User login
Clinical Progress Note: Myocardial Injury After Noncardiac Surgery
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
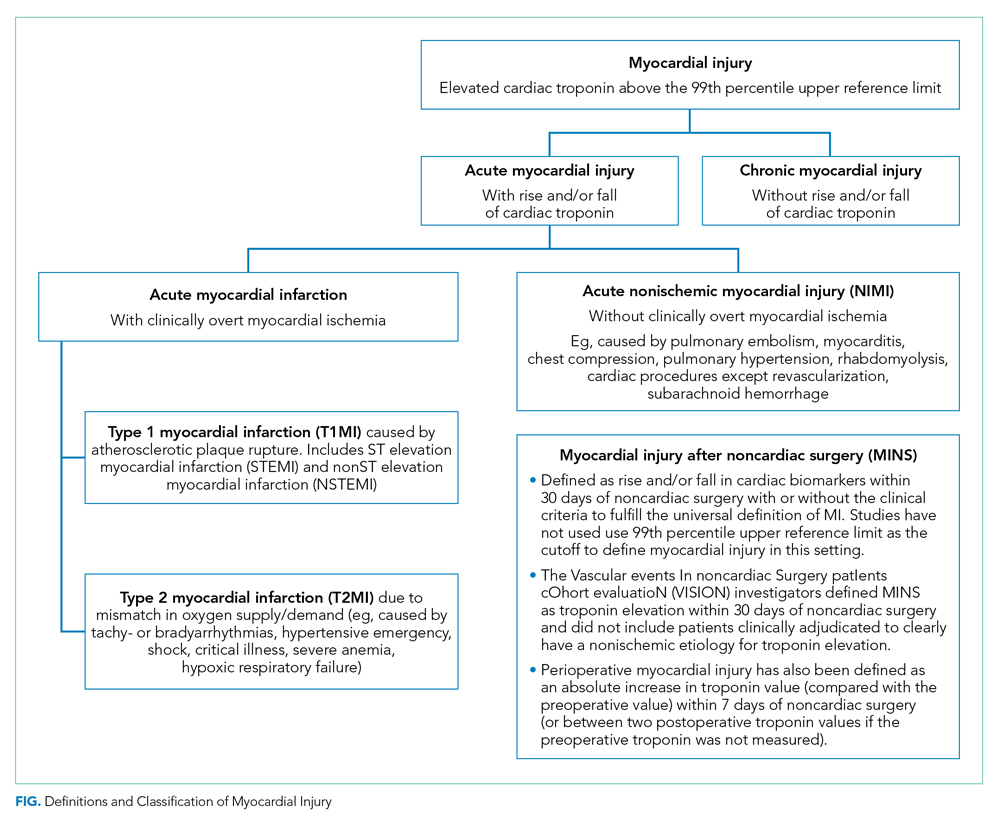
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
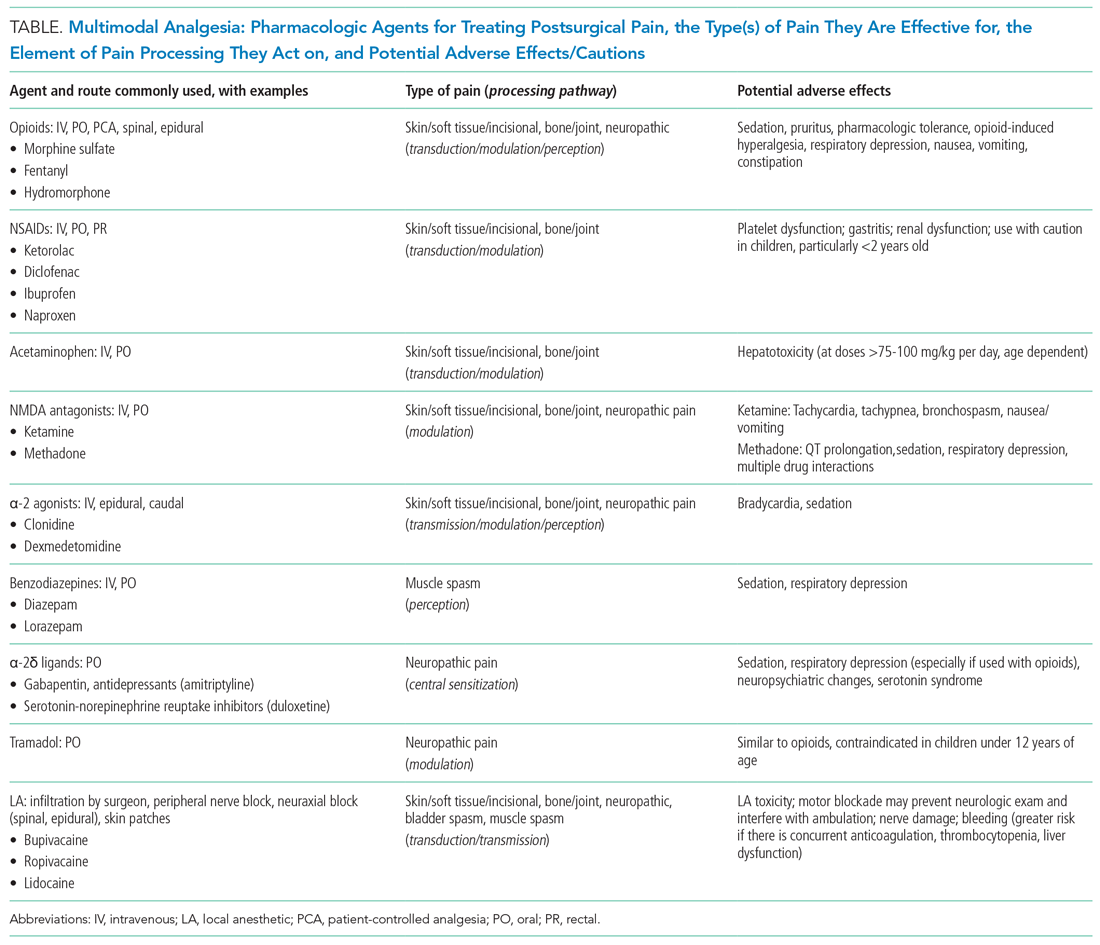
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8
EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
More than 200 million patients worldwide undergo major noncardiac surgery each year. Of these, more than 10 million patients suffer a major adverse cardiovascular event (MACE) within 30 days of surgery.1 Elevated troponins after noncardiac surgery have been associated with increased mortality, but the management of these patients and the indications for screening remain unclear. The nomenclature around myocardial injury also remains confusing. In this Progress Note, we aim to define myocardial injury after noncardiac surgery (MINS) and discuss the key questions on MINS and postoperative troponin elevation.
A PubMed search for medical subject headings and the terms “myocardial injury after noncardiac surgery,” “perioperative troponin,” and “postoperative troponin” restricted to humans, English language, and published in the past 5 years resulted in 144 articles. Articles most relevant to this progress note were included. Guidelines from major societies on perioperative cardiovascular assessment and management were also reviewed.
DEFINITION OF MYOCARDIAL INJURY AND MINS
The Fourth Universal Definition of Myocardial Infarction ( UDMI 4) defines myocardial injury as detection of an elevated cardiac troponin above the 99th percentile upper reference limit (URL).2 Different troponin assays are not comparable and institutions set their own thresholds for abnormal troponin. Per UDMI 4, myocardial injury is classified as (Figure)2-4:
- Acute Myocardial Infarction (MI): This is defined as “detection of a rise and/or fall of cardiac troponin with ≥1 value above the 99th percentile URL and ≥1 of the following: symptoms of acute myocardial ischemia, new ischemic electrocardiographic changes, development of pathological Q waves, or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.” If these patients have an acute atherosclerotic plaque rupture, they are classified as Type 1 MI (T1MI), and if they have a mismatch between oxygen supply/demand, they are classified as Type 2 MI (T2MI).
- Acute Nonischemic Myocardial Injury (NIMI): This is defined as detection of both a rise and/or fall of cardiac troponin and one or more cardiac troponin values above the 99th percentile URL, but no overt clinical evidence of myocardial ischemia.
- Chronic Myocardial Injury: This is defined as one or more cardiac troponin values above the 99th percentile URL but without a rise and/or fall pattern.
MINS is defined as a rise and/or fall of cardiac biomarkers of presumed ischemic etiology within 30 days of noncardiac surgery that may occur with or without the clinical criteria necessary to fulfill the universal definition of MI (Figure).5-8

EPIDEMIOLOGY AND OUTCOMES
A meta-analysis of 169 studies reported the overall incidence of MINS to be 17.9%; the incidence was 19.6% when systematic troponin screening was done versus 9.9% when troponins were ordered selectively based on the clinical context.5
That meta-analysis found that patients with MINS were more likely to be older, male, undergoing nonelective surgeries, and have hypertension, coronary artery disease (CAD), prior MI, heart failure, or kidney disease.5 Intraoperative hypotension (defined as systolic blood pressure <100 mm Hg or mean arterial pressure <55 mm Hg for up to 5 minutes or <60 mm Hg for 30 minutes or more) and intraoperative tachycardia (defined as heart rate >100 beats per minute) have been associated with MINS.5,9 The relationship between anesthesia type and MINS is uncertain.
MINS is associated with an increased risk of 30-day mortality, nonfatal cardiac arrest, heart failure, and stroke.In the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) studies, the majority of patients did not have ischemic symptoms.6,7 In this study, 30-day mortality rates were 8.5% to 13.5% in patients with ischemic symptoms or electrocardiographic changes and 2.9% to 7.7% in patients with asymptomatic troponin elevations. Among the patients without MINS, 30-day mortality was 0.6% to 1.1%. Higher levels of cardiac troponin were associated with higher mortality rates and shorter time to death.
SCREENING GUIDELINES
The recommendations for perioperative screening for MINS vary from society to society. Although MINS is associated with worse outcomes, and most patients with MINS are asymptomatic, perioperative screening for MINS in the absence of clinical signs or symptoms is currently not recommended by the American College of Cardiology/American Heart Association (ACC/AHA).10
ACC/AHA
“The usefulness of postoperative screening with troponin levels in patients at high risk for perioperative MI, but without signs or symptoms suggestive of myocardial ischemia or MI, is uncertain in the absence of established risks and benefits of a defined management strategy (Class IIb; level of evidence [LOE]–B).”10
European Society of Cardiology
“Measurement of B-type natriuretic peptides (BNP) and high-sensitivity troponins (hsTn) after surgery may be considered in high-risk patients to improve risk stratification (Class IIb; LOE-B). Preoperatively and postoperatively, patients who could most benefit from BNP or hsTn measurements are those with metabolic equivalents (METs) ≤4 or those with a revised cardiac risk index (RCRI) score >1 for vascular surgery and >2 for nonvascular surgery. Postoperatively, patients with a surgical Apgar score <7 should also be monitored with BNP or hsTn to detect complications early, independent of their RCRI values.”11
Canadian Cardiovascular Society
“We recommend obtaining daily troponins for 48-72 hours after noncardiac surgery in patients with a baseline risk of >5% for cardiovascular death or nonfatal MI at 30 days after surgery (ie, patients with an elevated N-terminal-proBNP (NT-proBNP)/BNP before surgery or, if there is no NT-proBNP/BNP before surgery, in those who have an RCRI score ≥1, age 45-64 years with significant cardiovascular disease, or age ≥65 years) (Strong recommendation; Moderate quality evidence).”1
MANAGEMENT OF MINS
Currently, evidence-based therapies are well established only for T1MI. However, it is often challenging to differentiate T1MI from other causes of troponin elevation in the perioperative setting in which anesthesia, sedation, or analgesia may mask ischemic symptoms that typically prompt further investigation. While peak troponin levels may be higher in T1MI than they are in T2MI, the initial or delta change in the troponin may provide poor discrimination between T1MI and T2MI.2 Management is complicated not only by the uncertainty about the underlying diagnosis (T1MI, T2MI, or NIMI) but also by the heterogeneity in the underlying pathophysiology of troponin elevation in patients with T2MI and NIMI. Patients with T2MI are generally sicker and have higher mortality than patients with T1MI, and management typically involves treating the underlying reason for oxygen supply/demand mismatch. Mortality in T2MI is more commonly caused by noncardiovascular causes, but underlying CAD is an independent predictor of cardiovascular death or recurrent MI in these patients.
The MANAGE trial (Management of Myocardial Injury After Noncardiac Surgery) had several methodological limitations to inform clinical practice but showed potential benefit of dabigatran in patients with MINS.12 In this trial, patients on dabigatran had significantly lower rates of the primary efficacy outcome (composite of vascular mortality and nonfatal MI, nonhemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism) without a significant increase in life-threatening, major, or critical organ bleeding. Of the secondary efficacy outcomes, only nonhemorrhagic stroke was significantly reduced with dabigatran, but the event rate was low. In the subgroup analysis, patients randomized to dabigatran within 5 days of MINS and those meeting the criteria for MI had significantly lower rates of the primary efficacy outcome.
Patients with T2MI with known CAD may benefit from long-term risk reduction strategies for secondary prevention. There are no definitive management strategies in the literature for T2MI with unknown or no CAD. The SWEDEHEART registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapy) enrolled 9,136 patients with MI with nonobstructive coronary arteries (MINOCA).13 Though MINOCA may include T1MI patients, the majority of these patients are classified as T2MI under UDMI 4. Therefore, it has been proposed that data from this registry may inform management on T2MI.14 Data from this registry showed that statins and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers were associated with lower incidence of MACE over a mean follow-up of 4.1 years. Dual-antiplatelet therapy or beta blockers did not significantly lower the incidence of MACE.13 In another study assessing 2-year mortality in patients with T2MI, beta blockers were beneficial.15
KEY QUESTIONS AND RECOMMENDATIONS
Who should be screened?
Screening can be performed if further risk stratification of high-risk patients or patients with poor functional status is desired. European Society of Cardiology and Canadian Cardiovascular Society guidelines provide guidance on the screening criteria. Troponin elevation in a low-risk group is associated with a low mortality rate, and many of these troponin elevations may be secondary to causes other than myocardial ischemia.
How should screening be conducted?
If planning to obtain postoperative troponins, then preoperative troponin should be obtained because 35% of the patients may have a chronic troponin elevation.
What is the risk if postoperative troponin screening is not performed?
Most patients with MINS are asymptomatic. Systematic screening with troponins (compared with selective screening based on clinical signs or symptoms) can detect T1MI that would otherwise remain occult and undiagnosed.
What is the risk if postoperative troponin screening is performed?
Detecting asymptomatic troponin elevations could lead to potentially harmful treatments (eg, increased risk of bleeding with antithrombotics in the postoperative setting, increased use of cardiac angiography, or addition of new medications such as statins and beta-blockers in the postoperative setting with the potential for adverse effects).
How should MINS be documented?
ST-elevation and non–ST elevation MI (STEMI and NSTEMI) should be reserved for T1MI only. T1MI should be documented when acute plaque rupture is strongly suspected. T2MI should be documented when oxygen supply/demand mismatch is strongly suspected as the etiology of acute MI (eg, T2MI secondary to tachyarrhythmia, hypertensive emergency, or septic shock). Documenting as “demand ischemia” or “unlikely acute coronary syndrome” for T2MI or NIMI should be avoided. Troponin elevations not meeting the criteria for acute MI should be documented as “non-MI troponin elevation” (eg, non-MI troponin elevation secondary to chronic kidney disease or left ventricular hypertrophy). Terms like “troponinitis” or “troponinemia” should be avoided.3
Can MINS be prevented?
There are no well-defined strategies for prevention of MINS, but cardiovascular risk factors should be optimized preoperatively for all patients. In a meta-analysis, preoperative aspirin was not associated with reduced incidence of MINS, and the role of preoperative statins remains speculative; however, nonacute initiation of beta-blockers preoperatively was associated with a lower incidence of MINS.5 Withholding angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers in the 24 hours prior to surgery has been associated with a lower incidence of MINS. Intraoperative hypotension or tachycardia should be avoided.
CONCLUSION
While MINS has been associated with increased 30-day mortality, there are currently no definitive evidence-based management strategies for these patients. Institutions should consider creating decision-support tools if considering screening for MINS based on patient- and surgery-specific risk factors.
Disclosures
The authors have nothing to disclose.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
1. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. https://doi.org/10.1016/j.cjca.2016.09.008.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231-2264. https://doi.org/10.1016/j.jacc.2018.08.1038.
3. Goyal A, Gluckman TJ, Levy A, et al. Translating the fourth universal definition of myocardial infarction into clinical documentation: ten pearls for frontline clinicians. Cardiology Magazine. 2018. https://www.acc.org/latest-in-cardiology/articles/2018/11/06/12/42/translating-the-fourth-universal-definition-of-myocardial-infarction-into-clinical-documentation-ten-pearls-for-frontline-clinicians. Accessed February 20, 2020.
4. King CJ, Levy AE, Trost JC. Clinical progress notes: updates from the 4th universal definition of myocardial infarction. J Hosp Med. 2019;14(9):555-557. https://doi.org/10.12788/jhm.3283.
5. Smilowitz NR, Redel-Traub G, Hausvater A, et al. Myocardial injury after noncardiac surgery: a systematic review and meta-analysis. Cardiol Rev. 2019;27(6):267-273. https://doi.org/10.1097/crd.0000000000000254.
6. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578. https://doi.org/10.1097/aln.0000000000000113.
7. Writing Committee for the VISION Study Investigators, Devereaux PJ, Biccard BM, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317(16):1642-1651. https://doi.org/10.1001/jama.2017.4360.
8. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation. 2018;137(12):1221-1232. https://doi.org/10.1161/circulationaha.117.030114.
9. Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126(6):1936-1945. https://doi.org/10.1213/ane.0000000000002560.
10. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77-e137. https://doi.org/10.1016/j.jacc.2014.07.944.
11. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. https://doi.org/10.1093/eurheartj/ehu282.
12. Devereaux PJ, Duceppe E, Guyatt G, et al. Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trial. Lancet. 2018;391(10137):2325-2334. https://doi.org/10.1016/s0140-6736(18)30832-8.
13. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135(16):1481-1489. https://doi.org/10.1161/circulationaha.116.026336.
14. DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. https://doi.org/10.1161/circulationaha.119.040631.
15. Sandoval Y, Smith SW, Sexter A, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130(12):1431-1439.e4. https://doi.org/10.1016/j.amjmed.2017.05.049.
© 2020 Society of Hospital Medicine
Clinical Progress Note: Point-of-Care Ultrasound Applications in COVID-19
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, was declared a pandemic on March 11, 2020. Although most patients (81%) develop mild illness, 14% develop severe illness, and 5% develop critical illness, including acute respiratory failure, septic shock, and multiorgan dysfunction.1
Point-of-care ultrasound (POCUS), or bedside ultrasound performed by a clinician caring for the patient, is being used to support the diagnosis and serially monitor patients with COVID-19. We performed a literature search of electronically discoverable peer-reviewed publications on POCUS use in COVID-19 from December 1, 2019, to April 10, 2020. We review key POCUS applications that are most relevant to frontline providers in the care of COVID-19 patients.
LUNG AND PLEURAL ULTRASOUND
Diagnosing COVID-19 disease by polymerase chain reaction is limited by availability of testing, delays in test positivity (mean 5.1 days), and high false-negative rate early in the course of the disease (sensitivity 81%).2 Chest computed tomography (CT) scans are often requested during the initial evaluation of suspected COVID-19, but the American College of Radiology has recommend against the routine use of CT scans for diagnosing COVID-19.3
The diagnostic accuracy of lung ultrasound (LUS) has been shown to be similar to chest CT scans in patients presenting with respiratory complaints, such as dyspnea and hypoxemia, caused by non–COVID-19 pneumonia (sensitivity, 85%; specificity, 93%).4 Normal LUS findings correlate well with CT chest scans showing absence of typical ground glass opacities. This negative predictive value is very important.5 However, early in the course of COVID-19, similar to CT scans, LUS may be normal during the first 5 days or in patients with mild disease.2 Unique advantages of LUS in COVID-19 include immediate availability of results, repeatability over time, and performance at the bedside, which avoids transportation of patients to radiology suites and disinfection of large imaging equipment.
LUS findings in COVID-19 include (a) an irregular, thickened pleural line, (b) B-lines in various patterns (discrete and confluent), (c) small subpleural consolidations, and (d) absence of pleural effusions (Figure). Bilateral, multifocal disease is common, while lobar alveolar consolidation is less common.6,7 In addition to supporting the initial diagnosis, LUS is being used to serially monitor hospitalized COVID-19 patients. As lung interstitial fluid content increases, discrete B-lines become confluent, and the number of affected lung zones increases, which can guide decisions about escalation of care. LUS is often used to guide decisions about prone ventilation, extracorporeal membrane oxygenation, and weaning from mechanical ventilation in acute respiratory failure of non–COVID-19 patients,8 and these concepts are being applied to COVID-19 patients. During recovery, reappearance of A-lines can be seen, but normalization of the LUS pattern is gradual over several weeks based on our experience and one report.9 Multiple LUS protocols examining 6 to 12 lung zones have been published prior to the COVID-19 pandemic. We recommend continuing to use an institutional protocol and evaluating at least one to two rib interspaces on the anterior, lateral, and posterior chest wall.

FOCUSED CARDIAC ULTRASOUND
Myriad cardiac complications have been described in COVID-19 – including acute coronary syndrome, myocarditis, cardiomyopathy with heart failure, and arrhythmias – secondary to increased cardiac stress from hypoxia, direct myocardial infection, or indirect injury from a hyperinflammatory response. Mortality is higher in patients with hypertension, diabetes, and coronary artery disease.10,11 Cardiac POCUS is being used to evaluate COVID-19 patients when troponin and B-type natriuretic peptide (BNP) are elevated or when there are hemodynamic or electrocardiogram changes. Given the high incidence of venous thromboembolism (VTE) in COVID-19,12 cardiac POCUS is being used to rapidly assess for right ventricular (RV) dysfunction and acute pulmonary hypertension.
The American Society of Echocardiography has recommended the use of cardiac POCUS by frontline providers for detection or characterization of preexisting cardiovascular disease, early identification of worsening cardiac function, serial monitoring and examination, and elucidation of cardiovascular pathologies associated with COVID-19.13 Sharing cardiac POCUS images in real time or through an image archive can reduce the need for consultative echocardiography, which ultimately reduces staff exposure, conserves personal protective equipment, and reduces need for decontamination of echocardiographic equipment.
The minimum cardiac POCUS views recommended in COVID-19 patients include the parasternal long-axis and short-axis views (midventricular level), either the apical or subcostal four-chamber view, and the subcostal long-axis view of the inferior vena cava.13 The goal of a cardiac POCUS exam is to qualitatively assess left ventricular (LV) systolic function, RV size and contractility, gross valvular and regional wall motion abnormalities, and pericardial effusion. In prone position ventilation, the swimmer’s position with one arm elevated above the shoulder may permit acquisition of apical views. Finally, integrated cardiopulmonary ultrasonography, including evaluation for deep vein thrombosis (DVT; see below), is ideal for proper characterization of underlying LV and RV function, volume status, and titration of vasopressor and inotropic support.
VENOUS THROMBOEMBOLISM
COVID-19 has been associated with a proinflammatory and hypercoagulable state with elevated
A POCUS exam for LE DVT consists of two-dimensional venous compression alone and yields results similar to formal vascular studies in both critically ill and noncritically ill patients. Because proximal LE thrombi have the highest risk of embolization, evaluation of the common femoral vein, femoral vein, and popliteal vein is most important.15 Either inability to compress a vein completely with wall-to-wall apposition or visualization of echogenic thrombus within the vein is diagnostic of DVT. Acute thrombi are gelatinous and may appear anechoic, while subacute or chronic thrombi are echogenic, but all veins with a DVT will not compress completely.
VASCULAR ACCESS
Ultrasound guidance for central venous catheter (CVC) insertion has been shown to increase procedure success rates and decrease mechanical complications, primarily arterial puncture and pneumothorax. Similarly, higher success rates and fewer insertion attempts have been observed with ultrasound-guided peripheral intravenous line and arterial line placement.17 Ultrasound-guided PIV placement can reduce referrals for midlines and peripherally inserted central catheters in hospitalized patients.18
In COVID-19 patients, use of ultrasound guidance for vascular access has distinct advantages. First, given the high incidence of DVT in COVID-19 patients,12 POCUS allows preprocedural evaluation of the target vessel for thrombosis, as well as anatomic variations and stenosis. Second, visualizing the needle tip and guidewire within the target vein prior to dilation nearly eliminates the risk of arterial puncture and inadvertent arterial dilation, which is particularly important in COVID-19 patients receiving high-dose prophylactic or therapeutic anticoagulation. Third, when inserting internal jugular and subclavian CVCs, visualization of normal lung sliding before and after the procedure safely rules out pneumothorax. However, if lung sliding is not seen before the procedure, it cannot be used to rule out pneumothorax afterward. Additionally, visualizing absence of the catheter tip in the right atrium and presence of a rapid atrial swirl sign within 2 seconds of briskly injecting 10 mL of saline confirms catheter tip placement near the superior vena cava/right atrial junction, which can eliminate the need for a postprocedure chest radiograph.17
ENDOTRACHEAL INTUBATION
POCUS can be used to rapidly confirm endotracheal tube (ETT) placement, which can reduce reliance on postintubation chest radiographs. A meta-analysis of prospective and randomized trials showed transtracheal ultrasonography had high sensitivity (98.7%) and specificity (97.1%) for confirming tracheal placement of ETTs.19 Confirming endotracheal intubation involves two steps: First, a linear transducer is placed transversely over the suprasternal notch to visualize the ETT passing through the trachea, and not the esophagus, during insertion. Second, after the ETT cuff has been inflated, bilateral lung sliding should be seen in sync with the respiratory cycle if the ETT is in the trachea. Absent lung sliding, but preserved lung pulse, on the anterior hemithorax is likely caused by main stem bronchial intubation, and withdrawing the ETT until bilateral lung sliding is seen confirms tracheal placement. Additionally, the following steps are recommended to reduce the risk of exposure to healthcare workers: minimizing use of bag-valve-mask ventilation, performing rapid sequence intubation using video laryngoscopy, and connecting the ETT to the ventilator immediately.
ULTRASOUND DEVICES AND DISINFECTION
Important considerations when selecting an ultrasound machine for use in COVID-19 patients include image quality, portability, functionality, and ease of disinfection. Advantages of handheld devices include portability and ease of disinfection, whereas cart-based systems generally have better image quality and functionality. To minimize the risk of cross contamination, an ultrasound machine should be dedicated exclusively for use on patients with confirmed COVID-19 and not shared with patients with suspected COVID-19.20 To minimize exposure to COVID-19 patients, frontline providers should perform POCUS exams only when findings may change management, and timing of the exam and views acquired should be selected deliberately.
Ultrasound machine disinfection should be integrated into routine donning and doffing procedures. When possible, both handheld and cart-based machines should be draped with protective covers during aerosol-generating procedures. Single use ultrasound gel packets are recommended in order to decrease the risk of nosocomial infection.20 After every use of an ultrasound machine on intact skin or for percutaneous procedures, low-level disinfection should be performed with an Environmental Protection Agency–recommended product that is effective against coronavirus.
Some ultrasound manufacturers have added teleultrasound software that allows remote training of novice POCUS users and remote guidance in actual patient care. Teleultrasound can be utilized to share images in real time with consultants or expert providers.
CONCLUSION
POCUS is uniquely poised to improve patient care during the COVID-19 pandemic. POCUS can be used to support the diagnosis of COVID-19 patients and monitor patients with confirmed disease. Common POCUS applications used in COVID-19 patients include evaluation of the lungs, heart, and deep veins, as well as performance of bedside procedures. Ultrasound machine portability and disinfection are important considerations in COVID-19 patients.
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648.
2. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. https://doi.org/10.1148/radiol.2020200642.
3. American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed April 10, 2020.
4. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y.
5. Hew M, Corcoran JP, Harriss EK, Rahman NM, Mallett S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open. 2015;5(5):e007838. https://doi.org/10.1136/bmjopen-2015-007838.
6. Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05996-6.
7. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Soc Sci Res Netw (SSRN). 2020. http://doi.org/10.2139/ssrn.3544750.
8. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701-714. https://doi.org/10.1164/rccm.201802-0236ci.
9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID-19 patient. Qjm. 2020. https://doi.org/10.1093/qjmed/hcaa141.
10. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.1286.
11. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;e201017. https://doi.org/10.1001/jamacardio.2020.1017.
12. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013.
13. Johri AM, Galen B, Kirkpatrick J, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020. https://doi.org/10.1016/j.echo.2020.04.017.
14. American Society of Hematology. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. April 9, 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism. Accessed April 10, 2020.
15. Fischer EA, Kinnear B, Sall D, et al. Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS). J Gen Intern Med. 2019;34(10):2062-2067. https://doi.org/10.1007/s11606-019-05120-5.
16. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;1-3. https://doi.org/10.1007/s00134-020-06040-3.
17. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
18. Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. https://doi.org/10.1136/bmjqs-2019-009923.
19. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med. 2018;72(6):627-636. https://doi.org/10.1016/j.annemergmed.2018.06.024.
20. Abramowicz J, Basseal J. WFUMB Position Statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020. https://doi.org/10.1016/j.ultrasmedbio.2020.03.033.
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, was declared a pandemic on March 11, 2020. Although most patients (81%) develop mild illness, 14% develop severe illness, and 5% develop critical illness, including acute respiratory failure, septic shock, and multiorgan dysfunction.1
Point-of-care ultrasound (POCUS), or bedside ultrasound performed by a clinician caring for the patient, is being used to support the diagnosis and serially monitor patients with COVID-19. We performed a literature search of electronically discoverable peer-reviewed publications on POCUS use in COVID-19 from December 1, 2019, to April 10, 2020. We review key POCUS applications that are most relevant to frontline providers in the care of COVID-19 patients.
LUNG AND PLEURAL ULTRASOUND
Diagnosing COVID-19 disease by polymerase chain reaction is limited by availability of testing, delays in test positivity (mean 5.1 days), and high false-negative rate early in the course of the disease (sensitivity 81%).2 Chest computed tomography (CT) scans are often requested during the initial evaluation of suspected COVID-19, but the American College of Radiology has recommend against the routine use of CT scans for diagnosing COVID-19.3
The diagnostic accuracy of lung ultrasound (LUS) has been shown to be similar to chest CT scans in patients presenting with respiratory complaints, such as dyspnea and hypoxemia, caused by non–COVID-19 pneumonia (sensitivity, 85%; specificity, 93%).4 Normal LUS findings correlate well with CT chest scans showing absence of typical ground glass opacities. This negative predictive value is very important.5 However, early in the course of COVID-19, similar to CT scans, LUS may be normal during the first 5 days or in patients with mild disease.2 Unique advantages of LUS in COVID-19 include immediate availability of results, repeatability over time, and performance at the bedside, which avoids transportation of patients to radiology suites and disinfection of large imaging equipment.
LUS findings in COVID-19 include (a) an irregular, thickened pleural line, (b) B-lines in various patterns (discrete and confluent), (c) small subpleural consolidations, and (d) absence of pleural effusions (Figure). Bilateral, multifocal disease is common, while lobar alveolar consolidation is less common.6,7 In addition to supporting the initial diagnosis, LUS is being used to serially monitor hospitalized COVID-19 patients. As lung interstitial fluid content increases, discrete B-lines become confluent, and the number of affected lung zones increases, which can guide decisions about escalation of care. LUS is often used to guide decisions about prone ventilation, extracorporeal membrane oxygenation, and weaning from mechanical ventilation in acute respiratory failure of non–COVID-19 patients,8 and these concepts are being applied to COVID-19 patients. During recovery, reappearance of A-lines can be seen, but normalization of the LUS pattern is gradual over several weeks based on our experience and one report.9 Multiple LUS protocols examining 6 to 12 lung zones have been published prior to the COVID-19 pandemic. We recommend continuing to use an institutional protocol and evaluating at least one to two rib interspaces on the anterior, lateral, and posterior chest wall.

FOCUSED CARDIAC ULTRASOUND
Myriad cardiac complications have been described in COVID-19 – including acute coronary syndrome, myocarditis, cardiomyopathy with heart failure, and arrhythmias – secondary to increased cardiac stress from hypoxia, direct myocardial infection, or indirect injury from a hyperinflammatory response. Mortality is higher in patients with hypertension, diabetes, and coronary artery disease.10,11 Cardiac POCUS is being used to evaluate COVID-19 patients when troponin and B-type natriuretic peptide (BNP) are elevated or when there are hemodynamic or electrocardiogram changes. Given the high incidence of venous thromboembolism (VTE) in COVID-19,12 cardiac POCUS is being used to rapidly assess for right ventricular (RV) dysfunction and acute pulmonary hypertension.
The American Society of Echocardiography has recommended the use of cardiac POCUS by frontline providers for detection or characterization of preexisting cardiovascular disease, early identification of worsening cardiac function, serial monitoring and examination, and elucidation of cardiovascular pathologies associated with COVID-19.13 Sharing cardiac POCUS images in real time or through an image archive can reduce the need for consultative echocardiography, which ultimately reduces staff exposure, conserves personal protective equipment, and reduces need for decontamination of echocardiographic equipment.
The minimum cardiac POCUS views recommended in COVID-19 patients include the parasternal long-axis and short-axis views (midventricular level), either the apical or subcostal four-chamber view, and the subcostal long-axis view of the inferior vena cava.13 The goal of a cardiac POCUS exam is to qualitatively assess left ventricular (LV) systolic function, RV size and contractility, gross valvular and regional wall motion abnormalities, and pericardial effusion. In prone position ventilation, the swimmer’s position with one arm elevated above the shoulder may permit acquisition of apical views. Finally, integrated cardiopulmonary ultrasonography, including evaluation for deep vein thrombosis (DVT; see below), is ideal for proper characterization of underlying LV and RV function, volume status, and titration of vasopressor and inotropic support.
VENOUS THROMBOEMBOLISM
COVID-19 has been associated with a proinflammatory and hypercoagulable state with elevated
A POCUS exam for LE DVT consists of two-dimensional venous compression alone and yields results similar to formal vascular studies in both critically ill and noncritically ill patients. Because proximal LE thrombi have the highest risk of embolization, evaluation of the common femoral vein, femoral vein, and popliteal vein is most important.15 Either inability to compress a vein completely with wall-to-wall apposition or visualization of echogenic thrombus within the vein is diagnostic of DVT. Acute thrombi are gelatinous and may appear anechoic, while subacute or chronic thrombi are echogenic, but all veins with a DVT will not compress completely.
VASCULAR ACCESS
Ultrasound guidance for central venous catheter (CVC) insertion has been shown to increase procedure success rates and decrease mechanical complications, primarily arterial puncture and pneumothorax. Similarly, higher success rates and fewer insertion attempts have been observed with ultrasound-guided peripheral intravenous line and arterial line placement.17 Ultrasound-guided PIV placement can reduce referrals for midlines and peripherally inserted central catheters in hospitalized patients.18
In COVID-19 patients, use of ultrasound guidance for vascular access has distinct advantages. First, given the high incidence of DVT in COVID-19 patients,12 POCUS allows preprocedural evaluation of the target vessel for thrombosis, as well as anatomic variations and stenosis. Second, visualizing the needle tip and guidewire within the target vein prior to dilation nearly eliminates the risk of arterial puncture and inadvertent arterial dilation, which is particularly important in COVID-19 patients receiving high-dose prophylactic or therapeutic anticoagulation. Third, when inserting internal jugular and subclavian CVCs, visualization of normal lung sliding before and after the procedure safely rules out pneumothorax. However, if lung sliding is not seen before the procedure, it cannot be used to rule out pneumothorax afterward. Additionally, visualizing absence of the catheter tip in the right atrium and presence of a rapid atrial swirl sign within 2 seconds of briskly injecting 10 mL of saline confirms catheter tip placement near the superior vena cava/right atrial junction, which can eliminate the need for a postprocedure chest radiograph.17
ENDOTRACHEAL INTUBATION
POCUS can be used to rapidly confirm endotracheal tube (ETT) placement, which can reduce reliance on postintubation chest radiographs. A meta-analysis of prospective and randomized trials showed transtracheal ultrasonography had high sensitivity (98.7%) and specificity (97.1%) for confirming tracheal placement of ETTs.19 Confirming endotracheal intubation involves two steps: First, a linear transducer is placed transversely over the suprasternal notch to visualize the ETT passing through the trachea, and not the esophagus, during insertion. Second, after the ETT cuff has been inflated, bilateral lung sliding should be seen in sync with the respiratory cycle if the ETT is in the trachea. Absent lung sliding, but preserved lung pulse, on the anterior hemithorax is likely caused by main stem bronchial intubation, and withdrawing the ETT until bilateral lung sliding is seen confirms tracheal placement. Additionally, the following steps are recommended to reduce the risk of exposure to healthcare workers: minimizing use of bag-valve-mask ventilation, performing rapid sequence intubation using video laryngoscopy, and connecting the ETT to the ventilator immediately.
ULTRASOUND DEVICES AND DISINFECTION
Important considerations when selecting an ultrasound machine for use in COVID-19 patients include image quality, portability, functionality, and ease of disinfection. Advantages of handheld devices include portability and ease of disinfection, whereas cart-based systems generally have better image quality and functionality. To minimize the risk of cross contamination, an ultrasound machine should be dedicated exclusively for use on patients with confirmed COVID-19 and not shared with patients with suspected COVID-19.20 To minimize exposure to COVID-19 patients, frontline providers should perform POCUS exams only when findings may change management, and timing of the exam and views acquired should be selected deliberately.
Ultrasound machine disinfection should be integrated into routine donning and doffing procedures. When possible, both handheld and cart-based machines should be draped with protective covers during aerosol-generating procedures. Single use ultrasound gel packets are recommended in order to decrease the risk of nosocomial infection.20 After every use of an ultrasound machine on intact skin or for percutaneous procedures, low-level disinfection should be performed with an Environmental Protection Agency–recommended product that is effective against coronavirus.
Some ultrasound manufacturers have added teleultrasound software that allows remote training of novice POCUS users and remote guidance in actual patient care. Teleultrasound can be utilized to share images in real time with consultants or expert providers.
CONCLUSION
POCUS is uniquely poised to improve patient care during the COVID-19 pandemic. POCUS can be used to support the diagnosis of COVID-19 patients and monitor patients with confirmed disease. Common POCUS applications used in COVID-19 patients include evaluation of the lungs, heart, and deep veins, as well as performance of bedside procedures. Ultrasound machine portability and disinfection are important considerations in COVID-19 patients.
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, was declared a pandemic on March 11, 2020. Although most patients (81%) develop mild illness, 14% develop severe illness, and 5% develop critical illness, including acute respiratory failure, septic shock, and multiorgan dysfunction.1
Point-of-care ultrasound (POCUS), or bedside ultrasound performed by a clinician caring for the patient, is being used to support the diagnosis and serially monitor patients with COVID-19. We performed a literature search of electronically discoverable peer-reviewed publications on POCUS use in COVID-19 from December 1, 2019, to April 10, 2020. We review key POCUS applications that are most relevant to frontline providers in the care of COVID-19 patients.
LUNG AND PLEURAL ULTRASOUND
Diagnosing COVID-19 disease by polymerase chain reaction is limited by availability of testing, delays in test positivity (mean 5.1 days), and high false-negative rate early in the course of the disease (sensitivity 81%).2 Chest computed tomography (CT) scans are often requested during the initial evaluation of suspected COVID-19, but the American College of Radiology has recommend against the routine use of CT scans for diagnosing COVID-19.3
The diagnostic accuracy of lung ultrasound (LUS) has been shown to be similar to chest CT scans in patients presenting with respiratory complaints, such as dyspnea and hypoxemia, caused by non–COVID-19 pneumonia (sensitivity, 85%; specificity, 93%).4 Normal LUS findings correlate well with CT chest scans showing absence of typical ground glass opacities. This negative predictive value is very important.5 However, early in the course of COVID-19, similar to CT scans, LUS may be normal during the first 5 days or in patients with mild disease.2 Unique advantages of LUS in COVID-19 include immediate availability of results, repeatability over time, and performance at the bedside, which avoids transportation of patients to radiology suites and disinfection of large imaging equipment.
LUS findings in COVID-19 include (a) an irregular, thickened pleural line, (b) B-lines in various patterns (discrete and confluent), (c) small subpleural consolidations, and (d) absence of pleural effusions (Figure). Bilateral, multifocal disease is common, while lobar alveolar consolidation is less common.6,7 In addition to supporting the initial diagnosis, LUS is being used to serially monitor hospitalized COVID-19 patients. As lung interstitial fluid content increases, discrete B-lines become confluent, and the number of affected lung zones increases, which can guide decisions about escalation of care. LUS is often used to guide decisions about prone ventilation, extracorporeal membrane oxygenation, and weaning from mechanical ventilation in acute respiratory failure of non–COVID-19 patients,8 and these concepts are being applied to COVID-19 patients. During recovery, reappearance of A-lines can be seen, but normalization of the LUS pattern is gradual over several weeks based on our experience and one report.9 Multiple LUS protocols examining 6 to 12 lung zones have been published prior to the COVID-19 pandemic. We recommend continuing to use an institutional protocol and evaluating at least one to two rib interspaces on the anterior, lateral, and posterior chest wall.

FOCUSED CARDIAC ULTRASOUND
Myriad cardiac complications have been described in COVID-19 – including acute coronary syndrome, myocarditis, cardiomyopathy with heart failure, and arrhythmias – secondary to increased cardiac stress from hypoxia, direct myocardial infection, or indirect injury from a hyperinflammatory response. Mortality is higher in patients with hypertension, diabetes, and coronary artery disease.10,11 Cardiac POCUS is being used to evaluate COVID-19 patients when troponin and B-type natriuretic peptide (BNP) are elevated or when there are hemodynamic or electrocardiogram changes. Given the high incidence of venous thromboembolism (VTE) in COVID-19,12 cardiac POCUS is being used to rapidly assess for right ventricular (RV) dysfunction and acute pulmonary hypertension.
The American Society of Echocardiography has recommended the use of cardiac POCUS by frontline providers for detection or characterization of preexisting cardiovascular disease, early identification of worsening cardiac function, serial monitoring and examination, and elucidation of cardiovascular pathologies associated with COVID-19.13 Sharing cardiac POCUS images in real time or through an image archive can reduce the need for consultative echocardiography, which ultimately reduces staff exposure, conserves personal protective equipment, and reduces need for decontamination of echocardiographic equipment.
The minimum cardiac POCUS views recommended in COVID-19 patients include the parasternal long-axis and short-axis views (midventricular level), either the apical or subcostal four-chamber view, and the subcostal long-axis view of the inferior vena cava.13 The goal of a cardiac POCUS exam is to qualitatively assess left ventricular (LV) systolic function, RV size and contractility, gross valvular and regional wall motion abnormalities, and pericardial effusion. In prone position ventilation, the swimmer’s position with one arm elevated above the shoulder may permit acquisition of apical views. Finally, integrated cardiopulmonary ultrasonography, including evaluation for deep vein thrombosis (DVT; see below), is ideal for proper characterization of underlying LV and RV function, volume status, and titration of vasopressor and inotropic support.
VENOUS THROMBOEMBOLISM
COVID-19 has been associated with a proinflammatory and hypercoagulable state with elevated
A POCUS exam for LE DVT consists of two-dimensional venous compression alone and yields results similar to formal vascular studies in both critically ill and noncritically ill patients. Because proximal LE thrombi have the highest risk of embolization, evaluation of the common femoral vein, femoral vein, and popliteal vein is most important.15 Either inability to compress a vein completely with wall-to-wall apposition or visualization of echogenic thrombus within the vein is diagnostic of DVT. Acute thrombi are gelatinous and may appear anechoic, while subacute or chronic thrombi are echogenic, but all veins with a DVT will not compress completely.
VASCULAR ACCESS
Ultrasound guidance for central venous catheter (CVC) insertion has been shown to increase procedure success rates and decrease mechanical complications, primarily arterial puncture and pneumothorax. Similarly, higher success rates and fewer insertion attempts have been observed with ultrasound-guided peripheral intravenous line and arterial line placement.17 Ultrasound-guided PIV placement can reduce referrals for midlines and peripherally inserted central catheters in hospitalized patients.18
In COVID-19 patients, use of ultrasound guidance for vascular access has distinct advantages. First, given the high incidence of DVT in COVID-19 patients,12 POCUS allows preprocedural evaluation of the target vessel for thrombosis, as well as anatomic variations and stenosis. Second, visualizing the needle tip and guidewire within the target vein prior to dilation nearly eliminates the risk of arterial puncture and inadvertent arterial dilation, which is particularly important in COVID-19 patients receiving high-dose prophylactic or therapeutic anticoagulation. Third, when inserting internal jugular and subclavian CVCs, visualization of normal lung sliding before and after the procedure safely rules out pneumothorax. However, if lung sliding is not seen before the procedure, it cannot be used to rule out pneumothorax afterward. Additionally, visualizing absence of the catheter tip in the right atrium and presence of a rapid atrial swirl sign within 2 seconds of briskly injecting 10 mL of saline confirms catheter tip placement near the superior vena cava/right atrial junction, which can eliminate the need for a postprocedure chest radiograph.17
ENDOTRACHEAL INTUBATION
POCUS can be used to rapidly confirm endotracheal tube (ETT) placement, which can reduce reliance on postintubation chest radiographs. A meta-analysis of prospective and randomized trials showed transtracheal ultrasonography had high sensitivity (98.7%) and specificity (97.1%) for confirming tracheal placement of ETTs.19 Confirming endotracheal intubation involves two steps: First, a linear transducer is placed transversely over the suprasternal notch to visualize the ETT passing through the trachea, and not the esophagus, during insertion. Second, after the ETT cuff has been inflated, bilateral lung sliding should be seen in sync with the respiratory cycle if the ETT is in the trachea. Absent lung sliding, but preserved lung pulse, on the anterior hemithorax is likely caused by main stem bronchial intubation, and withdrawing the ETT until bilateral lung sliding is seen confirms tracheal placement. Additionally, the following steps are recommended to reduce the risk of exposure to healthcare workers: minimizing use of bag-valve-mask ventilation, performing rapid sequence intubation using video laryngoscopy, and connecting the ETT to the ventilator immediately.
ULTRASOUND DEVICES AND DISINFECTION
Important considerations when selecting an ultrasound machine for use in COVID-19 patients include image quality, portability, functionality, and ease of disinfection. Advantages of handheld devices include portability and ease of disinfection, whereas cart-based systems generally have better image quality and functionality. To minimize the risk of cross contamination, an ultrasound machine should be dedicated exclusively for use on patients with confirmed COVID-19 and not shared with patients with suspected COVID-19.20 To minimize exposure to COVID-19 patients, frontline providers should perform POCUS exams only when findings may change management, and timing of the exam and views acquired should be selected deliberately.
Ultrasound machine disinfection should be integrated into routine donning and doffing procedures. When possible, both handheld and cart-based machines should be draped with protective covers during aerosol-generating procedures. Single use ultrasound gel packets are recommended in order to decrease the risk of nosocomial infection.20 After every use of an ultrasound machine on intact skin or for percutaneous procedures, low-level disinfection should be performed with an Environmental Protection Agency–recommended product that is effective against coronavirus.
Some ultrasound manufacturers have added teleultrasound software that allows remote training of novice POCUS users and remote guidance in actual patient care. Teleultrasound can be utilized to share images in real time with consultants or expert providers.
CONCLUSION
POCUS is uniquely poised to improve patient care during the COVID-19 pandemic. POCUS can be used to support the diagnosis of COVID-19 patients and monitor patients with confirmed disease. Common POCUS applications used in COVID-19 patients include evaluation of the lungs, heart, and deep veins, as well as performance of bedside procedures. Ultrasound machine portability and disinfection are important considerations in COVID-19 patients.
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648.
2. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. https://doi.org/10.1148/radiol.2020200642.
3. American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed April 10, 2020.
4. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y.
5. Hew M, Corcoran JP, Harriss EK, Rahman NM, Mallett S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open. 2015;5(5):e007838. https://doi.org/10.1136/bmjopen-2015-007838.
6. Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05996-6.
7. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Soc Sci Res Netw (SSRN). 2020. http://doi.org/10.2139/ssrn.3544750.
8. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701-714. https://doi.org/10.1164/rccm.201802-0236ci.
9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID-19 patient. Qjm. 2020. https://doi.org/10.1093/qjmed/hcaa141.
10. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.1286.
11. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;e201017. https://doi.org/10.1001/jamacardio.2020.1017.
12. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013.
13. Johri AM, Galen B, Kirkpatrick J, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020. https://doi.org/10.1016/j.echo.2020.04.017.
14. American Society of Hematology. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. April 9, 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism. Accessed April 10, 2020.
15. Fischer EA, Kinnear B, Sall D, et al. Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS). J Gen Intern Med. 2019;34(10):2062-2067. https://doi.org/10.1007/s11606-019-05120-5.
16. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;1-3. https://doi.org/10.1007/s00134-020-06040-3.
17. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
18. Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. https://doi.org/10.1136/bmjqs-2019-009923.
19. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med. 2018;72(6):627-636. https://doi.org/10.1016/j.annemergmed.2018.06.024.
20. Abramowicz J, Basseal J. WFUMB Position Statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020. https://doi.org/10.1016/j.ultrasmedbio.2020.03.033.
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. https://doi.org/10.1001/jama.2020.2648.
2. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. https://doi.org/10.1148/radiol.2020200642.
3. American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. March 11, 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed April 10, 2020.
4. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y.
5. Hew M, Corcoran JP, Harriss EK, Rahman NM, Mallett S. The diagnostic accuracy of chest ultrasound for CT-detected radiographic consolidation in hospitalised adults with acute respiratory failure: a systematic review. BMJ Open. 2015;5(5):e007838. https://doi.org/10.1136/bmjopen-2015-007838.
6. Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-05996-6.
7. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19). Soc Sci Res Netw (SSRN). 2020. http://doi.org/10.2139/ssrn.3544750.
8. Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit Care Med. 2019;199(6):701-714. https://doi.org/10.1164/rccm.201802-0236ci.
9. Ji L, Cao C, Lv Q, Li Y, Xie M. Serial bedside lung ultrasonography in a critically ill COVID-19 patient. Qjm. 2020. https://doi.org/10.1093/qjmed/hcaa141.
10. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. https://doi.org/10.1001/jamacardio.2020.1286.
11. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;e201017. https://doi.org/10.1001/jamacardio.2020.1017.
12. Klok F, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Throm Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013.
13. Johri AM, Galen B, Kirkpatrick J, Lanspa M, Mulvagh S, Thamman R. ASE statement on point-of-care ultrasound (POCUS) during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020. https://doi.org/10.1016/j.echo.2020.04.017.
14. American Society of Hematology. COVID-19 and Pulmonary Embolism: Frequently Asked Questions. April 9, 2020. https://www.hematology.org/covid-19/covid-19-and-pulmonary-embolism. Accessed April 10, 2020.
15. Fischer EA, Kinnear B, Sall D, et al. Hospitalist-Operated Compression Ultrasonography: a Point-of-Care Ultrasound Study (HOCUS-POCUS). J Gen Intern Med. 2019;34(10):2062-2067. https://doi.org/10.1007/s11606-019-05120-5.
16. Tavazzi G, Civardi L, Caneva L, Mongodi S, Mojoli F. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;1-3. https://doi.org/10.1007/s00134-020-06040-3.
17. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
18. Galen B, Baron S, Young S, Hall A, Berger-Spivack L, Southern W. Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf. 2020;29(3):245-249. https://doi.org/10.1136/bmjqs-2019-009923.
19. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med. 2018;72(6):627-636. https://doi.org/10.1016/j.annemergmed.2018.06.024.
20. Abramowicz J, Basseal J. WFUMB Position Statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020. https://doi.org/10.1016/j.ultrasmedbio.2020.03.033.
© 2020 Society of Hospital Medicine
Clinical Progress Note: Perioperative Pain Control in Hospitalized Pediatric Patients
Pediatric hospitalists play an increasingly significant role in perioperative pain management.1 Advances in pediatric surgical comanagement may improve quality of care and reduce the length of hospitalization.2 This review is based on queries of the PubMed and Cochrane databases between January 1, 2014, and July 15, 2019, using the search terms “perioperative pain management,” “postoperative pain,” “pediatric,” and “children.” In addition, the authors reviewed key position statements from the American Academy of Pediatrics (AAP), the American Pain Society (APS), the Centers for Disease Control and Prevention (CDC), and the Society of Hospital Medicine (SHM) regarding pain management.3 This update is intended to be relevant for practicing pediatric hospitalists, with a focus on recently expanded options for pain management and judicious opioid use in hospitalized children.
PERIOPERATIVE PAIN MANAGEMENT
Postoperative pain management begins preoperatively according to the concept of the perioperative surgical home (PSH).4 The preoperative history should identify the patient’s previous positive (eg, good pain control) and negative (eg, adverse reactions) experiences with pain medications. Family and patient expectations should be discussed regarding types and sources of pain, pain duration, exacerbating/alleviating factors, and modalities available for realistic pain control because preoperative information can limit anxiety and improve outcomes. Pain specialists can perform risk assessments preoperatively and develop plans to address pharmacologic tolerance, withdrawal, and opioid-induced hyperalgesia after surgery.5 Children with chronic pain and on preoperative opioids may require more analgesia for a longer duration postoperatively. Early recognition of variability of patient’s pain perception and differences in responses to pain need to be clearly communicated across the disciplines in a collaborative model of care.
Children with medical complexity and/or cognitive, emotional, or behavioral impairments may benefit from preoperative psychosocial treatments and utilization of pain self-management training and strategies that could further reduce anxiety and optimize postoperative care because patient and parental preoperative anxiety may be associated with adverse outcomes. Validated pain assessment tools like Revised FLACC (Face, Leg, Activity, Cry, and Consolability) Scale and Individualized Numeric Rating Scale could be particularly useful in children with limitations in communication or altered pain perception; therefore, medical teams and family members should discuss their utilization preoperatively.
MULTIMODAL ANALGESIA
Multimodal analgesia (MMA) is a strategy that synergistically uses pharmacologic and nonpharmacologic modalities to target pain at multiple points of the pain processing pathway (Table).6 MMA can optimize pain control by addressing different types of pain (eg, incisional pain, muscle spasm, or neuropathic pain), expedite recovery, reduce potential pharmacologic side effects, and decrease opioid consumption. Patients taking opioids are at an increased risk of developing opioid-related side effects such as respiratory depression, medication tolerance, and anxiety, with resultant longer hospital stay, increased readmissions, and higher costs of care.7 Treatment for postoperative pain should prioritize appropriately dosed and precisely scheduled MMA before opioid-focused analgesia with the goals of decreasing opioid-related adverse effects, intentional misuse, diversion, and accidental ingestions. The AAP, APS, CDC, and SHM endorse the use of MMA and recommend nonpharmacologic measures and regional anesthesia.8,9 The most used modalities in MMA are discussed below.
Acetaminophen
Acetaminophen has central-acting analgesic and antipyretic properties and readily crosses the blood brain barrier, which makes it particularly useful in spine and neurological surgeries. Oral administration is preferred when feasible. The AAP recommends refraining from rectal administration of acetaminophen as analgesia in children because of concerns about toxic effects and erratic, variable absorption.10 A systematic review of six studies found no benefit in pain control between intravenous (IV) and oral (PO) administration of acetaminophen in adults.11 There is a paucity of studies in children comparing PO with IV acetaminophen perioperative efficacy. Children may benefit from IV formulations in the early postoperative period, in cases with frequent nausea and vomiting, and in those with oral medication intolerance. Since infants have greater risk of respiratory depression from opioids, IV acetaminophen may have utility in this age group. Because of the cost associated with IV formulation, some institutions restrict IV acetaminophen. However, rapidly well-controlled pain and minimization of opioid-related side effects with shorter hospital stays may lower healthcare costs despite the cost of acetaminophen itself.
NSAIDs
NSAIDs possess anti-inflammatory properties through the inhibition of cyclooxygenase and blockade of prostaglandin production. NSAID risks include bleeding, renal and gastrointestinal toxicities, and potentially delayed wound and bone healing. Ketorolac is an NSAID that continues to be widely used with demonstrated opioid-sparing effects. Many retrospective studies including large numbers of pediatric patients have not demonstrated increased risks of bleeding nor poor wound healing with short postoperative use. A Cochrane review, however, concluded that there is insufficient data to either support or reject the efficacy or safety of ketorolac for postoperative pain treatment in children, mostly because of the very low quality of evidence.12
Regional Anesthesia
Regional anesthesia, which includes central (spinal/epidural/caudal) and peripheral blocks, decreases postoperative pain and opioid-associated side effects. Blocks typically consist of local anesthetic with or without the addition of adjuncts (eg, clonidine, dexamethasone). Regional anesthesia may also improve pulmonary function, compared with that of nonregional MMA use, in patients who have thoracic or upper abdominal surgeries. While having broad applications, the utility of regional anesthesia is greatest in preterm infants/neonates and in those with underlying respiratory pathology. A systematic review of randomized controlled trials demonstrated that regional anesthesia decreased opioid consumption and minimized postoperative pain with no significant complications attributed to its use.13 Additional studies are needed to better delineate specific surgical procedures and subpopulations of pediatric patients in which regional anesthesia may provide the most benefit.
Gabapentinoids
Children receiving gabapentinoids perioperatively have been shown to have fewer adverse reactions, decreased opioid consumption, and less anxiety, as well as improved pain scores. Gabapentin is increasingly being utilized for children with idiopathic scoliosis undergoing posterior spinal fusion, and there is some evidence for improving pain control and reducing opioid use. However, a recent systematic review found a paucity of data supporting its clinical use.14 Both gabapentin and pregabalin may further increase risks of respiratory depression, especially in synergy with opioids and benzodiazepines.
Opioids
Opioids should be used with caution in pediatric patients and are reserved primarily for the management of severe acute pain. The shortest duration of the lowest effective dose of opioids should be encouraged. Patient-controlled opioid analgesia (PCA) offers benefits when parenteral postoperative analgesia is indicated: It maximizes pain relief, minimizes risk of overdose, and improves psychological well-being through self-administration of pain medicines. Basal-infusion PCA should not be routinely used because it is associated with nausea, vomiting, and respiratory depression without having superior analgesia compared with demand use only. Monitoring of side stream end-tidal capnography can readily detect respiratory depression, especially if opioids, benzodiazepines, gabapentinoids, and diphenhydramine are used concomitantly. Patient education regarding opioid use, side effects, safe storage, and disposal practices is imperative because significant amounts of opioids remain in households after completion of treatment for pain and because opioid diversion and accidental ingestions account for significant morbidity. Providers need to balance efficient pain management with opioid stewardship, complying with state and federal policies to limit harm related to opioid diversion.15
Nonpharmacological Modalities
The use of nonpharmacologic therapies, along with pharmacologic modalities, for perioperative pain management has been shown to decrease opioid use and opioid-related side effects. Trials of acupressure have demonstrated improvement in nausea and vomiting, sleep quality, and pain and anxiety scores. Nonpharmacologic treatments currently serve as a complementary approach for pain and anxiety management in the perioperative setting including acupuncture, acupressure, osteopathic manipulative treatment, massage, meditation, biofeedback, hypnotherapy, and physical/occupational, relaxation, cognitive-behavioral, chiropractic, music, and art therapies. The Joint Commission suggests consideration of such modalities by hospitals.
FUTURE CONSIDERATIONS
Pediatric hospitalists have been traditionally involved in research and patient care improvements and should continue to actively contribute to establishing evidence-based guidelines for the treatment of acute postoperative pain in hospitalized children and adolescents. The sparsity of high-quality evidence prompts the need for more research. A standardized approach to perioperative pain management in the form of checklists, pathways, and protocols for specific procedures may be useful to educate providers and patients, while also standardizing available evidence-based interventions (eg, pediatric Enhanced Recovery After Surgery [ERAS] protocols).
CONCLUSION
Combining multimodal pharmacologic and integrative nonpharmacologic modalities can decrease opioid use and related side effects and improve the perioperative care of hospitalized children. Pediatric hospitalists have an opportunity to optimize care preoperatively, practice multimodal analgesia, and contribute to reducing risk of opioid diversion post operatively.
1. Society of Hospital Medicine Co-Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co-management. http://tools.hospitalmedicine.org/Implementation/Co-ManagementWhitePaper-final_5-10-10.pdf. Accessed October 11, 2019.
2. Rappaport DI, Rosenberg RE, Shaughnessy EE, et al. Pediatric hospitalist comanagement of surgical patients: Structural, quality, and financial considerations. J Hosp Med. 2014;9(11):737-742. https://doi.org/10.1002/jhm.2266.
3. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. http://www.nonpharmpaincare.org. Accessed on October 11, 2019.
4. Vetter TR, Kain ZN. Role of perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. https://doi.org/10.1213/ANE.0000000000002280.
5. Edwards DA, Hedrick TL, Jayaram J, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on perioperative management of patients on preoperative opioid therapy. Anesth Analg. 2019;129(2):553-566. http://doi.org/10.1213/ANE.0000000000004018.
6. Micromedex (electronic version). IBM Watson Health. Greenwood Village, Colorado, USA. https://www.micromedexsolutions.com. Accessed October 10, 2019.
7. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. https://doi.org/10.1016/j.jpain.2015.12.008.
8. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: A consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;3(4):263-266. https://doi.org/10.12788/jhm.2980.
9. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA. 2016;315(15):1624-1645. https://doi.org/10.1001/jama.2016.1464.
10. American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108 (4):1020-1024. https://doi.org/10.1542/peds.108.4.1020.
11. Jibril F, Sharaby S, Mohamed A, Wilby, KJ. Intravenous versus oral acetaminophen for pain: Systemic review of current evidence to support clinical decision-making. Can J Hosp Pharm. 2015;68(3):238-247. https://doi.org/10.4212/cjhp.v68i3.1458.
12. McNicol ED, Rowe E, Cooper TE. Ketorolac for postoperative pain in children. Cochrane Database Syst Rev. 2018;7(7). https://doi.org/10.1002/14651858.CD012294.pub2.
13. Kendall MC, Castro Alves LJ, Suh EI, McCormick ZL, De Oliveira GS. Regional anesthesia to ameliorate postoperative analgesia outcomes in pediatric surgical patients: an updated systematic review of randomized controlled trials. Local Reg Anesth. 2018;11:91-109. https://doi.org/10.2147/LRA.S185554.
14. Egunsola 0, Wylie CE, Chitty KM, et al. Systematic review of the efficacy and safety of gabapentin and pregabalin for pain in children and adolescents. Anesth Analg. 2019;128(4):811-819. https://doi.org/10.1213/ANE.0000000000003936.
15. Harbaugh C, Gadepalli SK. Pediatric postoperative opioid prescribing and the opioid crisis. Curr Opin Pediatr. 2019;31(3):377-385. https://doi.org/10.1097/MOP.0000000000000768.
Pediatric hospitalists play an increasingly significant role in perioperative pain management.1 Advances in pediatric surgical comanagement may improve quality of care and reduce the length of hospitalization.2 This review is based on queries of the PubMed and Cochrane databases between January 1, 2014, and July 15, 2019, using the search terms “perioperative pain management,” “postoperative pain,” “pediatric,” and “children.” In addition, the authors reviewed key position statements from the American Academy of Pediatrics (AAP), the American Pain Society (APS), the Centers for Disease Control and Prevention (CDC), and the Society of Hospital Medicine (SHM) regarding pain management.3 This update is intended to be relevant for practicing pediatric hospitalists, with a focus on recently expanded options for pain management and judicious opioid use in hospitalized children.
PERIOPERATIVE PAIN MANAGEMENT
Postoperative pain management begins preoperatively according to the concept of the perioperative surgical home (PSH).4 The preoperative history should identify the patient’s previous positive (eg, good pain control) and negative (eg, adverse reactions) experiences with pain medications. Family and patient expectations should be discussed regarding types and sources of pain, pain duration, exacerbating/alleviating factors, and modalities available for realistic pain control because preoperative information can limit anxiety and improve outcomes. Pain specialists can perform risk assessments preoperatively and develop plans to address pharmacologic tolerance, withdrawal, and opioid-induced hyperalgesia after surgery.5 Children with chronic pain and on preoperative opioids may require more analgesia for a longer duration postoperatively. Early recognition of variability of patient’s pain perception and differences in responses to pain need to be clearly communicated across the disciplines in a collaborative model of care.
Children with medical complexity and/or cognitive, emotional, or behavioral impairments may benefit from preoperative psychosocial treatments and utilization of pain self-management training and strategies that could further reduce anxiety and optimize postoperative care because patient and parental preoperative anxiety may be associated with adverse outcomes. Validated pain assessment tools like Revised FLACC (Face, Leg, Activity, Cry, and Consolability) Scale and Individualized Numeric Rating Scale could be particularly useful in children with limitations in communication or altered pain perception; therefore, medical teams and family members should discuss their utilization preoperatively.
MULTIMODAL ANALGESIA
Multimodal analgesia (MMA) is a strategy that synergistically uses pharmacologic and nonpharmacologic modalities to target pain at multiple points of the pain processing pathway (Table).6 MMA can optimize pain control by addressing different types of pain (eg, incisional pain, muscle spasm, or neuropathic pain), expedite recovery, reduce potential pharmacologic side effects, and decrease opioid consumption. Patients taking opioids are at an increased risk of developing opioid-related side effects such as respiratory depression, medication tolerance, and anxiety, with resultant longer hospital stay, increased readmissions, and higher costs of care.7 Treatment for postoperative pain should prioritize appropriately dosed and precisely scheduled MMA before opioid-focused analgesia with the goals of decreasing opioid-related adverse effects, intentional misuse, diversion, and accidental ingestions. The AAP, APS, CDC, and SHM endorse the use of MMA and recommend nonpharmacologic measures and regional anesthesia.8,9 The most used modalities in MMA are discussed below.
Acetaminophen
Acetaminophen has central-acting analgesic and antipyretic properties and readily crosses the blood brain barrier, which makes it particularly useful in spine and neurological surgeries. Oral administration is preferred when feasible. The AAP recommends refraining from rectal administration of acetaminophen as analgesia in children because of concerns about toxic effects and erratic, variable absorption.10 A systematic review of six studies found no benefit in pain control between intravenous (IV) and oral (PO) administration of acetaminophen in adults.11 There is a paucity of studies in children comparing PO with IV acetaminophen perioperative efficacy. Children may benefit from IV formulations in the early postoperative period, in cases with frequent nausea and vomiting, and in those with oral medication intolerance. Since infants have greater risk of respiratory depression from opioids, IV acetaminophen may have utility in this age group. Because of the cost associated with IV formulation, some institutions restrict IV acetaminophen. However, rapidly well-controlled pain and minimization of opioid-related side effects with shorter hospital stays may lower healthcare costs despite the cost of acetaminophen itself.
NSAIDs
NSAIDs possess anti-inflammatory properties through the inhibition of cyclooxygenase and blockade of prostaglandin production. NSAID risks include bleeding, renal and gastrointestinal toxicities, and potentially delayed wound and bone healing. Ketorolac is an NSAID that continues to be widely used with demonstrated opioid-sparing effects. Many retrospective studies including large numbers of pediatric patients have not demonstrated increased risks of bleeding nor poor wound healing with short postoperative use. A Cochrane review, however, concluded that there is insufficient data to either support or reject the efficacy or safety of ketorolac for postoperative pain treatment in children, mostly because of the very low quality of evidence.12
Regional Anesthesia
Regional anesthesia, which includes central (spinal/epidural/caudal) and peripheral blocks, decreases postoperative pain and opioid-associated side effects. Blocks typically consist of local anesthetic with or without the addition of adjuncts (eg, clonidine, dexamethasone). Regional anesthesia may also improve pulmonary function, compared with that of nonregional MMA use, in patients who have thoracic or upper abdominal surgeries. While having broad applications, the utility of regional anesthesia is greatest in preterm infants/neonates and in those with underlying respiratory pathology. A systematic review of randomized controlled trials demonstrated that regional anesthesia decreased opioid consumption and minimized postoperative pain with no significant complications attributed to its use.13 Additional studies are needed to better delineate specific surgical procedures and subpopulations of pediatric patients in which regional anesthesia may provide the most benefit.
Gabapentinoids
Children receiving gabapentinoids perioperatively have been shown to have fewer adverse reactions, decreased opioid consumption, and less anxiety, as well as improved pain scores. Gabapentin is increasingly being utilized for children with idiopathic scoliosis undergoing posterior spinal fusion, and there is some evidence for improving pain control and reducing opioid use. However, a recent systematic review found a paucity of data supporting its clinical use.14 Both gabapentin and pregabalin may further increase risks of respiratory depression, especially in synergy with opioids and benzodiazepines.
Opioids
Opioids should be used with caution in pediatric patients and are reserved primarily for the management of severe acute pain. The shortest duration of the lowest effective dose of opioids should be encouraged. Patient-controlled opioid analgesia (PCA) offers benefits when parenteral postoperative analgesia is indicated: It maximizes pain relief, minimizes risk of overdose, and improves psychological well-being through self-administration of pain medicines. Basal-infusion PCA should not be routinely used because it is associated with nausea, vomiting, and respiratory depression without having superior analgesia compared with demand use only. Monitoring of side stream end-tidal capnography can readily detect respiratory depression, especially if opioids, benzodiazepines, gabapentinoids, and diphenhydramine are used concomitantly. Patient education regarding opioid use, side effects, safe storage, and disposal practices is imperative because significant amounts of opioids remain in households after completion of treatment for pain and because opioid diversion and accidental ingestions account for significant morbidity. Providers need to balance efficient pain management with opioid stewardship, complying with state and federal policies to limit harm related to opioid diversion.15
Nonpharmacological Modalities
The use of nonpharmacologic therapies, along with pharmacologic modalities, for perioperative pain management has been shown to decrease opioid use and opioid-related side effects. Trials of acupressure have demonstrated improvement in nausea and vomiting, sleep quality, and pain and anxiety scores. Nonpharmacologic treatments currently serve as a complementary approach for pain and anxiety management in the perioperative setting including acupuncture, acupressure, osteopathic manipulative treatment, massage, meditation, biofeedback, hypnotherapy, and physical/occupational, relaxation, cognitive-behavioral, chiropractic, music, and art therapies. The Joint Commission suggests consideration of such modalities by hospitals.
FUTURE CONSIDERATIONS
Pediatric hospitalists have been traditionally involved in research and patient care improvements and should continue to actively contribute to establishing evidence-based guidelines for the treatment of acute postoperative pain in hospitalized children and adolescents. The sparsity of high-quality evidence prompts the need for more research. A standardized approach to perioperative pain management in the form of checklists, pathways, and protocols for specific procedures may be useful to educate providers and patients, while also standardizing available evidence-based interventions (eg, pediatric Enhanced Recovery After Surgery [ERAS] protocols).
CONCLUSION
Combining multimodal pharmacologic and integrative nonpharmacologic modalities can decrease opioid use and related side effects and improve the perioperative care of hospitalized children. Pediatric hospitalists have an opportunity to optimize care preoperatively, practice multimodal analgesia, and contribute to reducing risk of opioid diversion post operatively.
Pediatric hospitalists play an increasingly significant role in perioperative pain management.1 Advances in pediatric surgical comanagement may improve quality of care and reduce the length of hospitalization.2 This review is based on queries of the PubMed and Cochrane databases between January 1, 2014, and July 15, 2019, using the search terms “perioperative pain management,” “postoperative pain,” “pediatric,” and “children.” In addition, the authors reviewed key position statements from the American Academy of Pediatrics (AAP), the American Pain Society (APS), the Centers for Disease Control and Prevention (CDC), and the Society of Hospital Medicine (SHM) regarding pain management.3 This update is intended to be relevant for practicing pediatric hospitalists, with a focus on recently expanded options for pain management and judicious opioid use in hospitalized children.
PERIOPERATIVE PAIN MANAGEMENT
Postoperative pain management begins preoperatively according to the concept of the perioperative surgical home (PSH).4 The preoperative history should identify the patient’s previous positive (eg, good pain control) and negative (eg, adverse reactions) experiences with pain medications. Family and patient expectations should be discussed regarding types and sources of pain, pain duration, exacerbating/alleviating factors, and modalities available for realistic pain control because preoperative information can limit anxiety and improve outcomes. Pain specialists can perform risk assessments preoperatively and develop plans to address pharmacologic tolerance, withdrawal, and opioid-induced hyperalgesia after surgery.5 Children with chronic pain and on preoperative opioids may require more analgesia for a longer duration postoperatively. Early recognition of variability of patient’s pain perception and differences in responses to pain need to be clearly communicated across the disciplines in a collaborative model of care.
Children with medical complexity and/or cognitive, emotional, or behavioral impairments may benefit from preoperative psychosocial treatments and utilization of pain self-management training and strategies that could further reduce anxiety and optimize postoperative care because patient and parental preoperative anxiety may be associated with adverse outcomes. Validated pain assessment tools like Revised FLACC (Face, Leg, Activity, Cry, and Consolability) Scale and Individualized Numeric Rating Scale could be particularly useful in children with limitations in communication or altered pain perception; therefore, medical teams and family members should discuss their utilization preoperatively.
MULTIMODAL ANALGESIA
Multimodal analgesia (MMA) is a strategy that synergistically uses pharmacologic and nonpharmacologic modalities to target pain at multiple points of the pain processing pathway (Table).6 MMA can optimize pain control by addressing different types of pain (eg, incisional pain, muscle spasm, or neuropathic pain), expedite recovery, reduce potential pharmacologic side effects, and decrease opioid consumption. Patients taking opioids are at an increased risk of developing opioid-related side effects such as respiratory depression, medication tolerance, and anxiety, with resultant longer hospital stay, increased readmissions, and higher costs of care.7 Treatment for postoperative pain should prioritize appropriately dosed and precisely scheduled MMA before opioid-focused analgesia with the goals of decreasing opioid-related adverse effects, intentional misuse, diversion, and accidental ingestions. The AAP, APS, CDC, and SHM endorse the use of MMA and recommend nonpharmacologic measures and regional anesthesia.8,9 The most used modalities in MMA are discussed below.
Acetaminophen
Acetaminophen has central-acting analgesic and antipyretic properties and readily crosses the blood brain barrier, which makes it particularly useful in spine and neurological surgeries. Oral administration is preferred when feasible. The AAP recommends refraining from rectal administration of acetaminophen as analgesia in children because of concerns about toxic effects and erratic, variable absorption.10 A systematic review of six studies found no benefit in pain control between intravenous (IV) and oral (PO) administration of acetaminophen in adults.11 There is a paucity of studies in children comparing PO with IV acetaminophen perioperative efficacy. Children may benefit from IV formulations in the early postoperative period, in cases with frequent nausea and vomiting, and in those with oral medication intolerance. Since infants have greater risk of respiratory depression from opioids, IV acetaminophen may have utility in this age group. Because of the cost associated with IV formulation, some institutions restrict IV acetaminophen. However, rapidly well-controlled pain and minimization of opioid-related side effects with shorter hospital stays may lower healthcare costs despite the cost of acetaminophen itself.
NSAIDs
NSAIDs possess anti-inflammatory properties through the inhibition of cyclooxygenase and blockade of prostaglandin production. NSAID risks include bleeding, renal and gastrointestinal toxicities, and potentially delayed wound and bone healing. Ketorolac is an NSAID that continues to be widely used with demonstrated opioid-sparing effects. Many retrospective studies including large numbers of pediatric patients have not demonstrated increased risks of bleeding nor poor wound healing with short postoperative use. A Cochrane review, however, concluded that there is insufficient data to either support or reject the efficacy or safety of ketorolac for postoperative pain treatment in children, mostly because of the very low quality of evidence.12
Regional Anesthesia
Regional anesthesia, which includes central (spinal/epidural/caudal) and peripheral blocks, decreases postoperative pain and opioid-associated side effects. Blocks typically consist of local anesthetic with or without the addition of adjuncts (eg, clonidine, dexamethasone). Regional anesthesia may also improve pulmonary function, compared with that of nonregional MMA use, in patients who have thoracic or upper abdominal surgeries. While having broad applications, the utility of regional anesthesia is greatest in preterm infants/neonates and in those with underlying respiratory pathology. A systematic review of randomized controlled trials demonstrated that regional anesthesia decreased opioid consumption and minimized postoperative pain with no significant complications attributed to its use.13 Additional studies are needed to better delineate specific surgical procedures and subpopulations of pediatric patients in which regional anesthesia may provide the most benefit.
Gabapentinoids
Children receiving gabapentinoids perioperatively have been shown to have fewer adverse reactions, decreased opioid consumption, and less anxiety, as well as improved pain scores. Gabapentin is increasingly being utilized for children with idiopathic scoliosis undergoing posterior spinal fusion, and there is some evidence for improving pain control and reducing opioid use. However, a recent systematic review found a paucity of data supporting its clinical use.14 Both gabapentin and pregabalin may further increase risks of respiratory depression, especially in synergy with opioids and benzodiazepines.
Opioids
Opioids should be used with caution in pediatric patients and are reserved primarily for the management of severe acute pain. The shortest duration of the lowest effective dose of opioids should be encouraged. Patient-controlled opioid analgesia (PCA) offers benefits when parenteral postoperative analgesia is indicated: It maximizes pain relief, minimizes risk of overdose, and improves psychological well-being through self-administration of pain medicines. Basal-infusion PCA should not be routinely used because it is associated with nausea, vomiting, and respiratory depression without having superior analgesia compared with demand use only. Monitoring of side stream end-tidal capnography can readily detect respiratory depression, especially if opioids, benzodiazepines, gabapentinoids, and diphenhydramine are used concomitantly. Patient education regarding opioid use, side effects, safe storage, and disposal practices is imperative because significant amounts of opioids remain in households after completion of treatment for pain and because opioid diversion and accidental ingestions account for significant morbidity. Providers need to balance efficient pain management with opioid stewardship, complying with state and federal policies to limit harm related to opioid diversion.15
Nonpharmacological Modalities
The use of nonpharmacologic therapies, along with pharmacologic modalities, for perioperative pain management has been shown to decrease opioid use and opioid-related side effects. Trials of acupressure have demonstrated improvement in nausea and vomiting, sleep quality, and pain and anxiety scores. Nonpharmacologic treatments currently serve as a complementary approach for pain and anxiety management in the perioperative setting including acupuncture, acupressure, osteopathic manipulative treatment, massage, meditation, biofeedback, hypnotherapy, and physical/occupational, relaxation, cognitive-behavioral, chiropractic, music, and art therapies. The Joint Commission suggests consideration of such modalities by hospitals.
FUTURE CONSIDERATIONS
Pediatric hospitalists have been traditionally involved in research and patient care improvements and should continue to actively contribute to establishing evidence-based guidelines for the treatment of acute postoperative pain in hospitalized children and adolescents. The sparsity of high-quality evidence prompts the need for more research. A standardized approach to perioperative pain management in the form of checklists, pathways, and protocols for specific procedures may be useful to educate providers and patients, while also standardizing available evidence-based interventions (eg, pediatric Enhanced Recovery After Surgery [ERAS] protocols).
CONCLUSION
Combining multimodal pharmacologic and integrative nonpharmacologic modalities can decrease opioid use and related side effects and improve the perioperative care of hospitalized children. Pediatric hospitalists have an opportunity to optimize care preoperatively, practice multimodal analgesia, and contribute to reducing risk of opioid diversion post operatively.
1. Society of Hospital Medicine Co-Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co-management. http://tools.hospitalmedicine.org/Implementation/Co-ManagementWhitePaper-final_5-10-10.pdf. Accessed October 11, 2019.
2. Rappaport DI, Rosenberg RE, Shaughnessy EE, et al. Pediatric hospitalist comanagement of surgical patients: Structural, quality, and financial considerations. J Hosp Med. 2014;9(11):737-742. https://doi.org/10.1002/jhm.2266.
3. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. http://www.nonpharmpaincare.org. Accessed on October 11, 2019.
4. Vetter TR, Kain ZN. Role of perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. https://doi.org/10.1213/ANE.0000000000002280.
5. Edwards DA, Hedrick TL, Jayaram J, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on perioperative management of patients on preoperative opioid therapy. Anesth Analg. 2019;129(2):553-566. http://doi.org/10.1213/ANE.0000000000004018.
6. Micromedex (electronic version). IBM Watson Health. Greenwood Village, Colorado, USA. https://www.micromedexsolutions.com. Accessed October 10, 2019.
7. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. https://doi.org/10.1016/j.jpain.2015.12.008.
8. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: A consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;3(4):263-266. https://doi.org/10.12788/jhm.2980.
9. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA. 2016;315(15):1624-1645. https://doi.org/10.1001/jama.2016.1464.
10. American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108 (4):1020-1024. https://doi.org/10.1542/peds.108.4.1020.
11. Jibril F, Sharaby S, Mohamed A, Wilby, KJ. Intravenous versus oral acetaminophen for pain: Systemic review of current evidence to support clinical decision-making. Can J Hosp Pharm. 2015;68(3):238-247. https://doi.org/10.4212/cjhp.v68i3.1458.
12. McNicol ED, Rowe E, Cooper TE. Ketorolac for postoperative pain in children. Cochrane Database Syst Rev. 2018;7(7). https://doi.org/10.1002/14651858.CD012294.pub2.
13. Kendall MC, Castro Alves LJ, Suh EI, McCormick ZL, De Oliveira GS. Regional anesthesia to ameliorate postoperative analgesia outcomes in pediatric surgical patients: an updated systematic review of randomized controlled trials. Local Reg Anesth. 2018;11:91-109. https://doi.org/10.2147/LRA.S185554.
14. Egunsola 0, Wylie CE, Chitty KM, et al. Systematic review of the efficacy and safety of gabapentin and pregabalin for pain in children and adolescents. Anesth Analg. 2019;128(4):811-819. https://doi.org/10.1213/ANE.0000000000003936.
15. Harbaugh C, Gadepalli SK. Pediatric postoperative opioid prescribing and the opioid crisis. Curr Opin Pediatr. 2019;31(3):377-385. https://doi.org/10.1097/MOP.0000000000000768.
1. Society of Hospital Medicine Co-Management Advisory Panel. A white paper on a guide to hospitalist/orthopedic surgery co-management. http://tools.hospitalmedicine.org/Implementation/Co-ManagementWhitePaper-final_5-10-10.pdf. Accessed October 11, 2019.
2. Rappaport DI, Rosenberg RE, Shaughnessy EE, et al. Pediatric hospitalist comanagement of surgical patients: Structural, quality, and financial considerations. J Hosp Med. 2014;9(11):737-742. https://doi.org/10.1002/jhm.2266.
3. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. http://www.nonpharmpaincare.org. Accessed on October 11, 2019.
4. Vetter TR, Kain ZN. Role of perioperative surgical home in optimizing the perioperative use of opioids. Anesth Analg. 2017;125(5):1653-1657. https://doi.org/10.1213/ANE.0000000000002280.
5. Edwards DA, Hedrick TL, Jayaram J, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus statement on perioperative management of patients on preoperative opioid therapy. Anesth Analg. 2019;129(2):553-566. http://doi.org/10.1213/ANE.0000000000004018.
6. Micromedex (electronic version). IBM Watson Health. Greenwood Village, Colorado, USA. https://www.micromedexsolutions.com. Accessed October 10, 2019.
7. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. https://doi.org/10.1016/j.jpain.2015.12.008.
8. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: A consensus statement from the Society of Hospital Medicine. J Hosp Med. 2018;3(4):263-266. https://doi.org/10.12788/jhm.2980.
9. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA. 2016;315(15):1624-1645. https://doi.org/10.1001/jama.2016.1464.
10. American Academy of Pediatrics Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108 (4):1020-1024. https://doi.org/10.1542/peds.108.4.1020.
11. Jibril F, Sharaby S, Mohamed A, Wilby, KJ. Intravenous versus oral acetaminophen for pain: Systemic review of current evidence to support clinical decision-making. Can J Hosp Pharm. 2015;68(3):238-247. https://doi.org/10.4212/cjhp.v68i3.1458.
12. McNicol ED, Rowe E, Cooper TE. Ketorolac for postoperative pain in children. Cochrane Database Syst Rev. 2018;7(7). https://doi.org/10.1002/14651858.CD012294.pub2.
13. Kendall MC, Castro Alves LJ, Suh EI, McCormick ZL, De Oliveira GS. Regional anesthesia to ameliorate postoperative analgesia outcomes in pediatric surgical patients: an updated systematic review of randomized controlled trials. Local Reg Anesth. 2018;11:91-109. https://doi.org/10.2147/LRA.S185554.
14. Egunsola 0, Wylie CE, Chitty KM, et al. Systematic review of the efficacy and safety of gabapentin and pregabalin for pain in children and adolescents. Anesth Analg. 2019;128(4):811-819. https://doi.org/10.1213/ANE.0000000000003936.
15. Harbaugh C, Gadepalli SK. Pediatric postoperative opioid prescribing and the opioid crisis. Curr Opin Pediatr. 2019;31(3):377-385. https://doi.org/10.1097/MOP.0000000000000768.
© 2020 Society of Hospital Medicine
Methodological Progress Note: Classification and Regression Tree Analysis
Machine-learning is a type of artificial intelligence in which systems automatically learn and improve from experience without being explicitly programmed. Classification and Regression Tree (CART) analysis is a machine-learning algorithm that was developed to visually classify or segment populations into subgroups with similar characteristics and outcomes. CART analysis is a decision tree methodology that was initially developed in the 1960s for use in product marketing.1 Since then, a number of health disciplines have used it to isolate patient subgroups from larger populations to guide clinical decision-making by better identifying those most likely to benefit.2 The clinical utility of CART mirrors how most clinicians think, which is not in terms of coefficients (ie, regression output) but rather in terms of categories or classifications (eg, low vs high risk).
In this issue of the Journal of Hospital Medicine, Young and colleagues use classification trees to predict discharge placement (postacute care facility vs home) based on a patient’s hospital admission characteristics and mobility score. The resulting decision tree indicates that patients with the lowest mobility scores, as well as those 65 years and older, were most likely to be discharged to postacute care facilities.3 In this review, we orient the reader to the basics of CART analysis, discuss important intricacies, and weigh its pros, cons, and application as a statistical tool.
WHAT IS CART ANALYSIS?
CART is a nonparametric (ie, makes no assumptions about data distribution) statistical tool that identifies subgroups within a population whose members share common characteristics as defined by the independent variables included in the model. CART analysis is unique in that it yields a visual output of the data in the form of a multisegmented structure that resembles the branches of a tree (Figure). CART analysis consists of four basic steps: (1) tree-building (including splitting criteria and estimation of classification error), (2) stopping the tree-building process, (3) tree “pruning,” and (4) tree selection.
In general, CART analysis begins with a single “node” or group, which contains the entire sample population. This is referred to as the “parent node.” The CART procedure simultaneously examines all available independent variables and selects one that results in two groups that are the most distinct with respect to the outcome variable of interest. In Young et al’s example, posthospital discharge placement is the outcome.3 This parent node then branches into two “child nodes” according to the independent variable that was selected. Within each of these “child nodes,” the tree-growing methodology recursively assesses each of the remaining independent variables to determine which will result in the best split according to the chosen splitting criterion.2 Each subsequent “child node” will become a “parent node” to the two groups in which it splits. This process is repeated on the data in each subsequent “child node” and is stopped once a predefined stopping point is reached. Notably, while division into two groups is the most common application of CART modeling, there are models that can split data into more than two child nodes.
Since CART outcomes can be heavily dependent on the data being used (eg, electronic health records or administrative data), it is important to attempt to confirm results in a similar, but different, study cohort. Because obtaining separate data sources with similar cohorts can be difficult, many investigators using CART will utilize a “split sample approach” in which study data are split into separate training and validation sets.4 In the training set, which frequently comprises two-thirds of the available data, the algorithm is tested in exploratory analysis. Once the algorithm is defined and agreed upon, it is retested within a validation set, constructed from the remaining one-third of data. This approach, which Young et al utilize,3 allows for improved confidence and reduced risk of bias in the findings and allows for some degree of external validation. Further, the split sample approach supports more reliable measures of predictive accuracy: in Young et al’s case, the proportion of correctly classified patients discharged to a postacute care facility (sensitivity: 58%, 95% CI 49-68%) and the proportion of correctly classified patients discharged home (specificity: 84%, 95% CI 78-90%). Despite these advantages, the split sample approach is not universally used.
Classification Versus Regression Trees
While commonly grouped together, CARTs can be distinguished from one another based on the dependent, or outcome, variable. Categorical outcome variables require the use of a classification tree, while continuous outcomes utilize regression trees. Of note, the independent, or predictor, variables can be any combination of categorical or continuous variables. However, splitting at each node creates categorical output when using CART algorithms.
Splitting Criteria
The splitting of each node is based on reducing the degree of “impurity” (heterogeneity with respect to the outcome variable) within each node. For example, a node that has no impurity will have a zero error rate labeling its binary outcomes. While CART works well with categorical variables, continuous variables (eg, age) can also be assessed, though only with certain algorithms. Several different splitting criteria exist, each of which attempt to maximize the differences within each child node. While beyond the scope of this review, examples of popular splitting criteria are Gini, entropy, and minimum error.5
Stopping Rules
To manage the size of a tree, CART analysis allows for predefined stopping rules to minimize the extent of growth while also establishing a minimal degree of statistical difference between nodes that is considered meaningful. To accomplish this task, two stopping rules are often used. The first defines the minimum number of observations in child, or “terminal,” nodes. The second defines the maximum number of levels a tree may grow, thus allowing the investigator to decide the total number of predictor variables that can define a terminal node. While several other stopping rules exist, these are the most commonly utilized.
Pruning
To avoid missing important associations due to premature stoppage, investigators may use another mechanism to limit tree growth called “pruning.” For pruning, the first step is to grow a considerably large tree that includes many levels or nodes, possibly to the point where there are just a few observations per terminal node. Then, similar to the residual sum of squares in a regression, the investigator can calculate a misclassification cost (ie, goodness of fit) and select the tree with the smallest cost.2 Of note, stopping rules and pruning can be used simultaneously.
Classification Error
Similar to other forms of statistical inference it remains important to understand the uncertainty within the inference. In regression modeling, for example, classification errors can be calculated using standard errors of the parameter estimates. In CART analysis, because random samples from a population may produce different trees, measures of variability can be more complicated. One strategy is to generate a tree from a test sample and then use the remaining data to calculate a measure of the misclassification cost (a measure of how much additional accuracy a split must add to the entire tree to warrant the additional complexity). Alternatively, a “k-fold cross-validation” can be performed in which the data is broken down into k subsets from which a tree is created using all data except for one of the subsets. The computed tree is then applied to the remaining subset to determine a misclassification cost. These classification costs are important as they also impact the stopping and pruning processes. Ultimately, a final tree, which best limits classification errors, is selected.
WHEN WOULD YOU USE CART ANALYSIS?
This method can be useful in multiple settings in which an investigator wants to characterize a subpopulation from a larger cohort. Adaptation of this could include, but is not limited to, risk stratification,6 diagnostics,7 and patient identification for medical interventions.8 Moreover, CART analysis has the added benefit of creating visually interpretable predictive models that can be utilized for front-line clinical decision making.9,10
STRENGTHS OF CART ANALYSIS
CART analysis has been shown to have several advantages over other commonly used modeling methods. First, it is a nonparametric model that can handle highly skewed data and does not require that the predictor, or predictors, takes on a predetermined form (allowing them to be constructed from the data). This is helpful as many clinical variables can have wide degrees of variance.
Unlike other modeling techniques, CART can identify higher-order interactions between multiple variables, meaning it can handle interactions that occur whenever one variable affects the nature of an interaction between two other variables. Further, CART can handle multiple correlated independent variables, something logistic regression models classically cannot do.
From a clinical standpoint, the “logic” of the visual-based CART output can be easier to interpret than the probabilistic output (eg, odds ratio) associated with logistic regression modeling, making it more practical, applicable, and easier for clinicians to adopt.10,12 Finally, CART software is easy to use for those who do not have strong statistical backgrounds, and it is less resource intensive than other statistical methods.2
LIMITATIONS OF CART ANALYSIS
Despite these features, CART does have several disadvantages. First, due to the ease with which CART analysis can be performed, “data dredging” can be a significant concern. Its ideal use is with a priori consideration of independent variables.2 Second, while CART is most beneficial in describing links and cutoffs between variables, it may not be useful for hypothesis testing.2 Third, large data sets are needed to perform CART, especially if the investigator is using the split sample approach mentioned above.11 Finally, while CART is the most utilized decision tree methodology, several other types of decision tree methods exist: C4.5, CRUISE, Quick, Unbiased, Efficient Statistical Trees, Chi-square-Automatic-Interaction-Detection, and others. Many of these allow for splitting into more than two groups and have other features that may be more advantageous to one’s analysis.13
WHY DID THE AUTHORS USE CART?
Decision trees offer simple, interpretable results of multiple factors that can be easily applied to clinical scenarios. In this case, the authors specifically used classification tree analysis to take advantage of CART’s machine-learning ability to consider higher-order interactions to build their model—as they lacked a priori evidence to help guide them in traditional (ie, logistic regression) model construction. Furthermore, CART analysis created an output that logically and visually illustrates which combination of characteristics is most associated with discharge placement and can potentially be utilized to help facilitate discharge planning in future hospitalized patients. To sum up, this machine-learning methodology allowed the investigators to determine which variables taken together were the most suitable in predicting their outcome of interest and present these findings in a manner that busy clinicians can interpret and apply.
1. Magee JF. Decision Trees for Decision Making. Harvard Business Review. 1964. https://hbr.org/1964/07/decision-trees-for-decision-making. Accessed August 26, 2019.
2. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172-181. https://doi.org/10.1207/S15324796ABM2603_02
3. Young D, Colantuoni E, Seltzer D, et al. Prediction of disposition within 48-hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9):540-543. https://doi.org/10.12788/jhm.3332
4. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-1358. https://doi.org/10.1056/NEJMra1814259
5. Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer-Verlag; 1999. https://www.springer.com/gp/book/9781475730272. Accessed August 24, 2019.
6. Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, for the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. https://doi.org/10.1001/jama.293.5.572
7. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5(11):3403-3410.
8. Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23(19):4322-4329. https://doi.org/10.1200/JCO.2005.11.136
9. Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci. 2011;56(12):3664-3671. https://doi.org/10.1007/s10620-011-1849-x
10. Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Proceedings of Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, May 22-25, 2000; pp. 1–14.
11. Perlich C, Provost F, Simonoff JS. Tree induction vs logistic regression: a learning-curve analysis. J Mach Learn Res. 2003;4(Jun):211-255. https://doi.org/10.1162/153244304322972694
12. Woolever D. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31-36.
13. Loh WY. Classification and regression trees. Wires Data Min Know Disc. 2011;1(1):14-23. https://doi.org/10.1002/widm.8
Machine-learning is a type of artificial intelligence in which systems automatically learn and improve from experience without being explicitly programmed. Classification and Regression Tree (CART) analysis is a machine-learning algorithm that was developed to visually classify or segment populations into subgroups with similar characteristics and outcomes. CART analysis is a decision tree methodology that was initially developed in the 1960s for use in product marketing.1 Since then, a number of health disciplines have used it to isolate patient subgroups from larger populations to guide clinical decision-making by better identifying those most likely to benefit.2 The clinical utility of CART mirrors how most clinicians think, which is not in terms of coefficients (ie, regression output) but rather in terms of categories or classifications (eg, low vs high risk).
In this issue of the Journal of Hospital Medicine, Young and colleagues use classification trees to predict discharge placement (postacute care facility vs home) based on a patient’s hospital admission characteristics and mobility score. The resulting decision tree indicates that patients with the lowest mobility scores, as well as those 65 years and older, were most likely to be discharged to postacute care facilities.3 In this review, we orient the reader to the basics of CART analysis, discuss important intricacies, and weigh its pros, cons, and application as a statistical tool.
WHAT IS CART ANALYSIS?
CART is a nonparametric (ie, makes no assumptions about data distribution) statistical tool that identifies subgroups within a population whose members share common characteristics as defined by the independent variables included in the model. CART analysis is unique in that it yields a visual output of the data in the form of a multisegmented structure that resembles the branches of a tree (Figure). CART analysis consists of four basic steps: (1) tree-building (including splitting criteria and estimation of classification error), (2) stopping the tree-building process, (3) tree “pruning,” and (4) tree selection.
In general, CART analysis begins with a single “node” or group, which contains the entire sample population. This is referred to as the “parent node.” The CART procedure simultaneously examines all available independent variables and selects one that results in two groups that are the most distinct with respect to the outcome variable of interest. In Young et al’s example, posthospital discharge placement is the outcome.3 This parent node then branches into two “child nodes” according to the independent variable that was selected. Within each of these “child nodes,” the tree-growing methodology recursively assesses each of the remaining independent variables to determine which will result in the best split according to the chosen splitting criterion.2 Each subsequent “child node” will become a “parent node” to the two groups in which it splits. This process is repeated on the data in each subsequent “child node” and is stopped once a predefined stopping point is reached. Notably, while division into two groups is the most common application of CART modeling, there are models that can split data into more than two child nodes.
Since CART outcomes can be heavily dependent on the data being used (eg, electronic health records or administrative data), it is important to attempt to confirm results in a similar, but different, study cohort. Because obtaining separate data sources with similar cohorts can be difficult, many investigators using CART will utilize a “split sample approach” in which study data are split into separate training and validation sets.4 In the training set, which frequently comprises two-thirds of the available data, the algorithm is tested in exploratory analysis. Once the algorithm is defined and agreed upon, it is retested within a validation set, constructed from the remaining one-third of data. This approach, which Young et al utilize,3 allows for improved confidence and reduced risk of bias in the findings and allows for some degree of external validation. Further, the split sample approach supports more reliable measures of predictive accuracy: in Young et al’s case, the proportion of correctly classified patients discharged to a postacute care facility (sensitivity: 58%, 95% CI 49-68%) and the proportion of correctly classified patients discharged home (specificity: 84%, 95% CI 78-90%). Despite these advantages, the split sample approach is not universally used.
Classification Versus Regression Trees
While commonly grouped together, CARTs can be distinguished from one another based on the dependent, or outcome, variable. Categorical outcome variables require the use of a classification tree, while continuous outcomes utilize regression trees. Of note, the independent, or predictor, variables can be any combination of categorical or continuous variables. However, splitting at each node creates categorical output when using CART algorithms.
Splitting Criteria
The splitting of each node is based on reducing the degree of “impurity” (heterogeneity with respect to the outcome variable) within each node. For example, a node that has no impurity will have a zero error rate labeling its binary outcomes. While CART works well with categorical variables, continuous variables (eg, age) can also be assessed, though only with certain algorithms. Several different splitting criteria exist, each of which attempt to maximize the differences within each child node. While beyond the scope of this review, examples of popular splitting criteria are Gini, entropy, and minimum error.5
Stopping Rules
To manage the size of a tree, CART analysis allows for predefined stopping rules to minimize the extent of growth while also establishing a minimal degree of statistical difference between nodes that is considered meaningful. To accomplish this task, two stopping rules are often used. The first defines the minimum number of observations in child, or “terminal,” nodes. The second defines the maximum number of levels a tree may grow, thus allowing the investigator to decide the total number of predictor variables that can define a terminal node. While several other stopping rules exist, these are the most commonly utilized.
Pruning
To avoid missing important associations due to premature stoppage, investigators may use another mechanism to limit tree growth called “pruning.” For pruning, the first step is to grow a considerably large tree that includes many levels or nodes, possibly to the point where there are just a few observations per terminal node. Then, similar to the residual sum of squares in a regression, the investigator can calculate a misclassification cost (ie, goodness of fit) and select the tree with the smallest cost.2 Of note, stopping rules and pruning can be used simultaneously.
Classification Error
Similar to other forms of statistical inference it remains important to understand the uncertainty within the inference. In regression modeling, for example, classification errors can be calculated using standard errors of the parameter estimates. In CART analysis, because random samples from a population may produce different trees, measures of variability can be more complicated. One strategy is to generate a tree from a test sample and then use the remaining data to calculate a measure of the misclassification cost (a measure of how much additional accuracy a split must add to the entire tree to warrant the additional complexity). Alternatively, a “k-fold cross-validation” can be performed in which the data is broken down into k subsets from which a tree is created using all data except for one of the subsets. The computed tree is then applied to the remaining subset to determine a misclassification cost. These classification costs are important as they also impact the stopping and pruning processes. Ultimately, a final tree, which best limits classification errors, is selected.
WHEN WOULD YOU USE CART ANALYSIS?
This method can be useful in multiple settings in which an investigator wants to characterize a subpopulation from a larger cohort. Adaptation of this could include, but is not limited to, risk stratification,6 diagnostics,7 and patient identification for medical interventions.8 Moreover, CART analysis has the added benefit of creating visually interpretable predictive models that can be utilized for front-line clinical decision making.9,10
STRENGTHS OF CART ANALYSIS
CART analysis has been shown to have several advantages over other commonly used modeling methods. First, it is a nonparametric model that can handle highly skewed data and does not require that the predictor, or predictors, takes on a predetermined form (allowing them to be constructed from the data). This is helpful as many clinical variables can have wide degrees of variance.
Unlike other modeling techniques, CART can identify higher-order interactions between multiple variables, meaning it can handle interactions that occur whenever one variable affects the nature of an interaction between two other variables. Further, CART can handle multiple correlated independent variables, something logistic regression models classically cannot do.
From a clinical standpoint, the “logic” of the visual-based CART output can be easier to interpret than the probabilistic output (eg, odds ratio) associated with logistic regression modeling, making it more practical, applicable, and easier for clinicians to adopt.10,12 Finally, CART software is easy to use for those who do not have strong statistical backgrounds, and it is less resource intensive than other statistical methods.2
LIMITATIONS OF CART ANALYSIS
Despite these features, CART does have several disadvantages. First, due to the ease with which CART analysis can be performed, “data dredging” can be a significant concern. Its ideal use is with a priori consideration of independent variables.2 Second, while CART is most beneficial in describing links and cutoffs between variables, it may not be useful for hypothesis testing.2 Third, large data sets are needed to perform CART, especially if the investigator is using the split sample approach mentioned above.11 Finally, while CART is the most utilized decision tree methodology, several other types of decision tree methods exist: C4.5, CRUISE, Quick, Unbiased, Efficient Statistical Trees, Chi-square-Automatic-Interaction-Detection, and others. Many of these allow for splitting into more than two groups and have other features that may be more advantageous to one’s analysis.13
WHY DID THE AUTHORS USE CART?
Decision trees offer simple, interpretable results of multiple factors that can be easily applied to clinical scenarios. In this case, the authors specifically used classification tree analysis to take advantage of CART’s machine-learning ability to consider higher-order interactions to build their model—as they lacked a priori evidence to help guide them in traditional (ie, logistic regression) model construction. Furthermore, CART analysis created an output that logically and visually illustrates which combination of characteristics is most associated with discharge placement and can potentially be utilized to help facilitate discharge planning in future hospitalized patients. To sum up, this machine-learning methodology allowed the investigators to determine which variables taken together were the most suitable in predicting their outcome of interest and present these findings in a manner that busy clinicians can interpret and apply.
Machine-learning is a type of artificial intelligence in which systems automatically learn and improve from experience without being explicitly programmed. Classification and Regression Tree (CART) analysis is a machine-learning algorithm that was developed to visually classify or segment populations into subgroups with similar characteristics and outcomes. CART analysis is a decision tree methodology that was initially developed in the 1960s for use in product marketing.1 Since then, a number of health disciplines have used it to isolate patient subgroups from larger populations to guide clinical decision-making by better identifying those most likely to benefit.2 The clinical utility of CART mirrors how most clinicians think, which is not in terms of coefficients (ie, regression output) but rather in terms of categories or classifications (eg, low vs high risk).
In this issue of the Journal of Hospital Medicine, Young and colleagues use classification trees to predict discharge placement (postacute care facility vs home) based on a patient’s hospital admission characteristics and mobility score. The resulting decision tree indicates that patients with the lowest mobility scores, as well as those 65 years and older, were most likely to be discharged to postacute care facilities.3 In this review, we orient the reader to the basics of CART analysis, discuss important intricacies, and weigh its pros, cons, and application as a statistical tool.
WHAT IS CART ANALYSIS?
CART is a nonparametric (ie, makes no assumptions about data distribution) statistical tool that identifies subgroups within a population whose members share common characteristics as defined by the independent variables included in the model. CART analysis is unique in that it yields a visual output of the data in the form of a multisegmented structure that resembles the branches of a tree (Figure). CART analysis consists of four basic steps: (1) tree-building (including splitting criteria and estimation of classification error), (2) stopping the tree-building process, (3) tree “pruning,” and (4) tree selection.
In general, CART analysis begins with a single “node” or group, which contains the entire sample population. This is referred to as the “parent node.” The CART procedure simultaneously examines all available independent variables and selects one that results in two groups that are the most distinct with respect to the outcome variable of interest. In Young et al’s example, posthospital discharge placement is the outcome.3 This parent node then branches into two “child nodes” according to the independent variable that was selected. Within each of these “child nodes,” the tree-growing methodology recursively assesses each of the remaining independent variables to determine which will result in the best split according to the chosen splitting criterion.2 Each subsequent “child node” will become a “parent node” to the two groups in which it splits. This process is repeated on the data in each subsequent “child node” and is stopped once a predefined stopping point is reached. Notably, while division into two groups is the most common application of CART modeling, there are models that can split data into more than two child nodes.
Since CART outcomes can be heavily dependent on the data being used (eg, electronic health records or administrative data), it is important to attempt to confirm results in a similar, but different, study cohort. Because obtaining separate data sources with similar cohorts can be difficult, many investigators using CART will utilize a “split sample approach” in which study data are split into separate training and validation sets.4 In the training set, which frequently comprises two-thirds of the available data, the algorithm is tested in exploratory analysis. Once the algorithm is defined and agreed upon, it is retested within a validation set, constructed from the remaining one-third of data. This approach, which Young et al utilize,3 allows for improved confidence and reduced risk of bias in the findings and allows for some degree of external validation. Further, the split sample approach supports more reliable measures of predictive accuracy: in Young et al’s case, the proportion of correctly classified patients discharged to a postacute care facility (sensitivity: 58%, 95% CI 49-68%) and the proportion of correctly classified patients discharged home (specificity: 84%, 95% CI 78-90%). Despite these advantages, the split sample approach is not universally used.
Classification Versus Regression Trees
While commonly grouped together, CARTs can be distinguished from one another based on the dependent, or outcome, variable. Categorical outcome variables require the use of a classification tree, while continuous outcomes utilize regression trees. Of note, the independent, or predictor, variables can be any combination of categorical or continuous variables. However, splitting at each node creates categorical output when using CART algorithms.
Splitting Criteria
The splitting of each node is based on reducing the degree of “impurity” (heterogeneity with respect to the outcome variable) within each node. For example, a node that has no impurity will have a zero error rate labeling its binary outcomes. While CART works well with categorical variables, continuous variables (eg, age) can also be assessed, though only with certain algorithms. Several different splitting criteria exist, each of which attempt to maximize the differences within each child node. While beyond the scope of this review, examples of popular splitting criteria are Gini, entropy, and minimum error.5
Stopping Rules
To manage the size of a tree, CART analysis allows for predefined stopping rules to minimize the extent of growth while also establishing a minimal degree of statistical difference between nodes that is considered meaningful. To accomplish this task, two stopping rules are often used. The first defines the minimum number of observations in child, or “terminal,” nodes. The second defines the maximum number of levels a tree may grow, thus allowing the investigator to decide the total number of predictor variables that can define a terminal node. While several other stopping rules exist, these are the most commonly utilized.
Pruning
To avoid missing important associations due to premature stoppage, investigators may use another mechanism to limit tree growth called “pruning.” For pruning, the first step is to grow a considerably large tree that includes many levels or nodes, possibly to the point where there are just a few observations per terminal node. Then, similar to the residual sum of squares in a regression, the investigator can calculate a misclassification cost (ie, goodness of fit) and select the tree with the smallest cost.2 Of note, stopping rules and pruning can be used simultaneously.
Classification Error
Similar to other forms of statistical inference it remains important to understand the uncertainty within the inference. In regression modeling, for example, classification errors can be calculated using standard errors of the parameter estimates. In CART analysis, because random samples from a population may produce different trees, measures of variability can be more complicated. One strategy is to generate a tree from a test sample and then use the remaining data to calculate a measure of the misclassification cost (a measure of how much additional accuracy a split must add to the entire tree to warrant the additional complexity). Alternatively, a “k-fold cross-validation” can be performed in which the data is broken down into k subsets from which a tree is created using all data except for one of the subsets. The computed tree is then applied to the remaining subset to determine a misclassification cost. These classification costs are important as they also impact the stopping and pruning processes. Ultimately, a final tree, which best limits classification errors, is selected.
WHEN WOULD YOU USE CART ANALYSIS?
This method can be useful in multiple settings in which an investigator wants to characterize a subpopulation from a larger cohort. Adaptation of this could include, but is not limited to, risk stratification,6 diagnostics,7 and patient identification for medical interventions.8 Moreover, CART analysis has the added benefit of creating visually interpretable predictive models that can be utilized for front-line clinical decision making.9,10
STRENGTHS OF CART ANALYSIS
CART analysis has been shown to have several advantages over other commonly used modeling methods. First, it is a nonparametric model that can handle highly skewed data and does not require that the predictor, or predictors, takes on a predetermined form (allowing them to be constructed from the data). This is helpful as many clinical variables can have wide degrees of variance.
Unlike other modeling techniques, CART can identify higher-order interactions between multiple variables, meaning it can handle interactions that occur whenever one variable affects the nature of an interaction between two other variables. Further, CART can handle multiple correlated independent variables, something logistic regression models classically cannot do.
From a clinical standpoint, the “logic” of the visual-based CART output can be easier to interpret than the probabilistic output (eg, odds ratio) associated with logistic regression modeling, making it more practical, applicable, and easier for clinicians to adopt.10,12 Finally, CART software is easy to use for those who do not have strong statistical backgrounds, and it is less resource intensive than other statistical methods.2
LIMITATIONS OF CART ANALYSIS
Despite these features, CART does have several disadvantages. First, due to the ease with which CART analysis can be performed, “data dredging” can be a significant concern. Its ideal use is with a priori consideration of independent variables.2 Second, while CART is most beneficial in describing links and cutoffs between variables, it may not be useful for hypothesis testing.2 Third, large data sets are needed to perform CART, especially if the investigator is using the split sample approach mentioned above.11 Finally, while CART is the most utilized decision tree methodology, several other types of decision tree methods exist: C4.5, CRUISE, Quick, Unbiased, Efficient Statistical Trees, Chi-square-Automatic-Interaction-Detection, and others. Many of these allow for splitting into more than two groups and have other features that may be more advantageous to one’s analysis.13
WHY DID THE AUTHORS USE CART?
Decision trees offer simple, interpretable results of multiple factors that can be easily applied to clinical scenarios. In this case, the authors specifically used classification tree analysis to take advantage of CART’s machine-learning ability to consider higher-order interactions to build their model—as they lacked a priori evidence to help guide them in traditional (ie, logistic regression) model construction. Furthermore, CART analysis created an output that logically and visually illustrates which combination of characteristics is most associated with discharge placement and can potentially be utilized to help facilitate discharge planning in future hospitalized patients. To sum up, this machine-learning methodology allowed the investigators to determine which variables taken together were the most suitable in predicting their outcome of interest and present these findings in a manner that busy clinicians can interpret and apply.
1. Magee JF. Decision Trees for Decision Making. Harvard Business Review. 1964. https://hbr.org/1964/07/decision-trees-for-decision-making. Accessed August 26, 2019.
2. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172-181. https://doi.org/10.1207/S15324796ABM2603_02
3. Young D, Colantuoni E, Seltzer D, et al. Prediction of disposition within 48-hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9):540-543. https://doi.org/10.12788/jhm.3332
4. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-1358. https://doi.org/10.1056/NEJMra1814259
5. Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer-Verlag; 1999. https://www.springer.com/gp/book/9781475730272. Accessed August 24, 2019.
6. Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, for the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. https://doi.org/10.1001/jama.293.5.572
7. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5(11):3403-3410.
8. Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23(19):4322-4329. https://doi.org/10.1200/JCO.2005.11.136
9. Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci. 2011;56(12):3664-3671. https://doi.org/10.1007/s10620-011-1849-x
10. Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Proceedings of Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, May 22-25, 2000; pp. 1–14.
11. Perlich C, Provost F, Simonoff JS. Tree induction vs logistic regression: a learning-curve analysis. J Mach Learn Res. 2003;4(Jun):211-255. https://doi.org/10.1162/153244304322972694
12. Woolever D. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31-36.
13. Loh WY. Classification and regression trees. Wires Data Min Know Disc. 2011;1(1):14-23. https://doi.org/10.1002/widm.8
1. Magee JF. Decision Trees for Decision Making. Harvard Business Review. 1964. https://hbr.org/1964/07/decision-trees-for-decision-making. Accessed August 26, 2019.
2. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172-181. https://doi.org/10.1207/S15324796ABM2603_02
3. Young D, Colantuoni E, Seltzer D, et al. Prediction of disposition within 48-hours of hospital admission using patient mobility scores. J Hosp Med. 2020;15(9):540-543. https://doi.org/10.12788/jhm.3332
4. Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380(14):1347-1358. https://doi.org/10.1056/NEJMra1814259
5. Zhang H, Singer B. Recursive Partitioning in the Health Sciences. New York: Springer-Verlag; 1999. https://www.springer.com/gp/book/9781475730272. Accessed August 24, 2019.
6. Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ, for the ADHERE Scientific Advisory Committee SG. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580. https://doi.org/10.1001/jama.293.5.572
7. Hess KR, Abbruzzese MC, Lenzi R, Raber MN, Abbruzzese JL. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5(11):3403-3410.
8. Garzotto M, Beer TM, Hudson RG, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. 2005;23(19):4322-4329. https://doi.org/10.1200/JCO.2005.11.136
9. Hong W, Dong L, Huang Q, Wu W, Wu J, Wang Y. Prediction of severe acute pancreatitis using classification and regression tree analysis. Dig Dis Sci. 2011;56(12):3664-3671. https://doi.org/10.1007/s10620-011-1849-x
10. Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Proceedings of Annual Meeting of the Society for Academic Emergency Medicine, San Francisco, CA, USA, May 22-25, 2000; pp. 1–14.
11. Perlich C, Provost F, Simonoff JS. Tree induction vs logistic regression: a learning-curve analysis. J Mach Learn Res. 2003;4(Jun):211-255. https://doi.org/10.1162/153244304322972694
12. Woolever D. The art and science of clinical decision making. Fam Pract Manag. 2008;15(5):31-36.
13. Loh WY. Classification and regression trees. Wires Data Min Know Disc. 2011;1(1):14-23. https://doi.org/10.1002/widm.8
© 2020 Society of Hospital Medicine
Clinical Progress Note: Care of Children Hospitalized for Acute Asthma Exacerbation
Since the last National Heart, Lung, and Blood Institute’s (NHLBI) guidelines that were released in 2007, additional evidence has emerged in several areas of asthma care.1 To provide a concise clinical update relevant to the practice of pediatric hospital medicine, we searched PubMed for asthma publications in the last 10 years with a particular focus on articles published in the last 5 years. We used a validated pediatric search filter to identify pediatric studies, MeSH term for “Asthma,” and the following terms: “Clinical Pathways,” “Clinical Protocols,” “Dexamethasone,” and “Albuterol.” From these articles, we identified three areas of emerging evidence supporting practice change relative to the inpatient care of children with asthma, which are summarized in this brief review. This clinical practice update covers the emerging evidence supporting dexamethasone use for acute asthma exacerbations, the shift away from nebulized albuterol toward metered dose inhaler (MDI) albuterol, and the utility of asthma clinical pathways.
DEXAMETHASONE VS PREDNISONE FOR ACUTE ASTHMA EXACERBATIONS
In the last decade, emergency departments (EDs) have increasingly prescribed dexamethasone over prednisone because it is noninferior and has a superior side-effect profile, including less vomiting.2 However, the evidence for dexamethasone use in hospitalized children lagged behind ED practice change. This led to uncertainty among pediatric hospitalists regarding the most appropriate oral steroid to use, particularly for children who received dexamethasone in the ED prior to admission.3
Several studies have been published to address this gap in the literature. In 2015 Parikh et al. published a multicenter retrospective cohort study of dexamethasone vs prednisone among hospitalized children using the Pediatric Health Information Systems (PHIS) database. 4 The authors compared 1,166 patients who received dexamethasone only with a propensity-matched cohort of 1,284 patients receiving only prednisone/prednisolone. Outcomes included the proportion with a length of stay (LOS) greater than 3 days, all-cause readmission at 7 and 30 days, and cost of admission. A greater proportion of patients receiving prednisone/prednisolone had a LOS greater than 3 days when compared with those in the dexamethasone cohort. There were no significant differences in all cause 7- or 30-day readmission. The dexamethasone cohort had statistically significantly lower costs. The authors concluded that dexamethasone may be a viable alternative to prednisone/prednisolone for children admitted for acute asthma exacerbation not requiring admission to the pediatric intensive care unit (PICU).
In 2019, Tyler et al. published a single-center, retrospective, cohort study that used interrupted time series analysis to evaluate outcomes for inpatients with asthma before and after an ED’s protocol was changed to dexamethasone.5 Outcomes analyzed included LOS, hospital charges, and PICU transfer rates. The study included 1,015 subjects over a 36-month period. In the post–protocol change group, 65% of the subjects received dexamethasone only while 28% received a combination of dexamethasone and prednisone/prednisolone. The authors found no immediate significant differences in LOS, ICU transfers, or charges after the protocol change. However, they did see an overall 10% increased rate of PICU transfers in the period following the protocol change, a trend that could have been caused by difficult-to-measure differences in severity of patients before and after the protocol change. If the increase in PICU transfer rate was temporally associated with the ED protocol change, an immediate change in rate would be expected, and this was not seen. The authors speculated that dexamethasone may be inferior to prednisone for inpatients with the highest severity of asthma.
Combined with the practical benefit of dexamethasone’s shorter treatment course and decreased vomiting,2 these two studies support the use of dexamethasone in the inpatient setting for patients who don’t require ICU level care. A feasibility trial to determine noninferiority of dexamethasone vs prednisone is currently enrolling, according to clinicaltrials.gov.
NEBULIZED VS METERED-DOSE INHALER ALBUTEROL FOR ACUTE ASTHMA EXACERBATIONS
The 2007 NHLBI guidelines are clear that short-acting beta-2 agonists (SABA), delivered via nebulization or metered-dose inhaler (MDI) with a valved holding chamber (VHC), along with systemic steroids, should be the primary treatment in pediatric acute asthma exacerbations.1 The guidelines caution that nebulization therapy might be needed for patients who are ineffective in using MDIs because of age, level of agitation, or severity of asthma symptoms. Specific recommendations for management in the inpatient setting are brief but note that inpatient medication administration and care should mirror ED management strategies.1 Specific in-hospital management recommendations regarding nebulization vs MDI are not addressed.
A Cochrane Review by Cates et al. assessed pediatric and adult randomized trials comparing SABA delivery via MDI-VHC with that via nebulization.6 The analysis included 39 trials with a total of 729 adults and 1,897 children. Six of the included trials were conducted in an inpatient setting (207 enrolled children in these studies). The authors found that mechanism of SABA delivery did not affect ED admission rates or significantly influence other markers of treatment response (peak flow and forced expiratory volumes). In children, MDI-VHC use was associated with shorter ED length of stay, as well as a decreased frequency of common SABA side effects (ie, tachycardia and tremor). This review cites several areas in which research is needed, including MDI use in severe asthma exacerbations. This population often falls outside pediatric hospitalists’ scope of practice because these patients often require ICU-level care.
A recent systematic review of pediatric acute asthma management strategies by Castro-Rodriguez et al. found that using MDI-VHC to deliver SABA was superior to using nebulization as measured by decreased ED admission rates and ED length of stay, improved asthma clinical scores, and reduced SABA side effects.7 A 2016 prospective randomized trial of MDI-VHC vs nebulization in preschool-aged children presenting to an ED with asthma or virally mediated wheeze found that the SABA delivered via MDI-VHC was at least as effective as that delivered via nebulization.8
International asthma management guidelines more strongly recommend MDI-only treatment for pediatric patients admitted with moderate asthma.9 Despite this guidance, and the literature supporting transition in inpatient settings to bronchodilator administration via MDI, there are several barriers to exclusive MDI use in the inpatient setting. As mentioned by Cates et al., a recognized challenge in MDI-VHC adoption is overcoming the “nebulizer culture” in treating pediatric acute asthma symptoms.6 Perhaps not surprisingly, Press et al., in a retrospective secondary analysis of 25 institutions managing adults and children with acute asthma symptoms, found that 32% of all pediatric patients assessed received only nebulized SABA treatments during their hospitalization.10 Transitioning from nebulized albuterol to exclusively MDI-VHC albuterol will require significant systems changes.
UTILITY OF CLINICAL PATHWAYS
Clinical pathways operationalize practice guidelines and provide guidance on the treatments, testing, and management of an illness. Use of pediatric asthma pathways has increased steadily in the past decade, with one study of over 300 hospitals finding that, between 2005 to 2015, pathway implementation increased from 27% to 86%.11 This expanded use has coincided with a proliferation of publications evaluating the effects of these pathways. A systematic review examining the implementation and impact of asthma protocols identified over 100 articles published between 1986 and 2010, with the majority published after 2005.12 The study found implementation of guidelines through an asthma pathway generally improved patient care and provider performance regardless of implementation method.
Since that review, Kaiser et al. investigated the effects of pathway implementation at 42 children’s hospitals.13 They used interrupted time series to determine the effect of pathway implementation on LOS. Secondary outcomes included cost, use of bronchodilators, antibiotic use, and 30-day readmissions. This study found pathway implementation was associated with an 8.8% decrease in LOS and 3% decrease in hospital costs while increasing bronchodilator administration and decreasing antibiotic exposure. To determine the factors that allowed successful implementation of asthma pathways (as determined by reduction in LOS), Kaiser et al. performed qualitative interviews of key stakeholders at high- and low-performing hospitals.14 The most successful hospitals all used rigorous data-driven quality-improvement methodologies, set shared goals with key stakeholders, integrated the pathway into their electronic medical record, allowed nurses and respiratory therapists to titrate albuterol frequency, and engaged hospital leadership to secure needed resources.
Although in each of these studies, pathway implementation led to improvements in the acute management of patients, there was no reduction in pediatric asthma readmissions at 30 days.12,13 A meta-analysis of asthma-related quality improvement interventions also did not find an association between pathway implementation alone and decreased readmissions or ED revisits.15 The lack of improvement in these metrics may have been caused by the tendency for pathways to focus on the acute asthma management and lack of focus on chronic asthma severity. Asthma admissions are an opportunity for full evaluation of disease severity, allergen exposures, and education on medication and spacer technique. Refinement of pathways with a focus on chronic control and on transition from hospital to home may move the needle on decreasing the long-term morbidity of pediatric asthma.
CONCLUSION
Current evidence suggests pediatric hospitalists should consider transitioning from prednisolone/prednisone to dexamethasone and from nebulized albuterol delivery to MDI albuterol delivery for children admitted for acute asthma exacerbation who do not require ICU-level care. Implementing asthma clinical pathways that use rigorous quality improvement methods is an effective approach to adopt these and other evidence-based practice changes.
Disclosures
The authors have nothing to disclose.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.029.
2. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133(3):493-499. https://doi.org/10.1542/peds.2013-2273.
3. Cotter JM, Tyler A, Reese J, et al. Steroid variability in pediatric inpatient asthmatics: Survey on provider preferences of dexamethasone versus prednisone. J Asthma. 2019:1-7. https://doi.org/10.1080/02770903.2019.1622713.
4. Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639-644.e1. https://doi.org/10.1016/j.jpeds.2015.06.038.
5. Tyler A, Cotter JM, Moss A, et al. Outcomes for pediatric asthmatic inpatients after implementation of an emergency department dexamethasone treatment protocol. Hosp Pediatr. 2019;9(2):92-99. https://doi.org/10.1542/hpeds.2018-0099.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;(9):CD000052. https://doi.org/10.1002/14651858.CD000052.pub3.
7. Castro-Rodriguez JA, J Rodrigo G, E Rodriguea-Martinez C. Principal findings of systematic reviews of acute asthma treatment in childhood. J Asthma. 2015;52(10):1038-1045. https://doi.org/10.3109/02770903.2015.1033725.
8. Mitselou N, Hedlin G, Hederos CA. Spacers versus nebulizers in treatment of acute asthma - a prospective randomized study in preschool children. J Asthma. 2016;53(10):1059-1062. https://doi.org/10.1080/02770903.2016.1185114.
9. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. https://www.ginasthma.org. Accessed December 10, 2019.
10. Press VG, Hasegawa K, Heidt J, Bittner JC, Camargo CA Jr. Missed opportunities to transition from nebulizers to inhalers during hospitalization for acute asthma: A multicenter observational study. J Asthma. 2017;54(9):968-976. https://doi.org/10.1080/02770903.2017.
11. Kaiser SV, Rodean J, Bekmezian A, et al. Rising utilization of inpatient pediatric asthma pathways. J Asthma. 2018;55(2):196-207. https://doi.org/ 10.1080/02770903.2017.1316392.
12. Dexheimer JW, Borycki EM, Chiu KW, Johnson KB, Aronsky D. A systematic review of the implementation and impact of asthma protocols. BMC Med Inform Decis Mak. 2014;14:82. https://doi.org/10.1186/1472-6947-14-82.
13. Kaiser SV, Rodean J, Bekmezian A, et al. effectiveness of pediatric asthma pathways for hospitalized children: A multicenter, national analysis. J Pediatr. 2018;197:165-171.e2. https://doi.org/10.1016/j.jpeds.2018.01.084.
14. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
15. Parikh K, Keller S, Ralston S. Inpatient quality improvement interventions for asthma: A meta-analysis. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3334.
Since the last National Heart, Lung, and Blood Institute’s (NHLBI) guidelines that were released in 2007, additional evidence has emerged in several areas of asthma care.1 To provide a concise clinical update relevant to the practice of pediatric hospital medicine, we searched PubMed for asthma publications in the last 10 years with a particular focus on articles published in the last 5 years. We used a validated pediatric search filter to identify pediatric studies, MeSH term for “Asthma,” and the following terms: “Clinical Pathways,” “Clinical Protocols,” “Dexamethasone,” and “Albuterol.” From these articles, we identified three areas of emerging evidence supporting practice change relative to the inpatient care of children with asthma, which are summarized in this brief review. This clinical practice update covers the emerging evidence supporting dexamethasone use for acute asthma exacerbations, the shift away from nebulized albuterol toward metered dose inhaler (MDI) albuterol, and the utility of asthma clinical pathways.
DEXAMETHASONE VS PREDNISONE FOR ACUTE ASTHMA EXACERBATIONS
In the last decade, emergency departments (EDs) have increasingly prescribed dexamethasone over prednisone because it is noninferior and has a superior side-effect profile, including less vomiting.2 However, the evidence for dexamethasone use in hospitalized children lagged behind ED practice change. This led to uncertainty among pediatric hospitalists regarding the most appropriate oral steroid to use, particularly for children who received dexamethasone in the ED prior to admission.3
Several studies have been published to address this gap in the literature. In 2015 Parikh et al. published a multicenter retrospective cohort study of dexamethasone vs prednisone among hospitalized children using the Pediatric Health Information Systems (PHIS) database. 4 The authors compared 1,166 patients who received dexamethasone only with a propensity-matched cohort of 1,284 patients receiving only prednisone/prednisolone. Outcomes included the proportion with a length of stay (LOS) greater than 3 days, all-cause readmission at 7 and 30 days, and cost of admission. A greater proportion of patients receiving prednisone/prednisolone had a LOS greater than 3 days when compared with those in the dexamethasone cohort. There were no significant differences in all cause 7- or 30-day readmission. The dexamethasone cohort had statistically significantly lower costs. The authors concluded that dexamethasone may be a viable alternative to prednisone/prednisolone for children admitted for acute asthma exacerbation not requiring admission to the pediatric intensive care unit (PICU).
In 2019, Tyler et al. published a single-center, retrospective, cohort study that used interrupted time series analysis to evaluate outcomes for inpatients with asthma before and after an ED’s protocol was changed to dexamethasone.5 Outcomes analyzed included LOS, hospital charges, and PICU transfer rates. The study included 1,015 subjects over a 36-month period. In the post–protocol change group, 65% of the subjects received dexamethasone only while 28% received a combination of dexamethasone and prednisone/prednisolone. The authors found no immediate significant differences in LOS, ICU transfers, or charges after the protocol change. However, they did see an overall 10% increased rate of PICU transfers in the period following the protocol change, a trend that could have been caused by difficult-to-measure differences in severity of patients before and after the protocol change. If the increase in PICU transfer rate was temporally associated with the ED protocol change, an immediate change in rate would be expected, and this was not seen. The authors speculated that dexamethasone may be inferior to prednisone for inpatients with the highest severity of asthma.
Combined with the practical benefit of dexamethasone’s shorter treatment course and decreased vomiting,2 these two studies support the use of dexamethasone in the inpatient setting for patients who don’t require ICU level care. A feasibility trial to determine noninferiority of dexamethasone vs prednisone is currently enrolling, according to clinicaltrials.gov.
NEBULIZED VS METERED-DOSE INHALER ALBUTEROL FOR ACUTE ASTHMA EXACERBATIONS
The 2007 NHLBI guidelines are clear that short-acting beta-2 agonists (SABA), delivered via nebulization or metered-dose inhaler (MDI) with a valved holding chamber (VHC), along with systemic steroids, should be the primary treatment in pediatric acute asthma exacerbations.1 The guidelines caution that nebulization therapy might be needed for patients who are ineffective in using MDIs because of age, level of agitation, or severity of asthma symptoms. Specific recommendations for management in the inpatient setting are brief but note that inpatient medication administration and care should mirror ED management strategies.1 Specific in-hospital management recommendations regarding nebulization vs MDI are not addressed.
A Cochrane Review by Cates et al. assessed pediatric and adult randomized trials comparing SABA delivery via MDI-VHC with that via nebulization.6 The analysis included 39 trials with a total of 729 adults and 1,897 children. Six of the included trials were conducted in an inpatient setting (207 enrolled children in these studies). The authors found that mechanism of SABA delivery did not affect ED admission rates or significantly influence other markers of treatment response (peak flow and forced expiratory volumes). In children, MDI-VHC use was associated with shorter ED length of stay, as well as a decreased frequency of common SABA side effects (ie, tachycardia and tremor). This review cites several areas in which research is needed, including MDI use in severe asthma exacerbations. This population often falls outside pediatric hospitalists’ scope of practice because these patients often require ICU-level care.
A recent systematic review of pediatric acute asthma management strategies by Castro-Rodriguez et al. found that using MDI-VHC to deliver SABA was superior to using nebulization as measured by decreased ED admission rates and ED length of stay, improved asthma clinical scores, and reduced SABA side effects.7 A 2016 prospective randomized trial of MDI-VHC vs nebulization in preschool-aged children presenting to an ED with asthma or virally mediated wheeze found that the SABA delivered via MDI-VHC was at least as effective as that delivered via nebulization.8
International asthma management guidelines more strongly recommend MDI-only treatment for pediatric patients admitted with moderate asthma.9 Despite this guidance, and the literature supporting transition in inpatient settings to bronchodilator administration via MDI, there are several barriers to exclusive MDI use in the inpatient setting. As mentioned by Cates et al., a recognized challenge in MDI-VHC adoption is overcoming the “nebulizer culture” in treating pediatric acute asthma symptoms.6 Perhaps not surprisingly, Press et al., in a retrospective secondary analysis of 25 institutions managing adults and children with acute asthma symptoms, found that 32% of all pediatric patients assessed received only nebulized SABA treatments during their hospitalization.10 Transitioning from nebulized albuterol to exclusively MDI-VHC albuterol will require significant systems changes.
UTILITY OF CLINICAL PATHWAYS
Clinical pathways operationalize practice guidelines and provide guidance on the treatments, testing, and management of an illness. Use of pediatric asthma pathways has increased steadily in the past decade, with one study of over 300 hospitals finding that, between 2005 to 2015, pathway implementation increased from 27% to 86%.11 This expanded use has coincided with a proliferation of publications evaluating the effects of these pathways. A systematic review examining the implementation and impact of asthma protocols identified over 100 articles published between 1986 and 2010, with the majority published after 2005.12 The study found implementation of guidelines through an asthma pathway generally improved patient care and provider performance regardless of implementation method.
Since that review, Kaiser et al. investigated the effects of pathway implementation at 42 children’s hospitals.13 They used interrupted time series to determine the effect of pathway implementation on LOS. Secondary outcomes included cost, use of bronchodilators, antibiotic use, and 30-day readmissions. This study found pathway implementation was associated with an 8.8% decrease in LOS and 3% decrease in hospital costs while increasing bronchodilator administration and decreasing antibiotic exposure. To determine the factors that allowed successful implementation of asthma pathways (as determined by reduction in LOS), Kaiser et al. performed qualitative interviews of key stakeholders at high- and low-performing hospitals.14 The most successful hospitals all used rigorous data-driven quality-improvement methodologies, set shared goals with key stakeholders, integrated the pathway into their electronic medical record, allowed nurses and respiratory therapists to titrate albuterol frequency, and engaged hospital leadership to secure needed resources.
Although in each of these studies, pathway implementation led to improvements in the acute management of patients, there was no reduction in pediatric asthma readmissions at 30 days.12,13 A meta-analysis of asthma-related quality improvement interventions also did not find an association between pathway implementation alone and decreased readmissions or ED revisits.15 The lack of improvement in these metrics may have been caused by the tendency for pathways to focus on the acute asthma management and lack of focus on chronic asthma severity. Asthma admissions are an opportunity for full evaluation of disease severity, allergen exposures, and education on medication and spacer technique. Refinement of pathways with a focus on chronic control and on transition from hospital to home may move the needle on decreasing the long-term morbidity of pediatric asthma.
CONCLUSION
Current evidence suggests pediatric hospitalists should consider transitioning from prednisolone/prednisone to dexamethasone and from nebulized albuterol delivery to MDI albuterol delivery for children admitted for acute asthma exacerbation who do not require ICU-level care. Implementing asthma clinical pathways that use rigorous quality improvement methods is an effective approach to adopt these and other evidence-based practice changes.
Disclosures
The authors have nothing to disclose.
Since the last National Heart, Lung, and Blood Institute’s (NHLBI) guidelines that were released in 2007, additional evidence has emerged in several areas of asthma care.1 To provide a concise clinical update relevant to the practice of pediatric hospital medicine, we searched PubMed for asthma publications in the last 10 years with a particular focus on articles published in the last 5 years. We used a validated pediatric search filter to identify pediatric studies, MeSH term for “Asthma,” and the following terms: “Clinical Pathways,” “Clinical Protocols,” “Dexamethasone,” and “Albuterol.” From these articles, we identified three areas of emerging evidence supporting practice change relative to the inpatient care of children with asthma, which are summarized in this brief review. This clinical practice update covers the emerging evidence supporting dexamethasone use for acute asthma exacerbations, the shift away from nebulized albuterol toward metered dose inhaler (MDI) albuterol, and the utility of asthma clinical pathways.
DEXAMETHASONE VS PREDNISONE FOR ACUTE ASTHMA EXACERBATIONS
In the last decade, emergency departments (EDs) have increasingly prescribed dexamethasone over prednisone because it is noninferior and has a superior side-effect profile, including less vomiting.2 However, the evidence for dexamethasone use in hospitalized children lagged behind ED practice change. This led to uncertainty among pediatric hospitalists regarding the most appropriate oral steroid to use, particularly for children who received dexamethasone in the ED prior to admission.3
Several studies have been published to address this gap in the literature. In 2015 Parikh et al. published a multicenter retrospective cohort study of dexamethasone vs prednisone among hospitalized children using the Pediatric Health Information Systems (PHIS) database. 4 The authors compared 1,166 patients who received dexamethasone only with a propensity-matched cohort of 1,284 patients receiving only prednisone/prednisolone. Outcomes included the proportion with a length of stay (LOS) greater than 3 days, all-cause readmission at 7 and 30 days, and cost of admission. A greater proportion of patients receiving prednisone/prednisolone had a LOS greater than 3 days when compared with those in the dexamethasone cohort. There were no significant differences in all cause 7- or 30-day readmission. The dexamethasone cohort had statistically significantly lower costs. The authors concluded that dexamethasone may be a viable alternative to prednisone/prednisolone for children admitted for acute asthma exacerbation not requiring admission to the pediatric intensive care unit (PICU).
In 2019, Tyler et al. published a single-center, retrospective, cohort study that used interrupted time series analysis to evaluate outcomes for inpatients with asthma before and after an ED’s protocol was changed to dexamethasone.5 Outcomes analyzed included LOS, hospital charges, and PICU transfer rates. The study included 1,015 subjects over a 36-month period. In the post–protocol change group, 65% of the subjects received dexamethasone only while 28% received a combination of dexamethasone and prednisone/prednisolone. The authors found no immediate significant differences in LOS, ICU transfers, or charges after the protocol change. However, they did see an overall 10% increased rate of PICU transfers in the period following the protocol change, a trend that could have been caused by difficult-to-measure differences in severity of patients before and after the protocol change. If the increase in PICU transfer rate was temporally associated with the ED protocol change, an immediate change in rate would be expected, and this was not seen. The authors speculated that dexamethasone may be inferior to prednisone for inpatients with the highest severity of asthma.
Combined with the practical benefit of dexamethasone’s shorter treatment course and decreased vomiting,2 these two studies support the use of dexamethasone in the inpatient setting for patients who don’t require ICU level care. A feasibility trial to determine noninferiority of dexamethasone vs prednisone is currently enrolling, according to clinicaltrials.gov.
NEBULIZED VS METERED-DOSE INHALER ALBUTEROL FOR ACUTE ASTHMA EXACERBATIONS
The 2007 NHLBI guidelines are clear that short-acting beta-2 agonists (SABA), delivered via nebulization or metered-dose inhaler (MDI) with a valved holding chamber (VHC), along with systemic steroids, should be the primary treatment in pediatric acute asthma exacerbations.1 The guidelines caution that nebulization therapy might be needed for patients who are ineffective in using MDIs because of age, level of agitation, or severity of asthma symptoms. Specific recommendations for management in the inpatient setting are brief but note that inpatient medication administration and care should mirror ED management strategies.1 Specific in-hospital management recommendations regarding nebulization vs MDI are not addressed.
A Cochrane Review by Cates et al. assessed pediatric and adult randomized trials comparing SABA delivery via MDI-VHC with that via nebulization.6 The analysis included 39 trials with a total of 729 adults and 1,897 children. Six of the included trials were conducted in an inpatient setting (207 enrolled children in these studies). The authors found that mechanism of SABA delivery did not affect ED admission rates or significantly influence other markers of treatment response (peak flow and forced expiratory volumes). In children, MDI-VHC use was associated with shorter ED length of stay, as well as a decreased frequency of common SABA side effects (ie, tachycardia and tremor). This review cites several areas in which research is needed, including MDI use in severe asthma exacerbations. This population often falls outside pediatric hospitalists’ scope of practice because these patients often require ICU-level care.
A recent systematic review of pediatric acute asthma management strategies by Castro-Rodriguez et al. found that using MDI-VHC to deliver SABA was superior to using nebulization as measured by decreased ED admission rates and ED length of stay, improved asthma clinical scores, and reduced SABA side effects.7 A 2016 prospective randomized trial of MDI-VHC vs nebulization in preschool-aged children presenting to an ED with asthma or virally mediated wheeze found that the SABA delivered via MDI-VHC was at least as effective as that delivered via nebulization.8
International asthma management guidelines more strongly recommend MDI-only treatment for pediatric patients admitted with moderate asthma.9 Despite this guidance, and the literature supporting transition in inpatient settings to bronchodilator administration via MDI, there are several barriers to exclusive MDI use in the inpatient setting. As mentioned by Cates et al., a recognized challenge in MDI-VHC adoption is overcoming the “nebulizer culture” in treating pediatric acute asthma symptoms.6 Perhaps not surprisingly, Press et al., in a retrospective secondary analysis of 25 institutions managing adults and children with acute asthma symptoms, found that 32% of all pediatric patients assessed received only nebulized SABA treatments during their hospitalization.10 Transitioning from nebulized albuterol to exclusively MDI-VHC albuterol will require significant systems changes.
UTILITY OF CLINICAL PATHWAYS
Clinical pathways operationalize practice guidelines and provide guidance on the treatments, testing, and management of an illness. Use of pediatric asthma pathways has increased steadily in the past decade, with one study of over 300 hospitals finding that, between 2005 to 2015, pathway implementation increased from 27% to 86%.11 This expanded use has coincided with a proliferation of publications evaluating the effects of these pathways. A systematic review examining the implementation and impact of asthma protocols identified over 100 articles published between 1986 and 2010, with the majority published after 2005.12 The study found implementation of guidelines through an asthma pathway generally improved patient care and provider performance regardless of implementation method.
Since that review, Kaiser et al. investigated the effects of pathway implementation at 42 children’s hospitals.13 They used interrupted time series to determine the effect of pathway implementation on LOS. Secondary outcomes included cost, use of bronchodilators, antibiotic use, and 30-day readmissions. This study found pathway implementation was associated with an 8.8% decrease in LOS and 3% decrease in hospital costs while increasing bronchodilator administration and decreasing antibiotic exposure. To determine the factors that allowed successful implementation of asthma pathways (as determined by reduction in LOS), Kaiser et al. performed qualitative interviews of key stakeholders at high- and low-performing hospitals.14 The most successful hospitals all used rigorous data-driven quality-improvement methodologies, set shared goals with key stakeholders, integrated the pathway into their electronic medical record, allowed nurses and respiratory therapists to titrate albuterol frequency, and engaged hospital leadership to secure needed resources.
Although in each of these studies, pathway implementation led to improvements in the acute management of patients, there was no reduction in pediatric asthma readmissions at 30 days.12,13 A meta-analysis of asthma-related quality improvement interventions also did not find an association between pathway implementation alone and decreased readmissions or ED revisits.15 The lack of improvement in these metrics may have been caused by the tendency for pathways to focus on the acute asthma management and lack of focus on chronic asthma severity. Asthma admissions are an opportunity for full evaluation of disease severity, allergen exposures, and education on medication and spacer technique. Refinement of pathways with a focus on chronic control and on transition from hospital to home may move the needle on decreasing the long-term morbidity of pediatric asthma.
CONCLUSION
Current evidence suggests pediatric hospitalists should consider transitioning from prednisolone/prednisone to dexamethasone and from nebulized albuterol delivery to MDI albuterol delivery for children admitted for acute asthma exacerbation who do not require ICU-level care. Implementing asthma clinical pathways that use rigorous quality improvement methods is an effective approach to adopt these and other evidence-based practice changes.
Disclosures
The authors have nothing to disclose.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.029.
2. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133(3):493-499. https://doi.org/10.1542/peds.2013-2273.
3. Cotter JM, Tyler A, Reese J, et al. Steroid variability in pediatric inpatient asthmatics: Survey on provider preferences of dexamethasone versus prednisone. J Asthma. 2019:1-7. https://doi.org/10.1080/02770903.2019.1622713.
4. Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639-644.e1. https://doi.org/10.1016/j.jpeds.2015.06.038.
5. Tyler A, Cotter JM, Moss A, et al. Outcomes for pediatric asthmatic inpatients after implementation of an emergency department dexamethasone treatment protocol. Hosp Pediatr. 2019;9(2):92-99. https://doi.org/10.1542/hpeds.2018-0099.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;(9):CD000052. https://doi.org/10.1002/14651858.CD000052.pub3.
7. Castro-Rodriguez JA, J Rodrigo G, E Rodriguea-Martinez C. Principal findings of systematic reviews of acute asthma treatment in childhood. J Asthma. 2015;52(10):1038-1045. https://doi.org/10.3109/02770903.2015.1033725.
8. Mitselou N, Hedlin G, Hederos CA. Spacers versus nebulizers in treatment of acute asthma - a prospective randomized study in preschool children. J Asthma. 2016;53(10):1059-1062. https://doi.org/10.1080/02770903.2016.1185114.
9. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. https://www.ginasthma.org. Accessed December 10, 2019.
10. Press VG, Hasegawa K, Heidt J, Bittner JC, Camargo CA Jr. Missed opportunities to transition from nebulizers to inhalers during hospitalization for acute asthma: A multicenter observational study. J Asthma. 2017;54(9):968-976. https://doi.org/10.1080/02770903.2017.
11. Kaiser SV, Rodean J, Bekmezian A, et al. Rising utilization of inpatient pediatric asthma pathways. J Asthma. 2018;55(2):196-207. https://doi.org/ 10.1080/02770903.2017.1316392.
12. Dexheimer JW, Borycki EM, Chiu KW, Johnson KB, Aronsky D. A systematic review of the implementation and impact of asthma protocols. BMC Med Inform Decis Mak. 2014;14:82. https://doi.org/10.1186/1472-6947-14-82.
13. Kaiser SV, Rodean J, Bekmezian A, et al. effectiveness of pediatric asthma pathways for hospitalized children: A multicenter, national analysis. J Pediatr. 2018;197:165-171.e2. https://doi.org/10.1016/j.jpeds.2018.01.084.
14. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
15. Parikh K, Keller S, Ralston S. Inpatient quality improvement interventions for asthma: A meta-analysis. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3334.
1. National Asthma E, Prevention P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.029.
2. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133(3):493-499. https://doi.org/10.1542/peds.2013-2273.
3. Cotter JM, Tyler A, Reese J, et al. Steroid variability in pediatric inpatient asthmatics: Survey on provider preferences of dexamethasone versus prednisone. J Asthma. 2019:1-7. https://doi.org/10.1080/02770903.2019.1622713.
4. Parikh K, Hall M, Mittal V, et al. Comparative effectiveness of dexamethasone versus prednisone in children hospitalized with asthma. J Pediatr. 2015;167(3):639-644.e1. https://doi.org/10.1016/j.jpeds.2015.06.038.
5. Tyler A, Cotter JM, Moss A, et al. Outcomes for pediatric asthmatic inpatients after implementation of an emergency department dexamethasone treatment protocol. Hosp Pediatr. 2019;9(2):92-99. https://doi.org/10.1542/hpeds.2018-0099.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;(9):CD000052. https://doi.org/10.1002/14651858.CD000052.pub3.
7. Castro-Rodriguez JA, J Rodrigo G, E Rodriguea-Martinez C. Principal findings of systematic reviews of acute asthma treatment in childhood. J Asthma. 2015;52(10):1038-1045. https://doi.org/10.3109/02770903.2015.1033725.
8. Mitselou N, Hedlin G, Hederos CA. Spacers versus nebulizers in treatment of acute asthma - a prospective randomized study in preschool children. J Asthma. 2016;53(10):1059-1062. https://doi.org/10.1080/02770903.2016.1185114.
9. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. https://www.ginasthma.org. Accessed December 10, 2019.
10. Press VG, Hasegawa K, Heidt J, Bittner JC, Camargo CA Jr. Missed opportunities to transition from nebulizers to inhalers during hospitalization for acute asthma: A multicenter observational study. J Asthma. 2017;54(9):968-976. https://doi.org/10.1080/02770903.2017.
11. Kaiser SV, Rodean J, Bekmezian A, et al. Rising utilization of inpatient pediatric asthma pathways. J Asthma. 2018;55(2):196-207. https://doi.org/ 10.1080/02770903.2017.1316392.
12. Dexheimer JW, Borycki EM, Chiu KW, Johnson KB, Aronsky D. A systematic review of the implementation and impact of asthma protocols. BMC Med Inform Decis Mak. 2014;14:82. https://doi.org/10.1186/1472-6947-14-82.
13. Kaiser SV, Rodean J, Bekmezian A, et al. effectiveness of pediatric asthma pathways for hospitalized children: A multicenter, national analysis. J Pediatr. 2018;197:165-171.e2. https://doi.org/10.1016/j.jpeds.2018.01.084.
14. Kaiser SV, Lam R, Cabana MD, et al. Best practices in implementing inpatient pediatric asthma pathways: a qualitative study. J Asthma. 2019:1-11. https://doi.org/10.1080/02770903.2019.1606237.
15. Parikh K, Keller S, Ralston S. Inpatient quality improvement interventions for asthma: A meta-analysis. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3334.
© 2020 Society of Hospital Medicine
Methodologic Progress Note: Opportunistic Sampling for Pharmacology Studies in Hospitalized Children
Challenges in conducting and completing studies of drugs in vulnerable populations, such as hospitalized children, include weak study designs and lack of sufficient sample sizes to achieve adequate power.1 Limitations in the amount of blood that can be safely drawn in children and low parental consent rates due to concerns for anemia or pain, if venipuncture is required, lead to an insufficient number of patients enrolled in traditional clinical studies.2 Thus, sample size targets are often not met. Recognizing the limited pediatric data for many drugs routinely prescribed off-label in children, the Food and Drug Administration implemented the Best Pharmaceuticals Children Act and the Pediatric Research Equity Act (PREA) in 2003; these legislative acts require clinical studies to assess the safety and efficacy of new drugs in children.1 While studies conducted under these acts have provided important information for the clinical care of children, only one-third of mandatory pediatric postmarketing studies of the 114 new drugs and new indications subject to PREA requirements between 2007 and 2014 had been completed within seven years.3
Despite the challenges in conducting studies of drugs in children, robust pediatric data must be generated in children, especially in those with medical complexity or with chronic medical diseases and who have significant risk of experiencing adverse drug events. Data in adults cannot simply be extrapolated to children. In addition, studies from healthy children may not apply to hospitalized pediatric patients because of significant physiologic changes that occur in children who are ill enough to be hospitalized. Opportunistic sampling can provide robust drug disposition data and overcome some of the challenges encountered by traditional drug studies. In this Methodologic Progress Note, we describe the utility of opportunistic sampling as a research tool for hospitalists, in partnership with clinical pharmacologists, to study drug pharmacokinetics (PK) in hospitalized children.
OPPORTUNISTIC SAMPLING DEFINED
Opportunistic sampling relies on the use of blood samples that are ordered for clinical purposes, and its use is endorsed by the Pediatric Trials Network.2,5 Opportunistic sampling approaches have two types: sparse sampling and scavenged or remnant sampling. In sparse opportunistic sampling, additional blood is obtained at the same time clinical samples are ordered, avoiding the need for additional punctures.5 This approach requires bedside personnel to obtain additional blood that is sent to the research team for further processing. Scavenged sampling relies on leftover residual blood from clinical samples.2,5 After the clinical laboratory performs the laboratory test ordered by the clinical team, the research team can scavenge residual blood for measurement of drug concentrations. When drug concentrations are measured from multiple patients, clinical pharmacologists can perform population PK modeling to characterize the pharmacokinetics of the drug and its variability within the population level.
The Figure shows how scavenged samples from hypothetical patients could be used to generate a PK curve. In this example, three patients are admitted for osteomyelitis and treated with the same antibiotic administered every eight hours, as shown by the theoretical concentration versus time profiles in the top panel. To determine the effectiveness of treatment and timing of transition to enteral antibiotics, the clinical team orders C-reactive protein (CRP) approximately every 48 hours for each patient. Each patient has his/her first dose of antibiotic at a different time of day depending on the time of admission or surgical drainage. Therefore, the timing of the blood draw with respect to the most recent antibiotic dose varies between patients even if blood draws are ordered at the same time for all patients (ie, 4
Opportunistic sampling has advantages over traditional intensive PK studies that often require multiple blood draws (typically >8-10) within one dosing interval to adequately describe the phases of absorption, distribution, metabolism, and elimination. The number of vascular punctures can be painful if blood cannot be drawn from existing vascular access, and the large amount of blood (sometimes >1 mL/sample) required for these studies can be impractical, burdensome, and even dangerous in young children and neonates. Scavenged sampling reduces the risk of anemia because no additional blood is drawn beyond what is obtained for clinical purposes, and it does not disrupt nursing workflow or add to nursing workload. Approval to use scavenged blood requires approval from the institutional review board. At some institutions, the consent to treat form on admission may address the use of scavenged samples and therefore allow for waiver of consent. In addition, the consent process may occur retroactively after samples are collected. These methods lead to increased enrollment.
Limitations in this approach are that drugs may degrade over time in whole blood or processed samples. Therefore, the process by which the clinical laboratory stores the residual blood after clinical tests must be understood, and the stability of the drug or metabolite of interest in blood or plasma over time must be ensured. In addition, residual blood may not be present after a clinical test, and recording times of the drug administration and lab draws may be inaccurate.2,5
APPLICATIONS OF OPPORTUNISTIC SAMPLING IN CLINICAL PHARMACOLOGY RESEARCH
/section>Opportunistic sampling has been successfully used to study a variety of drugs in different pediatric populations but has been primarily used in neonates. The multicenter Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care trial has utilized this approach to evaluate the PK of over 30 drugs.5 Several antimicrobials have been studied through opportunistic sampling, including those frequently used in pediatric hospital medicine, such as ampicillin6 and clindamycin.7
This sampling approach may be most beneficial in studying select patients. Obese patients, who are often excluded in pediatric drug trials, have been previously included in opportunistic drug studies.8 The utility of opportunistic sampling to study antimicrobials, morphine and cardiac drugs has been demonstrated in neonates, both preterm and term, in whom additional blood draws can be challenging because of low total blood volume and limited vascular access.6,7,9-12
Although the frequency of blood draws from patients admitted to pediatric hospital medicine services is generally lower than that for patients on other subspecialty services, such as critical care, we can capitalize on the high volume of patients with common diagnoses (eg, pneumonia, skin, and soft tissue infections) who are admitted to hospital medicine. Using opportunistic sampling, we can study the PK of drugs frequently used in hospital medicine, such as antibiotics, antiepileptic drugs, steroids, and pain medications. In addition, we can measure drug concentrations to study the effects of route administration, oral versus enteric tube versus intravenous, to guide not only the dosing but also the timing of transition to enteral medications. Finally, we can study drugs that are commonly used in adult and pediatric patient populations cared for by hospitalists but who are often excluded from clinical drug trials, such as patients with medical complexity, patients with medical devices (eg, nervous system shunts and tracheostomies), patients taking concomitant medications, or patients on extracorporeal devices such as dialysis, to validate drug regimens.
CONCLUSION
Generating robust pediatric clinical pharmacology data has many inherent challenges because of the vulnerability of children. However, their vulnerability requires that medications be studied thoroughly in children to ensure their safety and effectiveness. Opportunistic sampling allows for rigorous studies to be conducted with adequate sample sizes while minimizing the risk of pain, anemia, and other adverse events related to clinical drug trials. Pediatric hospitalists should consider this approach to advance their knowledge of commonly used drugs that have not been adequately studied in hospitalized children and can expand the use of opportunistic sampling to study other aspects of disease, such as diagnostic or prognostic biomarkers.
1. Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC, USA: National Academies Press; 2012.
2. Laughon MM, Benjamin DK, Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643-652. https://doi.org/10.1586/ecp.11.43.
3. Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2018;173(1):68-74. https://doi.org/10.1001/jamapediatrics.2018.3416.
4. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9. https://doi.org/10.5863/1551-6776-24.1.4.
5. Balevic SJ, Cohen-Wolkowiez M. Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol. 2018;58(10):S58-S72. https://doi.org/10.1002/jcph.1053.
6. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013-3020. https://doi.org/10.1128/AAC.02374-13.
7. Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429-437. https://doi.org/10.1038/clpt.2014.134.
8. Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4). https://doi.org/10.1128/AAC.02014-16.
9. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: The utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273-1285. https://doi.org/10.1007/s40262-015-0291-1.
10. Dallefeld SH, Atz AM, Yogev R, et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn. 2018;45(3):419-430. https://doi.org/10.1007/s10928-018-9576-y.
11. Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744-747. https://doi.org/10.1038/jp.2016.79.
12. Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Vinks AA. Large variability in morphine concentrations in critically ill neonates receiving standard of care postoperative pain-management. Clin Pharmacol Ther. 2018;103:S45-S45.
Challenges in conducting and completing studies of drugs in vulnerable populations, such as hospitalized children, include weak study designs and lack of sufficient sample sizes to achieve adequate power.1 Limitations in the amount of blood that can be safely drawn in children and low parental consent rates due to concerns for anemia or pain, if venipuncture is required, lead to an insufficient number of patients enrolled in traditional clinical studies.2 Thus, sample size targets are often not met. Recognizing the limited pediatric data for many drugs routinely prescribed off-label in children, the Food and Drug Administration implemented the Best Pharmaceuticals Children Act and the Pediatric Research Equity Act (PREA) in 2003; these legislative acts require clinical studies to assess the safety and efficacy of new drugs in children.1 While studies conducted under these acts have provided important information for the clinical care of children, only one-third of mandatory pediatric postmarketing studies of the 114 new drugs and new indications subject to PREA requirements between 2007 and 2014 had been completed within seven years.3
Despite the challenges in conducting studies of drugs in children, robust pediatric data must be generated in children, especially in those with medical complexity or with chronic medical diseases and who have significant risk of experiencing adverse drug events. Data in adults cannot simply be extrapolated to children. In addition, studies from healthy children may not apply to hospitalized pediatric patients because of significant physiologic changes that occur in children who are ill enough to be hospitalized. Opportunistic sampling can provide robust drug disposition data and overcome some of the challenges encountered by traditional drug studies. In this Methodologic Progress Note, we describe the utility of opportunistic sampling as a research tool for hospitalists, in partnership with clinical pharmacologists, to study drug pharmacokinetics (PK) in hospitalized children.
OPPORTUNISTIC SAMPLING DEFINED
Opportunistic sampling relies on the use of blood samples that are ordered for clinical purposes, and its use is endorsed by the Pediatric Trials Network.2,5 Opportunistic sampling approaches have two types: sparse sampling and scavenged or remnant sampling. In sparse opportunistic sampling, additional blood is obtained at the same time clinical samples are ordered, avoiding the need for additional punctures.5 This approach requires bedside personnel to obtain additional blood that is sent to the research team for further processing. Scavenged sampling relies on leftover residual blood from clinical samples.2,5 After the clinical laboratory performs the laboratory test ordered by the clinical team, the research team can scavenge residual blood for measurement of drug concentrations. When drug concentrations are measured from multiple patients, clinical pharmacologists can perform population PK modeling to characterize the pharmacokinetics of the drug and its variability within the population level.
The Figure shows how scavenged samples from hypothetical patients could be used to generate a PK curve. In this example, three patients are admitted for osteomyelitis and treated with the same antibiotic administered every eight hours, as shown by the theoretical concentration versus time profiles in the top panel. To determine the effectiveness of treatment and timing of transition to enteral antibiotics, the clinical team orders C-reactive protein (CRP) approximately every 48 hours for each patient. Each patient has his/her first dose of antibiotic at a different time of day depending on the time of admission or surgical drainage. Therefore, the timing of the blood draw with respect to the most recent antibiotic dose varies between patients even if blood draws are ordered at the same time for all patients (ie, 4
Opportunistic sampling has advantages over traditional intensive PK studies that often require multiple blood draws (typically >8-10) within one dosing interval to adequately describe the phases of absorption, distribution, metabolism, and elimination. The number of vascular punctures can be painful if blood cannot be drawn from existing vascular access, and the large amount of blood (sometimes >1 mL/sample) required for these studies can be impractical, burdensome, and even dangerous in young children and neonates. Scavenged sampling reduces the risk of anemia because no additional blood is drawn beyond what is obtained for clinical purposes, and it does not disrupt nursing workflow or add to nursing workload. Approval to use scavenged blood requires approval from the institutional review board. At some institutions, the consent to treat form on admission may address the use of scavenged samples and therefore allow for waiver of consent. In addition, the consent process may occur retroactively after samples are collected. These methods lead to increased enrollment.
Limitations in this approach are that drugs may degrade over time in whole blood or processed samples. Therefore, the process by which the clinical laboratory stores the residual blood after clinical tests must be understood, and the stability of the drug or metabolite of interest in blood or plasma over time must be ensured. In addition, residual blood may not be present after a clinical test, and recording times of the drug administration and lab draws may be inaccurate.2,5
APPLICATIONS OF OPPORTUNISTIC SAMPLING IN CLINICAL PHARMACOLOGY RESEARCH
/section>Opportunistic sampling has been successfully used to study a variety of drugs in different pediatric populations but has been primarily used in neonates. The multicenter Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care trial has utilized this approach to evaluate the PK of over 30 drugs.5 Several antimicrobials have been studied through opportunistic sampling, including those frequently used in pediatric hospital medicine, such as ampicillin6 and clindamycin.7
This sampling approach may be most beneficial in studying select patients. Obese patients, who are often excluded in pediatric drug trials, have been previously included in opportunistic drug studies.8 The utility of opportunistic sampling to study antimicrobials, morphine and cardiac drugs has been demonstrated in neonates, both preterm and term, in whom additional blood draws can be challenging because of low total blood volume and limited vascular access.6,7,9-12
Although the frequency of blood draws from patients admitted to pediatric hospital medicine services is generally lower than that for patients on other subspecialty services, such as critical care, we can capitalize on the high volume of patients with common diagnoses (eg, pneumonia, skin, and soft tissue infections) who are admitted to hospital medicine. Using opportunistic sampling, we can study the PK of drugs frequently used in hospital medicine, such as antibiotics, antiepileptic drugs, steroids, and pain medications. In addition, we can measure drug concentrations to study the effects of route administration, oral versus enteric tube versus intravenous, to guide not only the dosing but also the timing of transition to enteral medications. Finally, we can study drugs that are commonly used in adult and pediatric patient populations cared for by hospitalists but who are often excluded from clinical drug trials, such as patients with medical complexity, patients with medical devices (eg, nervous system shunts and tracheostomies), patients taking concomitant medications, or patients on extracorporeal devices such as dialysis, to validate drug regimens.
CONCLUSION
Generating robust pediatric clinical pharmacology data has many inherent challenges because of the vulnerability of children. However, their vulnerability requires that medications be studied thoroughly in children to ensure their safety and effectiveness. Opportunistic sampling allows for rigorous studies to be conducted with adequate sample sizes while minimizing the risk of pain, anemia, and other adverse events related to clinical drug trials. Pediatric hospitalists should consider this approach to advance their knowledge of commonly used drugs that have not been adequately studied in hospitalized children and can expand the use of opportunistic sampling to study other aspects of disease, such as diagnostic or prognostic biomarkers.
Challenges in conducting and completing studies of drugs in vulnerable populations, such as hospitalized children, include weak study designs and lack of sufficient sample sizes to achieve adequate power.1 Limitations in the amount of blood that can be safely drawn in children and low parental consent rates due to concerns for anemia or pain, if venipuncture is required, lead to an insufficient number of patients enrolled in traditional clinical studies.2 Thus, sample size targets are often not met. Recognizing the limited pediatric data for many drugs routinely prescribed off-label in children, the Food and Drug Administration implemented the Best Pharmaceuticals Children Act and the Pediatric Research Equity Act (PREA) in 2003; these legislative acts require clinical studies to assess the safety and efficacy of new drugs in children.1 While studies conducted under these acts have provided important information for the clinical care of children, only one-third of mandatory pediatric postmarketing studies of the 114 new drugs and new indications subject to PREA requirements between 2007 and 2014 had been completed within seven years.3
Despite the challenges in conducting studies of drugs in children, robust pediatric data must be generated in children, especially in those with medical complexity or with chronic medical diseases and who have significant risk of experiencing adverse drug events. Data in adults cannot simply be extrapolated to children. In addition, studies from healthy children may not apply to hospitalized pediatric patients because of significant physiologic changes that occur in children who are ill enough to be hospitalized. Opportunistic sampling can provide robust drug disposition data and overcome some of the challenges encountered by traditional drug studies. In this Methodologic Progress Note, we describe the utility of opportunistic sampling as a research tool for hospitalists, in partnership with clinical pharmacologists, to study drug pharmacokinetics (PK) in hospitalized children.
OPPORTUNISTIC SAMPLING DEFINED
Opportunistic sampling relies on the use of blood samples that are ordered for clinical purposes, and its use is endorsed by the Pediatric Trials Network.2,5 Opportunistic sampling approaches have two types: sparse sampling and scavenged or remnant sampling. In sparse opportunistic sampling, additional blood is obtained at the same time clinical samples are ordered, avoiding the need for additional punctures.5 This approach requires bedside personnel to obtain additional blood that is sent to the research team for further processing. Scavenged sampling relies on leftover residual blood from clinical samples.2,5 After the clinical laboratory performs the laboratory test ordered by the clinical team, the research team can scavenge residual blood for measurement of drug concentrations. When drug concentrations are measured from multiple patients, clinical pharmacologists can perform population PK modeling to characterize the pharmacokinetics of the drug and its variability within the population level.
The Figure shows how scavenged samples from hypothetical patients could be used to generate a PK curve. In this example, three patients are admitted for osteomyelitis and treated with the same antibiotic administered every eight hours, as shown by the theoretical concentration versus time profiles in the top panel. To determine the effectiveness of treatment and timing of transition to enteral antibiotics, the clinical team orders C-reactive protein (CRP) approximately every 48 hours for each patient. Each patient has his/her first dose of antibiotic at a different time of day depending on the time of admission or surgical drainage. Therefore, the timing of the blood draw with respect to the most recent antibiotic dose varies between patients even if blood draws are ordered at the same time for all patients (ie, 4
Opportunistic sampling has advantages over traditional intensive PK studies that often require multiple blood draws (typically >8-10) within one dosing interval to adequately describe the phases of absorption, distribution, metabolism, and elimination. The number of vascular punctures can be painful if blood cannot be drawn from existing vascular access, and the large amount of blood (sometimes >1 mL/sample) required for these studies can be impractical, burdensome, and even dangerous in young children and neonates. Scavenged sampling reduces the risk of anemia because no additional blood is drawn beyond what is obtained for clinical purposes, and it does not disrupt nursing workflow or add to nursing workload. Approval to use scavenged blood requires approval from the institutional review board. At some institutions, the consent to treat form on admission may address the use of scavenged samples and therefore allow for waiver of consent. In addition, the consent process may occur retroactively after samples are collected. These methods lead to increased enrollment.
Limitations in this approach are that drugs may degrade over time in whole blood or processed samples. Therefore, the process by which the clinical laboratory stores the residual blood after clinical tests must be understood, and the stability of the drug or metabolite of interest in blood or plasma over time must be ensured. In addition, residual blood may not be present after a clinical test, and recording times of the drug administration and lab draws may be inaccurate.2,5
APPLICATIONS OF OPPORTUNISTIC SAMPLING IN CLINICAL PHARMACOLOGY RESEARCH
/section>Opportunistic sampling has been successfully used to study a variety of drugs in different pediatric populations but has been primarily used in neonates. The multicenter Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care trial has utilized this approach to evaluate the PK of over 30 drugs.5 Several antimicrobials have been studied through opportunistic sampling, including those frequently used in pediatric hospital medicine, such as ampicillin6 and clindamycin.7
This sampling approach may be most beneficial in studying select patients. Obese patients, who are often excluded in pediatric drug trials, have been previously included in opportunistic drug studies.8 The utility of opportunistic sampling to study antimicrobials, morphine and cardiac drugs has been demonstrated in neonates, both preterm and term, in whom additional blood draws can be challenging because of low total blood volume and limited vascular access.6,7,9-12
Although the frequency of blood draws from patients admitted to pediatric hospital medicine services is generally lower than that for patients on other subspecialty services, such as critical care, we can capitalize on the high volume of patients with common diagnoses (eg, pneumonia, skin, and soft tissue infections) who are admitted to hospital medicine. Using opportunistic sampling, we can study the PK of drugs frequently used in hospital medicine, such as antibiotics, antiepileptic drugs, steroids, and pain medications. In addition, we can measure drug concentrations to study the effects of route administration, oral versus enteric tube versus intravenous, to guide not only the dosing but also the timing of transition to enteral medications. Finally, we can study drugs that are commonly used in adult and pediatric patient populations cared for by hospitalists but who are often excluded from clinical drug trials, such as patients with medical complexity, patients with medical devices (eg, nervous system shunts and tracheostomies), patients taking concomitant medications, or patients on extracorporeal devices such as dialysis, to validate drug regimens.
CONCLUSION
Generating robust pediatric clinical pharmacology data has many inherent challenges because of the vulnerability of children. However, their vulnerability requires that medications be studied thoroughly in children to ensure their safety and effectiveness. Opportunistic sampling allows for rigorous studies to be conducted with adequate sample sizes while minimizing the risk of pain, anemia, and other adverse events related to clinical drug trials. Pediatric hospitalists should consider this approach to advance their knowledge of commonly used drugs that have not been adequately studied in hospitalized children and can expand the use of opportunistic sampling to study other aspects of disease, such as diagnostic or prognostic biomarkers.
1. Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC, USA: National Academies Press; 2012.
2. Laughon MM, Benjamin DK, Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643-652. https://doi.org/10.1586/ecp.11.43.
3. Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2018;173(1):68-74. https://doi.org/10.1001/jamapediatrics.2018.3416.
4. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9. https://doi.org/10.5863/1551-6776-24.1.4.
5. Balevic SJ, Cohen-Wolkowiez M. Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol. 2018;58(10):S58-S72. https://doi.org/10.1002/jcph.1053.
6. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013-3020. https://doi.org/10.1128/AAC.02374-13.
7. Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429-437. https://doi.org/10.1038/clpt.2014.134.
8. Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4). https://doi.org/10.1128/AAC.02014-16.
9. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: The utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273-1285. https://doi.org/10.1007/s40262-015-0291-1.
10. Dallefeld SH, Atz AM, Yogev R, et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn. 2018;45(3):419-430. https://doi.org/10.1007/s10928-018-9576-y.
11. Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744-747. https://doi.org/10.1038/jp.2016.79.
12. Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Vinks AA. Large variability in morphine concentrations in critically ill neonates receiving standard of care postoperative pain-management. Clin Pharmacol Ther. 2018;103:S45-S45.
1. Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC, USA: National Academies Press; 2012.
2. Laughon MM, Benjamin DK, Jr., Capparelli EV, et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4(5):643-652. https://doi.org/10.1586/ecp.11.43.
3. Hwang TJ, Orenstein L, Kesselheim AS, Bourgeois FT. Completion rate and reporting of mandatory pediatric postmarketing studies under the US Pediatric Research Equity Act. JAMA Pediatr. 2018;173(1):68-74. https://doi.org/10.1001/jamapediatrics.2018.3416.
4. Rieder M. Adverse drug reactions in children: pediatric pharmacy and drug safety. J Pediatr Pharmacol Ther. 2019;24(1):4-9. https://doi.org/10.5863/1551-6776-24.1.4.
5. Balevic SJ, Cohen-Wolkowiez M. Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol. 2018;58(10):S58-S72. https://doi.org/10.1002/jcph.1053.
6. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013-3020. https://doi.org/10.1128/AAC.02374-13.
7. Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429-437. https://doi.org/10.1038/clpt.2014.134.
8. Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61(4). https://doi.org/10.1128/AAC.02014-16.
9. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: The utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54(12):1273-1285. https://doi.org/10.1007/s40262-015-0291-1.
10. Dallefeld SH, Atz AM, Yogev R, et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn. 2018;45(3):419-430. https://doi.org/10.1007/s10928-018-9576-y.
11. Thakkar N, Gonzalez D, Cohen-Wolkowiez M, et al. An opportunistic study evaluating pharmacokinetics of sildenafil for the treatment of pulmonary hypertension in infants. J Perinatol. 2016;36(9):744-747. https://doi.org/10.1038/jp.2016.79.
12. Euteneuer JC, Mizuno T, Fukuda T, Zhao J, Setchell KD, Vinks AA. Large variability in morphine concentrations in critically ill neonates receiving standard of care postoperative pain-management. Clin Pharmacol Ther. 2018;103:S45-S45.
© 2021 Society of Hospital Medicine