User login
PCOS comes with high morbidity, medication use into late 40s
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
FROM ACTA OBSTETRICIA ET GYNECOLOGICA SCANDINAVICA
Endometriosis: Diagnosis, Surgical Management, and Overlapping Diagnosis.
As a gynecologist specializing in minimally invasive surgical techniques, what is your involvement in the process for diagnosing endometriosis?
Dr. Lager: At our multidisciplinary endometriosis center, we receive a range of referrals from excellent providers near San Francisco and beyond. As a result, patients will often have had extensive evaluations and multiple treatments. Nonetheless, it is important to take a thorough history, to gain an understanding of the progress of their disease, treatments they have taken and the results or side effects of those treatments, and the goals of the patient in order to guide next steps.
Reviewing previous operative reports, pathology, and surgical photos can also be helpful to guide next steps. Commonly, patients will present with dysmenorrhea, and depending on the severity of associated symptoms, such as dysuria, dyschezia, hematuria, or hematochezia, I may refer patients to our colleagues in urology or GI for further evaluation.
Patients will often have previous imaging such as an ultrasound or CT to evaluate anatomic etiology of the pain, but those studies are often negative. Depending on their history, I may order additional imaging, such as an MRI pelvis, and consideration of vaginal or rectal gel. We have worked closely with our radiologists who have developed a specific endometriosis protocol for deeply infiltrative endometriosis and have a multidisciplinary review committee to discuss complex cases.
Although the gold standard for diagnosis of endometriosis is surgical, this leads to a delay in treatment of 7 to 12 years.[1] So, if a patient presents with symptoms of endometriosis, I will discuss the likely diagnosis and start treatment.
Are there specific techniques that you prefer in your standard practice once a clear diagnosis is determined?
Dr. Lager: As I mentioned, although endometriosis is a surgical diagnosis, there may be findings on imaging which will lead to a diagnosis of endometriosis, including endometriomas, uterosacral thickening, a “kissing ovary” appearance, or hematosalpinx for example.
I discuss a broad range of treatment options based on the patient’s goals, from least invasive treatments to definitive surgery. I discuss dietary changes, integrative medicine (we are fortunate to have an integrative medicine gynecologist here at UCSF Osher Center), and pain psychology. Additionally, I review first-line hormonal management options such as: birth control pills, progestin-only pills, levenogestrol IUD, etonogestrel implant, and medroxyprogesterone acetate injection. In my practice, most patients have already tried initial treatment options, and are most interested in other options. I then review second-line options such as GnRH agonists, antagonists, danazol, and aromatase inhibitors. For patients that have had chronic pelvic pain, I also discuss peripheral and central sensitization, and overlapping diagnoses. Surgical management includes diagnostic laparoscopy and excision or ablation of endometriosis, hysterectomy, and oophorectomy.
Are there specific factors that you look for to help you decide whether surgical management is necessary?
Dr. Lager: There are several reasons why patients decide to proceed with surgical management. First, some patients are reticent to start treatment, particularly if they have had negative experiences with hormonal medications and desire a definitive diagnosis. Other patients choose to proceed with surgery for fertility reasons, and others have severe symptoms that are not managed by medications.
The goal of surgery is to remove all visible endometriotic lesions, restore normal anatomy and for pathologic diagnosis if there is atypical characteristics of an endometrial mass. The pelvic exam and imaging can often be helpful surgical planning. If there is a deeply infiltrative lesion in the bowel or bladder, I consult my urology and colorectal colleagues for surgical planning.
Endometriotic lesions are heterogenous, and can include superficial peritoneal lesions, clear vesicular lesions, “powder burn lesions”, endometriomas, and deep infiltrative lesions.
Additionally, I counsel patients on surgical options depending on the fertility desires. For patients with infertility and symptoms of endometriosis, primary surgery with excision or ablation increases pregnancy rates. One meta-analysis showed that operative laparoscopy improved live births and ongoing pregnancy rates.[2] This was found for the first laparoscopic surgery and not repeat surgery.
Can you talk a little bit more about some of the advancements and the controversies in surgical management, and how that impacts your practice or your treatment?
Dr. Lager: Controversy in surgical management includes excision versus ablation in surgical management of endometriosis. One randomized controlled trial showed an improvement with dyspareunia with excision versus ablation after 5 year follow up.[3] However, a recent meta-analysis from 2021 showed no difference in dysmenorrhea between excision and ablation.[4] I generally perform excision of endometriosis as it can provide a tissue for diagnosis and may allow for complete excision of a lesion that may have an underlying component not easily seen.
We also discussed some of the controversy related to fertility and endometriosis. Management of endometriomas in the face of desired fertility is unclear. Endometriomas that are >3 cm in diameter are associated with decreased anti-Mullerian hormone (AMH) levels, but ovarian cystectomy for endometriomas is also associated with decreased AMH levels. I will counsel patients regarding the risks and benefits of ovarian cystectomy and discuss with the reproductive endocrinologists if they recommend removal to improve oocyte retrieval.
Lastly, conservative versus definitive treatment is an important issue to discuss. Depending on a patient’s goals, conservative surgical management of endometriosis may be the most appropriate procedure. However, if a patient has multiple surgeries, does not desire to have children or has completed childbearing regardless of age and wants to decrease the risk of need for repeat surgery, I will discuss with patients that the risk of reoperation after hysterectomy versus conservative surgery is 8% vs 21% in 2 years and 59% vs 22% after 7 years, respectively.[5] Additionally, the patient may have an overlapping gynecological condition, such as adenomyosis or fibroids, and desire surgical management for those conditions as well. Management ultimately will depend on shared decision making,
You mentioned overlapping diagnosis. What are the impacts and barriers related to misdiagnosis or overlapping diagnosis, and what is your approach to recognizing those signs and symptoms?
Dr. Lager: The classic symptoms of endometriosis can overlap with several medical conditions. In addition to gynecologic issues such as adenomyosis and fibroids that I mentioned previously, symptoms such as pelvic pain, bloating, and dysuria can be associated with gastrointestinal conditions, painful bladder syndrome, neurologic, and musculoskeletal pain conditions. This is complex because the overlapping diagnoses can lead to misdiagnosis, and delay in diagnosis and missing an associated diagnosis can lead to inadequate treatment.
I approach the possibility of overlapping diagnoses in consultation with my colleagues who may recommend further testing, such as endoscopy and colonoscopy. Depending on the diagnoses, several treatments can be started concomitantly to address the multifactorial components of pain. For example, pelvic floor dysfunction related to pelvic pain can affect bowel habits, even without a diagnosis of IBS. Pelvic floor physical therapy can address one component of this. Similarly, even if we surgically or medically manage symptoms of endometriosis, the musculoskeletal pain can lead to persistent or worsening pain. The same goes for pain medicine and peripheral or central pain sensitization or neurological pain.
Was there anything else you’d like to share with your colleagues?
Dr. Lager: Endometriosis is a complex condition that requires a multifactorial approach that takes into consideration a patient’s goals. There is not a one-size fit for all patients with endometriosis due to all the issues we discussed. It will take time to address the varied components of pain and is an iterative process. Minimally invasive surgery has an important role in diagnosis and management of endometriosis but is one of several approaches to treat this complex condition. Thanks for taking the time to discuss this important condition that affects at least 10% of gynecological patients, and potentially more due to delayed and undiagnosed disease.
- Staal AH, van der Zanden M, Nap AW. Diagnostic delay of endometriosis in the Netherlands. Gynecol Obstet Invest. 2016;81(4):321-4. doi: 10.1159/000441911
- Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031. Update in: Cochrane Database Syst Rev. 2020;10:CD011031. doi:10.1002/14651858.CD011031.pub2
- Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004. doi: 10.1016/j.jmig.2014.04.002
- Burks C, Lee M, DeSarno M, Findley J, Flyckt R. Excision versus ablation for management of minimal to mild endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(3):587-597. doi:10.1016/j.jmig.2020.11.028
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-92. Erratum in: Obstet Gynecol. 2008;112(3):710. doi:10.1097/AOG.0b013e3181758ec6
As a gynecologist specializing in minimally invasive surgical techniques, what is your involvement in the process for diagnosing endometriosis?
Dr. Lager: At our multidisciplinary endometriosis center, we receive a range of referrals from excellent providers near San Francisco and beyond. As a result, patients will often have had extensive evaluations and multiple treatments. Nonetheless, it is important to take a thorough history, to gain an understanding of the progress of their disease, treatments they have taken and the results or side effects of those treatments, and the goals of the patient in order to guide next steps.
Reviewing previous operative reports, pathology, and surgical photos can also be helpful to guide next steps. Commonly, patients will present with dysmenorrhea, and depending on the severity of associated symptoms, such as dysuria, dyschezia, hematuria, or hematochezia, I may refer patients to our colleagues in urology or GI for further evaluation.
Patients will often have previous imaging such as an ultrasound or CT to evaluate anatomic etiology of the pain, but those studies are often negative. Depending on their history, I may order additional imaging, such as an MRI pelvis, and consideration of vaginal or rectal gel. We have worked closely with our radiologists who have developed a specific endometriosis protocol for deeply infiltrative endometriosis and have a multidisciplinary review committee to discuss complex cases.
Although the gold standard for diagnosis of endometriosis is surgical, this leads to a delay in treatment of 7 to 12 years.[1] So, if a patient presents with symptoms of endometriosis, I will discuss the likely diagnosis and start treatment.
Are there specific techniques that you prefer in your standard practice once a clear diagnosis is determined?
Dr. Lager: As I mentioned, although endometriosis is a surgical diagnosis, there may be findings on imaging which will lead to a diagnosis of endometriosis, including endometriomas, uterosacral thickening, a “kissing ovary” appearance, or hematosalpinx for example.
I discuss a broad range of treatment options based on the patient’s goals, from least invasive treatments to definitive surgery. I discuss dietary changes, integrative medicine (we are fortunate to have an integrative medicine gynecologist here at UCSF Osher Center), and pain psychology. Additionally, I review first-line hormonal management options such as: birth control pills, progestin-only pills, levenogestrol IUD, etonogestrel implant, and medroxyprogesterone acetate injection. In my practice, most patients have already tried initial treatment options, and are most interested in other options. I then review second-line options such as GnRH agonists, antagonists, danazol, and aromatase inhibitors. For patients that have had chronic pelvic pain, I also discuss peripheral and central sensitization, and overlapping diagnoses. Surgical management includes diagnostic laparoscopy and excision or ablation of endometriosis, hysterectomy, and oophorectomy.
Are there specific factors that you look for to help you decide whether surgical management is necessary?
Dr. Lager: There are several reasons why patients decide to proceed with surgical management. First, some patients are reticent to start treatment, particularly if they have had negative experiences with hormonal medications and desire a definitive diagnosis. Other patients choose to proceed with surgery for fertility reasons, and others have severe symptoms that are not managed by medications.
The goal of surgery is to remove all visible endometriotic lesions, restore normal anatomy and for pathologic diagnosis if there is atypical characteristics of an endometrial mass. The pelvic exam and imaging can often be helpful surgical planning. If there is a deeply infiltrative lesion in the bowel or bladder, I consult my urology and colorectal colleagues for surgical planning.
Endometriotic lesions are heterogenous, and can include superficial peritoneal lesions, clear vesicular lesions, “powder burn lesions”, endometriomas, and deep infiltrative lesions.
Additionally, I counsel patients on surgical options depending on the fertility desires. For patients with infertility and symptoms of endometriosis, primary surgery with excision or ablation increases pregnancy rates. One meta-analysis showed that operative laparoscopy improved live births and ongoing pregnancy rates.[2] This was found for the first laparoscopic surgery and not repeat surgery.
Can you talk a little bit more about some of the advancements and the controversies in surgical management, and how that impacts your practice or your treatment?
Dr. Lager: Controversy in surgical management includes excision versus ablation in surgical management of endometriosis. One randomized controlled trial showed an improvement with dyspareunia with excision versus ablation after 5 year follow up.[3] However, a recent meta-analysis from 2021 showed no difference in dysmenorrhea between excision and ablation.[4] I generally perform excision of endometriosis as it can provide a tissue for diagnosis and may allow for complete excision of a lesion that may have an underlying component not easily seen.
We also discussed some of the controversy related to fertility and endometriosis. Management of endometriomas in the face of desired fertility is unclear. Endometriomas that are >3 cm in diameter are associated with decreased anti-Mullerian hormone (AMH) levels, but ovarian cystectomy for endometriomas is also associated with decreased AMH levels. I will counsel patients regarding the risks and benefits of ovarian cystectomy and discuss with the reproductive endocrinologists if they recommend removal to improve oocyte retrieval.
Lastly, conservative versus definitive treatment is an important issue to discuss. Depending on a patient’s goals, conservative surgical management of endometriosis may be the most appropriate procedure. However, if a patient has multiple surgeries, does not desire to have children or has completed childbearing regardless of age and wants to decrease the risk of need for repeat surgery, I will discuss with patients that the risk of reoperation after hysterectomy versus conservative surgery is 8% vs 21% in 2 years and 59% vs 22% after 7 years, respectively.[5] Additionally, the patient may have an overlapping gynecological condition, such as adenomyosis or fibroids, and desire surgical management for those conditions as well. Management ultimately will depend on shared decision making,
You mentioned overlapping diagnosis. What are the impacts and barriers related to misdiagnosis or overlapping diagnosis, and what is your approach to recognizing those signs and symptoms?
Dr. Lager: The classic symptoms of endometriosis can overlap with several medical conditions. In addition to gynecologic issues such as adenomyosis and fibroids that I mentioned previously, symptoms such as pelvic pain, bloating, and dysuria can be associated with gastrointestinal conditions, painful bladder syndrome, neurologic, and musculoskeletal pain conditions. This is complex because the overlapping diagnoses can lead to misdiagnosis, and delay in diagnosis and missing an associated diagnosis can lead to inadequate treatment.
I approach the possibility of overlapping diagnoses in consultation with my colleagues who may recommend further testing, such as endoscopy and colonoscopy. Depending on the diagnoses, several treatments can be started concomitantly to address the multifactorial components of pain. For example, pelvic floor dysfunction related to pelvic pain can affect bowel habits, even without a diagnosis of IBS. Pelvic floor physical therapy can address one component of this. Similarly, even if we surgically or medically manage symptoms of endometriosis, the musculoskeletal pain can lead to persistent or worsening pain. The same goes for pain medicine and peripheral or central pain sensitization or neurological pain.
Was there anything else you’d like to share with your colleagues?
Dr. Lager: Endometriosis is a complex condition that requires a multifactorial approach that takes into consideration a patient’s goals. There is not a one-size fit for all patients with endometriosis due to all the issues we discussed. It will take time to address the varied components of pain and is an iterative process. Minimally invasive surgery has an important role in diagnosis and management of endometriosis but is one of several approaches to treat this complex condition. Thanks for taking the time to discuss this important condition that affects at least 10% of gynecological patients, and potentially more due to delayed and undiagnosed disease.
As a gynecologist specializing in minimally invasive surgical techniques, what is your involvement in the process for diagnosing endometriosis?
Dr. Lager: At our multidisciplinary endometriosis center, we receive a range of referrals from excellent providers near San Francisco and beyond. As a result, patients will often have had extensive evaluations and multiple treatments. Nonetheless, it is important to take a thorough history, to gain an understanding of the progress of their disease, treatments they have taken and the results or side effects of those treatments, and the goals of the patient in order to guide next steps.
Reviewing previous operative reports, pathology, and surgical photos can also be helpful to guide next steps. Commonly, patients will present with dysmenorrhea, and depending on the severity of associated symptoms, such as dysuria, dyschezia, hematuria, or hematochezia, I may refer patients to our colleagues in urology or GI for further evaluation.
Patients will often have previous imaging such as an ultrasound or CT to evaluate anatomic etiology of the pain, but those studies are often negative. Depending on their history, I may order additional imaging, such as an MRI pelvis, and consideration of vaginal or rectal gel. We have worked closely with our radiologists who have developed a specific endometriosis protocol for deeply infiltrative endometriosis and have a multidisciplinary review committee to discuss complex cases.
Although the gold standard for diagnosis of endometriosis is surgical, this leads to a delay in treatment of 7 to 12 years.[1] So, if a patient presents with symptoms of endometriosis, I will discuss the likely diagnosis and start treatment.
Are there specific techniques that you prefer in your standard practice once a clear diagnosis is determined?
Dr. Lager: As I mentioned, although endometriosis is a surgical diagnosis, there may be findings on imaging which will lead to a diagnosis of endometriosis, including endometriomas, uterosacral thickening, a “kissing ovary” appearance, or hematosalpinx for example.
I discuss a broad range of treatment options based on the patient’s goals, from least invasive treatments to definitive surgery. I discuss dietary changes, integrative medicine (we are fortunate to have an integrative medicine gynecologist here at UCSF Osher Center), and pain psychology. Additionally, I review first-line hormonal management options such as: birth control pills, progestin-only pills, levenogestrol IUD, etonogestrel implant, and medroxyprogesterone acetate injection. In my practice, most patients have already tried initial treatment options, and are most interested in other options. I then review second-line options such as GnRH agonists, antagonists, danazol, and aromatase inhibitors. For patients that have had chronic pelvic pain, I also discuss peripheral and central sensitization, and overlapping diagnoses. Surgical management includes diagnostic laparoscopy and excision or ablation of endometriosis, hysterectomy, and oophorectomy.
Are there specific factors that you look for to help you decide whether surgical management is necessary?
Dr. Lager: There are several reasons why patients decide to proceed with surgical management. First, some patients are reticent to start treatment, particularly if they have had negative experiences with hormonal medications and desire a definitive diagnosis. Other patients choose to proceed with surgery for fertility reasons, and others have severe symptoms that are not managed by medications.
The goal of surgery is to remove all visible endometriotic lesions, restore normal anatomy and for pathologic diagnosis if there is atypical characteristics of an endometrial mass. The pelvic exam and imaging can often be helpful surgical planning. If there is a deeply infiltrative lesion in the bowel or bladder, I consult my urology and colorectal colleagues for surgical planning.
Endometriotic lesions are heterogenous, and can include superficial peritoneal lesions, clear vesicular lesions, “powder burn lesions”, endometriomas, and deep infiltrative lesions.
Additionally, I counsel patients on surgical options depending on the fertility desires. For patients with infertility and symptoms of endometriosis, primary surgery with excision or ablation increases pregnancy rates. One meta-analysis showed that operative laparoscopy improved live births and ongoing pregnancy rates.[2] This was found for the first laparoscopic surgery and not repeat surgery.
Can you talk a little bit more about some of the advancements and the controversies in surgical management, and how that impacts your practice or your treatment?
Dr. Lager: Controversy in surgical management includes excision versus ablation in surgical management of endometriosis. One randomized controlled trial showed an improvement with dyspareunia with excision versus ablation after 5 year follow up.[3] However, a recent meta-analysis from 2021 showed no difference in dysmenorrhea between excision and ablation.[4] I generally perform excision of endometriosis as it can provide a tissue for diagnosis and may allow for complete excision of a lesion that may have an underlying component not easily seen.
We also discussed some of the controversy related to fertility and endometriosis. Management of endometriomas in the face of desired fertility is unclear. Endometriomas that are >3 cm in diameter are associated with decreased anti-Mullerian hormone (AMH) levels, but ovarian cystectomy for endometriomas is also associated with decreased AMH levels. I will counsel patients regarding the risks and benefits of ovarian cystectomy and discuss with the reproductive endocrinologists if they recommend removal to improve oocyte retrieval.
Lastly, conservative versus definitive treatment is an important issue to discuss. Depending on a patient’s goals, conservative surgical management of endometriosis may be the most appropriate procedure. However, if a patient has multiple surgeries, does not desire to have children or has completed childbearing regardless of age and wants to decrease the risk of need for repeat surgery, I will discuss with patients that the risk of reoperation after hysterectomy versus conservative surgery is 8% vs 21% in 2 years and 59% vs 22% after 7 years, respectively.[5] Additionally, the patient may have an overlapping gynecological condition, such as adenomyosis or fibroids, and desire surgical management for those conditions as well. Management ultimately will depend on shared decision making,
You mentioned overlapping diagnosis. What are the impacts and barriers related to misdiagnosis or overlapping diagnosis, and what is your approach to recognizing those signs and symptoms?
Dr. Lager: The classic symptoms of endometriosis can overlap with several medical conditions. In addition to gynecologic issues such as adenomyosis and fibroids that I mentioned previously, symptoms such as pelvic pain, bloating, and dysuria can be associated with gastrointestinal conditions, painful bladder syndrome, neurologic, and musculoskeletal pain conditions. This is complex because the overlapping diagnoses can lead to misdiagnosis, and delay in diagnosis and missing an associated diagnosis can lead to inadequate treatment.
I approach the possibility of overlapping diagnoses in consultation with my colleagues who may recommend further testing, such as endoscopy and colonoscopy. Depending on the diagnoses, several treatments can be started concomitantly to address the multifactorial components of pain. For example, pelvic floor dysfunction related to pelvic pain can affect bowel habits, even without a diagnosis of IBS. Pelvic floor physical therapy can address one component of this. Similarly, even if we surgically or medically manage symptoms of endometriosis, the musculoskeletal pain can lead to persistent or worsening pain. The same goes for pain medicine and peripheral or central pain sensitization or neurological pain.
Was there anything else you’d like to share with your colleagues?
Dr. Lager: Endometriosis is a complex condition that requires a multifactorial approach that takes into consideration a patient’s goals. There is not a one-size fit for all patients with endometriosis due to all the issues we discussed. It will take time to address the varied components of pain and is an iterative process. Minimally invasive surgery has an important role in diagnosis and management of endometriosis but is one of several approaches to treat this complex condition. Thanks for taking the time to discuss this important condition that affects at least 10% of gynecological patients, and potentially more due to delayed and undiagnosed disease.
- Staal AH, van der Zanden M, Nap AW. Diagnostic delay of endometriosis in the Netherlands. Gynecol Obstet Invest. 2016;81(4):321-4. doi: 10.1159/000441911
- Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031. Update in: Cochrane Database Syst Rev. 2020;10:CD011031. doi:10.1002/14651858.CD011031.pub2
- Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004. doi: 10.1016/j.jmig.2014.04.002
- Burks C, Lee M, DeSarno M, Findley J, Flyckt R. Excision versus ablation for management of minimal to mild endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(3):587-597. doi:10.1016/j.jmig.2020.11.028
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-92. Erratum in: Obstet Gynecol. 2008;112(3):710. doi:10.1097/AOG.0b013e3181758ec6
- Staal AH, van der Zanden M, Nap AW. Diagnostic delay of endometriosis in the Netherlands. Gynecol Obstet Invest. 2016;81(4):321-4. doi: 10.1159/000441911
- Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031. Update in: Cochrane Database Syst Rev. 2020;10:CD011031. doi:10.1002/14651858.CD011031.pub2
- Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004. doi: 10.1016/j.jmig.2014.04.002
- Burks C, Lee M, DeSarno M, Findley J, Flyckt R. Excision versus ablation for management of minimal to mild endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2021;28(3):587-597. doi:10.1016/j.jmig.2020.11.028
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-92. Erratum in: Obstet Gynecol. 2008;112(3):710. doi:10.1097/AOG.0b013e3181758ec6
Müllerian anomalies – old problem, new approach and classification
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at obnews@mdedge.com.
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at obnews@mdedge.com.
The American Society for Reproductive Medicine’s classification system for müllerian anomalies was the standard until the revision in 2021 by ASRM, which updated and expanded the classification presenting nine classes and imaging criteria: müllerian agenesis, cervical agenesis, unicornuate, uterus didelphys, bicornuate, septate, longitudinal vaginal septum, transverse vaginal septum, and complex anomalies. This month’s article addresses müllerian anomalies from embryology to treatment options.
The early embryo has the capability of developing a wolffian (internal male) or müllerian (internal female) system. Unless anti-müllerian hormone (formerly müllerian-inhibiting substance) is produced, the embryo develops a female reproductive system beginning with two lateral uterine anlagen that fuse in the midline and canalize. Müllerian anomalies occur because of accidents during fusion and canalization (see Table).
The incidence of müllerian anomalies is difficult to discern, given the potential for a normal reproductive outcome precluding an evaluation and based on the population studied. Müllerian anomalies are found in approximately 4.3% of fertile women, 3.5%-8% of infertile patients, 12.3%-13% of those with recurrent pregnancy losses, and 24.5% of patients with miscarriage and infertility. Of the müllerian anomalies, the most common is septate (35%), followed by bicornuate (26%), arcuate (18%), unicornuate (10%), didelphys (8%), and agenesis (3%) (Hum Reprod Update. 2001;7[2]:161; Hum Reprod Update. 2011;17[6]:761-71).
In 20%-30% of patients with müllerian anomalies, particularly in women with a unicornuate uterus, renal anomalies exist that are typically ipsilateral to the absent or rudimentary contralateral uterine horn (J Pediatr Adolesc Gynecol. 2021;34[2]:154-60). As there is no definitive evidence to suggest an association between a septate uterus and renal anomalies, the renal system evaluation can be deferred in this population (Fertil Steril. 2021 Nov;116[5]:1238-52).
Diagnosis
2-D ultrasound can be a screen for müllerian anomalies and genitourinary anatomic variants. The diagnostic accuracy of 3-D ultrasound with müllerian anomalies is reported to be 97.6% with sensitivity and specificity of 98.3% and 99.4%, respectively (Hum. Reprod. 2016;31[1]:2-7). As a result, office 3-D has essentially replaced MRI in the diagnosis of müllerian anomalies (Ultrasound Obstet Gynecol. 2015 Nov;46[5]:616-22), with one exception because of the avoidance of a transvaginal probe in the non–sexually active adult and younger adolescent/child. MRI is reserved for diagnosing complex müllerian anomalies or if there is a diagnostic challenge.
Criteria to diagnose müllerian anomalies by radiology begins with the “reference line,” i.e., a line joining both tubal ostia (interostial line). A septate uterus is diagnosed if the distance from the interostial line to the cephalad endometrium is more than 1 cm, otherwise it is considered normal or arcuate based on its appearance. An arcuate uterus has not been associated with impaired reproduction and can be viewed as a normal variant. Alternatively, a bicornuate uterus is diagnosed when the external fundal indentation is more than 1 cm (Fertil Steril. 2021 Nov;116[5]:1238-52).
Clinical course
Women with müllerian anomalies may experience pelvic pain and prolonged and/or abnormal bleeding at the time of menarche. While the ability to conceive may not be impaired from müllerian anomalies with the possible exception of the septate uterus, the pregnancy course can be affected, i.e., recurrent pregnancy loss, preterm birth, perinatal mortality, and malpresentation in labor (Reprod Biomed Online. 2014;29[6]:665). In women with septate, bicornuate, and uterine didelphys, fetal growth restriction appears to be increased. Spontaneous abortion rates of 32% and preterm birth rates of 28% have been reported in patients with uterus didelphys (Obstet Gynecol. 1990;75[6]:906).
Special consideration of the unicornuate is given because of the potential for a rudimentary horn that may communicate with the main uterine cavity and/or have functional endometrium which places the woman at risk of an ectopic pregnancy in the smaller horn. Patients with a unicornuate uterus are at higher risk for preterm labor and breech presentation. An obstructed (noncommunicating) functional rudimentary horn is a risk for endometriosis with cyclic pain because of outflow tract obstruction and an ectopic pregnancy prompting consideration for hemihysterectomy based on symptoms.
The septate uterus – old dogma revisited
The incidence of uterine septa is approximately 1-15 per 1,000. As the most common müllerian anomaly, the septate uterus has traditionally been associated with an increased risk for spontaneous abortion (21%-44%) and preterm birth (12%-33%). The live birth rate ranges from 50% to 72% (Hum Reprod Update. 2001;7[2]:161-74). A uterine septum is believed to develop as a result of failure of resorption of the tissue connecting the two paramesonephric (müllerian) ducts prior to the 20th embryonic week.
Incising the uterine septum (metroplasty) dates back to 1884 when Ruge described a blind transcervical metroplasty in a woman with two previous miscarriages who, postoperatively, delivered a healthy baby. In the early 1900s, Tompkins reported an abdominal metroplasty (Fertil Stertil. 2021;115:1140-2). The decision to proceed with metroplasty is based on only established observational studies (Fertil Steril. 2016;106:530-40). Until recently, the majority of studies suggested that metroplasty is associated with decreased spontaneous abortion rates and improved obstetrical outcomes. A retrospective case series of 361 patients with a septate uterus who had primary infertility of >2 years’ duration, a history of 1-2 spontaneous abortions, or recurrent pregnancy loss suggested a significant improvement in the live birth rate and reduction in miscarriage (Arch Gynecol Obstet. 2003;268:289-92). A meta-analysis found that the overall pregnancy rate after septum incision was 67.8% and the live-birth rate was 53.5% (J Minim Invas Gynecol. 2013;20:22-42).
Recently, two multinational studies question the prevailing dogma (Fertil Steril. 2021 Sep;116[3]:693-4). Both studies could not demonstrate any increase in live birth rate, reduction in preterm birth, or in pregnancy loss after metroplasty. A significant limitation was the lack of a uniform consensus on the definition of the septate uterus and allowing the discretion of the physician to diagnosis a septum (Hum Reprod. 2020;35:1578-88; Hum Reprod. 2021;36:1260-7).
Hysteroscopic metroplasty is not without complications. Uterine rupture during pregnancy or delivery, while rare, may be linked to significant entry into the myometrium and/or overzealous cauterization and perforation, which emphasizes the importance of appropriate techniques.
Conclusion
A diagnosis of müllerian anomalies justifies a comprehensive consultation with the patient given the risk of pregnancy complications. Management of the septate uterus has become controversial. In a patient with infertility, prior pregnancy loss, or poor obstetrical outcome, it is reasonable to consider metroplasty; otherwise, expectant management is an option.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Email him at obnews@mdedge.com.
Doxycycline bests azithromycin for anorectal chlamydia in women
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
NEW YORK (Reuters) – A one-week course of doxycycline was superior to a single dose of azithromycin in women with concurrent vaginal and anorectal chlamydia infection in an unblinded randomized controlled trial, mirroring previous results in men.
Researchers suggest that doxycycline should be the first-line therapy for chlamydia infection in women.
“It is clear we must consider that any woman with a urogenital infection must have an effective treatment for the anal infection, since nearly 80% of women have an anal infection concomitant with the vaginal infection,” Dr. Bertille de Barbeyrac of the University of Bordeaux, France, told Reuters Health by email.
However, she noted that “even [though] the study shows that doxycycline is more effective than azithromycin on anal infection, other studies are needed to prove that residual anal infection after treatment with azithromycin can be a source of vaginal contamination and therefore justify changing practices and eliminating azithromycin as a treatment for lower urogenital chlamydial infection in women.”
“There are other reasons [to make] this change,” she added, “such as the acquisition of macrolide resistance by M. genitalium following heavy use of azithromycin.”
As reported in The Lancet Infectious Diseases, Dr. Barbeyrac and colleagues randomly assigned 460 women (median age, 21) to either doxycycline or azithromycin in a multicenter, open-label superiority trial.
Participants received either azithromycin (a single 1-g dose, with or without food) or doxycycline (100 mg in the morning and evening at mealtimes for 7 days – that is, 100 mg of doxycycline twice daily).
The primary outcome was that the microbiological anorectal cure rate, defined as a C. trachomatis-negative nucleic acid amplification test (NAAT), resulted in anorectal specimens six weeks after treatment initiation among women who had a baseline positive result (about half the women in each treatment group).
Ninety-four percent of the doxycycline group versus 85% of the azithromycin group had an anorectal cure (adjusted odds ratio with imputation of missing values, 0.43).
Adverse events possibly related to treatment occurred in 11% of the doxycycline group versus 13% of the azithromycin group. Gastrointestinal disorders were most frequent, occurring in 8% of the doxycycline and 11% of the azithromycin groups.
Summing up, the authors write, “The microbiological anorectal cure rate was significantly lower among women who received a single dose of azithromycin than among those who received a 1-week course of doxycycline. This finding suggests that doxycycline should be the first-line therapy for C trachomatis infection in women.”
Dr. Meleen Chuang, medical director of women’s health at the Family Health Centers at NYU Langone, Brooklyn, commented in an email to Reuters Health that after reviewing this study “as well as CDC and WHO recommendations updated as of 2022, health care providers should be treating C. trachomatis infections with doxycycline 100 mg twice a day for seven days as first-line therapy rather than azithromycin, [given] concerns of increasing macrolide drug resistance against Mycoplasma genitalium and Neisseria gonorrhea.”
“Our clinicians also see the growing uptick of syphilis, gonorrhea, and chlamydia infections in our population, similarly to the rest of the United States since 2020,” she noted. “With the increase in STD infection ... treatment with doxycycline therapy with an important caveat to the patient to complete the one-week treatment regimen is extremely important.”
Dr. Latasha Murphy of the Gynecologic Care Institute at Mercy, Baltimore, also commented in an email to Reuters Health. She noted, “this study does not mirror my clinical experience. More patients have side effects from doxycycline than azithromycin in my experience. Also, anorectal screening is not routine in STD screening.”
“If any major changes to clinical care are made,” she said, “it may be for more consistent screening for anorectal disease. This may ultimately lead to doxycycline being the first line-treatment. More research is needed before making any definitive changes.”
Reuters Health Information © 2022
FDA-cleared panties could reduce STI risk during oral sex
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
.
The underwear, sold as Lorals for Protection, are single-use, vanilla-scented, natural latex panties that cover the genitals and anus and block the transfer of bodily fluids during oral sex, according to the company website. They sell in packages of four for $25.
The FDA didn’t run human clinical trials but granted authorization after the company gave it data about the product, The New York Times reported.
“The FDA’s authorization of this product gives people another option to protect against STIs during oral sex,” said Courtney Lias, PhD, director of the FDA office that led the review of the underwear.
Previously, the FDA authorized oral dams to prevent the spread of STIs during oral sex. Oral dams, sometimes called oral sex condoms, are thin latex barriers that go between one partner’s mouth and the other person’s genitals. The dams haven’t been widely used, partly because a person has to hold the dam in place during sex, unlike the panties.
“They’re extremely unpopular,” Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, told the Times. “I mean, honestly, could there be anything less sexy than a dental dam?”Melanie Cristol said she came up with the idea for the panties after discovering on her 2014 honeymoon that she had an infection that could be sexually transmitted.
“I wanted to feel sexy and confident and use something that was made with my body and actual sex in mind,” she told the Times.
The panties are made of material about as thin as a condom and form a seal on the thigh to keep fluids inside, she said.
Dr. Marrazzo said the panties are an advancement because there are few options for safe oral sex. She noted that some teenagers have their first sexual experience with oral sex and that the panties could reduce anxiety for people of all ages.
A version of this article first appeared on WebMD.com.
Sex toys for science
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California researchers are seeking women willing to use sex toys for science.
“We have not had good-quality studies with the use of modern vibrators,” Alexandra Dubinskaya, MD, an obstetrician who is leading the study, said in an interview.
Vibrators of various kinds have been used by women for centuries if not millennia. More than half of women in the United States have at least some experience with the devices.
Victorian-era physicians are said to have routinely prescribed multiple types of vibrators to treat “female hysteria,” although the frequency with which vibrators were recommended for therapeutic purposes has been questioned.
Still, Dr. Dubinskaya said vibrators have a long history of use as therapy – with some evidence of success.
She and her colleagues reviewed the medical literature and found that studies generally supported the use of vibrators for increased blood flow in pelvic tissues, improved sexual function, including orgasms, and possibly urinary incontinence by helping to strengthen the pelvic floor. They also appear to boost desire, arousal, and genital sensation.
For the new study, Dr. Dubinskaya and her colleagues hope to eventually include 100 women between the ages of 18 and 99 years. Each will receive a commercially available genital vibrator and instructions to use the device to reach orgasm three times per week for 3 to 4 months. The researchers will track any changes in sexual function, pelvic prolapse, urinary continence, and other measures of pelvic and sexual health.
The goal of the study, Dr. Dubinskaya said, is to provide prospective data for clinicians who might consider recommending vibrators to their patients – a list that includes urologists, gynecologists, and experts in sexual medicine.
These clinicians “are frequently the first to encounter questions on women’s sexual function, pelvic floor problems, and vulvar health,” Dr. Dubinskaya said. She noted that such questions are common.
Asking women to consider using vibrators might seem too sensitive a subject in a clinical setting, but Dr. Dubinskaya said data indicate that women are receptive to the suggestion.
Debra Lynne Herbenick, PhD, director of the Center for Sexual Health Promotion and a professor of public health at Indiana University, Indianapolis, who has studied vibrator use in the United States, said the research could make a valuable contribution to sexual health.
“This study is an important next step because it is a prospective study and will be able to assess changes in sexual and pelvic floor function over time in relation to vibrator use,” Dr. Herbenick said. Owing to the limited quality of the currently available evidence, these data have the potential “to support clinicians’ recommendations and also their communication with patients.”
Dr. Dubinskaya and Dr. Herbenick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study: Uterine polyp removal in office possible via ultrasound
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ultrasound-guided endometrial polypectomy could be a lower-cost, easily accessible alternative to hysteroscopy for women with abnormal uterine bleeding and polyps, researchers reported at the 2022 annual meeting of the American College of Obstetricians and Gynecologists.
The prospective study of 30 patients who underwent the experimental procedure showed that clinicians were able to remove all the polyps they identified quickly and without sedation.
The technique is a “clever way to address endometrial polyps,” said Lara Harvey, MD, MPH, a minimally invasive gynecologic surgeon at Vanderbilt University Medical Center, Nashville, Tenn., who was not involved in the study.
“If you’re a physician with access to in-office ultrasound and you’re familiar with saline infusion sonohysterogram, then this might be a useful approach without a lot of added expense, but more research is needed to validate the technique,” Dr. Harvey said in an interview.
The new technique was initially developed at the University of South Florida as an alternative to surgery for patients with medical comorbidities that placed them at an increased risk of complications with general anesthesia, according to Lauri Hochberg, MD, director of gynecologic imaging at the University of South Florida, Tampa.
However, “we found that it was effective and well-tolerated in general and began offering it to all patients with endometrial polyps, even if they were healthy and at low risk for surgical complications,” Dr. Hochberg told this news organization.
The procedure is performed by introducing pediatric grasping forceps into the uterus with ultrasound guidance. Doctors direct patients to take ibuprofen prior to the procedure, in addition to administering misoprostol intravaginally the night prior in cases of cervical stenosis. Lidocaine is also injected into the cervix and uterine cavity prior to polyp removal, both for anesthesia and to help visualize polyps on an ultrasound.
The 30 patients included in the study had polyps 5 cm or smaller in size and abnormal uterine bleeding. Dr. Hochberg said she chose 5 cm as a cut-off because larger lesions require more procedure time over potentially two visits to remove using the new approach. Patients were mean age 55 years, mean body mass index of 31, and 70% had postmenopausal bleeding.
According to Dr. Hochberg and Papri Sarkar, MD, a 4th-year resident working with her, procedures lasted an average of 12 minutes and allowed for complete polypectomy in all cases. The average polyp volume was 1.26 cm3 and pathologists found two cancerous lesions.
Patients reported median pain and satisfaction scores of 5 and 10 on 10-point scales, respectively. In addition, 13 of 16 patients who returned 3 months later for a saline infusion sonography showed no evidence of polyp recurrence and 14 patients reported complete resolution of symptoms.
Although a direct comparison of the in-office procedure and conventional hysteroscopy would help better define the role of the procedure, the findings indicate it is “safe and effective” and “would be a great tool to help patients” with abnormal uterine bleeding, Dr. Hochberg said.
“Physicians would need to learn the skill of ultrasound-guided removal, but this can be accomplished with study,” she added.
Dr. Harvey also expressed concern that because the new procedure does not allow for direct visualization of the base of the polyp, physicians may not excise the entire lesion. Providers interested in the procedure should “proceed with caution” until there are larger studies published, she said.
“I think widely deploying this technique for postmenopausal bleeding in particular, where there is a higher chance of endometrial cancer, would require really good data comparing it to the gold standard of hysteroscopy and showing that, yes, it is as good at removing polyps and also at diagnosing cancer,” Dr. Harvey said.
Dr. Harvey, Dr. Hochberg, and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IUD cuts heavy menses in nulliparous patients with obesity
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ACOG 2022
Society of Gynecologic Surgeons meeting champions training of future gynecologic surgeons
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
It was such a pleasure at the 48th Annual Meeting of the Society of Gynecologic Surgeons (SGS) to witness record meeting attendance and strong enthusiasm after 2 depressing years with the COVID-19 pandemic. Evidently, everyone was tired of virtual gatherings and presentations. As a dedicated surgical educator and a passionate vaginal surgeon, SGS President Carl Zimmerman, MD, chose “Gynecologic surgery training: Lessons from the past, looking to the future” as the theme for this year’s meeting. Our keynote speakers, Patricia Turner, MD, MBA, Executive Director of the American College of Surgeons, and Marta Crispens, MD, MBA, Professor and Division Director of Gynecologic Oncology at Vanderbilt, were spot on. They reviewed the current status of surgical training eloquently with convincing statistics. They mapped out the path forward by stressing collaboration and proposing strategies that might produce competent surgeons in all fields.
The meeting featured 2 panel discussions. The first, titled “Innovations in training gynecologic surgeons,” reviewed tracking in residency, use of simulation for surgical proficiency, and European perspective on training. The panelists emphasized the dwindling numbers of surgical procedures, especially vaginal hysterectomies. Cecile Ferrando, MD, suggested that tracking might be part of the answer, based on their experience, which provided a structure for residents to obtain concentrated training in their areas of interest. Douglas Miyazaki, MD, presented the prospects for his innovative, federally funded vaginal surgery simulation model. Oliver Preyer, MD, presented Austrian trainees’ low case volumes, showing that the grass was not actually greener on the other side. Finally, this panel reinvigorated ongoing debate about separating Obstetrics and Gynecology.
The second panel, “Operating room safety and efficiency,” shed light on human and nontechnical factors that might be as critical as surgeons’ skills and experience, and it highlighted an innovative technology that monitored and analyzed all operating room parameters to improve operational processes and surgical technique. Points by Jason Wright, MD, on the relationship between surgical volume and outcomes complemented the meeting theme and the first panel discussion. He underlined how much surgical volume of individual surgeons and hospitals mattered, but he also indicated that restrictive credentialing strategies might lead to unintended consequences.
Importantly, the SGS Women’s Council held a panel on the “Impact of Texas legislation on the physician/patient relationship” to provide a platform for members who had mixed feelings about attending this meeting in Texas.
The SGS meeting also included several popular postgraduate courses on multidisciplinary management of Müllerian anomalies, pelvic fistula treatment, surgical simulation, management modalities for uterine fibroids, and medical innovation and entrepreneurship. In this special section and in the next issue of OBG M
Commonly used antibiotics in ObGyn practice
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.
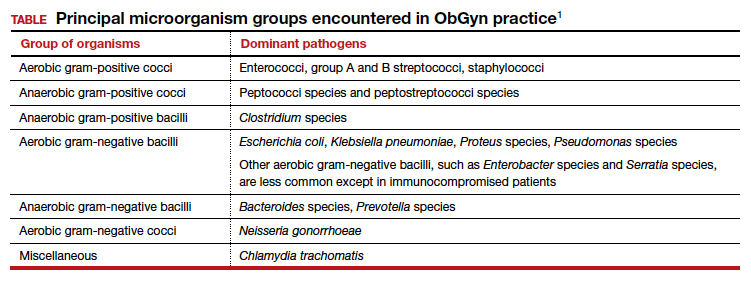
Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.

Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
In this article, I provide a simplified, practical review of the principal antibiotics that we use on a daily basis to treat bacterial infections. The antibiotics are listed in alphabetical order, either individually or by group. I focus first on the mechanism of action and spectrum of activity of the drugs used against the usual pelvic pathogens (TABLE).1 I then review their principal adverse effects, relative cost (categorized as low, intermediate, and high), and the key indications for these drugs in obstetrics and gynecology. In a forthcoming 2-part companion article, I will review how to select specific antibiotics and their dosing regimens for the most commonly encountered bacterial infections in our clinical practice.

Aminoglycoside antibiotics
The aminoglycosides include amikacin, gentamicin, plazomicin, and tobramycin.2,3 The 2 agents most commonly used in our specialty are amikacin and gentamicin. The drugs may be administered intramuscularly or intravenously, and they specifically target aerobic gram-negative bacilli. They also provide coverage against staphylococci and gonococci. Ototoxicity and nephrotoxicity are their principal adverse effects.
Aminoglycosides are used primarily as single agents to treat pyelonephritis caused by highly resistant bacteria and in combination with agents such as clindamycin and metronidazole to treat polymicrobial infections, including chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. Of all the aminoglycosides, gentamicin is clearly the least expensive.
Carbapenems
The original carbapenem widely introduced into clinical practice was imipenem-cilastatin. Imipenem, the active antibiotic, inhibits bacterial cell wall synthesis. Cilastatin inhibits renal dehydropeptidase I and, thereby, slows the metabolism of imipenem by the kidney. Other carbapenems include meropenem and ertapenem.
The carbapenems have the widest spectrum of activity against the pelvic pathogens of any antibiotic. They provide excellent coverage of aerobic and anaerobic gram-positive cocci and aerobic and anaerobic gram-negative bacilli. They do not cover methicillin-resistant Staphylococcus aureus (MRSA) and the enterococci very well.
A major adverse effect of the carbapenems is an allergic reaction, including anaphylaxis and Stevens-Johnson syndrome, and there is some minimal cross-sensitivity with the β-lactam antibiotics. Other important, but fortunately rare, adverse effects include neurotoxicity, hepatotoxicity, and Clostridium difficile colitis.4
As a group, the carbapenems are relatively more expensive than most other agents. Their principal application in our specialty is for single-agent treatment of serious polymicrobial infections, such as puerperal endometritis, pelvic cellulitis, and pelvic abscess, especially in patients who have a contraindication to the use of combination antibiotic regimens that include an aminoglycoside.1,2
Cephalosporins
The cephalosporins are β-lactam antibiotics that act by disrupting the synthesis of the bacterial cell wall. They may be administered orally, intramuscularly, and intravenously. The most common adverse effects associated with these agents are an allergic reaction, which can range from a mild rash to anaphylaxis and the Stevens-Johnson syndrome; central nervous system toxicity; and antibiotic-induced diarrhea, including C difficile colitis.1,2,4
This group of antibiotics can be confusing because it includes so many agents, and their spectrum of activity varies. I find it helpful to think about the coverage of these agents as limited spectrum versus intermediate spectrum versus extended spectrum.
The limited-spectrum cephalosporin prototypes are cephalexin (oral administration) and cefazolin (parenteral administration). This group of cephalosporins provides excellent coverage of aerobic and anaerobic gram-positive cocci. They are excellent against staphylococci, except for MRSA. Coverage is moderate for aerobic gram-negative bacilli but only limited for anaerobic gram-negative bacilli. They do not cover the enterococci. In our specialty, their principal application is for treatment of mastitis, urinary tract infections (UTIs), and wound infections and for prophylaxis against group B streptococcus (GBS) infection and post-cesarean infection.2,5 The cost of these drugs is relatively low.
The prototypes of the intermediate-spectrum cephalosporins are cefixime (oral) and ceftriaxone (parenteral). Both drugs have strong activity against aerobic and anaerobic streptococci, Neisseria gonorrhoeae, most aerobic gram-negative bacilli, and Treponema pallidum (principally, ceftriaxone). They are not consistently effective against staphylococci, particularly MRSA, and enterococci. Their key indications in obstetrics and gynecology are treatment of gonorrhea, syphilis (in penicillin-allergic patients), and acute pyelonephritis. Compared with the limited-spectrum cephalosporins, these antibiotics are moderately expensive.1,2
The 3 extended-spectrum cephalosporins used most commonly in our specialty are cefepime, cefotetan, and cefoxitin. These agents are administered intramuscularly and intravenously, and they provide very good coverage against aerobic and anaerobic gram-positive cocci, with the exception of staphylococci and enterococci. They have very good coverage against most gram-negative aerobic bacilli and excellent coverage against anerobic microorganisms. Their primary application in our specialty is for single-agent treatment of polymicrobial infections, such as puerperal endometritis and pelvic cellulitis. When used in combination with doxycycline, they are valuable in treating pelvic inflammatory disease. These drugs are more expensive than the limited-spectrum or intermediate-spectrum agents. They should not be used routinely as prophylaxis for pelvic surgery.1,2,5
Continue to: Fluorinated quinolones...
Fluorinated quinolones
The fluorinated quinolones include several agents, but the 3 most commonly used in our specialty are ciprofloxacin, ofloxacin, and levofloxacin. All 3 drugs can be administered orally; ciprofloxacin and levofloxacin also are available in intravenous formulations. These drugs interfere with bacterial protein synthesis by targeting DNA gyrase, an enzyme that introduces negative supertwists into DNA and separates interlocked DNA molecules.
These drugs provide excellent coverage against gram-negative bacilli, including Haemophilus influenzae; gram-negative cocci, such as N gonorrhoeae, Neisseria meningitidis, and Moraxella catarrhalis; and many staphylococci species. Levofloxacin, but not the other 2 drugs, provides moderate coverage against anaerobes. Ofloxacin and levofloxacin are active against chlamydia. Levofloxacin also covers the mycoplasma organisms that are responsible for atypical pneumonia.
As a group, the fluorinated quinolones are moderately expensive. The most likely adverse effects with these agents are gastrointestinal (GI) upset, headache, agitation, and sleep disturbance. Allergic reactions are rare. These drugs are of primary value in our specialty in treating gonorrhea, chlamydia, complicated UTIs, and respiratory tract infections.1,2,6
The penicillins
Penicillin
Penicillin, a β-lactam antibiotic, was one of the first antibiotics developed and employed in clinical practice. It may be administered orally, intramuscularly, and intravenously. Penicillin exerts its effect by interfering with bacterial cell wall synthesis. Its principal spectrum of activity is against aerobic streptococci, such as group A and B streptococcus; most anaerobic gram-positive cocci that are present in the vaginal flora; some anaerobic gram-negative bacilli; and T pallidum. Penicillin is not effective against the majority of staphylococci species, enterococci, or aerobic gram-negative bacilli, such as Escherichia coli.
Penicillin’s major adverse effect is an allergic reaction, experienced by less than 10% of recipients.7 Most reactions are mild and are characterized by a morbilliform skin rash. However, some reactions are severe and take the form of an urticarial skin eruption, laryngospasm, bronchospasm, and overt anaphylaxis. The cost of both oral and parenteral penicillin formulations is very low. In obstetrics and gynecology, penicillin is used primarily for the treatment of group A and B streptococci infections, clostridial infections, and syphilis.1,2
Ampicillin and amoxicillin
The β-lactam antibiotics ampicillin and amoxicillin also act by interfering with bacterial cell wall synthesis. Amoxicillin is administered orally; ampicillin may be administered orally, intramuscularly, and intravenously. Their spectrum of activity includes group A and B streptococci, enterococci, most anaerobic gram-positive cocci, some anaerobic gram-negative bacilli, many aerobic gram-negative bacilli, and clostridial organisms.
Like penicillin, ampicillin and amoxicillin may cause allergic reactions that range from mild rashes to anaphylaxis. Unlike the more narrow-spectrum penicillin, they may cause antibiotic-associated diarrhea, including C difficile colitis,4 and they may eliminate part of the normal vaginal flora and stimulate an overgrowth of yeast organisms in the vagina. The cost of ampicillin and amoxicillin is very low. These 2 agents are used primarily for treatment of group A and B streptococci infections and some UTIs, particularly those caused by enterococci.1,2
Dicloxacillin sodium
This penicillin derivative disrupts bacterial cell wall synthesis and targets primarily aerobic gram-positive cocci, particularly staphylococci species. The antibiotic is not active against MRSA. The principal adverse effects of dicloxacillin sodium are an allergic reaction and GI upset. The drug is very inexpensive.
The key application for dicloxacillin sodium in our specialty is for treatment of puerperal mastitis.1
Continue to: Extended-spectrum penicillins...
Extended-spectrum penicillins
Three interesting combination extended-spectrum penicillins are used widely in our specialty. They are ampicillin/sulbactam, amoxicillin/clavulanate, and piperacillin/tazobactam. Ampicillin/sulbactam may be administered intramuscularly and intravenously. Piperacillin/tazobactam is administered intravenously; amoxicillin/clavulanate is administered orally.
Clavulanate, sulbactam, and tazobactam are β-lactamase inhibitors. When added to the parent antibiotic (amoxicillin, ampicillin, and piperacillin, respectively), they significantly enhance the parent drug’s spectrum of activity. These agents interfere with bacterial cell wall synthesis. They provide excellent coverage of aerobic gram-positive cocci, including enterococci; anaerobic gram-positive cocci; anaerobic gram-negative bacilli; and aerobic gram-negative bacilli. Their principal adverse effects include allergic reactions and antibiotic-associated diarrhea. They are moderately expensive.
The principal application of ampicillin/sulbactam and piperacillin/tazobactam in our specialty is as single agents for treatment of puerperal endometritis, postoperative pelvic cellulitis, and pyelonephritis. The usual role for amoxicillin/clavulanate is for oral treatment of complicated UTIs, including pyelonephritis in early pregnancy, and for outpatient therapy of mild to moderately severe endometritis following delivery or pregnancy termination.
Macrolides, monobactams, and additional antibiotics
Azithromycin
Azithromycin is a macrolide antibiotic that is in the same class as erythromycin and clindamycin. In our specialty, it has largely replaced erythromycin because of its more convenient dosage schedule and its better tolerability. It inhibits bacterial protein synthesis, and it is available in both an oral and intravenous formulation.
Azithromycin has an excellent spectrum of activity against the 3 major microorganisms that cause otitis media, sinusitis, and bronchitis: Streptococcus pneumoniae, H influenzae, and M catarrhalis. It also provides excellent coverage of Chlamydia trachomatis, Mycoplasma pneumoniae, and genital mycoplasmas; in high doses it provides modest coverage against gonorrhea.8 Unlike erythromycin, it has minimal GI toxicity and is usually very well tolerated by most patients. One unusual, but very important, adverse effect of the drug is prolongation of the Q-T interval.9
Azithromycin is now available in generic form and is relatively inexpensive. As a single agent, its principal applications in our specialty are for treatment of respiratory tract infections such as otitis media, sinusitis, and acute bronchitis and for treatment of chlamydia urethritis and endocervicitis.8,10 In combination with ampicillin, azithromycin is used as prophylaxis in patients with preterm premature rupture of membranes (PPROM), and, in combination with cefazolin, it is used for prophylaxis in patients undergoing cesarean delivery.1,2,5
Aztreonam
Aztreonam is a monobactam antibiotic. Like the cephalosporins and penicillins, aztreonam inhibits bacterial cell wall synthesis. It may be administered intramuscularly and intravenously, and its principal spectrum of activity is against aerobic gram-negative bacilli, which is similar to the aminoglycosides’ spectrum.
Aztreonam’s most likely adverse effects include phlebitis at the injection site, allergy, GI upset, and diarrhea. The drug is moderately expensive. In our specialty, aztreonam could be used as a single agent, in lieu of an aminoglycoside, for treatment of pyelonephritis caused by an unusually resistant organism. It also could be used in combination with clindamycin or metronidazole plus ampicillin for treatment of polymicrobial infections, such as chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Continue to: Clindamycin...
Clindamycin
A macrolide antibiotic, clindamycin exerts its antibacterial effect by interfering with bacterial protein synthesis. It can be administered orally and intravenously. Its key spectrum of activity in our specialty includes GBS, staphylococci, and anaerobes. However, clindamycin is not active against enterococci or aerobic gram-negative bacilli. GI upset and antibiotic-induced diarrhea are its principal adverse effects, and clindamycin is one of the most important causes of C difficile colitis. Although it is available in a generic formulation, this drug is still relatively expensive.
Clindamycin’s principal application in our specialty is for treating staphylococcal infections, such as wound infections and mastitis. It is particularly effective against MRSA infections. When used in combination with an aminoglycoside such as gentamicin, clindamycin provides excellent treatment for chorioamnionitis, puerperal endometritis, and pelvic inflammatory disease. In fact, for many years, the combination of clindamycin plus gentamicin has been considered the gold standard for the treatment of polymicrobial, mixed aerobic-anaerobic pelvic infections.1,2
Doxycycline
Doxycycline, a tetracycline, exerts its antibacterial effect by inhibiting bacterial protein synthesis. The drug targets a broad range of pelvic pathogens, including C trachomatis and N gonorrhoeae, and it may be administered both orally and intravenously. Doxycycline’s principal adverse effects include headache, GI upset, and photosensitivity. By disrupting the normal bowel and vaginal flora, the drug also can cause diarrhea and vulvovaginal moniliasis. In addition, it can cause permanent discoloration of the teeth, and, for this reason, doxycycline should not be used in pregnant or lactating women or in young children.
Although doxycycline has been available in generic formulation for many years, it remains relatively expensive. As a single agent, its principal application in our specialty is for treatment of chlamydia infection. It may be used as prophylaxis for surgical procedures, such as hysterectomy and pregnancy terminations. In combination with an extended-spectrum cephalosporin, it also may be used to treat pelvic inflammatory disease.2,8,10
Metronidazole
Metronidazole, a nitroimidazole derivative, exerts its antibacterial effect by disrupting bacterial protein synthesis. The drug may be administered topically, orally, and intravenously. Its primary spectrum of activity is against anerobic microorganisms. It is also active against Giardia and Trichomonas vaginalis.
Metronidazole’s most common adverse effects are GI upset, a metallic taste in the mouth, and a disulfiram-like effect when taken with alcohol. The cost of oral and intravenous metronidazole is relatively low; ironically, the cost of topical metronidazole is relatively high. In our specialty, the principal applications of oral metronidazole are as a single agent for treatment of bacterial vaginosis and trichomoniasis. When combined with ampicillin plus an aminoglycoside, intravenous metronidazole provides excellent coverage against the diverse anaerobic microorganisms that cause chorioamnionitis, puerperal endometritis, and pelvic cellulitis.1,2
Trimethoprim-sulfamethoxazole (TMP-SMX)
This antibiotic combination (an antifolate and a sulfonamide) inhibits sequential steps in the synthesis of folic acid, an essential nutrient in bacterial metabolism. It is available in both an intravenous and oral formulation. TMP-SMX has a broad spectrum of activity against the aerobic gram-negative bacilli that cause UTIs in women. In addition, it provides excellent coverage against staphylococci, including MRSA; Pneumocystis jirovecii; and Toxoplasma gondii.
The medication’s principal toxicity is an allergic reaction. Some reactions are quite severe, such as the Stevens-Johnson syndrome. TMP-SMX is relatively inexpensive, particularly the oral formulation. The most common indications for TMP-SMX in our specialty are for treatment of UTIs, mastitis, and wound infections.1,2,11 In HIV-infected patients, the drug provides excellent prophylaxis against recurrent Pneumocystis and Toxoplasma infections. TMP-SMX should not be used in the first trimester of pregnancy because it has been linked to several birth defects, including neural tube defects, heart defects, choanal atresia, and diaphragmatic hernia.12
Nitrofurantoin
Usually administered orally as nitrofurantoin monohydrate macrocrystals, nitrofurantoin exerts its antibacterial effect primarily by inhibiting protein synthesis. Its principal spectrum of activity is against the aerobic gram-negative bacilli, with the exception of Proteus species. Nitrofurantoin’s most common adverse effects are GI upset, headache, vertigo, drowsiness, and allergic reactions. The drug is relatively inexpensive.
Nitrofurantoin is an excellent agent for the treatment of lower UTIs.11 It is not well concentrated in the renal parenchyma or blood, however, so it should not be used to treat pyelonephritis. As a general rule, nitrofurantoin should not be used in the first trimester of pregnancy because it has been associated with eye, heart, and facial cleft defects in the fetus.12
Vancomycin
Vancomycin exerts its antibacterial effect by inhibiting cell wall synthesis. It may be administered both orally and intravenously, and it specifically targets aerobic gram-positive cocci, particularly methicillin-sensitive and methicillin-resistant staphylococci. Vancomycin’s most important adverse effects include GI upset, nephrotoxicity, ototoxicity, and severe allergic reactions, such as anaphylaxis, Stevens-Johnson syndrome, and exfoliative dermatitis (the “red man” syndrome). The drug is moderately expensive.13
In its oral formulation, vancomycin’s principal application in our discipline is for treating C difficile colitis. In its intravenous formulation, it is used primarily as a single agent for GBS prophylaxis in penicillin-allergic patients, and it is used in combination with other antibiotics, such as clindamycin plus gentamicin, for treating patients with deep-seated incisional (wound) infections.1,2,13,14 ●
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2020: chapter 58.
- Duff P. Antibiotic selection in obstetrics: making cost-effective choices. Clin Obstet Gynecol. 2002;45:59-72.
- Wagenlehner FME, Cloutier DJ, Komirenko AS, et al; EPIC Study Group. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380:729-740.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539-1548.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384-394.
- Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019 381:2338-2351.
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
- Workowski KA, Bolan GA. Sexually transmitted disease treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects prevalence study. Arch Pediatr Adolesc Med. 2009;163:978985.
- Alvarez-Arango S, Ogunwole SM, Sequist TD, et al. Vancomycin infusion reaction—moving beyond “red man syndrome.” N Engl J Med. 2021;384:1283-1286.
- Finley TA, Duff P. Antibiotics for treatment of staphylococcal infections in the obstetric patient. Clin Obstet Gynecol. 2019;62:790-803.



