User login
Smaller, intrapericardial LVAD noninferior to HeartMate II
A smaller, centrifugal-flow left ventricular assist device that lies entirely within the pericardial space was found noninferior to the HeartMate II axial-flow device in patients with advanced heart failure who weren’t eligible for heart transplant, according to a report published online Feb. 2 in the New England Journal of Medicine.
The two LVADs were compared in ENDURANCE (A Clinical Trial to Evaluate the HeartWare Ventricular Assist System), a prospective, randomized trial in 445 patients who were treated at 48 U.S. sites and followed for 2 years. The study participants had an LV ejection fraction of 25% or less and high prevalences of abnormal renal function and dependence on intravenous inotropic support, said Joseph G. Rogers, MD, of Duke University, Durham, N.C., and his associates.
The study participants were randomly assigned to receive the HeartWare, an investigational centrifugal-flow LVAD (297 patients) or the standard axial-flow HeartMate II LVAD (148 patients). In the intention-to-treat analysis, the primary endpoint – a composite of survival free from disabling stroke and no removal of the device for malfunction or failure – was 55.4% with the new device and 59.1% in the control group. The results were similar in the per-protocol and the as-treated analyses, demonstrating that the new device was noninferior but not superior to the axial-flow LVAD, the investigators said (N Engl J Med. 2017 Feb 2. doi: 10.1056/NEJMoa1602954).
There were significantly more cases of device malfunction or failure requiring urgent surgery in the control group than in the centrifugal-flow group (16.2% vs 8.8%), but significantly more cases of stroke (29.7% vs 12.1%), sepsis, and right heart failure. Rates of major bleeding, cardiac arrhythmia, renal dysfunction, and infection were similar between the two study groups. Overall survival* also was not significantly different (60.2% with the new LVAD and 67.6% in the control group).
Both study groups showed significant and comparable improvement after LVAD implantation. Functional status improved to New York Heart Association class I or II in roughly 80% of patients. Mean 6-minute walk distance improved from 100.2 to 199.4 meters with the new device and from 91.9 to 190.1 meters in the control group, a change that was noted within 3 months of surgery and persisted through the end of follow-up. Similarly, mean scores on the Kansas City Cardiomyopathy Questionnaire improved by 25.8 points and 25.3 points, respectively, and mean scores on the European Quality of Life 5 Dimensions scale improved by 22.5 points and 25.5 points, respectively.
This trial was sponsored by HeartWare, which was also involved in data management and analysis. Dr. Rogers reported having no relevant financial disclosures; his associates reported ties to HeartWare (Medtronic), Thoratec (St. Jude Medical), Novartis, and GE HealthCare.
*Correction 2/2/17: An earlier version of this article misidentified survival rates as mortality rates.
The smaller, fully intrapericardial, centrifugal-flow LVAD met one of its goals: Compared with the existing LVAD, it significantly reduced the need for urgent reoperation due to device malfunction or failure.
However, it did not resolve some of the most important problems with LVAD support. It didn’t reduce stroke risk; in fact, the overall risk of stroke was higher with the new device. It also failed to reduce the risk of bleeding, sepsis, or right heart failure.
It appears that no LVAD is fully superior to the others.
Roland Hetzer, MD, PhD, and Eva M. Delmo Walter, MD, PhD, of Cardio Centrum in Berlin, made these remarks in an accompanying editorial (N Engl J Med. 2017 Feb 2. doi: 10.1056/NEJMe1613755). They reported having no relevant financial disclosures.
The smaller, fully intrapericardial, centrifugal-flow LVAD met one of its goals: Compared with the existing LVAD, it significantly reduced the need for urgent reoperation due to device malfunction or failure.
However, it did not resolve some of the most important problems with LVAD support. It didn’t reduce stroke risk; in fact, the overall risk of stroke was higher with the new device. It also failed to reduce the risk of bleeding, sepsis, or right heart failure.
It appears that no LVAD is fully superior to the others.
Roland Hetzer, MD, PhD, and Eva M. Delmo Walter, MD, PhD, of Cardio Centrum in Berlin, made these remarks in an accompanying editorial (N Engl J Med. 2017 Feb 2. doi: 10.1056/NEJMe1613755). They reported having no relevant financial disclosures.
The smaller, fully intrapericardial, centrifugal-flow LVAD met one of its goals: Compared with the existing LVAD, it significantly reduced the need for urgent reoperation due to device malfunction or failure.
However, it did not resolve some of the most important problems with LVAD support. It didn’t reduce stroke risk; in fact, the overall risk of stroke was higher with the new device. It also failed to reduce the risk of bleeding, sepsis, or right heart failure.
It appears that no LVAD is fully superior to the others.
Roland Hetzer, MD, PhD, and Eva M. Delmo Walter, MD, PhD, of Cardio Centrum in Berlin, made these remarks in an accompanying editorial (N Engl J Med. 2017 Feb 2. doi: 10.1056/NEJMe1613755). They reported having no relevant financial disclosures.
A smaller, centrifugal-flow left ventricular assist device that lies entirely within the pericardial space was found noninferior to the HeartMate II axial-flow device in patients with advanced heart failure who weren’t eligible for heart transplant, according to a report published online Feb. 2 in the New England Journal of Medicine.
The two LVADs were compared in ENDURANCE (A Clinical Trial to Evaluate the HeartWare Ventricular Assist System), a prospective, randomized trial in 445 patients who were treated at 48 U.S. sites and followed for 2 years. The study participants had an LV ejection fraction of 25% or less and high prevalences of abnormal renal function and dependence on intravenous inotropic support, said Joseph G. Rogers, MD, of Duke University, Durham, N.C., and his associates.
The study participants were randomly assigned to receive the HeartWare, an investigational centrifugal-flow LVAD (297 patients) or the standard axial-flow HeartMate II LVAD (148 patients). In the intention-to-treat analysis, the primary endpoint – a composite of survival free from disabling stroke and no removal of the device for malfunction or failure – was 55.4% with the new device and 59.1% in the control group. The results were similar in the per-protocol and the as-treated analyses, demonstrating that the new device was noninferior but not superior to the axial-flow LVAD, the investigators said (N Engl J Med. 2017 Feb 2. doi: 10.1056/NEJMoa1602954).
There were significantly more cases of device malfunction or failure requiring urgent surgery in the control group than in the centrifugal-flow group (16.2% vs 8.8%), but significantly more cases of stroke (29.7% vs 12.1%), sepsis, and right heart failure. Rates of major bleeding, cardiac arrhythmia, renal dysfunction, and infection were similar between the two study groups. Overall survival* also was not significantly different (60.2% with the new LVAD and 67.6% in the control group).
Both study groups showed significant and comparable improvement after LVAD implantation. Functional status improved to New York Heart Association class I or II in roughly 80% of patients. Mean 6-minute walk distance improved from 100.2 to 199.4 meters with the new device and from 91.9 to 190.1 meters in the control group, a change that was noted within 3 months of surgery and persisted through the end of follow-up. Similarly, mean scores on the Kansas City Cardiomyopathy Questionnaire improved by 25.8 points and 25.3 points, respectively, and mean scores on the European Quality of Life 5 Dimensions scale improved by 22.5 points and 25.5 points, respectively.
This trial was sponsored by HeartWare, which was also involved in data management and analysis. Dr. Rogers reported having no relevant financial disclosures; his associates reported ties to HeartWare (Medtronic), Thoratec (St. Jude Medical), Novartis, and GE HealthCare.
*Correction 2/2/17: An earlier version of this article misidentified survival rates as mortality rates.
A smaller, centrifugal-flow left ventricular assist device that lies entirely within the pericardial space was found noninferior to the HeartMate II axial-flow device in patients with advanced heart failure who weren’t eligible for heart transplant, according to a report published online Feb. 2 in the New England Journal of Medicine.
The two LVADs were compared in ENDURANCE (A Clinical Trial to Evaluate the HeartWare Ventricular Assist System), a prospective, randomized trial in 445 patients who were treated at 48 U.S. sites and followed for 2 years. The study participants had an LV ejection fraction of 25% or less and high prevalences of abnormal renal function and dependence on intravenous inotropic support, said Joseph G. Rogers, MD, of Duke University, Durham, N.C., and his associates.
The study participants were randomly assigned to receive the HeartWare, an investigational centrifugal-flow LVAD (297 patients) or the standard axial-flow HeartMate II LVAD (148 patients). In the intention-to-treat analysis, the primary endpoint – a composite of survival free from disabling stroke and no removal of the device for malfunction or failure – was 55.4% with the new device and 59.1% in the control group. The results were similar in the per-protocol and the as-treated analyses, demonstrating that the new device was noninferior but not superior to the axial-flow LVAD, the investigators said (N Engl J Med. 2017 Feb 2. doi: 10.1056/NEJMoa1602954).
There were significantly more cases of device malfunction or failure requiring urgent surgery in the control group than in the centrifugal-flow group (16.2% vs 8.8%), but significantly more cases of stroke (29.7% vs 12.1%), sepsis, and right heart failure. Rates of major bleeding, cardiac arrhythmia, renal dysfunction, and infection were similar between the two study groups. Overall survival* also was not significantly different (60.2% with the new LVAD and 67.6% in the control group).
Both study groups showed significant and comparable improvement after LVAD implantation. Functional status improved to New York Heart Association class I or II in roughly 80% of patients. Mean 6-minute walk distance improved from 100.2 to 199.4 meters with the new device and from 91.9 to 190.1 meters in the control group, a change that was noted within 3 months of surgery and persisted through the end of follow-up. Similarly, mean scores on the Kansas City Cardiomyopathy Questionnaire improved by 25.8 points and 25.3 points, respectively, and mean scores on the European Quality of Life 5 Dimensions scale improved by 22.5 points and 25.5 points, respectively.
This trial was sponsored by HeartWare, which was also involved in data management and analysis. Dr. Rogers reported having no relevant financial disclosures; his associates reported ties to HeartWare (Medtronic), Thoratec (St. Jude Medical), Novartis, and GE HealthCare.
*Correction 2/2/17: An earlier version of this article misidentified survival rates as mortality rates.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A smaller, centrifugal-flow left ventricular assist device was found noninferior to the existing FDA-approved axial-flow device in patients with advanced heart failure who weren’t eligible for heart transplant.
Major finding: The primary endpoint – a composite of survival free from disabling stroke and no removal of the device for malfunction or failure – was 55.4% with the new device and 59.1% in the control group.
Data source: ENDURANCE, a prospective multicenter randomized trial in 445 patients followed for 2 years.
Disclosures: This trial was sponsored by HeartWare, which was also involved in data management and analysis. Dr. Rogers reported having no relevant financial disclosures; his associates reported ties to HeartWare (Medtronic), Thoratec (St. Jude Medical), Novartis, and GE HealthCare.
STICHES boosts CABG role in severe LV dysfunction
SNOWMASS, COLO. – Coronary artery bypass graft surgery in patients with severe ischemic left ventricular dysfunction is overdue for an upgrade in status in the American College of Cardiology/American Heart Association guidelines on the strength of the landmark STICH trial and its extended follow-up stage known as STICHES, according to Vinod H. Thourani, MD.
Currently, the guidelines give CABG in this large and growing population a class IIb recommendation, meaning it “might be considered.” This undervalues the study’s core lesson: “STICHES showed a clear survival benefit with CABG, so this most likely should become a class IIa recommendation,” Dr. Thourani said at the Annual Cardiovascular Conference at Snowmass.
He went on to describe how he applies the key study findings to individual patients.
At 5 and 10 years of follow-up, the probability of all-cause mortality was reduced by 14% and 16%, respectively, in the CABG group. The surgery provided on average an 18-month extension of life. The price paid for the CABG benefit was a 3.6% mortality rate at 30 days; however, this was overcome by the 2-year mark, at which point survival in the CABG group surpassed that in controls. Thereafter, the all-cause mortality gap between the two groups continued to widen for the duration of follow-up.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization, the CABG group enjoyed a 26% relative risk reduction, compared with optimal medical management alone at 5 years, and a 28% reduction in risk at 10 years. The two study groups diverged in terms of risk of cardiovascular hospitalization after only 3 months.
CABG provided a reduction in the risk of cardiovascular death that was consistent across all ages. In contrast, the reduction in all-cause mortality was not, since a higher proportion of deaths in older patients came from cancer and other noncardiovascular causes (Circulation. 2016 Nov 1;134[18]:1314-24).
There have been no randomized, controlled trials of percutaneous coronary intervention in patients with heart failure.
“An interesting finding in STICHES was that medical therapy had a much higher 10-year all-cause mortality the younger the patient was. So CABG particularly benefits those who are at a younger age – in this study, age 60 or less. As you get older, say, at 80 years of age, I’m not sure there’s a huge benefit in all-cause mortality at that point,” said Dr. Thourani, professor of surgery and medicine, and codirector of the structural heart and valve center at Emory University in Atlanta.
In a STICH substudy, roughly half of participants underwent presurgical myocardial viability testing via single-photon emission CT and/or dobutamine echocardiography. The investigators found that the results didn’t predict mortality benefit for CABG (N Engl J Med. 2011 Apr 28;364[17]:1617-25).
More recently, however, other investigators have reported MRI to have prognostic value. For example, Belgian investigators showed that medical therapy in patients with ischemic heart failure and dysfunctional but viable myocardium on delayed-enhanced MRI was associated with a 4.56-fold increased likelihood of mortality during 3 years of follow-up, compared with complete revascularization via CABG (J Am Coll Cardiol. 2012 Feb 28;59[9]:825-35).
“This observation has been useful for me,” Dr. Thourani said. “My own personal practice is if I have good targets, I don’t do viability testing, but if I have really bad targets where I know I’m going to have a tough time sewing grafts, I try to get an MRI for viability testing.”
One important lesson of STICH is that all patients with heart failure and a low left ventricular ejection fraction should have a coronary angiogram, even if they are free of ischemia on noninvasive testing and have no angina. That’s because the patients enrolled in STICH had little or no angina, the surgeon continued.
These STICH-type patients will benefit greatly from a heart team assessment factoring in an individual’s Society of Thoracic Surgeons’ predicted risk score, based on age, comorbidities, and other factors. For example, if a patient’s STS risk score with CABG is 0.7%, that’s a strong argument for opting for the surgery, since the 30-day operative mortality in STICH was 3.6%. If, on the other hand, the STS score is greater than 7%, that’s a tougher call.
“I think it’s really important that a heart team assessment includes a noninvasive cardiologist as well as an interventional cardiologist and cardiac surgeon because I think interventionalists and cardiac surgeons sometimes get a little goofy in their assessment of these patients,” Dr. Thourani said.
Patients with a low ejection fraction and coronary artery disease who are deemed poor candidates for CABG should be evaluated for a mechanical circulatory support device or a heart transplant.
“I think that’s something we don’t think about enough, quite honestly,” he said.
Dr. Thourani reported serving as a consultant to Abbott Vascular, Edwards Lifesciences, and Gore, and receiving research grants from numerous companies.
SNOWMASS, COLO. – Coronary artery bypass graft surgery in patients with severe ischemic left ventricular dysfunction is overdue for an upgrade in status in the American College of Cardiology/American Heart Association guidelines on the strength of the landmark STICH trial and its extended follow-up stage known as STICHES, according to Vinod H. Thourani, MD.
Currently, the guidelines give CABG in this large and growing population a class IIb recommendation, meaning it “might be considered.” This undervalues the study’s core lesson: “STICHES showed a clear survival benefit with CABG, so this most likely should become a class IIa recommendation,” Dr. Thourani said at the Annual Cardiovascular Conference at Snowmass.
He went on to describe how he applies the key study findings to individual patients.
At 5 and 10 years of follow-up, the probability of all-cause mortality was reduced by 14% and 16%, respectively, in the CABG group. The surgery provided on average an 18-month extension of life. The price paid for the CABG benefit was a 3.6% mortality rate at 30 days; however, this was overcome by the 2-year mark, at which point survival in the CABG group surpassed that in controls. Thereafter, the all-cause mortality gap between the two groups continued to widen for the duration of follow-up.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization, the CABG group enjoyed a 26% relative risk reduction, compared with optimal medical management alone at 5 years, and a 28% reduction in risk at 10 years. The two study groups diverged in terms of risk of cardiovascular hospitalization after only 3 months.
CABG provided a reduction in the risk of cardiovascular death that was consistent across all ages. In contrast, the reduction in all-cause mortality was not, since a higher proportion of deaths in older patients came from cancer and other noncardiovascular causes (Circulation. 2016 Nov 1;134[18]:1314-24).
There have been no randomized, controlled trials of percutaneous coronary intervention in patients with heart failure.
“An interesting finding in STICHES was that medical therapy had a much higher 10-year all-cause mortality the younger the patient was. So CABG particularly benefits those who are at a younger age – in this study, age 60 or less. As you get older, say, at 80 years of age, I’m not sure there’s a huge benefit in all-cause mortality at that point,” said Dr. Thourani, professor of surgery and medicine, and codirector of the structural heart and valve center at Emory University in Atlanta.
In a STICH substudy, roughly half of participants underwent presurgical myocardial viability testing via single-photon emission CT and/or dobutamine echocardiography. The investigators found that the results didn’t predict mortality benefit for CABG (N Engl J Med. 2011 Apr 28;364[17]:1617-25).
More recently, however, other investigators have reported MRI to have prognostic value. For example, Belgian investigators showed that medical therapy in patients with ischemic heart failure and dysfunctional but viable myocardium on delayed-enhanced MRI was associated with a 4.56-fold increased likelihood of mortality during 3 years of follow-up, compared with complete revascularization via CABG (J Am Coll Cardiol. 2012 Feb 28;59[9]:825-35).
“This observation has been useful for me,” Dr. Thourani said. “My own personal practice is if I have good targets, I don’t do viability testing, but if I have really bad targets where I know I’m going to have a tough time sewing grafts, I try to get an MRI for viability testing.”
One important lesson of STICH is that all patients with heart failure and a low left ventricular ejection fraction should have a coronary angiogram, even if they are free of ischemia on noninvasive testing and have no angina. That’s because the patients enrolled in STICH had little or no angina, the surgeon continued.
These STICH-type patients will benefit greatly from a heart team assessment factoring in an individual’s Society of Thoracic Surgeons’ predicted risk score, based on age, comorbidities, and other factors. For example, if a patient’s STS risk score with CABG is 0.7%, that’s a strong argument for opting for the surgery, since the 30-day operative mortality in STICH was 3.6%. If, on the other hand, the STS score is greater than 7%, that’s a tougher call.
“I think it’s really important that a heart team assessment includes a noninvasive cardiologist as well as an interventional cardiologist and cardiac surgeon because I think interventionalists and cardiac surgeons sometimes get a little goofy in their assessment of these patients,” Dr. Thourani said.
Patients with a low ejection fraction and coronary artery disease who are deemed poor candidates for CABG should be evaluated for a mechanical circulatory support device or a heart transplant.
“I think that’s something we don’t think about enough, quite honestly,” he said.
Dr. Thourani reported serving as a consultant to Abbott Vascular, Edwards Lifesciences, and Gore, and receiving research grants from numerous companies.
SNOWMASS, COLO. – Coronary artery bypass graft surgery in patients with severe ischemic left ventricular dysfunction is overdue for an upgrade in status in the American College of Cardiology/American Heart Association guidelines on the strength of the landmark STICH trial and its extended follow-up stage known as STICHES, according to Vinod H. Thourani, MD.
Currently, the guidelines give CABG in this large and growing population a class IIb recommendation, meaning it “might be considered.” This undervalues the study’s core lesson: “STICHES showed a clear survival benefit with CABG, so this most likely should become a class IIa recommendation,” Dr. Thourani said at the Annual Cardiovascular Conference at Snowmass.
He went on to describe how he applies the key study findings to individual patients.
At 5 and 10 years of follow-up, the probability of all-cause mortality was reduced by 14% and 16%, respectively, in the CABG group. The surgery provided on average an 18-month extension of life. The price paid for the CABG benefit was a 3.6% mortality rate at 30 days; however, this was overcome by the 2-year mark, at which point survival in the CABG group surpassed that in controls. Thereafter, the all-cause mortality gap between the two groups continued to widen for the duration of follow-up.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization, the CABG group enjoyed a 26% relative risk reduction, compared with optimal medical management alone at 5 years, and a 28% reduction in risk at 10 years. The two study groups diverged in terms of risk of cardiovascular hospitalization after only 3 months.
CABG provided a reduction in the risk of cardiovascular death that was consistent across all ages. In contrast, the reduction in all-cause mortality was not, since a higher proportion of deaths in older patients came from cancer and other noncardiovascular causes (Circulation. 2016 Nov 1;134[18]:1314-24).
There have been no randomized, controlled trials of percutaneous coronary intervention in patients with heart failure.
“An interesting finding in STICHES was that medical therapy had a much higher 10-year all-cause mortality the younger the patient was. So CABG particularly benefits those who are at a younger age – in this study, age 60 or less. As you get older, say, at 80 years of age, I’m not sure there’s a huge benefit in all-cause mortality at that point,” said Dr. Thourani, professor of surgery and medicine, and codirector of the structural heart and valve center at Emory University in Atlanta.
In a STICH substudy, roughly half of participants underwent presurgical myocardial viability testing via single-photon emission CT and/or dobutamine echocardiography. The investigators found that the results didn’t predict mortality benefit for CABG (N Engl J Med. 2011 Apr 28;364[17]:1617-25).
More recently, however, other investigators have reported MRI to have prognostic value. For example, Belgian investigators showed that medical therapy in patients with ischemic heart failure and dysfunctional but viable myocardium on delayed-enhanced MRI was associated with a 4.56-fold increased likelihood of mortality during 3 years of follow-up, compared with complete revascularization via CABG (J Am Coll Cardiol. 2012 Feb 28;59[9]:825-35).
“This observation has been useful for me,” Dr. Thourani said. “My own personal practice is if I have good targets, I don’t do viability testing, but if I have really bad targets where I know I’m going to have a tough time sewing grafts, I try to get an MRI for viability testing.”
One important lesson of STICH is that all patients with heart failure and a low left ventricular ejection fraction should have a coronary angiogram, even if they are free of ischemia on noninvasive testing and have no angina. That’s because the patients enrolled in STICH had little or no angina, the surgeon continued.
These STICH-type patients will benefit greatly from a heart team assessment factoring in an individual’s Society of Thoracic Surgeons’ predicted risk score, based on age, comorbidities, and other factors. For example, if a patient’s STS risk score with CABG is 0.7%, that’s a strong argument for opting for the surgery, since the 30-day operative mortality in STICH was 3.6%. If, on the other hand, the STS score is greater than 7%, that’s a tougher call.
“I think it’s really important that a heart team assessment includes a noninvasive cardiologist as well as an interventional cardiologist and cardiac surgeon because I think interventionalists and cardiac surgeons sometimes get a little goofy in their assessment of these patients,” Dr. Thourani said.
Patients with a low ejection fraction and coronary artery disease who are deemed poor candidates for CABG should be evaluated for a mechanical circulatory support device or a heart transplant.
“I think that’s something we don’t think about enough, quite honestly,” he said.
Dr. Thourani reported serving as a consultant to Abbott Vascular, Edwards Lifesciences, and Gore, and receiving research grants from numerous companies.
EXPERT ANALYSIS AT THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Complex congenital heart conditions call for complex care in pregnancy
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
koakes@frontlinemedcom.com
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
koakes@frontlinemedcom.com
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
koakes@frontlinemedcom.com
On Twitter @karioakes
Curb AF recurrences through risk factor modification
SNOWMASS, COLO. – Overlooking the common modifiable risk factors in patients with atrial fibrillation is missing out on an excellent opportunity to help curb the growing global pandemic of the arrhythmia, Patrick T. O’Gara, MD, said at the Annual Cardiovascular Conference at Snowmass.
“My purpose here is a wake up call to improve screening for and treatment of modifiable risk factors in patients with atrial fibrillation,” declared Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
Overweight/obesity: Investigators at the University of Adelaide (Australia) demonstrated in the LEGACY trial that patients with atrial fibrillation (AF) and a BMI of 27 kg/m2 or more reduced their AF symptom burden in a dose-response fashion as they shed excess pounds as part of an intensive weight management program. Those who shed at least 10% of their baseline body weight had a 46% rate of 5-year freedom from AF without resort to rhythm control medications or ablation procedures of 46%. With 3%-9% weight loss, the rate was 22%. And with 3% weight loss, it was 13%.
The best results came from sustained linear weight loss. Weight fluctuations of greater than 5% – the classic yoyo dieting pattern – partially offset the overall benefit of weight loss with respect to recurrent AF (J Am Coll Cardiol. 2015 May 26;65(20):2159-69).
In a separate study, the same team of Australian investigators offered an opportunity to participate in a risk factor management program to patients with AF and a BMI of 27 kg/m2 or more who were undergoing radiofrequency ablation for their arrhythmia. Participants had significantly fewer repeat ablation procedures during followup and were also less likely to be on antiarrhythmic drugs than the patients who opted for usual care (J Am Coll Cardiol. 2014 Dec 2;64(21):2222-31).
Alcohol: The ‘holiday heart’ syndrome is well known, but alcohol consumption beyond binging can increase risk for AF. Dr. O’Gara noted that in a recent review article entitled “Alcohol and Atrial Fibrillation: A Sobering Review,” investigators at the University of Melbourne showed that while the relationship between the number of standard drinks per week and risk of cardiovascular mortality is J-shaped, with a nadir at 14-21 drinks per week in men and fewer in women, the risk of developing AF is linear over time and appears to increase incrementally with every additional drink per week (J Am Coll Cardiol. 2016 Dec 13;68(23):2567-76).
Also, a prospective study of nearly 80,000 Swedes free from AF at baseline, coupled with a meta-analysis of seven prospective studies found that for each additional drink per day consumed the risk of developing AF rose over time by roughly a further 10% compared to that of teetotalers (J Am Coll Cardiol. 2014; Jul 22;64(3):281-9).
Physical inactivity: In the prospective Tromso Study, in which more than 20,000 Norwegian adults were followed for 20 years, leisure time physical activity displayed a J-shaped relationship with the risk of developing AF. Moderately active subjects were an adjusted 19% less likely to develop AF than those with low physical activity, while the risk in subjects who regularly engaged in vigorous physical activity was 37% higher than in the low-activity group (Eur Heart J. 2016 Aug 1;37(29):2307-13).
“This effect of moderate exercise might be due to the associated weight loss, improved endothelial function, better sleep, perhaps a better balance between the sympathetic and parasympathetic nervous systems,” Dr. O’Gara observed.
How much physical activity is right for patients with AF? Dr. O’Gara said one of the best reviews he’s seen came from the University of Adelaide group (Circulation. 2016 Feb 2;133(5):457-9). They recommended a total of 120-200 minutes of exercise per week spread over three to five sessions. While the research base is strongest for moderate-intensity exercise, the Australians also noted the effectiveness and safety of a novel program of repeated 4-minute intervals of high-intensity exercise at 85%-95% of peak heart rate as demonstrated in a randomized controlled trial by investigators at the Norwegian University of Science and Technology in Trondheim. They showed this approach resulted in reduced time in AF and decreased AF symptoms coupled with improved quality of life and left atrial and ventricular function (Circulation. 2016 Feb 2;133(5):466-73).
“I think you could look at this review and feel very confident that there is some evidence base to substantiate your strong recommendation that patients actively engage in exercise as treatment for their atrial fibrillation,” the cardiologist said.
Sleep apnea: Investigators at Brigham and Women’s Hospital in Boston have demonstrated that effective treatment of sleep apnea with continuous positive airway pressure in patients with atrial fibrillation is associated with smaller atrial size and ventricular mass, lower blood pressure, and a significantly reduced risk of recurrent AF following an AF ablation procedure (J Am Heart Assoc. 2013 Nov 25;2(6):e000421).
“Sleep hygiene is one of the least attended aspects of cardiovascular health,” according to Dr. O’Gara. “We need to ask the partner or spouse, ‘How well does your partner sleep? Do you hear thrashing about, snoring, gagging, or notice restless legs?’ Heart failure folks are really tuned into this, but in the practice of seeing patients come into the emergency room with new-onset atrial fibrillation, it’s tenth on the list of five questions one would ask.”
Dr. O’Gara reported having no financial conflicts.
SNOWMASS, COLO. – Overlooking the common modifiable risk factors in patients with atrial fibrillation is missing out on an excellent opportunity to help curb the growing global pandemic of the arrhythmia, Patrick T. O’Gara, MD, said at the Annual Cardiovascular Conference at Snowmass.
“My purpose here is a wake up call to improve screening for and treatment of modifiable risk factors in patients with atrial fibrillation,” declared Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
Overweight/obesity: Investigators at the University of Adelaide (Australia) demonstrated in the LEGACY trial that patients with atrial fibrillation (AF) and a BMI of 27 kg/m2 or more reduced their AF symptom burden in a dose-response fashion as they shed excess pounds as part of an intensive weight management program. Those who shed at least 10% of their baseline body weight had a 46% rate of 5-year freedom from AF without resort to rhythm control medications or ablation procedures of 46%. With 3%-9% weight loss, the rate was 22%. And with 3% weight loss, it was 13%.
The best results came from sustained linear weight loss. Weight fluctuations of greater than 5% – the classic yoyo dieting pattern – partially offset the overall benefit of weight loss with respect to recurrent AF (J Am Coll Cardiol. 2015 May 26;65(20):2159-69).
In a separate study, the same team of Australian investigators offered an opportunity to participate in a risk factor management program to patients with AF and a BMI of 27 kg/m2 or more who were undergoing radiofrequency ablation for their arrhythmia. Participants had significantly fewer repeat ablation procedures during followup and were also less likely to be on antiarrhythmic drugs than the patients who opted for usual care (J Am Coll Cardiol. 2014 Dec 2;64(21):2222-31).
Alcohol: The ‘holiday heart’ syndrome is well known, but alcohol consumption beyond binging can increase risk for AF. Dr. O’Gara noted that in a recent review article entitled “Alcohol and Atrial Fibrillation: A Sobering Review,” investigators at the University of Melbourne showed that while the relationship between the number of standard drinks per week and risk of cardiovascular mortality is J-shaped, with a nadir at 14-21 drinks per week in men and fewer in women, the risk of developing AF is linear over time and appears to increase incrementally with every additional drink per week (J Am Coll Cardiol. 2016 Dec 13;68(23):2567-76).
Also, a prospective study of nearly 80,000 Swedes free from AF at baseline, coupled with a meta-analysis of seven prospective studies found that for each additional drink per day consumed the risk of developing AF rose over time by roughly a further 10% compared to that of teetotalers (J Am Coll Cardiol. 2014; Jul 22;64(3):281-9).
Physical inactivity: In the prospective Tromso Study, in which more than 20,000 Norwegian adults were followed for 20 years, leisure time physical activity displayed a J-shaped relationship with the risk of developing AF. Moderately active subjects were an adjusted 19% less likely to develop AF than those with low physical activity, while the risk in subjects who regularly engaged in vigorous physical activity was 37% higher than in the low-activity group (Eur Heart J. 2016 Aug 1;37(29):2307-13).
“This effect of moderate exercise might be due to the associated weight loss, improved endothelial function, better sleep, perhaps a better balance between the sympathetic and parasympathetic nervous systems,” Dr. O’Gara observed.
How much physical activity is right for patients with AF? Dr. O’Gara said one of the best reviews he’s seen came from the University of Adelaide group (Circulation. 2016 Feb 2;133(5):457-9). They recommended a total of 120-200 minutes of exercise per week spread over three to five sessions. While the research base is strongest for moderate-intensity exercise, the Australians also noted the effectiveness and safety of a novel program of repeated 4-minute intervals of high-intensity exercise at 85%-95% of peak heart rate as demonstrated in a randomized controlled trial by investigators at the Norwegian University of Science and Technology in Trondheim. They showed this approach resulted in reduced time in AF and decreased AF symptoms coupled with improved quality of life and left atrial and ventricular function (Circulation. 2016 Feb 2;133(5):466-73).
“I think you could look at this review and feel very confident that there is some evidence base to substantiate your strong recommendation that patients actively engage in exercise as treatment for their atrial fibrillation,” the cardiologist said.
Sleep apnea: Investigators at Brigham and Women’s Hospital in Boston have demonstrated that effective treatment of sleep apnea with continuous positive airway pressure in patients with atrial fibrillation is associated with smaller atrial size and ventricular mass, lower blood pressure, and a significantly reduced risk of recurrent AF following an AF ablation procedure (J Am Heart Assoc. 2013 Nov 25;2(6):e000421).
“Sleep hygiene is one of the least attended aspects of cardiovascular health,” according to Dr. O’Gara. “We need to ask the partner or spouse, ‘How well does your partner sleep? Do you hear thrashing about, snoring, gagging, or notice restless legs?’ Heart failure folks are really tuned into this, but in the practice of seeing patients come into the emergency room with new-onset atrial fibrillation, it’s tenth on the list of five questions one would ask.”
Dr. O’Gara reported having no financial conflicts.
SNOWMASS, COLO. – Overlooking the common modifiable risk factors in patients with atrial fibrillation is missing out on an excellent opportunity to help curb the growing global pandemic of the arrhythmia, Patrick T. O’Gara, MD, said at the Annual Cardiovascular Conference at Snowmass.
“My purpose here is a wake up call to improve screening for and treatment of modifiable risk factors in patients with atrial fibrillation,” declared Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
Overweight/obesity: Investigators at the University of Adelaide (Australia) demonstrated in the LEGACY trial that patients with atrial fibrillation (AF) and a BMI of 27 kg/m2 or more reduced their AF symptom burden in a dose-response fashion as they shed excess pounds as part of an intensive weight management program. Those who shed at least 10% of their baseline body weight had a 46% rate of 5-year freedom from AF without resort to rhythm control medications or ablation procedures of 46%. With 3%-9% weight loss, the rate was 22%. And with 3% weight loss, it was 13%.
The best results came from sustained linear weight loss. Weight fluctuations of greater than 5% – the classic yoyo dieting pattern – partially offset the overall benefit of weight loss with respect to recurrent AF (J Am Coll Cardiol. 2015 May 26;65(20):2159-69).
In a separate study, the same team of Australian investigators offered an opportunity to participate in a risk factor management program to patients with AF and a BMI of 27 kg/m2 or more who were undergoing radiofrequency ablation for their arrhythmia. Participants had significantly fewer repeat ablation procedures during followup and were also less likely to be on antiarrhythmic drugs than the patients who opted for usual care (J Am Coll Cardiol. 2014 Dec 2;64(21):2222-31).
Alcohol: The ‘holiday heart’ syndrome is well known, but alcohol consumption beyond binging can increase risk for AF. Dr. O’Gara noted that in a recent review article entitled “Alcohol and Atrial Fibrillation: A Sobering Review,” investigators at the University of Melbourne showed that while the relationship between the number of standard drinks per week and risk of cardiovascular mortality is J-shaped, with a nadir at 14-21 drinks per week in men and fewer in women, the risk of developing AF is linear over time and appears to increase incrementally with every additional drink per week (J Am Coll Cardiol. 2016 Dec 13;68(23):2567-76).
Also, a prospective study of nearly 80,000 Swedes free from AF at baseline, coupled with a meta-analysis of seven prospective studies found that for each additional drink per day consumed the risk of developing AF rose over time by roughly a further 10% compared to that of teetotalers (J Am Coll Cardiol. 2014; Jul 22;64(3):281-9).
Physical inactivity: In the prospective Tromso Study, in which more than 20,000 Norwegian adults were followed for 20 years, leisure time physical activity displayed a J-shaped relationship with the risk of developing AF. Moderately active subjects were an adjusted 19% less likely to develop AF than those with low physical activity, while the risk in subjects who regularly engaged in vigorous physical activity was 37% higher than in the low-activity group (Eur Heart J. 2016 Aug 1;37(29):2307-13).
“This effect of moderate exercise might be due to the associated weight loss, improved endothelial function, better sleep, perhaps a better balance between the sympathetic and parasympathetic nervous systems,” Dr. O’Gara observed.
How much physical activity is right for patients with AF? Dr. O’Gara said one of the best reviews he’s seen came from the University of Adelaide group (Circulation. 2016 Feb 2;133(5):457-9). They recommended a total of 120-200 minutes of exercise per week spread over three to five sessions. While the research base is strongest for moderate-intensity exercise, the Australians also noted the effectiveness and safety of a novel program of repeated 4-minute intervals of high-intensity exercise at 85%-95% of peak heart rate as demonstrated in a randomized controlled trial by investigators at the Norwegian University of Science and Technology in Trondheim. They showed this approach resulted in reduced time in AF and decreased AF symptoms coupled with improved quality of life and left atrial and ventricular function (Circulation. 2016 Feb 2;133(5):466-73).
“I think you could look at this review and feel very confident that there is some evidence base to substantiate your strong recommendation that patients actively engage in exercise as treatment for their atrial fibrillation,” the cardiologist said.
Sleep apnea: Investigators at Brigham and Women’s Hospital in Boston have demonstrated that effective treatment of sleep apnea with continuous positive airway pressure in patients with atrial fibrillation is associated with smaller atrial size and ventricular mass, lower blood pressure, and a significantly reduced risk of recurrent AF following an AF ablation procedure (J Am Heart Assoc. 2013 Nov 25;2(6):e000421).
“Sleep hygiene is one of the least attended aspects of cardiovascular health,” according to Dr. O’Gara. “We need to ask the partner or spouse, ‘How well does your partner sleep? Do you hear thrashing about, snoring, gagging, or notice restless legs?’ Heart failure folks are really tuned into this, but in the practice of seeing patients come into the emergency room with new-onset atrial fibrillation, it’s tenth on the list of five questions one would ask.”
Dr. O’Gara reported having no financial conflicts.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Cardiovascular complications in pregnancy quickly boost future risk
NEW ORLEANS – Women who experience peripartum cardiomyopathy or any of a variety of hypertensive disorders in pregnancy are at sharply increased risk of acute MI, stroke, or new-onset heart failure beginning within just a few years, Rima Arnaout, MD, reported at the American Heart Association scientific sessions.
“Our study supports the idea that women who have cardiovascular complications in pregnancy really need to be monitored closely for potential primary prevention of cardiovascular events,” said Dr. Arnaout of the University of California, San Francisco.
At a session devoted to “big data” studies in cardiovascular medicine, she presented one of the biggest: a retrospective cohort study of 1.66 million pregnancies during 2005-2009 in California women without any history of congenital or valvular heart disease or prepregnancy cardiovascular events. The California database was created as part of the U.S. Agency for Healthcare Research and Quality’s comprehensive Healthcare Cost and Utilization Project, which included more than 95% of the state’s hospitals. Women who experienced an MI or a stroke, or who were diagnosed with heart failure, during a median of 2.7 years and a maximum of 6 years of follow-up post pregnancy were identified via ICD-9 codes.
There were 111,202 cases of various forms of hypertension in pregnancy, for a 6.9% incidence. Peripartum cardiomyopathy was diagnosed in 562 women, for a rate of 3.5 cases per 10,000 pregnancies.
Indeed, in a multivariate Cox proportional hazards analysis adjusted for numerous potential confounders, peripartum cardiomyopathy was associated with a 16-fold increased risk of acute MI during the relatively short follow-up period, as well as 29-fold increased risks of stroke and heart failure, compared with women with no cardiovascular issues during their pregnancy.
Chronic hypertension, regardless of whether it occurred alone or in combination with preeclampsia or gestational hypertension, was associated with roughly a twofold increased risk of each of the three study outcomes, compared with women who didn’t experience a cardiovascular complication during pregnancy. De novo preeclampsia was also associated with roughly a twofold increased risk of later MI, heart failure, or stroke.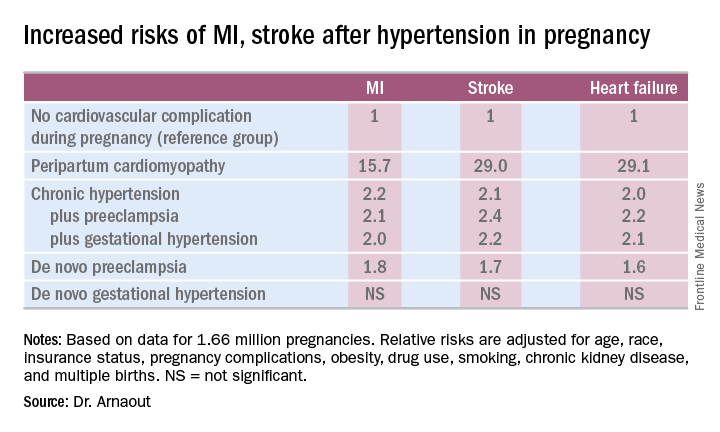
The only form of hypertension in pregnancy that wasn’t associated with a subsequent significantly increased risk of cardiovascular events was de novo gestational hypertension.
Audience member David C. Goff Jr., MD, head of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute in Bethesda, Md., rose to compliment Dr. Arnaout: “Great work and really important.”
He said that her findings are consistent with the notion that pregnancy constitutes a sort of early-life cardiovascular stress test. He said he wondered, however, just how comfortable Dr. Arnaout is in stating that gestational diabetes isn’t associated with increased subsequent cardiovascular risk, given the relatively short follow-up to date in this population of women who still remain several decades away from the age when cardiovascular event rates really start to climb.
“I completely agree with you,” she replied, noting that other investigators utilizing a different registry have reported an increased longer-term risk for women with gestational diabetes.
Dr. Arnaout said she and her coinvestigators plan to continue to follow the women who experienced peripartum cardiomyopathy or hypertension in pregnancy longer term. They’re also in the process of breaking down the data to look at the risks associated with specific subtypes of MI, stroke, and heart failure.
Dr. Arnaout reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
NEW ORLEANS – Women who experience peripartum cardiomyopathy or any of a variety of hypertensive disorders in pregnancy are at sharply increased risk of acute MI, stroke, or new-onset heart failure beginning within just a few years, Rima Arnaout, MD, reported at the American Heart Association scientific sessions.
“Our study supports the idea that women who have cardiovascular complications in pregnancy really need to be monitored closely for potential primary prevention of cardiovascular events,” said Dr. Arnaout of the University of California, San Francisco.
At a session devoted to “big data” studies in cardiovascular medicine, she presented one of the biggest: a retrospective cohort study of 1.66 million pregnancies during 2005-2009 in California women without any history of congenital or valvular heart disease or prepregnancy cardiovascular events. The California database was created as part of the U.S. Agency for Healthcare Research and Quality’s comprehensive Healthcare Cost and Utilization Project, which included more than 95% of the state’s hospitals. Women who experienced an MI or a stroke, or who were diagnosed with heart failure, during a median of 2.7 years and a maximum of 6 years of follow-up post pregnancy were identified via ICD-9 codes.
There were 111,202 cases of various forms of hypertension in pregnancy, for a 6.9% incidence. Peripartum cardiomyopathy was diagnosed in 562 women, for a rate of 3.5 cases per 10,000 pregnancies.
Indeed, in a multivariate Cox proportional hazards analysis adjusted for numerous potential confounders, peripartum cardiomyopathy was associated with a 16-fold increased risk of acute MI during the relatively short follow-up period, as well as 29-fold increased risks of stroke and heart failure, compared with women with no cardiovascular issues during their pregnancy.
Chronic hypertension, regardless of whether it occurred alone or in combination with preeclampsia or gestational hypertension, was associated with roughly a twofold increased risk of each of the three study outcomes, compared with women who didn’t experience a cardiovascular complication during pregnancy. De novo preeclampsia was also associated with roughly a twofold increased risk of later MI, heart failure, or stroke.
The only form of hypertension in pregnancy that wasn’t associated with a subsequent significantly increased risk of cardiovascular events was de novo gestational hypertension.
Audience member David C. Goff Jr., MD, head of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute in Bethesda, Md., rose to compliment Dr. Arnaout: “Great work and really important.”
He said that her findings are consistent with the notion that pregnancy constitutes a sort of early-life cardiovascular stress test. He said he wondered, however, just how comfortable Dr. Arnaout is in stating that gestational diabetes isn’t associated with increased subsequent cardiovascular risk, given the relatively short follow-up to date in this population of women who still remain several decades away from the age when cardiovascular event rates really start to climb.
“I completely agree with you,” she replied, noting that other investigators utilizing a different registry have reported an increased longer-term risk for women with gestational diabetes.
Dr. Arnaout said she and her coinvestigators plan to continue to follow the women who experienced peripartum cardiomyopathy or hypertension in pregnancy longer term. They’re also in the process of breaking down the data to look at the risks associated with specific subtypes of MI, stroke, and heart failure.
Dr. Arnaout reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
NEW ORLEANS – Women who experience peripartum cardiomyopathy or any of a variety of hypertensive disorders in pregnancy are at sharply increased risk of acute MI, stroke, or new-onset heart failure beginning within just a few years, Rima Arnaout, MD, reported at the American Heart Association scientific sessions.
“Our study supports the idea that women who have cardiovascular complications in pregnancy really need to be monitored closely for potential primary prevention of cardiovascular events,” said Dr. Arnaout of the University of California, San Francisco.
At a session devoted to “big data” studies in cardiovascular medicine, she presented one of the biggest: a retrospective cohort study of 1.66 million pregnancies during 2005-2009 in California women without any history of congenital or valvular heart disease or prepregnancy cardiovascular events. The California database was created as part of the U.S. Agency for Healthcare Research and Quality’s comprehensive Healthcare Cost and Utilization Project, which included more than 95% of the state’s hospitals. Women who experienced an MI or a stroke, or who were diagnosed with heart failure, during a median of 2.7 years and a maximum of 6 years of follow-up post pregnancy were identified via ICD-9 codes.
There were 111,202 cases of various forms of hypertension in pregnancy, for a 6.9% incidence. Peripartum cardiomyopathy was diagnosed in 562 women, for a rate of 3.5 cases per 10,000 pregnancies.
Indeed, in a multivariate Cox proportional hazards analysis adjusted for numerous potential confounders, peripartum cardiomyopathy was associated with a 16-fold increased risk of acute MI during the relatively short follow-up period, as well as 29-fold increased risks of stroke and heart failure, compared with women with no cardiovascular issues during their pregnancy.
Chronic hypertension, regardless of whether it occurred alone or in combination with preeclampsia or gestational hypertension, was associated with roughly a twofold increased risk of each of the three study outcomes, compared with women who didn’t experience a cardiovascular complication during pregnancy. De novo preeclampsia was also associated with roughly a twofold increased risk of later MI, heart failure, or stroke.
The only form of hypertension in pregnancy that wasn’t associated with a subsequent significantly increased risk of cardiovascular events was de novo gestational hypertension.
Audience member David C. Goff Jr., MD, head of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute in Bethesda, Md., rose to compliment Dr. Arnaout: “Great work and really important.”
He said that her findings are consistent with the notion that pregnancy constitutes a sort of early-life cardiovascular stress test. He said he wondered, however, just how comfortable Dr. Arnaout is in stating that gestational diabetes isn’t associated with increased subsequent cardiovascular risk, given the relatively short follow-up to date in this population of women who still remain several decades away from the age when cardiovascular event rates really start to climb.
“I completely agree with you,” she replied, noting that other investigators utilizing a different registry have reported an increased longer-term risk for women with gestational diabetes.
Dr. Arnaout said she and her coinvestigators plan to continue to follow the women who experienced peripartum cardiomyopathy or hypertension in pregnancy longer term. They’re also in the process of breaking down the data to look at the risks associated with specific subtypes of MI, stroke, and heart failure.
Dr. Arnaout reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: California women who developed peripartum cardiomyopathy were at 16- to 29-fold increased risk of experiencing an acute MI, stroke, or heart failure during a median 2.7 years of follow-up.
Data source: A retrospective cohort study of more than 95% of pregnancies in California during 2005-2009.
Disclosures: The presenter reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
Options narrow for acute decompensated heart failure
Acute decompensated heart failure is a condition that clinicians want to prevent rather than treat.
The idea that patients hospitalized with acute decompensated heart failure can have a substantial change in their prognosis from an acute intervention given in the hospital seemed to finally hit a brick wall in November at the American Heart Association scientific sessions.
Treatment of acute heart failure patients with the vasodilating natriuretic peptide ularitide failed to cut long-term cardiovascular mortality or improve several other acute and mid-term outcomes in the TRUE-AHF trial. In a second report, ATHENA-HF, acute treatment with high-dose spironolactone during acute heart failure hospitalizations failed to improve a marker of heart failure severity, N-terminal pro B-type natriuretic peptide.
What alternative interventions are left? Dr. Yancy, as well as Milton Packer, MD, the cardiologist who led TRUE-HF, had somewhat similar answers.
In his discussion of TRUE-AHF, Dr. Yancy cited two possibilities: greater use of the relatively new drug formulation sacubitril plus valsartan (Entresto), which showed strong benefit treating patients with chronic heart failure with reduced ejection fraction (HFrEF); and expanded use of pulmonary-artery pressure (PAP) monitoring using an implanted device that gives clinicians early warning when a heart failure patient’s fluid volume moves into the danger zone that precedes by days or weeks the acute decompensation that produces dyspnea and drives a patient to the hospital.
Increasing experience with PAP monitoring “continues to endorse the notion that having early warning [of fluid overload] is important,” Dr. Yancy said in an interview. “The point of acuity in acute decompensated heart failure predates hospital admission, and this monitoring system allows us to catch this before it causes an emergency department visit. The data are very persuasive.”
“PAP usually rises 2-4 weeks before a heart failure hospitalization. Perhaps we need to focus more on long-term treatment rather than treatment in the hospital.” That’s especially true for patients with heart failure with preserved ejection fraction (HFpEF) because right now no treatment is clearly proven to improve HFpEF outcomes (although several experts make a persuasive case for spironolactone).
“Tight regulation of volume status is our best chance to improve outcomes in HFpEF,” said Dr. Stevenson, who helped pioneer the idea of PAP monitoring for heart failure patients. “Maintaining good volume is very important for HFpEF patients.”
But success with PAP monitoring requires more than gathering the pressure data. Producing benefit for patients “is predicated on having an infrastructure to accommodate the influx of PAP data, being nimble enough to respond to the data and being very precise about which patients you use this in,” cautioned Dr. Yancy. “The tool is not beneficial if the infrastructure is not there,”
The idea is still so new (the first implanted PAP monitor received U.S. approval in 2014) that at his institution, Northwestern Medicine in Chicago, about a dozen patients now have a monitor, he said.
“We’re looking to use it in patients with HFpEF, whom we can’t offer anything else. I’m intrigued by what I see,” in this first wave of Northwestern patients. Dr. Yancy’s anecdotal experience with PAP monitoring so far “helps endorse what the trial results suggested” about providing incremental benefit to heart failure patients.
“The solution is to prevent hospitalization in the first place with the medications we already have, but they’re not used. The medications we already have are enough, but they need to be used,” Dr. Packer told me in an interview during the meeting. He speculated that perhaps 10%-15% of HFrEF patients currently receive the full guideline-directed regimen of heart failure drugs. “That’s unbelievable,” he exclaimed. If clinicians diligently treated advanced HFrEF patients with these four agents, “you’d see a 60%, 70% drop in hospitalizations,” he suggested.
Dr. Packer put some of the blame for underuse of guideline-directed medications on the low financial incentive physicians have to vigorously apply this strategy.
He backed PAP monitoring as a good additional step for selected patients with unstable heart failure. “But as a general approach to managing patients with class III HFrEF, first put them on optimal medical therapy; then we can talk about an invasive procedure to place a PAP monitoring device.”
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Acute decompensated heart failure is a condition that clinicians want to prevent rather than treat.
The idea that patients hospitalized with acute decompensated heart failure can have a substantial change in their prognosis from an acute intervention given in the hospital seemed to finally hit a brick wall in November at the American Heart Association scientific sessions.
Treatment of acute heart failure patients with the vasodilating natriuretic peptide ularitide failed to cut long-term cardiovascular mortality or improve several other acute and mid-term outcomes in the TRUE-AHF trial. In a second report, ATHENA-HF, acute treatment with high-dose spironolactone during acute heart failure hospitalizations failed to improve a marker of heart failure severity, N-terminal pro B-type natriuretic peptide.
What alternative interventions are left? Dr. Yancy, as well as Milton Packer, MD, the cardiologist who led TRUE-HF, had somewhat similar answers.
In his discussion of TRUE-AHF, Dr. Yancy cited two possibilities: greater use of the relatively new drug formulation sacubitril plus valsartan (Entresto), which showed strong benefit treating patients with chronic heart failure with reduced ejection fraction (HFrEF); and expanded use of pulmonary-artery pressure (PAP) monitoring using an implanted device that gives clinicians early warning when a heart failure patient’s fluid volume moves into the danger zone that precedes by days or weeks the acute decompensation that produces dyspnea and drives a patient to the hospital.
Increasing experience with PAP monitoring “continues to endorse the notion that having early warning [of fluid overload] is important,” Dr. Yancy said in an interview. “The point of acuity in acute decompensated heart failure predates hospital admission, and this monitoring system allows us to catch this before it causes an emergency department visit. The data are very persuasive.”
“PAP usually rises 2-4 weeks before a heart failure hospitalization. Perhaps we need to focus more on long-term treatment rather than treatment in the hospital.” That’s especially true for patients with heart failure with preserved ejection fraction (HFpEF) because right now no treatment is clearly proven to improve HFpEF outcomes (although several experts make a persuasive case for spironolactone).
“Tight regulation of volume status is our best chance to improve outcomes in HFpEF,” said Dr. Stevenson, who helped pioneer the idea of PAP monitoring for heart failure patients. “Maintaining good volume is very important for HFpEF patients.”
But success with PAP monitoring requires more than gathering the pressure data. Producing benefit for patients “is predicated on having an infrastructure to accommodate the influx of PAP data, being nimble enough to respond to the data and being very precise about which patients you use this in,” cautioned Dr. Yancy. “The tool is not beneficial if the infrastructure is not there,”
The idea is still so new (the first implanted PAP monitor received U.S. approval in 2014) that at his institution, Northwestern Medicine in Chicago, about a dozen patients now have a monitor, he said.
“We’re looking to use it in patients with HFpEF, whom we can’t offer anything else. I’m intrigued by what I see,” in this first wave of Northwestern patients. Dr. Yancy’s anecdotal experience with PAP monitoring so far “helps endorse what the trial results suggested” about providing incremental benefit to heart failure patients.
“The solution is to prevent hospitalization in the first place with the medications we already have, but they’re not used. The medications we already have are enough, but they need to be used,” Dr. Packer told me in an interview during the meeting. He speculated that perhaps 10%-15% of HFrEF patients currently receive the full guideline-directed regimen of heart failure drugs. “That’s unbelievable,” he exclaimed. If clinicians diligently treated advanced HFrEF patients with these four agents, “you’d see a 60%, 70% drop in hospitalizations,” he suggested.
Dr. Packer put some of the blame for underuse of guideline-directed medications on the low financial incentive physicians have to vigorously apply this strategy.
He backed PAP monitoring as a good additional step for selected patients with unstable heart failure. “But as a general approach to managing patients with class III HFrEF, first put them on optimal medical therapy; then we can talk about an invasive procedure to place a PAP monitoring device.”
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Acute decompensated heart failure is a condition that clinicians want to prevent rather than treat.
The idea that patients hospitalized with acute decompensated heart failure can have a substantial change in their prognosis from an acute intervention given in the hospital seemed to finally hit a brick wall in November at the American Heart Association scientific sessions.
Treatment of acute heart failure patients with the vasodilating natriuretic peptide ularitide failed to cut long-term cardiovascular mortality or improve several other acute and mid-term outcomes in the TRUE-AHF trial. In a second report, ATHENA-HF, acute treatment with high-dose spironolactone during acute heart failure hospitalizations failed to improve a marker of heart failure severity, N-terminal pro B-type natriuretic peptide.
What alternative interventions are left? Dr. Yancy, as well as Milton Packer, MD, the cardiologist who led TRUE-HF, had somewhat similar answers.
In his discussion of TRUE-AHF, Dr. Yancy cited two possibilities: greater use of the relatively new drug formulation sacubitril plus valsartan (Entresto), which showed strong benefit treating patients with chronic heart failure with reduced ejection fraction (HFrEF); and expanded use of pulmonary-artery pressure (PAP) monitoring using an implanted device that gives clinicians early warning when a heart failure patient’s fluid volume moves into the danger zone that precedes by days or weeks the acute decompensation that produces dyspnea and drives a patient to the hospital.
Increasing experience with PAP monitoring “continues to endorse the notion that having early warning [of fluid overload] is important,” Dr. Yancy said in an interview. “The point of acuity in acute decompensated heart failure predates hospital admission, and this monitoring system allows us to catch this before it causes an emergency department visit. The data are very persuasive.”
“PAP usually rises 2-4 weeks before a heart failure hospitalization. Perhaps we need to focus more on long-term treatment rather than treatment in the hospital.” That’s especially true for patients with heart failure with preserved ejection fraction (HFpEF) because right now no treatment is clearly proven to improve HFpEF outcomes (although several experts make a persuasive case for spironolactone).
“Tight regulation of volume status is our best chance to improve outcomes in HFpEF,” said Dr. Stevenson, who helped pioneer the idea of PAP monitoring for heart failure patients. “Maintaining good volume is very important for HFpEF patients.”
But success with PAP monitoring requires more than gathering the pressure data. Producing benefit for patients “is predicated on having an infrastructure to accommodate the influx of PAP data, being nimble enough to respond to the data and being very precise about which patients you use this in,” cautioned Dr. Yancy. “The tool is not beneficial if the infrastructure is not there,”
The idea is still so new (the first implanted PAP monitor received U.S. approval in 2014) that at his institution, Northwestern Medicine in Chicago, about a dozen patients now have a monitor, he said.
“We’re looking to use it in patients with HFpEF, whom we can’t offer anything else. I’m intrigued by what I see,” in this first wave of Northwestern patients. Dr. Yancy’s anecdotal experience with PAP monitoring so far “helps endorse what the trial results suggested” about providing incremental benefit to heart failure patients.
“The solution is to prevent hospitalization in the first place with the medications we already have, but they’re not used. The medications we already have are enough, but they need to be used,” Dr. Packer told me in an interview during the meeting. He speculated that perhaps 10%-15% of HFrEF patients currently receive the full guideline-directed regimen of heart failure drugs. “That’s unbelievable,” he exclaimed. If clinicians diligently treated advanced HFrEF patients with these four agents, “you’d see a 60%, 70% drop in hospitalizations,” he suggested.
Dr. Packer put some of the blame for underuse of guideline-directed medications on the low financial incentive physicians have to vigorously apply this strategy.
He backed PAP monitoring as a good additional step for selected patients with unstable heart failure. “But as a general approach to managing patients with class III HFrEF, first put them on optimal medical therapy; then we can talk about an invasive procedure to place a PAP monitoring device.”
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Entresto cuts LV mass in hypertensive patients
NEW ORLEANS – Hypertensive patients without heart failure treated with the heart failure formulation sacubitril plus valsartan had a significantly greater drop in their left ventricular mass than did patients treated with olmesartan in a randomized trial with 114 patients.
Treatment with sacubitril plus valsartan for both 3 and 12 months led to roughly twice as much reduction in left ventricular (LV) mass as did treatment with olmesartan, and this difference persisted after adjustment for between-group differences in blood pressure reduction, Roland E. Schmieder, MD, reported at the American Heart Association Scientific Sessions.
During clinical development of sacubitril plus valsartan (Entresto), the company that owns this compound, Novartis, ran a few small studies using the formulation to treat hypertension, but eventually those studies stopped, he said in an interview. “I hope this pushes development of other neprilysin inhibitor formulations for their blood pressure effects. I think this finding helps us understand why sacubitril plus valsartan was so effective for treating heart failure.” (N Engl J Med. 2014 Sep 11;371[11]:933-1004.)
The study enrolled patients with mild or moderate hypertension; the average blood pressure of enrolled patients was 155/92 mm Hg. They averaged 60 years of age, two-thirds were men, and their average LV mass index at baseline was 72 g/m2. The study excluded patients with heart failure. Patients randomized to receive sacubitril plus valsartan began on a dose of 200 mg/day, and after 2 weeks this rose to 400 mg/day, the maximum recommended dosage in the labeling. Patients randomized to olmesartan began on 20 mg/day, and after 2 weeks their dosage increased to 40 mg/day. Patients in both arms were also eligible to receive amlodipine for additional blood pressure lowering if deemed necessary by the treating physician.
The sacubitril plus valsartan group patients had an average cut in systolic blood pressure of about 26 mm Hg, both 3 and 12 months after the start of treatment. Patients in the olmesartan arm had decreases of 23 mm Hg and 21 mm Hg, respectively, at the two follow-up times. These between-group differences were statistically significant, Dr. Schmieder said.
Measurement of LV mass index using MRI scans showed an average reduction of LV mass index, compared with baseline of 6.4 g/m2 and 6.8 g/m2 after 3 and 12 months of treatment with sacubitril plus valsartan, and average reductions from baseline of 2.3 g/m2 and 3.5 g/m2 at the two follow-up examinations for patients treated with olmesartan. These statistically significant differences remained after adjustment for degree of blood pressure reduction at 3 and 12 months.
Additional measurements showed no between-group differences in aortic distensibility, but central pulse pressure also showed a significantly greater reduction with sacubitril plus valsartan, compared with olmesartan.
The trial was investigator initiated and received funding from Novartis. Dr. Schmieder has received honoraria from Novartis and also from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and Servier.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
This study produced very interesting and convincing data. The results suggested that treating hypertension with sacubitril plus valsartan produced a significant, incremental improvement in left ventricular mass beyond the formulation’s blood pressure effect. This could potentially have importance when treating patients with hypertension and left ventricular hypertrophy.
Olmesartan was a fair comparator to use. It arguably is the most potent angiotensin receptor blocker for reducing blood pressure. However, in routine practice we generally combine an angiotensin receptor blocker with a diuretic to get maximum blood pressure lowering. In addition, it is not new to show that blood pressure lowering reduces left ventricular size.
These data are too limited and the cost for prescribing sacubitril plus valsartan is so high, compared with most other antihypertensive drugs, that I would like to see additional study results documenting this effect before I’d be willing to prescribe sacubitril plus valsartan to patients with hypertension but no heart failure.
Dan J. Fintel, MD , a cardiologist and professor of medicine at Northwestern University in Chicago, made these comments in an interview. He has been a speaker on behalf of AstraZeneca, BMS, Daiichi Sankyo, Janssen, Merck, and Pfizer.
This study produced very interesting and convincing data. The results suggested that treating hypertension with sacubitril plus valsartan produced a significant, incremental improvement in left ventricular mass beyond the formulation’s blood pressure effect. This could potentially have importance when treating patients with hypertension and left ventricular hypertrophy.
Olmesartan was a fair comparator to use. It arguably is the most potent angiotensin receptor blocker for reducing blood pressure. However, in routine practice we generally combine an angiotensin receptor blocker with a diuretic to get maximum blood pressure lowering. In addition, it is not new to show that blood pressure lowering reduces left ventricular size.
These data are too limited and the cost for prescribing sacubitril plus valsartan is so high, compared with most other antihypertensive drugs, that I would like to see additional study results documenting this effect before I’d be willing to prescribe sacubitril plus valsartan to patients with hypertension but no heart failure.
Dan J. Fintel, MD , a cardiologist and professor of medicine at Northwestern University in Chicago, made these comments in an interview. He has been a speaker on behalf of AstraZeneca, BMS, Daiichi Sankyo, Janssen, Merck, and Pfizer.
This study produced very interesting and convincing data. The results suggested that treating hypertension with sacubitril plus valsartan produced a significant, incremental improvement in left ventricular mass beyond the formulation’s blood pressure effect. This could potentially have importance when treating patients with hypertension and left ventricular hypertrophy.
Olmesartan was a fair comparator to use. It arguably is the most potent angiotensin receptor blocker for reducing blood pressure. However, in routine practice we generally combine an angiotensin receptor blocker with a diuretic to get maximum blood pressure lowering. In addition, it is not new to show that blood pressure lowering reduces left ventricular size.
These data are too limited and the cost for prescribing sacubitril plus valsartan is so high, compared with most other antihypertensive drugs, that I would like to see additional study results documenting this effect before I’d be willing to prescribe sacubitril plus valsartan to patients with hypertension but no heart failure.
Dan J. Fintel, MD , a cardiologist and professor of medicine at Northwestern University in Chicago, made these comments in an interview. He has been a speaker on behalf of AstraZeneca, BMS, Daiichi Sankyo, Janssen, Merck, and Pfizer.
NEW ORLEANS – Hypertensive patients without heart failure treated with the heart failure formulation sacubitril plus valsartan had a significantly greater drop in their left ventricular mass than did patients treated with olmesartan in a randomized trial with 114 patients.
Treatment with sacubitril plus valsartan for both 3 and 12 months led to roughly twice as much reduction in left ventricular (LV) mass as did treatment with olmesartan, and this difference persisted after adjustment for between-group differences in blood pressure reduction, Roland E. Schmieder, MD, reported at the American Heart Association Scientific Sessions.
During clinical development of sacubitril plus valsartan (Entresto), the company that owns this compound, Novartis, ran a few small studies using the formulation to treat hypertension, but eventually those studies stopped, he said in an interview. “I hope this pushes development of other neprilysin inhibitor formulations for their blood pressure effects. I think this finding helps us understand why sacubitril plus valsartan was so effective for treating heart failure.” (N Engl J Med. 2014 Sep 11;371[11]:933-1004.)
The study enrolled patients with mild or moderate hypertension; the average blood pressure of enrolled patients was 155/92 mm Hg. They averaged 60 years of age, two-thirds were men, and their average LV mass index at baseline was 72 g/m2. The study excluded patients with heart failure. Patients randomized to receive sacubitril plus valsartan began on a dose of 200 mg/day, and after 2 weeks this rose to 400 mg/day, the maximum recommended dosage in the labeling. Patients randomized to olmesartan began on 20 mg/day, and after 2 weeks their dosage increased to 40 mg/day. Patients in both arms were also eligible to receive amlodipine for additional blood pressure lowering if deemed necessary by the treating physician.
The sacubitril plus valsartan group patients had an average cut in systolic blood pressure of about 26 mm Hg, both 3 and 12 months after the start of treatment. Patients in the olmesartan arm had decreases of 23 mm Hg and 21 mm Hg, respectively, at the two follow-up times. These between-group differences were statistically significant, Dr. Schmieder said.
Measurement of LV mass index using MRI scans showed an average reduction of LV mass index, compared with baseline of 6.4 g/m2 and 6.8 g/m2 after 3 and 12 months of treatment with sacubitril plus valsartan, and average reductions from baseline of 2.3 g/m2 and 3.5 g/m2 at the two follow-up examinations for patients treated with olmesartan. These statistically significant differences remained after adjustment for degree of blood pressure reduction at 3 and 12 months.
Additional measurements showed no between-group differences in aortic distensibility, but central pulse pressure also showed a significantly greater reduction with sacubitril plus valsartan, compared with olmesartan.
The trial was investigator initiated and received funding from Novartis. Dr. Schmieder has received honoraria from Novartis and also from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and Servier.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – Hypertensive patients without heart failure treated with the heart failure formulation sacubitril plus valsartan had a significantly greater drop in their left ventricular mass than did patients treated with olmesartan in a randomized trial with 114 patients.
Treatment with sacubitril plus valsartan for both 3 and 12 months led to roughly twice as much reduction in left ventricular (LV) mass as did treatment with olmesartan, and this difference persisted after adjustment for between-group differences in blood pressure reduction, Roland E. Schmieder, MD, reported at the American Heart Association Scientific Sessions.
During clinical development of sacubitril plus valsartan (Entresto), the company that owns this compound, Novartis, ran a few small studies using the formulation to treat hypertension, but eventually those studies stopped, he said in an interview. “I hope this pushes development of other neprilysin inhibitor formulations for their blood pressure effects. I think this finding helps us understand why sacubitril plus valsartan was so effective for treating heart failure.” (N Engl J Med. 2014 Sep 11;371[11]:933-1004.)
The study enrolled patients with mild or moderate hypertension; the average blood pressure of enrolled patients was 155/92 mm Hg. They averaged 60 years of age, two-thirds were men, and their average LV mass index at baseline was 72 g/m2. The study excluded patients with heart failure. Patients randomized to receive sacubitril plus valsartan began on a dose of 200 mg/day, and after 2 weeks this rose to 400 mg/day, the maximum recommended dosage in the labeling. Patients randomized to olmesartan began on 20 mg/day, and after 2 weeks their dosage increased to 40 mg/day. Patients in both arms were also eligible to receive amlodipine for additional blood pressure lowering if deemed necessary by the treating physician.
The sacubitril plus valsartan group patients had an average cut in systolic blood pressure of about 26 mm Hg, both 3 and 12 months after the start of treatment. Patients in the olmesartan arm had decreases of 23 mm Hg and 21 mm Hg, respectively, at the two follow-up times. These between-group differences were statistically significant, Dr. Schmieder said.
Measurement of LV mass index using MRI scans showed an average reduction of LV mass index, compared with baseline of 6.4 g/m2 and 6.8 g/m2 after 3 and 12 months of treatment with sacubitril plus valsartan, and average reductions from baseline of 2.3 g/m2 and 3.5 g/m2 at the two follow-up examinations for patients treated with olmesartan. These statistically significant differences remained after adjustment for degree of blood pressure reduction at 3 and 12 months.
Additional measurements showed no between-group differences in aortic distensibility, but central pulse pressure also showed a significantly greater reduction with sacubitril plus valsartan, compared with olmesartan.
The trial was investigator initiated and received funding from Novartis. Dr. Schmieder has received honoraria from Novartis and also from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and Servier.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: .
Major finding: After 1 year, average left ventricular mass index fell 6.8 g/m2 from baseline with sacubitril/valsartan and 3.5 g/m2 with olmesartan.
Data source: A multicenter, randomized trial with 114 patients with mild or moderate hypertension.
Disclosures: The trial was investigator initiated and received funding from Novartis, the company that markets sacubitril plus valsartan (Entresto). Dr. Schmieder has received honoraria from Novartis and also from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, and Servier.
Stem cell therapy for STEMI falls short at 2 years
NEW ORLEANS – Longer follow-up has not altered initial negative findings of the randomized phase II TIME trial, which tested the efficacy of early intracoronary stem cell therapy, given on day 3 or 7 after an ST-segment acute MI (STEMI). However, loss of the sickest patients from follow-up may have influenced the findings.
“When TIME (Transplantation in Myocardial Infarction Evaluation) was developed several years ago, the optimal timing for cell delivery following myocardial infarction was not known and had not been directly tested in a prospective clinical trial,” explained lead investigator Jay H. Traverse, MD, director of research at the Minneapolis Heart Institute Foundation and a senior consulting cardiologist at the Abbott Northwestern Hospital, Minneapolis.
With the new, additional follow-up out to 2 years in 85 patients, both groups saw further gains in LV ejection fraction and further reductions in LV infarct size, as well as stable LV end-diastolic volume index, but still with no significant differences between them.
Of note, however, 10 of the patients lost to follow-up were lost because they received implantable cardioverter defibrillators (ICDs) and, given technologic limitations at the time, could no longer undergo cardiac MRI, Dr. Traverse noted. “These 10 patients are important as we look at the long-term follow-up of TIME because these patients were more likely to have the most severe LV dysfunction and most remodeled left ventricles. This [subset] represents a limitation to long-term follow-up studies in this population.”
Challenges to research
Two challenging issues seen in many trials of cell therapy for cardiovascular disease are their underpowered nature and the changing natural history of the disease, according to session comoderator Timothy D. Henry, MD, head of cardiology at Cedars-Sinai in Los Angeles.
“One of the things with the TIME trial that’s striking, really, is that even though these are high-risk patients, there was almost no mortality in 2 years,” he commented. “Second of all... there were no differences in ejection fraction, but that’s because you are missing 30% of people. And your missing 30% of people are the sick people, so of course if you are just going to follow normal people for 2 years, you’re going to have normal results.”
“What you are seeing is a little bit illusory because all the sick patients got ICDs and we could no longer image them at that time,” Dr. Traverse agreed. “That will be less of an issue in some of our future trials that we have started now, like CONCERT and SENECA, where we are now able to do MRI analysis of people with devices. So that will certainly help.”
Nonetheless, all trials in similar patient populations are plagued by substantial rates of dropout over time and faced with improving outcomes generally, because of better medical therapy, he acknowledged. “You can see as far as the natural history, even taking into account the ICD changes that would have lowered volumes, people are pretty stable on medical therapy. Their ejection fractions and volumes out to 2 years were really quite stable. We have certainly impacted that.”
That issue raises the question of whether investigators should be using other endpoints going forward, according to session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute in Houston.
“One of the things I’ve seen over and over in this field is constantly evolving questions about which endpoints we should follow and what constitutes positive effects,” she commented. “So given the natural history, what are the endpoints we should be using?”
“Patients don’t care what their ejection fraction is per se. But they want to know if they have to get an ICD or if they will be hospitalized for heart failure, and their family is affected if they die,” Dr. Traverse replied. “We definitely need these hard endpoints. The problem is that you need so many patients with these hard endpoints that [the trials] are just financially not very doable. So that’s a big issue.”
Trial details
Patients enrolled in TIME had a first anterior STEMI, underwent reperfusion by angioplasty and stenting, and had LV dysfunction with an ejection fraction of 45% or lower. They all received either autologous bone marrow mononuclear cells or placebo on day 3 or day 7 after their MI by intracoronary infusion.
Of the initial 120 randomized patients, 10 patients were lost to follow-up because of receipt of an ICD, 3 had died (1 each from cardiovascular causes, pancreatitis, and hemorrhagic stroke), 7 were lost to follow-up for other reasons, and 15 had acquired other contraindications to MRI, according to Dr. Traverse.
At 2 years, the remaining patients in both the cell therapy and placebo groups had roughly 5% absolute increases in LV ejection fraction from baseline and roughly 45% reductions in infarct size from baseline, with no significant differences between groups.
When all patients were combined, about half were determined to have had microvascular obstruction at baseline. This finding was an adverse prognostic factor, associated with poorer recovery of LV function over time, greater adverse LV remodeling, and a higher likelihood of receiving an ICD, Dr. Traverse reported.
He has received a research grant from the National Heart, Lung, and Blood Institute, which sponsored the TIME trial.
NEW ORLEANS – Longer follow-up has not altered initial negative findings of the randomized phase II TIME trial, which tested the efficacy of early intracoronary stem cell therapy, given on day 3 or 7 after an ST-segment acute MI (STEMI). However, loss of the sickest patients from follow-up may have influenced the findings.
“When TIME (Transplantation in Myocardial Infarction Evaluation) was developed several years ago, the optimal timing for cell delivery following myocardial infarction was not known and had not been directly tested in a prospective clinical trial,” explained lead investigator Jay H. Traverse, MD, director of research at the Minneapolis Heart Institute Foundation and a senior consulting cardiologist at the Abbott Northwestern Hospital, Minneapolis.
With the new, additional follow-up out to 2 years in 85 patients, both groups saw further gains in LV ejection fraction and further reductions in LV infarct size, as well as stable LV end-diastolic volume index, but still with no significant differences between them.
Of note, however, 10 of the patients lost to follow-up were lost because they received implantable cardioverter defibrillators (ICDs) and, given technologic limitations at the time, could no longer undergo cardiac MRI, Dr. Traverse noted. “These 10 patients are important as we look at the long-term follow-up of TIME because these patients were more likely to have the most severe LV dysfunction and most remodeled left ventricles. This [subset] represents a limitation to long-term follow-up studies in this population.”
Challenges to research
Two challenging issues seen in many trials of cell therapy for cardiovascular disease are their underpowered nature and the changing natural history of the disease, according to session comoderator Timothy D. Henry, MD, head of cardiology at Cedars-Sinai in Los Angeles.
“One of the things with the TIME trial that’s striking, really, is that even though these are high-risk patients, there was almost no mortality in 2 years,” he commented. “Second of all... there were no differences in ejection fraction, but that’s because you are missing 30% of people. And your missing 30% of people are the sick people, so of course if you are just going to follow normal people for 2 years, you’re going to have normal results.”
“What you are seeing is a little bit illusory because all the sick patients got ICDs and we could no longer image them at that time,” Dr. Traverse agreed. “That will be less of an issue in some of our future trials that we have started now, like CONCERT and SENECA, where we are now able to do MRI analysis of people with devices. So that will certainly help.”
Nonetheless, all trials in similar patient populations are plagued by substantial rates of dropout over time and faced with improving outcomes generally, because of better medical therapy, he acknowledged. “You can see as far as the natural history, even taking into account the ICD changes that would have lowered volumes, people are pretty stable on medical therapy. Their ejection fractions and volumes out to 2 years were really quite stable. We have certainly impacted that.”
That issue raises the question of whether investigators should be using other endpoints going forward, according to session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute in Houston.
“One of the things I’ve seen over and over in this field is constantly evolving questions about which endpoints we should follow and what constitutes positive effects,” she commented. “So given the natural history, what are the endpoints we should be using?”
“Patients don’t care what their ejection fraction is per se. But they want to know if they have to get an ICD or if they will be hospitalized for heart failure, and their family is affected if they die,” Dr. Traverse replied. “We definitely need these hard endpoints. The problem is that you need so many patients with these hard endpoints that [the trials] are just financially not very doable. So that’s a big issue.”
Trial details
Patients enrolled in TIME had a first anterior STEMI, underwent reperfusion by angioplasty and stenting, and had LV dysfunction with an ejection fraction of 45% or lower. They all received either autologous bone marrow mononuclear cells or placebo on day 3 or day 7 after their MI by intracoronary infusion.
Of the initial 120 randomized patients, 10 patients were lost to follow-up because of receipt of an ICD, 3 had died (1 each from cardiovascular causes, pancreatitis, and hemorrhagic stroke), 7 were lost to follow-up for other reasons, and 15 had acquired other contraindications to MRI, according to Dr. Traverse.
At 2 years, the remaining patients in both the cell therapy and placebo groups had roughly 5% absolute increases in LV ejection fraction from baseline and roughly 45% reductions in infarct size from baseline, with no significant differences between groups.
When all patients were combined, about half were determined to have had microvascular obstruction at baseline. This finding was an adverse prognostic factor, associated with poorer recovery of LV function over time, greater adverse LV remodeling, and a higher likelihood of receiving an ICD, Dr. Traverse reported.
He has received a research grant from the National Heart, Lung, and Blood Institute, which sponsored the TIME trial.
NEW ORLEANS – Longer follow-up has not altered initial negative findings of the randomized phase II TIME trial, which tested the efficacy of early intracoronary stem cell therapy, given on day 3 or 7 after an ST-segment acute MI (STEMI). However, loss of the sickest patients from follow-up may have influenced the findings.
“When TIME (Transplantation in Myocardial Infarction Evaluation) was developed several years ago, the optimal timing for cell delivery following myocardial infarction was not known and had not been directly tested in a prospective clinical trial,” explained lead investigator Jay H. Traverse, MD, director of research at the Minneapolis Heart Institute Foundation and a senior consulting cardiologist at the Abbott Northwestern Hospital, Minneapolis.
With the new, additional follow-up out to 2 years in 85 patients, both groups saw further gains in LV ejection fraction and further reductions in LV infarct size, as well as stable LV end-diastolic volume index, but still with no significant differences between them.
Of note, however, 10 of the patients lost to follow-up were lost because they received implantable cardioverter defibrillators (ICDs) and, given technologic limitations at the time, could no longer undergo cardiac MRI, Dr. Traverse noted. “These 10 patients are important as we look at the long-term follow-up of TIME because these patients were more likely to have the most severe LV dysfunction and most remodeled left ventricles. This [subset] represents a limitation to long-term follow-up studies in this population.”
Challenges to research
Two challenging issues seen in many trials of cell therapy for cardiovascular disease are their underpowered nature and the changing natural history of the disease, according to session comoderator Timothy D. Henry, MD, head of cardiology at Cedars-Sinai in Los Angeles.
“One of the things with the TIME trial that’s striking, really, is that even though these are high-risk patients, there was almost no mortality in 2 years,” he commented. “Second of all... there were no differences in ejection fraction, but that’s because you are missing 30% of people. And your missing 30% of people are the sick people, so of course if you are just going to follow normal people for 2 years, you’re going to have normal results.”
“What you are seeing is a little bit illusory because all the sick patients got ICDs and we could no longer image them at that time,” Dr. Traverse agreed. “That will be less of an issue in some of our future trials that we have started now, like CONCERT and SENECA, where we are now able to do MRI analysis of people with devices. So that will certainly help.”
Nonetheless, all trials in similar patient populations are plagued by substantial rates of dropout over time and faced with improving outcomes generally, because of better medical therapy, he acknowledged. “You can see as far as the natural history, even taking into account the ICD changes that would have lowered volumes, people are pretty stable on medical therapy. Their ejection fractions and volumes out to 2 years were really quite stable. We have certainly impacted that.”
That issue raises the question of whether investigators should be using other endpoints going forward, according to session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute in Houston.
“One of the things I’ve seen over and over in this field is constantly evolving questions about which endpoints we should follow and what constitutes positive effects,” she commented. “So given the natural history, what are the endpoints we should be using?”
“Patients don’t care what their ejection fraction is per se. But they want to know if they have to get an ICD or if they will be hospitalized for heart failure, and their family is affected if they die,” Dr. Traverse replied. “We definitely need these hard endpoints. The problem is that you need so many patients with these hard endpoints that [the trials] are just financially not very doable. So that’s a big issue.”
Trial details
Patients enrolled in TIME had a first anterior STEMI, underwent reperfusion by angioplasty and stenting, and had LV dysfunction with an ejection fraction of 45% or lower. They all received either autologous bone marrow mononuclear cells or placebo on day 3 or day 7 after their MI by intracoronary infusion.
Of the initial 120 randomized patients, 10 patients were lost to follow-up because of receipt of an ICD, 3 had died (1 each from cardiovascular causes, pancreatitis, and hemorrhagic stroke), 7 were lost to follow-up for other reasons, and 15 had acquired other contraindications to MRI, according to Dr. Traverse.
At 2 years, the remaining patients in both the cell therapy and placebo groups had roughly 5% absolute increases in LV ejection fraction from baseline and roughly 45% reductions in infarct size from baseline, with no significant differences between groups.
When all patients were combined, about half were determined to have had microvascular obstruction at baseline. This finding was an adverse prognostic factor, associated with poorer recovery of LV function over time, greater adverse LV remodeling, and a higher likelihood of receiving an ICD, Dr. Traverse reported.
He has received a research grant from the National Heart, Lung, and Blood Institute, which sponsored the TIME trial.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: At 2 years, differences in LV ejection fraction, infarct size, and end-diastolic volume index between the cell therapy and placebo groups remained nonsignificant.
Data source: TIME, a randomized, phase II trial of intracoronary delivery of autologous bone marrow mononuclear cells in 120 patients with ST-segment acute MI and LV dysfunction.
Disclosures: Dr. Traverse has received a research grant from the National Heart, Lung, and Blood Institute.
Diabetes treatment costs doubled in Sweden since 2006
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
MUNICH – Sweden has experienced a doubling in its national costs for treating type 2 diabetes from €608 million in 2006 to €1.27 billion in 2014.
The increase is directly related to a surge of more than 100,000 in the number of patients with the disease and has been driven by increased hospitalizations for cardiovascular complications of diabetes, Almina Kalkan, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Costs jumped on a per-patient level as well, but the increase wasn’t related to diabetes treatment – in fact, antidiabetic medication costs remained stable at 4% over the entire study period. The real driver was the cost of treating heart failure and stroke, which increased by 92% and 73%, respectively, over the study period.
“You can really see that preventing these diabetes complications is of major importance, not only for patient quality of life but for reducing health care expenditures,” said Dr. Kalkan.
She and her colleagues searched the Swedish Prescribed Drug Registry to identify patients treated for type 2 diabetes, and linked those patients with annual hospital admissions, discharges, and hospital outpatient visits in the National Patient Register. This database doesn’t contain information on primary care visits, so this was imputed from prior studies, as were data on lost work productivity due to the disease.
According to national records, 206,183 Swedish citizens were treated for type 2 diabetes in 2006; by 2014, that number was 366,492. The mean patient age was unchanged (67 years). There was a significant increase of 2% in the number of patients who had cardiovascular disease (33%-35%). That was driven by increases in heart failure and atrial fibrillation; the proportion with myocardial infarction and stroke was unchanged.
Significantly more patients also had kidney disease by 2014 (1.5%-3.2%), although macrovascular disease had decreased by 4%. Lower limb amputations increased as well.
In the overall analysis, inpatient hospital visits accounted for the bulk of the spending, rising from €355 million in 2006 to €783 million in 2014. This was followed by spending on outpatient hospital care (from €112 million to €303 million). Spending on diabetes medications went from €39 million to €84 million, but the increase stayed proportional at just over 6%.
The total annual cost per patient increased as well, from just under €3,000/year to €3,500/year – an 18% increase.
“We still see that the main driver was inpatient and outpatient hospital care,“ Dr. Kalkan said. “Total inpatient costs increased by 24% per patient, and total outpatient costs increased by 52%.”
The proportion spent on inpatient and outpatient hospital care for each patient increased from 77% to 85% of total expenditures. Again, there was no change in the cost of diabetes medications or in the proportion of costs spent on such drugs.
Dr. Kalkan and her colleagues then conducted a societal cost analysis, which included data on primary care visits and lost job productivity related to diabetes. There was an overall 22% increase in national cost during the study period, rising from €4,200 to €5,300/patient-year.
“Inpatient visits increased by 72%, although length of stay decreased, from 13 to 11 days,” Dr. Kalkan said. “Despite this, the costs proportionately increased. This was directly due to the cost of treating the most common cardiovascular comorbidities of diabetes: heart failure, chest pain, myocardial infarction, and stroke.”
In this analysis, the cost of antidiabetic drugs was also quite small and remained stable, at 4% over the entire study period.
The cost of lost productivity was drawn from a 2015 report issued by the Swedish Institute for Health Economics. This report found that type 2 diabetes was related to a net per patient loss of €206/year in 2006 and €317/year in 2014 – a significant change.
The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT EASD 2016
Key clinical point:
Major finding: Treatment costs jumped from €608 million in 2006 to €1.27 billion in 2014.
Data source: The 8-year study used national health care data.
Disclosures: The cost analysis was a collaborative project of AstraZeneca, Uppsala University, and the Karolinksa Institute. Dr. Kalkan is an employee of AstraZeneca.
Dementia prevalence increased in heart failure patients
NEW ORLEANS – Elderly patients with heart failure had a significantly increased prevalence of both dementia and mild cognitive impairment, compared with similar people without heart failure, in an analysis of data collected from more than 6,000 U.S. residents enrolled in a long-term observational study.
Patients diagnosed with either heart failure with reduced ejection fraction or heart failure with preserved ejection fraction had an 89% increased prevalence of dementia and a 41% increased prevalence of mild cognitive impairment (MCI), compared with people from the same cohort who did not develop heart failure, in an analysis that adjusted for several demographic and clinical variables, Lucy S. Witt, MD, reported at the American Heart Association scientific sessions. She speculated that the link between heart failure and dementia and MCI might result from impaired cerebral perfusion in heart failure patients or from effects from heart failure medications.
The analysis used data collected for the Atherosclerosis Risk in Communities (ARIC) study, which began in 1987 and enrolled a randomly selected representative cohort of nearly 16,000 women and men aged 45-64 years old who resided in any of four U.S. communities. She specifically focused on the data collected from 6,431 of the participants who returned for a fifth follow-up examination during 2011-2013, including 5,490 people without heart failure, whose average age was 76 years, and 941 participants with heart failure, whose average age was 78 years.
Dementia prevalence at the fifth follow-up visit occurred at an adjusted rate of 5.6% among those without heart failure and 7.0% in those with heart failure. The examinations also found MCI in an adjusted 21.5% of those without heart failure and in 26.2% of those with heart failure, Dr. Witt reported. Adjustments included age, sex, location, education, hypertension, diabetes, depression, alcohol and tobacco use, cerebral vascular disease, marital status, and several other factors.
The relative risk for having dementia among the heart failure patients was roughly similar, regardless of whether ARIC participants with heart failure had a reduced or preserved left ventricular ejection fraction, she said.
ARIC is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – Elderly patients with heart failure had a significantly increased prevalence of both dementia and mild cognitive impairment, compared with similar people without heart failure, in an analysis of data collected from more than 6,000 U.S. residents enrolled in a long-term observational study.
Patients diagnosed with either heart failure with reduced ejection fraction or heart failure with preserved ejection fraction had an 89% increased prevalence of dementia and a 41% increased prevalence of mild cognitive impairment (MCI), compared with people from the same cohort who did not develop heart failure, in an analysis that adjusted for several demographic and clinical variables, Lucy S. Witt, MD, reported at the American Heart Association scientific sessions. She speculated that the link between heart failure and dementia and MCI might result from impaired cerebral perfusion in heart failure patients or from effects from heart failure medications.
The analysis used data collected for the Atherosclerosis Risk in Communities (ARIC) study, which began in 1987 and enrolled a randomly selected representative cohort of nearly 16,000 women and men aged 45-64 years old who resided in any of four U.S. communities. She specifically focused on the data collected from 6,431 of the participants who returned for a fifth follow-up examination during 2011-2013, including 5,490 people without heart failure, whose average age was 76 years, and 941 participants with heart failure, whose average age was 78 years.
Dementia prevalence at the fifth follow-up visit occurred at an adjusted rate of 5.6% among those without heart failure and 7.0% in those with heart failure. The examinations also found MCI in an adjusted 21.5% of those without heart failure and in 26.2% of those with heart failure, Dr. Witt reported. Adjustments included age, sex, location, education, hypertension, diabetes, depression, alcohol and tobacco use, cerebral vascular disease, marital status, and several other factors.
The relative risk for having dementia among the heart failure patients was roughly similar, regardless of whether ARIC participants with heart failure had a reduced or preserved left ventricular ejection fraction, she said.
ARIC is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – Elderly patients with heart failure had a significantly increased prevalence of both dementia and mild cognitive impairment, compared with similar people without heart failure, in an analysis of data collected from more than 6,000 U.S. residents enrolled in a long-term observational study.
Patients diagnosed with either heart failure with reduced ejection fraction or heart failure with preserved ejection fraction had an 89% increased prevalence of dementia and a 41% increased prevalence of mild cognitive impairment (MCI), compared with people from the same cohort who did not develop heart failure, in an analysis that adjusted for several demographic and clinical variables, Lucy S. Witt, MD, reported at the American Heart Association scientific sessions. She speculated that the link between heart failure and dementia and MCI might result from impaired cerebral perfusion in heart failure patients or from effects from heart failure medications.
The analysis used data collected for the Atherosclerosis Risk in Communities (ARIC) study, which began in 1987 and enrolled a randomly selected representative cohort of nearly 16,000 women and men aged 45-64 years old who resided in any of four U.S. communities. She specifically focused on the data collected from 6,431 of the participants who returned for a fifth follow-up examination during 2011-2013, including 5,490 people without heart failure, whose average age was 76 years, and 941 participants with heart failure, whose average age was 78 years.
Dementia prevalence at the fifth follow-up visit occurred at an adjusted rate of 5.6% among those without heart failure and 7.0% in those with heart failure. The examinations also found MCI in an adjusted 21.5% of those without heart failure and in 26.2% of those with heart failure, Dr. Witt reported. Adjustments included age, sex, location, education, hypertension, diabetes, depression, alcohol and tobacco use, cerebral vascular disease, marital status, and several other factors.
The relative risk for having dementia among the heart failure patients was roughly similar, regardless of whether ARIC participants with heart failure had a reduced or preserved left ventricular ejection fraction, she said.
ARIC is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Patients with heart failure had an adjusted 86% increased rate of dementia, compared with similar people without heart failure.
Data source: Analysis of 6,431 ARIC participants who returned for a fifth follow-up examination during 2011-2013.
Disclosures: The Atherosclerosis Risk in Commmunities (ARIC) study is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.























