User login
Spontaneous coronary artery dissection: New insights
Washington – Patients with spontaneous coronary artery dissection as their presentation of acute coronary syndrome have a markedly better long-term survival rate than do patients with ACS in its various other forms, Rahul Potluri, MD, reported at the annual meeting of the American College of Cardiology.
In his retrospective cohort study of 182 U.K. patients diagnosed with spontaneous coronary artery dissection (SCAD) as the cause of their ACS and 32,981 controls with ACS without SCAD, the 15-year all-cause mortality rate was 10.4% in the SCAD group vs. 32.1% in the controls.
Although SCAD was first described in 1931, half of the roughly 1,500 cases reported in the medical literature have been published within the past 5 years. That makes this series of 182 SCAD patients with 15-year outcome data unique in providing the longest follow-up to date reported in a sizable patient series, said Dr. Potluri of the University of Alberta, Edmonton, and Aston University in Birmingham, England.
SCAD is a rupture in a coronary artery wall not related to atherosclerotic heart disease, trauma, or iatrogenic causes. The dissection is thought to result from an intimal tear or medial hemorrhage from the vasa vasorum, with subsequent accumulation of blood in a false lumen.
It’s not entirely unexpected that patients with SCAD have a better long-term prognosis than do those with ACS without SCAD, Dr. Potluri said. After all, patients with SCAD tend to be considerably younger: In Dr. Potluri’s series, the average age was younger than 52 years, vs. 66 years in controls. Plus they had lower levels of cardiovascular risk factors, and by definition they were free of atherosclerotic heart disease. But SCAD often occurs in conjunction with fibromuscular dysplasia, and the long-term health implications of that combination have not been well studied.
In a multivariate logistic regression analysis adjusted for age, sex, ethnicity, and comorbid conditions, the likelihood of dying within 5 years was 89% greater in the non-SCAD group. During follow-up, the non-SCAD ACS group was 4.1-fold more likely to have another ACS and 32% more likely to be diagnosed with heart failure.
SCAD was typically managed conservatively in Dr. Potluri’s series: In the SCAD group, 11% underwent PCI and 2.7% had CABG, compared with 51% and 10.7%, respectively, of controls.
“Conservative treatment appears to be safe in the SCAD patient population,” he said.
The prevalence of SCAD in his series of more than 33,000 patients admitted for ACS to U.K. hospitals in 2000-2014 was 0.54%. Dr. Potluri said that low figure likely reflects considerable underreporting due to the fact that some data were from the early 2000s, when SCAD was still largely below physicians’ radar.
Dr. Potluri is an authority on big data analytics in medical research. He formed his 182-patient SCAD series using ACALM (Algorithm for Comorbidity, Associations, Length of Stay, and Mortality), an analytic tool he developed years ago as a medical student. At the time of his SCAD study, the ACALM registry included anonymous, deidentified data on 1.8 million U.K. patients. The ACALM methodology utilizes a form of artificial intelligence known as novel field derivation to analyze huge amalgamated data sets. This is made possible by the fact that all U.K. hospitals report patient data in an identical standardized way, which is not the case in the United States.
Dr. Potluri has previously applied the ACALM methodology to a variety of other health care issues, including studies of an association between hyperlipidemia and breast cancer, the relationship between cardiovascular disease and mental health, and the so-called weekend effect, whereby U.K. patients hospitalized for cardiovascular reasons on the weekend have higher mortality than do those admitted on weekdays.
THE ACALM registry now exceeds 4 million patients. Dr. Potluri said he plans to analyze this full group to develop a large, comprehensive SCAD patient registry. This will enable investigators to evaluate potential psychosocial risk factors for SCAD, the role of fibromuscular dysplasia and connective tissue diseases, and other practical questions.
He reported having no financial conflicts regarding his study.
Washington – Patients with spontaneous coronary artery dissection as their presentation of acute coronary syndrome have a markedly better long-term survival rate than do patients with ACS in its various other forms, Rahul Potluri, MD, reported at the annual meeting of the American College of Cardiology.
In his retrospective cohort study of 182 U.K. patients diagnosed with spontaneous coronary artery dissection (SCAD) as the cause of their ACS and 32,981 controls with ACS without SCAD, the 15-year all-cause mortality rate was 10.4% in the SCAD group vs. 32.1% in the controls.
Although SCAD was first described in 1931, half of the roughly 1,500 cases reported in the medical literature have been published within the past 5 years. That makes this series of 182 SCAD patients with 15-year outcome data unique in providing the longest follow-up to date reported in a sizable patient series, said Dr. Potluri of the University of Alberta, Edmonton, and Aston University in Birmingham, England.
SCAD is a rupture in a coronary artery wall not related to atherosclerotic heart disease, trauma, or iatrogenic causes. The dissection is thought to result from an intimal tear or medial hemorrhage from the vasa vasorum, with subsequent accumulation of blood in a false lumen.
It’s not entirely unexpected that patients with SCAD have a better long-term prognosis than do those with ACS without SCAD, Dr. Potluri said. After all, patients with SCAD tend to be considerably younger: In Dr. Potluri’s series, the average age was younger than 52 years, vs. 66 years in controls. Plus they had lower levels of cardiovascular risk factors, and by definition they were free of atherosclerotic heart disease. But SCAD often occurs in conjunction with fibromuscular dysplasia, and the long-term health implications of that combination have not been well studied.
In a multivariate logistic regression analysis adjusted for age, sex, ethnicity, and comorbid conditions, the likelihood of dying within 5 years was 89% greater in the non-SCAD group. During follow-up, the non-SCAD ACS group was 4.1-fold more likely to have another ACS and 32% more likely to be diagnosed with heart failure.
SCAD was typically managed conservatively in Dr. Potluri’s series: In the SCAD group, 11% underwent PCI and 2.7% had CABG, compared with 51% and 10.7%, respectively, of controls.
“Conservative treatment appears to be safe in the SCAD patient population,” he said.
The prevalence of SCAD in his series of more than 33,000 patients admitted for ACS to U.K. hospitals in 2000-2014 was 0.54%. Dr. Potluri said that low figure likely reflects considerable underreporting due to the fact that some data were from the early 2000s, when SCAD was still largely below physicians’ radar.
Dr. Potluri is an authority on big data analytics in medical research. He formed his 182-patient SCAD series using ACALM (Algorithm for Comorbidity, Associations, Length of Stay, and Mortality), an analytic tool he developed years ago as a medical student. At the time of his SCAD study, the ACALM registry included anonymous, deidentified data on 1.8 million U.K. patients. The ACALM methodology utilizes a form of artificial intelligence known as novel field derivation to analyze huge amalgamated data sets. This is made possible by the fact that all U.K. hospitals report patient data in an identical standardized way, which is not the case in the United States.
Dr. Potluri has previously applied the ACALM methodology to a variety of other health care issues, including studies of an association between hyperlipidemia and breast cancer, the relationship between cardiovascular disease and mental health, and the so-called weekend effect, whereby U.K. patients hospitalized for cardiovascular reasons on the weekend have higher mortality than do those admitted on weekdays.
THE ACALM registry now exceeds 4 million patients. Dr. Potluri said he plans to analyze this full group to develop a large, comprehensive SCAD patient registry. This will enable investigators to evaluate potential psychosocial risk factors for SCAD, the role of fibromuscular dysplasia and connective tissue diseases, and other practical questions.
He reported having no financial conflicts regarding his study.
Washington – Patients with spontaneous coronary artery dissection as their presentation of acute coronary syndrome have a markedly better long-term survival rate than do patients with ACS in its various other forms, Rahul Potluri, MD, reported at the annual meeting of the American College of Cardiology.
In his retrospective cohort study of 182 U.K. patients diagnosed with spontaneous coronary artery dissection (SCAD) as the cause of their ACS and 32,981 controls with ACS without SCAD, the 15-year all-cause mortality rate was 10.4% in the SCAD group vs. 32.1% in the controls.
Although SCAD was first described in 1931, half of the roughly 1,500 cases reported in the medical literature have been published within the past 5 years. That makes this series of 182 SCAD patients with 15-year outcome data unique in providing the longest follow-up to date reported in a sizable patient series, said Dr. Potluri of the University of Alberta, Edmonton, and Aston University in Birmingham, England.
SCAD is a rupture in a coronary artery wall not related to atherosclerotic heart disease, trauma, or iatrogenic causes. The dissection is thought to result from an intimal tear or medial hemorrhage from the vasa vasorum, with subsequent accumulation of blood in a false lumen.
It’s not entirely unexpected that patients with SCAD have a better long-term prognosis than do those with ACS without SCAD, Dr. Potluri said. After all, patients with SCAD tend to be considerably younger: In Dr. Potluri’s series, the average age was younger than 52 years, vs. 66 years in controls. Plus they had lower levels of cardiovascular risk factors, and by definition they were free of atherosclerotic heart disease. But SCAD often occurs in conjunction with fibromuscular dysplasia, and the long-term health implications of that combination have not been well studied.
In a multivariate logistic regression analysis adjusted for age, sex, ethnicity, and comorbid conditions, the likelihood of dying within 5 years was 89% greater in the non-SCAD group. During follow-up, the non-SCAD ACS group was 4.1-fold more likely to have another ACS and 32% more likely to be diagnosed with heart failure.
SCAD was typically managed conservatively in Dr. Potluri’s series: In the SCAD group, 11% underwent PCI and 2.7% had CABG, compared with 51% and 10.7%, respectively, of controls.
“Conservative treatment appears to be safe in the SCAD patient population,” he said.
The prevalence of SCAD in his series of more than 33,000 patients admitted for ACS to U.K. hospitals in 2000-2014 was 0.54%. Dr. Potluri said that low figure likely reflects considerable underreporting due to the fact that some data were from the early 2000s, when SCAD was still largely below physicians’ radar.
Dr. Potluri is an authority on big data analytics in medical research. He formed his 182-patient SCAD series using ACALM (Algorithm for Comorbidity, Associations, Length of Stay, and Mortality), an analytic tool he developed years ago as a medical student. At the time of his SCAD study, the ACALM registry included anonymous, deidentified data on 1.8 million U.K. patients. The ACALM methodology utilizes a form of artificial intelligence known as novel field derivation to analyze huge amalgamated data sets. This is made possible by the fact that all U.K. hospitals report patient data in an identical standardized way, which is not the case in the United States.
Dr. Potluri has previously applied the ACALM methodology to a variety of other health care issues, including studies of an association between hyperlipidemia and breast cancer, the relationship between cardiovascular disease and mental health, and the so-called weekend effect, whereby U.K. patients hospitalized for cardiovascular reasons on the weekend have higher mortality than do those admitted on weekdays.
THE ACALM registry now exceeds 4 million patients. Dr. Potluri said he plans to analyze this full group to develop a large, comprehensive SCAD patient registry. This will enable investigators to evaluate potential psychosocial risk factors for SCAD, the role of fibromuscular dysplasia and connective tissue diseases, and other practical questions.
He reported having no financial conflicts regarding his study.
At ACC 17
Key clinical point:
Major finding: The 15-year all-cause mortality rate was 10% in patients with spontaneous coronary artery dissection, compared with 32% in those with ACS without spontaneous coronary artery dissection.
Data source: A retrospective cohort study including 15-year outcomes for 182 patients with spontaneous coronary artery dissection and more than 32,000 controls with ACS without dissection.
Disclosures: The study presenter reported having no financial conflicts.
Transradial catheterizations boost operators’ radiation exposure
WASHINGTON – Coronary catheterization procedures done via transradial access produce significantly better patient outcomes, compared with transfemoral approaches, but transradial also leads to substantially higher radiation exposure to the operator, according to an analysis of 650 procedures.
When operators performed transradial coronary catheterizations they received on average nearly twice the radiation dose to their chest as they did when they performed transfemoral procedures, in a randomized trial that included 14 interventional cardiologists, Alessandro Sciahbasi, MD, reported at the annual meeting of the American College of Cardiology.
Radiation exposures to the operators’ wrists and eyes showed no significant differences between the two catheterization routes. The difference was limited to thorax exposure.
“These data worry me,” commented Sunil V. Rao, MD, a long-time advocate for increased transradial catheterizations in U.S. practice. “We are pretty obsessive about radiation detection in our catheterization laboratory,” said Dr. Rao, an interventional cardiologist at Duke University in Durham, N.C.
The RAD-MATRIX substudy focused on the radiation exposures received by 14 participating operators who wore radiation dosimeters on their wrist, chest, and near their eyes during each procedure. All operators performed both transradial and transfemoral catheterizations with the choice dictated by the randomization protocol. The transradial procedures underwent further randomization to access through either the patient’s left or right arm. The full analysis included 398 procedures performed by transfemoral access, and 252 with transradial access with 131 via the left forearm and 121 via the right forearm.
The analysis showed that, when the operators performed transradial catheterizations, they received an average, normalized, effective dose to their thorax of 2.3 mcSv per procedure and 1.2 mcSv when performing transfemoral catheterization, a statistically significant difference. The exposures from left and from right transradial access did not differ significantly. Concurrently with Dr. Sciahbasi’s report at the meeting the results also appeared in an online article (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.018).
The analysis also showed a 10-fold variability in exposure level among the 14 participating operators. Dr. Sciahbasi hypothesized that this reflected substantive differences in the degree of radiation protection used by each operator and suggested this implied that some interventionalists were not protecting themselves from exposure as thoroughly as possible.
RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures. Dr. Rao has been a consultant to Boehringer Ingelheim, Cardinal Health, Corindus, Cardiovascular Systems, and Medtronic.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
The findings from RAD-MATRIX are alarming. Although radiation exposure is generally acknowledged as an occupational hazard of interventional cardiology, it is rarely taken seriously. It is common to see individuals performing procedures without wearing leaded eyewear, or casually strolling into catheterization laboratories without wearing appropriate aprons. The topic is often approached jocularly with comments about childbearing with little thought given to cancer risk. However, the radiation-induced health risks to adults are nontrivial and outweigh by an order of magnitude any risk for hereditary disease.
Although the findings of RAD-MATRIX are unlikely to impede the swelling tide of the transradial approach, the implications are very important. They should prompt radial and femoral operators to heed radiation safety principles. In addition, we need rigorously collected registry data concerning the safety of percutaneous coronary interventions for operators and staff.
Neil S. Kleiman, MD , is professor of cardiology at Houston Methodist. Norman J. Kleiman, PhD , is director of the Eye Radiation on Environmental Research Laboratory at Columbia University in New York. They had no disclosures. These comments are from an editorial they wrote to accompany the published RAD-MATRIX results (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.016 ).
The findings from RAD-MATRIX are alarming. Although radiation exposure is generally acknowledged as an occupational hazard of interventional cardiology, it is rarely taken seriously. It is common to see individuals performing procedures without wearing leaded eyewear, or casually strolling into catheterization laboratories without wearing appropriate aprons. The topic is often approached jocularly with comments about childbearing with little thought given to cancer risk. However, the radiation-induced health risks to adults are nontrivial and outweigh by an order of magnitude any risk for hereditary disease.
Although the findings of RAD-MATRIX are unlikely to impede the swelling tide of the transradial approach, the implications are very important. They should prompt radial and femoral operators to heed radiation safety principles. In addition, we need rigorously collected registry data concerning the safety of percutaneous coronary interventions for operators and staff.
Neil S. Kleiman, MD , is professor of cardiology at Houston Methodist. Norman J. Kleiman, PhD , is director of the Eye Radiation on Environmental Research Laboratory at Columbia University in New York. They had no disclosures. These comments are from an editorial they wrote to accompany the published RAD-MATRIX results (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.016 ).
The findings from RAD-MATRIX are alarming. Although radiation exposure is generally acknowledged as an occupational hazard of interventional cardiology, it is rarely taken seriously. It is common to see individuals performing procedures without wearing leaded eyewear, or casually strolling into catheterization laboratories without wearing appropriate aprons. The topic is often approached jocularly with comments about childbearing with little thought given to cancer risk. However, the radiation-induced health risks to adults are nontrivial and outweigh by an order of magnitude any risk for hereditary disease.
Although the findings of RAD-MATRIX are unlikely to impede the swelling tide of the transradial approach, the implications are very important. They should prompt radial and femoral operators to heed radiation safety principles. In addition, we need rigorously collected registry data concerning the safety of percutaneous coronary interventions for operators and staff.
Neil S. Kleiman, MD , is professor of cardiology at Houston Methodist. Norman J. Kleiman, PhD , is director of the Eye Radiation on Environmental Research Laboratory at Columbia University in New York. They had no disclosures. These comments are from an editorial they wrote to accompany the published RAD-MATRIX results (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.016 ).
WASHINGTON – Coronary catheterization procedures done via transradial access produce significantly better patient outcomes, compared with transfemoral approaches, but transradial also leads to substantially higher radiation exposure to the operator, according to an analysis of 650 procedures.
When operators performed transradial coronary catheterizations they received on average nearly twice the radiation dose to their chest as they did when they performed transfemoral procedures, in a randomized trial that included 14 interventional cardiologists, Alessandro Sciahbasi, MD, reported at the annual meeting of the American College of Cardiology.
Radiation exposures to the operators’ wrists and eyes showed no significant differences between the two catheterization routes. The difference was limited to thorax exposure.
“These data worry me,” commented Sunil V. Rao, MD, a long-time advocate for increased transradial catheterizations in U.S. practice. “We are pretty obsessive about radiation detection in our catheterization laboratory,” said Dr. Rao, an interventional cardiologist at Duke University in Durham, N.C.
The RAD-MATRIX substudy focused on the radiation exposures received by 14 participating operators who wore radiation dosimeters on their wrist, chest, and near their eyes during each procedure. All operators performed both transradial and transfemoral catheterizations with the choice dictated by the randomization protocol. The transradial procedures underwent further randomization to access through either the patient’s left or right arm. The full analysis included 398 procedures performed by transfemoral access, and 252 with transradial access with 131 via the left forearm and 121 via the right forearm.
The analysis showed that, when the operators performed transradial catheterizations, they received an average, normalized, effective dose to their thorax of 2.3 mcSv per procedure and 1.2 mcSv when performing transfemoral catheterization, a statistically significant difference. The exposures from left and from right transradial access did not differ significantly. Concurrently with Dr. Sciahbasi’s report at the meeting the results also appeared in an online article (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.018).
The analysis also showed a 10-fold variability in exposure level among the 14 participating operators. Dr. Sciahbasi hypothesized that this reflected substantive differences in the degree of radiation protection used by each operator and suggested this implied that some interventionalists were not protecting themselves from exposure as thoroughly as possible.
RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures. Dr. Rao has been a consultant to Boehringer Ingelheim, Cardinal Health, Corindus, Cardiovascular Systems, and Medtronic.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Coronary catheterization procedures done via transradial access produce significantly better patient outcomes, compared with transfemoral approaches, but transradial also leads to substantially higher radiation exposure to the operator, according to an analysis of 650 procedures.
When operators performed transradial coronary catheterizations they received on average nearly twice the radiation dose to their chest as they did when they performed transfemoral procedures, in a randomized trial that included 14 interventional cardiologists, Alessandro Sciahbasi, MD, reported at the annual meeting of the American College of Cardiology.
Radiation exposures to the operators’ wrists and eyes showed no significant differences between the two catheterization routes. The difference was limited to thorax exposure.
“These data worry me,” commented Sunil V. Rao, MD, a long-time advocate for increased transradial catheterizations in U.S. practice. “We are pretty obsessive about radiation detection in our catheterization laboratory,” said Dr. Rao, an interventional cardiologist at Duke University in Durham, N.C.
The RAD-MATRIX substudy focused on the radiation exposures received by 14 participating operators who wore radiation dosimeters on their wrist, chest, and near their eyes during each procedure. All operators performed both transradial and transfemoral catheterizations with the choice dictated by the randomization protocol. The transradial procedures underwent further randomization to access through either the patient’s left or right arm. The full analysis included 398 procedures performed by transfemoral access, and 252 with transradial access with 131 via the left forearm and 121 via the right forearm.
The analysis showed that, when the operators performed transradial catheterizations, they received an average, normalized, effective dose to their thorax of 2.3 mcSv per procedure and 1.2 mcSv when performing transfemoral catheterization, a statistically significant difference. The exposures from left and from right transradial access did not differ significantly. Concurrently with Dr. Sciahbasi’s report at the meeting the results also appeared in an online article (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.018).
The analysis also showed a 10-fold variability in exposure level among the 14 participating operators. Dr. Sciahbasi hypothesized that this reflected substantive differences in the degree of radiation protection used by each operator and suggested this implied that some interventionalists were not protecting themselves from exposure as thoroughly as possible.
RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures. Dr. Rao has been a consultant to Boehringer Ingelheim, Cardinal Health, Corindus, Cardiovascular Systems, and Medtronic.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: Transradial catheterizations delivered an average effective thorax radiation dose of 2.3 mcSv, compared with 1.2 mcSv from transfemoral catheterizations.
Data source: RAD-MATRIX, a randomized study with 14 operators who performed 650 total procedures.
Disclosures: RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures.
Digoxin and heart failure mortality: The Swedes weigh in
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
AT ACC 17
Key clinical point:
Major finding: The use of digoxin in patients with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm.
Data source: An observational study of nearly 24,000 patients enrolled in the Swedish Heart Failure Registry, 18% of whom were on digoxin.
Disclosures: The study presenter reported having no financial conflicts.
What drives readmissions within 90 days after MI hospitalization
WASHINGTON – In a large population of Medicare patients hospitalized for acute MI, four factors stood out as predictors of increased likelihood of readmission within 90 days, Aaron D. Kugelmass, MD, reported at the annual meeting of the American College of Cardiology.
These four predictors of 90-day readmission were end-stage renal disease at the time of the initial admission for MI, which in a logistic regression model was independently associated with an 88% relative increase in readmission risk; no percutaneous coronary intervention (PCI) during the index hospitalization, which carried a 64% increase in risk; type 1 diabetes, with a 57% increased readmission rate; and heart failure at the initial hospitalization, with an associated 34% greater risk, according to Dr. Kugelmass, chief of cardiology and medical director of the heart and vascular center at Baystate Medical Center in Springfield, Mass.
“This is going to be a learning curve for everyone,” he said.
“The best way to deal with this change is to understand the factors driving costs and morbidity and mortality,” Dr. Kugelmass said, in explaining why he conducted a retrospective study of readmissions within 90 days in a population of 143,286 Medicare beneficiaries hospitalized for acute MI in 2014. The study focus was on readmissions because they add so much to total cost of care for a 90-day episode.
Twenty-eight percent of patients were readmitted at least once within 90 days of discharge following their acute MI. The Medicare bundled payment plan divides MI patients into two separate groups: those who undergo PCI during their initial hospitalization and those who receive medical management only. Thirty-one percent of the readmitted patients in Dr. Kugelmass’s study had undergone PCI during their index hospitalization, while the other 69% were managed medically.
Heart failure was the No. 1 reason for readmission within 90 days in patients who had PCI during the index hospitalization. It was the primary reason for 17.6% of readmissions. Next came recurrent angina or chest pain, which accounted for 6.6% of readmissions; chronic obstructive pulmonary disease or pneumonia, 6.3%; and GI bleeding with hemorrhage, which was the primary reason for 6.0% of readmissions. Together these four causes accounted for more than 36% of all readmissions in the PCI group.
“The GI bleeding data were really interesting,” the cardiologist said. “There’s a lot of talk now about reducing the duration of dual-antiplatelet therapy [DAPT] after PCI. This is an administrative data set that’s quite large, and it shows that GI bleeding in a post-PCI group early in the duration of DAPT is in fact a significant cause of readmission and poses significant hazard.”
Among patients who were medically managed during their index hospitalization, the top four reasons for readmission were heart failure, accounting for 20.6% of readmissions; cardiac surgery, 13.5%; sepsis, 7.8%; and chronic obstructive pulmonary disease/pneumonia, 6.3%. GI bleeding wasn’t a significant cause of readmission in this group.
“I think what we need to do next is dive deeper into the medically managed group. There is a cohort in there that’s incredibly sick and are likely to drive costs and be prone to readmission. And there’s another component of the medically managed group that had to be fairly healthy because they were able to undergo coronary artery bypass surgery within 90 days,” Dr. Kugelmass said.
He reported having no financial conflicts regarding his study.
WASHINGTON – In a large population of Medicare patients hospitalized for acute MI, four factors stood out as predictors of increased likelihood of readmission within 90 days, Aaron D. Kugelmass, MD, reported at the annual meeting of the American College of Cardiology.
These four predictors of 90-day readmission were end-stage renal disease at the time of the initial admission for MI, which in a logistic regression model was independently associated with an 88% relative increase in readmission risk; no percutaneous coronary intervention (PCI) during the index hospitalization, which carried a 64% increase in risk; type 1 diabetes, with a 57% increased readmission rate; and heart failure at the initial hospitalization, with an associated 34% greater risk, according to Dr. Kugelmass, chief of cardiology and medical director of the heart and vascular center at Baystate Medical Center in Springfield, Mass.
“This is going to be a learning curve for everyone,” he said.
“The best way to deal with this change is to understand the factors driving costs and morbidity and mortality,” Dr. Kugelmass said, in explaining why he conducted a retrospective study of readmissions within 90 days in a population of 143,286 Medicare beneficiaries hospitalized for acute MI in 2014. The study focus was on readmissions because they add so much to total cost of care for a 90-day episode.
Twenty-eight percent of patients were readmitted at least once within 90 days of discharge following their acute MI. The Medicare bundled payment plan divides MI patients into two separate groups: those who undergo PCI during their initial hospitalization and those who receive medical management only. Thirty-one percent of the readmitted patients in Dr. Kugelmass’s study had undergone PCI during their index hospitalization, while the other 69% were managed medically.
Heart failure was the No. 1 reason for readmission within 90 days in patients who had PCI during the index hospitalization. It was the primary reason for 17.6% of readmissions. Next came recurrent angina or chest pain, which accounted for 6.6% of readmissions; chronic obstructive pulmonary disease or pneumonia, 6.3%; and GI bleeding with hemorrhage, which was the primary reason for 6.0% of readmissions. Together these four causes accounted for more than 36% of all readmissions in the PCI group.
“The GI bleeding data were really interesting,” the cardiologist said. “There’s a lot of talk now about reducing the duration of dual-antiplatelet therapy [DAPT] after PCI. This is an administrative data set that’s quite large, and it shows that GI bleeding in a post-PCI group early in the duration of DAPT is in fact a significant cause of readmission and poses significant hazard.”
Among patients who were medically managed during their index hospitalization, the top four reasons for readmission were heart failure, accounting for 20.6% of readmissions; cardiac surgery, 13.5%; sepsis, 7.8%; and chronic obstructive pulmonary disease/pneumonia, 6.3%. GI bleeding wasn’t a significant cause of readmission in this group.
“I think what we need to do next is dive deeper into the medically managed group. There is a cohort in there that’s incredibly sick and are likely to drive costs and be prone to readmission. And there’s another component of the medically managed group that had to be fairly healthy because they were able to undergo coronary artery bypass surgery within 90 days,” Dr. Kugelmass said.
He reported having no financial conflicts regarding his study.
WASHINGTON – In a large population of Medicare patients hospitalized for acute MI, four factors stood out as predictors of increased likelihood of readmission within 90 days, Aaron D. Kugelmass, MD, reported at the annual meeting of the American College of Cardiology.
These four predictors of 90-day readmission were end-stage renal disease at the time of the initial admission for MI, which in a logistic regression model was independently associated with an 88% relative increase in readmission risk; no percutaneous coronary intervention (PCI) during the index hospitalization, which carried a 64% increase in risk; type 1 diabetes, with a 57% increased readmission rate; and heart failure at the initial hospitalization, with an associated 34% greater risk, according to Dr. Kugelmass, chief of cardiology and medical director of the heart and vascular center at Baystate Medical Center in Springfield, Mass.
“This is going to be a learning curve for everyone,” he said.
“The best way to deal with this change is to understand the factors driving costs and morbidity and mortality,” Dr. Kugelmass said, in explaining why he conducted a retrospective study of readmissions within 90 days in a population of 143,286 Medicare beneficiaries hospitalized for acute MI in 2014. The study focus was on readmissions because they add so much to total cost of care for a 90-day episode.
Twenty-eight percent of patients were readmitted at least once within 90 days of discharge following their acute MI. The Medicare bundled payment plan divides MI patients into two separate groups: those who undergo PCI during their initial hospitalization and those who receive medical management only. Thirty-one percent of the readmitted patients in Dr. Kugelmass’s study had undergone PCI during their index hospitalization, while the other 69% were managed medically.
Heart failure was the No. 1 reason for readmission within 90 days in patients who had PCI during the index hospitalization. It was the primary reason for 17.6% of readmissions. Next came recurrent angina or chest pain, which accounted for 6.6% of readmissions; chronic obstructive pulmonary disease or pneumonia, 6.3%; and GI bleeding with hemorrhage, which was the primary reason for 6.0% of readmissions. Together these four causes accounted for more than 36% of all readmissions in the PCI group.
“The GI bleeding data were really interesting,” the cardiologist said. “There’s a lot of talk now about reducing the duration of dual-antiplatelet therapy [DAPT] after PCI. This is an administrative data set that’s quite large, and it shows that GI bleeding in a post-PCI group early in the duration of DAPT is in fact a significant cause of readmission and poses significant hazard.”
Among patients who were medically managed during their index hospitalization, the top four reasons for readmission were heart failure, accounting for 20.6% of readmissions; cardiac surgery, 13.5%; sepsis, 7.8%; and chronic obstructive pulmonary disease/pneumonia, 6.3%. GI bleeding wasn’t a significant cause of readmission in this group.
“I think what we need to do next is dive deeper into the medically managed group. There is a cohort in there that’s incredibly sick and are likely to drive costs and be prone to readmission. And there’s another component of the medically managed group that had to be fairly healthy because they were able to undergo coronary artery bypass surgery within 90 days,” Dr. Kugelmass said.
He reported having no financial conflicts regarding his study.
Key clinical point:
Major finding: Twenty-eight percent of Medicare patients hospitalized for acute MI were readmitted within 90 days.
Data source: A retrospective study of readmissions within 90 days among more than 143,000 Medicare beneficiaries hospitalized for acute MI in 2014.
Disclosures: The study presenter reported having no financial conflicts.
CRT-D beneficial in mild HF with ejection fraction above 30%
WASHINGTON – Patients with mild heart failure symptoms, left bundle branch block, and a left ventricular ejection fraction of 31% to 44% who received cardiac resynchronization therapy with a built-in defibrillator experienced a significant reduction in all-cause mortality, compared with those randomized to an implantable cardioverter-defibrillator alone during 7 years of follow-up.
These results from a new MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) long-term follow-up substudy “suggest that patients with a relatively preserved ejection fraction greater than 30% benefit from CRT-D [cardiac resynchronization therapy defibrillator] and could potentially be considered for this therapy,” said Katherine Vermilye, MD, at the annual meeting of the American College of Cardiology.
In a subsequent publication, the MADIT-CRT investigators reported that, with extension of follow-up to 7 years, CRT-D also provided a significant benefit in terms of all-cause mortality in addition to the reduced rate of heart failure events (N Engl J Med. 2014 May 1;370[18]:1694-701).
However, even though an LVEF of 30% or less was a requirement for participation in MADIT-CRT, it turned out that, when the initial screening echocardiograms were eventually analyzed in a central core laboratory, one-third of study participants actually had a baseline LVEF of 31% to 44%, with the majority of excessive values being in the 31%-35% range.
Dr. Vermilye, of the University of Rochester in New York, presented a post hoc analysis of long-term outcomes in the subgroup having a baseline LVEF greater than 30%. They totaled 450 of 1,224 MADIT-CRT participants with left bundle branch block. They were significantly older and more likely to be female than the 824 subjects with an LVEF of 30% or less. They also had a shorter QRS duration – an average of 160 ms, versus 165 ms in patients with an LVEF of 30% or lower – and a smaller baseline left ventricular end systolic volume of 151 mL, compared with 196 mL in patients with a lower LVEF.
In a multivariate Cox regression analysis adjusted for potential confounders, CRT-D in patients with a baseline LVEF greater than 30% was associated with a 54% reduction in the risk of all-cause mortality at 7 years of follow-up, compared with receipt of an ICD-only device and with a smaller yet significant 31% reduction in risk in those with an LVEF of 30% or less. Worsening heart failure events were reduced by 64% in patients with a baseline LVEF greater than 30% who received CRT-D, compared with ICD-only, and by 54% in those with a lower baseline LVEF.
The reduction in all-cause mortality seen with CRT-D was confined to patients who were high responders to CRT as defined echocardiographically by at least a 35% change in left ventricular end systolic volume 1 year post implantation. They had an 85% reduction in the risk of death during 7 years of follow-up with CRT-D if their baseline LVEF was greater than 30% and a 58% relative risk reduction if their LVEF was 30% or less.
In contrast, CRT-D brought a significantly reduced risk of heart failure events regardless of whether a patient was a low or high responder, although the magnitude of benefit was greater in the high responders. Among patients with a baseline LVEF greater than 30%, CRT-D low responders had a 52% reduction in risk of heart failure events, compared with ICD recipients, while CRT-D high responders had an 81% relative risk reduction. Similarly, in patients with a baseline LVEF of 30% or less, CRT-D low responders had 48% reduction in heart failure events and high responders had a 79% risk reduction, compared with the ICD-only group.
Because this is a post hoc analysis, these new MADIT-CRT findings require validation in future studies, Dr. Vermilye observed.
MADIT-CRT was supported by Boston Scientific. Dr.. Vermilye reported having no financial conflicts.
WASHINGTON – Patients with mild heart failure symptoms, left bundle branch block, and a left ventricular ejection fraction of 31% to 44% who received cardiac resynchronization therapy with a built-in defibrillator experienced a significant reduction in all-cause mortality, compared with those randomized to an implantable cardioverter-defibrillator alone during 7 years of follow-up.
These results from a new MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) long-term follow-up substudy “suggest that patients with a relatively preserved ejection fraction greater than 30% benefit from CRT-D [cardiac resynchronization therapy defibrillator] and could potentially be considered for this therapy,” said Katherine Vermilye, MD, at the annual meeting of the American College of Cardiology.
In a subsequent publication, the MADIT-CRT investigators reported that, with extension of follow-up to 7 years, CRT-D also provided a significant benefit in terms of all-cause mortality in addition to the reduced rate of heart failure events (N Engl J Med. 2014 May 1;370[18]:1694-701).
However, even though an LVEF of 30% or less was a requirement for participation in MADIT-CRT, it turned out that, when the initial screening echocardiograms were eventually analyzed in a central core laboratory, one-third of study participants actually had a baseline LVEF of 31% to 44%, with the majority of excessive values being in the 31%-35% range.
Dr. Vermilye, of the University of Rochester in New York, presented a post hoc analysis of long-term outcomes in the subgroup having a baseline LVEF greater than 30%. They totaled 450 of 1,224 MADIT-CRT participants with left bundle branch block. They were significantly older and more likely to be female than the 824 subjects with an LVEF of 30% or less. They also had a shorter QRS duration – an average of 160 ms, versus 165 ms in patients with an LVEF of 30% or lower – and a smaller baseline left ventricular end systolic volume of 151 mL, compared with 196 mL in patients with a lower LVEF.
In a multivariate Cox regression analysis adjusted for potential confounders, CRT-D in patients with a baseline LVEF greater than 30% was associated with a 54% reduction in the risk of all-cause mortality at 7 years of follow-up, compared with receipt of an ICD-only device and with a smaller yet significant 31% reduction in risk in those with an LVEF of 30% or less. Worsening heart failure events were reduced by 64% in patients with a baseline LVEF greater than 30% who received CRT-D, compared with ICD-only, and by 54% in those with a lower baseline LVEF.
The reduction in all-cause mortality seen with CRT-D was confined to patients who were high responders to CRT as defined echocardiographically by at least a 35% change in left ventricular end systolic volume 1 year post implantation. They had an 85% reduction in the risk of death during 7 years of follow-up with CRT-D if their baseline LVEF was greater than 30% and a 58% relative risk reduction if their LVEF was 30% or less.
In contrast, CRT-D brought a significantly reduced risk of heart failure events regardless of whether a patient was a low or high responder, although the magnitude of benefit was greater in the high responders. Among patients with a baseline LVEF greater than 30%, CRT-D low responders had a 52% reduction in risk of heart failure events, compared with ICD recipients, while CRT-D high responders had an 81% relative risk reduction. Similarly, in patients with a baseline LVEF of 30% or less, CRT-D low responders had 48% reduction in heart failure events and high responders had a 79% risk reduction, compared with the ICD-only group.
Because this is a post hoc analysis, these new MADIT-CRT findings require validation in future studies, Dr. Vermilye observed.
MADIT-CRT was supported by Boston Scientific. Dr.. Vermilye reported having no financial conflicts.
WASHINGTON – Patients with mild heart failure symptoms, left bundle branch block, and a left ventricular ejection fraction of 31% to 44% who received cardiac resynchronization therapy with a built-in defibrillator experienced a significant reduction in all-cause mortality, compared with those randomized to an implantable cardioverter-defibrillator alone during 7 years of follow-up.
These results from a new MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) long-term follow-up substudy “suggest that patients with a relatively preserved ejection fraction greater than 30% benefit from CRT-D [cardiac resynchronization therapy defibrillator] and could potentially be considered for this therapy,” said Katherine Vermilye, MD, at the annual meeting of the American College of Cardiology.
In a subsequent publication, the MADIT-CRT investigators reported that, with extension of follow-up to 7 years, CRT-D also provided a significant benefit in terms of all-cause mortality in addition to the reduced rate of heart failure events (N Engl J Med. 2014 May 1;370[18]:1694-701).
However, even though an LVEF of 30% or less was a requirement for participation in MADIT-CRT, it turned out that, when the initial screening echocardiograms were eventually analyzed in a central core laboratory, one-third of study participants actually had a baseline LVEF of 31% to 44%, with the majority of excessive values being in the 31%-35% range.
Dr. Vermilye, of the University of Rochester in New York, presented a post hoc analysis of long-term outcomes in the subgroup having a baseline LVEF greater than 30%. They totaled 450 of 1,224 MADIT-CRT participants with left bundle branch block. They were significantly older and more likely to be female than the 824 subjects with an LVEF of 30% or less. They also had a shorter QRS duration – an average of 160 ms, versus 165 ms in patients with an LVEF of 30% or lower – and a smaller baseline left ventricular end systolic volume of 151 mL, compared with 196 mL in patients with a lower LVEF.
In a multivariate Cox regression analysis adjusted for potential confounders, CRT-D in patients with a baseline LVEF greater than 30% was associated with a 54% reduction in the risk of all-cause mortality at 7 years of follow-up, compared with receipt of an ICD-only device and with a smaller yet significant 31% reduction in risk in those with an LVEF of 30% or less. Worsening heart failure events were reduced by 64% in patients with a baseline LVEF greater than 30% who received CRT-D, compared with ICD-only, and by 54% in those with a lower baseline LVEF.
The reduction in all-cause mortality seen with CRT-D was confined to patients who were high responders to CRT as defined echocardiographically by at least a 35% change in left ventricular end systolic volume 1 year post implantation. They had an 85% reduction in the risk of death during 7 years of follow-up with CRT-D if their baseline LVEF was greater than 30% and a 58% relative risk reduction if their LVEF was 30% or less.
In contrast, CRT-D brought a significantly reduced risk of heart failure events regardless of whether a patient was a low or high responder, although the magnitude of benefit was greater in the high responders. Among patients with a baseline LVEF greater than 30%, CRT-D low responders had a 52% reduction in risk of heart failure events, compared with ICD recipients, while CRT-D high responders had an 81% relative risk reduction. Similarly, in patients with a baseline LVEF of 30% or less, CRT-D low responders had 48% reduction in heart failure events and high responders had a 79% risk reduction, compared with the ICD-only group.
Because this is a post hoc analysis, these new MADIT-CRT findings require validation in future studies, Dr. Vermilye observed.
MADIT-CRT was supported by Boston Scientific. Dr.. Vermilye reported having no financial conflicts.
AT ACC 2017
Key clinical point:
Major finding: The risk of all-cause mortality was reduced by 54% with CRT-D as compared with an ICD alone in MADIT-CRT participants with a baseline LVEF greater than 30% and by 31% in those with an LVEF of 30% or lower.
Data source: An analysis of 7-year rates of all-cause mortality and worsening heart failure events in 1,224 MADIT-CRT participants with left bundle branch block, 450 of whom had a baseline LVEF greater than 30%.
Disclosures: The MADIT-CRT study was supported by Boston Scientific. The presenter reported having no financial conflicts.
High readmits after peripheral arterial procedures
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
AT ACC 2017
Key clinical point:
Major finding: Readmission within 30 days after a peripheral arterial procedure occurred nationally in 17.4% of patients, with little between-hospital variation in rates.
Data source: A retrospective analysis of nearly 124,000 hospital admissions for lower extremity arterial endovascular or surgical procedures.
Disclosures: The study presenter reported having no financial conflicts of interest.
Refining SLE cardiovascular risk estimation
WASHINGTON – Red blood cell distribution width provides a novel tool for cardiovascular risk stratification in patients with systemic lupus erythematosus (SLE), Chang H. Kim, MD, reported at the annual meeting of the American College of Cardiology.
In a retrospective cohort study of nearly 70,000 patients with SLE, the 10-year rate of major adverse cardiovascular events (MACE) rose stepwise according to quintile of red cell distribution width (RDW) from 5.3% in patients with an RDW of 13.2% or less to 38.6% in those with an RDW of 15.8% or greater, according to Dr. Kim of Case Western Reserve University in Cleveland.
He utilized the Explorys database to determine the 10-year cumulative incidence of MACE – defined as acute MI, heart failure, or cerebrovascular accident – during 2007-2016 in 69,920 patients with SLE and 14,825,240 controls. Explorys is an 8-year-old Cleveland-based company that maintains a health care database incorporating 26 health care systems across the United States with nearly 50 million patients. It is part of IBM Watson Health.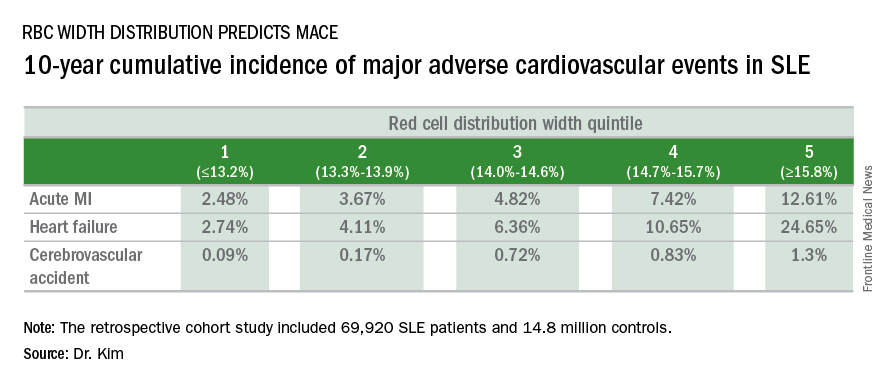
The MACE rate in patients with SLE displayed a graded increase in association with RDW quintile as measured in a routine CBC. (See table.) MACE rates were significantly higher in male than female SLE patients, but the graded relationship between RDW quintile and 10-year incidence of MACE persisted after adjustment for gender and the presence of anemia.
A graded association between RDW quintile and MACE also was noted in the control group of nearly 15 million individuals, but the absolute incidence of MACE in the non-SLE controls was far lower.
Dr. Kim reported having no financial conflicts regarding this unfunded study.
WASHINGTON – Red blood cell distribution width provides a novel tool for cardiovascular risk stratification in patients with systemic lupus erythematosus (SLE), Chang H. Kim, MD, reported at the annual meeting of the American College of Cardiology.
In a retrospective cohort study of nearly 70,000 patients with SLE, the 10-year rate of major adverse cardiovascular events (MACE) rose stepwise according to quintile of red cell distribution width (RDW) from 5.3% in patients with an RDW of 13.2% or less to 38.6% in those with an RDW of 15.8% or greater, according to Dr. Kim of Case Western Reserve University in Cleveland.
He utilized the Explorys database to determine the 10-year cumulative incidence of MACE – defined as acute MI, heart failure, or cerebrovascular accident – during 2007-2016 in 69,920 patients with SLE and 14,825,240 controls. Explorys is an 8-year-old Cleveland-based company that maintains a health care database incorporating 26 health care systems across the United States with nearly 50 million patients. It is part of IBM Watson Health.
The MACE rate in patients with SLE displayed a graded increase in association with RDW quintile as measured in a routine CBC. (See table.) MACE rates were significantly higher in male than female SLE patients, but the graded relationship between RDW quintile and 10-year incidence of MACE persisted after adjustment for gender and the presence of anemia.
A graded association between RDW quintile and MACE also was noted in the control group of nearly 15 million individuals, but the absolute incidence of MACE in the non-SLE controls was far lower.
Dr. Kim reported having no financial conflicts regarding this unfunded study.
WASHINGTON – Red blood cell distribution width provides a novel tool for cardiovascular risk stratification in patients with systemic lupus erythematosus (SLE), Chang H. Kim, MD, reported at the annual meeting of the American College of Cardiology.
In a retrospective cohort study of nearly 70,000 patients with SLE, the 10-year rate of major adverse cardiovascular events (MACE) rose stepwise according to quintile of red cell distribution width (RDW) from 5.3% in patients with an RDW of 13.2% or less to 38.6% in those with an RDW of 15.8% or greater, according to Dr. Kim of Case Western Reserve University in Cleveland.
He utilized the Explorys database to determine the 10-year cumulative incidence of MACE – defined as acute MI, heart failure, or cerebrovascular accident – during 2007-2016 in 69,920 patients with SLE and 14,825,240 controls. Explorys is an 8-year-old Cleveland-based company that maintains a health care database incorporating 26 health care systems across the United States with nearly 50 million patients. It is part of IBM Watson Health.
The MACE rate in patients with SLE displayed a graded increase in association with RDW quintile as measured in a routine CBC. (See table.) MACE rates were significantly higher in male than female SLE patients, but the graded relationship between RDW quintile and 10-year incidence of MACE persisted after adjustment for gender and the presence of anemia.
A graded association between RDW quintile and MACE also was noted in the control group of nearly 15 million individuals, but the absolute incidence of MACE in the non-SLE controls was far lower.
Dr. Kim reported having no financial conflicts regarding this unfunded study.
AT ACC 2017
Key clinical point:
Major finding: Systemic lupus erythematosus patients in the top quintile of RBC distribution width had a 10-year incidence of major adverse cardiovascular events of 39%.
Data source: This retrospective cohort study included nearly 70,000 SLE patients and 14.8 million controls.
Disclosures: The presenter reported no financial conflicts with regard to this unfunded study.
High early stroke risk for adult congenital heart disease
WASHINGTON – Adults with congenital heart disease are at fourfold greater risk of experiencing an ischemic stroke by age 60 than is the general population, Mette Glavind reported at the annual meeting of the American College of Cardiology.
She presented a population-based study that included all 14,710 Danish adults with congenital heart disease (ACHD) diagnosed in 1963-1994. Taking advantage of Denmark’s comprehensive system of linked national registries, she and her coinvestigators created a control group consisting of 144,735 age- and birth year–matched individuals from the general population.
During follow-up, a total of 2,868 Danes included in the study had an ischemic stroke. The cumulative incidence of ischemic stroke in the ACHD cohort was 0.8% by age 30 and 8.2% by age 60, compared with 0.09% and 2.9%, respectively, in controls, according to Ms. Glavind, a medical student at Aarhus (Denmark) University.
The median age at diagnosis of stroke was 52 years in the ACHD group and 69 years in controls. The risk of early ischemic stroke – defined as stroke at age 18-60 – was increased by 3.97-fold in the ACHD group, compared with controls. The risk of stroke after age 60 was increased by 1.68-fold.
Stroke was more likely to prove fatal in the ACHD group. Their 30-day stroke mortality rate was 10%, compared with 9.6% in controls. This corresponded to an adjusted 44% increased risk of stroke mortality, which was statistically significant.
The severity of congenital heart disease modified the stroke risk. Patients with mild or moderate ACHD had a 3.25-fold increased risk of early stroke, compared with controls, while those with severe or univentricular ACHD were at 5.97-fold greater risk.
For purposes of this study, mild ACHD was defined as a biventricular defect that was not repaired surgically or percutaneously. Moderate ACHD was considered to have biventricular pathophysiology with surgical or percutaneous intervention. The severe ACHD category was reserved for cases involving complex biventricular abnormalities.
By these definitions, 41% of patients had mild ACHD, 21% moderate, 22% severe, and 1% univentricular ACHD; the rest of the patients were unclassified.
This study was supported by Aarhus University and Cincinnati Children’s Hospital. Ms. Glavind reported having no financial conflicts.
WASHINGTON – Adults with congenital heart disease are at fourfold greater risk of experiencing an ischemic stroke by age 60 than is the general population, Mette Glavind reported at the annual meeting of the American College of Cardiology.
She presented a population-based study that included all 14,710 Danish adults with congenital heart disease (ACHD) diagnosed in 1963-1994. Taking advantage of Denmark’s comprehensive system of linked national registries, she and her coinvestigators created a control group consisting of 144,735 age- and birth year–matched individuals from the general population.
During follow-up, a total of 2,868 Danes included in the study had an ischemic stroke. The cumulative incidence of ischemic stroke in the ACHD cohort was 0.8% by age 30 and 8.2% by age 60, compared with 0.09% and 2.9%, respectively, in controls, according to Ms. Glavind, a medical student at Aarhus (Denmark) University.
The median age at diagnosis of stroke was 52 years in the ACHD group and 69 years in controls. The risk of early ischemic stroke – defined as stroke at age 18-60 – was increased by 3.97-fold in the ACHD group, compared with controls. The risk of stroke after age 60 was increased by 1.68-fold.
Stroke was more likely to prove fatal in the ACHD group. Their 30-day stroke mortality rate was 10%, compared with 9.6% in controls. This corresponded to an adjusted 44% increased risk of stroke mortality, which was statistically significant.
The severity of congenital heart disease modified the stroke risk. Patients with mild or moderate ACHD had a 3.25-fold increased risk of early stroke, compared with controls, while those with severe or univentricular ACHD were at 5.97-fold greater risk.
For purposes of this study, mild ACHD was defined as a biventricular defect that was not repaired surgically or percutaneously. Moderate ACHD was considered to have biventricular pathophysiology with surgical or percutaneous intervention. The severe ACHD category was reserved for cases involving complex biventricular abnormalities.
By these definitions, 41% of patients had mild ACHD, 21% moderate, 22% severe, and 1% univentricular ACHD; the rest of the patients were unclassified.
This study was supported by Aarhus University and Cincinnati Children’s Hospital. Ms. Glavind reported having no financial conflicts.
WASHINGTON – Adults with congenital heart disease are at fourfold greater risk of experiencing an ischemic stroke by age 60 than is the general population, Mette Glavind reported at the annual meeting of the American College of Cardiology.
She presented a population-based study that included all 14,710 Danish adults with congenital heart disease (ACHD) diagnosed in 1963-1994. Taking advantage of Denmark’s comprehensive system of linked national registries, she and her coinvestigators created a control group consisting of 144,735 age- and birth year–matched individuals from the general population.
During follow-up, a total of 2,868 Danes included in the study had an ischemic stroke. The cumulative incidence of ischemic stroke in the ACHD cohort was 0.8% by age 30 and 8.2% by age 60, compared with 0.09% and 2.9%, respectively, in controls, according to Ms. Glavind, a medical student at Aarhus (Denmark) University.
The median age at diagnosis of stroke was 52 years in the ACHD group and 69 years in controls. The risk of early ischemic stroke – defined as stroke at age 18-60 – was increased by 3.97-fold in the ACHD group, compared with controls. The risk of stroke after age 60 was increased by 1.68-fold.
Stroke was more likely to prove fatal in the ACHD group. Their 30-day stroke mortality rate was 10%, compared with 9.6% in controls. This corresponded to an adjusted 44% increased risk of stroke mortality, which was statistically significant.
The severity of congenital heart disease modified the stroke risk. Patients with mild or moderate ACHD had a 3.25-fold increased risk of early stroke, compared with controls, while those with severe or univentricular ACHD were at 5.97-fold greater risk.
For purposes of this study, mild ACHD was defined as a biventricular defect that was not repaired surgically or percutaneously. Moderate ACHD was considered to have biventricular pathophysiology with surgical or percutaneous intervention. The severe ACHD category was reserved for cases involving complex biventricular abnormalities.
By these definitions, 41% of patients had mild ACHD, 21% moderate, 22% severe, and 1% univentricular ACHD; the rest of the patients were unclassified.
This study was supported by Aarhus University and Cincinnati Children’s Hospital. Ms. Glavind reported having no financial conflicts.
AT ACC 2017
Key clinical point:
Major finding: Danish adults with complex biventricular congenital heart disease were sixfold more likely to have an ischemic stroke by age 60 years, compared with the general population.
Data source: A population-based registry study that included all Danish adults with congenital heart disease diagnosed in 1963-1994 and nearly 145,000 age- and birth year–matched controls drawn from the general Danish population.
Disclosures: This study was supported by Aarhus University and Cincinnati Children’s Hospital. The presenter reported having no financial conflicts.
Statins in HIV-positive patients are a missed opportunity
WASHINGTON – Only one in four U.S. adults with known HIV infection is on statin therapy, even though premature coronary heart disease has become a leading cause of morbidity and mortality for HIV-positive individuals in an era of greatly extended life expectancy due to highly active antiretroviral therapy, Robert S. Rosenson, MD, reported at the annual meeting of the American College of Cardiology.
Not only are statins greatly underutilized in HIV-positive patients, but in a large national observational study, 8% of those who were on a statin were on one that was contraindicated because of concomitant use of antiretroviral therapy or other agents posing a risk for major drug interactions, added Dr. Rosenson, professor of medicine and director of cardiometabolic disorders at Icahn School of Medicine at Mount Sinai, New York.
He presented a retrospective cohort study of 23,119 HIV-positive U.S. adults with commercial health insurance in 2014. The data source was the MarketScan database.
During the study year, 5,931 HIV-positive adults (26%) were taking a statin. In a multivariate regression analysis, factors associated with increased likelihood of statin therapy were age 50 years or older, male gender, known coronary heart disease, hypertension, and diabetes.
A total of 491 patients were taking a contraindicated statin or a statin at too high a dose to be safe, given the other medications they were on.
The protease inhibitors, nonnucleoside reverse transcriptase inhibitors, and some statins are metabolized by the cytochrome P450 isoenzyme CYP3A4. As a consequence, areas under the curve for simvastatin and lovastatin skyrocket when those statins are given with some protease inhibitors, placing patients at increased risk for myopathy, including rhabdomyolysis.
As a consequence, simvastatin and lovastatin are widely considered contraindicated in HIV-positive patients.
Infectious Diseases Society of America guidelines recommend pravastatin or low-dose atorvastatin at 10 mg/day as first-line therapy for lipid-lowering and cardiovascular risk reduction in HIV-positive patients, with fluvastatin a reasonable alternative.
Dr. Rosenson noted that in his study, 62% of the HIV-infected patients on a contraindicated statin were taking a protease inhibitor, 29% were on gemfibrozil (Lopid), and the remaining handful were on other agents posing significant risk of drug interactions, including azole antifungals, calcium channel blockers, or cobicistat (Tybost).
The statin study was jointly funded by Amgen, Mount Sinai, and the University of Alabama. Dr. Rosenson reported serving as a consultant to Amgen, Eli Lilly, Regeneron, and Sanofi.
WASHINGTON – Only one in four U.S. adults with known HIV infection is on statin therapy, even though premature coronary heart disease has become a leading cause of morbidity and mortality for HIV-positive individuals in an era of greatly extended life expectancy due to highly active antiretroviral therapy, Robert S. Rosenson, MD, reported at the annual meeting of the American College of Cardiology.
Not only are statins greatly underutilized in HIV-positive patients, but in a large national observational study, 8% of those who were on a statin were on one that was contraindicated because of concomitant use of antiretroviral therapy or other agents posing a risk for major drug interactions, added Dr. Rosenson, professor of medicine and director of cardiometabolic disorders at Icahn School of Medicine at Mount Sinai, New York.
He presented a retrospective cohort study of 23,119 HIV-positive U.S. adults with commercial health insurance in 2014. The data source was the MarketScan database.
During the study year, 5,931 HIV-positive adults (26%) were taking a statin. In a multivariate regression analysis, factors associated with increased likelihood of statin therapy were age 50 years or older, male gender, known coronary heart disease, hypertension, and diabetes.
A total of 491 patients were taking a contraindicated statin or a statin at too high a dose to be safe, given the other medications they were on.
The protease inhibitors, nonnucleoside reverse transcriptase inhibitors, and some statins are metabolized by the cytochrome P450 isoenzyme CYP3A4. As a consequence, areas under the curve for simvastatin and lovastatin skyrocket when those statins are given with some protease inhibitors, placing patients at increased risk for myopathy, including rhabdomyolysis.
As a consequence, simvastatin and lovastatin are widely considered contraindicated in HIV-positive patients.
Infectious Diseases Society of America guidelines recommend pravastatin or low-dose atorvastatin at 10 mg/day as first-line therapy for lipid-lowering and cardiovascular risk reduction in HIV-positive patients, with fluvastatin a reasonable alternative.
Dr. Rosenson noted that in his study, 62% of the HIV-infected patients on a contraindicated statin were taking a protease inhibitor, 29% were on gemfibrozil (Lopid), and the remaining handful were on other agents posing significant risk of drug interactions, including azole antifungals, calcium channel blockers, or cobicistat (Tybost).
The statin study was jointly funded by Amgen, Mount Sinai, and the University of Alabama. Dr. Rosenson reported serving as a consultant to Amgen, Eli Lilly, Regeneron, and Sanofi.
WASHINGTON – Only one in four U.S. adults with known HIV infection is on statin therapy, even though premature coronary heart disease has become a leading cause of morbidity and mortality for HIV-positive individuals in an era of greatly extended life expectancy due to highly active antiretroviral therapy, Robert S. Rosenson, MD, reported at the annual meeting of the American College of Cardiology.
Not only are statins greatly underutilized in HIV-positive patients, but in a large national observational study, 8% of those who were on a statin were on one that was contraindicated because of concomitant use of antiretroviral therapy or other agents posing a risk for major drug interactions, added Dr. Rosenson, professor of medicine and director of cardiometabolic disorders at Icahn School of Medicine at Mount Sinai, New York.
He presented a retrospective cohort study of 23,119 HIV-positive U.S. adults with commercial health insurance in 2014. The data source was the MarketScan database.
During the study year, 5,931 HIV-positive adults (26%) were taking a statin. In a multivariate regression analysis, factors associated with increased likelihood of statin therapy were age 50 years or older, male gender, known coronary heart disease, hypertension, and diabetes.
A total of 491 patients were taking a contraindicated statin or a statin at too high a dose to be safe, given the other medications they were on.
The protease inhibitors, nonnucleoside reverse transcriptase inhibitors, and some statins are metabolized by the cytochrome P450 isoenzyme CYP3A4. As a consequence, areas under the curve for simvastatin and lovastatin skyrocket when those statins are given with some protease inhibitors, placing patients at increased risk for myopathy, including rhabdomyolysis.
As a consequence, simvastatin and lovastatin are widely considered contraindicated in HIV-positive patients.
Infectious Diseases Society of America guidelines recommend pravastatin or low-dose atorvastatin at 10 mg/day as first-line therapy for lipid-lowering and cardiovascular risk reduction in HIV-positive patients, with fluvastatin a reasonable alternative.
Dr. Rosenson noted that in his study, 62% of the HIV-infected patients on a contraindicated statin were taking a protease inhibitor, 29% were on gemfibrozil (Lopid), and the remaining handful were on other agents posing significant risk of drug interactions, including azole antifungals, calcium channel blockers, or cobicistat (Tybost).
The statin study was jointly funded by Amgen, Mount Sinai, and the University of Alabama. Dr. Rosenson reported serving as a consultant to Amgen, Eli Lilly, Regeneron, and Sanofi.
AT ACC 2017
Key clinical point:
Major finding: Lipid-lowering in HIV-positive adults can be tricky: Eight percent of those who were on statin therapy were taking a statin contraindicated in HIV-infected individuals.
Data source: A retrospective study of more than 23,000 HIV-positive U.S. adults with commercial health insurance in 2014.
Disclosures: The statin study was jointly funded by Amgen and two U.S. medical schools. Dr. Rosenson reported serving as a consultant to Amgen, Eli Lilly, Regeneron, and Sanofi.
Antianginal agents underutilized after MI
WASHINGTON – Non–beta-blocker antianginal drugs are seriously underprescribed in patients with angina following percutaneous coronary intervention (PCI) for a myocardial infarction (MI), according to a large national study, Alexander C. Fanaroff, MD, said at the annual meeting of the American College of Cardiology.
Clinical practice in this regard appears to be largely out of touch with current evidence-based guidelines regarding angina management in patients with stable ischemic heart disease. Those ACC/American Heart Association guidelines recommend a stepwise approach beginning with a beta-blocker and sublingual nitroglycerin, adding a calcium channel blocker if the beta-blocker isn’t tolerated or effective, further adding a long-acting nitrate if symptoms persist, incorporating ranolazine (Ranexa) as needed, and finally turning to revascularization for symptomatic relief if multidrug therapy proves inadequate (J Am Coll Cardiol. 2012 Dec 18;60[24]:e44-e164).
“When you ask patients and physicians separately about the prevalence of angina, they tend to disagree, with physicians consistently thinking their patients have less angina than patients report themselves. Together, with our data, this indicates that strategies to improve clinician recognition and treatment of angina symptoms are needed,” he asserted.
TRANSLATE-ACS was a large, longitudinal, observational study of patients who underwent PCI at 233 U.S. hospitals during 2010-2012. Dr. Fanaroff focused on 12-month follow-up data on the 10,870 patients whose PCI was for treatment of an acute MI. In telephone interviews 6 weeks postdischarge which incorporated the validated Seattle Angina Questionnaire, 3,190 of these individuals (31%) with stable ischemic heart disease reported angina symptoms. Of those, 79% said their symptoms occurred at least monthly, 18% weekly, and 3% daily. Yet, only 1 in 5 patients with angina 6 weeks postdischarge were on a non–beta-blocker antianginal medication: 16% were on monotherapy with a calcium channel blocker or long-acting nitrate, 3% were on dual therapy, and less than 1% were on three non–beta-blockers for their angina.
The prevalence of angina dropped over time. At 6 months postdischarge, 40% of the original cohort of patients who had angina at 6 weeks still reported angina. At 12 months, it was 33%. Meanwhile, the use of non–beta-blocker antianginal agents crept up modestly over time, from 20% in patients with angina at 6 weeks postdischarge, to 22% in those with angina at 6 months, and 23% at 12 months.
On the other hand, 33% of patients with angina at 6 weeks had persistent angina through 12 months. Remarkably, 69% of these individuals never received a non–beta-blocker antianginal drug during all that time, including 61% of those who experienced daily or weekly angina.
The use of non–beta-blocker antianginal drugs was more frequent in patients with more severe angina: 25% of patients with daily or weekly angina at 6 weeks were on such medication, as were 34% at 6 months and 38% at 12 months, compared with 16%, 18%, and 22% of those with monthly angina. Still, most of these patients were on only a single non–beta-blocker antianginal agent.
Of patients with angina at 6 weeks, 12% subsequently underwent repeat PCI at some point during the first year postdischarge. Of these individuals, 19% were on a single non–beta-blocker antianginal drug at the time of their procedure, 6% were on two such drugs, and only 1% were on three.
Roughly 10% of patients with multivessel coronary disease who were identified angiographically at the time of their acute MI did not undergo multivessel PCI at that time. This was not predictive of angina 6 weeks post discharge, Dr. Fanaroff continued.
One audience member asked Dr. Fanaroff how he knows that the patients with self-reported angina didn’t actually have noncardiac chest pain, in which case their physicians’ decision not to prescribe second- and third-tier antianginal drugs would be appropriate.
Dr. Fanaroff responded that it’s possible, and even likely, that some patients with self-reported angina had a noncardiac source of their chest pain. However, they would be in the minority. The fact is that multiple studies have shown that patients who report experiencing angina after PCI for acute MI have about a 1.5-times increased risk of readmission and repeat revascularization, a 2-times increased risk of developing new depressive symptoms, and diminished quality of life scores.
The TRANSLATE-ACS study was sponsored by Eli Lilly. Dr. Fanaroff reported having no financial conflicts.
WASHINGTON – Non–beta-blocker antianginal drugs are seriously underprescribed in patients with angina following percutaneous coronary intervention (PCI) for a myocardial infarction (MI), according to a large national study, Alexander C. Fanaroff, MD, said at the annual meeting of the American College of Cardiology.
Clinical practice in this regard appears to be largely out of touch with current evidence-based guidelines regarding angina management in patients with stable ischemic heart disease. Those ACC/American Heart Association guidelines recommend a stepwise approach beginning with a beta-blocker and sublingual nitroglycerin, adding a calcium channel blocker if the beta-blocker isn’t tolerated or effective, further adding a long-acting nitrate if symptoms persist, incorporating ranolazine (Ranexa) as needed, and finally turning to revascularization for symptomatic relief if multidrug therapy proves inadequate (J Am Coll Cardiol. 2012 Dec 18;60[24]:e44-e164).
“When you ask patients and physicians separately about the prevalence of angina, they tend to disagree, with physicians consistently thinking their patients have less angina than patients report themselves. Together, with our data, this indicates that strategies to improve clinician recognition and treatment of angina symptoms are needed,” he asserted.
TRANSLATE-ACS was a large, longitudinal, observational study of patients who underwent PCI at 233 U.S. hospitals during 2010-2012. Dr. Fanaroff focused on 12-month follow-up data on the 10,870 patients whose PCI was for treatment of an acute MI. In telephone interviews 6 weeks postdischarge which incorporated the validated Seattle Angina Questionnaire, 3,190 of these individuals (31%) with stable ischemic heart disease reported angina symptoms. Of those, 79% said their symptoms occurred at least monthly, 18% weekly, and 3% daily. Yet, only 1 in 5 patients with angina 6 weeks postdischarge were on a non–beta-blocker antianginal medication: 16% were on monotherapy with a calcium channel blocker or long-acting nitrate, 3% were on dual therapy, and less than 1% were on three non–beta-blockers for their angina.
The prevalence of angina dropped over time. At 6 months postdischarge, 40% of the original cohort of patients who had angina at 6 weeks still reported angina. At 12 months, it was 33%. Meanwhile, the use of non–beta-blocker antianginal agents crept up modestly over time, from 20% in patients with angina at 6 weeks postdischarge, to 22% in those with angina at 6 months, and 23% at 12 months.
On the other hand, 33% of patients with angina at 6 weeks had persistent angina through 12 months. Remarkably, 69% of these individuals never received a non–beta-blocker antianginal drug during all that time, including 61% of those who experienced daily or weekly angina.
The use of non–beta-blocker antianginal drugs was more frequent in patients with more severe angina: 25% of patients with daily or weekly angina at 6 weeks were on such medication, as were 34% at 6 months and 38% at 12 months, compared with 16%, 18%, and 22% of those with monthly angina. Still, most of these patients were on only a single non–beta-blocker antianginal agent.
Of patients with angina at 6 weeks, 12% subsequently underwent repeat PCI at some point during the first year postdischarge. Of these individuals, 19% were on a single non–beta-blocker antianginal drug at the time of their procedure, 6% were on two such drugs, and only 1% were on three.
Roughly 10% of patients with multivessel coronary disease who were identified angiographically at the time of their acute MI did not undergo multivessel PCI at that time. This was not predictive of angina 6 weeks post discharge, Dr. Fanaroff continued.
One audience member asked Dr. Fanaroff how he knows that the patients with self-reported angina didn’t actually have noncardiac chest pain, in which case their physicians’ decision not to prescribe second- and third-tier antianginal drugs would be appropriate.
Dr. Fanaroff responded that it’s possible, and even likely, that some patients with self-reported angina had a noncardiac source of their chest pain. However, they would be in the minority. The fact is that multiple studies have shown that patients who report experiencing angina after PCI for acute MI have about a 1.5-times increased risk of readmission and repeat revascularization, a 2-times increased risk of developing new depressive symptoms, and diminished quality of life scores.
The TRANSLATE-ACS study was sponsored by Eli Lilly. Dr. Fanaroff reported having no financial conflicts.
WASHINGTON – Non–beta-blocker antianginal drugs are seriously underprescribed in patients with angina following percutaneous coronary intervention (PCI) for a myocardial infarction (MI), according to a large national study, Alexander C. Fanaroff, MD, said at the annual meeting of the American College of Cardiology.
Clinical practice in this regard appears to be largely out of touch with current evidence-based guidelines regarding angina management in patients with stable ischemic heart disease. Those ACC/American Heart Association guidelines recommend a stepwise approach beginning with a beta-blocker and sublingual nitroglycerin, adding a calcium channel blocker if the beta-blocker isn’t tolerated or effective, further adding a long-acting nitrate if symptoms persist, incorporating ranolazine (Ranexa) as needed, and finally turning to revascularization for symptomatic relief if multidrug therapy proves inadequate (J Am Coll Cardiol. 2012 Dec 18;60[24]:e44-e164).
“When you ask patients and physicians separately about the prevalence of angina, they tend to disagree, with physicians consistently thinking their patients have less angina than patients report themselves. Together, with our data, this indicates that strategies to improve clinician recognition and treatment of angina symptoms are needed,” he asserted.
TRANSLATE-ACS was a large, longitudinal, observational study of patients who underwent PCI at 233 U.S. hospitals during 2010-2012. Dr. Fanaroff focused on 12-month follow-up data on the 10,870 patients whose PCI was for treatment of an acute MI. In telephone interviews 6 weeks postdischarge which incorporated the validated Seattle Angina Questionnaire, 3,190 of these individuals (31%) with stable ischemic heart disease reported angina symptoms. Of those, 79% said their symptoms occurred at least monthly, 18% weekly, and 3% daily. Yet, only 1 in 5 patients with angina 6 weeks postdischarge were on a non–beta-blocker antianginal medication: 16% were on monotherapy with a calcium channel blocker or long-acting nitrate, 3% were on dual therapy, and less than 1% were on three non–beta-blockers for their angina.
The prevalence of angina dropped over time. At 6 months postdischarge, 40% of the original cohort of patients who had angina at 6 weeks still reported angina. At 12 months, it was 33%. Meanwhile, the use of non–beta-blocker antianginal agents crept up modestly over time, from 20% in patients with angina at 6 weeks postdischarge, to 22% in those with angina at 6 months, and 23% at 12 months.
On the other hand, 33% of patients with angina at 6 weeks had persistent angina through 12 months. Remarkably, 69% of these individuals never received a non–beta-blocker antianginal drug during all that time, including 61% of those who experienced daily or weekly angina.
The use of non–beta-blocker antianginal drugs was more frequent in patients with more severe angina: 25% of patients with daily or weekly angina at 6 weeks were on such medication, as were 34% at 6 months and 38% at 12 months, compared with 16%, 18%, and 22% of those with monthly angina. Still, most of these patients were on only a single non–beta-blocker antianginal agent.
Of patients with angina at 6 weeks, 12% subsequently underwent repeat PCI at some point during the first year postdischarge. Of these individuals, 19% were on a single non–beta-blocker antianginal drug at the time of their procedure, 6% were on two such drugs, and only 1% were on three.
Roughly 10% of patients with multivessel coronary disease who were identified angiographically at the time of their acute MI did not undergo multivessel PCI at that time. This was not predictive of angina 6 weeks post discharge, Dr. Fanaroff continued.
One audience member asked Dr. Fanaroff how he knows that the patients with self-reported angina didn’t actually have noncardiac chest pain, in which case their physicians’ decision not to prescribe second- and third-tier antianginal drugs would be appropriate.
Dr. Fanaroff responded that it’s possible, and even likely, that some patients with self-reported angina had a noncardiac source of their chest pain. However, they would be in the minority. The fact is that multiple studies have shown that patients who report experiencing angina after PCI for acute MI have about a 1.5-times increased risk of readmission and repeat revascularization, a 2-times increased risk of developing new depressive symptoms, and diminished quality of life scores.
The TRANSLATE-ACS study was sponsored by Eli Lilly. Dr. Fanaroff reported having no financial conflicts.
AT ACC 17
Key clinical point:
Major finding: Only 31% of patients who reported persistent angina for the first 12 months post PCI for acute MI reported taking any non–beta-blocker antianginal drugs during that period.
Data source: An analysis of nearly 11,000 patients who underwent PCI for an acute MI at 233 U.S. hospitals in the longitudinal observational TRANSLATE-ACS study.
Disclosures: The TRANSLATE-ACS study was sponsored by Eli Lilly. The presenter reported having no financial conflicts.













