User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
‘Bright future’ for growth factor therapy in osteoarthritis
LIVERPOOL, ENGLAND – (OA), according to David Hunter, MBBS, PhD.
Dr. Hunter, who is the Florance and Cope Chair of Rheumatology and professor of medicine at the University of Sydney and the Royal North Shore Hospital, Sydney, Australia, outlined some of the recent research with these agents in OA.
In 2013, a U.S. study (Arthritis Care Res. 2013;65[5]:703-11) showed that the 2007-2008 incidence of symptomatic knee OA was highest between the ages of 54 and 64 years, with the estimated median age at diagnosis of 55.8 years. Patients were substantially younger than were those who had been diagnosed at a median age of 68.5 years more than a decade earlier in 1991-1992.
“So, we’re developing [OA] at a much earlier age, but we’re obviously living longer and living with that morbidity for a substantial period of time,” Dr. Hunter said at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
“It’s within that context that we really need to think about both the individual and societal burden of osteoarthritis, and why we’re developing agents such as growth factors to help retard symptoms related to disease,” continued Dr. Hunter, chair of the Institute of Bone and Joint Research, deputy dean of the Northern Clinical School, and consultant rheumatologist at North Sydney Orthopaedic and Sports Medicine Centre, Sydney.
OA is characterized by articular cartilage damage, low-grade synovial inflammation, and hypertrophic bone changes that lead to pain and functional deterioration (Expert Opin Emerg Drugs. 2015;20[3]:361-78). Attempts to modify the disease process have thus focused on these three areas, aiming to regulate cartilage catabolism and anabolism, control inflammation, and remodel subchondral bone (Rheumatology [Oxford]. 2018;57[suppl. 4]:iv108-iv123).
Growth factors fall under the category of regulating cartilage catabolism and anabolism, playing a “significant role in musculoskeletal tissues’ homeostasis and repair,” Dr. Hunter explained. “They influence chemotaxis, differentiation, proliferation, and synthetic activity of cartilage and bone cells, and can regulate physiological remodeling and cartilage healing.”
Examples of growth factors of interest in OA include transforming growth factor-beta superfamily and bone morphogenic proteins 2 and 7, fibroblast growth factors 2 and 18, and insulin-like growth factor-1.
Two currently unproven and somewhat controversial ways of regulating cartilage catabolism and anabolism is the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP). Neither are ready for widespread use, Dr. Hunter observed, and more data on these approaches still need to be gathered.
The rationale behind the use of MSCs is that all joint tissues contain these cells, and they can potentially differentiate into cartilage, bone, or other tissues. There are changes in the quantity, phenotype, and differentiation potential of these cells in patients with OA, however, leading researchers to see if intra-articular injection of MSCs could be a possible therapeutic strategy. MSCs can be isolated from a number of different tissues and differentiate into multiple cells types. Animal model data suggest that their transplantation into an affected joint can stimulate articular repair.
“From a clinical perspective, there have been a vast array of clinical trials looking at different types of MSCs and different types of administration,” Dr. Hunter noted. Pooled data suggest that stem cell therapy does help to improve pain and function. However, the quality of trials has been called into question, with a positive publication bias suggested. There’s no clear evidence of any structural benefits, Dr. Hunter stated. “There’s a lot of stem cells being used for clinical purposes without the evidence to support their efficacy,” he cautioned. “We need much better regulation.”
There are several PRP agents, which deliver growth factors, cytokines, adhesive proteins, and other plasma proteins, such as clotting factors. It’s unknown how PRP therapy works in OA, whether it is just pain relieving or could affect the progression of joint damage. So far, studies have been done only in knee OA, so data in other joints need to be obtained.
“Whilst they have great potential, the literature on the use of PRP is conflicting,” Dr. Hunter said. “There are a lot of positive trials out there, but their quality in general is quite low.”
Better data are perhaps available for the recombinant human fibroblast growth factor–18 sprifermin, with recent 3-year data from an ongoing 5-year randomized, placebo-controlled, phase 2 study showing increased total cartilage thickness in the tibiofemoral joint relative to placebo. “There does appear to be a structure benefit,” Dr. Hunter said of sprifermin’s effects in the study versus intra-articular saline.
Although improvement in total Western Ontario and McMaster Universities Osteoarthritis Index scores were seen in the patients with knee OA, there was no real differentiation by dose or by placebo. The rationale for having a longer trial follow-up – the last dose of sprifermin was given at 18 months – is to see if there is a later effect.
In the later stages of development is Invossa TissueGene-C; this is drug that consists of patients’ normal and genetically-modified chondrocyte cells that express transforming growth factor-beta 1. Phase 2 findings have shown that it can improve pain and function scores in patients with moderate to advanced knee OA (BMC Musculoskelet Disord. 2017;18[1]:461). Based on those findings, two phase 3 trials are currently underway, with some further findings presented elsewhere at the OARSI meeting.
Even though there is still no disease-modifying osteoarthritis drug currently licensed for use, “I’m really positive about all of what we’ve learned, particularly with the application to future trials and ongoing trials in this space,” Dr. Hunter observed.
“Future strategies should differentiate between those agents aiming to reduce pain, and those targeting structural evolution, incorporating a combination of anabolic and anti-catabolic therapies,” Dr. Hunter suggested. One of the challenges, however, is to address the placebo effect, which can be exaggerated by the context of administration or surgery.
Furthermore, he proposed that new treatments should “fit into the natural progression of OA” by focusing on early disease where changes might still be reversible. “We’re getting more nuanced about where to intervene,” Dr. Hunter said.
Emerging treatments should also take the location of the disease and its heterogenic nature into account.
Dr. Hunter has consulted for Zynerba, TLC, Kolon TissueGene, and Merck Serono, and he disclosed receiving royalties from DJO for a patellofemoral brace patent.
SOURCE: Lohmander S et al. Osteoarthritis Cartilage. 2018:26(1):S6. Abstract I-18
LIVERPOOL, ENGLAND – (OA), according to David Hunter, MBBS, PhD.
Dr. Hunter, who is the Florance and Cope Chair of Rheumatology and professor of medicine at the University of Sydney and the Royal North Shore Hospital, Sydney, Australia, outlined some of the recent research with these agents in OA.
In 2013, a U.S. study (Arthritis Care Res. 2013;65[5]:703-11) showed that the 2007-2008 incidence of symptomatic knee OA was highest between the ages of 54 and 64 years, with the estimated median age at diagnosis of 55.8 years. Patients were substantially younger than were those who had been diagnosed at a median age of 68.5 years more than a decade earlier in 1991-1992.
“So, we’re developing [OA] at a much earlier age, but we’re obviously living longer and living with that morbidity for a substantial period of time,” Dr. Hunter said at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
“It’s within that context that we really need to think about both the individual and societal burden of osteoarthritis, and why we’re developing agents such as growth factors to help retard symptoms related to disease,” continued Dr. Hunter, chair of the Institute of Bone and Joint Research, deputy dean of the Northern Clinical School, and consultant rheumatologist at North Sydney Orthopaedic and Sports Medicine Centre, Sydney.
OA is characterized by articular cartilage damage, low-grade synovial inflammation, and hypertrophic bone changes that lead to pain and functional deterioration (Expert Opin Emerg Drugs. 2015;20[3]:361-78). Attempts to modify the disease process have thus focused on these three areas, aiming to regulate cartilage catabolism and anabolism, control inflammation, and remodel subchondral bone (Rheumatology [Oxford]. 2018;57[suppl. 4]:iv108-iv123).
Growth factors fall under the category of regulating cartilage catabolism and anabolism, playing a “significant role in musculoskeletal tissues’ homeostasis and repair,” Dr. Hunter explained. “They influence chemotaxis, differentiation, proliferation, and synthetic activity of cartilage and bone cells, and can regulate physiological remodeling and cartilage healing.”
Examples of growth factors of interest in OA include transforming growth factor-beta superfamily and bone morphogenic proteins 2 and 7, fibroblast growth factors 2 and 18, and insulin-like growth factor-1.
Two currently unproven and somewhat controversial ways of regulating cartilage catabolism and anabolism is the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP). Neither are ready for widespread use, Dr. Hunter observed, and more data on these approaches still need to be gathered.
The rationale behind the use of MSCs is that all joint tissues contain these cells, and they can potentially differentiate into cartilage, bone, or other tissues. There are changes in the quantity, phenotype, and differentiation potential of these cells in patients with OA, however, leading researchers to see if intra-articular injection of MSCs could be a possible therapeutic strategy. MSCs can be isolated from a number of different tissues and differentiate into multiple cells types. Animal model data suggest that their transplantation into an affected joint can stimulate articular repair.
“From a clinical perspective, there have been a vast array of clinical trials looking at different types of MSCs and different types of administration,” Dr. Hunter noted. Pooled data suggest that stem cell therapy does help to improve pain and function. However, the quality of trials has been called into question, with a positive publication bias suggested. There’s no clear evidence of any structural benefits, Dr. Hunter stated. “There’s a lot of stem cells being used for clinical purposes without the evidence to support their efficacy,” he cautioned. “We need much better regulation.”
There are several PRP agents, which deliver growth factors, cytokines, adhesive proteins, and other plasma proteins, such as clotting factors. It’s unknown how PRP therapy works in OA, whether it is just pain relieving or could affect the progression of joint damage. So far, studies have been done only in knee OA, so data in other joints need to be obtained.
“Whilst they have great potential, the literature on the use of PRP is conflicting,” Dr. Hunter said. “There are a lot of positive trials out there, but their quality in general is quite low.”
Better data are perhaps available for the recombinant human fibroblast growth factor–18 sprifermin, with recent 3-year data from an ongoing 5-year randomized, placebo-controlled, phase 2 study showing increased total cartilage thickness in the tibiofemoral joint relative to placebo. “There does appear to be a structure benefit,” Dr. Hunter said of sprifermin’s effects in the study versus intra-articular saline.
Although improvement in total Western Ontario and McMaster Universities Osteoarthritis Index scores were seen in the patients with knee OA, there was no real differentiation by dose or by placebo. The rationale for having a longer trial follow-up – the last dose of sprifermin was given at 18 months – is to see if there is a later effect.
In the later stages of development is Invossa TissueGene-C; this is drug that consists of patients’ normal and genetically-modified chondrocyte cells that express transforming growth factor-beta 1. Phase 2 findings have shown that it can improve pain and function scores in patients with moderate to advanced knee OA (BMC Musculoskelet Disord. 2017;18[1]:461). Based on those findings, two phase 3 trials are currently underway, with some further findings presented elsewhere at the OARSI meeting.
Even though there is still no disease-modifying osteoarthritis drug currently licensed for use, “I’m really positive about all of what we’ve learned, particularly with the application to future trials and ongoing trials in this space,” Dr. Hunter observed.
“Future strategies should differentiate between those agents aiming to reduce pain, and those targeting structural evolution, incorporating a combination of anabolic and anti-catabolic therapies,” Dr. Hunter suggested. One of the challenges, however, is to address the placebo effect, which can be exaggerated by the context of administration or surgery.
Furthermore, he proposed that new treatments should “fit into the natural progression of OA” by focusing on early disease where changes might still be reversible. “We’re getting more nuanced about where to intervene,” Dr. Hunter said.
Emerging treatments should also take the location of the disease and its heterogenic nature into account.
Dr. Hunter has consulted for Zynerba, TLC, Kolon TissueGene, and Merck Serono, and he disclosed receiving royalties from DJO for a patellofemoral brace patent.
SOURCE: Lohmander S et al. Osteoarthritis Cartilage. 2018:26(1):S6. Abstract I-18
LIVERPOOL, ENGLAND – (OA), according to David Hunter, MBBS, PhD.
Dr. Hunter, who is the Florance and Cope Chair of Rheumatology and professor of medicine at the University of Sydney and the Royal North Shore Hospital, Sydney, Australia, outlined some of the recent research with these agents in OA.
In 2013, a U.S. study (Arthritis Care Res. 2013;65[5]:703-11) showed that the 2007-2008 incidence of symptomatic knee OA was highest between the ages of 54 and 64 years, with the estimated median age at diagnosis of 55.8 years. Patients were substantially younger than were those who had been diagnosed at a median age of 68.5 years more than a decade earlier in 1991-1992.
“So, we’re developing [OA] at a much earlier age, but we’re obviously living longer and living with that morbidity for a substantial period of time,” Dr. Hunter said at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
“It’s within that context that we really need to think about both the individual and societal burden of osteoarthritis, and why we’re developing agents such as growth factors to help retard symptoms related to disease,” continued Dr. Hunter, chair of the Institute of Bone and Joint Research, deputy dean of the Northern Clinical School, and consultant rheumatologist at North Sydney Orthopaedic and Sports Medicine Centre, Sydney.
OA is characterized by articular cartilage damage, low-grade synovial inflammation, and hypertrophic bone changes that lead to pain and functional deterioration (Expert Opin Emerg Drugs. 2015;20[3]:361-78). Attempts to modify the disease process have thus focused on these three areas, aiming to regulate cartilage catabolism and anabolism, control inflammation, and remodel subchondral bone (Rheumatology [Oxford]. 2018;57[suppl. 4]:iv108-iv123).
Growth factors fall under the category of regulating cartilage catabolism and anabolism, playing a “significant role in musculoskeletal tissues’ homeostasis and repair,” Dr. Hunter explained. “They influence chemotaxis, differentiation, proliferation, and synthetic activity of cartilage and bone cells, and can regulate physiological remodeling and cartilage healing.”
Examples of growth factors of interest in OA include transforming growth factor-beta superfamily and bone morphogenic proteins 2 and 7, fibroblast growth factors 2 and 18, and insulin-like growth factor-1.
Two currently unproven and somewhat controversial ways of regulating cartilage catabolism and anabolism is the use of mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP). Neither are ready for widespread use, Dr. Hunter observed, and more data on these approaches still need to be gathered.
The rationale behind the use of MSCs is that all joint tissues contain these cells, and they can potentially differentiate into cartilage, bone, or other tissues. There are changes in the quantity, phenotype, and differentiation potential of these cells in patients with OA, however, leading researchers to see if intra-articular injection of MSCs could be a possible therapeutic strategy. MSCs can be isolated from a number of different tissues and differentiate into multiple cells types. Animal model data suggest that their transplantation into an affected joint can stimulate articular repair.
“From a clinical perspective, there have been a vast array of clinical trials looking at different types of MSCs and different types of administration,” Dr. Hunter noted. Pooled data suggest that stem cell therapy does help to improve pain and function. However, the quality of trials has been called into question, with a positive publication bias suggested. There’s no clear evidence of any structural benefits, Dr. Hunter stated. “There’s a lot of stem cells being used for clinical purposes without the evidence to support their efficacy,” he cautioned. “We need much better regulation.”
There are several PRP agents, which deliver growth factors, cytokines, adhesive proteins, and other plasma proteins, such as clotting factors. It’s unknown how PRP therapy works in OA, whether it is just pain relieving or could affect the progression of joint damage. So far, studies have been done only in knee OA, so data in other joints need to be obtained.
“Whilst they have great potential, the literature on the use of PRP is conflicting,” Dr. Hunter said. “There are a lot of positive trials out there, but their quality in general is quite low.”
Better data are perhaps available for the recombinant human fibroblast growth factor–18 sprifermin, with recent 3-year data from an ongoing 5-year randomized, placebo-controlled, phase 2 study showing increased total cartilage thickness in the tibiofemoral joint relative to placebo. “There does appear to be a structure benefit,” Dr. Hunter said of sprifermin’s effects in the study versus intra-articular saline.
Although improvement in total Western Ontario and McMaster Universities Osteoarthritis Index scores were seen in the patients with knee OA, there was no real differentiation by dose or by placebo. The rationale for having a longer trial follow-up – the last dose of sprifermin was given at 18 months – is to see if there is a later effect.
In the later stages of development is Invossa TissueGene-C; this is drug that consists of patients’ normal and genetically-modified chondrocyte cells that express transforming growth factor-beta 1. Phase 2 findings have shown that it can improve pain and function scores in patients with moderate to advanced knee OA (BMC Musculoskelet Disord. 2017;18[1]:461). Based on those findings, two phase 3 trials are currently underway, with some further findings presented elsewhere at the OARSI meeting.
Even though there is still no disease-modifying osteoarthritis drug currently licensed for use, “I’m really positive about all of what we’ve learned, particularly with the application to future trials and ongoing trials in this space,” Dr. Hunter observed.
“Future strategies should differentiate between those agents aiming to reduce pain, and those targeting structural evolution, incorporating a combination of anabolic and anti-catabolic therapies,” Dr. Hunter suggested. One of the challenges, however, is to address the placebo effect, which can be exaggerated by the context of administration or surgery.
Furthermore, he proposed that new treatments should “fit into the natural progression of OA” by focusing on early disease where changes might still be reversible. “We’re getting more nuanced about where to intervene,” Dr. Hunter said.
Emerging treatments should also take the location of the disease and its heterogenic nature into account.
Dr. Hunter has consulted for Zynerba, TLC, Kolon TissueGene, and Merck Serono, and he disclosed receiving royalties from DJO for a patellofemoral brace patent.
SOURCE: Lohmander S et al. Osteoarthritis Cartilage. 2018:26(1):S6. Abstract I-18
EXPERT ANALYSIS FROM OARSI 2018
TissueGene-C effects on knee OA seen at 3 years
LIVERPOOL, ENGLAND – TissueGene-C continues to show promise as a potential disease-modifying osteoarthritis drug, according to the long-term follow-up data of a phase 3 trial.
Within 2-3 years of receiving a single injection of the novel cell-gene therapy, patients with moderate knee OA were still experiencing significant improvement in the coprimary endpoints of knee symptoms, function, sports activity, and knee pain versus baseline values.
Differences in International Knee Documentation Committee (IKDC) scores from baseline to 2 and 3 years were a respective 15.3 and 14.8 points (both P less than .05 vs. baseline). Pain, assessed on a visual analog scale, was also significantly improved from baseline to 2 and 3 years (score changes –23.5 and –23.3; P less than .05 vs. baseline).
There were also significant improvements in the secondary endpoint of pain, stiffness, and physical function measured by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), with changes in scores of –19.8 and –17.4 versus baseline at 2 and 3 years, respectively (both P less than .05 vs. baseline).
“INVOISSA-K [TissueGene-C] is the first-in-class cell and gene therapy for the treatment of knee OA,” Dr. Lee reminded delegates at the congress, which is sponsored by the Osteoarthritis Research Society International. The novel treatment was made by collecting chondrocytes from a subject with polydactyl hands and then culturing these to create two subpopulations of cells, one with allogenic chondrocytes and the other with genetically modified chondrocytes that overexpress transforming growth factor-beta1. These subpopulations are then mixed in the ratio of 3:1 and delivered in a single intra-articular injection.
Dr. Lee, who is the president and chief executive officer of Kolon TissueGene, Rockville, Md., reported new findings of a phase 3 trial conducted exclusively in Korea at 12 study centers, the results of which were first presented at OARSI 2016.
At that time only the 12-month primary endpoint data were available, which showed that TissueGene-C improved IKDC scores from baseline by a significantly greater amount than a saline placebo, with changes in scores of 15.1 and 5 points, respectively, versus baseline values. Improvements in IKDC scores were seen as early as 3 months but only became significantly better than placebo at 6 months.
Similarly, visual analog scale pain scores had improved from baseline as early as 3 months but were only significantly different from placebo at 6 months (–23.4 change from baseline for TissueGene-C vs. –14.6 for placebo) and out to 12 months (–24.5 vs. –10.3). WOMAC scores with TissueGene-C were only significantly different from placebo at 12 months (–13.5 vs. –6.2 from baseline, respectively).
The 12-month OMERACT-OARSI responder rate was 84% for TissueGene-C and 45% for placebo. The most common adverse events seen with TissueGene-C were related to the injection site, with peripheral edema (9%), arthralgia (8%), joint swelling (6%), and injection-site pain (5%) reported.
A total of 163 patients were recruited into the study, all had Kellgren-Lawrence grade 3 knee OA, which was also graded as 3 or 4 on the International Cartilage Regeneration & Joint Preservation Society scale. Major lesions had be 6 cm2 or smaller. Active treatment was given to 78 patients, and 81 received placebo. The primary assessment period was at 12 months, but patients continued to be followed out to 5 years.
At OARSI 2018, Dr. Lee presented the findings of 12-month structural modification analyses for the first time. These showed that while the osteophyte score on MRI was not significantly different from baseline in the active-treatment arm, there was a significantly increased osteophyte score and total cartilage defect in the placebo-treated patients versus baseline values.
Subchondral bone changes at 12 months showed a trend for less bone area change with TissueGene-C than placebo and a trend for increased cartilage thickness. Greater reductions in serum CTX-1 and urine CTX-II were seen with active treatment than placebo, relative to screening values.
X-ray evaluation of joint-space narrowing showed that nonprogression was more likely in patients treated with TissueGene-C than with placebo (77% vs. 57%), although this was not significant. In addition, fewer patients treated with TissueGene-C than placebo who needed a total knee replacement at 2 years (0% and 7.5%) and at 3 years (2% and 14%).
TissueGene-C “has great potential” for being the first disease-modifying osteoarthritis drug to get to market, Dr. Lee suggested.
The clinical development program for TissueGene-C is further advanced in Korea than in the United States, where a phase 3 trial is about to start recruitment soon.
“We started our clinical trials in 2005 in Korea and in the U.S. simultaneously,” Dr. Lee said, noting that a biologics license application submitted in Korea in 2016 had been accepted by the Ministry of Food and Drug Safety on July 12, 2017, while only phase 1 and 2 trials have been completed in the United States
The study was funded by Kolon Life Science and TissueGene. Dr. Lee is an employee of TissueGene.
SOURCE: Lee B et al. Osteoarthritis Cartilage. 2018 Apr;26(1):S43-4.
LIVERPOOL, ENGLAND – TissueGene-C continues to show promise as a potential disease-modifying osteoarthritis drug, according to the long-term follow-up data of a phase 3 trial.
Within 2-3 years of receiving a single injection of the novel cell-gene therapy, patients with moderate knee OA were still experiencing significant improvement in the coprimary endpoints of knee symptoms, function, sports activity, and knee pain versus baseline values.
Differences in International Knee Documentation Committee (IKDC) scores from baseline to 2 and 3 years were a respective 15.3 and 14.8 points (both P less than .05 vs. baseline). Pain, assessed on a visual analog scale, was also significantly improved from baseline to 2 and 3 years (score changes –23.5 and –23.3; P less than .05 vs. baseline).
There were also significant improvements in the secondary endpoint of pain, stiffness, and physical function measured by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), with changes in scores of –19.8 and –17.4 versus baseline at 2 and 3 years, respectively (both P less than .05 vs. baseline).
“INVOISSA-K [TissueGene-C] is the first-in-class cell and gene therapy for the treatment of knee OA,” Dr. Lee reminded delegates at the congress, which is sponsored by the Osteoarthritis Research Society International. The novel treatment was made by collecting chondrocytes from a subject with polydactyl hands and then culturing these to create two subpopulations of cells, one with allogenic chondrocytes and the other with genetically modified chondrocytes that overexpress transforming growth factor-beta1. These subpopulations are then mixed in the ratio of 3:1 and delivered in a single intra-articular injection.
Dr. Lee, who is the president and chief executive officer of Kolon TissueGene, Rockville, Md., reported new findings of a phase 3 trial conducted exclusively in Korea at 12 study centers, the results of which were first presented at OARSI 2016.
At that time only the 12-month primary endpoint data were available, which showed that TissueGene-C improved IKDC scores from baseline by a significantly greater amount than a saline placebo, with changes in scores of 15.1 and 5 points, respectively, versus baseline values. Improvements in IKDC scores were seen as early as 3 months but only became significantly better than placebo at 6 months.
Similarly, visual analog scale pain scores had improved from baseline as early as 3 months but were only significantly different from placebo at 6 months (–23.4 change from baseline for TissueGene-C vs. –14.6 for placebo) and out to 12 months (–24.5 vs. –10.3). WOMAC scores with TissueGene-C were only significantly different from placebo at 12 months (–13.5 vs. –6.2 from baseline, respectively).
The 12-month OMERACT-OARSI responder rate was 84% for TissueGene-C and 45% for placebo. The most common adverse events seen with TissueGene-C were related to the injection site, with peripheral edema (9%), arthralgia (8%), joint swelling (6%), and injection-site pain (5%) reported.
A total of 163 patients were recruited into the study, all had Kellgren-Lawrence grade 3 knee OA, which was also graded as 3 or 4 on the International Cartilage Regeneration & Joint Preservation Society scale. Major lesions had be 6 cm2 or smaller. Active treatment was given to 78 patients, and 81 received placebo. The primary assessment period was at 12 months, but patients continued to be followed out to 5 years.
At OARSI 2018, Dr. Lee presented the findings of 12-month structural modification analyses for the first time. These showed that while the osteophyte score on MRI was not significantly different from baseline in the active-treatment arm, there was a significantly increased osteophyte score and total cartilage defect in the placebo-treated patients versus baseline values.
Subchondral bone changes at 12 months showed a trend for less bone area change with TissueGene-C than placebo and a trend for increased cartilage thickness. Greater reductions in serum CTX-1 and urine CTX-II were seen with active treatment than placebo, relative to screening values.
X-ray evaluation of joint-space narrowing showed that nonprogression was more likely in patients treated with TissueGene-C than with placebo (77% vs. 57%), although this was not significant. In addition, fewer patients treated with TissueGene-C than placebo who needed a total knee replacement at 2 years (0% and 7.5%) and at 3 years (2% and 14%).
TissueGene-C “has great potential” for being the first disease-modifying osteoarthritis drug to get to market, Dr. Lee suggested.
The clinical development program for TissueGene-C is further advanced in Korea than in the United States, where a phase 3 trial is about to start recruitment soon.
“We started our clinical trials in 2005 in Korea and in the U.S. simultaneously,” Dr. Lee said, noting that a biologics license application submitted in Korea in 2016 had been accepted by the Ministry of Food and Drug Safety on July 12, 2017, while only phase 1 and 2 trials have been completed in the United States
The study was funded by Kolon Life Science and TissueGene. Dr. Lee is an employee of TissueGene.
SOURCE: Lee B et al. Osteoarthritis Cartilage. 2018 Apr;26(1):S43-4.
LIVERPOOL, ENGLAND – TissueGene-C continues to show promise as a potential disease-modifying osteoarthritis drug, according to the long-term follow-up data of a phase 3 trial.
Within 2-3 years of receiving a single injection of the novel cell-gene therapy, patients with moderate knee OA were still experiencing significant improvement in the coprimary endpoints of knee symptoms, function, sports activity, and knee pain versus baseline values.
Differences in International Knee Documentation Committee (IKDC) scores from baseline to 2 and 3 years were a respective 15.3 and 14.8 points (both P less than .05 vs. baseline). Pain, assessed on a visual analog scale, was also significantly improved from baseline to 2 and 3 years (score changes –23.5 and –23.3; P less than .05 vs. baseline).
There were also significant improvements in the secondary endpoint of pain, stiffness, and physical function measured by the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), with changes in scores of –19.8 and –17.4 versus baseline at 2 and 3 years, respectively (both P less than .05 vs. baseline).
“INVOISSA-K [TissueGene-C] is the first-in-class cell and gene therapy for the treatment of knee OA,” Dr. Lee reminded delegates at the congress, which is sponsored by the Osteoarthritis Research Society International. The novel treatment was made by collecting chondrocytes from a subject with polydactyl hands and then culturing these to create two subpopulations of cells, one with allogenic chondrocytes and the other with genetically modified chondrocytes that overexpress transforming growth factor-beta1. These subpopulations are then mixed in the ratio of 3:1 and delivered in a single intra-articular injection.
Dr. Lee, who is the president and chief executive officer of Kolon TissueGene, Rockville, Md., reported new findings of a phase 3 trial conducted exclusively in Korea at 12 study centers, the results of which were first presented at OARSI 2016.
At that time only the 12-month primary endpoint data were available, which showed that TissueGene-C improved IKDC scores from baseline by a significantly greater amount than a saline placebo, with changes in scores of 15.1 and 5 points, respectively, versus baseline values. Improvements in IKDC scores were seen as early as 3 months but only became significantly better than placebo at 6 months.
Similarly, visual analog scale pain scores had improved from baseline as early as 3 months but were only significantly different from placebo at 6 months (–23.4 change from baseline for TissueGene-C vs. –14.6 for placebo) and out to 12 months (–24.5 vs. –10.3). WOMAC scores with TissueGene-C were only significantly different from placebo at 12 months (–13.5 vs. –6.2 from baseline, respectively).
The 12-month OMERACT-OARSI responder rate was 84% for TissueGene-C and 45% for placebo. The most common adverse events seen with TissueGene-C were related to the injection site, with peripheral edema (9%), arthralgia (8%), joint swelling (6%), and injection-site pain (5%) reported.
A total of 163 patients were recruited into the study, all had Kellgren-Lawrence grade 3 knee OA, which was also graded as 3 or 4 on the International Cartilage Regeneration & Joint Preservation Society scale. Major lesions had be 6 cm2 or smaller. Active treatment was given to 78 patients, and 81 received placebo. The primary assessment period was at 12 months, but patients continued to be followed out to 5 years.
At OARSI 2018, Dr. Lee presented the findings of 12-month structural modification analyses for the first time. These showed that while the osteophyte score on MRI was not significantly different from baseline in the active-treatment arm, there was a significantly increased osteophyte score and total cartilage defect in the placebo-treated patients versus baseline values.
Subchondral bone changes at 12 months showed a trend for less bone area change with TissueGene-C than placebo and a trend for increased cartilage thickness. Greater reductions in serum CTX-1 and urine CTX-II were seen with active treatment than placebo, relative to screening values.
X-ray evaluation of joint-space narrowing showed that nonprogression was more likely in patients treated with TissueGene-C than with placebo (77% vs. 57%), although this was not significant. In addition, fewer patients treated with TissueGene-C than placebo who needed a total knee replacement at 2 years (0% and 7.5%) and at 3 years (2% and 14%).
TissueGene-C “has great potential” for being the first disease-modifying osteoarthritis drug to get to market, Dr. Lee suggested.
The clinical development program for TissueGene-C is further advanced in Korea than in the United States, where a phase 3 trial is about to start recruitment soon.
“We started our clinical trials in 2005 in Korea and in the U.S. simultaneously,” Dr. Lee said, noting that a biologics license application submitted in Korea in 2016 had been accepted by the Ministry of Food and Drug Safety on July 12, 2017, while only phase 1 and 2 trials have been completed in the United States
The study was funded by Kolon Life Science and TissueGene. Dr. Lee is an employee of TissueGene.
SOURCE: Lee B et al. Osteoarthritis Cartilage. 2018 Apr;26(1):S43-4.
REPORTING FROM OARSI 2018
Key clinical point: A single intra-articular injection of TissueGene C produced significant and long-term relief of knee osteoarthritis.
Major finding: Changes in baseline International Knee Documentation Committee and visual analog scale pain scores from baseline to 2 and 3 years were 15.3 and 14.8 points (P less than .05), and –23.5 and –23.3 (P less than .05), respectively.
Study details: A multicenter, phase 3, randomized, double-blind, placebo-controlled trial of 163 patients with knee osteoarthritis.
Disclosures: The study was funded by Kolon Life Science and TissueGene. Dr. Lee is an employee of TissueGene.
Source: Lee B et al. Osteoarthritis Cartilage. 2018 Apr;26(1):S43-4.
Wearing lateral wedge insoles reduces osteoarthritis knee pain
LIVERPOOL, ENGLAND – Lateral wedge insoles reduced osteoarthritis knee pain to a greater extent than did wearing neutral insoles in a randomized, controlled, crossover trial.
An overall significant treatment difference of 0.7 points on a 0-10 numerical rating scale global pain score was observed when patients with painful medial osteoarthritis (OA) wore lateral wedge insoles compared with when they wore neutral insoles in their own shoes (95% confidence interval [CI], 0.1-1.2 points; P = .02).
The study’s findings are in contrast to previous research that has found no benefit of lateral wedge insoles in patients with medial compartment knee OA (JAMA. 2013;310[7]:722-30) because patients were specifically prescreened to find those who would be biomechanical responders, David T. Felson, MD, said at the World Congress on Osteoarthritis.
“There is increased medial load in medial knee OA, and that is thought to cause pain and medial joint damage,” Dr. Felson, of Boston University, said at the congress, sponsored by the Osteoarthritis Research Society International.
There is evidence, however, that by moving the center of pressure laterally during walking with the use of lateral wedge insoles, the external knee adduction movement (KAM) can be reduced by around 5%-6%, which in turn can reduce the load across the medial component.
“We asked the following question: ‘If patients were screened to remove those with painful patellofemoral OA and those who did not show a biomechanical response to lateral wedge insoles, would lateral wedge insoles actually reduce pain?’ ” Dr. Felson noted.
For inclusion in the trial, patients needed to be aged between 40 and 80 years and have x-rays showing Kellgren-Lawrence grade 2-4 OA with definite medial and no lateral joint space narrowing in the last 2 years. They then also needed to have experienced knee pain that was rated at least 4 or more on a 0-10 numerical rating scale in the last week. Patients then had to be examined by an experienced physiotherapist or have x-rays to exclude patellofemoral OA and inflammatory arthritis.
All patients then underwent biomechanical assessment which consisted of motion analysis screening. Patients who did not show at least a 2% decrease in KAM after wearing the lateral wedge insoles versus the neutral insoles were excluded.
Of 112 subjects who were screened for the study, there remained 62 with a mean age of 64 years and body mass index of 28 kg/m2 who could be randomized. The subjects, 73% of whom were male, were randomized to first wear either neutral insoles or insoles with a lateral five-degree wedge for 8 weeks and then, after an 8-week washout period, to wear the other type of insole for a further 8 weeks. Participants wore the insoles for at least 4 hours a day and for a median of 7 hours per day.
Results showed that the lateral wedge insoles reduced KAM by 7.5% versus their own shoes and by 6.6% versus a neutral insole, and of the 62 patients randomized, 56 completed the trial. Two patients in each group stopped participating in the trial because of adverse events, which with lateral wedge insoles were cramps in the calf and foot and increased pain in the knee, and with the neutral insoles included a blister and increased pain in knee and foot.
At baseline, the mean global knee pain score in the last week was 5.46. After use of the lateral wedge or neutral insoles, this fell to a respective 4.2 and 4.9.
Patients were asked to report on the pain experience during a nominated activity. The baseline value for pain was 6.18, which decreased to 4.9 and 5.8 in the two groups, respectively, with an overall treatment difference of 0.9 (P = .001) favoring the use of the lateral wedge insoles.
There was improvement in knee pain assessed by the Knee injury and Osteoarthritis Outcomes Score from a baseline of 55 points to a score of 60.6 for lateral wedge and 58.9 for the neutral insoles, a between group difference of –1.7 (P = .45).
“Lateral wedge insoles reduce knee pain in selected subjects with painful medial OA, those without patellofemoral OA, and those who are biomechanical responders to these insoles,” Dr. Felson said.
“I think the importance of this study is that it opens the door to a treatment that we were frustrated wasn’t working,” Dr. Felson said. Insoles are inexpensive, safe, and potentially widely useful, and the research paves the way for further study of these insoles and for perhaps for shoes that could modify knee loads.
The findings open the door to “stratified approaches to knee OA that may also offer us an avenue toward success in developing treatments,” he added.
The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
SOURCE: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
LIVERPOOL, ENGLAND – Lateral wedge insoles reduced osteoarthritis knee pain to a greater extent than did wearing neutral insoles in a randomized, controlled, crossover trial.
An overall significant treatment difference of 0.7 points on a 0-10 numerical rating scale global pain score was observed when patients with painful medial osteoarthritis (OA) wore lateral wedge insoles compared with when they wore neutral insoles in their own shoes (95% confidence interval [CI], 0.1-1.2 points; P = .02).
The study’s findings are in contrast to previous research that has found no benefit of lateral wedge insoles in patients with medial compartment knee OA (JAMA. 2013;310[7]:722-30) because patients were specifically prescreened to find those who would be biomechanical responders, David T. Felson, MD, said at the World Congress on Osteoarthritis.
“There is increased medial load in medial knee OA, and that is thought to cause pain and medial joint damage,” Dr. Felson, of Boston University, said at the congress, sponsored by the Osteoarthritis Research Society International.
There is evidence, however, that by moving the center of pressure laterally during walking with the use of lateral wedge insoles, the external knee adduction movement (KAM) can be reduced by around 5%-6%, which in turn can reduce the load across the medial component.
“We asked the following question: ‘If patients were screened to remove those with painful patellofemoral OA and those who did not show a biomechanical response to lateral wedge insoles, would lateral wedge insoles actually reduce pain?’ ” Dr. Felson noted.
For inclusion in the trial, patients needed to be aged between 40 and 80 years and have x-rays showing Kellgren-Lawrence grade 2-4 OA with definite medial and no lateral joint space narrowing in the last 2 years. They then also needed to have experienced knee pain that was rated at least 4 or more on a 0-10 numerical rating scale in the last week. Patients then had to be examined by an experienced physiotherapist or have x-rays to exclude patellofemoral OA and inflammatory arthritis.
All patients then underwent biomechanical assessment which consisted of motion analysis screening. Patients who did not show at least a 2% decrease in KAM after wearing the lateral wedge insoles versus the neutral insoles were excluded.
Of 112 subjects who were screened for the study, there remained 62 with a mean age of 64 years and body mass index of 28 kg/m2 who could be randomized. The subjects, 73% of whom were male, were randomized to first wear either neutral insoles or insoles with a lateral five-degree wedge for 8 weeks and then, after an 8-week washout period, to wear the other type of insole for a further 8 weeks. Participants wore the insoles for at least 4 hours a day and for a median of 7 hours per day.
Results showed that the lateral wedge insoles reduced KAM by 7.5% versus their own shoes and by 6.6% versus a neutral insole, and of the 62 patients randomized, 56 completed the trial. Two patients in each group stopped participating in the trial because of adverse events, which with lateral wedge insoles were cramps in the calf and foot and increased pain in the knee, and with the neutral insoles included a blister and increased pain in knee and foot.
At baseline, the mean global knee pain score in the last week was 5.46. After use of the lateral wedge or neutral insoles, this fell to a respective 4.2 and 4.9.
Patients were asked to report on the pain experience during a nominated activity. The baseline value for pain was 6.18, which decreased to 4.9 and 5.8 in the two groups, respectively, with an overall treatment difference of 0.9 (P = .001) favoring the use of the lateral wedge insoles.
There was improvement in knee pain assessed by the Knee injury and Osteoarthritis Outcomes Score from a baseline of 55 points to a score of 60.6 for lateral wedge and 58.9 for the neutral insoles, a between group difference of –1.7 (P = .45).
“Lateral wedge insoles reduce knee pain in selected subjects with painful medial OA, those without patellofemoral OA, and those who are biomechanical responders to these insoles,” Dr. Felson said.
“I think the importance of this study is that it opens the door to a treatment that we were frustrated wasn’t working,” Dr. Felson said. Insoles are inexpensive, safe, and potentially widely useful, and the research paves the way for further study of these insoles and for perhaps for shoes that could modify knee loads.
The findings open the door to “stratified approaches to knee OA that may also offer us an avenue toward success in developing treatments,” he added.
The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
SOURCE: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
LIVERPOOL, ENGLAND – Lateral wedge insoles reduced osteoarthritis knee pain to a greater extent than did wearing neutral insoles in a randomized, controlled, crossover trial.
An overall significant treatment difference of 0.7 points on a 0-10 numerical rating scale global pain score was observed when patients with painful medial osteoarthritis (OA) wore lateral wedge insoles compared with when they wore neutral insoles in their own shoes (95% confidence interval [CI], 0.1-1.2 points; P = .02).
The study’s findings are in contrast to previous research that has found no benefit of lateral wedge insoles in patients with medial compartment knee OA (JAMA. 2013;310[7]:722-30) because patients were specifically prescreened to find those who would be biomechanical responders, David T. Felson, MD, said at the World Congress on Osteoarthritis.
“There is increased medial load in medial knee OA, and that is thought to cause pain and medial joint damage,” Dr. Felson, of Boston University, said at the congress, sponsored by the Osteoarthritis Research Society International.
There is evidence, however, that by moving the center of pressure laterally during walking with the use of lateral wedge insoles, the external knee adduction movement (KAM) can be reduced by around 5%-6%, which in turn can reduce the load across the medial component.
“We asked the following question: ‘If patients were screened to remove those with painful patellofemoral OA and those who did not show a biomechanical response to lateral wedge insoles, would lateral wedge insoles actually reduce pain?’ ” Dr. Felson noted.
For inclusion in the trial, patients needed to be aged between 40 and 80 years and have x-rays showing Kellgren-Lawrence grade 2-4 OA with definite medial and no lateral joint space narrowing in the last 2 years. They then also needed to have experienced knee pain that was rated at least 4 or more on a 0-10 numerical rating scale in the last week. Patients then had to be examined by an experienced physiotherapist or have x-rays to exclude patellofemoral OA and inflammatory arthritis.
All patients then underwent biomechanical assessment which consisted of motion analysis screening. Patients who did not show at least a 2% decrease in KAM after wearing the lateral wedge insoles versus the neutral insoles were excluded.
Of 112 subjects who were screened for the study, there remained 62 with a mean age of 64 years and body mass index of 28 kg/m2 who could be randomized. The subjects, 73% of whom were male, were randomized to first wear either neutral insoles or insoles with a lateral five-degree wedge for 8 weeks and then, after an 8-week washout period, to wear the other type of insole for a further 8 weeks. Participants wore the insoles for at least 4 hours a day and for a median of 7 hours per day.
Results showed that the lateral wedge insoles reduced KAM by 7.5% versus their own shoes and by 6.6% versus a neutral insole, and of the 62 patients randomized, 56 completed the trial. Two patients in each group stopped participating in the trial because of adverse events, which with lateral wedge insoles were cramps in the calf and foot and increased pain in the knee, and with the neutral insoles included a blister and increased pain in knee and foot.
At baseline, the mean global knee pain score in the last week was 5.46. After use of the lateral wedge or neutral insoles, this fell to a respective 4.2 and 4.9.
Patients were asked to report on the pain experience during a nominated activity. The baseline value for pain was 6.18, which decreased to 4.9 and 5.8 in the two groups, respectively, with an overall treatment difference of 0.9 (P = .001) favoring the use of the lateral wedge insoles.
There was improvement in knee pain assessed by the Knee injury and Osteoarthritis Outcomes Score from a baseline of 55 points to a score of 60.6 for lateral wedge and 58.9 for the neutral insoles, a between group difference of –1.7 (P = .45).
“Lateral wedge insoles reduce knee pain in selected subjects with painful medial OA, those without patellofemoral OA, and those who are biomechanical responders to these insoles,” Dr. Felson said.
“I think the importance of this study is that it opens the door to a treatment that we were frustrated wasn’t working,” Dr. Felson said. Insoles are inexpensive, safe, and potentially widely useful, and the research paves the way for further study of these insoles and for perhaps for shoes that could modify knee loads.
The findings open the door to “stratified approaches to knee OA that may also offer us an avenue toward success in developing treatments,” he added.
The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
SOURCE: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
REPORTING FROM OARSI 2018
Key clinical point:
Major finding: There was a mean treatment difference of 0.7 points in a global pain score favoring lateral wedge over neutral insoles (P = .02).
Study details: Randomized, controlled, crossover study of 62 patients with painful medial knee OA.
Disclosures: The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
Source: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
Cathepsin K inhibitor exhibits bone protecting effects in osteoarthritis
LIVERPOOL, ENGLAND – The investigational cathepsin K inhibitor MIV-711 had positive effects on both bone and cartilage at 6 months in patients with knee osteoarthritis (OA) in a phase 2a study.
The MRI measures of the “area of bone in the medial femur region” and “average cartilage thickness” showed reduced progression with MIV-711 versus placebo.
MIV-711 also produced rapid and sustained reductions in the bone biomarkers CTX-I and CTX-II, study investigator Philip Conaghan, MBBS, PhD, reported at the World Congress on Osteoarthritis.
The primary endpoint of the trial – the change in average knee pain over 26 weeks assessed using an 11-point numerical rating scale (NRS) – was not met, however, with no differences between MIV-711 treatment and placebo.
While there were also no statistical differences in Western Ontario and McMaster Universities Osteoarthritis Index pain scores, one of several secondary endpoints assessed, there was a trend for less pain with MIV-711 than with placebo.
“The lack of symptom benefits may reflect that the study duration was not sufficient to see symptom reduction following structure modification,” Dr. Conaghan and his coauthors stated in their abstract.
“That’s something we’re seeing with all the drugs that are trying to get DMOAD [disease modifying osteoarthritis drug] licenses at present,“ Dr. Conaghan said at the Congress, sponsored by the Osteoarthritis Research Society International.
Dr. Conaghan, who is professor of musculoskeletal medicine and director of the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, noted: “Cathepsin K is a cysteine protease that degrades the collagen matrix and stops bone resorption, so it’s got a number of potential actions,” in the development of OA.
MIV-711 is a potent, selective, and reversible inhibitor of cathepsin K, he added, which has previously been shown to have bone structure–modifying properties in preclinical models. It also has been shown to reduce CTX-I and CTX-II in healthy volunteers.
The current randomized, double-blind, placebo-controlled phase 2a study included two active treatment (100 mg and 200 mg, once daily) arms and one placebo arm. For inclusion, patients had to have knee pain rated as 4 or higher on an 11-point NRS and Kellgren-Lawrence grade 2 or 3 knee OA.
Patients also were allowed to remain on any analgesic medication they were currently taking, provided this was stable. “That enables recruitment as a trialist, and it’s more like my usual practice as it’s unlikely I’d stop an analgesic before starting a new one,” Dr. Conaghan said.
In all, 240 patients were enrolled at six European sites. They had a mean age of 62 years, a body mass index of 32 kg/m2, and 77% were women. MRIs were assessed at baseline and at week 26, with additional clinic visits scheduled at 2, 4, 8, 14, and 20 weeks.
“Overall, there are not any great safety signals,” Dr. Conaghan said. The placebo, 100-mg, and 200-mg MIV-117 arms had similar rates of any adverse event (55%, 54.9%, and 52.4%) or treatment-related adverse events (21.2%, 20.7%, and 24.4%). There were more serious adverse events in the 100-mg and 200-mg MIV-711 treatment arms (3.7% and 2.4%) than in the placebo arm (1.3%), and more discontinuations (7.3%, 4.9%, and 3.8%).
“The primary endpoint of knee pain was not met, but there was a consistent trend, and it looks like there’s a benefit, but the statistically endpoint was not achieved,” Dr. Conaghan summarized.
“MRI measures do show structural modification in terms of bone shape and probably an effect on cartilage,” although the study was not powered to show an effect on the latter, Dr. Conaghan noted.
“The overall data, and from what we’ve seen regarding structural effects, do warrant this drug moving forward into further trials,” he added.
The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speaker’s bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
SOURCE: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
LIVERPOOL, ENGLAND – The investigational cathepsin K inhibitor MIV-711 had positive effects on both bone and cartilage at 6 months in patients with knee osteoarthritis (OA) in a phase 2a study.
The MRI measures of the “area of bone in the medial femur region” and “average cartilage thickness” showed reduced progression with MIV-711 versus placebo.
MIV-711 also produced rapid and sustained reductions in the bone biomarkers CTX-I and CTX-II, study investigator Philip Conaghan, MBBS, PhD, reported at the World Congress on Osteoarthritis.
The primary endpoint of the trial – the change in average knee pain over 26 weeks assessed using an 11-point numerical rating scale (NRS) – was not met, however, with no differences between MIV-711 treatment and placebo.
While there were also no statistical differences in Western Ontario and McMaster Universities Osteoarthritis Index pain scores, one of several secondary endpoints assessed, there was a trend for less pain with MIV-711 than with placebo.
“The lack of symptom benefits may reflect that the study duration was not sufficient to see symptom reduction following structure modification,” Dr. Conaghan and his coauthors stated in their abstract.
“That’s something we’re seeing with all the drugs that are trying to get DMOAD [disease modifying osteoarthritis drug] licenses at present,“ Dr. Conaghan said at the Congress, sponsored by the Osteoarthritis Research Society International.
Dr. Conaghan, who is professor of musculoskeletal medicine and director of the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, noted: “Cathepsin K is a cysteine protease that degrades the collagen matrix and stops bone resorption, so it’s got a number of potential actions,” in the development of OA.
MIV-711 is a potent, selective, and reversible inhibitor of cathepsin K, he added, which has previously been shown to have bone structure–modifying properties in preclinical models. It also has been shown to reduce CTX-I and CTX-II in healthy volunteers.
The current randomized, double-blind, placebo-controlled phase 2a study included two active treatment (100 mg and 200 mg, once daily) arms and one placebo arm. For inclusion, patients had to have knee pain rated as 4 or higher on an 11-point NRS and Kellgren-Lawrence grade 2 or 3 knee OA.
Patients also were allowed to remain on any analgesic medication they were currently taking, provided this was stable. “That enables recruitment as a trialist, and it’s more like my usual practice as it’s unlikely I’d stop an analgesic before starting a new one,” Dr. Conaghan said.
In all, 240 patients were enrolled at six European sites. They had a mean age of 62 years, a body mass index of 32 kg/m2, and 77% were women. MRIs were assessed at baseline and at week 26, with additional clinic visits scheduled at 2, 4, 8, 14, and 20 weeks.
“Overall, there are not any great safety signals,” Dr. Conaghan said. The placebo, 100-mg, and 200-mg MIV-117 arms had similar rates of any adverse event (55%, 54.9%, and 52.4%) or treatment-related adverse events (21.2%, 20.7%, and 24.4%). There were more serious adverse events in the 100-mg and 200-mg MIV-711 treatment arms (3.7% and 2.4%) than in the placebo arm (1.3%), and more discontinuations (7.3%, 4.9%, and 3.8%).
“The primary endpoint of knee pain was not met, but there was a consistent trend, and it looks like there’s a benefit, but the statistically endpoint was not achieved,” Dr. Conaghan summarized.
“MRI measures do show structural modification in terms of bone shape and probably an effect on cartilage,” although the study was not powered to show an effect on the latter, Dr. Conaghan noted.
“The overall data, and from what we’ve seen regarding structural effects, do warrant this drug moving forward into further trials,” he added.
The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speaker’s bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
SOURCE: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
LIVERPOOL, ENGLAND – The investigational cathepsin K inhibitor MIV-711 had positive effects on both bone and cartilage at 6 months in patients with knee osteoarthritis (OA) in a phase 2a study.
The MRI measures of the “area of bone in the medial femur region” and “average cartilage thickness” showed reduced progression with MIV-711 versus placebo.
MIV-711 also produced rapid and sustained reductions in the bone biomarkers CTX-I and CTX-II, study investigator Philip Conaghan, MBBS, PhD, reported at the World Congress on Osteoarthritis.
The primary endpoint of the trial – the change in average knee pain over 26 weeks assessed using an 11-point numerical rating scale (NRS) – was not met, however, with no differences between MIV-711 treatment and placebo.
While there were also no statistical differences in Western Ontario and McMaster Universities Osteoarthritis Index pain scores, one of several secondary endpoints assessed, there was a trend for less pain with MIV-711 than with placebo.
“The lack of symptom benefits may reflect that the study duration was not sufficient to see symptom reduction following structure modification,” Dr. Conaghan and his coauthors stated in their abstract.
“That’s something we’re seeing with all the drugs that are trying to get DMOAD [disease modifying osteoarthritis drug] licenses at present,“ Dr. Conaghan said at the Congress, sponsored by the Osteoarthritis Research Society International.
Dr. Conaghan, who is professor of musculoskeletal medicine and director of the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, noted: “Cathepsin K is a cysteine protease that degrades the collagen matrix and stops bone resorption, so it’s got a number of potential actions,” in the development of OA.
MIV-711 is a potent, selective, and reversible inhibitor of cathepsin K, he added, which has previously been shown to have bone structure–modifying properties in preclinical models. It also has been shown to reduce CTX-I and CTX-II in healthy volunteers.
The current randomized, double-blind, placebo-controlled phase 2a study included two active treatment (100 mg and 200 mg, once daily) arms and one placebo arm. For inclusion, patients had to have knee pain rated as 4 or higher on an 11-point NRS and Kellgren-Lawrence grade 2 or 3 knee OA.
Patients also were allowed to remain on any analgesic medication they were currently taking, provided this was stable. “That enables recruitment as a trialist, and it’s more like my usual practice as it’s unlikely I’d stop an analgesic before starting a new one,” Dr. Conaghan said.
In all, 240 patients were enrolled at six European sites. They had a mean age of 62 years, a body mass index of 32 kg/m2, and 77% were women. MRIs were assessed at baseline and at week 26, with additional clinic visits scheduled at 2, 4, 8, 14, and 20 weeks.
“Overall, there are not any great safety signals,” Dr. Conaghan said. The placebo, 100-mg, and 200-mg MIV-117 arms had similar rates of any adverse event (55%, 54.9%, and 52.4%) or treatment-related adverse events (21.2%, 20.7%, and 24.4%). There were more serious adverse events in the 100-mg and 200-mg MIV-711 treatment arms (3.7% and 2.4%) than in the placebo arm (1.3%), and more discontinuations (7.3%, 4.9%, and 3.8%).
“The primary endpoint of knee pain was not met, but there was a consistent trend, and it looks like there’s a benefit, but the statistically endpoint was not achieved,” Dr. Conaghan summarized.
“MRI measures do show structural modification in terms of bone shape and probably an effect on cartilage,” although the study was not powered to show an effect on the latter, Dr. Conaghan noted.
“The overall data, and from what we’ve seen regarding structural effects, do warrant this drug moving forward into further trials,” he added.
The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speaker’s bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
SOURCE: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
REPORTING FROM OARSI 2018
Key clinical point:
Major finding: A 63%-66% reduction in the medial femur bone area was observed with MIV-711, compared with placebo.
Study details: Randomized, double-blind, placebo-controlled phase 2a study of 240 patients with osteoarthritis knee pain.
Disclosures: The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speakers bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
Source: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
Patient-reported outcomes show impairment decades after acute knee injury
LIVERPOOL, ENGLAND – Decades after they were sustained, acute knee injuries caused clinically significant impairments in patient-reported outcomes, as well as upped the risk for knee osteoarthritis (OA) in an observational study.
Results of the study, which followed up individuals 32-37 years after they were treated for a ruptured anterior cruciate ligament (ACL) injury between 1980 and 1985, showed that, compared with the general population, they experienced greater levels of knee pain, participated less in physical activities, and had a reduced quality of life.
The link between OA and ACL injury is not new, with prior estimates suggesting that up to half of all patients with ACL injury develop OA within 10 years of the injury, said Stephanie Filbay, PhD, who presented the results of the study at the World Congress on Osteoarthritis. There have also been reports of knee pain and other symptoms, and poor quality of life more than 5 years later. What’s not been known until now, however, is what happens with even longer term follow-up, said Dr. Filbay, a postdoctoral research fellow in sport, exercise, and osteoarthritis at the University of Oxford, England.
The aims of the study were to compare patient-reported outcomes at 32-37-years’ follow-up against the general population, then to see if the baseline injury or treatment approach, or knee function 3-7 years after the initial injury had any influence on outcomes.
The study included 223 patients who were between aged 15 and 40 years at the time of the acute ACL injury between 1980 and 1985 and who had been seen within 2 weeks of ACL rupture at Linköping University Hospital in Linköping, Sweden. Patients had been allocated to early surgical or non-surgical treatment based on having an odd or even birth year. They had then been assessed 3-7 years later using a variety of tests to determine the strength of their quadriceps and hamstrings and the ability to hop on one leg.
All patients were then invited 32-37 years later after the initial injury to complete questionnaires and undergo clinical examination and X-rays. Only four people declined and 38 did not answer, leaving 181 (81%) people who agreed to participate and complete the Knee injury and Osteoarthritis Outcome Score (KOOS) and the ACL quality of life questionnaire (ACL-QOL).
The average age of participants at follow-up was 59 years (range, 47-74 years); 30% were female. 58% of all patients had been treated non-surgically initially, and 38% remained non-surgically treated at the longterm follow-up. At baseline, 58% had a meniscus injury.
Compared with an age- and sex-matched Swedish population, patients with ACL injuries had a lower KOOS for pain, sport/recreational activities, and quality of life. For example, KOOS for knee pain was around 65-70 for those with prior ACL injuries, compared with 80-90 for those without ACL injuries, where 100 indicates the best outcome or least pain and zero the worst.
KOOS was not affected by whether or not patients had initial ACL surgery or surgery at any point in their follow up. It also did not appear to matter if patients had a meniscal injury at baseline or not.
Quadriceps and hamstring strength at the 3-7 year postinjury assessment did not affect the longterm KOOS, but the ability to hop on one leg did: Those who were not able to hop on one leg for more than 90% of the time on the unaffected limb at the 3-7 years follow-up had worse pain, symptoms, function, and quality of life at the longterm follow-up point.
With regards to OA, “overall, more than one in two individuals had Kellgren-Lawrence grade 4 that could be considered severe radiographic changes in at least one compartment,” Dr. Filbay said at the meeting, which is sponsored by the Osteoarthritis Research Society International.
Severe radiographic changes were most common in the tibiofemoral joint, with around 47% having Kellgren-Lawrence (KL) grade 4. About 35% of tibiofemoral joints and about 60% of patellofemoral joints were KL grade 1.
Interestingly, different factors were found to be associated with OA in the tibiofemoral and patellofemoral joints, according to Dr. Filbay. Patients who had been treated non-surgically, whether initially or at any time during the 32-37 year follow-up, were more likely to have tibiofemoral OA, whereas those who had been treated surgically tended to have patellofemoral OA.
“Perhaps not surprisingly, meniscal injury at baseline was related to a higher percentage of tibiofemoral OA at long-term follow-up,” Dr. Filbay said.
Another finding was that patients with weaker hamstrings 3-7 years after the injury were more likely to develop patellofemoral joint OA.
Dr. Filbay had no disclosures.
SOURCE: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-3. Abstract 80.
LIVERPOOL, ENGLAND – Decades after they were sustained, acute knee injuries caused clinically significant impairments in patient-reported outcomes, as well as upped the risk for knee osteoarthritis (OA) in an observational study.
Results of the study, which followed up individuals 32-37 years after they were treated for a ruptured anterior cruciate ligament (ACL) injury between 1980 and 1985, showed that, compared with the general population, they experienced greater levels of knee pain, participated less in physical activities, and had a reduced quality of life.
The link between OA and ACL injury is not new, with prior estimates suggesting that up to half of all patients with ACL injury develop OA within 10 years of the injury, said Stephanie Filbay, PhD, who presented the results of the study at the World Congress on Osteoarthritis. There have also been reports of knee pain and other symptoms, and poor quality of life more than 5 years later. What’s not been known until now, however, is what happens with even longer term follow-up, said Dr. Filbay, a postdoctoral research fellow in sport, exercise, and osteoarthritis at the University of Oxford, England.
The aims of the study were to compare patient-reported outcomes at 32-37-years’ follow-up against the general population, then to see if the baseline injury or treatment approach, or knee function 3-7 years after the initial injury had any influence on outcomes.
The study included 223 patients who were between aged 15 and 40 years at the time of the acute ACL injury between 1980 and 1985 and who had been seen within 2 weeks of ACL rupture at Linköping University Hospital in Linköping, Sweden. Patients had been allocated to early surgical or non-surgical treatment based on having an odd or even birth year. They had then been assessed 3-7 years later using a variety of tests to determine the strength of their quadriceps and hamstrings and the ability to hop on one leg.
All patients were then invited 32-37 years later after the initial injury to complete questionnaires and undergo clinical examination and X-rays. Only four people declined and 38 did not answer, leaving 181 (81%) people who agreed to participate and complete the Knee injury and Osteoarthritis Outcome Score (KOOS) and the ACL quality of life questionnaire (ACL-QOL).
The average age of participants at follow-up was 59 years (range, 47-74 years); 30% were female. 58% of all patients had been treated non-surgically initially, and 38% remained non-surgically treated at the longterm follow-up. At baseline, 58% had a meniscus injury.
Compared with an age- and sex-matched Swedish population, patients with ACL injuries had a lower KOOS for pain, sport/recreational activities, and quality of life. For example, KOOS for knee pain was around 65-70 for those with prior ACL injuries, compared with 80-90 for those without ACL injuries, where 100 indicates the best outcome or least pain and zero the worst.
KOOS was not affected by whether or not patients had initial ACL surgery or surgery at any point in their follow up. It also did not appear to matter if patients had a meniscal injury at baseline or not.
Quadriceps and hamstring strength at the 3-7 year postinjury assessment did not affect the longterm KOOS, but the ability to hop on one leg did: Those who were not able to hop on one leg for more than 90% of the time on the unaffected limb at the 3-7 years follow-up had worse pain, symptoms, function, and quality of life at the longterm follow-up point.
With regards to OA, “overall, more than one in two individuals had Kellgren-Lawrence grade 4 that could be considered severe radiographic changes in at least one compartment,” Dr. Filbay said at the meeting, which is sponsored by the Osteoarthritis Research Society International.
Severe radiographic changes were most common in the tibiofemoral joint, with around 47% having Kellgren-Lawrence (KL) grade 4. About 35% of tibiofemoral joints and about 60% of patellofemoral joints were KL grade 1.
Interestingly, different factors were found to be associated with OA in the tibiofemoral and patellofemoral joints, according to Dr. Filbay. Patients who had been treated non-surgically, whether initially or at any time during the 32-37 year follow-up, were more likely to have tibiofemoral OA, whereas those who had been treated surgically tended to have patellofemoral OA.
“Perhaps not surprisingly, meniscal injury at baseline was related to a higher percentage of tibiofemoral OA at long-term follow-up,” Dr. Filbay said.
Another finding was that patients with weaker hamstrings 3-7 years after the injury were more likely to develop patellofemoral joint OA.
Dr. Filbay had no disclosures.
SOURCE: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-3. Abstract 80.
LIVERPOOL, ENGLAND – Decades after they were sustained, acute knee injuries caused clinically significant impairments in patient-reported outcomes, as well as upped the risk for knee osteoarthritis (OA) in an observational study.
Results of the study, which followed up individuals 32-37 years after they were treated for a ruptured anterior cruciate ligament (ACL) injury between 1980 and 1985, showed that, compared with the general population, they experienced greater levels of knee pain, participated less in physical activities, and had a reduced quality of life.
The link between OA and ACL injury is not new, with prior estimates suggesting that up to half of all patients with ACL injury develop OA within 10 years of the injury, said Stephanie Filbay, PhD, who presented the results of the study at the World Congress on Osteoarthritis. There have also been reports of knee pain and other symptoms, and poor quality of life more than 5 years later. What’s not been known until now, however, is what happens with even longer term follow-up, said Dr. Filbay, a postdoctoral research fellow in sport, exercise, and osteoarthritis at the University of Oxford, England.
The aims of the study were to compare patient-reported outcomes at 32-37-years’ follow-up against the general population, then to see if the baseline injury or treatment approach, or knee function 3-7 years after the initial injury had any influence on outcomes.
The study included 223 patients who were between aged 15 and 40 years at the time of the acute ACL injury between 1980 and 1985 and who had been seen within 2 weeks of ACL rupture at Linköping University Hospital in Linköping, Sweden. Patients had been allocated to early surgical or non-surgical treatment based on having an odd or even birth year. They had then been assessed 3-7 years later using a variety of tests to determine the strength of their quadriceps and hamstrings and the ability to hop on one leg.
All patients were then invited 32-37 years later after the initial injury to complete questionnaires and undergo clinical examination and X-rays. Only four people declined and 38 did not answer, leaving 181 (81%) people who agreed to participate and complete the Knee injury and Osteoarthritis Outcome Score (KOOS) and the ACL quality of life questionnaire (ACL-QOL).
The average age of participants at follow-up was 59 years (range, 47-74 years); 30% were female. 58% of all patients had been treated non-surgically initially, and 38% remained non-surgically treated at the longterm follow-up. At baseline, 58% had a meniscus injury.
Compared with an age- and sex-matched Swedish population, patients with ACL injuries had a lower KOOS for pain, sport/recreational activities, and quality of life. For example, KOOS for knee pain was around 65-70 for those with prior ACL injuries, compared with 80-90 for those without ACL injuries, where 100 indicates the best outcome or least pain and zero the worst.
KOOS was not affected by whether or not patients had initial ACL surgery or surgery at any point in their follow up. It also did not appear to matter if patients had a meniscal injury at baseline or not.
Quadriceps and hamstring strength at the 3-7 year postinjury assessment did not affect the longterm KOOS, but the ability to hop on one leg did: Those who were not able to hop on one leg for more than 90% of the time on the unaffected limb at the 3-7 years follow-up had worse pain, symptoms, function, and quality of life at the longterm follow-up point.
With regards to OA, “overall, more than one in two individuals had Kellgren-Lawrence grade 4 that could be considered severe radiographic changes in at least one compartment,” Dr. Filbay said at the meeting, which is sponsored by the Osteoarthritis Research Society International.
Severe radiographic changes were most common in the tibiofemoral joint, with around 47% having Kellgren-Lawrence (KL) grade 4. About 35% of tibiofemoral joints and about 60% of patellofemoral joints were KL grade 1.
Interestingly, different factors were found to be associated with OA in the tibiofemoral and patellofemoral joints, according to Dr. Filbay. Patients who had been treated non-surgically, whether initially or at any time during the 32-37 year follow-up, were more likely to have tibiofemoral OA, whereas those who had been treated surgically tended to have patellofemoral OA.
“Perhaps not surprisingly, meniscal injury at baseline was related to a higher percentage of tibiofemoral OA at long-term follow-up,” Dr. Filbay said.
Another finding was that patients with weaker hamstrings 3-7 years after the injury were more likely to develop patellofemoral joint OA.
Dr. Filbay had no disclosures.
SOURCE: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-3. Abstract 80.
REPORTING FROM OARSI 2018
Key clinical point: Decades after rupturing the anterior cruciate ligament (ACL), patients can experience significant impairments.
Major finding: 70% of 136 of the patients in the study developed knee osteoarthritis 32-37 years after an ACL injury.Study details: A population-based, observational follow-up study of 181 individuals who had an acute ACL injury in 1980-1985. Disclosures: Stephanie Filbay, PhD., had no disclosures. Source: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-53. Abstract 80.
Targeting inactivity, mood, and cognition could be key to reducing OA mortality
LIVERPOOL, ENGLAND – Osteoarthritis is associated with an increased risk in mortality, but three factors – inactivity, low mood, and cognitive ability – could be important targets to reduce this risk, according to the results of a study presented at the World Congress on Osteoarthritis.
“There’s recently been increasing interest in whether osteoarthritis (OA) is associated with mortality as the literature has failed to find a consistent link,” said Simran Parmar, a third-year medical student at Keele University, Newcastle-Under-Lyme, U.K.
Three years later, data from another study (BMJ. 2011;342:d1165) suggested an increased risk, with standardized mortality ratios calculated to be 1.55 for all-cause mortality and 1.71 for cardiovascular-specific mortality when comparing those with OA to those without OA in the general population.
However, more recent meta-analyses, performed in 2016, have failed to show a relationship between mortality and OA (Semin Arthritis Rheum. 2016;46[2]:160–7; Sci Rep. 2016;6:24393).
“This could be because of heterogeneity among the studies,” Mr. Parmar reasoned, adding that there was still an unclear relationship between OA and mortality.
So the aim of the current study was not only to take another look at the association to determine its strength but also to see what factors might be mediating the association in order to perhaps explain why OA might be associated with an increased risk of death.
The analysis used data on more than 8,000 individuals participating in the NorStOP (North Staffordshire Osteoarthritis Project). This is a large, population-based, prospective cohort initiated in 2002 that includes adults aged 50 years or older who are registered at any of six general practices.
At baseline, the mean age of participants was 65 years, 51% were female, and just under 30% had OA. During 10 years of follow-up, 1,188 (14.7%) participants died.
Osteoarthritis was significantly associated with mortality in both unadjusted and adjusted analyses.
“For the average person presenting to general practice in North Staffordshire, there’s a 39.4% increased risk of mortality if they have osteoarthritis compared to if they don’t,” Mr. Parmar said.
After adjustment for potential confounding factors, such as age, NSAID use, and common comorbidities, the increased mortality risk remained, with around a 15% increased risk of death for those with OA versus those without.
“We proposed six different mediators of this relationship,” Mr. Parmar said. These were depression, anxiety, low walking frequency, cognitive impairment, insomnia, and obesity. “The reason we chose these is because they can be targets for therapy in primary care.”
Three mediators significantly affected the relationship: low walking frequency, depression, and cognitive impairment; the respective hazard ratios and 95% confidence intervals were 1.12 (1.09-1.15), 1.11 (1.08-1.15), and 1.06 (1.03-1.09).
“This tells us that these could possibly be on the pathway between osteoarthritis and mortality, and this could provide further evidence that they could be used for targeted therapy of osteoarthritis,” Mr. Parmar suggested.
“This type of mediation analysis has not been done in the osteoarthritis field before,” Mr. Parmar observed. He conceded that the mediators found might actually have contributed to the development of OA and that pain interference used in the definition of OA could have been caused by other factors.
Nevertheless, these data suggest that there may be actionable factors that could be used in primary care to reduce mortality in OA.
Mr. Parmar suggested that “encouraging physical activity and considering the impact of comorbidities can help reduce the risk of mortality in adults with osteoarthritis.”
The study was funded by Arthritis Research UK, the North Staffordshire Primary Care Consortium, and the Medical Research Council. Mr. Parmar had no conflicts of interest to disclose.
SOURCE: Parmar S et al. Osteoarthritis Cartilage. 2018;26(1):S14-15.
LIVERPOOL, ENGLAND – Osteoarthritis is associated with an increased risk in mortality, but three factors – inactivity, low mood, and cognitive ability – could be important targets to reduce this risk, according to the results of a study presented at the World Congress on Osteoarthritis.
“There’s recently been increasing interest in whether osteoarthritis (OA) is associated with mortality as the literature has failed to find a consistent link,” said Simran Parmar, a third-year medical student at Keele University, Newcastle-Under-Lyme, U.K.
Three years later, data from another study (BMJ. 2011;342:d1165) suggested an increased risk, with standardized mortality ratios calculated to be 1.55 for all-cause mortality and 1.71 for cardiovascular-specific mortality when comparing those with OA to those without OA in the general population.
However, more recent meta-analyses, performed in 2016, have failed to show a relationship between mortality and OA (Semin Arthritis Rheum. 2016;46[2]:160–7; Sci Rep. 2016;6:24393).
“This could be because of heterogeneity among the studies,” Mr. Parmar reasoned, adding that there was still an unclear relationship between OA and mortality.
So the aim of the current study was not only to take another look at the association to determine its strength but also to see what factors might be mediating the association in order to perhaps explain why OA might be associated with an increased risk of death.
The analysis used data on more than 8,000 individuals participating in the NorStOP (North Staffordshire Osteoarthritis Project). This is a large, population-based, prospective cohort initiated in 2002 that includes adults aged 50 years or older who are registered at any of six general practices.
At baseline, the mean age of participants was 65 years, 51% were female, and just under 30% had OA. During 10 years of follow-up, 1,188 (14.7%) participants died.
Osteoarthritis was significantly associated with mortality in both unadjusted and adjusted analyses.
“For the average person presenting to general practice in North Staffordshire, there’s a 39.4% increased risk of mortality if they have osteoarthritis compared to if they don’t,” Mr. Parmar said.
After adjustment for potential confounding factors, such as age, NSAID use, and common comorbidities, the increased mortality risk remained, with around a 15% increased risk of death for those with OA versus those without.
“We proposed six different mediators of this relationship,” Mr. Parmar said. These were depression, anxiety, low walking frequency, cognitive impairment, insomnia, and obesity. “The reason we chose these is because they can be targets for therapy in primary care.”
Three mediators significantly affected the relationship: low walking frequency, depression, and cognitive impairment; the respective hazard ratios and 95% confidence intervals were 1.12 (1.09-1.15), 1.11 (1.08-1.15), and 1.06 (1.03-1.09).
“This tells us that these could possibly be on the pathway between osteoarthritis and mortality, and this could provide further evidence that they could be used for targeted therapy of osteoarthritis,” Mr. Parmar suggested.
“This type of mediation analysis has not been done in the osteoarthritis field before,” Mr. Parmar observed. He conceded that the mediators found might actually have contributed to the development of OA and that pain interference used in the definition of OA could have been caused by other factors.
Nevertheless, these data suggest that there may be actionable factors that could be used in primary care to reduce mortality in OA.
Mr. Parmar suggested that “encouraging physical activity and considering the impact of comorbidities can help reduce the risk of mortality in adults with osteoarthritis.”
The study was funded by Arthritis Research UK, the North Staffordshire Primary Care Consortium, and the Medical Research Council. Mr. Parmar had no conflicts of interest to disclose.
SOURCE: Parmar S et al. Osteoarthritis Cartilage. 2018;26(1):S14-15.
LIVERPOOL, ENGLAND – Osteoarthritis is associated with an increased risk in mortality, but three factors – inactivity, low mood, and cognitive ability – could be important targets to reduce this risk, according to the results of a study presented at the World Congress on Osteoarthritis.
“There’s recently been increasing interest in whether osteoarthritis (OA) is associated with mortality as the literature has failed to find a consistent link,” said Simran Parmar, a third-year medical student at Keele University, Newcastle-Under-Lyme, U.K.
Three years later, data from another study (BMJ. 2011;342:d1165) suggested an increased risk, with standardized mortality ratios calculated to be 1.55 for all-cause mortality and 1.71 for cardiovascular-specific mortality when comparing those with OA to those without OA in the general population.
However, more recent meta-analyses, performed in 2016, have failed to show a relationship between mortality and OA (Semin Arthritis Rheum. 2016;46[2]:160–7; Sci Rep. 2016;6:24393).
“This could be because of heterogeneity among the studies,” Mr. Parmar reasoned, adding that there was still an unclear relationship between OA and mortality.
So the aim of the current study was not only to take another look at the association to determine its strength but also to see what factors might be mediating the association in order to perhaps explain why OA might be associated with an increased risk of death.
The analysis used data on more than 8,000 individuals participating in the NorStOP (North Staffordshire Osteoarthritis Project). This is a large, population-based, prospective cohort initiated in 2002 that includes adults aged 50 years or older who are registered at any of six general practices.
At baseline, the mean age of participants was 65 years, 51% were female, and just under 30% had OA. During 10 years of follow-up, 1,188 (14.7%) participants died.
Osteoarthritis was significantly associated with mortality in both unadjusted and adjusted analyses.
“For the average person presenting to general practice in North Staffordshire, there’s a 39.4% increased risk of mortality if they have osteoarthritis compared to if they don’t,” Mr. Parmar said.
After adjustment for potential confounding factors, such as age, NSAID use, and common comorbidities, the increased mortality risk remained, with around a 15% increased risk of death for those with OA versus those without.
“We proposed six different mediators of this relationship,” Mr. Parmar said. These were depression, anxiety, low walking frequency, cognitive impairment, insomnia, and obesity. “The reason we chose these is because they can be targets for therapy in primary care.”
Three mediators significantly affected the relationship: low walking frequency, depression, and cognitive impairment; the respective hazard ratios and 95% confidence intervals were 1.12 (1.09-1.15), 1.11 (1.08-1.15), and 1.06 (1.03-1.09).
“This tells us that these could possibly be on the pathway between osteoarthritis and mortality, and this could provide further evidence that they could be used for targeted therapy of osteoarthritis,” Mr. Parmar suggested.
“This type of mediation analysis has not been done in the osteoarthritis field before,” Mr. Parmar observed. He conceded that the mediators found might actually have contributed to the development of OA and that pain interference used in the definition of OA could have been caused by other factors.
Nevertheless, these data suggest that there may be actionable factors that could be used in primary care to reduce mortality in OA.
Mr. Parmar suggested that “encouraging physical activity and considering the impact of comorbidities can help reduce the risk of mortality in adults with osteoarthritis.”
The study was funded by Arthritis Research UK, the North Staffordshire Primary Care Consortium, and the Medical Research Council. Mr. Parmar had no conflicts of interest to disclose.
SOURCE: Parmar S et al. Osteoarthritis Cartilage. 2018;26(1):S14-15.
REPORTING FROM OARSI 2018
Key clinical point:
Major finding: OA is associated with a 15% increased risk of mortality in the general population.
Study details: A large, prospective cohort study that included more than 8,000 adults older than 50 years in the general population who participated.
Disclosures: The study was funded by Arthritis Research UK, the North Staffordshire Primary Care Consortium, and the Medical Research Council. Mr. Parmar had no conflicts of interest to disclose.
Source: Parmar S et al. Osteoarthritis Cartilage. 2018;26(1):S14-15.
FDA advisory committee votes to recommend update to celecoxib safety labeling
SILVER SPRING, MD. – An FDA advisory committee voted (15 yes, 5 no, 1 abstention) to update the safety information in the label of celecoxib, a nonsteroidal anti-inflammatory drug (NSAID), for use in patients with osteoarthritis (OA) and rheumatoid arthritis, on the basis of results of the PRECISION trial.
A Joint Meeting ofthe Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee was convened April 24-25 to address two issues, the first being the consideration of Pfizer’s application for celecoxib and the second, to assess the safety of celecoxib and other common NSAIDs like ibuprofen and naproxen.
The randomized controlled PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen or Naproxen) trial compared celecoxib, naproxen, and ibuprofen and their cardiovascular outcomes. The PRECISION trial was undertaken after another selective COX-2 inhibitor, rofecoxib (Vioxx), was withdrawn from the market because of associated cardiovascular events. It compared the three drugs among more than 24,000 patients with painful arthritis and elevated cardiovascular risk.
Main results showed that the rates of cardiovascular events (cardiovascular death, myocardial infarction, or stroke) were 2.3% with celecoxib, 2.5% with naproxen, and 2.7% with ibuprofen during a follow-up approaching 3 years, showing noninferiority for celecoxib.
In a post hoc analysis presented by Steven Nissen, MD, patients taking ibuprofen and naproxen experienced adjudicated cardiovascular, GI, or renal events 28% and 15% more than did patients taking celecoxib.
The results of the study could inform clinical strategy, said Dr. Nissen, chair of cardiovascular medicine at the Cleveland Clinic in Ohio. “For arthritis patients who require NSAIDs to achieve an acceptable quality of life, particularly those at high cardiovascular, GI, or renal risk, the PRECISION trial suggests that a clinical strategy of starting patients on celecoxib 200 mg daily may be the safest approach, reserving full therapeutic doses of ibuprofen and naproxen for patients who do not respond to celecoxib."
SILVER SPRING, MD. – An FDA advisory committee voted (15 yes, 5 no, 1 abstention) to update the safety information in the label of celecoxib, a nonsteroidal anti-inflammatory drug (NSAID), for use in patients with osteoarthritis (OA) and rheumatoid arthritis, on the basis of results of the PRECISION trial.
A Joint Meeting ofthe Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee was convened April 24-25 to address two issues, the first being the consideration of Pfizer’s application for celecoxib and the second, to assess the safety of celecoxib and other common NSAIDs like ibuprofen and naproxen.
The randomized controlled PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen or Naproxen) trial compared celecoxib, naproxen, and ibuprofen and their cardiovascular outcomes. The PRECISION trial was undertaken after another selective COX-2 inhibitor, rofecoxib (Vioxx), was withdrawn from the market because of associated cardiovascular events. It compared the three drugs among more than 24,000 patients with painful arthritis and elevated cardiovascular risk.
Main results showed that the rates of cardiovascular events (cardiovascular death, myocardial infarction, or stroke) were 2.3% with celecoxib, 2.5% with naproxen, and 2.7% with ibuprofen during a follow-up approaching 3 years, showing noninferiority for celecoxib.
In a post hoc analysis presented by Steven Nissen, MD, patients taking ibuprofen and naproxen experienced adjudicated cardiovascular, GI, or renal events 28% and 15% more than did patients taking celecoxib.
The results of the study could inform clinical strategy, said Dr. Nissen, chair of cardiovascular medicine at the Cleveland Clinic in Ohio. “For arthritis patients who require NSAIDs to achieve an acceptable quality of life, particularly those at high cardiovascular, GI, or renal risk, the PRECISION trial suggests that a clinical strategy of starting patients on celecoxib 200 mg daily may be the safest approach, reserving full therapeutic doses of ibuprofen and naproxen for patients who do not respond to celecoxib."
SILVER SPRING, MD. – An FDA advisory committee voted (15 yes, 5 no, 1 abstention) to update the safety information in the label of celecoxib, a nonsteroidal anti-inflammatory drug (NSAID), for use in patients with osteoarthritis (OA) and rheumatoid arthritis, on the basis of results of the PRECISION trial.
A Joint Meeting ofthe Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee was convened April 24-25 to address two issues, the first being the consideration of Pfizer’s application for celecoxib and the second, to assess the safety of celecoxib and other common NSAIDs like ibuprofen and naproxen.
The randomized controlled PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen or Naproxen) trial compared celecoxib, naproxen, and ibuprofen and their cardiovascular outcomes. The PRECISION trial was undertaken after another selective COX-2 inhibitor, rofecoxib (Vioxx), was withdrawn from the market because of associated cardiovascular events. It compared the three drugs among more than 24,000 patients with painful arthritis and elevated cardiovascular risk.
Main results showed that the rates of cardiovascular events (cardiovascular death, myocardial infarction, or stroke) were 2.3% with celecoxib, 2.5% with naproxen, and 2.7% with ibuprofen during a follow-up approaching 3 years, showing noninferiority for celecoxib.
In a post hoc analysis presented by Steven Nissen, MD, patients taking ibuprofen and naproxen experienced adjudicated cardiovascular, GI, or renal events 28% and 15% more than did patients taking celecoxib.
The results of the study could inform clinical strategy, said Dr. Nissen, chair of cardiovascular medicine at the Cleveland Clinic in Ohio. “For arthritis patients who require NSAIDs to achieve an acceptable quality of life, particularly those at high cardiovascular, GI, or renal risk, the PRECISION trial suggests that a clinical strategy of starting patients on celecoxib 200 mg daily may be the safest approach, reserving full therapeutic doses of ibuprofen and naproxen for patients who do not respond to celecoxib."
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Arthritis limits physical activity the most in the South
“Physical activity is a proven strategy for managing arthritis symptoms,” but 35% of Americans with arthritis do not participate in any such activities or exercise, according to investigators who analyzed data from a national survey of more than 440,000 adults.
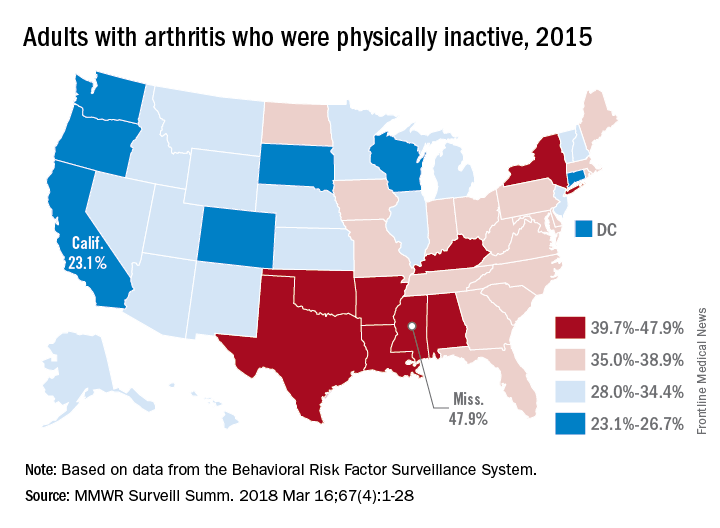
The low rates of inactivity in the western half of the country were topped by California’s 23.1% and South Dakota’s 23.4%, with Oregon (24.0%) and Wisconsin (24.6%) not too far behind, Dr. Barbour and his associates said based on data for 441,456 adults aged 18 years and older who were interviewed for the Behavioral Risk Factor Surveillance System.
Overall prevalence rates show that arthritis has the greatest effect in southern states, which, in addition to high inactivity, had more arthritis-attributable severe joint pain, more arthritis-attributable social participation restriction, and less leisure-time walking among adults with arthritis. This information, the investigators suggested, may help public health professionals “to better understand and target evidence-based nonpharmaceutical interventions, such as arthritis self-management education and physical activity.”
SOURCE: Barbour KE et al. MMWR Surveill Summ. 2018 Mar 16;67(4):1-28.
“Physical activity is a proven strategy for managing arthritis symptoms,” but 35% of Americans with arthritis do not participate in any such activities or exercise, according to investigators who analyzed data from a national survey of more than 440,000 adults.

The low rates of inactivity in the western half of the country were topped by California’s 23.1% and South Dakota’s 23.4%, with Oregon (24.0%) and Wisconsin (24.6%) not too far behind, Dr. Barbour and his associates said based on data for 441,456 adults aged 18 years and older who were interviewed for the Behavioral Risk Factor Surveillance System.
Overall prevalence rates show that arthritis has the greatest effect in southern states, which, in addition to high inactivity, had more arthritis-attributable severe joint pain, more arthritis-attributable social participation restriction, and less leisure-time walking among adults with arthritis. This information, the investigators suggested, may help public health professionals “to better understand and target evidence-based nonpharmaceutical interventions, such as arthritis self-management education and physical activity.”
SOURCE: Barbour KE et al. MMWR Surveill Summ. 2018 Mar 16;67(4):1-28.
“Physical activity is a proven strategy for managing arthritis symptoms,” but 35% of Americans with arthritis do not participate in any such activities or exercise, according to investigators who analyzed data from a national survey of more than 440,000 adults.

The low rates of inactivity in the western half of the country were topped by California’s 23.1% and South Dakota’s 23.4%, with Oregon (24.0%) and Wisconsin (24.6%) not too far behind, Dr. Barbour and his associates said based on data for 441,456 adults aged 18 years and older who were interviewed for the Behavioral Risk Factor Surveillance System.
Overall prevalence rates show that arthritis has the greatest effect in southern states, which, in addition to high inactivity, had more arthritis-attributable severe joint pain, more arthritis-attributable social participation restriction, and less leisure-time walking among adults with arthritis. This information, the investigators suggested, may help public health professionals “to better understand and target evidence-based nonpharmaceutical interventions, such as arthritis self-management education and physical activity.”
SOURCE: Barbour KE et al. MMWR Surveill Summ. 2018 Mar 16;67(4):1-28.
FROM MMWR SURVEILLANCE SUMMARIES
Intramuscular steroid injection reduced hip OA pain up to 12 weeks
Systemic treatment with an intramuscular glucocorticoid injection is effective, compared with placebo, in reducing pain in people with hip osteoarthritis for up to 12 weeks, a double-blinded, placebo-controlled, randomized trial suggests.
However, the study found benefit with intramuscular (IM) glucocorticoid injection at 2 weeks only when patients were at rest, and did not find any significant benefit with the injection in reducing pain while walking or in reducing Western Ontario and McMaster University Osteoarthritis Index (WOMAC) pain subscale scores. The report was published in Annals of the Rheumatic Diseases.
The multicenter, double-blinded, superiority trial randomized 106 patients with painful hip OA who were not responding to oral analgesics to either 40 mg triamcinolone acetate (n = 52) or placebo injection (n = 54) into the gluteus muscle. Overall, 73 patients (68%) were women, and the average age of the cohort was 64 years. Hip OA symptoms had occurred for at least 1 year in 70% of the patients.
The study’s three primary outcomes of hip pain severity 2 weeks after the injection on a 0-10 scale at rest and during walking and on the WOMAC pain subscale revealed inconsistent results with the treatment.
At the 2-week follow-up, patients who had received the IM glucocorticoid injection had a significant and clinically relevant difference in hip pain at rest (between-group difference = –1.3; 95% confidence interval, –2.3 to –0.3; P = .01). But at this time point there were no significant associations between glucocorticoid injection and hip pain during walking (difference = –0.9; 95% CI, –1.9 to 0.1; P = .07) and WOMAC pain subscale score (difference = –6.1; 95% CI, –13.4 to 1.2; P = .10), the researchers reported.
At 2-week follow-up, recipients of the glucocorticoid injection were significantly more likely to perceive improvement (relative risk = 1.7; 95% CI, 1.1 to 2.7; P = .02) or achieve OMERACT-OARSI level of response (RR = 2.0; 95% CI, 1.1 to 3.6; P = .03).
The authors described this finding as “surprising,” speculating that the 7-point Likert scale used to measure perceived improvement could have resulted in less power.
Nineteen patients in the glucocorticoid group reported 27 nonserious adverse events, compared with 13 patients in the placebo group who reported 18 adverse events.
The authors said the greatest effects of the glucocorticoid injection were seen at 4- to 12-week follow-up (the secondary outcomes of the study), instead of at the 2-week follow-up. For example, at 4-week follow-up, the glucocorticoid injection was associated with a significant hip pain reduction at rest (between-group difference = –1.2; 95% CI, –2.1 to –0.2; P = .01) and during walking (difference = –1.1; 95% CI, –2.0 to –0.2; P = .01). At 6 weeks, the corresponding figures for hip pain reduction were –1.4 at rest (95% CI, –2.4 to –0.5; P = .005) and –1.4 while walking (95% CI, –2.3 to –0.4; P = .004). The between-group differences were still significant at 12 weeks while at rest (difference = –1.2; 95% CI, –2.3 to –0.2; P = .02) and during walking (difference = –1.3; 95% CI, –2.2 to –0.3; P = .01).
Significant differences in favor of the glucocorticoid injection overall occurred on the WOMAC subscale scores for pain, function, and stiffness, as well as total Hip disability and Osteoarthritis Outcome Score for pain and total, intermittent, and constant pain measures on the Intermittent and Constant Osteoarthritis Pain scale. At 12 weeks, the between-group difference on the WOMAC total score was –9.4 (95% CI, –17.8 to –0.9; P = .03).
The researchers said it was surprising that hip pain reduction after IM glucocorticoid injection was still present at a similar degree at 12 weeks since previous studies had shown the effect usually peaked after 1-3 weeks.
“Our findings should be replicated in future research,” they said.
“An IM glucocorticoid injection showed effectiveness in patients with hip OA on one of the three primary outcomes at a 2 weeks post injection ... The effect is probably clinically relevant,” the authors concluded.
The investigators noted that in clinical practice patients are sometimes offered multiple injections per year, whereas in the current study patients received only one injection. There has also been concern that intra-articular glucocorticoid injections could cause toxicity to chondrocytes and potentially lead to OA progression, but the effect of a single IM injection is unknown.
Financial support for the study came from the Dutch Arthritis Foundation and the NutsOhra fund. Two of the authors reported receiving grants from several pharmaceutical companies, research consortia, and foundations.
SOURCE: Dorleijn D et al. Ann Rheum Dis. 2018 March 7. doi: 10.1136/annrheumdis-2017-212628
Systemic treatment with an intramuscular glucocorticoid injection is effective, compared with placebo, in reducing pain in people with hip osteoarthritis for up to 12 weeks, a double-blinded, placebo-controlled, randomized trial suggests.
However, the study found benefit with intramuscular (IM) glucocorticoid injection at 2 weeks only when patients were at rest, and did not find any significant benefit with the injection in reducing pain while walking or in reducing Western Ontario and McMaster University Osteoarthritis Index (WOMAC) pain subscale scores. The report was published in Annals of the Rheumatic Diseases.
The multicenter, double-blinded, superiority trial randomized 106 patients with painful hip OA who were not responding to oral analgesics to either 40 mg triamcinolone acetate (n = 52) or placebo injection (n = 54) into the gluteus muscle. Overall, 73 patients (68%) were women, and the average age of the cohort was 64 years. Hip OA symptoms had occurred for at least 1 year in 70% of the patients.
The study’s three primary outcomes of hip pain severity 2 weeks after the injection on a 0-10 scale at rest and during walking and on the WOMAC pain subscale revealed inconsistent results with the treatment.
At the 2-week follow-up, patients who had received the IM glucocorticoid injection had a significant and clinically relevant difference in hip pain at rest (between-group difference = –1.3; 95% confidence interval, –2.3 to –0.3; P = .01). But at this time point there were no significant associations between glucocorticoid injection and hip pain during walking (difference = –0.9; 95% CI, –1.9 to 0.1; P = .07) and WOMAC pain subscale score (difference = –6.1; 95% CI, –13.4 to 1.2; P = .10), the researchers reported.
At 2-week follow-up, recipients of the glucocorticoid injection were significantly more likely to perceive improvement (relative risk = 1.7; 95% CI, 1.1 to 2.7; P = .02) or achieve OMERACT-OARSI level of response (RR = 2.0; 95% CI, 1.1 to 3.6; P = .03).
The authors described this finding as “surprising,” speculating that the 7-point Likert scale used to measure perceived improvement could have resulted in less power.
Nineteen patients in the glucocorticoid group reported 27 nonserious adverse events, compared with 13 patients in the placebo group who reported 18 adverse events.
The authors said the greatest effects of the glucocorticoid injection were seen at 4- to 12-week follow-up (the secondary outcomes of the study), instead of at the 2-week follow-up. For example, at 4-week follow-up, the glucocorticoid injection was associated with a significant hip pain reduction at rest (between-group difference = –1.2; 95% CI, –2.1 to –0.2; P = .01) and during walking (difference = –1.1; 95% CI, –2.0 to –0.2; P = .01). At 6 weeks, the corresponding figures for hip pain reduction were –1.4 at rest (95% CI, –2.4 to –0.5; P = .005) and –1.4 while walking (95% CI, –2.3 to –0.4; P = .004). The between-group differences were still significant at 12 weeks while at rest (difference = –1.2; 95% CI, –2.3 to –0.2; P = .02) and during walking (difference = –1.3; 95% CI, –2.2 to –0.3; P = .01).
Significant differences in favor of the glucocorticoid injection overall occurred on the WOMAC subscale scores for pain, function, and stiffness, as well as total Hip disability and Osteoarthritis Outcome Score for pain and total, intermittent, and constant pain measures on the Intermittent and Constant Osteoarthritis Pain scale. At 12 weeks, the between-group difference on the WOMAC total score was –9.4 (95% CI, –17.8 to –0.9; P = .03).
The researchers said it was surprising that hip pain reduction after IM glucocorticoid injection was still present at a similar degree at 12 weeks since previous studies had shown the effect usually peaked after 1-3 weeks.
“Our findings should be replicated in future research,” they said.
“An IM glucocorticoid injection showed effectiveness in patients with hip OA on one of the three primary outcomes at a 2 weeks post injection ... The effect is probably clinically relevant,” the authors concluded.
The investigators noted that in clinical practice patients are sometimes offered multiple injections per year, whereas in the current study patients received only one injection. There has also been concern that intra-articular glucocorticoid injections could cause toxicity to chondrocytes and potentially lead to OA progression, but the effect of a single IM injection is unknown.
Financial support for the study came from the Dutch Arthritis Foundation and the NutsOhra fund. Two of the authors reported receiving grants from several pharmaceutical companies, research consortia, and foundations.
SOURCE: Dorleijn D et al. Ann Rheum Dis. 2018 March 7. doi: 10.1136/annrheumdis-2017-212628
Systemic treatment with an intramuscular glucocorticoid injection is effective, compared with placebo, in reducing pain in people with hip osteoarthritis for up to 12 weeks, a double-blinded, placebo-controlled, randomized trial suggests.
However, the study found benefit with intramuscular (IM) glucocorticoid injection at 2 weeks only when patients were at rest, and did not find any significant benefit with the injection in reducing pain while walking or in reducing Western Ontario and McMaster University Osteoarthritis Index (WOMAC) pain subscale scores. The report was published in Annals of the Rheumatic Diseases.
The multicenter, double-blinded, superiority trial randomized 106 patients with painful hip OA who were not responding to oral analgesics to either 40 mg triamcinolone acetate (n = 52) or placebo injection (n = 54) into the gluteus muscle. Overall, 73 patients (68%) were women, and the average age of the cohort was 64 years. Hip OA symptoms had occurred for at least 1 year in 70% of the patients.
The study’s three primary outcomes of hip pain severity 2 weeks after the injection on a 0-10 scale at rest and during walking and on the WOMAC pain subscale revealed inconsistent results with the treatment.
At the 2-week follow-up, patients who had received the IM glucocorticoid injection had a significant and clinically relevant difference in hip pain at rest (between-group difference = –1.3; 95% confidence interval, –2.3 to –0.3; P = .01). But at this time point there were no significant associations between glucocorticoid injection and hip pain during walking (difference = –0.9; 95% CI, –1.9 to 0.1; P = .07) and WOMAC pain subscale score (difference = –6.1; 95% CI, –13.4 to 1.2; P = .10), the researchers reported.
At 2-week follow-up, recipients of the glucocorticoid injection were significantly more likely to perceive improvement (relative risk = 1.7; 95% CI, 1.1 to 2.7; P = .02) or achieve OMERACT-OARSI level of response (RR = 2.0; 95% CI, 1.1 to 3.6; P = .03).
The authors described this finding as “surprising,” speculating that the 7-point Likert scale used to measure perceived improvement could have resulted in less power.
Nineteen patients in the glucocorticoid group reported 27 nonserious adverse events, compared with 13 patients in the placebo group who reported 18 adverse events.
The authors said the greatest effects of the glucocorticoid injection were seen at 4- to 12-week follow-up (the secondary outcomes of the study), instead of at the 2-week follow-up. For example, at 4-week follow-up, the glucocorticoid injection was associated with a significant hip pain reduction at rest (between-group difference = –1.2; 95% CI, –2.1 to –0.2; P = .01) and during walking (difference = –1.1; 95% CI, –2.0 to –0.2; P = .01). At 6 weeks, the corresponding figures for hip pain reduction were –1.4 at rest (95% CI, –2.4 to –0.5; P = .005) and –1.4 while walking (95% CI, –2.3 to –0.4; P = .004). The between-group differences were still significant at 12 weeks while at rest (difference = –1.2; 95% CI, –2.3 to –0.2; P = .02) and during walking (difference = –1.3; 95% CI, –2.2 to –0.3; P = .01).
Significant differences in favor of the glucocorticoid injection overall occurred on the WOMAC subscale scores for pain, function, and stiffness, as well as total Hip disability and Osteoarthritis Outcome Score for pain and total, intermittent, and constant pain measures on the Intermittent and Constant Osteoarthritis Pain scale. At 12 weeks, the between-group difference on the WOMAC total score was –9.4 (95% CI, –17.8 to –0.9; P = .03).
The researchers said it was surprising that hip pain reduction after IM glucocorticoid injection was still present at a similar degree at 12 weeks since previous studies had shown the effect usually peaked after 1-3 weeks.
“Our findings should be replicated in future research,” they said.
“An IM glucocorticoid injection showed effectiveness in patients with hip OA on one of the three primary outcomes at a 2 weeks post injection ... The effect is probably clinically relevant,” the authors concluded.
The investigators noted that in clinical practice patients are sometimes offered multiple injections per year, whereas in the current study patients received only one injection. There has also been concern that intra-articular glucocorticoid injections could cause toxicity to chondrocytes and potentially lead to OA progression, but the effect of a single IM injection is unknown.
Financial support for the study came from the Dutch Arthritis Foundation and the NutsOhra fund. Two of the authors reported receiving grants from several pharmaceutical companies, research consortia, and foundations.
SOURCE: Dorleijn D et al. Ann Rheum Dis. 2018 March 7. doi: 10.1136/annrheumdis-2017-212628
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: At 2 weeks, patients who had received the intramuscular glucocorticoid injection had a significant and clinically relevant difference in hip pain at rest (between-group difference = –1.3; 95% CI, –2.3 to –0.3; P = .01).
Study details: A 12-week, double blinded, placebo-controlled trial of 106 patients with hip OA randomized to 40 mg triamcinolone acetate or placebo injection.
Disclosures: Financial support for the study came from the Dutch Arthritis Foundation and the NutsOhra fund. Two of the authors reported receiving grants from several pharmaceutical companies, research consortia, and foundations.
Source: Dorleijn D et al. Ann Rheum Dis. 2018 Mar 7. doi: 10.1136/annrheumdis-2017-212628.
Nonopioid analgesics have no major disadvantages vs. opioids for chronic pain
Patients treated with opioids for moderate to severe chronic back pain or knee or hip osteoarthritis pain saw no significant improvement when results were compared with treatment using acetaminophen or nonsteroidal anti-inflammatory drugs in the randomized SPACE study.
These findings may help restructure how physicians treat patients with chronic pain in order to decrease the risk of opioid addiction in a population that is particularly susceptible.
“Long-term opioid therapy became a standard approach to managing chronic musculoskeletal pain despite a lack of high-quality data on benefits and harms,” wrote Erin E. Krebs, MD, MPH, core investigator at the Minneapolis Veterans Affairs Center for Chronic Disease Outcomes Research, and her colleagues. “Rising rates of opioid overdose deaths have raised questions about prescribing opioids for chronic pain management.”
In the 12-month SPACE (Strategies for Prescribing Analgesics Comparative Effectiveness) trial published March 6 in JAMA, the investigators reported randomizing a total of 240 patients to treatment with immediate-release opioids (morphine, oxycodone, or hydrocodone/acetaminophen) or acetaminophen or nonsteroidal anti-inflammatory drugs. The patients came from the Minneapolis VA system between June 2013 and December 2015. Patients in the opioid group were on average 57 years old, while those in the nonopioid group had an average age of 60 years. Men comprised 87% of all patients, and both groups were predominantly white (86%-88%) with chronic back pain (65%). The investigators excluded patients with physiological opioid dependence from ongoing opioid use.
Patients taking opioids saw no significant improvement in the primary outcome of pain-related function on the seven-item Brief Pain Inventory (BPI) interference scale over those taking nonopioids at the end of the 12-month period (P = .58), according to Dr. Krebs and her fellow investigators.
The main secondary outcome of pain intensity using the four-item BPI severity scale improved significantly more among the nonopioid group, which reported an average score of 3.5, compared with 4.0 in the opioid group (P = .03). However, the small difference of 0.5 is less than the minimal clinically important difference of 1.0, according to the investigators.
Anxiety control was the only secondary outcome measure that was significantly better among patients in the opioid group, which was unsurprising to the investigators. “This finding is consistent with the role of the endogenous opioid system in stress and emotional suffering,” they wrote.
The investigators were uncertain about the significance of this small difference because overall anxiety levels were low, with 9% of patients reporting moderate severity anxiety symptoms at baseline.
Patients in the opioid group took a mean of 1.7 analgesics during the study period, compared with 3.8 in the nonopioid group. In the nonopioid group, the mean number of months that nonopioid analgesics were prescribed ranged from 2.6 for acetaminophen to 5.9 with oral NSAIDs. In this group, tramadol was prescribed to no more than 11% of patients during any particular month and was dispensed for a mean of 0.4 months overall.
Patients in the opioid group took opioids for a mean of 8.1 months, with all other analgesics taken for a mean of 0.4 months or less. The authors noted that in “each 90-day follow-up period, fewer than 15% of patients in the opioid group had a mean dispensed dosage of 50 morphine-equivalent mg/day or more.” Patients could be titrated up to a maximum daily dosage of 100 morphine-equivalent mg/day.
Patients prescribed opioids were significantly more likely to report medication-related symptoms, and while misuse was not significantly higher in the opioid group, the investigators said that “Overall, opioids did not demonstrate any advantage over nonopioid medications that could potentially outweigh their greater risk of harms.”
Further studies will need to include a more diverse population, they noted.
The study was funded by an award from the U.S. Department of Veterans Affairs Health Services Research and Development Service. The investigators reported no relevant disclosures.
ezimmerman@frontlinemedcom.com
SOURCE: Krebs EE et al. JAMA. 2018 Mar 6;319(9):872-82
Patients treated with opioids for moderate to severe chronic back pain or knee or hip osteoarthritis pain saw no significant improvement when results were compared with treatment using acetaminophen or nonsteroidal anti-inflammatory drugs in the randomized SPACE study.
These findings may help restructure how physicians treat patients with chronic pain in order to decrease the risk of opioid addiction in a population that is particularly susceptible.
“Long-term opioid therapy became a standard approach to managing chronic musculoskeletal pain despite a lack of high-quality data on benefits and harms,” wrote Erin E. Krebs, MD, MPH, core investigator at the Minneapolis Veterans Affairs Center for Chronic Disease Outcomes Research, and her colleagues. “Rising rates of opioid overdose deaths have raised questions about prescribing opioids for chronic pain management.”
In the 12-month SPACE (Strategies for Prescribing Analgesics Comparative Effectiveness) trial published March 6 in JAMA, the investigators reported randomizing a total of 240 patients to treatment with immediate-release opioids (morphine, oxycodone, or hydrocodone/acetaminophen) or acetaminophen or nonsteroidal anti-inflammatory drugs. The patients came from the Minneapolis VA system between June 2013 and December 2015. Patients in the opioid group were on average 57 years old, while those in the nonopioid group had an average age of 60 years. Men comprised 87% of all patients, and both groups were predominantly white (86%-88%) with chronic back pain (65%). The investigators excluded patients with physiological opioid dependence from ongoing opioid use.
Patients taking opioids saw no significant improvement in the primary outcome of pain-related function on the seven-item Brief Pain Inventory (BPI) interference scale over those taking nonopioids at the end of the 12-month period (P = .58), according to Dr. Krebs and her fellow investigators.
The main secondary outcome of pain intensity using the four-item BPI severity scale improved significantly more among the nonopioid group, which reported an average score of 3.5, compared with 4.0 in the opioid group (P = .03). However, the small difference of 0.5 is less than the minimal clinically important difference of 1.0, according to the investigators.
Anxiety control was the only secondary outcome measure that was significantly better among patients in the opioid group, which was unsurprising to the investigators. “This finding is consistent with the role of the endogenous opioid system in stress and emotional suffering,” they wrote.
The investigators were uncertain about the significance of this small difference because overall anxiety levels were low, with 9% of patients reporting moderate severity anxiety symptoms at baseline.
Patients in the opioid group took a mean of 1.7 analgesics during the study period, compared with 3.8 in the nonopioid group. In the nonopioid group, the mean number of months that nonopioid analgesics were prescribed ranged from 2.6 for acetaminophen to 5.9 with oral NSAIDs. In this group, tramadol was prescribed to no more than 11% of patients during any particular month and was dispensed for a mean of 0.4 months overall.
Patients in the opioid group took opioids for a mean of 8.1 months, with all other analgesics taken for a mean of 0.4 months or less. The authors noted that in “each 90-day follow-up period, fewer than 15% of patients in the opioid group had a mean dispensed dosage of 50 morphine-equivalent mg/day or more.” Patients could be titrated up to a maximum daily dosage of 100 morphine-equivalent mg/day.
Patients prescribed opioids were significantly more likely to report medication-related symptoms, and while misuse was not significantly higher in the opioid group, the investigators said that “Overall, opioids did not demonstrate any advantage over nonopioid medications that could potentially outweigh their greater risk of harms.”
Further studies will need to include a more diverse population, they noted.
The study was funded by an award from the U.S. Department of Veterans Affairs Health Services Research and Development Service. The investigators reported no relevant disclosures.
ezimmerman@frontlinemedcom.com
SOURCE: Krebs EE et al. JAMA. 2018 Mar 6;319(9):872-82
Patients treated with opioids for moderate to severe chronic back pain or knee or hip osteoarthritis pain saw no significant improvement when results were compared with treatment using acetaminophen or nonsteroidal anti-inflammatory drugs in the randomized SPACE study.
These findings may help restructure how physicians treat patients with chronic pain in order to decrease the risk of opioid addiction in a population that is particularly susceptible.
“Long-term opioid therapy became a standard approach to managing chronic musculoskeletal pain despite a lack of high-quality data on benefits and harms,” wrote Erin E. Krebs, MD, MPH, core investigator at the Minneapolis Veterans Affairs Center for Chronic Disease Outcomes Research, and her colleagues. “Rising rates of opioid overdose deaths have raised questions about prescribing opioids for chronic pain management.”
In the 12-month SPACE (Strategies for Prescribing Analgesics Comparative Effectiveness) trial published March 6 in JAMA, the investigators reported randomizing a total of 240 patients to treatment with immediate-release opioids (morphine, oxycodone, or hydrocodone/acetaminophen) or acetaminophen or nonsteroidal anti-inflammatory drugs. The patients came from the Minneapolis VA system between June 2013 and December 2015. Patients in the opioid group were on average 57 years old, while those in the nonopioid group had an average age of 60 years. Men comprised 87% of all patients, and both groups were predominantly white (86%-88%) with chronic back pain (65%). The investigators excluded patients with physiological opioid dependence from ongoing opioid use.
Patients taking opioids saw no significant improvement in the primary outcome of pain-related function on the seven-item Brief Pain Inventory (BPI) interference scale over those taking nonopioids at the end of the 12-month period (P = .58), according to Dr. Krebs and her fellow investigators.
The main secondary outcome of pain intensity using the four-item BPI severity scale improved significantly more among the nonopioid group, which reported an average score of 3.5, compared with 4.0 in the opioid group (P = .03). However, the small difference of 0.5 is less than the minimal clinically important difference of 1.0, according to the investigators.
Anxiety control was the only secondary outcome measure that was significantly better among patients in the opioid group, which was unsurprising to the investigators. “This finding is consistent with the role of the endogenous opioid system in stress and emotional suffering,” they wrote.
The investigators were uncertain about the significance of this small difference because overall anxiety levels were low, with 9% of patients reporting moderate severity anxiety symptoms at baseline.
Patients in the opioid group took a mean of 1.7 analgesics during the study period, compared with 3.8 in the nonopioid group. In the nonopioid group, the mean number of months that nonopioid analgesics were prescribed ranged from 2.6 for acetaminophen to 5.9 with oral NSAIDs. In this group, tramadol was prescribed to no more than 11% of patients during any particular month and was dispensed for a mean of 0.4 months overall.
Patients in the opioid group took opioids for a mean of 8.1 months, with all other analgesics taken for a mean of 0.4 months or less. The authors noted that in “each 90-day follow-up period, fewer than 15% of patients in the opioid group had a mean dispensed dosage of 50 morphine-equivalent mg/day or more.” Patients could be titrated up to a maximum daily dosage of 100 morphine-equivalent mg/day.
Patients prescribed opioids were significantly more likely to report medication-related symptoms, and while misuse was not significantly higher in the opioid group, the investigators said that “Overall, opioids did not demonstrate any advantage over nonopioid medications that could potentially outweigh their greater risk of harms.”
Further studies will need to include a more diverse population, they noted.
The study was funded by an award from the U.S. Department of Veterans Affairs Health Services Research and Development Service. The investigators reported no relevant disclosures.
ezimmerman@frontlinemedcom.com
SOURCE: Krebs EE et al. JAMA. 2018 Mar 6;319(9):872-82
FROM JAMA
Key clinical point: Opioids and nonopioid analgesics provided similar improvements in pain-related function for patients with chronic pain.
Major finding: Patients taking opioids did not show significant improvement in pain-related function, compared with those taking nonopioids (P = .58).
Study details: A 12-month, randomized trial of 240 patients with chronic back, knee, or hip pain, gathered from a Veterans Affairs clinic between June 2013 to December 2015.
Disclosures: The study was funded by an award from the U.S. Department of Veterans Affairs Health Services Research and Development Service. The investigators reported no relevant disclosures.
Source: Krebs E et al. JAMA. 2018;319(9):872-82