User login
Variety is the spice of hospital medicine
Dr. Raj Sehgal enjoys a variety of roles
Unlike some children who wanted be firefighters or astronauts when they grew up, ever since Raj Sehgal, MD, FHM, was a boy, he dreamed of being a doctor.
Since earning his medical degree, Dr. Sehgal has kept himself involved in a wide variety of projects, driven by the desire to diversify his expertise.
Currently a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System, San Antonio, and University of Texas Health San Antonio, Dr. Sehgal has found his place as an educator as well as a clinician, earning the Division of Hospital Medicine Teaching Award in 2016.
As a member of the The Hospitalist’s volunteer editorial advisory board, Dr. Sehgal enjoys helping to educate and inform fellow hospitalists. He spoke with The Hospitalist to tell us more about himself.
How did you get into medicine?
I don’t know how old I was when I decided I was going to be a doctor, but it was at a very young age and I never really wavered in that desire. I guess I also would have wanted to be a baseball player or a musician, but I never had the talents for those, so it was doctor. That’s always what I was thinking of doing, straight through high school and college, and then after college I took a year off and joined AmeriCorps. I spent a year there and then went to medical school in Dallas at UT Southwestern. After medical school, I thought I should go somewhere as different from Dallas as possible, so I went to Portland, Ore., for my residency and then a fellowship in general internal medicine.
How did you end up in hospital medicine?
When I was doing my residency, I always enjoyed being a generalist. A lot of different areas of medicine interested me, but I like the breadth of things you encounter as a generalist, so I could never picture myself being a subspecialist, doing the same things every day, seeing the same things. I knew I wanted to keep practicing general internal medicine, so I took a fellowship where I was working both inpatient and outpatient, and when I was looking for a job, I sought out things that involved some inpatient and some outpatient work. It turned out hospital medicine was the best fit.
What would you say is your favorite part of hospital medicine?
My favorite part of the job is getting to teach, working with medical students and residents. I also like the variety of what I do as a hospitalist, so I’m about 50% clinical and the rest of the time I perform a variety of tasks, both administrative and educational.
What about your least favorite part of hospitalist work?
Sometimes, particularly if you’re doing clinical, educational, and administrative work, it can be a little overwhelming to try and do a little bit of everything. I think generally that’s a good thing, but sometimes it can feel like a little too much.
What is some of the best advice you have received regarding how to handle the stresses of hospital medicine?
Feel free to say no to things. When hospitalists are starting their careers, and particularly when they are new to a job and trying to express their desire to get involved, sometimes they can have too much thrown at them at once. People can get overloaded very quickly, so I think feeling like you’re able to say no to some requests, or to take some time to think before you accept an additional role. The other piece of advice I remember from my fellowship, is that, when you do something, make it count twice. For example, if you’re involved in a project, you get the practical clinical or educational benefits of whatever the project was. But also think about how you might write about your experience for research purposes, such as for a poster, article, or other presentation.
What is the worst advice you have been given?
I think it’s not necessarily bad advice, but I guess it’s advice that I haven’t really followed. Since I work in academic medicine, I’ve found that the people in academics fall into one of two categories: There are the people who find their niche and remain on that path, and they’re very clear about it and don’t really stray from it; and there are people who don’t find that niche right away. I think the advice I received when starting out was to try to find that niche, and if you’re building an academic career it is very helpful to have these things in which you have become the expert. But I’ve just tried to go where the job takes me. I don’t necessarily have a single academic niche or something that I spend all my time doing, but I do have my hand in a lot of different things. To me, that’s a lot more interesting because it adds to the variety of what you’re doing. Every day is a little different.
What else do you do professionally outside of hospital medicine?
I actually practice a little outpatient medicine. When I first started here, I wanted to keep some outpatient experience, and so I actually created my own clinic. It’s a procedure clinic where I do paracentesis on people who have cirrhosis. Then on the educational side, I sit on the admission committees for the medical school here, so I get to look through the applicants and choose who we interview, and then once we interview candidates, I help choose how we rank students for admission.
Where do you see yourself in the next 10 years?
I’ve never been one who looks at a particular job and says ‘Okay, I want to be the dean or have this position.’ I guess I just hope I’m better at the things I’m currently doing. I hope in 10 years that I’m a better teacher, that I’ll have learned more strategies to help more people, and that I have a better handle on the administrative side of the work. I hope I’ve progressed to a point in my career where I’m doing an even better job than I am now.
What are your goals as a member of the editorial board?
I have an interest not only in medicine but also in writing; I’ve gotten to do some writing both medical and nonmedical in the past. I’ve published a few articles in The Hospitalist, and hopefully, I can do more of that because writing is just another part of education.
Dr. Raj Sehgal enjoys a variety of roles
Dr. Raj Sehgal enjoys a variety of roles
Unlike some children who wanted be firefighters or astronauts when they grew up, ever since Raj Sehgal, MD, FHM, was a boy, he dreamed of being a doctor.
Since earning his medical degree, Dr. Sehgal has kept himself involved in a wide variety of projects, driven by the desire to diversify his expertise.
Currently a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System, San Antonio, and University of Texas Health San Antonio, Dr. Sehgal has found his place as an educator as well as a clinician, earning the Division of Hospital Medicine Teaching Award in 2016.
As a member of the The Hospitalist’s volunteer editorial advisory board, Dr. Sehgal enjoys helping to educate and inform fellow hospitalists. He spoke with The Hospitalist to tell us more about himself.
How did you get into medicine?
I don’t know how old I was when I decided I was going to be a doctor, but it was at a very young age and I never really wavered in that desire. I guess I also would have wanted to be a baseball player or a musician, but I never had the talents for those, so it was doctor. That’s always what I was thinking of doing, straight through high school and college, and then after college I took a year off and joined AmeriCorps. I spent a year there and then went to medical school in Dallas at UT Southwestern. After medical school, I thought I should go somewhere as different from Dallas as possible, so I went to Portland, Ore., for my residency and then a fellowship in general internal medicine.
How did you end up in hospital medicine?
When I was doing my residency, I always enjoyed being a generalist. A lot of different areas of medicine interested me, but I like the breadth of things you encounter as a generalist, so I could never picture myself being a subspecialist, doing the same things every day, seeing the same things. I knew I wanted to keep practicing general internal medicine, so I took a fellowship where I was working both inpatient and outpatient, and when I was looking for a job, I sought out things that involved some inpatient and some outpatient work. It turned out hospital medicine was the best fit.
What would you say is your favorite part of hospital medicine?
My favorite part of the job is getting to teach, working with medical students and residents. I also like the variety of what I do as a hospitalist, so I’m about 50% clinical and the rest of the time I perform a variety of tasks, both administrative and educational.
What about your least favorite part of hospitalist work?
Sometimes, particularly if you’re doing clinical, educational, and administrative work, it can be a little overwhelming to try and do a little bit of everything. I think generally that’s a good thing, but sometimes it can feel like a little too much.
What is some of the best advice you have received regarding how to handle the stresses of hospital medicine?
Feel free to say no to things. When hospitalists are starting their careers, and particularly when they are new to a job and trying to express their desire to get involved, sometimes they can have too much thrown at them at once. People can get overloaded very quickly, so I think feeling like you’re able to say no to some requests, or to take some time to think before you accept an additional role. The other piece of advice I remember from my fellowship, is that, when you do something, make it count twice. For example, if you’re involved in a project, you get the practical clinical or educational benefits of whatever the project was. But also think about how you might write about your experience for research purposes, such as for a poster, article, or other presentation.
What is the worst advice you have been given?
I think it’s not necessarily bad advice, but I guess it’s advice that I haven’t really followed. Since I work in academic medicine, I’ve found that the people in academics fall into one of two categories: There are the people who find their niche and remain on that path, and they’re very clear about it and don’t really stray from it; and there are people who don’t find that niche right away. I think the advice I received when starting out was to try to find that niche, and if you’re building an academic career it is very helpful to have these things in which you have become the expert. But I’ve just tried to go where the job takes me. I don’t necessarily have a single academic niche or something that I spend all my time doing, but I do have my hand in a lot of different things. To me, that’s a lot more interesting because it adds to the variety of what you’re doing. Every day is a little different.
What else do you do professionally outside of hospital medicine?
I actually practice a little outpatient medicine. When I first started here, I wanted to keep some outpatient experience, and so I actually created my own clinic. It’s a procedure clinic where I do paracentesis on people who have cirrhosis. Then on the educational side, I sit on the admission committees for the medical school here, so I get to look through the applicants and choose who we interview, and then once we interview candidates, I help choose how we rank students for admission.
Where do you see yourself in the next 10 years?
I’ve never been one who looks at a particular job and says ‘Okay, I want to be the dean or have this position.’ I guess I just hope I’m better at the things I’m currently doing. I hope in 10 years that I’m a better teacher, that I’ll have learned more strategies to help more people, and that I have a better handle on the administrative side of the work. I hope I’ve progressed to a point in my career where I’m doing an even better job than I am now.
What are your goals as a member of the editorial board?
I have an interest not only in medicine but also in writing; I’ve gotten to do some writing both medical and nonmedical in the past. I’ve published a few articles in The Hospitalist, and hopefully, I can do more of that because writing is just another part of education.
Unlike some children who wanted be firefighters or astronauts when they grew up, ever since Raj Sehgal, MD, FHM, was a boy, he dreamed of being a doctor.
Since earning his medical degree, Dr. Sehgal has kept himself involved in a wide variety of projects, driven by the desire to diversify his expertise.
Currently a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System, San Antonio, and University of Texas Health San Antonio, Dr. Sehgal has found his place as an educator as well as a clinician, earning the Division of Hospital Medicine Teaching Award in 2016.
As a member of the The Hospitalist’s volunteer editorial advisory board, Dr. Sehgal enjoys helping to educate and inform fellow hospitalists. He spoke with The Hospitalist to tell us more about himself.
How did you get into medicine?
I don’t know how old I was when I decided I was going to be a doctor, but it was at a very young age and I never really wavered in that desire. I guess I also would have wanted to be a baseball player or a musician, but I never had the talents for those, so it was doctor. That’s always what I was thinking of doing, straight through high school and college, and then after college I took a year off and joined AmeriCorps. I spent a year there and then went to medical school in Dallas at UT Southwestern. After medical school, I thought I should go somewhere as different from Dallas as possible, so I went to Portland, Ore., for my residency and then a fellowship in general internal medicine.
How did you end up in hospital medicine?
When I was doing my residency, I always enjoyed being a generalist. A lot of different areas of medicine interested me, but I like the breadth of things you encounter as a generalist, so I could never picture myself being a subspecialist, doing the same things every day, seeing the same things. I knew I wanted to keep practicing general internal medicine, so I took a fellowship where I was working both inpatient and outpatient, and when I was looking for a job, I sought out things that involved some inpatient and some outpatient work. It turned out hospital medicine was the best fit.
What would you say is your favorite part of hospital medicine?
My favorite part of the job is getting to teach, working with medical students and residents. I also like the variety of what I do as a hospitalist, so I’m about 50% clinical and the rest of the time I perform a variety of tasks, both administrative and educational.
What about your least favorite part of hospitalist work?
Sometimes, particularly if you’re doing clinical, educational, and administrative work, it can be a little overwhelming to try and do a little bit of everything. I think generally that’s a good thing, but sometimes it can feel like a little too much.
What is some of the best advice you have received regarding how to handle the stresses of hospital medicine?
Feel free to say no to things. When hospitalists are starting their careers, and particularly when they are new to a job and trying to express their desire to get involved, sometimes they can have too much thrown at them at once. People can get overloaded very quickly, so I think feeling like you’re able to say no to some requests, or to take some time to think before you accept an additional role. The other piece of advice I remember from my fellowship, is that, when you do something, make it count twice. For example, if you’re involved in a project, you get the practical clinical or educational benefits of whatever the project was. But also think about how you might write about your experience for research purposes, such as for a poster, article, or other presentation.
What is the worst advice you have been given?
I think it’s not necessarily bad advice, but I guess it’s advice that I haven’t really followed. Since I work in academic medicine, I’ve found that the people in academics fall into one of two categories: There are the people who find their niche and remain on that path, and they’re very clear about it and don’t really stray from it; and there are people who don’t find that niche right away. I think the advice I received when starting out was to try to find that niche, and if you’re building an academic career it is very helpful to have these things in which you have become the expert. But I’ve just tried to go where the job takes me. I don’t necessarily have a single academic niche or something that I spend all my time doing, but I do have my hand in a lot of different things. To me, that’s a lot more interesting because it adds to the variety of what you’re doing. Every day is a little different.
What else do you do professionally outside of hospital medicine?
I actually practice a little outpatient medicine. When I first started here, I wanted to keep some outpatient experience, and so I actually created my own clinic. It’s a procedure clinic where I do paracentesis on people who have cirrhosis. Then on the educational side, I sit on the admission committees for the medical school here, so I get to look through the applicants and choose who we interview, and then once we interview candidates, I help choose how we rank students for admission.
Where do you see yourself in the next 10 years?
I’ve never been one who looks at a particular job and says ‘Okay, I want to be the dean or have this position.’ I guess I just hope I’m better at the things I’m currently doing. I hope in 10 years that I’m a better teacher, that I’ll have learned more strategies to help more people, and that I have a better handle on the administrative side of the work. I hope I’ve progressed to a point in my career where I’m doing an even better job than I am now.
What are your goals as a member of the editorial board?
I have an interest not only in medicine but also in writing; I’ve gotten to do some writing both medical and nonmedical in the past. I’ve published a few articles in The Hospitalist, and hopefully, I can do more of that because writing is just another part of education.
VIDEO: Health advisers boost type 2 diabetes adherence
BOSTON – A company that partners patients and health advisers claims to help patients with type 2 diabetes to manage their symptoms and lower their HbA1c levels.
The Pack Health program connects each patient with a health adviser, who contacts them five times a week using a mix of phone calls, text messages, and emails. The goals include helping patients to find clinicians in their area, make appointments, and adhere to treatment, Dhiren Patel, PharmD, medical director at Pack Health, said at the annual meeting of the American Association of Clinical Endocrinologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients lost an average of 5 pounds, and annual eye and foot exams increased by 17% and 19%, respectively.
Similar remote health coach initiatives are being used to help other patients with chronic health conditions as part of the Pack Health services, which Dr. Patel discussed in a video interview.
SOURCE: Patel D et al. AACE 2018. Abstract 1209.
BOSTON – A company that partners patients and health advisers claims to help patients with type 2 diabetes to manage their symptoms and lower their HbA1c levels.
The Pack Health program connects each patient with a health adviser, who contacts them five times a week using a mix of phone calls, text messages, and emails. The goals include helping patients to find clinicians in their area, make appointments, and adhere to treatment, Dhiren Patel, PharmD, medical director at Pack Health, said at the annual meeting of the American Association of Clinical Endocrinologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients lost an average of 5 pounds, and annual eye and foot exams increased by 17% and 19%, respectively.
Similar remote health coach initiatives are being used to help other patients with chronic health conditions as part of the Pack Health services, which Dr. Patel discussed in a video interview.
SOURCE: Patel D et al. AACE 2018. Abstract 1209.
BOSTON – A company that partners patients and health advisers claims to help patients with type 2 diabetes to manage their symptoms and lower their HbA1c levels.
The Pack Health program connects each patient with a health adviser, who contacts them five times a week using a mix of phone calls, text messages, and emails. The goals include helping patients to find clinicians in their area, make appointments, and adhere to treatment, Dhiren Patel, PharmD, medical director at Pack Health, said at the annual meeting of the American Association of Clinical Endocrinologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients lost an average of 5 pounds, and annual eye and foot exams increased by 17% and 19%, respectively.
Similar remote health coach initiatives are being used to help other patients with chronic health conditions as part of the Pack Health services, which Dr. Patel discussed in a video interview.
SOURCE: Patel D et al. AACE 2018. Abstract 1209.
REPORTING FROM AACE 2018
Calcium, PTH predict permanent hypoparathyroidism
BOSTON – Low postoperative calcium serum and parathyroid hormone (PTH) levels may be strong predictors of permanent hypoparathyroidism in total thyroidectomy patients, according to results of a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
With incidence of transient hypoparathyroidism at 20-30%, being able to predict at-risk patients can significantly help clinicians with postoperative management, according to Steven Brown, DO, of the University of Arizona, Phoenix.
“It’s very important to draw the preoperative lab and postoperative lab in order to help those patients who are at risk,” said Dr. Brown in an oral abstract session.
To test the predictive accuracy of PTH and calcium levels, investigators conducted a single-center, retrospective study of 176 total thyroidectomy patients recorded during 1999-2013.
Patients with hypoparathyroidism had an average age of about 47 years, was almost entirely composed of females, was majority hispanic, and had mean postop calcium and PTH levels of 7.6 mg/dL and 8.0 pg/mL, respectively.
Those without hypoparathyroidism averaged about 51 years old, were equally hispanic and white, and had postop calcium and PTH levels of 8.08 mg/dL and 30.8 pg/dL, respectively.
Patients were split into four groups: Group 1 had low calcium and PTH levels (66), group 2 had low calcium and normal PTH levels (30), group 3 had normal calcium and low PTH levels (31), and group 4 had normal levels of both (49).
Over the study period, hypoparathyroidism developed in 30% of patients in group 1, 10% in group 2, 15% in group 3, and 2% in group 4.
Permanent hypothyroidism was defined as persistently low PTH (less than 12 pg/mL), low serum calcium (less than 8.0 mg/dL), and/or requiring calcitriol to maintain a normal calcium level for more than 6 months after total thyroidectomy.
Those with both low calcium and PTH levels were 4.3 times more likely to develop permanent hypoparathyroidism than those in the other groups, according to Dr. Brown.
Patients in the permanent hypoparathyroid group had a PTH drop of 70%, compared with 39% in the nonhypoparathyroid group. There was also a significant difference in respective drops of calcium levels (17.8% vs. 14.3%).
By comparing the levels before and after a thyroidectomy, physicians can act faster and more accurately when determining how best to treat patients to prevent hypoparathyroidism, a practice which, according to Dr. Brown, has already begun to be put into place.
“We’re starting to incorporate some of the practices into our patient care routine,” said Dr. Brown. “The next part of our project is going to be to actually do a prospective study at three different institution sites in order to evaluate this further.”
Dr. Brown reported no relevant financial disclosures.
SOURCE: Brown S et al. AACE 2018, Abstract 720.
BOSTON – Low postoperative calcium serum and parathyroid hormone (PTH) levels may be strong predictors of permanent hypoparathyroidism in total thyroidectomy patients, according to results of a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
With incidence of transient hypoparathyroidism at 20-30%, being able to predict at-risk patients can significantly help clinicians with postoperative management, according to Steven Brown, DO, of the University of Arizona, Phoenix.
“It’s very important to draw the preoperative lab and postoperative lab in order to help those patients who are at risk,” said Dr. Brown in an oral abstract session.
To test the predictive accuracy of PTH and calcium levels, investigators conducted a single-center, retrospective study of 176 total thyroidectomy patients recorded during 1999-2013.
Patients with hypoparathyroidism had an average age of about 47 years, was almost entirely composed of females, was majority hispanic, and had mean postop calcium and PTH levels of 7.6 mg/dL and 8.0 pg/mL, respectively.
Those without hypoparathyroidism averaged about 51 years old, were equally hispanic and white, and had postop calcium and PTH levels of 8.08 mg/dL and 30.8 pg/dL, respectively.
Patients were split into four groups: Group 1 had low calcium and PTH levels (66), group 2 had low calcium and normal PTH levels (30), group 3 had normal calcium and low PTH levels (31), and group 4 had normal levels of both (49).
Over the study period, hypoparathyroidism developed in 30% of patients in group 1, 10% in group 2, 15% in group 3, and 2% in group 4.
Permanent hypothyroidism was defined as persistently low PTH (less than 12 pg/mL), low serum calcium (less than 8.0 mg/dL), and/or requiring calcitriol to maintain a normal calcium level for more than 6 months after total thyroidectomy.
Those with both low calcium and PTH levels were 4.3 times more likely to develop permanent hypoparathyroidism than those in the other groups, according to Dr. Brown.
Patients in the permanent hypoparathyroid group had a PTH drop of 70%, compared with 39% in the nonhypoparathyroid group. There was also a significant difference in respective drops of calcium levels (17.8% vs. 14.3%).
By comparing the levels before and after a thyroidectomy, physicians can act faster and more accurately when determining how best to treat patients to prevent hypoparathyroidism, a practice which, according to Dr. Brown, has already begun to be put into place.
“We’re starting to incorporate some of the practices into our patient care routine,” said Dr. Brown. “The next part of our project is going to be to actually do a prospective study at three different institution sites in order to evaluate this further.”
Dr. Brown reported no relevant financial disclosures.
SOURCE: Brown S et al. AACE 2018, Abstract 720.
BOSTON – Low postoperative calcium serum and parathyroid hormone (PTH) levels may be strong predictors of permanent hypoparathyroidism in total thyroidectomy patients, according to results of a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
With incidence of transient hypoparathyroidism at 20-30%, being able to predict at-risk patients can significantly help clinicians with postoperative management, according to Steven Brown, DO, of the University of Arizona, Phoenix.
“It’s very important to draw the preoperative lab and postoperative lab in order to help those patients who are at risk,” said Dr. Brown in an oral abstract session.
To test the predictive accuracy of PTH and calcium levels, investigators conducted a single-center, retrospective study of 176 total thyroidectomy patients recorded during 1999-2013.
Patients with hypoparathyroidism had an average age of about 47 years, was almost entirely composed of females, was majority hispanic, and had mean postop calcium and PTH levels of 7.6 mg/dL and 8.0 pg/mL, respectively.
Those without hypoparathyroidism averaged about 51 years old, were equally hispanic and white, and had postop calcium and PTH levels of 8.08 mg/dL and 30.8 pg/dL, respectively.
Patients were split into four groups: Group 1 had low calcium and PTH levels (66), group 2 had low calcium and normal PTH levels (30), group 3 had normal calcium and low PTH levels (31), and group 4 had normal levels of both (49).
Over the study period, hypoparathyroidism developed in 30% of patients in group 1, 10% in group 2, 15% in group 3, and 2% in group 4.
Permanent hypothyroidism was defined as persistently low PTH (less than 12 pg/mL), low serum calcium (less than 8.0 mg/dL), and/or requiring calcitriol to maintain a normal calcium level for more than 6 months after total thyroidectomy.
Those with both low calcium and PTH levels were 4.3 times more likely to develop permanent hypoparathyroidism than those in the other groups, according to Dr. Brown.
Patients in the permanent hypoparathyroid group had a PTH drop of 70%, compared with 39% in the nonhypoparathyroid group. There was also a significant difference in respective drops of calcium levels (17.8% vs. 14.3%).
By comparing the levels before and after a thyroidectomy, physicians can act faster and more accurately when determining how best to treat patients to prevent hypoparathyroidism, a practice which, according to Dr. Brown, has already begun to be put into place.
“We’re starting to incorporate some of the practices into our patient care routine,” said Dr. Brown. “The next part of our project is going to be to actually do a prospective study at three different institution sites in order to evaluate this further.”
Dr. Brown reported no relevant financial disclosures.
SOURCE: Brown S et al. AACE 2018, Abstract 720.
REPORTING FROM AACE 18
Key clinical point:
Major finding: Patients with low calcium and low PTH were 4.3 times as likely as those without to develop permanent hypoparathyroidism after total thyroidectomy.
Study details: Retrospective, single center study of 176 total thyroidectomy patients during 1999-2013.
Disclosures: The presenter reported no relevant financial disclosures.
Source: Brown S et al. AACE 2018, Abstract 720.
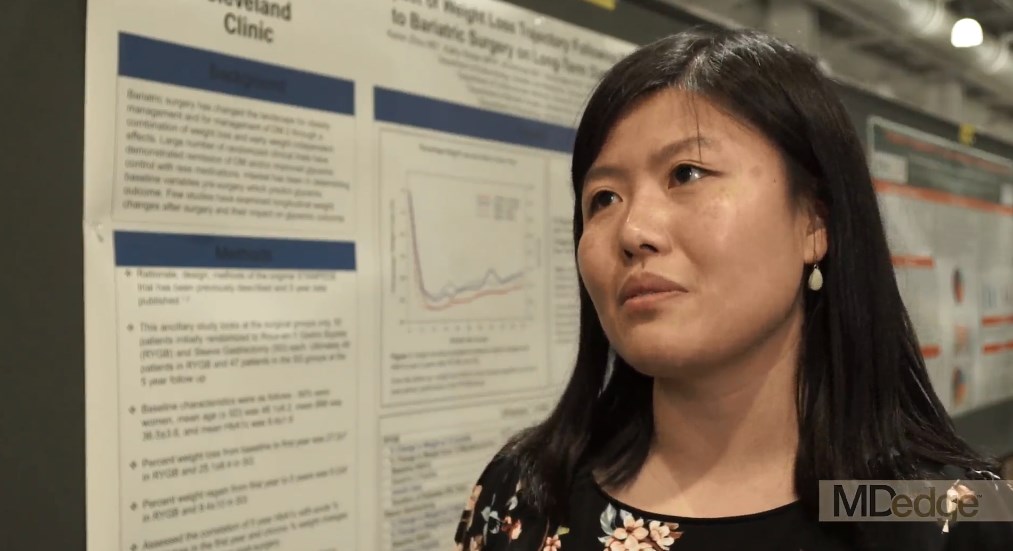
VIDEO: First year after bariatric surgery critical for HbA1c improvement
BOSTON – Acute weight loss during the first year after bariatric surgery has a significant effect on hemoglobin A1c level improvement at 5 years’ follow-up, according to a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
The data presented could help clinicians understand when and where to focus their efforts to help patients optimize weight loss in order to see the best long-term benefits of the procedure, according to presenter Keren Zhou, MD, an endocrinology fellow at the Cleveland Clinic.
“Clinicians need to really focus on that first year weight loss after bariatric surgery to try and optimize 5-year A1c outcomes,” said Dr. Zhou. “It also answers another question people have been having, which is how much does weight regain after bariatric surgery really matter? What we’ve been able to show here is that weight regain didn’t look very correlated at all.”
Dr. Zhou and her colleagues developed the ancillary study using data from the STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) trial, specifically looking at 96 patients: 49 who underwent bariatric surgery and 47 who had a sleeve gastrectomy.
Patients were majority female, on average 48 years old, with a mean body mass index of 36.5 and HbA1c level of 9.4.
Overall, bariatric surgery patients lost an average of 27.2% in the first year, and regained around 8.2% from the first to fifth year, while sleeve gastrectomy lost and regained 25.1% and 9.4% respectively.
When comparing weight loss in the first year and HbA1c levels, Dr. Zhou and her colleagues found a significant correlation for both bariatric surgery and sleeve gastrectomy patients (r +.34; P = .0006).
“It was interesting because when we graphically represented the weight changes in addition to the A1c over time, we found that they actually correlated quite closely, but it was only when we did the statistical analysis on the numbers that we found that [in both groups] people who lost less weight had a higher A1c at the 5-year mark,” said Dr. Zhou.
In the non–multivariable analysis, however, investigators found a more significant correlation between weight regain and HbA1c levels in gastrectomy patients, however these findings changed when Dr. Zhou and her fellow investigators controlled for insulin use and baseline C-peptide.
In continuing studies, Dr. Zhou and her team will dive deeper into why these correlations exist, as right now they can only speculate.
Dr. Zhou reported no relevant financial disclosures.
SOURCE: Zhou K et al. AACE 18. Abstract 240-F.
BOSTON – Acute weight loss during the first year after bariatric surgery has a significant effect on hemoglobin A1c level improvement at 5 years’ follow-up, according to a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
The data presented could help clinicians understand when and where to focus their efforts to help patients optimize weight loss in order to see the best long-term benefits of the procedure, according to presenter Keren Zhou, MD, an endocrinology fellow at the Cleveland Clinic.
“Clinicians need to really focus on that first year weight loss after bariatric surgery to try and optimize 5-year A1c outcomes,” said Dr. Zhou. “It also answers another question people have been having, which is how much does weight regain after bariatric surgery really matter? What we’ve been able to show here is that weight regain didn’t look very correlated at all.”
Dr. Zhou and her colleagues developed the ancillary study using data from the STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) trial, specifically looking at 96 patients: 49 who underwent bariatric surgery and 47 who had a sleeve gastrectomy.
Patients were majority female, on average 48 years old, with a mean body mass index of 36.5 and HbA1c level of 9.4.
Overall, bariatric surgery patients lost an average of 27.2% in the first year, and regained around 8.2% from the first to fifth year, while sleeve gastrectomy lost and regained 25.1% and 9.4% respectively.
When comparing weight loss in the first year and HbA1c levels, Dr. Zhou and her colleagues found a significant correlation for both bariatric surgery and sleeve gastrectomy patients (r +.34; P = .0006).
“It was interesting because when we graphically represented the weight changes in addition to the A1c over time, we found that they actually correlated quite closely, but it was only when we did the statistical analysis on the numbers that we found that [in both groups] people who lost less weight had a higher A1c at the 5-year mark,” said Dr. Zhou.
In the non–multivariable analysis, however, investigators found a more significant correlation between weight regain and HbA1c levels in gastrectomy patients, however these findings changed when Dr. Zhou and her fellow investigators controlled for insulin use and baseline C-peptide.
In continuing studies, Dr. Zhou and her team will dive deeper into why these correlations exist, as right now they can only speculate.
Dr. Zhou reported no relevant financial disclosures.
SOURCE: Zhou K et al. AACE 18. Abstract 240-F.
BOSTON – Acute weight loss during the first year after bariatric surgery has a significant effect on hemoglobin A1c level improvement at 5 years’ follow-up, according to a study presented at the annual meeting of the American Association of Clinical Endocrinologists.
The data presented could help clinicians understand when and where to focus their efforts to help patients optimize weight loss in order to see the best long-term benefits of the procedure, according to presenter Keren Zhou, MD, an endocrinology fellow at the Cleveland Clinic.
“Clinicians need to really focus on that first year weight loss after bariatric surgery to try and optimize 5-year A1c outcomes,” said Dr. Zhou. “It also answers another question people have been having, which is how much does weight regain after bariatric surgery really matter? What we’ve been able to show here is that weight regain didn’t look very correlated at all.”
Dr. Zhou and her colleagues developed the ancillary study using data from the STAMPEDE (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently) trial, specifically looking at 96 patients: 49 who underwent bariatric surgery and 47 who had a sleeve gastrectomy.
Patients were majority female, on average 48 years old, with a mean body mass index of 36.5 and HbA1c level of 9.4.
Overall, bariatric surgery patients lost an average of 27.2% in the first year, and regained around 8.2% from the first to fifth year, while sleeve gastrectomy lost and regained 25.1% and 9.4% respectively.
When comparing weight loss in the first year and HbA1c levels, Dr. Zhou and her colleagues found a significant correlation for both bariatric surgery and sleeve gastrectomy patients (r +.34; P = .0006).
“It was interesting because when we graphically represented the weight changes in addition to the A1c over time, we found that they actually correlated quite closely, but it was only when we did the statistical analysis on the numbers that we found that [in both groups] people who lost less weight had a higher A1c at the 5-year mark,” said Dr. Zhou.
In the non–multivariable analysis, however, investigators found a more significant correlation between weight regain and HbA1c levels in gastrectomy patients, however these findings changed when Dr. Zhou and her fellow investigators controlled for insulin use and baseline C-peptide.
In continuing studies, Dr. Zhou and her team will dive deeper into why these correlations exist, as right now they can only speculate.
Dr. Zhou reported no relevant financial disclosures.
SOURCE: Zhou K et al. AACE 18. Abstract 240-F.
REPORTING FROM AACE 18
Key clinical point:
Major finding: Change in weight within the first year was significantly correlated with lower HbA1c levels at 5 years (P = .0003).
Study details: Ancillary study of 96 patients who underwent either bariatric surgery or sleeve gastrectomy and participated in the STAMPEDE study.
Disclosures: Presenter reported no relevant financial disclosures.
Source: Zhou K et al. AACE 18. Abstract 240-F.
Elagolix shows long-term efficacy
AUSTIN, TEX. – A new treatment for endometriosis-related pain, Elagolix, showed evidence of being effective long term, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, manufactured by AbbVie, would be the first treatment of its kind if approved by the Food and Drug Administration, and would fulfill a needed relief for a more tolerable approach to severe endometriosis patients, according to presenter Eric S. Surrey, MD, medical director at the Colorado Center of Reproductive Medicine, Lone Tree.
“There have been no new medications approved for a long time for systematic endometriosis and there is a huge gap because the current options are expensive, and they are often injectable drugs,” said Dr. Surrey in an interview. “This would be an oral agent, which would be fabulous because it allows for a lot of flexibility and for many patients this could be much less concerning than using something long acting.”
To test the long-term effects of Elagolix, investigators studied 570 women with moderate to severe endometriosis-related pain who had gathered to participate in a previous phase 3, randomized, placebo-controlled trial concerning the drug’s effectiveness.
In the two extension studies, all participants were given either a 150- or 200-mg dose of Elagolix.
Average age of each patient group was between 31 and 34 years, and all groups were majority white, with a mean length of time from surgical diagnosis ranging from 45.5 to 56.6 months.
Patient improvements in dysmenorrhea and nonmenstrual pelvic pain continued between the first 6 months and 12 months of treatment, with a decrease of 46%-77% in the overall number of analgesics taken per day.
After 12 months of consecutive treatment, patients given 150 mg of Elagolix saw mean dysmenorrhea scores improve by 49%-53% from baseline, and by 82% for those at 200 mg, with certain expected adverse events, according to Dr. Surrey.
One of the most common adverse events associated with Elagolix was hot flashes, an unsurprising finding for Dr. Surrey and his colleagues considering Elagolix is a drug that lowers estrogen levels. However, any hot flashes patients experienced during the trial were still better than those associated with current medications, according to Dr. Surrey.
“In this extension study nobody dropped out because of hot flashes in the additional 6-month extension time,” Dr. Surrey explained. “If you look at the gold standard drug for endometriosis now, which is a GnRH agonist, which are highly available and are either injectable or implants, [patients taking these drugs] can have very severe hot flashes that require additional medication to alleviate the hot flashes at the same time.”
Patients did also experience some loss in bone density; however, Dr. Surrey argues the frequency and level of these adverse events is still better than current treatment options. One patient was required to discontinue the trial for bone density loss.
Currently, Elagolix is under FDA priority review, and if approved will be the first oral endometriosis treatment approved in over a decade, according to Dr. Surrey.
Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
SOURCE: Surrey ES et al. ACOG 2018, Abstract 11OP.
Having had the opportunity to review Dr. Eric Surrey's abstract for this year's annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, entitled "Long-term Safety and Efficacy of Elagolix Treatment in Women With Endometriosis-associated Pain," I believe use of Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, is a much-needed advancement in the long-term treatment of endometriosis-related pain. The fact that it is an oral medication, thus, not requiring a monthly or 3-month injection as does Lupron Depot (leuprolide acetate), the most popular GnRH agonist in the United States, is advantageous both for the patient and the busy office staff.
While I certainly understand that it is easy to compare data regarding bone loss in the use of an oral antagonist, Elagolix, with historical data with the GnRH agonist and note a lessening of bone loss in the Elagolix patients, it would be interesting to compare bone loss in patients utilizing Elagolix with bone loss in those treated with GnRH-agonist plus add-back therapy. Many practitioners will utilize progesterone supplementation or estrogen/progesterone supplementation when using GnRH-agonist therapy to decrease this risk. Furthermore, it would be interesting, in the future, to evaluate the impact on efficacy and bone loss if progesterone and estrogen/progesterone add-back were utilized in Elagolix therapy.
While I certainly realize and deeply respect Dr. Surrey's vast experience as both a clinical researcher and clinician utilizing a GnRH-agonist regimen, I am curious as to the basis of Dr. Surrey's comments regarding less severe hot flashes in comparison to GnRH-agonist treatment. I am not aware of any head-to-head studies comparing hot flashes between GnRH agonists (in particular, leuprolide acetate) and Elagolix.
Without a side-by-side comparison utilizing a validated scoring system, I find it hard to accept this conclusion.
Nevertheless, after reviewing this study and Dr. Surrey's comments, I look forward to utilizing Elagolix in my practice for long-term treatment of endometriosis-related pain.
Charles Miller, MD, is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He is a consultant and involved in research for AbbVie.
Having had the opportunity to review Dr. Eric Surrey's abstract for this year's annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, entitled "Long-term Safety and Efficacy of Elagolix Treatment in Women With Endometriosis-associated Pain," I believe use of Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, is a much-needed advancement in the long-term treatment of endometriosis-related pain. The fact that it is an oral medication, thus, not requiring a monthly or 3-month injection as does Lupron Depot (leuprolide acetate), the most popular GnRH agonist in the United States, is advantageous both for the patient and the busy office staff.
While I certainly understand that it is easy to compare data regarding bone loss in the use of an oral antagonist, Elagolix, with historical data with the GnRH agonist and note a lessening of bone loss in the Elagolix patients, it would be interesting to compare bone loss in patients utilizing Elagolix with bone loss in those treated with GnRH-agonist plus add-back therapy. Many practitioners will utilize progesterone supplementation or estrogen/progesterone supplementation when using GnRH-agonist therapy to decrease this risk. Furthermore, it would be interesting, in the future, to evaluate the impact on efficacy and bone loss if progesterone and estrogen/progesterone add-back were utilized in Elagolix therapy.
While I certainly realize and deeply respect Dr. Surrey's vast experience as both a clinical researcher and clinician utilizing a GnRH-agonist regimen, I am curious as to the basis of Dr. Surrey's comments regarding less severe hot flashes in comparison to GnRH-agonist treatment. I am not aware of any head-to-head studies comparing hot flashes between GnRH agonists (in particular, leuprolide acetate) and Elagolix.
Without a side-by-side comparison utilizing a validated scoring system, I find it hard to accept this conclusion.
Nevertheless, after reviewing this study and Dr. Surrey's comments, I look forward to utilizing Elagolix in my practice for long-term treatment of endometriosis-related pain.
Charles Miller, MD, is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He is a consultant and involved in research for AbbVie.
Having had the opportunity to review Dr. Eric Surrey's abstract for this year's annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, entitled "Long-term Safety and Efficacy of Elagolix Treatment in Women With Endometriosis-associated Pain," I believe use of Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, is a much-needed advancement in the long-term treatment of endometriosis-related pain. The fact that it is an oral medication, thus, not requiring a monthly or 3-month injection as does Lupron Depot (leuprolide acetate), the most popular GnRH agonist in the United States, is advantageous both for the patient and the busy office staff.
While I certainly understand that it is easy to compare data regarding bone loss in the use of an oral antagonist, Elagolix, with historical data with the GnRH agonist and note a lessening of bone loss in the Elagolix patients, it would be interesting to compare bone loss in patients utilizing Elagolix with bone loss in those treated with GnRH-agonist plus add-back therapy. Many practitioners will utilize progesterone supplementation or estrogen/progesterone supplementation when using GnRH-agonist therapy to decrease this risk. Furthermore, it would be interesting, in the future, to evaluate the impact on efficacy and bone loss if progesterone and estrogen/progesterone add-back were utilized in Elagolix therapy.
While I certainly realize and deeply respect Dr. Surrey's vast experience as both a clinical researcher and clinician utilizing a GnRH-agonist regimen, I am curious as to the basis of Dr. Surrey's comments regarding less severe hot flashes in comparison to GnRH-agonist treatment. I am not aware of any head-to-head studies comparing hot flashes between GnRH agonists (in particular, leuprolide acetate) and Elagolix.
Without a side-by-side comparison utilizing a validated scoring system, I find it hard to accept this conclusion.
Nevertheless, after reviewing this study and Dr. Surrey's comments, I look forward to utilizing Elagolix in my practice for long-term treatment of endometriosis-related pain.
Charles Miller, MD, is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL. He is a consultant and involved in research for AbbVie.
AUSTIN, TEX. – A new treatment for endometriosis-related pain, Elagolix, showed evidence of being effective long term, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, manufactured by AbbVie, would be the first treatment of its kind if approved by the Food and Drug Administration, and would fulfill a needed relief for a more tolerable approach to severe endometriosis patients, according to presenter Eric S. Surrey, MD, medical director at the Colorado Center of Reproductive Medicine, Lone Tree.
“There have been no new medications approved for a long time for systematic endometriosis and there is a huge gap because the current options are expensive, and they are often injectable drugs,” said Dr. Surrey in an interview. “This would be an oral agent, which would be fabulous because it allows for a lot of flexibility and for many patients this could be much less concerning than using something long acting.”
To test the long-term effects of Elagolix, investigators studied 570 women with moderate to severe endometriosis-related pain who had gathered to participate in a previous phase 3, randomized, placebo-controlled trial concerning the drug’s effectiveness.
In the two extension studies, all participants were given either a 150- or 200-mg dose of Elagolix.
Average age of each patient group was between 31 and 34 years, and all groups were majority white, with a mean length of time from surgical diagnosis ranging from 45.5 to 56.6 months.
Patient improvements in dysmenorrhea and nonmenstrual pelvic pain continued between the first 6 months and 12 months of treatment, with a decrease of 46%-77% in the overall number of analgesics taken per day.
After 12 months of consecutive treatment, patients given 150 mg of Elagolix saw mean dysmenorrhea scores improve by 49%-53% from baseline, and by 82% for those at 200 mg, with certain expected adverse events, according to Dr. Surrey.
One of the most common adverse events associated with Elagolix was hot flashes, an unsurprising finding for Dr. Surrey and his colleagues considering Elagolix is a drug that lowers estrogen levels. However, any hot flashes patients experienced during the trial were still better than those associated with current medications, according to Dr. Surrey.
“In this extension study nobody dropped out because of hot flashes in the additional 6-month extension time,” Dr. Surrey explained. “If you look at the gold standard drug for endometriosis now, which is a GnRH agonist, which are highly available and are either injectable or implants, [patients taking these drugs] can have very severe hot flashes that require additional medication to alleviate the hot flashes at the same time.”
Patients did also experience some loss in bone density; however, Dr. Surrey argues the frequency and level of these adverse events is still better than current treatment options. One patient was required to discontinue the trial for bone density loss.
Currently, Elagolix is under FDA priority review, and if approved will be the first oral endometriosis treatment approved in over a decade, according to Dr. Surrey.
Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
SOURCE: Surrey ES et al. ACOG 2018, Abstract 11OP.
AUSTIN, TEX. – A new treatment for endometriosis-related pain, Elagolix, showed evidence of being effective long term, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Elagolix, an oral nonpeptide gonadotropin-releasing hormone (GnRH) antagonist, manufactured by AbbVie, would be the first treatment of its kind if approved by the Food and Drug Administration, and would fulfill a needed relief for a more tolerable approach to severe endometriosis patients, according to presenter Eric S. Surrey, MD, medical director at the Colorado Center of Reproductive Medicine, Lone Tree.
“There have been no new medications approved for a long time for systematic endometriosis and there is a huge gap because the current options are expensive, and they are often injectable drugs,” said Dr. Surrey in an interview. “This would be an oral agent, which would be fabulous because it allows for a lot of flexibility and for many patients this could be much less concerning than using something long acting.”
To test the long-term effects of Elagolix, investigators studied 570 women with moderate to severe endometriosis-related pain who had gathered to participate in a previous phase 3, randomized, placebo-controlled trial concerning the drug’s effectiveness.
In the two extension studies, all participants were given either a 150- or 200-mg dose of Elagolix.
Average age of each patient group was between 31 and 34 years, and all groups were majority white, with a mean length of time from surgical diagnosis ranging from 45.5 to 56.6 months.
Patient improvements in dysmenorrhea and nonmenstrual pelvic pain continued between the first 6 months and 12 months of treatment, with a decrease of 46%-77% in the overall number of analgesics taken per day.
After 12 months of consecutive treatment, patients given 150 mg of Elagolix saw mean dysmenorrhea scores improve by 49%-53% from baseline, and by 82% for those at 200 mg, with certain expected adverse events, according to Dr. Surrey.
One of the most common adverse events associated with Elagolix was hot flashes, an unsurprising finding for Dr. Surrey and his colleagues considering Elagolix is a drug that lowers estrogen levels. However, any hot flashes patients experienced during the trial were still better than those associated with current medications, according to Dr. Surrey.
“In this extension study nobody dropped out because of hot flashes in the additional 6-month extension time,” Dr. Surrey explained. “If you look at the gold standard drug for endometriosis now, which is a GnRH agonist, which are highly available and are either injectable or implants, [patients taking these drugs] can have very severe hot flashes that require additional medication to alleviate the hot flashes at the same time.”
Patients did also experience some loss in bone density; however, Dr. Surrey argues the frequency and level of these adverse events is still better than current treatment options. One patient was required to discontinue the trial for bone density loss.
Currently, Elagolix is under FDA priority review, and if approved will be the first oral endometriosis treatment approved in over a decade, according to Dr. Surrey.
Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
SOURCE: Surrey ES et al. ACOG 2018, Abstract 11OP.
REPORTING FROM ACOG 2018
Key clinical point: New treatment for endometriosis-related pain shows long-term efficacy.
Major finding: Pain significantly decreased in test groups, compared with placebo (P less than .05).
Data source: A phase 3, randomized trial of 570 women with moderate to severe endometriosis.
Disclosures: Dr. Surrey and several coauthors receive financial support from AbbVie as consultants, board members, and/or employees. Dr. Surrey and Dr. Taylor receive additional support from companies including Pfizer, Bayer, and Obseva.
Source: Surrey ES et al. ACOG 2018, Abstract 11OP.
FDA approves subcutaneous tocilizumab for polyarticular JIA
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
in patients aged 2 years and older, according to a statement released May 14 by the drug’s manufacturer, Genentech.
While a intravenous formulation of the treatment was approved in 2013, this new delivery method may help make this treatment more accessible to the approximately 30 in every 100,000 children affected by PJIA, according to the release.
Doses were determined based on weight. Patients under 30 kg received 162 mg of tocilizumab every 3 weeks, while those 30 kg and over received 162 mg tocilizumab every 2 weeks.
Overall, safety of the subcutaneous delivery method was consistent with the IV study, as was the efficacy of the drug, the company said. A total of 28.8% of patients reported injection-site reactions – all moderate – and 15.4% reported neutrophil counts below 1 x 109 per liter.Tocilizumab can be taken either by itself or with methotrexate.
Marketing perks increased opioid prescriptions
, according to a study published in JAMA Internal Medicine.
With 40% of opioid-related deaths still coming from prescription opioids, understanding how marketing influences prescriber habits could lead to the creation of specific policies to lower the number of prescription drugs exchanging hands and save lives.
Dr. Hadland and his colleagues conducted a comparative analysis of opioid prescriptions from 2014 and 2015, retrieved from the Medicare Part D Opioid Prescriber Summary File and cross referenced that information with all recorded transactions from companies to physicians in 2014 from the Open Payments database.
In 2015, a total of 369,139 physicians were recorded prescribing opioids to Medicare patients. About 25,767 (7%) received a combined total of 105,368 “nonresearch opioid-related payments” with a sum total of $9,071,976 in 2014.
While Medicare opioid claims went down in 2015, physicians who received these payments, on average, prescribed 9.3% more opioids than those who did not, according to investigators.
INSYS Therapeutics, Teva Pharmaceuticals USA, and Janssen Pharmaceuticals were the three highest-paying companies, contributing $4.5 million, $869,155, and $854,251, respectively.
Payments included speaking fees ($6.2 million), meals ($1.8 million), travel ($730,824), consulting fees ($290,395), and education ($79,660). Investigators estimated that, with each meal a physician received, there was an associated 0.7% increase in opioid claims.
Dr. Hadland and fellow investigators do acknowledge the possibility of reverse causality, with physicians who already prescribe more opioids being more likely to receive industry payments.
Dr. Hadland and his team report no relevant financial disclosures.
SOURCE: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
, according to a study published in JAMA Internal Medicine.
With 40% of opioid-related deaths still coming from prescription opioids, understanding how marketing influences prescriber habits could lead to the creation of specific policies to lower the number of prescription drugs exchanging hands and save lives.
Dr. Hadland and his colleagues conducted a comparative analysis of opioid prescriptions from 2014 and 2015, retrieved from the Medicare Part D Opioid Prescriber Summary File and cross referenced that information with all recorded transactions from companies to physicians in 2014 from the Open Payments database.
In 2015, a total of 369,139 physicians were recorded prescribing opioids to Medicare patients. About 25,767 (7%) received a combined total of 105,368 “nonresearch opioid-related payments” with a sum total of $9,071,976 in 2014.
While Medicare opioid claims went down in 2015, physicians who received these payments, on average, prescribed 9.3% more opioids than those who did not, according to investigators.
INSYS Therapeutics, Teva Pharmaceuticals USA, and Janssen Pharmaceuticals were the three highest-paying companies, contributing $4.5 million, $869,155, and $854,251, respectively.
Payments included speaking fees ($6.2 million), meals ($1.8 million), travel ($730,824), consulting fees ($290,395), and education ($79,660). Investigators estimated that, with each meal a physician received, there was an associated 0.7% increase in opioid claims.
Dr. Hadland and fellow investigators do acknowledge the possibility of reverse causality, with physicians who already prescribe more opioids being more likely to receive industry payments.
Dr. Hadland and his team report no relevant financial disclosures.
SOURCE: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
, according to a study published in JAMA Internal Medicine.
With 40% of opioid-related deaths still coming from prescription opioids, understanding how marketing influences prescriber habits could lead to the creation of specific policies to lower the number of prescription drugs exchanging hands and save lives.
Dr. Hadland and his colleagues conducted a comparative analysis of opioid prescriptions from 2014 and 2015, retrieved from the Medicare Part D Opioid Prescriber Summary File and cross referenced that information with all recorded transactions from companies to physicians in 2014 from the Open Payments database.
In 2015, a total of 369,139 physicians were recorded prescribing opioids to Medicare patients. About 25,767 (7%) received a combined total of 105,368 “nonresearch opioid-related payments” with a sum total of $9,071,976 in 2014.
While Medicare opioid claims went down in 2015, physicians who received these payments, on average, prescribed 9.3% more opioids than those who did not, according to investigators.
INSYS Therapeutics, Teva Pharmaceuticals USA, and Janssen Pharmaceuticals were the three highest-paying companies, contributing $4.5 million, $869,155, and $854,251, respectively.
Payments included speaking fees ($6.2 million), meals ($1.8 million), travel ($730,824), consulting fees ($290,395), and education ($79,660). Investigators estimated that, with each meal a physician received, there was an associated 0.7% increase in opioid claims.
Dr. Hadland and fellow investigators do acknowledge the possibility of reverse causality, with physicians who already prescribe more opioids being more likely to receive industry payments.
Dr. Hadland and his team report no relevant financial disclosures.
SOURCE: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Doctors who received nonresearch payments on average had a higher number of opioid prescriptions than those who did not.
Major finding: Receiving a nonresearch payment from a pharmaceutical company was associated with about 9.3% more opioid claims.
Study details: Record analysis of 369,139 physicians collected from the Open Payments database and the Medicare Part D Opioid Prescriber Summary File for the years 2014 and 2015.
Disclosures: Dr. Hadland and his team report no relevant financial disclosures.
Source: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
IV superior to oral treatment for iron deficiency during pregnancy
AUSTIN, TEX. – Utilizing intravenous treatment for iron deficiency in anemic pregnant women was more efficacious than oral iron supplements, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
With 42% of pregnancies worldwide affected by anemia, according to the World Health Organization, improving treatment beyond the standard oral treatment could have a large effect on decreasing pregnancy complications.
“Women with bariatric surgery and inflammatory bowel disease are at higher risk of failure,” said Shravya Govindappagari, MD, a gynecologist affiliated with New York–Presbyterian Hospital. “Intravenous iron overcomes the limited intestinal absorption of oral formulations, and may increase iron stores more quickly.”
Dr. Govindappagari and her colleagues conducted a meta-analysis of 11 randomly controlled trials published between 2002 and 2017 to uncover the possible benefits of intravenous iron over oral treatment.
Studies were conducted in India, Egypt, France, and Turkey, with one additional multicenter study that gathered patients from seven different countries. Participants were given iron sucrose, ferric carboxymaltose, or low molecular weight iron dextran, according to Dr. Govindappagari.
In an overall assessment of subjects who achieved target hemoglobin levels, patients receiving intravenous iron were 2.66 times more likely to reach target levels than those given oral treatment (P less than .001). After 4 weeks of treatment, patients in the intravenous groups had a mean hemoglobin increase of 0.84 g/dl higher than those in the oral group (P less than .001).
Some clinicians may be wary about switching treatment modality from oral to intravenous; however, Dr. Govindappagari and fellow investigators found those taking oral treatment were 35% more likely to experience adverse effects than those receiving intravenous treatment.
While the analysis, according to Dr. Govindappagari, has merit, she and her team did not have access to relevant blinded, randomly controlled trials, which may have affected the findings. Maternal and neonatal outcomes were also not included in any of the studies analyzed, nor was a cost analysis of the financial burden of switching from oral to intravenous treatment.
Despite these limitations, Dr. Govindappagari and her colleagues assert the use of intravenous iron could have a significant effect on this problem.
“Intravenous iron compared to oral iron has a higher number reach target, a greater increase in hemoglobin, and has fewer side effects,” Dr. Govindappagari said to attendees. “This could be particularly useful in women in labor, during the third trimester, and women who are iron deficient and are at risk for postpartum hemorrhage.”
Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
SOURCE: Govindappagari S et al. ACOG 2018, Abstract 10OP.
AUSTIN, TEX. – Utilizing intravenous treatment for iron deficiency in anemic pregnant women was more efficacious than oral iron supplements, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
With 42% of pregnancies worldwide affected by anemia, according to the World Health Organization, improving treatment beyond the standard oral treatment could have a large effect on decreasing pregnancy complications.
“Women with bariatric surgery and inflammatory bowel disease are at higher risk of failure,” said Shravya Govindappagari, MD, a gynecologist affiliated with New York–Presbyterian Hospital. “Intravenous iron overcomes the limited intestinal absorption of oral formulations, and may increase iron stores more quickly.”
Dr. Govindappagari and her colleagues conducted a meta-analysis of 11 randomly controlled trials published between 2002 and 2017 to uncover the possible benefits of intravenous iron over oral treatment.
Studies were conducted in India, Egypt, France, and Turkey, with one additional multicenter study that gathered patients from seven different countries. Participants were given iron sucrose, ferric carboxymaltose, or low molecular weight iron dextran, according to Dr. Govindappagari.
In an overall assessment of subjects who achieved target hemoglobin levels, patients receiving intravenous iron were 2.66 times more likely to reach target levels than those given oral treatment (P less than .001). After 4 weeks of treatment, patients in the intravenous groups had a mean hemoglobin increase of 0.84 g/dl higher than those in the oral group (P less than .001).
Some clinicians may be wary about switching treatment modality from oral to intravenous; however, Dr. Govindappagari and fellow investigators found those taking oral treatment were 35% more likely to experience adverse effects than those receiving intravenous treatment.
While the analysis, according to Dr. Govindappagari, has merit, she and her team did not have access to relevant blinded, randomly controlled trials, which may have affected the findings. Maternal and neonatal outcomes were also not included in any of the studies analyzed, nor was a cost analysis of the financial burden of switching from oral to intravenous treatment.
Despite these limitations, Dr. Govindappagari and her colleagues assert the use of intravenous iron could have a significant effect on this problem.
“Intravenous iron compared to oral iron has a higher number reach target, a greater increase in hemoglobin, and has fewer side effects,” Dr. Govindappagari said to attendees. “This could be particularly useful in women in labor, during the third trimester, and women who are iron deficient and are at risk for postpartum hemorrhage.”
Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
SOURCE: Govindappagari S et al. ACOG 2018, Abstract 10OP.
AUSTIN, TEX. – Utilizing intravenous treatment for iron deficiency in anemic pregnant women was more efficacious than oral iron supplements, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
With 42% of pregnancies worldwide affected by anemia, according to the World Health Organization, improving treatment beyond the standard oral treatment could have a large effect on decreasing pregnancy complications.
“Women with bariatric surgery and inflammatory bowel disease are at higher risk of failure,” said Shravya Govindappagari, MD, a gynecologist affiliated with New York–Presbyterian Hospital. “Intravenous iron overcomes the limited intestinal absorption of oral formulations, and may increase iron stores more quickly.”
Dr. Govindappagari and her colleagues conducted a meta-analysis of 11 randomly controlled trials published between 2002 and 2017 to uncover the possible benefits of intravenous iron over oral treatment.
Studies were conducted in India, Egypt, France, and Turkey, with one additional multicenter study that gathered patients from seven different countries. Participants were given iron sucrose, ferric carboxymaltose, or low molecular weight iron dextran, according to Dr. Govindappagari.
In an overall assessment of subjects who achieved target hemoglobin levels, patients receiving intravenous iron were 2.66 times more likely to reach target levels than those given oral treatment (P less than .001). After 4 weeks of treatment, patients in the intravenous groups had a mean hemoglobin increase of 0.84 g/dl higher than those in the oral group (P less than .001).
Some clinicians may be wary about switching treatment modality from oral to intravenous; however, Dr. Govindappagari and fellow investigators found those taking oral treatment were 35% more likely to experience adverse effects than those receiving intravenous treatment.
While the analysis, according to Dr. Govindappagari, has merit, she and her team did not have access to relevant blinded, randomly controlled trials, which may have affected the findings. Maternal and neonatal outcomes were also not included in any of the studies analyzed, nor was a cost analysis of the financial burden of switching from oral to intravenous treatment.
Despite these limitations, Dr. Govindappagari and her colleagues assert the use of intravenous iron could have a significant effect on this problem.
“Intravenous iron compared to oral iron has a higher number reach target, a greater increase in hemoglobin, and has fewer side effects,” Dr. Govindappagari said to attendees. “This could be particularly useful in women in labor, during the third trimester, and women who are iron deficient and are at risk for postpartum hemorrhage.”
Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
SOURCE: Govindappagari S et al. ACOG 2018, Abstract 10OP.
REPORTING FROM ACOG 2018
Key clinical point: Intravenous iron treatment is better for pregnant women with anemia.
Major finding: Hemoglobin levels in women with intravenous iron increased by 1.2 g/dl more than in those using oral supplements after 4 weeks (P less than .001).
Data source: A meta-analysis of 11 randomized, controlled trials comparing intravenous with oral iron treatment.
Disclosures: Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
Source: Govindappagari S et al. ACOG 2018, Abstract 10OP.
VIDEO: Postpartum care gets a new look
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
ezimmerman@mdedge.com
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
ezimmerman@mdedge.com
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
ezimmerman@mdedge.com
REPORTING FROM ACOG 2018
VIDEO: Novel postpartum depression drug effective in phase 3 trial
AUSTIN, TEXAS – A novel therapeutic agent shows promise for postpartum depression in a phase 3 trial presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
, according to presenter Christine Clemson, PhD, senior medical director at Sage Therapeutics, the company developing brexanolone.
The randomized, placebo-controlled, double-blind study enrolled 138 women who were 6 months postpartum or less, and had been diagnosed with a major depressive episode during the third trimester or at 4 or fewer weeks postpartum, and had a 17-item Hamilton Rating Scale for Depression (HAM-D) score of 26 or greater.
They were randomized to either brexanolone 60 mcg/kg/hour or 90 mcg/kg/hour administered intravenously over 60 hours as inpatients, or placebo. All three groups were an average aged 27 years old, the majority were white, and they had a HAM-D score between 28.4 and 29.1 at baseline.
After the first 60 hours of treatment, patients in the brexanolone group had mean reductions in the HAM-D score of about 20 in the 60 mcg group (P less than .01) and 18 in the 90 mcg group (P less than .05), compared with almost 14 in the placebo group. This was the primary endpoint,
Patients retained improvement through day 30, while those in the placebo group experienced a slight swing in the opposite direction.
Adverse effects in the brexanolone-treated groups were minimal; the majority of events reported were headaches or dizziness. However, Dr. Clemson said that some patients had to stop breastfeeding for a week.
An application for brexanolone for treating postpartum depression was submitted to the Food and Drug Administration on April 23; if approved, it would be the first drug of its kind to become available to treat postpartum depression.
The study was funded by Sage Therapeutics; two of the six authors are company employees. Two authors, including the lead author, are from the department of psychiatry, at the University of North Carolina, Chapel Hill.
SOURCE: S. Meltzer-Brody S et al. ACOG 2018, Poster 29B.
AUSTIN, TEXAS – A novel therapeutic agent shows promise for postpartum depression in a phase 3 trial presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
, according to presenter Christine Clemson, PhD, senior medical director at Sage Therapeutics, the company developing brexanolone.
The randomized, placebo-controlled, double-blind study enrolled 138 women who were 6 months postpartum or less, and had been diagnosed with a major depressive episode during the third trimester or at 4 or fewer weeks postpartum, and had a 17-item Hamilton Rating Scale for Depression (HAM-D) score of 26 or greater.
They were randomized to either brexanolone 60 mcg/kg/hour or 90 mcg/kg/hour administered intravenously over 60 hours as inpatients, or placebo. All three groups were an average aged 27 years old, the majority were white, and they had a HAM-D score between 28.4 and 29.1 at baseline.
After the first 60 hours of treatment, patients in the brexanolone group had mean reductions in the HAM-D score of about 20 in the 60 mcg group (P less than .01) and 18 in the 90 mcg group (P less than .05), compared with almost 14 in the placebo group. This was the primary endpoint,
Patients retained improvement through day 30, while those in the placebo group experienced a slight swing in the opposite direction.
Adverse effects in the brexanolone-treated groups were minimal; the majority of events reported were headaches or dizziness. However, Dr. Clemson said that some patients had to stop breastfeeding for a week.
An application for brexanolone for treating postpartum depression was submitted to the Food and Drug Administration on April 23; if approved, it would be the first drug of its kind to become available to treat postpartum depression.
The study was funded by Sage Therapeutics; two of the six authors are company employees. Two authors, including the lead author, are from the department of psychiatry, at the University of North Carolina, Chapel Hill.
SOURCE: S. Meltzer-Brody S et al. ACOG 2018, Poster 29B.
AUSTIN, TEXAS – A novel therapeutic agent shows promise for postpartum depression in a phase 3 trial presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
, according to presenter Christine Clemson, PhD, senior medical director at Sage Therapeutics, the company developing brexanolone.
The randomized, placebo-controlled, double-blind study enrolled 138 women who were 6 months postpartum or less, and had been diagnosed with a major depressive episode during the third trimester or at 4 or fewer weeks postpartum, and had a 17-item Hamilton Rating Scale for Depression (HAM-D) score of 26 or greater.
They were randomized to either brexanolone 60 mcg/kg/hour or 90 mcg/kg/hour administered intravenously over 60 hours as inpatients, or placebo. All three groups were an average aged 27 years old, the majority were white, and they had a HAM-D score between 28.4 and 29.1 at baseline.
After the first 60 hours of treatment, patients in the brexanolone group had mean reductions in the HAM-D score of about 20 in the 60 mcg group (P less than .01) and 18 in the 90 mcg group (P less than .05), compared with almost 14 in the placebo group. This was the primary endpoint,
Patients retained improvement through day 30, while those in the placebo group experienced a slight swing in the opposite direction.
Adverse effects in the brexanolone-treated groups were minimal; the majority of events reported were headaches or dizziness. However, Dr. Clemson said that some patients had to stop breastfeeding for a week.
An application for brexanolone for treating postpartum depression was submitted to the Food and Drug Administration on April 23; if approved, it would be the first drug of its kind to become available to treat postpartum depression.
The study was funded by Sage Therapeutics; two of the six authors are company employees. Two authors, including the lead author, are from the department of psychiatry, at the University of North Carolina, Chapel Hill.
SOURCE: S. Meltzer-Brody S et al. ACOG 2018, Poster 29B.
REPORTING FROM ACOG 2018