User login
Menstrual phase impacts exercise effects in type 1 diabetes
Women with type 1 diabetes may need additional glucose after exercise during the luteal phase of the menstrual cycle, compared with other times, according to a study in nine women.
“We know that exercise is very beneficial for people with type 1 diabetes; we also know that fear of hypoglycemia is a major barrier to exercise in this population,” said Jane E. Yardley, PhD, in a presentation at the annual scientific sessions of the American Diabetes Association, New Orleans. Women with type 1 diabetes (T1D) perceive more barriers, compared with men, she added.
The menstrual cycle could be an additional barrier to exercise for women with T1D because it increases glucose fluctuations that have not been well documented in the literature to date, said Dr. Yardley, of the University of Alberta, Augustana.
The follicular phase of the menstrual cycle lasts from menses to the midcycle, about 14 days later. This is followed by the luteal phase, which lasts until approximately day 28, Dr. Yardley explained. Data on insulin sensitivity have shown that the late luteal phase is associated with “a little less insulin sensitivity” in women with T1D, she noted.
To assess the relationship between menstrual cycle, glucose control, and exercise, Dr. Yardley and colleagues compared the effects of a moderate aerobic exercise on glycemic responses between the early follicular and late luteal phases of the menstrual cycle in nine female participants with T1D.
The exercise involved 45 minutes of aerobic cycling at 50% of predetermined peak oxygen uptake (VO2peak) for 45 min. The mean age of the participants was 30.2 years, the mean hemoglobin A1C was 7.4%, and the mean VO2peak was 32.5 mL/kg per min. The women reported regular menstrual cycles, and none were using oral contraceptives.
Blood samples were collected before and immediately after exercise and after an hour of recovery. Participants wore continuous glucose monitors for at least 1 hour before and after exercise.
Menstrual cycle was confirmed via estrogen, estradiol, and progesterone.
Insulin levels varied greatly among the study participants, but the differences were not significant, Dr. Yardley said. Glucose levels consistently decreased during exercise and increased after exercise, she noted.
No significant difference in glucose was observed between the follicular and luteal phases.
However, “this needs to be interpreted in the context of the safety profiles that are in place in our lab,” which include carbohydrate supplements for individuals whose blood glucose levels drop below 4.5 mmol/L, she said.
In the current study, 6 of 9 participants required additional carbohydrates during the luteal phase, but only 1 participant needed additional carbohydrates during the follicular phase, she noted. For this reason, no differences were noted. “We actually prevented changes,” she said.
No significant differences were noted in mean glucose levels or number of hypoglycemic episodes at any of the time points between the two phases.
“One place where we did see a difference was in hyperglycemia 24 hours after exercise,” Dr. Yardley said. Level 1 hyperglycemia 24 hours after exercise was significantly more frequent in the follicular phase, compared with the luteal phase (P = .028).
The study findings were limited by the small sample size and homogenous population, and more research is needed to interpret the data, said Dr. Yardley.
However, the need for more glucose supplementation to prevent hypoglycemia during the luteal phase suggests a higher hypoglycemic risk associated with aerobic exercise during this time, she said.
In addition, the results suggest that the menstrual cycle should be taken into consideration when female participants are involved in exercise studies, she noted.
Study supports personalized exercise plans
“It is important to evaluate effects of exercise in people with type 1 diabetes and evaluate whether there is a difference those effects in men and women,” said Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., in an interview. “There is also a need to evaluate to what extent the changes in blood glucose patterns in women in response to exercise differ depending on the phase of the ovarian cycle,” said Dr. Rodbard, who was not involved in the study.
In the current study, “the researchers observed a decline in glucose during a 45-minute period of moderate aerobic exercise, cycling at 50% VO2peak followed by an increase during a 60-minute recovery period. There was a suggestive finding, in the nine subjects, that more carbohydrate supplementation was needed during the late luteal phase of the menstrual cycle than during the follicular phase,” Dr. Rodbard noted. “In contrast, the authors reported a significantly increased degree of hyperglycemia during the recovery phase for subjects during the follicular phase. These findings are consistent with and extend several recent studies from Dr. Yardley and coworkers, who have been focused on this area of research,” she said.
“This study provides provocative evidence that glucose responses to aerobic exercise in women may depend on the timing in relationship to their ovarian cycle,” said Dr. Rodbard. “These findings are based on a small group of subjects and were present in some but not all subjects. Clinicians should encourage women to evaluate and record their experiences during and after exercise in terms of need for carbohydrate supplementation for documented or symptomatic hypoglycemia and in terms of glucose changes as recorded using continuous glucose monitoring (CGM), both in relation to type of exercise and in relation to time in the menstrual cycle,” she said.
The findings also highlight the importance of individualized therapy that is “based on subjective inputs combined with analysis of CGM data during and following exercise,” said Dr. Rodbard. “It is likely that use of Automated Insulin Delivery (AID) will be helpful in achieving this level of individualization in view of the wide range of types, intensity, and duration of physical activity and exercise in which people with T1D engage and the myriad factors that can influence the glycemic response,” she said.
Looking ahead, “the authors and others should expand the present series of subjects using aerobic exercise and examine other types of exercise as well,” Dr. Rodbard noted. “It will be important to evaluate the consistency of these changes in glucose patterns within individuals on multiple occasions, and it would be helpful to repeat the studies in women using oral contraceptives.”
Dr. Yardley disclosed research support from Abbott, Dexcom, and LifeScan and disclosed serving on the speaker’s bureau for Abbott Diabetes. Dr. Rodbard had no financial conflicts to disclose. She serves on the Editorial Advisory Board of Clinical Endocrinology News.
Women with type 1 diabetes may need additional glucose after exercise during the luteal phase of the menstrual cycle, compared with other times, according to a study in nine women.
“We know that exercise is very beneficial for people with type 1 diabetes; we also know that fear of hypoglycemia is a major barrier to exercise in this population,” said Jane E. Yardley, PhD, in a presentation at the annual scientific sessions of the American Diabetes Association, New Orleans. Women with type 1 diabetes (T1D) perceive more barriers, compared with men, she added.
The menstrual cycle could be an additional barrier to exercise for women with T1D because it increases glucose fluctuations that have not been well documented in the literature to date, said Dr. Yardley, of the University of Alberta, Augustana.
The follicular phase of the menstrual cycle lasts from menses to the midcycle, about 14 days later. This is followed by the luteal phase, which lasts until approximately day 28, Dr. Yardley explained. Data on insulin sensitivity have shown that the late luteal phase is associated with “a little less insulin sensitivity” in women with T1D, she noted.
To assess the relationship between menstrual cycle, glucose control, and exercise, Dr. Yardley and colleagues compared the effects of a moderate aerobic exercise on glycemic responses between the early follicular and late luteal phases of the menstrual cycle in nine female participants with T1D.
The exercise involved 45 minutes of aerobic cycling at 50% of predetermined peak oxygen uptake (VO2peak) for 45 min. The mean age of the participants was 30.2 years, the mean hemoglobin A1C was 7.4%, and the mean VO2peak was 32.5 mL/kg per min. The women reported regular menstrual cycles, and none were using oral contraceptives.
Blood samples were collected before and immediately after exercise and after an hour of recovery. Participants wore continuous glucose monitors for at least 1 hour before and after exercise.
Menstrual cycle was confirmed via estrogen, estradiol, and progesterone.
Insulin levels varied greatly among the study participants, but the differences were not significant, Dr. Yardley said. Glucose levels consistently decreased during exercise and increased after exercise, she noted.
No significant difference in glucose was observed between the follicular and luteal phases.
However, “this needs to be interpreted in the context of the safety profiles that are in place in our lab,” which include carbohydrate supplements for individuals whose blood glucose levels drop below 4.5 mmol/L, she said.
In the current study, 6 of 9 participants required additional carbohydrates during the luteal phase, but only 1 participant needed additional carbohydrates during the follicular phase, she noted. For this reason, no differences were noted. “We actually prevented changes,” she said.
No significant differences were noted in mean glucose levels or number of hypoglycemic episodes at any of the time points between the two phases.
“One place where we did see a difference was in hyperglycemia 24 hours after exercise,” Dr. Yardley said. Level 1 hyperglycemia 24 hours after exercise was significantly more frequent in the follicular phase, compared with the luteal phase (P = .028).
The study findings were limited by the small sample size and homogenous population, and more research is needed to interpret the data, said Dr. Yardley.
However, the need for more glucose supplementation to prevent hypoglycemia during the luteal phase suggests a higher hypoglycemic risk associated with aerobic exercise during this time, she said.
In addition, the results suggest that the menstrual cycle should be taken into consideration when female participants are involved in exercise studies, she noted.
Study supports personalized exercise plans
“It is important to evaluate effects of exercise in people with type 1 diabetes and evaluate whether there is a difference those effects in men and women,” said Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., in an interview. “There is also a need to evaluate to what extent the changes in blood glucose patterns in women in response to exercise differ depending on the phase of the ovarian cycle,” said Dr. Rodbard, who was not involved in the study.
In the current study, “the researchers observed a decline in glucose during a 45-minute period of moderate aerobic exercise, cycling at 50% VO2peak followed by an increase during a 60-minute recovery period. There was a suggestive finding, in the nine subjects, that more carbohydrate supplementation was needed during the late luteal phase of the menstrual cycle than during the follicular phase,” Dr. Rodbard noted. “In contrast, the authors reported a significantly increased degree of hyperglycemia during the recovery phase for subjects during the follicular phase. These findings are consistent with and extend several recent studies from Dr. Yardley and coworkers, who have been focused on this area of research,” she said.
“This study provides provocative evidence that glucose responses to aerobic exercise in women may depend on the timing in relationship to their ovarian cycle,” said Dr. Rodbard. “These findings are based on a small group of subjects and were present in some but not all subjects. Clinicians should encourage women to evaluate and record their experiences during and after exercise in terms of need for carbohydrate supplementation for documented or symptomatic hypoglycemia and in terms of glucose changes as recorded using continuous glucose monitoring (CGM), both in relation to type of exercise and in relation to time in the menstrual cycle,” she said.
The findings also highlight the importance of individualized therapy that is “based on subjective inputs combined with analysis of CGM data during and following exercise,” said Dr. Rodbard. “It is likely that use of Automated Insulin Delivery (AID) will be helpful in achieving this level of individualization in view of the wide range of types, intensity, and duration of physical activity and exercise in which people with T1D engage and the myriad factors that can influence the glycemic response,” she said.
Looking ahead, “the authors and others should expand the present series of subjects using aerobic exercise and examine other types of exercise as well,” Dr. Rodbard noted. “It will be important to evaluate the consistency of these changes in glucose patterns within individuals on multiple occasions, and it would be helpful to repeat the studies in women using oral contraceptives.”
Dr. Yardley disclosed research support from Abbott, Dexcom, and LifeScan and disclosed serving on the speaker’s bureau for Abbott Diabetes. Dr. Rodbard had no financial conflicts to disclose. She serves on the Editorial Advisory Board of Clinical Endocrinology News.
Women with type 1 diabetes may need additional glucose after exercise during the luteal phase of the menstrual cycle, compared with other times, according to a study in nine women.
“We know that exercise is very beneficial for people with type 1 diabetes; we also know that fear of hypoglycemia is a major barrier to exercise in this population,” said Jane E. Yardley, PhD, in a presentation at the annual scientific sessions of the American Diabetes Association, New Orleans. Women with type 1 diabetes (T1D) perceive more barriers, compared with men, she added.
The menstrual cycle could be an additional barrier to exercise for women with T1D because it increases glucose fluctuations that have not been well documented in the literature to date, said Dr. Yardley, of the University of Alberta, Augustana.
The follicular phase of the menstrual cycle lasts from menses to the midcycle, about 14 days later. This is followed by the luteal phase, which lasts until approximately day 28, Dr. Yardley explained. Data on insulin sensitivity have shown that the late luteal phase is associated with “a little less insulin sensitivity” in women with T1D, she noted.
To assess the relationship between menstrual cycle, glucose control, and exercise, Dr. Yardley and colleagues compared the effects of a moderate aerobic exercise on glycemic responses between the early follicular and late luteal phases of the menstrual cycle in nine female participants with T1D.
The exercise involved 45 minutes of aerobic cycling at 50% of predetermined peak oxygen uptake (VO2peak) for 45 min. The mean age of the participants was 30.2 years, the mean hemoglobin A1C was 7.4%, and the mean VO2peak was 32.5 mL/kg per min. The women reported regular menstrual cycles, and none were using oral contraceptives.
Blood samples were collected before and immediately after exercise and after an hour of recovery. Participants wore continuous glucose monitors for at least 1 hour before and after exercise.
Menstrual cycle was confirmed via estrogen, estradiol, and progesterone.
Insulin levels varied greatly among the study participants, but the differences were not significant, Dr. Yardley said. Glucose levels consistently decreased during exercise and increased after exercise, she noted.
No significant difference in glucose was observed between the follicular and luteal phases.
However, “this needs to be interpreted in the context of the safety profiles that are in place in our lab,” which include carbohydrate supplements for individuals whose blood glucose levels drop below 4.5 mmol/L, she said.
In the current study, 6 of 9 participants required additional carbohydrates during the luteal phase, but only 1 participant needed additional carbohydrates during the follicular phase, she noted. For this reason, no differences were noted. “We actually prevented changes,” she said.
No significant differences were noted in mean glucose levels or number of hypoglycemic episodes at any of the time points between the two phases.
“One place where we did see a difference was in hyperglycemia 24 hours after exercise,” Dr. Yardley said. Level 1 hyperglycemia 24 hours after exercise was significantly more frequent in the follicular phase, compared with the luteal phase (P = .028).
The study findings were limited by the small sample size and homogenous population, and more research is needed to interpret the data, said Dr. Yardley.
However, the need for more glucose supplementation to prevent hypoglycemia during the luteal phase suggests a higher hypoglycemic risk associated with aerobic exercise during this time, she said.
In addition, the results suggest that the menstrual cycle should be taken into consideration when female participants are involved in exercise studies, she noted.
Study supports personalized exercise plans
“It is important to evaluate effects of exercise in people with type 1 diabetes and evaluate whether there is a difference those effects in men and women,” said Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., in an interview. “There is also a need to evaluate to what extent the changes in blood glucose patterns in women in response to exercise differ depending on the phase of the ovarian cycle,” said Dr. Rodbard, who was not involved in the study.
In the current study, “the researchers observed a decline in glucose during a 45-minute period of moderate aerobic exercise, cycling at 50% VO2peak followed by an increase during a 60-minute recovery period. There was a suggestive finding, in the nine subjects, that more carbohydrate supplementation was needed during the late luteal phase of the menstrual cycle than during the follicular phase,” Dr. Rodbard noted. “In contrast, the authors reported a significantly increased degree of hyperglycemia during the recovery phase for subjects during the follicular phase. These findings are consistent with and extend several recent studies from Dr. Yardley and coworkers, who have been focused on this area of research,” she said.
“This study provides provocative evidence that glucose responses to aerobic exercise in women may depend on the timing in relationship to their ovarian cycle,” said Dr. Rodbard. “These findings are based on a small group of subjects and were present in some but not all subjects. Clinicians should encourage women to evaluate and record their experiences during and after exercise in terms of need for carbohydrate supplementation for documented or symptomatic hypoglycemia and in terms of glucose changes as recorded using continuous glucose monitoring (CGM), both in relation to type of exercise and in relation to time in the menstrual cycle,” she said.
The findings also highlight the importance of individualized therapy that is “based on subjective inputs combined with analysis of CGM data during and following exercise,” said Dr. Rodbard. “It is likely that use of Automated Insulin Delivery (AID) will be helpful in achieving this level of individualization in view of the wide range of types, intensity, and duration of physical activity and exercise in which people with T1D engage and the myriad factors that can influence the glycemic response,” she said.
Looking ahead, “the authors and others should expand the present series of subjects using aerobic exercise and examine other types of exercise as well,” Dr. Rodbard noted. “It will be important to evaluate the consistency of these changes in glucose patterns within individuals on multiple occasions, and it would be helpful to repeat the studies in women using oral contraceptives.”
Dr. Yardley disclosed research support from Abbott, Dexcom, and LifeScan and disclosed serving on the speaker’s bureau for Abbott Diabetes. Dr. Rodbard had no financial conflicts to disclose. She serves on the Editorial Advisory Board of Clinical Endocrinology News.
FROM ADA 2022
Alabama cites Roe decision in call to ban transgender health care
Alabama urged a federal court on June 28 to drop its block on the state’s ban on gender-affirming care for transgender youth, citing the Supreme Court’s recent decision to overturn Roe v. Wade.
Alabama Attorney General Steve Marshall said the high court ruled that abortion isn’t protected under the 14th Amendment because it’s not “deeply rooted” in the nation’s history, which he noted could be said about access to gender-affirming care as well, according to Axios.
“No one – adult or child – has a right to transitioning treatments that is deeply rooted in our Nation’s history and tradition,” he wrote in a court document.
“The State can thus regulate or prohibit those interventions for children, even if an adult wants the drugs for his child,” he wrote.
In May, a federal judge blocked part of Alabama’s Senate Bill 184, which makes it a felony for someone to “engage in or cause” certain types of medical care for transgender youths. The law, which was put in place in April, allows for criminal prosecution against doctors, parents, guardians, and anyone else who provides care to a minor. The penalties could result in up to 10 years in prison and up to $15,000 in fines.
At that time, U.S. District Judge Liles Burke issued an injunction to stop Alabama from enforcing the law and allow challenges, including one filed by the Department of Justice. Mr. Burke said the state provided “no credible evidence to show that transitioning medications are ‘experimental.’ ”
“While Defendants offer some evidence that transitioning medications pose certain risks, the uncontradicted record evidence is that at least twenty-two major medical associations in the United States endorse transitioning medications as well-established, evidence-based treatments for gender dysphoria in minors,” he wrote in the ruling.
Medical organizations such as the American Academy of Pediatrics, American Psychological Association, and American Medical Association have urged governors to oppose legislation this year that would restrict gender-affirming medical care, saying that such laws could have negative effects on the mental health of transgender youths.
But on June 28, Mr. Marshall focused on the Constitution and what he believes the recent overturn of Roe implies.
“Just as the parental relationship does not unlock a Due Process right allowing parents to obtain medical marijuana or abortions for their children, neither does it unlock a right to transitioning treatments,” he wrote.
“The Constitution reserves to the State – not courts or medical interest groups – the authority to determine that these sterilizing interventions are too dangerous for minors,” he said.
Since the Supreme Court overturned Roe, people have expressed concerns that lawsuits could now target several rights that are protected under the 14th Amendment, including same-sex relationships, marriage equality, and access to contraceptives.
Justice Clarence Thomas, who wrote a concurring opinion to the majority decision, said the Supreme Court, “in future cases” should reconsider “substantive due process precedents” under previous landmark cases such as Griswold v. Connecticut, Lawrence v. Texas, and Obergefell v. Hodges.
At the same time, Justice Brett Kavanaugh, who also wrote a concurring opinion, said the decision to overturn Roe was only focused on abortion, saying it “does not mean the overruling of those precedents, and does not threaten or cast doubt on those precedents.”
A version of this article first appeared on WebMD.com.
Alabama urged a federal court on June 28 to drop its block on the state’s ban on gender-affirming care for transgender youth, citing the Supreme Court’s recent decision to overturn Roe v. Wade.
Alabama Attorney General Steve Marshall said the high court ruled that abortion isn’t protected under the 14th Amendment because it’s not “deeply rooted” in the nation’s history, which he noted could be said about access to gender-affirming care as well, according to Axios.
“No one – adult or child – has a right to transitioning treatments that is deeply rooted in our Nation’s history and tradition,” he wrote in a court document.
“The State can thus regulate or prohibit those interventions for children, even if an adult wants the drugs for his child,” he wrote.
In May, a federal judge blocked part of Alabama’s Senate Bill 184, which makes it a felony for someone to “engage in or cause” certain types of medical care for transgender youths. The law, which was put in place in April, allows for criminal prosecution against doctors, parents, guardians, and anyone else who provides care to a minor. The penalties could result in up to 10 years in prison and up to $15,000 in fines.
At that time, U.S. District Judge Liles Burke issued an injunction to stop Alabama from enforcing the law and allow challenges, including one filed by the Department of Justice. Mr. Burke said the state provided “no credible evidence to show that transitioning medications are ‘experimental.’ ”
“While Defendants offer some evidence that transitioning medications pose certain risks, the uncontradicted record evidence is that at least twenty-two major medical associations in the United States endorse transitioning medications as well-established, evidence-based treatments for gender dysphoria in minors,” he wrote in the ruling.
Medical organizations such as the American Academy of Pediatrics, American Psychological Association, and American Medical Association have urged governors to oppose legislation this year that would restrict gender-affirming medical care, saying that such laws could have negative effects on the mental health of transgender youths.
But on June 28, Mr. Marshall focused on the Constitution and what he believes the recent overturn of Roe implies.
“Just as the parental relationship does not unlock a Due Process right allowing parents to obtain medical marijuana or abortions for their children, neither does it unlock a right to transitioning treatments,” he wrote.
“The Constitution reserves to the State – not courts or medical interest groups – the authority to determine that these sterilizing interventions are too dangerous for minors,” he said.
Since the Supreme Court overturned Roe, people have expressed concerns that lawsuits could now target several rights that are protected under the 14th Amendment, including same-sex relationships, marriage equality, and access to contraceptives.
Justice Clarence Thomas, who wrote a concurring opinion to the majority decision, said the Supreme Court, “in future cases” should reconsider “substantive due process precedents” under previous landmark cases such as Griswold v. Connecticut, Lawrence v. Texas, and Obergefell v. Hodges.
At the same time, Justice Brett Kavanaugh, who also wrote a concurring opinion, said the decision to overturn Roe was only focused on abortion, saying it “does not mean the overruling of those precedents, and does not threaten or cast doubt on those precedents.”
A version of this article first appeared on WebMD.com.
Alabama urged a federal court on June 28 to drop its block on the state’s ban on gender-affirming care for transgender youth, citing the Supreme Court’s recent decision to overturn Roe v. Wade.
Alabama Attorney General Steve Marshall said the high court ruled that abortion isn’t protected under the 14th Amendment because it’s not “deeply rooted” in the nation’s history, which he noted could be said about access to gender-affirming care as well, according to Axios.
“No one – adult or child – has a right to transitioning treatments that is deeply rooted in our Nation’s history and tradition,” he wrote in a court document.
“The State can thus regulate or prohibit those interventions for children, even if an adult wants the drugs for his child,” he wrote.
In May, a federal judge blocked part of Alabama’s Senate Bill 184, which makes it a felony for someone to “engage in or cause” certain types of medical care for transgender youths. The law, which was put in place in April, allows for criminal prosecution against doctors, parents, guardians, and anyone else who provides care to a minor. The penalties could result in up to 10 years in prison and up to $15,000 in fines.
At that time, U.S. District Judge Liles Burke issued an injunction to stop Alabama from enforcing the law and allow challenges, including one filed by the Department of Justice. Mr. Burke said the state provided “no credible evidence to show that transitioning medications are ‘experimental.’ ”
“While Defendants offer some evidence that transitioning medications pose certain risks, the uncontradicted record evidence is that at least twenty-two major medical associations in the United States endorse transitioning medications as well-established, evidence-based treatments for gender dysphoria in minors,” he wrote in the ruling.
Medical organizations such as the American Academy of Pediatrics, American Psychological Association, and American Medical Association have urged governors to oppose legislation this year that would restrict gender-affirming medical care, saying that such laws could have negative effects on the mental health of transgender youths.
But on June 28, Mr. Marshall focused on the Constitution and what he believes the recent overturn of Roe implies.
“Just as the parental relationship does not unlock a Due Process right allowing parents to obtain medical marijuana or abortions for their children, neither does it unlock a right to transitioning treatments,” he wrote.
“The Constitution reserves to the State – not courts or medical interest groups – the authority to determine that these sterilizing interventions are too dangerous for minors,” he said.
Since the Supreme Court overturned Roe, people have expressed concerns that lawsuits could now target several rights that are protected under the 14th Amendment, including same-sex relationships, marriage equality, and access to contraceptives.
Justice Clarence Thomas, who wrote a concurring opinion to the majority decision, said the Supreme Court, “in future cases” should reconsider “substantive due process precedents” under previous landmark cases such as Griswold v. Connecticut, Lawrence v. Texas, and Obergefell v. Hodges.
At the same time, Justice Brett Kavanaugh, who also wrote a concurring opinion, said the decision to overturn Roe was only focused on abortion, saying it “does not mean the overruling of those precedents, and does not threaten or cast doubt on those precedents.”
A version of this article first appeared on WebMD.com.
Are ObGyns comfortable performing operative vaginal delivery as an alternative to cesarean delivery?
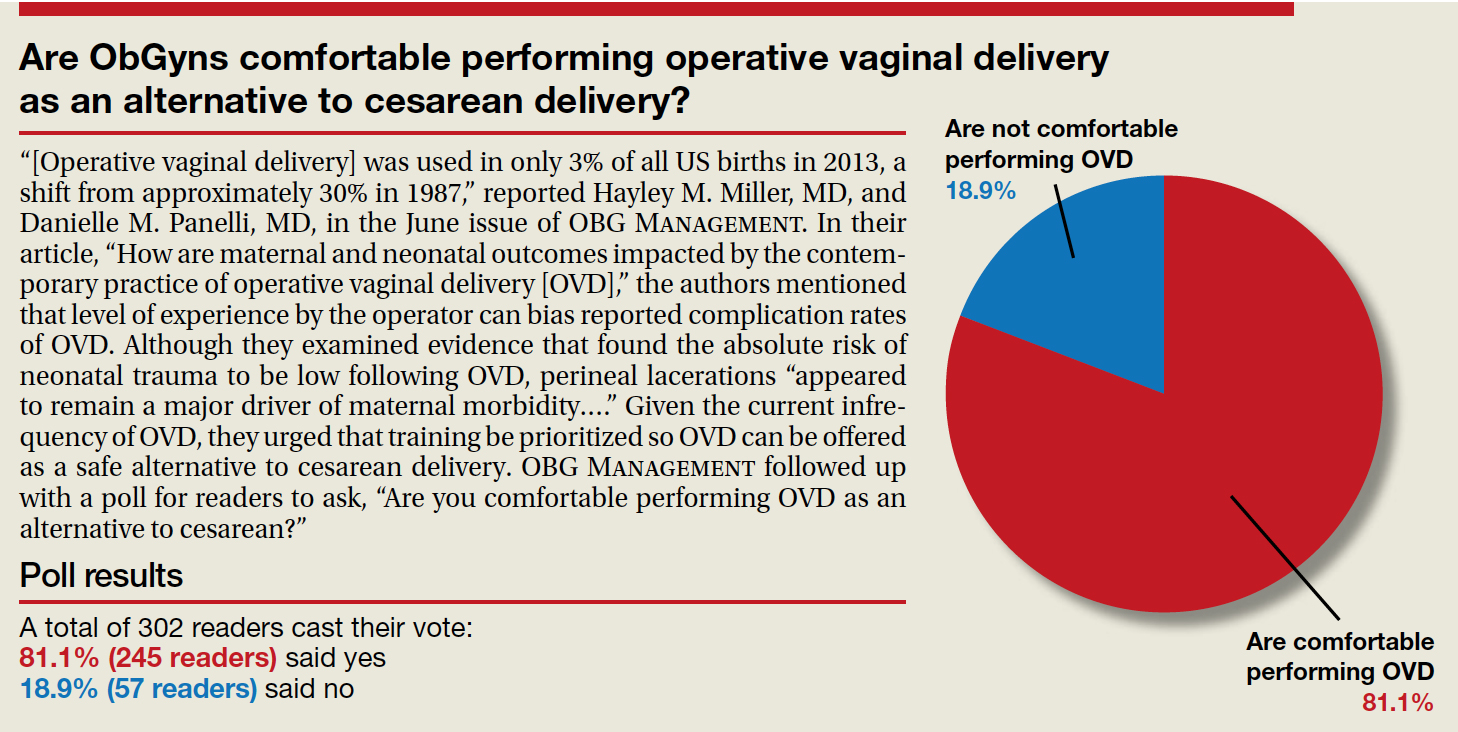
“[Operative vaginal delivery] was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987,” reported Hayley M. Miller, MD, and Danielle M. Panelli, MD, in the June issue of OBG Management. In their article, “How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery [OVD],” the authors mentioned that level of experience by the operator can bias reported complication rates of OVD. Although they examined evidence that found the absolute risk of neonatal trauma to be low following OVD, perineal lacerations “appeared to remain a major driver of maternal morbidity….” Given the current infrequency of OVD, they urged that training be prioritized so OVD can be offered as a safe alternative to cesarean delivery. OBG Management followed up with a poll for readers to ask, “Are you comfortable performing OVD as an alternative to cesarean?”
A total of 302 readers cast their vote:
81.1% (245 readers) said yes
18.9% (57 readers) said no
“[Operative vaginal delivery] was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987,” reported Hayley M. Miller, MD, and Danielle M. Panelli, MD, in the June issue of OBG Management. In their article, “How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery [OVD],” the authors mentioned that level of experience by the operator can bias reported complication rates of OVD. Although they examined evidence that found the absolute risk of neonatal trauma to be low following OVD, perineal lacerations “appeared to remain a major driver of maternal morbidity….” Given the current infrequency of OVD, they urged that training be prioritized so OVD can be offered as a safe alternative to cesarean delivery. OBG Management followed up with a poll for readers to ask, “Are you comfortable performing OVD as an alternative to cesarean?”
A total of 302 readers cast their vote:
81.1% (245 readers) said yes
18.9% (57 readers) said no

“[Operative vaginal delivery] was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987,” reported Hayley M. Miller, MD, and Danielle M. Panelli, MD, in the June issue of OBG Management. In their article, “How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery [OVD],” the authors mentioned that level of experience by the operator can bias reported complication rates of OVD. Although they examined evidence that found the absolute risk of neonatal trauma to be low following OVD, perineal lacerations “appeared to remain a major driver of maternal morbidity….” Given the current infrequency of OVD, they urged that training be prioritized so OVD can be offered as a safe alternative to cesarean delivery. OBG Management followed up with a poll for readers to ask, “Are you comfortable performing OVD as an alternative to cesarean?”
A total of 302 readers cast their vote:
81.1% (245 readers) said yes
18.9% (57 readers) said no

COVID-19 Pandemic stress affected ovulation, not menstruation
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ENDO 2022
HPV vaccination with Cervarix ‘unmasks’ cervical lesions from non-vax strains
Vaccines against human papillomavirus have been hailed as a success: they have been shown to decrease the incidence of cervical lesions associated with the HPV types that are in the vaccine.
However,
An expert not involved in the research said the new data “tell us to be a little bit careful.” Although the HPV types not included in the vaccine are rarer and less aggressive, they can still cause cancer.
The data come from the Costa Rica HPV Vaccine Trial, which involved more than 10,000 women aged 18-25 years. The HPV vaccine used in the trial was Cervarix, from GlaxoSmithKline. It covers the two leading causes of cervical cancer, HPV-16 and -18, and provides partial protection against three other genotypes.
After a follow-up of 11 years, among vaccinated women, there was an excess of precancerous cervical lesions caused by genotypes not included in the vaccine, resulting in negative vaccine efficacy for those HPV variants.
The increase wasn’t enough to offset the overall benefit of vaccination when all genotypes were considered, said the researchers, led by Jaimie Shing, PhD, a postdoctoral research fellow at the National Cancer Institute in Bethesda, Md.
Vaccinated women “still had long-term absolute reductions in high-grade lesions,” they pointed out.
The net protection “remained considerable, emphasizing the importance of HPV vaccination for cervical cancer prevention,” the team concluded.
The findings were published online in The Lancet Oncology.
The results are likely the first evidence to date of “clinical unmasking” with HPV vaccination, meaning that protection against the strains covered by the vaccine leaves women more prone to attack from other carcinogenic HPV variants.
This phenomenon “could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs,” the investigators commented.
Highlighting a need for caution
The take-home message from the trial is that “we have to be careful,” said Marc Steben, MD, co-President of HPV Global Action and a professor at the University of Montreal.
He noted that the Cervarix HPV vaccine used in the trial is not the vaccine that is used now in developed nations.
The current standard HPV vaccine is Gardasil 9 (Merck), which offers broader coverage against nine HPV types (types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
There are 12 main carcinogenic HPV genotypes, so unmasking of other strains is still possible with Gardasil 9, he said.
There is another issue, Dr. Steben added. The success of HPV vaccinations - a nearly 90% reduction in invasive cervical cancer in women who are vaccinated at a young age – has led to questions about the future role of routine cervical cancer screening.
“Some people are saying that if we achieve 90% coverage, we might” eliminate community transmission and no longer need to screen, he said.
These trial results “tell us to be a little bit careful,” Dr. Steben continued. Those HPV types that are less aggressive and rarer than HPV-16 and -18 “can still cause cancer and might be there and surprise us. It could take more time than we thought” to get to the point where screening can be eliminated.
“There might be a little problem if we stop too early,” he said.
Study details
During the period 2004-2005, the investigators randomly assigned 3,727 women aged 18-25 years to receive Cervarix and 3,739 to a control group that received the hepatitis A vaccine; after 4 years, the control group also received Cervarix and exited the study. They were replaced by an unvaccinated control group of 2,836 women. The new control group and the original HPV vaccine group were followed for an additional 7 years.
In years 7-11 of the trial, the investigators found 9.2 additional cervical intraepithelial neoplasias of grade 2 or worse (CIN2+) from HPV types not covered by Cervarix per 1,000 vaccinated women in comparison with unvaccinated participants. This corresponds to –71.2% negative vaccine efficacy against CIN2+ lesions of HPV types not covered by the vaccine.
There were 8.3 additional CIN3+ lesions from nontargeted HPV strains per 1,000 vaccinated women in comparison with unvaccinated participants, which corresponds to –135% negative vaccine efficacy.
Overall, however, there was a net benefit of vaccination, with 27 fewer CIN2+ lesions when all HPV genotypes – vaccine covered or not – were considered per 1,000 vaccinated women over the entire 11 years of follow-up.
There were also 8.7 fewer CIN3+ lesions across all genotypes per 1,000 vaccinated women, but the benefit was not statistically significant.
Among the study limits, the team was unable to evaluate the effect of clinical unmasking on cervical cancer, because women were treated for high-grade cervical lesions before cases could progress to cervical cancer.
The trial was funded by the National Cancer Institute and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline provided the Cervarix vaccine and supported aspects of the trial. Two authors are named inventors on U.S. government–owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. Dr. Steben is an adviser/speaker for many companies, including GlaxoSmithKline and Merck.
A version of this article first appeared on Medscape.com.
Vaccines against human papillomavirus have been hailed as a success: they have been shown to decrease the incidence of cervical lesions associated with the HPV types that are in the vaccine.
However,
An expert not involved in the research said the new data “tell us to be a little bit careful.” Although the HPV types not included in the vaccine are rarer and less aggressive, they can still cause cancer.
The data come from the Costa Rica HPV Vaccine Trial, which involved more than 10,000 women aged 18-25 years. The HPV vaccine used in the trial was Cervarix, from GlaxoSmithKline. It covers the two leading causes of cervical cancer, HPV-16 and -18, and provides partial protection against three other genotypes.
After a follow-up of 11 years, among vaccinated women, there was an excess of precancerous cervical lesions caused by genotypes not included in the vaccine, resulting in negative vaccine efficacy for those HPV variants.
The increase wasn’t enough to offset the overall benefit of vaccination when all genotypes were considered, said the researchers, led by Jaimie Shing, PhD, a postdoctoral research fellow at the National Cancer Institute in Bethesda, Md.
Vaccinated women “still had long-term absolute reductions in high-grade lesions,” they pointed out.
The net protection “remained considerable, emphasizing the importance of HPV vaccination for cervical cancer prevention,” the team concluded.
The findings were published online in The Lancet Oncology.
The results are likely the first evidence to date of “clinical unmasking” with HPV vaccination, meaning that protection against the strains covered by the vaccine leaves women more prone to attack from other carcinogenic HPV variants.
This phenomenon “could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs,” the investigators commented.
Highlighting a need for caution
The take-home message from the trial is that “we have to be careful,” said Marc Steben, MD, co-President of HPV Global Action and a professor at the University of Montreal.
He noted that the Cervarix HPV vaccine used in the trial is not the vaccine that is used now in developed nations.
The current standard HPV vaccine is Gardasil 9 (Merck), which offers broader coverage against nine HPV types (types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
There are 12 main carcinogenic HPV genotypes, so unmasking of other strains is still possible with Gardasil 9, he said.
There is another issue, Dr. Steben added. The success of HPV vaccinations - a nearly 90% reduction in invasive cervical cancer in women who are vaccinated at a young age – has led to questions about the future role of routine cervical cancer screening.
“Some people are saying that if we achieve 90% coverage, we might” eliminate community transmission and no longer need to screen, he said.
These trial results “tell us to be a little bit careful,” Dr. Steben continued. Those HPV types that are less aggressive and rarer than HPV-16 and -18 “can still cause cancer and might be there and surprise us. It could take more time than we thought” to get to the point where screening can be eliminated.
“There might be a little problem if we stop too early,” he said.
Study details
During the period 2004-2005, the investigators randomly assigned 3,727 women aged 18-25 years to receive Cervarix and 3,739 to a control group that received the hepatitis A vaccine; after 4 years, the control group also received Cervarix and exited the study. They were replaced by an unvaccinated control group of 2,836 women. The new control group and the original HPV vaccine group were followed for an additional 7 years.
In years 7-11 of the trial, the investigators found 9.2 additional cervical intraepithelial neoplasias of grade 2 or worse (CIN2+) from HPV types not covered by Cervarix per 1,000 vaccinated women in comparison with unvaccinated participants. This corresponds to –71.2% negative vaccine efficacy against CIN2+ lesions of HPV types not covered by the vaccine.
There were 8.3 additional CIN3+ lesions from nontargeted HPV strains per 1,000 vaccinated women in comparison with unvaccinated participants, which corresponds to –135% negative vaccine efficacy.
Overall, however, there was a net benefit of vaccination, with 27 fewer CIN2+ lesions when all HPV genotypes – vaccine covered or not – were considered per 1,000 vaccinated women over the entire 11 years of follow-up.
There were also 8.7 fewer CIN3+ lesions across all genotypes per 1,000 vaccinated women, but the benefit was not statistically significant.
Among the study limits, the team was unable to evaluate the effect of clinical unmasking on cervical cancer, because women were treated for high-grade cervical lesions before cases could progress to cervical cancer.
The trial was funded by the National Cancer Institute and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline provided the Cervarix vaccine and supported aspects of the trial. Two authors are named inventors on U.S. government–owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. Dr. Steben is an adviser/speaker for many companies, including GlaxoSmithKline and Merck.
A version of this article first appeared on Medscape.com.
Vaccines against human papillomavirus have been hailed as a success: they have been shown to decrease the incidence of cervical lesions associated with the HPV types that are in the vaccine.
However,
An expert not involved in the research said the new data “tell us to be a little bit careful.” Although the HPV types not included in the vaccine are rarer and less aggressive, they can still cause cancer.
The data come from the Costa Rica HPV Vaccine Trial, which involved more than 10,000 women aged 18-25 years. The HPV vaccine used in the trial was Cervarix, from GlaxoSmithKline. It covers the two leading causes of cervical cancer, HPV-16 and -18, and provides partial protection against three other genotypes.
After a follow-up of 11 years, among vaccinated women, there was an excess of precancerous cervical lesions caused by genotypes not included in the vaccine, resulting in negative vaccine efficacy for those HPV variants.
The increase wasn’t enough to offset the overall benefit of vaccination when all genotypes were considered, said the researchers, led by Jaimie Shing, PhD, a postdoctoral research fellow at the National Cancer Institute in Bethesda, Md.
Vaccinated women “still had long-term absolute reductions in high-grade lesions,” they pointed out.
The net protection “remained considerable, emphasizing the importance of HPV vaccination for cervical cancer prevention,” the team concluded.
The findings were published online in The Lancet Oncology.
The results are likely the first evidence to date of “clinical unmasking” with HPV vaccination, meaning that protection against the strains covered by the vaccine leaves women more prone to attack from other carcinogenic HPV variants.
This phenomenon “could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs,” the investigators commented.
Highlighting a need for caution
The take-home message from the trial is that “we have to be careful,” said Marc Steben, MD, co-President of HPV Global Action and a professor at the University of Montreal.
He noted that the Cervarix HPV vaccine used in the trial is not the vaccine that is used now in developed nations.
The current standard HPV vaccine is Gardasil 9 (Merck), which offers broader coverage against nine HPV types (types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
There are 12 main carcinogenic HPV genotypes, so unmasking of other strains is still possible with Gardasil 9, he said.
There is another issue, Dr. Steben added. The success of HPV vaccinations - a nearly 90% reduction in invasive cervical cancer in women who are vaccinated at a young age – has led to questions about the future role of routine cervical cancer screening.
“Some people are saying that if we achieve 90% coverage, we might” eliminate community transmission and no longer need to screen, he said.
These trial results “tell us to be a little bit careful,” Dr. Steben continued. Those HPV types that are less aggressive and rarer than HPV-16 and -18 “can still cause cancer and might be there and surprise us. It could take more time than we thought” to get to the point where screening can be eliminated.
“There might be a little problem if we stop too early,” he said.
Study details
During the period 2004-2005, the investigators randomly assigned 3,727 women aged 18-25 years to receive Cervarix and 3,739 to a control group that received the hepatitis A vaccine; after 4 years, the control group also received Cervarix and exited the study. They were replaced by an unvaccinated control group of 2,836 women. The new control group and the original HPV vaccine group were followed for an additional 7 years.
In years 7-11 of the trial, the investigators found 9.2 additional cervical intraepithelial neoplasias of grade 2 or worse (CIN2+) from HPV types not covered by Cervarix per 1,000 vaccinated women in comparison with unvaccinated participants. This corresponds to –71.2% negative vaccine efficacy against CIN2+ lesions of HPV types not covered by the vaccine.
There were 8.3 additional CIN3+ lesions from nontargeted HPV strains per 1,000 vaccinated women in comparison with unvaccinated participants, which corresponds to –135% negative vaccine efficacy.
Overall, however, there was a net benefit of vaccination, with 27 fewer CIN2+ lesions when all HPV genotypes – vaccine covered or not – were considered per 1,000 vaccinated women over the entire 11 years of follow-up.
There were also 8.7 fewer CIN3+ lesions across all genotypes per 1,000 vaccinated women, but the benefit was not statistically significant.
Among the study limits, the team was unable to evaluate the effect of clinical unmasking on cervical cancer, because women were treated for high-grade cervical lesions before cases could progress to cervical cancer.
The trial was funded by the National Cancer Institute and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline provided the Cervarix vaccine and supported aspects of the trial. Two authors are named inventors on U.S. government–owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. Dr. Steben is an adviser/speaker for many companies, including GlaxoSmithKline and Merck.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Can the ketogenic diet treat polycystic ovary syndrome?
MADRID – During the International Scientific Symposium “New Frontiers in Scientific Research” that recently took place in Barcelona, specialists analyzed the role of the very-low-calorie ketogenic diet. This analysis was in relation to three comorbidities that have a higher incidence among overweight and obese patients: polycystic ovary syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. The experts’ aim? To analyze and update the latest evidence on the benefits of this dietary choice.
Polycystic ovary syndrome
Alessandra Gambineri, MD, PhD, associate professor at the department of medicine and surgery (DIMEC) at the University of Bologna, Italy, addressed the link between obesity and polycystic ovary syndrome, which she described as a chronic disease that affects about 10% of women of childbearing age and that presents diverse phenotypes with different characteristics.
“The pathophysiology of this syndrome is characterized by the interaction of three factors: androgen excess, adipose tissue dysfunction, and insulin resistance. These factors interact with each other and are expressed differently in each phenotype,” said Dr. Gambineri.
She indicated that adipose tissue dysfunction is central to this pathology. This centrality results from its association with secretions, such as free fatty acids, proinflammatory cytokines, certain adipokines that promote insulin resistance, glucocorticosteroids, androgens, and oxidative stress.
“Similarly, the oxidative stress that characterizes this syndrome is increasingly present in obese individuals,” said Dr. Gambineri. “This oxidative stress also produces ovary hypotoxicity that aggravates ovulatory function. In this context, the very-low-calorie ketogenic diet can be useful in several ways: weight reduction; promoting the loss of mainly visceral/abdominal fat; decreasing lipotoxicity; and improving inflammation, hyperinsulinemia, and insulin resistance.”
This was the path followed to carry out a study that aimed to analyze the effects of the very-low-calorie ketogenic diet on manifestations of polycystic ovary syndrome in the obesity phenotype. Dr. Gambineri presented its results.
“The objective was to compare the effects of a very-low-calorie ketogenic diet and the standard low-calorie (hypocaloric) diet as a control group,” she said. “The effects studied include body weight, insulin resistance, menstrual cycle, ovulation, ovarian morphology, and hyperandrogenism in a population of 30 obese women with polycystic ovary syndrome and insulin resistance.”
Study participants had a diagnosis of polycystic ovary syndrome as defined by the National Institutes of Health criteria and were aged 18-45 years. These women were randomly assigned to two groups of equal size: experimental (very-low-calorie ketogenic diet) and control (hypocaloric diet). “The women assigned to the experimental group followed the ketogenic stage for eight weeks and then moved to the second, low-calorie diet phase for an additional eight weeks, while those in the control group (hypocaloric diet) followed the low-calorie diet for all 16 weeks.”
The primary outcomes were changes in weight and body composition, specifically fat mass and lean mass, measured by bioimpedance. “The changes observed in the following aspects were considered secondary outcomes: abdominal fat distribution, metabolic parameters, ovulation, ovarian morphology, hirsutism, hyperandrogenism, psychological well-being, and psychological distress,” said Dr. Gambineri. “Any reduction in the ovarian stroma, the area where androgens are synthesized, was also analyzed.”
The study authors found that although BMI decreased in both groups, this reduction was greater in the group that followed the very-low-calorie ketogenic diet. Significant weight loss was observed in both groups, 12.4 kg versus 4.7 kg. Significant differences were also observed in waist circumference (−8.1% in the experimental group vs. −2.2% in the control group), fat mass (−15.1% vs. −8.5%), and free testosterone (−30.3% vs. +10.6%). Only the experimental group saw a reduction in insulin.
“A key point regarding hyperandrogenism, especially regarding what’s referred to as free testosterone, there was only a significant reduction in the very-low-calorie ketogenic diet group,” said Dr. Gambineri. “This reduction was especially evident in the first part of the study, coinciding with the ketogenic period. The reason for this effect lies in the significant increase in the concentration of sex hormone-binding globulins, SHB6. Said globulins bind to the testosterone present in female blood, producing a reduction in free testosterone, a very important effect considering that this syndrome is an androgenic disorder. Furthermore, current treatments for polycystic ovary syndrome do not reduce free testosterone as much as this dietary approach does.”
For the specialist, among all these positive effects in these patients, perhaps most important is the notable improvement that occurs in ovulation. “At the beginning of the study, only 38.5% of the participants in the experimental group and 14.3% of those in the control group had ovulatory cycles. After the intervention, 84.6% managed to ovulate, compared to 35.7% who achieved this goal in the other group.”
Dr. Gambineri suggested that this method is “valid for reducing fat mass and rapidly improving hyperandrogenism and ovulatory dysfunction in women with obesity and polycystic ovary syndrome.”
Reversing type 2 diabetes?
Daniela Sofrà, MD, an endocrinologist specializing in diabetology at La Source Clinic, Lausanne, Switzerland, reviewed the current evidence on the role of the very-low-calorie ketogenic diet in the management of type 2 diabetes.
“It’s time to rethink diabetes treatment and focus efforts on managing obesity as an associated factor,” she said. “One of the hypotheses being examined in this regard is the twin cycle, which postulates that type 2 diabetes is the result of excess fat in the liver. This in turn is associated with insulin resistance with pancreas dysfunction.”
Dr. Sofrà added that there is a study documenting for the first time the reversibility of the morphology of the diabetic pancreas after caloric restriction with the very-low-calorie ketogenic diet. “The reason for this effect is the use of visceral and intrahepatic fat, which can lead to the remission of the clinical manifestation of type 2 diabetes, understanding as such the definition made by the American Diabetes Association: glycosylated hemoglobin < 6.5% without pharmacological therapy.”
Specifically, the results of this research showed that after following the very-low-calorie ketogenic diet and achieving a 15% weight loss (mean weight loss of the participants), liver glucose levels returned to normal levels within 7 days. Beta cell function returned to near normal within 8 weeks.
“Subsequent studies have shown the durability of remission of type 2 diabetes, thanks to the reactivation of the insulin-secreting function of beta cells that had become dedifferentiated in the face of chronic nutrient excess. Specifically, 6 out of 10 patients maintained glycosylated hemoglobin < 6% after 6 months without the need for pharmacological therapy,” Dr. Sofrà added.
Likewise, she highlighted that the probability of achieving remission is mainly determined by the duration of the disease. “The years with diabetes are one of the main predictors of the response that the patient will have with this dietary intervention. Studies have shown that remission is possible in patients with diabetes for less than 6 years, although there are other projects that indicate that it can be achieved with up to 10 years’ duration.”
Based on these data, Dr. Sofrà emphasized the pleiotropic effects of the very-low-calorie ketogenic diet on glycemic control, favoring the possible remission of diabetes or the reduction of drugs, as well as the reduction of the HOMA-IR index (insulin resistance) and waist circumference in people with type 2 diabetes.
Nonalcoholic fatty liver disease
The third comorbidity of obesity that may benefit from the very-low-calorie ketogenic diet is hepatic steatosis, or nonalcoholic fatty liver disease, said Hardy Walle, MD, an internal medicine specialist and director/founder of the Bodymed center, Kirkel, Germany, and one of the authors of this research.
“Recent research shows that ectopic fat and nonalcoholic fatty liver disease could be considered a cause, or at least one of the causes, of most of the diseases that affect the population as a consequence of overweight and obesity,” said Dr. Walle. “Some authors have stated that without fatty liver, there is no type 2 diabetes.”
Dr. Walle pointed out that between 30% and 40% of the adult population has nonalcoholic fatty liver disease, a percentage that increases considerably in people with obesity, reaching 70% prevalence and increasing, in the case of type 2 diabetes, to almost 90%. “Even normal weight does not rule out fatty liver; in fact, about 15% of people with nonalcoholic fatty liver disease are not overweight.”
In a setting where there are no approved drugs for the treatment of fatty liver (the current standard approach focuses on lifestyle interventions), a short-term hypocaloric diet (or liver fasting) is considered an effective method for management of this pathology. This principle was demonstrated by a study by the Saarland University, Saarbrücken, Germany, that Dr. Walle used to illustrate this statement.
“The participants (60 patients with hepatic steatosis) followed a hypocaloric diet (less than 1,000 kcal/day) for 14 days with a formula rich in protein and fiber specially developed for the treatment of nonalcoholic fatty liver disease. A fibroscan was then performed with controlled attenuation parameter measurement to quantify fatty liver disease. The results showed not only a significant improvement in nonalcoholic fatty liver disease parameters but also a marked improvement in all relevant metabolic parameters (serum lipids, liver enzymes),” explained Dr. Walle.
“This evidence leads us to affirm that the concept of hepatic fasting (by means of a hypocaloric diet) marks a point of reference for a future treatment approach for nonalcoholic fatty liver disease,” he concluded.
The study that Dr. Gambineri presented was carried out with the collaboration of the Pronokal Group (Nestlé Health Science). Dr. Gambineri, Dr. Sofrà, and Dr. Walle disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MADRID – During the International Scientific Symposium “New Frontiers in Scientific Research” that recently took place in Barcelona, specialists analyzed the role of the very-low-calorie ketogenic diet. This analysis was in relation to three comorbidities that have a higher incidence among overweight and obese patients: polycystic ovary syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. The experts’ aim? To analyze and update the latest evidence on the benefits of this dietary choice.
Polycystic ovary syndrome
Alessandra Gambineri, MD, PhD, associate professor at the department of medicine and surgery (DIMEC) at the University of Bologna, Italy, addressed the link between obesity and polycystic ovary syndrome, which she described as a chronic disease that affects about 10% of women of childbearing age and that presents diverse phenotypes with different characteristics.
“The pathophysiology of this syndrome is characterized by the interaction of three factors: androgen excess, adipose tissue dysfunction, and insulin resistance. These factors interact with each other and are expressed differently in each phenotype,” said Dr. Gambineri.
She indicated that adipose tissue dysfunction is central to this pathology. This centrality results from its association with secretions, such as free fatty acids, proinflammatory cytokines, certain adipokines that promote insulin resistance, glucocorticosteroids, androgens, and oxidative stress.
“Similarly, the oxidative stress that characterizes this syndrome is increasingly present in obese individuals,” said Dr. Gambineri. “This oxidative stress also produces ovary hypotoxicity that aggravates ovulatory function. In this context, the very-low-calorie ketogenic diet can be useful in several ways: weight reduction; promoting the loss of mainly visceral/abdominal fat; decreasing lipotoxicity; and improving inflammation, hyperinsulinemia, and insulin resistance.”
This was the path followed to carry out a study that aimed to analyze the effects of the very-low-calorie ketogenic diet on manifestations of polycystic ovary syndrome in the obesity phenotype. Dr. Gambineri presented its results.
“The objective was to compare the effects of a very-low-calorie ketogenic diet and the standard low-calorie (hypocaloric) diet as a control group,” she said. “The effects studied include body weight, insulin resistance, menstrual cycle, ovulation, ovarian morphology, and hyperandrogenism in a population of 30 obese women with polycystic ovary syndrome and insulin resistance.”
Study participants had a diagnosis of polycystic ovary syndrome as defined by the National Institutes of Health criteria and were aged 18-45 years. These women were randomly assigned to two groups of equal size: experimental (very-low-calorie ketogenic diet) and control (hypocaloric diet). “The women assigned to the experimental group followed the ketogenic stage for eight weeks and then moved to the second, low-calorie diet phase for an additional eight weeks, while those in the control group (hypocaloric diet) followed the low-calorie diet for all 16 weeks.”
The primary outcomes were changes in weight and body composition, specifically fat mass and lean mass, measured by bioimpedance. “The changes observed in the following aspects were considered secondary outcomes: abdominal fat distribution, metabolic parameters, ovulation, ovarian morphology, hirsutism, hyperandrogenism, psychological well-being, and psychological distress,” said Dr. Gambineri. “Any reduction in the ovarian stroma, the area where androgens are synthesized, was also analyzed.”
The study authors found that although BMI decreased in both groups, this reduction was greater in the group that followed the very-low-calorie ketogenic diet. Significant weight loss was observed in both groups, 12.4 kg versus 4.7 kg. Significant differences were also observed in waist circumference (−8.1% in the experimental group vs. −2.2% in the control group), fat mass (−15.1% vs. −8.5%), and free testosterone (−30.3% vs. +10.6%). Only the experimental group saw a reduction in insulin.
“A key point regarding hyperandrogenism, especially regarding what’s referred to as free testosterone, there was only a significant reduction in the very-low-calorie ketogenic diet group,” said Dr. Gambineri. “This reduction was especially evident in the first part of the study, coinciding with the ketogenic period. The reason for this effect lies in the significant increase in the concentration of sex hormone-binding globulins, SHB6. Said globulins bind to the testosterone present in female blood, producing a reduction in free testosterone, a very important effect considering that this syndrome is an androgenic disorder. Furthermore, current treatments for polycystic ovary syndrome do not reduce free testosterone as much as this dietary approach does.”
For the specialist, among all these positive effects in these patients, perhaps most important is the notable improvement that occurs in ovulation. “At the beginning of the study, only 38.5% of the participants in the experimental group and 14.3% of those in the control group had ovulatory cycles. After the intervention, 84.6% managed to ovulate, compared to 35.7% who achieved this goal in the other group.”
Dr. Gambineri suggested that this method is “valid for reducing fat mass and rapidly improving hyperandrogenism and ovulatory dysfunction in women with obesity and polycystic ovary syndrome.”
Reversing type 2 diabetes?
Daniela Sofrà, MD, an endocrinologist specializing in diabetology at La Source Clinic, Lausanne, Switzerland, reviewed the current evidence on the role of the very-low-calorie ketogenic diet in the management of type 2 diabetes.
“It’s time to rethink diabetes treatment and focus efforts on managing obesity as an associated factor,” she said. “One of the hypotheses being examined in this regard is the twin cycle, which postulates that type 2 diabetes is the result of excess fat in the liver. This in turn is associated with insulin resistance with pancreas dysfunction.”
Dr. Sofrà added that there is a study documenting for the first time the reversibility of the morphology of the diabetic pancreas after caloric restriction with the very-low-calorie ketogenic diet. “The reason for this effect is the use of visceral and intrahepatic fat, which can lead to the remission of the clinical manifestation of type 2 diabetes, understanding as such the definition made by the American Diabetes Association: glycosylated hemoglobin < 6.5% without pharmacological therapy.”
Specifically, the results of this research showed that after following the very-low-calorie ketogenic diet and achieving a 15% weight loss (mean weight loss of the participants), liver glucose levels returned to normal levels within 7 days. Beta cell function returned to near normal within 8 weeks.
“Subsequent studies have shown the durability of remission of type 2 diabetes, thanks to the reactivation of the insulin-secreting function of beta cells that had become dedifferentiated in the face of chronic nutrient excess. Specifically, 6 out of 10 patients maintained glycosylated hemoglobin < 6% after 6 months without the need for pharmacological therapy,” Dr. Sofrà added.
Likewise, she highlighted that the probability of achieving remission is mainly determined by the duration of the disease. “The years with diabetes are one of the main predictors of the response that the patient will have with this dietary intervention. Studies have shown that remission is possible in patients with diabetes for less than 6 years, although there are other projects that indicate that it can be achieved with up to 10 years’ duration.”
Based on these data, Dr. Sofrà emphasized the pleiotropic effects of the very-low-calorie ketogenic diet on glycemic control, favoring the possible remission of diabetes or the reduction of drugs, as well as the reduction of the HOMA-IR index (insulin resistance) and waist circumference in people with type 2 diabetes.
Nonalcoholic fatty liver disease
The third comorbidity of obesity that may benefit from the very-low-calorie ketogenic diet is hepatic steatosis, or nonalcoholic fatty liver disease, said Hardy Walle, MD, an internal medicine specialist and director/founder of the Bodymed center, Kirkel, Germany, and one of the authors of this research.
“Recent research shows that ectopic fat and nonalcoholic fatty liver disease could be considered a cause, or at least one of the causes, of most of the diseases that affect the population as a consequence of overweight and obesity,” said Dr. Walle. “Some authors have stated that without fatty liver, there is no type 2 diabetes.”
Dr. Walle pointed out that between 30% and 40% of the adult population has nonalcoholic fatty liver disease, a percentage that increases considerably in people with obesity, reaching 70% prevalence and increasing, in the case of type 2 diabetes, to almost 90%. “Even normal weight does not rule out fatty liver; in fact, about 15% of people with nonalcoholic fatty liver disease are not overweight.”
In a setting where there are no approved drugs for the treatment of fatty liver (the current standard approach focuses on lifestyle interventions), a short-term hypocaloric diet (or liver fasting) is considered an effective method for management of this pathology. This principle was demonstrated by a study by the Saarland University, Saarbrücken, Germany, that Dr. Walle used to illustrate this statement.
“The participants (60 patients with hepatic steatosis) followed a hypocaloric diet (less than 1,000 kcal/day) for 14 days with a formula rich in protein and fiber specially developed for the treatment of nonalcoholic fatty liver disease. A fibroscan was then performed with controlled attenuation parameter measurement to quantify fatty liver disease. The results showed not only a significant improvement in nonalcoholic fatty liver disease parameters but also a marked improvement in all relevant metabolic parameters (serum lipids, liver enzymes),” explained Dr. Walle.
“This evidence leads us to affirm that the concept of hepatic fasting (by means of a hypocaloric diet) marks a point of reference for a future treatment approach for nonalcoholic fatty liver disease,” he concluded.
The study that Dr. Gambineri presented was carried out with the collaboration of the Pronokal Group (Nestlé Health Science). Dr. Gambineri, Dr. Sofrà, and Dr. Walle disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MADRID – During the International Scientific Symposium “New Frontiers in Scientific Research” that recently took place in Barcelona, specialists analyzed the role of the very-low-calorie ketogenic diet. This analysis was in relation to three comorbidities that have a higher incidence among overweight and obese patients: polycystic ovary syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. The experts’ aim? To analyze and update the latest evidence on the benefits of this dietary choice.
Polycystic ovary syndrome
Alessandra Gambineri, MD, PhD, associate professor at the department of medicine and surgery (DIMEC) at the University of Bologna, Italy, addressed the link between obesity and polycystic ovary syndrome, which she described as a chronic disease that affects about 10% of women of childbearing age and that presents diverse phenotypes with different characteristics.
“The pathophysiology of this syndrome is characterized by the interaction of three factors: androgen excess, adipose tissue dysfunction, and insulin resistance. These factors interact with each other and are expressed differently in each phenotype,” said Dr. Gambineri.
She indicated that adipose tissue dysfunction is central to this pathology. This centrality results from its association with secretions, such as free fatty acids, proinflammatory cytokines, certain adipokines that promote insulin resistance, glucocorticosteroids, androgens, and oxidative stress.
“Similarly, the oxidative stress that characterizes this syndrome is increasingly present in obese individuals,” said Dr. Gambineri. “This oxidative stress also produces ovary hypotoxicity that aggravates ovulatory function. In this context, the very-low-calorie ketogenic diet can be useful in several ways: weight reduction; promoting the loss of mainly visceral/abdominal fat; decreasing lipotoxicity; and improving inflammation, hyperinsulinemia, and insulin resistance.”
This was the path followed to carry out a study that aimed to analyze the effects of the very-low-calorie ketogenic diet on manifestations of polycystic ovary syndrome in the obesity phenotype. Dr. Gambineri presented its results.
“The objective was to compare the effects of a very-low-calorie ketogenic diet and the standard low-calorie (hypocaloric) diet as a control group,” she said. “The effects studied include body weight, insulin resistance, menstrual cycle, ovulation, ovarian morphology, and hyperandrogenism in a population of 30 obese women with polycystic ovary syndrome and insulin resistance.”
Study participants had a diagnosis of polycystic ovary syndrome as defined by the National Institutes of Health criteria and were aged 18-45 years. These women were randomly assigned to two groups of equal size: experimental (very-low-calorie ketogenic diet) and control (hypocaloric diet). “The women assigned to the experimental group followed the ketogenic stage for eight weeks and then moved to the second, low-calorie diet phase for an additional eight weeks, while those in the control group (hypocaloric diet) followed the low-calorie diet for all 16 weeks.”
The primary outcomes were changes in weight and body composition, specifically fat mass and lean mass, measured by bioimpedance. “The changes observed in the following aspects were considered secondary outcomes: abdominal fat distribution, metabolic parameters, ovulation, ovarian morphology, hirsutism, hyperandrogenism, psychological well-being, and psychological distress,” said Dr. Gambineri. “Any reduction in the ovarian stroma, the area where androgens are synthesized, was also analyzed.”
The study authors found that although BMI decreased in both groups, this reduction was greater in the group that followed the very-low-calorie ketogenic diet. Significant weight loss was observed in both groups, 12.4 kg versus 4.7 kg. Significant differences were also observed in waist circumference (−8.1% in the experimental group vs. −2.2% in the control group), fat mass (−15.1% vs. −8.5%), and free testosterone (−30.3% vs. +10.6%). Only the experimental group saw a reduction in insulin.
“A key point regarding hyperandrogenism, especially regarding what’s referred to as free testosterone, there was only a significant reduction in the very-low-calorie ketogenic diet group,” said Dr. Gambineri. “This reduction was especially evident in the first part of the study, coinciding with the ketogenic period. The reason for this effect lies in the significant increase in the concentration of sex hormone-binding globulins, SHB6. Said globulins bind to the testosterone present in female blood, producing a reduction in free testosterone, a very important effect considering that this syndrome is an androgenic disorder. Furthermore, current treatments for polycystic ovary syndrome do not reduce free testosterone as much as this dietary approach does.”
For the specialist, among all these positive effects in these patients, perhaps most important is the notable improvement that occurs in ovulation. “At the beginning of the study, only 38.5% of the participants in the experimental group and 14.3% of those in the control group had ovulatory cycles. After the intervention, 84.6% managed to ovulate, compared to 35.7% who achieved this goal in the other group.”
Dr. Gambineri suggested that this method is “valid for reducing fat mass and rapidly improving hyperandrogenism and ovulatory dysfunction in women with obesity and polycystic ovary syndrome.”
Reversing type 2 diabetes?
Daniela Sofrà, MD, an endocrinologist specializing in diabetology at La Source Clinic, Lausanne, Switzerland, reviewed the current evidence on the role of the very-low-calorie ketogenic diet in the management of type 2 diabetes.
“It’s time to rethink diabetes treatment and focus efforts on managing obesity as an associated factor,” she said. “One of the hypotheses being examined in this regard is the twin cycle, which postulates that type 2 diabetes is the result of excess fat in the liver. This in turn is associated with insulin resistance with pancreas dysfunction.”
Dr. Sofrà added that there is a study documenting for the first time the reversibility of the morphology of the diabetic pancreas after caloric restriction with the very-low-calorie ketogenic diet. “The reason for this effect is the use of visceral and intrahepatic fat, which can lead to the remission of the clinical manifestation of type 2 diabetes, understanding as such the definition made by the American Diabetes Association: glycosylated hemoglobin < 6.5% without pharmacological therapy.”
Specifically, the results of this research showed that after following the very-low-calorie ketogenic diet and achieving a 15% weight loss (mean weight loss of the participants), liver glucose levels returned to normal levels within 7 days. Beta cell function returned to near normal within 8 weeks.
“Subsequent studies have shown the durability of remission of type 2 diabetes, thanks to the reactivation of the insulin-secreting function of beta cells that had become dedifferentiated in the face of chronic nutrient excess. Specifically, 6 out of 10 patients maintained glycosylated hemoglobin < 6% after 6 months without the need for pharmacological therapy,” Dr. Sofrà added.
Likewise, she highlighted that the probability of achieving remission is mainly determined by the duration of the disease. “The years with diabetes are one of the main predictors of the response that the patient will have with this dietary intervention. Studies have shown that remission is possible in patients with diabetes for less than 6 years, although there are other projects that indicate that it can be achieved with up to 10 years’ duration.”
Based on these data, Dr. Sofrà emphasized the pleiotropic effects of the very-low-calorie ketogenic diet on glycemic control, favoring the possible remission of diabetes or the reduction of drugs, as well as the reduction of the HOMA-IR index (insulin resistance) and waist circumference in people with type 2 diabetes.
Nonalcoholic fatty liver disease
The third comorbidity of obesity that may benefit from the very-low-calorie ketogenic diet is hepatic steatosis, or nonalcoholic fatty liver disease, said Hardy Walle, MD, an internal medicine specialist and director/founder of the Bodymed center, Kirkel, Germany, and one of the authors of this research.
“Recent research shows that ectopic fat and nonalcoholic fatty liver disease could be considered a cause, or at least one of the causes, of most of the diseases that affect the population as a consequence of overweight and obesity,” said Dr. Walle. “Some authors have stated that without fatty liver, there is no type 2 diabetes.”
Dr. Walle pointed out that between 30% and 40% of the adult population has nonalcoholic fatty liver disease, a percentage that increases considerably in people with obesity, reaching 70% prevalence and increasing, in the case of type 2 diabetes, to almost 90%. “Even normal weight does not rule out fatty liver; in fact, about 15% of people with nonalcoholic fatty liver disease are not overweight.”
In a setting where there are no approved drugs for the treatment of fatty liver (the current standard approach focuses on lifestyle interventions), a short-term hypocaloric diet (or liver fasting) is considered an effective method for management of this pathology. This principle was demonstrated by a study by the Saarland University, Saarbrücken, Germany, that Dr. Walle used to illustrate this statement.
“The participants (60 patients with hepatic steatosis) followed a hypocaloric diet (less than 1,000 kcal/day) for 14 days with a formula rich in protein and fiber specially developed for the treatment of nonalcoholic fatty liver disease. A fibroscan was then performed with controlled attenuation parameter measurement to quantify fatty liver disease. The results showed not only a significant improvement in nonalcoholic fatty liver disease parameters but also a marked improvement in all relevant metabolic parameters (serum lipids, liver enzymes),” explained Dr. Walle.
“This evidence leads us to affirm that the concept of hepatic fasting (by means of a hypocaloric diet) marks a point of reference for a future treatment approach for nonalcoholic fatty liver disease,” he concluded.
The study that Dr. Gambineri presented was carried out with the collaboration of the Pronokal Group (Nestlé Health Science). Dr. Gambineri, Dr. Sofrà, and Dr. Walle disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tampon shortage linked to supply chain, inflation issues
Social media users have posted about bare shelves and higher costs for months, marking the latest products to face stress under global supply chain concerns after baby formula, cars, and appliances.
Other menstrual products have increased in price as well because of inflation, according to Bloomberg News. The average price for a package of menstrual pads has increased about 8% this year, and the price of a box of tampons has increased about 10%.
Andre Schulten, the chief financial officer for Procter & Gamble, which makes and sells 4.5 billion boxes of Tampax each year, said on a recent earnings call that it has been “costly and highly volatile” to acquire the raw materials needed for production.
Raw materials such as cotton, rayon, and plastic, for instance, have been used to produce personal protective gear during the pandemic, which has led to shortages. The cost of transportation for consumer goods has also nearly tripled, and pandemic policies at ports have led to shipping delays.
Edgewell Personal Care, which makes the brands Playtex and o.b., has had a severe staff shortage at its Delaware facility where tampons are made, according to Time. The FDA has classified tampons as class II medical devices, which require certain quality-control regulations and qualified workers on the assembly line, the news outlet reported.
Representatives for CVS and Walgreens confirmed that they’ve had shortages in recent weeks, according to The Washington Post. Procter & Gamble said it is working with retail partners to make feminine care products more available.
“We understand it is frustrating for consumers when they can’t find what they need,” the company told the newspaper. “We can assure you this is a temporary situation.”
Kimberly-Clark, which makes U by Kotex tampons, told the Post that it “has not experienced a product or supply shortage” in the United States, saying it is “working closely with our retail partners to keep shelves stocked.”
But the shortage may grow worse as the year goes on and the peak season for shipping approaches, the newspaper reported.
“Capacity is only going to get tighter as we move toward the end of the year,” Vaughn Moore, chief executive of AIT Worldwide Logistics, told the Post.
While the situation is being straightened out, gynecologists have recommended against extending supply at home by wearing tampons for longer stretches of time, according to The New York Times. Toxic shock syndrome is a rare but potentially fatal condition that can occur when tampons are worn for more than 8 hours.
There are other options, such as reusable menstrual pads, period underwear, and menstrual cups and discs, the Times reported. But some of these may be less appealing to use, or they may cost too much.
A version of this article first appeared on WebMD.com.
Social media users have posted about bare shelves and higher costs for months, marking the latest products to face stress under global supply chain concerns after baby formula, cars, and appliances.
Other menstrual products have increased in price as well because of inflation, according to Bloomberg News. The average price for a package of menstrual pads has increased about 8% this year, and the price of a box of tampons has increased about 10%.
Andre Schulten, the chief financial officer for Procter & Gamble, which makes and sells 4.5 billion boxes of Tampax each year, said on a recent earnings call that it has been “costly and highly volatile” to acquire the raw materials needed for production.
Raw materials such as cotton, rayon, and plastic, for instance, have been used to produce personal protective gear during the pandemic, which has led to shortages. The cost of transportation for consumer goods has also nearly tripled, and pandemic policies at ports have led to shipping delays.
Edgewell Personal Care, which makes the brands Playtex and o.b., has had a severe staff shortage at its Delaware facility where tampons are made, according to Time. The FDA has classified tampons as class II medical devices, which require certain quality-control regulations and qualified workers on the assembly line, the news outlet reported.
Representatives for CVS and Walgreens confirmed that they’ve had shortages in recent weeks, according to The Washington Post. Procter & Gamble said it is working with retail partners to make feminine care products more available.
“We understand it is frustrating for consumers when they can’t find what they need,” the company told the newspaper. “We can assure you this is a temporary situation.”
Kimberly-Clark, which makes U by Kotex tampons, told the Post that it “has not experienced a product or supply shortage” in the United States, saying it is “working closely with our retail partners to keep shelves stocked.”
But the shortage may grow worse as the year goes on and the peak season for shipping approaches, the newspaper reported.
“Capacity is only going to get tighter as we move toward the end of the year,” Vaughn Moore, chief executive of AIT Worldwide Logistics, told the Post.
While the situation is being straightened out, gynecologists have recommended against extending supply at home by wearing tampons for longer stretches of time, according to The New York Times. Toxic shock syndrome is a rare but potentially fatal condition that can occur when tampons are worn for more than 8 hours.
There are other options, such as reusable menstrual pads, period underwear, and menstrual cups and discs, the Times reported. But some of these may be less appealing to use, or they may cost too much.
A version of this article first appeared on WebMD.com.
Social media users have posted about bare shelves and higher costs for months, marking the latest products to face stress under global supply chain concerns after baby formula, cars, and appliances.
Other menstrual products have increased in price as well because of inflation, according to Bloomberg News. The average price for a package of menstrual pads has increased about 8% this year, and the price of a box of tampons has increased about 10%.
Andre Schulten, the chief financial officer for Procter & Gamble, which makes and sells 4.5 billion boxes of Tampax each year, said on a recent earnings call that it has been “costly and highly volatile” to acquire the raw materials needed for production.
Raw materials such as cotton, rayon, and plastic, for instance, have been used to produce personal protective gear during the pandemic, which has led to shortages. The cost of transportation for consumer goods has also nearly tripled, and pandemic policies at ports have led to shipping delays.
Edgewell Personal Care, which makes the brands Playtex and o.b., has had a severe staff shortage at its Delaware facility where tampons are made, according to Time. The FDA has classified tampons as class II medical devices, which require certain quality-control regulations and qualified workers on the assembly line, the news outlet reported.
Representatives for CVS and Walgreens confirmed that they’ve had shortages in recent weeks, according to The Washington Post. Procter & Gamble said it is working with retail partners to make feminine care products more available.
“We understand it is frustrating for consumers when they can’t find what they need,” the company told the newspaper. “We can assure you this is a temporary situation.”
Kimberly-Clark, which makes U by Kotex tampons, told the Post that it “has not experienced a product or supply shortage” in the United States, saying it is “working closely with our retail partners to keep shelves stocked.”
But the shortage may grow worse as the year goes on and the peak season for shipping approaches, the newspaper reported.
“Capacity is only going to get tighter as we move toward the end of the year,” Vaughn Moore, chief executive of AIT Worldwide Logistics, told the Post.
While the situation is being straightened out, gynecologists have recommended against extending supply at home by wearing tampons for longer stretches of time, according to The New York Times. Toxic shock syndrome is a rare but potentially fatal condition that can occur when tampons are worn for more than 8 hours.
There are other options, such as reusable menstrual pads, period underwear, and menstrual cups and discs, the Times reported. But some of these may be less appealing to use, or they may cost too much.
A version of this article first appeared on WebMD.com.
MS and family planning: Bring it up at every office visit
NATIONAL HARBOR, MD – Just 2 days before she spoke in a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers, University of Colorado neurologist Anna Shah, MD, asked a 26-year-old patient with MS about whether she planned to have children. Absolutely not, the young woman replied. “I read online that I can give birth to a baby with MS, which is crazy.”
The patient didn’t understand the risk of having a child with MS – it’s thought to be 2%-5% if one parent has the condition – but she wouldn’t have learned the facts if Dr. Shah hadn’t asked the right questions. “It’s really important for us as a community to know how to be proactive with discussions [about pregnancy],” she said.
As she noted, an estimated 75% of patients with MS are women, most are diagnosed during prime child-bearing years, and many pregnancies in general – an estimated half – are not planned. And while a higher percentage of women with MS are having children than in the past, she said, misinformation remains common. In fact, physicians can be part of the problem.
Dr. Shaw highlighted a 2019 Italian survey that found that 16% of 395 people with MS reported that they were discouraged from having children, mainly by medical professionals, after their diagnosis. Seven percent said they never wanted to become parents because of their MS. A 2021 survey of 332 patients with MS in the United States, United Kingdom, France, Germany, Italy, and Spain, found that 56% reported that MS played a role in their decisions about family planning, and 14% of those decided not to have children.
In regard to women of child-bearing age, Dr. Shah recommends that Open-ended, individualized questions are key. “We don’t know what patients don’t share with us,” she said.
Make sure to consider the timing of any plans to have children, she said. If the patient wants to have children within a year, talk about matters such as whether disease activity is well-controlled (6-12 months of good control is ideal) and whether current disease-modifying therapies are safe. Make sure to get a baseline prepartum MRI scan, she said.
If the patients don’t want to have children, make sure they are using a reliable strategy to avoid conception. Be aware that modafinil – “not one that immediately comes to mind” – may decrease the efficacy of oral contraceptives, she said, as can anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, topiramate, and primidone). Oral contraceptives, meanwhile, may decrease levels of lamotrigine.
What if a patient has trouble conceiving? There are some hints in research that MS may boost the risk of infertility in women, Dr. Shah said. That’s why she recommends that colleagues consider referring a patient to an infertility specialist after attempting conception for 6 months as opposed to the general recommendation for 12 months.
Dr. Shah disclosed advisory board service (Genentech) and development of nonbranded educational programming through Novartis and the National Committee for Quality Assurance.
NATIONAL HARBOR, MD – Just 2 days before she spoke in a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers, University of Colorado neurologist Anna Shah, MD, asked a 26-year-old patient with MS about whether she planned to have children. Absolutely not, the young woman replied. “I read online that I can give birth to a baby with MS, which is crazy.”
The patient didn’t understand the risk of having a child with MS – it’s thought to be 2%-5% if one parent has the condition – but she wouldn’t have learned the facts if Dr. Shah hadn’t asked the right questions. “It’s really important for us as a community to know how to be proactive with discussions [about pregnancy],” she said.
As she noted, an estimated 75% of patients with MS are women, most are diagnosed during prime child-bearing years, and many pregnancies in general – an estimated half – are not planned. And while a higher percentage of women with MS are having children than in the past, she said, misinformation remains common. In fact, physicians can be part of the problem.
Dr. Shaw highlighted a 2019 Italian survey that found that 16% of 395 people with MS reported that they were discouraged from having children, mainly by medical professionals, after their diagnosis. Seven percent said they never wanted to become parents because of their MS. A 2021 survey of 332 patients with MS in the United States, United Kingdom, France, Germany, Italy, and Spain, found that 56% reported that MS played a role in their decisions about family planning, and 14% of those decided not to have children.
In regard to women of child-bearing age, Dr. Shah recommends that Open-ended, individualized questions are key. “We don’t know what patients don’t share with us,” she said.
Make sure to consider the timing of any plans to have children, she said. If the patient wants to have children within a year, talk about matters such as whether disease activity is well-controlled (6-12 months of good control is ideal) and whether current disease-modifying therapies are safe. Make sure to get a baseline prepartum MRI scan, she said.
If the patients don’t want to have children, make sure they are using a reliable strategy to avoid conception. Be aware that modafinil – “not one that immediately comes to mind” – may decrease the efficacy of oral contraceptives, she said, as can anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, topiramate, and primidone). Oral contraceptives, meanwhile, may decrease levels of lamotrigine.
What if a patient has trouble conceiving? There are some hints in research that MS may boost the risk of infertility in women, Dr. Shah said. That’s why she recommends that colleagues consider referring a patient to an infertility specialist after attempting conception for 6 months as opposed to the general recommendation for 12 months.
Dr. Shah disclosed advisory board service (Genentech) and development of nonbranded educational programming through Novartis and the National Committee for Quality Assurance.
NATIONAL HARBOR, MD – Just 2 days before she spoke in a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers, University of Colorado neurologist Anna Shah, MD, asked a 26-year-old patient with MS about whether she planned to have children. Absolutely not, the young woman replied. “I read online that I can give birth to a baby with MS, which is crazy.”
The patient didn’t understand the risk of having a child with MS – it’s thought to be 2%-5% if one parent has the condition – but she wouldn’t have learned the facts if Dr. Shah hadn’t asked the right questions. “It’s really important for us as a community to know how to be proactive with discussions [about pregnancy],” she said.
As she noted, an estimated 75% of patients with MS are women, most are diagnosed during prime child-bearing years, and many pregnancies in general – an estimated half – are not planned. And while a higher percentage of women with MS are having children than in the past, she said, misinformation remains common. In fact, physicians can be part of the problem.
Dr. Shaw highlighted a 2019 Italian survey that found that 16% of 395 people with MS reported that they were discouraged from having children, mainly by medical professionals, after their diagnosis. Seven percent said they never wanted to become parents because of their MS. A 2021 survey of 332 patients with MS in the United States, United Kingdom, France, Germany, Italy, and Spain, found that 56% reported that MS played a role in their decisions about family planning, and 14% of those decided not to have children.
In regard to women of child-bearing age, Dr. Shah recommends that Open-ended, individualized questions are key. “We don’t know what patients don’t share with us,” she said.
Make sure to consider the timing of any plans to have children, she said. If the patient wants to have children within a year, talk about matters such as whether disease activity is well-controlled (6-12 months of good control is ideal) and whether current disease-modifying therapies are safe. Make sure to get a baseline prepartum MRI scan, she said.
If the patients don’t want to have children, make sure they are using a reliable strategy to avoid conception. Be aware that modafinil – “not one that immediately comes to mind” – may decrease the efficacy of oral contraceptives, she said, as can anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, topiramate, and primidone). Oral contraceptives, meanwhile, may decrease levels of lamotrigine.
What if a patient has trouble conceiving? There are some hints in research that MS may boost the risk of infertility in women, Dr. Shah said. That’s why she recommends that colleagues consider referring a patient to an infertility specialist after attempting conception for 6 months as opposed to the general recommendation for 12 months.
Dr. Shah disclosed advisory board service (Genentech) and development of nonbranded educational programming through Novartis and the National Committee for Quality Assurance.
At CMSC 2022
What are the perinatal risks of SARS-CoV-2 infection in pregnancy?
Ferrara A, Hedderson MM, Zhu Y, et al. Perinatal complications in individuals in California with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. 2022;182:503-512. doi:10.1001/jamainternmed.2022.0330
Expert Commentary
SARS-CoV-2 infection is associated with several adverse outcomes, with the magnitude of specific risks varying by population studied and study design used. Early Centers for Disease Control and Prevention (CDC) data demonstrated that pregnant women were at increased risk for severe illness, including risks of intensive care unit (ICU) admission, invasive ventilation, and extracorporeal membrane oxygenation, compared with non–pregnant women.1 Since then, other groups have confirmed the increased risks of severe COVID-19, and also identified pregnancy-specific risks, such as preeclampsia, cesarean delivery (CD), prematurity, venous thromboembolic (VTE) disease, and stillbirth.2-6
The recent study by Ferrara and colleagues adds more granular data to help refine understanding of COVID-19 in pregnancy and counsel patients.
Details of the study
The authors conducted a retrospective cohort study between March 1, 2020, and March 16, 2021, using the electronic health records (EHRs) from Kaiser Permanente Northern California, an integrated managed care organization that serves 4.5 million patients annually. Universal testing for SARS-CoV-2 upon admission for delivery began December 1, 2020; prior to this date, asymptomatic pregnant women were tested only for certain criteria (such as being a health care worker or having high-risk medical conditions).
Pregnant women were identified with SARS-CoV-2 based on 1) a positive polymerase chain reaction test result between 30 days prior to the last menstrual period up to 7 days after delivery or 2) an ICD-10 diagnosis of SARS-CoV-2 infection. Pregnant women not meeting these criteria were classified as SARS-CoV-2 negative. Women were followed through pregnancy to understand if they experienced preterm birth (spontaneous and medically indicated), gestational hypertension, preeclampsia/eclampsia, VTE disease, gestational diabetes, severe maternal morbidity (as defined by the CDC), hospitalization, and livebirth (or stillbirth), in order to consider the timing of SARS-CoV-2 infection relative to each of these outcomes (and ascertain whether SARS-CoV-2 infection preceded any of these outcomes more commonly than not). Management of pregnancies with COVID-19 across this large organization and multiple hospitals was not specified.
Identified perinatal risks
Among 43,886 pregnant women included in the cohort, 1,332 (3.0%) were diagnosed with SARS-CoV-2, with the vast majority of positive tests in the third trimester. Significant sociodemographic differences were noted between those with and without SARS-CoV-2, including differences in age, self-reported race/ethnicity, neighborhood deprivation index, and pre-pregnancy body mass index; no differences were noted for other pre-existing comorbidities, gestational week at delivery, or smoking in pregnancy.
In multivariable models, SARS-CoV-2 infection in pregnancy was associated with severe maternal morbidity, preterm birth, and VTE disease. It was not associated with stillbirth, any hypertensive disorder of pregnancy, CD, or any neonatal complication.
The prevalence of SARS-CoV-2 was 1.3% prior to and 8.0% after implementation of universal testing in pregnancy
A total of 307 of the 1,332 pregant women with SARS-CoV-2 were admitted to the hospital for symptomatic infection; 3 required noninvasive positive-pressure ventilation, and 1 required mechanical ventilation.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
Strengths include the large, EHR-based dataset from a single organization, allowing for granular analysis on patient comorbidities and outcomes (rather than only based on diagnosis codes, as is true of many other large databases), as well as focus on relevant perinatal outcomes and thoughtful statistical modeling. However, a significant challenge with this, and many other studies, is ascertainment of SARS-CoV-2 infections throughout pregnancy. Asymptomatic and mildly symptomatic women, who may not be as likely to have adverse pregnancy outcomes, can often be counted in the unaffected population, biasing study results toward increased risks of SARS-CoV-2. Although the findings stratified by implementation of universal testing (which captures a greater fraction of asymptomatic patients at admission for delivery), do not suggest risk mitigation with asymptomatic status, this analysis did not capture asymptomatic infections earlier in pregnancy, many of which might not be associated with perinatal risk.
Another challenge with such a dataset is that one cannot determine the severity of illness of each patient without manual review of each chart; however, other data that are easily abstracted from the EHR may serve as a proxy. For instance, of the 307 women with symptomatic COVID-19, 4 required respiratory support above nasal cannula. This suggests a low rate of severely ill women, and may explain some of the findings in the study, such as no differences in the rate of CD, hypertensive disorders of pregnancy, or stillbirth, but does not explain the increased risk of both medically indicated and spontaneous preterm birth, or the rates of acute respiratory distress syndrome and sepsis that drive the increased risk of severe maternal morbidity.
The CDC has published data on the risks of stillbirth from a large hospital-based administrative database for COVID-19 from Premier Healthcare.2 In a cohort of over 1.2 million women admitted for delivery, including the timeframe of Ferrara et al’s study, COVID-19 was associated with a 2-fold increased risk of stillbirth, with higher risks noted with the delta variant. A rare outcome, stillbirth occurs in 6/1,000 births,7 which was the rate seen in Ferrara’s publication for both women with and without SARS-CoV-2 infection. The rare nature of the outcome may explain why a signal was not noted in the article of interest.
Translating data to patient counseling
Ferrara and colleagues’ study clearly confirms that COVID-19 infection has risks. Although many women with a COVID-19 infection in pregnancy may have an uncomplicated course, a favorable outcome is hard to predict with certainty. Risks of prematurity, VTE, organ dysfunction, and stillbirth from COVID-19 are rare but devastating complications. However, vaccinated women tend to incur far fewer adverse outcomes of COVID-19 in pregnancy, namely a 90% risk reduction in severe or critical COVID-19, with lower rates of ICU admissions and stillbirths.8 While these data strongly favor vaccination, we remain ill-advised on management strategies specifically to mitigate risk for the pregnancy once affected by COVID-19 infection. Thus, prevention with vaccination, mask wearing, and physical distancing remains a cornerstone of prenatal care in the current day. ●
These data continue to support that SARS-CoV-2 infection is associated with prematurity, VTE, and severe maternal adverse outcomes. As sports fanatics often state, the best defense is a good offense. In the case of SARS-CoV-2, COVID-19 vaccination, mask wearing, and physical distancing are likely the best offense against COVID-19 infection in pregnancy.
- Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3.
- DeSisto CL. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70. doi:10.15585/mmwr.mm7047e1.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and metaanalysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320.
- Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181:714-717. doi:10.1001/jamainternmed.2020.9241.
- Katz D, Bateman BT, Kjaer K, et al. The Society for Obstetric Anesthesia and Perinatology Coronavirus Disease 2019 Registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome Coronavirus-2 outbreak in the United States. Anesth Analg. 2021;133:462-473. doi:10.1213/ANE.0000000000005592.
- Metz TD, Clifton RG, Hughes BL, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748759. doi:10.1001/jama.2022.1190.
- Management of stillbirth. https ://www.acog.org/en/clinical/clinical-guidance/obstetric-care-consensus/articles/2020/03/management-of-stillbirth. Accessed May 23, 2022.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109. doi:10.1097/AOG.0000000000004621.
Ferrara A, Hedderson MM, Zhu Y, et al. Perinatal complications in individuals in California with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. 2022;182:503-512. doi:10.1001/jamainternmed.2022.0330
Expert Commentary
SARS-CoV-2 infection is associated with several adverse outcomes, with the magnitude of specific risks varying by population studied and study design used. Early Centers for Disease Control and Prevention (CDC) data demonstrated that pregnant women were at increased risk for severe illness, including risks of intensive care unit (ICU) admission, invasive ventilation, and extracorporeal membrane oxygenation, compared with non–pregnant women.1 Since then, other groups have confirmed the increased risks of severe COVID-19, and also identified pregnancy-specific risks, such as preeclampsia, cesarean delivery (CD), prematurity, venous thromboembolic (VTE) disease, and stillbirth.2-6
The recent study by Ferrara and colleagues adds more granular data to help refine understanding of COVID-19 in pregnancy and counsel patients.
Details of the study
The authors conducted a retrospective cohort study between March 1, 2020, and March 16, 2021, using the electronic health records (EHRs) from Kaiser Permanente Northern California, an integrated managed care organization that serves 4.5 million patients annually. Universal testing for SARS-CoV-2 upon admission for delivery began December 1, 2020; prior to this date, asymptomatic pregnant women were tested only for certain criteria (such as being a health care worker or having high-risk medical conditions).
Pregnant women were identified with SARS-CoV-2 based on 1) a positive polymerase chain reaction test result between 30 days prior to the last menstrual period up to 7 days after delivery or 2) an ICD-10 diagnosis of SARS-CoV-2 infection. Pregnant women not meeting these criteria were classified as SARS-CoV-2 negative. Women were followed through pregnancy to understand if they experienced preterm birth (spontaneous and medically indicated), gestational hypertension, preeclampsia/eclampsia, VTE disease, gestational diabetes, severe maternal morbidity (as defined by the CDC), hospitalization, and livebirth (or stillbirth), in order to consider the timing of SARS-CoV-2 infection relative to each of these outcomes (and ascertain whether SARS-CoV-2 infection preceded any of these outcomes more commonly than not). Management of pregnancies with COVID-19 across this large organization and multiple hospitals was not specified.
Identified perinatal risks
Among 43,886 pregnant women included in the cohort, 1,332 (3.0%) were diagnosed with SARS-CoV-2, with the vast majority of positive tests in the third trimester. Significant sociodemographic differences were noted between those with and without SARS-CoV-2, including differences in age, self-reported race/ethnicity, neighborhood deprivation index, and pre-pregnancy body mass index; no differences were noted for other pre-existing comorbidities, gestational week at delivery, or smoking in pregnancy.
In multivariable models, SARS-CoV-2 infection in pregnancy was associated with severe maternal morbidity, preterm birth, and VTE disease. It was not associated with stillbirth, any hypertensive disorder of pregnancy, CD, or any neonatal complication.
The prevalence of SARS-CoV-2 was 1.3% prior to and 8.0% after implementation of universal testing in pregnancy
A total of 307 of the 1,332 pregant women with SARS-CoV-2 were admitted to the hospital for symptomatic infection; 3 required noninvasive positive-pressure ventilation, and 1 required mechanical ventilation.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
Strengths include the large, EHR-based dataset from a single organization, allowing for granular analysis on patient comorbidities and outcomes (rather than only based on diagnosis codes, as is true of many other large databases), as well as focus on relevant perinatal outcomes and thoughtful statistical modeling. However, a significant challenge with this, and many other studies, is ascertainment of SARS-CoV-2 infections throughout pregnancy. Asymptomatic and mildly symptomatic women, who may not be as likely to have adverse pregnancy outcomes, can often be counted in the unaffected population, biasing study results toward increased risks of SARS-CoV-2. Although the findings stratified by implementation of universal testing (which captures a greater fraction of asymptomatic patients at admission for delivery), do not suggest risk mitigation with asymptomatic status, this analysis did not capture asymptomatic infections earlier in pregnancy, many of which might not be associated with perinatal risk.
Another challenge with such a dataset is that one cannot determine the severity of illness of each patient without manual review of each chart; however, other data that are easily abstracted from the EHR may serve as a proxy. For instance, of the 307 women with symptomatic COVID-19, 4 required respiratory support above nasal cannula. This suggests a low rate of severely ill women, and may explain some of the findings in the study, such as no differences in the rate of CD, hypertensive disorders of pregnancy, or stillbirth, but does not explain the increased risk of both medically indicated and spontaneous preterm birth, or the rates of acute respiratory distress syndrome and sepsis that drive the increased risk of severe maternal morbidity.
The CDC has published data on the risks of stillbirth from a large hospital-based administrative database for COVID-19 from Premier Healthcare.2 In a cohort of over 1.2 million women admitted for delivery, including the timeframe of Ferrara et al’s study, COVID-19 was associated with a 2-fold increased risk of stillbirth, with higher risks noted with the delta variant. A rare outcome, stillbirth occurs in 6/1,000 births,7 which was the rate seen in Ferrara’s publication for both women with and without SARS-CoV-2 infection. The rare nature of the outcome may explain why a signal was not noted in the article of interest.
Translating data to patient counseling
Ferrara and colleagues’ study clearly confirms that COVID-19 infection has risks. Although many women with a COVID-19 infection in pregnancy may have an uncomplicated course, a favorable outcome is hard to predict with certainty. Risks of prematurity, VTE, organ dysfunction, and stillbirth from COVID-19 are rare but devastating complications. However, vaccinated women tend to incur far fewer adverse outcomes of COVID-19 in pregnancy, namely a 90% risk reduction in severe or critical COVID-19, with lower rates of ICU admissions and stillbirths.8 While these data strongly favor vaccination, we remain ill-advised on management strategies specifically to mitigate risk for the pregnancy once affected by COVID-19 infection. Thus, prevention with vaccination, mask wearing, and physical distancing remains a cornerstone of prenatal care in the current day. ●
These data continue to support that SARS-CoV-2 infection is associated with prematurity, VTE, and severe maternal adverse outcomes. As sports fanatics often state, the best defense is a good offense. In the case of SARS-CoV-2, COVID-19 vaccination, mask wearing, and physical distancing are likely the best offense against COVID-19 infection in pregnancy.
Ferrara A, Hedderson MM, Zhu Y, et al. Perinatal complications in individuals in California with or without SARS-CoV-2 infection during pregnancy. JAMA Intern Med. 2022;182:503-512. doi:10.1001/jamainternmed.2022.0330
Expert Commentary
SARS-CoV-2 infection is associated with several adverse outcomes, with the magnitude of specific risks varying by population studied and study design used. Early Centers for Disease Control and Prevention (CDC) data demonstrated that pregnant women were at increased risk for severe illness, including risks of intensive care unit (ICU) admission, invasive ventilation, and extracorporeal membrane oxygenation, compared with non–pregnant women.1 Since then, other groups have confirmed the increased risks of severe COVID-19, and also identified pregnancy-specific risks, such as preeclampsia, cesarean delivery (CD), prematurity, venous thromboembolic (VTE) disease, and stillbirth.2-6
The recent study by Ferrara and colleagues adds more granular data to help refine understanding of COVID-19 in pregnancy and counsel patients.
Details of the study
The authors conducted a retrospective cohort study between March 1, 2020, and March 16, 2021, using the electronic health records (EHRs) from Kaiser Permanente Northern California, an integrated managed care organization that serves 4.5 million patients annually. Universal testing for SARS-CoV-2 upon admission for delivery began December 1, 2020; prior to this date, asymptomatic pregnant women were tested only for certain criteria (such as being a health care worker or having high-risk medical conditions).
Pregnant women were identified with SARS-CoV-2 based on 1) a positive polymerase chain reaction test result between 30 days prior to the last menstrual period up to 7 days after delivery or 2) an ICD-10 diagnosis of SARS-CoV-2 infection. Pregnant women not meeting these criteria were classified as SARS-CoV-2 negative. Women were followed through pregnancy to understand if they experienced preterm birth (spontaneous and medically indicated), gestational hypertension, preeclampsia/eclampsia, VTE disease, gestational diabetes, severe maternal morbidity (as defined by the CDC), hospitalization, and livebirth (or stillbirth), in order to consider the timing of SARS-CoV-2 infection relative to each of these outcomes (and ascertain whether SARS-CoV-2 infection preceded any of these outcomes more commonly than not). Management of pregnancies with COVID-19 across this large organization and multiple hospitals was not specified.
Identified perinatal risks
Among 43,886 pregnant women included in the cohort, 1,332 (3.0%) were diagnosed with SARS-CoV-2, with the vast majority of positive tests in the third trimester. Significant sociodemographic differences were noted between those with and without SARS-CoV-2, including differences in age, self-reported race/ethnicity, neighborhood deprivation index, and pre-pregnancy body mass index; no differences were noted for other pre-existing comorbidities, gestational week at delivery, or smoking in pregnancy.
In multivariable models, SARS-CoV-2 infection in pregnancy was associated with severe maternal morbidity, preterm birth, and VTE disease. It was not associated with stillbirth, any hypertensive disorder of pregnancy, CD, or any neonatal complication.
The prevalence of SARS-CoV-2 was 1.3% prior to and 8.0% after implementation of universal testing in pregnancy
A total of 307 of the 1,332 pregant women with SARS-CoV-2 were admitted to the hospital for symptomatic infection; 3 required noninvasive positive-pressure ventilation, and 1 required mechanical ventilation.
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
Strengths include the large, EHR-based dataset from a single organization, allowing for granular analysis on patient comorbidities and outcomes (rather than only based on diagnosis codes, as is true of many other large databases), as well as focus on relevant perinatal outcomes and thoughtful statistical modeling. However, a significant challenge with this, and many other studies, is ascertainment of SARS-CoV-2 infections throughout pregnancy. Asymptomatic and mildly symptomatic women, who may not be as likely to have adverse pregnancy outcomes, can often be counted in the unaffected population, biasing study results toward increased risks of SARS-CoV-2. Although the findings stratified by implementation of universal testing (which captures a greater fraction of asymptomatic patients at admission for delivery), do not suggest risk mitigation with asymptomatic status, this analysis did not capture asymptomatic infections earlier in pregnancy, many of which might not be associated with perinatal risk.
Another challenge with such a dataset is that one cannot determine the severity of illness of each patient without manual review of each chart; however, other data that are easily abstracted from the EHR may serve as a proxy. For instance, of the 307 women with symptomatic COVID-19, 4 required respiratory support above nasal cannula. This suggests a low rate of severely ill women, and may explain some of the findings in the study, such as no differences in the rate of CD, hypertensive disorders of pregnancy, or stillbirth, but does not explain the increased risk of both medically indicated and spontaneous preterm birth, or the rates of acute respiratory distress syndrome and sepsis that drive the increased risk of severe maternal morbidity.
The CDC has published data on the risks of stillbirth from a large hospital-based administrative database for COVID-19 from Premier Healthcare.2 In a cohort of over 1.2 million women admitted for delivery, including the timeframe of Ferrara et al’s study, COVID-19 was associated with a 2-fold increased risk of stillbirth, with higher risks noted with the delta variant. A rare outcome, stillbirth occurs in 6/1,000 births,7 which was the rate seen in Ferrara’s publication for both women with and without SARS-CoV-2 infection. The rare nature of the outcome may explain why a signal was not noted in the article of interest.
Translating data to patient counseling
Ferrara and colleagues’ study clearly confirms that COVID-19 infection has risks. Although many women with a COVID-19 infection in pregnancy may have an uncomplicated course, a favorable outcome is hard to predict with certainty. Risks of prematurity, VTE, organ dysfunction, and stillbirth from COVID-19 are rare but devastating complications. However, vaccinated women tend to incur far fewer adverse outcomes of COVID-19 in pregnancy, namely a 90% risk reduction in severe or critical COVID-19, with lower rates of ICU admissions and stillbirths.8 While these data strongly favor vaccination, we remain ill-advised on management strategies specifically to mitigate risk for the pregnancy once affected by COVID-19 infection. Thus, prevention with vaccination, mask wearing, and physical distancing remains a cornerstone of prenatal care in the current day. ●
These data continue to support that SARS-CoV-2 infection is associated with prematurity, VTE, and severe maternal adverse outcomes. As sports fanatics often state, the best defense is a good offense. In the case of SARS-CoV-2, COVID-19 vaccination, mask wearing, and physical distancing are likely the best offense against COVID-19 infection in pregnancy.
- Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3.
- DeSisto CL. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70. doi:10.15585/mmwr.mm7047e1.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and metaanalysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320.
- Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181:714-717. doi:10.1001/jamainternmed.2020.9241.
- Katz D, Bateman BT, Kjaer K, et al. The Society for Obstetric Anesthesia and Perinatology Coronavirus Disease 2019 Registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome Coronavirus-2 outbreak in the United States. Anesth Analg. 2021;133:462-473. doi:10.1213/ANE.0000000000005592.
- Metz TD, Clifton RG, Hughes BL, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748759. doi:10.1001/jama.2022.1190.
- Management of stillbirth. https ://www.acog.org/en/clinical/clinical-guidance/obstetric-care-consensus/articles/2020/03/management-of-stillbirth. Accessed May 23, 2022.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109. doi:10.1097/AOG.0000000000004621.
- Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3.
- DeSisto CL. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70. doi:10.15585/mmwr.mm7047e1.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and metaanalysis. BMJ. 2020;370:m3320. doi:10.1136/bmj.m3320.
- Jering KS, Claggett BL, Cunningham JW, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med. 2021;181:714-717. doi:10.1001/jamainternmed.2020.9241.
- Katz D, Bateman BT, Kjaer K, et al. The Society for Obstetric Anesthesia and Perinatology Coronavirus Disease 2019 Registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome Coronavirus-2 outbreak in the United States. Anesth Analg. 2021;133:462-473. doi:10.1213/ANE.0000000000005592.
- Metz TD, Clifton RG, Hughes BL, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748759. doi:10.1001/jama.2022.1190.
- Management of stillbirth. https ://www.acog.org/en/clinical/clinical-guidance/obstetric-care-consensus/articles/2020/03/management-of-stillbirth. Accessed May 23, 2022.
- Morgan JA, Biggio JRJ, Martin JK, et al. Maternal outcomes after severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection in vaccinated compared with unvaccinated pregnant patients. Obstet Gynecol. 2022;139:107-109. doi:10.1097/AOG.0000000000004621.
Defending access to reproductive health care
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
The 1973 Supreme Court of the United States (SCOTUS) decision in Roe v Wade was a landmark ruling,1 establishing that the United States Constitution provides a fundamental “right to privacy,” protecting pregnant people’s freedom to access all available reproductive health care options. Recognizing that the right to abortion was not absolute, the majority of justices supported a trimester system. In the first trimester, decisions about abortion care are fully controlled by patients and clinicians, and no government could place restrictions on access to abortion. In the second trimester, SCOTUS ruled that states may choose to regulate abortion to protect maternal health. (As an example of such state restrictions, in Massachusetts, for many years, but no longer, the state required that abortions occur in a hospital when the patient was between 18 and 24 weeks’ gestation in order to facilitate comprehensive emergency care for complications.) Beginning in the third trimester, a point at which a fetus could be viable, the Court ruled that a government could prohibit abortion except when an abortion was necessary to protect the life or health of the pregnant person. In 1992, the SCOTUS decision in Planned Parenthood v Casey2 rejected the trimester system, reaffirming the right to an abortion before fetal viability, and adopting a new standard that states may not create an undue burden on a person seeking an abortion b
If, as anticipated, the 2022 SCOTUS decision in Dobbs v Jackson Women’s Health Organization3 overturns the precedents set in Roe v Wade and Planned Parenthood v Casey, decisions on abortion law will be relegated to elected legislators and state courts.4 It is expected that at least 26 state legislatures and governors will enact stringent new restrictions on access to abortion. This cataclysmic reversal of judicial opinion creates a historic challenge to obstetrician-gynecologists and their patients and could threaten access to other vital reproductive services beyond abortion, like contraception. We will be fighting, state by state, for people’s right to access all available reproductive health procedures. This will also significantly affect the ability for providers in women’s reproductive health to obtain appropriate and necessary education and training in a critical skills. If access to safe abortion is restricted, we fear patients may be forced to consider unsafe abortion, raising the specter of a return to the 1960s, when an epidemic of unsafe abortion caused countless injuries and deaths.5,6
How do we best prepare for these challenges?
- We will need to be flexible and continually evolve our clinical practices to be adherent with state and local legislation and regulation.
- To reduce unintended pregnancies, we need to strengthen our efforts to ensure that every patient has ready access to all available contraceptive options with no out-of-pocket cost.
- When a contraceptive is desired, we will focus on educating people about effectiveness, and offering them highly reliable contraception, such as the implant or intrauterine devices.
- We need to ensure timely access to abortion if state-based laws permit abortion before 6 or 7 weeks’ gestation. Providing medication abortion without an in-person visit using a telehealth option would be one option to expand rapid access to early first trimester abortion.
- Clinicians in states with access to abortion services will need to collaborate with colleagues in states with restrictions on abortion services to improve patient access across state borders.
On a national level, advancing our effective advocacy in Congress may lead to national legislation passed and signed by the President. This could supersede most state laws prohibiting access to comprehensive women’s reproductive health and create a unified, national approach to abortion care, allowing for the appropriate training of all obstetrician-gynecologists. We will also need to develop teams in every state capable of advocating for laws that ensure access to all reproductive health care options. The American College of Obstetricians and Gynecologists has leaders trained and tasked with legislative advocacy in every state.7 This network will be a foundation upon which to build additional advocacy efforts.
As women’s health care professionals, our responsibility to our patients, is to work to ensure universal access to safe and effective comprehensive reproductive options, and to ensure that our workforce is prepared to meet the needs of our patients by defending the patient-clinician relationship. Abortion care saves lives of pregnant patients and reduces maternal morbidity.8 Access to safe abortion care as part of comprehensive reproductive services is an important component of health care. ●
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.
- Roe v Wade, 410 U.S. 113 (1973).
- Planned Parenthood v Casey, 505 U.S. 833 (1992).
- Dobbs v Jackson Women’s Health Organization, 19-1392. https://www.supremecourt.gov/search .aspx?filename=/docket/docketfiles/html /public/19-1392.html. Accessed May 18, 2022.
- Gerstein J, Ward A. Supreme Court has voted to overturn abortion rights, draft opinion shows. Politico. May 5, 2022. Updated May 3, 2022.
- Gold RB. Lessons from before Roe: will past be prologue? Guttmacher Institute. March 1, 2003. https://www.guttmacher.org/gpr/2003/03 /lessons-roe-will-past-be-prologue. Accessed May 18, 2022.
- Edelin KC. Broken Justice: A True Story of Race, Sex and Revenge in a Boston Courtroom. Pond View Press; 2007.
- The American College of Obstetricians and Gynecologists. Get involved in your state. ACOG web site. https://www.acog.org/advocacy /get-involved/get-involved-in-your-state. Accessed May 18, 2022.
- Institute of Medicine (US) Committee on Improving Birth Outcomes. Bale JR, Stoll BJ, Lucas AO, eds. Reducing maternal mortality and morbidity. In: Improving Birth Outcomes: Meeting the Challenge in the Developing World. Washington, DC: National Academies Press (US); 2003.