User login
Leg ulceration guidelines expected to soon include endovascular ablation
NEW YORK – Guidelines for the management of leg ulcerations will be changed to accommodate the results of the Early Venous Reflux Ablation trial, according to this video interview with the senior author, Alun H Davies, DSc, professor of vascular surgery, Imperial College, London.
In this video interview, conducted at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation, Dr. Davies recaps the major results of the study, which associated immediate endovascular ablation (early intervention) with significantly faster healing than did compression therapy with ablation, considered only after 6 months (delayed intervention).
These data have been published (N Engl J Med 2018 May 31;378:2105-14), but Dr. Davies focused in this interview on the cost efficacy of early intervention with endovascular ablation. In the United Kingdom, where the study was conducted, the data support the cost efficacy, but Dr. Davies predicted even greater savings in the United States because of the expense of frequent wound care visits.
Based on data from a randomized trial, he expects guidelines, including those in the United States, to be revised to list early endovascular ablation as a 1b or 1A recommendation, thereby establishing this intervention as a standard.
If follow-up after 3 years confirms a lower rate of recurrence, an advantage previously shown for open surgery relative to compression healing, the case for early endovascular intervention will be even stronger, according to Dr. Davies.
NEW YORK – Guidelines for the management of leg ulcerations will be changed to accommodate the results of the Early Venous Reflux Ablation trial, according to this video interview with the senior author, Alun H Davies, DSc, professor of vascular surgery, Imperial College, London.
In this video interview, conducted at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation, Dr. Davies recaps the major results of the study, which associated immediate endovascular ablation (early intervention) with significantly faster healing than did compression therapy with ablation, considered only after 6 months (delayed intervention).
These data have been published (N Engl J Med 2018 May 31;378:2105-14), but Dr. Davies focused in this interview on the cost efficacy of early intervention with endovascular ablation. In the United Kingdom, where the study was conducted, the data support the cost efficacy, but Dr. Davies predicted even greater savings in the United States because of the expense of frequent wound care visits.
Based on data from a randomized trial, he expects guidelines, including those in the United States, to be revised to list early endovascular ablation as a 1b or 1A recommendation, thereby establishing this intervention as a standard.
If follow-up after 3 years confirms a lower rate of recurrence, an advantage previously shown for open surgery relative to compression healing, the case for early endovascular intervention will be even stronger, according to Dr. Davies.
NEW YORK – Guidelines for the management of leg ulcerations will be changed to accommodate the results of the Early Venous Reflux Ablation trial, according to this video interview with the senior author, Alun H Davies, DSc, professor of vascular surgery, Imperial College, London.
In this video interview, conducted at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation, Dr. Davies recaps the major results of the study, which associated immediate endovascular ablation (early intervention) with significantly faster healing than did compression therapy with ablation, considered only after 6 months (delayed intervention).
These data have been published (N Engl J Med 2018 May 31;378:2105-14), but Dr. Davies focused in this interview on the cost efficacy of early intervention with endovascular ablation. In the United Kingdom, where the study was conducted, the data support the cost efficacy, but Dr. Davies predicted even greater savings in the United States because of the expense of frequent wound care visits.
Based on data from a randomized trial, he expects guidelines, including those in the United States, to be revised to list early endovascular ablation as a 1b or 1A recommendation, thereby establishing this intervention as a standard.
If follow-up after 3 years confirms a lower rate of recurrence, an advantage previously shown for open surgery relative to compression healing, the case for early endovascular intervention will be even stronger, according to Dr. Davies.
REPORTING FROM VEITHSYMPOSIUM
SVS guidelines address scope of practice concerns
NEW YORK – Vascular surgeons are the only specialty qualified to treat all vascular disorders with open surgery and/or endovascular treatment, including the thoracic aorta, according to the updated “Guidelines for hospital privileges in vascular surgery and endovascular interventions: Recommendations of the Society for Vascular Surgery.”
The guidelines, published in May’s Journal of Vascular Surgery, were last updated in 2008, said Keith D. Calligaro, MD, who spoke on their importance and potential benefits to vascular surgeons during his presentation at the VEITHsymposium.
The thoracic aorta component of the guidelines addresses scope of practice concerns between vascular and thoracic surgeons, said Dr. Calligaro, who is a clinical professor of surgery, University of Pennsylvania, and chief of vascular surgery and endovascular therapy at Pennsylvania Hospital, both in Philadelphia.
The guidelines relied on training requirements to provide some of the data to define vascular surgeons and privileges. The open vascular surgery training requirements still are defined by the Residency Review Committee for surgery, and those requirements include 250 major open vascular cases during training, including 30 open abdominal operations, 25 carotid, 45 peripheral open surgery cases, and 10 complex vascular surgeries, said Dr. Calligaro. “In terms of endovascular treatment, the training requirements are over 100 diagnostic caths and over 80 therapeutic interventions, and during training you would have had to have done more than 20 EVARs [endovascular aneurysm repairs].” That number jumped up from five EVARs in the previous guidelines.
Ultimately, “the SVS is basically saying ‘you need to be a vascular surgeon to perform vascular surgery,’ and you need have to have completed an Accreditation Council for Graduate Medical Education–accredited vascular residency.
“So if you are a general surgeon or a heart surgeon and you go to a new hospital and say ‘I want to do vascular,’ the vascular surgeon at that institution can refer to this document and say ‘no, the SVS is saying [the surgeon doesn’t] have the training.’ And I think that’s a pretty gutsy and important call,” said Dr. Calligaro.
It is a different case for endovascular surgery, he said. In this case, the requirement is to have completed an ACGME-accredited program in either vascular surgery, interventional radiology, or interventional cardiology to indicate the appropriate level of training. But SVS agreed with the recommendation by the American College of Cardiology that cardiologists not only had to complete 1 year of coronary interventions but also 1 year of peripheral intervention training, as well.
“So if you are at your hospital and have a cardiologist who is starting to do peripheral vascular stuff, now at least you can wave part of this document and say ‘Hey, look, the most important vascular society in the country is saying that, unless this individual had a year of peripheral training, this cardiologist should not be allowed to do endovascular peripheral interventions,’ ” Dr. Calligaro said.
SOURCE: Calligaro KD et al. J Vasc Surg. 2018 May;67(5):1337-44.
NEW YORK – Vascular surgeons are the only specialty qualified to treat all vascular disorders with open surgery and/or endovascular treatment, including the thoracic aorta, according to the updated “Guidelines for hospital privileges in vascular surgery and endovascular interventions: Recommendations of the Society for Vascular Surgery.”
The guidelines, published in May’s Journal of Vascular Surgery, were last updated in 2008, said Keith D. Calligaro, MD, who spoke on their importance and potential benefits to vascular surgeons during his presentation at the VEITHsymposium.
The thoracic aorta component of the guidelines addresses scope of practice concerns between vascular and thoracic surgeons, said Dr. Calligaro, who is a clinical professor of surgery, University of Pennsylvania, and chief of vascular surgery and endovascular therapy at Pennsylvania Hospital, both in Philadelphia.
The guidelines relied on training requirements to provide some of the data to define vascular surgeons and privileges. The open vascular surgery training requirements still are defined by the Residency Review Committee for surgery, and those requirements include 250 major open vascular cases during training, including 30 open abdominal operations, 25 carotid, 45 peripheral open surgery cases, and 10 complex vascular surgeries, said Dr. Calligaro. “In terms of endovascular treatment, the training requirements are over 100 diagnostic caths and over 80 therapeutic interventions, and during training you would have had to have done more than 20 EVARs [endovascular aneurysm repairs].” That number jumped up from five EVARs in the previous guidelines.
Ultimately, “the SVS is basically saying ‘you need to be a vascular surgeon to perform vascular surgery,’ and you need have to have completed an Accreditation Council for Graduate Medical Education–accredited vascular residency.
“So if you are a general surgeon or a heart surgeon and you go to a new hospital and say ‘I want to do vascular,’ the vascular surgeon at that institution can refer to this document and say ‘no, the SVS is saying [the surgeon doesn’t] have the training.’ And I think that’s a pretty gutsy and important call,” said Dr. Calligaro.
It is a different case for endovascular surgery, he said. In this case, the requirement is to have completed an ACGME-accredited program in either vascular surgery, interventional radiology, or interventional cardiology to indicate the appropriate level of training. But SVS agreed with the recommendation by the American College of Cardiology that cardiologists not only had to complete 1 year of coronary interventions but also 1 year of peripheral intervention training, as well.
“So if you are at your hospital and have a cardiologist who is starting to do peripheral vascular stuff, now at least you can wave part of this document and say ‘Hey, look, the most important vascular society in the country is saying that, unless this individual had a year of peripheral training, this cardiologist should not be allowed to do endovascular peripheral interventions,’ ” Dr. Calligaro said.
SOURCE: Calligaro KD et al. J Vasc Surg. 2018 May;67(5):1337-44.
NEW YORK – Vascular surgeons are the only specialty qualified to treat all vascular disorders with open surgery and/or endovascular treatment, including the thoracic aorta, according to the updated “Guidelines for hospital privileges in vascular surgery and endovascular interventions: Recommendations of the Society for Vascular Surgery.”
The guidelines, published in May’s Journal of Vascular Surgery, were last updated in 2008, said Keith D. Calligaro, MD, who spoke on their importance and potential benefits to vascular surgeons during his presentation at the VEITHsymposium.
The thoracic aorta component of the guidelines addresses scope of practice concerns between vascular and thoracic surgeons, said Dr. Calligaro, who is a clinical professor of surgery, University of Pennsylvania, and chief of vascular surgery and endovascular therapy at Pennsylvania Hospital, both in Philadelphia.
The guidelines relied on training requirements to provide some of the data to define vascular surgeons and privileges. The open vascular surgery training requirements still are defined by the Residency Review Committee for surgery, and those requirements include 250 major open vascular cases during training, including 30 open abdominal operations, 25 carotid, 45 peripheral open surgery cases, and 10 complex vascular surgeries, said Dr. Calligaro. “In terms of endovascular treatment, the training requirements are over 100 diagnostic caths and over 80 therapeutic interventions, and during training you would have had to have done more than 20 EVARs [endovascular aneurysm repairs].” That number jumped up from five EVARs in the previous guidelines.
Ultimately, “the SVS is basically saying ‘you need to be a vascular surgeon to perform vascular surgery,’ and you need have to have completed an Accreditation Council for Graduate Medical Education–accredited vascular residency.
“So if you are a general surgeon or a heart surgeon and you go to a new hospital and say ‘I want to do vascular,’ the vascular surgeon at that institution can refer to this document and say ‘no, the SVS is saying [the surgeon doesn’t] have the training.’ And I think that’s a pretty gutsy and important call,” said Dr. Calligaro.
It is a different case for endovascular surgery, he said. In this case, the requirement is to have completed an ACGME-accredited program in either vascular surgery, interventional radiology, or interventional cardiology to indicate the appropriate level of training. But SVS agreed with the recommendation by the American College of Cardiology that cardiologists not only had to complete 1 year of coronary interventions but also 1 year of peripheral intervention training, as well.
“So if you are at your hospital and have a cardiologist who is starting to do peripheral vascular stuff, now at least you can wave part of this document and say ‘Hey, look, the most important vascular society in the country is saying that, unless this individual had a year of peripheral training, this cardiologist should not be allowed to do endovascular peripheral interventions,’ ” Dr. Calligaro said.
SOURCE: Calligaro KD et al. J Vasc Surg. 2018 May;67(5):1337-44.
REPORTING FROM THE VEITHSYMPOSIUM
VQI-VVR registry data eyed for guiding development of ethical standards
NEW YORK – Registry data can be used to craft guidance for determining the appropriateness of procedures at vein centers, based on data presented by Thomas W. Wakefield, MD at the 2018 Veith Symposium.
The Vascular Quality Initiative Varicose Vein Registry (VQI-VVR), initiated in 2014 by the Society for Vascular Surgery in conjunction with the American Venous Forum, captures procedures that are performed in vein centers, office-based practices, and ambulatory or inpatient settings. The VVR looks at ablation and phlebectomy techniques and captures data including patient demographics, history, procedure data, plus early and late office-based and patient-reported follow-up in order to benchmark and improve outcomes and develop best practices and to help meet vein center certification requirements. The VVR includes 39 centers and more than 23,000 procedures.
Dr. Wakefield, who heads the VVR, used this registry as a means to illustrate how VQIs could be used to establish whether “the expected health benefit exceeds the expected negative consequences by a sufficiently wide margin that the procedure is worth doing.” This can be considered to be “appropriateness, which is part of ethical treatment.” Dr. Wakefield is the Stanley Professor of Vascular Surgery at the University of Michigan and section head, vascular surgery, University of Michigan Cardiovascular Center, Ann Arbor.
Data from the VQI registry (of which the VVR is a component) are now being used to generate appropriateness reports, said Dr. Wakefield.
The VQI represents a large comprehensive database of long-term data to define appropriate care. In addition, the VQI infrastructure is already geared to producing these reports both at a center and at a surgeon level. One disadvantage of the VVR registry, however, is low participation – only the 39 centers – and that it doesn’t capture cosmetic procedures and lesser (C1) disease. Further, it’s “likely the VQI participants are the ‘good actors,’ ” he added.
Targets for appropriateness include the proportion of patients undergoing ablation C2 or C4 disease or greater, the mean number of ablations per patient, the mean number of ablations per limb, and the proportion of perforated ablations for greater than C4 disease. Plotting out the data for these procedures at the center level can be assessed against current thinking on best practices in the various areas. For example, “the mean number of ablations per patient has been suggested at 1.8 to be about the right number,” and he used the graph of the center performance in this area to show that most of the centers were below this objective.
In an even more appropriate example of how this kind of data could be used to determine appropriateness, Dr. Wakefield described how perforated ablations should be performed for greater than C4 disease, but not for C2 disease. He described how, according to the actual data in the registry, there have been 870 total perforated treatments recorded, 38% for C2 disease, and of these 332 procedures, almost half of these were performed at one center only, with two other centers reporting 30 such procedures. “So clearly there are three centers that are doing perforated ablations for patients that are outside the guidelines,” Dr. Wakefield pointed out.
In future, payer demand is likely to demand that each treating physician provide evidence of the appropriateness of procedures performed, as well as appropriate patient selection and adherence to best practices, and good outcomes, which is part of what a society-based registry such as the VVR can provide.
“I believe the VQI-VVR is well-positioned to meet these needs. And if we ask the question ‘can VQI be used as a benchmark for setting ethical standards,’ I think it can certainly be used to help set appropriate standards, and since appropriateness is one part of ethical standards, I believe it has a role,” he concluded.
Dr. Wakefield reported that he had no disclosures.
NEW YORK – Registry data can be used to craft guidance for determining the appropriateness of procedures at vein centers, based on data presented by Thomas W. Wakefield, MD at the 2018 Veith Symposium.
The Vascular Quality Initiative Varicose Vein Registry (VQI-VVR), initiated in 2014 by the Society for Vascular Surgery in conjunction with the American Venous Forum, captures procedures that are performed in vein centers, office-based practices, and ambulatory or inpatient settings. The VVR looks at ablation and phlebectomy techniques and captures data including patient demographics, history, procedure data, plus early and late office-based and patient-reported follow-up in order to benchmark and improve outcomes and develop best practices and to help meet vein center certification requirements. The VVR includes 39 centers and more than 23,000 procedures.
Dr. Wakefield, who heads the VVR, used this registry as a means to illustrate how VQIs could be used to establish whether “the expected health benefit exceeds the expected negative consequences by a sufficiently wide margin that the procedure is worth doing.” This can be considered to be “appropriateness, which is part of ethical treatment.” Dr. Wakefield is the Stanley Professor of Vascular Surgery at the University of Michigan and section head, vascular surgery, University of Michigan Cardiovascular Center, Ann Arbor.
Data from the VQI registry (of which the VVR is a component) are now being used to generate appropriateness reports, said Dr. Wakefield.
The VQI represents a large comprehensive database of long-term data to define appropriate care. In addition, the VQI infrastructure is already geared to producing these reports both at a center and at a surgeon level. One disadvantage of the VVR registry, however, is low participation – only the 39 centers – and that it doesn’t capture cosmetic procedures and lesser (C1) disease. Further, it’s “likely the VQI participants are the ‘good actors,’ ” he added.
Targets for appropriateness include the proportion of patients undergoing ablation C2 or C4 disease or greater, the mean number of ablations per patient, the mean number of ablations per limb, and the proportion of perforated ablations for greater than C4 disease. Plotting out the data for these procedures at the center level can be assessed against current thinking on best practices in the various areas. For example, “the mean number of ablations per patient has been suggested at 1.8 to be about the right number,” and he used the graph of the center performance in this area to show that most of the centers were below this objective.
In an even more appropriate example of how this kind of data could be used to determine appropriateness, Dr. Wakefield described how perforated ablations should be performed for greater than C4 disease, but not for C2 disease. He described how, according to the actual data in the registry, there have been 870 total perforated treatments recorded, 38% for C2 disease, and of these 332 procedures, almost half of these were performed at one center only, with two other centers reporting 30 such procedures. “So clearly there are three centers that are doing perforated ablations for patients that are outside the guidelines,” Dr. Wakefield pointed out.
In future, payer demand is likely to demand that each treating physician provide evidence of the appropriateness of procedures performed, as well as appropriate patient selection and adherence to best practices, and good outcomes, which is part of what a society-based registry such as the VVR can provide.
“I believe the VQI-VVR is well-positioned to meet these needs. And if we ask the question ‘can VQI be used as a benchmark for setting ethical standards,’ I think it can certainly be used to help set appropriate standards, and since appropriateness is one part of ethical standards, I believe it has a role,” he concluded.
Dr. Wakefield reported that he had no disclosures.
NEW YORK – Registry data can be used to craft guidance for determining the appropriateness of procedures at vein centers, based on data presented by Thomas W. Wakefield, MD at the 2018 Veith Symposium.
The Vascular Quality Initiative Varicose Vein Registry (VQI-VVR), initiated in 2014 by the Society for Vascular Surgery in conjunction with the American Venous Forum, captures procedures that are performed in vein centers, office-based practices, and ambulatory or inpatient settings. The VVR looks at ablation and phlebectomy techniques and captures data including patient demographics, history, procedure data, plus early and late office-based and patient-reported follow-up in order to benchmark and improve outcomes and develop best practices and to help meet vein center certification requirements. The VVR includes 39 centers and more than 23,000 procedures.
Dr. Wakefield, who heads the VVR, used this registry as a means to illustrate how VQIs could be used to establish whether “the expected health benefit exceeds the expected negative consequences by a sufficiently wide margin that the procedure is worth doing.” This can be considered to be “appropriateness, which is part of ethical treatment.” Dr. Wakefield is the Stanley Professor of Vascular Surgery at the University of Michigan and section head, vascular surgery, University of Michigan Cardiovascular Center, Ann Arbor.
Data from the VQI registry (of which the VVR is a component) are now being used to generate appropriateness reports, said Dr. Wakefield.
The VQI represents a large comprehensive database of long-term data to define appropriate care. In addition, the VQI infrastructure is already geared to producing these reports both at a center and at a surgeon level. One disadvantage of the VVR registry, however, is low participation – only the 39 centers – and that it doesn’t capture cosmetic procedures and lesser (C1) disease. Further, it’s “likely the VQI participants are the ‘good actors,’ ” he added.
Targets for appropriateness include the proportion of patients undergoing ablation C2 or C4 disease or greater, the mean number of ablations per patient, the mean number of ablations per limb, and the proportion of perforated ablations for greater than C4 disease. Plotting out the data for these procedures at the center level can be assessed against current thinking on best practices in the various areas. For example, “the mean number of ablations per patient has been suggested at 1.8 to be about the right number,” and he used the graph of the center performance in this area to show that most of the centers were below this objective.
In an even more appropriate example of how this kind of data could be used to determine appropriateness, Dr. Wakefield described how perforated ablations should be performed for greater than C4 disease, but not for C2 disease. He described how, according to the actual data in the registry, there have been 870 total perforated treatments recorded, 38% for C2 disease, and of these 332 procedures, almost half of these were performed at one center only, with two other centers reporting 30 such procedures. “So clearly there are three centers that are doing perforated ablations for patients that are outside the guidelines,” Dr. Wakefield pointed out.
In future, payer demand is likely to demand that each treating physician provide evidence of the appropriateness of procedures performed, as well as appropriate patient selection and adherence to best practices, and good outcomes, which is part of what a society-based registry such as the VVR can provide.
“I believe the VQI-VVR is well-positioned to meet these needs. And if we ask the question ‘can VQI be used as a benchmark for setting ethical standards,’ I think it can certainly be used to help set appropriate standards, and since appropriateness is one part of ethical standards, I believe it has a role,” he concluded.
Dr. Wakefield reported that he had no disclosures.
REPORTING FROM THE 2018 VEITH SYMPOSIUM
Growing the pool of academic vascular surgeons
NEW YORK – Strategies for growing the pool of academic vascular surgeons might help avert the expected scarcity of physicians in this specialty, according to Peter K. Henke, MD, a professor of vascular surgery at the University of Michigan, Ann Arbor.
Dr. Henke recounted in a video interview key messages he delivered at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. He argued for going back to basics to enlist residents and fellows completing their training to stay in the specialty and consider an academic position.
Many of these steps are known, such as verifying that mentors are available to encourage skill acquisition and providing adequate time to achieve an acceptable balance of research and clinical work.
However, a successful program would not solely focus on luring young and promising junior faculty, he said. A supportive atmosphere requires collaboration and support to flow both up and down the ranks of seniority where everyone benefits.As an example, he singled out midlevel faculty as vulnerable when programs are not developed to ensure support is equally distributed. He explained that midlevel faculty members denied the encouragement available to surgeons just initiating their career can feel abandoned when they are skilled but not yet leaders in their program.
The Society of Vascular Surgery is pursing several initiatives to address the projected shortage within this specialty, according to Dr. Henke, but he argues that leaders of academic programs have a role to play in helping make the specialty attractive, particularly for those considering an academic career.
NEW YORK – Strategies for growing the pool of academic vascular surgeons might help avert the expected scarcity of physicians in this specialty, according to Peter K. Henke, MD, a professor of vascular surgery at the University of Michigan, Ann Arbor.
Dr. Henke recounted in a video interview key messages he delivered at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. He argued for going back to basics to enlist residents and fellows completing their training to stay in the specialty and consider an academic position.
Many of these steps are known, such as verifying that mentors are available to encourage skill acquisition and providing adequate time to achieve an acceptable balance of research and clinical work.
However, a successful program would not solely focus on luring young and promising junior faculty, he said. A supportive atmosphere requires collaboration and support to flow both up and down the ranks of seniority where everyone benefits.As an example, he singled out midlevel faculty as vulnerable when programs are not developed to ensure support is equally distributed. He explained that midlevel faculty members denied the encouragement available to surgeons just initiating their career can feel abandoned when they are skilled but not yet leaders in their program.
The Society of Vascular Surgery is pursing several initiatives to address the projected shortage within this specialty, according to Dr. Henke, but he argues that leaders of academic programs have a role to play in helping make the specialty attractive, particularly for those considering an academic career.
NEW YORK – Strategies for growing the pool of academic vascular surgeons might help avert the expected scarcity of physicians in this specialty, according to Peter K. Henke, MD, a professor of vascular surgery at the University of Michigan, Ann Arbor.
Dr. Henke recounted in a video interview key messages he delivered at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. He argued for going back to basics to enlist residents and fellows completing their training to stay in the specialty and consider an academic position.
Many of these steps are known, such as verifying that mentors are available to encourage skill acquisition and providing adequate time to achieve an acceptable balance of research and clinical work.
However, a successful program would not solely focus on luring young and promising junior faculty, he said. A supportive atmosphere requires collaboration and support to flow both up and down the ranks of seniority where everyone benefits.As an example, he singled out midlevel faculty as vulnerable when programs are not developed to ensure support is equally distributed. He explained that midlevel faculty members denied the encouragement available to surgeons just initiating their career can feel abandoned when they are skilled but not yet leaders in their program.
The Society of Vascular Surgery is pursing several initiatives to address the projected shortage within this specialty, according to Dr. Henke, but he argues that leaders of academic programs have a role to play in helping make the specialty attractive, particularly for those considering an academic career.
REPORTING FROM VEITHSYMPOSIUM
Drug-coated balloon advantage persists in femoral artery disease
NEW YORK – John Laird, MD, of the Adventist Heart Institute, St. Helena, Calif., presented the data at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
The data were drawn from the IN.PACT trial. In this trial, 331 patients were randomized to a paclitaxel-coated DCB device or standard percutaneous balloon angioplasty (PCBA), Dr. Laird explained.
The 5-year results are consistent with those previously reported at 1, 2, and 3 years. According to Dr. Laird, DCB continues to show an advantage for major outcomes over PCBA, and adverse events remain low.
Three DCB devices now available in the United States for dilatation of narrowed SFA. Although all have been associated with a reduced risk of target lesion revascularization relative to standard PCBA, the long-term follow-up presented from IN.PACT by Dr. Laird are the first to document 5-year outcomes.
In a video interview, Dr. Laird reported that there have been no thrombotic events since the 3-year results were presented.
Overall, he explains that the long-term outcomes provide additional confirmation that DCB is a safe procedure that reduces the need for stenting in SFA occlusions. Although he believes there might be clinically significant differences between available DCB devices, he concludes that DCB can be considered the first-line therapy for treating occluded femoral-popliteal arteries.
NEW YORK – John Laird, MD, of the Adventist Heart Institute, St. Helena, Calif., presented the data at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
The data were drawn from the IN.PACT trial. In this trial, 331 patients were randomized to a paclitaxel-coated DCB device or standard percutaneous balloon angioplasty (PCBA), Dr. Laird explained.
The 5-year results are consistent with those previously reported at 1, 2, and 3 years. According to Dr. Laird, DCB continues to show an advantage for major outcomes over PCBA, and adverse events remain low.
Three DCB devices now available in the United States for dilatation of narrowed SFA. Although all have been associated with a reduced risk of target lesion revascularization relative to standard PCBA, the long-term follow-up presented from IN.PACT by Dr. Laird are the first to document 5-year outcomes.
In a video interview, Dr. Laird reported that there have been no thrombotic events since the 3-year results were presented.
Overall, he explains that the long-term outcomes provide additional confirmation that DCB is a safe procedure that reduces the need for stenting in SFA occlusions. Although he believes there might be clinically significant differences between available DCB devices, he concludes that DCB can be considered the first-line therapy for treating occluded femoral-popliteal arteries.
NEW YORK – John Laird, MD, of the Adventist Heart Institute, St. Helena, Calif., presented the data at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
The data were drawn from the IN.PACT trial. In this trial, 331 patients were randomized to a paclitaxel-coated DCB device or standard percutaneous balloon angioplasty (PCBA), Dr. Laird explained.
The 5-year results are consistent with those previously reported at 1, 2, and 3 years. According to Dr. Laird, DCB continues to show an advantage for major outcomes over PCBA, and adverse events remain low.
Three DCB devices now available in the United States for dilatation of narrowed SFA. Although all have been associated with a reduced risk of target lesion revascularization relative to standard PCBA, the long-term follow-up presented from IN.PACT by Dr. Laird are the first to document 5-year outcomes.
In a video interview, Dr. Laird reported that there have been no thrombotic events since the 3-year results were presented.
Overall, he explains that the long-term outcomes provide additional confirmation that DCB is a safe procedure that reduces the need for stenting in SFA occlusions. Although he believes there might be clinically significant differences between available DCB devices, he concludes that DCB can be considered the first-line therapy for treating occluded femoral-popliteal arteries.
REPORTING FROM VEITHSYMPOSIUM
What are the barriers to solving the upcoming vascular surgeon shortage?
NEW YORK – Increasing the number of 0+5 integrated vascular surgery residency programs would help to alleviate a projected shortage of vascular surgeons, according to William D. Jordan, Jr., MD, professor of surgery, Emory University, Atlanta.*
“Ultimately the question is whether the workforce pipeline is large enough,” Dr. Jordan said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “When you consider that there are little more than 600 vascular trainees right now, and almost 600 planned retirements over the next 5 years, the answer to the question is no. Our workforce pipeline is not big enough.”
Dr. Jordan pointed out that, in addition, if one considers the current geographic distribution of vascular surgeons across the country, and go with the new standard that 1.4 surgeons are needed per 100,000 population, there is not a single state in the country that matches up to that goal. “So we are clearly going to have a shortage,” he commented. The only way to fill that shortage is to produce more vascular surgeons. But how does the change to a 0+5 residency program model impact that need?
In a survey conducted by the Association of Program Directors in Vascular Surgery in 2016, regarding challenges as perceived by the trainees, the top two concerns expressed were regarding competing specialties and physician burnout. Statistics bear out the concern regarding competing specialties, for example, there is an increase of 85% in interventional cardiology trainees being produced and a nearly 50% increase in interventional radiology trainees. However, in vascular, it is only 18%. With regard to the goals of those vascular trainees, 90% indicated that they wanted to be attached to some academic or teaching environment. “They don’t want to be the lone wolf out there,” Dr. Jordan said, and this is from concerns regarding workload, mentorship, and camaraderie, as well as regulatory and administrative obligations that are steadily increasing and can be handled more easily in a large institution. This will not fill the need for vascular surgeons in community hospitals, creating a shortage of distribution as well as actual numbers.
One key problem with current training is the fact that the new form of student comes with almost no real surgical skills and there is a dearth of vascular surgery cases available to fully accommodate many of them throughout their training career. This is a problem exacerbated by some residency review committees, which are loathe to give vascular surgery cases to new trainees.
Integrated vascular surgery residency programs have grown and there is a substantially greater interest in them, receiving even more applicants than orthopedics or neurosurgery. U.S. interest exceeds the number of 0+5 positions available. One way to deal with the projected 31% deficit in vascular surgeons by 2025 would thus be to increase the number of these training positions. The financial accommodations to do this would be large, but perhaps the creation of an independent vascular surgery specialty board would facilitate dealing with that issue, he concluded.
Dr. Jordan reported no disclosures relevant to his talk.
Correction, 11/19/18: An earlier version of this article misidentified the speaker in the session. The speaker was William D. Jordan, Jr., MD.
NEW YORK – Increasing the number of 0+5 integrated vascular surgery residency programs would help to alleviate a projected shortage of vascular surgeons, according to William D. Jordan, Jr., MD, professor of surgery, Emory University, Atlanta.*
“Ultimately the question is whether the workforce pipeline is large enough,” Dr. Jordan said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “When you consider that there are little more than 600 vascular trainees right now, and almost 600 planned retirements over the next 5 years, the answer to the question is no. Our workforce pipeline is not big enough.”
Dr. Jordan pointed out that, in addition, if one considers the current geographic distribution of vascular surgeons across the country, and go with the new standard that 1.4 surgeons are needed per 100,000 population, there is not a single state in the country that matches up to that goal. “So we are clearly going to have a shortage,” he commented. The only way to fill that shortage is to produce more vascular surgeons. But how does the change to a 0+5 residency program model impact that need?
In a survey conducted by the Association of Program Directors in Vascular Surgery in 2016, regarding challenges as perceived by the trainees, the top two concerns expressed were regarding competing specialties and physician burnout. Statistics bear out the concern regarding competing specialties, for example, there is an increase of 85% in interventional cardiology trainees being produced and a nearly 50% increase in interventional radiology trainees. However, in vascular, it is only 18%. With regard to the goals of those vascular trainees, 90% indicated that they wanted to be attached to some academic or teaching environment. “They don’t want to be the lone wolf out there,” Dr. Jordan said, and this is from concerns regarding workload, mentorship, and camaraderie, as well as regulatory and administrative obligations that are steadily increasing and can be handled more easily in a large institution. This will not fill the need for vascular surgeons in community hospitals, creating a shortage of distribution as well as actual numbers.
One key problem with current training is the fact that the new form of student comes with almost no real surgical skills and there is a dearth of vascular surgery cases available to fully accommodate many of them throughout their training career. This is a problem exacerbated by some residency review committees, which are loathe to give vascular surgery cases to new trainees.
Integrated vascular surgery residency programs have grown and there is a substantially greater interest in them, receiving even more applicants than orthopedics or neurosurgery. U.S. interest exceeds the number of 0+5 positions available. One way to deal with the projected 31% deficit in vascular surgeons by 2025 would thus be to increase the number of these training positions. The financial accommodations to do this would be large, but perhaps the creation of an independent vascular surgery specialty board would facilitate dealing with that issue, he concluded.
Dr. Jordan reported no disclosures relevant to his talk.
Correction, 11/19/18: An earlier version of this article misidentified the speaker in the session. The speaker was William D. Jordan, Jr., MD.
NEW YORK – Increasing the number of 0+5 integrated vascular surgery residency programs would help to alleviate a projected shortage of vascular surgeons, according to William D. Jordan, Jr., MD, professor of surgery, Emory University, Atlanta.*
“Ultimately the question is whether the workforce pipeline is large enough,” Dr. Jordan said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “When you consider that there are little more than 600 vascular trainees right now, and almost 600 planned retirements over the next 5 years, the answer to the question is no. Our workforce pipeline is not big enough.”
Dr. Jordan pointed out that, in addition, if one considers the current geographic distribution of vascular surgeons across the country, and go with the new standard that 1.4 surgeons are needed per 100,000 population, there is not a single state in the country that matches up to that goal. “So we are clearly going to have a shortage,” he commented. The only way to fill that shortage is to produce more vascular surgeons. But how does the change to a 0+5 residency program model impact that need?
In a survey conducted by the Association of Program Directors in Vascular Surgery in 2016, regarding challenges as perceived by the trainees, the top two concerns expressed were regarding competing specialties and physician burnout. Statistics bear out the concern regarding competing specialties, for example, there is an increase of 85% in interventional cardiology trainees being produced and a nearly 50% increase in interventional radiology trainees. However, in vascular, it is only 18%. With regard to the goals of those vascular trainees, 90% indicated that they wanted to be attached to some academic or teaching environment. “They don’t want to be the lone wolf out there,” Dr. Jordan said, and this is from concerns regarding workload, mentorship, and camaraderie, as well as regulatory and administrative obligations that are steadily increasing and can be handled more easily in a large institution. This will not fill the need for vascular surgeons in community hospitals, creating a shortage of distribution as well as actual numbers.
One key problem with current training is the fact that the new form of student comes with almost no real surgical skills and there is a dearth of vascular surgery cases available to fully accommodate many of them throughout their training career. This is a problem exacerbated by some residency review committees, which are loathe to give vascular surgery cases to new trainees.
Integrated vascular surgery residency programs have grown and there is a substantially greater interest in them, receiving even more applicants than orthopedics or neurosurgery. U.S. interest exceeds the number of 0+5 positions available. One way to deal with the projected 31% deficit in vascular surgeons by 2025 would thus be to increase the number of these training positions. The financial accommodations to do this would be large, but perhaps the creation of an independent vascular surgery specialty board would facilitate dealing with that issue, he concluded.
Dr. Jordan reported no disclosures relevant to his talk.
Correction, 11/19/18: An earlier version of this article misidentified the speaker in the session. The speaker was William D. Jordan, Jr., MD.
REPORTING FROM THE VEITHSYMPOSIUM
A bovine arch predicts worse outcomes with type B aortic dissections
NEW YORK – The presence of a bovine arch predicts higher mortality in patients with a type B aortic dissection (TBAD), according to a study presented by Jan S. Brunkwall, MD, at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
The bovine arch is a congenital interruption in the evolution of the arch, and is a misnomer because it does not actually reflect the arch branching pattern found in cattle. It represents the most common variation of the aortic arch, with a prevalence of 1%-41%, depending on the literature, according to a study published by Dr. Brunkwall, chairman of the department of vascular and endovascular surgery at the University of Cologne (Germany), and his colleagues (Eur J Vasc Endovasc Surg. 2018; 55:385-391).
In order to assess the effect of the bovine arch on survival, Dr. Brunkwall and his colleagues performed a retrospective cohort analysis of patients with TBAD admitted at two centers. CT angiograms (CTAs) of patients referred because of aortic dissection were also reevaluated with regard to the presence of a bovine arch.
A total of 154 patients with TBAD and 168 with type A aortic dissection were assessed, and 110 oncologic patients who had undergone a chest CTA for disease staging during the study period acted as a control group.
There was an overall prevalence of 17.6% for bovine arch variants, with no statistical difference in prevalence between patients with a dissection and those in the control group, or between patients with a type A or type B dissection. However, mortality was 34.5% in patients with TBAD who had a bovine arch versus 16% in patients without a bovine arch. This was a significant difference (P =.04), according to Dr. Brunkwall.
Multivariate analysis showed that the presence of a bovine arch with TBAD was an independent predictor of mortality. “The reason for the high mortality cannot be explained by our data,” said Dr. Brunkwall, “but there has been a suggestion that the shear stress is different and higher in patients with a bovine arch leading to a stiffer aorta and more endothelial damage.”
Dr. Brunkwall reported that he had no disclosures.
NEW YORK – The presence of a bovine arch predicts higher mortality in patients with a type B aortic dissection (TBAD), according to a study presented by Jan S. Brunkwall, MD, at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
The bovine arch is a congenital interruption in the evolution of the arch, and is a misnomer because it does not actually reflect the arch branching pattern found in cattle. It represents the most common variation of the aortic arch, with a prevalence of 1%-41%, depending on the literature, according to a study published by Dr. Brunkwall, chairman of the department of vascular and endovascular surgery at the University of Cologne (Germany), and his colleagues (Eur J Vasc Endovasc Surg. 2018; 55:385-391).
In order to assess the effect of the bovine arch on survival, Dr. Brunkwall and his colleagues performed a retrospective cohort analysis of patients with TBAD admitted at two centers. CT angiograms (CTAs) of patients referred because of aortic dissection were also reevaluated with regard to the presence of a bovine arch.
A total of 154 patients with TBAD and 168 with type A aortic dissection were assessed, and 110 oncologic patients who had undergone a chest CTA for disease staging during the study period acted as a control group.
There was an overall prevalence of 17.6% for bovine arch variants, with no statistical difference in prevalence between patients with a dissection and those in the control group, or between patients with a type A or type B dissection. However, mortality was 34.5% in patients with TBAD who had a bovine arch versus 16% in patients without a bovine arch. This was a significant difference (P =.04), according to Dr. Brunkwall.
Multivariate analysis showed that the presence of a bovine arch with TBAD was an independent predictor of mortality. “The reason for the high mortality cannot be explained by our data,” said Dr. Brunkwall, “but there has been a suggestion that the shear stress is different and higher in patients with a bovine arch leading to a stiffer aorta and more endothelial damage.”
Dr. Brunkwall reported that he had no disclosures.
NEW YORK – The presence of a bovine arch predicts higher mortality in patients with a type B aortic dissection (TBAD), according to a study presented by Jan S. Brunkwall, MD, at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
The bovine arch is a congenital interruption in the evolution of the arch, and is a misnomer because it does not actually reflect the arch branching pattern found in cattle. It represents the most common variation of the aortic arch, with a prevalence of 1%-41%, depending on the literature, according to a study published by Dr. Brunkwall, chairman of the department of vascular and endovascular surgery at the University of Cologne (Germany), and his colleagues (Eur J Vasc Endovasc Surg. 2018; 55:385-391).
In order to assess the effect of the bovine arch on survival, Dr. Brunkwall and his colleagues performed a retrospective cohort analysis of patients with TBAD admitted at two centers. CT angiograms (CTAs) of patients referred because of aortic dissection were also reevaluated with regard to the presence of a bovine arch.
A total of 154 patients with TBAD and 168 with type A aortic dissection were assessed, and 110 oncologic patients who had undergone a chest CTA for disease staging during the study period acted as a control group.
There was an overall prevalence of 17.6% for bovine arch variants, with no statistical difference in prevalence between patients with a dissection and those in the control group, or between patients with a type A or type B dissection. However, mortality was 34.5% in patients with TBAD who had a bovine arch versus 16% in patients without a bovine arch. This was a significant difference (P =.04), according to Dr. Brunkwall.
Multivariate analysis showed that the presence of a bovine arch with TBAD was an independent predictor of mortality. “The reason for the high mortality cannot be explained by our data,” said Dr. Brunkwall, “but there has been a suggestion that the shear stress is different and higher in patients with a bovine arch leading to a stiffer aorta and more endothelial damage.”
Dr. Brunkwall reported that he had no disclosures.
REPORTING FROM VEITHSYMPOSIUM
Renal vein thrombosis and pulmonary embolism
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
A 49-year-old man developed nephrotic-range proteinuria (urine protein–creatinine ratio 4.1 g/g), and primary membranous nephropathy was diagnosed by kidney biopsy. He declined therapy apart from angiotensin receptor blockade.
Five months after undergoing the biopsy, he presented to the emergency room with marked dyspnea, cough, and epigastric discomfort. His blood pressure was 160/100 mm Hg, heart rate 95 beats/minute, and oxygen saturation by pulse oximetry 97% at rest on ambient air, decreasing to 92% with ambulation.
Initial laboratory testing results were as follows:
- Sodium 135 mmol/L (reference range 136–144)
- Potassium 3.9 mmol/L (3.7–5.1)
- Chloride 104 mmol/L (97–105)
- Bicarbonate 21 mmol/L (22–30)
- Blood urea nitrogen 14 mg/dL (9–24)
- Serum creatinine 1.1 mg/dL (0.73–1.22)
- Albumin 2.1 g/dL (3.4–4.9).
Urinalysis revealed the following:
- 5 red blood cells per high-power field, compared with 1 to 2 previously
- 3+ proteinuria
- Urine protein–creatinine ratio 11 g/g
- No glucosuria.
Electrocardiography revealed normal sinus rhythm without ischemic changes. Chest radiography did not show consolidation.
At 7 months after the thrombotic event, there was no evidence of residual renal vein thrombosis on magnetic resonance venography, and at 14 months his serum creatinine level was 0.9 mg/dL, albumin 4.0 g/dL, and urine protein–creatinine ratio 0.8 g/g.
RENAL VEIN THROMBOSIS: RISK FACTORS AND CLINICAL FEATURES
Severe hypoalbuminemia in the setting of nephrotic syndrome due to membranous nephropathy is associated with the highest risk of venous thromboembolic events, with renal vein thrombus being the classic complication.1 Venous thromboembolic events also occur in other nephrotic syndromes, albeit at a lower frequency.2
Venous thromboembolic events are estimated to occur in 7% to 33% of patients with membranous glomerulopathy, with albumin levels less than 2.8 g/dL considered a notable risk factor.1,2
While often a chronic complication, acute renal vein thrombosis may present with flank pain and hematuria.3 In our patient, the dramatic increase in proteinuria and possibly the increase in hematuria suggested renal vein thrombosis. Proximal tubular dysfunction, such as glucosuria, can be seen on occasion.
DIAGNOSIS AND TREATMENT
Screening asymptomatic patients for renal vein thrombosis is not recommended, and the decision to start prophylactic anticoagulation must be individualized.4
Although renal venography historically was the gold standard test to diagnose renal vein thrombosis, it has been replaced by noninvasive imaging such as computed tomography and magnetic resonance venography.
While anticoagulation remains the treatment of choice, catheter-directed thrombectomy or surgical thrombectomy can be considered for some patients with acute renal vein thrombosis.5
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol 2017; 12(6):983–997. doi:10.2215/CJN.11761116
- Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81(2):190–195. doi:10.1038/ki.2011.312
- Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7(1):43–51. doi:10.2215/CJN.04250511
- Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85(6):1412–1420. doi:10.1038/ki.2013.476
- Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG. Percutaneous mechanical thrombectomy: a new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 2002; 17(6):1122–1125. pmid:12032209
Back pain as a sign of inferior vena cava filter complications
A 63-year-old woman presented with an acute exacerbation of chronic back pain after a fall. She was taking warfarin because of a history of factor V Leiden, deep vein thrombosis, and pulmonary embolism, for which a temporary inferior vena cava (IVC) filter had been placed 8 years ago. Her physicians had subsequently tried to remove the filter, without success. Some time after that, 1 of the filter struts had been removed after it migrated through her abdominal wall.
Laboratory testing revealed a supratherapeutic international normalized ratio of 8.5.
The patient underwent endovascular aneurysm repair with adequate placement of a vascular graft. She was discharged on therapeutic anticoagulation, and her back pain had notably improved.
COMPLICATIONS OF IVC FILTERS
In the United States, the use of IVC filters has increased significantly over the last decade, with placement rates ranging from 12% to 17% in patients with venous thromboembolism.1
The American Heart Association recommends filter placement for patients with venous thromboembolism for whom anticoagulation has failed or is contraindicated, patients unable to withstand pulmonary embolism, and patients who are hemodynamically unstable.2 While indications vary in the guidelines released by different societies, filters are most often placed in patients who have an acute bleed, significant surgery after admission for venous thromboembolism, metastatic cancer, and severe illness.3
Complications can occur during and after insertion and during removal. They are more frequent with temporary than with permanent filters, and include filter movement and fracture as well as occlusion and penetration.4,5
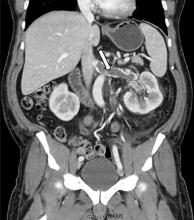
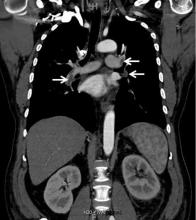
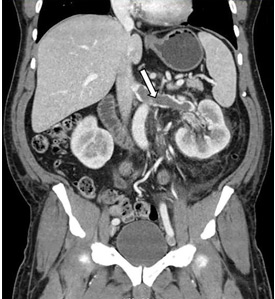
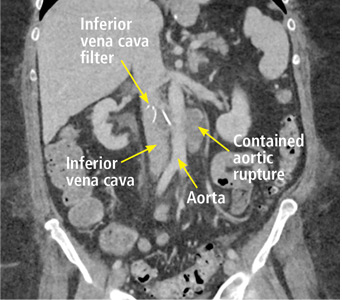
In our patient, we believe that the 3 remaining filter struts likely penetrated the wall of the IVC to the extent that they encountered adjacent structures (aorta, duodenum, kidney).
Of cases of IVC filter penetration reported to a US Food and Drug Administration database, 13.1% involved small bowel perforation, 6.5% involved aortic perforation, and 4.2% involved retroperitoneal bleeding. Symptoms such as abdominal and back pain were present in 38.3% of cases involving IVC penetration.5
Therefore, the differential diagnosis for patients with a history of IVC filter placement presenting with these symptoms should address filter complications, including occlusion, incorrect placement, fracture, migration, and penetration of the filter.4 If complications occur, treatment options include anticoagulation, endovascular repair, and surgical intervention.
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014; 177(3):742–743. doi:10.1016/j.ijcard.2014.08.010
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
- White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013; 173(7):506–512. doi:10.1001/jamainternmed.2013.2352
- Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26(1):23–28. doi:10.1053/j.semvascsurg.2013.04.005
- Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014; 25(8):1181–1185. doi:10.1016/j.jvir.2014.04.016
A 63-year-old woman presented with an acute exacerbation of chronic back pain after a fall. She was taking warfarin because of a history of factor V Leiden, deep vein thrombosis, and pulmonary embolism, for which a temporary inferior vena cava (IVC) filter had been placed 8 years ago. Her physicians had subsequently tried to remove the filter, without success. Some time after that, 1 of the filter struts had been removed after it migrated through her abdominal wall.
Laboratory testing revealed a supratherapeutic international normalized ratio of 8.5.
The patient underwent endovascular aneurysm repair with adequate placement of a vascular graft. She was discharged on therapeutic anticoagulation, and her back pain had notably improved.
COMPLICATIONS OF IVC FILTERS
In the United States, the use of IVC filters has increased significantly over the last decade, with placement rates ranging from 12% to 17% in patients with venous thromboembolism.1
The American Heart Association recommends filter placement for patients with venous thromboembolism for whom anticoagulation has failed or is contraindicated, patients unable to withstand pulmonary embolism, and patients who are hemodynamically unstable.2 While indications vary in the guidelines released by different societies, filters are most often placed in patients who have an acute bleed, significant surgery after admission for venous thromboembolism, metastatic cancer, and severe illness.3
Complications can occur during and after insertion and during removal. They are more frequent with temporary than with permanent filters, and include filter movement and fracture as well as occlusion and penetration.4,5
In our patient, we believe that the 3 remaining filter struts likely penetrated the wall of the IVC to the extent that they encountered adjacent structures (aorta, duodenum, kidney).
Of cases of IVC filter penetration reported to a US Food and Drug Administration database, 13.1% involved small bowel perforation, 6.5% involved aortic perforation, and 4.2% involved retroperitoneal bleeding. Symptoms such as abdominal and back pain were present in 38.3% of cases involving IVC penetration.5
Therefore, the differential diagnosis for patients with a history of IVC filter placement presenting with these symptoms should address filter complications, including occlusion, incorrect placement, fracture, migration, and penetration of the filter.4 If complications occur, treatment options include anticoagulation, endovascular repair, and surgical intervention.
A 63-year-old woman presented with an acute exacerbation of chronic back pain after a fall. She was taking warfarin because of a history of factor V Leiden, deep vein thrombosis, and pulmonary embolism, for which a temporary inferior vena cava (IVC) filter had been placed 8 years ago. Her physicians had subsequently tried to remove the filter, without success. Some time after that, 1 of the filter struts had been removed after it migrated through her abdominal wall.
Laboratory testing revealed a supratherapeutic international normalized ratio of 8.5.
The patient underwent endovascular aneurysm repair with adequate placement of a vascular graft. She was discharged on therapeutic anticoagulation, and her back pain had notably improved.
COMPLICATIONS OF IVC FILTERS
In the United States, the use of IVC filters has increased significantly over the last decade, with placement rates ranging from 12% to 17% in patients with venous thromboembolism.1
The American Heart Association recommends filter placement for patients with venous thromboembolism for whom anticoagulation has failed or is contraindicated, patients unable to withstand pulmonary embolism, and patients who are hemodynamically unstable.2 While indications vary in the guidelines released by different societies, filters are most often placed in patients who have an acute bleed, significant surgery after admission for venous thromboembolism, metastatic cancer, and severe illness.3
Complications can occur during and after insertion and during removal. They are more frequent with temporary than with permanent filters, and include filter movement and fracture as well as occlusion and penetration.4,5
In our patient, we believe that the 3 remaining filter struts likely penetrated the wall of the IVC to the extent that they encountered adjacent structures (aorta, duodenum, kidney).
Of cases of IVC filter penetration reported to a US Food and Drug Administration database, 13.1% involved small bowel perforation, 6.5% involved aortic perforation, and 4.2% involved retroperitoneal bleeding. Symptoms such as abdominal and back pain were present in 38.3% of cases involving IVC penetration.5
Therefore, the differential diagnosis for patients with a history of IVC filter placement presenting with these symptoms should address filter complications, including occlusion, incorrect placement, fracture, migration, and penetration of the filter.4 If complications occur, treatment options include anticoagulation, endovascular repair, and surgical intervention.
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014; 177(3):742–743. doi:10.1016/j.ijcard.2014.08.010
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
- White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013; 173(7):506–512. doi:10.1001/jamainternmed.2013.2352
- Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26(1):23–28. doi:10.1053/j.semvascsurg.2013.04.005
- Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014; 25(8):1181–1185. doi:10.1016/j.jvir.2014.04.016
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014; 177(3):742–743. doi:10.1016/j.ijcard.2014.08.010
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16):1788–1830. doi:10.1161/CIR.0b013e318214914f
- White RH, Geraghty EM, Brunson A, et al. High variation between hospitals in vena cava filter use for venous thromboembolism. JAMA Intern Med 2013; 173(7):506–512. doi:10.1001/jamainternmed.2013.2352
- Sella DM, Oldenburg WA. Complications of inferior vena cava filters. Semin Vasc Surg 2013; 26(1):23–28. doi:10.1053/j.semvascsurg.2013.04.005
- Andreoli JM, Lewandowski RJ, Vogelzang RL, Ryu RK. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014; 25(8):1181–1185. doi:10.1016/j.jvir.2014.04.016
Which patients with pulmonary embolism need echocardiography?
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
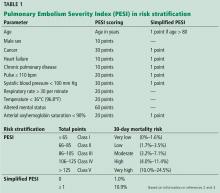
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
Most patients admitted with pulmonary embolism (PE) do not need transthoracic echocardiography (TTE); it should be performed in hemodynamically unstable patients, as well as in hemodynamically stable patients with specific elevated cardiac biomarkers and imaging features.
The decision to perform TTE should be based on clinical presentation, PE burden, and imaging findings (eg, computed tomographic angiography). TTE helps to stratify risk, guide management, monitor response to therapy, and give prognostic information for a subset of patients at increased risk for PE-related adverse events.
RISK STRATIFICATION IN PULMONARY EMBOLISM
PE has a spectrum of presentations ranging from no symptoms to shock. Based on the clinical presentation, PE can be categorized as high, intermediate, or low risk.
High-risk PE, often referred to as “massive” PE, is defined in current American Heart Association guidelines as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for at least 15 minutes or requiring inotropic support), persistent profound bradycardia (heart rate < 40 beats per minute with signs or symptoms of shock), syncope, or cardiac arrest.1
Intermediate-risk or “submassive” PE is more challenging to identify because patients are more hemodynamically stable, yet have evidence on electrocardiography, TTE, computed tomography, or cardiac biomarker testing—ie, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or troponin—that indicates myocardial injury or volume overload.1
Low-risk PE is acute PE in the absence of clinical markers of adverse prognosis that define massive or submassive PE.1
ECHOCARDIOGRAPHIC FEATURES OF HIGH-RISK PULMONARY EMBOLISM
Certain TTE findings suggest increased risk of a poor outcome and may warrant therapy that is more invasive and aggressive. High-risk features include the following:
- Impaired right ventricular function
- Interventricular septum bulging into the left ventricle (“D-shaped” septum)
- Dilated proximal pulmonary arteries
- Increased severity of tricuspid regurgitation
- Elevated right atrial pressure
- Elevated pulmonary artery pressure
- Free-floating right ventricular thrombi, which are associated with a mortality rate of up to 45% and can be detected in 7% to 18% of patients6
- Tricuspid annular plane systolic excursion, an echocardiographic measure of right ventricular function1; a value less than 17 mm suggests impaired right ventricular systolic function7
- The McConnell sign, a feature of acute massive PE: akinesia of the mid-free wall of the right ventricle and hypercontractility of the apex.
These TTE findings often lead to treatment with thrombolysis, transfer to the intensive care unit, and activation of the interventional team for catheter-based therapies.1,8 Free-floating right heart thrombi or thrombus straddling the interatrial septum (“thrombus in transit”) through a patent foramen ovale may require surgical embolectomy.8
PATIENT SELECTION AND INDICATIONS FOR ECHOCARDIOGRAPHY
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123:1788–1830. doi:10.1161/CIR.0b013e318214914f
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389. doi:10.1001/archinternmed.2010.199
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046. doi:10.1164/rccm.200506-862OC
- Bova C, Pesavento R, Marchiori A, et al; TELESIO Study Group. Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism. J Thromb Haemost 2009; 7:938–944. doi:10.1111/j.1538-7836.2009.03345.x
- Fernandez C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest 2015; 148:211–218. doi:10.1378/chest.14-2551
- Chartier L, Bera J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999; 99:2779–2783. pmid:10351972
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults. J Am Soc Echocardiogr 2010; 23:685–713. doi:10.1016/j.echo.2010.05.010
- Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033–3069a–k. doi:10.1093/eurheartj/ehu283
- Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29:1–42. doi:10.1016/j.echo.2015.09.011