User login
Immune dysregulation may drive long-term postpartum depression
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
Postpartum depression, anxiety, and posttraumatic stress disorder that persist 2-3 years after birth are associated with a dysregulated immune system that is characterized by increased inflammatory signaling, according to investigators.
These findings suggest that mental health screening for women who have given birth should continue beyond the first year post partum, reported lead author Jennifer M. Nicoloro-SantaBarbara, PhD, of Brigham and Women’s Hospital, Harvard Medical School, Boston, and colleagues.
“Delayed postpartum depression, also known as late-onset postpartum depression, can affect women up to 18 months after delivery,” the investigators wrote in the American Journal of Reproductive Immunology. “It can appear even later in some women, depending on the hormonal changes that occur after having a baby (for example, timing of weaning). However, the majority of research on maternal mental health focuses on the first year post birth, leaving a gap in research beyond 12 months post partum.”
To address this gap, the investigators enrolled 33 women who were 2-3 years post partum. Participants completed self-guided questionnaires on PTSD, depression, and anxiety, and provided blood samples for gene expression analysis.
Sixteen of the 33 women had clinically significant mood disturbances. and significantly reduced activation of genes associated with viral response.
“The results provide preliminary evidence of a mechanism (e.g., immune dysregulation) that might be contributing to mood disorders and bring us closer to the goal of identifying targetable biomarkers for mood disorders,” Dr. Nicoloro-SantaBarbara said in a written comment. “This work highlights the need for standardized and continual depression and anxiety screening in ob.gyn. and primary care settings that extends beyond the 6-week maternal visit and possibly beyond the first postpartum year.”
Findings draw skepticism
“The authors argue that mothers need to be screened for depression/anxiety longer than the first year post partum, and this is true, but it has nothing to do with their findings,” said Jennifer L. Payne, MD, an expert in reproductive psychiatry at the University of Virginia, Charlottesville.
In a written comment, she explained that the cross-sectional design makes it impossible to know whether the mood disturbances were linked with delivery at all.
“It is unclear if the depression/anxiety symptoms began after delivery or not,” Dr. Payne said. “In addition, it is unclear if the findings are causative or a result of depression/anxiety symptoms (the authors admit this in the limitations section). It is likely that the findings are not specific or even related to having delivered a child, but rather reflect a more general process related to depression/anxiety outside of the postpartum time period.”
Only prospective studies can answer these questions, she said.
Dr. Nicoloro-SantaBarbara agreed that further research is needed.
“Our findings are exciting, but still need to be replicated in larger samples with diverse women in order to make sure they generalize,” she said. “More work is needed to understand why inflammation plays a role in postpartum mental illness for some women and not others.”
The study was supported by a Cedars-Sinai Precision Health Grant, the Cousins Center for Psychoneuroimmunology, University of California, Los Angeles, and the National Institute of Mental Health. The investigators and Dr. Payne disclosed no relevant conflicts of interest.
FROM THE AMERICAN JOURNAL OF REPRODUCTIVE IMMUNOLOGY
Digital treatment may help relieve PTSD, panic disorder
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.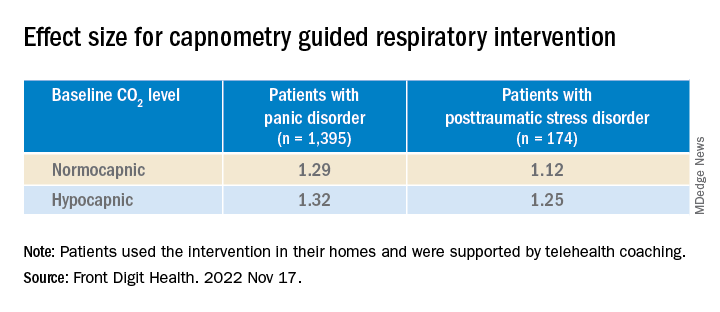
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The 28-day home-based treatment, known as the capnometry guided respiratory intervention (CGRI), uses an app-based feedback protocol to normalize respiration and increase patients’ ability to cope with symptoms of stress, anxiety, and panic by providing real time breath-to-breath feedback of respiratory rate and carbon dioxide (CO2) levels via a nasal cannula.
Results from the large real-world study showed that over 65% of patients with PD and over 72% of those with PTSD responded to the treatment. In addition, almost 75% of participants adhered to the study protocol, with low dropout rates.
“The brief duration of treatment, high adherence rates, and clinical benefit suggests that CGRI provides an important addition to treatment options for PD and PTSD,” the investigators write.
The study was published online in Frontiers in Digital Health.
‘New kid on the block’
The “respiratory dysregulation hypothesis” links CO2 sensitivity to panic attacks and PD, and similar reactivity has been identified in PTSD, but a “common limitation of psychotherapeutic and pharmacologic approaches to PD and PTSD is that neither address the role of respiratory physiology and breathing style,” the investigators note.
The most widely studied treatment for PTSD is trauma-focused psychotherapy, in which the patient reviews and revisits the trauma, but it has a high dropout rate, study investigator Michael Telch, PhD, director of the Laboratory for the Study of Anxiety Disorders, University of Texas, Austin, told this news organization.
He described CGRI for PTSD as a “relatively new kid on the block, so to speak.” The intervention was cleared by the U.S. Food and Drug Administration for treatment of PD and PTSD in 2013 and 2018, respectively, and is currently available through the Veterans Administration for veterans with PTSD. It is also covered by some commercial insurance plans.
“The underlying assumption [of CGRI] is that a person can learn to develop skills for controlling some of their physiological reactions that are triggered as a result of trauma,” said Dr. Telch.
The device uses a biofeedback approach to give patients “greater control over their physiological reactions, such as hyperventilation and increased respiration rate, and the focus is on providing a sense of mastery,” he said.
Participants with PTSD were assigned to a health coach. The device was delivered to the patient’s home, and patients met with the trained coach weekly and could check in between visits via text or e-mail. Twice-daily sessions were recommended.
“The coach gets feedback about what’s happening with the patient’s respiration and end-tidal CO2 levels [etCO2] and instructs participants how to keep their respiration rate and etCO2 at a more normal level,” said Dr. Telch.
The CGRI “teaches a specific breathing style via a system providing real-time feedback of respiratory rate (RR) and exhaled carbon dioxide levels facilitated by data capture,” the authors note.
Sense of mastery
Of the 1,569 participants, 1,395 had PD and 174 had PTSD (mean age, 39.2 [standard deviation, 13.9] years and 40.9 [SD, 14.9] years, respectively; 76% and 73% female, respectively). Those with PD completed the Panic Disorder Severity Scale (PDSS) and those with PTSD completed the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5), before and after the intervention.
The treatment response rate for PD was defined as a 40% or greater reduction in PDSS total scores, whereas treatment response rate for PTSD was defined as a 10-point or greater reduction in PCL-5 scores.
At baseline, patients were classified either as normocapnic or hypocapnic (etCO2 ≥ 37 or < 37, respectively), with 65% classified as normocapnic and 35% classified as hypocapnic.
Among patients with PD, there was a 50.2% mean pre- to posttreatment reduction in total PDSS scores (P < .001; d = 1.31), with a treatment response rate of 65.3% of patients.
Among patients with PTSD, there was a 41.1% pre- to posttreatment reduction in total PCL-5 scores (P < .001; d = 1.16), with a treatment response rate of 72.4%.
When investigators analyzed the response at the individual level, they found that 55.7% of patients with PD and 53.5% of those with PTSD were classified as treatment responders. This determination was based on a two-pronged approach that first calculated the Reliable Change Index (RCI) for each participant, and, in participants showing statistically reliable improvement, whether the posttreatment score was closer to the distribution of scores for patients without or with the given disorder.
“Patients with both normal and below-normal baseline exhaled CO2 levels experienced comparable benefit,” the authors report.
There were high levels of adherence across the full treatment period in both the PD and the PTSD groups (74.8% and 74.9%, respectively), with low dropout rates (10% and 11%, respectively).
“Not every single patient who undergoes any treatment has a perfect response, but the response rates to this treatment have, surprisingly, been quite positive and there have been no negative side effects,” Dr. Telch remarked.
He noted that one of the effects of PTSD is that the “patient has negative beliefs about their ability to control the world. ‘I can’t control my reactions. At any time, I could have a flashback.’ Helping the patient to develop any sense of mastery over some of their reactions can spill over and give them a greater sense of mastery and control, which can have a positive effect in reducing PTSD symptoms.”
‘A viable alternative’
Commenting on the research, Charles Marmar, MD, chair and Peter H. Schub Professor of Psychiatry, department of psychiatry, New York University, said that the study has some limitations, probably the most significant of which is that most participants had normal baseline CO2 levels.
“The treatment is fundamentally designed for people who hyperventilate and blow off too much CO2 so they can breathe in a more calm, relaxed way, but most people in the trial had normal CO2 to begin with,” said Dr. Marmar, who was not involved with the study.
“It’s likely that the major benefits were the relaxation from doing the breathing exercises rather than the change in CO2 levels,” he speculated.
The treatment is “probably a good thing for those patients who actually have abnormal CO2 levels. This treatment could be used in precision medicine, where you tailor treatments to those who actually need them rather than giving the same treatment to everyone,” he said.
“For patients who don’t respond to trauma-focused therapy or it’s too aversive for them to undergo, this new intervention provides a viable alternative,” Dr. Telch added.
The study was internally funded by Freespira. Dr. Telch is a scientific advisor at Freespira and receives compensation by way of stock options. The other authors’ disclosures are listed on the original paper. Dr. Marmar has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN DIGITAL HEALTH
Psychedelics for treating psychiatric disorders: Are they safe?
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
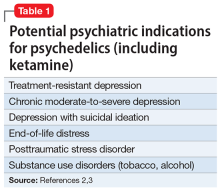
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
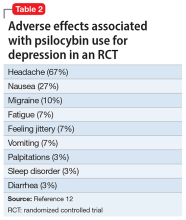
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40
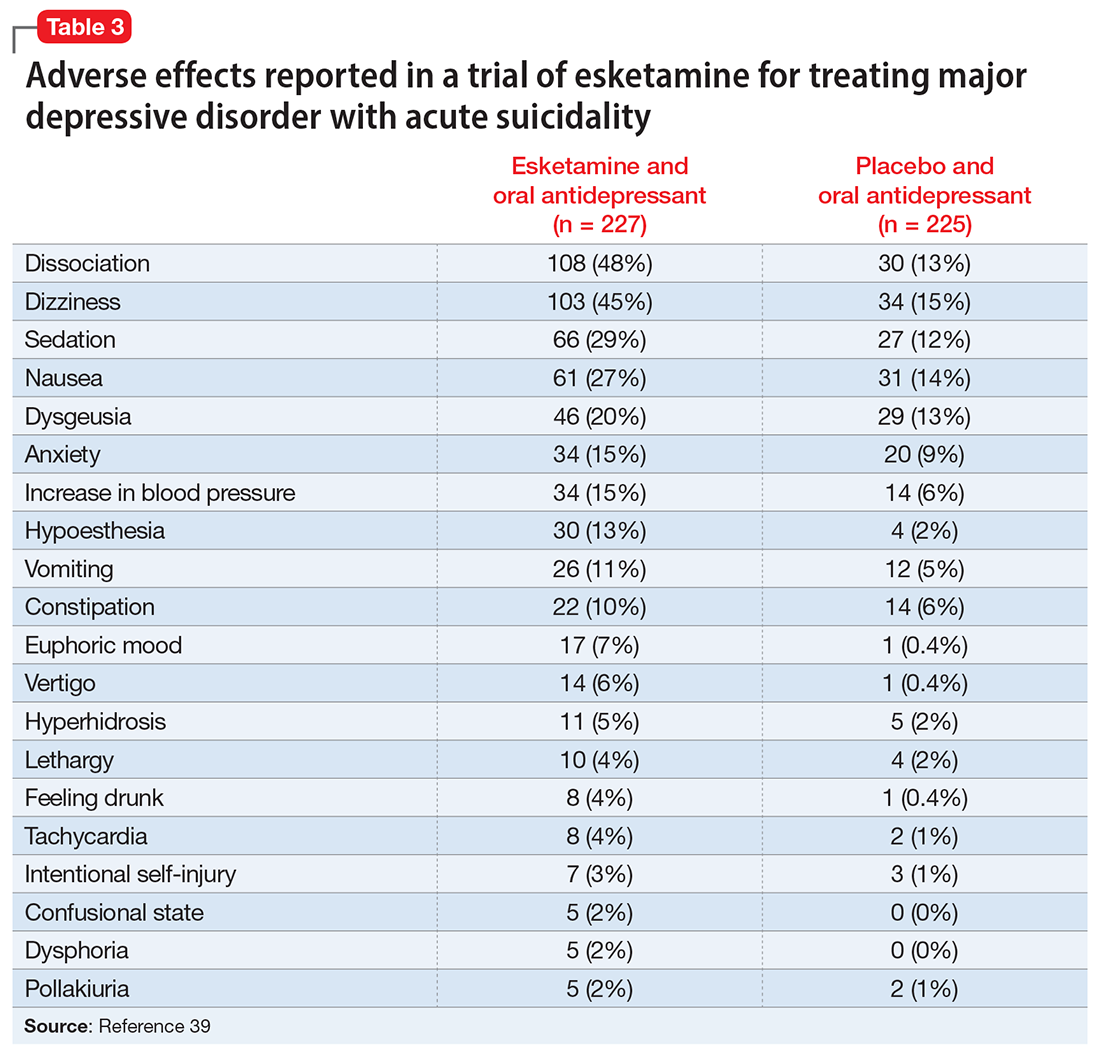
Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
Psychedelics are a class of substances known to produce alterations in consciousness and perception. In the last 2 decades, psychedelic research has garnered increasing attention from scientists, therapists, entrepreneurs, and the public. While many of these compounds remain illegal in the United States and in many parts of the world (Box1), a recent resurrection of psychedelic research has motivated the FDA to designate multiple psychedelic compounds as “breakthrough therapies,” thereby expediting the investigation, development, and review of psychedelic treatments.
Box
The legal landscape of psychedelics is rapidly evolving. Psilocybin use has been decriminalized in many cities in the United States (such as Denver), and some states (such as Oregon) have legalized it for therapeutic use.
It is important to understand the difference between decriminalization and legalization. Decriminalization means the substance is still prohibited under existing laws, but the legal system will choose not to enforce the prohibition. Legalization is the rescinding of laws prohibiting the use of the substance. In the United States, these laws may be state or federal. Despite psilocybin legalization for therapeutic use in Oregon and decriminalization in various cities, psychedelics currently remain illegal under federal law.
Source: Reference 1
There is growing evidence that psychedelics may be efficacious for treating a range of psychiatric disorders. Potential clinical indications for psychedelics include some forms of depression, posttraumatic stress disorder (PTSD), and substance use disorders (Table 12,3). In most instances, the clinical use of psychedelics is being investigated and offered in the context of psychedelic-assisted psychotherapy, though ketamine is a prominent exception. Ketamine and esketamine are already being used to treat depression, and FDA approval is anticipated for other psychedelics.
This article examines the adverse effect profile of classical (psilocybin [“mushrooms”], lysergic acid diethylamide [LSD], and N,N-dimethyltryptamine [DMT]/ayahuasca) and nonclassical (the entactogen 3,4-methylenedioxymethamphetamine [MDMA, known as “ecstasy”] and the dissociative anesthetic ketamine) psychedelics.
Psilocybin
Psilocybin is typically administered as a single dose of 10 to 30 mg and used in conjunction with preintegration and postintegration psychotherapy. Administration of psilocybin typically produces perceptual distortions and mind-altering effects, which are mediated through 5-HT2A brain receptor agonistic action.4 The acute effects last approximately 6 hours.5 While psilocybin has generated promising results in early clinical trials,3 the adverse effects of these agents have received less attention.
The adverse effect profile of psilocybin in adults appears promising but its powerful psychoactive effects necessitate cautious use.6 It has a very wide therapeutic index, and in a recent meta-analysis of psilocybin for depression, no serious adverse effects were reported in any of the 7 included studies.7 Common adverse effects in the context of clinical use include anxiety, dysphoria, confusion, and an increase in blood pressure and heart rate.6 Due to potential cardiac effects, psilocybin is contraindicated in individuals with cardiovascular and cerebrovascular disease.8 In recreational/nonclinical use, reactions such as suicidality, violence, convulsions, panic attacks, paranoia, confusion, prolonged dissociation, and mania have been reported.9,10 Animal and human studies indicate the risk of abuse and physical dependence is low. Major national surveys indicate low rates of abuse, treatment-seeking, and harm.11 In a recent 6-week randomized controlled trial (RCT) of psilocybin vs escitalopram for depression,12 no serious adverse events were reported. Adverse events reported in the psilocybin group in this trial are listed in Table 2.12
A recent phase 2 double-blind trial of single-dose psilocybin (1 mg, 10 mg, and 25 mg) for treatment-resistant depression (N = 233) sheds more light on the risk of adverse effects.13 The percentage of individuals experiencing adverse effects on Day 1 of administration was high: 61% in the 25 mg psilocybin group. Headache, nausea, fatigue, and dizziness were the most common effects. The incidence of any adverse event in the 25 mg group was 56% from Day 2 to Week 3, and 29% from Week 3 to Week 12. Suicidal ideation, suicidal behavior, or self-injury occurred in all 3 dose groups. Overall, 14% in the 25 mg group, 17% in the 10 mg group, and 9% in the 1 mg group showed worsening of suicidality from baseline to Week 3. Suicidal behavior was reported by 3 individuals in the 25 mg group after Week 3. The new-onset or worsening of preexisting suicidality with psilocybin reported in this study requires further investigation.
Lysergic acid diethylamide
LSD is similar to psilocybin in its agonistic action at the 5-HT2A brain receptors.4 It is typically administered as a single 100 to 200 μg dose and is used in conjunction with preintegration and postintegration psychotherapy.14 Its acute effects last approximately 12 hours.15
Continue to: Like psilocybin...
Like psilocybin, LSD has a wide therapeutic index. Commonly reported adverse effects of LSD are increased anxiety, dysphoria, and confusion. LSD can also lead to physiological adverse effects, such as increased blood pressure and heart rate, and thus is contraindicated in patients with severe heart disease.6 In a systematic review of the therapeutic use of LSD that included 567 participants,16 2 cases of serious adverse events were reported: a tonic-clonic seizure in a patient with a prior history of seizures, and a case of prolonged psychosis in a 21-year-old with a history of psychotic disorder.
Though few psychedelic studies have examined the adverse effects of these agents in older adults, a recent phase 1 study that recruited 48 healthy older adults (age 55 to 75) found that, compared to placebo, low doses (5 to 20 μg) of LSD 2 times a week for 3 weeks had similar adverse effects, cognitive impairment, or balance impairment.17 The only adverse effect noted to be different between the placebo group and active treatment groups was headache (50% for LSD 10 μg, 25% for LSD 20 μg, and 8% for placebo). Because the dose range (5 to 20 μg) used in this study was substantially lower than the typical therapeutic dose range of 100 to 200 μg, these results should not be interpreted as supporting the safety of LSD at higher doses in older adults.
DMT/ayahuasca
Ayahuasca is a plant-based psychedelic that contains an admixture of substances, including DMT, which acts as a 5-HT2A receptor agonist. In addition to DMT, ayahuasca also contains the alkaloid harmaline, which acts as a monoamine inhibitor. Use of ayahuasca can therefore pose a particular risk for individuals taking other serotonergic or noradrenergic medications or substances. The acute effects of DMT last approximately 4 hours,18 and acute administration of ayahuasca leads to a transient modified state of consciousness that is characterized by introspection, visions, enhanced emotions, and recall of personal memories.19 Research shows ayahuasca has been dosed at approximately 0.36 mg/kg of DMT for 1 dosing session alongside 6 2-hour therapy sessions.20
A recent review by Orsolini et al21 consolidated 40 preclinical, observational, and experimental studies of ayahuasca, and this compound appeared to be safe and well-tolerated; the most common adverse effects were transient emesis and nausea. In an RCT by Palhano-Fontes et al,20 nausea was observed in 71% of participants in the ayahuasca group (vs 26% placebo), vomiting in 57% of participants (vs 0% placebo), and restlessness in 50% of participants (vs 20% placebo). The authors noted that for some participants the ayahuasca session “was not necessarily a pleasant experience,” and was accompanied by psychological distress.20 Vomiting is traditionally viewed as an expected part of the purging process of ayahuasca religious ceremonies. Another review found that there appears to be good long-term tolerability of ayahuasca consumption among individuals who use this compound in religious ceremonies.22
MDMA
Entactogens (or empathogens) are a class of psychoactive substances that produce experiences of emotional openness and connection. MDMA is an entactogen known to release serotonin, norepinephrine, and dopamine by inhibiting reuptake.23 This process leads to the stimulation of neurohormonal signaling of oxytocin, cortisol, and other signaling molecules such as brain-derived neurotrophic factor.24 Memory reconsolidation and fear extinction may also play a therapeutic role, enabled by reduced activity in the amygdala and insula, and increased connectivity between the amygdala and hippocampus.24 MDMA has been reported to enhance feelings of well-being and increase prosocial behavior.25 In the therapeutic setting, MDMA has been generally dosed at 75 to 125 mg in 2 to 3 sessions alongside 10 therapy sessions. Administration of MDMA gives the user a subjective experience of energy and distortions in time and perception.26 These acute effects last approximately 2 to 4 hours.27
Continue to: A meta-analysis...
A meta-analysis of 5 RCTs of MDMA-assisted therapy for PTSD in adults demonstrated that MDMA was well-tolerated, and few serious adverse events were reported.28 Two trials from 2018 that were included in this meta-analysis—Mithoefer et al29 and Ot’alora et al30—illustrate the incidence of specific adverse effects. In a randomized, double-blind trial of 26 veterans and first responders with chronic PTSD, Mithoefer et al29 found the most commonly reported reactions during experimental sessions with MDMA were anxiety (81%), headache (69%), fatigue (62%), muscle tension (62%), and jaw clenching or tight jaw (50%). The most commonly reported reactions during 7 days of contact were fatigue (88%), anxiety (73%), insomnia (69%), headache (46%), muscle tension (46%), and increased irritability (46%). One instance of suicidal ideation was severe enough to require psychiatric hospitalization (this was the only instance of suicidal ideation among the 106 patients in the meta-analysis by Bahji et al28); the patient subsequently completed the trial. Transient elevation in pulse, blood pressure, and body temperature were noted during sessions that did not require medical intervention.29 Ot’alora et al30 found similar common adverse reactions: anxiety, dizziness, fatigue, headache, jaw clenching, muscle tension, and irritability. There were no serious adverse effects.
While the use of MDMA in controlled interventional settings has resulted in relatively few adverse events, robust literature describes the risks associated with the nonclinical/recreational use of MDMA. In cases of MDMA toxicity, death has been reported.31 Acutely, MDMA may lead to sympathomimetic effects, including serotonin syndrome.31 Longer-term studies of MDMA users have found chronic recreational use to be associated with worse sleep, poor mood, anxiety disturbances, memory deficits, and attention problems.32 MDMA has also been found to have moderate potential for abuse.33
Ketamine/esketamine
Ketamine is a dissociative anesthetic with some hallucinogenic effects. It is an N-methyl-
Esketamine, the S(+)-enantiomer of ketamine, is also an NDMA antagonist. It has been developed as an intranasal formulation, typically dosed between 56 and 84 mg 2 times a week for 1 month, once a week for the following month, and once every 1 to 2 weeks thereafter.35 In most ketamine and esketamine trials, these compounds have been used without psychotherapy, although some interventions have integrated psychotherapy with ketamine treatment.36
Bennett et al37 elaborated on 3 paradigms for ketamine treatment: biochemical, psychotherapeutic, and psychedelic. The biochemical model examines the neurobiological effects of the medication. The psychotherapeutic model views ketamine as a way of assisting the psychotherapy process. The psychedelic model utilizes ketamine’s dissociative and psychedelic properties to induce an altered state of consciousness for therapeutic purposes and psychospiritual exploration.
Continue to: A systematic review...
A systematic review of the common adverse effects associated with ketamine use in clinical trials for depression reported dissociation, sedation, perceptual disturbances, anxiety, agitation, euphoria, hypertension, tachycardia, headache, and dizziness.38 Adverse effects experienced with esketamine in clinical trials include dissociation, dizziness, sedation, hypertension, hypoesthesia, gastrointestinal symptoms, and euphoric mood (Table 339). A recent systemic review found both ketamine and esketamine demonstrated higher adverse events than control conditions. IV ketamine also demonstrated lower dropouts and adverse events when compared to intranasal esketamine.40

Nonclinical/recreational use of ketamine is notable for urinary toxicity; 20% to 30% of frequent users of ketamine experience urinary problems that can range from ketamine-induced cystitis to hydronephrosis and kidney failure.41 Liver toxicity has also been reported with chronic use of high-dose ketamine. Ketamine is liable to abuse, dependence, and tolerance. There is evidence that nonclinical use of ketamine may lead to morbidity; impairment of memory, cognition, and attention; and urinary, gastric, and hepatic pathology.42
The FDA prescribing information for esketamine lists aneurysmal vascular disease, arteriovenous malformation, and intracerebral hemorrhage as contraindications.39 Patients with cardiovascular and cerebrovascular conditions and risk factors may be at increased risk of adverse effects due to an increase in blood pressure. Esketamine can impair attention, judgment, thinking, reaction speed, and motor skills. Other adverse effects of esketamine noted in the prescribing information include dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, vomiting, feeling drunk, and euphoric mood.39A study of postmarketing safety concerns with esketamine using reports submitted to the FDA Adverse Event Reporting System (FAERS) revealed signals for suicidal ideation (reporting odds ratio [ROR] 24.03; 95% CI, 18.72 to 30.84), and completed suicide (ROR 5.75; 95% CI, 3.18 to 10.41).43 The signals for suicidal and self-injurious ideation remained significant when compared to venlafaxine in the FAERS database, while suicide attempts and fatal suicide attempts were no longer significant.43 Concerns regarding acute ketamine withdrawal have also been described in case reports.44
Other safety considerations of psychedelics
Hallucinogen persisting perception disorder
Hallucinogen persisting perception disorder (HPPD) is a rare condition associated with hallucinogen use. It is characterized by the recurrence of perceptual disturbances that an individual experienced while using hallucinogenic substances that creates significant distress or impairment.45 Because HPPD is a rare disorder, the exact prevalence is not well characterized, but DSM-5 suggests it is approximately 4.2%.46 HPPD is associated with numerous psychoactive substances, including psilocybin, ayahuasca, MDMA, and ketamine, but is most associated with LSD.45 HPPD is more likely to arise in individuals with histories of psychiatric illness or substance use disorders.47
Serotonin toxicity and other serotonergic interactions
Serotonin toxicity is a risk of serotonergic psychedelics, particularly when such agents are used in combination with serotonergic psychotropic medications. The most severe manifestation of serotonin toxicity is serotonin syndrome, which manifests as a life-threatening condition characterized by myoclonus, rigidity, agitation, delirium, and unstable cardiovascular functioning. Many psychedelic compounds have transient serotonin-related adverse effects, but serotonin toxicity due to psychedelic use is rare.48 Due to their mechanism of action, classical psychedelics are relatively safe in combination with monoamine oxidase inhibitors (MAOIs) and selective serotonin reuptake inhibitors. MDMA is a serotonin-releasing agent that has a higher risk of serotonin syndrome or hypertensive crisis when used in combination with MAOIs.48
Boundary violations in psychedelic-assisted psychotherapy
A key task facing psychedelic research is to establish parameters for the safe and ethical use of these agents. This is particularly relevant given the hype that surrounds the psychedelic resurgence and what we know about the controversial history of these substances. Anderson et al49 argued that “psychedelics can have lingering effects that include increased suggestibility and affective instability, as well as altered ego structure, social behaviour, and philosophical worldview. Stated simply, psychedelics can induce a vulnerable state both during and after treatment sessions.”
Continue to: Psychedelic treatment...
Psychedelic treatments such as psilocybin and MDMA are typically offered within the context of psychedelic-assisted psychotherapy, and some researchers have raised concerns regarding boundary violations,50 given the patients’ particularly vulnerable states. In addition to concerns about sexual harassment, the financial exploitation of older adults is also a possible risk.51
Caveats to consider
Novel psychedelics therapies have demonstrated promising preliminary results for a broad range of psychiatric indications, including depression, end-of-life distress, substance use disorders, PTSD, and improving well-being. To date, psychedelics are generally well-tolerated in adults in clinical trials.
However, when it comes to adverse effects, there are challenges in regards to interpreting the psychedelic state.52 Some consider any unpleasant or unsettling psychedelic experience as an adverse reaction, while others consider it part of the therapeutic process. This is exemplified by the case of vomiting during ayahuasca ceremonies, which is generally considered part of the ritual. In such instances, it is essential to obtain informed consent and ensure participants are aware of these aspects of the experience. Compared to substances such as alcohol, opioids, and cocaine, psychedelics are remarkably safe from a physiological perspective, especially with regards to the risks of toxicity, mortality, and dependence.53 Their psychological safety is less established, and more caution and research is needed. The high incidence of adverse effects and suicidality noted in the recent phase 2 trial of psilocybin in treatment resistant depression are a reminder of this.13
There is uncertainty regarding the magnitude of risk in real-world clinical practice, particularly regarding addiction, suicidality, and precipitation or worsening of psychotic disorders. For example, note the extensive exclusion criteria used in the psilocybin vs escitalopram RCT by Carhart-Harris et al12: currently or previously diagnosed psychotic disorder, immediate family member with a diagnosed psychotic disorder, significant medical comorbidity (eg, diabetes, epilepsy, severe cardiovascular disease, hepatic or renal failure), history of suicide attempts requiring hospitalization, history of mania, pregnancy, and abnormal QT interval prolongation, among others. It would be prudent to keep these contraindications in mind regarding the clinical use of psychedelics in the future. This is particularly important in older adults because such patients often have substantial medical comorbidities and are at greater risk for adverse effects. For ketamine, research has implicated the role of mu opioid agonism in mediating ketamine’s antidepressant effects.54 This raises concerns about abuse, dependence, and addiction, especially with long-term use. There are also concerns regarding protracted withdrawal symptoms and associated suicidality.55
The therapeutic use of psychedelics is an exciting and promising avenue, with ongoing research and a rapidly evolving literature. An attitude of cautious optimism is warranted, but efficacy and safety should be demonstrated in well-designed and rigorous trials with adequate long-term follow-up before routine clinical use is recommended.
Bottom Line
In clinical trials for psychiatric disorders, psychedelics have been associated with a range of cognitive, psychiatric, and psychoactive adverse effects but generally have been well-tolerated, with a low incidence of serious adverse effects.
Related Resources
- American Psychiatric Association. Position Statement on the Use of Psychedelic and Empathogenic Agents for Mental Health Conditions. Updated July 2022. Accessed October 24, 2022. https://www.psychiatry.org/getattachment/d5c13619-ca1f-491f-a7a8-b7141c800904/Position-Use-of-Psychedelic-Empathogenic-Agents.pdf
- Johns Hopkins Center for Psychedelic & Consciousness Research. https://hopkinspsychedelic.org/
- Multidisciplinary Association for Psychedelic Studies (MAPS). https://maps.org/
Drug Brand Names
Esketamine • Spravato
Ketamine • Ketalar
Venlafaxine • Effexor
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
1. The current legal status of psychedelics in the United States. Investing News Network. August 23, 2022. Accessed August 26, 2022. https://investingnews.com/legal-status-of-psychedelics-in-the-united-states/
2. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
3. Nutt D, Carhart-Harris R. The current status of psychedelics in psychiatry. JAMA Psychiatry. 2021;78(2):121-122.
4. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
5. Hasler F, Grimberg U, Benz MA et al. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145-156.
6. Johnson MW, Hendricks PS, Barrett FS, et al. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther. 2019;197:83-102.
7. Li NX, Hu YR, Chen WN, et al. Dose effect of psilocybin on primary and secondary depression: a preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26-34.
8. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
9. Carhart-Harris RL, Nutt DJ. User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study. J Subst Use. 2010;15(4):283-300.
10. van Amsterdam J, Opperhuizen A, van den Brink W. Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol. 2011;59(3):423-429.
11. Johnson MW, Griffiths RR, Hendricks PS, et al. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology. 2018;142:143-166.
12. Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl Med. 2021;384(15):1402-1411.
13. Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant Episode of major depression. N Engl J Med. 2022;387(18):1637-1648.
14. Galvão-Coelho NL, Marx W, Gonzalez M, et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology (Berl). 2021;238(2):341-354.
15. Schmid Y, Enzler F, Gasser P, et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78(8):544-553.
16. Fuentes JJ, Fonseca F, Elices M, et al. Therapeutic use of LSD in psychiatry: a systematic review of randomized-controlled clinical trials. Front Psychiatry. 2020;10:943.
17. Family N, Maillet EL, Williams LTJ, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology (Berl). 2020;237(3):841-853.
18. Frecska E, Bokor P, Winkelman M. The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Front Pharmacol. 2016;7:35.
19. Domínguez-Clavé E, Solar J, Elices M, et al. Ayahuasca: pharmacology, neuroscience and therapeutic potential. Brain Res Bull. 2016;126(Pt 1):89-101.
20. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655-663.
21. Orsolini L, Chiappini S, Papanti D, et al. How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy. Hum Psychopharmacol: Clin Exp. 2020;35(3):e2728.
22. Durante Í, Dos Santos RG, Bouso JC, et al. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2021;43(4):362-369.
23. Sessa B, Higbed L, Nutt D. A review of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy. Front Psychiatry. 2019;10:138.
24. Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Progress Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):221-228.
25. Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affective Neurosc. 2014;9(11):1645-1652.
26. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165(7):917-928.
27. Dumont GJ, Verkes RJ. A review of acute effects of 3, 4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20(2):176-187.
28. Bahji A, Forsyth A, Groll D, et al. Efficacy of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109735.
29. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
30. Ot’alora GM, Grigsby J, Poulter B, et al. 3,4-methylenedioxymethamphetamine-assisted psychotherapy for treatment of chronic posttraumatic stress disorder: a randomized phase 2 controlled trial. J Psychopharmacol. 2018;32(12):1295-1307.
31. Steinkellner T, Freissmuth M, Sitte HH, et al. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem. 2011;392(1-2):103-115.
32. Montoya AG, Sorrentino R, Lukas SE, et al. Long-term neuropsychiatric consequences of “ecstasy” (MDMA): a review. Harvard Rev Psychiatry. 2002;10(4):212-220.
33. Yazar‐Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. 2017;101(2):194-196.
34. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405.
35. Thase M, Connolly KR. Ketamine and esketamine for treating unipolar depression in adults: administration, efficacy, and adverse effects. Wolters Kluwer; 2019. Accessed August 26, 2022. https://www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects
36. Dore J, Turnispeed B, Dwyer S, et al. Ketamine assisted psychotherapy (KAP): patient demographics, clinical data and outcomes in three large practices administering ketamine with psychotherapy. J Psychoactive Drugs. 2019;51(2):189-198.
37. Bennett R, Yavorsky C, Bravo G. Ketamine for bipolar depression: biochemical, psychotherapeutic, and psychedelic approaches. Front Psychiatry. 2022;13:867484.
38. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78.
39. U.S. Food and Drug Administration. SPRAVATO® (esketamine). Prescribing information. Janssen; 2020. Accessed August 26, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf
40. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affective Disord. 2021;278:542-555.
41. Castellani D, Pirola GM, Gubbiotti M, et al. What urologists need to know about ketamine-induced uropathy: a systematic review. Neurourol Urodyn. 2020;39(4):1049-1062.
42. Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract. 2014;27(6):582-586.
43. Gastaldon, C, Raschi E, Kane JM, et al. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Psychother Psychosom. 2021;90(1):41-48.
44. Roxas N, Ahuja C, Isom J, et al. A potential case of acute ketamine withdrawal: clinical implications for the treatment of refractory depression. Am J Psychiatry. 2021;178(7):588-591.
45. Orsolini L, Papanti GD, De Berardis D, et al. The “Endless Trip” among the NPS users: psychopathology and psychopharmacology in the hallucinogen-persisting perception disorder. A systematic review. Front Psychiatry. 2017;8:240.
46. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatry Association; 2013.
47. Martinotti G, Santacroce R, Pettorruso M, et al. Hallucinogen persisting perception disorder: etiology, clinical features, and therapeutic perspectives. Brain Sci. 2018;8(3):47.
48. Malcolm B, Thomas K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology (Berl). 2022;239(6):1881-1891.
49. Anderson BT, Danforth AL, Grob CS. Psychedelic medicine: safety and ethical concerns. Lancet Psychiatry, 2020;7(10):829-830.
50. Goldhill O. Psychedelic therapy has a sexual abuse problem. QUARTZ. March 3, 2020. Accessed August 26, 2022. https://qz.com/1809184/psychedelic-therapy-has-a-sexual-abuse-problem-3/
51. Goldhill O. A psychedelic therapist allegedly took millions from a Holocaust survivor, highlighting worries about elders taking hallucinogens. STAT News. April 21, 2022. Accessed August 26, 2022. https://www.statnews.com/2022/04/21/psychedelic-therapist-allegedly-took-millions-from-holocaust-survivor-highlighting-worries-about-elders-taking-hallucinogens/
52. Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172(10):577-595.
53. Nutt D. Drugs Without the Hot Air: Minimising the Harms of Legal and Illegal Drugs. UIT Cambridge Ltd; 2012.
54. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
55. Schatzberg AF. A word to the wise about intranasal esketamine. Am J Psychiatry. 2019;176(6):422-424.
Postpartum posttraumatic stress disorder: An underestimated reality?
PAU, FRANCE – Postpartum posttraumatic stress disorder tends to get worse over the months following the birth of a child. Therefore, it’s necessary to screen for it as early on as possible and to ensure that women who are affected are given the proper treatment. This was the message delivered during the Infogyn 2022 conference by Ludivine Franchitto, MD, a child psychiatrist at Toulouse University Hospital, France. Because postpartum PTSD is still not fully recognized, treatment remains inadequate and poorly documented.
Impact on the caregivers as well
“The situation is the same as what we saw with postpartum depression. The debate went on for 20 years before its existence was formally declared,” Dr. Franchitto noted. But for her, the important thing is not knowing whether a traumatic stress state may be experienced by the mother who had complications during pregnancy or delivery. Instead, it’s about focusing on the repercussions for the child.
During her presentation, Dr. Franchitto also pointed out that it’s necessary to recognize that caregivers who work in maternity wards may also be negatively impacted, as they routinely see the complications that women have during pregnancy and delivery. These workers may also develop a PTSD state, requiring support so that they can properly carry out their duties.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder arises after exposure to actual (or threatened) death, serious injury, or sexual violence. Individuals who have witnessed a traumatic event in person or who have experienced repeated (or extreme) exposure to aversive details of traumatic events may also develop PTSD.
Dr. Franchitto mentioned some of the criteria needed to make the diagnosis. “Intrusive distressing memories of the event, recurrent distressing dreams related to the event, persistent avoidance of stimuli associated with the traumatic event, or negative alterations in cognitions and mood associated with the traumatic event. And the duration of the disturbance is more than 1 month.” There may also be marked alterations in arousal and reactivity associated with the traumatic event (for example, irritable behavior, loss of awareness of present surroundings).
Prevalent in 18% of women in high-risk groups
According to the studies, there is a wide variability of PTSD rates. If referring only to traumatic symptoms (for example, depressive syndrome, suicidal ideation, hyperreactivity, and persistent avoidance), the rate could reach up to 40%. A 2016 meta-analysis of 59 studies found that the prevalence of childbirth-related PTSD was 5.9%.
The authors distinguished two groups of women: those without complications during pregnancy or during delivery and those with severe complications related to the pregnancy, a fear of giving birth, a difficult delivery, an emergency C-section, a baby born prematurely with birth defects, etc. Their analysis showed PTSD rates of 4% and 18.5%, respectively.
Surprisingly, the major risk factor for PTSD turned out to be uncontrollable vomiting during pregnancy (seen in 40% of postpartum PTSD cases). The birth of a baby with birth defects was the second risk factor (35%), and the third, a history of violence in the mother’s childhood (34%). Women who experienced depression during the delivery were also at higher risk.
Other risk factors identified were lack of communication with the health care team, lack of consent, lack of support from the medical staff, and a long labor. Conversely, a sense of control and the support of a partner play a protective role.
Early screening
“If the symptoms of posttraumatic stress disorder aren’t treated after delivery, they tend to get worse over the period of 1 to 6 months following the child’s birth,” Dr. Franchitto indicated. This is why it’s necessary to screen for it as early as possible – in particular, by having the women fill out the City Birth Trauma Scale questionnaire – and provide proper treatment accordingly. When seeking to limit the effects of stress, early intervention by a psychologist may be beneficial.
Psychotherapy is the recommended first-line treatment for PTSD, especially cognitive behavioral therapy and Eye Movement Desensitization and Reprocessing therapy. This approach aims to limit the mental and behavioral avoidance that prevents the traumatic memory from being integrated and processed as a regular memory.
The consequences that the mother’s PTSD state has on the child are well documented. “Children whose mothers had PTSD during pregnancy have a lower birth weight and a shorter breast-feeding duration,” Dr. Franchitto reported. With respect to the quality of the mother-child relationship and the long-term development of the child, “the studies have highly conflicting findings.”
At the end of the presentation, Professor Israël Nisand, MD, an ob.gyn. at the American Hospital of Paris and the former president of the National College of French Gynecologists and Obstetricians, made the following comment: “I often think that we underestimate the consequences that the mother’s posttraumatic stress has on the child postpartum.” He added, “Postpartum posttraumatic stress disorder is a reality. Yet it isn’t screened for, let alone treated, even though it has serious consequences for the child.”
Dr. Franchitto also brought up the impact on members of the health care staff, the “second victims” of the traumatic events that occur while caring for the women in the maternity ward. “The estimated prevalence of PTSD symptoms among midwives is 22.9%,” which could lead to “a loss of confidence and a desire to leave the profession.”
Providing psychoeducation to health care staff
Dr. Franchitto believes that it’s essential to also protect caregivers who work in maternity wards. “It’s important to have the support of colleagues” – in particular, of team leaders – “and to share one’s experiences,” as long as one knows how to recognize the symptoms of posttraumatic stress through one’s emotions and is able to verbalize them.
She went on to say that providing psychoeducation to health care staff is therefore to be encouraged, as is “simulation-based training, for learning how to manage problematic situations.”
This content was originally published on Medscape French edition. A translated version appeared on Medscape.com.
PAU, FRANCE – Postpartum posttraumatic stress disorder tends to get worse over the months following the birth of a child. Therefore, it’s necessary to screen for it as early on as possible and to ensure that women who are affected are given the proper treatment. This was the message delivered during the Infogyn 2022 conference by Ludivine Franchitto, MD, a child psychiatrist at Toulouse University Hospital, France. Because postpartum PTSD is still not fully recognized, treatment remains inadequate and poorly documented.
Impact on the caregivers as well
“The situation is the same as what we saw with postpartum depression. The debate went on for 20 years before its existence was formally declared,” Dr. Franchitto noted. But for her, the important thing is not knowing whether a traumatic stress state may be experienced by the mother who had complications during pregnancy or delivery. Instead, it’s about focusing on the repercussions for the child.
During her presentation, Dr. Franchitto also pointed out that it’s necessary to recognize that caregivers who work in maternity wards may also be negatively impacted, as they routinely see the complications that women have during pregnancy and delivery. These workers may also develop a PTSD state, requiring support so that they can properly carry out their duties.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder arises after exposure to actual (or threatened) death, serious injury, or sexual violence. Individuals who have witnessed a traumatic event in person or who have experienced repeated (or extreme) exposure to aversive details of traumatic events may also develop PTSD.
Dr. Franchitto mentioned some of the criteria needed to make the diagnosis. “Intrusive distressing memories of the event, recurrent distressing dreams related to the event, persistent avoidance of stimuli associated with the traumatic event, or negative alterations in cognitions and mood associated with the traumatic event. And the duration of the disturbance is more than 1 month.” There may also be marked alterations in arousal and reactivity associated with the traumatic event (for example, irritable behavior, loss of awareness of present surroundings).
Prevalent in 18% of women in high-risk groups
According to the studies, there is a wide variability of PTSD rates. If referring only to traumatic symptoms (for example, depressive syndrome, suicidal ideation, hyperreactivity, and persistent avoidance), the rate could reach up to 40%. A 2016 meta-analysis of 59 studies found that the prevalence of childbirth-related PTSD was 5.9%.
The authors distinguished two groups of women: those without complications during pregnancy or during delivery and those with severe complications related to the pregnancy, a fear of giving birth, a difficult delivery, an emergency C-section, a baby born prematurely with birth defects, etc. Their analysis showed PTSD rates of 4% and 18.5%, respectively.
Surprisingly, the major risk factor for PTSD turned out to be uncontrollable vomiting during pregnancy (seen in 40% of postpartum PTSD cases). The birth of a baby with birth defects was the second risk factor (35%), and the third, a history of violence in the mother’s childhood (34%). Women who experienced depression during the delivery were also at higher risk.
Other risk factors identified were lack of communication with the health care team, lack of consent, lack of support from the medical staff, and a long labor. Conversely, a sense of control and the support of a partner play a protective role.
Early screening
“If the symptoms of posttraumatic stress disorder aren’t treated after delivery, they tend to get worse over the period of 1 to 6 months following the child’s birth,” Dr. Franchitto indicated. This is why it’s necessary to screen for it as early as possible – in particular, by having the women fill out the City Birth Trauma Scale questionnaire – and provide proper treatment accordingly. When seeking to limit the effects of stress, early intervention by a psychologist may be beneficial.
Psychotherapy is the recommended first-line treatment for PTSD, especially cognitive behavioral therapy and Eye Movement Desensitization and Reprocessing therapy. This approach aims to limit the mental and behavioral avoidance that prevents the traumatic memory from being integrated and processed as a regular memory.
The consequences that the mother’s PTSD state has on the child are well documented. “Children whose mothers had PTSD during pregnancy have a lower birth weight and a shorter breast-feeding duration,” Dr. Franchitto reported. With respect to the quality of the mother-child relationship and the long-term development of the child, “the studies have highly conflicting findings.”
At the end of the presentation, Professor Israël Nisand, MD, an ob.gyn. at the American Hospital of Paris and the former president of the National College of French Gynecologists and Obstetricians, made the following comment: “I often think that we underestimate the consequences that the mother’s posttraumatic stress has on the child postpartum.” He added, “Postpartum posttraumatic stress disorder is a reality. Yet it isn’t screened for, let alone treated, even though it has serious consequences for the child.”
Dr. Franchitto also brought up the impact on members of the health care staff, the “second victims” of the traumatic events that occur while caring for the women in the maternity ward. “The estimated prevalence of PTSD symptoms among midwives is 22.9%,” which could lead to “a loss of confidence and a desire to leave the profession.”
Providing psychoeducation to health care staff
Dr. Franchitto believes that it’s essential to also protect caregivers who work in maternity wards. “It’s important to have the support of colleagues” – in particular, of team leaders – “and to share one’s experiences,” as long as one knows how to recognize the symptoms of posttraumatic stress through one’s emotions and is able to verbalize them.
She went on to say that providing psychoeducation to health care staff is therefore to be encouraged, as is “simulation-based training, for learning how to manage problematic situations.”
This content was originally published on Medscape French edition. A translated version appeared on Medscape.com.
PAU, FRANCE – Postpartum posttraumatic stress disorder tends to get worse over the months following the birth of a child. Therefore, it’s necessary to screen for it as early on as possible and to ensure that women who are affected are given the proper treatment. This was the message delivered during the Infogyn 2022 conference by Ludivine Franchitto, MD, a child psychiatrist at Toulouse University Hospital, France. Because postpartum PTSD is still not fully recognized, treatment remains inadequate and poorly documented.
Impact on the caregivers as well
“The situation is the same as what we saw with postpartum depression. The debate went on for 20 years before its existence was formally declared,” Dr. Franchitto noted. But for her, the important thing is not knowing whether a traumatic stress state may be experienced by the mother who had complications during pregnancy or delivery. Instead, it’s about focusing on the repercussions for the child.
During her presentation, Dr. Franchitto also pointed out that it’s necessary to recognize that caregivers who work in maternity wards may also be negatively impacted, as they routinely see the complications that women have during pregnancy and delivery. These workers may also develop a PTSD state, requiring support so that they can properly carry out their duties.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder arises after exposure to actual (or threatened) death, serious injury, or sexual violence. Individuals who have witnessed a traumatic event in person or who have experienced repeated (or extreme) exposure to aversive details of traumatic events may also develop PTSD.
Dr. Franchitto mentioned some of the criteria needed to make the diagnosis. “Intrusive distressing memories of the event, recurrent distressing dreams related to the event, persistent avoidance of stimuli associated with the traumatic event, or negative alterations in cognitions and mood associated with the traumatic event. And the duration of the disturbance is more than 1 month.” There may also be marked alterations in arousal and reactivity associated with the traumatic event (for example, irritable behavior, loss of awareness of present surroundings).
Prevalent in 18% of women in high-risk groups
According to the studies, there is a wide variability of PTSD rates. If referring only to traumatic symptoms (for example, depressive syndrome, suicidal ideation, hyperreactivity, and persistent avoidance), the rate could reach up to 40%. A 2016 meta-analysis of 59 studies found that the prevalence of childbirth-related PTSD was 5.9%.
The authors distinguished two groups of women: those without complications during pregnancy or during delivery and those with severe complications related to the pregnancy, a fear of giving birth, a difficult delivery, an emergency C-section, a baby born prematurely with birth defects, etc. Their analysis showed PTSD rates of 4% and 18.5%, respectively.
Surprisingly, the major risk factor for PTSD turned out to be uncontrollable vomiting during pregnancy (seen in 40% of postpartum PTSD cases). The birth of a baby with birth defects was the second risk factor (35%), and the third, a history of violence in the mother’s childhood (34%). Women who experienced depression during the delivery were also at higher risk.
Other risk factors identified were lack of communication with the health care team, lack of consent, lack of support from the medical staff, and a long labor. Conversely, a sense of control and the support of a partner play a protective role.
Early screening
“If the symptoms of posttraumatic stress disorder aren’t treated after delivery, they tend to get worse over the period of 1 to 6 months following the child’s birth,” Dr. Franchitto indicated. This is why it’s necessary to screen for it as early as possible – in particular, by having the women fill out the City Birth Trauma Scale questionnaire – and provide proper treatment accordingly. When seeking to limit the effects of stress, early intervention by a psychologist may be beneficial.
Psychotherapy is the recommended first-line treatment for PTSD, especially cognitive behavioral therapy and Eye Movement Desensitization and Reprocessing therapy. This approach aims to limit the mental and behavioral avoidance that prevents the traumatic memory from being integrated and processed as a regular memory.
The consequences that the mother’s PTSD state has on the child are well documented. “Children whose mothers had PTSD during pregnancy have a lower birth weight and a shorter breast-feeding duration,” Dr. Franchitto reported. With respect to the quality of the mother-child relationship and the long-term development of the child, “the studies have highly conflicting findings.”
At the end of the presentation, Professor Israël Nisand, MD, an ob.gyn. at the American Hospital of Paris and the former president of the National College of French Gynecologists and Obstetricians, made the following comment: “I often think that we underestimate the consequences that the mother’s posttraumatic stress has on the child postpartum.” He added, “Postpartum posttraumatic stress disorder is a reality. Yet it isn’t screened for, let alone treated, even though it has serious consequences for the child.”
Dr. Franchitto also brought up the impact on members of the health care staff, the “second victims” of the traumatic events that occur while caring for the women in the maternity ward. “The estimated prevalence of PTSD symptoms among midwives is 22.9%,” which could lead to “a loss of confidence and a desire to leave the profession.”
Providing psychoeducation to health care staff
Dr. Franchitto believes that it’s essential to also protect caregivers who work in maternity wards. “It’s important to have the support of colleagues” – in particular, of team leaders – “and to share one’s experiences,” as long as one knows how to recognize the symptoms of posttraumatic stress through one’s emotions and is able to verbalize them.
She went on to say that providing psychoeducation to health care staff is therefore to be encouraged, as is “simulation-based training, for learning how to manage problematic situations.”
This content was originally published on Medscape French edition. A translated version appeared on Medscape.com.
Imaging IDs brain activity related to dissociative symptoms
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a neuroimaging study showed that different dissociative symptoms were linked to hyperconnectivity within several key regions of the brain, including the central executive, default, and salience networks as well as decreased connectivity of the central executive and salience networks with other brain areas.
Depersonalization/derealization showed a different brain signature than partially dissociated intrusions, and participants with posttraumatic stress disorder showed a different brain signature, compared with those who had dissociative identity disorder (DID).
“Dissociation is a complex, subjective set of symptoms that are largely experienced internally and, contrary to media portrayal, are not usually overtly observable,” lead author Lauren Lebois, PhD, director of the Dissociative Disorders and Trauma Research Program, McLean Hospital, Belmont, Mass., and assistant professor of psychiatry at Harvard Medical School, Boston, told this news organization.
“However, we have shown that you can objectively measure dissociation and link it to robust brain signatures. We hope these results will encourage clinicians to screen for dissociation and approach reports of these experiences seriously, empathetically, and with awareness that they can be treated effectively,” Dr. Lebois said.
The findings were published online in Neuropsychopharmacology.
Detachment, discontinuity
Pathological dissociation is “the experience of detachment from or discontinuity in one’s internal experience, sense of self, or surroundings” and is common in the aftermath of trauma, the investigators write.
Previous research into trauma-related pathological dissociation suggests it encompasses a range of experiences or “subtypes,” some of which frequently occur in PTSD and DID.
“Depersonalization and derealization involve feelings of detachment or disconnection from one’s sense of self, body, and environment,” the current researchers write. “Individuals report feeling like their body or surroundings are unreal or like they are in a movie.”
Dissociation also includes “experiences of self-alteration common in DID, in which people lose a sense of agency and ownership over their thoughts, emotions, actions, and body [and] experience some thoughts, emotions, etc. as partially dissociated intrusions,” Dr. Lebois said.
She added that dissociative symptoms are “common and disabling.” And dissociation and severe dissociative disorders such as DID “remain at best underappreciated and, at worst, frequently go undiagnosed or misdiagnosed,” with a high cost of stigmatization and misunderstanding preventing individuals from accessing effective treatment.
In addition, “given that DID disproportionately affects women, gender disparity is an important issue in this context,” Dr. Lebois noted.
Her team was motivated to conduct the study “to learn more about how different types of dissociation manifest in brain activity and to help combat the stigma around dissociation and DID.”
Filling the gap
The investigators drew on the “Triple Network” model of psychopathology, which “offers an integrative framework based in systems neuroscience for understanding cognitive and affective dysfunction across psychiatric conditions,” they write.
This model “implicates altered intrinsic organization and interactions between three large-scale brain networks across disorders,” they add.
The brain networks included in the study were the right-lateralized central executive network (rCEN), with the lateral frontoparietal brain region; the medial temporal subnetwork of the default network (tDN), with the medial frontoparietal brain region; and the cingulo-opercular subnetwork (cSN), with the midcingulo-insular brain region.
Previous neuroimaging research into dissociative disorders has implicated altered connectivity in these regions. However, although previous studies covered dissociation subtypes, they did not directly compare these subtypes. This study was designed to fill that gap, the investigators note.
They assessed 91 women with and without a history of childhood trauma, current PTSD, and with varying degrees of dissociation.
This included 19 with conventional PTSD (mean age, 33.4 years), 18 with PTSD dissociative subtype (mean age, 29.5 years), 26 with DID (mean age, 37.4 years), and 28 who acted as the healthy control group (mean age, 32 years).
Participants completed several scales regarding symptoms of PTSD, dissociation, and childhood trauma. They also underwent functional magnetic resonance imaging. Covariates included age, childhood maltreatment, and PTSD severity.
Connectivity alterations
Results showed the rCEN was “most impacted” by pathological dissociation, with 39 clusters linked to connectivity alterations.
Ten clusters within tDN exhibited within-network hyperconnectivity related to dissociation but only of the depersonalization/derealization subtype.
Eight clusters within cSN were linked to dissociation – specifically, within-network hyperconnectivity and decreased connectivity between regions in rCEN with cSN, with “no significant unique contributions of dissociation subtypes,” the researchers report.
“Depersonalization and derealization symptoms were associated with increased communication between a brain network involved in reasoning, attention, inhibition, and working memory and a brain region implicated in out-of-body experiences. This may, in part, contribute to depersonalization/derealization feelings of detachment, strangeness or unreality experienced with your body and surroundings,” Dr. Lebois said.
“In contrast, partially dissociated intrusion symptoms central to DID were linked to increased communication between a brain network involved in autobiographical memory and your sense of self and a brain network involved in reasoning, attention, inhibition, and working memory,” she added.
She noted that this matches how patients with DID describe their mental experiences: as sometimes feeling as if they lost a sense of ownership over their own thoughts and feelings, which can “intrude into their mental landscape.”
In the future, Dr. Lebois hopes that “we may be able to monitor dissociative brain signatures during psychotherapy to help assess recovery or relapse, or we could target brain activity directly with neurofeedback or neuromodulatory techniques as a dissociation treatment in and of itself.”
A first step?
Commenting on the study, Richard Loewenstein, MD, adjunct professor, department of psychiatry, University of Maryland School of Medicine, Baltimore, called the paper a “first step in more sophisticated studies of pathological dissociation using cutting-edge concepts of brain connectivity, methodology based on naturalistic, dimensional symptoms categories, and innovative statistical methods.”
Dr. Loewenstein, who was not involved with the current study, added that there is an “oversimplified conflation of hallucinations and other symptoms of dissociation with psychosis.” So studies may “incorrectly relate phenomena such as racism-based trauma to psychosis, rather than pathological dissociation and racism-based PTSD,” he said.
He noted that the implications are “profound, as pathological dissociation is not treatable with antipsychotic medications and requires treatment with psychotherapy specifically targeting symptoms of pathological dissociation.”
The study was funded by the Julia Kasparian Fund for Neuroscience Research and the National Institute of Mental Health. Dr. Lebois reported unpaid membership on the Scientific Committee for the International Society for the Study of Trauma and Dissociation, grant support from the NIMH and the Julia Kasparian Fund for Neuroscience Research, and spousal IP payments from Vanderbilt University for technology licensed to Acadia Pharmaceuticals unrelated to the present work. The other investigators’ disclosures are listed in the original paper. Dr. Loewenstein has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Higher rates of PTSD, BPD in transgender vs. cisgender psych patients
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL PSYCHIATRY
Sexual assault–related visits to the ED are on the rise
Data from the Federal Bureau of Investigation show an increase in reported rapes and sexual assaults (SAs) since 2006, and studies of victims show an increased risk of conditions such as suicidal ideation, PTSD, depression, substance use, and chronic conditions, write Emily L. Vogt of the University of Michigan, Ann Arbor, and colleagues.
However, trends and disparities in ED use by adults seeking care following SA have not been explored, they said.
For a study that was published in JAMA Network Open, researchers reviewed data from the Nationwide Emergency Department Sample (NEDS), a large, nationally representative database managed by the Agency for Healthcare Research and Quality. The dataset consisted of 120 million to 143 million weighted ED visits reported annually from 2006 through 2016. The study population included adults aged 18-65 years who had made an ED visit that was recorded in the NEDS and that was coded as an SA. SA was defined using ICD-9 codes until the fourth quarter of 2015, at which time ICD-10 codes came into use.
Overall, the number of SA-related ED visits increased by 1,533.0% during the study period, from 3,607 in 2006 to 55,296 in 2019. The average annual percentage change was 23.0% (P < .001). The greatest increase occurred from 2015 to 2016, when annual visits increased from 17,709 to 47,732. This increase likely reflected the updated ICD-10 codes, in which there are categories for suspected adult rape, confirmed adult rape, and adult forced sexual exploitation, the researchers note.
Patients presenting to the ED after an SA were mainly women (91.5%). Individuals aged 18-25 years accounted for nearly half of the presentations. Individuals in the lowest and second-lowest income quartiles also were overrepresented.
Despite the increased presentation to EDs, admission rates for SA decreased, from 12.6% to 4.3%, the researchers note. Patients who were older and were insured through Medicaid were more likely to be admitted than persons of other demographic groups.
The researchers also found that increases in ED presentations outpaced increases in SA reports to law enforcement. They compared the ED trends with FBI-reported rapes/SAs from 2015 to 2019 and found increases of 7% and 22% during the times of ICD-9 and ICD-10 codes, respectively. However, in 2019, the number of SA survivors who sought ED care remained below the number who reported to law enforcement (55,296 vs. 139,815, as determined on the basis of revised SA definitions).
“Although the association between increased coding specificity and documentation of SA is still unclear, ICD-10 likely contributed to increased ED documentation of SA,” but the data show steady increases that are independent of the coding change, the researchers write.
The study findings were limited by several factors, including the potential for multiple representations of patients, coding errors associated with the NEDS database, and the reliance on voluntary reports in the NEDS and FBI datasets, the researchers note. The results were strengthened by the large, diverse sample size and by the inclusion of hospital admissions and crime data for comparison, they say.
“As few as 21% of survivors seek medical care after SA, meaning that the survivors captured in this study represent a fraction of total SA-related care need,” the researchers write. “Our finding that most SA ED visits are by young, female, and low-income survivors can inform policy changes to better support these individuals,” which could include the development of outpatient and longitudinal care settings to better serve these populations, they conclude.
Better understanding not only of the trends underlying SA reporting but also of the demographics of survivors who seek treatment and evaluation after SA is vital, said Robert Glatter, MD, in an interview.
“Being able to better understand how social and societal movements affect a patient’s comfort in reporting an SA is vital in tracking the numbers of people who seek care in the ED,” said Dr. Glatter, an emergency medicine physician at Lenox Hill Hospital at Northwell Health, New York, and also of Hofstra University, Hempstead, N.Y.
Dr. Glatter said he was not surprised by the significant increase in sexual assault presentations, especially in light of increased awareness and the influence of the #MeToo movement and other social justice movements over the past decade.
“While I believe that victims of sexual violence may now feel more empowered to report an assault, the volume of SA that go unreported remains a serious public health issue and concern” in the United States and globally, he emphasized.
A key message from the current study is that there is a need for investment in “compassionate and comprehensive care for all survivors of SA,” Dr. Glatter said. “This includes recognition of the extensive mental health consequences of SA that can lead to not only depression, PTSD, and anxiety but also to suicidal ideation and suicide. The longer-term medical effects become life altering, permeating families and future generations,” he emphasized.
“As a society, we must also place a strong emphasis on caring for all SA survivors, but particularly those who come from economically or socially disadvantaged backgrounds who are uninsured or underinsured,” Dr. Glatter said. Issues of race, gender identity, and sexual identity among SA survivors also must be taken into consideration, he added.
“We need to better understand how our health care system can provide more nuanced follow-up care and reporting for survivors in outpatient settings. … Making access easier, while ensuring confidentiality, will allow more survivors of SA to seek treatment and care,” he said. “We also need to understand how using forensic nurses in this capacity, and beyond the ED, can better serve minority and racially diverse communities” and to increase the recruitment and training of such specialized nurses to care for SA victims, Dr. Glatter noted.
The study was supported by internal funding from the University of Michigan and the department of obstetrics and gynecology. Corresponding author Erica C. Marsh, MD, has received personal fees from Myovant Sciences and Pfizer unrelated to the current study. Dr. Glatter has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the Federal Bureau of Investigation show an increase in reported rapes and sexual assaults (SAs) since 2006, and studies of victims show an increased risk of conditions such as suicidal ideation, PTSD, depression, substance use, and chronic conditions, write Emily L. Vogt of the University of Michigan, Ann Arbor, and colleagues.
However, trends and disparities in ED use by adults seeking care following SA have not been explored, they said.
For a study that was published in JAMA Network Open, researchers reviewed data from the Nationwide Emergency Department Sample (NEDS), a large, nationally representative database managed by the Agency for Healthcare Research and Quality. The dataset consisted of 120 million to 143 million weighted ED visits reported annually from 2006 through 2016. The study population included adults aged 18-65 years who had made an ED visit that was recorded in the NEDS and that was coded as an SA. SA was defined using ICD-9 codes until the fourth quarter of 2015, at which time ICD-10 codes came into use.
Overall, the number of SA-related ED visits increased by 1,533.0% during the study period, from 3,607 in 2006 to 55,296 in 2019. The average annual percentage change was 23.0% (P < .001). The greatest increase occurred from 2015 to 2016, when annual visits increased from 17,709 to 47,732. This increase likely reflected the updated ICD-10 codes, in which there are categories for suspected adult rape, confirmed adult rape, and adult forced sexual exploitation, the researchers note.
Patients presenting to the ED after an SA were mainly women (91.5%). Individuals aged 18-25 years accounted for nearly half of the presentations. Individuals in the lowest and second-lowest income quartiles also were overrepresented.
Despite the increased presentation to EDs, admission rates for SA decreased, from 12.6% to 4.3%, the researchers note. Patients who were older and were insured through Medicaid were more likely to be admitted than persons of other demographic groups.
The researchers also found that increases in ED presentations outpaced increases in SA reports to law enforcement. They compared the ED trends with FBI-reported rapes/SAs from 2015 to 2019 and found increases of 7% and 22% during the times of ICD-9 and ICD-10 codes, respectively. However, in 2019, the number of SA survivors who sought ED care remained below the number who reported to law enforcement (55,296 vs. 139,815, as determined on the basis of revised SA definitions).
“Although the association between increased coding specificity and documentation of SA is still unclear, ICD-10 likely contributed to increased ED documentation of SA,” but the data show steady increases that are independent of the coding change, the researchers write.
The study findings were limited by several factors, including the potential for multiple representations of patients, coding errors associated with the NEDS database, and the reliance on voluntary reports in the NEDS and FBI datasets, the researchers note. The results were strengthened by the large, diverse sample size and by the inclusion of hospital admissions and crime data for comparison, they say.
“As few as 21% of survivors seek medical care after SA, meaning that the survivors captured in this study represent a fraction of total SA-related care need,” the researchers write. “Our finding that most SA ED visits are by young, female, and low-income survivors can inform policy changes to better support these individuals,” which could include the development of outpatient and longitudinal care settings to better serve these populations, they conclude.
Better understanding not only of the trends underlying SA reporting but also of the demographics of survivors who seek treatment and evaluation after SA is vital, said Robert Glatter, MD, in an interview.
“Being able to better understand how social and societal movements affect a patient’s comfort in reporting an SA is vital in tracking the numbers of people who seek care in the ED,” said Dr. Glatter, an emergency medicine physician at Lenox Hill Hospital at Northwell Health, New York, and also of Hofstra University, Hempstead, N.Y.
Dr. Glatter said he was not surprised by the significant increase in sexual assault presentations, especially in light of increased awareness and the influence of the #MeToo movement and other social justice movements over the past decade.
“While I believe that victims of sexual violence may now feel more empowered to report an assault, the volume of SA that go unreported remains a serious public health issue and concern” in the United States and globally, he emphasized.
A key message from the current study is that there is a need for investment in “compassionate and comprehensive care for all survivors of SA,” Dr. Glatter said. “This includes recognition of the extensive mental health consequences of SA that can lead to not only depression, PTSD, and anxiety but also to suicidal ideation and suicide. The longer-term medical effects become life altering, permeating families and future generations,” he emphasized.
“As a society, we must also place a strong emphasis on caring for all SA survivors, but particularly those who come from economically or socially disadvantaged backgrounds who are uninsured or underinsured,” Dr. Glatter said. Issues of race, gender identity, and sexual identity among SA survivors also must be taken into consideration, he added.
“We need to better understand how our health care system can provide more nuanced follow-up care and reporting for survivors in outpatient settings. … Making access easier, while ensuring confidentiality, will allow more survivors of SA to seek treatment and care,” he said. “We also need to understand how using forensic nurses in this capacity, and beyond the ED, can better serve minority and racially diverse communities” and to increase the recruitment and training of such specialized nurses to care for SA victims, Dr. Glatter noted.
The study was supported by internal funding from the University of Michigan and the department of obstetrics and gynecology. Corresponding author Erica C. Marsh, MD, has received personal fees from Myovant Sciences and Pfizer unrelated to the current study. Dr. Glatter has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the Federal Bureau of Investigation show an increase in reported rapes and sexual assaults (SAs) since 2006, and studies of victims show an increased risk of conditions such as suicidal ideation, PTSD, depression, substance use, and chronic conditions, write Emily L. Vogt of the University of Michigan, Ann Arbor, and colleagues.
However, trends and disparities in ED use by adults seeking care following SA have not been explored, they said.
For a study that was published in JAMA Network Open, researchers reviewed data from the Nationwide Emergency Department Sample (NEDS), a large, nationally representative database managed by the Agency for Healthcare Research and Quality. The dataset consisted of 120 million to 143 million weighted ED visits reported annually from 2006 through 2016. The study population included adults aged 18-65 years who had made an ED visit that was recorded in the NEDS and that was coded as an SA. SA was defined using ICD-9 codes until the fourth quarter of 2015, at which time ICD-10 codes came into use.
Overall, the number of SA-related ED visits increased by 1,533.0% during the study period, from 3,607 in 2006 to 55,296 in 2019. The average annual percentage change was 23.0% (P < .001). The greatest increase occurred from 2015 to 2016, when annual visits increased from 17,709 to 47,732. This increase likely reflected the updated ICD-10 codes, in which there are categories for suspected adult rape, confirmed adult rape, and adult forced sexual exploitation, the researchers note.
Patients presenting to the ED after an SA were mainly women (91.5%). Individuals aged 18-25 years accounted for nearly half of the presentations. Individuals in the lowest and second-lowest income quartiles also were overrepresented.
Despite the increased presentation to EDs, admission rates for SA decreased, from 12.6% to 4.3%, the researchers note. Patients who were older and were insured through Medicaid were more likely to be admitted than persons of other demographic groups.
The researchers also found that increases in ED presentations outpaced increases in SA reports to law enforcement. They compared the ED trends with FBI-reported rapes/SAs from 2015 to 2019 and found increases of 7% and 22% during the times of ICD-9 and ICD-10 codes, respectively. However, in 2019, the number of SA survivors who sought ED care remained below the number who reported to law enforcement (55,296 vs. 139,815, as determined on the basis of revised SA definitions).
“Although the association between increased coding specificity and documentation of SA is still unclear, ICD-10 likely contributed to increased ED documentation of SA,” but the data show steady increases that are independent of the coding change, the researchers write.
The study findings were limited by several factors, including the potential for multiple representations of patients, coding errors associated with the NEDS database, and the reliance on voluntary reports in the NEDS and FBI datasets, the researchers note. The results were strengthened by the large, diverse sample size and by the inclusion of hospital admissions and crime data for comparison, they say.
“As few as 21% of survivors seek medical care after SA, meaning that the survivors captured in this study represent a fraction of total SA-related care need,” the researchers write. “Our finding that most SA ED visits are by young, female, and low-income survivors can inform policy changes to better support these individuals,” which could include the development of outpatient and longitudinal care settings to better serve these populations, they conclude.
Better understanding not only of the trends underlying SA reporting but also of the demographics of survivors who seek treatment and evaluation after SA is vital, said Robert Glatter, MD, in an interview.
“Being able to better understand how social and societal movements affect a patient’s comfort in reporting an SA is vital in tracking the numbers of people who seek care in the ED,” said Dr. Glatter, an emergency medicine physician at Lenox Hill Hospital at Northwell Health, New York, and also of Hofstra University, Hempstead, N.Y.
Dr. Glatter said he was not surprised by the significant increase in sexual assault presentations, especially in light of increased awareness and the influence of the #MeToo movement and other social justice movements over the past decade.
“While I believe that victims of sexual violence may now feel more empowered to report an assault, the volume of SA that go unreported remains a serious public health issue and concern” in the United States and globally, he emphasized.
A key message from the current study is that there is a need for investment in “compassionate and comprehensive care for all survivors of SA,” Dr. Glatter said. “This includes recognition of the extensive mental health consequences of SA that can lead to not only depression, PTSD, and anxiety but also to suicidal ideation and suicide. The longer-term medical effects become life altering, permeating families and future generations,” he emphasized.
“As a society, we must also place a strong emphasis on caring for all SA survivors, but particularly those who come from economically or socially disadvantaged backgrounds who are uninsured or underinsured,” Dr. Glatter said. Issues of race, gender identity, and sexual identity among SA survivors also must be taken into consideration, he added.
“We need to better understand how our health care system can provide more nuanced follow-up care and reporting for survivors in outpatient settings. … Making access easier, while ensuring confidentiality, will allow more survivors of SA to seek treatment and care,” he said. “We also need to understand how using forensic nurses in this capacity, and beyond the ED, can better serve minority and racially diverse communities” and to increase the recruitment and training of such specialized nurses to care for SA victims, Dr. Glatter noted.
The study was supported by internal funding from the University of Michigan and the department of obstetrics and gynecology. Corresponding author Erica C. Marsh, MD, has received personal fees from Myovant Sciences and Pfizer unrelated to the current study. Dr. Glatter has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Complex trauma in the perinatal period
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5
Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.
Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5

Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.

Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
Complex posttraumatic stress disorder (CPTSD) is a condition characterized by classic trauma-related symptoms in addition to disturbances in self organization (DSO).1-3 DSO symptoms include negative self-concept, emotional dysregulation, and interpersonal problems. CPTSD differs from PTSD in that it includes symptoms of DSO, and differs from borderline personality disorder (BPD) in that it does not include extreme self-injurious behavior, a complete lack of sense of self, and avoidance of rejection or abandonment (Table1,2). The maladaptive traits of CPTSD are often the result of a chronic lack of safety in early childhood, particularly childhood sexual abuse (CSA). CSA may affect up to 20% of women and is defined by the CDC as “any completed or attempted sexual act, sexual contact with, or exploitation of a child by a caregiver.”4,5

Maternal lifetime trauma is more common among women who are in low-income minority groups and can lead to adverse birth outcomes in this vulnerable patient population.6 Recent research has found that trauma can increase cortisol levels during pregnancy, leading to increased placental permeability, inflammatory response, and longstanding alterations in the fetal hypothalamic-pituitary adrenal axis.6 A CPTSD diagnosis is of particular interest during the perinatal period because CPTSD is often a response to interpersonal trauma and attachment adversity, which can be reactivated during the perinatal period.7 CPTSD in survivors of CSA can be exacerbated due to feelings of disempowerment secondary to loss of bodily control throughout pregnancy, childbirth, breastfeeding, and obstetrical exams.5,8 Little is known about perinatal CPTSD, but we can extrapolate from trauma research that it is likely associated with the worsening of other maternal mental health conditions, suicidality, physical complaints, quality of life, maternal-child bonding outcomes, and low birth weight in offspring.5,9,10
Although there are no consensus guidelines on how to diagnose and treat CPTSD during the perinatal period, or how to promote family functioning thereafter, there are many opportunities for intervention. Mental health clinicians are in a particularly important position to care for women in the perinatal period, as collaborative work with obstetricians, pediatricians, and social services can have long-lasting effects.
In this article, we present cases of 3 CSA survivors who experienced worsening of CPTSD symptoms during the perinatal period and received psychiatric care via telehealth during the COVID-19 pandemic. We also identify best practice approaches and highlight areas for future research.
Case descriptions
Case 1
Ms. A, age 33, is married, has 3 children, has asthma, and is vaccinated against COVID-19. Her psychiatric history includes self-reported dissociative identity disorder and bulimia nervosa. At 2 months postpartum following an unplanned yet desired pregnancy, Ms. A presents to the outpatient clinic after a violent episode toward her husband during sexual intercourse. Since the first trimester of her pregnancy, she has expressed increased anxiety and difficulty sleeping, hypervigilance, intimacy avoidance, and negative views of herself and the world, yet she denies persistent depressive, manic, or psychotic symptoms, other maladaptive personality traits, or substance use. She recalls experiencing similar symptoms during her 2 previous peripartum periods, and attributes it to worsening memories of sexual abuse during childhood. Ms. A has a history of psychiatric hospitalizations during adolescence and young adulthood for suicidal ideation. She had been treated with various medications, including chlorpromazine, lamotrigine, carbamazepine, and clonazepam, but self-discontinued these medications in 2016 because she felt they were ineffective. Since becoming a mother, she has consistently denied depressive symptoms or suicidal ideation, and intermittently engaged in interpersonal psychotherapy targeting her conflictual relationship with her husband and parenting struggles.
Ms. A underwent an induced vaginal delivery at 36 weeks gestation due to preeclampsia and had success with breastfeeding. While engaging in sexual activity for the first time postpartum, she dissociated and later learned she had forcefully grabbed her husband’s neck for several seconds but did not cause any longstanding physical damage. Upon learning of this episode, Ms. A’s psychiatrist asks her to complete the International Trauma Questionnaire (ITQ), a brief self-report measure developed for the assessment of the ICD-11 diagnosis of CPTSD (Figure11). Ms. A also completes the PTSD Checklist for DSM-5 (PCL-5), the Dissociative Experiences Scale, and the Edinburgh Postnatal Depression Scale (EPDS) to assist with assessing her symptoms.12-15 The psychiatrist uses ICD-11 criteria to diagnose Ms. A with CPTSD, given her functional impairment associated with both PTSD and DSO symptoms, which have acutely worsened during the perinatal period.

Ms. A initially engages in extensive trauma psychoeducation and supportive psychotherapy for 3 months. She later pursues prolonged exposure psychotherapy targeting intimacy, and after 6 months of treatment, improves her avoidance behaviors and marriage.
Continue to: Case 2
Case 2
Ms. R, age 35, is a partnered mother expecting her third child. She has no relevant medical history and is not vaccinated against COVID-19. Her psychiatric history includes self-reported panic attacks and bipolar affective disorder (BPAD). During the second trimester of a desired, unplanned pregnancy, Ms. R presents to an outpatient psychiatry clinic with symptoms of worsening dysphoria and insomnia. She endorses frequent nightmares and flashbacks of CSA as well as remote intimate partner violence. These symptoms, along with hypervigilance, insomnia, anxiety, dysphoria, negative views of herself and her surroundings, and hallucinations of a shadow that whispers “come” when she is alone, worsened during the first trimester of her pregnancy. She recalls experiencing similar trauma-related symptoms during a previous pregnancy but denies a history of pervasive depressive, manic, or psychotic symptoms. She has no other maladaptive personality traits, denies prior substance use or suicidal behavior, and has never been psychiatrically hospitalized or taken psychotropic medications.
Ms. R completes the PCL-5, ITQ, EPDS, and Mood Disorder Questionnaire (MDQ). The results are notable for significant functional impairment related to PTSD and DSO symptoms with minimal concern for BPAD symptoms. The psychiatrist uses ICD-11 criteria to diagnose Ms. R with CPTSD and discusses treatment options with her and her obstetrician. Ms. R is reluctant to take medication until she delivers her baby. She intermittently attends supportive therapy while pregnant. Her pregnancy is complicated by gestational diabetes, and she often misses appointments with her obstetrician and nutritionist.
Ms. R has an uncomplicated vaginal delivery at 38 weeks gestation and success with breastfeeding, but continues to have CPTSD symptoms. She is prescribed quetiapine 25 mg/d for anxiety, insomnia, mood, and psychotic symptoms, but stops taking the medication after 3 days due to excessive sedation. Ms. R is then prescribed sertraline 50 mg/d, which she finds helpful, but has intermittent adherence. She misses multiple virtual appointments with the psychiatrist and does not want to attend in-person sessions due to fear of contracting COVID-19. The psychiatrist encourages Ms. R to get vaccinated, focuses on organizational skills during sessions to promote attendance, and recommends in-person appointments to increase her motivation for treatment and alliance building. Despite numerous outreach attempts, Ms. R is lost to follow-up at 10 months postpartum.
Case 3
Ms. S, age 29, is a partnered mother expecting her fourth child. Her medical history includes chronic back pain. She is not vaccinated against COVID-19, and her psychiatric history includes BPAD. During the first trimester of an undesired, unplanned pregnancy, Ms. S presents to an outpatient psychiatric clinic following an episode where she held a knife over her gravid abdomen during a fight with her partner. She recounts that she became dysregulated and held a knife to her body to communicate her distress, but she did not cut herself, and adamantly denies wanting to hurt herself or the fetus. Ms. S struggles with affective instability, poor frustration tolerance, and irritability. After 1 month of treatment, she discloses surviving prolonged CSA that led to her current nightmares and flashbacks. She also endorses impaired sleep, intimacy avoidance, hypervigilance, impulsive reckless behaviors (including excessive gambling), and negative views about herself and the world that worsened since she learned she was pregnant. Ms. S reports that these same symptoms were aggravated during prior perinatal periods and recalls 2 episodes of severe dysregulation that led to an interrupted suicide attempt and a violent episode toward a loved one. She denies other self-harm behaviors, substance use, or psychotic symptoms, and denies having a history of psychiatric hospitalizations. Ms. S recalls receiving a brief trial of topiramate for BPAD and migraine when she was last in outpatient psychiatric care 8 years ago.
Her psychiatrist administers the PCL-5, ITQ, MDQ, EPDS, and Borderline Symptoms List 23 (BLS-23). The results are notable for significant PTSD and DSO symptoms.16 The psychiatrist diagnoses Ms. S with CPTSD and bipolar II disorder, exacerbated during the peripartum period. Throughout the remainder of her pregnancy, she endorses mood instability with significant irritability but declines pharmacotherapy. Ms. S intermittently engages in psychotherapy using dialectical behavioral therapy (DBT) focusing on distress tolerance because she is unable to tolerate trauma-focused psychotherapy.
Continue to: Ms. S maintains the pregnancy...
Ms. S maintains the pregnancy without any additional complications and has a vaginal delivery at 39 weeks gestation. She initiates breastfeeding but chooses not to continue after 1 month due to fatigue, insomnia, and worsening mood. Her psychiatrist wants to contact Ms. S’s partner to discuss childcare support at night to promote better sleep conditions for Ms. S, but Ms. S declines. Ms. S intermittently attends virtual appointments, adamantly refuses the COVID-19 vaccine, and is fearful of starting a mood stabilizer despite extensive psychoeducation. At 5 months postpartum, Ms. S reports that she is in a worse mood and does not want to continue the appointment or further treatment, and abruptly ends the telepsychiatry session. Her psychiatrist reaches out the following week to schedule an in-person session if Ms. S agrees to wear personal protective equipment, which she is amenable to. During that appointment, the psychiatrist discusses the risks of bipolar depression and CPTSD on both her and her childrens’ development, against the risk of lamotrigine. Ms. S begins taking lamotrigine, which she tolerates without adverse effects, and quickly notices improvement in her mood as the medication is titrated up slowly to 200 mg/d. Ms. S then engages more consistently in psychotherapy and her CPTSD and bipolar II disorder symptoms much improve at 9 months postpartum.
Ensuring an accurate CPTSD diagnosis
These 3 cases illustrate the diversity and complexity of presentations for perinatal CPTSD following CSA. A CPTSD diagnosis is complicated because the differential is broad for those reporting PTSD and DSO symptoms, and CPTSD is commonly comorbid with other disorders such as anxiety and depression.17 While various scales can facilitate PTSD screening, the ITQ is helpful because it catalogs the symptoms of disturbances in self organization and functional impairment inherent in CPTSD. The ITQ can help clinicians and patients conceptualize symptoms and track progress (Figure11).
Once a patient screens positive, a CPTSD diagnosis is best made by the clinician after a full psychiatric interview, similar to other diagnoses. Psychiatrists must use ICD-11 criteria,1 as currently there are no formal DSM-5 criteria for CPTSD.2 Additional scales facilitate CPTSD symptom inventory, such as the PCL-5 to screen and monitor for PTSD symptoms and the BLS-23 to delineate between BPD or DSO symptoms.18 Furthermore, clinicians should screen for other comorbid conditions using additional scales such as the MDQ for BPAD and the EPDS for perinatal mood and anxiety disorders. Sharing a CPTSD diagnosis with a patient is an essential step when initiating treatment. Sensitive psychoeducation on the condition and its application to the perinatal period is key to establishing safety and trust, while also empowering survivors to make their own choices regarding treatment, all essential elements to trauma-informed care.19
A range of treatment options
Once CPTSD is appropriately diagnosed, clinicians must determine whether to use pharmacotherapy, psychotherapy, or both. A meta-analysis by Coventry et al20 sought to determine the best treatment strategies for complex traumatic events such as CSA, Multicomponent interventions were most promising, and psychological interventions were associated with larger effect sizes than pharmacologic interventions for managing PTSD, mood, and sleep. Therapeutic targets include trauma memory processing, self-perception, and dissociation, along with emotion, interpersonal, and somatic regulation.21
Psychotherapy. While there are no standardized guidelines for treating CPTSD, PTSD guidelines suggest using trauma-focused cognitive-behavioral therapy (TF-CBT) as a first-line therapy, though a longer course may be needed to resolve CPTSD symptoms compared to PTSD symptoms.3 DBT for PTSD can be particularly helpful in targeting DSO symptoms.22 Narrative therapy focused on identity, embodiment, and parenting has also shown to be effective for survivors of CSA in the perinatal period, specifically with the goal of meaning-making.5 Therapy can also be effective in a group setting (ie, a “Victim to Survivor” TF-CBT group).23 Sex and couples therapy may be indicated to reestablish trust, especially when it is evident there is sexual inhibition from trauma that influences the relationship, as seen in Case 1.24
Continue to: Pharmacotherapy
Pharmacotherapy. Case 2 and Case 3 both demonstrate that while the peripartum period presents an increased risk for exacerbation of psychiatric symptoms, patients and clinicians may be reluctant to start medications due to concerns for safety during pregnancy or lactation.25 Clinicians must weigh the risks of medication exposure against the risks of exposing the fetus or newborn to untreated psychiatric disease and consult an expert in reproductive psychiatry if questions or concerns arise.26
Adverse effects of psychotropic medications must be considered, especially sedation. Medications that lead to sedation may not be safe or feasible for a mother following delivery, especially if she is breastfeeding. This was exemplified in Case 2, when Ms. R was having troubling hallucinations for which the clinician prescribed quetiapine. The medication resulted in excessive sedation and Ms. R did not feel comfortable performing childcare duties while taking the medication, which greatly influenced future therapy decisions.
Making the decision to prescribe a certain medication for CPTSD is highly influenced by the patient’s most troubling symptoms and their comorbid diagnoses. Selective serotonin reuptake inhibitors (SSRIs) generally are considered safe during pregnancy and breastfeeding, and should be considered as a first-line intervention for PTSD, mood disorders, and anxiety disorders during the perinatal period.27 While prazosin is effective for PTSD symptoms outside of pregnancy, there is limited data regarding its safety during pregnancy and lactation, and it may lead to maternal hypotension and subsequent fetal adverse effects.28
Many patients with a history of CSA experience hallucinations and dissociative symptoms, as demonstrated by Case 1 and Case 2.29 In Case 3, Ms. S displayed features of BPAD with significant hypomanic symptoms and worsening suicidality during prior postpartum periods. The clinician felt comfortable prescribing lamotrigine, a relatively safe medication during the perinatal period compared to other mood stabilizers. Ms. S was amenable to taking lamotrigine, and her clinician avoided the use of an SSRI due to a concern of worsening a bipolar diathesis in this high-risk case.30 Case 2 and Case 3 both highlight the need to closely screen for comorbid conditions such as BPAD and using caution when considering an SSRI in light of the risk of precipitating mania, especially as the patient population is younger and at higher risk for antidepressant-associated mania.31,32
Help patients tap into their sources for strength
Other therapeutic strategies when treating patients with perinatal CPTSD include encouraging survivors to mobilize their support network and sources for strength. Chamberlain et al8 suggest incorporating socioecological and cultural contexts when considering outlets for social support systems and encourage collaborating with families, especially partners, along with community and spiritual networks. As seen in Case 3, clinicians should attempt to speak to family members on behalf of their patients to promote better sleeping conditions, which can greatly alleviate CPTSD and comorbid mood symptoms, and thus reduce suicide risk.33 Sources for strength should be accentuated and clinicians may need to advocate with child protective services to support parenting rights. As demonstrated in Case 1, motherhood can greatly reduce suicide risk, and should be promoted if a child’s safety is not in danger.34
Continue to: Clinicians must recognize...
Clinicians must recognize that patients in the perinatal period face barriers to obtaining health care, especially those with CPTSD, as these patients can be difficult to engage and retain. Each case described in this article challenged the psychiatrist with engagement and alliance-building, stemming from the patient’s CPTSD symptoms of interpersonal difficulties and negative views of surroundings. Case 2 demonstrates how the diagnosis can prevent patients from receiving appropriate prenatal care, while Case 3 shows how clinicians may need more flexible attendance policies and assertive outreach attempts to deliver the mental health care these patients deserve.
These vignettes highlight the psychosocial barriers women face during the perinatal period, such as caring for their child, financial stressors, and COVID-19 pandemic–related factors that can hinder treatment, which can be compounded by trauma. The uncertainty, unpredictability, loss of control, and loss of support structures collectively experienced during the pandemic can be triggering and precipitate worsening CPTSD symptoms.35 Women who experience trauma are less likely to obtain the COVID-19 vaccine for themselves or their children, and this hesitancy is often driven by institutional distrust.36 Policy leaders and clinicians should consider these factors to promote trauma-informed COVID-19 vaccine initiatives and expand mental health access using less orthodox treatment settings, such as telepsychiatry. Telepsychiatry can serve as a bridge to in-person care as patients may feel a higher sense of control when in a familiar home environment. Case 2 and Case 3 exemplify the difficulties of delivering mental health care to perinatal women with CPTSD during the pandemic, especially those who are vaccine-hesitant, and illustrate the importance of adapting a patient’s treatment plan in a personalized and trauma-informed way.
Psychiatrists can help obstetricians and pediatricians by explaining that avoidance patterns and distrust in the clinical setting may be related to trauma and are not grounds for conscious or subconscious punishment or abandonment. Educating other clinicians about trauma-informed care, precautions to use for perinatal patients, and ways to effectively support survivors of CSA can greatly improve health outcomes for perinatal women and their offspring.37
Bottom Line
Complex posttraumatic stress disorder (CPTSD) is characterized by classic PTSD symptoms as well as disturbances in self organization, which can include mood symptoms, psychotic symptoms, and maladaptive personality traits. CPTSD resulting from childhood sexual abuse is of particular concern for women, especially during the perinatal period. Clinicians must know how to recognize the signs and symptoms of CPTSD so they can tailor a trauma-informed treatment plan and promote treatment access in this highly vulnerable patient population.
Related Resources
- Tillman B, Sloan N, Westmoreland P. How COVID-19 affects peripartum women’s mental health. Current Psychiatry. 2021;20(6):18-22. doi:10.12788/cp.0129
- Keswani M, Nemcek S, Rashid N. Is it bipolar disorder, or a complex form of PTSD? Current Psychiatry. 2021;20(12):42-49. doi:10.12788/cp.0188
Drug Brand Names
Carbamazepine • Carbatrol
Clonazepam • Klonopin
Lamotrigine • Lamictal
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Topiramate • Topamax
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
1. World Health Organization. International Classification of Diseases, 11th Revision (ICD-11). Complex posttraumatic stress disorder. Accessed November 6, 2021. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/585833559
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Cloitre M, Garvert DW, Brewin CR, et al. Evidence for proposed ICD-11 PTSD and complexPTSD: a latent profile analysis. Eur J Psychotraumatol. 2013;4:10.3402/ejpt.v4i0.20706. doi:10.3402/ejpt.v4i0.20706
4. Leeb RT, Paulozzi LJ, Melanson C, et al. Child Maltreatment Surveillance: Uniform Definitions for Public Health and Recommended Data Elements, Version 1.0. Centers for Disease Control and Prevention, Department of Health & Human Services; 2008. Accessed August 24, 2022. https://www.cdc.gov/violenceprevention/pdf/cm_surveillance-a.pdf
5. Byrne J, Smart C, Watson G. “I felt like I was being abused all over again”: how survivors of child sexual abuse make sense of the perinatal period through their narratives. J Child Sex Abus. 2017;26(4):465-486. doi:10.1080/10538712.2017.1297880
6. Flom JD, Chiu YM, Hsu HL, et al. Maternal lifetime trauma and birthweight: effect modification by in utero cortisol and child sex. J Pediatr. 2018;203:301-308. doi:10.1016/j.jpeds.2018.07.069
7. Spinazzola J, van der Kolk B, Ford JD. When nowhere is safe: interpersonal trauma and attachment adversity as antecedents of posttraumatic stress disorder and developmental trauma disorder. J Trauma Stress. 2018;31(5):631-642. doi:10.1002/jts.22320
8. Chamberlain C, Gee G, Harfield S, et al. Parenting after a history of childhood maltreatment: a scoping review and map of evidence in the perinatal period. PloS One. 2019;14(3):e0213460. doi:10.1371/journal.pone.0213460
9. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi:10.1016/j.jad.2017.07.045
10. Gavin AR, Morris J. The association between maternal early life forced sexual intercourse and offspring birth weight: the role of socioeconomic status. J Womens Health (Larchmt). 2017;26(5):442-449. doi:10.1089/jwh.2016.5789
11. Cloitre M, Shevlin M, Brewin CR, et al. The international trauma questionnaire: development of a self-report measure of ICD-11 PTSD and complex PTSD. Acta Psychiatr Scand. 2018;138(6):536-546.
12. Cloitre M, Hyland P, Prins A, et al. The international trauma questionnaire (ITQ) measures reliable and clinically significant treatment-related change in PTSD and complex PTSD. Eur J Psychotraumatol. 2021;12(1):1930961. doi:10.1080/20008198.2021.1930961
13. Weathers FW, Litz BT, Keane TM, et al. PTSD Checklist for DSM-5 (PCL-5). US Department of Veterans Affairs. April 11, 2018. Accessed November 25, 2021. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.PDF
14. Dissociative Experiences Scale – II. TraumaDissociation.com. Accessed November 25, 2021. http://traumadissociation.com/des
15. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. doi:10.1192/bjp.150.6.782
16. Mood Disorder Questionnaire (MDQ). Oregon Health & Science University. Accessed November 7, 2021. https://www.ohsu.edu/sites/default/files/2019-06/cms-quality-bipolar_disorder_mdq_screener.pdf
17. Karatzias T, Hyland P, Bradley A, et al. Risk factors and comorbidity of ICD-11 PTSD and complex PTSD: findings from a trauma-exposed population based sample of adults in the United Kingdom. Depress Anxiety. 2019;36(9):887-894. doi:10.1002/da.22934
18. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
19. Fallot RD, Harris M. A trauma-informed approach to screening and assessment. New Dir Ment Health Serv. 2001;(89):23-31. doi:10.1002/yd.23320018904
20. Coventry PA, Meader N, Melton H, et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: systematic review and component network meta-analysis. PLoS Med. 2020;17(8):e1003262. doi:10.1371/journal.pmed.1003262
21. Ford JD. Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Curr Treat Options Psychiatry. 2021;8:1-17. doi:10.1007/s40501-020-00236-6
22. Becker-Sadzio J, Gundel F, Kroczek A, et al. Trauma exposure therapy in a pregnant woman suffering from complex posttraumatic stress disorder after childhood sexual abuse: risk or benefit? Eur J Psychotraumatol. 2020;11(1):1697581. doi:10.1080/20008198.2019.1697581
23. Mendelsohn M, Zachary RS, Harney PA. Group therapy as an ecological bridge to new community for trauma survivors. J Aggress Maltreat Trauma. 2007;14(1-2):227-243. doi:10.1300/J146v14n01_12
24. Macintosh HB, Vaillancourt-Morel MP, Bergeron S. Sex and couple therapy with survivors of childhood trauma. In: Hall KS, Binik YM, eds. Principles and Practice of Sex Therapy. 6th ed. Guilford Press; 2020.
25. Dresner N, Byatt N, Gopalan P, et al. Psychiatric care of peripartum women. Psychiatric Times. 2015;32(12).
26. Zagorski N. How to manage meds before, during, and after pregnancy. Psychiatric News. 2019;54(14):13. https://doi.org/10.1176/APPI.PN.2019.6B36
27. Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397-2407. doi:10.1056/NEJMoa1312828
28. Davidson AD, Bhat A, Chu F, et al. A systematic review of the use of prazosin in pregnancy and lactation. Gen Hosp Psychiatry. 2021;71:134-136. doi:10.1016/j.genhosppsych.2021.03.012
29. Shinn AK, Wolff JD, Hwang M, et al. Assessing voice hearing in trauma spectrum disorders: a comparison of two measures and a review of the literature. Front Psychiatry. 2020;10:1011. doi:10.3389/fpsyt.2019.01011
30. Raffi ER, Nonacs R, Cohen LS. Safety of psychotropic medications during pregnancy. Clin Perinatol. 2019;46(2):215-234. doi:10.1016/j.clp.2019.02.004
31. Martin A, Young C, Leckman JF, et al. Age effects on antidepressant-induced manic conversion. Arch Pediatr Adoles Med. 2004;158(8):773-780. doi:10.1001/archpedi.158.8.773
32. Gill N, Bayes A, Parker G. A review of antidepressant-associated hypomania in those diagnosed with unipolar depression-risk factors, conceptual models, and management. Curr Psychiatry Rep. 2020;22(4):20. doi:10.1007/s11920-020-01143-6
33. Harris LM, Huang X, Linthicum KP, et al. Sleep disturbances as risk factors for suicidal thoughts and behaviours: a meta-analysis of longitudinal studies. Sci Rep. 2020;10(1):13888. doi:10.1038/s41598-020-70866-6
34. Dehara M, Wells MB, Sjöqvist H, et al. Parenthood is associated with lower suicide risk: a register-based cohort study of 1.5 million Swedes. Acta Psychiatr Scand. 2021;143(3):206-215. doi:10.1111/acps.13240
35. Iyengar U, Jaiprakash B, Haitsuka H, et al. One year into the pandemic: a systematic review of perinatal mental health outcomes during COVID-19. Front Psychiatry. 2021;12:674194. doi:10.3389/fpsyt.2021.674194
36. Milan S, Dáu ALBT. The role of trauma in mothers’ COVID-19 vaccine beliefs and intentions. J Pediatr Psychol. 2021;46(5):526-535. doi:10.1093/jpepsy/jsab043
37. Coles J, Jones K. “Universal precautions”: perinatal touch and examination after childhood sexual abuse. Birth. 2009;36(3):230-236. doi:10.1111/j.1523-536X.2009.00327
Sleep kits help foster children manage effects of trauma
A stuffed animal, aromatherapy, a night light. A kit containing these and other items can help children in foster care who have experienced trauma sleep more soundly, a critical step in helping them cope with their emotional distress.
In a new study, researchers at the Children’s Hospital of Philadelphia reported that sleep kits specially tailored to foster children appeared to be helpful in most cases. The kits can be distributed by pediatricians in the office or clinic setting.
“Children who have experienced trauma can have issues with behavior, it can impact their school, and they have difficulties sleeping,” said Kristine Fortin, MD, MPH, director of the fostering health program at Safe Place: Center for Child Protection and Health at CHOP. “I thought, what could a pediatrician do in the office in one visit to help children with sleep?”
Dr. Fortin and colleagues designed sleep kits for both younger children and adolescents.
The version for teenagers contained a sound machine, aromatherapy spray, and a sleep mask. The kits for younger children contained matching stuffed toys to share with someone they felt connected to, and a rechargeable night-light. All kits included written materials about sleep hygiene, a journal, and directions for downloading a free age-appropriate relaxation app for belly breathing or a PTSD Coach app from the Department of Veterans Affairs to manage symptoms of trauma.
In a pilot study presented at the annual meeting of the American Academy of Pediatrics, Dr. Fortin and colleagues surveyed caregivers in foster homes about their use of the kits.
Of the 20 foster parents who responded to the survey, 11 said the kits helped “very much,” 5 others reported they helped “somewhat,” another 2 reported no improvements in sleep, and 2 said they didn’t know the effect. The children for whom results were unknown moved from the home without the sleep kit or had difficulties communicating with the foster parents, resulting in incomplete assessment, according to the researchers.
Night-lights were used most in the kits, followed by the stuffed toys, sound machines, and sleep journals.
Dr. Fortin said existing resources like sleep therapy or medication can be costly or difficult to find, and many pediatricians don’t have enough time during wellness visits to address symptoms like sleep deprivation. She said the sleep kits could be an alternative to other forms of sleep therapy. “If these sleep kits were effective, and could really help them sleep, then maybe less children would need something like medication.”
Dr. Fortin said the kits her group has designed are tailored specifically for children with symptoms of trauma, or with difficult emotions associated with foster care.
“We’ve tried to design something that can be really practical and easy to use in a pediatric visit, where there’s a lot of written information that can be discussed with the child and their family,” Dr. Fortin said.
She added that she would like to see clinicians give out the sleep kits during in-office visits.
“These sleep issues are common in foster children,” she said. “We felt it was important to do an intervention.”
Kristina Lenker, PhD, a sleep psychologist at Penn State Health, Hershey, said children in foster care often struggle with falling or staying asleep, an inability to sleep alone, nightmares, and bed wetting.
“Sleep kits can be particularly helpful for these children, given how they can help caregivers to provide a safe sleep environment and predictable routine, and send messages of safety and comfort at bedtime, with tangible objects, and enable children to feel a sense of control,” she said.
Charitable organizations like Pajama Program and Sleeping Children Around the World provide sleep kits to children from underserved backgrounds. But Candice Alfano, PhD, director of the University of Houston’s Sleep and Anxiety Center of Houston, said the CHOP kits are the first to specifically target sleeping difficulties in foster children.
“Sleep is a largely neglected yet essential area of health, development, and well-being in this highly-vulnerable population of youth, so I am very excited to see this work being done,” Dr. Alfano said in an interview.
Dr. Fortin and Dr. Lenker reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A stuffed animal, aromatherapy, a night light. A kit containing these and other items can help children in foster care who have experienced trauma sleep more soundly, a critical step in helping them cope with their emotional distress.
In a new study, researchers at the Children’s Hospital of Philadelphia reported that sleep kits specially tailored to foster children appeared to be helpful in most cases. The kits can be distributed by pediatricians in the office or clinic setting.
“Children who have experienced trauma can have issues with behavior, it can impact their school, and they have difficulties sleeping,” said Kristine Fortin, MD, MPH, director of the fostering health program at Safe Place: Center for Child Protection and Health at CHOP. “I thought, what could a pediatrician do in the office in one visit to help children with sleep?”
Dr. Fortin and colleagues designed sleep kits for both younger children and adolescents.
The version for teenagers contained a sound machine, aromatherapy spray, and a sleep mask. The kits for younger children contained matching stuffed toys to share with someone they felt connected to, and a rechargeable night-light. All kits included written materials about sleep hygiene, a journal, and directions for downloading a free age-appropriate relaxation app for belly breathing or a PTSD Coach app from the Department of Veterans Affairs to manage symptoms of trauma.
In a pilot study presented at the annual meeting of the American Academy of Pediatrics, Dr. Fortin and colleagues surveyed caregivers in foster homes about their use of the kits.
Of the 20 foster parents who responded to the survey, 11 said the kits helped “very much,” 5 others reported they helped “somewhat,” another 2 reported no improvements in sleep, and 2 said they didn’t know the effect. The children for whom results were unknown moved from the home without the sleep kit or had difficulties communicating with the foster parents, resulting in incomplete assessment, according to the researchers.
Night-lights were used most in the kits, followed by the stuffed toys, sound machines, and sleep journals.
Dr. Fortin said existing resources like sleep therapy or medication can be costly or difficult to find, and many pediatricians don’t have enough time during wellness visits to address symptoms like sleep deprivation. She said the sleep kits could be an alternative to other forms of sleep therapy. “If these sleep kits were effective, and could really help them sleep, then maybe less children would need something like medication.”
Dr. Fortin said the kits her group has designed are tailored specifically for children with symptoms of trauma, or with difficult emotions associated with foster care.
“We’ve tried to design something that can be really practical and easy to use in a pediatric visit, where there’s a lot of written information that can be discussed with the child and their family,” Dr. Fortin said.
She added that she would like to see clinicians give out the sleep kits during in-office visits.
“These sleep issues are common in foster children,” she said. “We felt it was important to do an intervention.”
Kristina Lenker, PhD, a sleep psychologist at Penn State Health, Hershey, said children in foster care often struggle with falling or staying asleep, an inability to sleep alone, nightmares, and bed wetting.
“Sleep kits can be particularly helpful for these children, given how they can help caregivers to provide a safe sleep environment and predictable routine, and send messages of safety and comfort at bedtime, with tangible objects, and enable children to feel a sense of control,” she said.
Charitable organizations like Pajama Program and Sleeping Children Around the World provide sleep kits to children from underserved backgrounds. But Candice Alfano, PhD, director of the University of Houston’s Sleep and Anxiety Center of Houston, said the CHOP kits are the first to specifically target sleeping difficulties in foster children.
“Sleep is a largely neglected yet essential area of health, development, and well-being in this highly-vulnerable population of youth, so I am very excited to see this work being done,” Dr. Alfano said in an interview.
Dr. Fortin and Dr. Lenker reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A stuffed animal, aromatherapy, a night light. A kit containing these and other items can help children in foster care who have experienced trauma sleep more soundly, a critical step in helping them cope with their emotional distress.
In a new study, researchers at the Children’s Hospital of Philadelphia reported that sleep kits specially tailored to foster children appeared to be helpful in most cases. The kits can be distributed by pediatricians in the office or clinic setting.
“Children who have experienced trauma can have issues with behavior, it can impact their school, and they have difficulties sleeping,” said Kristine Fortin, MD, MPH, director of the fostering health program at Safe Place: Center for Child Protection and Health at CHOP. “I thought, what could a pediatrician do in the office in one visit to help children with sleep?”
Dr. Fortin and colleagues designed sleep kits for both younger children and adolescents.
The version for teenagers contained a sound machine, aromatherapy spray, and a sleep mask. The kits for younger children contained matching stuffed toys to share with someone they felt connected to, and a rechargeable night-light. All kits included written materials about sleep hygiene, a journal, and directions for downloading a free age-appropriate relaxation app for belly breathing or a PTSD Coach app from the Department of Veterans Affairs to manage symptoms of trauma.
In a pilot study presented at the annual meeting of the American Academy of Pediatrics, Dr. Fortin and colleagues surveyed caregivers in foster homes about their use of the kits.
Of the 20 foster parents who responded to the survey, 11 said the kits helped “very much,” 5 others reported they helped “somewhat,” another 2 reported no improvements in sleep, and 2 said they didn’t know the effect. The children for whom results were unknown moved from the home without the sleep kit or had difficulties communicating with the foster parents, resulting in incomplete assessment, according to the researchers.
Night-lights were used most in the kits, followed by the stuffed toys, sound machines, and sleep journals.
Dr. Fortin said existing resources like sleep therapy or medication can be costly or difficult to find, and many pediatricians don’t have enough time during wellness visits to address symptoms like sleep deprivation. She said the sleep kits could be an alternative to other forms of sleep therapy. “If these sleep kits were effective, and could really help them sleep, then maybe less children would need something like medication.”
Dr. Fortin said the kits her group has designed are tailored specifically for children with symptoms of trauma, or with difficult emotions associated with foster care.
“We’ve tried to design something that can be really practical and easy to use in a pediatric visit, where there’s a lot of written information that can be discussed with the child and their family,” Dr. Fortin said.
She added that she would like to see clinicians give out the sleep kits during in-office visits.
“These sleep issues are common in foster children,” she said. “We felt it was important to do an intervention.”
Kristina Lenker, PhD, a sleep psychologist at Penn State Health, Hershey, said children in foster care often struggle with falling or staying asleep, an inability to sleep alone, nightmares, and bed wetting.
“Sleep kits can be particularly helpful for these children, given how they can help caregivers to provide a safe sleep environment and predictable routine, and send messages of safety and comfort at bedtime, with tangible objects, and enable children to feel a sense of control,” she said.
Charitable organizations like Pajama Program and Sleeping Children Around the World provide sleep kits to children from underserved backgrounds. But Candice Alfano, PhD, director of the University of Houston’s Sleep and Anxiety Center of Houston, said the CHOP kits are the first to specifically target sleeping difficulties in foster children.
“Sleep is a largely neglected yet essential area of health, development, and well-being in this highly-vulnerable population of youth, so I am very excited to see this work being done,” Dr. Alfano said in an interview.
Dr. Fortin and Dr. Lenker reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAP 2022
‘Disturbing’ lack of follow-up care after psychiatric crises
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is a concerning lack of follow-up care for young people who experience a mental health crisis, new research suggests.
The follow-up rate was less than 30% for those who had visited an ED.
The strongest predictor of follow-up was having received both primary and mental health care during the 6 months prior to using the acute service.
“For people discharging folks after a psychiatric crisis, whether it be in a hospital or emergency room setting, connecting them with their outpatient provider to ensure the transfer of care and continuity of care is vitally important to reduce risks for this population,” coinvestigator Brian Skehan, MD, PhD, assistant professor and psychiatrist, University of Massachusetts, Worcester, said during a press briefing.
If these discharged patients do not have a provider, “make sure they get one,” Lisa Dixon, MD, editor-in-chief of Psychiatric Services, added during the same briefing. “That’s the gift of life potentially for these young people.”
The findings were published online in Psychiatric Services.
Alarming trends
The alarming suicide trends among youths were exacerbated by the COVID-19 pandemic, Dr. Skehan noted.
He cited a 2021 study that showed more than 44% of high school students experienced persistent sadness or hopelessness over the previous year, 1 in 5 seriously considered suicide, and almost 1 in 10 actually attempted suicide.
“When we look at the number of young adults and adolescents struggling with behavioral health issues, the data trend is disturbing nationwide,” Dr. Skehan said.
The current study included participants aged 12-27 years who had private insurance. Many youth in this age category are experiencing significant changes, such as moving from high school to college and from pediatric providers to adult providers – and some “get lost in this transition,” said Dr. Skehan.
He noted many inpatient psychiatric units are not geared to young adults. “They may miss out on some aspects of inpatient care because it’s not geared to their developmental stage,” he said.
Assessing U.S. patient data in the IBM MarketScan commercial database (2013-2018), the researchers created two study samples: 95,153 inpatients and 108,576 patients who used the ED. All had an acute event stemming from a mental health condition.
The investigators explored the role of “established” outpatient care, defined as having had at least one visit with a provider of primary or mental health care in the 6 months prior to the acute psychiatric event.
Covariates included age at time of service (aged 12-17 years or 18-27 years), gender, health care plan type, psychiatric diagnosis, whether the acute event was self-harm or suicide related, and medical complexity.
Low follow-up rates
In the inpatient group, the average age was 18.9 years, the most common length of hospital stay was 4-6 days, and 1.5% left against medical advice. The most common primary diagnosis was major depression (53.7%), followed by bipolar disorder (22.3%). The least common disorders were PTSD, comorbid eating disorders, and disruptive disorders.
About one-third of participants had used both primary and mental health care during the 6 months before hospitalization, whereas 22.8% had no established outpatient care. Established care was most common among those with comorbid eating disorders and least common among those with psychotic disorders.
Results showed 42.7% of the hospitalized patients received follow up within 7 days and 67.4% received follow up within 30 days.
The strongest predictor of mental health follow-up care was established outpatient care. Compared with those who had no such care, those who had received both primary care and mental health care before the acute event had the highest odds of receiving follow-up (within 7 days, adjusted odds ratio, 2.81; 95% confidence interval, 2.68-2.94).
Older age and leaving against medical advice were associated with decreased likelihood of follow-up. Female sex, hospitalizations related to self-harm or suicidality, and longer length of stay were associated with increased likelihood of mental health follow-up care.
Compared with those hospitalized for major depression, those hospitalized for schizophrenia, bipolar disorder, PTSD, disruptive disorders, or comorbid substance use disorder were less likely to receive mental health follow-up. For example, only 23.7% of youth with comorbid substance use discharged from the hospital had follow-up within 7 days.
Similar patterns were observed for 30-day follow-up care.
‘Accessible and appealing’ options needed
In the ED-visit group, the average age was 19.5 years (58% female). Most (70.4%) had no chronic health conditions other than a psychiatric disorder. The primary diagnoses were anxiety disorders or phobias (44.1%) and major depression (23%).
One in four visits included a code for self-harm, suicidal ideation, or suicide attempt. And almost one third lacked established outpatient care before the ED visit.
Results showed 28.6% of the ED group received mental health care follow-up within 7 days and 46.4% received it within 30 days.
Again, the strongest predictor of mental health follow-up was prior outpatient care. For example, compared with participants with no established outpatient care, those with both primary care and mental health care were the most likely to receive follow-up within 7 days (aOR, 4.06; 95% CI, 3.72-4.42).
These numbers “are far from the goal of making sure everybody is getting follow-up care within 7 days of an acute psychiatric event,” Dr. Skehan said.
He stressed the need for “accessible and appealing options for youth.” These could include telehealth services, improved communication among health care providers in the ED, and reducing barriers to access follow-up care.
“This probably highlights the need to have more case management and referral services, and maybe make sure patients have a follow-up appointment before they leave the emergency room,” said Dr. Skehan. “This doesn’t necessarily guarantee they’ll get there but hopefully it makes it more likely they will have that access should they need it.”
The study was funded by grants from the National Institute of General Medical Sciences and the National Center for Advancing Translational Sciences, from the National Institutes of Health. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES