User login
ESC: CERTITUDE casts doubt on defibrillator benefit in CRT
LONDON – Heart failure patients who are candidates for cardiac resynchronization therapy in a routine clinical practice setting will likely not benefit from the addition of a defibrillator to a pacemaker, according to results of the CERTITUDE cohort study.
In CERTITUDE, most of the deaths in patients fitted with a cardiac resynchronization therapy–pacemaker (CRT-P) device were predominantly from causes other than sudden cardiac death (SCD), which is the main rationale for using a CRT-defibrillator (CRT-D) device, said lead investigator Jean-Yves Le Heuzey at the annual congress of the European Society of Cardiology.
“Our results should not be interpreted as a general lack of benefit from CRT-D vs. CRT-P or vice versa. Rather we demonstrate that given currently selected CRT-P patients in the French population, addition of a defibrillator may not significantly add to survival.” Therefore, patients who may be eligible for CRT should not “automatically” be considered as requiring a CRT-D, suggested Dr. Le Heuzey and his coinvestigators in an article that was published online at the time of the study’s presentation (Eur Heart J. 2015 Sep 1. doi: 10.1093/eurheartj/ehv455).
Current ESC guidelines on cardiac pacing and cardiac resynchronization therapy “leave flexibility for the physician” on the use of CRT-P and CRT-D” because there was no evidence of a superior effect of the latter over CRT alone, said Dr. Le Heuzey of René Descartes University in Paris at the meeting. A randomized, controlled trial would be the only way to determine this, but such a trial is unlikely to ever be conducted, he noted.
Despite the lack of evidence, however, CRT-D is widely used, more so in the United States than in Europe, Dr. Le Heuzey observed, where more than 90% of patients needing CRT would likely have a CRT-D rather than a CRT-P device implanted.
The aims of the CERTITUDE cohort study were to look at the extent to which CRT-P patients differ from CRT-D patients in a real-life setting, and to also see if there were patients in the CRT-P group that might have benefited from CRT-D.
The prospective, observational study involved 1,705 patients who were recruited at 41 centers throughout France over a 2-year period starting in January 2008. Of these, 31% were fitted with a CRT-P device and 69% with a CRT-D.
Results showed that patients who had a CRT-P versus a CRT-D implanted were significantly older, more often female, and more symptomatic. They were also significantly less likely to have coronary artery disease, more likely to have wider QRS intervals and to have atrial fibrillation, and more often had at least two comorbidities.
Analysis of the causes of death was performed at 2 years’ follow-up, which Dr. Le Heuzey conceded was a short period of time. At this point, 267 of 1,611 patients with complete follow-up data had died, giving an overall mortality rate of 8.4% per 100 patient-years for the entire cohort.
The crude mortality rate was found to be higher among CRT-P than CRT-D patients, at 13.1% versus 6.5% per 100 patient-years (relative risk, 2.01; 95% confidence interval, 1.56-2.58; P less than .0001). But when the cause of death was examined more closely, there was no significant difference in the number of SCDs between the groups (RR, 1.57; 95% CI, 0.71-3.46; P = .42) and no significant difference when a specific cause of death analysis was performed.
The main reasons for the almost doubled risk of death in the CRT-P group was an increase in non-SCD cardiovascular mortality, mainly progressive heart failure (RR, 0.27; 95% CI, 1.62-3.18) and other cardiovascular causes (RR, 4.4; 95% CI, 1.29-15.03), Dr. Le Heuzey and associates noted in the article.
“Overall, 95% of the excess mortality among CRT-P recipients was not related to SCD,” they wrote, noting that this “suggests that the presence of a back-up defibrillator would probably not have been beneficial in terms of improving survival for these patients.”
So where does this leave clinicians? Dr. Le Heuzey referred to Table 17 in the European guidelines (Eur Heart J. 2013;34:2281-2329) with guidance on factors favoring CRT-P or CRT-D. For instance, CRT-P might be favorable in a patient with advanced heart failure or one with severe renal insufficiency or on dialysis. Other major comorbidities or frailty or cachexia might veer a decision towards CRT without a defibrillator. On the other hand, factors favoring CRT-D include a life expectancy of more than 1 year; stable, moderate (New York Heart Association class II) disease; or low to moderate–risk ischemic heart disease, with a lack of comorbidities.
CERTITUDE was funded by grants from the French Institute of Health and Medical Research (INSERM) and from the French Cardiology Society. The latter received specific grants from Biotronik, Boston Scientific, Medtronic, Saint Jude Medical, and Sorin in order to perform the study. Dr. Le Heuzey disclosed receiving fees for participation in advisory boards and conferences from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb/Pfizer, Correvio, Daiichi-Sankyo, Meda, Sanofi, and Servier.
LONDON – Heart failure patients who are candidates for cardiac resynchronization therapy in a routine clinical practice setting will likely not benefit from the addition of a defibrillator to a pacemaker, according to results of the CERTITUDE cohort study.
In CERTITUDE, most of the deaths in patients fitted with a cardiac resynchronization therapy–pacemaker (CRT-P) device were predominantly from causes other than sudden cardiac death (SCD), which is the main rationale for using a CRT-defibrillator (CRT-D) device, said lead investigator Jean-Yves Le Heuzey at the annual congress of the European Society of Cardiology.
“Our results should not be interpreted as a general lack of benefit from CRT-D vs. CRT-P or vice versa. Rather we demonstrate that given currently selected CRT-P patients in the French population, addition of a defibrillator may not significantly add to survival.” Therefore, patients who may be eligible for CRT should not “automatically” be considered as requiring a CRT-D, suggested Dr. Le Heuzey and his coinvestigators in an article that was published online at the time of the study’s presentation (Eur Heart J. 2015 Sep 1. doi: 10.1093/eurheartj/ehv455).
Current ESC guidelines on cardiac pacing and cardiac resynchronization therapy “leave flexibility for the physician” on the use of CRT-P and CRT-D” because there was no evidence of a superior effect of the latter over CRT alone, said Dr. Le Heuzey of René Descartes University in Paris at the meeting. A randomized, controlled trial would be the only way to determine this, but such a trial is unlikely to ever be conducted, he noted.
Despite the lack of evidence, however, CRT-D is widely used, more so in the United States than in Europe, Dr. Le Heuzey observed, where more than 90% of patients needing CRT would likely have a CRT-D rather than a CRT-P device implanted.
The aims of the CERTITUDE cohort study were to look at the extent to which CRT-P patients differ from CRT-D patients in a real-life setting, and to also see if there were patients in the CRT-P group that might have benefited from CRT-D.
The prospective, observational study involved 1,705 patients who were recruited at 41 centers throughout France over a 2-year period starting in January 2008. Of these, 31% were fitted with a CRT-P device and 69% with a CRT-D.
Results showed that patients who had a CRT-P versus a CRT-D implanted were significantly older, more often female, and more symptomatic. They were also significantly less likely to have coronary artery disease, more likely to have wider QRS intervals and to have atrial fibrillation, and more often had at least two comorbidities.
Analysis of the causes of death was performed at 2 years’ follow-up, which Dr. Le Heuzey conceded was a short period of time. At this point, 267 of 1,611 patients with complete follow-up data had died, giving an overall mortality rate of 8.4% per 100 patient-years for the entire cohort.
The crude mortality rate was found to be higher among CRT-P than CRT-D patients, at 13.1% versus 6.5% per 100 patient-years (relative risk, 2.01; 95% confidence interval, 1.56-2.58; P less than .0001). But when the cause of death was examined more closely, there was no significant difference in the number of SCDs between the groups (RR, 1.57; 95% CI, 0.71-3.46; P = .42) and no significant difference when a specific cause of death analysis was performed.
The main reasons for the almost doubled risk of death in the CRT-P group was an increase in non-SCD cardiovascular mortality, mainly progressive heart failure (RR, 0.27; 95% CI, 1.62-3.18) and other cardiovascular causes (RR, 4.4; 95% CI, 1.29-15.03), Dr. Le Heuzey and associates noted in the article.
“Overall, 95% of the excess mortality among CRT-P recipients was not related to SCD,” they wrote, noting that this “suggests that the presence of a back-up defibrillator would probably not have been beneficial in terms of improving survival for these patients.”
So where does this leave clinicians? Dr. Le Heuzey referred to Table 17 in the European guidelines (Eur Heart J. 2013;34:2281-2329) with guidance on factors favoring CRT-P or CRT-D. For instance, CRT-P might be favorable in a patient with advanced heart failure or one with severe renal insufficiency or on dialysis. Other major comorbidities or frailty or cachexia might veer a decision towards CRT without a defibrillator. On the other hand, factors favoring CRT-D include a life expectancy of more than 1 year; stable, moderate (New York Heart Association class II) disease; or low to moderate–risk ischemic heart disease, with a lack of comorbidities.
CERTITUDE was funded by grants from the French Institute of Health and Medical Research (INSERM) and from the French Cardiology Society. The latter received specific grants from Biotronik, Boston Scientific, Medtronic, Saint Jude Medical, and Sorin in order to perform the study. Dr. Le Heuzey disclosed receiving fees for participation in advisory boards and conferences from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb/Pfizer, Correvio, Daiichi-Sankyo, Meda, Sanofi, and Servier.
LONDON – Heart failure patients who are candidates for cardiac resynchronization therapy in a routine clinical practice setting will likely not benefit from the addition of a defibrillator to a pacemaker, according to results of the CERTITUDE cohort study.
In CERTITUDE, most of the deaths in patients fitted with a cardiac resynchronization therapy–pacemaker (CRT-P) device were predominantly from causes other than sudden cardiac death (SCD), which is the main rationale for using a CRT-defibrillator (CRT-D) device, said lead investigator Jean-Yves Le Heuzey at the annual congress of the European Society of Cardiology.
“Our results should not be interpreted as a general lack of benefit from CRT-D vs. CRT-P or vice versa. Rather we demonstrate that given currently selected CRT-P patients in the French population, addition of a defibrillator may not significantly add to survival.” Therefore, patients who may be eligible for CRT should not “automatically” be considered as requiring a CRT-D, suggested Dr. Le Heuzey and his coinvestigators in an article that was published online at the time of the study’s presentation (Eur Heart J. 2015 Sep 1. doi: 10.1093/eurheartj/ehv455).
Current ESC guidelines on cardiac pacing and cardiac resynchronization therapy “leave flexibility for the physician” on the use of CRT-P and CRT-D” because there was no evidence of a superior effect of the latter over CRT alone, said Dr. Le Heuzey of René Descartes University in Paris at the meeting. A randomized, controlled trial would be the only way to determine this, but such a trial is unlikely to ever be conducted, he noted.
Despite the lack of evidence, however, CRT-D is widely used, more so in the United States than in Europe, Dr. Le Heuzey observed, where more than 90% of patients needing CRT would likely have a CRT-D rather than a CRT-P device implanted.
The aims of the CERTITUDE cohort study were to look at the extent to which CRT-P patients differ from CRT-D patients in a real-life setting, and to also see if there were patients in the CRT-P group that might have benefited from CRT-D.
The prospective, observational study involved 1,705 patients who were recruited at 41 centers throughout France over a 2-year period starting in January 2008. Of these, 31% were fitted with a CRT-P device and 69% with a CRT-D.
Results showed that patients who had a CRT-P versus a CRT-D implanted were significantly older, more often female, and more symptomatic. They were also significantly less likely to have coronary artery disease, more likely to have wider QRS intervals and to have atrial fibrillation, and more often had at least two comorbidities.
Analysis of the causes of death was performed at 2 years’ follow-up, which Dr. Le Heuzey conceded was a short period of time. At this point, 267 of 1,611 patients with complete follow-up data had died, giving an overall mortality rate of 8.4% per 100 patient-years for the entire cohort.
The crude mortality rate was found to be higher among CRT-P than CRT-D patients, at 13.1% versus 6.5% per 100 patient-years (relative risk, 2.01; 95% confidence interval, 1.56-2.58; P less than .0001). But when the cause of death was examined more closely, there was no significant difference in the number of SCDs between the groups (RR, 1.57; 95% CI, 0.71-3.46; P = .42) and no significant difference when a specific cause of death analysis was performed.
The main reasons for the almost doubled risk of death in the CRT-P group was an increase in non-SCD cardiovascular mortality, mainly progressive heart failure (RR, 0.27; 95% CI, 1.62-3.18) and other cardiovascular causes (RR, 4.4; 95% CI, 1.29-15.03), Dr. Le Heuzey and associates noted in the article.
“Overall, 95% of the excess mortality among CRT-P recipients was not related to SCD,” they wrote, noting that this “suggests that the presence of a back-up defibrillator would probably not have been beneficial in terms of improving survival for these patients.”
So where does this leave clinicians? Dr. Le Heuzey referred to Table 17 in the European guidelines (Eur Heart J. 2013;34:2281-2329) with guidance on factors favoring CRT-P or CRT-D. For instance, CRT-P might be favorable in a patient with advanced heart failure or one with severe renal insufficiency or on dialysis. Other major comorbidities or frailty or cachexia might veer a decision towards CRT without a defibrillator. On the other hand, factors favoring CRT-D include a life expectancy of more than 1 year; stable, moderate (New York Heart Association class II) disease; or low to moderate–risk ischemic heart disease, with a lack of comorbidities.
CERTITUDE was funded by grants from the French Institute of Health and Medical Research (INSERM) and from the French Cardiology Society. The latter received specific grants from Biotronik, Boston Scientific, Medtronic, Saint Jude Medical, and Sorin in order to perform the study. Dr. Le Heuzey disclosed receiving fees for participation in advisory boards and conferences from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb/Pfizer, Correvio, Daiichi-Sankyo, Meda, Sanofi, and Servier.
AT THE ESC CONGRESS 2015
Key clinical point:There appears to be no survival benefit of cardiac resynchronization therapy with a defibrillator CRT-D over a pacemaker (CRT-P).
Major finding: Although there was a higher death rate among CRT-P recipients, 95% of the excess mortality, compared with CRT-D recipients, was not related to sudden cardiac death.
Data source: The prospective, observational CERTITUDE cohort study in 1,705 French patients fitted with a CRT-P or CRT-D.
Disclosures: CERTITUDE was funded by grants from the French Institute of Health and Medical Research (INSERM) and from the French Cardiology Society. The latter received specific grants from Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and Sorin in order to perform the study. Dr. Le Heuzey disclosed receiving fees for participation in advisory boards and conferences from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb/Pfizer, Correvio, Daiichi-Sankyo, Meda, Sanofi, and Servier.
VIDEO: Adverse ventilation effect means rethinking Cheyne-Stokes respiration
LONDON – The management of Cheyne-Stokes respiration in patients with heart failure with reduced ejection fraction needs to be reconsidered following the troubling outcome of a major trial that tested adaptive servo-ventilation as treatment for this symptom, Dr. Lars Køber commented during an interview at the annual congress of the European Society of Cardiology.
Cheyne-Stokes respiration, a form of central sleep apnea, differs from obstructive sleep apnea in that heart failure patients do not seem to derive symptomatic benefit from adaptive servo-ventilation treatment, but “physicians have thought they could treat this sleep apnea [with ventilation] and it would change prognosis,” said Dr. Køber. “Treatment of sleep apnea is possible, so physicians had started doing it.” But instead of helping patients, the trial results strongly suggested that patients were harmed by treatment, which was significantly linked with increased rates of both all-cause and cardiovascular mortality (N Engl J Med. 2015 Sep 1. doi: 10.1056/NEJMoa1506459).
In our video interview, Dr. Køber, professor of cardiology at Rigshospitalet and the University of Copenhagen, discusses the results and what the findings imply for future treatment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
LONDON – The management of Cheyne-Stokes respiration in patients with heart failure with reduced ejection fraction needs to be reconsidered following the troubling outcome of a major trial that tested adaptive servo-ventilation as treatment for this symptom, Dr. Lars Køber commented during an interview at the annual congress of the European Society of Cardiology.
Cheyne-Stokes respiration, a form of central sleep apnea, differs from obstructive sleep apnea in that heart failure patients do not seem to derive symptomatic benefit from adaptive servo-ventilation treatment, but “physicians have thought they could treat this sleep apnea [with ventilation] and it would change prognosis,” said Dr. Køber. “Treatment of sleep apnea is possible, so physicians had started doing it.” But instead of helping patients, the trial results strongly suggested that patients were harmed by treatment, which was significantly linked with increased rates of both all-cause and cardiovascular mortality (N Engl J Med. 2015 Sep 1. doi: 10.1056/NEJMoa1506459).
In our video interview, Dr. Køber, professor of cardiology at Rigshospitalet and the University of Copenhagen, discusses the results and what the findings imply for future treatment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
LONDON – The management of Cheyne-Stokes respiration in patients with heart failure with reduced ejection fraction needs to be reconsidered following the troubling outcome of a major trial that tested adaptive servo-ventilation as treatment for this symptom, Dr. Lars Køber commented during an interview at the annual congress of the European Society of Cardiology.
Cheyne-Stokes respiration, a form of central sleep apnea, differs from obstructive sleep apnea in that heart failure patients do not seem to derive symptomatic benefit from adaptive servo-ventilation treatment, but “physicians have thought they could treat this sleep apnea [with ventilation] and it would change prognosis,” said Dr. Køber. “Treatment of sleep apnea is possible, so physicians had started doing it.” But instead of helping patients, the trial results strongly suggested that patients were harmed by treatment, which was significantly linked with increased rates of both all-cause and cardiovascular mortality (N Engl J Med. 2015 Sep 1. doi: 10.1056/NEJMoa1506459).
In our video interview, Dr. Køber, professor of cardiology at Rigshospitalet and the University of Copenhagen, discusses the results and what the findings imply for future treatment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2015
Heart failure’s surprises keep coming
Heart failure has historically been one of the most poorly understood and hard to treat cardiovascular diseases, and many reports at the annual congress of the European Society of Cardiology in London hammered home how many mysteries remain about heart failure and how many tricks it still keeps up its sleeves.
Probably the most high-profile example was the stunning result of the SERVE-HF trial, reported as a hot-line talk and published concurrently (N Engl J Med. 2015 Sep 1. doi: 10.1056/NEJMoa1506459). The trial randomized more than 1,300 patients with heart failure with reduced ejection fraction and central sleep apnea presenting as Cheyne-Stokes respiration to nocturnal treatment with adaptive servo-ventilation. The idea was simple: These heart failure patients have trouble breathing while asleep, so help them with a ventilator.
“We had a technology that could alleviate central sleep apnea, and we just wanted to prove how large the benefit was,” said Dr. Martin R. Cowie, the trial’s lead investigator. But in results that “surprised us completely,” said Dr. Cowie, ventilation not only failed to produce a significant benefit for the trial’s primary, combined endpoint, it also showed a significant deleterious effect for the secondary endpoints of death by any cause and for cardiovascular death. In short, treating these patients with a ventilator was killing them. “We don’t understand what’s going on here,” Dr. Cowie said. He characterized the outcome as a “game changer” because many physicians had already begun using this form of ventilation on patients as it made such perfect sense.
“It’s unbelievable. We thought it was a slam dunk,” commented Dr. Frank Rushitka when he summed up the congress’ heart failure program at the end of the meeting.
Dr. Athena Poppas, who delivered the invited commentary on SERVE-HF, highlighted the “complexity” of heart failure and how “poorly understood” it is. “We’ve seen so many times where we saw something that we thought was bad [and treated] without fully understanding the mechanisms,” she said in an interview.
A great example was the misbegotten flirtation a couple of decades ago with inotropic therapy. It was another no-brainer: drugs that stimulate the heart’s pumping function will help patients with impaired cardiac output. But then trial results showed that chronic inotropic treatment actually hastened patients’ demise. “Patients felt better but they died faster,” Dr. Poppas noted.
Heart failure’s surprises keep coming. During the congress, heart failure expert Dr. Marriel L. Jessup said, “We used to think the reason why patients with acute heart failure were diuretic resistant was because of poor cardiac output. Now we know that they have a lot of venous congestion that impacts the kidneys and liver.”
Another researcher, Dr. John C. Burnett Jr. added, “It’s not just plumbing” that harms the kidneys and liver, but also “deleterious molecules” released because of venous congestion that causes organ damage. The identity of those molecules is only now being explored. And recognition that liver damage is an important part of the heart failure syndrome occurred only recently.
Heart failure continues to reveal its mysteries slowly and reluctantly. Clinicians who believe they know all they need about how to safely and effectively treat it are fooling themselves and run the risk of hurting their patients.
On Twitter @mitchelzoler
Heart failure has historically been one of the most poorly understood and hard to treat cardiovascular diseases, and many reports at the annual congress of the European Society of Cardiology in London hammered home how many mysteries remain about heart failure and how many tricks it still keeps up its sleeves.
Probably the most high-profile example was the stunning result of the SERVE-HF trial, reported as a hot-line talk and published concurrently (N Engl J Med. 2015 Sep 1. doi: 10.1056/NEJMoa1506459). The trial randomized more than 1,300 patients with heart failure with reduced ejection fraction and central sleep apnea presenting as Cheyne-Stokes respiration to nocturnal treatment with adaptive servo-ventilation. The idea was simple: These heart failure patients have trouble breathing while asleep, so help them with a ventilator.
“We had a technology that could alleviate central sleep apnea, and we just wanted to prove how large the benefit was,” said Dr. Martin R. Cowie, the trial’s lead investigator. But in results that “surprised us completely,” said Dr. Cowie, ventilation not only failed to produce a significant benefit for the trial’s primary, combined endpoint, it also showed a significant deleterious effect for the secondary endpoints of death by any cause and for cardiovascular death. In short, treating these patients with a ventilator was killing them. “We don’t understand what’s going on here,” Dr. Cowie said. He characterized the outcome as a “game changer” because many physicians had already begun using this form of ventilation on patients as it made such perfect sense.
“It’s unbelievable. We thought it was a slam dunk,” commented Dr. Frank Rushitka when he summed up the congress’ heart failure program at the end of the meeting.
Dr. Athena Poppas, who delivered the invited commentary on SERVE-HF, highlighted the “complexity” of heart failure and how “poorly understood” it is. “We’ve seen so many times where we saw something that we thought was bad [and treated] without fully understanding the mechanisms,” she said in an interview.
A great example was the misbegotten flirtation a couple of decades ago with inotropic therapy. It was another no-brainer: drugs that stimulate the heart’s pumping function will help patients with impaired cardiac output. But then trial results showed that chronic inotropic treatment actually hastened patients’ demise. “Patients felt better but they died faster,” Dr. Poppas noted.
Heart failure’s surprises keep coming. During the congress, heart failure expert Dr. Marriel L. Jessup said, “We used to think the reason why patients with acute heart failure were diuretic resistant was because of poor cardiac output. Now we know that they have a lot of venous congestion that impacts the kidneys and liver.”
Another researcher, Dr. John C. Burnett Jr. added, “It’s not just plumbing” that harms the kidneys and liver, but also “deleterious molecules” released because of venous congestion that causes organ damage. The identity of those molecules is only now being explored. And recognition that liver damage is an important part of the heart failure syndrome occurred only recently.
Heart failure continues to reveal its mysteries slowly and reluctantly. Clinicians who believe they know all they need about how to safely and effectively treat it are fooling themselves and run the risk of hurting their patients.
On Twitter @mitchelzoler
Heart failure has historically been one of the most poorly understood and hard to treat cardiovascular diseases, and many reports at the annual congress of the European Society of Cardiology in London hammered home how many mysteries remain about heart failure and how many tricks it still keeps up its sleeves.
Probably the most high-profile example was the stunning result of the SERVE-HF trial, reported as a hot-line talk and published concurrently (N Engl J Med. 2015 Sep 1. doi: 10.1056/NEJMoa1506459). The trial randomized more than 1,300 patients with heart failure with reduced ejection fraction and central sleep apnea presenting as Cheyne-Stokes respiration to nocturnal treatment with adaptive servo-ventilation. The idea was simple: These heart failure patients have trouble breathing while asleep, so help them with a ventilator.
“We had a technology that could alleviate central sleep apnea, and we just wanted to prove how large the benefit was,” said Dr. Martin R. Cowie, the trial’s lead investigator. But in results that “surprised us completely,” said Dr. Cowie, ventilation not only failed to produce a significant benefit for the trial’s primary, combined endpoint, it also showed a significant deleterious effect for the secondary endpoints of death by any cause and for cardiovascular death. In short, treating these patients with a ventilator was killing them. “We don’t understand what’s going on here,” Dr. Cowie said. He characterized the outcome as a “game changer” because many physicians had already begun using this form of ventilation on patients as it made such perfect sense.
“It’s unbelievable. We thought it was a slam dunk,” commented Dr. Frank Rushitka when he summed up the congress’ heart failure program at the end of the meeting.
Dr. Athena Poppas, who delivered the invited commentary on SERVE-HF, highlighted the “complexity” of heart failure and how “poorly understood” it is. “We’ve seen so many times where we saw something that we thought was bad [and treated] without fully understanding the mechanisms,” she said in an interview.
A great example was the misbegotten flirtation a couple of decades ago with inotropic therapy. It was another no-brainer: drugs that stimulate the heart’s pumping function will help patients with impaired cardiac output. But then trial results showed that chronic inotropic treatment actually hastened patients’ demise. “Patients felt better but they died faster,” Dr. Poppas noted.
Heart failure’s surprises keep coming. During the congress, heart failure expert Dr. Marriel L. Jessup said, “We used to think the reason why patients with acute heart failure were diuretic resistant was because of poor cardiac output. Now we know that they have a lot of venous congestion that impacts the kidneys and liver.”
Another researcher, Dr. John C. Burnett Jr. added, “It’s not just plumbing” that harms the kidneys and liver, but also “deleterious molecules” released because of venous congestion that causes organ damage. The identity of those molecules is only now being explored. And recognition that liver damage is an important part of the heart failure syndrome occurred only recently.
Heart failure continues to reveal its mysteries slowly and reluctantly. Clinicians who believe they know all they need about how to safely and effectively treat it are fooling themselves and run the risk of hurting their patients.
On Twitter @mitchelzoler
ESC: New review yields reassuring digoxin data
LONDON – The best-quality available evidence demonstrates that digoxin reduces hospital admissions and has no effect upon all-cause mortality in patients with heart failure with or without atrial fibrillation, Dr. Dipak Kotecha reported at the annual congress of the European Society of Cardiology.
His meta-analysis of all observational and randomized controlled studies published since 1960 included 41 studies with 260,335 digoxin-treated patients, roughly 1 million controls, and more than 4 million person-years of follow-up.
What was unique about this meta-analysis is Dr. Kotecha and coworkers assessed each study in terms of its degree of bias due to confounding by indication and other limitations using two standardized bias scoring systems: the Cochrane Collaboration’s risk of bias tool for randomized controlled trials and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS).
The level of bias varied greatly among the observational studies. Moreover, the higher an observational study’s bias score, the greater the reported association between digoxin and mortality. In contrast, the seven randomized controlled trials all scored very low on bias and carried a consistent message that digoxin had a neutral effect on mortality, according to Dr. Kotecha of the University of Birmingham (England).
He noted that digoxin was first introduced into clinical cardiology back in 1785 in the form of digitalis. And although digoxin has seen widespread use for heart rate control and symptom reduction in patients with heart failure and/or atrial fibrillation over the years, prescriptions for the venerable drug have markedly declined recently in response to observational studies reporting a link with increased mortality. That purported association, he asserted, is highly dubious.
“Our comprehensive systematic review would suggest that confounding is the main reason why observational studies continue to show increases in mortality associated with digoxin. And this suggests that digoxin should continue to be considered as a treatment option to avoid hospital admissions in patients with heart failure and to achieve heart rate control in those with atrial fibrillation until better randomized data become available,” the cardiologist explained.
“I think that there’s one further important point to make here, and that’s that observational data, particularly when there are systematic differences in the two groups, should not be used to determine clinical efficacy. Propensity matching does not replace a randomized controlled trial in the assessment of efficacy,” he emphasized. “We’ve shown that observational data should be taken with extreme caution because digoxin is a second-line therapy and, like most of us, I tend to give digoxin to patients who are sicker, so we’re bound to see an increase in mortality and hospitalizations in those patients.”
Across all 41 studies, digoxin was associated with a small but clinically meaningful 8% reduction in the rate of all-cause hospitalization.
Asked what data are available regarding optimal dosing of digoxin, the cardiologist replied that the data are limited. “What data there are would suggest lower digoxin doses are better and higher doses tend to cause mortality. So our suggestion would be to continue using low digoxin doses, in combination if necessary with other drugs,” he said.
All of the randomized controlled trials have focused on patients with heart failure, alone or with comorbid atrial fibrillation. None have evaluated the impact of digoxin in patients with atrial fibrillation without heart failure. Given that atrial fibrillation is an emerging modern epidemic and a large chunk of digoxin-prescribing today is for rate control in such patients, this lack of high-quality data constitutes a major unmet need, Dr. Kotecha observed.
The RATE-AF trial, designed to advance knowledge in this area, is due to begin next year. The study, based at the University of Birmingham, will enroll patients with atrial fibrillation without heart failure who need rate control, randomizing them to a beta-blocker or digoxin.
Dr. Kotecha reported having no financial conflicts regarding his meta-analysis, which was funded by a grant from the university’s Arthur Thompson Trust.
Simultaneously with his presentation at the ESC congress, the meta-analysis was published online (BMJ 2105;351:h4451 doi:10.1136/bmj.h4451).
LONDON – The best-quality available evidence demonstrates that digoxin reduces hospital admissions and has no effect upon all-cause mortality in patients with heart failure with or without atrial fibrillation, Dr. Dipak Kotecha reported at the annual congress of the European Society of Cardiology.
His meta-analysis of all observational and randomized controlled studies published since 1960 included 41 studies with 260,335 digoxin-treated patients, roughly 1 million controls, and more than 4 million person-years of follow-up.
What was unique about this meta-analysis is Dr. Kotecha and coworkers assessed each study in terms of its degree of bias due to confounding by indication and other limitations using two standardized bias scoring systems: the Cochrane Collaboration’s risk of bias tool for randomized controlled trials and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS).
The level of bias varied greatly among the observational studies. Moreover, the higher an observational study’s bias score, the greater the reported association between digoxin and mortality. In contrast, the seven randomized controlled trials all scored very low on bias and carried a consistent message that digoxin had a neutral effect on mortality, according to Dr. Kotecha of the University of Birmingham (England).
He noted that digoxin was first introduced into clinical cardiology back in 1785 in the form of digitalis. And although digoxin has seen widespread use for heart rate control and symptom reduction in patients with heart failure and/or atrial fibrillation over the years, prescriptions for the venerable drug have markedly declined recently in response to observational studies reporting a link with increased mortality. That purported association, he asserted, is highly dubious.
“Our comprehensive systematic review would suggest that confounding is the main reason why observational studies continue to show increases in mortality associated with digoxin. And this suggests that digoxin should continue to be considered as a treatment option to avoid hospital admissions in patients with heart failure and to achieve heart rate control in those with atrial fibrillation until better randomized data become available,” the cardiologist explained.
“I think that there’s one further important point to make here, and that’s that observational data, particularly when there are systematic differences in the two groups, should not be used to determine clinical efficacy. Propensity matching does not replace a randomized controlled trial in the assessment of efficacy,” he emphasized. “We’ve shown that observational data should be taken with extreme caution because digoxin is a second-line therapy and, like most of us, I tend to give digoxin to patients who are sicker, so we’re bound to see an increase in mortality and hospitalizations in those patients.”
Across all 41 studies, digoxin was associated with a small but clinically meaningful 8% reduction in the rate of all-cause hospitalization.
Asked what data are available regarding optimal dosing of digoxin, the cardiologist replied that the data are limited. “What data there are would suggest lower digoxin doses are better and higher doses tend to cause mortality. So our suggestion would be to continue using low digoxin doses, in combination if necessary with other drugs,” he said.
All of the randomized controlled trials have focused on patients with heart failure, alone or with comorbid atrial fibrillation. None have evaluated the impact of digoxin in patients with atrial fibrillation without heart failure. Given that atrial fibrillation is an emerging modern epidemic and a large chunk of digoxin-prescribing today is for rate control in such patients, this lack of high-quality data constitutes a major unmet need, Dr. Kotecha observed.
The RATE-AF trial, designed to advance knowledge in this area, is due to begin next year. The study, based at the University of Birmingham, will enroll patients with atrial fibrillation without heart failure who need rate control, randomizing them to a beta-blocker or digoxin.
Dr. Kotecha reported having no financial conflicts regarding his meta-analysis, which was funded by a grant from the university’s Arthur Thompson Trust.
Simultaneously with his presentation at the ESC congress, the meta-analysis was published online (BMJ 2105;351:h4451 doi:10.1136/bmj.h4451).
LONDON – The best-quality available evidence demonstrates that digoxin reduces hospital admissions and has no effect upon all-cause mortality in patients with heart failure with or without atrial fibrillation, Dr. Dipak Kotecha reported at the annual congress of the European Society of Cardiology.
His meta-analysis of all observational and randomized controlled studies published since 1960 included 41 studies with 260,335 digoxin-treated patients, roughly 1 million controls, and more than 4 million person-years of follow-up.
What was unique about this meta-analysis is Dr. Kotecha and coworkers assessed each study in terms of its degree of bias due to confounding by indication and other limitations using two standardized bias scoring systems: the Cochrane Collaboration’s risk of bias tool for randomized controlled trials and the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS).
The level of bias varied greatly among the observational studies. Moreover, the higher an observational study’s bias score, the greater the reported association between digoxin and mortality. In contrast, the seven randomized controlled trials all scored very low on bias and carried a consistent message that digoxin had a neutral effect on mortality, according to Dr. Kotecha of the University of Birmingham (England).
He noted that digoxin was first introduced into clinical cardiology back in 1785 in the form of digitalis. And although digoxin has seen widespread use for heart rate control and symptom reduction in patients with heart failure and/or atrial fibrillation over the years, prescriptions for the venerable drug have markedly declined recently in response to observational studies reporting a link with increased mortality. That purported association, he asserted, is highly dubious.
“Our comprehensive systematic review would suggest that confounding is the main reason why observational studies continue to show increases in mortality associated with digoxin. And this suggests that digoxin should continue to be considered as a treatment option to avoid hospital admissions in patients with heart failure and to achieve heart rate control in those with atrial fibrillation until better randomized data become available,” the cardiologist explained.
“I think that there’s one further important point to make here, and that’s that observational data, particularly when there are systematic differences in the two groups, should not be used to determine clinical efficacy. Propensity matching does not replace a randomized controlled trial in the assessment of efficacy,” he emphasized. “We’ve shown that observational data should be taken with extreme caution because digoxin is a second-line therapy and, like most of us, I tend to give digoxin to patients who are sicker, so we’re bound to see an increase in mortality and hospitalizations in those patients.”
Across all 41 studies, digoxin was associated with a small but clinically meaningful 8% reduction in the rate of all-cause hospitalization.
Asked what data are available regarding optimal dosing of digoxin, the cardiologist replied that the data are limited. “What data there are would suggest lower digoxin doses are better and higher doses tend to cause mortality. So our suggestion would be to continue using low digoxin doses, in combination if necessary with other drugs,” he said.
All of the randomized controlled trials have focused on patients with heart failure, alone or with comorbid atrial fibrillation. None have evaluated the impact of digoxin in patients with atrial fibrillation without heart failure. Given that atrial fibrillation is an emerging modern epidemic and a large chunk of digoxin-prescribing today is for rate control in such patients, this lack of high-quality data constitutes a major unmet need, Dr. Kotecha observed.
The RATE-AF trial, designed to advance knowledge in this area, is due to begin next year. The study, based at the University of Birmingham, will enroll patients with atrial fibrillation without heart failure who need rate control, randomizing them to a beta-blocker or digoxin.
Dr. Kotecha reported having no financial conflicts regarding his meta-analysis, which was funded by a grant from the university’s Arthur Thompson Trust.
Simultaneously with his presentation at the ESC congress, the meta-analysis was published online (BMJ 2105;351:h4451 doi:10.1136/bmj.h4451).
AT THE ESC CONGRESS 2015
Key clinical point: Digoxin remains safe and effective in patients with heart failure and/or atrial fibrillation, according to a major new review.
Major finding: Using digoxin in patients with heart failure and/or atrial fibrillation reduces all-cause hospitalizations by 8% and has no impact on mortality.
Data source: A meta-analysis of all studies of the impact of digoxin on death and clinical outcomes published since 1960, including 41 studies with more than a quarter million digoxin-treated patients, 1 million controls, and more than 4 million person-years of follow-up.
Disclosures: The presenter reported having no financial conflicts regarding this study, funded by a university grant.
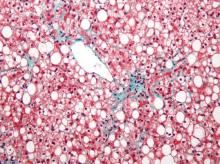
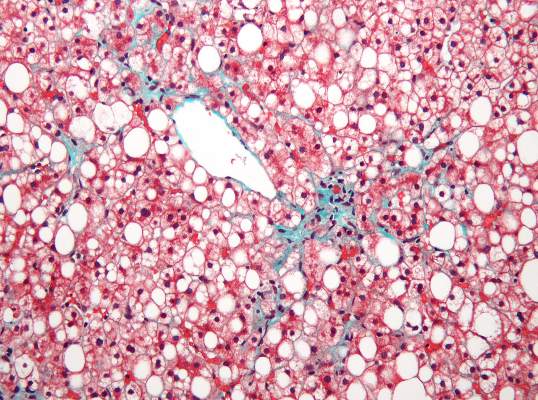
Subclinical heart dysfunction, fatty liver linked
Researchers found an association between nonalcoholic fatty liver disease (NAFLD) and myocardial dysfunction and remodeling, according to a new study published in Hepatology.
“Both NAFLD and heart failure (particularly heart failure with preserved ejection fraction) are obesity-related conditions that have reached epidemic proportions. We know from epidemiologic studies that persons with NAFLD are more likely to die from cardiovascular disease than from liver-related death. This risk seems to be proportional to the amount of fat in the liver and is independent of the presence of nonalcoholic steatohepatitis (NASH). There have been numerous studies that have focused on the relationship between NAFLD and coronary artery disease, but very little work has been done to determine relationships with heart failure,” Dr. Lisa B. VanWagner of Northwestern University in Chicago noted. She continued, “There are several well-established major risk factors for the development of clinical heart failure, including coronary artery disease, diabetes, and hypertension, all of which are also closely associated with NAFLD. However, whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction that may lead to the development of clinical heart failure is unknown.”
NAFLD and heart failure are both associated with obesity. Likewise, there is evidence that NAFLD may also be related to endothelial dysfunction, coronary plaques, coronary artery calcifications, as well as being an independent risk factor for cardiovascular disease.
Dr. VanWagner and her colleagues conducted a cross-sectional study of 2,713 patients from the CARDIA (Coronary Artery Risk Development in Young Adults) study to understand any associations between NAFLD and abnormalities in left ventricular (LV) function and structure. Study participants completed CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study (Hepatology 2015;62:773-83 [doi:10.1002/hep.27869]). Participants were excluded from analysis if they had missing or incomplete imaging, pregnancy, a history of MI or heart failure, cirrhosis, hepatitis, or chronic liver disease risk factors, or if they weighed more than 450 pounds,
Of the 2,713 subjects included in analysis, 48% were black and 58.8% were female. NAFLD was detected in 10% (n = 271) of participants. Those with NAFLD were more likely to be white males with metabolic syndrome and who were obese and had higher CT-measured levels of visceral adipose tissue, and an increased waist circumference and waist-to-hip ratio. Insulin resistance markers such as elevated fasting glucose, elevated C-reactive protein, and hypertriglyceridemia were more common in the participants with NAFLD.
Study participants with NAFLD had signs of myocardial remodeling such as more left ventricular wall thickness, LV end-diastolic volume, left aortic volume index, and LV mass index. Likewise, NAFLD was associated with more circumferential strain and global longitudinal strain but no differences in ejection fraction.
Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (34.6% vs. 23.6%; P less than .0001), and elevated LV filling pressures (33.3% vs. 23.7%; P less than .001) was more common in NAFLD participants, compared with non-NAFLD subjects.
After researchers adjusted for health behaviors and demographic factors, evidence of NAFLD was associated with worse GLS (P less than .0001). Finally, NAFLD was associated with subclinical cardiac remodeling and dysfunction even after adjustment for body mass index and heart failure risk factors (P less than .01).
Dr. VanWagner summarized, “NAFLD may not necessarily be a ‘benign condition’ as previously thought. In our study, we determined liver fat by CT scan, which admittedly detects fat at a higher level (typically greater than 30%) than for example on MRI, which can detect fat as low as 5%. A fatty liver detected on CT or even on [ultrasound], which has similar sensitivity as CT for detecting liver fat should prompt evaluation for additional cardiovascular risk factors and treatment of identified abnormalities to reduce [atherosclerotic cardiovascular disease] and [heart failure] risk. Currently, our study only shows associations between liver fat and subclinical changes in the myocardium and causality cannot be determined. [On the basis] of our data, we cannot recommend screening for HF [heart failure] in this population, but future studies are needed to determine if NAFLD in fact lies in the casual pathway for the development of clinical HF.”
The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
Researchers found an association between nonalcoholic fatty liver disease (NAFLD) and myocardial dysfunction and remodeling, according to a new study published in Hepatology.
“Both NAFLD and heart failure (particularly heart failure with preserved ejection fraction) are obesity-related conditions that have reached epidemic proportions. We know from epidemiologic studies that persons with NAFLD are more likely to die from cardiovascular disease than from liver-related death. This risk seems to be proportional to the amount of fat in the liver and is independent of the presence of nonalcoholic steatohepatitis (NASH). There have been numerous studies that have focused on the relationship between NAFLD and coronary artery disease, but very little work has been done to determine relationships with heart failure,” Dr. Lisa B. VanWagner of Northwestern University in Chicago noted. She continued, “There are several well-established major risk factors for the development of clinical heart failure, including coronary artery disease, diabetes, and hypertension, all of which are also closely associated with NAFLD. However, whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction that may lead to the development of clinical heart failure is unknown.”
NAFLD and heart failure are both associated with obesity. Likewise, there is evidence that NAFLD may also be related to endothelial dysfunction, coronary plaques, coronary artery calcifications, as well as being an independent risk factor for cardiovascular disease.
Dr. VanWagner and her colleagues conducted a cross-sectional study of 2,713 patients from the CARDIA (Coronary Artery Risk Development in Young Adults) study to understand any associations between NAFLD and abnormalities in left ventricular (LV) function and structure. Study participants completed CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study (Hepatology 2015;62:773-83 [doi:10.1002/hep.27869]). Participants were excluded from analysis if they had missing or incomplete imaging, pregnancy, a history of MI or heart failure, cirrhosis, hepatitis, or chronic liver disease risk factors, or if they weighed more than 450 pounds,
Of the 2,713 subjects included in analysis, 48% were black and 58.8% were female. NAFLD was detected in 10% (n = 271) of participants. Those with NAFLD were more likely to be white males with metabolic syndrome and who were obese and had higher CT-measured levels of visceral adipose tissue, and an increased waist circumference and waist-to-hip ratio. Insulin resistance markers such as elevated fasting glucose, elevated C-reactive protein, and hypertriglyceridemia were more common in the participants with NAFLD.
Study participants with NAFLD had signs of myocardial remodeling such as more left ventricular wall thickness, LV end-diastolic volume, left aortic volume index, and LV mass index. Likewise, NAFLD was associated with more circumferential strain and global longitudinal strain but no differences in ejection fraction.
Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (34.6% vs. 23.6%; P less than .0001), and elevated LV filling pressures (33.3% vs. 23.7%; P less than .001) was more common in NAFLD participants, compared with non-NAFLD subjects.
After researchers adjusted for health behaviors and demographic factors, evidence of NAFLD was associated with worse GLS (P less than .0001). Finally, NAFLD was associated with subclinical cardiac remodeling and dysfunction even after adjustment for body mass index and heart failure risk factors (P less than .01).
Dr. VanWagner summarized, “NAFLD may not necessarily be a ‘benign condition’ as previously thought. In our study, we determined liver fat by CT scan, which admittedly detects fat at a higher level (typically greater than 30%) than for example on MRI, which can detect fat as low as 5%. A fatty liver detected on CT or even on [ultrasound], which has similar sensitivity as CT for detecting liver fat should prompt evaluation for additional cardiovascular risk factors and treatment of identified abnormalities to reduce [atherosclerotic cardiovascular disease] and [heart failure] risk. Currently, our study only shows associations between liver fat and subclinical changes in the myocardium and causality cannot be determined. [On the basis] of our data, we cannot recommend screening for HF [heart failure] in this population, but future studies are needed to determine if NAFLD in fact lies in the casual pathway for the development of clinical HF.”
The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
Researchers found an association between nonalcoholic fatty liver disease (NAFLD) and myocardial dysfunction and remodeling, according to a new study published in Hepatology.
“Both NAFLD and heart failure (particularly heart failure with preserved ejection fraction) are obesity-related conditions that have reached epidemic proportions. We know from epidemiologic studies that persons with NAFLD are more likely to die from cardiovascular disease than from liver-related death. This risk seems to be proportional to the amount of fat in the liver and is independent of the presence of nonalcoholic steatohepatitis (NASH). There have been numerous studies that have focused on the relationship between NAFLD and coronary artery disease, but very little work has been done to determine relationships with heart failure,” Dr. Lisa B. VanWagner of Northwestern University in Chicago noted. She continued, “There are several well-established major risk factors for the development of clinical heart failure, including coronary artery disease, diabetes, and hypertension, all of which are also closely associated with NAFLD. However, whether NAFLD is independently associated with subclinical myocardial remodeling or dysfunction that may lead to the development of clinical heart failure is unknown.”
NAFLD and heart failure are both associated with obesity. Likewise, there is evidence that NAFLD may also be related to endothelial dysfunction, coronary plaques, coronary artery calcifications, as well as being an independent risk factor for cardiovascular disease.
Dr. VanWagner and her colleagues conducted a cross-sectional study of 2,713 patients from the CARDIA (Coronary Artery Risk Development in Young Adults) study to understand any associations between NAFLD and abnormalities in left ventricular (LV) function and structure. Study participants completed CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study (Hepatology 2015;62:773-83 [doi:10.1002/hep.27869]). Participants were excluded from analysis if they had missing or incomplete imaging, pregnancy, a history of MI or heart failure, cirrhosis, hepatitis, or chronic liver disease risk factors, or if they weighed more than 450 pounds,
Of the 2,713 subjects included in analysis, 48% were black and 58.8% were female. NAFLD was detected in 10% (n = 271) of participants. Those with NAFLD were more likely to be white males with metabolic syndrome and who were obese and had higher CT-measured levels of visceral adipose tissue, and an increased waist circumference and waist-to-hip ratio. Insulin resistance markers such as elevated fasting glucose, elevated C-reactive protein, and hypertriglyceridemia were more common in the participants with NAFLD.
Study participants with NAFLD had signs of myocardial remodeling such as more left ventricular wall thickness, LV end-diastolic volume, left aortic volume index, and LV mass index. Likewise, NAFLD was associated with more circumferential strain and global longitudinal strain but no differences in ejection fraction.
Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (34.6% vs. 23.6%; P less than .0001), and elevated LV filling pressures (33.3% vs. 23.7%; P less than .001) was more common in NAFLD participants, compared with non-NAFLD subjects.
After researchers adjusted for health behaviors and demographic factors, evidence of NAFLD was associated with worse GLS (P less than .0001). Finally, NAFLD was associated with subclinical cardiac remodeling and dysfunction even after adjustment for body mass index and heart failure risk factors (P less than .01).
Dr. VanWagner summarized, “NAFLD may not necessarily be a ‘benign condition’ as previously thought. In our study, we determined liver fat by CT scan, which admittedly detects fat at a higher level (typically greater than 30%) than for example on MRI, which can detect fat as low as 5%. A fatty liver detected on CT or even on [ultrasound], which has similar sensitivity as CT for detecting liver fat should prompt evaluation for additional cardiovascular risk factors and treatment of identified abnormalities to reduce [atherosclerotic cardiovascular disease] and [heart failure] risk. Currently, our study only shows associations between liver fat and subclinical changes in the myocardium and causality cannot be determined. [On the basis] of our data, we cannot recommend screening for HF [heart failure] in this population, but future studies are needed to determine if NAFLD in fact lies in the casual pathway for the development of clinical HF.”
The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
FROM HEPATOLOGY
Key clinical point: Researchers found an association between nonalcoholic fatty liver disease and myocardial dysfunction and remodeling.
Major finding: Subclinical systolic dysfunction (P less than .001 for the trend), subclinical diastolic dysfunction with impaired left ventricular relaxation (P less than .0001), and elevated LV filling pressures (P less than .001) was more common in participants with NAFLD than in those without.
Data source: A cross-sectional study of 2,713 patients from the CARDIA study using CT quantification of liver fat and echocardiography with Doppler during the 25-year follow-up to the initial study.
Disclosures: The investigators reported multiple supporting sources, including the National Institutes of Health, American Association for the Study of Liver Disease Foundation, and the American Heart Association. Dr. Lewis reported receiving grants from Novo Nordisk.
ESC: Novel apnea treatment not helpful, possibly harmful in heart failure
Adaptive servo-ventilation is not beneficial and may even be harmful for patients who have predominantly central sleep apnea accompanying heart failure with reduced ejection fraction, Dr. Martin R. Cowie reported at the annual congress of the European Society of Cardiology.
The noninvasive therapy did control central sleep apnea in a large international randomized controlled trial, but nevertheless did not affect the composite end point of death from any cause, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF. Moreover, it unexpectedly raised the risk of cardiovascular death by 34%, and significantly increased all-cause mortality as well, said Dr. Cowie of Imperial College London.
Adaptive servo-ventilation delivers servo-controlled inspiratory pressure on top of expiratory positive airway pressure during sleep, to alleviate central sleep apnea. This form of sleep-disordered breathing, which may manifest as Cheyne-Stokes respiration in patients who have HF with reduced ejection fraction, is reported to affect up to 40% of this patient population. Its prevalence rises as the severity of HF increases, and it is an independent risk marker for poor prognosis and death in HF.
A recent trial showed that continuous positive airway pressure (CPAP) did not improve morbidity or mortality in patients who had HF with central sleep apnea, but suggested that a treatment that could reduce the apnea-hypopnea index (AHI) – the number of apnea or hypopnea events per hour of sleep – to below 15 might be effective. Adaptive servo-ventilation can accomplish this, and small studies and meta-analyses have shown that the treatment improves surrogate markers including plasma concentration of brain natriuretic peptide, left ventricular ejection fraction (LVEF), and functional outcomes in heart failure.
Dr. Cowie and his associates conducted the SERVE-HF trial, assessing the effect of adding adaptive servo-ventilation to guideline-based medical therapy on survival and cardiovascular outcomes. He presented the trial results at the meeting, and they were simultaneously published online (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMoa1506459).
The industry-sponsored study comprised 1,325 patients aged 22 and older treated and followed at 91 medical centers for a median of 31 months (range, 0-80 months). They were randomly assigned to receive medical therapy plus adaptive servo-ventilation delivered through a face mask for at least 5 hours every night (666 intervention subjects) or medical therapy alone (659 control subjects).
Central sleep apnea was well controlled only in the intervention group. At 1 year, their mean AHI was 6.6 events per hour, and the oxygen desaturation index – the number of times per hour that the blood oxygen level dropped by 3 or more percentage points from baseline level – was 8.6.
Yet the primary composite end point was not significantly different between the two study groups: The rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it. The treatment also had no significant effect on a broad spectrum of secondary measures such as symptoms and quality of life. Six-minute walk distance gradually declined in both groups, but that decline was significantly more pronounced in the intervention group, the investigator said.
Even more worrisome was the significant increase in mortality associated with adaptive servo-ventilation. Cardiovascular mortality was 29.9% with the treatment, compared with 24.0% without it, for a hazard ratio of 1.34. All-cause mortality was 34.8% with the treatment and 29.3% without it, for an HR of 1.28.
The reason for this unexpected result is not yet known. One explanation is that central sleep apnea may be a compensatory mechanism with potentially beneficial effects in patients who have HF. Attenuating those effects with adaptive servo-ventilation may then have been detrimental. For example, central sleep apnea, and particularly Cheyne-Stokes breathing, may beneficially activate the respiratory muscles, increase sympathetic nervous system activity, induce hypercapnic acidosis, increase end-expiratory lung volume, and raise intrinsic positive airway pressure.
Another possibility is that applying positive airway pressure with adaptive servo-ventilation may impair cardiac function in at least a portion of patients who have HF by decreasing cardiac output and stroke volume during treatment.
ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
Adaptive servo-ventilation should not be used outside of clinical trials in heart failure patients who have predominantly central sleep apnea, at least until the reason for the unexpected 34% increase in cardiovascular mortality is understood.
The issue is important because at least one new technique to abolish Cheyne-Stokes respiration that doesn’t use positive pressure therapy – phrenic-nerve stimulation – has already been developed and is being assessed in a clinical trial. If Cheyne-Stokes respiration is actually beneficial in HF, this strategy may prove harmful.
Dr. Ulysses J. Magalang is in the division of pulmonary, allergy, critical care, and sleep medicine at Ohio State University Wexner Medical Center, Columbus. Dr. Allan I. Pack is at the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania, Philadelphia. Dr. Magalang reported grants support from the Rudi Schulte Family Foundation, Hill-Rom, and the Tzagournis Medical Research Endowment; Dr. Pack reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the SERVE-HF report (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMe1510397Th).
Adaptive servo-ventilation should not be used outside of clinical trials in heart failure patients who have predominantly central sleep apnea, at least until the reason for the unexpected 34% increase in cardiovascular mortality is understood.
The issue is important because at least one new technique to abolish Cheyne-Stokes respiration that doesn’t use positive pressure therapy – phrenic-nerve stimulation – has already been developed and is being assessed in a clinical trial. If Cheyne-Stokes respiration is actually beneficial in HF, this strategy may prove harmful.
Dr. Ulysses J. Magalang is in the division of pulmonary, allergy, critical care, and sleep medicine at Ohio State University Wexner Medical Center, Columbus. Dr. Allan I. Pack is at the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania, Philadelphia. Dr. Magalang reported grants support from the Rudi Schulte Family Foundation, Hill-Rom, and the Tzagournis Medical Research Endowment; Dr. Pack reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the SERVE-HF report (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMe1510397Th).
Adaptive servo-ventilation should not be used outside of clinical trials in heart failure patients who have predominantly central sleep apnea, at least until the reason for the unexpected 34% increase in cardiovascular mortality is understood.
The issue is important because at least one new technique to abolish Cheyne-Stokes respiration that doesn’t use positive pressure therapy – phrenic-nerve stimulation – has already been developed and is being assessed in a clinical trial. If Cheyne-Stokes respiration is actually beneficial in HF, this strategy may prove harmful.
Dr. Ulysses J. Magalang is in the division of pulmonary, allergy, critical care, and sleep medicine at Ohio State University Wexner Medical Center, Columbus. Dr. Allan I. Pack is at the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania, Philadelphia. Dr. Magalang reported grants support from the Rudi Schulte Family Foundation, Hill-Rom, and the Tzagournis Medical Research Endowment; Dr. Pack reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the SERVE-HF report (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMe1510397Th).
Adaptive servo-ventilation is not beneficial and may even be harmful for patients who have predominantly central sleep apnea accompanying heart failure with reduced ejection fraction, Dr. Martin R. Cowie reported at the annual congress of the European Society of Cardiology.
The noninvasive therapy did control central sleep apnea in a large international randomized controlled trial, but nevertheless did not affect the composite end point of death from any cause, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF. Moreover, it unexpectedly raised the risk of cardiovascular death by 34%, and significantly increased all-cause mortality as well, said Dr. Cowie of Imperial College London.
Adaptive servo-ventilation delivers servo-controlled inspiratory pressure on top of expiratory positive airway pressure during sleep, to alleviate central sleep apnea. This form of sleep-disordered breathing, which may manifest as Cheyne-Stokes respiration in patients who have HF with reduced ejection fraction, is reported to affect up to 40% of this patient population. Its prevalence rises as the severity of HF increases, and it is an independent risk marker for poor prognosis and death in HF.
A recent trial showed that continuous positive airway pressure (CPAP) did not improve morbidity or mortality in patients who had HF with central sleep apnea, but suggested that a treatment that could reduce the apnea-hypopnea index (AHI) – the number of apnea or hypopnea events per hour of sleep – to below 15 might be effective. Adaptive servo-ventilation can accomplish this, and small studies and meta-analyses have shown that the treatment improves surrogate markers including plasma concentration of brain natriuretic peptide, left ventricular ejection fraction (LVEF), and functional outcomes in heart failure.
Dr. Cowie and his associates conducted the SERVE-HF trial, assessing the effect of adding adaptive servo-ventilation to guideline-based medical therapy on survival and cardiovascular outcomes. He presented the trial results at the meeting, and they were simultaneously published online (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMoa1506459).
The industry-sponsored study comprised 1,325 patients aged 22 and older treated and followed at 91 medical centers for a median of 31 months (range, 0-80 months). They were randomly assigned to receive medical therapy plus adaptive servo-ventilation delivered through a face mask for at least 5 hours every night (666 intervention subjects) or medical therapy alone (659 control subjects).
Central sleep apnea was well controlled only in the intervention group. At 1 year, their mean AHI was 6.6 events per hour, and the oxygen desaturation index – the number of times per hour that the blood oxygen level dropped by 3 or more percentage points from baseline level – was 8.6.
Yet the primary composite end point was not significantly different between the two study groups: The rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it. The treatment also had no significant effect on a broad spectrum of secondary measures such as symptoms and quality of life. Six-minute walk distance gradually declined in both groups, but that decline was significantly more pronounced in the intervention group, the investigator said.
Even more worrisome was the significant increase in mortality associated with adaptive servo-ventilation. Cardiovascular mortality was 29.9% with the treatment, compared with 24.0% without it, for a hazard ratio of 1.34. All-cause mortality was 34.8% with the treatment and 29.3% without it, for an HR of 1.28.
The reason for this unexpected result is not yet known. One explanation is that central sleep apnea may be a compensatory mechanism with potentially beneficial effects in patients who have HF. Attenuating those effects with adaptive servo-ventilation may then have been detrimental. For example, central sleep apnea, and particularly Cheyne-Stokes breathing, may beneficially activate the respiratory muscles, increase sympathetic nervous system activity, induce hypercapnic acidosis, increase end-expiratory lung volume, and raise intrinsic positive airway pressure.
Another possibility is that applying positive airway pressure with adaptive servo-ventilation may impair cardiac function in at least a portion of patients who have HF by decreasing cardiac output and stroke volume during treatment.
ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
Adaptive servo-ventilation is not beneficial and may even be harmful for patients who have predominantly central sleep apnea accompanying heart failure with reduced ejection fraction, Dr. Martin R. Cowie reported at the annual congress of the European Society of Cardiology.
The noninvasive therapy did control central sleep apnea in a large international randomized controlled trial, but nevertheless did not affect the composite end point of death from any cause, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening HF. Moreover, it unexpectedly raised the risk of cardiovascular death by 34%, and significantly increased all-cause mortality as well, said Dr. Cowie of Imperial College London.
Adaptive servo-ventilation delivers servo-controlled inspiratory pressure on top of expiratory positive airway pressure during sleep, to alleviate central sleep apnea. This form of sleep-disordered breathing, which may manifest as Cheyne-Stokes respiration in patients who have HF with reduced ejection fraction, is reported to affect up to 40% of this patient population. Its prevalence rises as the severity of HF increases, and it is an independent risk marker for poor prognosis and death in HF.
A recent trial showed that continuous positive airway pressure (CPAP) did not improve morbidity or mortality in patients who had HF with central sleep apnea, but suggested that a treatment that could reduce the apnea-hypopnea index (AHI) – the number of apnea or hypopnea events per hour of sleep – to below 15 might be effective. Adaptive servo-ventilation can accomplish this, and small studies and meta-analyses have shown that the treatment improves surrogate markers including plasma concentration of brain natriuretic peptide, left ventricular ejection fraction (LVEF), and functional outcomes in heart failure.
Dr. Cowie and his associates conducted the SERVE-HF trial, assessing the effect of adding adaptive servo-ventilation to guideline-based medical therapy on survival and cardiovascular outcomes. He presented the trial results at the meeting, and they were simultaneously published online (N Engl J Med. 2015 Sep 1. doi:10.1056/NEJMoa1506459).
The industry-sponsored study comprised 1,325 patients aged 22 and older treated and followed at 91 medical centers for a median of 31 months (range, 0-80 months). They were randomly assigned to receive medical therapy plus adaptive servo-ventilation delivered through a face mask for at least 5 hours every night (666 intervention subjects) or medical therapy alone (659 control subjects).
Central sleep apnea was well controlled only in the intervention group. At 1 year, their mean AHI was 6.6 events per hour, and the oxygen desaturation index – the number of times per hour that the blood oxygen level dropped by 3 or more percentage points from baseline level – was 8.6.
Yet the primary composite end point was not significantly different between the two study groups: The rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it. The treatment also had no significant effect on a broad spectrum of secondary measures such as symptoms and quality of life. Six-minute walk distance gradually declined in both groups, but that decline was significantly more pronounced in the intervention group, the investigator said.
Even more worrisome was the significant increase in mortality associated with adaptive servo-ventilation. Cardiovascular mortality was 29.9% with the treatment, compared with 24.0% without it, for a hazard ratio of 1.34. All-cause mortality was 34.8% with the treatment and 29.3% without it, for an HR of 1.28.
The reason for this unexpected result is not yet known. One explanation is that central sleep apnea may be a compensatory mechanism with potentially beneficial effects in patients who have HF. Attenuating those effects with adaptive servo-ventilation may then have been detrimental. For example, central sleep apnea, and particularly Cheyne-Stokes breathing, may beneficially activate the respiratory muscles, increase sympathetic nervous system activity, induce hypercapnic acidosis, increase end-expiratory lung volume, and raise intrinsic positive airway pressure.
Another possibility is that applying positive airway pressure with adaptive servo-ventilation may impair cardiac function in at least a portion of patients who have HF by decreasing cardiac output and stroke volume during treatment.
ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
FROM THE ESC CONGRESS 2015
Key clinical point: Adaptive servo-ventilation is not beneficial and may even be harmful for central sleep apnea accompanying heart failure.
Major finding: The composite rate of death from any cause, lifesaving cardiovascular intervention, and unplanned hospitalization for worsening HF was 54.1% with adaptive servo-ventilation and 50.8% without it, a nonsignificant difference.
Data source: An international randomized clinical trial involving 1,325 adults followed for a median of 31 months.
Disclosures: ResMed, maker of the AutoSet adaptive servo-ventilator, sponsored SERVE-HF, which was also supported by the National Institute for Health Research and the National Institutes of Health. Dr. Cowie disclosed ties with Servier, Novartis, Pfizer, St. Jude Medical, Boston Scientific, Respicardia,Medtronic, and Bayer; his associates reported ties to numerous industry sources.
VIDEO: Newer type 2 diabetes drugs pose no significant heart failure risk
LONDON – Neither the GLP-1 receptor agonist lixisenatide nor the DPP-4 inhibitor sitagliptin significantly increased the risk of heart failure or associated hospitalizations in patients with type 2 diabetes, according to data from two large cardiovascular safety trials.
Further analysis from ELIXA (Evaluation of Lixisenatide [Lyxumia] in Acute Coronary Syndrome) showed that the hazard ratios for several secondary heart failure endpoints were 1.00 or below. Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure than in those who did not, there was there was no difference between treatment with the GLP-1 (glucagon-like peptide 1) agonist or placebo.
The findings add to those already presented in June at the annual scientific sessions of the American Diabetes Association from the 6,068-patient trial, which enrolled patients with type 2 diabetes who had experienced an acute coronary syndrome within the past 6 months.
Other reassuring data on the safety of newer diabetes medicines came from an updated analysis of TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin [Januvia]). The time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms, and there were no differences between the treatments in terms of hospitalization for heart failure or cardiovascular death or hospitalization for heart failure or all-cause death with the DPP-4 (dipeptyl peptidase 4) inhibitor. TECOS enrolled nearly 15,000 patients with type 2 diabetes and concurrent cardiovascular disease who were aged 50 years or older and the primary findings have been published (N Engl J Med. 2015 July 15;373:232-42).
These trials provide accumulating evidence that there is no heart failure risk with these particular diabetes medications, Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, commented in an interview at the annual congress of the European Society of Cardiology. Dr. Rydén added that the trial results everyone is waiting to hear about concern the cardiovascular safety of the selective sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance).
Advance information issued by manufacturer Boehringer-Ingelheim suggests that this drug actually may lower cardiovascular risk in patients at high risk for cardiovascular events, Dr. Rydén observed. “Now, if that is true, it is the only modern glucose-lowering drug to have shown such a capacity.” Results of the EMPA-REG OUTCOME study will be presented in September at the annual meeting of the European Association for the Study of Diabetes in Stockholm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Neither the GLP-1 receptor agonist lixisenatide nor the DPP-4 inhibitor sitagliptin significantly increased the risk of heart failure or associated hospitalizations in patients with type 2 diabetes, according to data from two large cardiovascular safety trials.
Further analysis from ELIXA (Evaluation of Lixisenatide [Lyxumia] in Acute Coronary Syndrome) showed that the hazard ratios for several secondary heart failure endpoints were 1.00 or below. Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure than in those who did not, there was there was no difference between treatment with the GLP-1 (glucagon-like peptide 1) agonist or placebo.
The findings add to those already presented in June at the annual scientific sessions of the American Diabetes Association from the 6,068-patient trial, which enrolled patients with type 2 diabetes who had experienced an acute coronary syndrome within the past 6 months.
Other reassuring data on the safety of newer diabetes medicines came from an updated analysis of TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin [Januvia]). The time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms, and there were no differences between the treatments in terms of hospitalization for heart failure or cardiovascular death or hospitalization for heart failure or all-cause death with the DPP-4 (dipeptyl peptidase 4) inhibitor. TECOS enrolled nearly 15,000 patients with type 2 diabetes and concurrent cardiovascular disease who were aged 50 years or older and the primary findings have been published (N Engl J Med. 2015 July 15;373:232-42).
These trials provide accumulating evidence that there is no heart failure risk with these particular diabetes medications, Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, commented in an interview at the annual congress of the European Society of Cardiology. Dr. Rydén added that the trial results everyone is waiting to hear about concern the cardiovascular safety of the selective sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance).
Advance information issued by manufacturer Boehringer-Ingelheim suggests that this drug actually may lower cardiovascular risk in patients at high risk for cardiovascular events, Dr. Rydén observed. “Now, if that is true, it is the only modern glucose-lowering drug to have shown such a capacity.” Results of the EMPA-REG OUTCOME study will be presented in September at the annual meeting of the European Association for the Study of Diabetes in Stockholm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – Neither the GLP-1 receptor agonist lixisenatide nor the DPP-4 inhibitor sitagliptin significantly increased the risk of heart failure or associated hospitalizations in patients with type 2 diabetes, according to data from two large cardiovascular safety trials.
Further analysis from ELIXA (Evaluation of Lixisenatide [Lyxumia] in Acute Coronary Syndrome) showed that the hazard ratios for several secondary heart failure endpoints were 1.00 or below. Although the risk for heart failure hospitalization was found to be nine times higher in patients who had a prior history of heart failure than in those who did not, there was there was no difference between treatment with the GLP-1 (glucagon-like peptide 1) agonist or placebo.
The findings add to those already presented in June at the annual scientific sessions of the American Diabetes Association from the 6,068-patient trial, which enrolled patients with type 2 diabetes who had experienced an acute coronary syndrome within the past 6 months.
Other reassuring data on the safety of newer diabetes medicines came from an updated analysis of TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin [Januvia]). The time to first hospitalization for heart failure did not differ between the placebo and sitagliptin arms, and there were no differences between the treatments in terms of hospitalization for heart failure or cardiovascular death or hospitalization for heart failure or all-cause death with the DPP-4 (dipeptyl peptidase 4) inhibitor. TECOS enrolled nearly 15,000 patients with type 2 diabetes and concurrent cardiovascular disease who were aged 50 years or older and the primary findings have been published (N Engl J Med. 2015 July 15;373:232-42).
These trials provide accumulating evidence that there is no heart failure risk with these particular diabetes medications, Dr. Lars Rydén, professor of cardiology at the Karolinska Institute in Stockholm, commented in an interview at the annual congress of the European Society of Cardiology. Dr. Rydén added that the trial results everyone is waiting to hear about concern the cardiovascular safety of the selective sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin (Jardiance).
Advance information issued by manufacturer Boehringer-Ingelheim suggests that this drug actually may lower cardiovascular risk in patients at high risk for cardiovascular events, Dr. Rydén observed. “Now, if that is true, it is the only modern glucose-lowering drug to have shown such a capacity.” Results of the EMPA-REG OUTCOME study will be presented in September at the annual meeting of the European Association for the Study of Diabetes in Stockholm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Heart failure readmission-reducing device debuts slowly
In an era when rehospitalization of patients with heart failure for episodes of acute decompensation has become a top target for reduction in the U.S. health care system via Medicare’s Readmissions Reduction Program CardioMEMS looks like the tool that every U.S. health care system and medical center has dreamed of having.
A wireless and battery-less implanted device, CardioMEMS guides management of stage III heart failure patients by allowing daily monitoring of patients’ pulmonary-artery pressure (PAP). Using these measures to fine-tune patient treatment with diuretics and vasodilators led to a statistically significant and clinically meaningful 37% relative reduction in heart failure rehospitalizations over an average 15-month follow-up, based on results from CardioMEMS’ pivotal U.S. trial as well as from subsequent secondary analyses. All-cause mortality was reduced and patients’ quality of life improved. And device-driven therapy yielded these benefits equally well in patients with either preserved or reduced left ventricular function as well as in a variety of other subgroups including patients with cardiac resynchronization devices or implantable cardioverter defibrillators, chronic obstructive pulmonary disease, chronic kidney disease, atrial fibrillation, pulmonary hypertension, or a history of myocardial infarction.
Yet in the first year after CardioMEMS received Food and Drug Administration approval and came onto the U.S. market, the device has taken a strikingly slow path into routine heart failure practice, according to heart failure cardiologists at several U.S. centers. A representative of St. Jude, the company that markets CardioMEMS, saidin July that sales of the device during the first half of 2015 had involved more than 200 U.S. customers and slightly surpassed earlier expectations, and the company now anticipated full 2015 sales to run roughly 25% ahead of projections made at the start of this year. Despite that, the company’s second quarter report acknowledged the challenges that CardioMEMS faced during its first year on the U.S. market.
Numbers show a slow rollout
“I belong to a consortium of academic heart failure physicians who come from many of the major U.S. academic medical centers, and a recent straw poll of the members showed that close to no one was using it [CardioMEMS] on a regular basis, and the majority said they were not yet using it at all or in the process of starting their program,” said Dr. Javed Butler, a heart failure specialist who is professor of medicine, chief of cardiology, and codirector of the Stony Brook (N.Y.) University Heart Center.
At Stony Brook, no heart failure patient had received the device as of July 2015, although Dr. Butler said that his program’s use of CardioMEMS will probably start soon. Cardiologists at a handful of other U.S. centers report similarly slow starts.
At Massachusetts General Hospital (MGH) in Boston not a single patient has received CardioMEMS, though the heart failure staff there hopes to soon launch pilot use in 10 patients, said Dr. Kimberly A. Parks, associate director of the resynchronization and advanced cardiac therapeutics program. At the University of Colorado Hospital in Aurora, three patients have received the device since their first implant in the late winter, and the program is now on track to place devices in another one or two patients each month, said Dr. Natasha L. Altman, a cardiologist who heads the center’s CardioMEMS program. At Brigham and Women’s Hospital in Boston, which participated in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) pivotal trial that led to CardioMEMS approval, so far a “handful, fewer than 10 patients” received the device since marketing began, said Dr. Akshay S. Desai, associate director of Brigham Cardiovascular Consultants. Even at Ohio State’s Wexner Medical Center in Columbus, the program run by Dr. William T. Abraham, coprincipal investigator for CHAMPION, a relatively modest number of roughly 50 patients had received CardioMEMS in routine practice since the first patient received a device there in June 2014 following the FDA’s approval, Dr. Abraham said in an interview.
Contrasting with this level of use is the substantial number of patients who meet the CHAMPION enrollment criteria of New York Heart Association class III heart failure and a heart failure hospitalization within the prior year. Experts estimate that throughout the United States this group must number at least a half-million patients, and at the level of individual medical centers that provide advanced heart failure care, the numbers likely reach several hundred patients at each site.
Several challenges slow CardioMEMS implantation
Why such a slow start for what seems to be such an attractive device that clinicians uniformly praise for having a strong evidence base in CHAMPION and a compelling medical rationale? The answer seems multifactorial, including the need to convince skeptical hospital administrators and insurance payers to provide and pay for the device and follow-up care, a requirement to ramp up the patient-monitoring and management infrastructure, and the challenge of transitioning a relatively complex therapeutic formula from a successful clinical trial model into routine care.
“If this was a pill that you could prescribe to patients at hospital discharge, people would jump on it. But CardioMEMS is not as simple as prescribing a pill. There are a lot of logistical issues that make it very difficult,” said Stony Brook’s Dr. Butler in an interview.
This sentiment was shared not only by other cardiologists but also by St. Jude itself, as a company spokesman itemized several challenges the company encountered once it began trying to sell CardioMEMS commercially. “Developing the market for CardioMEMS will continue to take time,” said the St. Jude staffer during a July webcast on the company’s second-quarter 2015 performance. CardioMEMS’ sales were affected by the need to educate multiple constituencies, satisfy new-technology review committees, address reimbursement, access capital budgets, and create consensus among disparate stakeholders, the webcast said. In addition, the early St. Jude experience selling CardioMEMS showed that once a new customer signs a contract, “we find that customers tend to introduce CardioMEMS ... [on] a pilot basis to gain experience with the technology and the reimbursement process.”
First is the challenge of selling a hospital’s administrative leadership on making an upfront capital investment in CardioMEMS equipment, giving the green light to performing procedures that just about break even relative to reimbursement, and then waiting to recoup the initial expenditure and perhaps make some money in the long term by avoiding readmissions and cutting lengths of stay. According to an analysis run by Dr. Parks of MGH, based on the CHAMPION results, for every 10 patients managed using CardioMEMS for 6 months, a center could expect to prevent nearly 15 patient-days in the hospital.
“Our administration is in support, but skeptical; I think that’s why it’s been slow to start,” said Dr. Parks. “The biggest limitation is the upfront cost of the device, and it’s not clear that the reimbursement will allow you to break even” when putting in devices, she said in an interview. “You could justify this by saying you’ll reduce hospitalizations, but the first impression from our administrators was that we were already doing a pretty good job limiting rehospitalizations so why do we need to add this?” The MGH leadership and clinicians eventually agreed on a plan to start the program with 10 implants and then analyze the results to decide if it makes sense to continue. Dr. Parks said she and her colleagues hope to have their first 10 patients implanted with a CardioMEMS before the end of this year.
Another hurdle at MGH was setting up the infrastructure so that a nurse could monitor patients and set in motion the alerts and treatment changes designed to normalize PAP normalized when it falls out of the target range. “It’s a lot of work to put the system in place to manage the devices,” Dr. Parks noted.
Dr. Butler echoed both these challenges. “You need to convince the hospital administrators and make a case based on the cost savings [later on during ongoing management] rather than positive revenue when you do the procedure. If you can expect future cost savings it’s a viable case to make, but a more difficult case to make,” he said. “You also take on the liability of monitoring patients” long term. “If you can follow several hundred patients there may be enough [follow-up] interventions to pay the salaries of staff ” who monitor the patients, but it is very difficult if you have a nurse who is monitoring five patients,” he said. Another issue complicating the economics is that the physicians who supervise monitoring are mostly not the same ones who performed the CardioMEMS placement procedure and received the procedure’s reimbursement. “These are the system barriers that are out there,” Dr. Butler said.
Dr. Altman in Colorado faced a different challenge. “We had good buy-in from our administration. Everyone is interested in reducing rehospitalizations so the administrators were very supportive. The major roadblock has been insurers. Medicare covers it, but so far Colorado Medicaid and several private insurers do not,” Dr. Altman said in an interview. The inconsistent pattern of insurance coverage has already meant that some heart failure patients in her program who were good clinical candidates for CardioMEMS could not receive the device. “I’ve had at least six or seven good candidates, but only three received the device because of insurance reimbursement issues,” she said.
But Dr. Altman expressed optimism that the coverage situation would improve as more programs start using CarioMEMS and insurers grow more familiar with daily PAP monitoring of heart failure patients. She noted that a new CardioMEMS program will soon start at a second Denver-based medical center, and she expressed confidence that ongoing pressure from physicians and administrators at both institutions will change the mind of officials at Colorado’s Medicaid program to provide reimbursement, and once that happens she expects the private insurers will change their policies as well.
To Dr. Abraham at Ohio State, one of the developers of the concept of using an implanted PAP monitor to guide management of heart failure patients, CardioMEMS slow take off is not surprising. “It provides physicians with daily information on a patient’s hemodynamics, which is something they never had before except in the catheterization laboratory, intensive care unit or cardiac care unit. Managing patients based on hemodynamics even in the absence of worsening signs and symptoms is a paradigm shift. It takes time to adopt new things.” He noted that most hospitals and health systems already have in place case managers or nurse navigators who run systems that have relied on the insensitive parameters of signs, symptoms, and patients’ weights. The same infrastructure should be able to fairly easily switch to focusing instead on PAP, said Dr. Abraham, professor and director of cardiovascular medicine at Wexner Medical Center.
He acknowledged that the upfront capital cost required to start a CardioMEMS program can poses a financial barrier at many U.S. centers, but once that start-up price is paid the actual implantation into each patient comes close to breaking even with existing reimbursements and the system should eventually result in a return on the investment in the form of reduced readmissions and keeping patients stable, he said. Dr. Abraham suggested that the possibility also exists that maintaining better stability in patients with class III heart failure and preventing episodes of acute decompensation through better-titrated fluid-volume control could produce a long-term change in the natural history of these patients, whose disease historically has been marked by progression to class IV heart failure and the eventual need for a left ventricular assist device, heart transplant, and death. Although this potential impact of refined treatment based on daily PAP monitoring remains to be proven, a secondary analysis from CHAMPION showing a 57% relative reduction in all-cause mortality over an average of 17 months of follow-up that Dr. Abraham reported at the American College of Cardiology annual meeting in March
The hurdle of routine practice
Transitioning CardioMEMS from its successful research track record to everyday clinical practice may pose the trickiest barrier of all. The consensus among heart failure experts seems to be that the best approach to do this successfully is to start slow, focus on the most rational patients within the broad enrollment criteria used in CHAMPION, and then gradually expand from that presuming the first wave of results from routine use at a particular site look similar to those from the trial.
“The next big question for PAP monitoring is can we replicate the trial’s success in routine practice? Can this be scaled up?” said Dr. Desai from Brigham and Women’s. “We’re still learning how to identify the right patients, the ones who’ll benefit,”
“The two types of patients we are primarily implanting now are those with classic class III heart failure who were hospitalized during the past 12 months and now are hospitalized again and are right in front of us. They are the lowest-hanging fruit because they are in the hospital today,” said Dr. Abraham. “The second group are the patients who come into the clinic complaining about their symptoms. What we are not yet doing is calling in all patients” with stage III heart failure and a history of at least one hospitalization.”
Other groups are taking a much more selective approach. Dr. Altman and her colleagues in Denver are only targeting patients who have been hospitalized at least twice within the past year, and more specifically patients recently rehospitalized within 30 days of their prior hospitalization. They are also focusing on patients expected to have a high level of compliance with the daily data-collection demands of PAP monitoring and the need to regularly adjust their medication dosages after receiving call backs from their clinicians. This criteria means that they are ruling out patients with a history of substance abuse, she said.
She also noted the need to tailor the target PAP to the specific clinical status of individual patients. Patients with mitral regurgitation, for example, will have a higher “normal” diastolic PAP and hence require a somewhat different target for stability maintenance. “You need to understand each patient’s baseline pressures and adjust their medications based on that,” Dr. Altman said.
“In real life patients tend to be older and sicker, so their benefit may be even greater than what was seen in CHAMPION, but perhaps the results will also be diluted because of comorbidities like renal failure or chronic obstructive pulmonary disease,” said Dr. Butler, although post hoc subanalyses of CHAMPION data showed that these comorbidities did not blunt the positive impact of PAP-guided treatment. “I don’t know if I’ll be successful in selecting the right patients, and whether my interventions in real life will produce the same good outcomes” seen in CHAMPION. “We’ll select patients who are close to the CHAMPION criteria, but not patients on dialysis [who were excluded from CHAMPION]; we’ll select patients with a modest degree of comorbidity and reasonable expected survival,” Dr. Butler said.
“If you had a drug that reduced rehospitalization rates it would be pretty clear how to proceed, but in this case it is not the device that reduces readmissions, it just gives you data and you need to act on the data,” Dr. Butler added. He acknowledged that the CHAMPION investigators used a relatively simple, straightforward management algorithm for patients whose PAP fell out of the target range, such as a diastolic PAP greater than 20 mm Hg or less than 8 mm Hg. The first management step is to raise or reduce the patient’s diuretic dosage, and if that fails to quickly normalize PAP the next step is to adjust the vasodilator dosage.
Managing patients in CHAMPION was “not totally an art” but neither was it “totally a science,” Dr. Butler said. Managing patients based on daily data collected using a CardioMEMS device will “require a little finesse,” he said. “It’s not straight science.”
Programs that have already used PAP monitoring on a routine basis, like those in Denver and at Brigham and Women’s, consider their experience too small in size and short in duration to draw any substantive conclusions regarding their success compared with the CHAMPION results. But Dr. Abraham, whose program has now placed CardioMEMS routinely in a few dozen patients and followed them for as long as a year, said that in his center’s patients the device and management system has produced outcomes that roughly match what he saw in the pivotal trial.
“The ongoing concern is that what was achieved in the CHAMPION trial may not be what is achieved in routine practice,” said Dr. Desai. The care that patients received in the trial is “a little of a black box,” he said, and during the FDA’s approval process some reviewers raised concerns that patients in the active-treatment arm simply received more intensive surveillance, although this concern eventually resolved. “There is not a lot of skepticism” among clinicians about this treatment, but there is uncertainly about exactly whom are the best patients to treat and whether their responses be as good as in CHAMPION, Dr. Desai said in an interview.
“The proof [of efficacy] will be in each individual center’s experience” using CardioMEMS, Dr. Altman said.
CHAMPION was initially funded by CardioMEMS, which then was acquired by St. Jude which is the company marketing the CardioMEMS device and associated hardware. Dr. Butler said that he had no current relevant disclosures, although in the past he had been a consultant to CardioMEMS (prior to its acquisition by St. Jude). Dr. Parks and Dr. Altman had no disclosures. Dr. Desai has been a consultant to and has received honoraria as a speaker on behalf of CardioMEMS and St. Jude. Dr. Abraham has been a consultant to CardioMEMS and St. Jude, was co-principal investigator on the CHAMPION trial, and is the principal investigator on a new St. Jude-funded trial studying CardioMEMS.
On Twitter @mitchelzoler
In an era when rehospitalization of patients with heart failure for episodes of acute decompensation has become a top target for reduction in the U.S. health care system via Medicare’s Readmissions Reduction Program CardioMEMS looks like the tool that every U.S. health care system and medical center has dreamed of having.
A wireless and battery-less implanted device, CardioMEMS guides management of stage III heart failure patients by allowing daily monitoring of patients’ pulmonary-artery pressure (PAP). Using these measures to fine-tune patient treatment with diuretics and vasodilators led to a statistically significant and clinically meaningful 37% relative reduction in heart failure rehospitalizations over an average 15-month follow-up, based on results from CardioMEMS’ pivotal U.S. trial as well as from subsequent secondary analyses. All-cause mortality was reduced and patients’ quality of life improved. And device-driven therapy yielded these benefits equally well in patients with either preserved or reduced left ventricular function as well as in a variety of other subgroups including patients with cardiac resynchronization devices or implantable cardioverter defibrillators, chronic obstructive pulmonary disease, chronic kidney disease, atrial fibrillation, pulmonary hypertension, or a history of myocardial infarction.
Yet in the first year after CardioMEMS received Food and Drug Administration approval and came onto the U.S. market, the device has taken a strikingly slow path into routine heart failure practice, according to heart failure cardiologists at several U.S. centers. A representative of St. Jude, the company that markets CardioMEMS, saidin July that sales of the device during the first half of 2015 had involved more than 200 U.S. customers and slightly surpassed earlier expectations, and the company now anticipated full 2015 sales to run roughly 25% ahead of projections made at the start of this year. Despite that, the company’s second quarter report acknowledged the challenges that CardioMEMS faced during its first year on the U.S. market.
Numbers show a slow rollout
“I belong to a consortium of academic heart failure physicians who come from many of the major U.S. academic medical centers, and a recent straw poll of the members showed that close to no one was using it [CardioMEMS] on a regular basis, and the majority said they were not yet using it at all or in the process of starting their program,” said Dr. Javed Butler, a heart failure specialist who is professor of medicine, chief of cardiology, and codirector of the Stony Brook (N.Y.) University Heart Center.
At Stony Brook, no heart failure patient had received the device as of July 2015, although Dr. Butler said that his program’s use of CardioMEMS will probably start soon. Cardiologists at a handful of other U.S. centers report similarly slow starts.
At Massachusetts General Hospital (MGH) in Boston not a single patient has received CardioMEMS, though the heart failure staff there hopes to soon launch pilot use in 10 patients, said Dr. Kimberly A. Parks, associate director of the resynchronization and advanced cardiac therapeutics program. At the University of Colorado Hospital in Aurora, three patients have received the device since their first implant in the late winter, and the program is now on track to place devices in another one or two patients each month, said Dr. Natasha L. Altman, a cardiologist who heads the center’s CardioMEMS program. At Brigham and Women’s Hospital in Boston, which participated in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) pivotal trial that led to CardioMEMS approval, so far a “handful, fewer than 10 patients” received the device since marketing began, said Dr. Akshay S. Desai, associate director of Brigham Cardiovascular Consultants. Even at Ohio State’s Wexner Medical Center in Columbus, the program run by Dr. William T. Abraham, coprincipal investigator for CHAMPION, a relatively modest number of roughly 50 patients had received CardioMEMS in routine practice since the first patient received a device there in June 2014 following the FDA’s approval, Dr. Abraham said in an interview.
Contrasting with this level of use is the substantial number of patients who meet the CHAMPION enrollment criteria of New York Heart Association class III heart failure and a heart failure hospitalization within the prior year. Experts estimate that throughout the United States this group must number at least a half-million patients, and at the level of individual medical centers that provide advanced heart failure care, the numbers likely reach several hundred patients at each site.
Several challenges slow CardioMEMS implantation
Why such a slow start for what seems to be such an attractive device that clinicians uniformly praise for having a strong evidence base in CHAMPION and a compelling medical rationale? The answer seems multifactorial, including the need to convince skeptical hospital administrators and insurance payers to provide and pay for the device and follow-up care, a requirement to ramp up the patient-monitoring and management infrastructure, and the challenge of transitioning a relatively complex therapeutic formula from a successful clinical trial model into routine care.
“If this was a pill that you could prescribe to patients at hospital discharge, people would jump on it. But CardioMEMS is not as simple as prescribing a pill. There are a lot of logistical issues that make it very difficult,” said Stony Brook’s Dr. Butler in an interview.
This sentiment was shared not only by other cardiologists but also by St. Jude itself, as a company spokesman itemized several challenges the company encountered once it began trying to sell CardioMEMS commercially. “Developing the market for CardioMEMS will continue to take time,” said the St. Jude staffer during a July webcast on the company’s second-quarter 2015 performance. CardioMEMS’ sales were affected by the need to educate multiple constituencies, satisfy new-technology review committees, address reimbursement, access capital budgets, and create consensus among disparate stakeholders, the webcast said. In addition, the early St. Jude experience selling CardioMEMS showed that once a new customer signs a contract, “we find that customers tend to introduce CardioMEMS ... [on] a pilot basis to gain experience with the technology and the reimbursement process.”
First is the challenge of selling a hospital’s administrative leadership on making an upfront capital investment in CardioMEMS equipment, giving the green light to performing procedures that just about break even relative to reimbursement, and then waiting to recoup the initial expenditure and perhaps make some money in the long term by avoiding readmissions and cutting lengths of stay. According to an analysis run by Dr. Parks of MGH, based on the CHAMPION results, for every 10 patients managed using CardioMEMS for 6 months, a center could expect to prevent nearly 15 patient-days in the hospital.
“Our administration is in support, but skeptical; I think that’s why it’s been slow to start,” said Dr. Parks. “The biggest limitation is the upfront cost of the device, and it’s not clear that the reimbursement will allow you to break even” when putting in devices, she said in an interview. “You could justify this by saying you’ll reduce hospitalizations, but the first impression from our administrators was that we were already doing a pretty good job limiting rehospitalizations so why do we need to add this?” The MGH leadership and clinicians eventually agreed on a plan to start the program with 10 implants and then analyze the results to decide if it makes sense to continue. Dr. Parks said she and her colleagues hope to have their first 10 patients implanted with a CardioMEMS before the end of this year.
Another hurdle at MGH was setting up the infrastructure so that a nurse could monitor patients and set in motion the alerts and treatment changes designed to normalize PAP normalized when it falls out of the target range. “It’s a lot of work to put the system in place to manage the devices,” Dr. Parks noted.
Dr. Butler echoed both these challenges. “You need to convince the hospital administrators and make a case based on the cost savings [later on during ongoing management] rather than positive revenue when you do the procedure. If you can expect future cost savings it’s a viable case to make, but a more difficult case to make,” he said. “You also take on the liability of monitoring patients” long term. “If you can follow several hundred patients there may be enough [follow-up] interventions to pay the salaries of staff ” who monitor the patients, but it is very difficult if you have a nurse who is monitoring five patients,” he said. Another issue complicating the economics is that the physicians who supervise monitoring are mostly not the same ones who performed the CardioMEMS placement procedure and received the procedure’s reimbursement. “These are the system barriers that are out there,” Dr. Butler said.
Dr. Altman in Colorado faced a different challenge. “We had good buy-in from our administration. Everyone is interested in reducing rehospitalizations so the administrators were very supportive. The major roadblock has been insurers. Medicare covers it, but so far Colorado Medicaid and several private insurers do not,” Dr. Altman said in an interview. The inconsistent pattern of insurance coverage has already meant that some heart failure patients in her program who were good clinical candidates for CardioMEMS could not receive the device. “I’ve had at least six or seven good candidates, but only three received the device because of insurance reimbursement issues,” she said.
But Dr. Altman expressed optimism that the coverage situation would improve as more programs start using CarioMEMS and insurers grow more familiar with daily PAP monitoring of heart failure patients. She noted that a new CardioMEMS program will soon start at a second Denver-based medical center, and she expressed confidence that ongoing pressure from physicians and administrators at both institutions will change the mind of officials at Colorado’s Medicaid program to provide reimbursement, and once that happens she expects the private insurers will change their policies as well.
To Dr. Abraham at Ohio State, one of the developers of the concept of using an implanted PAP monitor to guide management of heart failure patients, CardioMEMS slow take off is not surprising. “It provides physicians with daily information on a patient’s hemodynamics, which is something they never had before except in the catheterization laboratory, intensive care unit or cardiac care unit. Managing patients based on hemodynamics even in the absence of worsening signs and symptoms is a paradigm shift. It takes time to adopt new things.” He noted that most hospitals and health systems already have in place case managers or nurse navigators who run systems that have relied on the insensitive parameters of signs, symptoms, and patients’ weights. The same infrastructure should be able to fairly easily switch to focusing instead on PAP, said Dr. Abraham, professor and director of cardiovascular medicine at Wexner Medical Center.
He acknowledged that the upfront capital cost required to start a CardioMEMS program can poses a financial barrier at many U.S. centers, but once that start-up price is paid the actual implantation into each patient comes close to breaking even with existing reimbursements and the system should eventually result in a return on the investment in the form of reduced readmissions and keeping patients stable, he said. Dr. Abraham suggested that the possibility also exists that maintaining better stability in patients with class III heart failure and preventing episodes of acute decompensation through better-titrated fluid-volume control could produce a long-term change in the natural history of these patients, whose disease historically has been marked by progression to class IV heart failure and the eventual need for a left ventricular assist device, heart transplant, and death. Although this potential impact of refined treatment based on daily PAP monitoring remains to be proven, a secondary analysis from CHAMPION showing a 57% relative reduction in all-cause mortality over an average of 17 months of follow-up that Dr. Abraham reported at the American College of Cardiology annual meeting in March
The hurdle of routine practice
Transitioning CardioMEMS from its successful research track record to everyday clinical practice may pose the trickiest barrier of all. The consensus among heart failure experts seems to be that the best approach to do this successfully is to start slow, focus on the most rational patients within the broad enrollment criteria used in CHAMPION, and then gradually expand from that presuming the first wave of results from routine use at a particular site look similar to those from the trial.
“The next big question for PAP monitoring is can we replicate the trial’s success in routine practice? Can this be scaled up?” said Dr. Desai from Brigham and Women’s. “We’re still learning how to identify the right patients, the ones who’ll benefit,”
“The two types of patients we are primarily implanting now are those with classic class III heart failure who were hospitalized during the past 12 months and now are hospitalized again and are right in front of us. They are the lowest-hanging fruit because they are in the hospital today,” said Dr. Abraham. “The second group are the patients who come into the clinic complaining about their symptoms. What we are not yet doing is calling in all patients” with stage III heart failure and a history of at least one hospitalization.”
Other groups are taking a much more selective approach. Dr. Altman and her colleagues in Denver are only targeting patients who have been hospitalized at least twice within the past year, and more specifically patients recently rehospitalized within 30 days of their prior hospitalization. They are also focusing on patients expected to have a high level of compliance with the daily data-collection demands of PAP monitoring and the need to regularly adjust their medication dosages after receiving call backs from their clinicians. This criteria means that they are ruling out patients with a history of substance abuse, she said.
She also noted the need to tailor the target PAP to the specific clinical status of individual patients. Patients with mitral regurgitation, for example, will have a higher “normal” diastolic PAP and hence require a somewhat different target for stability maintenance. “You need to understand each patient’s baseline pressures and adjust their medications based on that,” Dr. Altman said.
“In real life patients tend to be older and sicker, so their benefit may be even greater than what was seen in CHAMPION, but perhaps the results will also be diluted because of comorbidities like renal failure or chronic obstructive pulmonary disease,” said Dr. Butler, although post hoc subanalyses of CHAMPION data showed that these comorbidities did not blunt the positive impact of PAP-guided treatment. “I don’t know if I’ll be successful in selecting the right patients, and whether my interventions in real life will produce the same good outcomes” seen in CHAMPION. “We’ll select patients who are close to the CHAMPION criteria, but not patients on dialysis [who were excluded from CHAMPION]; we’ll select patients with a modest degree of comorbidity and reasonable expected survival,” Dr. Butler said.
“If you had a drug that reduced rehospitalization rates it would be pretty clear how to proceed, but in this case it is not the device that reduces readmissions, it just gives you data and you need to act on the data,” Dr. Butler added. He acknowledged that the CHAMPION investigators used a relatively simple, straightforward management algorithm for patients whose PAP fell out of the target range, such as a diastolic PAP greater than 20 mm Hg or less than 8 mm Hg. The first management step is to raise or reduce the patient’s diuretic dosage, and if that fails to quickly normalize PAP the next step is to adjust the vasodilator dosage.
Managing patients in CHAMPION was “not totally an art” but neither was it “totally a science,” Dr. Butler said. Managing patients based on daily data collected using a CardioMEMS device will “require a little finesse,” he said. “It’s not straight science.”
Programs that have already used PAP monitoring on a routine basis, like those in Denver and at Brigham and Women’s, consider their experience too small in size and short in duration to draw any substantive conclusions regarding their success compared with the CHAMPION results. But Dr. Abraham, whose program has now placed CardioMEMS routinely in a few dozen patients and followed them for as long as a year, said that in his center’s patients the device and management system has produced outcomes that roughly match what he saw in the pivotal trial.
“The ongoing concern is that what was achieved in the CHAMPION trial may not be what is achieved in routine practice,” said Dr. Desai. The care that patients received in the trial is “a little of a black box,” he said, and during the FDA’s approval process some reviewers raised concerns that patients in the active-treatment arm simply received more intensive surveillance, although this concern eventually resolved. “There is not a lot of skepticism” among clinicians about this treatment, but there is uncertainly about exactly whom are the best patients to treat and whether their responses be as good as in CHAMPION, Dr. Desai said in an interview.
“The proof [of efficacy] will be in each individual center’s experience” using CardioMEMS, Dr. Altman said.
CHAMPION was initially funded by CardioMEMS, which then was acquired by St. Jude which is the company marketing the CardioMEMS device and associated hardware. Dr. Butler said that he had no current relevant disclosures, although in the past he had been a consultant to CardioMEMS (prior to its acquisition by St. Jude). Dr. Parks and Dr. Altman had no disclosures. Dr. Desai has been a consultant to and has received honoraria as a speaker on behalf of CardioMEMS and St. Jude. Dr. Abraham has been a consultant to CardioMEMS and St. Jude, was co-principal investigator on the CHAMPION trial, and is the principal investigator on a new St. Jude-funded trial studying CardioMEMS.
On Twitter @mitchelzoler
In an era when rehospitalization of patients with heart failure for episodes of acute decompensation has become a top target for reduction in the U.S. health care system via Medicare’s Readmissions Reduction Program CardioMEMS looks like the tool that every U.S. health care system and medical center has dreamed of having.
A wireless and battery-less implanted device, CardioMEMS guides management of stage III heart failure patients by allowing daily monitoring of patients’ pulmonary-artery pressure (PAP). Using these measures to fine-tune patient treatment with diuretics and vasodilators led to a statistically significant and clinically meaningful 37% relative reduction in heart failure rehospitalizations over an average 15-month follow-up, based on results from CardioMEMS’ pivotal U.S. trial as well as from subsequent secondary analyses. All-cause mortality was reduced and patients’ quality of life improved. And device-driven therapy yielded these benefits equally well in patients with either preserved or reduced left ventricular function as well as in a variety of other subgroups including patients with cardiac resynchronization devices or implantable cardioverter defibrillators, chronic obstructive pulmonary disease, chronic kidney disease, atrial fibrillation, pulmonary hypertension, or a history of myocardial infarction.
Yet in the first year after CardioMEMS received Food and Drug Administration approval and came onto the U.S. market, the device has taken a strikingly slow path into routine heart failure practice, according to heart failure cardiologists at several U.S. centers. A representative of St. Jude, the company that markets CardioMEMS, saidin July that sales of the device during the first half of 2015 had involved more than 200 U.S. customers and slightly surpassed earlier expectations, and the company now anticipated full 2015 sales to run roughly 25% ahead of projections made at the start of this year. Despite that, the company’s second quarter report acknowledged the challenges that CardioMEMS faced during its first year on the U.S. market.
Numbers show a slow rollout
“I belong to a consortium of academic heart failure physicians who come from many of the major U.S. academic medical centers, and a recent straw poll of the members showed that close to no one was using it [CardioMEMS] on a regular basis, and the majority said they were not yet using it at all or in the process of starting their program,” said Dr. Javed Butler, a heart failure specialist who is professor of medicine, chief of cardiology, and codirector of the Stony Brook (N.Y.) University Heart Center.
At Stony Brook, no heart failure patient had received the device as of July 2015, although Dr. Butler said that his program’s use of CardioMEMS will probably start soon. Cardiologists at a handful of other U.S. centers report similarly slow starts.
At Massachusetts General Hospital (MGH) in Boston not a single patient has received CardioMEMS, though the heart failure staff there hopes to soon launch pilot use in 10 patients, said Dr. Kimberly A. Parks, associate director of the resynchronization and advanced cardiac therapeutics program. At the University of Colorado Hospital in Aurora, three patients have received the device since their first implant in the late winter, and the program is now on track to place devices in another one or two patients each month, said Dr. Natasha L. Altman, a cardiologist who heads the center’s CardioMEMS program. At Brigham and Women’s Hospital in Boston, which participated in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) pivotal trial that led to CardioMEMS approval, so far a “handful, fewer than 10 patients” received the device since marketing began, said Dr. Akshay S. Desai, associate director of Brigham Cardiovascular Consultants. Even at Ohio State’s Wexner Medical Center in Columbus, the program run by Dr. William T. Abraham, coprincipal investigator for CHAMPION, a relatively modest number of roughly 50 patients had received CardioMEMS in routine practice since the first patient received a device there in June 2014 following the FDA’s approval, Dr. Abraham said in an interview.
Contrasting with this level of use is the substantial number of patients who meet the CHAMPION enrollment criteria of New York Heart Association class III heart failure and a heart failure hospitalization within the prior year. Experts estimate that throughout the United States this group must number at least a half-million patients, and at the level of individual medical centers that provide advanced heart failure care, the numbers likely reach several hundred patients at each site.
Several challenges slow CardioMEMS implantation
Why such a slow start for what seems to be such an attractive device that clinicians uniformly praise for having a strong evidence base in CHAMPION and a compelling medical rationale? The answer seems multifactorial, including the need to convince skeptical hospital administrators and insurance payers to provide and pay for the device and follow-up care, a requirement to ramp up the patient-monitoring and management infrastructure, and the challenge of transitioning a relatively complex therapeutic formula from a successful clinical trial model into routine care.
“If this was a pill that you could prescribe to patients at hospital discharge, people would jump on it. But CardioMEMS is not as simple as prescribing a pill. There are a lot of logistical issues that make it very difficult,” said Stony Brook’s Dr. Butler in an interview.
This sentiment was shared not only by other cardiologists but also by St. Jude itself, as a company spokesman itemized several challenges the company encountered once it began trying to sell CardioMEMS commercially. “Developing the market for CardioMEMS will continue to take time,” said the St. Jude staffer during a July webcast on the company’s second-quarter 2015 performance. CardioMEMS’ sales were affected by the need to educate multiple constituencies, satisfy new-technology review committees, address reimbursement, access capital budgets, and create consensus among disparate stakeholders, the webcast said. In addition, the early St. Jude experience selling CardioMEMS showed that once a new customer signs a contract, “we find that customers tend to introduce CardioMEMS ... [on] a pilot basis to gain experience with the technology and the reimbursement process.”
First is the challenge of selling a hospital’s administrative leadership on making an upfront capital investment in CardioMEMS equipment, giving the green light to performing procedures that just about break even relative to reimbursement, and then waiting to recoup the initial expenditure and perhaps make some money in the long term by avoiding readmissions and cutting lengths of stay. According to an analysis run by Dr. Parks of MGH, based on the CHAMPION results, for every 10 patients managed using CardioMEMS for 6 months, a center could expect to prevent nearly 15 patient-days in the hospital.
“Our administration is in support, but skeptical; I think that’s why it’s been slow to start,” said Dr. Parks. “The biggest limitation is the upfront cost of the device, and it’s not clear that the reimbursement will allow you to break even” when putting in devices, she said in an interview. “You could justify this by saying you’ll reduce hospitalizations, but the first impression from our administrators was that we were already doing a pretty good job limiting rehospitalizations so why do we need to add this?” The MGH leadership and clinicians eventually agreed on a plan to start the program with 10 implants and then analyze the results to decide if it makes sense to continue. Dr. Parks said she and her colleagues hope to have their first 10 patients implanted with a CardioMEMS before the end of this year.
Another hurdle at MGH was setting up the infrastructure so that a nurse could monitor patients and set in motion the alerts and treatment changes designed to normalize PAP normalized when it falls out of the target range. “It’s a lot of work to put the system in place to manage the devices,” Dr. Parks noted.
Dr. Butler echoed both these challenges. “You need to convince the hospital administrators and make a case based on the cost savings [later on during ongoing management] rather than positive revenue when you do the procedure. If you can expect future cost savings it’s a viable case to make, but a more difficult case to make,” he said. “You also take on the liability of monitoring patients” long term. “If you can follow several hundred patients there may be enough [follow-up] interventions to pay the salaries of staff ” who monitor the patients, but it is very difficult if you have a nurse who is monitoring five patients,” he said. Another issue complicating the economics is that the physicians who supervise monitoring are mostly not the same ones who performed the CardioMEMS placement procedure and received the procedure’s reimbursement. “These are the system barriers that are out there,” Dr. Butler said.
Dr. Altman in Colorado faced a different challenge. “We had good buy-in from our administration. Everyone is interested in reducing rehospitalizations so the administrators were very supportive. The major roadblock has been insurers. Medicare covers it, but so far Colorado Medicaid and several private insurers do not,” Dr. Altman said in an interview. The inconsistent pattern of insurance coverage has already meant that some heart failure patients in her program who were good clinical candidates for CardioMEMS could not receive the device. “I’ve had at least six or seven good candidates, but only three received the device because of insurance reimbursement issues,” she said.
But Dr. Altman expressed optimism that the coverage situation would improve as more programs start using CarioMEMS and insurers grow more familiar with daily PAP monitoring of heart failure patients. She noted that a new CardioMEMS program will soon start at a second Denver-based medical center, and she expressed confidence that ongoing pressure from physicians and administrators at both institutions will change the mind of officials at Colorado’s Medicaid program to provide reimbursement, and once that happens she expects the private insurers will change their policies as well.
To Dr. Abraham at Ohio State, one of the developers of the concept of using an implanted PAP monitor to guide management of heart failure patients, CardioMEMS slow take off is not surprising. “It provides physicians with daily information on a patient’s hemodynamics, which is something they never had before except in the catheterization laboratory, intensive care unit or cardiac care unit. Managing patients based on hemodynamics even in the absence of worsening signs and symptoms is a paradigm shift. It takes time to adopt new things.” He noted that most hospitals and health systems already have in place case managers or nurse navigators who run systems that have relied on the insensitive parameters of signs, symptoms, and patients’ weights. The same infrastructure should be able to fairly easily switch to focusing instead on PAP, said Dr. Abraham, professor and director of cardiovascular medicine at Wexner Medical Center.
He acknowledged that the upfront capital cost required to start a CardioMEMS program can poses a financial barrier at many U.S. centers, but once that start-up price is paid the actual implantation into each patient comes close to breaking even with existing reimbursements and the system should eventually result in a return on the investment in the form of reduced readmissions and keeping patients stable, he said. Dr. Abraham suggested that the possibility also exists that maintaining better stability in patients with class III heart failure and preventing episodes of acute decompensation through better-titrated fluid-volume control could produce a long-term change in the natural history of these patients, whose disease historically has been marked by progression to class IV heart failure and the eventual need for a left ventricular assist device, heart transplant, and death. Although this potential impact of refined treatment based on daily PAP monitoring remains to be proven, a secondary analysis from CHAMPION showing a 57% relative reduction in all-cause mortality over an average of 17 months of follow-up that Dr. Abraham reported at the American College of Cardiology annual meeting in March
The hurdle of routine practice
Transitioning CardioMEMS from its successful research track record to everyday clinical practice may pose the trickiest barrier of all. The consensus among heart failure experts seems to be that the best approach to do this successfully is to start slow, focus on the most rational patients within the broad enrollment criteria used in CHAMPION, and then gradually expand from that presuming the first wave of results from routine use at a particular site look similar to those from the trial.
“The next big question for PAP monitoring is can we replicate the trial’s success in routine practice? Can this be scaled up?” said Dr. Desai from Brigham and Women’s. “We’re still learning how to identify the right patients, the ones who’ll benefit,”
“The two types of patients we are primarily implanting now are those with classic class III heart failure who were hospitalized during the past 12 months and now are hospitalized again and are right in front of us. They are the lowest-hanging fruit because they are in the hospital today,” said Dr. Abraham. “The second group are the patients who come into the clinic complaining about their symptoms. What we are not yet doing is calling in all patients” with stage III heart failure and a history of at least one hospitalization.”
Other groups are taking a much more selective approach. Dr. Altman and her colleagues in Denver are only targeting patients who have been hospitalized at least twice within the past year, and more specifically patients recently rehospitalized within 30 days of their prior hospitalization. They are also focusing on patients expected to have a high level of compliance with the daily data-collection demands of PAP monitoring and the need to regularly adjust their medication dosages after receiving call backs from their clinicians. This criteria means that they are ruling out patients with a history of substance abuse, she said.
She also noted the need to tailor the target PAP to the specific clinical status of individual patients. Patients with mitral regurgitation, for example, will have a higher “normal” diastolic PAP and hence require a somewhat different target for stability maintenance. “You need to understand each patient’s baseline pressures and adjust their medications based on that,” Dr. Altman said.
“In real life patients tend to be older and sicker, so their benefit may be even greater than what was seen in CHAMPION, but perhaps the results will also be diluted because of comorbidities like renal failure or chronic obstructive pulmonary disease,” said Dr. Butler, although post hoc subanalyses of CHAMPION data showed that these comorbidities did not blunt the positive impact of PAP-guided treatment. “I don’t know if I’ll be successful in selecting the right patients, and whether my interventions in real life will produce the same good outcomes” seen in CHAMPION. “We’ll select patients who are close to the CHAMPION criteria, but not patients on dialysis [who were excluded from CHAMPION]; we’ll select patients with a modest degree of comorbidity and reasonable expected survival,” Dr. Butler said.
“If you had a drug that reduced rehospitalization rates it would be pretty clear how to proceed, but in this case it is not the device that reduces readmissions, it just gives you data and you need to act on the data,” Dr. Butler added. He acknowledged that the CHAMPION investigators used a relatively simple, straightforward management algorithm for patients whose PAP fell out of the target range, such as a diastolic PAP greater than 20 mm Hg or less than 8 mm Hg. The first management step is to raise or reduce the patient’s diuretic dosage, and if that fails to quickly normalize PAP the next step is to adjust the vasodilator dosage.
Managing patients in CHAMPION was “not totally an art” but neither was it “totally a science,” Dr. Butler said. Managing patients based on daily data collected using a CardioMEMS device will “require a little finesse,” he said. “It’s not straight science.”
Programs that have already used PAP monitoring on a routine basis, like those in Denver and at Brigham and Women’s, consider their experience too small in size and short in duration to draw any substantive conclusions regarding their success compared with the CHAMPION results. But Dr. Abraham, whose program has now placed CardioMEMS routinely in a few dozen patients and followed them for as long as a year, said that in his center’s patients the device and management system has produced outcomes that roughly match what he saw in the pivotal trial.
“The ongoing concern is that what was achieved in the CHAMPION trial may not be what is achieved in routine practice,” said Dr. Desai. The care that patients received in the trial is “a little of a black box,” he said, and during the FDA’s approval process some reviewers raised concerns that patients in the active-treatment arm simply received more intensive surveillance, although this concern eventually resolved. “There is not a lot of skepticism” among clinicians about this treatment, but there is uncertainly about exactly whom are the best patients to treat and whether their responses be as good as in CHAMPION, Dr. Desai said in an interview.
“The proof [of efficacy] will be in each individual center’s experience” using CardioMEMS, Dr. Altman said.
CHAMPION was initially funded by CardioMEMS, which then was acquired by St. Jude which is the company marketing the CardioMEMS device and associated hardware. Dr. Butler said that he had no current relevant disclosures, although in the past he had been a consultant to CardioMEMS (prior to its acquisition by St. Jude). Dr. Parks and Dr. Altman had no disclosures. Dr. Desai has been a consultant to and has received honoraria as a speaker on behalf of CardioMEMS and St. Jude. Dr. Abraham has been a consultant to CardioMEMS and St. Jude, was co-principal investigator on the CHAMPION trial, and is the principal investigator on a new St. Jude-funded trial studying CardioMEMS.
On Twitter @mitchelzoler
VIDEO: Hospitalized heart failure patients susceptible to C. difficile
LONDON – U.S. patients hospitalized for heart failure and treated with antibiotics during their hospital stay had an increased rate both for developing Clostridium difficile infection and dying from it, based on nationwide data collected from nearly six million patients.
“Heart failure was an independent risk factor” in multivariate analyses that controlled for demographic factors as well as for several comorbidities of heart failure, Dr. Petra Mamic said in a video interview at the annual congress of the European Society of Cardiology.
She and her associates used data collected by the National Inpatient Sample during 2012 in more than 5.8 million U.S. hospitalized patients who received antibiotic treatment for a urinary tract infection, pneumonia, or sepsis. They compared the rate of subsequent infection with C. difficile in the roughly 1.4 million of these patients who had heart failure and the nearly 4.5 million without heart failure. In a multivariate analysis heart failure patients were 13% more likely to develop C. difficile infection compared with patients without heart failure. In a second multivariate analysis that focused just on patients with heart failure those who had become infected by C. difficile were 81% more likely to die in hospital compared with heart failure patients without this type of infection.
“Heart failure patients are frequently hospitalized and have a lot of bacterial infections, and so receive treatment with a lot of antibiotics,” said Dr. Mamic, an internal medicine physician at Stanford (Calif.) University. “What is important is that C. difficile infection is preventable. Our ultimate goal is to prevent C. difficile in these patients who have such high morbidity and mortality,”
Dr. Mamic had no disclosures.
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – U.S. patients hospitalized for heart failure and treated with antibiotics during their hospital stay had an increased rate both for developing Clostridium difficile infection and dying from it, based on nationwide data collected from nearly six million patients.
“Heart failure was an independent risk factor” in multivariate analyses that controlled for demographic factors as well as for several comorbidities of heart failure, Dr. Petra Mamic said in a video interview at the annual congress of the European Society of Cardiology.
She and her associates used data collected by the National Inpatient Sample during 2012 in more than 5.8 million U.S. hospitalized patients who received antibiotic treatment for a urinary tract infection, pneumonia, or sepsis. They compared the rate of subsequent infection with C. difficile in the roughly 1.4 million of these patients who had heart failure and the nearly 4.5 million without heart failure. In a multivariate analysis heart failure patients were 13% more likely to develop C. difficile infection compared with patients without heart failure. In a second multivariate analysis that focused just on patients with heart failure those who had become infected by C. difficile were 81% more likely to die in hospital compared with heart failure patients without this type of infection.
“Heart failure patients are frequently hospitalized and have a lot of bacterial infections, and so receive treatment with a lot of antibiotics,” said Dr. Mamic, an internal medicine physician at Stanford (Calif.) University. “What is important is that C. difficile infection is preventable. Our ultimate goal is to prevent C. difficile in these patients who have such high morbidity and mortality,”
Dr. Mamic had no disclosures.
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LONDON – U.S. patients hospitalized for heart failure and treated with antibiotics during their hospital stay had an increased rate both for developing Clostridium difficile infection and dying from it, based on nationwide data collected from nearly six million patients.
“Heart failure was an independent risk factor” in multivariate analyses that controlled for demographic factors as well as for several comorbidities of heart failure, Dr. Petra Mamic said in a video interview at the annual congress of the European Society of Cardiology.
She and her associates used data collected by the National Inpatient Sample during 2012 in more than 5.8 million U.S. hospitalized patients who received antibiotic treatment for a urinary tract infection, pneumonia, or sepsis. They compared the rate of subsequent infection with C. difficile in the roughly 1.4 million of these patients who had heart failure and the nearly 4.5 million without heart failure. In a multivariate analysis heart failure patients were 13% more likely to develop C. difficile infection compared with patients without heart failure. In a second multivariate analysis that focused just on patients with heart failure those who had become infected by C. difficile were 81% more likely to die in hospital compared with heart failure patients without this type of infection.
“Heart failure patients are frequently hospitalized and have a lot of bacterial infections, and so receive treatment with a lot of antibiotics,” said Dr. Mamic, an internal medicine physician at Stanford (Calif.) University. “What is important is that C. difficile infection is preventable. Our ultimate goal is to prevent C. difficile in these patients who have such high morbidity and mortality,”
Dr. Mamic had no disclosures.
On Twitter @mitchelzoler
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2015
ESC: Cyclosporine intervention failed to benefit STEMI patients
Intravenous administration of cyclosporine just before percutaneous coronary intervention was not associated with a reduced risk of adverse outcomes at 1 year in a study of patients who underwent the procedure after experiencing an acute anterior ST-segment elevation myocardial infarction.
Findings from the multicenter, randomized study, known as the Does Cyclosporine Improve Clinical Outcome in ST Elevation Myocardial Infarction Patients (CIRCUS) trial, were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1505489).
For the phase 3 trial, Dr. Michel Ovize and associates investigated whether a single intravenous dose of cyclosporine, administered just before PCI, would improve clinical outcomes and prevent left adverse left ventricular modeling at 1 year in patients with anterior STEMI.
“Growing evidence from experimental studies and small-size proof-of-concept clinical trials shows that reperfusion injury contributes greatly to the final infarct size,” the researchers wrote. “Preclinical studies indicate that the opening of the mitochondrial permeability transition pore (PTP) in the inner mitochondrial membrane plays a major role in reperfusion injury. Either genetic or pharmacologic inhibition of cyclophilin D, a major component of the PTP, reduces the severity of myocardial reperfusion injury.”
Dr. Ovize of the Clinical Investigation Center of Lyon, France, and his associates chose to test cyclosporine, a pharmacologic inhibitor of cyclophilin D, after a phase 2 trial conducted by the same researchers found that administration of cyclosporine immediately before PCI reduced the myocardial infarct size in patients with STEMI. In CIRCUS, which was conducted from April 2011 through February 2014 at 42 hospitals in three countries, 475 patients with an acute anterior STEMI who were undergoing PCI within 12 hours of symptom onset received IV cyclosporine at a dose of 2.5 mg/kg of body weight, while 495 patients received placebo. The primary outcome was a composite of death from any cause, worsening of heart failure during initial hospitalization, rehospitalization for heart failure, or adverse left ventricular remodeling at 1 year.
At baseline, the mean age of patients was 60 years, 82% were men, and their mean body mass index was 27 kg/m2. One year of follow-up data was available for 395 patients in the cyclosporine group and 396 in the placebo group. The researchers found no significant differences between the two groups in the proportion who experienced a primary outcome event (59% in the cyclosporine group vs. 58% in the control group) or in the rate of all-cause mortality (7.1% vs. 6.6%).
There were also no differences between groups in the rate of initial worsening of heart failure or rehospitalization for heart failure at 1 year (22.8% vs. 22.7%). In addition, a similar proportion of patients in both groups experienced adverse left ventricular remodeling (42.8% vs. 40.7%).
“One important limitation of this trial is that we included in the primary outcome the nonclinical outcome of adverse left ventricular remodeling, which accounted for a substantial proportion of the primary outcome rate,” the researchers wrote. “Left ventricular remodeling is a surrogate outcome – one that was not successfully measured in 17.4% of the trial participants. However, in secondary analyses, no beneficial effect of cyclosporine was detected on any of the hard clinical outcomes.”
They went on to note that nearly one of four patients in CIRCUS died or was hospitalized for heart failure, “a reminder of the substantial residual risk in this population and the persistent room for improvement in the medical treatment of high-risk patients with STEMI. The results of this trial do not challenge the concept that reperfusion injury is clinically important. Recent phase 2 trials that have used either ischemic or pharmacologic postconditioning suggest that interventions applied at the time of reperfusion can limit infarct size and improve functional recovery. Their effect on clinical outcome remains to be determined in phase 3 studies.”
The study was supported by grants from the French Ministry of Health and Research National Program and NeuroVive Pharmaceutical. Dr. Ovize disclosed that he is a consultant for NueroVive Pharmaceutical.
Why, despite the promising experimental and clinical data, did cyclosporine have no effect on clinical outcomes? Several factors may be considered. First, although the experimental data supporting the cardioprotective effect of cyclosporine are extensive, not all the studies have been positive. The clinical evidence in favor of a cardioprotective effect is limited to one study in this patient group. Another study involving patients with STEMI who were treated with thrombolysis showed no effect with cyclosporine.
Second, the use of an increase in left ventricular end-diastolic volume as one of the primary outcomes has to be queried. This outcome, for which data were missing in 17% of patients, is not a clinical one but a surrogate marker of adverse left ventricular modeling.
Finally, the use of CicloMulsion (NeuroVive Pharmaceutical), a new formulation of cyclosporine that was used in this study, instead of Sandimmune (Novartis), which was used in the original study by Piot et al., may have contributed to the neutral results.
CicloMulsion contains a lipid emulsion carrier vehicle, thereby avoiding the risk of anaphylaxis that has been associated with Sandimmune, which uses an ethanol and polyoxyethylated castor oil (Cremophor EL) carrier vehicle.
Although the results of the CIRCUS study are disappointing, they do not disprove the existence or clinical significance of myocardial reperfusion injury, because it appears that the formulation of cyclosporine used in the study might not have been effective at preventing myocardial reperfusion injury. If the benefits of PTP inhibition are to be harnessed, more specific inhibitors will need to be discovered. Therefore, the search to find an effective therapy for preventing myocardial reperfusion injury and improving clinical outcomes in patients with reperfused STEMI should continue.
Dr. Derek J. Hausenloy and Derek M. Yellon, D.Sc., are with the Hatter Cardiovascular Institute, University College London, and the National Institute of Health Research University College London Hospitals Biomedical Research Centre. Both reported having no financial disclosures. This text was extracted from an editorial that appeared online Aug. 30, 2015 in the New England Journal of Medicine (doi: 10.1056/NEJMe1509718).
Why, despite the promising experimental and clinical data, did cyclosporine have no effect on clinical outcomes? Several factors may be considered. First, although the experimental data supporting the cardioprotective effect of cyclosporine are extensive, not all the studies have been positive. The clinical evidence in favor of a cardioprotective effect is limited to one study in this patient group. Another study involving patients with STEMI who were treated with thrombolysis showed no effect with cyclosporine.
Second, the use of an increase in left ventricular end-diastolic volume as one of the primary outcomes has to be queried. This outcome, for which data were missing in 17% of patients, is not a clinical one but a surrogate marker of adverse left ventricular modeling.
Finally, the use of CicloMulsion (NeuroVive Pharmaceutical), a new formulation of cyclosporine that was used in this study, instead of Sandimmune (Novartis), which was used in the original study by Piot et al., may have contributed to the neutral results.
CicloMulsion contains a lipid emulsion carrier vehicle, thereby avoiding the risk of anaphylaxis that has been associated with Sandimmune, which uses an ethanol and polyoxyethylated castor oil (Cremophor EL) carrier vehicle.
Although the results of the CIRCUS study are disappointing, they do not disprove the existence or clinical significance of myocardial reperfusion injury, because it appears that the formulation of cyclosporine used in the study might not have been effective at preventing myocardial reperfusion injury. If the benefits of PTP inhibition are to be harnessed, more specific inhibitors will need to be discovered. Therefore, the search to find an effective therapy for preventing myocardial reperfusion injury and improving clinical outcomes in patients with reperfused STEMI should continue.
Dr. Derek J. Hausenloy and Derek M. Yellon, D.Sc., are with the Hatter Cardiovascular Institute, University College London, and the National Institute of Health Research University College London Hospitals Biomedical Research Centre. Both reported having no financial disclosures. This text was extracted from an editorial that appeared online Aug. 30, 2015 in the New England Journal of Medicine (doi: 10.1056/NEJMe1509718).
Why, despite the promising experimental and clinical data, did cyclosporine have no effect on clinical outcomes? Several factors may be considered. First, although the experimental data supporting the cardioprotective effect of cyclosporine are extensive, not all the studies have been positive. The clinical evidence in favor of a cardioprotective effect is limited to one study in this patient group. Another study involving patients with STEMI who were treated with thrombolysis showed no effect with cyclosporine.
Second, the use of an increase in left ventricular end-diastolic volume as one of the primary outcomes has to be queried. This outcome, for which data were missing in 17% of patients, is not a clinical one but a surrogate marker of adverse left ventricular modeling.
Finally, the use of CicloMulsion (NeuroVive Pharmaceutical), a new formulation of cyclosporine that was used in this study, instead of Sandimmune (Novartis), which was used in the original study by Piot et al., may have contributed to the neutral results.
CicloMulsion contains a lipid emulsion carrier vehicle, thereby avoiding the risk of anaphylaxis that has been associated with Sandimmune, which uses an ethanol and polyoxyethylated castor oil (Cremophor EL) carrier vehicle.
Although the results of the CIRCUS study are disappointing, they do not disprove the existence or clinical significance of myocardial reperfusion injury, because it appears that the formulation of cyclosporine used in the study might not have been effective at preventing myocardial reperfusion injury. If the benefits of PTP inhibition are to be harnessed, more specific inhibitors will need to be discovered. Therefore, the search to find an effective therapy for preventing myocardial reperfusion injury and improving clinical outcomes in patients with reperfused STEMI should continue.
Dr. Derek J. Hausenloy and Derek M. Yellon, D.Sc., are with the Hatter Cardiovascular Institute, University College London, and the National Institute of Health Research University College London Hospitals Biomedical Research Centre. Both reported having no financial disclosures. This text was extracted from an editorial that appeared online Aug. 30, 2015 in the New England Journal of Medicine (doi: 10.1056/NEJMe1509718).
Intravenous administration of cyclosporine just before percutaneous coronary intervention was not associated with a reduced risk of adverse outcomes at 1 year in a study of patients who underwent the procedure after experiencing an acute anterior ST-segment elevation myocardial infarction.
Findings from the multicenter, randomized study, known as the Does Cyclosporine Improve Clinical Outcome in ST Elevation Myocardial Infarction Patients (CIRCUS) trial, were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1505489).
For the phase 3 trial, Dr. Michel Ovize and associates investigated whether a single intravenous dose of cyclosporine, administered just before PCI, would improve clinical outcomes and prevent left adverse left ventricular modeling at 1 year in patients with anterior STEMI.
“Growing evidence from experimental studies and small-size proof-of-concept clinical trials shows that reperfusion injury contributes greatly to the final infarct size,” the researchers wrote. “Preclinical studies indicate that the opening of the mitochondrial permeability transition pore (PTP) in the inner mitochondrial membrane plays a major role in reperfusion injury. Either genetic or pharmacologic inhibition of cyclophilin D, a major component of the PTP, reduces the severity of myocardial reperfusion injury.”
Dr. Ovize of the Clinical Investigation Center of Lyon, France, and his associates chose to test cyclosporine, a pharmacologic inhibitor of cyclophilin D, after a phase 2 trial conducted by the same researchers found that administration of cyclosporine immediately before PCI reduced the myocardial infarct size in patients with STEMI. In CIRCUS, which was conducted from April 2011 through February 2014 at 42 hospitals in three countries, 475 patients with an acute anterior STEMI who were undergoing PCI within 12 hours of symptom onset received IV cyclosporine at a dose of 2.5 mg/kg of body weight, while 495 patients received placebo. The primary outcome was a composite of death from any cause, worsening of heart failure during initial hospitalization, rehospitalization for heart failure, or adverse left ventricular remodeling at 1 year.
At baseline, the mean age of patients was 60 years, 82% were men, and their mean body mass index was 27 kg/m2. One year of follow-up data was available for 395 patients in the cyclosporine group and 396 in the placebo group. The researchers found no significant differences between the two groups in the proportion who experienced a primary outcome event (59% in the cyclosporine group vs. 58% in the control group) or in the rate of all-cause mortality (7.1% vs. 6.6%).
There were also no differences between groups in the rate of initial worsening of heart failure or rehospitalization for heart failure at 1 year (22.8% vs. 22.7%). In addition, a similar proportion of patients in both groups experienced adverse left ventricular remodeling (42.8% vs. 40.7%).
“One important limitation of this trial is that we included in the primary outcome the nonclinical outcome of adverse left ventricular remodeling, which accounted for a substantial proportion of the primary outcome rate,” the researchers wrote. “Left ventricular remodeling is a surrogate outcome – one that was not successfully measured in 17.4% of the trial participants. However, in secondary analyses, no beneficial effect of cyclosporine was detected on any of the hard clinical outcomes.”
They went on to note that nearly one of four patients in CIRCUS died or was hospitalized for heart failure, “a reminder of the substantial residual risk in this population and the persistent room for improvement in the medical treatment of high-risk patients with STEMI. The results of this trial do not challenge the concept that reperfusion injury is clinically important. Recent phase 2 trials that have used either ischemic or pharmacologic postconditioning suggest that interventions applied at the time of reperfusion can limit infarct size and improve functional recovery. Their effect on clinical outcome remains to be determined in phase 3 studies.”
The study was supported by grants from the French Ministry of Health and Research National Program and NeuroVive Pharmaceutical. Dr. Ovize disclosed that he is a consultant for NueroVive Pharmaceutical.
Intravenous administration of cyclosporine just before percutaneous coronary intervention was not associated with a reduced risk of adverse outcomes at 1 year in a study of patients who underwent the procedure after experiencing an acute anterior ST-segment elevation myocardial infarction.
Findings from the multicenter, randomized study, known as the Does Cyclosporine Improve Clinical Outcome in ST Elevation Myocardial Infarction Patients (CIRCUS) trial, were presented at the annual congress of the European Society of Cardiology and published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1505489).
For the phase 3 trial, Dr. Michel Ovize and associates investigated whether a single intravenous dose of cyclosporine, administered just before PCI, would improve clinical outcomes and prevent left adverse left ventricular modeling at 1 year in patients with anterior STEMI.
“Growing evidence from experimental studies and small-size proof-of-concept clinical trials shows that reperfusion injury contributes greatly to the final infarct size,” the researchers wrote. “Preclinical studies indicate that the opening of the mitochondrial permeability transition pore (PTP) in the inner mitochondrial membrane plays a major role in reperfusion injury. Either genetic or pharmacologic inhibition of cyclophilin D, a major component of the PTP, reduces the severity of myocardial reperfusion injury.”
Dr. Ovize of the Clinical Investigation Center of Lyon, France, and his associates chose to test cyclosporine, a pharmacologic inhibitor of cyclophilin D, after a phase 2 trial conducted by the same researchers found that administration of cyclosporine immediately before PCI reduced the myocardial infarct size in patients with STEMI. In CIRCUS, which was conducted from April 2011 through February 2014 at 42 hospitals in three countries, 475 patients with an acute anterior STEMI who were undergoing PCI within 12 hours of symptom onset received IV cyclosporine at a dose of 2.5 mg/kg of body weight, while 495 patients received placebo. The primary outcome was a composite of death from any cause, worsening of heart failure during initial hospitalization, rehospitalization for heart failure, or adverse left ventricular remodeling at 1 year.
At baseline, the mean age of patients was 60 years, 82% were men, and their mean body mass index was 27 kg/m2. One year of follow-up data was available for 395 patients in the cyclosporine group and 396 in the placebo group. The researchers found no significant differences between the two groups in the proportion who experienced a primary outcome event (59% in the cyclosporine group vs. 58% in the control group) or in the rate of all-cause mortality (7.1% vs. 6.6%).
There were also no differences between groups in the rate of initial worsening of heart failure or rehospitalization for heart failure at 1 year (22.8% vs. 22.7%). In addition, a similar proportion of patients in both groups experienced adverse left ventricular remodeling (42.8% vs. 40.7%).
“One important limitation of this trial is that we included in the primary outcome the nonclinical outcome of adverse left ventricular remodeling, which accounted for a substantial proportion of the primary outcome rate,” the researchers wrote. “Left ventricular remodeling is a surrogate outcome – one that was not successfully measured in 17.4% of the trial participants. However, in secondary analyses, no beneficial effect of cyclosporine was detected on any of the hard clinical outcomes.”
They went on to note that nearly one of four patients in CIRCUS died or was hospitalized for heart failure, “a reminder of the substantial residual risk in this population and the persistent room for improvement in the medical treatment of high-risk patients with STEMI. The results of this trial do not challenge the concept that reperfusion injury is clinically important. Recent phase 2 trials that have used either ischemic or pharmacologic postconditioning suggest that interventions applied at the time of reperfusion can limit infarct size and improve functional recovery. Their effect on clinical outcome remains to be determined in phase 3 studies.”
The study was supported by grants from the French Ministry of Health and Research National Program and NeuroVive Pharmaceutical. Dr. Ovize disclosed that he is a consultant for NueroVive Pharmaceutical.
FROM THE ESC CONGRESS 2015
Key clinical point: Cyclosporine administration prior to PCI did not benefit STEMI patients.
Major finding: There were no significant differences between intervention and control groups in the proportion who experienced a primary outcome event (59% in the cyclosporine group vs. 58% in the control group).
Data source: A randomized, placebo-controlled trial of 970 patients with an acute anterior ST-segment elevation myocardial infarction who were undergoing percutaneous coronary intervention within 12 hours after symptom onset.
Disclosures: The study was supported by grants from the French Ministry of Health and Research National Program and NeuroVive Pharmaceutical. Dr. Ovize disclosed that he is a consultant for NueroVive Pharmaceutical.