User login
Insomnia diagnosis and treatment across the lifespan
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings,and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59
Pharmacologic interventions
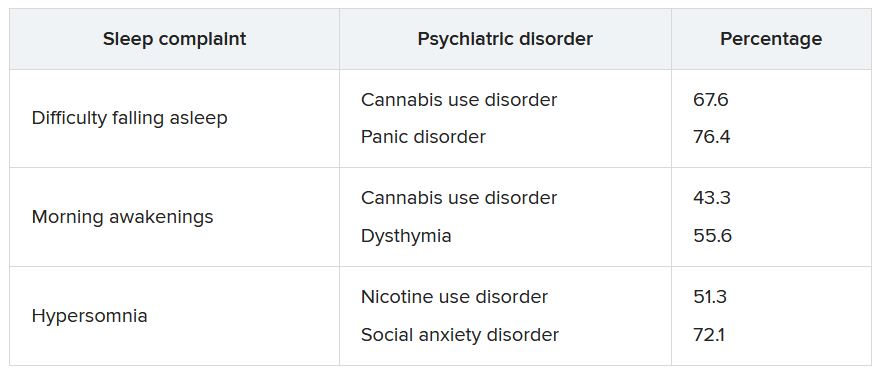
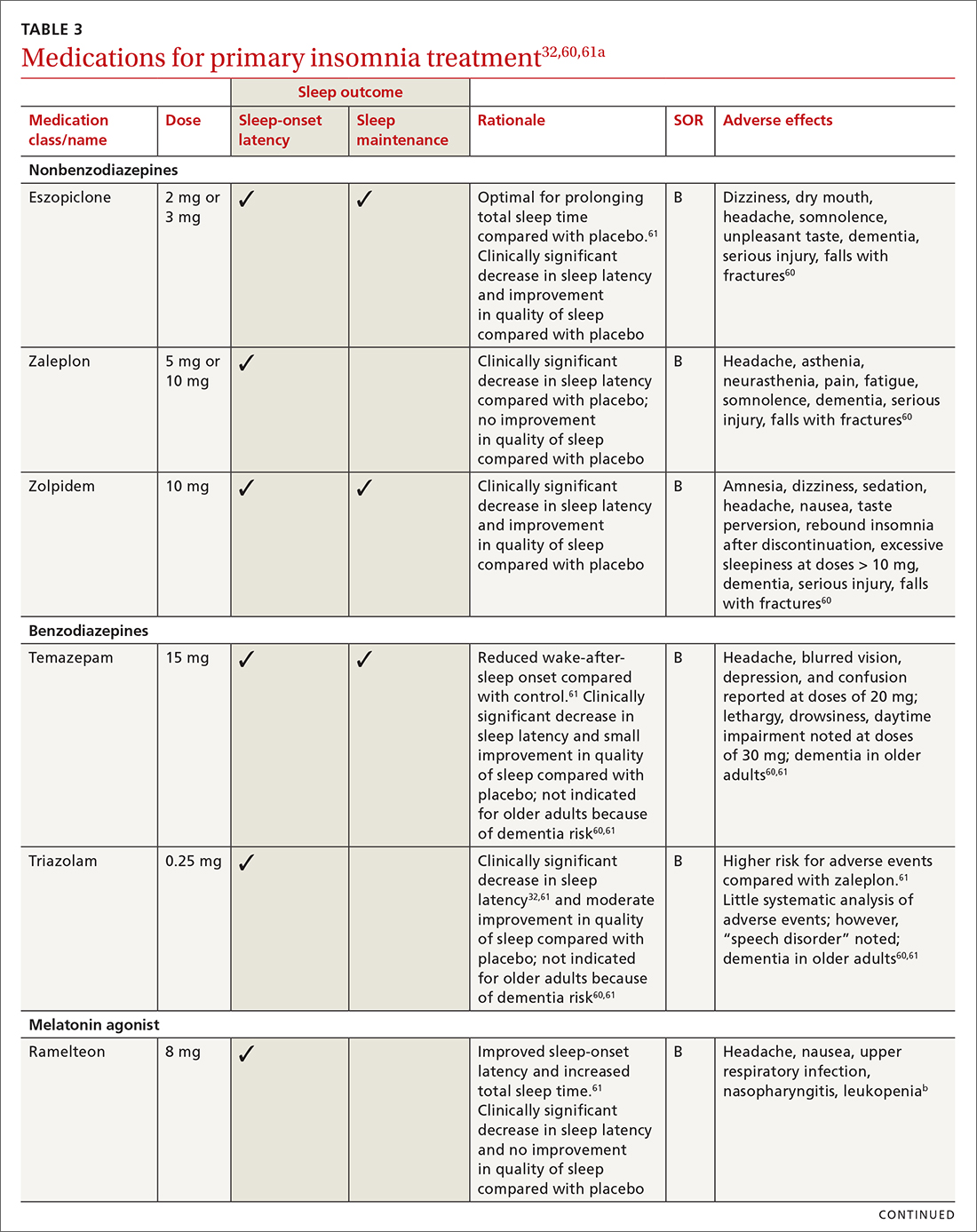
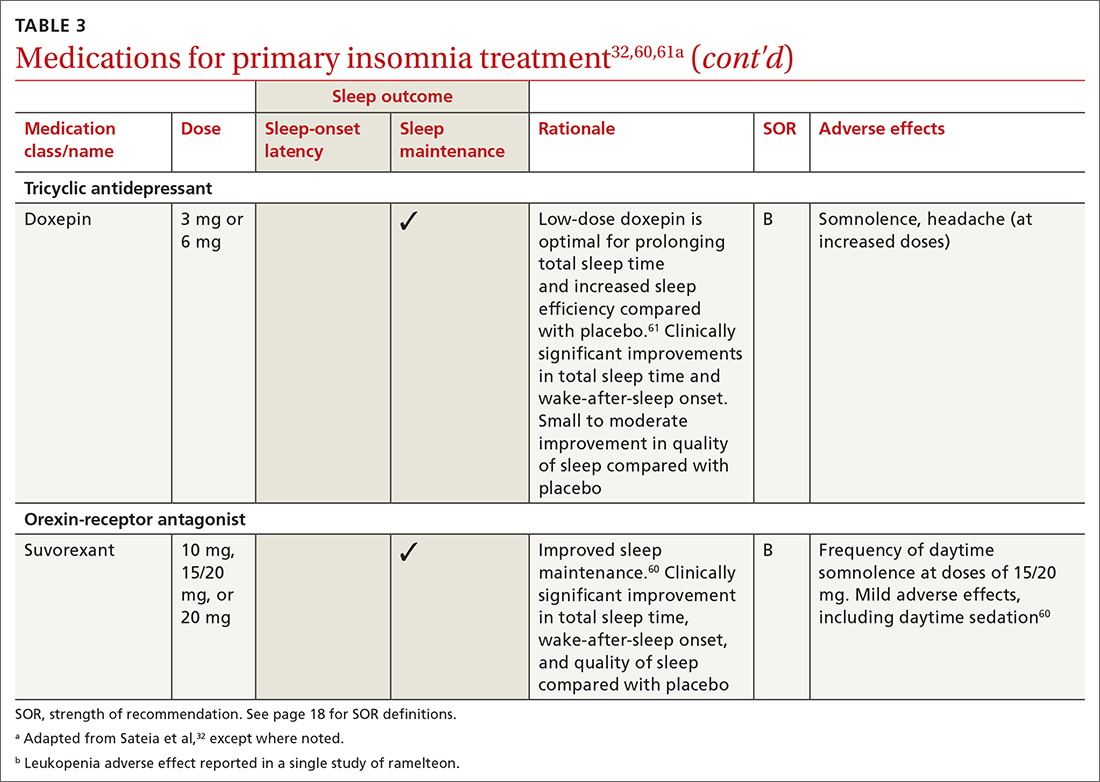
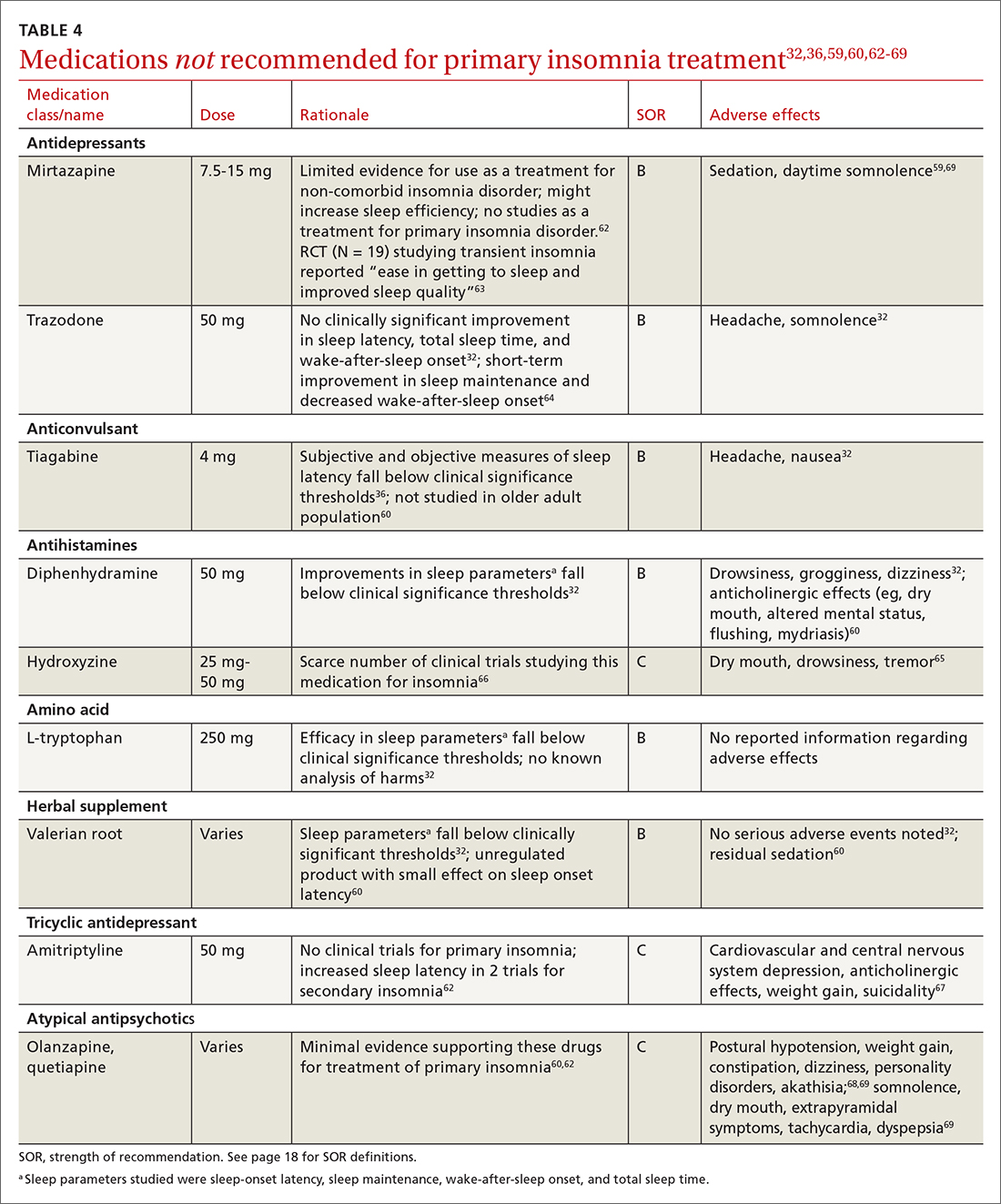
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61)are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32
Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; angela.colistra@lvhn.org
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings,and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59


Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61)are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32

Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; angela.colistra@lvhn.org
Insomnia disorder is common throughout the lifespan, affecting up to 22% of the population.1 Insomnia has a negative effect on patients’ quality of life and is associated with reported worse health-related quality of life, greater overall work impairment, and higher utilization of health care resources compared to patients without insomnia.2
Fortunately, many validated diagnostic tools are available to support physicians in the care of affected patients. In addition, many pharmacologic and nonpharmacologic treatment options exist. This review endeavors to help you refine the care you provide to patients across the lifespan by reviewing the evidence-based strategies for the diagnosis and treatment of insomnia in children, adolescents, and adults.

Defining insomnia
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) defines insomnia disorder as a predominant complaint of dissatisfaction with sleep quantity or quality, associated with 1 or more of the following3:
1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.)
2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.)
3. Early-morning awakening with inability to return to sleep.
Sleep difficulty must be present for at least 3 months and must occur at least 3 nights per week to be classified as persistent insomnia.3 If symptoms last fewer than 3 months, insomnia is considered acute, which has a different DSM-5 code ("other specified insomnia disorder").3 Primary insomnia is its own diagnosis that cannot be defined by other sleep-wake cycle disorders, mental health conditions, or medical diagnoses that cause sleep disturbances, nor is it attributable to the physiologic effects of a substance (eg, substance use disorders, medication effects).3
The International Classification of Sleep Disorders, 3rd edition (ICSD-3) notably consolidates all insomnia diagnoses (ie, “primary” and “comorbid”) under a single diagnosis (“chronic insomnia disorder”), which is a distinction from the DSM-5 diagnosis in terms of classification.4 Diagnosis of insomnia requires the presence of 3 criteria: (1) persistence of sleep difficulty, (2) adequate opportunity for sleep, and (3) associated daytime dysfunction.5
How insomnia affects specific patient populations
Children and adolescents. Appropriate screening, diagnosis, and interventions for insomnia in children and adolescents are associated with better health outcomes, including improved attention, behavior, learning, memory, emotional regulation, quality of life, and mental and physical health.6 In one study of insomnia in the pediatric population (N = 1038), 41% of parents reported symptoms of sleep disturbances in their children.7 Pediatric insomnia can lead to impaired attention, poor academic performance, and behavioral disturbances.7 In addition, there is a high prevalence of sleep disturbances in children with neurodevelopmental disorders.8
Insomnia is the most prevalent sleep disorder in adolescents but frequently goes unrecognized, and therefore is underdiagnosed and undertreated.9 Insomnia in adolescents is associated with depression and suicidality.9-12 Growing evidence also links it to anorexia nervosa,13 substance use disorders,14 and impaired neurocognitive function.15
Continue to: Pregnant women
Pregnant women. Sleep disorders in pregnancy are common and influenced by multiple factors. A meta-analysis found that 57% to 74% of women in various trimesters of pregnancy reported subthreshold symptoms of insomnia16; however, changes in sleep duration and sleep quality during pregnancy may be related to hormonal, physiologic, metabolic, psychological, and posture mechanisms.17,18
Sleep quality also worsens as pregnancy progresses.16 Insomnia coupled with poor sleep quality has been shown to increase the risk for postpartum depression, premature delivery, prolonged labor, and cesarean delivery, as well as preeclampsia, gestational hypertension, stillbirth, and large-for-gestational-age infants.19,20
Older adults. Insomnia is a common complaint in the geriatric population and is associated with significant morbidity, as well as higher rates of depression and suicidality.21 Circadian rhythms change and sleep cycles advance as people age, leading to a decrease in total sleep time, earlier sleep onset, earlier awakenings,and increased frequency of waking after sleep onset.21,22 Advanced age, polypharmacy, and high medical comorbidity increase insomnia prevalence.23
Studies have shown that older adults who sleep fewer than 5 hours per night have an increased risk for diabetes and metabolic syndrome.21 Sleep loss also has been linked to increased rates of hypertension, coronary artery disease, myocardial infarction, and possibly stroke.21,22 Poor sleep has been associated with increased rates of cortical atrophy in community-dwelling older adults.21 Daytime drowsiness increases fall risk.22 Older adults with self-reported decreased physical function also had increased rates of insomnia and increased rates of daytime sleepiness.22
Making the diagnosis: What to ask, tools to use
Clinical evaluation is most helpful for diagnosing insomnia.24 A complete work-up includes physical examination, review of medications and supplements, evaluation of a 2-week sleep diary (kept by the patient, parent, or caregiver), and assessment using a validated sleep-quality rating scale.24 Be sure to obtain a complete health history, including medical events, substance use, and psychiatric history.24
Continue to: Inquire about sleep initiation...
Inquire about sleep initiation, sleep maintenance, and early awakening, as well as behavioral and environmental factors that may contribute to sleep concerns.10,18 Consider medical sleep disorders that have overlapping symptoms with insomnia, including obstructive sleep apnea (OSA), restless leg syndrome (RLS), or circadian rhythm sleep-wake disorders. If there are co-occurring chronic medical problems, reassess insomnia symptoms after the other medical diagnoses are controlled.
TABLE 125-29 includes a list of validated screening tools for insomnia and where they can be accessed. Recommended screening tools for children and adolescents include daytime sleepiness questionnaires, comprehensive sleep instruments, and self-assessments.25,30 Although several studies of insomnia in pregnancy have used tools listed in TABLE 1,25-29 only the Insomnia Severity Index has been validated for use with this population.26,27 Diagnosis of insomnia in older adults requires a comprehensive sleep history collected from the patient, partners, or caregivers.21

Measuring sleep performance
Several aspects of insomnia (defined in TABLE 231-33) are targeted as outcome measures when treating patients. Sleep-onset latency, total sleep time, and wake-after-sleep onset are all formally measured by polysomnography.31-33 Use polysomnography when you suspect OSA, narcolepsy, idiopathic hypersomnia, periodic limb movement disorder, RLS, REM behavior disorder (characterized by the loss of normal muscle atonia and dream enactment behavior that is violent in nature34), or parasomnias. Home polysomnography testing is appropriate for adult patients who meet criteria for OSA and have uncomplicated insomnia.35 Self-reporting (use of sleep logs) and actigraphy (measurement by wearable monitoring devices) may be more accessible methods for gathering sleep data from patients. Use of wearable consumer sleep technology such as heart rate monitors with corresponding smartphone applications (eg, Fitbit, Jawbone Up devices, and the Whoop device) are increasing as a means of monitoring sleep as well as delivering insomnia interventions.36

Actigraphy has been shown to produce significantly distinct results from self-reporting when measuring total sleep time, sleep-onset latency, wake-after-sleep onset, and sleep efficiency in adult and pediatric patients with insomnia.37 Actigraphy yields distinct estimates of sleep patterns when compared to sleep logs, which suggests that while both measures are often correlated, actigraphy has utility in assessing sleep continuity in conjunction with sleep logs in terms of diagnostic and posttreatment assessment.37
Continue to: Treatment options
Treatment options: Start with the nonpharmacologic
Both nonpharmacologic and pharmacologic interventions are available for the treatment of insomnia. Starting with nonpharmacologic options is preferred.
Nonpharmacologic interventions
Sleep hygiene. Poor sleep hygiene can contribute to insomnia but does not cause it.31 Healthy sleep habits include keeping the sleep environment quiet, free of interruptions, and at an adequate temperature; adhering to a regular sleep schedule; avoiding naps; going to bed when drowsy; getting out of bed if not asleep within 15 to 20 minutes and returning when drowsy; exercising regularly; and avoiding caffeine, nicotine, alcohol, and other substances that interfere with sleep.24 Technology use prior to bedtime is prevalent and associated with sleep and circadian rhythm disturbances.38
Sleep hygiene education is often insufficient on its own.31 But it has been shown to benefit older adults with insomnia.19,32
Sleep hygiene during pregnancy emphasizes drinking fluids only in the daytime to avoid awakening to urinate at night, avoiding specific foods to decrease heartburn, napping only in the early part of the day, and sleeping on either the left or the right side of the body with knees and hips bent and a pillow under pressure points in the second and third trimesters.18,39
Pediatric insomnia. Sleep hygiene is an important first-line treatment for pediatric insomnia, especially among children with attention-deficit/hyperactivity disorder.40
Continue to: CBT-I
Cognitive behavioral therapy for insomnia (CBT-I). US and European guidelines recommend CBT-I—a multicomponent, nonpharmacologic, insomnia-focused psychotherapy—as a first-line treatment for short- and long-term insomnia32,41,42 across a wide range of patient demographics.17,43-47 CBT-I is a multiweek intensive treatment that combines sleep hygiene practices with cognitive therapy and behavioral interventions, including stimulus control, sleep restriction, and relaxation training.32,48 CBT-I monotherapy has been shown to have greater efficacy than sleep hygiene education for patients with insomnia, especially for those with medical or psychiatric comorbidities.49 It also has been shown to be effective when delivered in person or even digitally.50-52 For example, CBT-I Coach is a mobile application for people who are already engaged in CBT-I with a health care provider; it provides a structured program to alleviate symptoms.53
Although CBT-I methods are appropriate for adolescents and school-aged children, evaluations of the efficacy of the individual components (stimulus control, arousal reduction, cognitive therapy, improved sleep hygiene practices, and sleep restriction) are needed to understand what methods are most effective in this population.9
Cognitive and/or behavioral Interventions. Cognitive therapy (to change negative thoughts about sleep) and behavioral interventions (eg, changes to sleep routines, sleep restriction, moving the child’s bedtime to match the time of falling asleep [bedtime fading],41 stimulus control)9,43,54-56 may be used independently. Separate meta-analyses support the use of cognitive and behavioral interventions for adolescent insomnia,9,43 school-aged children with insomnia and sleep difficulties,43,49 and adolescents with sleep difficulties and daytime fatigue.41 The trials for children and adolescents followed the same recommendations for treatment as CBT-I but often used fewer components of the treatment, resulting in focused cognitive or behavioral interventions.
One controlled evaluation showed support for separate cognitive and behavioral techniques for insomnia in children.54 A meta-analysis (6 studies; N = 529) found that total sleep time, as measured with actigraphy, improved among school-aged children and adolescents with insomnia after treatment with 4 or more types of cognitive or behavioral therapy sessions.43 Sleep-onset latency, measured by actigraphy and sleep diaries, decreased in the intervention group.43
A controlled evaluation of CBT for behavioral insomnia in school-aged children (N = 42) randomized participants to CBT (n = 21) or waitlist control (n = 21).54 The 6 CBT sessions combined behavioral sleep medicine techniques (ie, sleep restriction) with anxiety treatment techniques (eg, cognitive restructuring).54 Those in the intervention group showed statistically significant improvement in sleep latency, wake-after-sleep onset, and sleep efficiency (all P ≤ .003), compared with controls.54 Total sleep time was unaffected by the intervention. A notable change was the number of patients who still had an insomnia diagnosis postintervention. Among children in the CBT group, 14.3% met diagnostic criteria vs 95% of children in the control group.54 Similarly, at the 1-month follow-up, 9.5% of CBT group members still had insomnia, compared with 86.7% of the control group participants.54
Continue to: Multiple randomized and nonranomized studies...
Multiple randomized and nonrandomized studies have found that infants also respond to behavioral interventions, such as establishing regular daytime and sleep routines, reducing environmental noises or distractions, and allowing for self-soothing at bedtime.55 A controlled trial (N = 279) of newborns and their mothers evaluated sleep interventions that included guidance on bedtime sleep routines, starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine.56 The intervention group demonstrated longer sleep duration (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01) at 40 weeks postintervention compared with the control group.56
The clinically significant outcomes of this study are related to the guidance offered to parents to help infants achieve longer sleep. More intervention-group infants were allowed to self-soothe to sleep without being held or fed, had earlier bedtimes, and fell asleep ≤ 15 minutes after being put into bed than their counterparts in the control group.56
Exercise. As a sole intervention, exercise for insomnia is readily available and low cost, but it is not universally effective. One study of patients older than 60 years (N = 43) showed that a 16-week moderate exercise regimen slightly improved total sleep time by an average of 42 minutes (P = .05), sleep-onset latency improved an average of 11.5 minutes (P = .007), and global sleep quality improved by 3.4 points as measured by the Pittsburgh Sleep Quality Index (PSQI; P ≤ .01).57 No significant improvements occurred in sleep efficiency. Exercise is one of several nonpharmacologic alternatives for treating insomnia in pregnancy.58
A lack of uniformity in patient populations, intervention protocols, and outcome measures confounded results of 2 systematic reviews that included comparisons of yoga or tai chi as standalone alternatives to CBT-I for insomnia treatment.58,59 Other interventions, such as mindfulness or relaxation training, have been studied as insomnia interventions, but no conclusive evidence about their efficacy exists.45,59


Pharmacologic interventions
Pharmacologic treatment should not be the sole intervention for the treatment of insomnia but should be used in combination with nonpharmacologic interventions.32 Of note, only low-quality evidence exists for any pharmacologic interventions for insomnia.32 The decision to prescribe medications should rely on the predominant sleep complaint, with sleep maintenance and sleep-onset latency as the guiding factors.32 Medications used for insomnia treatment (TABLE 332,60,61)are classified according to these and other sleep outcomes described in TABLE 1.25-29 Prescribe them at the lowest dose and for the shortest amount of time possible.32,62 Avoid medications listed in TABLE 432,36,59,60,62-69 because data showing clinically significant improvements in insomnia are lacking, and analysis for potential harms is inadequate.32

Continue to: Melatonin is not recommended
Melatonin is not recommended for treating insomnia in adults, pregnant patients, older adults, or most children because its effects are clinically insignificant,32 residual sedation has been reported,60 and no analysis of harms has been undertaken.32 Despite this, melatonin is frequently utilized for insomnia, and patients take over-the-counter melatonin for a myriad of sleep complaints. Melatonin is indicated in the treatment of insomnia in children with neurodevelopmental disorders. (See discussion in "Prescribing for children.")
Hypnotics are medications licensed for short-term sleep promotion in adults and can induce tolerance and dependence.32 Nonbenzodiazepine-receptor agonists at clinical doses do not appear to suppress REM sleep, although there are reports of increases in latency to REM sleep.70
Antidepressants. Although treatment of insomnia with antidepressants is widespread, evidence of their efficacy is unclear.32,62 The tolerability and safety of antidepressants for insomnia also are uncertain due to limited reporting of adverse events.32
The use of sedating antidepressants may be driven by concern over the longer-term use of hypnotics and the limited availability of psychological treatments including CBT-I.32 Sedating antidepressants are indicated for comorbid or secondary insomnia (attributable to mental health conditions, medical conditions, other sleep disorders, or substance use or misuse); however, there are few clinical trials studying them for primary insomnia treatment.62 Antidepressants—tricyclic antidepressants included—can reduce the amount of REM sleep and increase REM sleep-onset latency.71,72
Antihistamines and antipsychotics. Although antihistamines (eg, hydroxyzine, diphenhydramine) and antipsychotics frequently are prescribed off-label for primary insomnia, there is a lack of evidence to support either type of medication for this purpose.36,62,73 H1-antihistamines such as hydroxyzine increase REM-onset latency and reduce the duration of REM sleep.73 Depending on the specific medication, second-generation antipsychotics such as olanzapine and quetiapine have mixed effects on REM sleep parameters.65
Continue to: Prescribing for children
Prescribing for children. There is no FDA-approved medication for the treatment of insomnia in children.52 However, melatonin has shown promising results for treating insomnia in children with neurodevelopmental disorders. A systematic review (13 trials; N = 682) with meta-analysis (9 studies; n = 541) showed that melatonin significantly improved total sleep time compared with placebo (mean difference [MD] = 48.26 minutes; 95% CI, 36.78-59.73).8 In 11 studies (n = 581), sleep-onset latency improved significantly with melatonin use.8 No difference was noted in the frequency of wake-after-sleep onset.8 No medication-related adverse events were reported. Heterogeneity (I2 = 31%) and inconsistency among included studies shed doubt on the findings; therefore, further research is needed.8
Prescribing in pregnancy. Prescribing medications to treat insomnia in pregnancy is complex and controversial. No consistency exists among guidelines and recommendations for treating insomnia in the pregnant population. Pharmacotherapy for insomnia is frequently prescribed off-label in pregnant patients. Examples include benzodiazepine-receptor agonists, antidepressants, and gamma-aminobutyric acid–reuptake inhibitors.45
Pharmacotherapy in pregnancy is a unique challenge, wherein clinicians consider not only the potential drug toxicity to the fetus but also the potential changes in the pregnant patient’s pharmacokinetics that influence appropriate medication doses.39,74 Worth noting: Zolpidem has been associated with preterm birth, cesarean birth, and low-birth-weight infants.45,74 The lack of clinical trials of pharmacotherapy in pregnant patients results in a limited understanding of medication effects on long-term health and safety outcomes in this population.39,74
A review of 3 studies with small sample sizes found that when antidepressants or antihistamines were taken during pregnancy, neither had significant adverse effects on mother or child.68 Weigh the risks of medications with the risk for disease burden and apply a shared decision-making approach with the patient, including providing an accurate assessment of risks and safety information regarding medication use.39 Online resources such as ReproTox (www.reprotox.org) and MotherToBaby (https://mothertobaby.org) are available to support clinicians treating pregnant and lactating patients.39
Prescribing for older adults. Treatment of insomnia in older adults requires a multifactorial approach.22 For all older adults, start interventions with nonpharmacologic treatments for insomnia followed by treatment of any underlying medical and psychiatric disorders that affect sleep.21 If medications are required, start with the lowest dose and titrate upward slowly. Use sedating low-dose antidepressants for insomnia only when the older patient has comorbid depression.60 Although nonbenzodiazepine-receptor agonists have improved safety profiles compared with benzodiazepines, their use for older adults should be limited because of adverse effects that include dementia, serious injury, and falls with fractures.60
Keep these points in mind
Poor sleep has many detrimental health effects and can significantly affect quality of life for patients across the lifespan. Use nonpharmacologic interventions—such as sleep hygiene education, CBT-I, and cognitive/behavioral therapies—as first-line treatments. When utilizing pharmacotherapy for insomnia, consider the patient’s distressing symptoms of insomnia as guideposts for prescribing. Use pharmacologic treatments intermittently, short term, and in conjunction with nonpharmacologic options.
CORRESPONDENCE
Angela L. Colistra, PhD, LPC, CAADC, CCS, 707 Hamilton Street, 8th floor, LVHN Department of Family Medicine, Allentown, PA 18101; angela.colistra@lvhn.org
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
1. Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592-600. doi: 10.1016/j.biopsych.2010.10.023
2. DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health Status, work productivity, and healthcare resource use. PloS One. 2015;10:e0137117. doi: 10.1371/journal.pone.0137117
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013: 362-368.
4. Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146:1387-1394. doi: 10.1378/chest.14-0970
5. American Academy of Sleep Medicine. International Classification of Sleep Disorders. American Academy of Sleep Medicine, 3d ed; 2014.
6. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
7. Archbold KH, Pituch KJ, Panahi P, et al. Symptoms of sleep disturbances among children at two general pediatric clinics. J Pediatr. 2002;140:97-102. doi: 10.1067/mpd.2002.119990
8. Abdelgadir IS, Gordon MA, Akobeng AK. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: a systematic review and meta-analysis. Arch Dis Child. 2018;103:1155-1162. doi: 10.1136/archdischild-2017-314181
9. de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12-24. doi: 10.1016/j.smrv.2017.06.009
10. Roberts RE, Duong HT. Depression and insomnia among adolescents: a prospective perspective. J Affect Disord. 2013;148:66-71. doi: 10.1016/j.jad.2012.11.049
11. Sivertsen B, Harvey AG, Lundervold AJ, et al. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16-18 years. Eur Child Adolesc Psychiatry. 2014;23:681-689. doi: 10.1007/s00787-013-0502-y
12. Alvaro PK, Roberts RM, Harris JK, et al. The direction of the relationship between symptoms of insomnia and psychiatric disorders in adolescents. J Affect Disord. 2017;207:167-174. doi: 10.1016/j.jad.2016.08.032
13. Allison KC, Spaeth A, Hopkins CM. Sleep and eating disorders. Curr Psychiatry Rep. 2016;18:92. doi: 10.1007/s11920-016-0728-8
14. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the Future: National Results on Drug Use: 1975-2013. Institute for Social Research, The University of Michigan; 2014.
15. Kuula L, Pesonen AK, Martikainen S, et al. Poor sleep and neurocognitive function in early adolescence. Sleep Med. 2015;16:1207-1212. doi: 10.1016/j.sleep.2015.06.017
16. Sedov ID, Anderson NJ, Dhillon AK. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30:e13207. doi: 10.1111/jsr.13207
17. Oyiengo D, Louis M, Hott B, et al. Sleep disorders in pregnancy. Clin Chest Med. 2014;35:571-587. doi: 10.1016/j.ccm.2014.06.012
18. Hashmi AM, Bhatia SK, Bhatia SK, et al. Insomnia during pregnancy: diagnosis and rational interventions. Pak J Med Sci. 2016; 32:1030-1037. doi: 10.12669/pjms.324.10421
19. Abbott SM, Attarian H, Zee PC. Sleep disorders in perinatal women. Best Pract Res Clin Obstet Gynaecol. 2014;28:159-168. doi: 10.1016/j.bpobgyn.2013.09.003
20. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436
21. Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14:1017-1024. doi: 10.5664/jcsm.7172
22. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12:31-38. doi: 10.1016/j.jsmc.2016.10.008
23. Miner B, Gill TM, Yaggi HK, et al. Insomnia in community-living persons with advanced age. J Am Geriatr Soc. 2018;66:1592-1597. doi: 10.1111/jgs.15414
24. Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487-504.
25. Owens JA, Dalzell V. Use of the ‘BEARS’ sleep screening tool in a pediatric residents’ continuity clinic: a pilot study. Sleep Med. 2005;6:63-69. doi: 10.1016/j.sleep.2004.07.015
26. Okun ML, Buysse DJ, Hall MH. Identifying insomnia in early pregnancy: validation of the Insomnia Symptoms Questionnaire (ISQ) in pregnant women. J Clin Sleep Med. 2015;11:645-54. doi: 10.5664/jcsm.4776
27. Morin CM, Belleville G, Bélanger L. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. doi: 10.1093/sleep/34.5.601
28. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. doi: 10.1016/0165-1781(89)90047-4
29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540-545. doi: 10.1093/sleep/14.6.540
30. Baddam SKR, Canapari CA, Van de Grift J, et al. Screening and evaluation of sleep disturbances and sleep disorders in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30:65-84. doi: 10.1016/j.chc.2020.09.005
31. De Crescenzo F, Foti F, Ciabattini M, et al. Comparative efficacy and acceptability of pharmacological treatments for insomnia in adults: a systematic review and network meta‐analysis. Cochrane Database Syst Rev. 2016;2016(9):CD012364. doi: 10.1002/14651858.CD012364
32. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
33. Morin AK, Jarvis CI, Lynch AM. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharmacother. 2007; 27:89-110. doi: 10.1592/phco.27.1.89
34. Berry RB, Wagner MH. Sleep Medicine Pearls. 3rd ed. Elsevier/Saunders; 2015:533-541.
35. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479-504. doi: 10.5664/jcsm.6506
36. Glazer Baron K, Culnan E, Duffecy J, et al. How are consumer sleep technology data being used to deliver behavioral sleep medicine interventions? A systematic review. Behav Sleep Med. 2022;20:173-187. doi: 10.1080/15402002.2021.1898397
37. Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14:1209-1230.
38. Gradisar M, Wolfson AR, Harvey AG, et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. doi: 10.5664/jcsm.3272
39. Miller MA, Mehta N, Clark-Bilodeau C, et al. Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 2020;157:184-197. doi: 10.1016/j.chest.2019.09.026
40. Nikles J, Mitchell GK, de Miranda Araújo R, et al. A systematic review of the effectiveness of sleep hygiene in children with ADHD. Psychol Health Med. 2020;25:497-518. doi: 10.1080/13548506.2020.1732431
41. Baglioni C, Altena E, Bjorvatn B, et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res. 2019;29. doi: 10.1111/jsr.12967
42. Jernelöv S, Blom K, Hentati Isacsson N, et al. Very long-term outcome of cognitive behavioral therapy for insomnia: one- and ten-year follow-up of a randomized controlled trial. Cogn Behav Ther. 2022;51:72-88. doi: 10.1080/16506073.2021.2009019
43. Åslund L, Arnberg F, Kanstrup M, et al. Cognitive and behavioral interventions to improve sleep in school-age children and adolescents: a systematic review and meta-analysis. J Clin Sleep Med. 2018;14:1937-1947. doi: 10.5664/jcsm.7498
44. Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133:911-919. doi: 10.1097/AOG.0000000000003216
45. Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234
46. Hinrichsen GA, Leipzig RM. Efficacy of cognitive behavioral therapy for insomnia in geriatric primary care patients. J Am Geriatr Soc. 2021;69:2993-2995. doi: 10.1111/jgs.17319
47. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41:1-12. doi: 10.1093/sleep/zsy104
48. American Sleep Association. Cognitive behavioral therapy (CBT): treatment for insomnia. Accessed May 4, 2022. www.sleepassociation.org/sleep-treatments/cognitive-behavioral-therapy/#:~:text=Cognitive%20Behavioral%20Therapy%20for%20Insomnia%2C%20also%20known%20as
49. Zhou FC, Yang Y, Wang YY, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatry Q. 2020;91:1209-1224. doi: 10.1007/s11126-020-09820-8
50. Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49:491-500. doi: 10.1017/S0033291718001113
51. Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psych. 2020;77:484-492. doi: 10.1001/jamapsychiatry.2019.4491
52. de Bruin EJ, Bögels SM, Oort FJ, et al. Improvements of adolescent psychopathology after insomnia treatment: results from a randomized controlled trial over 1 year. J Child Psychol Psych. 2018;59:509-522. doi: 10.1111/jcpp.12834
53. Hoffman JE, Taylor K, Manber R, et al. CBT-I Coach (version 1.0). [Mobile application software]. Accessed December 9, 2022. https://itunes.apple.com
54. Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49:379-88. doi: 10.1016/j.brat.2011.03.008
55. Hungenberg M, Houss B, Narayan M, et al. Do behavioral interventions improve nighttime sleep in children < 1 year old? J Fam Pract. 2022;71:E16-E17. doi: 10.12788/jfp.0446
56. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT Responsive Parenting Intervention and Infant Sleep. Pediatrics. 2016;138:e20160762. doi: 10.1542/peds.2016-0762
57. Montgomery P, Dennis J. Physical exercise for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002; 2002(4):CD003404. doi:10.1002/14651858.CD003404
58. Yang SY, Lan SJ, Yen YY, et al. Effects of exercise on sleep quality in pregnant women: a systematic review and meta-analysis of randomized controlled trials. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14:1-10. doi: 10.1016/j.anr.2020.01.003
59. Wang F, Eun-Kyoung Lee O, Feng F, et al. The effect of meditative movement on sleep quality: a systematic review. Sleep Med Rev. 2016;30:43-52. doi: 10.1016/j.smrv.2015.12.001
60. Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38:2340-2372. doi: 10.1016/j.clinthera.2016.09.010
61. Chiu HY, Lee HC, Liu JW, et al. Comparative efficacy and safety of hypnotics for insomnia in older adults: a systematic review and network meta-analysis. Sleep. 2021;44(5):zsaa260. doi: 10.1093/sleep/zsaa260
62. Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70:197-245. doi: 10.1124/pr.117.014381
63. Karsten J, Hagenauw LA, Kamphuis J, et al. Low doses of mirtazapine or quetiapine for transient insomnia: a randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31:327-337. doi: 10.1177/0269881116681399
64. Yi X-Y, Ni S-F, Ghadami MR, et al. Trazodone for the treatment of insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018;45:25-32. doi: 10.1016/j.sleep.2018.01.010
65. Monti JM, Torterolo P, Pandi Perumal SR. The effects of second generation antipsychotic drugs on sleep variables in healthy subjects and patients with schizophrenia. Sleep Med Rev. 2017;33:51-57. doi: 10.1016/j.smrv.2016.05.002
66. Krzystanek M, Krysta K, Pałasz A. First generation antihistaminic drugs used in the treatment of insomnia—superstitions and evidence. Pharmacother Psychiatry Neurol. 2020;36:33-40.
67. Amitriptyline hydrochloride. NIH US National Library of Medicine: DailyMed. Updated October 6, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d012a4-cd95-46c6-a6b7-b15d6fd5269d
68. Olanzapine. NIH US National Library of Medicine: DailyMed. Updated October 23, 2015. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e8626e68-088d-47ff-bf06-489a778815aa
69. Quetiapine extended release. NIH US National Library of Medicine: DailyMed. Updated January 28, 2021. Accessed July 27, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07e4f3f4-42cb-4b22-bf8d-8c3279d26e9
70. Roehrs T, Roth T. Drug-related sleep stage changes: functional significance and clinical relevance. Sleep Med Clin. 2010;5:559-570. doi: 10.1016/j.jsmc.2010.08.002
71. Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927-947. doi: 10.2165/00003495-200565070-00003
72. Winokur A, Gary KA, Rodner S, et al. Depression, sleep physiology, and antidepressant drugs. Depress Anxiety. 2001;14:19-28. doi: 10.1002/da.1043
73. Ozdemir PG, Karadag AS, Selvi Y, et al. Assessment of the effects of antihistamine drugs on mood, sleep quality, sleepiness, and dream anxiety. Int J Psychiatry Clin Pract. 2014;18:161-168. doi: 10.3109/13651501.2014.907919
74. Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol. 2015;212:428-441. doi:10.1016/j.ajog.2014.10.1106
PRACTICE RECOMMENDATIONS
› Use a standard validated screening tool for the diagnosis of insomnia in all age groups. A
› Employ nonpharmacologic interventions as first-line treatment for insomnia in all populations. A
› Utilize sleep hygiene or cognitive behavioral therapy for insomnia in adolescents and all adults. A
› Initiate independent cognitive or behavioral therapies with younger children. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The longevity gene: Healthy mutant reverses heart aging
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
Will your smartphone be the next doctor’s office?
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
A fingertip pressed against a phone’s camera lens can measure a heart rate. The microphone, kept by the bedside, can screen for sleep apnea. Even the speaker is being tapped, to monitor breathing using sonar technology.
In the best of this new world, the data is conveyed remotely to a medical professional for the convenience and comfort of the patient or, in some cases, to support a clinician without the need for costly hardware.
But using smartphones as diagnostic tools is a work in progress, experts say. Although doctors and their patients have found some real-world success in deploying the phone as a medical device, the overall potential remains unfulfilled and uncertain.
Smartphones come packed with sensors capable of monitoring a patient’s vital signs. They can help assess people for concussions, watch for atrial fibrillation, and conduct mental health wellness checks, to name the uses of a few nascent applications.
Companies and researchers eager to find medical applications for smartphone technology are tapping into modern phones’ built-in cameras and light sensors; microphones; accelerometers, which detect body movements; gyroscopes; and even speakers. The apps then use artificial intelligence software to analyze the collected sights and sounds to create an easy connection between patients and physicians. Earning potential and marketability are evidenced by the more than 350,000 digital health products available in app stores, according to a Grand View Research report.
“It’s very hard to put devices into the patient home or in the hospital, but everybody is just walking around with a cellphone that has a network connection,” said Dr. Andrew Gostine, CEO of the sensor network company Artisight. Most Americans own a smartphone, including more than 60% of people 65 and over, an increase from just 13% a decade ago, according the Pew Research Center. The COVID-19 pandemic has also pushed people to become more comfortable with virtual care.
Some of these products have sought FDA clearance to be marketed as a medical device. That way, if patients must pay to use the software, health insurers are more likely to cover at least part of the cost. Other products are designated as exempt from this regulatory process, placed in the same clinical classification as a Band-Aid. But how the agency handles AI and machine learning–based medical devices is still being adjusted to reflect software’s adaptive nature.
Ensuring accuracy and clinical validation is crucial to securing buy-in from health care providers. And many tools still need fine-tuning, said Eugene Yang, MD, a professor of medicine at the University of Washington, Seattle. Currently, Dr. Yang is testing contactless measurement of blood pressure, heart rate, and oxygen saturation gleaned remotely via Zoom camera footage of a patient’s face.
Judging these new technologies is difficult because they rely on algorithms built by machine learning and artificial intelligence to collect data, rather than the physical tools typically used in hospitals. So researchers cannot “compare apples to apples” with medical industry standards, Dr. Yang said. Failure to build in such assurances undermines the technology’s ultimate goals of easing costs and access because a doctor still must verify results.
“False positives and false negatives lead to more testing and more cost to the health care system,” he said.
Big tech companies like Google have heavily invested in researching this kind of technology, catering to clinicians and in-home caregivers, as well as consumers. Currently, in the Google Fit app, users can check their heart rate by placing their finger on the rear-facing camera lens or track their breathing rate using the front-facing camera.
“If you took the sensor out of the phone and out of a clinical device, they are probably the same thing,” said Shwetak Patel, director of health technologies at Google and a professor of electrical and computer engineering at the University of Washington.
Google’s research uses machine learning and computer vision, a field within AI based on information from visual inputs like videos or images. So instead of using a blood pressure cuff, for example, the algorithm can interpret slight visual changes to the body that serve as proxies and biosignals for a patient’s blood pressure, Mr. Patel said.
Google is also investigating the effectiveness of the built-in microphone for detecting heartbeats and murmurs and using the camera to preserve eyesight by screening for diabetic eye disease, according to information the company published last year.
The tech giant recently purchased Sound Life Sciences, a Seattle startup with an FDA-cleared sonar technology app. It uses a smart device’s speaker to bounce inaudible pulses off a patient’s body to identify movement and monitor breathing.
Binah.ai, based in Israel, is another company using the smartphone camera to calculate vital signs. Its software looks at the region around the eyes, where the skin is a bit thinner, and analyzes the light reflecting off blood vessels back to the lens. The company is wrapping up a U.S. clinical trial and marketing its wellness app directly to insurers and other health companies, said company spokesperson Mona Popilian-Yona.
The applications even reach into disciplines such as optometry and mental health:
- With the microphone, Canary Speech uses the same underlying technology as Amazon’s Alexa to analyze patients’ voices for mental health conditions. The software can integrate with telemedicine appointments and allow clinicians to screen for anxiety and depression using a library of vocal biomarkers and predictive analytics, said Henry O’Connell, the company’s CEO.
- Australia-based ResApp Health last year for its iPhone app that screens for moderate to severe obstructive sleep apnea by listening to breathing and snoring. SleepCheckRx, which will require a prescription, is minimally invasive compared with sleep studies currently used to diagnose sleep apnea. Those can cost thousands of dollars and require an array of tests.
- Brightlamp’s Reflex app is a clinical decision support tool for helping manage concussions and vision rehabilitation, among other things. Using an iPad’s or iPhone’s camera, the mobile app measures how a person’s pupils react to changes in light. Through machine learning analysis, the imagery gives practitioners data points for evaluating patients. Brightlamp sells directly to health care providers and is being used in more than 230 clinics. Clinicians pay a $400 standard annual fee per account, which is currently not covered by insurance. The Department of Defense has an ongoing clinical trial using Reflex.
In some cases, such as with the Reflex app, the data is processed directly on the phone – rather than in the cloud, Brightlamp CEO Kurtis Sluss said. By processing everything on the device, the app avoids running into privacy issues, as streaming data elsewhere requires patient consent.
But algorithms need to be trained and tested by collecting reams of data, and that is an ongoing process.
Researchers, for example, have found that some computer vision applications, like heart rate or blood pressure monitoring, can be less accurate for darker skin. Studies are underway to find better solutions.
Small algorithm glitches can also produce false alarms and frighten patients enough to keep widespread adoption out of reach. For example, Apple’s new car-crash detection feature, available on both the latest iPhone and Apple Watch, was set off when people were riding roller coasters and automatically dialed 911.
“We’re not there yet,” Dr. Yang said. “That’s the bottom line.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Sleep complaints in major depression flag risk for other psychiatric disorders
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Serum trace metals relate to lower risk of sleep disorders
, based on data from 3,660 individuals.
Previous research has shown an association between trace metals and sleep and sleep patterns, but data on the impact of serum trace metals on sleep disorders have been limited, wrote Ming-Gang Deng, MD, of Wuhan (China) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) 2011-2016 to calculate the odds ratios of sleep disorders and serum zinc (Zn), copper (Cu), and selenium (Se). The study population included adults aged 18 years and older, with an average age of 47.6 years. Approximately half of the participants were men, and the majority was non-Hispanic white. Serum Zn, Cu, and Se were identified at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health. The lower limits of detection for Zn, Cu, and Se were 2.9 mcg/dL, 2.5 mcg/dL, and 4.5 mcg/L, respectively. Sleep disorders were assessed based on self-reports of discussions with health professionals about sleep disorders, and via the Sleep Disorder Questionnaire.
After adjusting for sociodemographic, behavioral characteristics, and health characteristics, adults in the highest tertiles of serum Zn had a 30% reduced risk of sleep disorders, compared with those in the lowest tertiles of serum Zn (odds ratio, 0.70; P = .035). In measures of trace metals ratios, serum Zn/Cu and Zn/Se also were significantly associated with reduced risk of sleep disorders for individuals in the highest tertiles, compared with those in the lowest tertiles (OR, 0.62 and OR, 0.68, respectively).
However, serum Cu, Se and Cu/Se were not associated with sleep disorder risk.
Sociodemographic factors included age, sex, race, education level, family income level; behavioral characteristics included smoking, alcohol consumption, physical activity, and caffeine intake.
The researchers also used a restricted cubic spline model to examine the dose-response relationships between serum trace metals, serum trace metals ratios, and sleep disorders. In this analysis, higher levels of serum Zn, Zn/Cu, and Zn/Se were related to reduced risk of sleep disorders, while no significant association appeared between serum Cu, Se, or Cu/Se and sleep disorders risk.
The findings showing a lack of association between Se and sleep disorders were not consistent with previous studies, the researchers wrote in their discussion. Previous research has shown that a higher Se was less likely to be associated with trouble falling asleep, and has shown a potential treatment effect of Se on obstructive sleep apnea, they said.
“Although serum Cu and Se levels were not correlated to sleep disorders in our study, the Zn/Cu and Zn/Se may provide some novel insights,” they wrote. For example, Zn/Cu has been used as a predictor of several clinical complications related to an increased risk of sleep disorders including cardiovascular disease, cancer, and major depressive disorder, they noted.
The findings were limited by several factors including the cross-sectional design, use of self-reports, and the inability to examine relationships between trace metals and specific sleep disorder symptoms, such as restless legs syndrome, insomnia, and obstructive sleep apnea, the researchers noted.
However, the results were strengthened by the large national sample, and support data from previous studies, they said.
“The inverse associations of serum Zn, and Zn/Cu, Zn/Se with sleep disorders enlightened us that increasing Zn intake may be an excellent approach to prevent sleep disorders due to its benefits from these three aspects,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, based on data from 3,660 individuals.
Previous research has shown an association between trace metals and sleep and sleep patterns, but data on the impact of serum trace metals on sleep disorders have been limited, wrote Ming-Gang Deng, MD, of Wuhan (China) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) 2011-2016 to calculate the odds ratios of sleep disorders and serum zinc (Zn), copper (Cu), and selenium (Se). The study population included adults aged 18 years and older, with an average age of 47.6 years. Approximately half of the participants were men, and the majority was non-Hispanic white. Serum Zn, Cu, and Se were identified at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health. The lower limits of detection for Zn, Cu, and Se were 2.9 mcg/dL, 2.5 mcg/dL, and 4.5 mcg/L, respectively. Sleep disorders were assessed based on self-reports of discussions with health professionals about sleep disorders, and via the Sleep Disorder Questionnaire.
After adjusting for sociodemographic, behavioral characteristics, and health characteristics, adults in the highest tertiles of serum Zn had a 30% reduced risk of sleep disorders, compared with those in the lowest tertiles of serum Zn (odds ratio, 0.70; P = .035). In measures of trace metals ratios, serum Zn/Cu and Zn/Se also were significantly associated with reduced risk of sleep disorders for individuals in the highest tertiles, compared with those in the lowest tertiles (OR, 0.62 and OR, 0.68, respectively).
However, serum Cu, Se and Cu/Se were not associated with sleep disorder risk.
Sociodemographic factors included age, sex, race, education level, family income level; behavioral characteristics included smoking, alcohol consumption, physical activity, and caffeine intake.
The researchers also used a restricted cubic spline model to examine the dose-response relationships between serum trace metals, serum trace metals ratios, and sleep disorders. In this analysis, higher levels of serum Zn, Zn/Cu, and Zn/Se were related to reduced risk of sleep disorders, while no significant association appeared between serum Cu, Se, or Cu/Se and sleep disorders risk.
The findings showing a lack of association between Se and sleep disorders were not consistent with previous studies, the researchers wrote in their discussion. Previous research has shown that a higher Se was less likely to be associated with trouble falling asleep, and has shown a potential treatment effect of Se on obstructive sleep apnea, they said.
“Although serum Cu and Se levels were not correlated to sleep disorders in our study, the Zn/Cu and Zn/Se may provide some novel insights,” they wrote. For example, Zn/Cu has been used as a predictor of several clinical complications related to an increased risk of sleep disorders including cardiovascular disease, cancer, and major depressive disorder, they noted.
The findings were limited by several factors including the cross-sectional design, use of self-reports, and the inability to examine relationships between trace metals and specific sleep disorder symptoms, such as restless legs syndrome, insomnia, and obstructive sleep apnea, the researchers noted.
However, the results were strengthened by the large national sample, and support data from previous studies, they said.
“The inverse associations of serum Zn, and Zn/Cu, Zn/Se with sleep disorders enlightened us that increasing Zn intake may be an excellent approach to prevent sleep disorders due to its benefits from these three aspects,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
, based on data from 3,660 individuals.
Previous research has shown an association between trace metals and sleep and sleep patterns, but data on the impact of serum trace metals on sleep disorders have been limited, wrote Ming-Gang Deng, MD, of Wuhan (China) University and colleagues.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from the National Health and Nutrition Examination Survey (NHANES) 2011-2016 to calculate the odds ratios of sleep disorders and serum zinc (Zn), copper (Cu), and selenium (Se). The study population included adults aged 18 years and older, with an average age of 47.6 years. Approximately half of the participants were men, and the majority was non-Hispanic white. Serum Zn, Cu, and Se were identified at the Environmental Health Sciences Laboratory of the Centers for Disease Control and Prevention National Center for Environmental Health. The lower limits of detection for Zn, Cu, and Se were 2.9 mcg/dL, 2.5 mcg/dL, and 4.5 mcg/L, respectively. Sleep disorders were assessed based on self-reports of discussions with health professionals about sleep disorders, and via the Sleep Disorder Questionnaire.
After adjusting for sociodemographic, behavioral characteristics, and health characteristics, adults in the highest tertiles of serum Zn had a 30% reduced risk of sleep disorders, compared with those in the lowest tertiles of serum Zn (odds ratio, 0.70; P = .035). In measures of trace metals ratios, serum Zn/Cu and Zn/Se also were significantly associated with reduced risk of sleep disorders for individuals in the highest tertiles, compared with those in the lowest tertiles (OR, 0.62 and OR, 0.68, respectively).
However, serum Cu, Se and Cu/Se were not associated with sleep disorder risk.
Sociodemographic factors included age, sex, race, education level, family income level; behavioral characteristics included smoking, alcohol consumption, physical activity, and caffeine intake.
The researchers also used a restricted cubic spline model to examine the dose-response relationships between serum trace metals, serum trace metals ratios, and sleep disorders. In this analysis, higher levels of serum Zn, Zn/Cu, and Zn/Se were related to reduced risk of sleep disorders, while no significant association appeared between serum Cu, Se, or Cu/Se and sleep disorders risk.
The findings showing a lack of association between Se and sleep disorders were not consistent with previous studies, the researchers wrote in their discussion. Previous research has shown that a higher Se was less likely to be associated with trouble falling asleep, and has shown a potential treatment effect of Se on obstructive sleep apnea, they said.
“Although serum Cu and Se levels were not correlated to sleep disorders in our study, the Zn/Cu and Zn/Se may provide some novel insights,” they wrote. For example, Zn/Cu has been used as a predictor of several clinical complications related to an increased risk of sleep disorders including cardiovascular disease, cancer, and major depressive disorder, they noted.
The findings were limited by several factors including the cross-sectional design, use of self-reports, and the inability to examine relationships between trace metals and specific sleep disorder symptoms, such as restless legs syndrome, insomnia, and obstructive sleep apnea, the researchers noted.
However, the results were strengthened by the large national sample, and support data from previous studies, they said.
“The inverse associations of serum Zn, and Zn/Cu, Zn/Se with sleep disorders enlightened us that increasing Zn intake may be an excellent approach to prevent sleep disorders due to its benefits from these three aspects,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Sleep-disordered breathing promotes elevated arterial stiffness and preeclampsia
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
, based on data from 181 individuals.
The intermittent hypoxia resulting from sleep-disordered breathing (SDB) has been linked to cardiovascular disease and hypertension, wrote Kim Phan, PhD, of McGill University, Montreal, and colleagues.
SDB has been associated with increased preeclampsia risk, and women with preeclampsia show increased arterial stiffness, but an association between SDB and arterial stiffness in pregnancy has not been explored, they said.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers reviewed data from 181 women with high-risk singleton pregnancies recruited from two tertiary obstetrics clinics in Montreal. High-risk pregnancy was defined as meeting at least one of the following criteria: age 35 years and older, body mass index 25 kg/m2 or higher, chronic hypertension, preexisting diabetes mellitus, preexisting renal disease, or personal or first-degree relative with a history of preeclampsia.
Participants were assessed at each trimester via the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, and Restless Legs Syndrome questionnaire. Sleep-disordered breathing was defined as loud snoring or witnessed sleep apneas at least three times a week. Arterial stiffness was assessed via applanation tonometry every 4 weeks from baseline throughout pregnancy.
Overall, 23% of the study population met the criteria for SDB; SDB in the first or second trimester was associated with a significantly increased risk of preeclampsia (odds ratio 3.4). The effect of SDB on preeclampsia was increased in women who reported excessive daytime sleepiness, defined as scores higher than 10 on the Epworth Sleepiness Scale. The odds ratio for preeclampsia in the first or second trimester increased to 5.7 in women with hypersomnolence in addition to SDB. The risk of preeclampsia was even higher (OR 8.2) in the third trimester.
Self-reported total sleep time decreased in the second and third trimesters compared with the first, but reports of excessive daytime sleepiness remained consistent throughout the pregnancies, the researchers noted.
The results highlight the need to screen pregnant women for SDB in all three trimesters; however, “future studies will need to assess the incremental benefit of integrating SDB into risk assessment calculators in pregnancy,” the researchers wrote in their discussion. Randomized trials are needed to determine the value of interventions such as continuous positive airway pressure to reduce arterial stiffness and the risks of hypertensive disorders of pregnancy, they said. More data also are needed to examine the role of excessive daytime sleepiness as a modifier of arterial stiffness and preeclampsia risk, they noted.
The findings were limited by the prospective design, which prevents conclusions of causality, the researchers noted. Other limitations included the focus on high-risk pregnancy, which may limit generalizability, and the use of symptoms, not sleep recordings, to identify SDB, they said.
However, the results show an independent association between SDB and arterial stiffness during pregnancy, and offer potentially useful insights into the mechanisms of SDB-associated cardiovascular conditions, they noted.
“This work may inform future studies exploring the value of using arterial stiffness, as an early noninvasive indicator of subclinical vascular dysfunction in pregnant women with SDB,” they concluded.
The study was supported by the Fonds de recherche du Quebec – Sante (FRQS), Heart and Stroke Foundation of Canada, McGill University’s academic enrichment fund, and the Canadian Foundation for Women’s Health. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Severe OSA tied to poor prognoses in stroke patients
Patients with acute ischemic stroke had a worse prognosis if they had also experienced severe obstructive sleep apnea (OSA), based on data from 125 individuals.
OSA is on the rise, and is associated with pathophysiological changes, and data from previous studies suggest that severe OSA doubles the risk of stroke and increases risk of stroke recurrence, according to Juan Xu, PhD, of Soochow University, Suzhou, China, and colleagues.
“There is a high comorbidity between stroke and OSA,” and effective sleep is important to cerebral function recovery, the researchers wrote. Early prediction of stroke prognosis may inform treatment in stroke patients, but the value of OSA as a predictor of functional prognosis has not been explored.
In a study published in Sleep Medicine, the researchers analyzed data from 125 adults with mild to moderate ischemic stroke and OSA. The participants underwent polysomnography within a week of stroke onset between January 2015 and June 2020 and were grouped by severity according to apnea-hypopnea index (AHI) of either less than 30/h (not severe) or 30/h or higher (severe). The mean age of the patients was 58 years, and 87% were men. Approximately one-third of the participants met the criteria for severe OSA.
The researchers assessed the impact of OSA on functional prognosis in the acute phase of stroke, and reviewed quantitative electroencephalography (EEG) markers in stroke patients during sleep.
Overall, individuals with severe OSA were significantly more likely than those with less severe OSA to have comorbid hypertension (85.4% vs. 56%; P = .002) and a higher body mass index (28 vs. 24; P < .001). Other factors including blood pressure, smoking history, alcohol use, and comorbid diabetes were similar between the groups.
Quantitative EEG among patients with severe OSA showed lower relative power of high-frequency bands (alpha, beta, and sigma). The EEG also showed higher delta/alpha power ratio and slowing ratio, and higher delta relative power (delta RP) in severe OSA (P < .05 for all).
In addition, severe OSA was associated with more than triple the risk (3.6-fold increase) of poor prognosis, defined as a Modified Rankin Scale score of 3 or higher (24.4% for severe OSA vs. 8.3% for nonsevere OSA; P = .03).
the researchers wrote. “Integrating the alteration of quantitative EEG parameters may improve the accuracy of early predictions of functional prognosis in patients with stroke.”
The findings were limited by several factors including the retrospective design and the lack of a sizable non-OSA control group, the researchers noted. Other limitations included the use of an AHI of 30/h or higher to define severity and the use of data from medical histories, with the potential for information bias, and the use of only 30-second continuous polysomnography segments.
However, the results suggest that increased delta RP and TSR, and decreased alpha, beta, and sigma RP, may be independent predictors of a poor functional prognosis in stroke patients with OSA, and that the prognosis could be improved by treating the OSA, they concluded.
The study was supported by the Natural Science Foundation of China and the Discipline Construction Program of the Second Affiliated Hospital of Soochow University. The researchers reported no financial conflicts.
A version of this article first appeared on Medscape.com.
Patients with acute ischemic stroke had a worse prognosis if they had also experienced severe obstructive sleep apnea (OSA), based on data from 125 individuals.
OSA is on the rise, and is associated with pathophysiological changes, and data from previous studies suggest that severe OSA doubles the risk of stroke and increases risk of stroke recurrence, according to Juan Xu, PhD, of Soochow University, Suzhou, China, and colleagues.
“There is a high comorbidity between stroke and OSA,” and effective sleep is important to cerebral function recovery, the researchers wrote. Early prediction of stroke prognosis may inform treatment in stroke patients, but the value of OSA as a predictor of functional prognosis has not been explored.
In a study published in Sleep Medicine, the researchers analyzed data from 125 adults with mild to moderate ischemic stroke and OSA. The participants underwent polysomnography within a week of stroke onset between January 2015 and June 2020 and were grouped by severity according to apnea-hypopnea index (AHI) of either less than 30/h (not severe) or 30/h or higher (severe). The mean age of the patients was 58 years, and 87% were men. Approximately one-third of the participants met the criteria for severe OSA.
The researchers assessed the impact of OSA on functional prognosis in the acute phase of stroke, and reviewed quantitative electroencephalography (EEG) markers in stroke patients during sleep.
Overall, individuals with severe OSA were significantly more likely than those with less severe OSA to have comorbid hypertension (85.4% vs. 56%; P = .002) and a higher body mass index (28 vs. 24; P < .001). Other factors including blood pressure, smoking history, alcohol use, and comorbid diabetes were similar between the groups.
Quantitative EEG among patients with severe OSA showed lower relative power of high-frequency bands (alpha, beta, and sigma). The EEG also showed higher delta/alpha power ratio and slowing ratio, and higher delta relative power (delta RP) in severe OSA (P < .05 for all).
In addition, severe OSA was associated with more than triple the risk (3.6-fold increase) of poor prognosis, defined as a Modified Rankin Scale score of 3 or higher (24.4% for severe OSA vs. 8.3% for nonsevere OSA; P = .03).
the researchers wrote. “Integrating the alteration of quantitative EEG parameters may improve the accuracy of early predictions of functional prognosis in patients with stroke.”
The findings were limited by several factors including the retrospective design and the lack of a sizable non-OSA control group, the researchers noted. Other limitations included the use of an AHI of 30/h or higher to define severity and the use of data from medical histories, with the potential for information bias, and the use of only 30-second continuous polysomnography segments.
However, the results suggest that increased delta RP and TSR, and decreased alpha, beta, and sigma RP, may be independent predictors of a poor functional prognosis in stroke patients with OSA, and that the prognosis could be improved by treating the OSA, they concluded.
The study was supported by the Natural Science Foundation of China and the Discipline Construction Program of the Second Affiliated Hospital of Soochow University. The researchers reported no financial conflicts.
A version of this article first appeared on Medscape.com.
Patients with acute ischemic stroke had a worse prognosis if they had also experienced severe obstructive sleep apnea (OSA), based on data from 125 individuals.
OSA is on the rise, and is associated with pathophysiological changes, and data from previous studies suggest that severe OSA doubles the risk of stroke and increases risk of stroke recurrence, according to Juan Xu, PhD, of Soochow University, Suzhou, China, and colleagues.
“There is a high comorbidity between stroke and OSA,” and effective sleep is important to cerebral function recovery, the researchers wrote. Early prediction of stroke prognosis may inform treatment in stroke patients, but the value of OSA as a predictor of functional prognosis has not been explored.
In a study published in Sleep Medicine, the researchers analyzed data from 125 adults with mild to moderate ischemic stroke and OSA. The participants underwent polysomnography within a week of stroke onset between January 2015 and June 2020 and were grouped by severity according to apnea-hypopnea index (AHI) of either less than 30/h (not severe) or 30/h or higher (severe). The mean age of the patients was 58 years, and 87% were men. Approximately one-third of the participants met the criteria for severe OSA.
The researchers assessed the impact of OSA on functional prognosis in the acute phase of stroke, and reviewed quantitative electroencephalography (EEG) markers in stroke patients during sleep.
Overall, individuals with severe OSA were significantly more likely than those with less severe OSA to have comorbid hypertension (85.4% vs. 56%; P = .002) and a higher body mass index (28 vs. 24; P < .001). Other factors including blood pressure, smoking history, alcohol use, and comorbid diabetes were similar between the groups.
Quantitative EEG among patients with severe OSA showed lower relative power of high-frequency bands (alpha, beta, and sigma). The EEG also showed higher delta/alpha power ratio and slowing ratio, and higher delta relative power (delta RP) in severe OSA (P < .05 for all).
In addition, severe OSA was associated with more than triple the risk (3.6-fold increase) of poor prognosis, defined as a Modified Rankin Scale score of 3 or higher (24.4% for severe OSA vs. 8.3% for nonsevere OSA; P = .03).
the researchers wrote. “Integrating the alteration of quantitative EEG parameters may improve the accuracy of early predictions of functional prognosis in patients with stroke.”
The findings were limited by several factors including the retrospective design and the lack of a sizable non-OSA control group, the researchers noted. Other limitations included the use of an AHI of 30/h or higher to define severity and the use of data from medical histories, with the potential for information bias, and the use of only 30-second continuous polysomnography segments.
However, the results suggest that increased delta RP and TSR, and decreased alpha, beta, and sigma RP, may be independent predictors of a poor functional prognosis in stroke patients with OSA, and that the prognosis could be improved by treating the OSA, they concluded.
The study was supported by the Natural Science Foundation of China and the Discipline Construction Program of the Second Affiliated Hospital of Soochow University. The researchers reported no financial conflicts.
A version of this article first appeared on Medscape.com.
FROM SLEEP MEDICINE
Mothers’ sleep issues promote poor outcomes for infants
or insomnia, based on data from approximately 5,000 infants.
Sleep disturbance is common during pregnancy, and “sleep disorders during pregnancy can have significant consequences for both the pregnant person and their infant,” write Jennifer N. Felder, PhD, of the University of California, San Francisco, and colleagues.
However, data on the impact of maternal insomnia on specific infant outcomes are limited, they said.
In a study published recently in the journal Sleep Health, the researchers reviewed data from 3,371 pregnant women diagnosed with sleep apnea and 3,213 with insomnia. Of these, 2,357 and 2,212 were matched with controls in a propensity-score analysis. The referent controls were matched for maternal characteristics, obstetric factors, and infant factors among individuals without a sleep disorder. All were singleton pregnancies.
Adverse infant outcomes included the following:
- One- and 5-minute Apgar scores less than 7.
- Respiratory distress syndrome.
- Neonatal intensive care unit admission.
- Hypoglycemia.
- Infant death.
- Hospital stay of longer than 2 days for vaginal delivery or longer than 4 days for cesarean delivery.
- Emergency department visit before 3 months of age.
- Emergency department visit in the first year of life.
- Composite measure of adverse infant outcomes.
Compared with matched controls, the infants born to mothers with sleep apnea had a significantly increased risk for any adverse outcome (50.1% vs. 53.5%) and of the specific outcomes of low 1-minute Apgar scores (6.3% vs. 9.6%), neonatal ICU stays (6.3% vs. 8.4%), and an emergency department visit in the first year of life (33.6% vs. 36.9%).
For infants born to mothers with insomnia, the only significant difference in outcomes compared with controls was an increased likelihood of an emergency department visit (37.2% vs. 32.3%).
“Research on possible mechanisms of the relation between maternal prenatal sleep apnea and poorer birth and infant outcomes associations is small but growing, implicating systemic inflammation and late or prolonged fetal heart rate decelerations,” the researchers write in their discussion.
Research on insomnia during pregnancy and adverse infant outcomes is limited, and the largest studies have been complicated by the effects of insomnia medication; therefore, “our finding that infants born to mothers with an insomnia diagnosis were at increased risk of only emergency room visit, but no other analyzed infant outcomes, is important and novel,” they note.
The findings were limited by several factors, including the reliance on medical records, which may lack details on how routinely health care professionals assessed sleep disorders, the researchers noted. “Consequently, the findings presented here may reflect more severe cases of insomnia and sleep apnea, and may not represent the population of individuals with diagnosed sleep apnea or insomnia during pregnancy generally,” the authors say. Other limitations included a lack of information on treatment of sleep disorders and on the timing of diagnosis (before pregnancy or during pregnancy).
However, the results were strengthened by the large, population-based sample and use of codes to highlight research questions, the researchers said.
In light of the health consequences of sleep disorders in pregnancy, the data suggest that sleep apnea and insomnia in pregnant women may serve as targets for risk assessment of adverse infant outcomes, and more research is needed to determine whether addressing sleep issues reduces these outcomes, they concluded.
The study was supported by the University of California, San Francisco, Preterm Birth Initiative and by grants to lead author Dr. Felder from the National Center for Complementary and Integrative Health and to a coauthor from the National Heart, Lung, and Blood Institute. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or insomnia, based on data from approximately 5,000 infants.
Sleep disturbance is common during pregnancy, and “sleep disorders during pregnancy can have significant consequences for both the pregnant person and their infant,” write Jennifer N. Felder, PhD, of the University of California, San Francisco, and colleagues.
However, data on the impact of maternal insomnia on specific infant outcomes are limited, they said.
In a study published recently in the journal Sleep Health, the researchers reviewed data from 3,371 pregnant women diagnosed with sleep apnea and 3,213 with insomnia. Of these, 2,357 and 2,212 were matched with controls in a propensity-score analysis. The referent controls were matched for maternal characteristics, obstetric factors, and infant factors among individuals without a sleep disorder. All were singleton pregnancies.
Adverse infant outcomes included the following:
- One- and 5-minute Apgar scores less than 7.
- Respiratory distress syndrome.
- Neonatal intensive care unit admission.
- Hypoglycemia.
- Infant death.
- Hospital stay of longer than 2 days for vaginal delivery or longer than 4 days for cesarean delivery.
- Emergency department visit before 3 months of age.
- Emergency department visit in the first year of life.
- Composite measure of adverse infant outcomes.
Compared with matched controls, the infants born to mothers with sleep apnea had a significantly increased risk for any adverse outcome (50.1% vs. 53.5%) and of the specific outcomes of low 1-minute Apgar scores (6.3% vs. 9.6%), neonatal ICU stays (6.3% vs. 8.4%), and an emergency department visit in the first year of life (33.6% vs. 36.9%).
For infants born to mothers with insomnia, the only significant difference in outcomes compared with controls was an increased likelihood of an emergency department visit (37.2% vs. 32.3%).
“Research on possible mechanisms of the relation between maternal prenatal sleep apnea and poorer birth and infant outcomes associations is small but growing, implicating systemic inflammation and late or prolonged fetal heart rate decelerations,” the researchers write in their discussion.
Research on insomnia during pregnancy and adverse infant outcomes is limited, and the largest studies have been complicated by the effects of insomnia medication; therefore, “our finding that infants born to mothers with an insomnia diagnosis were at increased risk of only emergency room visit, but no other analyzed infant outcomes, is important and novel,” they note.
The findings were limited by several factors, including the reliance on medical records, which may lack details on how routinely health care professionals assessed sleep disorders, the researchers noted. “Consequently, the findings presented here may reflect more severe cases of insomnia and sleep apnea, and may not represent the population of individuals with diagnosed sleep apnea or insomnia during pregnancy generally,” the authors say. Other limitations included a lack of information on treatment of sleep disorders and on the timing of diagnosis (before pregnancy or during pregnancy).
However, the results were strengthened by the large, population-based sample and use of codes to highlight research questions, the researchers said.
In light of the health consequences of sleep disorders in pregnancy, the data suggest that sleep apnea and insomnia in pregnant women may serve as targets for risk assessment of adverse infant outcomes, and more research is needed to determine whether addressing sleep issues reduces these outcomes, they concluded.
The study was supported by the University of California, San Francisco, Preterm Birth Initiative and by grants to lead author Dr. Felder from the National Center for Complementary and Integrative Health and to a coauthor from the National Heart, Lung, and Blood Institute. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
or insomnia, based on data from approximately 5,000 infants.
Sleep disturbance is common during pregnancy, and “sleep disorders during pregnancy can have significant consequences for both the pregnant person and their infant,” write Jennifer N. Felder, PhD, of the University of California, San Francisco, and colleagues.
However, data on the impact of maternal insomnia on specific infant outcomes are limited, they said.
In a study published recently in the journal Sleep Health, the researchers reviewed data from 3,371 pregnant women diagnosed with sleep apnea and 3,213 with insomnia. Of these, 2,357 and 2,212 were matched with controls in a propensity-score analysis. The referent controls were matched for maternal characteristics, obstetric factors, and infant factors among individuals without a sleep disorder. All were singleton pregnancies.
Adverse infant outcomes included the following:
- One- and 5-minute Apgar scores less than 7.
- Respiratory distress syndrome.
- Neonatal intensive care unit admission.
- Hypoglycemia.
- Infant death.
- Hospital stay of longer than 2 days for vaginal delivery or longer than 4 days for cesarean delivery.
- Emergency department visit before 3 months of age.
- Emergency department visit in the first year of life.
- Composite measure of adverse infant outcomes.
Compared with matched controls, the infants born to mothers with sleep apnea had a significantly increased risk for any adverse outcome (50.1% vs. 53.5%) and of the specific outcomes of low 1-minute Apgar scores (6.3% vs. 9.6%), neonatal ICU stays (6.3% vs. 8.4%), and an emergency department visit in the first year of life (33.6% vs. 36.9%).
For infants born to mothers with insomnia, the only significant difference in outcomes compared with controls was an increased likelihood of an emergency department visit (37.2% vs. 32.3%).
“Research on possible mechanisms of the relation between maternal prenatal sleep apnea and poorer birth and infant outcomes associations is small but growing, implicating systemic inflammation and late or prolonged fetal heart rate decelerations,” the researchers write in their discussion.
Research on insomnia during pregnancy and adverse infant outcomes is limited, and the largest studies have been complicated by the effects of insomnia medication; therefore, “our finding that infants born to mothers with an insomnia diagnosis were at increased risk of only emergency room visit, but no other analyzed infant outcomes, is important and novel,” they note.
The findings were limited by several factors, including the reliance on medical records, which may lack details on how routinely health care professionals assessed sleep disorders, the researchers noted. “Consequently, the findings presented here may reflect more severe cases of insomnia and sleep apnea, and may not represent the population of individuals with diagnosed sleep apnea or insomnia during pregnancy generally,” the authors say. Other limitations included a lack of information on treatment of sleep disorders and on the timing of diagnosis (before pregnancy or during pregnancy).
However, the results were strengthened by the large, population-based sample and use of codes to highlight research questions, the researchers said.
In light of the health consequences of sleep disorders in pregnancy, the data suggest that sleep apnea and insomnia in pregnant women may serve as targets for risk assessment of adverse infant outcomes, and more research is needed to determine whether addressing sleep issues reduces these outcomes, they concluded.
The study was supported by the University of California, San Francisco, Preterm Birth Initiative and by grants to lead author Dr. Felder from the National Center for Complementary and Integrative Health and to a coauthor from the National Heart, Lung, and Blood Institute. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP HEALTH
Improving sleep boosts cognition in refractory temporal lobe epilepsy
NASHVILLE, TENN. – Targeting relevant sleep problems for patients with refractory temporal lobe epilepsy (TLE) improves cognition, results of a new, double-blind, randomized controlled trial suggest.
Study findings show significant improvement in REM sleep and language scores for patients with TLE who took the cholinesterase inhibitor donepezil and better slow-wave sleep and memory scores for those who took the sleep aid zolpidem.
, study investigator Garima Shukla, MBBS, MD, DM, professor, division of neurology, department of medicine, Queens University, Kingston, Ont., told this news organization.
Daytime sleepiness could be a red flag in these patients, although it could mean they just have treatable sleep apnea, said Dr. Shukla. “But if they have very poor slow-wave sleep, we could try increasing its percentage by prescribing zolpidem.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Sleep, cognitive disturbances common
Sleep disturbances and cognitive disturbances are common among patients with TLE. Executive function is affected in almost all patients with refractory epilepsy, and it’s “super common” that TLE patients have memory disturbances, said Dr. Shukla.
The study included 108 patients with refractory TLE who were awaiting surgery. The patients, who had no severe comorbidities, were randomly assigned to three groups; the final number in each group was 36.
Patients in group 1 received donepezil 10 mg in the morning and a placebo at night. (Donepezil is used to treat memory loss associated with Alzheimer’s disease.)
Those in group 2 received a placebo in the morning and zolpidem 6.25 mg at night. Group 3 patients received a placebo in the morning and again at night.
The mean age of the patients was 25.4, 27.1, and 27.6 years, and the percentage of men was 63.8%, 72.2%, and 63.8% in groups 1, 2, and 3, respectively.
In all groups, patients had been experiencing about three seizures per month. The median number of antiseizure medications was two in group 1 and three in both groups 2 and 3.
Researchers evaluated sleep using the Pittsburgh Sleep Quality Index, the Epsworth Sleepiness Scale, and video polysomnography and electroencephalography.
To assess executive function, they used the Trail A & B, Stroop, and forward and backward Digit Span tests. For memory, they used the Weschler Memory Scale, and for language, the Western Aphasia Battery. They conducted follow-up evaluations at 6 months.
The results showed significant improvement in the percentage of rapid eye movement (REM) sleep in group 1 (from 14.81 at baseline to 18.21 at 6 months). In this group, the number of patients whose REM sleep percentage was less than 15 dropped significantly from 29 (of 36) to 10.
In group 2, sleep-onset latency significantly improved, and the percentage of N3 (slow-wave) sleep stage increased significantly from 25.27 to 28.74.
Regarding cognitive outcomes, backward Digit Span was significantly improved for patients in group 1. In this group, there was also a significant reduction in the time taken for Stroop A test, and there was significant improvement in language.
In group 2, there was a significant improvement in verbal and visual memory scores. There were no significant changes in group 3.
The increase in REM sleep percentage in group 1 strongly correlated with increased language and executive function scores. Similarly, in group 2, the increase in N3 sleep percentage strongly correlated with an increase in verbal memory scores.
On the basis of these observations, giving a small dose of zolpidem to a patient with “acceptable” REM sleep but very little slow-wave sleep may boost the patient’s non-REM sleep, said Dr. Shukla. “By improving non-REM sleep percentage, we will possibly help memory consolidation.”
Dr. Shukla sees this study as “a stepping-stone” to larger, multicenter trials testing “the effect of zolpidem through its impact on improving non-REM sleep percentage consolidation and its impact on memory.”
This idea veers somewhat from the traditional idea that REM sleep plays a greater role in memory consolidation, she said. “We actually found it correlates very well with language, which we have also seen in some of our anecdotal case reports.”
Patients whose language scores are very poor are “the population I would pick to target REM sleep through donepezil,” said Dr. Shukla.
‘Encouraging’ findings
Commenting for this news organization, Daniel Goldenholz, MD, PhD, assistant professor, Harvard Beth Israel Deaconess Medical Center, Boston, praised the study design.
“It allows for comparison between different treatments, as well as a placebo control group,” said Dr. Goldenholz, who added, “There appears to be good follow-up” as well.
The fact that medication may provide some cognitive benefit for patients with TLE is “very encouraging,” he said.
He noted many patients with TLE complain of memory or language problems. “So, this is a major concern.”
However, he cautioned about side effects. “Putting all temporal lobe epilepsy patients who say that they have memory problems or language problems on these medications could have some serious consequences.”
The study was funded by a Department of Health Research grant from the government of India. Dr. Goldenholz is on the advisory board for epilepsy AI, Eyzs, and Magic Leap.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – Targeting relevant sleep problems for patients with refractory temporal lobe epilepsy (TLE) improves cognition, results of a new, double-blind, randomized controlled trial suggest.
Study findings show significant improvement in REM sleep and language scores for patients with TLE who took the cholinesterase inhibitor donepezil and better slow-wave sleep and memory scores for those who took the sleep aid zolpidem.
, study investigator Garima Shukla, MBBS, MD, DM, professor, division of neurology, department of medicine, Queens University, Kingston, Ont., told this news organization.
Daytime sleepiness could be a red flag in these patients, although it could mean they just have treatable sleep apnea, said Dr. Shukla. “But if they have very poor slow-wave sleep, we could try increasing its percentage by prescribing zolpidem.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Sleep, cognitive disturbances common
Sleep disturbances and cognitive disturbances are common among patients with TLE. Executive function is affected in almost all patients with refractory epilepsy, and it’s “super common” that TLE patients have memory disturbances, said Dr. Shukla.
The study included 108 patients with refractory TLE who were awaiting surgery. The patients, who had no severe comorbidities, were randomly assigned to three groups; the final number in each group was 36.
Patients in group 1 received donepezil 10 mg in the morning and a placebo at night. (Donepezil is used to treat memory loss associated with Alzheimer’s disease.)
Those in group 2 received a placebo in the morning and zolpidem 6.25 mg at night. Group 3 patients received a placebo in the morning and again at night.
The mean age of the patients was 25.4, 27.1, and 27.6 years, and the percentage of men was 63.8%, 72.2%, and 63.8% in groups 1, 2, and 3, respectively.
In all groups, patients had been experiencing about three seizures per month. The median number of antiseizure medications was two in group 1 and three in both groups 2 and 3.
Researchers evaluated sleep using the Pittsburgh Sleep Quality Index, the Epsworth Sleepiness Scale, and video polysomnography and electroencephalography.
To assess executive function, they used the Trail A & B, Stroop, and forward and backward Digit Span tests. For memory, they used the Weschler Memory Scale, and for language, the Western Aphasia Battery. They conducted follow-up evaluations at 6 months.
The results showed significant improvement in the percentage of rapid eye movement (REM) sleep in group 1 (from 14.81 at baseline to 18.21 at 6 months). In this group, the number of patients whose REM sleep percentage was less than 15 dropped significantly from 29 (of 36) to 10.
In group 2, sleep-onset latency significantly improved, and the percentage of N3 (slow-wave) sleep stage increased significantly from 25.27 to 28.74.
Regarding cognitive outcomes, backward Digit Span was significantly improved for patients in group 1. In this group, there was also a significant reduction in the time taken for Stroop A test, and there was significant improvement in language.
In group 2, there was a significant improvement in verbal and visual memory scores. There were no significant changes in group 3.
The increase in REM sleep percentage in group 1 strongly correlated with increased language and executive function scores. Similarly, in group 2, the increase in N3 sleep percentage strongly correlated with an increase in verbal memory scores.
On the basis of these observations, giving a small dose of zolpidem to a patient with “acceptable” REM sleep but very little slow-wave sleep may boost the patient’s non-REM sleep, said Dr. Shukla. “By improving non-REM sleep percentage, we will possibly help memory consolidation.”
Dr. Shukla sees this study as “a stepping-stone” to larger, multicenter trials testing “the effect of zolpidem through its impact on improving non-REM sleep percentage consolidation and its impact on memory.”
This idea veers somewhat from the traditional idea that REM sleep plays a greater role in memory consolidation, she said. “We actually found it correlates very well with language, which we have also seen in some of our anecdotal case reports.”
Patients whose language scores are very poor are “the population I would pick to target REM sleep through donepezil,” said Dr. Shukla.
‘Encouraging’ findings
Commenting for this news organization, Daniel Goldenholz, MD, PhD, assistant professor, Harvard Beth Israel Deaconess Medical Center, Boston, praised the study design.
“It allows for comparison between different treatments, as well as a placebo control group,” said Dr. Goldenholz, who added, “There appears to be good follow-up” as well.
The fact that medication may provide some cognitive benefit for patients with TLE is “very encouraging,” he said.
He noted many patients with TLE complain of memory or language problems. “So, this is a major concern.”
However, he cautioned about side effects. “Putting all temporal lobe epilepsy patients who say that they have memory problems or language problems on these medications could have some serious consequences.”
The study was funded by a Department of Health Research grant from the government of India. Dr. Goldenholz is on the advisory board for epilepsy AI, Eyzs, and Magic Leap.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – Targeting relevant sleep problems for patients with refractory temporal lobe epilepsy (TLE) improves cognition, results of a new, double-blind, randomized controlled trial suggest.
Study findings show significant improvement in REM sleep and language scores for patients with TLE who took the cholinesterase inhibitor donepezil and better slow-wave sleep and memory scores for those who took the sleep aid zolpidem.
, study investigator Garima Shukla, MBBS, MD, DM, professor, division of neurology, department of medicine, Queens University, Kingston, Ont., told this news organization.
Daytime sleepiness could be a red flag in these patients, although it could mean they just have treatable sleep apnea, said Dr. Shukla. “But if they have very poor slow-wave sleep, we could try increasing its percentage by prescribing zolpidem.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Sleep, cognitive disturbances common
Sleep disturbances and cognitive disturbances are common among patients with TLE. Executive function is affected in almost all patients with refractory epilepsy, and it’s “super common” that TLE patients have memory disturbances, said Dr. Shukla.
The study included 108 patients with refractory TLE who were awaiting surgery. The patients, who had no severe comorbidities, were randomly assigned to three groups; the final number in each group was 36.
Patients in group 1 received donepezil 10 mg in the morning and a placebo at night. (Donepezil is used to treat memory loss associated with Alzheimer’s disease.)
Those in group 2 received a placebo in the morning and zolpidem 6.25 mg at night. Group 3 patients received a placebo in the morning and again at night.
The mean age of the patients was 25.4, 27.1, and 27.6 years, and the percentage of men was 63.8%, 72.2%, and 63.8% in groups 1, 2, and 3, respectively.
In all groups, patients had been experiencing about three seizures per month. The median number of antiseizure medications was two in group 1 and three in both groups 2 and 3.
Researchers evaluated sleep using the Pittsburgh Sleep Quality Index, the Epsworth Sleepiness Scale, and video polysomnography and electroencephalography.
To assess executive function, they used the Trail A & B, Stroop, and forward and backward Digit Span tests. For memory, they used the Weschler Memory Scale, and for language, the Western Aphasia Battery. They conducted follow-up evaluations at 6 months.
The results showed significant improvement in the percentage of rapid eye movement (REM) sleep in group 1 (from 14.81 at baseline to 18.21 at 6 months). In this group, the number of patients whose REM sleep percentage was less than 15 dropped significantly from 29 (of 36) to 10.
In group 2, sleep-onset latency significantly improved, and the percentage of N3 (slow-wave) sleep stage increased significantly from 25.27 to 28.74.
Regarding cognitive outcomes, backward Digit Span was significantly improved for patients in group 1. In this group, there was also a significant reduction in the time taken for Stroop A test, and there was significant improvement in language.
In group 2, there was a significant improvement in verbal and visual memory scores. There were no significant changes in group 3.
The increase in REM sleep percentage in group 1 strongly correlated with increased language and executive function scores. Similarly, in group 2, the increase in N3 sleep percentage strongly correlated with an increase in verbal memory scores.
On the basis of these observations, giving a small dose of zolpidem to a patient with “acceptable” REM sleep but very little slow-wave sleep may boost the patient’s non-REM sleep, said Dr. Shukla. “By improving non-REM sleep percentage, we will possibly help memory consolidation.”
Dr. Shukla sees this study as “a stepping-stone” to larger, multicenter trials testing “the effect of zolpidem through its impact on improving non-REM sleep percentage consolidation and its impact on memory.”
This idea veers somewhat from the traditional idea that REM sleep plays a greater role in memory consolidation, she said. “We actually found it correlates very well with language, which we have also seen in some of our anecdotal case reports.”
Patients whose language scores are very poor are “the population I would pick to target REM sleep through donepezil,” said Dr. Shukla.
‘Encouraging’ findings
Commenting for this news organization, Daniel Goldenholz, MD, PhD, assistant professor, Harvard Beth Israel Deaconess Medical Center, Boston, praised the study design.
“It allows for comparison between different treatments, as well as a placebo control group,” said Dr. Goldenholz, who added, “There appears to be good follow-up” as well.
The fact that medication may provide some cognitive benefit for patients with TLE is “very encouraging,” he said.
He noted many patients with TLE complain of memory or language problems. “So, this is a major concern.”
However, he cautioned about side effects. “Putting all temporal lobe epilepsy patients who say that they have memory problems or language problems on these medications could have some serious consequences.”
The study was funded by a Department of Health Research grant from the government of India. Dr. Goldenholz is on the advisory board for epilepsy AI, Eyzs, and Magic Leap.
A version of this article first appeared on Medscape.com.
AT AES 2022