User login
Imaging after bariatric surgery appears overdone
Nearly 70% of bariatric surgery patients received postoperative imaging, with more than one-third receiving CT imaging. This high level of screening resulted in symptom-related findings in only 23% of cases, and may be excessive, according to researchers who studied nearly 600 adults who underwent bariatric surgery.
As the volume of bariatric surgery has increased, so has the role of postoperative imaging, wrote Dana Haddad, MD, and her colleagues at Harlem Hospital Center, New York.
“However, there is a lack of well-defined postoperative imaging guidelines,” they said. “Detrimental aspects of postoperative imaging include the potential for false-positive findings leading to further and often unnecessary investigations, radiation exposure, and additional cost,” they added.
The primary outcomes were the numbers of initial postimaging studies and whether the findings supported subsequent studies.
The study population included 399 adults who underwent laparoscopic bypass and 144 who underwent sleeve gastrectomy. The average age of the patients was 41 years and 90% were women.
The researchers identified 907 imaging studies performed in 400 patients (69% of the study population). Of these, 38% were ultrasound, 36% were CT, 15% were x-ray, 6.6% were fluoroscopy, 3.3% were MRI, and .6% were nuclear medicine.
On review of the imaging findings, the researchers found that half (50%) were unremarkable, while 13% were either surgery related or symptom related, 6.8% were not related to surgery but might have explained patients’ symptoms, 4.3% were surgery-related but not likely to explain symptoms, and 26% were incidental. “Interestingly, no incidental findings were found to be of major clinical importance; all were benign,” according to the researchers.
However, incidental findings led to a total of 71 additional studies, and to 5 laparoscopic cholecystectomies.
A univariate analysis showed that the factors with a significant impact a patient’s odds of undergoing postoperative abdominal imaging included having a bypass procedure vs. a sleeve procedure, older age, and lower baseline body mass index. In addition, patients with a history of abdominal surgery or dyspepsia or those who had a routine postoperative upper gastrointestinal series were significantly more likely to undergo CT scans. Patients with history of ulcer or reflux were significantly less likely to undergo CT scans.
Although the study was limited by the retrospective design and lack of information about possible imaging of patients at other centers, “results suggest that nonroutine postoperative abdominal imaging in the bariatric population is common and requires streamlined protocols, with almost 70% of patients undergoing imaging and greater than 70% of findings being unrelated to symptoms or negative,” the researchers said.
A clinical algorithm for imaging of bariatric patients should be based on clinical parameters collected during a physical exam. “Once an algorithm is in place, further studies will be needed to validate its accuracy and efficiency,” the researchers stated.
Dr. Haddad and her colleagues had no financial conflicts to disclose.
Nearly 70% of bariatric surgery patients received postoperative imaging, with more than one-third receiving CT imaging. This high level of screening resulted in symptom-related findings in only 23% of cases, and may be excessive, according to researchers who studied nearly 600 adults who underwent bariatric surgery.
As the volume of bariatric surgery has increased, so has the role of postoperative imaging, wrote Dana Haddad, MD, and her colleagues at Harlem Hospital Center, New York.
“However, there is a lack of well-defined postoperative imaging guidelines,” they said. “Detrimental aspects of postoperative imaging include the potential for false-positive findings leading to further and often unnecessary investigations, radiation exposure, and additional cost,” they added.
The primary outcomes were the numbers of initial postimaging studies and whether the findings supported subsequent studies.
The study population included 399 adults who underwent laparoscopic bypass and 144 who underwent sleeve gastrectomy. The average age of the patients was 41 years and 90% were women.
The researchers identified 907 imaging studies performed in 400 patients (69% of the study population). Of these, 38% were ultrasound, 36% were CT, 15% were x-ray, 6.6% were fluoroscopy, 3.3% were MRI, and .6% were nuclear medicine.
On review of the imaging findings, the researchers found that half (50%) were unremarkable, while 13% were either surgery related or symptom related, 6.8% were not related to surgery but might have explained patients’ symptoms, 4.3% were surgery-related but not likely to explain symptoms, and 26% were incidental. “Interestingly, no incidental findings were found to be of major clinical importance; all were benign,” according to the researchers.
However, incidental findings led to a total of 71 additional studies, and to 5 laparoscopic cholecystectomies.
A univariate analysis showed that the factors with a significant impact a patient’s odds of undergoing postoperative abdominal imaging included having a bypass procedure vs. a sleeve procedure, older age, and lower baseline body mass index. In addition, patients with a history of abdominal surgery or dyspepsia or those who had a routine postoperative upper gastrointestinal series were significantly more likely to undergo CT scans. Patients with history of ulcer or reflux were significantly less likely to undergo CT scans.
Although the study was limited by the retrospective design and lack of information about possible imaging of patients at other centers, “results suggest that nonroutine postoperative abdominal imaging in the bariatric population is common and requires streamlined protocols, with almost 70% of patients undergoing imaging and greater than 70% of findings being unrelated to symptoms or negative,” the researchers said.
A clinical algorithm for imaging of bariatric patients should be based on clinical parameters collected during a physical exam. “Once an algorithm is in place, further studies will be needed to validate its accuracy and efficiency,” the researchers stated.
Dr. Haddad and her colleagues had no financial conflicts to disclose.
Nearly 70% of bariatric surgery patients received postoperative imaging, with more than one-third receiving CT imaging. This high level of screening resulted in symptom-related findings in only 23% of cases, and may be excessive, according to researchers who studied nearly 600 adults who underwent bariatric surgery.
As the volume of bariatric surgery has increased, so has the role of postoperative imaging, wrote Dana Haddad, MD, and her colleagues at Harlem Hospital Center, New York.
“However, there is a lack of well-defined postoperative imaging guidelines,” they said. “Detrimental aspects of postoperative imaging include the potential for false-positive findings leading to further and often unnecessary investigations, radiation exposure, and additional cost,” they added.
The primary outcomes were the numbers of initial postimaging studies and whether the findings supported subsequent studies.
The study population included 399 adults who underwent laparoscopic bypass and 144 who underwent sleeve gastrectomy. The average age of the patients was 41 years and 90% were women.
The researchers identified 907 imaging studies performed in 400 patients (69% of the study population). Of these, 38% were ultrasound, 36% were CT, 15% were x-ray, 6.6% were fluoroscopy, 3.3% were MRI, and .6% were nuclear medicine.
On review of the imaging findings, the researchers found that half (50%) were unremarkable, while 13% were either surgery related or symptom related, 6.8% were not related to surgery but might have explained patients’ symptoms, 4.3% were surgery-related but not likely to explain symptoms, and 26% were incidental. “Interestingly, no incidental findings were found to be of major clinical importance; all were benign,” according to the researchers.
However, incidental findings led to a total of 71 additional studies, and to 5 laparoscopic cholecystectomies.
A univariate analysis showed that the factors with a significant impact a patient’s odds of undergoing postoperative abdominal imaging included having a bypass procedure vs. a sleeve procedure, older age, and lower baseline body mass index. In addition, patients with a history of abdominal surgery or dyspepsia or those who had a routine postoperative upper gastrointestinal series were significantly more likely to undergo CT scans. Patients with history of ulcer or reflux were significantly less likely to undergo CT scans.
Although the study was limited by the retrospective design and lack of information about possible imaging of patients at other centers, “results suggest that nonroutine postoperative abdominal imaging in the bariatric population is common and requires streamlined protocols, with almost 70% of patients undergoing imaging and greater than 70% of findings being unrelated to symptoms or negative,” the researchers said.
A clinical algorithm for imaging of bariatric patients should be based on clinical parameters collected during a physical exam. “Once an algorithm is in place, further studies will be needed to validate its accuracy and efficiency,” the researchers stated.
Dr. Haddad and her colleagues had no financial conflicts to disclose.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: No well-defined guidelines exist for when to use postoperative imaging in bariatric surgery patients.
Major finding: Approximately 70% of postoperative imaging findings were not symptom related, and incidental findings led to 71 additional studies.
Data source: A review of 578 patients who underwent gastric bypass or sleeve gastrectomy.
Disclosures: The researchers had no financial conflicts to disclose.
Rural patients less likely to have bariatric surgery
Obese patients living in rural areas of West Virginia were substantially less likely than were their urban and suburban counterparts to have bariatric surgery, according to findings from a study comparing outcomes in two patient groups.
This discrepancy is attributed to a difference between rural and nonrural patients in type of insurance coverage. In this 2-year study, rural patients were nearly five times more likely to be covered by West Virginia Medicaid than were patients living in nonrural areas of the state, said Kristie L. Bergmann, PhD, of the department of behavioral medicine and psychiatry, West Virginia University, Morgantown, and her associates. The findings were published in Surgery for Obesity and Related Diseases (2017;13[4]:632-6), the journal of the American Society for Metabolic and Bariatric Surgery.
Previous research has identified rural patients’ lack of insurance as a barrier to health care. “Our results suggest that insurance denial represents a successive barrier. Despite being insured, rural individuals may be barred from surgery if insurance carriers do not offer it as a covered benefit, deny approval, or require indomitable prerequisites for surgery,” Dr. Bergmann and her associates said.
They examined the associations among rural status, access to bariatric surgery, and surgical outcomes in West Virginia in part because the state’s residents “have been consistently ranked as the most obese population in the United States, with approximately 35.1% of residents meeting criteria for obesity.” West Virginia also has the highest rates of diabetes (13%) and hypertension (41%) in the United States.
At the same time, rural populations are known to have decreased access to all forms of health care and specifically to bariatric surgery. This makes rural West Virginians “a particularly vulnerable population of interest,” the investigators said.
They performed a retrospective cohort study involving 122 obese patients seeking bariatric surgery at their university’s medical center during 2012-2014. A total of 97% of these patients were white, 83% were women, and the mean age was 47 years. Only 82 of the 122 study participants underwent bariatric surgery: 77 had Roux-en-Y gastric bypass and 5 had sleeve gastrectomy.
Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference. When Medicaid patients were excluded from the analysis, nonrural status no longer predicted the use of bariatric surgery.
Moreover, when Medicaid coverage was controlled for, rural status had no effect on the effectiveness of bariatric surgery. Patients residing in rural areas had the same attendance at follow-up visits and the same reduction in body mass index at 6 months and at 12 months as did nonrural patients.
In addition, patients who had higher levels of education and who worked full-time were more likely to undergo bariatric surgery, but overall, rural patients were more likely to have comorbidities, disability, and lower rates of full-time work. “An argument can be made that rural individuals may have a greater medical need for bariatric surgery, as obesity and associated health conditions may contribute to lower rates of employment. Unfortunately, barring these patients from receiving care may reinforce a cycle of disability and declining health status,” Dr. Bergmann and her associates noted.
This study was limited in that it had a relatively small sample size, particularly in analyses that excluded Medicaid recipients. It also had a follow-up of only 1 year, so longer-term outcomes of bariatric surgery could not be assessed. “Our sample is also predominantly Caucasian and may have unique culturally-based characteristics” that limit the generalizability of the study findings, they added.
No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
Obese patients living in rural areas of West Virginia were substantially less likely than were their urban and suburban counterparts to have bariatric surgery, according to findings from a study comparing outcomes in two patient groups.
This discrepancy is attributed to a difference between rural and nonrural patients in type of insurance coverage. In this 2-year study, rural patients were nearly five times more likely to be covered by West Virginia Medicaid than were patients living in nonrural areas of the state, said Kristie L. Bergmann, PhD, of the department of behavioral medicine and psychiatry, West Virginia University, Morgantown, and her associates. The findings were published in Surgery for Obesity and Related Diseases (2017;13[4]:632-6), the journal of the American Society for Metabolic and Bariatric Surgery.
Previous research has identified rural patients’ lack of insurance as a barrier to health care. “Our results suggest that insurance denial represents a successive barrier. Despite being insured, rural individuals may be barred from surgery if insurance carriers do not offer it as a covered benefit, deny approval, or require indomitable prerequisites for surgery,” Dr. Bergmann and her associates said.
They examined the associations among rural status, access to bariatric surgery, and surgical outcomes in West Virginia in part because the state’s residents “have been consistently ranked as the most obese population in the United States, with approximately 35.1% of residents meeting criteria for obesity.” West Virginia also has the highest rates of diabetes (13%) and hypertension (41%) in the United States.
At the same time, rural populations are known to have decreased access to all forms of health care and specifically to bariatric surgery. This makes rural West Virginians “a particularly vulnerable population of interest,” the investigators said.
They performed a retrospective cohort study involving 122 obese patients seeking bariatric surgery at their university’s medical center during 2012-2014. A total of 97% of these patients were white, 83% were women, and the mean age was 47 years. Only 82 of the 122 study participants underwent bariatric surgery: 77 had Roux-en-Y gastric bypass and 5 had sleeve gastrectomy.
Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference. When Medicaid patients were excluded from the analysis, nonrural status no longer predicted the use of bariatric surgery.
Moreover, when Medicaid coverage was controlled for, rural status had no effect on the effectiveness of bariatric surgery. Patients residing in rural areas had the same attendance at follow-up visits and the same reduction in body mass index at 6 months and at 12 months as did nonrural patients.
In addition, patients who had higher levels of education and who worked full-time were more likely to undergo bariatric surgery, but overall, rural patients were more likely to have comorbidities, disability, and lower rates of full-time work. “An argument can be made that rural individuals may have a greater medical need for bariatric surgery, as obesity and associated health conditions may contribute to lower rates of employment. Unfortunately, barring these patients from receiving care may reinforce a cycle of disability and declining health status,” Dr. Bergmann and her associates noted.
This study was limited in that it had a relatively small sample size, particularly in analyses that excluded Medicaid recipients. It also had a follow-up of only 1 year, so longer-term outcomes of bariatric surgery could not be assessed. “Our sample is also predominantly Caucasian and may have unique culturally-based characteristics” that limit the generalizability of the study findings, they added.
No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
Obese patients living in rural areas of West Virginia were substantially less likely than were their urban and suburban counterparts to have bariatric surgery, according to findings from a study comparing outcomes in two patient groups.
This discrepancy is attributed to a difference between rural and nonrural patients in type of insurance coverage. In this 2-year study, rural patients were nearly five times more likely to be covered by West Virginia Medicaid than were patients living in nonrural areas of the state, said Kristie L. Bergmann, PhD, of the department of behavioral medicine and psychiatry, West Virginia University, Morgantown, and her associates. The findings were published in Surgery for Obesity and Related Diseases (2017;13[4]:632-6), the journal of the American Society for Metabolic and Bariatric Surgery.
Previous research has identified rural patients’ lack of insurance as a barrier to health care. “Our results suggest that insurance denial represents a successive barrier. Despite being insured, rural individuals may be barred from surgery if insurance carriers do not offer it as a covered benefit, deny approval, or require indomitable prerequisites for surgery,” Dr. Bergmann and her associates said.
They examined the associations among rural status, access to bariatric surgery, and surgical outcomes in West Virginia in part because the state’s residents “have been consistently ranked as the most obese population in the United States, with approximately 35.1% of residents meeting criteria for obesity.” West Virginia also has the highest rates of diabetes (13%) and hypertension (41%) in the United States.
At the same time, rural populations are known to have decreased access to all forms of health care and specifically to bariatric surgery. This makes rural West Virginians “a particularly vulnerable population of interest,” the investigators said.
They performed a retrospective cohort study involving 122 obese patients seeking bariatric surgery at their university’s medical center during 2012-2014. A total of 97% of these patients were white, 83% were women, and the mean age was 47 years. Only 82 of the 122 study participants underwent bariatric surgery: 77 had Roux-en-Y gastric bypass and 5 had sleeve gastrectomy.
Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference. When Medicaid patients were excluded from the analysis, nonrural status no longer predicted the use of bariatric surgery.
Moreover, when Medicaid coverage was controlled for, rural status had no effect on the effectiveness of bariatric surgery. Patients residing in rural areas had the same attendance at follow-up visits and the same reduction in body mass index at 6 months and at 12 months as did nonrural patients.
In addition, patients who had higher levels of education and who worked full-time were more likely to undergo bariatric surgery, but overall, rural patients were more likely to have comorbidities, disability, and lower rates of full-time work. “An argument can be made that rural individuals may have a greater medical need for bariatric surgery, as obesity and associated health conditions may contribute to lower rates of employment. Unfortunately, barring these patients from receiving care may reinforce a cycle of disability and declining health status,” Dr. Bergmann and her associates noted.
This study was limited in that it had a relatively small sample size, particularly in analyses that excluded Medicaid recipients. It also had a follow-up of only 1 year, so longer-term outcomes of bariatric surgery could not be assessed. “Our sample is also predominantly Caucasian and may have unique culturally-based characteristics” that limit the generalizability of the study findings, they added.
No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: Obese patients living in rural areas of West Virginia are substantially less likely than are their urban and suburban counterparts to have bariatric surgery.
Major finding: Rural residents were significantly less likely to undergo bariatric surgery than were nonrural patients, but coverage by West Virginia Medicaid explained 83.6% of this difference.
Data source: A retrospective single-center cohort study involving 122 obese West Virginians seeking bariatric surgery in 2012-2014.
Disclosures: No specific sponsor was cited for this study. Dr. Bergmann and her associates reported having no relevant financial disclosures.
Gastric bands hit with high reoperation rates, rising costs
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).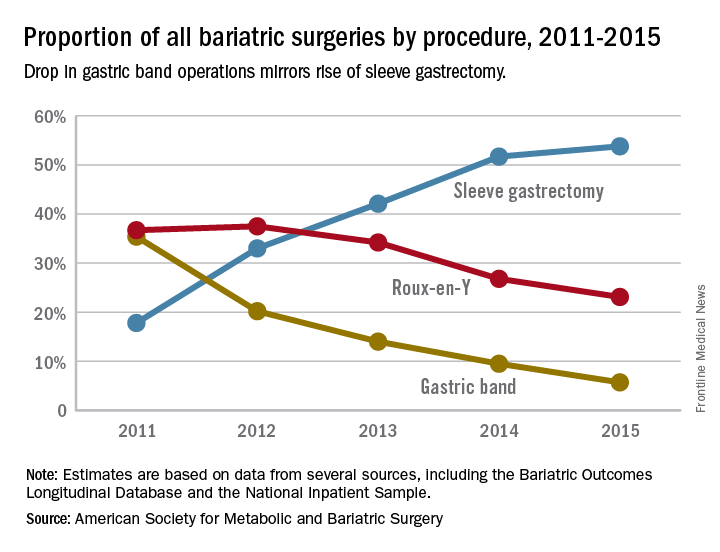
The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
Dr. Ibrahim and his colleagues have suggested that payers reconsider covering the adjustable laparoscopic gastric band. I disagree and feel that this device still has a role, albeit limited in the modern bariatric surgical program. Many patients do well for a long period. A committed surgeon and program, and the ideal patient with a similar level of commitment, are needed to achieve these best outcomes. Now that patients and surgeons are better informed of the drawbacks to the device, use has decreased without external regulations or policies to drive this change. No single bariatric procedure is appropriate for all patients. Patients need options, and we need better data to help guide their decisions. Do not throw the baby out with the bathwater.
Jon C. Gould, MD, FACS, is with the Medical College of Wisconsin, Milwaukee. Dr. Gould made these comments in an editorial (JAMA Surg. 2017 May 17; doi: 10.1001/jamasurg.2017.1082) that accompanied the study. He has no disclosures.
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).
The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
About one in five laparoscopic gastric band surgeries result in device-related reoperations and reoperations account for almost half of all Medicare expenditures for gastric band surgery, a large retrospective study has found.
The laparoscopic adjustable gastric band for treatment of morbid obesity, approved in 2001 by the Food and Drug Administration, was once a common choice for bariatric patients. Although its use has declined from in recent years, the American Society for Metabolic and Bariatric Surgery estimated that 11,000 bands were placed in 2015 and many others remain in place (ASMBS, Estimate of bariatric surgery numbers, 2011-2015, https://goo.gl/f8iByl). Many of these gastric bands will need to be removed, replaced, or revised in a series of procedures over the coming years.
Of the 24,042 gastric band patients in this study group, 4,636 (18.5%) underwent reoperation, defined as band removal, band replacement, or revision to a different bariatric procedure, but not including band size adjustment. Patients who had reoperations were more likely to be women, to be white, and to have slightly lower rates of hypertension and diabetes. But they were also more likely to have received a psychiatric, anemia, or electrolyte disorder diagnosis at the time of their index operations.
Among the 4,636 patients who had reoperations, 17,539 such procedures were performed, an average of 3.8 procedures per patient, in addition to the index operation, over an average follow-up of 4.5 years. The most common reoperation was for band removal (41.8%). Other reasons included conversion to laparoscopic Roux-en-Y gastric bypass (13.1%) or laparoscopic sleeve gastrectomy (5.3%).
The study also looked at the regional differences, reflecting the comparative success of some programs in managing laparoscopic gastric band placement. Reoperation rates across the referral hospitals ranged from 5% to 95.5%, The study found a nearly a threefold variation in reoperation rates across geographic regions. The bottom quartile of hospital referral regions had an average reoperation rate of 13.3% (0.3 standard deviation) and the top quartile had an average reoperation rate of 39.1% (0.21 SD). Top-quartile regions were concentrated in the West, but were otherwise distributed throughout the country.
Most reoperations were elective admissions (79.9%), while 10% were classified as urgent and another 10.1% as emergency. So although previous studies have documented complications such as band slippage and gastric erosion, the preponderance of elective admissions suggests patient and clinician preferences, or weight loss failure, rather than emergency situations, may be the driving force in the reoperation trend.
The investigators concluded that patients should be fully informed about the likelihood of reoperation with the gastric band. In addition, the wide range of reoperation rates across regions and institutions suggests that more training or better patient selection may be needed to improve outcomes. However, they suggested that “taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to the payers, which raises concerns about its safety, effectiveness, and value.” They added that “payers should reconsider their coverage of the gastric band device.”
Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
FROM JAMA SURGERY
Key clinical point: Reoperations after gastric band placement are common and raise concerns about the safety, effectiveness, and value of the device.
Major finding: During the study period, reoperations accounted for 47.6% of Medicare payments for laparoscopic gastric band procedures.
Data source: Medicare Provider Analysis and Review file of 25,042 beneficiaries who had gastric band procedures between 2006 and 2013.
Disclosures: Coauthor Justin B. Dimick, MD, disclosed a financial interest in ArborMetrix. The other coauthors reported having no financial disclosures. The Robert Wood Johnson Foundation, U.S. Department of Veterans Affairs, National Institute on Aging, and National Institute of Diabetes and Digestive and Kidney Diseases provided funding.
Bariatric patients can conquer obesity, but few achieve BMI < 25
Around one-quarter of obese individuals who undergo Roux-en-Y gastric bypass had sustained, long-term remission of obesity, but far fewer achieved body mass index of under 25 kg/m2 or maintained it over 5 years, new research suggests.
Researchers reported on the outcomes of a retrospective cohort study of 219 patients who underwent Roux-en-Y gastric bypass surgery at a single center between 2008 and 2010 and were followed for up to 7 years after the procedure.
Only 16.9% of female patients achieved a BMI of under 25 kg/m2 – during the study period, and only 2.7% reached this BMI by year 2 and sustained it at least to year 5 of follow-up.
Two males in the study achieved BMI of under 25 during follow-up. One was recorded as having maintained this weight at year 1 and the other at year 4, but no further measurements were available for either.
“Given the low number of patients achieving BMI of less than 25 kg/m2, we also wanted to focus on another important clinical goal of obesity remission (BMI less than 30),” wrote Corey J. Lager, MD, of the University of Michigan, Brehm Center for Diabetes, Ann Arbor, and his coauthors. “Taking into account that the mean BMI prior to surgery in our cohort was 47.1 kg/m2, this target is associated with significant estimated health benefits and likely brings a mortality benefit for patients undergoing gastric bypass.”
The authors said that a conservative estimate of the probability of achieving and sustaining BMI 25 or less after Roux-en-Y gastric bypass was just 2.3%. However, they offered a more liberal estimate – based on the number of patients who were at BMI 25 or under at the last available data point – of 6.8%.
Achieving weight loss to a BMI less than 30 was significantly influenced by age. The group who achieved this weight were on average 3 years younger at baseline than those who did not.
Similarly, initial BMI played a role in outcomes. The women who achieved a BMI below 30 had an initial mean BMI of 43.5, compared with 50.4 in the women who did not achieve this weight (P less than .0001). In males, the mean baseline BMI in those who got their weight below 30 was 44.6, compared with 48.1 in those who did not (P = .18).
Roux-en-Y gastric bypass was also associated with significant and sustained decreases in both systolic and diastolic blood pressure that was similar for both sexes. The maximum mean decrease of 14 ± 7 mm Hg was achieved at 1 year after surgery, and, at 5 years, the mean decrease was 11 ± 3 mm Hg.
The authors commented that, despite “excellent” weight loss being achieved by a majority of patients, the findings show the challenge of weight loss and maintenance in patients with a very high BMI. However, they also pointed to the encouragingly low rates of significant weight regain and the fact that fewer than 1% of patients returned to a weight greater than their preoperative weight. Higher preoperative BMI was correlated with greater weight loss but also negatively correlated with achieving BMI under 30.
The authors concluded with two takeaway messages. First, realistic goals should be set for patients undergoing gastric bypass surgery, with an emphasis on remission of obesity and with a reduced expectation of achievement of BMI under 25 over the long run. In addition, because the higher the initial BMI, the less likely that weight loss will not be maintained, “we should also carefully examine the option of pursuing surgery at lower BMI cutoffs, at which point patients have a greater likelihood of obesity remission.”
The study was supported by the University of Michigan Health System, the National Institutes of Health, and the Nutrition Obesity Research Centers. One author declared grant support and advisory positions with pharmaceutical companies and intellectual property unrelated to the study. Another author is an investigator on a sponsored clinical study. No other conflicts of interest were declared.
Around one-quarter of obese individuals who undergo Roux-en-Y gastric bypass had sustained, long-term remission of obesity, but far fewer achieved body mass index of under 25 kg/m2 or maintained it over 5 years, new research suggests.
Researchers reported on the outcomes of a retrospective cohort study of 219 patients who underwent Roux-en-Y gastric bypass surgery at a single center between 2008 and 2010 and were followed for up to 7 years after the procedure.
Only 16.9% of female patients achieved a BMI of under 25 kg/m2 – during the study period, and only 2.7% reached this BMI by year 2 and sustained it at least to year 5 of follow-up.
Two males in the study achieved BMI of under 25 during follow-up. One was recorded as having maintained this weight at year 1 and the other at year 4, but no further measurements were available for either.
“Given the low number of patients achieving BMI of less than 25 kg/m2, we also wanted to focus on another important clinical goal of obesity remission (BMI less than 30),” wrote Corey J. Lager, MD, of the University of Michigan, Brehm Center for Diabetes, Ann Arbor, and his coauthors. “Taking into account that the mean BMI prior to surgery in our cohort was 47.1 kg/m2, this target is associated with significant estimated health benefits and likely brings a mortality benefit for patients undergoing gastric bypass.”
The authors said that a conservative estimate of the probability of achieving and sustaining BMI 25 or less after Roux-en-Y gastric bypass was just 2.3%. However, they offered a more liberal estimate – based on the number of patients who were at BMI 25 or under at the last available data point – of 6.8%.
Achieving weight loss to a BMI less than 30 was significantly influenced by age. The group who achieved this weight were on average 3 years younger at baseline than those who did not.
Similarly, initial BMI played a role in outcomes. The women who achieved a BMI below 30 had an initial mean BMI of 43.5, compared with 50.4 in the women who did not achieve this weight (P less than .0001). In males, the mean baseline BMI in those who got their weight below 30 was 44.6, compared with 48.1 in those who did not (P = .18).
Roux-en-Y gastric bypass was also associated with significant and sustained decreases in both systolic and diastolic blood pressure that was similar for both sexes. The maximum mean decrease of 14 ± 7 mm Hg was achieved at 1 year after surgery, and, at 5 years, the mean decrease was 11 ± 3 mm Hg.
The authors commented that, despite “excellent” weight loss being achieved by a majority of patients, the findings show the challenge of weight loss and maintenance in patients with a very high BMI. However, they also pointed to the encouragingly low rates of significant weight regain and the fact that fewer than 1% of patients returned to a weight greater than their preoperative weight. Higher preoperative BMI was correlated with greater weight loss but also negatively correlated with achieving BMI under 30.
The authors concluded with two takeaway messages. First, realistic goals should be set for patients undergoing gastric bypass surgery, with an emphasis on remission of obesity and with a reduced expectation of achievement of BMI under 25 over the long run. In addition, because the higher the initial BMI, the less likely that weight loss will not be maintained, “we should also carefully examine the option of pursuing surgery at lower BMI cutoffs, at which point patients have a greater likelihood of obesity remission.”
The study was supported by the University of Michigan Health System, the National Institutes of Health, and the Nutrition Obesity Research Centers. One author declared grant support and advisory positions with pharmaceutical companies and intellectual property unrelated to the study. Another author is an investigator on a sponsored clinical study. No other conflicts of interest were declared.
Around one-quarter of obese individuals who undergo Roux-en-Y gastric bypass had sustained, long-term remission of obesity, but far fewer achieved body mass index of under 25 kg/m2 or maintained it over 5 years, new research suggests.
Researchers reported on the outcomes of a retrospective cohort study of 219 patients who underwent Roux-en-Y gastric bypass surgery at a single center between 2008 and 2010 and were followed for up to 7 years after the procedure.
Only 16.9% of female patients achieved a BMI of under 25 kg/m2 – during the study period, and only 2.7% reached this BMI by year 2 and sustained it at least to year 5 of follow-up.
Two males in the study achieved BMI of under 25 during follow-up. One was recorded as having maintained this weight at year 1 and the other at year 4, but no further measurements were available for either.
“Given the low number of patients achieving BMI of less than 25 kg/m2, we also wanted to focus on another important clinical goal of obesity remission (BMI less than 30),” wrote Corey J. Lager, MD, of the University of Michigan, Brehm Center for Diabetes, Ann Arbor, and his coauthors. “Taking into account that the mean BMI prior to surgery in our cohort was 47.1 kg/m2, this target is associated with significant estimated health benefits and likely brings a mortality benefit for patients undergoing gastric bypass.”
The authors said that a conservative estimate of the probability of achieving and sustaining BMI 25 or less after Roux-en-Y gastric bypass was just 2.3%. However, they offered a more liberal estimate – based on the number of patients who were at BMI 25 or under at the last available data point – of 6.8%.
Achieving weight loss to a BMI less than 30 was significantly influenced by age. The group who achieved this weight were on average 3 years younger at baseline than those who did not.
Similarly, initial BMI played a role in outcomes. The women who achieved a BMI below 30 had an initial mean BMI of 43.5, compared with 50.4 in the women who did not achieve this weight (P less than .0001). In males, the mean baseline BMI in those who got their weight below 30 was 44.6, compared with 48.1 in those who did not (P = .18).
Roux-en-Y gastric bypass was also associated with significant and sustained decreases in both systolic and diastolic blood pressure that was similar for both sexes. The maximum mean decrease of 14 ± 7 mm Hg was achieved at 1 year after surgery, and, at 5 years, the mean decrease was 11 ± 3 mm Hg.
The authors commented that, despite “excellent” weight loss being achieved by a majority of patients, the findings show the challenge of weight loss and maintenance in patients with a very high BMI. However, they also pointed to the encouragingly low rates of significant weight regain and the fact that fewer than 1% of patients returned to a weight greater than their preoperative weight. Higher preoperative BMI was correlated with greater weight loss but also negatively correlated with achieving BMI under 30.
The authors concluded with two takeaway messages. First, realistic goals should be set for patients undergoing gastric bypass surgery, with an emphasis on remission of obesity and with a reduced expectation of achievement of BMI under 25 over the long run. In addition, because the higher the initial BMI, the less likely that weight loss will not be maintained, “we should also carefully examine the option of pursuing surgery at lower BMI cutoffs, at which point patients have a greater likelihood of obesity remission.”
The study was supported by the University of Michigan Health System, the National Institutes of Health, and the Nutrition Obesity Research Centers. One author declared grant support and advisory positions with pharmaceutical companies and intellectual property unrelated to the study. Another author is an investigator on a sponsored clinical study. No other conflicts of interest were declared.
FROM OBESITY SURGERY
Key clinical point: Roux-en-Y gastric bypass is associated with sustained, long-term remission of obesity in around one-quarter of patients.
Major finding: Nearly one-quarter of patients who underwent Roux-en-Y gastric bypass achieved a BMI below 30 kg/m2 that was sustained at their last follow-up, but healthy weight was sustained at 5 years’ follow-up in far fewer patients.
Data source: Retrospective cohort study of 219 patients.
Disclosures: The study was supported by the University of Michigan Health System, the National Institutes of Health, and the Nutrition Obesity Research Centers. One author declared grant support and advisory positions with pharmaceutical companies and intellectual property unrelated to the study. Another author is an investigator on a sponsored clinical study. No other conflicts of interest were declared.
Roux-en-Y bests sleeve gastrectomy for weight loss
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
koakes@frontlinemedcom.com
On Twitter @karioakes
AT ENDO 2017
ORLANDO – Roux-en-Y gastric bypass resulted in greater weight loss than sleeve gastrectomy in a study that followed more than 700 patients, an effect that was sustained over time.
However, surgical complications were more common than with sleeve gastrectomy, and patients were more likely to have an extended hospital stay.
The study, conducted by Corey Lager, MD, and his collaborators at the University of Michigan Medical Center, Ann Arbor, looked at 5-year outcomes for 380 patients who had Roux-en-Y gastric bypass (RYGB), compared with those for 336 patients who received sleeve gastrectomy (SG).
Specific outcomes examined included the amount of absolute weight loss and excess body weight loss over the 5-year study period, whether obesity-related comorbidities resolved, and the type and number of complications seen with each procedure.
Sleeve gastrectomy is becoming increasingly popular, even as RYGB and adjustable gastric banding procedures have become more and more rare, Dr. Lager said at the annual meeting of the Endocrine Society. Duodenal switch procedures have continued to represent a very small proportion of surgical weight loss surgeries. Of the four, SG accounted for nearly 80% of the procedures performed in 2013; RYGB, which accounted for about 60% of procedures in 2006, fell to about 30% of procedures by 2013.
The investigators conducted a retrospective analysis of patients undergoing RYGB or SG from January 2008 to November 2013. Patients were seen annually in postoperative follow-up, so the study was able to track body mass index (BMI), weight, excess body weight loss, hemoglobin A1c levels, blood pressure, and serum lipid and vitamin levels over the 5-year period. Additionally, the study captured 30-day postoperative complications for each procedure.
Although about 80% of patients undergoing each procedure were female and baseline lab values and characteristics were similar in many respects, patients undergoing sleeve gastrectomy had higher body weight (mean, 143 kg) and BMI (mean, 50 kg/m2), compared with those who received RYGB (weight, 133 kg; BMI, 47; P less than .001 for both). The average age in both groups was about 45 years.
Sleeve gastrectomy patients were less likely to continue for the full 5 years of follow-up. Of 336 SG patients originally enrolled, 93 had 5-year data. Of the 380 RYGB patients, 188 returned for the 5-year follow-up.
At all time points, the RYGB patients had significantly more total weight loss than the SG patients (P less than .05); the initial weight loss for RYGB patients approached 28% of body weight at year 1, compared with about 23% for the SG patients. By the end of the 5-year period, RYGB patients had maintained about a 24% weight loss, compared with almost 20% for the SG group.
This pattern was mirrored for BMI in each cohort: At year 1, the RYGB patients were down about 14 points, compared with about 12 points for the SG group. By year 5, the difference had narrowed so that each group had lost a mean of between 11 and 12 points from their original BMI, but the difference was still statistically significant (P less than .05).
The final measure of weight loss was excess body weight lost, and again, RYGB patients lost significantly more of their excess body weight at all time points than did the SG patients. At the end of the first year, RYGB had lost more than 65% of their excess body weight, compared with about 48% for the SG patients. By 5 years, the SG patients had regained enough weight that their net excess weight loss was a little less than 40%, while the RYGB patients’ regain put them at about 55% excess weight loss by the end of the study period.
In terms of biomarkers, systolic blood pressure did not differ significantly between the three groups except at study year 3, though the RYGB group had numerically slightly lower systolic blood pressures at all time points. Total cholesterol was lower at 1, 2, 4, and 5 years after surgery for the RYGB group.
Sleeve gastrectomy, as expected, had lower rates of grade I surgical complications, including hemorrhage and infection. Also, the SG patients had fewer postsurgical emergency department visits and a shorter length of stay.
The study results were consistent with those of a 2016 meta-analysis that favored RYGB in terms of excess weight lost, readmission for diabetes-related complications, and resolution of hypertension (Obes Surg. 2016 Feb;26[2]:429-42).
Although this was a large study, it was limited by its retrospective nature and by the lack of randomization, said Dr. Lager. Retaining patients for long-term follow-up was also an issue: Of the original 719 patients, 507 were followed at 3 years and 281 at 5 years, so a significant number weren’t tracked for the full 5 years.
Dr. Lager reported no conflicts of interest, and the study had no outside sources of funding.
koakes@frontlinemedcom.com
On Twitter @karioakes
Key clinical point:
Major finding: At 5 years post surgery, Roux-en-Y recipients had kept off 25% of their body weight, compared with 20% for sleeve gastrectomy patients (P less than .05).
Data source: Longitudinal follow-up of 716 patients who had one of two surgical procedures for weight loss.
Disclosures: None of the study authors reported relevant disclosures, and no external source of funding was reported.
Nomogram may direct diabetes patients to best operation
PHILADELPHIA – A nomogram that assigns a disease severity score to individuals with type 2 diabetes may provide a tool that helps surgeons, endocrinologists, and primary care physicians determine which weight-loss surgical procedure would be most effective, according to an analysis of 900 patients from Cleveland Clinic and University Hospital Clinic, Barcelona, reported at the annual meeting of the American Surgical Association.
“This is the largest reported cohort with long-term glycemic follow-up data that categorizes diabetes into three validated stages of severity to guide procedure selection,” said Ali Aminian, MD, of Cleveland Clinic. The study also highlighted the importance of surgery in early diabetes. The study involved a modeling cohort of 659 patients who had bariatric procedures at Cleveland Clinic from 2005 to 2011 and a separate data set of 241 patients from Barcelona to validate the findings. Roux-en-Y gastric bypass (RYGB) was performed in 78% of the Cleveland Clinic group and 49% of the Barcelona group, with the remainder having sleeve gastrectomy (SG).
RYGB and SG account for more than 95% of all bariatric procedures in people with type 2 diabetes, Dr. Aminian said, but outcomes of clinical trials have been variable, some reporting up to half of patients having long-term relapses. The Cleveland Clinic study involved all patients with type 2 diabetes who had RYGB or SG from 2005 to 2011 with 5 years or more of glycemic data, with a median follow-up of 7 years. The study used American Diabetes Association targets to define remission and glycemic control.
“Long-term response after bariatric surgery in patients with diabetes significantly differs according to diabetes severity,” Dr. Aminian said. “For example, the outcome of surgery in a patient who has diabetes for 2 years is significantly different than a patient who has diabetes for 15 years taking three medications, including insulin.”
The researchers generated the nomogram based on these four independent preoperative factors:
- Number of preoperative diabetes medications (P less than .0001).
- Insulin use (P = .002).
- Duration of diabetes (P less than .0001).
- Glycemic control (P = .002).
“These factors are readily available in clinical practice and are considered a proxy of functional pancreatic beta-cell reserve,” Dr. Aminian said. The nomogram scores the severity of each factor on a scale of 0 to 100. For example, one preoperative medication scores 12, but five scores 63; duration of diabetes of 1 year scores five points, but 16 years scores 60. The patient’s total points represent the Individualized Metabolic Surgery score.
The researchers assigned three categories: A score of 25 or less represents mild disease, a score of 25-95 represents moderate, and 95 to the maximum 180 is severe disease.
Based on the Cleveland Clinic and Barcelona cohorts, the researchers next developed recommendations for average risk patients in each category.
RYGB is “suggested” in mild disease based on remission rates of 92% in the Cleveland subgroup and 91% in the Barcelona subgroup vs. SG remission rates of 74% and 91%, respectively. On the other end of the spectrum, in patients with severe diabetes, both procedures were less effective in achieving long-term diabetes remission. The disparities were more pronounced for the moderate group: 60% and 70% for RYGB and 25% and 56% for SG in the Cleveland Clinic and Barcelona subgroups, respectively. Hence the nomogram highly “recommends” RYGB. An online calculator to determine Individualized Metabolic Surgery score is available at http://riskcalc.org/Metabolic_Surgery_Score/.
“Obviously, this is the first attempt toward individualized procedure selection and more work needs to be done,” Dr. Aminian said. “Our findings also highlight the importance of surgical intervention in early stages of diabetes in order to achieve sustainable remission.”
In his discussion, Matthew M. Hutter, MD, FACS, of Harvard Medical School, Boston, offered to “quibble” with Dr. Aminian’s conclusions. “I challenge you on your conclusion for the mild and severe categories,” Dr. Hutter said. “My rate-of-cure data makes me want to recommend bypass for any patient with diabetes – mild, moderate or severe.”
Dr. Aminian acknowledged that the number of SG cases in the severe subgroup – 51 – was not great, and long-term diabetes remission was comparable between the two procedures. The key distinguishing measure in the severe category was a net 8% difference between two procedures in glycemic control at last follow-up. “This is the difference that we must decide whether it’s clinically important or not – whether we’re willing to recommend a riskier procedure for an extra 8% achieving glycemic control,” Dr. Aminian said. “If someone thinks it’s worth the risk, then they may suggest gastric bypass. If someone thinks it’s not worth the risk, then they may suggest a sleeve gastrectomy. But, we should remember that patients in the severe group are very high-risk patients.”
Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – A nomogram that assigns a disease severity score to individuals with type 2 diabetes may provide a tool that helps surgeons, endocrinologists, and primary care physicians determine which weight-loss surgical procedure would be most effective, according to an analysis of 900 patients from Cleveland Clinic and University Hospital Clinic, Barcelona, reported at the annual meeting of the American Surgical Association.
“This is the largest reported cohort with long-term glycemic follow-up data that categorizes diabetes into three validated stages of severity to guide procedure selection,” said Ali Aminian, MD, of Cleveland Clinic. The study also highlighted the importance of surgery in early diabetes. The study involved a modeling cohort of 659 patients who had bariatric procedures at Cleveland Clinic from 2005 to 2011 and a separate data set of 241 patients from Barcelona to validate the findings. Roux-en-Y gastric bypass (RYGB) was performed in 78% of the Cleveland Clinic group and 49% of the Barcelona group, with the remainder having sleeve gastrectomy (SG).
RYGB and SG account for more than 95% of all bariatric procedures in people with type 2 diabetes, Dr. Aminian said, but outcomes of clinical trials have been variable, some reporting up to half of patients having long-term relapses. The Cleveland Clinic study involved all patients with type 2 diabetes who had RYGB or SG from 2005 to 2011 with 5 years or more of glycemic data, with a median follow-up of 7 years. The study used American Diabetes Association targets to define remission and glycemic control.
“Long-term response after bariatric surgery in patients with diabetes significantly differs according to diabetes severity,” Dr. Aminian said. “For example, the outcome of surgery in a patient who has diabetes for 2 years is significantly different than a patient who has diabetes for 15 years taking three medications, including insulin.”
The researchers generated the nomogram based on these four independent preoperative factors:
- Number of preoperative diabetes medications (P less than .0001).
- Insulin use (P = .002).
- Duration of diabetes (P less than .0001).
- Glycemic control (P = .002).
“These factors are readily available in clinical practice and are considered a proxy of functional pancreatic beta-cell reserve,” Dr. Aminian said. The nomogram scores the severity of each factor on a scale of 0 to 100. For example, one preoperative medication scores 12, but five scores 63; duration of diabetes of 1 year scores five points, but 16 years scores 60. The patient’s total points represent the Individualized Metabolic Surgery score.
The researchers assigned three categories: A score of 25 or less represents mild disease, a score of 25-95 represents moderate, and 95 to the maximum 180 is severe disease.
Based on the Cleveland Clinic and Barcelona cohorts, the researchers next developed recommendations for average risk patients in each category.
RYGB is “suggested” in mild disease based on remission rates of 92% in the Cleveland subgroup and 91% in the Barcelona subgroup vs. SG remission rates of 74% and 91%, respectively. On the other end of the spectrum, in patients with severe diabetes, both procedures were less effective in achieving long-term diabetes remission. The disparities were more pronounced for the moderate group: 60% and 70% for RYGB and 25% and 56% for SG in the Cleveland Clinic and Barcelona subgroups, respectively. Hence the nomogram highly “recommends” RYGB. An online calculator to determine Individualized Metabolic Surgery score is available at http://riskcalc.org/Metabolic_Surgery_Score/.
“Obviously, this is the first attempt toward individualized procedure selection and more work needs to be done,” Dr. Aminian said. “Our findings also highlight the importance of surgical intervention in early stages of diabetes in order to achieve sustainable remission.”
In his discussion, Matthew M. Hutter, MD, FACS, of Harvard Medical School, Boston, offered to “quibble” with Dr. Aminian’s conclusions. “I challenge you on your conclusion for the mild and severe categories,” Dr. Hutter said. “My rate-of-cure data makes me want to recommend bypass for any patient with diabetes – mild, moderate or severe.”
Dr. Aminian acknowledged that the number of SG cases in the severe subgroup – 51 – was not great, and long-term diabetes remission was comparable between the two procedures. The key distinguishing measure in the severe category was a net 8% difference between two procedures in glycemic control at last follow-up. “This is the difference that we must decide whether it’s clinically important or not – whether we’re willing to recommend a riskier procedure for an extra 8% achieving glycemic control,” Dr. Aminian said. “If someone thinks it’s worth the risk, then they may suggest gastric bypass. If someone thinks it’s not worth the risk, then they may suggest a sleeve gastrectomy. But, we should remember that patients in the severe group are very high-risk patients.”
Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
PHILADELPHIA – A nomogram that assigns a disease severity score to individuals with type 2 diabetes may provide a tool that helps surgeons, endocrinologists, and primary care physicians determine which weight-loss surgical procedure would be most effective, according to an analysis of 900 patients from Cleveland Clinic and University Hospital Clinic, Barcelona, reported at the annual meeting of the American Surgical Association.
“This is the largest reported cohort with long-term glycemic follow-up data that categorizes diabetes into three validated stages of severity to guide procedure selection,” said Ali Aminian, MD, of Cleveland Clinic. The study also highlighted the importance of surgery in early diabetes. The study involved a modeling cohort of 659 patients who had bariatric procedures at Cleveland Clinic from 2005 to 2011 and a separate data set of 241 patients from Barcelona to validate the findings. Roux-en-Y gastric bypass (RYGB) was performed in 78% of the Cleveland Clinic group and 49% of the Barcelona group, with the remainder having sleeve gastrectomy (SG).
RYGB and SG account for more than 95% of all bariatric procedures in people with type 2 diabetes, Dr. Aminian said, but outcomes of clinical trials have been variable, some reporting up to half of patients having long-term relapses. The Cleveland Clinic study involved all patients with type 2 diabetes who had RYGB or SG from 2005 to 2011 with 5 years or more of glycemic data, with a median follow-up of 7 years. The study used American Diabetes Association targets to define remission and glycemic control.
“Long-term response after bariatric surgery in patients with diabetes significantly differs according to diabetes severity,” Dr. Aminian said. “For example, the outcome of surgery in a patient who has diabetes for 2 years is significantly different than a patient who has diabetes for 15 years taking three medications, including insulin.”
The researchers generated the nomogram based on these four independent preoperative factors:
- Number of preoperative diabetes medications (P less than .0001).
- Insulin use (P = .002).
- Duration of diabetes (P less than .0001).
- Glycemic control (P = .002).
“These factors are readily available in clinical practice and are considered a proxy of functional pancreatic beta-cell reserve,” Dr. Aminian said. The nomogram scores the severity of each factor on a scale of 0 to 100. For example, one preoperative medication scores 12, but five scores 63; duration of diabetes of 1 year scores five points, but 16 years scores 60. The patient’s total points represent the Individualized Metabolic Surgery score.
The researchers assigned three categories: A score of 25 or less represents mild disease, a score of 25-95 represents moderate, and 95 to the maximum 180 is severe disease.
Based on the Cleveland Clinic and Barcelona cohorts, the researchers next developed recommendations for average risk patients in each category.
RYGB is “suggested” in mild disease based on remission rates of 92% in the Cleveland subgroup and 91% in the Barcelona subgroup vs. SG remission rates of 74% and 91%, respectively. On the other end of the spectrum, in patients with severe diabetes, both procedures were less effective in achieving long-term diabetes remission. The disparities were more pronounced for the moderate group: 60% and 70% for RYGB and 25% and 56% for SG in the Cleveland Clinic and Barcelona subgroups, respectively. Hence the nomogram highly “recommends” RYGB. An online calculator to determine Individualized Metabolic Surgery score is available at http://riskcalc.org/Metabolic_Surgery_Score/.
“Obviously, this is the first attempt toward individualized procedure selection and more work needs to be done,” Dr. Aminian said. “Our findings also highlight the importance of surgical intervention in early stages of diabetes in order to achieve sustainable remission.”
In his discussion, Matthew M. Hutter, MD, FACS, of Harvard Medical School, Boston, offered to “quibble” with Dr. Aminian’s conclusions. “I challenge you on your conclusion for the mild and severe categories,” Dr. Hutter said. “My rate-of-cure data makes me want to recommend bypass for any patient with diabetes – mild, moderate or severe.”
Dr. Aminian acknowledged that the number of SG cases in the severe subgroup – 51 – was not great, and long-term diabetes remission was comparable between the two procedures. The key distinguishing measure in the severe category was a net 8% difference between two procedures in glycemic control at last follow-up. “This is the difference that we must decide whether it’s clinically important or not – whether we’re willing to recommend a riskier procedure for an extra 8% achieving glycemic control,” Dr. Aminian said. “If someone thinks it’s worth the risk, then they may suggest gastric bypass. If someone thinks it’s not worth the risk, then they may suggest a sleeve gastrectomy. But, we should remember that patients in the severe group are very high-risk patients.”
Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is to be published in Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: A nomogram has been developed that assigns an Individualized Metabolic Surgery score to individuals with type 2 diabetes to help determine which type of bariatric procedure would provide best outcomes.
Major finding: In mild diabetes (Individualized Metabolic Surgery score less than or equal to 25), Roux-en-Y gastric bypass and sleeve gastrectomy significantly improve diabetes. For patients with severe diabetes (IMS Score greater than 95), both procedures have similarly low efficacy for diabetes remission.
Data source: Analysis of 900 patients with type 2 diabetes who had either Roux-en-Y gastric bypass or sleeve gastrectomy with a minimum 5-year follow-up.
Disclosure: Dr. Aminian reported no financial disclosures. Dr. Hutter disclosed receiving conference reimbursement from Olympus.
How bariatric surgery improves knee osteoarthritis
LAS VEGAS – Most of the improvement in knee pain that occurs following bariatric surgery in obese patients with knee osteoarthritis happens in the first month after surgery, well before the bulk of the weight loss takes place, Jonathan Samuels, MD, reported at the World Congress on Osteoarthritis.
This observation suggests that bariatric surgery’s mechanism of benefit in patients with knee osteoarthritis (OA) isn’t simply a matter of reduced mechanical load on the joints caused by a lessened weight burden, Dr. Samuels observed at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
Indeed, his prospective study of 150 obese patients with comorbid knee OA points to metabolic factors as likely playing a key role.
His study, featuring 2 years of follow-up to date, showed that bariatric surgery improved knee OA proportionate to the percentage of excess weight loss achieved. The greatest reduction in knee pain as well as the most profound weight loss occurred in the 35 patients who underwent gastric bypass and the 97 who opted for sleeve gastrectomy; patients who underwent laparoscopic adjustable gastric banding had more modest outcomes on both scores.
The disparate timing of the reductions in excess weight and knee pain was particularly eye catching. With all three forms of bariatric surgery, weight loss continued steadily for roughly the first 12 months. It then plateaued and was generally maintained at the new body mass index for the second 12 months.
In contrast, improvement in knee pain according to the validated Knee Injury and Osteoarthritis Outcome Score (KOOS) leveled out after just 1 month post surgery and was then sustained through 23 months. Levels of the inflammatory cytokines and adipokines interleukin-6, interleukin-1 receptor antagonist, and lipopolysaccharides were elevated at baseline but dropped steadily in concert with the reduction in excess body weight during the first 12 months after surgery. In contrast, levels of the anti-inflammatory cytokine sRAGE (soluble receptor for advanced glycation end products) were abnormally low prior to surgery but increased sharply for the first 3 months afterward before leveling off. And levels of serum leptin, which were roughly sevenfold greater than in normal controls at baseline, fell precipitously during the first month after bariatric surgery before plateauing, following the same pattern as the improvement in knee pain.
“This suggests that perhaps leptin is the key mediator in this OA population,” said Dr. Samuels.
Obese patients with knee OA are in a catch-22 situation. Obese individuals are at greatly increased lifetime risk of developing knee OA, and patients with chronic knee pain have a tough time losing weight.
“The treatments that might work with either obesity or knee pain alone often fail when both of these are present,” he observed.
That’s why bariatric surgery is becoming an increasingly popular treatment strategy in these patients. Sleeve gastrectomy, gastric bypass, and laparoscopic adjustable gastric banding are all Food and Drug Administration approved treatments for obesity in the presence of at least one qualifying comorbid condition, and knee OA qualifies.
Dr. Samuels reported having no financial conflicts regarding his study.
LAS VEGAS – Most of the improvement in knee pain that occurs following bariatric surgery in obese patients with knee osteoarthritis happens in the first month after surgery, well before the bulk of the weight loss takes place, Jonathan Samuels, MD, reported at the World Congress on Osteoarthritis.
This observation suggests that bariatric surgery’s mechanism of benefit in patients with knee osteoarthritis (OA) isn’t simply a matter of reduced mechanical load on the joints caused by a lessened weight burden, Dr. Samuels observed at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
Indeed, his prospective study of 150 obese patients with comorbid knee OA points to metabolic factors as likely playing a key role.
His study, featuring 2 years of follow-up to date, showed that bariatric surgery improved knee OA proportionate to the percentage of excess weight loss achieved. The greatest reduction in knee pain as well as the most profound weight loss occurred in the 35 patients who underwent gastric bypass and the 97 who opted for sleeve gastrectomy; patients who underwent laparoscopic adjustable gastric banding had more modest outcomes on both scores.
The disparate timing of the reductions in excess weight and knee pain was particularly eye catching. With all three forms of bariatric surgery, weight loss continued steadily for roughly the first 12 months. It then plateaued and was generally maintained at the new body mass index for the second 12 months.
In contrast, improvement in knee pain according to the validated Knee Injury and Osteoarthritis Outcome Score (KOOS) leveled out after just 1 month post surgery and was then sustained through 23 months. Levels of the inflammatory cytokines and adipokines interleukin-6, interleukin-1 receptor antagonist, and lipopolysaccharides were elevated at baseline but dropped steadily in concert with the reduction in excess body weight during the first 12 months after surgery. In contrast, levels of the anti-inflammatory cytokine sRAGE (soluble receptor for advanced glycation end products) were abnormally low prior to surgery but increased sharply for the first 3 months afterward before leveling off. And levels of serum leptin, which were roughly sevenfold greater than in normal controls at baseline, fell precipitously during the first month after bariatric surgery before plateauing, following the same pattern as the improvement in knee pain.
“This suggests that perhaps leptin is the key mediator in this OA population,” said Dr. Samuels.
Obese patients with knee OA are in a catch-22 situation. Obese individuals are at greatly increased lifetime risk of developing knee OA, and patients with chronic knee pain have a tough time losing weight.
“The treatments that might work with either obesity or knee pain alone often fail when both of these are present,” he observed.
That’s why bariatric surgery is becoming an increasingly popular treatment strategy in these patients. Sleeve gastrectomy, gastric bypass, and laparoscopic adjustable gastric banding are all Food and Drug Administration approved treatments for obesity in the presence of at least one qualifying comorbid condition, and knee OA qualifies.
Dr. Samuels reported having no financial conflicts regarding his study.
LAS VEGAS – Most of the improvement in knee pain that occurs following bariatric surgery in obese patients with knee osteoarthritis happens in the first month after surgery, well before the bulk of the weight loss takes place, Jonathan Samuels, MD, reported at the World Congress on Osteoarthritis.
This observation suggests that bariatric surgery’s mechanism of benefit in patients with knee osteoarthritis (OA) isn’t simply a matter of reduced mechanical load on the joints caused by a lessened weight burden, Dr. Samuels observed at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
Indeed, his prospective study of 150 obese patients with comorbid knee OA points to metabolic factors as likely playing a key role.
His study, featuring 2 years of follow-up to date, showed that bariatric surgery improved knee OA proportionate to the percentage of excess weight loss achieved. The greatest reduction in knee pain as well as the most profound weight loss occurred in the 35 patients who underwent gastric bypass and the 97 who opted for sleeve gastrectomy; patients who underwent laparoscopic adjustable gastric banding had more modest outcomes on both scores.
The disparate timing of the reductions in excess weight and knee pain was particularly eye catching. With all three forms of bariatric surgery, weight loss continued steadily for roughly the first 12 months. It then plateaued and was generally maintained at the new body mass index for the second 12 months.
In contrast, improvement in knee pain according to the validated Knee Injury and Osteoarthritis Outcome Score (KOOS) leveled out after just 1 month post surgery and was then sustained through 23 months. Levels of the inflammatory cytokines and adipokines interleukin-6, interleukin-1 receptor antagonist, and lipopolysaccharides were elevated at baseline but dropped steadily in concert with the reduction in excess body weight during the first 12 months after surgery. In contrast, levels of the anti-inflammatory cytokine sRAGE (soluble receptor for advanced glycation end products) were abnormally low prior to surgery but increased sharply for the first 3 months afterward before leveling off. And levels of serum leptin, which were roughly sevenfold greater than in normal controls at baseline, fell precipitously during the first month after bariatric surgery before plateauing, following the same pattern as the improvement in knee pain.
“This suggests that perhaps leptin is the key mediator in this OA population,” said Dr. Samuels.
Obese patients with knee OA are in a catch-22 situation. Obese individuals are at greatly increased lifetime risk of developing knee OA, and patients with chronic knee pain have a tough time losing weight.
“The treatments that might work with either obesity or knee pain alone often fail when both of these are present,” he observed.
That’s why bariatric surgery is becoming an increasingly popular treatment strategy in these patients. Sleeve gastrectomy, gastric bypass, and laparoscopic adjustable gastric banding are all Food and Drug Administration approved treatments for obesity in the presence of at least one qualifying comorbid condition, and knee OA qualifies.
Dr. Samuels reported having no financial conflicts regarding his study.
AT OARSI 2017
Key clinical point:
Major finding: Most of the improvement in knee pain – and most of the accompanying drop in serum leptin – happens in the first month following bariatric surgery, well before most weight loss has occurred.
Data source: A prospective observational study of 150 obese patients with knee osteoarthritis who underwent bariatric surgery.
Disclosures: The study presenter reported having no financial conflicts.
Endoscopic weight loss surgery cuts costs, side effects
Obese patients who underwent endoscopic sleeve gastroplasty had significantly fewer complications and shorter hospital stays than did those who had laparoscopic sleeve gastrectomy or laparoscopic band placement, according to results from a study of 278 adults. The data were presented at the annual Digestive Disease Week®.
Overall, 1% of patients who underwent endoscopic sleeve gastroplasty (ESG) experienced adverse events, compared with 8% of those who underwent laparoscopic sleeve gastrectomy (LSG) and 9% of those who underwent laparoscopic band placement (LAGB).
ESG, which reduces gastric volume by use of an endoscopic suturing system of full-thickness sutures through the greater curvature of the stomach, is becoming a popular weight-loss procedure for patients with a body mass index greater than 30 kg/m2 who are poor candidates for laparoscopic surgery or who would prefer a less invasive procedure, according to Reem Z. Sharaiha, MD, of Cornell University, New York.
Dr. Sharaiha and her colleagues randomized 91 patients to ESG, 120 to LSG, and 67 to LAGB. Patient demographic characteristics, including age, gender, and diabetes, were similar among the three groups. However, patients in the LSG group had a higher average BMI than did the LAGB and ESG groups (47.3 kg/m2, 45.7 kg/m2, and 38.8 kg/m2, respectively). In addition, the incidence of hypertension, and hyperlipidemia was significantly higher in each of the surgical groups compared to the ESG group (P less than .01).
The average postprocedure hospital stay was 0.13 days for ESG patients compared with 3.09 days for LSG patients and 1.68 days for LAGB patients. ESG also had the lowest cost of the three procedures, averaging $12,000 for the procedure compared to $22,000 for LSG and $15,000 for LAGB.
After 1 year, patients in the LSG group had the greatest percentage of total body weight loss (29.3%), followed by ESG patients (17.6%), and LAGB patients (14.5%). Rates of leaks, pulmonary embolism events, and 90-day readmission were not significantly different among the groups.
The study results do not imply that ESG will replace either LAGB or LSG for weight loss, Dr. Sharaiha noted, but the results suggest that ESG is a viable option for some patients.
Dr. Sharaiha had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
Obese patients who underwent endoscopic sleeve gastroplasty had significantly fewer complications and shorter hospital stays than did those who had laparoscopic sleeve gastrectomy or laparoscopic band placement, according to results from a study of 278 adults. The data were presented at the annual Digestive Disease Week®.
Overall, 1% of patients who underwent endoscopic sleeve gastroplasty (ESG) experienced adverse events, compared with 8% of those who underwent laparoscopic sleeve gastrectomy (LSG) and 9% of those who underwent laparoscopic band placement (LAGB).
ESG, which reduces gastric volume by use of an endoscopic suturing system of full-thickness sutures through the greater curvature of the stomach, is becoming a popular weight-loss procedure for patients with a body mass index greater than 30 kg/m2 who are poor candidates for laparoscopic surgery or who would prefer a less invasive procedure, according to Reem Z. Sharaiha, MD, of Cornell University, New York.
Dr. Sharaiha and her colleagues randomized 91 patients to ESG, 120 to LSG, and 67 to LAGB. Patient demographic characteristics, including age, gender, and diabetes, were similar among the three groups. However, patients in the LSG group had a higher average BMI than did the LAGB and ESG groups (47.3 kg/m2, 45.7 kg/m2, and 38.8 kg/m2, respectively). In addition, the incidence of hypertension, and hyperlipidemia was significantly higher in each of the surgical groups compared to the ESG group (P less than .01).
The average postprocedure hospital stay was 0.13 days for ESG patients compared with 3.09 days for LSG patients and 1.68 days for LAGB patients. ESG also had the lowest cost of the three procedures, averaging $12,000 for the procedure compared to $22,000 for LSG and $15,000 for LAGB.
After 1 year, patients in the LSG group had the greatest percentage of total body weight loss (29.3%), followed by ESG patients (17.6%), and LAGB patients (14.5%). Rates of leaks, pulmonary embolism events, and 90-day readmission were not significantly different among the groups.
The study results do not imply that ESG will replace either LAGB or LSG for weight loss, Dr. Sharaiha noted, but the results suggest that ESG is a viable option for some patients.
Dr. Sharaiha had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
Obese patients who underwent endoscopic sleeve gastroplasty had significantly fewer complications and shorter hospital stays than did those who had laparoscopic sleeve gastrectomy or laparoscopic band placement, according to results from a study of 278 adults. The data were presented at the annual Digestive Disease Week®.
Overall, 1% of patients who underwent endoscopic sleeve gastroplasty (ESG) experienced adverse events, compared with 8% of those who underwent laparoscopic sleeve gastrectomy (LSG) and 9% of those who underwent laparoscopic band placement (LAGB).
ESG, which reduces gastric volume by use of an endoscopic suturing system of full-thickness sutures through the greater curvature of the stomach, is becoming a popular weight-loss procedure for patients with a body mass index greater than 30 kg/m2 who are poor candidates for laparoscopic surgery or who would prefer a less invasive procedure, according to Reem Z. Sharaiha, MD, of Cornell University, New York.
Dr. Sharaiha and her colleagues randomized 91 patients to ESG, 120 to LSG, and 67 to LAGB. Patient demographic characteristics, including age, gender, and diabetes, were similar among the three groups. However, patients in the LSG group had a higher average BMI than did the LAGB and ESG groups (47.3 kg/m2, 45.7 kg/m2, and 38.8 kg/m2, respectively). In addition, the incidence of hypertension, and hyperlipidemia was significantly higher in each of the surgical groups compared to the ESG group (P less than .01).
The average postprocedure hospital stay was 0.13 days for ESG patients compared with 3.09 days for LSG patients and 1.68 days for LAGB patients. ESG also had the lowest cost of the three procedures, averaging $12,000 for the procedure compared to $22,000 for LSG and $15,000 for LAGB.
After 1 year, patients in the LSG group had the greatest percentage of total body weight loss (29.3%), followed by ESG patients (17.6%), and LAGB patients (14.5%). Rates of leaks, pulmonary embolism events, and 90-day readmission were not significantly different among the groups.
The study results do not imply that ESG will replace either LAGB or LSG for weight loss, Dr. Sharaiha noted, but the results suggest that ESG is a viable option for some patients.
Dr. Sharaiha had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
FROM DDW
Key clinical point: Endoscopic sleeve gastroplasty is a viable option for patients seeking weight loss but wishing to avoid major surgery.
Major finding: After 1 year, 1% of patients who underwent endoscopic sleeve gastroplasty experienced adverse events, compared with 8% of laparoscopic sleeve gastrectomy patients, and 9% of laparoscopic band placement patients.
Data source: A randomized trial of 278 obese adults who underwent one of three weight loss procedures.
Disclosures: Dr. Sharaiha had no relevant financial conflicts to disclose.
Series supports viability of ambulatory laparoscopic sleeve gastrectomy
HOUSTON – An ambulatory approach to laparoscopic sleeve gastrectomy is a safe and viable option to improve patient satisfaction and soften the economic blow of these procedures on patients, based on a large series at one surgery center in Cincinnati.
“With proper patient selection, utilization of enhanced recovery pathways with an overall low readmission rate and the complication profile point to the feasibility of laparoscopic sleeve gastrectomy [LSG] as a safe outpatient procedure,” said Sepehr Lalezari, MD, now a surgical fellow at Johns Hopkins University, Baltimore.
About 105,000 LSG operations were performed in the United States in 2015, representing 54% of all bariatric operations, according to the American Society for Metabolic and Bariatric Surgery.
Patient selection and strict adherence to protocols are keys to success for ambulatory LSG, Dr. Lalezari said. Suitable patients were found to be ambulatory, between ages 18 and 65 years; had a body mass index (BMI) less than 55 kg/m2 for males and less than 60 kg/m2 for females; weighed less than 500 lb; had an American Society of Anesthesiologists’ classification score less than 4; and had no significant cardiopulmonary impairment, had no history of renal failure or organ transplant, and were not on a transplant wait list.
In this series, 71% of patients (579) were female, and the average BMI was 43. The total complication rate was 2.3% (19); 17 of these patients required hospital admission.
Postoperative complications included gastric leaks (seven, 0.9%); intra-abdominal abscess requiring percutaneous drainage (four, 0.5%); dehydration, nausea, and/or vomiting (four, 0.5%); and one of each of the following: acute cholecystitis, postoperative bleeding, surgical site infection (SSI), and portal vein thrombosis/pulmonary embolism.
The two complications managed on an outpatient basis were the SSI and one intra-abdominal abscess, Dr. Lalezari said.
“The only readmissions in our series that could have been possibly prevented with an overnight stay in the hospital were the four cases of nausea, vomiting, and/or dehydration,” he said. “These only accounted for 0.5% of the total cases performed.”
The readmission rates for ambulatory LSG in this series compared favorably with large trials that did not distinguish between ambulatory and inpatient LSG procedures, Dr. Lalezari noted. A 2016 analysis of 35,655 patients in the American College of Surgeons National Surgical Quality Improvement Program database reported a readmission rate of 3.7% for LSG (Surg Endosc. 2016 Jun;30[6]:2342-50).
A larger study of 130,000 patients who had bariatric surgery reported an LSG readmission rate of 2.8% (Ann Surg. 2016 Nov 15. doi: 10.1097/SLA.0000000000002079). The most common cause for readmissions these trials reported were nausea, vomiting, and/or dehydration.
Bariatric surgeons have embraced enhanced recovery pathways and fast-track surgery, with good results, Dr. Lalezari said, citing work by Zhamak Khorgami, MD, and colleagues at the Cleveland Clinic (Surg Obes Relat Dis. 2017 Feb;13[2]:273-80).
“Looking at fast-track surgery, they found that patients discharged on postoperative day 1 vs. day 2 or 3 did not change outcomes”; those discharged later than postoperative day 1 trended toward a higher readmission rate of 2.8% vs. 3.6%, Dr. Lalezari said.
The enhanced recovery/fast track protocol Dr. Lalezari and his coauthors used involves placing intravenous lines and infusing 1 L crystalloid before starting the procedure, and administration of famotidine and metoclopramide prior to anesthesia. The protocol utilizes sequential compression devices and avoids Foley catheters and intra-abdominal drains. Patients receive dexamethasone and ondansetron during the operation. The protocol emphasizes early ambulation and resumption of oral intake.
The operation uses a 36-French bougie starting about 5 cm from the pylorus, and all staple lines are reinforced with buttress material. At the end of the surgery, all incisions are infiltrated with 30 cc of 0.5% bupivacaine with epinephrine.
Patients are ambulating about 90 minutes after surgery and are monitored for 3-4 hours. They receive a total volume of 3-4 L crystalloids. When they’re tolerating clear liquids, voiding spontaneously, and walking independently, and their pain is well controlled (pain score less than 5/10) and vital signs are within normal limits, they’re discharged.
Postoperative follow-up involves a call at 48 hours and in-clinic follow-up at weeks 1 and 4. Additional follow-up is scheduled at 3-month intervals for 1 year, then at 6 months for up to 2 years, and then yearly afterward.
“With proper patient selection and utilization of enhanced recovery pathways, the low overall readmission rate (2.1%) and complication profile (2.3%) in our series point to the feasibility of laparoscopic sleeve gastrectomy as a safe outpatient procedure,” Dr. Lalezari said.
He reported having no relevant financial disclosures.
HOUSTON – An ambulatory approach to laparoscopic sleeve gastrectomy is a safe and viable option to improve patient satisfaction and soften the economic blow of these procedures on patients, based on a large series at one surgery center in Cincinnati.
“With proper patient selection, utilization of enhanced recovery pathways with an overall low readmission rate and the complication profile point to the feasibility of laparoscopic sleeve gastrectomy [LSG] as a safe outpatient procedure,” said Sepehr Lalezari, MD, now a surgical fellow at Johns Hopkins University, Baltimore.
About 105,000 LSG operations were performed in the United States in 2015, representing 54% of all bariatric operations, according to the American Society for Metabolic and Bariatric Surgery.
Patient selection and strict adherence to protocols are keys to success for ambulatory LSG, Dr. Lalezari said. Suitable patients were found to be ambulatory, between ages 18 and 65 years; had a body mass index (BMI) less than 55 kg/m2 for males and less than 60 kg/m2 for females; weighed less than 500 lb; had an American Society of Anesthesiologists’ classification score less than 4; and had no significant cardiopulmonary impairment, had no history of renal failure or organ transplant, and were not on a transplant wait list.
In this series, 71% of patients (579) were female, and the average BMI was 43. The total complication rate was 2.3% (19); 17 of these patients required hospital admission.
Postoperative complications included gastric leaks (seven, 0.9%); intra-abdominal abscess requiring percutaneous drainage (four, 0.5%); dehydration, nausea, and/or vomiting (four, 0.5%); and one of each of the following: acute cholecystitis, postoperative bleeding, surgical site infection (SSI), and portal vein thrombosis/pulmonary embolism.
The two complications managed on an outpatient basis were the SSI and one intra-abdominal abscess, Dr. Lalezari said.
“The only readmissions in our series that could have been possibly prevented with an overnight stay in the hospital were the four cases of nausea, vomiting, and/or dehydration,” he said. “These only accounted for 0.5% of the total cases performed.”
The readmission rates for ambulatory LSG in this series compared favorably with large trials that did not distinguish between ambulatory and inpatient LSG procedures, Dr. Lalezari noted. A 2016 analysis of 35,655 patients in the American College of Surgeons National Surgical Quality Improvement Program database reported a readmission rate of 3.7% for LSG (Surg Endosc. 2016 Jun;30[6]:2342-50).
A larger study of 130,000 patients who had bariatric surgery reported an LSG readmission rate of 2.8% (Ann Surg. 2016 Nov 15. doi: 10.1097/SLA.0000000000002079). The most common cause for readmissions these trials reported were nausea, vomiting, and/or dehydration.
Bariatric surgeons have embraced enhanced recovery pathways and fast-track surgery, with good results, Dr. Lalezari said, citing work by Zhamak Khorgami, MD, and colleagues at the Cleveland Clinic (Surg Obes Relat Dis. 2017 Feb;13[2]:273-80).
“Looking at fast-track surgery, they found that patients discharged on postoperative day 1 vs. day 2 or 3 did not change outcomes”; those discharged later than postoperative day 1 trended toward a higher readmission rate of 2.8% vs. 3.6%, Dr. Lalezari said.
The enhanced recovery/fast track protocol Dr. Lalezari and his coauthors used involves placing intravenous lines and infusing 1 L crystalloid before starting the procedure, and administration of famotidine and metoclopramide prior to anesthesia. The protocol utilizes sequential compression devices and avoids Foley catheters and intra-abdominal drains. Patients receive dexamethasone and ondansetron during the operation. The protocol emphasizes early ambulation and resumption of oral intake.
The operation uses a 36-French bougie starting about 5 cm from the pylorus, and all staple lines are reinforced with buttress material. At the end of the surgery, all incisions are infiltrated with 30 cc of 0.5% bupivacaine with epinephrine.
Patients are ambulating about 90 minutes after surgery and are monitored for 3-4 hours. They receive a total volume of 3-4 L crystalloids. When they’re tolerating clear liquids, voiding spontaneously, and walking independently, and their pain is well controlled (pain score less than 5/10) and vital signs are within normal limits, they’re discharged.
Postoperative follow-up involves a call at 48 hours and in-clinic follow-up at weeks 1 and 4. Additional follow-up is scheduled at 3-month intervals for 1 year, then at 6 months for up to 2 years, and then yearly afterward.
“With proper patient selection and utilization of enhanced recovery pathways, the low overall readmission rate (2.1%) and complication profile (2.3%) in our series point to the feasibility of laparoscopic sleeve gastrectomy as a safe outpatient procedure,” Dr. Lalezari said.
He reported having no relevant financial disclosures.
HOUSTON – An ambulatory approach to laparoscopic sleeve gastrectomy is a safe and viable option to improve patient satisfaction and soften the economic blow of these procedures on patients, based on a large series at one surgery center in Cincinnati.
“With proper patient selection, utilization of enhanced recovery pathways with an overall low readmission rate and the complication profile point to the feasibility of laparoscopic sleeve gastrectomy [LSG] as a safe outpatient procedure,” said Sepehr Lalezari, MD, now a surgical fellow at Johns Hopkins University, Baltimore.
About 105,000 LSG operations were performed in the United States in 2015, representing 54% of all bariatric operations, according to the American Society for Metabolic and Bariatric Surgery.
Patient selection and strict adherence to protocols are keys to success for ambulatory LSG, Dr. Lalezari said. Suitable patients were found to be ambulatory, between ages 18 and 65 years; had a body mass index (BMI) less than 55 kg/m2 for males and less than 60 kg/m2 for females; weighed less than 500 lb; had an American Society of Anesthesiologists’ classification score less than 4; and had no significant cardiopulmonary impairment, had no history of renal failure or organ transplant, and were not on a transplant wait list.
In this series, 71% of patients (579) were female, and the average BMI was 43. The total complication rate was 2.3% (19); 17 of these patients required hospital admission.
Postoperative complications included gastric leaks (seven, 0.9%); intra-abdominal abscess requiring percutaneous drainage (four, 0.5%); dehydration, nausea, and/or vomiting (four, 0.5%); and one of each of the following: acute cholecystitis, postoperative bleeding, surgical site infection (SSI), and portal vein thrombosis/pulmonary embolism.
The two complications managed on an outpatient basis were the SSI and one intra-abdominal abscess, Dr. Lalezari said.
“The only readmissions in our series that could have been possibly prevented with an overnight stay in the hospital were the four cases of nausea, vomiting, and/or dehydration,” he said. “These only accounted for 0.5% of the total cases performed.”
The readmission rates for ambulatory LSG in this series compared favorably with large trials that did not distinguish between ambulatory and inpatient LSG procedures, Dr. Lalezari noted. A 2016 analysis of 35,655 patients in the American College of Surgeons National Surgical Quality Improvement Program database reported a readmission rate of 3.7% for LSG (Surg Endosc. 2016 Jun;30[6]:2342-50).
A larger study of 130,000 patients who had bariatric surgery reported an LSG readmission rate of 2.8% (Ann Surg. 2016 Nov 15. doi: 10.1097/SLA.0000000000002079). The most common cause for readmissions these trials reported were nausea, vomiting, and/or dehydration.
Bariatric surgeons have embraced enhanced recovery pathways and fast-track surgery, with good results, Dr. Lalezari said, citing work by Zhamak Khorgami, MD, and colleagues at the Cleveland Clinic (Surg Obes Relat Dis. 2017 Feb;13[2]:273-80).
“Looking at fast-track surgery, they found that patients discharged on postoperative day 1 vs. day 2 or 3 did not change outcomes”; those discharged later than postoperative day 1 trended toward a higher readmission rate of 2.8% vs. 3.6%, Dr. Lalezari said.
The enhanced recovery/fast track protocol Dr. Lalezari and his coauthors used involves placing intravenous lines and infusing 1 L crystalloid before starting the procedure, and administration of famotidine and metoclopramide prior to anesthesia. The protocol utilizes sequential compression devices and avoids Foley catheters and intra-abdominal drains. Patients receive dexamethasone and ondansetron during the operation. The protocol emphasizes early ambulation and resumption of oral intake.
The operation uses a 36-French bougie starting about 5 cm from the pylorus, and all staple lines are reinforced with buttress material. At the end of the surgery, all incisions are infiltrated with 30 cc of 0.5% bupivacaine with epinephrine.
Patients are ambulating about 90 minutes after surgery and are monitored for 3-4 hours. They receive a total volume of 3-4 L crystalloids. When they’re tolerating clear liquids, voiding spontaneously, and walking independently, and their pain is well controlled (pain score less than 5/10) and vital signs are within normal limits, they’re discharged.
Postoperative follow-up involves a call at 48 hours and in-clinic follow-up at weeks 1 and 4. Additional follow-up is scheduled at 3-month intervals for 1 year, then at 6 months for up to 2 years, and then yearly afterward.
“With proper patient selection and utilization of enhanced recovery pathways, the low overall readmission rate (2.1%) and complication profile (2.3%) in our series point to the feasibility of laparoscopic sleeve gastrectomy as a safe outpatient procedure,” Dr. Lalezari said.
He reported having no relevant financial disclosures.
AT SAGES 2017
Key clinical point: Laparoscopic sleeve gastrectomy is a safe outpatient procedure – with strict adherence to enhanced recovery pathways and fast-track protocols.
Major finding: This series reported an overall readmission rate of 2.1% and a complication rate of 2.3% in patients who had outpatient LSG.
Data source: A retrospective review of 821 patients who had ambulatory LSG by a single surgeon from 2011 to 2015.
Disclosures: Dr. Lalezari reported having no relevant financial disclosures.
Study underscores antipsoriatic effect of gastric bypass surgery
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Gastric bypass surgery was associated with more than a 50% drop in baseline rates of psoriasis, and with about a 70% decrease in the incidence of psoriatic arthritis, investigators reported.
In contrast, gastric banding did not appear to affect baselines rates of either of these autoimmune conditions, Alexander Egeberg, MD, of Herlev and Gentofte Hospital, Hellerup, Denmark, and associates reported in JAMA Surgery. “Although speculative, these findings may be the result of post-operative differences in weight loss and nutrient uptake, as well as differences in the postsurgical secretion of a number of gut hormones, including [glucagon-like peptide-1],” they wrote.
Psoriasis strongly correlates with obesity, and weight loss appears to mitigate psoriatic symptoms, the investigators noted. Previously, small studies and case series indicated that bariatric surgery might induce remission of psoriasis. To further investigate this possibility, Dr. Egeberg and his associates conducted a longitudinal cohort study of all 12,364 patients who underwent gastric bypass surgery and all 1,071 patients who underwent gastric banding in Denmark between 1997 and 2012 (JAMA Surg. 2017;152:344-349). No patient had psoriasis symptoms at the start of the study. A total of 272 (2%) gastric bypass patients developed psoriasis before their surgery, while only 0.5% did so afterward. In contrast, gastric banding was not tied to a significant change in the incidence of psoriasis – the preoperative rate was 0.5%, and the postoperative rate was 0.4%. Similarly, respective rates of psoriatic arthritis were 0.5% and 0.1% before and after gastric bypass, but were 0.3% and 0.6% before and after gastric banding. Additionally, respective rates of severe psoriasis were 0.8% and 0% before and after gastric bypass, but were about 0.2% and 0.5% before and after gastric banding.
After adjusting for age, sex, alcohol abuse, socioeconomic status, smoking, and diabetes status, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis (P = .004), with about a 56% drop in the rate of severe psoriasis (P = .02), and with about a 71% drop in the rate of psoriatic arthritis (P = .01). In contrast, neither crude nor adjusted models linked gastric banding to a decrease in the incidence of psoriasis, severe psoriasis, or psoriatic arthritis, the researchers said.
Gastric banding is “a purely restrictive procedure,” while gastric bypass – especially Roux-en-Y bypass – diverts nutrients to the distal small intestine, where enteroendocrine cells secrete GLP-1, the researchers wrote.
“These postoperative hormonal changes may, in addition to the weight loss, be important for the antipsoriatic effect of gastric bypass,” they added. “Both gastric bypass and gastric banding have been shown to lead to sustained weight loss, suggesting that the observed differences in our study might be caused by factors other than weight loss.”
An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies.
Key clinical point: Gastric bypass, but not gastric banding, was associated with significant drops in rates of psoriasis and psoriatic arthritis.
Major finding: In an adjusted model, gastric bypass was associated with about a 48% drop in the incidence of any type of psoriasis, with a 56% drop in the rate of severe psoriasis, and with a 71% drop in the rate of psoriatic arthritis.
Data source: A population-based cohort study of 12,364 gastric bypass patients and 1,071 gastric banding patients.
Disclosures: An unrestricted research grant from the Novo Nordisk Foundation funded the work. Dr. Egeberg disclosed ties to Pfizer and Eli Lilly. One coinvestigator disclosed ties to these and several other pharmaceutical companies. The other coinvestigators reported having no ties to industry.