User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Postpartum Treatment of Metastatic Recurrent Giant Cell Tumor of Capitate Bone of Wrist
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
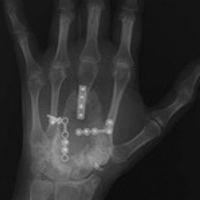
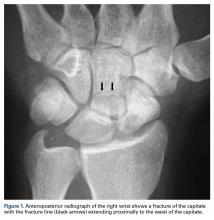
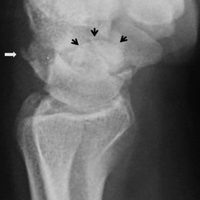
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
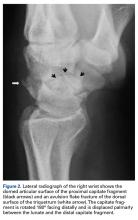
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
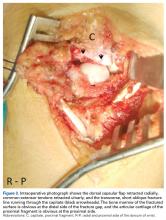
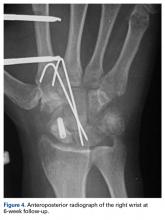
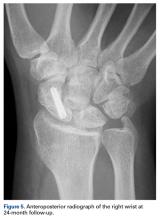
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
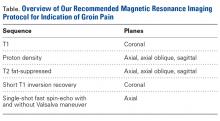
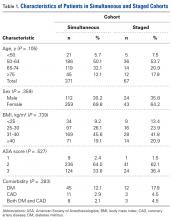
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
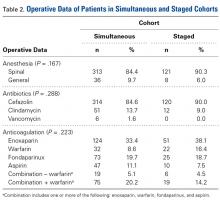
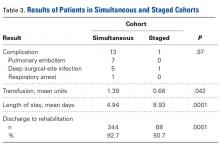
Patient Preference Before and After Arthroscopic Rotator Cuff Repair: Which Is More Important, Pain Relief or Strength Return?
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Pain relief and return of strength are important satisfaction variables for patients undergoing ARCR.
- Pain relief and strength return are equally desirable in the majority (50%) of the patients before and after ARCR.
- Overall, patient preference for strength return dominates pain relief in long-term.
- Increasing age is associated with a stronger preference for pain relief.
- Improved understanding of patient expectations after ARCR will promote meaningful changes in patient satisfaction.
A rotator cuff tear (RCT) can cause significant pain, weakness, stiffness, and loss of function in the shoulder. In most patients, arthroscopic rotator cuff repair (ARCR) provides significant and reproducible pain relief and variable return of shoulder strength and function.1-4 ARCR outcomes are well described and well represented by validated outcome measures.5-9 However, these outcomes do not always correlate with patient satisfaction. For example, after ARCR, 2 patients with similar outcome scores may have different satisfaction levels.
Patient satisfaction involves multiple factors and varies with the patient’s preoperative expectations and the degree to which the surgery matches the patient’s desired outcomes.10-15 In clinical studies, Tashjian and colleagues,10 Henn and colleagues,11 and O’Holleran and colleagues12 found patient satisfaction correlated most highly with postoperative shoulder pain, shoulder function, general health status, and outcome scores. However, our understanding of patients’ desired outcomes and expectations of ARCR is limited, particularly regarding the importance of pain relief and strength return relative to each other. We believe patients’ preoperative expectations are influenced by their self-assessments of symptom severity and by their understanding of the outcomes of surgical procedures and of the information they receive from their surgeons during preoperative evaluation.
We conducted an observational study to determine patients’ preoperative preferences and the importance of post-ARCR pain relief and strength return relative to each other. After surgery, preferences and ratings of pain relief and strength return were reevaluated to determine if they were altered by outcomes. We also studied the influence of multiple factors, including severity of preoperative symptoms (pain, weakness), age, sex, occupation, and active sports involvement, on patients’ preoperative ratings of the importance of post-ARCR improvements in pain relief and strength return. We hypothesized that patients would vary in how they preoperatively value and desire post-ARCR pain relief and strength return.
Materials and Methods
The simple shoulder questionnaire (Figure) designed for this study had 12 items. Patients subjectively assessed the severity of their symptoms (pain level, shoulder weakness) and rated the importance of both pain relief and strength return to their occupational and personal life.
Before patients underwent surgery for symptomatic suspected RCTs, they were approached to participate in this prospective study. Sixty-five patients provided informed consent on forms approved by an Institutional Review Board. Inclusion criteria were suspected unilateral rotator cuff pathology and willingness to participate. Of the 65 patients, 60 underwent ARCR without another procedure, such as shoulder instability repair, SLAP (superior labrum anterior-to-posterior) repair, or distal clavicle excision; the other 5 patients elected nonoperative treatment and were excluded from review. At a mean (SD) follow-up of 5.2 (0.2) years, the 60 patients who had surgery completed the questionnaire again and rated the importance of pain relief and strength return relative to each other.
Patients with RCTs were divided according to age, sex, shoulder dominance, occupation type, and active sports involvement. Standard definitions for occupation types were used: blue-collar, manual labor jobs; white-collar, salaried/educated positions; and retired.
Matched-pairs t tests were used to compare preoperative and postoperative continuous variables (strength return preference, pain relief preference, SPD). One-way analysis of variance (ANOVA) was used to compare categorical variables (sex, shoulder dominance, active sports involvement) with continuous variables (SPD), and bivariate regression was used to compare groups with continuous data (age, SPD). In cases involving more than 2 groups (occupation types), the Tukey honestly significant difference (HSD) test was used to evaluate intergroup differences. P < .05 was used for statistical significance.
Results
ARCR Outcomes
After ARCR, there was significant improvement in patient-reported pain and subjective strength scores. Mean (SD) pain score improved from 5.9 (2.3) to 1.3 (2.3) after ARCR (P < .001), and mean (SD) strength improved from 46% (22%) of normal to 84% (17%) of normal (P < .001).
Importance of Post-ARCR Pain Relief and Strength Return
Analysis of preoperative questionnaire responses
revealed that, of 60 patients, 29 (48.3%) considered pain relief and strength return equally important, 20 (33.3%) valued postoperative strength return was more important, and 11 patients (18.3%) rated pain relief was more important than strength return. After a mean (SD) follow-up of 5.2 (0.2) years, 33 patients (55 %) valued pain relief and strength return as equally important, 17 patients (28.3%) preferred a strength recovery, and 10 patients (16.7%) preferred pain relief.
Overall patient ratings were significantly higher for strength return compared to pain relief before surgery, mean (SD), 9.2 (2.1) and 8.6 (2.3) (P = .02), and afterward, 8.9 (1.9) and 8.2 (3.1) (P = .03) (Table 1).
Subgroup Analyses
Sex and Age. Of the 60 patients, 43 were male and 17 female. Mean (SD) preoperative SPD was 1.0 (2.7) for males and 0.7 (2.3) females; the difference was not significant (P = .61). After surgery, females emphasized strength return over pain relief more than males did: Mean (SD) SPD was significantly higher (P = .04) for females, 1.7 (3.0), than for males, 0.4 (2.5). There were no preoperative–postoperative differences (P = .33) for males or females (Table 2).
Hand Dominance. RCT was found in the dominant shoulder of 31 patients (52%). Shoulder dominance did not affect SPD: Mean (SD) preoperative SPD was 1.3 (2.3) for dominant shoulders and 0.5 (2.7) for nondominant shoulders (P = .21), and postoperative SPD was 0.7 (2.6) for dominant and 0.9 (2.8) for nondominant (P = .79). SPD did not change from before surgery to after surgery for dominant (P = .14) or nondominant (P = .28) shoulders (Table 2).
Active Sports Participation. Thirty-two patients (53%) reported preoperative involvement in sports; 35 (58%) reported postoperative involvement (P = .37). Mean (SD) preoperative SPD was 1.4 (3.0) for involved patients and 0.3 (1.7) for uninvolved patients (P = .09), and postoperative SPD was 0.6 (2.8) for involved patients and 1.0 (2.6) for uninvolved patients (P = .53). SPD did not change from before surgery to after surgery for involved (P = .17) or uninvolved (P = .26) patients (Table 2).
Occupation Type. There were 9 blue-collar workers (15%), 32 white-collar workers (53%), and 19 retirees (32%). Mean (SD) preoperative SPD was 2.8 (4.2) for blue-collar workers, 1.2 (2.1) for white-collar workers, and –0.4 (0.4) for retirees. There were no significant differences in preoperative SPD between blue-collar and white-collar workers (P = .19) or between white-collar workers and retirees (P = .06), but there was a significant difference between blue-collar workers and retirees (P = .004). Mean (SD) postoperative SPD was 1.3 (2.7) for blue-collar workers, 1.2 (3.1) for white-collar workers, and –0.3 (1.6) for retirees. There were no significant differences between blue-collar and white-collar workers (P = .99), white-collar workers and retirees (P = .13), or blue-collar workers and retirees (P = .3).
Discussion
In this study, we wanted to determine patients’ pre- and postoperative preferences for pain relief and strength return after ARCR. Preoperative and postoperative preference analysis of the 60 patients who underwent ARCR revealed that the majority valued pain relief and strength return equally. However, overall, there was higher ratings for strength return in long term after ARCR, irrespective of age, sex, preoperative levels of shoulder pain and weakness, and preoperative and postoperative sports involvement.
Patients’ preoperative expectations are a function of their assessment of their symptoms, their perceptions of expected surgical outcomes, and their understanding of preoperative discussion with their surgeons. In this study, patients self-assessed their shoulder symptoms and their effect on their occupational and personal life. They also rated the importance of post-ARCR pain relief and strength return relative to each other. To assess whether surgical outcomes affected perceptions of pain relief and strength return, patients completed the questionnaire before and after surgery. Overall, patients rated postoperative strength return over pain relief on long-term (5 years).
Subgroup analysis revealed a weak positive correlation between patient-reported preoperative pain scores and ratings of the importance of pain relief after surgery, but there was no correlation between postoperative pain scores and ratings of the importance of pain relief after surgery. This finding was surprising because we thought pain relief would be more important than strength return for patients with higher pain scores.1-3,16-21 We would like to clarify a point about this study: That patients preferred strength return over pain relief does not mean they did not care about pain relief. A substantial subset of patients (~50%) valued pain relief and strength return equally. In rotator cuff pathology, pain and weakness are to an extent interrelated. Shoulder pain that limits a patient’s ability to perform a strenuous task can be perceived as shoulder weakness, which may explain why, despite having higher pain scores, patients preferred strength return over pain relief. Increasing age showed a positive correlation with preference for pain relief, which explains the finding that retirees preferred pain relief over strength return. We used SPD to express the preference for strength return over pain relief before and after ARCR. Unfortunately, SPD may not be used to quantitatively define the preference for strength return over pain relief.
Patient satisfaction after RCR involves multiple factors and has been well studied. In a retrospective analysis of 112 patients, Tashjian and colleagues10 found that patient satisfaction was affected by preoperative expectations, marital status, disability status, preoperative pain function, and general health status after RCR. They also found a positive but weak correlation between patient satisfaction and functional outcome scores, including visual analog scale (VAS), Simple Shoulder Test (SST), and Disabilities of the Arm, Shoulder, and Hand (DASH) scores. Henn and colleagues11 evaluated 125 patients who underwent primary RCR for a chronic RCT. Higher preoperative expectations correlated with better postoperative VAS, SST, DASH, and Short Form 36 performance, irrespective of worker compensation status, symptom duration, number of patient comorbidities, tear size, repair technique, and number of previous operations. In a prospective cohort analysis of 311 RCR patients, O’Holleran and colleagues12 found that decreased patient satisfaction was associated with postoperative pain and dysfunction. Furthermore, willingness to recommend surgery to another person was significantly related to patient satisfaction. In the present study, we did not correlate preoperative expectations with postoperative outcome scores or evaluate the effect of other known factors on RCR outcomes. Our main goal was to understand ARCR patients’ preoperative and postoperative evaluations of the importance of pain relief and strength return relative to each other. Improved understanding of patients’ expectations will allow us to identify disparities between expectations and outcomes.
Our study had several limitations. First, our questionnaire was not validated. However, we used it only as an assessment tool, to collect data, and do not propose using it to assess ARCR outcomes. Second, objective strength measurements were not performed, before or after surgery, and therefore patients’ perceptions of weakness were not tested. Third, we did not correlate preoperative or postoperative shoulder outcome scores with patients’ expectations. Our intention was to understand how ARCR patients rate the importance of pain relief and strength return relative to each other. Fourth, we did not correlate patients’ expectations of strength return and pain relief with preoperative tear size or postoperative retear status.
Our observational study results showed that, before undergoing ARCR, most patients valued postoperative pain relief and strength return equally. However, there was an overall preference for strength return over pain relief. Furthermore, this preference held up irrespective of age, sex, sports involvement, or preoperative symptom severity. These findings add to our understanding of patients’ preoperative expectations of ARCR.
Am J Orthop. 2017;46(4):E244-E250. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
1. Cole BJ, McCarty LP 3rd, Kang RW, Alford W, Lewis PB, Hayden JK. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg. 2007;16(5):579-585.
2. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
3. Wilson F, Hinov V, Adams G. Arthroscopic repair of full-thickness tears of the rotator cuff: 2- to 14-year follow-up. Arthroscopy. 2002;18(2):136-144.
4. Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012;28(11):1587-1591.
5. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143-149.
7. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
8. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587-594.
9. Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med. 1996;24(4):472-476.
10. Tashjian RZ, Bradley MP, Tocci S, Rey J, Henn RF, Green A. Factors influencing patient satisfaction after rotator cuff repair. J Shoulder Elbow Surg. 2007;16(6):752-758.
11. Henn RF 3rd, Kang L, Tashjian RZ, Green A. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89(9):1913-1919.
12. O’Holleran JD, Kocher MS, Horan MP, Briggs KK, Hawkins RJ. Determinants of patient satisfaction with outcome after rotator cuff surgery. J Bone Joint Surg Am. 2005;87(1):121-126.
13. Namdari S, Donegan RP, Chamberlain AM, Galatz LM, Yamaguchi K, Keener JD. Factors affecting outcome after structural failure of repaired rotator cuff tears. J Bone Joint Surg Am. 2014;96(2):99-105.
14. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
15. Sonnabend DH, Watson EM. Structural factors affecting the outcome of rotator cuff repair. J Shoulder Elbow Surg. 2002;11(3):212-218.
16. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953-960.
18. DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16(6):759-765.
19. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
20. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
21. Romeo AA, Hang DW, Bach BR Jr, Shott S. Repair of full thickness rotator cuff tears. Gender, age, and other factors affecting outcome. Clin Orthop Relat Res. 1999;(367):243-255.
Percutaneous Trigger Finger Release
Comparison of Anterior and Posterior Corticosteroid Injections for Pain Relief and Functional Improvement in Shoulder Impingement Syndrome
Take-Home Points
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months
CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used.
Clinical response to CSI may not depend on injection accuracy.
Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Shoulder pain, a common clinical problem, occurs in 7% to 34% of the general population and in 21% of people older than 70 years.1Subacromial impingement refers to shoulder pain resulting from mechanical impingement of the rotator cuff underneath the coracoacromial arch between the acromion and the humeral head.2,3 Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain, resulting in significant functional deficits and disability.3
Treatment options for SIS include conservative modalities such as use of nonsteroidal anti-inflammatory drugs, physical therapy (PT), and subacromial corticosteroid injections (CSIs). Studies have found short- and long-term improvement in pain, function, and range of motion after CSI.4-8 Subacromial CSI can be administered through an anterior or a posterior route.4,9 There have been several studies of the accuracy of anterior and posterior CSIs,10-12 with 2 studies finding similar accuracy for these routes.10,11 However, there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12
Although the accuracy of anterior and posterior routes has been studied, their effect on clinical outcomes has not. We conducted a study to understand the effects of anterior and posterior CSIs on SIS. As one of the accuracy studies suggested anterior CSI is more accurate—the anterior route was theorized to provide easier access to the subacromial space12—we hypothesized patients treated with anterior CSI would have superior clinical outcomes 6 months after injection.12,13
Materials and Methods
Study Participants and Randomization
After this study received Institutional Review Board approval, patients with shoulder pain of more than 3 months’ duration and consistent with SIS were screened for inclusion. Eligible patients had pain in the anterior biceps and over the top of the shoulder with overhead activities as well as one or more clinical findings on physical examination: Hawkins-Kennedy sign, Neer sign, painful arc, and infraspinatus pain (pain with external rotation).
Patients were excluded if their history included prior subacromial CSI, adhesive capsulitis (inability to passively abduct shoulder to 90° with scapular stabilization), calcific tendonitis, radiographic evidence of os acromiale, cervical radiculopathy, Spurling sign, neck pain, radiating arm pain or numbness, sensory deficits, or neck and upper extremity motor dysfunction. Also excluded were patients with full-thickness rotator cuff tear, weakness on arm elevation, positive "drop arm sign," or high-riding humerus on standing shoulder radiograph. Patients who had work-related injuries or who were involved in worker compensation were excluded as well.
Enrolled patients were randomly assigned (with use of a computer-based random number generator) to receive either anterior CSI or posterior CSI.
Injection Procedures
All patients were administered 5 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone by 2 board-certified orthopedic surgeons using a 22-gauge 1½-inch needle. For patients who received their subacromial CSI by the anterior route, the arm was held in 0° of abduction and 20° of external rotation. The needle was inserted medial to the humeral head, lateral to the coracoid process, beginning 1 cm inferior to the clavicle with the needle directed posteriorly and laterally toward the acromion.10 For patients who received their CSI by the posterior route, the arm was held in 0° of abduction, the posterolateral corner of the acromion was identified by palpation, and the needle was inserted 1 cm inferior and medial to this point with the needle directed anteriorly and laterally toward the acromion.10,12 In both groups, the subacromial space was identified when a drop in pressure was felt during needle insertion. Accuracy was assessed post hoc by asking patients to grade their response to the injection on a visual analog scale (VAS); VAS score was used as a surrogate for improvement. All patients had a positive Neer test: Pain decreased with impingement maneuvers immediately after injection.
All patients were referred for PT provided according to an evidence-based rehabilitation protocol.14 This program emphasized range of motion with shoulder shrugs, scapular retraction, and pendulum exercises; flexibility with stretching exercises targeting the anterior and posterior aspects of the shoulder and cane stretching for forward elevation and external rotation; and strength with strengthening exercises involving the rotator cuff and scapular stabilizers.
Outcome Measures
Pain was measured with VAS scores and function with Single Assessment Numeric Evaluation (SANE) scores. The VAS is a validated outcome measure of pain intensity. A numeric version of the VAS was used: Patients selected the whole number, from 0 (no pain) to 10 (worst possible pain), that best reflected their pain intensity. On SANE, another validated outcome measure, patients rated their shoulder function as a percentage of normal, from 0% (no function possible) to 100% (perfect).15 Before injection, all patients were administered the VAS and SANE questionnaires to establish their baseline pain level and opinion of shoulder function. These measures were repeated 1, 3, and 6 months after injection. Telephone interviews were conducted at 1 month and 6 months. Patients were asked to return to clinic 3 months after injection as part of the standard of care. At 3 months, 47 (86%) of the 55 patients returned for follow-up and were administered the VAS and SANE questionnaires; the other 8 completed the questionnaires by telephone. At each time point, patients were asked to report on their participation in PT and/or adherence to their home exercise program.
Statistical Analysis
Power analysis performed with Student t test and a 2-sided level of P = .05 compared VAS pain scores between the anterior and posterior injection routes and found a mean (SD) difference of 1.4 (1.7).16 Power calculations made with nQuery Advisor Version 7.0 (Statistical Solutions) indicated a total sample size of 60 patients (30/group) would provide 80% power for detecting a significant difference assuming a 20% dropout rate.
Two-way mixed-model analysis of variance (ANOVA) was used to compare the anterior and posterior routes for statistical differences in both VAS pain scores and SANE function scores at baseline and 1, 3, and 6 months after injection. Likewise, time at baseline (just before injection)was compared with follow-up (1, 3, 6 months) with 2-way mixed-model ANOVA adjusting for anterior or posterior route. Multivariate analysis was performed to evaluate differences between baseline and 6-month follow-up with respect to anterior and posterior injection routes, controlling for age, sex, and body mass index (BMI) for VAS and SANE scores. Parametric testing methods were used for statistical analysis, which was performed with IBM SPSS Statistics Version 21.0 (IBM Corp). Significance was set at P < .05.
Results
Patient Characteristics
Of the 55 patients enrolled, 25 (46%) received anterior subacromial CSI and 30 (54%) received posterior CSI. All enrolled patients had a positive Neer impingement test immediately after injection. Mean (SD) age was 48 (9.3) years for anterior group patients and 48 (9.0) years for posterior group patients. There was no significant difference in age or BMI between the 2 groups (Table).
Five patients (9%) were excluded from the study after randomization and CSI: 2 for a full-thickness rotator cuff tear, 1 for a Bankart lesion, 1 for adhesive capsulitis, and 1 for a worker compensation claim.
One month after injection, 41 patients (75%) reported having engaged in PT as prescribed. Of the 47 patients (86%) who returned for the 3-month follow-up, 25 (46%) reported having engaged in PT between 1 month and 3 months after injection. Fourteen patients (26%) reported attending PT between 3 and 6 months post-injection.
Outcome Measures
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in VAS scores between the anterior and posterior groups at any time point (P = .45). Both groups had highly significant pain reductions (anterior, F = 9.71, P < .001; posterior, F = 13.46, P < .001). Figure 1 shows mean VAS scores and significant reductions in pain 1, 3, and 6 months after injection (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of pain reduction over time, as indicated by a nonsignificant (P = .50) difference in slopes. These pain score reductions were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in SANE scores between the anterior and posterior groups, except for a higher mean score in the anterior group at 3 months
(P = .02). There were no other group differences (P > .10 for all). Both groups had highly significant improvements in function (anterior, F = 17.34,
P < .001; posterior, F = 13.57, P < .001). Figure 2 shows mean SANE scores and significant improvement at 1, 3, and 6 months (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of improved function over time, as indicated by a nonsignificant (P = .51) difference in slopes. These function score improvements were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
From the results of this prospective randomized study, we concluded subacromial CSI significantly reduces pain and improves function regardless of route used. In addition, age, sex, and BMI do not significantly affect the efficacy of either anterior CSI or posterior CSI.
Discussion
In patients with SIS, anterior CSI and posterior CSI provided significant improvements in pain and function 1, 3, and 6 months after injection. These effects were independent of age, sex, BMI, and PT participation. There were no significant differences in outcomes between injection routes.
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.4-8 Although clinical outcomes are inconsistent, CSI can be used to address SIS symptoms in appropriate patients. Specifically, Blair and colleagues6 found that, CSI consisting of 4 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone was effective in alleviating shoulder pain and improving shoulder range of motion. Other authors have similarly reported improved outcomes after subacromial injection and short-term follow-up with PT.4,7,8 Our findings are consistent with these reports: CSI coupled with a structured rehabilitation program is effective in alleviating symptoms associated with acute or subacute SIS.
Numerous studies have found improved clinical outcomes after anterior CSI and after posterior CSI,6-8 but no study has directly compared the clinical impact of anterior CSI with that of posterior CSI—which suggests injection route may not affect ultimate clinical outcomes.
CSI accuracy has been studied extensively.10-12,17-20 Although 2 studies found similar accuracy for anterior and posterior routes,10,11 there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12 Collectively, these studies expose the inherent difficulty in treating shoulder pain with localized subacromial injection. Therapy may fail because of errant needle positioning. Two prospective studies found improved clinical outcomes with successful delivery of medication into the subacromial space.17,18 Poor clinical outcomes may result from inaccurate CSI.
In contrast to other clinical studies, our study found that injection route was not associated with differences in clinical response. In a prospective randomized clinical trial in which 75 patients received a subacromial injection, Marder and colleagues12 found anterior routes 84% accurate and posterior routes 56% accurate; they concluded acromion anatomy and subacromial bursa anatomy make posterior injections more difficult. As theorized by Gruson and colleagues,13 with use of an anterior route, the needle enters inferior to the concavity of the acromion and provides easier access to the subacromial space. This idea is in line with Marder and colleagues’12 conclusion that subacromial bursa anatomy provides a favorable environment for accurate CSI.
If accuracy is positively correlated with clinical improvement and anterior routes are more accurate, there should be a difference in response to posterior injections. Our results provide evidence that clinical response to CSI may not depend on injection accuracy. Perhaps merely placing the corticosteroid near the bursa is adequate for improving symptoms or perhaps some of the clinical improvement is due to the systemic effect of corticosteroids. These possibilities require further analysis.
Establishing the efficacy of CSI in SIS is difficult. The literature includes various study designs, different CSI indications and medication formulations, and varying emphasis on the role of organized PT. Rehabilitation has been found to alleviate joint pain by reducing inflammation,14 but data do not universally support this finding.21,22 Nevertheless, use of PT might explain the divergence in clinical outcomes reported by Marder and colleagues,12 who found anterior CSI more accurate than posterior CSI. In our practice, PT is recommended for all SIS patients, not only those who have CSI. Thus, our findings are framed within the context of successful CSI but may include patients who improved with PT alone. This issue raises the question of whether subacromial CSI should be guided by ultrasound. Ultrasound guidance can improve CSI accuracy and clinical outcomes,23-25 though the value of this benefit is debated.26
This study had several limitations. First, pain relief was patient reported. Second, the treatment plan involved CSI with PT but did not control for CSI used alone. PT, which is part of the standard of care for patients with SIS, added another degree of complexity to the study. Third, there may have been some variability in SIS severity (stage 1, 2, or 3). Fourth, although the study design controlled for various shoulder pathologies, advanced imaging, which could have provided diagnosis confirmation, was not available for all patients. Therefore, concurrent conditions may have confounded results. However, randomization was used to try to minimize this effect. Fifth, although injection routes were randomly assigned, the trial was not blinded. Sixth, the study was underpowered by 1 patient, as there was an estimated 20% dropout rate over 3 and 6 months of follow-up. However, we do not think our results were significantly affected.
Although more research is needed to fully describe the role of subacromial CSI in SIS, our study findings suggested that CSI using either an anterior or a posterior route creates a window of symptomatic relief in which patients may be able to engage in PT.
Conclusion
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months. No differences were found between anterior and posterior CSIs. In the context of this study, CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used. Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):CD004016.
2. Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59(10):1178-1186.
3. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech. 2003;18(5):369-379.
4. Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23(6):496-500.
5. Bhagra A, Syed H, Reed DA, et al. Efficacy of musculoskeletal injections by primary care providers in the office: a retrospective cohort study. Int J Gen Med. 2013;6:237-243.
6. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78(11):1685-1689.
7. Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment.
J Shoulder Elbow Surg. 2009;18(2):172-177.
8. Yu C, Chen CH, Liu HT, Dai MH, Wang IC, Wang KC. Subacromial injections of corticosteroids and Xylocaine for painful subacromial impingement syndrome. Chang Gung Med J. 2006;29(5):474-478.
9. Codsi MJ. The painful shoulder: when to inject and when to refer. Cleve Clin J Med. 2007;74(7):473-474, 477-478, 480-482 passim.
10. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(15):61S-66S.
12. Marder RA, Kim SH, Labson JD, Hunter JC. Injection of the subacromial bursa in patients with rotator cuff syndrome: a prospective, randomized study comparing the effectiveness of different routes. J Bone Joint Surg Am. 2012;94(16):
1442-1447.
13. Gruson, KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008;17(1 suppl):118S-130S.
14. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160.
15. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
16. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease.
J Shoulder Elbow Surg. 2009;88(6):927-932.
17. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
18. Esenyel CZ, Esenyel M, Yeiltepe R, et al. The correlation between the accuracy of steroid injections and subsequent shoulder pain and function in subacromial impingement
syndrome [in Turkish]. Acta Orthop Traumatol Turc. 2003;37(1):
41-45.
19. Powell SE, Davis SM, Lee EH, et al. Accuracy of palpation-directed intra-articular glenohumeral injection confirmed by magnetic resonance arthrography. Arthroscopy. 2015;31(2):205-208.
20. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
21. Desmeules F, Côté CH, Frémont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: a systematic review. Clin J Sport Med. 2003;13(3):176-182.
22. Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320-1325.
23. Chen MJ, Lew HL, Hsu TC, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
24. Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013;45(12):
2205-2213.
25. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
26. Hall S, Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.
Take-Home Points
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months
CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used.
Clinical response to CSI may not depend on injection accuracy.
Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Shoulder pain, a common clinical problem, occurs in 7% to 34% of the general population and in 21% of people older than 70 years.1Subacromial impingement refers to shoulder pain resulting from mechanical impingement of the rotator cuff underneath the coracoacromial arch between the acromion and the humeral head.2,3 Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain, resulting in significant functional deficits and disability.3
Treatment options for SIS include conservative modalities such as use of nonsteroidal anti-inflammatory drugs, physical therapy (PT), and subacromial corticosteroid injections (CSIs). Studies have found short- and long-term improvement in pain, function, and range of motion after CSI.4-8 Subacromial CSI can be administered through an anterior or a posterior route.4,9 There have been several studies of the accuracy of anterior and posterior CSIs,10-12 with 2 studies finding similar accuracy for these routes.10,11 However, there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12
Although the accuracy of anterior and posterior routes has been studied, their effect on clinical outcomes has not. We conducted a study to understand the effects of anterior and posterior CSIs on SIS. As one of the accuracy studies suggested anterior CSI is more accurate—the anterior route was theorized to provide easier access to the subacromial space12—we hypothesized patients treated with anterior CSI would have superior clinical outcomes 6 months after injection.12,13
Materials and Methods
Study Participants and Randomization
After this study received Institutional Review Board approval, patients with shoulder pain of more than 3 months’ duration and consistent with SIS were screened for inclusion. Eligible patients had pain in the anterior biceps and over the top of the shoulder with overhead activities as well as one or more clinical findings on physical examination: Hawkins-Kennedy sign, Neer sign, painful arc, and infraspinatus pain (pain with external rotation).
Patients were excluded if their history included prior subacromial CSI, adhesive capsulitis (inability to passively abduct shoulder to 90° with scapular stabilization), calcific tendonitis, radiographic evidence of os acromiale, cervical radiculopathy, Spurling sign, neck pain, radiating arm pain or numbness, sensory deficits, or neck and upper extremity motor dysfunction. Also excluded were patients with full-thickness rotator cuff tear, weakness on arm elevation, positive "drop arm sign," or high-riding humerus on standing shoulder radiograph. Patients who had work-related injuries or who were involved in worker compensation were excluded as well.
Enrolled patients were randomly assigned (with use of a computer-based random number generator) to receive either anterior CSI or posterior CSI.
Injection Procedures
All patients were administered 5 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone by 2 board-certified orthopedic surgeons using a 22-gauge 1½-inch needle. For patients who received their subacromial CSI by the anterior route, the arm was held in 0° of abduction and 20° of external rotation. The needle was inserted medial to the humeral head, lateral to the coracoid process, beginning 1 cm inferior to the clavicle with the needle directed posteriorly and laterally toward the acromion.10 For patients who received their CSI by the posterior route, the arm was held in 0° of abduction, the posterolateral corner of the acromion was identified by palpation, and the needle was inserted 1 cm inferior and medial to this point with the needle directed anteriorly and laterally toward the acromion.10,12 In both groups, the subacromial space was identified when a drop in pressure was felt during needle insertion. Accuracy was assessed post hoc by asking patients to grade their response to the injection on a visual analog scale (VAS); VAS score was used as a surrogate for improvement. All patients had a positive Neer test: Pain decreased with impingement maneuvers immediately after injection.
All patients were referred for PT provided according to an evidence-based rehabilitation protocol.14 This program emphasized range of motion with shoulder shrugs, scapular retraction, and pendulum exercises; flexibility with stretching exercises targeting the anterior and posterior aspects of the shoulder and cane stretching for forward elevation and external rotation; and strength with strengthening exercises involving the rotator cuff and scapular stabilizers.
Outcome Measures
Pain was measured with VAS scores and function with Single Assessment Numeric Evaluation (SANE) scores. The VAS is a validated outcome measure of pain intensity. A numeric version of the VAS was used: Patients selected the whole number, from 0 (no pain) to 10 (worst possible pain), that best reflected their pain intensity. On SANE, another validated outcome measure, patients rated their shoulder function as a percentage of normal, from 0% (no function possible) to 100% (perfect).15 Before injection, all patients were administered the VAS and SANE questionnaires to establish their baseline pain level and opinion of shoulder function. These measures were repeated 1, 3, and 6 months after injection. Telephone interviews were conducted at 1 month and 6 months. Patients were asked to return to clinic 3 months after injection as part of the standard of care. At 3 months, 47 (86%) of the 55 patients returned for follow-up and were administered the VAS and SANE questionnaires; the other 8 completed the questionnaires by telephone. At each time point, patients were asked to report on their participation in PT and/or adherence to their home exercise program.
Statistical Analysis
Power analysis performed with Student t test and a 2-sided level of P = .05 compared VAS pain scores between the anterior and posterior injection routes and found a mean (SD) difference of 1.4 (1.7).16 Power calculations made with nQuery Advisor Version 7.0 (Statistical Solutions) indicated a total sample size of 60 patients (30/group) would provide 80% power for detecting a significant difference assuming a 20% dropout rate.
Two-way mixed-model analysis of variance (ANOVA) was used to compare the anterior and posterior routes for statistical differences in both VAS pain scores and SANE function scores at baseline and 1, 3, and 6 months after injection. Likewise, time at baseline (just before injection)was compared with follow-up (1, 3, 6 months) with 2-way mixed-model ANOVA adjusting for anterior or posterior route. Multivariate analysis was performed to evaluate differences between baseline and 6-month follow-up with respect to anterior and posterior injection routes, controlling for age, sex, and body mass index (BMI) for VAS and SANE scores. Parametric testing methods were used for statistical analysis, which was performed with IBM SPSS Statistics Version 21.0 (IBM Corp). Significance was set at P < .05.
Results
Patient Characteristics
Of the 55 patients enrolled, 25 (46%) received anterior subacromial CSI and 30 (54%) received posterior CSI. All enrolled patients had a positive Neer impingement test immediately after injection. Mean (SD) age was 48 (9.3) years for anterior group patients and 48 (9.0) years for posterior group patients. There was no significant difference in age or BMI between the 2 groups (Table).
Five patients (9%) were excluded from the study after randomization and CSI: 2 for a full-thickness rotator cuff tear, 1 for a Bankart lesion, 1 for adhesive capsulitis, and 1 for a worker compensation claim.
One month after injection, 41 patients (75%) reported having engaged in PT as prescribed. Of the 47 patients (86%) who returned for the 3-month follow-up, 25 (46%) reported having engaged in PT between 1 month and 3 months after injection. Fourteen patients (26%) reported attending PT between 3 and 6 months post-injection.
Outcome Measures
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in VAS scores between the anterior and posterior groups at any time point (P = .45). Both groups had highly significant pain reductions (anterior, F = 9.71, P < .001; posterior, F = 13.46, P < .001). Figure 1 shows mean VAS scores and significant reductions in pain 1, 3, and 6 months after injection (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of pain reduction over time, as indicated by a nonsignificant (P = .50) difference in slopes. These pain score reductions were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in SANE scores between the anterior and posterior groups, except for a higher mean score in the anterior group at 3 months
(P = .02). There were no other group differences (P > .10 for all). Both groups had highly significant improvements in function (anterior, F = 17.34,
P < .001; posterior, F = 13.57, P < .001). Figure 2 shows mean SANE scores and significant improvement at 1, 3, and 6 months (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of improved function over time, as indicated by a nonsignificant (P = .51) difference in slopes. These function score improvements were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
From the results of this prospective randomized study, we concluded subacromial CSI significantly reduces pain and improves function regardless of route used. In addition, age, sex, and BMI do not significantly affect the efficacy of either anterior CSI or posterior CSI.
Discussion
In patients with SIS, anterior CSI and posterior CSI provided significant improvements in pain and function 1, 3, and 6 months after injection. These effects were independent of age, sex, BMI, and PT participation. There were no significant differences in outcomes between injection routes.
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.4-8 Although clinical outcomes are inconsistent, CSI can be used to address SIS symptoms in appropriate patients. Specifically, Blair and colleagues6 found that, CSI consisting of 4 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone was effective in alleviating shoulder pain and improving shoulder range of motion. Other authors have similarly reported improved outcomes after subacromial injection and short-term follow-up with PT.4,7,8 Our findings are consistent with these reports: CSI coupled with a structured rehabilitation program is effective in alleviating symptoms associated with acute or subacute SIS.
Numerous studies have found improved clinical outcomes after anterior CSI and after posterior CSI,6-8 but no study has directly compared the clinical impact of anterior CSI with that of posterior CSI—which suggests injection route may not affect ultimate clinical outcomes.
CSI accuracy has been studied extensively.10-12,17-20 Although 2 studies found similar accuracy for anterior and posterior routes,10,11 there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12 Collectively, these studies expose the inherent difficulty in treating shoulder pain with localized subacromial injection. Therapy may fail because of errant needle positioning. Two prospective studies found improved clinical outcomes with successful delivery of medication into the subacromial space.17,18 Poor clinical outcomes may result from inaccurate CSI.
In contrast to other clinical studies, our study found that injection route was not associated with differences in clinical response. In a prospective randomized clinical trial in which 75 patients received a subacromial injection, Marder and colleagues12 found anterior routes 84% accurate and posterior routes 56% accurate; they concluded acromion anatomy and subacromial bursa anatomy make posterior injections more difficult. As theorized by Gruson and colleagues,13 with use of an anterior route, the needle enters inferior to the concavity of the acromion and provides easier access to the subacromial space. This idea is in line with Marder and colleagues’12 conclusion that subacromial bursa anatomy provides a favorable environment for accurate CSI.
If accuracy is positively correlated with clinical improvement and anterior routes are more accurate, there should be a difference in response to posterior injections. Our results provide evidence that clinical response to CSI may not depend on injection accuracy. Perhaps merely placing the corticosteroid near the bursa is adequate for improving symptoms or perhaps some of the clinical improvement is due to the systemic effect of corticosteroids. These possibilities require further analysis.
Establishing the efficacy of CSI in SIS is difficult. The literature includes various study designs, different CSI indications and medication formulations, and varying emphasis on the role of organized PT. Rehabilitation has been found to alleviate joint pain by reducing inflammation,14 but data do not universally support this finding.21,22 Nevertheless, use of PT might explain the divergence in clinical outcomes reported by Marder and colleagues,12 who found anterior CSI more accurate than posterior CSI. In our practice, PT is recommended for all SIS patients, not only those who have CSI. Thus, our findings are framed within the context of successful CSI but may include patients who improved with PT alone. This issue raises the question of whether subacromial CSI should be guided by ultrasound. Ultrasound guidance can improve CSI accuracy and clinical outcomes,23-25 though the value of this benefit is debated.26
This study had several limitations. First, pain relief was patient reported. Second, the treatment plan involved CSI with PT but did not control for CSI used alone. PT, which is part of the standard of care for patients with SIS, added another degree of complexity to the study. Third, there may have been some variability in SIS severity (stage 1, 2, or 3). Fourth, although the study design controlled for various shoulder pathologies, advanced imaging, which could have provided diagnosis confirmation, was not available for all patients. Therefore, concurrent conditions may have confounded results. However, randomization was used to try to minimize this effect. Fifth, although injection routes were randomly assigned, the trial was not blinded. Sixth, the study was underpowered by 1 patient, as there was an estimated 20% dropout rate over 3 and 6 months of follow-up. However, we do not think our results were significantly affected.
Although more research is needed to fully describe the role of subacromial CSI in SIS, our study findings suggested that CSI using either an anterior or a posterior route creates a window of symptomatic relief in which patients may be able to engage in PT.
Conclusion
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months. No differences were found between anterior and posterior CSIs. In the context of this study, CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used. Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Take-Home Points
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months
CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used.
Clinical response to CSI may not depend on injection accuracy.
Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
Shoulder pain, a common clinical problem, occurs in 7% to 34% of the general population and in 21% of people older than 70 years.1Subacromial impingement refers to shoulder pain resulting from mechanical impingement of the rotator cuff underneath the coracoacromial arch between the acromion and the humeral head.2,3 Subacromial impingement syndrome (SIS) is the most common cause of shoulder pain, resulting in significant functional deficits and disability.3
Treatment options for SIS include conservative modalities such as use of nonsteroidal anti-inflammatory drugs, physical therapy (PT), and subacromial corticosteroid injections (CSIs). Studies have found short- and long-term improvement in pain, function, and range of motion after CSI.4-8 Subacromial CSI can be administered through an anterior or a posterior route.4,9 There have been several studies of the accuracy of anterior and posterior CSIs,10-12 with 2 studies finding similar accuracy for these routes.10,11 However, there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12
Although the accuracy of anterior and posterior routes has been studied, their effect on clinical outcomes has not. We conducted a study to understand the effects of anterior and posterior CSIs on SIS. As one of the accuracy studies suggested anterior CSI is more accurate—the anterior route was theorized to provide easier access to the subacromial space12—we hypothesized patients treated with anterior CSI would have superior clinical outcomes 6 months after injection.12,13
Materials and Methods
Study Participants and Randomization
After this study received Institutional Review Board approval, patients with shoulder pain of more than 3 months’ duration and consistent with SIS were screened for inclusion. Eligible patients had pain in the anterior biceps and over the top of the shoulder with overhead activities as well as one or more clinical findings on physical examination: Hawkins-Kennedy sign, Neer sign, painful arc, and infraspinatus pain (pain with external rotation).
Patients were excluded if their history included prior subacromial CSI, adhesive capsulitis (inability to passively abduct shoulder to 90° with scapular stabilization), calcific tendonitis, radiographic evidence of os acromiale, cervical radiculopathy, Spurling sign, neck pain, radiating arm pain or numbness, sensory deficits, or neck and upper extremity motor dysfunction. Also excluded were patients with full-thickness rotator cuff tear, weakness on arm elevation, positive "drop arm sign," or high-riding humerus on standing shoulder radiograph. Patients who had work-related injuries or who were involved in worker compensation were excluded as well.
Enrolled patients were randomly assigned (with use of a computer-based random number generator) to receive either anterior CSI or posterior CSI.
Injection Procedures
All patients were administered 5 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone by 2 board-certified orthopedic surgeons using a 22-gauge 1½-inch needle. For patients who received their subacromial CSI by the anterior route, the arm was held in 0° of abduction and 20° of external rotation. The needle was inserted medial to the humeral head, lateral to the coracoid process, beginning 1 cm inferior to the clavicle with the needle directed posteriorly and laterally toward the acromion.10 For patients who received their CSI by the posterior route, the arm was held in 0° of abduction, the posterolateral corner of the acromion was identified by palpation, and the needle was inserted 1 cm inferior and medial to this point with the needle directed anteriorly and laterally toward the acromion.10,12 In both groups, the subacromial space was identified when a drop in pressure was felt during needle insertion. Accuracy was assessed post hoc by asking patients to grade their response to the injection on a visual analog scale (VAS); VAS score was used as a surrogate for improvement. All patients had a positive Neer test: Pain decreased with impingement maneuvers immediately after injection.
All patients were referred for PT provided according to an evidence-based rehabilitation protocol.14 This program emphasized range of motion with shoulder shrugs, scapular retraction, and pendulum exercises; flexibility with stretching exercises targeting the anterior and posterior aspects of the shoulder and cane stretching for forward elevation and external rotation; and strength with strengthening exercises involving the rotator cuff and scapular stabilizers.
Outcome Measures
Pain was measured with VAS scores and function with Single Assessment Numeric Evaluation (SANE) scores. The VAS is a validated outcome measure of pain intensity. A numeric version of the VAS was used: Patients selected the whole number, from 0 (no pain) to 10 (worst possible pain), that best reflected their pain intensity. On SANE, another validated outcome measure, patients rated their shoulder function as a percentage of normal, from 0% (no function possible) to 100% (perfect).15 Before injection, all patients were administered the VAS and SANE questionnaires to establish their baseline pain level and opinion of shoulder function. These measures were repeated 1, 3, and 6 months after injection. Telephone interviews were conducted at 1 month and 6 months. Patients were asked to return to clinic 3 months after injection as part of the standard of care. At 3 months, 47 (86%) of the 55 patients returned for follow-up and were administered the VAS and SANE questionnaires; the other 8 completed the questionnaires by telephone. At each time point, patients were asked to report on their participation in PT and/or adherence to their home exercise program.
Statistical Analysis
Power analysis performed with Student t test and a 2-sided level of P = .05 compared VAS pain scores between the anterior and posterior injection routes and found a mean (SD) difference of 1.4 (1.7).16 Power calculations made with nQuery Advisor Version 7.0 (Statistical Solutions) indicated a total sample size of 60 patients (30/group) would provide 80% power for detecting a significant difference assuming a 20% dropout rate.
Two-way mixed-model analysis of variance (ANOVA) was used to compare the anterior and posterior routes for statistical differences in both VAS pain scores and SANE function scores at baseline and 1, 3, and 6 months after injection. Likewise, time at baseline (just before injection)was compared with follow-up (1, 3, 6 months) with 2-way mixed-model ANOVA adjusting for anterior or posterior route. Multivariate analysis was performed to evaluate differences between baseline and 6-month follow-up with respect to anterior and posterior injection routes, controlling for age, sex, and body mass index (BMI) for VAS and SANE scores. Parametric testing methods were used for statistical analysis, which was performed with IBM SPSS Statistics Version 21.0 (IBM Corp). Significance was set at P < .05.
Results
Patient Characteristics
Of the 55 patients enrolled, 25 (46%) received anterior subacromial CSI and 30 (54%) received posterior CSI. All enrolled patients had a positive Neer impingement test immediately after injection. Mean (SD) age was 48 (9.3) years for anterior group patients and 48 (9.0) years for posterior group patients. There was no significant difference in age or BMI between the 2 groups (Table).
Five patients (9%) were excluded from the study after randomization and CSI: 2 for a full-thickness rotator cuff tear, 1 for a Bankart lesion, 1 for adhesive capsulitis, and 1 for a worker compensation claim.
One month after injection, 41 patients (75%) reported having engaged in PT as prescribed. Of the 47 patients (86%) who returned for the 3-month follow-up, 25 (46%) reported having engaged in PT between 1 month and 3 months after injection. Fourteen patients (26%) reported attending PT between 3 and 6 months post-injection.
Outcome Measures
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in VAS scores between the anterior and posterior groups at any time point (P = .45). Both groups had highly significant pain reductions (anterior, F = 9.71, P < .001; posterior, F = 13.46, P < .001). Figure 1 shows mean VAS scores and significant reductions in pain 1, 3, and 6 months after injection (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of pain reduction over time, as indicated by a nonsignificant (P = .50) difference in slopes. These pain score reductions were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
Two-way repeated-measures ANOVA with age, sex, and BMI included as covariates revealed no significant differences in SANE scores between the anterior and posterior groups, except for a higher mean score in the anterior group at 3 months
(P = .02). There were no other group differences (P > .10 for all). Both groups had highly significant improvements in function (anterior, F = 17.34,
P < .001; posterior, F = 13.57, P < .001). Figure 2 shows mean SANE scores and significant improvement at 1, 3, and 6 months (see asterisks for anterior and posterior groups; P < .001 for all). The groups had parallel rates of improved function over time, as indicated by a nonsignificant (P = .51) difference in slopes. These function score improvements were significant for both injection routes and were independent of age, sex, and BMI (P > .05 for all).
From the results of this prospective randomized study, we concluded subacromial CSI significantly reduces pain and improves function regardless of route used. In addition, age, sex, and BMI do not significantly affect the efficacy of either anterior CSI or posterior CSI.
Discussion
In patients with SIS, anterior CSI and posterior CSI provided significant improvements in pain and function 1, 3, and 6 months after injection. These effects were independent of age, sex, BMI, and PT participation. There were no significant differences in outcomes between injection routes.
When conservative treatments for SIS do not resolve symptoms, inflammation and pain can be reduced with use of subacromial CSI.4-8 Although clinical outcomes are inconsistent, CSI can be used to address SIS symptoms in appropriate patients. Specifically, Blair and colleagues6 found that, CSI consisting of 4 mL of lidocaine 1% (without epinephrine) and 2 mL (80 mg) of triamcinolone was effective in alleviating shoulder pain and improving shoulder range of motion. Other authors have similarly reported improved outcomes after subacromial injection and short-term follow-up with PT.4,7,8 Our findings are consistent with these reports: CSI coupled with a structured rehabilitation program is effective in alleviating symptoms associated with acute or subacute SIS.
Numerous studies have found improved clinical outcomes after anterior CSI and after posterior CSI,6-8 but no study has directly compared the clinical impact of anterior CSI with that of posterior CSI—which suggests injection route may not affect ultimate clinical outcomes.
CSI accuracy has been studied extensively.10-12,17-20 Although 2 studies found similar accuracy for anterior and posterior routes,10,11 there may be a sex difference: In women, a posterior route may be less accurate than an anterior or a lateral route.12 Collectively, these studies expose the inherent difficulty in treating shoulder pain with localized subacromial injection. Therapy may fail because of errant needle positioning. Two prospective studies found improved clinical outcomes with successful delivery of medication into the subacromial space.17,18 Poor clinical outcomes may result from inaccurate CSI.
In contrast to other clinical studies, our study found that injection route was not associated with differences in clinical response. In a prospective randomized clinical trial in which 75 patients received a subacromial injection, Marder and colleagues12 found anterior routes 84% accurate and posterior routes 56% accurate; they concluded acromion anatomy and subacromial bursa anatomy make posterior injections more difficult. As theorized by Gruson and colleagues,13 with use of an anterior route, the needle enters inferior to the concavity of the acromion and provides easier access to the subacromial space. This idea is in line with Marder and colleagues’12 conclusion that subacromial bursa anatomy provides a favorable environment for accurate CSI.
If accuracy is positively correlated with clinical improvement and anterior routes are more accurate, there should be a difference in response to posterior injections. Our results provide evidence that clinical response to CSI may not depend on injection accuracy. Perhaps merely placing the corticosteroid near the bursa is adequate for improving symptoms or perhaps some of the clinical improvement is due to the systemic effect of corticosteroids. These possibilities require further analysis.
Establishing the efficacy of CSI in SIS is difficult. The literature includes various study designs, different CSI indications and medication formulations, and varying emphasis on the role of organized PT. Rehabilitation has been found to alleviate joint pain by reducing inflammation,14 but data do not universally support this finding.21,22 Nevertheless, use of PT might explain the divergence in clinical outcomes reported by Marder and colleagues,12 who found anterior CSI more accurate than posterior CSI. In our practice, PT is recommended for all SIS patients, not only those who have CSI. Thus, our findings are framed within the context of successful CSI but may include patients who improved with PT alone. This issue raises the question of whether subacromial CSI should be guided by ultrasound. Ultrasound guidance can improve CSI accuracy and clinical outcomes,23-25 though the value of this benefit is debated.26
This study had several limitations. First, pain relief was patient reported. Second, the treatment plan involved CSI with PT but did not control for CSI used alone. PT, which is part of the standard of care for patients with SIS, added another degree of complexity to the study. Third, there may have been some variability in SIS severity (stage 1, 2, or 3). Fourth, although the study design controlled for various shoulder pathologies, advanced imaging, which could have provided diagnosis confirmation, was not available for all patients. Therefore, concurrent conditions may have confounded results. However, randomization was used to try to minimize this effect. Fifth, although injection routes were randomly assigned, the trial was not blinded. Sixth, the study was underpowered by 1 patient, as there was an estimated 20% dropout rate over 3 and 6 months of follow-up. However, we do not think our results were significantly affected.
Although more research is needed to fully describe the role of subacromial CSI in SIS, our study findings suggested that CSI using either an anterior or a posterior route creates a window of symptomatic relief in which patients may be able to engage in PT.
Conclusion
Both anterior CSI and posterior CSI significantly improved pain and function for up to 6 months. No differences were found between anterior and posterior CSIs. In the context of this study, CSI combined with structured PT produced significant improvement in pain and function in patients with SIS, regardless of injection route used. Clinicians should rely on their clinical acumen when selecting injection routes, as anterior and posterior are both beneficial.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):CD004016.
2. Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59(10):1178-1186.
3. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech. 2003;18(5):369-379.
4. Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23(6):496-500.
5. Bhagra A, Syed H, Reed DA, et al. Efficacy of musculoskeletal injections by primary care providers in the office: a retrospective cohort study. Int J Gen Med. 2013;6:237-243.
6. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78(11):1685-1689.
7. Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment.
J Shoulder Elbow Surg. 2009;18(2):172-177.
8. Yu C, Chen CH, Liu HT, Dai MH, Wang IC, Wang KC. Subacromial injections of corticosteroids and Xylocaine for painful subacromial impingement syndrome. Chang Gung Med J. 2006;29(5):474-478.
9. Codsi MJ. The painful shoulder: when to inject and when to refer. Cleve Clin J Med. 2007;74(7):473-474, 477-478, 480-482 passim.
10. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(15):61S-66S.
12. Marder RA, Kim SH, Labson JD, Hunter JC. Injection of the subacromial bursa in patients with rotator cuff syndrome: a prospective, randomized study comparing the effectiveness of different routes. J Bone Joint Surg Am. 2012;94(16):
1442-1447.
13. Gruson, KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008;17(1 suppl):118S-130S.
14. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160.
15. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
16. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease.
J Shoulder Elbow Surg. 2009;88(6):927-932.
17. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
18. Esenyel CZ, Esenyel M, Yeiltepe R, et al. The correlation between the accuracy of steroid injections and subsequent shoulder pain and function in subacromial impingement
syndrome [in Turkish]. Acta Orthop Traumatol Turc. 2003;37(1):
41-45.
19. Powell SE, Davis SM, Lee EH, et al. Accuracy of palpation-directed intra-articular glenohumeral injection confirmed by magnetic resonance arthrography. Arthroscopy. 2015;31(2):205-208.
20. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
21. Desmeules F, Côté CH, Frémont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: a systematic review. Clin J Sport Med. 2003;13(3):176-182.
22. Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320-1325.
23. Chen MJ, Lew HL, Hsu TC, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
24. Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013;45(12):
2205-2213.
25. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
26. Hall S, Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003;(1):CD004016.
2. Bell AD, Conaway D. Corticosteroid injections for painful shoulders. Int J Clin Pract. 2005;59(10):1178-1186.
3. Michener LA, McClure PW, Karduna AR. Anatomical and biomechanical mechanisms of subacromial impingement syndrome. Clin Biomech. 2003;18(5):369-379.
4. Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23(6):496-500.
5. Bhagra A, Syed H, Reed DA, et al. Efficacy of musculoskeletal injections by primary care providers in the office: a retrospective cohort study. Int J Gen Med. 2013;6:237-243.
6. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am. 1996;78(11):1685-1689.
7. Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of nonoperative treatment.
J Shoulder Elbow Surg. 2009;18(2):172-177.
8. Yu C, Chen CH, Liu HT, Dai MH, Wang IC, Wang KC. Subacromial injections of corticosteroids and Xylocaine for painful subacromial impingement syndrome. Chang Gung Med J. 2006;29(5):474-478.
9. Codsi MJ. The painful shoulder: when to inject and when to refer. Cleve Clin J Med. 2007;74(7):473-474, 477-478, 480-482 passim.
10. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(15):61S-66S.
12. Marder RA, Kim SH, Labson JD, Hunter JC. Injection of the subacromial bursa in patients with rotator cuff syndrome: a prospective, randomized study comparing the effectiveness of different routes. J Bone Joint Surg Am. 2012;94(16):
1442-1447.
13. Gruson, KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg. 2008;17(1 suppl):118S-130S.
14. Kuhn JE. Exercise in the treatment of rotator cuff impingement: a systematic review and a synthesized evidence-based rehabilitation protocol. J Shoulder Elbow Surg. 2009;18(1):138-160.
15. Williams GN, Gangel TJ, Arciero RA, Uhorchak JM, Taylor DC. Comparison of the Single Assessment Numeric Evaluation method and two shoulder rating scales. Outcomes measures after shoulder surgery. Am J Sports Med. 1999;27(2):214-221.
16. Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease.
J Shoulder Elbow Surg. 2009;88(6):927-932.
17. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
18. Esenyel CZ, Esenyel M, Yeiltepe R, et al. The correlation between the accuracy of steroid injections and subsequent shoulder pain and function in subacromial impingement
syndrome [in Turkish]. Acta Orthop Traumatol Turc. 2003;37(1):
41-45.
19. Powell SE, Davis SM, Lee EH, et al. Accuracy of palpation-directed intra-articular glenohumeral injection confirmed by magnetic resonance arthrography. Arthroscopy. 2015;31(2):205-208.
20. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
21. Desmeules F, Côté CH, Frémont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: a systematic review. Clin J Sport Med. 2003;13(3):176-182.
22. Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-de Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: randomised, single blind study. BMJ. 1997;314(7090):1320-1325.
23. Chen MJ, Lew HL, Hsu TC, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil. 2006;85(1):31-35.
24. Hsieh LF, Hsu WC, Lin YJ, Wu SH, Chang KC, Chang HL. Is ultrasound-guided injection more effective in chronic subacromial bursitis? Med Sci Sports Exerc. 2013;45(12):
2205-2213.
25. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
26. Hall S, Buchbinder R. Do imaging methods that guide needle placement improve outcome? Ann Rheum Dis. 2004;63(9):1007-1008.
Referral Patterns for Chronic Groin Pain and Athletic Pubalgia/Sports Hernia: Magnetic Resonance Imaging Findings, Treatment, and Outcomes
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
The past 3 decades have seen an evolution in the understanding, diagnosis, and treatment of groin pain, both chronic and acute, in athletes and non-athletes alike. Groin pain and groin injury are common. Most cases are transient, with patients returning to their activities within weeks or months. There has also been increasing awareness of a definitive population of patients who do not get better, or who improve and plateau before reaching preinjury level of performance.1-3 Several authors have brought more attention to the injury, introducing vocabulary, theories, diagnostic testing, and diagnoses, which now constitute a knowledge base.1,3-5
As stated in almost every article on groin pain and diagnosis, lack of cohesive agreement and vocabulary, and consistent protocols and procedures, has abounded, making general understanding and agreement in this area inconsistent.1,6-8In this article, members of a tertiary-care group specializing in chronic groin pain, athletic pubalgia (sports hernia), and inguinal herniorrhaphy outline their clinical examination, diagnostic algorithm, imaging protocol, treatment strategy, and outcomes for a population of patients referred by physicians and allied health professionals for a suspected diagnosis of athletic pubalgia.
Background
The pubic symphysis acts as a stabilizing central anchor with elaborate involvement of the anterior structures, including the rectus abdominis, adductor longus, and inguinal ligaments.3,7,9 Literature from Europe, Australia, and the United States has described groin pain, mostly in professional athletes, involving these pubic structures and attachments. Several publications have been addressing chronic groin pain, and each has its own diagnostic algorithm, imaging protocol, and treatment strategy.3,6,9-18
Terminology specific to groin pain in athletes is not new, and has a varied history dating to the early 20th century. Terms such as sportsman hernia19 and subsequently sports hernia20, have recently been embraced by the lay population. In 1999, Gibbon21 described shearing of the common adductor–rectus abdominis anatomical and functional unit and referenced a 1902 anatomical text that describes vertical ligamentous fibers contiguous with rectus sheath and adductor muscles, both attaching to the pubis. Injury to this region is the basis of pubalgia, a term originally used in 1984 by Brunet to describe a pain syndrome at the pubis.22
Many authors have proposed replacing sports hernia with athletic pubalgia.1,3,6,7,10,14,18,23 These terms refer to a group of musculoskeletal processes that occur in and around the pubic symphysis and that share similar mechanisms of injury and common clinical manifestations. The condition was originally described in high-performance athletes, and at one point the term sports hernia was reserved for this patient population.5 According to many authors, presence of an inguinal hernia excludes the diagnosis.1,2,5Magnetic resonance imaging (MRI) has helped to advance and define our understanding of the injury.10 As the history of the literature suggests, earlier concepts of chronic pain focused either on the medial aspect of the inguinal canal and its structures or on the pubic attachments. Many specialists in the area have concluded that the chronic groin pain injury can and often does embody both elements.3,9 Correlation with MRI findings, injury seen during surgical procedures, and cadaveric studies have directed our understanding to a structure, the pre-pubic aponeurotic complex (P-PAC), or rectus aponeurotic plate.12,24,25 Anatomically, the P-PAC, which has several fascial components, attaches posteriorly to the pubic bone and, to a degree, the pubic symphyseal cartilaginous disc. Major contributions to the P-PAC are fibers from the rectus abdominis tendon, the medial aspect of the transversalis and internal oblique muscles (the conjoint tendon, according to some), the inguinal ligament, and the adductor longus tendon.26When communicating with referring physicians, we use the term athletic pubalgia to indicate a specific injury. The athletic pubalgia injury can be defined as serial microtearing,1 or complete tearing, of the posterior attachment of the P-PAC off the anterior pubis.3,10 Complete tearing or displacement can occur unilaterally or across the midline to the other side. As athletic pubalgia is a specific anatomical injury rather than a broad category of findings, an additional pathologic diagnosis, such as inguinal hernia, does not exclude the diagnosis of athletic pubalgia. Unfortunately, the terms sports hernia and sportsman hernia, commonly used in the media and in professional communities, have largely confused the broader understanding of nuances and of the differences between the specific injuries and MRI findings.18
Our Experience
In our practice, we see groin pain patients referred by internists, physiatrists, physical therapists, trainers, general surgeons, urologists, gynecologists, and orthopedic surgeons. In many cases, patients have been through several consultations and work-ups, as their pain syndrome does not fall under a specific category. Patients without inguinal hernia, hip injury, urologic, or gynecologic issues typically are referred to a physiatrist or a physical therapist. Often, there are marginal improvements with physical therapy, but in some cases the injury never completely resolves, and the patient continues to have pain with activity or return to sports.
Most of our patients are nonprofessional athletes, men and women who range widely in age and participate casually or regularly in sporting events. Most lack the rigorous training, conditioning, and close supervision that professional athletes receive. Many other patients are nonprofessional but elite athletes who train 7 days a week for marathons, ultramarathons, triathlons, obstacle course races (“mudders”), and similar events.
Work-Up
A single algorithm is used for all patients initially referred to the surgeon’s office for pelvic or groin pain. The initial interview directs attention to injury onset and mechanism, duration of rest or physical therapy after surgery, pain quality and pain levels, and antagonistic movements and positions. Examination starts with assessment for inguinal, femoral, and umbilical hernias. Resisted sit-up, leg-raise, adduction, and hip assessment tests are performed. The P-PAC is examined with a maneuver similar to the one used for inguinal hernia, as it allows for better assessment of the transversalis fascia (over the direct space) to determine if the inguinal canal floor is attenuated and bulges forward with the Valsalva maneuver. Then, the lateral aspect of the rectus muscle is assessed for pain, usually with the head raised to contract the muscle, to determine tenderness along the lateral border. The rectus edge is traced down to the pubis at its attachment, the superolateral border of the P-PAC. Examination proceeds medially, over the rectus attachment, toward the pubic symphysis, continuing the assessment for tenderness. Laterally, the conjoint tendon and inguinal ligament medial attachments are assessed at the level of the pubic tubercle, which represents the lateral border of the P-PAC. Finally, the examination continues to the inferior border with assessment of the adductor longus attachment, which is best performed with the leg in an adducted position. In the acute or semiacute setting (pain within 1 year of injury onset), tenderness is often elicited. With long-standing injuries, pain is often not elicited, but the patient experiences pain along this axis during activity or afterward.
Patients with positive history and physical examination findings proceed through an MRI protocol designed to detect pathology of the pubic symphysis, hips, and inguinal canals (Figures 1A-1D).
Treatment
Patients who report sustaining an acute groin injury within the previous 6 months are treated nonoperatively. A combination of rest, nonsteroidal anti-inflammatory drugs, and physical therapy is generally recommended.2,10 In cases of failed nonoperative management, patients are evaluated for surgery. No single operation is recommended for all patients.1,6,14,27,28 (Larson26 recently reviewed results from several trials involving a variety of surgical repairs and found return-to-sports rates ranging from 80% to 100%.) Findings from the physical examination and from the properly protocolled MRI examination are used in planning surgery to correct any pathology that could be contributing to symptoms or destabilization of the structures attaching to the pubis. Disruption of the P-PAC from the pubis would be repaired, for example. Additional injuries, such as partial or complete detachment of the conjoint tendon or inguinal ligament, may be repaired as well. If the transversalis fascia is attenuated and bulging forward, the inguinal floor is closed. Adductor longus tendon pathology is addressed, most commonly with partial tendinolysis. Often, concomitant inguinal hernias are found, and these may be repaired in open fashion while other maneuvers are being performed, or laparoscopically.
Materials and Methods
After receiving study approval from our Institutional Review Board, we retrospectively searched for all MRIs performed by our radiology department between March 1, 2011 and March 31, 2013 on patients referred for an indication of groin pain, sports hernia, or athletic pubalgia. Patients were excluded if they were younger than 18 years any time during their care. Some patients previously or subsequently underwent computed tomography or ultrasonography. MRIs were reviewed and positive findings were compiled in a database. Charts were reviewed to identify which patients in the dataset underwent surgery, after MRI, to address their presenting chief complaint. Surgery date and procedure(s) performed were recorded. Patients were interviewed by telephone as part of the in-office postoperative follow-up.
Results
One hundred nineteen MRIs were performed on 117 patients (97 men, 83%). Mean age was 39.8 years. Seventy-nine patients (68%) had an MRI finding of athletic pubalgia, 67 (57%) had an acetabular labral tear in one or both hip joints, and 41 (35%) had a true inguinal hernia. Concomitant findings were common: 47 cases of athletic pubalgia and labral tear(s), 28 cases of athletic pubalgia and inguinal hernia, and 15 cases of all 3 (athletic pubalgia, labral tear, inguinal hernia).
Use of breath-hold axial single-shot fast spin-echo sequences with and without the Valsalva maneuver increased sensitivity in detecting pathologies—inguinal hernia and Gilmore groin in particular. On 24 of the 119 MRIs, the Valsalva maneuver either revealed the finding or made it significantly more apparent.
Of all patients referred for MRI for chronic groin pain, 48 (41%) subsequently underwent surgery. In 29 surgeries, the rectus abdominis, adductor longus, and/or pre-pubic aponeurotic plate were repaired; in 13 cases, herniorrhaphy was performed as well; in 2 cases, masses involving the spermatic cord were removed.
The most common surgery (30 cases) was herniorrhaphy, which was performed as a single procedure, multiple procedures, or in combination with procedures not related to a true hernia. Eighteen patients underwent surgery only for hernia repair.
Of the 79 patients with MRI-positive athletic pubalgia, 39 subsequently underwent surgery, and 31 (79%) of these were followed up by telephone. Mean duration of rest after surgery was 6.2 weeks. Twelve patients (39%) had physical therapy after surgery, some as early as 4 weeks, and some have continued their therapy since surgery. Of the 31 patients who were followed up after surgery, 23 (74%) resumed previous activity levels. Return to previous activity level took these patients a mean of 17.9 weeks. When asked if outcomes satisfied their expectations, 28 patients (90%) said yes, and 3 said no.
Forty patients with MRI-positive athletic pubalgia were nonoperatively treated, and 28 (70%) of these patients were followed up. In this group, mean duration of rest after surgery was 6.9 weeks. Thirteen patients (46%) participated in physical therapy, for a mean duration of 10.8 weeks. Of the patients followed up, 19 (68%) returned to previous activity level. Twenty-one patients (75%) were satisfied with their outcome.
Discussion
Diagnosis and treatment of chronic groin pain have had a long, confusing, and frustrating history for both patients and the medical professionals who provide them with care.3,6,7,10 Historically, the problem has been, in part, the lack of diagnostic capabilities. Currently, however, pubalgia MRI protocol allows the exact pathology to be demonstrated.3 As already noted, concomitant hip pathology or inguinal hernia is not unusual8; any structural abnormality in the area is a potential destabilizer of the structures attached to the pubis.18 Solving only one of these issues may offer only partial resolution of symptoms and thereby reduce the rate of successful treatment of groin pain.
Diagnostic algorithms are being developed. In addition, nonoperative treatments are being tried for some of the issues. Physicians are giving diagnostic and therapeutic steroid injections in the pubic cleft, along the rectus abdominis/adductor longus complex, or posterior to the P-PAC. Platelet-rich plasma injection therapy has had limited success.29This article provides a snapshot of what a tertiary-care group of physicians specializing in chronic groin pain sees in an unfiltered setting. We think this is instructive for several reasons.
First, many patients in our population have visited a multitude of specialists without receiving a diagnosis or being referred appropriately. Simply, many specialists do not know the next step in treating groin pain and thus do not make the appropriate referral. Until recently, the literature has not been helpful. It has poorly described the constellation of injuries comprising chronic groin pain. More significantly, groin injuries have been presented as ambiguous injuries lacking effective treatment. Over the past decade, however, abundant literature on the correlation of these injuries with specific MRI findings has made the case otherwise.
Second, a specific MRI pubalgia protocol is needed. Inability to make a correct diagnosis, because of improper MRI, continues to add to the confusion surrounding the injury and undoubtedly prolongs the general medical community’s thinking that diagnosis and treatment of chronic groin pain are elusive. Our data support this point in many ways. Although all patients in this study were seen by a medical professional before coming to our office, none had received a diagnosis of occult hernia or attenuated transversalis fascia; nevertheless, we identified inguinal hernia, Gilmore groin, or both in 44% of these patients. These findings are not surprising, as MRI was the crucial link in diagnosis. In addition, the point made by other groin pain specialists—that a hernia precludes a pubalgia diagnosis1,2,5—is not supported by our data. Inguinal hernia can and does exist in conjunction with pubalgia. More than half the patients in our study had a combined diagnosis. We contend that, much as hip labral pathology occurs concomitantly with pubalgia,23 inguinal hernia may be a predisposing factor as well. A defect in the direct or indirect space can destabilize the area and place additional strain on the pubic attachments.
In our experience, the dynamic Valsalva sequence improves detection of true hernias and anterior abdominal wall deficiencies and should be included in each protocol for the evaluation of acute or chronic groin pain.
Shear forces and injury at the pubis can occur outside professional athletics. Our patient population is nonprofessional athletes, teenagers to retirees, and all can develop athletic pubalgia. Ninety percent of surveyed patients who received a diagnosis and were treated surgically were satisfied with their outcomes.
Am J Orthop. 2017;46(4):E251-E256. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
1. Meyers WC, Lanfranco A, Castellanos A. Surgical management of chronic lower abdominal and groin pain in high-performance athletes. Curr Sports Med Rep. 2002;1(5):301-305.
2. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
3. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and “sports hernia”: optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438.
4. Gilmore OJA. Gilmore’s groin: ten years experience of groin disruption—a previously unsolved problem in sportsmen. Sports Med Soft Tissue Trauma. 1991;3:12-14.
5. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
6. Kavanagh EC, Koulouris G, Ford S, McMahon P, Johnson C, Eustace SJ. MR imaging of groin pain in the athlete. Semin Musculoskelet Radiol. 2006;10(3):197-207.
7. Cunningham PM, Brennan D, O’Connell M, MacMahon P, O’Neill P, Eustace S. Patterns of bone and soft-tissue injury at the symphysis pubis in soccer players: observations at MRI. AJR Am J Roentgenol. 2007;188(3):W291-W296.
8. Zoga AC, Kavanagh EC, Omar IM, et al. Athletic pubalgia and the “sports hernia”: MR imaging findings. Radiology. 2008;247(3):797-807.
9. Koulouris G. Imaging review of groin pain in elite athletes: an anatomic approach to imaging findings. AJR Am J Roentgenol. 2008;191(4):962-972.
10. Albers SL, Spritzer CE, Garrett WE Jr, Meyers WC. MR findings in athletes with pubalgia. Skeletal Radiol. 2001;30(5):270-277.
11. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167.
12. Robinson P, Salehi F, Grainger A, et al. Cadaveric and MRI study of the musculotendinous contributions to the capsule of the symphysis pubis. AJR Am J Roentgenol. 2007;188(5):W440-W445.
13. Schilders E, Talbot JC, Robinson P, Dimitrakopoulou A, Gibbon WW, Bismil Q. Adductor-related groin pain in recreational athletes. J Bone Joint Surg Am. 2009;91(10):2455-2460.
14. Davies AG, Clarke AW, Gilmore J, Wotherspoon M, Connell DA. Review: imaging of groin pain in the athlete. Skeletal Radiol. 2010;39(7):629-644.
15. Mullens FE, Zoga AC, Morrison WB, Meyers WC. Review of MRI technique and imaging findings in athletic pubalgia and the “sports hernia.” Eur J Radiol. 2012;81(12):3780-3792.
16. Zoga AC, Meyers WC. Magnetic resonance imaging for pain after surgical treatment for athletic pubalgia and the “sports hernia.” Semin Musculoskelet Radiol. 2011;15(4):372-382.
17. Beer E. Periostitis of symphysis and descending rami of pubes following suprapubic operations. Int J Med Surg. 1924;37(5):224-225.
18. MacMahon PJ, Hogan BA, Shelly MJ, Eustace SJ, Kavanagh EC. Imaging of groin pain. Magn Reson Imaging Clin N Am. 2009;17(4):655-666.
19. Malycha P, Lovell G. Inguinal surgery in athletes with chronic groin pain: the ‘sportsman’s’ hernia. Aust N Z J Surg. 1992;62(2):123-125.
20. Hackney RG. The sports hernia: a cause of chronic groin pain. Br J Sports Med. 1993;27(1):58-62.
21. Gibbon WW. Groin pain in athletes. Lancet. 1999;353(9162):1444-1445.
22. Brunet B, Brunet-Geudj E, Genety J. La pubalgie: syndrome “fourre-tout” pur une plus grande riguer diagnostique et therapeutique. Intantanes Medicaux. 1984;55:25-30.
23. Lischuk AW, Dorantes TM, Wong W, Haims AH. Imaging of sports-related hip and groin injuries. Sports Health. 2010;2(3):252-261.
24. Gibbon WW, Hession PR. Diseases of the pubis and pubic symphysis: MR imaging appearances. AJR Am J Roentgenol. 1997;169(3):849-853.
25. Gamble JG, Simmons SC, Freedman M. The symphysis pubis. Anatomic and pathologic considerations. Clin Orthop Relat Res. 1986;(203):261-272.
26. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
27. Maffulli N, Loppini M, Longo UG, Denaro V. Bilateral mini-invasive adductor tenotomy for the management of chronic unilateral adductor longus tendinopathy in athletes. Am J Sports Med. 2012;40(8):1880-1886.
28. Schilders E, Dimitrakopoulou A, Cooke M, Bismil Q, Cooke C. Effectiveness of a selective partial adductor release for chronic adductor-related groin pain in professional athletes. Am J Sports Med. 2013;41(3):603-607.
29. Scholten PM, Massimi S, Dahmen N, Diamond J, Wyss J. Successful treatment of athletic pubalgia in a lacrosse player with ultrasound-guided needle tenotomy and platelet-rich plasma injection: a case report. PM R. 2015;7(1):79-83.
Severity Weighting of Postoperative Adverse Events in Orthopedic Surgery
Take-Home Points
- Studies of AEs after orthopedic surgery commonly use composite AE outcomes.
- These types of outcomes treat AEs with different clinical significance similarly.
- This study created a single severity-weighted outcome that can be used to characterize the overall severity of a given patient’s postoperative course.
- Future studies may benefit from using this new severity-weighted outcome score.
Recently there has been an increase in the use of national databases for orthopedic surgery research.1-4 Studies commonly compare rates of postoperative adverse events (AEs) across different demographic, comorbidity, and procedural characteristics.5-23 Their conclusions often highlight different modifiable and/or nonmodifiable risk factors associated with the occurrence of postoperative events.
The several dozen AEs that have been investigated range from very severe (eg, death, myocardial infarction, coma) to less severe (eg, urinary tract infection [UTI], anemia requiring blood transfusion). A common approach for these studies is to consider many AEs together in the same analysis, asking a question such as, “What are risk factors for the occurrence of ‘adverse events’ after spine surgery?” Such studies test for associations with the occurrence of “any adverse event,” the occurrence of any “serious adverse event,” or similar composite outcomes. How common this type of study has become is indicated by the fact that in 2013 and 2014, at least 12 such studies were published in Clinical Orthopaedics and Related Research and the Journal of Bone and Joint Surgery,5-14,21-23 and many more in other orthopedic journals.15-20 However, there is a problem in using this type of composite outcome to perform such analyses: AEs with highly varying degrees of severity have identical impacts on the outcome variable, changing it from negative (“no adverse event”) to positive (“at least one adverse event”). As a result, the system may treat a very severe AE such as death and a very minor AE such as UTI similarly. Even in studies that use the slightly more specific composite outcome of “serious adverse events,” death and a nonlethal thromboembolic event would be treated similarly. Failure to differentiate these AEs in terms of their clinical significance detracts from the clinical applicability of conclusions drawn from studies using these types of composite AE outcomes.
In one of many examples that can be considered, a retrospective cohort study compared general and spinal anesthesia used in total knee arthroplasty.10 The rate of any AEs was higher with general anesthesia than with spinal anesthesia (12.34% vs 10.72%; P = .003). However, the only 2 specific AEs that had statistically significant differences were anemia requiring blood transfusion (6.07% vs 5.02%; P = .009) and superficial surgical-site infection (SSI; 0.92% vs 0.68%; P < .001). These 2 AEs are of relatively low severity; nevertheless, because these AEs are common, their differences constituted the majority of the difference in the rate of any AEs. In contrast, differences in the more severe AEs, such as death (0.11% vs 0.22%; P > .05), septic shock (0.14% vs 0.12%; P > .05), and myocardial infarction (0.20% vs 0.20%; P > .05), were small and not statistically significant. Had more weight been given to these more severe events, the outcome of the study likely would have been “no difference.”
To address this shortcoming in orthopedic research methodology, we created a severity-weighted outcome score that can be used to determine the overall “severity” of any given patient’s postoperative course. We also tested this novel outcome score for correlation with procedure type and patient characteristics using orthopedic patients from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP). Our intention is for database investigators to be able to use this outcome score in place of the composite outcomes that are dominating this type of research.
Methods
Generation of Severity Weights
Our method is described generally as utility weighting, assigning value weights reflective of overall impact to differing outcome states.24 Parallel methods have been used to generate the disability weights used to determine disability-adjusted life years for the Global Burden of Disease project25 and many other areas of health, economic, and policy research.
All orthopedic faculty members at 2 geographically disparate, large US academic institutions were invited to participate in a severity-weighting exercise. Each surgeon who agreed to participate performed the exercise independently.
- STEP 1: Please reorder the AE cards by your perception of “severity” for a patient experiencing that event after an orthopedic procedure.
- STEP 2: Once your cards are in order, please determine how many postoperative occurrences of each event you would “trade” for 1 patient experiencing postoperative death. Place this number of occurrences in the box in the upper right corner of each card.
- NOTES: As you consider each AE:
- Please consider an “average” occurrence of that AE, but note that in no case does the AE result in perioperative death.
- Please consider only the “severity” for the patient. (Do not consider the extent to which the event may be related to surgical error.)
- Please consider that the numbers you assign are relative to each other. Hence, if you would trade 20 of “event A” for 1 death, and if you would trade 40 of “event B” for 1 death, the implication is that you would trade 20 of “event A” for 40 of “event B.”
- You may readjust the order of your cards at any point.
Participants’ responses were recorded. For each number provided by each participant, the inverse (reciprocal) was taken and multiplied by 100%. This new number was taken to be the percentage severity of death that the given participant considered the given AE to embody. For example, as a hypothetical on one end of the spectrum, if a participant reported 1 (he/she would trade 1 AE X for 1 death), then the severity would be 1/1 × 100% = 100% of death, a very severe AE. Conversely, if a participant reported a very large number like 100,000 (he/she would trade 100,000 AEs X for 1 death), then the severity would be 1/100,000 × 100% = 0.001% of death, a very minor AE. More commonly, a participant will report a number like 25, which would translate to 4% of death (1/25 × 100% = 4%). For each AE, weights were then averaged across participants to derive a mean severity weight to be used to generate a novel composite outcome score.
Definition of Novel Composite Outcome Score
The novel composite outcome score would be expressed as a percentage to be interpreted as percentage severity of death, which we termed severity-weighted outcome relative to death (SWORD). For each patient, SWORD was defined as no AE (0%) or postoperative death (100%), with other AEs assigned mean severity weights based on faculty members’ survey responses. A patient with multiple AEs would be assigned the weight for the more severe AE. This method was chosen over summing the AE weights because in many cases the AEs were thought to overlap; hence, summing would be inappropriate. For example, generally a deep SSI would result in a return to the operating room, and one would not want to double-count this AE. Similarly, it would not make sense for a patient who died of a complication to have a SWORD of >100%, which would be the summing result.
Application to ACS-NSQIP Patients
ACS-NSQIP is a surgical registry that prospectively identifies patients undergoing major surgery at any of >500 institutions nationwide.26,27 Patients are characterized at baseline and are followed for AEs over the first 30 postoperative days.
First, mean SWORD was calculated and reported for patients undergoing each of the 8 procedures. Analysis of variance (ANOVA) was used to test for associations of mean SWORD with type of procedure both before and after multivariate adjustment for demographics (sex; age in years, <40, 40-49, 50-59, 60-69, 70-79, 80-89, ≥90) and comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, exertional dyspnea, end-stage renal disease, congestive heart failure).
Second, patients undergoing the procedure with the highest mean SWORD (hip fracture surgery) were examined in depth. Among only these patients, multivariate ANOVA was used to test for associations of mean SWORD with the same demographics and comorbidities.
All statistical tests were 2-tailed. Significance was set at α = 0.05 (P < .05).
All 23 institution A faculty members (100%) and 24 (89%) of the 27 institution B faculty members completed the exercise.
In the ACS-NSQIP database, 85,109 patients were identified on the basis of the initial inclusion criteria.
Results
Figure 1 shows mean severity weights and standard errors generated from faculty responses. Mean (standard error) severity weight for UTI was 0.23% (0.08%); blood transfusion, 0.28% (0.09%); pneumonia, 0.55% (0.15%); hospital readmission, 0.59% (0.23%); wound dehiscence, 0.64% (0.17%); deep vein thrombosis, 0.64% (0.19%); superficial SSI, 0.68% (0.23%); return to operating room, 0.91% (0.29%); progressive renal insufficiency, 0.93% (0.27%); graft/prosthesis/flap failure, 1.20% (0.34%); unplanned intubation, 1.38% (0.53%); deep SSI, 1.45% (0.38%); failure to wean from ventilator, 1.45% (0.48%); organ/space SSI, 1.76% (0.46%); sepsis without shock, 1.77% (0.42%); peripheral nerve injury, 1.83% (0.47%); pulmonary embolism, 2.99% (0.76%); acute renal failure, 3.95% (0.85%); myocardial infarction, 4.16% (0.98%); septic shock, 7.17% (1.36%); stroke, 8.73% (1.74%); cardiac arrest requiring cardiopulmonary resuscitation, 9.97% (2.46%); and coma, 15.14% (3.04%).
Among ACS-NSQIP patients, mean SWORD ranged from 0.2% (elective anterior cervical decompression and fusion) to 6.0% (hip fracture surgery) (Figure 2).
Discussion
The use of national databases in studies has become increasingly common in orthopedic surgery.1-4
The academic orthopedic surgeons who participated in our severity-weighting exercise thought the various AEs have markedly different severities. The least severe AE (UTI) was considered 0.23% as severe as postoperative death, with other events spanning the range up to 15.14% as severe as death. This wide range of severities demonstrates the problem with composite outcomes that implicitly consider all AEs similarly severe. Use of these markedly disparate weights in the development of SWORD enables this outcome to be more clinically applicable than outcomes such as “any adverse events.”
SWORD was highly associated with procedure type both before and after adjustment for demographics and comorbidities. Among patients undergoing the highest SWORD procedure (hip fracture surgery), SWORD was also associated with age, sex, and 4 of 6 tested comorbidities. Together, our findings show how SWORD is intended to be used in studies: to identify demographic, comorbidity, and procedural risk factors for an adverse postoperative course. We propose that researchers use our weighted outcome as their primary outcome—it is more meaningful than the simpler composite outcomes commonly used.
Outside orthopedic surgery, a small series of studies has addressed severity weighting of postoperative AEs.25,28-30 However, their approach was very different, as they were not designed to generate weights that could be transferred to future studies; rather, they simply compared severities of postoperative courses for patients within each individual study. In each study, a review of each original patient record was required, as the severity of each patient’s postoperative course was characterized according to the degree of any postoperative intervention—from no intervention to minor interventions such as placement of an intravenous catheter and major interventions such as endoscopic, radiologic, and surgical procedures. Only after the degree of intervention was defined could an outcome score be assigned to a given patient. However, databases do not depict the degree of intervention with nearly enough detail for this type of approach; they typically identify only occurrence or nonoccurrence of each event. Our work, which arose independently from this body of literature, enables an entirely different type of analysis. SWORD, which is not based on degree of intervention but on perceived severity of an “average” event, enables direct application of severity weights to large databases that store simple information on occurrence and nonoccurrence of specific AEs.
This study had several limitations. Most significantly, the generated severity weights were based on the surgeons’ subjective perceptions of severity, not on definitive assessments of the impacts of specific AEs on actual patients. We did not query the specialists who treat the complications or who present data on the costs and disabilities that may arise from these AEs. In addition, to develop our severity weighting scale, we queried faculty at only 2 institutions. A survey of surgeons throughout the United States would be more representative and would minimize selection bias. This is a potential research area. Another limitation is that scoring was subjective, based on surgeons’ perceptions of patients—in contrast to the Global Burden of Disease project, in which severity was based more objectively on epidemiologic data from >150 countries.
Orthopedic database research itself has often-noted limitations, including inability to sufficiently control for confounders, potential inaccuracies in data coding, limited follow-up, and lack of orthopedic-specific outcomes.1-4,31-33 However, this research also has much to offer, has increased tremendously over the past several years, and is expected to continue to expand. Many of the limitations of database studies cannot be entirely reversed. In providing a system for weighting postoperative AEs, our study fills a methodologic void. Future studies in orthopedics may benefit from using the severity-weighted outcome score presented here. Other fields with growth in database research may consider using similar methods to create severity-weighting systems of their own.
Am J Orthop. 2017;46(4):E235-E243. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
2. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193.
3. Bohl DD, Grauer JN, Leopold SS. Editor’s spotlight/Take 5: Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671.
4. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: “Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198.
5. Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387-1394.
6. Edelstein AI, Lovecchio FC, Saha S, Hsu WK, Kim JY. Impact of resident involvement on orthopaedic surgery outcomes: an analysis of 30,628 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2014;96(15):e131.
7. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
8. Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes S. Thirty-day morbidity after single-level anterior cervical discectomy and fusion: identification of risk factors and emphasis on the safety of outpatient procedures. J Bone Joint Surg Am. 2014;96(15):1288-1294.
9. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10.
10. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
11. Odum SM, Springer BD. In-hospital complication rates and associated factors after simultaneous bilateral versus unilateral total knee arthroplasty. J Bone Joint Surg Am. 2014;96(13):1058-1065.
12. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191.
13. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
14. Mednick RE, Alvi HM, Krishnan V, Lovecchio F, Manning DW. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Joint Surg Am. 2014;96(14):1201-1209.
15. Pugely AJ, Martin CT, Gao Y, Ilgenfritz R, Weinstein SL. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine. 2014;39(15):1225-1234.
16. Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924.
17. Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025-2030.
18. Bohl DD, Fu MC, Golinvaux NS, Basques BA, Gruskay JA, Grauer JN. The “July effect” in primary total hip and knee arthroplasty: analysis of 21,434 cases from the ACS-NSQIP database. J Arthroplasty. 2014;29(7):1332-1338.
19. Bohl DD, Fu MC, Gruskay JA, Basques BA, Golinvaux NS, Grauer JN. “July effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(7):603-611.
20. Babu R, Thomas S, Hazzard MA, et al. Morbidity, mortality, and health care costs for patients undergoing spine surgery following the ACGME resident duty-hour reform: clinical article. J Neurosurg Spine. 2014;21(4):502-515.
21. Lovecchio F, Beal M, Kwasny M, Manning D. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472(11):3570-3575.
22. Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300.
23. Easterlin MC, Chang DG, Talamini M, Chang DC. Older age increases short-term surgical complications after primary knee arthroplasty. Clin Orthop Relat Res. 2013;471(8):2611-2620.
24. Morimoto T, Fukui T. Utilities measured by rating scale, time trade-off, and standard gamble: review and reference for health care professionals. J Epidemiology. 2002;12(2):160-178.
25. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143.
26. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2011 Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug11.ashx. Published October 2012. Accessed December 1, 2013.
27. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574-1581.
28. Strasberg SM, Hall BL. Postoperative Morbidity Index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626.
29. Beilan J, Strakosha R, Palacios DA, Rosser CJ. The Postoperative Morbidity Index: a quantitative weighing of postoperative complications applied to urological procedures. BMC Urol. 2014;14:1.
30. Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion Severity Grading System: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298.
31. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: a study in obesity. Spine J. 2014;14(12):2923-2928.
32. Golinvaux NS, Bohl DD, Basques BA, Grauer JN. Administrative database concerns: accuracy of International Classification of Diseases, Ninth Revision coding is poor for preoperative anemia in patients undergoing spinal fusion. Spine. 2014;39(24):2019-2023.
33. Bekkers S, Bot AG, Makarawung D, Neuhaus V, Ring D. The National Hospital Discharge Survey and Nationwide Inpatient Sample: the databases used affect results in THA research. Clin Orthop Relat Res. 2014;472(11):3441-3449.
Take-Home Points
- Studies of AEs after orthopedic surgery commonly use composite AE outcomes.
- These types of outcomes treat AEs with different clinical significance similarly.
- This study created a single severity-weighted outcome that can be used to characterize the overall severity of a given patient’s postoperative course.
- Future studies may benefit from using this new severity-weighted outcome score.
Recently there has been an increase in the use of national databases for orthopedic surgery research.1-4 Studies commonly compare rates of postoperative adverse events (AEs) across different demographic, comorbidity, and procedural characteristics.5-23 Their conclusions often highlight different modifiable and/or nonmodifiable risk factors associated with the occurrence of postoperative events.
The several dozen AEs that have been investigated range from very severe (eg, death, myocardial infarction, coma) to less severe (eg, urinary tract infection [UTI], anemia requiring blood transfusion). A common approach for these studies is to consider many AEs together in the same analysis, asking a question such as, “What are risk factors for the occurrence of ‘adverse events’ after spine surgery?” Such studies test for associations with the occurrence of “any adverse event,” the occurrence of any “serious adverse event,” or similar composite outcomes. How common this type of study has become is indicated by the fact that in 2013 and 2014, at least 12 such studies were published in Clinical Orthopaedics and Related Research and the Journal of Bone and Joint Surgery,5-14,21-23 and many more in other orthopedic journals.15-20 However, there is a problem in using this type of composite outcome to perform such analyses: AEs with highly varying degrees of severity have identical impacts on the outcome variable, changing it from negative (“no adverse event”) to positive (“at least one adverse event”). As a result, the system may treat a very severe AE such as death and a very minor AE such as UTI similarly. Even in studies that use the slightly more specific composite outcome of “serious adverse events,” death and a nonlethal thromboembolic event would be treated similarly. Failure to differentiate these AEs in terms of their clinical significance detracts from the clinical applicability of conclusions drawn from studies using these types of composite AE outcomes.
In one of many examples that can be considered, a retrospective cohort study compared general and spinal anesthesia used in total knee arthroplasty.10 The rate of any AEs was higher with general anesthesia than with spinal anesthesia (12.34% vs 10.72%; P = .003). However, the only 2 specific AEs that had statistically significant differences were anemia requiring blood transfusion (6.07% vs 5.02%; P = .009) and superficial surgical-site infection (SSI; 0.92% vs 0.68%; P < .001). These 2 AEs are of relatively low severity; nevertheless, because these AEs are common, their differences constituted the majority of the difference in the rate of any AEs. In contrast, differences in the more severe AEs, such as death (0.11% vs 0.22%; P > .05), septic shock (0.14% vs 0.12%; P > .05), and myocardial infarction (0.20% vs 0.20%; P > .05), were small and not statistically significant. Had more weight been given to these more severe events, the outcome of the study likely would have been “no difference.”
To address this shortcoming in orthopedic research methodology, we created a severity-weighted outcome score that can be used to determine the overall “severity” of any given patient’s postoperative course. We also tested this novel outcome score for correlation with procedure type and patient characteristics using orthopedic patients from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP). Our intention is for database investigators to be able to use this outcome score in place of the composite outcomes that are dominating this type of research.
Methods
Generation of Severity Weights
Our method is described generally as utility weighting, assigning value weights reflective of overall impact to differing outcome states.24 Parallel methods have been used to generate the disability weights used to determine disability-adjusted life years for the Global Burden of Disease project25 and many other areas of health, economic, and policy research.
All orthopedic faculty members at 2 geographically disparate, large US academic institutions were invited to participate in a severity-weighting exercise. Each surgeon who agreed to participate performed the exercise independently.
- STEP 1: Please reorder the AE cards by your perception of “severity” for a patient experiencing that event after an orthopedic procedure.
- STEP 2: Once your cards are in order, please determine how many postoperative occurrences of each event you would “trade” for 1 patient experiencing postoperative death. Place this number of occurrences in the box in the upper right corner of each card.
- NOTES: As you consider each AE:
- Please consider an “average” occurrence of that AE, but note that in no case does the AE result in perioperative death.
- Please consider only the “severity” for the patient. (Do not consider the extent to which the event may be related to surgical error.)
- Please consider that the numbers you assign are relative to each other. Hence, if you would trade 20 of “event A” for 1 death, and if you would trade 40 of “event B” for 1 death, the implication is that you would trade 20 of “event A” for 40 of “event B.”
- You may readjust the order of your cards at any point.
Participants’ responses were recorded. For each number provided by each participant, the inverse (reciprocal) was taken and multiplied by 100%. This new number was taken to be the percentage severity of death that the given participant considered the given AE to embody. For example, as a hypothetical on one end of the spectrum, if a participant reported 1 (he/she would trade 1 AE X for 1 death), then the severity would be 1/1 × 100% = 100% of death, a very severe AE. Conversely, if a participant reported a very large number like 100,000 (he/she would trade 100,000 AEs X for 1 death), then the severity would be 1/100,000 × 100% = 0.001% of death, a very minor AE. More commonly, a participant will report a number like 25, which would translate to 4% of death (1/25 × 100% = 4%). For each AE, weights were then averaged across participants to derive a mean severity weight to be used to generate a novel composite outcome score.
Definition of Novel Composite Outcome Score
The novel composite outcome score would be expressed as a percentage to be interpreted as percentage severity of death, which we termed severity-weighted outcome relative to death (SWORD). For each patient, SWORD was defined as no AE (0%) or postoperative death (100%), with other AEs assigned mean severity weights based on faculty members’ survey responses. A patient with multiple AEs would be assigned the weight for the more severe AE. This method was chosen over summing the AE weights because in many cases the AEs were thought to overlap; hence, summing would be inappropriate. For example, generally a deep SSI would result in a return to the operating room, and one would not want to double-count this AE. Similarly, it would not make sense for a patient who died of a complication to have a SWORD of >100%, which would be the summing result.
Application to ACS-NSQIP Patients
ACS-NSQIP is a surgical registry that prospectively identifies patients undergoing major surgery at any of >500 institutions nationwide.26,27 Patients are characterized at baseline and are followed for AEs over the first 30 postoperative days.
First, mean SWORD was calculated and reported for patients undergoing each of the 8 procedures. Analysis of variance (ANOVA) was used to test for associations of mean SWORD with type of procedure both before and after multivariate adjustment for demographics (sex; age in years, <40, 40-49, 50-59, 60-69, 70-79, 80-89, ≥90) and comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, exertional dyspnea, end-stage renal disease, congestive heart failure).
Second, patients undergoing the procedure with the highest mean SWORD (hip fracture surgery) were examined in depth. Among only these patients, multivariate ANOVA was used to test for associations of mean SWORD with the same demographics and comorbidities.
All statistical tests were 2-tailed. Significance was set at α = 0.05 (P < .05).
All 23 institution A faculty members (100%) and 24 (89%) of the 27 institution B faculty members completed the exercise.
In the ACS-NSQIP database, 85,109 patients were identified on the basis of the initial inclusion criteria.
Results
Figure 1 shows mean severity weights and standard errors generated from faculty responses. Mean (standard error) severity weight for UTI was 0.23% (0.08%); blood transfusion, 0.28% (0.09%); pneumonia, 0.55% (0.15%); hospital readmission, 0.59% (0.23%); wound dehiscence, 0.64% (0.17%); deep vein thrombosis, 0.64% (0.19%); superficial SSI, 0.68% (0.23%); return to operating room, 0.91% (0.29%); progressive renal insufficiency, 0.93% (0.27%); graft/prosthesis/flap failure, 1.20% (0.34%); unplanned intubation, 1.38% (0.53%); deep SSI, 1.45% (0.38%); failure to wean from ventilator, 1.45% (0.48%); organ/space SSI, 1.76% (0.46%); sepsis without shock, 1.77% (0.42%); peripheral nerve injury, 1.83% (0.47%); pulmonary embolism, 2.99% (0.76%); acute renal failure, 3.95% (0.85%); myocardial infarction, 4.16% (0.98%); septic shock, 7.17% (1.36%); stroke, 8.73% (1.74%); cardiac arrest requiring cardiopulmonary resuscitation, 9.97% (2.46%); and coma, 15.14% (3.04%).
Among ACS-NSQIP patients, mean SWORD ranged from 0.2% (elective anterior cervical decompression and fusion) to 6.0% (hip fracture surgery) (Figure 2).
Discussion
The use of national databases in studies has become increasingly common in orthopedic surgery.1-4
The academic orthopedic surgeons who participated in our severity-weighting exercise thought the various AEs have markedly different severities. The least severe AE (UTI) was considered 0.23% as severe as postoperative death, with other events spanning the range up to 15.14% as severe as death. This wide range of severities demonstrates the problem with composite outcomes that implicitly consider all AEs similarly severe. Use of these markedly disparate weights in the development of SWORD enables this outcome to be more clinically applicable than outcomes such as “any adverse events.”
SWORD was highly associated with procedure type both before and after adjustment for demographics and comorbidities. Among patients undergoing the highest SWORD procedure (hip fracture surgery), SWORD was also associated with age, sex, and 4 of 6 tested comorbidities. Together, our findings show how SWORD is intended to be used in studies: to identify demographic, comorbidity, and procedural risk factors for an adverse postoperative course. We propose that researchers use our weighted outcome as their primary outcome—it is more meaningful than the simpler composite outcomes commonly used.
Outside orthopedic surgery, a small series of studies has addressed severity weighting of postoperative AEs.25,28-30 However, their approach was very different, as they were not designed to generate weights that could be transferred to future studies; rather, they simply compared severities of postoperative courses for patients within each individual study. In each study, a review of each original patient record was required, as the severity of each patient’s postoperative course was characterized according to the degree of any postoperative intervention—from no intervention to minor interventions such as placement of an intravenous catheter and major interventions such as endoscopic, radiologic, and surgical procedures. Only after the degree of intervention was defined could an outcome score be assigned to a given patient. However, databases do not depict the degree of intervention with nearly enough detail for this type of approach; they typically identify only occurrence or nonoccurrence of each event. Our work, which arose independently from this body of literature, enables an entirely different type of analysis. SWORD, which is not based on degree of intervention but on perceived severity of an “average” event, enables direct application of severity weights to large databases that store simple information on occurrence and nonoccurrence of specific AEs.
This study had several limitations. Most significantly, the generated severity weights were based on the surgeons’ subjective perceptions of severity, not on definitive assessments of the impacts of specific AEs on actual patients. We did not query the specialists who treat the complications or who present data on the costs and disabilities that may arise from these AEs. In addition, to develop our severity weighting scale, we queried faculty at only 2 institutions. A survey of surgeons throughout the United States would be more representative and would minimize selection bias. This is a potential research area. Another limitation is that scoring was subjective, based on surgeons’ perceptions of patients—in contrast to the Global Burden of Disease project, in which severity was based more objectively on epidemiologic data from >150 countries.
Orthopedic database research itself has often-noted limitations, including inability to sufficiently control for confounders, potential inaccuracies in data coding, limited follow-up, and lack of orthopedic-specific outcomes.1-4,31-33 However, this research also has much to offer, has increased tremendously over the past several years, and is expected to continue to expand. Many of the limitations of database studies cannot be entirely reversed. In providing a system for weighting postoperative AEs, our study fills a methodologic void. Future studies in orthopedics may benefit from using the severity-weighted outcome score presented here. Other fields with growth in database research may consider using similar methods to create severity-weighting systems of their own.
Am J Orthop. 2017;46(4):E235-E243. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Studies of AEs after orthopedic surgery commonly use composite AE outcomes.
- These types of outcomes treat AEs with different clinical significance similarly.
- This study created a single severity-weighted outcome that can be used to characterize the overall severity of a given patient’s postoperative course.
- Future studies may benefit from using this new severity-weighted outcome score.
Recently there has been an increase in the use of national databases for orthopedic surgery research.1-4 Studies commonly compare rates of postoperative adverse events (AEs) across different demographic, comorbidity, and procedural characteristics.5-23 Their conclusions often highlight different modifiable and/or nonmodifiable risk factors associated with the occurrence of postoperative events.
The several dozen AEs that have been investigated range from very severe (eg, death, myocardial infarction, coma) to less severe (eg, urinary tract infection [UTI], anemia requiring blood transfusion). A common approach for these studies is to consider many AEs together in the same analysis, asking a question such as, “What are risk factors for the occurrence of ‘adverse events’ after spine surgery?” Such studies test for associations with the occurrence of “any adverse event,” the occurrence of any “serious adverse event,” or similar composite outcomes. How common this type of study has become is indicated by the fact that in 2013 and 2014, at least 12 such studies were published in Clinical Orthopaedics and Related Research and the Journal of Bone and Joint Surgery,5-14,21-23 and many more in other orthopedic journals.15-20 However, there is a problem in using this type of composite outcome to perform such analyses: AEs with highly varying degrees of severity have identical impacts on the outcome variable, changing it from negative (“no adverse event”) to positive (“at least one adverse event”). As a result, the system may treat a very severe AE such as death and a very minor AE such as UTI similarly. Even in studies that use the slightly more specific composite outcome of “serious adverse events,” death and a nonlethal thromboembolic event would be treated similarly. Failure to differentiate these AEs in terms of their clinical significance detracts from the clinical applicability of conclusions drawn from studies using these types of composite AE outcomes.
In one of many examples that can be considered, a retrospective cohort study compared general and spinal anesthesia used in total knee arthroplasty.10 The rate of any AEs was higher with general anesthesia than with spinal anesthesia (12.34% vs 10.72%; P = .003). However, the only 2 specific AEs that had statistically significant differences were anemia requiring blood transfusion (6.07% vs 5.02%; P = .009) and superficial surgical-site infection (SSI; 0.92% vs 0.68%; P < .001). These 2 AEs are of relatively low severity; nevertheless, because these AEs are common, their differences constituted the majority of the difference in the rate of any AEs. In contrast, differences in the more severe AEs, such as death (0.11% vs 0.22%; P > .05), septic shock (0.14% vs 0.12%; P > .05), and myocardial infarction (0.20% vs 0.20%; P > .05), were small and not statistically significant. Had more weight been given to these more severe events, the outcome of the study likely would have been “no difference.”
To address this shortcoming in orthopedic research methodology, we created a severity-weighted outcome score that can be used to determine the overall “severity” of any given patient’s postoperative course. We also tested this novel outcome score for correlation with procedure type and patient characteristics using orthopedic patients from the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP). Our intention is for database investigators to be able to use this outcome score in place of the composite outcomes that are dominating this type of research.
Methods
Generation of Severity Weights
Our method is described generally as utility weighting, assigning value weights reflective of overall impact to differing outcome states.24 Parallel methods have been used to generate the disability weights used to determine disability-adjusted life years for the Global Burden of Disease project25 and many other areas of health, economic, and policy research.
All orthopedic faculty members at 2 geographically disparate, large US academic institutions were invited to participate in a severity-weighting exercise. Each surgeon who agreed to participate performed the exercise independently.
- STEP 1: Please reorder the AE cards by your perception of “severity” for a patient experiencing that event after an orthopedic procedure.
- STEP 2: Once your cards are in order, please determine how many postoperative occurrences of each event you would “trade” for 1 patient experiencing postoperative death. Place this number of occurrences in the box in the upper right corner of each card.
- NOTES: As you consider each AE:
- Please consider an “average” occurrence of that AE, but note that in no case does the AE result in perioperative death.
- Please consider only the “severity” for the patient. (Do not consider the extent to which the event may be related to surgical error.)
- Please consider that the numbers you assign are relative to each other. Hence, if you would trade 20 of “event A” for 1 death, and if you would trade 40 of “event B” for 1 death, the implication is that you would trade 20 of “event A” for 40 of “event B.”
- You may readjust the order of your cards at any point.
Participants’ responses were recorded. For each number provided by each participant, the inverse (reciprocal) was taken and multiplied by 100%. This new number was taken to be the percentage severity of death that the given participant considered the given AE to embody. For example, as a hypothetical on one end of the spectrum, if a participant reported 1 (he/she would trade 1 AE X for 1 death), then the severity would be 1/1 × 100% = 100% of death, a very severe AE. Conversely, if a participant reported a very large number like 100,000 (he/she would trade 100,000 AEs X for 1 death), then the severity would be 1/100,000 × 100% = 0.001% of death, a very minor AE. More commonly, a participant will report a number like 25, which would translate to 4% of death (1/25 × 100% = 4%). For each AE, weights were then averaged across participants to derive a mean severity weight to be used to generate a novel composite outcome score.
Definition of Novel Composite Outcome Score
The novel composite outcome score would be expressed as a percentage to be interpreted as percentage severity of death, which we termed severity-weighted outcome relative to death (SWORD). For each patient, SWORD was defined as no AE (0%) or postoperative death (100%), with other AEs assigned mean severity weights based on faculty members’ survey responses. A patient with multiple AEs would be assigned the weight for the more severe AE. This method was chosen over summing the AE weights because in many cases the AEs were thought to overlap; hence, summing would be inappropriate. For example, generally a deep SSI would result in a return to the operating room, and one would not want to double-count this AE. Similarly, it would not make sense for a patient who died of a complication to have a SWORD of >100%, which would be the summing result.
Application to ACS-NSQIP Patients
ACS-NSQIP is a surgical registry that prospectively identifies patients undergoing major surgery at any of >500 institutions nationwide.26,27 Patients are characterized at baseline and are followed for AEs over the first 30 postoperative days.
First, mean SWORD was calculated and reported for patients undergoing each of the 8 procedures. Analysis of variance (ANOVA) was used to test for associations of mean SWORD with type of procedure both before and after multivariate adjustment for demographics (sex; age in years, <40, 40-49, 50-59, 60-69, 70-79, 80-89, ≥90) and comorbidities (diabetes, hypertension, chronic obstructive pulmonary disease, exertional dyspnea, end-stage renal disease, congestive heart failure).
Second, patients undergoing the procedure with the highest mean SWORD (hip fracture surgery) were examined in depth. Among only these patients, multivariate ANOVA was used to test for associations of mean SWORD with the same demographics and comorbidities.
All statistical tests were 2-tailed. Significance was set at α = 0.05 (P < .05).
All 23 institution A faculty members (100%) and 24 (89%) of the 27 institution B faculty members completed the exercise.
In the ACS-NSQIP database, 85,109 patients were identified on the basis of the initial inclusion criteria.
Results
Figure 1 shows mean severity weights and standard errors generated from faculty responses. Mean (standard error) severity weight for UTI was 0.23% (0.08%); blood transfusion, 0.28% (0.09%); pneumonia, 0.55% (0.15%); hospital readmission, 0.59% (0.23%); wound dehiscence, 0.64% (0.17%); deep vein thrombosis, 0.64% (0.19%); superficial SSI, 0.68% (0.23%); return to operating room, 0.91% (0.29%); progressive renal insufficiency, 0.93% (0.27%); graft/prosthesis/flap failure, 1.20% (0.34%); unplanned intubation, 1.38% (0.53%); deep SSI, 1.45% (0.38%); failure to wean from ventilator, 1.45% (0.48%); organ/space SSI, 1.76% (0.46%); sepsis without shock, 1.77% (0.42%); peripheral nerve injury, 1.83% (0.47%); pulmonary embolism, 2.99% (0.76%); acute renal failure, 3.95% (0.85%); myocardial infarction, 4.16% (0.98%); septic shock, 7.17% (1.36%); stroke, 8.73% (1.74%); cardiac arrest requiring cardiopulmonary resuscitation, 9.97% (2.46%); and coma, 15.14% (3.04%).
Among ACS-NSQIP patients, mean SWORD ranged from 0.2% (elective anterior cervical decompression and fusion) to 6.0% (hip fracture surgery) (Figure 2).
Discussion
The use of national databases in studies has become increasingly common in orthopedic surgery.1-4
The academic orthopedic surgeons who participated in our severity-weighting exercise thought the various AEs have markedly different severities. The least severe AE (UTI) was considered 0.23% as severe as postoperative death, with other events spanning the range up to 15.14% as severe as death. This wide range of severities demonstrates the problem with composite outcomes that implicitly consider all AEs similarly severe. Use of these markedly disparate weights in the development of SWORD enables this outcome to be more clinically applicable than outcomes such as “any adverse events.”
SWORD was highly associated with procedure type both before and after adjustment for demographics and comorbidities. Among patients undergoing the highest SWORD procedure (hip fracture surgery), SWORD was also associated with age, sex, and 4 of 6 tested comorbidities. Together, our findings show how SWORD is intended to be used in studies: to identify demographic, comorbidity, and procedural risk factors for an adverse postoperative course. We propose that researchers use our weighted outcome as their primary outcome—it is more meaningful than the simpler composite outcomes commonly used.
Outside orthopedic surgery, a small series of studies has addressed severity weighting of postoperative AEs.25,28-30 However, their approach was very different, as they were not designed to generate weights that could be transferred to future studies; rather, they simply compared severities of postoperative courses for patients within each individual study. In each study, a review of each original patient record was required, as the severity of each patient’s postoperative course was characterized according to the degree of any postoperative intervention—from no intervention to minor interventions such as placement of an intravenous catheter and major interventions such as endoscopic, radiologic, and surgical procedures. Only after the degree of intervention was defined could an outcome score be assigned to a given patient. However, databases do not depict the degree of intervention with nearly enough detail for this type of approach; they typically identify only occurrence or nonoccurrence of each event. Our work, which arose independently from this body of literature, enables an entirely different type of analysis. SWORD, which is not based on degree of intervention but on perceived severity of an “average” event, enables direct application of severity weights to large databases that store simple information on occurrence and nonoccurrence of specific AEs.
This study had several limitations. Most significantly, the generated severity weights were based on the surgeons’ subjective perceptions of severity, not on definitive assessments of the impacts of specific AEs on actual patients. We did not query the specialists who treat the complications or who present data on the costs and disabilities that may arise from these AEs. In addition, to develop our severity weighting scale, we queried faculty at only 2 institutions. A survey of surgeons throughout the United States would be more representative and would minimize selection bias. This is a potential research area. Another limitation is that scoring was subjective, based on surgeons’ perceptions of patients—in contrast to the Global Burden of Disease project, in which severity was based more objectively on epidemiologic data from >150 countries.
Orthopedic database research itself has often-noted limitations, including inability to sufficiently control for confounders, potential inaccuracies in data coding, limited follow-up, and lack of orthopedic-specific outcomes.1-4,31-33 However, this research also has much to offer, has increased tremendously over the past several years, and is expected to continue to expand. Many of the limitations of database studies cannot be entirely reversed. In providing a system for weighting postoperative AEs, our study fills a methodologic void. Future studies in orthopedics may benefit from using the severity-weighted outcome score presented here. Other fields with growth in database research may consider using similar methods to create severity-weighting systems of their own.
Am J Orthop. 2017;46(4):E235-E243. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
2. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193.
3. Bohl DD, Grauer JN, Leopold SS. Editor’s spotlight/Take 5: Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671.
4. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: “Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198.
5. Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387-1394.
6. Edelstein AI, Lovecchio FC, Saha S, Hsu WK, Kim JY. Impact of resident involvement on orthopaedic surgery outcomes: an analysis of 30,628 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2014;96(15):e131.
7. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
8. Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes S. Thirty-day morbidity after single-level anterior cervical discectomy and fusion: identification of risk factors and emphasis on the safety of outpatient procedures. J Bone Joint Surg Am. 2014;96(15):1288-1294.
9. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10.
10. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
11. Odum SM, Springer BD. In-hospital complication rates and associated factors after simultaneous bilateral versus unilateral total knee arthroplasty. J Bone Joint Surg Am. 2014;96(13):1058-1065.
12. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191.
13. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
14. Mednick RE, Alvi HM, Krishnan V, Lovecchio F, Manning DW. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Joint Surg Am. 2014;96(14):1201-1209.
15. Pugely AJ, Martin CT, Gao Y, Ilgenfritz R, Weinstein SL. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine. 2014;39(15):1225-1234.
16. Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924.
17. Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025-2030.
18. Bohl DD, Fu MC, Golinvaux NS, Basques BA, Gruskay JA, Grauer JN. The “July effect” in primary total hip and knee arthroplasty: analysis of 21,434 cases from the ACS-NSQIP database. J Arthroplasty. 2014;29(7):1332-1338.
19. Bohl DD, Fu MC, Gruskay JA, Basques BA, Golinvaux NS, Grauer JN. “July effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(7):603-611.
20. Babu R, Thomas S, Hazzard MA, et al. Morbidity, mortality, and health care costs for patients undergoing spine surgery following the ACGME resident duty-hour reform: clinical article. J Neurosurg Spine. 2014;21(4):502-515.
21. Lovecchio F, Beal M, Kwasny M, Manning D. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472(11):3570-3575.
22. Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300.
23. Easterlin MC, Chang DG, Talamini M, Chang DC. Older age increases short-term surgical complications after primary knee arthroplasty. Clin Orthop Relat Res. 2013;471(8):2611-2620.
24. Morimoto T, Fukui T. Utilities measured by rating scale, time trade-off, and standard gamble: review and reference for health care professionals. J Epidemiology. 2002;12(2):160-178.
25. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143.
26. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2011 Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug11.ashx. Published October 2012. Accessed December 1, 2013.
27. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574-1581.
28. Strasberg SM, Hall BL. Postoperative Morbidity Index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626.
29. Beilan J, Strakosha R, Palacios DA, Rosser CJ. The Postoperative Morbidity Index: a quantitative weighing of postoperative complications applied to urological procedures. BMC Urol. 2014;14:1.
30. Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion Severity Grading System: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298.
31. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: a study in obesity. Spine J. 2014;14(12):2923-2928.
32. Golinvaux NS, Bohl DD, Basques BA, Grauer JN. Administrative database concerns: accuracy of International Classification of Diseases, Ninth Revision coding is poor for preoperative anemia in patients undergoing spinal fusion. Spine. 2014;39(24):2019-2023.
33. Bekkers S, Bot AG, Makarawung D, Neuhaus V, Ring D. The National Hospital Discharge Survey and Nationwide Inpatient Sample: the databases used affect results in THA research. Clin Orthop Relat Res. 2014;472(11):3441-3449.
1. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680.
2. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193.
3. Bohl DD, Grauer JN, Leopold SS. Editor’s spotlight/Take 5: Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671.
4. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: “Variations in data collection methods between national databases affect study results: a comparison of the Nationwide Inpatient Sample and National Surgical Quality Improvement Program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198.
5. Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am. 2014;96(16):1387-1394.
6. Edelstein AI, Lovecchio FC, Saha S, Hsu WK, Kim JY. Impact of resident involvement on orthopaedic surgery outcomes: an analysis of 30,628 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2014;96(15):e131.
7. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
8. Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes S. Thirty-day morbidity after single-level anterior cervical discectomy and fusion: identification of risk factors and emphasis on the safety of outpatient procedures. J Bone Joint Surg Am. 2014;96(15):1288-1294.
9. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10.
10. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
11. Odum SM, Springer BD. In-hospital complication rates and associated factors after simultaneous bilateral versus unilateral total knee arthroplasty. J Bone Joint Surg Am. 2014;96(13):1058-1065.
12. Yoshihara H, Yoneoka D. Trends in the incidence and in-hospital outcomes of elective major orthopaedic surgery in patients eighty years of age and older in the United States from 2000 to 2009. J Bone Joint Surg Am. 2014;96(14):1185-1191.
13. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
14. Mednick RE, Alvi HM, Krishnan V, Lovecchio F, Manning DW. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Joint Surg Am. 2014;96(14):1201-1209.
15. Pugely AJ, Martin CT, Gao Y, Ilgenfritz R, Weinstein SL. The incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric database. Spine. 2014;39(15):1225-1234.
16. Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924.
17. Belmont PJ Jr, Goodman GP, Hamilton W, Waterman BR, Bader JO, Schoenfeld AJ. Morbidity and mortality in the thirty-day period following total hip arthroplasty: risk factors and incidence. J Arthroplasty. 2014;29(10):2025-2030.
18. Bohl DD, Fu MC, Golinvaux NS, Basques BA, Gruskay JA, Grauer JN. The “July effect” in primary total hip and knee arthroplasty: analysis of 21,434 cases from the ACS-NSQIP database. J Arthroplasty. 2014;29(7):1332-1338.
19. Bohl DD, Fu MC, Gruskay JA, Basques BA, Golinvaux NS, Grauer JN. “July effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Spine. 2014;39(7):603-611.
20. Babu R, Thomas S, Hazzard MA, et al. Morbidity, mortality, and health care costs for patients undergoing spine surgery following the ACGME resident duty-hour reform: clinical article. J Neurosurg Spine. 2014;21(4):502-515.
21. Lovecchio F, Beal M, Kwasny M, Manning D. Do patients with insulin-dependent and noninsulin-dependent diabetes have different risks for complications after arthroplasty? Clin Orthop Relat Res. 2014;472(11):3570-3575.
22. Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300.
23. Easterlin MC, Chang DG, Talamini M, Chang DC. Older age increases short-term surgical complications after primary knee arthroplasty. Clin Orthop Relat Res. 2013;471(8):2611-2620.
24. Morimoto T, Fukui T. Utilities measured by rating scale, time trade-off, and standard gamble: review and reference for health care professionals. J Epidemiology. 2002;12(2):160-178.
25. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143.
26. American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2011 Participant Use Data File. https://www.facs.org/~/media/files/quality%20programs/nsqip/ug11.ashx. Published October 2012. Accessed December 1, 2013.
27. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574-1581.
28. Strasberg SM, Hall BL. Postoperative Morbidity Index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213(5):616-626.
29. Beilan J, Strakosha R, Palacios DA, Rosser CJ. The Postoperative Morbidity Index: a quantitative weighing of postoperative complications applied to urological procedures. BMC Urol. 2014;14:1.
30. Porembka MR, Hall BL, Hirbe M, Strasberg SM. Quantitative weighting of postoperative complications based on the Accordion Severity Grading System: demonstration of potential impact using the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(3):286-298.
31. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: a study in obesity. Spine J. 2014;14(12):2923-2928.
32. Golinvaux NS, Bohl DD, Basques BA, Grauer JN. Administrative database concerns: accuracy of International Classification of Diseases, Ninth Revision coding is poor for preoperative anemia in patients undergoing spinal fusion. Spine. 2014;39(24):2019-2023.
33. Bekkers S, Bot AG, Makarawung D, Neuhaus V, Ring D. The National Hospital Discharge Survey and Nationwide Inpatient Sample: the databases used affect results in THA research. Clin Orthop Relat Res. 2014;472(11):3441-3449.
Trans-Scaphoid Transcapitate Perilunate Fracture-Dislocation
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- TSTC-PLFD is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.
- TSTC-PLFD is associated by a complex ligamentous injury of the wrist.
- Impaction of the wrist in extension seems to be the most important predictor of this injury.
- Optimal treatment for TSTC-PLFD is open reduction, anatomical alignment, and ligamentous and osseous stabilization.
- The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.
Trans-scaphoid transcapitate (TSTC) perilunate fracture-dislocation (PLFD) is a rare hyperextension wrist injury characterized by fracture of both the scaphoid and the capitate and rotation of the proximal bone fragment of the capitate.1 Isolated capitate fractures with or without rotation of its proximal fragment have been well described.2,3 Obviously, this specific type of injury represents just the osseous part of a more complex ligamentous wrist injury.2,3
TSTC-PLFD was first described by Nicholson4 in 1940. In 1956, Fenton5 coined the term scaphocapitate syndrome, which became widely known. With PLFD, accurate diagnosis may be delayed. Usually, only the scaphoid fracture is identified by radiologic examination, and thus the severity of the injury is underestimated and appropriate treatment delayed.3,6,7 The English literature includes only case reports and small series on this rare perilunate injury.6-9 In this article, we report the case of an adult with TSTC-PLFD. We describe the radiographic and intraoperative findings, review the current surgical principles for reduction and stabilization of this injury, and assess the clinical and radiologic outcomes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 32-year-old man sustained an isolated injury of his right (dominant) hand after falling from a height of 6 feet and landing on his outstretched right arm with the wrist in extension.
With the patient under general anesthesia and a humerus tourniquet applied, an external fixator was placed for spanning of the wrist joint. The dorsal aspect of the wrist joint was approached through a midline longitudinal 5-cm incision, centered over the Lister tubercle. For adequate exposure of the dorsal wrist, a flap of the dorsal capsule was raised with the apex at the triquetrum and a radial broad base, as previously described.9 An avulsion fracture at the insertion of the dorsal capsule to the triquetrum was observed. The dorsal surface of the hamate and lunate showed a small area of bone contusion with hemorrhagic infiltration. The scapholunate and lunotriquetral ligaments were intact. The proximal fragment of the capitate was identified deep into the space between the lunate and distal capitate fragment; the articular surface of the bone fragment was rotated 180° distally (Figure 3).
Skin sutures were removed 2 weeks after surgery, K-wires 6 weeks after surgery, and the external fixator 8 weeks after surgery. At 8 weeks, radiographs showed healing of both fractures, scaphoid and capitate. The patient was allowed gradual passive and active-assisted range-of-motion exercises of the wrist at 8 weeks, and he returned to work 3 months after surgery. At 12-month follow-up, all fractures were completely healed, and the wrist was stable and pain-free.
Discussion
The exact biomechanism of TSTC-PLFD is unclear. Impaction of the wrist in extension seems to be the most important predictor of this injury.5,7,9-11 According to Stein and Siegel,10 scaphoid fractures first allow hyperextension of the wrist; the lunate and the capitate rotate dorsally, and the dorsal surface of the capitate impacts the dorsal edge of the distal radius, causing a fracture of the neck of the capitate. If the wrist continues to rotate into further hyperextension, the unsupported, proximal part of the capitate rotates 90° around itself.9,10 When the carpus returns to neutral position, the bone fragment of the capitate rotates further, reaching a position of 180°, with its proximal articular surface facing distally. In this type of injury, the axis of rotation is transverse (radioulnar), in contrast to the perpendicular (anteroposterior) axis of rotation suggested by the initial report by Fenton.5 The scaphoid is fractured by impaction of the radial styloid process. Monahan and Galasko11 reported a case of capitate fracture with palmar displacement and 90° rotation of the proximal bone fragment; the fragmented surface was facing dorsally. A transverse axis of rotation, as in our patient’s case, could explain this type of displacement supporting the mechanism of injury proposed by Stein and Siegel.10 Vance and colleagues7 described various patterns of scaphocapitate fractures and concluded that no single mechanism of injury accounts for these types of injuries. Other authors have considered scaphocapitate syndrome as a specific type of TSTC-PLFD, one that reduces either spontaneously or with manipulation.1,3,12 Detailed evaluation of standard anteroposterior and lateral wrist radiographs can provide enough evidence for the diagnosis of this injury. Computed tomography may define further the type and extent of injury.7 In our patient’s case, wrist impaction caused the scaphoid and capitate fractures and the avulsion of the capsule attachment to the triquetrum. The distal fragment of the capitate subluxated dorsally in relation to the lunate. The lateral radiograph of the wrist showed its position in the lunate fossa. According to the classification of Herzberg and colleagues12 and Mayfield and colleagues,13 this represents a dorsal PLFD of the greater carpal bones arc.
Conservative treatment is not recommended for PLFD because closed reduction usually is not possible, and poor functional outcomes are common. Instead, optimal treatment is open reduction, anatomical alignment, and ligamentous and osseous stabilization.7,12,14,15 Dorsal, palmar, and combined approaches have been used in surgery for perilunate injuries. A dorsal approach through a radius-based capsular flap allows excellent exposure of the dorsal wrist and facilitates reduction of fractures.9 Capitate reduction should precede scaphoid reduction because scaphoid reduction cannot be easily maintained, especially when the fracture interface is comminuted.7 In addition, scaphoid reduction may be guided from the radial surface of the capitate. Moreover, when the scaphoid is fixated first, reduction of the rotated head of the capitate usually is difficult. In our patient’s case, traction applied through the external fixator facilitated reduction and K-wire fixation of the capitate fracture. After scaphoid fixation, the K-wires were advanced through the capitate to the lunate to stabilize the capitolunate joint. The wrist must be immobilized for 6 to 8 weeks after surgical repair of PLFD. A cast can be used, but, as with our patient, an external fixator facilitates fracture reduction and wrist stability during osteosynthesis. During immobilization, the wrist should be maintained in neutral position to avoid stretching the dorsal and palmar wrist capsule and ligaments.16The most important complications of scaphoid and capitate fractures and PLFD are osteonecrosis and nonunion.17-20 Similar to scaphoid fractures, capitate fractures proximal to the waist of the capitate are associated with increased risk of osteonecrosis. Therefore, anatomical reduction and stabilization favor revascularization of the proximal bone fragment. Moreover, any osteonecrosis that occurs in the proximal part of the capitate is not an indication for further surgery as long as wrist height is maintained. Nonunion is not common after open reduction and internal fixation of PLFD (eg, our patient’s fractures healed completely).17 Radiographically, nonunion is characterized by bone absorption and sclerosis of the ends of the bone. Treatment of capitate nonunion depends on symptom severity, bone fragment size, and radiographic evidence of arthritic changes.3,7,21-23 Treatment options include resection of sclerotic edges, bone grafting, and stabilization21 and removal of the proximal capitate fragment and limited arthrodesis,22 as arthritic changes likely are inevitable.22,23TSTC-PLFD is a rare wrist injury. Careful radiographic evaluation of the carpal bones and their relationships on both anteroposterior and lateral views is mandatory in making the correct diagnosis. Open reduction (preferably with use of an external fixator) and internal fixation are recommended for optimal healing and functional outcomes.
Am J Orthop. 2017;46(4):E230-E234. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
1. Johnson RP. The acutely injured wrist and its residuals. Clin Orthop Relat Res. 1980;(149):33-44.
2. Volk AG, Schnall SB, Merkle P, Stevanovic M. Unusual capitate fracture: a case report. J Hand Surg Am. 1995;20(4):581-582.
3. Apergis E, Darmanis S, Kastanis G, Papanikolaou A. Does the term scaphocapitate syndrome need to be revised? A report of 6 cases. J Hand Surg Br. 2001;26(5):441-445.
4. Nicholson CB. Fracture dislocation of the os magnum. J Roy Navy Med Serv. 1940;26:289-291.
5. Fenton RL. The naviculo-capitate fracture syndrome. J Bone Joint Surg Am. 1956;38(3):681-684.
6. Strohm PC, Laier P, Müller CA, Gutorski S, Pfister U. Scaphocapitate fracture syndrome of both hands—first description of a bilateral occurrence of a rare carpal injury [in German]. Unfallchirurg. 2003;106(4):339-342.
7. Vance RM, Gelberman R, Evans EF. Scaphocapitate fractures. Patterns of dislocation, mechanisms of injury, and preliminary results of treatment. J Bone Joint Surg Am. 1980;62(2):271-276.
8. Apostolides JG, Lifchez SD, Christy MR. Complex and rare fracture patterns in perilunate dislocations. Hand. 2011;6(3):287-294.
9. Berger RA, Bishop AT, Bettinger PC. New dorsal capsulotomy for the surgical exposure of the wrist. Ann Plast Surg. 1995;35(1):54-59.
10. Stein F, Siegel MW. Naviculocapitate fracture syndrome. A case report: new thoughts on the mechanism of injury. J Bone Joint Surg Am. 1969;51(2):391-395.
11. Monahan PR, Galasko CS. The scapho-capitate fracture syndrome. A mechanism of injury. J Bone Joint Surg Br. 1972;54(1):122-124.
12. Herzberg G, Comtet JJ, Linscheid RL, Amadio PC, Cooney WP, Stalder J. Perilunate dislocations and fracture-dislocations: a multicenter study. J Hand Surg Am. 1993;18(5):768-779.
13. Mayfield JK, Johnson RP, Kilcoyne RK. Carpal dislocations: pathomechanics and progressive perilunar instability. J Hand Surg Am. 1980;5(3):226-241.
14. Moneim MS, Hofammann KE 3rd, Omer GE. Transscaphoid perilunate fracture-dislocation. Result of open reduction and pin fixation. Clin Orthop Relat Res. 1984;(190):227-235.
15. Andreasi A, Coppo M, Danda F. Trans-scapho-capitate perilunar dislocation of the carpus. Ital J Orthop Traumatol. 1986;12(4):461-466.
16. Song D, Goodman S, Gilula LA, Wollstein R. Ulnocarpal translation in perilunate dislocations. J Hand Surg Eur. 2009;34(3):388-390.
17. Rand JA, Linscheid RL, Dobyns JH. Capitate fractures: a long-term follow-up. Clin Orthop Relat Res. 1982;(165):209-216.
18. Panagis JS, Gelberman RH, Taleisnik J, Baumgaertner M. The arterial anatomy of the human carpus. Part II: the intraosseous vascularity. J Hand Surg Am. 1983;8(4):375-382.
19. Freedman DM, Botte MJ, Gelberman RH. Vascularity of the carpus. Clin Orthop Relat Res. 2001;(383):47-59.
20. Vander Grend R, Dell PC, Glowczewskie F, Leslie B, Ruby LK. Intraosseous blood supply of the capitate and its correlation with aseptic necrosis. J Hand Surg Am. 1984;9(5):677-683.
21. Rico AA, Holguin PH, Martin JG. Pseudarthrosis of the capitate. J Hand Surg Br. 1999;24(3):382-384.
22. Kumar A, Olney DB. Multiple carpometacarpal dislocations. J Accid Emerg Med. 1994;11(4):257-258.
23. Kohut GN. Extra-articular fractures of the distal radius in young adults. A technique of closed reposition and stabilisation by mono-segmental, radio-radial external fixator. Ann Chir Main Memb Super. 1995;14(1):14-19.
Six Steps to Reduce Taxes on Investments: Minimizing What You Pay in a Tough Environment
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Is Simultaneous Bilateral Total Knee Arthroplasty (BTKA) as Safe as Staged BTKA?
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.