User login
Age, skin cancer risks for ICI-induced bullous pemphigoid identified
that may result in treatment interruption or cessation.
Investigators in Boston report that among patients receiving ICIs, being aged 70 years or older and having skin cancer are both significant risk factors for bullous pemphigoid. On the plus side, ICI-induced bullous pemphigoid also appears to be a marker for improved tumor responses to therapy.
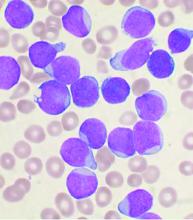
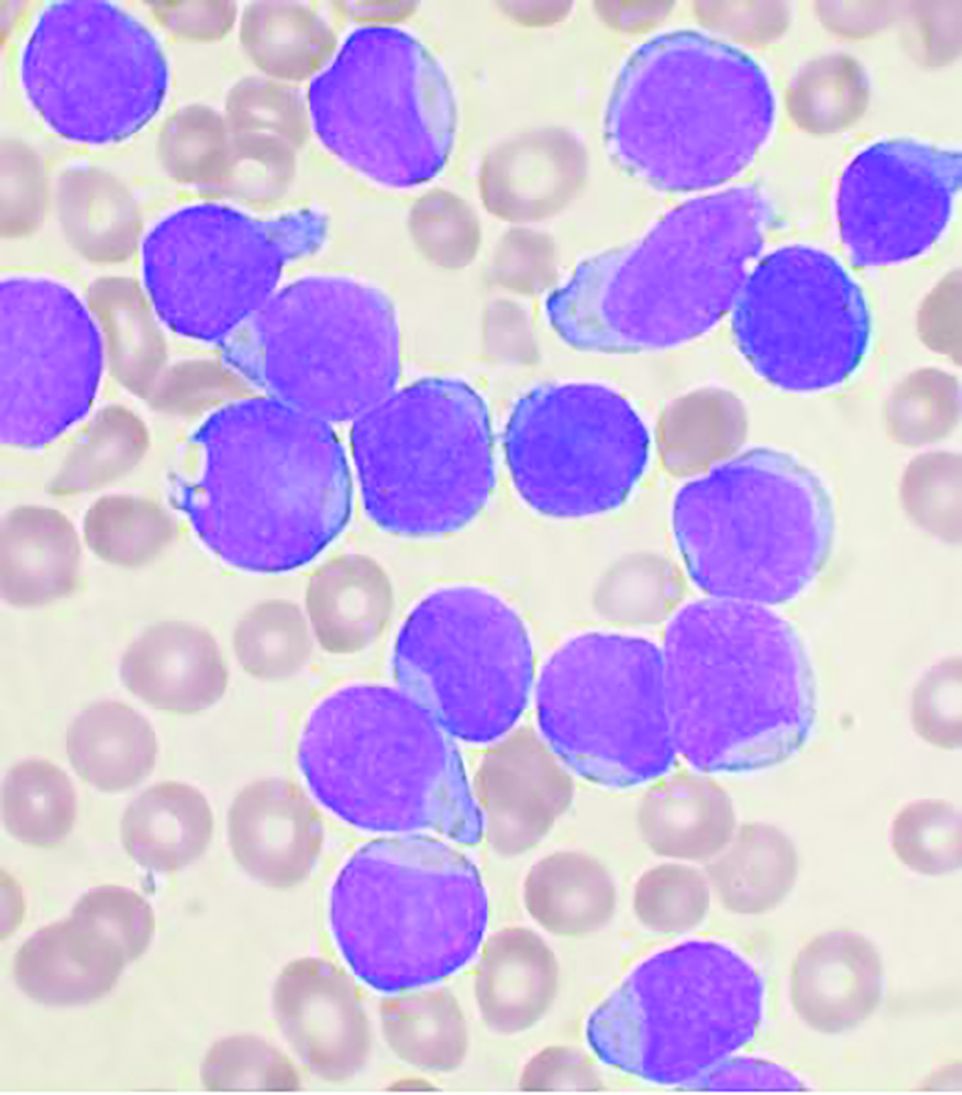
In a nested case-control study of 5,636 patients with cancer who received either a programmed death 1 inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo) or a cytotoxic T-lymphocyte–associated protein 4 inhibitor such as ipilimumab (Yervoy), 35 patients (0.6%) developed bullous pemphigoid. The study by Nicole R. LeBoeuf, MD, MPH, from Brigham and Women’s Hospital in Boston and colleagues was published online in JAMA Dermatology.
“What is interesting is that 0.6 is a small number, but we’re seeing bullous pemphigoid at considerably higher frequency than is expected in the general population,” Dr. LeBoeuf said in an interview.
And although bullous pemphigoid has the potential to disrupt ICI therapy, it also appears to be a marker for a favorable tumor response, the investigators found.
Their findings suggest that management of bullous pemphigoid for patients receiving ICIs should focus on early identification and management with therapies directed at the specific toxicity, Dr. LeBoeuf said.
“When you make a specific diagnosis like bullous pemphigoid, then you can treat that specific disease with very targeted therapies, such as omalizumab or dupilumab or rituximab – things that are not globally immune suppressing like steroid or other T-cell–depleting agents. Studies have shown that depleting B cells with anti-CD20 agents is not detrimental to immune checkpoint inhibitor therapy,” she said.
Dermatologic AEs common
About 40% of patients with cancer treated with ICIs experience immune-related dermatologic adverse events (AEs) that can range from mild rashes and hair and nail changes to uncommon but life-threatening complications, such as Stevens-Johnson syndrome, a form of toxic epidermal necrolysis, according to members of a European Academy of Dermatology and Venereology task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they wrote in a position statement on the management of ICI-derived dermatologic adverse events.
Dr. LeBoeuf and colleagues note that, while reported risk factors for idiopathic bullous pemphigoid include advanced age, type 2 diabetes, use of dipeptidyl peptidase-4 inhibitors, cerebrovascular disease, and neurocognitive disease, risk factors for bullous pemphigoid and other adverse dermatologic events associated with ICIs are less well known.
Study details
To identify risk factors for bullous pemphigoid in patients receiving ICI, the investigators performed a case-control study nested within a retrospective cohort study.
They evaluated records for all patients in the three Harvard-affiliated hospitals to identify patients with ICI-associated bullous pemphigoid from October 2014 through December 2020. Control persons were all patients in the Dana-Farber cancer registry who received ICIs during the study period.
The investigators chose age at ICI initiation (69 years and younger or 70 years and older), sex, ICI agents, and cancer type as potential risk factors.
They used propensity score matching based on age, cancer type, ICI agent, and number of ICI cycles to match two control persons with each case patient.
Of the 5,636 patients treated with ICIs during the study period, 35 (0.6%) developed bullous pemphigoid. The median age was 72.8 years, and 71.4% were men.
In a multivariate logistic regression model that included 2,955 patients with complete data in the cancer registry, factors significantly associated with developing bullous pemphigoid included age 70 years or older (odds ratio, 2.32; P = .01), having melanoma (OR, 3.21; P < .001), and having nonmelanoma skin cancer (OR, 8.32; P < .001).
In comparing the 35 case patients with their matched control patients, a complete or partial response at first restaging imaging was significantly associated with developing bullous pemphigoid (OR, 3.37; P = .01). In addition, there was a higher likelihood of tumor responses to ICIs among patients with bullous pemphigoid, compared with matched control patients (objective response rate, 82.9% vs. 61.4%; P = .03).
Prudent toxicity management
Ryan Sullivan, MD, who treats patients with skin cancer at Massachusetts General Hospital Cancer Center, Boston, but was not involved in the study, commented that the findings raise questions about the relationship between skin cancers and immune-related adverse events.
“It is compelling that bullous pemphigoid is a skin toxicity and is more common to happen in skin cancer patients,” he noted. “That’s a very interesting finding, and the reason that it’s interesting is that it’s harder to understand why a presumably antibody-mediated side effect would be more likely to have that cross-reactivity where the tumor started and where the toxicity happened,” he said in an interview.
He noted that the benefits of ICIs for patients with skin cancers far outweigh the risks of dermatologic adverse events such as bullous pemphigoid and that ICI-associated events require judicious management.
“This is true across the spectrum of toxicities: There are clear manifestations of toxicity that we should be more thoughtful about what’s driving them, more thoughtful about what it is, and more thoughtful about treating them, other than just pouring steroids into patients in industrial doses and hoping that everything’s going to be OK,” he said.
No funding source for the study was reported. Dr. LeBoeuf reported receiving grants from the National Institutes of Health National Cancer Institute during the conduct of the study and personal fees for serving as a consultant for several companies outside the study. Coauthor Arash Mostaghimi, MD, MPA, MPH, is associate editor of JAMA Dermatology but was not involved in study selection or evaluation for publication. Dr. Sullivan disclosed consulting for ICI makers Bristol-Myers Squibb and Merck.
A version of this article first appeared on Medscape.com.
that may result in treatment interruption or cessation.
Investigators in Boston report that among patients receiving ICIs, being aged 70 years or older and having skin cancer are both significant risk factors for bullous pemphigoid. On the plus side, ICI-induced bullous pemphigoid also appears to be a marker for improved tumor responses to therapy.
In a nested case-control study of 5,636 patients with cancer who received either a programmed death 1 inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo) or a cytotoxic T-lymphocyte–associated protein 4 inhibitor such as ipilimumab (Yervoy), 35 patients (0.6%) developed bullous pemphigoid. The study by Nicole R. LeBoeuf, MD, MPH, from Brigham and Women’s Hospital in Boston and colleagues was published online in JAMA Dermatology.
“What is interesting is that 0.6 is a small number, but we’re seeing bullous pemphigoid at considerably higher frequency than is expected in the general population,” Dr. LeBoeuf said in an interview.
And although bullous pemphigoid has the potential to disrupt ICI therapy, it also appears to be a marker for a favorable tumor response, the investigators found.
Their findings suggest that management of bullous pemphigoid for patients receiving ICIs should focus on early identification and management with therapies directed at the specific toxicity, Dr. LeBoeuf said.
“When you make a specific diagnosis like bullous pemphigoid, then you can treat that specific disease with very targeted therapies, such as omalizumab or dupilumab or rituximab – things that are not globally immune suppressing like steroid or other T-cell–depleting agents. Studies have shown that depleting B cells with anti-CD20 agents is not detrimental to immune checkpoint inhibitor therapy,” she said.
Dermatologic AEs common
About 40% of patients with cancer treated with ICIs experience immune-related dermatologic adverse events (AEs) that can range from mild rashes and hair and nail changes to uncommon but life-threatening complications, such as Stevens-Johnson syndrome, a form of toxic epidermal necrolysis, according to members of a European Academy of Dermatology and Venereology task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they wrote in a position statement on the management of ICI-derived dermatologic adverse events.
Dr. LeBoeuf and colleagues note that, while reported risk factors for idiopathic bullous pemphigoid include advanced age, type 2 diabetes, use of dipeptidyl peptidase-4 inhibitors, cerebrovascular disease, and neurocognitive disease, risk factors for bullous pemphigoid and other adverse dermatologic events associated with ICIs are less well known.
Study details
To identify risk factors for bullous pemphigoid in patients receiving ICI, the investigators performed a case-control study nested within a retrospective cohort study.
They evaluated records for all patients in the three Harvard-affiliated hospitals to identify patients with ICI-associated bullous pemphigoid from October 2014 through December 2020. Control persons were all patients in the Dana-Farber cancer registry who received ICIs during the study period.
The investigators chose age at ICI initiation (69 years and younger or 70 years and older), sex, ICI agents, and cancer type as potential risk factors.
They used propensity score matching based on age, cancer type, ICI agent, and number of ICI cycles to match two control persons with each case patient.
Of the 5,636 patients treated with ICIs during the study period, 35 (0.6%) developed bullous pemphigoid. The median age was 72.8 years, and 71.4% were men.
In a multivariate logistic regression model that included 2,955 patients with complete data in the cancer registry, factors significantly associated with developing bullous pemphigoid included age 70 years or older (odds ratio, 2.32; P = .01), having melanoma (OR, 3.21; P < .001), and having nonmelanoma skin cancer (OR, 8.32; P < .001).
In comparing the 35 case patients with their matched control patients, a complete or partial response at first restaging imaging was significantly associated with developing bullous pemphigoid (OR, 3.37; P = .01). In addition, there was a higher likelihood of tumor responses to ICIs among patients with bullous pemphigoid, compared with matched control patients (objective response rate, 82.9% vs. 61.4%; P = .03).
Prudent toxicity management
Ryan Sullivan, MD, who treats patients with skin cancer at Massachusetts General Hospital Cancer Center, Boston, but was not involved in the study, commented that the findings raise questions about the relationship between skin cancers and immune-related adverse events.
“It is compelling that bullous pemphigoid is a skin toxicity and is more common to happen in skin cancer patients,” he noted. “That’s a very interesting finding, and the reason that it’s interesting is that it’s harder to understand why a presumably antibody-mediated side effect would be more likely to have that cross-reactivity where the tumor started and where the toxicity happened,” he said in an interview.
He noted that the benefits of ICIs for patients with skin cancers far outweigh the risks of dermatologic adverse events such as bullous pemphigoid and that ICI-associated events require judicious management.
“This is true across the spectrum of toxicities: There are clear manifestations of toxicity that we should be more thoughtful about what’s driving them, more thoughtful about what it is, and more thoughtful about treating them, other than just pouring steroids into patients in industrial doses and hoping that everything’s going to be OK,” he said.
No funding source for the study was reported. Dr. LeBoeuf reported receiving grants from the National Institutes of Health National Cancer Institute during the conduct of the study and personal fees for serving as a consultant for several companies outside the study. Coauthor Arash Mostaghimi, MD, MPA, MPH, is associate editor of JAMA Dermatology but was not involved in study selection or evaluation for publication. Dr. Sullivan disclosed consulting for ICI makers Bristol-Myers Squibb and Merck.
A version of this article first appeared on Medscape.com.
that may result in treatment interruption or cessation.
Investigators in Boston report that among patients receiving ICIs, being aged 70 years or older and having skin cancer are both significant risk factors for bullous pemphigoid. On the plus side, ICI-induced bullous pemphigoid also appears to be a marker for improved tumor responses to therapy.
In a nested case-control study of 5,636 patients with cancer who received either a programmed death 1 inhibitor such as pembrolizumab (Keytruda) or nivolumab (Opdivo) or a cytotoxic T-lymphocyte–associated protein 4 inhibitor such as ipilimumab (Yervoy), 35 patients (0.6%) developed bullous pemphigoid. The study by Nicole R. LeBoeuf, MD, MPH, from Brigham and Women’s Hospital in Boston and colleagues was published online in JAMA Dermatology.
“What is interesting is that 0.6 is a small number, but we’re seeing bullous pemphigoid at considerably higher frequency than is expected in the general population,” Dr. LeBoeuf said in an interview.
And although bullous pemphigoid has the potential to disrupt ICI therapy, it also appears to be a marker for a favorable tumor response, the investigators found.
Their findings suggest that management of bullous pemphigoid for patients receiving ICIs should focus on early identification and management with therapies directed at the specific toxicity, Dr. LeBoeuf said.
“When you make a specific diagnosis like bullous pemphigoid, then you can treat that specific disease with very targeted therapies, such as omalizumab or dupilumab or rituximab – things that are not globally immune suppressing like steroid or other T-cell–depleting agents. Studies have shown that depleting B cells with anti-CD20 agents is not detrimental to immune checkpoint inhibitor therapy,” she said.
Dermatologic AEs common
About 40% of patients with cancer treated with ICIs experience immune-related dermatologic adverse events (AEs) that can range from mild rashes and hair and nail changes to uncommon but life-threatening complications, such as Stevens-Johnson syndrome, a form of toxic epidermal necrolysis, according to members of a European Academy of Dermatology and Venereology task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they wrote in a position statement on the management of ICI-derived dermatologic adverse events.
Dr. LeBoeuf and colleagues note that, while reported risk factors for idiopathic bullous pemphigoid include advanced age, type 2 diabetes, use of dipeptidyl peptidase-4 inhibitors, cerebrovascular disease, and neurocognitive disease, risk factors for bullous pemphigoid and other adverse dermatologic events associated with ICIs are less well known.
Study details
To identify risk factors for bullous pemphigoid in patients receiving ICI, the investigators performed a case-control study nested within a retrospective cohort study.
They evaluated records for all patients in the three Harvard-affiliated hospitals to identify patients with ICI-associated bullous pemphigoid from October 2014 through December 2020. Control persons were all patients in the Dana-Farber cancer registry who received ICIs during the study period.
The investigators chose age at ICI initiation (69 years and younger or 70 years and older), sex, ICI agents, and cancer type as potential risk factors.
They used propensity score matching based on age, cancer type, ICI agent, and number of ICI cycles to match two control persons with each case patient.
Of the 5,636 patients treated with ICIs during the study period, 35 (0.6%) developed bullous pemphigoid. The median age was 72.8 years, and 71.4% were men.
In a multivariate logistic regression model that included 2,955 patients with complete data in the cancer registry, factors significantly associated with developing bullous pemphigoid included age 70 years or older (odds ratio, 2.32; P = .01), having melanoma (OR, 3.21; P < .001), and having nonmelanoma skin cancer (OR, 8.32; P < .001).
In comparing the 35 case patients with their matched control patients, a complete or partial response at first restaging imaging was significantly associated with developing bullous pemphigoid (OR, 3.37; P = .01). In addition, there was a higher likelihood of tumor responses to ICIs among patients with bullous pemphigoid, compared with matched control patients (objective response rate, 82.9% vs. 61.4%; P = .03).
Prudent toxicity management
Ryan Sullivan, MD, who treats patients with skin cancer at Massachusetts General Hospital Cancer Center, Boston, but was not involved in the study, commented that the findings raise questions about the relationship between skin cancers and immune-related adverse events.
“It is compelling that bullous pemphigoid is a skin toxicity and is more common to happen in skin cancer patients,” he noted. “That’s a very interesting finding, and the reason that it’s interesting is that it’s harder to understand why a presumably antibody-mediated side effect would be more likely to have that cross-reactivity where the tumor started and where the toxicity happened,” he said in an interview.
He noted that the benefits of ICIs for patients with skin cancers far outweigh the risks of dermatologic adverse events such as bullous pemphigoid and that ICI-associated events require judicious management.
“This is true across the spectrum of toxicities: There are clear manifestations of toxicity that we should be more thoughtful about what’s driving them, more thoughtful about what it is, and more thoughtful about treating them, other than just pouring steroids into patients in industrial doses and hoping that everything’s going to be OK,” he said.
No funding source for the study was reported. Dr. LeBoeuf reported receiving grants from the National Institutes of Health National Cancer Institute during the conduct of the study and personal fees for serving as a consultant for several companies outside the study. Coauthor Arash Mostaghimi, MD, MPA, MPH, is associate editor of JAMA Dermatology but was not involved in study selection or evaluation for publication. Dr. Sullivan disclosed consulting for ICI makers Bristol-Myers Squibb and Merck.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
New injectable gel can deliver immune cells directly to cancer tumors
A simple, two-ingredient gel may boost the fighting power of a groundbreaking cancer treatment, say Stanford University engineers.
The gel – made from water and a plant-based polymer – delivers targeted T cells adjacent to a cancer growth, taking aim at solid tumors.
It’s the latest development in CAR T-cell therapy, a type of immunotherapy that involves collecting the patient’s T cells, reengineering them to be stronger, and returning them to the patient’s body.
Results have been promising in blood cancers, such as leukemia and lymphoma, but less so in solid tumors, such as brain, breast, or kidney cancer, according to the National Cancer Institute.
The gel “is a really exciting step forward,” says Abigail Grosskopf, a PhD candidate at Stanford (Calif.) University, who is the lead study author, “because it can change the delivery of these cells and expand this kind of treatment to other cancers.”
CAR T-cell therapy: Limits in solid tumors
Currently available CAR T-cell therapies are administered by intravenous infusion. But that doesn’t do much against tumors in specific locations because the cells enter the bloodstream and flow throughout the body. The cancer-fighting effort exhausts the T cells, weakening their ability to infiltrate dense tumors.
CAR T cells need cytokines to tell them when to attack, Ms. Grosskopf explains. If delivered through an IV drip, the number of cytokines required to destroy a solid tumor would be toxic to other, healthy parts of the body.
So
In their study, which was published in Science Advances, the injections wiped out mouse tumors in 12 days. The gel degraded harmlessly a few weeks later.
A “leaky pen” that fights cancer
The reason a gel works better than a liquid is because of its staying power, says Ms. Grosskopf, who compares the method to a leaky pen.
The gel acts as the “pen,” releasing activated CAR T cells at regular intervals to attack the cancerous growth. Whereas liquid dissipates quickly, the gel’s structure is strong enough to stay in place for weeks, Ms. Grosskopf says. Plus, it’s biocompatible and harmless within the body, she adds.
More preclinical studies are needed before human clinical trials can occur, Ms. Grosskopf says.
“Not only could this be a way to deliver T cells and cytokines,” Ms. Grosskopf says, “but it may be used for other targeted therapy cancer drugs that are in development. So we see this as running parallel to those efforts.”
Taking an even broader view, the gel could have applications across medical specialties, such as slow-release delivery of vaccines.
A version of this article first appeared on Medscape.com.
A simple, two-ingredient gel may boost the fighting power of a groundbreaking cancer treatment, say Stanford University engineers.
The gel – made from water and a plant-based polymer – delivers targeted T cells adjacent to a cancer growth, taking aim at solid tumors.
It’s the latest development in CAR T-cell therapy, a type of immunotherapy that involves collecting the patient’s T cells, reengineering them to be stronger, and returning them to the patient’s body.
Results have been promising in blood cancers, such as leukemia and lymphoma, but less so in solid tumors, such as brain, breast, or kidney cancer, according to the National Cancer Institute.
The gel “is a really exciting step forward,” says Abigail Grosskopf, a PhD candidate at Stanford (Calif.) University, who is the lead study author, “because it can change the delivery of these cells and expand this kind of treatment to other cancers.”
CAR T-cell therapy: Limits in solid tumors
Currently available CAR T-cell therapies are administered by intravenous infusion. But that doesn’t do much against tumors in specific locations because the cells enter the bloodstream and flow throughout the body. The cancer-fighting effort exhausts the T cells, weakening their ability to infiltrate dense tumors.
CAR T cells need cytokines to tell them when to attack, Ms. Grosskopf explains. If delivered through an IV drip, the number of cytokines required to destroy a solid tumor would be toxic to other, healthy parts of the body.
So
In their study, which was published in Science Advances, the injections wiped out mouse tumors in 12 days. The gel degraded harmlessly a few weeks later.
A “leaky pen” that fights cancer
The reason a gel works better than a liquid is because of its staying power, says Ms. Grosskopf, who compares the method to a leaky pen.
The gel acts as the “pen,” releasing activated CAR T cells at regular intervals to attack the cancerous growth. Whereas liquid dissipates quickly, the gel’s structure is strong enough to stay in place for weeks, Ms. Grosskopf says. Plus, it’s biocompatible and harmless within the body, she adds.
More preclinical studies are needed before human clinical trials can occur, Ms. Grosskopf says.
“Not only could this be a way to deliver T cells and cytokines,” Ms. Grosskopf says, “but it may be used for other targeted therapy cancer drugs that are in development. So we see this as running parallel to those efforts.”
Taking an even broader view, the gel could have applications across medical specialties, such as slow-release delivery of vaccines.
A version of this article first appeared on Medscape.com.
A simple, two-ingredient gel may boost the fighting power of a groundbreaking cancer treatment, say Stanford University engineers.
The gel – made from water and a plant-based polymer – delivers targeted T cells adjacent to a cancer growth, taking aim at solid tumors.
It’s the latest development in CAR T-cell therapy, a type of immunotherapy that involves collecting the patient’s T cells, reengineering them to be stronger, and returning them to the patient’s body.
Results have been promising in blood cancers, such as leukemia and lymphoma, but less so in solid tumors, such as brain, breast, or kidney cancer, according to the National Cancer Institute.
The gel “is a really exciting step forward,” says Abigail Grosskopf, a PhD candidate at Stanford (Calif.) University, who is the lead study author, “because it can change the delivery of these cells and expand this kind of treatment to other cancers.”
CAR T-cell therapy: Limits in solid tumors
Currently available CAR T-cell therapies are administered by intravenous infusion. But that doesn’t do much against tumors in specific locations because the cells enter the bloodstream and flow throughout the body. The cancer-fighting effort exhausts the T cells, weakening their ability to infiltrate dense tumors.
CAR T cells need cytokines to tell them when to attack, Ms. Grosskopf explains. If delivered through an IV drip, the number of cytokines required to destroy a solid tumor would be toxic to other, healthy parts of the body.
So
In their study, which was published in Science Advances, the injections wiped out mouse tumors in 12 days. The gel degraded harmlessly a few weeks later.
A “leaky pen” that fights cancer
The reason a gel works better than a liquid is because of its staying power, says Ms. Grosskopf, who compares the method to a leaky pen.
The gel acts as the “pen,” releasing activated CAR T cells at regular intervals to attack the cancerous growth. Whereas liquid dissipates quickly, the gel’s structure is strong enough to stay in place for weeks, Ms. Grosskopf says. Plus, it’s biocompatible and harmless within the body, she adds.
More preclinical studies are needed before human clinical trials can occur, Ms. Grosskopf says.
“Not only could this be a way to deliver T cells and cytokines,” Ms. Grosskopf says, “but it may be used for other targeted therapy cancer drugs that are in development. So we see this as running parallel to those efforts.”
Taking an even broader view, the gel could have applications across medical specialties, such as slow-release delivery of vaccines.
A version of this article first appeared on Medscape.com.
FROM SCIENCE ADVANCES
Adverse skin effects of cancer immunotherapy reviewed
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Immune checkpoint inhibitors (ICIs) have unquestionably revolutionized the care of patients with malignant melanoma, non-small cell lung cancer, and other types of cancer.
, according to members of a European Academy of Dermatology and Venereology (EADV) task force.
“The desirable, immune-mediated oncologic response is often achieved at the cost of immune-related adverse events (irAEs) that may potentially affect any organ system,” they write in a position statement on the management of ICI-derived dermatologic adverse events.
Recommendations from the EADV “Dermatology for Cancer Patients” task force have been published in the Journal of the European Academy of Dermatology and Venereology.
Task force members developed the recommendations based on clinical experience from published data and came up with specific recommendations for treating cutaneous toxicities associated with dermatologic immune-related adverse events (dirAEs) that occur in patients receiving immunotherapy with an ICI.
ICIs include the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) inhibitor ipilimumab (Yervoy, Bristol Myers Squibb), and inhibitors of programmed death protein 1 (PD-1) and its ligand (PD-L1), including nivolumab (Opdivo, Bristol Myers Squibb), pembrolizumab (Keytruda, Merck), and other agents.
“The basic principle of management is that the interventions should be tailored to serve the equilibrium between patients’ relief from the symptoms and signs of skin toxicity and the preservation of an unimpeded oncologic treatment,” they write.
The recommendations are in line with those included in a 2021 update of the American Society of Clinical Oncology (ASCO) guidelines on the management of irAEs in patients treated with ICIs across the whole range of organ systems, said Milan J. Anadkat, MD, professor of dermatology and director of dermatology clinical trials at Washington University School of Medicine, St. Louis. Dr. Anadkat was a coauthor of the ASCO guideline update.
Although the European recommendations focus only on dermatologic side effects of ICIs in patients with cancer, “that doesn’t diminish their importance. They do a good job of summarizing how to approach and how to manage it depending on the severity of the toxicities and the various types of toxicities,” he told this news organization.
Having a paper focused exclusively on the dermatologic side effects of ICIs allows the inclusion of photographs that can help clinicians identify specific conditions that may require referral to a dermatologist, he said.
Both Dr. Anadkat and the authors of the European recommendations noted that dermatologic irAEs are more common with CTLA-4 inhibition than with PD-1/PD-L1 inhibition.
“It has to do with where the target is,” Dr. Anadkat said. “CTLA-4 inhibition works on a central aspect of the immune system, so it’s a much less specific site, whereas PD-1 affects an interaction at the site of the tumor cell itself, so it’s a little more specific.”
Pruritus
ICI-induced pruritus can occur without apparent skin changes, they write, noting that in a recent study of patients with dirAEs, about one-third had isolated pruritus.
The task force members cite a meta-analysis indicating a pruritus incidence of 13.2% for patients treated with nivolumab and 20.2% for patients treated with pembrolizumab but respective grade 3 pruritus rates of only 0.5% and 2.3%. The reported incidence of pruritus with ipilimumab was 47% in a different study.
Recommended treatments include topical moisturizers with or without medium-to-high potency corticosteroids for grade 1 reactions, non-sedating histamines and/or GABA agonists such as pregabalin, or gabapentin for grade 2 pruritus, and suspension of ICIs until pruritus improves in patients with grade 3 pruritus.
Maculopapular rash
Maculopapular or eczema-like rashes may occur in up to 68% of patients who receive a CTLA-4 inhibitor and up to 20% of those who receive a PD1/PD-L1 inhibitor, the authors note. Rashes commonly appear within 3-6 weeks of initiating therapy.
“The clinical presentation is nonspecific and consists of a rapid onset of multiple minimally scaly, erythematous macules and papules, congregating into plaques. Lesions are mostly located on trunk and extensor surfaces of the extremities and the face is generally spared,” they write.
Maculopapular rashes are typically accompanied by itching but could be asymptomatic, they noted.
Mild (grade 1) rashes may respond to moisturizers and topical potent or super-potent corticosteroids. Patients with grade 2 rash should also receive oral antihistamines. Systemic corticosteroids may be considered for patients with grade 3 rashes but only after other dirAEs that may require specific management, such as psoriasis, are ruled out.
Psoriasis-like rash
The most common form of psoriasis seen in patients treated with ICIs is psoriasis vulgaris with plaques, but other clinical variants are also seen, the authors note.
“Topical agents (corticosteroids, Vitamin D analogues) are prescribed in Grades 1/2 and supplementary” to systemic treatment for patients with grade 3 or recalcitrant lesions, they write. “If skin-directed therapies fail to provide symptomatic control,” systemic treatment and narrow band UVB phototherapy “should be considered,” they add.
Evidence regarding the use of systemic therapies to treat psoriasis-like rash associated with ICIs is sparse. Acitretin can be safely used in patients with cancer. Low-dose methotrexate is also safe to use except in patients with non-melanoma skin cancers. Cyclosporine, however, should be avoided because of the potential for tumor-promoting effects, they emphasized.
The recommendations also cover treatment of lichen planus-like and vitiligo-like rashes, as well as hair and nail changes, autoimmune bullous disorders, and oral mucosal dirAEs.
In addition, the recommendations cover severe cutaneous adverse reactions as well as serious, potentially life-threatening dirAEs, including Stevens-Johnson syndrome/TEN, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome (DRESS/DIHS).
“The dose of corticosteroids may be adapted to the severity of DRESS. The therapeutic benefit of systemic corticosteroids in the management of SJS/TEN remains controversial, and some authors favor treatment with cyclosporine. However, the use of corticosteroids in this context of ICI treatment appears reasonable and should be proposed. Short courses of steroids seem also effective in AGEP,” the task force members write.
The recommendations did not have outside funding. Of the 19 authors, 6 disclosed relationships with various pharmaceutical companies, including AbbVie, Leo Pharma, Boehringer Ingelheim, Bristol Myers Squibb, and/or Janssen. Dr. Anadkat disclosed previous relationships with Merck, Bristol Myers Squibb, and current relationships with others.
A version of this article first appeared on Medscape.com.
Women at higher risk of serious adverse events from cancer therapy
and this is seen with chemotherapy, targeted agents, and especially with immunotherapy.
The finding comes from a review of more than 23,000 participants across 202 trials of various cancers (excluding sex-related cancers) that has been conducted over the past 40 years.
The investigators found a 34% increased risk of severe AEs among women, compared with men, climbing to a 49% higher risk with immunotherapy.
Women had a substantially greater risk of severe symptomatic AEs, including with immune checkpoint inhibitors and targeted tyrosine kinase inhibitors, and were more likely to experience severe hematologic AEs with chemotherapy and immunotherapy.
The particularly large sex differences with immunotherapy suggest “that studying AEs from these agents is a priority,” the investigators comment.
The article was published online on Feb. 4 in the Journal of Clinical Oncology.
“It has been understood that women have more toxicity from chemotherapy than men, but almost no research has aimed to understand whether that pattern held for novel treatments like immunotherapy or targeted therapies. We found similar large differences, especially for immune treatments,” said lead investigator Joseph Unger, PhD, a biostatistician and health services researcher at the Fred Hutchinson Cancer Research Center, Seattle, in an institutional press release.
A “better understanding of the nature of the underlying mechanisms could potentially lead to interventions or delivery modifications to reduce toxicity in women,” the investigators comment in their article.
Among a sea of possible explanations for the finding, there could be differences in how men and women metabolize cancer therapies or differences in how they perceive symptoms. Women may also receive relatively higher doses because of their body size or have higher adherence to treatment.
Whatever the case, as cancer treatment becomes increasingly individualized, “sex may be an important consideration,” Dr. Unger said.
Study details
The study involved 8,838 women and 14,458 men across the trials, which were phase 2 or 3 investigations conducted by the SWOG Cancer Research Network from 1980 to 2019. Trials including sex-related cancers were excluded. In the trials included in the review, the most common cancers were gastrointestinal and lung, followed by leukemia.
Seventy-five percent of the subjects received chemotherapy, and the rest received either targeted therapy or immunotherapy.
Two-thirds of the subjects had at least one grade 3 or higher AE; women had a 25% higher risk than men of having AEs of grade 5 or higher.
After adjusting for age, race, disease prognosis, and other factors, women were at increased risk of severe symptomatic AEs, such as nausea and pain, across all treatment lines and especially with immunotherapy, for which reports of symptomatic AEs were 66% higher.
Women also had a higher risk of symptomatic gastrointestinal AEs with all three treatments and a higher risk of sleep-related AEs with chemotherapy and immunotherapy, which “could be a function of hormonal effects interacting with cancer treatment,” the investigators said.
As for readily measurable AEs, women were at higher risk than men for objective hematologic AEs with chemotherapy, immunotherapy, and targeted therapy. There were no statistically significant sex differences in the risk of nonhematologic objective AEs.
The team notes that increased toxicity among women has been associated with improved survival, which may give AEs more time to develop. Higher rates of AEs might also signal increased delivery or efficacy of cancer treatments.
However, a previous study found that men may have a better response to immunotherapy than women. Immune checkpoint inhibitors were twice as effective as standard cancer therapies in the treatment of men with advanced solid tumors compared to their female counterparts, concluded a team that carried out a meta-analysis of 20 randomized controlled trials involving more than 11,351 patients.
The study was funded by the National Cancer Institute and others. Dr. Unger has disclosed no relevant financial relationships. Several coauthors have reported ties to a handful of companies, including Johnson & Johnson and Seattle Genetics. One is an employee of AIM Specialty Health.
A version of this article first appeared on Medscape.com.
and this is seen with chemotherapy, targeted agents, and especially with immunotherapy.
The finding comes from a review of more than 23,000 participants across 202 trials of various cancers (excluding sex-related cancers) that has been conducted over the past 40 years.
The investigators found a 34% increased risk of severe AEs among women, compared with men, climbing to a 49% higher risk with immunotherapy.
Women had a substantially greater risk of severe symptomatic AEs, including with immune checkpoint inhibitors and targeted tyrosine kinase inhibitors, and were more likely to experience severe hematologic AEs with chemotherapy and immunotherapy.
The particularly large sex differences with immunotherapy suggest “that studying AEs from these agents is a priority,” the investigators comment.
The article was published online on Feb. 4 in the Journal of Clinical Oncology.
“It has been understood that women have more toxicity from chemotherapy than men, but almost no research has aimed to understand whether that pattern held for novel treatments like immunotherapy or targeted therapies. We found similar large differences, especially for immune treatments,” said lead investigator Joseph Unger, PhD, a biostatistician and health services researcher at the Fred Hutchinson Cancer Research Center, Seattle, in an institutional press release.
A “better understanding of the nature of the underlying mechanisms could potentially lead to interventions or delivery modifications to reduce toxicity in women,” the investigators comment in their article.
Among a sea of possible explanations for the finding, there could be differences in how men and women metabolize cancer therapies or differences in how they perceive symptoms. Women may also receive relatively higher doses because of their body size or have higher adherence to treatment.
Whatever the case, as cancer treatment becomes increasingly individualized, “sex may be an important consideration,” Dr. Unger said.
Study details
The study involved 8,838 women and 14,458 men across the trials, which were phase 2 or 3 investigations conducted by the SWOG Cancer Research Network from 1980 to 2019. Trials including sex-related cancers were excluded. In the trials included in the review, the most common cancers were gastrointestinal and lung, followed by leukemia.
Seventy-five percent of the subjects received chemotherapy, and the rest received either targeted therapy or immunotherapy.
Two-thirds of the subjects had at least one grade 3 or higher AE; women had a 25% higher risk than men of having AEs of grade 5 or higher.
After adjusting for age, race, disease prognosis, and other factors, women were at increased risk of severe symptomatic AEs, such as nausea and pain, across all treatment lines and especially with immunotherapy, for which reports of symptomatic AEs were 66% higher.
Women also had a higher risk of symptomatic gastrointestinal AEs with all three treatments and a higher risk of sleep-related AEs with chemotherapy and immunotherapy, which “could be a function of hormonal effects interacting with cancer treatment,” the investigators said.
As for readily measurable AEs, women were at higher risk than men for objective hematologic AEs with chemotherapy, immunotherapy, and targeted therapy. There were no statistically significant sex differences in the risk of nonhematologic objective AEs.
The team notes that increased toxicity among women has been associated with improved survival, which may give AEs more time to develop. Higher rates of AEs might also signal increased delivery or efficacy of cancer treatments.
However, a previous study found that men may have a better response to immunotherapy than women. Immune checkpoint inhibitors were twice as effective as standard cancer therapies in the treatment of men with advanced solid tumors compared to their female counterparts, concluded a team that carried out a meta-analysis of 20 randomized controlled trials involving more than 11,351 patients.
The study was funded by the National Cancer Institute and others. Dr. Unger has disclosed no relevant financial relationships. Several coauthors have reported ties to a handful of companies, including Johnson & Johnson and Seattle Genetics. One is an employee of AIM Specialty Health.
A version of this article first appeared on Medscape.com.
and this is seen with chemotherapy, targeted agents, and especially with immunotherapy.
The finding comes from a review of more than 23,000 participants across 202 trials of various cancers (excluding sex-related cancers) that has been conducted over the past 40 years.
The investigators found a 34% increased risk of severe AEs among women, compared with men, climbing to a 49% higher risk with immunotherapy.
Women had a substantially greater risk of severe symptomatic AEs, including with immune checkpoint inhibitors and targeted tyrosine kinase inhibitors, and were more likely to experience severe hematologic AEs with chemotherapy and immunotherapy.
The particularly large sex differences with immunotherapy suggest “that studying AEs from these agents is a priority,” the investigators comment.
The article was published online on Feb. 4 in the Journal of Clinical Oncology.
“It has been understood that women have more toxicity from chemotherapy than men, but almost no research has aimed to understand whether that pattern held for novel treatments like immunotherapy or targeted therapies. We found similar large differences, especially for immune treatments,” said lead investigator Joseph Unger, PhD, a biostatistician and health services researcher at the Fred Hutchinson Cancer Research Center, Seattle, in an institutional press release.
A “better understanding of the nature of the underlying mechanisms could potentially lead to interventions or delivery modifications to reduce toxicity in women,” the investigators comment in their article.
Among a sea of possible explanations for the finding, there could be differences in how men and women metabolize cancer therapies or differences in how they perceive symptoms. Women may also receive relatively higher doses because of their body size or have higher adherence to treatment.
Whatever the case, as cancer treatment becomes increasingly individualized, “sex may be an important consideration,” Dr. Unger said.
Study details
The study involved 8,838 women and 14,458 men across the trials, which were phase 2 or 3 investigations conducted by the SWOG Cancer Research Network from 1980 to 2019. Trials including sex-related cancers were excluded. In the trials included in the review, the most common cancers were gastrointestinal and lung, followed by leukemia.
Seventy-five percent of the subjects received chemotherapy, and the rest received either targeted therapy or immunotherapy.
Two-thirds of the subjects had at least one grade 3 or higher AE; women had a 25% higher risk than men of having AEs of grade 5 or higher.
After adjusting for age, race, disease prognosis, and other factors, women were at increased risk of severe symptomatic AEs, such as nausea and pain, across all treatment lines and especially with immunotherapy, for which reports of symptomatic AEs were 66% higher.
Women also had a higher risk of symptomatic gastrointestinal AEs with all three treatments and a higher risk of sleep-related AEs with chemotherapy and immunotherapy, which “could be a function of hormonal effects interacting with cancer treatment,” the investigators said.
As for readily measurable AEs, women were at higher risk than men for objective hematologic AEs with chemotherapy, immunotherapy, and targeted therapy. There were no statistically significant sex differences in the risk of nonhematologic objective AEs.
The team notes that increased toxicity among women has been associated with improved survival, which may give AEs more time to develop. Higher rates of AEs might also signal increased delivery or efficacy of cancer treatments.
However, a previous study found that men may have a better response to immunotherapy than women. Immune checkpoint inhibitors were twice as effective as standard cancer therapies in the treatment of men with advanced solid tumors compared to their female counterparts, concluded a team that carried out a meta-analysis of 20 randomized controlled trials involving more than 11,351 patients.
The study was funded by the National Cancer Institute and others. Dr. Unger has disclosed no relevant financial relationships. Several coauthors have reported ties to a handful of companies, including Johnson & Johnson and Seattle Genetics. One is an employee of AIM Specialty Health.
A version of this article first appeared on Medscape.com.
Review eyes nail unit toxicities secondary to targeted cancer therapy
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Hyperprogression on immunotherapy: When outcomes are much worse
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
Immunotherapy with checkpoint inhibitors has ushered in a new era of cancer therapy, with some patients showing dramatic responses and significantly better outcomes than with other therapies across many cancer types. But some patients do worse, sometimes much worse.
A subset of patients who undergo immunotherapy experience unexpected, rapid disease progression, with a dramatic acceleration of disease trajectory. They also have a shorter progression-free survival and overall survival than would have been expected.
This has been described as hyperprogression and has been termed “hyperprogressive disease” (HPD). It has been seen in a variety of cancers; the incidence ranges from 4% to 29% in the studies reported to date.
There has been some debate over whether this is a real phenomenon or whether it is part of the natural course of disease.
HPD is a “provocative phenomenon,” wrote the authors of a recent commentary entitled “Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact?”
“This phenomenon has polarized oncologists who debate that this could still reflect the natural history of the disease,” said the author of another commentary.
But the tide is now turning toward acceptance of HPD, said Kartik Sehgal, MD, an oncologist at Dana-Farber Cancer Institute and Harvard University, both in Boston.
“With publication of multiple clinical reports of different cancer types worldwide, hyperprogression is now accepted by most oncologists to be a true phenomenon rather than natural progression of disease,” Dr. Sehgal said.
He authored an invited commentary in JAMA Network Openabout one of the latest meta-analyses (JAMA Netw Open. 2021;4[3]:e211136) to investigate HPD during immunotherapy. One of the biggest issues is that the studies that have reported on HPD have been retrospective, with a lack of comparator groups and a lack of a standardized definition of hyperprogression. Dr. Sehgal emphasized the need to study hyperprogression in well-designed prospective studies.
Existing data on HPD
HPD was described as “a new pattern of progression” seen in patients undergoing immune checkpoint inhibitor therapy in a 2017 article published in Clinical Cancer Research. Authors Stephane Champiat, MD, PhD, of Institut Gustave Roussy, Universite Paris Saclay, Villejuif, France, and colleagues cited “anecdotal occurrences” of HPD among patients in phase 1 trials of anti–PD-1/PD-L1 agents.
In that study, HPD was defined by tumor growth rate ratio. The incidence was 9% among 213 patients.
The findings raised concerns about treating elderly patients with anti–PD-1/PD-L1 monotherapy, according to the authors, who called for further study.
That same year, Roberto Ferrara, MD, and colleagues from the Insitut Gustave Roussy reported additional data indicating an incidence of HPD of 16% among 333 patients with non–small cell lung cancer who underwent immunotherapy at eight centers from 2012 to 2017. The findings, which were presented at the 2017 World Conference on Lung Cancer and reported at the time by this news organization, also showed that the incidence of HPD was higher with immunotherapy than with single-agent chemotherapy (5%).
Median overall survival (OS) was just 3.4 months among those with HPD, compared with 13 months in the overall study population – worse, even, than the median 5.4-month OS observed among patients with progressive disease who received immunotherapy.
In the wake of these findings, numerous researchers have attempted to better define HPD, its incidence, and patient factors associated with developing HPD while undergoing immunotherapy.
However, there is little so far to show for those efforts, Vivek Subbiah, MD, of the University of Texas MD Anderson Cancer Center, Houston, said in an interview.
“Many questions remain to be answered,” said Dr. Subbiah, clinical medical director of the Clinical Center for Targeted Therapy in the division of cancer medicine at MD Anderson. He was the senior author of the “Fact, Fiction, or Alternative Fact?” commentary.
Work is underway to elucidate biological mechanisms. Some groups have implicated the Fc region of antibodies. Another group has reported EGFR and MDM2/MDM4 amplifications in patients with HPD, Dr. Subbiah and colleagues noted.
Other “proposed contributing pathological mechanisms include modulation of tumor immune microenvironment through macrophages and regulatory T cells as well as activation of oncogenic signaling pathways,” noted Dr. Sehgal.
Both groups of authors emphasize the urgent need for prospective studies.
It is imperative to confirm underlying biology, predict which patients are at risk, and identify therapeutic directions for patients who experience HPD, Dr. Subbiah said.
The main challenge is defining HPD, he added. Definitions that have been proposed include tumor growth at least two times greater than in control persons, a 15% increase in tumor burden in a set period, and disease progression of 50% from the first evaluation before treatment, he said.
The recent meta-analysis by Hyo Jung Park, MD, PhD, and colleagues, which Dr. Sehgal addressed in his invited commentary, highlights the many approaches used for defining HPD.
Depending on the definition used, the incidence of HPD across 24 studies involving more than 3,100 patients ranged from 5.9% to 43.1%.
“Hyperprogressive disease could be overestimated or underestimated based on current assessment,” Dr. Park and colleagues concluded. They highlighted the importance of “establishing uniform and clinically relevant criteria based on currently available evidence.”
Steps for solving the HPD mystery
“I think we need to come up with consensus criteria for an HPD definition. We need a unified definition,” Dr. Subbiah said. “We also need to design prospective studies to prove or disprove the immunotherapy-HPD association.”
Prospective registries with independent review of patients with suspected immunotherapy-related HPD would be useful for assessing the true incidence and the biology of HPD among patients undergoing immunotherapy, he suggested.
“We need to know the immunologic signals of HPD. This can give us an idea if patients can be prospectively identified for being at risk,” he said. “We also need to know what to do if they are at risk.”
Dr. Sehgal also called for consensus on an HPD definition, with input from a multidisciplinary group that includes “colleagues from radiology, medical oncology, radiation oncology. Getting expertise from different disciplines would be helpful,” he said.
Dr. Park and colleagues suggested several key requirements for an optimal HP definition, such as the inclusion of multiple variables for measuring tumor growth acceleration, “sufficiently quantitative” criteria for determining time to failure, and establishment of a standardized measure of tumor growth acceleration.
The agreed-upon definition of HPD could be applied to patients in a prospective registry and to existing trial data, Dr. Sehgal said.
“Eventually, the goal of this exercise is to [determine] how we can help our patients the best, having a biomarker that can at least inform us in terms of being aware and being proactive in terms of looking for this ... so that interventions can be brought on earlier,” he said.
“If we know what may be a biological mechanism, we can design trials that are designed to look at how to overcome that HPD,” he said.
Dr. Sehgal said he believes HPD is triggered in some way by treatment, including immunotherapy, chemotherapy, and targeted therapy, but perhaps in different ways for each.
He estimated the true incidence of immunotherapy-related HPD will be in the 9%-10% range.
“This is a substantial number of patients, so it’s important that we try to understand this phenomenon, using, again, uniform criteria,” he said.
Current treatment decision-making
Until more is known, Dr. Sehgal said he considers the potential risk factors when treating patients with immunotherapy.
For example, the presence of MDM2 or MDM4 amplification on a genomic profile may factor into his treatment decision-making when it comes to using immunotherapy or immunotherapy in combination with chemotherapy, he said.
“Is that the only factor that is going to make me choose one thing or another? No,” Dr. Sehgal said. However, he said it would make him more “proactive in making sure the patient is doing clinically okay” and in determining when to obtain on-treatment imaging studies.
Dr. Subbiah emphasized the relative benefit of immunotherapy, noting that survival with chemotherapy for many difficult-to-treat cancers in the relapsed/refractory metastatic setting is less than 2 years.
Immunotherapy with checkpoint inhibitors has allowed some of these patients to live longer (with survival reported to be more than 10 years for patients with metastatic melanoma).
“Immunotherapy has been a game changer; it has been transformative in the lives of these patients,” Dr. Subbiah said. “So unless there is any other contraindication, the benefit of receiving immunotherapy for an approved indication far outweighs the risk of HPD.”
A version of this article first appeared on Medscape.com.
FDA panel votes against 2 cancer indications but backs 4 of 6
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
Federal advisers have supported the efforts of pharmaceutical companies in four of six cases in which these firms are fighting to maintain cancer indications for approved drugs. The advisers voted against the companies in two cases.
The staff of the Food and Drug Administration will now consider these votes as they decide what to do regarding the six cases of what they have termed “dangling” accelerated approvals.
“One of the reasons I think we’re convening today is to prevent these accelerated approvals from dangling ad infinitum,” commented one of the members of the advisory panel.
In these cases, companies have been unable to prove the expected benefits that led the FDA to grant accelerated approvals for these indications.
These accelerated approvals, which are often based on surrogate endpoints, such as overall response rates, are granted on the condition that further findings show a clinical benefit – such as in progression-free survival or overall survival – in larger trials.
The FDA tasked its Oncologic Drugs Advisory Committee (ODAC) with conducting the review of the six accelerated approvals for cancer indications at a 3-day meeting (April 27-29).
These reviews were only for specific cancer indications and will not lead to the removal of drugs from the market. These drugs have already been approved for several cancer indications. For example, one of the drugs that was reviewed, pembrolizumab (Keytruda), is approved in the United States for 28 indications.
The FDA is facing growing pains in its efforts to manage the rapidly changing landscape for these immune checkpoint inhibitors. This field of medicine has experienced an “unprecedented level of drug development” in recent years, FDA officials said in briefing materials, owing in part to the agency’s willingness to accept surrogate markers for accelerated approvals. Although some companies have struggled with these, others have built strong cases for the use of their checkpoint inhibitors for these indications.
The ODAC panelists, for example, noted the emergence of nivolumab (Opdivo) as an option for patients with gastric cancer as a reason for seeking to withdraw an indication for pembrolizumab (Keytruda) for this disease.
Just weeks before the meeting, on April 16, the FDA approved nivolumab plus chemotherapy as a first-line treatment for advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma. This was a full approval based on data showing an overall survival benefit from a phase 3 trial.
Votes by indication
On April 29, the last day of the meeting, the ODAC panel voted 6-2 against maintaining pembrolizumab’s indication as monotherapy for an advanced form of gastric cancer. This was an accelerated approval (granted in 2017) that was based on overall response rates from an open-label trial.
That last day of the meeting also saw another negative vote. On April 29, the ODAC panel voted 5-4 against maintaining an indication for nivolumab in patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib (Nexavar).
This accelerated approval for nivolumab was granted in 2017. The FDA said it had requested ODAC’s feedback on this indication because of the recent full approval of another checkpoint inhibitor for HCC, atezolizumab (Tecentriq), in combination with bevacizumab (Avastin) for patients with unresectable or metastatic diseases who have not received prior systemic therapy. This full approval (in May 2020) was based on an overall survival benefit.
There was one last vote on the third day of the meeting, and it was positive. The ODAC panel voted 8-0 in favor of maintaining the indication for the use of pembrolizumab as monotherapy for patients with HCC who have previously been treated with sorafenib.
The FDA altered the composition of the ODAC panel during the week, adding members in some cases who had expertise in particular cancers. That led to different totals for the week’s ODAC votes, as shown in the tallies summarized below.
On the first day of the meeting (April 27), the ODAC panel voted 7-2 in favor of maintaining a breast cancer indication for atezolizumab (Tecentriq). This covered use of the immunotherapy in combination with nab-paclitaxel for patients with unresectable locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1.
The second day of the meeting (April 28) also saw two positive votes. The ODAC panel voted 10-1 for maintaining the indication for atezolizumab for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial. The panel also voted 5-3 for maintaining the indication for pembrolizumab in patients with locally advanced or metastatic urothelial carcinoma who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1.
The FDA is not bound to follow the voting and recommendations of its advisory panels, but it usually does so.
Managing shifts in treatment
In both of the cases in which ODAC voted against maintaining indications, Richard Pazdur, MD, the FDA’s top regulator for cancer medicines, jumped into the debate. Dr. Pazdur countered arguments put forward by representatives of the manufacturers as they sought to maintain indications for their drugs.
Merck officials and representatives argued for pembrolizumab, saying that maintaining the gastric cancer indication might help patients whose disease has progressed despite earlier treatment.
Dr. Pazdur emphasized that the agency would help Merck and physicians to have access to pembrolizumab for these patients even if this one indication were to be withdrawn. But Dr. Pazdur and ODAC members also noted the recent shift in the landscape for gastric cancer, with the recent approval of a new indication for nivolumab.
“I want to emphasize to the patient community out there [that] we firmly believe in the role of checkpoint inhibitors in this disease,” Dr. Pazdur said during the discussion of the indication for pembrolizumab for gastric cancer. “We have to be cognizant of what is the appropriate setting for that, and it currently is in the first line.”
Dr. Pazdur noted that two studies had failed to confirm the expected benefit from pembrolizumab for patients with more advanced disease. Still, if “small numbers” of patients with advanced disease wanted access to Merck’s drug, the FDA and the company could accommodate them. The FDA could delay the removal of the gastric indication to allow patients to continue receiving it. The FDA also could work with physicians on other routes to provide the medicine, such as through single-patient investigational new drug applications or an expanded access program.
“Or Merck can alternatively give the drug gratis to patients,” Dr. Pazdur said.
#ProjectFacilitate for expanded access
One of Merck’s speakers at the ODAC meeting, Peter Enzinger, MD, of the Dana-Farber Cancer Institute, Boston, objected to Dr. Pazdur’s plan.
A loss of the gastric indication for pembrolizumab would result in patients with advanced cancer missing out on a chance to try this therapy. Some patients will not have had a chance to try a checkpoint inhibitor earlier in their treatment, and a loss of the indication would cost them that opportunity, he said.
“An expanded-access program sounds very nice, but the reality is that our patients are incredibly sick and that weeks matter,” Dr. Enzinger said, citing administrative hurdles as a barrier to treatment.
“Our patients just don’t have the time for that, and therefore I don’t think an expanded access program is the way to go,” Dr. Enzinger said.
Dr. Pazdur responded to these objections by highlighting an initiative called Project Facilitate at the FDA’s Oncology Center for Excellence. During the meeting, Dr. Pazdur’s division used its @FDAOncology Twitter handle to draw attention to this project.
ODAC panelist Diane Reidy-Lagunes, MD, of Memorial Sloan Kettering Cancer Center, New York, said she had struggled with this vote. She was one of the two panelists to vote in favor of keeping the indication.
“This is also incredibly hard for me. I actually changed it at the last minute,” she said of her vote.
But Dr. Reidy-Lagunes said she was concerned that some patients with advanced disease might not be able to get a checkpoint inhibitor.
“With disparities in healthcare and differences in the way that patients are treated throughout our country, I was nervous that they may not be able to get treated,” she said, noting that she shared her fellow panelists’ doubts about use of pembrolizumab as third-line treatment, owing to negative results in trials.
ODAC member David Mitchell, who served as a consumer representative, also said he found the vote on the gastric indication for pembrolizumab to be a difficult decision.
“As a patient with incurable cancer who’s now being given all three major classes of drugs to treat my disease in combination, these issues really cut close to home,” Mr. Mitchell said.
He said the expectation that the FDA’s expanded access program could help patients with advanced disease try pembrolizumab helped him decide to vote with the 6-2 majority against maintaining this gastric cancer approval.
His vote was based on “the changing treatment landscape.” There is general agreement that the patients in question should receive checkpoint inhibitors as first-line treatment, not third-line treatment, Mr. Mitchell said. The FDA should delay a withdrawal of the approval for pembrolizumab in this case and should allow a transition for those who missed out on treatment with a checkpoint inhibitor earlier in the disease course, he suggested.
“To protect the safety and well-being of patients, we have to base decisions on data,” Mr. Mitchell said. “The data don’t support maintaining the indication” for pembrolizumab.
Close split on nivolumab
In contrast to the 6-2 vote against maintaining the pembrolizumab indication, the ODAC panel split more closely, 5-4, on the question of maintaining an indication for the use as monotherapy of nivolumab in HCC.
ODAC panelist Philip C. Hoffman, MD, of the University of Chicago was among those who supported keeping the indication.
“There’s still an unmet need for second-line immunotherapy because there will always be some patients who are poor candidates for bevacizumab or who are not tolerating or responding to sorafenib,” he said.
ODAC panelist Mark A. Lewis, MD, of Intermountain Healthcare, Salt Lake City, said he voted “no” in part because he doubted that Bristol-Myers Squibb would be able to soon produce data for nivolumab that was needed to support this indication.
A version of this article first appeared on Medscape.com.
To stay: Two more cancer indications with ‘dangling approvals’
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Two more cancer indications that had been granted accelerated approval by the Food and Drug Administration are going to stay in place, at least for now. This was the verdict after the second day of a historic 3-day meeting (April 27-29) and follows a similar verdict from day 1.
Federal advisers so far have supported the idea of maintaining conditional approvals of some cancer indications for a number of immune checkpoint inhibitors, despite poor results in studies that were meant to confirm the benefit of these medicines for certain patients.
On the second day (April 28) of the FDA meeting, the Oncologic Drugs Advisory Committee (ODAC) supported the views of pharmaceutical companies in two more cases of what top agency staff call “dangling accelerated approvals.”
ODAC voted 10-1 in favor of maintaining the indication for atezolizumab (Tecentriq) for the first-line treatment of cisplatin-ineligible patients with advanced/metastatic urothelial carcinoma, pending final overall survival results from the IMvigor130 trial.
ODAC also voted 5-3 that day in favor of maintaining accelerated approval for pembrolizumab (Keytruda) for first-line cisplatin- and carboplatin-ineligible patients with advanced/metastatic urothelial carcinoma.
The FDA often follows the advice of its panels, but it is not bound to do so. If the FDA were to decide to strip the indications in question from these PD-1 medicines, such decisions would not remove these drugs from the market. The three drugs have already been approved for a number of other cancer indications.
Off-label prescribing is not uncommon in oncology, but a loss of an approved indication would affect reimbursement for these medicines, Scot Ebbinghaus, MD, vice president of oncology clinical research at Merck (the manufacturer of pembrolizumab), told ODAC members during a discussion of the possible consequences of removing the indications in question.
“Access to those treatments may end up being substantially limited, and really the best way to ensure that there’s access is to maintain FDA approval,” Dr. Ebbinghaus said.
Another participant at the meeting asked the panel and the FDA to consider the burden on patients in paying for medicines that have not yet proved to be beneficial.
Diana Zuckerman, PhD, of the nonprofit National Center for Health Research, noted that the ODAC panel included physicians who see cancer patients.
“You’re used to trying different types of treatments in hopes that something will work,” she said. “Shouldn’t cancer patients be eligible for free treatment in clinical trials instead of paying for treatment that isn’t proven to work?”
Rapid development of PD-1 drugs
Top officials at the FDA framed the challenges with accelerated approvals for immunotherapy drugs in an April 21 article in The New England Journal of Medicine. Over the course of about 6 years, the FDA approved six of these PD-1 or PD-L1 drugs for more than 75 indications in oncology, wrote Richard Pazdur, MD, and Julia A. Beaver, MD, of the FDA.
“Development of drugs in this class occurred more rapidly than that in any other therapeutic area in history,” they wrote.
In 10 cases, the required follow-up trials did not confirm the expected benefit, and yet marketing authorization for these drugs continued, leading Dr. Pazdur and Dr. Beaver to dub these “dangling” accelerated approvals. Four of these indications were voluntarily withdrawn. For the other six indications, the FDA sought feedback from ODAC during the 3-day meeting. Over the first 2 days of the meeting, ODAC recommended that three of these cancer indications remain. Three more will be considered on the last day of the meeting.
A version of this article first appeared on Medscape.com.
Allo-HSCT plus monoclonal antibody treatment can improve survival in patients with r/r B-ALL
The use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in minimal residual disease (MRD)–negative remission patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) after the start of monoclonal antibody treatment, according to the results of a landmark analysis presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Previous studies have indicated that allo-HSCT improves the results of treatment in r/r B-ALL patients, compared with chemotherapy alone. In addition, it has been found that the monoclonal antibodies (Mab), anti-CD19-blinatumomab and anti-CD22-inotuzumab ozogamicin, induced remission in a significant proportion of such patients.
To determine if the use of allo-HSCT improves the outcome of patients in MRD-negative remission with or without Mab treatment, researchers performed a landmark analysis of 110 patients who achieved MRD-negative status after Mab treatment. The analysis examined results at 2, 4, and 6 months subsequent to the initiation of Mab treatment, according to poster presentation by Inna V. Markova, MD, and colleagues at Pavlov University, Saint Petersburg, Russian Federation.
Study details
The researchers included 110 patients who achieved MRD-negative status outside of clinical trials at a single institution in the analysis. Forty of the patients (36%) were children and 70 (64%) were adults. The median age for all patients was 23 years and the median follow up was 24 months. Fifty-seven (52%) and 53 (48%) patients received Mab for hematological relapse and persistent measurable residual disease or for molecular relapse, respectively. Therapy with Mab alone without subsequent allo-HSCT was used in 36 (31%) patients (30 received blinatumomab and 6 received inotuzumab ozogamicin). A total of 74 (69%) patients received allo-HSCT from a matched related or unrelated donor (MD-HSCT, n = 38) or haploidentical donor (Haplo-HSCT, n = 36). All patients received posttransplantation cyclophosphamide (PTCY)–based graft-versus host disease (GVHD) prophylaxis. Landmark analysis was performed at 2, 4, and 6 months after Mab therapy initiation to determine the effect of allo-HSCT on the outcome and the optimal timing of HSCT. Overall survival and disease-free survival (DFS) were used as outcomes.
Promising results
No significant differences between the MD-HSCT, Mab alone, and Haplo-HSCT groups were observed in 2-month landmark analysis (P = .4 for OS and P =.65 for DFS). However, the 4-month landmark analysis demonstrated superior overall survival and DFS in patients after MD-HSCT, but not Haplo-HSCT, compared with Mab alone: 2-year OS was 75%, 50%, and 27,7% (P = .032) and DFS was 53.5%, 51.3%, and 16.6% (P = .02) for MD-HSCT, Mab alone and Haplo-HSCT groups, respectively. In addition, 6-month analysis showed that there was no benefit from subsequent transplantation, according to the authors, with regard to overall survival (P = .11).
“Our study demonstrated that at least MD-HSCT with PTCY platform improves survival in MRD-negative remission if performed during the first 4 months after Mab initiation. Haplo-HSCT or MD-HSCT beyond 4 months are not associated with improved outcomes in this groups of patients,” the researchers concluded.
The researchers reported they had no conflicts of interest to declare.
The use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in minimal residual disease (MRD)–negative remission patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) after the start of monoclonal antibody treatment, according to the results of a landmark analysis presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Previous studies have indicated that allo-HSCT improves the results of treatment in r/r B-ALL patients, compared with chemotherapy alone. In addition, it has been found that the monoclonal antibodies (Mab), anti-CD19-blinatumomab and anti-CD22-inotuzumab ozogamicin, induced remission in a significant proportion of such patients.
To determine if the use of allo-HSCT improves the outcome of patients in MRD-negative remission with or without Mab treatment, researchers performed a landmark analysis of 110 patients who achieved MRD-negative status after Mab treatment. The analysis examined results at 2, 4, and 6 months subsequent to the initiation of Mab treatment, according to poster presentation by Inna V. Markova, MD, and colleagues at Pavlov University, Saint Petersburg, Russian Federation.
Study details
The researchers included 110 patients who achieved MRD-negative status outside of clinical trials at a single institution in the analysis. Forty of the patients (36%) were children and 70 (64%) were adults. The median age for all patients was 23 years and the median follow up was 24 months. Fifty-seven (52%) and 53 (48%) patients received Mab for hematological relapse and persistent measurable residual disease or for molecular relapse, respectively. Therapy with Mab alone without subsequent allo-HSCT was used in 36 (31%) patients (30 received blinatumomab and 6 received inotuzumab ozogamicin). A total of 74 (69%) patients received allo-HSCT from a matched related or unrelated donor (MD-HSCT, n = 38) or haploidentical donor (Haplo-HSCT, n = 36). All patients received posttransplantation cyclophosphamide (PTCY)–based graft-versus host disease (GVHD) prophylaxis. Landmark analysis was performed at 2, 4, and 6 months after Mab therapy initiation to determine the effect of allo-HSCT on the outcome and the optimal timing of HSCT. Overall survival and disease-free survival (DFS) were used as outcomes.
Promising results
No significant differences between the MD-HSCT, Mab alone, and Haplo-HSCT groups were observed in 2-month landmark analysis (P = .4 for OS and P =.65 for DFS). However, the 4-month landmark analysis demonstrated superior overall survival and DFS in patients after MD-HSCT, but not Haplo-HSCT, compared with Mab alone: 2-year OS was 75%, 50%, and 27,7% (P = .032) and DFS was 53.5%, 51.3%, and 16.6% (P = .02) for MD-HSCT, Mab alone and Haplo-HSCT groups, respectively. In addition, 6-month analysis showed that there was no benefit from subsequent transplantation, according to the authors, with regard to overall survival (P = .11).
“Our study demonstrated that at least MD-HSCT with PTCY platform improves survival in MRD-negative remission if performed during the first 4 months after Mab initiation. Haplo-HSCT or MD-HSCT beyond 4 months are not associated with improved outcomes in this groups of patients,” the researchers concluded.
The researchers reported they had no conflicts of interest to declare.
The use of allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve survival in minimal residual disease (MRD)–negative remission patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) after the start of monoclonal antibody treatment, according to the results of a landmark analysis presented at the virtual meeting of the European Society for Blood and Marrow Transplantation.
Previous studies have indicated that allo-HSCT improves the results of treatment in r/r B-ALL patients, compared with chemotherapy alone. In addition, it has been found that the monoclonal antibodies (Mab), anti-CD19-blinatumomab and anti-CD22-inotuzumab ozogamicin, induced remission in a significant proportion of such patients.
To determine if the use of allo-HSCT improves the outcome of patients in MRD-negative remission with or without Mab treatment, researchers performed a landmark analysis of 110 patients who achieved MRD-negative status after Mab treatment. The analysis examined results at 2, 4, and 6 months subsequent to the initiation of Mab treatment, according to poster presentation by Inna V. Markova, MD, and colleagues at Pavlov University, Saint Petersburg, Russian Federation.
Study details
The researchers included 110 patients who achieved MRD-negative status outside of clinical trials at a single institution in the analysis. Forty of the patients (36%) were children and 70 (64%) were adults. The median age for all patients was 23 years and the median follow up was 24 months. Fifty-seven (52%) and 53 (48%) patients received Mab for hematological relapse and persistent measurable residual disease or for molecular relapse, respectively. Therapy with Mab alone without subsequent allo-HSCT was used in 36 (31%) patients (30 received blinatumomab and 6 received inotuzumab ozogamicin). A total of 74 (69%) patients received allo-HSCT from a matched related or unrelated donor (MD-HSCT, n = 38) or haploidentical donor (Haplo-HSCT, n = 36). All patients received posttransplantation cyclophosphamide (PTCY)–based graft-versus host disease (GVHD) prophylaxis. Landmark analysis was performed at 2, 4, and 6 months after Mab therapy initiation to determine the effect of allo-HSCT on the outcome and the optimal timing of HSCT. Overall survival and disease-free survival (DFS) were used as outcomes.
Promising results
No significant differences between the MD-HSCT, Mab alone, and Haplo-HSCT groups were observed in 2-month landmark analysis (P = .4 for OS and P =.65 for DFS). However, the 4-month landmark analysis demonstrated superior overall survival and DFS in patients after MD-HSCT, but not Haplo-HSCT, compared with Mab alone: 2-year OS was 75%, 50%, and 27,7% (P = .032) and DFS was 53.5%, 51.3%, and 16.6% (P = .02) for MD-HSCT, Mab alone and Haplo-HSCT groups, respectively. In addition, 6-month analysis showed that there was no benefit from subsequent transplantation, according to the authors, with regard to overall survival (P = .11).
“Our study demonstrated that at least MD-HSCT with PTCY platform improves survival in MRD-negative remission if performed during the first 4 months after Mab initiation. Haplo-HSCT or MD-HSCT beyond 4 months are not associated with improved outcomes in this groups of patients,” the researchers concluded.
The researchers reported they had no conflicts of interest to declare.
FROM EBMT 2021
Checkpoint inhibitor–induced rheumatic complications often arise late
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
Most checkpoint inhibitor–induced rheumatic complications in cancer patients can be treated successfully with corticosteroids, albeit often at considerably higher doses than rheumatologists typically use in managing rheumatoid arthritis, Eric M. Ruderman, MD, observed at the 2021 Rheumatology Winter Clinical Symposium.
“In RA, we’re all used to the idea that 5 or 10 mg of corticosteroids per day can make a tremendous difference. That’s not always the case here. Patients who develop rheumatic immunotherapy-related adverse events often require 20-30 mg/day to get symptoms under control,” according to Dr. Ruderman, professor of medicine (rheumatology) at Northwestern University, Chicago.
This may be in part because oncologists typically don’t refer affected patients to rheumatologists early on. Guidelines from the National Comprehensive Cancer Network and other oncology groups suggest referral only once a patient develops grade 3 immunotherapy-related rheumatic adverse events, meaning the symptoms significantly impair daily activities, he explained.
Checkpoint inhibitors, which induce T-cell activation to fight the patient’s malignancy, can produce a plethora of off-target effects. These adverse events may involve the skin, heart, lungs, kidneys, eyes, blood, GI tract, and endocrine organs. The drugs also can cause rheumatic or neurologic complications. The most common of these adverse events are colitis and rash. Next most common are arthritis and arthralgia. Rheumatic side effects are most common as a consequence of immunotherapy using a CTLA4 (cytotoxic T-lymphocyte-associated protein 4) inhibitor, but can also occur in association with programmed cell death protein 1 (PD-1) inhibitors and PD-ligand 1 inhibitors. Arthritis and other rheumatic adverse events are more common in patients undergoing combination therapy.
Some form of frank inflammatory arthritis occurs in 5%-10% of cancer patients undergoing checkpoint inhibitor therapy. This can manifest as an RA-like polyarthritis, spondyloarthritis, polymyalgia rheumatica, necrotizing myositis, or vasculitis. Arthralgia occurs in up to 40% of treated patients.
This immunotherapy-related arthritis is typically more inflammatory than RA. It also has a much more abrupt onset. It is usually seronegative and has no gender predisposition, and the limited available evidence to date suggests there is no increased risk of this complication in checkpoint inhibitor–treated patients with a history of prior rheumatic disease, according to Dr. Ruderman.
Delayed onset and resolution of rheumatologic immune-related adverse events
“Onset and resolution of rheumatologic adverse events with immunotherapy may be delayed. This is an important point: While skin rash and colitis often show up pretty early in the course of immunotherapy, some of the arthritic events can happen later. They can actually continue after the immunotherapy is stopped,” the rheumatologist said.
Indeed, a retrospective nationwide Canadian study of 117 patients at nine academic centers who developed 136 rheumatic immune-related adverse events in conjunction with cancer immunotherapy found that the mean time to the first such event was 6.8 months into checkpoint inhibitor therapy. The most common rheumatic complication was symmetric polyarthritis, affecting 45 patients. Other rheumatologic immune-related complications included polymyalgia rheumatica in 17 patients, noninflammatory musculoskeletal symptoms in 18, and myositis in 9.
Seventy-six patients were treated with prednisone for a mean of 8.4 months at a maximum dose of 60 mg/day. Forty-two moved up the treatment ladder to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) to manage their symptoms. Only two patients required escalation to biologic therapy. A reassuring finding in this relatively small study was that treatment of the patients’ rheumatic complications didn’t appear to worsen the tumor response to immunotherapy: Twenty-three patients experienced tumor progression prior to treatment of their rheumatic disorder, and 14 did so following treatment.
Flares of preexisting rheumatic diseases
These tend to occur much earlier in the course of immune checkpoint inhibitor therapy for cancer than de novo immunotherapy-related rheumatic adverse events. In a retrospective Australian study of 12 cancer patients with preexisting rheumatic disease before going on a PD-1 inhibitor and 24 others with no such history, all of whom developed rheumatic adverse events while on the checkpoint inhibitor, the mean time to a flare of preexisting rheumatic disease was 6.2 weeks, compared to 21.5 weeks in patients who experienced a de novo rheumatic adverse event.
Dr. Ruderman supports recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of rheumatic immune-related adverse events due to cancer immunotherapy, even though the underlying level of evidence is fairly weak. The recommendations call for the use of csDMARDs when corticosteroids don’t adequately control symptoms. And when the response to csDMARDs is insufficient, the next step is a biologic, preferably a tumor necrosis factor inhibitor or interleukin-6 inhibitor.
“At our institution, the oncologists are a little bit nervous about using biologics in cancer patients, but I think more and more they’re going to have to accept it. And so far there isn’t a ton of evidence that suggests the addition of biologics interferes with the efficacy of the immunotherapy,” the rheumatologist said.
He underscored the critical importance of one of the overarching principles of the EULAR guidelines: the need for interdisciplinary coordination between rheumatologists and oncologists regarding the problem of rheumatologic immune-related adverse events.
“Oncologists aren’t good at managing inflammatory arthritis. I think they really need us,” he said.
Dr. Ruderman reported serving as a consultant to and/or receiving a research grant from nine pharmaceutical companies.
FROM RWCS 2021