiding the US healthcare system through this extraordinary crisis.
The rapid onset of the novel coronavirus disease 2019 (COVID-19) pandemic forced the US healthcare system to scramble to prepare for a health crisis with many unknowns. Early on, it was unclear exactly how the virus was transmitted, how many people would fall ill or how ill they would get, what treatments would be most efficacious, and what resources were needed to care for patients.1 Given the short window the healthcare system had to prepare, many initial and important decisions were made quickly and often at a local level, with limited coordination and standardization across localities and organizations. These decisions included what services could be offered, how best to allocate potentially scarce resources (such as personal protective equipment and ventilators), and how much surge capacity to build.2,3 In short, many of the early decisions about the pandemic were understandably varied, and the lack of standardized metrics to help guide decision-making did not help the situation.
CHALLENGES WITH MANAGING THE PANDEMIC WITHOUT STANDARDIZED METRICS
Unfortunately, as the COVID-19 pandemic continues, there has been insufficient movement toward standardizing definitions for many key measures needed to manage the public health response. Even small differences in definitions can have important implications for decision-making.4 For example, public health officials have recommended communities achieve a positivity rate of 5% or lower for 14 straight days before easing virus-related restrictions.5 In Maryland, two different entities are calculating positivity rates for the state using different methodologies and producing different results, which can have significant public health and economic implications for the state. Johns Hopkins University’s Resource Center calculates the positivity rate by comparing the number of people who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to all people who were tested. This method consistently produces a positivity rate for Maryland above the 5% threshold. In contrast, the state of Maryland calculates the positivity rate by comparing the number of positive tests for SARS-CoV-2 to the number of tests conducted, even if the same person had multiple tests (unless the tests are performed the same day at the same location). This method consistently produces a positivity rate for Maryland below the 5% threshold.6
THE POLITICIZATION OF THE DATA
The lack of standardized definitions leads not only to debate and confusion over what steps to take next, but also opens the door to politicization of pandemic data. This is readily apparent when considering mortality due to COVID-19. For example, different states use different definitions for COVID-19 mortality. Alabama defines COVID-19 mortality by only including patients who tested positive for the SARS-CoV-2 virus and the cause of death was attributed to COVID-19. In contrast, Colorado’s COVID-19 mortality definition includes those patients who are believed to have died of COVID-19, but does not require confirmation of SARS-CoV-2 infection by a positive test.7 Further compounding the challenge, some politicians reference the COVID-19 mortality rate as a comparison of those who died from COVID-19 with those who were sick with COVID-19, reflecting the success rate of treating patients with COVID-19, an area in which the United States has done relatively well compared with other countries. This definition of the mortality rate suits a narrative of successful pandemic management.8 However, many public health officials suggest the COVID-19 mortality rate should be defined by comparing the number of deaths from COVID-19 as a percentage of the population, which reflects the percentage of the population dying from the disease. In this regard, the United States has not done as well relative to other countries.9 These different definitions highlight how the United States lacks a standardized way to compare its performance across states and with other countries, even on a straightforward measure like mortality.
CURRENT METRICS THAT NEED STANDARDIZATION
The lack of clarity on, and politicization of, pandemic data demonstrate the need to take stock of what metrics require standardization to help public health officials and health system leaders manage the pandemic response moving forward. The Table provides examples of currently used metrics that would benefit from better standardization to inform decision-making across a broad range of settings, including public health, hospitals, physician clinics, and nursing homes. For example, a commonly referenced metric during the pandemic has been a moving average of the incidence rate of positive COVID-19 cases in a defined geographic area (eg, a state).10,11 This data point is helpful to healthcare delivery organizations for understanding the change in COVID-19 cases in their cities and states, which can inform planning on whether or not to continue elective surgeries or how many beds need to be kept in reserve status for a potential surge of hospitalizations. But there has not been a consensus around whether the reporting of COVID-19 positive tests should reflect the day the test was performed or the day the test results were available. The day the test results were available can be influenced by lengthy or uneven turnaround times for the results (eg, backlogs in labs) and can paint a false picture of trends with the virus.

As another example, knowing the percentage of the population that has tested positive for COVID-19 can help inform both resource planning and reopening decisions. But there has been variation in whether counts of positive COVID-19 tests should only include antigen tests, or antibody tests as well. This exact question played out when the Centers for Disease Control and Prevention (CDC) made decisions that differed from those of many states about whether to include antibody tests in their publicly announced COVID-19 testing numbers,12 perhaps undermining public confidence in the reported data.
MOVING FORWARD WITH STANDARDIZING DEFINITIONS
To capture currently unstandardized metrics with broad applicability, the United States should form a consensus task force to identify and define metrics and, over time, refine them based on current science and public health priorities. The task force would require a mix of individuals with various skill sets, such as expertise in infectious diseases and epidemiology, healthcare operations, statistics, performance measurement, and public health. The US Department of Health and Human Services is likely the appropriate sponsor, with representation from the National Institutes of Health, the CDC, and the Agency for Healthcare Research and Quality, in partnership with national provider and public health group representatives.
Once standardized definitions for metrics have been agreed upon, the metric definitions will need to be made readily available to the public and healthcare organizations. Standardization will permit collection of electronic health records for quick calculation and review, with an output of dashboards for reporting. It would also prevent every public health and healthcare delivery organization from having to define its own metrics, freeing them up to focus on planning. Several metrics already have standard definitions, and those metrics have proven useful for decision-making. For example, there is agreement that the turnaround time for a SARS-CoV-2 test is measured by the difference in time between when the test was performed and when the test results were available. This standard definition allows for performance comparisons across different laboratories within the same service area and comparisons across different regions of the country. Once the metrics are standardized, public health leaders and healthcare organizations can use variation in performance and outcomes to identify leading indicators for planning.
CONCLUSION
Amid the COVID-19 pandemic, the US healthcare system finds itself in a state of managing uncertainty for a prolonged period of time. The unprecedented nature of this crisis means that best practices will not always be clear. Providing access to clearly defined, standardized metrics will be essential to public health officials and healthcare organization leaders’ ability to manage through this pandemic. The risk of not moving in this direction means forcing leaders to make decisions without the best information available. Good data will be essential to guiding the US healthcare system through this extraordinary crisis.
- Weston S, Frieman MB. COVID-19: knowns, unknowns, and questions. mSphere. 2020;5(2):e00203-20. https://doi.org/10.1128/mSphere.00203-20
- Griffin KM, Karas MG, Ivascu NS, Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020;201(11):1337-1344. https://doi.org/10.1164/rccm.202004-1037CP
- De Georgeo MR, De Georgeo JM, Egan TM, et al. Containing SARS-CoV-2 in hospitals facing finite PPE, limited testing, and physical space variability: navigating resource constrained enhanced traffic control bundling. J Microbiol Immunol. 2020;S1684-1182(20)30166-3. https://doi.org/10.1016/j.jmii.2020.07.009
- Fischhoff B. Making decisions in a COVID-19 world. JAMA. 2020;324(2):139-140. https://doi.org/10.1001/jama.2020.10178
- Collins K. Is your state doing enough coronavirus testing? New York Times. October 14, 2020. Updated October 29, 2020. Accessed October 14, 2020. https://www.nytimes.com/interactive/2020/us/coronavirus-testing.html
- Ruiz N. Why is Maryland’s coronavirus positivity rate always lower than what Johns Hopkins says it is — and does it matter? Baltimore Sun. September 10, 2020. Accessed October 14, 2020. https://www.baltimoresun.com/coronavirus/bs-md-maryland-coronavirus-positivity-rate-hopkins-20200817-zoepxdjlxbazdm6kabrjehbemq-story.html
- Brown E, Reinhard B, Thebault R. Which deaths count toward the covid-19 death toll? It depends on the state. Washington Post. April 16, 2020. Accessed July 23, 2020. https://www.washingtonpost.com/investigations/which-deaths-count-toward-the-covid-19-death-toll-it-depends-on-the-state/2020/04/16/bca84ae0-7991-11ea-a130-df573469f094_story.html
- Carlisle M. Here’s what Trump got wrong about America’s COVID-19 death rate. Time. August 4, 2020. Accessed October 14, 2020. https://time.com/5875411/trump-covid-19-death-rate-interview/
- Mortality analyses. Johns Hopkins University & Medicine Coronavirus Resource Center. October 14, 2020. Updated October 29, 2020. Accessed October 14, 2020. https://coronavirus.jhu.edu/data/mortality
- COVID-19 daily case incidence rate maps. Kentucky Cabinet for Health and Family Services. Accessed October 14, 2020. https://chfs.ky.gov/Pages/cv19maps.aspx
- COVID-19 trajectory animations. Pennsylvania Department of Health. Accessed October 14, 2020. https://www.health.pa.gov/topics/disease/coronavirus/Pages/Data-Animations.aspx
- Stolberg SG, Kaplan S, Mervosh S. CDC test counting error leaves epidemiologists ‘really baffled.’ New York Times. May 22, 2020. Updated June 3, 2020. Accessed July 23, 2020. https://www.nytimes.com/2020/05/22/us/politics/coronavirus-tests-cdc.html
- Weston S, Frieman MB. COVID-19: knowns, unknowns, and questions. mSphere. 2020;5(2):e00203-20. https://doi.org/10.1128/mSphere.00203-20
- Griffin KM, Karas MG, Ivascu NS, Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020;201(11):1337-1344. https://doi.org/10.1164/rccm.202004-1037CP
- De Georgeo MR, De Georgeo JM, Egan TM, et al. Containing SARS-CoV-2 in hospitals facing finite PPE, limited testing, and physical space variability: navigating resource constrained enhanced traffic control bundling. J Microbiol Immunol. 2020;S1684-1182(20)30166-3. https://doi.org/10.1016/j.jmii.2020.07.009
- Fischhoff B. Making decisions in a COVID-19 world. JAMA. 2020;324(2):139-140. https://doi.org/10.1001/jama.2020.10178
- Collins K. Is your state doing enough coronavirus testing? New York Times. October 14, 2020. Updated October 29, 2020. Accessed October 14, 2020. https://www.nytimes.com/interactive/2020/us/coronavirus-testing.html
- Ruiz N. Why is Maryland’s coronavirus positivity rate always lower than what Johns Hopkins says it is — and does it matter? Baltimore Sun. September 10, 2020. Accessed October 14, 2020. https://www.baltimoresun.com/coronavirus/bs-md-maryland-coronavirus-positivity-rate-hopkins-20200817-zoepxdjlxbazdm6kabrjehbemq-story.html
- Brown E, Reinhard B, Thebault R. Which deaths count toward the covid-19 death toll? It depends on the state. Washington Post. April 16, 2020. Accessed July 23, 2020. https://www.washingtonpost.com/investigations/which-deaths-count-toward-the-covid-19-death-toll-it-depends-on-the-state/2020/04/16/bca84ae0-7991-11ea-a130-df573469f094_story.html
- Carlisle M. Here’s what Trump got wrong about America’s COVID-19 death rate. Time. August 4, 2020. Accessed October 14, 2020. https://time.com/5875411/trump-covid-19-death-rate-interview/
- Mortality analyses. Johns Hopkins University & Medicine Coronavirus Resource Center. October 14, 2020. Updated October 29, 2020. Accessed October 14, 2020. https://coronavirus.jhu.edu/data/mortality
- COVID-19 daily case incidence rate maps. Kentucky Cabinet for Health and Family Services. Accessed October 14, 2020. https://chfs.ky.gov/Pages/cv19maps.aspx
- COVID-19 trajectory animations. Pennsylvania Department of Health. Accessed October 14, 2020. https://www.health.pa.gov/topics/disease/coronavirus/Pages/Data-Animations.aspx
- Stolberg SG, Kaplan S, Mervosh S. CDC test counting error leaves epidemiologists ‘really baffled.’ New York Times. May 22, 2020. Updated June 3, 2020. Accessed July 23, 2020. https://www.nytimes.com/2020/05/22/us/politics/coronavirus-tests-cdc.html
© 2021 Society of Hospital Medicine
Email: jmaustin@jhmi.edu; Telephone: 832-816-5618; Twitter: @JMatthewAustin.
Opportunities for Improving Population Health in the Post–COVID-19 Era
The novel coronavirus disease of 2019 (COVID-19), caused by the SARS-CoV-2 pathogen, has resulted in a health crisis unlike any other experienced in the past century, with millions of people infected and over one million people dying from COVID-19 worldwide. The pandemic has disproportionately impacted historically marginalized groups, resulting in higher rates of infection, hospitalization, and death in racial/ethnic minority populations, including Black, Hispanic/Latinx, and Native American populations, compared with the White population.1 Statistics suggest that it is not just socioeconomic differences but also structural racism that has played a role in worse health outcomes in minority populations. However, the health inequities uncovered by the pandemic represent an opportunity—a “plastic hour” in which improvements at the population level may be uniquely possible.2 As healthcare providers, we must take advantage of this moment and work toward improving healthcare and increasing health equity in the post–COVID-19 era. We highlight three strategies to guide us toward achieving this goal: (1) prioritizing health system equity and government improvements to population health, (2) fostering community resilience, and (3) promoting equity in economic sustainability.
HEALTH SYSTEM AND GOVERNMENT IMPROVEMENTS TO POPULATION HEALTH
The COVID-19 pandemic has revealed deep-seated structural and medical vulnerabilities in the US healthcare system, with distressing racial/ethnic differences in COVID-19 infection continuing to emerge.3 Despite variation in the availability and quality of these data, disparities observed in COVID-19 have tracked closely with historical inequities in access to healthcare and discrimination within the healthcare system.4 Any approach to addressing these inequities must appreciate the intersection between social and medical vulnerabilities.
It is notable that healthcare systems serving the most vulnerable populations have borne the brunt of the economic toll of COVID-19. Hospitals in socioeconomically challenged areas lost millions of dollars due to the postponement of elective procedures and reallocation of most resources to COVID-related hospital admissions. Many community-based practices, already stretched in caring for medically and socially complex patients, had to shut their doors. These losses have left patients without the support of their network of healthcare and community service organizations—at the same time that many of them have also lost support for food and housing, employer-based health insurance, and in-person schooling and childcare.
The current circumstances due to the COVID-19 pandemic, therefore, require us to reconsider many aspects of both healthcare and the social safety net, including the reliance on financial penalties as a strategy to improve health quality, which ultimately has a disproportionate impact on communities of color.5 The present situation may also allow for the federal, state, and local governments, as well as health systems and payers, to make targeted investments in healthcare, public health, and community programs. For example, an increased healthcare system investment on preventive and primary care will be essential to reducing the chronic risk factors that underlie COVID-19 infection and death. Efforts by payers to reduce economic incentives for unnecessary elective procedures, while simultaneously providing incentives to increase the focus on preventive care, would further stimulate this effort. Although there is controversy over the inclusion of social risk in financial and value-based health system payment models, novel approaches to this problem (eg, consideration of improvement over achievement of static targets) may provide an opportunity for struggling health systems to invest in new strategies for underserved populations. Additionally, investing in a care system that allows racial, language, and cultural concordance between clinicians and patients would both promote a diverse workforce and improve quality of care. Health system equity will also depend upon bold policy advances such as expansion of Medicaid to all states, separation of health insurance from employment, and targeted government and health system investments around social risk (eg, food and housing insecurity). These programs will help vulnerable communities close the gap on disparities in health outcomes that have been so persistent.
Some of these specific concerns were addressed by the Coronavirus Aid, Relief, and Economic Security (CARES) Act that was implemented by the US Congress to address the broad needs of Americans during the acute crisis.6 The CARES Act provided supplementary funding to community health centers and healthcare systems caring for the uninsured. Cash assistance was provided to most US taxpayers along with financial support to those experiencing unemployment through July 31, 2020, measures that have yet to be extended. In addition to the CARES Act, policymakers proposed establishing a COVID-19 Racial and Ethnic Disparities Task Force Act to drive equitable recommendations and provide oversight to the nation’s response to COVID-19.7
While these measures were critical to the immediate pandemic response, future US congressional relief plans are needed to ensure equity remains a tenet of state and federal policy post COVID-19, particularly with respect to social determinants of health. Additional recommendations for federal relief include rent assistance for low-income families, eviction stoppages, and increased funding for short-term food insecurity. With respect to long-term goals, this is the time to address broader injustices, such as lack of affordable housing, lack of a sensible national strategy around food security, and a lack of equitable educational and justice systems. This moment also offers an opportunity to consider the best way to address the impact of centuries of structural racism. If we place equity at the center of policy implementation, we will certainly see downstream health consequences—ones that would begin to address the health disparities present long before the current pandemic.
FOSTERING COMMUNITY RESILIENCE
While national, state, and local responses to COVID-19 are required to bolster population health when we emerge from the COVID-19 crisis, a focus on community resilience is also needed. Community resilience, or the ability to prevent, withstand, and mitigate the stress of a disaster like COVID-19, requires integration of emergency preparedness practices into community disaster programs, with ongoing efforts to mitigate disparities in chronic disease management. A framework for community resilience includes (1) engaging with communities in planning, response, and post–COVID-19 recovery, (2) ensuring communities have access to high quality, culturally concordant health and social services, and (3) developing robust community networks to mobilize individuals, community services, and public health infrastructure in times of emergency.8
After seeing the devastating effects of Hurricane Katrina in 2005, researchers, public health officials, and community leaders founded the Los Angeles County Community Disaster Resilience (LACCDR) project. Through this collaborative effort, the LACCDR established partnerships across 16 communities to foster community resilience during health emergencies against the backdrop of daily chronic stressors such as violence, segregation, poverty, and homelessness.8 A model such as this to improve health systems and public health integration post-COVID will support health provisions and help build trust in communities wherein there is a high distrust of the healthcare system. Engaging with community partners early to ensure that its members have access to basic needs (eg, food, water, shelter), public health needs (eg, timely information, personal protective equipment such as face coverings and cleaning supplies), and affordable testing and vaccination will help prevent disparities that could affect the most vulnerable in future phases of the COVID-19 crisis.
PROMOTING EQUITY AS A SUSTAINABLE ECONOMIC STRATEGY
Over 40 million Americans were seeking unemployment benefits at the peak of the economic repercussions of the COVID-19 pandemic. Unfortunately, low-income, rural, and minority communities disproportionately experienced this economic shock. Given the relationship between wealth and health, successfully achieving equity post-COVID-19 will require deeper financial investments in underserved communities.9 Healthcare organizations, which represent 18% of the United States gross domestic product and employ nearly 9% of all working individuals, are uniquely positioned to have a direct influence on this strategy.
One equity-based strategy is for healthcare institutions to pursue an anchor mission. Anchor missions have increased a health system’s investment in social services, including providing housing and food resources.10 Additionally, hospitals such as Brigham and Women’s, Boston Children’s Hospital, and Bon Secours Health System, are working with a diverse group of entrepreneurs to create jobs and build wealth in underserved communities by employing local and minority-owned businesses to support critical supply chain purchasing decisions regarding food, maintenance, and construction projects.11 These local and inclusive hiring and procurement measures can be bolstered by continued place-based investments by all health system leaders in vulnerable communities.
CONCLUSION
Since the first enslaved Africans were brought to America over 400 years ago, racial and ethnic minorities have experienced struggle and triumph, sadness and joy. The bonds of a long legacy of discrimination are so deep that we must be intentional in our pursuit of equity—during and beyond the COVID-19 pandemic. Placing equity at the center of healthcare system practice and policy implementation, fostering community resilience and emergency preparedness, and prioritizing equity in economic strategic planning are key steps toward addressing the population-level inequities exposed by the COVID-19 pandemic. As the once touted “great equalizer” rages on, we must remember that we are all jointly affected by the distress caused by the novel coronavirus and we also must be more aware than ever of our interconnectedness. We can use this time of pandemic to fight more than ever to ensure that all populations can enjoy just and optimal health.
Acknowledgments
The authors would like to thank Dr Denise Polit for her review of this manuscript.
- Williams DR, Cooper LA. COVID–19 and health equity–a new kind of “herd immunity”. JAMA. 2020;323(24):2478-2480. https://doi.org/10.1001/jama.2020.8051
- Packer G. America’s plastic hour is upon us. The Atlantic. October 2020. Accessed September 28, 2020. https://www.theatlantic.com/magazine/archive/2020/10/make-america-again/615478/
- Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med. 2020;35(10):3097-3099. https://doi.org/10.1007/s11606-020-06081-w
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press (US); 2003. https://doi.org/10.17226/12875
- Zuckerman RB, Joynt Maddox KE, Sheingold SH, Chen LM, Epstein AM. Effect of a hospital-wide measure on the readmissions reduction program. N Engl J Med. 2017;377(16):1551-1558. https://doi.org/10.1056/nejmsa1701791
- Cochrane E. House passes relief for small businesses and aid for hospitals and testing. New York Times. April 23, 2020. Accessed May 21, 2020. https://www.nytimes.com/2020/04/23/us/politics/house-passes-relief-for-small-businesses-and-aid-for-hospitals-and-testing.html
- Harris announces legislation to establish task force to combat racial and ethnic disparities in COVID-19. News release. Kamala D. Harris US Senator for California; April 30, 2020. Accessed May 21, 2020. https://www.harris.senate.gov/news/press-releases/harris-announces-legislation-to-establish-task-force-to-combat-racial-and-ethnic-disparities-in-covid-19
- Chandra A, Williams M, Plough A, et al. Getting actionable about community resilience: the Los Angeles County Community Disaster Resilience project. Am J Public Health. 2013;103(7):1181-1189. https://doi.org/10.2105/ajph.2013.301270
- Rawshani A, Svensson AM, Zethelius B, Eliasson B, Rosengren A, Gudbjörnsdottir S. Association between socioeconomic status and mortality, cardiovascular disease, and cancer in patients with type 2 diabetes. JAMA Intern Med. 2016;176(8):1146-1154. https://doi.org/10.1001/jamainternmed.2016.2940
- Horwitz LI, Chang C, Arcilla HN, Knickman JR. Quantifying health systems’ investment in social determinants of health, by sector, 2017-19. Health Aff (Millwood). 2020;39(2):192-198. https://doi.org/10.1377/hlthaff.2019.01246
- Nanos J. Diverse, locally owned food start-ups make the menus at Harvard, UMass, and BC. Boston Globe. January 24, 2020. Accessed September 28, 2020. https://www.bostonglobe.com/business/2020/01/24/diverse-locally-owned-food-start-ups-make-menus-harvard-umass-and/WwJFew6KVgXu1NyIK1BNqI/story.html
The novel coronavirus disease of 2019 (COVID-19), caused by the SARS-CoV-2 pathogen, has resulted in a health crisis unlike any other experienced in the past century, with millions of people infected and over one million people dying from COVID-19 worldwide. The pandemic has disproportionately impacted historically marginalized groups, resulting in higher rates of infection, hospitalization, and death in racial/ethnic minority populations, including Black, Hispanic/Latinx, and Native American populations, compared with the White population.1 Statistics suggest that it is not just socioeconomic differences but also structural racism that has played a role in worse health outcomes in minority populations. However, the health inequities uncovered by the pandemic represent an opportunity—a “plastic hour” in which improvements at the population level may be uniquely possible.2 As healthcare providers, we must take advantage of this moment and work toward improving healthcare and increasing health equity in the post–COVID-19 era. We highlight three strategies to guide us toward achieving this goal: (1) prioritizing health system equity and government improvements to population health, (2) fostering community resilience, and (3) promoting equity in economic sustainability.
HEALTH SYSTEM AND GOVERNMENT IMPROVEMENTS TO POPULATION HEALTH
The COVID-19 pandemic has revealed deep-seated structural and medical vulnerabilities in the US healthcare system, with distressing racial/ethnic differences in COVID-19 infection continuing to emerge.3 Despite variation in the availability and quality of these data, disparities observed in COVID-19 have tracked closely with historical inequities in access to healthcare and discrimination within the healthcare system.4 Any approach to addressing these inequities must appreciate the intersection between social and medical vulnerabilities.
It is notable that healthcare systems serving the most vulnerable populations have borne the brunt of the economic toll of COVID-19. Hospitals in socioeconomically challenged areas lost millions of dollars due to the postponement of elective procedures and reallocation of most resources to COVID-related hospital admissions. Many community-based practices, already stretched in caring for medically and socially complex patients, had to shut their doors. These losses have left patients without the support of their network of healthcare and community service organizations—at the same time that many of them have also lost support for food and housing, employer-based health insurance, and in-person schooling and childcare.
The current circumstances due to the COVID-19 pandemic, therefore, require us to reconsider many aspects of both healthcare and the social safety net, including the reliance on financial penalties as a strategy to improve health quality, which ultimately has a disproportionate impact on communities of color.5 The present situation may also allow for the federal, state, and local governments, as well as health systems and payers, to make targeted investments in healthcare, public health, and community programs. For example, an increased healthcare system investment on preventive and primary care will be essential to reducing the chronic risk factors that underlie COVID-19 infection and death. Efforts by payers to reduce economic incentives for unnecessary elective procedures, while simultaneously providing incentives to increase the focus on preventive care, would further stimulate this effort. Although there is controversy over the inclusion of social risk in financial and value-based health system payment models, novel approaches to this problem (eg, consideration of improvement over achievement of static targets) may provide an opportunity for struggling health systems to invest in new strategies for underserved populations. Additionally, investing in a care system that allows racial, language, and cultural concordance between clinicians and patients would both promote a diverse workforce and improve quality of care. Health system equity will also depend upon bold policy advances such as expansion of Medicaid to all states, separation of health insurance from employment, and targeted government and health system investments around social risk (eg, food and housing insecurity). These programs will help vulnerable communities close the gap on disparities in health outcomes that have been so persistent.
Some of these specific concerns were addressed by the Coronavirus Aid, Relief, and Economic Security (CARES) Act that was implemented by the US Congress to address the broad needs of Americans during the acute crisis.6 The CARES Act provided supplementary funding to community health centers and healthcare systems caring for the uninsured. Cash assistance was provided to most US taxpayers along with financial support to those experiencing unemployment through July 31, 2020, measures that have yet to be extended. In addition to the CARES Act, policymakers proposed establishing a COVID-19 Racial and Ethnic Disparities Task Force Act to drive equitable recommendations and provide oversight to the nation’s response to COVID-19.7
While these measures were critical to the immediate pandemic response, future US congressional relief plans are needed to ensure equity remains a tenet of state and federal policy post COVID-19, particularly with respect to social determinants of health. Additional recommendations for federal relief include rent assistance for low-income families, eviction stoppages, and increased funding for short-term food insecurity. With respect to long-term goals, this is the time to address broader injustices, such as lack of affordable housing, lack of a sensible national strategy around food security, and a lack of equitable educational and justice systems. This moment also offers an opportunity to consider the best way to address the impact of centuries of structural racism. If we place equity at the center of policy implementation, we will certainly see downstream health consequences—ones that would begin to address the health disparities present long before the current pandemic.
FOSTERING COMMUNITY RESILIENCE
While national, state, and local responses to COVID-19 are required to bolster population health when we emerge from the COVID-19 crisis, a focus on community resilience is also needed. Community resilience, or the ability to prevent, withstand, and mitigate the stress of a disaster like COVID-19, requires integration of emergency preparedness practices into community disaster programs, with ongoing efforts to mitigate disparities in chronic disease management. A framework for community resilience includes (1) engaging with communities in planning, response, and post–COVID-19 recovery, (2) ensuring communities have access to high quality, culturally concordant health and social services, and (3) developing robust community networks to mobilize individuals, community services, and public health infrastructure in times of emergency.8
After seeing the devastating effects of Hurricane Katrina in 2005, researchers, public health officials, and community leaders founded the Los Angeles County Community Disaster Resilience (LACCDR) project. Through this collaborative effort, the LACCDR established partnerships across 16 communities to foster community resilience during health emergencies against the backdrop of daily chronic stressors such as violence, segregation, poverty, and homelessness.8 A model such as this to improve health systems and public health integration post-COVID will support health provisions and help build trust in communities wherein there is a high distrust of the healthcare system. Engaging with community partners early to ensure that its members have access to basic needs (eg, food, water, shelter), public health needs (eg, timely information, personal protective equipment such as face coverings and cleaning supplies), and affordable testing and vaccination will help prevent disparities that could affect the most vulnerable in future phases of the COVID-19 crisis.
PROMOTING EQUITY AS A SUSTAINABLE ECONOMIC STRATEGY
Over 40 million Americans were seeking unemployment benefits at the peak of the economic repercussions of the COVID-19 pandemic. Unfortunately, low-income, rural, and minority communities disproportionately experienced this economic shock. Given the relationship between wealth and health, successfully achieving equity post-COVID-19 will require deeper financial investments in underserved communities.9 Healthcare organizations, which represent 18% of the United States gross domestic product and employ nearly 9% of all working individuals, are uniquely positioned to have a direct influence on this strategy.
One equity-based strategy is for healthcare institutions to pursue an anchor mission. Anchor missions have increased a health system’s investment in social services, including providing housing and food resources.10 Additionally, hospitals such as Brigham and Women’s, Boston Children’s Hospital, and Bon Secours Health System, are working with a diverse group of entrepreneurs to create jobs and build wealth in underserved communities by employing local and minority-owned businesses to support critical supply chain purchasing decisions regarding food, maintenance, and construction projects.11 These local and inclusive hiring and procurement measures can be bolstered by continued place-based investments by all health system leaders in vulnerable communities.
CONCLUSION
Since the first enslaved Africans were brought to America over 400 years ago, racial and ethnic minorities have experienced struggle and triumph, sadness and joy. The bonds of a long legacy of discrimination are so deep that we must be intentional in our pursuit of equity—during and beyond the COVID-19 pandemic. Placing equity at the center of healthcare system practice and policy implementation, fostering community resilience and emergency preparedness, and prioritizing equity in economic strategic planning are key steps toward addressing the population-level inequities exposed by the COVID-19 pandemic. As the once touted “great equalizer” rages on, we must remember that we are all jointly affected by the distress caused by the novel coronavirus and we also must be more aware than ever of our interconnectedness. We can use this time of pandemic to fight more than ever to ensure that all populations can enjoy just and optimal health.
Acknowledgments
The authors would like to thank Dr Denise Polit for her review of this manuscript.
The novel coronavirus disease of 2019 (COVID-19), caused by the SARS-CoV-2 pathogen, has resulted in a health crisis unlike any other experienced in the past century, with millions of people infected and over one million people dying from COVID-19 worldwide. The pandemic has disproportionately impacted historically marginalized groups, resulting in higher rates of infection, hospitalization, and death in racial/ethnic minority populations, including Black, Hispanic/Latinx, and Native American populations, compared with the White population.1 Statistics suggest that it is not just socioeconomic differences but also structural racism that has played a role in worse health outcomes in minority populations. However, the health inequities uncovered by the pandemic represent an opportunity—a “plastic hour” in which improvements at the population level may be uniquely possible.2 As healthcare providers, we must take advantage of this moment and work toward improving healthcare and increasing health equity in the post–COVID-19 era. We highlight three strategies to guide us toward achieving this goal: (1) prioritizing health system equity and government improvements to population health, (2) fostering community resilience, and (3) promoting equity in economic sustainability.
HEALTH SYSTEM AND GOVERNMENT IMPROVEMENTS TO POPULATION HEALTH
The COVID-19 pandemic has revealed deep-seated structural and medical vulnerabilities in the US healthcare system, with distressing racial/ethnic differences in COVID-19 infection continuing to emerge.3 Despite variation in the availability and quality of these data, disparities observed in COVID-19 have tracked closely with historical inequities in access to healthcare and discrimination within the healthcare system.4 Any approach to addressing these inequities must appreciate the intersection between social and medical vulnerabilities.
It is notable that healthcare systems serving the most vulnerable populations have borne the brunt of the economic toll of COVID-19. Hospitals in socioeconomically challenged areas lost millions of dollars due to the postponement of elective procedures and reallocation of most resources to COVID-related hospital admissions. Many community-based practices, already stretched in caring for medically and socially complex patients, had to shut their doors. These losses have left patients without the support of their network of healthcare and community service organizations—at the same time that many of them have also lost support for food and housing, employer-based health insurance, and in-person schooling and childcare.
The current circumstances due to the COVID-19 pandemic, therefore, require us to reconsider many aspects of both healthcare and the social safety net, including the reliance on financial penalties as a strategy to improve health quality, which ultimately has a disproportionate impact on communities of color.5 The present situation may also allow for the federal, state, and local governments, as well as health systems and payers, to make targeted investments in healthcare, public health, and community programs. For example, an increased healthcare system investment on preventive and primary care will be essential to reducing the chronic risk factors that underlie COVID-19 infection and death. Efforts by payers to reduce economic incentives for unnecessary elective procedures, while simultaneously providing incentives to increase the focus on preventive care, would further stimulate this effort. Although there is controversy over the inclusion of social risk in financial and value-based health system payment models, novel approaches to this problem (eg, consideration of improvement over achievement of static targets) may provide an opportunity for struggling health systems to invest in new strategies for underserved populations. Additionally, investing in a care system that allows racial, language, and cultural concordance between clinicians and patients would both promote a diverse workforce and improve quality of care. Health system equity will also depend upon bold policy advances such as expansion of Medicaid to all states, separation of health insurance from employment, and targeted government and health system investments around social risk (eg, food and housing insecurity). These programs will help vulnerable communities close the gap on disparities in health outcomes that have been so persistent.
Some of these specific concerns were addressed by the Coronavirus Aid, Relief, and Economic Security (CARES) Act that was implemented by the US Congress to address the broad needs of Americans during the acute crisis.6 The CARES Act provided supplementary funding to community health centers and healthcare systems caring for the uninsured. Cash assistance was provided to most US taxpayers along with financial support to those experiencing unemployment through July 31, 2020, measures that have yet to be extended. In addition to the CARES Act, policymakers proposed establishing a COVID-19 Racial and Ethnic Disparities Task Force Act to drive equitable recommendations and provide oversight to the nation’s response to COVID-19.7
While these measures were critical to the immediate pandemic response, future US congressional relief plans are needed to ensure equity remains a tenet of state and federal policy post COVID-19, particularly with respect to social determinants of health. Additional recommendations for federal relief include rent assistance for low-income families, eviction stoppages, and increased funding for short-term food insecurity. With respect to long-term goals, this is the time to address broader injustices, such as lack of affordable housing, lack of a sensible national strategy around food security, and a lack of equitable educational and justice systems. This moment also offers an opportunity to consider the best way to address the impact of centuries of structural racism. If we place equity at the center of policy implementation, we will certainly see downstream health consequences—ones that would begin to address the health disparities present long before the current pandemic.
FOSTERING COMMUNITY RESILIENCE
While national, state, and local responses to COVID-19 are required to bolster population health when we emerge from the COVID-19 crisis, a focus on community resilience is also needed. Community resilience, or the ability to prevent, withstand, and mitigate the stress of a disaster like COVID-19, requires integration of emergency preparedness practices into community disaster programs, with ongoing efforts to mitigate disparities in chronic disease management. A framework for community resilience includes (1) engaging with communities in planning, response, and post–COVID-19 recovery, (2) ensuring communities have access to high quality, culturally concordant health and social services, and (3) developing robust community networks to mobilize individuals, community services, and public health infrastructure in times of emergency.8
After seeing the devastating effects of Hurricane Katrina in 2005, researchers, public health officials, and community leaders founded the Los Angeles County Community Disaster Resilience (LACCDR) project. Through this collaborative effort, the LACCDR established partnerships across 16 communities to foster community resilience during health emergencies against the backdrop of daily chronic stressors such as violence, segregation, poverty, and homelessness.8 A model such as this to improve health systems and public health integration post-COVID will support health provisions and help build trust in communities wherein there is a high distrust of the healthcare system. Engaging with community partners early to ensure that its members have access to basic needs (eg, food, water, shelter), public health needs (eg, timely information, personal protective equipment such as face coverings and cleaning supplies), and affordable testing and vaccination will help prevent disparities that could affect the most vulnerable in future phases of the COVID-19 crisis.
PROMOTING EQUITY AS A SUSTAINABLE ECONOMIC STRATEGY
Over 40 million Americans were seeking unemployment benefits at the peak of the economic repercussions of the COVID-19 pandemic. Unfortunately, low-income, rural, and minority communities disproportionately experienced this economic shock. Given the relationship between wealth and health, successfully achieving equity post-COVID-19 will require deeper financial investments in underserved communities.9 Healthcare organizations, which represent 18% of the United States gross domestic product and employ nearly 9% of all working individuals, are uniquely positioned to have a direct influence on this strategy.
One equity-based strategy is for healthcare institutions to pursue an anchor mission. Anchor missions have increased a health system’s investment in social services, including providing housing and food resources.10 Additionally, hospitals such as Brigham and Women’s, Boston Children’s Hospital, and Bon Secours Health System, are working with a diverse group of entrepreneurs to create jobs and build wealth in underserved communities by employing local and minority-owned businesses to support critical supply chain purchasing decisions regarding food, maintenance, and construction projects.11 These local and inclusive hiring and procurement measures can be bolstered by continued place-based investments by all health system leaders in vulnerable communities.
CONCLUSION
Since the first enslaved Africans were brought to America over 400 years ago, racial and ethnic minorities have experienced struggle and triumph, sadness and joy. The bonds of a long legacy of discrimination are so deep that we must be intentional in our pursuit of equity—during and beyond the COVID-19 pandemic. Placing equity at the center of healthcare system practice and policy implementation, fostering community resilience and emergency preparedness, and prioritizing equity in economic strategic planning are key steps toward addressing the population-level inequities exposed by the COVID-19 pandemic. As the once touted “great equalizer” rages on, we must remember that we are all jointly affected by the distress caused by the novel coronavirus and we also must be more aware than ever of our interconnectedness. We can use this time of pandemic to fight more than ever to ensure that all populations can enjoy just and optimal health.
Acknowledgments
The authors would like to thank Dr Denise Polit for her review of this manuscript.
- Williams DR, Cooper LA. COVID–19 and health equity–a new kind of “herd immunity”. JAMA. 2020;323(24):2478-2480. https://doi.org/10.1001/jama.2020.8051
- Packer G. America’s plastic hour is upon us. The Atlantic. October 2020. Accessed September 28, 2020. https://www.theatlantic.com/magazine/archive/2020/10/make-america-again/615478/
- Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med. 2020;35(10):3097-3099. https://doi.org/10.1007/s11606-020-06081-w
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press (US); 2003. https://doi.org/10.17226/12875
- Zuckerman RB, Joynt Maddox KE, Sheingold SH, Chen LM, Epstein AM. Effect of a hospital-wide measure on the readmissions reduction program. N Engl J Med. 2017;377(16):1551-1558. https://doi.org/10.1056/nejmsa1701791
- Cochrane E. House passes relief for small businesses and aid for hospitals and testing. New York Times. April 23, 2020. Accessed May 21, 2020. https://www.nytimes.com/2020/04/23/us/politics/house-passes-relief-for-small-businesses-and-aid-for-hospitals-and-testing.html
- Harris announces legislation to establish task force to combat racial and ethnic disparities in COVID-19. News release. Kamala D. Harris US Senator for California; April 30, 2020. Accessed May 21, 2020. https://www.harris.senate.gov/news/press-releases/harris-announces-legislation-to-establish-task-force-to-combat-racial-and-ethnic-disparities-in-covid-19
- Chandra A, Williams M, Plough A, et al. Getting actionable about community resilience: the Los Angeles County Community Disaster Resilience project. Am J Public Health. 2013;103(7):1181-1189. https://doi.org/10.2105/ajph.2013.301270
- Rawshani A, Svensson AM, Zethelius B, Eliasson B, Rosengren A, Gudbjörnsdottir S. Association between socioeconomic status and mortality, cardiovascular disease, and cancer in patients with type 2 diabetes. JAMA Intern Med. 2016;176(8):1146-1154. https://doi.org/10.1001/jamainternmed.2016.2940
- Horwitz LI, Chang C, Arcilla HN, Knickman JR. Quantifying health systems’ investment in social determinants of health, by sector, 2017-19. Health Aff (Millwood). 2020;39(2):192-198. https://doi.org/10.1377/hlthaff.2019.01246
- Nanos J. Diverse, locally owned food start-ups make the menus at Harvard, UMass, and BC. Boston Globe. January 24, 2020. Accessed September 28, 2020. https://www.bostonglobe.com/business/2020/01/24/diverse-locally-owned-food-start-ups-make-menus-harvard-umass-and/WwJFew6KVgXu1NyIK1BNqI/story.html
- Williams DR, Cooper LA. COVID–19 and health equity–a new kind of “herd immunity”. JAMA. 2020;323(24):2478-2480. https://doi.org/10.1001/jama.2020.8051
- Packer G. America’s plastic hour is upon us. The Atlantic. October 2020. Accessed September 28, 2020. https://www.theatlantic.com/magazine/archive/2020/10/make-america-again/615478/
- Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med. 2020;35(10):3097-3099. https://doi.org/10.1007/s11606-020-06081-w
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press (US); 2003. https://doi.org/10.17226/12875
- Zuckerman RB, Joynt Maddox KE, Sheingold SH, Chen LM, Epstein AM. Effect of a hospital-wide measure on the readmissions reduction program. N Engl J Med. 2017;377(16):1551-1558. https://doi.org/10.1056/nejmsa1701791
- Cochrane E. House passes relief for small businesses and aid for hospitals and testing. New York Times. April 23, 2020. Accessed May 21, 2020. https://www.nytimes.com/2020/04/23/us/politics/house-passes-relief-for-small-businesses-and-aid-for-hospitals-and-testing.html
- Harris announces legislation to establish task force to combat racial and ethnic disparities in COVID-19. News release. Kamala D. Harris US Senator for California; April 30, 2020. Accessed May 21, 2020. https://www.harris.senate.gov/news/press-releases/harris-announces-legislation-to-establish-task-force-to-combat-racial-and-ethnic-disparities-in-covid-19
- Chandra A, Williams M, Plough A, et al. Getting actionable about community resilience: the Los Angeles County Community Disaster Resilience project. Am J Public Health. 2013;103(7):1181-1189. https://doi.org/10.2105/ajph.2013.301270
- Rawshani A, Svensson AM, Zethelius B, Eliasson B, Rosengren A, Gudbjörnsdottir S. Association between socioeconomic status and mortality, cardiovascular disease, and cancer in patients with type 2 diabetes. JAMA Intern Med. 2016;176(8):1146-1154. https://doi.org/10.1001/jamainternmed.2016.2940
- Horwitz LI, Chang C, Arcilla HN, Knickman JR. Quantifying health systems’ investment in social determinants of health, by sector, 2017-19. Health Aff (Millwood). 2020;39(2):192-198. https://doi.org/10.1377/hlthaff.2019.01246
- Nanos J. Diverse, locally owned food start-ups make the menus at Harvard, UMass, and BC. Boston Globe. January 24, 2020. Accessed September 28, 2020. https://www.bostonglobe.com/business/2020/01/24/diverse-locally-owned-food-start-ups-make-menus-harvard-umass-and/WwJFew6KVgXu1NyIK1BNqI/story.html
© 2021 Society of Hospital Medicine
Email: uessien@pitt.edu.
Language Barriers, Equity, and COVID-19: The Impact of a Novel Spanish Language Care Group
Our knowledge of how natural catastrophes affect vulnerable populations should have helped us anticipate how coronavirus disease 2019 (COVID-19) would strike the United States. This disaster has followed the well-heeled path of its predecessors, predictably bending to the influence of social determinants of health,1 structural inequality, and limited access to healthcare. Communities of color were hit early, hit hard,2 and yet again, became our nation’s canary in the coal mine. Hospitals across the country have had a front seat to this novel coronavirus’ disproportionate effect across the diverse communities we serve. Several of the cities and neighborhoods adjacent to our hospital are home to the area’s highest density of limited English proficient (LEP), immigrant, Spanish-speaking individuals.3,4 Our neighbors in these areas are more likely to have lower socioeconomic status, live in crowded housing, work in service industries deemed to be essential, and depend on shared and mass transit to get to work.5,6 As became clear, many in these communities could not work from home, get groceries delivered, or adequately social distance; these were pandemic luxuries afforded to other, more affluent areas.7
THE COVID-19 SURGE
In the weeks between March 25, 2020, and April 13, 2020, the Massachusetts General Hospital in Boston entered a COVID-19 surge now familiar to hospitals across the world. Like our peer institutions, we made broad and creative structural changes to inpatient services to meet the surge and we followed the numbers with anticipation. Over that 2-week period, we indeed saw the COVID-19–positive inpatient population swell as we had feared. However, with each page from the Emergency Department a disturbing trend was borne out:
ADMIT: 53-year-old Spanish-speaker with tachypnea.
ADMIT: 57-year-old factory worker, Spanish-speaking, sick for 10 days, intubated in the ED.
ADMIT: 58-year-old bodega employee, Spanish-speaking, febrile and breathless.
It buzzed across the medical floors and intensive care units: “What is going on in our Spanish speaking neighborhoods?” In fact, our shared anecdotal view was soon confirmed by admission statistics. Over the interval that our total COVID-19 census alarmingly rose sevenfold, the LEP Spanish-speaking census traced a striking curve, increasing nearly 20 times, to constitute over 40% of all COVID-19 patients (Figure). These communities were bearing a disproportionate share of the local burden of the pandemic.
There is consensus in the health care community about the impact of LEP on quality of care, and how, if unaddressed, significant disparities emerge.8 In fact, there is a broadly accepted professional,9 ethical,10 and legal11,12 imperative for hospitals to address the language needs of LEP patients using interpreter services. However, clinicians often feel forced to rely on their own limited language skills to bridge the communication divide, especially in time-limited, critical situations.13 And regrettably, the highly problematic strategy of relying upon family members to aid with communication is still commonly used. The ideal approach, however, is to invest in developing care models that recognize language as an asset and leverage the skills of multilingual clinicians who care for patients in their own language, in a culturally and linguistically competent way.14 It is not surprising that, when clinicians and patients communicate in the same language, there is demonstrably improved adherence to treatment plans,15 increased patient insight into health conditions,16 and improved delivery of health education.17
FORMATION OF THE SPANISH LANGUAGE CARE GROUP
COVID-19 created unique challenges to our interpreter services. The overwhelming number of LEP Spanish-speaking patients made it difficult for our existing interpreter staff to provide in-person translation. Virtual interpreter services were always available; however, using telephone interpretation in full personal protective equipment with patients who were already isolated and dealing with a scary diagnosis did not feel adequate to the need. In response to what we were seeing, on April 13, 2020, the idea emerged from the Chief Equity and Inclusion Officer, a native Spanish speaker, to assemble a team of native Spanish-speaking doctors, deploying them to assist in the clinical care of those LEP Spanish-speaking patients admitted with COVID-19. Out of this idea grew a creative and novel care delivery model, fashioned to prioritize culturally and linguistically competent care. It was deployed a few days later as the Spanish Language Care Group (SLCG). The belief was that this group’s members were uniquely equipped to work directly with existing frontline teams on the floors, intensive care units and the emergency department. As doctors, they were able to act as extensions of those teams, independently carrying out patient-facing clinical tasks, in Spanish, on an ad hoc basis. They took on history taking, procedural consents, clinical updates, discharge instructions, serious illness conversations and family meetings. They comforted and educated the frightened, connected with families, and unearthed relevant patient history that would have otherwise gone unnoticed. In many cases the SLCG member was the main figure communicating with patients as their clinical status deteriorated, as they were intubated, as they faced their worst fears about COVID-19.
At the time the group was assembled, each SLCG physician was verified as Qualified Bilingual Staff, already clinically credentialled at the hospital, and ready to volunteer to meet the need on the medicine COVID surge services. They practiced in virtually every division and department, including Anesthesia, Cardiology, Dermatology, Emergency Medicine, Gastroenterology, General Medicine, Neurology, Pediatrics, Psychiatry, and Radiology. With the assistance of leadership in Hospital Medicine, this team was rapidly deployed to inpatient teams to assist with the clinical care of COVID-19 patients. In total, 51 physicians—representing 14 countries of origin—participated in the effort, and their titles ranged from intern to full professor. Fourteen of them were formally deployed in the COVID surge context with approval of their departmental and divisional leadership. With such a robust response and institutional support, the SLCG was able to provide 24-hour coverage in support of the Medicine teams. During the peak of this hospital’s COVID surge, seven SLCG members were deployed daily 7
For those patients in their most vulnerable moments, the impact of the SLCG’s work is hard to overestimate, and it has also been measured by overwhelmingly positive feedback from surge care teams: “The quality of care we provided today would have been impossible without [the SLCG]. I’m so grateful and was nearly moved to tears realizing how stunted our relationships with these patients have been due to language barrier.” Another team said that the SLCG doctor was able to “care for the patient in the same way I would have if I could speak Spanish” and “it is like day and night.”After the spring 2020 surge of COVID-19, procedural work resumed, so the SLCG doctors—many of whose usual clinical activity was suspended by the pandemic—returned to their proper perch on the organization chart. But as they reflect on their experience with the group, they report that it stirred a strong and very personal sense of purpose and vocation. Should a subsequent surge of COVID-19 occur, they are committed to building on the foundation that they have laid.
DEPLOYING A LANGUAGE CARE GROUP TEAM
For hospitals that may consider deploying a team such as the SLCG, we can offer a number of concrete actions and policy recommendations. First, in preparation for the COVID surge we identified hospital clinicians with multilingual skills through the deployment of a multilingual registry. Such a registry is critical to understanding which clinicians among existing staff have these skills and who can be approached to join the team. Second, the inpatient medicine surge leadership team at our hospital, immediately recognizing the importance of this effort, developed a staffing strategy to integrate the SLCG into the institutional surge response. The benefit that the team offers needs to be made clear to those at the highest levels of operations and planning. Third, a strong and well-established Center for Diversity and Inclusion, and its leadership, helped facilitate our group’s staffing and organization. For hospitals looking to embrace the strength that their diversity-oriented recruitment efforts have afforded them, we recommend creating a centralized space in which professional relationships can grow and deepen, diverse perspectives can be explored, and embedded cultural and language skills can be championed.
The US healthcare system has much to learn from this phase of the COVID-19 era. Our experience with the Spanish Language Care Group has highlighted the value of language-concordant care, the power of cultural and linguistic competency, and the resiliency that diversity brings to a hospital’s professional staff. Our urgent response to COVID-19 has unroofed a long-simmering challenge: the detriment to care that arises when language becomes an obstacle. We are bringing a new focus to this issue and learning to view it through an equity lens. This is lending new energy to an ongoing conversation about how this hospital thinks about diversity, equity, and healthcare access in these pandemic times and into the hoped-for beyond.
Acknowledgments
The authors wish to express their profound gratitude to the members of the Spanish Language Care Group who brought such humanity and professionalism to the care of our patients during a uniquely vulnerable time.
- Social Determinants of Health. World Health Organization. Accessed November 10, 2020. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1
- Buchanan L, Patel JK, Rosenthal BM, Singhvi A. A month of coronavirus in New York City: see the hardest-hit areas. New York Times. April 1, 2020. Accessed November 10, 2020. https://www.nytimes.com/interactive/2020/04/01/nyregion/nyc-coronavirus-cases-map.html
- QuickFacts: Chelsea city, Massachusetts. United States Census Bureau. Accessed November 10, 2020. https://www.census.gov/quickfacts/chelseacitymassachusetts
- Boston by the Numbers 2018. Research Division, Boston Planning & Development Agency. September 2018. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/3e8bfacf-27c1-4b55-adee-29c5d79f4a38
- Demographic Profile of Adult Limited English Speakers in Massachusetts. Research Division, Boston Planning & Development Agency. February 2019. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/dfe1117a-af16-4257-b0f5-1d95dbd575fe
- Boston in Context: Neighborhoods 2012-2016 American Community Survey. Research Division, Boston Planning & Development Agency. March 2018. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/55f2d86f-eccf-4f68-8d8d-c631fefb0161
- Canipe C. The social distancing of America. Reuters Graphics. April 2, 2020. Accessed November 10, 2020. https://graphics.reuters.com/HEALTH-CORONAVIRUS/USA/qmypmkmwpra/
- Betancourt J, Green AR, Carrillo JE, Park ER. Cultural competency and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
- Racial and Ethnic Disparities in Health Care, Updated 2010. American College of Physicians; 2010. Accessed November 10, 2020. https://www.acponline.org/system/files/documents/advocacy/current_policy_papers/assets/racial_disparities.pdf
- 1.1.3 Patient rights. In: Chapter 1: Opinions on Patient-Physician Relationships. Code of Medical Ethics. American Medical Association; 2016. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf
- Title VI of the Civil Rights Act of 1964, as amended, 42 USC §2000d et seq. July 2, 1964.
- Patient Protection and Affordable Care Act of 2010, Pub L No. 111-148, 124 Stat 119 (2010) §1557.
- Regenstein M, Andres E, Wynia MK. Appropriate use of non-English-language skills in clinical care. JAMA. 2013;309(2):145-146. https://doi.org/10.1001/jama.2012.116984
- Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007;22(Suppl) 2:324-330.
- Manson A. Language concordance as a determinant of patient compliance and emergency room use in patients with asthma. Med Care. 1988;26(12):1119-1128. https://doi.org/10.1097/00005650-198812000-00003
- Seijo R, Gomez H, Garcia M, Shelton D. Acculturation, access to care, and use of preventive services by Hispanics: findings from HANES 1982-84. Am J Public Health. 1991;80(suppl):11-19
- Shapiro J, Saltzer EB. Cross-cultural aspects of physician-patient communications patterns. Urban Health. 1981;10(10):10-15.
Our knowledge of how natural catastrophes affect vulnerable populations should have helped us anticipate how coronavirus disease 2019 (COVID-19) would strike the United States. This disaster has followed the well-heeled path of its predecessors, predictably bending to the influence of social determinants of health,1 structural inequality, and limited access to healthcare. Communities of color were hit early, hit hard,2 and yet again, became our nation’s canary in the coal mine. Hospitals across the country have had a front seat to this novel coronavirus’ disproportionate effect across the diverse communities we serve. Several of the cities and neighborhoods adjacent to our hospital are home to the area’s highest density of limited English proficient (LEP), immigrant, Spanish-speaking individuals.3,4 Our neighbors in these areas are more likely to have lower socioeconomic status, live in crowded housing, work in service industries deemed to be essential, and depend on shared and mass transit to get to work.5,6 As became clear, many in these communities could not work from home, get groceries delivered, or adequately social distance; these were pandemic luxuries afforded to other, more affluent areas.7
THE COVID-19 SURGE
In the weeks between March 25, 2020, and April 13, 2020, the Massachusetts General Hospital in Boston entered a COVID-19 surge now familiar to hospitals across the world. Like our peer institutions, we made broad and creative structural changes to inpatient services to meet the surge and we followed the numbers with anticipation. Over that 2-week period, we indeed saw the COVID-19–positive inpatient population swell as we had feared. However, with each page from the Emergency Department a disturbing trend was borne out:
ADMIT: 53-year-old Spanish-speaker with tachypnea.
ADMIT: 57-year-old factory worker, Spanish-speaking, sick for 10 days, intubated in the ED.
ADMIT: 58-year-old bodega employee, Spanish-speaking, febrile and breathless.
It buzzed across the medical floors and intensive care units: “What is going on in our Spanish speaking neighborhoods?” In fact, our shared anecdotal view was soon confirmed by admission statistics. Over the interval that our total COVID-19 census alarmingly rose sevenfold, the LEP Spanish-speaking census traced a striking curve, increasing nearly 20 times, to constitute over 40% of all COVID-19 patients (Figure). These communities were bearing a disproportionate share of the local burden of the pandemic.

There is consensus in the health care community about the impact of LEP on quality of care, and how, if unaddressed, significant disparities emerge.8 In fact, there is a broadly accepted professional,9 ethical,10 and legal11,12 imperative for hospitals to address the language needs of LEP patients using interpreter services. However, clinicians often feel forced to rely on their own limited language skills to bridge the communication divide, especially in time-limited, critical situations.13 And regrettably, the highly problematic strategy of relying upon family members to aid with communication is still commonly used. The ideal approach, however, is to invest in developing care models that recognize language as an asset and leverage the skills of multilingual clinicians who care for patients in their own language, in a culturally and linguistically competent way.14 It is not surprising that, when clinicians and patients communicate in the same language, there is demonstrably improved adherence to treatment plans,15 increased patient insight into health conditions,16 and improved delivery of health education.17
FORMATION OF THE SPANISH LANGUAGE CARE GROUP
COVID-19 created unique challenges to our interpreter services. The overwhelming number of LEP Spanish-speaking patients made it difficult for our existing interpreter staff to provide in-person translation. Virtual interpreter services were always available; however, using telephone interpretation in full personal protective equipment with patients who were already isolated and dealing with a scary diagnosis did not feel adequate to the need. In response to what we were seeing, on April 13, 2020, the idea emerged from the Chief Equity and Inclusion Officer, a native Spanish speaker, to assemble a team of native Spanish-speaking doctors, deploying them to assist in the clinical care of those LEP Spanish-speaking patients admitted with COVID-19. Out of this idea grew a creative and novel care delivery model, fashioned to prioritize culturally and linguistically competent care. It was deployed a few days later as the Spanish Language Care Group (SLCG). The belief was that this group’s members were uniquely equipped to work directly with existing frontline teams on the floors, intensive care units and the emergency department. As doctors, they were able to act as extensions of those teams, independently carrying out patient-facing clinical tasks, in Spanish, on an ad hoc basis. They took on history taking, procedural consents, clinical updates, discharge instructions, serious illness conversations and family meetings. They comforted and educated the frightened, connected with families, and unearthed relevant patient history that would have otherwise gone unnoticed. In many cases the SLCG member was the main figure communicating with patients as their clinical status deteriorated, as they were intubated, as they faced their worst fears about COVID-19.
At the time the group was assembled, each SLCG physician was verified as Qualified Bilingual Staff, already clinically credentialled at the hospital, and ready to volunteer to meet the need on the medicine COVID surge services. They practiced in virtually every division and department, including Anesthesia, Cardiology, Dermatology, Emergency Medicine, Gastroenterology, General Medicine, Neurology, Pediatrics, Psychiatry, and Radiology. With the assistance of leadership in Hospital Medicine, this team was rapidly deployed to inpatient teams to assist with the clinical care of COVID-19 patients. In total, 51 physicians—representing 14 countries of origin—participated in the effort, and their titles ranged from intern to full professor. Fourteen of them were formally deployed in the COVID surge context with approval of their departmental and divisional leadership. With such a robust response and institutional support, the SLCG was able to provide 24-hour coverage in support of the Medicine teams. During the peak of this hospital’s COVID surge, seven SLCG members were deployed daily 7
For those patients in their most vulnerable moments, the impact of the SLCG’s work is hard to overestimate, and it has also been measured by overwhelmingly positive feedback from surge care teams: “The quality of care we provided today would have been impossible without [the SLCG]. I’m so grateful and was nearly moved to tears realizing how stunted our relationships with these patients have been due to language barrier.” Another team said that the SLCG doctor was able to “care for the patient in the same way I would have if I could speak Spanish” and “it is like day and night.”After the spring 2020 surge of COVID-19, procedural work resumed, so the SLCG doctors—many of whose usual clinical activity was suspended by the pandemic—returned to their proper perch on the organization chart. But as they reflect on their experience with the group, they report that it stirred a strong and very personal sense of purpose and vocation. Should a subsequent surge of COVID-19 occur, they are committed to building on the foundation that they have laid.
DEPLOYING A LANGUAGE CARE GROUP TEAM
For hospitals that may consider deploying a team such as the SLCG, we can offer a number of concrete actions and policy recommendations. First, in preparation for the COVID surge we identified hospital clinicians with multilingual skills through the deployment of a multilingual registry. Such a registry is critical to understanding which clinicians among existing staff have these skills and who can be approached to join the team. Second, the inpatient medicine surge leadership team at our hospital, immediately recognizing the importance of this effort, developed a staffing strategy to integrate the SLCG into the institutional surge response. The benefit that the team offers needs to be made clear to those at the highest levels of operations and planning. Third, a strong and well-established Center for Diversity and Inclusion, and its leadership, helped facilitate our group’s staffing and organization. For hospitals looking to embrace the strength that their diversity-oriented recruitment efforts have afforded them, we recommend creating a centralized space in which professional relationships can grow and deepen, diverse perspectives can be explored, and embedded cultural and language skills can be championed.
The US healthcare system has much to learn from this phase of the COVID-19 era. Our experience with the Spanish Language Care Group has highlighted the value of language-concordant care, the power of cultural and linguistic competency, and the resiliency that diversity brings to a hospital’s professional staff. Our urgent response to COVID-19 has unroofed a long-simmering challenge: the detriment to care that arises when language becomes an obstacle. We are bringing a new focus to this issue and learning to view it through an equity lens. This is lending new energy to an ongoing conversation about how this hospital thinks about diversity, equity, and healthcare access in these pandemic times and into the hoped-for beyond.
Acknowledgments
The authors wish to express their profound gratitude to the members of the Spanish Language Care Group who brought such humanity and professionalism to the care of our patients during a uniquely vulnerable time.
Our knowledge of how natural catastrophes affect vulnerable populations should have helped us anticipate how coronavirus disease 2019 (COVID-19) would strike the United States. This disaster has followed the well-heeled path of its predecessors, predictably bending to the influence of social determinants of health,1 structural inequality, and limited access to healthcare. Communities of color were hit early, hit hard,2 and yet again, became our nation’s canary in the coal mine. Hospitals across the country have had a front seat to this novel coronavirus’ disproportionate effect across the diverse communities we serve. Several of the cities and neighborhoods adjacent to our hospital are home to the area’s highest density of limited English proficient (LEP), immigrant, Spanish-speaking individuals.3,4 Our neighbors in these areas are more likely to have lower socioeconomic status, live in crowded housing, work in service industries deemed to be essential, and depend on shared and mass transit to get to work.5,6 As became clear, many in these communities could not work from home, get groceries delivered, or adequately social distance; these were pandemic luxuries afforded to other, more affluent areas.7
THE COVID-19 SURGE
In the weeks between March 25, 2020, and April 13, 2020, the Massachusetts General Hospital in Boston entered a COVID-19 surge now familiar to hospitals across the world. Like our peer institutions, we made broad and creative structural changes to inpatient services to meet the surge and we followed the numbers with anticipation. Over that 2-week period, we indeed saw the COVID-19–positive inpatient population swell as we had feared. However, with each page from the Emergency Department a disturbing trend was borne out:
ADMIT: 53-year-old Spanish-speaker with tachypnea.
ADMIT: 57-year-old factory worker, Spanish-speaking, sick for 10 days, intubated in the ED.
ADMIT: 58-year-old bodega employee, Spanish-speaking, febrile and breathless.
It buzzed across the medical floors and intensive care units: “What is going on in our Spanish speaking neighborhoods?” In fact, our shared anecdotal view was soon confirmed by admission statistics. Over the interval that our total COVID-19 census alarmingly rose sevenfold, the LEP Spanish-speaking census traced a striking curve, increasing nearly 20 times, to constitute over 40% of all COVID-19 patients (Figure). These communities were bearing a disproportionate share of the local burden of the pandemic.

There is consensus in the health care community about the impact of LEP on quality of care, and how, if unaddressed, significant disparities emerge.8 In fact, there is a broadly accepted professional,9 ethical,10 and legal11,12 imperative for hospitals to address the language needs of LEP patients using interpreter services. However, clinicians often feel forced to rely on their own limited language skills to bridge the communication divide, especially in time-limited, critical situations.13 And regrettably, the highly problematic strategy of relying upon family members to aid with communication is still commonly used. The ideal approach, however, is to invest in developing care models that recognize language as an asset and leverage the skills of multilingual clinicians who care for patients in their own language, in a culturally and linguistically competent way.14 It is not surprising that, when clinicians and patients communicate in the same language, there is demonstrably improved adherence to treatment plans,15 increased patient insight into health conditions,16 and improved delivery of health education.17
FORMATION OF THE SPANISH LANGUAGE CARE GROUP
COVID-19 created unique challenges to our interpreter services. The overwhelming number of LEP Spanish-speaking patients made it difficult for our existing interpreter staff to provide in-person translation. Virtual interpreter services were always available; however, using telephone interpretation in full personal protective equipment with patients who were already isolated and dealing with a scary diagnosis did not feel adequate to the need. In response to what we were seeing, on April 13, 2020, the idea emerged from the Chief Equity and Inclusion Officer, a native Spanish speaker, to assemble a team of native Spanish-speaking doctors, deploying them to assist in the clinical care of those LEP Spanish-speaking patients admitted with COVID-19. Out of this idea grew a creative and novel care delivery model, fashioned to prioritize culturally and linguistically competent care. It was deployed a few days later as the Spanish Language Care Group (SLCG). The belief was that this group’s members were uniquely equipped to work directly with existing frontline teams on the floors, intensive care units and the emergency department. As doctors, they were able to act as extensions of those teams, independently carrying out patient-facing clinical tasks, in Spanish, on an ad hoc basis. They took on history taking, procedural consents, clinical updates, discharge instructions, serious illness conversations and family meetings. They comforted and educated the frightened, connected with families, and unearthed relevant patient history that would have otherwise gone unnoticed. In many cases the SLCG member was the main figure communicating with patients as their clinical status deteriorated, as they were intubated, as they faced their worst fears about COVID-19.
At the time the group was assembled, each SLCG physician was verified as Qualified Bilingual Staff, already clinically credentialled at the hospital, and ready to volunteer to meet the need on the medicine COVID surge services. They practiced in virtually every division and department, including Anesthesia, Cardiology, Dermatology, Emergency Medicine, Gastroenterology, General Medicine, Neurology, Pediatrics, Psychiatry, and Radiology. With the assistance of leadership in Hospital Medicine, this team was rapidly deployed to inpatient teams to assist with the clinical care of COVID-19 patients. In total, 51 physicians—representing 14 countries of origin—participated in the effort, and their titles ranged from intern to full professor. Fourteen of them were formally deployed in the COVID surge context with approval of their departmental and divisional leadership. With such a robust response and institutional support, the SLCG was able to provide 24-hour coverage in support of the Medicine teams. During the peak of this hospital’s COVID surge, seven SLCG members were deployed daily 7
For those patients in their most vulnerable moments, the impact of the SLCG’s work is hard to overestimate, and it has also been measured by overwhelmingly positive feedback from surge care teams: “The quality of care we provided today would have been impossible without [the SLCG]. I’m so grateful and was nearly moved to tears realizing how stunted our relationships with these patients have been due to language barrier.” Another team said that the SLCG doctor was able to “care for the patient in the same way I would have if I could speak Spanish” and “it is like day and night.”After the spring 2020 surge of COVID-19, procedural work resumed, so the SLCG doctors—many of whose usual clinical activity was suspended by the pandemic—returned to their proper perch on the organization chart. But as they reflect on their experience with the group, they report that it stirred a strong and very personal sense of purpose and vocation. Should a subsequent surge of COVID-19 occur, they are committed to building on the foundation that they have laid.
DEPLOYING A LANGUAGE CARE GROUP TEAM
For hospitals that may consider deploying a team such as the SLCG, we can offer a number of concrete actions and policy recommendations. First, in preparation for the COVID surge we identified hospital clinicians with multilingual skills through the deployment of a multilingual registry. Such a registry is critical to understanding which clinicians among existing staff have these skills and who can be approached to join the team. Second, the inpatient medicine surge leadership team at our hospital, immediately recognizing the importance of this effort, developed a staffing strategy to integrate the SLCG into the institutional surge response. The benefit that the team offers needs to be made clear to those at the highest levels of operations and planning. Third, a strong and well-established Center for Diversity and Inclusion, and its leadership, helped facilitate our group’s staffing and organization. For hospitals looking to embrace the strength that their diversity-oriented recruitment efforts have afforded them, we recommend creating a centralized space in which professional relationships can grow and deepen, diverse perspectives can be explored, and embedded cultural and language skills can be championed.
The US healthcare system has much to learn from this phase of the COVID-19 era. Our experience with the Spanish Language Care Group has highlighted the value of language-concordant care, the power of cultural and linguistic competency, and the resiliency that diversity brings to a hospital’s professional staff. Our urgent response to COVID-19 has unroofed a long-simmering challenge: the detriment to care that arises when language becomes an obstacle. We are bringing a new focus to this issue and learning to view it through an equity lens. This is lending new energy to an ongoing conversation about how this hospital thinks about diversity, equity, and healthcare access in these pandemic times and into the hoped-for beyond.
Acknowledgments
The authors wish to express their profound gratitude to the members of the Spanish Language Care Group who brought such humanity and professionalism to the care of our patients during a uniquely vulnerable time.
- Social Determinants of Health. World Health Organization. Accessed November 10, 2020. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1
- Buchanan L, Patel JK, Rosenthal BM, Singhvi A. A month of coronavirus in New York City: see the hardest-hit areas. New York Times. April 1, 2020. Accessed November 10, 2020. https://www.nytimes.com/interactive/2020/04/01/nyregion/nyc-coronavirus-cases-map.html
- QuickFacts: Chelsea city, Massachusetts. United States Census Bureau. Accessed November 10, 2020. https://www.census.gov/quickfacts/chelseacitymassachusetts
- Boston by the Numbers 2018. Research Division, Boston Planning & Development Agency. September 2018. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/3e8bfacf-27c1-4b55-adee-29c5d79f4a38
- Demographic Profile of Adult Limited English Speakers in Massachusetts. Research Division, Boston Planning & Development Agency. February 2019. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/dfe1117a-af16-4257-b0f5-1d95dbd575fe
- Boston in Context: Neighborhoods 2012-2016 American Community Survey. Research Division, Boston Planning & Development Agency. March 2018. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/55f2d86f-eccf-4f68-8d8d-c631fefb0161
- Canipe C. The social distancing of America. Reuters Graphics. April 2, 2020. Accessed November 10, 2020. https://graphics.reuters.com/HEALTH-CORONAVIRUS/USA/qmypmkmwpra/
- Betancourt J, Green AR, Carrillo JE, Park ER. Cultural competency and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
- Racial and Ethnic Disparities in Health Care, Updated 2010. American College of Physicians; 2010. Accessed November 10, 2020. https://www.acponline.org/system/files/documents/advocacy/current_policy_papers/assets/racial_disparities.pdf
- 1.1.3 Patient rights. In: Chapter 1: Opinions on Patient-Physician Relationships. Code of Medical Ethics. American Medical Association; 2016. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf
- Title VI of the Civil Rights Act of 1964, as amended, 42 USC §2000d et seq. July 2, 1964.
- Patient Protection and Affordable Care Act of 2010, Pub L No. 111-148, 124 Stat 119 (2010) §1557.
- Regenstein M, Andres E, Wynia MK. Appropriate use of non-English-language skills in clinical care. JAMA. 2013;309(2):145-146. https://doi.org/10.1001/jama.2012.116984
- Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007;22(Suppl) 2:324-330.
- Manson A. Language concordance as a determinant of patient compliance and emergency room use in patients with asthma. Med Care. 1988;26(12):1119-1128. https://doi.org/10.1097/00005650-198812000-00003
- Seijo R, Gomez H, Garcia M, Shelton D. Acculturation, access to care, and use of preventive services by Hispanics: findings from HANES 1982-84. Am J Public Health. 1991;80(suppl):11-19
- Shapiro J, Saltzer EB. Cross-cultural aspects of physician-patient communications patterns. Urban Health. 1981;10(10):10-15.
- Social Determinants of Health. World Health Organization. Accessed November 10, 2020. https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1
- Buchanan L, Patel JK, Rosenthal BM, Singhvi A. A month of coronavirus in New York City: see the hardest-hit areas. New York Times. April 1, 2020. Accessed November 10, 2020. https://www.nytimes.com/interactive/2020/04/01/nyregion/nyc-coronavirus-cases-map.html
- QuickFacts: Chelsea city, Massachusetts. United States Census Bureau. Accessed November 10, 2020. https://www.census.gov/quickfacts/chelseacitymassachusetts
- Boston by the Numbers 2018. Research Division, Boston Planning & Development Agency. September 2018. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/3e8bfacf-27c1-4b55-adee-29c5d79f4a38
- Demographic Profile of Adult Limited English Speakers in Massachusetts. Research Division, Boston Planning & Development Agency. February 2019. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/dfe1117a-af16-4257-b0f5-1d95dbd575fe
- Boston in Context: Neighborhoods 2012-2016 American Community Survey. Research Division, Boston Planning & Development Agency. March 2018. Accessed November 10, 2020. http://www.bostonplans.org/getattachment/55f2d86f-eccf-4f68-8d8d-c631fefb0161
- Canipe C. The social distancing of America. Reuters Graphics. April 2, 2020. Accessed November 10, 2020. https://graphics.reuters.com/HEALTH-CORONAVIRUS/USA/qmypmkmwpra/
- Betancourt J, Green AR, Carrillo JE, Park ER. Cultural competency and health care disparities: key perspectives and trends. Health Aff (Millwood). 2005;24(2):499-505. https://doi.org/10.1377/hlthaff.24.2.499
- Racial and Ethnic Disparities in Health Care, Updated 2010. American College of Physicians; 2010. Accessed November 10, 2020. https://www.acponline.org/system/files/documents/advocacy/current_policy_papers/assets/racial_disparities.pdf
- 1.1.3 Patient rights. In: Chapter 1: Opinions on Patient-Physician Relationships. Code of Medical Ethics. American Medical Association; 2016. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-1.pdf
- Title VI of the Civil Rights Act of 1964, as amended, 42 USC §2000d et seq. July 2, 1964.
- Patient Protection and Affordable Care Act of 2010, Pub L No. 111-148, 124 Stat 119 (2010) §1557.
- Regenstein M, Andres E, Wynia MK. Appropriate use of non-English-language skills in clinical care. JAMA. 2013;309(2):145-146. https://doi.org/10.1001/jama.2012.116984
- Ngo-Metzger Q, Sorkin DH, Phillips RS, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007;22(Suppl) 2:324-330.
- Manson A. Language concordance as a determinant of patient compliance and emergency room use in patients with asthma. Med Care. 1988;26(12):1119-1128. https://doi.org/10.1097/00005650-198812000-00003
- Seijo R, Gomez H, Garcia M, Shelton D. Acculturation, access to care, and use of preventive services by Hispanics: findings from HANES 1982-84. Am J Public Health. 1991;80(suppl):11-19
- Shapiro J, Saltzer EB. Cross-cultural aspects of physician-patient communications patterns. Urban Health. 1981;10(10):10-15.
© 2020 Society of Hospital Medicine
Email: sknuesel@mgh.harvard.edu; Telephone: 617-898-7722; Twitter: @StevenKnuesel
Racial Health Disparities, COVID-19, and a Way Forward for US Health Systems
The coronavirus disease 2019 (COVID-19) pandemic highlights long-standing inequities in health along racial/ethnic lines in the United States. Black, Hispanic, and Indigenous people have been disproportionately affected during the pandemic. For example, the age-adjusted mortality rate among Black people with COVID-19 is 3.4 times as high as that of White people.1
Structural racism shapes social forces, institutions, and ideologies that generate and reinforce racial inequities across different aspects of life. In this perspective, we discuss how, in the COVID-19 context, structural racism shapes access to and quality of care, as well as socioeconomic and health status. We offer guidance to health systems and healthcare providers on addressing health inequities.
HEALTHCARE QUALITY AND ACCESS
Disparities in access to and quality of care contribute to racial health disparities. At the onset of the COVID-19 pandemic in the United States, guidelines for COVID-19 testing were restrictive, only investigating those who had symptoms and had recently traveled to Wuhan, China, or had contact with someone who may have had the virus.2 News reports show disparities in access to testing, with testing sites favoring wealthier, Whiter communities, a feature of racial residential segregation.3 Residential segregation has also contributed to a concentration of closures among urban public hospitals, affecting access to care.4 In New York City (NYC) and Boston, early hotspots of the pandemic, Black and Hispanic patients and underinsured/uninsured patients were significantly less likely to access care from academic medical centers (AMCs) compared with White, privately insured patients.5 AMCs boast greater resources, and inequalities produced by this segregated system of care are often exacerbated by governmental allocation of resources. For instance, NYC’s public hospitals care for the city’s low-income residents (who are disproportionately insured by Medicaid), yet received far less federal aid from the Provider Relief Fund COVID-19 High Impact Payments, which favored larger, private hospitals in Manhattan. These public hospitals, however, face looming Medicaid cuts.6 Similarly, the federal government delayed the release of funds to health centers located on Native American reservations, adversely affecting the Indian Health Service’s preparedness to face the pandemic.7 In tandem with the effects of residential segregation, these data highlight the tiered nature of the US healthcare system, a structure that significantly impacts the quality of care patients receive along racial and socioeconomic lines. Furthermore, studies have documented racial disparities in the provision of advanced therapies: in the case of predicting algorithms that identify patients with complex illnesses, reliance on cost (thus, previous utilization data) rather than actual illness means that only 17.5% of Black patients receive additional help.8
SOCIOECONOMIC STATUS, OCCUPATIONAL AND RESIDENTIAL RISK
Healthcare alone does not explain the observed disparities. The disproportionately high risk of contracting the SARS-CoV-2 virus among Black, Hispanic and Indigenous people can be explained by factors that render physical distancing a luxury. First, in terms of occupational hazards, only 1 in 5 Black and 1 in 6 Hispanic workers can work remotely compared with 1 in 3 White workers. Additionally, Black and Hispanic workers are more likely to have jobs classified as critical in industries such as food retail, hospitality, and public transit. In NYC, Metropolitan Transportation Authority (MTA) employees reported using their own masks and home disinfectant at work, only to be reprimanded. By April 8, 2020, at least 41 MTA workers had died of COVID-19, and more than 6,000 were ill or self-quarantining, resulting in a transit crisis with increasingly long wait times and crowded subway platforms.9 Jason Hargrove, a Black bus driver in Detroit, shared a video underscoring the dangers of his work in which he says, “We’re out here as public workers, doing our job…but for you to get on the bus and stand on the bus, and cough several times without covering up your mouth . . . in the middle of a pandemic…some folks don’t care.” He died of COVID-19 complications 11 days after sharing his video.10 Such conditions likely also increased riders’ risk of contracting COVID-19. And while in aggregate, essential workers in healthcare receive more personal protective equipment (PPE) than those in other occupations, within NYC hospitals, the rationing of PPE was such that low-wage, nonmedical workers (79% of whom are Black or Hispanic) were given less PPE or none at all compared with nurses and physicians.11
Beyond occupational hazards, Black and Hispanic people are more likely to live in multigenerational homes, an identified risk factor of COVID-19 infection.12 Furthermore, Black and Hispanic people are overrepresented among homeless people as well as among those incarcerated. These social conditions, all products of structural racism, substantially and adversely affect the health status of Black, Hispanic, and Indigenous people, especially as it relates to comorbidities associated with higher COVID-19 mortality.
DISPARITIES IN HEALTH STATUS
Black people are disproportionately represented among COVID-19 patients requiring hospitalization, consistent with more severe disease or delayed presentation. For instance, among a cohort of 3,626 patients in a health system in Louisiana, 76.9% of COVID-19 patients hospitalized and 70.6% of those who died were Black, even though Black people comprise only 31% of this health system’s patient population.13 Conditions associated with COVID-19 mortality include heart failure, obesity, and chronic obstructive pulmonary disease. Black, Hispanic, and Indigenous people have higher rates of these chronic illnesses,14 increasing COVID-19 mortality risk. The increased prevalence of these illnesses is attributable to the aforementioned social conditions and environmental factors and to the additional stress associated with repeated exposure to discrimination.15
RECOMMENDATIONS
Although the disparities highlighted during the pandemic are staggering, this moment can serve as a portal to reimagine a more equitable healthcare system. Health systems and providers should (1) remain vigilant in addressing bias and its effects on patient care; (2) implement strategies to mitigate structural bias and use data to rapidly mitigate disparities in quality of care and transitions in care; and (3) address inequities, diversity, and inclusion across the entire healthcare workforce.
Addressing Provider Bias
At the patient care level, healthcare providers have a role in ensuring patients have positive experiences with the healthcare system; this is an opportunity to address medical distrust. Providers should recognize the burden of psychosocial stress and place-based risk that contributes to patients’ presentations and clinical courses. In patient encounters, this awareness should translate to action, acknowledging patients’ experiences and individuality and upholding their dignity. Under conditions of burnout, physicians’ biases are more likely to manifest in patient encounters,16 and although stress and burnout among providers are likely at an all-time high during the COVID-19 pandemic, patients of color must not suffer disproportionately.
Addressing Structural Bias in Care Provision
Health systems should establish checklist-based protocols in order to mitigate the impact of bias on patient care, such as on referrals for advanced therapies. Algorithms used to automate certain aspects of care should not be biased against Black, Hispanic, and Indigenous patients, as has been the case with algorithms that lead to Black patients receiving lower levels of care compared with White patients with similar clinical presentations.8 Health systems should therefore systematically collect racial and sociodemographic data and implement rapid-cycle evaluation of processes and outcomes to root out biases. In tracking their own performance in providing equitable care, health systems should create feedback systems that inform individual providers of their practices for improvement, and individual departments should hold frequent “morbidity and mortality” style reviews of practices and outcomes to continuously improve. Additionally, collaborations with and financial support of community-based organizations to ensure safe transitions of care and to contribute to addressing patients’ unmet social needs should become the norm. This is particularly relevant for COVID-19 survivors who may face long-term chronic physical and mental sequelae such as post–intensive care syndrome and require multidisciplinary care.17
Workforce Equity, Diversity, and Inclusion
Health systems should also examine and address the ways in which they contribute to racial health inequities beyond healthcare provision. Among healthcare organizations, hospitals employ the majority of low-wage healthcare workers, most of them Black or Hispanic women. Nearly half of Black and Hispanic female healthcare workers earn less than $15 hourly (cited as a living wage, which could help prevent a significant number of premature deaths),18 and a quarter are uninsured or on Medicaid. Raising the hourly minimum wage to at least $15 would reduce poverty among female healthcare workers by 27.1%.19 Mortality decreases as income increases, and the lowest-income healthcare workers have a nearly six-fold higher risk of death relative to their highest-earning counterparts, a gradient steeper compared with other fields.20 Health systems should guarantee occupational safety and adequate wages and benefits and provide employees with career-advancing opportunities that would facilitate upward mobility.
In addition to the aforementioned structural inequities embedded within the healthcare infrastructure, low-wage Black healthcare workers report experiencing interpersonal discrimination at work, such as being assigned more tasks compared with their White peers and having others higher up the hierarchy, such as supervisors, nurses, and physicians, assume they are incompetent. Workplace discrimination spans the organizational hierarchy. Black nurses and physicians report both interpersonal and organizational discrimination from patients and other healthcare workers and in terms of barriers to opportunities through hiring and credentialing processes.21 Black physicians are at greater risk of burnout and attrition, which is partly attributable to experiencing discrimination.22,23
To address these experiences, health systems should invest in creating a work climate that is inclusive and explicitly stands against racism and other forms of discrimination. The rise of the Black Lives Matter movement has contributed to improving people’s attitudes toward Black people over the past years,24 whereas implicit bias trainings, commonly employed to improve diversity and inclusion, may unwittingly further entrench the denial of the impact of racism (by attributing it to implicit rather than explicit attitudes)25 or heighten intergroup racial anxiety and reduce individuals’ intentions to engage in intergroup contact.26 Moreover, evidence shows interracial contact in medical school yields more positive explicit and implicit attitudes toward Black people among non–Black medical trainees, whereas bias trainings do not,27 and a positive racial climate in medical school yields a greater interest in serving underserved and minority populations among non–Black medical trainees.28 In other words, fostering a culture and structure that champions racial justice and diversifying the healthcare workforce would synergistically improve non–Black healthcare workers’ attitudes toward Black people while also improving the working conditions of Black healthcare workers and the experiences of Black patients. Healthcare is the fastest growing industry in the United States, and such initiatives would likely have a tremendous impact on moving the needle toward health equity.
CONCLUSION
The COVID-19 disparities were predictable. This pandemic may not end any time soon and certainly will not be the last we experience. Therefore, healthcare workers and health systems should recognize the societal barriers patients and workers face and implement strategies to eliminate biased practices in the provision of healthcare as well as through the compensation structure and workplace protection of healthcare workers, especially when the healthcare system experiences undue stress.
1. The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. APM Research Lab. October 15, 2020. Accessed October 24, 2020. https://www.apmresearchlab.org/covid/deaths-by-race
2. Wang J, Huth L, Umlauf T. How the CDC’s restrictive testing guidelines hid the coronavirus epidemic. Wall Street Journal. March 22, 2020. Accessed June 20, 2020. https://www.wsj.com/articles/how-the-cdcs-restrictive-testing-guidelines-hid-the-coronavirus-epidemic-11584882001
3. McMinn S, Carlsen A, Jaspers B, Talbot R, Adeline S. In large Texas cities, access to coronavirus testing may depend on where you live. NPR. May 27, 2020. Accessed June 20, 2020. https://www.npr.org/sections/health-shots/2020/05/27/862215848/across-texas-black-and-hispanic-neighborhoods-have-fewer-coronavirus-testing-sit
4. Ko M, Needleman J, Derose KP, Laugesen MJ, Ponce NA. Residential segregation and the survival of U.S. urban public hospitals. Med Care Res Rev. 2014;71(3):243-260. https://doi.org/10.1177/1077558713515079
5. Tikkanen RS, Woolhandler S, Himmelstein DU, et al. Hospital payer and racial/ethnic mix at private academic medical centers in Boston and New York City. Int J Health Serv. 2017;47(3):460-476. https://doi.org/10.1177/0020731416689549
6. Eisenbberg A. New York’s safety-net hospitals were the front lines of the coronavirus. Now they’re facing ruin. May 16, 2020. Accessed October 24, 2020. Politico. https://www.politico.com/states/new-york/albany/story/2020/05/16/new-yorks-safety-net-hospitals-were-the-front-lines-of-the-coronavirus-now-theyre-facing-ruin-1284316
7. Cancryn A. Exclusive: emergency coronavirus funds for American Indian health stalled. Politico. March 20, 2020. Accessed June 20, 2020. https://www.politico.com/news/2020/03/20/coronavirus-american-indian-health-138724
8. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
9. Goldbaum C. 41 transit workers dead: crisis takes staggering toll on subways. New York Times. April 8, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/08/nyregion/coronavirus-nyc-mta-subway.html
10. Levenson M. 11 days after fuming about a coughing passenger, a bus driver died from the coronavirus. New York Times. April 4, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/04/us/detroit-bus-driver-coronavirus.html
11. Hong N. 3 hospital workers gave out masks. Weeks later, they all were dead. New York Times. May 4, 2020. Accessed July 18, 2020. https://www.nytimes.com/2020/05/04/nyregion/coronavirus-ny-hospital-workers.html
12. Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324(4):390-392. https://doi.org/10.1001/jama.2020.11370
13. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/nejmsa2011686
14. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2010;1186(1):69-101. https://doi.org/10.1111/j.1749-6632.2009.05339.x
15. Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff. 2005;24(2):325-334. https://doi.org/10.1377/hlthaff.24.2.325
16. Dyrbye L, Herrin J, West CP, et al. Association of racial bias with burnout among resident physicians. JAMA Netw Open. 2019;2(7):e197457. https://doi.org/10.1001/jamanetworkopen.2019.7457
17. Johnson SF, Nguemeni Tiako MJ, Flash MJE, Lamas DJ, Alba GA. Disparities in the recovery from critical illness due to COVID-19 [correspondence]. Lancet Psychiatry. 2020;7(8):e54-e55. https://doi.org/10.1016/S2215-0366(20)30292-3
18. Tsao TY, Konty KJ, Van Wye G, et al. Estimating potential reductions in premature mortality in New York City from raising the minimum wage to $15. Am J Public Health. 2016;106(6):1036-1041. https://doi.org/10.2105/AJPH.2016.303188
19. Himmelstein KEW, Venkataramani AS. Economic vulnerability among US female health care workers: potential impact of a $15-per-hour minimum wage. Am J Public Health. 2019;109(2):198-205. https://doi.org/10.2105/AJPH.2018.304801
20. Matta S, Chatterjee P, Venkataramani AS. The income-based mortality gradient among US health care workers: cohort study. J Gen Intern Med. Ahead of print. June 2020:1-3. https://doi.org/10.1007/s11606-020-05989-7
21. Wingfield AH, Chavez K. Getting in, getting hired, getting sideways looks: organizational hierarchy and perceptions of racial discrimination. Am Sociol Rev. 2020;85(1):31-57. https://doi.org/10.1177/0003122419894335
22. Nuñez-Smith M, Pilgrim N, Wynia M, et al. Race/ethnicity and workplace discrimination: results of a national survey of physicians. J Gen Intern Med. 2009;24(11):1198-1204. https://doi.org/10.1007/s11606-009-1103-9
23. Nuñez-Smith M, Pilgrim N, Wynia M, et al. Health care workplace discrimination and physician turnover. J Natl Med Assoc. 2009;101(12):1274-1282. https://doi.org/10.1016/S0027-9684(15)31139-1
24. Sawyer J, Gampa A. Implicit and explicit racial attitudes changed during Black Lives Matter. Pers Soc Psychol Bull. 2018;44(7):1039-1059. https://doi.org/10.1177/0146167218757454
25. Daumeyer NM, Onyeador IN, Brown X, Richeson JA. Consequences of attributing discrimination to implicit vs. explicit bias. J Exp Soc Psychol. 2019;84. https://doi.org/10.1016/j.jesp.2019.04.010
26. Perry SP, Dovidio JF, Murphy MC, van Ryn M. The joint effect of bias awareness and self-reported prejudice on intergroup anxiety and intentions for intergroup contact. Cult Divers Ethn Minor Psychol. 2015;21(1):89-96. https://doi.org/10.1037/a0037147
27. Onyeador IN, Wittlin NM, Burke SE, et al. The value of interracial contact for reducing anti-Black bias among non-Black physicians: a Cognitive Habits and Growth Evaluation (CHANGE) study report. Psychol Sci. 2020;31(1):18-30. https://doi.org/10.1177/0956797619879139
28. Phelan SM, Burke SE, Cunningham BA, et al. The effects of racism in medical education on students’ decisions to practice in underserved or minority communities. Acad Med. 2019;94(8):1178-1189. https://doi.org/10.1097/ACM.0000000000002719
The coronavirus disease 2019 (COVID-19) pandemic highlights long-standing inequities in health along racial/ethnic lines in the United States. Black, Hispanic, and Indigenous people have been disproportionately affected during the pandemic. For example, the age-adjusted mortality rate among Black people with COVID-19 is 3.4 times as high as that of White people.1
Structural racism shapes social forces, institutions, and ideologies that generate and reinforce racial inequities across different aspects of life. In this perspective, we discuss how, in the COVID-19 context, structural racism shapes access to and quality of care, as well as socioeconomic and health status. We offer guidance to health systems and healthcare providers on addressing health inequities.
HEALTHCARE QUALITY AND ACCESS
Disparities in access to and quality of care contribute to racial health disparities. At the onset of the COVID-19 pandemic in the United States, guidelines for COVID-19 testing were restrictive, only investigating those who had symptoms and had recently traveled to Wuhan, China, or had contact with someone who may have had the virus.2 News reports show disparities in access to testing, with testing sites favoring wealthier, Whiter communities, a feature of racial residential segregation.3 Residential segregation has also contributed to a concentration of closures among urban public hospitals, affecting access to care.4 In New York City (NYC) and Boston, early hotspots of the pandemic, Black and Hispanic patients and underinsured/uninsured patients were significantly less likely to access care from academic medical centers (AMCs) compared with White, privately insured patients.5 AMCs boast greater resources, and inequalities produced by this segregated system of care are often exacerbated by governmental allocation of resources. For instance, NYC’s public hospitals care for the city’s low-income residents (who are disproportionately insured by Medicaid), yet received far less federal aid from the Provider Relief Fund COVID-19 High Impact Payments, which favored larger, private hospitals in Manhattan. These public hospitals, however, face looming Medicaid cuts.6 Similarly, the federal government delayed the release of funds to health centers located on Native American reservations, adversely affecting the Indian Health Service’s preparedness to face the pandemic.7 In tandem with the effects of residential segregation, these data highlight the tiered nature of the US healthcare system, a structure that significantly impacts the quality of care patients receive along racial and socioeconomic lines. Furthermore, studies have documented racial disparities in the provision of advanced therapies: in the case of predicting algorithms that identify patients with complex illnesses, reliance on cost (thus, previous utilization data) rather than actual illness means that only 17.5% of Black patients receive additional help.8
SOCIOECONOMIC STATUS, OCCUPATIONAL AND RESIDENTIAL RISK
Healthcare alone does not explain the observed disparities. The disproportionately high risk of contracting the SARS-CoV-2 virus among Black, Hispanic and Indigenous people can be explained by factors that render physical distancing a luxury. First, in terms of occupational hazards, only 1 in 5 Black and 1 in 6 Hispanic workers can work remotely compared with 1 in 3 White workers. Additionally, Black and Hispanic workers are more likely to have jobs classified as critical in industries such as food retail, hospitality, and public transit. In NYC, Metropolitan Transportation Authority (MTA) employees reported using their own masks and home disinfectant at work, only to be reprimanded. By April 8, 2020, at least 41 MTA workers had died of COVID-19, and more than 6,000 were ill or self-quarantining, resulting in a transit crisis with increasingly long wait times and crowded subway platforms.9 Jason Hargrove, a Black bus driver in Detroit, shared a video underscoring the dangers of his work in which he says, “We’re out here as public workers, doing our job…but for you to get on the bus and stand on the bus, and cough several times without covering up your mouth . . . in the middle of a pandemic…some folks don’t care.” He died of COVID-19 complications 11 days after sharing his video.10 Such conditions likely also increased riders’ risk of contracting COVID-19. And while in aggregate, essential workers in healthcare receive more personal protective equipment (PPE) than those in other occupations, within NYC hospitals, the rationing of PPE was such that low-wage, nonmedical workers (79% of whom are Black or Hispanic) were given less PPE or none at all compared with nurses and physicians.11
Beyond occupational hazards, Black and Hispanic people are more likely to live in multigenerational homes, an identified risk factor of COVID-19 infection.12 Furthermore, Black and Hispanic people are overrepresented among homeless people as well as among those incarcerated. These social conditions, all products of structural racism, substantially and adversely affect the health status of Black, Hispanic, and Indigenous people, especially as it relates to comorbidities associated with higher COVID-19 mortality.
DISPARITIES IN HEALTH STATUS
Black people are disproportionately represented among COVID-19 patients requiring hospitalization, consistent with more severe disease or delayed presentation. For instance, among a cohort of 3,626 patients in a health system in Louisiana, 76.9% of COVID-19 patients hospitalized and 70.6% of those who died were Black, even though Black people comprise only 31% of this health system’s patient population.13 Conditions associated with COVID-19 mortality include heart failure, obesity, and chronic obstructive pulmonary disease. Black, Hispanic, and Indigenous people have higher rates of these chronic illnesses,14 increasing COVID-19 mortality risk. The increased prevalence of these illnesses is attributable to the aforementioned social conditions and environmental factors and to the additional stress associated with repeated exposure to discrimination.15
RECOMMENDATIONS
Although the disparities highlighted during the pandemic are staggering, this moment can serve as a portal to reimagine a more equitable healthcare system. Health systems and providers should (1) remain vigilant in addressing bias and its effects on patient care; (2) implement strategies to mitigate structural bias and use data to rapidly mitigate disparities in quality of care and transitions in care; and (3) address inequities, diversity, and inclusion across the entire healthcare workforce.
Addressing Provider Bias
At the patient care level, healthcare providers have a role in ensuring patients have positive experiences with the healthcare system; this is an opportunity to address medical distrust. Providers should recognize the burden of psychosocial stress and place-based risk that contributes to patients’ presentations and clinical courses. In patient encounters, this awareness should translate to action, acknowledging patients’ experiences and individuality and upholding their dignity. Under conditions of burnout, physicians’ biases are more likely to manifest in patient encounters,16 and although stress and burnout among providers are likely at an all-time high during the COVID-19 pandemic, patients of color must not suffer disproportionately.
Addressing Structural Bias in Care Provision
Health systems should establish checklist-based protocols in order to mitigate the impact of bias on patient care, such as on referrals for advanced therapies. Algorithms used to automate certain aspects of care should not be biased against Black, Hispanic, and Indigenous patients, as has been the case with algorithms that lead to Black patients receiving lower levels of care compared with White patients with similar clinical presentations.8 Health systems should therefore systematically collect racial and sociodemographic data and implement rapid-cycle evaluation of processes and outcomes to root out biases. In tracking their own performance in providing equitable care, health systems should create feedback systems that inform individual providers of their practices for improvement, and individual departments should hold frequent “morbidity and mortality” style reviews of practices and outcomes to continuously improve. Additionally, collaborations with and financial support of community-based organizations to ensure safe transitions of care and to contribute to addressing patients’ unmet social needs should become the norm. This is particularly relevant for COVID-19 survivors who may face long-term chronic physical and mental sequelae such as post–intensive care syndrome and require multidisciplinary care.17
Workforce Equity, Diversity, and Inclusion
Health systems should also examine and address the ways in which they contribute to racial health inequities beyond healthcare provision. Among healthcare organizations, hospitals employ the majority of low-wage healthcare workers, most of them Black or Hispanic women. Nearly half of Black and Hispanic female healthcare workers earn less than $15 hourly (cited as a living wage, which could help prevent a significant number of premature deaths),18 and a quarter are uninsured or on Medicaid. Raising the hourly minimum wage to at least $15 would reduce poverty among female healthcare workers by 27.1%.19 Mortality decreases as income increases, and the lowest-income healthcare workers have a nearly six-fold higher risk of death relative to their highest-earning counterparts, a gradient steeper compared with other fields.20 Health systems should guarantee occupational safety and adequate wages and benefits and provide employees with career-advancing opportunities that would facilitate upward mobility.
In addition to the aforementioned structural inequities embedded within the healthcare infrastructure, low-wage Black healthcare workers report experiencing interpersonal discrimination at work, such as being assigned more tasks compared with their White peers and having others higher up the hierarchy, such as supervisors, nurses, and physicians, assume they are incompetent. Workplace discrimination spans the organizational hierarchy. Black nurses and physicians report both interpersonal and organizational discrimination from patients and other healthcare workers and in terms of barriers to opportunities through hiring and credentialing processes.21 Black physicians are at greater risk of burnout and attrition, which is partly attributable to experiencing discrimination.22,23
To address these experiences, health systems should invest in creating a work climate that is inclusive and explicitly stands against racism and other forms of discrimination. The rise of the Black Lives Matter movement has contributed to improving people’s attitudes toward Black people over the past years,24 whereas implicit bias trainings, commonly employed to improve diversity and inclusion, may unwittingly further entrench the denial of the impact of racism (by attributing it to implicit rather than explicit attitudes)25 or heighten intergroup racial anxiety and reduce individuals’ intentions to engage in intergroup contact.26 Moreover, evidence shows interracial contact in medical school yields more positive explicit and implicit attitudes toward Black people among non–Black medical trainees, whereas bias trainings do not,27 and a positive racial climate in medical school yields a greater interest in serving underserved and minority populations among non–Black medical trainees.28 In other words, fostering a culture and structure that champions racial justice and diversifying the healthcare workforce would synergistically improve non–Black healthcare workers’ attitudes toward Black people while also improving the working conditions of Black healthcare workers and the experiences of Black patients. Healthcare is the fastest growing industry in the United States, and such initiatives would likely have a tremendous impact on moving the needle toward health equity.
CONCLUSION
The COVID-19 disparities were predictable. This pandemic may not end any time soon and certainly will not be the last we experience. Therefore, healthcare workers and health systems should recognize the societal barriers patients and workers face and implement strategies to eliminate biased practices in the provision of healthcare as well as through the compensation structure and workplace protection of healthcare workers, especially when the healthcare system experiences undue stress.
The coronavirus disease 2019 (COVID-19) pandemic highlights long-standing inequities in health along racial/ethnic lines in the United States. Black, Hispanic, and Indigenous people have been disproportionately affected during the pandemic. For example, the age-adjusted mortality rate among Black people with COVID-19 is 3.4 times as high as that of White people.1
Structural racism shapes social forces, institutions, and ideologies that generate and reinforce racial inequities across different aspects of life. In this perspective, we discuss how, in the COVID-19 context, structural racism shapes access to and quality of care, as well as socioeconomic and health status. We offer guidance to health systems and healthcare providers on addressing health inequities.
HEALTHCARE QUALITY AND ACCESS
Disparities in access to and quality of care contribute to racial health disparities. At the onset of the COVID-19 pandemic in the United States, guidelines for COVID-19 testing were restrictive, only investigating those who had symptoms and had recently traveled to Wuhan, China, or had contact with someone who may have had the virus.2 News reports show disparities in access to testing, with testing sites favoring wealthier, Whiter communities, a feature of racial residential segregation.3 Residential segregation has also contributed to a concentration of closures among urban public hospitals, affecting access to care.4 In New York City (NYC) and Boston, early hotspots of the pandemic, Black and Hispanic patients and underinsured/uninsured patients were significantly less likely to access care from academic medical centers (AMCs) compared with White, privately insured patients.5 AMCs boast greater resources, and inequalities produced by this segregated system of care are often exacerbated by governmental allocation of resources. For instance, NYC’s public hospitals care for the city’s low-income residents (who are disproportionately insured by Medicaid), yet received far less federal aid from the Provider Relief Fund COVID-19 High Impact Payments, which favored larger, private hospitals in Manhattan. These public hospitals, however, face looming Medicaid cuts.6 Similarly, the federal government delayed the release of funds to health centers located on Native American reservations, adversely affecting the Indian Health Service’s preparedness to face the pandemic.7 In tandem with the effects of residential segregation, these data highlight the tiered nature of the US healthcare system, a structure that significantly impacts the quality of care patients receive along racial and socioeconomic lines. Furthermore, studies have documented racial disparities in the provision of advanced therapies: in the case of predicting algorithms that identify patients with complex illnesses, reliance on cost (thus, previous utilization data) rather than actual illness means that only 17.5% of Black patients receive additional help.8
SOCIOECONOMIC STATUS, OCCUPATIONAL AND RESIDENTIAL RISK
Healthcare alone does not explain the observed disparities. The disproportionately high risk of contracting the SARS-CoV-2 virus among Black, Hispanic and Indigenous people can be explained by factors that render physical distancing a luxury. First, in terms of occupational hazards, only 1 in 5 Black and 1 in 6 Hispanic workers can work remotely compared with 1 in 3 White workers. Additionally, Black and Hispanic workers are more likely to have jobs classified as critical in industries such as food retail, hospitality, and public transit. In NYC, Metropolitan Transportation Authority (MTA) employees reported using their own masks and home disinfectant at work, only to be reprimanded. By April 8, 2020, at least 41 MTA workers had died of COVID-19, and more than 6,000 were ill or self-quarantining, resulting in a transit crisis with increasingly long wait times and crowded subway platforms.9 Jason Hargrove, a Black bus driver in Detroit, shared a video underscoring the dangers of his work in which he says, “We’re out here as public workers, doing our job…but for you to get on the bus and stand on the bus, and cough several times without covering up your mouth . . . in the middle of a pandemic…some folks don’t care.” He died of COVID-19 complications 11 days after sharing his video.10 Such conditions likely also increased riders’ risk of contracting COVID-19. And while in aggregate, essential workers in healthcare receive more personal protective equipment (PPE) than those in other occupations, within NYC hospitals, the rationing of PPE was such that low-wage, nonmedical workers (79% of whom are Black or Hispanic) were given less PPE or none at all compared with nurses and physicians.11
Beyond occupational hazards, Black and Hispanic people are more likely to live in multigenerational homes, an identified risk factor of COVID-19 infection.12 Furthermore, Black and Hispanic people are overrepresented among homeless people as well as among those incarcerated. These social conditions, all products of structural racism, substantially and adversely affect the health status of Black, Hispanic, and Indigenous people, especially as it relates to comorbidities associated with higher COVID-19 mortality.
DISPARITIES IN HEALTH STATUS
Black people are disproportionately represented among COVID-19 patients requiring hospitalization, consistent with more severe disease or delayed presentation. For instance, among a cohort of 3,626 patients in a health system in Louisiana, 76.9% of COVID-19 patients hospitalized and 70.6% of those who died were Black, even though Black people comprise only 31% of this health system’s patient population.13 Conditions associated with COVID-19 mortality include heart failure, obesity, and chronic obstructive pulmonary disease. Black, Hispanic, and Indigenous people have higher rates of these chronic illnesses,14 increasing COVID-19 mortality risk. The increased prevalence of these illnesses is attributable to the aforementioned social conditions and environmental factors and to the additional stress associated with repeated exposure to discrimination.15
RECOMMENDATIONS
Although the disparities highlighted during the pandemic are staggering, this moment can serve as a portal to reimagine a more equitable healthcare system. Health systems and providers should (1) remain vigilant in addressing bias and its effects on patient care; (2) implement strategies to mitigate structural bias and use data to rapidly mitigate disparities in quality of care and transitions in care; and (3) address inequities, diversity, and inclusion across the entire healthcare workforce.
Addressing Provider Bias
At the patient care level, healthcare providers have a role in ensuring patients have positive experiences with the healthcare system; this is an opportunity to address medical distrust. Providers should recognize the burden of psychosocial stress and place-based risk that contributes to patients’ presentations and clinical courses. In patient encounters, this awareness should translate to action, acknowledging patients’ experiences and individuality and upholding their dignity. Under conditions of burnout, physicians’ biases are more likely to manifest in patient encounters,16 and although stress and burnout among providers are likely at an all-time high during the COVID-19 pandemic, patients of color must not suffer disproportionately.
Addressing Structural Bias in Care Provision
Health systems should establish checklist-based protocols in order to mitigate the impact of bias on patient care, such as on referrals for advanced therapies. Algorithms used to automate certain aspects of care should not be biased against Black, Hispanic, and Indigenous patients, as has been the case with algorithms that lead to Black patients receiving lower levels of care compared with White patients with similar clinical presentations.8 Health systems should therefore systematically collect racial and sociodemographic data and implement rapid-cycle evaluation of processes and outcomes to root out biases. In tracking their own performance in providing equitable care, health systems should create feedback systems that inform individual providers of their practices for improvement, and individual departments should hold frequent “morbidity and mortality” style reviews of practices and outcomes to continuously improve. Additionally, collaborations with and financial support of community-based organizations to ensure safe transitions of care and to contribute to addressing patients’ unmet social needs should become the norm. This is particularly relevant for COVID-19 survivors who may face long-term chronic physical and mental sequelae such as post–intensive care syndrome and require multidisciplinary care.17
Workforce Equity, Diversity, and Inclusion
Health systems should also examine and address the ways in which they contribute to racial health inequities beyond healthcare provision. Among healthcare organizations, hospitals employ the majority of low-wage healthcare workers, most of them Black or Hispanic women. Nearly half of Black and Hispanic female healthcare workers earn less than $15 hourly (cited as a living wage, which could help prevent a significant number of premature deaths),18 and a quarter are uninsured or on Medicaid. Raising the hourly minimum wage to at least $15 would reduce poverty among female healthcare workers by 27.1%.19 Mortality decreases as income increases, and the lowest-income healthcare workers have a nearly six-fold higher risk of death relative to their highest-earning counterparts, a gradient steeper compared with other fields.20 Health systems should guarantee occupational safety and adequate wages and benefits and provide employees with career-advancing opportunities that would facilitate upward mobility.
In addition to the aforementioned structural inequities embedded within the healthcare infrastructure, low-wage Black healthcare workers report experiencing interpersonal discrimination at work, such as being assigned more tasks compared with their White peers and having others higher up the hierarchy, such as supervisors, nurses, and physicians, assume they are incompetent. Workplace discrimination spans the organizational hierarchy. Black nurses and physicians report both interpersonal and organizational discrimination from patients and other healthcare workers and in terms of barriers to opportunities through hiring and credentialing processes.21 Black physicians are at greater risk of burnout and attrition, which is partly attributable to experiencing discrimination.22,23
To address these experiences, health systems should invest in creating a work climate that is inclusive and explicitly stands against racism and other forms of discrimination. The rise of the Black Lives Matter movement has contributed to improving people’s attitudes toward Black people over the past years,24 whereas implicit bias trainings, commonly employed to improve diversity and inclusion, may unwittingly further entrench the denial of the impact of racism (by attributing it to implicit rather than explicit attitudes)25 or heighten intergroup racial anxiety and reduce individuals’ intentions to engage in intergroup contact.26 Moreover, evidence shows interracial contact in medical school yields more positive explicit and implicit attitudes toward Black people among non–Black medical trainees, whereas bias trainings do not,27 and a positive racial climate in medical school yields a greater interest in serving underserved and minority populations among non–Black medical trainees.28 In other words, fostering a culture and structure that champions racial justice and diversifying the healthcare workforce would synergistically improve non–Black healthcare workers’ attitudes toward Black people while also improving the working conditions of Black healthcare workers and the experiences of Black patients. Healthcare is the fastest growing industry in the United States, and such initiatives would likely have a tremendous impact on moving the needle toward health equity.
CONCLUSION
The COVID-19 disparities were predictable. This pandemic may not end any time soon and certainly will not be the last we experience. Therefore, healthcare workers and health systems should recognize the societal barriers patients and workers face and implement strategies to eliminate biased practices in the provision of healthcare as well as through the compensation structure and workplace protection of healthcare workers, especially when the healthcare system experiences undue stress.
1. The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. APM Research Lab. October 15, 2020. Accessed October 24, 2020. https://www.apmresearchlab.org/covid/deaths-by-race
2. Wang J, Huth L, Umlauf T. How the CDC’s restrictive testing guidelines hid the coronavirus epidemic. Wall Street Journal. March 22, 2020. Accessed June 20, 2020. https://www.wsj.com/articles/how-the-cdcs-restrictive-testing-guidelines-hid-the-coronavirus-epidemic-11584882001
3. McMinn S, Carlsen A, Jaspers B, Talbot R, Adeline S. In large Texas cities, access to coronavirus testing may depend on where you live. NPR. May 27, 2020. Accessed June 20, 2020. https://www.npr.org/sections/health-shots/2020/05/27/862215848/across-texas-black-and-hispanic-neighborhoods-have-fewer-coronavirus-testing-sit
4. Ko M, Needleman J, Derose KP, Laugesen MJ, Ponce NA. Residential segregation and the survival of U.S. urban public hospitals. Med Care Res Rev. 2014;71(3):243-260. https://doi.org/10.1177/1077558713515079
5. Tikkanen RS, Woolhandler S, Himmelstein DU, et al. Hospital payer and racial/ethnic mix at private academic medical centers in Boston and New York City. Int J Health Serv. 2017;47(3):460-476. https://doi.org/10.1177/0020731416689549
6. Eisenbberg A. New York’s safety-net hospitals were the front lines of the coronavirus. Now they’re facing ruin. May 16, 2020. Accessed October 24, 2020. Politico. https://www.politico.com/states/new-york/albany/story/2020/05/16/new-yorks-safety-net-hospitals-were-the-front-lines-of-the-coronavirus-now-theyre-facing-ruin-1284316
7. Cancryn A. Exclusive: emergency coronavirus funds for American Indian health stalled. Politico. March 20, 2020. Accessed June 20, 2020. https://www.politico.com/news/2020/03/20/coronavirus-american-indian-health-138724
8. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
9. Goldbaum C. 41 transit workers dead: crisis takes staggering toll on subways. New York Times. April 8, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/08/nyregion/coronavirus-nyc-mta-subway.html
10. Levenson M. 11 days after fuming about a coughing passenger, a bus driver died from the coronavirus. New York Times. April 4, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/04/us/detroit-bus-driver-coronavirus.html
11. Hong N. 3 hospital workers gave out masks. Weeks later, they all were dead. New York Times. May 4, 2020. Accessed July 18, 2020. https://www.nytimes.com/2020/05/04/nyregion/coronavirus-ny-hospital-workers.html
12. Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324(4):390-392. https://doi.org/10.1001/jama.2020.11370
13. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/nejmsa2011686
14. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2010;1186(1):69-101. https://doi.org/10.1111/j.1749-6632.2009.05339.x
15. Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff. 2005;24(2):325-334. https://doi.org/10.1377/hlthaff.24.2.325
16. Dyrbye L, Herrin J, West CP, et al. Association of racial bias with burnout among resident physicians. JAMA Netw Open. 2019;2(7):e197457. https://doi.org/10.1001/jamanetworkopen.2019.7457
17. Johnson SF, Nguemeni Tiako MJ, Flash MJE, Lamas DJ, Alba GA. Disparities in the recovery from critical illness due to COVID-19 [correspondence]. Lancet Psychiatry. 2020;7(8):e54-e55. https://doi.org/10.1016/S2215-0366(20)30292-3
18. Tsao TY, Konty KJ, Van Wye G, et al. Estimating potential reductions in premature mortality in New York City from raising the minimum wage to $15. Am J Public Health. 2016;106(6):1036-1041. https://doi.org/10.2105/AJPH.2016.303188
19. Himmelstein KEW, Venkataramani AS. Economic vulnerability among US female health care workers: potential impact of a $15-per-hour minimum wage. Am J Public Health. 2019;109(2):198-205. https://doi.org/10.2105/AJPH.2018.304801
20. Matta S, Chatterjee P, Venkataramani AS. The income-based mortality gradient among US health care workers: cohort study. J Gen Intern Med. Ahead of print. June 2020:1-3. https://doi.org/10.1007/s11606-020-05989-7
21. Wingfield AH, Chavez K. Getting in, getting hired, getting sideways looks: organizational hierarchy and perceptions of racial discrimination. Am Sociol Rev. 2020;85(1):31-57. https://doi.org/10.1177/0003122419894335
22. Nuñez-Smith M, Pilgrim N, Wynia M, et al. Race/ethnicity and workplace discrimination: results of a national survey of physicians. J Gen Intern Med. 2009;24(11):1198-1204. https://doi.org/10.1007/s11606-009-1103-9
23. Nuñez-Smith M, Pilgrim N, Wynia M, et al. Health care workplace discrimination and physician turnover. J Natl Med Assoc. 2009;101(12):1274-1282. https://doi.org/10.1016/S0027-9684(15)31139-1
24. Sawyer J, Gampa A. Implicit and explicit racial attitudes changed during Black Lives Matter. Pers Soc Psychol Bull. 2018;44(7):1039-1059. https://doi.org/10.1177/0146167218757454
25. Daumeyer NM, Onyeador IN, Brown X, Richeson JA. Consequences of attributing discrimination to implicit vs. explicit bias. J Exp Soc Psychol. 2019;84. https://doi.org/10.1016/j.jesp.2019.04.010
26. Perry SP, Dovidio JF, Murphy MC, van Ryn M. The joint effect of bias awareness and self-reported prejudice on intergroup anxiety and intentions for intergroup contact. Cult Divers Ethn Minor Psychol. 2015;21(1):89-96. https://doi.org/10.1037/a0037147
27. Onyeador IN, Wittlin NM, Burke SE, et al. The value of interracial contact for reducing anti-Black bias among non-Black physicians: a Cognitive Habits and Growth Evaluation (CHANGE) study report. Psychol Sci. 2020;31(1):18-30. https://doi.org/10.1177/0956797619879139
28. Phelan SM, Burke SE, Cunningham BA, et al. The effects of racism in medical education on students’ decisions to practice in underserved or minority communities. Acad Med. 2019;94(8):1178-1189. https://doi.org/10.1097/ACM.0000000000002719
1. The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. APM Research Lab. October 15, 2020. Accessed October 24, 2020. https://www.apmresearchlab.org/covid/deaths-by-race
2. Wang J, Huth L, Umlauf T. How the CDC’s restrictive testing guidelines hid the coronavirus epidemic. Wall Street Journal. March 22, 2020. Accessed June 20, 2020. https://www.wsj.com/articles/how-the-cdcs-restrictive-testing-guidelines-hid-the-coronavirus-epidemic-11584882001
3. McMinn S, Carlsen A, Jaspers B, Talbot R, Adeline S. In large Texas cities, access to coronavirus testing may depend on where you live. NPR. May 27, 2020. Accessed June 20, 2020. https://www.npr.org/sections/health-shots/2020/05/27/862215848/across-texas-black-and-hispanic-neighborhoods-have-fewer-coronavirus-testing-sit
4. Ko M, Needleman J, Derose KP, Laugesen MJ, Ponce NA. Residential segregation and the survival of U.S. urban public hospitals. Med Care Res Rev. 2014;71(3):243-260. https://doi.org/10.1177/1077558713515079
5. Tikkanen RS, Woolhandler S, Himmelstein DU, et al. Hospital payer and racial/ethnic mix at private academic medical centers in Boston and New York City. Int J Health Serv. 2017;47(3):460-476. https://doi.org/10.1177/0020731416689549
6. Eisenbberg A. New York’s safety-net hospitals were the front lines of the coronavirus. Now they’re facing ruin. May 16, 2020. Accessed October 24, 2020. Politico. https://www.politico.com/states/new-york/albany/story/2020/05/16/new-yorks-safety-net-hospitals-were-the-front-lines-of-the-coronavirus-now-theyre-facing-ruin-1284316
7. Cancryn A. Exclusive: emergency coronavirus funds for American Indian health stalled. Politico. March 20, 2020. Accessed June 20, 2020. https://www.politico.com/news/2020/03/20/coronavirus-american-indian-health-138724
8. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
9. Goldbaum C. 41 transit workers dead: crisis takes staggering toll on subways. New York Times. April 8, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/08/nyregion/coronavirus-nyc-mta-subway.html
10. Levenson M. 11 days after fuming about a coughing passenger, a bus driver died from the coronavirus. New York Times. April 4, 2020. Accessed June 20, 2020. https://www.nytimes.com/2020/04/04/us/detroit-bus-driver-coronavirus.html
11. Hong N. 3 hospital workers gave out masks. Weeks later, they all were dead. New York Times. May 4, 2020. Accessed July 18, 2020. https://www.nytimes.com/2020/05/04/nyregion/coronavirus-ny-hospital-workers.html
12. Emeruwa UN, Ona S, Shaman JL, et al. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324(4):390-392. https://doi.org/10.1001/jama.2020.11370
13. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/nejmsa2011686
14. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2010;1186(1):69-101. https://doi.org/10.1111/j.1749-6632.2009.05339.x
15. Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff. 2005;24(2):325-334. https://doi.org/10.1377/hlthaff.24.2.325
16. Dyrbye L, Herrin J, West CP, et al. Association of racial bias with burnout among resident physicians. JAMA Netw Open. 2019;2(7):e197457. https://doi.org/10.1001/jamanetworkopen.2019.7457
17. Johnson SF, Nguemeni Tiako MJ, Flash MJE, Lamas DJ, Alba GA. Disparities in the recovery from critical illness due to COVID-19 [correspondence]. Lancet Psychiatry. 2020;7(8):e54-e55. https://doi.org/10.1016/S2215-0366(20)30292-3
18. Tsao TY, Konty KJ, Van Wye G, et al. Estimating potential reductions in premature mortality in New York City from raising the minimum wage to $15. Am J Public Health. 2016;106(6):1036-1041. https://doi.org/10.2105/AJPH.2016.303188
19. Himmelstein KEW, Venkataramani AS. Economic vulnerability among US female health care workers: potential impact of a $15-per-hour minimum wage. Am J Public Health. 2019;109(2):198-205. https://doi.org/10.2105/AJPH.2018.304801
20. Matta S, Chatterjee P, Venkataramani AS. The income-based mortality gradient among US health care workers: cohort study. J Gen Intern Med. Ahead of print. June 2020:1-3. https://doi.org/10.1007/s11606-020-05989-7
21. Wingfield AH, Chavez K. Getting in, getting hired, getting sideways looks: organizational hierarchy and perceptions of racial discrimination. Am Sociol Rev. 2020;85(1):31-57. https://doi.org/10.1177/0003122419894335
22. Nuñez-Smith M, Pilgrim N, Wynia M, et al. Race/ethnicity and workplace discrimination: results of a national survey of physicians. J Gen Intern Med. 2009;24(11):1198-1204. https://doi.org/10.1007/s11606-009-1103-9
23. Nuñez-Smith M, Pilgrim N, Wynia M, et al. Health care workplace discrimination and physician turnover. J Natl Med Assoc. 2009;101(12):1274-1282. https://doi.org/10.1016/S0027-9684(15)31139-1
24. Sawyer J, Gampa A. Implicit and explicit racial attitudes changed during Black Lives Matter. Pers Soc Psychol Bull. 2018;44(7):1039-1059. https://doi.org/10.1177/0146167218757454
25. Daumeyer NM, Onyeador IN, Brown X, Richeson JA. Consequences of attributing discrimination to implicit vs. explicit bias. J Exp Soc Psychol. 2019;84. https://doi.org/10.1016/j.jesp.2019.04.010
26. Perry SP, Dovidio JF, Murphy MC, van Ryn M. The joint effect of bias awareness and self-reported prejudice on intergroup anxiety and intentions for intergroup contact. Cult Divers Ethn Minor Psychol. 2015;21(1):89-96. https://doi.org/10.1037/a0037147
27. Onyeador IN, Wittlin NM, Burke SE, et al. The value of interracial contact for reducing anti-Black bias among non-Black physicians: a Cognitive Habits and Growth Evaluation (CHANGE) study report. Psychol Sci. 2020;31(1):18-30. https://doi.org/10.1177/0956797619879139
28. Phelan SM, Burke SE, Cunningham BA, et al. The effects of racism in medical education on students’ decisions to practice in underserved or minority communities. Acad Med. 2019;94(8):1178-1189. https://doi.org/10.1097/ACM.0000000000002719
© 2021 Society of Hospital Medicine