User login
Diet, exercise improved peak oxygen intake in obese HFpEF patients
Diet and exercise, either in combination or alone, improved exercise capacity in older obese patients with clinically stable heart failure with preserved ejection fraction in a controlled trial reported Jan. 5 in JAMA.
In a controlled trial of 100 obese persons aged 60 years or over, 81 of whom were women, all with stable but chronic HFpEF, participants were randomly assigned to a diet intervention, exercise, both, or to a control group that did not modify behavior. The diet and exercise combination arm had double the increased oxygen intake capacity (VO2) when compared with the other test arms. The groups assigned to either diet or exercise saw virtually the same increase in their peak VO2 compared with controls, although, across the study, there were no statistically significant changes in quality-of-life scores as measured by the Minnesota Living with Heart Failure Questionnaire.
The trial was the first to explore a nonpharmaceutical approach to improving this cohort’s capacity to exercise, as well as its overall heart function and reduced symptoms of HFpEF.
The results are “intriguing, and worthy of further investigation in a community population with longer follow-up,” Dr. Nanette K. Wenger, professor of medicine in cardiology at Emory University, Atlanta, wrote in an accompanying editorial (JAMA. 2016;315[1]:31-3. doi: 10.1001/jama.2015.17347).
Despite HFpEF being the most common form of heart failure in the United States, primarily affecting older women, there are no treatment guidelines specifically for its management. Exercise intolerance, typically with exertional dyspnea leading to a reduced quality of life, is HFpEF’s primary symptom. Because of the so-called heart failure “obesity paradox” that indicates persons who are slightly overweight or obese have less mortality from heart failure than do their average or underweight counterparts, diet is a controversial aspect of treating HFpEF (Circ Heart Fail. 2011 May;4[3]:324-31).
Although the condition is nearly always associated with being overweight or obese, the 2013 American Heart Association/American College of Cardiology Guideline for the Management of Heart Failure does not include dietary recommendations for weight loss as a HFpEF management strategy, but it does recommend treating the condition by way of lowering hypertension pharmaceutically.
“Diet to achieve weight loss could be an important new strategy to help patients with HFpEF, particularly those afflicted with severe exercise intolerance,” said the study’s lead author, Dr. Dalane W. Kitzman, professor of internal medicine in cardiology and geriatrics at Wake Forest University, Winston-Salem, N.C.
The study’s focus on diet alone and in combination with exercise for the sake of impacting peak oxygen intake was to help clarify the role of diet in heart failure. In the non-HFpEF older, obese population, restricting calories has been shown to improve left ventricular function and exercise capacity, lower inflammation markers, and improve skeletal muscular function.
Over the course of the study’s 20 weeks, the group that was assigned a hypocaloric dietary intervention had an increase in its peak oxygen consumption, the study’s primary endpoint, of 1.3 mL/kg per min. The exercise-only group increased by 1.2 mL/kg per min. Meanwhile, the group assigned to a reduced-calorie diet and exercise increased by 2.5 mL/kg per min (JAMA. 2016;315[1]:36-46. doi: 10.1001/jama.2015.17346). The accepted clinically meaningful increase in peak oxygen consumption is 1.0 mL/kg per min.
Diet and exercise together also produced the most significant weight loss, with the combination group dropping an average of 22 pounds while the diet-alone group lost an average of 15 pounds. The exercise-only group lost an average of 6.5 pounds. Exercise for the groups assigned to it consisted of 1-hour supervised sessions, three times weekly.
As for why there was no notable improvement in quality of life, the other endpoint of the study, Dr. Wenger theorized in her editorial that “patients with HFpEF test their newly acquired activity tolerance to the precipitation of exertional dyspnea, [in other words], they remain symptomatic.”
Dr. Kitzman and his colleagues, however, wrote that upward trends in two other quality-of-life measures – the Kansas City Cardiomyopathy Questionnaire score and the Rand SF-36 – showed a possible effect on quality of life.
The overall findings indicate that intentional weight loss via calorie restriction is “feasible, appeared safe, and significantly improved the coprimary outcome of exercise capacity,” according to the authors.
Also in this study, the change in peak VO2 levels positively correlated with the change in the percentage of lean body mass (P = .003) and the change in the ratio between thigh muscle and intermuscular fat. This seemed to equal an increase in leg muscle and leg muscle quality.
Because higher levels of adipose tissue are associated with inflammation, hypertension, insulin resistance, and dyslipidemia, the study authors theorized that, as the body mass ratios of study participants improved, their bodies became more efficient at extracting oxygen from the blood, and thus could better sustain physical activity.
Although there were few adverse events observed in the study, including acute shortness of breath in a member of the exercise-only group, Dr. Kitzman said in a JAMA podcast that, of some concern was that a third of the body mass lost by participants was muscle tissue. “That’s important because persons with heart failure have less-than-normal amounts of muscle tissue as part of their heart failure syndrome and as part of growing old. It could have adverse long-term consequences,” he said.
Dr. Kitzman said in an interview that he and his colleagues have decided next to focus on resistance training’s impact on VO2, after this study showed that overall muscle mass decreased mildly with diet, even with aerobic exercise. The new study will seek to establish a correlation between improved retention of skeletal muscle, strength, and overall function and resistance training combined with diet and aerobic exercise in HFpEF.
“We chose resistance training because it is known to increase skeletal muscle mass. On the other hand, resistance training can temporarily increase blood pressure and left ventricular afterload, which are already increased in HFpEF,” he said.
The net effect is that, at least for now, “resistance training in HFpEF should be formally tested before applying to patients,” Dr. Kitzman noted. The same is true, he added, for recommending dietary restrictions in HFpEF patients.
On Twitter @whitneymcknight
Because this study of increased exercise capacity in persons who have heart failure with preserved ejection fraction focuses on the changes in symptoms, rather than the cardiac output, the results are paradigm shifting. That’s exciting for our older populations because we want to avoid medications and because exercise is so important in older adults for so many reasons, including self-confidence, stability, and function.
 |
Dr. Susan Zieman |
In the past, most of the drugs used in heart failure targeted either volume or pace. But diuretics to lessen volume and beta-blockers or calcium-channel blockers to slow the heart haven’t really helped HFpEF patients a great deal.
I always keep in the back of my mind that no drug is better than any drug, particularly in our older populations that are more vulnerable to side effects.
Although clinicians should not necessarily advise HFpEF patients to begin a diet and exercise regime just yet, the study points toward these interventions as being safe and effective. We still need more data on the study participants who were on diet and exercise and lost muscle mass.
I expect Dr. Kitzman’s current investigation into resistance training in HFpEF to elucidate just how much diet, aerobic exercise, and strength can improve outcomes.
“I think all could easily be added to the treatment regimen. It might actually be that these interventions will prove better than medications,” Dr. Zieman said.
Dr. Susan Zieman, medical officer, National Institute on Aging, Division of Geriatrics and Clinical Gerontology, made these remarks in an interview.
Because this study of increased exercise capacity in persons who have heart failure with preserved ejection fraction focuses on the changes in symptoms, rather than the cardiac output, the results are paradigm shifting. That’s exciting for our older populations because we want to avoid medications and because exercise is so important in older adults for so many reasons, including self-confidence, stability, and function.
 |
Dr. Susan Zieman |
In the past, most of the drugs used in heart failure targeted either volume or pace. But diuretics to lessen volume and beta-blockers or calcium-channel blockers to slow the heart haven’t really helped HFpEF patients a great deal.
I always keep in the back of my mind that no drug is better than any drug, particularly in our older populations that are more vulnerable to side effects.
Although clinicians should not necessarily advise HFpEF patients to begin a diet and exercise regime just yet, the study points toward these interventions as being safe and effective. We still need more data on the study participants who were on diet and exercise and lost muscle mass.
I expect Dr. Kitzman’s current investigation into resistance training in HFpEF to elucidate just how much diet, aerobic exercise, and strength can improve outcomes.
“I think all could easily be added to the treatment regimen. It might actually be that these interventions will prove better than medications,” Dr. Zieman said.
Dr. Susan Zieman, medical officer, National Institute on Aging, Division of Geriatrics and Clinical Gerontology, made these remarks in an interview.
Because this study of increased exercise capacity in persons who have heart failure with preserved ejection fraction focuses on the changes in symptoms, rather than the cardiac output, the results are paradigm shifting. That’s exciting for our older populations because we want to avoid medications and because exercise is so important in older adults for so many reasons, including self-confidence, stability, and function.
 |
Dr. Susan Zieman |
In the past, most of the drugs used in heart failure targeted either volume or pace. But diuretics to lessen volume and beta-blockers or calcium-channel blockers to slow the heart haven’t really helped HFpEF patients a great deal.
I always keep in the back of my mind that no drug is better than any drug, particularly in our older populations that are more vulnerable to side effects.
Although clinicians should not necessarily advise HFpEF patients to begin a diet and exercise regime just yet, the study points toward these interventions as being safe and effective. We still need more data on the study participants who were on diet and exercise and lost muscle mass.
I expect Dr. Kitzman’s current investigation into resistance training in HFpEF to elucidate just how much diet, aerobic exercise, and strength can improve outcomes.
“I think all could easily be added to the treatment regimen. It might actually be that these interventions will prove better than medications,” Dr. Zieman said.
Dr. Susan Zieman, medical officer, National Institute on Aging, Division of Geriatrics and Clinical Gerontology, made these remarks in an interview.
Diet and exercise, either in combination or alone, improved exercise capacity in older obese patients with clinically stable heart failure with preserved ejection fraction in a controlled trial reported Jan. 5 in JAMA.
In a controlled trial of 100 obese persons aged 60 years or over, 81 of whom were women, all with stable but chronic HFpEF, participants were randomly assigned to a diet intervention, exercise, both, or to a control group that did not modify behavior. The diet and exercise combination arm had double the increased oxygen intake capacity (VO2) when compared with the other test arms. The groups assigned to either diet or exercise saw virtually the same increase in their peak VO2 compared with controls, although, across the study, there were no statistically significant changes in quality-of-life scores as measured by the Minnesota Living with Heart Failure Questionnaire.
The trial was the first to explore a nonpharmaceutical approach to improving this cohort’s capacity to exercise, as well as its overall heart function and reduced symptoms of HFpEF.
The results are “intriguing, and worthy of further investigation in a community population with longer follow-up,” Dr. Nanette K. Wenger, professor of medicine in cardiology at Emory University, Atlanta, wrote in an accompanying editorial (JAMA. 2016;315[1]:31-3. doi: 10.1001/jama.2015.17347).
Despite HFpEF being the most common form of heart failure in the United States, primarily affecting older women, there are no treatment guidelines specifically for its management. Exercise intolerance, typically with exertional dyspnea leading to a reduced quality of life, is HFpEF’s primary symptom. Because of the so-called heart failure “obesity paradox” that indicates persons who are slightly overweight or obese have less mortality from heart failure than do their average or underweight counterparts, diet is a controversial aspect of treating HFpEF (Circ Heart Fail. 2011 May;4[3]:324-31).
Although the condition is nearly always associated with being overweight or obese, the 2013 American Heart Association/American College of Cardiology Guideline for the Management of Heart Failure does not include dietary recommendations for weight loss as a HFpEF management strategy, but it does recommend treating the condition by way of lowering hypertension pharmaceutically.
“Diet to achieve weight loss could be an important new strategy to help patients with HFpEF, particularly those afflicted with severe exercise intolerance,” said the study’s lead author, Dr. Dalane W. Kitzman, professor of internal medicine in cardiology and geriatrics at Wake Forest University, Winston-Salem, N.C.
The study’s focus on diet alone and in combination with exercise for the sake of impacting peak oxygen intake was to help clarify the role of diet in heart failure. In the non-HFpEF older, obese population, restricting calories has been shown to improve left ventricular function and exercise capacity, lower inflammation markers, and improve skeletal muscular function.
Over the course of the study’s 20 weeks, the group that was assigned a hypocaloric dietary intervention had an increase in its peak oxygen consumption, the study’s primary endpoint, of 1.3 mL/kg per min. The exercise-only group increased by 1.2 mL/kg per min. Meanwhile, the group assigned to a reduced-calorie diet and exercise increased by 2.5 mL/kg per min (JAMA. 2016;315[1]:36-46. doi: 10.1001/jama.2015.17346). The accepted clinically meaningful increase in peak oxygen consumption is 1.0 mL/kg per min.
Diet and exercise together also produced the most significant weight loss, with the combination group dropping an average of 22 pounds while the diet-alone group lost an average of 15 pounds. The exercise-only group lost an average of 6.5 pounds. Exercise for the groups assigned to it consisted of 1-hour supervised sessions, three times weekly.
As for why there was no notable improvement in quality of life, the other endpoint of the study, Dr. Wenger theorized in her editorial that “patients with HFpEF test their newly acquired activity tolerance to the precipitation of exertional dyspnea, [in other words], they remain symptomatic.”
Dr. Kitzman and his colleagues, however, wrote that upward trends in two other quality-of-life measures – the Kansas City Cardiomyopathy Questionnaire score and the Rand SF-36 – showed a possible effect on quality of life.
The overall findings indicate that intentional weight loss via calorie restriction is “feasible, appeared safe, and significantly improved the coprimary outcome of exercise capacity,” according to the authors.
Also in this study, the change in peak VO2 levels positively correlated with the change in the percentage of lean body mass (P = .003) and the change in the ratio between thigh muscle and intermuscular fat. This seemed to equal an increase in leg muscle and leg muscle quality.
Because higher levels of adipose tissue are associated with inflammation, hypertension, insulin resistance, and dyslipidemia, the study authors theorized that, as the body mass ratios of study participants improved, their bodies became more efficient at extracting oxygen from the blood, and thus could better sustain physical activity.
Although there were few adverse events observed in the study, including acute shortness of breath in a member of the exercise-only group, Dr. Kitzman said in a JAMA podcast that, of some concern was that a third of the body mass lost by participants was muscle tissue. “That’s important because persons with heart failure have less-than-normal amounts of muscle tissue as part of their heart failure syndrome and as part of growing old. It could have adverse long-term consequences,” he said.
Dr. Kitzman said in an interview that he and his colleagues have decided next to focus on resistance training’s impact on VO2, after this study showed that overall muscle mass decreased mildly with diet, even with aerobic exercise. The new study will seek to establish a correlation between improved retention of skeletal muscle, strength, and overall function and resistance training combined with diet and aerobic exercise in HFpEF.
“We chose resistance training because it is known to increase skeletal muscle mass. On the other hand, resistance training can temporarily increase blood pressure and left ventricular afterload, which are already increased in HFpEF,” he said.
The net effect is that, at least for now, “resistance training in HFpEF should be formally tested before applying to patients,” Dr. Kitzman noted. The same is true, he added, for recommending dietary restrictions in HFpEF patients.
On Twitter @whitneymcknight
Diet and exercise, either in combination or alone, improved exercise capacity in older obese patients with clinically stable heart failure with preserved ejection fraction in a controlled trial reported Jan. 5 in JAMA.
In a controlled trial of 100 obese persons aged 60 years or over, 81 of whom were women, all with stable but chronic HFpEF, participants were randomly assigned to a diet intervention, exercise, both, or to a control group that did not modify behavior. The diet and exercise combination arm had double the increased oxygen intake capacity (VO2) when compared with the other test arms. The groups assigned to either diet or exercise saw virtually the same increase in their peak VO2 compared with controls, although, across the study, there were no statistically significant changes in quality-of-life scores as measured by the Minnesota Living with Heart Failure Questionnaire.
The trial was the first to explore a nonpharmaceutical approach to improving this cohort’s capacity to exercise, as well as its overall heart function and reduced symptoms of HFpEF.
The results are “intriguing, and worthy of further investigation in a community population with longer follow-up,” Dr. Nanette K. Wenger, professor of medicine in cardiology at Emory University, Atlanta, wrote in an accompanying editorial (JAMA. 2016;315[1]:31-3. doi: 10.1001/jama.2015.17347).
Despite HFpEF being the most common form of heart failure in the United States, primarily affecting older women, there are no treatment guidelines specifically for its management. Exercise intolerance, typically with exertional dyspnea leading to a reduced quality of life, is HFpEF’s primary symptom. Because of the so-called heart failure “obesity paradox” that indicates persons who are slightly overweight or obese have less mortality from heart failure than do their average or underweight counterparts, diet is a controversial aspect of treating HFpEF (Circ Heart Fail. 2011 May;4[3]:324-31).
Although the condition is nearly always associated with being overweight or obese, the 2013 American Heart Association/American College of Cardiology Guideline for the Management of Heart Failure does not include dietary recommendations for weight loss as a HFpEF management strategy, but it does recommend treating the condition by way of lowering hypertension pharmaceutically.
“Diet to achieve weight loss could be an important new strategy to help patients with HFpEF, particularly those afflicted with severe exercise intolerance,” said the study’s lead author, Dr. Dalane W. Kitzman, professor of internal medicine in cardiology and geriatrics at Wake Forest University, Winston-Salem, N.C.
The study’s focus on diet alone and in combination with exercise for the sake of impacting peak oxygen intake was to help clarify the role of diet in heart failure. In the non-HFpEF older, obese population, restricting calories has been shown to improve left ventricular function and exercise capacity, lower inflammation markers, and improve skeletal muscular function.
Over the course of the study’s 20 weeks, the group that was assigned a hypocaloric dietary intervention had an increase in its peak oxygen consumption, the study’s primary endpoint, of 1.3 mL/kg per min. The exercise-only group increased by 1.2 mL/kg per min. Meanwhile, the group assigned to a reduced-calorie diet and exercise increased by 2.5 mL/kg per min (JAMA. 2016;315[1]:36-46. doi: 10.1001/jama.2015.17346). The accepted clinically meaningful increase in peak oxygen consumption is 1.0 mL/kg per min.
Diet and exercise together also produced the most significant weight loss, with the combination group dropping an average of 22 pounds while the diet-alone group lost an average of 15 pounds. The exercise-only group lost an average of 6.5 pounds. Exercise for the groups assigned to it consisted of 1-hour supervised sessions, three times weekly.
As for why there was no notable improvement in quality of life, the other endpoint of the study, Dr. Wenger theorized in her editorial that “patients with HFpEF test their newly acquired activity tolerance to the precipitation of exertional dyspnea, [in other words], they remain symptomatic.”
Dr. Kitzman and his colleagues, however, wrote that upward trends in two other quality-of-life measures – the Kansas City Cardiomyopathy Questionnaire score and the Rand SF-36 – showed a possible effect on quality of life.
The overall findings indicate that intentional weight loss via calorie restriction is “feasible, appeared safe, and significantly improved the coprimary outcome of exercise capacity,” according to the authors.
Also in this study, the change in peak VO2 levels positively correlated with the change in the percentage of lean body mass (P = .003) and the change in the ratio between thigh muscle and intermuscular fat. This seemed to equal an increase in leg muscle and leg muscle quality.
Because higher levels of adipose tissue are associated with inflammation, hypertension, insulin resistance, and dyslipidemia, the study authors theorized that, as the body mass ratios of study participants improved, their bodies became more efficient at extracting oxygen from the blood, and thus could better sustain physical activity.
Although there were few adverse events observed in the study, including acute shortness of breath in a member of the exercise-only group, Dr. Kitzman said in a JAMA podcast that, of some concern was that a third of the body mass lost by participants was muscle tissue. “That’s important because persons with heart failure have less-than-normal amounts of muscle tissue as part of their heart failure syndrome and as part of growing old. It could have adverse long-term consequences,” he said.
Dr. Kitzman said in an interview that he and his colleagues have decided next to focus on resistance training’s impact on VO2, after this study showed that overall muscle mass decreased mildly with diet, even with aerobic exercise. The new study will seek to establish a correlation between improved retention of skeletal muscle, strength, and overall function and resistance training combined with diet and aerobic exercise in HFpEF.
“We chose resistance training because it is known to increase skeletal muscle mass. On the other hand, resistance training can temporarily increase blood pressure and left ventricular afterload, which are already increased in HFpEF,” he said.
The net effect is that, at least for now, “resistance training in HFpEF should be formally tested before applying to patients,” Dr. Kitzman noted. The same is true, he added, for recommending dietary restrictions in HFpEF patients.
On Twitter @whitneymcknight
FROM JAMA
Key clinical point: Diet and exercise, alone or in concert, could help increase exercise tolerance in obese patients with heart failure with preserved ejection fraction (HFpEF).
Major finding: Diet improved peak VO2 by 1.3 mL/kg per min, exercise by 1.2 mL/kg per min, and both by 2.5 mL/kg per min in obese older patients with HFpEF.
Data source: Controlled study of 100 obese patients aged 60 years and older with chronic, stable HFpEF, randomly assigned to 20 weeks of diet intervention, exercise, both, or control.
Disclosures: Dr. Kitzman reported receiving consulting fees from a variety of pharmaceutical companies and grant support from Novartis. This trial was supported in part by the National Institute on Aging and the National Heart, Lung, and Blood Institute.
The palliative path: Talking with elderly patients facing emergency surgery
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
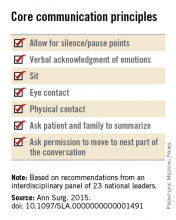
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
FROM ANNALS OF SURGERY
Chagas disease: Neither foreign nor untreatable
Chagas disease is a vector-borne parasitic disease, endemic to the Americas, that remains as little recognized by U.S. patients and practitioners as the obscure winged insects that transmit it.
Transmission occurs when triatomine bugs, commonly called “kissing bugs,” pierce the skin to feed and leave behind parasite-infected feces that can enter the bloodstream; pregnant women can also transmit Chagas to their newborns.
About a third of patients infected with Trypanosoma cruzi, the protozoan parasite that causes Chagas, will develop cardiac abnormalities such as cardiomyopathy, arrhythmias, and heart failure – often decades after becoming infected. In the United States, where blood banks began screening for Chagas in 2007, patients without symptoms are likely to learn they are positive only after donating blood.
Conventional wisdom has long maintained that Chagas is limited to Central and South America. But immigration from Chagas-endemic countries, such as El Salvador, Mexico, and Bolivia, means more people are living with the disease in the United States.
“One percent of the Latin American immigrant population we screen [in Los Angeles] has Chagas,” said Dr. Sheba K. Meymandi, cardiologist and director of Center of Excellence for Chagas Disease at Olive View–UCLA Medical Center in Los Angeles, who also works with the city’s health department to detect Chagas. “That’s huge.”
Meanwhile, blood banks are discovering more cases among people without ties to Latin America, and species of kissing bugs native to the southern United States are increasingly recognized as a non-negligible source of Chagas transmission. Of 39 Chagas cases reported to Texas health authorities in 2013 and 2014, 12 were thought to be locally acquired.
Dr. Heather Yun, an infectious disease specialist at the San Antonio Military Medical Center, said risk factors for local transmission are not well established, but “we think people who are living in poverty in substandard housing, people who spend a lot of time outdoors, especially at night, and people involved with direct blood contact with wild game in Southern parts of the United States” may be at higher risk.
A U.S. disease
Evidence is amassing quickly that Chagas is a U.S. disease. But U.S. clinicians still lag in their knowledge of it, say physicians treating Chagas cases. “In medical school we get a 2-hour lecture on it, and it’s always been presented as an exotic disease and one you don’t treat,” Dr. Meymandi said.
The persistent perception of Chagas as a foreign disease means clinicians are inclined to dismiss positive results from a blood screening, particularly from someone who is not from Latin America. Yet cardiologists, ID practitioners, obstetricians, and primary care physicians all need to be aware that cases do occur in the United States and are potentially treatable.
Dr. Laila Woc-Colburn, an infectious disease and tropical medicine specialist at Baylor College of Medicine in Houston, said many people with Chagas never make it to an infectious disease specialist or cardiologist for a work-up. “When you test positive on serology [after a blood donation], you get a letter recommending you consult your physician. Most will go to their primary care doctors, who might say ‘this isn’t a disease in the United States.’ In Houston, that is often the case.”
Dr. Meymandi, who has treated hundreds of patients with Chagas with and without cardiac involvement, said any physician with a potential Chagas case must act. “If you get someone that’s positive, it’s your duty as a physician to confirm the positivity with CDC,” she said.
Dr. Yun concurred. “The most important message is, do something,” she said. “Don’t just assume it’s a false positive.”
Diagnosis is not simple and requires testing beyond the initial ELISA assay used in blood-bank screening. Confirmatory tests must be carried out in coordination with the Centers for Disease Control and Prevention. Also, with no agents approved by the Food and Drug Administration to treat Chagas, treatment is available only through the CDC’s investigational drugs protocol. Both drugs used in Chagas, benznidazole and nifurtimox, come with serious adverse effects that must be closely monitored.
“It’s time consuming, filling out the forms, getting the consent, tracking and sending back lab results to CDC in order to get drugs – it’s not like you can just write a prescription,” Dr. Meymandi said. But, “if you don’t know how to treat the patient or don’t have time, find someone like me,” she noted, adding that she is available to counsel any physician daunted by a potential Chagas case.
Treatment options
No formal clinical algorithm exists for Chagas, but Dr. Meymandi, Dr. Yun, and Dr. Woc-Colburn all pointed to a 2007 JAMA article, which describes diagnosis and treatment protocols, as an important reference for clinicians to start with. It’s “the best approximation of a clinical guideline we have,” Dr. Yun said (JAMA. 2007;298[18]:2171-81. doi:10.1001/jama.298.18.2171).
Dr. Meymandi, who has treated more Chagas patients than has any other U.S. clinician, said that treatment has changed somewhat since the JAMA article was published. In 2007, she said, nifurtimox was the main drug available through CDC, while benznidazole, which is somewhat better tolerated and has shorter treatment duration, has since become the first-line agent.
“We’ve lowered the dose of benznidazole, maxing out at 400 mg/day to decrease the toxicity,” she said. Also, treatment is now being extended to some patients aged 60 years and older.
The decision to treat or not treat, clinicians say, depends on the patient’s age, disease progression, comorbidities and potential serious drug interactions, and willingness to tolerate side effects that, with nifurtimox especially, can include skin sloughing, rash, and psychological and neurologic symptoms including depression and peripheral neuropathy.
“If you don’t have side effects, you’re not taking the drugs,” Dr. Meymandi said. Dr. Woc-Colburn noted that polypharmacy was a major consideration when treating older adults for Chagas. “If I have a patient who has diabetes, obesity, [and] end-stage renal disease, it’s not going to be ideal to give [benznidazole].”
Recent, highly anticipated results from BENEFIT, a large randomized trial (n = 2,854) showed that benznidazole reduced parasite load but was not helpful in halting cardiac damage at 5 years’ follow-up in patients with established Chagas cardiomyopathy (N Engl J Med. 2015 Oct;373:1295-306. doi:10.1056/NEJMoa1507574).
Dr. Meymandi, whose earlier research established that Chagas cardiomyopathy carries significantly higher morbidity and mortality than does non–Chagas cardiomyopathy (Circulation. 2012;126:A18171), said that the BENEFIT results underscore the need for physicians to be bullish in their approach to treating Chagas soon after diagnosis.
“It doesn’t matter if they’re symptomatic or asymptomatic. You can’t wait till they progress to treat. If you wait for the progression of disease you’ve lost the battle. You can’t wait and follow conservatively until you see the complications, because once those complications have started the parasitic load is too high for you to have an impact,” she said.
Dr. Yun said that given the toxicity of current treatment, she hoped to see more studies show clearer evidence of clinical benefit, “either reductions in mortality or reductions in end organ disease.” Most studies “have focused on clearance of parasite, which is important, but it’s not as important decreasing the risk of death or cardiomyopathy or heart failure.”
Rick Tarleton, Ph.D., a biologist the University of Georgia, in Athens, who has worked on Chagas for more than 30 years, said that because Chagas pathology is directly tied to parasite load – and not, as people have suggested in the past, an autoimmune reaction resulting from parasite exposure – drug treatment may prove to be worthwhile even in patients with significant cardiac involvement.
“You get rid of the parasite, you get rid of the progression of the disease,” Dr. Tarleton said. Even the findings from the BENEFIT trial, he said, did not lead him to conclude that treatment in people with established cardiac disease was futile.
“If you’re treating people who are already chronically infected and showing symptoms, the question is not have you reversed the damage, it’s have you stopped accumulating damage,” he noted. “And a 5-year follow-up is probably not long enough to know whether you’ve stopped accumulating.”
“We have drugs, they’re not great, they do have side effects, they don’t always work,” Dr. Tarleton said. “But they’re better than nothing. And they ought to be more widely used.”
Dr. Meymandi said that current supplies of benznidazole at CDC are low and that a dozen patients at her clinic are awaiting treatment. Meanwhile, access may soon be complicated further by the announcement, this month, that KaloBios Pharmaceuticals had bought the rights to seek FDA approval of benznidazole and market it in the United States.
The same company’s CEO came under fire in recent months for acquiring rights to an inexpensive drug to treat toxoplasmosis in AIDS patients, then announcing a price increase from $13.50 to $750 a pill.
“Everyone’s really concerned,” Dr. Meymandi said, “because Chagas is a disease of the poor.”
Chagas disease is a vector-borne parasitic disease, endemic to the Americas, that remains as little recognized by U.S. patients and practitioners as the obscure winged insects that transmit it.
Transmission occurs when triatomine bugs, commonly called “kissing bugs,” pierce the skin to feed and leave behind parasite-infected feces that can enter the bloodstream; pregnant women can also transmit Chagas to their newborns.
About a third of patients infected with Trypanosoma cruzi, the protozoan parasite that causes Chagas, will develop cardiac abnormalities such as cardiomyopathy, arrhythmias, and heart failure – often decades after becoming infected. In the United States, where blood banks began screening for Chagas in 2007, patients without symptoms are likely to learn they are positive only after donating blood.
Conventional wisdom has long maintained that Chagas is limited to Central and South America. But immigration from Chagas-endemic countries, such as El Salvador, Mexico, and Bolivia, means more people are living with the disease in the United States.
“One percent of the Latin American immigrant population we screen [in Los Angeles] has Chagas,” said Dr. Sheba K. Meymandi, cardiologist and director of Center of Excellence for Chagas Disease at Olive View–UCLA Medical Center in Los Angeles, who also works with the city’s health department to detect Chagas. “That’s huge.”
Meanwhile, blood banks are discovering more cases among people without ties to Latin America, and species of kissing bugs native to the southern United States are increasingly recognized as a non-negligible source of Chagas transmission. Of 39 Chagas cases reported to Texas health authorities in 2013 and 2014, 12 were thought to be locally acquired.
Dr. Heather Yun, an infectious disease specialist at the San Antonio Military Medical Center, said risk factors for local transmission are not well established, but “we think people who are living in poverty in substandard housing, people who spend a lot of time outdoors, especially at night, and people involved with direct blood contact with wild game in Southern parts of the United States” may be at higher risk.
A U.S. disease
Evidence is amassing quickly that Chagas is a U.S. disease. But U.S. clinicians still lag in their knowledge of it, say physicians treating Chagas cases. “In medical school we get a 2-hour lecture on it, and it’s always been presented as an exotic disease and one you don’t treat,” Dr. Meymandi said.
The persistent perception of Chagas as a foreign disease means clinicians are inclined to dismiss positive results from a blood screening, particularly from someone who is not from Latin America. Yet cardiologists, ID practitioners, obstetricians, and primary care physicians all need to be aware that cases do occur in the United States and are potentially treatable.
Dr. Laila Woc-Colburn, an infectious disease and tropical medicine specialist at Baylor College of Medicine in Houston, said many people with Chagas never make it to an infectious disease specialist or cardiologist for a work-up. “When you test positive on serology [after a blood donation], you get a letter recommending you consult your physician. Most will go to their primary care doctors, who might say ‘this isn’t a disease in the United States.’ In Houston, that is often the case.”
Dr. Meymandi, who has treated hundreds of patients with Chagas with and without cardiac involvement, said any physician with a potential Chagas case must act. “If you get someone that’s positive, it’s your duty as a physician to confirm the positivity with CDC,” she said.
Dr. Yun concurred. “The most important message is, do something,” she said. “Don’t just assume it’s a false positive.”
Diagnosis is not simple and requires testing beyond the initial ELISA assay used in blood-bank screening. Confirmatory tests must be carried out in coordination with the Centers for Disease Control and Prevention. Also, with no agents approved by the Food and Drug Administration to treat Chagas, treatment is available only through the CDC’s investigational drugs protocol. Both drugs used in Chagas, benznidazole and nifurtimox, come with serious adverse effects that must be closely monitored.
“It’s time consuming, filling out the forms, getting the consent, tracking and sending back lab results to CDC in order to get drugs – it’s not like you can just write a prescription,” Dr. Meymandi said. But, “if you don’t know how to treat the patient or don’t have time, find someone like me,” she noted, adding that she is available to counsel any physician daunted by a potential Chagas case.
Treatment options
No formal clinical algorithm exists for Chagas, but Dr. Meymandi, Dr. Yun, and Dr. Woc-Colburn all pointed to a 2007 JAMA article, which describes diagnosis and treatment protocols, as an important reference for clinicians to start with. It’s “the best approximation of a clinical guideline we have,” Dr. Yun said (JAMA. 2007;298[18]:2171-81. doi:10.1001/jama.298.18.2171).
Dr. Meymandi, who has treated more Chagas patients than has any other U.S. clinician, said that treatment has changed somewhat since the JAMA article was published. In 2007, she said, nifurtimox was the main drug available through CDC, while benznidazole, which is somewhat better tolerated and has shorter treatment duration, has since become the first-line agent.
“We’ve lowered the dose of benznidazole, maxing out at 400 mg/day to decrease the toxicity,” she said. Also, treatment is now being extended to some patients aged 60 years and older.
The decision to treat or not treat, clinicians say, depends on the patient’s age, disease progression, comorbidities and potential serious drug interactions, and willingness to tolerate side effects that, with nifurtimox especially, can include skin sloughing, rash, and psychological and neurologic symptoms including depression and peripheral neuropathy.
“If you don’t have side effects, you’re not taking the drugs,” Dr. Meymandi said. Dr. Woc-Colburn noted that polypharmacy was a major consideration when treating older adults for Chagas. “If I have a patient who has diabetes, obesity, [and] end-stage renal disease, it’s not going to be ideal to give [benznidazole].”
Recent, highly anticipated results from BENEFIT, a large randomized trial (n = 2,854) showed that benznidazole reduced parasite load but was not helpful in halting cardiac damage at 5 years’ follow-up in patients with established Chagas cardiomyopathy (N Engl J Med. 2015 Oct;373:1295-306. doi:10.1056/NEJMoa1507574).
Dr. Meymandi, whose earlier research established that Chagas cardiomyopathy carries significantly higher morbidity and mortality than does non–Chagas cardiomyopathy (Circulation. 2012;126:A18171), said that the BENEFIT results underscore the need for physicians to be bullish in their approach to treating Chagas soon after diagnosis.
“It doesn’t matter if they’re symptomatic or asymptomatic. You can’t wait till they progress to treat. If you wait for the progression of disease you’ve lost the battle. You can’t wait and follow conservatively until you see the complications, because once those complications have started the parasitic load is too high for you to have an impact,” she said.
Dr. Yun said that given the toxicity of current treatment, she hoped to see more studies show clearer evidence of clinical benefit, “either reductions in mortality or reductions in end organ disease.” Most studies “have focused on clearance of parasite, which is important, but it’s not as important decreasing the risk of death or cardiomyopathy or heart failure.”
Rick Tarleton, Ph.D., a biologist the University of Georgia, in Athens, who has worked on Chagas for more than 30 years, said that because Chagas pathology is directly tied to parasite load – and not, as people have suggested in the past, an autoimmune reaction resulting from parasite exposure – drug treatment may prove to be worthwhile even in patients with significant cardiac involvement.
“You get rid of the parasite, you get rid of the progression of the disease,” Dr. Tarleton said. Even the findings from the BENEFIT trial, he said, did not lead him to conclude that treatment in people with established cardiac disease was futile.
“If you’re treating people who are already chronically infected and showing symptoms, the question is not have you reversed the damage, it’s have you stopped accumulating damage,” he noted. “And a 5-year follow-up is probably not long enough to know whether you’ve stopped accumulating.”
“We have drugs, they’re not great, they do have side effects, they don’t always work,” Dr. Tarleton said. “But they’re better than nothing. And they ought to be more widely used.”
Dr. Meymandi said that current supplies of benznidazole at CDC are low and that a dozen patients at her clinic are awaiting treatment. Meanwhile, access may soon be complicated further by the announcement, this month, that KaloBios Pharmaceuticals had bought the rights to seek FDA approval of benznidazole and market it in the United States.
The same company’s CEO came under fire in recent months for acquiring rights to an inexpensive drug to treat toxoplasmosis in AIDS patients, then announcing a price increase from $13.50 to $750 a pill.
“Everyone’s really concerned,” Dr. Meymandi said, “because Chagas is a disease of the poor.”
Chagas disease is a vector-borne parasitic disease, endemic to the Americas, that remains as little recognized by U.S. patients and practitioners as the obscure winged insects that transmit it.
Transmission occurs when triatomine bugs, commonly called “kissing bugs,” pierce the skin to feed and leave behind parasite-infected feces that can enter the bloodstream; pregnant women can also transmit Chagas to their newborns.
About a third of patients infected with Trypanosoma cruzi, the protozoan parasite that causes Chagas, will develop cardiac abnormalities such as cardiomyopathy, arrhythmias, and heart failure – often decades after becoming infected. In the United States, where blood banks began screening for Chagas in 2007, patients without symptoms are likely to learn they are positive only after donating blood.
Conventional wisdom has long maintained that Chagas is limited to Central and South America. But immigration from Chagas-endemic countries, such as El Salvador, Mexico, and Bolivia, means more people are living with the disease in the United States.
“One percent of the Latin American immigrant population we screen [in Los Angeles] has Chagas,” said Dr. Sheba K. Meymandi, cardiologist and director of Center of Excellence for Chagas Disease at Olive View–UCLA Medical Center in Los Angeles, who also works with the city’s health department to detect Chagas. “That’s huge.”
Meanwhile, blood banks are discovering more cases among people without ties to Latin America, and species of kissing bugs native to the southern United States are increasingly recognized as a non-negligible source of Chagas transmission. Of 39 Chagas cases reported to Texas health authorities in 2013 and 2014, 12 were thought to be locally acquired.
Dr. Heather Yun, an infectious disease specialist at the San Antonio Military Medical Center, said risk factors for local transmission are not well established, but “we think people who are living in poverty in substandard housing, people who spend a lot of time outdoors, especially at night, and people involved with direct blood contact with wild game in Southern parts of the United States” may be at higher risk.
A U.S. disease
Evidence is amassing quickly that Chagas is a U.S. disease. But U.S. clinicians still lag in their knowledge of it, say physicians treating Chagas cases. “In medical school we get a 2-hour lecture on it, and it’s always been presented as an exotic disease and one you don’t treat,” Dr. Meymandi said.
The persistent perception of Chagas as a foreign disease means clinicians are inclined to dismiss positive results from a blood screening, particularly from someone who is not from Latin America. Yet cardiologists, ID practitioners, obstetricians, and primary care physicians all need to be aware that cases do occur in the United States and are potentially treatable.
Dr. Laila Woc-Colburn, an infectious disease and tropical medicine specialist at Baylor College of Medicine in Houston, said many people with Chagas never make it to an infectious disease specialist or cardiologist for a work-up. “When you test positive on serology [after a blood donation], you get a letter recommending you consult your physician. Most will go to their primary care doctors, who might say ‘this isn’t a disease in the United States.’ In Houston, that is often the case.”
Dr. Meymandi, who has treated hundreds of patients with Chagas with and without cardiac involvement, said any physician with a potential Chagas case must act. “If you get someone that’s positive, it’s your duty as a physician to confirm the positivity with CDC,” she said.
Dr. Yun concurred. “The most important message is, do something,” she said. “Don’t just assume it’s a false positive.”
Diagnosis is not simple and requires testing beyond the initial ELISA assay used in blood-bank screening. Confirmatory tests must be carried out in coordination with the Centers for Disease Control and Prevention. Also, with no agents approved by the Food and Drug Administration to treat Chagas, treatment is available only through the CDC’s investigational drugs protocol. Both drugs used in Chagas, benznidazole and nifurtimox, come with serious adverse effects that must be closely monitored.
“It’s time consuming, filling out the forms, getting the consent, tracking and sending back lab results to CDC in order to get drugs – it’s not like you can just write a prescription,” Dr. Meymandi said. But, “if you don’t know how to treat the patient or don’t have time, find someone like me,” she noted, adding that she is available to counsel any physician daunted by a potential Chagas case.
Treatment options
No formal clinical algorithm exists for Chagas, but Dr. Meymandi, Dr. Yun, and Dr. Woc-Colburn all pointed to a 2007 JAMA article, which describes diagnosis and treatment protocols, as an important reference for clinicians to start with. It’s “the best approximation of a clinical guideline we have,” Dr. Yun said (JAMA. 2007;298[18]:2171-81. doi:10.1001/jama.298.18.2171).
Dr. Meymandi, who has treated more Chagas patients than has any other U.S. clinician, said that treatment has changed somewhat since the JAMA article was published. In 2007, she said, nifurtimox was the main drug available through CDC, while benznidazole, which is somewhat better tolerated and has shorter treatment duration, has since become the first-line agent.
“We’ve lowered the dose of benznidazole, maxing out at 400 mg/day to decrease the toxicity,” she said. Also, treatment is now being extended to some patients aged 60 years and older.
The decision to treat or not treat, clinicians say, depends on the patient’s age, disease progression, comorbidities and potential serious drug interactions, and willingness to tolerate side effects that, with nifurtimox especially, can include skin sloughing, rash, and psychological and neurologic symptoms including depression and peripheral neuropathy.
“If you don’t have side effects, you’re not taking the drugs,” Dr. Meymandi said. Dr. Woc-Colburn noted that polypharmacy was a major consideration when treating older adults for Chagas. “If I have a patient who has diabetes, obesity, [and] end-stage renal disease, it’s not going to be ideal to give [benznidazole].”
Recent, highly anticipated results from BENEFIT, a large randomized trial (n = 2,854) showed that benznidazole reduced parasite load but was not helpful in halting cardiac damage at 5 years’ follow-up in patients with established Chagas cardiomyopathy (N Engl J Med. 2015 Oct;373:1295-306. doi:10.1056/NEJMoa1507574).
Dr. Meymandi, whose earlier research established that Chagas cardiomyopathy carries significantly higher morbidity and mortality than does non–Chagas cardiomyopathy (Circulation. 2012;126:A18171), said that the BENEFIT results underscore the need for physicians to be bullish in their approach to treating Chagas soon after diagnosis.
“It doesn’t matter if they’re symptomatic or asymptomatic. You can’t wait till they progress to treat. If you wait for the progression of disease you’ve lost the battle. You can’t wait and follow conservatively until you see the complications, because once those complications have started the parasitic load is too high for you to have an impact,” she said.
Dr. Yun said that given the toxicity of current treatment, she hoped to see more studies show clearer evidence of clinical benefit, “either reductions in mortality or reductions in end organ disease.” Most studies “have focused on clearance of parasite, which is important, but it’s not as important decreasing the risk of death or cardiomyopathy or heart failure.”
Rick Tarleton, Ph.D., a biologist the University of Georgia, in Athens, who has worked on Chagas for more than 30 years, said that because Chagas pathology is directly tied to parasite load – and not, as people have suggested in the past, an autoimmune reaction resulting from parasite exposure – drug treatment may prove to be worthwhile even in patients with significant cardiac involvement.
“You get rid of the parasite, you get rid of the progression of the disease,” Dr. Tarleton said. Even the findings from the BENEFIT trial, he said, did not lead him to conclude that treatment in people with established cardiac disease was futile.
“If you’re treating people who are already chronically infected and showing symptoms, the question is not have you reversed the damage, it’s have you stopped accumulating damage,” he noted. “And a 5-year follow-up is probably not long enough to know whether you’ve stopped accumulating.”
“We have drugs, they’re not great, they do have side effects, they don’t always work,” Dr. Tarleton said. “But they’re better than nothing. And they ought to be more widely used.”
Dr. Meymandi said that current supplies of benznidazole at CDC are low and that a dozen patients at her clinic are awaiting treatment. Meanwhile, access may soon be complicated further by the announcement, this month, that KaloBios Pharmaceuticals had bought the rights to seek FDA approval of benznidazole and market it in the United States.
The same company’s CEO came under fire in recent months for acquiring rights to an inexpensive drug to treat toxoplasmosis in AIDS patients, then announcing a price increase from $13.50 to $750 a pill.
“Everyone’s really concerned,” Dr. Meymandi said, “because Chagas is a disease of the poor.”
Beta-blocker prevents trastuzumab-related LVEF drop
SAN ANTONIO – Prophylactic beta-blockade with bisoprolol during adjuvant trastuzumab therapy for HER2-positive breast cancer prevented trastuzumab-induced decline in left ventricular ejection fraction in MANTICORE, a randomized trial presented at the San Antonio Breast Cancer Symposium.
“MANTICORE provides the first intervention proven in a randomized, double-blind, placebo-controlled, multicenter way to be an effective means of preventing trastuzumab-associated left ventricular dysfunction,” said Edith Pituskin, Ph.D., of the University of Alberta, Edmonton.
MANTICORE (Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research) randomized 99 patients with HER2-positive early breast cancer and a normal-range left ventricular ejection fraction (LVEF) at baseline to bisoprolol (Zebeta), the ACE inhibitor perindopril (Aceon), or placebo shortly before starting a planned 1-year course of adjuvant trastuzumab (Herceptin). The patients had low background levels of cardiovascular risk factors. Roughly three-quarters of subjects were able to titrate up to the target dose of 10 mg once daily for bisoprolol or 8 mg once daily for perindopril.
Cardiac MRI assessments at baseline, 3, and 12 months – the point when trastuzumab and the cardioprotective medications were stopped – showed that neither bisoprolol nor perindopril prevented trastuzumab-related left ventricular remodeling, which was a disappointment, given that this was the prespecified primary endpoint.
“Our results hint that, potentially, trastuzumab exposure–related left ventricular remodeling is not reversible,” Dr. Pituskin said.
The average reduction from baseline in LVEF over the course of a year of adjuvant trastuzumab therapy was 5% with placebo, 3% with perindopril, and 1% with bisoprolol, with the differences between bisoprolol and the other two study arms being highly statistically significant.
On the plus side, both of the once-daily cardiac medications displayed an important side benefit in MANTICORE: an eightfold reduction in the number of interruptions of trastuzumab therapy mandated by a significant drop in LVEF. There were eight such interruptions in the control group versus one each in the bisoprolol and perindopril arms. That’s of practical value in terms of patient convenience, cost of care, and possibly even the efficacy of the adjuvant cancer regimen, she noted.
Audience members raised several questions: what’s the clinical significance of the asymptomatic reduction in LVEF seen in the control group in MANTICORE? Does it place affected patients at risk for overt heart failure as time passes? And since LVEF is just one aspect of cardiac function, isn’t it possible that the cardiac medications were simply propping up LVEF while the underlying cardiotoxic effects of trastuzumab remained unchecked, such that the LVEF will drop once patients are off therapy?
Dr. Pituskin replied that these are good questions, the answers to which may be forthcoming at the planned follow-up cardiac MRI to be conducted at 24 months, a full year after discontinuation of the therapies.
Asked to compare the MANTICORE findings with those of the PRADA trial presented at this year’s annual meeting of the American Heart Association, in which an LVEF drop in breast cancer patients on adjuvant anthracycline and trastuzumab was prevented by prophylactic use of the angiotensin receptor blocker candesartan (Atacand) but not the beta-blocker metoprolol, Dr. Pituskin said she can’t make a definitive comparison until PRADA is published, but that it’s her understanding PRADA was a single-center trial without serial cardiac MRIs, and it included many more participants on an anthracycline-containing regimen, long recognized as a major hazard in terms of cardiotoxicity, and one thought to have a different cardiotoxic mechanism than trastuzumab.
By way of background, she noted that roughly 20% of women with breast cancer have HER2 receptor–overexpressing tumors. In such patients it’s well established that trastuzumab reduces mortality by one-third. However, it’s also well established that one in five patients on trastuzumab experience left ventricular dysfunction, and 1%-5% of patients develop heart failure, an “extremely devastating” complication carrying a 50% 5-year mortality. Beta-blockers and ACE inhibitors or angiotensin receptor blockers are standard, guideline-recommended treatments for patients with established heart failure.
SAN ANTONIO – Prophylactic beta-blockade with bisoprolol during adjuvant trastuzumab therapy for HER2-positive breast cancer prevented trastuzumab-induced decline in left ventricular ejection fraction in MANTICORE, a randomized trial presented at the San Antonio Breast Cancer Symposium.
“MANTICORE provides the first intervention proven in a randomized, double-blind, placebo-controlled, multicenter way to be an effective means of preventing trastuzumab-associated left ventricular dysfunction,” said Edith Pituskin, Ph.D., of the University of Alberta, Edmonton.
MANTICORE (Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research) randomized 99 patients with HER2-positive early breast cancer and a normal-range left ventricular ejection fraction (LVEF) at baseline to bisoprolol (Zebeta), the ACE inhibitor perindopril (Aceon), or placebo shortly before starting a planned 1-year course of adjuvant trastuzumab (Herceptin). The patients had low background levels of cardiovascular risk factors. Roughly three-quarters of subjects were able to titrate up to the target dose of 10 mg once daily for bisoprolol or 8 mg once daily for perindopril.
Cardiac MRI assessments at baseline, 3, and 12 months – the point when trastuzumab and the cardioprotective medications were stopped – showed that neither bisoprolol nor perindopril prevented trastuzumab-related left ventricular remodeling, which was a disappointment, given that this was the prespecified primary endpoint.
“Our results hint that, potentially, trastuzumab exposure–related left ventricular remodeling is not reversible,” Dr. Pituskin said.
The average reduction from baseline in LVEF over the course of a year of adjuvant trastuzumab therapy was 5% with placebo, 3% with perindopril, and 1% with bisoprolol, with the differences between bisoprolol and the other two study arms being highly statistically significant.
On the plus side, both of the once-daily cardiac medications displayed an important side benefit in MANTICORE: an eightfold reduction in the number of interruptions of trastuzumab therapy mandated by a significant drop in LVEF. There were eight such interruptions in the control group versus one each in the bisoprolol and perindopril arms. That’s of practical value in terms of patient convenience, cost of care, and possibly even the efficacy of the adjuvant cancer regimen, she noted.
Audience members raised several questions: what’s the clinical significance of the asymptomatic reduction in LVEF seen in the control group in MANTICORE? Does it place affected patients at risk for overt heart failure as time passes? And since LVEF is just one aspect of cardiac function, isn’t it possible that the cardiac medications were simply propping up LVEF while the underlying cardiotoxic effects of trastuzumab remained unchecked, such that the LVEF will drop once patients are off therapy?
Dr. Pituskin replied that these are good questions, the answers to which may be forthcoming at the planned follow-up cardiac MRI to be conducted at 24 months, a full year after discontinuation of the therapies.
Asked to compare the MANTICORE findings with those of the PRADA trial presented at this year’s annual meeting of the American Heart Association, in which an LVEF drop in breast cancer patients on adjuvant anthracycline and trastuzumab was prevented by prophylactic use of the angiotensin receptor blocker candesartan (Atacand) but not the beta-blocker metoprolol, Dr. Pituskin said she can’t make a definitive comparison until PRADA is published, but that it’s her understanding PRADA was a single-center trial without serial cardiac MRIs, and it included many more participants on an anthracycline-containing regimen, long recognized as a major hazard in terms of cardiotoxicity, and one thought to have a different cardiotoxic mechanism than trastuzumab.
By way of background, she noted that roughly 20% of women with breast cancer have HER2 receptor–overexpressing tumors. In such patients it’s well established that trastuzumab reduces mortality by one-third. However, it’s also well established that one in five patients on trastuzumab experience left ventricular dysfunction, and 1%-5% of patients develop heart failure, an “extremely devastating” complication carrying a 50% 5-year mortality. Beta-blockers and ACE inhibitors or angiotensin receptor blockers are standard, guideline-recommended treatments for patients with established heart failure.
SAN ANTONIO – Prophylactic beta-blockade with bisoprolol during adjuvant trastuzumab therapy for HER2-positive breast cancer prevented trastuzumab-induced decline in left ventricular ejection fraction in MANTICORE, a randomized trial presented at the San Antonio Breast Cancer Symposium.
“MANTICORE provides the first intervention proven in a randomized, double-blind, placebo-controlled, multicenter way to be an effective means of preventing trastuzumab-associated left ventricular dysfunction,” said Edith Pituskin, Ph.D., of the University of Alberta, Edmonton.
MANTICORE (Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research) randomized 99 patients with HER2-positive early breast cancer and a normal-range left ventricular ejection fraction (LVEF) at baseline to bisoprolol (Zebeta), the ACE inhibitor perindopril (Aceon), or placebo shortly before starting a planned 1-year course of adjuvant trastuzumab (Herceptin). The patients had low background levels of cardiovascular risk factors. Roughly three-quarters of subjects were able to titrate up to the target dose of 10 mg once daily for bisoprolol or 8 mg once daily for perindopril.
Cardiac MRI assessments at baseline, 3, and 12 months – the point when trastuzumab and the cardioprotective medications were stopped – showed that neither bisoprolol nor perindopril prevented trastuzumab-related left ventricular remodeling, which was a disappointment, given that this was the prespecified primary endpoint.
“Our results hint that, potentially, trastuzumab exposure–related left ventricular remodeling is not reversible,” Dr. Pituskin said.
The average reduction from baseline in LVEF over the course of a year of adjuvant trastuzumab therapy was 5% with placebo, 3% with perindopril, and 1% with bisoprolol, with the differences between bisoprolol and the other two study arms being highly statistically significant.
On the plus side, both of the once-daily cardiac medications displayed an important side benefit in MANTICORE: an eightfold reduction in the number of interruptions of trastuzumab therapy mandated by a significant drop in LVEF. There were eight such interruptions in the control group versus one each in the bisoprolol and perindopril arms. That’s of practical value in terms of patient convenience, cost of care, and possibly even the efficacy of the adjuvant cancer regimen, she noted.
Audience members raised several questions: what’s the clinical significance of the asymptomatic reduction in LVEF seen in the control group in MANTICORE? Does it place affected patients at risk for overt heart failure as time passes? And since LVEF is just one aspect of cardiac function, isn’t it possible that the cardiac medications were simply propping up LVEF while the underlying cardiotoxic effects of trastuzumab remained unchecked, such that the LVEF will drop once patients are off therapy?
Dr. Pituskin replied that these are good questions, the answers to which may be forthcoming at the planned follow-up cardiac MRI to be conducted at 24 months, a full year after discontinuation of the therapies.
Asked to compare the MANTICORE findings with those of the PRADA trial presented at this year’s annual meeting of the American Heart Association, in which an LVEF drop in breast cancer patients on adjuvant anthracycline and trastuzumab was prevented by prophylactic use of the angiotensin receptor blocker candesartan (Atacand) but not the beta-blocker metoprolol, Dr. Pituskin said she can’t make a definitive comparison until PRADA is published, but that it’s her understanding PRADA was a single-center trial without serial cardiac MRIs, and it included many more participants on an anthracycline-containing regimen, long recognized as a major hazard in terms of cardiotoxicity, and one thought to have a different cardiotoxic mechanism than trastuzumab.
By way of background, she noted that roughly 20% of women with breast cancer have HER2 receptor–overexpressing tumors. In such patients it’s well established that trastuzumab reduces mortality by one-third. However, it’s also well established that one in five patients on trastuzumab experience left ventricular dysfunction, and 1%-5% of patients develop heart failure, an “extremely devastating” complication carrying a 50% 5-year mortality. Beta-blockers and ACE inhibitors or angiotensin receptor blockers are standard, guideline-recommended treatments for patients with established heart failure.
AT SABCS 2015
Key clinical point: Bisoprolol prevents the trastuzumab-related decline in left ventricular ejection fraction in breast cancer patients.
Major finding: The average reduction from baseline in LVEF over the course of a year of adjuvant trastuzumab therapy was 5% with placebo, 3% with perindopril, and 1% with bisoprolol, with the differences between bisoprolol and the other two study arms being highly statistically significant. .
Data source: MANTICORE was a randomized, double-blind, multicenter study in which 99 patients with HER2-positive breast cancer were placed on prophylactic bisoprolol, perindopril, or placebo before starting adjuvant trastuzumab.
Disclosures: MANTICORE was funded by the Canadian Institutes for Health Research and the Alberta Cancer Foundation. The study presenter reported having no financial conflicts of interest.
FDA cancels REMS for rosiglitazone
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
The thiazolidinedione agent rosiglitazone now can be prescribed without complying with a Risk Evaluation and Mitigation Strategy (REMS), according to the Food and Drug Administration.
It’s been a long, strange trip for the drug that was approved in 1999 to lower blood sugar in patients with type 2 diabetes and is sold under a number of brands, including Avandia, Avandamet, and Avandaryl, as well as generics. Over the first years it was marketed, there were reports that its use was associated with an increased risk for heart failure and myocardial infarctions.
In May 2007, the FDA issued an alert that use of rosiglitazone was associated with ischemic cardiovascular events, which eventually led the agency to add a black box warning to the drug’s label in August 2007 that its use was associated with heart failure in patients with type 2 diabetes. In November 2007 the FDA extended the warning to cover an increased risk for myocardial infarction, based on “a meta-analysis of 42 clinical studies (mean duration 6 months; 14,237 total patients), most of which compared Avandia to placebo, showed Avandia to be associated with an increased risk of myocardial ischemic events such as angina or myocardial infarction. Three other studies (mean duration 41 months; 14,067 patients), comparing Avandia to some other approved oral antidiabetic agents or placebo, have not confirmed or excluded this risk. In their entirety, the available data on the risk of myocardial ischemia are inconclusive.”
In May 2011, the FDA issued a requirement that physicians who prescribed rosiglitazone participate in a REMS program. But by November 2013 the agency had withdrawn its restrictions on prescription of the drug to patients with diabetes.
Today’s action may be the final step in returning the drug to use. However, the number of new drugs that have been approved for management of type 2 diabetes in the years since rosiglitazone first appeared problematic may make this FDA action irrelevant.
FROM THE FOOD AND DRUG ADMINISTRATION
VIDEO: Beta-blocker prevented trastuzumab-related drop in LVEF
SAN ANTONIO – Prophylactic beta-blockade with bisoprolol during adjuvant trastuzumab therapy for HER2-positive breast cancer prevented trastuzumab-induced decline in left ventricular ejection fraction in a randomized trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Edie Pituskin of the University of Alberta, Edmonton, explained why the double-blind, placebo-controlled MANTICORE trial may change clinical practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Prophylactic beta-blockade with bisoprolol during adjuvant trastuzumab therapy for HER2-positive breast cancer prevented trastuzumab-induced decline in left ventricular ejection fraction in a randomized trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Edie Pituskin of the University of Alberta, Edmonton, explained why the double-blind, placebo-controlled MANTICORE trial may change clinical practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Prophylactic beta-blockade with bisoprolol during adjuvant trastuzumab therapy for HER2-positive breast cancer prevented trastuzumab-induced decline in left ventricular ejection fraction in a randomized trial.
In an interview at the San Antonio Breast Cancer Symposium, Dr. Edie Pituskin of the University of Alberta, Edmonton, explained why the double-blind, placebo-controlled MANTICORE trial may change clinical practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2015
AHA: Should BP targets be higher in asymptomatic aortic stenosis?
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
ORLANDO – Optimal blood pressure in patients with asymptomatic mild to moderate aortic stenosis is 140-159 mm Hg for systolic and 70-89 mm Hg for diastolic, according to an analysis of all-cause mortality in the world’s largest data set of such patients with longitudinal follow-up and endpoint evaluation.
Within those target blood pressure ranges, the nadir in terms of all-cause mortality – and thus the true optimal blood pressure – is about 145/82 mm Hg, Dr. Kristian Wachtell reported at the American Heart Association scientific sessions.
He presented an analysis of 1,873 asymptomatic patients with mild to moderate aortic stenosis (AS) and a peak aortic-jet velocity of 2.5-4.0 M/sec upon enrollment in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) trial. SEAS was a double-blind, multicenter study in which participants were randomized to 40 mg of simvastatin plus 10 mg of ezetimibe per day or placebo and followed for a mean of 4.3 years. Half of them had a history of hypertension at baseline.
As previously reported (N Engl J Med. 2008 Sep 25;359[13]:1343-56), SEAS was a negative trial. Lipid-lowering therapy didn’t affect AS progression or aortic valve–related outcomes. On the plus side, the SEAS cohort is the world’s largest population of patients with asymptomatic AS followed prospectively for clinical endpoints, noted Dr. Wachtell of Oslo University.
He and his coinvestigators decided to plot all-cause mortality versus average blood pressure in the SEAS cohort because of the paucity of data regarding blood pressure and antihypertensive therapy in patients with asymptomatic AS. Neither the 2014 ACC/AHA guidelines for management of valvular heart disease (Circulation. 2014 Jun 10;129[23]:e521-643) nor the European guidelines provide recommendations for optimal blood pressure targets in patients with asymptomatic AS, even though AS is the third most common cardiac disease, behind hypertension and coronary artery disease.
Moreover, nearly 100,000 aortic valve replacements are done per year in the United States as a consequence of AS. And AS and hypertension go hand in hand, with the prevalence of hypertension among patients with AS cited as 50% or more in multiple studies, the cardiologist continued.
In a multivariate analysis adjusted for aortic valve area index and peak velocity, heart failure, MI, and aortic valve replacement during follow-up, all-cause mortality showed a U-shaped relationship with blood pressure. A systolic blood pressure below 120 mm Hg was associated with a 5-fold increased risk of mortality, a systolic of 120-139 mm Hg carried a 1.5-fold increased risk, and a diastolic blood pressure of 90 mm Hg or more was associated with a 1.9-fold increased risk.
“Patients with low systolic blood pressure had an increased mortality risk and should probably undertake individual clinical assessment for blood pressure control and evaluation of their AS,” Dr. Wachtell said.
The first question audience members asked was, “What about SPRINT?” The SPRINT trial, presented elsewhere at the meeting, was a practice-changing study that was the talk of the conference. It redefined the systolic blood pressure treatment target as less than 120 mm Hg instead of less than 140 mm Hg in hypertensive patients (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939).
“I think it’s most likely that for blood pressure, one size does not fit all,” Dr. Wachtell replied. “The extent of target organ damage – and you could say that aortic stenosis is actually target organ damage, like atrial fibrillation or left ventricular hypertrophy – warrants a different level of blood pressure.”
He added, however, that the SEAS analysis was based on observational data, and that’s a limitation.
“This is a qualified guess as to what blood pressures should be in patients with aortic stenosis,” he cautioned.
Dr. Wachtell reported having no conflicts of interest regarding his presentation.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Optimal blood pressures in patients with asymptomatic aortic stenosis appear to be significantly higher than those identified for the general hypertensive population in the recent SPRINT trial.
Major finding: All-cause mortality in patients with asymptomatic mild to moderate aortic stenosis is lowest at a blood pressure of 145/82 mm Hg.
Data source: This was an analysis of all-cause mortality over a mean follow-up of 4.3 years in 1,873 patients with asymptomatic mild to moderate aortic stenosis.
Disclosures: This secondary analysis of a completed clinical trial was conducted without commercial support. The presenter reported having no financial conflicts.
AHA: Three measures risk stratify acute heart failure
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.
The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.

The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Three simple, routinely collected measurements together provide a lot of insight into the risk faced by community-dwelling patients hospitalized for acute decompensated heart failure, according to data collected from 3,628 patients at one U.S. center.
The three measures are blood urea nitrogen (BUN), systolic blood pressure, and serum creatinine. Using dichotomous cutoffs first calculated a decade ago, these three parameters distinguish up to an eightfold range of postdischarge mortality during the 30 or 90 days following an index hospitalization, and up to a fourfold range of risk for rehospitalization for heart failure during the ensuing 30 or 90 days, Dr. Sithu Win said at the American Heart Association scientific sessions.
Applying this three-measure assessment to patients hospitalized with acute decompensated heart failure “may guide care-transition planning and promote efficient allocation of limited resources,” said Dr. Win, a cardiologist at the Mayo Clinic in Rochester, Minn. The next step is to try to figure out the best way to use this risk prognostication in routine practice, he added.
Researchers published the original analysis that identified BUN, systolic BP, and serum creatinine as key prognostic measures in 2005 using data taken from more than 65,000 U.S. heart failure patients enrolled in ADHERE (Acute Decompensated Heart Failure National Registry) (JAMA. 2005 Feb 2;293[5]:572-80). Using a classification and regression tree analysis, the 2005 study verified dichotomous cutoffs for these three parameters that identified patients at highest risk for in-hospital mortality.
The 2005 study prioritized the application of these cutoffs to define in-hospital mortality risk: first BUN, then the systolic BP criterion, and lastly the serum creatinine criterion. This resulted in five risk levels: Highest-risk patients had a BUN of at least 43 mg/dL, a systolic BP of less than 115 mm Hg, and a serum creatinine of at least 2.75 mg/dL. Lowest-risk patients had a BUN of less than 43 mg/dL and a systolic BP of more than 115 mm Hg. (In lower-risk patients, serum-creatinine level dropped out as a risk determinant.) The analysis also created three categories of patients with intermediate risk based on various combinations of the three measures.

The new study run by Dr. Win and his associates evaluated how this risk-assessment tool developed to predict in-hospital mortality performed for predicting event rates among community-based heart failure patients who had a total of 5,918 hospitalizations for acute decompensated heart failure at the Mayo Clinic during 2000-2013. They averaged 78 years old, half were women, and 48% had heart failure with preserved ejection fraction.
The risk-level distribution of the 3,628 Mayo patients closely matched the pattern seen in the original ADHERE registry: 63% were low risk, 17% were at intermediate level 3 (the lowest risk level in the intermediate range), 13% were at intermediate level 2, 5% at intermediate level 1, and 2% were categorized as high risk.
For 30-day mortality post hospitalization, patients at the highest risk level had a mortality rate eightfold higher than did the lowest-risk patients, those rated intermediate level 1 had a fivefold higher mortality rate, intermediate level 2 patients had a threefold higher rate, and those at intermediate 3 had a 50% higher rate, Dr. Win reported. During the 90 days after discharge, mortality rates relative to the lowest risk level ranged from a sixfold higher rate among the highest-risk patients to a 50% higher rate among patients with an intermediate 3 designation.
Analysis of rehospitalizations for heart failure showed that, by 30 days after hospitalization, the readmission rate ran threefold higher in the highest-risk patients, compared with those at the lowest risk and fourfold higher among those at intermediate risk level 1. Heart failure readmissions by 90 days following the index hospitalization ran threefold higher for both the highest-risk patients as well as those at intermediate level 1, compared with the patients at lowest risk.
The new analyses also showed that roughly similar risk patterns occurred regardless of whether patients had heart failure with reduced or preserved ejection fraction during their index hospitalization, although the relatively increased rate of 30-day mortality with a worse risk profile was most dramatic among patients with reduced ejection fraction. Age, sex, and comorbidity severity did not have a marked effect on the relationships between event rates and risk levels, Dr. Win said.
Dr. Win had no disclosures.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Dichotomous cutoffs for BUN, systolic BP, and serum creatinine together robustly risk stratified patients hospitalized for acute decompensated heart failure.
Major finding: Three baseline measures together stratified patients over an eightfold range for 30-day mortality following hospital discharge.
Data source: Application of the risk-stratification formula to 3,628 heart failure patients hospitalized at one U.S. center during 2000-2013.
Disclosures: Dr. Win had no disclosures.
AHA: Spirometry identifies mortality risk in asymptomatic adults
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
On Twitter@mitchelzoler
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
On Twitter@mitchelzoler
ORLANDO – Unselected people from the general population without clinically apparent lung disease but with low lung function had significantly increased mortality during follow-up that was independent of cardiac function, in results from more than 13,000 middle-aged Germans.
“Subtle, subclinical pulmonary impairment is a risk indicator for increased mortality independent of cardiac performance,” Dr. Christina Baum said at the American Heart Association scientific sessions.
The researchers used spirometry to measure each subject’s forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The results showed that “spirometry is a good screening tool that is not very expensive,” making spirometry an effective risk assessment tool for use in the general adult population, said Dr. Baum of the department of general and interventional cardiology at the University Heart Center in Hamburg, Germany.
She and her associates used data collected in the Gutenberg Health Study, which enrolled more than 15,000 German women and men aged 35-74 years during 2007-2012. The investigators excluded people with a history of pulmonary disease, resulting in a study cohort of 13,191, who averaged 55 years old, with 51% men.
At enrollment into the study, all people underwent screening spirometry and echocardiography. Their average baseline FEV1 was 2.9 L and their average FVC was 3.7 L, and 4% had heart failure based on assessments of left ventricular size and function by echocardiography. The first 5,000 enrollees also had measurements taken of their serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I through use of a high-sensitivity assay. The researchers used data from patients followed for a median of 5.5 years.
During follow-up, people in the lowest tertile for FEV1 and those in the lowest tertile for FVC had higher rates of all-cause mortality, compared with those in the highest tertile for each of these two parameters.
In a multivariate analysis that adjusted for age, sex, body mass index, smoking status, hypertension, dyslipidemia, heart failure status, serum levels of N-terminal probrain natriuretic peptide and cardiac troponin I, and other parameters, people with lower FEV1 and FVC readings had significantly worse survival, Dr. Baum said. Every 1–standard deviation increase in FEV1 was linked with a statistically significant, 38% reduced mortality rate; furthermore, a similar significant inverse association existed between FVC and mortality, she reported.
On Twitter@mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Measurement of low FEV1 or low FVC by spirometry identified people at increased mortality risk independent of their cardiac function.
Major finding: For each standard deviation rise in FEV1, mortality fell by 38%.
Data source: The Gutenberg Heart Study, which enrolled 15,010 German residents aged 35-74 years old, including 13,191 without prevalent pulmonary disease.
Disclosures: Dr. Baum had no relevant financial disclosures.
AHA: Empagliflozin for T2D reduces heart failure endpoints
ORLANDO – The SGLT2 inhibitor empagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or death due to cardiovascular disease by 34% in patients with type 2 diabetes and prior cardiovascular events in the landmark EMPA-REG OUTCOME trial, Dr. Silvio E. Inzucchi reported at the American Heart Association scientific sessions.
The benefit was consistent regardless of whether or not patients had heart failure at enrollment, added Dr. Inzucchi, professor of medicine and clinical director of the endocrinology section at Yale University in New Haven, Conn.
He presented a prespecified secondary analysis from EMPA-REG OUTCOME that focused on heart failure–related endpoints. He had previously presented the primary outcome in Stockholm at the annual meeting of the European Association for the Study of Diabetes: a 14% relative risk reduction for empagliflozin in the composite of cardiovascular death, MI, or stroke, compared with placebo, driven largely by a 38% relative risk reduction in cardiovascular death.
The phase III, randomized, double-blind study has taken the worlds of cardiology and endocrinology by storm because it has convincingly demonstrated that empagliflozin (Jardiance) is the first glucose-lowering agent that also prevents cardiovascular complications.
“There are 12 different types of medications for lowering blood sugar levels in patients with type 2 diabetes – more than there are for lowering high blood pressure,” he noted. “We have been searching for decades for a diabetes medicine that will not only lower blood sugar but also reduce cardiovascular complications.”
The EMPA-REG OUTCOME trial included 7,020 patients with type 2 diabetes and a history of cardiovascular disease who were followed for a median of 3.1 years. Participants had a high rate of background optimal medical therapy for secondary cardiovascular prevention. They were randomized to empagliflozin at 10 or 25 mg/day or placebo. Results with the two doses of empagliflozin were pooled because the outcomes were so similar.
In addition to the 34% relative risk reduction in the combined endpoint of heart failure hospitalization or heart failure death, the empagliflozin group also experienced a 39% reduction in the composite of heart failure hospitalization or death due to heart failure.
These advantages for empagliflozin held true for all prespecified patient subgroups, including those based upon age, renal function, use of insulin, and background cardioprotective medications, Dr. Inzucchi noted.
Of study participants, 10% already had heart failure at baseline. Their rate of heart failure hospitalization or cardiovascular death was 20.1% on placebo and 16.2% with empagliflozin, a 28% relative risk reduction. In patients without heart failure at enrollment, the rates of this composite endpoint were 7.1% with placebo and 4.5% with the SGLT2 (sodium-glucose transporter 2) inhibitor, for a 37% relative risk reduction.
The only adverse event that occurred more frequently in empagliflozin-treated patients with or without heart failure than in controls was a threefold increase in genital infections. These easily treatable infections are a consequence of empagliflozin’s mechanism of action in reducing blood glucose, which entails increasing urinary excretion, the endocrinologist explained.
Discussant Dr. Allison B. Goldfine called Dr. Inzucchi’s update “really exciting.”
“Up to this time there has been little within the diabetes therapeutic pharmacologic options that has established clear safety, specifically with regard to heart failure. While we may not fully understand the mechanism behind the observed cardiovascular risk reduction, the uniformity of the findings is really remarkably consistent,” said Dr. Goldfine, head of the section of endocrinology at the Joslin Diabetes Center and an endocrinologist at Harvard Medical School, Boston.
“This is very promising,” Dr. David Goff, dean of the Colorado School of Public Health, declared in an interview. “We’ve had a lot of disappointments in the field of cardiovascular disease prevention for patients with diabetes mellitus. I think this medication should change the way we take care of people with diabetes.”
Metformin is widely accepted within endocrinology as the first-line agent for the treatment of type 2 diabetes, Dr. Inzucchi said. But he added that cardiovascular disease is a major problem in patients with diabetes. Heart failure, for example, is present in more than one in five type 2 diabetic patients over age 65.
Asked if he believes the cardiovascular benefits seen with empagliflozin in EMPA-REG OUTCOME are likely to be due to a class effect for SGLT2 inhibitors, Dr. Inzucchi replied that it’s impossible to say, since empagliflozin’s mechanism of cardiovascular benefit is unknown.
“Large randomized trials with hard cardiovascular endpoints are ongoing for the other two SGLT2 inhibitors, canagliflozin and dapagliflozin. Results should be available in 2-3 years. Then we’ll know,” he said.
Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
ORLANDO – The SGLT2 inhibitor empagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or death due to cardiovascular disease by 34% in patients with type 2 diabetes and prior cardiovascular events in the landmark EMPA-REG OUTCOME trial, Dr. Silvio E. Inzucchi reported at the American Heart Association scientific sessions.
The benefit was consistent regardless of whether or not patients had heart failure at enrollment, added Dr. Inzucchi, professor of medicine and clinical director of the endocrinology section at Yale University in New Haven, Conn.
He presented a prespecified secondary analysis from EMPA-REG OUTCOME that focused on heart failure–related endpoints. He had previously presented the primary outcome in Stockholm at the annual meeting of the European Association for the Study of Diabetes: a 14% relative risk reduction for empagliflozin in the composite of cardiovascular death, MI, or stroke, compared with placebo, driven largely by a 38% relative risk reduction in cardiovascular death.
The phase III, randomized, double-blind study has taken the worlds of cardiology and endocrinology by storm because it has convincingly demonstrated that empagliflozin (Jardiance) is the first glucose-lowering agent that also prevents cardiovascular complications.
“There are 12 different types of medications for lowering blood sugar levels in patients with type 2 diabetes – more than there are for lowering high blood pressure,” he noted. “We have been searching for decades for a diabetes medicine that will not only lower blood sugar but also reduce cardiovascular complications.”
The EMPA-REG OUTCOME trial included 7,020 patients with type 2 diabetes and a history of cardiovascular disease who were followed for a median of 3.1 years. Participants had a high rate of background optimal medical therapy for secondary cardiovascular prevention. They were randomized to empagliflozin at 10 or 25 mg/day or placebo. Results with the two doses of empagliflozin were pooled because the outcomes were so similar.
In addition to the 34% relative risk reduction in the combined endpoint of heart failure hospitalization or heart failure death, the empagliflozin group also experienced a 39% reduction in the composite of heart failure hospitalization or death due to heart failure.
These advantages for empagliflozin held true for all prespecified patient subgroups, including those based upon age, renal function, use of insulin, and background cardioprotective medications, Dr. Inzucchi noted.
Of study participants, 10% already had heart failure at baseline. Their rate of heart failure hospitalization or cardiovascular death was 20.1% on placebo and 16.2% with empagliflozin, a 28% relative risk reduction. In patients without heart failure at enrollment, the rates of this composite endpoint were 7.1% with placebo and 4.5% with the SGLT2 (sodium-glucose transporter 2) inhibitor, for a 37% relative risk reduction.
The only adverse event that occurred more frequently in empagliflozin-treated patients with or without heart failure than in controls was a threefold increase in genital infections. These easily treatable infections are a consequence of empagliflozin’s mechanism of action in reducing blood glucose, which entails increasing urinary excretion, the endocrinologist explained.
Discussant Dr. Allison B. Goldfine called Dr. Inzucchi’s update “really exciting.”
“Up to this time there has been little within the diabetes therapeutic pharmacologic options that has established clear safety, specifically with regard to heart failure. While we may not fully understand the mechanism behind the observed cardiovascular risk reduction, the uniformity of the findings is really remarkably consistent,” said Dr. Goldfine, head of the section of endocrinology at the Joslin Diabetes Center and an endocrinologist at Harvard Medical School, Boston.
“This is very promising,” Dr. David Goff, dean of the Colorado School of Public Health, declared in an interview. “We’ve had a lot of disappointments in the field of cardiovascular disease prevention for patients with diabetes mellitus. I think this medication should change the way we take care of people with diabetes.”
Metformin is widely accepted within endocrinology as the first-line agent for the treatment of type 2 diabetes, Dr. Inzucchi said. But he added that cardiovascular disease is a major problem in patients with diabetes. Heart failure, for example, is present in more than one in five type 2 diabetic patients over age 65.
Asked if he believes the cardiovascular benefits seen with empagliflozin in EMPA-REG OUTCOME are likely to be due to a class effect for SGLT2 inhibitors, Dr. Inzucchi replied that it’s impossible to say, since empagliflozin’s mechanism of cardiovascular benefit is unknown.
“Large randomized trials with hard cardiovascular endpoints are ongoing for the other two SGLT2 inhibitors, canagliflozin and dapagliflozin. Results should be available in 2-3 years. Then we’ll know,” he said.
Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
ORLANDO – The SGLT2 inhibitor empagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or death due to cardiovascular disease by 34% in patients with type 2 diabetes and prior cardiovascular events in the landmark EMPA-REG OUTCOME trial, Dr. Silvio E. Inzucchi reported at the American Heart Association scientific sessions.
The benefit was consistent regardless of whether or not patients had heart failure at enrollment, added Dr. Inzucchi, professor of medicine and clinical director of the endocrinology section at Yale University in New Haven, Conn.
He presented a prespecified secondary analysis from EMPA-REG OUTCOME that focused on heart failure–related endpoints. He had previously presented the primary outcome in Stockholm at the annual meeting of the European Association for the Study of Diabetes: a 14% relative risk reduction for empagliflozin in the composite of cardiovascular death, MI, or stroke, compared with placebo, driven largely by a 38% relative risk reduction in cardiovascular death.
The phase III, randomized, double-blind study has taken the worlds of cardiology and endocrinology by storm because it has convincingly demonstrated that empagliflozin (Jardiance) is the first glucose-lowering agent that also prevents cardiovascular complications.
“There are 12 different types of medications for lowering blood sugar levels in patients with type 2 diabetes – more than there are for lowering high blood pressure,” he noted. “We have been searching for decades for a diabetes medicine that will not only lower blood sugar but also reduce cardiovascular complications.”
The EMPA-REG OUTCOME trial included 7,020 patients with type 2 diabetes and a history of cardiovascular disease who were followed for a median of 3.1 years. Participants had a high rate of background optimal medical therapy for secondary cardiovascular prevention. They were randomized to empagliflozin at 10 or 25 mg/day or placebo. Results with the two doses of empagliflozin were pooled because the outcomes were so similar.
In addition to the 34% relative risk reduction in the combined endpoint of heart failure hospitalization or heart failure death, the empagliflozin group also experienced a 39% reduction in the composite of heart failure hospitalization or death due to heart failure.
These advantages for empagliflozin held true for all prespecified patient subgroups, including those based upon age, renal function, use of insulin, and background cardioprotective medications, Dr. Inzucchi noted.
Of study participants, 10% already had heart failure at baseline. Their rate of heart failure hospitalization or cardiovascular death was 20.1% on placebo and 16.2% with empagliflozin, a 28% relative risk reduction. In patients without heart failure at enrollment, the rates of this composite endpoint were 7.1% with placebo and 4.5% with the SGLT2 (sodium-glucose transporter 2) inhibitor, for a 37% relative risk reduction.
The only adverse event that occurred more frequently in empagliflozin-treated patients with or without heart failure than in controls was a threefold increase in genital infections. These easily treatable infections are a consequence of empagliflozin’s mechanism of action in reducing blood glucose, which entails increasing urinary excretion, the endocrinologist explained.
Discussant Dr. Allison B. Goldfine called Dr. Inzucchi’s update “really exciting.”
“Up to this time there has been little within the diabetes therapeutic pharmacologic options that has established clear safety, specifically with regard to heart failure. While we may not fully understand the mechanism behind the observed cardiovascular risk reduction, the uniformity of the findings is really remarkably consistent,” said Dr. Goldfine, head of the section of endocrinology at the Joslin Diabetes Center and an endocrinologist at Harvard Medical School, Boston.
“This is very promising,” Dr. David Goff, dean of the Colorado School of Public Health, declared in an interview. “We’ve had a lot of disappointments in the field of cardiovascular disease prevention for patients with diabetes mellitus. I think this medication should change the way we take care of people with diabetes.”
Metformin is widely accepted within endocrinology as the first-line agent for the treatment of type 2 diabetes, Dr. Inzucchi said. But he added that cardiovascular disease is a major problem in patients with diabetes. Heart failure, for example, is present in more than one in five type 2 diabetic patients over age 65.
Asked if he believes the cardiovascular benefits seen with empagliflozin in EMPA-REG OUTCOME are likely to be due to a class effect for SGLT2 inhibitors, Dr. Inzucchi replied that it’s impossible to say, since empagliflozin’s mechanism of cardiovascular benefit is unknown.
“Large randomized trials with hard cardiovascular endpoints are ongoing for the other two SGLT2 inhibitors, canagliflozin and dapagliflozin. Results should be available in 2-3 years. Then we’ll know,” he said.
Dr. Inzucchi disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Empagliflozin is the first glucose-lowering medication ever shown to reduce heart failure hospitalizations or cardiovascular death in patients with type 2 diabetes.
Major finding: The composite rate of heart failure hospitalization or death due to heart failure was reduced by 39% in type 2 diabetic patients on empagliflozin compared with placebo.
Data source: This was a secondary analysis of the EMPA-REG OUTCOME trial, a phase-III, double-blind, randomized trial of 7,020 patients with type 2 diabetes and prior cardiovascular disease.
Disclosures: The presenter disclosed ties with Boehringer Ingelheim, Merck, Janssen, Novo Nordisk, Sanofi/Regeron, Intarcia, Lexicon, Paxel, Takeda, and Eli Lilly. He acknowledged CME-funding to Yale University from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbot, Merck Sharp & Dohme, and Sanofi.