User login
Stress and infertility – is it a proven cause and effect?
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.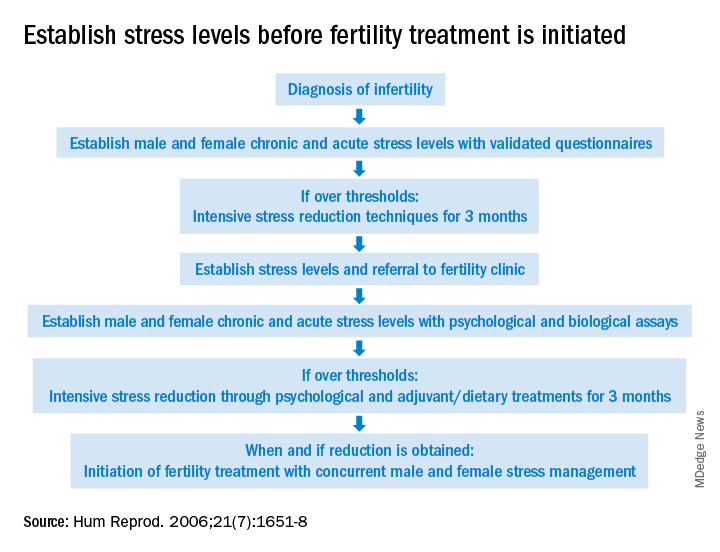
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
“Just relax, stop thinking about it and, more than likely, it will happen.” If ever there was a controversial subject in medicine, especially in reproduction, the relationship between stress and infertility would be high on the list. Who among us has not overheard or even personally shared with an infertility patient that they should try and reduce their stress to improve fertility? The theory is certainly not new. Hippocrates, back in the 5th century B.C., was one of the first to associate a woman’s psychological state with her reproductive potential. His contention was that a physical sign of psychological stress in women (which scholars later dubbed “hysteria”) could result in sterility. In medieval times, a German abbess and mystic named Hildegard of Bingen posited women suffering from melancholy – a condition that we today might call depression – were infertile as a result.
The deeper meaning behind the flippant advice to relax is implicit blame; that is, a woman interprets the link of stress and infertility as a declaration that she is sabotaging reproduction. Not only is this assumption flawed, but it does further damage to a woman’s emotional fragility. To provide the presumption of stress affecting reproduction, a recent survey of over 5,000 infertility patients found, remarkably, 98% considered emotional stress as either a cause or a contributor to infertility, and 31% believed stress was a cause of miscarriage, although racial differences existed (J Assist Reprod Genet. 2021 Apr;38[4]:877-87). This relationship was mostly seen in women who used complementary and alternative medicine, Black women, and those who frequented Internet search engines. Whereas women who had a professional degree, had more infertility insurance coverage, and were nonreligious were less likely to attribute stress to infertility. Intriguingly, the more engaged the physicians, the less patients linked stress with infertility, while the contrary also applied.
The power of stress can be exemplified by the pathophysiology of amenorrhea. Functional hypothalamic amenorrhea is the most common cause of the female athlete triad of secondary amenorrhea in women of childbearing age. It is a reversible disorder caused by stress related to weight loss, excessive exercise and/or traumatic mental experiences (Endocrines. 2021;2:203-11). Stress of infertility has also been demonstrated to be equivalent to a diagnosis of cancer and other major medical morbidities (J Psychosom Obstet Gynaecol. 1993;14[Suppl]:45-52).
A definitive link between stress and infertility is evasive because of the lack of controlled, prospective longitudinal studies and the challenge of reducing variables in the analysis. The question remains which developed initially – the stress or the infertility? Infertility treatment is a physical, emotional, and financial investment. Stress and the duration of infertility are correlative. The additive factor is that poor insurance coverage for costly fertility treatment can not only heighten stress but, concurrently, subject the patient to the risk of exploitation driven by desperation whereby they accept unproven “add-ons” offered with assisted reproductive technologies (ART).
Both acute and chronic stress affect the number of oocytes retrieved and fertilized with ART as well as live birth delivery and birth weights (Fertil Steril. 2001;76:675-87). Men are also affected by stress, which is manifested by decreased libido and impaired semen, further compromised as the duration of infertility continues. The gut-derived hormone ghrelin appears to play a role with stress and reproduction (Endocr Rev. 2017;38:432-67).
As the relationship between stress and infertility is far from proven, there are conflicting study results. Two meta-analyses failed to show any association between stress and the outcomes of ART cycles (Hum Reprod. 2011;26:2763-76; BMJ. 2011;342:d223). In contrast, a recent study suggested stress during infertility treatment was contributed by the variables of low spousal support, financial constraints, and social coercion in the early years of marriage (J Hum Reprod Sci. 2018;11:172-9). Emotional distress was found to be three times greater in women whose families had unrealistic expectations from treatments.
Fortunately, psychotherapy during the ART cycle has demonstrated a benefit in outcomes. Domar revealed psychological support and cognitive behavior therapy resulted in higher pregnancy rates than in the control group (Fertil Steril. 2000;73:805-12). Another recent study appears to support stress reduction improving reproductive potential (Dialogues Clin Neurosci. 2018;20[1]:41-7).
Given the evidence provided in this article, it behooves infertility clinics to address baseline (chronic) stress and acute stress (because of infertility) prior to initiating treatment (see Figure). While the definitive answer addressing the impact of stress on reproduction remains unknown, we may share with our patients a definition in which they may find enlightenment, “Stress is trying to control an event in which one is incapable.”
Dr. Mark P Trolice is director of Fertility CARE: The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
PCOS common in adolescent girls with type 2 diabetes
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
FROM JAMA NETWORK OPEN
Testes may ‘serve as viral sanctuary’ for SARS-CoV-2, small study shows
, raising questions about potential consequences for reproductive health among those infected.
The study, published online Feb. 8 on the preprint server MedRxiv, found that “patients who become critically ill exhibit severe damages and may harbor the active virus in testes,” which can “serve as a viral sanctuary.”
Guilherme M.J. Costa, PhD, a professor at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, led the study, which has not yet been peer-reviewed.
“A critical point of this article is that the virus was active in the patient’s testis after a long period of infection, indicating that the testis is able to maintain the viable virus for extended periods. It happens for many kinds of viruses in this genital organ,” Dr. Costa said in an interview.
Brian Keith McNeil, MD, vice-chair, department of urology at SUNY Downstate Health Sciences University in New York, told this news organization that the topic of COVID-19 and fertility has been discussed but data are sparse on the subject.
“The question this raises is whether or not COVID can live in the testes, and based on this it seems it can,” he said, adding that it also raises the question of whether COVID-19 could be transmitted through semen. “It leads one to wonder whether this could have a long-term impact on fertility in men and women.”
The authors wrote that deep testicular evaluation of patients who have been infected with COVID-19 is critical because the testes have one of the highest expressions of angiotensin converting enzyme 2 (ACE2) receptors, which play a large role in entrance of the virus into cells.
“A direct influence of SARS-CoV-2 in testicular cells might deregulate ACE2, elevating the levels of angiotensin II, a potent pro-inflammatory and angiogenic peptide,” the authors wrote.
Sperm-producing cells infected
In 2021, the researchers enrolled 11 male patients deceased from COVID-19 complications; none had received a vaccine. Infection was confirmed by SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) performed during their hospital stay. All 11 patients were admitted to the intensive care unit with severe pulmonary symptoms.
All but one of the patients had children and none had scrotal symptoms or complaints during their time in the hospital. Their clinical histories revealed no testicular disorders.
Dr. Costa said they found that detecting SARS-CoV-2 mRNA in testes is difficult in a conventional RT-PCR test.
Therefore, “We modified the protocol of the RT-PCR and used nanosensors. We observed that SARS-CoV-2 has a huge tropism for the testes in this context,” he said.
He said the team performed stainings and “discovered that macrophages and germ cells are highly infected.”
That’s important, he said, because an immune cell, which is supposed to fight the virus, is infected in the tissue. Also, the germ cell, responsible for sperm production, is infected.
“This reopens the worries about the presence of SARS-CoV-2 in semen, as other authors mentioned,” he said.
New findings
The team also found that the testes are a good place for viral replication.
The authors say they are the first to show:
- The longer the severe condition, the lower the number of surviving germ cells.
- There was fluctuation in several essential testicular genes.
- The intratesticular testosterone levels are 30 times reduced in the testes of COVID-19 patients.
The control group was composed of six patients who had undergone testicle removal after prostate cancer was suspected. Collection of both testicles from the test group was performed within 3 hours of death after a family member signed an informed consent document.
Recent research on semen demonstrates that patients who recovered from COVID-19 reestablish their sperm quality after 3 months of infection.
That study, in Fertility and Sterility, found that sperm quality was initially reduced for months in some men after recovery from COVID-19.
The team studied semen samples from 120 Belgian men (mean age, 35 years) at an average 52 days after their last COVID-19 symptoms. The semen was not found to be infectious.
But among 35 men who provided samples within a month after infection, reductions in sperm motility were evident in 60% and sperm counts were reduced in 37%, according to the report.
Testicular damage
The results [of the Costa et al. paper] emphasize the importance of testicular damage in severe COVID-19,” Rafael Kroon Campos, PhD, a postdoctoral fellow in the department of microbiology & immunology at the University of Texas Medical Branch at Galveston, said in an interview.
He noted that other viruses have also been shown to infect or otherwise cause testicular damage or orchitis, such as Zika, Ebola, and the closely related SARS-CoV-1. Sexual transmission has been documented for Zika and Ebola viruses.
Dr. Campos said with SARS-CoV-2, it is unclear whether sexual transmission plays a role.
“Some reports found evidence of viral RNA in semen, but these were rare occurrences. The study by Costa and colleagues used a combination of sensitive techniques and they were able to detect a small amount of viral RNA and viral protein in the testicular tissue of the deceased patients, as well as show viral factories indicating replication of the virus by electron microscopy,” he said.
Dr. Campos said the findings are particularly important and concerning because of the large number of severe cases of COVID-19.
“It is critical to continue to investigate the impact of the disease in testes, including the impact of different variants of concern on testicular damage,” he said.
Dr. McNeil and Dr. Campos have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, raising questions about potential consequences for reproductive health among those infected.
The study, published online Feb. 8 on the preprint server MedRxiv, found that “patients who become critically ill exhibit severe damages and may harbor the active virus in testes,” which can “serve as a viral sanctuary.”
Guilherme M.J. Costa, PhD, a professor at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, led the study, which has not yet been peer-reviewed.
“A critical point of this article is that the virus was active in the patient’s testis after a long period of infection, indicating that the testis is able to maintain the viable virus for extended periods. It happens for many kinds of viruses in this genital organ,” Dr. Costa said in an interview.
Brian Keith McNeil, MD, vice-chair, department of urology at SUNY Downstate Health Sciences University in New York, told this news organization that the topic of COVID-19 and fertility has been discussed but data are sparse on the subject.
“The question this raises is whether or not COVID can live in the testes, and based on this it seems it can,” he said, adding that it also raises the question of whether COVID-19 could be transmitted through semen. “It leads one to wonder whether this could have a long-term impact on fertility in men and women.”
The authors wrote that deep testicular evaluation of patients who have been infected with COVID-19 is critical because the testes have one of the highest expressions of angiotensin converting enzyme 2 (ACE2) receptors, which play a large role in entrance of the virus into cells.
“A direct influence of SARS-CoV-2 in testicular cells might deregulate ACE2, elevating the levels of angiotensin II, a potent pro-inflammatory and angiogenic peptide,” the authors wrote.
Sperm-producing cells infected
In 2021, the researchers enrolled 11 male patients deceased from COVID-19 complications; none had received a vaccine. Infection was confirmed by SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) performed during their hospital stay. All 11 patients were admitted to the intensive care unit with severe pulmonary symptoms.
All but one of the patients had children and none had scrotal symptoms or complaints during their time in the hospital. Their clinical histories revealed no testicular disorders.
Dr. Costa said they found that detecting SARS-CoV-2 mRNA in testes is difficult in a conventional RT-PCR test.
Therefore, “We modified the protocol of the RT-PCR and used nanosensors. We observed that SARS-CoV-2 has a huge tropism for the testes in this context,” he said.
He said the team performed stainings and “discovered that macrophages and germ cells are highly infected.”
That’s important, he said, because an immune cell, which is supposed to fight the virus, is infected in the tissue. Also, the germ cell, responsible for sperm production, is infected.
“This reopens the worries about the presence of SARS-CoV-2 in semen, as other authors mentioned,” he said.
New findings
The team also found that the testes are a good place for viral replication.
The authors say they are the first to show:
- The longer the severe condition, the lower the number of surviving germ cells.
- There was fluctuation in several essential testicular genes.
- The intratesticular testosterone levels are 30 times reduced in the testes of COVID-19 patients.
The control group was composed of six patients who had undergone testicle removal after prostate cancer was suspected. Collection of both testicles from the test group was performed within 3 hours of death after a family member signed an informed consent document.
Recent research on semen demonstrates that patients who recovered from COVID-19 reestablish their sperm quality after 3 months of infection.
That study, in Fertility and Sterility, found that sperm quality was initially reduced for months in some men after recovery from COVID-19.
The team studied semen samples from 120 Belgian men (mean age, 35 years) at an average 52 days after their last COVID-19 symptoms. The semen was not found to be infectious.
But among 35 men who provided samples within a month after infection, reductions in sperm motility were evident in 60% and sperm counts were reduced in 37%, according to the report.
Testicular damage
The results [of the Costa et al. paper] emphasize the importance of testicular damage in severe COVID-19,” Rafael Kroon Campos, PhD, a postdoctoral fellow in the department of microbiology & immunology at the University of Texas Medical Branch at Galveston, said in an interview.
He noted that other viruses have also been shown to infect or otherwise cause testicular damage or orchitis, such as Zika, Ebola, and the closely related SARS-CoV-1. Sexual transmission has been documented for Zika and Ebola viruses.
Dr. Campos said with SARS-CoV-2, it is unclear whether sexual transmission plays a role.
“Some reports found evidence of viral RNA in semen, but these were rare occurrences. The study by Costa and colleagues used a combination of sensitive techniques and they were able to detect a small amount of viral RNA and viral protein in the testicular tissue of the deceased patients, as well as show viral factories indicating replication of the virus by electron microscopy,” he said.
Dr. Campos said the findings are particularly important and concerning because of the large number of severe cases of COVID-19.
“It is critical to continue to investigate the impact of the disease in testes, including the impact of different variants of concern on testicular damage,” he said.
Dr. McNeil and Dr. Campos have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, raising questions about potential consequences for reproductive health among those infected.
The study, published online Feb. 8 on the preprint server MedRxiv, found that “patients who become critically ill exhibit severe damages and may harbor the active virus in testes,” which can “serve as a viral sanctuary.”
Guilherme M.J. Costa, PhD, a professor at Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, led the study, which has not yet been peer-reviewed.
“A critical point of this article is that the virus was active in the patient’s testis after a long period of infection, indicating that the testis is able to maintain the viable virus for extended periods. It happens for many kinds of viruses in this genital organ,” Dr. Costa said in an interview.
Brian Keith McNeil, MD, vice-chair, department of urology at SUNY Downstate Health Sciences University in New York, told this news organization that the topic of COVID-19 and fertility has been discussed but data are sparse on the subject.
“The question this raises is whether or not COVID can live in the testes, and based on this it seems it can,” he said, adding that it also raises the question of whether COVID-19 could be transmitted through semen. “It leads one to wonder whether this could have a long-term impact on fertility in men and women.”
The authors wrote that deep testicular evaluation of patients who have been infected with COVID-19 is critical because the testes have one of the highest expressions of angiotensin converting enzyme 2 (ACE2) receptors, which play a large role in entrance of the virus into cells.
“A direct influence of SARS-CoV-2 in testicular cells might deregulate ACE2, elevating the levels of angiotensin II, a potent pro-inflammatory and angiogenic peptide,” the authors wrote.
Sperm-producing cells infected
In 2021, the researchers enrolled 11 male patients deceased from COVID-19 complications; none had received a vaccine. Infection was confirmed by SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) performed during their hospital stay. All 11 patients were admitted to the intensive care unit with severe pulmonary symptoms.
All but one of the patients had children and none had scrotal symptoms or complaints during their time in the hospital. Their clinical histories revealed no testicular disorders.
Dr. Costa said they found that detecting SARS-CoV-2 mRNA in testes is difficult in a conventional RT-PCR test.
Therefore, “We modified the protocol of the RT-PCR and used nanosensors. We observed that SARS-CoV-2 has a huge tropism for the testes in this context,” he said.
He said the team performed stainings and “discovered that macrophages and germ cells are highly infected.”
That’s important, he said, because an immune cell, which is supposed to fight the virus, is infected in the tissue. Also, the germ cell, responsible for sperm production, is infected.
“This reopens the worries about the presence of SARS-CoV-2 in semen, as other authors mentioned,” he said.
New findings
The team also found that the testes are a good place for viral replication.
The authors say they are the first to show:
- The longer the severe condition, the lower the number of surviving germ cells.
- There was fluctuation in several essential testicular genes.
- The intratesticular testosterone levels are 30 times reduced in the testes of COVID-19 patients.
The control group was composed of six patients who had undergone testicle removal after prostate cancer was suspected. Collection of both testicles from the test group was performed within 3 hours of death after a family member signed an informed consent document.
Recent research on semen demonstrates that patients who recovered from COVID-19 reestablish their sperm quality after 3 months of infection.
That study, in Fertility and Sterility, found that sperm quality was initially reduced for months in some men after recovery from COVID-19.
The team studied semen samples from 120 Belgian men (mean age, 35 years) at an average 52 days after their last COVID-19 symptoms. The semen was not found to be infectious.
But among 35 men who provided samples within a month after infection, reductions in sperm motility were evident in 60% and sperm counts were reduced in 37%, according to the report.
Testicular damage
The results [of the Costa et al. paper] emphasize the importance of testicular damage in severe COVID-19,” Rafael Kroon Campos, PhD, a postdoctoral fellow in the department of microbiology & immunology at the University of Texas Medical Branch at Galveston, said in an interview.
He noted that other viruses have also been shown to infect or otherwise cause testicular damage or orchitis, such as Zika, Ebola, and the closely related SARS-CoV-1. Sexual transmission has been documented for Zika and Ebola viruses.
Dr. Campos said with SARS-CoV-2, it is unclear whether sexual transmission plays a role.
“Some reports found evidence of viral RNA in semen, but these were rare occurrences. The study by Costa and colleagues used a combination of sensitive techniques and they were able to detect a small amount of viral RNA and viral protein in the testicular tissue of the deceased patients, as well as show viral factories indicating replication of the virus by electron microscopy,” he said.
Dr. Campos said the findings are particularly important and concerning because of the large number of severe cases of COVID-19.
“It is critical to continue to investigate the impact of the disease in testes, including the impact of different variants of concern on testicular damage,” he said.
Dr. McNeil and Dr. Campos have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Endocrine Society and others to FDA: Restrict BPA
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
The chemical is used to make plastics in items such as food containers, pitchers, and inner linings of metal products. Small amounts of BPA can leak into food and beverages.
The petition points to a December 2021 report by the European Food Safety Authority titled: “Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs,” which summarizes evidence gathered since 2013.
It concludes that “there is a health concern from BPA exposure for all age groups.” Specific concerns include harm to the immune system and male and female reproductive systems.
Average American exposed to 5,000 times the safe level of BPA
The EFSA established a new “tolerable daily intake” of BPA of 0.04 ng/kg of body weight per day. By contrast, in 2014 the FDA estimated that the mean BPA intake for the U.S. population older than 2 years was 200 ng/kg bw/day and that the 90th percentile for BPA intake was 500 ng/kg of body weight per day.
“Using FDA’s own exposure estimates, the average American is exposed to more than 5000 times the safe level of 0.04 ng BPA/kg [body weight per day] set by the EFSA expert panel. Without a doubt, these values constitute a high health risk and support the conclusion that uses of BPA are not safe ... Given the magnitude of the overexposure, we request an expedited review by FDA,” the petition reads.
In addition to the Endocrine Society, which has long warned about the dangers of endocrine-disrupting chemicals, other signatories to the petition include the Environmental Defense Fund, Breast Cancer Prevention Partners, Clean Water Action/Clean Water Fund, Consumer Reports, Environmental Working Group, Healthy Babies Bright Futures, and the former director of the National Institute of Environmental Health Sciences and National Toxicology Program.
In a statement, Endocrine Society BPA expert Heather Patisaul, PhD, of North Carolina University, Raleigh, said the report’s findings “are extremely concerning and prove the point that even very low levels of BPA exposure can be harmful and lead to issues with reproductive health, breast cancer risk, behavior, and metabolism.”
“The FDA needs to acknowledge the science behind endocrine-disrupting chemicals and act accordingly to protect public health,” she urged.
The FDA is expected to decide within the next few days whether to open a docket to accept comments.
A final decision could take 6 months or longer, an Endocrine Society spokesperson told this news organization.
A version of this article first appeared on Medscape.com.
Updated endometriosis guidelines emphasize less laparoscopy, more hormone therapy
Updated guidelines for the management and treatment of endometriosis reflect changes in clinical practice to guide clinician and patient decision-making, according to a statement from the European Society of Human Reproduction and Embryology, which issued the guidelines in February 2022.
Although the exact prevalence of endometriosis remains unclear, estimates suggest that approximately 190 million women and adolescent girls are affected by endometriosis during their reproductive years, and women continue to suffer beyond menopause, according to the authors. Endometriosis has a significant impact on society through both direct and indirect health care costs comparable to those of type 2 diabetes, rheumatoid arthritis, and Crohn’s disease, they noted.
The guidelines are the first update on the topic of endometriosis since 2014, and include more than 100 recommendations, according to the European Society of Human Reproduction and Embryology (ESHRE). The target audience, according to the authors, is secondary and tertiary health care providers who treat women with endometriosis. The recommendations were based on research papers published up to Dec. 1, 2020.
Although most of the recent studies confirm previous ESHRE recommendations, several topics reflect significant changes in clinical practice.
Notably, laparoscopy is no longer recommended as the diagnostic gold standard, and should be used only in patients with negative imaging for whom empirical treatment was unsuccessful.
For pain management, studies support the use of GnRH antagonists as a second-line treatment, while laparoscopic uterosacral nerve ablation and presacral neurectomy are no longer included in the recommendations.
The guidelines include new information on pregnancy and fertility preservation for women with endometriosis. The Endometriosis Fertility Index (EFI) was added to support joint decision-making for women seeking pregnancy after surgery. However, the extended use of GnRH antagonist prior to assisted reproductive technology treatments to improve live birth rate is not recommended.
Endometriosis in adolescent patients is included in the guidelines for the first time, and strong recommendations include taking a careful history and using ultrasound if appropriate, but the use of serum biomarkers is not recommended for diagnosis. Strong recommendations for treatment strategies for adolescents include hormonal contraceptives or progestins as a first-line therapy.
Recommendations for managing endometriosis in menopause are more extensive than in previous guidelines and the strongest update is against the use of estrogen-only treatment in these patients. However, the guidelines continue to recommend treating women with a history of endometriosis after surgical menopause with combined estrogen-progestogen therapy “at least up to the age of natural menopause.”
Expanded recommendations related to endometriosis and cancer begin with a strong recommendation for clinicians to advise women that endometriosis is not associated with a significantly higher risk of cancer overall. “Although endometriosis is associated with a higher risk of ovarian, breast, and thyroid cancers in particular, the increase in absolute risk compared with women in the general population is low,” the authors wrote. Other strong recommendations include reassuring women with endometriosis of the low risk of malignancy associated with hormonal contraceptive use, and performing cancer screening according to the existing population-based guidelines without additional screening. Epidemiologic data show that complete excision of visible endometriosis may reduce the risk of ovarian cancer, but the potential benefits must be weighed against the risks of surgery, including morbidity, pain, and ovarian reserve, the authors said.
The guidelines include recommendations related to asymptomatic endometriosis, extrapelvic endometriosis, and primary prevention of endometriosis, but without major changes to the 2014 guidelines.
Guidelines expand strategies, but research gaps remain
In 2021, an international working group of the American Association of Gynecologic Laparoscopists, the European Society for Gynecologic Endoscopy, ESHRE, and the World Endometriosis Society defined endometriosis as “a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process,” Mark P. Trolice, MD, director of The IVF Center, Orlando, Fla., and professor of obstetrics and gynecology at the University of Central Florida, said in an interview.
Although the current guidelines represent the second update since 2005, many unanswered questions remain, Dr. Trolice said. “There is a large diagnostic void between the onset of symptoms and the time to a reliable diagnosis averaging between 8 and 12 years,” he emphasized.
Dr. Trolice noted the change of the addition of an oral GnRH antagonist, “now FDA approved for the treatment of pain associated with endometriosis,” he said. However, “Extended GnRH agonist prior to ART is not recommended due to the lack of any clear benefit,” he noted.
Dr. Trolice noted the inclusion of the Endometriosis Fertility Index (EFI), published in 2010, “as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and IUI [intrauterine insemination]) based on patient characteristics, revised ASRM staging, and ‘least function score of the adnexa.’ ” He agreed with the need for expanded information on the topics of endometriosis and adolescence and endometriosis and cancer.
The most important changes for clinical practice include reducing unnecessary laparoscopy and procedures without benefit, such as laparoscopic uterosacral nerve ablation and presacral neurectomy, and GnRH suppression using an oral antagonist, said Dr. Trolice. Other especially practical guidance includes the recommendation to discontinue advising patients that pregnancy will reduce symptoms of endometriosis, and to avoid prescribing estrogen-only treatment in menopause given the risk of malignant transformation of endometriosis, he said.
Another clinically useful recommendation, though not a significant update, is the need to identify extrapelvic endometriosis symptoms, such as cyclical shoulder pain, cyclical spontaneous pneumothorax, cyclical cough, or nodules that enlarge during menses, Dr. Trolice added.
Barriers to implementing the updated guidelines include lack of education of clinicians, including primary care providers, and the lack of definitive evidence for many areas, he noted.
As for additional research, more data are needed to explore the genetic, mutational, and epigenetic profile of endometriosis, and to identify biomarkers to noninvasively detect and provide a prognosis for endometriosis, and optimal methods for prevention and management, said Dr. Trolice. Other research gaps include “definitive medical and surgical treatment of endometriosis for improvement of fertility, quality of life, and reduction of pain,” he noted. From a fertility standpoint, more studies are needed on “the use of ovarian tissue or oocytes cryopreservation in adolescents and adults who undergo ovarian surgery for endometriomas, and the role of the EFI as a presurgical triage tool and to predict IUI outcomes,” said Dr. Trolice.
Overall, society recommendations such as these from ESHRE “serve as guides for physicians by providing evidence-based medicine and dispelling prior unproven practices so patients may receive the most effective care of endometriosis, throughout a woman’s life,” Dr. Trolice emphasized.
The current guideline will be considered for revision in 2025, and the full version is available on the ESHRE website.
Members of the ESHRE guideline development group received no payment for participating in the development process, although they were reimbursed for travel expenses related to guideline meetings.
Dr. Trolice had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Updated guidelines for the management and treatment of endometriosis reflect changes in clinical practice to guide clinician and patient decision-making, according to a statement from the European Society of Human Reproduction and Embryology, which issued the guidelines in February 2022.
Although the exact prevalence of endometriosis remains unclear, estimates suggest that approximately 190 million women and adolescent girls are affected by endometriosis during their reproductive years, and women continue to suffer beyond menopause, according to the authors. Endometriosis has a significant impact on society through both direct and indirect health care costs comparable to those of type 2 diabetes, rheumatoid arthritis, and Crohn’s disease, they noted.
The guidelines are the first update on the topic of endometriosis since 2014, and include more than 100 recommendations, according to the European Society of Human Reproduction and Embryology (ESHRE). The target audience, according to the authors, is secondary and tertiary health care providers who treat women with endometriosis. The recommendations were based on research papers published up to Dec. 1, 2020.
Although most of the recent studies confirm previous ESHRE recommendations, several topics reflect significant changes in clinical practice.
Notably, laparoscopy is no longer recommended as the diagnostic gold standard, and should be used only in patients with negative imaging for whom empirical treatment was unsuccessful.
For pain management, studies support the use of GnRH antagonists as a second-line treatment, while laparoscopic uterosacral nerve ablation and presacral neurectomy are no longer included in the recommendations.
The guidelines include new information on pregnancy and fertility preservation for women with endometriosis. The Endometriosis Fertility Index (EFI) was added to support joint decision-making for women seeking pregnancy after surgery. However, the extended use of GnRH antagonist prior to assisted reproductive technology treatments to improve live birth rate is not recommended.
Endometriosis in adolescent patients is included in the guidelines for the first time, and strong recommendations include taking a careful history and using ultrasound if appropriate, but the use of serum biomarkers is not recommended for diagnosis. Strong recommendations for treatment strategies for adolescents include hormonal contraceptives or progestins as a first-line therapy.
Recommendations for managing endometriosis in menopause are more extensive than in previous guidelines and the strongest update is against the use of estrogen-only treatment in these patients. However, the guidelines continue to recommend treating women with a history of endometriosis after surgical menopause with combined estrogen-progestogen therapy “at least up to the age of natural menopause.”
Expanded recommendations related to endometriosis and cancer begin with a strong recommendation for clinicians to advise women that endometriosis is not associated with a significantly higher risk of cancer overall. “Although endometriosis is associated with a higher risk of ovarian, breast, and thyroid cancers in particular, the increase in absolute risk compared with women in the general population is low,” the authors wrote. Other strong recommendations include reassuring women with endometriosis of the low risk of malignancy associated with hormonal contraceptive use, and performing cancer screening according to the existing population-based guidelines without additional screening. Epidemiologic data show that complete excision of visible endometriosis may reduce the risk of ovarian cancer, but the potential benefits must be weighed against the risks of surgery, including morbidity, pain, and ovarian reserve, the authors said.
The guidelines include recommendations related to asymptomatic endometriosis, extrapelvic endometriosis, and primary prevention of endometriosis, but without major changes to the 2014 guidelines.
Guidelines expand strategies, but research gaps remain
In 2021, an international working group of the American Association of Gynecologic Laparoscopists, the European Society for Gynecologic Endoscopy, ESHRE, and the World Endometriosis Society defined endometriosis as “a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process,” Mark P. Trolice, MD, director of The IVF Center, Orlando, Fla., and professor of obstetrics and gynecology at the University of Central Florida, said in an interview.
Although the current guidelines represent the second update since 2005, many unanswered questions remain, Dr. Trolice said. “There is a large diagnostic void between the onset of symptoms and the time to a reliable diagnosis averaging between 8 and 12 years,” he emphasized.
Dr. Trolice noted the change of the addition of an oral GnRH antagonist, “now FDA approved for the treatment of pain associated with endometriosis,” he said. However, “Extended GnRH agonist prior to ART is not recommended due to the lack of any clear benefit,” he noted.
Dr. Trolice noted the inclusion of the Endometriosis Fertility Index (EFI), published in 2010, “as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and IUI [intrauterine insemination]) based on patient characteristics, revised ASRM staging, and ‘least function score of the adnexa.’ ” He agreed with the need for expanded information on the topics of endometriosis and adolescence and endometriosis and cancer.
The most important changes for clinical practice include reducing unnecessary laparoscopy and procedures without benefit, such as laparoscopic uterosacral nerve ablation and presacral neurectomy, and GnRH suppression using an oral antagonist, said Dr. Trolice. Other especially practical guidance includes the recommendation to discontinue advising patients that pregnancy will reduce symptoms of endometriosis, and to avoid prescribing estrogen-only treatment in menopause given the risk of malignant transformation of endometriosis, he said.
Another clinically useful recommendation, though not a significant update, is the need to identify extrapelvic endometriosis symptoms, such as cyclical shoulder pain, cyclical spontaneous pneumothorax, cyclical cough, or nodules that enlarge during menses, Dr. Trolice added.
Barriers to implementing the updated guidelines include lack of education of clinicians, including primary care providers, and the lack of definitive evidence for many areas, he noted.
As for additional research, more data are needed to explore the genetic, mutational, and epigenetic profile of endometriosis, and to identify biomarkers to noninvasively detect and provide a prognosis for endometriosis, and optimal methods for prevention and management, said Dr. Trolice. Other research gaps include “definitive medical and surgical treatment of endometriosis for improvement of fertility, quality of life, and reduction of pain,” he noted. From a fertility standpoint, more studies are needed on “the use of ovarian tissue or oocytes cryopreservation in adolescents and adults who undergo ovarian surgery for endometriomas, and the role of the EFI as a presurgical triage tool and to predict IUI outcomes,” said Dr. Trolice.
Overall, society recommendations such as these from ESHRE “serve as guides for physicians by providing evidence-based medicine and dispelling prior unproven practices so patients may receive the most effective care of endometriosis, throughout a woman’s life,” Dr. Trolice emphasized.
The current guideline will be considered for revision in 2025, and the full version is available on the ESHRE website.
Members of the ESHRE guideline development group received no payment for participating in the development process, although they were reimbursed for travel expenses related to guideline meetings.
Dr. Trolice had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Updated guidelines for the management and treatment of endometriosis reflect changes in clinical practice to guide clinician and patient decision-making, according to a statement from the European Society of Human Reproduction and Embryology, which issued the guidelines in February 2022.
Although the exact prevalence of endometriosis remains unclear, estimates suggest that approximately 190 million women and adolescent girls are affected by endometriosis during their reproductive years, and women continue to suffer beyond menopause, according to the authors. Endometriosis has a significant impact on society through both direct and indirect health care costs comparable to those of type 2 diabetes, rheumatoid arthritis, and Crohn’s disease, they noted.
The guidelines are the first update on the topic of endometriosis since 2014, and include more than 100 recommendations, according to the European Society of Human Reproduction and Embryology (ESHRE). The target audience, according to the authors, is secondary and tertiary health care providers who treat women with endometriosis. The recommendations were based on research papers published up to Dec. 1, 2020.
Although most of the recent studies confirm previous ESHRE recommendations, several topics reflect significant changes in clinical practice.
Notably, laparoscopy is no longer recommended as the diagnostic gold standard, and should be used only in patients with negative imaging for whom empirical treatment was unsuccessful.
For pain management, studies support the use of GnRH antagonists as a second-line treatment, while laparoscopic uterosacral nerve ablation and presacral neurectomy are no longer included in the recommendations.
The guidelines include new information on pregnancy and fertility preservation for women with endometriosis. The Endometriosis Fertility Index (EFI) was added to support joint decision-making for women seeking pregnancy after surgery. However, the extended use of GnRH antagonist prior to assisted reproductive technology treatments to improve live birth rate is not recommended.
Endometriosis in adolescent patients is included in the guidelines for the first time, and strong recommendations include taking a careful history and using ultrasound if appropriate, but the use of serum biomarkers is not recommended for diagnosis. Strong recommendations for treatment strategies for adolescents include hormonal contraceptives or progestins as a first-line therapy.
Recommendations for managing endometriosis in menopause are more extensive than in previous guidelines and the strongest update is against the use of estrogen-only treatment in these patients. However, the guidelines continue to recommend treating women with a history of endometriosis after surgical menopause with combined estrogen-progestogen therapy “at least up to the age of natural menopause.”
Expanded recommendations related to endometriosis and cancer begin with a strong recommendation for clinicians to advise women that endometriosis is not associated with a significantly higher risk of cancer overall. “Although endometriosis is associated with a higher risk of ovarian, breast, and thyroid cancers in particular, the increase in absolute risk compared with women in the general population is low,” the authors wrote. Other strong recommendations include reassuring women with endometriosis of the low risk of malignancy associated with hormonal contraceptive use, and performing cancer screening according to the existing population-based guidelines without additional screening. Epidemiologic data show that complete excision of visible endometriosis may reduce the risk of ovarian cancer, but the potential benefits must be weighed against the risks of surgery, including morbidity, pain, and ovarian reserve, the authors said.
The guidelines include recommendations related to asymptomatic endometriosis, extrapelvic endometriosis, and primary prevention of endometriosis, but without major changes to the 2014 guidelines.
Guidelines expand strategies, but research gaps remain
In 2021, an international working group of the American Association of Gynecologic Laparoscopists, the European Society for Gynecologic Endoscopy, ESHRE, and the World Endometriosis Society defined endometriosis as “a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process,” Mark P. Trolice, MD, director of The IVF Center, Orlando, Fla., and professor of obstetrics and gynecology at the University of Central Florida, said in an interview.
Although the current guidelines represent the second update since 2005, many unanswered questions remain, Dr. Trolice said. “There is a large diagnostic void between the onset of symptoms and the time to a reliable diagnosis averaging between 8 and 12 years,” he emphasized.
Dr. Trolice noted the change of the addition of an oral GnRH antagonist, “now FDA approved for the treatment of pain associated with endometriosis,” he said. However, “Extended GnRH agonist prior to ART is not recommended due to the lack of any clear benefit,” he noted.
Dr. Trolice noted the inclusion of the Endometriosis Fertility Index (EFI), published in 2010, “as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and IUI [intrauterine insemination]) based on patient characteristics, revised ASRM staging, and ‘least function score of the adnexa.’ ” He agreed with the need for expanded information on the topics of endometriosis and adolescence and endometriosis and cancer.
The most important changes for clinical practice include reducing unnecessary laparoscopy and procedures without benefit, such as laparoscopic uterosacral nerve ablation and presacral neurectomy, and GnRH suppression using an oral antagonist, said Dr. Trolice. Other especially practical guidance includes the recommendation to discontinue advising patients that pregnancy will reduce symptoms of endometriosis, and to avoid prescribing estrogen-only treatment in menopause given the risk of malignant transformation of endometriosis, he said.
Another clinically useful recommendation, though not a significant update, is the need to identify extrapelvic endometriosis symptoms, such as cyclical shoulder pain, cyclical spontaneous pneumothorax, cyclical cough, or nodules that enlarge during menses, Dr. Trolice added.
Barriers to implementing the updated guidelines include lack of education of clinicians, including primary care providers, and the lack of definitive evidence for many areas, he noted.
As for additional research, more data are needed to explore the genetic, mutational, and epigenetic profile of endometriosis, and to identify biomarkers to noninvasively detect and provide a prognosis for endometriosis, and optimal methods for prevention and management, said Dr. Trolice. Other research gaps include “definitive medical and surgical treatment of endometriosis for improvement of fertility, quality of life, and reduction of pain,” he noted. From a fertility standpoint, more studies are needed on “the use of ovarian tissue or oocytes cryopreservation in adolescents and adults who undergo ovarian surgery for endometriomas, and the role of the EFI as a presurgical triage tool and to predict IUI outcomes,” said Dr. Trolice.
Overall, society recommendations such as these from ESHRE “serve as guides for physicians by providing evidence-based medicine and dispelling prior unproven practices so patients may receive the most effective care of endometriosis, throughout a woman’s life,” Dr. Trolice emphasized.
The current guideline will be considered for revision in 2025, and the full version is available on the ESHRE website.
Members of the ESHRE guideline development group received no payment for participating in the development process, although they were reimbursed for travel expenses related to guideline meetings.
Dr. Trolice had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Intensive weight loss fails to help women with obesity and infertility
An intensive weight-loss intervention prior to conception had no effect on birth rates in women with obesity and unexplained infertility, compared with a standard weight-maintenance program, based on data from nearly 400 women.
Obese women experiencing infertility are often counseled to lose weight before attempting fertility treatments in order to improve outcomes based on epidemiologic evidence of an association between obesity and infertility, but data to support this advice are limited, wrote Richard S. Legro, MD, of Penn State University, Hershey, and colleagues.
The researchers proposed that a more intensive preconception weight loss intervention followed by infertility treatment would be more likely to yield a healthy live birth, compared with a standard weight maintenance intervention.
In an open-label study published in PLOS Medicine, the researchers randomized 379 women at nine academic centers to a standard lifestyle group that followed a weight-maintenance plan focused on physical activity, but not weight loss; or an intensive intervention of diet and medication with a target weight loss of 7%. Both interventions lasted for 16 weeks between July 2015 and July 2018. After the interventions, patients in both groups underwent standardized empiric fertility treatment with three cycles of ovarian stimulation and intrauterine insemination.
The primary outcome was a live birth at 37 weeks’ gestation or later, with no congenital abnormalities and a birth weight between 2,500 g and 4,000 g. Baseline characteristics including age, education level, race, and body mass index (BMI) were similar between the groups.
The incidence of healthy live births was not significantly different between the standard treatment and intensive treatment groups (15.2% vs. 12.2%; P = 0.40) by the final follow-up time of September 2019. However, women in the intensive group had significantly greater weight loss, compared with the standard group (–6.6% vs. –0.3%; P < .001). Women in the intensive group also showed improvements in metabolic health. Notably, the incidence of metabolic syndrome dropped from 53.6% to 49.4% in the standard group, compared with a decrease from 52.8% to 32.2% in the intensive group over the 16-week study period, the researchers wrote.
Gastrointestinal side effects were significantly more common in the intensive group, but these were consistent with documented side effects of the weight loss medication used (Orlistat).
First-trimester pregnancy loss was higher in the intensive group, compared with the standard group (33.3% vs. 23.7%), but the difference was not significant. Most pregnancy complications, including preterm labor, premature rupture of membranes, preeclampsia, and gestational diabetes had nonsignificant improvements in the intensive group, compared with the standard group. Similarly, nonsignificant improvements were noted in the intervention group for intrauterine growth restriction and admission to the neonatal ICU.
Limitations of the study included the relatively small number of pregnancies, which prevented assessment of rare complications in subgroups, and the challenge of matching control interventions, the researchers noted.
However, the results were strengthened by the focus on women with unexplained infertility, the inclusion of a comparison group, and the collection of data on complications after conception, they wrote.
Avenues for future research include interventions of different duration and intensity prior to conception, which may improve outcomes, the researchers said in their discussion of the findings. “A period of weight stabilization and maintenance after a weight-loss intervention prior to commencing infertility therapy is worth exploring,” they noted, but couples eager to conceive may be reluctant to wait for a weight-loss intervention, they added.
“Our findings directly impact current standards of clinical care, where women who are obese with unexplained infertility are to our knowledge routinely counseled to lose weight prior to initiation of infertility treatment,” they concluded.
Data may inform patient discussions
The current study is important because a large amount of previous research has shown an association between obesity and decreased fecundity in women and men, Mark P. Trolice, MD, of the University of Central Florida, Orlando, and director of the IVF Center in Winter Park, Fla., said in an interview.
According to the Centers for Disease Control and Prevention, the prevalence of obesity in the United States remains more than 40%, said Dr. Trolice. “Patients and physicians would benefit from clarity of obesity’s effect, if any, on reproduction,” he noted.
In contrast to the authors’ hypothesis, “the study did not find a difference in the live birth rate following up to three cycles of intrauterine insemination (IUI) between an intensive weight loss group [and] women who exercised without weight loss,” said Dr. Trolice. “Prior to this study, many reports suggested a decline in fertility with elevations in BMI, particularly during fertility treatment,” he added.
The take-home message from the current study is a that an elevated BMI, while possibly increasing the risks of metabolic disorders, did not appear to impact fecundity, he said.
The authors therefore concluded, “There is not strong evidence to recommend weight loss prior to conception in women who are obese with unexplained infertility,” Dr. Trolice said.
Regardless of the potential effect of preconception weight loss on fertility, barriers to starting a weight loss program include a woman’s eagerness to move forward with fertility treatments without waiting for weight loss, Dr. Trolice noted. “By the time a woman reaches an infertility specialist, she has been trying to conceive for at least 1 year,” he said. “At the initial consultation, these patients are anxious to undergo necessary additional diagnostic testing followed by treatment. Consequently, initiation of a weight-loss program is viewed as a delay toward the goal of family building,” he explained.
“More research is needed to demonstrate the safety of intensive weight loss preconception,” said Dr. Trolice. However, he said, “the issue of elevated BMI and increased risk of pregnancy complications remains, but this study provides important information for providers regarding counseling their patients desiring pregnancy.”
The study was supported by multiple grants from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Nutrisystem provided discounted coupons for food allotments in the standardized treatment group, and FitBit provided the study organizers with discounted Fitbits for activity monitoring. Lead author Dr. Legro disclosed consulting fees from InSupp, Ferring, Bayer, Abbvie and Fractyl, and research sponsorship from Guerbet and the National Institutes of Health. Dr. Trolice had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn News.
An intensive weight-loss intervention prior to conception had no effect on birth rates in women with obesity and unexplained infertility, compared with a standard weight-maintenance program, based on data from nearly 400 women.
Obese women experiencing infertility are often counseled to lose weight before attempting fertility treatments in order to improve outcomes based on epidemiologic evidence of an association between obesity and infertility, but data to support this advice are limited, wrote Richard S. Legro, MD, of Penn State University, Hershey, and colleagues.
The researchers proposed that a more intensive preconception weight loss intervention followed by infertility treatment would be more likely to yield a healthy live birth, compared with a standard weight maintenance intervention.
In an open-label study published in PLOS Medicine, the researchers randomized 379 women at nine academic centers to a standard lifestyle group that followed a weight-maintenance plan focused on physical activity, but not weight loss; or an intensive intervention of diet and medication with a target weight loss of 7%. Both interventions lasted for 16 weeks between July 2015 and July 2018. After the interventions, patients in both groups underwent standardized empiric fertility treatment with three cycles of ovarian stimulation and intrauterine insemination.
The primary outcome was a live birth at 37 weeks’ gestation or later, with no congenital abnormalities and a birth weight between 2,500 g and 4,000 g. Baseline characteristics including age, education level, race, and body mass index (BMI) were similar between the groups.
The incidence of healthy live births was not significantly different between the standard treatment and intensive treatment groups (15.2% vs. 12.2%; P = 0.40) by the final follow-up time of September 2019. However, women in the intensive group had significantly greater weight loss, compared with the standard group (–6.6% vs. –0.3%; P < .001). Women in the intensive group also showed improvements in metabolic health. Notably, the incidence of metabolic syndrome dropped from 53.6% to 49.4% in the standard group, compared with a decrease from 52.8% to 32.2% in the intensive group over the 16-week study period, the researchers wrote.
Gastrointestinal side effects were significantly more common in the intensive group, but these were consistent with documented side effects of the weight loss medication used (Orlistat).
First-trimester pregnancy loss was higher in the intensive group, compared with the standard group (33.3% vs. 23.7%), but the difference was not significant. Most pregnancy complications, including preterm labor, premature rupture of membranes, preeclampsia, and gestational diabetes had nonsignificant improvements in the intensive group, compared with the standard group. Similarly, nonsignificant improvements were noted in the intervention group for intrauterine growth restriction and admission to the neonatal ICU.
Limitations of the study included the relatively small number of pregnancies, which prevented assessment of rare complications in subgroups, and the challenge of matching control interventions, the researchers noted.
However, the results were strengthened by the focus on women with unexplained infertility, the inclusion of a comparison group, and the collection of data on complications after conception, they wrote.
Avenues for future research include interventions of different duration and intensity prior to conception, which may improve outcomes, the researchers said in their discussion of the findings. “A period of weight stabilization and maintenance after a weight-loss intervention prior to commencing infertility therapy is worth exploring,” they noted, but couples eager to conceive may be reluctant to wait for a weight-loss intervention, they added.
“Our findings directly impact current standards of clinical care, where women who are obese with unexplained infertility are to our knowledge routinely counseled to lose weight prior to initiation of infertility treatment,” they concluded.
Data may inform patient discussions
The current study is important because a large amount of previous research has shown an association between obesity and decreased fecundity in women and men, Mark P. Trolice, MD, of the University of Central Florida, Orlando, and director of the IVF Center in Winter Park, Fla., said in an interview.
According to the Centers for Disease Control and Prevention, the prevalence of obesity in the United States remains more than 40%, said Dr. Trolice. “Patients and physicians would benefit from clarity of obesity’s effect, if any, on reproduction,” he noted.
In contrast to the authors’ hypothesis, “the study did not find a difference in the live birth rate following up to three cycles of intrauterine insemination (IUI) between an intensive weight loss group [and] women who exercised without weight loss,” said Dr. Trolice. “Prior to this study, many reports suggested a decline in fertility with elevations in BMI, particularly during fertility treatment,” he added.
The take-home message from the current study is a that an elevated BMI, while possibly increasing the risks of metabolic disorders, did not appear to impact fecundity, he said.
The authors therefore concluded, “There is not strong evidence to recommend weight loss prior to conception in women who are obese with unexplained infertility,” Dr. Trolice said.
Regardless of the potential effect of preconception weight loss on fertility, barriers to starting a weight loss program include a woman’s eagerness to move forward with fertility treatments without waiting for weight loss, Dr. Trolice noted. “By the time a woman reaches an infertility specialist, she has been trying to conceive for at least 1 year,” he said. “At the initial consultation, these patients are anxious to undergo necessary additional diagnostic testing followed by treatment. Consequently, initiation of a weight-loss program is viewed as a delay toward the goal of family building,” he explained.
“More research is needed to demonstrate the safety of intensive weight loss preconception,” said Dr. Trolice. However, he said, “the issue of elevated BMI and increased risk of pregnancy complications remains, but this study provides important information for providers regarding counseling their patients desiring pregnancy.”
The study was supported by multiple grants from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Nutrisystem provided discounted coupons for food allotments in the standardized treatment group, and FitBit provided the study organizers with discounted Fitbits for activity monitoring. Lead author Dr. Legro disclosed consulting fees from InSupp, Ferring, Bayer, Abbvie and Fractyl, and research sponsorship from Guerbet and the National Institutes of Health. Dr. Trolice had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn News.
An intensive weight-loss intervention prior to conception had no effect on birth rates in women with obesity and unexplained infertility, compared with a standard weight-maintenance program, based on data from nearly 400 women.
Obese women experiencing infertility are often counseled to lose weight before attempting fertility treatments in order to improve outcomes based on epidemiologic evidence of an association between obesity and infertility, but data to support this advice are limited, wrote Richard S. Legro, MD, of Penn State University, Hershey, and colleagues.
The researchers proposed that a more intensive preconception weight loss intervention followed by infertility treatment would be more likely to yield a healthy live birth, compared with a standard weight maintenance intervention.
In an open-label study published in PLOS Medicine, the researchers randomized 379 women at nine academic centers to a standard lifestyle group that followed a weight-maintenance plan focused on physical activity, but not weight loss; or an intensive intervention of diet and medication with a target weight loss of 7%. Both interventions lasted for 16 weeks between July 2015 and July 2018. After the interventions, patients in both groups underwent standardized empiric fertility treatment with three cycles of ovarian stimulation and intrauterine insemination.
The primary outcome was a live birth at 37 weeks’ gestation or later, with no congenital abnormalities and a birth weight between 2,500 g and 4,000 g. Baseline characteristics including age, education level, race, and body mass index (BMI) were similar between the groups.
The incidence of healthy live births was not significantly different between the standard treatment and intensive treatment groups (15.2% vs. 12.2%; P = 0.40) by the final follow-up time of September 2019. However, women in the intensive group had significantly greater weight loss, compared with the standard group (–6.6% vs. –0.3%; P < .001). Women in the intensive group also showed improvements in metabolic health. Notably, the incidence of metabolic syndrome dropped from 53.6% to 49.4% in the standard group, compared with a decrease from 52.8% to 32.2% in the intensive group over the 16-week study period, the researchers wrote.
Gastrointestinal side effects were significantly more common in the intensive group, but these were consistent with documented side effects of the weight loss medication used (Orlistat).
First-trimester pregnancy loss was higher in the intensive group, compared with the standard group (33.3% vs. 23.7%), but the difference was not significant. Most pregnancy complications, including preterm labor, premature rupture of membranes, preeclampsia, and gestational diabetes had nonsignificant improvements in the intensive group, compared with the standard group. Similarly, nonsignificant improvements were noted in the intervention group for intrauterine growth restriction and admission to the neonatal ICU.
Limitations of the study included the relatively small number of pregnancies, which prevented assessment of rare complications in subgroups, and the challenge of matching control interventions, the researchers noted.
However, the results were strengthened by the focus on women with unexplained infertility, the inclusion of a comparison group, and the collection of data on complications after conception, they wrote.
Avenues for future research include interventions of different duration and intensity prior to conception, which may improve outcomes, the researchers said in their discussion of the findings. “A period of weight stabilization and maintenance after a weight-loss intervention prior to commencing infertility therapy is worth exploring,” they noted, but couples eager to conceive may be reluctant to wait for a weight-loss intervention, they added.
“Our findings directly impact current standards of clinical care, where women who are obese with unexplained infertility are to our knowledge routinely counseled to lose weight prior to initiation of infertility treatment,” they concluded.
Data may inform patient discussions
The current study is important because a large amount of previous research has shown an association between obesity and decreased fecundity in women and men, Mark P. Trolice, MD, of the University of Central Florida, Orlando, and director of the IVF Center in Winter Park, Fla., said in an interview.
According to the Centers for Disease Control and Prevention, the prevalence of obesity in the United States remains more than 40%, said Dr. Trolice. “Patients and physicians would benefit from clarity of obesity’s effect, if any, on reproduction,” he noted.
In contrast to the authors’ hypothesis, “the study did not find a difference in the live birth rate following up to three cycles of intrauterine insemination (IUI) between an intensive weight loss group [and] women who exercised without weight loss,” said Dr. Trolice. “Prior to this study, many reports suggested a decline in fertility with elevations in BMI, particularly during fertility treatment,” he added.
The take-home message from the current study is a that an elevated BMI, while possibly increasing the risks of metabolic disorders, did not appear to impact fecundity, he said.
The authors therefore concluded, “There is not strong evidence to recommend weight loss prior to conception in women who are obese with unexplained infertility,” Dr. Trolice said.
Regardless of the potential effect of preconception weight loss on fertility, barriers to starting a weight loss program include a woman’s eagerness to move forward with fertility treatments without waiting for weight loss, Dr. Trolice noted. “By the time a woman reaches an infertility specialist, she has been trying to conceive for at least 1 year,” he said. “At the initial consultation, these patients are anxious to undergo necessary additional diagnostic testing followed by treatment. Consequently, initiation of a weight-loss program is viewed as a delay toward the goal of family building,” he explained.
“More research is needed to demonstrate the safety of intensive weight loss preconception,” said Dr. Trolice. However, he said, “the issue of elevated BMI and increased risk of pregnancy complications remains, but this study provides important information for providers regarding counseling their patients desiring pregnancy.”
The study was supported by multiple grants from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Nutrisystem provided discounted coupons for food allotments in the standardized treatment group, and FitBit provided the study organizers with discounted Fitbits for activity monitoring. Lead author Dr. Legro disclosed consulting fees from InSupp, Ferring, Bayer, Abbvie and Fractyl, and research sponsorship from Guerbet and the National Institutes of Health. Dr. Trolice had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn News.
FROM PLOS MEDICINE
Preschool boys’ behaviors traced back to moms’ thyroid hormones
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women’s thyroid hormone trajectories (levels in the first, second, and third trimester) may predict whether their male offspring are aggressive or withdrawn at age 4.
Certain maternal thyroid hormone trajectories were associated with problem behaviors in preschool boys in a study of close to 2,000 mother-child pairs in China.
The researchers identified low, moderate, and high thyroid-stimulating hormone (TSH) and free thyroxine (FT4) trajectories.
Most women had a low TSH trajectory and moderate FT4 trajectory, which the researchers deemed to be reference (normal) trajectories.
The children’s primary caregiver (parent or grandparent) completed an extensive questionnaire about their child’s behavior at age 4.
The 4-year-old boys whose mothers had a high TSH trajectory during pregnancy were more likely to be withdrawn and to externalize problems (odds ratio, 2.01 and 2.69, respectively).
Boys whose mothers had a high FT4 trajectory during pregnancy were more likely to be anxious/depressed (OR, 2.22).
And boys whose mothers had a moderate TSH trajectory or low FT4 trajectory were more likely to show aggressive behavior (OR, 3.76 and 4.17, respectively), compared with boys whose mothers had normal TSH and FT4 trajectories, after adjusting for potential confounders.
However, there was no association between abnormal maternal thyroid hormone trajectories and behavior problems in 4-year-old girls.
The study by Peixuan Li, BM, and colleagues was published online Jan. 6 in the Journal of Clinical Endocrinology & Metabolism.
‘Study supports monitoring thyroid function in pregnancy’
“Our findings highlight the significance of close monitoring and management of maternal thyroid function during pregnancy,” senior author Kun Huang, PhD, said in a press release from the Endocrine Society.
“This research presents a new perspective in early intervention of children’s emotional and behavioral problems,” added Dr. Huang, from Anhui Medical University, Hefei, China.
The results add to a growing body of literature about a controversial link between maternal thyroid hormones in pregnancy, when the fetal brain is developing, and subsequent behavior in preschool children, Caroline T. Nguyen, MD, who was not involved with this research, commented in an email.
“Some studies show an association between thyroid levels and behavioral outcomes, others not,” added Dr. Nguyen, assistant professor of clinical medicine, Keck School of Medicine, University of Southern California, Los Angeles. And “some studies have found sex-specific associations with maternal thyroid levels and neurocognitive/behavioral outcomes, others have not.”
Women considering pregnancy should be evaluated for possible thyroid disease, she continued. Currently, no universal screening mandates exist for thyroid disease in pregnancy, but the 2017 American Thyroid Association guidelines do recommend screening women at risk for thyroid dysfunction.
“I think screening for thyroid peroxidase antibody (TPOAb) positivity is helpful in women desiring pregnancy,” Dr. Nguyen continued, “because we know that patients with TPOAb positivity are at increased risk for miscarriage and have a blunted response to the increased demands of pregnancy for thyroid hormone production.”
TPOAb positivity is also associated with the increased risk of postpartum and long-term thyroid dysfunction.
This current study, Dr. Nguyen summarized, “adds to a growing body of research of the relationship of thyroid hormone levels and neurocognitive outcomes [in offspring] and supports the monitoring of thyroid disease in pregnancy.”
“However, we do not have sufficient data to demonstrate the benefits of intervention with levothyroxine treatment,” she noted.
Nevertheless, the lack of positive data does not suggest there is no theoretical benefit of intervention, she said, as such studies are very challenging to do.
“Physicians can help reduce stress and anxiety in patients desiring pregnancy by [recommending] preconception counseling, screening patients at risk for thyroid disease, and optimizing thyroid hormone levels before and during pregnancy,” according to Dr. Nguyen.
Maternal TSH and FT4 trajectories and preschoolers’ behaviors
Previous studies have reported that during pregnancy, maternal subclinical hypothyroidism (elevated TSH with normal FT4) as well as isolated hypothyroxinemia (decreased FT4 with normal TSH) are associated with adverse maternal and child outcomes, including preterm delivery and low birth weight.
However, most studies have not determined maternal thyroid hormone levels in different trimesters.
Researchers recruited pregnant women going for their first antenatal checkup at the Ma’anshan Maternal and Child Health Hospital in China from May 2013 to September 2014 and identified 1,860 mother-child pairs.
They determined maternal thyroid hormone levels from blood samples taken during the first, second, and third trimester: on average, gestational week 10, 25, and 34, respectively.
The researchers found that TSH levels increased somewhat from trimester 1 to trimester 2 and then decreased slightly in trimester 3. Most women (68%) had a low TSH trajectory, 28% had a moderate TSH trajectory, and 4% had a high TSH trajectory.
FT4 levels dropped sharply from trimester 1 to trimester 2 and then increased somewhat in trimester 3. About half of the women (52%) had a moderate FT4 trajectory, 33% had a low FT4 trajectory, and 15% had a high FT4 trajectory.
Most women (96.5%) had a low and stable TPOAb level, and the rest (3.5%) had high and decreasing TPOAb levels.
When the children in the study were 4 years old, their main caregiver (parent or grandparent) completed the 100-question Achenbach Child Behavior checklist to identify whether the child often, sometimes, or never displayed three internalizing problem behaviors (emotionally reactive, anxious/depressed, or withdrawn) and/or two externalizing problem behaviors (attention problems or aggressive behavior).
Study limitations, more research needed
It is not clear why the associations between maternal hormones and offspring behavior were only seen in boys. Perhaps male fetuses are more sensitive than female fetuses to changing maternal thyroid hormone levels in pregnancy, the researchers speculate.
They acknowledge that study limitations include there were few children with aggressive behavior, so the confidence interval for the association of the moderate TSH trajectory with aggressive behavior was very wide.
In addition, evaluation of children’s behavior by caregivers was subjective. Also, the researchers did not have information about iodine levels, and low iodine levels can impair child brain development.
And there may have been residual confounders that researchers did not account for, such as differences in family upbringing, parental marital status, and the mother’s exposure to endocrine disruptors.
Therefore, further research is needed.
The study was supported by grants from the National Natural Science Foundation of China, the University Synergy Innovation Program of Anhui Province, the Sci-Tech Basic Resources Research Program of China, the National Key Research and Development Program, the Chinese Academy of Medical Sciences, and the Research Fund of Anhui Institute of Translational Medicine. The researchers and Dr. Nguyen have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
FDA approves levoketoconazole for Cushing syndrome
The Food and Drug Administration has approved levoketoconazole (Recorlev, Xeris Biopharma) for the treatment of endogenous hypercortisolemia in adults with Cushing syndrome for whom surgery is not possible or was not curative.
Endogenous Cushing syndrome is a relatively rare condition characterized by chronically elevated cortisol levels, typically arising from a benign pituitary tumor. Left untreated, it can lead to reproductive problems and hirsutism in women, as well as serious complications, including diabetes, hypertension, tissue fragility, and mood disorders. Half of patients will die within 5 years if left untreated.
Levoketoconazole inhibits cortisol synthesis. The FDA approval was based on efficacy and safety data from two phase 3 studies involving a total of 166 patients with endogenous Cushing syndrome. In both the open-label, single-arm SONICS study and the randomized, placebo-controlled LOGICS trial, the drug significantly reduced and normalized mean urinary free cortisol levels and improved several secondary endpoints. The ongoing open-label OPTICS study will gather long-term data.
The Recorlev label includes boxed warnings about the potential for life-threatening hepatotoxicity and QT prolongation. Prior to and during treatment, patients should undergo liver enzyme testing, ECG, and correction of hypokalemia and hypomagnesemia.
The most common adverse reactions (occurring in less than 20%) include nausea/vomiting, hypokalemia, hemorrhage/contusion, systemic hypertension, headache, hepatic injury, abnormal uterine bleeding, erythema, fatigue, abdominal pain/dyspepsia, arthritis, upper respiratory infection, myalgia, arrhythmia, back pain, insomnia/sleep disturbances, and peripheral edema.
“Cushing syndrome is a rare disease that can be physically and emotionally devastating to the patient. Most patients endure years of symptoms prior to obtaining a diagnosis and are then faced with limited effective treatment options ... We are excited to see that the long and complicated path of rare drug development has reached FDA approval on a new therapeutic option for our underserved Cushing’s community,” Leslie Edwin, president of the Cushing’s Support & Research Foundation, said in a Xeris statement.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved levoketoconazole (Recorlev, Xeris Biopharma) for the treatment of endogenous hypercortisolemia in adults with Cushing syndrome for whom surgery is not possible or was not curative.
Endogenous Cushing syndrome is a relatively rare condition characterized by chronically elevated cortisol levels, typically arising from a benign pituitary tumor. Left untreated, it can lead to reproductive problems and hirsutism in women, as well as serious complications, including diabetes, hypertension, tissue fragility, and mood disorders. Half of patients will die within 5 years if left untreated.
Levoketoconazole inhibits cortisol synthesis. The FDA approval was based on efficacy and safety data from two phase 3 studies involving a total of 166 patients with endogenous Cushing syndrome. In both the open-label, single-arm SONICS study and the randomized, placebo-controlled LOGICS trial, the drug significantly reduced and normalized mean urinary free cortisol levels and improved several secondary endpoints. The ongoing open-label OPTICS study will gather long-term data.
The Recorlev label includes boxed warnings about the potential for life-threatening hepatotoxicity and QT prolongation. Prior to and during treatment, patients should undergo liver enzyme testing, ECG, and correction of hypokalemia and hypomagnesemia.
The most common adverse reactions (occurring in less than 20%) include nausea/vomiting, hypokalemia, hemorrhage/contusion, systemic hypertension, headache, hepatic injury, abnormal uterine bleeding, erythema, fatigue, abdominal pain/dyspepsia, arthritis, upper respiratory infection, myalgia, arrhythmia, back pain, insomnia/sleep disturbances, and peripheral edema.
“Cushing syndrome is a rare disease that can be physically and emotionally devastating to the patient. Most patients endure years of symptoms prior to obtaining a diagnosis and are then faced with limited effective treatment options ... We are excited to see that the long and complicated path of rare drug development has reached FDA approval on a new therapeutic option for our underserved Cushing’s community,” Leslie Edwin, president of the Cushing’s Support & Research Foundation, said in a Xeris statement.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved levoketoconazole (Recorlev, Xeris Biopharma) for the treatment of endogenous hypercortisolemia in adults with Cushing syndrome for whom surgery is not possible or was not curative.
Endogenous Cushing syndrome is a relatively rare condition characterized by chronically elevated cortisol levels, typically arising from a benign pituitary tumor. Left untreated, it can lead to reproductive problems and hirsutism in women, as well as serious complications, including diabetes, hypertension, tissue fragility, and mood disorders. Half of patients will die within 5 years if left untreated.
Levoketoconazole inhibits cortisol synthesis. The FDA approval was based on efficacy and safety data from two phase 3 studies involving a total of 166 patients with endogenous Cushing syndrome. In both the open-label, single-arm SONICS study and the randomized, placebo-controlled LOGICS trial, the drug significantly reduced and normalized mean urinary free cortisol levels and improved several secondary endpoints. The ongoing open-label OPTICS study will gather long-term data.
The Recorlev label includes boxed warnings about the potential for life-threatening hepatotoxicity and QT prolongation. Prior to and during treatment, patients should undergo liver enzyme testing, ECG, and correction of hypokalemia and hypomagnesemia.
The most common adverse reactions (occurring in less than 20%) include nausea/vomiting, hypokalemia, hemorrhage/contusion, systemic hypertension, headache, hepatic injury, abnormal uterine bleeding, erythema, fatigue, abdominal pain/dyspepsia, arthritis, upper respiratory infection, myalgia, arrhythmia, back pain, insomnia/sleep disturbances, and peripheral edema.
“Cushing syndrome is a rare disease that can be physically and emotionally devastating to the patient. Most patients endure years of symptoms prior to obtaining a diagnosis and are then faced with limited effective treatment options ... We are excited to see that the long and complicated path of rare drug development has reached FDA approval on a new therapeutic option for our underserved Cushing’s community,” Leslie Edwin, president of the Cushing’s Support & Research Foundation, said in a Xeris statement.
A version of this article first appeared on Medscape.com.
Hyperprolactinemia – When, why, and how to evaluate prolactin
Because of the increasing popularity and success of in vitro fertilization, the field of reproductive endocrinology and infertility has steadily morphed toward the treatment of infertility. Nevertheless, a physician board certified in reproductive endocrinology and infertility is the referring physician of choice regarding prolactin disorders and gynecologists should be familiar with the symptoms and sequela of prolactin elevations. This month’s column will address when to obtain a serum prolactin and how to appropriately manage hyperprolactinemia.
Of all the anterior pituitary hormones (adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone ), prolactin is the only one under tonic inhibition by dopamine. Disturbances in this dopaminergic pathway result in elevated serum prolactin. The normal range for prolactin is approximately 5-20 ng/mL.
In the nonpregnant state, little is known regarding the purpose of prolactin, which is produced by the anterior pituitary cluster of cells called lactotrophs. To prepare the breast for postpartum lactation, increases in prolactin are necessary and sustained throughout pregnancy. Second to pregnancy, amenorrhea can occur in 10%-20% of cases of hyperprolactinemia. Outside of pregnancy, elevations in prolactin result in hypogonadism, through gonadotropin-releasing hormone suppression, resulting in infertility (48%), headache (39%), oligomenorrhea (29%) and galactorrhea (24%).1 Most hypogonadal symptoms are more likely to occur with prolactin levels greater than 100 ng/mL, whereas infertility and ovulation dysfunction can occur with mild to moderate hyperprolactinemia, respectively. Prolonged amenorrhea can risk bone mineral density loss.
While the focus of our discussion is the effect of prolactin on women, men with hyperprolactinemia can experience hypogonadotropic hypogonadism with resultant decreased libido, impotence, infertility, gynecomastia, or, rarely, galactorrhea.2
The three Ps – physiological, pharmacologic, pathological
Physiological causes of hyperprolactinemia include rising estradiol during the late follicular phase and into the secretory phase of the menstrual cycle or while taking combined oral contraception, nipple stimulation, pregnancy, lactation, meals, sleep, and stress.
Drugs can interrupt the dopaminergic pathway, thereby elevating serum prolactin but usually not above 100 ng/mL, except for the antipsychotic drug risperidone, which can cause marked elevation up to 300 or even 400 ng/mL. Medications that can cause hyperprolactinemia are estrogens, neuroleptic drugs such as risperidone, metoclopramide, antidepressant drugs, cimetidine, methyldopa, and verapamil.
A pituitary MRI can diagnose an adenoma, that is, a collection of cells in the pituitary that are responsible for hyperprolactinemia and is named based on its size. Microadenomas are less than 1 cm and are typically associated with serum prolactin values below 200 ng/mL. Macroadenomas can worsen while a patient is on combined oral contraception and during pregnancy; fortunately, this is not the case with a microadenoma.
Hypothyroidism can elevate serum prolactin since thyrotropin releasing hormone is known to stimulate prolactin secretion.3 Consequently, when a patient presents with both hypothyroidism and hyperprolactinemia, thyroid replacement should be initiated for thyroid regulation and potential restoration of prolactin levels. If hyperprolactinemia persists, then further evaluation is required. Chronic renal impairment can also elevate prolactin levels due to decreased clearance.
Management
The appropriate evaluation of hyperprolactinemia consists of a history to disclose medications, identify galactorrhea, and visual changes. Because of an adenoma compressing the optic chiasm, partial blindness may occur where vision is lost in the outer half of both the right and left visual field, called bitemporal hemianopsia. Mild elevations in prolactin should be tested at a time when physiological influences are at a minimum, that is, during menses, fasting, and in late morning.4 Persistent elevations should be appropriately evaluated rather than by using the empiric “shotgun” approach of prescribing a dopamine agonist. Laboratory testing for repeated elevations in prolactin includes a pituitary MRI looking for a mass in the hypothalamic-pituitary region that interrupts dopamine suppression.
Treatment of hyperprolactinemia begins with a dopamine agonist and is indicated when there is hypogonadism or intolerable galactorrhea. Cabergoline is the first choice because of effectiveness (reduced adenoma size in greater than 90% of patients) and lesser side effects, particularly nausea, than bromocriptine. Dopamine agonists, such as bromocriptine and cabergoline, belong to the category of ergot-derived dopamine agonists and have been used to treat Parkinson’s disease. At high doses used to treat Parkinson’s, cabergoline is associated with an increased risk of valvular heart disease. In the United States, pergolide was voluntarily withdrawn from the market in March 2007 because of this risk. At the lower doses generally used for the treatment of hyperprolactinemia, cabergoline is probably not associated with excess risk.5
Newer dopamine agonists are known as nonergot. These are pramipexole, ropinirole, rotigotine, and apomorphine. They have not been associated with a risk of heart damage and can be prescribed.
The initial prescribing dose of cabergoline should be 0.25 mg twice a week or 0.5 mg once a week. If bromocriptine is used, the starting dose is 1.25 mg after dinner or at bedtime for 1 week, then increasing to 1.25 mg twice a day (after breakfast and after dinner or at bedtime to reduce nausea and fatigue). After 1 month of a dopamine agonist, the patient should be evaluated for side effects and a serum prolactin level should be obtained. With a normal prolactin level, gonadal function will probably return within a few months. The dopamine agonist should typically be discontinued with pregnancy as pregnancy increases prolactin physiologically.
Treatment of a macroadenoma is essential when the tumor is large enough to cause neurologic symptoms, such as visual impairment or headache, and is preferable when it is invasive or when there are enlarging microadenomas since they are likely to continue to grow and become symptomatic. About 95% of microadenomas have not been shown to increase in size during 4-6 years of observation.6
Transsphenoidal surgery should be considered when there is:
- Persistent hyperprolactinemia and/or size of the adenoma, with associated symptoms or signs despite several months of dopamine agonist treatment at high doses.
- Presence of a giant lactotroph adenoma (e.g., >3 cm) with pregnancy desired including those whose adenoma responds to a dopamine agonist – to avoid significant growth during pregnancy while off medication.
Data from over 6,000 pregnancies suggest that the administration of bromocriptine during the first month of pregnancy does not harm the fetus.7
Discontinuing treatment
Three scenarios may allow for cessation of dopamine agonist therapy. The first is when a patient has had a normal serum prolactin test following 2 years of low-dose dopamine agonist. Another is the patient who had hyperprolactinemia and a microadenoma that responded to treatment with a normal prolactin level and no further evidence of an adenoma by MRI for at least 2 years. Lastly, the patient who had a macroadenoma prior to treatment and a subsequent normal serum prolactin level without an adenoma for at least 2 years.
Like the management of thyroid dysfunction, our field must be aware of prolactin disorders for early detection, prompt referral, and appropriate management to minimize long-term consequences.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Bayrak A et al. Fertil Steril. 2005 Jul;84(1):181-5.
2. Carter JN et al. N Engl J Med. 1978 Oct 19;299(16):847-52.
3. Sachson R et al. N Engl J Med. 1972;287:972.
4. Singh SP and Singh TP. Ann Endocrinol (Paris). 1984;45(2):137-41.
5. Valassi E et al. J Clin Endocrinol Metab. 2010 Mar;95(3):1025-33.
6. Sisam DA et al. Fertil Steril. 1987 Jul;48(1):67-71.
7. Molitch ME. Best Pract Res Clin Endocrinol Metab. 2011 Dec;25(6):885-96.
Because of the increasing popularity and success of in vitro fertilization, the field of reproductive endocrinology and infertility has steadily morphed toward the treatment of infertility. Nevertheless, a physician board certified in reproductive endocrinology and infertility is the referring physician of choice regarding prolactin disorders and gynecologists should be familiar with the symptoms and sequela of prolactin elevations. This month’s column will address when to obtain a serum prolactin and how to appropriately manage hyperprolactinemia.
Of all the anterior pituitary hormones (adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone ), prolactin is the only one under tonic inhibition by dopamine. Disturbances in this dopaminergic pathway result in elevated serum prolactin. The normal range for prolactin is approximately 5-20 ng/mL.
In the nonpregnant state, little is known regarding the purpose of prolactin, which is produced by the anterior pituitary cluster of cells called lactotrophs. To prepare the breast for postpartum lactation, increases in prolactin are necessary and sustained throughout pregnancy. Second to pregnancy, amenorrhea can occur in 10%-20% of cases of hyperprolactinemia. Outside of pregnancy, elevations in prolactin result in hypogonadism, through gonadotropin-releasing hormone suppression, resulting in infertility (48%), headache (39%), oligomenorrhea (29%) and galactorrhea (24%).1 Most hypogonadal symptoms are more likely to occur with prolactin levels greater than 100 ng/mL, whereas infertility and ovulation dysfunction can occur with mild to moderate hyperprolactinemia, respectively. Prolonged amenorrhea can risk bone mineral density loss.
While the focus of our discussion is the effect of prolactin on women, men with hyperprolactinemia can experience hypogonadotropic hypogonadism with resultant decreased libido, impotence, infertility, gynecomastia, or, rarely, galactorrhea.2
The three Ps – physiological, pharmacologic, pathological
Physiological causes of hyperprolactinemia include rising estradiol during the late follicular phase and into the secretory phase of the menstrual cycle or while taking combined oral contraception, nipple stimulation, pregnancy, lactation, meals, sleep, and stress.
Drugs can interrupt the dopaminergic pathway, thereby elevating serum prolactin but usually not above 100 ng/mL, except for the antipsychotic drug risperidone, which can cause marked elevation up to 300 or even 400 ng/mL. Medications that can cause hyperprolactinemia are estrogens, neuroleptic drugs such as risperidone, metoclopramide, antidepressant drugs, cimetidine, methyldopa, and verapamil.
A pituitary MRI can diagnose an adenoma, that is, a collection of cells in the pituitary that are responsible for hyperprolactinemia and is named based on its size. Microadenomas are less than 1 cm and are typically associated with serum prolactin values below 200 ng/mL. Macroadenomas can worsen while a patient is on combined oral contraception and during pregnancy; fortunately, this is not the case with a microadenoma.
Hypothyroidism can elevate serum prolactin since thyrotropin releasing hormone is known to stimulate prolactin secretion.3 Consequently, when a patient presents with both hypothyroidism and hyperprolactinemia, thyroid replacement should be initiated for thyroid regulation and potential restoration of prolactin levels. If hyperprolactinemia persists, then further evaluation is required. Chronic renal impairment can also elevate prolactin levels due to decreased clearance.
Management
The appropriate evaluation of hyperprolactinemia consists of a history to disclose medications, identify galactorrhea, and visual changes. Because of an adenoma compressing the optic chiasm, partial blindness may occur where vision is lost in the outer half of both the right and left visual field, called bitemporal hemianopsia. Mild elevations in prolactin should be tested at a time when physiological influences are at a minimum, that is, during menses, fasting, and in late morning.4 Persistent elevations should be appropriately evaluated rather than by using the empiric “shotgun” approach of prescribing a dopamine agonist. Laboratory testing for repeated elevations in prolactin includes a pituitary MRI looking for a mass in the hypothalamic-pituitary region that interrupts dopamine suppression.
Treatment of hyperprolactinemia begins with a dopamine agonist and is indicated when there is hypogonadism or intolerable galactorrhea. Cabergoline is the first choice because of effectiveness (reduced adenoma size in greater than 90% of patients) and lesser side effects, particularly nausea, than bromocriptine. Dopamine agonists, such as bromocriptine and cabergoline, belong to the category of ergot-derived dopamine agonists and have been used to treat Parkinson’s disease. At high doses used to treat Parkinson’s, cabergoline is associated with an increased risk of valvular heart disease. In the United States, pergolide was voluntarily withdrawn from the market in March 2007 because of this risk. At the lower doses generally used for the treatment of hyperprolactinemia, cabergoline is probably not associated with excess risk.5
Newer dopamine agonists are known as nonergot. These are pramipexole, ropinirole, rotigotine, and apomorphine. They have not been associated with a risk of heart damage and can be prescribed.
The initial prescribing dose of cabergoline should be 0.25 mg twice a week or 0.5 mg once a week. If bromocriptine is used, the starting dose is 1.25 mg after dinner or at bedtime for 1 week, then increasing to 1.25 mg twice a day (after breakfast and after dinner or at bedtime to reduce nausea and fatigue). After 1 month of a dopamine agonist, the patient should be evaluated for side effects and a serum prolactin level should be obtained. With a normal prolactin level, gonadal function will probably return within a few months. The dopamine agonist should typically be discontinued with pregnancy as pregnancy increases prolactin physiologically.
Treatment of a macroadenoma is essential when the tumor is large enough to cause neurologic symptoms, such as visual impairment or headache, and is preferable when it is invasive or when there are enlarging microadenomas since they are likely to continue to grow and become symptomatic. About 95% of microadenomas have not been shown to increase in size during 4-6 years of observation.6
Transsphenoidal surgery should be considered when there is:
- Persistent hyperprolactinemia and/or size of the adenoma, with associated symptoms or signs despite several months of dopamine agonist treatment at high doses.
- Presence of a giant lactotroph adenoma (e.g., >3 cm) with pregnancy desired including those whose adenoma responds to a dopamine agonist – to avoid significant growth during pregnancy while off medication.
Data from over 6,000 pregnancies suggest that the administration of bromocriptine during the first month of pregnancy does not harm the fetus.7
Discontinuing treatment
Three scenarios may allow for cessation of dopamine agonist therapy. The first is when a patient has had a normal serum prolactin test following 2 years of low-dose dopamine agonist. Another is the patient who had hyperprolactinemia and a microadenoma that responded to treatment with a normal prolactin level and no further evidence of an adenoma by MRI for at least 2 years. Lastly, the patient who had a macroadenoma prior to treatment and a subsequent normal serum prolactin level without an adenoma for at least 2 years.
Like the management of thyroid dysfunction, our field must be aware of prolactin disorders for early detection, prompt referral, and appropriate management to minimize long-term consequences.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Bayrak A et al. Fertil Steril. 2005 Jul;84(1):181-5.
2. Carter JN et al. N Engl J Med. 1978 Oct 19;299(16):847-52.
3. Sachson R et al. N Engl J Med. 1972;287:972.
4. Singh SP and Singh TP. Ann Endocrinol (Paris). 1984;45(2):137-41.
5. Valassi E et al. J Clin Endocrinol Metab. 2010 Mar;95(3):1025-33.
6. Sisam DA et al. Fertil Steril. 1987 Jul;48(1):67-71.
7. Molitch ME. Best Pract Res Clin Endocrinol Metab. 2011 Dec;25(6):885-96.
Because of the increasing popularity and success of in vitro fertilization, the field of reproductive endocrinology and infertility has steadily morphed toward the treatment of infertility. Nevertheless, a physician board certified in reproductive endocrinology and infertility is the referring physician of choice regarding prolactin disorders and gynecologists should be familiar with the symptoms and sequela of prolactin elevations. This month’s column will address when to obtain a serum prolactin and how to appropriately manage hyperprolactinemia.
Of all the anterior pituitary hormones (adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone ), prolactin is the only one under tonic inhibition by dopamine. Disturbances in this dopaminergic pathway result in elevated serum prolactin. The normal range for prolactin is approximately 5-20 ng/mL.
In the nonpregnant state, little is known regarding the purpose of prolactin, which is produced by the anterior pituitary cluster of cells called lactotrophs. To prepare the breast for postpartum lactation, increases in prolactin are necessary and sustained throughout pregnancy. Second to pregnancy, amenorrhea can occur in 10%-20% of cases of hyperprolactinemia. Outside of pregnancy, elevations in prolactin result in hypogonadism, through gonadotropin-releasing hormone suppression, resulting in infertility (48%), headache (39%), oligomenorrhea (29%) and galactorrhea (24%).1 Most hypogonadal symptoms are more likely to occur with prolactin levels greater than 100 ng/mL, whereas infertility and ovulation dysfunction can occur with mild to moderate hyperprolactinemia, respectively. Prolonged amenorrhea can risk bone mineral density loss.
While the focus of our discussion is the effect of prolactin on women, men with hyperprolactinemia can experience hypogonadotropic hypogonadism with resultant decreased libido, impotence, infertility, gynecomastia, or, rarely, galactorrhea.2
The three Ps – physiological, pharmacologic, pathological
Physiological causes of hyperprolactinemia include rising estradiol during the late follicular phase and into the secretory phase of the menstrual cycle or while taking combined oral contraception, nipple stimulation, pregnancy, lactation, meals, sleep, and stress.
Drugs can interrupt the dopaminergic pathway, thereby elevating serum prolactin but usually not above 100 ng/mL, except for the antipsychotic drug risperidone, which can cause marked elevation up to 300 or even 400 ng/mL. Medications that can cause hyperprolactinemia are estrogens, neuroleptic drugs such as risperidone, metoclopramide, antidepressant drugs, cimetidine, methyldopa, and verapamil.
A pituitary MRI can diagnose an adenoma, that is, a collection of cells in the pituitary that are responsible for hyperprolactinemia and is named based on its size. Microadenomas are less than 1 cm and are typically associated with serum prolactin values below 200 ng/mL. Macroadenomas can worsen while a patient is on combined oral contraception and during pregnancy; fortunately, this is not the case with a microadenoma.
Hypothyroidism can elevate serum prolactin since thyrotropin releasing hormone is known to stimulate prolactin secretion.3 Consequently, when a patient presents with both hypothyroidism and hyperprolactinemia, thyroid replacement should be initiated for thyroid regulation and potential restoration of prolactin levels. If hyperprolactinemia persists, then further evaluation is required. Chronic renal impairment can also elevate prolactin levels due to decreased clearance.
Management
The appropriate evaluation of hyperprolactinemia consists of a history to disclose medications, identify galactorrhea, and visual changes. Because of an adenoma compressing the optic chiasm, partial blindness may occur where vision is lost in the outer half of both the right and left visual field, called bitemporal hemianopsia. Mild elevations in prolactin should be tested at a time when physiological influences are at a minimum, that is, during menses, fasting, and in late morning.4 Persistent elevations should be appropriately evaluated rather than by using the empiric “shotgun” approach of prescribing a dopamine agonist. Laboratory testing for repeated elevations in prolactin includes a pituitary MRI looking for a mass in the hypothalamic-pituitary region that interrupts dopamine suppression.
Treatment of hyperprolactinemia begins with a dopamine agonist and is indicated when there is hypogonadism or intolerable galactorrhea. Cabergoline is the first choice because of effectiveness (reduced adenoma size in greater than 90% of patients) and lesser side effects, particularly nausea, than bromocriptine. Dopamine agonists, such as bromocriptine and cabergoline, belong to the category of ergot-derived dopamine agonists and have been used to treat Parkinson’s disease. At high doses used to treat Parkinson’s, cabergoline is associated with an increased risk of valvular heart disease. In the United States, pergolide was voluntarily withdrawn from the market in March 2007 because of this risk. At the lower doses generally used for the treatment of hyperprolactinemia, cabergoline is probably not associated with excess risk.5
Newer dopamine agonists are known as nonergot. These are pramipexole, ropinirole, rotigotine, and apomorphine. They have not been associated with a risk of heart damage and can be prescribed.
The initial prescribing dose of cabergoline should be 0.25 mg twice a week or 0.5 mg once a week. If bromocriptine is used, the starting dose is 1.25 mg after dinner or at bedtime for 1 week, then increasing to 1.25 mg twice a day (after breakfast and after dinner or at bedtime to reduce nausea and fatigue). After 1 month of a dopamine agonist, the patient should be evaluated for side effects and a serum prolactin level should be obtained. With a normal prolactin level, gonadal function will probably return within a few months. The dopamine agonist should typically be discontinued with pregnancy as pregnancy increases prolactin physiologically.
Treatment of a macroadenoma is essential when the tumor is large enough to cause neurologic symptoms, such as visual impairment or headache, and is preferable when it is invasive or when there are enlarging microadenomas since they are likely to continue to grow and become symptomatic. About 95% of microadenomas have not been shown to increase in size during 4-6 years of observation.6
Transsphenoidal surgery should be considered when there is:
- Persistent hyperprolactinemia and/or size of the adenoma, with associated symptoms or signs despite several months of dopamine agonist treatment at high doses.
- Presence of a giant lactotroph adenoma (e.g., >3 cm) with pregnancy desired including those whose adenoma responds to a dopamine agonist – to avoid significant growth during pregnancy while off medication.
Data from over 6,000 pregnancies suggest that the administration of bromocriptine during the first month of pregnancy does not harm the fetus.7
Discontinuing treatment
Three scenarios may allow for cessation of dopamine agonist therapy. The first is when a patient has had a normal serum prolactin test following 2 years of low-dose dopamine agonist. Another is the patient who had hyperprolactinemia and a microadenoma that responded to treatment with a normal prolactin level and no further evidence of an adenoma by MRI for at least 2 years. Lastly, the patient who had a macroadenoma prior to treatment and a subsequent normal serum prolactin level without an adenoma for at least 2 years.
Like the management of thyroid dysfunction, our field must be aware of prolactin disorders for early detection, prompt referral, and appropriate management to minimize long-term consequences.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Bayrak A et al. Fertil Steril. 2005 Jul;84(1):181-5.
2. Carter JN et al. N Engl J Med. 1978 Oct 19;299(16):847-52.
3. Sachson R et al. N Engl J Med. 1972;287:972.
4. Singh SP and Singh TP. Ann Endocrinol (Paris). 1984;45(2):137-41.
5. Valassi E et al. J Clin Endocrinol Metab. 2010 Mar;95(3):1025-33.
6. Sisam DA et al. Fertil Steril. 1987 Jul;48(1):67-71.
7. Molitch ME. Best Pract Res Clin Endocrinol Metab. 2011 Dec;25(6):885-96.
EMA panel backs linzagolix for uterine fibroid symptoms
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) on December 17 recommended approval of linzagolix (Yselty, ObsEva), an oral gonadotropin-releasing hormone (GnRH) antagonist, for the management of moderate to severe symptoms of uterine fibroids (UF) in adult women of reproductive age.
If approved, linzagolix – which is taken once per day – would become the first GnRH receptor antagonist with a nonhormonal option to reach the market. The U.S. Food and Drug Administration in November accepted ObsEva’s new drug application for the medication, with a decision expected by September 2022.
“The positive CHMP opinion is an important milestone for millions of women in the EU living with UF to address the diverse medical needs of the women who suffer from this condition,” said Brian O’Callaghan, CEO of ObsEva, in a statement. “We will continue our productive, ongoing dialogue with [the] EMA toward potential marketing authorization in the EU and, in parallel, continue to work with the FDA to advance linzagolix through the U.S. regulatory process.”
The committee’s positive opinion was based on 52-week results from PRIMROSE 1 and PRIMROSE 2 phase 3 trials, involving more than 1,000 patients in the United States and Europe, as well as results from 76-week follow-up studies of patients in those trials. The two phase 3 trials assessed a 200-mg and 100-mg dose of linzagolix, with and without hormone add-back therapy (ABT; 1 mg estradiol and 0.5 mg norethisterone acetate).
According to ObsEVA, both trials met their primary endpoints, with all doses showing statistically significant and clinically relevant reductions in heavy menstrual bleeding (HMB) compared to placebo. The trials also achieved several secondary endpoints, including reduction in pain, rates of amenorrhea, time to reduced HMB, and amenorrhea and for the high dose without ABT, reductions in uterine and fibroid volume, the company said.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) on December 17 recommended approval of linzagolix (Yselty, ObsEva), an oral gonadotropin-releasing hormone (GnRH) antagonist, for the management of moderate to severe symptoms of uterine fibroids (UF) in adult women of reproductive age.
If approved, linzagolix – which is taken once per day – would become the first GnRH receptor antagonist with a nonhormonal option to reach the market. The U.S. Food and Drug Administration in November accepted ObsEva’s new drug application for the medication, with a decision expected by September 2022.
“The positive CHMP opinion is an important milestone for millions of women in the EU living with UF to address the diverse medical needs of the women who suffer from this condition,” said Brian O’Callaghan, CEO of ObsEva, in a statement. “We will continue our productive, ongoing dialogue with [the] EMA toward potential marketing authorization in the EU and, in parallel, continue to work with the FDA to advance linzagolix through the U.S. regulatory process.”
The committee’s positive opinion was based on 52-week results from PRIMROSE 1 and PRIMROSE 2 phase 3 trials, involving more than 1,000 patients in the United States and Europe, as well as results from 76-week follow-up studies of patients in those trials. The two phase 3 trials assessed a 200-mg and 100-mg dose of linzagolix, with and without hormone add-back therapy (ABT; 1 mg estradiol and 0.5 mg norethisterone acetate).
According to ObsEVA, both trials met their primary endpoints, with all doses showing statistically significant and clinically relevant reductions in heavy menstrual bleeding (HMB) compared to placebo. The trials also achieved several secondary endpoints, including reduction in pain, rates of amenorrhea, time to reduced HMB, and amenorrhea and for the high dose without ABT, reductions in uterine and fibroid volume, the company said.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) on December 17 recommended approval of linzagolix (Yselty, ObsEva), an oral gonadotropin-releasing hormone (GnRH) antagonist, for the management of moderate to severe symptoms of uterine fibroids (UF) in adult women of reproductive age.
If approved, linzagolix – which is taken once per day – would become the first GnRH receptor antagonist with a nonhormonal option to reach the market. The U.S. Food and Drug Administration in November accepted ObsEva’s new drug application for the medication, with a decision expected by September 2022.
“The positive CHMP opinion is an important milestone for millions of women in the EU living with UF to address the diverse medical needs of the women who suffer from this condition,” said Brian O’Callaghan, CEO of ObsEva, in a statement. “We will continue our productive, ongoing dialogue with [the] EMA toward potential marketing authorization in the EU and, in parallel, continue to work with the FDA to advance linzagolix through the U.S. regulatory process.”
The committee’s positive opinion was based on 52-week results from PRIMROSE 1 and PRIMROSE 2 phase 3 trials, involving more than 1,000 patients in the United States and Europe, as well as results from 76-week follow-up studies of patients in those trials. The two phase 3 trials assessed a 200-mg and 100-mg dose of linzagolix, with and without hormone add-back therapy (ABT; 1 mg estradiol and 0.5 mg norethisterone acetate).
According to ObsEVA, both trials met their primary endpoints, with all doses showing statistically significant and clinically relevant reductions in heavy menstrual bleeding (HMB) compared to placebo. The trials also achieved several secondary endpoints, including reduction in pain, rates of amenorrhea, time to reduced HMB, and amenorrhea and for the high dose without ABT, reductions in uterine and fibroid volume, the company said.
A version of this article first appeared on Medscape.com.









