User login
Pharmacist-Led Management of HIV PrEP Within the Veterans Health Administration
The US Department of Health and Human Services (HHS) Ending the HIV Epidemic framework aims to decrease HIV infections in the United States by 90% before 2030.1 Achieving this goal requires identifying persons at high risk for HIV and ensuring timely and efficient access to HIV preexposure prophylaxis (PrEP).2-5 However, despite its commercial availability since 2012, community uptake of PrEP is low.6 In 2019, < 25% of Americans who could benefit from PrEP were using this preventive therapy.7 Poor uptake of PrEP has also been documented among veterans and US military service members. National data on men in the military and men who have sex with men (MSM) in the military suggest that about 12,000 service members are eligible for PrEP; however, only 2000 service members and their beneficiaries accessed PrEP in February 2017.8
A review of health records of US military service members conducted from 2014 to 2016 indicated that most patients who received PrEP did not receive recommended monitoring in accordance with the Centers for Disease Control and Prevention (CDC) guidelines. Furthermore, 16% of these individuals did not have HIV testing within 14 days of initiating PrEP, and 13% were never evaluated for hepatitis B infection.8
Pharmacists are highly accessible health care professionals (HCPs): More than 90% of Americans live within 5 miles of a community pharmacy.9 Pharmacists play an integral role within the outpatient health care team and have been responsible for improvements in health care outcomes for a variety of chronic conditions and immunization practices.10-13 Additionally, community pharmacists have provided vital access to care during the COVID-19 pandemic.14 The clinical pharmacist practitioner (CPP) is an innovative and advanced role within the Veterans Health Administration (VHA), functioning with a scope of practice and prescribing privileges to provide direct patient care.15
CPPs are well suited to address the need for increased access, capacity, and timely provision of PrEP, especially in areas where HIV acquisition rates are high or in areas with reduced access to care. We describe a model for a pharmacist-led HIV PrEP program (Pharm-PrEP) to increase access to PrEP. A similar program could be adapted to further expand the use of PrEP in other health care systems and community settings.
Pharm-PrEP Program Description
The Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHCS) provides health care services at 11 locations in southern California and serves > 86,000 veterans. The VAGLAHCS pharmacy staff includes 33 CPPs who practice in more than 9 clinical service lines. HIV PrEP services are available through the infectious diseases (ID) service for veterans wishing to begin or continue PrEP or for those identified as high risk. HIV PrEP consultations are placed by the referring HCP to the ID service for scheduling and evaluation. Prior to implementation of the pharmacist-managed PrEP clinic, 2 ID physician assistants (PAs) were responsible for PrEP evaluation, initiation, and follow-up. Each PA had 1 half-day face-to-face clinic and 1 PA had an additional half-day telehealth clinic. About 100 PrEP patients were followed by the ID group.
In July 2019, through collaboration with the ID service, a pharmacy PrEP clinic was created to increase access for veterans to initiate or continue PrEP. This clinic included 1 ID-trained CPP and 1 postgraduate-year-2 pharmacy resident. The CPP initiates and monitors veterans for HIV PrEP with prescribing privileges under a defined scope of practice.
Awareness of this novel service was raised through in-service training sessions for primary care and women’s health clinics. Referrals are generated directly from primary care practitioners (PCPs) or emergency department (ED) visits and are accepted on a continuing basis. Visits with the CPP are conducted in person or through telehealth services based on patient preference. Direct CPP patient care appointments involve a standardized assessment and discussion of patient HIV transmission risk, a review of social and sexual history, sexual practices and HIV risk, clinical evidence of acute HIV or other sexually transmitted infection (STI) symptoms, follow-up PrEP monitoring requirements, and counseling on appropriate PrEP use. CPPs can order laboratory tests, bone densitometry (DEXA scan), immunizations, PrEP, and STI treatment as required. ID service physicians are available during CPP visits for further assessment or consultation. While initially most visits are conducted in person, follow-up visits by telehealth or video have become predominant; most patients prefer these modalities, citing convenience, flexibility, and the ability to obtain laboratory tests in advance. Use of telephone and video is intended to reduce patient loss to follow-up.
All required baseline laboratory panels for PrEP monitoring are ordered and interpreted by the CPP in accordance with CDC guidelines.16 These include screening for syphilis, gonorrhea, and chlamydia; fourth-generation antibody-antigen HIV tests; renal function; viral hepatitis; and pregnancy. After reviewing screening results, the CPP will prescribe tenofovir disproxil fumarate/emtricitabine (TDF/FTC) or tenofovir alafenamide/emtricitabine (TAF/FTC) based on individual patient clinical characteristics, US Food and Drug Administration–approved labeling, and VA Pharmacy Benefits Management Criteria for Use. Initial prescriptions are for a 30-day supply with subsequent prescriptions for 90 days (no refills), providing follow-up HIV testing is completed.
Follow-up PrEP visits are scheduled about every 3 months with some overlap to avoid gaps in medication due to late laboratory testing or delayed receipt of mailed medications. The only laboratory testing strictly required each quarter before PrEP renewal is HIV and pregnancy testing. Other screenings, including STIs and renal function are completed at least every 6 months or more frequently, if indicated, based on individual risk factors. Hepatitis C antibody testing is conducted annually if the patient has ongoing risk factors. Treatment of gonorrhea/chlamydia and syphilis for patients with positive test results is also initiated by the CPP, including recommending antimicrobial regimens. Additional interventions conducted as part of the clinic include indicated vaccinations (meningococcal, human papillomavirus, hepatitis A and B), and DEXA scans. Collaboration with ID service attendings and PAs is conducted on an as-needed basis via direct consultation in the colocated clinic or through email or messaging.
Periodic surveillance of a local dashboard of veterans eligible for HIV PrEP is conducted to re-engage veterans in care who may have been lost to follow-up, along with periodic review of a local STI dashboard. These dashboards capture population-based data to identify patients who may benefit from additional STI screenings as well as potential candidates for HIV PrEP. Clinicians can review their patient panel to target individuals who may be due for specific actions. Patients are identified as needing cotesting if they screen positive for ≥ 1 STIs but have not had a concurrent or subsequent full screening panel (gonorrhea, chlamydia, syphilis, and HIV). Cotesting for bacterial STIs and HIV at the time of an encounter has been promoted to expedite STI identification and treatment and limit community transmission. These reports also identify patients who may be potential candidates for HIV PrEP, based on a history of positive screenings, frequent STI testing, recent prescriptions for postexposure prophylaxis (PEP) or encounters with specific International Classification of Diseases codes associated with high-risk practices.
Clinic Quality of Care
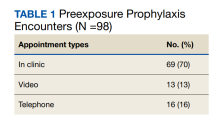
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
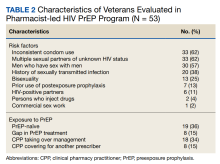
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.
STI screening is a vital component of the Pharm-PrEP clinic and helped identify 4 patients with gonorrhea/chlamydia at baseline and 1 with syphilis after initiation of PrEP. All patients were prescribed antibiotics at the screening. Los Angeles County has high rates of STI transmission;thus implementation of clinic processes allowing the CPP to screen for STIs, interpret test results, and treat patients with STIs is vital to limit spread in the community.17
Selection of PrEP Regimen
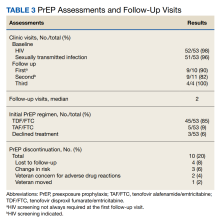
The majority of individuals in the cohort received TDF/FTC for PrEP; TAF/FTC was restricted to individuals who had documented renal dysfunction or bone loss (Table 3).
Six DEXA scans were completed by the end of the evaluation period and 2 had abnormal results. One patient discontinued TDF/FTC and reinitiated with TAF/FTC. The other was switched to TAF/FTC 1 month after initiation.
Follow-Up Visits
The median number of visits per patient was 2. The median time between visits was in accordance with recommended follow-up intervals with 35 days between visits 1 and 2, 60 days between visits 2 and 3, and 88 days thereafter. In all, 10 veterans (20%) stopped PrEP: 4 (8%) were lost to follow-up; 3 (6%) had sustained behavior changes decreasing their HIV exposure risk; 2 (4%) were concerned about ADRs; and 1 (2%) moved out of state. Even after including those patients with a decrease in HIV exposure risk who no longer required PrEP, our 20% discontinuation rate was lower than those reported in other studies that showed a wide variation in PrEP discontinuation rates ranging from 33% to 62%.18-20
Challenges
Some challenges with the implementation of the clinic included logistic and operational barriers, such as developing clinical pathways and managing workflow to facilitate vaccinations or STI treatment for individuals using video or telehealth services, as well as encouraging referrals from PCPs. These challenges were addressed by providing periodic targeted in-service training sessions to primary care teams to increase awareness of the Pharm-PrEP clinic. Collaboration with the ID service and ED allowed implementation of a direct pathway for patients initiated on nonoccupational HIV PEP after a high-risk exposure to be evaluated for transition to HIV PrEP. This PEP-2-PrEP pathway was designed to decrease barriers to follow-up for high-risk individuals who had recently received PEP in the ED. The CPP plays an active, integral role in managing patient care in the PEP-2-PrEP pathway.
Pharmacist-Led PrEP Care
The implementation of the VAGLAHCS Pharm-PrEP clinic demonstrates how CPPs can expand access and manage HIV PrEP with high reliability. Key factors for successfully integrating CPPs as PrEP prescribers include identifying physician champions; using in-services or other training platforms to raise awareness among potential referring HCPs and stimulate referrals; and developing processes to identify high-risk veterans. Also, nontraditional modes of care, such as video or telehealth appointments, can increase access and expand the volume of patient care visits. Such modalities are useful for PrEP management when combined with a well-defined operational process for laboratory specimen collection before appointments. This system is particularly well suited to increasing access to PrEP for patients who live in rural or highly rural areas that are medically underserved or who have difficulty traveling to a clinical facility for an in-person visit.
Although community health care organizations and HCPs face pay barriers not present in the VHA system, several studies have demonstrated feasability of pharmacist-led clinics in private health care systems.21-24 Havens and colleagues described a PrEP program affilitated with an university that assessed patient satisfaction and pharmacist acceptability with this new service.22 The results of surveys reported high patient satisfaction and pharmacist acceptability.23 Tung and colleagues described a PrEP clinic located in a community pharmacy with the ability to bill for pharmacist and laboratory services in addition to medication costs.24 These studies, along with our findings, demonstrate that CPPs are well positioned to manage HIV PrEP in the community. Leveraging the skills and experience of CPPs to address poor uptake and access to PrEP should be a central component in achieving the goals of the Ending the HIV Epidemic initiative, given that pharmacists are one of the most accessible groups of HCPs nationally.
Pharmacist prescriptive authority varies across different states and may depend on collaborative practice agreements, statewide protocols, or class-specific prescribing.25 For example, California was among the first states to authorize initiation and prescription of HIV PrEP and PEP by pharmacists in specified amounts after appropriate training.26 Nationwide support for similar policies in the community and within health care systems will be critical to the successful implementation and functioning of pharmacy-led PrEP clinics.
Conclusions
The success of this Pharm-PrEP clinic was largely due to a collaborative, interdisciplinary effort to implement this new clinic process and incorporate the CPP into the general ID outpatient clinic, while allowing flexibility in scheduling and use of different encounter modalities for patients. Deploying pharmacists as PrEP prescribers can help health care systems increase PrEP access and capacity and improve efforts to achieve the goals of the Ending the HIV Epidemic. This type of program can be a model for other health care organizations and systems to implement pharmacy-led PrEP clinics and expand telehealth modalities to deliver PrEP.
Acknowledgments
The infectious diseases service at the Veterans Affairs Greater Los Angeles Healthcare System and the veterans we serve.
1. Centers for Disease Control and Prevention. About Ending the HIV Epidemic in the U.S. Initiative. Updated September 7, 2021. Accessed April 3, 2023. https://www.cdc.gov/endhiv/about.html
2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. doi:10.1016/S0140-6736(15)00056-2 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi:10.1056/NEJMoa1108524
4. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090. doi:10.1016/S0140-6736(13)61127-7
5. Effectivenesss of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention. Updated June 17, 2022. Accessed April 3, 2023. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html
6. Centers for Disease Control and Prevention. HIV prevention pill not reaching most American who could benefit- especially people of color. Press release. Updated March 6, 2018. Accessed April 3, 2023. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
7. America’s HIV Epidemic Analysis Dashboard (AHEAD).The Six EHE Indicators: PrEP coverage. Accessed April 3, 2023. https://ahead.hiv.gov
8. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. Military Services - 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67(20):569-574. Published 2018 May 25. doi:10.15585/mmwr.mm6720a1
9. National Association of Chain Drug Stores (NACDS) Foundation. Face-to-Face with Community Pharmacies. Accessed April 14, 2023. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf
10. Newman TV, San-Juan-Rodriguez A, Parekh N, et al. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: an umbrella review. Res Social Adm Pharm. 2020;16(9):1155-1165. doi:10.1016/j.sapharm. 2019.12.016
11. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. doi:10.1002/phar.2083
12. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm. 2018;75(14):1039-1047. doi:10.2146/ajhp170789
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi:10.1001/jama.2013.6549.
14. Strand MA, Bratberg J, Eukel H, Hardy M, Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi:10.5888/pcd17.200317
15. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415. doi:10.2146/ajhp150778
16. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. March 2018. Accessed April 3, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
17. County of Los Angeles Public Health. Sexually transmitted diseases in Los Angeles County, 2019. May 2021. Accessed April 3, 2023. http://publichealth.lacounty.gov/dhsp/Reports/STD/2019_LAC_STD_Snapshot_051921Update.pdf
18. Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi:10.1002/jia2.25250
19. Morgan E, Ryan DT, Newcomb ME, Mustanski B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018;22(11):3645-3648. doi:10.1007/s10461-018-2125-2
20. Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. Published 2019 Feb 26. doi:10.1093/ofid/ofz101
21. Ryan K, Lewis J, Sanchez D, Anderson B, Mercier RC. 1293. The next step in PrEP: evaluating outcomes of a pharmacist-run HIV pre-exposure prophylaxis (PrEP) clinic. Open Forum Infect Dis. 2018;5(suppl 1):S395. doi:10.1093/ofid/ofy210.1126
22. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365. doi:10.1093/ofid/ofz365
23. Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS Behav. 2022;26(5):1377-1392. doi:10.1007/s10461-021-03494-4
24. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561. doi:10.1071/SH18084
25. Sachdev G, Kliethermes MA, Vernon V, Leal S, Crabtree G. Current status of prescriptive authority by pharmacists in the United States. J Am Coll Clin Pharm. 2020;3(4):807-817. doi:10.1002/jac5.1245
26. California legislation information: SB-159 HIV: preexposure and postexposure prophylaxis. Accessed April 14, 2023. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159
The US Department of Health and Human Services (HHS) Ending the HIV Epidemic framework aims to decrease HIV infections in the United States by 90% before 2030.1 Achieving this goal requires identifying persons at high risk for HIV and ensuring timely and efficient access to HIV preexposure prophylaxis (PrEP).2-5 However, despite its commercial availability since 2012, community uptake of PrEP is low.6 In 2019, < 25% of Americans who could benefit from PrEP were using this preventive therapy.7 Poor uptake of PrEP has also been documented among veterans and US military service members. National data on men in the military and men who have sex with men (MSM) in the military suggest that about 12,000 service members are eligible for PrEP; however, only 2000 service members and their beneficiaries accessed PrEP in February 2017.8
A review of health records of US military service members conducted from 2014 to 2016 indicated that most patients who received PrEP did not receive recommended monitoring in accordance with the Centers for Disease Control and Prevention (CDC) guidelines. Furthermore, 16% of these individuals did not have HIV testing within 14 days of initiating PrEP, and 13% were never evaluated for hepatitis B infection.8
Pharmacists are highly accessible health care professionals (HCPs): More than 90% of Americans live within 5 miles of a community pharmacy.9 Pharmacists play an integral role within the outpatient health care team and have been responsible for improvements in health care outcomes for a variety of chronic conditions and immunization practices.10-13 Additionally, community pharmacists have provided vital access to care during the COVID-19 pandemic.14 The clinical pharmacist practitioner (CPP) is an innovative and advanced role within the Veterans Health Administration (VHA), functioning with a scope of practice and prescribing privileges to provide direct patient care.15
CPPs are well suited to address the need for increased access, capacity, and timely provision of PrEP, especially in areas where HIV acquisition rates are high or in areas with reduced access to care. We describe a model for a pharmacist-led HIV PrEP program (Pharm-PrEP) to increase access to PrEP. A similar program could be adapted to further expand the use of PrEP in other health care systems and community settings.
Pharm-PrEP Program Description
The Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHCS) provides health care services at 11 locations in southern California and serves > 86,000 veterans. The VAGLAHCS pharmacy staff includes 33 CPPs who practice in more than 9 clinical service lines. HIV PrEP services are available through the infectious diseases (ID) service for veterans wishing to begin or continue PrEP or for those identified as high risk. HIV PrEP consultations are placed by the referring HCP to the ID service for scheduling and evaluation. Prior to implementation of the pharmacist-managed PrEP clinic, 2 ID physician assistants (PAs) were responsible for PrEP evaluation, initiation, and follow-up. Each PA had 1 half-day face-to-face clinic and 1 PA had an additional half-day telehealth clinic. About 100 PrEP patients were followed by the ID group.
In July 2019, through collaboration with the ID service, a pharmacy PrEP clinic was created to increase access for veterans to initiate or continue PrEP. This clinic included 1 ID-trained CPP and 1 postgraduate-year-2 pharmacy resident. The CPP initiates and monitors veterans for HIV PrEP with prescribing privileges under a defined scope of practice.
Awareness of this novel service was raised through in-service training sessions for primary care and women’s health clinics. Referrals are generated directly from primary care practitioners (PCPs) or emergency department (ED) visits and are accepted on a continuing basis. Visits with the CPP are conducted in person or through telehealth services based on patient preference. Direct CPP patient care appointments involve a standardized assessment and discussion of patient HIV transmission risk, a review of social and sexual history, sexual practices and HIV risk, clinical evidence of acute HIV or other sexually transmitted infection (STI) symptoms, follow-up PrEP monitoring requirements, and counseling on appropriate PrEP use. CPPs can order laboratory tests, bone densitometry (DEXA scan), immunizations, PrEP, and STI treatment as required. ID service physicians are available during CPP visits for further assessment or consultation. While initially most visits are conducted in person, follow-up visits by telehealth or video have become predominant; most patients prefer these modalities, citing convenience, flexibility, and the ability to obtain laboratory tests in advance. Use of telephone and video is intended to reduce patient loss to follow-up.
All required baseline laboratory panels for PrEP monitoring are ordered and interpreted by the CPP in accordance with CDC guidelines.16 These include screening for syphilis, gonorrhea, and chlamydia; fourth-generation antibody-antigen HIV tests; renal function; viral hepatitis; and pregnancy. After reviewing screening results, the CPP will prescribe tenofovir disproxil fumarate/emtricitabine (TDF/FTC) or tenofovir alafenamide/emtricitabine (TAF/FTC) based on individual patient clinical characteristics, US Food and Drug Administration–approved labeling, and VA Pharmacy Benefits Management Criteria for Use. Initial prescriptions are for a 30-day supply with subsequent prescriptions for 90 days (no refills), providing follow-up HIV testing is completed.
Follow-up PrEP visits are scheduled about every 3 months with some overlap to avoid gaps in medication due to late laboratory testing or delayed receipt of mailed medications. The only laboratory testing strictly required each quarter before PrEP renewal is HIV and pregnancy testing. Other screenings, including STIs and renal function are completed at least every 6 months or more frequently, if indicated, based on individual risk factors. Hepatitis C antibody testing is conducted annually if the patient has ongoing risk factors. Treatment of gonorrhea/chlamydia and syphilis for patients with positive test results is also initiated by the CPP, including recommending antimicrobial regimens. Additional interventions conducted as part of the clinic include indicated vaccinations (meningococcal, human papillomavirus, hepatitis A and B), and DEXA scans. Collaboration with ID service attendings and PAs is conducted on an as-needed basis via direct consultation in the colocated clinic or through email or messaging.
Periodic surveillance of a local dashboard of veterans eligible for HIV PrEP is conducted to re-engage veterans in care who may have been lost to follow-up, along with periodic review of a local STI dashboard. These dashboards capture population-based data to identify patients who may benefit from additional STI screenings as well as potential candidates for HIV PrEP. Clinicians can review their patient panel to target individuals who may be due for specific actions. Patients are identified as needing cotesting if they screen positive for ≥ 1 STIs but have not had a concurrent or subsequent full screening panel (gonorrhea, chlamydia, syphilis, and HIV). Cotesting for bacterial STIs and HIV at the time of an encounter has been promoted to expedite STI identification and treatment and limit community transmission. These reports also identify patients who may be potential candidates for HIV PrEP, based on a history of positive screenings, frequent STI testing, recent prescriptions for postexposure prophylaxis (PEP) or encounters with specific International Classification of Diseases codes associated with high-risk practices.
Clinic Quality of Care
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.
STI screening is a vital component of the Pharm-PrEP clinic and helped identify 4 patients with gonorrhea/chlamydia at baseline and 1 with syphilis after initiation of PrEP. All patients were prescribed antibiotics at the screening. Los Angeles County has high rates of STI transmission;thus implementation of clinic processes allowing the CPP to screen for STIs, interpret test results, and treat patients with STIs is vital to limit spread in the community.17
Selection of PrEP Regimen
The majority of individuals in the cohort received TDF/FTC for PrEP; TAF/FTC was restricted to individuals who had documented renal dysfunction or bone loss (Table 3).
Six DEXA scans were completed by the end of the evaluation period and 2 had abnormal results. One patient discontinued TDF/FTC and reinitiated with TAF/FTC. The other was switched to TAF/FTC 1 month after initiation.
Follow-Up Visits
The median number of visits per patient was 2. The median time between visits was in accordance with recommended follow-up intervals with 35 days between visits 1 and 2, 60 days between visits 2 and 3, and 88 days thereafter. In all, 10 veterans (20%) stopped PrEP: 4 (8%) were lost to follow-up; 3 (6%) had sustained behavior changes decreasing their HIV exposure risk; 2 (4%) were concerned about ADRs; and 1 (2%) moved out of state. Even after including those patients with a decrease in HIV exposure risk who no longer required PrEP, our 20% discontinuation rate was lower than those reported in other studies that showed a wide variation in PrEP discontinuation rates ranging from 33% to 62%.18-20
Challenges
Some challenges with the implementation of the clinic included logistic and operational barriers, such as developing clinical pathways and managing workflow to facilitate vaccinations or STI treatment for individuals using video or telehealth services, as well as encouraging referrals from PCPs. These challenges were addressed by providing periodic targeted in-service training sessions to primary care teams to increase awareness of the Pharm-PrEP clinic. Collaboration with the ID service and ED allowed implementation of a direct pathway for patients initiated on nonoccupational HIV PEP after a high-risk exposure to be evaluated for transition to HIV PrEP. This PEP-2-PrEP pathway was designed to decrease barriers to follow-up for high-risk individuals who had recently received PEP in the ED. The CPP plays an active, integral role in managing patient care in the PEP-2-PrEP pathway.
Pharmacist-Led PrEP Care
The implementation of the VAGLAHCS Pharm-PrEP clinic demonstrates how CPPs can expand access and manage HIV PrEP with high reliability. Key factors for successfully integrating CPPs as PrEP prescribers include identifying physician champions; using in-services or other training platforms to raise awareness among potential referring HCPs and stimulate referrals; and developing processes to identify high-risk veterans. Also, nontraditional modes of care, such as video or telehealth appointments, can increase access and expand the volume of patient care visits. Such modalities are useful for PrEP management when combined with a well-defined operational process for laboratory specimen collection before appointments. This system is particularly well suited to increasing access to PrEP for patients who live in rural or highly rural areas that are medically underserved or who have difficulty traveling to a clinical facility for an in-person visit.
Although community health care organizations and HCPs face pay barriers not present in the VHA system, several studies have demonstrated feasability of pharmacist-led clinics in private health care systems.21-24 Havens and colleagues described a PrEP program affilitated with an university that assessed patient satisfaction and pharmacist acceptability with this new service.22 The results of surveys reported high patient satisfaction and pharmacist acceptability.23 Tung and colleagues described a PrEP clinic located in a community pharmacy with the ability to bill for pharmacist and laboratory services in addition to medication costs.24 These studies, along with our findings, demonstrate that CPPs are well positioned to manage HIV PrEP in the community. Leveraging the skills and experience of CPPs to address poor uptake and access to PrEP should be a central component in achieving the goals of the Ending the HIV Epidemic initiative, given that pharmacists are one of the most accessible groups of HCPs nationally.
Pharmacist prescriptive authority varies across different states and may depend on collaborative practice agreements, statewide protocols, or class-specific prescribing.25 For example, California was among the first states to authorize initiation and prescription of HIV PrEP and PEP by pharmacists in specified amounts after appropriate training.26 Nationwide support for similar policies in the community and within health care systems will be critical to the successful implementation and functioning of pharmacy-led PrEP clinics.
Conclusions
The success of this Pharm-PrEP clinic was largely due to a collaborative, interdisciplinary effort to implement this new clinic process and incorporate the CPP into the general ID outpatient clinic, while allowing flexibility in scheduling and use of different encounter modalities for patients. Deploying pharmacists as PrEP prescribers can help health care systems increase PrEP access and capacity and improve efforts to achieve the goals of the Ending the HIV Epidemic. This type of program can be a model for other health care organizations and systems to implement pharmacy-led PrEP clinics and expand telehealth modalities to deliver PrEP.
Acknowledgments
The infectious diseases service at the Veterans Affairs Greater Los Angeles Healthcare System and the veterans we serve.
The US Department of Health and Human Services (HHS) Ending the HIV Epidemic framework aims to decrease HIV infections in the United States by 90% before 2030.1 Achieving this goal requires identifying persons at high risk for HIV and ensuring timely and efficient access to HIV preexposure prophylaxis (PrEP).2-5 However, despite its commercial availability since 2012, community uptake of PrEP is low.6 In 2019, < 25% of Americans who could benefit from PrEP were using this preventive therapy.7 Poor uptake of PrEP has also been documented among veterans and US military service members. National data on men in the military and men who have sex with men (MSM) in the military suggest that about 12,000 service members are eligible for PrEP; however, only 2000 service members and their beneficiaries accessed PrEP in February 2017.8
A review of health records of US military service members conducted from 2014 to 2016 indicated that most patients who received PrEP did not receive recommended monitoring in accordance with the Centers for Disease Control and Prevention (CDC) guidelines. Furthermore, 16% of these individuals did not have HIV testing within 14 days of initiating PrEP, and 13% were never evaluated for hepatitis B infection.8
Pharmacists are highly accessible health care professionals (HCPs): More than 90% of Americans live within 5 miles of a community pharmacy.9 Pharmacists play an integral role within the outpatient health care team and have been responsible for improvements in health care outcomes for a variety of chronic conditions and immunization practices.10-13 Additionally, community pharmacists have provided vital access to care during the COVID-19 pandemic.14 The clinical pharmacist practitioner (CPP) is an innovative and advanced role within the Veterans Health Administration (VHA), functioning with a scope of practice and prescribing privileges to provide direct patient care.15
CPPs are well suited to address the need for increased access, capacity, and timely provision of PrEP, especially in areas where HIV acquisition rates are high or in areas with reduced access to care. We describe a model for a pharmacist-led HIV PrEP program (Pharm-PrEP) to increase access to PrEP. A similar program could be adapted to further expand the use of PrEP in other health care systems and community settings.
Pharm-PrEP Program Description
The Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHCS) provides health care services at 11 locations in southern California and serves > 86,000 veterans. The VAGLAHCS pharmacy staff includes 33 CPPs who practice in more than 9 clinical service lines. HIV PrEP services are available through the infectious diseases (ID) service for veterans wishing to begin or continue PrEP or for those identified as high risk. HIV PrEP consultations are placed by the referring HCP to the ID service for scheduling and evaluation. Prior to implementation of the pharmacist-managed PrEP clinic, 2 ID physician assistants (PAs) were responsible for PrEP evaluation, initiation, and follow-up. Each PA had 1 half-day face-to-face clinic and 1 PA had an additional half-day telehealth clinic. About 100 PrEP patients were followed by the ID group.
In July 2019, through collaboration with the ID service, a pharmacy PrEP clinic was created to increase access for veterans to initiate or continue PrEP. This clinic included 1 ID-trained CPP and 1 postgraduate-year-2 pharmacy resident. The CPP initiates and monitors veterans for HIV PrEP with prescribing privileges under a defined scope of practice.
Awareness of this novel service was raised through in-service training sessions for primary care and women’s health clinics. Referrals are generated directly from primary care practitioners (PCPs) or emergency department (ED) visits and are accepted on a continuing basis. Visits with the CPP are conducted in person or through telehealth services based on patient preference. Direct CPP patient care appointments involve a standardized assessment and discussion of patient HIV transmission risk, a review of social and sexual history, sexual practices and HIV risk, clinical evidence of acute HIV or other sexually transmitted infection (STI) symptoms, follow-up PrEP monitoring requirements, and counseling on appropriate PrEP use. CPPs can order laboratory tests, bone densitometry (DEXA scan), immunizations, PrEP, and STI treatment as required. ID service physicians are available during CPP visits for further assessment or consultation. While initially most visits are conducted in person, follow-up visits by telehealth or video have become predominant; most patients prefer these modalities, citing convenience, flexibility, and the ability to obtain laboratory tests in advance. Use of telephone and video is intended to reduce patient loss to follow-up.
All required baseline laboratory panels for PrEP monitoring are ordered and interpreted by the CPP in accordance with CDC guidelines.16 These include screening for syphilis, gonorrhea, and chlamydia; fourth-generation antibody-antigen HIV tests; renal function; viral hepatitis; and pregnancy. After reviewing screening results, the CPP will prescribe tenofovir disproxil fumarate/emtricitabine (TDF/FTC) or tenofovir alafenamide/emtricitabine (TAF/FTC) based on individual patient clinical characteristics, US Food and Drug Administration–approved labeling, and VA Pharmacy Benefits Management Criteria for Use. Initial prescriptions are for a 30-day supply with subsequent prescriptions for 90 days (no refills), providing follow-up HIV testing is completed.
Follow-up PrEP visits are scheduled about every 3 months with some overlap to avoid gaps in medication due to late laboratory testing or delayed receipt of mailed medications. The only laboratory testing strictly required each quarter before PrEP renewal is HIV and pregnancy testing. Other screenings, including STIs and renal function are completed at least every 6 months or more frequently, if indicated, based on individual risk factors. Hepatitis C antibody testing is conducted annually if the patient has ongoing risk factors. Treatment of gonorrhea/chlamydia and syphilis for patients with positive test results is also initiated by the CPP, including recommending antimicrobial regimens. Additional interventions conducted as part of the clinic include indicated vaccinations (meningococcal, human papillomavirus, hepatitis A and B), and DEXA scans. Collaboration with ID service attendings and PAs is conducted on an as-needed basis via direct consultation in the colocated clinic or through email or messaging.
Periodic surveillance of a local dashboard of veterans eligible for HIV PrEP is conducted to re-engage veterans in care who may have been lost to follow-up, along with periodic review of a local STI dashboard. These dashboards capture population-based data to identify patients who may benefit from additional STI screenings as well as potential candidates for HIV PrEP. Clinicians can review their patient panel to target individuals who may be due for specific actions. Patients are identified as needing cotesting if they screen positive for ≥ 1 STIs but have not had a concurrent or subsequent full screening panel (gonorrhea, chlamydia, syphilis, and HIV). Cotesting for bacterial STIs and HIV at the time of an encounter has been promoted to expedite STI identification and treatment and limit community transmission. These reports also identify patients who may be potential candidates for HIV PrEP, based on a history of positive screenings, frequent STI testing, recent prescriptions for postexposure prophylaxis (PEP) or encounters with specific International Classification of Diseases codes associated with high-risk practices.
Clinic Quality of Care
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.
STI screening is a vital component of the Pharm-PrEP clinic and helped identify 4 patients with gonorrhea/chlamydia at baseline and 1 with syphilis after initiation of PrEP. All patients were prescribed antibiotics at the screening. Los Angeles County has high rates of STI transmission;thus implementation of clinic processes allowing the CPP to screen for STIs, interpret test results, and treat patients with STIs is vital to limit spread in the community.17
Selection of PrEP Regimen
The majority of individuals in the cohort received TDF/FTC for PrEP; TAF/FTC was restricted to individuals who had documented renal dysfunction or bone loss (Table 3).
Six DEXA scans were completed by the end of the evaluation period and 2 had abnormal results. One patient discontinued TDF/FTC and reinitiated with TAF/FTC. The other was switched to TAF/FTC 1 month after initiation.
Follow-Up Visits
The median number of visits per patient was 2. The median time between visits was in accordance with recommended follow-up intervals with 35 days between visits 1 and 2, 60 days between visits 2 and 3, and 88 days thereafter. In all, 10 veterans (20%) stopped PrEP: 4 (8%) were lost to follow-up; 3 (6%) had sustained behavior changes decreasing their HIV exposure risk; 2 (4%) were concerned about ADRs; and 1 (2%) moved out of state. Even after including those patients with a decrease in HIV exposure risk who no longer required PrEP, our 20% discontinuation rate was lower than those reported in other studies that showed a wide variation in PrEP discontinuation rates ranging from 33% to 62%.18-20
Challenges
Some challenges with the implementation of the clinic included logistic and operational barriers, such as developing clinical pathways and managing workflow to facilitate vaccinations or STI treatment for individuals using video or telehealth services, as well as encouraging referrals from PCPs. These challenges were addressed by providing periodic targeted in-service training sessions to primary care teams to increase awareness of the Pharm-PrEP clinic. Collaboration with the ID service and ED allowed implementation of a direct pathway for patients initiated on nonoccupational HIV PEP after a high-risk exposure to be evaluated for transition to HIV PrEP. This PEP-2-PrEP pathway was designed to decrease barriers to follow-up for high-risk individuals who had recently received PEP in the ED. The CPP plays an active, integral role in managing patient care in the PEP-2-PrEP pathway.
Pharmacist-Led PrEP Care
The implementation of the VAGLAHCS Pharm-PrEP clinic demonstrates how CPPs can expand access and manage HIV PrEP with high reliability. Key factors for successfully integrating CPPs as PrEP prescribers include identifying physician champions; using in-services or other training platforms to raise awareness among potential referring HCPs and stimulate referrals; and developing processes to identify high-risk veterans. Also, nontraditional modes of care, such as video or telehealth appointments, can increase access and expand the volume of patient care visits. Such modalities are useful for PrEP management when combined with a well-defined operational process for laboratory specimen collection before appointments. This system is particularly well suited to increasing access to PrEP for patients who live in rural or highly rural areas that are medically underserved or who have difficulty traveling to a clinical facility for an in-person visit.
Although community health care organizations and HCPs face pay barriers not present in the VHA system, several studies have demonstrated feasability of pharmacist-led clinics in private health care systems.21-24 Havens and colleagues described a PrEP program affilitated with an university that assessed patient satisfaction and pharmacist acceptability with this new service.22 The results of surveys reported high patient satisfaction and pharmacist acceptability.23 Tung and colleagues described a PrEP clinic located in a community pharmacy with the ability to bill for pharmacist and laboratory services in addition to medication costs.24 These studies, along with our findings, demonstrate that CPPs are well positioned to manage HIV PrEP in the community. Leveraging the skills and experience of CPPs to address poor uptake and access to PrEP should be a central component in achieving the goals of the Ending the HIV Epidemic initiative, given that pharmacists are one of the most accessible groups of HCPs nationally.
Pharmacist prescriptive authority varies across different states and may depend on collaborative practice agreements, statewide protocols, or class-specific prescribing.25 For example, California was among the first states to authorize initiation and prescription of HIV PrEP and PEP by pharmacists in specified amounts after appropriate training.26 Nationwide support for similar policies in the community and within health care systems will be critical to the successful implementation and functioning of pharmacy-led PrEP clinics.
Conclusions
The success of this Pharm-PrEP clinic was largely due to a collaborative, interdisciplinary effort to implement this new clinic process and incorporate the CPP into the general ID outpatient clinic, while allowing flexibility in scheduling and use of different encounter modalities for patients. Deploying pharmacists as PrEP prescribers can help health care systems increase PrEP access and capacity and improve efforts to achieve the goals of the Ending the HIV Epidemic. This type of program can be a model for other health care organizations and systems to implement pharmacy-led PrEP clinics and expand telehealth modalities to deliver PrEP.
Acknowledgments
The infectious diseases service at the Veterans Affairs Greater Los Angeles Healthcare System and the veterans we serve.
1. Centers for Disease Control and Prevention. About Ending the HIV Epidemic in the U.S. Initiative. Updated September 7, 2021. Accessed April 3, 2023. https://www.cdc.gov/endhiv/about.html
2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. doi:10.1016/S0140-6736(15)00056-2 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi:10.1056/NEJMoa1108524
4. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090. doi:10.1016/S0140-6736(13)61127-7
5. Effectivenesss of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention. Updated June 17, 2022. Accessed April 3, 2023. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html
6. Centers for Disease Control and Prevention. HIV prevention pill not reaching most American who could benefit- especially people of color. Press release. Updated March 6, 2018. Accessed April 3, 2023. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
7. America’s HIV Epidemic Analysis Dashboard (AHEAD).The Six EHE Indicators: PrEP coverage. Accessed April 3, 2023. https://ahead.hiv.gov
8. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. Military Services - 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67(20):569-574. Published 2018 May 25. doi:10.15585/mmwr.mm6720a1
9. National Association of Chain Drug Stores (NACDS) Foundation. Face-to-Face with Community Pharmacies. Accessed April 14, 2023. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf
10. Newman TV, San-Juan-Rodriguez A, Parekh N, et al. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: an umbrella review. Res Social Adm Pharm. 2020;16(9):1155-1165. doi:10.1016/j.sapharm. 2019.12.016
11. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. doi:10.1002/phar.2083
12. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm. 2018;75(14):1039-1047. doi:10.2146/ajhp170789
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi:10.1001/jama.2013.6549.
14. Strand MA, Bratberg J, Eukel H, Hardy M, Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi:10.5888/pcd17.200317
15. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415. doi:10.2146/ajhp150778
16. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. March 2018. Accessed April 3, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
17. County of Los Angeles Public Health. Sexually transmitted diseases in Los Angeles County, 2019. May 2021. Accessed April 3, 2023. http://publichealth.lacounty.gov/dhsp/Reports/STD/2019_LAC_STD_Snapshot_051921Update.pdf
18. Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi:10.1002/jia2.25250
19. Morgan E, Ryan DT, Newcomb ME, Mustanski B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018;22(11):3645-3648. doi:10.1007/s10461-018-2125-2
20. Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. Published 2019 Feb 26. doi:10.1093/ofid/ofz101
21. Ryan K, Lewis J, Sanchez D, Anderson B, Mercier RC. 1293. The next step in PrEP: evaluating outcomes of a pharmacist-run HIV pre-exposure prophylaxis (PrEP) clinic. Open Forum Infect Dis. 2018;5(suppl 1):S395. doi:10.1093/ofid/ofy210.1126
22. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365. doi:10.1093/ofid/ofz365
23. Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS Behav. 2022;26(5):1377-1392. doi:10.1007/s10461-021-03494-4
24. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561. doi:10.1071/SH18084
25. Sachdev G, Kliethermes MA, Vernon V, Leal S, Crabtree G. Current status of prescriptive authority by pharmacists in the United States. J Am Coll Clin Pharm. 2020;3(4):807-817. doi:10.1002/jac5.1245
26. California legislation information: SB-159 HIV: preexposure and postexposure prophylaxis. Accessed April 14, 2023. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159
1. Centers for Disease Control and Prevention. About Ending the HIV Epidemic in the U.S. Initiative. Updated September 7, 2021. Accessed April 3, 2023. https://www.cdc.gov/endhiv/about.html
2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. doi:10.1016/S0140-6736(15)00056-2 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi:10.1056/NEJMoa1108524
4. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090. doi:10.1016/S0140-6736(13)61127-7
5. Effectivenesss of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention. Updated June 17, 2022. Accessed April 3, 2023. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html
6. Centers for Disease Control and Prevention. HIV prevention pill not reaching most American who could benefit- especially people of color. Press release. Updated March 6, 2018. Accessed April 3, 2023. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
7. America’s HIV Epidemic Analysis Dashboard (AHEAD).The Six EHE Indicators: PrEP coverage. Accessed April 3, 2023. https://ahead.hiv.gov
8. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. Military Services - 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67(20):569-574. Published 2018 May 25. doi:10.15585/mmwr.mm6720a1
9. National Association of Chain Drug Stores (NACDS) Foundation. Face-to-Face with Community Pharmacies. Accessed April 14, 2023. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf
10. Newman TV, San-Juan-Rodriguez A, Parekh N, et al. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: an umbrella review. Res Social Adm Pharm. 2020;16(9):1155-1165. doi:10.1016/j.sapharm. 2019.12.016
11. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. doi:10.1002/phar.2083
12. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm. 2018;75(14):1039-1047. doi:10.2146/ajhp170789
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi:10.1001/jama.2013.6549.
14. Strand MA, Bratberg J, Eukel H, Hardy M, Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi:10.5888/pcd17.200317
15. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415. doi:10.2146/ajhp150778
16. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. March 2018. Accessed April 3, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
17. County of Los Angeles Public Health. Sexually transmitted diseases in Los Angeles County, 2019. May 2021. Accessed April 3, 2023. http://publichealth.lacounty.gov/dhsp/Reports/STD/2019_LAC_STD_Snapshot_051921Update.pdf
18. Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi:10.1002/jia2.25250
19. Morgan E, Ryan DT, Newcomb ME, Mustanski B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018;22(11):3645-3648. doi:10.1007/s10461-018-2125-2
20. Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. Published 2019 Feb 26. doi:10.1093/ofid/ofz101
21. Ryan K, Lewis J, Sanchez D, Anderson B, Mercier RC. 1293. The next step in PrEP: evaluating outcomes of a pharmacist-run HIV pre-exposure prophylaxis (PrEP) clinic. Open Forum Infect Dis. 2018;5(suppl 1):S395. doi:10.1093/ofid/ofy210.1126
22. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365. doi:10.1093/ofid/ofz365
23. Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS Behav. 2022;26(5):1377-1392. doi:10.1007/s10461-021-03494-4
24. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561. doi:10.1071/SH18084
25. Sachdev G, Kliethermes MA, Vernon V, Leal S, Crabtree G. Current status of prescriptive authority by pharmacists in the United States. J Am Coll Clin Pharm. 2020;3(4):807-817. doi:10.1002/jac5.1245
26. California legislation information: SB-159 HIV: preexposure and postexposure prophylaxis. Accessed April 14, 2023. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159
Dried blood spot test validated for HIV, hep B, and hep C
A test that uses a single drop of dried blood to detect HIV, hepatitis B virus, and HCV has been validated and is now in use in some high-risk settings in Denmark, according to research presented at the annual European Congress of Clinical Microbiology & Infectious Diseases.
Molecular biologist Stephen Nilsson-Møller, MSc, and colleagues at the department of clinical microbiology, Copenhagen University Hospital, developed and validated the test, known as the Dried Blood Spot (DBS), for HIV, HBV, and HCV.
The “test that can detect low viral loads for all three viruses from a single drop of blood, and can be done using existing hospital equipment,” Mr. Nilsson-Møller said in an interview. “Importantly, it does not require venipuncture, but can be done from a drop of dried blood from the finger.”
He highlighted the utility of the new test in more challenging settings. “This method is particularly useful in high-risk settings such as homeless shelters, drug rehabilitation centers, and prisons, where needles might be misused, and it can be difficult to convince people to have the more invasive test.”
“Also, in some places – such as in low- and middle-income settings – there is a distinct risk of ruining blood samples before analysis due to limited refrigeration for transit and storage,” he added. “[Standard] blood samples need to be analyzed within 6 hours when kept at room temperature, while dried blood spots can last for 9 months at room temperature and can be mailed to a laboratory with the right equipment to analyze it.”
Tiny amounts of virus detected
Mr. Nilsson-Møller was tasked with developing a test for use by the university’s department of infectious diseases to screen people in high-risk settings in the capital region of Copenhagen. The work forms part of a PhD project by Jonas Demant at the University of Copenhagen, for which he is screening for HIV, HBV, and HCV in drug rehabilitation centers, prisons, and homeless shelters.
The study is the first to use the Hologic Panther system (a nucleic acid amplification test) combining all three viruses, Mr. Nilsson-Møller pointed out. “A tiny amount of virus can be detected because it is a very sensitive platform using transcription-mediated amplification.”
“If it detects low amounts of virus, it will create many copies very quickly, creating a signal that tells us that the sample is positive,” he explained.
The researchers collected whole blood from a finger prick, dried it out on a protein saver card (filter paper), and cut out a 1.2-cm diameter dry blood spot which was then prepared for analysis.
Twenty blood samples with known amounts of HIV, HBV, and HCV were analyzed via the DBS method (60 in total) and the viruses were detected in all of the samples.
To validate the method, the researchers used plasma with a known viral load, and a series of dilutions were performed to determine the lower limit for positive detection of all three viruses.
“Untreated patients typically have above 1 million IU/mL of viral loads in their plasma, and we found that we can detect much lower levels,” said Mr. Nilsson-Møller. “Ideally, 40 mcL of blood is good, but less should be sufficient if the test is on untreated patients.”
Early testing and treatment reduces morbidity and mortality
Elimination of HBV, HCV, and HIV by 2030 is a global health strategy set by the World Health Organization, but to meet this goal, new approaches for diagnostic testing are required. The DBS test for HIV, HBV, and HCV promises to make a significant contribution toward this goal.
“One in two people currently living with HIV is diagnosed late in the course of their infection, and an even larger proportion of the estimated 6 million Europeans living with chronic hepatitis B or C are not aware that they are infected,” said Anastasia Pharris, PhD, from the European Center for Disease Prevention and Control Principal Expert Infectious Diseases.
“Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HBV, HCV, and HIV in the European Union and European Economic Area,” she pointed out.
Dr. Pharris also highlighted that, while HIV, and often HBV infection, require lifelong treatment, HCV infection is now curable within a few weeks. “To maximize the benefits of individual treatment for all three infections, it is critical to test and diagnose people as soon as possible – in itself a challenge given that these infections can typically be asymptomatic for years.
“Early diagnosis of HBV, HCV, or HIV is vital as it allows people to access treatment, which significantly reduces associated long-term morbidity and mortality.
“In many cases, those most at risk of one of these infections are also more vulnerable to infection with one or both of the other viruses, making the argument for integrated testing even stronger,” she said in an interview.
Mr. Nilsson-Møller and Dr. Pharris reported no relevant financial relationships. Aptima kits for validation were provided by Hologic.
A version of this article first appeared on Medscape.com.
A test that uses a single drop of dried blood to detect HIV, hepatitis B virus, and HCV has been validated and is now in use in some high-risk settings in Denmark, according to research presented at the annual European Congress of Clinical Microbiology & Infectious Diseases.
Molecular biologist Stephen Nilsson-Møller, MSc, and colleagues at the department of clinical microbiology, Copenhagen University Hospital, developed and validated the test, known as the Dried Blood Spot (DBS), for HIV, HBV, and HCV.
The “test that can detect low viral loads for all three viruses from a single drop of blood, and can be done using existing hospital equipment,” Mr. Nilsson-Møller said in an interview. “Importantly, it does not require venipuncture, but can be done from a drop of dried blood from the finger.”
He highlighted the utility of the new test in more challenging settings. “This method is particularly useful in high-risk settings such as homeless shelters, drug rehabilitation centers, and prisons, where needles might be misused, and it can be difficult to convince people to have the more invasive test.”
“Also, in some places – such as in low- and middle-income settings – there is a distinct risk of ruining blood samples before analysis due to limited refrigeration for transit and storage,” he added. “[Standard] blood samples need to be analyzed within 6 hours when kept at room temperature, while dried blood spots can last for 9 months at room temperature and can be mailed to a laboratory with the right equipment to analyze it.”
Tiny amounts of virus detected
Mr. Nilsson-Møller was tasked with developing a test for use by the university’s department of infectious diseases to screen people in high-risk settings in the capital region of Copenhagen. The work forms part of a PhD project by Jonas Demant at the University of Copenhagen, for which he is screening for HIV, HBV, and HCV in drug rehabilitation centers, prisons, and homeless shelters.
The study is the first to use the Hologic Panther system (a nucleic acid amplification test) combining all three viruses, Mr. Nilsson-Møller pointed out. “A tiny amount of virus can be detected because it is a very sensitive platform using transcription-mediated amplification.”
“If it detects low amounts of virus, it will create many copies very quickly, creating a signal that tells us that the sample is positive,” he explained.
The researchers collected whole blood from a finger prick, dried it out on a protein saver card (filter paper), and cut out a 1.2-cm diameter dry blood spot which was then prepared for analysis.
Twenty blood samples with known amounts of HIV, HBV, and HCV were analyzed via the DBS method (60 in total) and the viruses were detected in all of the samples.
To validate the method, the researchers used plasma with a known viral load, and a series of dilutions were performed to determine the lower limit for positive detection of all three viruses.
“Untreated patients typically have above 1 million IU/mL of viral loads in their plasma, and we found that we can detect much lower levels,” said Mr. Nilsson-Møller. “Ideally, 40 mcL of blood is good, but less should be sufficient if the test is on untreated patients.”
Early testing and treatment reduces morbidity and mortality
Elimination of HBV, HCV, and HIV by 2030 is a global health strategy set by the World Health Organization, but to meet this goal, new approaches for diagnostic testing are required. The DBS test for HIV, HBV, and HCV promises to make a significant contribution toward this goal.
“One in two people currently living with HIV is diagnosed late in the course of their infection, and an even larger proportion of the estimated 6 million Europeans living with chronic hepatitis B or C are not aware that they are infected,” said Anastasia Pharris, PhD, from the European Center for Disease Prevention and Control Principal Expert Infectious Diseases.
“Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HBV, HCV, and HIV in the European Union and European Economic Area,” she pointed out.
Dr. Pharris also highlighted that, while HIV, and often HBV infection, require lifelong treatment, HCV infection is now curable within a few weeks. “To maximize the benefits of individual treatment for all three infections, it is critical to test and diagnose people as soon as possible – in itself a challenge given that these infections can typically be asymptomatic for years.
“Early diagnosis of HBV, HCV, or HIV is vital as it allows people to access treatment, which significantly reduces associated long-term morbidity and mortality.
“In many cases, those most at risk of one of these infections are also more vulnerable to infection with one or both of the other viruses, making the argument for integrated testing even stronger,” she said in an interview.
Mr. Nilsson-Møller and Dr. Pharris reported no relevant financial relationships. Aptima kits for validation were provided by Hologic.
A version of this article first appeared on Medscape.com.
A test that uses a single drop of dried blood to detect HIV, hepatitis B virus, and HCV has been validated and is now in use in some high-risk settings in Denmark, according to research presented at the annual European Congress of Clinical Microbiology & Infectious Diseases.
Molecular biologist Stephen Nilsson-Møller, MSc, and colleagues at the department of clinical microbiology, Copenhagen University Hospital, developed and validated the test, known as the Dried Blood Spot (DBS), for HIV, HBV, and HCV.
The “test that can detect low viral loads for all three viruses from a single drop of blood, and can be done using existing hospital equipment,” Mr. Nilsson-Møller said in an interview. “Importantly, it does not require venipuncture, but can be done from a drop of dried blood from the finger.”
He highlighted the utility of the new test in more challenging settings. “This method is particularly useful in high-risk settings such as homeless shelters, drug rehabilitation centers, and prisons, where needles might be misused, and it can be difficult to convince people to have the more invasive test.”
“Also, in some places – such as in low- and middle-income settings – there is a distinct risk of ruining blood samples before analysis due to limited refrigeration for transit and storage,” he added. “[Standard] blood samples need to be analyzed within 6 hours when kept at room temperature, while dried blood spots can last for 9 months at room temperature and can be mailed to a laboratory with the right equipment to analyze it.”
Tiny amounts of virus detected
Mr. Nilsson-Møller was tasked with developing a test for use by the university’s department of infectious diseases to screen people in high-risk settings in the capital region of Copenhagen. The work forms part of a PhD project by Jonas Demant at the University of Copenhagen, for which he is screening for HIV, HBV, and HCV in drug rehabilitation centers, prisons, and homeless shelters.
The study is the first to use the Hologic Panther system (a nucleic acid amplification test) combining all three viruses, Mr. Nilsson-Møller pointed out. “A tiny amount of virus can be detected because it is a very sensitive platform using transcription-mediated amplification.”
“If it detects low amounts of virus, it will create many copies very quickly, creating a signal that tells us that the sample is positive,” he explained.
The researchers collected whole blood from a finger prick, dried it out on a protein saver card (filter paper), and cut out a 1.2-cm diameter dry blood spot which was then prepared for analysis.
Twenty blood samples with known amounts of HIV, HBV, and HCV were analyzed via the DBS method (60 in total) and the viruses were detected in all of the samples.
To validate the method, the researchers used plasma with a known viral load, and a series of dilutions were performed to determine the lower limit for positive detection of all three viruses.
“Untreated patients typically have above 1 million IU/mL of viral loads in their plasma, and we found that we can detect much lower levels,” said Mr. Nilsson-Møller. “Ideally, 40 mcL of blood is good, but less should be sufficient if the test is on untreated patients.”
Early testing and treatment reduces morbidity and mortality
Elimination of HBV, HCV, and HIV by 2030 is a global health strategy set by the World Health Organization, but to meet this goal, new approaches for diagnostic testing are required. The DBS test for HIV, HBV, and HCV promises to make a significant contribution toward this goal.
“One in two people currently living with HIV is diagnosed late in the course of their infection, and an even larger proportion of the estimated 6 million Europeans living with chronic hepatitis B or C are not aware that they are infected,” said Anastasia Pharris, PhD, from the European Center for Disease Prevention and Control Principal Expert Infectious Diseases.
“Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HBV, HCV, and HIV in the European Union and European Economic Area,” she pointed out.
Dr. Pharris also highlighted that, while HIV, and often HBV infection, require lifelong treatment, HCV infection is now curable within a few weeks. “To maximize the benefits of individual treatment for all three infections, it is critical to test and diagnose people as soon as possible – in itself a challenge given that these infections can typically be asymptomatic for years.
“Early diagnosis of HBV, HCV, or HIV is vital as it allows people to access treatment, which significantly reduces associated long-term morbidity and mortality.
“In many cases, those most at risk of one of these infections are also more vulnerable to infection with one or both of the other viruses, making the argument for integrated testing even stronger,” she said in an interview.
Mr. Nilsson-Møller and Dr. Pharris reported no relevant financial relationships. Aptima kits for validation were provided by Hologic.
A version of this article first appeared on Medscape.com.
FROM ECCMID 2023
Perinatal HIV nearly eradicated in U.S.
, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
“This country has been really aggressive about counseling women who are pregnant and getting mothers in care,” Dr. Gandhi said.
The treatment method was discovered more than 30 years ago. Prior to the therapy and ensuing awareness campaigns to prevent transmission, mothers with HIV would typically pass the virus to their child in utero, during delivery, or while breastfeeding.
“There should be zero children born with HIV, given that we’ve had these drugs for so long,” Dr. Ghandi said.
Disparities persist
But challenges remain in some communities, where babies born to Black mothers are disproportionately affected by the disease, the new study found. “Racial and ethnic differences in perinatal HIV diagnoses persisted through the 10-year period,” the report’s authors concluded. “The highest rates of perinatal HIV diagnoses were seen among infants born to Black women.”
Although rates of perinatal HIV declined for babies born to Black mothers over the decade-long study, the diagnosis rate was above the goal of elimination at 3.1 for every 100,000 live births, according to the data.
Meanwhile, transmission rates hovered around 1%-2% for Latinx and Hispanic women and mothers who identified as “other races,” including Native American.
Despite the availability of medication, expectant mothers may face several hurdles to getting the daily treatment they need to prevent transmission to their fetus, according to Jennifer Jao, MD, MPH, a physician of infectious diseases at Lurie Children’s Hospital of Chicago.
They might have trouble securing health insurance or finding transportation to doctor’s appointments, or face other problems like lacking secure housing or food – all factors that prevent them from prioritizing the care.
“All of those things play into the mix,” Dr. Jao said. “We see over and over again that closing the gap means you’ve got to reach the women who are pregnant and who don’t have resources.”
Progress in ‘danger’
Experts said they’re not sure what the impact of the COVID-19 pandemic, accompanied by a recent uptick in sexually transmitted diseases, will be on rates of perinatal HIV. Some women were unable to access prenatal health care during the pandemic because they couldn’t access public transportation or childcare, the U.S. Government Accountability Office said in 2022.
Globally, a decline in rates of HIV and AIDS rates has slowed, prompting the World Health Organization to warn last year that progress on the disease is in danger. Researchers only included HIV rates in the United States through 2019, so the data are outdated, Dr. Gandhi noted.
“All of this put together means we don’t know where we are with perinatal transmission over the last 3 years,” she said.
In an accompanying editorial, coauthors Nahida Chakhtoura, MD, MsGH, and Bill Kapogiannis, MD, both with the National Institutes of Health, urge health care professionals to take an active role in eliminating these racial and ethnic disparities in an effort to – as the title of their editorial proclaims – achieve a “road to zero perinatal HIV transmission” in the United States.
“The more proactive we are in identifying and promptly addressing systematic deficiencies that exacerbate health inequities in cutting-edge research innovations and optimal clinical service provision,” they write, “the less reactive we will need to be when new transmissible infections appear at our doorstep.”
A version of this article first appeared on Medscape.com.
, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
“This country has been really aggressive about counseling women who are pregnant and getting mothers in care,” Dr. Gandhi said.
The treatment method was discovered more than 30 years ago. Prior to the therapy and ensuing awareness campaigns to prevent transmission, mothers with HIV would typically pass the virus to their child in utero, during delivery, or while breastfeeding.
“There should be zero children born with HIV, given that we’ve had these drugs for so long,” Dr. Ghandi said.
Disparities persist
But challenges remain in some communities, where babies born to Black mothers are disproportionately affected by the disease, the new study found. “Racial and ethnic differences in perinatal HIV diagnoses persisted through the 10-year period,” the report’s authors concluded. “The highest rates of perinatal HIV diagnoses were seen among infants born to Black women.”
Although rates of perinatal HIV declined for babies born to Black mothers over the decade-long study, the diagnosis rate was above the goal of elimination at 3.1 for every 100,000 live births, according to the data.
Meanwhile, transmission rates hovered around 1%-2% for Latinx and Hispanic women and mothers who identified as “other races,” including Native American.
Despite the availability of medication, expectant mothers may face several hurdles to getting the daily treatment they need to prevent transmission to their fetus, according to Jennifer Jao, MD, MPH, a physician of infectious diseases at Lurie Children’s Hospital of Chicago.
They might have trouble securing health insurance or finding transportation to doctor’s appointments, or face other problems like lacking secure housing or food – all factors that prevent them from prioritizing the care.
“All of those things play into the mix,” Dr. Jao said. “We see over and over again that closing the gap means you’ve got to reach the women who are pregnant and who don’t have resources.”
Progress in ‘danger’
Experts said they’re not sure what the impact of the COVID-19 pandemic, accompanied by a recent uptick in sexually transmitted diseases, will be on rates of perinatal HIV. Some women were unable to access prenatal health care during the pandemic because they couldn’t access public transportation or childcare, the U.S. Government Accountability Office said in 2022.
Globally, a decline in rates of HIV and AIDS rates has slowed, prompting the World Health Organization to warn last year that progress on the disease is in danger. Researchers only included HIV rates in the United States through 2019, so the data are outdated, Dr. Gandhi noted.
“All of this put together means we don’t know where we are with perinatal transmission over the last 3 years,” she said.
In an accompanying editorial, coauthors Nahida Chakhtoura, MD, MsGH, and Bill Kapogiannis, MD, both with the National Institutes of Health, urge health care professionals to take an active role in eliminating these racial and ethnic disparities in an effort to – as the title of their editorial proclaims – achieve a “road to zero perinatal HIV transmission” in the United States.
“The more proactive we are in identifying and promptly addressing systematic deficiencies that exacerbate health inequities in cutting-edge research innovations and optimal clinical service provision,” they write, “the less reactive we will need to be when new transmissible infections appear at our doorstep.”
A version of this article first appeared on Medscape.com.
, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
“This country has been really aggressive about counseling women who are pregnant and getting mothers in care,” Dr. Gandhi said.
The treatment method was discovered more than 30 years ago. Prior to the therapy and ensuing awareness campaigns to prevent transmission, mothers with HIV would typically pass the virus to their child in utero, during delivery, or while breastfeeding.
“There should be zero children born with HIV, given that we’ve had these drugs for so long,” Dr. Ghandi said.
Disparities persist
But challenges remain in some communities, where babies born to Black mothers are disproportionately affected by the disease, the new study found. “Racial and ethnic differences in perinatal HIV diagnoses persisted through the 10-year period,” the report’s authors concluded. “The highest rates of perinatal HIV diagnoses were seen among infants born to Black women.”
Although rates of perinatal HIV declined for babies born to Black mothers over the decade-long study, the diagnosis rate was above the goal of elimination at 3.1 for every 100,000 live births, according to the data.
Meanwhile, transmission rates hovered around 1%-2% for Latinx and Hispanic women and mothers who identified as “other races,” including Native American.
Despite the availability of medication, expectant mothers may face several hurdles to getting the daily treatment they need to prevent transmission to their fetus, according to Jennifer Jao, MD, MPH, a physician of infectious diseases at Lurie Children’s Hospital of Chicago.
They might have trouble securing health insurance or finding transportation to doctor’s appointments, or face other problems like lacking secure housing or food – all factors that prevent them from prioritizing the care.
“All of those things play into the mix,” Dr. Jao said. “We see over and over again that closing the gap means you’ve got to reach the women who are pregnant and who don’t have resources.”
Progress in ‘danger’
Experts said they’re not sure what the impact of the COVID-19 pandemic, accompanied by a recent uptick in sexually transmitted diseases, will be on rates of perinatal HIV. Some women were unable to access prenatal health care during the pandemic because they couldn’t access public transportation or childcare, the U.S. Government Accountability Office said in 2022.
Globally, a decline in rates of HIV and AIDS rates has slowed, prompting the World Health Organization to warn last year that progress on the disease is in danger. Researchers only included HIV rates in the United States through 2019, so the data are outdated, Dr. Gandhi noted.
“All of this put together means we don’t know where we are with perinatal transmission over the last 3 years,” she said.
In an accompanying editorial, coauthors Nahida Chakhtoura, MD, MsGH, and Bill Kapogiannis, MD, both with the National Institutes of Health, urge health care professionals to take an active role in eliminating these racial and ethnic disparities in an effort to – as the title of their editorial proclaims – achieve a “road to zero perinatal HIV transmission” in the United States.
“The more proactive we are in identifying and promptly addressing systematic deficiencies that exacerbate health inequities in cutting-edge research innovations and optimal clinical service provision,” they write, “the less reactive we will need to be when new transmissible infections appear at our doorstep.”
A version of this article first appeared on Medscape.com.
HIV testing still suboptimal
from the Centers for Disease Control and Prevention. The reasons are complex and could jeopardize goals of ending the AIDS epidemic by 2030.
Patients and doctors alike face system challenges, including stigma, confidentiality concerns, racism, and inequitable access. Yet doctors, public health authorities, and even some patients agree that testing does work: In 2022, 81% of people diagnosed with HIV were linked to care within 30 days. Moreover, many patients are aware of where and how they wish to be tested. So, what would it take to achieve what ostensibly should be the lowest hanging fruit in the HIV care continuum?
“We didn’t look at the reasons for not testing,” Marc Pitasi, MPH, CDC epidemiologist and coauthor of the CDC study said in an interview. But “we found that the majority of people prefer the test in a clinical setting, so that’s a huge important piece of the puzzle,” he said.
The “never-tested” populations (4,334 of 6,072) in the study were predominantly aged 18-29 years (79.7%) and 50 years plus (78.1%). A total of 48% of never-tested adults also indicated that they had engaged in past-year risky behaviors (that is, injection drug use, treated for a sexually transmitted disease, exchanged sex/drugs for money, engaged in condomless anal sex, or had more than four sex partners). However, the difference between never-tested adults who live in EHE (Ending the HIV Epidemic in the U.S.)–designated jurisdictions (comprising 50 areas and 7 U.S. states responsible for more than 50% of new HIV infections) and those residing in non-EHE areas was only about 5 percentage points (69.1% vs. 74.5%, respectively), underscoring the need for broader engagement.
“There’s definitely a lack of testing across the board,” explained Lina Rosengren-Hovee, MD, MPH, MS, an infectious disease epidemiologist at the University of North Carolina at Chapel Hill. “There are all sorts of biases on how we make decisions and how we stratify … and these heuristics that we have in our minds to identify who is at risk and who needs testing,” she said.
“If we just look at the need for HIV testing based on who is at risk, I think that we are always going to fall short.”
Conflicting priorities
Seventeen years have passed since the CDC recommended that HIV testing and screening be offered at least once to all people aged 13-64 years in a routine clinical setting, with an opt-out option and without a separate written consent. People at higher risk (sexually active gay, bisexual, and other men who have sex with men) should be rescreened at least annually.
These recommendations were subsequently reinforced by numerous organizations, including the U.S. Preventive Services Task Force in 2013 and again in 2019, and the American Academy of Pediatrics in 2021.
But Dr. Rosengren-Hovee said that some clinicians remain unaware of the guidelines; for others, they’re usually not top-of-mind because of conflicting priorities.
This is especially true of pediatricians, who, despite data demonstrating that adolescents account for roughly 21% of new HIV diagnoses, rarely recognize or take advantage of HIV-testing opportunities during routine clinical visits.
“Pediatricians want to do the right thing for their patients but at the same time, they want to do the right thing on so many different fronts,” said Sarah Wood, MD, of the University of Pennsylvania, Philadelphia, and attending physician of adolescent medicine at Children’s Hospital of Philadelphia.
Dr. Wood is coauthor of a study published in Implementation Science Communicationsexamining pediatrician perspectives on implementing HIV testing and prevention. Participants identified confidentiality and time constraints as the most important challenges across every step of their workflow, which in turn, influenced perceptions about patients’ perceived risks for acquiring HIV – perceptions that Dr. Wood believes can be overcome.
“We need to really push pediatricians (through guideline-making societies like AAP and USPSTF) that screening should be universal and not linked to sexual activity or pinned to behavior, so the offer of testing is a universal opt-out,” she said. Additionally, “we need to make it easier for pediatricians to order the test,” for example, “through an office rapid test … and a redesigned workflow that moves the conversation away from physicians and nurse practitioners to medical assistants.”
Dr. Wood also pointed out that any effort would require pediatricians and other types of providers to overcome discomfort around sexual health conversations, noting that, while pediatricians are ideally positioned to work with parents to do education around sexual health, training and impetus are needed.
A fractured system
A fractured, often ill-funded U.S. health care system might also be at play according to Scott Harris, MD, MPH, state health officer of the Alabama Department of Public Health in Montgomery, and Association of State and Territorial Health Officials’ Infectious Disease Policy Committee chair.
“There’s a general consensus among everyone in public health that [HIV testing] is an important issue that we’re not addressing as well as we’d like to,” he said.
Dr. Harris acknowledged that, while COVID diverted attention away from HIV, some states have prioritized HIV more than others.
“We don’t have a national public health program; we have a nationwide public health program,” he said. “Everyone’s different and has different responsibilities and authorities ... depending on where their funding streams come from.”
The White House recently announced that it proposed a measure in its Fiscal Year 2023 budget to increase funding for HIV a further $313 million to accelerate efforts to end HIV by 2030, also adding a mandatory program to increase preexposure prophylaxis (PrEP) access. Without congressional approval, the measures are doomed to fail, leaving many states without the proper tools to enhance existing programs, and further painting overworked clinicians into a corner.
For patients, the ramifications are even greater.
“The majority of folks [in the CDC study] that were not tested said that if they were to get tested, they’d prefer to do that within the context of their primary care setting,” said Justin C. Smith, MS, MPH, director of the Campaign to End AIDS, Positive Impact Health Centers; a behavioral scientist at Emory University’s Rollins School of Public Health in Atlanta; and a member of the Presidential Advisory Council on HIV/AIDS.
“When you create a more responsive system that really speaks to the needs that people are expressing, that can provide better outcomes,” Dr. Smith said.
“It’s vital that we create health care and public health interventions that change the dynamics ... and make sure that we’re designing systems with the people that we’re trying to serve at the center.”
Mr. Pitasi, Dr. Rosengren-Hovee, Dr. Wood, Dr. Harris, and Dr. Smith have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the Centers for Disease Control and Prevention. The reasons are complex and could jeopardize goals of ending the AIDS epidemic by 2030.
Patients and doctors alike face system challenges, including stigma, confidentiality concerns, racism, and inequitable access. Yet doctors, public health authorities, and even some patients agree that testing does work: In 2022, 81% of people diagnosed with HIV were linked to care within 30 days. Moreover, many patients are aware of where and how they wish to be tested. So, what would it take to achieve what ostensibly should be the lowest hanging fruit in the HIV care continuum?
“We didn’t look at the reasons for not testing,” Marc Pitasi, MPH, CDC epidemiologist and coauthor of the CDC study said in an interview. But “we found that the majority of people prefer the test in a clinical setting, so that’s a huge important piece of the puzzle,” he said.
The “never-tested” populations (4,334 of 6,072) in the study were predominantly aged 18-29 years (79.7%) and 50 years plus (78.1%). A total of 48% of never-tested adults also indicated that they had engaged in past-year risky behaviors (that is, injection drug use, treated for a sexually transmitted disease, exchanged sex/drugs for money, engaged in condomless anal sex, or had more than four sex partners). However, the difference between never-tested adults who live in EHE (Ending the HIV Epidemic in the U.S.)–designated jurisdictions (comprising 50 areas and 7 U.S. states responsible for more than 50% of new HIV infections) and those residing in non-EHE areas was only about 5 percentage points (69.1% vs. 74.5%, respectively), underscoring the need for broader engagement.
“There’s definitely a lack of testing across the board,” explained Lina Rosengren-Hovee, MD, MPH, MS, an infectious disease epidemiologist at the University of North Carolina at Chapel Hill. “There are all sorts of biases on how we make decisions and how we stratify … and these heuristics that we have in our minds to identify who is at risk and who needs testing,” she said.
“If we just look at the need for HIV testing based on who is at risk, I think that we are always going to fall short.”
Conflicting priorities
Seventeen years have passed since the CDC recommended that HIV testing and screening be offered at least once to all people aged 13-64 years in a routine clinical setting, with an opt-out option and without a separate written consent. People at higher risk (sexually active gay, bisexual, and other men who have sex with men) should be rescreened at least annually.
These recommendations were subsequently reinforced by numerous organizations, including the U.S. Preventive Services Task Force in 2013 and again in 2019, and the American Academy of Pediatrics in 2021.
But Dr. Rosengren-Hovee said that some clinicians remain unaware of the guidelines; for others, they’re usually not top-of-mind because of conflicting priorities.
This is especially true of pediatricians, who, despite data demonstrating that adolescents account for roughly 21% of new HIV diagnoses, rarely recognize or take advantage of HIV-testing opportunities during routine clinical visits.
“Pediatricians want to do the right thing for their patients but at the same time, they want to do the right thing on so many different fronts,” said Sarah Wood, MD, of the University of Pennsylvania, Philadelphia, and attending physician of adolescent medicine at Children’s Hospital of Philadelphia.
Dr. Wood is coauthor of a study published in Implementation Science Communicationsexamining pediatrician perspectives on implementing HIV testing and prevention. Participants identified confidentiality and time constraints as the most important challenges across every step of their workflow, which in turn, influenced perceptions about patients’ perceived risks for acquiring HIV – perceptions that Dr. Wood believes can be overcome.
“We need to really push pediatricians (through guideline-making societies like AAP and USPSTF) that screening should be universal and not linked to sexual activity or pinned to behavior, so the offer of testing is a universal opt-out,” she said. Additionally, “we need to make it easier for pediatricians to order the test,” for example, “through an office rapid test … and a redesigned workflow that moves the conversation away from physicians and nurse practitioners to medical assistants.”
Dr. Wood also pointed out that any effort would require pediatricians and other types of providers to overcome discomfort around sexual health conversations, noting that, while pediatricians are ideally positioned to work with parents to do education around sexual health, training and impetus are needed.
A fractured system
A fractured, often ill-funded U.S. health care system might also be at play according to Scott Harris, MD, MPH, state health officer of the Alabama Department of Public Health in Montgomery, and Association of State and Territorial Health Officials’ Infectious Disease Policy Committee chair.
“There’s a general consensus among everyone in public health that [HIV testing] is an important issue that we’re not addressing as well as we’d like to,” he said.
Dr. Harris acknowledged that, while COVID diverted attention away from HIV, some states have prioritized HIV more than others.
“We don’t have a national public health program; we have a nationwide public health program,” he said. “Everyone’s different and has different responsibilities and authorities ... depending on where their funding streams come from.”
The White House recently announced that it proposed a measure in its Fiscal Year 2023 budget to increase funding for HIV a further $313 million to accelerate efforts to end HIV by 2030, also adding a mandatory program to increase preexposure prophylaxis (PrEP) access. Without congressional approval, the measures are doomed to fail, leaving many states without the proper tools to enhance existing programs, and further painting overworked clinicians into a corner.
For patients, the ramifications are even greater.
“The majority of folks [in the CDC study] that were not tested said that if they were to get tested, they’d prefer to do that within the context of their primary care setting,” said Justin C. Smith, MS, MPH, director of the Campaign to End AIDS, Positive Impact Health Centers; a behavioral scientist at Emory University’s Rollins School of Public Health in Atlanta; and a member of the Presidential Advisory Council on HIV/AIDS.
“When you create a more responsive system that really speaks to the needs that people are expressing, that can provide better outcomes,” Dr. Smith said.
“It’s vital that we create health care and public health interventions that change the dynamics ... and make sure that we’re designing systems with the people that we’re trying to serve at the center.”
Mr. Pitasi, Dr. Rosengren-Hovee, Dr. Wood, Dr. Harris, and Dr. Smith have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the Centers for Disease Control and Prevention. The reasons are complex and could jeopardize goals of ending the AIDS epidemic by 2030.
Patients and doctors alike face system challenges, including stigma, confidentiality concerns, racism, and inequitable access. Yet doctors, public health authorities, and even some patients agree that testing does work: In 2022, 81% of people diagnosed with HIV were linked to care within 30 days. Moreover, many patients are aware of where and how they wish to be tested. So, what would it take to achieve what ostensibly should be the lowest hanging fruit in the HIV care continuum?
“We didn’t look at the reasons for not testing,” Marc Pitasi, MPH, CDC epidemiologist and coauthor of the CDC study said in an interview. But “we found that the majority of people prefer the test in a clinical setting, so that’s a huge important piece of the puzzle,” he said.
The “never-tested” populations (4,334 of 6,072) in the study were predominantly aged 18-29 years (79.7%) and 50 years plus (78.1%). A total of 48% of never-tested adults also indicated that they had engaged in past-year risky behaviors (that is, injection drug use, treated for a sexually transmitted disease, exchanged sex/drugs for money, engaged in condomless anal sex, or had more than four sex partners). However, the difference between never-tested adults who live in EHE (Ending the HIV Epidemic in the U.S.)–designated jurisdictions (comprising 50 areas and 7 U.S. states responsible for more than 50% of new HIV infections) and those residing in non-EHE areas was only about 5 percentage points (69.1% vs. 74.5%, respectively), underscoring the need for broader engagement.
“There’s definitely a lack of testing across the board,” explained Lina Rosengren-Hovee, MD, MPH, MS, an infectious disease epidemiologist at the University of North Carolina at Chapel Hill. “There are all sorts of biases on how we make decisions and how we stratify … and these heuristics that we have in our minds to identify who is at risk and who needs testing,” she said.
“If we just look at the need for HIV testing based on who is at risk, I think that we are always going to fall short.”
Conflicting priorities
Seventeen years have passed since the CDC recommended that HIV testing and screening be offered at least once to all people aged 13-64 years in a routine clinical setting, with an opt-out option and without a separate written consent. People at higher risk (sexually active gay, bisexual, and other men who have sex with men) should be rescreened at least annually.
These recommendations were subsequently reinforced by numerous organizations, including the U.S. Preventive Services Task Force in 2013 and again in 2019, and the American Academy of Pediatrics in 2021.
But Dr. Rosengren-Hovee said that some clinicians remain unaware of the guidelines; for others, they’re usually not top-of-mind because of conflicting priorities.
This is especially true of pediatricians, who, despite data demonstrating that adolescents account for roughly 21% of new HIV diagnoses, rarely recognize or take advantage of HIV-testing opportunities during routine clinical visits.
“Pediatricians want to do the right thing for their patients but at the same time, they want to do the right thing on so many different fronts,” said Sarah Wood, MD, of the University of Pennsylvania, Philadelphia, and attending physician of adolescent medicine at Children’s Hospital of Philadelphia.
Dr. Wood is coauthor of a study published in Implementation Science Communicationsexamining pediatrician perspectives on implementing HIV testing and prevention. Participants identified confidentiality and time constraints as the most important challenges across every step of their workflow, which in turn, influenced perceptions about patients’ perceived risks for acquiring HIV – perceptions that Dr. Wood believes can be overcome.
“We need to really push pediatricians (through guideline-making societies like AAP and USPSTF) that screening should be universal and not linked to sexual activity or pinned to behavior, so the offer of testing is a universal opt-out,” she said. Additionally, “we need to make it easier for pediatricians to order the test,” for example, “through an office rapid test … and a redesigned workflow that moves the conversation away from physicians and nurse practitioners to medical assistants.”
Dr. Wood also pointed out that any effort would require pediatricians and other types of providers to overcome discomfort around sexual health conversations, noting that, while pediatricians are ideally positioned to work with parents to do education around sexual health, training and impetus are needed.
A fractured system
A fractured, often ill-funded U.S. health care system might also be at play according to Scott Harris, MD, MPH, state health officer of the Alabama Department of Public Health in Montgomery, and Association of State and Territorial Health Officials’ Infectious Disease Policy Committee chair.
“There’s a general consensus among everyone in public health that [HIV testing] is an important issue that we’re not addressing as well as we’d like to,” he said.
Dr. Harris acknowledged that, while COVID diverted attention away from HIV, some states have prioritized HIV more than others.
“We don’t have a national public health program; we have a nationwide public health program,” he said. “Everyone’s different and has different responsibilities and authorities ... depending on where their funding streams come from.”
The White House recently announced that it proposed a measure in its Fiscal Year 2023 budget to increase funding for HIV a further $313 million to accelerate efforts to end HIV by 2030, also adding a mandatory program to increase preexposure prophylaxis (PrEP) access. Without congressional approval, the measures are doomed to fail, leaving many states without the proper tools to enhance existing programs, and further painting overworked clinicians into a corner.
For patients, the ramifications are even greater.
“The majority of folks [in the CDC study] that were not tested said that if they were to get tested, they’d prefer to do that within the context of their primary care setting,” said Justin C. Smith, MS, MPH, director of the Campaign to End AIDS, Positive Impact Health Centers; a behavioral scientist at Emory University’s Rollins School of Public Health in Atlanta; and a member of the Presidential Advisory Council on HIV/AIDS.
“When you create a more responsive system that really speaks to the needs that people are expressing, that can provide better outcomes,” Dr. Smith said.
“It’s vital that we create health care and public health interventions that change the dynamics ... and make sure that we’re designing systems with the people that we’re trying to serve at the center.”
Mr. Pitasi, Dr. Rosengren-Hovee, Dr. Wood, Dr. Harris, and Dr. Smith have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-acting ART effective without viral suppression
as is typically required before initiation, results from two new studies suggest.
Meanwhile, a third study shows early promise of a long-acting ART regimen that could require injections only twice yearly.
“Instead of having the burden of taking a pill every day, patients with HIV can now have these choices of being able to forget about their treatment for up to 2 months at a time,” Moti N. Ramgopal, MD, first author of one of the three studies, said in a press conference at the Conference on Retroviruses and Opportunistic Infections. “My patients tell me that it’s game-changing for them.”
First head-to-head comparison
The injectable intramuscular combination of cabotegravir and rilpivirine, administered once every 1 or 2 months, is the first and only long-acting ART to be approved by the Food and Drug Administration for people with HIV. At the meeting, Dr. Ramgopal, from the Midway Immunology and Research Center in Fort Pierce, Fla., reported the results from the first head-to-head study comparing the regimen with the standard daily oral regimen of bictegravir/emtricitabine/tenofovir alafenamide (B/FTC/TAF).
For the phase 3b SOLAR trial, adults with HIV who were already virologically suppressed and treated with daily B/FTC/TAF were randomly assigned to remain on the oral regimen (n = 223) or switch to the long-acting injections every 2 months, either with an oral lead-in (n = 173) or without a lead-in (n = 274).
The results after 12 months showed noninferiority between the groups, with 90% of the long-acting ART group and 93% in the oral daily pill group maintaining viral suppression, defined as plasma HIV-1 RNA less than 50 copies/mL.
Two patients (0.4%) receiving the long-acting ART in a modified-intention to treat and 3 (0.6%) in the intention-to-treat populations had confirmed virologic failure, and rates of adverse events leading to withdrawal were 6% with the long-acting ART group versus 1% with the oral treatment group.
As many as 90% of subjects reported preferring the long-acting ART over their previous oral therapy at the end of the study, and those in the long-acting group also reported significantly greater improvements in quality of life on the HIV Treatment Satisfaction Questionnaire status version compared with the oral therapy group at the end of the study (P < .001).
Prior to the randomization, as many as 47% of patients on the oral regimen reported “always” or “often” having psychosocial challenges with the daily therapy, including worries of having their HIV status revealed and forgetting to take the medication.
“These data demonstrate that [the long-acting regimen] addresses important unmet needs for people with HIV who are virally suppressed on oral daily HIV therapy, while improving the quality of life,” said Dr. Ramgopal.
Suppression at onset not necessarily required
For all of its benefits, a key caveat of the cabotegravir and rilpivirine long-acting regimen is that it is approved only for patients who have already achieved viral suppression and are currently on oral ART, meaning some of the people most in need, including those with unstable housing, mental illness, or substance abuse disorders, may be excluded.
To evaluate the therapy’s efficacy among those patient types, Monica Gandhi, MD, MPH, professor of medicine and associate division chief at the University of California, San Francisco, enrolled 133 participants between June 2021 and November 2022 at the Ward 86 HIV Clinic, a safety-net clinic in San Francisco, to initiate long-acting ART. Participants included 57 patients (43%) with untreated or unsuppressed HIV, and 76 who were virally suppressed on oral ART.
Of the whole study group, 66% reported unstable housing, 8% reported experiencing homelessness, 38% reported having a mental illness, and 33% reported substance use.
Although all of the subjects (100%) who started with viral suppression remained suppressed over the study’s 26-week follow-up, the rate of viral suppression at the study’s end was nearly as high – 55 of 57 subjects (96.5%) – among those who started long-acting ART without having viral suppression.
Of note, the overall rate of study participants who did not achieve or maintain viral suppression (1.5%) was consistent with rates reported in clinical trials of long-acting ART in people with HIV who had previously achieved viral suppression on daily oral ART.
“Our patient population does not look like the patient population that got enrolled in the clinical trials to determine the approval criteria for long-acting ART,” Dr. Gandhi said in presenting the findings.
“If 10% of the population carries 90% of the HIV virus – which we see in modeling – then we need innovations for this population if we want to end the HIV epidemic,” she added.
“We tried long-acting ART in our diverse, urban, low-income population and we saw very high virologic suppression rates equal to those that were seen in the clinical trials,” Dr. Ghandi reported. “This shows that long-acting ART, used creatively and used boldly, could really make a dent in the [efforts] to end the HIV epidemic movement.”
Commenting on the study in a press statement, Nora Volkow, MD, director of the National Institute on Drug Abuse, said “Dr. Gandhi and her team have made state-of-the-art HIV treatment finally available to people with unique challenges, like those who use drugs, and have found success.”
“This is the sweet spot for addressing HIV – thinking outside the box to deliver care in a way that meets people’s needs, even when that means it happens outside the clinic walls, by phone, or on neighborhood streets,” she said. “This can be done, but it requires creativity and resolve.”
Twice-yearly dosing option?
Looking ahead, an even more intriguing scenario of a long-acting ART requiring injections only once every 6 months may be getting closer to fruition. Researchers at CROI 2023 reported early but promising safety and efficacy results of an innovative combination of the first-in-class HIV-1 capsid inhibitor lenacapavir with teropavimab and zinlirvimab, two broadly neutralizing antibodies (bNAbs).
To achieve the goal of the longer-acting therapy, Joseph J. Eron, MD, of the University of North Carolina at Chapel Hill, and colleagues modified both antibodies to extend their half-lives and allow less-frequent dosing.
For the phase 1b trial, 20 adult patients with virologically suppressed HIV for at least 18 months were randomly assigned to one of two doses of the ART, both groups receiving lenacapavir at 927 mg subcutaneous after oral loading, plus teropavimab (30 mg/kg IV) and zinlirvimab at either 10 mg/kg or 30 mg/kg.
Patients had to have a CD4 count greater than 500 and CD4 nadir greater than 350, and importantly, patients had to demonstrate sensitivity on DNA phenotyping to both bNAbs at baseline.
After 26 weeks, 18 of the 20 participants (90%) maintained a viral suppression of HIV-1 RNA less than 50 copies/mL.
Of the remaining two patients, one in the 10–mg/kg zinlirvimab group had a confirmed HIV RNA of 50 c/mL (155 copies/mL, confirmed 524 copies/mL) at week 16 and was able to be resuppressed with reinitiation of baseline ART, and one participant in the zinlirvimab 30 mg/kg group withdrew consent at week 12, with viral suppression, and chose to go back on oral therapy
The safety profile looked favorable, with no serious adverse events and two patients with grade 3 AEs, including one experiencing an injection site cellulitis and one with injection site erythema.
Dr. Eron noted that “bNAb sensitivity is an important issue and a limitation for broad use, [because] only about 50% of people with HIV in the U.S. would be sensitive to both antibodies.”
However, “we are doing a pilot of only 10 participants looking to see if it works with sensitivity to a single antibody, which would increase [applicability] to about 90% of people with HIV,” he said in an interview.
At a press conference, Dr. Eron commented on how far HIV treatment has come, from the early days of patients having to wake up every 4 hours to take their medication, then to having to take 15-20 pills a day, to the current option of long-acting ART every other month, and now the potential of just a twice-yearly treatment.
“This is a very preliminary proof-of-concept study and not a very large study, but I think it’s incredibly important,” he said.
The SOLAR study was funded by ViiV Healthcare. Dr. Ramgopal has received speaking and/or consulting fees from AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Dr. Eron’s study was funded by Gilead Sciences.
A version of this article first appeared on Medscape.com.
as is typically required before initiation, results from two new studies suggest.
Meanwhile, a third study shows early promise of a long-acting ART regimen that could require injections only twice yearly.
“Instead of having the burden of taking a pill every day, patients with HIV can now have these choices of being able to forget about their treatment for up to 2 months at a time,” Moti N. Ramgopal, MD, first author of one of the three studies, said in a press conference at the Conference on Retroviruses and Opportunistic Infections. “My patients tell me that it’s game-changing for them.”
First head-to-head comparison
The injectable intramuscular combination of cabotegravir and rilpivirine, administered once every 1 or 2 months, is the first and only long-acting ART to be approved by the Food and Drug Administration for people with HIV. At the meeting, Dr. Ramgopal, from the Midway Immunology and Research Center in Fort Pierce, Fla., reported the results from the first head-to-head study comparing the regimen with the standard daily oral regimen of bictegravir/emtricitabine/tenofovir alafenamide (B/FTC/TAF).
For the phase 3b SOLAR trial, adults with HIV who were already virologically suppressed and treated with daily B/FTC/TAF were randomly assigned to remain on the oral regimen (n = 223) or switch to the long-acting injections every 2 months, either with an oral lead-in (n = 173) or without a lead-in (n = 274).
The results after 12 months showed noninferiority between the groups, with 90% of the long-acting ART group and 93% in the oral daily pill group maintaining viral suppression, defined as plasma HIV-1 RNA less than 50 copies/mL.
Two patients (0.4%) receiving the long-acting ART in a modified-intention to treat and 3 (0.6%) in the intention-to-treat populations had confirmed virologic failure, and rates of adverse events leading to withdrawal were 6% with the long-acting ART group versus 1% with the oral treatment group.
As many as 90% of subjects reported preferring the long-acting ART over their previous oral therapy at the end of the study, and those in the long-acting group also reported significantly greater improvements in quality of life on the HIV Treatment Satisfaction Questionnaire status version compared with the oral therapy group at the end of the study (P < .001).
Prior to the randomization, as many as 47% of patients on the oral regimen reported “always” or “often” having psychosocial challenges with the daily therapy, including worries of having their HIV status revealed and forgetting to take the medication.
“These data demonstrate that [the long-acting regimen] addresses important unmet needs for people with HIV who are virally suppressed on oral daily HIV therapy, while improving the quality of life,” said Dr. Ramgopal.
Suppression at onset not necessarily required
For all of its benefits, a key caveat of the cabotegravir and rilpivirine long-acting regimen is that it is approved only for patients who have already achieved viral suppression and are currently on oral ART, meaning some of the people most in need, including those with unstable housing, mental illness, or substance abuse disorders, may be excluded.
To evaluate the therapy’s efficacy among those patient types, Monica Gandhi, MD, MPH, professor of medicine and associate division chief at the University of California, San Francisco, enrolled 133 participants between June 2021 and November 2022 at the Ward 86 HIV Clinic, a safety-net clinic in San Francisco, to initiate long-acting ART. Participants included 57 patients (43%) with untreated or unsuppressed HIV, and 76 who were virally suppressed on oral ART.
Of the whole study group, 66% reported unstable housing, 8% reported experiencing homelessness, 38% reported having a mental illness, and 33% reported substance use.
Although all of the subjects (100%) who started with viral suppression remained suppressed over the study’s 26-week follow-up, the rate of viral suppression at the study’s end was nearly as high – 55 of 57 subjects (96.5%) – among those who started long-acting ART without having viral suppression.
Of note, the overall rate of study participants who did not achieve or maintain viral suppression (1.5%) was consistent with rates reported in clinical trials of long-acting ART in people with HIV who had previously achieved viral suppression on daily oral ART.
“Our patient population does not look like the patient population that got enrolled in the clinical trials to determine the approval criteria for long-acting ART,” Dr. Gandhi said in presenting the findings.
“If 10% of the population carries 90% of the HIV virus – which we see in modeling – then we need innovations for this population if we want to end the HIV epidemic,” she added.
“We tried long-acting ART in our diverse, urban, low-income population and we saw very high virologic suppression rates equal to those that were seen in the clinical trials,” Dr. Ghandi reported. “This shows that long-acting ART, used creatively and used boldly, could really make a dent in the [efforts] to end the HIV epidemic movement.”
Commenting on the study in a press statement, Nora Volkow, MD, director of the National Institute on Drug Abuse, said “Dr. Gandhi and her team have made state-of-the-art HIV treatment finally available to people with unique challenges, like those who use drugs, and have found success.”
“This is the sweet spot for addressing HIV – thinking outside the box to deliver care in a way that meets people’s needs, even when that means it happens outside the clinic walls, by phone, or on neighborhood streets,” she said. “This can be done, but it requires creativity and resolve.”
Twice-yearly dosing option?
Looking ahead, an even more intriguing scenario of a long-acting ART requiring injections only once every 6 months may be getting closer to fruition. Researchers at CROI 2023 reported early but promising safety and efficacy results of an innovative combination of the first-in-class HIV-1 capsid inhibitor lenacapavir with teropavimab and zinlirvimab, two broadly neutralizing antibodies (bNAbs).
To achieve the goal of the longer-acting therapy, Joseph J. Eron, MD, of the University of North Carolina at Chapel Hill, and colleagues modified both antibodies to extend their half-lives and allow less-frequent dosing.
For the phase 1b trial, 20 adult patients with virologically suppressed HIV for at least 18 months were randomly assigned to one of two doses of the ART, both groups receiving lenacapavir at 927 mg subcutaneous after oral loading, plus teropavimab (30 mg/kg IV) and zinlirvimab at either 10 mg/kg or 30 mg/kg.
Patients had to have a CD4 count greater than 500 and CD4 nadir greater than 350, and importantly, patients had to demonstrate sensitivity on DNA phenotyping to both bNAbs at baseline.
After 26 weeks, 18 of the 20 participants (90%) maintained a viral suppression of HIV-1 RNA less than 50 copies/mL.
Of the remaining two patients, one in the 10–mg/kg zinlirvimab group had a confirmed HIV RNA of 50 c/mL (155 copies/mL, confirmed 524 copies/mL) at week 16 and was able to be resuppressed with reinitiation of baseline ART, and one participant in the zinlirvimab 30 mg/kg group withdrew consent at week 12, with viral suppression, and chose to go back on oral therapy
The safety profile looked favorable, with no serious adverse events and two patients with grade 3 AEs, including one experiencing an injection site cellulitis and one with injection site erythema.
Dr. Eron noted that “bNAb sensitivity is an important issue and a limitation for broad use, [because] only about 50% of people with HIV in the U.S. would be sensitive to both antibodies.”
However, “we are doing a pilot of only 10 participants looking to see if it works with sensitivity to a single antibody, which would increase [applicability] to about 90% of people with HIV,” he said in an interview.
At a press conference, Dr. Eron commented on how far HIV treatment has come, from the early days of patients having to wake up every 4 hours to take their medication, then to having to take 15-20 pills a day, to the current option of long-acting ART every other month, and now the potential of just a twice-yearly treatment.
“This is a very preliminary proof-of-concept study and not a very large study, but I think it’s incredibly important,” he said.
The SOLAR study was funded by ViiV Healthcare. Dr. Ramgopal has received speaking and/or consulting fees from AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Dr. Eron’s study was funded by Gilead Sciences.
A version of this article first appeared on Medscape.com.
as is typically required before initiation, results from two new studies suggest.
Meanwhile, a third study shows early promise of a long-acting ART regimen that could require injections only twice yearly.
“Instead of having the burden of taking a pill every day, patients with HIV can now have these choices of being able to forget about their treatment for up to 2 months at a time,” Moti N. Ramgopal, MD, first author of one of the three studies, said in a press conference at the Conference on Retroviruses and Opportunistic Infections. “My patients tell me that it’s game-changing for them.”
First head-to-head comparison
The injectable intramuscular combination of cabotegravir and rilpivirine, administered once every 1 or 2 months, is the first and only long-acting ART to be approved by the Food and Drug Administration for people with HIV. At the meeting, Dr. Ramgopal, from the Midway Immunology and Research Center in Fort Pierce, Fla., reported the results from the first head-to-head study comparing the regimen with the standard daily oral regimen of bictegravir/emtricitabine/tenofovir alafenamide (B/FTC/TAF).
For the phase 3b SOLAR trial, adults with HIV who were already virologically suppressed and treated with daily B/FTC/TAF were randomly assigned to remain on the oral regimen (n = 223) or switch to the long-acting injections every 2 months, either with an oral lead-in (n = 173) or without a lead-in (n = 274).
The results after 12 months showed noninferiority between the groups, with 90% of the long-acting ART group and 93% in the oral daily pill group maintaining viral suppression, defined as plasma HIV-1 RNA less than 50 copies/mL.
Two patients (0.4%) receiving the long-acting ART in a modified-intention to treat and 3 (0.6%) in the intention-to-treat populations had confirmed virologic failure, and rates of adverse events leading to withdrawal were 6% with the long-acting ART group versus 1% with the oral treatment group.
As many as 90% of subjects reported preferring the long-acting ART over their previous oral therapy at the end of the study, and those in the long-acting group also reported significantly greater improvements in quality of life on the HIV Treatment Satisfaction Questionnaire status version compared with the oral therapy group at the end of the study (P < .001).
Prior to the randomization, as many as 47% of patients on the oral regimen reported “always” or “often” having psychosocial challenges with the daily therapy, including worries of having their HIV status revealed and forgetting to take the medication.
“These data demonstrate that [the long-acting regimen] addresses important unmet needs for people with HIV who are virally suppressed on oral daily HIV therapy, while improving the quality of life,” said Dr. Ramgopal.
Suppression at onset not necessarily required
For all of its benefits, a key caveat of the cabotegravir and rilpivirine long-acting regimen is that it is approved only for patients who have already achieved viral suppression and are currently on oral ART, meaning some of the people most in need, including those with unstable housing, mental illness, or substance abuse disorders, may be excluded.
To evaluate the therapy’s efficacy among those patient types, Monica Gandhi, MD, MPH, professor of medicine and associate division chief at the University of California, San Francisco, enrolled 133 participants between June 2021 and November 2022 at the Ward 86 HIV Clinic, a safety-net clinic in San Francisco, to initiate long-acting ART. Participants included 57 patients (43%) with untreated or unsuppressed HIV, and 76 who were virally suppressed on oral ART.
Of the whole study group, 66% reported unstable housing, 8% reported experiencing homelessness, 38% reported having a mental illness, and 33% reported substance use.
Although all of the subjects (100%) who started with viral suppression remained suppressed over the study’s 26-week follow-up, the rate of viral suppression at the study’s end was nearly as high – 55 of 57 subjects (96.5%) – among those who started long-acting ART without having viral suppression.
Of note, the overall rate of study participants who did not achieve or maintain viral suppression (1.5%) was consistent with rates reported in clinical trials of long-acting ART in people with HIV who had previously achieved viral suppression on daily oral ART.
“Our patient population does not look like the patient population that got enrolled in the clinical trials to determine the approval criteria for long-acting ART,” Dr. Gandhi said in presenting the findings.
“If 10% of the population carries 90% of the HIV virus – which we see in modeling – then we need innovations for this population if we want to end the HIV epidemic,” she added.
“We tried long-acting ART in our diverse, urban, low-income population and we saw very high virologic suppression rates equal to those that were seen in the clinical trials,” Dr. Ghandi reported. “This shows that long-acting ART, used creatively and used boldly, could really make a dent in the [efforts] to end the HIV epidemic movement.”
Commenting on the study in a press statement, Nora Volkow, MD, director of the National Institute on Drug Abuse, said “Dr. Gandhi and her team have made state-of-the-art HIV treatment finally available to people with unique challenges, like those who use drugs, and have found success.”
“This is the sweet spot for addressing HIV – thinking outside the box to deliver care in a way that meets people’s needs, even when that means it happens outside the clinic walls, by phone, or on neighborhood streets,” she said. “This can be done, but it requires creativity and resolve.”
Twice-yearly dosing option?
Looking ahead, an even more intriguing scenario of a long-acting ART requiring injections only once every 6 months may be getting closer to fruition. Researchers at CROI 2023 reported early but promising safety and efficacy results of an innovative combination of the first-in-class HIV-1 capsid inhibitor lenacapavir with teropavimab and zinlirvimab, two broadly neutralizing antibodies (bNAbs).
To achieve the goal of the longer-acting therapy, Joseph J. Eron, MD, of the University of North Carolina at Chapel Hill, and colleagues modified both antibodies to extend their half-lives and allow less-frequent dosing.
For the phase 1b trial, 20 adult patients with virologically suppressed HIV for at least 18 months were randomly assigned to one of two doses of the ART, both groups receiving lenacapavir at 927 mg subcutaneous after oral loading, plus teropavimab (30 mg/kg IV) and zinlirvimab at either 10 mg/kg or 30 mg/kg.
Patients had to have a CD4 count greater than 500 and CD4 nadir greater than 350, and importantly, patients had to demonstrate sensitivity on DNA phenotyping to both bNAbs at baseline.
After 26 weeks, 18 of the 20 participants (90%) maintained a viral suppression of HIV-1 RNA less than 50 copies/mL.
Of the remaining two patients, one in the 10–mg/kg zinlirvimab group had a confirmed HIV RNA of 50 c/mL (155 copies/mL, confirmed 524 copies/mL) at week 16 and was able to be resuppressed with reinitiation of baseline ART, and one participant in the zinlirvimab 30 mg/kg group withdrew consent at week 12, with viral suppression, and chose to go back on oral therapy
The safety profile looked favorable, with no serious adverse events and two patients with grade 3 AEs, including one experiencing an injection site cellulitis and one with injection site erythema.
Dr. Eron noted that “bNAb sensitivity is an important issue and a limitation for broad use, [because] only about 50% of people with HIV in the U.S. would be sensitive to both antibodies.”
However, “we are doing a pilot of only 10 participants looking to see if it works with sensitivity to a single antibody, which would increase [applicability] to about 90% of people with HIV,” he said in an interview.
At a press conference, Dr. Eron commented on how far HIV treatment has come, from the early days of patients having to wake up every 4 hours to take their medication, then to having to take 15-20 pills a day, to the current option of long-acting ART every other month, and now the potential of just a twice-yearly treatment.
“This is a very preliminary proof-of-concept study and not a very large study, but I think it’s incredibly important,” he said.
The SOLAR study was funded by ViiV Healthcare. Dr. Ramgopal has received speaking and/or consulting fees from AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Dr. Eron’s study was funded by Gilead Sciences.
A version of this article first appeared on Medscape.com.
FROM CROI 2023
Doxy PEP does not lower risk of STIs in cisgender women
The benefits of doxycycline postexposure prophylaxis (Doxy PEP) in preventing the transmission of sexually transmitted infections (STIs) in men and transgender women do not appear to extend to cisgender women, who have disproportionately high rates of infection in many regions.
“This was the first trial to evaluate doxycycline PEP for cisgender women,” said first author Jenell Stewart, DO, of the University of Minnesota, Minneapolis, in discussing the findings at a press conference at the Conference on Retroviruses & Opportunistic Infections.
“Unfortunately, our primary outcome was not statistically significant – we did not see a reduction in STIs among cisgender women, which is in stark contrast to [reported effects] among cisgender men and transgender women,” she said.
The findings are from a study of 449 nonpregnant cisgender women (mean age, 24 years) in Kenya who had been taking daily oral HIV preexposure prophylaxis (PrEP) for a median of about 7 months.
The women were randomly assigned to receive either Doxy PEP 200 mg, to be taken within 72 hours of sex (n = 224), or standard care, which included quarterly screening and treatment of STIs (n = 225).
Of the women, 36.7% reported transactional sex at enrollment; their baseline prevalence of STIs was 17.9%, including 14.1% with chlamydia, 3.8% gonorrhea, and 0.4% syphilis. There were no differences between the study groups.
In surveys, 78% of the women reported adherence to the use of Doxy PEP; they took the prophylaxis at least as many days as they had sex.
Nevertheless, there was no significant difference in the incidence of STIs, reported over 1 year, at quarterly visits that included genital STI testing, between groups, with 50 patients in the Doxy PEP group and 59 in the standard screening group developing STIs (relative risk, 0.88; P = .51).
Of the infections, 85 were chlamydia, including 35 in the Doxy PEP group and 50 with standard of care, while 31 were gonorrhea, including 19 in the Doxy PEP group and 12 with standard of care; 8 had both infections, and there was 1 syphilis infection.
The results were consistent across subanalyses of patients grouped according to STI, who became pregnant (n = 80), or sorted by other factors including age, contraceptive use, transactional sex, and STI at baseline.
None of the women developed HIV, and there were no serious events associated with the Doxy PEP treatment.
Cisgender women bear ‘highest burden’ of STIs
The findings are disappointing in light of the higher rates of STIs among cisgender women, with the Centers for Disease Control and Prevention reporting that women also disproportionately bear the long-term consequences of STIs.
“For example, each year, untreated sexually transmitted diseases cause infertility in at least 20,000 women in the United States, and a pregnant woman is highly likely to pass syphilis unto her unborn baby if left untested or untreated,” the CDC reports.
The STI rates are particularly high for women taking HIV PrEP in regions like East Africa, where rates of STIs among cisgender women in many cases are higher than rates for men taking PrEP in high income countries, Dr. Stewart said.
Previous studies of Doxy PEP in men and transgender women taking HIV PrEP, including new research presented at CROI, have shown highly encouraging reductions in STIs, at rates of up to approximately 80% for chlamydia and syphilis.
Adherence, anatomy, resistance
The key theories for the lack of a prevention of infections in cisgender women surround the issues of resistances, as well as anatomy and adherence, said Dr. Stewart.
In terms of bacterial resistances, while initial testing in a limited number of samples the study found no evidence of markers of resistance for chlamydia, all of the gonorrhea samples did show tetracycline-resistant N gonorrhea at baseline and follow-up in both groups.
Regarding anatomic differences, doxycycline may not prevent STIs in endocervical tissue among cisgender women, Dr. Stewart noted. Women are known to be at higher risk of infection because the lining of the vagina is thinner than the skin of the penis, allowing for easier penetration of bacteria and viruses.
The study was designed to optimize adherence to Doxy PEP. Measures included monitoring with weekly text message surveys, in which the women reported a high rate of adherence.
The overall retention rate in the study was high; as many as 97% of the quarterly follow-up visits were completed, including 95% in the Doxy PEP group and 98% of the standard care group. The response rate for the weekly surveys was 81%.
Of note, women reported the use of the treatment to be “imperfect,” suggesting social problems, such as biases toward the use of the prophylaxis.
The results underscore the need for ongoing efforts to make sure no groups of patients are left behind as interventions advance, Dr. Stewart said.
“The burden of STIs on cisgender women is large and growing,” she concluded. “STI prevention interventions are needed.”
Commenting on the study, Renee A. Heffron, PhD, MPH, said the findings “are somewhat surprising because results from trials in other populations have been positive.
“But cisgender women are exposed through the cervix, and this tissue is different from rectal or urethral tissue,” Dr. Heffron, a professor at the department of medicine and director of the Center for AIDS Research at the University of Alabama, Birmingham, told this news organization.
Further findings from the research should help shed light on key issues of adherence and drug concentration levels in cervical tissue, she added.
“For cisgender women, these data are the first and the beginning of understanding whether this is a viable strategy,” Dr. Heffron said.
“We have more to learn to better understand the results from the trial main outcomes, and if there are tweaks to this strategy that would improve efficacy.”
The authors and Dr. Heffron have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The benefits of doxycycline postexposure prophylaxis (Doxy PEP) in preventing the transmission of sexually transmitted infections (STIs) in men and transgender women do not appear to extend to cisgender women, who have disproportionately high rates of infection in many regions.
“This was the first trial to evaluate doxycycline PEP for cisgender women,” said first author Jenell Stewart, DO, of the University of Minnesota, Minneapolis, in discussing the findings at a press conference at the Conference on Retroviruses & Opportunistic Infections.
“Unfortunately, our primary outcome was not statistically significant – we did not see a reduction in STIs among cisgender women, which is in stark contrast to [reported effects] among cisgender men and transgender women,” she said.
The findings are from a study of 449 nonpregnant cisgender women (mean age, 24 years) in Kenya who had been taking daily oral HIV preexposure prophylaxis (PrEP) for a median of about 7 months.
The women were randomly assigned to receive either Doxy PEP 200 mg, to be taken within 72 hours of sex (n = 224), or standard care, which included quarterly screening and treatment of STIs (n = 225).
Of the women, 36.7% reported transactional sex at enrollment; their baseline prevalence of STIs was 17.9%, including 14.1% with chlamydia, 3.8% gonorrhea, and 0.4% syphilis. There were no differences between the study groups.
In surveys, 78% of the women reported adherence to the use of Doxy PEP; they took the prophylaxis at least as many days as they had sex.
Nevertheless, there was no significant difference in the incidence of STIs, reported over 1 year, at quarterly visits that included genital STI testing, between groups, with 50 patients in the Doxy PEP group and 59 in the standard screening group developing STIs (relative risk, 0.88; P = .51).
Of the infections, 85 were chlamydia, including 35 in the Doxy PEP group and 50 with standard of care, while 31 were gonorrhea, including 19 in the Doxy PEP group and 12 with standard of care; 8 had both infections, and there was 1 syphilis infection.
The results were consistent across subanalyses of patients grouped according to STI, who became pregnant (n = 80), or sorted by other factors including age, contraceptive use, transactional sex, and STI at baseline.
None of the women developed HIV, and there were no serious events associated with the Doxy PEP treatment.
Cisgender women bear ‘highest burden’ of STIs
The findings are disappointing in light of the higher rates of STIs among cisgender women, with the Centers for Disease Control and Prevention reporting that women also disproportionately bear the long-term consequences of STIs.
“For example, each year, untreated sexually transmitted diseases cause infertility in at least 20,000 women in the United States, and a pregnant woman is highly likely to pass syphilis unto her unborn baby if left untested or untreated,” the CDC reports.
The STI rates are particularly high for women taking HIV PrEP in regions like East Africa, where rates of STIs among cisgender women in many cases are higher than rates for men taking PrEP in high income countries, Dr. Stewart said.
Previous studies of Doxy PEP in men and transgender women taking HIV PrEP, including new research presented at CROI, have shown highly encouraging reductions in STIs, at rates of up to approximately 80% for chlamydia and syphilis.
Adherence, anatomy, resistance
The key theories for the lack of a prevention of infections in cisgender women surround the issues of resistances, as well as anatomy and adherence, said Dr. Stewart.
In terms of bacterial resistances, while initial testing in a limited number of samples the study found no evidence of markers of resistance for chlamydia, all of the gonorrhea samples did show tetracycline-resistant N gonorrhea at baseline and follow-up in both groups.
Regarding anatomic differences, doxycycline may not prevent STIs in endocervical tissue among cisgender women, Dr. Stewart noted. Women are known to be at higher risk of infection because the lining of the vagina is thinner than the skin of the penis, allowing for easier penetration of bacteria and viruses.
The study was designed to optimize adherence to Doxy PEP. Measures included monitoring with weekly text message surveys, in which the women reported a high rate of adherence.
The overall retention rate in the study was high; as many as 97% of the quarterly follow-up visits were completed, including 95% in the Doxy PEP group and 98% of the standard care group. The response rate for the weekly surveys was 81%.
Of note, women reported the use of the treatment to be “imperfect,” suggesting social problems, such as biases toward the use of the prophylaxis.
The results underscore the need for ongoing efforts to make sure no groups of patients are left behind as interventions advance, Dr. Stewart said.
“The burden of STIs on cisgender women is large and growing,” she concluded. “STI prevention interventions are needed.”
Commenting on the study, Renee A. Heffron, PhD, MPH, said the findings “are somewhat surprising because results from trials in other populations have been positive.
“But cisgender women are exposed through the cervix, and this tissue is different from rectal or urethral tissue,” Dr. Heffron, a professor at the department of medicine and director of the Center for AIDS Research at the University of Alabama, Birmingham, told this news organization.
Further findings from the research should help shed light on key issues of adherence and drug concentration levels in cervical tissue, she added.
“For cisgender women, these data are the first and the beginning of understanding whether this is a viable strategy,” Dr. Heffron said.
“We have more to learn to better understand the results from the trial main outcomes, and if there are tweaks to this strategy that would improve efficacy.”
The authors and Dr. Heffron have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The benefits of doxycycline postexposure prophylaxis (Doxy PEP) in preventing the transmission of sexually transmitted infections (STIs) in men and transgender women do not appear to extend to cisgender women, who have disproportionately high rates of infection in many regions.
“This was the first trial to evaluate doxycycline PEP for cisgender women,” said first author Jenell Stewart, DO, of the University of Minnesota, Minneapolis, in discussing the findings at a press conference at the Conference on Retroviruses & Opportunistic Infections.
“Unfortunately, our primary outcome was not statistically significant – we did not see a reduction in STIs among cisgender women, which is in stark contrast to [reported effects] among cisgender men and transgender women,” she said.
The findings are from a study of 449 nonpregnant cisgender women (mean age, 24 years) in Kenya who had been taking daily oral HIV preexposure prophylaxis (PrEP) for a median of about 7 months.
The women were randomly assigned to receive either Doxy PEP 200 mg, to be taken within 72 hours of sex (n = 224), or standard care, which included quarterly screening and treatment of STIs (n = 225).
Of the women, 36.7% reported transactional sex at enrollment; their baseline prevalence of STIs was 17.9%, including 14.1% with chlamydia, 3.8% gonorrhea, and 0.4% syphilis. There were no differences between the study groups.
In surveys, 78% of the women reported adherence to the use of Doxy PEP; they took the prophylaxis at least as many days as they had sex.
Nevertheless, there was no significant difference in the incidence of STIs, reported over 1 year, at quarterly visits that included genital STI testing, between groups, with 50 patients in the Doxy PEP group and 59 in the standard screening group developing STIs (relative risk, 0.88; P = .51).
Of the infections, 85 were chlamydia, including 35 in the Doxy PEP group and 50 with standard of care, while 31 were gonorrhea, including 19 in the Doxy PEP group and 12 with standard of care; 8 had both infections, and there was 1 syphilis infection.
The results were consistent across subanalyses of patients grouped according to STI, who became pregnant (n = 80), or sorted by other factors including age, contraceptive use, transactional sex, and STI at baseline.
None of the women developed HIV, and there were no serious events associated with the Doxy PEP treatment.
Cisgender women bear ‘highest burden’ of STIs
The findings are disappointing in light of the higher rates of STIs among cisgender women, with the Centers for Disease Control and Prevention reporting that women also disproportionately bear the long-term consequences of STIs.
“For example, each year, untreated sexually transmitted diseases cause infertility in at least 20,000 women in the United States, and a pregnant woman is highly likely to pass syphilis unto her unborn baby if left untested or untreated,” the CDC reports.
The STI rates are particularly high for women taking HIV PrEP in regions like East Africa, where rates of STIs among cisgender women in many cases are higher than rates for men taking PrEP in high income countries, Dr. Stewart said.
Previous studies of Doxy PEP in men and transgender women taking HIV PrEP, including new research presented at CROI, have shown highly encouraging reductions in STIs, at rates of up to approximately 80% for chlamydia and syphilis.
Adherence, anatomy, resistance
The key theories for the lack of a prevention of infections in cisgender women surround the issues of resistances, as well as anatomy and adherence, said Dr. Stewart.
In terms of bacterial resistances, while initial testing in a limited number of samples the study found no evidence of markers of resistance for chlamydia, all of the gonorrhea samples did show tetracycline-resistant N gonorrhea at baseline and follow-up in both groups.
Regarding anatomic differences, doxycycline may not prevent STIs in endocervical tissue among cisgender women, Dr. Stewart noted. Women are known to be at higher risk of infection because the lining of the vagina is thinner than the skin of the penis, allowing for easier penetration of bacteria and viruses.
The study was designed to optimize adherence to Doxy PEP. Measures included monitoring with weekly text message surveys, in which the women reported a high rate of adherence.
The overall retention rate in the study was high; as many as 97% of the quarterly follow-up visits were completed, including 95% in the Doxy PEP group and 98% of the standard care group. The response rate for the weekly surveys was 81%.
Of note, women reported the use of the treatment to be “imperfect,” suggesting social problems, such as biases toward the use of the prophylaxis.
The results underscore the need for ongoing efforts to make sure no groups of patients are left behind as interventions advance, Dr. Stewart said.
“The burden of STIs on cisgender women is large and growing,” she concluded. “STI prevention interventions are needed.”
Commenting on the study, Renee A. Heffron, PhD, MPH, said the findings “are somewhat surprising because results from trials in other populations have been positive.
“But cisgender women are exposed through the cervix, and this tissue is different from rectal or urethral tissue,” Dr. Heffron, a professor at the department of medicine and director of the Center for AIDS Research at the University of Alabama, Birmingham, told this news organization.
Further findings from the research should help shed light on key issues of adherence and drug concentration levels in cervical tissue, she added.
“For cisgender women, these data are the first and the beginning of understanding whether this is a viable strategy,” Dr. Heffron said.
“We have more to learn to better understand the results from the trial main outcomes, and if there are tweaks to this strategy that would improve efficacy.”
The authors and Dr. Heffron have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CROI 2023
Scientists create ‘vagina on a chip’: What to know
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
For years, women’s health advocates have argued that far more research is needed on women’s bodies and health. The world’s first-ever “vagina on a chip,” recently developed at Harvard’s Wyss Institute for Biologically Inspired Engineering in Boston, could go a long way to making that happen.
“Women’s health has not received the attention it deserves,” says Don Ingber, MD, PhD, who led the team that created the vagina chip. The advance quickly drew media attention after it was reported in the journal Microbiome. But researchers hope for more than headlines. They see the chip as a way to facilitate vaginal health research and open the door to vital new treatments.
By now, you may have heard of “organs on chips”: tiny devices about the size of a flash drive that are designed to mimic the biological activity of human organs. These glass chips contain living human cells within grooves that allow the passage of fluid, to either maintain or disrupt the cells’ function. So far, Dr. Ingber and his team at the Wyss Institute have developed more than 15 organ chip models, including chips that mimic the lung, intestine, kidney, and bone marrow.
The idea to develop a vagina chip grew out of research, funded by the Gates Foundation, on a childhood disease called environmental enteric dysfunction, an intestinal disease most commonly found in low-resource nations that is the second leading cause of death in children under 5. That’s when Dr. Ingber discovered just how much the child’s microbiome influences this disease.
Stemming from that work, the Gates Foundation turned its attention to newborn health – in particular, the impact of bacterial vaginosis, an imbalance in the vagina’s bacterial makeup. Bacterial vaginosis occurs in one out of four women worldwide and has been linked to premature birth as well as HIV, HPV persistence, and cervical cancer.
The goal was to test “live biotherapeutic products,” or living microbes like probiotics, that might restore the vagina’s microbiome to health.
No other preclinical model exists to perform tests like that, says Dr. Ingber.
“The vagina chip is a way to help make some advances,” he says.
The Gates Foundation recognized that women’s reproductive health is a major issue, not only in low-income nations, but everywhere around the world. As the project evolved, Dr. Ingber began to hear from female colleagues about how neglected women’s reproductive health is in medical science.
“It is something I became sensitive to and realized this is just the starting point,” Dr. Ingber says.
Take bacterial vaginosis, for example. Since 1982, treatment has revolved around the same two antibiotics. That’s partly because there is no animal model to study. No other species has the same vaginal bacterial community as humans do.
That makes developing any new therapy “incredibly challenging,” explains Caroline Mitchell, MD, MPH, an ob.gyn. at Massachusetts General Hospital, Boston, and a member of the consortium.
It turns out, replicating the vagina in a lab dish is, to use the technical term, very hard.
“That’s where a vagina chip offers an opportunity,” Dr. Mitchell says. “It’s not super-high throughput, but it’s way more high throughput than a [human] clinical trial.”
As such, the vagina chip could help scientists find new treatments much faster.
Like Dr. Ingber, Dr. Mitchell also sees the chip as a way to bring more attention to the largely unmet needs in female reproductive medicine.
“Women’s reproductive health has been under-resourced, under-prioritized, and largely disregarded for decades,” she says. And the time may be ripe for change: Dr. Mitchell says she was encouraged by the National Institutes of Health’s Advancing NIH Research on the Health of Women conference, held in 2021 in response to a congressional request to address women’s health research efforts.
Beyond bacterial vaginosis, Dr. Mitchell imagines the chip could help scientists find new treatments for vaginal yeast infection (candidiasis), chlamydia, and endometriosis. As with bacterial vaginosis, medicines for vaginal yeast infections have not advanced in decades, Dr. Mitchell says. Efforts to develop a vaccine for chlamydia – which can cause permanent damage to a woman’s reproductive system – have dragged on for many years. And endometriosis, an often painful condition in which the tissue that makes up the uterine lining grows outside the uterus, remains under-researched despite affecting 10% of childbearing-age women.
While some mouse models are used in chlamydia research, it’s hard to say if they’ll translate to humans, given the vaginal and cervical bacterial differences.
“Our understanding of the basic physiology of the environment of the vagina and cervix is another area where we’re woefully ignorant,” Dr. Mitchell says.
To that end, Dr. Ingber’s team is developing more complex chips mimicking the vagina and the cervix. One of his team members wants to use the chips to study infertility. The researchers have already used the chips to see how bacterial vaginosis and mucous changes impact the way sperm migrates up the reproductive tract.
The lab is now linking vagina and cervix chips together to study viral infections of the cervix, like HPV, and all types of bacterial diseases of the vaginal tract. By applying cervical mucus to the vagina chip, they hope to learn more about how female reproductive tissues respond to infection and inflammation.
“I always say that organ chips are like synthetic biology at the cell tissue and organ level,” says Dr. Ingber. “You start simple and see if you [can] mimic a clinical situation.”
As they make the chips more complex – perhaps by adding blood vessel cells and female hormones – Dr. Ingber foresees being able to study the response to hormonal changes during the menstrual cycle.
“We can begin to explore the effects of cycling over time as well as other types of hormonal effects,” he says.
Dr. Ingber also envisions linking the vagina chip to other organ chips – he’s already succeeded in linking eight different organ types together. But for now, the team hopes the vagina chip will enhance our understanding of basic female reproductive biology and speed up the process of developing new treatments for women’s health.
A version of this article first appeared on WebMD.com.
FROM MICROBIOME
Highly anticipated HIV vaccine fails in large trial
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
officials announced Wednesday.
The vaccine had been in development since 2019 and was given to 3,900 study participants through October 2022, but data shows it does not protect against HIV compared with a placebo, according to developer Janssen Pharmaceutical.
Experts estimate the failure means there won’t be another potential vaccine on the horizon for 3 to 5 years, the New York Times reported.
“It’s obviously disappointing,” Anthony Fauci, MD, former head of the National Institute of Allergy and Infectious Diseases, told MSNBC, noting that other areas of HIV treatment research are promising. “I don’t think that people should give up on the field of the HIV vaccine.”
No safety issues had been identified with the vaccine during the trial, which studied the experimental treatment in men who have sex with men or with transgender people.
There is no cure for HIV, but disease progression can be managed with existing treatments. HIV attacks the body’s immune system and destroys white blood cells, increasing the risk of other infections. More than 1.5 million people worldwide were infected with HIV in 2021 and 38.4 million people are living with the virus, according to UNAIDS.
A version of this article first appeared on WebMD.com.
Kaposi’s sarcoma: Antiretroviral-related improvements in survival measured
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
FDA approves first-in-class drug for HIV
The U.S. Food and Drug Administration has approved the medication lenacapavir (Sunlenca) for adults living with multidrug resistant HIV-1 infection. .
“Following today’s decision from the FDA, lenacapavir helps to fill a critical unmet need for people with complex prior treatment histories and offers physicians a long-awaited twice-yearly option for these patients who otherwise have limited therapy choices,” said site principal investigator Sorana Segal-Maurer, MD, a professor of clinical medicine at Weill Cornell Medicine, New York, in a statement.
HIV drug regimens generally consist of two or three HIV medicines combined in a daily pill. In 2021, the FDA approved the first injectable complete drug regimen for HIV-1, Cabenuva, which can be administered monthly or every other month. Lenacapavir is administered only twice annually, but it is also combined with other antiretrovirals. The injections and oral tablets of lenacapavir are estimated to cost $42,250 in the first year of treatment and then $39,000 annually in the subsequent years, Reuters reported.
Lenacapavir is the first of a new class of drug called capsid inhibitors to be FDA-approved for treating HIV-1. The drug blocks the HIV-1 virus’s protein shell and interferes with essential steps of the virus’s evolution. The approval, announced today, was based on a multicenter clinical trial of 72 patients with multidrug resistant HIV-1 infection. After a year of the medication, 30 (83%) of the 36 patients randomly assigned to take lenacapavir, in combination with other HIV medications, had undetectable viral loads.
“Today’s approval ushers in a new class of antiretroviral drugs that may help patients with HIV who have run out of treatment options,” said Debra Birnkrant, MD, director of the division of antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “The availability of new classes of antiretroviral medications may possibly help these patients live longer, healthier lives.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the medication lenacapavir (Sunlenca) for adults living with multidrug resistant HIV-1 infection. .
“Following today’s decision from the FDA, lenacapavir helps to fill a critical unmet need for people with complex prior treatment histories and offers physicians a long-awaited twice-yearly option for these patients who otherwise have limited therapy choices,” said site principal investigator Sorana Segal-Maurer, MD, a professor of clinical medicine at Weill Cornell Medicine, New York, in a statement.
HIV drug regimens generally consist of two or three HIV medicines combined in a daily pill. In 2021, the FDA approved the first injectable complete drug regimen for HIV-1, Cabenuva, which can be administered monthly or every other month. Lenacapavir is administered only twice annually, but it is also combined with other antiretrovirals. The injections and oral tablets of lenacapavir are estimated to cost $42,250 in the first year of treatment and then $39,000 annually in the subsequent years, Reuters reported.
Lenacapavir is the first of a new class of drug called capsid inhibitors to be FDA-approved for treating HIV-1. The drug blocks the HIV-1 virus’s protein shell and interferes with essential steps of the virus’s evolution. The approval, announced today, was based on a multicenter clinical trial of 72 patients with multidrug resistant HIV-1 infection. After a year of the medication, 30 (83%) of the 36 patients randomly assigned to take lenacapavir, in combination with other HIV medications, had undetectable viral loads.
“Today’s approval ushers in a new class of antiretroviral drugs that may help patients with HIV who have run out of treatment options,” said Debra Birnkrant, MD, director of the division of antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “The availability of new classes of antiretroviral medications may possibly help these patients live longer, healthier lives.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the medication lenacapavir (Sunlenca) for adults living with multidrug resistant HIV-1 infection. .
“Following today’s decision from the FDA, lenacapavir helps to fill a critical unmet need for people with complex prior treatment histories and offers physicians a long-awaited twice-yearly option for these patients who otherwise have limited therapy choices,” said site principal investigator Sorana Segal-Maurer, MD, a professor of clinical medicine at Weill Cornell Medicine, New York, in a statement.
HIV drug regimens generally consist of two or three HIV medicines combined in a daily pill. In 2021, the FDA approved the first injectable complete drug regimen for HIV-1, Cabenuva, which can be administered monthly or every other month. Lenacapavir is administered only twice annually, but it is also combined with other antiretrovirals. The injections and oral tablets of lenacapavir are estimated to cost $42,250 in the first year of treatment and then $39,000 annually in the subsequent years, Reuters reported.
Lenacapavir is the first of a new class of drug called capsid inhibitors to be FDA-approved for treating HIV-1. The drug blocks the HIV-1 virus’s protein shell and interferes with essential steps of the virus’s evolution. The approval, announced today, was based on a multicenter clinical trial of 72 patients with multidrug resistant HIV-1 infection. After a year of the medication, 30 (83%) of the 36 patients randomly assigned to take lenacapavir, in combination with other HIV medications, had undetectable viral loads.
“Today’s approval ushers in a new class of antiretroviral drugs that may help patients with HIV who have run out of treatment options,” said Debra Birnkrant, MD, director of the division of antivirals in the FDA’s Center for Drug Evaluation and Research, in a press release. “The availability of new classes of antiretroviral medications may possibly help these patients live longer, healthier lives.”
A version of this article first appeared on Medscape.com.