User login
Are single-incision mini-slings the new gold standard for stress urinary incontinence?
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Monkeypox: Another emerging threat?
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
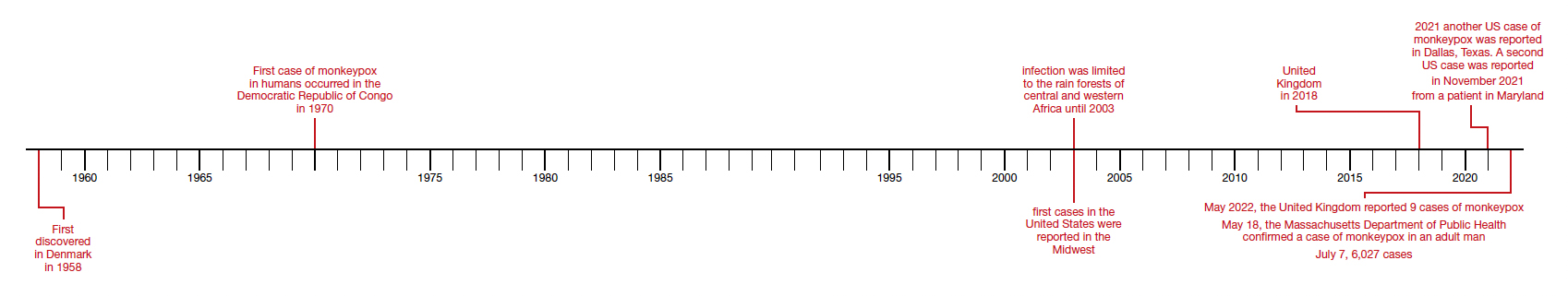
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16
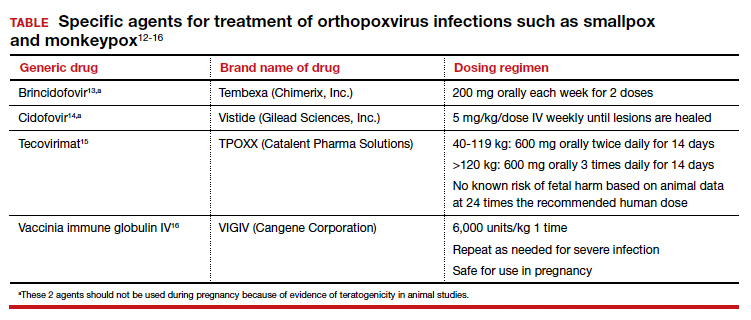
Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
Antibiotic-resistant bacteria emerging in community settings
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from the Centers for Disease Control and Prevention found that
Traditionally, CRE has been thought of as a nosocomial infection, acquired in a hospital or other health care facility (nursing home, long-term acute care hospital, dialysis center, etc.). This is the first population-level study to show otherwise, with fully 10% of the CRE isolates found to be community acquired.
CREs are a group of multidrug-resistant bacteria considered an urgent health threat by the CDC because they can rapidly spread between patients, especially those who are most seriously ill and vulnerable, and because they are so difficult to treat. These patients often require treatment with toxic antibiotics, such as colistin, and carry a high mortality rate – up to 50% in some studies.
Overall, 30% of CREs carry a carbapenemase – an enzyme that can make them resistant to carbapenem antibiotics. The genes for this are readily transferable between bacteria and help account for their spread in hospitals.
But in this study, published in the American Journal of Infection Control, of the 12 isolates that underwent whole-genome sequencing, 42% of the CA-CRE isolates carried the carbapenemase gene. Lead author Sandra Bulens, MPH, a health scientist in the CDC’s division of health care quality promotion, said in an interview, “The findings highlight the potential for CP-CRE to move from health care settings into the community. The fact that 5 of the 12 isolates harbored a carbapenemase gene introduces new challenges for controlling spread of CP-CRE.”
CDC researchers analyzed data from eight U.S. metropolitan areas between 2012 and 2015 as part of the CDC’s Emerging Infections Program (EIP) health care–associated infections – community interface activity, which conducts surveillance for CRE and other drug-resistant gram-negative bacteria. Cases of CA-CRE were compared with HCA-CRE, with 1499 cases in 1,194 case-patients being analyzed. Though Klebsiella pneumoniae was the most common isolate, there were some differences between metropolitan areas.
The incidence of CRE cases per 100,000 population was 2.96 (95% confidence interval, 2.81-3.11) overall and 0.29 (95% CI, 0.25-0.25) for CA-CRE. Most CA-CRE cases were in White persons (73%) and women (84%). Urine cultures were the source of 98% of all CA-CRE cases, compared with 86% of HCA-CRE cases (P < .001). Though small numbers, the numbers of patients with CA-CRE without apparent underlying medical condition (n = 51; 37%) was greater when compared with patients with HCA-CRE (n = 36; 3%; P < .001).
Asked for independent comment, Lance Price, PhD, of George Washington University and the founding director of GW’s Antibiotic Resistance Action Center, Washington, said, “what’s striking about these data is that: ‘Who is the front line, at least in the United States for CRE?’ It’s women, older women. ... At some point, we have to frame drug resistance as a women’s health issue.”
Dr. Price noted that the 10% of patients with CA-CRE acquired it in the community. “I would argue that probably none of them had any idea, because there’s this silent community epidemic,” he said. “It’s asymptomatic carriage and transmission in the community. Somebody can be this walking reservoir of these really dangerous bacteria and have no idea.”
This is an increasingly serious problem for women, Dr. Price said, because, “with a community-acquired bladder infection, you’re going to call your doctor or go to an urgent care, and they’re not going to test you. They’re going to guess what you have, and they’re going to prescribe an antibiotic, and that antibiotic is going to fail. So then your bladder infection continues, and then you wait a few more days, and you start to get flank pain and kidney infection. ... If you start getting a fever, they might admit you. They are going to start treating you immediately, and they might miss it because you’ve got this organism that’s resistant to all the best antibiotics. ... The gateway to the blood is the UTI.”
Because of such empiric treatment and increasing resistance, the risk for treatment failure is quite high, especially for older women. Ms. Bulens, however, said that, “[although] 10% of CRE were in persons without health care risk factors, the proportion of all UTIs in this population that are CRE is going to be very, very small.”
This study involved cultures from 2012 to 2015. Before the pandemic, from 2012 to 2017, U.S. deaths from antibiotic resistance fell by 18% overall and by 30% in hospitals.
But in the first year of the COVID-19 pandemic, there was a 15% increase in infections and deaths from antibiotic-resistant (AMR), hospital-acquired bacteria. In 2020, 29,400 patients died from AMR infections. There was a 78% increase in carbapenem-resistant Acinetobacter baumannii health care–associated infections, a 35% increase in carbapenem-resistant Enterobacterales, and 32% increases in both multidrug-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase–producing Enterobacterales. Aside from gram-negative bacteria, methicillin-resistant Staphylococcus aureus rose 13%, and Candida auris rose 60%. But owing to limited surveillance, recent sound figures are lacking.
Ms. Bulens and Dr. Price reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
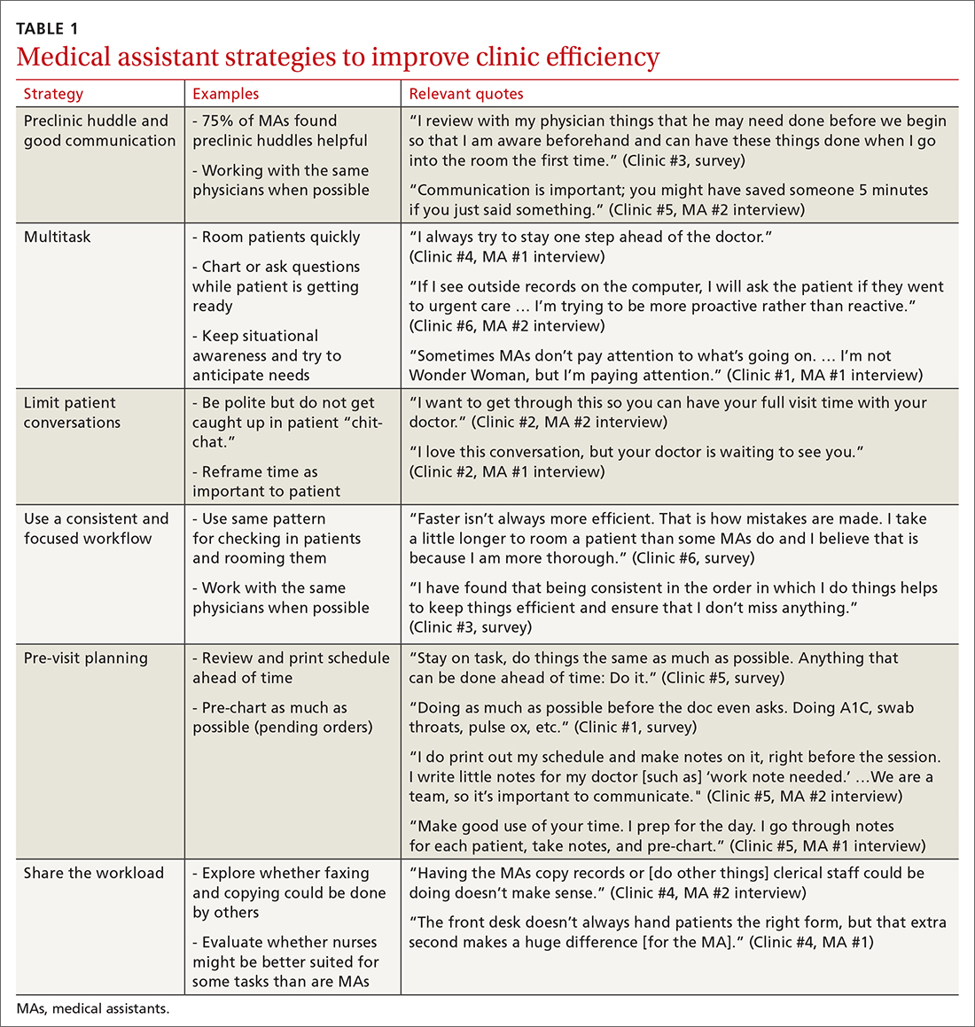
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
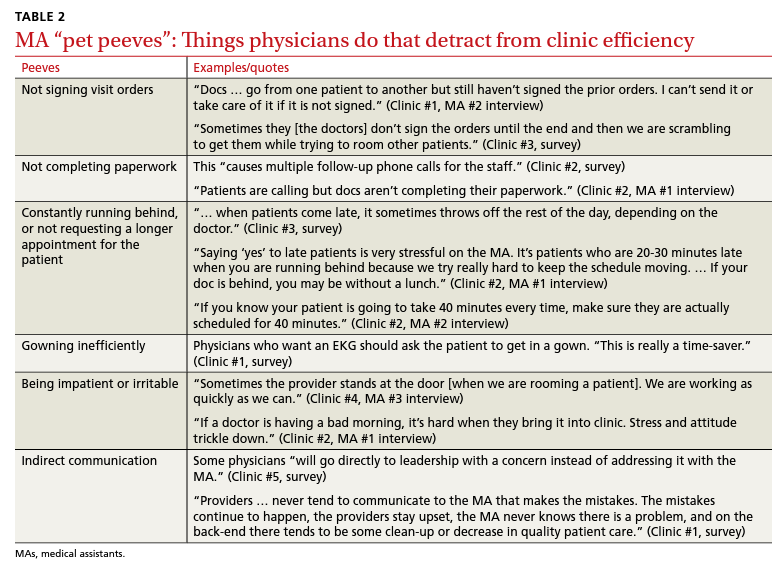
Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; ktgold@umich.edu
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; ktgold@umich.edu
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; ktgold@umich.edu
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Nurses’ cohort study: Endometriosis elevates stroke risk
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
FROM STROKE
PCOS in mothers tied to health problems in children
Children whose mothers have polycystic ovary syndrome (PCOS) have increased rates of hospitalization for various conditions, including asthma, pneumonia, and ear infection, a study of more than 1 million children shows.
The associations were not particularly strong, according to the researchers. But they raise questions about the reasons for the increased risk and whether interventions such as diet, exercise, or medications could lead to healthier outcomes for children whose mothers have PCOS.
“The findings suggest that maternal PCOS may have a negative impact on offspring development, enough to lead to a measurable increase in the risk of childhood hospitalization,” study coauthor Nathalie Auger, MD, associate professor of epidemiology at University of Montreal, and colleagues reported in Human Reproduction.
“They are minor differences, just enough that we can statistically identify them. They’re not something where everyone should be worrying at this point,” Dr. Auger told this news organization.
Still, some of the hospitalizations, such as those related to infection or allergy, could be prevented with earlier ambulatory care, so some degree of greater awareness among parents and clinicians may be warranted, she said.
Thirteen years of follow-up
PCOS – a reproductive disorder characterized by irregular periods, increased male hormones, and metabolic complications – affects some 10% of women. People with the condition are at increased risk for obesity, type 2 diabetes, and cardiovascular disease.
Although prior research has shown that maternal PCOS may be associated with higher body mass index and attention deficit disorder in children, data on long-term childhood health outcomes have been limited, Dr. Auger’s group noted.
To examine illness in children exposed to maternal PCOS, the investigators analyzed hospitalization rates for nearly 1.04 million children in Quebec between 2006 and 2020; 7,160 of the children had mothers with PCOS.
In all, 275,354 children were hospitalized during 13 years of follow-up, including 2,314 whose mothers had PCOS.
Children exposed to PCOS were hospitalized at a rate of 68.9 per 1,000 person-years – roughly 50% more often than the rate of 45.3 per 1,000 person-years for children not exposed to maternal PCOS.
In an analysis that adjusted for maternal characteristics, childhood hospitalization for any reason was 1.32 times more likely for children exposed to maternal PCOS.
Hospitalizations linked to infectious diseases – such as for bronchitis, bronchiolitis, pneumonia, nephritis, otitis media, or meningitis – were 1.31 times more likely among children exposed to PCOS. Allergy-related hospitalizations, such as for allergic asthma and anaphylaxis, were 1.47 times more likely, according to the researchers.
Metabolic hospitalizations were 1.59 times more likely. For gastrointestinal hospitalizations, the hazard ratio was 1.72. For central nervous system hospitalizations, it was 1.74.
The associations were stronger in earlier childhood, and results were similar for boys and girls, the investigators reported.
Hospitalizations for cardiovascular disease, musculoskeletal conditions, or malignancy were not increased.
‘Surprising’ links
“The findings are surprising in that some of the conditions that they showed increased risk for, like asthma and some infections, are not conditions that we think of as being typically associated with PCOS,” said Andrea E. Dunaif, MD, chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease at Mount Sinai Health System, New York, who was not part of the study team.
Earlier studies of offspring of women with PCOS have suggested that children may be at increased risk for insulin resistance and obesity.
Differences in genetics, intrauterine environments, patterns of health care use by women with PCOS, and behavioral factors, such as diet and how children are raised, are variables that could have contributed to the different hospitalization rates among children exposed to maternal PCOS, Dr. Auger said.
“Everything is interconnected,” she said.
The study was supported by a grant from the Canadian Institutes of Health Research. Dr. Auger has received a career award from Fonds de Recherche du Québec-Santé. Dr. Dunaif has consulted for Novo Nordisk and Fractyl Laboratories (now Fractyl Health).
A version of this article first appeared on Medscape.com.
Children whose mothers have polycystic ovary syndrome (PCOS) have increased rates of hospitalization for various conditions, including asthma, pneumonia, and ear infection, a study of more than 1 million children shows.
The associations were not particularly strong, according to the researchers. But they raise questions about the reasons for the increased risk and whether interventions such as diet, exercise, or medications could lead to healthier outcomes for children whose mothers have PCOS.
“The findings suggest that maternal PCOS may have a negative impact on offspring development, enough to lead to a measurable increase in the risk of childhood hospitalization,” study coauthor Nathalie Auger, MD, associate professor of epidemiology at University of Montreal, and colleagues reported in Human Reproduction.
“They are minor differences, just enough that we can statistically identify them. They’re not something where everyone should be worrying at this point,” Dr. Auger told this news organization.
Still, some of the hospitalizations, such as those related to infection or allergy, could be prevented with earlier ambulatory care, so some degree of greater awareness among parents and clinicians may be warranted, she said.
Thirteen years of follow-up
PCOS – a reproductive disorder characterized by irregular periods, increased male hormones, and metabolic complications – affects some 10% of women. People with the condition are at increased risk for obesity, type 2 diabetes, and cardiovascular disease.
Although prior research has shown that maternal PCOS may be associated with higher body mass index and attention deficit disorder in children, data on long-term childhood health outcomes have been limited, Dr. Auger’s group noted.
To examine illness in children exposed to maternal PCOS, the investigators analyzed hospitalization rates for nearly 1.04 million children in Quebec between 2006 and 2020; 7,160 of the children had mothers with PCOS.
In all, 275,354 children were hospitalized during 13 years of follow-up, including 2,314 whose mothers had PCOS.
Children exposed to PCOS were hospitalized at a rate of 68.9 per 1,000 person-years – roughly 50% more often than the rate of 45.3 per 1,000 person-years for children not exposed to maternal PCOS.
In an analysis that adjusted for maternal characteristics, childhood hospitalization for any reason was 1.32 times more likely for children exposed to maternal PCOS.
Hospitalizations linked to infectious diseases – such as for bronchitis, bronchiolitis, pneumonia, nephritis, otitis media, or meningitis – were 1.31 times more likely among children exposed to PCOS. Allergy-related hospitalizations, such as for allergic asthma and anaphylaxis, were 1.47 times more likely, according to the researchers.
Metabolic hospitalizations were 1.59 times more likely. For gastrointestinal hospitalizations, the hazard ratio was 1.72. For central nervous system hospitalizations, it was 1.74.
The associations were stronger in earlier childhood, and results were similar for boys and girls, the investigators reported.
Hospitalizations for cardiovascular disease, musculoskeletal conditions, or malignancy were not increased.
‘Surprising’ links
“The findings are surprising in that some of the conditions that they showed increased risk for, like asthma and some infections, are not conditions that we think of as being typically associated with PCOS,” said Andrea E. Dunaif, MD, chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease at Mount Sinai Health System, New York, who was not part of the study team.
Earlier studies of offspring of women with PCOS have suggested that children may be at increased risk for insulin resistance and obesity.
Differences in genetics, intrauterine environments, patterns of health care use by women with PCOS, and behavioral factors, such as diet and how children are raised, are variables that could have contributed to the different hospitalization rates among children exposed to maternal PCOS, Dr. Auger said.
“Everything is interconnected,” she said.
The study was supported by a grant from the Canadian Institutes of Health Research. Dr. Auger has received a career award from Fonds de Recherche du Québec-Santé. Dr. Dunaif has consulted for Novo Nordisk and Fractyl Laboratories (now Fractyl Health).
A version of this article first appeared on Medscape.com.
Children whose mothers have polycystic ovary syndrome (PCOS) have increased rates of hospitalization for various conditions, including asthma, pneumonia, and ear infection, a study of more than 1 million children shows.
The associations were not particularly strong, according to the researchers. But they raise questions about the reasons for the increased risk and whether interventions such as diet, exercise, or medications could lead to healthier outcomes for children whose mothers have PCOS.
“The findings suggest that maternal PCOS may have a negative impact on offspring development, enough to lead to a measurable increase in the risk of childhood hospitalization,” study coauthor Nathalie Auger, MD, associate professor of epidemiology at University of Montreal, and colleagues reported in Human Reproduction.
“They are minor differences, just enough that we can statistically identify them. They’re not something where everyone should be worrying at this point,” Dr. Auger told this news organization.
Still, some of the hospitalizations, such as those related to infection or allergy, could be prevented with earlier ambulatory care, so some degree of greater awareness among parents and clinicians may be warranted, she said.
Thirteen years of follow-up
PCOS – a reproductive disorder characterized by irregular periods, increased male hormones, and metabolic complications – affects some 10% of women. People with the condition are at increased risk for obesity, type 2 diabetes, and cardiovascular disease.
Although prior research has shown that maternal PCOS may be associated with higher body mass index and attention deficit disorder in children, data on long-term childhood health outcomes have been limited, Dr. Auger’s group noted.
To examine illness in children exposed to maternal PCOS, the investigators analyzed hospitalization rates for nearly 1.04 million children in Quebec between 2006 and 2020; 7,160 of the children had mothers with PCOS.
In all, 275,354 children were hospitalized during 13 years of follow-up, including 2,314 whose mothers had PCOS.
Children exposed to PCOS were hospitalized at a rate of 68.9 per 1,000 person-years – roughly 50% more often than the rate of 45.3 per 1,000 person-years for children not exposed to maternal PCOS.
In an analysis that adjusted for maternal characteristics, childhood hospitalization for any reason was 1.32 times more likely for children exposed to maternal PCOS.
Hospitalizations linked to infectious diseases – such as for bronchitis, bronchiolitis, pneumonia, nephritis, otitis media, or meningitis – were 1.31 times more likely among children exposed to PCOS. Allergy-related hospitalizations, such as for allergic asthma and anaphylaxis, were 1.47 times more likely, according to the researchers.
Metabolic hospitalizations were 1.59 times more likely. For gastrointestinal hospitalizations, the hazard ratio was 1.72. For central nervous system hospitalizations, it was 1.74.
The associations were stronger in earlier childhood, and results were similar for boys and girls, the investigators reported.
Hospitalizations for cardiovascular disease, musculoskeletal conditions, or malignancy were not increased.
‘Surprising’ links
“The findings are surprising in that some of the conditions that they showed increased risk for, like asthma and some infections, are not conditions that we think of as being typically associated with PCOS,” said Andrea E. Dunaif, MD, chief of the Hilda and J. Lester Gabrilove Division of Endocrinology, Diabetes, and Bone Disease at Mount Sinai Health System, New York, who was not part of the study team.
Earlier studies of offspring of women with PCOS have suggested that children may be at increased risk for insulin resistance and obesity.
Differences in genetics, intrauterine environments, patterns of health care use by women with PCOS, and behavioral factors, such as diet and how children are raised, are variables that could have contributed to the different hospitalization rates among children exposed to maternal PCOS, Dr. Auger said.
“Everything is interconnected,” she said.
The study was supported by a grant from the Canadian Institutes of Health Research. Dr. Auger has received a career award from Fonds de Recherche du Québec-Santé. Dr. Dunaif has consulted for Novo Nordisk and Fractyl Laboratories (now Fractyl Health).
A version of this article first appeared on Medscape.com.
FROM HUMAN REPRODUCTION
Some have heavier periods after COVID vaccine
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
Many women who got a COVID-19 vaccine have reported heavier bleeding during their periods since they had the shots.
A team of researchers investigated the trend and set out to find out who among the vaccinated were more likely to experience the menstruation changes.
The researchers were led by Katharine M.N. Lee, PhD, MS, of the division of public health sciences at Washington University in St. Louis. Their findings were published ahead of print in Science Advances.
The investigators analyzed more than 139,000 responses from an online survey from both currently and formerly menstruating women.
They found that, among people who have regular periods, about the same percentage had heavier bleeding after they got a COVID vaccine as had no change in bleeding after the vaccine (44% vs. 42%, respectively).
“A much smaller portion had lighter periods,” they write.
The phenomenon has been difficult to study because questions about changes in menstruation are not a standard part of vaccine trials.
Date of last period is often tracked in clinical trials to make sure a participant is not pregnant, but the questions about periods often stop there.
Additionally, periods are different for everyone and can be influenced by all sorts of environmental factors, so making associations regarding exposures is problematic.
No changes found to fertility
The authors emphasized that, generally, changes to menstrual bleeding are not uncommon nor dangerous. They also emphasized that the changes in bleeding don’t mean changes to fertility.
The uterine reproductive system is flexible when the body is under stress, they note.
“We know that running a marathon may influence hormone concentrations in the short term while not rendering that person infertile,” the authors write.
However, they acknowledge that investigating these reports is critical in building trust in medicine.
This report includes information that hasn’t been available through the clinical trial follow-up process.
For instance, the authors write, “To the best of our knowledge, our work is the first to examine breakthrough bleeding after vaccination in either pre- or postmenopausal people.”
Reports of changes to periods after vaccination started emerging in 2021. But without data, reports were largely dismissed, fueling criticism from those waging campaigns against COVID vaccines.
Dr. Lee and colleagues gathered data from those who responded to the online survey and detailed some trends.
People who were bleeding more heavily after vaccination were more likely to be older, Hispanic, had vaccine side effects of fever and fatigue, had been pregnant at some point, or had given birth.
People with regular periods who had endometriosis, prolonged bleeding during their periods, polycystic ovarian syndrome (PCOS) or fibroids were also more likely to have increased bleeding after a COVID vaccine.
Breakthrough bleeding
For people who don’t menstruate, but have not reached menopause, breakthrough bleeding happened more often in women who had been pregnant and/or had given birth.
Among respondents who were postmenopausal, breakthrough bleeding happened more often in younger people and/or those who are Hispanic.
More than a third of the respondents (39%) who use gender-affirming hormones that eliminate menstruation reported breakthrough bleeding after vaccination.
The majority of premenopausal people on long-acting, reversible contraception (71%) and the majority of postmenopausal respondents (66%) had breakthrough bleeding as well.
The authors note that you can’t compare the percentages who report these experiences in the survey with the incidence of those who would experience changes in menstrual bleeding in the general population.
The nature of the online survey means it may be naturally biased because the people who responded may be more often those who noted some change in their own menstrual experiences, particularly if that involved discomfort, pain, or fear.
Researchers also acknowledge that Black, Indigenous, Latinx, and other respondents of color are underrepresented in this research and that represents a limitation in the work.
Alison Edelman, MD, MPH, with the department of obstetrics and gynecology at Oregon Health & Science University in Portland, was not involved with Dr. Lee and associates’ study but has also studied the relationship between COVID vaccines and menstruation.
Her team’s study found that COVID vaccination is associated with a small change in time between periods but not length of periods.
She said about the work by Dr. Lee and colleagues, “This work really elevates the voices of the public and what they’re experiencing.”
The association makes sense, Dr. Edelman says, in that the reproductive system and the immune system talk to each other and inflammation in the immune system is going to be noticed by the system governing periods.
Lack of data on the relationship between exposures and menstruation didn’t start with COVID. “There has been a signal in the population before with other vaccines that’s been dismissed,” she said.
Tracking menstruation information in clinical trials can help physicians counsel women on what may be coming with any vaccine and alleviate fears and vaccine hesitancy, Dr. Edelman explained. It can also help vaccine developers know what to include in information about their product.
“When you are counseled about what to expect, it’s not as scary. That provides trust in the system,” she said. She likened it to original lack of data on whether COVID-19 vaccines would affect pregnancy.
“We have great science now that COVID vaccine does not affect fertility and [vaccine] does not impact pregnancy.”
Another important aspect of this paper is that it included subgroups not studied before regarding menstruation and breakthrough bleeding, such as those taking gender-affirming hormones, she added.
Menstruation has been often overlooked as important in clinical trial exposures but Dr. Edelman hopes this recent attention and question will escalate and prompt more research.
“I’m hoping with the immense outpouring from the public about how important this is, that future studies will look at this a little bit better,” she says.
She said when the National Institutes of Health opened up funding for trials on COVID-19 vaccines and menstruation, researchers got flooded with requests from women to share their stories.
“As a researcher – I’ve been doing research for over 20 years – that’s not something that usually happens. I would love to have that happen for every research project.”
The authors and Dr. Edelman declare that they have no competing interests. This research was supported in part by the University of Illinois Beckman Institute for Advanced Science and Technology, the University of Illinois Interdisciplinary Health Sciences Institute, the National Institutes of Health, the Foundation for Barnes-Jewish Hospital, and the Siteman Cancer Center.
FROM SCIENCE ADVANCES
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
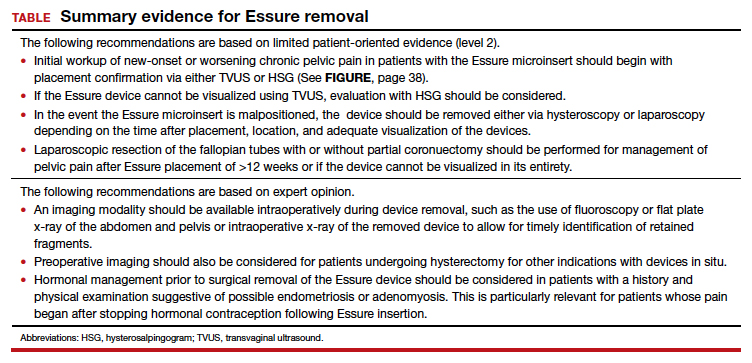
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
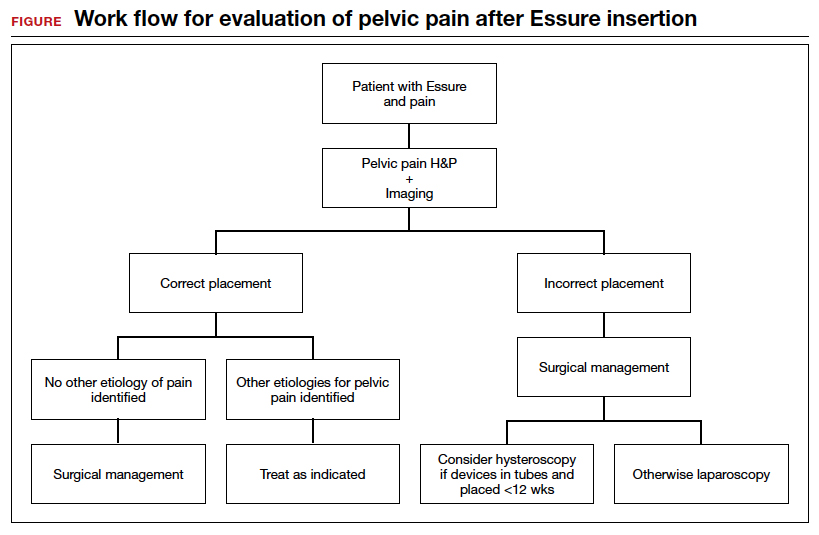
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
Misoprostol: Clinical pharmacology in obstetrics and gynecology
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.
Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Pregnancy, breastfeeding, and more linked to lower CRC risk
Estrogen exposure helps protect against colorectal cancer (CRC), and in some instances, the protection is site specific, a new analysis finds.
In a 17-year study involving almost 5,000 women, researchers from Germany found that hormone replacement therapy, oral contraceptive use, pregnancy, breastfeeding, and menopause at age 50 or older were all significantly associated with reductions in CRC risk.
Interestingly, the reduced risk of CRC observed for pregnancy and breastfeeding only applied to proximal colon cancer, while the association with oral contraceptive use was confined to the distal colon and rectum.
The results were published online in JNCI Cancer Spectrum.
CRC is the second most common cause of cancer death. It is responsible for more than one million deaths globally, according to the latest figures from the Global Burden of Disease 2019 Cancer Collaboration.
And sex seems to make a difference. The Global Burden analysis, echoing previous data, found that CRC is less common among women and that fewer women die from the disease.
Little, however, is known about the mechanisms of estrogen signaling in CRC or the impact of reproductive factors on CRC, despite a large amount of literature linking CRC risk to exogenous estrogens, such as hormone replacement therapy and oral contraceptives.
In the current analysis, the team recruited 2,650 patients with CRC from 20 German cancer centers between 2003 and 2020. Researchers used standardized questionnaires to garner the women’s reproductive histories.
A matched control group of 2,175 participants who did not have a history of CRC was randomly selected from population registries. All analyses were adjusted for known CRC risk factors, such as age; body mass index; education level; family history; having previously undergone large-bowel endoscopy; diabetes; and smoking status.
The researchers found that each pregnancy was associated with a small but significant 9% reduction in CRC risk (odds ratio, 0.91), specifically in the proximal colon (OR, 0.86).
Overall, breastfeeding for a year or longer was associated with a significantly lower CRC risk, compared with never breastfeeding (OR, 0.74), but the results were only significant for the proximal colon (OR, 0.58).
Oral contraceptive use for 9 years or longer was associated with a lower CRC risk (OR, 0.75) but was only significant for the distal colon (OR, 0.63). Hormone replacement therapy was associated with a lower risk of CRC irrespective of tumor location (OR, 0.76). And using both was linked to a 42% CRC risk reduction (OR, 0.58).
Although age at menarche was not associated with CRC risk, menopause at age 50 or older was associated with a significant 17% lower risk of CRC.
In an email interview, lead author Tobias Niedermaier, PhD, expressed surprise at two of the findings. The first was the small association between pregnancies and CRC risk, “despite the strong increase in estrogen levels during pregnancy,” he said. He speculated that pregnancy-related increases in insulin levels may have “largely offset the protection effects of estrogen exposure during pregnancy.”
The second surprise was that the age at menarche did not have a bearing on CRC risk, which could be because “exposure to estrogen levels in younger ages [is] less relevant with respect to CRC risk, because CRC typically develops at comparably old age.”
John Marshall, MD, who was not involved in the research, commented that such studies “put a lot of pressure on people to perform in a certain way to modify their personal risk of something.” However, “we would not recommend people alter their life choices for reproduction for this,” said Dr. Marshall, chief of the Division of Hematology/Oncology at Georgetown University, Washington, D.C.
Dr. Niedermaier agreed that “while this knowledge will certainly not change a woman’s decision on family planning,” he noted that the findings “could influence current CRC screening strategies, for example, by risk-adapted screening intervals [and] start and stop ages of screening.”
Dr. Niedermaier and colleagues’ work was funded by the German Research Council, the German Federal Ministry of Education and Research, and the Interdisciplinary Research Program of the National Center for Tumor Diseases. Dr. Niedermaier has disclosed no relevant financial relationships. Dr. Marshall writes a column that appears regularly on Medscape: Marshall on Oncology. He has served as speaker or member of a speakers’ bureau for Genentech, Amgen, Bayer, Celgene Corporation, and Caris Life Sciences.
A version of this article first appeared on Medscape.com.
Estrogen exposure helps protect against colorectal cancer (CRC), and in some instances, the protection is site specific, a new analysis finds.
In a 17-year study involving almost 5,000 women, researchers from Germany found that hormone replacement therapy, oral contraceptive use, pregnancy, breastfeeding, and menopause at age 50 or older were all significantly associated with reductions in CRC risk.
Interestingly, the reduced risk of CRC observed for pregnancy and breastfeeding only applied to proximal colon cancer, while the association with oral contraceptive use was confined to the distal colon and rectum.
The results were published online in JNCI Cancer Spectrum.
CRC is the second most common cause of cancer death. It is responsible for more than one million deaths globally, according to the latest figures from the Global Burden of Disease 2019 Cancer Collaboration.
And sex seems to make a difference. The Global Burden analysis, echoing previous data, found that CRC is less common among women and that fewer women die from the disease.
Little, however, is known about the mechanisms of estrogen signaling in CRC or the impact of reproductive factors on CRC, despite a large amount of literature linking CRC risk to exogenous estrogens, such as hormone replacement therapy and oral contraceptives.
In the current analysis, the team recruited 2,650 patients with CRC from 20 German cancer centers between 2003 and 2020. Researchers used standardized questionnaires to garner the women’s reproductive histories.
A matched control group of 2,175 participants who did not have a history of CRC was randomly selected from population registries. All analyses were adjusted for known CRC risk factors, such as age; body mass index; education level; family history; having previously undergone large-bowel endoscopy; diabetes; and smoking status.
The researchers found that each pregnancy was associated with a small but significant 9% reduction in CRC risk (odds ratio, 0.91), specifically in the proximal colon (OR, 0.86).
Overall, breastfeeding for a year or longer was associated with a significantly lower CRC risk, compared with never breastfeeding (OR, 0.74), but the results were only significant for the proximal colon (OR, 0.58).
Oral contraceptive use for 9 years or longer was associated with a lower CRC risk (OR, 0.75) but was only significant for the distal colon (OR, 0.63). Hormone replacement therapy was associated with a lower risk of CRC irrespective of tumor location (OR, 0.76). And using both was linked to a 42% CRC risk reduction (OR, 0.58).
Although age at menarche was not associated with CRC risk, menopause at age 50 or older was associated with a significant 17% lower risk of CRC.
In an email interview, lead author Tobias Niedermaier, PhD, expressed surprise at two of the findings. The first was the small association between pregnancies and CRC risk, “despite the strong increase in estrogen levels during pregnancy,” he said. He speculated that pregnancy-related increases in insulin levels may have “largely offset the protection effects of estrogen exposure during pregnancy.”
The second surprise was that the age at menarche did not have a bearing on CRC risk, which could be because “exposure to estrogen levels in younger ages [is] less relevant with respect to CRC risk, because CRC typically develops at comparably old age.”
John Marshall, MD, who was not involved in the research, commented that such studies “put a lot of pressure on people to perform in a certain way to modify their personal risk of something.” However, “we would not recommend people alter their life choices for reproduction for this,” said Dr. Marshall, chief of the Division of Hematology/Oncology at Georgetown University, Washington, D.C.
Dr. Niedermaier agreed that “while this knowledge will certainly not change a woman’s decision on family planning,” he noted that the findings “could influence current CRC screening strategies, for example, by risk-adapted screening intervals [and] start and stop ages of screening.”
Dr. Niedermaier and colleagues’ work was funded by the German Research Council, the German Federal Ministry of Education and Research, and the Interdisciplinary Research Program of the National Center for Tumor Diseases. Dr. Niedermaier has disclosed no relevant financial relationships. Dr. Marshall writes a column that appears regularly on Medscape: Marshall on Oncology. He has served as speaker or member of a speakers’ bureau for Genentech, Amgen, Bayer, Celgene Corporation, and Caris Life Sciences.
A version of this article first appeared on Medscape.com.
Estrogen exposure helps protect against colorectal cancer (CRC), and in some instances, the protection is site specific, a new analysis finds.
In a 17-year study involving almost 5,000 women, researchers from Germany found that hormone replacement therapy, oral contraceptive use, pregnancy, breastfeeding, and menopause at age 50 or older were all significantly associated with reductions in CRC risk.
Interestingly, the reduced risk of CRC observed for pregnancy and breastfeeding only applied to proximal colon cancer, while the association with oral contraceptive use was confined to the distal colon and rectum.
The results were published online in JNCI Cancer Spectrum.
CRC is the second most common cause of cancer death. It is responsible for more than one million deaths globally, according to the latest figures from the Global Burden of Disease 2019 Cancer Collaboration.
And sex seems to make a difference. The Global Burden analysis, echoing previous data, found that CRC is less common among women and that fewer women die from the disease.
Little, however, is known about the mechanisms of estrogen signaling in CRC or the impact of reproductive factors on CRC, despite a large amount of literature linking CRC risk to exogenous estrogens, such as hormone replacement therapy and oral contraceptives.
In the current analysis, the team recruited 2,650 patients with CRC from 20 German cancer centers between 2003 and 2020. Researchers used standardized questionnaires to garner the women’s reproductive histories.
A matched control group of 2,175 participants who did not have a history of CRC was randomly selected from population registries. All analyses were adjusted for known CRC risk factors, such as age; body mass index; education level; family history; having previously undergone large-bowel endoscopy; diabetes; and smoking status.
The researchers found that each pregnancy was associated with a small but significant 9% reduction in CRC risk (odds ratio, 0.91), specifically in the proximal colon (OR, 0.86).
Overall, breastfeeding for a year or longer was associated with a significantly lower CRC risk, compared with never breastfeeding (OR, 0.74), but the results were only significant for the proximal colon (OR, 0.58).
Oral contraceptive use for 9 years or longer was associated with a lower CRC risk (OR, 0.75) but was only significant for the distal colon (OR, 0.63). Hormone replacement therapy was associated with a lower risk of CRC irrespective of tumor location (OR, 0.76). And using both was linked to a 42% CRC risk reduction (OR, 0.58).
Although age at menarche was not associated with CRC risk, menopause at age 50 or older was associated with a significant 17% lower risk of CRC.
In an email interview, lead author Tobias Niedermaier, PhD, expressed surprise at two of the findings. The first was the small association between pregnancies and CRC risk, “despite the strong increase in estrogen levels during pregnancy,” he said. He speculated that pregnancy-related increases in insulin levels may have “largely offset the protection effects of estrogen exposure during pregnancy.”
The second surprise was that the age at menarche did not have a bearing on CRC risk, which could be because “exposure to estrogen levels in younger ages [is] less relevant with respect to CRC risk, because CRC typically develops at comparably old age.”
John Marshall, MD, who was not involved in the research, commented that such studies “put a lot of pressure on people to perform in a certain way to modify their personal risk of something.” However, “we would not recommend people alter their life choices for reproduction for this,” said Dr. Marshall, chief of the Division of Hematology/Oncology at Georgetown University, Washington, D.C.
Dr. Niedermaier agreed that “while this knowledge will certainly not change a woman’s decision on family planning,” he noted that the findings “could influence current CRC screening strategies, for example, by risk-adapted screening intervals [and] start and stop ages of screening.”
Dr. Niedermaier and colleagues’ work was funded by the German Research Council, the German Federal Ministry of Education and Research, and the Interdisciplinary Research Program of the National Center for Tumor Diseases. Dr. Niedermaier has disclosed no relevant financial relationships. Dr. Marshall writes a column that appears regularly on Medscape: Marshall on Oncology. He has served as speaker or member of a speakers’ bureau for Genentech, Amgen, Bayer, Celgene Corporation, and Caris Life Sciences.
A version of this article first appeared on Medscape.com.





