User login
Check for neuromyelitis optica spectrum disorder in suspect HIV patients
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
HIV-associated neuromyelitis optica spectrum disorder (NMOSD) is a recently recognized entity and high index of suspicion is needed to diagnose these patients, according to Thomas Mathew, MD, and his colleagues at St. John’s Medical College Hospital, Bengaluru, India.
“NMOSD can be associated with a wide range of autoimmune diseases but clinicians rarely diagnose NMOSD in cases of HIV infection and HIV-associated NMOSD is rarely mentioned in the conventional classification of NMOSD,” they stated.
Dr. Mathew and his colleagues reported the results of a case study they made of six cases of HIV-NMOSD identified from the literature and 1 HIV-infected patient from a registry for NMOSD that they had established, which had a total of 25 patients with the condition.
There were four men and three women in the study, ranging from 8 years to 49 years of age. The duration of HIV infection in these patients ranged from newly detected to 15 years, according to the report, published in Multiple Sclerosis and Related Disorders (2019 Jan;27:289-93).
Optic neuritis followed by myelitis was the commonest presentation, occurring in five of the seven patients. Of these, six patients were assayed for anti–aquaporin 4 antibodies, which are considered a serological marker of neuromyelitis optica; three patients were positive and three were negative.
All patients received immunomodulatory treatment. Five of the seven patients had a poor recovery from acute attacks, but no patient had further relapses while on immunomodulatory treatment and antiretroviral therapy.
Dr. Mathew and his colleagues suggested that all patients with HIV infection presenting with optic neuritis or/and myelitis, should have their anti–aquaporin 4 antibody status checked and in all patients of NMOSD, HIV infection should be ruled out.
“Prognosis of these patients is variable; residual neurological deficits were common but treatment prevented further attacks. Increased awareness of this association will lead to earlier diagnosis, early treatment and prevention of disability,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Mathew T et al. Mult Scler Relat Disord. 2019 Jan;27:289-93.
FROM MULTIPLE SCLEROSIS AND RELATED DISORDERS
T marneffei Infection: Risk Extends to Patients Without HIV
Patients with HIV/AIDs are vulnerable to Talaromyces marneffei (T marneffei) infection, formerly penicilliosis. But in recent years more cases have been seen in patients not infected with HIV, too. Most cases originate in Southeast Asia: 10% of patients with AIDS in Hong Kong and 30% of patients in North Thailand, for example, have T marneffei infections. But patients with AIDS and penicilliosis travel, as do other immunocompromised patients. Thus, the first reported case of a patient with long-standing pulmonary sarcoidosis who developed T marneffei infection may have significance for clinicians caring for people with, or without, HIV.
Most patients with T marneffei infection have fever, weight loss, and malaise. Subcutaneous abscesses and papulelike ulcers are common (sometimes the lesions are very small). Anemia, hepatosplenomegaly, lymphadenopathy, and diarrhea also are relatively common. However, while cough is a notable symptom, pneumonia is rare—even though the organism is inhaled.
The patient in this report, a native of Cangnan County (an endemic fungal area) in Southeast China, was admitted to the hospital with a 3-week history of daily hyperpyrexia and coughing sputum. When antibiotics did not help, a fungal culture revealed why: He had T marneffei infection. The clinicians say the preexisting pulmonary sarcoidosis covered the clinical features of T marneffei and initially misled them.
After 3 months of antifungal treatment, the patient’s physical condition improved. And the lung lesions were “markedly absorbed” after 3 months. The respiratory signs and skin lesions disappeared gradually after 8 days of treatment.
T marneffei infection is fatal if untreated. Early diagnosis and treatment with antifungals can be life saving.
Source:
Yu X, Miao K, Zhou C, et al. BMC Infect Dis. 2018;18(1):390.
Patients with HIV/AIDs are vulnerable to Talaromyces marneffei (T marneffei) infection, formerly penicilliosis. But in recent years more cases have been seen in patients not infected with HIV, too. Most cases originate in Southeast Asia: 10% of patients with AIDS in Hong Kong and 30% of patients in North Thailand, for example, have T marneffei infections. But patients with AIDS and penicilliosis travel, as do other immunocompromised patients. Thus, the first reported case of a patient with long-standing pulmonary sarcoidosis who developed T marneffei infection may have significance for clinicians caring for people with, or without, HIV.
Most patients with T marneffei infection have fever, weight loss, and malaise. Subcutaneous abscesses and papulelike ulcers are common (sometimes the lesions are very small). Anemia, hepatosplenomegaly, lymphadenopathy, and diarrhea also are relatively common. However, while cough is a notable symptom, pneumonia is rare—even though the organism is inhaled.
The patient in this report, a native of Cangnan County (an endemic fungal area) in Southeast China, was admitted to the hospital with a 3-week history of daily hyperpyrexia and coughing sputum. When antibiotics did not help, a fungal culture revealed why: He had T marneffei infection. The clinicians say the preexisting pulmonary sarcoidosis covered the clinical features of T marneffei and initially misled them.
After 3 months of antifungal treatment, the patient’s physical condition improved. And the lung lesions were “markedly absorbed” after 3 months. The respiratory signs and skin lesions disappeared gradually after 8 days of treatment.
T marneffei infection is fatal if untreated. Early diagnosis and treatment with antifungals can be life saving.
Source:
Yu X, Miao K, Zhou C, et al. BMC Infect Dis. 2018;18(1):390.
Patients with HIV/AIDs are vulnerable to Talaromyces marneffei (T marneffei) infection, formerly penicilliosis. But in recent years more cases have been seen in patients not infected with HIV, too. Most cases originate in Southeast Asia: 10% of patients with AIDS in Hong Kong and 30% of patients in North Thailand, for example, have T marneffei infections. But patients with AIDS and penicilliosis travel, as do other immunocompromised patients. Thus, the first reported case of a patient with long-standing pulmonary sarcoidosis who developed T marneffei infection may have significance for clinicians caring for people with, or without, HIV.
Most patients with T marneffei infection have fever, weight loss, and malaise. Subcutaneous abscesses and papulelike ulcers are common (sometimes the lesions are very small). Anemia, hepatosplenomegaly, lymphadenopathy, and diarrhea also are relatively common. However, while cough is a notable symptom, pneumonia is rare—even though the organism is inhaled.
The patient in this report, a native of Cangnan County (an endemic fungal area) in Southeast China, was admitted to the hospital with a 3-week history of daily hyperpyrexia and coughing sputum. When antibiotics did not help, a fungal culture revealed why: He had T marneffei infection. The clinicians say the preexisting pulmonary sarcoidosis covered the clinical features of T marneffei and initially misled them.
After 3 months of antifungal treatment, the patient’s physical condition improved. And the lung lesions were “markedly absorbed” after 3 months. The respiratory signs and skin lesions disappeared gradually after 8 days of treatment.
T marneffei infection is fatal if untreated. Early diagnosis and treatment with antifungals can be life saving.
Source:
Yu X, Miao K, Zhou C, et al. BMC Infect Dis. 2018;18(1):390.
People with HIV still at increased cardiovascular risk
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
CHICAGO – HIV infection remained linked with an increased risk for developing a cardiovascular disease event among U.S. patients, even in a recent era of antiretroviral therapy.
U.S. health insurance beneficiaries diagnosed with an HIV infection and likely put on antiretroviral therapy sometime during 2011-2015 had a statistically significant, 21% increased risk for the combination of MIs, coronary revascularizations, stroke, and lower-extremity peripheral artery disease (PAD) in a case-control, retrospective analysis, Robert S. Rosenson, MD, said in a poster he presented at the American Heart Association scientific sessions.
“We looked at a contemporary population of people with HIV treated with antiretroviral therapy, and we looked at stroke and lower-extremity PAD [peripheral artery disease] as well as MI, while most prior studies only looked at MIs,” noted Dr. Rosenson, a professor of medicine and director of cardiometabolic disorders at the Icahn School of Medicine at Mount Sinai Medical Center in New York.
The analysis found no significant differences in outcomes that linked with the specific type of antiretroviral therapy patients received. The most commonly used antiretroviral drug was a non–nucleoside reverse transcriptase inhibitor, taken by about 80% of the HIV-infected patients, Dr. Rosenson said. The 2011-2015 period examined in the study largely predated the more recent era, when integrase strand transfer inhibitor drugs have increasingly become the core agent for treating HIV infection.
Another key finding in the study was that a scant 19% of the people infected with HIV received statin treatment, and only 4% were on a high-intensity dosage. The 2018 guideline on cholesterol management identifies HIV infection as one of several “risk enhancers” that boost a person’s cardiovascular disease (CVD) risk and intensify their need for statin treatment (Circulation. 2018 Nov 10. doi: 10.1161/CIR.0000000000000625).
“Hopefully use of statins will increase in people with HIV, but of course we need evidence because so far the evidence does not show benefit,” he noted. In the data Dr. Rosenson reported, the HIV-infected patients who received a statin had roughly the same elevated risk for a CVD event as did HIV-infected patients who did not get a statin.
His study used data from a U.S. commercial database that combined Medicare patients with patients covered by commercial insurers. The analysis identified 82,426 people presumed recently infected by HIV based on either a hospitalization discharge with a diagnostic code for HIV or after filling at least two prescriptions for an antiretroviral drug during January 2011–June 2015. The researchers matched these cases on a 4:1 basis with 329,704 controls from the database matched by age, sex, and year for their index date. The total study cohort averaged about 45 years old, but the people infected by HIV averaged a couple of years older and also had at baseline an increased prevalence of several CVD risk factors and comorbidities. The people with HIV had a more than threefold higher rate of tobacco use, chronic kidney disease, and liver disease, and double the rate of diagnosed depression.
In a multivariate analysis that controlled for many demographic, social, and clinical variables, the results showed that the HIV-infected people had statistically significant higher rates of every individual element in the CVD composite. They had a 26% higher rate of MIs, a 17% higher rate of MIs plus coronary revascularization, a 30% higher rate of stroke, and a doubled rate of lower-extremity PAD.
SOURCE: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: U.S. insurance beneficiaries newly diagnosed with HIV had a significantly higher rate of CVD events than people without HIV.
Major finding: The adjusted rate of cardiovascular disease events was 21% higher in people infected with HIV, compared with matched, uninfected people.
Study details: A retrospective, case control study of 412,130 U.S. health insurance beneficiaries.
Disclosures: The study received partial funding from Amgen. Dr. Rosenson has received honoraria from Amgen, Akcaa, and Kowa; he has been an advisor to Amgen, Regeneron, and Sanofi; and he has received research funding from Amgen, Akcaa, AstraZeneca, and The Medicines Company.
Source: Rosenson RS et al. Circulation. 2018 Nov 6;138[suppl 1]:A14410.
HIV prevention: Mandating insurance coverage of PrEP
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.
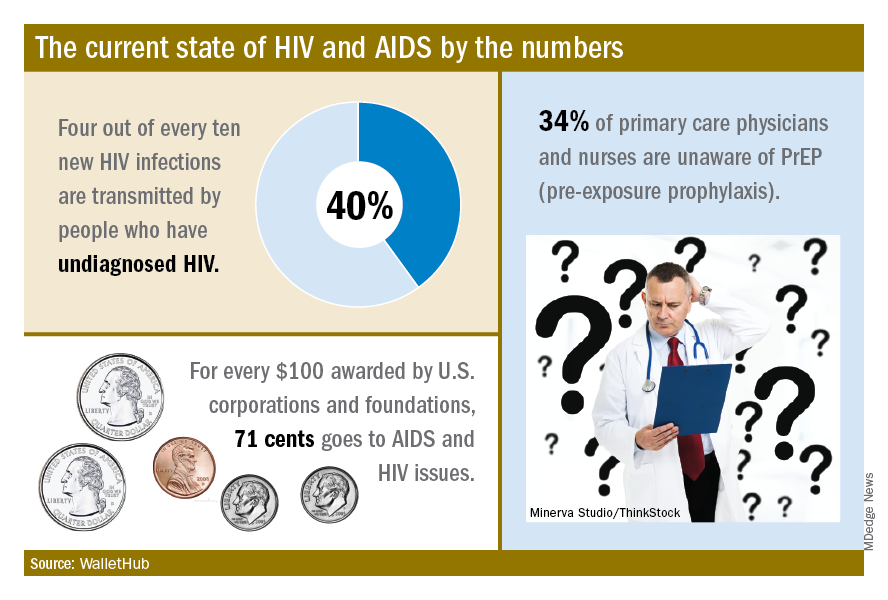
The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.

The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
Pre-exposure prophylaxis (PrEP) for HIV is valuable enough for the federal government to mandate insurance coverage, a group of experts told the personal finance website WalletHub, but individuals who are at risk for infection may be missing out for other reasons.

The effectiveness of PrEP is clear, those experts said, but 34% of primary care physicians and nurses in the United States are unaware of the preventive regimen, according to the WalletHub report, which also noted that the majority of Americans with AIDS (61%) are not seeing a specialist.
“Even among [men who have sex with men] in the U.S., coverage is only about 10%, which is abysmal. We can and need to do better. If we don’t pay now, we’ll pay later,” Steffanie Strathdee, PhD, associate dean of global health sciences and Harold Simon Professor at the University of California, San Diego, told WalletHub.
Those taking PrEP have a 90% chance of avoiding HIV infection, the report noted.
“Making PrEP available to all is a giant step forward in the fight against HIV. Mandating this critical prevention be covered by all insurance plans makes it part of mainstream medicine and will only increase its use and help prevent HIV acquisition in exposed populations. I can’t think of other low-risk, high-reward prophylaxis for a lifelong disease,” said Sharon Nachman, MD, professor of pediatrics and associate dean for research at the State University of New York at Stony Brook.
To get PrEP covered, the U.S. Preventive Services Task Force needs to act, explained Gerald M. Oppenheimer, PhD, MPH, of the department of health policy and management at the City University of New York.
“Under the Affordable Care Act, if the [USPSTF] finds that PrEP serves as an effective prevention to disease and gives it a grade of A or B, all insurers must offer it free. That, of course, may lead to an increase in premiums. This is another example of pharmaceutical companies charging high prices in the U.S., compared to what other countries pay, and cries out for an amendment to Medicare Part D, allowing the federal government to negotiate lower drug prices,” he said.
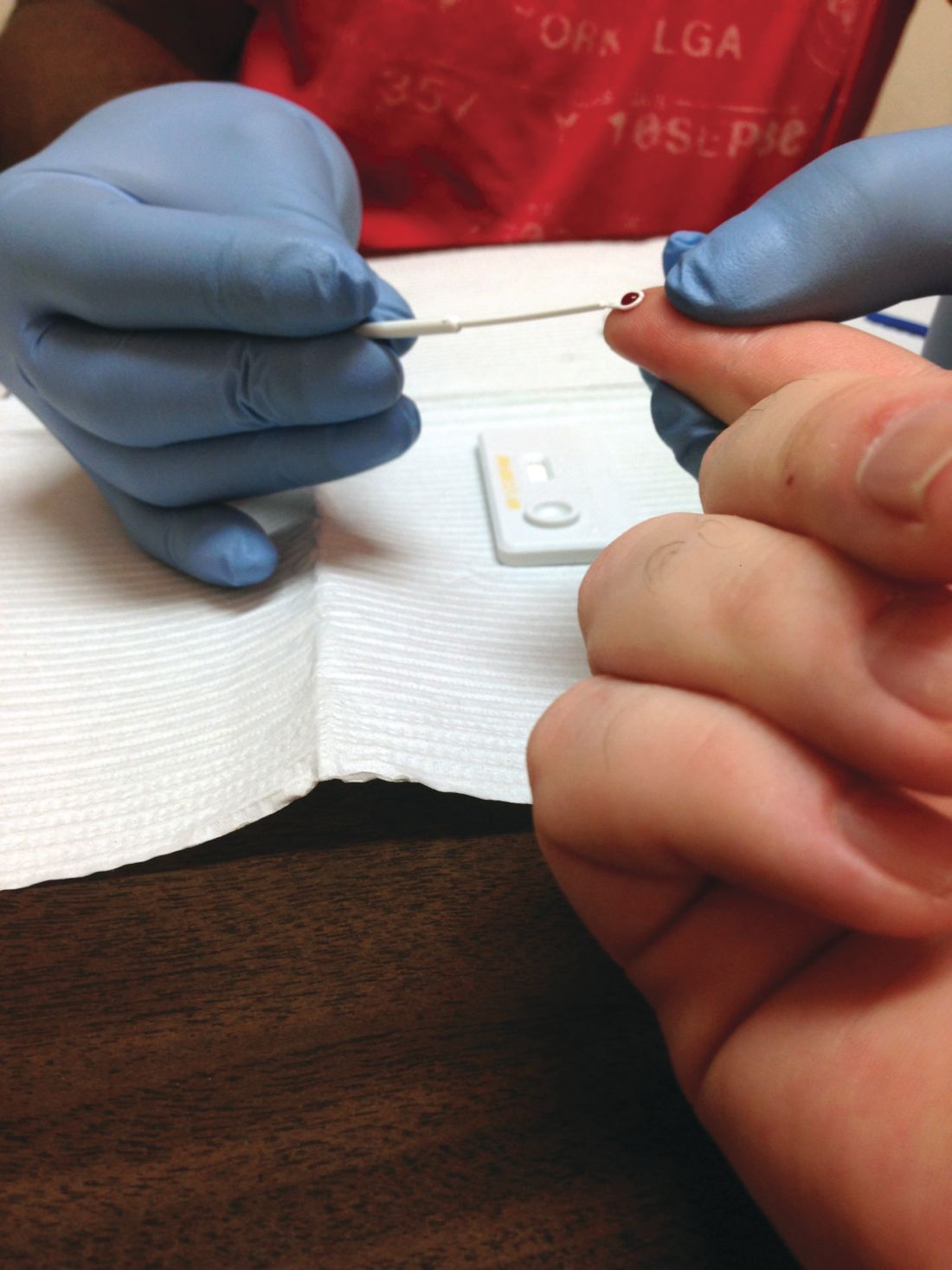
Missed HIV screening opportunities found among subsequently infected youth
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
In the year prior to HIV diagnosis, there were high rates of missed opportunities for HIV testing and sexual history documentation, according to a retrospective study of youth with HIV aged 14-26 years who were treated at an HIV clinic. These results demonstrate a failed need for routine HIV screening and counseling in adolescents, according to Nellie Riendeau Lazar, MPH, of Children’s Hospital of Philadelphia, and her colleagues.
The researchers retrospectively identified 301 subjects between January 2009 and April 2015 who met their study criteria. A total of 58 of these (19%) had at least one visit in the care network in the year prior to diagnosis and their entry into the adolescent HIV clinic, and they were analyzed for missed diagnosis. The adolescent HIV clinic is part of a large care network in the Philadelphia area that includes a pediatric emergency department and a tertiary care hospital. At the time of the study, there were 31 primary care sites, according to the authors.
The mean age of the subjects in the study was 17. The majority (80%) were young men, African-American (93%), and men who have sex with men (81%). There were no significant differences seen in demographics between those with and without prior visits in the health system (J Adolesc Health. 2018;63:799-802).
The 58 subjects were seen in 179 health care visits in the year prior to their diagnosis: 56% outpatient, 40% emergency department, and 4% inpatient visits. Only 59% of these visits had any documentation of sexual history and “the overwhelming majority of those noting sexual activity included no other information,” such as number of partners, sex of partners, or condom use, according to the researchers.
Among the total cohort, 183 of 301 had never had an HIV test prior to their first positive test, even though 26% had been seen in the care network in the 3 years prior to their diagnosis. Among the 58 in the missed opportunity analysis, only 48% had HIV testing, even though 88% (51) had documented symptoms in their visits that could have been consistent with acute infection.
“Our findings support the most recent guidelines from the Centers for Disease Control and Prevention, American Academy of Pediatrics (AAP), and United States Preventive Services Task Force (USPSTF), recommending routine HIV screening for all adolescents, regardless of risk,” the researchers stated. “Adolescents may not always disclose sexual activity during routine assessment, and provider level barriers limit the reach of risk-based testing algorithms,” they added.
The authors reported that they had no conflicts of interest.
SOURCE: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
FROM THE JOURNAL OF ADOLESCENT HEALTH
Key clinical point: Only 51% of youth with symptoms suggesting acute retroviral syndrome were tested.
Major finding: HIV testing was performed in only 48% of the subjects seen in the year prior to their diagnosis.
Study details: Retrospective review of subjects with HIV aged 14-26 years, comparing those with and without HIV screening within the year prior to diagnosis.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Lazar NR et al. J Adolesc Health. 2018;63:799-802.
Temixys plus other antiretrovirals approved for HIV-1
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved the combination of lamivudine (3TC) and tenofovir disoproxil fumarate (TDF) known as Temixys for treatment of HIV-1 when used with other antiretrovirals. The approval is for adult and pediatric patients with HIV-1 who weigh at least 35 kg.
The approval is based on data through 144 weeks in a double-blind, active-controlled, multicenter trial in 600 antiretroviral-naive patients. The trial compared TDF/3TC plus efavirenz (EFV) with 3TC/EFV plus stavudine (d4T). The results showed similar responses at 144 weeks between both groups: 62% of patients taking TDF/3TC/EFV and 58% of patients taking d4T/3TC/EFV achieved and maintained fewer than 50 copies/mL of HIV-1 RNA.
The most common adverse events include headache, pain, depression, rash, and diarrhea. Prior to initiating treatment, patients should be tested for hepatitis B virus because there have been reports of 3TC-resistant strains of hepatitis B virus associated with treatment of HIV-1 with 3TC-containing regimens in coinfected patients. Patients should also be tested for estimated creatinine clearance, urine glucose, and urine protein because TDF/3TC is not recommended for patients with renal impairment.
The full prescribing information can be found on the FDA website.
Promising Results From Anti-HIV Combination Treatment
Reliable treatment with broadly neutralizing antibodies—bNAbs—could change the future for people living with HIV. But studies have found that infusions of a single bNAb did not suppress HIV because some patients developed resistance.
Rockefeller University researchers, however, theorized that combining multiple antibodies that target distinct regions of HIV would both suppress the virus and prevent resistance. So in an NIH-supported pilot study, researhcers recruited 15 volunteers whose HIV was suppressed with antiretroviral treatment (ART) and who were sensitive to 3BNC117 and 10-1074, both potent bNAbs.
Participants received infusions of both bNAbs, stopped taking ART 2 days later, and received additional infusions 3 and 6 weeks later.
Of the 11 people who completed the study, 9 maintained viral suppression without ART for an average of 15 weeks, until the amount of bNAbs in their bodies fell below protective levels. In 2 of the 9, virus was controlled through the end of the 30-week follow-up period. The remaining 2 participants were found to harbor HIV resistant to at least 1 bNAb and experienced viral rebound before 12 weeks after stopping ART.
The researchers are enrolling people with HIV in a larger study to determine an optimal regimen of bNAbs.
Reliable treatment with broadly neutralizing antibodies—bNAbs—could change the future for people living with HIV. But studies have found that infusions of a single bNAb did not suppress HIV because some patients developed resistance.
Rockefeller University researchers, however, theorized that combining multiple antibodies that target distinct regions of HIV would both suppress the virus and prevent resistance. So in an NIH-supported pilot study, researhcers recruited 15 volunteers whose HIV was suppressed with antiretroviral treatment (ART) and who were sensitive to 3BNC117 and 10-1074, both potent bNAbs.
Participants received infusions of both bNAbs, stopped taking ART 2 days later, and received additional infusions 3 and 6 weeks later.
Of the 11 people who completed the study, 9 maintained viral suppression without ART for an average of 15 weeks, until the amount of bNAbs in their bodies fell below protective levels. In 2 of the 9, virus was controlled through the end of the 30-week follow-up period. The remaining 2 participants were found to harbor HIV resistant to at least 1 bNAb and experienced viral rebound before 12 weeks after stopping ART.
The researchers are enrolling people with HIV in a larger study to determine an optimal regimen of bNAbs.
Reliable treatment with broadly neutralizing antibodies—bNAbs—could change the future for people living with HIV. But studies have found that infusions of a single bNAb did not suppress HIV because some patients developed resistance.
Rockefeller University researchers, however, theorized that combining multiple antibodies that target distinct regions of HIV would both suppress the virus and prevent resistance. So in an NIH-supported pilot study, researhcers recruited 15 volunteers whose HIV was suppressed with antiretroviral treatment (ART) and who were sensitive to 3BNC117 and 10-1074, both potent bNAbs.
Participants received infusions of both bNAbs, stopped taking ART 2 days later, and received additional infusions 3 and 6 weeks later.
Of the 11 people who completed the study, 9 maintained viral suppression without ART for an average of 15 weeks, until the amount of bNAbs in their bodies fell below protective levels. In 2 of the 9, virus was controlled through the end of the 30-week follow-up period. The remaining 2 participants were found to harbor HIV resistant to at least 1 bNAb and experienced viral rebound before 12 weeks after stopping ART.
The researchers are enrolling people with HIV in a larger study to determine an optimal regimen of bNAbs.
Draft guidelines advise HIV screening for most teens and adults
Individuals aged 15-65 years, including pregnant women, should be screened for HIV infection, and those at risk should be given prophylaxis, according to draft recommendations issued by the U.S. Preventive Services Task Force. The screening recommendation extends to younger adolescents and older adults at increased risk for HIV infection. The recommendations are level A.
HIV remains a significant public health issue in the United States, with rates rising among individuals aged 25-29 years, although the overall number of cases has dropped slightly, according to the USPSTF report.
HIV prevention is a multistep process that includes not only screening but also wearing condoms during sex and using clean needles and syringes if injecting drugs, the researchers noted.
However, those at high risk for HIV, such as intravenous drug users, can help reduce their risk by taking a daily pill, the researchers wrote.
In an evidence report submitted to the Agency for Healthcare Research and Quality, researchers reviewed the Cochrane databases, MEDLINE, and Embase for studies up to June 2018. Based on data from 11 trials, pre-exposure prophylaxis (PrEP) consisting of antiretroviral therapy was associated with decreased risk of HIV infection, compared with placebo or no PrEP, with consistent effects across risk categories, the investigators noted.
The most common HIV risk factors include man-to-man sexual contact, injection drug use, having sex without a condom, exchanging sex for drugs or money, and having sex with an HIV-infected partner, according to the USPSTF report.
Although PrEP was associated with renal and gastrointestinal adverse effects, most were mild and resolved when the therapy either ended or continued long term. The use of PrEP does not absolve high-risk individuals from observing safety in sex activity and intravenous drug use, the researchers noted.
The Task Force’s draft recommendation statements and draft evidence reviews are available for public comment and are posted on the Task Force website at www.uspreventiveservicestaskforce.org. Comments can be submitted from Nov. 20, 2018, to Dec. 26, 2018, at www.uspreventiveservicestaskforce.org/tfcomment.htm.
Individuals aged 15-65 years, including pregnant women, should be screened for HIV infection, and those at risk should be given prophylaxis, according to draft recommendations issued by the U.S. Preventive Services Task Force. The screening recommendation extends to younger adolescents and older adults at increased risk for HIV infection. The recommendations are level A.
HIV remains a significant public health issue in the United States, with rates rising among individuals aged 25-29 years, although the overall number of cases has dropped slightly, according to the USPSTF report.
HIV prevention is a multistep process that includes not only screening but also wearing condoms during sex and using clean needles and syringes if injecting drugs, the researchers noted.
However, those at high risk for HIV, such as intravenous drug users, can help reduce their risk by taking a daily pill, the researchers wrote.
In an evidence report submitted to the Agency for Healthcare Research and Quality, researchers reviewed the Cochrane databases, MEDLINE, and Embase for studies up to June 2018. Based on data from 11 trials, pre-exposure prophylaxis (PrEP) consisting of antiretroviral therapy was associated with decreased risk of HIV infection, compared with placebo or no PrEP, with consistent effects across risk categories, the investigators noted.
The most common HIV risk factors include man-to-man sexual contact, injection drug use, having sex without a condom, exchanging sex for drugs or money, and having sex with an HIV-infected partner, according to the USPSTF report.
Although PrEP was associated with renal and gastrointestinal adverse effects, most were mild and resolved when the therapy either ended or continued long term. The use of PrEP does not absolve high-risk individuals from observing safety in sex activity and intravenous drug use, the researchers noted.
The Task Force’s draft recommendation statements and draft evidence reviews are available for public comment and are posted on the Task Force website at www.uspreventiveservicestaskforce.org. Comments can be submitted from Nov. 20, 2018, to Dec. 26, 2018, at www.uspreventiveservicestaskforce.org/tfcomment.htm.
Individuals aged 15-65 years, including pregnant women, should be screened for HIV infection, and those at risk should be given prophylaxis, according to draft recommendations issued by the U.S. Preventive Services Task Force. The screening recommendation extends to younger adolescents and older adults at increased risk for HIV infection. The recommendations are level A.
HIV remains a significant public health issue in the United States, with rates rising among individuals aged 25-29 years, although the overall number of cases has dropped slightly, according to the USPSTF report.
HIV prevention is a multistep process that includes not only screening but also wearing condoms during sex and using clean needles and syringes if injecting drugs, the researchers noted.
However, those at high risk for HIV, such as intravenous drug users, can help reduce their risk by taking a daily pill, the researchers wrote.
In an evidence report submitted to the Agency for Healthcare Research and Quality, researchers reviewed the Cochrane databases, MEDLINE, and Embase for studies up to June 2018. Based on data from 11 trials, pre-exposure prophylaxis (PrEP) consisting of antiretroviral therapy was associated with decreased risk of HIV infection, compared with placebo or no PrEP, with consistent effects across risk categories, the investigators noted.
The most common HIV risk factors include man-to-man sexual contact, injection drug use, having sex without a condom, exchanging sex for drugs or money, and having sex with an HIV-infected partner, according to the USPSTF report.
Although PrEP was associated with renal and gastrointestinal adverse effects, most were mild and resolved when the therapy either ended or continued long term. The use of PrEP does not absolve high-risk individuals from observing safety in sex activity and intravenous drug use, the researchers noted.
The Task Force’s draft recommendation statements and draft evidence reviews are available for public comment and are posted on the Task Force website at www.uspreventiveservicestaskforce.org. Comments can be submitted from Nov. 20, 2018, to Dec. 26, 2018, at www.uspreventiveservicestaskforce.org/tfcomment.htm.
Are Doctors Willing to PrEP Young Patients?
In 2015, young people aged 13 to 24 years—disproportionately young men and boys—accounted for 23% of new HIV infections. Pre-exposure prophylaxis (PrEP) can prevent HIV, and has been found safe and effective for young people, but are health care providers who treat adolescents willing to prescribe it?
Researchers from University of California, San Francisco say internal medicine and infectious disease providers have expressed concerns about adherence, development of resistant HIV strains, higher risk sexual behavior, cost, toxicity, and lack of evidence. Data are lacking, though, among youth providers. To find out how aware those clinicians are about PrEP, and how willing they are to prescribe it, the researchers conducted an online survey of members of the Society of Adolescent Health and Medicine.
Almost all of the 162 respondents had heard of PrEP, and agreed that it prevents HIV. Of the respondents, 57 (35%) had prescribed PrEP. Although 73% said they had treated few to no young patients with HIV, 65% were willing to prescribe PrEP to adolescents (aged 13-17 years) and young adults. Only 30 providers said they would refer adolescents and 25 would refer young adults.
Among the providers who would refer or were not willing to prescribe to adolescents, 35 (67%) would prescribe PrEP if it were FDA-approved for adolescents.
Willingness to prescribe was associated with the provider having enough knowledge to safely provide PrEP to adolescents and a belief that adolescents would adhere to a daily medication regimen. Some also said they would prefer to know that they could ensure confidentiality.
The researchers say their findings highlight potential opportunities to reduce HIV incidence among young people by shaping educational and implementation tools to improve provider self-efficacy and youth adherence.
In 2015, young people aged 13 to 24 years—disproportionately young men and boys—accounted for 23% of new HIV infections. Pre-exposure prophylaxis (PrEP) can prevent HIV, and has been found safe and effective for young people, but are health care providers who treat adolescents willing to prescribe it?
Researchers from University of California, San Francisco say internal medicine and infectious disease providers have expressed concerns about adherence, development of resistant HIV strains, higher risk sexual behavior, cost, toxicity, and lack of evidence. Data are lacking, though, among youth providers. To find out how aware those clinicians are about PrEP, and how willing they are to prescribe it, the researchers conducted an online survey of members of the Society of Adolescent Health and Medicine.
Almost all of the 162 respondents had heard of PrEP, and agreed that it prevents HIV. Of the respondents, 57 (35%) had prescribed PrEP. Although 73% said they had treated few to no young patients with HIV, 65% were willing to prescribe PrEP to adolescents (aged 13-17 years) and young adults. Only 30 providers said they would refer adolescents and 25 would refer young adults.
Among the providers who would refer or were not willing to prescribe to adolescents, 35 (67%) would prescribe PrEP if it were FDA-approved for adolescents.
Willingness to prescribe was associated with the provider having enough knowledge to safely provide PrEP to adolescents and a belief that adolescents would adhere to a daily medication regimen. Some also said they would prefer to know that they could ensure confidentiality.
The researchers say their findings highlight potential opportunities to reduce HIV incidence among young people by shaping educational and implementation tools to improve provider self-efficacy and youth adherence.
In 2015, young people aged 13 to 24 years—disproportionately young men and boys—accounted for 23% of new HIV infections. Pre-exposure prophylaxis (PrEP) can prevent HIV, and has been found safe and effective for young people, but are health care providers who treat adolescents willing to prescribe it?
Researchers from University of California, San Francisco say internal medicine and infectious disease providers have expressed concerns about adherence, development of resistant HIV strains, higher risk sexual behavior, cost, toxicity, and lack of evidence. Data are lacking, though, among youth providers. To find out how aware those clinicians are about PrEP, and how willing they are to prescribe it, the researchers conducted an online survey of members of the Society of Adolescent Health and Medicine.
Almost all of the 162 respondents had heard of PrEP, and agreed that it prevents HIV. Of the respondents, 57 (35%) had prescribed PrEP. Although 73% said they had treated few to no young patients with HIV, 65% were willing to prescribe PrEP to adolescents (aged 13-17 years) and young adults. Only 30 providers said they would refer adolescents and 25 would refer young adults.
Among the providers who would refer or were not willing to prescribe to adolescents, 35 (67%) would prescribe PrEP if it were FDA-approved for adolescents.
Willingness to prescribe was associated with the provider having enough knowledge to safely provide PrEP to adolescents and a belief that adolescents would adhere to a daily medication regimen. Some also said they would prefer to know that they could ensure confidentiality.
The researchers say their findings highlight potential opportunities to reduce HIV incidence among young people by shaping educational and implementation tools to improve provider self-efficacy and youth adherence.
ICYMI: Prednisone lowers IRIS risk in patients with HIV
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
In patients with HIV, treatment with prednisone for 4 weeks after antiretroviral therapy initiation significantly reduced the risk of tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS). The results from the randomized, double-blind, placebo-controlled trial were published in the New England Journal of Medicine (2018 Nov 14. doi: 10.1056/NEJMoa1800762).
A total of 240 patients were enrolled in the study, with a median age of 36 years; 60% were men, and 73% had microbiologically confirmed tuberculosis. The median CD4 count of the patients was 49 cells/mcL and the median HIV type 1 RNA viral load was 5.5 log10 copies/mL. A total of 120 patients were assigned to each group, with 18 patients lost to follow-up or withdrawn. Tuberculosis-associated IRIS was diagnosed in 39 patients (32.5%) in the prednisone group and in 56 (46.7%) in the placebo group, yielding a relative IRIS risk of 0.70 (95% confidence interval, 0.51-0.96; P = .03), according to the researchers for the PredART (Preventing TB-IRIS in High-Risk Patients: a Randomized Placebo-Controlled Trial of Prednisone) study team.
We covered this story before it was published in the journal. Find our coverage from the Conference on Retroviruses & Opportunistic Infections at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE