User login
FDA approves Sapien 3 transcatheter valve for bioprosthetic valve failure
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
A prescription for heart failure success: Change the name
PARIS – Does heart failure’s name doom any progress against the disease?
That was the provocative premise advanced by Lynne Warner Stevenson, MD, who suggested that efforts to prevent, diagnose, and treat the disease would go better if it could only jettison that unfortunate word “failure,” its hard-wired albatross.
Dr. Stevenson offered several potentially superior alternatives, including cardiac insufficiency, heart dysfunction, and her favorite, cardiomyopathy.
“Is heart failure still the best diagnosis” for the entire spectrum of disease that most patients progress through ,including the many patients in earlier stages of the disease who do not have a truly failing heart? “Perhaps cardiomyopathy is the condition and heart failure is the transition,” she proposed.
To Dr. Stevenson, it’s more than just semantics.
“Words are hugely powerful,” she explained in an interview following her talk. “I think patients do not want to be seen as having heart failure. They don’t want to think of themselves as having heart failure. I think it can make them delay getting care, and it makes them ignore the disease. I worry about that a lot. I also worry that patients don’t provide support to each other that they could. Patients tend to hide that they have heart failure. We need to come up with a term that does not make patients ashamed of their disease.”
Part of the problem, Dr. Stevenson said, is that the name heart failure can be very misleading depending on the stage of the disease that patients have. Patients with stage B (presymptomatic) disease and those with mild stage C disease “don’t see themselves as having heart failure,” as having a heart that has failed them. “We need to be able to convince these patients that they have a disease that we need to treat carefully and aggressively.”
Additionally, labeling tens of millions of people as having stage A heart failure, which is presymptomatic and occurs before the heart shows any sign of damage or dysfunction, is also counterproductive, maintained Dr. Stevenson, professor of medicine at Harvard Medical School and director of the Cardiomyopathy and Heart Failure Program at Brigham and Women’s Hospital in Boston.
“So many people are at risk of developing heart failure,” she noted, including patients with hypertension, diabetes, or coronary artery disease. To label them all as already also having heart failure at that stage “tends to make them ignore the disease that we are trying to get them to pay attention to. Telling patients they have the disease that we are trying to prevent doesn’t help.”
Calling the whole range of the disease heart failure also confuses patients and others. “Patients ask me, ‘How can I have heart failure without any symptoms?’ ‘My ejection fraction improved to almost normal; do I still have heart failure?’ and ‘I don’t understand how my heart muscle is strong but my heart is failing,’ ” she said
For Dr. Stevenson, perhaps the biggest problem is the stigma of failure and the way that word ties a huge weight to the disease that prompts patients and caregivers alike to relegate it to a hidden and neglected place.
“It’s failure. Who is proud to have heart failure? Where are the marches for heart failure? Where are the celebrity champions for heart failure? We have celebrities who are happy to admit that they have Parkinson’s disease, ALS [amyotrophic lateral sclerosis], drug addiction, and even erectile dysfunction, but no one wants to say they have heart failure. We can’t get any traction behind heart failure. It doesn’t sound very inspiring,” an issue that even percolates down to dissuading clinicians from pursuing a career in heart failure care. Young people do not aspire to go into failure, she said.
“We need to call it something else.”
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Does heart failure’s name doom any progress against the disease?
That was the provocative premise advanced by Lynne Warner Stevenson, MD, who suggested that efforts to prevent, diagnose, and treat the disease would go better if it could only jettison that unfortunate word “failure,” its hard-wired albatross.
Dr. Stevenson offered several potentially superior alternatives, including cardiac insufficiency, heart dysfunction, and her favorite, cardiomyopathy.
“Is heart failure still the best diagnosis” for the entire spectrum of disease that most patients progress through ,including the many patients in earlier stages of the disease who do not have a truly failing heart? “Perhaps cardiomyopathy is the condition and heart failure is the transition,” she proposed.
To Dr. Stevenson, it’s more than just semantics.
“Words are hugely powerful,” she explained in an interview following her talk. “I think patients do not want to be seen as having heart failure. They don’t want to think of themselves as having heart failure. I think it can make them delay getting care, and it makes them ignore the disease. I worry about that a lot. I also worry that patients don’t provide support to each other that they could. Patients tend to hide that they have heart failure. We need to come up with a term that does not make patients ashamed of their disease.”
Part of the problem, Dr. Stevenson said, is that the name heart failure can be very misleading depending on the stage of the disease that patients have. Patients with stage B (presymptomatic) disease and those with mild stage C disease “don’t see themselves as having heart failure,” as having a heart that has failed them. “We need to be able to convince these patients that they have a disease that we need to treat carefully and aggressively.”
Additionally, labeling tens of millions of people as having stage A heart failure, which is presymptomatic and occurs before the heart shows any sign of damage or dysfunction, is also counterproductive, maintained Dr. Stevenson, professor of medicine at Harvard Medical School and director of the Cardiomyopathy and Heart Failure Program at Brigham and Women’s Hospital in Boston.
“So many people are at risk of developing heart failure,” she noted, including patients with hypertension, diabetes, or coronary artery disease. To label them all as already also having heart failure at that stage “tends to make them ignore the disease that we are trying to get them to pay attention to. Telling patients they have the disease that we are trying to prevent doesn’t help.”
Calling the whole range of the disease heart failure also confuses patients and others. “Patients ask me, ‘How can I have heart failure without any symptoms?’ ‘My ejection fraction improved to almost normal; do I still have heart failure?’ and ‘I don’t understand how my heart muscle is strong but my heart is failing,’ ” she said
For Dr. Stevenson, perhaps the biggest problem is the stigma of failure and the way that word ties a huge weight to the disease that prompts patients and caregivers alike to relegate it to a hidden and neglected place.
“It’s failure. Who is proud to have heart failure? Where are the marches for heart failure? Where are the celebrity champions for heart failure? We have celebrities who are happy to admit that they have Parkinson’s disease, ALS [amyotrophic lateral sclerosis], drug addiction, and even erectile dysfunction, but no one wants to say they have heart failure. We can’t get any traction behind heart failure. It doesn’t sound very inspiring,” an issue that even percolates down to dissuading clinicians from pursuing a career in heart failure care. Young people do not aspire to go into failure, she said.
“We need to call it something else.”
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Does heart failure’s name doom any progress against the disease?
That was the provocative premise advanced by Lynne Warner Stevenson, MD, who suggested that efforts to prevent, diagnose, and treat the disease would go better if it could only jettison that unfortunate word “failure,” its hard-wired albatross.
Dr. Stevenson offered several potentially superior alternatives, including cardiac insufficiency, heart dysfunction, and her favorite, cardiomyopathy.
“Is heart failure still the best diagnosis” for the entire spectrum of disease that most patients progress through ,including the many patients in earlier stages of the disease who do not have a truly failing heart? “Perhaps cardiomyopathy is the condition and heart failure is the transition,” she proposed.
To Dr. Stevenson, it’s more than just semantics.
“Words are hugely powerful,” she explained in an interview following her talk. “I think patients do not want to be seen as having heart failure. They don’t want to think of themselves as having heart failure. I think it can make them delay getting care, and it makes them ignore the disease. I worry about that a lot. I also worry that patients don’t provide support to each other that they could. Patients tend to hide that they have heart failure. We need to come up with a term that does not make patients ashamed of their disease.”
Part of the problem, Dr. Stevenson said, is that the name heart failure can be very misleading depending on the stage of the disease that patients have. Patients with stage B (presymptomatic) disease and those with mild stage C disease “don’t see themselves as having heart failure,” as having a heart that has failed them. “We need to be able to convince these patients that they have a disease that we need to treat carefully and aggressively.”
Additionally, labeling tens of millions of people as having stage A heart failure, which is presymptomatic and occurs before the heart shows any sign of damage or dysfunction, is also counterproductive, maintained Dr. Stevenson, professor of medicine at Harvard Medical School and director of the Cardiomyopathy and Heart Failure Program at Brigham and Women’s Hospital in Boston.
“So many people are at risk of developing heart failure,” she noted, including patients with hypertension, diabetes, or coronary artery disease. To label them all as already also having heart failure at that stage “tends to make them ignore the disease that we are trying to get them to pay attention to. Telling patients they have the disease that we are trying to prevent doesn’t help.”
Calling the whole range of the disease heart failure also confuses patients and others. “Patients ask me, ‘How can I have heart failure without any symptoms?’ ‘My ejection fraction improved to almost normal; do I still have heart failure?’ and ‘I don’t understand how my heart muscle is strong but my heart is failing,’ ” she said
For Dr. Stevenson, perhaps the biggest problem is the stigma of failure and the way that word ties a huge weight to the disease that prompts patients and caregivers alike to relegate it to a hidden and neglected place.
“It’s failure. Who is proud to have heart failure? Where are the marches for heart failure? Where are the celebrity champions for heart failure? We have celebrities who are happy to admit that they have Parkinson’s disease, ALS [amyotrophic lateral sclerosis], drug addiction, and even erectile dysfunction, but no one wants to say they have heart failure. We can’t get any traction behind heart failure. It doesn’t sound very inspiring,” an issue that even percolates down to dissuading clinicians from pursuing a career in heart failure care. Young people do not aspire to go into failure, she said.
“We need to call it something else.”
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2017
Spironolactone’s HFpEF benefit happens mostly in women
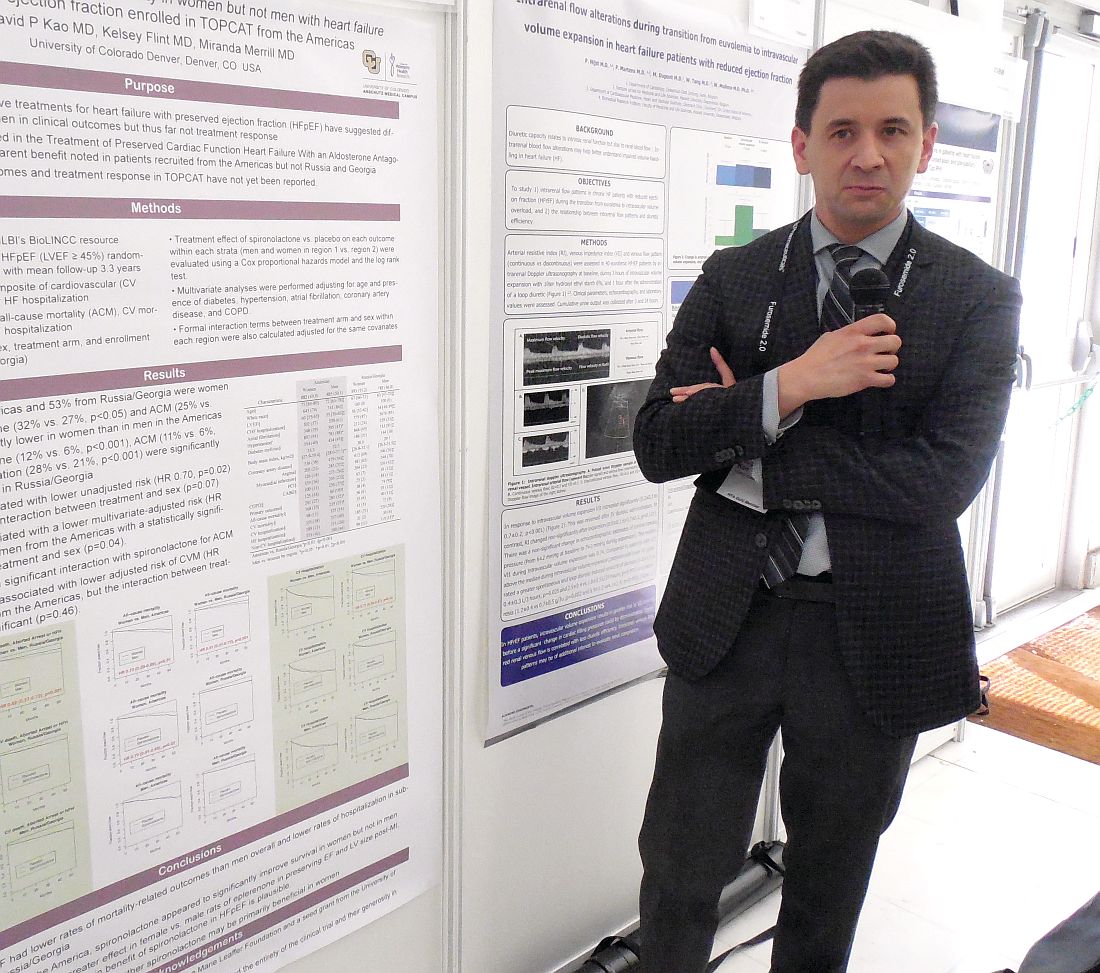
PARIS – Just when the aldosterone receptor antagonists spironolactone and eplerenone received official recognition in the 2017 U.S. heart failure guidelines as the only drug class that benefits patients with heart failure with preserved ejection fraction (HFpEF), a new post-hoc analysis of the pivotal evidence suggests the benefit is mostly in women, with little benefit to men.
The new analysis used data collected from TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), which randomized patients with HFpEF to treatment with spironolactone or placebo. Results from the overall study were neutral for the primary outcome of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization (N Engl J Med. 2014 April 10;370[15]:1383-92). However, a series of post-hoc analyses showed that a high percentage of patients enrolled at centers in Russia or Georgia did not match the expected HFpEF profile, and these patients had poor responses to spironolactone. In contrast, patients enrolled at centers in the Americas more frequently matched the study’s target HFpEF profile, and they showed significant improvement for the primary endpoint (Circulation. 2015 Jan 6;131[1]:34-42).
“The observation that most of the benefit [from spironolactone treatment] may have been in women is interesting, but I don’t think that it would stop me from using [an aldosterone receptor antagonist] in men,” said Dr. Kao while presenting his report. The outcomes in both men and women “head in the same direction. It’s just that the mortality benefit is much clearer in women,” said Dr. Kao, a cardiologist at the University of Colorado at Denver, Aurora.
Among the patients enrolled at centers in North and South America, 882 were women, and 885 were men. Dr. Kao used the data collected in TOPCAT to calculate the impact of spironolactone treatment relative to placebo on outcomes just among women in the Americas and just among men.
The difference in all-cause mortality associated with spironolactone treatment had an even sharper sex disparity that in the primary outcome. Overall, all-cause mortality was 28% less common among women, compared with men, in the Americas. Among women, spironolactone treatment linked with a 30% reduced all-cause mortality rate, compared with placebo. Among men, the survival curves of those on spironolactone or placebo superimposed.
Dr. Kao said that published study results in rats had suggested that eplerenone (Inspra), an aldosterone receptor antagonist like spironolactone, had a more potent effect in females rats, compared with male rats, for preserving left ventricular function and size following myocardial damage. In addition, women with HFpEF often have more left ventricular hypertrophy, while men often have more diastolic dysfunction, and prior findings had suggested that aldosterone plays a role in left ventricular hypertrophy.
TOPCAT received no commercial funding. Dr. Kao had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Just when the aldosterone receptor antagonists spironolactone and eplerenone received official recognition in the 2017 U.S. heart failure guidelines as the only drug class that benefits patients with heart failure with preserved ejection fraction (HFpEF), a new post-hoc analysis of the pivotal evidence suggests the benefit is mostly in women, with little benefit to men.
The new analysis used data collected from TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), which randomized patients with HFpEF to treatment with spironolactone or placebo. Results from the overall study were neutral for the primary outcome of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization (N Engl J Med. 2014 April 10;370[15]:1383-92). However, a series of post-hoc analyses showed that a high percentage of patients enrolled at centers in Russia or Georgia did not match the expected HFpEF profile, and these patients had poor responses to spironolactone. In contrast, patients enrolled at centers in the Americas more frequently matched the study’s target HFpEF profile, and they showed significant improvement for the primary endpoint (Circulation. 2015 Jan 6;131[1]:34-42).
“The observation that most of the benefit [from spironolactone treatment] may have been in women is interesting, but I don’t think that it would stop me from using [an aldosterone receptor antagonist] in men,” said Dr. Kao while presenting his report. The outcomes in both men and women “head in the same direction. It’s just that the mortality benefit is much clearer in women,” said Dr. Kao, a cardiologist at the University of Colorado at Denver, Aurora.
Among the patients enrolled at centers in North and South America, 882 were women, and 885 were men. Dr. Kao used the data collected in TOPCAT to calculate the impact of spironolactone treatment relative to placebo on outcomes just among women in the Americas and just among men.
The difference in all-cause mortality associated with spironolactone treatment had an even sharper sex disparity that in the primary outcome. Overall, all-cause mortality was 28% less common among women, compared with men, in the Americas. Among women, spironolactone treatment linked with a 30% reduced all-cause mortality rate, compared with placebo. Among men, the survival curves of those on spironolactone or placebo superimposed.
Dr. Kao said that published study results in rats had suggested that eplerenone (Inspra), an aldosterone receptor antagonist like spironolactone, had a more potent effect in females rats, compared with male rats, for preserving left ventricular function and size following myocardial damage. In addition, women with HFpEF often have more left ventricular hypertrophy, while men often have more diastolic dysfunction, and prior findings had suggested that aldosterone plays a role in left ventricular hypertrophy.
TOPCAT received no commercial funding. Dr. Kao had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Just when the aldosterone receptor antagonists spironolactone and eplerenone received official recognition in the 2017 U.S. heart failure guidelines as the only drug class that benefits patients with heart failure with preserved ejection fraction (HFpEF), a new post-hoc analysis of the pivotal evidence suggests the benefit is mostly in women, with little benefit to men.
The new analysis used data collected from TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), which randomized patients with HFpEF to treatment with spironolactone or placebo. Results from the overall study were neutral for the primary outcome of cardiovascular death, aborted cardiac arrest, and heart failure hospitalization (N Engl J Med. 2014 April 10;370[15]:1383-92). However, a series of post-hoc analyses showed that a high percentage of patients enrolled at centers in Russia or Georgia did not match the expected HFpEF profile, and these patients had poor responses to spironolactone. In contrast, patients enrolled at centers in the Americas more frequently matched the study’s target HFpEF profile, and they showed significant improvement for the primary endpoint (Circulation. 2015 Jan 6;131[1]:34-42).
“The observation that most of the benefit [from spironolactone treatment] may have been in women is interesting, but I don’t think that it would stop me from using [an aldosterone receptor antagonist] in men,” said Dr. Kao while presenting his report. The outcomes in both men and women “head in the same direction. It’s just that the mortality benefit is much clearer in women,” said Dr. Kao, a cardiologist at the University of Colorado at Denver, Aurora.
Among the patients enrolled at centers in North and South America, 882 were women, and 885 were men. Dr. Kao used the data collected in TOPCAT to calculate the impact of spironolactone treatment relative to placebo on outcomes just among women in the Americas and just among men.
The difference in all-cause mortality associated with spironolactone treatment had an even sharper sex disparity that in the primary outcome. Overall, all-cause mortality was 28% less common among women, compared with men, in the Americas. Among women, spironolactone treatment linked with a 30% reduced all-cause mortality rate, compared with placebo. Among men, the survival curves of those on spironolactone or placebo superimposed.
Dr. Kao said that published study results in rats had suggested that eplerenone (Inspra), an aldosterone receptor antagonist like spironolactone, had a more potent effect in females rats, compared with male rats, for preserving left ventricular function and size following myocardial damage. In addition, women with HFpEF often have more left ventricular hypertrophy, while men often have more diastolic dysfunction, and prior findings had suggested that aldosterone plays a role in left ventricular hypertrophy.
TOPCAT received no commercial funding. Dr. Kao had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: Women from the Americas in TOPCAT had an 18% lower rate of primary endpoint events, compared with men in the trial.
Data source: TOPCAT, a multicenter, randomized study with 3,445 patients.
Disclosures: TOPCAT received no commercial funding. Dr. Kao had no disclosures.
New heart failure guidelines prioritize prevention
The latest update to U.S. guidelines for heart failure management, released at the end of April , puts unprecedented emphasis on heart failure prevention and also shines a brighter light on patients at risk for developing heart failure – people with hypertension, diabetes, or coronary artery disease.
“We have embraced the fact that heart failure can be prevented and that the progression of heart failure can be interrupted, and we articulated how we can use biomarkers to screen patients with asymptomatic left ventricular dysfunction,” said Clyde W. Yancy, MD, chair of the writing group that issued the 2017 focused update to the heart failure management guidelines on behalf of the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America. (Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509).
This means that, for the first time, these guidelines focus on stage A heart failure patients, those without symptoms or detectable left ventricular dysfunction but at risk for heart failure, a heart failure subgroup that was ignored in the past. Prevention is most immediate for stage A patients. The new guidelines cite the stage A definition from the 2009 guidelines: patients with “risk factors that clearly predispose toward the development of heart failure. For example, patients with coronary artery disease, hypertension, or diabetes mellitus who do not yet demonstrate impaired left ventricular function.”
The number of patients with coronary artery disease, hypertension, or diabetes is pretty large. The new heart failure guidelines apply to many people.
“The mindset [on heart failure] has been on treatment, not prevention. There is far more focus [in the new guidelines] on prevention than ever before,” commented Javed Butler, MD, professor and chief of cardiology at Stony Brook (N.Y.) University and a member of the guideline writing panel.
“One reason why heart failure prevention has not been a focus was because people thought that, if you prevented coronary artery disease, you prevented heart failure. What we’ve learned is that a lot of heart failure is not ischemic and not from overt coronary disease, especially age-related HFpEF [heart failure with preserved ejection fraction]. Hopefully, these guidelines will spur more interest in prevention and risk factor control,” Dr. Butler said in an interview.
It starts with blood pressure
The guidelines contain an entirely new section devoted to blood pressure, and, while part of the section deals with a target blood pressure for symptomatic (stage C) heart failure patients (a goal systolic pressure of less than 130 mm Hg for patients with either a preserved or reduced ejection fraction), the first entry is a target blood pressure of less than 130/80 mm Hg for all stage A heart failure patients.
Because stage A is defined to include any adult with hypertension, the new heart failure guidelines set a new blood pressure treatment goal for all U.S. adults with hypertension at a time when the long-awaited revision to U.S. hypertension management guidelines from the ACC and AHA are still pending. Until the new hypertension guidelines come out – they’re expected later this year – the blood pressure target set in the heart failure guidelines will have to suffice.
Indeed, the less than 130/80-mm Hg target for on-treatment blood pressure set for heart failure prevention in the new guidelines was picked to “harmonize” with the guidelines that the ACC and AHA hypertension panel will soon release, Dr. Jessup said in an interview.
The main evidence for this target, lower than in most prior U.S. hypertension guidelines, comes from SPRINT (Systolic Blood Pressure Intervention Trial) (N Engl J Med. 2015 Nov 26;373[22]:2103-16). In that trial, the goal blood pressure that linked with the best outcomes was less than 120/80 mm Hg, although the average achieved systolic blood pressure was above that goal with a mean systolic pressure of 121.5 mm Hg. One reason for setting a higher goal systolic pressure for practice was that analyses have shown that blood pressure measurement in SPRINT did not perform like conventional measurements in routine practice. SPRINT patients appeared to have lower measured pressures than they would have recorded had they been measured by more conventional means (Hypertension. 2017 January;69[1]:15-9).
“The way that blood pressures were measured in SPRINT, a pressure of 120/80 mm Hg in the trial was akin to a pressure of 130/80 mm Hg in an office,” Dr. Yancy, chief of cardiology at Northwestern University, Chicago, said in an interview. “To avoid dangerous hypotension and to approximate SPRINT, an office pressure of less than 130 mm Hg is a reasonable number.”
New role for BNP screening
Stage A patients are more than just the target for more aggressive hypertension control. They are now also potential candidates for screening for an elevated blood level of brain natriuretic peptide (BNP) or N-terminal (NT)–proBNP. The guidelines panel makes this a level IIa recommendation, saying that a screening BNP test in patients at risk for developing heart failure can be useful if followed by team-based care and optimized guideline directed medical therapy.
This guideline follows the lead of two successful controlled trials that focused more aggressive preventive treatments on stage A patients with an elevated level of BNP or NT-proBNP – the STOP-HF (JAMA. 2013 July 3;310[1]:66-74) and PONTIAC (J Am Coll Cardiol. 2013 Oct;62[15]:1365-72) trials. The target population for some type of BNP screening are patients with cardiovascular disease, vascular disease, diabetes, obesity, or hypertension, Dr. Yancy said. “It was evident in STOP-HF that, if you screened and intervened, you could make a difference” in the development of heart failure.
The STOP-HF intervention included “optimal risk factor management” and “coaching by a specialist nurse who emphasized individual risk status and the importance of adherence to medication and healthy lifestyle behaviors.”
The guidelines aren’t clear on which patients at risk for developing heart failure, stage A patients, should get screened with BNP or NT-proBNP. Dr. Jessup said that it’s for patients in whom a positive result would trigger more aggressive management.
Getting a BNP on a suspect patient can raise a red flag to the patient, as well as to the physician, that more intervention is needed. “It’s easy for a physician to ignore a high-risk patient who looks okay and feels okay.” A BNP or NT-proBNP test can pick out the patients who shouldn’t be ignored, Dr. Januzzi said.
HFpEF treatment now possible
Another groundbreaking change in the guidelines is inclusion, for the first time, of a medical treatment specific for HFpEF. The aldosterone receptor antagonists (ARAs) spironolactone and eplerenone received a class IIb recommendation: An ARA might be considered to decrease hospitalizations in patients with HFpEF with an ejection fraction of at least 45%, an elevated BNP or recent hospitalization, and good renal function and potassium level.
The “might be considered” recommendation is guarded but understandable given that the evidence comes from the somewhat controversial, post-hoc analysis of data from the pivotal TOPCAT trial (N Engl J Med. 2014 Apr 10;370[15]:1383-92) that focused on just the roughly half of patients seen at centers in North or South America (Circulation. 2015 Jan 6;131[1]:34-42).
“It would be irresponsible to overlook the potential that [ARAs] may help patients who looks like the ones enrolled in TOPCAT in the Americas,” said Dr. Yancy. “We blended evidence and pragmatism and said that the field needs this” treatment. He said that an ARA was a reasonable option for HFpEF patients with symptoms of heart failure and a positive biomarker test result.
Dr. Butler largely agreed. ARA treatment is for HFpEF patients with symptomatic heart failure and either a history of hospitalization or a high BNP level, he said.
“I was surprised by how strongly the committee felt there was a reasonable signal of help from ARAs in HFpEF,” said Dr. Jessup. “I believe in them too,” she added.
Dr. Jessup suggested targeting an ARA to a HFpEF patient with some hypertension, some volume problem, some peripheral edema, and a lot of breathlessness but with no underlying ischemia. “I use an ARA on these patients pretty quickly,” Dr. Jessup said. It’s best to start with a low dosage and see how the patient responds. “The best responders have a really stiff heart” and are usually not the more elderly HFpEF patients. ARA treatment also provides more steady volume control, superior to furosemide, she said.
Yet more additions
The revised guidelines contain even more changes. “We say that checking for anemia is important and how iron is an intervention that might make a difference,” said Dr. Jessup. Also, primary care physicians and cardiologists “should look for obstructive sleep apnea” in heart failure patients for whom “intervention with weight loss might help,” she said.
Another feature is the focus on tailored treatment, with many treatment elements that need customizing to each different type of heart failure patient. “Not every drug needs to be given to every patient,” Dr. Yancy warned.
The specifics of how to orchestrate all the guidelines into a coherent management plan may become clearer later this year, when the ACC/AHA group will release a follow-up “Heart Failure Pathways” document, aimed at bridging the gap between guidelines and actual clinical practice, Dr. Yancy said. “We want more value from writing the guidelines. The biggest obstacle is how to implement them,” and that’s what the pathways follow-up will address.
“The biggest challenge the societies have is how to motivate physicians and nurses to more aggressively treat heart failure,” said Dr. Butler.
Dr. Yancy and Dr. Jessup had no disclosures. Dr. Butler has been a consultant to 11 companies. Dr. Januzzi has been a consultant to Critical Diagnostics, Novartis, Phillips, Roche Diagnostics, and Sphingotec, and he has received research support from Amgen, Boehringer Ingelheim, Janssen, and Prevencio.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
The latest update to U.S. guidelines for heart failure management, released at the end of April , puts unprecedented emphasis on heart failure prevention and also shines a brighter light on patients at risk for developing heart failure – people with hypertension, diabetes, or coronary artery disease.
“We have embraced the fact that heart failure can be prevented and that the progression of heart failure can be interrupted, and we articulated how we can use biomarkers to screen patients with asymptomatic left ventricular dysfunction,” said Clyde W. Yancy, MD, chair of the writing group that issued the 2017 focused update to the heart failure management guidelines on behalf of the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America. (Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509).
This means that, for the first time, these guidelines focus on stage A heart failure patients, those without symptoms or detectable left ventricular dysfunction but at risk for heart failure, a heart failure subgroup that was ignored in the past. Prevention is most immediate for stage A patients. The new guidelines cite the stage A definition from the 2009 guidelines: patients with “risk factors that clearly predispose toward the development of heart failure. For example, patients with coronary artery disease, hypertension, or diabetes mellitus who do not yet demonstrate impaired left ventricular function.”
The number of patients with coronary artery disease, hypertension, or diabetes is pretty large. The new heart failure guidelines apply to many people.
“The mindset [on heart failure] has been on treatment, not prevention. There is far more focus [in the new guidelines] on prevention than ever before,” commented Javed Butler, MD, professor and chief of cardiology at Stony Brook (N.Y.) University and a member of the guideline writing panel.
“One reason why heart failure prevention has not been a focus was because people thought that, if you prevented coronary artery disease, you prevented heart failure. What we’ve learned is that a lot of heart failure is not ischemic and not from overt coronary disease, especially age-related HFpEF [heart failure with preserved ejection fraction]. Hopefully, these guidelines will spur more interest in prevention and risk factor control,” Dr. Butler said in an interview.
It starts with blood pressure
The guidelines contain an entirely new section devoted to blood pressure, and, while part of the section deals with a target blood pressure for symptomatic (stage C) heart failure patients (a goal systolic pressure of less than 130 mm Hg for patients with either a preserved or reduced ejection fraction), the first entry is a target blood pressure of less than 130/80 mm Hg for all stage A heart failure patients.
Because stage A is defined to include any adult with hypertension, the new heart failure guidelines set a new blood pressure treatment goal for all U.S. adults with hypertension at a time when the long-awaited revision to U.S. hypertension management guidelines from the ACC and AHA are still pending. Until the new hypertension guidelines come out – they’re expected later this year – the blood pressure target set in the heart failure guidelines will have to suffice.
Indeed, the less than 130/80-mm Hg target for on-treatment blood pressure set for heart failure prevention in the new guidelines was picked to “harmonize” with the guidelines that the ACC and AHA hypertension panel will soon release, Dr. Jessup said in an interview.
The main evidence for this target, lower than in most prior U.S. hypertension guidelines, comes from SPRINT (Systolic Blood Pressure Intervention Trial) (N Engl J Med. 2015 Nov 26;373[22]:2103-16). In that trial, the goal blood pressure that linked with the best outcomes was less than 120/80 mm Hg, although the average achieved systolic blood pressure was above that goal with a mean systolic pressure of 121.5 mm Hg. One reason for setting a higher goal systolic pressure for practice was that analyses have shown that blood pressure measurement in SPRINT did not perform like conventional measurements in routine practice. SPRINT patients appeared to have lower measured pressures than they would have recorded had they been measured by more conventional means (Hypertension. 2017 January;69[1]:15-9).
“The way that blood pressures were measured in SPRINT, a pressure of 120/80 mm Hg in the trial was akin to a pressure of 130/80 mm Hg in an office,” Dr. Yancy, chief of cardiology at Northwestern University, Chicago, said in an interview. “To avoid dangerous hypotension and to approximate SPRINT, an office pressure of less than 130 mm Hg is a reasonable number.”
New role for BNP screening
Stage A patients are more than just the target for more aggressive hypertension control. They are now also potential candidates for screening for an elevated blood level of brain natriuretic peptide (BNP) or N-terminal (NT)–proBNP. The guidelines panel makes this a level IIa recommendation, saying that a screening BNP test in patients at risk for developing heart failure can be useful if followed by team-based care and optimized guideline directed medical therapy.
This guideline follows the lead of two successful controlled trials that focused more aggressive preventive treatments on stage A patients with an elevated level of BNP or NT-proBNP – the STOP-HF (JAMA. 2013 July 3;310[1]:66-74) and PONTIAC (J Am Coll Cardiol. 2013 Oct;62[15]:1365-72) trials. The target population for some type of BNP screening are patients with cardiovascular disease, vascular disease, diabetes, obesity, or hypertension, Dr. Yancy said. “It was evident in STOP-HF that, if you screened and intervened, you could make a difference” in the development of heart failure.
The STOP-HF intervention included “optimal risk factor management” and “coaching by a specialist nurse who emphasized individual risk status and the importance of adherence to medication and healthy lifestyle behaviors.”
The guidelines aren’t clear on which patients at risk for developing heart failure, stage A patients, should get screened with BNP or NT-proBNP. Dr. Jessup said that it’s for patients in whom a positive result would trigger more aggressive management.
Getting a BNP on a suspect patient can raise a red flag to the patient, as well as to the physician, that more intervention is needed. “It’s easy for a physician to ignore a high-risk patient who looks okay and feels okay.” A BNP or NT-proBNP test can pick out the patients who shouldn’t be ignored, Dr. Januzzi said.
HFpEF treatment now possible
Another groundbreaking change in the guidelines is inclusion, for the first time, of a medical treatment specific for HFpEF. The aldosterone receptor antagonists (ARAs) spironolactone and eplerenone received a class IIb recommendation: An ARA might be considered to decrease hospitalizations in patients with HFpEF with an ejection fraction of at least 45%, an elevated BNP or recent hospitalization, and good renal function and potassium level.
The “might be considered” recommendation is guarded but understandable given that the evidence comes from the somewhat controversial, post-hoc analysis of data from the pivotal TOPCAT trial (N Engl J Med. 2014 Apr 10;370[15]:1383-92) that focused on just the roughly half of patients seen at centers in North or South America (Circulation. 2015 Jan 6;131[1]:34-42).
“It would be irresponsible to overlook the potential that [ARAs] may help patients who looks like the ones enrolled in TOPCAT in the Americas,” said Dr. Yancy. “We blended evidence and pragmatism and said that the field needs this” treatment. He said that an ARA was a reasonable option for HFpEF patients with symptoms of heart failure and a positive biomarker test result.
Dr. Butler largely agreed. ARA treatment is for HFpEF patients with symptomatic heart failure and either a history of hospitalization or a high BNP level, he said.
“I was surprised by how strongly the committee felt there was a reasonable signal of help from ARAs in HFpEF,” said Dr. Jessup. “I believe in them too,” she added.
Dr. Jessup suggested targeting an ARA to a HFpEF patient with some hypertension, some volume problem, some peripheral edema, and a lot of breathlessness but with no underlying ischemia. “I use an ARA on these patients pretty quickly,” Dr. Jessup said. It’s best to start with a low dosage and see how the patient responds. “The best responders have a really stiff heart” and are usually not the more elderly HFpEF patients. ARA treatment also provides more steady volume control, superior to furosemide, she said.
Yet more additions
The revised guidelines contain even more changes. “We say that checking for anemia is important and how iron is an intervention that might make a difference,” said Dr. Jessup. Also, primary care physicians and cardiologists “should look for obstructive sleep apnea” in heart failure patients for whom “intervention with weight loss might help,” she said.
Another feature is the focus on tailored treatment, with many treatment elements that need customizing to each different type of heart failure patient. “Not every drug needs to be given to every patient,” Dr. Yancy warned.
The specifics of how to orchestrate all the guidelines into a coherent management plan may become clearer later this year, when the ACC/AHA group will release a follow-up “Heart Failure Pathways” document, aimed at bridging the gap between guidelines and actual clinical practice, Dr. Yancy said. “We want more value from writing the guidelines. The biggest obstacle is how to implement them,” and that’s what the pathways follow-up will address.
“The biggest challenge the societies have is how to motivate physicians and nurses to more aggressively treat heart failure,” said Dr. Butler.
Dr. Yancy and Dr. Jessup had no disclosures. Dr. Butler has been a consultant to 11 companies. Dr. Januzzi has been a consultant to Critical Diagnostics, Novartis, Phillips, Roche Diagnostics, and Sphingotec, and he has received research support from Amgen, Boehringer Ingelheim, Janssen, and Prevencio.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
The latest update to U.S. guidelines for heart failure management, released at the end of April , puts unprecedented emphasis on heart failure prevention and also shines a brighter light on patients at risk for developing heart failure – people with hypertension, diabetes, or coronary artery disease.
“We have embraced the fact that heart failure can be prevented and that the progression of heart failure can be interrupted, and we articulated how we can use biomarkers to screen patients with asymptomatic left ventricular dysfunction,” said Clyde W. Yancy, MD, chair of the writing group that issued the 2017 focused update to the heart failure management guidelines on behalf of the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America. (Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509).
This means that, for the first time, these guidelines focus on stage A heart failure patients, those without symptoms or detectable left ventricular dysfunction but at risk for heart failure, a heart failure subgroup that was ignored in the past. Prevention is most immediate for stage A patients. The new guidelines cite the stage A definition from the 2009 guidelines: patients with “risk factors that clearly predispose toward the development of heart failure. For example, patients with coronary artery disease, hypertension, or diabetes mellitus who do not yet demonstrate impaired left ventricular function.”
The number of patients with coronary artery disease, hypertension, or diabetes is pretty large. The new heart failure guidelines apply to many people.
“The mindset [on heart failure] has been on treatment, not prevention. There is far more focus [in the new guidelines] on prevention than ever before,” commented Javed Butler, MD, professor and chief of cardiology at Stony Brook (N.Y.) University and a member of the guideline writing panel.
“One reason why heart failure prevention has not been a focus was because people thought that, if you prevented coronary artery disease, you prevented heart failure. What we’ve learned is that a lot of heart failure is not ischemic and not from overt coronary disease, especially age-related HFpEF [heart failure with preserved ejection fraction]. Hopefully, these guidelines will spur more interest in prevention and risk factor control,” Dr. Butler said in an interview.
It starts with blood pressure
The guidelines contain an entirely new section devoted to blood pressure, and, while part of the section deals with a target blood pressure for symptomatic (stage C) heart failure patients (a goal systolic pressure of less than 130 mm Hg for patients with either a preserved or reduced ejection fraction), the first entry is a target blood pressure of less than 130/80 mm Hg for all stage A heart failure patients.
Because stage A is defined to include any adult with hypertension, the new heart failure guidelines set a new blood pressure treatment goal for all U.S. adults with hypertension at a time when the long-awaited revision to U.S. hypertension management guidelines from the ACC and AHA are still pending. Until the new hypertension guidelines come out – they’re expected later this year – the blood pressure target set in the heart failure guidelines will have to suffice.
Indeed, the less than 130/80-mm Hg target for on-treatment blood pressure set for heart failure prevention in the new guidelines was picked to “harmonize” with the guidelines that the ACC and AHA hypertension panel will soon release, Dr. Jessup said in an interview.
The main evidence for this target, lower than in most prior U.S. hypertension guidelines, comes from SPRINT (Systolic Blood Pressure Intervention Trial) (N Engl J Med. 2015 Nov 26;373[22]:2103-16). In that trial, the goal blood pressure that linked with the best outcomes was less than 120/80 mm Hg, although the average achieved systolic blood pressure was above that goal with a mean systolic pressure of 121.5 mm Hg. One reason for setting a higher goal systolic pressure for practice was that analyses have shown that blood pressure measurement in SPRINT did not perform like conventional measurements in routine practice. SPRINT patients appeared to have lower measured pressures than they would have recorded had they been measured by more conventional means (Hypertension. 2017 January;69[1]:15-9).
“The way that blood pressures were measured in SPRINT, a pressure of 120/80 mm Hg in the trial was akin to a pressure of 130/80 mm Hg in an office,” Dr. Yancy, chief of cardiology at Northwestern University, Chicago, said in an interview. “To avoid dangerous hypotension and to approximate SPRINT, an office pressure of less than 130 mm Hg is a reasonable number.”
New role for BNP screening
Stage A patients are more than just the target for more aggressive hypertension control. They are now also potential candidates for screening for an elevated blood level of brain natriuretic peptide (BNP) or N-terminal (NT)–proBNP. The guidelines panel makes this a level IIa recommendation, saying that a screening BNP test in patients at risk for developing heart failure can be useful if followed by team-based care and optimized guideline directed medical therapy.
This guideline follows the lead of two successful controlled trials that focused more aggressive preventive treatments on stage A patients with an elevated level of BNP or NT-proBNP – the STOP-HF (JAMA. 2013 July 3;310[1]:66-74) and PONTIAC (J Am Coll Cardiol. 2013 Oct;62[15]:1365-72) trials. The target population for some type of BNP screening are patients with cardiovascular disease, vascular disease, diabetes, obesity, or hypertension, Dr. Yancy said. “It was evident in STOP-HF that, if you screened and intervened, you could make a difference” in the development of heart failure.
The STOP-HF intervention included “optimal risk factor management” and “coaching by a specialist nurse who emphasized individual risk status and the importance of adherence to medication and healthy lifestyle behaviors.”
The guidelines aren’t clear on which patients at risk for developing heart failure, stage A patients, should get screened with BNP or NT-proBNP. Dr. Jessup said that it’s for patients in whom a positive result would trigger more aggressive management.
Getting a BNP on a suspect patient can raise a red flag to the patient, as well as to the physician, that more intervention is needed. “It’s easy for a physician to ignore a high-risk patient who looks okay and feels okay.” A BNP or NT-proBNP test can pick out the patients who shouldn’t be ignored, Dr. Januzzi said.
HFpEF treatment now possible
Another groundbreaking change in the guidelines is inclusion, for the first time, of a medical treatment specific for HFpEF. The aldosterone receptor antagonists (ARAs) spironolactone and eplerenone received a class IIb recommendation: An ARA might be considered to decrease hospitalizations in patients with HFpEF with an ejection fraction of at least 45%, an elevated BNP or recent hospitalization, and good renal function and potassium level.
The “might be considered” recommendation is guarded but understandable given that the evidence comes from the somewhat controversial, post-hoc analysis of data from the pivotal TOPCAT trial (N Engl J Med. 2014 Apr 10;370[15]:1383-92) that focused on just the roughly half of patients seen at centers in North or South America (Circulation. 2015 Jan 6;131[1]:34-42).
“It would be irresponsible to overlook the potential that [ARAs] may help patients who looks like the ones enrolled in TOPCAT in the Americas,” said Dr. Yancy. “We blended evidence and pragmatism and said that the field needs this” treatment. He said that an ARA was a reasonable option for HFpEF patients with symptoms of heart failure and a positive biomarker test result.
Dr. Butler largely agreed. ARA treatment is for HFpEF patients with symptomatic heart failure and either a history of hospitalization or a high BNP level, he said.
“I was surprised by how strongly the committee felt there was a reasonable signal of help from ARAs in HFpEF,” said Dr. Jessup. “I believe in them too,” she added.
Dr. Jessup suggested targeting an ARA to a HFpEF patient with some hypertension, some volume problem, some peripheral edema, and a lot of breathlessness but with no underlying ischemia. “I use an ARA on these patients pretty quickly,” Dr. Jessup said. It’s best to start with a low dosage and see how the patient responds. “The best responders have a really stiff heart” and are usually not the more elderly HFpEF patients. ARA treatment also provides more steady volume control, superior to furosemide, she said.
Yet more additions
The revised guidelines contain even more changes. “We say that checking for anemia is important and how iron is an intervention that might make a difference,” said Dr. Jessup. Also, primary care physicians and cardiologists “should look for obstructive sleep apnea” in heart failure patients for whom “intervention with weight loss might help,” she said.
Another feature is the focus on tailored treatment, with many treatment elements that need customizing to each different type of heart failure patient. “Not every drug needs to be given to every patient,” Dr. Yancy warned.
The specifics of how to orchestrate all the guidelines into a coherent management plan may become clearer later this year, when the ACC/AHA group will release a follow-up “Heart Failure Pathways” document, aimed at bridging the gap between guidelines and actual clinical practice, Dr. Yancy said. “We want more value from writing the guidelines. The biggest obstacle is how to implement them,” and that’s what the pathways follow-up will address.
“The biggest challenge the societies have is how to motivate physicians and nurses to more aggressively treat heart failure,” said Dr. Butler.
Dr. Yancy and Dr. Jessup had no disclosures. Dr. Butler has been a consultant to 11 companies. Dr. Januzzi has been a consultant to Critical Diagnostics, Novartis, Phillips, Roche Diagnostics, and Sphingotec, and he has received research support from Amgen, Boehringer Ingelheim, Janssen, and Prevencio.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Oral iron of no benefit in heart failure with iron deficiency
High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure who also have iron deficiency, according to a report published online May 16 in JAMA.
Iron deficiency in patients with HF, regardless of their hemoglobin status, is associated with reduced functional capacity, poorer quality of life, and increased mortality. Iron plays a crucial role in the delivery and utilization of oxygen, and “cells with high-energy demands, including skeletal and cardiac myocytes, are particularly sensitive to depleted iron stores,” said Gregory D. Lewis, MD, of the pulmonary critical care unit of Massachusetts General Hospital, Boston, and his associates.
The IRONOUT study was conducted at 23 U.S. medical centers, where outcomes after 16 weeks of oral iron therapy (150 mg twice daily) were compared against matching placebo in 225 patients. The median patient age was 63 years, and the median duration of HF was 5.7 years. Ischemic heart disease was the primary cause of HF in 78% of the study participants.
These patients had low LVEF and poor exercise capacity, despite having high rates of guideline-directed treatment with medications.
The primary endpoint was a change in peak oxygen uptake (peak VO2) at the conclusion of treatment, a measure that “reflects the multiple mechanisms by which iron repletion is expected to improve systemic oxygen delivery and utilization.” Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min), the investigators wrote (JAMA Pediatr. 2017 May 16. doi: 10.1001/jama.2017.5427).
In subgroup analyses, oral iron also failed to improve peak VO2 in any subgroup of patients: neither men nor women; neither those with decreased hemoglobin nor those with normal hemoglobin levels; nor patients with or without venous congestion at baseline. Oral iron also failed to improve secondary endpoints including 6-minute walk distance, quality of life scores, NT-proBNP levels, and ventilatory efficiency.
In contrast to previous studies of IV iron repletion, oral iron supplementation “produced minimal improvement in iron stores, implicating the route of administration rather than the strategy of iron repletion in the lack of clinical benefit,” Dr. Lewis and his associates said.
This study was funded by the National Heart, Lung, and Blood Institute, which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure who also have iron deficiency, according to a report published online May 16 in JAMA.
Iron deficiency in patients with HF, regardless of their hemoglobin status, is associated with reduced functional capacity, poorer quality of life, and increased mortality. Iron plays a crucial role in the delivery and utilization of oxygen, and “cells with high-energy demands, including skeletal and cardiac myocytes, are particularly sensitive to depleted iron stores,” said Gregory D. Lewis, MD, of the pulmonary critical care unit of Massachusetts General Hospital, Boston, and his associates.
The IRONOUT study was conducted at 23 U.S. medical centers, where outcomes after 16 weeks of oral iron therapy (150 mg twice daily) were compared against matching placebo in 225 patients. The median patient age was 63 years, and the median duration of HF was 5.7 years. Ischemic heart disease was the primary cause of HF in 78% of the study participants.
These patients had low LVEF and poor exercise capacity, despite having high rates of guideline-directed treatment with medications.
The primary endpoint was a change in peak oxygen uptake (peak VO2) at the conclusion of treatment, a measure that “reflects the multiple mechanisms by which iron repletion is expected to improve systemic oxygen delivery and utilization.” Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min), the investigators wrote (JAMA Pediatr. 2017 May 16. doi: 10.1001/jama.2017.5427).
In subgroup analyses, oral iron also failed to improve peak VO2 in any subgroup of patients: neither men nor women; neither those with decreased hemoglobin nor those with normal hemoglobin levels; nor patients with or without venous congestion at baseline. Oral iron also failed to improve secondary endpoints including 6-minute walk distance, quality of life scores, NT-proBNP levels, and ventilatory efficiency.
In contrast to previous studies of IV iron repletion, oral iron supplementation “produced minimal improvement in iron stores, implicating the route of administration rather than the strategy of iron repletion in the lack of clinical benefit,” Dr. Lewis and his associates said.
This study was funded by the National Heart, Lung, and Blood Institute, which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure who also have iron deficiency, according to a report published online May 16 in JAMA.
Iron deficiency in patients with HF, regardless of their hemoglobin status, is associated with reduced functional capacity, poorer quality of life, and increased mortality. Iron plays a crucial role in the delivery and utilization of oxygen, and “cells with high-energy demands, including skeletal and cardiac myocytes, are particularly sensitive to depleted iron stores,” said Gregory D. Lewis, MD, of the pulmonary critical care unit of Massachusetts General Hospital, Boston, and his associates.
The IRONOUT study was conducted at 23 U.S. medical centers, where outcomes after 16 weeks of oral iron therapy (150 mg twice daily) were compared against matching placebo in 225 patients. The median patient age was 63 years, and the median duration of HF was 5.7 years. Ischemic heart disease was the primary cause of HF in 78% of the study participants.
These patients had low LVEF and poor exercise capacity, despite having high rates of guideline-directed treatment with medications.
The primary endpoint was a change in peak oxygen uptake (peak VO2) at the conclusion of treatment, a measure that “reflects the multiple mechanisms by which iron repletion is expected to improve systemic oxygen delivery and utilization.” Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min), the investigators wrote (JAMA Pediatr. 2017 May 16. doi: 10.1001/jama.2017.5427).
In subgroup analyses, oral iron also failed to improve peak VO2 in any subgroup of patients: neither men nor women; neither those with decreased hemoglobin nor those with normal hemoglobin levels; nor patients with or without venous congestion at baseline. Oral iron also failed to improve secondary endpoints including 6-minute walk distance, quality of life scores, NT-proBNP levels, and ventilatory efficiency.
In contrast to previous studies of IV iron repletion, oral iron supplementation “produced minimal improvement in iron stores, implicating the route of administration rather than the strategy of iron repletion in the lack of clinical benefit,” Dr. Lewis and his associates said.
This study was funded by the National Heart, Lung, and Blood Institute, which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
Key clinical point: High-dose oral iron therapy doesn’t improve exercise capacity in the estimated 50% of patients with symptomatic heart failure and iron deficiency.
Major finding: Change in peak VO2 was not significantly different between the 111 participants who took oral iron supplements (+23 mL/min) and the 114 who took placebo (–2 mL/min).
Data source: A multicenter, randomized, double-blind, placebo-controlled phase II trial involving 225 patients treated for 16 weeks.
Disclosures: This study was funded by the National Heart, Lung, and Blood Institute (NCT02188784), which also conceived, designed, and conducted the trial. Dr. Lewis reported ties to Abbott, Novartis, Shape Systems, Stealth Bio Therapeutics, Ironwood, Cheetah Medical, Luitpold, and SoniVie. His associates reported ties to numerous industry sources.
PPIs triple heart failure hospitalization risk in atrial fib patients
PARIS – Unwarranted prescriptions for proton pump inhibitors tripled the rate at which patients with atrial fibrillation needed hospitalization for a first episode of acute heart failure, in a retrospective study of 172 patients at a single center in Portugal.
About a third of the atrial fibrillation patients received a proton pump inhibitor (PPI) without a clear indication, and the PPI recipients developed heart failure at 2.9 times the rate as patients not on a PPI, a statistically significant difference, João B. Augusto, MD, reported at a meeting held by the Heart Failure Association of the European Society of Cardiology. Dr. Augusto believes that these patients largely had no need for PPI treatment, and the drug may have cut iron and vitamin B12 absorption by lowering gastric acid, resulting in deficiencies that produced anemia, and following that, heart failure, he suggested.
The study focused on 423 patients admitted to Fernando da Fonseca Hospital during January 2014–June 2015 with a primary or secondary diagnosis of atrial fibrillation. He excluded 101 patients with a history of heart failure, 109 patients on antiplatelet therapy, and 33 patients with a clear need for PPI treatment because of a gastrointestinal condition. Another 11 patients were lost to follow-up, leaving 172 patients followed for 1 year.
At the time of their initial hospitalization, 53 patients (31%) received a prescription for a PPI despite having no gastrointestinal diagnosis, likely a prophylactic step for patients receiving an oral anticoagulant, Dr. Augusto said. The patients averaged 69 years old, and nearly two-thirds were men.
During 1-year follow-up, the incidence of hospitalization for acute heart failure was 8% in the patients not on a PPI and 23% among those on a PPI, a statistically significant difference. In a regression analysis that controlled for age and chronic kidney disease, the incidence of acute heart failure was 2.9 times more common among patients on a PPI, Dr. Augusto said. He and his associates used these findings to educate their hospital’s staff to not needlessly prescribe a PPI to atrial fibrillation patients.
Dr. Augusto had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Unwarranted prescriptions for proton pump inhibitors tripled the rate at which patients with atrial fibrillation needed hospitalization for a first episode of acute heart failure, in a retrospective study of 172 patients at a single center in Portugal.
About a third of the atrial fibrillation patients received a proton pump inhibitor (PPI) without a clear indication, and the PPI recipients developed heart failure at 2.9 times the rate as patients not on a PPI, a statistically significant difference, João B. Augusto, MD, reported at a meeting held by the Heart Failure Association of the European Society of Cardiology. Dr. Augusto believes that these patients largely had no need for PPI treatment, and the drug may have cut iron and vitamin B12 absorption by lowering gastric acid, resulting in deficiencies that produced anemia, and following that, heart failure, he suggested.
The study focused on 423 patients admitted to Fernando da Fonseca Hospital during January 2014–June 2015 with a primary or secondary diagnosis of atrial fibrillation. He excluded 101 patients with a history of heart failure, 109 patients on antiplatelet therapy, and 33 patients with a clear need for PPI treatment because of a gastrointestinal condition. Another 11 patients were lost to follow-up, leaving 172 patients followed for 1 year.
At the time of their initial hospitalization, 53 patients (31%) received a prescription for a PPI despite having no gastrointestinal diagnosis, likely a prophylactic step for patients receiving an oral anticoagulant, Dr. Augusto said. The patients averaged 69 years old, and nearly two-thirds were men.
During 1-year follow-up, the incidence of hospitalization for acute heart failure was 8% in the patients not on a PPI and 23% among those on a PPI, a statistically significant difference. In a regression analysis that controlled for age and chronic kidney disease, the incidence of acute heart failure was 2.9 times more common among patients on a PPI, Dr. Augusto said. He and his associates used these findings to educate their hospital’s staff to not needlessly prescribe a PPI to atrial fibrillation patients.
Dr. Augusto had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Unwarranted prescriptions for proton pump inhibitors tripled the rate at which patients with atrial fibrillation needed hospitalization for a first episode of acute heart failure, in a retrospective study of 172 patients at a single center in Portugal.
About a third of the atrial fibrillation patients received a proton pump inhibitor (PPI) without a clear indication, and the PPI recipients developed heart failure at 2.9 times the rate as patients not on a PPI, a statistically significant difference, João B. Augusto, MD, reported at a meeting held by the Heart Failure Association of the European Society of Cardiology. Dr. Augusto believes that these patients largely had no need for PPI treatment, and the drug may have cut iron and vitamin B12 absorption by lowering gastric acid, resulting in deficiencies that produced anemia, and following that, heart failure, he suggested.
The study focused on 423 patients admitted to Fernando da Fonseca Hospital during January 2014–June 2015 with a primary or secondary diagnosis of atrial fibrillation. He excluded 101 patients with a history of heart failure, 109 patients on antiplatelet therapy, and 33 patients with a clear need for PPI treatment because of a gastrointestinal condition. Another 11 patients were lost to follow-up, leaving 172 patients followed for 1 year.
At the time of their initial hospitalization, 53 patients (31%) received a prescription for a PPI despite having no gastrointestinal diagnosis, likely a prophylactic step for patients receiving an oral anticoagulant, Dr. Augusto said. The patients averaged 69 years old, and nearly two-thirds were men.
During 1-year follow-up, the incidence of hospitalization for acute heart failure was 8% in the patients not on a PPI and 23% among those on a PPI, a statistically significant difference. In a regression analysis that controlled for age and chronic kidney disease, the incidence of acute heart failure was 2.9 times more common among patients on a PPI, Dr. Augusto said. He and his associates used these findings to educate their hospital’s staff to not needlessly prescribe a PPI to atrial fibrillation patients.
Dr. Augusto had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: Atrial fibrillation patients had a 2.9 times higher acute heart failure rate on a proton pump inhibitor, compared with no PPI.
Data source: Retrospective review of 172 atrial fibrillation patients seen during 2014-2015 at a single center in Portugal.
Disclosures: Dr. Augusto had no disclosures.
Bromocriptine shows efficacy, safety for peripartum cardiomyopathy
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: At 6-month follow-up, more than 80% of patients had full or partial restoration of their left ventricular function.
Data source: The PPCM trial, which enrolled 63 women at 12 German centers.
Disclosures: The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
Noncardiovascular comorbidities spike in acute heart failure
PARIS – Patients hospitalized for heart failure increasingly present with a growing number of noncardiovascular comorbidities, according to registry data from more than 300 U.S. hospitals.
During the decade of 2005-2014, the percentage of patients hospitalized for heart failure diagnosed with three or more noncardiovascular comorbidities (NCCs) jumped from abut 17% of these patients in 2005 to about 28% in 2015, Abhinav Sharma, MD, said at a meeting held by the Heart Failure Association of the ESC. This increase occurred as the percentages of hospitalized heart failure patients with none or one NCC showed clear decreases.
U.S. patients hospitalized for heart failure “appear to now be sicker and more medically complex. Probably, a large number of the noncardiovascular comorbidities are not being recognized when the focus is on treating the patient’s heart failure,” he said in an interview. “If we can identify the noncardiovascular comorbidities and target appropriate treatment, it may potentially decrease the risk of readmissions.”
He included five NCCs in his analysis: chronic obstructive pulmonary disease, anemia, diabetes, chronic kidney disease, and obesity.
His analysis showed that a higher rate of readmissions, as well as increased mortality both in hospital and during the 30 days following discharge, are outcomes that all connect with increased numbers of NCCs. Patients with three or more NCCs at the time of their heart failure admission were about 50% more likely to die in hospital, about 65% more likely to die during the 30 days following admission, about 35% more likely to be readmitted, and about half as likely to be discharged home following hospitalization, when compared with patients with no NCC in multivariate analyses that adjusted for demographic and other clinical variables. Patients with three or more NCCs were also about 67% more likely to have an index hospitalization of at least 4 days, compared with patients with no NCC.
Dr. Sharma speculated that the increased prevalence of multiple NCCs in acute heart failure patients may result, in part, from secular trends in the rates of diabetes and obesity and the noncardiovascular comorbidities associated with these conditions. All five of the NCCs included in his analysis showed increased prevalence rates from 2005 to 2014 in the patients he studied. The biggest jump occurred in the prevalence of chronic obstructive pulmonary disease, which rose from about 27% in 2005 to about 35% in 2014.
His study used data collected in the Get With the Guidelines–Heart Failure Registry, which began in 2005, and included just under 208,000 total patients. He acknowledged that it is hard to know how representative these patients are of the entire population of U.S. patients hospitalized for heart failure during the study period, because the patients he studied came from a self-selected group of more than 300 hospitals that opted to participate in the registry. “We need to see if this can be extrapolated to all U.S. hospitals,” Dr. Sharma said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Patients hospitalized for heart failure increasingly present with a growing number of noncardiovascular comorbidities, according to registry data from more than 300 U.S. hospitals.
During the decade of 2005-2014, the percentage of patients hospitalized for heart failure diagnosed with three or more noncardiovascular comorbidities (NCCs) jumped from abut 17% of these patients in 2005 to about 28% in 2015, Abhinav Sharma, MD, said at a meeting held by the Heart Failure Association of the ESC. This increase occurred as the percentages of hospitalized heart failure patients with none or one NCC showed clear decreases.
U.S. patients hospitalized for heart failure “appear to now be sicker and more medically complex. Probably, a large number of the noncardiovascular comorbidities are not being recognized when the focus is on treating the patient’s heart failure,” he said in an interview. “If we can identify the noncardiovascular comorbidities and target appropriate treatment, it may potentially decrease the risk of readmissions.”
He included five NCCs in his analysis: chronic obstructive pulmonary disease, anemia, diabetes, chronic kidney disease, and obesity.
His analysis showed that a higher rate of readmissions, as well as increased mortality both in hospital and during the 30 days following discharge, are outcomes that all connect with increased numbers of NCCs. Patients with three or more NCCs at the time of their heart failure admission were about 50% more likely to die in hospital, about 65% more likely to die during the 30 days following admission, about 35% more likely to be readmitted, and about half as likely to be discharged home following hospitalization, when compared with patients with no NCC in multivariate analyses that adjusted for demographic and other clinical variables. Patients with three or more NCCs were also about 67% more likely to have an index hospitalization of at least 4 days, compared with patients with no NCC.
Dr. Sharma speculated that the increased prevalence of multiple NCCs in acute heart failure patients may result, in part, from secular trends in the rates of diabetes and obesity and the noncardiovascular comorbidities associated with these conditions. All five of the NCCs included in his analysis showed increased prevalence rates from 2005 to 2014 in the patients he studied. The biggest jump occurred in the prevalence of chronic obstructive pulmonary disease, which rose from about 27% in 2005 to about 35% in 2014.
His study used data collected in the Get With the Guidelines–Heart Failure Registry, which began in 2005, and included just under 208,000 total patients. He acknowledged that it is hard to know how representative these patients are of the entire population of U.S. patients hospitalized for heart failure during the study period, because the patients he studied came from a self-selected group of more than 300 hospitals that opted to participate in the registry. “We need to see if this can be extrapolated to all U.S. hospitals,” Dr. Sharma said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Patients hospitalized for heart failure increasingly present with a growing number of noncardiovascular comorbidities, according to registry data from more than 300 U.S. hospitals.
During the decade of 2005-2014, the percentage of patients hospitalized for heart failure diagnosed with three or more noncardiovascular comorbidities (NCCs) jumped from abut 17% of these patients in 2005 to about 28% in 2015, Abhinav Sharma, MD, said at a meeting held by the Heart Failure Association of the ESC. This increase occurred as the percentages of hospitalized heart failure patients with none or one NCC showed clear decreases.
U.S. patients hospitalized for heart failure “appear to now be sicker and more medically complex. Probably, a large number of the noncardiovascular comorbidities are not being recognized when the focus is on treating the patient’s heart failure,” he said in an interview. “If we can identify the noncardiovascular comorbidities and target appropriate treatment, it may potentially decrease the risk of readmissions.”
He included five NCCs in his analysis: chronic obstructive pulmonary disease, anemia, diabetes, chronic kidney disease, and obesity.
His analysis showed that a higher rate of readmissions, as well as increased mortality both in hospital and during the 30 days following discharge, are outcomes that all connect with increased numbers of NCCs. Patients with three or more NCCs at the time of their heart failure admission were about 50% more likely to die in hospital, about 65% more likely to die during the 30 days following admission, about 35% more likely to be readmitted, and about half as likely to be discharged home following hospitalization, when compared with patients with no NCC in multivariate analyses that adjusted for demographic and other clinical variables. Patients with three or more NCCs were also about 67% more likely to have an index hospitalization of at least 4 days, compared with patients with no NCC.
Dr. Sharma speculated that the increased prevalence of multiple NCCs in acute heart failure patients may result, in part, from secular trends in the rates of diabetes and obesity and the noncardiovascular comorbidities associated with these conditions. All five of the NCCs included in his analysis showed increased prevalence rates from 2005 to 2014 in the patients he studied. The biggest jump occurred in the prevalence of chronic obstructive pulmonary disease, which rose from about 27% in 2005 to about 35% in 2014.
His study used data collected in the Get With the Guidelines–Heart Failure Registry, which began in 2005, and included just under 208,000 total patients. He acknowledged that it is hard to know how representative these patients are of the entire population of U.S. patients hospitalized for heart failure during the study period, because the patients he studied came from a self-selected group of more than 300 hospitals that opted to participate in the registry. “We need to see if this can be extrapolated to all U.S. hospitals,” Dr. Sharma said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: The prevalence of three or more noncardiovascular comorbidities jumped from about 17% in 2005 to about 28% in 2014.
Data source: Review of 207,984 U.S. hospitalized heart failure patients included in the Get With the Guidelines–Heart Failure Registry during 2005-2014.
Disclosures: Dr. Sharma has received research support from Roche Diagnostics and Takeda.
VIDEO: Setbacks of serelaxin, ularitide prompt rethinking acute heart failure strategies
PARIS – Serelaxin’s failure to meet its primary endpoints in an acute heart failure trial with more than 6,500 patients, coupled with a similar failure by ularitide in the same patient population in pivotal trial results first reported in November 2016, led some experts to rethink their conception of potential interventions for patients hospitalized for acute heart failure decompensations.
“We learned in TRUE-AHF that giving a drug very early [in acute heart failure] does not prevent [long-term] death. It means that early is not early enough,” Alexandre Mebazaa, MD, said in a video interview at a meeting held by the Heart Failure Association of the European Society of Cardiology.
In terms of finding new management strategies for patients who develop acute decompensations, “we need to better understand acute heart failure and the best subset of patients who might benefit” from existing or new drugs, he said.
The RELAX-AHF-2 trial enrolled and analyzed 6,545 patients hospitalized with an acute heart failure decompensation at more than 500 sites in 34 countries. The study compared the impact of a 48-hour IV infusion of serelaxin with placebo when begun within 16 hours of hospitalization for acute heart failure and added to standard treatment.
These findings closely matched the performance of ularitide in a similar study design, TRUE-AHF (New Engl J Med. 2017 Apr 12. doi: 10.1056/NEJMoa1601895).
At the 2016 meeting of the Heart Failure Association of the ESC, the organization released revised guidelines for diagnosing and managing heart failure that stressed the importance of rapid response to acute heart failure, including possible treatment with vasodilator drugs. The guidelines acknowledged that while “Vasodilators are the second most often used agents in acute heart failure for symptomatic relief; however, there is no robust evidence confirming their beneficial effects” (Eur Heart J. 2016 Jul 14;37[27]:2129-200).
Both ularitide and serelaxin are potent IV vasodilators, and their failure to meet their efficacy endpoints in these two trials put vasodilation and rapid decongestion into question as strategies to improve midterm prognosis in heart failure patients following acute decompensation episodes.
Serelaxin has been developed by Novartis, and ularitide has been developed by Cardiorentis. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis, as well as from several other companies. Dr. Metra has been a consultant to Novartis. She has also served as consultant or spokesperson for Abbott Vascular, Amgen, AstraZeneca, Fresenius, Relypsa, and Servier.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Serelaxin’s failure to meet its primary endpoints in an acute heart failure trial with more than 6,500 patients, coupled with a similar failure by ularitide in the same patient population in pivotal trial results first reported in November 2016, led some experts to rethink their conception of potential interventions for patients hospitalized for acute heart failure decompensations.
“We learned in TRUE-AHF that giving a drug very early [in acute heart failure] does not prevent [long-term] death. It means that early is not early enough,” Alexandre Mebazaa, MD, said in a video interview at a meeting held by the Heart Failure Association of the European Society of Cardiology.
In terms of finding new management strategies for patients who develop acute decompensations, “we need to better understand acute heart failure and the best subset of patients who might benefit” from existing or new drugs, he said.
The RELAX-AHF-2 trial enrolled and analyzed 6,545 patients hospitalized with an acute heart failure decompensation at more than 500 sites in 34 countries. The study compared the impact of a 48-hour IV infusion of serelaxin with placebo when begun within 16 hours of hospitalization for acute heart failure and added to standard treatment.
These findings closely matched the performance of ularitide in a similar study design, TRUE-AHF (New Engl J Med. 2017 Apr 12. doi: 10.1056/NEJMoa1601895).
At the 2016 meeting of the Heart Failure Association of the ESC, the organization released revised guidelines for diagnosing and managing heart failure that stressed the importance of rapid response to acute heart failure, including possible treatment with vasodilator drugs. The guidelines acknowledged that while “Vasodilators are the second most often used agents in acute heart failure for symptomatic relief; however, there is no robust evidence confirming their beneficial effects” (Eur Heart J. 2016 Jul 14;37[27]:2129-200).
Both ularitide and serelaxin are potent IV vasodilators, and their failure to meet their efficacy endpoints in these two trials put vasodilation and rapid decongestion into question as strategies to improve midterm prognosis in heart failure patients following acute decompensation episodes.
Serelaxin has been developed by Novartis, and ularitide has been developed by Cardiorentis. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis, as well as from several other companies. Dr. Metra has been a consultant to Novartis. She has also served as consultant or spokesperson for Abbott Vascular, Amgen, AstraZeneca, Fresenius, Relypsa, and Servier.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
PARIS – Serelaxin’s failure to meet its primary endpoints in an acute heart failure trial with more than 6,500 patients, coupled with a similar failure by ularitide in the same patient population in pivotal trial results first reported in November 2016, led some experts to rethink their conception of potential interventions for patients hospitalized for acute heart failure decompensations.
“We learned in TRUE-AHF that giving a drug very early [in acute heart failure] does not prevent [long-term] death. It means that early is not early enough,” Alexandre Mebazaa, MD, said in a video interview at a meeting held by the Heart Failure Association of the European Society of Cardiology.
In terms of finding new management strategies for patients who develop acute decompensations, “we need to better understand acute heart failure and the best subset of patients who might benefit” from existing or new drugs, he said.
The RELAX-AHF-2 trial enrolled and analyzed 6,545 patients hospitalized with an acute heart failure decompensation at more than 500 sites in 34 countries. The study compared the impact of a 48-hour IV infusion of serelaxin with placebo when begun within 16 hours of hospitalization for acute heart failure and added to standard treatment.
These findings closely matched the performance of ularitide in a similar study design, TRUE-AHF (New Engl J Med. 2017 Apr 12. doi: 10.1056/NEJMoa1601895).
At the 2016 meeting of the Heart Failure Association of the ESC, the organization released revised guidelines for diagnosing and managing heart failure that stressed the importance of rapid response to acute heart failure, including possible treatment with vasodilator drugs. The guidelines acknowledged that while “Vasodilators are the second most often used agents in acute heart failure for symptomatic relief; however, there is no robust evidence confirming their beneficial effects” (Eur Heart J. 2016 Jul 14;37[27]:2129-200).
Both ularitide and serelaxin are potent IV vasodilators, and their failure to meet their efficacy endpoints in these two trials put vasodilation and rapid decongestion into question as strategies to improve midterm prognosis in heart failure patients following acute decompensation episodes.
Serelaxin has been developed by Novartis, and ularitide has been developed by Cardiorentis. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis, as well as from several other companies. Dr. Metra has been a consultant to Novartis. She has also served as consultant or spokesperson for Abbott Vascular, Amgen, AstraZeneca, Fresenius, Relypsa, and Servier.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2017
Digoxin and heart failure mortality: The Swedes weigh in
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
AT ACC 17
Key clinical point:
Major finding: The use of digoxin in patients with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm.
Data source: An observational study of nearly 24,000 patients enrolled in the Swedish Heart Failure Registry, 18% of whom were on digoxin.
Disclosures: The study presenter reported having no financial conflicts.