User login
Pertussis vaccine at birth shows immune response, tolerability
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
Pertussis is most likely to cause morbidity or kill neonates between birth and when they are given their first pertussis vaccine at 6-8 weeks of age. This is well known.
In the current study giving the acellular pertussis (aP) vaccine at birth led to “significantly higher antibody titers to pertussis antigens at 10 weeks of age,” compared with those who did not receive it. Those infants who received the birth dose of aP vaccine also had higher pertussis antibodies at 6 weeks, whether or not their mothers had received Tdap within 5 years prior to delivery.
When this study began in 2009, maternal immunization was not a well accepted concept, but this attitude has changed, in part due to the safe vaccination of pregnant women with the pandemic flu vaccine. Despite this, Centers for Disease Control and Prevention 2016 data showed that only 49% of pregnant women in the United Stated received Tdap. These rates need to increase.
Administering the aP vaccine with the existing hepatitis B vaccine at birth to infants whose mothers who did not receive Tdap during pregnancy would be a practical solution, if the aP vaccine were universally available.
But the aP vaccine currently is not available in the United States and many other countries as a standalone vaccine, and the administration of DTaP as a birth dose has been linked with “significant immune interference.” The aP vaccine could have a place in countries where it is available, and there is no maternal immunization program. Otherwise, boosting maternal immunization appears to be the primary approach for now.
Kathryn M. Edwards, MD, is the Sarah H. Sell and Cornelius Vanderbilt Chair in Pediatrics at Vanderbilt University, Nashville. She specializes in pediatric infectious diseases. These comments are a summary of her editorial accompanying the article by Wood et al. (Pediatrics. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2363). Dr. Edwards said she had no conflicts of interest.
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
compared with a group receiving only the hepatitis B vaccine, a randomized clinical trial from Australia has found.
“These results indicate that a birth dose of aP vaccine is immunogenic in newborns and significantly narrows the immunity gap between birth and 14 days after receipt of DTaP at 6 or 8 weeks of age, marking the critical period when infants are most vulnerable to severe pertussis infection,” reported Nicholas Wood, PhD, of the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases in New South Wales, Australia, and his colleagues.
“Administration of the acellular pertussis vaccine at birth has the potential to reduce severe morbidity from Bordetella pertussis infection in the first 3 months of life, especially for infants of mothers who have not received a pertussis vaccine during pregnancy,” the researchers concluded in JAMA Pediatrics.
The researchers enrolled 417 infants from Sydney, Melbourne, Adelaide, and Perth between June 2010 and March 2013 and randomized them to receive either the hepatitis B vaccine alone (n = 205) or the hepatitis B vaccine with a monovalent acellular pertussis vaccine (n = 212) within the first 5 days after birth. The randomization was stratified for mothers’ receipt of the Tdap before pregnancy.
The Centers for Disease Control and Prevention currently recommends all newborns receive the hepatitis B vaccine shortly after birth and that pregnant women receive the Tdap vaccine during each pregnancy. There is not currently a monovalent acellular pertussis vaccine licensed in the United States.
The study infants then received the hexavalent DTaP-Hib-hep B-polio vaccine and the 10-valent pneumococcal conjugate vaccine at 6 weeks, 4 months, and 6 months.
The primary outcome was detectable levels of IgG antibody to pertussis toxin and pertactin at 10 weeks old.
Of the 206 infants receiving the pertussis vaccine at birth, 93% had detectable antibodies to pertussis toxin and pertactin at 10 weeks, compared with 51% of the 193 infants who received only the hepatitis B shot (P less than .001). Geometric mean concentration for pertussis toxin IgG also was four times higher in infants who received the pertussis vaccine at birth.
Adverse events were similar in the two groups both at birth and at 32 weeks, demonstrating that the pertussis birth dose is safe and tolerable.
“More important, in this study, the prevalence of fever after receipt of the birth dose, which can mistakenly be associated with potential sepsis and result in additional investigations in the neonatal period, was similar in both the group that received the aP vaccine at birth and the control group,” the authors reported.
A remaining question is the potential impact of maternal antibodies on protection from pertussis.
“The presence of maternal pertussis antibodies at birth can negatively affect postprimary responses to pertussis, diphtheria, and diphtheria-related CRM197 conjugate vaccines with a variety of infant immunization schedules and vaccines,” the authors noted. “The clinical significance of reductions in pertussis antibody related to maternal interference will require ongoing clinical evaluation, because there are no accepted serologic correlates of protection.”
The research was funded by a Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reported having no conflicts of interest.
SOURCE: Wood N et al, JAMA Pediatr. 2018 Sep 10. doi: 10.1001/jamapediatrics.2018.2349.
FROM JAMA PEDIATRICS
Key clinical point: A monovalent acellular pertussis vaccine dose at birth appears safe, tolerable, and effective.
Major finding: 93% of 212 newborns receiving an acellular pertussis vaccine at birth showed antibodies against pertussis toxin and pertactin at 10 weeks, compared with 51% of 205 newborns without the birth dose.
Study details: The findings are based on a randomized controlled trial involving 417 healthy term newborns in four Australian cities from June 2010 to March 2013.
Disclosures: The research was funded by an Australian National Health and Medical Research Council (NHMRC) grant, and several authors received NHMRC grants. One author also was supported by a Murdoch Children’s Research Institute Career Development Award. GlaxoSmithKline provided the vaccine and conducted the serologic assays. The authors reporting having no conflicts of interest.
Source: Wood N et al. JAMA Pediatr. 2018 Sep. 10. doi: 10.1001/jamapediatrics.2018.2349.
Eat/sleep/console approach almost eliminates morphine for NAS
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
ATLANTA – In just 7 months, the University of North Carolina Children’s Hospital, Chapel Hill, dropped the length of stay for neonatal abstinence syndrome from about 11 days to 5 days by moving from scheduled to PRN morphine dosing and abandoning the Finnegan score, according to a report at the Pediatric Hospital Medicine meeting.
The use of morphine fell from 93% of infants transferred to the hospital’s inpatient floors for neonatal abstinence syndrome (NAS) to just 12%, with no downsides for infants or moms.
“Our results have been incredibly encouraging,” said lead investigator and pediatrics resident Thomas Blount, MD. The take-home message is to treat the infant, rather than relying on the Finnegan score.
UNC Children’s, which treats about 50 infants a year for NAS on its inpatient floors, had been using the traditional approach: babies were automatically scheduled for morphine and Finnegan scoring – a withdrawal symptom checklist – every 4 hours, regardless of need. Sometimes infants weren’t even assessed to see if they actually needed morphine before the next dose was given.
“Waking babies up every 4 hours just seemed crazy; of course, they were going to have heightened neurologic signs and symptoms.” Meanwhile, families and providers were frustrated that infants who were otherwise doing well were held for an extra week or more to wean them off morphine, Dr. Blount said at the meeting.
In Nov. 2017, the hospital switched to the eat/sleep/console (ESC) model for NAS on its inpatient floors. The model emphasizes what’s been shown to work in recent years: keeping the infant with the mother; encouraging breast feeding, skin-on-skin contact, and other comfort measures; and supplementing feeds to help with weight gain. Morphine is reserved for when those measures fail and given only with a needs assessment (Hosp Pediatr. 2018 Jan;8(1):1-6).
The hospital ditched Finnegan scoring on its inpatient floors. Nurses were asked instead to check if infants were feeding adequately, sleeping at least an hour between feedings, and able to be consoled within 10 minutes when upset. If the infants met all three of those ESC criteria, providers moved on. They left the baby swaddled, relied on ambient white noise of ocean waves, and checked back on them later. “They didn’t mess with them,” Dr. Blount said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Finnegan scoring “was causing so much anxiety. Staff and families became hypervigilant,” set off by every little twitch and yawn the baby made. It was a good thing when it was abandoned; everyone relaxed, he said.
The changes made a huge difference. The average number of morphine doses dropped from 39 per infant to just 7 total doses among 23 infants in the first 7 months of the ESC initiative. Currently, morphine is used in only about 1 of 10 cases. “We estimate that we’ve given over 900 fewer doses” since ESC was put in place, Dr. Blount said.

There’s been no change in 30-day readmission rates – just one since the changes were made, for bronchiolitis – and no change in weight loss among infants with NAS. Babies are meeting all the ESC criteria to thrive.
“We had a lot of pushback initially, mostly from nursing staff and residents wondering how this was going to work. It quickly went away,” Dr. Blount said.
His team is now considering rolling ESC out to the newborn nursery and NICU.
There was no industry funding for the work, and Dr. Blount didn’t have any disclosures.
REPORTING FROM PHM 2018
Key clinical point: When it comes to neonatal abstinence syndrome, treat the infant, not the Finnegan score.
Major finding: The University of North Carolina Children’s Hospital dropped the length of stay for neonatal abstinence syndrome from about 11 to 5 days by moving from scheduled to PRN morphine and abandoning Finnegan scoring. Morphine use fell more than 80%.
Study details: Review of a 7-month quality improvement project
Disclosures: There was no industry funding for the work. The lead investigator didn’t have any disclosures.
AAP cautions against marijuana use during pregnancy, breastfeeding
, according to a recent clinical report published in the journal Pediatrics.
“The fact that marijuana is legal in many states may give the impression the drug is harmless during pregnancy, especially with stories swirling on social media about using it for nausea with morning sickness,” Sheryl A. Ryan, MD, FAAP, Chair of the American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention, stated in a press release. “But in fact, this is still a big question. We do not have good safety data on prenatal exposure to marijuana. Based on the limited data that do exist, as pediatricians, we believe there is cause to be concerned about how the drug will impact the long-term development of children.”
The rate of marijuana use is increasing among pregnant women 18 years to 44 years old is increasing, the committee said, with 3.84% of women in 2014 within that age range using marijuana within the past month compared with 2.37% in 2002. Among women who were between 18 years and 25 years old, the rate of marijuana use within the past month was 7.47% in 2014.
The committee also noted research has shown cannabidiol exposure in the short term may impact placental permeability to “pharmacologic agents and recreational substances, potentially placing the fetus at risk from these agents or drugs.” A more well-known substance in marijuana, delta-9-tetrahydrocannabinol (THC) crosses the placental barrier and can appear in fetal blood. Studies have reported any level of marijuana use among pregnant women put the mothers at risk of anemia, while their newborns had an increased risk of low-birth weight and neonatal intensive care unit (NICU) use. Further research has shown impaired mental development, executive function deficits, increased impulsivity and hyperactivity, behavioral problems, depressive symptoms, and greater rates of substance abuse among children exposed to marijuana.
“Many of these effects may not show up right away, but they can impact how well a child can maneuver in the world,” Dr. Ryan stated in the release. “Children’s and teens’ cognitive ability to manage their time and school work might be harmed down the line from marijuana use during their mother’s pregnancy.”
In a related study, Kerri A. Bertrand, MPH, from the department of pediatrics at the University of California in San Diego, Calif., and her colleagues studied cannabinoid concentrations in breastmilk donated to a human milk biorepository. The investigators analyzed 54 samples donated by 50 women who used marijuana while breastfeeding between 2014 and 2017 and determined whether substances such as delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), cannabidiol, and cannabinol were present in breastmilk by performing liquid chromatography mass spectrometry electrospray ionization on the samples.
They found 34 of 54 samples (64%) had detectable delta-9-THC approximately 6 days after marijuana use (median concentration, 9.47 ng/mL; range, 1.01-323 ng/mL), while 5 of 54 samples (9%) had measurable concentrations of 11-OH-THC (range, 1.33-12.80 ng/mL) and 5 of 54 samples (9%) contained measurable cannabidiol (range, 1.32-8.56 ng/mL). Predictors of log delta-9-THC concentrations included number of hours since last use (-0.03; 95% confidence interval, -0.04 to -0.01; P equals .005), the number of times per day marijuana was used (0.51; 95% CI, 0.03-0.99; P equals .039), and the amount of time between sample donation and analysis (0.08; 95% CI, 0.00-0.15; P equals .038), researchers said.
“Because marijuana is the most commonly used recreational drug among breastfeeding women, information regarding risks to breastfeeding infants is urgently needed,” Dr. Bertrand and colleagues wrote in their study.
The authors of the AAP clinical report acknowledge no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
SOURCE: Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
The study by Bertrand and colleagues should be commended for being among the first to analyze cannabinoids in breast milk, but there are still very important questions to be answered about marijuana use among women who breast-feed, Sheryl A. Ryan, MD, FAAP, wrote in a related editorial.
Questions remain about why one-third of participants in the study had no detectable cannabinoids in their breast milk, and a frame of reference is needed for the levels that did appear in the study, Dr. Ryan said. Data are also needed on how the cannabinoids “accumulate in the infant, how the infant metabolizes these substances, how quickly they are excreted, whether they accumulate, and thus how long these metabolites remain in the infant,” she said.
Dr. Ryan also questioned what to tell mothers who use marijuana but want to breastfeed their newborns, and noted guidelines from the AAP and the American College of Obstetricians currently recommend avoiding marijuana use entirely while breastfeeding.
“With their study, Bertrand et al. have provided additional and valuable support for those current recommendations. But the picture is incomplete without our understanding of what is happening at the level of those infants exposed to cannabinoid–containing breast milk,” Dr. Ryan said. “Hopefully, the calls for research to answer these important questions will not go unheeded.”
Dr. Ryan is from the Division of Adolescent Medicine and Department of Pediatrics at Penn State Health Children’s Hospital in Hershey, Penn. These comments summarize her editorial in response to Bertrand and colleagues. She reports no relevant conflicts of interest (Ryan SA. Pediatrics. 2018;142[3]:e20181921).
The study by Bertrand and colleagues should be commended for being among the first to analyze cannabinoids in breast milk, but there are still very important questions to be answered about marijuana use among women who breast-feed, Sheryl A. Ryan, MD, FAAP, wrote in a related editorial.
Questions remain about why one-third of participants in the study had no detectable cannabinoids in their breast milk, and a frame of reference is needed for the levels that did appear in the study, Dr. Ryan said. Data are also needed on how the cannabinoids “accumulate in the infant, how the infant metabolizes these substances, how quickly they are excreted, whether they accumulate, and thus how long these metabolites remain in the infant,” she said.
Dr. Ryan also questioned what to tell mothers who use marijuana but want to breastfeed their newborns, and noted guidelines from the AAP and the American College of Obstetricians currently recommend avoiding marijuana use entirely while breastfeeding.
“With their study, Bertrand et al. have provided additional and valuable support for those current recommendations. But the picture is incomplete without our understanding of what is happening at the level of those infants exposed to cannabinoid–containing breast milk,” Dr. Ryan said. “Hopefully, the calls for research to answer these important questions will not go unheeded.”
Dr. Ryan is from the Division of Adolescent Medicine and Department of Pediatrics at Penn State Health Children’s Hospital in Hershey, Penn. These comments summarize her editorial in response to Bertrand and colleagues. She reports no relevant conflicts of interest (Ryan SA. Pediatrics. 2018;142[3]:e20181921).
The study by Bertrand and colleagues should be commended for being among the first to analyze cannabinoids in breast milk, but there are still very important questions to be answered about marijuana use among women who breast-feed, Sheryl A. Ryan, MD, FAAP, wrote in a related editorial.
Questions remain about why one-third of participants in the study had no detectable cannabinoids in their breast milk, and a frame of reference is needed for the levels that did appear in the study, Dr. Ryan said. Data are also needed on how the cannabinoids “accumulate in the infant, how the infant metabolizes these substances, how quickly they are excreted, whether they accumulate, and thus how long these metabolites remain in the infant,” she said.
Dr. Ryan also questioned what to tell mothers who use marijuana but want to breastfeed their newborns, and noted guidelines from the AAP and the American College of Obstetricians currently recommend avoiding marijuana use entirely while breastfeeding.
“With their study, Bertrand et al. have provided additional and valuable support for those current recommendations. But the picture is incomplete without our understanding of what is happening at the level of those infants exposed to cannabinoid–containing breast milk,” Dr. Ryan said. “Hopefully, the calls for research to answer these important questions will not go unheeded.”
Dr. Ryan is from the Division of Adolescent Medicine and Department of Pediatrics at Penn State Health Children’s Hospital in Hershey, Penn. These comments summarize her editorial in response to Bertrand and colleagues. She reports no relevant conflicts of interest (Ryan SA. Pediatrics. 2018;142[3]:e20181921).
, according to a recent clinical report published in the journal Pediatrics.
“The fact that marijuana is legal in many states may give the impression the drug is harmless during pregnancy, especially with stories swirling on social media about using it for nausea with morning sickness,” Sheryl A. Ryan, MD, FAAP, Chair of the American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention, stated in a press release. “But in fact, this is still a big question. We do not have good safety data on prenatal exposure to marijuana. Based on the limited data that do exist, as pediatricians, we believe there is cause to be concerned about how the drug will impact the long-term development of children.”
The rate of marijuana use is increasing among pregnant women 18 years to 44 years old is increasing, the committee said, with 3.84% of women in 2014 within that age range using marijuana within the past month compared with 2.37% in 2002. Among women who were between 18 years and 25 years old, the rate of marijuana use within the past month was 7.47% in 2014.
The committee also noted research has shown cannabidiol exposure in the short term may impact placental permeability to “pharmacologic agents and recreational substances, potentially placing the fetus at risk from these agents or drugs.” A more well-known substance in marijuana, delta-9-tetrahydrocannabinol (THC) crosses the placental barrier and can appear in fetal blood. Studies have reported any level of marijuana use among pregnant women put the mothers at risk of anemia, while their newborns had an increased risk of low-birth weight and neonatal intensive care unit (NICU) use. Further research has shown impaired mental development, executive function deficits, increased impulsivity and hyperactivity, behavioral problems, depressive symptoms, and greater rates of substance abuse among children exposed to marijuana.
“Many of these effects may not show up right away, but they can impact how well a child can maneuver in the world,” Dr. Ryan stated in the release. “Children’s and teens’ cognitive ability to manage their time and school work might be harmed down the line from marijuana use during their mother’s pregnancy.”
In a related study, Kerri A. Bertrand, MPH, from the department of pediatrics at the University of California in San Diego, Calif., and her colleagues studied cannabinoid concentrations in breastmilk donated to a human milk biorepository. The investigators analyzed 54 samples donated by 50 women who used marijuana while breastfeeding between 2014 and 2017 and determined whether substances such as delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), cannabidiol, and cannabinol were present in breastmilk by performing liquid chromatography mass spectrometry electrospray ionization on the samples.
They found 34 of 54 samples (64%) had detectable delta-9-THC approximately 6 days after marijuana use (median concentration, 9.47 ng/mL; range, 1.01-323 ng/mL), while 5 of 54 samples (9%) had measurable concentrations of 11-OH-THC (range, 1.33-12.80 ng/mL) and 5 of 54 samples (9%) contained measurable cannabidiol (range, 1.32-8.56 ng/mL). Predictors of log delta-9-THC concentrations included number of hours since last use (-0.03; 95% confidence interval, -0.04 to -0.01; P equals .005), the number of times per day marijuana was used (0.51; 95% CI, 0.03-0.99; P equals .039), and the amount of time between sample donation and analysis (0.08; 95% CI, 0.00-0.15; P equals .038), researchers said.
“Because marijuana is the most commonly used recreational drug among breastfeeding women, information regarding risks to breastfeeding infants is urgently needed,” Dr. Bertrand and colleagues wrote in their study.
The authors of the AAP clinical report acknowledge no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
SOURCE: Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
, according to a recent clinical report published in the journal Pediatrics.
“The fact that marijuana is legal in many states may give the impression the drug is harmless during pregnancy, especially with stories swirling on social media about using it for nausea with morning sickness,” Sheryl A. Ryan, MD, FAAP, Chair of the American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention, stated in a press release. “But in fact, this is still a big question. We do not have good safety data on prenatal exposure to marijuana. Based on the limited data that do exist, as pediatricians, we believe there is cause to be concerned about how the drug will impact the long-term development of children.”
The rate of marijuana use is increasing among pregnant women 18 years to 44 years old is increasing, the committee said, with 3.84% of women in 2014 within that age range using marijuana within the past month compared with 2.37% in 2002. Among women who were between 18 years and 25 years old, the rate of marijuana use within the past month was 7.47% in 2014.
The committee also noted research has shown cannabidiol exposure in the short term may impact placental permeability to “pharmacologic agents and recreational substances, potentially placing the fetus at risk from these agents or drugs.” A more well-known substance in marijuana, delta-9-tetrahydrocannabinol (THC) crosses the placental barrier and can appear in fetal blood. Studies have reported any level of marijuana use among pregnant women put the mothers at risk of anemia, while their newborns had an increased risk of low-birth weight and neonatal intensive care unit (NICU) use. Further research has shown impaired mental development, executive function deficits, increased impulsivity and hyperactivity, behavioral problems, depressive symptoms, and greater rates of substance abuse among children exposed to marijuana.
“Many of these effects may not show up right away, but they can impact how well a child can maneuver in the world,” Dr. Ryan stated in the release. “Children’s and teens’ cognitive ability to manage their time and school work might be harmed down the line from marijuana use during their mother’s pregnancy.”
In a related study, Kerri A. Bertrand, MPH, from the department of pediatrics at the University of California in San Diego, Calif., and her colleagues studied cannabinoid concentrations in breastmilk donated to a human milk biorepository. The investigators analyzed 54 samples donated by 50 women who used marijuana while breastfeeding between 2014 and 2017 and determined whether substances such as delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), cannabidiol, and cannabinol were present in breastmilk by performing liquid chromatography mass spectrometry electrospray ionization on the samples.
They found 34 of 54 samples (64%) had detectable delta-9-THC approximately 6 days after marijuana use (median concentration, 9.47 ng/mL; range, 1.01-323 ng/mL), while 5 of 54 samples (9%) had measurable concentrations of 11-OH-THC (range, 1.33-12.80 ng/mL) and 5 of 54 samples (9%) contained measurable cannabidiol (range, 1.32-8.56 ng/mL). Predictors of log delta-9-THC concentrations included number of hours since last use (-0.03; 95% confidence interval, -0.04 to -0.01; P equals .005), the number of times per day marijuana was used (0.51; 95% CI, 0.03-0.99; P equals .039), and the amount of time between sample donation and analysis (0.08; 95% CI, 0.00-0.15; P equals .038), researchers said.
“Because marijuana is the most commonly used recreational drug among breastfeeding women, information regarding risks to breastfeeding infants is urgently needed,” Dr. Bertrand and colleagues wrote in their study.
The authors of the AAP clinical report acknowledge no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
SOURCE: Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
PEDIATRICS
Key clinical point: More studies are needed to analyze the long-term effects marijuana has on mother and child during pregnancy and while breastfeeding.
Major finding: Of women between 18 years and 44 years old, 3.84% used marijuana during pregnancy in 2014 compared with 2.37% in 2002; 64% of samples in Bertrand and colleagues’ study had THC traceable in breastmilk approximately 6 days after marijuana use.
Study details:A clinical report on marijuana use during pregnancy and while breastfeeding, and a study of 50 women who used marijuana while breastfeeding and donated samples to a human milk biorepository.
Disclosures:The authors of the AAP clinical report no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
Source: Ryan SA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1889. Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
In the U.S., breastfeeding starts out strong, but drops off fast
More than half the infants born in the United States in 2015 (57.6%) were still breastfeeding at 6 months old – an improvement over 2014 survey results, and another step toward the 61% goal set forth in Healthy People 2020.
The CDC Breastfeeding Report Card for 2018 found that about 83% of infants born in 2015 started to breastfeed at birth. Further, five of eight Healthy People 2020 breastfeeding goals were met in 2015.
Yet, the numbers also paint a picture familiar to many clinicians and families: By 1 year, only 36% of infants were still breastfeeding, and just 25% of infants were exclusively breastfed through 6 months, as the American Academy of Pediatrics recommends. This puts the U.S. on the lower end of the global breastfeeding scale, with most countries reporting rates of 30% and higher, according to World Health Organization data. Globally, 41% of mothers breastfeed exclusively through 6 months, according to the latest UNICEF data.
The report concludes that U.S. mothers may not be getting the support they need from health care providers, family members, and employers to meet their breastfeeding goals.
“High breastfeeding initiation rates show that most mothers in the United States want to breastfeed and start out doing so,” the report states. “However, despite the recommendation to breastfeed exclusively for about the first 6 months, less than 50% of infants were exclusively breastfed through 3 months and about 25% were exclusively breastfed through 6 months.”
The CDC Breastfeeding Report Card provides national- and state-level data to help clinicians, child care providers, and families promote breastfeeding and support the women who choose it. In addition to providing an in-depth look at the numbers, the report compares current findings to the breastfeeding goals outlined in Healthy People 2020. This year, it looked at data on breastfeeding practices and support in all 50 states, the District of Columbia, Puerto Rico, the Virgin Islands, and – for the first time – Guam.
The report is published every 2 years, but the data comprising it are updated annually. The latest rates reflect breastfeeding practices among U.S. infants born in 2015 and are based on results of the 2016 and 2017 U.S. National Immunization Surveys.
Since the first report card in 2007, rates of exclusive breastfeeding at 3 and 6 months have increased. However, although rates for any breastfeeding at 6 and 12 months increased in 2015 from 2014 rates, there was no appreciable increase in exclusive breastfeeding at 3 and 6 months, the report noted. And about 17% of infants who were breastfed at birth still got some formula supplementation in the first 2 days of life.
Recognizing that breastfeeding support at the birth facility is a key driver of success, Healthy People 2020 tracks the proportion of live births in facilities that provided the recommended support. These “baby-friendly hospitals” receive a special designation from the WHO/UNICEF Baby-Friendly Hospital Initiative, and their numbers are increasing, the report noted. In 12 states, more than 40% of births occurred in such facilities, comprising more than 1 million infants in 2015 (26%), and far exceeding the HP2020 8% goal.
In a nation in which many new mothers must return to employment outside the home, breastfeeding support at work is crucial. Just 49% of employers provide breastfeeding facilities for these women, the report found. While this may seem less than optimal, it still exceeds the HP2020 goal of 38%.
“All sectors of society – family and friends, hospitals, health care offices/clinics, childcare facilities, community-based organizations, and workplaces – can play a role in improving the health of families by supporting breastfeeding,” the report said. “To reach their breastfeeding goals, mothers need continuity of care, which is achieved by consistent, collaborative, and high-quality breastfeeding services and support.”
SOURCE: CDC Breastfeeding Report Card
More than half the infants born in the United States in 2015 (57.6%) were still breastfeeding at 6 months old – an improvement over 2014 survey results, and another step toward the 61% goal set forth in Healthy People 2020.
The CDC Breastfeeding Report Card for 2018 found that about 83% of infants born in 2015 started to breastfeed at birth. Further, five of eight Healthy People 2020 breastfeeding goals were met in 2015.
Yet, the numbers also paint a picture familiar to many clinicians and families: By 1 year, only 36% of infants were still breastfeeding, and just 25% of infants were exclusively breastfed through 6 months, as the American Academy of Pediatrics recommends. This puts the U.S. on the lower end of the global breastfeeding scale, with most countries reporting rates of 30% and higher, according to World Health Organization data. Globally, 41% of mothers breastfeed exclusively through 6 months, according to the latest UNICEF data.
The report concludes that U.S. mothers may not be getting the support they need from health care providers, family members, and employers to meet their breastfeeding goals.
“High breastfeeding initiation rates show that most mothers in the United States want to breastfeed and start out doing so,” the report states. “However, despite the recommendation to breastfeed exclusively for about the first 6 months, less than 50% of infants were exclusively breastfed through 3 months and about 25% were exclusively breastfed through 6 months.”
The CDC Breastfeeding Report Card provides national- and state-level data to help clinicians, child care providers, and families promote breastfeeding and support the women who choose it. In addition to providing an in-depth look at the numbers, the report compares current findings to the breastfeeding goals outlined in Healthy People 2020. This year, it looked at data on breastfeeding practices and support in all 50 states, the District of Columbia, Puerto Rico, the Virgin Islands, and – for the first time – Guam.
The report is published every 2 years, but the data comprising it are updated annually. The latest rates reflect breastfeeding practices among U.S. infants born in 2015 and are based on results of the 2016 and 2017 U.S. National Immunization Surveys.
Since the first report card in 2007, rates of exclusive breastfeeding at 3 and 6 months have increased. However, although rates for any breastfeeding at 6 and 12 months increased in 2015 from 2014 rates, there was no appreciable increase in exclusive breastfeeding at 3 and 6 months, the report noted. And about 17% of infants who were breastfed at birth still got some formula supplementation in the first 2 days of life.
Recognizing that breastfeeding support at the birth facility is a key driver of success, Healthy People 2020 tracks the proportion of live births in facilities that provided the recommended support. These “baby-friendly hospitals” receive a special designation from the WHO/UNICEF Baby-Friendly Hospital Initiative, and their numbers are increasing, the report noted. In 12 states, more than 40% of births occurred in such facilities, comprising more than 1 million infants in 2015 (26%), and far exceeding the HP2020 8% goal.
In a nation in which many new mothers must return to employment outside the home, breastfeeding support at work is crucial. Just 49% of employers provide breastfeeding facilities for these women, the report found. While this may seem less than optimal, it still exceeds the HP2020 goal of 38%.
“All sectors of society – family and friends, hospitals, health care offices/clinics, childcare facilities, community-based organizations, and workplaces – can play a role in improving the health of families by supporting breastfeeding,” the report said. “To reach their breastfeeding goals, mothers need continuity of care, which is achieved by consistent, collaborative, and high-quality breastfeeding services and support.”
SOURCE: CDC Breastfeeding Report Card
More than half the infants born in the United States in 2015 (57.6%) were still breastfeeding at 6 months old – an improvement over 2014 survey results, and another step toward the 61% goal set forth in Healthy People 2020.
The CDC Breastfeeding Report Card for 2018 found that about 83% of infants born in 2015 started to breastfeed at birth. Further, five of eight Healthy People 2020 breastfeeding goals were met in 2015.
Yet, the numbers also paint a picture familiar to many clinicians and families: By 1 year, only 36% of infants were still breastfeeding, and just 25% of infants were exclusively breastfed through 6 months, as the American Academy of Pediatrics recommends. This puts the U.S. on the lower end of the global breastfeeding scale, with most countries reporting rates of 30% and higher, according to World Health Organization data. Globally, 41% of mothers breastfeed exclusively through 6 months, according to the latest UNICEF data.
The report concludes that U.S. mothers may not be getting the support they need from health care providers, family members, and employers to meet their breastfeeding goals.
“High breastfeeding initiation rates show that most mothers in the United States want to breastfeed and start out doing so,” the report states. “However, despite the recommendation to breastfeed exclusively for about the first 6 months, less than 50% of infants were exclusively breastfed through 3 months and about 25% were exclusively breastfed through 6 months.”
The CDC Breastfeeding Report Card provides national- and state-level data to help clinicians, child care providers, and families promote breastfeeding and support the women who choose it. In addition to providing an in-depth look at the numbers, the report compares current findings to the breastfeeding goals outlined in Healthy People 2020. This year, it looked at data on breastfeeding practices and support in all 50 states, the District of Columbia, Puerto Rico, the Virgin Islands, and – for the first time – Guam.
The report is published every 2 years, but the data comprising it are updated annually. The latest rates reflect breastfeeding practices among U.S. infants born in 2015 and are based on results of the 2016 and 2017 U.S. National Immunization Surveys.
Since the first report card in 2007, rates of exclusive breastfeeding at 3 and 6 months have increased. However, although rates for any breastfeeding at 6 and 12 months increased in 2015 from 2014 rates, there was no appreciable increase in exclusive breastfeeding at 3 and 6 months, the report noted. And about 17% of infants who were breastfed at birth still got some formula supplementation in the first 2 days of life.
Recognizing that breastfeeding support at the birth facility is a key driver of success, Healthy People 2020 tracks the proportion of live births in facilities that provided the recommended support. These “baby-friendly hospitals” receive a special designation from the WHO/UNICEF Baby-Friendly Hospital Initiative, and their numbers are increasing, the report noted. In 12 states, more than 40% of births occurred in such facilities, comprising more than 1 million infants in 2015 (26%), and far exceeding the HP2020 8% goal.
In a nation in which many new mothers must return to employment outside the home, breastfeeding support at work is crucial. Just 49% of employers provide breastfeeding facilities for these women, the report found. While this may seem less than optimal, it still exceeds the HP2020 goal of 38%.
“All sectors of society – family and friends, hospitals, health care offices/clinics, childcare facilities, community-based organizations, and workplaces – can play a role in improving the health of families by supporting breastfeeding,” the report said. “To reach their breastfeeding goals, mothers need continuity of care, which is achieved by consistent, collaborative, and high-quality breastfeeding services and support.”
SOURCE: CDC Breastfeeding Report Card
Lung ultrasound predicts need for first-dose surfactant in neonates
Lung ultrasound score (LUS) is an effective means of predicting whether extremely preterm neonates undergoing continuous positive airway pressure (CPAP) treatment for respiratory distress syndrome (RDS) require surfactant, according to results of study published in Pediatrics.
Lucia De Martino, MD, of the division of pediatrics and neonatal critical care at the A. Béclère Medical Centre of the South Paris University Hospital and her associates enrolled 133 neonates of 30 weeks’ gestation or less born between 2015 and 2016. They designed the prospective diagnostic accuracy cohort study, which was conducted in an academic tertiary care referral neonatal ICU.
The first dose of surfactant was administered at a mean 4 hours of life. Those that required further treatment received a second dose of surfactant at a mean 28 hours of life. Each patient received a single lung ultrasound lasting an average of 3 minutes. In each case, the procedure was well tolerated.
In particular, the study demonstrated that LUS is able to accurately predict the need for a first dose and “reveals fair accuracy when it comes to predicting surfactant retreatment,” they observed. The authors speculate that using LUS to predict retreatment is somewhat less reliable because of either the lower number of patients requiring retreatment or reasons related to the biology of surfactant.
“A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose, whereas a cutoff value of 10 predicts the need for surfactant retreatment,” Dr. De Martino and her colleagues advised.
Of key importance was the finding that LUS is of greatest value to preterm infants less than 34 weeks’ gestation; the authors observed that LUS had significantly lower diagnostic accuracy in infants who were either late term or term. They offered that this outcome was likely attributable to the homogeneous nature of preterm neonates, who are commonly affected by RDS and tend to present with a variety of respiratory disorders and surfactant injury to differing degrees.
At present, international guidelines only recommend surfactant replacement in cases where CPAP has failed, but administering surfactant within the first 2-3 hours of life is key to reducing bronchopulmonary dysplasia as well as the risk of death, they said.
Current surfactant replacement is determined solely by fraction of inspired oxygen cutoff levels, which can result in delayed or even unnecessary treatment. Because neonates who are extremely preterm benefit the most from treatment, “both situations are potentially harmful because late surfactant replacement is less efficacious and giving surfactant when it is not needed may be invasive and seems to increase lung inflammation in animal models,” Dr. De Martino and her associates cautioned.
The authors had no relevant financial disclosures.
SOURCE: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
Point-of-care ultrasound (POCUS) has been recognized for years for its value in assessing sick neonates, but a recent survey showed that less than one-third of U.S. neonatal-perinatal medicine programs actually use bedside ultrasound for health care diagnosis and management. Although its use historically has been confined to pediatric cardiology and radiology, it has gained more of a foothold in acute pediatric care settings, and its use in evaluating neonate lungs is a “relatively new and potentially revolutionary approach,” Maria V. Fraga, MD, and her associates wrote in an accompanying editorial.
A growing body of data over the past 2 decades is available to help radiologists and bedside providers to better understand the applications and limitations of POCUS. Findings in similar studies looking at the use of LUS in neonates “make the article by De Martino et al. so important,” Dr. Fraga and her associates emphasized. Dr. De Martino and her colleagues were able to use POCUS of the lung “to develop reliable predictive models for the need for surfactant treatment and re-dosing” in a group of preterm infants.
Although it would seem reasonable to expect the potential benefits of POCUS to have worldwide application, implementation is inconsistent. Clinicians in Australia, New Zealand, and Canada are trained and use POCUS daily, but this is not the case in other countries such as the United States. Concern over legal risks and training, as well as turf disputes with cardiology and radiology, the lack of clinicians actively using ultrasound, and scarce evidence showing benefit of its use could be to blame.
“The development of a POCUS program requires an accessible dedicated ultrasound machine kept in close proximity to clinical areas, a core group of interested clinicians, and a training and accreditation program with a commitment to continuing professional development,” advised Dr. Fraga and her associates.
“It is important to understand the limitation of bedside ultrasound, which should always be performed for a specific clinical purpose and to answer a clinical question and does not always mandate a full comprehensive study,” they added.
Dr. Fraga and her associates are affiliated with the department of pediatrics at the University of Pennsylvania, Philadelphia. There was no external funding and the authors had no relevant financial disclosures. These comments are adapted from an editorial accompanying the article by De Martino et al. (Pediatrics. 2018. doi: 10.1542/peds.2018-1621).
Point-of-care ultrasound (POCUS) has been recognized for years for its value in assessing sick neonates, but a recent survey showed that less than one-third of U.S. neonatal-perinatal medicine programs actually use bedside ultrasound for health care diagnosis and management. Although its use historically has been confined to pediatric cardiology and radiology, it has gained more of a foothold in acute pediatric care settings, and its use in evaluating neonate lungs is a “relatively new and potentially revolutionary approach,” Maria V. Fraga, MD, and her associates wrote in an accompanying editorial.
A growing body of data over the past 2 decades is available to help radiologists and bedside providers to better understand the applications and limitations of POCUS. Findings in similar studies looking at the use of LUS in neonates “make the article by De Martino et al. so important,” Dr. Fraga and her associates emphasized. Dr. De Martino and her colleagues were able to use POCUS of the lung “to develop reliable predictive models for the need for surfactant treatment and re-dosing” in a group of preterm infants.
Although it would seem reasonable to expect the potential benefits of POCUS to have worldwide application, implementation is inconsistent. Clinicians in Australia, New Zealand, and Canada are trained and use POCUS daily, but this is not the case in other countries such as the United States. Concern over legal risks and training, as well as turf disputes with cardiology and radiology, the lack of clinicians actively using ultrasound, and scarce evidence showing benefit of its use could be to blame.
“The development of a POCUS program requires an accessible dedicated ultrasound machine kept in close proximity to clinical areas, a core group of interested clinicians, and a training and accreditation program with a commitment to continuing professional development,” advised Dr. Fraga and her associates.
“It is important to understand the limitation of bedside ultrasound, which should always be performed for a specific clinical purpose and to answer a clinical question and does not always mandate a full comprehensive study,” they added.
Dr. Fraga and her associates are affiliated with the department of pediatrics at the University of Pennsylvania, Philadelphia. There was no external funding and the authors had no relevant financial disclosures. These comments are adapted from an editorial accompanying the article by De Martino et al. (Pediatrics. 2018. doi: 10.1542/peds.2018-1621).
Point-of-care ultrasound (POCUS) has been recognized for years for its value in assessing sick neonates, but a recent survey showed that less than one-third of U.S. neonatal-perinatal medicine programs actually use bedside ultrasound for health care diagnosis and management. Although its use historically has been confined to pediatric cardiology and radiology, it has gained more of a foothold in acute pediatric care settings, and its use in evaluating neonate lungs is a “relatively new and potentially revolutionary approach,” Maria V. Fraga, MD, and her associates wrote in an accompanying editorial.
A growing body of data over the past 2 decades is available to help radiologists and bedside providers to better understand the applications and limitations of POCUS. Findings in similar studies looking at the use of LUS in neonates “make the article by De Martino et al. so important,” Dr. Fraga and her associates emphasized. Dr. De Martino and her colleagues were able to use POCUS of the lung “to develop reliable predictive models for the need for surfactant treatment and re-dosing” in a group of preterm infants.
Although it would seem reasonable to expect the potential benefits of POCUS to have worldwide application, implementation is inconsistent. Clinicians in Australia, New Zealand, and Canada are trained and use POCUS daily, but this is not the case in other countries such as the United States. Concern over legal risks and training, as well as turf disputes with cardiology and radiology, the lack of clinicians actively using ultrasound, and scarce evidence showing benefit of its use could be to blame.
“The development of a POCUS program requires an accessible dedicated ultrasound machine kept in close proximity to clinical areas, a core group of interested clinicians, and a training and accreditation program with a commitment to continuing professional development,” advised Dr. Fraga and her associates.
“It is important to understand the limitation of bedside ultrasound, which should always be performed for a specific clinical purpose and to answer a clinical question and does not always mandate a full comprehensive study,” they added.
Dr. Fraga and her associates are affiliated with the department of pediatrics at the University of Pennsylvania, Philadelphia. There was no external funding and the authors had no relevant financial disclosures. These comments are adapted from an editorial accompanying the article by De Martino et al. (Pediatrics. 2018. doi: 10.1542/peds.2018-1621).
Lung ultrasound score (LUS) is an effective means of predicting whether extremely preterm neonates undergoing continuous positive airway pressure (CPAP) treatment for respiratory distress syndrome (RDS) require surfactant, according to results of study published in Pediatrics.
Lucia De Martino, MD, of the division of pediatrics and neonatal critical care at the A. Béclère Medical Centre of the South Paris University Hospital and her associates enrolled 133 neonates of 30 weeks’ gestation or less born between 2015 and 2016. They designed the prospective diagnostic accuracy cohort study, which was conducted in an academic tertiary care referral neonatal ICU.
The first dose of surfactant was administered at a mean 4 hours of life. Those that required further treatment received a second dose of surfactant at a mean 28 hours of life. Each patient received a single lung ultrasound lasting an average of 3 minutes. In each case, the procedure was well tolerated.
In particular, the study demonstrated that LUS is able to accurately predict the need for a first dose and “reveals fair accuracy when it comes to predicting surfactant retreatment,” they observed. The authors speculate that using LUS to predict retreatment is somewhat less reliable because of either the lower number of patients requiring retreatment or reasons related to the biology of surfactant.
“A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose, whereas a cutoff value of 10 predicts the need for surfactant retreatment,” Dr. De Martino and her colleagues advised.
Of key importance was the finding that LUS is of greatest value to preterm infants less than 34 weeks’ gestation; the authors observed that LUS had significantly lower diagnostic accuracy in infants who were either late term or term. They offered that this outcome was likely attributable to the homogeneous nature of preterm neonates, who are commonly affected by RDS and tend to present with a variety of respiratory disorders and surfactant injury to differing degrees.
At present, international guidelines only recommend surfactant replacement in cases where CPAP has failed, but administering surfactant within the first 2-3 hours of life is key to reducing bronchopulmonary dysplasia as well as the risk of death, they said.
Current surfactant replacement is determined solely by fraction of inspired oxygen cutoff levels, which can result in delayed or even unnecessary treatment. Because neonates who are extremely preterm benefit the most from treatment, “both situations are potentially harmful because late surfactant replacement is less efficacious and giving surfactant when it is not needed may be invasive and seems to increase lung inflammation in animal models,” Dr. De Martino and her associates cautioned.
The authors had no relevant financial disclosures.
SOURCE: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
Lung ultrasound score (LUS) is an effective means of predicting whether extremely preterm neonates undergoing continuous positive airway pressure (CPAP) treatment for respiratory distress syndrome (RDS) require surfactant, according to results of study published in Pediatrics.
Lucia De Martino, MD, of the division of pediatrics and neonatal critical care at the A. Béclère Medical Centre of the South Paris University Hospital and her associates enrolled 133 neonates of 30 weeks’ gestation or less born between 2015 and 2016. They designed the prospective diagnostic accuracy cohort study, which was conducted in an academic tertiary care referral neonatal ICU.
The first dose of surfactant was administered at a mean 4 hours of life. Those that required further treatment received a second dose of surfactant at a mean 28 hours of life. Each patient received a single lung ultrasound lasting an average of 3 minutes. In each case, the procedure was well tolerated.
In particular, the study demonstrated that LUS is able to accurately predict the need for a first dose and “reveals fair accuracy when it comes to predicting surfactant retreatment,” they observed. The authors speculate that using LUS to predict retreatment is somewhat less reliable because of either the lower number of patients requiring retreatment or reasons related to the biology of surfactant.
“A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose, whereas a cutoff value of 10 predicts the need for surfactant retreatment,” Dr. De Martino and her colleagues advised.
Of key importance was the finding that LUS is of greatest value to preterm infants less than 34 weeks’ gestation; the authors observed that LUS had significantly lower diagnostic accuracy in infants who were either late term or term. They offered that this outcome was likely attributable to the homogeneous nature of preterm neonates, who are commonly affected by RDS and tend to present with a variety of respiratory disorders and surfactant injury to differing degrees.
At present, international guidelines only recommend surfactant replacement in cases where CPAP has failed, but administering surfactant within the first 2-3 hours of life is key to reducing bronchopulmonary dysplasia as well as the risk of death, they said.
Current surfactant replacement is determined solely by fraction of inspired oxygen cutoff levels, which can result in delayed or even unnecessary treatment. Because neonates who are extremely preterm benefit the most from treatment, “both situations are potentially harmful because late surfactant replacement is less efficacious and giving surfactant when it is not needed may be invasive and seems to increase lung inflammation in animal models,” Dr. De Martino and her associates cautioned.
The authors had no relevant financial disclosures.
SOURCE: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
FROM PEDIATRICS
Key clinical point:
Major finding: A LUS cutoff value between 6 and 8 provides optimal sensitivity and specificity for predicting the need for the first surfactant dose.
Study details: Prospective cohort diagnostic accuracy study that included 133 infants.
Disclosures: The authors had no relevant financial disclosures.
Source: De Martino L et al. Pediatrics. 2018. doi: 10.1542/peds.2018-0463.
More deliveries now include opioid use disorder
according to the Centers for Disease Control and Prevention.
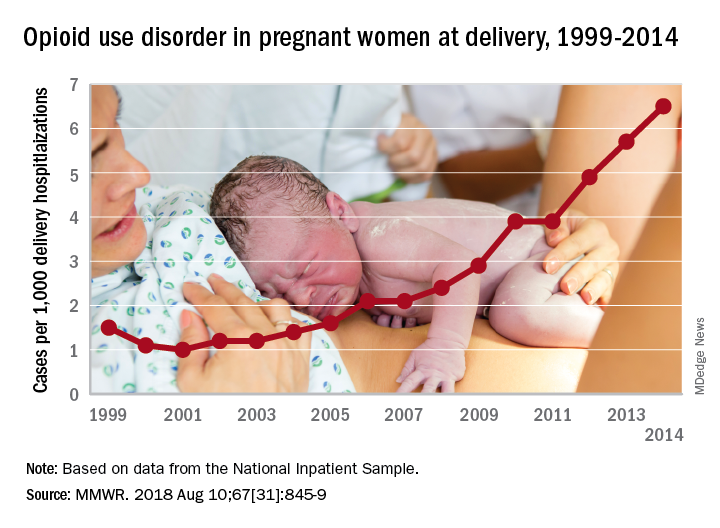
The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
FROM MMWR
Short-course IV antibiotics okay for newborn bacteremic UTI
ATLANTA – A short course of IV antibiotics – 7 days or less – is fine for most infants with uncomplicated bacteremic urinary tract infections, according to a review of 116 children younger than 60 days.
How long to treat bacteremic UTIs in the very young has been debated in pediatrics for a while, with some centers opting for a few days and others for 2 weeks or more. Shorter courses reduce length of stay, costs, and complications, but there hasn’t been much research to see whether they work as well.
The new investigation has suggested they do. “Young infants with bacteremic UTI who received less than or equal to 7 days of IV antibiotic therapy did not have more recurrent UTIs,” compared “to infants who received longer courses. Short course IV therapy with early conversion to oral antibiotics may be considered in this population,” said lead investigator Sanyukta Desai, MD, at the Pediatric Hospital Medicine meeting.
The team compared outcomes of 58 infants treated for 7 days or less to outcomes of 58 infants treated for more than 7 days at 11 children’s hospitals scattered across the United States.
Urine was collected by catheter, and each child grew the same organism in their blood and urine cultures, confirming the diagnosis of bacteremic UTI. Children with bacterial meningitis, or suspected of having it, were excluded. The subjects had all been admitted through the ED.
There was quite a bit of variation among the 11 hospitals, with the proportion of children treated with short courses ranging from 10% to 81%.
As for the results, two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days. None of them developed meningitis, and none required ICU admission. Propensity-score matching revealed an odds ratio for recurrence that favored shorter treatment, but it wasn’t statistically significant.
The mean length of stay was 5 days in the short-course arm and 11 days in the long-course arm. There were no serious adverse events within 30 days of the index admission in either group.
Among the recurrences, the two children in the short-course arm were initially treated for 3 and 5 days. Both were older than 28 days at their initial presentation, and both had vesicoureteral reflux of at least grade 2, which was not diagnosed in one child until after the recurrence. The other child had been on prophylactic trimethoprim/sulfamethoxazole before the recurrence.
The four recurrent cases in the long arm initially received either 10 or 14 days of IV antibiotics. Two children had grade 4 vesicoureteral reflux and had been on prophylactic amoxicillin.
Infants treated with longer antibiotic courses were more likely to be under 28 days old, appear ill at presentation, have had bacteremia for more than 24 hours, and have and grow out pathogens other than Escherichia coli. The two groups were otherwise balanced for sex, prematurity, complex chronic conditions, and known genitourinary anomalies.
With such low event rates, the study wasn’t powered to detect small but potentially meaningful differences in outcomes, and further work is needed to define which children would benefit from longer treatment courses. Even so, “it was reassuring that patients did well in both arms,” said Dr. Desai, a clinical fellow in the division of hospital medicine at Cincinnati Children’s Hospital.
“At our institution with uncomplicated UTI, we wait to see what the culture grows.” If there’s an oral antibiotic that will work, “we send [infants] home in 3-4 days. We haven’t had any poor outcomes, even when they’re bacteremic,” she said.
The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
ATLANTA – A short course of IV antibiotics – 7 days or less – is fine for most infants with uncomplicated bacteremic urinary tract infections, according to a review of 116 children younger than 60 days.
How long to treat bacteremic UTIs in the very young has been debated in pediatrics for a while, with some centers opting for a few days and others for 2 weeks or more. Shorter courses reduce length of stay, costs, and complications, but there hasn’t been much research to see whether they work as well.
The new investigation has suggested they do. “Young infants with bacteremic UTI who received less than or equal to 7 days of IV antibiotic therapy did not have more recurrent UTIs,” compared “to infants who received longer courses. Short course IV therapy with early conversion to oral antibiotics may be considered in this population,” said lead investigator Sanyukta Desai, MD, at the Pediatric Hospital Medicine meeting.
The team compared outcomes of 58 infants treated for 7 days or less to outcomes of 58 infants treated for more than 7 days at 11 children’s hospitals scattered across the United States.
Urine was collected by catheter, and each child grew the same organism in their blood and urine cultures, confirming the diagnosis of bacteremic UTI. Children with bacterial meningitis, or suspected of having it, were excluded. The subjects had all been admitted through the ED.
There was quite a bit of variation among the 11 hospitals, with the proportion of children treated with short courses ranging from 10% to 81%.
As for the results, two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days. None of them developed meningitis, and none required ICU admission. Propensity-score matching revealed an odds ratio for recurrence that favored shorter treatment, but it wasn’t statistically significant.
The mean length of stay was 5 days in the short-course arm and 11 days in the long-course arm. There were no serious adverse events within 30 days of the index admission in either group.
Among the recurrences, the two children in the short-course arm were initially treated for 3 and 5 days. Both were older than 28 days at their initial presentation, and both had vesicoureteral reflux of at least grade 2, which was not diagnosed in one child until after the recurrence. The other child had been on prophylactic trimethoprim/sulfamethoxazole before the recurrence.
The four recurrent cases in the long arm initially received either 10 or 14 days of IV antibiotics. Two children had grade 4 vesicoureteral reflux and had been on prophylactic amoxicillin.
Infants treated with longer antibiotic courses were more likely to be under 28 days old, appear ill at presentation, have had bacteremia for more than 24 hours, and have and grow out pathogens other than Escherichia coli. The two groups were otherwise balanced for sex, prematurity, complex chronic conditions, and known genitourinary anomalies.
With such low event rates, the study wasn’t powered to detect small but potentially meaningful differences in outcomes, and further work is needed to define which children would benefit from longer treatment courses. Even so, “it was reassuring that patients did well in both arms,” said Dr. Desai, a clinical fellow in the division of hospital medicine at Cincinnati Children’s Hospital.
“At our institution with uncomplicated UTI, we wait to see what the culture grows.” If there’s an oral antibiotic that will work, “we send [infants] home in 3-4 days. We haven’t had any poor outcomes, even when they’re bacteremic,” she said.
The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
ATLANTA – A short course of IV antibiotics – 7 days or less – is fine for most infants with uncomplicated bacteremic urinary tract infections, according to a review of 116 children younger than 60 days.
How long to treat bacteremic UTIs in the very young has been debated in pediatrics for a while, with some centers opting for a few days and others for 2 weeks or more. Shorter courses reduce length of stay, costs, and complications, but there hasn’t been much research to see whether they work as well.
The new investigation has suggested they do. “Young infants with bacteremic UTI who received less than or equal to 7 days of IV antibiotic therapy did not have more recurrent UTIs,” compared “to infants who received longer courses. Short course IV therapy with early conversion to oral antibiotics may be considered in this population,” said lead investigator Sanyukta Desai, MD, at the Pediatric Hospital Medicine meeting.
The team compared outcomes of 58 infants treated for 7 days or less to outcomes of 58 infants treated for more than 7 days at 11 children’s hospitals scattered across the United States.
Urine was collected by catheter, and each child grew the same organism in their blood and urine cultures, confirming the diagnosis of bacteremic UTI. Children with bacterial meningitis, or suspected of having it, were excluded. The subjects had all been admitted through the ED.
There was quite a bit of variation among the 11 hospitals, with the proportion of children treated with short courses ranging from 10% to 81%.
As for the results, two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days. None of them developed meningitis, and none required ICU admission. Propensity-score matching revealed an odds ratio for recurrence that favored shorter treatment, but it wasn’t statistically significant.
The mean length of stay was 5 days in the short-course arm and 11 days in the long-course arm. There were no serious adverse events within 30 days of the index admission in either group.
Among the recurrences, the two children in the short-course arm were initially treated for 3 and 5 days. Both were older than 28 days at their initial presentation, and both had vesicoureteral reflux of at least grade 2, which was not diagnosed in one child until after the recurrence. The other child had been on prophylactic trimethoprim/sulfamethoxazole before the recurrence.
The four recurrent cases in the long arm initially received either 10 or 14 days of IV antibiotics. Two children had grade 4 vesicoureteral reflux and had been on prophylactic amoxicillin.
Infants treated with longer antibiotic courses were more likely to be under 28 days old, appear ill at presentation, have had bacteremia for more than 24 hours, and have and grow out pathogens other than Escherichia coli. The two groups were otherwise balanced for sex, prematurity, complex chronic conditions, and known genitourinary anomalies.
With such low event rates, the study wasn’t powered to detect small but potentially meaningful differences in outcomes, and further work is needed to define which children would benefit from longer treatment courses. Even so, “it was reassuring that patients did well in both arms,” said Dr. Desai, a clinical fellow in the division of hospital medicine at Cincinnati Children’s Hospital.
“At our institution with uncomplicated UTI, we wait to see what the culture grows.” If there’s an oral antibiotic that will work, “we send [infants] home in 3-4 days. We haven’t had any poor outcomes, even when they’re bacteremic,” she said.
The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
REPORTING FROM PHM 2018
Key clinical point:
Major finding: Two patients in the short-course group (3%) and four in the long-course group (7%) had recurrent UTIs within 30 days.
Study details: Review of 116 infants.
Disclosures: The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
Preterm birth rate ‘is on the rise again’
After several years of decline, the incidence of preterm births in the United States “is on the rise again,” according to the National Center for Health Statistics.
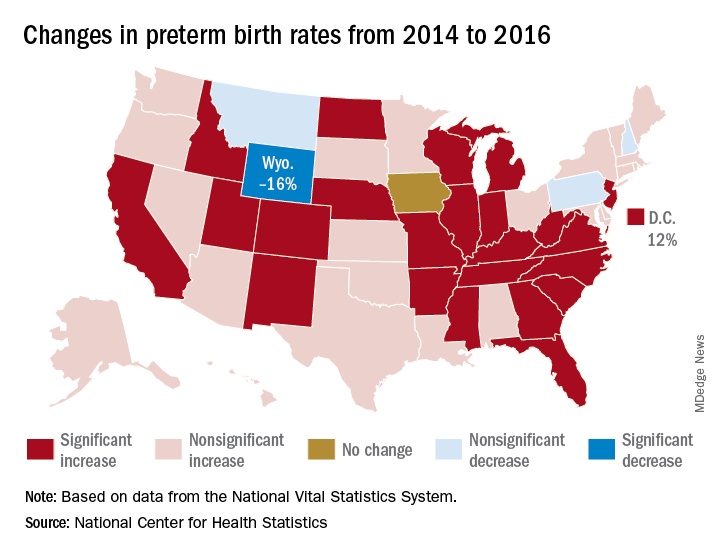
That 3% increase was spread pretty evenly: 23 states and the District of Columbia experienced statistically significant increases from 2014 to 2016, and 22 other states also had increases, although these were not statistically significant. One state, Iowa, had no change; three states – Montana, New Hampshire, and Pennsylvania – had nonsignificant declines, and Wyoming was the only state with a statistically significant drop (16%) in preterm birth incidence, they said based on data from the National Vital Statistics System.
The largest increase, 12%, was seen in the District of Columbia, followed by Idaho and North Dakota at 10% and Arkansas, New Mexico, and West Virginia at 9%, the researchers reported.
After several years of decline, the incidence of preterm births in the United States “is on the rise again,” according to the National Center for Health Statistics.

That 3% increase was spread pretty evenly: 23 states and the District of Columbia experienced statistically significant increases from 2014 to 2016, and 22 other states also had increases, although these were not statistically significant. One state, Iowa, had no change; three states – Montana, New Hampshire, and Pennsylvania – had nonsignificant declines, and Wyoming was the only state with a statistically significant drop (16%) in preterm birth incidence, they said based on data from the National Vital Statistics System.
The largest increase, 12%, was seen in the District of Columbia, followed by Idaho and North Dakota at 10% and Arkansas, New Mexico, and West Virginia at 9%, the researchers reported.
After several years of decline, the incidence of preterm births in the United States “is on the rise again,” according to the National Center for Health Statistics.

That 3% increase was spread pretty evenly: 23 states and the District of Columbia experienced statistically significant increases from 2014 to 2016, and 22 other states also had increases, although these were not statistically significant. One state, Iowa, had no change; three states – Montana, New Hampshire, and Pennsylvania – had nonsignificant declines, and Wyoming was the only state with a statistically significant drop (16%) in preterm birth incidence, they said based on data from the National Vital Statistics System.
The largest increase, 12%, was seen in the District of Columbia, followed by Idaho and North Dakota at 10% and Arkansas, New Mexico, and West Virginia at 9%, the researchers reported.
Spinal muscular atrophy added to newborn screening panel recommendations
Spinal muscular atrophy (SMA) is now among the disorders officially included in the Recommended Uniform Screening Panel (RUSP), which is used by state public health departments to screen newborns for genetic disorders.
Secretary of the Department of Health and Human Services Alex M. Azar II formally added SMA to the panel July 2 on the recommendation of the Advisory Committee on Heritable Disorders in Newborns and Children.
“Adding SMA to the list will help ensure that babies born with SMA are identified, so that they have the opportunity to benefit from early treatment and intervention,” according to a statement from the Muscular Dystrophy Association about the decision. “This testing can also provide families with a genetic diagnosis – information that often is required to determine whether their child is eligible to participate in clinical trials.”
Adding SMA to the RUSP does not mean states must screen newborns for the disorder. Each state’s public health apparatus decides independently whether to accept the recommendation and which disorders on the RUSP to screen for. Most states screen for most disorders on the RUSP. Evidence compiled by the advisory committee suggested wide variation in resources, infrastructure, funding, and time to implementation among states.
An estimated 1 in 11,000 newborns have SMA, a disorder caused by mutations in the survival motor neuron 1 (SMN1) gene. SMA affects motor neurons in the brain stem and spinal cord leading to motor weakness and atrophy. The only treatment for SMA had been palliative care until the Food and Drug Administration approved nusinersen (Spinraza) for the disorder in December 2016, although the drug’s approval has raised some ethical questions.1-3
After reviewing the evidence at their February 8, 2018 meeting, the advisory committee recommended the addition of spinal muscular atrophy screening to the RUSP in a March 8, 2018, letter from committee chair Joseph A. Bocchini Jr., MD, who is a professor and the chairman of the department of pediatrics at Louisiana State University Health in Shreveport.
Secretary Azar accepted the recommendation based on the evidence the committee provided; he also requested a follow-up report within 2 years “describing the status of implementing newborn screening for SMA and clinical outcomes of early treatment, including any potential harms, for infants diagnosed with SMA.”
The advisory committee makes its recommendations to the HHS on which heritable disorders to include in the RUSP after they have assessed a systematic, evidence-based review assigned by the committee to an external independent group. Alex R. Kemper, MD, MPH, a professor of pediatrics at the Ohio State University and division chief of ambulatory pediatrics at Nationwide Children’s Hospital, both in Columbus, led the review group for SMA. Dr. Kemper is also deputy editor of the journal Pediatrics and a member of the U.S. Preventive Services Task Force.
According to Secretary Azar’s summary in his July 2, 2018, letter of acceptance, the evidence review suggested that “early screening and treatment can lead to decreased mortality for individuals with SMA and improved motor milestones.”
Dr. Kemper elaborated in an interview that, “SMA can be detected through newborn screening, and treatment is now available that can not only reduce the risk of death but decrease the development of neurologic impairment. As with adding any condition to newborn screening, public health laboratories will need to develop strategies to incorporate the screening test. The current FDA-approved treatment, nusinersen, is delivered by lumbar puncture into the spinal fluid. In addition, there are exciting advances in gene therapy leading to new treatment approaches.”
Approximately 95% of SMA cases result from the deletion of exon 7 from both alleles of SMN1. (Other rarer cases are caused by mutations in different genes.) Without the SMN protein produced by SMN1, a person gradually loses muscle function.
A similar gene, SMN2, also can produce the SMN protein but in much lower amounts, typically less than 10% of what a person needs. People can, however, have multiple copies of SMN2, which can produce slightly more SMN protein for a slower disease process.
The five types of spinal muscular atrophy are determined according to symptom onset, which directly correlates with disorder severity and prognosis. Just over half (54%) of SMA cases are Type I, in which progressive weakness occurs over the first 6 months of life and results in early death. Only 18% of children with Type I live past age 4 years, and 68% die by age 2 years. Type 0 is rarer but more severe, usually causing fetal loss or early infant death.
Type II represents 18% of SMA cases and causes progressive weakness by age 15 months. Most people with Type II survive to their 30s but then experience respiratory failure and rarely reach their fourth decade. Individuals with Types III and IV typically have a normal lifespan and only begin to see progressive muscle weakness after 1 year old or in adulthood.
Dr. Kemper’s group focused on the three types diagnosed in infancy: types I, II, and III.
Dr. Kemper emphasized in an interview that “it will be critical to make sure that infants diagnosed with SMA through newborn screening receive follow-up shortly afterward to determine whether they would benefit from nusinersen. More information is needed about the long-term outcomes of those infants who begin treatment following newborn screening so we not only know about outcomes in later childhood and adolescence but treatment approaches can be further refined and personalized.”
Nusinersen works by altering the splicing of precursor messenger RNA in SMN2 so that the mRNA strands are longer, which thereby increases how much SMN protein is produced. Concerns about the medication, however, have included its cost – $750,000 in the first year and $375,000 every following year for life – and potential adverse events from repeated administration. Nusinersen is injected into the spinal canal four times in the first year and then once annually, and the painful injections require patient immobilization. Potential adverse events include thrombocytopenia and nephrotoxicity, along with potential complications from repeated lumbar punctures over time.2
Other concerns about the drug include its limited evidence base, lack of long-term data, associated costs with administration (for example, travel costs), the potential for patients taking nusinersen to be excluded from future clinical trials on other treatments, and ensuring parents have enough information on the drug’s limitations and potential risks to provide adequate informed consent.2
Yet evidence to date is favorable in children with early onset. Dr. Bocchini wrote in the letter to Secretary Azar that “limited data suggest that treatment effect is greater when the treatment is initiated before symptoms develop and when the individual has more copies of SMN2.”
Dr. Kemper’s group concluded that screening can detect SMA in newborns and that treatment can modify disease course. “Grey literature suggests those with total disease duration less than or equal to 12 weeks before nusinersen treatment were more likely to have better outcomes than those with longer periods of disease duration.”
“Presymptomatic treatment alters the natural history” of the disorder, the group found, although outcome data past 1 year of age are not yet available. Based on findings from a New York pilot program, they predicted that nationwide newborn screening would avert 33 deaths and 48 cases of children who were dependent on a ventilator among an annual cohort of 4 million births.
At the time of the evidence review, Massachusetts, Minnesota, Missouri, North Carolina, New York, Utah, and Wisconsin initiated pilot programs or whole-population mandated screening for SMA. Of the three states that reported costs, all reported costs at $1 or less per screen.
The research for the evidence review was funded by a Health Resources and Services Administration grant to Duke University, Durham, N.C. No disclosures were provided for evidence review group members.
References
1. Gene Ther. 2017 Sep;24(9):534-8.
2. JAMA Intern Med. 2018 Jun 1;178(6):743-44.
3. JAMA Pediatr. 2018 Feb 1;172(2):188-92.
Spinal muscular atrophy (SMA) is now among the disorders officially included in the Recommended Uniform Screening Panel (RUSP), which is used by state public health departments to screen newborns for genetic disorders.
Secretary of the Department of Health and Human Services Alex M. Azar II formally added SMA to the panel July 2 on the recommendation of the Advisory Committee on Heritable Disorders in Newborns and Children.
“Adding SMA to the list will help ensure that babies born with SMA are identified, so that they have the opportunity to benefit from early treatment and intervention,” according to a statement from the Muscular Dystrophy Association about the decision. “This testing can also provide families with a genetic diagnosis – information that often is required to determine whether their child is eligible to participate in clinical trials.”
Adding SMA to the RUSP does not mean states must screen newborns for the disorder. Each state’s public health apparatus decides independently whether to accept the recommendation and which disorders on the RUSP to screen for. Most states screen for most disorders on the RUSP. Evidence compiled by the advisory committee suggested wide variation in resources, infrastructure, funding, and time to implementation among states.
An estimated 1 in 11,000 newborns have SMA, a disorder caused by mutations in the survival motor neuron 1 (SMN1) gene. SMA affects motor neurons in the brain stem and spinal cord leading to motor weakness and atrophy. The only treatment for SMA had been palliative care until the Food and Drug Administration approved nusinersen (Spinraza) for the disorder in December 2016, although the drug’s approval has raised some ethical questions.1-3
After reviewing the evidence at their February 8, 2018 meeting, the advisory committee recommended the addition of spinal muscular atrophy screening to the RUSP in a March 8, 2018, letter from committee chair Joseph A. Bocchini Jr., MD, who is a professor and the chairman of the department of pediatrics at Louisiana State University Health in Shreveport.
Secretary Azar accepted the recommendation based on the evidence the committee provided; he also requested a follow-up report within 2 years “describing the status of implementing newborn screening for SMA and clinical outcomes of early treatment, including any potential harms, for infants diagnosed with SMA.”
The advisory committee makes its recommendations to the HHS on which heritable disorders to include in the RUSP after they have assessed a systematic, evidence-based review assigned by the committee to an external independent group. Alex R. Kemper, MD, MPH, a professor of pediatrics at the Ohio State University and division chief of ambulatory pediatrics at Nationwide Children’s Hospital, both in Columbus, led the review group for SMA. Dr. Kemper is also deputy editor of the journal Pediatrics and a member of the U.S. Preventive Services Task Force.
According to Secretary Azar’s summary in his July 2, 2018, letter of acceptance, the evidence review suggested that “early screening and treatment can lead to decreased mortality for individuals with SMA and improved motor milestones.”
Dr. Kemper elaborated in an interview that, “SMA can be detected through newborn screening, and treatment is now available that can not only reduce the risk of death but decrease the development of neurologic impairment. As with adding any condition to newborn screening, public health laboratories will need to develop strategies to incorporate the screening test. The current FDA-approved treatment, nusinersen, is delivered by lumbar puncture into the spinal fluid. In addition, there are exciting advances in gene therapy leading to new treatment approaches.”
Approximately 95% of SMA cases result from the deletion of exon 7 from both alleles of SMN1. (Other rarer cases are caused by mutations in different genes.) Without the SMN protein produced by SMN1, a person gradually loses muscle function.
A similar gene, SMN2, also can produce the SMN protein but in much lower amounts, typically less than 10% of what a person needs. People can, however, have multiple copies of SMN2, which can produce slightly more SMN protein for a slower disease process.
The five types of spinal muscular atrophy are determined according to symptom onset, which directly correlates with disorder severity and prognosis. Just over half (54%) of SMA cases are Type I, in which progressive weakness occurs over the first 6 months of life and results in early death. Only 18% of children with Type I live past age 4 years, and 68% die by age 2 years. Type 0 is rarer but more severe, usually causing fetal loss or early infant death.
Type II represents 18% of SMA cases and causes progressive weakness by age 15 months. Most people with Type II survive to their 30s but then experience respiratory failure and rarely reach their fourth decade. Individuals with Types III and IV typically have a normal lifespan and only begin to see progressive muscle weakness after 1 year old or in adulthood.
Dr. Kemper’s group focused on the three types diagnosed in infancy: types I, II, and III.
Dr. Kemper emphasized in an interview that “it will be critical to make sure that infants diagnosed with SMA through newborn screening receive follow-up shortly afterward to determine whether they would benefit from nusinersen. More information is needed about the long-term outcomes of those infants who begin treatment following newborn screening so we not only know about outcomes in later childhood and adolescence but treatment approaches can be further refined and personalized.”
Nusinersen works by altering the splicing of precursor messenger RNA in SMN2 so that the mRNA strands are longer, which thereby increases how much SMN protein is produced. Concerns about the medication, however, have included its cost – $750,000 in the first year and $375,000 every following year for life – and potential adverse events from repeated administration. Nusinersen is injected into the spinal canal four times in the first year and then once annually, and the painful injections require patient immobilization. Potential adverse events include thrombocytopenia and nephrotoxicity, along with potential complications from repeated lumbar punctures over time.2
Other concerns about the drug include its limited evidence base, lack of long-term data, associated costs with administration (for example, travel costs), the potential for patients taking nusinersen to be excluded from future clinical trials on other treatments, and ensuring parents have enough information on the drug’s limitations and potential risks to provide adequate informed consent.2
Yet evidence to date is favorable in children with early onset. Dr. Bocchini wrote in the letter to Secretary Azar that “limited data suggest that treatment effect is greater when the treatment is initiated before symptoms develop and when the individual has more copies of SMN2.”
Dr. Kemper’s group concluded that screening can detect SMA in newborns and that treatment can modify disease course. “Grey literature suggests those with total disease duration less than or equal to 12 weeks before nusinersen treatment were more likely to have better outcomes than those with longer periods of disease duration.”
“Presymptomatic treatment alters the natural history” of the disorder, the group found, although outcome data past 1 year of age are not yet available. Based on findings from a New York pilot program, they predicted that nationwide newborn screening would avert 33 deaths and 48 cases of children who were dependent on a ventilator among an annual cohort of 4 million births.
At the time of the evidence review, Massachusetts, Minnesota, Missouri, North Carolina, New York, Utah, and Wisconsin initiated pilot programs or whole-population mandated screening for SMA. Of the three states that reported costs, all reported costs at $1 or less per screen.
The research for the evidence review was funded by a Health Resources and Services Administration grant to Duke University, Durham, N.C. No disclosures were provided for evidence review group members.
References
1. Gene Ther. 2017 Sep;24(9):534-8.
2. JAMA Intern Med. 2018 Jun 1;178(6):743-44.
3. JAMA Pediatr. 2018 Feb 1;172(2):188-92.
Spinal muscular atrophy (SMA) is now among the disorders officially included in the Recommended Uniform Screening Panel (RUSP), which is used by state public health departments to screen newborns for genetic disorders.
Secretary of the Department of Health and Human Services Alex M. Azar II formally added SMA to the panel July 2 on the recommendation of the Advisory Committee on Heritable Disorders in Newborns and Children.
“Adding SMA to the list will help ensure that babies born with SMA are identified, so that they have the opportunity to benefit from early treatment and intervention,” according to a statement from the Muscular Dystrophy Association about the decision. “This testing can also provide families with a genetic diagnosis – information that often is required to determine whether their child is eligible to participate in clinical trials.”
Adding SMA to the RUSP does not mean states must screen newborns for the disorder. Each state’s public health apparatus decides independently whether to accept the recommendation and which disorders on the RUSP to screen for. Most states screen for most disorders on the RUSP. Evidence compiled by the advisory committee suggested wide variation in resources, infrastructure, funding, and time to implementation among states.
An estimated 1 in 11,000 newborns have SMA, a disorder caused by mutations in the survival motor neuron 1 (SMN1) gene. SMA affects motor neurons in the brain stem and spinal cord leading to motor weakness and atrophy. The only treatment for SMA had been palliative care until the Food and Drug Administration approved nusinersen (Spinraza) for the disorder in December 2016, although the drug’s approval has raised some ethical questions.1-3
After reviewing the evidence at their February 8, 2018 meeting, the advisory committee recommended the addition of spinal muscular atrophy screening to the RUSP in a March 8, 2018, letter from committee chair Joseph A. Bocchini Jr., MD, who is a professor and the chairman of the department of pediatrics at Louisiana State University Health in Shreveport.
Secretary Azar accepted the recommendation based on the evidence the committee provided; he also requested a follow-up report within 2 years “describing the status of implementing newborn screening for SMA and clinical outcomes of early treatment, including any potential harms, for infants diagnosed with SMA.”
The advisory committee makes its recommendations to the HHS on which heritable disorders to include in the RUSP after they have assessed a systematic, evidence-based review assigned by the committee to an external independent group. Alex R. Kemper, MD, MPH, a professor of pediatrics at the Ohio State University and division chief of ambulatory pediatrics at Nationwide Children’s Hospital, both in Columbus, led the review group for SMA. Dr. Kemper is also deputy editor of the journal Pediatrics and a member of the U.S. Preventive Services Task Force.
According to Secretary Azar’s summary in his July 2, 2018, letter of acceptance, the evidence review suggested that “early screening and treatment can lead to decreased mortality for individuals with SMA and improved motor milestones.”
Dr. Kemper elaborated in an interview that, “SMA can be detected through newborn screening, and treatment is now available that can not only reduce the risk of death but decrease the development of neurologic impairment. As with adding any condition to newborn screening, public health laboratories will need to develop strategies to incorporate the screening test. The current FDA-approved treatment, nusinersen, is delivered by lumbar puncture into the spinal fluid. In addition, there are exciting advances in gene therapy leading to new treatment approaches.”
Approximately 95% of SMA cases result from the deletion of exon 7 from both alleles of SMN1. (Other rarer cases are caused by mutations in different genes.) Without the SMN protein produced by SMN1, a person gradually loses muscle function.
A similar gene, SMN2, also can produce the SMN protein but in much lower amounts, typically less than 10% of what a person needs. People can, however, have multiple copies of SMN2, which can produce slightly more SMN protein for a slower disease process.
The five types of spinal muscular atrophy are determined according to symptom onset, which directly correlates with disorder severity and prognosis. Just over half (54%) of SMA cases are Type I, in which progressive weakness occurs over the first 6 months of life and results in early death. Only 18% of children with Type I live past age 4 years, and 68% die by age 2 years. Type 0 is rarer but more severe, usually causing fetal loss or early infant death.
Type II represents 18% of SMA cases and causes progressive weakness by age 15 months. Most people with Type II survive to their 30s but then experience respiratory failure and rarely reach their fourth decade. Individuals with Types III and IV typically have a normal lifespan and only begin to see progressive muscle weakness after 1 year old or in adulthood.
Dr. Kemper’s group focused on the three types diagnosed in infancy: types I, II, and III.
Dr. Kemper emphasized in an interview that “it will be critical to make sure that infants diagnosed with SMA through newborn screening receive follow-up shortly afterward to determine whether they would benefit from nusinersen. More information is needed about the long-term outcomes of those infants who begin treatment following newborn screening so we not only know about outcomes in later childhood and adolescence but treatment approaches can be further refined and personalized.”
Nusinersen works by altering the splicing of precursor messenger RNA in SMN2 so that the mRNA strands are longer, which thereby increases how much SMN protein is produced. Concerns about the medication, however, have included its cost – $750,000 in the first year and $375,000 every following year for life – and potential adverse events from repeated administration. Nusinersen is injected into the spinal canal four times in the first year and then once annually, and the painful injections require patient immobilization. Potential adverse events include thrombocytopenia and nephrotoxicity, along with potential complications from repeated lumbar punctures over time.2
Other concerns about the drug include its limited evidence base, lack of long-term data, associated costs with administration (for example, travel costs), the potential for patients taking nusinersen to be excluded from future clinical trials on other treatments, and ensuring parents have enough information on the drug’s limitations and potential risks to provide adequate informed consent.2
Yet evidence to date is favorable in children with early onset. Dr. Bocchini wrote in the letter to Secretary Azar that “limited data suggest that treatment effect is greater when the treatment is initiated before symptoms develop and when the individual has more copies of SMN2.”
Dr. Kemper’s group concluded that screening can detect SMA in newborns and that treatment can modify disease course. “Grey literature suggests those with total disease duration less than or equal to 12 weeks before nusinersen treatment were more likely to have better outcomes than those with longer periods of disease duration.”
“Presymptomatic treatment alters the natural history” of the disorder, the group found, although outcome data past 1 year of age are not yet available. Based on findings from a New York pilot program, they predicted that nationwide newborn screening would avert 33 deaths and 48 cases of children who were dependent on a ventilator among an annual cohort of 4 million births.
At the time of the evidence review, Massachusetts, Minnesota, Missouri, North Carolina, New York, Utah, and Wisconsin initiated pilot programs or whole-population mandated screening for SMA. Of the three states that reported costs, all reported costs at $1 or less per screen.
The research for the evidence review was funded by a Health Resources and Services Administration grant to Duke University, Durham, N.C. No disclosures were provided for evidence review group members.
References
1. Gene Ther. 2017 Sep;24(9):534-8.
2. JAMA Intern Med. 2018 Jun 1;178(6):743-44.
3. JAMA Pediatr. 2018 Feb 1;172(2):188-92.
More testing of febrile infants at teaching vs. community hospitals, but similar outcomes
TORONTO – according to a study presented at the Pediatric Academic Societies annual meeting.
“The community hospitals are doing less procedures on the infants, but with basically the exact same outcomes,” said Beth C. Natt, MD, MPH, director of pediatric hospital medicine at Bridgeport (Conn.) Hospital.
Babies who presented to university-affiliated hospitals were more likely to be hospitalized (70% vs. 67%; P = .001) than were those at community hospitals, but had a similar likelihood of being diagnosed with bacteremia, meningitis, or urinary tract infection. The rates of missed bacterial infection were 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
“There is some thought that in community settings, because we’re not completing the workup in the standard, protocolized way seen at teaching hospitals, we might be doing wrong by the children, but these data show we’re actually doing just fine,” Dr. Natt said in an interview.
She and her colleagues reviewed 9,884 febrile infant evaluations occurring at 132 hospitals participating in the Reducing Excessive Variation in the Infant Sepsis Evaluation (REVISE) quality improvement project. Two-thirds of the infants (n = 6,479) were evaluated across 78 university-affiliated hospitals and 3,405 (or 34%) were seen at 54 community hospitals. Hospital status was self-reported.
The teaching hospitals more often had at least one pediatric emergency medicine provider, compared with community hospitals (90% vs. 57%; P = .001) and were more likely to see babies between 7 and 30 days old (90% vs. 57%; P = .001). They also were more likely to obtain urine cultures (92% vs. 88%; P = 0.001), blood cultures (84% vs. 80%; P = .001), and cerebral spinal fluid cultures (62% vs. 57%; P = .001).
On the other hand, community hospitals were significantly more likely to see children presenting with respiratory symptoms (39% vs. 36% for teaching hospitals; P = .014), and were more likely to order chest x-rays on febrile infants (32% vs. 24% for university-affiliated hospitals; P = .001).
“As a community hospitalist, the results weren’t that surprising to me,” said Dr. Natt. “If anything was surprising it was how often we were doing chest x-rays, but I think that had to do with the fact that we had more children with respiratory symptoms coming to community hospitals.
“The American Academy of Pediatrics guidelines for fever were written last in 1993, when I was in high school, so they are very due to be revised,” said Dr. Natt. “I suspect the new guidelines will have us doing fewer spinal taps in children and more watchful waiting.”
TORONTO – according to a study presented at the Pediatric Academic Societies annual meeting.
“The community hospitals are doing less procedures on the infants, but with basically the exact same outcomes,” said Beth C. Natt, MD, MPH, director of pediatric hospital medicine at Bridgeport (Conn.) Hospital.
Babies who presented to university-affiliated hospitals were more likely to be hospitalized (70% vs. 67%; P = .001) than were those at community hospitals, but had a similar likelihood of being diagnosed with bacteremia, meningitis, or urinary tract infection. The rates of missed bacterial infection were 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
“There is some thought that in community settings, because we’re not completing the workup in the standard, protocolized way seen at teaching hospitals, we might be doing wrong by the children, but these data show we’re actually doing just fine,” Dr. Natt said in an interview.
She and her colleagues reviewed 9,884 febrile infant evaluations occurring at 132 hospitals participating in the Reducing Excessive Variation in the Infant Sepsis Evaluation (REVISE) quality improvement project. Two-thirds of the infants (n = 6,479) were evaluated across 78 university-affiliated hospitals and 3,405 (or 34%) were seen at 54 community hospitals. Hospital status was self-reported.
The teaching hospitals more often had at least one pediatric emergency medicine provider, compared with community hospitals (90% vs. 57%; P = .001) and were more likely to see babies between 7 and 30 days old (90% vs. 57%; P = .001). They also were more likely to obtain urine cultures (92% vs. 88%; P = 0.001), blood cultures (84% vs. 80%; P = .001), and cerebral spinal fluid cultures (62% vs. 57%; P = .001).
On the other hand, community hospitals were significantly more likely to see children presenting with respiratory symptoms (39% vs. 36% for teaching hospitals; P = .014), and were more likely to order chest x-rays on febrile infants (32% vs. 24% for university-affiliated hospitals; P = .001).
“As a community hospitalist, the results weren’t that surprising to me,” said Dr. Natt. “If anything was surprising it was how often we were doing chest x-rays, but I think that had to do with the fact that we had more children with respiratory symptoms coming to community hospitals.
“The American Academy of Pediatrics guidelines for fever were written last in 1993, when I was in high school, so they are very due to be revised,” said Dr. Natt. “I suspect the new guidelines will have us doing fewer spinal taps in children and more watchful waiting.”
TORONTO – according to a study presented at the Pediatric Academic Societies annual meeting.
“The community hospitals are doing less procedures on the infants, but with basically the exact same outcomes,” said Beth C. Natt, MD, MPH, director of pediatric hospital medicine at Bridgeport (Conn.) Hospital.
Babies who presented to university-affiliated hospitals were more likely to be hospitalized (70% vs. 67%; P = .001) than were those at community hospitals, but had a similar likelihood of being diagnosed with bacteremia, meningitis, or urinary tract infection. The rates of missed bacterial infection were 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
“There is some thought that in community settings, because we’re not completing the workup in the standard, protocolized way seen at teaching hospitals, we might be doing wrong by the children, but these data show we’re actually doing just fine,” Dr. Natt said in an interview.
She and her colleagues reviewed 9,884 febrile infant evaluations occurring at 132 hospitals participating in the Reducing Excessive Variation in the Infant Sepsis Evaluation (REVISE) quality improvement project. Two-thirds of the infants (n = 6,479) were evaluated across 78 university-affiliated hospitals and 3,405 (or 34%) were seen at 54 community hospitals. Hospital status was self-reported.
The teaching hospitals more often had at least one pediatric emergency medicine provider, compared with community hospitals (90% vs. 57%; P = .001) and were more likely to see babies between 7 and 30 days old (90% vs. 57%; P = .001). They also were more likely to obtain urine cultures (92% vs. 88%; P = 0.001), blood cultures (84% vs. 80%; P = .001), and cerebral spinal fluid cultures (62% vs. 57%; P = .001).
On the other hand, community hospitals were significantly more likely to see children presenting with respiratory symptoms (39% vs. 36% for teaching hospitals; P = .014), and were more likely to order chest x-rays on febrile infants (32% vs. 24% for university-affiliated hospitals; P = .001).
“As a community hospitalist, the results weren’t that surprising to me,” said Dr. Natt. “If anything was surprising it was how often we were doing chest x-rays, but I think that had to do with the fact that we had more children with respiratory symptoms coming to community hospitals.
“The American Academy of Pediatrics guidelines for fever were written last in 1993, when I was in high school, so they are very due to be revised,” said Dr. Natt. “I suspect the new guidelines will have us doing fewer spinal taps in children and more watchful waiting.”
AT PAS 18
Key clinical point: University-affiliated hospitals do more invasive testing in febrile infants, but have outcomes similar to those of community hospitals.
Major finding: The rate of missed bacterial infection did not differ between hospital types: 0.8% for teaching hospitals and 1% for community hospitals (P = .346).
Study details: Review of 9,884 febrile infant evaluations occurring at 132 hospitals, 66% of which were university-affiliated hospitals and 34% of which were community hospitals.
Disclosures: The investigators reported no conflicts of interest.