User login
CV events scuttle bardoxolone for diabetic kidney disease
Bardoxolone methyl may have reduced the risk of end-stage renal disease in patients with type 2 diabetes and stage 4 chronic kidney disease in a phase III clinical trial, but researchers stopped the study early after patients taking the drug had an increased rate of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke.
The BEACON trial had only an estimated 40% statistical power to determine bardoxolone methyl’s true effects, given its termination because of safety concerns, according to data reported at the annual Kidney Week meeting sponsored by the American Society of Nephrology.
The findings were simultaneously reported online Nov. 9 in the New England Journal of Medicine (doi:10.1056/NEJMoa1306033).
Bardoxolone methyl, the most potent known activator of a transcription factor that regulates antioxidant genes, was previously shown to raise the estimated glomerular filtration rate (eGFR) in patients with diabetes-related kidney disease. However, it also increased the incidence of albuminuria and induced unintended weight loss.
To determine whether longer-term treatment with bardoxolone methyl might translate that eGFR benefit into a slower progression to end-stage renal disease (ESRD), Dr. Dick de Zeeuw of the University of Groningen (the Netherlands) and his associates performed the double-blind BEACON trial (Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events).
BEACON was sponsored by Reata Pharmaceuticals and included patients from the United States, Europe, Australia, Canada, Israel, and Mexico, resulting in a diverse patient population with regard to age, race/ethnicity, and area of residence. Both diabetic retinopathy and neuropathy were common comorbidities, as was cardiovascular disease.
The 2,185 study participants had type 2 diabetes and moderate to severe chronic kidney diseases, with a baseline eGFR of 15 to less than 30 mL/min per 1.73 m2 of body surface area. They were randomly assigned to receive either once-daily bardoxolone methyl 20 mg (1,088 patients) or a matching placebo (1,097 patients), along with conventional background therapies given at the discretion of their treating physicians. Those included inhibitors of the renin-angiotensin-aldosterone system, insulin or other hypoglycemic agents, and appropriate cardiovascular medications.
The median duration of exposure was 7 months for bardoxolone methyl and 8 months for placebo, and the median follow-up for both was 9 months.
Bardoxolone significantly improved eGFR, compared with placebo, and fewer patients who took the drug progressed to ESRD. But BEACON was terminated early because of an excess of CV events among patients receiving bardoxolone methyl. That "truncated" study duration limited the trial’s statistical power.
The primary endpoint was a composite of progression to ESRD or cardiovascular death, and it occurred in 6% of both study groups. However, deaths from CV causes were significantly more frequent in the active treatment group (27 patients) than in the placebo group (19 patients), with a hazard ratio of 1.44, Dr. de Zeeuw reported.
In particular, 96 patients in the bardoxolone group had heart failure (HF) events, compared with only 55 patients in the placebo group. Moreover, significantly more of the HF events in the active treatment group were severe enough to require hospitalization or cause death.
Similarly, significantly more patients taking bardoxolone methyl had a composite outcome of nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, or CV death. And the number of deaths from any cause was greater – though not significantly – with bardoxolone methyl (44 deaths) than with placebo (31 deaths) (HR, 1.47; P = .10).
Compared with placebo, bardoxolone methyl also raised blood pressure and heart rate, increased levels of B-type natriuretic peptide, raised the rate of albuminuria, and caused substantial unintended weight loss. The investigators were unable to determine whether there was a loss of body fat, intracellular (that is, skeletal muscle) water, or extracellular (interstitial) water. There was a concomitant fall in serum albumin and hemoglobin levels, which may reflect hemodilution caused by fluid retention, the investigators found.
The increases in blood pressure and heart rate "constitute a potentially potent combination of factors that are likely to precipitate HF in an at-risk population." and the increase in B-type natriuretic peptide "is consistent with an increase in left ventricular wall stress," Dr. de Zeeuw said.
The investigators attempted to identify patient characteristics that might be associated with the development of HF in the bardoxolone methyl recipients, but were unable to do so.
Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
*This article was updated November 11, 2013.
The adverse events linked to bardoxolone methyl in this study included excess HF and cardiovascular events, as well as increased rates of high blood pressure, high heart rate, albuminuria, GI symptoms, and muscle-related symptoms, said Dr. Jonathan Himmelfarb and Dr. Katherine R. Tuttle.
"The authors speculate that fluid retention, increased afterload, and higher heart rate contributed to heart failure," but it also is possible that bardoxolone methyl may exert direct toxic effects on the heart, Dr. Himmelfarb and Dr. Tuttle said.
In any case, "caution should be exercised whenever any drug for diabetic kidney disease increases, rather than decreases, albuminuria," they noted.
Dr. Himmelfarb and Dr. Tuttle are at the Kidney Research Institute and the division of nephrology at the University of Washington, Seattle. Dr. Tuttle also is at Providence Sacred Heart Medical Center and Children’s Hospital, Spokane. Dr. Himmelfarb reported ties to Abbott Laboratories, and Dr. Tuttle reported ties to Eli Lilly. These remarks were taken from their editorial accompanying Dr. de Zeeuw’s report (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1313104]).
The adverse events linked to bardoxolone methyl in this study included excess HF and cardiovascular events, as well as increased rates of high blood pressure, high heart rate, albuminuria, GI symptoms, and muscle-related symptoms, said Dr. Jonathan Himmelfarb and Dr. Katherine R. Tuttle.
"The authors speculate that fluid retention, increased afterload, and higher heart rate contributed to heart failure," but it also is possible that bardoxolone methyl may exert direct toxic effects on the heart, Dr. Himmelfarb and Dr. Tuttle said.
In any case, "caution should be exercised whenever any drug for diabetic kidney disease increases, rather than decreases, albuminuria," they noted.
Dr. Himmelfarb and Dr. Tuttle are at the Kidney Research Institute and the division of nephrology at the University of Washington, Seattle. Dr. Tuttle also is at Providence Sacred Heart Medical Center and Children’s Hospital, Spokane. Dr. Himmelfarb reported ties to Abbott Laboratories, and Dr. Tuttle reported ties to Eli Lilly. These remarks were taken from their editorial accompanying Dr. de Zeeuw’s report (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1313104]).
The adverse events linked to bardoxolone methyl in this study included excess HF and cardiovascular events, as well as increased rates of high blood pressure, high heart rate, albuminuria, GI symptoms, and muscle-related symptoms, said Dr. Jonathan Himmelfarb and Dr. Katherine R. Tuttle.
"The authors speculate that fluid retention, increased afterload, and higher heart rate contributed to heart failure," but it also is possible that bardoxolone methyl may exert direct toxic effects on the heart, Dr. Himmelfarb and Dr. Tuttle said.
In any case, "caution should be exercised whenever any drug for diabetic kidney disease increases, rather than decreases, albuminuria," they noted.
Dr. Himmelfarb and Dr. Tuttle are at the Kidney Research Institute and the division of nephrology at the University of Washington, Seattle. Dr. Tuttle also is at Providence Sacred Heart Medical Center and Children’s Hospital, Spokane. Dr. Himmelfarb reported ties to Abbott Laboratories, and Dr. Tuttle reported ties to Eli Lilly. These remarks were taken from their editorial accompanying Dr. de Zeeuw’s report (N. Engl. J. Med. 2013 Nov. 9 [doi:10.1056/NEJMe1313104]).
Bardoxolone methyl may have reduced the risk of end-stage renal disease in patients with type 2 diabetes and stage 4 chronic kidney disease in a phase III clinical trial, but researchers stopped the study early after patients taking the drug had an increased rate of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke.
The BEACON trial had only an estimated 40% statistical power to determine bardoxolone methyl’s true effects, given its termination because of safety concerns, according to data reported at the annual Kidney Week meeting sponsored by the American Society of Nephrology.
The findings were simultaneously reported online Nov. 9 in the New England Journal of Medicine (doi:10.1056/NEJMoa1306033).
Bardoxolone methyl, the most potent known activator of a transcription factor that regulates antioxidant genes, was previously shown to raise the estimated glomerular filtration rate (eGFR) in patients with diabetes-related kidney disease. However, it also increased the incidence of albuminuria and induced unintended weight loss.
To determine whether longer-term treatment with bardoxolone methyl might translate that eGFR benefit into a slower progression to end-stage renal disease (ESRD), Dr. Dick de Zeeuw of the University of Groningen (the Netherlands) and his associates performed the double-blind BEACON trial (Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events).
BEACON was sponsored by Reata Pharmaceuticals and included patients from the United States, Europe, Australia, Canada, Israel, and Mexico, resulting in a diverse patient population with regard to age, race/ethnicity, and area of residence. Both diabetic retinopathy and neuropathy were common comorbidities, as was cardiovascular disease.
The 2,185 study participants had type 2 diabetes and moderate to severe chronic kidney diseases, with a baseline eGFR of 15 to less than 30 mL/min per 1.73 m2 of body surface area. They were randomly assigned to receive either once-daily bardoxolone methyl 20 mg (1,088 patients) or a matching placebo (1,097 patients), along with conventional background therapies given at the discretion of their treating physicians. Those included inhibitors of the renin-angiotensin-aldosterone system, insulin or other hypoglycemic agents, and appropriate cardiovascular medications.
The median duration of exposure was 7 months for bardoxolone methyl and 8 months for placebo, and the median follow-up for both was 9 months.
Bardoxolone significantly improved eGFR, compared with placebo, and fewer patients who took the drug progressed to ESRD. But BEACON was terminated early because of an excess of CV events among patients receiving bardoxolone methyl. That "truncated" study duration limited the trial’s statistical power.
The primary endpoint was a composite of progression to ESRD or cardiovascular death, and it occurred in 6% of both study groups. However, deaths from CV causes were significantly more frequent in the active treatment group (27 patients) than in the placebo group (19 patients), with a hazard ratio of 1.44, Dr. de Zeeuw reported.
In particular, 96 patients in the bardoxolone group had heart failure (HF) events, compared with only 55 patients in the placebo group. Moreover, significantly more of the HF events in the active treatment group were severe enough to require hospitalization or cause death.
Similarly, significantly more patients taking bardoxolone methyl had a composite outcome of nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, or CV death. And the number of deaths from any cause was greater – though not significantly – with bardoxolone methyl (44 deaths) than with placebo (31 deaths) (HR, 1.47; P = .10).
Compared with placebo, bardoxolone methyl also raised blood pressure and heart rate, increased levels of B-type natriuretic peptide, raised the rate of albuminuria, and caused substantial unintended weight loss. The investigators were unable to determine whether there was a loss of body fat, intracellular (that is, skeletal muscle) water, or extracellular (interstitial) water. There was a concomitant fall in serum albumin and hemoglobin levels, which may reflect hemodilution caused by fluid retention, the investigators found.
The increases in blood pressure and heart rate "constitute a potentially potent combination of factors that are likely to precipitate HF in an at-risk population." and the increase in B-type natriuretic peptide "is consistent with an increase in left ventricular wall stress," Dr. de Zeeuw said.
The investigators attempted to identify patient characteristics that might be associated with the development of HF in the bardoxolone methyl recipients, but were unable to do so.
Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
*This article was updated November 11, 2013.
Bardoxolone methyl may have reduced the risk of end-stage renal disease in patients with type 2 diabetes and stage 4 chronic kidney disease in a phase III clinical trial, but researchers stopped the study early after patients taking the drug had an increased rate of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke.
The BEACON trial had only an estimated 40% statistical power to determine bardoxolone methyl’s true effects, given its termination because of safety concerns, according to data reported at the annual Kidney Week meeting sponsored by the American Society of Nephrology.
The findings were simultaneously reported online Nov. 9 in the New England Journal of Medicine (doi:10.1056/NEJMoa1306033).
Bardoxolone methyl, the most potent known activator of a transcription factor that regulates antioxidant genes, was previously shown to raise the estimated glomerular filtration rate (eGFR) in patients with diabetes-related kidney disease. However, it also increased the incidence of albuminuria and induced unintended weight loss.
To determine whether longer-term treatment with bardoxolone methyl might translate that eGFR benefit into a slower progression to end-stage renal disease (ESRD), Dr. Dick de Zeeuw of the University of Groningen (the Netherlands) and his associates performed the double-blind BEACON trial (Bardoxolone Methyl Evaluation in Patients With Chronic Kidney Disease and Type 2 Diabetes Mellitus: The Occurrence of Renal Events).
BEACON was sponsored by Reata Pharmaceuticals and included patients from the United States, Europe, Australia, Canada, Israel, and Mexico, resulting in a diverse patient population with regard to age, race/ethnicity, and area of residence. Both diabetic retinopathy and neuropathy were common comorbidities, as was cardiovascular disease.
The 2,185 study participants had type 2 diabetes and moderate to severe chronic kidney diseases, with a baseline eGFR of 15 to less than 30 mL/min per 1.73 m2 of body surface area. They were randomly assigned to receive either once-daily bardoxolone methyl 20 mg (1,088 patients) or a matching placebo (1,097 patients), along with conventional background therapies given at the discretion of their treating physicians. Those included inhibitors of the renin-angiotensin-aldosterone system, insulin or other hypoglycemic agents, and appropriate cardiovascular medications.
The median duration of exposure was 7 months for bardoxolone methyl and 8 months for placebo, and the median follow-up for both was 9 months.
Bardoxolone significantly improved eGFR, compared with placebo, and fewer patients who took the drug progressed to ESRD. But BEACON was terminated early because of an excess of CV events among patients receiving bardoxolone methyl. That "truncated" study duration limited the trial’s statistical power.
The primary endpoint was a composite of progression to ESRD or cardiovascular death, and it occurred in 6% of both study groups. However, deaths from CV causes were significantly more frequent in the active treatment group (27 patients) than in the placebo group (19 patients), with a hazard ratio of 1.44, Dr. de Zeeuw reported.
In particular, 96 patients in the bardoxolone group had heart failure (HF) events, compared with only 55 patients in the placebo group. Moreover, significantly more of the HF events in the active treatment group were severe enough to require hospitalization or cause death.
Similarly, significantly more patients taking bardoxolone methyl had a composite outcome of nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, or CV death. And the number of deaths from any cause was greater – though not significantly – with bardoxolone methyl (44 deaths) than with placebo (31 deaths) (HR, 1.47; P = .10).
Compared with placebo, bardoxolone methyl also raised blood pressure and heart rate, increased levels of B-type natriuretic peptide, raised the rate of albuminuria, and caused substantial unintended weight loss. The investigators were unable to determine whether there was a loss of body fat, intracellular (that is, skeletal muscle) water, or extracellular (interstitial) water. There was a concomitant fall in serum albumin and hemoglobin levels, which may reflect hemodilution caused by fluid retention, the investigators found.
The increases in blood pressure and heart rate "constitute a potentially potent combination of factors that are likely to precipitate HF in an at-risk population." and the increase in B-type natriuretic peptide "is consistent with an increase in left ventricular wall stress," Dr. de Zeeuw said.
The investigators attempted to identify patient characteristics that might be associated with the development of HF in the bardoxolone methyl recipients, but were unable to do so.
Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
*This article was updated November 11, 2013.
FROM KIDNEY WEEK
Major Finding: Bardoxolone methyl improved eGFR and may have delayed progression to ESRD, but that result was inconclusive because the trial was terminated early as a result of an excess of cardiovascular deaths, heart failure events, nonfatal myocardial infarction, and nonfatal stroke in patients given the drug.
Data Source: An international phase III double-blind trial involving 2,185 patients with type 2 diabetes and stage 4 chronic kidney disease who were randomly assigned to receive daily oral bardoxolone methyl or placebo and were followed for a median of 9 months.
Disclosures: Reata Pharmaceuticals funded the BEACON trial. Dr. de Zeeuw reported ties to AbbVie, Astellas, Chemocentryx, Johnson & Johnson, and Reata, and his associates reported ties to numerous industry sources.
Heart irradiation is lower with contemporary breast radiotherapy
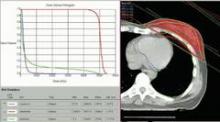
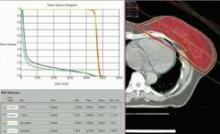
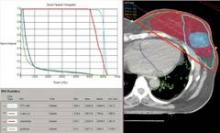
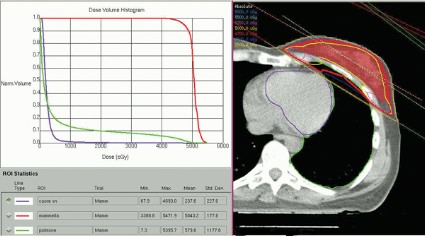
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.
 |
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.
 |
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.
 |
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In an Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more.
Data source: Retrospective review of 100 consecutive patients treated with radiotherapy for left-sided breast cancer at one institution in 2011.
Disclosures: Dr. Lonardi reported having no financial disclosures.
Trial explores ranolazine in pulmonary hypertension-linked heart failure
CHICAGO – The antianginal drug ranolazine improved hemodynamics and functional status in patients with pulmonary hypertension associated with heart failure with preserved ejection fraction in a proof-of-concept trial of just 10 patients.
Six months of twice-daily ranolazine (Ranexa) decreased baseline mean pulmonary arterial pressure (PAP) by 41% and mean pulmonary capillary wedge pressure (PCWP) by 40% in this hard-to-treat population.
Half of the patients improved from World Health Organization functional class III to II, and no patient deteriorated.
"This pilot study suggests ranolazine might be a promising treatment for pulmonary hypertension associated with heart failure with preserved EF [ejection fraction]," Dr. Harrison Farber said during a late-breaking abstract session at the annual meeting of the American College of Chest Physicians.
Pulmonary hypertension (PH) associated with heart failure with preserved ejection fraction (HFpEF) is an increasingly common condition, but currently no effective treatment exists for HFpEF alone or PH associated with it. Disappointing results were reported earlier this year for sildenafil (Viagra) in patients with diastolic heart failure in the RELAX trial.
"This pilot study suggests ranolazine might be a promising treatment for pulmonary hypertension associated with heart failure with preserved EF [ejection fraction]..."
Ranolazine is approved for the treatment of chronic angina, and was selected because animal and human data suggest it reduces left ventricular wall stiffness and mechanical dysfunction in conditions associated with diastolic dysfunction, said Dr. Farber, director of the pulmonary hypertension center, Boston University.
To ensure patients in the single-center, open-label, prospective, non–placebo controlled trial truly had HFpEF, entry criteria were fairly strict. They included left ventricular ejection fraction (LVEF) greater than 50% by echocardiogram, mean PAP of at least 25 mm Hg, PCWP between 18 mm Hg and 30 mm Hg, and a pulmonary arterial diastolic pressure-PCWP gradient of 10 mm Hg or less.
The eight women and two men received twice-daily ranolazine 500 mg, titrated to 1,000 mg twice daily, as tolerated. Their mean age was 63 years, mean body mass index 41.6 kg/m2, and 80% had diabetes.
At 6 months, mean PAP dropped from 39 mm Hg to 23 mm Hg, mean PCWP from 22 mm Hg to 13 mm HG, and 6-minute walk distances increased from 286 meters to 319 meters, Dr. Farber said.
"Seven of the subjects have actually continued ranolazine for a year or greater and the 6-minute-walk and functional class improvements have actually been maintained to this point," he said.
No significant changes were seen in cardiac index, pulmonary vascular resistance, echocardiogram parameters, brain natriuretic peptide, N-terminal pro-BNP, or troponin.
There was, however, a significant reduction in LV mass at 6 months on blinded MRI evaluation, and a trend for improved right ventricular ejection fraction and decrease in RV mass, he said.
Session comoderator Dr. Namita Sood with Ohio State University in Columbus congratulated the authors on "a courageous study" in an understudied population and asked whether the drop in PAP and PCWP was a consequence of improved LV and diastolic dysfunction or whether ranolazine may have a direct effect on pulmonary vasculature.
Dr. Farber responded, "To be honest, the answer is I don’t know, but since the two were sort of in concert, my guess would be – although it was a small study and I can’t tell you for sure – it was more of an effect on the left ventricle and a concomitant drop in the pulmonary pressures."
Some audience members questioned whether the investigators targeted the right population since patients did not appear to be sufficiently diuresed at entry. Dr. Farber said the exploratory analysis was done to determine if a signal was present and that tighter controls would be needed if they go forward. Diuretics were not controlled in the 10 patients, although no dramatic changes in doses occurred. No patients were on nitrates.
One patient discontinued treatment because of the worsening of an existing symptomatic bradyarrhythmia. Otherwise, the drug was well tolerated, with the only other side effect being headache that went away in the first week of treatment, he said.
Dr. Farber and his coauthors reported grant support from Gilead Sciences, which makes ranolazine.
This article was updated 11/7/13.
CHICAGO – The antianginal drug ranolazine improved hemodynamics and functional status in patients with pulmonary hypertension associated with heart failure with preserved ejection fraction in a proof-of-concept trial of just 10 patients.
Six months of twice-daily ranolazine (Ranexa) decreased baseline mean pulmonary arterial pressure (PAP) by 41% and mean pulmonary capillary wedge pressure (PCWP) by 40% in this hard-to-treat population.
Half of the patients improved from World Health Organization functional class III to II, and no patient deteriorated.
"This pilot study suggests ranolazine might be a promising treatment for pulmonary hypertension associated with heart failure with preserved EF [ejection fraction]," Dr. Harrison Farber said during a late-breaking abstract session at the annual meeting of the American College of Chest Physicians.
Pulmonary hypertension (PH) associated with heart failure with preserved ejection fraction (HFpEF) is an increasingly common condition, but currently no effective treatment exists for HFpEF alone or PH associated with it. Disappointing results were reported earlier this year for sildenafil (Viagra) in patients with diastolic heart failure in the RELAX trial.
"This pilot study suggests ranolazine might be a promising treatment for pulmonary hypertension associated with heart failure with preserved EF [ejection fraction]..."
Ranolazine is approved for the treatment of chronic angina, and was selected because animal and human data suggest it reduces left ventricular wall stiffness and mechanical dysfunction in conditions associated with diastolic dysfunction, said Dr. Farber, director of the pulmonary hypertension center, Boston University.
To ensure patients in the single-center, open-label, prospective, non–placebo controlled trial truly had HFpEF, entry criteria were fairly strict. They included left ventricular ejection fraction (LVEF) greater than 50% by echocardiogram, mean PAP of at least 25 mm Hg, PCWP between 18 mm Hg and 30 mm Hg, and a pulmonary arterial diastolic pressure-PCWP gradient of 10 mm Hg or less.
The eight women and two men received twice-daily ranolazine 500 mg, titrated to 1,000 mg twice daily, as tolerated. Their mean age was 63 years, mean body mass index 41.6 kg/m2, and 80% had diabetes.
At 6 months, mean PAP dropped from 39 mm Hg to 23 mm Hg, mean PCWP from 22 mm Hg to 13 mm HG, and 6-minute walk distances increased from 286 meters to 319 meters, Dr. Farber said.
"Seven of the subjects have actually continued ranolazine for a year or greater and the 6-minute-walk and functional class improvements have actually been maintained to this point," he said.
No significant changes were seen in cardiac index, pulmonary vascular resistance, echocardiogram parameters, brain natriuretic peptide, N-terminal pro-BNP, or troponin.
There was, however, a significant reduction in LV mass at 6 months on blinded MRI evaluation, and a trend for improved right ventricular ejection fraction and decrease in RV mass, he said.
Session comoderator Dr. Namita Sood with Ohio State University in Columbus congratulated the authors on "a courageous study" in an understudied population and asked whether the drop in PAP and PCWP was a consequence of improved LV and diastolic dysfunction or whether ranolazine may have a direct effect on pulmonary vasculature.
Dr. Farber responded, "To be honest, the answer is I don’t know, but since the two were sort of in concert, my guess would be – although it was a small study and I can’t tell you for sure – it was more of an effect on the left ventricle and a concomitant drop in the pulmonary pressures."
Some audience members questioned whether the investigators targeted the right population since patients did not appear to be sufficiently diuresed at entry. Dr. Farber said the exploratory analysis was done to determine if a signal was present and that tighter controls would be needed if they go forward. Diuretics were not controlled in the 10 patients, although no dramatic changes in doses occurred. No patients were on nitrates.
One patient discontinued treatment because of the worsening of an existing symptomatic bradyarrhythmia. Otherwise, the drug was well tolerated, with the only other side effect being headache that went away in the first week of treatment, he said.
Dr. Farber and his coauthors reported grant support from Gilead Sciences, which makes ranolazine.
This article was updated 11/7/13.
CHICAGO – The antianginal drug ranolazine improved hemodynamics and functional status in patients with pulmonary hypertension associated with heart failure with preserved ejection fraction in a proof-of-concept trial of just 10 patients.
Six months of twice-daily ranolazine (Ranexa) decreased baseline mean pulmonary arterial pressure (PAP) by 41% and mean pulmonary capillary wedge pressure (PCWP) by 40% in this hard-to-treat population.
Half of the patients improved from World Health Organization functional class III to II, and no patient deteriorated.
"This pilot study suggests ranolazine might be a promising treatment for pulmonary hypertension associated with heart failure with preserved EF [ejection fraction]," Dr. Harrison Farber said during a late-breaking abstract session at the annual meeting of the American College of Chest Physicians.
Pulmonary hypertension (PH) associated with heart failure with preserved ejection fraction (HFpEF) is an increasingly common condition, but currently no effective treatment exists for HFpEF alone or PH associated with it. Disappointing results were reported earlier this year for sildenafil (Viagra) in patients with diastolic heart failure in the RELAX trial.
"This pilot study suggests ranolazine might be a promising treatment for pulmonary hypertension associated with heart failure with preserved EF [ejection fraction]..."
Ranolazine is approved for the treatment of chronic angina, and was selected because animal and human data suggest it reduces left ventricular wall stiffness and mechanical dysfunction in conditions associated with diastolic dysfunction, said Dr. Farber, director of the pulmonary hypertension center, Boston University.
To ensure patients in the single-center, open-label, prospective, non–placebo controlled trial truly had HFpEF, entry criteria were fairly strict. They included left ventricular ejection fraction (LVEF) greater than 50% by echocardiogram, mean PAP of at least 25 mm Hg, PCWP between 18 mm Hg and 30 mm Hg, and a pulmonary arterial diastolic pressure-PCWP gradient of 10 mm Hg or less.
The eight women and two men received twice-daily ranolazine 500 mg, titrated to 1,000 mg twice daily, as tolerated. Their mean age was 63 years, mean body mass index 41.6 kg/m2, and 80% had diabetes.
At 6 months, mean PAP dropped from 39 mm Hg to 23 mm Hg, mean PCWP from 22 mm Hg to 13 mm HG, and 6-minute walk distances increased from 286 meters to 319 meters, Dr. Farber said.
"Seven of the subjects have actually continued ranolazine for a year or greater and the 6-minute-walk and functional class improvements have actually been maintained to this point," he said.
No significant changes were seen in cardiac index, pulmonary vascular resistance, echocardiogram parameters, brain natriuretic peptide, N-terminal pro-BNP, or troponin.
There was, however, a significant reduction in LV mass at 6 months on blinded MRI evaluation, and a trend for improved right ventricular ejection fraction and decrease in RV mass, he said.
Session comoderator Dr. Namita Sood with Ohio State University in Columbus congratulated the authors on "a courageous study" in an understudied population and asked whether the drop in PAP and PCWP was a consequence of improved LV and diastolic dysfunction or whether ranolazine may have a direct effect on pulmonary vasculature.
Dr. Farber responded, "To be honest, the answer is I don’t know, but since the two were sort of in concert, my guess would be – although it was a small study and I can’t tell you for sure – it was more of an effect on the left ventricle and a concomitant drop in the pulmonary pressures."
Some audience members questioned whether the investigators targeted the right population since patients did not appear to be sufficiently diuresed at entry. Dr. Farber said the exploratory analysis was done to determine if a signal was present and that tighter controls would be needed if they go forward. Diuretics were not controlled in the 10 patients, although no dramatic changes in doses occurred. No patients were on nitrates.
One patient discontinued treatment because of the worsening of an existing symptomatic bradyarrhythmia. Otherwise, the drug was well tolerated, with the only other side effect being headache that went away in the first week of treatment, he said.
Dr. Farber and his coauthors reported grant support from Gilead Sciences, which makes ranolazine.
This article was updated 11/7/13.
AT CHEST 2013
Major finding: At 6 months, ranolazine decreased baseline mean pulmonary arterial pressure 41% and mean pulmonary wedge pressure by 40%.
Data source: A prospective open-label trial in 10 patients with pulmonary hypertension associated with heart failure with preserved ejection fraction.
Disclosures: Dr. Farber and his coauthors reported grant support from Gilead Sciences, which makes ranolazine.
Esmolol stabilizes heart rate in septic shock
The short-acting, intravenous beta-blocker esmolol has been shown to reduce and stabilize heart rates without adverse effects in patients with severe septic shock, a new phase II study has found.
In an open-label study that randomized 154 patients with septic shock and a heart rate of 95 or higher to standard care or titrated esmolol, the beta-blocker was associated with successful reductions in heart rate to between 80 and 94 beats per minute over a 96-hour period: a median of –28 BPM for the esmolol group compared with –6 for controls (P less than .001).
For their research, Dr. Andrea Morelli of the University of Rome La Sapienza and colleagues recruited from the hospital’s intensive care unit patients with septic shock and a heart rate of 95 BPM or above (JAMA 2013;310:1683-91).
Patients with lower heart rates or with previous beta-blocker use were excluded. Subjects in both groups required norepinephrine to maintain a mean arterial pressure of 65 mm Hg or higher. The primary outcome measure was heart rate stabilization at between 80 and 94 BPM.
The esmolol group, which received a median continuous infusion of 100 mg/hr during the treatment period, also saw improved stroke work index and left ventricular stroke work, which investigators suspected was a result of improved diastolic filling. Esmolol treatment was associated with maintenance of mean arterial pressure and reduced need for norepinephrine. It was not associated with higher hepatic, renal, or myocardial injury compared with controls. Importantly, mortality at 28 days was considerably and significantly lower in the esmolol group than in controls: 49.4% vs. 80.5%. Each group comprised 77 patients.
In an editorial, Dr. Michael R. Pinsky of the department of critical care at the University of Pittsburgh called the findings "consistent with selective blockage of beta-adrenergic hyperactivity causing improved myocardial performance and decreased metabolic demand without compromising peripheral vascular function." Nonetheless, he cautioned clinicians against applying these results to all patients in septic shock (JAMA 2013;310:1677-8). "The reasons for this caution involve the limitations of this study and limitations in the current understanding of how beta-blocker therapy can cause such effects."
Dr. Morelli and colleagues acknowledged several limitations of their study. One was its single-center, open-label design. (As Dr. Pinsky noted in his editorial, a blinded study would be almost impossible to carry out because heart rate titration would be difficult to mask). The results should be replicated in a larger, multicenter trial, the researchers wrote. They noted that they had used "an arbitrary predefined heart rate threshold rather than an individualized approach titrated to specific myocardial characteristics or other biomarkers." Finally, the researchers allowed that the unexpectedly large mortality difference seen in the study could have been the result of confounding.
The study was funded by the University of Rome La Sapienza. Dr. Morelli disclosed honoraria from Baxter, the manufacturer of esmolol. A coauthor, Dr. Mervyn Singer, reported ties with Baxter. Dr. Pinsky did not report any disclosures relevant to his editorial.
The short-acting, intravenous beta-blocker esmolol has been shown to reduce and stabilize heart rates without adverse effects in patients with severe septic shock, a new phase II study has found.
In an open-label study that randomized 154 patients with septic shock and a heart rate of 95 or higher to standard care or titrated esmolol, the beta-blocker was associated with successful reductions in heart rate to between 80 and 94 beats per minute over a 96-hour period: a median of –28 BPM for the esmolol group compared with –6 for controls (P less than .001).
For their research, Dr. Andrea Morelli of the University of Rome La Sapienza and colleagues recruited from the hospital’s intensive care unit patients with septic shock and a heart rate of 95 BPM or above (JAMA 2013;310:1683-91).
Patients with lower heart rates or with previous beta-blocker use were excluded. Subjects in both groups required norepinephrine to maintain a mean arterial pressure of 65 mm Hg or higher. The primary outcome measure was heart rate stabilization at between 80 and 94 BPM.
The esmolol group, which received a median continuous infusion of 100 mg/hr during the treatment period, also saw improved stroke work index and left ventricular stroke work, which investigators suspected was a result of improved diastolic filling. Esmolol treatment was associated with maintenance of mean arterial pressure and reduced need for norepinephrine. It was not associated with higher hepatic, renal, or myocardial injury compared with controls. Importantly, mortality at 28 days was considerably and significantly lower in the esmolol group than in controls: 49.4% vs. 80.5%. Each group comprised 77 patients.
In an editorial, Dr. Michael R. Pinsky of the department of critical care at the University of Pittsburgh called the findings "consistent with selective blockage of beta-adrenergic hyperactivity causing improved myocardial performance and decreased metabolic demand without compromising peripheral vascular function." Nonetheless, he cautioned clinicians against applying these results to all patients in septic shock (JAMA 2013;310:1677-8). "The reasons for this caution involve the limitations of this study and limitations in the current understanding of how beta-blocker therapy can cause such effects."
Dr. Morelli and colleagues acknowledged several limitations of their study. One was its single-center, open-label design. (As Dr. Pinsky noted in his editorial, a blinded study would be almost impossible to carry out because heart rate titration would be difficult to mask). The results should be replicated in a larger, multicenter trial, the researchers wrote. They noted that they had used "an arbitrary predefined heart rate threshold rather than an individualized approach titrated to specific myocardial characteristics or other biomarkers." Finally, the researchers allowed that the unexpectedly large mortality difference seen in the study could have been the result of confounding.
The study was funded by the University of Rome La Sapienza. Dr. Morelli disclosed honoraria from Baxter, the manufacturer of esmolol. A coauthor, Dr. Mervyn Singer, reported ties with Baxter. Dr. Pinsky did not report any disclosures relevant to his editorial.
The short-acting, intravenous beta-blocker esmolol has been shown to reduce and stabilize heart rates without adverse effects in patients with severe septic shock, a new phase II study has found.
In an open-label study that randomized 154 patients with septic shock and a heart rate of 95 or higher to standard care or titrated esmolol, the beta-blocker was associated with successful reductions in heart rate to between 80 and 94 beats per minute over a 96-hour period: a median of –28 BPM for the esmolol group compared with –6 for controls (P less than .001).
For their research, Dr. Andrea Morelli of the University of Rome La Sapienza and colleagues recruited from the hospital’s intensive care unit patients with septic shock and a heart rate of 95 BPM or above (JAMA 2013;310:1683-91).
Patients with lower heart rates or with previous beta-blocker use were excluded. Subjects in both groups required norepinephrine to maintain a mean arterial pressure of 65 mm Hg or higher. The primary outcome measure was heart rate stabilization at between 80 and 94 BPM.
The esmolol group, which received a median continuous infusion of 100 mg/hr during the treatment period, also saw improved stroke work index and left ventricular stroke work, which investigators suspected was a result of improved diastolic filling. Esmolol treatment was associated with maintenance of mean arterial pressure and reduced need for norepinephrine. It was not associated with higher hepatic, renal, or myocardial injury compared with controls. Importantly, mortality at 28 days was considerably and significantly lower in the esmolol group than in controls: 49.4% vs. 80.5%. Each group comprised 77 patients.
In an editorial, Dr. Michael R. Pinsky of the department of critical care at the University of Pittsburgh called the findings "consistent with selective blockage of beta-adrenergic hyperactivity causing improved myocardial performance and decreased metabolic demand without compromising peripheral vascular function." Nonetheless, he cautioned clinicians against applying these results to all patients in septic shock (JAMA 2013;310:1677-8). "The reasons for this caution involve the limitations of this study and limitations in the current understanding of how beta-blocker therapy can cause such effects."
Dr. Morelli and colleagues acknowledged several limitations of their study. One was its single-center, open-label design. (As Dr. Pinsky noted in his editorial, a blinded study would be almost impossible to carry out because heart rate titration would be difficult to mask). The results should be replicated in a larger, multicenter trial, the researchers wrote. They noted that they had used "an arbitrary predefined heart rate threshold rather than an individualized approach titrated to specific myocardial characteristics or other biomarkers." Finally, the researchers allowed that the unexpectedly large mortality difference seen in the study could have been the result of confounding.
The study was funded by the University of Rome La Sapienza. Dr. Morelli disclosed honoraria from Baxter, the manufacturer of esmolol. A coauthor, Dr. Mervyn Singer, reported ties with Baxter. Dr. Pinsky did not report any disclosures relevant to his editorial.
FROM JAMA
Main finding: A short-acting beta-blocker, esmolol, is effective in reducing heart rates among patients with septic shock and tachycardia.
Data source: A single-center, open-label randomized trial (n = 154).
Disclosures: The study was funded by the University of Rome La Sapienza. Dr. Morelli disclosed honoraria from Baxter, the manufacturer of esmolol. A coauthor, Dr. Mervyn Singer, reported ties with Baxter. Dr. Pinsky did not report any disclosures relevant to his editorial.
On heart failure and beta-blocker dosages
Getting the right therapeutic dose of any drug is not always easy. Using antibiotics to treat infection or antihypertensive drugs to lower blood pressure can be measured easily by simple physiologic measurements.
The treatment of heart failure with beta-blockers or ACE inhibitors, however, has been largely defined by clinical trials, which by their nature use one dosage and usually provide the clinician with limited information about the range of the best and most effective dosages. The rigor of choosing the correct dosage in clinical trials is often limited to small, underpowered phase II studies carried out well before the major phase III trials, which are designed to support efficacy and safety, usually at that one dosage. And still, physicians usually pick the lowest dose, following Hippocrates’ dictum to "do no harm." This dilemma has particular importance in picking the best dose of a beta-blocker in heart failure.
A recent presentation at the annual congress of the European Society of Cardiology by Dr. L. Brent Mitchell ("Full-dose beta-blockers still show benefit," October 2013, p. 26) sheds some important light on the benefit of maximum dosing with beta-blockers in heart failure patients treated with cardiac resynchronization therapy (CRT) or implantable cardiac defibrillators (ICDs) in whom bradycardia escape pacing was present.
Although all patients received standard drug therapy, patients receiving less than 50% of the full recommended dose of beta-blocker had a worse outcome in regard to mortality and rehospitalization when compared with patients receiving the full recommended dose, regardless of the beta-blocker used. Roughly one-half of these heart failure ICD/CRT patients were receiving less than half of the recommended dose for heart failure therapy. Older patients and those with more advance heart failure tended to receive the lower dose. In this patient population with pacemaker-controlled low heart rate, the issue of beta-blocker–induced bradycardia is no longer an issue: the higher the better.
In patients with atrial-controlled heart rates with sinus rhythm or atrial fibrillation, however, the induction of bradycardia has been an issue as physicians up-titrate dosages. The effect on morbidity and mortality of varying doses of metoprolol succinate (Toprol) was examined in the MERIT-HF trial (J. Am. Coll. Cardiol. 2002;40:491-8), in which physicians were encouraged to up-titrate to the highest dose. The limitation of up-titration was bradycardia. The high-dose (greater than 100 mg/day) and low-dose (100 mg/day or less) patients received 192 mg and 76 mg/day, respectively. Despite the different maximal doses, the final heart rate achieved with the up-titration was 68 beats/min. Patients receiving the high dose and low dose achieved the same relative benefit of therapy. The low-dose patient group was older and had a higher New York Heart Association functional class.
These observations suggest that there was a significant variability in the patient’s sensitivity to beta-blocker therapy, but the achievement of a low heart rate, regardless of dose, was effective in achieving the best therapeutic benefit. In a small dose-ranging study, patients were randomized to receive 50 or 200 mg/day of Toprol. The patients receiving 200 mg demonstrated an increase in ejection fraction and a decrease in end systolic volume, compared with the 50 mg–dose patients, who failed to evidence any hemodynamic improvement (Circulation 2007;116:49-56).
These observations emphasize the uncertainties of drug dosing in heart failure with our standard therapy. The benefit of high doses of beta-blockers in the ICD/CRT trial in patients whose heart rate was controlled with bradycardia pacing provides important support for the use of high doses in these individuals. In patients whose heart rate was controlled by atrial rhythms in the MERIT-HF trial, heart rate became the major limitation of drug therapy. In these patients, up-titration to maximal heart rate expressed the presence of a variable sensitivity to beta-blockade. The achievement of a slow heart rate, regardless of dose, appeared to achieve a similar benefit on heart failure outcomes.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies, and was the co-principal investigator of the MERIT-HF trial.
Getting the right therapeutic dose of any drug is not always easy. Using antibiotics to treat infection or antihypertensive drugs to lower blood pressure can be measured easily by simple physiologic measurements.
The treatment of heart failure with beta-blockers or ACE inhibitors, however, has been largely defined by clinical trials, which by their nature use one dosage and usually provide the clinician with limited information about the range of the best and most effective dosages. The rigor of choosing the correct dosage in clinical trials is often limited to small, underpowered phase II studies carried out well before the major phase III trials, which are designed to support efficacy and safety, usually at that one dosage. And still, physicians usually pick the lowest dose, following Hippocrates’ dictum to "do no harm." This dilemma has particular importance in picking the best dose of a beta-blocker in heart failure.
A recent presentation at the annual congress of the European Society of Cardiology by Dr. L. Brent Mitchell ("Full-dose beta-blockers still show benefit," October 2013, p. 26) sheds some important light on the benefit of maximum dosing with beta-blockers in heart failure patients treated with cardiac resynchronization therapy (CRT) or implantable cardiac defibrillators (ICDs) in whom bradycardia escape pacing was present.
Although all patients received standard drug therapy, patients receiving less than 50% of the full recommended dose of beta-blocker had a worse outcome in regard to mortality and rehospitalization when compared with patients receiving the full recommended dose, regardless of the beta-blocker used. Roughly one-half of these heart failure ICD/CRT patients were receiving less than half of the recommended dose for heart failure therapy. Older patients and those with more advance heart failure tended to receive the lower dose. In this patient population with pacemaker-controlled low heart rate, the issue of beta-blocker–induced bradycardia is no longer an issue: the higher the better.
In patients with atrial-controlled heart rates with sinus rhythm or atrial fibrillation, however, the induction of bradycardia has been an issue as physicians up-titrate dosages. The effect on morbidity and mortality of varying doses of metoprolol succinate (Toprol) was examined in the MERIT-HF trial (J. Am. Coll. Cardiol. 2002;40:491-8), in which physicians were encouraged to up-titrate to the highest dose. The limitation of up-titration was bradycardia. The high-dose (greater than 100 mg/day) and low-dose (100 mg/day or less) patients received 192 mg and 76 mg/day, respectively. Despite the different maximal doses, the final heart rate achieved with the up-titration was 68 beats/min. Patients receiving the high dose and low dose achieved the same relative benefit of therapy. The low-dose patient group was older and had a higher New York Heart Association functional class.
These observations suggest that there was a significant variability in the patient’s sensitivity to beta-blocker therapy, but the achievement of a low heart rate, regardless of dose, was effective in achieving the best therapeutic benefit. In a small dose-ranging study, patients were randomized to receive 50 or 200 mg/day of Toprol. The patients receiving 200 mg demonstrated an increase in ejection fraction and a decrease in end systolic volume, compared with the 50 mg–dose patients, who failed to evidence any hemodynamic improvement (Circulation 2007;116:49-56).
These observations emphasize the uncertainties of drug dosing in heart failure with our standard therapy. The benefit of high doses of beta-blockers in the ICD/CRT trial in patients whose heart rate was controlled with bradycardia pacing provides important support for the use of high doses in these individuals. In patients whose heart rate was controlled by atrial rhythms in the MERIT-HF trial, heart rate became the major limitation of drug therapy. In these patients, up-titration to maximal heart rate expressed the presence of a variable sensitivity to beta-blockade. The achievement of a slow heart rate, regardless of dose, appeared to achieve a similar benefit on heart failure outcomes.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies, and was the co-principal investigator of the MERIT-HF trial.
Getting the right therapeutic dose of any drug is not always easy. Using antibiotics to treat infection or antihypertensive drugs to lower blood pressure can be measured easily by simple physiologic measurements.
The treatment of heart failure with beta-blockers or ACE inhibitors, however, has been largely defined by clinical trials, which by their nature use one dosage and usually provide the clinician with limited information about the range of the best and most effective dosages. The rigor of choosing the correct dosage in clinical trials is often limited to small, underpowered phase II studies carried out well before the major phase III trials, which are designed to support efficacy and safety, usually at that one dosage. And still, physicians usually pick the lowest dose, following Hippocrates’ dictum to "do no harm." This dilemma has particular importance in picking the best dose of a beta-blocker in heart failure.
A recent presentation at the annual congress of the European Society of Cardiology by Dr. L. Brent Mitchell ("Full-dose beta-blockers still show benefit," October 2013, p. 26) sheds some important light on the benefit of maximum dosing with beta-blockers in heart failure patients treated with cardiac resynchronization therapy (CRT) or implantable cardiac defibrillators (ICDs) in whom bradycardia escape pacing was present.
Although all patients received standard drug therapy, patients receiving less than 50% of the full recommended dose of beta-blocker had a worse outcome in regard to mortality and rehospitalization when compared with patients receiving the full recommended dose, regardless of the beta-blocker used. Roughly one-half of these heart failure ICD/CRT patients were receiving less than half of the recommended dose for heart failure therapy. Older patients and those with more advance heart failure tended to receive the lower dose. In this patient population with pacemaker-controlled low heart rate, the issue of beta-blocker–induced bradycardia is no longer an issue: the higher the better.
In patients with atrial-controlled heart rates with sinus rhythm or atrial fibrillation, however, the induction of bradycardia has been an issue as physicians up-titrate dosages. The effect on morbidity and mortality of varying doses of metoprolol succinate (Toprol) was examined in the MERIT-HF trial (J. Am. Coll. Cardiol. 2002;40:491-8), in which physicians were encouraged to up-titrate to the highest dose. The limitation of up-titration was bradycardia. The high-dose (greater than 100 mg/day) and low-dose (100 mg/day or less) patients received 192 mg and 76 mg/day, respectively. Despite the different maximal doses, the final heart rate achieved with the up-titration was 68 beats/min. Patients receiving the high dose and low dose achieved the same relative benefit of therapy. The low-dose patient group was older and had a higher New York Heart Association functional class.
These observations suggest that there was a significant variability in the patient’s sensitivity to beta-blocker therapy, but the achievement of a low heart rate, regardless of dose, was effective in achieving the best therapeutic benefit. In a small dose-ranging study, patients were randomized to receive 50 or 200 mg/day of Toprol. The patients receiving 200 mg demonstrated an increase in ejection fraction and a decrease in end systolic volume, compared with the 50 mg–dose patients, who failed to evidence any hemodynamic improvement (Circulation 2007;116:49-56).
These observations emphasize the uncertainties of drug dosing in heart failure with our standard therapy. The benefit of high doses of beta-blockers in the ICD/CRT trial in patients whose heart rate was controlled with bradycardia pacing provides important support for the use of high doses in these individuals. In patients whose heart rate was controlled by atrial rhythms in the MERIT-HF trial, heart rate became the major limitation of drug therapy. In these patients, up-titration to maximal heart rate expressed the presence of a variable sensitivity to beta-blockade. The achievement of a slow heart rate, regardless of dose, appeared to achieve a similar benefit on heart failure outcomes.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies, and was the co-principal investigator of the MERIT-HF trial.
Life-saving therapies could eliminate wait-list disparities
ORLANDO – More women than men died during the first year of being on the heart transplant waiting list, and the disparity may be influenced by the difference in the use of life-saving therapies, according to a 12-year analysis of a national database.
After adjusting for several variables, researchers found that female gender was associated with a 10% increased risk of being removed from the waiting list because the women died or were deemed too sick during the first year. When the researchers added implantable cardioverter defibrillator use to the analysis, that risk was attenuated to 8%, although still significant, but adding ICDs and left ventricular assist devices (LVADs) eliminated the risk associated with the female gender, said Dr. Alanna Morris of Emory University, Atlanta.
The study, which looked at the Organ Procurement and Transplantation Network (OPTN) database, showed that women on the waiting list were significantly less likely to have an ICD (55% vs. 64%; P less than .001), or a ventricular assist device (24% vs. 30%; P less than .001) than were men, said Dr. Morris, who presented her unpublished abstract at the annual meeting of the Heart Failure Society of America.
The finding is in line with studies that have shown significantly lower rates of ICD implantation among women with end-stage heart failure, compared with men. Research has also shown that women are less likely to be referred for LVAD implantation, even though no survival difference between genders while on LVAD support has been observed, the authors noted.
Meanwhile, the proportion of women on the wait list has increased by more than 4% in the past decade, but studies on women’s survival while on wait lists have shown conflicting results, the researchers added.
They identified nearly 27,000 adult patients (23% were women) in the OPTN database between January 2000 and September 2012, who were listed for their first heart transplant.
There were several statistically significant differences between genders at baseline, aside from ICD and LVAD use. Female heart transplant candidates were younger (52 vs. 56 years), were less likely to have diabetes (21% vs. 27%), were less likely to have a normal glomerular filtration rate (49% vs. 53%), had a lower pulmonary capillary wedge pressure (20 vs. 21 mm Hg), and had fewer median days (67 vs. 84) on a wait list (P less than .001 for all).
The 1-year unadjusted survival rate in women on the waiting list was significantly lower, at 70%, than in men, at 73% (P = .006).
After adjustment for age, race, blood type, and support with extracorporeal membrane oxygenation or intra-aortic balloon pump, female gender was still associated with a higher risk of the primary end point, which was removal from the wait list due to death or being deemed too sick to transplant at 1 year (hazard ratio 1.10; P = .026), the authors reported.
But, after adjustment for ICD and LVAD use, the gender gap was eliminated (HR 1.06; P = .2).
Dr. Morris said that a more aggressive use of these life-saving therapies can eliminate wait-list disparities. The findings also point to the importance of educating community physicians and heart failure patients about the current standards of care, she said.
Changes in the allocation algorithm and improvements in LVAD technology have led to advancements in management of patients on heart transplant waiting lists, the authors said. Between 2001 and 2011, wait list mortality declined from 17 deaths per 100 wait-list years, to 12 deaths. During the same period, wait-list death among patients with an LVAD declined from 102 deaths per 100 wait-list years to 13.
Dr. Morris had no financial relationships to disclose.
On Twitter @NaseemSMiller
ORLANDO – More women than men died during the first year of being on the heart transplant waiting list, and the disparity may be influenced by the difference in the use of life-saving therapies, according to a 12-year analysis of a national database.
After adjusting for several variables, researchers found that female gender was associated with a 10% increased risk of being removed from the waiting list because the women died or were deemed too sick during the first year. When the researchers added implantable cardioverter defibrillator use to the analysis, that risk was attenuated to 8%, although still significant, but adding ICDs and left ventricular assist devices (LVADs) eliminated the risk associated with the female gender, said Dr. Alanna Morris of Emory University, Atlanta.
The study, which looked at the Organ Procurement and Transplantation Network (OPTN) database, showed that women on the waiting list were significantly less likely to have an ICD (55% vs. 64%; P less than .001), or a ventricular assist device (24% vs. 30%; P less than .001) than were men, said Dr. Morris, who presented her unpublished abstract at the annual meeting of the Heart Failure Society of America.
The finding is in line with studies that have shown significantly lower rates of ICD implantation among women with end-stage heart failure, compared with men. Research has also shown that women are less likely to be referred for LVAD implantation, even though no survival difference between genders while on LVAD support has been observed, the authors noted.
Meanwhile, the proportion of women on the wait list has increased by more than 4% in the past decade, but studies on women’s survival while on wait lists have shown conflicting results, the researchers added.
They identified nearly 27,000 adult patients (23% were women) in the OPTN database between January 2000 and September 2012, who were listed for their first heart transplant.
There were several statistically significant differences between genders at baseline, aside from ICD and LVAD use. Female heart transplant candidates were younger (52 vs. 56 years), were less likely to have diabetes (21% vs. 27%), were less likely to have a normal glomerular filtration rate (49% vs. 53%), had a lower pulmonary capillary wedge pressure (20 vs. 21 mm Hg), and had fewer median days (67 vs. 84) on a wait list (P less than .001 for all).
The 1-year unadjusted survival rate in women on the waiting list was significantly lower, at 70%, than in men, at 73% (P = .006).
After adjustment for age, race, blood type, and support with extracorporeal membrane oxygenation or intra-aortic balloon pump, female gender was still associated with a higher risk of the primary end point, which was removal from the wait list due to death or being deemed too sick to transplant at 1 year (hazard ratio 1.10; P = .026), the authors reported.
But, after adjustment for ICD and LVAD use, the gender gap was eliminated (HR 1.06; P = .2).
Dr. Morris said that a more aggressive use of these life-saving therapies can eliminate wait-list disparities. The findings also point to the importance of educating community physicians and heart failure patients about the current standards of care, she said.
Changes in the allocation algorithm and improvements in LVAD technology have led to advancements in management of patients on heart transplant waiting lists, the authors said. Between 2001 and 2011, wait list mortality declined from 17 deaths per 100 wait-list years, to 12 deaths. During the same period, wait-list death among patients with an LVAD declined from 102 deaths per 100 wait-list years to 13.
Dr. Morris had no financial relationships to disclose.
On Twitter @NaseemSMiller
ORLANDO – More women than men died during the first year of being on the heart transplant waiting list, and the disparity may be influenced by the difference in the use of life-saving therapies, according to a 12-year analysis of a national database.
After adjusting for several variables, researchers found that female gender was associated with a 10% increased risk of being removed from the waiting list because the women died or were deemed too sick during the first year. When the researchers added implantable cardioverter defibrillator use to the analysis, that risk was attenuated to 8%, although still significant, but adding ICDs and left ventricular assist devices (LVADs) eliminated the risk associated with the female gender, said Dr. Alanna Morris of Emory University, Atlanta.
The study, which looked at the Organ Procurement and Transplantation Network (OPTN) database, showed that women on the waiting list were significantly less likely to have an ICD (55% vs. 64%; P less than .001), or a ventricular assist device (24% vs. 30%; P less than .001) than were men, said Dr. Morris, who presented her unpublished abstract at the annual meeting of the Heart Failure Society of America.
The finding is in line with studies that have shown significantly lower rates of ICD implantation among women with end-stage heart failure, compared with men. Research has also shown that women are less likely to be referred for LVAD implantation, even though no survival difference between genders while on LVAD support has been observed, the authors noted.
Meanwhile, the proportion of women on the wait list has increased by more than 4% in the past decade, but studies on women’s survival while on wait lists have shown conflicting results, the researchers added.
They identified nearly 27,000 adult patients (23% were women) in the OPTN database between January 2000 and September 2012, who were listed for their first heart transplant.
There were several statistically significant differences between genders at baseline, aside from ICD and LVAD use. Female heart transplant candidates were younger (52 vs. 56 years), were less likely to have diabetes (21% vs. 27%), were less likely to have a normal glomerular filtration rate (49% vs. 53%), had a lower pulmonary capillary wedge pressure (20 vs. 21 mm Hg), and had fewer median days (67 vs. 84) on a wait list (P less than .001 for all).
The 1-year unadjusted survival rate in women on the waiting list was significantly lower, at 70%, than in men, at 73% (P = .006).
After adjustment for age, race, blood type, and support with extracorporeal membrane oxygenation or intra-aortic balloon pump, female gender was still associated with a higher risk of the primary end point, which was removal from the wait list due to death or being deemed too sick to transplant at 1 year (hazard ratio 1.10; P = .026), the authors reported.
But, after adjustment for ICD and LVAD use, the gender gap was eliminated (HR 1.06; P = .2).
Dr. Morris said that a more aggressive use of these life-saving therapies can eliminate wait-list disparities. The findings also point to the importance of educating community physicians and heart failure patients about the current standards of care, she said.
Changes in the allocation algorithm and improvements in LVAD technology have led to advancements in management of patients on heart transplant waiting lists, the authors said. Between 2001 and 2011, wait list mortality declined from 17 deaths per 100 wait-list years, to 12 deaths. During the same period, wait-list death among patients with an LVAD declined from 102 deaths per 100 wait-list years to 13.
Dr. Morris had no financial relationships to disclose.
On Twitter @NaseemSMiller
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Major finding: Female gender was associated with a 10% increased risk of being removed from the waiting list due to death or being too sick during the first year. When ICD was added to the analysis, risk was attenuated to 8%, but adding ICDs and LVADs eliminated the risk associated with the female gender.
Data source: Analysis of Organ Procurement and Transplantation Network database during 2000-2012.
Disclosures: Dr. Morris had no financial relationships to disclose.
Remote monitoring reduced death in advanced heart failure
AMSTERDAM – An implant-based remote monitoring system in patients with advanced heart failure significantly reduced the worsening of their condition, overnight hospitalization, and total mortality, according to a trial conducted by the device-maker.
The IN-TIME study, a multicenter, prospective, randomized, controlled trial, showed that the mortality at 1 year follow-up was 3.4% in the home monitoring group, compared with 8.7% in patients with standard care. This is the first time a home monitoring trial has shown a significant drop in death rate.
"IN-TIME is really a timely study," said Dr. Angelo Auricchio of Lugano, Switzerland, because the study coincides with the most recent ESC guidelines for CRT and pacemakers, which for the very first time contain recommendation for remote monitoring. Dr. Auricchio was one of the guideline’s authors.
Rehospitalization or death resulting from worsening heart failure are often preceded by clinical events or specific trends in clinical parameters. Use of home monitoring can detect some of these events and trends early, "offering the possibility to intervene and prevent hospitalization for worsening heart failure," said Dr. Gerhard Hindricks, the lead investigator of the trial, who presented the results at the annual congress of the European Society of Cardiology.
Findings from other trials, including TRUST and REFORM have hinted at the benefits of remote monitoring, but they didn’t show the significant results seen with IN-TIME.
Patients received Biotronik’s ICD or CRT-D equipped with home monitoring software. The device detects biological signals, such as heart rate, occurrence of arrhythmias, onset of arrhythmia or shocks, and transmits the signals to a small external patient communicator called Cardio Messenger, which in turn sends the signals to a centralized monitoring unit in Heart Center Leipzig, Germany.
"Trained nursing staff took the incoming data, and then activated a chain of action according to prespecified workflows,\" said Dr. Hindricks of University of Leipzig. In some cases, if the physician was not available, the providers would directly contact the patient to advise a follow-up with a general practitioner or appropriate clinic. The physician would also enter the information about the measure taken into the centralized monitoring system.
Researchers randomized 664 patients – 1:1, with no crossover – to either home monitoring (333) or standard of care (331). All patients were receiving optimal medical therapy.
The only significant difference in the use of ACE inhibitors between home monitoring and control groups (92% v. 86%, respectively), said Dr. Hindricks.
Patients were on average 65 years old and the majority was male. On average, 60% had an implanted CRT-D, and 40%, an ICD. Follow-up was 1 year.
The primary endpoint was the modified Packer Score, and 19% of the patients in the home monitoring group suffered from a worsening of their condition, compared with close to 28% in the group who were receiving the current standard of care only (P less than .05).
The secondary endpoint of all-cause mortality, also showed highly significant reduction in the home monitoring group (10 deaths), compared with the control arm (27 deaths) (P = .004). And, while 80% of all deaths were due to cardiovascular causes, there was a significant reduction in the cardiovascular mortality among the home monitoring group (8 deaths vs. 21 deaths in control; P = .012).
The home monitoring transmission rate was 85%, and there were 1,311 home monitoring observations, 66% of which were related to home monitoring issues; for instance, the patient’s device hadn’t transmitted data for 3 consecutive days, triggering an alert. The rest resulted from clinical events, with atrial fibrillation events being the most common (8.5%).
A total of 696 of patient contacts were made as a result of home monitoring alerts. Preliminary analysis of the data showed that 13% were from drug incompliance and 16% triggered additional visit to physician.
Dr. Hindricks said that one of the keys to the success of the program was minimal patient involvement.
Dr. Auricchio said that IN-TIME was a very important study "but we should have a cautionary note about the fact that the technology used is quite proprietary to one company, so the question is if it applies to other providers." He added that the study was done in a very defined patient population, and it’s yet to be seen whether its findings apply to other heart failure patients.
Dr. Hindricks has received honoraria for lectures and has been an adviser/consultant for Biosense, Biotronik, Stereotaxis, and St. Jude Medical. He has also been an adviser/consultant for CyberHeart. Dr. Auricchio has received payments from Biotronik, St. Jude Medical, Medtronic, Abbott, Philips, and several other companies.
On Twitter @NaseemSMiller
AMSTERDAM – An implant-based remote monitoring system in patients with advanced heart failure significantly reduced the worsening of their condition, overnight hospitalization, and total mortality, according to a trial conducted by the device-maker.
The IN-TIME study, a multicenter, prospective, randomized, controlled trial, showed that the mortality at 1 year follow-up was 3.4% in the home monitoring group, compared with 8.7% in patients with standard care. This is the first time a home monitoring trial has shown a significant drop in death rate.
"IN-TIME is really a timely study," said Dr. Angelo Auricchio of Lugano, Switzerland, because the study coincides with the most recent ESC guidelines for CRT and pacemakers, which for the very first time contain recommendation for remote monitoring. Dr. Auricchio was one of the guideline’s authors.
Rehospitalization or death resulting from worsening heart failure are often preceded by clinical events or specific trends in clinical parameters. Use of home monitoring can detect some of these events and trends early, "offering the possibility to intervene and prevent hospitalization for worsening heart failure," said Dr. Gerhard Hindricks, the lead investigator of the trial, who presented the results at the annual congress of the European Society of Cardiology.
Findings from other trials, including TRUST and REFORM have hinted at the benefits of remote monitoring, but they didn’t show the significant results seen with IN-TIME.
Patients received Biotronik’s ICD or CRT-D equipped with home monitoring software. The device detects biological signals, such as heart rate, occurrence of arrhythmias, onset of arrhythmia or shocks, and transmits the signals to a small external patient communicator called Cardio Messenger, which in turn sends the signals to a centralized monitoring unit in Heart Center Leipzig, Germany.
"Trained nursing staff took the incoming data, and then activated a chain of action according to prespecified workflows,\" said Dr. Hindricks of University of Leipzig. In some cases, if the physician was not available, the providers would directly contact the patient to advise a follow-up with a general practitioner or appropriate clinic. The physician would also enter the information about the measure taken into the centralized monitoring system.
Researchers randomized 664 patients – 1:1, with no crossover – to either home monitoring (333) or standard of care (331). All patients were receiving optimal medical therapy.
The only significant difference in the use of ACE inhibitors between home monitoring and control groups (92% v. 86%, respectively), said Dr. Hindricks.
Patients were on average 65 years old and the majority was male. On average, 60% had an implanted CRT-D, and 40%, an ICD. Follow-up was 1 year.
The primary endpoint was the modified Packer Score, and 19% of the patients in the home monitoring group suffered from a worsening of their condition, compared with close to 28% in the group who were receiving the current standard of care only (P less than .05).
The secondary endpoint of all-cause mortality, also showed highly significant reduction in the home monitoring group (10 deaths), compared with the control arm (27 deaths) (P = .004). And, while 80% of all deaths were due to cardiovascular causes, there was a significant reduction in the cardiovascular mortality among the home monitoring group (8 deaths vs. 21 deaths in control; P = .012).
The home monitoring transmission rate was 85%, and there were 1,311 home monitoring observations, 66% of which were related to home monitoring issues; for instance, the patient’s device hadn’t transmitted data for 3 consecutive days, triggering an alert. The rest resulted from clinical events, with atrial fibrillation events being the most common (8.5%).
A total of 696 of patient contacts were made as a result of home monitoring alerts. Preliminary analysis of the data showed that 13% were from drug incompliance and 16% triggered additional visit to physician.
Dr. Hindricks said that one of the keys to the success of the program was minimal patient involvement.
Dr. Auricchio said that IN-TIME was a very important study "but we should have a cautionary note about the fact that the technology used is quite proprietary to one company, so the question is if it applies to other providers." He added that the study was done in a very defined patient population, and it’s yet to be seen whether its findings apply to other heart failure patients.
Dr. Hindricks has received honoraria for lectures and has been an adviser/consultant for Biosense, Biotronik, Stereotaxis, and St. Jude Medical. He has also been an adviser/consultant for CyberHeart. Dr. Auricchio has received payments from Biotronik, St. Jude Medical, Medtronic, Abbott, Philips, and several other companies.
On Twitter @NaseemSMiller
AMSTERDAM – An implant-based remote monitoring system in patients with advanced heart failure significantly reduced the worsening of their condition, overnight hospitalization, and total mortality, according to a trial conducted by the device-maker.
The IN-TIME study, a multicenter, prospective, randomized, controlled trial, showed that the mortality at 1 year follow-up was 3.4% in the home monitoring group, compared with 8.7% in patients with standard care. This is the first time a home monitoring trial has shown a significant drop in death rate.
"IN-TIME is really a timely study," said Dr. Angelo Auricchio of Lugano, Switzerland, because the study coincides with the most recent ESC guidelines for CRT and pacemakers, which for the very first time contain recommendation for remote monitoring. Dr. Auricchio was one of the guideline’s authors.
Rehospitalization or death resulting from worsening heart failure are often preceded by clinical events or specific trends in clinical parameters. Use of home monitoring can detect some of these events and trends early, "offering the possibility to intervene and prevent hospitalization for worsening heart failure," said Dr. Gerhard Hindricks, the lead investigator of the trial, who presented the results at the annual congress of the European Society of Cardiology.
Findings from other trials, including TRUST and REFORM have hinted at the benefits of remote monitoring, but they didn’t show the significant results seen with IN-TIME.
Patients received Biotronik’s ICD or CRT-D equipped with home monitoring software. The device detects biological signals, such as heart rate, occurrence of arrhythmias, onset of arrhythmia or shocks, and transmits the signals to a small external patient communicator called Cardio Messenger, which in turn sends the signals to a centralized monitoring unit in Heart Center Leipzig, Germany.
"Trained nursing staff took the incoming data, and then activated a chain of action according to prespecified workflows,\" said Dr. Hindricks of University of Leipzig. In some cases, if the physician was not available, the providers would directly contact the patient to advise a follow-up with a general practitioner or appropriate clinic. The physician would also enter the information about the measure taken into the centralized monitoring system.
Researchers randomized 664 patients – 1:1, with no crossover – to either home monitoring (333) or standard of care (331). All patients were receiving optimal medical therapy.
The only significant difference in the use of ACE inhibitors between home monitoring and control groups (92% v. 86%, respectively), said Dr. Hindricks.
Patients were on average 65 years old and the majority was male. On average, 60% had an implanted CRT-D, and 40%, an ICD. Follow-up was 1 year.
The primary endpoint was the modified Packer Score, and 19% of the patients in the home monitoring group suffered from a worsening of their condition, compared with close to 28% in the group who were receiving the current standard of care only (P less than .05).
The secondary endpoint of all-cause mortality, also showed highly significant reduction in the home monitoring group (10 deaths), compared with the control arm (27 deaths) (P = .004). And, while 80% of all deaths were due to cardiovascular causes, there was a significant reduction in the cardiovascular mortality among the home monitoring group (8 deaths vs. 21 deaths in control; P = .012).
The home monitoring transmission rate was 85%, and there were 1,311 home monitoring observations, 66% of which were related to home monitoring issues; for instance, the patient’s device hadn’t transmitted data for 3 consecutive days, triggering an alert. The rest resulted from clinical events, with atrial fibrillation events being the most common (8.5%).
A total of 696 of patient contacts were made as a result of home monitoring alerts. Preliminary analysis of the data showed that 13% were from drug incompliance and 16% triggered additional visit to physician.
Dr. Hindricks said that one of the keys to the success of the program was minimal patient involvement.
Dr. Auricchio said that IN-TIME was a very important study "but we should have a cautionary note about the fact that the technology used is quite proprietary to one company, so the question is if it applies to other providers." He added that the study was done in a very defined patient population, and it’s yet to be seen whether its findings apply to other heart failure patients.
Dr. Hindricks has received honoraria for lectures and has been an adviser/consultant for Biosense, Biotronik, Stereotaxis, and St. Jude Medical. He has also been an adviser/consultant for CyberHeart. Dr. Auricchio has received payments from Biotronik, St. Jude Medical, Medtronic, Abbott, Philips, and several other companies.
On Twitter @NaseemSMiller
AT THE ESC CONGRESS 2013
Major finding: At 1-year, the mortality of home monitoring group was 3.4%, compared with 8.7% in patients receiving standard care (P = .004).
Data source: A prospective randomized, controlled trial of nearly 700 patients.
Disclosures: Dr. Hindricks has received honoraria for lectures and has been an adviser/consultant for Biosense, Biotronik, Stereotaxis, and St. Jude Medical. He has also been an adviser/consultant for CyberHeart. Dr. Auricchio has received payments from Biotronik, St. Jude Medical, Medtronic, Abbott, Philips, and several other companies.
Macitentan approved for pulmonary arterial hypertension
Another endothelin receptor blocker, macitentan, has been approved for treating pulmonary arterial hypertension, the Food and Drug Administration announced on Oct. 18.
In a study of 742 patients with pulmonary arterial hypertension (PAH), macitentan over an average of 2 years was "effective in delaying disease progression, a finding that included a decline in exercise ability, worsening symptoms of PAH or need for additional PAH medication," according to the FDA statement issued on Oct. 18. Anemia, nasopharyngitis, sore throat, bronchitis, headache, and urinary tract infection were among the common side effects associated with treatment, the statement said.
It will be marketed as Opsumit by Actelion Pharmaceuticals US. The approved indication is for the treatment of PAH, WHO Group I, to delay disease progression, which includes death, initiation of intravenous or subcutaneous prostanoids, or clinical worsening of PAH (decreased 6-minute walk distance, worsened PAH symptoms and need for additional PAH treatment, according to Actelion). The approved dose is 10 mg daily; it is an oral medication.
Like the other endothelin receptor blockers, macitentan’s label includes a boxed warning that it is a teratogen and should not be used in pregnant women, and that women can receive the drug only through a REMS (Risk Evaluation and Mitigation Strategy) program that will restrict the drug’s distribution. Under the Opsumit REMS, distribution of the drug will be restricted and prescribers will have to enroll in the REMS and become certified to prescribe the drug. Female patients will also need to be enrolled and must comply with pregnancy testing and contraception requirements before starting treatment. Pharmacies that dispense the drug will need to be certified and will dispense the drug only to authorized patients, the FDA said.
The study of 742 patients was SERAPHIN (Study With an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome), which compared treatment with 3 mg or 10 mg of macitentan once a day, or placebo, and were allowed to be treated with phosphodiesterase-5 inhibitors or oral or inhaled prostanoids, according to Actelion.
The primary end point, a composite of a PAH event or death from any cause, was reached by 38% of patients receiving 3-mg macitentan and 31% of those receiving 10-mg macitentan, compared with 46% of patients receiving placebo. The hazard ratios of 0.7 for the 3-mg dose and 0.55 for the 10-mg dose were statistically significant.
Macitentan is under review in Europe, Canada, Switzerland, Australia, Taiwan, Korea and Mexico, according to Actelion.
Actelion also markets bosentan (Tracleer), another endothelin receptor antagonist, which was approved for treating PAH in 2001. Ambrisentan (Letairis; Gilead), also an endothelin receptor antagonist, was approved in 2007.
Another endothelin receptor blocker, macitentan, has been approved for treating pulmonary arterial hypertension, the Food and Drug Administration announced on Oct. 18.
In a study of 742 patients with pulmonary arterial hypertension (PAH), macitentan over an average of 2 years was "effective in delaying disease progression, a finding that included a decline in exercise ability, worsening symptoms of PAH or need for additional PAH medication," according to the FDA statement issued on Oct. 18. Anemia, nasopharyngitis, sore throat, bronchitis, headache, and urinary tract infection were among the common side effects associated with treatment, the statement said.
It will be marketed as Opsumit by Actelion Pharmaceuticals US. The approved indication is for the treatment of PAH, WHO Group I, to delay disease progression, which includes death, initiation of intravenous or subcutaneous prostanoids, or clinical worsening of PAH (decreased 6-minute walk distance, worsened PAH symptoms and need for additional PAH treatment, according to Actelion). The approved dose is 10 mg daily; it is an oral medication.
Like the other endothelin receptor blockers, macitentan’s label includes a boxed warning that it is a teratogen and should not be used in pregnant women, and that women can receive the drug only through a REMS (Risk Evaluation and Mitigation Strategy) program that will restrict the drug’s distribution. Under the Opsumit REMS, distribution of the drug will be restricted and prescribers will have to enroll in the REMS and become certified to prescribe the drug. Female patients will also need to be enrolled and must comply with pregnancy testing and contraception requirements before starting treatment. Pharmacies that dispense the drug will need to be certified and will dispense the drug only to authorized patients, the FDA said.
The study of 742 patients was SERAPHIN (Study With an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome), which compared treatment with 3 mg or 10 mg of macitentan once a day, or placebo, and were allowed to be treated with phosphodiesterase-5 inhibitors or oral or inhaled prostanoids, according to Actelion.
The primary end point, a composite of a PAH event or death from any cause, was reached by 38% of patients receiving 3-mg macitentan and 31% of those receiving 10-mg macitentan, compared with 46% of patients receiving placebo. The hazard ratios of 0.7 for the 3-mg dose and 0.55 for the 10-mg dose were statistically significant.
Macitentan is under review in Europe, Canada, Switzerland, Australia, Taiwan, Korea and Mexico, according to Actelion.
Actelion also markets bosentan (Tracleer), another endothelin receptor antagonist, which was approved for treating PAH in 2001. Ambrisentan (Letairis; Gilead), also an endothelin receptor antagonist, was approved in 2007.
Another endothelin receptor blocker, macitentan, has been approved for treating pulmonary arterial hypertension, the Food and Drug Administration announced on Oct. 18.
In a study of 742 patients with pulmonary arterial hypertension (PAH), macitentan over an average of 2 years was "effective in delaying disease progression, a finding that included a decline in exercise ability, worsening symptoms of PAH or need for additional PAH medication," according to the FDA statement issued on Oct. 18. Anemia, nasopharyngitis, sore throat, bronchitis, headache, and urinary tract infection were among the common side effects associated with treatment, the statement said.
It will be marketed as Opsumit by Actelion Pharmaceuticals US. The approved indication is for the treatment of PAH, WHO Group I, to delay disease progression, which includes death, initiation of intravenous or subcutaneous prostanoids, or clinical worsening of PAH (decreased 6-minute walk distance, worsened PAH symptoms and need for additional PAH treatment, according to Actelion). The approved dose is 10 mg daily; it is an oral medication.
Like the other endothelin receptor blockers, macitentan’s label includes a boxed warning that it is a teratogen and should not be used in pregnant women, and that women can receive the drug only through a REMS (Risk Evaluation and Mitigation Strategy) program that will restrict the drug’s distribution. Under the Opsumit REMS, distribution of the drug will be restricted and prescribers will have to enroll in the REMS and become certified to prescribe the drug. Female patients will also need to be enrolled and must comply with pregnancy testing and contraception requirements before starting treatment. Pharmacies that dispense the drug will need to be certified and will dispense the drug only to authorized patients, the FDA said.
The study of 742 patients was SERAPHIN (Study With an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome), which compared treatment with 3 mg or 10 mg of macitentan once a day, or placebo, and were allowed to be treated with phosphodiesterase-5 inhibitors or oral or inhaled prostanoids, according to Actelion.
The primary end point, a composite of a PAH event or death from any cause, was reached by 38% of patients receiving 3-mg macitentan and 31% of those receiving 10-mg macitentan, compared with 46% of patients receiving placebo. The hazard ratios of 0.7 for the 3-mg dose and 0.55 for the 10-mg dose were statistically significant.
Macitentan is under review in Europe, Canada, Switzerland, Australia, Taiwan, Korea and Mexico, according to Actelion.
Actelion also markets bosentan (Tracleer), another endothelin receptor antagonist, which was approved for treating PAH in 2001. Ambrisentan (Letairis; Gilead), also an endothelin receptor antagonist, was approved in 2007.
Bariatric surgery may prevent heart failure
AMSTERDAM – New findings from the landmark Swedish Obese Subjects study "strongly suggest" that bariatric surgery reduces by roughly half the long-term risk of developing heart failure, Dr. Kristjan Karason reported at the annual congress of the European Society of Cardiology.
"This is the first study to look at the effect of bariatric surgery on heart failure risk," observed Dr. Karason of the University of Gothenburg (Sweden). "Our findings support a modulating role of excess body fat in the pathogenesis of heart failure."
The SOS (Swedish Obese Subjects) study has been a major driver of the growing enthusiasm for bariatric surgery, not only as a means of achieving sustained weight loss far beyond what can typically be accomplished medically, but also as a means of reducing obese patients’ elevated risks for a variety of serious chronic comorbid diseases.
For example, the SOS investigators have previously reported that bariatric surgery reduced the long-term risk of developing type 2 diabetes by 87% compared to matched obese controls who didn’t undergo weight-loss surgery (N. Engl. J. Med. 2012;367:695-704), that it reduced the risk of fatal or nonfatal acute MI or stroke by one-third during a median 14.7 years of follow-up (JAMA 2012;307:56-65), and it resulted in a 42% decrease in cancer incidence in women (Lancet Oncol. 2009;10:653-62).
The SOS is a nonrandomized, prospective, observational study involving 2,010 obese subjects who underwent bariatric surgery in 1987-2001, when they were 37-60 years old. A total of 68% of the bariatric surgery recipients had vertical band gastroplasty, 19% underwent gastric banding, and 13% had a Roux en-Y gastric bypass. They were extensively matched by 18 variables to 2,037 obese controls. The SOS study is being conducted at 25 surgical departments and 480 primary care clinics across Sweden. Follow-up is ongoing.
It has been known for more than a decade that increased body mass index is associated with greater risk of developing heart failure. The mechanism involved is not well defined but is probably multifactorial. Obesity imposes a greater hemodynamic load on the heart, both preload and afterload, with resultant left ventricular hypertrophy and diastolic dysfunction. Obesity is also associated with higher levels of cardiovascular risk factors and an increased risk of atrial fibrillation, Dr. Karason noted.
Mean weight loss after a median of 14.7 years of prospective follow-up in the SOS study was 18% in the bariatric surgery group and 1% in controls. During follow-up, 91 bariatric surgery patients and 152 controls were diagnosed with heart failure, for an incidence rate of 3.1 as compared with 5.2 cases per 1,000 person-years in the control subjects.
This translated to a 48% relative risk reduction in new-onset heart failure in a multivariate regression analysis adjusted for age, sex, baseline body mass index, waist circumference, blood glucose, lipids, prior cardiovascular disease, and smoking status.
One audience member asked how to interpret the new SOS findings in light of the heart failure obesity paradox, which is the observation in multiple studies that overweight and obese heart failure patients tends to fare better than leaner ones.
"There have been no studies of weight loss in obese patients with heart failure. There really should be," Dr. Karason replied. "But my feeling is that they would reduce their risk because they will improve several risk factors, and their hemodynamic situation is also improved. So my recommendation would be for those heart failure patients to lose weight. I don’t have any studies to support that."
The SOS study is funded by the Swedish Research Council. Dr. Karason reported having no germane financial interests.
AMSTERDAM – New findings from the landmark Swedish Obese Subjects study "strongly suggest" that bariatric surgery reduces by roughly half the long-term risk of developing heart failure, Dr. Kristjan Karason reported at the annual congress of the European Society of Cardiology.
"This is the first study to look at the effect of bariatric surgery on heart failure risk," observed Dr. Karason of the University of Gothenburg (Sweden). "Our findings support a modulating role of excess body fat in the pathogenesis of heart failure."
The SOS (Swedish Obese Subjects) study has been a major driver of the growing enthusiasm for bariatric surgery, not only as a means of achieving sustained weight loss far beyond what can typically be accomplished medically, but also as a means of reducing obese patients’ elevated risks for a variety of serious chronic comorbid diseases.
For example, the SOS investigators have previously reported that bariatric surgery reduced the long-term risk of developing type 2 diabetes by 87% compared to matched obese controls who didn’t undergo weight-loss surgery (N. Engl. J. Med. 2012;367:695-704), that it reduced the risk of fatal or nonfatal acute MI or stroke by one-third during a median 14.7 years of follow-up (JAMA 2012;307:56-65), and it resulted in a 42% decrease in cancer incidence in women (Lancet Oncol. 2009;10:653-62).
The SOS is a nonrandomized, prospective, observational study involving 2,010 obese subjects who underwent bariatric surgery in 1987-2001, when they were 37-60 years old. A total of 68% of the bariatric surgery recipients had vertical band gastroplasty, 19% underwent gastric banding, and 13% had a Roux en-Y gastric bypass. They were extensively matched by 18 variables to 2,037 obese controls. The SOS study is being conducted at 25 surgical departments and 480 primary care clinics across Sweden. Follow-up is ongoing.
It has been known for more than a decade that increased body mass index is associated with greater risk of developing heart failure. The mechanism involved is not well defined but is probably multifactorial. Obesity imposes a greater hemodynamic load on the heart, both preload and afterload, with resultant left ventricular hypertrophy and diastolic dysfunction. Obesity is also associated with higher levels of cardiovascular risk factors and an increased risk of atrial fibrillation, Dr. Karason noted.
Mean weight loss after a median of 14.7 years of prospective follow-up in the SOS study was 18% in the bariatric surgery group and 1% in controls. During follow-up, 91 bariatric surgery patients and 152 controls were diagnosed with heart failure, for an incidence rate of 3.1 as compared with 5.2 cases per 1,000 person-years in the control subjects.
This translated to a 48% relative risk reduction in new-onset heart failure in a multivariate regression analysis adjusted for age, sex, baseline body mass index, waist circumference, blood glucose, lipids, prior cardiovascular disease, and smoking status.
One audience member asked how to interpret the new SOS findings in light of the heart failure obesity paradox, which is the observation in multiple studies that overweight and obese heart failure patients tends to fare better than leaner ones.
"There have been no studies of weight loss in obese patients with heart failure. There really should be," Dr. Karason replied. "But my feeling is that they would reduce their risk because they will improve several risk factors, and their hemodynamic situation is also improved. So my recommendation would be for those heart failure patients to lose weight. I don’t have any studies to support that."
The SOS study is funded by the Swedish Research Council. Dr. Karason reported having no germane financial interests.
AMSTERDAM – New findings from the landmark Swedish Obese Subjects study "strongly suggest" that bariatric surgery reduces by roughly half the long-term risk of developing heart failure, Dr. Kristjan Karason reported at the annual congress of the European Society of Cardiology.
"This is the first study to look at the effect of bariatric surgery on heart failure risk," observed Dr. Karason of the University of Gothenburg (Sweden). "Our findings support a modulating role of excess body fat in the pathogenesis of heart failure."
The SOS (Swedish Obese Subjects) study has been a major driver of the growing enthusiasm for bariatric surgery, not only as a means of achieving sustained weight loss far beyond what can typically be accomplished medically, but also as a means of reducing obese patients’ elevated risks for a variety of serious chronic comorbid diseases.
For example, the SOS investigators have previously reported that bariatric surgery reduced the long-term risk of developing type 2 diabetes by 87% compared to matched obese controls who didn’t undergo weight-loss surgery (N. Engl. J. Med. 2012;367:695-704), that it reduced the risk of fatal or nonfatal acute MI or stroke by one-third during a median 14.7 years of follow-up (JAMA 2012;307:56-65), and it resulted in a 42% decrease in cancer incidence in women (Lancet Oncol. 2009;10:653-62).
The SOS is a nonrandomized, prospective, observational study involving 2,010 obese subjects who underwent bariatric surgery in 1987-2001, when they were 37-60 years old. A total of 68% of the bariatric surgery recipients had vertical band gastroplasty, 19% underwent gastric banding, and 13% had a Roux en-Y gastric bypass. They were extensively matched by 18 variables to 2,037 obese controls. The SOS study is being conducted at 25 surgical departments and 480 primary care clinics across Sweden. Follow-up is ongoing.
It has been known for more than a decade that increased body mass index is associated with greater risk of developing heart failure. The mechanism involved is not well defined but is probably multifactorial. Obesity imposes a greater hemodynamic load on the heart, both preload and afterload, with resultant left ventricular hypertrophy and diastolic dysfunction. Obesity is also associated with higher levels of cardiovascular risk factors and an increased risk of atrial fibrillation, Dr. Karason noted.
Mean weight loss after a median of 14.7 years of prospective follow-up in the SOS study was 18% in the bariatric surgery group and 1% in controls. During follow-up, 91 bariatric surgery patients and 152 controls were diagnosed with heart failure, for an incidence rate of 3.1 as compared with 5.2 cases per 1,000 person-years in the control subjects.
This translated to a 48% relative risk reduction in new-onset heart failure in a multivariate regression analysis adjusted for age, sex, baseline body mass index, waist circumference, blood glucose, lipids, prior cardiovascular disease, and smoking status.
One audience member asked how to interpret the new SOS findings in light of the heart failure obesity paradox, which is the observation in multiple studies that overweight and obese heart failure patients tends to fare better than leaner ones.
"There have been no studies of weight loss in obese patients with heart failure. There really should be," Dr. Karason replied. "But my feeling is that they would reduce their risk because they will improve several risk factors, and their hemodynamic situation is also improved. So my recommendation would be for those heart failure patients to lose weight. I don’t have any studies to support that."
The SOS study is funded by the Swedish Research Council. Dr. Karason reported having no germane financial interests.
AT THE ESC CONGRESS 2013
Major finding: The incidence rate of heart failure during a median 15 years of prospective follow-up after bariatric surgery was 3.1 cases per 1,000 person-years, compared with 5.2/1,000 person-years in obese controls.
Data source: The Swedish Obese Subjects study included 2,010 obese subjects who underwent bariatric surgery in 1987-2001 and 2,037 closely matched obese controls. It is a nonrandomized, prospective, observational study.
Disclosures: The study is funded by the Swedish Research Council. The presenter reported having no financial conflicts.
Hunting for cardiovascular signals from diabetes drugs
Diabetes drug development entered a new era in 2007 when a meta-analysis of 42 studies that had compared rosiglitazone with other drugs in a total of nearly 28,000 patients suggested that rosiglitazone treatment led to significantly increased rates of cardiovascular death and myocardial infarction.
Based largely on that, the Food and Drug Administration in 2008 issued recommendations to companies developing new diabetes drugs to run large-scale trials aimed at assessing their cardiovascular effects.
In 2011, the FDA placed restrictions on prescribing rosiglitazone, and U.S. use of the drug plummeted. (An FDA panel voted in June to relax some of the restrictions the agency had imposed.)
Some consequences of the long shadow cast by the rosiglitazone experience played out in talks at the annual congress of the European Society for the Study of Diabetes in September, as well as at the annual congress of the European Society of Cardiology a few weeks before that. The new data show just how painstaking investigators have become in trying to parse out hints of cardiovascular danger lurking in masses of megatrial data.
Heart failure haunts DPP-4 inhibitors
At ESC, as well as in a pair of simultaneously published articles, researchers reported results from two large trials designed to follow the FDA recommendations and assess cardiovascular safety for two new selective dipeptidyl peptidase-4 (DPP-4) inhibitors, saxagliptin (Onglyza) and alogliptin, in patients with type 2 diabetes.
Saxagliptin was the drug with the red-flag signal, while alogliptin faced guilt by association. The saxagliptin study, SAVOR-TIMI 53, used patients with either an established history of cardiovascular disease (CVD) or multiple CVD risk factors. The study’s primary safety endpoint, the combined rate of CVD death, nonfatal myocardial infarction, or nonfatal stroke during 2 years of follow-up, was virtually identical in the two groups, 7.3% in the saxagliptin patients and 7.2% in the controls.
The study’s secondary safety endpoint, which included the primary combined plus other endpoints such as need for revascularization and hospitalization for heart failure, was also similar in the two study arms, with this expanded combined endpoint occurring in 12.8% of the saxagliptin patients and 12.4% of the controls (N. Engl. J. Med. 2013;369:1317-26).
The whisper of a saxagliptin problem was in just one secondary, individual safety endpoint, hospitalizations for heart failure. These occurred in 3.5% of the saxagliptin patients and in 2.8% of controls, a statistically significant difference. Further data presented at EASD showed that this difference clustered predominantly in the subgroup of patients who entered the study with presumed heart failure, based on having a blood level of NT-pro brain natriuretic peptide of at least 333, about 25% of patients in both treatment arms (although the investigators identified heart failure in only 13% of enrolled patients). Within this subgroup, heart failure–associated hospitalizations occurred in 10.9% of the saxagliptin patients and 8.9% of the controls, a statistically significant difference, reported Dr. Deepak L. Bhatt, one of the investigators for the SAVOR-TIMI 53 study, which randomized more than 16,000 patients.
In contrast, the alogliptin study, EXAMINE, which randomized more than 5,000 patients with type 2 diabetes and a recent acute coronary syndrome event to treatment with alogliptin or placebo, found no real confirmation of this problem (N. Engl. J. Med. 2013;369:1327-35).
After the early-September report of this saxagliptin and heart failure hospitalization signal, the EXAMINE researchers went back to their data to specifically look at this issue, Dr. William B. White, the lead investigator, said when he presented the new findings at EASD. During a median of 18 months of follow-up, the rate of hospitalizations for heart failure was 3.1% in the alogliptin group and 2.9% in the placebo group, a difference that was not statistically significant, although it trended just slightly toward an alogliptin problem. Among the subgroup of patients who entered EXAMINE with a history of heart failure, the rate of the combined cardiovascular safety endpoint of CV death, nonfatal MI, or nonfatal stroke was 18% in the alogliptin group and 22% in the placebo group, a difference that was not statistically significant.
Despite the absence of any apparent heart failure–hospitalization effect in EXAMINE, Dr. Naveed Sattar, the invited discussant for the two reports at EASD, meta-analyzed the heart failure–hospitalization results from both studies, and found a combined, 24% relative increase in this outcome for the two DPP-4 inhibitors combined, a statistically significant effect largely driven toward significance by the underlying statistical significance in the saxagliptin study; the alogliptin results couldn’t resolve this difference since they trended in the same direction. Dr. Sattar concluded that it raised enough doubt about the safety of this entire drug class in patients with pre-existing heart failure to recommend not using drugs from this class in patients with any indication of heart failure – regardless of severity – until the apparent link received further scrutiny. Dr. Bhatt promised a report with further information on the heart failure issue at the American Heart Association Scientific Sessions in November.
Signals for canagliflozin, too?
SAVOR-TIMI 53 and EXAMINE provide the first results from what are more than a dozen large studies – involving more than 100,000 patients – launched recently to look for adverse cardiovascular effects from drugs in six different diabetes drug classes, according to Dr. Sanjay Kaul, who also spoke at EASD. He presented results from a meta-analysis he performed on results from nine phase III and phase II trials of canagliflozin (Invokana), an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class and approved by the FDA last March for treatment of type 2 diabetes, the first drug in that class to get U.S. marketing approval.
In the largest of these trials, CANVAS – the cardiovascular-assessment study for canagliflozin set up by the drug’s developer, Janssen – treatment with canagliflozin linked with a statistically significant, sixfold increase in cardiovascular events during the first 30 days of treatment, a link that then disappeared with longer-term treatment in patients with high CV risk. (Complete results from CANVAS have not yet been reported.) But Dr. Kaul did not see a similar excess in the other eight studies.
The canagliflozin meta-analysis also showed a nonsignificant, 46% increased risk of stroke in patients on the drug, a signal not seen in any other individual cardiovascular endpoint. Dr. Kaul analyzed data from 14 phase II and III trials for a second SGLT2 inhibitor drug, dapagliflozin, which showed no suggestion of causing any cardiovascular risk.
The canagliflozin risk signals are not reasons to stop using the drug or to withdraw it from the market, said Dr. Kaul, a member of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee. The early signal of risk in CANVAS was not seen in any other study, and it may have been a statistical artifact, he said.
He said that signals of risk must be considered relative to a drug’s benefit. Applying the same standard of cardiovascular harm to drugs that are highly effective and to drugs that are not very effective "doesn’t make sense," he said. "A risk-benefit trade-off depends on the benefit. If you have a diabetes drug that lowers hemoglobin A1c by 1.5%-2% I’m willing to accept greater harm than I would from a drug that produced a 0.5% drop in hemoglobin A1c."
A final rosiglitazone thought
Dr. Kaul also made this observation about the rosiglitazone experience, which in retrospect produced "insufficient evidence to either incriminate or exonerate rosiglitazone," he said.
"That a diabetes drug [rosiglitazone] could have become a blockbuster without any established outcome benefits does not reflect well on the drug development and approval process. Equally lamentable is that this drug was virtually killed based on insufficient evidence and entrenched opinions."
Dr. Bhatt has received research grants from several companies, including AstraZeneca and Bristol-Myers Squibb (BMS), which market saxagliptin, and Takeda, maker of alogliptin. Dr. White is a safety consultant for several companies, including AstraZeneca and Takeda. Dr. Sattar is a consultant to several pharmaceutical firms, including AstraZeneca and BMS. Dr. Kaul is a consultant to Novo Nordisk, Sanofi, and Boehringer Ingelheim, and owns stock in Johnson & Johnson.
On Twitter @mitchelzoler
Diabetes drug development entered a new era in 2007 when a meta-analysis of 42 studies that had compared rosiglitazone with other drugs in a total of nearly 28,000 patients suggested that rosiglitazone treatment led to significantly increased rates of cardiovascular death and myocardial infarction.
Based largely on that, the Food and Drug Administration in 2008 issued recommendations to companies developing new diabetes drugs to run large-scale trials aimed at assessing their cardiovascular effects.
In 2011, the FDA placed restrictions on prescribing rosiglitazone, and U.S. use of the drug plummeted. (An FDA panel voted in June to relax some of the restrictions the agency had imposed.)
Some consequences of the long shadow cast by the rosiglitazone experience played out in talks at the annual congress of the European Society for the Study of Diabetes in September, as well as at the annual congress of the European Society of Cardiology a few weeks before that. The new data show just how painstaking investigators have become in trying to parse out hints of cardiovascular danger lurking in masses of megatrial data.
Heart failure haunts DPP-4 inhibitors
At ESC, as well as in a pair of simultaneously published articles, researchers reported results from two large trials designed to follow the FDA recommendations and assess cardiovascular safety for two new selective dipeptidyl peptidase-4 (DPP-4) inhibitors, saxagliptin (Onglyza) and alogliptin, in patients with type 2 diabetes.
Saxagliptin was the drug with the red-flag signal, while alogliptin faced guilt by association. The saxagliptin study, SAVOR-TIMI 53, used patients with either an established history of cardiovascular disease (CVD) or multiple CVD risk factors. The study’s primary safety endpoint, the combined rate of CVD death, nonfatal myocardial infarction, or nonfatal stroke during 2 years of follow-up, was virtually identical in the two groups, 7.3% in the saxagliptin patients and 7.2% in the controls.
The study’s secondary safety endpoint, which included the primary combined plus other endpoints such as need for revascularization and hospitalization for heart failure, was also similar in the two study arms, with this expanded combined endpoint occurring in 12.8% of the saxagliptin patients and 12.4% of the controls (N. Engl. J. Med. 2013;369:1317-26).
The whisper of a saxagliptin problem was in just one secondary, individual safety endpoint, hospitalizations for heart failure. These occurred in 3.5% of the saxagliptin patients and in 2.8% of controls, a statistically significant difference. Further data presented at EASD showed that this difference clustered predominantly in the subgroup of patients who entered the study with presumed heart failure, based on having a blood level of NT-pro brain natriuretic peptide of at least 333, about 25% of patients in both treatment arms (although the investigators identified heart failure in only 13% of enrolled patients). Within this subgroup, heart failure–associated hospitalizations occurred in 10.9% of the saxagliptin patients and 8.9% of the controls, a statistically significant difference, reported Dr. Deepak L. Bhatt, one of the investigators for the SAVOR-TIMI 53 study, which randomized more than 16,000 patients.
In contrast, the alogliptin study, EXAMINE, which randomized more than 5,000 patients with type 2 diabetes and a recent acute coronary syndrome event to treatment with alogliptin or placebo, found no real confirmation of this problem (N. Engl. J. Med. 2013;369:1327-35).
After the early-September report of this saxagliptin and heart failure hospitalization signal, the EXAMINE researchers went back to their data to specifically look at this issue, Dr. William B. White, the lead investigator, said when he presented the new findings at EASD. During a median of 18 months of follow-up, the rate of hospitalizations for heart failure was 3.1% in the alogliptin group and 2.9% in the placebo group, a difference that was not statistically significant, although it trended just slightly toward an alogliptin problem. Among the subgroup of patients who entered EXAMINE with a history of heart failure, the rate of the combined cardiovascular safety endpoint of CV death, nonfatal MI, or nonfatal stroke was 18% in the alogliptin group and 22% in the placebo group, a difference that was not statistically significant.
Despite the absence of any apparent heart failure–hospitalization effect in EXAMINE, Dr. Naveed Sattar, the invited discussant for the two reports at EASD, meta-analyzed the heart failure–hospitalization results from both studies, and found a combined, 24% relative increase in this outcome for the two DPP-4 inhibitors combined, a statistically significant effect largely driven toward significance by the underlying statistical significance in the saxagliptin study; the alogliptin results couldn’t resolve this difference since they trended in the same direction. Dr. Sattar concluded that it raised enough doubt about the safety of this entire drug class in patients with pre-existing heart failure to recommend not using drugs from this class in patients with any indication of heart failure – regardless of severity – until the apparent link received further scrutiny. Dr. Bhatt promised a report with further information on the heart failure issue at the American Heart Association Scientific Sessions in November.
Signals for canagliflozin, too?
SAVOR-TIMI 53 and EXAMINE provide the first results from what are more than a dozen large studies – involving more than 100,000 patients – launched recently to look for adverse cardiovascular effects from drugs in six different diabetes drug classes, according to Dr. Sanjay Kaul, who also spoke at EASD. He presented results from a meta-analysis he performed on results from nine phase III and phase II trials of canagliflozin (Invokana), an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class and approved by the FDA last March for treatment of type 2 diabetes, the first drug in that class to get U.S. marketing approval.
In the largest of these trials, CANVAS – the cardiovascular-assessment study for canagliflozin set up by the drug’s developer, Janssen – treatment with canagliflozin linked with a statistically significant, sixfold increase in cardiovascular events during the first 30 days of treatment, a link that then disappeared with longer-term treatment in patients with high CV risk. (Complete results from CANVAS have not yet been reported.) But Dr. Kaul did not see a similar excess in the other eight studies.
The canagliflozin meta-analysis also showed a nonsignificant, 46% increased risk of stroke in patients on the drug, a signal not seen in any other individual cardiovascular endpoint. Dr. Kaul analyzed data from 14 phase II and III trials for a second SGLT2 inhibitor drug, dapagliflozin, which showed no suggestion of causing any cardiovascular risk.
The canagliflozin risk signals are not reasons to stop using the drug or to withdraw it from the market, said Dr. Kaul, a member of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee. The early signal of risk in CANVAS was not seen in any other study, and it may have been a statistical artifact, he said.
He said that signals of risk must be considered relative to a drug’s benefit. Applying the same standard of cardiovascular harm to drugs that are highly effective and to drugs that are not very effective "doesn’t make sense," he said. "A risk-benefit trade-off depends on the benefit. If you have a diabetes drug that lowers hemoglobin A1c by 1.5%-2% I’m willing to accept greater harm than I would from a drug that produced a 0.5% drop in hemoglobin A1c."
A final rosiglitazone thought
Dr. Kaul also made this observation about the rosiglitazone experience, which in retrospect produced "insufficient evidence to either incriminate or exonerate rosiglitazone," he said.
"That a diabetes drug [rosiglitazone] could have become a blockbuster without any established outcome benefits does not reflect well on the drug development and approval process. Equally lamentable is that this drug was virtually killed based on insufficient evidence and entrenched opinions."
Dr. Bhatt has received research grants from several companies, including AstraZeneca and Bristol-Myers Squibb (BMS), which market saxagliptin, and Takeda, maker of alogliptin. Dr. White is a safety consultant for several companies, including AstraZeneca and Takeda. Dr. Sattar is a consultant to several pharmaceutical firms, including AstraZeneca and BMS. Dr. Kaul is a consultant to Novo Nordisk, Sanofi, and Boehringer Ingelheim, and owns stock in Johnson & Johnson.
On Twitter @mitchelzoler
Diabetes drug development entered a new era in 2007 when a meta-analysis of 42 studies that had compared rosiglitazone with other drugs in a total of nearly 28,000 patients suggested that rosiglitazone treatment led to significantly increased rates of cardiovascular death and myocardial infarction.
Based largely on that, the Food and Drug Administration in 2008 issued recommendations to companies developing new diabetes drugs to run large-scale trials aimed at assessing their cardiovascular effects.
In 2011, the FDA placed restrictions on prescribing rosiglitazone, and U.S. use of the drug plummeted. (An FDA panel voted in June to relax some of the restrictions the agency had imposed.)
Some consequences of the long shadow cast by the rosiglitazone experience played out in talks at the annual congress of the European Society for the Study of Diabetes in September, as well as at the annual congress of the European Society of Cardiology a few weeks before that. The new data show just how painstaking investigators have become in trying to parse out hints of cardiovascular danger lurking in masses of megatrial data.
Heart failure haunts DPP-4 inhibitors
At ESC, as well as in a pair of simultaneously published articles, researchers reported results from two large trials designed to follow the FDA recommendations and assess cardiovascular safety for two new selective dipeptidyl peptidase-4 (DPP-4) inhibitors, saxagliptin (Onglyza) and alogliptin, in patients with type 2 diabetes.
Saxagliptin was the drug with the red-flag signal, while alogliptin faced guilt by association. The saxagliptin study, SAVOR-TIMI 53, used patients with either an established history of cardiovascular disease (CVD) or multiple CVD risk factors. The study’s primary safety endpoint, the combined rate of CVD death, nonfatal myocardial infarction, or nonfatal stroke during 2 years of follow-up, was virtually identical in the two groups, 7.3% in the saxagliptin patients and 7.2% in the controls.
The study’s secondary safety endpoint, which included the primary combined plus other endpoints such as need for revascularization and hospitalization for heart failure, was also similar in the two study arms, with this expanded combined endpoint occurring in 12.8% of the saxagliptin patients and 12.4% of the controls (N. Engl. J. Med. 2013;369:1317-26).
The whisper of a saxagliptin problem was in just one secondary, individual safety endpoint, hospitalizations for heart failure. These occurred in 3.5% of the saxagliptin patients and in 2.8% of controls, a statistically significant difference. Further data presented at EASD showed that this difference clustered predominantly in the subgroup of patients who entered the study with presumed heart failure, based on having a blood level of NT-pro brain natriuretic peptide of at least 333, about 25% of patients in both treatment arms (although the investigators identified heart failure in only 13% of enrolled patients). Within this subgroup, heart failure–associated hospitalizations occurred in 10.9% of the saxagliptin patients and 8.9% of the controls, a statistically significant difference, reported Dr. Deepak L. Bhatt, one of the investigators for the SAVOR-TIMI 53 study, which randomized more than 16,000 patients.
In contrast, the alogliptin study, EXAMINE, which randomized more than 5,000 patients with type 2 diabetes and a recent acute coronary syndrome event to treatment with alogliptin or placebo, found no real confirmation of this problem (N. Engl. J. Med. 2013;369:1327-35).
After the early-September report of this saxagliptin and heart failure hospitalization signal, the EXAMINE researchers went back to their data to specifically look at this issue, Dr. William B. White, the lead investigator, said when he presented the new findings at EASD. During a median of 18 months of follow-up, the rate of hospitalizations for heart failure was 3.1% in the alogliptin group and 2.9% in the placebo group, a difference that was not statistically significant, although it trended just slightly toward an alogliptin problem. Among the subgroup of patients who entered EXAMINE with a history of heart failure, the rate of the combined cardiovascular safety endpoint of CV death, nonfatal MI, or nonfatal stroke was 18% in the alogliptin group and 22% in the placebo group, a difference that was not statistically significant.
Despite the absence of any apparent heart failure–hospitalization effect in EXAMINE, Dr. Naveed Sattar, the invited discussant for the two reports at EASD, meta-analyzed the heart failure–hospitalization results from both studies, and found a combined, 24% relative increase in this outcome for the two DPP-4 inhibitors combined, a statistically significant effect largely driven toward significance by the underlying statistical significance in the saxagliptin study; the alogliptin results couldn’t resolve this difference since they trended in the same direction. Dr. Sattar concluded that it raised enough doubt about the safety of this entire drug class in patients with pre-existing heart failure to recommend not using drugs from this class in patients with any indication of heart failure – regardless of severity – until the apparent link received further scrutiny. Dr. Bhatt promised a report with further information on the heart failure issue at the American Heart Association Scientific Sessions in November.
Signals for canagliflozin, too?
SAVOR-TIMI 53 and EXAMINE provide the first results from what are more than a dozen large studies – involving more than 100,000 patients – launched recently to look for adverse cardiovascular effects from drugs in six different diabetes drug classes, according to Dr. Sanjay Kaul, who also spoke at EASD. He presented results from a meta-analysis he performed on results from nine phase III and phase II trials of canagliflozin (Invokana), an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class and approved by the FDA last March for treatment of type 2 diabetes, the first drug in that class to get U.S. marketing approval.
In the largest of these trials, CANVAS – the cardiovascular-assessment study for canagliflozin set up by the drug’s developer, Janssen – treatment with canagliflozin linked with a statistically significant, sixfold increase in cardiovascular events during the first 30 days of treatment, a link that then disappeared with longer-term treatment in patients with high CV risk. (Complete results from CANVAS have not yet been reported.) But Dr. Kaul did not see a similar excess in the other eight studies.
The canagliflozin meta-analysis also showed a nonsignificant, 46% increased risk of stroke in patients on the drug, a signal not seen in any other individual cardiovascular endpoint. Dr. Kaul analyzed data from 14 phase II and III trials for a second SGLT2 inhibitor drug, dapagliflozin, which showed no suggestion of causing any cardiovascular risk.
The canagliflozin risk signals are not reasons to stop using the drug or to withdraw it from the market, said Dr. Kaul, a member of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee. The early signal of risk in CANVAS was not seen in any other study, and it may have been a statistical artifact, he said.
He said that signals of risk must be considered relative to a drug’s benefit. Applying the same standard of cardiovascular harm to drugs that are highly effective and to drugs that are not very effective "doesn’t make sense," he said. "A risk-benefit trade-off depends on the benefit. If you have a diabetes drug that lowers hemoglobin A1c by 1.5%-2% I’m willing to accept greater harm than I would from a drug that produced a 0.5% drop in hemoglobin A1c."
A final rosiglitazone thought
Dr. Kaul also made this observation about the rosiglitazone experience, which in retrospect produced "insufficient evidence to either incriminate or exonerate rosiglitazone," he said.
"That a diabetes drug [rosiglitazone] could have become a blockbuster without any established outcome benefits does not reflect well on the drug development and approval process. Equally lamentable is that this drug was virtually killed based on insufficient evidence and entrenched opinions."
Dr. Bhatt has received research grants from several companies, including AstraZeneca and Bristol-Myers Squibb (BMS), which market saxagliptin, and Takeda, maker of alogliptin. Dr. White is a safety consultant for several companies, including AstraZeneca and Takeda. Dr. Sattar is a consultant to several pharmaceutical firms, including AstraZeneca and BMS. Dr. Kaul is a consultant to Novo Nordisk, Sanofi, and Boehringer Ingelheim, and owns stock in Johnson & Johnson.
On Twitter @mitchelzoler