User login
Nearly half of infants don’t sleep through the night at 1 year
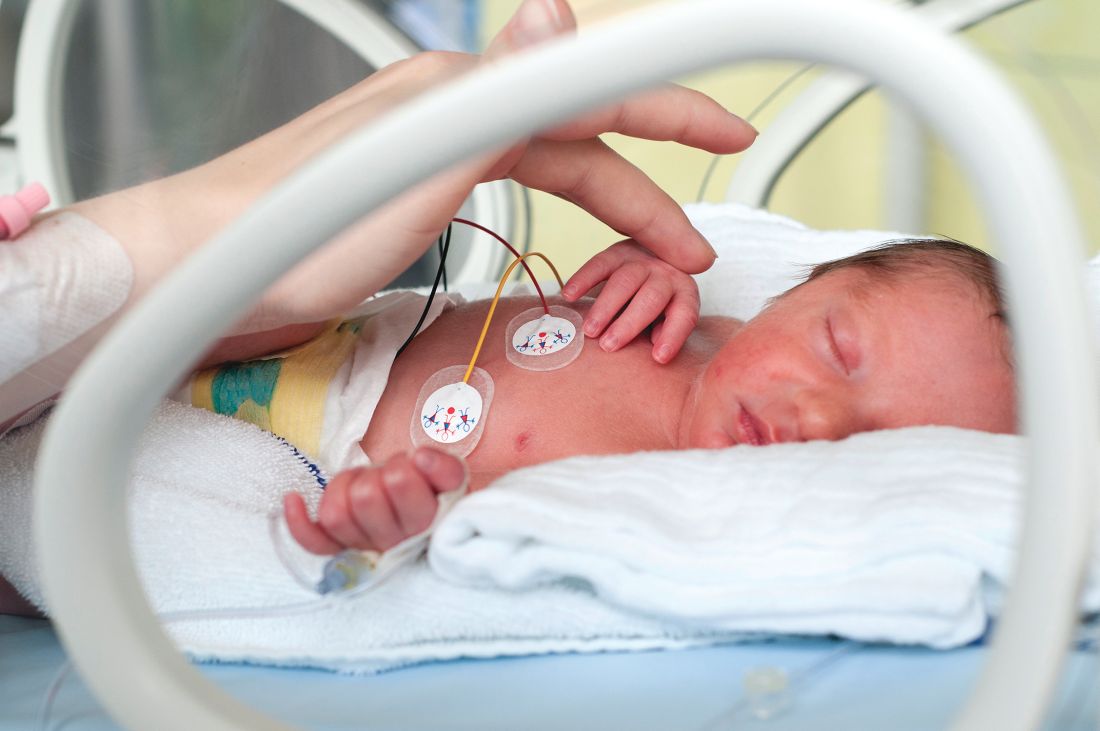
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
FROM PEDIATRICS
Key clinical point: Sleeping through the night in infancy was not significantly associated with any variations in development or maternal mood.
Major finding: There were no associations between 6 or 8 hours of uninterrupted sleep and concurrent mental or psychomotor development or reported depressive symptoms.
Study details: An analysis of 388 mother-infant dyads in a longitudinal birth cohort study.
Disclosures: Authors said they had no financial relationships or potential conflicts of interest to disclose.
Source: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Platelet transfusion threshold matters for preterm infants
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
A lower threshold for platelet transfusions in preterm infants with severe thrombocytopenia is associated with significantly lower incidence of death and major bleeding, compared with a higher threshold, a new study suggests.
A new major bleeding episode or death occurred in 26% of infants in the high-threshold group, compared with 19% in the low-threshold group, representing a 57% higher risk of poor outcomes even after researchers adjusted for gestational age and intrauterine growth restriction (odds ratio, 1.57; P = .02).
Researchers reported the results of a trial in 660 infants with a mean gestational age of 26.6 weeks, who were randomized to a platelet infusion either at a high platelet–count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
“Although retrospective studies have suggested that platelet transfusions may cause harm in neonates independently of the disease process, data from randomized controlled trials to support this are lacking,” Anna Curley, MD, of the National Maternity Hospital in Dublin and her coauthors reported in the New England Journal of Medicine.
The rates of minor or worse bleeding were similar between the two groups, and the percentage of infants surviving with bronchopulmonary dysplasia at 36 weeks of corrected age was higher in the high-threshold group (63% vs. 54%; OR, 1.54).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups.
The outcomes of transfusions were not influenced by other factors such as intrauterine growth restriction, gestational age, or postnatal age at randomization.
“Our trial highlights the importance of trials of platelet transfusion involving patients with conditions other than haematological malignancies,” the authors wrote.
However they acknowledged that the reasons for the differences in mortality and outcomes between the two study groups were unknown.
“Platelets have recognized immunological and inflammatory effects, outside of effects on hemostasis,” they wrote. “The effect of transfusing adult platelets to a delicately balance neonatal hemostatic system with relatively hypofunctional platelets is also poorly understood.”
The study was supported by the National Health Service Blood and Transplant Research and Development Committee and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
SOURCE: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The odds of new major bleeding or death were 57% higher in preterm infants who received a platelet transfusion at a higher threshold of 50,000 per mm3 than at a lower threshold of 25,000 mm3 (P = .02).Study details: Randomized study in 660 preterm infants with severe thrombocytopenia.
Disclosures: The study was supported by the National Health Service Blood and Transplant Research and Development Committee, and other foundations. Authors reported financial disclosures related to Sanquin and Cerus.
Source: Curley A et al. N Engl J Med. 2018 Nov 2. doi: 10.1056/NEJMoa1807320.
In pediatric ICU, being underweight can be deadly
SAN DIEGO – Underweight people don’t get much attention amid the obesity epidemic. But a new analysis of worldwide data finds that underweight pediatric ICU patients worldwide face a higher risk of death within 28 days than all their counterparts, even the overweight and obese.
While the report suggests that underweight patients weren’t sicker than the other children and young adults, they also faced a higher risk of fluid accumulation and all-stage acute kidney injury, compared with overweight children, study lead author Rajit K. Basu, MD, MS, of Emory University and Children’s Healthcare of Atlanta, said in an interview. His team’s findings were released at Kidney Week 2018, sponsored by the American Society of Nephrology.
“Obesity gets the lion’s share of the spotlight, but there is a large and likely growing population of children who, for reasons left to be fully parsed out, are underweight,” Dr. Basu said. “These patients have increased attributable risks for poor outcome.”
The new report is a follow-up analysis of a 2017 prospective study by the same team that tracked acute kidney injury and mortality in 4,683 pediatric ICU patients at 32 clinics in Asia, Australia, Europe, and North America. The patients, aged from 3 months to 25 years, were recruited over 3 months in 2014 (N Engl J Med 2017;376:11-20).
The researchers launched the study to better understand the risk facing underweight pediatric patients. “There is a paucity of data linking mortality to weight classification in children,” Dr. Basu said. “There are only a few reports, and there is a suggestion that the ‘obesity paradox’ – protection from morbidity and mortality because of excessive weight – exists.”
For the new analysis, researchers tracked 3,719 patients: 29% were underweight, 44% had normal weight, 11% were overweight, and 16% were obese.
The 28-day mortality rate was 4% overall and highest in the underweight patients at 6%, compared with normal (3%), overweight (2%), and obese patients (2%) (P less than .0001). Underweight patients had a higher adjusted risk of mortality, compared with normal-weight patients (adjusted odds ratio, 1.8; 95% confidence interval, 1.2-2.8).
Underweight patients also had “a higher risk of fluid accumulation and a higher incidence of all-stage acute kidney injury, compared to overweight children,” Dr. Basu said.
The study authors also examined mortality rates in the 14% of patients (n = 542) who had sepsis. Again, underweight patients had the highest risk of 28-day mortality (15%), compared with normal weight (7%), overweight (4%), and obese patients (5%) (P = 0.003).
Who are the underweight children? “Analysis of the comorbidities reveals that nearly one-third of these children had some neuromuscular and/or pulmonary comorbidities, implying that these children were most likely static cerebral palsy children or had neuromuscular developmental disorder,” Dr. Basu said. “The demographic data also interestingly pointed out that the underweight population was predominantly Eastern Asian in origin.”
But there wasn’t a sign of increased illness in the underweight patients. “We can say that these kids were no sicker compared to the overweight kids as assessed by objective severity-of-illness scoring tools used in the critically ill population,” he said.
Is there a link between fluid overload and higher mortality numbers in underweight children? “There is a preponderance of data now, particularly in children, associating excessive fluid accumulation and poor outcome,” Dr. Basu said, who pointed to a 2018 systematic review and analysis that linked fluid overload to a higher risk of in-hospital mortality (OR, 4.34; 95% CI, 3.01-6.26) (JAMA Pediatr. 2018;172[3]:257-68).
Fluid accumulation disrupts organs “via hydrostatic pressure overregulation, causing an imbalance in local mediators of hormonal homeostasis and through vascular congestion,” he said. However, best practices regarding fluid are not yet clear.
“Fluid accumulation does occur frequently,” he said, “and it is likely a very important and relevant part of practice for bedside providers to be mindful on a multiple-times-a-day basis of what is happening with net fluid balance and how that relates to end-organ function, particularly the lungs and the kidneys.”
The National Institutes of Health provided partial funding for the study. One of the authors received fellowship funding from Gambro/Baxter Healthcare.
SAN DIEGO – Underweight people don’t get much attention amid the obesity epidemic. But a new analysis of worldwide data finds that underweight pediatric ICU patients worldwide face a higher risk of death within 28 days than all their counterparts, even the overweight and obese.
While the report suggests that underweight patients weren’t sicker than the other children and young adults, they also faced a higher risk of fluid accumulation and all-stage acute kidney injury, compared with overweight children, study lead author Rajit K. Basu, MD, MS, of Emory University and Children’s Healthcare of Atlanta, said in an interview. His team’s findings were released at Kidney Week 2018, sponsored by the American Society of Nephrology.
“Obesity gets the lion’s share of the spotlight, but there is a large and likely growing population of children who, for reasons left to be fully parsed out, are underweight,” Dr. Basu said. “These patients have increased attributable risks for poor outcome.”
The new report is a follow-up analysis of a 2017 prospective study by the same team that tracked acute kidney injury and mortality in 4,683 pediatric ICU patients at 32 clinics in Asia, Australia, Europe, and North America. The patients, aged from 3 months to 25 years, were recruited over 3 months in 2014 (N Engl J Med 2017;376:11-20).
The researchers launched the study to better understand the risk facing underweight pediatric patients. “There is a paucity of data linking mortality to weight classification in children,” Dr. Basu said. “There are only a few reports, and there is a suggestion that the ‘obesity paradox’ – protection from morbidity and mortality because of excessive weight – exists.”
For the new analysis, researchers tracked 3,719 patients: 29% were underweight, 44% had normal weight, 11% were overweight, and 16% were obese.
The 28-day mortality rate was 4% overall and highest in the underweight patients at 6%, compared with normal (3%), overweight (2%), and obese patients (2%) (P less than .0001). Underweight patients had a higher adjusted risk of mortality, compared with normal-weight patients (adjusted odds ratio, 1.8; 95% confidence interval, 1.2-2.8).
Underweight patients also had “a higher risk of fluid accumulation and a higher incidence of all-stage acute kidney injury, compared to overweight children,” Dr. Basu said.
The study authors also examined mortality rates in the 14% of patients (n = 542) who had sepsis. Again, underweight patients had the highest risk of 28-day mortality (15%), compared with normal weight (7%), overweight (4%), and obese patients (5%) (P = 0.003).
Who are the underweight children? “Analysis of the comorbidities reveals that nearly one-third of these children had some neuromuscular and/or pulmonary comorbidities, implying that these children were most likely static cerebral palsy children or had neuromuscular developmental disorder,” Dr. Basu said. “The demographic data also interestingly pointed out that the underweight population was predominantly Eastern Asian in origin.”
But there wasn’t a sign of increased illness in the underweight patients. “We can say that these kids were no sicker compared to the overweight kids as assessed by objective severity-of-illness scoring tools used in the critically ill population,” he said.
Is there a link between fluid overload and higher mortality numbers in underweight children? “There is a preponderance of data now, particularly in children, associating excessive fluid accumulation and poor outcome,” Dr. Basu said, who pointed to a 2018 systematic review and analysis that linked fluid overload to a higher risk of in-hospital mortality (OR, 4.34; 95% CI, 3.01-6.26) (JAMA Pediatr. 2018;172[3]:257-68).
Fluid accumulation disrupts organs “via hydrostatic pressure overregulation, causing an imbalance in local mediators of hormonal homeostasis and through vascular congestion,” he said. However, best practices regarding fluid are not yet clear.
“Fluid accumulation does occur frequently,” he said, “and it is likely a very important and relevant part of practice for bedside providers to be mindful on a multiple-times-a-day basis of what is happening with net fluid balance and how that relates to end-organ function, particularly the lungs and the kidneys.”
The National Institutes of Health provided partial funding for the study. One of the authors received fellowship funding from Gambro/Baxter Healthcare.
SAN DIEGO – Underweight people don’t get much attention amid the obesity epidemic. But a new analysis of worldwide data finds that underweight pediatric ICU patients worldwide face a higher risk of death within 28 days than all their counterparts, even the overweight and obese.
While the report suggests that underweight patients weren’t sicker than the other children and young adults, they also faced a higher risk of fluid accumulation and all-stage acute kidney injury, compared with overweight children, study lead author Rajit K. Basu, MD, MS, of Emory University and Children’s Healthcare of Atlanta, said in an interview. His team’s findings were released at Kidney Week 2018, sponsored by the American Society of Nephrology.
“Obesity gets the lion’s share of the spotlight, but there is a large and likely growing population of children who, for reasons left to be fully parsed out, are underweight,” Dr. Basu said. “These patients have increased attributable risks for poor outcome.”
The new report is a follow-up analysis of a 2017 prospective study by the same team that tracked acute kidney injury and mortality in 4,683 pediatric ICU patients at 32 clinics in Asia, Australia, Europe, and North America. The patients, aged from 3 months to 25 years, were recruited over 3 months in 2014 (N Engl J Med 2017;376:11-20).
The researchers launched the study to better understand the risk facing underweight pediatric patients. “There is a paucity of data linking mortality to weight classification in children,” Dr. Basu said. “There are only a few reports, and there is a suggestion that the ‘obesity paradox’ – protection from morbidity and mortality because of excessive weight – exists.”
For the new analysis, researchers tracked 3,719 patients: 29% were underweight, 44% had normal weight, 11% were overweight, and 16% were obese.
The 28-day mortality rate was 4% overall and highest in the underweight patients at 6%, compared with normal (3%), overweight (2%), and obese patients (2%) (P less than .0001). Underweight patients had a higher adjusted risk of mortality, compared with normal-weight patients (adjusted odds ratio, 1.8; 95% confidence interval, 1.2-2.8).
Underweight patients also had “a higher risk of fluid accumulation and a higher incidence of all-stage acute kidney injury, compared to overweight children,” Dr. Basu said.
The study authors also examined mortality rates in the 14% of patients (n = 542) who had sepsis. Again, underweight patients had the highest risk of 28-day mortality (15%), compared with normal weight (7%), overweight (4%), and obese patients (5%) (P = 0.003).
Who are the underweight children? “Analysis of the comorbidities reveals that nearly one-third of these children had some neuromuscular and/or pulmonary comorbidities, implying that these children were most likely static cerebral palsy children or had neuromuscular developmental disorder,” Dr. Basu said. “The demographic data also interestingly pointed out that the underweight population was predominantly Eastern Asian in origin.”
But there wasn’t a sign of increased illness in the underweight patients. “We can say that these kids were no sicker compared to the overweight kids as assessed by objective severity-of-illness scoring tools used in the critically ill population,” he said.
Is there a link between fluid overload and higher mortality numbers in underweight children? “There is a preponderance of data now, particularly in children, associating excessive fluid accumulation and poor outcome,” Dr. Basu said, who pointed to a 2018 systematic review and analysis that linked fluid overload to a higher risk of in-hospital mortality (OR, 4.34; 95% CI, 3.01-6.26) (JAMA Pediatr. 2018;172[3]:257-68).
Fluid accumulation disrupts organs “via hydrostatic pressure overregulation, causing an imbalance in local mediators of hormonal homeostasis and through vascular congestion,” he said. However, best practices regarding fluid are not yet clear.
“Fluid accumulation does occur frequently,” he said, “and it is likely a very important and relevant part of practice for bedside providers to be mindful on a multiple-times-a-day basis of what is happening with net fluid balance and how that relates to end-organ function, particularly the lungs and the kidneys.”
The National Institutes of Health provided partial funding for the study. One of the authors received fellowship funding from Gambro/Baxter Healthcare.
REPORTING FROM KIDNEY WEEK 2018
Key clinical point:
Major finding: Underweight patients had a higher adjusted risk of 28-day mortality than normal-weight patients (adjusted odds ratio, 1.8; 95% confidence interval, 1.2-2.8).
Study details: A follow-up analysis of 3,719 pediatric ICU patients, aged from 3 months to 25 years, recruited in a prospective study over 3 months in 2014 at 32 worldwide centers.
Disclosures: The National Institutes of Health provided partial funding for the study. One of the authors received fellowship funding from Gambro/Baxter Healthcare.
How to help crying infants
Babies evolved to cry to get their needs met and adults evolved to be aroused by the sound. Perfect match, right? But crying/fussing was rated the No. 1 hardest part of parenting for 0- to 3-year-olds in our data from more than 68,000 parents.
As clinicians, we become amazingly immune to the crying in our offices, but hopefully not to crying as a concern of parents. Our training directs us to look for pathology such as under- or overfeeding, infection, gastroesophageal reflux disease (GERD), volvulus, a hair tourniquet, or an injury as causes of crying. Having ruled these out, the bigger task is making sure that parents learn to read their infants’ crying and find ways to console them. Learning to handle crying can be tense, frustrating, and upsetting for parents, but success is ultimately satisfying and an important part of the reciprocal interaction that builds attachment for both parent and child.
“Developmental crying” is a great term for explaining crying in the first 3 months to families, as it is age related. The acronym PURPLE was created to teach about this normal crying.
- “P” is for peak of crying – babies may cry more each week, most in month 2, then less in months 3-5.
- “U” is for unexpected – crying can come and go without explanation.
- “R” is for resists soothing – babies may not stop crying no matter what is tried.
- “P” is for pain-like face – babies appear to be in pain, even if they are not.
- “L” is for long lasting – crying can last 5 hours a day or more.
- “E” is for evening – the baby might cry more in the late afternoon and evening.
The 1- to 2-week visit is a key time to teach parents about the expected upcoming crying and ways to manage it. I lean on the evidence-based steps for soothing, using the 5 S’s described by Harvey Karp, MD, based on the wisdom of T. Berry Brazelton, MD. These include:
- Swaddling in a wrap that constrains arms and legs.
- Side or stomach holding (but not for sleeping).
- Shushing sounds of voice, radio static, fan, air conditioner, or car ride.
- Swinging gently (point out to never shake a baby).
- Sucking on a pacifier, finger, or hand.
Because babies change state slowly and also respond to high caregiver emotion such as anxiety, it is important for parents to take some deep breaths and give each “S” several minutes to have an effect.
Some of our patients will go beyond typical crying to colic, defined as crying for at least 3 hours per day, at least 3 days per week, starting before 3 months post term. While typically easing by age 3 months, colic can in nightmare cases persist to age 1 year. Needless to say, prolonged crying can be an enormous stress for families. Researchers in the Fussy Baby Network consulting on infant crying have found value in a family prescription for REST: Reassurance, Empathy, Support, and Time away. Reassurance that the child is not ill should be provided after a careful history, including asking why parents think the baby is crying, and a physical exam, even if we think we can tell at a glance that the baby is okay. Remember that every parent’s first concern is whether the baby is okay, and crying indicates otherwise. Parents are counting on us for a thorough exam before we reassure them. Exhausted new parents deserve empathy – acknowledgment of how difficult, scary, and maddening it is to not be able to console their newborns.
While friends are saying “You must be so happy!” after a child is born, ambivalence (What have I done to my life?) is very common, but not easy to admit. We can point out that the typical age at which parents report “loving” their infants is actually more like 6 weeks, when they finally smile! To acknowledge ambivalence, I may say, “When you feel like throwing him out the window, it’s okay to lay him in the crib and put on headphones for a few minutes.” Music, meditation, yoga, or exercise are break activities that also can reduce parent stress.
Support for the parents is one of the most important protections for children at all ages, but can’t be assumed. Unfortunately, crying tends to increase beginning at 2 weeks post term, right when the partner returns to work or relatives leave. It is important to ask, “Who is helping you with the baby?” Just having a partner doesn’t mean that person is taking a turn! Fathers may be afraid of holding a baby, or may have no experience and try to defer. We need to encourage fathers in all aspects of care but especially to “find at least one way to console your baby.” Sometimes mothers “rescue” dads too quickly. While mothers may have both more experience with infants and the magic of breastfeeding for consoling, depriving fathers of working through the struggle of managing crying can result in their developing less confidence in caregiving. It also can cause them to miss out on supporting the mother at this delicate time, a chance to profoundly strengthen the partnership.
Taking into account the importance for parents of finding ways to console crying babies, getting some “time away” can be helpful, especially if crying goes on for months. Maybe those friends who asked, “What can I do to help?” could come over for an hour in the evening (peak crying time) to hold the baby. This also can be a chance for parent time together, a newly rare commodity.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Babies evolved to cry to get their needs met and adults evolved to be aroused by the sound. Perfect match, right? But crying/fussing was rated the No. 1 hardest part of parenting for 0- to 3-year-olds in our data from more than 68,000 parents.
As clinicians, we become amazingly immune to the crying in our offices, but hopefully not to crying as a concern of parents. Our training directs us to look for pathology such as under- or overfeeding, infection, gastroesophageal reflux disease (GERD), volvulus, a hair tourniquet, or an injury as causes of crying. Having ruled these out, the bigger task is making sure that parents learn to read their infants’ crying and find ways to console them. Learning to handle crying can be tense, frustrating, and upsetting for parents, but success is ultimately satisfying and an important part of the reciprocal interaction that builds attachment for both parent and child.
“Developmental crying” is a great term for explaining crying in the first 3 months to families, as it is age related. The acronym PURPLE was created to teach about this normal crying.
- “P” is for peak of crying – babies may cry more each week, most in month 2, then less in months 3-5.
- “U” is for unexpected – crying can come and go without explanation.
- “R” is for resists soothing – babies may not stop crying no matter what is tried.
- “P” is for pain-like face – babies appear to be in pain, even if they are not.
- “L” is for long lasting – crying can last 5 hours a day or more.
- “E” is for evening – the baby might cry more in the late afternoon and evening.
The 1- to 2-week visit is a key time to teach parents about the expected upcoming crying and ways to manage it. I lean on the evidence-based steps for soothing, using the 5 S’s described by Harvey Karp, MD, based on the wisdom of T. Berry Brazelton, MD. These include:
- Swaddling in a wrap that constrains arms and legs.
- Side or stomach holding (but not for sleeping).
- Shushing sounds of voice, radio static, fan, air conditioner, or car ride.
- Swinging gently (point out to never shake a baby).
- Sucking on a pacifier, finger, or hand.
Because babies change state slowly and also respond to high caregiver emotion such as anxiety, it is important for parents to take some deep breaths and give each “S” several minutes to have an effect.
Some of our patients will go beyond typical crying to colic, defined as crying for at least 3 hours per day, at least 3 days per week, starting before 3 months post term. While typically easing by age 3 months, colic can in nightmare cases persist to age 1 year. Needless to say, prolonged crying can be an enormous stress for families. Researchers in the Fussy Baby Network consulting on infant crying have found value in a family prescription for REST: Reassurance, Empathy, Support, and Time away. Reassurance that the child is not ill should be provided after a careful history, including asking why parents think the baby is crying, and a physical exam, even if we think we can tell at a glance that the baby is okay. Remember that every parent’s first concern is whether the baby is okay, and crying indicates otherwise. Parents are counting on us for a thorough exam before we reassure them. Exhausted new parents deserve empathy – acknowledgment of how difficult, scary, and maddening it is to not be able to console their newborns.
While friends are saying “You must be so happy!” after a child is born, ambivalence (What have I done to my life?) is very common, but not easy to admit. We can point out that the typical age at which parents report “loving” their infants is actually more like 6 weeks, when they finally smile! To acknowledge ambivalence, I may say, “When you feel like throwing him out the window, it’s okay to lay him in the crib and put on headphones for a few minutes.” Music, meditation, yoga, or exercise are break activities that also can reduce parent stress.
Support for the parents is one of the most important protections for children at all ages, but can’t be assumed. Unfortunately, crying tends to increase beginning at 2 weeks post term, right when the partner returns to work or relatives leave. It is important to ask, “Who is helping you with the baby?” Just having a partner doesn’t mean that person is taking a turn! Fathers may be afraid of holding a baby, or may have no experience and try to defer. We need to encourage fathers in all aspects of care but especially to “find at least one way to console your baby.” Sometimes mothers “rescue” dads too quickly. While mothers may have both more experience with infants and the magic of breastfeeding for consoling, depriving fathers of working through the struggle of managing crying can result in their developing less confidence in caregiving. It also can cause them to miss out on supporting the mother at this delicate time, a chance to profoundly strengthen the partnership.
Taking into account the importance for parents of finding ways to console crying babies, getting some “time away” can be helpful, especially if crying goes on for months. Maybe those friends who asked, “What can I do to help?” could come over for an hour in the evening (peak crying time) to hold the baby. This also can be a chance for parent time together, a newly rare commodity.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Babies evolved to cry to get their needs met and adults evolved to be aroused by the sound. Perfect match, right? But crying/fussing was rated the No. 1 hardest part of parenting for 0- to 3-year-olds in our data from more than 68,000 parents.
As clinicians, we become amazingly immune to the crying in our offices, but hopefully not to crying as a concern of parents. Our training directs us to look for pathology such as under- or overfeeding, infection, gastroesophageal reflux disease (GERD), volvulus, a hair tourniquet, or an injury as causes of crying. Having ruled these out, the bigger task is making sure that parents learn to read their infants’ crying and find ways to console them. Learning to handle crying can be tense, frustrating, and upsetting for parents, but success is ultimately satisfying and an important part of the reciprocal interaction that builds attachment for both parent and child.
“Developmental crying” is a great term for explaining crying in the first 3 months to families, as it is age related. The acronym PURPLE was created to teach about this normal crying.
- “P” is for peak of crying – babies may cry more each week, most in month 2, then less in months 3-5.
- “U” is for unexpected – crying can come and go without explanation.
- “R” is for resists soothing – babies may not stop crying no matter what is tried.
- “P” is for pain-like face – babies appear to be in pain, even if they are not.
- “L” is for long lasting – crying can last 5 hours a day or more.
- “E” is for evening – the baby might cry more in the late afternoon and evening.
The 1- to 2-week visit is a key time to teach parents about the expected upcoming crying and ways to manage it. I lean on the evidence-based steps for soothing, using the 5 S’s described by Harvey Karp, MD, based on the wisdom of T. Berry Brazelton, MD. These include:
- Swaddling in a wrap that constrains arms and legs.
- Side or stomach holding (but not for sleeping).
- Shushing sounds of voice, radio static, fan, air conditioner, or car ride.
- Swinging gently (point out to never shake a baby).
- Sucking on a pacifier, finger, or hand.
Because babies change state slowly and also respond to high caregiver emotion such as anxiety, it is important for parents to take some deep breaths and give each “S” several minutes to have an effect.
Some of our patients will go beyond typical crying to colic, defined as crying for at least 3 hours per day, at least 3 days per week, starting before 3 months post term. While typically easing by age 3 months, colic can in nightmare cases persist to age 1 year. Needless to say, prolonged crying can be an enormous stress for families. Researchers in the Fussy Baby Network consulting on infant crying have found value in a family prescription for REST: Reassurance, Empathy, Support, and Time away. Reassurance that the child is not ill should be provided after a careful history, including asking why parents think the baby is crying, and a physical exam, even if we think we can tell at a glance that the baby is okay. Remember that every parent’s first concern is whether the baby is okay, and crying indicates otherwise. Parents are counting on us for a thorough exam before we reassure them. Exhausted new parents deserve empathy – acknowledgment of how difficult, scary, and maddening it is to not be able to console their newborns.
While friends are saying “You must be so happy!” after a child is born, ambivalence (What have I done to my life?) is very common, but not easy to admit. We can point out that the typical age at which parents report “loving” their infants is actually more like 6 weeks, when they finally smile! To acknowledge ambivalence, I may say, “When you feel like throwing him out the window, it’s okay to lay him in the crib and put on headphones for a few minutes.” Music, meditation, yoga, or exercise are break activities that also can reduce parent stress.
Support for the parents is one of the most important protections for children at all ages, but can’t be assumed. Unfortunately, crying tends to increase beginning at 2 weeks post term, right when the partner returns to work or relatives leave. It is important to ask, “Who is helping you with the baby?” Just having a partner doesn’t mean that person is taking a turn! Fathers may be afraid of holding a baby, or may have no experience and try to defer. We need to encourage fathers in all aspects of care but especially to “find at least one way to console your baby.” Sometimes mothers “rescue” dads too quickly. While mothers may have both more experience with infants and the magic of breastfeeding for consoling, depriving fathers of working through the struggle of managing crying can result in their developing less confidence in caregiving. It also can cause them to miss out on supporting the mother at this delicate time, a chance to profoundly strengthen the partnership.
Taking into account the importance for parents of finding ways to console crying babies, getting some “time away” can be helpful, especially if crying goes on for months. Maybe those friends who asked, “What can I do to help?” could come over for an hour in the evening (peak crying time) to hold the baby. This also can be a chance for parent time together, a newly rare commodity.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She has no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Endocrine Society updates guidelines for congenital adrenal hyperplasia
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
It’s time for universal CMV screening at birth
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
REPORTING FROM IDWEEK 2018
The devil is in the headlines
“Breast Milk From Bottle May Not Be As Beneficial As Feeding Directly From The Breast, Researchers Say.” This was the headline on the AAP Daily Briefing that was sent to American Academy of Pediatrics members on Sept 25, 2018.
I suspect that this finding doesn’t surprise you. I can imagine a dozen factors that could make bottled breast milk less advantageous for a baby than milk received directly from the mother’s breast. Antibodies might adhere to the glass surface. A few degrees above or below body temperature could interfere with gastric emptying. Or a temptation to focus on the level in the bottle and inadvertently overfeed could inflate the baby’s body mass index at 3 months, as was found in the study published online in the September 2018 issue of Pediatrics (“Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food”).
I agree that the title of the actual paper is rather dry; it’s a scientific research paper. But the distillation chosen by the folks at AAP Daily Briefing seems ill advised. They were not alone. CCN-Health chose “Breastfeeding better for babies’ weight gain than pumping, new study says” (Michael Nedelman, Sept. 24, 2018). HealthDay News headlined its story with “Milk straight from breast best for baby’s weight” (Sept. 24, 2018).
The articles themselves were well balanced and accurately described this research based on more than 2,500 Canadian mother-infant dyads. But not everyone – including mothers who are struggling with or considering breastfeeding – reads beyond the headlines. How many realize that “better for babies’ weight gain” means a slower weight gain? For the mother who has found that, for a variety of reasons, pumping is the only way she can provide her baby the benefits of breast milk, what these headlines suggest is another blow to her already fragile sense of self-worth.
This research article is excellent and should be read by all of us who counsel young families. It suggests that one of the contributors to our epidemic of childhood obesity may be that bottle-feeding discourages the infant’s own self-regulation skills. It should prompt us to ask every parent who is bottle-feeding his or her baby – regardless of what is in the bottle – exactly how they decide how much to put in the bottle and how long a feeding takes. Even if we are comfortable with the infant’s weight gain, we should caution parents to be more aware of the baby’s cues that he or she has had enough. Not every baby provides cues that are obvious, and parents may need our coaching in deciding how much to feed. This research paper also suggests that as long as breastfeeding was continued, introduction of solids as early as 5 months was not associated with an unhealthy BMI trajectory.
Unfortunately, the reporting of this research article is another example of the hazards of the explosive growth of the Internet. There really is no reason to keep the results of well-crafted research from the lay public, particularly if they are explained in common sense language. However, this places a burden of responsibility on the editors of websites to consider the damage that can be done by a poorly chosen headline.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
“Breast Milk From Bottle May Not Be As Beneficial As Feeding Directly From The Breast, Researchers Say.” This was the headline on the AAP Daily Briefing that was sent to American Academy of Pediatrics members on Sept 25, 2018.
I suspect that this finding doesn’t surprise you. I can imagine a dozen factors that could make bottled breast milk less advantageous for a baby than milk received directly from the mother’s breast. Antibodies might adhere to the glass surface. A few degrees above or below body temperature could interfere with gastric emptying. Or a temptation to focus on the level in the bottle and inadvertently overfeed could inflate the baby’s body mass index at 3 months, as was found in the study published online in the September 2018 issue of Pediatrics (“Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food”).
I agree that the title of the actual paper is rather dry; it’s a scientific research paper. But the distillation chosen by the folks at AAP Daily Briefing seems ill advised. They were not alone. CCN-Health chose “Breastfeeding better for babies’ weight gain than pumping, new study says” (Michael Nedelman, Sept. 24, 2018). HealthDay News headlined its story with “Milk straight from breast best for baby’s weight” (Sept. 24, 2018).
The articles themselves were well balanced and accurately described this research based on more than 2,500 Canadian mother-infant dyads. But not everyone – including mothers who are struggling with or considering breastfeeding – reads beyond the headlines. How many realize that “better for babies’ weight gain” means a slower weight gain? For the mother who has found that, for a variety of reasons, pumping is the only way she can provide her baby the benefits of breast milk, what these headlines suggest is another blow to her already fragile sense of self-worth.
This research article is excellent and should be read by all of us who counsel young families. It suggests that one of the contributors to our epidemic of childhood obesity may be that bottle-feeding discourages the infant’s own self-regulation skills. It should prompt us to ask every parent who is bottle-feeding his or her baby – regardless of what is in the bottle – exactly how they decide how much to put in the bottle and how long a feeding takes. Even if we are comfortable with the infant’s weight gain, we should caution parents to be more aware of the baby’s cues that he or she has had enough. Not every baby provides cues that are obvious, and parents may need our coaching in deciding how much to feed. This research paper also suggests that as long as breastfeeding was continued, introduction of solids as early as 5 months was not associated with an unhealthy BMI trajectory.
Unfortunately, the reporting of this research article is another example of the hazards of the explosive growth of the Internet. There really is no reason to keep the results of well-crafted research from the lay public, particularly if they are explained in common sense language. However, this places a burden of responsibility on the editors of websites to consider the damage that can be done by a poorly chosen headline.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
“Breast Milk From Bottle May Not Be As Beneficial As Feeding Directly From The Breast, Researchers Say.” This was the headline on the AAP Daily Briefing that was sent to American Academy of Pediatrics members on Sept 25, 2018.
I suspect that this finding doesn’t surprise you. I can imagine a dozen factors that could make bottled breast milk less advantageous for a baby than milk received directly from the mother’s breast. Antibodies might adhere to the glass surface. A few degrees above or below body temperature could interfere with gastric emptying. Or a temptation to focus on the level in the bottle and inadvertently overfeed could inflate the baby’s body mass index at 3 months, as was found in the study published online in the September 2018 issue of Pediatrics (“Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food”).
I agree that the title of the actual paper is rather dry; it’s a scientific research paper. But the distillation chosen by the folks at AAP Daily Briefing seems ill advised. They were not alone. CCN-Health chose “Breastfeeding better for babies’ weight gain than pumping, new study says” (Michael Nedelman, Sept. 24, 2018). HealthDay News headlined its story with “Milk straight from breast best for baby’s weight” (Sept. 24, 2018).
The articles themselves were well balanced and accurately described this research based on more than 2,500 Canadian mother-infant dyads. But not everyone – including mothers who are struggling with or considering breastfeeding – reads beyond the headlines. How many realize that “better for babies’ weight gain” means a slower weight gain? For the mother who has found that, for a variety of reasons, pumping is the only way she can provide her baby the benefits of breast milk, what these headlines suggest is another blow to her already fragile sense of self-worth.
This research article is excellent and should be read by all of us who counsel young families. It suggests that one of the contributors to our epidemic of childhood obesity may be that bottle-feeding discourages the infant’s own self-regulation skills. It should prompt us to ask every parent who is bottle-feeding his or her baby – regardless of what is in the bottle – exactly how they decide how much to put in the bottle and how long a feeding takes. Even if we are comfortable with the infant’s weight gain, we should caution parents to be more aware of the baby’s cues that he or she has had enough. Not every baby provides cues that are obvious, and parents may need our coaching in deciding how much to feed. This research paper also suggests that as long as breastfeeding was continued, introduction of solids as early as 5 months was not associated with an unhealthy BMI trajectory.
Unfortunately, the reporting of this research article is another example of the hazards of the explosive growth of the Internet. There really is no reason to keep the results of well-crafted research from the lay public, particularly if they are explained in common sense language. However, this places a burden of responsibility on the editors of websites to consider the damage that can be done by a poorly chosen headline.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Methadone may shorten NICU stays for neonatal abstinence syndrome
Lengths of stay in the hospital and in the neonatal ICU (NICU) for infants with neonatal abstinence syndrome (NAS) were significantly shorter with methadone treatment, compared with morphine treatment, according to a study published in the Journal of Pediatrics.
Veeral N. Tolia, MD, of Baylor University in Dallas, and his associates, gathered data from NICUs participating in the Clinical Data Warehouse and identified 7,667 singleton infants with no congenital abnormalities treated for NAS with either methadone (15%) or morphine (85%) in the first 7 days of life during 2011-2015.
The median hospital length of stay (LOS) was 18 days (interquartile range, 11-30 days) for infants treated with methadone and 23 days (IQR, 16-33 days) for those treated with morphine (P less than .001). The methadone-treated infants also had a shorter median LOS in the NICU than did the morphine-treated infants: 17 days (IQR, 10-29 days) versus 21 days (IQR, 14-36 days; P less than .001). In multivariable analysis, methadone treatment still was associated with a significantly shorter LOS in both cases. The methadone-treated infants also were significantly less likely to require two medications or three or more medications, compared with the morphine treated infants.
Although there was only a modest difference in LOS, Dr. Tolia and associates consider their findings relevant to clinical practice and are important given that “NAS incidence continues to rise and accounts for a substantial portion of health care utilization, including a fourfold increase in the proportion of neonatal hospital costs of all births covered by Medicaid.”
One of the strengths of this study is the size of its cohort; among its weaknesses is that the Clinical Data Warehouse does not include information on symptom severity, indication for starting therapy, or weaning practices.
The researchers declared no conflicts of interest.
SOURCE: Tolia VN et al. J Pediatr. 2018 Sep. doi: 10.1016/j.jpeds.2018.07.061.
Lengths of stay in the hospital and in the neonatal ICU (NICU) for infants with neonatal abstinence syndrome (NAS) were significantly shorter with methadone treatment, compared with morphine treatment, according to a study published in the Journal of Pediatrics.
Veeral N. Tolia, MD, of Baylor University in Dallas, and his associates, gathered data from NICUs participating in the Clinical Data Warehouse and identified 7,667 singleton infants with no congenital abnormalities treated for NAS with either methadone (15%) or morphine (85%) in the first 7 days of life during 2011-2015.
The median hospital length of stay (LOS) was 18 days (interquartile range, 11-30 days) for infants treated with methadone and 23 days (IQR, 16-33 days) for those treated with morphine (P less than .001). The methadone-treated infants also had a shorter median LOS in the NICU than did the morphine-treated infants: 17 days (IQR, 10-29 days) versus 21 days (IQR, 14-36 days; P less than .001). In multivariable analysis, methadone treatment still was associated with a significantly shorter LOS in both cases. The methadone-treated infants also were significantly less likely to require two medications or three or more medications, compared with the morphine treated infants.
Although there was only a modest difference in LOS, Dr. Tolia and associates consider their findings relevant to clinical practice and are important given that “NAS incidence continues to rise and accounts for a substantial portion of health care utilization, including a fourfold increase in the proportion of neonatal hospital costs of all births covered by Medicaid.”
One of the strengths of this study is the size of its cohort; among its weaknesses is that the Clinical Data Warehouse does not include information on symptom severity, indication for starting therapy, or weaning practices.
The researchers declared no conflicts of interest.
SOURCE: Tolia VN et al. J Pediatr. 2018 Sep. doi: 10.1016/j.jpeds.2018.07.061.
Lengths of stay in the hospital and in the neonatal ICU (NICU) for infants with neonatal abstinence syndrome (NAS) were significantly shorter with methadone treatment, compared with morphine treatment, according to a study published in the Journal of Pediatrics.
Veeral N. Tolia, MD, of Baylor University in Dallas, and his associates, gathered data from NICUs participating in the Clinical Data Warehouse and identified 7,667 singleton infants with no congenital abnormalities treated for NAS with either methadone (15%) or morphine (85%) in the first 7 days of life during 2011-2015.
The median hospital length of stay (LOS) was 18 days (interquartile range, 11-30 days) for infants treated with methadone and 23 days (IQR, 16-33 days) for those treated with morphine (P less than .001). The methadone-treated infants also had a shorter median LOS in the NICU than did the morphine-treated infants: 17 days (IQR, 10-29 days) versus 21 days (IQR, 14-36 days; P less than .001). In multivariable analysis, methadone treatment still was associated with a significantly shorter LOS in both cases. The methadone-treated infants also were significantly less likely to require two medications or three or more medications, compared with the morphine treated infants.
Although there was only a modest difference in LOS, Dr. Tolia and associates consider their findings relevant to clinical practice and are important given that “NAS incidence continues to rise and accounts for a substantial portion of health care utilization, including a fourfold increase in the proportion of neonatal hospital costs of all births covered by Medicaid.”
One of the strengths of this study is the size of its cohort; among its weaknesses is that the Clinical Data Warehouse does not include information on symptom severity, indication for starting therapy, or weaning practices.
The researchers declared no conflicts of interest.
SOURCE: Tolia VN et al. J Pediatr. 2018 Sep. doi: 10.1016/j.jpeds.2018.07.061.
FROM THE JOURNAL OF PEDIATRICS
Congenital syphilis rates continue skyrocketing alongside other STDs
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Key clinical point: Newborn syphilis cases have more than doubled in 5 years along with substantial increases in chlamydia, gonorrhea, and syphilis.
Major finding: 918 cases of newborn syphilis were reported in 37 states in 2017.
Study details: The findings are based on data from public health notifiable disease reports and multiple federal and private surveillance projects.
Disclosures: The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
Source: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017.
How to screen for, manage FASD in a medical home
providing early intervention and accessing community resources, according to a clinical report from the American Academy of Pediatrics.
After the AAP released its guidelines on fetal alcohol spectrum disorder (FASD) in 2015, some pediatricians asked for further guidance on how to care for patients with FASD within the medical home, as many had a knowledge gap on how to best manage these patients.
“For some pediatricians, it can seem like a daunting task to care for an individual with an FASD, but there are aspects of integrated care and providing a medical home that can be instituted as with all children with complex medical diagnoses,” wrote Renee M. Turchi, MD, MPH, of the department of pediatrics at St. Christopher’s Hospital for Children and Drexel Dornsife School of Public Health in Philadelphia, and her colleagues on the AAP Committee on Substance Abuse and the Council on Children with Disabilities. Their report is in Pediatrics. “In addition, not recognizing an FASD can lead to inadequate treatment and less-than-optimal outcomes for the patient and family.”
Dr. Turchi and her colleagues released the FASD clinical report with “strategies to support families who are interacting with early intervention services, the educational system, the behavioral and/or mental health system, other community resources, and the transition to adult-oriented heath care systems when appropriate.” They noted the prevalence of FASD is increasing, with 1 in 10 pregnant women using alcohol within the past 30 days and 1 in 33 pregnant women reporting binge drinking in the past 30 days. They reaffirmed the AAP’s endorsement from the 2015 clinical report on FASD regarding abstinence of alcohol for pregnant women, emphasizing that there is no amount or kind of alcohol that is risk free during pregnancy, nor is there a time in pregnancy when drinking alcohol is risk free.
Providers in a medical home should communicate any prenatal alcohol exposure (PAE) to obstetric providers so they can review risk factors, optimize screening, and monitor children, Dr. Turchi and her colleagues said. They also should understand the diagnostic criteria and classifications for FASDs, including physical features such as low weight, short palpebral features, smooth philtrum, a thin upper lip, abnormalities in the central nervous system, and any alcohol use during pregnancy. Any child – regardless of age – is a candidate for universal PAE screening at initial visits or when “additional cognitive and behavioral concerns arise.”
The federal Child Abuse Prevention and Treatment Act “does not require clinicians to report to child protective services if a child has been exposed prenatally to alcohol (i.e., for a positive PAE screening result). Referral to child protective services is required if the child has been diagnosed with an FASD in the period between birth and 3 years. The intent of this referral is to develop safe care and possible treatment plans for the infant and caregiver if needed, not to initiate punitive actions,” according to the report. States have their own definitions about child abuse and neglect, so the report encourages providers to know the mandates and reporting laws in the states where they practice.
Monitoring children in a medical home for the signs and symptoms of FASD is important, the authors said, because research has shown an increased chance at reducing adverse life outcomes if a child is diagnosed before age 6 and is in a stable home with access to support services.
Management of children with FASD is individual, as symptoms for each child will uniquely present not just in terms of physical issues such as growth or congenital defects affecting the heart, eyes, kidneys, or bones, but also as developmental, cognitive, and behavioral problems. Children with FASD also may receive a concomitant diagnosis when evaluated, such as ADHD or depression, that will require additional accommodation. The use of evidence-based diagnostic and standard screening approaches and referring when necessary will help reevaluate whether a child has a condition such as ADHD, oppositional defiant disorder, or another diagnosis, or is displaying symptoms of FASD such as a receptive or expressive language disorder.
Pediatricians must work together with the families, educational professionals, the mental health community, and therapists to help manage FASD in children. In cases where a child is in foster care, partnering with the foster care partners and child welfare agencies to gain access to the medical information of the biological parents is important to determine whether there is parental history of substance abuse and to provide appropriate treatment and interventions.
“Given the complex array of systems and services requiring navigation and coordination for children with an FASD and their families, a high-quality primary care medical home with partnerships with families, specialists, therapists, mental and/or behavioral health professionals, and community partners is critical, as it is for all children with special health care needs,” Dr. Turchi and her colleagues said.
The authors reported no relevant conflicts of interest.
SOURCE: Turchi RM et al. Pediatrics. 2018 Sept 10. doi:10.1542/peds.2018-2333.
providing early intervention and accessing community resources, according to a clinical report from the American Academy of Pediatrics.
After the AAP released its guidelines on fetal alcohol spectrum disorder (FASD) in 2015, some pediatricians asked for further guidance on how to care for patients with FASD within the medical home, as many had a knowledge gap on how to best manage these patients.
“For some pediatricians, it can seem like a daunting task to care for an individual with an FASD, but there are aspects of integrated care and providing a medical home that can be instituted as with all children with complex medical diagnoses,” wrote Renee M. Turchi, MD, MPH, of the department of pediatrics at St. Christopher’s Hospital for Children and Drexel Dornsife School of Public Health in Philadelphia, and her colleagues on the AAP Committee on Substance Abuse and the Council on Children with Disabilities. Their report is in Pediatrics. “In addition, not recognizing an FASD can lead to inadequate treatment and less-than-optimal outcomes for the patient and family.”
Dr. Turchi and her colleagues released the FASD clinical report with “strategies to support families who are interacting with early intervention services, the educational system, the behavioral and/or mental health system, other community resources, and the transition to adult-oriented heath care systems when appropriate.” They noted the prevalence of FASD is increasing, with 1 in 10 pregnant women using alcohol within the past 30 days and 1 in 33 pregnant women reporting binge drinking in the past 30 days. They reaffirmed the AAP’s endorsement from the 2015 clinical report on FASD regarding abstinence of alcohol for pregnant women, emphasizing that there is no amount or kind of alcohol that is risk free during pregnancy, nor is there a time in pregnancy when drinking alcohol is risk free.
Providers in a medical home should communicate any prenatal alcohol exposure (PAE) to obstetric providers so they can review risk factors, optimize screening, and monitor children, Dr. Turchi and her colleagues said. They also should understand the diagnostic criteria and classifications for FASDs, including physical features such as low weight, short palpebral features, smooth philtrum, a thin upper lip, abnormalities in the central nervous system, and any alcohol use during pregnancy. Any child – regardless of age – is a candidate for universal PAE screening at initial visits or when “additional cognitive and behavioral concerns arise.”
The federal Child Abuse Prevention and Treatment Act “does not require clinicians to report to child protective services if a child has been exposed prenatally to alcohol (i.e., for a positive PAE screening result). Referral to child protective services is required if the child has been diagnosed with an FASD in the period between birth and 3 years. The intent of this referral is to develop safe care and possible treatment plans for the infant and caregiver if needed, not to initiate punitive actions,” according to the report. States have their own definitions about child abuse and neglect, so the report encourages providers to know the mandates and reporting laws in the states where they practice.
Monitoring children in a medical home for the signs and symptoms of FASD is important, the authors said, because research has shown an increased chance at reducing adverse life outcomes if a child is diagnosed before age 6 and is in a stable home with access to support services.
Management of children with FASD is individual, as symptoms for each child will uniquely present not just in terms of physical issues such as growth or congenital defects affecting the heart, eyes, kidneys, or bones, but also as developmental, cognitive, and behavioral problems. Children with FASD also may receive a concomitant diagnosis when evaluated, such as ADHD or depression, that will require additional accommodation. The use of evidence-based diagnostic and standard screening approaches and referring when necessary will help reevaluate whether a child has a condition such as ADHD, oppositional defiant disorder, or another diagnosis, or is displaying symptoms of FASD such as a receptive or expressive language disorder.
Pediatricians must work together with the families, educational professionals, the mental health community, and therapists to help manage FASD in children. In cases where a child is in foster care, partnering with the foster care partners and child welfare agencies to gain access to the medical information of the biological parents is important to determine whether there is parental history of substance abuse and to provide appropriate treatment and interventions.
“Given the complex array of systems and services requiring navigation and coordination for children with an FASD and their families, a high-quality primary care medical home with partnerships with families, specialists, therapists, mental and/or behavioral health professionals, and community partners is critical, as it is for all children with special health care needs,” Dr. Turchi and her colleagues said.
The authors reported no relevant conflicts of interest.
SOURCE: Turchi RM et al. Pediatrics. 2018 Sept 10. doi:10.1542/peds.2018-2333.
providing early intervention and accessing community resources, according to a clinical report from the American Academy of Pediatrics.
After the AAP released its guidelines on fetal alcohol spectrum disorder (FASD) in 2015, some pediatricians asked for further guidance on how to care for patients with FASD within the medical home, as many had a knowledge gap on how to best manage these patients.
“For some pediatricians, it can seem like a daunting task to care for an individual with an FASD, but there are aspects of integrated care and providing a medical home that can be instituted as with all children with complex medical diagnoses,” wrote Renee M. Turchi, MD, MPH, of the department of pediatrics at St. Christopher’s Hospital for Children and Drexel Dornsife School of Public Health in Philadelphia, and her colleagues on the AAP Committee on Substance Abuse and the Council on Children with Disabilities. Their report is in Pediatrics. “In addition, not recognizing an FASD can lead to inadequate treatment and less-than-optimal outcomes for the patient and family.”
Dr. Turchi and her colleagues released the FASD clinical report with “strategies to support families who are interacting with early intervention services, the educational system, the behavioral and/or mental health system, other community resources, and the transition to adult-oriented heath care systems when appropriate.” They noted the prevalence of FASD is increasing, with 1 in 10 pregnant women using alcohol within the past 30 days and 1 in 33 pregnant women reporting binge drinking in the past 30 days. They reaffirmed the AAP’s endorsement from the 2015 clinical report on FASD regarding abstinence of alcohol for pregnant women, emphasizing that there is no amount or kind of alcohol that is risk free during pregnancy, nor is there a time in pregnancy when drinking alcohol is risk free.
Providers in a medical home should communicate any prenatal alcohol exposure (PAE) to obstetric providers so they can review risk factors, optimize screening, and monitor children, Dr. Turchi and her colleagues said. They also should understand the diagnostic criteria and classifications for FASDs, including physical features such as low weight, short palpebral features, smooth philtrum, a thin upper lip, abnormalities in the central nervous system, and any alcohol use during pregnancy. Any child – regardless of age – is a candidate for universal PAE screening at initial visits or when “additional cognitive and behavioral concerns arise.”
The federal Child Abuse Prevention and Treatment Act “does not require clinicians to report to child protective services if a child has been exposed prenatally to alcohol (i.e., for a positive PAE screening result). Referral to child protective services is required if the child has been diagnosed with an FASD in the period between birth and 3 years. The intent of this referral is to develop safe care and possible treatment plans for the infant and caregiver if needed, not to initiate punitive actions,” according to the report. States have their own definitions about child abuse and neglect, so the report encourages providers to know the mandates and reporting laws in the states where they practice.
Monitoring children in a medical home for the signs and symptoms of FASD is important, the authors said, because research has shown an increased chance at reducing adverse life outcomes if a child is diagnosed before age 6 and is in a stable home with access to support services.
Management of children with FASD is individual, as symptoms for each child will uniquely present not just in terms of physical issues such as growth or congenital defects affecting the heart, eyes, kidneys, or bones, but also as developmental, cognitive, and behavioral problems. Children with FASD also may receive a concomitant diagnosis when evaluated, such as ADHD or depression, that will require additional accommodation. The use of evidence-based diagnostic and standard screening approaches and referring when necessary will help reevaluate whether a child has a condition such as ADHD, oppositional defiant disorder, or another diagnosis, or is displaying symptoms of FASD such as a receptive or expressive language disorder.
Pediatricians must work together with the families, educational professionals, the mental health community, and therapists to help manage FASD in children. In cases where a child is in foster care, partnering with the foster care partners and child welfare agencies to gain access to the medical information of the biological parents is important to determine whether there is parental history of substance abuse and to provide appropriate treatment and interventions.
“Given the complex array of systems and services requiring navigation and coordination for children with an FASD and their families, a high-quality primary care medical home with partnerships with families, specialists, therapists, mental and/or behavioral health professionals, and community partners is critical, as it is for all children with special health care needs,” Dr. Turchi and her colleagues said.
The authors reported no relevant conflicts of interest.
SOURCE: Turchi RM et al. Pediatrics. 2018 Sept 10. doi:10.1542/peds.2018-2333.
FROM PEDIATRICS