User login
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
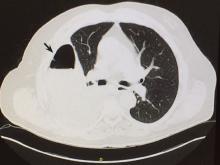
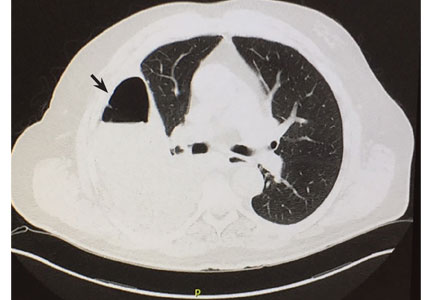
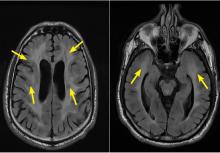
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
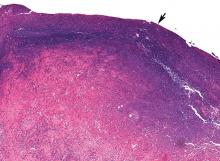
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
Frailty tied to higher mortality after major vascular surgery
Frailty defined as functional dependence is a predictor of mortality risk in elderly patients having major vascular surgery, a meta-analysis of studies has found
“Functional dependency may be recommended for use in rapid screening for frailty in major vascular surgery because of the high quality of associated evidence. Additionally, information on central muscle mass also adds incremental predictive value to long-term survival of elderly patients after major vascular surgery,” the study investigaters stated. However, they pointed out that “other newly developed frailty tools require further validation in more studies” before they should be adopted.
The report, published in the European Journal of Vascular and Endovascular Surgery, evaluated the effect of frailty in major vascular surgery from a search of MEDLINE, Embase, Cochrane Database, and Scopus through May 2018. Data were extracted from the articles related to surgery for abdominal aortic aneurysms (AAA) and lower extremity artery disease (LEAD), and a modified Newcastle-Ottawa scale was used to assess the quality of the included studies, according to Jiarong Wang, MD, of the department of vascular surgery, Sichuan University, Sichuan Province, China, and colleagues. A total of 22 cohort studies and one randomized controlled trial was used in the final analysis. The reviewers expressed the impact of frailty on outcomes as odds ratios (OR) or hazard ratios (HR) using a random effects model.
The researchers found that frailty, in terms of functional dependence, was associated with a significantly increased 30-day mortality risk in patients with AAA without heterogeneity (OR 5.15) and also in LEAD patients (OR 3.29). Functionally dependent patients also had a significantly increased 30-day mortality risk, compared with independent patients (OR 4.49), and similar results were observed after stratifying those who underwent AAA repair (OR 5.14) or lower extremity revascularization (OR 4.18). Even for patients who underwent endovascular procedures rather than open surgery, functional dependency was also associated with a significantly increased 30-day mortality risk (OR 4.90). In addition, with regard to 30-day morbidity, frailty was associated with a significantly increased risk in both AAA (OR 2.79) and LEAD (OR 1.40) patients.
As far as long-term outcomes were concerned, frailty was associated with a significantly increased risk of long-term all-cause mortality in the overall studied population (HR 2.22), as well as in patients with AAA repair (HR 2.10) and LEAD revascularization (HR 2.46). Dr. Wang and colleagues found that central muscle mass was the only tool with moderate quality of evidence predicting long-term survival after major vascular surgery (HR .48), with other single-domain tools such as nutrition or cognition scoring being of low quality. The modified Frailty Index was the only multi-domain tool with moderate quality in predicting mortality for AAA, while others were scored as low or very low, the authors added.
“Future research is warranted to establish consensus on how to select the optimal frailty tool for certain clinical settings,” they concluded.
The authors reported that they had no conflicts of interest and no funding sources for the study.
SOURCE: Wang, J et al. Eur J Vasc Endovasc Surg. 2018;56:591-602.
Frailty defined as functional dependence is a predictor of mortality risk in elderly patients having major vascular surgery, a meta-analysis of studies has found
“Functional dependency may be recommended for use in rapid screening for frailty in major vascular surgery because of the high quality of associated evidence. Additionally, information on central muscle mass also adds incremental predictive value to long-term survival of elderly patients after major vascular surgery,” the study investigaters stated. However, they pointed out that “other newly developed frailty tools require further validation in more studies” before they should be adopted.
The report, published in the European Journal of Vascular and Endovascular Surgery, evaluated the effect of frailty in major vascular surgery from a search of MEDLINE, Embase, Cochrane Database, and Scopus through May 2018. Data were extracted from the articles related to surgery for abdominal aortic aneurysms (AAA) and lower extremity artery disease (LEAD), and a modified Newcastle-Ottawa scale was used to assess the quality of the included studies, according to Jiarong Wang, MD, of the department of vascular surgery, Sichuan University, Sichuan Province, China, and colleagues. A total of 22 cohort studies and one randomized controlled trial was used in the final analysis. The reviewers expressed the impact of frailty on outcomes as odds ratios (OR) or hazard ratios (HR) using a random effects model.
The researchers found that frailty, in terms of functional dependence, was associated with a significantly increased 30-day mortality risk in patients with AAA without heterogeneity (OR 5.15) and also in LEAD patients (OR 3.29). Functionally dependent patients also had a significantly increased 30-day mortality risk, compared with independent patients (OR 4.49), and similar results were observed after stratifying those who underwent AAA repair (OR 5.14) or lower extremity revascularization (OR 4.18). Even for patients who underwent endovascular procedures rather than open surgery, functional dependency was also associated with a significantly increased 30-day mortality risk (OR 4.90). In addition, with regard to 30-day morbidity, frailty was associated with a significantly increased risk in both AAA (OR 2.79) and LEAD (OR 1.40) patients.
As far as long-term outcomes were concerned, frailty was associated with a significantly increased risk of long-term all-cause mortality in the overall studied population (HR 2.22), as well as in patients with AAA repair (HR 2.10) and LEAD revascularization (HR 2.46). Dr. Wang and colleagues found that central muscle mass was the only tool with moderate quality of evidence predicting long-term survival after major vascular surgery (HR .48), with other single-domain tools such as nutrition or cognition scoring being of low quality. The modified Frailty Index was the only multi-domain tool with moderate quality in predicting mortality for AAA, while others were scored as low or very low, the authors added.
“Future research is warranted to establish consensus on how to select the optimal frailty tool for certain clinical settings,” they concluded.
The authors reported that they had no conflicts of interest and no funding sources for the study.
SOURCE: Wang, J et al. Eur J Vasc Endovasc Surg. 2018;56:591-602.
Frailty defined as functional dependence is a predictor of mortality risk in elderly patients having major vascular surgery, a meta-analysis of studies has found
“Functional dependency may be recommended for use in rapid screening for frailty in major vascular surgery because of the high quality of associated evidence. Additionally, information on central muscle mass also adds incremental predictive value to long-term survival of elderly patients after major vascular surgery,” the study investigaters stated. However, they pointed out that “other newly developed frailty tools require further validation in more studies” before they should be adopted.
The report, published in the European Journal of Vascular and Endovascular Surgery, evaluated the effect of frailty in major vascular surgery from a search of MEDLINE, Embase, Cochrane Database, and Scopus through May 2018. Data were extracted from the articles related to surgery for abdominal aortic aneurysms (AAA) and lower extremity artery disease (LEAD), and a modified Newcastle-Ottawa scale was used to assess the quality of the included studies, according to Jiarong Wang, MD, of the department of vascular surgery, Sichuan University, Sichuan Province, China, and colleagues. A total of 22 cohort studies and one randomized controlled trial was used in the final analysis. The reviewers expressed the impact of frailty on outcomes as odds ratios (OR) or hazard ratios (HR) using a random effects model.
The researchers found that frailty, in terms of functional dependence, was associated with a significantly increased 30-day mortality risk in patients with AAA without heterogeneity (OR 5.15) and also in LEAD patients (OR 3.29). Functionally dependent patients also had a significantly increased 30-day mortality risk, compared with independent patients (OR 4.49), and similar results were observed after stratifying those who underwent AAA repair (OR 5.14) or lower extremity revascularization (OR 4.18). Even for patients who underwent endovascular procedures rather than open surgery, functional dependency was also associated with a significantly increased 30-day mortality risk (OR 4.90). In addition, with regard to 30-day morbidity, frailty was associated with a significantly increased risk in both AAA (OR 2.79) and LEAD (OR 1.40) patients.
As far as long-term outcomes were concerned, frailty was associated with a significantly increased risk of long-term all-cause mortality in the overall studied population (HR 2.22), as well as in patients with AAA repair (HR 2.10) and LEAD revascularization (HR 2.46). Dr. Wang and colleagues found that central muscle mass was the only tool with moderate quality of evidence predicting long-term survival after major vascular surgery (HR .48), with other single-domain tools such as nutrition or cognition scoring being of low quality. The modified Frailty Index was the only multi-domain tool with moderate quality in predicting mortality for AAA, while others were scored as low or very low, the authors added.
“Future research is warranted to establish consensus on how to select the optimal frailty tool for certain clinical settings,” they concluded.
The authors reported that they had no conflicts of interest and no funding sources for the study.
SOURCE: Wang, J et al. Eur J Vasc Endovasc Surg. 2018;56:591-602.
FROM EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point: Frailty was associated with increased short- and long-term mortality in major vascular surgery.
Major finding: Frailty was associated with a fourfold increased risk of 30-day mortality and a doubled increased risk of long-term mortality after major vascular surgery.
Study details: A meta-analysis of 22 cohort studies and one randomized controlled trial.
Disclosures: The authors reported that they had no conflicts of interest and no funding sources for the study.
Source: Wang, J et al., 2018. Eur J Vasc Endovasc Surg. 56:591-602.
Vascular emergencies on the rise, but more patients surviving
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
ST. LOUIS – A patient with a nontraumatic vascular emergency is significantly less likely to die today than a decade ago, with few exceptions, according to a new national analysis looking at 10 years of data. Unsurprisingly, endovascular surgery rates climbed over the study period, as did rates of acute limb ischemia, said Todd Vogel, MD, who discussed the study at the annual meeting of the Midwestern Vascular Surgical Society.
With an objective of evaluating trends for management of nontraumatic vascular emergencies in the United States, Dr. Vogel, who is chief of vascular and endovascular surgery at the University of Missouri–Columbia, and his colleagues examined frequencies of vascular emergencies, mortality rates, and how open versus endoscopic procedure technique affected the data.
To do this, the investigators used the U.S. National Inpatient Sample from 2005 to 2014 to identify nontraumatic vascular emergencies.
Using ICD-9 clinical management diagnosis and procedure codes allowed the investigators to capture a wide array of vascular emergencies, Dr. Vogel said. These included ruptured abdominal, thoracic, and thoracoabdominal aortic aneurysms (rAAAs, rTAAs, and rTAAAs, respectively), as well as acute limb ischemia, acute mesenteric ischemia, and ruptured visceral artery aneurysms.
Among the outcomes analyzed in the study were a trend analysis looking at how outcomes changed over time and an analysis of in-hospital mortality. Dr. Vogel and his colleagues also examined hospital resource utilization including length of stay and total hospital cost, inflation adjusted to 2014 costs.
The prevalence of endovascular intervention increased sharply over the study period, as one would expect, Dr. Vogel said. “At the beginning, we had about 24% of patients getting endovascular intervention for vascular emergencies, and currently, it’s 36%.” (P for trend, less than .0001).
Mortality dropped steeply overall, with overall mortality going from 13.80% to 9.14% during the study period (P less than .0001). Much of this decrease could be attributed to mortality for open procedures decreasing by over a third, from 16.5% to 10.7%, over the study period (P less than .0001). Endovascular procedure–related mortality decreased from 8.3% to 7.9% (P = .03).
Ruptured abdominal and thoracic aortic aneurysms were much less likely to be fatal in 2014 than in 2005. The overall mortality rate for rAAA went from 41.4% to 27.6% (P less than .0001) and rates for rTAAs dropped overall from 41.2% to 23.0% (P = .002).
However, endovascular rTAA repair mortality jumped from 14.9% to 27.4% (P = .0003) while mortality for open procedures plummeted from 51.3% to 16.7% (P less than .0001).
In-hospital mortality for some conditions didn’t change much over time: rTAAA mortality, for example, increased, but by a nonsignificant amount (44.7% vs. 47.6%; P = .06). “Mortality rates for rTAAA have remained static, despite the advances in treatment,” Dr. Vogel said.
Discussing these “concerning” results, Dr. Vogel noted that the increase in mortality “suggests an increased use of endovascular repair on higher-risk patients.” The mortality rate for ruptured visceral artery aneurysms did not change significantly either (16.7% vs. 6.7%, P = .09).
Overall, patients were 44% female and 66% white. “Over half of the patients were aged 70 or greater,” he said.
Acute limb ischemia was by far the most common vascular emergency, accounting for 82.4% of the total. Next most common were rAAAs, which made up just 10.79% of the vascular emergencies studied.
Looking at hospitalization trends over time, acute limb ischemia showed a slight trend up over the study period, from an occurrence rate of about 8.2 per 100,000 individuals at the beginning to about 9.0 per 100,000 by 2014.
Acute mesenteric ischemia also trended up, from an occurrence rate of about 4 per 1 million individuals in 2005 to about 6 per 1 million in 2014; rAAAs trended down, from about 13 per 1 million to a little over 9 per 1 million over the study period.
Among the other vascular emergencies incurring hospitalization, rTAAAs and ruptured visceral artery aneurysms were both rare, occurring in fewer than 7 per 10 million individuals, but both showed a slight upward trend over the study period. Slightly more common were rTAAs, which occurred at a rate of about 12 per 10 million individuals at the beginning of the study period and at slightly less than 15 per 10 million by the end.
Looking at hospital resource utilization, length of stay dropped significantly (P less than .004), but costs, unsurprisingly, increased over the study period, from about $25,000 to about $30,000 per occurrence (P less than .0001).
“The overall frequency of vascular emergencies has significantly increased over time,” Dr. Vogel said, “but in subgroup analysis ruptured abdominal [aortic] aneurysms are decreasing.” As endovascular procedures have increased, “The overall mortality has decreased, so we actually are doing better.” Some of this drop “may be due to improved perioperative care” as well as the increase in endovascular utilization, he noted.
In sum, though mortality has generally improved as endovascular procedures have become more common in vascular emergencies, “increased implementation of endovascular repair may not always improve outcomes,” Dr. Vogel said, especially in the context of an increasingly complex and aging patient population.
Dr. Vogel reported no conflicts of interest and no outside sources of funding.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Rates of endovascular repair for nontraumatic vascular emergencies rose sharply.
Major finding: Endovascular repair rates for nontraumatic vascular emergencies climbed from 24% to 36% of cases from 2005 to 2014 (P for trend, less than .0001).
Study details: A 10-year sample of hospitalizations for nontraumatic vascular emergencies from the U.S. National Inpatient Sample.
Disclosures: Dr. Vogel reported no outside sources of funding and no conflicts of interest.
Claudication, CLI differ significantly in hospital readmission, costs, mortality
Patients treated for claudication vs. critical limb ischemia (CLI) differed significantly in their initial cost of admission, readmission costs, length of stay (LOS), days to readmission, and mortality (during initial admission, as well as any admission), according to the results of a database analysis of more than 90,000 patients in the Nationwide Readmission Database.
Readmissions were influenced not only by the admission diagnosis and intervention performed “but more importantly and significantly by the patient’s characteristics such as age, sex, CCI [Charlson Comorbidity Index], and various other demographic factors,” wrote Rennier A. Martinez, MD, of JFK Medical Center, Atlantis, Fla., and his colleagues. The report was published in the October issue of Annals of Vascular Surgery.
The study used the International Classification of Diseases, Ninth Revision (ICD-9) codes and queried the Nationwide Readmission Database for 2013 and 2014 for all 92,769 adult patients admitted with the principal diagnosis of claudication (ICD-9 code 440.21; n = 33,055 patients) or CLI (ICD-9 code 440.22e440.24; n = 59,714 patients) who underwent percutaneous angioplasty (ICD-9 code 39.50, 39.90), peripheral bypass (ICD-9 code 39.29), or aortofemoral bypass (ICD-9 code 39.25).
The 30-day readmission rates were 9.0% for claudication and 19.3% for CLI. Similarly, the any readmission rates were 21.5% and 40.4% for claudication vs. CLI.
Significant differences were found for claudication and CLI, respectively, on initial cost of admission ($18,548 vs. $29,148), readmission costs ($14,726 vs. $17,681), LOS (4 days vs. 9 days), days to readmission (73 days vs. 59 days), mortality during initial admission (256 vs. 1,363), and mortality during any admission (538 vs. 3,838), all P less than .001.
Univariate and multivariate logistic regression analysis found that claudication, CLI, angioplasty, peripheral bypass, aortofemoral bypass, female sex, age younger than 65, Charlson Comorbidity Index, LOS, and primary expected payer status were all significant predictors of 30-day and overall readmissions at varying degrees.
The researchers also found that the five most common disease readmission groups were other vascular procedures (12.6%), amputation of lower limb except toes (6.3%), sepsis (5.4%), heart failure (4.9%), and postoperative or other device infections (4.8%) (Ann Vasc Surg. 2018;52:96-107).
The increased costs and higher levels of morbidity and mortality seen with CLI vs. claudication are not surprising given previous research showing that there are higher rates of complications in patients with CLI. A previous review showed there was a threefold higher risk of myocardial infarction, stroke, and vascular death in patients with CLI compared with patients with claudication, according Dr. Martinez and his colleagues.
“Readmissions after lower extremity procedures for patients admitted for claudication or CLI are influenced not only by the admission diagnosis and intervention performed but more importantly and significantly by the patient’s characteristics such as age, sex, CCI, and various other demographic factors,” the researchers wrote. “It is paramount to continue to perform this kind of study to better identify patients at risk for readmission and work toward prevention,” they concluded.
Dr. Martinez and his colleagues did not report disclosures, but indicated that the study did not receive any outside funding.
SOURCE: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
Patients treated for claudication vs. critical limb ischemia (CLI) differed significantly in their initial cost of admission, readmission costs, length of stay (LOS), days to readmission, and mortality (during initial admission, as well as any admission), according to the results of a database analysis of more than 90,000 patients in the Nationwide Readmission Database.
Readmissions were influenced not only by the admission diagnosis and intervention performed “but more importantly and significantly by the patient’s characteristics such as age, sex, CCI [Charlson Comorbidity Index], and various other demographic factors,” wrote Rennier A. Martinez, MD, of JFK Medical Center, Atlantis, Fla., and his colleagues. The report was published in the October issue of Annals of Vascular Surgery.
The study used the International Classification of Diseases, Ninth Revision (ICD-9) codes and queried the Nationwide Readmission Database for 2013 and 2014 for all 92,769 adult patients admitted with the principal diagnosis of claudication (ICD-9 code 440.21; n = 33,055 patients) or CLI (ICD-9 code 440.22e440.24; n = 59,714 patients) who underwent percutaneous angioplasty (ICD-9 code 39.50, 39.90), peripheral bypass (ICD-9 code 39.29), or aortofemoral bypass (ICD-9 code 39.25).
The 30-day readmission rates were 9.0% for claudication and 19.3% for CLI. Similarly, the any readmission rates were 21.5% and 40.4% for claudication vs. CLI.
Significant differences were found for claudication and CLI, respectively, on initial cost of admission ($18,548 vs. $29,148), readmission costs ($14,726 vs. $17,681), LOS (4 days vs. 9 days), days to readmission (73 days vs. 59 days), mortality during initial admission (256 vs. 1,363), and mortality during any admission (538 vs. 3,838), all P less than .001.
Univariate and multivariate logistic regression analysis found that claudication, CLI, angioplasty, peripheral bypass, aortofemoral bypass, female sex, age younger than 65, Charlson Comorbidity Index, LOS, and primary expected payer status were all significant predictors of 30-day and overall readmissions at varying degrees.
The researchers also found that the five most common disease readmission groups were other vascular procedures (12.6%), amputation of lower limb except toes (6.3%), sepsis (5.4%), heart failure (4.9%), and postoperative or other device infections (4.8%) (Ann Vasc Surg. 2018;52:96-107).
The increased costs and higher levels of morbidity and mortality seen with CLI vs. claudication are not surprising given previous research showing that there are higher rates of complications in patients with CLI. A previous review showed there was a threefold higher risk of myocardial infarction, stroke, and vascular death in patients with CLI compared with patients with claudication, according Dr. Martinez and his colleagues.
“Readmissions after lower extremity procedures for patients admitted for claudication or CLI are influenced not only by the admission diagnosis and intervention performed but more importantly and significantly by the patient’s characteristics such as age, sex, CCI, and various other demographic factors,” the researchers wrote. “It is paramount to continue to perform this kind of study to better identify patients at risk for readmission and work toward prevention,” they concluded.
Dr. Martinez and his colleagues did not report disclosures, but indicated that the study did not receive any outside funding.
SOURCE: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
Patients treated for claudication vs. critical limb ischemia (CLI) differed significantly in their initial cost of admission, readmission costs, length of stay (LOS), days to readmission, and mortality (during initial admission, as well as any admission), according to the results of a database analysis of more than 90,000 patients in the Nationwide Readmission Database.
Readmissions were influenced not only by the admission diagnosis and intervention performed “but more importantly and significantly by the patient’s characteristics such as age, sex, CCI [Charlson Comorbidity Index], and various other demographic factors,” wrote Rennier A. Martinez, MD, of JFK Medical Center, Atlantis, Fla., and his colleagues. The report was published in the October issue of Annals of Vascular Surgery.
The study used the International Classification of Diseases, Ninth Revision (ICD-9) codes and queried the Nationwide Readmission Database for 2013 and 2014 for all 92,769 adult patients admitted with the principal diagnosis of claudication (ICD-9 code 440.21; n = 33,055 patients) or CLI (ICD-9 code 440.22e440.24; n = 59,714 patients) who underwent percutaneous angioplasty (ICD-9 code 39.50, 39.90), peripheral bypass (ICD-9 code 39.29), or aortofemoral bypass (ICD-9 code 39.25).
The 30-day readmission rates were 9.0% for claudication and 19.3% for CLI. Similarly, the any readmission rates were 21.5% and 40.4% for claudication vs. CLI.
Significant differences were found for claudication and CLI, respectively, on initial cost of admission ($18,548 vs. $29,148), readmission costs ($14,726 vs. $17,681), LOS (4 days vs. 9 days), days to readmission (73 days vs. 59 days), mortality during initial admission (256 vs. 1,363), and mortality during any admission (538 vs. 3,838), all P less than .001.
Univariate and multivariate logistic regression analysis found that claudication, CLI, angioplasty, peripheral bypass, aortofemoral bypass, female sex, age younger than 65, Charlson Comorbidity Index, LOS, and primary expected payer status were all significant predictors of 30-day and overall readmissions at varying degrees.
The researchers also found that the five most common disease readmission groups were other vascular procedures (12.6%), amputation of lower limb except toes (6.3%), sepsis (5.4%), heart failure (4.9%), and postoperative or other device infections (4.8%) (Ann Vasc Surg. 2018;52:96-107).
The increased costs and higher levels of morbidity and mortality seen with CLI vs. claudication are not surprising given previous research showing that there are higher rates of complications in patients with CLI. A previous review showed there was a threefold higher risk of myocardial infarction, stroke, and vascular death in patients with CLI compared with patients with claudication, according Dr. Martinez and his colleagues.
“Readmissions after lower extremity procedures for patients admitted for claudication or CLI are influenced not only by the admission diagnosis and intervention performed but more importantly and significantly by the patient’s characteristics such as age, sex, CCI, and various other demographic factors,” the researchers wrote. “It is paramount to continue to perform this kind of study to better identify patients at risk for readmission and work toward prevention,” they concluded.
Dr. Martinez and his colleagues did not report disclosures, but indicated that the study did not receive any outside funding.
SOURCE: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
FROM ANNALS OF VASCULAR SURGERY
Key clinical point: CLI was significantly more expensive and showed higher mortality rates compared with claudication.
Major finding: The 30-day readmission/any readmission rate was 9.0%/21.5% and 19.3%/40.4%, for claudication and CLI, respectively.
Study details: An analysis of more than 90,000 patients in the Nationwide Readmission Database in 2013 and 2014.
Disclosures: The authors did not report disclosures but indicated that the study did not receive any outside funding.
Source: Martinez RA et al. Ann Vasc Surg. 2018;52:96-107.
When stroke runs in the family
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
A 54-year-old man presented to our hospital with acute-onset left-sided weakness and right facial droop. Three days earlier he had also had migraine-like headaches, which he had never experienced before. He also reported a change in behavior during the past week, which his family had described as inappropriate laughter.
He had no history of hypertension, diabetes, or dyslipidemia. He did not smoke or drink alcohol. However, he had an extensive family history of stroke. His mother had a stroke at age 50, his brother a stroke at age 57, and his sister had been admitted for a stroke 1 month earlier at the age of 52.
On examination, he had weakness of the left arm and leg, right facial droop, and hyperactive reflexes on the left side. He had no sensory or cerebellar deficits. He had episodes of laughter during the examination.
We learned that the patient’s sister had undergone a workup showing mutations in the NOTCH3 gene and a skin biopsy study consistent with CADASIL.
Our patient was started on antiplatelet and high-intensity statin therapy. His symptoms improved, and he was discharged to an acute inpatient rehabilitation facility. He was referred to a CADASIL registry.
STROKE AND HEREDITY
CADASIL is a rare hereditary vascular disorder inherited in an autosomal dominant manner. It is the most common inherited form of small-vessel disease and results from a mutation in the NOTCH3 gene that leads to degeneration of smooth muscle in cerebral blood vessels. It can manifest as migraine with aura, vascular dementia, cognitive impairment, or ischemic stroke.
The diagnosis is based on a clinical picture that typically includes stroke at a young age (age 40 to 50) in the absence of stroke risk factors, or frequent lacunar infarction episodes that can manifest as migraine, lacunar infarct, or dementia.1 Some patients, such as ours, may have subtle nonspecific behavioral changes such as inappropriate laughter, which may herald the development of an infarct.
Characteristic findings on MRI are white matter hyperintensities that tend to be bilateral and symmetrical in the periventricular areas. Symmetrical involvement in the temporal lobes has high sensitivity and specificity for CADASIL.2 Biopsy study of the skin, muscle, or sural nerve shows small-vessel changes that include thickening of the media, granular material positive on periodic acid-Schiff staining, and narrowing of the lumen. However, the gold standard for diagnosis is confirmation of the NOTCH3 mutation on chromosome 19.1,2
There is no known treatment for CADASIL.
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
- Davous P. CADASIL: a review with proposed diagnostic criteria. Eur J Neurol 1998; 5(3):219–233. pmid:10210836
- Stojanov D, Vojinovic S, Aracki-Trenkic A, et al. Imaging characteristics of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL). Bosn J Basic Med Sci 2015; 15(1):1–8. doi:10.17305/bjbms.2015.247
Opioids don’t treat pain better than ibuprofen after venous ablation surgery
ST. LOUIS – Compared with ibuprofen, opioid pain medication offered little benefit for pain control after venous ablation surgery, in the experience of one surgical center.
Sharing study results at a poster session at the annual meeting of the Midwestern Vascular Surgery Society, Jana Sacco, MD, and her colleagues found that patients who received opioid prescriptions after venous ablations did not have significantly different postsurgical pain than did those who received ibuprofen alone.
The study, conducted against the national backdrop of greater scrutiny of postsurgical opioid prescribing, was the first to look at post–venous ablation pain management strategies, said Dr. Sacco, a resident physician at Henry Ford Hospital, Detroit. Venous ablation surgery can improve quality of life for patients with varicose veins, but best practices for managing postprocedure discomfort had not been clear; some patients receive opioid pain medications, while others are directed to use ibuprofen as needed for pain control.
The retrospective, single-center study assessed pre- and postoperative pain for patients undergoing venous ablation procedures over a 2-year period, said Dr. Sacco.
Patients who were prescribed opioids were compared with patients who were simply asked to take ibuprofen for pain control.
Comparing preoperative to postoperative pain scores, Dr. Sacco and her colleagues defined a change of 2-3 points on a 0-10 Likert scale as “good” improvement; a change of 1 point was defined as “mild” improvement, and no change or worsening was defined as no improvement.
Of the 268 patients for whom postoperative follow-up data were available, 142 received opioid prescriptions, while 126 did not.
Across the entire group of patients studied, those who had moderate to severe preoperative pain had significant improvement in pain after their procedures.
Whether patients received opioid pain medication after their venous ablation was not correlated with the degree of improvement in postprocedure pain scores. Of those who saw no improvement, 30 patients (45%) received opioids and 36 (55%) did not. Of the 89 patients who saw mild postprocedure improvement in pain, 35 (40%) were not discharged on opioids, and of 65 patients who had good improvement in postprocedure pain, 44% were not discharged on opioids (P = .7 for difference across groups).
When Dr. Sacco and her fellow researchers examined such patient characteristics as sex, race, body mass index, smoking status, and CEAP venous severity classification, they did not see any significant differences in pain scores. Similarly, neither the type of procedure (radiofrequency or laser ablation) nor information on whether compression treatment was used was associated with a difference in pain scores.
Dr. Sacco and her coauthors noted that the study was limited by its retrospective nature and the fact that patients were all drawn from a single institution. Additionally, the investigators were only able to ascertain whether opioids had been prescribed, not whether – or how much – medication was actually taken by patients.
“Most patients report an improvement in symptoms after undergoing vein ablation procedures,” reported Dr. Sacco and her colleagues, and most patients also do well with nonopioid pain control regimens. “Overprescribing opioids exposes patients to the risk of narcotic overdose and chronic opioid use and should be used with caution for patients undergoing vein ablation surgery,” they wrote.
Dr. Sacco reported no outside sources of funding and no conflicts of interest.
ST. LOUIS – Compared with ibuprofen, opioid pain medication offered little benefit for pain control after venous ablation surgery, in the experience of one surgical center.
Sharing study results at a poster session at the annual meeting of the Midwestern Vascular Surgery Society, Jana Sacco, MD, and her colleagues found that patients who received opioid prescriptions after venous ablations did not have significantly different postsurgical pain than did those who received ibuprofen alone.
The study, conducted against the national backdrop of greater scrutiny of postsurgical opioid prescribing, was the first to look at post–venous ablation pain management strategies, said Dr. Sacco, a resident physician at Henry Ford Hospital, Detroit. Venous ablation surgery can improve quality of life for patients with varicose veins, but best practices for managing postprocedure discomfort had not been clear; some patients receive opioid pain medications, while others are directed to use ibuprofen as needed for pain control.
The retrospective, single-center study assessed pre- and postoperative pain for patients undergoing venous ablation procedures over a 2-year period, said Dr. Sacco.
Patients who were prescribed opioids were compared with patients who were simply asked to take ibuprofen for pain control.
Comparing preoperative to postoperative pain scores, Dr. Sacco and her colleagues defined a change of 2-3 points on a 0-10 Likert scale as “good” improvement; a change of 1 point was defined as “mild” improvement, and no change or worsening was defined as no improvement.
Of the 268 patients for whom postoperative follow-up data were available, 142 received opioid prescriptions, while 126 did not.
Across the entire group of patients studied, those who had moderate to severe preoperative pain had significant improvement in pain after their procedures.
Whether patients received opioid pain medication after their venous ablation was not correlated with the degree of improvement in postprocedure pain scores. Of those who saw no improvement, 30 patients (45%) received opioids and 36 (55%) did not. Of the 89 patients who saw mild postprocedure improvement in pain, 35 (40%) were not discharged on opioids, and of 65 patients who had good improvement in postprocedure pain, 44% were not discharged on opioids (P = .7 for difference across groups).
When Dr. Sacco and her fellow researchers examined such patient characteristics as sex, race, body mass index, smoking status, and CEAP venous severity classification, they did not see any significant differences in pain scores. Similarly, neither the type of procedure (radiofrequency or laser ablation) nor information on whether compression treatment was used was associated with a difference in pain scores.
Dr. Sacco and her coauthors noted that the study was limited by its retrospective nature and the fact that patients were all drawn from a single institution. Additionally, the investigators were only able to ascertain whether opioids had been prescribed, not whether – or how much – medication was actually taken by patients.
“Most patients report an improvement in symptoms after undergoing vein ablation procedures,” reported Dr. Sacco and her colleagues, and most patients also do well with nonopioid pain control regimens. “Overprescribing opioids exposes patients to the risk of narcotic overdose and chronic opioid use and should be used with caution for patients undergoing vein ablation surgery,” they wrote.
Dr. Sacco reported no outside sources of funding and no conflicts of interest.
ST. LOUIS – Compared with ibuprofen, opioid pain medication offered little benefit for pain control after venous ablation surgery, in the experience of one surgical center.
Sharing study results at a poster session at the annual meeting of the Midwestern Vascular Surgery Society, Jana Sacco, MD, and her colleagues found that patients who received opioid prescriptions after venous ablations did not have significantly different postsurgical pain than did those who received ibuprofen alone.
The study, conducted against the national backdrop of greater scrutiny of postsurgical opioid prescribing, was the first to look at post–venous ablation pain management strategies, said Dr. Sacco, a resident physician at Henry Ford Hospital, Detroit. Venous ablation surgery can improve quality of life for patients with varicose veins, but best practices for managing postprocedure discomfort had not been clear; some patients receive opioid pain medications, while others are directed to use ibuprofen as needed for pain control.
The retrospective, single-center study assessed pre- and postoperative pain for patients undergoing venous ablation procedures over a 2-year period, said Dr. Sacco.
Patients who were prescribed opioids were compared with patients who were simply asked to take ibuprofen for pain control.
Comparing preoperative to postoperative pain scores, Dr. Sacco and her colleagues defined a change of 2-3 points on a 0-10 Likert scale as “good” improvement; a change of 1 point was defined as “mild” improvement, and no change or worsening was defined as no improvement.
Of the 268 patients for whom postoperative follow-up data were available, 142 received opioid prescriptions, while 126 did not.
Across the entire group of patients studied, those who had moderate to severe preoperative pain had significant improvement in pain after their procedures.
Whether patients received opioid pain medication after their venous ablation was not correlated with the degree of improvement in postprocedure pain scores. Of those who saw no improvement, 30 patients (45%) received opioids and 36 (55%) did not. Of the 89 patients who saw mild postprocedure improvement in pain, 35 (40%) were not discharged on opioids, and of 65 patients who had good improvement in postprocedure pain, 44% were not discharged on opioids (P = .7 for difference across groups).
When Dr. Sacco and her fellow researchers examined such patient characteristics as sex, race, body mass index, smoking status, and CEAP venous severity classification, they did not see any significant differences in pain scores. Similarly, neither the type of procedure (radiofrequency or laser ablation) nor information on whether compression treatment was used was associated with a difference in pain scores.
Dr. Sacco and her coauthors noted that the study was limited by its retrospective nature and the fact that patients were all drawn from a single institution. Additionally, the investigators were only able to ascertain whether opioids had been prescribed, not whether – or how much – medication was actually taken by patients.
“Most patients report an improvement in symptoms after undergoing vein ablation procedures,” reported Dr. Sacco and her colleagues, and most patients also do well with nonopioid pain control regimens. “Overprescribing opioids exposes patients to the risk of narcotic overdose and chronic opioid use and should be used with caution for patients undergoing vein ablation surgery,” they wrote.
Dr. Sacco reported no outside sources of funding and no conflicts of interest.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Prescribing opioids after venous ablation surgery didn’t improve pain control over ibuprofen.
Major finding:
Study details: Retrospective, single-institution study of 268 patients undergoing venous ablation surgery.
Disclosures: Dr. Sacco reported no conflicts of interest and no outside sources of funding.
Vascular programs without NIVL curriculum leave trainees feeling unprepared
ST. LOUIS – Many vascular surgery trainees felt unprepared to take the Registered Physician in Vascular Interpretation (RPVI) exam, according to a recent survey. However, trainees in a program without a structured noninvasive vascular laboratory (NIVL) curriculum felt particularly unprepared, said Daisy Chou, MD.
“There is wide variation in NIVL experience amongst vascular surgery training programs,” noted Dr. Chou, a vascular surgery fellow at the Ohio State University, Columbus. She presented survey results at the annual meeting of the Midwestern Vascular Surgical Society. The survey constructed by Dr. Chou and her colleagues went out to trainees in both 0+5 and 5+2 vascular surgery training programs in September, 2017, in 114 unique programs.
Eventually, trainees from just over half of the programs responded (N = 61 programs, 53.5%), said Dr. Chou. Using responses from individual trainees, the authors grouped programs into one of two categories: those whose trainees felt well prepared for the RPVI, and those whose trainees felt unprepared for the RPVI.
In addition to a yes/no question about preparedness, the survey also asked whether training programs had a structured curriculum; respondents were asked to identify specific NIVL-related training activities. The survey asked about individual didactic components, as well as whether the trainee spent individual time with an attending physician and hands-on time with vascular technologists. Respondents were asked about the amount of time, measured in half days per week, spent in the vascular laboratory.
Finally, the survey asked whether trainees took a pre-RPVI exam review course, and whether they passed the RPVI exam on their first attempt.
Overall, 34 of the programs with respondents (55.7%) had structured curricula; the same number included lectures. Twenty programs (32.8%) provided video content, and 29 (47.5%) used textbooks. Just 18 programs (29.5%) assigned articles.
One-on-one time spent with an attending physician and focused on NIVL techniques was reported for 32 programs (52.5%). More programs (n = 37; 60.7%) provided trainees hands-on experience with vascular technologists.
Most programs (n = 32; 52.5%) had trainees spending less than one half day per week in the vascular laboratory, according to survey respondents.
In terms of preparedness, respondents for over half of the programs did not respond to the question asking whether they felt prepared for the RPVI, presumably because they had not yet taken the exam. This, acknowledged Dr. Chou, was a significant limitation of the survey. There was a timing problem: Trainees were surveyed at the start of the 2017-2018 academic year, but the RPVI exam isn’t usually taken until the end of the final year of training, with review courses taken not long before that.
Of the 32 programs with trainees who reported taking the RPVI exam, 18 had trainees who felt unprepared, and 14 program had trainees who felt well prepared. About a quarter of programs (N = 15; 24.6%) had trainees who took a review course prior to taking the exam.
Dr. Chou and her colleagues then examined the survey responses another way, seeing what differentiated the programs whose trainees felt well prepared from those with trainees who felt unprepared.
Statistically, the clear standout was whether the program had a structured curriculum: The 14 programs with a structured curriculum all had students who reported feeling well prepared. Just one-third of the 18 programs with unprepared students had a structured curriculum, which was a significant difference (P = .0001).
Also, programs that assigned articles and those that gave formal lectures were more likely to have students who felt prepared to sit for the RPVI exam (P = .002 and .004, respectively). A higher number of programs that gave trainees hands-on time with vascular technologists had trainees who felt prepared, but the difference wasn’t quite statistically significant (P = .05).
Having taken a review course prior to the exam was associated with feeling well prepared (P = .03).
Dr. Chou and her colleagues performed a logistic regression analysis to arrive at the educational components associated with the highest odds for trainees feeling well prepared. Lectures and articles came out on top in this analysis (odds ratios for feeling well prepared, 15.88 and 15.97, respectively). Hands-on time with vascular technologists had an odds ratio of 5.12 for feeling prepared.
Taking a review course boosted preparedness as well, with an odds ratio of 11.85 for feeling well prepared for the RPVI exam. This created a bit of a conundrum for the investigators, said Dr. Chou: “All well prepared programs had a structured NIVL curriculum, but most of their trainees still took an RPVI review course, so it’s unclear if the structured curriculum or the review course is responsible for trainees feeling well prepared for the RPVI exam,” she said.
An important caveat to the analysis of survey results, said Dr. Chou, is that “It’s unknown how these results will translate into pass rates.
“Vascular surgery leadership should not leave NIVL education to review courses,” said Dr. Chou. The ultimate goal, she said, should be to achieve expertise in the service of providing better patient care. To this end, Dr. Chou and her coauthors recommend that a structured NIVL curriculum be incorporated into vascular surgery training, and that the program include time spent with vascular technologists, a formal lecture-based component, and structured reading, as is provided by a journal club.
Dr. Chou reported no conflicts of interest, and no external sources of funding.
ST. LOUIS – Many vascular surgery trainees felt unprepared to take the Registered Physician in Vascular Interpretation (RPVI) exam, according to a recent survey. However, trainees in a program without a structured noninvasive vascular laboratory (NIVL) curriculum felt particularly unprepared, said Daisy Chou, MD.
“There is wide variation in NIVL experience amongst vascular surgery training programs,” noted Dr. Chou, a vascular surgery fellow at the Ohio State University, Columbus. She presented survey results at the annual meeting of the Midwestern Vascular Surgical Society. The survey constructed by Dr. Chou and her colleagues went out to trainees in both 0+5 and 5+2 vascular surgery training programs in September, 2017, in 114 unique programs.
Eventually, trainees from just over half of the programs responded (N = 61 programs, 53.5%), said Dr. Chou. Using responses from individual trainees, the authors grouped programs into one of two categories: those whose trainees felt well prepared for the RPVI, and those whose trainees felt unprepared for the RPVI.
In addition to a yes/no question about preparedness, the survey also asked whether training programs had a structured curriculum; respondents were asked to identify specific NIVL-related training activities. The survey asked about individual didactic components, as well as whether the trainee spent individual time with an attending physician and hands-on time with vascular technologists. Respondents were asked about the amount of time, measured in half days per week, spent in the vascular laboratory.
Finally, the survey asked whether trainees took a pre-RPVI exam review course, and whether they passed the RPVI exam on their first attempt.
Overall, 34 of the programs with respondents (55.7%) had structured curricula; the same number included lectures. Twenty programs (32.8%) provided video content, and 29 (47.5%) used textbooks. Just 18 programs (29.5%) assigned articles.
One-on-one time spent with an attending physician and focused on NIVL techniques was reported for 32 programs (52.5%). More programs (n = 37; 60.7%) provided trainees hands-on experience with vascular technologists.
Most programs (n = 32; 52.5%) had trainees spending less than one half day per week in the vascular laboratory, according to survey respondents.
In terms of preparedness, respondents for over half of the programs did not respond to the question asking whether they felt prepared for the RPVI, presumably because they had not yet taken the exam. This, acknowledged Dr. Chou, was a significant limitation of the survey. There was a timing problem: Trainees were surveyed at the start of the 2017-2018 academic year, but the RPVI exam isn’t usually taken until the end of the final year of training, with review courses taken not long before that.
Of the 32 programs with trainees who reported taking the RPVI exam, 18 had trainees who felt unprepared, and 14 program had trainees who felt well prepared. About a quarter of programs (N = 15; 24.6%) had trainees who took a review course prior to taking the exam.
Dr. Chou and her colleagues then examined the survey responses another way, seeing what differentiated the programs whose trainees felt well prepared from those with trainees who felt unprepared.
Statistically, the clear standout was whether the program had a structured curriculum: The 14 programs with a structured curriculum all had students who reported feeling well prepared. Just one-third of the 18 programs with unprepared students had a structured curriculum, which was a significant difference (P = .0001).
Also, programs that assigned articles and those that gave formal lectures were more likely to have students who felt prepared to sit for the RPVI exam (P = .002 and .004, respectively). A higher number of programs that gave trainees hands-on time with vascular technologists had trainees who felt prepared, but the difference wasn’t quite statistically significant (P = .05).
Having taken a review course prior to the exam was associated with feeling well prepared (P = .03).
Dr. Chou and her colleagues performed a logistic regression analysis to arrive at the educational components associated with the highest odds for trainees feeling well prepared. Lectures and articles came out on top in this analysis (odds ratios for feeling well prepared, 15.88 and 15.97, respectively). Hands-on time with vascular technologists had an odds ratio of 5.12 for feeling prepared.
Taking a review course boosted preparedness as well, with an odds ratio of 11.85 for feeling well prepared for the RPVI exam. This created a bit of a conundrum for the investigators, said Dr. Chou: “All well prepared programs had a structured NIVL curriculum, but most of their trainees still took an RPVI review course, so it’s unclear if the structured curriculum or the review course is responsible for trainees feeling well prepared for the RPVI exam,” she said.
An important caveat to the analysis of survey results, said Dr. Chou, is that “It’s unknown how these results will translate into pass rates.
“Vascular surgery leadership should not leave NIVL education to review courses,” said Dr. Chou. The ultimate goal, she said, should be to achieve expertise in the service of providing better patient care. To this end, Dr. Chou and her coauthors recommend that a structured NIVL curriculum be incorporated into vascular surgery training, and that the program include time spent with vascular technologists, a formal lecture-based component, and structured reading, as is provided by a journal club.
Dr. Chou reported no conflicts of interest, and no external sources of funding.
ST. LOUIS – Many vascular surgery trainees felt unprepared to take the Registered Physician in Vascular Interpretation (RPVI) exam, according to a recent survey. However, trainees in a program without a structured noninvasive vascular laboratory (NIVL) curriculum felt particularly unprepared, said Daisy Chou, MD.
“There is wide variation in NIVL experience amongst vascular surgery training programs,” noted Dr. Chou, a vascular surgery fellow at the Ohio State University, Columbus. She presented survey results at the annual meeting of the Midwestern Vascular Surgical Society. The survey constructed by Dr. Chou and her colleagues went out to trainees in both 0+5 and 5+2 vascular surgery training programs in September, 2017, in 114 unique programs.
Eventually, trainees from just over half of the programs responded (N = 61 programs, 53.5%), said Dr. Chou. Using responses from individual trainees, the authors grouped programs into one of two categories: those whose trainees felt well prepared for the RPVI, and those whose trainees felt unprepared for the RPVI.
In addition to a yes/no question about preparedness, the survey also asked whether training programs had a structured curriculum; respondents were asked to identify specific NIVL-related training activities. The survey asked about individual didactic components, as well as whether the trainee spent individual time with an attending physician and hands-on time with vascular technologists. Respondents were asked about the amount of time, measured in half days per week, spent in the vascular laboratory.
Finally, the survey asked whether trainees took a pre-RPVI exam review course, and whether they passed the RPVI exam on their first attempt.
Overall, 34 of the programs with respondents (55.7%) had structured curricula; the same number included lectures. Twenty programs (32.8%) provided video content, and 29 (47.5%) used textbooks. Just 18 programs (29.5%) assigned articles.
One-on-one time spent with an attending physician and focused on NIVL techniques was reported for 32 programs (52.5%). More programs (n = 37; 60.7%) provided trainees hands-on experience with vascular technologists.
Most programs (n = 32; 52.5%) had trainees spending less than one half day per week in the vascular laboratory, according to survey respondents.
In terms of preparedness, respondents for over half of the programs did not respond to the question asking whether they felt prepared for the RPVI, presumably because they had not yet taken the exam. This, acknowledged Dr. Chou, was a significant limitation of the survey. There was a timing problem: Trainees were surveyed at the start of the 2017-2018 academic year, but the RPVI exam isn’t usually taken until the end of the final year of training, with review courses taken not long before that.
Of the 32 programs with trainees who reported taking the RPVI exam, 18 had trainees who felt unprepared, and 14 program had trainees who felt well prepared. About a quarter of programs (N = 15; 24.6%) had trainees who took a review course prior to taking the exam.
Dr. Chou and her colleagues then examined the survey responses another way, seeing what differentiated the programs whose trainees felt well prepared from those with trainees who felt unprepared.
Statistically, the clear standout was whether the program had a structured curriculum: The 14 programs with a structured curriculum all had students who reported feeling well prepared. Just one-third of the 18 programs with unprepared students had a structured curriculum, which was a significant difference (P = .0001).
Also, programs that assigned articles and those that gave formal lectures were more likely to have students who felt prepared to sit for the RPVI exam (P = .002 and .004, respectively). A higher number of programs that gave trainees hands-on time with vascular technologists had trainees who felt prepared, but the difference wasn’t quite statistically significant (P = .05).
Having taken a review course prior to the exam was associated with feeling well prepared (P = .03).
Dr. Chou and her colleagues performed a logistic regression analysis to arrive at the educational components associated with the highest odds for trainees feeling well prepared. Lectures and articles came out on top in this analysis (odds ratios for feeling well prepared, 15.88 and 15.97, respectively). Hands-on time with vascular technologists had an odds ratio of 5.12 for feeling prepared.
Taking a review course boosted preparedness as well, with an odds ratio of 11.85 for feeling well prepared for the RPVI exam. This created a bit of a conundrum for the investigators, said Dr. Chou: “All well prepared programs had a structured NIVL curriculum, but most of their trainees still took an RPVI review course, so it’s unclear if the structured curriculum or the review course is responsible for trainees feeling well prepared for the RPVI exam,” she said.
An important caveat to the analysis of survey results, said Dr. Chou, is that “It’s unknown how these results will translate into pass rates.
“Vascular surgery leadership should not leave NIVL education to review courses,” said Dr. Chou. The ultimate goal, she said, should be to achieve expertise in the service of providing better patient care. To this end, Dr. Chou and her coauthors recommend that a structured NIVL curriculum be incorporated into vascular surgery training, and that the program include time spent with vascular technologists, a formal lecture-based component, and structured reading, as is provided by a journal club.
Dr. Chou reported no conflicts of interest, and no external sources of funding.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Many vascular surgery trainees do not feel prepared to take the RPVI exam.
Major finding: Lectures and textbook reading were highly associated with feeling prepared (P = .002 and .004, respectively).
Study details: Survey of trainees in 114 vascular surgery training programs.
Disclosures: The author reported no outside sources of funding, and no conflicts of interest.
Tracking 90-day vascular surgery outcomes: The coming new normal?
The Centers for Medicare and Medicaid Services is test driving a new quality measurement model that pushes hospital readmissions measures out from 30 to 90 days.
Previous research has identified vascular surgery as having twice as high rates of 90-day readmissions, compared with 30-day readmissions (Am J Manag Care. 2014;20[9]:e432-e438), and this could prove problematic in light of the CMS pilot project currently underway, according to Donald E. Fry, MD, of MPA Healthcare Solutions, Chicago, and his colleagues.
They performed a study that found a high level of adverse outcomes for common vascular procedures and that there was a significant variability in risk-adjusted outcomes among best- and poorest-performing hospitals in all major vascular procedures, indicating that a large opportunity exists for improvement in results.
Medicare’s value-based care Readmissions Reduction Model developed financial penalties for hospitals that fail to achieve acceptable performance scores, and in doing so shifted some of the financial risks of care to the providers based on a 30-day readmission model. In contrast, the pilot Bundled Payments for Care Improvement (BPCI) Advanced Program, which the CMS plans to launch in October 2018, will follow a 90-day period of postoperative care as its duration of financial accountability.
“While BPCI Advanced, has, until now, focused upon orthopedics, cardiovascular procedures, and high-volume medical admissions areas, it is anticipated that vascular surgery will be included in the future,” according to the investigators. Therefore, the researchers performed an in-depth analysis to examine the 90-day outcomes of common vascular surgeries across hospitals as a prelude to the vascular surgery field having to potentially confront this new CMS model (Surgery 2018 Jun 22 doi: 10.1016/j.surg.2018.03.025).
Dr. Fry and his colleagues used the Medicare Limited Data Set for 2012-2014 to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postoperative care. A pool of more than 500 aggregated and individual candidate risk factors, including age and sex, was used in model development, based upon data from 359 hospitals with 10,815 patients in the Medicare Limited Data Set.
The researchers examined the risk-adjusted outcomes of four major groupings of vascular surgery procedures: elective open aortic; open peripheral vascular procedures; endovascular aortic; and percutaneous angioplasty procedures.
They found that the total adverse-outcome rate (AO) was 27.8% for open aortic procedures, 31.5% for open peripheral vascular procedures, 19.6% for endovascular aortic procedures, and 36.4% for percutaneous angioplasty procedures. The difference in risk-adjusted adverse-outcome rates between the best- and the poorest- performing deciles was 32.2% for open aortic procedures, 29.5% for open peripheral vascular procedures, 21.5% for endovascular aortic procedures, and 37.1% for percutaneous angioplasty procedures.
The model determined significant risk factors (P less than .001) for inpatient death (including malnutrition, intestinal ischemia, supplemental oxygen, and age greater than or equal to 85 years); prolonged length of stay (including supplemental oxygen, peritoneal adhesions, and chronic lung obstructive disease); 90-day postdischarge death (including heart failure, chronic infection, psychosis, and primary head/neck cancer); and 90-day postdischarge readmission (malnutrition, chronic obstructive lung disease, upper aerodigestive tract cancer, and skin ulceration) for these procedures.
For all cases, the total 90-day postdischarge mortality rate exceeded the inpatient death rate, and readmissions were the major driver of the total AO. They found that 22% of all patients readmitted across the entire 90-day interval had not seen a physician for follow-up after discharge. [This] “begs the question of whether more frequent physician or physician-extender follow-up can reduce this AO,” according to Dr. Fry and his colleagues. “Importantly, first readmissions during days 31-90 following discharge were almost as common as those occurring during the initial 30 days. Over 20% of total readmissions were subsequently repeat events during the 90-day interval,” they added.
They also found that the variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures and indicates a large opportunity for improvement in results.
“Understanding variables associated with higher risk can be used as a decision support tool to identify which patients will need increased vigilance to avoid AOs. Identification of very high risk may become a consideration in the assessment of the appropriateness of the surgical intervention. If providers know their outcomes and those outcomes are benchmarked against the whole population of hospitals, then clinical performance can be improved by specific care redesign initiatives,” the researchers concluded.
Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
SOURCE: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
The Centers for Medicare and Medicaid Services is test driving a new quality measurement model that pushes hospital readmissions measures out from 30 to 90 days.
Previous research has identified vascular surgery as having twice as high rates of 90-day readmissions, compared with 30-day readmissions (Am J Manag Care. 2014;20[9]:e432-e438), and this could prove problematic in light of the CMS pilot project currently underway, according to Donald E. Fry, MD, of MPA Healthcare Solutions, Chicago, and his colleagues.
They performed a study that found a high level of adverse outcomes for common vascular procedures and that there was a significant variability in risk-adjusted outcomes among best- and poorest-performing hospitals in all major vascular procedures, indicating that a large opportunity exists for improvement in results.
Medicare’s value-based care Readmissions Reduction Model developed financial penalties for hospitals that fail to achieve acceptable performance scores, and in doing so shifted some of the financial risks of care to the providers based on a 30-day readmission model. In contrast, the pilot Bundled Payments for Care Improvement (BPCI) Advanced Program, which the CMS plans to launch in October 2018, will follow a 90-day period of postoperative care as its duration of financial accountability.
“While BPCI Advanced, has, until now, focused upon orthopedics, cardiovascular procedures, and high-volume medical admissions areas, it is anticipated that vascular surgery will be included in the future,” according to the investigators. Therefore, the researchers performed an in-depth analysis to examine the 90-day outcomes of common vascular surgeries across hospitals as a prelude to the vascular surgery field having to potentially confront this new CMS model (Surgery 2018 Jun 22 doi: 10.1016/j.surg.2018.03.025).
Dr. Fry and his colleagues used the Medicare Limited Data Set for 2012-2014 to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postoperative care. A pool of more than 500 aggregated and individual candidate risk factors, including age and sex, was used in model development, based upon data from 359 hospitals with 10,815 patients in the Medicare Limited Data Set.
The researchers examined the risk-adjusted outcomes of four major groupings of vascular surgery procedures: elective open aortic; open peripheral vascular procedures; endovascular aortic; and percutaneous angioplasty procedures.
They found that the total adverse-outcome rate (AO) was 27.8% for open aortic procedures, 31.5% for open peripheral vascular procedures, 19.6% for endovascular aortic procedures, and 36.4% for percutaneous angioplasty procedures. The difference in risk-adjusted adverse-outcome rates between the best- and the poorest- performing deciles was 32.2% for open aortic procedures, 29.5% for open peripheral vascular procedures, 21.5% for endovascular aortic procedures, and 37.1% for percutaneous angioplasty procedures.
The model determined significant risk factors (P less than .001) for inpatient death (including malnutrition, intestinal ischemia, supplemental oxygen, and age greater than or equal to 85 years); prolonged length of stay (including supplemental oxygen, peritoneal adhesions, and chronic lung obstructive disease); 90-day postdischarge death (including heart failure, chronic infection, psychosis, and primary head/neck cancer); and 90-day postdischarge readmission (malnutrition, chronic obstructive lung disease, upper aerodigestive tract cancer, and skin ulceration) for these procedures.
For all cases, the total 90-day postdischarge mortality rate exceeded the inpatient death rate, and readmissions were the major driver of the total AO. They found that 22% of all patients readmitted across the entire 90-day interval had not seen a physician for follow-up after discharge. [This] “begs the question of whether more frequent physician or physician-extender follow-up can reduce this AO,” according to Dr. Fry and his colleagues. “Importantly, first readmissions during days 31-90 following discharge were almost as common as those occurring during the initial 30 days. Over 20% of total readmissions were subsequently repeat events during the 90-day interval,” they added.
They also found that the variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures and indicates a large opportunity for improvement in results.
“Understanding variables associated with higher risk can be used as a decision support tool to identify which patients will need increased vigilance to avoid AOs. Identification of very high risk may become a consideration in the assessment of the appropriateness of the surgical intervention. If providers know their outcomes and those outcomes are benchmarked against the whole population of hospitals, then clinical performance can be improved by specific care redesign initiatives,” the researchers concluded.
Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
SOURCE: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
The Centers for Medicare and Medicaid Services is test driving a new quality measurement model that pushes hospital readmissions measures out from 30 to 90 days.
Previous research has identified vascular surgery as having twice as high rates of 90-day readmissions, compared with 30-day readmissions (Am J Manag Care. 2014;20[9]:e432-e438), and this could prove problematic in light of the CMS pilot project currently underway, according to Donald E. Fry, MD, of MPA Healthcare Solutions, Chicago, and his colleagues.
They performed a study that found a high level of adverse outcomes for common vascular procedures and that there was a significant variability in risk-adjusted outcomes among best- and poorest-performing hospitals in all major vascular procedures, indicating that a large opportunity exists for improvement in results.
Medicare’s value-based care Readmissions Reduction Model developed financial penalties for hospitals that fail to achieve acceptable performance scores, and in doing so shifted some of the financial risks of care to the providers based on a 30-day readmission model. In contrast, the pilot Bundled Payments for Care Improvement (BPCI) Advanced Program, which the CMS plans to launch in October 2018, will follow a 90-day period of postoperative care as its duration of financial accountability.
“While BPCI Advanced, has, until now, focused upon orthopedics, cardiovascular procedures, and high-volume medical admissions areas, it is anticipated that vascular surgery will be included in the future,” according to the investigators. Therefore, the researchers performed an in-depth analysis to examine the 90-day outcomes of common vascular surgeries across hospitals as a prelude to the vascular surgery field having to potentially confront this new CMS model (Surgery 2018 Jun 22 doi: 10.1016/j.surg.2018.03.025).
Dr. Fry and his colleagues used the Medicare Limited Data Set for 2012-2014 to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postoperative care. A pool of more than 500 aggregated and individual candidate risk factors, including age and sex, was used in model development, based upon data from 359 hospitals with 10,815 patients in the Medicare Limited Data Set.
The researchers examined the risk-adjusted outcomes of four major groupings of vascular surgery procedures: elective open aortic; open peripheral vascular procedures; endovascular aortic; and percutaneous angioplasty procedures.
They found that the total adverse-outcome rate (AO) was 27.8% for open aortic procedures, 31.5% for open peripheral vascular procedures, 19.6% for endovascular aortic procedures, and 36.4% for percutaneous angioplasty procedures. The difference in risk-adjusted adverse-outcome rates between the best- and the poorest- performing deciles was 32.2% for open aortic procedures, 29.5% for open peripheral vascular procedures, 21.5% for endovascular aortic procedures, and 37.1% for percutaneous angioplasty procedures.
The model determined significant risk factors (P less than .001) for inpatient death (including malnutrition, intestinal ischemia, supplemental oxygen, and age greater than or equal to 85 years); prolonged length of stay (including supplemental oxygen, peritoneal adhesions, and chronic lung obstructive disease); 90-day postdischarge death (including heart failure, chronic infection, psychosis, and primary head/neck cancer); and 90-day postdischarge readmission (malnutrition, chronic obstructive lung disease, upper aerodigestive tract cancer, and skin ulceration) for these procedures.
For all cases, the total 90-day postdischarge mortality rate exceeded the inpatient death rate, and readmissions were the major driver of the total AO. They found that 22% of all patients readmitted across the entire 90-day interval had not seen a physician for follow-up after discharge. [This] “begs the question of whether more frequent physician or physician-extender follow-up can reduce this AO,” according to Dr. Fry and his colleagues. “Importantly, first readmissions during days 31-90 following discharge were almost as common as those occurring during the initial 30 days. Over 20% of total readmissions were subsequently repeat events during the 90-day interval,” they added.
They also found that the variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures and indicates a large opportunity for improvement in results.
“Understanding variables associated with higher risk can be used as a decision support tool to identify which patients will need increased vigilance to avoid AOs. Identification of very high risk may become a consideration in the assessment of the appropriateness of the surgical intervention. If providers know their outcomes and those outcomes are benchmarked against the whole population of hospitals, then clinical performance can be improved by specific care redesign initiatives,” the researchers concluded.
Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
SOURCE: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
FROM SURGERY
Key clinical point: The variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures.
Major finding: The total adverse-outcome rate was 27.8% for open aortic procedures, 31.5% for open peripheral artery, 19.6% for endovascular aortic, and 36.4% for percutaneous angioplasty.
Study details: The Medicare Limited Data Set for 2012-2014 was used to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postop care.
Disclosures: Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
Source: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
Risk factors for postop cardiac events differ between vascular and general surgery
Predictive risk factors for cardiac events (CEs) after general and vascular surgery differed significantly, according to a large retrospective study. However, there was no significant difference seen in the overall incidence of CEs between the two types of surgery, reported Derrick Acheampong, MD, and his colleagues at the Icahn School of Medicine at Mount Sinai, New York.
They performed a retrospective data analysis of 8,441 adult patients at their large urban teaching hospital; these patients had undergone general or vascular surgery during 2013-2016 and, in the analysis, were grouped by whether they experienced postoperative CEs.
Univariate and multivariate analyses identified predictors of postoperative CE and the association of CEs with adverse postoperative outcomes. CEs were defined as myocardial infarction or cardiac arrest within the 30-day postoperative period.
A total of 157 patients (1.9%) experienced CEs after major general and vascular surgery, with no significant difference in incidence between the two types of surgery (P = .44), according to their report, published online in the Annals of Medicine and Surgery. CE-associated mortality among this group was high, at 55.4%.
The occurrence of a CE following surgery in both groups was significantly associated with increased mortality, as well as pulmonary, renal, and neurological complications, in addition to systemic sepsis, postoperative red blood cell transfusion, unplanned return to the operating room, and prolonged hospitalization, according to the researchers.
However, predictors of CEs risk between vascular and general surgery were significantly different.
For general surgery, American Society of Anesthesiologists (ASA) status greater than 3, dependent functional status, acute renal failure or dialysis, weight loss, creatinine greater than 1.2 mg/dL, international normalized ratio (INR) greater than 1.5, and partial thromboplastin time (PTT) less than 35 seconds were all unique independent predictors of postoperative CEs.
For vascular surgery, the unique significant predictors of postoperative CEs were age greater than 65 years, emergency surgery, diabetes, congestive heart failure, systemic sepsis, and operative time greater than 240 minutes.
The only common predictive risk factors for postoperative CEs for the two forms of surgery were hematocrit less than 34% and ventilator dependence.
“The present study corroborates reported studies that recommend separate predictive CE risk indices and risk stratification among different surgical specialties. Predictors for CE greatly differed between general and vascular surgery patients in our patient population,” the authors stated.
They concluded with the hope that their study “provides useful information to surgeons and allows for the necessary resources to be focused on identified at-risk patients to improve surgical outcomes.”
Dr. Acheampong and his colleagues reported having no disclosures.
SOURCE: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
Predictive risk factors for cardiac events (CEs) after general and vascular surgery differed significantly, according to a large retrospective study. However, there was no significant difference seen in the overall incidence of CEs between the two types of surgery, reported Derrick Acheampong, MD, and his colleagues at the Icahn School of Medicine at Mount Sinai, New York.
They performed a retrospective data analysis of 8,441 adult patients at their large urban teaching hospital; these patients had undergone general or vascular surgery during 2013-2016 and, in the analysis, were grouped by whether they experienced postoperative CEs.
Univariate and multivariate analyses identified predictors of postoperative CE and the association of CEs with adverse postoperative outcomes. CEs were defined as myocardial infarction or cardiac arrest within the 30-day postoperative period.
A total of 157 patients (1.9%) experienced CEs after major general and vascular surgery, with no significant difference in incidence between the two types of surgery (P = .44), according to their report, published online in the Annals of Medicine and Surgery. CE-associated mortality among this group was high, at 55.4%.
The occurrence of a CE following surgery in both groups was significantly associated with increased mortality, as well as pulmonary, renal, and neurological complications, in addition to systemic sepsis, postoperative red blood cell transfusion, unplanned return to the operating room, and prolonged hospitalization, according to the researchers.
However, predictors of CEs risk between vascular and general surgery were significantly different.
For general surgery, American Society of Anesthesiologists (ASA) status greater than 3, dependent functional status, acute renal failure or dialysis, weight loss, creatinine greater than 1.2 mg/dL, international normalized ratio (INR) greater than 1.5, and partial thromboplastin time (PTT) less than 35 seconds were all unique independent predictors of postoperative CEs.
For vascular surgery, the unique significant predictors of postoperative CEs were age greater than 65 years, emergency surgery, diabetes, congestive heart failure, systemic sepsis, and operative time greater than 240 minutes.
The only common predictive risk factors for postoperative CEs for the two forms of surgery were hematocrit less than 34% and ventilator dependence.
“The present study corroborates reported studies that recommend separate predictive CE risk indices and risk stratification among different surgical specialties. Predictors for CE greatly differed between general and vascular surgery patients in our patient population,” the authors stated.
They concluded with the hope that their study “provides useful information to surgeons and allows for the necessary resources to be focused on identified at-risk patients to improve surgical outcomes.”
Dr. Acheampong and his colleagues reported having no disclosures.
SOURCE: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
Predictive risk factors for cardiac events (CEs) after general and vascular surgery differed significantly, according to a large retrospective study. However, there was no significant difference seen in the overall incidence of CEs between the two types of surgery, reported Derrick Acheampong, MD, and his colleagues at the Icahn School of Medicine at Mount Sinai, New York.
They performed a retrospective data analysis of 8,441 adult patients at their large urban teaching hospital; these patients had undergone general or vascular surgery during 2013-2016 and, in the analysis, were grouped by whether they experienced postoperative CEs.
Univariate and multivariate analyses identified predictors of postoperative CE and the association of CEs with adverse postoperative outcomes. CEs were defined as myocardial infarction or cardiac arrest within the 30-day postoperative period.
A total of 157 patients (1.9%) experienced CEs after major general and vascular surgery, with no significant difference in incidence between the two types of surgery (P = .44), according to their report, published online in the Annals of Medicine and Surgery. CE-associated mortality among this group was high, at 55.4%.
The occurrence of a CE following surgery in both groups was significantly associated with increased mortality, as well as pulmonary, renal, and neurological complications, in addition to systemic sepsis, postoperative red blood cell transfusion, unplanned return to the operating room, and prolonged hospitalization, according to the researchers.
However, predictors of CEs risk between vascular and general surgery were significantly different.
For general surgery, American Society of Anesthesiologists (ASA) status greater than 3, dependent functional status, acute renal failure or dialysis, weight loss, creatinine greater than 1.2 mg/dL, international normalized ratio (INR) greater than 1.5, and partial thromboplastin time (PTT) less than 35 seconds were all unique independent predictors of postoperative CEs.
For vascular surgery, the unique significant predictors of postoperative CEs were age greater than 65 years, emergency surgery, diabetes, congestive heart failure, systemic sepsis, and operative time greater than 240 minutes.
The only common predictive risk factors for postoperative CEs for the two forms of surgery were hematocrit less than 34% and ventilator dependence.
“The present study corroborates reported studies that recommend separate predictive CE risk indices and risk stratification among different surgical specialties. Predictors for CE greatly differed between general and vascular surgery patients in our patient population,” the authors stated.
They concluded with the hope that their study “provides useful information to surgeons and allows for the necessary resources to be focused on identified at-risk patients to improve surgical outcomes.”
Dr. Acheampong and his colleagues reported having no disclosures.
SOURCE: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
FROM ANNALS OF MEDICINE AND SURGERY
Key clinical point: There was a significant difference in predictive risk factors for postoperative cardiac events between vascular and general surgery.
Major finding: The 1.9% incidence of cardiac events following general or vascular surgery was associated with a mortality rate of 55%.
Study details: Retrospective study of 8,441 patients who underwent vascular or general surgery during 2013-2015.
Disclosures: The authors reported having no disclosures.
Source: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
Fournier gangrene
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
An 88-year-old man with a 1-day history of fever and altered mental status was transferred to the emergency department. He had been receiving conservative management for low-risk localized prostate cancer but had no previous cardiovascular or gastrointestinal problems.
Based on these findings, the diagnosis was Fournier gangrene. Despite aggressive treatment, the patient’s condition deteriorated rapidly, and he died 2 hours after admission.
FOURNIER GANGRENE: NECROTIZING FASCIITIS OF THE PERINEUM
Fournier gangrene is a rare but rapidly progressive necrotizing fasciitis of the perineum with a high death rate.
Predisposing factors for Fournier gangrene include older age, diabetes mellitus, morbid obesity, cardiovascular disorders, chronic alcoholism, long-term corticosteroid treatment, malignancy, and human immunodeficiency virus infection.1,2 Urethral obstruction, instrumentation, urinary extravasation, and trauma have also been associated with this condition.3
In general, organisms from the urinary tract spread along the fascial planes to involve the penis and scrotum.
The differential diagnosis of Fournier gangrene includes scrotal and perineal disorders, as well as intra-abdominal disorders such as cellulitis, abscess, strangulated hernia, pyoderma gangrenosum, allergic vasculitis, vascular occlusion syndromes, and warfarin necrosis.
Delay in the diagnosis of Fournier gangrene leads to an extremely high death rate due to rapid progression of the disease, leading to sepsis, multiple organ failure, and disseminated intravascular coagulation. Immediate diagnosis and appropriate treatment such as broad-spectrum antibiotics and extensive surgical debridement reduce morbidity and control the infection. Antibiotics for methicillin-resistant Staphylococcus aureus should be considered if there is a history of or risk factors for this organism.4
Necrotizing fasciitis, including Fournier gangrene, is a common indication for intravenous immunoglobulin, and this treatment has been reported to be effective in a few cases. However, a double-blind, placebo-controlled trial that evaluated the benefit of this treatment was terminated early due to slow patient recruitment.5
A delay of even a few hours from suspicion of Fournier gangrene to surgical debridement significantly increases the risk of death.6 Thus, when it is suspected, immediate surgical intervention may be necessary to confirm the diagnosis and to treat it. The usual combination of antibiotic therapy for Fournier gangrene includes penicillin for the streptococcal species, a third-generation cephalosporin with or without an aminoglycoside for the gram-negative organisms, and metronidazole for anaerobic bacteria.
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157
- Wang YK, Li YH, Wu ST, Meng E. Fournier’s gangrene. QJM 2017; 110(10):671–672. doi:10.1093/qjmed/hcx124
- Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006; 30(9):1750–1754. doi:10.1007/s00268-005-0777-3
- Paonam SS, Bag S. Fournier gangrene with extensive necrosis of urethra and bladder mucosa: a rare occurrence in a patient with advanced prostate cancer. Urol Ann 2015; 7(4):507–509. doi:10.4103/0974-7796.157975
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg 2008; 6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
- Koch C, Hecker A, Grau V, Padberg W, Wolff M, Henrich M. Intravenous immunoglobulin in necrotizing fasciitis—a case report and review of recent literature. Ann Med Surg (Lond) 2015; 4(3):260–263. doi:10.1016/j.amsu.2015.07.017
- Singh A, Ahmed K, Aydin A, Khan MS, Dasgupta P. Fournier's gangrene. A clinical review. Arch Ital Urol Androl 2016; 88(3):157–164. doi:10.4081/aiua.2016.3.157