User login
Surgery better than medical therapy in some GERD patients
Surgery may be more effective than medical therapy, according to results from a randomized trial in 78 patients with reflux-related heartburn refractory to proton pump inhibitors (PPIs).
Stuart J. Spechler, MD, from Baylor University Medical Center, Dallas, and coauthors wrote in the New England Journal of Medicine that, for these patients, there were no medical treatment options that had been shown to have long-term benefit, so PPIs were often continued despite not offering adequate symptom relief. Other medical options such as baclofen and neuromodulators often have unacceptable side effects, and studies of their efficacy were few and of short duration.
In this study, patients were randomized either to laparoscopic Nissen fundoplication, treatment with omeprazole plus baclofen with desipramine depending on symptoms, or a control treatment of omeprazole plus placebo.
At 1 year, researchers saw a significantly higher rate of treatment success – defined as 50% or greater improvement in gastroesophageal reflux disease health-related quality of life score – in the surgery group (67%), compared with the medical-treatment group (28%) and control-medical group (12%).
This translated to an unadjusted 138% greater chance of treatment success with surgery, compared with active medical treatment, and a greater than 400% increase for surgery, compared with the control medical treatment.
Researchers also did a prespecified subgroup analysis among people with reflex hypersensitivity or abnormal acid reflux, and found the incidence of success with surgery was 71% and 62%, respectively.
They described this finding as “noteworthy,” given that reflux hypersensitivity was considered a functional disorder that would not be expected to improve with a procedure that didn’t alter abnormal esophageal pain perception.
However, they acknowledged that, as the study did not include a sham-surgery group, they couldn’t determine how much the placebo effect might have contributed to the treatment success of surgery.
They also stressed that the randomized group was a highly selected group of patients, and that the systematic work-up including esophageal multichannel intraluminal impedance pH monitoring could identify a subgroup that might have a better response to surgery than to medical treatment.
Four patients in the surgery group experienced a total of five serious adverse events, including one patients who had a herniated fundoplication treated with repeat surgery; four patients in the active-medical group experienced four serious adverse events; and three patients in the control-medical group experienced five serious adverse events.
The authors noted that 366 patients with PPI-refractory heartburn were originally enrolled in the study, then treated with 20 mg of omeprazole twice daily for 2 weeks with strict instructions to take 20 minutes before breakfast and dinner. Of these patients, 42 had their symptoms relieved by the omeprazole treatment and so were excluded from the randomization.
The “strict instructions” on how to take omeprazole were important, because PPIs only bind to gastric proton pumps that are actively secreting acid, the authors wrote. They also commented that the relative potencies of individual PPIs can vary, so patients not on omeprazole before the study may have responded better to this than other PPIs.
Before randomizations, patients also underwent endoscopy, esophageal biopsy, esophageal manometry, and multichannel intraluminal impedance pH monitoring. This excluded another 23 patients who were found to have non–gastroesophageal reflux disease, including eosinophilic esophagitis, other endoscopic or histologic abnormalities, and manometric abnormalities.
“This trial highlights the critical importance of systematic evaluation, similar to that recommended by Gyawali and Fass for managing the care of patients with PPI-refractory heartburn,” they wrote. “Many patients would not complete this rigorous evaluation, and among those who did, the cause of heartburn in most of them was not [gastroesophageal reflux disease].”
The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
SOURCE: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
Around 40% of troublesome heartburn fails to respond to proton pump inhibitor therapy, which may reflect a diverse range of underlying causes of the condition. Therefore we cannot treat it as a single disease process that will respond to higher and higher doses of acid suppression.
The results of a study of surgical intervention in a carefully selected group of patients are striking in showing surgery’s superiority to medical treatment, but it is important to note that 79% of patients enrolled in the study did not meet the criteria for surgery. Therefore these findings cannot be generalized to all patients with refractory heartburn, and each case should only be considered for surgery after extended trials of medical therapy.
Nicholas J. Talley, MD, PhD, is from the faculty of health and medicine at the University of Newcastle (Australia) and Hunter Medical Research Institute, also in Newcastle. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 17. doi: 10.1056/NEJMe1911623). Dr. Talley declared a range of consultancies, grants, personal fees, and patents unrelated to the study.
Around 40% of troublesome heartburn fails to respond to proton pump inhibitor therapy, which may reflect a diverse range of underlying causes of the condition. Therefore we cannot treat it as a single disease process that will respond to higher and higher doses of acid suppression.
The results of a study of surgical intervention in a carefully selected group of patients are striking in showing surgery’s superiority to medical treatment, but it is important to note that 79% of patients enrolled in the study did not meet the criteria for surgery. Therefore these findings cannot be generalized to all patients with refractory heartburn, and each case should only be considered for surgery after extended trials of medical therapy.
Nicholas J. Talley, MD, PhD, is from the faculty of health and medicine at the University of Newcastle (Australia) and Hunter Medical Research Institute, also in Newcastle. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 17. doi: 10.1056/NEJMe1911623). Dr. Talley declared a range of consultancies, grants, personal fees, and patents unrelated to the study.
Around 40% of troublesome heartburn fails to respond to proton pump inhibitor therapy, which may reflect a diverse range of underlying causes of the condition. Therefore we cannot treat it as a single disease process that will respond to higher and higher doses of acid suppression.
The results of a study of surgical intervention in a carefully selected group of patients are striking in showing surgery’s superiority to medical treatment, but it is important to note that 79% of patients enrolled in the study did not meet the criteria for surgery. Therefore these findings cannot be generalized to all patients with refractory heartburn, and each case should only be considered for surgery after extended trials of medical therapy.
Nicholas J. Talley, MD, PhD, is from the faculty of health and medicine at the University of Newcastle (Australia) and Hunter Medical Research Institute, also in Newcastle. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Oct 17. doi: 10.1056/NEJMe1911623). Dr. Talley declared a range of consultancies, grants, personal fees, and patents unrelated to the study.
Surgery may be more effective than medical therapy, according to results from a randomized trial in 78 patients with reflux-related heartburn refractory to proton pump inhibitors (PPIs).
Stuart J. Spechler, MD, from Baylor University Medical Center, Dallas, and coauthors wrote in the New England Journal of Medicine that, for these patients, there were no medical treatment options that had been shown to have long-term benefit, so PPIs were often continued despite not offering adequate symptom relief. Other medical options such as baclofen and neuromodulators often have unacceptable side effects, and studies of their efficacy were few and of short duration.
In this study, patients were randomized either to laparoscopic Nissen fundoplication, treatment with omeprazole plus baclofen with desipramine depending on symptoms, or a control treatment of omeprazole plus placebo.
At 1 year, researchers saw a significantly higher rate of treatment success – defined as 50% or greater improvement in gastroesophageal reflux disease health-related quality of life score – in the surgery group (67%), compared with the medical-treatment group (28%) and control-medical group (12%).
This translated to an unadjusted 138% greater chance of treatment success with surgery, compared with active medical treatment, and a greater than 400% increase for surgery, compared with the control medical treatment.
Researchers also did a prespecified subgroup analysis among people with reflex hypersensitivity or abnormal acid reflux, and found the incidence of success with surgery was 71% and 62%, respectively.
They described this finding as “noteworthy,” given that reflux hypersensitivity was considered a functional disorder that would not be expected to improve with a procedure that didn’t alter abnormal esophageal pain perception.
However, they acknowledged that, as the study did not include a sham-surgery group, they couldn’t determine how much the placebo effect might have contributed to the treatment success of surgery.
They also stressed that the randomized group was a highly selected group of patients, and that the systematic work-up including esophageal multichannel intraluminal impedance pH monitoring could identify a subgroup that might have a better response to surgery than to medical treatment.
Four patients in the surgery group experienced a total of five serious adverse events, including one patients who had a herniated fundoplication treated with repeat surgery; four patients in the active-medical group experienced four serious adverse events; and three patients in the control-medical group experienced five serious adverse events.
The authors noted that 366 patients with PPI-refractory heartburn were originally enrolled in the study, then treated with 20 mg of omeprazole twice daily for 2 weeks with strict instructions to take 20 minutes before breakfast and dinner. Of these patients, 42 had their symptoms relieved by the omeprazole treatment and so were excluded from the randomization.
The “strict instructions” on how to take omeprazole were important, because PPIs only bind to gastric proton pumps that are actively secreting acid, the authors wrote. They also commented that the relative potencies of individual PPIs can vary, so patients not on omeprazole before the study may have responded better to this than other PPIs.
Before randomizations, patients also underwent endoscopy, esophageal biopsy, esophageal manometry, and multichannel intraluminal impedance pH monitoring. This excluded another 23 patients who were found to have non–gastroesophageal reflux disease, including eosinophilic esophagitis, other endoscopic or histologic abnormalities, and manometric abnormalities.
“This trial highlights the critical importance of systematic evaluation, similar to that recommended by Gyawali and Fass for managing the care of patients with PPI-refractory heartburn,” they wrote. “Many patients would not complete this rigorous evaluation, and among those who did, the cause of heartburn in most of them was not [gastroesophageal reflux disease].”
The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
SOURCE: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
Surgery may be more effective than medical therapy, according to results from a randomized trial in 78 patients with reflux-related heartburn refractory to proton pump inhibitors (PPIs).
Stuart J. Spechler, MD, from Baylor University Medical Center, Dallas, and coauthors wrote in the New England Journal of Medicine that, for these patients, there were no medical treatment options that had been shown to have long-term benefit, so PPIs were often continued despite not offering adequate symptom relief. Other medical options such as baclofen and neuromodulators often have unacceptable side effects, and studies of their efficacy were few and of short duration.
In this study, patients were randomized either to laparoscopic Nissen fundoplication, treatment with omeprazole plus baclofen with desipramine depending on symptoms, or a control treatment of omeprazole plus placebo.
At 1 year, researchers saw a significantly higher rate of treatment success – defined as 50% or greater improvement in gastroesophageal reflux disease health-related quality of life score – in the surgery group (67%), compared with the medical-treatment group (28%) and control-medical group (12%).
This translated to an unadjusted 138% greater chance of treatment success with surgery, compared with active medical treatment, and a greater than 400% increase for surgery, compared with the control medical treatment.
Researchers also did a prespecified subgroup analysis among people with reflex hypersensitivity or abnormal acid reflux, and found the incidence of success with surgery was 71% and 62%, respectively.
They described this finding as “noteworthy,” given that reflux hypersensitivity was considered a functional disorder that would not be expected to improve with a procedure that didn’t alter abnormal esophageal pain perception.
However, they acknowledged that, as the study did not include a sham-surgery group, they couldn’t determine how much the placebo effect might have contributed to the treatment success of surgery.
They also stressed that the randomized group was a highly selected group of patients, and that the systematic work-up including esophageal multichannel intraluminal impedance pH monitoring could identify a subgroup that might have a better response to surgery than to medical treatment.
Four patients in the surgery group experienced a total of five serious adverse events, including one patients who had a herniated fundoplication treated with repeat surgery; four patients in the active-medical group experienced four serious adverse events; and three patients in the control-medical group experienced five serious adverse events.
The authors noted that 366 patients with PPI-refractory heartburn were originally enrolled in the study, then treated with 20 mg of omeprazole twice daily for 2 weeks with strict instructions to take 20 minutes before breakfast and dinner. Of these patients, 42 had their symptoms relieved by the omeprazole treatment and so were excluded from the randomization.
The “strict instructions” on how to take omeprazole were important, because PPIs only bind to gastric proton pumps that are actively secreting acid, the authors wrote. They also commented that the relative potencies of individual PPIs can vary, so patients not on omeprazole before the study may have responded better to this than other PPIs.
Before randomizations, patients also underwent endoscopy, esophageal biopsy, esophageal manometry, and multichannel intraluminal impedance pH monitoring. This excluded another 23 patients who were found to have non–gastroesophageal reflux disease, including eosinophilic esophagitis, other endoscopic or histologic abnormalities, and manometric abnormalities.
“This trial highlights the critical importance of systematic evaluation, similar to that recommended by Gyawali and Fass for managing the care of patients with PPI-refractory heartburn,” they wrote. “Many patients would not complete this rigorous evaluation, and among those who did, the cause of heartburn in most of them was not [gastroesophageal reflux disease].”
The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
SOURCE: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Surgery may be more effective than medical therapy in some patients with treatment-refractory gastroesophageal reflux disease.
Major finding: Patients with treatment-refractory gastroesophageal reflux disease treated with surgery were 2.38 times more likely to respond to surgery than medical treatment.
Study details: A randomized, controlled trial in 78 patients with gastroesophageal reflux disease.
Disclosures: The study was funded by the Department of Veterans Affairs Cooperative Studies Program. Four authors declared consultancies with and/or grants from the pharmaceutical sector.
Source: Spechler SJ et al. N Engl J Med. 2019 Oct 16. doi: 10.1056/NEJMoa1811424.
Dupilumab may reduce dysphagia in adults with eosinophilic esophagitis
Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis enrolled in a randomized trial, investigators reported.
Treatment with this monoclonal antibody also improved histologic disease features and abnormal endoscopic features, compared with placebo, according to investigators in the phase 2 trial, which included 47 patients enrolled at 14 U.S. study sites.
Injection-site erythema and nasopharyngitis were more common among dupilumab-treated versus placebo-treated patients, and there were no serious adverse events or deaths observed, according to cofirst authors Ikuo Hirano, MD, of Northwestern University, Chicago, and Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill.
“Dupilumab is the first targeted biologic agent to improve dysphagia, histologic and endoscopic measures of disease, as well as esophageal function, and have an acceptable safety profile in adult patients with active eosinophilic esophagitis,” said Dr. Hirano and Dr. Dellon and associates in the journal Gastroenterology.
The report on the phase 2 trial included 47 adults with active eosinophilic esophagitis randomized to weekly subcutaneous injections of dupilumab at a dose of 300 mg or placebo. All participants had a score of 5 or higher on the Straumann Dysphagia Instrument (SDI), a patient-reported outcome measure.
Change in SDI score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab, according to investigators, who reported a least-squares mean change of –3.0 from baseline, versus –1.3 for placebo (P = .0304).
The original plan was to measure dupilumab’s effect on SDI out to week 12 of treatment, but because of technical problems with an electronic diary system used in the trial, there was significant data loss, and this primary endpoint was instead evaluated at week 10, investigators said in their report.
Improvements in SDI scores were apparent as early as week 1 after dupilumab treatment started, they added, noting that 39% of dupilumab-treated patients had an improvement in SDI score of at least 3, compared with just 13% of placebo-treated patients (P = .490).
Dupilumab also improved outcomes measured by the eosinophilic esophagitis histology scoring system (EoE-HSS), including a 68.3% improvement in severity and 54.6% in extent of disease from baseline to week 12, investigators said.
Likewise, dupilumab improved endoscopic outcomes at week 12 as measured by the eosinophilic esophagitis Endoscopic Reference Score (EREFS), and improved esophageal distensibility plateau, a measure of esophageal function, by 18%, compared with placebo, according to the report.
The Food and Drug Administration has approved dupilumab for use in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, and has granted orphan drug designation for its use in the treatment of eosinophilic esophagitis, according to Sanofi and Regeneron Pharmaceuticals.
Dupilumab antagonizes the interleukin (IL)–4 receptor-alpha component of the type 2 receptor, thereby inhibiting signaling of IL-4 and IL-13, the investigators noted in their report.
“These results demonstrate that interleukin-4 and interleukin-13 are central pathological mediators of esophageal inflammation and dysfunction in adult patients with active eosinophilic esophagitis,” said investigators in their report.
The anti-IgE monoclonal antibody omalizumab (Xolair) failed to improve dysphagia and histologic features of eosinophilic esophagitis, suggesting the pathogenesis of this disease is not mediated by IgE, they added.
A number of other targeted biologic agents, including the anti–IL-5 agents mepolizumab and reslizumab, have failed to significantly improve dysphagia versus placebo in patients with eosinophilic esophagitis, they added.
The research was sponsored by Sanofi and Regeneron. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Adare, Allakos, Banner, Calypso, Enumeral, EsoCap, GlaxoSmithKline, Meritage, Regeneron, Robarts, and Shire, and among others.
SOURCE: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
AGA patient education on eosinophilic esophagitis can help your patients better understand the condition. Visit https://www.gastro.org/practice-guidance/gi-patient-center/topic/eosinophilic-esophagitis-eoe.
Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis enrolled in a randomized trial, investigators reported.
Treatment with this monoclonal antibody also improved histologic disease features and abnormal endoscopic features, compared with placebo, according to investigators in the phase 2 trial, which included 47 patients enrolled at 14 U.S. study sites.
Injection-site erythema and nasopharyngitis were more common among dupilumab-treated versus placebo-treated patients, and there were no serious adverse events or deaths observed, according to cofirst authors Ikuo Hirano, MD, of Northwestern University, Chicago, and Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill.
“Dupilumab is the first targeted biologic agent to improve dysphagia, histologic and endoscopic measures of disease, as well as esophageal function, and have an acceptable safety profile in adult patients with active eosinophilic esophagitis,” said Dr. Hirano and Dr. Dellon and associates in the journal Gastroenterology.
The report on the phase 2 trial included 47 adults with active eosinophilic esophagitis randomized to weekly subcutaneous injections of dupilumab at a dose of 300 mg or placebo. All participants had a score of 5 or higher on the Straumann Dysphagia Instrument (SDI), a patient-reported outcome measure.
Change in SDI score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab, according to investigators, who reported a least-squares mean change of –3.0 from baseline, versus –1.3 for placebo (P = .0304).
The original plan was to measure dupilumab’s effect on SDI out to week 12 of treatment, but because of technical problems with an electronic diary system used in the trial, there was significant data loss, and this primary endpoint was instead evaluated at week 10, investigators said in their report.
Improvements in SDI scores were apparent as early as week 1 after dupilumab treatment started, they added, noting that 39% of dupilumab-treated patients had an improvement in SDI score of at least 3, compared with just 13% of placebo-treated patients (P = .490).
Dupilumab also improved outcomes measured by the eosinophilic esophagitis histology scoring system (EoE-HSS), including a 68.3% improvement in severity and 54.6% in extent of disease from baseline to week 12, investigators said.
Likewise, dupilumab improved endoscopic outcomes at week 12 as measured by the eosinophilic esophagitis Endoscopic Reference Score (EREFS), and improved esophageal distensibility plateau, a measure of esophageal function, by 18%, compared with placebo, according to the report.
The Food and Drug Administration has approved dupilumab for use in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, and has granted orphan drug designation for its use in the treatment of eosinophilic esophagitis, according to Sanofi and Regeneron Pharmaceuticals.
Dupilumab antagonizes the interleukin (IL)–4 receptor-alpha component of the type 2 receptor, thereby inhibiting signaling of IL-4 and IL-13, the investigators noted in their report.
“These results demonstrate that interleukin-4 and interleukin-13 are central pathological mediators of esophageal inflammation and dysfunction in adult patients with active eosinophilic esophagitis,” said investigators in their report.
The anti-IgE monoclonal antibody omalizumab (Xolair) failed to improve dysphagia and histologic features of eosinophilic esophagitis, suggesting the pathogenesis of this disease is not mediated by IgE, they added.
A number of other targeted biologic agents, including the anti–IL-5 agents mepolizumab and reslizumab, have failed to significantly improve dysphagia versus placebo in patients with eosinophilic esophagitis, they added.
The research was sponsored by Sanofi and Regeneron. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Adare, Allakos, Banner, Calypso, Enumeral, EsoCap, GlaxoSmithKline, Meritage, Regeneron, Robarts, and Shire, and among others.
SOURCE: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
AGA patient education on eosinophilic esophagitis can help your patients better understand the condition. Visit https://www.gastro.org/practice-guidance/gi-patient-center/topic/eosinophilic-esophagitis-eoe.
Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis enrolled in a randomized trial, investigators reported.
Treatment with this monoclonal antibody also improved histologic disease features and abnormal endoscopic features, compared with placebo, according to investigators in the phase 2 trial, which included 47 patients enrolled at 14 U.S. study sites.
Injection-site erythema and nasopharyngitis were more common among dupilumab-treated versus placebo-treated patients, and there were no serious adverse events or deaths observed, according to cofirst authors Ikuo Hirano, MD, of Northwestern University, Chicago, and Evan S. Dellon, MD, MPH, of the University of North Carolina at Chapel Hill.
“Dupilumab is the first targeted biologic agent to improve dysphagia, histologic and endoscopic measures of disease, as well as esophageal function, and have an acceptable safety profile in adult patients with active eosinophilic esophagitis,” said Dr. Hirano and Dr. Dellon and associates in the journal Gastroenterology.
The report on the phase 2 trial included 47 adults with active eosinophilic esophagitis randomized to weekly subcutaneous injections of dupilumab at a dose of 300 mg or placebo. All participants had a score of 5 or higher on the Straumann Dysphagia Instrument (SDI), a patient-reported outcome measure.
Change in SDI score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab, according to investigators, who reported a least-squares mean change of –3.0 from baseline, versus –1.3 for placebo (P = .0304).
The original plan was to measure dupilumab’s effect on SDI out to week 12 of treatment, but because of technical problems with an electronic diary system used in the trial, there was significant data loss, and this primary endpoint was instead evaluated at week 10, investigators said in their report.
Improvements in SDI scores were apparent as early as week 1 after dupilumab treatment started, they added, noting that 39% of dupilumab-treated patients had an improvement in SDI score of at least 3, compared with just 13% of placebo-treated patients (P = .490).
Dupilumab also improved outcomes measured by the eosinophilic esophagitis histology scoring system (EoE-HSS), including a 68.3% improvement in severity and 54.6% in extent of disease from baseline to week 12, investigators said.
Likewise, dupilumab improved endoscopic outcomes at week 12 as measured by the eosinophilic esophagitis Endoscopic Reference Score (EREFS), and improved esophageal distensibility plateau, a measure of esophageal function, by 18%, compared with placebo, according to the report.
The Food and Drug Administration has approved dupilumab for use in atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyposis, and has granted orphan drug designation for its use in the treatment of eosinophilic esophagitis, according to Sanofi and Regeneron Pharmaceuticals.
Dupilumab antagonizes the interleukin (IL)–4 receptor-alpha component of the type 2 receptor, thereby inhibiting signaling of IL-4 and IL-13, the investigators noted in their report.
“These results demonstrate that interleukin-4 and interleukin-13 are central pathological mediators of esophageal inflammation and dysfunction in adult patients with active eosinophilic esophagitis,” said investigators in their report.
The anti-IgE monoclonal antibody omalizumab (Xolair) failed to improve dysphagia and histologic features of eosinophilic esophagitis, suggesting the pathogenesis of this disease is not mediated by IgE, they added.
A number of other targeted biologic agents, including the anti–IL-5 agents mepolizumab and reslizumab, have failed to significantly improve dysphagia versus placebo in patients with eosinophilic esophagitis, they added.
The research was sponsored by Sanofi and Regeneron. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Adare, Allakos, Banner, Calypso, Enumeral, EsoCap, GlaxoSmithKline, Meritage, Regeneron, Robarts, and Shire, and among others.
SOURCE: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
AGA patient education on eosinophilic esophagitis can help your patients better understand the condition. Visit https://www.gastro.org/practice-guidance/gi-patient-center/topic/eosinophilic-esophagitis-eoe.
FROM GASTROENTEROLOGY
Key clinical point: Dupilumab (Dupixent) significantly reduced patient-reported dysphagia among adults with eosinophilic esophagitis.
Major finding: Change in the Straumann Dysphagia Instrument (SDI) score from baseline to week 10, the study primary endpoint, was significantly improved for dupilumab (least squares mean change of –3.0 from baseline, versus –1.3 for placebo; P = .0304).
Study details: A phase 2 trial including 47 adults with EoE randomized to dupilumab or placebo.
Disclosures: The research was sponsored by Sanofi and Regeneron Pharmaceuticals. Several study coauthors indicated that they were current or former employees of those companies. Other study authors provided disclosures related to Regeneron, Adare, Allakos, Receptos/Celgene, Meritage, Shire, Alivio, Banner, Calypso, Enumeral, EsoCap, Glax-oSmithKline, and Robarts, among others.
Source: Hirano I et al. Gastroenterology. 2019 Oct 5. doi: 10.1053/j.gastro.2019.09.042.
Both POEM approaches successful, safe as treatment for achalasia
Despite theoretical preferences for either the anterior or the posterior approach to peroral endoscopic myotomy (POEM) in patients with achalasia, a new study has found no significant difference between the two in regard to clinical success or safety.
“Both approaches are equivalently safe when performed by experienced operators,” wrote Mouen A. Khashab, MD, of Johns Hopkins Medicine in Baltimore and coauthors, adding that the most notable difference was “closure was rated as easier during the posterior approach,” and fewer clips were needed. The study was published in Gastrointestinal Endoscopy.
To analyze and compare the efficacy of the two POEM approaches, the researchers conducted a multicenter controlled clinical trial of 150 patients with achalasia. They were randomized into two groups: those receiving POEM with the anterior approach (n = 73) or the posterior approach (n = 77). Of those patients, 148 received POEM and 138 completed 1-year follow-up. At 3, 6, and 12 months’ follow-up by phone call, patients were evaluated via outcomes that included Eckardt and dysphagia scores, quality of life scales, and gastroesophageal reflux disease questionnaire score.
Technical success was achieved in all 77 patients in the posterior group compared with 71 patients (97.3%) in the anterior group (P = .23). Both groups had a median length of hospital stay post procedure of 2 days. Adverse events occurred in seven patients (9%) in the posterior group and in eight patients (11%) in the anterior group (P = .703).
Though no significant differences were found between the two groups in time to perform mucosal incision, submucosal tunneling, myotomy, or closure, the median difficulty of closure in the posterior group was lower than in the anterior group (P = .002). In addition, fewer clips were needed during closure in the posterior approach.
After per-protocol analysis, clinical success at 1 year was achieved in 89% of patients in the posterior group (95% confidence interval, 81%-96%) and 90% of patients in the anterior group (95% CI, 82%-97%). At 1-year follow-up, both groups had an Eckardt score of 0 (P = .994) and their median gastroesophageal reflux disease score was 6 (P = .73). All patients who completed quality of life questionnaires reported improvements, with a median change in pain of 23 in the anterior group and 34 in the posterior group (P = .49). The posterior group also reported a greater median change in social functioning (50 vs. 38; P = .02).
The authors noted their study’s potential limitations, including relying on the Eckardt scoring system – one that was recently questioned in terms of validity – to determine clinical success. However, they also offered an argument in favor of clinical scoring because of “the importance of symptom improvement from the patient perspective.” Also, because of the lack of prestudy data comparing the anterior and posterior approaches, they chose 15% as the noninferiority margin for clinical efficacy, which could be regarded as a limitation as well.
Four of the authors reported potential conflicts of interest, including serving as consultants for various medical companies, serving on medical advisory boards, and receiving research support and personal fees. The other authors reported no conflicts of interest.
SOURCE: Khashab MA et al. Gastrointest Endosc. 2019 Aug 10. doi: 10.1016/j.gie.2019.07.034.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process. To learn more visit www.gastro.org/CIGT.
Despite theoretical preferences for either the anterior or the posterior approach to peroral endoscopic myotomy (POEM) in patients with achalasia, a new study has found no significant difference between the two in regard to clinical success or safety.
“Both approaches are equivalently safe when performed by experienced operators,” wrote Mouen A. Khashab, MD, of Johns Hopkins Medicine in Baltimore and coauthors, adding that the most notable difference was “closure was rated as easier during the posterior approach,” and fewer clips were needed. The study was published in Gastrointestinal Endoscopy.
To analyze and compare the efficacy of the two POEM approaches, the researchers conducted a multicenter controlled clinical trial of 150 patients with achalasia. They were randomized into two groups: those receiving POEM with the anterior approach (n = 73) or the posterior approach (n = 77). Of those patients, 148 received POEM and 138 completed 1-year follow-up. At 3, 6, and 12 months’ follow-up by phone call, patients were evaluated via outcomes that included Eckardt and dysphagia scores, quality of life scales, and gastroesophageal reflux disease questionnaire score.
Technical success was achieved in all 77 patients in the posterior group compared with 71 patients (97.3%) in the anterior group (P = .23). Both groups had a median length of hospital stay post procedure of 2 days. Adverse events occurred in seven patients (9%) in the posterior group and in eight patients (11%) in the anterior group (P = .703).
Though no significant differences were found between the two groups in time to perform mucosal incision, submucosal tunneling, myotomy, or closure, the median difficulty of closure in the posterior group was lower than in the anterior group (P = .002). In addition, fewer clips were needed during closure in the posterior approach.
After per-protocol analysis, clinical success at 1 year was achieved in 89% of patients in the posterior group (95% confidence interval, 81%-96%) and 90% of patients in the anterior group (95% CI, 82%-97%). At 1-year follow-up, both groups had an Eckardt score of 0 (P = .994) and their median gastroesophageal reflux disease score was 6 (P = .73). All patients who completed quality of life questionnaires reported improvements, with a median change in pain of 23 in the anterior group and 34 in the posterior group (P = .49). The posterior group also reported a greater median change in social functioning (50 vs. 38; P = .02).
The authors noted their study’s potential limitations, including relying on the Eckardt scoring system – one that was recently questioned in terms of validity – to determine clinical success. However, they also offered an argument in favor of clinical scoring because of “the importance of symptom improvement from the patient perspective.” Also, because of the lack of prestudy data comparing the anterior and posterior approaches, they chose 15% as the noninferiority margin for clinical efficacy, which could be regarded as a limitation as well.
Four of the authors reported potential conflicts of interest, including serving as consultants for various medical companies, serving on medical advisory boards, and receiving research support and personal fees. The other authors reported no conflicts of interest.
SOURCE: Khashab MA et al. Gastrointest Endosc. 2019 Aug 10. doi: 10.1016/j.gie.2019.07.034.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process. To learn more visit www.gastro.org/CIGT.
Despite theoretical preferences for either the anterior or the posterior approach to peroral endoscopic myotomy (POEM) in patients with achalasia, a new study has found no significant difference between the two in regard to clinical success or safety.
“Both approaches are equivalently safe when performed by experienced operators,” wrote Mouen A. Khashab, MD, of Johns Hopkins Medicine in Baltimore and coauthors, adding that the most notable difference was “closure was rated as easier during the posterior approach,” and fewer clips were needed. The study was published in Gastrointestinal Endoscopy.
To analyze and compare the efficacy of the two POEM approaches, the researchers conducted a multicenter controlled clinical trial of 150 patients with achalasia. They were randomized into two groups: those receiving POEM with the anterior approach (n = 73) or the posterior approach (n = 77). Of those patients, 148 received POEM and 138 completed 1-year follow-up. At 3, 6, and 12 months’ follow-up by phone call, patients were evaluated via outcomes that included Eckardt and dysphagia scores, quality of life scales, and gastroesophageal reflux disease questionnaire score.
Technical success was achieved in all 77 patients in the posterior group compared with 71 patients (97.3%) in the anterior group (P = .23). Both groups had a median length of hospital stay post procedure of 2 days. Adverse events occurred in seven patients (9%) in the posterior group and in eight patients (11%) in the anterior group (P = .703).
Though no significant differences were found between the two groups in time to perform mucosal incision, submucosal tunneling, myotomy, or closure, the median difficulty of closure in the posterior group was lower than in the anterior group (P = .002). In addition, fewer clips were needed during closure in the posterior approach.
After per-protocol analysis, clinical success at 1 year was achieved in 89% of patients in the posterior group (95% confidence interval, 81%-96%) and 90% of patients in the anterior group (95% CI, 82%-97%). At 1-year follow-up, both groups had an Eckardt score of 0 (P = .994) and their median gastroesophageal reflux disease score was 6 (P = .73). All patients who completed quality of life questionnaires reported improvements, with a median change in pain of 23 in the anterior group and 34 in the posterior group (P = .49). The posterior group also reported a greater median change in social functioning (50 vs. 38; P = .02).
The authors noted their study’s potential limitations, including relying on the Eckardt scoring system – one that was recently questioned in terms of validity – to determine clinical success. However, they also offered an argument in favor of clinical scoring because of “the importance of symptom improvement from the patient perspective.” Also, because of the lack of prestudy data comparing the anterior and posterior approaches, they chose 15% as the noninferiority margin for clinical efficacy, which could be regarded as a limitation as well.
Four of the authors reported potential conflicts of interest, including serving as consultants for various medical companies, serving on medical advisory boards, and receiving research support and personal fees. The other authors reported no conflicts of interest.
SOURCE: Khashab MA et al. Gastrointest Endosc. 2019 Aug 10. doi: 10.1016/j.gie.2019.07.034.
The AGA Center for GI Innovation and Technology supports innovation and the development of new technology in gastroenterology, hepatology, nutrition and obesity by guiding medical device and therapeutics innovators through the technology development and adoption process. To learn more visit www.gastro.org/CIGT.
FROM GASTROINTESTINAL ENDOSCOPY
AGA Clinical Practice Update on the utility of endoscopic submucosal dissection in T1b esophageal cancer: Expert review
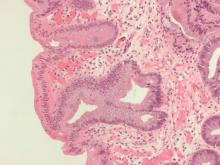
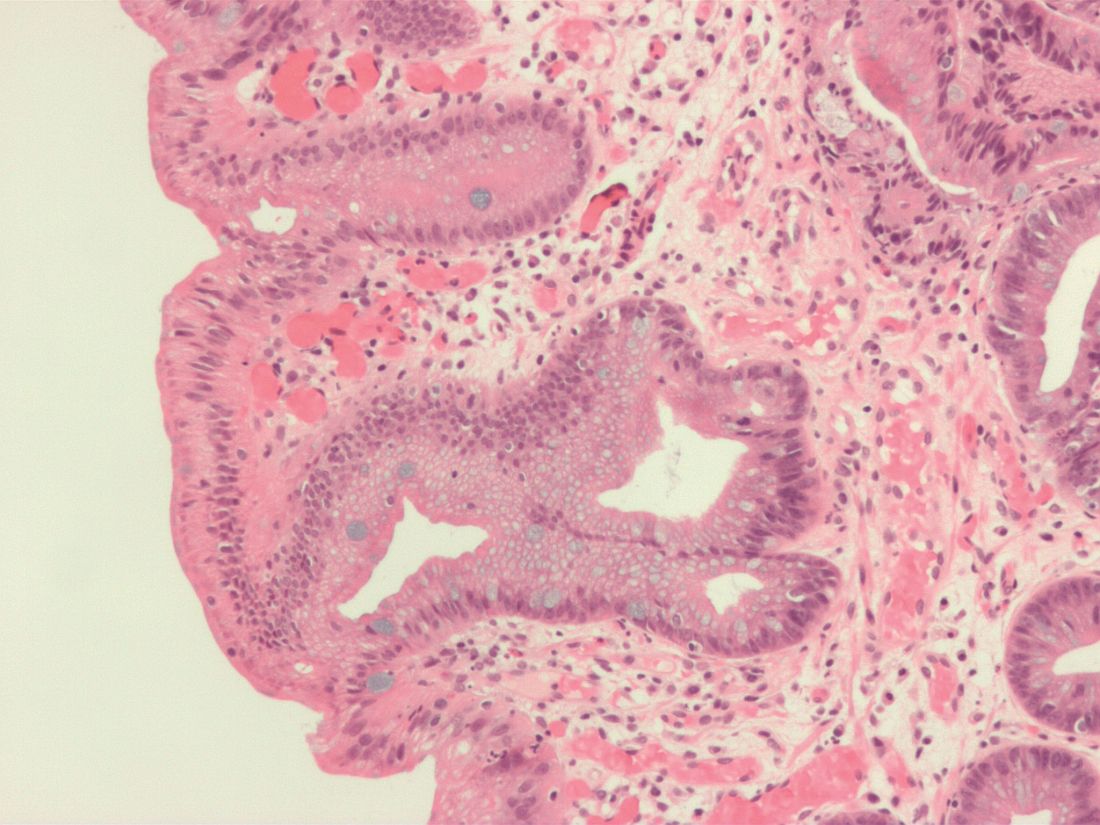
Endoscopic submucosal dissection (ESD) is a viable treatment option for patients with submucosal (T1b) esophageal cancer who have a low risk of lymph node metastasis, according to an expert review.
Among patients with T1b esophageal cancer, ideal candidates for ESD have small (less than 2 cm), well-differentiated tumors that do not invade beyond the superficial submucosa (SM1) and lack lymphovascular invasion, reported lead author Mohamed O. Othman, MD, of Baylor College of Medicine in Houston, and colleagues. The literature review was recently commissioned by the American Gastroenterological Association (AGA), because of high clinical relevance.
“[ESD] has been gaining momentum as an alternative to surgery in treating early gastrointestinal neoplasms,” the investigators wrote in Clinical Gastroenterology and Hepatology.
Most patients who undergo surgical resection develop gastroesophageal reflux, the investigators noted, and many others develop serious complications or do not survive the procedure.
“Even a high-volume center such as Mayo Clinic reported a surgical mortality of 4% for T1a esophageal cancer,” the investigators wrote. “Moreover, 34% of patients developed postoperative complications such as anastomotic leaks, anastomotic strictures, cardiopulmonary complications, and feeding jejunostomy leaks. ... Therefore, a less-invasive alternative to esophagectomy would be extremely valuable in the management of early stage [esophageal cancer] if proven effective.”
The investigators reviewed studies evaluating safety and efficacy of surgical and endoscopic techniques, as well as available data for chemoradiation and radiofrequency ablation combinations, which could potentially optimize outcomes of endoscopic resection.
They concluded that most patients with esophageal cancer that does not extend beyond the mucosa (T1a) can be cured with endoscopic resection, based on 5-year survival rates from several Japanese trials. For patients with T1b disease, however, ESD is best suited for those with a low risk of lymph node metastasis. Unfortunately, identifying these candidates can be challenging, according to the investigators.
“The risk of lymph node metastasis depends on the depth of invasion, histologic type, and molecular characterization of the tumor,” the investigators explained, noting that depth of invasion is the trickiest to discern. Although endoscopic ultrasound (EUS) is still recommended for submucosal imaging, the review showed that EUS may overstage cancer in Barrett’s esophagus. The investigators suggested that volume laser endoscopy with infrared light could be a more accurate alternative, but it is not yet a clinical reality.
The review also showed potential for combining ESD with other modalities. For example, a study by Hamada and colleagues involving 66 patients with submucosal (T1b) esophageal squamous cell carcinoma found that a combination of ESD with chemoradiation led to similar 3- and 5-year survival rates as radical esophagectomy. The investigators highlighted the importance of lymph node metastasis in this study, as none of the 30 patients lacking lymph node involvement had metastatic recurrence, compared with 6 of the 36 patients who exhibited lymph node metastasis. According to the investigators, promising data are also anticipated for this combination among those with adenocarcinoma. And for patients with intestinal metaplasia and/or dysplasia, adding radiofrequency ablation after ESD appears to be an effective option; one recent study by Sharmila Subramaniam, BMBS, and colleagues found that this strategy led to clearance rates of 85% and 96% for metaplasia and dysplasia, respectively.
“Additional treatment should be determined by factors such as tumor grade, status of lymphovascular invasion, and depth of tumor, which have a direct influence on metastatic potential,” the investigators wrote.
The investigators suggested that, in the future, better diagnostics will be needed to characterize T1b disease, as this could streamline patient selection. “Future research should focus on novel biological and immunohistochemistry markers that can aid in the prediction of tumor behavior and [lymph node metastasis] in T1b esophageal cancer,” they concluded.
The study was commissioned by the American Gastroenterological Association. The investigators disclosed additional relationships with Boston Scientific, Olympus, Lumendi, and others.
SOURCE: Othman MO et al. CGH. 2019 Jun 4. doi: 10.1016/j.cgh.2019.05.045.
Endoscopic submucosal dissection (ESD) is a viable treatment option for patients with submucosal (T1b) esophageal cancer who have a low risk of lymph node metastasis, according to an expert review.
Among patients with T1b esophageal cancer, ideal candidates for ESD have small (less than 2 cm), well-differentiated tumors that do not invade beyond the superficial submucosa (SM1) and lack lymphovascular invasion, reported lead author Mohamed O. Othman, MD, of Baylor College of Medicine in Houston, and colleagues. The literature review was recently commissioned by the American Gastroenterological Association (AGA), because of high clinical relevance.
“[ESD] has been gaining momentum as an alternative to surgery in treating early gastrointestinal neoplasms,” the investigators wrote in Clinical Gastroenterology and Hepatology.
Most patients who undergo surgical resection develop gastroesophageal reflux, the investigators noted, and many others develop serious complications or do not survive the procedure.
“Even a high-volume center such as Mayo Clinic reported a surgical mortality of 4% for T1a esophageal cancer,” the investigators wrote. “Moreover, 34% of patients developed postoperative complications such as anastomotic leaks, anastomotic strictures, cardiopulmonary complications, and feeding jejunostomy leaks. ... Therefore, a less-invasive alternative to esophagectomy would be extremely valuable in the management of early stage [esophageal cancer] if proven effective.”
The investigators reviewed studies evaluating safety and efficacy of surgical and endoscopic techniques, as well as available data for chemoradiation and radiofrequency ablation combinations, which could potentially optimize outcomes of endoscopic resection.
They concluded that most patients with esophageal cancer that does not extend beyond the mucosa (T1a) can be cured with endoscopic resection, based on 5-year survival rates from several Japanese trials. For patients with T1b disease, however, ESD is best suited for those with a low risk of lymph node metastasis. Unfortunately, identifying these candidates can be challenging, according to the investigators.
“The risk of lymph node metastasis depends on the depth of invasion, histologic type, and molecular characterization of the tumor,” the investigators explained, noting that depth of invasion is the trickiest to discern. Although endoscopic ultrasound (EUS) is still recommended for submucosal imaging, the review showed that EUS may overstage cancer in Barrett’s esophagus. The investigators suggested that volume laser endoscopy with infrared light could be a more accurate alternative, but it is not yet a clinical reality.
The review also showed potential for combining ESD with other modalities. For example, a study by Hamada and colleagues involving 66 patients with submucosal (T1b) esophageal squamous cell carcinoma found that a combination of ESD with chemoradiation led to similar 3- and 5-year survival rates as radical esophagectomy. The investigators highlighted the importance of lymph node metastasis in this study, as none of the 30 patients lacking lymph node involvement had metastatic recurrence, compared with 6 of the 36 patients who exhibited lymph node metastasis. According to the investigators, promising data are also anticipated for this combination among those with adenocarcinoma. And for patients with intestinal metaplasia and/or dysplasia, adding radiofrequency ablation after ESD appears to be an effective option; one recent study by Sharmila Subramaniam, BMBS, and colleagues found that this strategy led to clearance rates of 85% and 96% for metaplasia and dysplasia, respectively.
“Additional treatment should be determined by factors such as tumor grade, status of lymphovascular invasion, and depth of tumor, which have a direct influence on metastatic potential,” the investigators wrote.
The investigators suggested that, in the future, better diagnostics will be needed to characterize T1b disease, as this could streamline patient selection. “Future research should focus on novel biological and immunohistochemistry markers that can aid in the prediction of tumor behavior and [lymph node metastasis] in T1b esophageal cancer,” they concluded.
The study was commissioned by the American Gastroenterological Association. The investigators disclosed additional relationships with Boston Scientific, Olympus, Lumendi, and others.
SOURCE: Othman MO et al. CGH. 2019 Jun 4. doi: 10.1016/j.cgh.2019.05.045.
Endoscopic submucosal dissection (ESD) is a viable treatment option for patients with submucosal (T1b) esophageal cancer who have a low risk of lymph node metastasis, according to an expert review.
Among patients with T1b esophageal cancer, ideal candidates for ESD have small (less than 2 cm), well-differentiated tumors that do not invade beyond the superficial submucosa (SM1) and lack lymphovascular invasion, reported lead author Mohamed O. Othman, MD, of Baylor College of Medicine in Houston, and colleagues. The literature review was recently commissioned by the American Gastroenterological Association (AGA), because of high clinical relevance.
“[ESD] has been gaining momentum as an alternative to surgery in treating early gastrointestinal neoplasms,” the investigators wrote in Clinical Gastroenterology and Hepatology.
Most patients who undergo surgical resection develop gastroesophageal reflux, the investigators noted, and many others develop serious complications or do not survive the procedure.
“Even a high-volume center such as Mayo Clinic reported a surgical mortality of 4% for T1a esophageal cancer,” the investigators wrote. “Moreover, 34% of patients developed postoperative complications such as anastomotic leaks, anastomotic strictures, cardiopulmonary complications, and feeding jejunostomy leaks. ... Therefore, a less-invasive alternative to esophagectomy would be extremely valuable in the management of early stage [esophageal cancer] if proven effective.”
The investigators reviewed studies evaluating safety and efficacy of surgical and endoscopic techniques, as well as available data for chemoradiation and radiofrequency ablation combinations, which could potentially optimize outcomes of endoscopic resection.
They concluded that most patients with esophageal cancer that does not extend beyond the mucosa (T1a) can be cured with endoscopic resection, based on 5-year survival rates from several Japanese trials. For patients with T1b disease, however, ESD is best suited for those with a low risk of lymph node metastasis. Unfortunately, identifying these candidates can be challenging, according to the investigators.
“The risk of lymph node metastasis depends on the depth of invasion, histologic type, and molecular characterization of the tumor,” the investigators explained, noting that depth of invasion is the trickiest to discern. Although endoscopic ultrasound (EUS) is still recommended for submucosal imaging, the review showed that EUS may overstage cancer in Barrett’s esophagus. The investigators suggested that volume laser endoscopy with infrared light could be a more accurate alternative, but it is not yet a clinical reality.
The review also showed potential for combining ESD with other modalities. For example, a study by Hamada and colleagues involving 66 patients with submucosal (T1b) esophageal squamous cell carcinoma found that a combination of ESD with chemoradiation led to similar 3- and 5-year survival rates as radical esophagectomy. The investigators highlighted the importance of lymph node metastasis in this study, as none of the 30 patients lacking lymph node involvement had metastatic recurrence, compared with 6 of the 36 patients who exhibited lymph node metastasis. According to the investigators, promising data are also anticipated for this combination among those with adenocarcinoma. And for patients with intestinal metaplasia and/or dysplasia, adding radiofrequency ablation after ESD appears to be an effective option; one recent study by Sharmila Subramaniam, BMBS, and colleagues found that this strategy led to clearance rates of 85% and 96% for metaplasia and dysplasia, respectively.
“Additional treatment should be determined by factors such as tumor grade, status of lymphovascular invasion, and depth of tumor, which have a direct influence on metastatic potential,” the investigators wrote.
The investigators suggested that, in the future, better diagnostics will be needed to characterize T1b disease, as this could streamline patient selection. “Future research should focus on novel biological and immunohistochemistry markers that can aid in the prediction of tumor behavior and [lymph node metastasis] in T1b esophageal cancer,” they concluded.
The study was commissioned by the American Gastroenterological Association. The investigators disclosed additional relationships with Boston Scientific, Olympus, Lumendi, and others.
SOURCE: Othman MO et al. CGH. 2019 Jun 4. doi: 10.1016/j.cgh.2019.05.045.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Magnetic sphincter augmentation controls regurgitation
Adults with moderate to severe regurgitation showed significant improvement after magnetic sphincter augmentation, compared with increased proton pump inhibitor therapy, based on data from 152 patients.
Proton pump inhibitors (PPIs) are often prescribed for patients with refractory gastroesophageal reflux disease (GERD), but these medications do not address the weakness in the lower esophageal sphincter that often contributes to refractory regurgitative GERD, wrote Reginald Bell, MD, of the Institute of Esophageal and Reflux Surgery in Englewood, Colo., and colleagues.
Magnetic sphincter augmentation (MSA) is “an alternative to fundoplication that uses magnetic attraction from inside a series of titanium beads to augment the weak [lower esophageal sphincter] and reestablish the body’s natural barrier to reflux,” the researchers wrote.
In the CALIBER study, published in Clinical Gastroenterology and Hepatology, the researchers randomized 102 patients to twice-daily PPI (20 mg omeprazole) and 50 patients to laparoscopic MSA. Treatment was assessed at 6 months, and patients in the PPI group with persistent regurgitation were invited to cross into the MSA group, with 25 patients doing so. The patients were spread across 20 sites and treated between July 2015 and February 2017. Outcomes including regurgitation, foregut scores, esophageal acid exposure, and adverse events were assessed after 1 year.
MSA controlled regurgitation in 72 of 75 patients (96%) at 1 year, while 8 of 43 PPI patients (19%) reported control of regurgitation. In addition, 81% of the MSA patients reported improvement in GERD health-related quality of life, and 91% discontinued daily use of PPIs. Significant numbers of patients in the MSA group reported decreased dysphagia, bloating, and esophageal acid exposure, and 70% had normal pH levels at the end of the study.
No serious perioperative adverse events occurred in either group during the study period; 19 original MSA patients and 10 MSA crossover patients reported dysphagia, but they reported less at 6 months and 12 months, compared with baseline.
The study findings were limited by several factors, including the relatively short follow-up period and the different methods of pH testing at 6 months (transnasal impedance) and at 12 months (telemetry capsule), the researchers noted. However, the results support MSA as an effective option for patients with medically refractory regurgitative GERD that was superior to PPI for controlling regurgitation.
“Regurgitation and associated heartburn symptoms responded to MSA even when completely nonresponsive to PPI therapy, in line with the mechanical, volume origin of regurgitative symptoms,” they concluded.
Dr. Bell and several coauthors disclosed honoraria from Ethicon for teaching services. The study was supported in part by Ethicon.
SOURCE: Bell R et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.056.
Adults with moderate to severe regurgitation showed significant improvement after magnetic sphincter augmentation, compared with increased proton pump inhibitor therapy, based on data from 152 patients.
Proton pump inhibitors (PPIs) are often prescribed for patients with refractory gastroesophageal reflux disease (GERD), but these medications do not address the weakness in the lower esophageal sphincter that often contributes to refractory regurgitative GERD, wrote Reginald Bell, MD, of the Institute of Esophageal and Reflux Surgery in Englewood, Colo., and colleagues.
Magnetic sphincter augmentation (MSA) is “an alternative to fundoplication that uses magnetic attraction from inside a series of titanium beads to augment the weak [lower esophageal sphincter] and reestablish the body’s natural barrier to reflux,” the researchers wrote.
In the CALIBER study, published in Clinical Gastroenterology and Hepatology, the researchers randomized 102 patients to twice-daily PPI (20 mg omeprazole) and 50 patients to laparoscopic MSA. Treatment was assessed at 6 months, and patients in the PPI group with persistent regurgitation were invited to cross into the MSA group, with 25 patients doing so. The patients were spread across 20 sites and treated between July 2015 and February 2017. Outcomes including regurgitation, foregut scores, esophageal acid exposure, and adverse events were assessed after 1 year.
MSA controlled regurgitation in 72 of 75 patients (96%) at 1 year, while 8 of 43 PPI patients (19%) reported control of regurgitation. In addition, 81% of the MSA patients reported improvement in GERD health-related quality of life, and 91% discontinued daily use of PPIs. Significant numbers of patients in the MSA group reported decreased dysphagia, bloating, and esophageal acid exposure, and 70% had normal pH levels at the end of the study.
No serious perioperative adverse events occurred in either group during the study period; 19 original MSA patients and 10 MSA crossover patients reported dysphagia, but they reported less at 6 months and 12 months, compared with baseline.
The study findings were limited by several factors, including the relatively short follow-up period and the different methods of pH testing at 6 months (transnasal impedance) and at 12 months (telemetry capsule), the researchers noted. However, the results support MSA as an effective option for patients with medically refractory regurgitative GERD that was superior to PPI for controlling regurgitation.
“Regurgitation and associated heartburn symptoms responded to MSA even when completely nonresponsive to PPI therapy, in line with the mechanical, volume origin of regurgitative symptoms,” they concluded.
Dr. Bell and several coauthors disclosed honoraria from Ethicon for teaching services. The study was supported in part by Ethicon.
SOURCE: Bell R et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.056.
Adults with moderate to severe regurgitation showed significant improvement after magnetic sphincter augmentation, compared with increased proton pump inhibitor therapy, based on data from 152 patients.
Proton pump inhibitors (PPIs) are often prescribed for patients with refractory gastroesophageal reflux disease (GERD), but these medications do not address the weakness in the lower esophageal sphincter that often contributes to refractory regurgitative GERD, wrote Reginald Bell, MD, of the Institute of Esophageal and Reflux Surgery in Englewood, Colo., and colleagues.
Magnetic sphincter augmentation (MSA) is “an alternative to fundoplication that uses magnetic attraction from inside a series of titanium beads to augment the weak [lower esophageal sphincter] and reestablish the body’s natural barrier to reflux,” the researchers wrote.
In the CALIBER study, published in Clinical Gastroenterology and Hepatology, the researchers randomized 102 patients to twice-daily PPI (20 mg omeprazole) and 50 patients to laparoscopic MSA. Treatment was assessed at 6 months, and patients in the PPI group with persistent regurgitation were invited to cross into the MSA group, with 25 patients doing so. The patients were spread across 20 sites and treated between July 2015 and February 2017. Outcomes including regurgitation, foregut scores, esophageal acid exposure, and adverse events were assessed after 1 year.
MSA controlled regurgitation in 72 of 75 patients (96%) at 1 year, while 8 of 43 PPI patients (19%) reported control of regurgitation. In addition, 81% of the MSA patients reported improvement in GERD health-related quality of life, and 91% discontinued daily use of PPIs. Significant numbers of patients in the MSA group reported decreased dysphagia, bloating, and esophageal acid exposure, and 70% had normal pH levels at the end of the study.
No serious perioperative adverse events occurred in either group during the study period; 19 original MSA patients and 10 MSA crossover patients reported dysphagia, but they reported less at 6 months and 12 months, compared with baseline.
The study findings were limited by several factors, including the relatively short follow-up period and the different methods of pH testing at 6 months (transnasal impedance) and at 12 months (telemetry capsule), the researchers noted. However, the results support MSA as an effective option for patients with medically refractory regurgitative GERD that was superior to PPI for controlling regurgitation.
“Regurgitation and associated heartburn symptoms responded to MSA even when completely nonresponsive to PPI therapy, in line with the mechanical, volume origin of regurgitative symptoms,” they concluded.
Dr. Bell and several coauthors disclosed honoraria from Ethicon for teaching services. The study was supported in part by Ethicon.
SOURCE: Bell R et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.056.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
FDA: Sandoz recalls ranitidine capsules with NDMA
, according to a news release from the agency.
The recall applies to 14 lots in which NDMA, a probable human carcinogen and nitrosamine impurity formed as a byproduct of several industrial and natural processes, has been detected at levels above those set by the FDA, according to a company announcement on Sept. 23 from Sandoz. According to the announcement, which also specifies the affected lots, the company has not received any reports of adverse events related to use of the products in the recall.
According to the FDA release, so far, only the specified lots of ranitidine are known to be contaminated, and patients can continue taking this stomach acid–reducing histamine2 blocker from lots that are not affected by the recall.
“When we identify lapses in the quality of drugs that pose potential risks for patients, the FDA makes all efforts to understand the issue and provide our best recommendation to the public as quickly and accurately as possible,” said acting FDA Commissioner Norman E. Sharpless, MD.
As part of this ongoing investigation, the FDA recently posted a testing protocol for detecting NDMA in ranitidine; the agency hopes regulators and industry will use this protocol to begin their own laboratory testing as well and send samples to the FDA for further testing.
More information about the recall, as well as instructions for patients and health care professionals, can be found in the full news release on the FDA website. The agency also encourages any adverse reactions be reported to its MedWatch program.
, according to a news release from the agency.
The recall applies to 14 lots in which NDMA, a probable human carcinogen and nitrosamine impurity formed as a byproduct of several industrial and natural processes, has been detected at levels above those set by the FDA, according to a company announcement on Sept. 23 from Sandoz. According to the announcement, which also specifies the affected lots, the company has not received any reports of adverse events related to use of the products in the recall.
According to the FDA release, so far, only the specified lots of ranitidine are known to be contaminated, and patients can continue taking this stomach acid–reducing histamine2 blocker from lots that are not affected by the recall.
“When we identify lapses in the quality of drugs that pose potential risks for patients, the FDA makes all efforts to understand the issue and provide our best recommendation to the public as quickly and accurately as possible,” said acting FDA Commissioner Norman E. Sharpless, MD.
As part of this ongoing investigation, the FDA recently posted a testing protocol for detecting NDMA in ranitidine; the agency hopes regulators and industry will use this protocol to begin their own laboratory testing as well and send samples to the FDA for further testing.
More information about the recall, as well as instructions for patients and health care professionals, can be found in the full news release on the FDA website. The agency also encourages any adverse reactions be reported to its MedWatch program.
, according to a news release from the agency.
The recall applies to 14 lots in which NDMA, a probable human carcinogen and nitrosamine impurity formed as a byproduct of several industrial and natural processes, has been detected at levels above those set by the FDA, according to a company announcement on Sept. 23 from Sandoz. According to the announcement, which also specifies the affected lots, the company has not received any reports of adverse events related to use of the products in the recall.
According to the FDA release, so far, only the specified lots of ranitidine are known to be contaminated, and patients can continue taking this stomach acid–reducing histamine2 blocker from lots that are not affected by the recall.
“When we identify lapses in the quality of drugs that pose potential risks for patients, the FDA makes all efforts to understand the issue and provide our best recommendation to the public as quickly and accurately as possible,” said acting FDA Commissioner Norman E. Sharpless, MD.
As part of this ongoing investigation, the FDA recently posted a testing protocol for detecting NDMA in ranitidine; the agency hopes regulators and industry will use this protocol to begin their own laboratory testing as well and send samples to the FDA for further testing.
More information about the recall, as well as instructions for patients and health care professionals, can be found in the full news release on the FDA website. The agency also encourages any adverse reactions be reported to its MedWatch program.
NDMA found in samples of ranitidine, FDA says
According to the Food and Drug Administration,
“NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a prepared statement issued on Sept. 13, 2019. “The FDA has been investigating NDMA and other nitrosamine impurities in blood pressure and heart failure medicines called Angiotensin II Receptor Blockers (ARBs) since last year. In the case of ARBs, the FDA has recommended numerous recalls as it discovered unacceptable levels of nitrosamines.”
Dr. Woodcock said that the agency is working with industry partners to determine whether the low levels of NDMA in ranitidine pose a risk to patients, and it plans to post that information when it becomes available. For now, “patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health,” she said. “Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
Dr. Woodcock emphasized that the FDA is not suggesting that individuals stop taking ranitidine at this time. “However, patients taking prescription ranitidine who wish to discontinue use should talk to their health care professional about other treatment options,” she said. “People taking OTC ranitidine could consider using other OTC medicines approved for their condition. There are multiple drugs on the market that are approved for the same or similar uses as ranitidine.”
She advised consumers and health care professionals to report any adverse reactions with ranitidine to the FDA’s MedWatch program to help the agency better understand the problem.
Visit the AGA GI Patient Center for education to share with your patients about GERD, including symptoms, testing, lifestyle modifications and drug treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
According to the Food and Drug Administration,
“NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a prepared statement issued on Sept. 13, 2019. “The FDA has been investigating NDMA and other nitrosamine impurities in blood pressure and heart failure medicines called Angiotensin II Receptor Blockers (ARBs) since last year. In the case of ARBs, the FDA has recommended numerous recalls as it discovered unacceptable levels of nitrosamines.”
Dr. Woodcock said that the agency is working with industry partners to determine whether the low levels of NDMA in ranitidine pose a risk to patients, and it plans to post that information when it becomes available. For now, “patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health,” she said. “Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
Dr. Woodcock emphasized that the FDA is not suggesting that individuals stop taking ranitidine at this time. “However, patients taking prescription ranitidine who wish to discontinue use should talk to their health care professional about other treatment options,” she said. “People taking OTC ranitidine could consider using other OTC medicines approved for their condition. There are multiple drugs on the market that are approved for the same or similar uses as ranitidine.”
She advised consumers and health care professionals to report any adverse reactions with ranitidine to the FDA’s MedWatch program to help the agency better understand the problem.
Visit the AGA GI Patient Center for education to share with your patients about GERD, including symptoms, testing, lifestyle modifications and drug treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
According to the Food and Drug Administration,
“NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a prepared statement issued on Sept. 13, 2019. “The FDA has been investigating NDMA and other nitrosamine impurities in blood pressure and heart failure medicines called Angiotensin II Receptor Blockers (ARBs) since last year. In the case of ARBs, the FDA has recommended numerous recalls as it discovered unacceptable levels of nitrosamines.”
Dr. Woodcock said that the agency is working with industry partners to determine whether the low levels of NDMA in ranitidine pose a risk to patients, and it plans to post that information when it becomes available. For now, “patients should be able to trust that their medicines are as safe as they can be and that the benefits of taking them outweigh any risk to their health,” she said. “Although NDMA may cause harm in large amounts, the levels the FDA is finding in ranitidine from preliminary tests barely exceed amounts you might expect to find in common foods.”
Dr. Woodcock emphasized that the FDA is not suggesting that individuals stop taking ranitidine at this time. “However, patients taking prescription ranitidine who wish to discontinue use should talk to their health care professional about other treatment options,” she said. “People taking OTC ranitidine could consider using other OTC medicines approved for their condition. There are multiple drugs on the market that are approved for the same or similar uses as ranitidine.”
She advised consumers and health care professionals to report any adverse reactions with ranitidine to the FDA’s MedWatch program to help the agency better understand the problem.
Visit the AGA GI Patient Center for education to share with your patients about GERD, including symptoms, testing, lifestyle modifications and drug treatments at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroesophageal-reflux-disease-gerd.
Diagnosis and management of gastroparesis and functional dyspepsia pose challenges
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
Help educate your patients about gastroparesis, its symptoms and causes, as well as testing and treatment using AGA patient education, which can be found in the GI Patient Center at https://www.gastro.org/practice-guidance/gi-patient-center/topic/gastroparesis.
EXPERT ANALYSIS FROM FRESTON CONFERENCE 2019
Endoscopic therapy decreases recurrence of intestinal metaplasia, dysplasia in patients with Barrett’s esophagus
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
A study of patients with Barrett’s esophagus found that, although intestinal metaplasia and dysplasia in the cardia were common before treatment, they were more frequently present at higher levels and successful endoscopic eradication therapy lessened the risk.
“The results of this study provide evidence to suggest that, in Barrett’s esophagus patients who have achieved CEIM [complete eradication of intestinal metaplasia], it is sufficient to perform a close examination of the cardia and, in the absence of visible abnormalities, to randomly biopsy only at the level of TGF [top of gastric folds], rather than deeper into the cardia, during surveillance exams,” wrote Swathi Eluri, MD, of the University of North Carolina in Chapel Hill and coauthors. The study was published in Clinical Gastroenterology and Hepatology.
To determine the prevalence of intestinal metaplasia or dysplasia in the cardia of patients with Barrett’s esophagus who successfully underwent endoscopic eradication therapy (EET), along with the incidence of cardia intestinal metaplasia or dysplasia in patients undergoing EET, this single-center study examined two groups: a cross-sectional group of 116 patients who had achieved CEIM, and a longitudinal group of 42 treatment-naive patients who were receiving EET and subsequently achieved CEIM.
Along with clinical biopsies, the cross-sectional group underwent standardized biopsies from four quadrants in four locations: the distal esophagus, 1 cm proximal to top of gastric folds (TGF–1); at TGF; 1 cm into the gastric cardia (TGF+1); and 2 cm into the cardia (TGF+2). The longitudinal group also underwent 16 biopsies in the same areas; after CEIM was achieved, they underwent standard research biopsies of the distal esophagus and cardia at 6- and 18-month follow-ups.
Within the cross-sectional group, 15% of patients (n = 17) had intestinal metaplasia or dysplasia in the cardia after CEIM. Of those 17 patients, 12 had intestinal metaplasia, 2 were indefinite for dysplasia, and 3 had low-grade dysplasia. Of the 12 patients with cardia intestinal metaplasia, 83% had it at the level of TGF; 50% at TGF+1; and 25% at TGF+2.
Within the longitudinal group, 28% of patients (n = 12) had intestinal metaplasia or dysplasia in the cardia before ablation. Of those 12 patients, 9 had dysplastic intestinal metaplasia. Cases of pretreatment dysplasia were all found at the level of TGF, with one case extended to TGF+1. All patients achieved CEIM; at 18 months post CEIM, two patients had intestinal metaplasia and none had dysplasia.
The authors shared their study’s limitations, which included the lack of generalizability of a single-center study and a notable number of dropouts in the longitudinal group. They also acknowledged using multiple ablation modalities, although they added that most patients in both groups underwent radiofrequency ablation, the most commonly used treatment method and one that made “the results of the study more applicable to real-world practice.”
In turn, the authors noted their study’s strengths, which included the collection of data in a standardized manner and the availability of complete ablation history for all patients. Theirs was also the first study to systematically sample the cardia at multiple levels, which allows for “a more granular understanding of the location of initial and incident cardia lesions, which can guide depth of ablation during EET.”
The study was funded by an American Gastroenterological Association Research Scholar Award and CSA Medical. The authors reported no conflicts of interest.
SOURCE: Eluri S et al. Clin Gastroenterol Hepatol. 2019 May 8. doi: 10.1016/j.cgh.2019.04.065.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Can dietary therapies treat GERD effectively?
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
CHICAGO – , according to an overview presented at the meeting, sponsored by the American Gastroenterological Association. Modifying diet may reduce lower esophageal sphincter (LES) pressure and decrease the number of reflux events. Prescribing an overly restrictive diet, however, can promote hypervigilance and overwhelm patients. Successful dietary therapy requires balancing expectations and maintaining cognitive flexibility, said John E. Pandolfino, MD, Hans Popper Professor of Medicine at Northwestern University in Chicago.
When a patient presents with GERD and does not have warning signs such as dysphagia or odynophagia, the initial treatment typically is a proton pump inhibitor (PPI). This therapy effectively reduces the acidity of the gastric juice and improves acid clearance. It does not, however, change the number of reflux events or affect tissue permeability, said Dr. Pandolfino. Dietary therapy has the potential to address these outcomes.
Diets can facilitate weight loss
The first mechanism by which dietary therapies reduce GERD is by facilitating weight loss. “Obesity is associated with reflux. If you reduce that gastroesophageal pressure gradient that is generated by truncal obesity, you will improve reflux,” said Dr. Pandolfino. Second, reducing the intake of alcohol, coffee, or carbohydrates can decrease the acidity of the gastric juice. Certain foods can reduce the number of reflux events, and others can strengthen the LES.
The increasing incidence of obesity is associated with increasing incidence of GERD. Exacerbations of GERD increase the number of transient LES relaxations (TLESRs), increase the amount of liquid refluxate, and promote the formation of a hiatus hernia, said Dr. Pandolfino. One study found that moderate weight gain can cause or worsen reflux symptoms among patients of normal weight (N Engl J Med. 2006;354[22]:2340-8.). Weight loss was associated with a decreased risk of GERD symptoms. Another analysis found that reducing body mass index by 3.5 points is associated with “a dramatic reduction in overall symptoms,” said Dr. Pandolfino (Am J Gastroenterol. 2013;108[3]:376-82). Weight loss enhanced the effects of medication and reduced the gastroesophageal pressure gradient.
Dr. Pandolfino and colleagues developed and studied the Reflux Improvement and Monitoring (TRIM) program as a treatment for GERD. In this program, patients with GERD who had a BMI above 30 and were taking a PPI were referred to health coaches for weight loss treatment. Participants’ GERD Q scores decreased from 8.7 at baseline to 7.5 at 3 months and 7.4 at 6 months. Furthermore, percentage of excess body weight continued to decline for 12 months among patients who participated in TRIM, compared with controls (Am J Gastroenterol. 2018;113[1]:23-30.).
“These patients learn healthier habits [such as] walking a little bit more and watching the overall volume of food that they’re taking in,” said Dr. Pandolfino. “This was a simple thing to focus on, diet and exercise, that dramatically reduced overall severity of reflux. The interesting thing here is that we got 30% of people off their PPI therapy.”
Lifestyle changes may benefit patients
Several common lifestyle recommendations for patients with GERD relate to diet. Such recommendations include avoiding alcohol; eating smaller, more frequent meals; and avoiding food within 3 hours of bedtime. But data suggest that it is not effective to recommend the avoidance of acidic or irritative foods (e.g., citrus fruits, tomatoes, and carbonated beverages) or refluxogenic foods (e.g., fatty or fried foods, coffee, and chocolate) to all patients. Genetic predispositions may cause these foods to be irritants to certain patients, but “I don’t globally tell people to avoid things unless they irritate them,” said Dr. Pandolfino.
Understanding the mechanism by which certain foods trigger GERD can aid in appropriate therapy. For example, coffee can reduce LES pressure and increase gastric acid production. “If you have someone who already has low LES pressure, reducing coffee consumption might help that patient,” said Dr. Pandolfino. Data suggest that certain elimination diets are ineffective, however. Clinical trials do not suggest that eliminating carbonated beverages affects symptoms, and the data about eliminating alcohol, citrus, spicy foods, and chocolate are conflicting (Curr Gastroenterol Rep. 2017;19[8]:38.).
In a 2018 study, investigators gave patients with GERD 5 g of psyllium t.i.d. They performed physiologic testing on the patients at baseline and after 10 days of the diet. The intervention was associated with a significant increase in LES pressure and a reduction in overall reflux (World J Gastroenterol. 2018;24[21]:2291-9.). “This was one of the first studies that showed a dramatic improvement in physiology,” said Dr. Pandolfino. “Certainly, this is provocative, and I think that this is not an unreasonable thing to do in someone who’s not getting enough fiber.”
In addition to improving cardiovascular disease and diabetes, the Mediterranean diet reduces reflux symptoms and complications. When the researchers controlled for eating habits, the association persisted (Dis Esophagus. 2016;29[7]:794-800.).
Optimal GERD therapy follows from an analysis of patient-centered foci, such as obesity and triggers, and specific functional defects. In the quest for personalized therapy, a clinician should not discount the underlying pathogenesis, because some patients may require medications or surgery, said Dr. Pandolfino.
REPORTING FROM FRESTON CONFERENCE 2019