User login
Clinical Progress Note: Point-of-Care Ultrasound in the Evaluation of the Dyspneic Adult
Point-of-care ultrasound (POCUS) continues to gain traction in contemporary clinical practice both as a diagnostic tool and as an extension of the physical examination. Hospital Medicine (HM) lags behind Emergency Medicine (EM) and Critical Care (CC) in our uptake of such technology, although momentum is gaining. Leaders in HM have published frameworks for competency and credentialing, and the Society for Hospital Medicine has created a pathway for certification.1 POCUS use is the standard of care for several bedside procedures, but evidence for diagnostic applications is changing rapidly as the literature expands. However, the applicability of this evidence to HM patients can be challenging as most published studies are still from EM and CC settings. This Progress Note focuses on how a hospitalist might incorporate POCUS in the evaluation of adult patients with dyspnea. This topic was chosen after reviewing several relevant studies published in the past five years and recognizing the importance of dyspnea in HM. The Progress Note begins with a review of POCUS for undifferentiated dyspnea before exploring studies of common diagnoses that present with dyspnea, including pneumonia, pleural effusion, and acute decompensated heart failure (ADHF), aiming to update the knowledge of HM providers regarding this technology as well as to stimulate further study in this field.
SEARCH STRATEGY
In collaboration with an academic librarian in March 2019, PubMed was searched for studies published within the past five years using several MESH search terms for POCUS. The search was originally focused to the field of HM using specific search terms, but this yielded a very limited number of studies. Therefore, the search strategy was expanded to include EM and CC studies. This final search generated 346 papers that were supplemented with additional literature searches using references from studies found in the initial search.
UNDIFFERENTIATED DYSPNEA
Dyspnea is common in HM, both as the reason for a patient’s admission and as a symptom that develops during hospitalization such as after intravenous fluid resuscitation, a possible aspiration event, or central line placement. The differential diagnosis is broad, and multiple studies suggest that POCUS can aid in the evaluation of undifferentiated dyspnea while also being cost effective and avoiding the potential radiation of other testing modalities. The pulmonary POCUS evaluation incorporates a combination of several findings, including “A-lines” or horizontal artifacts from normal aerated lung; “B-lines”, vertical artifacts generated by extra-alveolar fluid, consolidation or “tissue-like pattern”; air bronchograms, consolidated lung surrounding airways; anechoic or hypoechoic areas in dependent zones of the lung; and the presence or absence of pleural sliding.2
In one prospective observational study of five internal medicine residents with no prior POCUS experience and three hours of training, the addition of handheld POCUS devices to usual clinical information improved the diagnostic accuracy for pneumonia, pulmonary edema, pleural effusion, and obstructive lung disease when evaluating patients with a primary complaint of dyspnea (area under the curve [AUC] 0.81 vs 0.87, P < .01).2 However, the largest improvements in the operating characteristics were observed with the two residents who received an extended two-week elective of training.
In another study of 383 consecutive patients presenting to the ED with dyspnea, physicians with basic and advanced POCUS training were blinded to all clinical information and recorded a diagnosis after performing a lung POCUS examination. The “ultrasound physician’s” diagnosis was then compared to the treating emergency department (ED) physician’s diagnosis using history, physical, and other diagnostic data. Lung POCUS had a sensitivity and a specificity of 87.6% and 96.2% for pulmonary edema, 85.7% and 99% for pneumonia, 98.2% and 67.3% for asthma/chronic obstructive pulmonary disease (COPD), 46.2% and 100% for pulmonary embolus (PE), and 71.4% and 100% for pneumothorax, respectively.3 The scanning protocol used, the BLUE (Bedside Lung Ultrasound Examination) protocol, was focused on ruling out significant pulmonary etiologies of dyspnea. The protocol classified the finding of normal lung ultrasound (A-line profile) as COPD or asthma since these conditions will have a normal sonographic appearance. This approach could lead to incorrect labeling of other extrapulmonary causes of dyspnea as COPD or asthma. The findings of this study suggest that POCUS is most effective at ruling in pulmonary edema and pneumonia while being most effective at ruling out asthma or COPD as causes of dyspnea. It is both sensitive and specific for pneumothorax. However, as other studies have found, the sensitivity of POCUS for COPD, asthma, and PE was inferior to traditional clinical evaluation.4 One of the few studies looking specifically at hospitalized ward patients compared a blinded lung POCUS diagnosis and a discharge clinical diagnosis classified as cardiac, pulmonary, or mixed dyspnea. The authors of that study found an “interstitial pattern” (two areas with more than two B-lines) in 94% of those classified as cardiac on discharge, but POCUS findings were less precise for those discharged with a pulmonary etiology of dyspnea.5 Identifying B-lines on lung POCUS appears to be helpful in rapidly differentiating cardiac from pulmonary etiologies of dyspnea.
An additional advantage of POCUS is that multiple organ systems can be evaluated in rapid succession when the etiology of dyspnea is unknown. In a smaller ED study of patients presenting with undifferentiated dyspnea, a diagnosis was recorded after history-taking and physical examination and then recorded again after lung, cardiac, and inferior vena cava POCUS. Clinician diagnostic accuracy improved from 53% to 77% with the use of POCUS (P = .003) compared with the final diagnosis.6 The treating physician’s primary impression changed in almost 50% of cases after using POCUS, most of which was driven by improved sensitivity and specificity of ADHF. In another study of 2,700 patients presenting to the ED with dyspnea, cardiopulmonary POCUS shortened the time to diagnosis (186 ± 72 minutes vs 24 ± 10 minutes, P = .025).4 These studies suggest that the use of POCUS in the initial evaluation of patients with undifferentiated dyspnea is a valuable tool with respect to diagnostic accuracy and timeliness.
PNEUMONIA
There are several different sonographic findings that can indicate pneumonia, such as consolidation or “hepatization”, the “shred” sign of an irregular border between consolidated lung and aerated lung, unilateral B-lines, and dynamic air bronchograms. Several recent systematic reviews and meta-analyses have investigated the operating characteristics of POCUS for the diagnosis of pneumonia. These reviews are limited by heterogeneity with respect to different patient populations, sonographers, and reference standards, but all three reviews found similar results, with the pooled AUC values ranging from 95% to 98%.7-9 This recent evidence along with other reviews suggests that lung ultrasound can serve as a primary diagnostic tool in pneumonia and is probably superior to chest radiography.
PLEURAL EFFUSION
Pleural effusions are observed with POCUS as anechoic or hypoechoic areas, generally in dependent lung zones. POCUS may provide additional benefit by better characterizing the effusion as having septations or floating fibrin strands. One recent systematic review and meta-analysis including 1,554 patients found that POCUS had excellent sensitivity and specificity (94% and 98%, respectively) in detecting pleural effusion versus chest radiography (51% and 91%, respectively), both compared with reference standard imaging such as computed tomography. The subgroup analysis found that sensitivity was higher for scanners who were intensivists or radiologists than for other physicians (97% vs 90%; P ≤ .001) and also found a nonstatistically significant trend toward reduced sensitivity when pocket-sized devices were used (90% vs 95%, P = .09).10
ACUTE DECOMPENSATED HEART FAILURE
It is extremely important to recognize that a POCUS finding of decreased left ventricular ejection fraction is not synonymous with a diagnosis of ADHF. Bedside providers can use POCUS to estimate cardiac function, but other clinical information is required to determine whether the syndrome of ADHF is present. In one study, examinations performed by 10 internists with approximately 18 hours of training in focused cardiac POCUS had a sensitivity and a specificity of 91% and 88%, respectively, for classifying left ventricular systolic function as normal or mildly, moderately, or severely depressed with “good/substantial” agreement (k = 0.77) compared with formal echocardiography.11 The presence of bilateral B-lines as a sign of pulmonary edema suggests accompanying functional decompensation. A meta-analysis of seven articles including 1075 patients in various clinical settings (ED, ICU, and inpatient wards) found a sensitivity of 94.1% and a specificity of 92.4% for using B-lines to diagnose acute cardiogenic pulmonary edema compared with the final clinical diagnosis.12 Al Deeb et al. examined 226 patients and found similar sensitivity (95.3%) and specificity (88.2%) for diagnosing acute cardiogenic pulmonary edema when nurses were trained to evaluate for bilateral B-lines in dyspneic patients admitted to the hospital, also compared with the adjudicated final diagnosis.13 Carlino et al. evaluated dyspneic patients using a three-minute pocket-sized device scan of the heart, lungs, and inferior vena cava and found that no single view offered a substantial improvement in diagnostic accuracy; however, the combination of bilateral B-lines and/or pleural effusion and either a dilated left atrium or left ventricular ejection fraction (LVEF) of <40% had a very high diagnostic accuracy (AUC 0.97).14 Russell et al. performed a secondary analysis of a prospective observational study of patients with dyspnea and found that a simple three-view scanning protocol looking for the presence of B-lines on the right and left anterior superior lung zones and an LVEF of <45% took an average of one minute and 32 seconds to perform and had 100% specificity for ADHF if all three were positive.15 Another recent systematic review and meta-analysis of six studies and 1,827 patients found a sensitivity of 88% (CI 75%-95%) for lung POCUS compared with a chest radiography at a sensitivity of 73% (70%-76%) for the diagnosis of ADHF.16 All these studies suggest that improving the diagnosis of ADHF does not require complex echocardiographic views and is probably more feasible and accessible than many expect.
SUMMARY
POCUS continues to show promise for evaluating patients with dyspnea. It is clear that adding a few POCUS examination maneuvers to a provider’s toolbox, such as looking for B-lines and overall cardiac function, can improve
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. Published online only January 2, 2019. https://doi.org/10.12788/jhm.3079.
2. Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9(9):594-597. https://doi.org/10.1002/jhm.2219.
3. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.02.028.
4. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295-1301. https://doi.org/10.1016/j.chest.2017.02.003.
5. Perrone T, Maggi A, Sgarlata C, et al. Lung ultrasound in internal medicine: a bedside help to increase accuracy in the diagnosis of dyspnea. Eur J Intern Med. 2017;46:61-65. https://doi.org/10.1016/j.ejim.2017.07.034.
6. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. https://doi.org/10.5811/westjem.2015.11.28525.
7. Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018;25(5):312-321. https://doi.org/10.1097/MEJ.0000000000000517.
8. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y
9. Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine . 2017;96(3):e5713. https://doi.org/10.1097/MD.0000000000005713.
10. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1-10.
11. Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92-99. https://doi.org/10.1002/jcu.22272.
12. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. https://doi.org/10.1111/acem.12435.
13. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea: a prospective observational study. Medicine (Baltimore). 2016;95(9):e2925. https://doi.org/10.1097/MD.0000000000002925.
14. Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the emergency department. Echocardiography (Mount Kisco, NY). 2018;35(6):785-791. https://doi.org/10.1111/echo.13851.
15. Russell FM, Ehrman RR. A modified lung and cardiac ultrasound protocol saves time and rules in the diagnosis of acute heart failure. J Emerg Med. 2017;52(6):839-845. https://doi.org/10.1016/j.jemermed.2017.02.003.
16. Maw AM, Hassanin A, Ho PM, et al. diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703. https://doi.org/10.1001/jamanetworkopen.2019.0703.
Point-of-care ultrasound (POCUS) continues to gain traction in contemporary clinical practice both as a diagnostic tool and as an extension of the physical examination. Hospital Medicine (HM) lags behind Emergency Medicine (EM) and Critical Care (CC) in our uptake of such technology, although momentum is gaining. Leaders in HM have published frameworks for competency and credentialing, and the Society for Hospital Medicine has created a pathway for certification.1 POCUS use is the standard of care for several bedside procedures, but evidence for diagnostic applications is changing rapidly as the literature expands. However, the applicability of this evidence to HM patients can be challenging as most published studies are still from EM and CC settings. This Progress Note focuses on how a hospitalist might incorporate POCUS in the evaluation of adult patients with dyspnea. This topic was chosen after reviewing several relevant studies published in the past five years and recognizing the importance of dyspnea in HM. The Progress Note begins with a review of POCUS for undifferentiated dyspnea before exploring studies of common diagnoses that present with dyspnea, including pneumonia, pleural effusion, and acute decompensated heart failure (ADHF), aiming to update the knowledge of HM providers regarding this technology as well as to stimulate further study in this field.
SEARCH STRATEGY
In collaboration with an academic librarian in March 2019, PubMed was searched for studies published within the past five years using several MESH search terms for POCUS. The search was originally focused to the field of HM using specific search terms, but this yielded a very limited number of studies. Therefore, the search strategy was expanded to include EM and CC studies. This final search generated 346 papers that were supplemented with additional literature searches using references from studies found in the initial search.
UNDIFFERENTIATED DYSPNEA
Dyspnea is common in HM, both as the reason for a patient’s admission and as a symptom that develops during hospitalization such as after intravenous fluid resuscitation, a possible aspiration event, or central line placement. The differential diagnosis is broad, and multiple studies suggest that POCUS can aid in the evaluation of undifferentiated dyspnea while also being cost effective and avoiding the potential radiation of other testing modalities. The pulmonary POCUS evaluation incorporates a combination of several findings, including “A-lines” or horizontal artifacts from normal aerated lung; “B-lines”, vertical artifacts generated by extra-alveolar fluid, consolidation or “tissue-like pattern”; air bronchograms, consolidated lung surrounding airways; anechoic or hypoechoic areas in dependent zones of the lung; and the presence or absence of pleural sliding.2
In one prospective observational study of five internal medicine residents with no prior POCUS experience and three hours of training, the addition of handheld POCUS devices to usual clinical information improved the diagnostic accuracy for pneumonia, pulmonary edema, pleural effusion, and obstructive lung disease when evaluating patients with a primary complaint of dyspnea (area under the curve [AUC] 0.81 vs 0.87, P < .01).2 However, the largest improvements in the operating characteristics were observed with the two residents who received an extended two-week elective of training.
In another study of 383 consecutive patients presenting to the ED with dyspnea, physicians with basic and advanced POCUS training were blinded to all clinical information and recorded a diagnosis after performing a lung POCUS examination. The “ultrasound physician’s” diagnosis was then compared to the treating emergency department (ED) physician’s diagnosis using history, physical, and other diagnostic data. Lung POCUS had a sensitivity and a specificity of 87.6% and 96.2% for pulmonary edema, 85.7% and 99% for pneumonia, 98.2% and 67.3% for asthma/chronic obstructive pulmonary disease (COPD), 46.2% and 100% for pulmonary embolus (PE), and 71.4% and 100% for pneumothorax, respectively.3 The scanning protocol used, the BLUE (Bedside Lung Ultrasound Examination) protocol, was focused on ruling out significant pulmonary etiologies of dyspnea. The protocol classified the finding of normal lung ultrasound (A-line profile) as COPD or asthma since these conditions will have a normal sonographic appearance. This approach could lead to incorrect labeling of other extrapulmonary causes of dyspnea as COPD or asthma. The findings of this study suggest that POCUS is most effective at ruling in pulmonary edema and pneumonia while being most effective at ruling out asthma or COPD as causes of dyspnea. It is both sensitive and specific for pneumothorax. However, as other studies have found, the sensitivity of POCUS for COPD, asthma, and PE was inferior to traditional clinical evaluation.4 One of the few studies looking specifically at hospitalized ward patients compared a blinded lung POCUS diagnosis and a discharge clinical diagnosis classified as cardiac, pulmonary, or mixed dyspnea. The authors of that study found an “interstitial pattern” (two areas with more than two B-lines) in 94% of those classified as cardiac on discharge, but POCUS findings were less precise for those discharged with a pulmonary etiology of dyspnea.5 Identifying B-lines on lung POCUS appears to be helpful in rapidly differentiating cardiac from pulmonary etiologies of dyspnea.
An additional advantage of POCUS is that multiple organ systems can be evaluated in rapid succession when the etiology of dyspnea is unknown. In a smaller ED study of patients presenting with undifferentiated dyspnea, a diagnosis was recorded after history-taking and physical examination and then recorded again after lung, cardiac, and inferior vena cava POCUS. Clinician diagnostic accuracy improved from 53% to 77% with the use of POCUS (P = .003) compared with the final diagnosis.6 The treating physician’s primary impression changed in almost 50% of cases after using POCUS, most of which was driven by improved sensitivity and specificity of ADHF. In another study of 2,700 patients presenting to the ED with dyspnea, cardiopulmonary POCUS shortened the time to diagnosis (186 ± 72 minutes vs 24 ± 10 minutes, P = .025).4 These studies suggest that the use of POCUS in the initial evaluation of patients with undifferentiated dyspnea is a valuable tool with respect to diagnostic accuracy and timeliness.
PNEUMONIA
There are several different sonographic findings that can indicate pneumonia, such as consolidation or “hepatization”, the “shred” sign of an irregular border between consolidated lung and aerated lung, unilateral B-lines, and dynamic air bronchograms. Several recent systematic reviews and meta-analyses have investigated the operating characteristics of POCUS for the diagnosis of pneumonia. These reviews are limited by heterogeneity with respect to different patient populations, sonographers, and reference standards, but all three reviews found similar results, with the pooled AUC values ranging from 95% to 98%.7-9 This recent evidence along with other reviews suggests that lung ultrasound can serve as a primary diagnostic tool in pneumonia and is probably superior to chest radiography.
PLEURAL EFFUSION
Pleural effusions are observed with POCUS as anechoic or hypoechoic areas, generally in dependent lung zones. POCUS may provide additional benefit by better characterizing the effusion as having septations or floating fibrin strands. One recent systematic review and meta-analysis including 1,554 patients found that POCUS had excellent sensitivity and specificity (94% and 98%, respectively) in detecting pleural effusion versus chest radiography (51% and 91%, respectively), both compared with reference standard imaging such as computed tomography. The subgroup analysis found that sensitivity was higher for scanners who were intensivists or radiologists than for other physicians (97% vs 90%; P ≤ .001) and also found a nonstatistically significant trend toward reduced sensitivity when pocket-sized devices were used (90% vs 95%, P = .09).10
ACUTE DECOMPENSATED HEART FAILURE
It is extremely important to recognize that a POCUS finding of decreased left ventricular ejection fraction is not synonymous with a diagnosis of ADHF. Bedside providers can use POCUS to estimate cardiac function, but other clinical information is required to determine whether the syndrome of ADHF is present. In one study, examinations performed by 10 internists with approximately 18 hours of training in focused cardiac POCUS had a sensitivity and a specificity of 91% and 88%, respectively, for classifying left ventricular systolic function as normal or mildly, moderately, or severely depressed with “good/substantial” agreement (k = 0.77) compared with formal echocardiography.11 The presence of bilateral B-lines as a sign of pulmonary edema suggests accompanying functional decompensation. A meta-analysis of seven articles including 1075 patients in various clinical settings (ED, ICU, and inpatient wards) found a sensitivity of 94.1% and a specificity of 92.4% for using B-lines to diagnose acute cardiogenic pulmonary edema compared with the final clinical diagnosis.12 Al Deeb et al. examined 226 patients and found similar sensitivity (95.3%) and specificity (88.2%) for diagnosing acute cardiogenic pulmonary edema when nurses were trained to evaluate for bilateral B-lines in dyspneic patients admitted to the hospital, also compared with the adjudicated final diagnosis.13 Carlino et al. evaluated dyspneic patients using a three-minute pocket-sized device scan of the heart, lungs, and inferior vena cava and found that no single view offered a substantial improvement in diagnostic accuracy; however, the combination of bilateral B-lines and/or pleural effusion and either a dilated left atrium or left ventricular ejection fraction (LVEF) of <40% had a very high diagnostic accuracy (AUC 0.97).14 Russell et al. performed a secondary analysis of a prospective observational study of patients with dyspnea and found that a simple three-view scanning protocol looking for the presence of B-lines on the right and left anterior superior lung zones and an LVEF of <45% took an average of one minute and 32 seconds to perform and had 100% specificity for ADHF if all three were positive.15 Another recent systematic review and meta-analysis of six studies and 1,827 patients found a sensitivity of 88% (CI 75%-95%) for lung POCUS compared with a chest radiography at a sensitivity of 73% (70%-76%) for the diagnosis of ADHF.16 All these studies suggest that improving the diagnosis of ADHF does not require complex echocardiographic views and is probably more feasible and accessible than many expect.
SUMMARY
POCUS continues to show promise for evaluating patients with dyspnea. It is clear that adding a few POCUS examination maneuvers to a provider’s toolbox, such as looking for B-lines and overall cardiac function, can improve
Point-of-care ultrasound (POCUS) continues to gain traction in contemporary clinical practice both as a diagnostic tool and as an extension of the physical examination. Hospital Medicine (HM) lags behind Emergency Medicine (EM) and Critical Care (CC) in our uptake of such technology, although momentum is gaining. Leaders in HM have published frameworks for competency and credentialing, and the Society for Hospital Medicine has created a pathway for certification.1 POCUS use is the standard of care for several bedside procedures, but evidence for diagnostic applications is changing rapidly as the literature expands. However, the applicability of this evidence to HM patients can be challenging as most published studies are still from EM and CC settings. This Progress Note focuses on how a hospitalist might incorporate POCUS in the evaluation of adult patients with dyspnea. This topic was chosen after reviewing several relevant studies published in the past five years and recognizing the importance of dyspnea in HM. The Progress Note begins with a review of POCUS for undifferentiated dyspnea before exploring studies of common diagnoses that present with dyspnea, including pneumonia, pleural effusion, and acute decompensated heart failure (ADHF), aiming to update the knowledge of HM providers regarding this technology as well as to stimulate further study in this field.
SEARCH STRATEGY
In collaboration with an academic librarian in March 2019, PubMed was searched for studies published within the past five years using several MESH search terms for POCUS. The search was originally focused to the field of HM using specific search terms, but this yielded a very limited number of studies. Therefore, the search strategy was expanded to include EM and CC studies. This final search generated 346 papers that were supplemented with additional literature searches using references from studies found in the initial search.
UNDIFFERENTIATED DYSPNEA
Dyspnea is common in HM, both as the reason for a patient’s admission and as a symptom that develops during hospitalization such as after intravenous fluid resuscitation, a possible aspiration event, or central line placement. The differential diagnosis is broad, and multiple studies suggest that POCUS can aid in the evaluation of undifferentiated dyspnea while also being cost effective and avoiding the potential radiation of other testing modalities. The pulmonary POCUS evaluation incorporates a combination of several findings, including “A-lines” or horizontal artifacts from normal aerated lung; “B-lines”, vertical artifacts generated by extra-alveolar fluid, consolidation or “tissue-like pattern”; air bronchograms, consolidated lung surrounding airways; anechoic or hypoechoic areas in dependent zones of the lung; and the presence or absence of pleural sliding.2
In one prospective observational study of five internal medicine residents with no prior POCUS experience and three hours of training, the addition of handheld POCUS devices to usual clinical information improved the diagnostic accuracy for pneumonia, pulmonary edema, pleural effusion, and obstructive lung disease when evaluating patients with a primary complaint of dyspnea (area under the curve [AUC] 0.81 vs 0.87, P < .01).2 However, the largest improvements in the operating characteristics were observed with the two residents who received an extended two-week elective of training.
In another study of 383 consecutive patients presenting to the ED with dyspnea, physicians with basic and advanced POCUS training were blinded to all clinical information and recorded a diagnosis after performing a lung POCUS examination. The “ultrasound physician’s” diagnosis was then compared to the treating emergency department (ED) physician’s diagnosis using history, physical, and other diagnostic data. Lung POCUS had a sensitivity and a specificity of 87.6% and 96.2% for pulmonary edema, 85.7% and 99% for pneumonia, 98.2% and 67.3% for asthma/chronic obstructive pulmonary disease (COPD), 46.2% and 100% for pulmonary embolus (PE), and 71.4% and 100% for pneumothorax, respectively.3 The scanning protocol used, the BLUE (Bedside Lung Ultrasound Examination) protocol, was focused on ruling out significant pulmonary etiologies of dyspnea. The protocol classified the finding of normal lung ultrasound (A-line profile) as COPD or asthma since these conditions will have a normal sonographic appearance. This approach could lead to incorrect labeling of other extrapulmonary causes of dyspnea as COPD or asthma. The findings of this study suggest that POCUS is most effective at ruling in pulmonary edema and pneumonia while being most effective at ruling out asthma or COPD as causes of dyspnea. It is both sensitive and specific for pneumothorax. However, as other studies have found, the sensitivity of POCUS for COPD, asthma, and PE was inferior to traditional clinical evaluation.4 One of the few studies looking specifically at hospitalized ward patients compared a blinded lung POCUS diagnosis and a discharge clinical diagnosis classified as cardiac, pulmonary, or mixed dyspnea. The authors of that study found an “interstitial pattern” (two areas with more than two B-lines) in 94% of those classified as cardiac on discharge, but POCUS findings were less precise for those discharged with a pulmonary etiology of dyspnea.5 Identifying B-lines on lung POCUS appears to be helpful in rapidly differentiating cardiac from pulmonary etiologies of dyspnea.
An additional advantage of POCUS is that multiple organ systems can be evaluated in rapid succession when the etiology of dyspnea is unknown. In a smaller ED study of patients presenting with undifferentiated dyspnea, a diagnosis was recorded after history-taking and physical examination and then recorded again after lung, cardiac, and inferior vena cava POCUS. Clinician diagnostic accuracy improved from 53% to 77% with the use of POCUS (P = .003) compared with the final diagnosis.6 The treating physician’s primary impression changed in almost 50% of cases after using POCUS, most of which was driven by improved sensitivity and specificity of ADHF. In another study of 2,700 patients presenting to the ED with dyspnea, cardiopulmonary POCUS shortened the time to diagnosis (186 ± 72 minutes vs 24 ± 10 minutes, P = .025).4 These studies suggest that the use of POCUS in the initial evaluation of patients with undifferentiated dyspnea is a valuable tool with respect to diagnostic accuracy and timeliness.
PNEUMONIA
There are several different sonographic findings that can indicate pneumonia, such as consolidation or “hepatization”, the “shred” sign of an irregular border between consolidated lung and aerated lung, unilateral B-lines, and dynamic air bronchograms. Several recent systematic reviews and meta-analyses have investigated the operating characteristics of POCUS for the diagnosis of pneumonia. These reviews are limited by heterogeneity with respect to different patient populations, sonographers, and reference standards, but all three reviews found similar results, with the pooled AUC values ranging from 95% to 98%.7-9 This recent evidence along with other reviews suggests that lung ultrasound can serve as a primary diagnostic tool in pneumonia and is probably superior to chest radiography.
PLEURAL EFFUSION
Pleural effusions are observed with POCUS as anechoic or hypoechoic areas, generally in dependent lung zones. POCUS may provide additional benefit by better characterizing the effusion as having septations or floating fibrin strands. One recent systematic review and meta-analysis including 1,554 patients found that POCUS had excellent sensitivity and specificity (94% and 98%, respectively) in detecting pleural effusion versus chest radiography (51% and 91%, respectively), both compared with reference standard imaging such as computed tomography. The subgroup analysis found that sensitivity was higher for scanners who were intensivists or radiologists than for other physicians (97% vs 90%; P ≤ .001) and also found a nonstatistically significant trend toward reduced sensitivity when pocket-sized devices were used (90% vs 95%, P = .09).10
ACUTE DECOMPENSATED HEART FAILURE
It is extremely important to recognize that a POCUS finding of decreased left ventricular ejection fraction is not synonymous with a diagnosis of ADHF. Bedside providers can use POCUS to estimate cardiac function, but other clinical information is required to determine whether the syndrome of ADHF is present. In one study, examinations performed by 10 internists with approximately 18 hours of training in focused cardiac POCUS had a sensitivity and a specificity of 91% and 88%, respectively, for classifying left ventricular systolic function as normal or mildly, moderately, or severely depressed with “good/substantial” agreement (k = 0.77) compared with formal echocardiography.11 The presence of bilateral B-lines as a sign of pulmonary edema suggests accompanying functional decompensation. A meta-analysis of seven articles including 1075 patients in various clinical settings (ED, ICU, and inpatient wards) found a sensitivity of 94.1% and a specificity of 92.4% for using B-lines to diagnose acute cardiogenic pulmonary edema compared with the final clinical diagnosis.12 Al Deeb et al. examined 226 patients and found similar sensitivity (95.3%) and specificity (88.2%) for diagnosing acute cardiogenic pulmonary edema when nurses were trained to evaluate for bilateral B-lines in dyspneic patients admitted to the hospital, also compared with the adjudicated final diagnosis.13 Carlino et al. evaluated dyspneic patients using a three-minute pocket-sized device scan of the heart, lungs, and inferior vena cava and found that no single view offered a substantial improvement in diagnostic accuracy; however, the combination of bilateral B-lines and/or pleural effusion and either a dilated left atrium or left ventricular ejection fraction (LVEF) of <40% had a very high diagnostic accuracy (AUC 0.97).14 Russell et al. performed a secondary analysis of a prospective observational study of patients with dyspnea and found that a simple three-view scanning protocol looking for the presence of B-lines on the right and left anterior superior lung zones and an LVEF of <45% took an average of one minute and 32 seconds to perform and had 100% specificity for ADHF if all three were positive.15 Another recent systematic review and meta-analysis of six studies and 1,827 patients found a sensitivity of 88% (CI 75%-95%) for lung POCUS compared with a chest radiography at a sensitivity of 73% (70%-76%) for the diagnosis of ADHF.16 All these studies suggest that improving the diagnosis of ADHF does not require complex echocardiographic views and is probably more feasible and accessible than many expect.
SUMMARY
POCUS continues to show promise for evaluating patients with dyspnea. It is clear that adding a few POCUS examination maneuvers to a provider’s toolbox, such as looking for B-lines and overall cardiac function, can improve
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. Published online only January 2, 2019. https://doi.org/10.12788/jhm.3079.
2. Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9(9):594-597. https://doi.org/10.1002/jhm.2219.
3. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.02.028.
4. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295-1301. https://doi.org/10.1016/j.chest.2017.02.003.
5. Perrone T, Maggi A, Sgarlata C, et al. Lung ultrasound in internal medicine: a bedside help to increase accuracy in the diagnosis of dyspnea. Eur J Intern Med. 2017;46:61-65. https://doi.org/10.1016/j.ejim.2017.07.034.
6. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. https://doi.org/10.5811/westjem.2015.11.28525.
7. Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018;25(5):312-321. https://doi.org/10.1097/MEJ.0000000000000517.
8. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y
9. Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine . 2017;96(3):e5713. https://doi.org/10.1097/MD.0000000000005713.
10. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1-10.
11. Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92-99. https://doi.org/10.1002/jcu.22272.
12. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. https://doi.org/10.1111/acem.12435.
13. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea: a prospective observational study. Medicine (Baltimore). 2016;95(9):e2925. https://doi.org/10.1097/MD.0000000000002925.
14. Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the emergency department. Echocardiography (Mount Kisco, NY). 2018;35(6):785-791. https://doi.org/10.1111/echo.13851.
15. Russell FM, Ehrman RR. A modified lung and cardiac ultrasound protocol saves time and rules in the diagnosis of acute heart failure. J Emerg Med. 2017;52(6):839-845. https://doi.org/10.1016/j.jemermed.2017.02.003.
16. Maw AM, Hassanin A, Ho PM, et al. diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703. https://doi.org/10.1001/jamanetworkopen.2019.0703.
1. Soni NJ, Schnobrich D, Matthews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. Published online only January 2, 2019. https://doi.org/10.12788/jhm.3079.
2. Filopei J, Siedenburg H, Rattner P, Fukaya E, Kory P. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9(9):594-597. https://doi.org/10.1002/jhm.2219.
3. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019. https://doi.org/10.1016/j.ajem.2019.02.028.
4. Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295-1301. https://doi.org/10.1016/j.chest.2017.02.003.
5. Perrone T, Maggi A, Sgarlata C, et al. Lung ultrasound in internal medicine: a bedside help to increase accuracy in the diagnosis of dyspnea. Eur J Intern Med. 2017;46:61-65. https://doi.org/10.1016/j.ejim.2017.07.034.
6. Mantuani D, Frazee BW, Fahimi J, Nagdev A. Point-of-care multi-organ ultrasound improves diagnostic accuracy in adults presenting to the emergency department with acute dyspnea. West J Emerg Med. 2016;17(1):46-53. https://doi.org/10.5811/westjem.2015.11.28525.
7. Orso D, Guglielmo N, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med. 2018;25(5):312-321. https://doi.org/10.1097/MEJ.0000000000000517.
8. Alzahrani SA, Al-Salamah MA, Al-Madani WH, Elbarbary MA. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit Ultrasound J. 2017;9(1):6. https://doi.org/10.1186/s13089-017-0059-y
9. Long L, Zhao HT, Zhang ZY, Wang GY, Zhao HL. Lung ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Medicine . 2017;96(3):e5713. https://doi.org/10.1097/MD.0000000000005713.
10. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1-10.
11. Johnson BK, Tierney DM, Rosborough TK, Harris KM, Newell MC. Internal medicine point-of-care ultrasound assessment of left ventricular function correlates with formal echocardiography. J Clin Ultrasound. 2016;44(2):92-99. https://doi.org/10.1002/jcu.22272.
12. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843-852. https://doi.org/10.1111/acem.12435.
13. Mumoli N, Vitale J, Giorgi-Pierfranceschi M, et al. Accuracy of nurse-performed lung ultrasound in patients with acute dyspnea: a prospective observational study. Medicine (Baltimore). 2016;95(9):e2925. https://doi.org/10.1097/MD.0000000000002925.
14. Carlino MV, Paladino F, Sforza A, et al. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the emergency department. Echocardiography (Mount Kisco, NY). 2018;35(6):785-791. https://doi.org/10.1111/echo.13851.
15. Russell FM, Ehrman RR. A modified lung and cardiac ultrasound protocol saves time and rules in the diagnosis of acute heart failure. J Emerg Med. 2017;52(6):839-845. https://doi.org/10.1016/j.jemermed.2017.02.003.
16. Maw AM, Hassanin A, Ho PM, et al. diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3):e190703. https://doi.org/10.1001/jamanetworkopen.2019.0703.
© 2020 Society of Hospital Medicine
Clinical Progress Note: High Flow Nasal Cannula Therapy for Bronchiolitis Outside the ICU in Infants
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
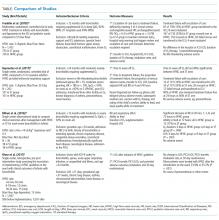
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
Viral bronchiolitis is the most common indication for infant hospitalization in the United States.1 The treatment mainstay remains supportive care, including supplemental oxygen when indicated.1 High flow nasal cannula (HFNC) therapy delivers humidified, heated air blended with oxygen, allowing much higher flow rates than standard nasal cannula therapy and is being used more frequently in inpatient settings.
OVERVIEW AND CLINICAL QUESTION
Infants and toddlers with bronchiolitis develop increased work of breathing to preserve oxygenation and ventilation in the setting of altered airway resistance and lung compliance.2,3 In addition to oxygen supplementation, HFNC is used to reduce work of breathing through several mechanisms:2-6 (1) Nasopharyngeal dead space washout clears oxygen-depleted gas at the end of expiration, facilitating alveolar ventilation (ie, carbon dioxide retention improves); (2) High flow rates match increased inspiratory flow demands of acutely ill patients, reducing nasopharyngeal inspiratory resistance and optimizing dead space washout, thus decreasing work of breathing; (3) Adequate flow rates generate distending pressure, which prevents pharyngeal collapse, supports lung recruitment, and reduces respiratory effort (demonstrated in younger infants); and (4) HFNC systems heat and humidify the breathing gas, reducing the metabolic work required to condition cool, dry gas and improving conductance and pulmonary compliance.2-5
HFNC therapy is used more commonly in acute care units despite limited literature on its effectiveness outside the intensive care unit (ICU).7,8 We asked the question, “Does use of HFNC therapy for infants with bronchiolitis hospitalized in acute care units result in improved outcomes when compared with standard nasal cannula oxygen therapy, including length of stay (LOS), oxygen therapy duration, and preventing escalations of care such as ICU transfer, positive pressure ventilation, and intubation?” Also, do published studies provide guidance for the initiation and management of HFNC? We focused our search on studies published in the last five years that included patients with bronchiolitis treated with HFNC outside the ICU; here, we review those studies most relevant to pediatric hospitalists.
RECENT LITERATURE REVIEW
No guideline exists for initiating flow or fraction of inspired oxygen (FiO2). HFNC may be initiated for hypoxia, increased work of breathing, or both in patients with bronchiolitis. To achieve optimal dead space washout, inspiratory flow, and distending pressure, initial flow rates should be 1.5 to 2 L/kg/min, particularly for infants and young children.2-5 Weiler et al.3 evaluated the breathing effort of ICU patients at 0.5, 1, 1.5, and 2 L/kg/min and found optimal flow rates for improved work of breathing were 1.5-2 L/kg/min. The smallest patients, ≤8 kg, saw the greatest benefit, a finding likely explained by larger anatomic dead space in infants/small children compared with older children.3 For older/larger children (>20 kg), an initial flow closer to 1 L/kg/min is often appropriate.5 When used for hypoxia, initiating flow without supplemental FiO2 may improve oxygenation by flushing nasopharyngeal dead space. FiO2 should be titrated to achieve the goal set by the treatment team, often ≥90%. Improvement in heart rate and peripheral oxygen saturation (SpO2) can be observed within 60 minutes of initiating HFNC in patients responsive to therapy.6
HFNC therapy is safe when used correctly.6,9,10Potential adverse effects include pneumothorax, pressure injury, mucosal injury/bleeding, and delayed escalation to invasive ventilation. While difficult to quantify, recent studies report low rates or no serious HFNC complications. For example, only 2 of 1,127 patients supported with HFNC developed a pneumothorax and neither required evacuation.2,9-12
Inclusion criteria and HFNC protocols vary among published studies. Most HFNC protocols reviewed may not have optimally supported all of the patients in their HFNC groups, often by limiting flow to <2 L/kg/min.6-9,11,12 These variables may explain the disparate results, with some studies demonstrating apparent benefits and others no difference.7,9,10,12
Two studies of infants with bronchiolitis showed HFNC therapy may prevent ICU transfer, but this benefit may be limited to rescue when standard oxygen therapy fails, rather than as a superior initial support modality.7,9 Kepreotes et al.9 reported a single-center, randomized controlled trial comparing HFNC with standard oxygen therapy with 101 patients in each treatment arm. The primary outcome, median time to wean off oxygen, was not significantly different between the two groups: 24 hours (95% CI: 18-28) in the HFNC group versus 20 hours in the standard therapy group (95% CI: 17-34). The HFNC group had fewer treatment failures (abnormal heart rate, respiratory rate, SpO2 <90%, or severe respiratory distress score while on maximum therapy) than the standard therapy group, and 20 (63%) of the 33 patients who failed standard therapy were rescued with HFNC, avoiding transfer to the ICU. Fourteen patients from the HFNC group and 12 from the standard oxygen group required transfer to the ICU for support escalation. Although this study did not show a significant difference in oxygen weaning time between groups, it appears to support HFNC use as a rescue modality to reduce or prevent ICU transfer.9 Franklin et al.10 conducted a multicenter, randomized, controlled trial to compare standard nasal cannula oxygen therapy with HFNC (2 L/kg/min) in 1,472 patients. Patients receiving HFNC had lower care escalation rates due to treatment failure, defined as the presence of at least three of four clinical criteria and the clinician determining escalation was indicated. Oxygen therapy duration, ICU admission rates, and LOS were not significantly different between groups. Similar to the previous study, a large portion of the standard therapy patients who failed treatment (102 of 167) crossed over to the HFNC arm in an attempt to avoid ICU transfer. Twelve patients required intubation: 8 (1%) receiving HFNC and 4 (0.5%) receiving the standard therapy.10
Two additional studies, both with study design limitations, did not demonstrate differences in ICU transfer rates and had variable differences in outcomes. Riese et al.7 retrospectively assessed HFNC use outside the ICU at one institution and included 936 patients admitted before and 1,001 patients admitted after HFNC guideline implementation on the wards. Flow rates were based on age and not weight. They found no difference in LOS, ICU transfer rate, ICU LOS, intubation rates, or 30-day readmission rates, though HFNC use increased over time. The HFNC guideline is a potentially significant limitation as it may not have provided optimal flow rates to all subjects given it was based on age rather than weight. Milani et al.12 performed a single-center observational study of 36 infants aged <12 months, treated for bronchiolitis on the ward, who were informally assigned to HFNC or standard therapy based upon HFNC device availability. HFNC flow rate was determined by the equation: L/min = 8 mL/kg × respiratory rate × 0.3. Using mean weight and respiratory rate for patients in this group, it appears patients in the HFNC group were treated with flow rates less than the 1.5-2 L/kg/min recommended to be effective.2,3,12 Despite this, clinical improvement was faster in the HFNC group, including respiratory rate and effort, ability to feed, days on oxygen supplementation, and hospital LOS. ICU admission was not different between the two groups.12 The Table compares the four studies discussed above.
Given increasing use of HFNC outside the ICU, institutions risk overuse and increased healthcare costs.13 Limited data on HFNC overuse exist, but several studies report increased use after implementation on the wards without robust evidence indicating it improves outcomes.7,14 Overuse of HFNC is a concern that should be considered as institutions develop HFNC protocols. Another important consideration is safe feeding. One study examined 132 children ages one month to two years with bronchiolitis who were receiving HFNC and enteral nutrition.15 Only one patient had aspiration respiratory failure, and 12 had nutrition interruptions, demonstrating oral nutrition is generally well tolerated15 and should be considered in patients with stable respiratory status on HFNC.
CONCLUSIONS
Many children’s hospitals have extended the use of HFNC outside the ICU for children with bronchiolitis despite the paucity of evidence demonstrating its benefit over standard flow oxygen. Given variation in protocols, study designs, outcomes, and number of patients studied, it is difficult to assess its efficacy outside the ICU. However, based on the studies reviewed herein, HFNC therapy does not appear to decrease LOS, time on oxygen, or escalations of care, such as ICU transfers, positive pressure ventilation, or intubation, when used as a primary therapy.7,9,11,12 Future research will ideally use optimal flow rates to determine the effectiveness of HFNC on acute care units. Although not addressed in the above studies, additional benefits to be considered in future studies include: (1) increased critical care capacity by allowing patients to be supported on the floor and (2) the ability for patients to remain closer to home when HFNC is used in the community hospital setting.
In each of the large, randomized studies reviewed, most (66%-75%) patients treated with standard low flow oxygen were supported successfully and did not require escalation to HFNC.9,10 Hospitalists should continue to use standard low flow oxygen as first-line respiratory support for patients with bronchiolitis.1 No evidence supports the use of HFNC therapy early in a child’s inpatient course; rather, it should be used when standard oxygen therapy fails. Future research should focus on better elucidating which patients will benefit most from HFNC to prevent overuse.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-1502. https://doi.org/10.1542/peds.2014-2742.
2. Milesi C, Baleine J, Matecki S, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013;39(6):1088-1094. https://doi.org/10.1007/s00134-013-2879-y.
3. Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The relationship between high flow nasal cannula flow rate and effort of breathing in children. J Pediatr. 2017;189:66-71. https://doi.org/10.1016/j.jpeds.2017.06.006.
4. Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400-1405. https://doi.org/10.1016/j.rmed.2009.04.007.
5. Milesi C, Boubal M, Jacquot A, et al. High-flow nasal cannula: recommendations for daily practice in pediatrics. Ann Intensive Care. 2014;4(1):29. https://doi.org/10.1186/s13613-014-0029-5.
6. Heikkila P, Sokuri P, Mecklin M, et al. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018;107(11):1971-1976. https://doi.org/10.1111/apa.14421.
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195.
8. Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017;52(6):806-812. https://doi.org/10.1002/ppul.23626.
9. Kepreotes E, Whitehead B, Attia J, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017;389(10072):930-939. https://doi.org/10.1016/S0140-6736(17)30061-2.
10. Franklin D, Babl FE, Schibler A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2446-2447. https://doi.org/10.1056/NEJMc1805312.
11. Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014;50(5):373-378. https://doi.org/10.1111/jpc.12509.
12. Milani GP, Plebani AM, Arturi E, et al. Using a high-flow nasal cannula provided superior results to low-flow oxygen delivery in moderate to severe bronchiolitis. Acta Paediatr. 2016;105(8):e368-e372. https://doi.org/10.1111/apa.13444.
13. Modesto i Alapont V, Garcia Cusco M, Medina A. High-flow oxygen therapy in infants with bronchiolitis. N Engl J Med. 2018;378(25):2444. https://doi.org/10.1056/NEJMc1805312.
14. Mace AO, Gibbons J, Schultz A, Knight G, Martin AC. Humidified high-flow nasal cannula oxygen for bronchiolitis: should we go with the flow? Arch Dis Child. 2018;103(3):303. https://doi.org/10.1136/archdischild-2017-313950.
15. Sochet AA, McGee JA, October TW. Oral nutrition in children with bronchiolitis on high-flow nasal cannula is well tolerated. Hosp Pediatr. 2017;7(5):249-255. https://doi.org/10.1542/hpeds.2016-0131.
© 2020 Society of Hospital Medicine
Clinical Progress Note: Point-of-Care Ultrasound for the Pediatric Hospitalist
The recent designation of Pediatric Hospital Medicine (PHM) as a board-certified subspecialty has provided the opportunity to define which skills are core to hospitalist practice. One skill that is novel to the field and gaining traction is point-of-care ultrasonography (POCUS). POCUS differs from traditional ultrasonography in that it is performed at the bedside by the primary clinician and aims to answer a focused clinical question (eg, does this patient have a skin abscess?) rather than to provide a comprehensive evaluation of the anatomy and physiology. The proposed advantages of POCUS include real-time image interpretation, cost savings, procedural guidance to minimize complications, and reduction of ionizing radiation. Although specialties such as Critical Care (CC) and Emergency Medicine (EM) have integrated POCUS into their practice and training, best practices in PHM have not been defined. This Progress Note is a summary of recent evidence to update past reviews and set the stage for future PHM POCUS research and education.
LITERATURE SEARCH STRATEGY AND TOPIC SELECTION
We met with an academic librarian in March 2019 and performed a search of PubMed using Medical Subject Headings (MESH) terms associated with POCUS as well as Pediatrics. We limited our search to studies published within the past five years. The search was originally focused to the field of PHM before expanding to a broader search since very few studies were found that focused on Hospital Medicine or general pediatric ward populations. This initial search generated 274 publications. We then performed a supplemental literature search using references from studies found in our initial search, as well as further ad hoc searching in Embase and Google Scholar.
After our literature search, we reviewed the PHM core competencies and identified the common clinical diagnoses and core skills for which there is POCUS literature published in the past five years. These included acute abdominal pain, bronchiolitis, pneumonia, skin and soft tissue infection, newborn care/delivery room management, bladder catheterization, fluid management, intravenous access, and lumbar puncture (LP). We chose to focus on one skill and two diagnoses that were generalizable to pediatric hospitalists across different settings and for which there was compelling evidence for POCUS use, such as pneumonia, skin abscess, and LP. We found few studies that included general pediatric ward patients, but we considered EM and CC studies to be relevant as several pediatric hospitalists practice in these clinical settings and with these patient populations.
PNEUMONIA
POCUS can be useful for diagnosing pneumonia by direct visualization of lung consolidation or by identification of various sonographic artifacts that suggest pathology. For example, “B-lines” are vertical artifacts that extend from the pleura and suggest interstitial fluid or pneumonia when they are present in abnormally high numbers or density. POCUS can also be used to diagnose parapneumonic effusions by scanning dependent areas of the lung (eg, the diaphragm in children sitting upright) and looking for anechoic or hypoechoic areas.
Three recent meta-analyses found favorable operating characteristics when using POCUS for the diagnosis of pneumonia in children, with summary sensitivities of 93%-94% and specificities of 92%-96%.1-3 However, these meta-analyses were limited by high heterogeneity due to the inclusion of multiple different care settings and the use of variable reference standards and sonographic criteria for diagnosing pneumonia. POCUS is superior to chest radiography for evaluating parapneumonic pleural effusions,4 allowing for rapid identification of loculations, fibrin strands, and proteinaceous material, and for serial bedside evaluation of effusion size and characteristics.
Additional advantages of POCUS include avoidance of ionizing radiation and the potential for cost and time savings. Two studies demonstrated reductions in radiography use and improved cost, although they were not conducted on hospitalized patients. One randomized controlled trial (RCT) conducted in a pediatric emergency department (ED) demonstrated a 38.8% reduction in chest radiography use without increasing the ED length of stay (EDLOS), antibiotic use, or unscheduled follow-up visits.5 A retrospective matched cohort study conducted in another pediatric ED reported that when compared with patients evaluated by chest radiography, those evaluated by POCUS had significantly shorter EDLOS (−60.9 min) and mean health systems savings ($187 per patient).6 We believe that POCUS has value in the evaluation and management of pneumonia and parapneumonic effusions, although further studies investigating patient outcomes and involving inpatient populations are required.
SKIN ABSCESS
POCUS can augment the physical examination, helping to both avoid unnecessary incision and drainage (I+D) procedures and detect drainable fluid collections. Abscess is suggested when hypoechoic material without vascular flow is detected, and although other structures such as vessels, cysts, and lymph nodes can mimic skin abscesses, this is a relatively straightforward examination for clinicians to learn.
Two meta-analyses found that POCUS had high sensitivity for diagnosing skin abscesses in the ED.7,8 A pediatric subgroup analysis conducted in a study by Barbic et al. found a sensitivity and a specificity of 94% (95% C: 88%-98%) and 83% (95% C: 47%-97%), respectively.7 Subramaniam et al. included six studies (four pediatric) with 800 patients (653 ≤ 18 years old) and found an overall pooled sensitivity of 97% (95% C: 94%-98%) and a specificity of 83% (95% C: 75%-88%).8 No subgroup analysis was performed, but the included pediatric studies reported sensitivities and specificities between 90%-98% and 68%-87%, respectively.
Although POCUS performs better than physical examination for the diagnosis of drainable abscesses, evidence regarding patient outcomes is mixed. A retrospective review from four pediatric EDs found that integration of POCUS lowered treatment failure rates, defined as any incision and drainage (I+D) or surgical manipulation after discharge from the initial ED visit (4.4% vs 15.6%, P < .005).9 A single-center retrospective cohort study found that POCUS reduced EDLOS by a median of 73 minutes (95% C: 52-94 min) when compared with radiology-performed studies.10 The aforementioned study conducted by Barbic et al. found that in pediatric studies, POCUS led to a change in management (eg, whether or not to attempt I+D) in 14%-27% of patients.7 However, a multicenter prospective observational cohort study involving seven pediatric EDs found that despite changing the management in 22.9% of cases, POCUS was not associated with any statistically significant differences in treatment failure rates, EDLOS, discharge rates, use of sedation, or use of alternative imaging.11 These studies were limited by a lack of randomization or control group and emphasize the need for RCTs that measure patient outcomes. Future studies should investigate how POCUS can be used in inpatient settings both for initial diagnosis of drainable abscesses and for serial evaluation of evolving phlegmon or incompletely drained collections.
LUMBAR PUNCTURE
LP is commonly performed by pediatric hospitalists, although success can be influenced by numerous factors, including provider and staff expertise, patient anatomy, and body habitus. Requiring multiple attempts can increase patient discomfort and parental anxiety. Failure to obtain cerebrospinal fluid can delay diagnosis or leave providers in uncertain clinical situations that may commit patients to prolonged antibiotic courses. POCUS can be used to identify anatomic markers such as interspinous processes, anatomic midline, and depth of the ligamentum flavum.12 It can also be used to identify epidural hematomas after failed LPs to avoid additional unsuccessful attempts.13 POCUS guidance for LP has been described using both static (preprocedural marking) and dynamic (scanning during the procedure) techniques, although most of the studies use the static approach. The Society for Hospital Medicine POCUS Task Force has recently released a position statement recommending that POCUS should be used for site selection before performing LP in adult patients when providers are adequately trained.12 Although this position statement was for adult patients, recent evidence suggests that there is also benefit in Pediatrics.
Two recent meta-analyses have investigated POCUS use for pediatric LPs.14,15 Olowoyeye et al. included four studies with a total of 277 patients and found that POCUS use was associated with a reduction in traumatic taps (risk ratio [RR] = 0.53, 95% C: 0.13-0.82) when compared with landmark approaches.14 However, there was no statistically significant reduction in LP failure, number of needle insertion attempts, or procedure length. A more recent meta-analysis performed a pediatric subgroup analysis of six studies including 452 patients and found a statistically significant reduction in traumatic taps (13.7% vs 31.8%, risk difference = −21.3%, 95% C: −38.2% to −4.3%) and number of needle insertion attempts (1.53 vs 2.07, mean difference = −0.47, 95% C: −0.73 to −0.21).15 The primary outcome of LP success trended toward favoring POCUS, but it was not statistically significant (88.4% vs 74.0%, OR = 2.55, 95% C: 0.99-6.52). We believe t
CONCLUSION
Evidence accumulated in the past five years has built on previous work suggesting that POCUS has a role in the diagnosis of pneumonia and skin abscess and in the performance of LPs. However, gaps in the literature remain when applying POCUS in PHM. Only a few studies to date were conducted in non-CC inpatient settings, and although several pediatric hospitalists work in EDs or care for critically ill children, our largest population comprises general pediatric ward patients. Studies have also used ultrasonographers with variable POCUS training and clinical experience, which makes comparing or combining studies challenging since POCUS is dependent on provider skills. Studies involving PHM providers and inpatient populations are needed. Additional studies evaluating the process and outcome measures are also needed to understand whether the theoretical advantages are consistently realized in real-world PHM practice. Finally, PHM-specific curricula should be designed in collaboration with various PHM stakeholders and with specialties who already have robust POCUS training pathways. There is opportunity within PHM for multi institutional research collaboration, identification of best practices, and development of PHM-specific training for fellowship and faculty development programs.
1. Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21(3):183-195. https://doi.org/10.1007/s40477-018-0306-5.
2. Najgrodzka P, Buda N, Zamojska A, Marciniewicz E, Lewandowicz-Uszynska A. Lung ultrasonography in the diagnosis of pneumonia in children-a metaanalysis and a review of pediatric lung imaging. Ultrasound Q. 2019; 35(2):157-163. https://doi.org/10.1097/RUQ.0000000000000411.
3. Xin H, Li J, Hu HY. Is lung ultrasound useful for diagnosing pneumonia in children?: a meta-analysis and systematic review. Ultrasound Q. 2018;34(1):3-10. https://doi.org/10.1097/RUQ.0000000000000330.
4. Esposito S, Papa SS, Borzani I, et al. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital J Pediatr. 2014;40(1):37. https://doi.org/10.1186/1824-7288-40-37.
5. Jones BP, Tay ET, Elikashvili I, et al. Feasibility and safety of substituting lung ultrasonography for chest radiography when diagnosing pneumonia in children: a randomized controlled trial. Chest. 2016;150(1):131-138. https://doi.org/10.1016/j.chest.2016.02.643.
6. Harel‐Sterling M, Diallo M, Santhirakumaran S, Maxim T, Tessaro M. Emergency department resource use in pediatric pneumonia: point‐of‐care lung ultrasonography versus chest radiography. J Ultrasound Med. 2019;38(2):407-414. https://doi.org/10.1002/jum.14703.
7. Barbic D, Chenkin J, Cho DD, Jelic T, Scheuermeyer FX. In patients presenting to the emergency department with skin and soft tissue infections what is the diagnostic accuracy of point-of-care ultrasonography for the diagnosis of abscess compared to the current standard of care? A systematic review and meta-analysis. BMJ Open. 2017;7(1):e013688. https://doi.org/10.1136/bmjopen-2016-013688.
8. Subramaniam S, Bober J, Chao J, Zehtabchi S. Point-of-care ultrasound for diagnosis of abscess in skin and soft tissue infections. Acad Emerg Med. 2016;23(11):1298-1306. https://doi.org/10.1111/acem.13049.
9. Gaspari RJ, Sanseverino A. Ultrasound-guided drainage for pediatric soft tissue abscesses decreases clinical failure rates compared to drainage without ultrasound: a retrospective study. J Ultrasound Med. 2018;37(1):131-136. https://doi.org/10.1002/jum.14318.
10. Lin MJ, Neuman M, Rempell R, Monuteaux M, Levy J. Point-of-care ultrasound is associated with decreased length of stay in children presenting to the emergency department with soft tissue infection. J Emerg Med. 2018;54(1):96-101. https://doi.org/10.1016/j.jemermed.2017.09.017.
11. Lam SHF, Sivitz A, Alade K, et al. Comparison of ultrasound guidance vs. clinical assessment alone for management of pediatric skin and soft tissue infections. J Emerg Med. 2018;55(5):693-701. https://doi.org/10.1016/j.jemermed.2018.07.010.
12. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019. J Hosp Med. 2019;14:E1-E11. https://doi.org/10.12788/jhm.3197.
13. Kusulas MP, Eutsler EP, DePiero AD. Bedside ultrasound for the evaluation of epidural hematoma after infant lumbar puncture [published online ahead of print January 2, 2018]. Pediatr Emerg Care. 2018. https://doi.org/10.1097/PEC.0000000000001383.
14. Olowoyeye A, Fadahunsi O, Okudo J, Opaneye O, Okwundu C. Ultrasound imaging versus palpation method for diagnostic lumbar puncture in neonates and infants: a systematic review and meta-analysis. BMJ Paediatr Open. 2019;3(1):e000412. https://doi.org/10.1136/bmjpo-2018-000412
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
The recent designation of Pediatric Hospital Medicine (PHM) as a board-certified subspecialty has provided the opportunity to define which skills are core to hospitalist practice. One skill that is novel to the field and gaining traction is point-of-care ultrasonography (POCUS). POCUS differs from traditional ultrasonography in that it is performed at the bedside by the primary clinician and aims to answer a focused clinical question (eg, does this patient have a skin abscess?) rather than to provide a comprehensive evaluation of the anatomy and physiology. The proposed advantages of POCUS include real-time image interpretation, cost savings, procedural guidance to minimize complications, and reduction of ionizing radiation. Although specialties such as Critical Care (CC) and Emergency Medicine (EM) have integrated POCUS into their practice and training, best practices in PHM have not been defined. This Progress Note is a summary of recent evidence to update past reviews and set the stage for future PHM POCUS research and education.
LITERATURE SEARCH STRATEGY AND TOPIC SELECTION
We met with an academic librarian in March 2019 and performed a search of PubMed using Medical Subject Headings (MESH) terms associated with POCUS as well as Pediatrics. We limited our search to studies published within the past five years. The search was originally focused to the field of PHM before expanding to a broader search since very few studies were found that focused on Hospital Medicine or general pediatric ward populations. This initial search generated 274 publications. We then performed a supplemental literature search using references from studies found in our initial search, as well as further ad hoc searching in Embase and Google Scholar.
After our literature search, we reviewed the PHM core competencies and identified the common clinical diagnoses and core skills for which there is POCUS literature published in the past five years. These included acute abdominal pain, bronchiolitis, pneumonia, skin and soft tissue infection, newborn care/delivery room management, bladder catheterization, fluid management, intravenous access, and lumbar puncture (LP). We chose to focus on one skill and two diagnoses that were generalizable to pediatric hospitalists across different settings and for which there was compelling evidence for POCUS use, such as pneumonia, skin abscess, and LP. We found few studies that included general pediatric ward patients, but we considered EM and CC studies to be relevant as several pediatric hospitalists practice in these clinical settings and with these patient populations.
PNEUMONIA
POCUS can be useful for diagnosing pneumonia by direct visualization of lung consolidation or by identification of various sonographic artifacts that suggest pathology. For example, “B-lines” are vertical artifacts that extend from the pleura and suggest interstitial fluid or pneumonia when they are present in abnormally high numbers or density. POCUS can also be used to diagnose parapneumonic effusions by scanning dependent areas of the lung (eg, the diaphragm in children sitting upright) and looking for anechoic or hypoechoic areas.
Three recent meta-analyses found favorable operating characteristics when using POCUS for the diagnosis of pneumonia in children, with summary sensitivities of 93%-94% and specificities of 92%-96%.1-3 However, these meta-analyses were limited by high heterogeneity due to the inclusion of multiple different care settings and the use of variable reference standards and sonographic criteria for diagnosing pneumonia. POCUS is superior to chest radiography for evaluating parapneumonic pleural effusions,4 allowing for rapid identification of loculations, fibrin strands, and proteinaceous material, and for serial bedside evaluation of effusion size and characteristics.
Additional advantages of POCUS include avoidance of ionizing radiation and the potential for cost and time savings. Two studies demonstrated reductions in radiography use and improved cost, although they were not conducted on hospitalized patients. One randomized controlled trial (RCT) conducted in a pediatric emergency department (ED) demonstrated a 38.8% reduction in chest radiography use without increasing the ED length of stay (EDLOS), antibiotic use, or unscheduled follow-up visits.5 A retrospective matched cohort study conducted in another pediatric ED reported that when compared with patients evaluated by chest radiography, those evaluated by POCUS had significantly shorter EDLOS (−60.9 min) and mean health systems savings ($187 per patient).6 We believe that POCUS has value in the evaluation and management of pneumonia and parapneumonic effusions, although further studies investigating patient outcomes and involving inpatient populations are required.
SKIN ABSCESS
POCUS can augment the physical examination, helping to both avoid unnecessary incision and drainage (I+D) procedures and detect drainable fluid collections. Abscess is suggested when hypoechoic material without vascular flow is detected, and although other structures such as vessels, cysts, and lymph nodes can mimic skin abscesses, this is a relatively straightforward examination for clinicians to learn.
Two meta-analyses found that POCUS had high sensitivity for diagnosing skin abscesses in the ED.7,8 A pediatric subgroup analysis conducted in a study by Barbic et al. found a sensitivity and a specificity of 94% (95% C: 88%-98%) and 83% (95% C: 47%-97%), respectively.7 Subramaniam et al. included six studies (four pediatric) with 800 patients (653 ≤ 18 years old) and found an overall pooled sensitivity of 97% (95% C: 94%-98%) and a specificity of 83% (95% C: 75%-88%).8 No subgroup analysis was performed, but the included pediatric studies reported sensitivities and specificities between 90%-98% and 68%-87%, respectively.
Although POCUS performs better than physical examination for the diagnosis of drainable abscesses, evidence regarding patient outcomes is mixed. A retrospective review from four pediatric EDs found that integration of POCUS lowered treatment failure rates, defined as any incision and drainage (I+D) or surgical manipulation after discharge from the initial ED visit (4.4% vs 15.6%, P < .005).9 A single-center retrospective cohort study found that POCUS reduced EDLOS by a median of 73 minutes (95% C: 52-94 min) when compared with radiology-performed studies.10 The aforementioned study conducted by Barbic et al. found that in pediatric studies, POCUS led to a change in management (eg, whether or not to attempt I+D) in 14%-27% of patients.7 However, a multicenter prospective observational cohort study involving seven pediatric EDs found that despite changing the management in 22.9% of cases, POCUS was not associated with any statistically significant differences in treatment failure rates, EDLOS, discharge rates, use of sedation, or use of alternative imaging.11 These studies were limited by a lack of randomization or control group and emphasize the need for RCTs that measure patient outcomes. Future studies should investigate how POCUS can be used in inpatient settings both for initial diagnosis of drainable abscesses and for serial evaluation of evolving phlegmon or incompletely drained collections.
LUMBAR PUNCTURE
LP is commonly performed by pediatric hospitalists, although success can be influenced by numerous factors, including provider and staff expertise, patient anatomy, and body habitus. Requiring multiple attempts can increase patient discomfort and parental anxiety. Failure to obtain cerebrospinal fluid can delay diagnosis or leave providers in uncertain clinical situations that may commit patients to prolonged antibiotic courses. POCUS can be used to identify anatomic markers such as interspinous processes, anatomic midline, and depth of the ligamentum flavum.12 It can also be used to identify epidural hematomas after failed LPs to avoid additional unsuccessful attempts.13 POCUS guidance for LP has been described using both static (preprocedural marking) and dynamic (scanning during the procedure) techniques, although most of the studies use the static approach. The Society for Hospital Medicine POCUS Task Force has recently released a position statement recommending that POCUS should be used for site selection before performing LP in adult patients when providers are adequately trained.12 Although this position statement was for adult patients, recent evidence suggests that there is also benefit in Pediatrics.
Two recent meta-analyses have investigated POCUS use for pediatric LPs.14,15 Olowoyeye et al. included four studies with a total of 277 patients and found that POCUS use was associated with a reduction in traumatic taps (risk ratio [RR] = 0.53, 95% C: 0.13-0.82) when compared with landmark approaches.14 However, there was no statistically significant reduction in LP failure, number of needle insertion attempts, or procedure length. A more recent meta-analysis performed a pediatric subgroup analysis of six studies including 452 patients and found a statistically significant reduction in traumatic taps (13.7% vs 31.8%, risk difference = −21.3%, 95% C: −38.2% to −4.3%) and number of needle insertion attempts (1.53 vs 2.07, mean difference = −0.47, 95% C: −0.73 to −0.21).15 The primary outcome of LP success trended toward favoring POCUS, but it was not statistically significant (88.4% vs 74.0%, OR = 2.55, 95% C: 0.99-6.52). We believe t
CONCLUSION
Evidence accumulated in the past five years has built on previous work suggesting that POCUS has a role in the diagnosis of pneumonia and skin abscess and in the performance of LPs. However, gaps in the literature remain when applying POCUS in PHM. Only a few studies to date were conducted in non-CC inpatient settings, and although several pediatric hospitalists work in EDs or care for critically ill children, our largest population comprises general pediatric ward patients. Studies have also used ultrasonographers with variable POCUS training and clinical experience, which makes comparing or combining studies challenging since POCUS is dependent on provider skills. Studies involving PHM providers and inpatient populations are needed. Additional studies evaluating the process and outcome measures are also needed to understand whether the theoretical advantages are consistently realized in real-world PHM practice. Finally, PHM-specific curricula should be designed in collaboration with various PHM stakeholders and with specialties who already have robust POCUS training pathways. There is opportunity within PHM for multi institutional research collaboration, identification of best practices, and development of PHM-specific training for fellowship and faculty development programs.
The recent designation of Pediatric Hospital Medicine (PHM) as a board-certified subspecialty has provided the opportunity to define which skills are core to hospitalist practice. One skill that is novel to the field and gaining traction is point-of-care ultrasonography (POCUS). POCUS differs from traditional ultrasonography in that it is performed at the bedside by the primary clinician and aims to answer a focused clinical question (eg, does this patient have a skin abscess?) rather than to provide a comprehensive evaluation of the anatomy and physiology. The proposed advantages of POCUS include real-time image interpretation, cost savings, procedural guidance to minimize complications, and reduction of ionizing radiation. Although specialties such as Critical Care (CC) and Emergency Medicine (EM) have integrated POCUS into their practice and training, best practices in PHM have not been defined. This Progress Note is a summary of recent evidence to update past reviews and set the stage for future PHM POCUS research and education.
LITERATURE SEARCH STRATEGY AND TOPIC SELECTION
We met with an academic librarian in March 2019 and performed a search of PubMed using Medical Subject Headings (MESH) terms associated with POCUS as well as Pediatrics. We limited our search to studies published within the past five years. The search was originally focused to the field of PHM before expanding to a broader search since very few studies were found that focused on Hospital Medicine or general pediatric ward populations. This initial search generated 274 publications. We then performed a supplemental literature search using references from studies found in our initial search, as well as further ad hoc searching in Embase and Google Scholar.
After our literature search, we reviewed the PHM core competencies and identified the common clinical diagnoses and core skills for which there is POCUS literature published in the past five years. These included acute abdominal pain, bronchiolitis, pneumonia, skin and soft tissue infection, newborn care/delivery room management, bladder catheterization, fluid management, intravenous access, and lumbar puncture (LP). We chose to focus on one skill and two diagnoses that were generalizable to pediatric hospitalists across different settings and for which there was compelling evidence for POCUS use, such as pneumonia, skin abscess, and LP. We found few studies that included general pediatric ward patients, but we considered EM and CC studies to be relevant as several pediatric hospitalists practice in these clinical settings and with these patient populations.
PNEUMONIA
POCUS can be useful for diagnosing pneumonia by direct visualization of lung consolidation or by identification of various sonographic artifacts that suggest pathology. For example, “B-lines” are vertical artifacts that extend from the pleura and suggest interstitial fluid or pneumonia when they are present in abnormally high numbers or density. POCUS can also be used to diagnose parapneumonic effusions by scanning dependent areas of the lung (eg, the diaphragm in children sitting upright) and looking for anechoic or hypoechoic areas.
Three recent meta-analyses found favorable operating characteristics when using POCUS for the diagnosis of pneumonia in children, with summary sensitivities of 93%-94% and specificities of 92%-96%.1-3 However, these meta-analyses were limited by high heterogeneity due to the inclusion of multiple different care settings and the use of variable reference standards and sonographic criteria for diagnosing pneumonia. POCUS is superior to chest radiography for evaluating parapneumonic pleural effusions,4 allowing for rapid identification of loculations, fibrin strands, and proteinaceous material, and for serial bedside evaluation of effusion size and characteristics.
Additional advantages of POCUS include avoidance of ionizing radiation and the potential for cost and time savings. Two studies demonstrated reductions in radiography use and improved cost, although they were not conducted on hospitalized patients. One randomized controlled trial (RCT) conducted in a pediatric emergency department (ED) demonstrated a 38.8% reduction in chest radiography use without increasing the ED length of stay (EDLOS), antibiotic use, or unscheduled follow-up visits.5 A retrospective matched cohort study conducted in another pediatric ED reported that when compared with patients evaluated by chest radiography, those evaluated by POCUS had significantly shorter EDLOS (−60.9 min) and mean health systems savings ($187 per patient).6 We believe that POCUS has value in the evaluation and management of pneumonia and parapneumonic effusions, although further studies investigating patient outcomes and involving inpatient populations are required.
SKIN ABSCESS
POCUS can augment the physical examination, helping to both avoid unnecessary incision and drainage (I+D) procedures and detect drainable fluid collections. Abscess is suggested when hypoechoic material without vascular flow is detected, and although other structures such as vessels, cysts, and lymph nodes can mimic skin abscesses, this is a relatively straightforward examination for clinicians to learn.
Two meta-analyses found that POCUS had high sensitivity for diagnosing skin abscesses in the ED.7,8 A pediatric subgroup analysis conducted in a study by Barbic et al. found a sensitivity and a specificity of 94% (95% C: 88%-98%) and 83% (95% C: 47%-97%), respectively.7 Subramaniam et al. included six studies (four pediatric) with 800 patients (653 ≤ 18 years old) and found an overall pooled sensitivity of 97% (95% C: 94%-98%) and a specificity of 83% (95% C: 75%-88%).8 No subgroup analysis was performed, but the included pediatric studies reported sensitivities and specificities between 90%-98% and 68%-87%, respectively.
Although POCUS performs better than physical examination for the diagnosis of drainable abscesses, evidence regarding patient outcomes is mixed. A retrospective review from four pediatric EDs found that integration of POCUS lowered treatment failure rates, defined as any incision and drainage (I+D) or surgical manipulation after discharge from the initial ED visit (4.4% vs 15.6%, P < .005).9 A single-center retrospective cohort study found that POCUS reduced EDLOS by a median of 73 minutes (95% C: 52-94 min) when compared with radiology-performed studies.10 The aforementioned study conducted by Barbic et al. found that in pediatric studies, POCUS led to a change in management (eg, whether or not to attempt I+D) in 14%-27% of patients.7 However, a multicenter prospective observational cohort study involving seven pediatric EDs found that despite changing the management in 22.9% of cases, POCUS was not associated with any statistically significant differences in treatment failure rates, EDLOS, discharge rates, use of sedation, or use of alternative imaging.11 These studies were limited by a lack of randomization or control group and emphasize the need for RCTs that measure patient outcomes. Future studies should investigate how POCUS can be used in inpatient settings both for initial diagnosis of drainable abscesses and for serial evaluation of evolving phlegmon or incompletely drained collections.
LUMBAR PUNCTURE
LP is commonly performed by pediatric hospitalists, although success can be influenced by numerous factors, including provider and staff expertise, patient anatomy, and body habitus. Requiring multiple attempts can increase patient discomfort and parental anxiety. Failure to obtain cerebrospinal fluid can delay diagnosis or leave providers in uncertain clinical situations that may commit patients to prolonged antibiotic courses. POCUS can be used to identify anatomic markers such as interspinous processes, anatomic midline, and depth of the ligamentum flavum.12 It can also be used to identify epidural hematomas after failed LPs to avoid additional unsuccessful attempts.13 POCUS guidance for LP has been described using both static (preprocedural marking) and dynamic (scanning during the procedure) techniques, although most of the studies use the static approach. The Society for Hospital Medicine POCUS Task Force has recently released a position statement recommending that POCUS should be used for site selection before performing LP in adult patients when providers are adequately trained.12 Although this position statement was for adult patients, recent evidence suggests that there is also benefit in Pediatrics.
Two recent meta-analyses have investigated POCUS use for pediatric LPs.14,15 Olowoyeye et al. included four studies with a total of 277 patients and found that POCUS use was associated with a reduction in traumatic taps (risk ratio [RR] = 0.53, 95% C: 0.13-0.82) when compared with landmark approaches.14 However, there was no statistically significant reduction in LP failure, number of needle insertion attempts, or procedure length. A more recent meta-analysis performed a pediatric subgroup analysis of six studies including 452 patients and found a statistically significant reduction in traumatic taps (13.7% vs 31.8%, risk difference = −21.3%, 95% C: −38.2% to −4.3%) and number of needle insertion attempts (1.53 vs 2.07, mean difference = −0.47, 95% C: −0.73 to −0.21).15 The primary outcome of LP success trended toward favoring POCUS, but it was not statistically significant (88.4% vs 74.0%, OR = 2.55, 95% C: 0.99-6.52). We believe t
CONCLUSION
Evidence accumulated in the past five years has built on previous work suggesting that POCUS has a role in the diagnosis of pneumonia and skin abscess and in the performance of LPs. However, gaps in the literature remain when applying POCUS in PHM. Only a few studies to date were conducted in non-CC inpatient settings, and although several pediatric hospitalists work in EDs or care for critically ill children, our largest population comprises general pediatric ward patients. Studies have also used ultrasonographers with variable POCUS training and clinical experience, which makes comparing or combining studies challenging since POCUS is dependent on provider skills. Studies involving PHM providers and inpatient populations are needed. Additional studies evaluating the process and outcome measures are also needed to understand whether the theoretical advantages are consistently realized in real-world PHM practice. Finally, PHM-specific curricula should be designed in collaboration with various PHM stakeholders and with specialties who already have robust POCUS training pathways. There is opportunity within PHM for multi institutional research collaboration, identification of best practices, and development of PHM-specific training for fellowship and faculty development programs.
1. Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21(3):183-195. https://doi.org/10.1007/s40477-018-0306-5.
2. Najgrodzka P, Buda N, Zamojska A, Marciniewicz E, Lewandowicz-Uszynska A. Lung ultrasonography in the diagnosis of pneumonia in children-a metaanalysis and a review of pediatric lung imaging. Ultrasound Q. 2019; 35(2):157-163. https://doi.org/10.1097/RUQ.0000000000000411.
3. Xin H, Li J, Hu HY. Is lung ultrasound useful for diagnosing pneumonia in children?: a meta-analysis and systematic review. Ultrasound Q. 2018;34(1):3-10. https://doi.org/10.1097/RUQ.0000000000000330.
4. Esposito S, Papa SS, Borzani I, et al. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital J Pediatr. 2014;40(1):37. https://doi.org/10.1186/1824-7288-40-37.
5. Jones BP, Tay ET, Elikashvili I, et al. Feasibility and safety of substituting lung ultrasonography for chest radiography when diagnosing pneumonia in children: a randomized controlled trial. Chest. 2016;150(1):131-138. https://doi.org/10.1016/j.chest.2016.02.643.
6. Harel‐Sterling M, Diallo M, Santhirakumaran S, Maxim T, Tessaro M. Emergency department resource use in pediatric pneumonia: point‐of‐care lung ultrasonography versus chest radiography. J Ultrasound Med. 2019;38(2):407-414. https://doi.org/10.1002/jum.14703.
7. Barbic D, Chenkin J, Cho DD, Jelic T, Scheuermeyer FX. In patients presenting to the emergency department with skin and soft tissue infections what is the diagnostic accuracy of point-of-care ultrasonography for the diagnosis of abscess compared to the current standard of care? A systematic review and meta-analysis. BMJ Open. 2017;7(1):e013688. https://doi.org/10.1136/bmjopen-2016-013688.
8. Subramaniam S, Bober J, Chao J, Zehtabchi S. Point-of-care ultrasound for diagnosis of abscess in skin and soft tissue infections. Acad Emerg Med. 2016;23(11):1298-1306. https://doi.org/10.1111/acem.13049.
9. Gaspari RJ, Sanseverino A. Ultrasound-guided drainage for pediatric soft tissue abscesses decreases clinical failure rates compared to drainage without ultrasound: a retrospective study. J Ultrasound Med. 2018;37(1):131-136. https://doi.org/10.1002/jum.14318.
10. Lin MJ, Neuman M, Rempell R, Monuteaux M, Levy J. Point-of-care ultrasound is associated with decreased length of stay in children presenting to the emergency department with soft tissue infection. J Emerg Med. 2018;54(1):96-101. https://doi.org/10.1016/j.jemermed.2017.09.017.
11. Lam SHF, Sivitz A, Alade K, et al. Comparison of ultrasound guidance vs. clinical assessment alone for management of pediatric skin and soft tissue infections. J Emerg Med. 2018;55(5):693-701. https://doi.org/10.1016/j.jemermed.2018.07.010.
12. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019. J Hosp Med. 2019;14:E1-E11. https://doi.org/10.12788/jhm.3197.
13. Kusulas MP, Eutsler EP, DePiero AD. Bedside ultrasound for the evaluation of epidural hematoma after infant lumbar puncture [published online ahead of print January 2, 2018]. Pediatr Emerg Care. 2018. https://doi.org/10.1097/PEC.0000000000001383.
14. Olowoyeye A, Fadahunsi O, Okudo J, Opaneye O, Okwundu C. Ultrasound imaging versus palpation method for diagnostic lumbar puncture in neonates and infants: a systematic review and meta-analysis. BMJ Paediatr Open. 2019;3(1):e000412. https://doi.org/10.1136/bmjpo-2018-000412
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
1. Orso D, Ban A, Guglielmo N. Lung ultrasound in diagnosing pneumonia in childhood: a systematic review and meta-analysis. J Ultrasound. 2018;21(3):183-195. https://doi.org/10.1007/s40477-018-0306-5.
2. Najgrodzka P, Buda N, Zamojska A, Marciniewicz E, Lewandowicz-Uszynska A. Lung ultrasonography in the diagnosis of pneumonia in children-a metaanalysis and a review of pediatric lung imaging. Ultrasound Q. 2019; 35(2):157-163. https://doi.org/10.1097/RUQ.0000000000000411.
3. Xin H, Li J, Hu HY. Is lung ultrasound useful for diagnosing pneumonia in children?: a meta-analysis and systematic review. Ultrasound Q. 2018;34(1):3-10. https://doi.org/10.1097/RUQ.0000000000000330.
4. Esposito S, Papa SS, Borzani I, et al. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital J Pediatr. 2014;40(1):37. https://doi.org/10.1186/1824-7288-40-37.
5. Jones BP, Tay ET, Elikashvili I, et al. Feasibility and safety of substituting lung ultrasonography for chest radiography when diagnosing pneumonia in children: a randomized controlled trial. Chest. 2016;150(1):131-138. https://doi.org/10.1016/j.chest.2016.02.643.
6. Harel‐Sterling M, Diallo M, Santhirakumaran S, Maxim T, Tessaro M. Emergency department resource use in pediatric pneumonia: point‐of‐care lung ultrasonography versus chest radiography. J Ultrasound Med. 2019;38(2):407-414. https://doi.org/10.1002/jum.14703.
7. Barbic D, Chenkin J, Cho DD, Jelic T, Scheuermeyer FX. In patients presenting to the emergency department with skin and soft tissue infections what is the diagnostic accuracy of point-of-care ultrasonography for the diagnosis of abscess compared to the current standard of care? A systematic review and meta-analysis. BMJ Open. 2017;7(1):e013688. https://doi.org/10.1136/bmjopen-2016-013688.
8. Subramaniam S, Bober J, Chao J, Zehtabchi S. Point-of-care ultrasound for diagnosis of abscess in skin and soft tissue infections. Acad Emerg Med. 2016;23(11):1298-1306. https://doi.org/10.1111/acem.13049.
9. Gaspari RJ, Sanseverino A. Ultrasound-guided drainage for pediatric soft tissue abscesses decreases clinical failure rates compared to drainage without ultrasound: a retrospective study. J Ultrasound Med. 2018;37(1):131-136. https://doi.org/10.1002/jum.14318.
10. Lin MJ, Neuman M, Rempell R, Monuteaux M, Levy J. Point-of-care ultrasound is associated with decreased length of stay in children presenting to the emergency department with soft tissue infection. J Emerg Med. 2018;54(1):96-101. https://doi.org/10.1016/j.jemermed.2017.09.017.
11. Lam SHF, Sivitz A, Alade K, et al. Comparison of ultrasound guidance vs. clinical assessment alone for management of pediatric skin and soft tissue infections. J Emerg Med. 2018;55(5):693-701. https://doi.org/10.1016/j.jemermed.2018.07.010.
12. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the society of hospital medicine [published online ahead of print June 10, 2019. J Hosp Med. 2019;14:E1-E11. https://doi.org/10.12788/jhm.3197.
13. Kusulas MP, Eutsler EP, DePiero AD. Bedside ultrasound for the evaluation of epidural hematoma after infant lumbar puncture [published online ahead of print January 2, 2018]. Pediatr Emerg Care. 2018. https://doi.org/10.1097/PEC.0000000000001383.
14. Olowoyeye A, Fadahunsi O, Okudo J, Opaneye O, Okwundu C. Ultrasound imaging versus palpation method for diagnostic lumbar puncture in neonates and infants: a systematic review and meta-analysis. BMJ Paediatr Open. 2019;3(1):e000412. https://doi.org/10.1136/bmjpo-2018-000412
15. Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85-96. https://doi.org/10.1111/acem.13558.
© 2020 Society of Hospital Medicine
Methodolgical Progress Note: Handling Missing Data in Clinical Research
Research, in the field of Hospital Medicine, often leverages data collected for reasons other than research. For example, electronic medical record data or patient satisfaction survey results can be used to answer questions that are relevant to the practice of hospital medicine. In these types of datasets, data will inevitably be missing. Such missing data can compromise our ability to draw definitive conclusions from our research study. This review introduces the concept of missing data, describes patterns and mechanisms of missing data, and discusses common approaches for the handling of missing data, including sensitivity analyses for determining how robust the results are despite assumptions made about the missing data.
CONSEQUENCES OF MISSING DATA
Missing data create a host of problems for researchers. First, missing data result in a loss of information and can diminish the power of the proposed study. Second, the irregular data complicate the analysis because many of the standard software procedures used have been developed for fully observed or “complete” data (ie, each subject has a value for all measures of interest). Finally, missing data may introduce bias due to the systematic difference between the observed and the unobserved data. For example, if men are less likely than women to complete all questions in a patient satisfaction survey when they are not satisfied, then hospital satisfaction analyses that rely on completed surveys would tend to provide biased estimates of the satisfaction males have with their care.
MINIMIZING MISSING DATA WITH STUDY DESIGN
The ideal approach to mitigating problems caused by missing data is to anticipate and incorporate strategies to minimize missing data into the study design (ie, when planning data collection protocols for prospective studies). This plan should provide strategies for minimizing nonresponse and estimating the magnitude of anticipated missing data to ensure that the study achieves sufficient strength despite the missing data.
Strategies for minimizing nonresponse include (1) informing potential study participants, at initial contact, about the implications of missing data on the ability to answer the research question; (2) collecting several phone numbers, addresses, preferred method of contact and calling times, as well as an alternative contact, in case the primary study contact is unable to be reached; (3) specifying the number of call backs, as well as the time of contact; and (4) piloting data capture questions for phrasing, clarity, and sensitivity, in order to resolve problems before initiating the study. One approach that can be used to mitigate the impact of missing data in surveys is to contact a sample of the initial nonrespondents using a more intensive follow-up approach (eg, a nonresponse to a mailed survey is followed up by a telephone call in order to conduct the survey again over the phone), and this is referred to as “nonresponse two-phase sampling.” The additional data, captured in the second phase, not only reduces the nonresponse rate but can also provide important information on the missing data mechanism.1,2 In longitudinal studies with dropouts, one can measure participants’ intent to drop out in order to evaluate how much the probability of dropping out depends on missing responses.3 One may also choose to determine the power and implications of sample size under different missing data assumptions.4
UNDERSTANDING THE REASONS FOR MISSING DATA
Different data sources are likely to have unique reasons for missing values due to the workflows involved in how the data are collected. In research involving the use of data from electronic medical records, missing data on specific diagnoses involving patients who are regularly engaged in care are often considered to be “not present” or “normal”, since clinical documentation workflows are largely governed by the concept of “documentation by exception” in which diagnoses are documented only when there is an exception to the expectation that these are not present. For example, “diabetes mellitus” is commonly documented, but “diabetes mellitus not present” is rarely documented in electronic medical records which are used for clinical care. Thus, lack of explicit documentation is likely to indicate that diabetes mellitus is, in fact, not present.
Certain variables may be missing simply because there is no quantifiable value—ie, the data do not exist. Structural missingness refers to a value that does not exist for a logical reason (eg, “What is the gender of your first child?” for those who do not have a child). Censoring, which occurs during “time to event” analysis, refers to a situation where information about a subject stops before the event of interest happens, for example, when a subject in a study involving a 30-day outcome dies at day 14. The term “limit of detection” refers to the lowest or highest level at which two distinct values can reasonably be distinguished (eg, the lower limit of detection of a C-reactive protein assay may be 1 mg/dL, so lower values might simply be reported by the lab as <1 mg/dL).5 These types of missing data require specific methods that are not discussed in this review.
These examples illustrate that approaches to dealing with missing data vary depending on what data sources are used and how data are collected. Understanding the reasons missing data are present is a necessary step in formulating a robust analytic approach to handling missing data.
MISSING DATA PATTERNS AND MECHANISMS
Missing Data Patterns
Evaluating missing data patterns provides information on the degree and complexity of the missing data problem and can aid in choosing an appropriate missing data handling method. This is because some analytic methods work well for a general pattern (nonmonotone) and other methods work for special patterns (eg, monotone, file matching). In longitudinal studies, missing data is commonly missing in a monotone pattern, where once one variable is missing then all subsequent variables are also missing for a particular subject. This occurs when a study participant is lost to follow-up. For example, a monotone missing data pattern may occur in a study that requires a series of follow-up visits for laboratory blood tests. If a patient drops out, it results in a monotone missing data pattern, as no data on blood test results are available once the patient drops out. If the patient just skips an intermediate visit but returns for the final blood test, this would show a nonmonotone missing data pattern. A file-matching pattern occurs when variables are never observed together. This pattern can occur when data from several studies are merged and some variables are not collected in all studies. For example, three studies are merged and all three collect blood pressure, but only one study collects age and only one study collects sex.
Missing Data Mechanisms
The missing data mechanism relates to the underlying reasons for missing values and the relationships between variables with and without missing data. In general, missing data can be either random or nonrandom with distinctions in randomness made by three types: (1) data missing completely at random (MCAR); (2) data missing at random (MAR); and (3) data missing not at random (MNAR).6 As with the missing data pattern, understanding the missing data mechanism can aid in selecting an appropriate approach to handling the missing data.
Data are MCAR if the missingness does not depend on any study variables, meaning that all subjects are equally likely to be missing certain data elements. When the data are MCAR, those with missing values can be viewed as a simple random sample from the complete (but never actually observed) data and can be dropped from analysis without causing bias in the results. If the values of some diagnostic tests were missing for some patients due to equipment malfunction or electricity outage, for example, then the missingness may be considered MCAR.
Data are MAR if the missingness depends on the observed characteristics but not the unobserved characteristics, meaning that the relationships observed in the data can be used to predict the occurrence of missing values. Because the “randomness” of MAR is conditional on observed characteristics, which distinguishes it from the “completely at random” type of MCAR, dropping or omitting those cases with missing values from the analysis may lead to biased results.7 In a study of quality of life (QOL) for patients with mild to moderate traumatic brain injury, if health-related QOL questions were not answered by some patients with high pain levels (even though the pain levels were recorded), the missingness of QOL may be considered as MAR. This is due to the fact that within subjects grouped by the observed characteristic of pain (that is, conditional on similar levels of pain) the missingness of QOL is the result of chance and does not depend on the values (observed or unobserved) of QOL. It follows then, that once grouped into a high (or low) pain stratum, if QOL is considered MAR, then, whether or not it is observed, is random.
Data are considered MNAR if their missingness depends on characteristics that are not observed and cannot be fully explained by the observed characteristics. Systematic differences between missing and nonmissing data exist for data that is MNAR. For example, if a survey of household income had an increased probability of missing incomes from the low-income families then the data would be considered as MNAR.
Randomness in the missing data mechanism may be ignored without affecting the inference in some circumstances.8 Both MCAR and MAR can be considered as “ignorable” in the sense that a proper method (eg, multiple imputation) may recover the missing information without modeling (ie, accounting for) the random process of the missing data mechanism (Table).9 In contrast, the MNAR mechanism requires a method that takes into account the missing data mechanism in order to make inferences about the complete (and partially unobserved) data; or in other words, a model for the missing data mechanism cannot be ignored. It is for this reason that the MNAR mechanism is often called “nonignorable”. Nonignorable missing data present a challenge to researchers because the mechanism underlying the missingness must be included in the analysis. Yet researchers rarely know what the missingness mechanism is, and the data needed to validate any putative mechanism is, in fact, missing. In cases when more than one variable is subject to missingness, researchers need to assess the missingness mechanism for each variable and tailor their approach to the specific missing data problems.9
ANALYTIC APPROACHES
There is no universally accepted standard to guide when statistical methods should be applied to account for missing data. The amount of missing data alone cannot fully assess the missing data problem; missing data patterns and mechanisms can have greater impact on research results than the proportion of missing data alone. A good statistical method for handling missing data should provide an unbiased estimate of the quantity that the investigators intend to estimate; make use of the partial information in the incomplete cases to improve efficiency (and in most cases also to reduce bias); and provide valid estimates of the standard errors, confidence intervals, and P values for statistical tests. There are generally four broadly defined classes of methods for handling missing data in clinical research: (1) the complete-case analysis, (2) single imputation methods, (3) the weighted estimating-equation approach, and (4) the model-based approach including maximum likelihood (ML) and multiple imputation (Table and Appendix).10
Since missing data mechanisms cannot be conclusively verified, it is good practice to conduct some sensitivity analyses to test the robustness of the primary results. For this purpose, pattern-mixture models provide a flexible framework for implementing sensitivity analyses to missing data assumptions and can be used to evaluate the possibility of the data being MNAR. In this framework, the missing data distribution is modeled and then incorporated into the outcome model of interest. Tipping-point analysis is a sensitivity analysis where the missing data is replaced with a range of values to determine how much the values must change for the results of the study to tip from significant to not significant. If the same general conclusions remain valid over a range of assumptions about the missing data values, then one can have greater confidence in the study conclusions.
SUMMARY AND RECOMMENDATIONS
In dealing with missing data from clinical research, clinicians and statisticians need to work together to minimize missingness at the data collection stage, document the reasons for missingness, use substantive knowledge, if possible, to assess the missing data mechanism, perform primary analysis based on a defensible missing data mechanism, and conduct a sensitivity analysis to assess whether the primary result is robust despite departure from the assumed missing data mechanism.
Acknowledgments
The following members of the Journal of Hospital Medicine Leadership team contributed to this review: Mel L. Anderson, MD; Peter Cram, MD, MBA; JoAnna K. Leyenaar, MD, PhD, MPH; Brian P. Lucas, MD, MS; Oanh Nguyen, MD, MAS; Samir S. Shah, MD, MSCE; Erin E. Shaughnessy, MD, MSHCM; and Heidi J. Sucharew, PhD.
1. Zhang N, Chen H, Elliott MR. Nonrespondent subsample multiple imputation in two-phase sampling for nonresponse. J Off Stat. 2016;32(3):769-785. https://doi.org/10.1515/jos-2016-0039
2. Zhang Y, Chen H, Zhang N. Bayesian inference for nonresponse two-phase sampling. Stat Sin. 2018;28(4):2167-2187. https://doi.org/10.5705/ss.202017.0016
3. Demirtas H, Schafer JL. On the performance of random-coefficient pattern-mixture models for non-ignorable drop-out. Stat Med. 2003;22(16):2553-2575. https://doi.org/10.1002/sim.1475
4. Davey A, Savla J. Estimating statistical power with incomplete data. Org Res Methods. 2009;12(2):320-346. https://doi.org/10.1177/1094428107300366
5. Harel O, Perkins N, Schisterman EF. The use of multiple imputation for data subject to limits of detection. Sri Lankan J Appl Stat. 2014;5(4):227. https://doi.org/10.4038/sljastats.v5i4.7792
6. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. https://doi.org/10.2307/2335739
7. Van der Heijden GJ, Donders ART, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol.2006;59(10):1102-1109. https://doi.org/10.1016/j.jclinepi.2006.01.015
8. Little RJ, Rubin DB. Statistical analysis with missing data: Wiley; 2019. Hoboken, New Jersey.
9. Little RJ, Zhang N. Subsample ignorable likelihood for regression analysis with missing data. J Royal Stat Soc. 2011;60(4):591-605. https://doi.org/10.1111/j.1467-9876.2011.00763.x
10. Little RJ, D’agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355-1360. https://doi.org/10.1056/NEJMsr1203730
11. Little RJ, Rubin DB. Single imputation methods. Statistical analysis with missing data 2002:59-74. Hoboken, New Jersey.
12. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. https://doi.org/10.1177/0962280210395740
13. Han P. Multiply robust estimation in regression analysis with missing data. J Am Stat Assoc. 2014;109(504):1159-1173. https://doi.org/10.1080/01621459.2014.880058
14. Yucel RM. State of the multiple imputation software. J Stat Softw. 2011;45(1). https://doi.org/10.18637/jss.v045.i01
Research, in the field of Hospital Medicine, often leverages data collected for reasons other than research. For example, electronic medical record data or patient satisfaction survey results can be used to answer questions that are relevant to the practice of hospital medicine. In these types of datasets, data will inevitably be missing. Such missing data can compromise our ability to draw definitive conclusions from our research study. This review introduces the concept of missing data, describes patterns and mechanisms of missing data, and discusses common approaches for the handling of missing data, including sensitivity analyses for determining how robust the results are despite assumptions made about the missing data.
CONSEQUENCES OF MISSING DATA
Missing data create a host of problems for researchers. First, missing data result in a loss of information and can diminish the power of the proposed study. Second, the irregular data complicate the analysis because many of the standard software procedures used have been developed for fully observed or “complete” data (ie, each subject has a value for all measures of interest). Finally, missing data may introduce bias due to the systematic difference between the observed and the unobserved data. For example, if men are less likely than women to complete all questions in a patient satisfaction survey when they are not satisfied, then hospital satisfaction analyses that rely on completed surveys would tend to provide biased estimates of the satisfaction males have with their care.
MINIMIZING MISSING DATA WITH STUDY DESIGN
The ideal approach to mitigating problems caused by missing data is to anticipate and incorporate strategies to minimize missing data into the study design (ie, when planning data collection protocols for prospective studies). This plan should provide strategies for minimizing nonresponse and estimating the magnitude of anticipated missing data to ensure that the study achieves sufficient strength despite the missing data.
Strategies for minimizing nonresponse include (1) informing potential study participants, at initial contact, about the implications of missing data on the ability to answer the research question; (2) collecting several phone numbers, addresses, preferred method of contact and calling times, as well as an alternative contact, in case the primary study contact is unable to be reached; (3) specifying the number of call backs, as well as the time of contact; and (4) piloting data capture questions for phrasing, clarity, and sensitivity, in order to resolve problems before initiating the study. One approach that can be used to mitigate the impact of missing data in surveys is to contact a sample of the initial nonrespondents using a more intensive follow-up approach (eg, a nonresponse to a mailed survey is followed up by a telephone call in order to conduct the survey again over the phone), and this is referred to as “nonresponse two-phase sampling.” The additional data, captured in the second phase, not only reduces the nonresponse rate but can also provide important information on the missing data mechanism.1,2 In longitudinal studies with dropouts, one can measure participants’ intent to drop out in order to evaluate how much the probability of dropping out depends on missing responses.3 One may also choose to determine the power and implications of sample size under different missing data assumptions.4
UNDERSTANDING THE REASONS FOR MISSING DATA
Different data sources are likely to have unique reasons for missing values due to the workflows involved in how the data are collected. In research involving the use of data from electronic medical records, missing data on specific diagnoses involving patients who are regularly engaged in care are often considered to be “not present” or “normal”, since clinical documentation workflows are largely governed by the concept of “documentation by exception” in which diagnoses are documented only when there is an exception to the expectation that these are not present. For example, “diabetes mellitus” is commonly documented, but “diabetes mellitus not present” is rarely documented in electronic medical records which are used for clinical care. Thus, lack of explicit documentation is likely to indicate that diabetes mellitus is, in fact, not present.
Certain variables may be missing simply because there is no quantifiable value—ie, the data do not exist. Structural missingness refers to a value that does not exist for a logical reason (eg, “What is the gender of your first child?” for those who do not have a child). Censoring, which occurs during “time to event” analysis, refers to a situation where information about a subject stops before the event of interest happens, for example, when a subject in a study involving a 30-day outcome dies at day 14. The term “limit of detection” refers to the lowest or highest level at which two distinct values can reasonably be distinguished (eg, the lower limit of detection of a C-reactive protein assay may be 1 mg/dL, so lower values might simply be reported by the lab as <1 mg/dL).5 These types of missing data require specific methods that are not discussed in this review.
These examples illustrate that approaches to dealing with missing data vary depending on what data sources are used and how data are collected. Understanding the reasons missing data are present is a necessary step in formulating a robust analytic approach to handling missing data.
MISSING DATA PATTERNS AND MECHANISMS
Missing Data Patterns
Evaluating missing data patterns provides information on the degree and complexity of the missing data problem and can aid in choosing an appropriate missing data handling method. This is because some analytic methods work well for a general pattern (nonmonotone) and other methods work for special patterns (eg, monotone, file matching). In longitudinal studies, missing data is commonly missing in a monotone pattern, where once one variable is missing then all subsequent variables are also missing for a particular subject. This occurs when a study participant is lost to follow-up. For example, a monotone missing data pattern may occur in a study that requires a series of follow-up visits for laboratory blood tests. If a patient drops out, it results in a monotone missing data pattern, as no data on blood test results are available once the patient drops out. If the patient just skips an intermediate visit but returns for the final blood test, this would show a nonmonotone missing data pattern. A file-matching pattern occurs when variables are never observed together. This pattern can occur when data from several studies are merged and some variables are not collected in all studies. For example, three studies are merged and all three collect blood pressure, but only one study collects age and only one study collects sex.
Missing Data Mechanisms
The missing data mechanism relates to the underlying reasons for missing values and the relationships between variables with and without missing data. In general, missing data can be either random or nonrandom with distinctions in randomness made by three types: (1) data missing completely at random (MCAR); (2) data missing at random (MAR); and (3) data missing not at random (MNAR).6 As with the missing data pattern, understanding the missing data mechanism can aid in selecting an appropriate approach to handling the missing data.
Data are MCAR if the missingness does not depend on any study variables, meaning that all subjects are equally likely to be missing certain data elements. When the data are MCAR, those with missing values can be viewed as a simple random sample from the complete (but never actually observed) data and can be dropped from analysis without causing bias in the results. If the values of some diagnostic tests were missing for some patients due to equipment malfunction or electricity outage, for example, then the missingness may be considered MCAR.
Data are MAR if the missingness depends on the observed characteristics but not the unobserved characteristics, meaning that the relationships observed in the data can be used to predict the occurrence of missing values. Because the “randomness” of MAR is conditional on observed characteristics, which distinguishes it from the “completely at random” type of MCAR, dropping or omitting those cases with missing values from the analysis may lead to biased results.7 In a study of quality of life (QOL) for patients with mild to moderate traumatic brain injury, if health-related QOL questions were not answered by some patients with high pain levels (even though the pain levels were recorded), the missingness of QOL may be considered as MAR. This is due to the fact that within subjects grouped by the observed characteristic of pain (that is, conditional on similar levels of pain) the missingness of QOL is the result of chance and does not depend on the values (observed or unobserved) of QOL. It follows then, that once grouped into a high (or low) pain stratum, if QOL is considered MAR, then, whether or not it is observed, is random.
Data are considered MNAR if their missingness depends on characteristics that are not observed and cannot be fully explained by the observed characteristics. Systematic differences between missing and nonmissing data exist for data that is MNAR. For example, if a survey of household income had an increased probability of missing incomes from the low-income families then the data would be considered as MNAR.
Randomness in the missing data mechanism may be ignored without affecting the inference in some circumstances.8 Both MCAR and MAR can be considered as “ignorable” in the sense that a proper method (eg, multiple imputation) may recover the missing information without modeling (ie, accounting for) the random process of the missing data mechanism (Table).9 In contrast, the MNAR mechanism requires a method that takes into account the missing data mechanism in order to make inferences about the complete (and partially unobserved) data; or in other words, a model for the missing data mechanism cannot be ignored. It is for this reason that the MNAR mechanism is often called “nonignorable”. Nonignorable missing data present a challenge to researchers because the mechanism underlying the missingness must be included in the analysis. Yet researchers rarely know what the missingness mechanism is, and the data needed to validate any putative mechanism is, in fact, missing. In cases when more than one variable is subject to missingness, researchers need to assess the missingness mechanism for each variable and tailor their approach to the specific missing data problems.9
ANALYTIC APPROACHES
There is no universally accepted standard to guide when statistical methods should be applied to account for missing data. The amount of missing data alone cannot fully assess the missing data problem; missing data patterns and mechanisms can have greater impact on research results than the proportion of missing data alone. A good statistical method for handling missing data should provide an unbiased estimate of the quantity that the investigators intend to estimate; make use of the partial information in the incomplete cases to improve efficiency (and in most cases also to reduce bias); and provide valid estimates of the standard errors, confidence intervals, and P values for statistical tests. There are generally four broadly defined classes of methods for handling missing data in clinical research: (1) the complete-case analysis, (2) single imputation methods, (3) the weighted estimating-equation approach, and (4) the model-based approach including maximum likelihood (ML) and multiple imputation (Table and Appendix).10
Since missing data mechanisms cannot be conclusively verified, it is good practice to conduct some sensitivity analyses to test the robustness of the primary results. For this purpose, pattern-mixture models provide a flexible framework for implementing sensitivity analyses to missing data assumptions and can be used to evaluate the possibility of the data being MNAR. In this framework, the missing data distribution is modeled and then incorporated into the outcome model of interest. Tipping-point analysis is a sensitivity analysis where the missing data is replaced with a range of values to determine how much the values must change for the results of the study to tip from significant to not significant. If the same general conclusions remain valid over a range of assumptions about the missing data values, then one can have greater confidence in the study conclusions.
SUMMARY AND RECOMMENDATIONS
In dealing with missing data from clinical research, clinicians and statisticians need to work together to minimize missingness at the data collection stage, document the reasons for missingness, use substantive knowledge, if possible, to assess the missing data mechanism, perform primary analysis based on a defensible missing data mechanism, and conduct a sensitivity analysis to assess whether the primary result is robust despite departure from the assumed missing data mechanism.
Acknowledgments
The following members of the Journal of Hospital Medicine Leadership team contributed to this review: Mel L. Anderson, MD; Peter Cram, MD, MBA; JoAnna K. Leyenaar, MD, PhD, MPH; Brian P. Lucas, MD, MS; Oanh Nguyen, MD, MAS; Samir S. Shah, MD, MSCE; Erin E. Shaughnessy, MD, MSHCM; and Heidi J. Sucharew, PhD.
Research, in the field of Hospital Medicine, often leverages data collected for reasons other than research. For example, electronic medical record data or patient satisfaction survey results can be used to answer questions that are relevant to the practice of hospital medicine. In these types of datasets, data will inevitably be missing. Such missing data can compromise our ability to draw definitive conclusions from our research study. This review introduces the concept of missing data, describes patterns and mechanisms of missing data, and discusses common approaches for the handling of missing data, including sensitivity analyses for determining how robust the results are despite assumptions made about the missing data.
CONSEQUENCES OF MISSING DATA
Missing data create a host of problems for researchers. First, missing data result in a loss of information and can diminish the power of the proposed study. Second, the irregular data complicate the analysis because many of the standard software procedures used have been developed for fully observed or “complete” data (ie, each subject has a value for all measures of interest). Finally, missing data may introduce bias due to the systematic difference between the observed and the unobserved data. For example, if men are less likely than women to complete all questions in a patient satisfaction survey when they are not satisfied, then hospital satisfaction analyses that rely on completed surveys would tend to provide biased estimates of the satisfaction males have with their care.
MINIMIZING MISSING DATA WITH STUDY DESIGN
The ideal approach to mitigating problems caused by missing data is to anticipate and incorporate strategies to minimize missing data into the study design (ie, when planning data collection protocols for prospective studies). This plan should provide strategies for minimizing nonresponse and estimating the magnitude of anticipated missing data to ensure that the study achieves sufficient strength despite the missing data.
Strategies for minimizing nonresponse include (1) informing potential study participants, at initial contact, about the implications of missing data on the ability to answer the research question; (2) collecting several phone numbers, addresses, preferred method of contact and calling times, as well as an alternative contact, in case the primary study contact is unable to be reached; (3) specifying the number of call backs, as well as the time of contact; and (4) piloting data capture questions for phrasing, clarity, and sensitivity, in order to resolve problems before initiating the study. One approach that can be used to mitigate the impact of missing data in surveys is to contact a sample of the initial nonrespondents using a more intensive follow-up approach (eg, a nonresponse to a mailed survey is followed up by a telephone call in order to conduct the survey again over the phone), and this is referred to as “nonresponse two-phase sampling.” The additional data, captured in the second phase, not only reduces the nonresponse rate but can also provide important information on the missing data mechanism.1,2 In longitudinal studies with dropouts, one can measure participants’ intent to drop out in order to evaluate how much the probability of dropping out depends on missing responses.3 One may also choose to determine the power and implications of sample size under different missing data assumptions.4
UNDERSTANDING THE REASONS FOR MISSING DATA
Different data sources are likely to have unique reasons for missing values due to the workflows involved in how the data are collected. In research involving the use of data from electronic medical records, missing data on specific diagnoses involving patients who are regularly engaged in care are often considered to be “not present” or “normal”, since clinical documentation workflows are largely governed by the concept of “documentation by exception” in which diagnoses are documented only when there is an exception to the expectation that these are not present. For example, “diabetes mellitus” is commonly documented, but “diabetes mellitus not present” is rarely documented in electronic medical records which are used for clinical care. Thus, lack of explicit documentation is likely to indicate that diabetes mellitus is, in fact, not present.
Certain variables may be missing simply because there is no quantifiable value—ie, the data do not exist. Structural missingness refers to a value that does not exist for a logical reason (eg, “What is the gender of your first child?” for those who do not have a child). Censoring, which occurs during “time to event” analysis, refers to a situation where information about a subject stops before the event of interest happens, for example, when a subject in a study involving a 30-day outcome dies at day 14. The term “limit of detection” refers to the lowest or highest level at which two distinct values can reasonably be distinguished (eg, the lower limit of detection of a C-reactive protein assay may be 1 mg/dL, so lower values might simply be reported by the lab as <1 mg/dL).5 These types of missing data require specific methods that are not discussed in this review.
These examples illustrate that approaches to dealing with missing data vary depending on what data sources are used and how data are collected. Understanding the reasons missing data are present is a necessary step in formulating a robust analytic approach to handling missing data.
MISSING DATA PATTERNS AND MECHANISMS
Missing Data Patterns
Evaluating missing data patterns provides information on the degree and complexity of the missing data problem and can aid in choosing an appropriate missing data handling method. This is because some analytic methods work well for a general pattern (nonmonotone) and other methods work for special patterns (eg, monotone, file matching). In longitudinal studies, missing data is commonly missing in a monotone pattern, where once one variable is missing then all subsequent variables are also missing for a particular subject. This occurs when a study participant is lost to follow-up. For example, a monotone missing data pattern may occur in a study that requires a series of follow-up visits for laboratory blood tests. If a patient drops out, it results in a monotone missing data pattern, as no data on blood test results are available once the patient drops out. If the patient just skips an intermediate visit but returns for the final blood test, this would show a nonmonotone missing data pattern. A file-matching pattern occurs when variables are never observed together. This pattern can occur when data from several studies are merged and some variables are not collected in all studies. For example, three studies are merged and all three collect blood pressure, but only one study collects age and only one study collects sex.
Missing Data Mechanisms
The missing data mechanism relates to the underlying reasons for missing values and the relationships between variables with and without missing data. In general, missing data can be either random or nonrandom with distinctions in randomness made by three types: (1) data missing completely at random (MCAR); (2) data missing at random (MAR); and (3) data missing not at random (MNAR).6 As with the missing data pattern, understanding the missing data mechanism can aid in selecting an appropriate approach to handling the missing data.
Data are MCAR if the missingness does not depend on any study variables, meaning that all subjects are equally likely to be missing certain data elements. When the data are MCAR, those with missing values can be viewed as a simple random sample from the complete (but never actually observed) data and can be dropped from analysis without causing bias in the results. If the values of some diagnostic tests were missing for some patients due to equipment malfunction or electricity outage, for example, then the missingness may be considered MCAR.
Data are MAR if the missingness depends on the observed characteristics but not the unobserved characteristics, meaning that the relationships observed in the data can be used to predict the occurrence of missing values. Because the “randomness” of MAR is conditional on observed characteristics, which distinguishes it from the “completely at random” type of MCAR, dropping or omitting those cases with missing values from the analysis may lead to biased results.7 In a study of quality of life (QOL) for patients with mild to moderate traumatic brain injury, if health-related QOL questions were not answered by some patients with high pain levels (even though the pain levels were recorded), the missingness of QOL may be considered as MAR. This is due to the fact that within subjects grouped by the observed characteristic of pain (that is, conditional on similar levels of pain) the missingness of QOL is the result of chance and does not depend on the values (observed or unobserved) of QOL. It follows then, that once grouped into a high (or low) pain stratum, if QOL is considered MAR, then, whether or not it is observed, is random.
Data are considered MNAR if their missingness depends on characteristics that are not observed and cannot be fully explained by the observed characteristics. Systematic differences between missing and nonmissing data exist for data that is MNAR. For example, if a survey of household income had an increased probability of missing incomes from the low-income families then the data would be considered as MNAR.
Randomness in the missing data mechanism may be ignored without affecting the inference in some circumstances.8 Both MCAR and MAR can be considered as “ignorable” in the sense that a proper method (eg, multiple imputation) may recover the missing information without modeling (ie, accounting for) the random process of the missing data mechanism (Table).9 In contrast, the MNAR mechanism requires a method that takes into account the missing data mechanism in order to make inferences about the complete (and partially unobserved) data; or in other words, a model for the missing data mechanism cannot be ignored. It is for this reason that the MNAR mechanism is often called “nonignorable”. Nonignorable missing data present a challenge to researchers because the mechanism underlying the missingness must be included in the analysis. Yet researchers rarely know what the missingness mechanism is, and the data needed to validate any putative mechanism is, in fact, missing. In cases when more than one variable is subject to missingness, researchers need to assess the missingness mechanism for each variable and tailor their approach to the specific missing data problems.9
ANALYTIC APPROACHES
There is no universally accepted standard to guide when statistical methods should be applied to account for missing data. The amount of missing data alone cannot fully assess the missing data problem; missing data patterns and mechanisms can have greater impact on research results than the proportion of missing data alone. A good statistical method for handling missing data should provide an unbiased estimate of the quantity that the investigators intend to estimate; make use of the partial information in the incomplete cases to improve efficiency (and in most cases also to reduce bias); and provide valid estimates of the standard errors, confidence intervals, and P values for statistical tests. There are generally four broadly defined classes of methods for handling missing data in clinical research: (1) the complete-case analysis, (2) single imputation methods, (3) the weighted estimating-equation approach, and (4) the model-based approach including maximum likelihood (ML) and multiple imputation (Table and Appendix).10
Since missing data mechanisms cannot be conclusively verified, it is good practice to conduct some sensitivity analyses to test the robustness of the primary results. For this purpose, pattern-mixture models provide a flexible framework for implementing sensitivity analyses to missing data assumptions and can be used to evaluate the possibility of the data being MNAR. In this framework, the missing data distribution is modeled and then incorporated into the outcome model of interest. Tipping-point analysis is a sensitivity analysis where the missing data is replaced with a range of values to determine how much the values must change for the results of the study to tip from significant to not significant. If the same general conclusions remain valid over a range of assumptions about the missing data values, then one can have greater confidence in the study conclusions.
SUMMARY AND RECOMMENDATIONS
In dealing with missing data from clinical research, clinicians and statisticians need to work together to minimize missingness at the data collection stage, document the reasons for missingness, use substantive knowledge, if possible, to assess the missing data mechanism, perform primary analysis based on a defensible missing data mechanism, and conduct a sensitivity analysis to assess whether the primary result is robust despite departure from the assumed missing data mechanism.
Acknowledgments
The following members of the Journal of Hospital Medicine Leadership team contributed to this review: Mel L. Anderson, MD; Peter Cram, MD, MBA; JoAnna K. Leyenaar, MD, PhD, MPH; Brian P. Lucas, MD, MS; Oanh Nguyen, MD, MAS; Samir S. Shah, MD, MSCE; Erin E. Shaughnessy, MD, MSHCM; and Heidi J. Sucharew, PhD.
1. Zhang N, Chen H, Elliott MR. Nonrespondent subsample multiple imputation in two-phase sampling for nonresponse. J Off Stat. 2016;32(3):769-785. https://doi.org/10.1515/jos-2016-0039
2. Zhang Y, Chen H, Zhang N. Bayesian inference for nonresponse two-phase sampling. Stat Sin. 2018;28(4):2167-2187. https://doi.org/10.5705/ss.202017.0016
3. Demirtas H, Schafer JL. On the performance of random-coefficient pattern-mixture models for non-ignorable drop-out. Stat Med. 2003;22(16):2553-2575. https://doi.org/10.1002/sim.1475
4. Davey A, Savla J. Estimating statistical power with incomplete data. Org Res Methods. 2009;12(2):320-346. https://doi.org/10.1177/1094428107300366
5. Harel O, Perkins N, Schisterman EF. The use of multiple imputation for data subject to limits of detection. Sri Lankan J Appl Stat. 2014;5(4):227. https://doi.org/10.4038/sljastats.v5i4.7792
6. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. https://doi.org/10.2307/2335739
7. Van der Heijden GJ, Donders ART, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol.2006;59(10):1102-1109. https://doi.org/10.1016/j.jclinepi.2006.01.015
8. Little RJ, Rubin DB. Statistical analysis with missing data: Wiley; 2019. Hoboken, New Jersey.
9. Little RJ, Zhang N. Subsample ignorable likelihood for regression analysis with missing data. J Royal Stat Soc. 2011;60(4):591-605. https://doi.org/10.1111/j.1467-9876.2011.00763.x
10. Little RJ, D’agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355-1360. https://doi.org/10.1056/NEJMsr1203730
11. Little RJ, Rubin DB. Single imputation methods. Statistical analysis with missing data 2002:59-74. Hoboken, New Jersey.
12. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. https://doi.org/10.1177/0962280210395740
13. Han P. Multiply robust estimation in regression analysis with missing data. J Am Stat Assoc. 2014;109(504):1159-1173. https://doi.org/10.1080/01621459.2014.880058
14. Yucel RM. State of the multiple imputation software. J Stat Softw. 2011;45(1). https://doi.org/10.18637/jss.v045.i01
1. Zhang N, Chen H, Elliott MR. Nonrespondent subsample multiple imputation in two-phase sampling for nonresponse. J Off Stat. 2016;32(3):769-785. https://doi.org/10.1515/jos-2016-0039
2. Zhang Y, Chen H, Zhang N. Bayesian inference for nonresponse two-phase sampling. Stat Sin. 2018;28(4):2167-2187. https://doi.org/10.5705/ss.202017.0016
3. Demirtas H, Schafer JL. On the performance of random-coefficient pattern-mixture models for non-ignorable drop-out. Stat Med. 2003;22(16):2553-2575. https://doi.org/10.1002/sim.1475
4. Davey A, Savla J. Estimating statistical power with incomplete data. Org Res Methods. 2009;12(2):320-346. https://doi.org/10.1177/1094428107300366
5. Harel O, Perkins N, Schisterman EF. The use of multiple imputation for data subject to limits of detection. Sri Lankan J Appl Stat. 2014;5(4):227. https://doi.org/10.4038/sljastats.v5i4.7792
6. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. https://doi.org/10.2307/2335739
7. Van der Heijden GJ, Donders ART, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol.2006;59(10):1102-1109. https://doi.org/10.1016/j.jclinepi.2006.01.015
8. Little RJ, Rubin DB. Statistical analysis with missing data: Wiley; 2019. Hoboken, New Jersey.
9. Little RJ, Zhang N. Subsample ignorable likelihood for regression analysis with missing data. J Royal Stat Soc. 2011;60(4):591-605. https://doi.org/10.1111/j.1467-9876.2011.00763.x
10. Little RJ, D’agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355-1360. https://doi.org/10.1056/NEJMsr1203730
11. Little RJ, Rubin DB. Single imputation methods. Statistical analysis with missing data 2002:59-74. Hoboken, New Jersey.
12. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. https://doi.org/10.1177/0962280210395740
13. Han P. Multiply robust estimation in regression analysis with missing data. J Am Stat Assoc. 2014;109(504):1159-1173. https://doi.org/10.1080/01621459.2014.880058
14. Yucel RM. State of the multiple imputation software. J Stat Softw. 2011;45(1). https://doi.org/10.18637/jss.v045.i01
© 2019 Society of Hospital Medicine
Clinical Progress Note: Addressing Prognosis in Advanced Dementia
Advanced dementia (AD) is a serious terminal illness. Some features of AD include significant memory deficits (inability to recognize family members), inability to ambulate, very limited verbal communication, and needing assistance with all activities of daily living.1 AD carries a six-month mortality of 25% and a median survival of 1.3 years.1
Despite a limited life expectancy, patients with AD face increasingly significant symptom burden and use of burdensome interventions2 near the end of life. Among the most common interventions hospitalists routinely navigate during a hospitalization is tube feeding for enteral artificial nutrition, which has not been shown to prolong survival, improve quality of life, decrease risk of aspiration pneumonia, or decrease the risk of pressure ulcers.3,4 Recent data show that rates of hospitalizations in the last 90 days of life, especially in the last three days of life, are increasing.5 These late transitions can have significant negative impact on family perceptions of quality of care, including not being treated with respect, receiving care inconsistent with goals, receiving inadequate communication about care decisions, and not being fully informed of the medical conditions.6
Therefore, hospitalization in AD, especially a readmission, indicates a critical change in a patient’s illness, marking an opportune time to have discussions on prognosis and improve care at the end of life. While determining and sharing prognosis can be challenging in the setting of many chronic diseases, resources exist to help clinicians share prognosis in AD and understand the goals of care for each patient.7 The aim of this paper is to assist hospitalists in addressing prognosis in the setting of AD. We identify and present key knowledge and recommendations from relevant articles identified from a hand-search of articles, published in 2018, from leading palliative care journals, as well as a MEDLINE search from 2003 through December 2018 using the key words “dementia” and “prognosis.” Final presented articles and recommendations were determined based on scientific rigor and relevance to hospital-based care of patients with AD.
IMPORTANCE OF PROGNOSIS DISCUSSIONS IN ADVANCED DEMENTIA
For a myriad of reasons, most AD caregivers do not receive adequate information on the complications of dementia or prognosis.2 Conversations that provide prognostic estimates and aim to understand the goals, preferences, and values of AD patients and their surrogates can help in providing goal-concordant care. A prospective study of nursing-home patients with AD showed that having goals of care discussions was strongly associated with surrogates’ likelihood of estimating a life expectancy of less than six months in AD patients.8 Having this perception was associated with a lower likelihood of patients with AD undergoing burdensome interventions such as hospitalizations, parenteral therapy, venipuncture, feeding tube, or urinary catheterization.8 To help improve goal-concordant care, it is important that hospitalists be prepared to have prognostic conversations with patients and their caregivers.
“FORESEEING” PROGNOSIS IN ADVANCED DEMENTIA
Offering a clinical prognosis involves components of foreseeing (estimating prognosis) and foretelling (sharing prognosis).9 Foreseeing prognosis in AD can be complex due to the highly variable but slow, dwindling clinical course of AD. As a practical matter, determining if a patient has a six-month prognosis is most helpful as eligibility for hospice services may allow for a discharge to a supportive home setting instead of a transfer to an institution.10 An evidence-based clinical prediction rule, the Advanced Dementia Prognostic Tool (ADEPT, Appendix Table),11 can be used to estimate prognosis by a composite of 12 risk factors in nursing-home patients. Although the consensus-based National Hospice and Palliative Care Organization (NHPCO) guidelines for Medicare hospice eligibility12 (Table) do not perform well in predicting individual mortality, they are used as criteria for hospice enrollment. Given the variability of course of AD, evaluating the mortality risk for acute illnesses leading to hospitalization, like pneumonia or hip fracture, can further help estimate prognosis. The website, www.eprognosis.com, combines various prediction tools to help estimate prognosis. Although using these tools can often help clinicians satisfy the entry requirements to offer hospice, the ADEPT tool and the NHPCO criteria both perform poorly in discriminating those who will or will not actually die in six months. ADEPT, as a prognostic tool, has not yet been validated for community-dwelling patients. Clinicians should exercise caution in making a highly specific estimate of survival in AD, but can and should communicate the expected decline in function over time.
“FORETELLING” PROGNOSIS IN ADVANCED DEMENTIA
Having goals of care conversations and sharing prognosis has many benefits. A large multistate cohort study showed that having goals of care conversations among patients with terminal cancer was associated with less use of intensive care units, mechanical ventilation, and cardio-pulmonary resuscitation.13 Caregivers may also benefit from prognosis discussions through identifying resources to care for the patient at home and by potentially limiting their risk of major depressive order and regret, common among those witnessing patients undergoing aggressive treatment at the end of life.13 How to share prognosis can be challenging; however, tools such as the Serious Illness Conversation Guide14 can provide step-by-step guidance for providers. The key aspects of the guide are asking permission, assessing illness understanding, and exploring goals, fears, worries, and tradeoffs.
Before exploring goals, it is helpful to explain the serious illness by using “I wish,” “I worry,” and “I wonder” statements such as “I wish I had better news for you; your mom’s dementia has progressed given the recent complication of aspiration,” “I worry that she will not be able to eat on her own and will develop another serious infection very soon,” or “I wonder whether it is a good time to talk about what your mom would want if she cannot eat on her own.”
An example of an effective conversation about artificial nutrition and hydration with a surrogate of a patient with AD with recurrent aspirations may include the following elements:14,15
- Obtain the caregiver’s and/or patient’s perception of illness: “Is it OK if we have a conversation about what may lie ahead with your mother? Is there anyone else that should be present? What is your understanding of your mother’s illness?”
- Give relevant data: “Based on her current level of decline with complications and repetitive hospitalizations, I am worried that her life expectancy is likely measured in months rather than years.”
- Address emotions: “This must be very hard to hear. I cannot imagine how difficult it must be to see her in the hospital so often.”
- Elicit concerns and goals based on understanding key values: “Tell me what you are hoping for regarding your mother’s future care and what worries you have. Tell me what your mother would say if she could fully understand her current situation.”
- Present goals based on patient and caregiver values: “Based on what you have told me about your mother, she valued her interactions with family and her independence, and she would not want measures that would cause distress, especially when facing a terminal illness.”
- Be mindful of prognostic uncertainty: “While we cannot know for certain what will happen next, I am very worried that your mother will continue to aspirate even with a feeding tube.”
- Make a recommendation with permission: “From our conversation, I have an idea of what treatment might make sense to your mom. May I share my recommendation with you?” If they are willing, you might say: “As evidence shows that feeding tubes do not improve the level of family interaction or independence in patients with dementia and as your mother would not want any distressing procedures, I recommend that we do not place a feeding-tube.”
- Balance realism and hope: “Instead, we can focus on other ways to maintain dignity and quality of life for her even without a feeding tube.”
RESPONDING TO CHALLENGES
Conversations about goals of care and prognosis can be challenging and time consuming. At times, the conversations can be strained. The following tips are based on authors’ shared experiences to help in those challenging situations:
- Caregivers may show signs of emotional and/or cognitive strain: Recognize and name the emotional response and consider asking the family if they need a break to avoid overtaxing them.
“I can see that this is very difficult for you. Do you want to take a break and meet again?”
- Caregivers may have unrealistic hopes: Confirm the caregivers’ understanding of the situation, before assuming their hope is unrealistic. Try to reframe what they/we can hope for by validating their goals while avoiding unnecessary burdens or discomfort.
“I want to be sure that I have explained your mom’s situation clearly. Can you tell me in your words, what I have told you?” as this gives you an opportunity to clarify misunderstandings that may manifest as “false hope”.
“Together we can hope for the best and see if your mother can tolerate hand-feeding safely without causing any harm or distress.”
- Avoid assumptions about cultural and religious beliefs: Be curious and demonstrate cultural humility to all patients.
“Are there any cultural or spiritual beliefs that are important to you or your mother?”
- Avoid spending too much time on clinical details: Give families time to share stories about the patient in better days as this gives you an opportunity to get to know the patient.
“Tell me more about what your mother was like when she was healthy.”
- Listen first, recommend second: Refrain from making recommendations about the patient’s care before you understand his/her values and preferences.
“What would your mother say is most important to her as her health worsens?”
- Use active listening techniques: Using reflection statements can confirm your understanding of the caregiver’s view point.
“So, I hear that your mother valued being at home and being comfortable. Is that correct?”
These conversations are often an iterative process of helping the patient and family traverse the course of AD. Therefore, starting the process even during a hospitalization earlier in the course of AD can help engage in preparedness planning to provide goal-concordant care and help optimize the patient’s quality of life.
CONCLUSION
Hospitalization among patients with AD can signal a significant change in prognosis and represents an important opportunity for further dialogue. A patient- and caregiver-centered conversation, sharing prognosis and learning about values important to the patient and family, has the potential to lead to less burdensome interventions. Doing so can minimize harm, promote quality of life, and reduce unnecessary care transitions near the end of life.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Havyer was supported, in part, by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics grant. Dr. Abedini was supported by the National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI.
1. Mitchell SL. Advanced dementia. N Engl J Med. 2015;373(13):1276-1277. https://doi.org/10.1056/NEJMcp1412652.
2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. https://doi.org/10.1056/NEJMoa0902234.
3. Teno JM, Gozalo PL, Mitchell SL, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60(10):1918-1921. https://doi.org/10.1111/j.1532-5415.2012.04148.x.
4. Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding tubes and the prevention or healing of pressure ulcers. Arch Intern Med. 2012;172(9):697-701. https://doi.org/10.1001/archinternmed.2012.1200.
5. Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. https://doi.org/10.1001/jama.2012.207624.
6. Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late transitions and bereaved family member perceptions of quality of end-of-life care. J Am Geriatr Soc. 2018;66(9):1730-1736. https://doi.org/10.1111/jgs.15455.
7. Ansari AA, Pomerantz DH, Jayes RL, Aguirre EA, Havyer RD. Promoting primary palliative care in severe chronic obstructive pulmonary disease: symptom management and preparedness planning. J Palliat Care. 2019;34(2):85-91. https://doi.org/10.1177/0825859718819437.
8. Loizeau AJ, Shaffer ML, Habtemariam DA, Hanson LC, Volandes AE, Mitchell SL. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922-929. https://doi.org/10.1001/jamainternmed.2018.1413.
9. Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med. 2008;11(1):84-103. https://doi.org/10.1089/jpm.2008.9992.
10. Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44(5):750-756. https://doi.org/10.1016/j.jpainsymman.2012.08.004.
11. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929-1935. https://doi.org/10.1001/jama.2010.1572.
12. Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Amer J Hospice & Pall Care. 2003;20(2):105-113. https://doi.org/10.1177/104990910302000208
13. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. https://doi.org/10.1001/jama.300.14.1665.
14. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032-2015-009032. https://doi.org/10.1136/bmjopen-2015-009032
15. Ansari A, Pomerantz D, Smith K. Being mindful: difficult decisions in advanced dementia and end stage renal disease. SGIM Forum. 2017;40(3):4, 13.
Advanced dementia (AD) is a serious terminal illness. Some features of AD include significant memory deficits (inability to recognize family members), inability to ambulate, very limited verbal communication, and needing assistance with all activities of daily living.1 AD carries a six-month mortality of 25% and a median survival of 1.3 years.1
Despite a limited life expectancy, patients with AD face increasingly significant symptom burden and use of burdensome interventions2 near the end of life. Among the most common interventions hospitalists routinely navigate during a hospitalization is tube feeding for enteral artificial nutrition, which has not been shown to prolong survival, improve quality of life, decrease risk of aspiration pneumonia, or decrease the risk of pressure ulcers.3,4 Recent data show that rates of hospitalizations in the last 90 days of life, especially in the last three days of life, are increasing.5 These late transitions can have significant negative impact on family perceptions of quality of care, including not being treated with respect, receiving care inconsistent with goals, receiving inadequate communication about care decisions, and not being fully informed of the medical conditions.6
Therefore, hospitalization in AD, especially a readmission, indicates a critical change in a patient’s illness, marking an opportune time to have discussions on prognosis and improve care at the end of life. While determining and sharing prognosis can be challenging in the setting of many chronic diseases, resources exist to help clinicians share prognosis in AD and understand the goals of care for each patient.7 The aim of this paper is to assist hospitalists in addressing prognosis in the setting of AD. We identify and present key knowledge and recommendations from relevant articles identified from a hand-search of articles, published in 2018, from leading palliative care journals, as well as a MEDLINE search from 2003 through December 2018 using the key words “dementia” and “prognosis.” Final presented articles and recommendations were determined based on scientific rigor and relevance to hospital-based care of patients with AD.
IMPORTANCE OF PROGNOSIS DISCUSSIONS IN ADVANCED DEMENTIA
For a myriad of reasons, most AD caregivers do not receive adequate information on the complications of dementia or prognosis.2 Conversations that provide prognostic estimates and aim to understand the goals, preferences, and values of AD patients and their surrogates can help in providing goal-concordant care. A prospective study of nursing-home patients with AD showed that having goals of care discussions was strongly associated with surrogates’ likelihood of estimating a life expectancy of less than six months in AD patients.8 Having this perception was associated with a lower likelihood of patients with AD undergoing burdensome interventions such as hospitalizations, parenteral therapy, venipuncture, feeding tube, or urinary catheterization.8 To help improve goal-concordant care, it is important that hospitalists be prepared to have prognostic conversations with patients and their caregivers.
“FORESEEING” PROGNOSIS IN ADVANCED DEMENTIA
Offering a clinical prognosis involves components of foreseeing (estimating prognosis) and foretelling (sharing prognosis).9 Foreseeing prognosis in AD can be complex due to the highly variable but slow, dwindling clinical course of AD. As a practical matter, determining if a patient has a six-month prognosis is most helpful as eligibility for hospice services may allow for a discharge to a supportive home setting instead of a transfer to an institution.10 An evidence-based clinical prediction rule, the Advanced Dementia Prognostic Tool (ADEPT, Appendix Table),11 can be used to estimate prognosis by a composite of 12 risk factors in nursing-home patients. Although the consensus-based National Hospice and Palliative Care Organization (NHPCO) guidelines for Medicare hospice eligibility12 (Table) do not perform well in predicting individual mortality, they are used as criteria for hospice enrollment. Given the variability of course of AD, evaluating the mortality risk for acute illnesses leading to hospitalization, like pneumonia or hip fracture, can further help estimate prognosis. The website, www.eprognosis.com, combines various prediction tools to help estimate prognosis. Although using these tools can often help clinicians satisfy the entry requirements to offer hospice, the ADEPT tool and the NHPCO criteria both perform poorly in discriminating those who will or will not actually die in six months. ADEPT, as a prognostic tool, has not yet been validated for community-dwelling patients. Clinicians should exercise caution in making a highly specific estimate of survival in AD, but can and should communicate the expected decline in function over time.
“FORETELLING” PROGNOSIS IN ADVANCED DEMENTIA
Having goals of care conversations and sharing prognosis has many benefits. A large multistate cohort study showed that having goals of care conversations among patients with terminal cancer was associated with less use of intensive care units, mechanical ventilation, and cardio-pulmonary resuscitation.13 Caregivers may also benefit from prognosis discussions through identifying resources to care for the patient at home and by potentially limiting their risk of major depressive order and regret, common among those witnessing patients undergoing aggressive treatment at the end of life.13 How to share prognosis can be challenging; however, tools such as the Serious Illness Conversation Guide14 can provide step-by-step guidance for providers. The key aspects of the guide are asking permission, assessing illness understanding, and exploring goals, fears, worries, and tradeoffs.
Before exploring goals, it is helpful to explain the serious illness by using “I wish,” “I worry,” and “I wonder” statements such as “I wish I had better news for you; your mom’s dementia has progressed given the recent complication of aspiration,” “I worry that she will not be able to eat on her own and will develop another serious infection very soon,” or “I wonder whether it is a good time to talk about what your mom would want if she cannot eat on her own.”
An example of an effective conversation about artificial nutrition and hydration with a surrogate of a patient with AD with recurrent aspirations may include the following elements:14,15
- Obtain the caregiver’s and/or patient’s perception of illness: “Is it OK if we have a conversation about what may lie ahead with your mother? Is there anyone else that should be present? What is your understanding of your mother’s illness?”
- Give relevant data: “Based on her current level of decline with complications and repetitive hospitalizations, I am worried that her life expectancy is likely measured in months rather than years.”
- Address emotions: “This must be very hard to hear. I cannot imagine how difficult it must be to see her in the hospital so often.”
- Elicit concerns and goals based on understanding key values: “Tell me what you are hoping for regarding your mother’s future care and what worries you have. Tell me what your mother would say if she could fully understand her current situation.”
- Present goals based on patient and caregiver values: “Based on what you have told me about your mother, she valued her interactions with family and her independence, and she would not want measures that would cause distress, especially when facing a terminal illness.”
- Be mindful of prognostic uncertainty: “While we cannot know for certain what will happen next, I am very worried that your mother will continue to aspirate even with a feeding tube.”
- Make a recommendation with permission: “From our conversation, I have an idea of what treatment might make sense to your mom. May I share my recommendation with you?” If they are willing, you might say: “As evidence shows that feeding tubes do not improve the level of family interaction or independence in patients with dementia and as your mother would not want any distressing procedures, I recommend that we do not place a feeding-tube.”
- Balance realism and hope: “Instead, we can focus on other ways to maintain dignity and quality of life for her even without a feeding tube.”
RESPONDING TO CHALLENGES
Conversations about goals of care and prognosis can be challenging and time consuming. At times, the conversations can be strained. The following tips are based on authors’ shared experiences to help in those challenging situations:
- Caregivers may show signs of emotional and/or cognitive strain: Recognize and name the emotional response and consider asking the family if they need a break to avoid overtaxing them.
“I can see that this is very difficult for you. Do you want to take a break and meet again?”
- Caregivers may have unrealistic hopes: Confirm the caregivers’ understanding of the situation, before assuming their hope is unrealistic. Try to reframe what they/we can hope for by validating their goals while avoiding unnecessary burdens or discomfort.
“I want to be sure that I have explained your mom’s situation clearly. Can you tell me in your words, what I have told you?” as this gives you an opportunity to clarify misunderstandings that may manifest as “false hope”.
“Together we can hope for the best and see if your mother can tolerate hand-feeding safely without causing any harm or distress.”
- Avoid assumptions about cultural and religious beliefs: Be curious and demonstrate cultural humility to all patients.
“Are there any cultural or spiritual beliefs that are important to you or your mother?”
- Avoid spending too much time on clinical details: Give families time to share stories about the patient in better days as this gives you an opportunity to get to know the patient.
“Tell me more about what your mother was like when she was healthy.”
- Listen first, recommend second: Refrain from making recommendations about the patient’s care before you understand his/her values and preferences.
“What would your mother say is most important to her as her health worsens?”
- Use active listening techniques: Using reflection statements can confirm your understanding of the caregiver’s view point.
“So, I hear that your mother valued being at home and being comfortable. Is that correct?”
These conversations are often an iterative process of helping the patient and family traverse the course of AD. Therefore, starting the process even during a hospitalization earlier in the course of AD can help engage in preparedness planning to provide goal-concordant care and help optimize the patient’s quality of life.
CONCLUSION
Hospitalization among patients with AD can signal a significant change in prognosis and represents an important opportunity for further dialogue. A patient- and caregiver-centered conversation, sharing prognosis and learning about values important to the patient and family, has the potential to lead to less burdensome interventions. Doing so can minimize harm, promote quality of life, and reduce unnecessary care transitions near the end of life.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Havyer was supported, in part, by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics grant. Dr. Abedini was supported by the National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI.
Advanced dementia (AD) is a serious terminal illness. Some features of AD include significant memory deficits (inability to recognize family members), inability to ambulate, very limited verbal communication, and needing assistance with all activities of daily living.1 AD carries a six-month mortality of 25% and a median survival of 1.3 years.1
Despite a limited life expectancy, patients with AD face increasingly significant symptom burden and use of burdensome interventions2 near the end of life. Among the most common interventions hospitalists routinely navigate during a hospitalization is tube feeding for enteral artificial nutrition, which has not been shown to prolong survival, improve quality of life, decrease risk of aspiration pneumonia, or decrease the risk of pressure ulcers.3,4 Recent data show that rates of hospitalizations in the last 90 days of life, especially in the last three days of life, are increasing.5 These late transitions can have significant negative impact on family perceptions of quality of care, including not being treated with respect, receiving care inconsistent with goals, receiving inadequate communication about care decisions, and not being fully informed of the medical conditions.6
Therefore, hospitalization in AD, especially a readmission, indicates a critical change in a patient’s illness, marking an opportune time to have discussions on prognosis and improve care at the end of life. While determining and sharing prognosis can be challenging in the setting of many chronic diseases, resources exist to help clinicians share prognosis in AD and understand the goals of care for each patient.7 The aim of this paper is to assist hospitalists in addressing prognosis in the setting of AD. We identify and present key knowledge and recommendations from relevant articles identified from a hand-search of articles, published in 2018, from leading palliative care journals, as well as a MEDLINE search from 2003 through December 2018 using the key words “dementia” and “prognosis.” Final presented articles and recommendations were determined based on scientific rigor and relevance to hospital-based care of patients with AD.
IMPORTANCE OF PROGNOSIS DISCUSSIONS IN ADVANCED DEMENTIA
For a myriad of reasons, most AD caregivers do not receive adequate information on the complications of dementia or prognosis.2 Conversations that provide prognostic estimates and aim to understand the goals, preferences, and values of AD patients and their surrogates can help in providing goal-concordant care. A prospective study of nursing-home patients with AD showed that having goals of care discussions was strongly associated with surrogates’ likelihood of estimating a life expectancy of less than six months in AD patients.8 Having this perception was associated with a lower likelihood of patients with AD undergoing burdensome interventions such as hospitalizations, parenteral therapy, venipuncture, feeding tube, or urinary catheterization.8 To help improve goal-concordant care, it is important that hospitalists be prepared to have prognostic conversations with patients and their caregivers.
“FORESEEING” PROGNOSIS IN ADVANCED DEMENTIA
Offering a clinical prognosis involves components of foreseeing (estimating prognosis) and foretelling (sharing prognosis).9 Foreseeing prognosis in AD can be complex due to the highly variable but slow, dwindling clinical course of AD. As a practical matter, determining if a patient has a six-month prognosis is most helpful as eligibility for hospice services may allow for a discharge to a supportive home setting instead of a transfer to an institution.10 An evidence-based clinical prediction rule, the Advanced Dementia Prognostic Tool (ADEPT, Appendix Table),11 can be used to estimate prognosis by a composite of 12 risk factors in nursing-home patients. Although the consensus-based National Hospice and Palliative Care Organization (NHPCO) guidelines for Medicare hospice eligibility12 (Table) do not perform well in predicting individual mortality, they are used as criteria for hospice enrollment. Given the variability of course of AD, evaluating the mortality risk for acute illnesses leading to hospitalization, like pneumonia or hip fracture, can further help estimate prognosis. The website, www.eprognosis.com, combines various prediction tools to help estimate prognosis. Although using these tools can often help clinicians satisfy the entry requirements to offer hospice, the ADEPT tool and the NHPCO criteria both perform poorly in discriminating those who will or will not actually die in six months. ADEPT, as a prognostic tool, has not yet been validated for community-dwelling patients. Clinicians should exercise caution in making a highly specific estimate of survival in AD, but can and should communicate the expected decline in function over time.
“FORETELLING” PROGNOSIS IN ADVANCED DEMENTIA
Having goals of care conversations and sharing prognosis has many benefits. A large multistate cohort study showed that having goals of care conversations among patients with terminal cancer was associated with less use of intensive care units, mechanical ventilation, and cardio-pulmonary resuscitation.13 Caregivers may also benefit from prognosis discussions through identifying resources to care for the patient at home and by potentially limiting their risk of major depressive order and regret, common among those witnessing patients undergoing aggressive treatment at the end of life.13 How to share prognosis can be challenging; however, tools such as the Serious Illness Conversation Guide14 can provide step-by-step guidance for providers. The key aspects of the guide are asking permission, assessing illness understanding, and exploring goals, fears, worries, and tradeoffs.
Before exploring goals, it is helpful to explain the serious illness by using “I wish,” “I worry,” and “I wonder” statements such as “I wish I had better news for you; your mom’s dementia has progressed given the recent complication of aspiration,” “I worry that she will not be able to eat on her own and will develop another serious infection very soon,” or “I wonder whether it is a good time to talk about what your mom would want if she cannot eat on her own.”
An example of an effective conversation about artificial nutrition and hydration with a surrogate of a patient with AD with recurrent aspirations may include the following elements:14,15
- Obtain the caregiver’s and/or patient’s perception of illness: “Is it OK if we have a conversation about what may lie ahead with your mother? Is there anyone else that should be present? What is your understanding of your mother’s illness?”
- Give relevant data: “Based on her current level of decline with complications and repetitive hospitalizations, I am worried that her life expectancy is likely measured in months rather than years.”
- Address emotions: “This must be very hard to hear. I cannot imagine how difficult it must be to see her in the hospital so often.”
- Elicit concerns and goals based on understanding key values: “Tell me what you are hoping for regarding your mother’s future care and what worries you have. Tell me what your mother would say if she could fully understand her current situation.”
- Present goals based on patient and caregiver values: “Based on what you have told me about your mother, she valued her interactions with family and her independence, and she would not want measures that would cause distress, especially when facing a terminal illness.”
- Be mindful of prognostic uncertainty: “While we cannot know for certain what will happen next, I am very worried that your mother will continue to aspirate even with a feeding tube.”
- Make a recommendation with permission: “From our conversation, I have an idea of what treatment might make sense to your mom. May I share my recommendation with you?” If they are willing, you might say: “As evidence shows that feeding tubes do not improve the level of family interaction or independence in patients with dementia and as your mother would not want any distressing procedures, I recommend that we do not place a feeding-tube.”
- Balance realism and hope: “Instead, we can focus on other ways to maintain dignity and quality of life for her even without a feeding tube.”
RESPONDING TO CHALLENGES
Conversations about goals of care and prognosis can be challenging and time consuming. At times, the conversations can be strained. The following tips are based on authors’ shared experiences to help in those challenging situations:
- Caregivers may show signs of emotional and/or cognitive strain: Recognize and name the emotional response and consider asking the family if they need a break to avoid overtaxing them.
“I can see that this is very difficult for you. Do you want to take a break and meet again?”
- Caregivers may have unrealistic hopes: Confirm the caregivers’ understanding of the situation, before assuming their hope is unrealistic. Try to reframe what they/we can hope for by validating their goals while avoiding unnecessary burdens or discomfort.
“I want to be sure that I have explained your mom’s situation clearly. Can you tell me in your words, what I have told you?” as this gives you an opportunity to clarify misunderstandings that may manifest as “false hope”.
“Together we can hope for the best and see if your mother can tolerate hand-feeding safely without causing any harm or distress.”
- Avoid assumptions about cultural and religious beliefs: Be curious and demonstrate cultural humility to all patients.
“Are there any cultural or spiritual beliefs that are important to you or your mother?”
- Avoid spending too much time on clinical details: Give families time to share stories about the patient in better days as this gives you an opportunity to get to know the patient.
“Tell me more about what your mother was like when she was healthy.”
- Listen first, recommend second: Refrain from making recommendations about the patient’s care before you understand his/her values and preferences.
“What would your mother say is most important to her as her health worsens?”
- Use active listening techniques: Using reflection statements can confirm your understanding of the caregiver’s view point.
“So, I hear that your mother valued being at home and being comfortable. Is that correct?”
These conversations are often an iterative process of helping the patient and family traverse the course of AD. Therefore, starting the process even during a hospitalization earlier in the course of AD can help engage in preparedness planning to provide goal-concordant care and help optimize the patient’s quality of life.
CONCLUSION
Hospitalization among patients with AD can signal a significant change in prognosis and represents an important opportunity for further dialogue. A patient- and caregiver-centered conversation, sharing prognosis and learning about values important to the patient and family, has the potential to lead to less burdensome interventions. Doing so can minimize harm, promote quality of life, and reduce unnecessary care transitions near the end of life.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Havyer was supported, in part, by the Mayo Clinic Department of Medicine Catalyst for Advancing in Academics grant. Dr. Abedini was supported by the National Clinician Scholars Program at the Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI.
1. Mitchell SL. Advanced dementia. N Engl J Med. 2015;373(13):1276-1277. https://doi.org/10.1056/NEJMcp1412652.
2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. https://doi.org/10.1056/NEJMoa0902234.
3. Teno JM, Gozalo PL, Mitchell SL, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60(10):1918-1921. https://doi.org/10.1111/j.1532-5415.2012.04148.x.
4. Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding tubes and the prevention or healing of pressure ulcers. Arch Intern Med. 2012;172(9):697-701. https://doi.org/10.1001/archinternmed.2012.1200.
5. Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. https://doi.org/10.1001/jama.2012.207624.
6. Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late transitions and bereaved family member perceptions of quality of end-of-life care. J Am Geriatr Soc. 2018;66(9):1730-1736. https://doi.org/10.1111/jgs.15455.
7. Ansari AA, Pomerantz DH, Jayes RL, Aguirre EA, Havyer RD. Promoting primary palliative care in severe chronic obstructive pulmonary disease: symptom management and preparedness planning. J Palliat Care. 2019;34(2):85-91. https://doi.org/10.1177/0825859718819437.
8. Loizeau AJ, Shaffer ML, Habtemariam DA, Hanson LC, Volandes AE, Mitchell SL. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922-929. https://doi.org/10.1001/jamainternmed.2018.1413.
9. Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med. 2008;11(1):84-103. https://doi.org/10.1089/jpm.2008.9992.
10. Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44(5):750-756. https://doi.org/10.1016/j.jpainsymman.2012.08.004.
11. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929-1935. https://doi.org/10.1001/jama.2010.1572.
12. Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Amer J Hospice & Pall Care. 2003;20(2):105-113. https://doi.org/10.1177/104990910302000208
13. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. https://doi.org/10.1001/jama.300.14.1665.
14. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032-2015-009032. https://doi.org/10.1136/bmjopen-2015-009032
15. Ansari A, Pomerantz D, Smith K. Being mindful: difficult decisions in advanced dementia and end stage renal disease. SGIM Forum. 2017;40(3):4, 13.
1. Mitchell SL. Advanced dementia. N Engl J Med. 2015;373(13):1276-1277. https://doi.org/10.1056/NEJMcp1412652.
2. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529-1538. https://doi.org/10.1056/NEJMoa0902234.
3. Teno JM, Gozalo PL, Mitchell SL, et al. Does feeding tube insertion and its timing improve survival? J Am Geriatr Soc. 2012;60(10):1918-1921. https://doi.org/10.1111/j.1532-5415.2012.04148.x.
4. Teno JM, Gozalo P, Mitchell SL, Kuo S, Fulton AT, Mor V. Feeding tubes and the prevention or healing of pressure ulcers. Arch Intern Med. 2012;172(9):697-701. https://doi.org/10.1001/archinternmed.2012.1200.
5. Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. https://doi.org/10.1001/jama.2012.207624.
6. Makaroun LK, Teno JM, Freedman VA, Kasper JD, Gozalo P, Mor V. Late transitions and bereaved family member perceptions of quality of end-of-life care. J Am Geriatr Soc. 2018;66(9):1730-1736. https://doi.org/10.1111/jgs.15455.
7. Ansari AA, Pomerantz DH, Jayes RL, Aguirre EA, Havyer RD. Promoting primary palliative care in severe chronic obstructive pulmonary disease: symptom management and preparedness planning. J Palliat Care. 2019;34(2):85-91. https://doi.org/10.1177/0825859718819437.
8. Loizeau AJ, Shaffer ML, Habtemariam DA, Hanson LC, Volandes AE, Mitchell SL. Association of prognostic estimates with burdensome interventions in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178(7):922-929. https://doi.org/10.1001/jamainternmed.2018.1413.
9. Glare PA, Sinclair CT. Palliative medicine review: Prognostication. J Palliat Med. 2008;11(1):84-103. https://doi.org/10.1089/jpm.2008.9992.
10. Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44(5):750-756. https://doi.org/10.1016/j.jpainsymman.2012.08.004.
11. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304(17):1929-1935. https://doi.org/10.1001/jama.2010.1572.
12. Schonwetter RS, Han B, Small BJ, et al. Predictors of six-month survival among patients with dementia: an evaluation of hospice Medicare guidelines. Amer J Hospice & Pall Care. 2003;20(2):105-113. https://doi.org/10.1177/104990910302000208
13. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. https://doi.org/10.1001/jama.300.14.1665.
14. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: a randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5(10):e009032-2015-009032. https://doi.org/10.1136/bmjopen-2015-009032
15. Ansari A, Pomerantz D, Smith K. Being mindful: difficult decisions in advanced dementia and end stage renal disease. SGIM Forum. 2017;40(3):4, 13.
© 2019 Society of Hospital Medicine
Clinical Progress Note: Procalcitonin in the Management of Pediatric Lower Respiratory Tract Infection
Procalcitonin (PCT) is a biomarker that has shown promise to identify bacterial etiology in acute infections, including bacterial lower respiratory tract infection (LRTI). In 2017, the United States Food and Drug Administration (FDA) approved the use of PCT as a diagnostic aid to guide the decisions around antibiotic therapy in acute LRTI.1 Although most of the data supporting the use of PCT for LRTI stems from adult studies, the high disease burden, predominance of viral etiologies, and frequent diagnostic uncertainty resulting in antibiotic overuse make pediatric LRTI an ideal target for the use of PCT as a diagnostic aid. This review evaluates and summarizes the current evidence regarding the role of PCT in the clinical care of pediatric LRTI, including its use in guiding antibiotic use and prognosticating disease severity.
THE ROLE OF PROCALCITONIN IN GUIDING INITIATION OF ANTIBIOTICS
The commonly used PCT cut points for withholding or stopping antibiotics in adults and children are 0.1 µg/L (very low risk of bacterial etiology) or 0.25 µg/L (low risk of bacterial etiology).2-4 Among the 532 children enrolled in the multicenter study of Etiology of Pneumonia in the Community (EPIC), a PCT threshold of 0.25 µg/L demonstrated an approximate sensitivity of 85%, specificity of 45%, positive likelihood ratio of 1.55, and negative likelihood ratio of 0.33 for community acquired pneumonia (CAP) caused by typical bacterial pathogens.5 Lowering the cutoff to <0.1 µg/L increased PCT sensitivity to 100%, decreased specificity, positive likelihood ratio, and negative likelihood ratio to 20%, 1.26, and 0, respectively. Although the EPIC study obtained culture and performed PCR testing on any blood sample, pleural fluid specimen, endotracheal aspirate, or bronchoalveolar–lavage specimens obtained during the study period, currently available laboratory methods show poor sensitivity for defining bacterial LRTI. Thus, bacterial etiologies may have been underestimated. The highly negative predictive value demonstrated in this study highlights the potential of PCT as a biomarker for ruling out bacterial diseases, including LRTI.
Multiple studies have evaluated the potential utility of PCT in guiding antibiotic initiation in adults with LRTI, but data on pediatric patients are sparse.4 In a randomized, single-center Italian study comparing a PCT-guided algorithm (withholding antibiotics when PCT < 0.25 µg/L) versus usual care among 319 hospitalized children with pneumonia, the PCT group experienced fewer antibiotic initiations (15.5% vs 100%, P < .05) without significant differences in recurrence of respiratory symptoms or new antibiotic prescriptions in the month following enrollment.2
A similar randomized trial using a PCT-guided algorithm for the initiation of antibiotics conducted among 337 Swiss children presented to the emergency department (ED) with pneumonia and other LRTIs failed to demonstrate decreases in antibiotic initiation.3 This study used an algorithm that categorized the likelihood of requiring antibiotic treatment for bacterial LRTI as “definitely” if PCT was >0.5 µg/L, “probably” if PCT was 0.26–0.5 µg/L, “probably not” if PCT was 0.1–0.25 µg/L, and “definitely not” if PCT was <0.1 µg/L. In the PCT group, 104 out of 168 (62%) patients received antibiotics within 14 days compared with 93 out of 165 (56%) patients in the control group (odds ratio [OR]: 1.26, 95% CI: 0.81, 1.95). In the subgroup analyses, the odds of administering antibiotics to those with nonpneumonia LRTI was significantly higher than those of the PCT group and control group (OR: 4.09, 95% CI: 1.8, 9.93); the odds of receiving antibiotics also showed no difference in the subgroup of children with pneumonia (OR: 0.66, 95% CI: 0.35, 1.23).
The benefit of PCT for informing decisions around the initiation of antibiotics likely varies based on perceived risk of bacterial diseases. When the pretest probability of bacterial disease is extremely high, the use of PCT is unlikely to alter treatment decisions. Similarly, PCT should not be used in situations where the pretest probability for bacterial pneumonia is very low—in these instances, an elevated PCT may lead to unnecessary antibiotic use among children presenting to the ED. However, the risk of bacterial pneumonia is often equivocal, and in these situations, PCT may provide clinicians with useful insights, primarily for ruling out bacterial disease.
THE ROLE OF PROCALCITONIN IN GUIDING DISCONTINUATION OF ANTIBIOTICS
In the study by Esposito et al., the PCT levels were additionally measured every two days until discharge and during two scheduled follow-up visits; the antibiotics were discontinued when PCT < 0.25 µg/L.2 The PCT-guided group experienced shorter antibiotic duration (mean 5.4 vs 11.0 days, P < .05), shorter length of hospital stay (mean 4.7 vs 5.61 days for mild LRTI and 5.01 vs 5.93 for severe LRTI), and fewer antibiotic-related adverse events (3.9% vs 25.2%, P < .05). Similarly, in the study by Baer et al., the PCT-guided group had PCT levels repeated on days three and five after enrollment, and the antibiotics were discontinued when PCT was less than 0.25 µg/L. The duration of antibiotic administration was significantly lower in the PCT-guided group (mean difference: 1.8 days, 95% CI: −3.1, −0.).3 The rates of hospitalization, duration of hospital stay, and mean impairment of daily activities attributable to LRTI were similar between groups.
Considering the adult studies and the small number of pediatric LRTI research published to date, the use of PCT to safely reduce antibiotic treatment duration is encouraging.4 Although the studies on the kinetics of PCT are limited, the biomarker has been shown to rise two to four hours after a bacterial stimulus, peak in 24-48 hours and achieve a half-life of 24-36 hours.6,7 As such, serial PCT measurements at 24-hour intervals for three to five days may be more beneficial than stand-alone PCT tests. Nonetheless, additional studies are needed to better define groups of patients who will most likely benefit from PCT testing and to understand how to best integrate testing into clinical practice.
PROCALCITONIN FOR SEVERITY PREDICTION OF LRTI
PCT has also been explored as a marker of LRTI disease severity. In a 2008 multicenter cohort encompassing 1,651 adults with pneumonia, PCT < 0.1 µg/L was associated with a decreased 30-day mortality, shorter length of stay, and decreased admission to the intensive care unit (ICU) compared with those with PCT>0.1 µg/L.8 In a 2017 study of 317 adults hospitalized with pneumonia, the PCT level was significantly higher in those with bacteremia and in those admitted to intensive care.9 When used in combination with the pneumonia severity index (PSI), the addition of PCT resulted in improved prognostic performance compared with the PSI alone for both outcomes, increasing the area under the receiver operating characteristic curve from 0.67 to 0.85 for bacteremia and from 0.58 to 0.64 for intensive care. Similarly, in the adult EPIC cohort, the addition of PCT contributed significant prognostic information beyond existing severity scores for predicting the need for invasive respiratory or vasopressor support; each 1 µg/L increase in PCT was associated with a 1% to 2% absolute increase in the need for this outcome.10
A European study of 100 children with pneumonia also demonstrated higher PCT values among hospitalized children (n = 26, median PCT 17.8 µg/L) compared with outpatient children (n = 73, median PCT 0.72 µg/L, P < .01).11 Among the 532 children from the EPIC study, a PCT < 0.25 µg/L was associated with the reduced odds of ICU admission (adjusted OR: 0.48; 95% CI: 0.30, 0.78) and a 2.3-day (95% CI: 1.4, 3.2) decrease in the average length of stay compared with those with higher PCT concentrations.5 Of the 34 children with empyema requiring drainage, 28 (82%) showed a PCT concentration ≥0.5 µg/L. Additional pediatric studies are needed, but the limited data to date suggest that PCT may play a role in predicting pediatric LRTI disease severity, including the need for mechanical ventilatory support and ICU-level care.
LIMITATIONS TO CLINICAL APPLICATION
Although PCT shows promise as a biomarker to reliably rule out bacterial infection, several potential limitations exist in assessing its role in pediatric LRTI. Atypical bacterial infections (ie, Mycoplasma pneumoniae) and localized bacterial infection may not induce significant PCT production, as has been shown in adults and children with tonsillitis, localized skin infections, endocarditis, or empyema (Table).12 The majority of clinical trials in LRTI have been conducted in the adult population,4 with the number of pediatric trials remaining small.2,3 Given the predominance of viral LRTI in children compared with adults, the utility of PCT may differ in these populations.13,14 Furthermore, existing studies demonstrate mixed results regarding the magnitude of benefits that PCT may provide in terms of limiting antibiotic use. Another concern is the potential of PCT to increase unnecessary antibiotic use in those with viral LRTI,3 as PCT may also be increased in populations with systemic inflammation from nonbacterial causes.12,15
CONCLUSIONS AND CLINICAL APPLICATION
The misuse of antibiotics is a public health crisis resulting in the emergence of antibiotic-resistant pathogens and adverse outcomes, including Clostridioides difficile infection, drug toxicities, and increased healthcare costs.16 Pneumonia is responsible for more days of antibiotics than any other disease in children’s hospitals and is an important target for stewardship efforts.17 PCT is a promising biomarker for distinguishing bacterial from viral infection, and its use may help in making informed antibiotic decisions and predicting disease outcomes in pediatric LRTI. Although PCT has been cleared by the FDA for assisting with antibiotic decisions in pediatric LRTI, the majority of evidence supporting this indication is drawn from adults. Additional studies are needed prior to the widespread implementation in the pediatric population, but the results of available pediatric studies show promise. The clinical context and severity of patient presentation are important when considering whether or not to use PCT and how to best interpret PCT levels when making clinical management decisions. The utility of PCT for antibiotic initiation in the pediatric population is encouraging given the predominance of viral etiologies in pediatric LRTI. Currently available data demonstrate the value of serial PCT measurements in antibiotic de-escalation and promoting antibiotic stewardship for children and adults.2-4 As with all new diagnostic modalities, provider education is paramount to ensure a safe and value-driven implementation.
Disclosures
Dr. Katz received investigator-initiated grant funding from Roche and bioMérieux to conduct research involving procalcitonin in the past three years. Dr. Sartori has nothing to disclose. Dr. Williams received investigator-initiated grant funding from bioMérieux to conduct research involving procalcitonin in the past three years.
Funding
This work was supported by the National Institute of Health (1T32AI095202-07).
Disclaimer
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Roche, or bioMérieux.
1. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. US Food and Drug Administration. [Press Release]. Silver Spring, MD, February 23 2017.
2. Esposito S, Tagliabue C, Picciolli I, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939-1945. https://doi.org/10.1016/j.rmed.2011.09.003.
3. Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419. https://doi.org/10.1371/journal.pone.0068419.
4. Choi JJ MM, Simon MS, Evans AT, Self WH, Glesby MJ. Procalcitonin in the diagnosis and management of community-acquired pneumonia in hospitalized adults. J Hosp Med. 2019;18(X);XXX-XXX. https://doi.org/10.12788/jhm.3272.
5. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2017;7(1): 46-53. https://doi.org/10.1093/jpids/piw091.
6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605-1608. https://doi.org/10.1210/jcem.79.6.7989463.
7. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888-889.
8. Huang DT, Weissfeld LA, Kellum JA, et al; GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48-58 e42. https://doi.org/10.1016/j.annemergmed.2008.01.003.
9. McCluskey SM, Schuetz P, Abers MS, et al. Serial procalcitonin as a predictor of pacteremia and peed for intensive care unit care in adults with pneumonia, including those with highest severity: A Prospective Cohort Study. Open Forum Infect Dis. 2017;4(1):ofw238. https://doi.org/10.1093/ofid/ofw238.
10. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819-828. https://doi.org/10.1016/j.chest.2016.04.010.
11. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129-137. https://doi.org/10.1080/00365540600951283.
12. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. https://doi.org/10.3343/alm.2014.34.4.263.
13. Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
14. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/NEJMoa1500245.
15. Aloisio E, Dolci A, Panteghini M. Procalcitonin: Between evidence and critical issues. Clin Chim Acta. 2019;496:7-12. https://doi.org/10.1016/j.cca.2019.06.010.
16. Society for Healthcare Epidemiology of A, Infectious Diseases Society of A, Pediatric Infectious Diseases S. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. https://doi.org/10.1086/665010.
17. Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. https://doi.org/10.1086/673982.
Procalcitonin (PCT) is a biomarker that has shown promise to identify bacterial etiology in acute infections, including bacterial lower respiratory tract infection (LRTI). In 2017, the United States Food and Drug Administration (FDA) approved the use of PCT as a diagnostic aid to guide the decisions around antibiotic therapy in acute LRTI.1 Although most of the data supporting the use of PCT for LRTI stems from adult studies, the high disease burden, predominance of viral etiologies, and frequent diagnostic uncertainty resulting in antibiotic overuse make pediatric LRTI an ideal target for the use of PCT as a diagnostic aid. This review evaluates and summarizes the current evidence regarding the role of PCT in the clinical care of pediatric LRTI, including its use in guiding antibiotic use and prognosticating disease severity.
THE ROLE OF PROCALCITONIN IN GUIDING INITIATION OF ANTIBIOTICS
The commonly used PCT cut points for withholding or stopping antibiotics in adults and children are 0.1 µg/L (very low risk of bacterial etiology) or 0.25 µg/L (low risk of bacterial etiology).2-4 Among the 532 children enrolled in the multicenter study of Etiology of Pneumonia in the Community (EPIC), a PCT threshold of 0.25 µg/L demonstrated an approximate sensitivity of 85%, specificity of 45%, positive likelihood ratio of 1.55, and negative likelihood ratio of 0.33 for community acquired pneumonia (CAP) caused by typical bacterial pathogens.5 Lowering the cutoff to <0.1 µg/L increased PCT sensitivity to 100%, decreased specificity, positive likelihood ratio, and negative likelihood ratio to 20%, 1.26, and 0, respectively. Although the EPIC study obtained culture and performed PCR testing on any blood sample, pleural fluid specimen, endotracheal aspirate, or bronchoalveolar–lavage specimens obtained during the study period, currently available laboratory methods show poor sensitivity for defining bacterial LRTI. Thus, bacterial etiologies may have been underestimated. The highly negative predictive value demonstrated in this study highlights the potential of PCT as a biomarker for ruling out bacterial diseases, including LRTI.
Multiple studies have evaluated the potential utility of PCT in guiding antibiotic initiation in adults with LRTI, but data on pediatric patients are sparse.4 In a randomized, single-center Italian study comparing a PCT-guided algorithm (withholding antibiotics when PCT < 0.25 µg/L) versus usual care among 319 hospitalized children with pneumonia, the PCT group experienced fewer antibiotic initiations (15.5% vs 100%, P < .05) without significant differences in recurrence of respiratory symptoms or new antibiotic prescriptions in the month following enrollment.2
A similar randomized trial using a PCT-guided algorithm for the initiation of antibiotics conducted among 337 Swiss children presented to the emergency department (ED) with pneumonia and other LRTIs failed to demonstrate decreases in antibiotic initiation.3 This study used an algorithm that categorized the likelihood of requiring antibiotic treatment for bacterial LRTI as “definitely” if PCT was >0.5 µg/L, “probably” if PCT was 0.26–0.5 µg/L, “probably not” if PCT was 0.1–0.25 µg/L, and “definitely not” if PCT was <0.1 µg/L. In the PCT group, 104 out of 168 (62%) patients received antibiotics within 14 days compared with 93 out of 165 (56%) patients in the control group (odds ratio [OR]: 1.26, 95% CI: 0.81, 1.95). In the subgroup analyses, the odds of administering antibiotics to those with nonpneumonia LRTI was significantly higher than those of the PCT group and control group (OR: 4.09, 95% CI: 1.8, 9.93); the odds of receiving antibiotics also showed no difference in the subgroup of children with pneumonia (OR: 0.66, 95% CI: 0.35, 1.23).
The benefit of PCT for informing decisions around the initiation of antibiotics likely varies based on perceived risk of bacterial diseases. When the pretest probability of bacterial disease is extremely high, the use of PCT is unlikely to alter treatment decisions. Similarly, PCT should not be used in situations where the pretest probability for bacterial pneumonia is very low—in these instances, an elevated PCT may lead to unnecessary antibiotic use among children presenting to the ED. However, the risk of bacterial pneumonia is often equivocal, and in these situations, PCT may provide clinicians with useful insights, primarily for ruling out bacterial disease.
THE ROLE OF PROCALCITONIN IN GUIDING DISCONTINUATION OF ANTIBIOTICS
In the study by Esposito et al., the PCT levels were additionally measured every two days until discharge and during two scheduled follow-up visits; the antibiotics were discontinued when PCT < 0.25 µg/L.2 The PCT-guided group experienced shorter antibiotic duration (mean 5.4 vs 11.0 days, P < .05), shorter length of hospital stay (mean 4.7 vs 5.61 days for mild LRTI and 5.01 vs 5.93 for severe LRTI), and fewer antibiotic-related adverse events (3.9% vs 25.2%, P < .05). Similarly, in the study by Baer et al., the PCT-guided group had PCT levels repeated on days three and five after enrollment, and the antibiotics were discontinued when PCT was less than 0.25 µg/L. The duration of antibiotic administration was significantly lower in the PCT-guided group (mean difference: 1.8 days, 95% CI: −3.1, −0.).3 The rates of hospitalization, duration of hospital stay, and mean impairment of daily activities attributable to LRTI were similar between groups.
Considering the adult studies and the small number of pediatric LRTI research published to date, the use of PCT to safely reduce antibiotic treatment duration is encouraging.4 Although the studies on the kinetics of PCT are limited, the biomarker has been shown to rise two to four hours after a bacterial stimulus, peak in 24-48 hours and achieve a half-life of 24-36 hours.6,7 As such, serial PCT measurements at 24-hour intervals for three to five days may be more beneficial than stand-alone PCT tests. Nonetheless, additional studies are needed to better define groups of patients who will most likely benefit from PCT testing and to understand how to best integrate testing into clinical practice.
PROCALCITONIN FOR SEVERITY PREDICTION OF LRTI
PCT has also been explored as a marker of LRTI disease severity. In a 2008 multicenter cohort encompassing 1,651 adults with pneumonia, PCT < 0.1 µg/L was associated with a decreased 30-day mortality, shorter length of stay, and decreased admission to the intensive care unit (ICU) compared with those with PCT>0.1 µg/L.8 In a 2017 study of 317 adults hospitalized with pneumonia, the PCT level was significantly higher in those with bacteremia and in those admitted to intensive care.9 When used in combination with the pneumonia severity index (PSI), the addition of PCT resulted in improved prognostic performance compared with the PSI alone for both outcomes, increasing the area under the receiver operating characteristic curve from 0.67 to 0.85 for bacteremia and from 0.58 to 0.64 for intensive care. Similarly, in the adult EPIC cohort, the addition of PCT contributed significant prognostic information beyond existing severity scores for predicting the need for invasive respiratory or vasopressor support; each 1 µg/L increase in PCT was associated with a 1% to 2% absolute increase in the need for this outcome.10
A European study of 100 children with pneumonia also demonstrated higher PCT values among hospitalized children (n = 26, median PCT 17.8 µg/L) compared with outpatient children (n = 73, median PCT 0.72 µg/L, P < .01).11 Among the 532 children from the EPIC study, a PCT < 0.25 µg/L was associated with the reduced odds of ICU admission (adjusted OR: 0.48; 95% CI: 0.30, 0.78) and a 2.3-day (95% CI: 1.4, 3.2) decrease in the average length of stay compared with those with higher PCT concentrations.5 Of the 34 children with empyema requiring drainage, 28 (82%) showed a PCT concentration ≥0.5 µg/L. Additional pediatric studies are needed, but the limited data to date suggest that PCT may play a role in predicting pediatric LRTI disease severity, including the need for mechanical ventilatory support and ICU-level care.
LIMITATIONS TO CLINICAL APPLICATION
Although PCT shows promise as a biomarker to reliably rule out bacterial infection, several potential limitations exist in assessing its role in pediatric LRTI. Atypical bacterial infections (ie, Mycoplasma pneumoniae) and localized bacterial infection may not induce significant PCT production, as has been shown in adults and children with tonsillitis, localized skin infections, endocarditis, or empyema (Table).12 The majority of clinical trials in LRTI have been conducted in the adult population,4 with the number of pediatric trials remaining small.2,3 Given the predominance of viral LRTI in children compared with adults, the utility of PCT may differ in these populations.13,14 Furthermore, existing studies demonstrate mixed results regarding the magnitude of benefits that PCT may provide in terms of limiting antibiotic use. Another concern is the potential of PCT to increase unnecessary antibiotic use in those with viral LRTI,3 as PCT may also be increased in populations with systemic inflammation from nonbacterial causes.12,15
CONCLUSIONS AND CLINICAL APPLICATION
The misuse of antibiotics is a public health crisis resulting in the emergence of antibiotic-resistant pathogens and adverse outcomes, including Clostridioides difficile infection, drug toxicities, and increased healthcare costs.16 Pneumonia is responsible for more days of antibiotics than any other disease in children’s hospitals and is an important target for stewardship efforts.17 PCT is a promising biomarker for distinguishing bacterial from viral infection, and its use may help in making informed antibiotic decisions and predicting disease outcomes in pediatric LRTI. Although PCT has been cleared by the FDA for assisting with antibiotic decisions in pediatric LRTI, the majority of evidence supporting this indication is drawn from adults. Additional studies are needed prior to the widespread implementation in the pediatric population, but the results of available pediatric studies show promise. The clinical context and severity of patient presentation are important when considering whether or not to use PCT and how to best interpret PCT levels when making clinical management decisions. The utility of PCT for antibiotic initiation in the pediatric population is encouraging given the predominance of viral etiologies in pediatric LRTI. Currently available data demonstrate the value of serial PCT measurements in antibiotic de-escalation and promoting antibiotic stewardship for children and adults.2-4 As with all new diagnostic modalities, provider education is paramount to ensure a safe and value-driven implementation.
Disclosures
Dr. Katz received investigator-initiated grant funding from Roche and bioMérieux to conduct research involving procalcitonin in the past three years. Dr. Sartori has nothing to disclose. Dr. Williams received investigator-initiated grant funding from bioMérieux to conduct research involving procalcitonin in the past three years.
Funding
This work was supported by the National Institute of Health (1T32AI095202-07).
Disclaimer
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Roche, or bioMérieux.
Procalcitonin (PCT) is a biomarker that has shown promise to identify bacterial etiology in acute infections, including bacterial lower respiratory tract infection (LRTI). In 2017, the United States Food and Drug Administration (FDA) approved the use of PCT as a diagnostic aid to guide the decisions around antibiotic therapy in acute LRTI.1 Although most of the data supporting the use of PCT for LRTI stems from adult studies, the high disease burden, predominance of viral etiologies, and frequent diagnostic uncertainty resulting in antibiotic overuse make pediatric LRTI an ideal target for the use of PCT as a diagnostic aid. This review evaluates and summarizes the current evidence regarding the role of PCT in the clinical care of pediatric LRTI, including its use in guiding antibiotic use and prognosticating disease severity.
THE ROLE OF PROCALCITONIN IN GUIDING INITIATION OF ANTIBIOTICS
The commonly used PCT cut points for withholding or stopping antibiotics in adults and children are 0.1 µg/L (very low risk of bacterial etiology) or 0.25 µg/L (low risk of bacterial etiology).2-4 Among the 532 children enrolled in the multicenter study of Etiology of Pneumonia in the Community (EPIC), a PCT threshold of 0.25 µg/L demonstrated an approximate sensitivity of 85%, specificity of 45%, positive likelihood ratio of 1.55, and negative likelihood ratio of 0.33 for community acquired pneumonia (CAP) caused by typical bacterial pathogens.5 Lowering the cutoff to <0.1 µg/L increased PCT sensitivity to 100%, decreased specificity, positive likelihood ratio, and negative likelihood ratio to 20%, 1.26, and 0, respectively. Although the EPIC study obtained culture and performed PCR testing on any blood sample, pleural fluid specimen, endotracheal aspirate, or bronchoalveolar–lavage specimens obtained during the study period, currently available laboratory methods show poor sensitivity for defining bacterial LRTI. Thus, bacterial etiologies may have been underestimated. The highly negative predictive value demonstrated in this study highlights the potential of PCT as a biomarker for ruling out bacterial diseases, including LRTI.
Multiple studies have evaluated the potential utility of PCT in guiding antibiotic initiation in adults with LRTI, but data on pediatric patients are sparse.4 In a randomized, single-center Italian study comparing a PCT-guided algorithm (withholding antibiotics when PCT < 0.25 µg/L) versus usual care among 319 hospitalized children with pneumonia, the PCT group experienced fewer antibiotic initiations (15.5% vs 100%, P < .05) without significant differences in recurrence of respiratory symptoms or new antibiotic prescriptions in the month following enrollment.2
A similar randomized trial using a PCT-guided algorithm for the initiation of antibiotics conducted among 337 Swiss children presented to the emergency department (ED) with pneumonia and other LRTIs failed to demonstrate decreases in antibiotic initiation.3 This study used an algorithm that categorized the likelihood of requiring antibiotic treatment for bacterial LRTI as “definitely” if PCT was >0.5 µg/L, “probably” if PCT was 0.26–0.5 µg/L, “probably not” if PCT was 0.1–0.25 µg/L, and “definitely not” if PCT was <0.1 µg/L. In the PCT group, 104 out of 168 (62%) patients received antibiotics within 14 days compared with 93 out of 165 (56%) patients in the control group (odds ratio [OR]: 1.26, 95% CI: 0.81, 1.95). In the subgroup analyses, the odds of administering antibiotics to those with nonpneumonia LRTI was significantly higher than those of the PCT group and control group (OR: 4.09, 95% CI: 1.8, 9.93); the odds of receiving antibiotics also showed no difference in the subgroup of children with pneumonia (OR: 0.66, 95% CI: 0.35, 1.23).
The benefit of PCT for informing decisions around the initiation of antibiotics likely varies based on perceived risk of bacterial diseases. When the pretest probability of bacterial disease is extremely high, the use of PCT is unlikely to alter treatment decisions. Similarly, PCT should not be used in situations where the pretest probability for bacterial pneumonia is very low—in these instances, an elevated PCT may lead to unnecessary antibiotic use among children presenting to the ED. However, the risk of bacterial pneumonia is often equivocal, and in these situations, PCT may provide clinicians with useful insights, primarily for ruling out bacterial disease.
THE ROLE OF PROCALCITONIN IN GUIDING DISCONTINUATION OF ANTIBIOTICS
In the study by Esposito et al., the PCT levels were additionally measured every two days until discharge and during two scheduled follow-up visits; the antibiotics were discontinued when PCT < 0.25 µg/L.2 The PCT-guided group experienced shorter antibiotic duration (mean 5.4 vs 11.0 days, P < .05), shorter length of hospital stay (mean 4.7 vs 5.61 days for mild LRTI and 5.01 vs 5.93 for severe LRTI), and fewer antibiotic-related adverse events (3.9% vs 25.2%, P < .05). Similarly, in the study by Baer et al., the PCT-guided group had PCT levels repeated on days three and five after enrollment, and the antibiotics were discontinued when PCT was less than 0.25 µg/L. The duration of antibiotic administration was significantly lower in the PCT-guided group (mean difference: 1.8 days, 95% CI: −3.1, −0.).3 The rates of hospitalization, duration of hospital stay, and mean impairment of daily activities attributable to LRTI were similar between groups.
Considering the adult studies and the small number of pediatric LRTI research published to date, the use of PCT to safely reduce antibiotic treatment duration is encouraging.4 Although the studies on the kinetics of PCT are limited, the biomarker has been shown to rise two to four hours after a bacterial stimulus, peak in 24-48 hours and achieve a half-life of 24-36 hours.6,7 As such, serial PCT measurements at 24-hour intervals for three to five days may be more beneficial than stand-alone PCT tests. Nonetheless, additional studies are needed to better define groups of patients who will most likely benefit from PCT testing and to understand how to best integrate testing into clinical practice.
PROCALCITONIN FOR SEVERITY PREDICTION OF LRTI
PCT has also been explored as a marker of LRTI disease severity. In a 2008 multicenter cohort encompassing 1,651 adults with pneumonia, PCT < 0.1 µg/L was associated with a decreased 30-day mortality, shorter length of stay, and decreased admission to the intensive care unit (ICU) compared with those with PCT>0.1 µg/L.8 In a 2017 study of 317 adults hospitalized with pneumonia, the PCT level was significantly higher in those with bacteremia and in those admitted to intensive care.9 When used in combination with the pneumonia severity index (PSI), the addition of PCT resulted in improved prognostic performance compared with the PSI alone for both outcomes, increasing the area under the receiver operating characteristic curve from 0.67 to 0.85 for bacteremia and from 0.58 to 0.64 for intensive care. Similarly, in the adult EPIC cohort, the addition of PCT contributed significant prognostic information beyond existing severity scores for predicting the need for invasive respiratory or vasopressor support; each 1 µg/L increase in PCT was associated with a 1% to 2% absolute increase in the need for this outcome.10
A European study of 100 children with pneumonia also demonstrated higher PCT values among hospitalized children (n = 26, median PCT 17.8 µg/L) compared with outpatient children (n = 73, median PCT 0.72 µg/L, P < .01).11 Among the 532 children from the EPIC study, a PCT < 0.25 µg/L was associated with the reduced odds of ICU admission (adjusted OR: 0.48; 95% CI: 0.30, 0.78) and a 2.3-day (95% CI: 1.4, 3.2) decrease in the average length of stay compared with those with higher PCT concentrations.5 Of the 34 children with empyema requiring drainage, 28 (82%) showed a PCT concentration ≥0.5 µg/L. Additional pediatric studies are needed, but the limited data to date suggest that PCT may play a role in predicting pediatric LRTI disease severity, including the need for mechanical ventilatory support and ICU-level care.
LIMITATIONS TO CLINICAL APPLICATION
Although PCT shows promise as a biomarker to reliably rule out bacterial infection, several potential limitations exist in assessing its role in pediatric LRTI. Atypical bacterial infections (ie, Mycoplasma pneumoniae) and localized bacterial infection may not induce significant PCT production, as has been shown in adults and children with tonsillitis, localized skin infections, endocarditis, or empyema (Table).12 The majority of clinical trials in LRTI have been conducted in the adult population,4 with the number of pediatric trials remaining small.2,3 Given the predominance of viral LRTI in children compared with adults, the utility of PCT may differ in these populations.13,14 Furthermore, existing studies demonstrate mixed results regarding the magnitude of benefits that PCT may provide in terms of limiting antibiotic use. Another concern is the potential of PCT to increase unnecessary antibiotic use in those with viral LRTI,3 as PCT may also be increased in populations with systemic inflammation from nonbacterial causes.12,15
CONCLUSIONS AND CLINICAL APPLICATION
The misuse of antibiotics is a public health crisis resulting in the emergence of antibiotic-resistant pathogens and adverse outcomes, including Clostridioides difficile infection, drug toxicities, and increased healthcare costs.16 Pneumonia is responsible for more days of antibiotics than any other disease in children’s hospitals and is an important target for stewardship efforts.17 PCT is a promising biomarker for distinguishing bacterial from viral infection, and its use may help in making informed antibiotic decisions and predicting disease outcomes in pediatric LRTI. Although PCT has been cleared by the FDA for assisting with antibiotic decisions in pediatric LRTI, the majority of evidence supporting this indication is drawn from adults. Additional studies are needed prior to the widespread implementation in the pediatric population, but the results of available pediatric studies show promise. The clinical context and severity of patient presentation are important when considering whether or not to use PCT and how to best interpret PCT levels when making clinical management decisions. The utility of PCT for antibiotic initiation in the pediatric population is encouraging given the predominance of viral etiologies in pediatric LRTI. Currently available data demonstrate the value of serial PCT measurements in antibiotic de-escalation and promoting antibiotic stewardship for children and adults.2-4 As with all new diagnostic modalities, provider education is paramount to ensure a safe and value-driven implementation.
Disclosures
Dr. Katz received investigator-initiated grant funding from Roche and bioMérieux to conduct research involving procalcitonin in the past three years. Dr. Sartori has nothing to disclose. Dr. Williams received investigator-initiated grant funding from bioMérieux to conduct research involving procalcitonin in the past three years.
Funding
This work was supported by the National Institute of Health (1T32AI095202-07).
Disclaimer
This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Roche, or bioMérieux.
1. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. US Food and Drug Administration. [Press Release]. Silver Spring, MD, February 23 2017.
2. Esposito S, Tagliabue C, Picciolli I, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939-1945. https://doi.org/10.1016/j.rmed.2011.09.003.
3. Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419. https://doi.org/10.1371/journal.pone.0068419.
4. Choi JJ MM, Simon MS, Evans AT, Self WH, Glesby MJ. Procalcitonin in the diagnosis and management of community-acquired pneumonia in hospitalized adults. J Hosp Med. 2019;18(X);XXX-XXX. https://doi.org/10.12788/jhm.3272.
5. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2017;7(1): 46-53. https://doi.org/10.1093/jpids/piw091.
6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605-1608. https://doi.org/10.1210/jcem.79.6.7989463.
7. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888-889.
8. Huang DT, Weissfeld LA, Kellum JA, et al; GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48-58 e42. https://doi.org/10.1016/j.annemergmed.2008.01.003.
9. McCluskey SM, Schuetz P, Abers MS, et al. Serial procalcitonin as a predictor of pacteremia and peed for intensive care unit care in adults with pneumonia, including those with highest severity: A Prospective Cohort Study. Open Forum Infect Dis. 2017;4(1):ofw238. https://doi.org/10.1093/ofid/ofw238.
10. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819-828. https://doi.org/10.1016/j.chest.2016.04.010.
11. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129-137. https://doi.org/10.1080/00365540600951283.
12. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. https://doi.org/10.3343/alm.2014.34.4.263.
13. Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
14. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/NEJMoa1500245.
15. Aloisio E, Dolci A, Panteghini M. Procalcitonin: Between evidence and critical issues. Clin Chim Acta. 2019;496:7-12. https://doi.org/10.1016/j.cca.2019.06.010.
16. Society for Healthcare Epidemiology of A, Infectious Diseases Society of A, Pediatric Infectious Diseases S. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. https://doi.org/10.1086/665010.
17. Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. https://doi.org/10.1086/673982.
1. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. US Food and Drug Administration. [Press Release]. Silver Spring, MD, February 23 2017.
2. Esposito S, Tagliabue C, Picciolli I, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir Med. 2011;105(12):1939-1945. https://doi.org/10.1016/j.rmed.2011.09.003.
3. Baer G, Baumann P, Buettcher M, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013;8(8):e68419. https://doi.org/10.1371/journal.pone.0068419.
4. Choi JJ MM, Simon MS, Evans AT, Self WH, Glesby MJ. Procalcitonin in the diagnosis and management of community-acquired pneumonia in hospitalized adults. J Hosp Med. 2019;18(X);XXX-XXX. https://doi.org/10.12788/jhm.3272.
5. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2017;7(1): 46-53. https://doi.org/10.1093/jpids/piw091.
6. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79(6):1605-1608. https://doi.org/10.1210/jcem.79.6.7989463.
7. Brunkhorst FM, Heinz U, Forycki ZF. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med. 1998;24(8):888-889.
8. Huang DT, Weissfeld LA, Kellum JA, et al; GenIMS Investigators. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48-58 e42. https://doi.org/10.1016/j.annemergmed.2008.01.003.
9. McCluskey SM, Schuetz P, Abers MS, et al. Serial procalcitonin as a predictor of pacteremia and peed for intensive care unit care in adults with pneumonia, including those with highest severity: A Prospective Cohort Study. Open Forum Infect Dis. 2017;4(1):ofw238. https://doi.org/10.1093/ofid/ofw238.
10. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest. 2016;150(4):819-828. https://doi.org/10.1016/j.chest.2016.04.010.
11. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129-137. https://doi.org/10.1080/00365540600951283.
12. Meisner M. Update on procalcitonin measurements. Ann Lab Med. 2014;34(4):263-273. https://doi.org/10.3343/alm.2014.34.4.263.
13. Jain S, Williams DJ, Arnold SR, et al; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
14. Jain S, Self WH, Wunderink RG, et al; CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/NEJMoa1500245.
15. Aloisio E, Dolci A, Panteghini M. Procalcitonin: Between evidence and critical issues. Clin Chim Acta. 2019;496:7-12. https://doi.org/10.1016/j.cca.2019.06.010.
16. Society for Healthcare Epidemiology of A, Infectious Diseases Society of A, Pediatric Infectious Diseases S. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. https://doi.org/10.1086/665010.
17. Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. https://doi.org/10.1086/673982.
© 2019 Society of Hospital Medicine