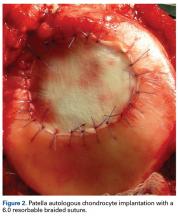
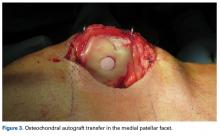
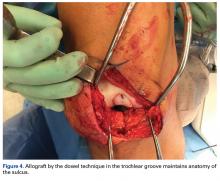
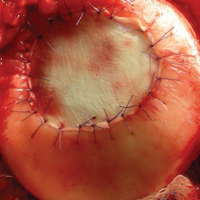
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
5 Points on Stiff Elbow
Take-Home Points
- Proper patient selection is critical as extensive postoperative rehabilitation is required to obtain an excellent outcome.
- Open and arthroscopic approaches are effective treatment options for elbow contractures.
- Elbow stability must be restored to obtain a successful outcome.
- Knowledge of neurovascular anatomy is essential to prevent neurologic complications.
- Prophylactic ulnar nerve release should be considered, especially in patients with limited flexion.
Elbow stiffness has several etiologies, posttraumatic being the most common. Elbow stiffness can have debilitating functional effects necessitating treatment. In a biomechanical study of normal elbow function, Morrey and colleagues1 determined that a flexion extension arc of 100° (30°-130°) and a forearm rotation arc of 100° (50° pronation-50° supination) are required in 90% of activities of daily living. Similarly, elbow flexion of <105° was poorly tolerated, whereas patients could easier adapt to flexion contractures up to 40°.2
The goal of initial evaluation should be to establish the cause of the contracture and the patient’s functional demands and ability to cooperate in the extensive postoperative rehabilitation that is essential in achieving an excellent functional outcome. In a thorough clinical examination, the clinician must note skin, range of motion (ROM), ligamentous stability, and neurovascular structures and give special attention to ulnar nerve function and symptoms. Mid-arc pain suggests additional intra-articular pathology, as stiffness typically causes pain only at the limits of motion as osteophytes impinge and soft tissue is under maximal tension. Routine elbow radiographs are required in all cases, and computed tomography (CT) can be useful in evaluating osseous sources of contracture. Suspected ligamentous instability and cartilaginous defects particularly in the setting of mid-arc pain are best evaluated with magnetic resonance imaging.3
In this 5-point review, we evaluate treatment options as well as rehabilitation protocols in the management of elbow stiffness.
1 Anatomy of Contracture: The Usual Suspects
The cause of elbow stiffness is incompletely understood. Several posited contributing factors include biology, complex intra-articular anatomy, capsular distention favoring a flexed position, and tenuous postoperative fixation necessitating prolonged immobilization. Identifying intrinsic and extrinsic anatomical sources of stiffness can help guide treatment.4 Intrinsic pathology includes intra-articular malunion, osteophytes, loose bodies, and adhesions; extrinsic pathology includes soft-tissue contracture, heterotopic ossification, and extra-articular malunion.
Compared with the normal elbow, the capsule becomes thickened and fibrotic and thereby prevents motion. Severe contractures, and extension contractures in particular, may require release of the posterior medial capsule and the posterior medial collateral ligament (MCL) to regain motion. In a series of 42 patients with flexion <100°, Park and colleagues5 noted that all patients required release of the posterior band of the MCL to regain flexion. Other muscular impediments to motion include contracture of the brachialis and scarring of the triceps to the posterior humerus. Scarring of the triceps to the humerus can limit flexion.
In the posttrauma setting, intra-articular and extra-articular malunion must be considered. Extension malunion of the distal humerus can reduce flexion,6 and shortening with compromise of the olecranon and coronoid fossae can limit both flexion and extension.
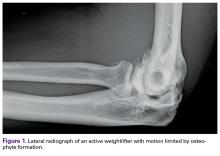
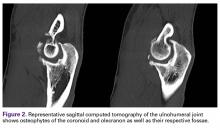
Last, heterotopic ossification and osteophytes should be assessed as potential causes of limited ROM. Both the coronoid process and the olecranon can develop osteophytes, and their respective fossae should be assessed with CT. Posterior impingement is rare at the tip of the olecranon; it occurs because of "widening" of the olecranon by "Mickey Mouse ear" osteophytes and bony encroachment along the medial and lateral columns. Thus, the olecranon must be narrowed and the fossa widened and deepened.
In case of concomitant ligament instability, we prefer to reconstruct the ligament first, and then perform contracture release as a staged procedure. We favor a staged approach because the rehabilitation regimens for instability and contracture release are diametrically opposed: Instability requires immobilization, and contracture release requires immediate motion. Last, incision placement and ulnar nerve management are crucial in minimizing the potential complications of the second procedure.
2 Nonoperative Treatment
In the absence of significant bony impediments to motion—such as heterotopic ossification or malunion—initial treatment should commence with nonoperative therapy. Therapy should be initiated as soon as concern for stiffness arises in order to prevent contracture. Initial nonoperative treatment can also serve as an important litmus test of postoperative adherence. Adequate patient relaxation is crucial in avoiding co-contracture resisting stretching forces. Passive ROM exercises use sustained force to allow time-dependent stress relaxation to increase tissue length as well as fatigue antagonist muscles. In addition, hold-and-relax techniques apply isometric resistance to induce relaxation of antagonist muscles.7 Active ROM should emphasize triceps isolation and elbow extension to prevent scarring of the triceps to the posterior humerus.
Corrective splinting can be an effective adjuvant to physiotherapy. Static progressive turnbuckle splints was described as an effective treatment for both elbow flexion and extension contractures, effecting an average 43° increase in elbow motion in a series of 15 patients.8 Similarly, Gelinas and colleagues9 noted improvement among 22 patients treated with turnbuckle splinting for an average of 4.5 months. In addition, serial extension splints may be used in the treatment of elbow flexion contractures.
3 Open Contacture Release and Surgical Approach
When nonoperative therapies fail to restore the functional arc of motion, patients with flexion contractures or extension contractures of >30° may be indicated for contracture release. Surgical approach should be determined by meticulous preoperative planning that notes prior incisions and CT findings. It can be helpful to organize common offending structures and their effects on flexion and extension (Table).
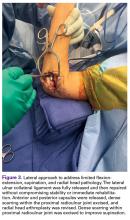
A medial over-the-top approach uses the medial supracondylar ridge as a landmark, subperiosteally reflecting the brachialis anteriorly.10 The ulnar nerve is neurolyzed and protected posteriorly. The flexor-pronator mass is split distally and elevated along with the brachialis as a single sleeve of muscle. The coronal plane of dissection should be the anterior half of the lateral epicondyle to avoid injury to the MCL. Large Bennett or Hohmann retractors can hinge on the lateral border of the humerus and provide clear visualization of the anterior capsule and the ulnohumeral joint. Exposure of the radiocapitellar joint is possible, but this joint is very deep in the operative field, and caution should be taken excising the anterolateral capsule because of the risk of radial nerve injury. The ulnar nerve can be temporarily transposed anteriorly to dissect posteriorly along the supracondylar ridge of the humerus. The triceps is reflected off the distal humerus. Occasionally, the posterior band of the MCL must be resected in severe extension contractures. If possible, the anterior bundle should be preserved. With this approach, the anterior capsule, distal humerus, coronoid process, posterior MCL, posterior capsule, and triceps can be addressed. The zone anterior to the radial head and the anterolateral and posterolateral capsule cannot be safely exposed with a medial approach. As described by Wada and colleagues,11 a primarily medial approach resulted in an average 64° increase in arc of motion.
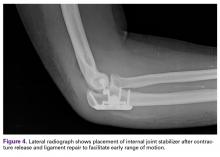
an internal joint stabilizer (Skeletal Dynamics) (Figure 4) and to initiate motion therapy immediately. External fixation (hinged or unhinged is rarely used in our practice.
4 Arthroscopic Contracture Release and Technique
Recently, arthroscopic elbow contracture release, a technically demanding but effective treatment option, has gained popularity. Knowledge of neurovascular anatomy is a prerequisite to the prevention of devastating neurologic complications (ulnar, median, and radial nerve transections have been described14,15). Relative contraindications include extensive heterotopic ossification, ulnar nerve transposition, and limited arthroscopic experience. Functional improvements as well as average 26° to 42° increases in arc of motion have been described with arthroscopic release.16-18 In thin-framed patients with dense elbow capsular scarring (severe loss of elbow motion with hard block) and small joint space, arthroscopic release and particularly arthroscope insertion are notoriously difficult.
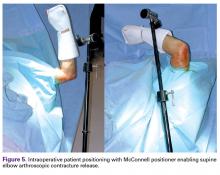
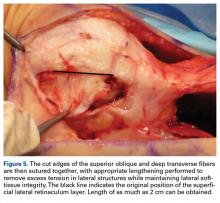
The patient may be placed in the prone, lateral decubitus, or supine position, depending on surgeon preference (Figure 5). Before surgery, portals and the ulnar nerve should be carefully outlined.19
We prefer to start by entering the posterior compartment and using the shaver to create a working space. All bone work and resectioning should be performed before capsular resection. After the joint and the olecranon fossa are identified, soft-tissue and bony débridement of the olecranon and the fossa can be performed. Care should be taken to protect the ulnar nerve when the posteromedial corner or medial gutter is approached.
5 Additional Considerations
After surgery, the elbow is immobilized in maximal extension and supination with an anterior splint, and therapy is initiated either immediately or after temporary immobilization.16,19,20 Regional anesthesia is crucial in obtaining adequate pain control and establishing an immediate postoperative therapy program. The utility of continuous passive motion (CPM) in postoperative protocols is controversial. A retrospective case-control study of 32 patients matched on age, diagnosis, and contraction severity found no benefit of CPM use, and increased costs and hospital length of stay, leading the authors to recommend against CPM use.20
Neurovascular risks are associated with both open and arthroscopic elbow contracture release. Particularly concerning is the risk of traction ulnar neuropathy, described in upward of 20% of patients.21 Anatomical studies have found decreases in cubital tunnel and ulnar nerve area as elbow flexion increases with corresponding increased intraneural pressure,22 leading some authors to recommend prophylactic ulnar nerve release with limited preoperative flexion.15 Nevertheless, despite transposition, ulnar nerve symptoms were noted in 8 of 40 patients who underwent open contracture release for posttraumatic loss of elbow flexion.5 In a retrospective review of 164 open and arthroscopic elbow contracture releases, Williams and colleagues21 noted an 8.1% rate of postoperative new-onset ulnar nerve symptoms. The rate of ulnar neuropathy was nonsignificantly elevated among patients with preoperative flexion of <100° (15.2% vs 3.7%; P = .057). Recently, a retrospective review of 564 consecutive arthroscopic contracture releases found a significantly higher rate of delayed-onset ulnar neuritis among patients without prophylactic ulnar nerve decompression or transposition (11% vs 3%; P < .001).23 Further analysis revealed that, compared with decompression, ulnar nerve transposition did not offer additional benefit but was associated with a significantly higher rate of wound complications (19% vs 4%; P = .03). We favor prophylactic release, particularly in the setting of preoperative extension contracture. For open contracture release from the lateral approach, however, we do not routinely release the ulnar nerve unless there were preoperative symptoms.
Although open and arthroscopic contracture releases can provide durable outcomes in the setting of painless elbow stiffness, options are more limited in the treatment of the painful stiff elbow. Total elbow arthroplasty remains an option in low-demand elderly patients but is not without significant risk of complications.24 In addition, durability concerns and postoperative restrictions make total elbow arthroplasty less attractive to younger patients. Interposition arthroplasty may be indicated as a salvage procedure in the treatment of a young or high-demand patient with a stiff painful elbow.25 Elbow stability is crucial in obtaining a successful outcome, and data on optimal graft choices are limited.
Conclusion
Elbow stiffness, a common complication of trauma, significantly impairs activities of daily living. Early after trauma, therapy should be initiated to prevent contracture. In the absence of symptomatic arthritis, both open and arthroscopic contracture releases are effective surgical treatments in properly selected and motivated patients. Although more research is needed to establish the optimal surgical approach, severity and anatomical cause of contracture should guide decisions as to which approach to use. Having a thorough understanding of neurovascular anatomy and of prophylactic ulnar nerve decompression in the setting of limited preoperative flexion can mitigate complications.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Hotchkiss RN. Elbow contracture. In: Green DP, Rotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. New York, NY: Churchill-Livingstone; 2005:667-682.
3. Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19(2):13-19.
4. Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601-618.
5. Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692-2699.
6. Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg Am. 2009;34(7):
1256-1260.
7. Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300-309.
8. Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092-1095.
9. Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74-78.
10. Hotchkiss RN, Kasparyan GN. The medial "over the top" approach to the elbow. Tech Orthop. 2000;15(2):105-112.
11. Wada T, Ishii S, Usui M, Miyano S. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Br. 2000;82(1):68-73.
12. Husband JB, Hastings H. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353-1358.
13. Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603-1605.
14. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83(1):25-34.
16. Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11(6):624-629.
17. Ćefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434-439.
18. Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842-849.
19. Sahajpal D, Choi T, Wright TW. Arthroscopic release of the stiff elbow. J Hand Surg. 2009;34(3):540-544.
20. Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg. 2007;32(10):1605-1623.
21. Williams BG, Sotereanos DG, Baratz ME, Jarrett CD, Venouziou AI, Miller MC. The contracted elbow: is ulnar nerve release necessary? J Shoulder Elbow Surg. 2012;21(12):
1632-1636.
22. Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492-501.
23. Blonna D, O’Driscoll SW. Delayed-onset ulnar neuritis after release of elbow contracture: preventive strategies derived from a study of 563 cases. Arthroscopy. 2014;30(8):947-956.
24. Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg. 2000;82(9):1260-1268.
25. Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181-188.
Take-Home Points
- Proper patient selection is critical as extensive postoperative rehabilitation is required to obtain an excellent outcome.
- Open and arthroscopic approaches are effective treatment options for elbow contractures.
- Elbow stability must be restored to obtain a successful outcome.
- Knowledge of neurovascular anatomy is essential to prevent neurologic complications.
- Prophylactic ulnar nerve release should be considered, especially in patients with limited flexion.
Elbow stiffness has several etiologies, posttraumatic being the most common. Elbow stiffness can have debilitating functional effects necessitating treatment. In a biomechanical study of normal elbow function, Morrey and colleagues1 determined that a flexion extension arc of 100° (30°-130°) and a forearm rotation arc of 100° (50° pronation-50° supination) are required in 90% of activities of daily living. Similarly, elbow flexion of <105° was poorly tolerated, whereas patients could easier adapt to flexion contractures up to 40°.2
The goal of initial evaluation should be to establish the cause of the contracture and the patient’s functional demands and ability to cooperate in the extensive postoperative rehabilitation that is essential in achieving an excellent functional outcome. In a thorough clinical examination, the clinician must note skin, range of motion (ROM), ligamentous stability, and neurovascular structures and give special attention to ulnar nerve function and symptoms. Mid-arc pain suggests additional intra-articular pathology, as stiffness typically causes pain only at the limits of motion as osteophytes impinge and soft tissue is under maximal tension. Routine elbow radiographs are required in all cases, and computed tomography (CT) can be useful in evaluating osseous sources of contracture. Suspected ligamentous instability and cartilaginous defects particularly in the setting of mid-arc pain are best evaluated with magnetic resonance imaging.3
In this 5-point review, we evaluate treatment options as well as rehabilitation protocols in the management of elbow stiffness.
1 Anatomy of Contracture: The Usual Suspects
The cause of elbow stiffness is incompletely understood. Several posited contributing factors include biology, complex intra-articular anatomy, capsular distention favoring a flexed position, and tenuous postoperative fixation necessitating prolonged immobilization. Identifying intrinsic and extrinsic anatomical sources of stiffness can help guide treatment.4 Intrinsic pathology includes intra-articular malunion, osteophytes, loose bodies, and adhesions; extrinsic pathology includes soft-tissue contracture, heterotopic ossification, and extra-articular malunion.
Compared with the normal elbow, the capsule becomes thickened and fibrotic and thereby prevents motion. Severe contractures, and extension contractures in particular, may require release of the posterior medial capsule and the posterior medial collateral ligament (MCL) to regain motion. In a series of 42 patients with flexion <100°, Park and colleagues5 noted that all patients required release of the posterior band of the MCL to regain flexion. Other muscular impediments to motion include contracture of the brachialis and scarring of the triceps to the posterior humerus. Scarring of the triceps to the humerus can limit flexion.
In the posttrauma setting, intra-articular and extra-articular malunion must be considered. Extension malunion of the distal humerus can reduce flexion,6 and shortening with compromise of the olecranon and coronoid fossae can limit both flexion and extension.
Last, heterotopic ossification and osteophytes should be assessed as potential causes of limited ROM. Both the coronoid process and the olecranon can develop osteophytes, and their respective fossae should be assessed with CT. Posterior impingement is rare at the tip of the olecranon; it occurs because of "widening" of the olecranon by "Mickey Mouse ear" osteophytes and bony encroachment along the medial and lateral columns. Thus, the olecranon must be narrowed and the fossa widened and deepened.
In case of concomitant ligament instability, we prefer to reconstruct the ligament first, and then perform contracture release as a staged procedure. We favor a staged approach because the rehabilitation regimens for instability and contracture release are diametrically opposed: Instability requires immobilization, and contracture release requires immediate motion. Last, incision placement and ulnar nerve management are crucial in minimizing the potential complications of the second procedure.
2 Nonoperative Treatment
In the absence of significant bony impediments to motion—such as heterotopic ossification or malunion—initial treatment should commence with nonoperative therapy. Therapy should be initiated as soon as concern for stiffness arises in order to prevent contracture. Initial nonoperative treatment can also serve as an important litmus test of postoperative adherence. Adequate patient relaxation is crucial in avoiding co-contracture resisting stretching forces. Passive ROM exercises use sustained force to allow time-dependent stress relaxation to increase tissue length as well as fatigue antagonist muscles. In addition, hold-and-relax techniques apply isometric resistance to induce relaxation of antagonist muscles.7 Active ROM should emphasize triceps isolation and elbow extension to prevent scarring of the triceps to the posterior humerus.
Corrective splinting can be an effective adjuvant to physiotherapy. Static progressive turnbuckle splints was described as an effective treatment for both elbow flexion and extension contractures, effecting an average 43° increase in elbow motion in a series of 15 patients.8 Similarly, Gelinas and colleagues9 noted improvement among 22 patients treated with turnbuckle splinting for an average of 4.5 months. In addition, serial extension splints may be used in the treatment of elbow flexion contractures.
3 Open Contacture Release and Surgical Approach
When nonoperative therapies fail to restore the functional arc of motion, patients with flexion contractures or extension contractures of >30° may be indicated for contracture release. Surgical approach should be determined by meticulous preoperative planning that notes prior incisions and CT findings. It can be helpful to organize common offending structures and their effects on flexion and extension (Table).
A medial over-the-top approach uses the medial supracondylar ridge as a landmark, subperiosteally reflecting the brachialis anteriorly.10 The ulnar nerve is neurolyzed and protected posteriorly. The flexor-pronator mass is split distally and elevated along with the brachialis as a single sleeve of muscle. The coronal plane of dissection should be the anterior half of the lateral epicondyle to avoid injury to the MCL. Large Bennett or Hohmann retractors can hinge on the lateral border of the humerus and provide clear visualization of the anterior capsule and the ulnohumeral joint. Exposure of the radiocapitellar joint is possible, but this joint is very deep in the operative field, and caution should be taken excising the anterolateral capsule because of the risk of radial nerve injury. The ulnar nerve can be temporarily transposed anteriorly to dissect posteriorly along the supracondylar ridge of the humerus. The triceps is reflected off the distal humerus. Occasionally, the posterior band of the MCL must be resected in severe extension contractures. If possible, the anterior bundle should be preserved. With this approach, the anterior capsule, distal humerus, coronoid process, posterior MCL, posterior capsule, and triceps can be addressed. The zone anterior to the radial head and the anterolateral and posterolateral capsule cannot be safely exposed with a medial approach. As described by Wada and colleagues,11 a primarily medial approach resulted in an average 64° increase in arc of motion.
an internal joint stabilizer (Skeletal Dynamics) (Figure 4) and to initiate motion therapy immediately. External fixation (hinged or unhinged is rarely used in our practice.
4 Arthroscopic Contracture Release and Technique
Recently, arthroscopic elbow contracture release, a technically demanding but effective treatment option, has gained popularity. Knowledge of neurovascular anatomy is a prerequisite to the prevention of devastating neurologic complications (ulnar, median, and radial nerve transections have been described14,15). Relative contraindications include extensive heterotopic ossification, ulnar nerve transposition, and limited arthroscopic experience. Functional improvements as well as average 26° to 42° increases in arc of motion have been described with arthroscopic release.16-18 In thin-framed patients with dense elbow capsular scarring (severe loss of elbow motion with hard block) and small joint space, arthroscopic release and particularly arthroscope insertion are notoriously difficult.
The patient may be placed in the prone, lateral decubitus, or supine position, depending on surgeon preference (Figure 5). Before surgery, portals and the ulnar nerve should be carefully outlined.19
We prefer to start by entering the posterior compartment and using the shaver to create a working space. All bone work and resectioning should be performed before capsular resection. After the joint and the olecranon fossa are identified, soft-tissue and bony débridement of the olecranon and the fossa can be performed. Care should be taken to protect the ulnar nerve when the posteromedial corner or medial gutter is approached.
5 Additional Considerations
After surgery, the elbow is immobilized in maximal extension and supination with an anterior splint, and therapy is initiated either immediately or after temporary immobilization.16,19,20 Regional anesthesia is crucial in obtaining adequate pain control and establishing an immediate postoperative therapy program. The utility of continuous passive motion (CPM) in postoperative protocols is controversial. A retrospective case-control study of 32 patients matched on age, diagnosis, and contraction severity found no benefit of CPM use, and increased costs and hospital length of stay, leading the authors to recommend against CPM use.20
Neurovascular risks are associated with both open and arthroscopic elbow contracture release. Particularly concerning is the risk of traction ulnar neuropathy, described in upward of 20% of patients.21 Anatomical studies have found decreases in cubital tunnel and ulnar nerve area as elbow flexion increases with corresponding increased intraneural pressure,22 leading some authors to recommend prophylactic ulnar nerve release with limited preoperative flexion.15 Nevertheless, despite transposition, ulnar nerve symptoms were noted in 8 of 40 patients who underwent open contracture release for posttraumatic loss of elbow flexion.5 In a retrospective review of 164 open and arthroscopic elbow contracture releases, Williams and colleagues21 noted an 8.1% rate of postoperative new-onset ulnar nerve symptoms. The rate of ulnar neuropathy was nonsignificantly elevated among patients with preoperative flexion of <100° (15.2% vs 3.7%; P = .057). Recently, a retrospective review of 564 consecutive arthroscopic contracture releases found a significantly higher rate of delayed-onset ulnar neuritis among patients without prophylactic ulnar nerve decompression or transposition (11% vs 3%; P < .001).23 Further analysis revealed that, compared with decompression, ulnar nerve transposition did not offer additional benefit but was associated with a significantly higher rate of wound complications (19% vs 4%; P = .03). We favor prophylactic release, particularly in the setting of preoperative extension contracture. For open contracture release from the lateral approach, however, we do not routinely release the ulnar nerve unless there were preoperative symptoms.
Although open and arthroscopic contracture releases can provide durable outcomes in the setting of painless elbow stiffness, options are more limited in the treatment of the painful stiff elbow. Total elbow arthroplasty remains an option in low-demand elderly patients but is not without significant risk of complications.24 In addition, durability concerns and postoperative restrictions make total elbow arthroplasty less attractive to younger patients. Interposition arthroplasty may be indicated as a salvage procedure in the treatment of a young or high-demand patient with a stiff painful elbow.25 Elbow stability is crucial in obtaining a successful outcome, and data on optimal graft choices are limited.
Conclusion
Elbow stiffness, a common complication of trauma, significantly impairs activities of daily living. Early after trauma, therapy should be initiated to prevent contracture. In the absence of symptomatic arthritis, both open and arthroscopic contracture releases are effective surgical treatments in properly selected and motivated patients. Although more research is needed to establish the optimal surgical approach, severity and anatomical cause of contracture should guide decisions as to which approach to use. Having a thorough understanding of neurovascular anatomy and of prophylactic ulnar nerve decompression in the setting of limited preoperative flexion can mitigate complications.
Take-Home Points
- Proper patient selection is critical as extensive postoperative rehabilitation is required to obtain an excellent outcome.
- Open and arthroscopic approaches are effective treatment options for elbow contractures.
- Elbow stability must be restored to obtain a successful outcome.
- Knowledge of neurovascular anatomy is essential to prevent neurologic complications.
- Prophylactic ulnar nerve release should be considered, especially in patients with limited flexion.
Elbow stiffness has several etiologies, posttraumatic being the most common. Elbow stiffness can have debilitating functional effects necessitating treatment. In a biomechanical study of normal elbow function, Morrey and colleagues1 determined that a flexion extension arc of 100° (30°-130°) and a forearm rotation arc of 100° (50° pronation-50° supination) are required in 90% of activities of daily living. Similarly, elbow flexion of <105° was poorly tolerated, whereas patients could easier adapt to flexion contractures up to 40°.2
The goal of initial evaluation should be to establish the cause of the contracture and the patient’s functional demands and ability to cooperate in the extensive postoperative rehabilitation that is essential in achieving an excellent functional outcome. In a thorough clinical examination, the clinician must note skin, range of motion (ROM), ligamentous stability, and neurovascular structures and give special attention to ulnar nerve function and symptoms. Mid-arc pain suggests additional intra-articular pathology, as stiffness typically causes pain only at the limits of motion as osteophytes impinge and soft tissue is under maximal tension. Routine elbow radiographs are required in all cases, and computed tomography (CT) can be useful in evaluating osseous sources of contracture. Suspected ligamentous instability and cartilaginous defects particularly in the setting of mid-arc pain are best evaluated with magnetic resonance imaging.3
In this 5-point review, we evaluate treatment options as well as rehabilitation protocols in the management of elbow stiffness.
1 Anatomy of Contracture: The Usual Suspects
The cause of elbow stiffness is incompletely understood. Several posited contributing factors include biology, complex intra-articular anatomy, capsular distention favoring a flexed position, and tenuous postoperative fixation necessitating prolonged immobilization. Identifying intrinsic and extrinsic anatomical sources of stiffness can help guide treatment.4 Intrinsic pathology includes intra-articular malunion, osteophytes, loose bodies, and adhesions; extrinsic pathology includes soft-tissue contracture, heterotopic ossification, and extra-articular malunion.
Compared with the normal elbow, the capsule becomes thickened and fibrotic and thereby prevents motion. Severe contractures, and extension contractures in particular, may require release of the posterior medial capsule and the posterior medial collateral ligament (MCL) to regain motion. In a series of 42 patients with flexion <100°, Park and colleagues5 noted that all patients required release of the posterior band of the MCL to regain flexion. Other muscular impediments to motion include contracture of the brachialis and scarring of the triceps to the posterior humerus. Scarring of the triceps to the humerus can limit flexion.
In the posttrauma setting, intra-articular and extra-articular malunion must be considered. Extension malunion of the distal humerus can reduce flexion,6 and shortening with compromise of the olecranon and coronoid fossae can limit both flexion and extension.
Last, heterotopic ossification and osteophytes should be assessed as potential causes of limited ROM. Both the coronoid process and the olecranon can develop osteophytes, and their respective fossae should be assessed with CT. Posterior impingement is rare at the tip of the olecranon; it occurs because of "widening" of the olecranon by "Mickey Mouse ear" osteophytes and bony encroachment along the medial and lateral columns. Thus, the olecranon must be narrowed and the fossa widened and deepened.
In case of concomitant ligament instability, we prefer to reconstruct the ligament first, and then perform contracture release as a staged procedure. We favor a staged approach because the rehabilitation regimens for instability and contracture release are diametrically opposed: Instability requires immobilization, and contracture release requires immediate motion. Last, incision placement and ulnar nerve management are crucial in minimizing the potential complications of the second procedure.
2 Nonoperative Treatment
In the absence of significant bony impediments to motion—such as heterotopic ossification or malunion—initial treatment should commence with nonoperative therapy. Therapy should be initiated as soon as concern for stiffness arises in order to prevent contracture. Initial nonoperative treatment can also serve as an important litmus test of postoperative adherence. Adequate patient relaxation is crucial in avoiding co-contracture resisting stretching forces. Passive ROM exercises use sustained force to allow time-dependent stress relaxation to increase tissue length as well as fatigue antagonist muscles. In addition, hold-and-relax techniques apply isometric resistance to induce relaxation of antagonist muscles.7 Active ROM should emphasize triceps isolation and elbow extension to prevent scarring of the triceps to the posterior humerus.
Corrective splinting can be an effective adjuvant to physiotherapy. Static progressive turnbuckle splints was described as an effective treatment for both elbow flexion and extension contractures, effecting an average 43° increase in elbow motion in a series of 15 patients.8 Similarly, Gelinas and colleagues9 noted improvement among 22 patients treated with turnbuckle splinting for an average of 4.5 months. In addition, serial extension splints may be used in the treatment of elbow flexion contractures.
3 Open Contacture Release and Surgical Approach
When nonoperative therapies fail to restore the functional arc of motion, patients with flexion contractures or extension contractures of >30° may be indicated for contracture release. Surgical approach should be determined by meticulous preoperative planning that notes prior incisions and CT findings. It can be helpful to organize common offending structures and their effects on flexion and extension (Table).
A medial over-the-top approach uses the medial supracondylar ridge as a landmark, subperiosteally reflecting the brachialis anteriorly.10 The ulnar nerve is neurolyzed and protected posteriorly. The flexor-pronator mass is split distally and elevated along with the brachialis as a single sleeve of muscle. The coronal plane of dissection should be the anterior half of the lateral epicondyle to avoid injury to the MCL. Large Bennett or Hohmann retractors can hinge on the lateral border of the humerus and provide clear visualization of the anterior capsule and the ulnohumeral joint. Exposure of the radiocapitellar joint is possible, but this joint is very deep in the operative field, and caution should be taken excising the anterolateral capsule because of the risk of radial nerve injury. The ulnar nerve can be temporarily transposed anteriorly to dissect posteriorly along the supracondylar ridge of the humerus. The triceps is reflected off the distal humerus. Occasionally, the posterior band of the MCL must be resected in severe extension contractures. If possible, the anterior bundle should be preserved. With this approach, the anterior capsule, distal humerus, coronoid process, posterior MCL, posterior capsule, and triceps can be addressed. The zone anterior to the radial head and the anterolateral and posterolateral capsule cannot be safely exposed with a medial approach. As described by Wada and colleagues,11 a primarily medial approach resulted in an average 64° increase in arc of motion.
an internal joint stabilizer (Skeletal Dynamics) (Figure 4) and to initiate motion therapy immediately. External fixation (hinged or unhinged is rarely used in our practice.
4 Arthroscopic Contracture Release and Technique
Recently, arthroscopic elbow contracture release, a technically demanding but effective treatment option, has gained popularity. Knowledge of neurovascular anatomy is a prerequisite to the prevention of devastating neurologic complications (ulnar, median, and radial nerve transections have been described14,15). Relative contraindications include extensive heterotopic ossification, ulnar nerve transposition, and limited arthroscopic experience. Functional improvements as well as average 26° to 42° increases in arc of motion have been described with arthroscopic release.16-18 In thin-framed patients with dense elbow capsular scarring (severe loss of elbow motion with hard block) and small joint space, arthroscopic release and particularly arthroscope insertion are notoriously difficult.
The patient may be placed in the prone, lateral decubitus, or supine position, depending on surgeon preference (Figure 5). Before surgery, portals and the ulnar nerve should be carefully outlined.19
We prefer to start by entering the posterior compartment and using the shaver to create a working space. All bone work and resectioning should be performed before capsular resection. After the joint and the olecranon fossa are identified, soft-tissue and bony débridement of the olecranon and the fossa can be performed. Care should be taken to protect the ulnar nerve when the posteromedial corner or medial gutter is approached.
5 Additional Considerations
After surgery, the elbow is immobilized in maximal extension and supination with an anterior splint, and therapy is initiated either immediately or after temporary immobilization.16,19,20 Regional anesthesia is crucial in obtaining adequate pain control and establishing an immediate postoperative therapy program. The utility of continuous passive motion (CPM) in postoperative protocols is controversial. A retrospective case-control study of 32 patients matched on age, diagnosis, and contraction severity found no benefit of CPM use, and increased costs and hospital length of stay, leading the authors to recommend against CPM use.20
Neurovascular risks are associated with both open and arthroscopic elbow contracture release. Particularly concerning is the risk of traction ulnar neuropathy, described in upward of 20% of patients.21 Anatomical studies have found decreases in cubital tunnel and ulnar nerve area as elbow flexion increases with corresponding increased intraneural pressure,22 leading some authors to recommend prophylactic ulnar nerve release with limited preoperative flexion.15 Nevertheless, despite transposition, ulnar nerve symptoms were noted in 8 of 40 patients who underwent open contracture release for posttraumatic loss of elbow flexion.5 In a retrospective review of 164 open and arthroscopic elbow contracture releases, Williams and colleagues21 noted an 8.1% rate of postoperative new-onset ulnar nerve symptoms. The rate of ulnar neuropathy was nonsignificantly elevated among patients with preoperative flexion of <100° (15.2% vs 3.7%; P = .057). Recently, a retrospective review of 564 consecutive arthroscopic contracture releases found a significantly higher rate of delayed-onset ulnar neuritis among patients without prophylactic ulnar nerve decompression or transposition (11% vs 3%; P < .001).23 Further analysis revealed that, compared with decompression, ulnar nerve transposition did not offer additional benefit but was associated with a significantly higher rate of wound complications (19% vs 4%; P = .03). We favor prophylactic release, particularly in the setting of preoperative extension contracture. For open contracture release from the lateral approach, however, we do not routinely release the ulnar nerve unless there were preoperative symptoms.
Although open and arthroscopic contracture releases can provide durable outcomes in the setting of painless elbow stiffness, options are more limited in the treatment of the painful stiff elbow. Total elbow arthroplasty remains an option in low-demand elderly patients but is not without significant risk of complications.24 In addition, durability concerns and postoperative restrictions make total elbow arthroplasty less attractive to younger patients. Interposition arthroplasty may be indicated as a salvage procedure in the treatment of a young or high-demand patient with a stiff painful elbow.25 Elbow stability is crucial in obtaining a successful outcome, and data on optimal graft choices are limited.
Conclusion
Elbow stiffness, a common complication of trauma, significantly impairs activities of daily living. Early after trauma, therapy should be initiated to prevent contracture. In the absence of symptomatic arthritis, both open and arthroscopic contracture releases are effective surgical treatments in properly selected and motivated patients. Although more research is needed to establish the optimal surgical approach, severity and anatomical cause of contracture should guide decisions as to which approach to use. Having a thorough understanding of neurovascular anatomy and of prophylactic ulnar nerve decompression in the setting of limited preoperative flexion can mitigate complications.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Hotchkiss RN. Elbow contracture. In: Green DP, Rotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. New York, NY: Churchill-Livingstone; 2005:667-682.
3. Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19(2):13-19.
4. Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601-618.
5. Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692-2699.
6. Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg Am. 2009;34(7):
1256-1260.
7. Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300-309.
8. Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092-1095.
9. Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74-78.
10. Hotchkiss RN, Kasparyan GN. The medial "over the top" approach to the elbow. Tech Orthop. 2000;15(2):105-112.
11. Wada T, Ishii S, Usui M, Miyano S. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Br. 2000;82(1):68-73.
12. Husband JB, Hastings H. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353-1358.
13. Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603-1605.
14. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83(1):25-34.
16. Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11(6):624-629.
17. Ćefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434-439.
18. Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842-849.
19. Sahajpal D, Choi T, Wright TW. Arthroscopic release of the stiff elbow. J Hand Surg. 2009;34(3):540-544.
20. Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg. 2007;32(10):1605-1623.
21. Williams BG, Sotereanos DG, Baratz ME, Jarrett CD, Venouziou AI, Miller MC. The contracted elbow: is ulnar nerve release necessary? J Shoulder Elbow Surg. 2012;21(12):
1632-1636.
22. Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492-501.
23. Blonna D, O’Driscoll SW. Delayed-onset ulnar neuritis after release of elbow contracture: preventive strategies derived from a study of 563 cases. Arthroscopy. 2014;30(8):947-956.
24. Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg. 2000;82(9):1260-1268.
25. Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181-188.
1. Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872-877.
2. Hotchkiss RN. Elbow contracture. In: Green DP, Rotchkiss RN, Pederson WC, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. New York, NY: Churchill-Livingstone; 2005:667-682.
3. Van Zeeland NL, Yamaguchi K. Arthroscopic capsular release of the elbow. J Shoulder Elbow Surg. 2010;19(2):13-19.
4. Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601-618.
5. Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692-2699.
6. Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg Am. 2009;34(7):
1256-1260.
7. Taylor DC, Dalton JD, Seaber AV, Garrett WE. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300-309.
8. Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092-1095.
9. Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74-78.
10. Hotchkiss RN, Kasparyan GN. The medial "over the top" approach to the elbow. Tech Orthop. 2000;15(2):105-112.
11. Wada T, Ishii S, Usui M, Miyano S. The medial approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Br. 2000;82(1):68-73.
12. Husband JB, Hastings H. The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353-1358.
13. Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603-1605.
14. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
15. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83(1):25-34.
16. Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 2002;11(6):624-629.
17. Ćefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434-439.
18. Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842-849.
19. Sahajpal D, Choi T, Wright TW. Arthroscopic release of the stiff elbow. J Hand Surg. 2009;34(3):540-544.
20. Lindenhovius AL, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg. 2007;32(10):1605-1623.
21. Williams BG, Sotereanos DG, Baratz ME, Jarrett CD, Venouziou AI, Miller MC. The contracted elbow: is ulnar nerve release necessary? J Shoulder Elbow Surg. 2012;21(12):
1632-1636.
22. Gelberman RH, Yamaguchi K, Hollstien SB, et al. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. an experimental study in human cadavera. J Bone Joint Surg Am. 1998;80(4):492-501.
23. Blonna D, O’Driscoll SW. Delayed-onset ulnar neuritis after release of elbow contracture: preventive strategies derived from a study of 563 cases. Arthroscopy. 2014;30(8):947-956.
24. Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg. 2000;82(9):1260-1268.
25. Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181-188.
Distal Radius Fractures: Reconstruction Approaches, Planning, and Principles
Take-Home Points
- Restore proper anatomic parameters; compare to the other side.
- Don't forget about the DRU joint.
- CT can aide in identifying subtle articular depression and severe comminution to change operative management.
- Remember, there still is a role for external fixators; an alternative remains an internal spanning plate.
- Respect the soft tissues, which can aide in reduction, however don't leave the operating room without feeling confident about your fixation.
Distal radius fracture (DRF), a common fracture, accounts for almost one sixth of all emergency department visits.1 With the advent of emerging technologies and refined technique, treatment options for DRFs have evolved. Although controversy remains regarding nonoperative vs operative treatment of DRFs in the elderly,2,3 select situations (open injuries, complex high-energy injuries, young age) warrant definitive fixation. Previously, internal fixation options were limited. Current technologies include locked fixed-angle plating, fragment-specific fixation, and locked variable-angle plating. These modalities aid in achieving and maintaining more anatomical fixation. This article summarizes tips, tricks, and planning for definitive external and internal fixation of complex DRFs.
Anatomical Considerations and Classification
The wrist joint, part of the complex articular network that begins at the forearm and ends at the distal interphalangeal joint, is the foundation for fine- and gross-motor skills. Understanding the anatomy of this network can provide a valuable roadmap for operative reconstruction.
At the wrist level, the radius bears most of the weight-bearing, and in some studies exhibits up to 80% of the load.1,4 The triangular distal radius bears this weight through a biconcave articular surface with facets for the lunate and scaphoid separated by an anteroposterior ridge.5-7 The radius also articulates with the ulnar head at the sigmoid notch to form the distal radioulnar (DRU) joint. Restoring the relationships of the DRU joint, the triangular fibrocartilage complex, and the ulnar variance is of paramount importance.1,8,9
Classical teaching calls for restoration of radial inclination to about 23°, volar tilt to 11° to 12°, and radial length to about 11 mm. Especially regarding volar tilt and radial length, however, cadaveric and clinical studies have found more variance, leading to use of the contralateral extremity as an operative template, particularly when closed reduction thought to be adequate deviates significantly from these parameters.1,4,7
DRF classification based on these principles has led to abundant representation in the literature.10-13 Many authors have focused on fracture lines, comminution degree, articular surface violation, and other anatomical or radiographic characteristics of DRF classification and operative fixation approach.10-13 In 2001, Fernandez9 proposed a classification system focused on energy or mechanism of injury. In comparisons,14 the Fernandez system had the highest interobserver reliability—higher than that of AO (Arbeitsgemeinschaft für Osteosynthesefragen).
Considerations for Operative Treatment: Column Theory
In the restoration of anatomical alignment in complex DRFs, it is important to consider the 3 joints and the 3 columns—radial, intermediate, and ulnar (Figure 1). [[{"fid":"201864","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]In addition, parallels between the distal radius and the tibial plateau can be considered because of similarities in operative goals. Restoration of mechanical axis, length, alignment, rotation, and articular surfaces is paramount.15 Considering multiple surgical approaches to address "bicolumnar injuries" and reconstructing the "simpler" columnar injury first are common principles.16
The goals of fracture fixation at the wrist are the same as at any other joint: anatomical reduction, stable fixation, and early range of motion (ROM). Column restoration can result in consistent achievement of those goals. Intuitively, there is a close correlation between anatomical alignment and functional results.17 Rebuilding the structural foundation of the columns with respect to buttressing and restoring the 3 radial articulations with the ulna, scaphoid, and lunate can consistently yield restoration of length, inclination, and tilt (Figure 2). [[{"fid":"201865","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Next, we discuss the options available and how to use each to an advantage, individually or in hybrid constructs.
External Fixation: Is There Still a Role?
In the setting of highly comminuted, complex fractures, external fixation with Kirschner wires (K-wires) is a reasonable choice, with restoration of motion and strength within 75% to 80% of the uninjured wrist.18 In a 2-year study of 113 patients with comminuted metaphyseal DRFs randomly assigned to either external fixation or casting, Kreder and colleagues19 found a trend toward better clinical, functional, and radiographic outcomes with external fixation with or without K-wire fixation. There was improved restoration of radial length and palmar tilt with external fixation. A study of unstable DRF in patients with osteoporosis found that redisplacement was more common after treatment with a cast than after treatment with an external fixator.20 Although closed reduction and casting continue to have a role in the treatment of DRF, Kreder and colleagues19 found that remanipulation was necessary in at least 9% of cases. According to a meta-analysis21 of the literature on DRF treatment, 4 articles directly address the question of the superiority of external fixation over closed reduction and casting, and 3 of the 4 found more favorable radiographic and functional outcomes with external fixation.
External fixation is useful in treating complex DRFs with metaphyseal comminution. It can also be effective in the presence of simple articular involvement without depression of the joint surface. External fixation devices can span areas of soft-
tissue injury and are useful as manipulation tools in achieving anatomical reduction. Although external fixation is effective, its complications include pin-tract infection, nerve injury, loss of reduction, and loss of digital ROM. In a meta-analysis, Li-hai and colleagues22 found that external fixators had a complication rate of 30.9%. With this technique, it is important to avoid midcarpal distraction, excessive ulnar deviation, and excessive palmar flexion. Papadonikolakis and colleagues23 found that distraction of as little as 2 mm to 5 mm significantly affected the function of the flexor digitorum superficialis at the metacarpophalangeal joint. Over-distraction in wrist flexion can lead to lengthening of the extensor tendons and loss of full digital ROM. Excessive flexion and ulnar deviation can lead to median nerve compression and associated symptoms, as well as poor extensor and radial tendon length. In addition, prolonged distraction in excessive flexion combined with swelling and inflammation during fracture healing causes digital stiffness and contracture.23 Biomechanical studies have found that proximal pin placement in the radius, along with distal pin fixation in 6 metacarpal cortices through the second and third metacarpals, helps provide the strongest fixation.24
As for technique, pins are placed in the second metacarpal and radial shaft. With respect to the radius, the incision is made just proximal to the edge of the abductor pollicis longus muscle in the "bare area." Ideal pin placement is between the extensor carpi radialis longus and the extensor carpi radialis brevis, with care taken to avoid the radial sensory nerve, which lies between the extensor carpi radialis longus and the brachialis and emerges 9 cm proximal to the radial styloid.25 Next, a 2.5-cm to 3-cm incision is made over the palpable edge of the index metacarpal near the base. During drilling, the guide is placed at intersecting 45° angles, and the distal pin is placed 2 cm to 3 cm from the proximal pin. The proximal metacarpal pin is placed at the base of the metacarpal. The second metacarpal pin can also be placed first, with the external fixator used to judge proximal placement of the radial pin within the bare area.
Various supplements to external fixation have positive outcomes. Wolfe and colleagues18 found that using K-wires with the external fixation construct added stability in flexion/extension, radial/ulnar deviation, and rotational motion. They noted that fixation stability may depend more on the augmentation to fixation than on the external fixator itself. In a prospective, randomized trial, Moroni and colleagues26,27 found that, compared with standard pins, hydroxyapatite-coated pins had higher extraction torque, which was associated with improved fixation. When combined with external fixation, calcium phosphate cement also provided additional stability, allowing the bone filler to help maintain articular reduction and cortical continuity.28,29
External fixation has its disadvantages and complications. It can be bulky, and theoretically it contributes to higher rates of stiffness in the wrist and fingers.30-32 Higher rates of pin-site infection have been reported, along with hardware failure and associated loss of reduction, in patients treated with external fixation (Figures 3A-3C).31-33[[{"fid":"201866","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]In addition, joint overdistraction can adversely affect the length-tension curve and contribute to potential reflex sympathetic dystrophy, which can be devastating (Figures 4A, 4B).1,21,31,33 Despite these complications, external fixation remains a powerful tool in the treatment of high-energy DRFs. [[{"fid":"201867","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]In many cases, authors who compared open reduction and internal fixation (ORIF) with external fixation found no significant differences in outcome scores or function.31-34 In a meta-analysis of 917 patients, Margaliot and colleagues33 found no differences in pain, grip strength, wrist ROM, or radiographic parameters. More recently, in prospective randomized trials, both Egol and colleagues31 and Grewal and colleagues34 compared hybrid external fixation with ORIF, and, though early outcomes favored ORIF, 1-year follow-up comparisons were even, and there were no significant differences. These consistently reproducible results reaffirm keeping external fixation in the orthopedic toolbox.
Definitive Reconstruction With ORIF
Early nonlocked dorsal plating options for DRF fixation had unacceptable rates of plate failure, poor cosmesis, and extensor tendon complications.17,35-37 Subsequent technologic advances—multiple approaches, lower profile plating, and rigid, fragment-specific fixation—have allowed even the most complex fracture patterns to be addressed (Table). In malunited fractures, bone graft may not be required if the fracture is extra-articular and treated with a volar locking plate. [[{"fid":"201868","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]Other options include corticocancellous autograft from the iliac crest, hydroxyapatite synthetic grafts, and osteoconductive bone graft substitutes, such as bone morphogenic proteins. In addition, healing times are similar in cases, regardless of whether a graft was used.38
Involvement of the radial and intermediate columns should be addressed first. Although some may prefer a single volar plate, others may use fragment-specific fixation to buttress a comminuted radial styloid (in orthogonal fashion) and/or a dorsal ulnar fragment to restore the intermediate column and thereby fully restore the radial articular surface.39,40 Typically, restoring the radial and intermediate columns for radial articular reduction subsequently and simultaneously restores the majority of radial height and length. After the radial and intermediate columns are reduced and stabilized, the need for ulna column fixation can be determined. Important factors in ulna column restoration are severe osteoporosis and ulna head and/or neck comminution. Significant comminution throughout the metaphysis of both the radius and the ulna may also warrant stabilizing the ulna with internal fixation. Finally, any DRU joint instability noted on examination should also favor fixing the ulnar side.
Assessment of the distal ulna in these complex fractures goes beyond the involvement of an ulnar styloid fracture. Typically, fractures at the base of the ulnar styloid have been reported to have little clinical relevance, including a low incidence of associated DRU joint problems.41-43 Decisions to address the ulnar column are largely swayed by any instability found on DRU joint testing, as laxity caused by severe comminution can dictate the need for distal ring fixation to provide support. Even in the presence of a high-energy fracture in severely osteoporotic bone, the argument can be made to prevent instability by supporting the ulnar column. Stabilization of the ulnar articular surface can also be made more facile by creating an easier "A" fracture pattern (per AO classification) from a complex "C" to further aid in achieving efficient anatomical reduction. After preoperative planning is completed, depending on which columns need to be addressed, several surgical approaches can be considered to achieve maximum exposure and soft-tissue mobilization in order to successfully complete the operative fixation goals.
Volar Approach
An approach is selected for ideal exposure of a facile environment for definitive fixation. Access to the radial column can be gained with the extended flexor carpi radialis (FCR) approach. This approach allows visualization and removal of the appropriate deforming forces on the radial column to allow for fracture reduction by "opening the book," similar to that of tibial plateau reconstruction.44,45 It may be prudent to perform a preincision Allen test as well as a preoperative DRU joint examination for comparison after ORIF is complete. Compared with the classic Henry approach near the distal radius, going through the volar sheath of the FCR avoids many of the perforating radial artery branches. Avoiding stripping the radial artery of its surrounding fat and lymphatics prevents postoperative "cold intolerance." Retracting the FCR ulnarly and then incising the dorsal FCR sheath provide ready access to the pronator quadratus after collective ulnar mobilization of both the FCR and the flexor pollicis longus.44 In addition, for work near the distal FCR sheath, care must be taken to avoid the branch of the palmar cutaneous nerve that emerges about 5 cm proximal to the wrist flexion crease.46
Once at the level of the pronator quadratus, an "L-shape" incision can be made to reflect the muscle off the radius. Care must be taken when working too distal to avoid transection of the inserting volar wrist ligaments.44 Leaving a cuff for repair of the pronator remains controversial. In a recent case-control series, however, Hershman and colleagues47 did not find significant differences in function or complication rate in patients with and without repair. After reflection, adequate exposure of the radial column should be achieved. Ready access to the radial styloid for orthogonal plating can be obtained by releasing the brachioradialis, which simultaneously releases one of the primary fracture deforming forces.44 With this incision and exposure, if needed, dorsal bone grafting can be achieved from the volar side; however, care must be taken to protect the first dorsal compartment.48 The cutaneous branch of the median nerve may be at risk with this exposure, but avoiding dissection ulnar to the FCR tendon can help to reduce this risk.49
Before surgery, if the fracture pattern dictates a more ulnar approach, we prefer the extended carpal tunnel approach. Using the plane between the palmaris longus and the flexor digitorum superficialis medially and the FCR laterally, the extended carpal tunnel approach provides an obvious release of the flexor retinaculum but, more important, allows for extensile access to the sigmoid notch, the DRU joint, and the ulnar column.
Dorsal Approach
The dorsal approach is necessary in a few select cases. With a focus on fragment-specific fixation, presence of a significant dorsal ulnar fragment should warrant a dorsal approach.50 In addition, in select, rare cases in which volar access is limited or unavailable, dorsal access is the only option.50 Finally, if direct articular visualization is required, the dorsal approach typically is favored as the stronger radiocarpal ligaments found on the volar side are maintained.
Access should begin with an incision centered over the dorsal distal radius; a safe access point is just ulnar to the Lister tubercle. On incision of the retinaculum through a full-thickness excision, the third dorsal compartment is opened and the extensor pollicis longus (EPL) mobilized, fully exposing the dorsal distal radius. Work can be performed on either side of the EPL between the second and fourth dorsal compartments. Exposure typically is not an issue because of the pliable soft tissue of the dorsum, with ready access from styloid to styloid.44 Here, low-profile plates and/or mini-fragment-specific plate options should be used to minimize potential tendon damage.51 Care must also be taken to avoid damaging the radiocarpal or scapholunate ligaments.49 On closure, the retinaculum is repaired primarily; however, though some proponents advocate relocating the EPL tendon into its groove, we prefer leaving the EPL free within the surrounding soft tissue to reduce tension and promote unhindered excursion. The dorsal approach, though controversial and used inconsistently, should remain an important tool in anatomical restoration, especially in cases of complex fracture patterns.
Conclusion
Controversy still marks the lack of consensus on deciding which DRF treatment is optimal. Some investigators question moving away from external fixation and cite the lack of significantly better data relative to ORIF.21,52 The same proponents note that the only advantage over external fixation is earlier return to function and cite reports of tendon rupture and complications with both dorsal and volar fixation options.34,53-58 Other investigators find that operative treatment generally does not provide a significant improvement over nonoperative treatment.59
With the advent of lower profile locked plating, fragment-specific fixation, and variable-angle devices, comparative clinical trials are finding it difficult to keep up.60-64 Results from ongoing prospective randomized trials like ORCHID (Open Reduction and Internal Fixation Versus Casting for Highly Comminuted Intra-Articular Fractures of the Distal Radius; 500 patients >65 years old, 15 centers) will provide more definitive answers about ideal treatment.65
Anatomical restoration involves a versatile array of fragment fixation and reconstruction. Careful preoperative planning and a consistent approach to restoring the radial, intermediate, and ulnar columns, along with a proper surgical approach, are ideal. Many advances in internal fixation have been exceedingly helpful. Use of external fixation, especially in a bridging fashion with or without supplementation, is still valuable in many situations.
1. Liporace FA, Adams MR, Capo JT, Koval KJ. Distal radius fractures. J Orthop Trauma. 2009;23(10):739-748.
2. Lee YS, Wei TY, Cheng YC, Hsu TL, Huang CR. A comparative study of Colles’ fractures in patients between fifty and seventy years of age: percutaneous K-wiring versus volar locking plating. Int Orthop. 2012;36(4):789-794.
3. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824-835.e2.
4. Ring D. Treatment of the neglected distal radius fracture. Clin Orthop Relat Res. 2005;(431):85-92.
5. Berger RA. Arthroscopic anatomy of the wrist and distal radioulnar joint. Hand Clin. 1999;15(3):393-413, vii.
6. Berger RA. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;(383):32-40.
7. McCann PA, Clarke D, Amirfeyz R, Bhatia R. The cadaveric anatomy of the distal radius: implications for the use of volar plates. Ann R Coll Surg Engl. 2012;94(2):116-120.
8. Ekenstam F. Osseous anatomy and articular relationships about the distal ulna. Hand Clin. 1998;14(2):161-164.
9. Fernandez DL. Distal radius fracture: the rationale of a classification. Chir Main. 2001;20(6):411-425.
10. Raskin KB, Melone CP Jr. Unstable articular fractures of the distal radius. Comparative techniques of ligamentotaxis. Orthop Clin North Am. 1993;24(2):275-286.
11. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
12. Jenkins NH. The unstable Colles’ fracture. J Hand Surg Br. 1989;14(2):149-154.
13. Cooney WP, Dobyns JH, Linscheid RL. Arthroscopy of the wrist: anatomy and classification of carpal instability. Arthroscopy. 1990;6(2):133-140.
14. Kural C, Sungur I, Kaya I, Ugras A, Ertürk A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33(11):801.
15. Lipton HA, Wollstein R. Operative treatment of intraarticular distal radial fractures. Clin Orthop Relat Res. 1996;(327):110-124.
16. Wolfe SW. Distal radius fractures. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011:561-638.
17. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):
588-592.
18. Wolfe SW, Austin G, Lorenze M, Swigart CR, Panjabi MM. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg Am. 1999;24(3):516-524.
19. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma. 2006;20(2):115-121.
20. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg. 2004;93(1):64-67.
21. Paksima N, Panchal A, Posner MA, Green SM, Mehiman CT, Hiebert R. A meta-analysis of the literature on distal radius fractures: review of 615 articles. Bull Hosp Jt Dis. 2004;62(1-2):40-46.
22. Li-hai Z, Ya-nan W, Zhi M, et al. Volar locking plate versus external fixation for the treatment of unstable distal radial fractures: a meta-analysis of randomized controlled trials.
J Surg Res. 2015;193(1):324-333.
23. Papadonikolakis A, Shen J, Garrett JP, Davis SM, Ruch DS. The effect of increasing distraction on digital motion after external fixation of the wrist. J Hand Surg Am. 2005;30(4):
773-779.
24. Seitz WH Jr, Froimson AI, Brooks DB, et al. Biomechanical analysis of pin placement and pin size for external fixation of distal radius fractures. Clin Orthop Relat Res. 1990;(251):
207-212.
25. Beldner S, Zlotolow DA, Melone CP Jr, Agnes AM, Jones MH. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am. 2005;30(6):1226-1230.
26. Moroni A, Faldini C, Marchetti S, Manca M, Consoli V, Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83(5):717-721.
27. Moroni A, Heikkila J, Magyar G, Toksvig-Larsen S, Giannini S. Fixation strength and pin tract infection of hydroxyapatite-coated tapered pins. Clin Orthop Relat Res. 2001;(388):209-217.
28. Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of fixation of intra-articular distal radial fractures with calcium-phosphate bone cement. J Bone Joint Surg Am. 2002;84(9):1579-1586.
29. Tobe M, Mizutani K, Tsubuku Y. Treatment of distal radius fracture with the use of calcium phosphate bone cement as a filler. Tech Hand Up Extrem Surg. 2004;8(2):95-101.
30. Capo JT, Rossy W, Henry P, Maurer RJ, Naidu S, Chen L.
External fixation of distal radius fractures: effect of distraction and duration. J Hand Surg Am. 2009;34(9):1605-1611.
31. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
32. Egol KA, Paksima N, Puopolo S, Klugman J, Hiebert R, Koval KJ. Treatment of external fixation pins about the wrist: a prospective, randomized trial. J Bone Joint Surg Am. 2006;88(2):349-354.
33. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
34. Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):
1899-1906.
35. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990;15(1):1-11.
36. Hove LM, Nilsen PT, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius. 31 patients followed for 3-7 years. Acta Orthop Scand. 1997;68(1):59-63.
37. Carter PR, Frederick HA, Laseter GF. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg Am. 1998;23(2):300-307.
38. Mugnai R, Tarallo L, Lancellotti E, et al. Corrective osteotomies of the radius: grafting or not? World J Orthop. 2016;7(2):128-135.
39. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149.
40. Helmerhorst GT, Kloen P. Orthogonal plating of intra-articular distal radius fractures with an associated radial column fracture via a single volar approach. Injury. 2012;43(8):1307-1312.
41. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
42. Souer JS, Ring D, Matschke S, Audige L, Marent-Huber M, Jupiter JB; AOCID Prospective ORIF Distal Radius Study Group. Effect of an unrepaired fracture of the ulnar styloid base on outcome after plate-and-screw fixation of a distal radial fracture. J Bone Joint Surg Am. 2009;91(4):830-838.
43. Noda K, Goto A, Murase T, Sugamoto K, Yoshikawa H, Moritomo H. Interosseous membrane of the forearm: an anatomical study of ligament attachment locations. J Hand Surg Am. 2009;34(3):415-422.
44. Catalano LW 3rd, Zlotolow DA, Hitchcock PB, Shah SN, Barron OA. Surgical exposures of the radius and ulna. J Am Acad Orthop Surg. 2011;19(7):430-438.
45. Orbay JL, Badia A, Indriago IR, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204-211.
46. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am. 1990;15(1):38-43.
47. Hershman SH, Immerman I, Bechtel C, Lekic N, Paksima N, Egol KA. The effects of pronator quadratus repair on outcomes after volar plating of distal radius fractures. J Orthop Trauma. 2013;27(3):130-133.
48. Prommersberger KJ, Lanz UB. Corrective osteotomy of the distal radius through volar approach. Tech Hand Up Extrem Surg. 2004;8(2):70-77.
49. Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011;6(1):8-17.
50. Tavakolian JD, Jupiter JB. Dorsal plating for distal radius fractures. Hand Clin. 2005;21(3):341-346.
51. Yu YR, Makhni MC, Tabrizi S, Rozental TD, Mundanthanam G, Day CS. Complications of low-profile dorsal versus volar locking plates in the distal radius: a comparative study. J Hand Surg Am. 2011;36(7):1135-1141.
52. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma. 2011;71(4):939-942.
53. Wilcke MK, Abbaszadegan H, Adolphson PY. Wrist function recovers more rapidly after volar locked plating than after external fixation but the outcomes are similar after 1 year. Acta Orthop. 2011;82(1):76-81.
54. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189.
55. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am. 2011;36(1):3-9.
56. Soong M, Earp BE, Bishop G, Leung A, Blazar P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg Am. 2011;93(4):328-335.
57. Jeudy J, Steiger V, Boyer P, Cronier P, Bizot P, Massin P. Treatment of complex fractures of the distal radius: a prospective randomised comparison of external fixation ‘versus’ locked volar plating. Injury. 2012;43(2):174-179.
58. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
59. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
60. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg Am. 2012;37(3):446-453.
61. Dahl WJ, Nassab PF, Burgess KM, et al. Biomechanical properties of fixed-angle volar distal radius plates under dynamic loading. J Hand Surg Am. 2012;37(7):1381-1387.
62. Park JH, Hagopian J, Ilyas AM. Variable-angle locking screw volar plating of distal radius fractures. Hand Clin. 2010;26(3):373-380, vi.
63. Pensy RA, Brunton LM, Parks BG, Higgins JP, Chhabra AB. Single-incision extensile volar approach to the distal radius and concurrent carpal tunnel release: cadaveric study. J Hand Surg Am. 2010;35(2):217-222.
64. Klos K, Rausch S, Löffler M, et al. A biomechanical comparison of a biodegradable volar locked plate with two titanium volar locked plates in a distal radius fracture model. J Trauma. 2010;68(4):984-991.
65. Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;12:84
Take-Home Points
- Restore proper anatomic parameters; compare to the other side.
- Don't forget about the DRU joint.
- CT can aide in identifying subtle articular depression and severe comminution to change operative management.
- Remember, there still is a role for external fixators; an alternative remains an internal spanning plate.
- Respect the soft tissues, which can aide in reduction, however don't leave the operating room without feeling confident about your fixation.
Distal radius fracture (DRF), a common fracture, accounts for almost one sixth of all emergency department visits.1 With the advent of emerging technologies and refined technique, treatment options for DRFs have evolved. Although controversy remains regarding nonoperative vs operative treatment of DRFs in the elderly,2,3 select situations (open injuries, complex high-energy injuries, young age) warrant definitive fixation. Previously, internal fixation options were limited. Current technologies include locked fixed-angle plating, fragment-specific fixation, and locked variable-angle plating. These modalities aid in achieving and maintaining more anatomical fixation. This article summarizes tips, tricks, and planning for definitive external and internal fixation of complex DRFs.
Anatomical Considerations and Classification
The wrist joint, part of the complex articular network that begins at the forearm and ends at the distal interphalangeal joint, is the foundation for fine- and gross-motor skills. Understanding the anatomy of this network can provide a valuable roadmap for operative reconstruction.
At the wrist level, the radius bears most of the weight-bearing, and in some studies exhibits up to 80% of the load.1,4 The triangular distal radius bears this weight through a biconcave articular surface with facets for the lunate and scaphoid separated by an anteroposterior ridge.5-7 The radius also articulates with the ulnar head at the sigmoid notch to form the distal radioulnar (DRU) joint. Restoring the relationships of the DRU joint, the triangular fibrocartilage complex, and the ulnar variance is of paramount importance.1,8,9
Classical teaching calls for restoration of radial inclination to about 23°, volar tilt to 11° to 12°, and radial length to about 11 mm. Especially regarding volar tilt and radial length, however, cadaveric and clinical studies have found more variance, leading to use of the contralateral extremity as an operative template, particularly when closed reduction thought to be adequate deviates significantly from these parameters.1,4,7
DRF classification based on these principles has led to abundant representation in the literature.10-13 Many authors have focused on fracture lines, comminution degree, articular surface violation, and other anatomical or radiographic characteristics of DRF classification and operative fixation approach.10-13 In 2001, Fernandez9 proposed a classification system focused on energy or mechanism of injury. In comparisons,14 the Fernandez system had the highest interobserver reliability—higher than that of AO (Arbeitsgemeinschaft für Osteosynthesefragen).
Considerations for Operative Treatment: Column Theory
In the restoration of anatomical alignment in complex DRFs, it is important to consider the 3 joints and the 3 columns—radial, intermediate, and ulnar (Figure 1). [[{"fid":"201864","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]In addition, parallels between the distal radius and the tibial plateau can be considered because of similarities in operative goals. Restoration of mechanical axis, length, alignment, rotation, and articular surfaces is paramount.15 Considering multiple surgical approaches to address "bicolumnar injuries" and reconstructing the "simpler" columnar injury first are common principles.16
The goals of fracture fixation at the wrist are the same as at any other joint: anatomical reduction, stable fixation, and early range of motion (ROM). Column restoration can result in consistent achievement of those goals. Intuitively, there is a close correlation between anatomical alignment and functional results.17 Rebuilding the structural foundation of the columns with respect to buttressing and restoring the 3 radial articulations with the ulna, scaphoid, and lunate can consistently yield restoration of length, inclination, and tilt (Figure 2). [[{"fid":"201865","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Next, we discuss the options available and how to use each to an advantage, individually or in hybrid constructs.
External Fixation: Is There Still a Role?
In the setting of highly comminuted, complex fractures, external fixation with Kirschner wires (K-wires) is a reasonable choice, with restoration of motion and strength within 75% to 80% of the uninjured wrist.18 In a 2-year study of 113 patients with comminuted metaphyseal DRFs randomly assigned to either external fixation or casting, Kreder and colleagues19 found a trend toward better clinical, functional, and radiographic outcomes with external fixation with or without K-wire fixation. There was improved restoration of radial length and palmar tilt with external fixation. A study of unstable DRF in patients with osteoporosis found that redisplacement was more common after treatment with a cast than after treatment with an external fixator.20 Although closed reduction and casting continue to have a role in the treatment of DRF, Kreder and colleagues19 found that remanipulation was necessary in at least 9% of cases. According to a meta-analysis21 of the literature on DRF treatment, 4 articles directly address the question of the superiority of external fixation over closed reduction and casting, and 3 of the 4 found more favorable radiographic and functional outcomes with external fixation.
External fixation is useful in treating complex DRFs with metaphyseal comminution. It can also be effective in the presence of simple articular involvement without depression of the joint surface. External fixation devices can span areas of soft-
tissue injury and are useful as manipulation tools in achieving anatomical reduction. Although external fixation is effective, its complications include pin-tract infection, nerve injury, loss of reduction, and loss of digital ROM. In a meta-analysis, Li-hai and colleagues22 found that external fixators had a complication rate of 30.9%. With this technique, it is important to avoid midcarpal distraction, excessive ulnar deviation, and excessive palmar flexion. Papadonikolakis and colleagues23 found that distraction of as little as 2 mm to 5 mm significantly affected the function of the flexor digitorum superficialis at the metacarpophalangeal joint. Over-distraction in wrist flexion can lead to lengthening of the extensor tendons and loss of full digital ROM. Excessive flexion and ulnar deviation can lead to median nerve compression and associated symptoms, as well as poor extensor and radial tendon length. In addition, prolonged distraction in excessive flexion combined with swelling and inflammation during fracture healing causes digital stiffness and contracture.23 Biomechanical studies have found that proximal pin placement in the radius, along with distal pin fixation in 6 metacarpal cortices through the second and third metacarpals, helps provide the strongest fixation.24
As for technique, pins are placed in the second metacarpal and radial shaft. With respect to the radius, the incision is made just proximal to the edge of the abductor pollicis longus muscle in the "bare area." Ideal pin placement is between the extensor carpi radialis longus and the extensor carpi radialis brevis, with care taken to avoid the radial sensory nerve, which lies between the extensor carpi radialis longus and the brachialis and emerges 9 cm proximal to the radial styloid.25 Next, a 2.5-cm to 3-cm incision is made over the palpable edge of the index metacarpal near the base. During drilling, the guide is placed at intersecting 45° angles, and the distal pin is placed 2 cm to 3 cm from the proximal pin. The proximal metacarpal pin is placed at the base of the metacarpal. The second metacarpal pin can also be placed first, with the external fixator used to judge proximal placement of the radial pin within the bare area.
Various supplements to external fixation have positive outcomes. Wolfe and colleagues18 found that using K-wires with the external fixation construct added stability in flexion/extension, radial/ulnar deviation, and rotational motion. They noted that fixation stability may depend more on the augmentation to fixation than on the external fixator itself. In a prospective, randomized trial, Moroni and colleagues26,27 found that, compared with standard pins, hydroxyapatite-coated pins had higher extraction torque, which was associated with improved fixation. When combined with external fixation, calcium phosphate cement also provided additional stability, allowing the bone filler to help maintain articular reduction and cortical continuity.28,29
External fixation has its disadvantages and complications. It can be bulky, and theoretically it contributes to higher rates of stiffness in the wrist and fingers.30-32 Higher rates of pin-site infection have been reported, along with hardware failure and associated loss of reduction, in patients treated with external fixation (Figures 3A-3C).31-33[[{"fid":"201866","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]In addition, joint overdistraction can adversely affect the length-tension curve and contribute to potential reflex sympathetic dystrophy, which can be devastating (Figures 4A, 4B).1,21,31,33 Despite these complications, external fixation remains a powerful tool in the treatment of high-energy DRFs. [[{"fid":"201867","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]In many cases, authors who compared open reduction and internal fixation (ORIF) with external fixation found no significant differences in outcome scores or function.31-34 In a meta-analysis of 917 patients, Margaliot and colleagues33 found no differences in pain, grip strength, wrist ROM, or radiographic parameters. More recently, in prospective randomized trials, both Egol and colleagues31 and Grewal and colleagues34 compared hybrid external fixation with ORIF, and, though early outcomes favored ORIF, 1-year follow-up comparisons were even, and there were no significant differences. These consistently reproducible results reaffirm keeping external fixation in the orthopedic toolbox.
Definitive Reconstruction With ORIF
Early nonlocked dorsal plating options for DRF fixation had unacceptable rates of plate failure, poor cosmesis, and extensor tendon complications.17,35-37 Subsequent technologic advances—multiple approaches, lower profile plating, and rigid, fragment-specific fixation—have allowed even the most complex fracture patterns to be addressed (Table). In malunited fractures, bone graft may not be required if the fracture is extra-articular and treated with a volar locking plate. [[{"fid":"201868","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]Other options include corticocancellous autograft from the iliac crest, hydroxyapatite synthetic grafts, and osteoconductive bone graft substitutes, such as bone morphogenic proteins. In addition, healing times are similar in cases, regardless of whether a graft was used.38
Involvement of the radial and intermediate columns should be addressed first. Although some may prefer a single volar plate, others may use fragment-specific fixation to buttress a comminuted radial styloid (in orthogonal fashion) and/or a dorsal ulnar fragment to restore the intermediate column and thereby fully restore the radial articular surface.39,40 Typically, restoring the radial and intermediate columns for radial articular reduction subsequently and simultaneously restores the majority of radial height and length. After the radial and intermediate columns are reduced and stabilized, the need for ulna column fixation can be determined. Important factors in ulna column restoration are severe osteoporosis and ulna head and/or neck comminution. Significant comminution throughout the metaphysis of both the radius and the ulna may also warrant stabilizing the ulna with internal fixation. Finally, any DRU joint instability noted on examination should also favor fixing the ulnar side.
Assessment of the distal ulna in these complex fractures goes beyond the involvement of an ulnar styloid fracture. Typically, fractures at the base of the ulnar styloid have been reported to have little clinical relevance, including a low incidence of associated DRU joint problems.41-43 Decisions to address the ulnar column are largely swayed by any instability found on DRU joint testing, as laxity caused by severe comminution can dictate the need for distal ring fixation to provide support. Even in the presence of a high-energy fracture in severely osteoporotic bone, the argument can be made to prevent instability by supporting the ulnar column. Stabilization of the ulnar articular surface can also be made more facile by creating an easier "A" fracture pattern (per AO classification) from a complex "C" to further aid in achieving efficient anatomical reduction. After preoperative planning is completed, depending on which columns need to be addressed, several surgical approaches can be considered to achieve maximum exposure and soft-tissue mobilization in order to successfully complete the operative fixation goals.
Volar Approach
An approach is selected for ideal exposure of a facile environment for definitive fixation. Access to the radial column can be gained with the extended flexor carpi radialis (FCR) approach. This approach allows visualization and removal of the appropriate deforming forces on the radial column to allow for fracture reduction by "opening the book," similar to that of tibial plateau reconstruction.44,45 It may be prudent to perform a preincision Allen test as well as a preoperative DRU joint examination for comparison after ORIF is complete. Compared with the classic Henry approach near the distal radius, going through the volar sheath of the FCR avoids many of the perforating radial artery branches. Avoiding stripping the radial artery of its surrounding fat and lymphatics prevents postoperative "cold intolerance." Retracting the FCR ulnarly and then incising the dorsal FCR sheath provide ready access to the pronator quadratus after collective ulnar mobilization of both the FCR and the flexor pollicis longus.44 In addition, for work near the distal FCR sheath, care must be taken to avoid the branch of the palmar cutaneous nerve that emerges about 5 cm proximal to the wrist flexion crease.46
Once at the level of the pronator quadratus, an "L-shape" incision can be made to reflect the muscle off the radius. Care must be taken when working too distal to avoid transection of the inserting volar wrist ligaments.44 Leaving a cuff for repair of the pronator remains controversial. In a recent case-control series, however, Hershman and colleagues47 did not find significant differences in function or complication rate in patients with and without repair. After reflection, adequate exposure of the radial column should be achieved. Ready access to the radial styloid for orthogonal plating can be obtained by releasing the brachioradialis, which simultaneously releases one of the primary fracture deforming forces.44 With this incision and exposure, if needed, dorsal bone grafting can be achieved from the volar side; however, care must be taken to protect the first dorsal compartment.48 The cutaneous branch of the median nerve may be at risk with this exposure, but avoiding dissection ulnar to the FCR tendon can help to reduce this risk.49
Before surgery, if the fracture pattern dictates a more ulnar approach, we prefer the extended carpal tunnel approach. Using the plane between the palmaris longus and the flexor digitorum superficialis medially and the FCR laterally, the extended carpal tunnel approach provides an obvious release of the flexor retinaculum but, more important, allows for extensile access to the sigmoid notch, the DRU joint, and the ulnar column.
Dorsal Approach
The dorsal approach is necessary in a few select cases. With a focus on fragment-specific fixation, presence of a significant dorsal ulnar fragment should warrant a dorsal approach.50 In addition, in select, rare cases in which volar access is limited or unavailable, dorsal access is the only option.50 Finally, if direct articular visualization is required, the dorsal approach typically is favored as the stronger radiocarpal ligaments found on the volar side are maintained.
Access should begin with an incision centered over the dorsal distal radius; a safe access point is just ulnar to the Lister tubercle. On incision of the retinaculum through a full-thickness excision, the third dorsal compartment is opened and the extensor pollicis longus (EPL) mobilized, fully exposing the dorsal distal radius. Work can be performed on either side of the EPL between the second and fourth dorsal compartments. Exposure typically is not an issue because of the pliable soft tissue of the dorsum, with ready access from styloid to styloid.44 Here, low-profile plates and/or mini-fragment-specific plate options should be used to minimize potential tendon damage.51 Care must also be taken to avoid damaging the radiocarpal or scapholunate ligaments.49 On closure, the retinaculum is repaired primarily; however, though some proponents advocate relocating the EPL tendon into its groove, we prefer leaving the EPL free within the surrounding soft tissue to reduce tension and promote unhindered excursion. The dorsal approach, though controversial and used inconsistently, should remain an important tool in anatomical restoration, especially in cases of complex fracture patterns.
Conclusion
Controversy still marks the lack of consensus on deciding which DRF treatment is optimal. Some investigators question moving away from external fixation and cite the lack of significantly better data relative to ORIF.21,52 The same proponents note that the only advantage over external fixation is earlier return to function and cite reports of tendon rupture and complications with both dorsal and volar fixation options.34,53-58 Other investigators find that operative treatment generally does not provide a significant improvement over nonoperative treatment.59
With the advent of lower profile locked plating, fragment-specific fixation, and variable-angle devices, comparative clinical trials are finding it difficult to keep up.60-64 Results from ongoing prospective randomized trials like ORCHID (Open Reduction and Internal Fixation Versus Casting for Highly Comminuted Intra-Articular Fractures of the Distal Radius; 500 patients >65 years old, 15 centers) will provide more definitive answers about ideal treatment.65
Anatomical restoration involves a versatile array of fragment fixation and reconstruction. Careful preoperative planning and a consistent approach to restoring the radial, intermediate, and ulnar columns, along with a proper surgical approach, are ideal. Many advances in internal fixation have been exceedingly helpful. Use of external fixation, especially in a bridging fashion with or without supplementation, is still valuable in many situations.
Take-Home Points
- Restore proper anatomic parameters; compare to the other side.
- Don't forget about the DRU joint.
- CT can aide in identifying subtle articular depression and severe comminution to change operative management.
- Remember, there still is a role for external fixators; an alternative remains an internal spanning plate.
- Respect the soft tissues, which can aide in reduction, however don't leave the operating room without feeling confident about your fixation.
Distal radius fracture (DRF), a common fracture, accounts for almost one sixth of all emergency department visits.1 With the advent of emerging technologies and refined technique, treatment options for DRFs have evolved. Although controversy remains regarding nonoperative vs operative treatment of DRFs in the elderly,2,3 select situations (open injuries, complex high-energy injuries, young age) warrant definitive fixation. Previously, internal fixation options were limited. Current technologies include locked fixed-angle plating, fragment-specific fixation, and locked variable-angle plating. These modalities aid in achieving and maintaining more anatomical fixation. This article summarizes tips, tricks, and planning for definitive external and internal fixation of complex DRFs.
Anatomical Considerations and Classification
The wrist joint, part of the complex articular network that begins at the forearm and ends at the distal interphalangeal joint, is the foundation for fine- and gross-motor skills. Understanding the anatomy of this network can provide a valuable roadmap for operative reconstruction.
At the wrist level, the radius bears most of the weight-bearing, and in some studies exhibits up to 80% of the load.1,4 The triangular distal radius bears this weight through a biconcave articular surface with facets for the lunate and scaphoid separated by an anteroposterior ridge.5-7 The radius also articulates with the ulnar head at the sigmoid notch to form the distal radioulnar (DRU) joint. Restoring the relationships of the DRU joint, the triangular fibrocartilage complex, and the ulnar variance is of paramount importance.1,8,9
Classical teaching calls for restoration of radial inclination to about 23°, volar tilt to 11° to 12°, and radial length to about 11 mm. Especially regarding volar tilt and radial length, however, cadaveric and clinical studies have found more variance, leading to use of the contralateral extremity as an operative template, particularly when closed reduction thought to be adequate deviates significantly from these parameters.1,4,7
DRF classification based on these principles has led to abundant representation in the literature.10-13 Many authors have focused on fracture lines, comminution degree, articular surface violation, and other anatomical or radiographic characteristics of DRF classification and operative fixation approach.10-13 In 2001, Fernandez9 proposed a classification system focused on energy or mechanism of injury. In comparisons,14 the Fernandez system had the highest interobserver reliability—higher than that of AO (Arbeitsgemeinschaft für Osteosynthesefragen).
Considerations for Operative Treatment: Column Theory
In the restoration of anatomical alignment in complex DRFs, it is important to consider the 3 joints and the 3 columns—radial, intermediate, and ulnar (Figure 1). [[{"fid":"201864","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]In addition, parallels between the distal radius and the tibial plateau can be considered because of similarities in operative goals. Restoration of mechanical axis, length, alignment, rotation, and articular surfaces is paramount.15 Considering multiple surgical approaches to address "bicolumnar injuries" and reconstructing the "simpler" columnar injury first are common principles.16
The goals of fracture fixation at the wrist are the same as at any other joint: anatomical reduction, stable fixation, and early range of motion (ROM). Column restoration can result in consistent achievement of those goals. Intuitively, there is a close correlation between anatomical alignment and functional results.17 Rebuilding the structural foundation of the columns with respect to buttressing and restoring the 3 radial articulations with the ulna, scaphoid, and lunate can consistently yield restoration of length, inclination, and tilt (Figure 2). [[{"fid":"201865","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Next, we discuss the options available and how to use each to an advantage, individually or in hybrid constructs.
External Fixation: Is There Still a Role?
In the setting of highly comminuted, complex fractures, external fixation with Kirschner wires (K-wires) is a reasonable choice, with restoration of motion and strength within 75% to 80% of the uninjured wrist.18 In a 2-year study of 113 patients with comminuted metaphyseal DRFs randomly assigned to either external fixation or casting, Kreder and colleagues19 found a trend toward better clinical, functional, and radiographic outcomes with external fixation with or without K-wire fixation. There was improved restoration of radial length and palmar tilt with external fixation. A study of unstable DRF in patients with osteoporosis found that redisplacement was more common after treatment with a cast than after treatment with an external fixator.20 Although closed reduction and casting continue to have a role in the treatment of DRF, Kreder and colleagues19 found that remanipulation was necessary in at least 9% of cases. According to a meta-analysis21 of the literature on DRF treatment, 4 articles directly address the question of the superiority of external fixation over closed reduction and casting, and 3 of the 4 found more favorable radiographic and functional outcomes with external fixation.
External fixation is useful in treating complex DRFs with metaphyseal comminution. It can also be effective in the presence of simple articular involvement without depression of the joint surface. External fixation devices can span areas of soft-
tissue injury and are useful as manipulation tools in achieving anatomical reduction. Although external fixation is effective, its complications include pin-tract infection, nerve injury, loss of reduction, and loss of digital ROM. In a meta-analysis, Li-hai and colleagues22 found that external fixators had a complication rate of 30.9%. With this technique, it is important to avoid midcarpal distraction, excessive ulnar deviation, and excessive palmar flexion. Papadonikolakis and colleagues23 found that distraction of as little as 2 mm to 5 mm significantly affected the function of the flexor digitorum superficialis at the metacarpophalangeal joint. Over-distraction in wrist flexion can lead to lengthening of the extensor tendons and loss of full digital ROM. Excessive flexion and ulnar deviation can lead to median nerve compression and associated symptoms, as well as poor extensor and radial tendon length. In addition, prolonged distraction in excessive flexion combined with swelling and inflammation during fracture healing causes digital stiffness and contracture.23 Biomechanical studies have found that proximal pin placement in the radius, along with distal pin fixation in 6 metacarpal cortices through the second and third metacarpals, helps provide the strongest fixation.24
As for technique, pins are placed in the second metacarpal and radial shaft. With respect to the radius, the incision is made just proximal to the edge of the abductor pollicis longus muscle in the "bare area." Ideal pin placement is between the extensor carpi radialis longus and the extensor carpi radialis brevis, with care taken to avoid the radial sensory nerve, which lies between the extensor carpi radialis longus and the brachialis and emerges 9 cm proximal to the radial styloid.25 Next, a 2.5-cm to 3-cm incision is made over the palpable edge of the index metacarpal near the base. During drilling, the guide is placed at intersecting 45° angles, and the distal pin is placed 2 cm to 3 cm from the proximal pin. The proximal metacarpal pin is placed at the base of the metacarpal. The second metacarpal pin can also be placed first, with the external fixator used to judge proximal placement of the radial pin within the bare area.
Various supplements to external fixation have positive outcomes. Wolfe and colleagues18 found that using K-wires with the external fixation construct added stability in flexion/extension, radial/ulnar deviation, and rotational motion. They noted that fixation stability may depend more on the augmentation to fixation than on the external fixator itself. In a prospective, randomized trial, Moroni and colleagues26,27 found that, compared with standard pins, hydroxyapatite-coated pins had higher extraction torque, which was associated with improved fixation. When combined with external fixation, calcium phosphate cement also provided additional stability, allowing the bone filler to help maintain articular reduction and cortical continuity.28,29
External fixation has its disadvantages and complications. It can be bulky, and theoretically it contributes to higher rates of stiffness in the wrist and fingers.30-32 Higher rates of pin-site infection have been reported, along with hardware failure and associated loss of reduction, in patients treated with external fixation (Figures 3A-3C).31-33[[{"fid":"201866","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]In addition, joint overdistraction can adversely affect the length-tension curve and contribute to potential reflex sympathetic dystrophy, which can be devastating (Figures 4A, 4B).1,21,31,33 Despite these complications, external fixation remains a powerful tool in the treatment of high-energy DRFs. [[{"fid":"201867","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]In many cases, authors who compared open reduction and internal fixation (ORIF) with external fixation found no significant differences in outcome scores or function.31-34 In a meta-analysis of 917 patients, Margaliot and colleagues33 found no differences in pain, grip strength, wrist ROM, or radiographic parameters. More recently, in prospective randomized trials, both Egol and colleagues31 and Grewal and colleagues34 compared hybrid external fixation with ORIF, and, though early outcomes favored ORIF, 1-year follow-up comparisons were even, and there were no significant differences. These consistently reproducible results reaffirm keeping external fixation in the orthopedic toolbox.
Definitive Reconstruction With ORIF
Early nonlocked dorsal plating options for DRF fixation had unacceptable rates of plate failure, poor cosmesis, and extensor tendon complications.17,35-37 Subsequent technologic advances—multiple approaches, lower profile plating, and rigid, fragment-specific fixation—have allowed even the most complex fracture patterns to be addressed (Table). In malunited fractures, bone graft may not be required if the fracture is extra-articular and treated with a volar locking plate. [[{"fid":"201868","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]Other options include corticocancellous autograft from the iliac crest, hydroxyapatite synthetic grafts, and osteoconductive bone graft substitutes, such as bone morphogenic proteins. In addition, healing times are similar in cases, regardless of whether a graft was used.38
Involvement of the radial and intermediate columns should be addressed first. Although some may prefer a single volar plate, others may use fragment-specific fixation to buttress a comminuted radial styloid (in orthogonal fashion) and/or a dorsal ulnar fragment to restore the intermediate column and thereby fully restore the radial articular surface.39,40 Typically, restoring the radial and intermediate columns for radial articular reduction subsequently and simultaneously restores the majority of radial height and length. After the radial and intermediate columns are reduced and stabilized, the need for ulna column fixation can be determined. Important factors in ulna column restoration are severe osteoporosis and ulna head and/or neck comminution. Significant comminution throughout the metaphysis of both the radius and the ulna may also warrant stabilizing the ulna with internal fixation. Finally, any DRU joint instability noted on examination should also favor fixing the ulnar side.
Assessment of the distal ulna in these complex fractures goes beyond the involvement of an ulnar styloid fracture. Typically, fractures at the base of the ulnar styloid have been reported to have little clinical relevance, including a low incidence of associated DRU joint problems.41-43 Decisions to address the ulnar column are largely swayed by any instability found on DRU joint testing, as laxity caused by severe comminution can dictate the need for distal ring fixation to provide support. Even in the presence of a high-energy fracture in severely osteoporotic bone, the argument can be made to prevent instability by supporting the ulnar column. Stabilization of the ulnar articular surface can also be made more facile by creating an easier "A" fracture pattern (per AO classification) from a complex "C" to further aid in achieving efficient anatomical reduction. After preoperative planning is completed, depending on which columns need to be addressed, several surgical approaches can be considered to achieve maximum exposure and soft-tissue mobilization in order to successfully complete the operative fixation goals.
Volar Approach
An approach is selected for ideal exposure of a facile environment for definitive fixation. Access to the radial column can be gained with the extended flexor carpi radialis (FCR) approach. This approach allows visualization and removal of the appropriate deforming forces on the radial column to allow for fracture reduction by "opening the book," similar to that of tibial plateau reconstruction.44,45 It may be prudent to perform a preincision Allen test as well as a preoperative DRU joint examination for comparison after ORIF is complete. Compared with the classic Henry approach near the distal radius, going through the volar sheath of the FCR avoids many of the perforating radial artery branches. Avoiding stripping the radial artery of its surrounding fat and lymphatics prevents postoperative "cold intolerance." Retracting the FCR ulnarly and then incising the dorsal FCR sheath provide ready access to the pronator quadratus after collective ulnar mobilization of both the FCR and the flexor pollicis longus.44 In addition, for work near the distal FCR sheath, care must be taken to avoid the branch of the palmar cutaneous nerve that emerges about 5 cm proximal to the wrist flexion crease.46
Once at the level of the pronator quadratus, an "L-shape" incision can be made to reflect the muscle off the radius. Care must be taken when working too distal to avoid transection of the inserting volar wrist ligaments.44 Leaving a cuff for repair of the pronator remains controversial. In a recent case-control series, however, Hershman and colleagues47 did not find significant differences in function or complication rate in patients with and without repair. After reflection, adequate exposure of the radial column should be achieved. Ready access to the radial styloid for orthogonal plating can be obtained by releasing the brachioradialis, which simultaneously releases one of the primary fracture deforming forces.44 With this incision and exposure, if needed, dorsal bone grafting can be achieved from the volar side; however, care must be taken to protect the first dorsal compartment.48 The cutaneous branch of the median nerve may be at risk with this exposure, but avoiding dissection ulnar to the FCR tendon can help to reduce this risk.49
Before surgery, if the fracture pattern dictates a more ulnar approach, we prefer the extended carpal tunnel approach. Using the plane between the palmaris longus and the flexor digitorum superficialis medially and the FCR laterally, the extended carpal tunnel approach provides an obvious release of the flexor retinaculum but, more important, allows for extensile access to the sigmoid notch, the DRU joint, and the ulnar column.
Dorsal Approach
The dorsal approach is necessary in a few select cases. With a focus on fragment-specific fixation, presence of a significant dorsal ulnar fragment should warrant a dorsal approach.50 In addition, in select, rare cases in which volar access is limited or unavailable, dorsal access is the only option.50 Finally, if direct articular visualization is required, the dorsal approach typically is favored as the stronger radiocarpal ligaments found on the volar side are maintained.
Access should begin with an incision centered over the dorsal distal radius; a safe access point is just ulnar to the Lister tubercle. On incision of the retinaculum through a full-thickness excision, the third dorsal compartment is opened and the extensor pollicis longus (EPL) mobilized, fully exposing the dorsal distal radius. Work can be performed on either side of the EPL between the second and fourth dorsal compartments. Exposure typically is not an issue because of the pliable soft tissue of the dorsum, with ready access from styloid to styloid.44 Here, low-profile plates and/or mini-fragment-specific plate options should be used to minimize potential tendon damage.51 Care must also be taken to avoid damaging the radiocarpal or scapholunate ligaments.49 On closure, the retinaculum is repaired primarily; however, though some proponents advocate relocating the EPL tendon into its groove, we prefer leaving the EPL free within the surrounding soft tissue to reduce tension and promote unhindered excursion. The dorsal approach, though controversial and used inconsistently, should remain an important tool in anatomical restoration, especially in cases of complex fracture patterns.
Conclusion
Controversy still marks the lack of consensus on deciding which DRF treatment is optimal. Some investigators question moving away from external fixation and cite the lack of significantly better data relative to ORIF.21,52 The same proponents note that the only advantage over external fixation is earlier return to function and cite reports of tendon rupture and complications with both dorsal and volar fixation options.34,53-58 Other investigators find that operative treatment generally does not provide a significant improvement over nonoperative treatment.59
With the advent of lower profile locked plating, fragment-specific fixation, and variable-angle devices, comparative clinical trials are finding it difficult to keep up.60-64 Results from ongoing prospective randomized trials like ORCHID (Open Reduction and Internal Fixation Versus Casting for Highly Comminuted Intra-Articular Fractures of the Distal Radius; 500 patients >65 years old, 15 centers) will provide more definitive answers about ideal treatment.65
Anatomical restoration involves a versatile array of fragment fixation and reconstruction. Careful preoperative planning and a consistent approach to restoring the radial, intermediate, and ulnar columns, along with a proper surgical approach, are ideal. Many advances in internal fixation have been exceedingly helpful. Use of external fixation, especially in a bridging fashion with or without supplementation, is still valuable in many situations.
1. Liporace FA, Adams MR, Capo JT, Koval KJ. Distal radius fractures. J Orthop Trauma. 2009;23(10):739-748.
2. Lee YS, Wei TY, Cheng YC, Hsu TL, Huang CR. A comparative study of Colles’ fractures in patients between fifty and seventy years of age: percutaneous K-wiring versus volar locking plating. Int Orthop. 2012;36(4):789-794.
3. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824-835.e2.
4. Ring D. Treatment of the neglected distal radius fracture. Clin Orthop Relat Res. 2005;(431):85-92.
5. Berger RA. Arthroscopic anatomy of the wrist and distal radioulnar joint. Hand Clin. 1999;15(3):393-413, vii.
6. Berger RA. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;(383):32-40.
7. McCann PA, Clarke D, Amirfeyz R, Bhatia R. The cadaveric anatomy of the distal radius: implications for the use of volar plates. Ann R Coll Surg Engl. 2012;94(2):116-120.
8. Ekenstam F. Osseous anatomy and articular relationships about the distal ulna. Hand Clin. 1998;14(2):161-164.
9. Fernandez DL. Distal radius fracture: the rationale of a classification. Chir Main. 2001;20(6):411-425.
10. Raskin KB, Melone CP Jr. Unstable articular fractures of the distal radius. Comparative techniques of ligamentotaxis. Orthop Clin North Am. 1993;24(2):275-286.
11. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
12. Jenkins NH. The unstable Colles’ fracture. J Hand Surg Br. 1989;14(2):149-154.
13. Cooney WP, Dobyns JH, Linscheid RL. Arthroscopy of the wrist: anatomy and classification of carpal instability. Arthroscopy. 1990;6(2):133-140.
14. Kural C, Sungur I, Kaya I, Ugras A, Ertürk A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33(11):801.
15. Lipton HA, Wollstein R. Operative treatment of intraarticular distal radial fractures. Clin Orthop Relat Res. 1996;(327):110-124.
16. Wolfe SW. Distal radius fractures. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011:561-638.
17. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):
588-592.
18. Wolfe SW, Austin G, Lorenze M, Swigart CR, Panjabi MM. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg Am. 1999;24(3):516-524.
19. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma. 2006;20(2):115-121.
20. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg. 2004;93(1):64-67.
21. Paksima N, Panchal A, Posner MA, Green SM, Mehiman CT, Hiebert R. A meta-analysis of the literature on distal radius fractures: review of 615 articles. Bull Hosp Jt Dis. 2004;62(1-2):40-46.
22. Li-hai Z, Ya-nan W, Zhi M, et al. Volar locking plate versus external fixation for the treatment of unstable distal radial fractures: a meta-analysis of randomized controlled trials.
J Surg Res. 2015;193(1):324-333.
23. Papadonikolakis A, Shen J, Garrett JP, Davis SM, Ruch DS. The effect of increasing distraction on digital motion after external fixation of the wrist. J Hand Surg Am. 2005;30(4):
773-779.
24. Seitz WH Jr, Froimson AI, Brooks DB, et al. Biomechanical analysis of pin placement and pin size for external fixation of distal radius fractures. Clin Orthop Relat Res. 1990;(251):
207-212.
25. Beldner S, Zlotolow DA, Melone CP Jr, Agnes AM, Jones MH. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am. 2005;30(6):1226-1230.
26. Moroni A, Faldini C, Marchetti S, Manca M, Consoli V, Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83(5):717-721.
27. Moroni A, Heikkila J, Magyar G, Toksvig-Larsen S, Giannini S. Fixation strength and pin tract infection of hydroxyapatite-coated tapered pins. Clin Orthop Relat Res. 2001;(388):209-217.
28. Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of fixation of intra-articular distal radial fractures with calcium-phosphate bone cement. J Bone Joint Surg Am. 2002;84(9):1579-1586.
29. Tobe M, Mizutani K, Tsubuku Y. Treatment of distal radius fracture with the use of calcium phosphate bone cement as a filler. Tech Hand Up Extrem Surg. 2004;8(2):95-101.
30. Capo JT, Rossy W, Henry P, Maurer RJ, Naidu S, Chen L.
External fixation of distal radius fractures: effect of distraction and duration. J Hand Surg Am. 2009;34(9):1605-1611.
31. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
32. Egol KA, Paksima N, Puopolo S, Klugman J, Hiebert R, Koval KJ. Treatment of external fixation pins about the wrist: a prospective, randomized trial. J Bone Joint Surg Am. 2006;88(2):349-354.
33. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
34. Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):
1899-1906.
35. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990;15(1):1-11.
36. Hove LM, Nilsen PT, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius. 31 patients followed for 3-7 years. Acta Orthop Scand. 1997;68(1):59-63.
37. Carter PR, Frederick HA, Laseter GF. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg Am. 1998;23(2):300-307.
38. Mugnai R, Tarallo L, Lancellotti E, et al. Corrective osteotomies of the radius: grafting or not? World J Orthop. 2016;7(2):128-135.
39. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149.
40. Helmerhorst GT, Kloen P. Orthogonal plating of intra-articular distal radius fractures with an associated radial column fracture via a single volar approach. Injury. 2012;43(8):1307-1312.
41. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
42. Souer JS, Ring D, Matschke S, Audige L, Marent-Huber M, Jupiter JB; AOCID Prospective ORIF Distal Radius Study Group. Effect of an unrepaired fracture of the ulnar styloid base on outcome after plate-and-screw fixation of a distal radial fracture. J Bone Joint Surg Am. 2009;91(4):830-838.
43. Noda K, Goto A, Murase T, Sugamoto K, Yoshikawa H, Moritomo H. Interosseous membrane of the forearm: an anatomical study of ligament attachment locations. J Hand Surg Am. 2009;34(3):415-422.
44. Catalano LW 3rd, Zlotolow DA, Hitchcock PB, Shah SN, Barron OA. Surgical exposures of the radius and ulna. J Am Acad Orthop Surg. 2011;19(7):430-438.
45. Orbay JL, Badia A, Indriago IR, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204-211.
46. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am. 1990;15(1):38-43.
47. Hershman SH, Immerman I, Bechtel C, Lekic N, Paksima N, Egol KA. The effects of pronator quadratus repair on outcomes after volar plating of distal radius fractures. J Orthop Trauma. 2013;27(3):130-133.
48. Prommersberger KJ, Lanz UB. Corrective osteotomy of the distal radius through volar approach. Tech Hand Up Extrem Surg. 2004;8(2):70-77.
49. Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011;6(1):8-17.
50. Tavakolian JD, Jupiter JB. Dorsal plating for distal radius fractures. Hand Clin. 2005;21(3):341-346.
51. Yu YR, Makhni MC, Tabrizi S, Rozental TD, Mundanthanam G, Day CS. Complications of low-profile dorsal versus volar locking plates in the distal radius: a comparative study. J Hand Surg Am. 2011;36(7):1135-1141.
52. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma. 2011;71(4):939-942.
53. Wilcke MK, Abbaszadegan H, Adolphson PY. Wrist function recovers more rapidly after volar locked plating than after external fixation but the outcomes are similar after 1 year. Acta Orthop. 2011;82(1):76-81.
54. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189.
55. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am. 2011;36(1):3-9.
56. Soong M, Earp BE, Bishop G, Leung A, Blazar P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg Am. 2011;93(4):328-335.
57. Jeudy J, Steiger V, Boyer P, Cronier P, Bizot P, Massin P. Treatment of complex fractures of the distal radius: a prospective randomised comparison of external fixation ‘versus’ locked volar plating. Injury. 2012;43(2):174-179.
58. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
59. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
60. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg Am. 2012;37(3):446-453.
61. Dahl WJ, Nassab PF, Burgess KM, et al. Biomechanical properties of fixed-angle volar distal radius plates under dynamic loading. J Hand Surg Am. 2012;37(7):1381-1387.
62. Park JH, Hagopian J, Ilyas AM. Variable-angle locking screw volar plating of distal radius fractures. Hand Clin. 2010;26(3):373-380, vi.
63. Pensy RA, Brunton LM, Parks BG, Higgins JP, Chhabra AB. Single-incision extensile volar approach to the distal radius and concurrent carpal tunnel release: cadaveric study. J Hand Surg Am. 2010;35(2):217-222.
64. Klos K, Rausch S, Löffler M, et al. A biomechanical comparison of a biodegradable volar locked plate with two titanium volar locked plates in a distal radius fracture model. J Trauma. 2010;68(4):984-991.
65. Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;12:84
1. Liporace FA, Adams MR, Capo JT, Koval KJ. Distal radius fractures. J Orthop Trauma. 2009;23(10):739-748.
2. Lee YS, Wei TY, Cheng YC, Hsu TL, Huang CR. A comparative study of Colles’ fractures in patients between fifty and seventy years of age: percutaneous K-wiring versus volar locking plating. Int Orthop. 2012;36(4):789-794.
3. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824-835.e2.
4. Ring D. Treatment of the neglected distal radius fracture. Clin Orthop Relat Res. 2005;(431):85-92.
5. Berger RA. Arthroscopic anatomy of the wrist and distal radioulnar joint. Hand Clin. 1999;15(3):393-413, vii.
6. Berger RA. The anatomy of the ligaments of the wrist and distal radioulnar joints. Clin Orthop Relat Res. 2001;(383):32-40.
7. McCann PA, Clarke D, Amirfeyz R, Bhatia R. The cadaveric anatomy of the distal radius: implications for the use of volar plates. Ann R Coll Surg Engl. 2012;94(2):116-120.
8. Ekenstam F. Osseous anatomy and articular relationships about the distal ulna. Hand Clin. 1998;14(2):161-164.
9. Fernandez DL. Distal radius fracture: the rationale of a classification. Chir Main. 2001;20(6):411-425.
10. Raskin KB, Melone CP Jr. Unstable articular fractures of the distal radius. Comparative techniques of ligamentotaxis. Orthop Clin North Am. 1993;24(2):275-286.
11. Melone CP Jr. Distal radius fractures: patterns of articular fragmentation. Orthop Clin North Am. 1993;24(2):239-253.
12. Jenkins NH. The unstable Colles’ fracture. J Hand Surg Br. 1989;14(2):149-154.
13. Cooney WP, Dobyns JH, Linscheid RL. Arthroscopy of the wrist: anatomy and classification of carpal instability. Arthroscopy. 1990;6(2):133-140.
14. Kural C, Sungur I, Kaya I, Ugras A, Ertürk A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33(11):801.
15. Lipton HA, Wollstein R. Operative treatment of intraarticular distal radial fractures. Clin Orthop Relat Res. 1996;(327):110-124.
16. Wolfe SW. Distal radius fractures. Green’s Operative Hand Surgery. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011:561-638.
17. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):
588-592.
18. Wolfe SW, Austin G, Lorenze M, Swigart CR, Panjabi MM. A biomechanical comparison of different wrist external fixators with and without K-wire augmentation. J Hand Surg Am. 1999;24(3):516-524.
19. Kreder HJ, Agel J, McKee MD, Schemitsch EH, Stephen D, Hanel DP. A randomized, controlled trial of distal radius fractures with metaphyseal displacement but without joint incongruity: closed reduction and casting versus closed reduction, spanning external fixation, and optional percutaneous K-wires. J Orthop Trauma. 2006;20(2):115-121.
20. Moroni A, Vannini F, Faldini C, Pegreffi F, Giannini S. Cast vs external fixation: a comparative study in elderly osteoporotic distal radial fracture patients. Scand J Surg. 2004;93(1):64-67.
21. Paksima N, Panchal A, Posner MA, Green SM, Mehiman CT, Hiebert R. A meta-analysis of the literature on distal radius fractures: review of 615 articles. Bull Hosp Jt Dis. 2004;62(1-2):40-46.
22. Li-hai Z, Ya-nan W, Zhi M, et al. Volar locking plate versus external fixation for the treatment of unstable distal radial fractures: a meta-analysis of randomized controlled trials.
J Surg Res. 2015;193(1):324-333.
23. Papadonikolakis A, Shen J, Garrett JP, Davis SM, Ruch DS. The effect of increasing distraction on digital motion after external fixation of the wrist. J Hand Surg Am. 2005;30(4):
773-779.
24. Seitz WH Jr, Froimson AI, Brooks DB, et al. Biomechanical analysis of pin placement and pin size for external fixation of distal radius fractures. Clin Orthop Relat Res. 1990;(251):
207-212.
25. Beldner S, Zlotolow DA, Melone CP Jr, Agnes AM, Jones MH. Anatomy of the lateral antebrachial cutaneous and superficial radial nerves in the forearm: a cadaveric and clinical study. J Hand Surg Am. 2005;30(6):1226-1230.
26. Moroni A, Faldini C, Marchetti S, Manca M, Consoli V, Giannini S. Improvement of the bone-pin interface strength in osteoporotic bone with use of hydroxyapatite-coated tapered external-fixation pins. A prospective, randomized clinical study of wrist fractures. J Bone Joint Surg Am. 2001;83(5):717-721.
27. Moroni A, Heikkila J, Magyar G, Toksvig-Larsen S, Giannini S. Fixation strength and pin tract infection of hydroxyapatite-coated tapered pins. Clin Orthop Relat Res. 2001;(388):209-217.
28. Higgins TF, Dodds SD, Wolfe SW. A biomechanical analysis of fixation of intra-articular distal radial fractures with calcium-phosphate bone cement. J Bone Joint Surg Am. 2002;84(9):1579-1586.
29. Tobe M, Mizutani K, Tsubuku Y. Treatment of distal radius fracture with the use of calcium phosphate bone cement as a filler. Tech Hand Up Extrem Surg. 2004;8(2):95-101.
30. Capo JT, Rossy W, Henry P, Maurer RJ, Naidu S, Chen L.
External fixation of distal radius fractures: effect of distraction and duration. J Hand Surg Am. 2009;34(9):1605-1611.
31. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary Kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: a randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221.
32. Egol KA, Paksima N, Puopolo S, Klugman J, Hiebert R, Koval KJ. Treatment of external fixation pins about the wrist: a prospective, randomized trial. J Bone Joint Surg Am. 2006;88(2):349-354.
33. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
34. Grewal R, MacDermid JC, King GJ, Faber KJ. Open reduction internal fixation versus percutaneous pinning with external fixation of distal radius fractures: a prospective, randomized clinical trial. J Hand Surg Am. 2011;36(12):
1899-1906.
35. Axelrod TS, McMurtry RY. Open reduction and internal fixation of comminuted, intraarticular fractures of the distal radius. J Hand Surg Am. 1990;15(1):1-11.
36. Hove LM, Nilsen PT, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius. 31 patients followed for 3-7 years. Acta Orthop Scand. 1997;68(1):59-63.
37. Carter PR, Frederick HA, Laseter GF. Open reduction and internal fixation of unstable distal radius fractures with a low-profile plate: a multicenter study of 73 fractures. J Hand Surg Am. 1998;23(2):300-307.
38. Mugnai R, Tarallo L, Lancellotti E, et al. Corrective osteotomies of the radius: grafting or not? World J Orthop. 2016;7(2):128-135.
39. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149.
40. Helmerhorst GT, Kloen P. Orthogonal plating of intra-articular distal radius fractures with an associated radial column fracture via a single volar approach. Injury. 2012;43(8):1307-1312.
41. May MM, Lawton JN, Blazar PE. Ulnar styloid fractures associated with distal radius fractures: incidence and implications for distal radioulnar joint instability. J Hand Surg Am. 2002;27(6):965-971.
42. Souer JS, Ring D, Matschke S, Audige L, Marent-Huber M, Jupiter JB; AOCID Prospective ORIF Distal Radius Study Group. Effect of an unrepaired fracture of the ulnar styloid base on outcome after plate-and-screw fixation of a distal radial fracture. J Bone Joint Surg Am. 2009;91(4):830-838.
43. Noda K, Goto A, Murase T, Sugamoto K, Yoshikawa H, Moritomo H. Interosseous membrane of the forearm: an anatomical study of ligament attachment locations. J Hand Surg Am. 2009;34(3):415-422.
44. Catalano LW 3rd, Zlotolow DA, Hitchcock PB, Shah SN, Barron OA. Surgical exposures of the radius and ulna. J Am Acad Orthop Surg. 2011;19(7):430-438.
45. Orbay JL, Badia A, Indriago IR, et al. The extended flexor carpi radialis approach: a new perspective for the distal radius fracture. Tech Hand Up Extrem Surg. 2001;5(4):204-211.
46. Hobbs RA, Magnussen PA, Tonkin MA. Palmar cutaneous branch of the median nerve. J Hand Surg Am. 1990;15(1):38-43.
47. Hershman SH, Immerman I, Bechtel C, Lekic N, Paksima N, Egol KA. The effects of pronator quadratus repair on outcomes after volar plating of distal radius fractures. J Orthop Trauma. 2013;27(3):130-133.
48. Prommersberger KJ, Lanz UB. Corrective osteotomy of the distal radius through volar approach. Tech Hand Up Extrem Surg. 2004;8(2):70-77.
49. Ilyas AM. Surgical approaches to the distal radius. Hand (N Y). 2011;6(1):8-17.
50. Tavakolian JD, Jupiter JB. Dorsal plating for distal radius fractures. Hand Clin. 2005;21(3):341-346.
51. Yu YR, Makhni MC, Tabrizi S, Rozental TD, Mundanthanam G, Day CS. Complications of low-profile dorsal versus volar locking plates in the distal radius: a comparative study. J Hand Surg Am. 2011;36(7):1135-1141.
52. Mattila VM, Huttunen TT, Sillanpää P, Niemi S, Pihlajamäki H, Kannus P. Significant change in the surgical treatment of distal radius fractures: a nationwide study between 1998 and 2008 in Finland. J Trauma. 2011;71(4):939-942.
53. Wilcke MK, Abbaszadegan H, Adolphson PY. Wrist function recovers more rapidly after volar locked plating than after external fixation but the outcomes are similar after 1 year. Acta Orthop. 2011;82(1):76-81.
54. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189.
55. Soong M, van Leerdam R, Guitton TG, Got C, Katarincic J, Ring D. Fracture of the distal radius: risk factors for complications after locked volar plate fixation. J Hand Surg Am. 2011;36(1):3-9.
56. Soong M, Earp BE, Bishop G, Leung A, Blazar P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg Am. 2011;93(4):328-335.
57. Jeudy J, Steiger V, Boyer P, Cronier P, Bizot P, Massin P. Treatment of complex fractures of the distal radius: a prospective randomised comparison of external fixation ‘versus’ locked volar plating. Injury. 2012;43(2):174-179.
58. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
59. Egol KA, Walsh M, Romo-Cardoso S, Dorsky S, Paksima N. Distal radial fractures in the elderly: operative compared with nonoperative treatment. J Bone Joint Surg Am. 2010;92(9):1851-1857.
60. Wall LB, Brodt MD, Silva MJ, Boyer MI, Calfee RP. The effects of screw length on stability of simulated osteoporotic distal radius fractures fixed with volar locking plates. J Hand Surg Am. 2012;37(3):446-453.
61. Dahl WJ, Nassab PF, Burgess KM, et al. Biomechanical properties of fixed-angle volar distal radius plates under dynamic loading. J Hand Surg Am. 2012;37(7):1381-1387.
62. Park JH, Hagopian J, Ilyas AM. Variable-angle locking screw volar plating of distal radius fractures. Hand Clin. 2010;26(3):373-380, vi.
63. Pensy RA, Brunton LM, Parks BG, Higgins JP, Chhabra AB. Single-incision extensile volar approach to the distal radius and concurrent carpal tunnel release: cadaveric study. J Hand Surg Am. 2010;35(2):217-222.
64. Klos K, Rausch S, Löffler M, et al. A biomechanical comparison of a biodegradable volar locked plate with two titanium volar locked plates in a distal radius fracture model. J Trauma. 2010;68(4):984-991.
65. Bartl C, Stengel D, Bruckner T, et al. Open reduction and internal fixation versus casting for highly comminuted and intra-articular fractures of the distal radius (ORCHID): protocol for a randomized clinical multi-center trial. Trials. 2011;12:84
Management and Prevention of Intraoperative Acetabular Fracture in Primary Total Hip Arthroplasty
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
Take Home Points
- IAF is an uncommon, but serious complication of primary THA.
- Small (<50 mm) cups are at higher risk for causing IAF.
- Prompt recognition is critical to prevent component migration and need for revision.
- Posterior column integrity is cirtical to a successful outcome when IAF occurs.
- Initial stable fixation, with or without intraoperative acetabular revision, is critical for successful outcome when IAF is identified.
Intraoperative acetabular fracture (IAF) is a rare complication of primary total hip arthroplasty (THA).1-3 IAFs commonly occur with impaction of the acetabular component. Studies have found that underreaming of the acetabulum and impaction of relatively large, elliptic, or monoblock components may increase the risk of IAFs.2-5 There is a paucity of literature on risk factors, treatment strategies, and outcomes of this potentially devastating complication.
In this article, we report on the incidence of IAF in primary THA at our high-volume institution and present strategies for managing and preventing this rare fracture.
Materials and Methods
Between 1997 and 2015, more than 20 fellowship-trained arthroplasty surgeons performed 21,519 primary THAs at our institution. After obtaining Institutional Review Board approval for this study, we retrospectively searched the hospital database and identified 16 patients (16 hips) who sustained an IAF in primary THA. Mean age of the cohort (13 women, 3 men) at time of surgery was 70 years (range, 42-89 years). Of the 16 patients, 13 had a preoperative diagnosis of osteoarthritis, 2 had posttraumatic arthritis, and 1 had rheumatoid arthritis. A posterolateral approach was used with 14 patients and a modified anterolateral approach with the other 2. Surgical technique and implant selection varied among surgeons. Thirteen THAs were performed with an all-press-fit technique and 3 with a hybrid technique (uncemented acetabular component, cemented femoral component). In 9 cases, the acetabular component underwent supplemental screw fixation. Whether to use acetabular component screws or cemented femoral components was decided intraoperatively by the surgeon.
The cohort’s acetabular components were either elliptic modular or hemispheric modular. The elliptic modular component used was the Peripheral Self-Locking (PSL) implant (Stryker Howmedica Osteonics), and the hemispheric modular components used were either the Trident implant (Stryker Howmedica Osteonics) or the ZTT-II implant (DePuy Synthes). Elliptic acetabular components have a peripheral flare, in contrast to true hemispheric acetabular components. Ten elliptic modular and 6 hemispheric modular components were implanted. In all cases, the difference between the final reamer used to prepare the acetabular bed and the true largest external diameter of the impacted shell was 2 mm or less.
The cohort’s 16 femoral components consisted of 8 Secur-Fit uncemented components (Stryker Howmedica Osteonics), 3 Accolade uncemented components (Stryker Howmedica Osteonics), 3 Omnifit EON cemented components (Stryker Howmedica Osteonics), and 2 S-ROM uncemented components (DePuy Synthes).
After surgery, all patients were followed up according to individual surgeon protocol for radiographic and physical examination.
Data on IAF incidence were obtained from a hospital database and were confirmed with electronic medical record (EMR) documentation. Also obtained were IAF causes and locations recorded in operative notes. For fractures identified after surgery, location was obtained from the immediate postoperative radiograph. Fracture management (eg, supplemental screw fixation, fracture reduction and fixation, bone grafting, acetabular component revision, protected weight-bearing) was determined from EMR documentation.
Results
Sixteen patients sustained an IAF in primary THA. All IAFs occurred in cases involving cementless acetabular components. The institution’s incidence of IAF with use of cementless components was 0.0007%.
Of the 5 IAFs (31%) identified during surgery, 4 were noted during impaction of the acetabular component, and 1 was noted during reaming. Eighty percent of these IAFs occurred directly posterior, and 60% were addressed at time of index procedure secondary to acetabular component instability. The other 11 fractures (69%) were identified on standard postoperative anteroposterior pelvis radiographs obtained in the postanesthesia care unit (PACU). Details of component characteristics, fracture location, immediate treatment, and weight-bearing precautions for all 16 patients are listed in the Table.
There were additional complications. One patient sustained an intraoperative proximal femur fracture, which was addressed at the index THA with application of a cerclage wire and reinsertion of the femoral component; no further surgical intervention was required, and the femur fracture healed uneventfully. Another patient had a postoperative ileus that required nasogastric tube decompression and monitoring in the intensive care unit; the ileus resolved spontaneously. A third patient, initially treated with bone grafting and cemented cup insertion, was diagnosed with a periprosthetic joint infection 3 weeks after the index THA and was treated with explantation of all components and girdlestone resection arthroplasty; 1 month after the resection arthroplasty, a persistently draining wound was treated with irrigation and débridement. There were no other medical complications, thromboembolic events, or dislocations.
One to 7 weeks after surgery, patients returned for initial follow-up, and radiographs were obtained for component stability assessment. Three patients presented with gross acetabular instability, and revisions were performed. Standard clinical follow-up continued for all patients per individual surgeon protocol. Mean follow-up was 4 years.
Discussion
IAF is an uncommon complication of THA. The rarity of IAFs makes it difficult to obtain a cohort large enough to study the problem. Given the increasing incidence of primary THAs and the almost ubiquitous use of press-fit acetabular components, surgeons who perform THAs undoubtedly will encounter IAFs in their own practice. In this article, we report our institution’s experience with periprosthetic IAFs and provide a framework for making decisions regarding these complications.
Anatomical locations of IAFs have been associated with variable outcomes. In a 2015 series, Laflamme and colleagues6 found posterior column stability a crucial factor in implant stability. Fractures with posterior column instability had a 67% failure rate, and patients with an intact posterior column reliably had osteointegration occur without further intervention.6 In our series, fractures that violated the posterior column had similar results. All these fractures required further operative intervention, either at the index procedure or in the early postoperative period. Loss of posterior column stability prevents secure fixation of the acetabular component, thereby preventing successful hip reconstruction. One posterior column fracture in our series was not recognized until after surgery, on a PACU radiograph, and 1 posterior column fracture was fully appreciated only after postoperative computed tomography (CT) was obtained during immediate hospitalization after the index procedure. In both cases, conservative management was unsuccessful. Revision arthroplasty (and in 1 case late posterior column fixation) was performed to achieve adequate reconstruction. There were no failures after posterior column fixation. In cases of posterior wall or column fracture, we recommend early aggressive treatment, preferably at the time of index arthroplasty, to prevent catastrophic failure.
Most commonly, periprosthetic IAFs go unnoticed until initial postoperative radiographs are examined.6 Eleven of the 16 IAFs in our series were first recognized on radiographs obtained in the PACU. Surgeons thus have difficult decisions to make. The literature has little discussion on managing early postoperative periprosthetic IAFs. Most recent studies, which consist of small series and case reports, have focused on late and often traumatic IAFs.7-9 These were initially classified by Peterson and Lewallen10 as type I, which are stable radiographically (no movement relative to previous radiographs) and do not produce pain with minor movement of the extremity, or type II, which are unstable radiographically (gross displacement of component) or produce pain with any hip motion. Type I fractures were more common and were often managed with protected weight-bearing and observation. The authors concluded that, in type I fractures, retaining the original acetabular component is difficult; however, when these fractures are treated appropriately, a functional prosthesis can be salvaged, and fracture union can be expected.
Less common are acetabular fractures detected during surgery, as in our study. In an outcome series, Haidukewych and colleagues3 reported on 21 periprosthetic acetabular fractures, all recognized during surgery and managed according to perceived stability of the component. All fractures healed uneventfully, and there were no other complications.
These studies provide a framework for addressing IAFs noticed in the early postoperative period. The diagnostic dilemma presented by these fractures was first discussed by Laflamme and colleagues.6 Nine of the 32 fractures in their series were classified as so-called type III fractures, recognized only after the early postoperative period. Additional radiographs (eg, Judet views) or CT scans were crucial in determining acetabular component stability, given the known poor outcomes associated with posterior column fracture. In our series, only 1 patient had CT performed after intraoperative recognition of fracture, and the extent of the fracture was not readily apparent on the patient’s postoperative radiograph. Given the successful recognition and treatment of these fractures in the early postoperative period in our series,
it is difficult to recommend advanced imaging for all periprosthetic IAFs. Perhaps this success is attributable to our almost universal use of screws for acetabular component fixation. Of the 11 patients with fractures recognized during the postoperative period, 8 had supplemental screw fixation at time of index surgery. If there is a question of fixation during component insertion, we recommend scrutinizing the acetabular rim for fracture and placing supplemental screw fixation. Screws placed for acetabular component fixation provide initial stability and may prevent early component failure in the setting of unrecognized medial or anterior fracture. In addition, when component stability is in question after impaction, we recommend using finger palpation to evaluate the sciatic notch for cortical step-off from an otherwise unrecognized fracture. Protected weight-bearing in the postoperative period may be left to the discretion of the surgeon, and the decision should be based on intraoperative stability of the acetabular component.
In our series, there was a disproportionate representation of fractures associated with elliptic acetabular components. All 5 of the fractures recognized during surgery and 5 of the 11 recognized after surgery occurred with elliptic components. The association between elliptic cup design and periprosthetic IAF was identified earlier, by Haidukewych and colleagues.3 Their series showed a statistically significant increase in fracture incidence with impaction of an elliptic cup into a bed prepared with a hemispheric reamer. In the present series, 75% of our acetabular components were impacted into a bed underreamed by 1 mm to 2 mm. It is typical of many surgeons at our institution to underream by 1 mm to 2 mm regardless of the type of component being implanted, though they show a growing trend to overream by only 1 mm with the PSL component, which has been both safe and reliable in preventing catastrophic posterior column fractures, especially with impaction of small (<50 mm) acetabular components. We have not observed early loosening or other evidence of failure with this technique. Cup impaction generates significant hoop stresses that can easily fracture sclerotic or otherwise poor-quality bone, and the dense bone around the acetabular rim experiences increased stress with impaction of elliptic components.2,11-15 Surgeons must understand the design traits of their components and be cognizant of the true difference between the diameter of the final reamer used and the real diameter of the acetabular component. We recommend having a difference of ≤1 mm to mitigate the risk of IAF occurring with cup insertion. With use of elliptic components, slight overreaming of the acetabular bed should be considered. More study is needed to better define these outcomes.
Study Limitations
Our study had several limitations, including the inherent biases of its retrospective design, small cohort size, and inclusion of multiple surgeons. Small cohort size is unavoidable given the low incidence of these injuries, and our study encompassed the experience of a high-volume hip arthroplasty service. There is the possibility that a subset of fractures may have persistently gone unrecognized, either during or after surgery, and the actual incidence of these complications may be higher. These outcomes represent our institutional experience addressing the complexities of these injuries. The lack of standardization in the management of these fractures in our series reflects the diagnostic dilemma they present, as well as the need for more study focused on their management and outcomes.
Conclusion
IAF, an uncommon complication of primary THA, most commonly occurs during component impaction. Acetabular component and surgical technique may influence the fracture rate. Intraoperative or prompt postoperative recognition of these fractures is crucial, as their location is associated with stability and outcome. Careful examination of postoperative radiographs, judicious use of advanced imaging, and close follow-up are needed to prevent early catastrophic failure. We argue against simply observing these unstable fractures and recommend early treatment with rigid fixation and, when necessary, acetabular component revision.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
1. Sharkey PF, Hozack WJ, Callaghan JJ, et al. Acetabular fractures associated with cementless acetabular cup insertion: a report of 13 cases. J Arthroplasty.1999;14(4):426-431.
2. Kim YS, Callaghan JJ, Ahn PB, Brown TD. Fracture of the acetabulum during insertion of an oversized hemispherical component. J Bone Joint Surg Am. 1995;77(1):111-117.
3. Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88(9):1952-1956.
4. Curtis MJ, Jinnah RH, Wilson VD, Hungerford DS. The initial stability of uncemented acetabular components. J Bone Joint Surg Br. 1992;74(3):372-376.
5. Lachiewicz PF, Suh PB, Gilbert JA. In vitro initial fixation of porous-coated acetabular total hip components. A biomechanical and comparative study. J Arthroplasty. 1989;4(3):201-205.
6. Laflamme GY, Belzile EL, Fernandes JC, Vendittoli PA, Hébert-Davies J. Periprosthetic fractures of the acetabulum during component insertion: posterior column stability
is crucial. J Arthroplasty. 2015;30(2):265-269.
7. Desai G, Reis MD. Early postoperative acetabular discontinuity after total hip arthroplasty. J Arthroplasty. 2011;26(8):1570.e17-e19.
8. Gelalis ID, Politis AN, Arnaoutoglou CM, Georgakopoulos N, Mitsiou D, Xenakis TA. Traumatic periprosthetic acetabular fracture treated by acute one-stage revision arthroplasty. A case report and review of the literature. Injury. 2010;41(4):421-424.
9. Gras F, Marintschev I, Klos K, Fujak A, Mückley T, Hofmann GO. Navigated percutaneous screw fixation of a periprosthetic acetabular fracture. J Arthroplasty. 2010;25(7):1169.e1-e4.
10. Peterson CA, Lewallen DG. Periprosthetic fracture of the acetabulum after total hip arthroplasty. J Bone Joint Surg Am. 1996;78(8):1206-1213.
11. Hansen TM, Koenman JB, Headley AK. 3-D FEM analysis of interface fixation of acetabular implants. Trans Orthop Res Soc. 1992;17:400.
12. Yerby SA, Taylor JK, Murzic WJ. Acetabular component interface: press-fit fixation. Trans Orthop Res Soc. 1992;17:384.
13. Callaghan JJ. The clinical results and basic science of total hip arthroplasty with porous-coated prostheses. J Bone Joint Surg Am. 1993;75(2):299-310.
14. Cheng SL, Binnington AG, Bragdon CR, Jasty M, Harris WH, Davey JR. The effect of sizing mismatch on bone ingrowth into uncemented porous coated acetabular components: an in vivo canine study. Trans Orthop Res Soc. 1990;15:442.
15. Morscher E, Bereiter H, Lampert C, Cementless press-fit cup: principles, experimental data, and three-year follow-up study. Clin Orthop Relat Res. 1989;(249):12-20.
Comparison of Lateral Retinaculum Release and Lengthening in the Treatment of Patellofemoral Disorders
Take-Home Points
- Understanding the indications for treatment is essential.
- Identifying the superficial (oblique fibers) and deep layers (transverse fibers) of the LR is very important and can lengthen the LR by as much as 20 mm.
- Open procedures reduce the risk of hematomas and related pain.
- The goal is to obtain 1 or 2 patellar quadrants of medial and lateral patellar glide in extensino and a neutral patella.
- If the Z-plasty is combined with the MPFL reconstruction or tibial tubercle transfer, the LR is set to length after the tubercle transfer and before the MPFL reconstruction (to avoid overconstraint).
Anterior knee pain is a common clinical problem that can be challenging to correct, in large part because of multiple causative factors, including structural/anatomical, functional, alignment, and neuroperception/pain pathway factors. One difficult aspect of anatomical assessment is judging the soft-tissue balance between the medial restraints (medial patellofemoral ligament [MPFL]; medial quadriceps tendon to femoral ligament; medial patellotibial and patellomeniscal ligaments) and the lateral restraints (lateral retinaculum [LR] specifically). Both LR tightness and patellar instability can be interpreted as anterior knee pain. Differentiating these entities is one of the most difficult clinical challenges in orthopedics.
LR release (LRR) has been found to improve patellar mobility and tracking.1 In the absence of clearly defined guidelines, the procedure quickly gained in popularity because of its technical simplicity and the enticing "one tool fits all" treatment approach suggested in early reviews. Injudicious use of LRR, alone or in combination with other procedures, led to iatrogenic instability and chronic pain. LR lengthening (LRL) was introduced to address LR tightness while maintaining lateral soft-tissue integrity and avoiding some of the severe complications of LRR.2
Today, isolated use of LRR/LRL is recommended only for treatment of LR tightness and pain secondary to lateral patellar hypercompression.3 It can also be used as an adjunct treatment in the setting of patellofemoral instability. LRR/LRL should never be used as primary treatment for patellofemoral instability.
In this review of treatments for LR tightness and patellofemoral disorders, we compare the use of LRR and LRL.
Discussion
LR procedures are indicated for LR tightness, which is assessed by taking a history, performing a physical examination, and obtaining diagnostic imaging. Decisions should be based on all findings considered together and never on imaging findings alone.
Physical Examination
The physical examination should include assessment of limb alignment, patellar mobility, muscle balance, and dynamic patellar tracking.
Limb Alignment. Abnormal valgus, rotational deformities, and increased Q-angle are associated with LR tightness. Valgus alignment can be assessed on standing inspection; rotational deformities with increased hip anteversion by hip motion with the patient in the prone position (increased hip internal rotation, decreased hip external rotation); and Q-angle on weight-bearing standing examination and with the patient flexing and extending the knee while seated.
Patellar Mobility. The patellar glide and tilt tests provide the most direct evaluations of LR tightness. Medial displacement of <1 quadrant is consistent with tightness, and displacement of >3 quadrants is consistent with laxity. In full extension, the patellar glide test evaluates only the soft-tissue restraints; at 30° flexion, it also evaluates patellofemoral engagement. The patellar tilt test measures the lifting of the lateral edge of the patella. With normal elevation being 0° to 20°, lack of patellar tilt means the LR is tight, and tilt of >20° means it is loose. MPFL patency can be examined with the Lachman test; the examiner rapidly moves the patella laterally while feeling for the characteristic hard endpoint of lateral translation.
Muscle Balance. The tone, strength, and tightness of the core (abdomen, dorsal, and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius) should be evaluated.
Dynamic Patellar Tracking. The J-sign is the course (shaped like an inverted J) that the patella takes when it is medialized into the trochlea from its laterally displaced resting position as the knee goes from full extension to flexion. The J-sign can be associated with LR tightness, trochlear dysplasia, and patella alta.
Imaging
Although we cannot provide a comprehensive review of the imaging literature, the following radiologic examinations should be used to assess the patellofemoral joint.
30° Lateral Radiograph. Increased tilt is seen when the lateral facet is not anterior to the patellar ridge. Also evaluated are trochlear anatomy, patellar height, and other factors involved in patellofemoral disorders.
30° Flexed Axial (Merchant) Radiograph. Patellar tilt, subluxation, and trochlear dysplasia are evaluated. Images obtained with progressive flexion can be very useful in verifying patellar tilt reduction. Lack of reduction during early flexion suggests LR tightness.4
Alignment Axial Radiographs (Scanogram). Valgus alignment is assessed with this full-length, standing, long-leg examination.
Computed Tomography/Magnetic Resonance Imaging. Many parameters of patellar alignment have been described. Basic assessment should include evaluation of patellar tilt, angle by the line across posterior condyles and a line through the greatest patellar width (>20° indicates abnormality and LR tightness) and tibial tubercle-trochlear groove distance (computed tomography or magnetic resonance imaging scan of the knee is used to measure this distance, and to confirm a significant amount in light of complex patellofemoral malalignment5).
Indications
Lateral compression syndrome with LR tightness is often successfully treated with isolated LRR, and results are reproducible and predictable.6 Surgical intervention for patellofemoral pain should be undertaken only after failed extensive nonoperative treatment with physical therapy and bracing/taping. Patients with LR tightness on preoperative examination, lateral patellar tilt on imaging, and normal Q-angle can obtain satisfactory results with this procedure. Patellar subluxation or dislocation history, high Q-angle (>20°), grade 3 or 4 chondral injury, and patellofemoral arthritis are associated with poorer outcomes when the procedure is performed in isolation.6International Patellofemoral Study Group members agreed that LRR/LRL is a valid treatment option when indicated, but it is rarely performed in isolation and constitutes only 1% to 2% of surgeries performed by this group of experts.7 When lateral compression syndrome progresses to arthritis, LRR/LRL can be performed with lateral patella facetectomy for maximal improvement.4 In the setting of patellofemoral instability, LRR/LRL can be combined with proximal and/or distal realignment surgery if the LR is tight. The LR is the last line of defense limiting lateral translation in the setting of an incompetent MPFL. Isolated LRR/LRL in the setting of instability further destabilizes the patella and worsens the instability. Therefore, LRR/LRL
is a poor surgical option as an isolated procedure for this condition and should be used only as an adjunct in cases of patellofemoral instability with LR tightness that does not allow the patella to be centralized into the trochlea.8 LRR/LRL can also be performed to improve patellar tracking in patellofemoral arthroplasty and total knee arthroplasty.
Lateral Retinaculum Release Versus Lengthening
LRR was first described for the treatment of patellar instability in 1891.9 It was also used for the treatment of lateral patellar hypercompression syndrome associated with LR tightness that led to lateral patellar tracking, joint overload, degeneration, and anterior knee pain.10 Metcalf10 further popularized the procedure by describing a minimally invasive arthroscopic version. However, the arthroscopic technique is as aggressive as the open technique and may be performed with less control, potentially making its results more variable. As proximal and distal releases are performed from the "inside out," more capsule and muscle disruption is needed to release the more superficial layers.
Z-plasty lengthening of the LR was described as an alternative for maintaining lateral patellar soft-tissue integrity while reducing the tension of the lateral tissue restraints.3 This is our preferred method.
Performing LRL instead of LRR avoids iatrogenic medial patellar instability, avoids overrelease and muscle injury, and improves soft-tissue balance.3 Open release or lengthening reduces inadvertent injury to the lateral superior/inferior geniculate arteries and allows direct hemostasis. Two prospective randomized studies found functional knee outcomes and return to athletic activities were improved more after LRL than LRR.11,12 These procedures had similar rates of postoperative knee stiffness, decreased muscle mass, and decreased strength. Each prospective study used an extensive LRR technique for LRR cases (various authors have recommended performing the release until the patella is perpendicular to the trochlea), which may have affected outcomes. In any case, with lengthening, the surgeon is less likely to excessively disrupt the lateral tissues.
Lateral Retinaculum Release. LRR can be openly performed by lateral parapatellar incision,1 a mini-open percutaneous technique, or arthroscopy. For these open techniques, incisions of various sizes have been used to access the LR and incise it about 1 cm lateral to the patella starting at the distal end of the vastus lateralis and extending distally until patellar tilt reduction is sufficient. If tightness in deep flexion persists, the LRR can be extended distally to the tibial tubercle. Open techniques have the advantage of sparing the joint capsule. All-arthroscopic techniques involve using electrocautery to cut through the capsule and access the LR.
Lateral Retinaculum Lengthening.
The LR is sharply divided into a superficial layer of superficial oblique fibers from the anterior iliotibial band and a deep layer of transverse fibers from the femur. For LRR, these 2 layers must be identified separate from the articular capsule.13
Complications
Complications of performing LRR/LRL to change the lateral restraint include medial patellar instability, increased lateral pain, repair failure, recurrent lateral instability, quadriceps weakness and atrophy, postoperative hemarthrosis, knee stiffness, wound complications, and thermal skin injury.7 These complications often result from poor surgical technique and too aggressive release. Although recommended patellar tilt historically has varied from 45° to 90°, the current goal is to normalize the tight soft-tissue restraints without creating secondary instability.
The most significant complication of LRR is medial patellar instability caused by muscle atrophy and loss of soft-tissue restraint.14 Medial instability can be difficult to diagnose and should be considered in any patient with patellofemoral pain, popping, or patellar instability after LRR.15 A positive medial subluxation test or medial patellar apprehension test suggests medial instability.
Medial patellar instability usually requires surgical treatment. Direct LR repair, lateral soft-tissue reconstruction, and other procedures can be used to restore lateral restraint.15 However, these are salvage techniques, and patients often remain significantly limited by pain or instability. Therefore, the LR must be carefully addressed and preferably should undergo lengthening rather than release.
1. Merchant AC, Mercer RL. Lateral release of the patella. A preliminary report. Clin Orthop Relat Res. 1974;(103):40-45.
2. Ceder LC, Larson RL. Z-plasty lateral retinacular release for the treatment of patellar compression syndrome. Clin Orthop Relat Res. 1979;(144):110-113.
3. Biedert R. Lateral patellar hypercompression, tilt and mild lateral subluxation. In: Biedert R, ed. Patellofemoral Disorders. Chichester, England: Wiley; 2004:161-166.
4. Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100-106.
5. Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle–posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
6. Lattermann C, Toth J, Bach BR Jr. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc. 2007;15(2):57-60.
7. Fithian DC, Paxton EW, Post WR, Panni AS; International Patellofemoral Study Group. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20(5):463-468.
8. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
9. Pollard B. Old dislocation of patella by intra-articular operation. Lancet. 1891;(988):17-22.
10. Metcalf RW. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
11. Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788-797.
12. O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759-1769.
13. Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br. 2008;90(4):527-534.
14. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
15. McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26-33.
Take-Home Points
- Understanding the indications for treatment is essential.
- Identifying the superficial (oblique fibers) and deep layers (transverse fibers) of the LR is very important and can lengthen the LR by as much as 20 mm.
- Open procedures reduce the risk of hematomas and related pain.
- The goal is to obtain 1 or 2 patellar quadrants of medial and lateral patellar glide in extensino and a neutral patella.
- If the Z-plasty is combined with the MPFL reconstruction or tibial tubercle transfer, the LR is set to length after the tubercle transfer and before the MPFL reconstruction (to avoid overconstraint).
Anterior knee pain is a common clinical problem that can be challenging to correct, in large part because of multiple causative factors, including structural/anatomical, functional, alignment, and neuroperception/pain pathway factors. One difficult aspect of anatomical assessment is judging the soft-tissue balance between the medial restraints (medial patellofemoral ligament [MPFL]; medial quadriceps tendon to femoral ligament; medial patellotibial and patellomeniscal ligaments) and the lateral restraints (lateral retinaculum [LR] specifically). Both LR tightness and patellar instability can be interpreted as anterior knee pain. Differentiating these entities is one of the most difficult clinical challenges in orthopedics.
LR release (LRR) has been found to improve patellar mobility and tracking.1 In the absence of clearly defined guidelines, the procedure quickly gained in popularity because of its technical simplicity and the enticing "one tool fits all" treatment approach suggested in early reviews. Injudicious use of LRR, alone or in combination with other procedures, led to iatrogenic instability and chronic pain. LR lengthening (LRL) was introduced to address LR tightness while maintaining lateral soft-tissue integrity and avoiding some of the severe complications of LRR.2
Today, isolated use of LRR/LRL is recommended only for treatment of LR tightness and pain secondary to lateral patellar hypercompression.3 It can also be used as an adjunct treatment in the setting of patellofemoral instability. LRR/LRL should never be used as primary treatment for patellofemoral instability.
In this review of treatments for LR tightness and patellofemoral disorders, we compare the use of LRR and LRL.
Discussion
LR procedures are indicated for LR tightness, which is assessed by taking a history, performing a physical examination, and obtaining diagnostic imaging. Decisions should be based on all findings considered together and never on imaging findings alone.
Physical Examination
The physical examination should include assessment of limb alignment, patellar mobility, muscle balance, and dynamic patellar tracking.
Limb Alignment. Abnormal valgus, rotational deformities, and increased Q-angle are associated with LR tightness. Valgus alignment can be assessed on standing inspection; rotational deformities with increased hip anteversion by hip motion with the patient in the prone position (increased hip internal rotation, decreased hip external rotation); and Q-angle on weight-bearing standing examination and with the patient flexing and extending the knee while seated.
Patellar Mobility. The patellar glide and tilt tests provide the most direct evaluations of LR tightness. Medial displacement of <1 quadrant is consistent with tightness, and displacement of >3 quadrants is consistent with laxity. In full extension, the patellar glide test evaluates only the soft-tissue restraints; at 30° flexion, it also evaluates patellofemoral engagement. The patellar tilt test measures the lifting of the lateral edge of the patella. With normal elevation being 0° to 20°, lack of patellar tilt means the LR is tight, and tilt of >20° means it is loose. MPFL patency can be examined with the Lachman test; the examiner rapidly moves the patella laterally while feeling for the characteristic hard endpoint of lateral translation.
Muscle Balance. The tone, strength, and tightness of the core (abdomen, dorsal, and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius) should be evaluated.
Dynamic Patellar Tracking. The J-sign is the course (shaped like an inverted J) that the patella takes when it is medialized into the trochlea from its laterally displaced resting position as the knee goes from full extension to flexion. The J-sign can be associated with LR tightness, trochlear dysplasia, and patella alta.
Imaging
Although we cannot provide a comprehensive review of the imaging literature, the following radiologic examinations should be used to assess the patellofemoral joint.
30° Lateral Radiograph. Increased tilt is seen when the lateral facet is not anterior to the patellar ridge. Also evaluated are trochlear anatomy, patellar height, and other factors involved in patellofemoral disorders.
30° Flexed Axial (Merchant) Radiograph. Patellar tilt, subluxation, and trochlear dysplasia are evaluated. Images obtained with progressive flexion can be very useful in verifying patellar tilt reduction. Lack of reduction during early flexion suggests LR tightness.4
Alignment Axial Radiographs (Scanogram). Valgus alignment is assessed with this full-length, standing, long-leg examination.
Computed Tomography/Magnetic Resonance Imaging. Many parameters of patellar alignment have been described. Basic assessment should include evaluation of patellar tilt, angle by the line across posterior condyles and a line through the greatest patellar width (>20° indicates abnormality and LR tightness) and tibial tubercle-trochlear groove distance (computed tomography or magnetic resonance imaging scan of the knee is used to measure this distance, and to confirm a significant amount in light of complex patellofemoral malalignment5).
Indications
Lateral compression syndrome with LR tightness is often successfully treated with isolated LRR, and results are reproducible and predictable.6 Surgical intervention for patellofemoral pain should be undertaken only after failed extensive nonoperative treatment with physical therapy and bracing/taping. Patients with LR tightness on preoperative examination, lateral patellar tilt on imaging, and normal Q-angle can obtain satisfactory results with this procedure. Patellar subluxation or dislocation history, high Q-angle (>20°), grade 3 or 4 chondral injury, and patellofemoral arthritis are associated with poorer outcomes when the procedure is performed in isolation.6International Patellofemoral Study Group members agreed that LRR/LRL is a valid treatment option when indicated, but it is rarely performed in isolation and constitutes only 1% to 2% of surgeries performed by this group of experts.7 When lateral compression syndrome progresses to arthritis, LRR/LRL can be performed with lateral patella facetectomy for maximal improvement.4 In the setting of patellofemoral instability, LRR/LRL can be combined with proximal and/or distal realignment surgery if the LR is tight. The LR is the last line of defense limiting lateral translation in the setting of an incompetent MPFL. Isolated LRR/LRL in the setting of instability further destabilizes the patella and worsens the instability. Therefore, LRR/LRL
is a poor surgical option as an isolated procedure for this condition and should be used only as an adjunct in cases of patellofemoral instability with LR tightness that does not allow the patella to be centralized into the trochlea.8 LRR/LRL can also be performed to improve patellar tracking in patellofemoral arthroplasty and total knee arthroplasty.
Lateral Retinaculum Release Versus Lengthening
LRR was first described for the treatment of patellar instability in 1891.9 It was also used for the treatment of lateral patellar hypercompression syndrome associated with LR tightness that led to lateral patellar tracking, joint overload, degeneration, and anterior knee pain.10 Metcalf10 further popularized the procedure by describing a minimally invasive arthroscopic version. However, the arthroscopic technique is as aggressive as the open technique and may be performed with less control, potentially making its results more variable. As proximal and distal releases are performed from the "inside out," more capsule and muscle disruption is needed to release the more superficial layers.
Z-plasty lengthening of the LR was described as an alternative for maintaining lateral patellar soft-tissue integrity while reducing the tension of the lateral tissue restraints.3 This is our preferred method.
Performing LRL instead of LRR avoids iatrogenic medial patellar instability, avoids overrelease and muscle injury, and improves soft-tissue balance.3 Open release or lengthening reduces inadvertent injury to the lateral superior/inferior geniculate arteries and allows direct hemostasis. Two prospective randomized studies found functional knee outcomes and return to athletic activities were improved more after LRL than LRR.11,12 These procedures had similar rates of postoperative knee stiffness, decreased muscle mass, and decreased strength. Each prospective study used an extensive LRR technique for LRR cases (various authors have recommended performing the release until the patella is perpendicular to the trochlea), which may have affected outcomes. In any case, with lengthening, the surgeon is less likely to excessively disrupt the lateral tissues.
Lateral Retinaculum Release. LRR can be openly performed by lateral parapatellar incision,1 a mini-open percutaneous technique, or arthroscopy. For these open techniques, incisions of various sizes have been used to access the LR and incise it about 1 cm lateral to the patella starting at the distal end of the vastus lateralis and extending distally until patellar tilt reduction is sufficient. If tightness in deep flexion persists, the LRR can be extended distally to the tibial tubercle. Open techniques have the advantage of sparing the joint capsule. All-arthroscopic techniques involve using electrocautery to cut through the capsule and access the LR.
Lateral Retinaculum Lengthening.
The LR is sharply divided into a superficial layer of superficial oblique fibers from the anterior iliotibial band and a deep layer of transverse fibers from the femur. For LRR, these 2 layers must be identified separate from the articular capsule.13
Complications
Complications of performing LRR/LRL to change the lateral restraint include medial patellar instability, increased lateral pain, repair failure, recurrent lateral instability, quadriceps weakness and atrophy, postoperative hemarthrosis, knee stiffness, wound complications, and thermal skin injury.7 These complications often result from poor surgical technique and too aggressive release. Although recommended patellar tilt historically has varied from 45° to 90°, the current goal is to normalize the tight soft-tissue restraints without creating secondary instability.
The most significant complication of LRR is medial patellar instability caused by muscle atrophy and loss of soft-tissue restraint.14 Medial instability can be difficult to diagnose and should be considered in any patient with patellofemoral pain, popping, or patellar instability after LRR.15 A positive medial subluxation test or medial patellar apprehension test suggests medial instability.
Medial patellar instability usually requires surgical treatment. Direct LR repair, lateral soft-tissue reconstruction, and other procedures can be used to restore lateral restraint.15 However, these are salvage techniques, and patients often remain significantly limited by pain or instability. Therefore, the LR must be carefully addressed and preferably should undergo lengthening rather than release.
Take-Home Points
- Understanding the indications for treatment is essential.
- Identifying the superficial (oblique fibers) and deep layers (transverse fibers) of the LR is very important and can lengthen the LR by as much as 20 mm.
- Open procedures reduce the risk of hematomas and related pain.
- The goal is to obtain 1 or 2 patellar quadrants of medial and lateral patellar glide in extensino and a neutral patella.
- If the Z-plasty is combined with the MPFL reconstruction or tibial tubercle transfer, the LR is set to length after the tubercle transfer and before the MPFL reconstruction (to avoid overconstraint).
Anterior knee pain is a common clinical problem that can be challenging to correct, in large part because of multiple causative factors, including structural/anatomical, functional, alignment, and neuroperception/pain pathway factors. One difficult aspect of anatomical assessment is judging the soft-tissue balance between the medial restraints (medial patellofemoral ligament [MPFL]; medial quadriceps tendon to femoral ligament; medial patellotibial and patellomeniscal ligaments) and the lateral restraints (lateral retinaculum [LR] specifically). Both LR tightness and patellar instability can be interpreted as anterior knee pain. Differentiating these entities is one of the most difficult clinical challenges in orthopedics.
LR release (LRR) has been found to improve patellar mobility and tracking.1 In the absence of clearly defined guidelines, the procedure quickly gained in popularity because of its technical simplicity and the enticing "one tool fits all" treatment approach suggested in early reviews. Injudicious use of LRR, alone or in combination with other procedures, led to iatrogenic instability and chronic pain. LR lengthening (LRL) was introduced to address LR tightness while maintaining lateral soft-tissue integrity and avoiding some of the severe complications of LRR.2
Today, isolated use of LRR/LRL is recommended only for treatment of LR tightness and pain secondary to lateral patellar hypercompression.3 It can also be used as an adjunct treatment in the setting of patellofemoral instability. LRR/LRL should never be used as primary treatment for patellofemoral instability.
In this review of treatments for LR tightness and patellofemoral disorders, we compare the use of LRR and LRL.
Discussion
LR procedures are indicated for LR tightness, which is assessed by taking a history, performing a physical examination, and obtaining diagnostic imaging. Decisions should be based on all findings considered together and never on imaging findings alone.
Physical Examination
The physical examination should include assessment of limb alignment, patellar mobility, muscle balance, and dynamic patellar tracking.
Limb Alignment. Abnormal valgus, rotational deformities, and increased Q-angle are associated with LR tightness. Valgus alignment can be assessed on standing inspection; rotational deformities with increased hip anteversion by hip motion with the patient in the prone position (increased hip internal rotation, decreased hip external rotation); and Q-angle on weight-bearing standing examination and with the patient flexing and extending the knee while seated.
Patellar Mobility. The patellar glide and tilt tests provide the most direct evaluations of LR tightness. Medial displacement of <1 quadrant is consistent with tightness, and displacement of >3 quadrants is consistent with laxity. In full extension, the patellar glide test evaluates only the soft-tissue restraints; at 30° flexion, it also evaluates patellofemoral engagement. The patellar tilt test measures the lifting of the lateral edge of the patella. With normal elevation being 0° to 20°, lack of patellar tilt means the LR is tight, and tilt of >20° means it is loose. MPFL patency can be examined with the Lachman test; the examiner rapidly moves the patella laterally while feeling for the characteristic hard endpoint of lateral translation.
Muscle Balance. The tone, strength, and tightness of the core (abdomen, dorsal, and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius) should be evaluated.
Dynamic Patellar Tracking. The J-sign is the course (shaped like an inverted J) that the patella takes when it is medialized into the trochlea from its laterally displaced resting position as the knee goes from full extension to flexion. The J-sign can be associated with LR tightness, trochlear dysplasia, and patella alta.
Imaging
Although we cannot provide a comprehensive review of the imaging literature, the following radiologic examinations should be used to assess the patellofemoral joint.
30° Lateral Radiograph. Increased tilt is seen when the lateral facet is not anterior to the patellar ridge. Also evaluated are trochlear anatomy, patellar height, and other factors involved in patellofemoral disorders.
30° Flexed Axial (Merchant) Radiograph. Patellar tilt, subluxation, and trochlear dysplasia are evaluated. Images obtained with progressive flexion can be very useful in verifying patellar tilt reduction. Lack of reduction during early flexion suggests LR tightness.4
Alignment Axial Radiographs (Scanogram). Valgus alignment is assessed with this full-length, standing, long-leg examination.
Computed Tomography/Magnetic Resonance Imaging. Many parameters of patellar alignment have been described. Basic assessment should include evaluation of patellar tilt, angle by the line across posterior condyles and a line through the greatest patellar width (>20° indicates abnormality and LR tightness) and tibial tubercle-trochlear groove distance (computed tomography or magnetic resonance imaging scan of the knee is used to measure this distance, and to confirm a significant amount in light of complex patellofemoral malalignment5).
Indications
Lateral compression syndrome with LR tightness is often successfully treated with isolated LRR, and results are reproducible and predictable.6 Surgical intervention for patellofemoral pain should be undertaken only after failed extensive nonoperative treatment with physical therapy and bracing/taping. Patients with LR tightness on preoperative examination, lateral patellar tilt on imaging, and normal Q-angle can obtain satisfactory results with this procedure. Patellar subluxation or dislocation history, high Q-angle (>20°), grade 3 or 4 chondral injury, and patellofemoral arthritis are associated with poorer outcomes when the procedure is performed in isolation.6International Patellofemoral Study Group members agreed that LRR/LRL is a valid treatment option when indicated, but it is rarely performed in isolation and constitutes only 1% to 2% of surgeries performed by this group of experts.7 When lateral compression syndrome progresses to arthritis, LRR/LRL can be performed with lateral patella facetectomy for maximal improvement.4 In the setting of patellofemoral instability, LRR/LRL can be combined with proximal and/or distal realignment surgery if the LR is tight. The LR is the last line of defense limiting lateral translation in the setting of an incompetent MPFL. Isolated LRR/LRL in the setting of instability further destabilizes the patella and worsens the instability. Therefore, LRR/LRL
is a poor surgical option as an isolated procedure for this condition and should be used only as an adjunct in cases of patellofemoral instability with LR tightness that does not allow the patella to be centralized into the trochlea.8 LRR/LRL can also be performed to improve patellar tracking in patellofemoral arthroplasty and total knee arthroplasty.
Lateral Retinaculum Release Versus Lengthening
LRR was first described for the treatment of patellar instability in 1891.9 It was also used for the treatment of lateral patellar hypercompression syndrome associated with LR tightness that led to lateral patellar tracking, joint overload, degeneration, and anterior knee pain.10 Metcalf10 further popularized the procedure by describing a minimally invasive arthroscopic version. However, the arthroscopic technique is as aggressive as the open technique and may be performed with less control, potentially making its results more variable. As proximal and distal releases are performed from the "inside out," more capsule and muscle disruption is needed to release the more superficial layers.
Z-plasty lengthening of the LR was described as an alternative for maintaining lateral patellar soft-tissue integrity while reducing the tension of the lateral tissue restraints.3 This is our preferred method.
Performing LRL instead of LRR avoids iatrogenic medial patellar instability, avoids overrelease and muscle injury, and improves soft-tissue balance.3 Open release or lengthening reduces inadvertent injury to the lateral superior/inferior geniculate arteries and allows direct hemostasis. Two prospective randomized studies found functional knee outcomes and return to athletic activities were improved more after LRL than LRR.11,12 These procedures had similar rates of postoperative knee stiffness, decreased muscle mass, and decreased strength. Each prospective study used an extensive LRR technique for LRR cases (various authors have recommended performing the release until the patella is perpendicular to the trochlea), which may have affected outcomes. In any case, with lengthening, the surgeon is less likely to excessively disrupt the lateral tissues.
Lateral Retinaculum Release. LRR can be openly performed by lateral parapatellar incision,1 a mini-open percutaneous technique, or arthroscopy. For these open techniques, incisions of various sizes have been used to access the LR and incise it about 1 cm lateral to the patella starting at the distal end of the vastus lateralis and extending distally until patellar tilt reduction is sufficient. If tightness in deep flexion persists, the LRR can be extended distally to the tibial tubercle. Open techniques have the advantage of sparing the joint capsule. All-arthroscopic techniques involve using electrocautery to cut through the capsule and access the LR.
Lateral Retinaculum Lengthening.
The LR is sharply divided into a superficial layer of superficial oblique fibers from the anterior iliotibial band and a deep layer of transverse fibers from the femur. For LRR, these 2 layers must be identified separate from the articular capsule.13
Complications
Complications of performing LRR/LRL to change the lateral restraint include medial patellar instability, increased lateral pain, repair failure, recurrent lateral instability, quadriceps weakness and atrophy, postoperative hemarthrosis, knee stiffness, wound complications, and thermal skin injury.7 These complications often result from poor surgical technique and too aggressive release. Although recommended patellar tilt historically has varied from 45° to 90°, the current goal is to normalize the tight soft-tissue restraints without creating secondary instability.
The most significant complication of LRR is medial patellar instability caused by muscle atrophy and loss of soft-tissue restraint.14 Medial instability can be difficult to diagnose and should be considered in any patient with patellofemoral pain, popping, or patellar instability after LRR.15 A positive medial subluxation test or medial patellar apprehension test suggests medial instability.
Medial patellar instability usually requires surgical treatment. Direct LR repair, lateral soft-tissue reconstruction, and other procedures can be used to restore lateral restraint.15 However, these are salvage techniques, and patients often remain significantly limited by pain or instability. Therefore, the LR must be carefully addressed and preferably should undergo lengthening rather than release.
1. Merchant AC, Mercer RL. Lateral release of the patella. A preliminary report. Clin Orthop Relat Res. 1974;(103):40-45.
2. Ceder LC, Larson RL. Z-plasty lateral retinacular release for the treatment of patellar compression syndrome. Clin Orthop Relat Res. 1979;(144):110-113.
3. Biedert R. Lateral patellar hypercompression, tilt and mild lateral subluxation. In: Biedert R, ed. Patellofemoral Disorders. Chichester, England: Wiley; 2004:161-166.
4. Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100-106.
5. Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle–posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
6. Lattermann C, Toth J, Bach BR Jr. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc. 2007;15(2):57-60.
7. Fithian DC, Paxton EW, Post WR, Panni AS; International Patellofemoral Study Group. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20(5):463-468.
8. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
9. Pollard B. Old dislocation of patella by intra-articular operation. Lancet. 1891;(988):17-22.
10. Metcalf RW. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
11. Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788-797.
12. O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759-1769.
13. Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br. 2008;90(4):527-534.
14. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
15. McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26-33.
1. Merchant AC, Mercer RL. Lateral release of the patella. A preliminary report. Clin Orthop Relat Res. 1974;(103):40-45.
2. Ceder LC, Larson RL. Z-plasty lateral retinacular release for the treatment of patellar compression syndrome. Clin Orthop Relat Res. 1979;(144):110-113.
3. Biedert R. Lateral patellar hypercompression, tilt and mild lateral subluxation. In: Biedert R, ed. Patellofemoral Disorders. Chichester, England: Wiley; 2004:161-166.
4. Hinckel BB, Arendt EA. Lateral retinaculum lengthening or release. Oper Tech Sports Med. 2015;23(2):100-106.
5. Seitlinger G, Scheurecker G, Högler R, Labey L, Innocenti B, Hofmann S. Tibial tubercle–posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med. 2012;40(5):1119-1125.
6. Lattermann C, Toth J, Bach BR Jr. The role of lateral retinacular release in the treatment of patellar instability. Sports Med Arthrosc. 2007;15(2):57-60.
7. Fithian DC, Paxton EW, Post WR, Panni AS; International Patellofemoral Study Group. Lateral retinacular release: a survey of the International Patellofemoral Study Group. Arthroscopy. 2004;20(5):463-468.
8. Christoforakis J, Bull AM, Strachan RK, Shymkiw R, Senavongse W, Amis AA. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273-277.
9. Pollard B. Old dislocation of patella by intra-articular operation. Lancet. 1891;(988):17-22.
10. Metcalf RW. An arthroscopic method for lateral release of subluxating or dislocating patella. Clin Orthop Relat Res. 1982;167:9-18.
11. Pagenstert G, Wolf N, Bachmann M, et al. Open lateral patellar retinacular lengthening versus open retinacular release in lateral patellar hypercompression syndrome: a prospective double-blinded comparative study on complications and outcome. Arthroscopy. 2012;28(6):788-797.
12. O’Neill DB. Open lateral retinacular lengthening compared with arthroscopic release. A prospective, randomized outcome study. J Bone Joint Surg Am. 1997;79(12):1759-1769.
13. Merican AM, Amis AA. Anatomy of the lateral retinaculum of the knee. J Bone Joint Surg Br. 2008;90(4):527-534.
14. Hughston JC, Deese M. Medial subluxation of the patella as a complication of lateral retinacular release. Am J Sports Med. 1988;16(4):383-388.
15. McCarthy MA, Bollier MJ. Medial patella subluxation: diagnosis and treatment. Iowa Orthop J. 2015;35:26-33.
Patella Alta Sees You, Do You See It?
Take-Home Points
The decision to adda TTDO to an MPFL reconstruction is dependent on patellar height as assessed with the CDI, as well as multiple other patient and anatomical factors.
TTDOs that include a complete detachment of the tibial tubercle (as required for distalization) have increased risk of nonunion and hardware failure.
Poor surgical technique (failure to make a flat osteotomy cut, cortical only bone block, poor bony apposition of the detached bone block- particularly at the location of any transverse plane cut, and failure to minimize thermal damage through copious irrigation) can increase nonunion risk.
Postoperative rehabilitation should include a 6-week period of limited weight-bearing.
Reconstruction of the MPFL should be performed after any TTO is performed.
Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased tibial tubercle-trochlear groove (TT-TG) or tibial tubercle-posterior cruciate ligament distance.2,5 Of all the factors, TT-TG distance and the medial patellofemoral ligament (MPFL) have received the ost attention. Patellar height remains a crucial yet underappreciated contributor that is amenable to surgical correction with tibial tubercle distalization osteotomy (TTDO). The obvious question is how severe patella alta must be to require surgical correction. In other words, when is patella alta so severe that isolated MPFL reconstruction is insufficient to restore patellar stability?
The indications for TTDO are not completely clear and depend on multiple factors. Patient factors, physical examination findings, and radiographic measures must be considered. In general, adding TTDO to MPFL reconstruction should be considered when the degree of patella alta exceeds 1.4 on the Caton-Deschamps Index (CDI). Presence of trochlear dysplasia, patellar maltracking (J-sign), lateral patellar apprehension that persists at higher flexion angles, and decreased patellotrochlear articular cartilage contact on sagittal magnetic resonance imaging may drive the decision to proceed with TTDO when the CDI is lower.
Why You Need To Know About Patella Alta
Recurrent lateral patellar dislocation is a debilitating knee condition that often involves young, active patients and significantly affects their quality of life. The MPFL is a primary restraint to lateral patellar dislocation, and an MPFL injury is a key contributor to loss of patellar stability. MPFL reconstruction is increasingly being performed to treat recurrent lateral patellar instability.6 Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased TT-TG distance.2,5 This review focuses on patella alta.
The classic teaching of the Lyon School of Knee Surgery in France, the menu à la carte, is that patella alta exceeding 1.2 on the CDI is an indication for TTDO.2 Although this teaching is an excellent guide for normal anatomy, we must keep in mind that the classic surgical menu does not consider the influence of MPFL reconstruction, as development of the menu predated this surgical option. At that time, the proximal soft-tissue procedures included vastus medialis obliquus plasty and advancement, which are performed to balance soft tissues and treat patellar tilt. These procedures and MPFL reconstruction have different functions, and the difference may be important. Furthermore, performing TTDO alongside MPFL reconstruction significantly increases the risk of complications and alters the rehabilitation protocol. However, significant untreated patella alta has been implicated as a cause of failure of isolated MPFL reconstruction.7 Establishing when MPFL reconstruction alone is sufficient is therefore crucial in avoiding the increased morbidity associated with the addition of TTDO.
Discussion
Above a certain degree of patella alta, isolated MPFL reconstruction fails to restore patellar stability. What remains unknown is the appropriate CDI cutoff (1.4) and whether the same cutoff can be used for all patients. In 2013, Wagner and colleagues8 assessed the influence of patella alta on isolated MPFL reconstruction outcomes and found no significant difference, though their study did not include many patients with significant alta and was underpowered. In 2014, Feller and colleagues9 reported on a series of patients who were successfully treated with isolated MPFL reconstruction despite patella alta significantly exceeding the traditional CDI cutoff of 1.2. Their indication for performing the isolated procedure was normal patellar tracking—in particular, absence of the J-sign. Further analysis of these patients revealed a preponderance of relatively normal TT-TG distances and low-grade, if any, trochlear dysplasia in comparison with other patients treated with a combination of MPFL reconstruction and tibial tubercle osteotomy.
Together, the work of Wagner and colleagues8 and Feller and colleagues9 suggests the historical use of the CDI of 1.2 as a hard and fast indication for adding TTDO is aggressive. In fact, it is probably the case that there really is no single CDI cutoff that is an appropriate indication for adding TTDO in all patients with instability. This decision is, and should be, influenced by a multitude of other factors, including other anatomical factors, physical examination findings, patient factors, and, of course, patient preference.
An interesting idea to consider in treating patellar instability is the interplay of patella alta and trochlear dysplasia. Patella alta is theorized as contributing to patellar instability in part by delaying entry of the patella into the TG as the knee flexes, therefore requiring less force to laterally displace
the patella.10 Similarly, in the setting of trochlear dysplasia, a shallow TG leads to less bony constraint of the patella, particularly in the groove’s superior portions, which are more involved in lower grade dysplasia. Because trochlear dysplasia and patella alta decrease patellar stability by similar mechanisms, they clearly interact, and a patient with both is at higher risk for instability than a patient who exhibits either in isolation.11 Therefore, trochlear dysplasia, particularly higher grade, may be an indication for adding TTDO at lower CDI.
Other imaging and physical examination factors can provide additional insight into the process of patellar engagement into the trochlea in each patient. The patellotrochlear index (PTI) directly measures the relationship between the patella and the trochlea, rather than relative to the tibia, as with other measures of patellar height.12[[{"fid":"201853","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]The PTI is correlated with tibia-based measures of height, but the correlation is not perfect. Lower degrees of overlap between the patella and the trochlea (PTI <0.15) and significant functional patella alta may warrant adding TTDO in cases of borderline CDI (1.2-1.4). Figures 1A, 1B and 2A, 2B show the imaging of 2 patients with relatively similar patellar height (assessed with CDI) but quite different degrees of overlap between the patella and trochlea. [[{"fid":"201854","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The patient with less overlap is more likely to have delayed patellar engagement and symptomatic patella alta and thus may be a poorer candidate for isolated MPFL reconstruction. For additional information, please refer to the work by Roland Biedert, MD, who has proposed trochlear lengthening in these situations.13
Physical examination (even in the era of advance imaging) continues to provide useful insight into whether to add TTDO. One physical examination test that can help in understanding patellar-
trochlear dynamics is the patellar apprehension and relief test. Patellar apprehension has been widely discussed, but equally important is the degree of knee flexion above which apprehension dissipates. As patella alta and trochlear dysplasia become more severe, more knee flexion is required to relieve apprehension. Apprehension that is relieved at 30° to 40° of flexion suggests that patellar stability stands a good chance of being restored with isolated MPFL reconstruction, whereas persistent instability >45° or especially 60° of knee flexion suggests that there is significant patella alta, trochlear dysplasia, or both and that TTDO should be added. A large J-sign during knee flexion and extension provides further evidence that entry of the patella into the TG is delayed, typically because of patella alta, trochlear dysplasia, or both, and possibly tight lateral structures or a lateralized tibial tubercle. This sign is another clue that isolated MPFL reconstruction may be insufficient to completely restore patellar stability.
1. Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45-54.
2. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
3. Geenen E, Molenaers G, Martens M. Patella alta in patellofemoral instability. Acta Orthop Belg. 1989;55(3):387-393.
4. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
5. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl) [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
6. Feller JA, Lind M, Nelson J, Diduch DR, Arendt E. Repair and reconstruction of the medial patellofemoral ligament for treatment of lateral patellar dislocations. In: Scott WN, ed. Insall & Scott—Surgery of the Knee. 5th ed. Philadelphia, PA: Churchill Livingstone; 2011:677-687.
7. Thaunat M, Erasmus PJ. Recurrent patellar dislocation after medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):40-43.
8. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
9. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
10. Ward SR, Terk MR, Powers CM. Patella alta: association
with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):
1749-1755.
11. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309
12. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
13. Biedert RM. Trochlear lengthening osteotomy with or without elevation of the lateral trochlear facet. In: Zaffagnini S, Dejour D, Arendt EA, eds. Patellofemoral Pain, Instability, and Arthritis. Germany: Springer-Verlag Berlin Heidelberg; 2010:
209-215.
Take-Home Points
The decision to adda TTDO to an MPFL reconstruction is dependent on patellar height as assessed with the CDI, as well as multiple other patient and anatomical factors.
TTDOs that include a complete detachment of the tibial tubercle (as required for distalization) have increased risk of nonunion and hardware failure.
Poor surgical technique (failure to make a flat osteotomy cut, cortical only bone block, poor bony apposition of the detached bone block- particularly at the location of any transverse plane cut, and failure to minimize thermal damage through copious irrigation) can increase nonunion risk.
Postoperative rehabilitation should include a 6-week period of limited weight-bearing.
Reconstruction of the MPFL should be performed after any TTO is performed.
Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased tibial tubercle-trochlear groove (TT-TG) or tibial tubercle-posterior cruciate ligament distance.2,5 Of all the factors, TT-TG distance and the medial patellofemoral ligament (MPFL) have received the ost attention. Patellar height remains a crucial yet underappreciated contributor that is amenable to surgical correction with tibial tubercle distalization osteotomy (TTDO). The obvious question is how severe patella alta must be to require surgical correction. In other words, when is patella alta so severe that isolated MPFL reconstruction is insufficient to restore patellar stability?
The indications for TTDO are not completely clear and depend on multiple factors. Patient factors, physical examination findings, and radiographic measures must be considered. In general, adding TTDO to MPFL reconstruction should be considered when the degree of patella alta exceeds 1.4 on the Caton-Deschamps Index (CDI). Presence of trochlear dysplasia, patellar maltracking (J-sign), lateral patellar apprehension that persists at higher flexion angles, and decreased patellotrochlear articular cartilage contact on sagittal magnetic resonance imaging may drive the decision to proceed with TTDO when the CDI is lower.
Why You Need To Know About Patella Alta
Recurrent lateral patellar dislocation is a debilitating knee condition that often involves young, active patients and significantly affects their quality of life. The MPFL is a primary restraint to lateral patellar dislocation, and an MPFL injury is a key contributor to loss of patellar stability. MPFL reconstruction is increasingly being performed to treat recurrent lateral patellar instability.6 Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased TT-TG distance.2,5 This review focuses on patella alta.
The classic teaching of the Lyon School of Knee Surgery in France, the menu à la carte, is that patella alta exceeding 1.2 on the CDI is an indication for TTDO.2 Although this teaching is an excellent guide for normal anatomy, we must keep in mind that the classic surgical menu does not consider the influence of MPFL reconstruction, as development of the menu predated this surgical option. At that time, the proximal soft-tissue procedures included vastus medialis obliquus plasty and advancement, which are performed to balance soft tissues and treat patellar tilt. These procedures and MPFL reconstruction have different functions, and the difference may be important. Furthermore, performing TTDO alongside MPFL reconstruction significantly increases the risk of complications and alters the rehabilitation protocol. However, significant untreated patella alta has been implicated as a cause of failure of isolated MPFL reconstruction.7 Establishing when MPFL reconstruction alone is sufficient is therefore crucial in avoiding the increased morbidity associated with the addition of TTDO.
Discussion
Above a certain degree of patella alta, isolated MPFL reconstruction fails to restore patellar stability. What remains unknown is the appropriate CDI cutoff (1.4) and whether the same cutoff can be used for all patients. In 2013, Wagner and colleagues8 assessed the influence of patella alta on isolated MPFL reconstruction outcomes and found no significant difference, though their study did not include many patients with significant alta and was underpowered. In 2014, Feller and colleagues9 reported on a series of patients who were successfully treated with isolated MPFL reconstruction despite patella alta significantly exceeding the traditional CDI cutoff of 1.2. Their indication for performing the isolated procedure was normal patellar tracking—in particular, absence of the J-sign. Further analysis of these patients revealed a preponderance of relatively normal TT-TG distances and low-grade, if any, trochlear dysplasia in comparison with other patients treated with a combination of MPFL reconstruction and tibial tubercle osteotomy.
Together, the work of Wagner and colleagues8 and Feller and colleagues9 suggests the historical use of the CDI of 1.2 as a hard and fast indication for adding TTDO is aggressive. In fact, it is probably the case that there really is no single CDI cutoff that is an appropriate indication for adding TTDO in all patients with instability. This decision is, and should be, influenced by a multitude of other factors, including other anatomical factors, physical examination findings, patient factors, and, of course, patient preference.
An interesting idea to consider in treating patellar instability is the interplay of patella alta and trochlear dysplasia. Patella alta is theorized as contributing to patellar instability in part by delaying entry of the patella into the TG as the knee flexes, therefore requiring less force to laterally displace
the patella.10 Similarly, in the setting of trochlear dysplasia, a shallow TG leads to less bony constraint of the patella, particularly in the groove’s superior portions, which are more involved in lower grade dysplasia. Because trochlear dysplasia and patella alta decrease patellar stability by similar mechanisms, they clearly interact, and a patient with both is at higher risk for instability than a patient who exhibits either in isolation.11 Therefore, trochlear dysplasia, particularly higher grade, may be an indication for adding TTDO at lower CDI.
Other imaging and physical examination factors can provide additional insight into the process of patellar engagement into the trochlea in each patient. The patellotrochlear index (PTI) directly measures the relationship between the patella and the trochlea, rather than relative to the tibia, as with other measures of patellar height.12[[{"fid":"201853","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]The PTI is correlated with tibia-based measures of height, but the correlation is not perfect. Lower degrees of overlap between the patella and the trochlea (PTI <0.15) and significant functional patella alta may warrant adding TTDO in cases of borderline CDI (1.2-1.4). Figures 1A, 1B and 2A, 2B show the imaging of 2 patients with relatively similar patellar height (assessed with CDI) but quite different degrees of overlap between the patella and trochlea. [[{"fid":"201854","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The patient with less overlap is more likely to have delayed patellar engagement and symptomatic patella alta and thus may be a poorer candidate for isolated MPFL reconstruction. For additional information, please refer to the work by Roland Biedert, MD, who has proposed trochlear lengthening in these situations.13
Physical examination (even in the era of advance imaging) continues to provide useful insight into whether to add TTDO. One physical examination test that can help in understanding patellar-
trochlear dynamics is the patellar apprehension and relief test. Patellar apprehension has been widely discussed, but equally important is the degree of knee flexion above which apprehension dissipates. As patella alta and trochlear dysplasia become more severe, more knee flexion is required to relieve apprehension. Apprehension that is relieved at 30° to 40° of flexion suggests that patellar stability stands a good chance of being restored with isolated MPFL reconstruction, whereas persistent instability >45° or especially 60° of knee flexion suggests that there is significant patella alta, trochlear dysplasia, or both and that TTDO should be added. A large J-sign during knee flexion and extension provides further evidence that entry of the patella into the TG is delayed, typically because of patella alta, trochlear dysplasia, or both, and possibly tight lateral structures or a lateralized tibial tubercle. This sign is another clue that isolated MPFL reconstruction may be insufficient to completely restore patellar stability.
Take-Home Points
The decision to adda TTDO to an MPFL reconstruction is dependent on patellar height as assessed with the CDI, as well as multiple other patient and anatomical factors.
TTDOs that include a complete detachment of the tibial tubercle (as required for distalization) have increased risk of nonunion and hardware failure.
Poor surgical technique (failure to make a flat osteotomy cut, cortical only bone block, poor bony apposition of the detached bone block- particularly at the location of any transverse plane cut, and failure to minimize thermal damage through copious irrigation) can increase nonunion risk.
Postoperative rehabilitation should include a 6-week period of limited weight-bearing.
Reconstruction of the MPFL should be performed after any TTO is performed.
Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased tibial tubercle-trochlear groove (TT-TG) or tibial tubercle-posterior cruciate ligament distance.2,5 Of all the factors, TT-TG distance and the medial patellofemoral ligament (MPFL) have received the ost attention. Patellar height remains a crucial yet underappreciated contributor that is amenable to surgical correction with tibial tubercle distalization osteotomy (TTDO). The obvious question is how severe patella alta must be to require surgical correction. In other words, when is patella alta so severe that isolated MPFL reconstruction is insufficient to restore patellar stability?
The indications for TTDO are not completely clear and depend on multiple factors. Patient factors, physical examination findings, and radiographic measures must be considered. In general, adding TTDO to MPFL reconstruction should be considered when the degree of patella alta exceeds 1.4 on the Caton-Deschamps Index (CDI). Presence of trochlear dysplasia, patellar maltracking (J-sign), lateral patellar apprehension that persists at higher flexion angles, and decreased patellotrochlear articular cartilage contact on sagittal magnetic resonance imaging may drive the decision to proceed with TTDO when the CDI is lower.
Why You Need To Know About Patella Alta
Recurrent lateral patellar dislocation is a debilitating knee condition that often involves young, active patients and significantly affects their quality of life. The MPFL is a primary restraint to lateral patellar dislocation, and an MPFL injury is a key contributor to loss of patellar stability. MPFL reconstruction is increasingly being performed to treat recurrent lateral patellar instability.6 Patellar instability is the result of numerous anatomical factors, including trochlear dysplasia,1,2 patella alta,2-4 and increased TT-TG distance.2,5 This review focuses on patella alta.
The classic teaching of the Lyon School of Knee Surgery in France, the menu à la carte, is that patella alta exceeding 1.2 on the CDI is an indication for TTDO.2 Although this teaching is an excellent guide for normal anatomy, we must keep in mind that the classic surgical menu does not consider the influence of MPFL reconstruction, as development of the menu predated this surgical option. At that time, the proximal soft-tissue procedures included vastus medialis obliquus plasty and advancement, which are performed to balance soft tissues and treat patellar tilt. These procedures and MPFL reconstruction have different functions, and the difference may be important. Furthermore, performing TTDO alongside MPFL reconstruction significantly increases the risk of complications and alters the rehabilitation protocol. However, significant untreated patella alta has been implicated as a cause of failure of isolated MPFL reconstruction.7 Establishing when MPFL reconstruction alone is sufficient is therefore crucial in avoiding the increased morbidity associated with the addition of TTDO.
Discussion
Above a certain degree of patella alta, isolated MPFL reconstruction fails to restore patellar stability. What remains unknown is the appropriate CDI cutoff (1.4) and whether the same cutoff can be used for all patients. In 2013, Wagner and colleagues8 assessed the influence of patella alta on isolated MPFL reconstruction outcomes and found no significant difference, though their study did not include many patients with significant alta and was underpowered. In 2014, Feller and colleagues9 reported on a series of patients who were successfully treated with isolated MPFL reconstruction despite patella alta significantly exceeding the traditional CDI cutoff of 1.2. Their indication for performing the isolated procedure was normal patellar tracking—in particular, absence of the J-sign. Further analysis of these patients revealed a preponderance of relatively normal TT-TG distances and low-grade, if any, trochlear dysplasia in comparison with other patients treated with a combination of MPFL reconstruction and tibial tubercle osteotomy.
Together, the work of Wagner and colleagues8 and Feller and colleagues9 suggests the historical use of the CDI of 1.2 as a hard and fast indication for adding TTDO is aggressive. In fact, it is probably the case that there really is no single CDI cutoff that is an appropriate indication for adding TTDO in all patients with instability. This decision is, and should be, influenced by a multitude of other factors, including other anatomical factors, physical examination findings, patient factors, and, of course, patient preference.
An interesting idea to consider in treating patellar instability is the interplay of patella alta and trochlear dysplasia. Patella alta is theorized as contributing to patellar instability in part by delaying entry of the patella into the TG as the knee flexes, therefore requiring less force to laterally displace
the patella.10 Similarly, in the setting of trochlear dysplasia, a shallow TG leads to less bony constraint of the patella, particularly in the groove’s superior portions, which are more involved in lower grade dysplasia. Because trochlear dysplasia and patella alta decrease patellar stability by similar mechanisms, they clearly interact, and a patient with both is at higher risk for instability than a patient who exhibits either in isolation.11 Therefore, trochlear dysplasia, particularly higher grade, may be an indication for adding TTDO at lower CDI.
Other imaging and physical examination factors can provide additional insight into the process of patellar engagement into the trochlea in each patient. The patellotrochlear index (PTI) directly measures the relationship between the patella and the trochlea, rather than relative to the tibia, as with other measures of patellar height.12[[{"fid":"201853","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]The PTI is correlated with tibia-based measures of height, but the correlation is not perfect. Lower degrees of overlap between the patella and the trochlea (PTI <0.15) and significant functional patella alta may warrant adding TTDO in cases of borderline CDI (1.2-1.4). Figures 1A, 1B and 2A, 2B show the imaging of 2 patients with relatively similar patellar height (assessed with CDI) but quite different degrees of overlap between the patella and trochlea. [[{"fid":"201854","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The patient with less overlap is more likely to have delayed patellar engagement and symptomatic patella alta and thus may be a poorer candidate for isolated MPFL reconstruction. For additional information, please refer to the work by Roland Biedert, MD, who has proposed trochlear lengthening in these situations.13
Physical examination (even in the era of advance imaging) continues to provide useful insight into whether to add TTDO. One physical examination test that can help in understanding patellar-
trochlear dynamics is the patellar apprehension and relief test. Patellar apprehension has been widely discussed, but equally important is the degree of knee flexion above which apprehension dissipates. As patella alta and trochlear dysplasia become more severe, more knee flexion is required to relieve apprehension. Apprehension that is relieved at 30° to 40° of flexion suggests that patellar stability stands a good chance of being restored with isolated MPFL reconstruction, whereas persistent instability >45° or especially 60° of knee flexion suggests that there is significant patella alta, trochlear dysplasia, or both and that TTDO should be added. A large J-sign during knee flexion and extension provides further evidence that entry of the patella into the TG is delayed, typically because of patella alta, trochlear dysplasia, or both, and possibly tight lateral structures or a lateralized tibial tubercle. This sign is another clue that isolated MPFL reconstruction may be insufficient to completely restore patellar stability.
1. Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45-54.
2. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
3. Geenen E, Molenaers G, Martens M. Patella alta in patellofemoral instability. Acta Orthop Belg. 1989;55(3):387-393.
4. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
5. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl) [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
6. Feller JA, Lind M, Nelson J, Diduch DR, Arendt E. Repair and reconstruction of the medial patellofemoral ligament for treatment of lateral patellar dislocations. In: Scott WN, ed. Insall & Scott—Surgery of the Knee. 5th ed. Philadelphia, PA: Churchill Livingstone; 2011:677-687.
7. Thaunat M, Erasmus PJ. Recurrent patellar dislocation after medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):40-43.
8. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
9. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
10. Ward SR, Terk MR, Powers CM. Patella alta: association
with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):
1749-1755.
11. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309
12. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
13. Biedert RM. Trochlear lengthening osteotomy with or without elevation of the lateral trochlear facet. In: Zaffagnini S, Dejour D, Arendt EA, eds. Patellofemoral Pain, Instability, and Arthritis. Germany: Springer-Verlag Berlin Heidelberg; 2010:
209-215.
1. Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45-54.
2. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
3. Geenen E, Molenaers G, Martens M. Patella alta in patellofemoral instability. Acta Orthop Belg. 1989;55(3):387-393.
4. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
5. Goutallier D, Bernageau J, Lecudonnec B. The measurement of the tibial tuberosity. Patella groove distanced technique and results (author’s transl) [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(5):423-428.
6. Feller JA, Lind M, Nelson J, Diduch DR, Arendt E. Repair and reconstruction of the medial patellofemoral ligament for treatment of lateral patellar dislocations. In: Scott WN, ed. Insall & Scott—Surgery of the Knee. 5th ed. Philadelphia, PA: Churchill Livingstone; 2011:677-687.
7. Thaunat M, Erasmus PJ. Recurrent patellar dislocation after medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(1):40-43.
8. Wagner D, Pfalzer F, Hingelbaum S, Huth J, Mauch F, Bauer G. The influence of risk factors on clinical outcomes following anatomical medial patellofemoral ligament (MPFL) reconstruction using the gracilis tendon. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):318-324.
9. Feller JA, Richmond AK, Wasiak J. Medial patellofemoral ligament reconstruction as an isolated or combined procedure for recurrent patellar instability. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2470-2476.
10. Ward SR, Terk MR, Powers CM. Patella alta: association
with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):
1749-1755.
11. Lewallen L, McIntosh A, Dahm D. First-time patellofemoral dislocation: risk factors for recurrent instability. J Knee Surg. 2015;28(4):303-309
12. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
13. Biedert RM. Trochlear lengthening osteotomy with or without elevation of the lateral trochlear facet. In: Zaffagnini S, Dejour D, Arendt EA, eds. Patellofemoral Pain, Instability, and Arthritis. Germany: Springer-Verlag Berlin Heidelberg; 2010:
209-215.
Cartilage Restoration in the Patellofemoral Joint
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
Take-Home Points
- Careful evaluation is key in attributing knee pain to patellofemoral cartilage lesions-that is, in making a "diagnosis by exclusion".
- Initial treatment is nonoperative management focused on weight loss and extensive "core-to-floor" rehabilitation.
- Optimization of anatomy and biomechanics is crucial.
- Factors important in surgical decision-making incude defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
- The most commonly used surgical procedures-autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft-have demonstrated improved intermediate-term outcomes.
Patellofemoral (PF) pain is often a component of more general anterior knee pain. One source of PF pain is chondral lesions. As these lesions are commonly seen on magnetic resonance imaging (MRI) and during arthroscopy, it is necessary to differentiate incidental and symptomatic lesions.1 In addition, the correlation between symptoms and lesion presence and severity is poor.
PF pain is multifactorial (structural lesions, malalignment, deconditioning, muscle imbalance and overuse) and can coexist with other lesions in the knee (ligament tears, meniscal injuries, and cartilage lesions in other compartments). Therefore, careful evaluation is key in attributing knee pain to PF cartilage lesions—that is, in making a "diagnosis by exclusion."
From the start, it must be appreciated that the vast majority of patients will not require surgery, and many who require surgery for pain will not require cartilage restoration. One key to success with PF patients is a good working relationship with an experienced physical therapist.
Etiology
The primary causes of PF cartilage lesions are patellar instability, chronic maltracking without instability, direct trauma, repetitive microtrauma, and idiopathic.
Patellar Instability
Patients with patellar instability often present with underlying anatomical risk factors (eg, trochlear dysplasia, increased Q-angle/tibial tubercle-trochlear groove [TT-TG] distance, patella alta, and unbalanced medial and lateral soft tissues2). These factors should be addressed before surgery.
Patellar instability can cause cartilage damage during the dislocation event or by chronic subluxation. Cartilage becomes damaged in up to 96% of patellar dislocations.3 Most commonly, the damage consists of fissuring and/or fibrillation, but chondral and osteochondral fractures can occur as well. During dislocation, the medial patella strikes the lateral aspect of the femur, and, as the knee collapses into flexion, the lateral aspect of the proximal lateral femoral condyle (weight-bearing area) can sustain damage. In the patella, typically the injury is distal-medial (occasionally crossing the median ridge). A shear lesion may involve the chondral surface or be osteochondral (Figure 1A).
Chronic Maltracking Without Instability
Chronic maltracking is usually related to anatomical abnormalities, which include the same factors that can cause patellar instability. A common combination is trochlear dysplasia, increased TT-TG or TT-posterior cruciate ligament distance, and lateral soft-tissue contracture. These are often seen in PF joints that progress to lateral PF arthritis. As lateral PF arthritis progresses, lateral soft-tissue contracture worsens, compounding symptoms of laterally based pain. With respect to cartilage repair, these joints can be treated if recognized early; however, once osteoarthritis is fully established in the joint, facetectomy or PF replacement may be necessary.
Direct Trauma
With the knee in flexion during a direct trauma over the patella (eg, fall or dashboard trauma), all zones of cartilage and subchondral bone in both patella and trochlea can be injured, leading to macrostructural damage, chondral/osteochondral fracture, or, with a subcritical force, microstructural damage and chondrocyte death, subsequently causing cartilage degeneration (cartilage may look normal initially; the matrix takes months to years to deteriorate). Direct trauma usually occurs with the knee flexed. Therefore, these lesions typically are located in the distal trochlea and superior pole of the patella.
Repetitive Microtrauma
Minor injuries, which by themselves do not immediately cause apparent chondral or osteochondral fractures, may eventually exceed the capacity of natural cartilage homeostasis and result in repetitive microtrauma. Common causes are repeated jumping (as in basketball and volleyball) and prolonged flexed-knee position (eg, what a baseball catcher experiences), which may also be associated with other lesions caused by extensor apparatus overload (eg, quadriceps tendon or patellar tendon tendinitis, and fat pad impingement syndrome).
Idiopathic
In a subset of patients with osteochondritis dissecans, the patella is the lesion site. In another subset, idiopathic lesions may be related to a genetic predisposition to osteoarthritis and may not be restricted to the PF joint. In some cases, the PF joint is the first compartment to degenerate and is the most symptomatic in a setting of truly tricompartmental disease. In these cases, treating only the PF lesion can result in functional failure, owing to disease progression in other compartments. Even mild disease in other compartments should be carefully evaluated.
History and Physical Examination
Patients often report a history of anterior knee pain that worsens with stair use, prolonged sitting, and flexed-knee activities (eg, squatting). Compared with pain alone, swelling, though not specific to cartilage disease, is more suspicious for a cartilage etiology. Identifying the cartilage defect as the sole source of pain is particularly difficult in patients with recurrent patellar instability. In these patients, pain and swelling, even between instability episodes, suggest that cartilage damage is at least a component of the symptomology.
Important diagnostic components of physical examination are gait analysis, tibiofemoral alignment, and patellar alignment in all 3 planes, both static and functional. Patella-specific measurements include medial-lateral position and quadrants of excursion, lateral tilt, and patella alta, as well as J-sign and subluxation with quadriceps contraction in extension.
It is also important to document effusion; crepitus; active and passive range of motion (spine, hips, knees); site of pain or tenderness to palpation (medial, lateral, distal, retropatellar) and whether it matches the complaints and the location of the cartilage lesion; results of the grind test (placing downward force on the patella during flexion and extension) and whether they match the flexion angle of the tenderness and the flexion angle in which the cartilage lesion has increased PF contact; ligamentous and soft-tissue stability or imbalance (tibiofemoral and patellar; apprehension test, glide test, tilt test); and muscle strength, flexibility, and atrophy of the core (abdomen, dorsal and hip muscles) and lower extremities (quadriceps, hamstrings, gastrocnemius).
Imaging
Imaging should be used to evaluate both PF alignment and the cartilage lesions. For alignment, standard radiographs (weight-bearing knee sequence and axial view; full limb length when needed), computed tomography, and MRI can be used.
Meaningful evaluation requires MRI with cartilage-specific sequences, including standard spin-echo (SE) and gradient-recalled echo (GRE), fast SE, and, for cartilage morphology, T2-weighted fat suppression (FS) and 3-dimensional SE and GRE.5 For evaluation of cartilage function and metabolism, the collagen network, and proteoglycan content in the knee cartilage matrix, consideration should be given to compositional assessment techniques, such as T2 mapping, delayed gadolinium-enhanced MRI of cartilage, T1ρ imaging, sodium imaging, and diffusion-weighted sequences.5 Use of the latter functional sequences is still debatable, and these sequences are not widely available.
Treatment
In general, the initial approach is nonoperative management focused on weight loss and extensive core-to-floor rehabilitation, unless surgery is specifically indicated (eg, for loose body removal or osteochondral fracture reattachment). Rehabilitation focuses on achieving adequate range of motion of the spine, hips, and knees along with muscle strength and flexibility of the core (abdomen, dorsal and hip muscles) and lower limbs (quadriceps, hamstrings, gastrocnemius). Rehabilitation is not defined by time but rather by development of an optimized soft-tissue envelope that decreases joint reactive forces. The full process can take 6 to 9 months, but there should be some improvement by 3 months.
Corticosteroid, hyaluronic acid,6 or platelet-rich plasma7 injections can provide temporary relief and facilitate rehabilitation in the setting of pain inhibition. As stand-alone treatment, injections are more suitable for more diffuse degenerative lesions in older and low-demand patients than for focal traumatic lesions in young and high-demand patients.
Surgery is indicated for full-thickness or nearly full-thickness lesions (International Cartilage Repair Society grade 3a or higher) >1 cm2 after failed conservative treatment.
Optimization of anatomy and biomechanics is crucial, as persistent abnormalities lead to high rates of failure of cartilage procedures, and correction of those factors results in outcomes similar to those of patients without such abnormal anatomy.8 The procedures most commonly used to improve patellar tracking or unloading in the PF compartment are lateral retinacular lengthening and TT transfer: medialization and/or distalization for correction of malalignment, and straight anteriorization or anteromedialization for unloading. These procedures can improve symptoms and function in lateral and distal patellar and trochlear lesions even without the addition of a cartilage restoration procedure.
Factors that are important in surgical decision-making include defect location and size, subchondral bone status, unipolar vs bipolar lesions, and previous cartilage procedure.
Location. The shapes of the patella and trochlea vary much more than the shapes of the condyles and plateaus. This variability complicates morphology matching, particularly with involvement of the central TG and median patellar ridge. Therefore, focal contained lesions of the patella and trochlea may be more technically amenable to cell therapy techniques than to osteochondral procedures, which require contour matching between donor and recipient
Size. Although small lesions in the femoral condyles can be considered for microfracture (MFx) or osteochondral autograft transfer (OAT), MFx is less suitable because of poor results in the PF joint, and OAT because of donor-site morbidity in the trochlea.
Subchondral bone status. When subchondral bone is compromised, such as with bone loss, cysts, or significant bone edema, the entire osteochondral unit should be treated. Here, OAT and osteochondral allograft (OCA) are the preferred treatments, depending on lesion size.
Unipolar vs bipolar lesions. Compared with unipolar lesions, bipolar lesions tend to have worse outcomes. Therefore, an associated unloading procedure (TT osteotomy) should be given special consideration. Autologous chondrocyte implantation (ACI) appears to have better outcomes than OCA for bipolar PF lesions.9,10
Previous surgery. Although a failed cartilage procedure can negatively affect ACI outcomes, particularly in the presence of intralesional osteophytes,11 it does not affect OCA outcomes.12 Therefore, after previous MFx, OCA instead of ACI may be considered.
Fragment Fixation
Viable fragments from traumatic lesions (direct trauma or patellar dislocation) or osteochondritis dissecans should be repaired if possible, particularly in young patients. In a fragment that contains a substantial amount of bone, compression screws provide stable fixation. More recently, it has been recognized that fixation of predominantly cartilaginous fragments can be successful13 (Figure 1B). Débridement of soft tissue in the lesion bed and on the fragment is important in facilitating healing, as is removal of sclerotic bone.
MFx
Although MFx can have good outcomes in small contained femoral condyle lesions, in the PF joint treatment has been more challenging, and clinical outcomes have been poor (increased subchondral edema, increased effusion).14 In addition, deterioration becomes significant after 36 months. Therefore, MFx should be restricted to small (<2 cm2), well-contained trochlear defects, particularly in low-demand patients.
ACI and Matrix-Induced ACI
As stated, ACI (Figure 2) is suitable for PF joints because it intrinsically respects the complex anatomy.
OAT
As mentioned, donor-site morbidity may compromise final outcomes of harvest and implantation in the PF joint. Nonetheless, in carefully selected patients with small lesions that are limited to 1 facet (not including the patellar ridge or the TG) and that require only 1 plug (Figure 3), OAT can have good clinical results.16
OCA
Two techniques can be used with OCA in the PF joint. The dowel technique, in which circular plugs are implanted, is predominantly used for defects that do not cross the midline (those located in their entirety on the medial or lateral aspect of the patella or trochlea). Central defects, which can be treated with the dowel technique as well, are technically more challenging to match perfectly, because of the complex geometry of the median ridge and the TG (Figure 4).
Experimental and Emerging Technologies
Biocartilage
Biocartilage, a dehydrated, micronized allogeneic cartilage scaffold implanted with platelet-rich plasma and fibrin glue added over a contained MFx-treated defect, can be used in the patella and trochlea and has the same indications as MFx (small lesions, contained lesions). There are limited clinical studies of short- or long-term outcomes.
Fresh and Viable OCA
Fresh OCA (ProChondrix; AlloSource) and viable/cryopreserved OCA (Cartiform; Arthrex) are thin osteochondral scaffolds that contain viable chondrocytes and growth factors. They can be implanted alone or used with MFx, and are indicated for lesions measuring 1 cm2 to 3 cm2. Aside from a case report,17 there are no clinical studies on outcomes.
Bone Marrow Aspirate Concentrate Implantation
Bone marrow aspirate concentrate from centrifuged iliac crest–harvested aspirate containing mesenchymal stem cells with chondrogenic potential is applied under a synthetic scaffold. Indications are the same as for ACI. Medium-term follow-up studies in the PF joint have shown good results, similar to those obtained with matrix-induced ACI.18
Particulated Juvenile Allograft Cartilage
Particulated juvenile allograft cartilage (DeNovo NT Graft; Zimmer Biomet) is minced cartilage allograft (from juvenile donors) that has been cut into cubes (~1 mm3). Indications are for patellar and trochlear lesions 1 cm2 to 6 cm2. For both the trochlea and the patella, short-term outcomes have been good.19,20
Rehabilitation After Surgery
Isolated PF cartilage restoration generally does not require prolonged weight-bearing restrictions, and ambulation with the knee locked in full extension is permitted as tolerated. Concurrent TT osteotomy, however, requires protection with 4 to 6 weeks of toe-touch weight-bearing to minimize the risk of tibial fracture.
Conclusion
Comprehensive preoperative assessment is essential and should include a thorough core-to-floor physical examination as well as PF-specific imaging. Treatment of symptomatic chondral lesions in the PF joint requires specific technical and postoperative management, which differs significantly from management involving the condyles. Attending to all these details makes the outcomes of PF cartilage treatment reproducible. These outcomes may rival those of condylar treatment.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460.
2. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
3. Nomura E, Inoue M. Cartilage lesions of the patella in recurrent patellar dislocation. Am J Sports Med. 2004;32(2):498-502.
4. Vollnberg B, Koehlitz T, Jung T, et al. Prevalence of cartilage lesions and early osteoarthritis in patients with patellar dislocation. Eur Radiol. 2012;22(11):2347-2356.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37-61.
6. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036-2045.e14.
7. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906-918.
8. Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-1081.
9. Meric G, Gracitelli GC, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med. 2015;43(3):709-714.
10. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-1124.
11. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-908.
12. Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-891.
13. Anderson CN, Magnussen RA, Block JJ, Anderson AF, Spindler KP. Operative fixation of chondral loose bodies in osteochondritis dissecans in the knee: a report of 5 cases. Orthop J Sports Med. 2013;1(2):2325967113496546.
14. Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14(11):1119-1125.
15. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):452-457.
16. Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am. 2014;96(10):816-823.
17. Hoffman JK, Geraghty S, Protzman NM. Articular cartilage repair using marrow simulation augmented with a viable chondral allograft: 9-month postoperative histological evaluation. Case Rep Orthop. 2015;2015:617365.
18. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82-97.
19. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-1425.
20. Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-1670.
Practice Makes Perfect?
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
It is human nature to practice things that we are already good at doing. If you’re a golfer, then you know what I’m talking about. I hit the driver over and over again on the range, but never practice hitting the bad lie in the bunker, or the half-swing wedge from a tight lie. I sink hundreds of 3 footers, but can’t putt into this range from 50 feet. I’ve gotten much better at golf since I started playing, but my scores have hardly gone down.
I think a similar thing happens in our orthopedic practices. I read everything I can on the anterior cruciate ligament, yet I already feel comfortable with my reconstruction technique. I skim, or avoid reading altogether, articles about topics I don’t like to treat, like the hand or spine. Yet, I still see these things every day in my practice and on call. If my depth of knowledge in these areas was as good as it is in sports medicine, I could provide better, more immediate care to my patients, rather than refer them to subspecialists.
A perfect orthopedic example would be the patellofemoral joint. One of the least enjoyable patient encounters for me is the young adult with normal alignment and intractable anterior knee pain that does not respond to nonoperative treatment. I’m concerned any surgical intervention may make them worse and I’m often left without much to offer the patient.
It’s for this reason AJO has partnered with Dr. Jack Farr to produce the patellofemoral issue; to provide a comprehensive guide to the latest thinking in the treatment of patellofemoral disorders (see the March/April 2017 issue). We solicited so much outstanding content, that a single issue could not hold all of the articles. In this issue, our patellofemoral series continues with 3 outstanding articles. Magnussen presents "Patella Alta Sees You, Do You See It?" and Hinckel and colleagues have authored a guide to patellofemoral cartilage restoration. Unal and colleagues follow-up with a review of the lateral retinaculum.
In our "Codes to Know" section, we reexamine diagnostic arthroscopy, a code most of us have billed infrequently. New technologies, however, have made it possible to peer into the joint in the office, and McMillan and colleagues teach us how to make it economically feasible, even for employed physicians.
Finally, we have a number of great articles on difficult problems—the stiff elbow, complex distal radius fractures, and intraoperative acetabular fractures during total hip arthroplasty.
Please enjoy this issue and think about what topics you tend to shy away from. I’m willing to bet you can add the most to your practice by studying up on these topics. As always, please provide your feedback to our editorial team so that we can continue to make improvements to our journal. We envision a change in the way orthopedists utilize a journal in their practice, and are continuously looking for ways to make AJO a more relevant tool for improving your patient care and workflow. We are working hard to give our readers the journal they deserve, but in my spare time, I’ll be brushing up on trochleoplasties and half-swing wedges.
Pronator Teres Myotendinous Tear
I read with interest the article "Pronator Teres Myotendinous Tear" by Drs. Qayyum, Villacis, and Jobin (Am J Orthop. 2017;46(2):E105-E107), and I commend the authors for their interesting exploration of this unusual injury.
I would like to note that this pathology was previously reported by our group in 2015.1 We now have a 2-year follow-up on this patient, and he has remained asymptomatic since his return to golf. Since this article was published, we have been contacted by 3 patients (one of whom is a radiologist who interpreted his own magnetic resonance imaging) describing similar mechanisms of injury, symptoms, imaging findings, and recovery with nonoperative management. This suggests that pronator teres rupture may have been previously unrecognized or underreported.
It is interesting that this patient was injured when his club stuck in the ground while our patient reported taking only a small divot during his injury. From these differing mechanisms it is unclear whether forceful contraction or sudden loading is the largest risk factor for obtaining this injury, and this could be a point for further research. As awareness of this injury pattern spreads, we look forward to seeing larger series and establishing the success rate of nonoperative treatment and the risk factors for its failure.
Brooks W. Ficke, MD
Brent A. Ponce, MD
Birmingham, AL
Authors' Response
We appreciate Dr. Ficke’s comments regarding his experience treating pronator teres injuries and agree that they are likely under-recognized and possibly underreported. We are uncertain which mechanisms during the golf swing strains the pronator teres to the point of injury, but it may be a combination of muscular fatigue, forceful contraction, and sudden resistance to concentric loading during the club striking the ground. In our experience, these injuries do appear to heal without observable deficit. Our patient is back golfing regularly without any arm symptoms and actually had an improvement in his golf handicap this season.
Charles M. Jobin, MD
Usama Qayyum, MBBS
Diego Villacis, MD
New York, NY
1. Ficke BW, Larrison MC, Ponce BA. Isolated rupture of the pronator teres in an amateur golfer: a case report. Int J Orthop. 2015;2(6):481-483.
I read with interest the article "Pronator Teres Myotendinous Tear" by Drs. Qayyum, Villacis, and Jobin (Am J Orthop. 2017;46(2):E105-E107), and I commend the authors for their interesting exploration of this unusual injury.
I would like to note that this pathology was previously reported by our group in 2015.1 We now have a 2-year follow-up on this patient, and he has remained asymptomatic since his return to golf. Since this article was published, we have been contacted by 3 patients (one of whom is a radiologist who interpreted his own magnetic resonance imaging) describing similar mechanisms of injury, symptoms, imaging findings, and recovery with nonoperative management. This suggests that pronator teres rupture may have been previously unrecognized or underreported.
It is interesting that this patient was injured when his club stuck in the ground while our patient reported taking only a small divot during his injury. From these differing mechanisms it is unclear whether forceful contraction or sudden loading is the largest risk factor for obtaining this injury, and this could be a point for further research. As awareness of this injury pattern spreads, we look forward to seeing larger series and establishing the success rate of nonoperative treatment and the risk factors for its failure.
Brooks W. Ficke, MD
Brent A. Ponce, MD
Birmingham, AL
Authors' Response
We appreciate Dr. Ficke’s comments regarding his experience treating pronator teres injuries and agree that they are likely under-recognized and possibly underreported. We are uncertain which mechanisms during the golf swing strains the pronator teres to the point of injury, but it may be a combination of muscular fatigue, forceful contraction, and sudden resistance to concentric loading during the club striking the ground. In our experience, these injuries do appear to heal without observable deficit. Our patient is back golfing regularly without any arm symptoms and actually had an improvement in his golf handicap this season.
Charles M. Jobin, MD
Usama Qayyum, MBBS
Diego Villacis, MD
New York, NY
I read with interest the article "Pronator Teres Myotendinous Tear" by Drs. Qayyum, Villacis, and Jobin (Am J Orthop. 2017;46(2):E105-E107), and I commend the authors for their interesting exploration of this unusual injury.
I would like to note that this pathology was previously reported by our group in 2015.1 We now have a 2-year follow-up on this patient, and he has remained asymptomatic since his return to golf. Since this article was published, we have been contacted by 3 patients (one of whom is a radiologist who interpreted his own magnetic resonance imaging) describing similar mechanisms of injury, symptoms, imaging findings, and recovery with nonoperative management. This suggests that pronator teres rupture may have been previously unrecognized or underreported.
It is interesting that this patient was injured when his club stuck in the ground while our patient reported taking only a small divot during his injury. From these differing mechanisms it is unclear whether forceful contraction or sudden loading is the largest risk factor for obtaining this injury, and this could be a point for further research. As awareness of this injury pattern spreads, we look forward to seeing larger series and establishing the success rate of nonoperative treatment and the risk factors for its failure.
Brooks W. Ficke, MD
Brent A. Ponce, MD
Birmingham, AL
Authors' Response
We appreciate Dr. Ficke’s comments regarding his experience treating pronator teres injuries and agree that they are likely under-recognized and possibly underreported. We are uncertain which mechanisms during the golf swing strains the pronator teres to the point of injury, but it may be a combination of muscular fatigue, forceful contraction, and sudden resistance to concentric loading during the club striking the ground. In our experience, these injuries do appear to heal without observable deficit. Our patient is back golfing regularly without any arm symptoms and actually had an improvement in his golf handicap this season.
Charles M. Jobin, MD
Usama Qayyum, MBBS
Diego Villacis, MD
New York, NY
1. Ficke BW, Larrison MC, Ponce BA. Isolated rupture of the pronator teres in an amateur golfer: a case report. Int J Orthop. 2015;2(6):481-483.
1. Ficke BW, Larrison MC, Ponce BA. Isolated rupture of the pronator teres in an amateur golfer: a case report. Int J Orthop. 2015;2(6):481-483.
Percutaneous Release of Trigger Digits
Take-Home Points
- The author had a 90% success rate with no complications in treating almost 600 trigger digits.
- All digits can be safely treated, including multiple fingers on one hand, all in an office setting.
- Percutaneous trigger release appears to be a safe and reliable alternative to open surgery.
- Success rate, discomfort, and cost may make a percutaneous trigger release preferable to even a trial of corticosteroid injection.
- A failed percutaneous release can be successfully treated with an open release, if needed.
Trigger finger, or stenosing flexor tenosynovitis, is a condition characterized by clicking or locking during finger movement, sometimes resulting in the freezing of a digit in flexion or extension1 (Figure 1). [[{"fid":"202300","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]Tendon inflammation is thought to cause constriction of the tendon sheath and bunching of the fibrous bundles of the first annular (A1) pulley, often creating a palpable nodule at the base of the digit.2,3 Many patients experience intermittent joint pain and swelling, which may progress to triggering or complete locking of the digit.1 One of the most common conditions treated by hand surgeons, trigger finger is most often reported in the dominant hand of women in their sixth decade of life and has been associated with several conditions, including diabetes and rheumatoid arthritis.4-6 Other researchers have indicated the thumb and ring finger are most commonly affected, though all fingers can potentially trigger.7,8
Initial treatment often involves injecting corticosteroid into the flexor tendon sheath, at or proximal to the annular pulley system, to reduce inflammation and the fibrous nodule.3 Another injection study found an initial success rate of 57% with a single injection, and 86% with a second injection, but patients were monitored for only 6 months, a period that may have been too short for symptom recurrence.7
On failure of steroid injections, patients typically are treated with open tendon sheath incision.9 This procedure, usually performed in a hospital or outpatient surgery setting, requires postoperative wound care, including dressing changes, suture removal, possible hand therapy, and follow-up physician visits. Operative treatment involves making a 1-cm to 2-cm incision, releasing the A1 pulley, and skin suturing.7,8,10 The most common postoperative complaint is incisional tenderness, though long-term scar pain, infection, nerve injury, and disease recurrence have been reported.8 Overall, the procedure is very successful, providing up to 100% symptom relief.7,8,10
Endoscopic release of trigger finger has also been described as an effective operative treatment. This technique involves passing a small cannula through a palmar incision—using an endoscope and retrograde knife within this 2.7-mm tunnel.10 With this treatment, reduced visibility may increase the risk of nerve injury.10 Although generally successful, endoscopic release requires anesthesia and expensive instruments and has a significant learning curve.8,10
More recently, percutaneous release of trigger finger has been described as a definitive, in-office treatment.5,6,11,12 Percutaneous release has the obvious advantages of no open incision, less scarring, less discomfort, and shorter recovery. Several studies have found comparable success rates for open and percutaneous procedures but consistently shorter recovery with the percutaneous technique.7,8,12 Given its lower recurrence rate (vs steroid injections) and shorter recovery and lower cost (vs a surgical procedure), percutaneous treatment of stenosing tenosynovitis appears to be a safe, highly successful, and minimally invasive treatment method.8 This study represents a single surgeon’s experience with percutaneous tendon sheath incision over a 10-year period.
Methods
Patients presented with symptoms of stenosing flexor tenosynovitis with severity ranging from intermittent triggering to frank locking of the digit. Most patients underwent prior conservative treatment, including corticosteroid injections and hand therapy. With each patient, the senior author discussed the pathophysiology of trigger digit; treatment options, including observation, hand therapy, corticosteroid injection, percutaneous release, and open release; and potential risks and complications. The treatment path—initial corticosteroid injection, percutaneous release, or open release—was left up to the patient. The only exclusion criterion was prior surgery to the involved digit, and there was no discrimination by finger, symptomatic period, or severity. Each released digit was recorded independently. In no case was anticoagulant therapy discontinued.
A complete medical history was obtained for each patient.
Over a 10-year period (March 2003-December 2013), percutaneous release was performed on 596 trigger fingers in 429 patients, 18 years old or older. Of these patients, 279 were female. Mean age was 62 years (range, 26-97 years). Of the 531 releases with handedness recorded, 56.3% were performed on trigger digits on dominant hands (Table 1). [[{"fid":"202302","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":""}}}]]Mean duration of symptoms before percutaneous release was 9.7 months (range, 0.5-132 months). Of the 596 digits, 69 were reported to have previously sustained trauma, and 161 had been unsuccessfully treated with one or more cortisone injections before undergoing release. Of the suspected comorbidities examined, carpal tunnel syndrome was previously diagnosed in 79 patients and diabetes in 56 patients.1
Of the 429 patients, 313 had a single digit released and 116 had multiple digits released. Of the 116 patients in the multiple-release group, 80 had 2 fingers released, 24 had 3 released, 7 had 4 released, and 5 had 5 released. The 596 released trigger fingers consisted of 188 thumbs, 41 index fingers, 185 middle fingers, 140 ring fingers, and 42 small fingers.
Surgical Technique
In-office percutaneous trigger finger releases were performed with a local anesthetic. One milliliter of lidocaine 1% injection was used to anesthetize the skin, the subcutaneous tissues, and the flexor tendon sheath at the level of the A1 pulley. As described by Pandey and colleagues,6 the proper location of the pulley was confirmed using specific surface landmarks on each digit. After waiting several minutes to allow the anesthetic to take effect, the surgeon inserted an 18-gauge needle into the center of the pulley with the digit held in extension (Figure 2). [[{"fid":"202303","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The needle was carefully moved longitudinally along the length of the pulley with the bevel of the needle parallel to the tendon. A grating sensation was felt as the fibers of the pulley were cut. Several needle passes were made until the pulley was felt to have been released. Complete release was determined by loss of the grating sensation, along with complete relief of any further symptoms of triggering. The puncture site was cleaned and covered with a light sterile dressing (watch the Video online). There was no postoperative immobilization, and patients were encouraged to immediately return to normal use of the digit. Hand therapy was not prescribed, and pain medications were not dispensed. A 1-week follow-up appointment was scheduled, and patients were advised to return for evaluation in the event of any recurring symptoms (eg, triggering, swelling, stiffness, pain).
Results
were successfully released with 1 percutaneous procedure (recurrence or failure rate, 9.9%). The thumb was the digit most reliably released (success rate, 94.7%) (Table 2). [[{"fid":"202306","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":""}}}]]Patients with recurrent or unresolved symptoms were given the options of a second percutaneous release or an open surgical procedure. Of the 59 digits unsuccessfully released, as identified by persistent triggering or locking of the digit, 17 were treated with a second percutaneous release (15 were successful), and 40 underwent open tendon sheath incision as a second procedure (success rate, 100%); triggering persisted in the remaining 2 digits, and these were considered failures (the 2 patients did not pursue further treatment).
There were no complications: infection; nerve, artery, or tendon injury; or chronic pain. Some patients had mild stiffness, swelling, or pain for a few days after the procedure, and these effects typically resolved without treatment. In 29 digits, persistent pain or swelling without triggering was successfully treated with a corticosteroid injection.
Discussion
[[{"fid":"202307","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":""}}}]]Over a 10-year period, 596 percutaneous trigger finger releases were sequentially performed by a single surgeon. The 90% success rate compares favorably with rates found in other studies (Table 3).5-9,12-14 The surgeon’s success rates for individual years vary and demonstrate no clear trend or learning curve with the procedure (Figure 3). There were no significant complications. Patient satisfaction with the procedure was high.[[{"fid":"202308","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]
There were no injuries to digital nerves, arteries, or flexor tendons, either early or late, and no reports of infections or long-term pain or loss of motion. Although it is quite probable that in some procedures the longitudinal passes of the 18-gauge needle may have also slightly cut into the flexor tendon after passing through the A1 pulley, the direction of the needle passes was in line with the direction of the collagen fibers of the tendon, and thus any inadvertent superficial abrasion would not have structurally weakened the tendon. Of the 40 digits that underwent open release after incomplete or failed percutaneous release, none showed significant longitudinal lacerations of the superficialis tendon. During these revision surgeries, the typical intraoperative finding was incomplete release of the A1 pulley, usually at the distal end. Although loss of the grating sensation or relief of further triggering symptoms was considered adequate evidence of a successful release in this study, small tendon attachments could remain and potentially could lead to recurrent triggering. Given the high success rate achieved with the large sample, however, these 2 factors are considered appropriate indicators of successful release.
It is unclear why there was a relatively consistent 10% failure rate and why it did not decrease over the 10-year study period. Although the technique used does not have a significant learning curve, it appears that digits are not actively triggering at time of procedure have a higher failure rate. When a patient’s digit is actively triggering, assessment of the success of the procedure is relatively straightforward, whereas when a digit intermittently triggers and locks and is not symptomatic in the office, success cannot be immediately determined.
No specific digit was significantly more prone to failed releases, though the small finger had the lowest success rate (85.7%). Given that only 56.4% of patients experienced triggering on the dominant hand, there is not enough evidence to suggest a significant relationship between likelihood of a trigger digit and a patient’s hand dominance. Similarly, there was no correlation between the duration of symptoms and the success of the percutaneous procedure.
Investigation of the relationship between the previously suggested comorbidities of carpal tunnel syndrome and diabetes was also inconclusive. Only 79 (18%) of 429 patients reported having carpal tunnel syndrome, and even fewer, 56 (13.0%), reported having diabetes. Only 69 of the 596 treated digits reportedly had sustained trauma before developing triggering symptoms, and only 12 of the 69 were unsuccessfully released. In addition, of the 161 digits in which one or more steroid injections failed to resolve triggering symptoms, 158 (87.3%) were successfully released with 1 percutaneous procedure. Collectively, these data show percutaneous release can effectively eliminate triggering symptoms in a digit that has sustained injury or that has been unsuccessfully treated with nonoperative methods. Failed percutaneous release subsequently can be reliably treated with an open procedure, and results are excellent.
This study had several limitations. It was retrospective, nonblinded, and did not compare outcomes of percutaneous release with those of an open procedure. Data are presented to support the efficacy and safety of percutaneous release as a treatment option. Another limitation is that pre-release treatment was not controlled. Patients had been treated with a variety of nonoperative methods, including use of anti-inflammatory medication, hand therapy, splinting, and one or more corticosteroid injections, both at our office and elsewhere.
Percutaneous release appears to have an advantage in terms of pain relief, but the study did not evaluate or control for procedure discomfort. However, patients who had been treated with a corticosteroid injection before percutaneous release consistently refused corticosteroid injections for subsequent trigger digits, citing the dramatic pain reduction achieved with release relative to injection. Similarly, all patients who had a trigger digit treated with open tendon sheath incision in the past indicated a strong preference for the percutaneous release.
Follow-up on this patient population was inconsistent and incomplete. Many patients did not return, presumably because they considered the procedure a success and thought follow-up was unnecessary. However, some patients may have had a recurrence or an incomplete release and gone elsewhere for treatment.
The results of this study, to date the largest study on percutaneous release of trigger finger, provide more evidence of the safety and efficacy of this procedure as a treatment option. The success rate of percutaneous release is high, surpasses that of nonoperative treatments such as steroid injections, and approaches that of open and endoscopic surgical alternatives. Some of the obvious advantages of percutaneous release are less visible scarring, fewer incision-related complications, and shorter rehabilitation.10 In addition, post-procedure pain is possibly reduced, symptom relief is comparable, operative time is significantly shorter,8 and percutaneous release is easily performed in the office setting.
Percutaneous release is a viable treatment option for stenosing flexor tenosynovitis, regardless of previously used nonoperative treatment methods, duration or severity of symptoms, or trigger digit treated.
1. Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1(2):92-96.
2. Fahey JJ, Bollinger JA. Trigger-finger in adults and children. J Bone Joint Surg Am. 1954;36(6):1200-1218.
3. Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722-727.
4. Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20(1):109-114.
5. Habbu R, Putman MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
6. Pandey BK, Sharma S, Manandhar RR, Pradhan RL, Lakhey S, Rijal KP. Percutaneous trigger finger release. Nepal Orthop Assoc J. 2010;1(1):1-5.
7. Sato ES, Gomes dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51(1):93-99.
8. Dierks U, Hoffmann R, Meek MF. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008;12(3):183-187.
9. Tanaka J. Percutaneous trigger finger release. Tech Hand Up Extrem Surg. 1999;3(1):52-57.
10. Pegoli L, Cavalli E, Cortese P, Parolo C, Pajardi G. A comparison of endoscopic and open trigger finger release. Hand Surg. 2008;13(3):147-151.
11. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31(1):135-146.
12. Schramm JM, Nguyen M, Wongworawat MD. The safety of percutaneous trigger finger release. Hand. 2008;3(1):44-46.
13. Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand. 2009;4(1):35-37.
14. Cihantimur B, Akin S, Ozcan M. Percutaneous treatment of trigger finger. 34 fingers followed 0.5-2 years. Acta Orthop Scand. 1998;69(2):167-168.
Take-Home Points
- The author had a 90% success rate with no complications in treating almost 600 trigger digits.
- All digits can be safely treated, including multiple fingers on one hand, all in an office setting.
- Percutaneous trigger release appears to be a safe and reliable alternative to open surgery.
- Success rate, discomfort, and cost may make a percutaneous trigger release preferable to even a trial of corticosteroid injection.
- A failed percutaneous release can be successfully treated with an open release, if needed.
Trigger finger, or stenosing flexor tenosynovitis, is a condition characterized by clicking or locking during finger movement, sometimes resulting in the freezing of a digit in flexion or extension1 (Figure 1). [[{"fid":"202300","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]Tendon inflammation is thought to cause constriction of the tendon sheath and bunching of the fibrous bundles of the first annular (A1) pulley, often creating a palpable nodule at the base of the digit.2,3 Many patients experience intermittent joint pain and swelling, which may progress to triggering or complete locking of the digit.1 One of the most common conditions treated by hand surgeons, trigger finger is most often reported in the dominant hand of women in their sixth decade of life and has been associated with several conditions, including diabetes and rheumatoid arthritis.4-6 Other researchers have indicated the thumb and ring finger are most commonly affected, though all fingers can potentially trigger.7,8
Initial treatment often involves injecting corticosteroid into the flexor tendon sheath, at or proximal to the annular pulley system, to reduce inflammation and the fibrous nodule.3 Another injection study found an initial success rate of 57% with a single injection, and 86% with a second injection, but patients were monitored for only 6 months, a period that may have been too short for symptom recurrence.7
On failure of steroid injections, patients typically are treated with open tendon sheath incision.9 This procedure, usually performed in a hospital or outpatient surgery setting, requires postoperative wound care, including dressing changes, suture removal, possible hand therapy, and follow-up physician visits. Operative treatment involves making a 1-cm to 2-cm incision, releasing the A1 pulley, and skin suturing.7,8,10 The most common postoperative complaint is incisional tenderness, though long-term scar pain, infection, nerve injury, and disease recurrence have been reported.8 Overall, the procedure is very successful, providing up to 100% symptom relief.7,8,10
Endoscopic release of trigger finger has also been described as an effective operative treatment. This technique involves passing a small cannula through a palmar incision—using an endoscope and retrograde knife within this 2.7-mm tunnel.10 With this treatment, reduced visibility may increase the risk of nerve injury.10 Although generally successful, endoscopic release requires anesthesia and expensive instruments and has a significant learning curve.8,10
More recently, percutaneous release of trigger finger has been described as a definitive, in-office treatment.5,6,11,12 Percutaneous release has the obvious advantages of no open incision, less scarring, less discomfort, and shorter recovery. Several studies have found comparable success rates for open and percutaneous procedures but consistently shorter recovery with the percutaneous technique.7,8,12 Given its lower recurrence rate (vs steroid injections) and shorter recovery and lower cost (vs a surgical procedure), percutaneous treatment of stenosing tenosynovitis appears to be a safe, highly successful, and minimally invasive treatment method.8 This study represents a single surgeon’s experience with percutaneous tendon sheath incision over a 10-year period.
Methods
Patients presented with symptoms of stenosing flexor tenosynovitis with severity ranging from intermittent triggering to frank locking of the digit. Most patients underwent prior conservative treatment, including corticosteroid injections and hand therapy. With each patient, the senior author discussed the pathophysiology of trigger digit; treatment options, including observation, hand therapy, corticosteroid injection, percutaneous release, and open release; and potential risks and complications. The treatment path—initial corticosteroid injection, percutaneous release, or open release—was left up to the patient. The only exclusion criterion was prior surgery to the involved digit, and there was no discrimination by finger, symptomatic period, or severity. Each released digit was recorded independently. In no case was anticoagulant therapy discontinued.
A complete medical history was obtained for each patient.
Over a 10-year period (March 2003-December 2013), percutaneous release was performed on 596 trigger fingers in 429 patients, 18 years old or older. Of these patients, 279 were female. Mean age was 62 years (range, 26-97 years). Of the 531 releases with handedness recorded, 56.3% were performed on trigger digits on dominant hands (Table 1). [[{"fid":"202302","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":""}}}]]Mean duration of symptoms before percutaneous release was 9.7 months (range, 0.5-132 months). Of the 596 digits, 69 were reported to have previously sustained trauma, and 161 had been unsuccessfully treated with one or more cortisone injections before undergoing release. Of the suspected comorbidities examined, carpal tunnel syndrome was previously diagnosed in 79 patients and diabetes in 56 patients.1
Of the 429 patients, 313 had a single digit released and 116 had multiple digits released. Of the 116 patients in the multiple-release group, 80 had 2 fingers released, 24 had 3 released, 7 had 4 released, and 5 had 5 released. The 596 released trigger fingers consisted of 188 thumbs, 41 index fingers, 185 middle fingers, 140 ring fingers, and 42 small fingers.
Surgical Technique
In-office percutaneous trigger finger releases were performed with a local anesthetic. One milliliter of lidocaine 1% injection was used to anesthetize the skin, the subcutaneous tissues, and the flexor tendon sheath at the level of the A1 pulley. As described by Pandey and colleagues,6 the proper location of the pulley was confirmed using specific surface landmarks on each digit. After waiting several minutes to allow the anesthetic to take effect, the surgeon inserted an 18-gauge needle into the center of the pulley with the digit held in extension (Figure 2). [[{"fid":"202303","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The needle was carefully moved longitudinally along the length of the pulley with the bevel of the needle parallel to the tendon. A grating sensation was felt as the fibers of the pulley were cut. Several needle passes were made until the pulley was felt to have been released. Complete release was determined by loss of the grating sensation, along with complete relief of any further symptoms of triggering. The puncture site was cleaned and covered with a light sterile dressing (watch the Video online). There was no postoperative immobilization, and patients were encouraged to immediately return to normal use of the digit. Hand therapy was not prescribed, and pain medications were not dispensed. A 1-week follow-up appointment was scheduled, and patients were advised to return for evaluation in the event of any recurring symptoms (eg, triggering, swelling, stiffness, pain).
Results
were successfully released with 1 percutaneous procedure (recurrence or failure rate, 9.9%). The thumb was the digit most reliably released (success rate, 94.7%) (Table 2). [[{"fid":"202306","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":""}}}]]Patients with recurrent or unresolved symptoms were given the options of a second percutaneous release or an open surgical procedure. Of the 59 digits unsuccessfully released, as identified by persistent triggering or locking of the digit, 17 were treated with a second percutaneous release (15 were successful), and 40 underwent open tendon sheath incision as a second procedure (success rate, 100%); triggering persisted in the remaining 2 digits, and these were considered failures (the 2 patients did not pursue further treatment).
There were no complications: infection; nerve, artery, or tendon injury; or chronic pain. Some patients had mild stiffness, swelling, or pain for a few days after the procedure, and these effects typically resolved without treatment. In 29 digits, persistent pain or swelling without triggering was successfully treated with a corticosteroid injection.
Discussion
[[{"fid":"202307","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":""}}}]]Over a 10-year period, 596 percutaneous trigger finger releases were sequentially performed by a single surgeon. The 90% success rate compares favorably with rates found in other studies (Table 3).5-9,12-14 The surgeon’s success rates for individual years vary and demonstrate no clear trend or learning curve with the procedure (Figure 3). There were no significant complications. Patient satisfaction with the procedure was high.[[{"fid":"202308","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]
There were no injuries to digital nerves, arteries, or flexor tendons, either early or late, and no reports of infections or long-term pain or loss of motion. Although it is quite probable that in some procedures the longitudinal passes of the 18-gauge needle may have also slightly cut into the flexor tendon after passing through the A1 pulley, the direction of the needle passes was in line with the direction of the collagen fibers of the tendon, and thus any inadvertent superficial abrasion would not have structurally weakened the tendon. Of the 40 digits that underwent open release after incomplete or failed percutaneous release, none showed significant longitudinal lacerations of the superficialis tendon. During these revision surgeries, the typical intraoperative finding was incomplete release of the A1 pulley, usually at the distal end. Although loss of the grating sensation or relief of further triggering symptoms was considered adequate evidence of a successful release in this study, small tendon attachments could remain and potentially could lead to recurrent triggering. Given the high success rate achieved with the large sample, however, these 2 factors are considered appropriate indicators of successful release.
It is unclear why there was a relatively consistent 10% failure rate and why it did not decrease over the 10-year study period. Although the technique used does not have a significant learning curve, it appears that digits are not actively triggering at time of procedure have a higher failure rate. When a patient’s digit is actively triggering, assessment of the success of the procedure is relatively straightforward, whereas when a digit intermittently triggers and locks and is not symptomatic in the office, success cannot be immediately determined.
No specific digit was significantly more prone to failed releases, though the small finger had the lowest success rate (85.7%). Given that only 56.4% of patients experienced triggering on the dominant hand, there is not enough evidence to suggest a significant relationship between likelihood of a trigger digit and a patient’s hand dominance. Similarly, there was no correlation between the duration of symptoms and the success of the percutaneous procedure.
Investigation of the relationship between the previously suggested comorbidities of carpal tunnel syndrome and diabetes was also inconclusive. Only 79 (18%) of 429 patients reported having carpal tunnel syndrome, and even fewer, 56 (13.0%), reported having diabetes. Only 69 of the 596 treated digits reportedly had sustained trauma before developing triggering symptoms, and only 12 of the 69 were unsuccessfully released. In addition, of the 161 digits in which one or more steroid injections failed to resolve triggering symptoms, 158 (87.3%) were successfully released with 1 percutaneous procedure. Collectively, these data show percutaneous release can effectively eliminate triggering symptoms in a digit that has sustained injury or that has been unsuccessfully treated with nonoperative methods. Failed percutaneous release subsequently can be reliably treated with an open procedure, and results are excellent.
This study had several limitations. It was retrospective, nonblinded, and did not compare outcomes of percutaneous release with those of an open procedure. Data are presented to support the efficacy and safety of percutaneous release as a treatment option. Another limitation is that pre-release treatment was not controlled. Patients had been treated with a variety of nonoperative methods, including use of anti-inflammatory medication, hand therapy, splinting, and one or more corticosteroid injections, both at our office and elsewhere.
Percutaneous release appears to have an advantage in terms of pain relief, but the study did not evaluate or control for procedure discomfort. However, patients who had been treated with a corticosteroid injection before percutaneous release consistently refused corticosteroid injections for subsequent trigger digits, citing the dramatic pain reduction achieved with release relative to injection. Similarly, all patients who had a trigger digit treated with open tendon sheath incision in the past indicated a strong preference for the percutaneous release.
Follow-up on this patient population was inconsistent and incomplete. Many patients did not return, presumably because they considered the procedure a success and thought follow-up was unnecessary. However, some patients may have had a recurrence or an incomplete release and gone elsewhere for treatment.
The results of this study, to date the largest study on percutaneous release of trigger finger, provide more evidence of the safety and efficacy of this procedure as a treatment option. The success rate of percutaneous release is high, surpasses that of nonoperative treatments such as steroid injections, and approaches that of open and endoscopic surgical alternatives. Some of the obvious advantages of percutaneous release are less visible scarring, fewer incision-related complications, and shorter rehabilitation.10 In addition, post-procedure pain is possibly reduced, symptom relief is comparable, operative time is significantly shorter,8 and percutaneous release is easily performed in the office setting.
Percutaneous release is a viable treatment option for stenosing flexor tenosynovitis, regardless of previously used nonoperative treatment methods, duration or severity of symptoms, or trigger digit treated.
Take-Home Points
- The author had a 90% success rate with no complications in treating almost 600 trigger digits.
- All digits can be safely treated, including multiple fingers on one hand, all in an office setting.
- Percutaneous trigger release appears to be a safe and reliable alternative to open surgery.
- Success rate, discomfort, and cost may make a percutaneous trigger release preferable to even a trial of corticosteroid injection.
- A failed percutaneous release can be successfully treated with an open release, if needed.
Trigger finger, or stenosing flexor tenosynovitis, is a condition characterized by clicking or locking during finger movement, sometimes resulting in the freezing of a digit in flexion or extension1 (Figure 1). [[{"fid":"202300","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]Tendon inflammation is thought to cause constriction of the tendon sheath and bunching of the fibrous bundles of the first annular (A1) pulley, often creating a palpable nodule at the base of the digit.2,3 Many patients experience intermittent joint pain and swelling, which may progress to triggering or complete locking of the digit.1 One of the most common conditions treated by hand surgeons, trigger finger is most often reported in the dominant hand of women in their sixth decade of life and has been associated with several conditions, including diabetes and rheumatoid arthritis.4-6 Other researchers have indicated the thumb and ring finger are most commonly affected, though all fingers can potentially trigger.7,8
Initial treatment often involves injecting corticosteroid into the flexor tendon sheath, at or proximal to the annular pulley system, to reduce inflammation and the fibrous nodule.3 Another injection study found an initial success rate of 57% with a single injection, and 86% with a second injection, but patients were monitored for only 6 months, a period that may have been too short for symptom recurrence.7
On failure of steroid injections, patients typically are treated with open tendon sheath incision.9 This procedure, usually performed in a hospital or outpatient surgery setting, requires postoperative wound care, including dressing changes, suture removal, possible hand therapy, and follow-up physician visits. Operative treatment involves making a 1-cm to 2-cm incision, releasing the A1 pulley, and skin suturing.7,8,10 The most common postoperative complaint is incisional tenderness, though long-term scar pain, infection, nerve injury, and disease recurrence have been reported.8 Overall, the procedure is very successful, providing up to 100% symptom relief.7,8,10
Endoscopic release of trigger finger has also been described as an effective operative treatment. This technique involves passing a small cannula through a palmar incision—using an endoscope and retrograde knife within this 2.7-mm tunnel.10 With this treatment, reduced visibility may increase the risk of nerve injury.10 Although generally successful, endoscopic release requires anesthesia and expensive instruments and has a significant learning curve.8,10
More recently, percutaneous release of trigger finger has been described as a definitive, in-office treatment.5,6,11,12 Percutaneous release has the obvious advantages of no open incision, less scarring, less discomfort, and shorter recovery. Several studies have found comparable success rates for open and percutaneous procedures but consistently shorter recovery with the percutaneous technique.7,8,12 Given its lower recurrence rate (vs steroid injections) and shorter recovery and lower cost (vs a surgical procedure), percutaneous treatment of stenosing tenosynovitis appears to be a safe, highly successful, and minimally invasive treatment method.8 This study represents a single surgeon’s experience with percutaneous tendon sheath incision over a 10-year period.
Methods
Patients presented with symptoms of stenosing flexor tenosynovitis with severity ranging from intermittent triggering to frank locking of the digit. Most patients underwent prior conservative treatment, including corticosteroid injections and hand therapy. With each patient, the senior author discussed the pathophysiology of trigger digit; treatment options, including observation, hand therapy, corticosteroid injection, percutaneous release, and open release; and potential risks and complications. The treatment path—initial corticosteroid injection, percutaneous release, or open release—was left up to the patient. The only exclusion criterion was prior surgery to the involved digit, and there was no discrimination by finger, symptomatic period, or severity. Each released digit was recorded independently. In no case was anticoagulant therapy discontinued.
A complete medical history was obtained for each patient.
Over a 10-year period (March 2003-December 2013), percutaneous release was performed on 596 trigger fingers in 429 patients, 18 years old or older. Of these patients, 279 were female. Mean age was 62 years (range, 26-97 years). Of the 531 releases with handedness recorded, 56.3% were performed on trigger digits on dominant hands (Table 1). [[{"fid":"202302","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 1.","field_file_image_credit[und][0][value]":""}}}]]Mean duration of symptoms before percutaneous release was 9.7 months (range, 0.5-132 months). Of the 596 digits, 69 were reported to have previously sustained trauma, and 161 had been unsuccessfully treated with one or more cortisone injections before undergoing release. Of the suspected comorbidities examined, carpal tunnel syndrome was previously diagnosed in 79 patients and diabetes in 56 patients.1
Of the 429 patients, 313 had a single digit released and 116 had multiple digits released. Of the 116 patients in the multiple-release group, 80 had 2 fingers released, 24 had 3 released, 7 had 4 released, and 5 had 5 released. The 596 released trigger fingers consisted of 188 thumbs, 41 index fingers, 185 middle fingers, 140 ring fingers, and 42 small fingers.
Surgical Technique
In-office percutaneous trigger finger releases were performed with a local anesthetic. One milliliter of lidocaine 1% injection was used to anesthetize the skin, the subcutaneous tissues, and the flexor tendon sheath at the level of the A1 pulley. As described by Pandey and colleagues,6 the proper location of the pulley was confirmed using specific surface landmarks on each digit. After waiting several minutes to allow the anesthetic to take effect, the surgeon inserted an 18-gauge needle into the center of the pulley with the digit held in extension (Figure 2). [[{"fid":"202303","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]The needle was carefully moved longitudinally along the length of the pulley with the bevel of the needle parallel to the tendon. A grating sensation was felt as the fibers of the pulley were cut. Several needle passes were made until the pulley was felt to have been released. Complete release was determined by loss of the grating sensation, along with complete relief of any further symptoms of triggering. The puncture site was cleaned and covered with a light sterile dressing (watch the Video online). There was no postoperative immobilization, and patients were encouraged to immediately return to normal use of the digit. Hand therapy was not prescribed, and pain medications were not dispensed. A 1-week follow-up appointment was scheduled, and patients were advised to return for evaluation in the event of any recurring symptoms (eg, triggering, swelling, stiffness, pain).
Results
were successfully released with 1 percutaneous procedure (recurrence or failure rate, 9.9%). The thumb was the digit most reliably released (success rate, 94.7%) (Table 2). [[{"fid":"202306","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table 2.","field_file_image_credit[und][0][value]":""}}}]]Patients with recurrent or unresolved symptoms were given the options of a second percutaneous release or an open surgical procedure. Of the 59 digits unsuccessfully released, as identified by persistent triggering or locking of the digit, 17 were treated with a second percutaneous release (15 were successful), and 40 underwent open tendon sheath incision as a second procedure (success rate, 100%); triggering persisted in the remaining 2 digits, and these were considered failures (the 2 patients did not pursue further treatment).
There were no complications: infection; nerve, artery, or tendon injury; or chronic pain. Some patients had mild stiffness, swelling, or pain for a few days after the procedure, and these effects typically resolved without treatment. In 29 digits, persistent pain or swelling without triggering was successfully treated with a corticosteroid injection.
Discussion
[[{"fid":"202307","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Table 3.","field_file_image_credit[und][0][value]":""}}}]]Over a 10-year period, 596 percutaneous trigger finger releases were sequentially performed by a single surgeon. The 90% success rate compares favorably with rates found in other studies (Table 3).5-9,12-14 The surgeon’s success rates for individual years vary and demonstrate no clear trend or learning curve with the procedure (Figure 3). There were no significant complications. Patient satisfaction with the procedure was high.[[{"fid":"202308","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]
There were no injuries to digital nerves, arteries, or flexor tendons, either early or late, and no reports of infections or long-term pain or loss of motion. Although it is quite probable that in some procedures the longitudinal passes of the 18-gauge needle may have also slightly cut into the flexor tendon after passing through the A1 pulley, the direction of the needle passes was in line with the direction of the collagen fibers of the tendon, and thus any inadvertent superficial abrasion would not have structurally weakened the tendon. Of the 40 digits that underwent open release after incomplete or failed percutaneous release, none showed significant longitudinal lacerations of the superficialis tendon. During these revision surgeries, the typical intraoperative finding was incomplete release of the A1 pulley, usually at the distal end. Although loss of the grating sensation or relief of further triggering symptoms was considered adequate evidence of a successful release in this study, small tendon attachments could remain and potentially could lead to recurrent triggering. Given the high success rate achieved with the large sample, however, these 2 factors are considered appropriate indicators of successful release.
It is unclear why there was a relatively consistent 10% failure rate and why it did not decrease over the 10-year study period. Although the technique used does not have a significant learning curve, it appears that digits are not actively triggering at time of procedure have a higher failure rate. When a patient’s digit is actively triggering, assessment of the success of the procedure is relatively straightforward, whereas when a digit intermittently triggers and locks and is not symptomatic in the office, success cannot be immediately determined.
No specific digit was significantly more prone to failed releases, though the small finger had the lowest success rate (85.7%). Given that only 56.4% of patients experienced triggering on the dominant hand, there is not enough evidence to suggest a significant relationship between likelihood of a trigger digit and a patient’s hand dominance. Similarly, there was no correlation between the duration of symptoms and the success of the percutaneous procedure.
Investigation of the relationship between the previously suggested comorbidities of carpal tunnel syndrome and diabetes was also inconclusive. Only 79 (18%) of 429 patients reported having carpal tunnel syndrome, and even fewer, 56 (13.0%), reported having diabetes. Only 69 of the 596 treated digits reportedly had sustained trauma before developing triggering symptoms, and only 12 of the 69 were unsuccessfully released. In addition, of the 161 digits in which one or more steroid injections failed to resolve triggering symptoms, 158 (87.3%) were successfully released with 1 percutaneous procedure. Collectively, these data show percutaneous release can effectively eliminate triggering symptoms in a digit that has sustained injury or that has been unsuccessfully treated with nonoperative methods. Failed percutaneous release subsequently can be reliably treated with an open procedure, and results are excellent.
This study had several limitations. It was retrospective, nonblinded, and did not compare outcomes of percutaneous release with those of an open procedure. Data are presented to support the efficacy and safety of percutaneous release as a treatment option. Another limitation is that pre-release treatment was not controlled. Patients had been treated with a variety of nonoperative methods, including use of anti-inflammatory medication, hand therapy, splinting, and one or more corticosteroid injections, both at our office and elsewhere.
Percutaneous release appears to have an advantage in terms of pain relief, but the study did not evaluate or control for procedure discomfort. However, patients who had been treated with a corticosteroid injection before percutaneous release consistently refused corticosteroid injections for subsequent trigger digits, citing the dramatic pain reduction achieved with release relative to injection. Similarly, all patients who had a trigger digit treated with open tendon sheath incision in the past indicated a strong preference for the percutaneous release.
Follow-up on this patient population was inconsistent and incomplete. Many patients did not return, presumably because they considered the procedure a success and thought follow-up was unnecessary. However, some patients may have had a recurrence or an incomplete release and gone elsewhere for treatment.
The results of this study, to date the largest study on percutaneous release of trigger finger, provide more evidence of the safety and efficacy of this procedure as a treatment option. The success rate of percutaneous release is high, surpasses that of nonoperative treatments such as steroid injections, and approaches that of open and endoscopic surgical alternatives. Some of the obvious advantages of percutaneous release are less visible scarring, fewer incision-related complications, and shorter rehabilitation.10 In addition, post-procedure pain is possibly reduced, symptom relief is comparable, operative time is significantly shorter,8 and percutaneous release is easily performed in the office setting.
Percutaneous release is a viable treatment option for stenosing flexor tenosynovitis, regardless of previously used nonoperative treatment methods, duration or severity of symptoms, or trigger digit treated.
1. Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1(2):92-96.
2. Fahey JJ, Bollinger JA. Trigger-finger in adults and children. J Bone Joint Surg Am. 1954;36(6):1200-1218.
3. Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722-727.
4. Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20(1):109-114.
5. Habbu R, Putman MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
6. Pandey BK, Sharma S, Manandhar RR, Pradhan RL, Lakhey S, Rijal KP. Percutaneous trigger finger release. Nepal Orthop Assoc J. 2010;1(1):1-5.
7. Sato ES, Gomes dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51(1):93-99.
8. Dierks U, Hoffmann R, Meek MF. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008;12(3):183-187.
9. Tanaka J. Percutaneous trigger finger release. Tech Hand Up Extrem Surg. 1999;3(1):52-57.
10. Pegoli L, Cavalli E, Cortese P, Parolo C, Pajardi G. A comparison of endoscopic and open trigger finger release. Hand Surg. 2008;13(3):147-151.
11. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31(1):135-146.
12. Schramm JM, Nguyen M, Wongworawat MD. The safety of percutaneous trigger finger release. Hand. 2008;3(1):44-46.
13. Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand. 2009;4(1):35-37.
14. Cihantimur B, Akin S, Ozcan M. Percutaneous treatment of trigger finger. 34 fingers followed 0.5-2 years. Acta Orthop Scand. 1998;69(2):167-168.
1. Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1(2):92-96.
2. Fahey JJ, Bollinger JA. Trigger-finger in adults and children. J Bone Joint Surg Am. 1954;36(6):1200-1218.
3. Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(4):722-727.
4. Chammas M, Bousquet P, Renard E, Poirier JL, Jaffiol C, Allieu Y. Dupuytren’s disease, carpal tunnel syndrome, trigger finger, and diabetes mellitus. J Hand Surg Am. 1995;20(1):109-114.
5. Habbu R, Putman MD, Adams JE. Percutaneous release of the A1 pulley: a cadaver study. J Hand Surg Am. 2012;37(11):2273-2277.
6. Pandey BK, Sharma S, Manandhar RR, Pradhan RL, Lakhey S, Rijal KP. Percutaneous trigger finger release. Nepal Orthop Assoc J. 2010;1(1):1-5.
7. Sato ES, Gomes dos Santos JB, Belloti JC, Albertoni WM, Faloppa F. Treatment of trigger finger: randomized clinical trial comparing the methods of corticosteroid injection, percutaneous release and open surgery. Rheumatology. 2012;51(1):93-99.
8. Dierks U, Hoffmann R, Meek MF. Open versus percutaneous release of the A1-pulley for stenosing tendovaginitis: a prospective randomized trial. Tech Hand Up Extrem Surg. 2008;12(3):183-187.
9. Tanaka J. Percutaneous trigger finger release. Tech Hand Up Extrem Surg. 1999;3(1):52-57.
10. Pegoli L, Cavalli E, Cortese P, Parolo C, Pajardi G. A comparison of endoscopic and open trigger finger release. Hand Surg. 2008;13(3):147-151.
11. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31(1):135-146.
12. Schramm JM, Nguyen M, Wongworawat MD. The safety of percutaneous trigger finger release. Hand. 2008;3(1):44-46.
13. Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand. 2009;4(1):35-37.
14. Cihantimur B, Akin S, Ozcan M. Percutaneous treatment of trigger finger. 34 fingers followed 0.5-2 years. Acta Orthop Scand. 1998;69(2):167-168.