User login
Restless legs a new modifiable risk factor for dementia?
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
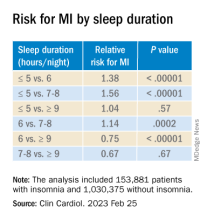
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY
Strong support for CBT as first-line treatment for insomnia in seniors
NEW ORLEANS –
“The lack of awareness among clinicians who take care of older adults that CBT for insomnia (CBT-I) is an effective treatment for insomnia is an issue,” Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry, Creighton University, Omaha, Neb., told this news organization.
Dr. Tampi was among the speakers during a session as part of the American Association for Geriatric Psychiatry annual meeting addressing the complex challenges of treating insomnia in older patients, who tend to have higher rates of insomnia than their younger counterparts.
The prevalence of insomnia in older adults is estimated to be 20%-40%, and medication is frequently the first treatment choice, a less than ideal approach, said Dr. Tampi.
“Prescribing sedatives and hypnotics, which can cause severe adverse effects, without a thorough assessment that includes comorbidities that may be causing the insomnia” is among the biggest mistakes clinicians make in the treatment of insomnia in older patients, Dr. Tampi said in an interview.
“It’s our duty as providers to first take a good assessment, talk about polymorbidity, and try to address those conditions, and judiciously use medications in conjunction with at least components of CBT-I,” he said.
Long-term safety, efficacy unclear
About one-third of older adults take at least one form of pharmacological treatment for insomnia symptoms, said Ebony Dix, MD, assistant professor of psychiatry at Yale University, New Haven, Conn., in a separate talk during the session. This, despite the low-risk profile of CBT and recommendations from various medical societies that CBT should be tried first.
Dr. Dix noted that medications approved for insomnia by the U.S. Food and Drug Administration, including melatonin receptor agonists, heterocyclics, and dual orexin receptor antagonists (DORAs), can play an important role in the short-term management of insomnia, but their long-term effects are unknown.
“Pharmacotherapeutic agents may be effective in the short term, but there is a lack of sufficient, statistically significant data to support the long-term safety and efficacy of any [sleep] medication, especially in aging adults, due to the impact of hypnotic drugs on sleep architecture, the impact of aging on pharmacokinetics, as well as polypharmacy and drug-to-drug interactions,” Dr. Dix said. She noted that clinical trials of insomnia drugs rarely include geriatric patients.
The American Academy of Sleep Medicine recommends CBT-I as first-line treatment for insomnia, with the key benefit being its exemplary safety profile, said Shilpa Srinivasan, MD, a professor of clinical psychiatry at the University of South Carolina, Columbia, who also presented during the session.
“The biggest [attribute] of CBT-I management strategies is the low risk of side effects,” she said. “How many medications can we say that about?”
The CBT-I intervention includes a focus on key components of lifestyle and mental health issues to improve sleep. These include the following:
- Strictly restricting sleep hours for bedtime and arising (with napping discouraged).
- Control of stimulus to disrupt falling asleep.
- Cognitive therapy to identify and replace maladaptive beliefs.
- Control of sleep hygiene for optimal sleep.
- Relaxation training.
Keys to success
Dr. Srinivasan noted one recent study of CBT-I among patients aged 60 and older with insomnia and depression. The 156 participants randomized to receive weekly 120-minute CBT-I sessions over 2 months were significantly less likely to develop new or recurrent major depression versus their counterparts randomized to receive sleep education (hazard ratio, 0.51; P = .02).
However, CBT-I is more labor intensive than medication and requires provider training and motivation, and commitment on the part of the patient, to be successful.
“We really need to ensure that even when patients are receiving pharmacologic interventions for insomnia that we provide psychoeducation. At the end of the day, some of these nonpharmacologic components can make or break the success of pharmacotherapy,” said Dr. Srinivasan.
Whether using CBT-I alone or in combination with pharmacotherapy, the intervention does not necessarily have to include all components to be beneficial, she said.
“I think one of the challenges in incorporating CBT-I is the misconception that it is an all-or-nothing approach wherein every modality must be utilized,” she said. “While multicomponent CBT-I has been shown to be effective, the individual components can be incorporated into patient encounters in a stepped approach.”
Informing patients that they have options other than medications and involving them in decision-making is key, she added.
“In the case of insomnia, this is particularly relevant because of the physical and emotional distress that it causes,” Dr. Srinivasan said. “Patients often seek over-the-counter medications or other nonprescribed agents to try to obtain relief even before seeking treatment in a health care setting. There is less awareness about evidence-based and effective nonpharmacologic treatments such as CBT-I.”
Dr. Tampi, Dr. Dix, and Dr. Srinivasan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“The lack of awareness among clinicians who take care of older adults that CBT for insomnia (CBT-I) is an effective treatment for insomnia is an issue,” Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry, Creighton University, Omaha, Neb., told this news organization.
Dr. Tampi was among the speakers during a session as part of the American Association for Geriatric Psychiatry annual meeting addressing the complex challenges of treating insomnia in older patients, who tend to have higher rates of insomnia than their younger counterparts.
The prevalence of insomnia in older adults is estimated to be 20%-40%, and medication is frequently the first treatment choice, a less than ideal approach, said Dr. Tampi.
“Prescribing sedatives and hypnotics, which can cause severe adverse effects, without a thorough assessment that includes comorbidities that may be causing the insomnia” is among the biggest mistakes clinicians make in the treatment of insomnia in older patients, Dr. Tampi said in an interview.
“It’s our duty as providers to first take a good assessment, talk about polymorbidity, and try to address those conditions, and judiciously use medications in conjunction with at least components of CBT-I,” he said.
Long-term safety, efficacy unclear
About one-third of older adults take at least one form of pharmacological treatment for insomnia symptoms, said Ebony Dix, MD, assistant professor of psychiatry at Yale University, New Haven, Conn., in a separate talk during the session. This, despite the low-risk profile of CBT and recommendations from various medical societies that CBT should be tried first.
Dr. Dix noted that medications approved for insomnia by the U.S. Food and Drug Administration, including melatonin receptor agonists, heterocyclics, and dual orexin receptor antagonists (DORAs), can play an important role in the short-term management of insomnia, but their long-term effects are unknown.
“Pharmacotherapeutic agents may be effective in the short term, but there is a lack of sufficient, statistically significant data to support the long-term safety and efficacy of any [sleep] medication, especially in aging adults, due to the impact of hypnotic drugs on sleep architecture, the impact of aging on pharmacokinetics, as well as polypharmacy and drug-to-drug interactions,” Dr. Dix said. She noted that clinical trials of insomnia drugs rarely include geriatric patients.
The American Academy of Sleep Medicine recommends CBT-I as first-line treatment for insomnia, with the key benefit being its exemplary safety profile, said Shilpa Srinivasan, MD, a professor of clinical psychiatry at the University of South Carolina, Columbia, who also presented during the session.
“The biggest [attribute] of CBT-I management strategies is the low risk of side effects,” she said. “How many medications can we say that about?”
The CBT-I intervention includes a focus on key components of lifestyle and mental health issues to improve sleep. These include the following:
- Strictly restricting sleep hours for bedtime and arising (with napping discouraged).
- Control of stimulus to disrupt falling asleep.
- Cognitive therapy to identify and replace maladaptive beliefs.
- Control of sleep hygiene for optimal sleep.
- Relaxation training.
Keys to success
Dr. Srinivasan noted one recent study of CBT-I among patients aged 60 and older with insomnia and depression. The 156 participants randomized to receive weekly 120-minute CBT-I sessions over 2 months were significantly less likely to develop new or recurrent major depression versus their counterparts randomized to receive sleep education (hazard ratio, 0.51; P = .02).
However, CBT-I is more labor intensive than medication and requires provider training and motivation, and commitment on the part of the patient, to be successful.
“We really need to ensure that even when patients are receiving pharmacologic interventions for insomnia that we provide psychoeducation. At the end of the day, some of these nonpharmacologic components can make or break the success of pharmacotherapy,” said Dr. Srinivasan.
Whether using CBT-I alone or in combination with pharmacotherapy, the intervention does not necessarily have to include all components to be beneficial, she said.
“I think one of the challenges in incorporating CBT-I is the misconception that it is an all-or-nothing approach wherein every modality must be utilized,” she said. “While multicomponent CBT-I has been shown to be effective, the individual components can be incorporated into patient encounters in a stepped approach.”
Informing patients that they have options other than medications and involving them in decision-making is key, she added.
“In the case of insomnia, this is particularly relevant because of the physical and emotional distress that it causes,” Dr. Srinivasan said. “Patients often seek over-the-counter medications or other nonprescribed agents to try to obtain relief even before seeking treatment in a health care setting. There is less awareness about evidence-based and effective nonpharmacologic treatments such as CBT-I.”
Dr. Tampi, Dr. Dix, and Dr. Srinivasan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“The lack of awareness among clinicians who take care of older adults that CBT for insomnia (CBT-I) is an effective treatment for insomnia is an issue,” Rajesh R. Tampi, MD, professor and chairman of the department of psychiatry, Creighton University, Omaha, Neb., told this news organization.
Dr. Tampi was among the speakers during a session as part of the American Association for Geriatric Psychiatry annual meeting addressing the complex challenges of treating insomnia in older patients, who tend to have higher rates of insomnia than their younger counterparts.
The prevalence of insomnia in older adults is estimated to be 20%-40%, and medication is frequently the first treatment choice, a less than ideal approach, said Dr. Tampi.
“Prescribing sedatives and hypnotics, which can cause severe adverse effects, without a thorough assessment that includes comorbidities that may be causing the insomnia” is among the biggest mistakes clinicians make in the treatment of insomnia in older patients, Dr. Tampi said in an interview.
“It’s our duty as providers to first take a good assessment, talk about polymorbidity, and try to address those conditions, and judiciously use medications in conjunction with at least components of CBT-I,” he said.
Long-term safety, efficacy unclear
About one-third of older adults take at least one form of pharmacological treatment for insomnia symptoms, said Ebony Dix, MD, assistant professor of psychiatry at Yale University, New Haven, Conn., in a separate talk during the session. This, despite the low-risk profile of CBT and recommendations from various medical societies that CBT should be tried first.
Dr. Dix noted that medications approved for insomnia by the U.S. Food and Drug Administration, including melatonin receptor agonists, heterocyclics, and dual orexin receptor antagonists (DORAs), can play an important role in the short-term management of insomnia, but their long-term effects are unknown.
“Pharmacotherapeutic agents may be effective in the short term, but there is a lack of sufficient, statistically significant data to support the long-term safety and efficacy of any [sleep] medication, especially in aging adults, due to the impact of hypnotic drugs on sleep architecture, the impact of aging on pharmacokinetics, as well as polypharmacy and drug-to-drug interactions,” Dr. Dix said. She noted that clinical trials of insomnia drugs rarely include geriatric patients.
The American Academy of Sleep Medicine recommends CBT-I as first-line treatment for insomnia, with the key benefit being its exemplary safety profile, said Shilpa Srinivasan, MD, a professor of clinical psychiatry at the University of South Carolina, Columbia, who also presented during the session.
“The biggest [attribute] of CBT-I management strategies is the low risk of side effects,” she said. “How many medications can we say that about?”
The CBT-I intervention includes a focus on key components of lifestyle and mental health issues to improve sleep. These include the following:
- Strictly restricting sleep hours for bedtime and arising (with napping discouraged).
- Control of stimulus to disrupt falling asleep.
- Cognitive therapy to identify and replace maladaptive beliefs.
- Control of sleep hygiene for optimal sleep.
- Relaxation training.
Keys to success
Dr. Srinivasan noted one recent study of CBT-I among patients aged 60 and older with insomnia and depression. The 156 participants randomized to receive weekly 120-minute CBT-I sessions over 2 months were significantly less likely to develop new or recurrent major depression versus their counterparts randomized to receive sleep education (hazard ratio, 0.51; P = .02).
However, CBT-I is more labor intensive than medication and requires provider training and motivation, and commitment on the part of the patient, to be successful.
“We really need to ensure that even when patients are receiving pharmacologic interventions for insomnia that we provide psychoeducation. At the end of the day, some of these nonpharmacologic components can make or break the success of pharmacotherapy,” said Dr. Srinivasan.
Whether using CBT-I alone or in combination with pharmacotherapy, the intervention does not necessarily have to include all components to be beneficial, she said.
“I think one of the challenges in incorporating CBT-I is the misconception that it is an all-or-nothing approach wherein every modality must be utilized,” she said. “While multicomponent CBT-I has been shown to be effective, the individual components can be incorporated into patient encounters in a stepped approach.”
Informing patients that they have options other than medications and involving them in decision-making is key, she added.
“In the case of insomnia, this is particularly relevant because of the physical and emotional distress that it causes,” Dr. Srinivasan said. “Patients often seek over-the-counter medications or other nonprescribed agents to try to obtain relief even before seeking treatment in a health care setting. There is less awareness about evidence-based and effective nonpharmacologic treatments such as CBT-I.”
Dr. Tampi, Dr. Dix, and Dr. Srinivasan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAGP 2023
Central Sleep Apnea in Adults: Diagnosis and Treatment
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring.If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring.If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring.If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
Childhood nightmares a prelude to cognitive problems, Parkinson’s?
new research shows.
Compared with children who never had distressing dreams between ages 7 and 11 years, those who had persistent distressing dreams were 76% more likely to develop cognitive impairment and roughly seven times more likely to develop PD by age 50 years.
It’s been shown previously that sleep problems in adulthood, including distressing dreams, can precede the onset of neurodegenerative diseases such as Alzheimer’s disease (AD) or PD by several years, and in some cases decades, study investigator Abidemi Otaiku, BMBS, University of Birmingham (England), told this news organization.
However, no studies have investigated whether distressing dreams during childhood might also be associated with increased risk for cognitive decline or PD.
“As such, these findings provide evidence for the first time that certain sleep problems in childhood (having regular distressing dreams) could be an early indicator of increased dementia and PD risk,” Dr. Otaiku said.
He noted that the findings build on previous studies which showed that regular nightmares in childhood could be an early indicator for psychiatric problems in adolescence, such as borderline personality disorder, attention-deficit/hyperactivity disorder, and psychosis.
The study was published online February 26 in The Lancet journal eClinicalMedicine.
Statistically significant
The prospective, longitudinal analysis used data from the 1958 British Birth Cohort Study, a prospective birth cohort which included all people born in Britain during a single week in 1958.
At age 7 years (in 1965) and 11 years (in 1969), mothers were asked to report whether their child experienced “bad dreams or night terrors” in the past 3 months, and cognitive impairment and PD were determined at age 50 (2008).
Among a total of 6,991 children (51% girls), 78.2% never had distressing dreams, 17.9% had transient distressing dreams (either at ages 7 or 11 years), and 3.8% had persistent distressing dreams (at both ages 7 and 11 years).
By age 50, 262 participants had developed cognitive impairment, and five had been diagnosed with PD.
After adjusting for all covariates, having more regular distressing dreams during childhood was “linearly and statistically significantly” associated with higher risk of developing cognitive impairment or PD by age 50 years (P = .037). This was the case in both boys and girls.
Compared with children who never had bad dreams, peers who had persistent distressing dreams (at ages 7 and 11 years) had an 85% increased risk for cognitive impairment or PD by age 50 (adjusted odds ratio, 1.85; 95% confidence interval, 1.10-3.11; P = .019).
The associations remained when incident cognitive impairment and incident PD were analyzed separately.
Compared with children who never had distressing dreams, children who had persistent distressing dreams were 76% more likely to develop cognitive impairment by age 50 years (aOR, 1.76; 95% CI, 1.03-2.99; P = .037), and were about seven times more likely to be diagnosed with PD by age 50 years (aOR, 7.35; 95% CI, 1.03-52.73; P = .047).
The linear association was statistically significant for PD (P = .050) and had a trend toward statistical significance for cognitive impairment (P = .074).
Mechanism unclear
“Early-life nightmares might be causally associated with cognitive impairment and PD, noncausally associated with cognitive impairment and PD, or both. At this stage it remains unclear which of the three options is correct. Therefore, further research on mechanisms is needed,” Dr. Otaiku told this news organization.
“One plausible noncausal explanation is that there are shared genetic factors which predispose individuals to having frequent nightmares in childhood, and to developing neurodegenerative diseases such as AD or PD in adulthood,” he added.
It’s also plausible that having regular nightmares throughout childhood could be a causal risk factor for cognitive impairment and PD by causing chronic sleep disruption, he noted.
“Chronic sleep disruption due to nightmares might lead to impaired glymphatic clearance during sleep – and thus greater accumulation of pathological proteins in the brain, such as amyloid-beta and alpha-synuclein,” Dr. Otaiku said.
Disrupted sleep throughout childhood might also impair normal brain development, which could make children’s brains less resilient to neuropathologic damage, he said.
Clinical implications?
There are established treatments for childhood nightmares, including nonpharmacologic approaches.
“For children who have regular nightmares that lead to impaired daytime functioning, it may well be a good idea for them to see a sleep physician to discuss whether treatment may be needed,” Dr. Otaiku said.
But should doctors treat children with persistent nightmares for the purpose of preventing neurodegenerative diseases in adulthood or psychiatric problems in adolescence?
“It’s an interesting possibility. However, more research is needed to confirm these epidemiological associations and to determine whether or not nightmares are a causal risk factor for these conditions,” Dr. Otaiku concluded.
The study received no external funding. Dr. Otaiku reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
Compared with children who never had distressing dreams between ages 7 and 11 years, those who had persistent distressing dreams were 76% more likely to develop cognitive impairment and roughly seven times more likely to develop PD by age 50 years.
It’s been shown previously that sleep problems in adulthood, including distressing dreams, can precede the onset of neurodegenerative diseases such as Alzheimer’s disease (AD) or PD by several years, and in some cases decades, study investigator Abidemi Otaiku, BMBS, University of Birmingham (England), told this news organization.
However, no studies have investigated whether distressing dreams during childhood might also be associated with increased risk for cognitive decline or PD.
“As such, these findings provide evidence for the first time that certain sleep problems in childhood (having regular distressing dreams) could be an early indicator of increased dementia and PD risk,” Dr. Otaiku said.
He noted that the findings build on previous studies which showed that regular nightmares in childhood could be an early indicator for psychiatric problems in adolescence, such as borderline personality disorder, attention-deficit/hyperactivity disorder, and psychosis.
The study was published online February 26 in The Lancet journal eClinicalMedicine.
Statistically significant
The prospective, longitudinal analysis used data from the 1958 British Birth Cohort Study, a prospective birth cohort which included all people born in Britain during a single week in 1958.
At age 7 years (in 1965) and 11 years (in 1969), mothers were asked to report whether their child experienced “bad dreams or night terrors” in the past 3 months, and cognitive impairment and PD were determined at age 50 (2008).
Among a total of 6,991 children (51% girls), 78.2% never had distressing dreams, 17.9% had transient distressing dreams (either at ages 7 or 11 years), and 3.8% had persistent distressing dreams (at both ages 7 and 11 years).
By age 50, 262 participants had developed cognitive impairment, and five had been diagnosed with PD.
After adjusting for all covariates, having more regular distressing dreams during childhood was “linearly and statistically significantly” associated with higher risk of developing cognitive impairment or PD by age 50 years (P = .037). This was the case in both boys and girls.
Compared with children who never had bad dreams, peers who had persistent distressing dreams (at ages 7 and 11 years) had an 85% increased risk for cognitive impairment or PD by age 50 (adjusted odds ratio, 1.85; 95% confidence interval, 1.10-3.11; P = .019).
The associations remained when incident cognitive impairment and incident PD were analyzed separately.
Compared with children who never had distressing dreams, children who had persistent distressing dreams were 76% more likely to develop cognitive impairment by age 50 years (aOR, 1.76; 95% CI, 1.03-2.99; P = .037), and were about seven times more likely to be diagnosed with PD by age 50 years (aOR, 7.35; 95% CI, 1.03-52.73; P = .047).
The linear association was statistically significant for PD (P = .050) and had a trend toward statistical significance for cognitive impairment (P = .074).
Mechanism unclear
“Early-life nightmares might be causally associated with cognitive impairment and PD, noncausally associated with cognitive impairment and PD, or both. At this stage it remains unclear which of the three options is correct. Therefore, further research on mechanisms is needed,” Dr. Otaiku told this news organization.
“One plausible noncausal explanation is that there are shared genetic factors which predispose individuals to having frequent nightmares in childhood, and to developing neurodegenerative diseases such as AD or PD in adulthood,” he added.
It’s also plausible that having regular nightmares throughout childhood could be a causal risk factor for cognitive impairment and PD by causing chronic sleep disruption, he noted.
“Chronic sleep disruption due to nightmares might lead to impaired glymphatic clearance during sleep – and thus greater accumulation of pathological proteins in the brain, such as amyloid-beta and alpha-synuclein,” Dr. Otaiku said.
Disrupted sleep throughout childhood might also impair normal brain development, which could make children’s brains less resilient to neuropathologic damage, he said.
Clinical implications?
There are established treatments for childhood nightmares, including nonpharmacologic approaches.
“For children who have regular nightmares that lead to impaired daytime functioning, it may well be a good idea for them to see a sleep physician to discuss whether treatment may be needed,” Dr. Otaiku said.
But should doctors treat children with persistent nightmares for the purpose of preventing neurodegenerative diseases in adulthood or psychiatric problems in adolescence?
“It’s an interesting possibility. However, more research is needed to confirm these epidemiological associations and to determine whether or not nightmares are a causal risk factor for these conditions,” Dr. Otaiku concluded.
The study received no external funding. Dr. Otaiku reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
Compared with children who never had distressing dreams between ages 7 and 11 years, those who had persistent distressing dreams were 76% more likely to develop cognitive impairment and roughly seven times more likely to develop PD by age 50 years.
It’s been shown previously that sleep problems in adulthood, including distressing dreams, can precede the onset of neurodegenerative diseases such as Alzheimer’s disease (AD) or PD by several years, and in some cases decades, study investigator Abidemi Otaiku, BMBS, University of Birmingham (England), told this news organization.
However, no studies have investigated whether distressing dreams during childhood might also be associated with increased risk for cognitive decline or PD.
“As such, these findings provide evidence for the first time that certain sleep problems in childhood (having regular distressing dreams) could be an early indicator of increased dementia and PD risk,” Dr. Otaiku said.
He noted that the findings build on previous studies which showed that regular nightmares in childhood could be an early indicator for psychiatric problems in adolescence, such as borderline personality disorder, attention-deficit/hyperactivity disorder, and psychosis.
The study was published online February 26 in The Lancet journal eClinicalMedicine.
Statistically significant
The prospective, longitudinal analysis used data from the 1958 British Birth Cohort Study, a prospective birth cohort which included all people born in Britain during a single week in 1958.
At age 7 years (in 1965) and 11 years (in 1969), mothers were asked to report whether their child experienced “bad dreams or night terrors” in the past 3 months, and cognitive impairment and PD were determined at age 50 (2008).
Among a total of 6,991 children (51% girls), 78.2% never had distressing dreams, 17.9% had transient distressing dreams (either at ages 7 or 11 years), and 3.8% had persistent distressing dreams (at both ages 7 and 11 years).
By age 50, 262 participants had developed cognitive impairment, and five had been diagnosed with PD.
After adjusting for all covariates, having more regular distressing dreams during childhood was “linearly and statistically significantly” associated with higher risk of developing cognitive impairment or PD by age 50 years (P = .037). This was the case in both boys and girls.
Compared with children who never had bad dreams, peers who had persistent distressing dreams (at ages 7 and 11 years) had an 85% increased risk for cognitive impairment or PD by age 50 (adjusted odds ratio, 1.85; 95% confidence interval, 1.10-3.11; P = .019).
The associations remained when incident cognitive impairment and incident PD were analyzed separately.
Compared with children who never had distressing dreams, children who had persistent distressing dreams were 76% more likely to develop cognitive impairment by age 50 years (aOR, 1.76; 95% CI, 1.03-2.99; P = .037), and were about seven times more likely to be diagnosed with PD by age 50 years (aOR, 7.35; 95% CI, 1.03-52.73; P = .047).
The linear association was statistically significant for PD (P = .050) and had a trend toward statistical significance for cognitive impairment (P = .074).
Mechanism unclear
“Early-life nightmares might be causally associated with cognitive impairment and PD, noncausally associated with cognitive impairment and PD, or both. At this stage it remains unclear which of the three options is correct. Therefore, further research on mechanisms is needed,” Dr. Otaiku told this news organization.
“One plausible noncausal explanation is that there are shared genetic factors which predispose individuals to having frequent nightmares in childhood, and to developing neurodegenerative diseases such as AD or PD in adulthood,” he added.
It’s also plausible that having regular nightmares throughout childhood could be a causal risk factor for cognitive impairment and PD by causing chronic sleep disruption, he noted.
“Chronic sleep disruption due to nightmares might lead to impaired glymphatic clearance during sleep – and thus greater accumulation of pathological proteins in the brain, such as amyloid-beta and alpha-synuclein,” Dr. Otaiku said.
Disrupted sleep throughout childhood might also impair normal brain development, which could make children’s brains less resilient to neuropathologic damage, he said.
Clinical implications?
There are established treatments for childhood nightmares, including nonpharmacologic approaches.
“For children who have regular nightmares that lead to impaired daytime functioning, it may well be a good idea for them to see a sleep physician to discuss whether treatment may be needed,” Dr. Otaiku said.
But should doctors treat children with persistent nightmares for the purpose of preventing neurodegenerative diseases in adulthood or psychiatric problems in adolescence?
“It’s an interesting possibility. However, more research is needed to confirm these epidemiological associations and to determine whether or not nightmares are a causal risk factor for these conditions,” Dr. Otaiku concluded.
The study received no external funding. Dr. Otaiku reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
Iron deficiency in psychiatric patients
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34
Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34

Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34

Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
Insomnia, short sleep linked to greater risk for MI
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Myths about smoking, diet, alcohol, and cancer persist
FRANCE – Conducted every 5 years since 2005, the Cancer Survey documents the knowledge, perceptions, and way of life of the French people in relation to cancer. The researchers analyzed responses to telephone interviews of a representative sample of almost 5,000 individuals aged 15-85 years.
This study shows how thinking has changed over time and how difficult it is to alter preconceived notions.
Is cancer hereditary?
The report shows that 67.7% of respondents believe that cancer is a hereditary disease. Respondents were asked to explain their answer. “Data show that medical practices for cancer treatment substantiate this belief [that cancer is hereditary],” wrote the authors of the report.
“Indeed, health care professionals almost systematically ask questions about family history of breast cancer and, when a family member has been diagnosed with cancer, medical monitoring of other family members is often sought out, thus reinforcing the belief that cancer is hereditary,” they said.
Furthermore, there seems to be confusion regarding the role of genes in the development of cancer. A person can inherit cancer-predisposing genes, not cancer itself. The authors highlighted their concern that this confusion may “lead people to think that prevention measures are unnecessary because cancer is inherited.”
Misconceptions about smoking
About 41% of smokers think that the length of time one has been smoking is the biggest determining factor for developing cancer; 58.1% think the number of cigarettes smoked per day has a bigger impact.
Experts at InCA and SPF put the debate to rest, stating that prolonged exposure to carcinogenic substances is far more toxic. As for the danger threshold concerning the number of cigarettes smoked per day, respondents believed this to be 9.2 cigarettes per day, on average. They believed that the danger threshold for the number of years as an active smoker is 13.4, on average.
“The [survey] respondents clearly understand that smoking carries a risk, but many smokers think that light smoking or smoking for a short period of time doesn’t carry any risks.” Yet it is understood that even occasional tobacco consumption increases mortality.
This was not the only misconception regarding smoking and its relationship with cancer. About 34% of survey respondents agreed with the following statement: “Smoking doesn’t cause cancer unless you’re a heavy smoker and have smoked for a long time.” Furthermore, 43.3% agreed with the statement, “Pollution is more likely to cause cancer than smoking,” 54.6% think that “exercising cleans your lungs of tobacco,” and 61.6% think that “a smoker can prevent developing cancer caused by smoking if they know to quit on time.”
Overweight and obesity
Although diet and excess weight represent the third and fourth biggest avoidable cancer risk factors, after smoking and alcohol, only 30% of survey respondents knew of this link.
“Among the causes of cancer known and cited by respondents without prompting, excessive weight and obesity were mentioned only 100 times out of 12,558 responses,” highlighted the authors of the report. The explanation put forward by the authors is that discourse about diet has been more focused on diet as a protective health factor, especially in preventing cardiovascular diseases. “The link between cancer and diet is less prominent in the public space,” they noted.
Breastfeeding and cancer
About 63% of survey respondents, which for the first time included both women and men, believe that breastfeeding does not affect mothers’ risk of breast cancer, but this is a misconception. And almost 1 in 3 respondents said that breastfeeding provides health benefits for the mother.
Artificial UV rays
Exposure to UV rays, whether of natural or artificial origin, is a major risk factor for skin cancer. However, 1 in 5 people (20.9%) think that a session in a tanning bed is less harmful than sun exposure.
Daily stress
Regarding psychological factors linked to cancer, the authors noted that risk factors not supported by scientific evidence were, ironically, cited more often by respondents than proven risk factors. There is a real knowledge gap between scientific data and the beliefs of the French people. For example, “working at night” is largely not seen as a risk factor, but data show that it presents a clear risk. However, “not being able to express one’s feelings,” “having been weakened by traumatic experiences,” and “being exposed to the stress of modern life” are seen as risk factors of cancer, without any scientific evidence.
Cigarettes and e-cigarettes
About 53% of respondents agreed that “e-cigarettes are just as harmful or more harmful than traditional cigarettes.” Nicotine and the flavors in e-cigarettes are largely perceived as “very” or “extremely” harmful to the health of a person. However, the authors note that “no published study on nicotine substitutes has shown harmful effects on the health of a person, let alone determined it a risk factor for cancer. The nicotine doses in e-cigarettes are similar to traditional nicotine substitutes, and no cytotoxic effect of nicotine in its inhaled form has been found.” There seems to be confusion between dependence and risk of cancer.
Alcohol consumption
Eight of 10 respondents believe that “some people can drink a lot of alcohol all their life without ever getting cancer,” which goes against the scientific literature. The authors of the report state that the negative effects of alcohol on health seem poorly understood. Although alcohol is the second biggest cause of cancer, only a third of survey respondents cited it without having been prompted as one of the main causes of cancer. And 23.5% even think that “in terms of decreasing your risk of cancer, it’s better to drink a little wine than to drink no wine at all.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
FRANCE – Conducted every 5 years since 2005, the Cancer Survey documents the knowledge, perceptions, and way of life of the French people in relation to cancer. The researchers analyzed responses to telephone interviews of a representative sample of almost 5,000 individuals aged 15-85 years.
This study shows how thinking has changed over time and how difficult it is to alter preconceived notions.
Is cancer hereditary?
The report shows that 67.7% of respondents believe that cancer is a hereditary disease. Respondents were asked to explain their answer. “Data show that medical practices for cancer treatment substantiate this belief [that cancer is hereditary],” wrote the authors of the report.
“Indeed, health care professionals almost systematically ask questions about family history of breast cancer and, when a family member has been diagnosed with cancer, medical monitoring of other family members is often sought out, thus reinforcing the belief that cancer is hereditary,” they said.
Furthermore, there seems to be confusion regarding the role of genes in the development of cancer. A person can inherit cancer-predisposing genes, not cancer itself. The authors highlighted their concern that this confusion may “lead people to think that prevention measures are unnecessary because cancer is inherited.”
Misconceptions about smoking
About 41% of smokers think that the length of time one has been smoking is the biggest determining factor for developing cancer; 58.1% think the number of cigarettes smoked per day has a bigger impact.
Experts at InCA and SPF put the debate to rest, stating that prolonged exposure to carcinogenic substances is far more toxic. As for the danger threshold concerning the number of cigarettes smoked per day, respondents believed this to be 9.2 cigarettes per day, on average. They believed that the danger threshold for the number of years as an active smoker is 13.4, on average.
“The [survey] respondents clearly understand that smoking carries a risk, but many smokers think that light smoking or smoking for a short period of time doesn’t carry any risks.” Yet it is understood that even occasional tobacco consumption increases mortality.
This was not the only misconception regarding smoking and its relationship with cancer. About 34% of survey respondents agreed with the following statement: “Smoking doesn’t cause cancer unless you’re a heavy smoker and have smoked for a long time.” Furthermore, 43.3% agreed with the statement, “Pollution is more likely to cause cancer than smoking,” 54.6% think that “exercising cleans your lungs of tobacco,” and 61.6% think that “a smoker can prevent developing cancer caused by smoking if they know to quit on time.”
Overweight and obesity
Although diet and excess weight represent the third and fourth biggest avoidable cancer risk factors, after smoking and alcohol, only 30% of survey respondents knew of this link.
“Among the causes of cancer known and cited by respondents without prompting, excessive weight and obesity were mentioned only 100 times out of 12,558 responses,” highlighted the authors of the report. The explanation put forward by the authors is that discourse about diet has been more focused on diet as a protective health factor, especially in preventing cardiovascular diseases. “The link between cancer and diet is less prominent in the public space,” they noted.
Breastfeeding and cancer
About 63% of survey respondents, which for the first time included both women and men, believe that breastfeeding does not affect mothers’ risk of breast cancer, but this is a misconception. And almost 1 in 3 respondents said that breastfeeding provides health benefits for the mother.
Artificial UV rays
Exposure to UV rays, whether of natural or artificial origin, is a major risk factor for skin cancer. However, 1 in 5 people (20.9%) think that a session in a tanning bed is less harmful than sun exposure.
Daily stress
Regarding psychological factors linked to cancer, the authors noted that risk factors not supported by scientific evidence were, ironically, cited more often by respondents than proven risk factors. There is a real knowledge gap between scientific data and the beliefs of the French people. For example, “working at night” is largely not seen as a risk factor, but data show that it presents a clear risk. However, “not being able to express one’s feelings,” “having been weakened by traumatic experiences,” and “being exposed to the stress of modern life” are seen as risk factors of cancer, without any scientific evidence.
Cigarettes and e-cigarettes
About 53% of respondents agreed that “e-cigarettes are just as harmful or more harmful than traditional cigarettes.” Nicotine and the flavors in e-cigarettes are largely perceived as “very” or “extremely” harmful to the health of a person. However, the authors note that “no published study on nicotine substitutes has shown harmful effects on the health of a person, let alone determined it a risk factor for cancer. The nicotine doses in e-cigarettes are similar to traditional nicotine substitutes, and no cytotoxic effect of nicotine in its inhaled form has been found.” There seems to be confusion between dependence and risk of cancer.
Alcohol consumption
Eight of 10 respondents believe that “some people can drink a lot of alcohol all their life without ever getting cancer,” which goes against the scientific literature. The authors of the report state that the negative effects of alcohol on health seem poorly understood. Although alcohol is the second biggest cause of cancer, only a third of survey respondents cited it without having been prompted as one of the main causes of cancer. And 23.5% even think that “in terms of decreasing your risk of cancer, it’s better to drink a little wine than to drink no wine at all.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
FRANCE – Conducted every 5 years since 2005, the Cancer Survey documents the knowledge, perceptions, and way of life of the French people in relation to cancer. The researchers analyzed responses to telephone interviews of a representative sample of almost 5,000 individuals aged 15-85 years.
This study shows how thinking has changed over time and how difficult it is to alter preconceived notions.
Is cancer hereditary?
The report shows that 67.7% of respondents believe that cancer is a hereditary disease. Respondents were asked to explain their answer. “Data show that medical practices for cancer treatment substantiate this belief [that cancer is hereditary],” wrote the authors of the report.
“Indeed, health care professionals almost systematically ask questions about family history of breast cancer and, when a family member has been diagnosed with cancer, medical monitoring of other family members is often sought out, thus reinforcing the belief that cancer is hereditary,” they said.
Furthermore, there seems to be confusion regarding the role of genes in the development of cancer. A person can inherit cancer-predisposing genes, not cancer itself. The authors highlighted their concern that this confusion may “lead people to think that prevention measures are unnecessary because cancer is inherited.”
Misconceptions about smoking
About 41% of smokers think that the length of time one has been smoking is the biggest determining factor for developing cancer; 58.1% think the number of cigarettes smoked per day has a bigger impact.
Experts at InCA and SPF put the debate to rest, stating that prolonged exposure to carcinogenic substances is far more toxic. As for the danger threshold concerning the number of cigarettes smoked per day, respondents believed this to be 9.2 cigarettes per day, on average. They believed that the danger threshold for the number of years as an active smoker is 13.4, on average.
“The [survey] respondents clearly understand that smoking carries a risk, but many smokers think that light smoking or smoking for a short period of time doesn’t carry any risks.” Yet it is understood that even occasional tobacco consumption increases mortality.
This was not the only misconception regarding smoking and its relationship with cancer. About 34% of survey respondents agreed with the following statement: “Smoking doesn’t cause cancer unless you’re a heavy smoker and have smoked for a long time.” Furthermore, 43.3% agreed with the statement, “Pollution is more likely to cause cancer than smoking,” 54.6% think that “exercising cleans your lungs of tobacco,” and 61.6% think that “a smoker can prevent developing cancer caused by smoking if they know to quit on time.”
Overweight and obesity
Although diet and excess weight represent the third and fourth biggest avoidable cancer risk factors, after smoking and alcohol, only 30% of survey respondents knew of this link.
“Among the causes of cancer known and cited by respondents without prompting, excessive weight and obesity were mentioned only 100 times out of 12,558 responses,” highlighted the authors of the report. The explanation put forward by the authors is that discourse about diet has been more focused on diet as a protective health factor, especially in preventing cardiovascular diseases. “The link between cancer and diet is less prominent in the public space,” they noted.
Breastfeeding and cancer
About 63% of survey respondents, which for the first time included both women and men, believe that breastfeeding does not affect mothers’ risk of breast cancer, but this is a misconception. And almost 1 in 3 respondents said that breastfeeding provides health benefits for the mother.
Artificial UV rays
Exposure to UV rays, whether of natural or artificial origin, is a major risk factor for skin cancer. However, 1 in 5 people (20.9%) think that a session in a tanning bed is less harmful than sun exposure.
Daily stress
Regarding psychological factors linked to cancer, the authors noted that risk factors not supported by scientific evidence were, ironically, cited more often by respondents than proven risk factors. There is a real knowledge gap between scientific data and the beliefs of the French people. For example, “working at night” is largely not seen as a risk factor, but data show that it presents a clear risk. However, “not being able to express one’s feelings,” “having been weakened by traumatic experiences,” and “being exposed to the stress of modern life” are seen as risk factors of cancer, without any scientific evidence.
Cigarettes and e-cigarettes
About 53% of respondents agreed that “e-cigarettes are just as harmful or more harmful than traditional cigarettes.” Nicotine and the flavors in e-cigarettes are largely perceived as “very” or “extremely” harmful to the health of a person. However, the authors note that “no published study on nicotine substitutes has shown harmful effects on the health of a person, let alone determined it a risk factor for cancer. The nicotine doses in e-cigarettes are similar to traditional nicotine substitutes, and no cytotoxic effect of nicotine in its inhaled form has been found.” There seems to be confusion between dependence and risk of cancer.
Alcohol consumption
Eight of 10 respondents believe that “some people can drink a lot of alcohol all their life without ever getting cancer,” which goes against the scientific literature. The authors of the report state that the negative effects of alcohol on health seem poorly understood. Although alcohol is the second biggest cause of cancer, only a third of survey respondents cited it without having been prompted as one of the main causes of cancer. And 23.5% even think that “in terms of decreasing your risk of cancer, it’s better to drink a little wine than to drink no wine at all.”
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Irregular sleep tied to markers of atherosclerosis
a new report suggests.
In particular, variation in sleep duration of more than 2 hours per night in the same week was tied to higher rates of atherosclerosis.
“Poor sleep is linked with several cardiovascular conditions, including heart disease, hypertension, and type 2 diabetes,” lead author Kelsie M. Full, PhD, MPH, assistant professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview.
“Overall, we found that participants who slept varying amounts of hours throughout the week (meaning that one night they slept less, one night they slept more) were more likely to have atherosclerosis than participants who slept about the same amount of time each night,” she said.
The study was published online in the Journal of the American Heart Association.
Analyzing associations
Dr. Full and colleagues examined data from 2032 participants in the Multi-Ethnic Study of Atherosclerosis Sleep Ancillary Study, which included adults aged between 45 and 84 years in six U.S. communities who completed 7-day wrist actigraphy assessment and kept a sleep diary between 2010 and 2013.
For subclinical markers of cardiovascular disease, participants underwent assessments of coronary artery calcium, carotid plaque presence, carotid intima-media thickness, and ankle-brachial index.
The research team assessed sleep duration, or the total number of minutes of sleep in a night, and sleep timing regularity, which was determined on the basis of the time someone initially fell asleep each night. They adjusted for cardiovascular disease risk factors and sleep characteristics, such as obstructive sleep apnea, sleep duration, and sleep fragmentation.
The average age of the participants was 68.6 years, and 53.6% were women. About 37.9% identified as White, 27.6% as Black or African American, 23.4% as Hispanic American, and 11.1% as Chinese American.
During the 7-day period, about 38% of participants experienced a change in sleep duration of more than 90 minutes, and 18% experienced a sleep duration change of more than 120 minutes. Those who had irregular sleep were more likely to be non-White, current smokers, have lower average annual incomes, have work shift schedules or did not work, and have a higher average body mass index.
For the study, sleep duration irregularity was defined as a standard deviation of more than 120 minutes. Those participants who had a greater degree of sleep irregularity were more likely to have high coronary artery calcium burden than those whose sleep duration was more more regular, defined as an SD of 60 minutes or less (> 300; prevalence ratio, 1.33; 95% confidence interval, 1.03-1.71), as well as abnormal ankle-brachial index (< 0.9, prevalence ratio, 1.75;95% CI, 1.03-2.95).
Further, those with irregular sleep timing (SD > 90 minutes) were more likely to have a high coronary artery calcium burden (prevalence ratio, 1.39; 95% CI, 1.07-1.82) in comparison with those with more regular sleep timing (SD < 30 minutes).
“The biggest surprise to me was that 30% of the participants in the study had total sleep times that varied by more than 90 minutes over the course of the week,” Dr. Full said. “This is consistent with prior studies that suggest that a large proportion of the general public have irregular sleep patterns, not just shift workers.”
Investigating next steps
In additional analyses, Dr. Full and colleagues found that sleep duration regularity continued to be associated with high coronary artery calcium burden and abnormal ankle-brachial index when accounting for severe obstructive sleep apnea, average nightly sleep duration, and average sleep fragmentation.
Notably, when sleep duration was added, all participants with more irregular sleep durations (SD > 60 minutes) were more likely to have a high coronary artery calcium burden, compared with those with regular sleep durations (SD < 60 minutes). The results remained when participants who reported shift work, including night shift work, were excluded.
Additional studies are needed to understand the mechanisms, the study authors wrote. Night-to-night variability in sleep duration and sleep timing can cause desynchronization in the sleep-wake timing and circadian disruption.
“A key issue highlighted in this study is that sleep irregularity itself, independent of how much sleep people were getting, was related to heart health. Sleep is a naturally recurring phenomenon, and maintaining regularity helps provide stability and predictability to the body,” Michael Grandner, PhD, associate professor of psychiatry and director of the sleep and health research program at the University of Arizona, Tucson, said in an interview.
Dr. Grandner, who wasn’t involved with this study, has researched sleep irregularity and associations with cardiovascular disease, diabetes, obesity, and many other adverse outcomes.
“When people have very irregular sleep schedules, it may make it harder for the body to optimally make good use of the sleep it is getting, since it such a moving target,” he said. “The unique angle here is the ability to focus on regularity of sleep.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health. One author received grants and consulting fees from pharmaceutical companies unrelated to the research. The other authors and Dr. Grandner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new report suggests.
In particular, variation in sleep duration of more than 2 hours per night in the same week was tied to higher rates of atherosclerosis.
“Poor sleep is linked with several cardiovascular conditions, including heart disease, hypertension, and type 2 diabetes,” lead author Kelsie M. Full, PhD, MPH, assistant professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview.
“Overall, we found that participants who slept varying amounts of hours throughout the week (meaning that one night they slept less, one night they slept more) were more likely to have atherosclerosis than participants who slept about the same amount of time each night,” she said.
The study was published online in the Journal of the American Heart Association.
Analyzing associations
Dr. Full and colleagues examined data from 2032 participants in the Multi-Ethnic Study of Atherosclerosis Sleep Ancillary Study, which included adults aged between 45 and 84 years in six U.S. communities who completed 7-day wrist actigraphy assessment and kept a sleep diary between 2010 and 2013.
For subclinical markers of cardiovascular disease, participants underwent assessments of coronary artery calcium, carotid plaque presence, carotid intima-media thickness, and ankle-brachial index.
The research team assessed sleep duration, or the total number of minutes of sleep in a night, and sleep timing regularity, which was determined on the basis of the time someone initially fell asleep each night. They adjusted for cardiovascular disease risk factors and sleep characteristics, such as obstructive sleep apnea, sleep duration, and sleep fragmentation.
The average age of the participants was 68.6 years, and 53.6% were women. About 37.9% identified as White, 27.6% as Black or African American, 23.4% as Hispanic American, and 11.1% as Chinese American.
During the 7-day period, about 38% of participants experienced a change in sleep duration of more than 90 minutes, and 18% experienced a sleep duration change of more than 120 minutes. Those who had irregular sleep were more likely to be non-White, current smokers, have lower average annual incomes, have work shift schedules or did not work, and have a higher average body mass index.
For the study, sleep duration irregularity was defined as a standard deviation of more than 120 minutes. Those participants who had a greater degree of sleep irregularity were more likely to have high coronary artery calcium burden than those whose sleep duration was more more regular, defined as an SD of 60 minutes or less (> 300; prevalence ratio, 1.33; 95% confidence interval, 1.03-1.71), as well as abnormal ankle-brachial index (< 0.9, prevalence ratio, 1.75;95% CI, 1.03-2.95).
Further, those with irregular sleep timing (SD > 90 minutes) were more likely to have a high coronary artery calcium burden (prevalence ratio, 1.39; 95% CI, 1.07-1.82) in comparison with those with more regular sleep timing (SD < 30 minutes).
“The biggest surprise to me was that 30% of the participants in the study had total sleep times that varied by more than 90 minutes over the course of the week,” Dr. Full said. “This is consistent with prior studies that suggest that a large proportion of the general public have irregular sleep patterns, not just shift workers.”
Investigating next steps
In additional analyses, Dr. Full and colleagues found that sleep duration regularity continued to be associated with high coronary artery calcium burden and abnormal ankle-brachial index when accounting for severe obstructive sleep apnea, average nightly sleep duration, and average sleep fragmentation.
Notably, when sleep duration was added, all participants with more irregular sleep durations (SD > 60 minutes) were more likely to have a high coronary artery calcium burden, compared with those with regular sleep durations (SD < 60 minutes). The results remained when participants who reported shift work, including night shift work, were excluded.
Additional studies are needed to understand the mechanisms, the study authors wrote. Night-to-night variability in sleep duration and sleep timing can cause desynchronization in the sleep-wake timing and circadian disruption.
“A key issue highlighted in this study is that sleep irregularity itself, independent of how much sleep people were getting, was related to heart health. Sleep is a naturally recurring phenomenon, and maintaining regularity helps provide stability and predictability to the body,” Michael Grandner, PhD, associate professor of psychiatry and director of the sleep and health research program at the University of Arizona, Tucson, said in an interview.
Dr. Grandner, who wasn’t involved with this study, has researched sleep irregularity and associations with cardiovascular disease, diabetes, obesity, and many other adverse outcomes.
“When people have very irregular sleep schedules, it may make it harder for the body to optimally make good use of the sleep it is getting, since it such a moving target,” he said. “The unique angle here is the ability to focus on regularity of sleep.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health. One author received grants and consulting fees from pharmaceutical companies unrelated to the research. The other authors and Dr. Grandner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new report suggests.
In particular, variation in sleep duration of more than 2 hours per night in the same week was tied to higher rates of atherosclerosis.
“Poor sleep is linked with several cardiovascular conditions, including heart disease, hypertension, and type 2 diabetes,” lead author Kelsie M. Full, PhD, MPH, assistant professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview.
“Overall, we found that participants who slept varying amounts of hours throughout the week (meaning that one night they slept less, one night they slept more) were more likely to have atherosclerosis than participants who slept about the same amount of time each night,” she said.
The study was published online in the Journal of the American Heart Association.
Analyzing associations
Dr. Full and colleagues examined data from 2032 participants in the Multi-Ethnic Study of Atherosclerosis Sleep Ancillary Study, which included adults aged between 45 and 84 years in six U.S. communities who completed 7-day wrist actigraphy assessment and kept a sleep diary between 2010 and 2013.
For subclinical markers of cardiovascular disease, participants underwent assessments of coronary artery calcium, carotid plaque presence, carotid intima-media thickness, and ankle-brachial index.
The research team assessed sleep duration, or the total number of minutes of sleep in a night, and sleep timing regularity, which was determined on the basis of the time someone initially fell asleep each night. They adjusted for cardiovascular disease risk factors and sleep characteristics, such as obstructive sleep apnea, sleep duration, and sleep fragmentation.
The average age of the participants was 68.6 years, and 53.6% were women. About 37.9% identified as White, 27.6% as Black or African American, 23.4% as Hispanic American, and 11.1% as Chinese American.
During the 7-day period, about 38% of participants experienced a change in sleep duration of more than 90 minutes, and 18% experienced a sleep duration change of more than 120 minutes. Those who had irregular sleep were more likely to be non-White, current smokers, have lower average annual incomes, have work shift schedules or did not work, and have a higher average body mass index.
For the study, sleep duration irregularity was defined as a standard deviation of more than 120 minutes. Those participants who had a greater degree of sleep irregularity were more likely to have high coronary artery calcium burden than those whose sleep duration was more more regular, defined as an SD of 60 minutes or less (> 300; prevalence ratio, 1.33; 95% confidence interval, 1.03-1.71), as well as abnormal ankle-brachial index (< 0.9, prevalence ratio, 1.75;95% CI, 1.03-2.95).
Further, those with irregular sleep timing (SD > 90 minutes) were more likely to have a high coronary artery calcium burden (prevalence ratio, 1.39; 95% CI, 1.07-1.82) in comparison with those with more regular sleep timing (SD < 30 minutes).
“The biggest surprise to me was that 30% of the participants in the study had total sleep times that varied by more than 90 minutes over the course of the week,” Dr. Full said. “This is consistent with prior studies that suggest that a large proportion of the general public have irregular sleep patterns, not just shift workers.”
Investigating next steps
In additional analyses, Dr. Full and colleagues found that sleep duration regularity continued to be associated with high coronary artery calcium burden and abnormal ankle-brachial index when accounting for severe obstructive sleep apnea, average nightly sleep duration, and average sleep fragmentation.
Notably, when sleep duration was added, all participants with more irregular sleep durations (SD > 60 minutes) were more likely to have a high coronary artery calcium burden, compared with those with regular sleep durations (SD < 60 minutes). The results remained when participants who reported shift work, including night shift work, were excluded.
Additional studies are needed to understand the mechanisms, the study authors wrote. Night-to-night variability in sleep duration and sleep timing can cause desynchronization in the sleep-wake timing and circadian disruption.
“A key issue highlighted in this study is that sleep irregularity itself, independent of how much sleep people were getting, was related to heart health. Sleep is a naturally recurring phenomenon, and maintaining regularity helps provide stability and predictability to the body,” Michael Grandner, PhD, associate professor of psychiatry and director of the sleep and health research program at the University of Arizona, Tucson, said in an interview.
Dr. Grandner, who wasn’t involved with this study, has researched sleep irregularity and associations with cardiovascular disease, diabetes, obesity, and many other adverse outcomes.
“When people have very irregular sleep schedules, it may make it harder for the body to optimally make good use of the sleep it is getting, since it such a moving target,” he said. “The unique angle here is the ability to focus on regularity of sleep.”
The study was supported by the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health. One author received grants and consulting fees from pharmaceutical companies unrelated to the research. The other authors and Dr. Grandner disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Sleep abnormalities common in all stages of psychosis
For example, compared with their healthy peers, participants in a chronic psychosis stage had reduced density, amplitude, and duration of spindles – or bursts of brainwave activity during sleep identified by electroencephalography.
“The results suggest sleep could be an important target [and] an area of research and clinical intervention that could make a difference” in the lives of patients at risk for psychosis, study investigator Fabio Ferrarelli, MD, PhD, associate professor of psychiatry and director of the Sleep and Schizophrenia Program, University of Pittsburgh School of Medicine, told this news organization.
The findings were published online in JAMA Psychiatry.
‘Window of opportunity’
Researchers separate psychosis into stages. During the “clinically high-risk for psychosis” (CHR-P) stage, patients have milder symptoms but do not have a diagnosable psychotic disorder. Those in the early psychosis (EP) stage have had a first episode of psychosis. When they reach a cut-off, often at 5 years, they are considered to have chronic psychosis (CP).
Previous studies have shown that altered sleep often precedes a psychotic episode in early psychosis, and disrupted sleep contributes to predicting transition to psychosis in youth at risk for the condition. Individuals with CP commonly report sleep disturbances, such as insomnia.
Following a literature search, the investigators for this current meta-analysis selected 21 studies assessing sleep disturbance prevalence in 5,135 patients. They also selected 39 studies measuring sleep alterations subjectively (for example, sleep quality) and/or objectively (for example, sleep architecture and sleep oscillation) in 1,575 patients and 977 healthy controls.
The included studies measured the prevalence of sleep disturbances and/or sleep characteristics at different psychosis stages using polysomnography, EEG, actigraphy, or self-reports.
The pooled prevalence of sleep disturbances was 50% across clinical stages (95% confidence interval, 40%-61%). The prevalence was 54% in CHR-P, 68% in EP, and 44% in CP.
The prevalence of insomnia as the primary sleep disturbance was 34% of pooled cases, 48% of the EP group, and 27% of the CP group.
“What’s interesting is the rate of sleep disturbances is relatively stable across stages,” said Dr. Ferrarelli. “This is important because you have a window of opportunity to do some early intervention in people who are at risk that can prevent things from getting worse.”
He suggests clinicians screen for insomnia in early-course patients and perhaps recommend cognitive behavioral therapy (CBT) for insomnia. As well, they should promote sleep hygiene measures for at-risk patients, including such things as avoiding caffeine, alcohol, and screen time before bedtime and adopting a regular sleep pattern.
“These are people at risk, which means they have a 20%-30% chance of eventually developing a psychotic disorder,” said Dr. Ferrarelli. “Maybe disrupted sleep is one of the factors that can make a difference.”
Altered sleep architecture
To compare sleep quality between clinical and control groups, studies used total scores on the Pittsburgh Sleep Quality Index (PSQI), where a score over 5 indicates a sleep problem.
There was a significant standardized mean difference in pooled cases versus controls (SMD, 1.0; 95% CI, 0.7-1.3; P < .001). Each clinical group showed poorer sleep quality, compared with controls.
When assessing sleep architecture abnormalities, stage-specific case-control comparisons showed these were driven by EP and CP stages.
Altered sleep characteristics in both these stages included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.
Compared with controls, CP was the only clinical group with more arousals. Patients with CP also had more arousals than the CHR-P group, and the number of arousals was significantly affected by medication.
The findings indicate the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, the investigators write.
They add that clinicians should consider medication adjustments, such as decreased doses or switches to another compound.
‘Robust’ spindle results
As for spindle parameters, pooled cases showed significantly decreased spindle density (SMD, –1.06), spindle amplitude (SMD, –1.08), and spindle duration (SMD, −1.21), compared with controls. Stage-specific comparisons revealed these deficits were present in both EP and CP relative to controls.
Dr. Ferrarelli noted the results for spindle abnormalities were among “the most robust” and show that these abnormalities “tend to get worse over the course of the illness.”
The spindle data are “a lot more informative” than that provided by other sleep parameters “in the sense they can yield what could be wrong, where it could be, and potentially what you can do about it,” said Dr. Ferrarelli.
“This might be an objective measure that could be used to identify individuals who have a psychosis disorder, monitor progression of illness, and for prognostic reasons,” he added.
He noted that spindles may also represent a promising target for treatment interventions and added that non-invasive transcranial magnetic stimulation has shown promise in restoring sleep oscillations, including spindles.
Another way to evoke target-brain activity may be through auditory tones – with a patient listening to a particular sound through headphones while asleep, Dr. Ferrarelli said.
Reaffirms previous data
Commenting on the study, Jeffrey A. Lieberman, MD, professor and chair in psychiatry at Columbia University, New York, and a past president of the American Psychiatric Association, noted that the review “just reaffirms what has been reported by individual studies for decades.”
That so many at-risk study subjects had a sleep abnormality is not surprising, said Dr. Lieberman, who was not involved with the current research.
“How many individuals in late adolescence or early adulthood have sleep problems?” he asked. “I would venture to say it’s probably a lot. So the question is: How distinctive is this from what occurs in people who don’t develop the illness?”
The aim of sleep research in the area of schizophrenia has long been to disentangle the effects of medication and environmental factors from the disease and to be able to treat patients to normalize their sleep, said Dr. Lieberman.
“But it’s not clear from these results how one would do that,” he added.
The authors “don’t fundamentally tell us anything about the underlying cause of the illness or the pathophysiology, and they don’t really offer any kind of clear direction for clinical intervention,” he said.
The study was supported by the National Institute of Mental Health. Dr. Ferrarelli reported grants from the National Institute of Mental Health during the conduct of the study. Dr. Lieberman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For example, compared with their healthy peers, participants in a chronic psychosis stage had reduced density, amplitude, and duration of spindles – or bursts of brainwave activity during sleep identified by electroencephalography.
“The results suggest sleep could be an important target [and] an area of research and clinical intervention that could make a difference” in the lives of patients at risk for psychosis, study investigator Fabio Ferrarelli, MD, PhD, associate professor of psychiatry and director of the Sleep and Schizophrenia Program, University of Pittsburgh School of Medicine, told this news organization.
The findings were published online in JAMA Psychiatry.
‘Window of opportunity’
Researchers separate psychosis into stages. During the “clinically high-risk for psychosis” (CHR-P) stage, patients have milder symptoms but do not have a diagnosable psychotic disorder. Those in the early psychosis (EP) stage have had a first episode of psychosis. When they reach a cut-off, often at 5 years, they are considered to have chronic psychosis (CP).
Previous studies have shown that altered sleep often precedes a psychotic episode in early psychosis, and disrupted sleep contributes to predicting transition to psychosis in youth at risk for the condition. Individuals with CP commonly report sleep disturbances, such as insomnia.
Following a literature search, the investigators for this current meta-analysis selected 21 studies assessing sleep disturbance prevalence in 5,135 patients. They also selected 39 studies measuring sleep alterations subjectively (for example, sleep quality) and/or objectively (for example, sleep architecture and sleep oscillation) in 1,575 patients and 977 healthy controls.
The included studies measured the prevalence of sleep disturbances and/or sleep characteristics at different psychosis stages using polysomnography, EEG, actigraphy, or self-reports.
The pooled prevalence of sleep disturbances was 50% across clinical stages (95% confidence interval, 40%-61%). The prevalence was 54% in CHR-P, 68% in EP, and 44% in CP.
The prevalence of insomnia as the primary sleep disturbance was 34% of pooled cases, 48% of the EP group, and 27% of the CP group.
“What’s interesting is the rate of sleep disturbances is relatively stable across stages,” said Dr. Ferrarelli. “This is important because you have a window of opportunity to do some early intervention in people who are at risk that can prevent things from getting worse.”
He suggests clinicians screen for insomnia in early-course patients and perhaps recommend cognitive behavioral therapy (CBT) for insomnia. As well, they should promote sleep hygiene measures for at-risk patients, including such things as avoiding caffeine, alcohol, and screen time before bedtime and adopting a regular sleep pattern.
“These are people at risk, which means they have a 20%-30% chance of eventually developing a psychotic disorder,” said Dr. Ferrarelli. “Maybe disrupted sleep is one of the factors that can make a difference.”
Altered sleep architecture
To compare sleep quality between clinical and control groups, studies used total scores on the Pittsburgh Sleep Quality Index (PSQI), where a score over 5 indicates a sleep problem.
There was a significant standardized mean difference in pooled cases versus controls (SMD, 1.0; 95% CI, 0.7-1.3; P < .001). Each clinical group showed poorer sleep quality, compared with controls.
When assessing sleep architecture abnormalities, stage-specific case-control comparisons showed these were driven by EP and CP stages.
Altered sleep characteristics in both these stages included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.
Compared with controls, CP was the only clinical group with more arousals. Patients with CP also had more arousals than the CHR-P group, and the number of arousals was significantly affected by medication.
The findings indicate the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, the investigators write.
They add that clinicians should consider medication adjustments, such as decreased doses or switches to another compound.
‘Robust’ spindle results
As for spindle parameters, pooled cases showed significantly decreased spindle density (SMD, –1.06), spindle amplitude (SMD, –1.08), and spindle duration (SMD, −1.21), compared with controls. Stage-specific comparisons revealed these deficits were present in both EP and CP relative to controls.
Dr. Ferrarelli noted the results for spindle abnormalities were among “the most robust” and show that these abnormalities “tend to get worse over the course of the illness.”
The spindle data are “a lot more informative” than that provided by other sleep parameters “in the sense they can yield what could be wrong, where it could be, and potentially what you can do about it,” said Dr. Ferrarelli.
“This might be an objective measure that could be used to identify individuals who have a psychosis disorder, monitor progression of illness, and for prognostic reasons,” he added.
He noted that spindles may also represent a promising target for treatment interventions and added that non-invasive transcranial magnetic stimulation has shown promise in restoring sleep oscillations, including spindles.
Another way to evoke target-brain activity may be through auditory tones – with a patient listening to a particular sound through headphones while asleep, Dr. Ferrarelli said.
Reaffirms previous data
Commenting on the study, Jeffrey A. Lieberman, MD, professor and chair in psychiatry at Columbia University, New York, and a past president of the American Psychiatric Association, noted that the review “just reaffirms what has been reported by individual studies for decades.”
That so many at-risk study subjects had a sleep abnormality is not surprising, said Dr. Lieberman, who was not involved with the current research.
“How many individuals in late adolescence or early adulthood have sleep problems?” he asked. “I would venture to say it’s probably a lot. So the question is: How distinctive is this from what occurs in people who don’t develop the illness?”
The aim of sleep research in the area of schizophrenia has long been to disentangle the effects of medication and environmental factors from the disease and to be able to treat patients to normalize their sleep, said Dr. Lieberman.
“But it’s not clear from these results how one would do that,” he added.
The authors “don’t fundamentally tell us anything about the underlying cause of the illness or the pathophysiology, and they don’t really offer any kind of clear direction for clinical intervention,” he said.
The study was supported by the National Institute of Mental Health. Dr. Ferrarelli reported grants from the National Institute of Mental Health during the conduct of the study. Dr. Lieberman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For example, compared with their healthy peers, participants in a chronic psychosis stage had reduced density, amplitude, and duration of spindles – or bursts of brainwave activity during sleep identified by electroencephalography.
“The results suggest sleep could be an important target [and] an area of research and clinical intervention that could make a difference” in the lives of patients at risk for psychosis, study investigator Fabio Ferrarelli, MD, PhD, associate professor of psychiatry and director of the Sleep and Schizophrenia Program, University of Pittsburgh School of Medicine, told this news organization.
The findings were published online in JAMA Psychiatry.
‘Window of opportunity’
Researchers separate psychosis into stages. During the “clinically high-risk for psychosis” (CHR-P) stage, patients have milder symptoms but do not have a diagnosable psychotic disorder. Those in the early psychosis (EP) stage have had a first episode of psychosis. When they reach a cut-off, often at 5 years, they are considered to have chronic psychosis (CP).
Previous studies have shown that altered sleep often precedes a psychotic episode in early psychosis, and disrupted sleep contributes to predicting transition to psychosis in youth at risk for the condition. Individuals with CP commonly report sleep disturbances, such as insomnia.
Following a literature search, the investigators for this current meta-analysis selected 21 studies assessing sleep disturbance prevalence in 5,135 patients. They also selected 39 studies measuring sleep alterations subjectively (for example, sleep quality) and/or objectively (for example, sleep architecture and sleep oscillation) in 1,575 patients and 977 healthy controls.
The included studies measured the prevalence of sleep disturbances and/or sleep characteristics at different psychosis stages using polysomnography, EEG, actigraphy, or self-reports.
The pooled prevalence of sleep disturbances was 50% across clinical stages (95% confidence interval, 40%-61%). The prevalence was 54% in CHR-P, 68% in EP, and 44% in CP.
The prevalence of insomnia as the primary sleep disturbance was 34% of pooled cases, 48% of the EP group, and 27% of the CP group.
“What’s interesting is the rate of sleep disturbances is relatively stable across stages,” said Dr. Ferrarelli. “This is important because you have a window of opportunity to do some early intervention in people who are at risk that can prevent things from getting worse.”
He suggests clinicians screen for insomnia in early-course patients and perhaps recommend cognitive behavioral therapy (CBT) for insomnia. As well, they should promote sleep hygiene measures for at-risk patients, including such things as avoiding caffeine, alcohol, and screen time before bedtime and adopting a regular sleep pattern.
“These are people at risk, which means they have a 20%-30% chance of eventually developing a psychotic disorder,” said Dr. Ferrarelli. “Maybe disrupted sleep is one of the factors that can make a difference.”
Altered sleep architecture
To compare sleep quality between clinical and control groups, studies used total scores on the Pittsburgh Sleep Quality Index (PSQI), where a score over 5 indicates a sleep problem.
There was a significant standardized mean difference in pooled cases versus controls (SMD, 1.0; 95% CI, 0.7-1.3; P < .001). Each clinical group showed poorer sleep quality, compared with controls.
When assessing sleep architecture abnormalities, stage-specific case-control comparisons showed these were driven by EP and CP stages.
Altered sleep characteristics in both these stages included increased sleep onset latency, increased wake after sleep onset, and reduced sleep efficiency.
Compared with controls, CP was the only clinical group with more arousals. Patients with CP also had more arousals than the CHR-P group, and the number of arousals was significantly affected by medication.
The findings indicate the effects of antipsychotic medications on sleep should be closely monitored, especially in CP, the investigators write.
They add that clinicians should consider medication adjustments, such as decreased doses or switches to another compound.
‘Robust’ spindle results
As for spindle parameters, pooled cases showed significantly decreased spindle density (SMD, –1.06), spindle amplitude (SMD, –1.08), and spindle duration (SMD, −1.21), compared with controls. Stage-specific comparisons revealed these deficits were present in both EP and CP relative to controls.
Dr. Ferrarelli noted the results for spindle abnormalities were among “the most robust” and show that these abnormalities “tend to get worse over the course of the illness.”
The spindle data are “a lot more informative” than that provided by other sleep parameters “in the sense they can yield what could be wrong, where it could be, and potentially what you can do about it,” said Dr. Ferrarelli.
“This might be an objective measure that could be used to identify individuals who have a psychosis disorder, monitor progression of illness, and for prognostic reasons,” he added.
He noted that spindles may also represent a promising target for treatment interventions and added that non-invasive transcranial magnetic stimulation has shown promise in restoring sleep oscillations, including spindles.
Another way to evoke target-brain activity may be through auditory tones – with a patient listening to a particular sound through headphones while asleep, Dr. Ferrarelli said.
Reaffirms previous data
Commenting on the study, Jeffrey A. Lieberman, MD, professor and chair in psychiatry at Columbia University, New York, and a past president of the American Psychiatric Association, noted that the review “just reaffirms what has been reported by individual studies for decades.”
That so many at-risk study subjects had a sleep abnormality is not surprising, said Dr. Lieberman, who was not involved with the current research.
“How many individuals in late adolescence or early adulthood have sleep problems?” he asked. “I would venture to say it’s probably a lot. So the question is: How distinctive is this from what occurs in people who don’t develop the illness?”
The aim of sleep research in the area of schizophrenia has long been to disentangle the effects of medication and environmental factors from the disease and to be able to treat patients to normalize their sleep, said Dr. Lieberman.
“But it’s not clear from these results how one would do that,” he added.
The authors “don’t fundamentally tell us anything about the underlying cause of the illness or the pathophysiology, and they don’t really offer any kind of clear direction for clinical intervention,” he said.
The study was supported by the National Institute of Mental Health. Dr. Ferrarelli reported grants from the National Institute of Mental Health during the conduct of the study. Dr. Lieberman has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Poor sleep quality as a teen may up MS risk in adulthood
Too little sleep or poor sleep quality during the teen years can significantly increase the risk for multiple sclerosis (MS) during adulthood, new research suggests.
In a large case-control study, individuals who slept less than 7 hours a night on average during adolescence were 40% more likely to develop MS later on. The risk was even higher for those who rated their sleep quality as bad.
On the other hand, MS was significantly less common among individuals who slept longer as teens – indicating a possible protective benefit.
While sleep duration has been associated with mortality or disease risk for other conditions, sleep quality usually has little to no effect on risk, lead investigator Torbjörn Åkerstedt, PhD, sleep researcher and professor of psychology, department of neuroscience, Karolinska Institutet, Stockholm, told this news organization.
“I hadn’t really expected that, but those results were quite strong, even stronger than sleep duration,” Dr. Åkerstedt said.
“We don’t really know why this is happening in young age, but the most suitable explanation is that the brain in still developing quite a bit, and you’re interfering with it,” he added.
The findings were published online in the Journal of Neurology, Neurosurgery and Psychiatry.
Strong association
Other studies have tied sleep deprivation to increased risk for serious illness, but the link between sleep and MS risk isn’t as well studied.
Previous research by Dr. Åkerstedt showed that the risk for MS was higher among individuals who took part in shift work before the age of 20. However, the impact of sleep duration or quality among teens was unknown.
The current Swedish population-based case-control study included 2,075 patients with MS and 3,164 without the disorder. All participants were asked to recall how many hours on average they slept per night between the ages of 15 and 19 years and to rate their sleep quality during that time.
Results showed that individuals who slept fewer than 7 hours a night during their teen years were 40% more likely to have MS as adults (odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1-1.7).
Poor sleep quality increased MS risk even more (OR, 1.5; 95% CI, 1.3-1.9).
The association remained strong even after adjustment for additional sleep on weekends and breaks and excluding shift workers.
Long sleep ‘apparently good’
The researchers also conducted several sensitivity studies to rule out confounders that might bias the association, such as excluding participants who reported currently experiencing less sleep or poor sleep.
“You would expect that people who are suffering from sleep problems today would be the people who reported sleep problems during their youth,” but that didn’t happen, Dr. Åkerstedt noted.
The investigators also entered data on sleep duration and sleep quality at the same time, thinking the data would cancel each other out. However, the association remained the same.
“Quite often you see that sleep duration would eliminate the effect of sleep complaints in the prediction of disease, but here both remain significant when they are entered at the same time,” Dr. Åkerstedt said. “You get the feeling that this might mean they act together to produce results,” he added.
“One other thing that surprised me is that long sleep was apparently good,” said Dr. Åkerstedt.
The investigators have conducted several studies on sleep duration and mortality. In recent research, they found that both short sleep and long sleep predicted mortality – “and often, long sleep is a stronger predictor than short sleep,” he said.
Underestimated problem?
Commenting on the findings, Kathleen Zackowski, PhD, associate vice president of research for the National Multiple Sclerosis Society in Baltimore, noted that participants were asked to rate their own sleep quality during adolescence, a subjective report that may mean sleep quality has an even larger association with MS risk.
“That they found a result with sleep quality says to me that there probably is a bigger problem, because I don’t know if people over- or underestimate their sleep quality,” said Dr. Zackowski, who was not involved with the research.
“If we could get to that sleep quality question a little more objectively, I bet that we’d find there’s a lot more to the story,” she said.
That’s a story the researchers would like to explore, Dr. Åkerstedt reported. Designing a prospective study that more closely tracks sleeping habits during adolescence and follows individuals through adulthood could provide valuable information about how sleep quality and duration affect immune system development and MS risk, he said.
Dr. Zackowski said clinicians know that MS is not caused just by a genetic abnormality and that other environmental lifestyle factors seem to play a part.
“If we find out that sleep is one of those lifestyle factors, this is very changeable,” she added.
The study was funded by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Brain Foundation, AFA Insurance, the European Aviation Safety Authority, the Tercentenary Fund of the Bank of Sweden, the Margaretha af Ugglas Foundation, the Swedish Foundation for MS Research, and NEURO Sweden. Dr. Åkerstadt has been supported by Tercentenary Fund of Bank of Sweden, AFA Insurance, and the European Aviation Safety Authority. Dr. Zackowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too little sleep or poor sleep quality during the teen years can significantly increase the risk for multiple sclerosis (MS) during adulthood, new research suggests.
In a large case-control study, individuals who slept less than 7 hours a night on average during adolescence were 40% more likely to develop MS later on. The risk was even higher for those who rated their sleep quality as bad.
On the other hand, MS was significantly less common among individuals who slept longer as teens – indicating a possible protective benefit.
While sleep duration has been associated with mortality or disease risk for other conditions, sleep quality usually has little to no effect on risk, lead investigator Torbjörn Åkerstedt, PhD, sleep researcher and professor of psychology, department of neuroscience, Karolinska Institutet, Stockholm, told this news organization.
“I hadn’t really expected that, but those results were quite strong, even stronger than sleep duration,” Dr. Åkerstedt said.
“We don’t really know why this is happening in young age, but the most suitable explanation is that the brain in still developing quite a bit, and you’re interfering with it,” he added.
The findings were published online in the Journal of Neurology, Neurosurgery and Psychiatry.
Strong association
Other studies have tied sleep deprivation to increased risk for serious illness, but the link between sleep and MS risk isn’t as well studied.
Previous research by Dr. Åkerstedt showed that the risk for MS was higher among individuals who took part in shift work before the age of 20. However, the impact of sleep duration or quality among teens was unknown.
The current Swedish population-based case-control study included 2,075 patients with MS and 3,164 without the disorder. All participants were asked to recall how many hours on average they slept per night between the ages of 15 and 19 years and to rate their sleep quality during that time.
Results showed that individuals who slept fewer than 7 hours a night during their teen years were 40% more likely to have MS as adults (odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1-1.7).
Poor sleep quality increased MS risk even more (OR, 1.5; 95% CI, 1.3-1.9).
The association remained strong even after adjustment for additional sleep on weekends and breaks and excluding shift workers.
Long sleep ‘apparently good’
The researchers also conducted several sensitivity studies to rule out confounders that might bias the association, such as excluding participants who reported currently experiencing less sleep or poor sleep.
“You would expect that people who are suffering from sleep problems today would be the people who reported sleep problems during their youth,” but that didn’t happen, Dr. Åkerstedt noted.
The investigators also entered data on sleep duration and sleep quality at the same time, thinking the data would cancel each other out. However, the association remained the same.
“Quite often you see that sleep duration would eliminate the effect of sleep complaints in the prediction of disease, but here both remain significant when they are entered at the same time,” Dr. Åkerstedt said. “You get the feeling that this might mean they act together to produce results,” he added.
“One other thing that surprised me is that long sleep was apparently good,” said Dr. Åkerstedt.
The investigators have conducted several studies on sleep duration and mortality. In recent research, they found that both short sleep and long sleep predicted mortality – “and often, long sleep is a stronger predictor than short sleep,” he said.
Underestimated problem?
Commenting on the findings, Kathleen Zackowski, PhD, associate vice president of research for the National Multiple Sclerosis Society in Baltimore, noted that participants were asked to rate their own sleep quality during adolescence, a subjective report that may mean sleep quality has an even larger association with MS risk.
“That they found a result with sleep quality says to me that there probably is a bigger problem, because I don’t know if people over- or underestimate their sleep quality,” said Dr. Zackowski, who was not involved with the research.
“If we could get to that sleep quality question a little more objectively, I bet that we’d find there’s a lot more to the story,” she said.
That’s a story the researchers would like to explore, Dr. Åkerstedt reported. Designing a prospective study that more closely tracks sleeping habits during adolescence and follows individuals through adulthood could provide valuable information about how sleep quality and duration affect immune system development and MS risk, he said.
Dr. Zackowski said clinicians know that MS is not caused just by a genetic abnormality and that other environmental lifestyle factors seem to play a part.
“If we find out that sleep is one of those lifestyle factors, this is very changeable,” she added.
The study was funded by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Brain Foundation, AFA Insurance, the European Aviation Safety Authority, the Tercentenary Fund of the Bank of Sweden, the Margaretha af Ugglas Foundation, the Swedish Foundation for MS Research, and NEURO Sweden. Dr. Åkerstadt has been supported by Tercentenary Fund of Bank of Sweden, AFA Insurance, and the European Aviation Safety Authority. Dr. Zackowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Too little sleep or poor sleep quality during the teen years can significantly increase the risk for multiple sclerosis (MS) during adulthood, new research suggests.
In a large case-control study, individuals who slept less than 7 hours a night on average during adolescence were 40% more likely to develop MS later on. The risk was even higher for those who rated their sleep quality as bad.
On the other hand, MS was significantly less common among individuals who slept longer as teens – indicating a possible protective benefit.
While sleep duration has been associated with mortality or disease risk for other conditions, sleep quality usually has little to no effect on risk, lead investigator Torbjörn Åkerstedt, PhD, sleep researcher and professor of psychology, department of neuroscience, Karolinska Institutet, Stockholm, told this news organization.
“I hadn’t really expected that, but those results were quite strong, even stronger than sleep duration,” Dr. Åkerstedt said.
“We don’t really know why this is happening in young age, but the most suitable explanation is that the brain in still developing quite a bit, and you’re interfering with it,” he added.
The findings were published online in the Journal of Neurology, Neurosurgery and Psychiatry.
Strong association
Other studies have tied sleep deprivation to increased risk for serious illness, but the link between sleep and MS risk isn’t as well studied.
Previous research by Dr. Åkerstedt showed that the risk for MS was higher among individuals who took part in shift work before the age of 20. However, the impact of sleep duration or quality among teens was unknown.
The current Swedish population-based case-control study included 2,075 patients with MS and 3,164 without the disorder. All participants were asked to recall how many hours on average they slept per night between the ages of 15 and 19 years and to rate their sleep quality during that time.
Results showed that individuals who slept fewer than 7 hours a night during their teen years were 40% more likely to have MS as adults (odds ratio [OR], 1.4; 95% confidence interval [CI], 1.1-1.7).
Poor sleep quality increased MS risk even more (OR, 1.5; 95% CI, 1.3-1.9).
The association remained strong even after adjustment for additional sleep on weekends and breaks and excluding shift workers.
Long sleep ‘apparently good’
The researchers also conducted several sensitivity studies to rule out confounders that might bias the association, such as excluding participants who reported currently experiencing less sleep or poor sleep.
“You would expect that people who are suffering from sleep problems today would be the people who reported sleep problems during their youth,” but that didn’t happen, Dr. Åkerstedt noted.
The investigators also entered data on sleep duration and sleep quality at the same time, thinking the data would cancel each other out. However, the association remained the same.
“Quite often you see that sleep duration would eliminate the effect of sleep complaints in the prediction of disease, but here both remain significant when they are entered at the same time,” Dr. Åkerstedt said. “You get the feeling that this might mean they act together to produce results,” he added.
“One other thing that surprised me is that long sleep was apparently good,” said Dr. Åkerstedt.
The investigators have conducted several studies on sleep duration and mortality. In recent research, they found that both short sleep and long sleep predicted mortality – “and often, long sleep is a stronger predictor than short sleep,” he said.
Underestimated problem?
Commenting on the findings, Kathleen Zackowski, PhD, associate vice president of research for the National Multiple Sclerosis Society in Baltimore, noted that participants were asked to rate their own sleep quality during adolescence, a subjective report that may mean sleep quality has an even larger association with MS risk.
“That they found a result with sleep quality says to me that there probably is a bigger problem, because I don’t know if people over- or underestimate their sleep quality,” said Dr. Zackowski, who was not involved with the research.
“If we could get to that sleep quality question a little more objectively, I bet that we’d find there’s a lot more to the story,” she said.
That’s a story the researchers would like to explore, Dr. Åkerstedt reported. Designing a prospective study that more closely tracks sleeping habits during adolescence and follows individuals through adulthood could provide valuable information about how sleep quality and duration affect immune system development and MS risk, he said.
Dr. Zackowski said clinicians know that MS is not caused just by a genetic abnormality and that other environmental lifestyle factors seem to play a part.
“If we find out that sleep is one of those lifestyle factors, this is very changeable,” she added.
The study was funded by the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, the Swedish Brain Foundation, AFA Insurance, the European Aviation Safety Authority, the Tercentenary Fund of the Bank of Sweden, the Margaretha af Ugglas Foundation, the Swedish Foundation for MS Research, and NEURO Sweden. Dr. Åkerstadt has been supported by Tercentenary Fund of Bank of Sweden, AFA Insurance, and the European Aviation Safety Authority. Dr. Zackowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.