User login
Asymptomatic carotid artery disease: A personalized approach to management
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
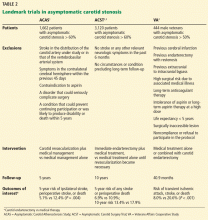
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
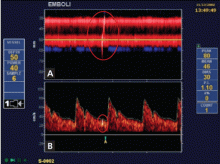
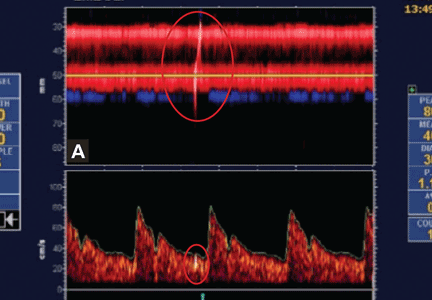
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
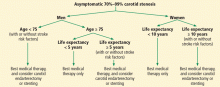
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
Carotid artery disease that is asymptomatic poses a dilemma: Should the patient undergo revascularization (surgical carotid endarterectomy or percutaneous stenting) or receive medical therapy alone?
On one hand, because one consequence of carotid atherosclerosis—ischemic stroke—can be devastating or deadly, many physicians and patients would rather “do something,” ie, proceed with surgery. Furthermore, several randomized trials1–4 found carotid endarterectomy superior to medical therapy.
On the other hand, these trials were conducted in the 1990s. Surgery has improved since then, but so has medical therapy. And if we re-examine the data from the trials in terms of the absolute risk reduction and number needed to treat, as opposed to the relative risk reduction, surgery may appear less beneficial.
Needed is a way to identify patients who would benefit from surgery and those who would more likely be harmed. Research in that direction is ongoing.
Here, we present a simple algorithmic approach to managing asymptomatic carotid artery stenosis based on the patient’s age, sex, and life expectancy. Our approach is based on a review of the best available evidence.
UP TO 8% OF ADULTS HAVE STENOSIS
Stroke is the third largest cause of death in the United States and the leading cause of disability.5 From 10% to 15% of strokes are associated with carotid artery stenosis.6,7
The prevalence of asymptomatic carotid disease, defined as stenosis greater than 50%, ranges from 4% to 8% in adults.8
However, major societies recommend against screening for carotid stenosis in the general population.9–12 Similarly, the US Preventive Services Task Force also discourages the use of carotid auscultation as screening in the general population (Table 1).13 Generally, cases of asymptomatic carotid stenosis are diagnosed by ultrasonography after the patient’s physician happens to hear a bruit during a routine examination, during a preoperative assessment, or after the patient suffers a transient ischemic attack or stroke on the contralateral side.
CLASS II RECOMMENDATIONS FOR SURGERY OR STENTING
There are well-established guidelines for managing symptomatic carotid disease,14 based on evidence from the North American Symptomatic Carotid Endarterectomy Trial15 and the European Carotid Surgery Trial,16 both from 1998. But how to manage asymptomatic carotid disease remains uncertain.
If stenosis of the internal carotid artery is greater than 70% on ultrasonography, computed tomography, or magnetic resonance imaging, and if the risk of perioperative stroke and death is low (< 3%), current guidelines14 give carotid endarterectomy a class IIa recommendation (ie, evidence is conflicting, but the weight of evidence is in favor), and they give prophylactic carotid artery stenting with optimal medical treatment a class IIb recommendation (efficacy is less well established).5
But medical management has improved, and new data suggest that this improvement may override the minimal net benefit of intervention in some patients.17 Some authors suggest that it is best to use patient characteristics and imaging features to guide treatment.18
EVIDENCE TO SUPPORT CAROTID REVASCULARIZATION
Three major trials (Table 2) published nearly 20 years ago provide the foundation of the current guidelines:
- the Endarterectomy for Asymptomatic Carotid Atherosclerosis Study (ACAS)1
- the Asymptomatic Carotid Surgery Trial (ACST)2,3
- the Veterans Affairs (VA) Cooperative Study.4
A Cochrane review of these trials,19 where medical therapy consisted only of aspirin and little use of statin therapy, found that carotid endarterectomy reduced the rate of perioperative stroke or death or any subsequent stroke in the next 3 years by 31% (relative risk 69%, 95% confidence interval [CI] 0.57–0.83). “Perioperative” was defined as the period from randomization until 30 days after surgery in the surgical group and an equivalent period in the medical group.
Moreover, carotid endarterectomy reduced the rate of disabling or fatal nonperioperative stroke by 50% compared with medical management alone.1,2,19 Patients who had contralateral symptomatic disease or who had undergone contralateral carotid endarterectomy seemed to benefit more from the procedure than those who had not.19
Also, the ACST investigators found that revascularization was associated with a reduction in contralateral strokes (which occurred in 39 vs 64 patients, P = .01) independent of contralateral symptoms or contralateral carotid endarterectomy.2,3 The exact mechanism is unknown but could be related to better blood pressure control and risk factor modification after carotid endarterectomy.
Another factor supporting revascularization is that the outcomes of revascularization have improved over time. In 2010, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST)20 reported a 30-day periprocedural incidence of death or stroke of only 1.4%, compared with 2.9% in the earlier landmark trials.
Stenting is a noninferior alternative
For patients who have asymptomatic stenosis greater than 80% on color duplex ultrasonography and a risk of stroke or death during carotid endarterectomy that is prohibitively high (> 3%), carotid stenting has proved to be a noninferior alternative.21,22
The Stenting and Angioplasty With Protection of Patients With High Risk for Endarterectomy (SAPPHIRE) trial21 reported a risk of death, stroke, or myocardial infarction of about 5% at 30 days and 10% at 1 year after stenting. A recent observational study revealed lower perioperative complication rates, with a risk of death or stroke of about 3%, which satisfy current guideline requirements.23
To be deemed at high surgical risk and therefore eligible for the SAPPHIRE trial,21 patients had to have clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal-nerve palsy, recurrent stenosis after carotid endarterectomy, previous radical neck surgery or radiation therapy to the neck, or age greater than 80.
EVIDENCE AGAINST CAROTID REVASCULARIZATION
Although carotid revascularization has evidence to support it, further interpretation of the data may lessen its apparent benefits.
Small absolute benefit, high number needed to treat
If we compare the relative risk reduction for the outcome of perioperative death or any stroke over 5 years (30% to 50%) vs the absolute risk reduction (4% to 5.9%), revascularization seems less attractive.19
The benefit may be further diminished if we consider only strokes related to large vessels, since up to 45% of strokes in patients with carotid disease are lacunar or cardioembolic.24 Assessing for prevention of large-vessel stroke using the ACAS data, the benefit of carotid endarterectomy for prevention of stroke is further decreased to a 3.5% absolute risk reduction, and the number needed to treat for 2 years increases from 62 to 111.24,25 Nevertheless, revascularization is necessary in appropriately selected patients, as a cerebrovascular event can cause life-altering changes to a patient’s cognitive, emotional, and physical condition.26
Medical therapy—and surgery—are evolving
The optimal medical management used in the landmark studies was significantly different from what is currently recommended. The ACAS trial18 used only aspirin as optimal medical management, with no mention of statins. In the ACST trial,2,3 the use of statins increased over time, from 7% to 11% at the beginning of the trial to 80% to 82% at the end.
On the other hand, the ACAS1 surgeons were required to have an excellent safety record to participate. This might have compromised the trial’s validity or our ability to generalize its conclusions.
Recent data from Abbott17 suggested a loss of a statistically significant surgical advantage in prevention of ipsilateral stroke and transient ischemic attack from the early 1990s. This is most likely explained by improved medical therapy, since there was a 22% increase in baseline proportion of patients receiving antiplatelet therapy from 1985 to 2007, with 60% of patients taking antihypertensive drugs and 30% of patients taking lipid-lowering drugs. Moreover, since 2001, the annual rates of ipsilateral stroke in patients receiving medical management alone fell below those of patients who underwent carotid endarterectomy in the ACAS trial.
The analysis by Abbott17 has major limitations: inclusion of small studies, many crossover patients, and heterogeneity. In support of this allegation, a small trial (33 patients) reported a risk of stroke ipsilateral to an asymptomatic carotid stenosis as low as 0.34% per year.25 Even when contrasting the outcomes of medical therapy against those of current carotid endarterectomy, in which the rate of perioperative stroke and death have fallen to 0.88% to 1.7%,17,27,28 there is concern that the risk associated with surgery may outweigh the long-term benefit.
Flaws in the landmark trials
Beyond the debate of the questionable benefit of revascularization, well-defined flaws in the landmark trials weaken or limit their influence on current treatment guidelines and protocols for deciding whether to revascularize.
No significant benefit was found for patients over age 75.2,3 This was thought to be due to decreased life expectancy, since the benefit from revascularization becomes significant after 3 years from intervention.1–3 Also, studies have shown that increasing age is associated with a higher risk of perioperative stroke and death.20,21
Women showed no benefit at 5 years and only a trend toward benefit at 10 years (P = .05),2 likely from a higher rate of periprocedural strokes.
Blacks and Hispanics were underrepresented in the landmark studies,19 while one observational study reported a higher incidence of in-hospital stroke after carotid endarterectomy in black patients (6.6%) than in white patients (2%).29
When associated with contralateral carotid occlusion, carotid endarterectomy carries a higher risk of perioperative stroke or death.23,30,31
Carotid revascularization failed to reduce the risk of death—the total number of deaths within 10 years was not significantly reduced by immediate carotid endarterectomy compared with deferring the procedure.2
EVIDENCE SUPPORTING OPTIMAL MEDICAL MANAGEMENT
Optimal medical therapy mainly consists of antiplatelet therapy, blood pressure management, diabetic glycemic control, and statin therapy along with lifestyle changes including smoking cessation, exercise, and weight loss (Table 3).9 Detailed recommendations are provided in the American Heart Association/American Stroke Association guidelines for primary prevention of stroke.32
Antiplatelet therapy has been shown to reduce the incidence of stroke by 25%. There is no added benefit in combining antiplatelet agents unless the patient has concomitant symptomatic coronary artery disease, recent coronary stenting, or severe peripheral artery disease.33,34
Blood pressure control can reduce the incidence of stroke by 30% to 40%, and recent data suggest that drugs working on the renin-angiotensin system offer more benefit than beta-blockers for the same reduction in blood pressure.34,35
Diabetic glycemic control is supported, as higher hemoglobin A1c and fasting glucose values are associated with higher relative risk of stroke.32,36,37 However, the stroke rate does not differ significantly between patients receiving intensive therapy and those receiving standard therapy.34
Statins actually shrink carotid plaques and reduce the risk of stroke by 15% for each 10% reduction in low-density lipoprotein cholesterol. It is estimated that statin therapy confers a 30% relative risk reduction of stroke over 20 years.34,38–41
Smoking increases the overall risk of stroke by 150%, making its cessation mandatory.42
HIGH-RISK FEATURES FOR STROKE IN ASYMPTOMATIC CAROTID STENOSIS
Studies have tried to identify risk factors for stroke, so that patients at high risk could undergo revascularization and benefit from it. However, no well-defined high-risk features have yet been described that would identify patients who would benefit from early surgery.
For instance, no correlation has been found between age, sex, diabetes mellitus, lipid levels, or smoking and progression of disease.43 In contrast, having either contralateral symptomatic carotid disease or contralateral total occlusion translated into a higher ipsilateral stroke risk.18 And in several studies, the 5-year risk of ipsilateral stroke was as high as 16.2% for those with 60% to 99% stenosis.1,2,18,24,43
Features of the plaque itself
More recently, there has been a focus on plaque evaluation to predict outcomes.
Percent stenosis. An increased risk of death or stroke has been reported with higher degrees of stenosis or plaque progression.44,45 The gross annual risk of ipsilateral stroke increases from 1.5% with stenosis of 60% to 70%, to 4.2% with stenosis of 71% to 90%, and to 7% with stenosis of 91% to 99%. Nevertheless, current data are insufficient to determine whether there is increasing benefit from surgery with increasing degree of stenosis in asymptomatic carotid disease.1,3,24,44
Plaque progression translates to a 7.2% absolute increase in the incidence of stroke (1.1% if the plaque is stable vs 8.3% if the plaque is progressing). Interestingly, plaque progression to greater than 80% stenosis results in worse outcomes (relative risk 3.4, 95% CI 1.5–7.8) compared with the same level of stenosis without recent progression.33
Intimal wall thickening of more than 1.15 mm confers a hazard ratio for stroke of 3 (95% CI 1.48–6.11).46
Increased echolucency also confers a hazard ratio for stroke of 3 (95% CI 1.4–8.0).46
A low gray-scale median (a surrogate of plaque composition) and plaque area have been identified as independent predictors of ipsilateral events.44
Embolic signals on transcranial Doppler ultrasonography (Figure 1) have been associated with a hazard ratio for stroke of 2.54 over 2 years.47
Carotid plaques predominantly composed of lipid-rich necrotic cores carry a higher risk of stroke (hazard ratio 7.2, 95% CI 1.12–46.20).48
High tensile stress (circumferential wall tension divided by the intima-media thickness), and fibrous cap thickening (< 500 µm) predict plaque rupture.49
Plaque ulceration. The risk of stroke increases with worsening degree of plaque ulceration: 0.4% per year for type A ulcerated plaques (small minimal excavations) compared with 12.5% for type B (large obvious excavations) and type C (multiple cavities or cavernous).50
Low cerebrovascular reactivity. Perfusion studies such as cerebrovascular reactivity evaluate changes in cerebral blood flow in response to a stimulus such as inhaled carbon dioxide, breath-holding, or acetazolamide. This may provide a useful index of cerebral vascular function. For instance, low reactivity has been associated with ipsilateral ischemic events (odds ratio 14.4, 95% CI 2.63–78.74, P = .0021).51,52 Silvestrini et al53 reported that the incidence of ipsilateral cerebrovascular ischemic events was 4.1% per year in patients who had normal cerebral vasoreactivity during breath-holding, vs 13.9% in those with low cerebral reactivity.
BEST MEDICAL THERAPY, ALONE OR COMBINED WITH REVASCULARIZATION
For carotid revascularization to be a viable option for asymptomatic carotid stenosis, the morbidity and mortality rates associated with the operation must be less than the incidence of neurologic events in patients who do not undergo the operation.54 An important caveat is that the longer a patient survives after carotid endarterectomy, the greater the potential benefit, since the adverse consequences of surgery are generally limited to the perioperative period.19
The current evidence regarding medical management of asymptomatic carotid stenosis suggests that the rate of ipsilateral stroke is now lower than it was in the control groups in the landmark trials.2,3,17,45,47,55,56 Ultimately, adherence to current best medical management takes priority over the decision to revascularize. The best current medical therapy includes, but is not limited to, antithrombotic therapy, statin therapy, blood pressure control, diabetes management, smoking cessation, and lifestyle changes (Table 3).
As noted above, stroke risk seems variable in the asymptomatic population according to the presence or absence of risk factors. Yet no well-defined “high-risk stroke profile” has been identified. Therefore, a patient-by-patient decision based on best available evidence should identify patients who may benefit from carotid revascularization. If asymptomatic carotid stenosis of 70% to 99% is found, factors that favor revascularization are male sex, younger age, and longer life expectancy (Figure 2).
For those with intermediate or high-risk surgical features, uncertainty exists in management since no studies have compared revascularization against medical management only in this group of patients.1 However, data from high-risk cohorts had high enough complication rates in both intervention arms to question the benefit of revascularization over medical therapy.20,21 Therefore, the individual perioperative risk of stroke, myocardial infarction, and death must be weighed against the potential benefit of revascularization for each patient.
If revascularization is pursued, studies have demonstrated that carotid artery stenting is not inferior to endarterectomy15,16 in high-surgical-risk patients. However, the revascularization approach must be tailored to the patient profile, since stenting demonstrated a lower risk of periprocedural myocardial infarction but a higher risk of stroke compared with endarteretomy.20
Finally, the current acceptable risks of perioperative stroke and death must be revised if revascularization is elected. Current data suggest that a lower threshold—around 1.4%—can be used.20 Moreover, further guidelines must determine the impact of adding myocardial infarction to the tolerable perioperative risks, since it has been excluded from main trials and guidelines.20
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010; 376:1074–1084.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: Asymptomatic Carotid Surgery Trial. Stroke 2004; 35:2425–2427.
- Hobson RW 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke 2011; 42:227–276.
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24:35–41.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209.
- Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke 1992; 23:818–822.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Am Coll Cardiol 2011; 57:1002–1044.
- Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 2006; 37:1583–1633.
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW 2nd, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease. J Neuroimaging 2007; 17:19–47.
- Bates ER, Babb JD, Casey DE Jr, et al. ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol 2007; 49:126–170.
- US Preventive Services Task Force. Screening for carotid artery stenosis: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007; 147:854–859.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Circulation 2006; 113:e409–e449.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009; 40:e573–e583.
- Venkatachalam S. Asymptomatic carotid stenosis: immediate revascularization or watchful waiting? Curr Cardiol Rep 2014; 16:440.
- Chambers BR, Donnan GA. Carotid endarterectomy for asymptomatic carotid stenosis. Cochrane Database Syst Rev 2005; 4:CD001923.
- Brott TG, Hobson RW 2nd, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Yadav JS, Wholey MH, Kuntz RE, et al; for the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351:1493–1501.
- Aksoy O, Kapadia SR, Bajzer C, Clark WM, Shishehbor MH. Carotid stenting vs surgery: parsing the risk of stroke and MI. Cleve Clin J Med 2010; 77:892–902.
- Gray WA, Rosenfield KA, Jaff MR, Chaturvedi S, Peng L, Verta P. Influence of site and operator characteristics on carotid artery stent outcomes: analysis of the CAPTURE 2 (Carotid ACCULINK/ACCUNET Post Approval Trial to Uncover Rare Events) clinical study. JACC Cardiovasc Interv 2011; 4:235–246.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Walkup MH, Faries PL. Update on surgical management for asymptomatic carotid stenosis. Curr Cardiol Rep 2011; 13:24–29.
- Halliday A, Bulbulia R, Gray W, et al. Status update and interim results from the asymptomatic carotid surgery trial-2 (ACST-2). Eur J Vasc Endovasc Surg 2013; 46:510–518.
- Chaturvedi S, Madhavan R, Santhakumar S, Mehri-Basha M, Raje N. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke 2008; 39:2966–2968.
- Maatz W, Köhler J, Botsios S, John V, Walterbusch G. Risk of stroke for carotid endarterectomy patients with contralateral carotid occlusion. Ann Vasc Surg 2008; 22:45–51.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006; 37:577–617.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sillesen H. What does ‘best medical therapy’ really mean? Eur J Vasc Endovasc Surg 2008; 35:139–144.
- Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005; 366:1545–1553.
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Predictors of stroke in middle-aged patients with non-insulin-dependent diabetes. Stroke 1996; 27:63–68.
- Selvin E, Coresh J, Shahar E, Zhang L, Steffes M, Sharrett AR. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol 2005; 4:821–826.
- Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg 2007; 46:373–386.
- Hegland O, Dickstein K, Larsen JP. Effect of simvastatin in preventing progression of carotid artery stenosis. Am J Cardiol 2001; 87:643–645, A10.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–2445.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989; 298:789–794.
- AbuRahma AF, Cook CC, Metz MJ, Wulu JT Jr, Bartolucci A. Natural history of carotid artery stenosis contralateral to endarterectomy: results from two randomized prospective trials. J Vasc Surg 2003; 38:1154–1161.
- Nicolaides AN, Kakkos SK, Griffin M, et al. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg 2005; 30:275–284.
- Lewis RF, Abrahamowicz M, Côté R, Battista RN. Predictive power of duplex ultrasonography in asymptomatic carotid disease. Ann Intern Med 1997; 127:13–20.
- Silvestrini M, Altamura C, Cerqua R, et al. Ultrasonographic markers of vascular risk in patients with asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2013; 33:619–624.
- Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010; 9:663–671.
- Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012; 34:343–350.
- Makris GC, Nicolaides AN, Xu XY, Geroulakos G. Introduction to the biomechanics of carotid plaque pathogenesis and rupture: review of the clinical evidence. Br J Radiol 2010; 83:729–735.
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978; 113:1352–1359.
- Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996; 27:2188–2190.
- Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001; 124:457–467.
- Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000; 283:2122–2127.
- Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med 1998; 3:101–108.
- Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38:1470–1475.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
KEY POINTS
- Current guidelines are based on outdated data that may not represent the best evidence regarding the management of asymptomatic carotid disease.
- Stroke is a devastating outcome of carotid disease, and most patients and physicians are wary of deferring revascularization until a stroke occurs.
- Given the inherent risk associated with revascularization (endarterectomy or stenting) and the paucity of data, the approach should be personalized on the basis of life expectancy, sex, risk factors for stroke, and clinical acumen.
- Future research should focus on noninvasive tools to determine which patients are at high risk of stroke and may benefit from revascularization.
AHA: Mixed results for mitral valve replacement vs. repair
Patients undergoing mitral valve replacement had a lower risk of regurgitation and heart failure–related adverse events at 2 years than those undergoing valve repair, according to the results of a trial presented at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
The results of the trial appear to associate mitral valve replacement with clinical advantages over mitral valve repair after 2 years of follow-up. However, replacement held no significant advantages over repair in the primary endpoint of left ventricular end-systolic volume index (LVESVI) or in overall survival, said Dr. Daniel Goldstein of the department of cardiothoracic surgery at Montefiore Medical Center, New York.
In the trial conducted by the Cardiothoracic Surgical Trials Network (CTSN), 251 patients with chronic severe ischemic mitral regurgitation were randomly assigned to undergo surgical repair of the mitral valve or to receive a mitral valve replacement with a prosthetic and procedure selected at the discretion of the surgeon.
In addition to the primary endpoint of LVESVI, the two approaches were also compared for survival, regurgitation recurrence, and heart failure events.
At 2 years, the mean change from baseline in LVESVI, a measure of remodeling, did not differ significantly between the repair and replacement arms (–9.0 vs. –6.5 mL/m2, respectively). In addition, although the 2-year mortality rate was numerically lower in the repair arm relative to the replacement arm (19% vs. 23.2%, respectively), it was also not statistically different (P = .39).
However, the rate of recurrence of moderate or severe mitral regurgitation favored replacement over repair and was significant (3.8% vs. 58.8%, respectively; P less than .001). In addition, the rate of cardiovascular readmissions was significantly lower in the replacement group (P = .01).
For those in the repair group, there were significant trends for more serious adverse events related to heart failure (P = .05) and for a lower quality of life improvement (P = .07) on the Minnesota Living With Heart Failure questionnaire. There were no significant differences in rates of all serious adverse events or overall readmissions.
All of the differences between groups observed at 2 years amplify differences previously reported after 12 months (N Engl J Med. 2014 Jan 2;370[1]:23-32). For example, the difference in the rate of moderate to severe regurgitation favoring replacement over repair was already significant at that time (2.3% vs. 32.6%, respectively; P less than .001), even though the mortality rates were then, as now, numerically lower in the repair group versus the replacement group (14.3% vs. 17.6%, respectively; P = .45).
Dr. Goldstein reported no relevant financial relationships.
Patients undergoing mitral valve replacement had a lower risk of regurgitation and heart failure–related adverse events at 2 years than those undergoing valve repair, according to the results of a trial presented at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
The results of the trial appear to associate mitral valve replacement with clinical advantages over mitral valve repair after 2 years of follow-up. However, replacement held no significant advantages over repair in the primary endpoint of left ventricular end-systolic volume index (LVESVI) or in overall survival, said Dr. Daniel Goldstein of the department of cardiothoracic surgery at Montefiore Medical Center, New York.
In the trial conducted by the Cardiothoracic Surgical Trials Network (CTSN), 251 patients with chronic severe ischemic mitral regurgitation were randomly assigned to undergo surgical repair of the mitral valve or to receive a mitral valve replacement with a prosthetic and procedure selected at the discretion of the surgeon.
In addition to the primary endpoint of LVESVI, the two approaches were also compared for survival, regurgitation recurrence, and heart failure events.
At 2 years, the mean change from baseline in LVESVI, a measure of remodeling, did not differ significantly between the repair and replacement arms (–9.0 vs. –6.5 mL/m2, respectively). In addition, although the 2-year mortality rate was numerically lower in the repair arm relative to the replacement arm (19% vs. 23.2%, respectively), it was also not statistically different (P = .39).
However, the rate of recurrence of moderate or severe mitral regurgitation favored replacement over repair and was significant (3.8% vs. 58.8%, respectively; P less than .001). In addition, the rate of cardiovascular readmissions was significantly lower in the replacement group (P = .01).
For those in the repair group, there were significant trends for more serious adverse events related to heart failure (P = .05) and for a lower quality of life improvement (P = .07) on the Minnesota Living With Heart Failure questionnaire. There were no significant differences in rates of all serious adverse events or overall readmissions.
All of the differences between groups observed at 2 years amplify differences previously reported after 12 months (N Engl J Med. 2014 Jan 2;370[1]:23-32). For example, the difference in the rate of moderate to severe regurgitation favoring replacement over repair was already significant at that time (2.3% vs. 32.6%, respectively; P less than .001), even though the mortality rates were then, as now, numerically lower in the repair group versus the replacement group (14.3% vs. 17.6%, respectively; P = .45).
Dr. Goldstein reported no relevant financial relationships.
Patients undergoing mitral valve replacement had a lower risk of regurgitation and heart failure–related adverse events at 2 years than those undergoing valve repair, according to the results of a trial presented at the American Heart Association scientific sessions and published simultaneously in the New England Journal of Medicine.
The results of the trial appear to associate mitral valve replacement with clinical advantages over mitral valve repair after 2 years of follow-up. However, replacement held no significant advantages over repair in the primary endpoint of left ventricular end-systolic volume index (LVESVI) or in overall survival, said Dr. Daniel Goldstein of the department of cardiothoracic surgery at Montefiore Medical Center, New York.
In the trial conducted by the Cardiothoracic Surgical Trials Network (CTSN), 251 patients with chronic severe ischemic mitral regurgitation were randomly assigned to undergo surgical repair of the mitral valve or to receive a mitral valve replacement with a prosthetic and procedure selected at the discretion of the surgeon.
In addition to the primary endpoint of LVESVI, the two approaches were also compared for survival, regurgitation recurrence, and heart failure events.
At 2 years, the mean change from baseline in LVESVI, a measure of remodeling, did not differ significantly between the repair and replacement arms (–9.0 vs. –6.5 mL/m2, respectively). In addition, although the 2-year mortality rate was numerically lower in the repair arm relative to the replacement arm (19% vs. 23.2%, respectively), it was also not statistically different (P = .39).
However, the rate of recurrence of moderate or severe mitral regurgitation favored replacement over repair and was significant (3.8% vs. 58.8%, respectively; P less than .001). In addition, the rate of cardiovascular readmissions was significantly lower in the replacement group (P = .01).
For those in the repair group, there were significant trends for more serious adverse events related to heart failure (P = .05) and for a lower quality of life improvement (P = .07) on the Minnesota Living With Heart Failure questionnaire. There were no significant differences in rates of all serious adverse events or overall readmissions.
All of the differences between groups observed at 2 years amplify differences previously reported after 12 months (N Engl J Med. 2014 Jan 2;370[1]:23-32). For example, the difference in the rate of moderate to severe regurgitation favoring replacement over repair was already significant at that time (2.3% vs. 32.6%, respectively; P less than .001), even though the mortality rates were then, as now, numerically lower in the repair group versus the replacement group (14.3% vs. 17.6%, respectively; P = .45).
Dr. Goldstein reported no relevant financial relationships.
FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Mitral valve replacement reduced regurgitation better than valve repair, but it didn’t significantly improve left ventricular function or survival.
Major finding: In patients with severe ischemic mitral regurgitation, regurgitation occurred more frequently after mitral valve repair than after valve replacement (58.8% vs. 3.8%; P less than .001), but left ventricular end-systolic volume indexes and survival rates were not significantly different.
Data source: A randomized, multicenter trial with 251 patients.
Disclosures: Dr. Goldstein reported no relevant financial relationships.
Fish oil, aspirin didn’t reduce AVF failure in kidney disease
SAN DIEGO – Neither fish oil nor aspirin can be recommended for prevention of primary arteriovenous fistula failure in patients with advanced renal disease, a randomized trial showed.
“AVF failure increases patient morbidity and health provider costs,” Dr. Ashley B. Irish said during a press briefing at a meeting sponsored by the American Society of Nephrology. “Therefore, therapies to reduce AVF failure may reduce patient outcomes and reduce costs. But it remains uncertain whether targeting antiplatelet agents to affect thrombosis is going to improve the numbers of AVFs you can actually use.”
In a randomized trial known as Fish Oils and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED), Dr. Irish and his associates set out to determine whether omega-3 polyunsaturated fatty acids (fish oil) can reduce new AVF access failure at 12 months. The secondary outcome was to determine if aspirin would have the same effect.
The intervention consisted of 12 weeks of fish oil 4 g or placebo (olive oil) divided in two doses. A subset of patients were randomized to aspirin 100 mg or matching placebo in addition to fish oil or placebo in a factorial design, said Dr, Irish, of the department of nephrology at Fiona Stanley Hospital, Perth, Australia. AVF failure was defined as AVF thrombosis and/or abandonment and/or cannulation formation.
The study was carried out at centers in Australia, Malaysia, and the United Kingdom, and included 567 patients with stage 4 or stage 5 chronic kidney disease who were either on dialysis or who were expected to start within 12 months. Their mean age was 55 years, 63% were male, 46% had diabetes, but only 16% were on vascular dialysis. “So, these patients were a little bit healthier than you might expect in usual populations of people on dialysis,” Dr. Irish said.
The researchers found that fish oil had no effect on preventing AVF failure. In fact, 47% of patients who received fish oil had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk after adjusting for aspirin use was 1.03).
“So, nearly half of all patients in this trial had no usable AVF at 12 months after surgery,” Dr. Irish said. “It didn’t matter whether you were old or young, had vascular disease, or were diabetic or not. Fish oil didn’t help.”
Aspirin didn’t work, either. In fact, 45% of those who received aspirin had a failed AVF at 12 months, compared with 43% of those who received placebo (RR, 1.05).
Dr. Irish noted that the proportion of serious adverse events was low and similar between patients who received fish oil and those who received or aspirin. “The treatment was safe; it just didn’t work,” he said.
“This study and others have shown that trying to prevent AVFs from clotting or promoting their development by inhibiting platelets and blood vessel changes is not effective,” Dr. Irish explained. “The focus might need to shift to other strategies, such as preserving blood vessels from damage, surgical techniques, and modification of vessel walls by infrared and other pharmacological treatments.”
Dr. Irish characterized the study results as “disappointing, because these therapies are cheap, safe, and readily available. But it’s not a waste of time, because now we know that this avenue of approach in research is not going to lead us anywhere. It’s time to move on. This information will help us inform practice and plan new trials.”
The researchers reported having no financial disclosures.
SAN DIEGO – Neither fish oil nor aspirin can be recommended for prevention of primary arteriovenous fistula failure in patients with advanced renal disease, a randomized trial showed.
“AVF failure increases patient morbidity and health provider costs,” Dr. Ashley B. Irish said during a press briefing at a meeting sponsored by the American Society of Nephrology. “Therefore, therapies to reduce AVF failure may reduce patient outcomes and reduce costs. But it remains uncertain whether targeting antiplatelet agents to affect thrombosis is going to improve the numbers of AVFs you can actually use.”
In a randomized trial known as Fish Oils and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED), Dr. Irish and his associates set out to determine whether omega-3 polyunsaturated fatty acids (fish oil) can reduce new AVF access failure at 12 months. The secondary outcome was to determine if aspirin would have the same effect.
The intervention consisted of 12 weeks of fish oil 4 g or placebo (olive oil) divided in two doses. A subset of patients were randomized to aspirin 100 mg or matching placebo in addition to fish oil or placebo in a factorial design, said Dr, Irish, of the department of nephrology at Fiona Stanley Hospital, Perth, Australia. AVF failure was defined as AVF thrombosis and/or abandonment and/or cannulation formation.
The study was carried out at centers in Australia, Malaysia, and the United Kingdom, and included 567 patients with stage 4 or stage 5 chronic kidney disease who were either on dialysis or who were expected to start within 12 months. Their mean age was 55 years, 63% were male, 46% had diabetes, but only 16% were on vascular dialysis. “So, these patients were a little bit healthier than you might expect in usual populations of people on dialysis,” Dr. Irish said.
The researchers found that fish oil had no effect on preventing AVF failure. In fact, 47% of patients who received fish oil had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk after adjusting for aspirin use was 1.03).
“So, nearly half of all patients in this trial had no usable AVF at 12 months after surgery,” Dr. Irish said. “It didn’t matter whether you were old or young, had vascular disease, or were diabetic or not. Fish oil didn’t help.”
Aspirin didn’t work, either. In fact, 45% of those who received aspirin had a failed AVF at 12 months, compared with 43% of those who received placebo (RR, 1.05).
Dr. Irish noted that the proportion of serious adverse events was low and similar between patients who received fish oil and those who received or aspirin. “The treatment was safe; it just didn’t work,” he said.
“This study and others have shown that trying to prevent AVFs from clotting or promoting their development by inhibiting platelets and blood vessel changes is not effective,” Dr. Irish explained. “The focus might need to shift to other strategies, such as preserving blood vessels from damage, surgical techniques, and modification of vessel walls by infrared and other pharmacological treatments.”
Dr. Irish characterized the study results as “disappointing, because these therapies are cheap, safe, and readily available. But it’s not a waste of time, because now we know that this avenue of approach in research is not going to lead us anywhere. It’s time to move on. This information will help us inform practice and plan new trials.”
The researchers reported having no financial disclosures.
SAN DIEGO – Neither fish oil nor aspirin can be recommended for prevention of primary arteriovenous fistula failure in patients with advanced renal disease, a randomized trial showed.
“AVF failure increases patient morbidity and health provider costs,” Dr. Ashley B. Irish said during a press briefing at a meeting sponsored by the American Society of Nephrology. “Therefore, therapies to reduce AVF failure may reduce patient outcomes and reduce costs. But it remains uncertain whether targeting antiplatelet agents to affect thrombosis is going to improve the numbers of AVFs you can actually use.”
In a randomized trial known as Fish Oils and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED), Dr. Irish and his associates set out to determine whether omega-3 polyunsaturated fatty acids (fish oil) can reduce new AVF access failure at 12 months. The secondary outcome was to determine if aspirin would have the same effect.
The intervention consisted of 12 weeks of fish oil 4 g or placebo (olive oil) divided in two doses. A subset of patients were randomized to aspirin 100 mg or matching placebo in addition to fish oil or placebo in a factorial design, said Dr, Irish, of the department of nephrology at Fiona Stanley Hospital, Perth, Australia. AVF failure was defined as AVF thrombosis and/or abandonment and/or cannulation formation.
The study was carried out at centers in Australia, Malaysia, and the United Kingdom, and included 567 patients with stage 4 or stage 5 chronic kidney disease who were either on dialysis or who were expected to start within 12 months. Their mean age was 55 years, 63% were male, 46% had diabetes, but only 16% were on vascular dialysis. “So, these patients were a little bit healthier than you might expect in usual populations of people on dialysis,” Dr. Irish said.
The researchers found that fish oil had no effect on preventing AVF failure. In fact, 47% of patients who received fish oil had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk after adjusting for aspirin use was 1.03).
“So, nearly half of all patients in this trial had no usable AVF at 12 months after surgery,” Dr. Irish said. “It didn’t matter whether you were old or young, had vascular disease, or were diabetic or not. Fish oil didn’t help.”
Aspirin didn’t work, either. In fact, 45% of those who received aspirin had a failed AVF at 12 months, compared with 43% of those who received placebo (RR, 1.05).
Dr. Irish noted that the proportion of serious adverse events was low and similar between patients who received fish oil and those who received or aspirin. “The treatment was safe; it just didn’t work,” he said.
“This study and others have shown that trying to prevent AVFs from clotting or promoting their development by inhibiting platelets and blood vessel changes is not effective,” Dr. Irish explained. “The focus might need to shift to other strategies, such as preserving blood vessels from damage, surgical techniques, and modification of vessel walls by infrared and other pharmacological treatments.”
Dr. Irish characterized the study results as “disappointing, because these therapies are cheap, safe, and readily available. But it’s not a waste of time, because now we know that this avenue of approach in research is not going to lead us anywhere. It’s time to move on. This information will help us inform practice and plan new trials.”
The researchers reported having no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Three months of fish oil or aspirin intake had no impact in reducing arteriovenous fistula failure at 12 months.
Major finding: Nearly half of patients who received fish oil (47%) had a failed AVF at 12 months, compared with 47% of those who received placebo (relative risk, 1.03). Results were similar for patients who received aspirin.
Data source: A study of 567 patients with stage 4 or stage 5 chronic kidney disease who were randomized to receive 12 weeks of fish oil 4 g or placebo (olive oil), or to aspirin 100 mg or matching placebo.
Disclosures: The researchers reported having no financial disclosures.
Stellate ulceration in a nonuremic patient
A 64-year-old man was admitted for extensive painful ulceration of the left lower leg (Figure 1) that occurred after a fall and that had worsened over the last 4 months.
His medical history included hyperuricemia, hypertension, and type 2 diabetes mellitus. He had no known cardiac or renal disease.
Results of initial laboratory testing showed the following:
- Hemoglobin 10.9 g/dL (reference range 13.5–17.5); red blood cells were normocytic and normochromic
- White blood cell count 10.2 × 109/L (4.5–11.0)
- Neutrophil count 9.11 × 109/L (2.0–8.5)
- C-reactive protein 259 mg/L (< 5)
- Creatinine, urea, sodium, potassium, calcium, and phosphate were within normal limits.
Doppler ultrasonography of the legs showed mild diffuse atheromatous arterial disease without significant blockage of blood flow, in addition to mild bilateral venous insufficiency.
Cutaneous biopsy showed intravascular calcium deposition in the hypodermis (Figure 2) and reticular dermis, erythrocyte extravasation in the superficial dermis, and epidermal necrosis, thus establishing the diagnosis of nonuremic calciphylaxis. The vascular occlusion with spreading necrosis gave the characteristic stellate appearance.
Aside from diabetes, our patient had none of the conditions usually associated with nonuremic calciphylaxis—namely, hyperparathyroidism, previous corticosteroid therapy, warfarin use, connective tissue disease, or malignancy.
A POORLY UNDERSTOOD SMALL-VESSEL VASCULOPATHY
Calciphylaxis is a poorly understood small-vessel vasculopathy, most often associated with end-stage renal disease, with a prevalence of 1% to 4% in patients on dialysis.1 It carries a high risk of death, most often from sepsis.
The cause is still unclear, but several conditions have been implicated, including primary hyperparathyroidism, malignancies, alcoholic liver disease, connective tissue disease, and diabetes.2
Making the diagnosis may be challenging, especially in nonuremic patients. It is a rare condition, the presentation is not always typical, and it can occur with fully normal kidney function and normal indicators of calcium and phosphate metabolism.
The differential diagnosis includes:
- Vasculitis, either primary or secondary to an autoimmune disorder such as rheumatoid arthritis, systemic lupus erythematosus, or cryoglobulinemia
- Peripheral vascular disease
- Other inflammatory conditions such as pyoderma gangrenosum and panniculitis
- Infections such as cellulitis and necrotizing fasciitis
- Iatrogenic disorders such as warfarin necrosis and early-stage nephrogenic systemic fibrosis.3,4
The current approach to treatment is multidisciplinary and is based only on case reports and small case series, since no randomized prospective trial has been done. The goal is optimal control of calcium and phosphate homeostasis and correction of hypercoagulability.5 Available data4,5 support appropriate wound care and surgical debridement.4,5 Intravenous sodium thiosulfate is the most widely used medical treatment and can be given regardless of the level of renal function. Resolution rates have been greater than 90% in patients with normal renal function, whereas improvement in cutaneous ulcers and pain has been observed in 70% of hemodialysis patients.4 However, it does not reduce the associated mortality rate.4
Awareness of nonuremic calciphylaxis and a high index of suspicion are needed when any patient presents with a leg ulcer and no clear cause. It should be considered in the differential diagnosis of leg ulcer in patients with chronic renal failure even if they have risk factors for more common causes of ulcers, and even occasionally in patients such as ours without chronic kidney disease or other risk factors for this condition.
OUR PATIENT’S MANAGEMENT
The patient developed profuse diarrhea, and infection with Clostridium difficile was confirmed. Despite treatment with metronidazole and vancomycin, he died several days later. No treatment directed to calciphylaxis was ever started because of the patient’s unstable condition during the entire hospitalization.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med 2008; 75:772–777.
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3:1139–1143.
- Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: An emerging mimicker of vasculitis. Autoimmun Rev 2008; 7:638–643.
- Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol 2013; 58:87–92.
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
A 64-year-old man was admitted for extensive painful ulceration of the left lower leg (Figure 1) that occurred after a fall and that had worsened over the last 4 months.
His medical history included hyperuricemia, hypertension, and type 2 diabetes mellitus. He had no known cardiac or renal disease.
Results of initial laboratory testing showed the following:
- Hemoglobin 10.9 g/dL (reference range 13.5–17.5); red blood cells were normocytic and normochromic
- White blood cell count 10.2 × 109/L (4.5–11.0)
- Neutrophil count 9.11 × 109/L (2.0–8.5)
- C-reactive protein 259 mg/L (< 5)
- Creatinine, urea, sodium, potassium, calcium, and phosphate were within normal limits.
Doppler ultrasonography of the legs showed mild diffuse atheromatous arterial disease without significant blockage of blood flow, in addition to mild bilateral venous insufficiency.
Cutaneous biopsy showed intravascular calcium deposition in the hypodermis (Figure 2) and reticular dermis, erythrocyte extravasation in the superficial dermis, and epidermal necrosis, thus establishing the diagnosis of nonuremic calciphylaxis. The vascular occlusion with spreading necrosis gave the characteristic stellate appearance.
Aside from diabetes, our patient had none of the conditions usually associated with nonuremic calciphylaxis—namely, hyperparathyroidism, previous corticosteroid therapy, warfarin use, connective tissue disease, or malignancy.
A POORLY UNDERSTOOD SMALL-VESSEL VASCULOPATHY
Calciphylaxis is a poorly understood small-vessel vasculopathy, most often associated with end-stage renal disease, with a prevalence of 1% to 4% in patients on dialysis.1 It carries a high risk of death, most often from sepsis.
The cause is still unclear, but several conditions have been implicated, including primary hyperparathyroidism, malignancies, alcoholic liver disease, connective tissue disease, and diabetes.2
Making the diagnosis may be challenging, especially in nonuremic patients. It is a rare condition, the presentation is not always typical, and it can occur with fully normal kidney function and normal indicators of calcium and phosphate metabolism.
The differential diagnosis includes:
- Vasculitis, either primary or secondary to an autoimmune disorder such as rheumatoid arthritis, systemic lupus erythematosus, or cryoglobulinemia
- Peripheral vascular disease
- Other inflammatory conditions such as pyoderma gangrenosum and panniculitis
- Infections such as cellulitis and necrotizing fasciitis
- Iatrogenic disorders such as warfarin necrosis and early-stage nephrogenic systemic fibrosis.3,4
The current approach to treatment is multidisciplinary and is based only on case reports and small case series, since no randomized prospective trial has been done. The goal is optimal control of calcium and phosphate homeostasis and correction of hypercoagulability.5 Available data4,5 support appropriate wound care and surgical debridement.4,5 Intravenous sodium thiosulfate is the most widely used medical treatment and can be given regardless of the level of renal function. Resolution rates have been greater than 90% in patients with normal renal function, whereas improvement in cutaneous ulcers and pain has been observed in 70% of hemodialysis patients.4 However, it does not reduce the associated mortality rate.4
Awareness of nonuremic calciphylaxis and a high index of suspicion are needed when any patient presents with a leg ulcer and no clear cause. It should be considered in the differential diagnosis of leg ulcer in patients with chronic renal failure even if they have risk factors for more common causes of ulcers, and even occasionally in patients such as ours without chronic kidney disease or other risk factors for this condition.
OUR PATIENT’S MANAGEMENT
The patient developed profuse diarrhea, and infection with Clostridium difficile was confirmed. Despite treatment with metronidazole and vancomycin, he died several days later. No treatment directed to calciphylaxis was ever started because of the patient’s unstable condition during the entire hospitalization.
A 64-year-old man was admitted for extensive painful ulceration of the left lower leg (Figure 1) that occurred after a fall and that had worsened over the last 4 months.
His medical history included hyperuricemia, hypertension, and type 2 diabetes mellitus. He had no known cardiac or renal disease.
Results of initial laboratory testing showed the following:
- Hemoglobin 10.9 g/dL (reference range 13.5–17.5); red blood cells were normocytic and normochromic
- White blood cell count 10.2 × 109/L (4.5–11.0)
- Neutrophil count 9.11 × 109/L (2.0–8.5)
- C-reactive protein 259 mg/L (< 5)
- Creatinine, urea, sodium, potassium, calcium, and phosphate were within normal limits.
Doppler ultrasonography of the legs showed mild diffuse atheromatous arterial disease without significant blockage of blood flow, in addition to mild bilateral venous insufficiency.
Cutaneous biopsy showed intravascular calcium deposition in the hypodermis (Figure 2) and reticular dermis, erythrocyte extravasation in the superficial dermis, and epidermal necrosis, thus establishing the diagnosis of nonuremic calciphylaxis. The vascular occlusion with spreading necrosis gave the characteristic stellate appearance.
Aside from diabetes, our patient had none of the conditions usually associated with nonuremic calciphylaxis—namely, hyperparathyroidism, previous corticosteroid therapy, warfarin use, connective tissue disease, or malignancy.
A POORLY UNDERSTOOD SMALL-VESSEL VASCULOPATHY
Calciphylaxis is a poorly understood small-vessel vasculopathy, most often associated with end-stage renal disease, with a prevalence of 1% to 4% in patients on dialysis.1 It carries a high risk of death, most often from sepsis.
The cause is still unclear, but several conditions have been implicated, including primary hyperparathyroidism, malignancies, alcoholic liver disease, connective tissue disease, and diabetes.2
Making the diagnosis may be challenging, especially in nonuremic patients. It is a rare condition, the presentation is not always typical, and it can occur with fully normal kidney function and normal indicators of calcium and phosphate metabolism.
The differential diagnosis includes:
- Vasculitis, either primary or secondary to an autoimmune disorder such as rheumatoid arthritis, systemic lupus erythematosus, or cryoglobulinemia
- Peripheral vascular disease
- Other inflammatory conditions such as pyoderma gangrenosum and panniculitis
- Infections such as cellulitis and necrotizing fasciitis
- Iatrogenic disorders such as warfarin necrosis and early-stage nephrogenic systemic fibrosis.3,4
The current approach to treatment is multidisciplinary and is based only on case reports and small case series, since no randomized prospective trial has been done. The goal is optimal control of calcium and phosphate homeostasis and correction of hypercoagulability.5 Available data4,5 support appropriate wound care and surgical debridement.4,5 Intravenous sodium thiosulfate is the most widely used medical treatment and can be given regardless of the level of renal function. Resolution rates have been greater than 90% in patients with normal renal function, whereas improvement in cutaneous ulcers and pain has been observed in 70% of hemodialysis patients.4 However, it does not reduce the associated mortality rate.4
Awareness of nonuremic calciphylaxis and a high index of suspicion are needed when any patient presents with a leg ulcer and no clear cause. It should be considered in the differential diagnosis of leg ulcer in patients with chronic renal failure even if they have risk factors for more common causes of ulcers, and even occasionally in patients such as ours without chronic kidney disease or other risk factors for this condition.
OUR PATIENT’S MANAGEMENT
The patient developed profuse diarrhea, and infection with Clostridium difficile was confirmed. Despite treatment with metronidazole and vancomycin, he died several days later. No treatment directed to calciphylaxis was ever started because of the patient’s unstable condition during the entire hospitalization.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med 2008; 75:772–777.
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3:1139–1143.
- Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: An emerging mimicker of vasculitis. Autoimmun Rev 2008; 7:638–643.
- Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol 2013; 58:87–92.
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med 2008; 75:772–777.
- Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol 2008; 3:1139–1143.
- Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: An emerging mimicker of vasculitis. Autoimmun Rev 2008; 7:638–643.
- Wollina U. Update on cutaneous calciphylaxis. Indian J Dermatol 2013; 58:87–92.
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
EADV: Best treatments for great saphenous vein reflux
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Key clinical point: Long-term outcomes for treatment of great saphenous vein reflux were significantly better with conventional surgery or endovenous laser ablation than with ultrasound-guided foam sclerotherapy.
Major finding: Obliteration or absence of the treated great saphenous vein segment was achieved with conventional surgery in 85% of treated cases, with endovenous laser ablation (EVLA) in 77% of legs treated, and with ultrasound-guided foam sclerotherapy (UGFS) in 23%.
Data source: This multicenter clinical trial with 5-year follow-up included 224 legs randomized to one of three popular treatments for great saphenous varicose veins.
Disclosures: The study was sponsored by Erasmus University. The presenter reported having no financial conflicts of interest.
TCT: Paclitaxel-coated balloon delivers durable SFA patency
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.
 |
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.
 |
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
The IN.PACT Admiral paclitaxel-covered balloon provides a powerful new tool for treating superficial femoral artery and popliteal artery disease that works better than does a conventional balloon and avoids using a stent.
Not all drug-coated balloons (DCBs) are alike, even if they use the same antiproliferative drug, paclitaxel. The evidence suggests that the IN.PACT Admiral drug-coated balloon is superior to the performance of the Lutonix 035 DCB, although this has only been assessed in separate studies and not as a head-to-head comparison.
 |
Dr. Gary Gershony |
Another option for treating superficial femoropopliteal disease is with any of a variety of stents. I think the general feeling among peripheral-artery specialists is that it’s better for patients to avoid having a stent permanently in their leg when other, equally-good options are available to try first. Sometimes placing a stent is unavoidable to produce a substantially better revascularization outcome, for example when a dissection occurs or for treating a significant residual stenosis.
The IN.PACT Admiral DCB has not yet been tested on complex or calcified lesions so its performance in those settings is not yet know. The basic message from this 2-year follow-up is that this paclitaxel-coated balloon had better results out to 2-years than a conventional balloon for lesions that were not especially complex and with an average length of 9 cm. For many patients with lesions like these a DCB is a good option because it may produce a durable result while maintaining the option to use a stent later if necessary.
Vascular specialists have been concerned about longer-term follow-up of the results from the IN.PACT SFA trial to see if a signal appeared of catchup restenosis between years 1 and 2. The results showed no evidence of this. It is reassuring to see this DCB technology can produce an effect that’s durable for 2 years without leaving behind a permanent implant. It strengthens the case for this particular DCB but should not be extrapolated to all drug-coated balloons or to all types of femoropopliteal lesions.
Dr. Gary Gershony is an interventional cardiologist and medical director of cardiovascular research, education and technology at John Muir Cardiovascular Institute of John Muir Health in Concord, Calif. He had no relevant disclosures. He made these comments as a discussant for the report and in an interview.
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
SAN FRANCISCO – Treatment of femoropopliteal arterial disease with a paclitaxel-coated balloon produced durable, 2-year benefits compared with conventional balloon angioplasty during extended follow-up of the pivotal trial that led to U.S. approval of this drug-coated balloon.
The durability of the benefit first seen after 1 year when follow-up continued out to 2 years was an important finding that distinguishes the IN.PACT Admiral paclitaxel-covered balloon used in the current study from the first and only other drug-covered balloon (DCB) approved for U.S. practice, the Lutonix 035 DCB.
“Not all drug-coated balloons are the same,” Dr. John R. Laird said while reporting the IN.PACT Admiral DCB results at the Transcatheter Cardiovascular Therapeutics annual meeting.
Although both the IN.PACT Admiral and Lutonix 035 DCB have paclitaxel coatings, the two devices differ by paclitaxel dose density on the balloon’s surface (3.5 mcg/mm2 and 2.0 mcg/mm2, respectively), type of excipient (carrier) used, and the balloon coating, noted Dr. Laird, professor and medical director of the Vascular Center at the University of California, Davis in Sacramento.
After the first year, primary patency ran 82% among the 220 patients randomized to the DCB and 52% in patients treated with percutaneous transluminal angioplasty, a statistically significant 30 percentage point difference in favor of the DCB. After 2 years, the rates were 79% in the DCB arm and 50% with a conventional balloon. “We saw no late catch-up that reduced the patency rate,” said Dr. Laird.
The INPACT SFA I(Randomized Trial of IN.PACT Admiral Drug Coated Balloon vs. Standard PTA for the Treatment of SFA and Proximal Popliteal Arterial Disease) trial enrolled 331 patients at 57 centers in the United States and Europe. Researchers reported the study’s primary efficacy and safety endpoints with 1-year follow-up earlier this year (Circulation. 2015 Feb 3;131:495-502). Concurrent with Dr. Laird’s report at the meeting, the 2-year results appeared online (J Amer Coll Card. 2015.doi:10.1016/j.jacc.2015.09.063).
Dr. Laird acknowledged that some types of stents also have shown good efficacy for treating femoropopliteal disease, but he had reservations about placing a stent when the DCB option exists.
“A lot of people have the sense that if we can avoid placing a stent in a femoral artery it helps preserve future treatment options for the patient. The problem with a stent is that once in-stent restenosis occurs in a leg artery, then the chances of getting a good result with an intravascular approach are poor,” Dr. Laird said at the meeting, sponsored by the Cardiovascular Research Foundation.
One potentially concerning finding from the 2-year follow-up was a statistically significant excess of all-cause mortality in the patients who received the DCB, with 16 deaths in the DCB arm and 1 death in the control, angioplasty arm. Dr. Laird dismissed the clinical importance of the finding, noting that all the deaths in the DCB arm had been independently adjudicated with none judged related to the device or procedure. In addition, the deaths occurred an average of 560 days following the procedure.
On Twitter @mitchelzoler
AT TCT 2015
Key clinical point: Two-year follow-up of paclitaxel-coated balloon treatment of femoropopliteal lesions showed durable and substantially better patency, compared with conventional balloon treatment.
Major finding: Two-year primary patency rate was 79% after treatment with the IN.PACT Admiral balloon and 50% with a conventional balloon.
Data source: INPACT SFA 1, a multicenter, randomized trial with 331 enrolled patients.
Disclosures: INPACT SFA I was sponsored by Medtronic, the company that markets the IN.PACT Admiral drug-coated balloon. Dr. Laird has been a consultant to Medtronic as well as to Bard, Abbott Vascular, Boston Scientific and Cordis. He also owns stock in several device companies.
Low incidence of DVT reported after percutaneous EVAR
Completely percutaneous endovascular aortic aneurysm repair (PEVAR) has become more common, using the suture-mediated “preclose” technique. The rate of periprocedural, iatrogenic, acute deep vein thrombosis (DVT), hitherto unknown, was found to be low for this approach, according to a study reported by Dr. Courtney E. Morgan and her colleagues at the Northwestern University, Chicago.
The researchers assessed 52 consecutive patients (44 men) with a mean age of 73 years, who underwent PEVAR at their center. Only 6% had a prior history of DVT (J Vasc Surg. 2015 Aug; 62:351-4).
Acute DVT was seen in four patients on postoperative day 1. These four DVTs comprised one femoropopliteal, and three calf DVTs. Three of these patients had associated risk factors: history of DVT (two patients); active smokers (one patient); and obesity (body mass index greater than 30 kg/m2 in all three patients).
At 2 weeks postoperatively, 75% of the DVTs had resolved.
“We found an overall rate of proximal DVT of 4% after PEVAR, which increased to 13% when calf-vein DVTs were included. Most patients with postoperative DVT had preexisting risk factors, which suggests that routine duplex ultrasound screening after PEVAR is not necessary unless there exist preclinical risk factors or postprocedural clinical indications suggestive of DVT,” the authors concluded.
Two of the researchers have received funding and/or served as speakers/consultants for device companies involved in EVAR.
Read the full study online in the Journal of Vascular Surgery.
Completely percutaneous endovascular aortic aneurysm repair (PEVAR) has become more common, using the suture-mediated “preclose” technique. The rate of periprocedural, iatrogenic, acute deep vein thrombosis (DVT), hitherto unknown, was found to be low for this approach, according to a study reported by Dr. Courtney E. Morgan and her colleagues at the Northwestern University, Chicago.
The researchers assessed 52 consecutive patients (44 men) with a mean age of 73 years, who underwent PEVAR at their center. Only 6% had a prior history of DVT (J Vasc Surg. 2015 Aug; 62:351-4).
Acute DVT was seen in four patients on postoperative day 1. These four DVTs comprised one femoropopliteal, and three calf DVTs. Three of these patients had associated risk factors: history of DVT (two patients); active smokers (one patient); and obesity (body mass index greater than 30 kg/m2 in all three patients).
At 2 weeks postoperatively, 75% of the DVTs had resolved.
“We found an overall rate of proximal DVT of 4% after PEVAR, which increased to 13% when calf-vein DVTs were included. Most patients with postoperative DVT had preexisting risk factors, which suggests that routine duplex ultrasound screening after PEVAR is not necessary unless there exist preclinical risk factors or postprocedural clinical indications suggestive of DVT,” the authors concluded.
Two of the researchers have received funding and/or served as speakers/consultants for device companies involved in EVAR.
Read the full study online in the Journal of Vascular Surgery.
Completely percutaneous endovascular aortic aneurysm repair (PEVAR) has become more common, using the suture-mediated “preclose” technique. The rate of periprocedural, iatrogenic, acute deep vein thrombosis (DVT), hitherto unknown, was found to be low for this approach, according to a study reported by Dr. Courtney E. Morgan and her colleagues at the Northwestern University, Chicago.
The researchers assessed 52 consecutive patients (44 men) with a mean age of 73 years, who underwent PEVAR at their center. Only 6% had a prior history of DVT (J Vasc Surg. 2015 Aug; 62:351-4).
Acute DVT was seen in four patients on postoperative day 1. These four DVTs comprised one femoropopliteal, and three calf DVTs. Three of these patients had associated risk factors: history of DVT (two patients); active smokers (one patient); and obesity (body mass index greater than 30 kg/m2 in all three patients).
At 2 weeks postoperatively, 75% of the DVTs had resolved.
“We found an overall rate of proximal DVT of 4% after PEVAR, which increased to 13% when calf-vein DVTs were included. Most patients with postoperative DVT had preexisting risk factors, which suggests that routine duplex ultrasound screening after PEVAR is not necessary unless there exist preclinical risk factors or postprocedural clinical indications suggestive of DVT,” the authors concluded.
Two of the researchers have received funding and/or served as speakers/consultants for device companies involved in EVAR.
Read the full study online in the Journal of Vascular Surgery.
FROM THE JOURNAL OF VASCULAR SURGERY
Hybrid approach tackles critical limb ischemia
CHICAGO – A hybrid approach combining external iliac endarterectomy with stenting may offer vascular surgeons a more robust option to stenting alone or aortofemoral bypass in patients with critical limb ischemia.
“Hybrid-based iliofemoral endarterectomy provides a minimally invasive option for revascularization, producing robust inflow restoration and low perioperative morbidity,” study author Dr. Crystal Kavanagh of St. Joseph Mercy Health Center in Ann Arbor, Mich., said.
The 5-year retrospective series, presented here at the annual meeting of the Midwestern Vascular Surgical Society, earned the prestigious Szilagyi Award for best clinical research.
Dr. Kavanagh and her colleagues crafted the hybrid technique because conventional open approaches in managing external iliac occlusive disease are associated with considerable morbidity. At the same time, long or multisegmental external iliac-to-femoral arterial lesions treated with stenting alone have produced poor patency and typically require additional outflow procedures, she explained.
The technique uses external iliac endarterectomy, aided with a traditional moll-ring stripper. A longitudinal, femoral cut-down is completed. A wire is advanced through the ipsilateral external iliac artery into the aorta after heparinization and obtaining access via an 18-gauge micropuncture in the common femoral artery. Intraluminal positioning is confirmed and a moll-ring endarterectomy is completed over the wire using a balloon to create the distal transection point, Dr. Kavanagh explained. The moll-ring is sized to the maximum diameter that will be accommodated by the ring.
After partially deflating the balloon, the plaque is extracted. A long-segment endarterectomy is typically completed, leaving a widely patent external iliac artery, she said.
In cases where adjunct iliac stenting is required, such as a proximal dissection flap, the stent size is larger than what is typically placed with stenting alone, Dr. Kavanagh observed.
The 2007 TASC (TransAtlantic InterSociety Consensus) recommendations suggest that TASC A lesions should undergo endovascular treatment as first-line therapy, while TASC D lesions should undergo traditional open surgical bypass.
Consensus has been slow to form for TASC B and C lesions, although most TASC B lesions undergo endovascular treatment and most TASC C lesions undergo open bypass.
Among the 40 limbs in the series, a common iliac (CI) artery stent (mean diameter, 8 mm: mean length, 59 mm) was placed in 19 limbs; a CI-to-external iliac (EI) stent (mean diameter, 10 mm; mean length, 100 mm) in 7 limbs; and an EI stent (mean diameter, 10 mm; mean length, 100 mm) in 21 limbs.
None of the iliac lesions were TASC category A or B, 17% were TASC C, and 83% TASC D. Concomitant infrainguinal disease of these patients had femoral/popliteal lesions, of which 16% were type A, 33% type B, 19% type C, and 32% type D.
Half of the 33 patients had three-vessel runoff, 33% two-vessel runoff, and 17% single-vessel runoff.
The hybrid procedure was completed as planned in all 40 limbs, Dr. Kavanagh said. There was no intraoperative or 90-day mortality.
Perioperative complications were minimal, with a 30-day readmission rate of only 12%, she said. This included one patient with one-vessel run-off who re-presented with ischemia requiring common femoral-to-below-the-knee popliteal bypass.
A second patient was admitted at postoperative day 47 with an infected pseudoaneurysm requiring patch angioplasty revision, for a 90-day readmission rate of 15%.
“Concerns about potential plaque rupture or hemorrhage can easily be dealt with via a covered stent graft, given intraluminal wire access throughout the procedure,” senior author Dr. Abdulhameed Aziz said in an interview.
Significant gains were made from baseline in postoperative ankle-brachial index (mean, 0.4 vs. 076; P less than .001), as well as in toe pressures (mean, 32 mm Hg vs. 60 mm Hg; P less than .001), Dr. Kavanagh said.
After a median follow-up of 13 months, primary patency was 100%.
“Combined common femoral endarterectomy with iliac stenting has demonstrated comparable patency to operative bypass in the short term,” she said.
“We theorize that the longer-segment endarterectomy, in our case essentially going from the iliac bifurcation to the common femoral, may produce a more durable result ... Stenting the proximal transection point may prevent restenosis.”
The authors reported no financial disclosures.
The combination of open surgical procedures with endovascular interventions has enriched the spectrum of vascular reconstructions significantly. These so-called hybrid procedures are especially worthwhile if pros and cons of both approaches could be combined and the groin could be considered as the hub. Technically spoken, the groin is the ideal hub for these kind of procedures. Why is that the case? Usually the surgical access to the common femoral artery (CFA) is easy. Furthermore the long-term results of femoral/retrograde iliac endarterectomy (often in combination with profundoplasty) are undoubtedly excellent. For the endovascular world, the (almost) NO-GO for any metal in the groin is still valid, and balloon dilatation of the femoral arteries is hemodynamically insufficient in most cases. However, PTA [percutaneous transluminal angioplasty] and stenting of the iliac arteries comes with good long-term results and avoids the sometimes-extended surgical access via the abdomen or the retroperitoneum.
Technically, it is advisable to perform the procedure in the following way: exposure of the CFA up to the inguinal ligament and down to the proximal superficial and deep femoral artery; puncture of the CFA in a noncalcified area and retrograde guide-wire access to the distal aorta (confirmation by angiography); balloon blockage of the proximal iliac artery (if technically possible; open endarterectomy of the CFA (including the proximal superficial and deep femoral artery; and retrograde ring-stripper endarterectomy of the iliac arteries and reconstruction of the femoral arteries (patchplasty, femoral transposition, profundoplasty). Balloon dilatation and stenting will be performed at the end of the procedure via a 7F or 9F sheath. We prefer balloon-expandable stents for the common and self-expandable stents for the external iliac artery, respectively. The contralateral groin should also be prepared for kissing stenting of both iliac arteries. Very rarely, an antegrade iliac access (via contralateral or brachial) access) is necessary. Whether or not covered stents have better long-term results is an open issue, however, covered stents should always be available to treat rare complications like an iatrogenic iliac rupture.
Especially Rutherford stage 5 or 6 patients very often present with multisegment disease including the femoropopliteal and the crural arteries. Since an even perfect inguinal inflow might not be sufficient in CLI [critical limb ischemia], these patients often need additional open or endovascular procedures. Again, the latter can be performed simultaneously via the hub femoral artery.
Even though hybrid procedures have been an essential part of vascular surgical practice for some years now, the Midwestern Vascular Surgical Society and Dr. Kavanagh have to be congratulated for raising this clinically very important topic again.
Dr. Hans-Henning Eckstein is a Professor at the Department for Vascular and Endovascular Surgery, Klinikum rechts der Isar, Technical University Munich, and is an associate medical editor for Vascular Specialist.
The combination of open surgical procedures with endovascular interventions has enriched the spectrum of vascular reconstructions significantly. These so-called hybrid procedures are especially worthwhile if pros and cons of both approaches could be combined and the groin could be considered as the hub. Technically spoken, the groin is the ideal hub for these kind of procedures. Why is that the case? Usually the surgical access to the common femoral artery (CFA) is easy. Furthermore the long-term results of femoral/retrograde iliac endarterectomy (often in combination with profundoplasty) are undoubtedly excellent. For the endovascular world, the (almost) NO-GO for any metal in the groin is still valid, and balloon dilatation of the femoral arteries is hemodynamically insufficient in most cases. However, PTA [percutaneous transluminal angioplasty] and stenting of the iliac arteries comes with good long-term results and avoids the sometimes-extended surgical access via the abdomen or the retroperitoneum.
Technically, it is advisable to perform the procedure in the following way: exposure of the CFA up to the inguinal ligament and down to the proximal superficial and deep femoral artery; puncture of the CFA in a noncalcified area and retrograde guide-wire access to the distal aorta (confirmation by angiography); balloon blockage of the proximal iliac artery (if technically possible; open endarterectomy of the CFA (including the proximal superficial and deep femoral artery; and retrograde ring-stripper endarterectomy of the iliac arteries and reconstruction of the femoral arteries (patchplasty, femoral transposition, profundoplasty). Balloon dilatation and stenting will be performed at the end of the procedure via a 7F or 9F sheath. We prefer balloon-expandable stents for the common and self-expandable stents for the external iliac artery, respectively. The contralateral groin should also be prepared for kissing stenting of both iliac arteries. Very rarely, an antegrade iliac access (via contralateral or brachial) access) is necessary. Whether or not covered stents have better long-term results is an open issue, however, covered stents should always be available to treat rare complications like an iatrogenic iliac rupture.
Especially Rutherford stage 5 or 6 patients very often present with multisegment disease including the femoropopliteal and the crural arteries. Since an even perfect inguinal inflow might not be sufficient in CLI [critical limb ischemia], these patients often need additional open or endovascular procedures. Again, the latter can be performed simultaneously via the hub femoral artery.
Even though hybrid procedures have been an essential part of vascular surgical practice for some years now, the Midwestern Vascular Surgical Society and Dr. Kavanagh have to be congratulated for raising this clinically very important topic again.
Dr. Hans-Henning Eckstein is a Professor at the Department for Vascular and Endovascular Surgery, Klinikum rechts der Isar, Technical University Munich, and is an associate medical editor for Vascular Specialist.
The combination of open surgical procedures with endovascular interventions has enriched the spectrum of vascular reconstructions significantly. These so-called hybrid procedures are especially worthwhile if pros and cons of both approaches could be combined and the groin could be considered as the hub. Technically spoken, the groin is the ideal hub for these kind of procedures. Why is that the case? Usually the surgical access to the common femoral artery (CFA) is easy. Furthermore the long-term results of femoral/retrograde iliac endarterectomy (often in combination with profundoplasty) are undoubtedly excellent. For the endovascular world, the (almost) NO-GO for any metal in the groin is still valid, and balloon dilatation of the femoral arteries is hemodynamically insufficient in most cases. However, PTA [percutaneous transluminal angioplasty] and stenting of the iliac arteries comes with good long-term results and avoids the sometimes-extended surgical access via the abdomen or the retroperitoneum.
Technically, it is advisable to perform the procedure in the following way: exposure of the CFA up to the inguinal ligament and down to the proximal superficial and deep femoral artery; puncture of the CFA in a noncalcified area and retrograde guide-wire access to the distal aorta (confirmation by angiography); balloon blockage of the proximal iliac artery (if technically possible; open endarterectomy of the CFA (including the proximal superficial and deep femoral artery; and retrograde ring-stripper endarterectomy of the iliac arteries and reconstruction of the femoral arteries (patchplasty, femoral transposition, profundoplasty). Balloon dilatation and stenting will be performed at the end of the procedure via a 7F or 9F sheath. We prefer balloon-expandable stents for the common and self-expandable stents for the external iliac artery, respectively. The contralateral groin should also be prepared for kissing stenting of both iliac arteries. Very rarely, an antegrade iliac access (via contralateral or brachial) access) is necessary. Whether or not covered stents have better long-term results is an open issue, however, covered stents should always be available to treat rare complications like an iatrogenic iliac rupture.
Especially Rutherford stage 5 or 6 patients very often present with multisegment disease including the femoropopliteal and the crural arteries. Since an even perfect inguinal inflow might not be sufficient in CLI [critical limb ischemia], these patients often need additional open or endovascular procedures. Again, the latter can be performed simultaneously via the hub femoral artery.
Even though hybrid procedures have been an essential part of vascular surgical practice for some years now, the Midwestern Vascular Surgical Society and Dr. Kavanagh have to be congratulated for raising this clinically very important topic again.
Dr. Hans-Henning Eckstein is a Professor at the Department for Vascular and Endovascular Surgery, Klinikum rechts der Isar, Technical University Munich, and is an associate medical editor for Vascular Specialist.
CHICAGO – A hybrid approach combining external iliac endarterectomy with stenting may offer vascular surgeons a more robust option to stenting alone or aortofemoral bypass in patients with critical limb ischemia.
“Hybrid-based iliofemoral endarterectomy provides a minimally invasive option for revascularization, producing robust inflow restoration and low perioperative morbidity,” study author Dr. Crystal Kavanagh of St. Joseph Mercy Health Center in Ann Arbor, Mich., said.
The 5-year retrospective series, presented here at the annual meeting of the Midwestern Vascular Surgical Society, earned the prestigious Szilagyi Award for best clinical research.
Dr. Kavanagh and her colleagues crafted the hybrid technique because conventional open approaches in managing external iliac occlusive disease are associated with considerable morbidity. At the same time, long or multisegmental external iliac-to-femoral arterial lesions treated with stenting alone have produced poor patency and typically require additional outflow procedures, she explained.
The technique uses external iliac endarterectomy, aided with a traditional moll-ring stripper. A longitudinal, femoral cut-down is completed. A wire is advanced through the ipsilateral external iliac artery into the aorta after heparinization and obtaining access via an 18-gauge micropuncture in the common femoral artery. Intraluminal positioning is confirmed and a moll-ring endarterectomy is completed over the wire using a balloon to create the distal transection point, Dr. Kavanagh explained. The moll-ring is sized to the maximum diameter that will be accommodated by the ring.
After partially deflating the balloon, the plaque is extracted. A long-segment endarterectomy is typically completed, leaving a widely patent external iliac artery, she said.
In cases where adjunct iliac stenting is required, such as a proximal dissection flap, the stent size is larger than what is typically placed with stenting alone, Dr. Kavanagh observed.
The 2007 TASC (TransAtlantic InterSociety Consensus) recommendations suggest that TASC A lesions should undergo endovascular treatment as first-line therapy, while TASC D lesions should undergo traditional open surgical bypass.
Consensus has been slow to form for TASC B and C lesions, although most TASC B lesions undergo endovascular treatment and most TASC C lesions undergo open bypass.
Among the 40 limbs in the series, a common iliac (CI) artery stent (mean diameter, 8 mm: mean length, 59 mm) was placed in 19 limbs; a CI-to-external iliac (EI) stent (mean diameter, 10 mm; mean length, 100 mm) in 7 limbs; and an EI stent (mean diameter, 10 mm; mean length, 100 mm) in 21 limbs.
None of the iliac lesions were TASC category A or B, 17% were TASC C, and 83% TASC D. Concomitant infrainguinal disease of these patients had femoral/popliteal lesions, of which 16% were type A, 33% type B, 19% type C, and 32% type D.
Half of the 33 patients had three-vessel runoff, 33% two-vessel runoff, and 17% single-vessel runoff.
The hybrid procedure was completed as planned in all 40 limbs, Dr. Kavanagh said. There was no intraoperative or 90-day mortality.
Perioperative complications were minimal, with a 30-day readmission rate of only 12%, she said. This included one patient with one-vessel run-off who re-presented with ischemia requiring common femoral-to-below-the-knee popliteal bypass.
A second patient was admitted at postoperative day 47 with an infected pseudoaneurysm requiring patch angioplasty revision, for a 90-day readmission rate of 15%.
“Concerns about potential plaque rupture or hemorrhage can easily be dealt with via a covered stent graft, given intraluminal wire access throughout the procedure,” senior author Dr. Abdulhameed Aziz said in an interview.
Significant gains were made from baseline in postoperative ankle-brachial index (mean, 0.4 vs. 076; P less than .001), as well as in toe pressures (mean, 32 mm Hg vs. 60 mm Hg; P less than .001), Dr. Kavanagh said.
After a median follow-up of 13 months, primary patency was 100%.
“Combined common femoral endarterectomy with iliac stenting has demonstrated comparable patency to operative bypass in the short term,” she said.
“We theorize that the longer-segment endarterectomy, in our case essentially going from the iliac bifurcation to the common femoral, may produce a more durable result ... Stenting the proximal transection point may prevent restenosis.”
The authors reported no financial disclosures.
CHICAGO – A hybrid approach combining external iliac endarterectomy with stenting may offer vascular surgeons a more robust option to stenting alone or aortofemoral bypass in patients with critical limb ischemia.
“Hybrid-based iliofemoral endarterectomy provides a minimally invasive option for revascularization, producing robust inflow restoration and low perioperative morbidity,” study author Dr. Crystal Kavanagh of St. Joseph Mercy Health Center in Ann Arbor, Mich., said.
The 5-year retrospective series, presented here at the annual meeting of the Midwestern Vascular Surgical Society, earned the prestigious Szilagyi Award for best clinical research.
Dr. Kavanagh and her colleagues crafted the hybrid technique because conventional open approaches in managing external iliac occlusive disease are associated with considerable morbidity. At the same time, long or multisegmental external iliac-to-femoral arterial lesions treated with stenting alone have produced poor patency and typically require additional outflow procedures, she explained.
The technique uses external iliac endarterectomy, aided with a traditional moll-ring stripper. A longitudinal, femoral cut-down is completed. A wire is advanced through the ipsilateral external iliac artery into the aorta after heparinization and obtaining access via an 18-gauge micropuncture in the common femoral artery. Intraluminal positioning is confirmed and a moll-ring endarterectomy is completed over the wire using a balloon to create the distal transection point, Dr. Kavanagh explained. The moll-ring is sized to the maximum diameter that will be accommodated by the ring.
After partially deflating the balloon, the plaque is extracted. A long-segment endarterectomy is typically completed, leaving a widely patent external iliac artery, she said.
In cases where adjunct iliac stenting is required, such as a proximal dissection flap, the stent size is larger than what is typically placed with stenting alone, Dr. Kavanagh observed.
The 2007 TASC (TransAtlantic InterSociety Consensus) recommendations suggest that TASC A lesions should undergo endovascular treatment as first-line therapy, while TASC D lesions should undergo traditional open surgical bypass.
Consensus has been slow to form for TASC B and C lesions, although most TASC B lesions undergo endovascular treatment and most TASC C lesions undergo open bypass.
Among the 40 limbs in the series, a common iliac (CI) artery stent (mean diameter, 8 mm: mean length, 59 mm) was placed in 19 limbs; a CI-to-external iliac (EI) stent (mean diameter, 10 mm; mean length, 100 mm) in 7 limbs; and an EI stent (mean diameter, 10 mm; mean length, 100 mm) in 21 limbs.
None of the iliac lesions were TASC category A or B, 17% were TASC C, and 83% TASC D. Concomitant infrainguinal disease of these patients had femoral/popliteal lesions, of which 16% were type A, 33% type B, 19% type C, and 32% type D.
Half of the 33 patients had three-vessel runoff, 33% two-vessel runoff, and 17% single-vessel runoff.
The hybrid procedure was completed as planned in all 40 limbs, Dr. Kavanagh said. There was no intraoperative or 90-day mortality.
Perioperative complications were minimal, with a 30-day readmission rate of only 12%, she said. This included one patient with one-vessel run-off who re-presented with ischemia requiring common femoral-to-below-the-knee popliteal bypass.
A second patient was admitted at postoperative day 47 with an infected pseudoaneurysm requiring patch angioplasty revision, for a 90-day readmission rate of 15%.
“Concerns about potential plaque rupture or hemorrhage can easily be dealt with via a covered stent graft, given intraluminal wire access throughout the procedure,” senior author Dr. Abdulhameed Aziz said in an interview.
Significant gains were made from baseline in postoperative ankle-brachial index (mean, 0.4 vs. 076; P less than .001), as well as in toe pressures (mean, 32 mm Hg vs. 60 mm Hg; P less than .001), Dr. Kavanagh said.
After a median follow-up of 13 months, primary patency was 100%.
“Combined common femoral endarterectomy with iliac stenting has demonstrated comparable patency to operative bypass in the short term,” she said.
“We theorize that the longer-segment endarterectomy, in our case essentially going from the iliac bifurcation to the common femoral, may produce a more durable result ... Stenting the proximal transection point may prevent restenosis.”
The authors reported no financial disclosures.
AT MIDWESTERN VASCULAR 2015
Key clinical point: Hybrid-based iliofemoral endarterectomy provides robust inflow restoration comparable to aortofemoral bypass, with minimal perioperative morbidity.
Major finding: Primary patency was 100% with a mean follow-up of 13 months.
Data source: Five-year retrospective study in 40 limbs in 33 patients with critical limb ischemia.
Disclosures: The authors reported having no financial disclosures.
Endologix announces FDA approval of AFX2 Bifurcated Endograft
The U.S. Food and Drug Administration has approved the AFX2 Bifurcated Endograft System for the treatment of abdominal aortic aneurysms (AAA), the device’s manufacturer, Endologix, announced in a statement.
Endologix also touts the AFX2 as a way to facilitate percutaneous endovascular aneurysm repair (EVAR) by providing low-profile contralateral access through a 7F introducer. The device incorporates Endologix’s ActiveSeal technology, DuraPly expanded polytetrafluoroethylene graft material, and the Vela proximal endograft.
The AFX2 is expected to hit the market in the United States in the first quarter of 2016.
The U.S. Food and Drug Administration has approved the AFX2 Bifurcated Endograft System for the treatment of abdominal aortic aneurysms (AAA), the device’s manufacturer, Endologix, announced in a statement.
Endologix also touts the AFX2 as a way to facilitate percutaneous endovascular aneurysm repair (EVAR) by providing low-profile contralateral access through a 7F introducer. The device incorporates Endologix’s ActiveSeal technology, DuraPly expanded polytetrafluoroethylene graft material, and the Vela proximal endograft.
The AFX2 is expected to hit the market in the United States in the first quarter of 2016.
The U.S. Food and Drug Administration has approved the AFX2 Bifurcated Endograft System for the treatment of abdominal aortic aneurysms (AAA), the device’s manufacturer, Endologix, announced in a statement.
Endologix also touts the AFX2 as a way to facilitate percutaneous endovascular aneurysm repair (EVAR) by providing low-profile contralateral access through a 7F introducer. The device incorporates Endologix’s ActiveSeal technology, DuraPly expanded polytetrafluoroethylene graft material, and the Vela proximal endograft.
The AFX2 is expected to hit the market in the United States in the first quarter of 2016.
Reattaching intercostals fails to squelch spinal cord ischemia in TAAA repairs
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.
 |
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.
 |
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.
 |
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
AT MIDWESTERN VASCULAR 2015
Key clinical point: Intercostal artery reimplantation (ICAR) did not produce a significant reduction in spinal cord ischemia following thoracoabdominal aortic aneurysm repair, even in the highest risk patients.
Major finding: ICAR was not a significant predictor of spinal cord ischemia (OR, 0.78; P = .55).
Data source: Retrospective analysis of 805 patients undergoing TAAA with or without ICAR.
Disclosures: The authors reported having no conflicts of interest.