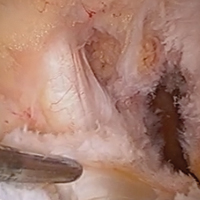
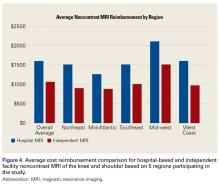
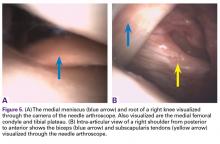
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
The Role of Synovial Cytokines in the Diagnosis of Periprosthetic Joint Infections: Current Concepts
Take-Home Points
- In cases of failed TJA, it is important to differentiate between septic and aseptic etiologies.
- Chronic and low-grade infections are challenging for orthopedic surgeons, as the symptoms often overlap with aseptic etiologies.
- Verification of infection eradication before beginning the second-stage reimplantation surgery is extremely important, but pre- and intraoperative findings can be unreliable.
- Synovial fluid cytokines have been shown to accurately diagnose PJIs.
- Synovial fluid cytokines may help surgeons differentiate between septic and aseptic cases of failed TJA.
Total joint arthroplasty (TJA) is an effective procedure that has been extensively used to relieve pain and improve quality of life in patients with various forms of joint disease. Although advances in technology and surgical technique have improved the success of TJA, periprosthetic joint infection (PJI) remains a serious complication. In the United States, it is estimated that PJI is the most common reason for total knee arthroplasty failure and the third most common reason for total hip arthroplasty revision.1 Although the incidence of PJI is 1% to 2%, the dramatic increase in TJA volume is expected to be accompanied by a similar rise in the number of infected TJAs; that number is expected to exceed 60,000 in the United States by 2020.2 Moreover, management of PJI is expensive and imposes a heavy burden on the healthcare system, with costs expected to hit $20 billion by 2020 in the US.2 Therefore, treating asepsis cases as infections imposes a heavy burden on the healthcare system and may result in excessive morbidity.3 At the same time, inadequate management of a PJI may result in recurrences that require infection treatment with morbid procedures, such as arthrodesis or amputation. Accurate diagnosis of PJI is of paramount importance in preventing potential implications of a misdiagnosed case. Unfortunately, the PJI diagnosis is extremely challenging, and the available diagnostic tests are often unreliable.4 Thus, research has recently focused on use of several synovial fluid cytokines in the detection of PJI.5-7 In this article, we provide an overview of the synovial biomarkers being used to diagnose PJI.
Diagnosis of Periprosthetic Joint Infection
Differentiating between septic and aseptic failed TJA is important, as the treatment options differ considerably. PJI can be broadly classified as acute or early postoperative (<6 weeks), late chronic (indolent onset), and acute-on-chronic (acute onset in well-functioning prosthesis, secondary to hematogenous spread).8 The acute and acute-on-chronic presentations are often associated with obvious signs of infection.9 However, chronic and low-grade infections pose a challenge to modern orthopedic practice, as the symptoms often overlap with that of aseptic causes of TJA failure.10 As a result, the International Consensus Group on Periprosthetic Joint Infection developed complex criteria using the Musculoskeletal Infection Society definition of PJI and involving a battery of tests for PJI diagnosis.11 According to these criteria, PJI is diagnosed when 1 of the 2 major criteria or 3 of the 5 minor criteria are met (Table 1).
Although these criteria constitute the most agreed on and widely used standard for PJI diagnosis, the definition is complex and often incomplete until surgical intervention. An ideal diagnostic test would aid in managing a PJI and provide results before a treatment decision is made. Many revision surgeries are being performed with insufficient information about the true diagnosis, and the diagnosis might change during or after surgery. About 10% of the revisions presumed to be aseptic may unexpectedly grow cultures during surgery and thereby satisfy the criteria for PJI after surgery.12 Moreover, with the use of novel methods such as polymerase chain reaction, microorganisms were identified in more than three-fourths of the presumed aseptic revisions.13 The optimal management of such cases is controversial, and it is unclear whether positive cultures should be treated as possible contaminants or true infection.12,14
Verification of Infection Eradication
A 2-stage revision procedure, widely accepted as the standard treatment for PJI, has success rates approaching 94%.15 In this procedure, it is important to verify infection eradication before beginning the second-stage reimplantation. Verification is crucial in avoiding reimplantation of an infected joint.16 After the first stage, patients are usually administered intravenous antibiotics for at least 6 weeks; these antibiotics are then withheld, and systemic inflammatory markers are evaluated for infection eradication. Although reliable criteria have been established for PJI diagnosis, guidelines for detecting eradication of infection are rudimentary. Most surgeons monitor the decrease in serologic markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) level, to assess the response to treatment. However, noninfectious etiologies may result in continued elevation of these markers.17 Even though aspirations are often performed to diagnose persistent infection before the second-stage procedure, their diagnostic utility may be limited.18 Use of cultures is also limited, as presence of antibiotic-loaded spacers can decrease the sensitivity of culture.19 Inadequate diagnosis often leads to unnecessary continuation of antimicrobial therapy or additional surgical débridement. Nuclear scans often remain positive because of aseptic inflammation related to surgery and are not useful in documenting sepsis arrest.20 Given the limitations of available tests, novel strategies for identifying the presence of infection at the second stage are being tested.
Synovial Fluid Cytokines
PJI pathogenesis begins with colonization of the implant surfaces with microorganisms and subsequent formation of biofilms.21 The human immune system is activated by the microbial products, cell wall components, and various biofilm proteins. Immune cells are recruited to the site, where they secrete a myriad of inflammatory biomarkers, such as cytokines, which promote further recruitment of inflammatory cells and aid in the eradication of pathogens.9 These inflammatory cytokines and cells are involved in aseptic inflammatory joint conditions, such as rheumatoid arthritis22,23; however, some are specifically involved in immune pathways combating pathogens.24 This action is the basis for increasing interest in using various synovial fluid cytokines and other biomarkers in the diagnosis of PJI. Here we describe some of the commonly studied cytokines.
Interleukin 1β
Interleukin 1β (IL-1β) is a major proinflammatory cytokine that is synthesized by multiple cells, including macrophages and monocytes.25 IL-1β is produced in response to microorganisms, other cytokines, antigen-presenting cells, and immune complexes; stimulates production of acute-phase proteins by the liver; and is an important pyrogen.25 Deirmengian and colleagues5 found that synovial IL-1β increased 258-fold in patients with a PJI. Studies have found that synovial IL-1β has sensitivity ranging from 66.7% to 100% and specificity ranging from 87% to 100%, with 1 study reporting an accuracy of 100%.5,6,26,27
Interleukin 6
Also produced by macrophages and monocytes, interleukin 6 (IL-6) is a potent stimulator of acute-phase proteins.28,29 IL-6 has a role as a chemoattractant and helps with cell differentiation when changing from innate to acquired immunity.30 It is also used as an aid in diagnosing PJI; it has sensitivity ranging from 62% to 100% and specificity ranging from 85% to 100%.5,6,26,31,32 Synovial IL-6 measurements were more accurate than serum IL-6 measurements.26 Furthermore, synovial IL-6 can be increased up to 27-fold in PJI cases.5 In one study, synovial IL-6 levels >2100 pg/mL had sensitivity of 62.5% and specificity of 85.7% in PJI diagnosis26; in another study, an IL-6 threshold of 4270 pg/mL had sensitivity of 87.1%, specificity of 100%, and accuracy of 94.6%.31
C-Reactive Protein
CRP is an acute-phase reactant. Blood levels increase in response to aseptic inflammatory processes and systemic infection.33 CRP plays an important role in host defense by activating complement and helping mediate phagocytosis.33,34 Although serum CRP levels have been used in diagnosing PJIs,6 they can yield false-negative results.35,36 Therefore, attention turned to synovial CRP levels, which were found to be increased 13-fold in PJI cases.5 It has been shown that synovial CRP levels are significantly higher in infected vs noninfected prosthetic joints34 and had diagnostic accuracy better than that of serum CRP levels in diagnosing PJI.37 One study found that CRP at a threshold of 3.7 mg/L had sensitivity of 84%, specificity of 97.1%, and accuracy of 91.5%,37 whereas another study found that CRP at a threshold of 3.61 mg/L had sensitivity of 87.1%, specificity of 97.7%, and accuracy of 93.3%.31
α-Defensin
α-Defensin, a natural peptide produced and secreted by neutrophils in response to pathogens, has antimicrobial and cytotoxic properties,38-40 signals for the secretion of various cytokines, and acts as a chemoattractant for various immune cells.41 Deirmengian and colleagues6 found that α-defensin was consistently elevated in patients with PJI. α-Defensin is extremely accurate in diagnosing PJI; it has sensitivity ranging from 97% to 100% and specificity ranging from 96% to 100%.6,27,42 Moreover, α-defensin was effective in diagnosing PJI caused by a wide spectrum of organisms, including various low-virulence bacteria and fungi.43
Leukocyte Esterase
Leukocyte esterase is an enzyme produced and secreted by neutrophils at sites of active infection.7,44 Testing for this enzyme is performed with a colorimetric strip and was originally performed for the diagnosis of urinary tract infections.44,45 In a study conducted by Parvizi and colleagues,7 this strip was used to test for leukocyte esterase in synovial fluid samples; a ++ reading was found to have sensitivity of 80.6% and specificity of 100% in diagnosing knee PJI. Similarly, De Vecchi and colleagues45 found sensitivity of 92.6% and specificity of 97%.
Other Synovial Markers
Research has identified numerous molecular biomarkers that may be associated with the pathogenesis of PJI. Although several (eg, cytokines) have demonstrated higher levels in synovial fluid in patients with PJI than in normal controls, only a few have had clinically relevant diagnostic utility.6 Deirmengian and colleagues6 screened 43 synovial fluid biomarkers that potentially could be used in the diagnosis of PJI. Besides the cytokine α-defensin, 4 other biomarkers—lactoferrin, neutrophil gelatinase-associated lipocalcin, neutrophil elastase 2, and bactericidal/permeability-increasing protein—had accuracy of 100%. In addition, 8 cytokines and biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1β, IL-6, IL-10, IL-1α) had area under the curve values higher than 0.9. Studies have also evaluated the diagnostic utility of metabolic products such as lactate, lactate dehydrogenase, and glucose; their accuracy was comparable to that of serum CRP.32
Serum Markers
In addition to the synovial fluid cytokines, several serum inflammatory cytokines have been studied as potential targets in diagnosing infection. Serum IL-6 has had excellent diagnostic accuracy46 and, when combined with CRP, could increase sensitivity in diagnosing PJI; such a combination (vs either test alone) could be useful in screening patients.47,48 Biomarkers such as tumor necrosis factor α and procalcitonin are considered very specific for PJI and may be useful in confirmatory testing.48 Evidence also suggests that toll-like receptor 2 proteins are elevated in the serum of patients with PJI and therefore are a potential diagnostic tool.49
Limitations of Synovial Cytokines
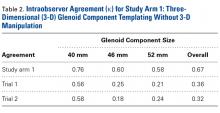
The literature suggests that some synovial fluid cytokines have promise.6 However, the best biomarker or combination of biomarkers is yet to be determined. Results have been consistent with α-defensin and other cytokines but mixed with IL-6 and still others32,42,50 (Table 2).
Information on the utility of synovial biomarkers in detecting persistent infection is limited. Frangiamore and colleagues50 found that IL-1 and IL-6 levels decreased between the stages of 2-stage revision. Unfortunately, none of the synovial fluid cytokines investigated (IL-1, IL-2, IL-6, IL-8, Il-10, interferon γ, granulocyte macrophage-colony stimulating factor, tumor necrosis factor α, IL-12p70) satisfactorily detected resolution of infection in the setting of prior treatment for PJI. Although cytokines are expected to be elevated in the presence of infection, the internal milieu at the time of stage 2 of the revision makes diagnosis of infection difficult. In addition, presence of spacer particles and recent surgery may activate immune pathways and yield false-positive results. Furthermore, antibiotic cement spacers may suppress the microorganisms to very low levels and yield false-negative results even if these organisms remain virulent.19
Even though the synovial molecular markers can detect the presence of infection, they are unable to identify pathogens. As identifying the pathogen is important in the treatment of PJI, there has been interest in using polymerase chain reaction (PCR) techniques.51 These tests may also provide specific information about the pathogen, such as its antibiotic sensitivity. A recently developed technology, the Ibis T5000 Universal Biosensor (Ibis Biosciences), uses novel pan-domain primers in a series of PCRs. This biosensor is useful in diagnosing infections when cultures are negative and appears to be more accurate than conventional PCR.13 As reported by Jacovides and colleagues,13 this novel PCR technique identified an organism in about 88% of presumed cases of aseptic revision.
Conclusion
PJI poses an extreme challenge to the healthcare system. Given the morbidity associated with improper management of PJI, accurate diagnosis is of paramount importance. Given the limitations of current tests, synovial fluid cytokines hold promise in the diagnosis of PJIs. However, these cytokines are expensive, and their clinical utility in PJI management is not well established. More research is needed before guidelines for synovial fluid cytokines and biomarkers can replace or be incorporated into guidelines for the treatment of PJIs.
1 Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
3. Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85(6):1000-1004.
4. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869-882.
5. Deirmengian C, Hallab N, Tarabishy A, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017-2023.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
7. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242-2248.
8. Kuiper JW, Willink RT, Moojen DJF, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667-676.
9. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654.
10. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
11. Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
12. Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29(11):2181-2186.
13. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)–based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
14. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):94-99.
15. Macheras GA, Koutsostathis SD, Kateros K, Papadakis S, Anastasopoulos P. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int. 2012;22(suppl 8):S54-S61.
16. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res. 2016;474(7):1619-1626.
17. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002-1008.
18. Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305-309.
19. Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552-1557.
20. Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66-78.
21. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158-168.
22. Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49-53.
23. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585-592.
24. Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545-594.
25. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075-1079.
26. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644-651.
27. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
28. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
29. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621-636.
30. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888.
31. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
32. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
33. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24(2):163-176.
34. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27(8 suppl):12-16.
35. Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444-e449.
36. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-1626.
37. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54-60.
38. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105-128.
39. Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427-1435.
40. Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104(6):1778-1783.
41. Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249.
42. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
43. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229-2235.
44. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
45. De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555-560.
46. Di Cesare PE, Chang E, Preston CF, Liu C. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921-1927.
47. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61(3):332-341.
48. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94-99.
49. Galliera E, Drago L, Vassena C, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620-623.
50. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630-1639.
51. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
52. Omar M, Ettinger M, Reichling M, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97(2):173-176.
53. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997-4003.
54. Omar M, Ettinger M, Reichling M, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. J Bone Joint Surg Am. 2014;96(24):2032-2037.
Take-Home Points
- In cases of failed TJA, it is important to differentiate between septic and aseptic etiologies.
- Chronic and low-grade infections are challenging for orthopedic surgeons, as the symptoms often overlap with aseptic etiologies.
- Verification of infection eradication before beginning the second-stage reimplantation surgery is extremely important, but pre- and intraoperative findings can be unreliable.
- Synovial fluid cytokines have been shown to accurately diagnose PJIs.
- Synovial fluid cytokines may help surgeons differentiate between septic and aseptic cases of failed TJA.
Total joint arthroplasty (TJA) is an effective procedure that has been extensively used to relieve pain and improve quality of life in patients with various forms of joint disease. Although advances in technology and surgical technique have improved the success of TJA, periprosthetic joint infection (PJI) remains a serious complication. In the United States, it is estimated that PJI is the most common reason for total knee arthroplasty failure and the third most common reason for total hip arthroplasty revision.1 Although the incidence of PJI is 1% to 2%, the dramatic increase in TJA volume is expected to be accompanied by a similar rise in the number of infected TJAs; that number is expected to exceed 60,000 in the United States by 2020.2 Moreover, management of PJI is expensive and imposes a heavy burden on the healthcare system, with costs expected to hit $20 billion by 2020 in the US.2 Therefore, treating asepsis cases as infections imposes a heavy burden on the healthcare system and may result in excessive morbidity.3 At the same time, inadequate management of a PJI may result in recurrences that require infection treatment with morbid procedures, such as arthrodesis or amputation. Accurate diagnosis of PJI is of paramount importance in preventing potential implications of a misdiagnosed case. Unfortunately, the PJI diagnosis is extremely challenging, and the available diagnostic tests are often unreliable.4 Thus, research has recently focused on use of several synovial fluid cytokines in the detection of PJI.5-7 In this article, we provide an overview of the synovial biomarkers being used to diagnose PJI.
Diagnosis of Periprosthetic Joint Infection
Differentiating between septic and aseptic failed TJA is important, as the treatment options differ considerably. PJI can be broadly classified as acute or early postoperative (<6 weeks), late chronic (indolent onset), and acute-on-chronic (acute onset in well-functioning prosthesis, secondary to hematogenous spread).8 The acute and acute-on-chronic presentations are often associated with obvious signs of infection.9 However, chronic and low-grade infections pose a challenge to modern orthopedic practice, as the symptoms often overlap with that of aseptic causes of TJA failure.10 As a result, the International Consensus Group on Periprosthetic Joint Infection developed complex criteria using the Musculoskeletal Infection Society definition of PJI and involving a battery of tests for PJI diagnosis.11 According to these criteria, PJI is diagnosed when 1 of the 2 major criteria or 3 of the 5 minor criteria are met (Table 1).
Although these criteria constitute the most agreed on and widely used standard for PJI diagnosis, the definition is complex and often incomplete until surgical intervention. An ideal diagnostic test would aid in managing a PJI and provide results before a treatment decision is made. Many revision surgeries are being performed with insufficient information about the true diagnosis, and the diagnosis might change during or after surgery. About 10% of the revisions presumed to be aseptic may unexpectedly grow cultures during surgery and thereby satisfy the criteria for PJI after surgery.12 Moreover, with the use of novel methods such as polymerase chain reaction, microorganisms were identified in more than three-fourths of the presumed aseptic revisions.13 The optimal management of such cases is controversial, and it is unclear whether positive cultures should be treated as possible contaminants or true infection.12,14
Verification of Infection Eradication
A 2-stage revision procedure, widely accepted as the standard treatment for PJI, has success rates approaching 94%.15 In this procedure, it is important to verify infection eradication before beginning the second-stage reimplantation. Verification is crucial in avoiding reimplantation of an infected joint.16 After the first stage, patients are usually administered intravenous antibiotics for at least 6 weeks; these antibiotics are then withheld, and systemic inflammatory markers are evaluated for infection eradication. Although reliable criteria have been established for PJI diagnosis, guidelines for detecting eradication of infection are rudimentary. Most surgeons monitor the decrease in serologic markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) level, to assess the response to treatment. However, noninfectious etiologies may result in continued elevation of these markers.17 Even though aspirations are often performed to diagnose persistent infection before the second-stage procedure, their diagnostic utility may be limited.18 Use of cultures is also limited, as presence of antibiotic-loaded spacers can decrease the sensitivity of culture.19 Inadequate diagnosis often leads to unnecessary continuation of antimicrobial therapy or additional surgical débridement. Nuclear scans often remain positive because of aseptic inflammation related to surgery and are not useful in documenting sepsis arrest.20 Given the limitations of available tests, novel strategies for identifying the presence of infection at the second stage are being tested.
Synovial Fluid Cytokines
PJI pathogenesis begins with colonization of the implant surfaces with microorganisms and subsequent formation of biofilms.21 The human immune system is activated by the microbial products, cell wall components, and various biofilm proteins. Immune cells are recruited to the site, where they secrete a myriad of inflammatory biomarkers, such as cytokines, which promote further recruitment of inflammatory cells and aid in the eradication of pathogens.9 These inflammatory cytokines and cells are involved in aseptic inflammatory joint conditions, such as rheumatoid arthritis22,23; however, some are specifically involved in immune pathways combating pathogens.24 This action is the basis for increasing interest in using various synovial fluid cytokines and other biomarkers in the diagnosis of PJI. Here we describe some of the commonly studied cytokines.
Interleukin 1β
Interleukin 1β (IL-1β) is a major proinflammatory cytokine that is synthesized by multiple cells, including macrophages and monocytes.25 IL-1β is produced in response to microorganisms, other cytokines, antigen-presenting cells, and immune complexes; stimulates production of acute-phase proteins by the liver; and is an important pyrogen.25 Deirmengian and colleagues5 found that synovial IL-1β increased 258-fold in patients with a PJI. Studies have found that synovial IL-1β has sensitivity ranging from 66.7% to 100% and specificity ranging from 87% to 100%, with 1 study reporting an accuracy of 100%.5,6,26,27
Interleukin 6
Also produced by macrophages and monocytes, interleukin 6 (IL-6) is a potent stimulator of acute-phase proteins.28,29 IL-6 has a role as a chemoattractant and helps with cell differentiation when changing from innate to acquired immunity.30 It is also used as an aid in diagnosing PJI; it has sensitivity ranging from 62% to 100% and specificity ranging from 85% to 100%.5,6,26,31,32 Synovial IL-6 measurements were more accurate than serum IL-6 measurements.26 Furthermore, synovial IL-6 can be increased up to 27-fold in PJI cases.5 In one study, synovial IL-6 levels >2100 pg/mL had sensitivity of 62.5% and specificity of 85.7% in PJI diagnosis26; in another study, an IL-6 threshold of 4270 pg/mL had sensitivity of 87.1%, specificity of 100%, and accuracy of 94.6%.31
C-Reactive Protein
CRP is an acute-phase reactant. Blood levels increase in response to aseptic inflammatory processes and systemic infection.33 CRP plays an important role in host defense by activating complement and helping mediate phagocytosis.33,34 Although serum CRP levels have been used in diagnosing PJIs,6 they can yield false-negative results.35,36 Therefore, attention turned to synovial CRP levels, which were found to be increased 13-fold in PJI cases.5 It has been shown that synovial CRP levels are significantly higher in infected vs noninfected prosthetic joints34 and had diagnostic accuracy better than that of serum CRP levels in diagnosing PJI.37 One study found that CRP at a threshold of 3.7 mg/L had sensitivity of 84%, specificity of 97.1%, and accuracy of 91.5%,37 whereas another study found that CRP at a threshold of 3.61 mg/L had sensitivity of 87.1%, specificity of 97.7%, and accuracy of 93.3%.31
α-Defensin
α-Defensin, a natural peptide produced and secreted by neutrophils in response to pathogens, has antimicrobial and cytotoxic properties,38-40 signals for the secretion of various cytokines, and acts as a chemoattractant for various immune cells.41 Deirmengian and colleagues6 found that α-defensin was consistently elevated in patients with PJI. α-Defensin is extremely accurate in diagnosing PJI; it has sensitivity ranging from 97% to 100% and specificity ranging from 96% to 100%.6,27,42 Moreover, α-defensin was effective in diagnosing PJI caused by a wide spectrum of organisms, including various low-virulence bacteria and fungi.43
Leukocyte Esterase
Leukocyte esterase is an enzyme produced and secreted by neutrophils at sites of active infection.7,44 Testing for this enzyme is performed with a colorimetric strip and was originally performed for the diagnosis of urinary tract infections.44,45 In a study conducted by Parvizi and colleagues,7 this strip was used to test for leukocyte esterase in synovial fluid samples; a ++ reading was found to have sensitivity of 80.6% and specificity of 100% in diagnosing knee PJI. Similarly, De Vecchi and colleagues45 found sensitivity of 92.6% and specificity of 97%.
Other Synovial Markers
Research has identified numerous molecular biomarkers that may be associated with the pathogenesis of PJI. Although several (eg, cytokines) have demonstrated higher levels in synovial fluid in patients with PJI than in normal controls, only a few have had clinically relevant diagnostic utility.6 Deirmengian and colleagues6 screened 43 synovial fluid biomarkers that potentially could be used in the diagnosis of PJI. Besides the cytokine α-defensin, 4 other biomarkers—lactoferrin, neutrophil gelatinase-associated lipocalcin, neutrophil elastase 2, and bactericidal/permeability-increasing protein—had accuracy of 100%. In addition, 8 cytokines and biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1β, IL-6, IL-10, IL-1α) had area under the curve values higher than 0.9. Studies have also evaluated the diagnostic utility of metabolic products such as lactate, lactate dehydrogenase, and glucose; their accuracy was comparable to that of serum CRP.32
Serum Markers
In addition to the synovial fluid cytokines, several serum inflammatory cytokines have been studied as potential targets in diagnosing infection. Serum IL-6 has had excellent diagnostic accuracy46 and, when combined with CRP, could increase sensitivity in diagnosing PJI; such a combination (vs either test alone) could be useful in screening patients.47,48 Biomarkers such as tumor necrosis factor α and procalcitonin are considered very specific for PJI and may be useful in confirmatory testing.48 Evidence also suggests that toll-like receptor 2 proteins are elevated in the serum of patients with PJI and therefore are a potential diagnostic tool.49
Limitations of Synovial Cytokines
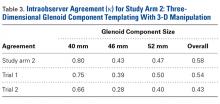
The literature suggests that some synovial fluid cytokines have promise.6 However, the best biomarker or combination of biomarkers is yet to be determined. Results have been consistent with α-defensin and other cytokines but mixed with IL-6 and still others32,42,50 (Table 2).
Information on the utility of synovial biomarkers in detecting persistent infection is limited. Frangiamore and colleagues50 found that IL-1 and IL-6 levels decreased between the stages of 2-stage revision. Unfortunately, none of the synovial fluid cytokines investigated (IL-1, IL-2, IL-6, IL-8, Il-10, interferon γ, granulocyte macrophage-colony stimulating factor, tumor necrosis factor α, IL-12p70) satisfactorily detected resolution of infection in the setting of prior treatment for PJI. Although cytokines are expected to be elevated in the presence of infection, the internal milieu at the time of stage 2 of the revision makes diagnosis of infection difficult. In addition, presence of spacer particles and recent surgery may activate immune pathways and yield false-positive results. Furthermore, antibiotic cement spacers may suppress the microorganisms to very low levels and yield false-negative results even if these organisms remain virulent.19
Even though the synovial molecular markers can detect the presence of infection, they are unable to identify pathogens. As identifying the pathogen is important in the treatment of PJI, there has been interest in using polymerase chain reaction (PCR) techniques.51 These tests may also provide specific information about the pathogen, such as its antibiotic sensitivity. A recently developed technology, the Ibis T5000 Universal Biosensor (Ibis Biosciences), uses novel pan-domain primers in a series of PCRs. This biosensor is useful in diagnosing infections when cultures are negative and appears to be more accurate than conventional PCR.13 As reported by Jacovides and colleagues,13 this novel PCR technique identified an organism in about 88% of presumed cases of aseptic revision.
Conclusion
PJI poses an extreme challenge to the healthcare system. Given the morbidity associated with improper management of PJI, accurate diagnosis is of paramount importance. Given the limitations of current tests, synovial fluid cytokines hold promise in the diagnosis of PJIs. However, these cytokines are expensive, and their clinical utility in PJI management is not well established. More research is needed before guidelines for synovial fluid cytokines and biomarkers can replace or be incorporated into guidelines for the treatment of PJIs.
Take-Home Points
- In cases of failed TJA, it is important to differentiate between septic and aseptic etiologies.
- Chronic and low-grade infections are challenging for orthopedic surgeons, as the symptoms often overlap with aseptic etiologies.
- Verification of infection eradication before beginning the second-stage reimplantation surgery is extremely important, but pre- and intraoperative findings can be unreliable.
- Synovial fluid cytokines have been shown to accurately diagnose PJIs.
- Synovial fluid cytokines may help surgeons differentiate between septic and aseptic cases of failed TJA.
Total joint arthroplasty (TJA) is an effective procedure that has been extensively used to relieve pain and improve quality of life in patients with various forms of joint disease. Although advances in technology and surgical technique have improved the success of TJA, periprosthetic joint infection (PJI) remains a serious complication. In the United States, it is estimated that PJI is the most common reason for total knee arthroplasty failure and the third most common reason for total hip arthroplasty revision.1 Although the incidence of PJI is 1% to 2%, the dramatic increase in TJA volume is expected to be accompanied by a similar rise in the number of infected TJAs; that number is expected to exceed 60,000 in the United States by 2020.2 Moreover, management of PJI is expensive and imposes a heavy burden on the healthcare system, with costs expected to hit $20 billion by 2020 in the US.2 Therefore, treating asepsis cases as infections imposes a heavy burden on the healthcare system and may result in excessive morbidity.3 At the same time, inadequate management of a PJI may result in recurrences that require infection treatment with morbid procedures, such as arthrodesis or amputation. Accurate diagnosis of PJI is of paramount importance in preventing potential implications of a misdiagnosed case. Unfortunately, the PJI diagnosis is extremely challenging, and the available diagnostic tests are often unreliable.4 Thus, research has recently focused on use of several synovial fluid cytokines in the detection of PJI.5-7 In this article, we provide an overview of the synovial biomarkers being used to diagnose PJI.
Diagnosis of Periprosthetic Joint Infection
Differentiating between septic and aseptic failed TJA is important, as the treatment options differ considerably. PJI can be broadly classified as acute or early postoperative (<6 weeks), late chronic (indolent onset), and acute-on-chronic (acute onset in well-functioning prosthesis, secondary to hematogenous spread).8 The acute and acute-on-chronic presentations are often associated with obvious signs of infection.9 However, chronic and low-grade infections pose a challenge to modern orthopedic practice, as the symptoms often overlap with that of aseptic causes of TJA failure.10 As a result, the International Consensus Group on Periprosthetic Joint Infection developed complex criteria using the Musculoskeletal Infection Society definition of PJI and involving a battery of tests for PJI diagnosis.11 According to these criteria, PJI is diagnosed when 1 of the 2 major criteria or 3 of the 5 minor criteria are met (Table 1).
Although these criteria constitute the most agreed on and widely used standard for PJI diagnosis, the definition is complex and often incomplete until surgical intervention. An ideal diagnostic test would aid in managing a PJI and provide results before a treatment decision is made. Many revision surgeries are being performed with insufficient information about the true diagnosis, and the diagnosis might change during or after surgery. About 10% of the revisions presumed to be aseptic may unexpectedly grow cultures during surgery and thereby satisfy the criteria for PJI after surgery.12 Moreover, with the use of novel methods such as polymerase chain reaction, microorganisms were identified in more than three-fourths of the presumed aseptic revisions.13 The optimal management of such cases is controversial, and it is unclear whether positive cultures should be treated as possible contaminants or true infection.12,14
Verification of Infection Eradication
A 2-stage revision procedure, widely accepted as the standard treatment for PJI, has success rates approaching 94%.15 In this procedure, it is important to verify infection eradication before beginning the second-stage reimplantation. Verification is crucial in avoiding reimplantation of an infected joint.16 After the first stage, patients are usually administered intravenous antibiotics for at least 6 weeks; these antibiotics are then withheld, and systemic inflammatory markers are evaluated for infection eradication. Although reliable criteria have been established for PJI diagnosis, guidelines for detecting eradication of infection are rudimentary. Most surgeons monitor the decrease in serologic markers, such as erythrocyte sedimentation rate and C-reactive protein (CRP) level, to assess the response to treatment. However, noninfectious etiologies may result in continued elevation of these markers.17 Even though aspirations are often performed to diagnose persistent infection before the second-stage procedure, their diagnostic utility may be limited.18 Use of cultures is also limited, as presence of antibiotic-loaded spacers can decrease the sensitivity of culture.19 Inadequate diagnosis often leads to unnecessary continuation of antimicrobial therapy or additional surgical débridement. Nuclear scans often remain positive because of aseptic inflammation related to surgery and are not useful in documenting sepsis arrest.20 Given the limitations of available tests, novel strategies for identifying the presence of infection at the second stage are being tested.
Synovial Fluid Cytokines
PJI pathogenesis begins with colonization of the implant surfaces with microorganisms and subsequent formation of biofilms.21 The human immune system is activated by the microbial products, cell wall components, and various biofilm proteins. Immune cells are recruited to the site, where they secrete a myriad of inflammatory biomarkers, such as cytokines, which promote further recruitment of inflammatory cells and aid in the eradication of pathogens.9 These inflammatory cytokines and cells are involved in aseptic inflammatory joint conditions, such as rheumatoid arthritis22,23; however, some are specifically involved in immune pathways combating pathogens.24 This action is the basis for increasing interest in using various synovial fluid cytokines and other biomarkers in the diagnosis of PJI. Here we describe some of the commonly studied cytokines.
Interleukin 1β
Interleukin 1β (IL-1β) is a major proinflammatory cytokine that is synthesized by multiple cells, including macrophages and monocytes.25 IL-1β is produced in response to microorganisms, other cytokines, antigen-presenting cells, and immune complexes; stimulates production of acute-phase proteins by the liver; and is an important pyrogen.25 Deirmengian and colleagues5 found that synovial IL-1β increased 258-fold in patients with a PJI. Studies have found that synovial IL-1β has sensitivity ranging from 66.7% to 100% and specificity ranging from 87% to 100%, with 1 study reporting an accuracy of 100%.5,6,26,27
Interleukin 6
Also produced by macrophages and monocytes, interleukin 6 (IL-6) is a potent stimulator of acute-phase proteins.28,29 IL-6 has a role as a chemoattractant and helps with cell differentiation when changing from innate to acquired immunity.30 It is also used as an aid in diagnosing PJI; it has sensitivity ranging from 62% to 100% and specificity ranging from 85% to 100%.5,6,26,31,32 Synovial IL-6 measurements were more accurate than serum IL-6 measurements.26 Furthermore, synovial IL-6 can be increased up to 27-fold in PJI cases.5 In one study, synovial IL-6 levels >2100 pg/mL had sensitivity of 62.5% and specificity of 85.7% in PJI diagnosis26; in another study, an IL-6 threshold of 4270 pg/mL had sensitivity of 87.1%, specificity of 100%, and accuracy of 94.6%.31
C-Reactive Protein
CRP is an acute-phase reactant. Blood levels increase in response to aseptic inflammatory processes and systemic infection.33 CRP plays an important role in host defense by activating complement and helping mediate phagocytosis.33,34 Although serum CRP levels have been used in diagnosing PJIs,6 they can yield false-negative results.35,36 Therefore, attention turned to synovial CRP levels, which were found to be increased 13-fold in PJI cases.5 It has been shown that synovial CRP levels are significantly higher in infected vs noninfected prosthetic joints34 and had diagnostic accuracy better than that of serum CRP levels in diagnosing PJI.37 One study found that CRP at a threshold of 3.7 mg/L had sensitivity of 84%, specificity of 97.1%, and accuracy of 91.5%,37 whereas another study found that CRP at a threshold of 3.61 mg/L had sensitivity of 87.1%, specificity of 97.7%, and accuracy of 93.3%.31
α-Defensin
α-Defensin, a natural peptide produced and secreted by neutrophils in response to pathogens, has antimicrobial and cytotoxic properties,38-40 signals for the secretion of various cytokines, and acts as a chemoattractant for various immune cells.41 Deirmengian and colleagues6 found that α-defensin was consistently elevated in patients with PJI. α-Defensin is extremely accurate in diagnosing PJI; it has sensitivity ranging from 97% to 100% and specificity ranging from 96% to 100%.6,27,42 Moreover, α-defensin was effective in diagnosing PJI caused by a wide spectrum of organisms, including various low-virulence bacteria and fungi.43
Leukocyte Esterase
Leukocyte esterase is an enzyme produced and secreted by neutrophils at sites of active infection.7,44 Testing for this enzyme is performed with a colorimetric strip and was originally performed for the diagnosis of urinary tract infections.44,45 In a study conducted by Parvizi and colleagues,7 this strip was used to test for leukocyte esterase in synovial fluid samples; a ++ reading was found to have sensitivity of 80.6% and specificity of 100% in diagnosing knee PJI. Similarly, De Vecchi and colleagues45 found sensitivity of 92.6% and specificity of 97%.
Other Synovial Markers
Research has identified numerous molecular biomarkers that may be associated with the pathogenesis of PJI. Although several (eg, cytokines) have demonstrated higher levels in synovial fluid in patients with PJI than in normal controls, only a few have had clinically relevant diagnostic utility.6 Deirmengian and colleagues6 screened 43 synovial fluid biomarkers that potentially could be used in the diagnosis of PJI. Besides the cytokine α-defensin, 4 other biomarkers—lactoferrin, neutrophil gelatinase-associated lipocalcin, neutrophil elastase 2, and bactericidal/permeability-increasing protein—had accuracy of 100%. In addition, 8 cytokines and biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1β, IL-6, IL-10, IL-1α) had area under the curve values higher than 0.9. Studies have also evaluated the diagnostic utility of metabolic products such as lactate, lactate dehydrogenase, and glucose; their accuracy was comparable to that of serum CRP.32
Serum Markers
In addition to the synovial fluid cytokines, several serum inflammatory cytokines have been studied as potential targets in diagnosing infection. Serum IL-6 has had excellent diagnostic accuracy46 and, when combined with CRP, could increase sensitivity in diagnosing PJI; such a combination (vs either test alone) could be useful in screening patients.47,48 Biomarkers such as tumor necrosis factor α and procalcitonin are considered very specific for PJI and may be useful in confirmatory testing.48 Evidence also suggests that toll-like receptor 2 proteins are elevated in the serum of patients with PJI and therefore are a potential diagnostic tool.49
Limitations of Synovial Cytokines
The literature suggests that some synovial fluid cytokines have promise.6 However, the best biomarker or combination of biomarkers is yet to be determined. Results have been consistent with α-defensin and other cytokines but mixed with IL-6 and still others32,42,50 (Table 2).
Information on the utility of synovial biomarkers in detecting persistent infection is limited. Frangiamore and colleagues50 found that IL-1 and IL-6 levels decreased between the stages of 2-stage revision. Unfortunately, none of the synovial fluid cytokines investigated (IL-1, IL-2, IL-6, IL-8, Il-10, interferon γ, granulocyte macrophage-colony stimulating factor, tumor necrosis factor α, IL-12p70) satisfactorily detected resolution of infection in the setting of prior treatment for PJI. Although cytokines are expected to be elevated in the presence of infection, the internal milieu at the time of stage 2 of the revision makes diagnosis of infection difficult. In addition, presence of spacer particles and recent surgery may activate immune pathways and yield false-positive results. Furthermore, antibiotic cement spacers may suppress the microorganisms to very low levels and yield false-negative results even if these organisms remain virulent.19
Even though the synovial molecular markers can detect the presence of infection, they are unable to identify pathogens. As identifying the pathogen is important in the treatment of PJI, there has been interest in using polymerase chain reaction (PCR) techniques.51 These tests may also provide specific information about the pathogen, such as its antibiotic sensitivity. A recently developed technology, the Ibis T5000 Universal Biosensor (Ibis Biosciences), uses novel pan-domain primers in a series of PCRs. This biosensor is useful in diagnosing infections when cultures are negative and appears to be more accurate than conventional PCR.13 As reported by Jacovides and colleagues,13 this novel PCR technique identified an organism in about 88% of presumed cases of aseptic revision.
Conclusion
PJI poses an extreme challenge to the healthcare system. Given the morbidity associated with improper management of PJI, accurate diagnosis is of paramount importance. Given the limitations of current tests, synovial fluid cytokines hold promise in the diagnosis of PJIs. However, these cytokines are expensive, and their clinical utility in PJI management is not well established. More research is needed before guidelines for synovial fluid cytokines and biomarkers can replace or be incorporated into guidelines for the treatment of PJIs.
1 Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
3. Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85(6):1000-1004.
4. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869-882.
5. Deirmengian C, Hallab N, Tarabishy A, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017-2023.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
7. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242-2248.
8. Kuiper JW, Willink RT, Moojen DJF, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667-676.
9. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654.
10. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
11. Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
12. Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29(11):2181-2186.
13. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)–based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
14. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):94-99.
15. Macheras GA, Koutsostathis SD, Kateros K, Papadakis S, Anastasopoulos P. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int. 2012;22(suppl 8):S54-S61.
16. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res. 2016;474(7):1619-1626.
17. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002-1008.
18. Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305-309.
19. Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552-1557.
20. Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66-78.
21. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158-168.
22. Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49-53.
23. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585-592.
24. Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545-594.
25. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075-1079.
26. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644-651.
27. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
28. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
29. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621-636.
30. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888.
31. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
32. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
33. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24(2):163-176.
34. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27(8 suppl):12-16.
35. Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444-e449.
36. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-1626.
37. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54-60.
38. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105-128.
39. Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427-1435.
40. Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104(6):1778-1783.
41. Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249.
42. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
43. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229-2235.
44. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
45. De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555-560.
46. Di Cesare PE, Chang E, Preston CF, Liu C. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921-1927.
47. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61(3):332-341.
48. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94-99.
49. Galliera E, Drago L, Vassena C, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620-623.
50. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630-1639.
51. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
52. Omar M, Ettinger M, Reichling M, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97(2):173-176.
53. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997-4003.
54. Omar M, Ettinger M, Reichling M, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. J Bone Joint Surg Am. 2014;96(24):2032-2037.
1 Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61-65.e1.
3. Sierra RJ, Trousdale RT, Pagnano MW. Above-the-knee amputation after a total knee replacement: prevalence, etiology, and functional outcome. J Bone Joint Surg Am. 2003;85(6):1000-1004.
4. Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88(4):869-882.
5. Deirmengian C, Hallab N, Tarabishy A, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017-2023.
6. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254-3262.
7. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242-2248.
8. Kuiper JW, Willink RT, Moojen DJF, van den Bekerom MP, Colen S. Treatment of acute periprosthetic infections with prosthesis retention: review of current concepts. World J Orthop. 2014;5(5):667-676.
9. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-1654.
10. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1-e25.
11. Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
12. Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29(11):2181-2186.
13. Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)–based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94(24):2247-2254.
14. Barrack RL, Aggarwal A, Burnett RS, et al. The fate of the unexpected positive intraoperative cultures after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):94-99.
15. Macheras GA, Koutsostathis SD, Kateros K, Papadakis S, Anastasopoulos P. A two stage re-implantation protocol for the treatment of deep periprosthetic hip infection. Mid to long-term results. Hip Int. 2012;22(suppl 8):S54-S61.
16. George J, Kwiecien G, Klika AK, et al. Are frozen sections and MSIS criteria reliable at the time of reimplantation of two-stage revision arthroplasty? Clin Orthop Relat Res. 2016;474(7):1619-1626.
17. Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469(4):1002-1008.
18. Lonner JH, Siliski JM, Della Valle C, DiCesare P, Lotke PA. Role of knee aspiration after resection of the infected total knee arthroplasty. Am J Orthop. 2001;30(4):305-309.
19. Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82(11):1552-1557.
20. Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39(1):66-78.
21. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol. 2012;65(2):158-168.
22. Fontana A, Hengartner H, Weber E, Fehr K, Grob PJ, Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49-53.
23. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83(2):585-592.
24. Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals (Basel). 2014;7(5):545-594.
25. Stylianou E, Saklatvala J. Interleukin-1. Int J Biochem Cell Biol. 1998;30(10):1075-1079.
26. Gollwitzer H, Dombrowski Y, Prodinger PM, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644-651.
27. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439-1445.
28. Randau TM, Friedrich MJ, Wimmer MD, et al. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9(2):e89045.
29. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621-636.
30. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888.
31. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 suppl):99-103.e1.
32. Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29(6):1105-1109.
33. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24(2):163-176.
34. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27(8 suppl):12-16.
35. Ghanem E, Antoci V, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444-e449.
36. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-1626.
37. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ. Mark B. Coventry award: synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54-60.
38. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105-128.
39. Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427-1435.
40. Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104(6):1778-1783.
41. Ulm H, Wilmes M, Shai Y, Sahl HG. Antimicrobial host defensins—specific antibiotic activities and innate defense modulation. Front Immunol. 2012;3:249.
42. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006-4009.
43. Deirmengian C, Kardos K, Kilmartin P, Gulati S, Citrano P, Booth RE. The alpha-defensin test for periprosthetic joint infection responds to a wide spectrum of organisms. Clin Orthop Relat Res. 2015;473(7):2229-2235.
44. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96(22):1917-1920.
45. De Vecchi E, Villa F, Bortolin M, et al. Leucocyte esterase, glucose and C-reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555-560.
46. Di Cesare PE, Chang E, Preston CF, Liu C. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921-1927.
47. Ettinger M, Calliess T, Kielstein JT, et al. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis. 2015;61(3):332-341.
48. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Götze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94-99.
49. Galliera E, Drago L, Vassena C, et al. Toll-like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620-623.
50. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630-1639.
51. Rasouli MR, Harandi AA, Adeli B, Purtill JJ, Parvizi J. Revision total knee arthroplasty: infection should be ruled out in all cases. J Arthroplasty. 2012;27(6):1239-1243.e1-e2.
52. Omar M, Ettinger M, Reichling M, et al. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97(2):173-176.
53. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997-4003.
54. Omar M, Ettinger M, Reichling M, et al. Preliminary results of a new test for rapid diagnosis of septic arthritis with use of leukocyte esterase and glucose reagent strips. J Bone Joint Surg Am. 2014;96(24):2032-2037.
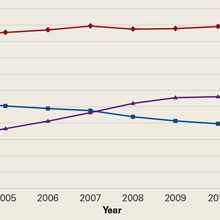
Paraskiing Crash and Knee Dislocation With Multiligament Reconstruction and Iliotibial Band Repair
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
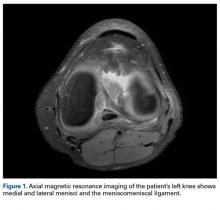
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
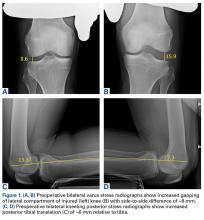
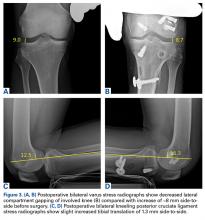
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
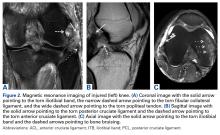
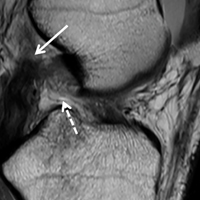
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
Take-Home Points
- Reconstruction of a torn ITB is important in restoration of native anatomy and function given its properties in anterolateral stabilization and resistance to varus stress and internal tibial rotation.
- Restoration of posterolateral instability primarily involves reconstructing the FCL, PLT, and popliteofibular ligament.
- For combined PLC injuries, concurrent reconstruction of the cruciate ligaments in one stage is highly recommended.
- Post-surgery, a 6-week non-weight-bearing, limited flexion rehab protocol utilizing a dynamic PCL brace, such as the PCL Rebound brace, is recommended to prevent posterior tibial sag.
- Arthrofibrosis and decreased ROM can be seen following a violent knee injury which requires extensive multiligament reconstruction surgeries, occasionally requiring a secondary surgery for further restoration of knee motion.
Tibiofemoral knee dislocations are uncommon injuries that have devastating complications and potentially result in complex surgeries.1 Knee dislocations (KDs) can be classified with the Schenck system.2 KD-I is a multiligament injury involving the anterior cruciate ligament (ACL) or the posterior cruciate ligament (PCL), and the scale increases in severity/number of ligaments involved, with KD-V being a multiligament injury with periarticular fracture.2
In this article, we report the case of a complex multiligament knee reconstruction performed with a midsubstance iliotibial band (ITB) repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old man presented 12 days after a paraskiing crash in which he collided with a tree at 45 mph and fell 40 feet before hitting snow. Physical examination revealed a large hemarthrosis of the left lower extremity and ecchymosis about the posterolateral aspect of the knee and popliteal fossa. Range of motion (ROM) was limited from 5° of hyperextension to 90° of flexion. Additional motion was deferred secondary to pain. Varus stress testing at 0° and 30° of knee flexion demonstrated significant side-to-side differences. The Lachman test, posterior drawer test, and posterolateral drawer test were all 3+. The dial test was 3 to 4+ compared with the contralateral knee. Valgus stress testing at 0° and 30° of flexion did not reveal any side-to-side laxity. The calf was nontender, and all compartments were soft. The patient reported no neurovascular symptoms and had no neuromotor deficits other than mild common peroneal nerve dysesthesias.
Varus stress radiographs showed increased side-to-side gapping (8 mm) of the lateral compartment of the injured knee. Kneeling posterior stress radiographs, limited because of the patient’s inability to apply full stress on the injured knee secondary to pain, showed a difference of 6 mm in increased posterior translation on the uninjured leg (Figures 1A-1D).
First Surgery
1. PLC Approach. A lateral hockey-stick skin incision was made along the ITB and extended distally between the fibular head and the Gerdy tubercle. The subcutaneous tissue was then dissected, and a posteriorly based flap was developed for preservation of vascular support to the superficial tissues. The ITB and the lateral capsule had completely torn off of the femur, allowing exposure directly into the joint. The long and short heads of the biceps femoris were exposed, with about 50% of the biceps attachment torn. The FCL was torn midsubstance, and the PLT had no remnant attachment left on the femur.
2. ITB and Lateral Capsule Tag Stitched. The torn ends of the ITB were dissected and tag stitches placed in each end. Tag stitches were also placed in the lateral capsule in preparation for a direct repair.
3. Neurolysis. The common peroneal nerve was found encased in a significant amount of scar tissue, and extensive neurolysis was required. Slow, methodical dissection was performed under the partially torn long head of the biceps femoris and was continued through the scar tissue and adhesions. Distally, 5 mm to 7 mm of the peroneus longus fascia was incised as part of the neurolysis in order to prevent nerve irritation or foot drop caused by postoperative swelling.
4. PLC Tunnels. The margin between the lateral gastrocnemius tendon and the soleus muscle was identified by blunt dissection that allowed palpation of the posteromedial aspect of the fibular styloid and the popliteus musculotendinous junction. The underlying biceps bursa was incised in order to locate the midportion of the FCL remnant, which typically is tag-stitched with No. 2 FiberWire to help identify the femoral attachment (this was not done because of the complete tear at the midsubstance of the FCL).
Subperiosteal dissection of the lateral aspect of the fibular head was performed anterior to posterior and distally extended to the champagne-glass drop-off of the fibular head. Continuing the dissection distally beyond this point can endanger the common peroneal nerve. A small sulcus can be palpated where the distal FCL inserts on the fibular head. Posteriorly, a small elevator was used to dissect the soleus muscle off of the posteromedial aspect of the fibular head, where the fibular tunnel would later be created.
A Chandler retractor was placed posterior to the fibular head to protect the neurovascular bundle. With the aid of a collateral ligament aiming device, a guide pin was drilled from the lateral aspect of the fibular head (FCL attachment) to the posteromedial downslope of the fibular styloid (popliteofibular ligament attachment). The entry point of the guide pin was immediately above the champagne- glass drop-off, at the distal insertion site of the FCL, which was described as being 28.4 mm from the styloid tip and 8.2 mm posterior to the anterior margin of the fibular head.3 Care should be taken not to ream the tunnel too proximal, as doing so increases the risk of iatrogenic fracture. A 7-mm reamer was then used to drill the fibular tunnel. To facilitate later passage of the graft, a passing suture was placed through the tunnel, leaving the loop anterolateral.
Next, the starting point for the tibial tunnel was located on the flat spot of the anterolateral tibia distal and medial to the Gerdy tubercle, just lateral to the tibial tubercle. The tibial popliteal sulcus was identified by palpation of the posterolateral tibial plateau to localize the site of the popliteus musculotendinous junction, which is the ideal location of the posterior aperture of the tibial tunnel. This point is 1 cm proximal and 1 cm medial to the posteromedial exit of the fibular tunnel. A Chandler retractor was placed anterior to the lateral gastrocnemius to protect the neurovascular bundle. In the locations described earlier, a cruciate aiming device was used to place a guide pin anterior to posterior. A 9-mm tunnel was overreamed and a passing suture placed, leaving the loop posterior to facilitate graft passage.
The femoral insertions of the FCL and the PLT were then identified. ITB splitting was not necessary, given the complete midsubstance tear of this structure. The FCL attachment was identified 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle.3 Sharp dissection was performed in this location, proximal to distal, exposing the lateral epicondyle and the small sulcus at the FCL attachment site. A collateral ligament reconstruction aiming sleeve was used to drill a guide pin over the FCL femoral attachment site and out the medial aspect of the distal thigh, about 5 cm proximal and anterior to the adductor tubercle.
The femoral attachment of the PLT was reported located 18.5 mm anterior to the FCL insertion, in the anterior fifth of the popliteal sulcus.3 Although arthrotomy is usually required in order to access the PLT attachment, it was not necessary in this case, given the lateral capsule tear. A guide pin was inserted at the PLT attachment site, parallel to the FCL pin. After proper placement was verified, a 9-mm reamer was used to drill the FCL and PLT tunnels to a depth of 25 mm (socket), and a passing suture was placed into each tunnel to facilitate graft passage.
5. ACL Graft Harvest. The central third of the ipsilateral patellar tendon was harvested for use in the ACL reconstruction. Included were a 10-mm × 20-mm bone plug from the patella and a 10-mm × 25-mm bone plug from the tibial tubercle. The patella defect was then bone-grafted, and the patellar tendon closed side-to-side.
6. Graft Preparation. For the PLC, we used a split Achilles tendon allograft that had two 9-mm × 25-mm bone plugs proximally and were tubularized distally. For the PCL, we used an anterolateral bundle (ALB), which consisted of an Achilles tendon allograft that had an 11-mm × 25-mm bone plug proximally and was tubularized distally, and a posteromedial bundle (PMB), which consisted of a tibialis anterior allograft that was tubularized at both ends. For the ACL, we used a bone–patellar tendon–bone autograft 10 mm in diameter with a 20-mm femoral bone plug and a 25-mm tibial bone plug distally.
7. Arthroscopy. We created standard anterolateral and anteromedial parapatellar portals and performed arthroscopy, including lysis of adhesions. Cartilage and menisci were lesion-free.
8. PCL Femoral Tunnels. The ALB attachment was identified and outlined with a coagulator between the trochlear point and the medial arch point, adjacent to the edge of the articular cartilage. Similarly, the PMB attachment was marked about 8 mm or 9 mm posterior to the edge of the articular cartilage of the medial femoral condyle and slightly posterior to the ALB tunnel.4
In the anterolateral tunnel, an acorn reamer 11 mm in diameter was used to score the entry point of the ALB femoral tunnel. An eyelet pin was then drilled through the reamer anteromedially out the knee. Then a closed socket tunnel was reamed over the eyelet pin to a depth of 25 mm. A passing suture was pulled through the tunnel in preparation for graft passage.
With use of the same technique, a 7-mm reamer was placed against the outline of the PMB attachment site, and an eyelet pin was drilled through this reamer and out the anteromedial aspect of the knee. Again, a 25-mm deep closed socket was reamed. A bone bridge distance of 2 mm was maintained between the 2 femoral PCL bundle tunnels.
9. ACL Femoral Tunnel. The femoral ACL attachment was identified and outlined. An over-the-top guide was used to determine proper placement of the 10-mm low-profile reamer. A guide pin was drilled through the center of the reamer. The reamer was used to create a 25-mm deep closed socket tunnel, and a passing stitch was placed.
10. PCL Tibial Tunnel. With use of a 70° arthroscope for visualization, a posteromedial arthroscopic portal was created, and a shaver and a coagulator were used to identify the tibial PCL attachment, located distally along the PCL facet, until the proximal aspect of the popliteus muscle fibers were visualized. A guide pin was drilled starting at the anteromedial aspect of the tibia, about 6 cm distal to the joint line and centered between the anterior tibial crest and the medial tibial border. The pin exited posteriorly at the center of the PCL tibial attachment along the PCL bundle ridge, which was reported located between the ALB and the PMB on the tibia.5 Pin placement was verified with intraoperative lateral and anteroposterior radiographs. On the lateral radiograph, the pin should be about 6 mm or 7 mm proximal to the champagne-glass drop-off at the PCL facet on the posterior aspect of the tibia. On the anteroposterior radiograph, the pin should be 1 mm to 2 mm distal to the joint line and at the medial aspect of the lateral tibial eminence. A large curette was passed through the posteromedial arthroscopic portal both to retract the posterior tissues away from the reamer and to protect against guide-pin protrusion The guide pin was then overreamed with a 12-mm acorn reamer.
A large smoother was passed proximally up the tibial tunnel and then pulled out the anteromedial portal with a grasper. The smoother was gently cycled to smooth the intra-articular tibial tunnel aperture to remove any bony spicules that could interfere with graft passage. The smoother was then pulled back into the joint, passed out the anterolateral arthroscopic portal, and secured with a small clamp.4
11. ACL Tibial Tunnel. The ACL tibial attachment site was identified and cleaned of soft tissue. A guide pin was placed and then overreamed with a 10-mm acorn reamer.
12. PCL Femoral Fixation. The PMB graft was passed into its tunnel and secured with a 7-mm × 23-mm titanium screw. Next, the ALB was secured to the femur with a 7-mm × 20-mm titanium screw. The smoother was used to pull both grafts down through the tibial tunnel.
13. ACL Femoral Fixation. A 7-mm × 20-mm titanium screw was then used to fix the ACL autograft inside the femur. Traction was applied to the 3 cruciate grafts. There was no sign of impingement.
14. PLC Femoral Fixation. The FCL and the popliteus bone plugs were passed into their respective femoral sockets and secured with 7-mm × 20-mm titanium screws.
15. Lateral Capsule Femoral Anchors. Two suture anchors were placed into the femur, and the sutures were passed through the femoral portion of the lateral capsule for later repair.
16. PCL Tibial Fixation. Both grafts were fixed with a fully threaded bicortical 6.5-mm × 40-mm cannulated cancellous screw and an 18-mm spiked washer. The ALB was fixed first, with the knee flexed to 90°, traction on the graft, and the tibia in neutral rotation. Restoration of the normal tibiofemoral step-off was verified. The PMB was then fixed with the knee in full extension. A posterior drawer test was performed to verify restoration of stability.
17. PLC Fibula Fixation. The PLT graft was passed down the popliteal hiatus, and the FCL graft was passed under the remnant of the biceps bursa on the fibular head and then through the fibular head, anterolateral to posteromedial. The FCL graft was fixed in the fibular tunnel with the knee in 20° of flexion, a slight valgus reduction force, the tibia in neutral rotation, and traction on the graft. A 7-mm × 23-mm bioabsorbable screw was used.
18. Lateral Capsular Repair. The lateral capsule was directly repaired with the previously placed sutures. The sutures were tied with the knee in 20° of flexion.
19. PLC Tibial Fixation. The grafts were passed together, posterior to anterior, through the tibial tunnel. The knee was cycled several times through complete flexion/extension ROM. A 9-mm × 23-mm bioabsorbable screw was then used to fix the grafts to the tibia. During this fixation, the knee was kept in 60° of flexion and neutral rotation while traction was being applied to the distal end of both grafts.
20. ACL Tibial Fixation. A 9-mm × 20-mm titanium screw was used to fix the ACL graft with the knee in full extension. The graft was then viewed intra-articularly to confirm there was no impingement. The Lachman, posterior drawer, posterolateral drawer, dial, and varus stress tests were performed to ensure restoration of stability.
21. ITB Repair. A portion of the remaining Achilles tendon allograft was used to perform ITB reconstruction (reconstitution of the gaped portion of the ITB). Orthocord (DePuy Synthes) and Vicryl (Ethicon) sutures were used for this reconstruction. Knee stability was deemed restored, and the incisions were closed in standard layered fashion.
First Surgery: Postoperative Management
The patient remained non-weight-bearing the first 6 weeks after surgery, with prone knee flexion limited (0°-90°) the first 2 weeks. In addition, a PCL Jack brace (Albrecht) was placed 1 week after surgery and was to be worn at all times to decrease stress on the PCL grafts.
As ROM was not progressing as expected, the patient was instructed to use a continuous passive motion (CPM) machine 2 hours 3 times a day. About 4 weeks after surgery, with ROM still not progressing, the frequency of use of this machine was increased.
Despite continued physical therapy, use of the CPM machine, and pain management, ROM was limited (11°-90° of flexion) 5.5 months after left knee multiligament reconstruction. However, stress radiographs showed excellent stability. Varus stress radiographs showed a side-to-side difference of 0.3 mm less on the left (injured) knee, and kneeling PCL stress radiographs showed a side-to-side difference of 1.3 mm more on the left knee (Figures 3A-3D).
Second Surgery and Postoperative Management
As gentle manipulation under anesthesia was unsuccessful, the patient underwent knee arthroscopy, including 4-compartment lysis of adhesions, arthroscopically assisted posteromedial capsular release, and post-débridement manipulation under anesthesia. During manipulation, full extension and knee flexion up to 135° were achieved. ACL, PCL, and popliteus grafts were visualized and confirmed to be intact.
After this second surgery, the patient was to resume physical therapy and begin weight- bearing as tolerated. Active ROM was prioritized in an attempt to reach full ROM. In addition, a CPM machine was to be used from 0° to 135° of knee flexion 4 hours 3 times a day for 6 weeks.
Two weeks after surgery, the patient had continued pain, and extracapsular swelling in the left knee. However, ROM (0°-115° of flexion) was improved relative to before surgery (11°-90° of flexion), though it remained below the range on the contralateral side. Of note, the patient reported having a flexion contracture (~10°) in the immediate postoperative period. He had woken up with it after sleeping with the CPM machine the night before. The contracture delayed his physical therapy for several hours and resulted in a redesign of his therapy protocol to emphasize full, active knee extension and patellar mobilization, as well as discontinuation of use of the CPM machine. Corticosteroids were initiated to help with the extracapsular swelling, and the new therapy regimen brought adequate progress in ROM. Four months after the second surgery, the patient had full extension and 135° of flexion and was transitioned into wearing the PCL Rebound brace.
Discussion
This case was unique because of the midsubstance ITB tear and simultaneous multiligament injury caused by a KD-IIIL, a KD involving the ACL, the PCL, and the PLC with the medial side intact. There is limited research on ITB repair generally, with or without KD involvement. In a retrospective review of acute knee trauma cases, ITB pathologies were seen on 45% of reviewed MRI scans, and only 3% of the injuries were grade III; in addition, only 9 (5%) of the 200 cases involved both ITB and multiligament (ACL, PCL) knee injuries.6
After our patient’s ACL, PCL, and PLC were reconstructed, a fan piece of the Achilles tendon allograft from the PLC reconstruction was used to repair the ITB. The graft was used to reconstitute the torn gapped portion of the band in multiple locations, and this repair helped restore stability. The literature has reported numerous surgical uses for a portion of the ITB but few studies on repairing this anatomical structure. Preservation of the ITB is important to restoration of native anatomy and function. The ITB helps with anterolateral stabilization of the knee and with resistance of varus stress and internal tibial rotation.
The PLC reconstruction used in this case has been biomechanically validated as restoring the knee to near native stability through anatomical reconstruction of the PLC’s 3 main static stabilizers: the FCL, the PLT, and the popliteofibular ligament.7-9 First described in 2004,7 this anatomical PLC reconstruction technique has improved subjective and objective patient outcomes.10,11 For combined PLC injuries (eg, our patient’s injuries), Geeslin and LaPrade10 recommended concurrent reconstruction of the cruciate ligaments. In addition to the PLC reconstruction, the anatomical double-bundle PCL reconstruction used in this case has demonstrated significant improvements in subjective and objective outcome scores and objective knee stability.12
Although the stability and anatomy of this patient’s injured knee were reestablished, his development of arthrofibrosis is important. Many have discussed the commonality of arthrofibrosis or decreased ROM after extensive multiligament reconstruction surgeries.13,14 One study involving surgical management and outcomes of multiligament knee injuries found that, in more than half of its cases, restoration of full ROM required at least one operation after the initial one.13 Therefore, it is not unusual that our patient required a second operation for decreased ROM.
Conclusion
After surgery, excellent stabilization was achieved. Although the patient had setbacks related to pain and decreased ROM, his second surgery and continued physical therapy likely will help him return to his preoperative recreational activity levels.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
1. Delos D, Warren RF, Marx RG. Multiligament knee injuries and their treatment. Oper Tech Sports Med. 2010;18(4):219-226.
2. Hobby B, Treme G, Wascher DC, Schenck RC. How I manage knee dislocations. Oper Tech Sports Med. 2010;18(4):227-234.
3. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860.
4. Chahla J, Nitri M, Civitarese D, Dean CS, Moulton SG, LaPrade RF. Anatomic double-bundle posterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(1):e149-e156.
5. Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF. Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am. 2012;94(21):1936-1945.
6. Mansour R, Yoong P, McKean D, Teh JL. The iliotibial band in acute knee trauma: patterns of injury on MR imaging. Skeletal Radiol. 2014;43(10):1369-1375.
7. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414.
8. McCarthy M, Camarda L, Wijdicks CA, Johansen S, Engebretsen L, LaPrade RF. Anatomic posterolateral knee reconstructions require a popliteofibular ligament reconstruction through a tibial tunnel. Am J Sports Med. 2010;38(8):1674-1681.
9. LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543-549.
10. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683.
11. LaPrade RF, Johansen S, Agel J, Risberg MA, Moksnes H, Engebretsen L. Outcomes of an anatomic posterolateral knee reconstruction. J Bone Joint Surg Am. 2010;92(1):16-22.
12. Spiridonov SI, Slinkard NJ, LaPrade RF. Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am. 2011;93(19):1773-1780.
13. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
14. Yenchak AJ, Wilk KE, Arrigo CA, Simpson CD, Andrews JR. Criteria-based management of an acute multistructure knee injury in a professional football player: a case report. J Orthop Sports Phys Ther. 2011;41(9):675-686.
Risk of Osteoporotic Fracture After Steroid Injections in Patients With Medicare
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
Take-Home Points
Analysis of patients in the Medicare database showed that each successive ESI decreased the risk of an osteoporotic spine fracture by 2%, and that each successive LJSI decreases it by 4%.
Although statistically significant, this may not be clinically relevant.
Successive ESI did not influence the risk of developing an osteoporotic hip or wrist fracture, but that each additional LJSI reduced the risk.
Prolonged steroid exposure was found to increase the risk of spine fracture for ESI and LJSI patients.
Acute exposure to exogenous steroids via the epidural space, transforaminal space, or large joints does not seem to increase the risk of an osteoporotic fracture of the spine, hip, or wrist.
Epidural steroid injections (ESIs) are widely used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. The treatment rationale is that locally injected anti-inflammatory drugs, such as steroids, reduce inflammation by inhibiting formation and release of inflammatory cytokines, leading to pain reduction.1,2 According to 4 systematic reviews, the best available evidence of the efficacy of ESIs is less than robust.3-6 These reviews were limited by the heterogeneity of patient selection, delivery mode, type and dose of steroid used, number and frequency of ESIs, and outcome measures.
The association of chronic oral steroid use and the development of osteoporosis was previously established.7,8 One concern is that acute exposure to steroids in the form of lumbar ESIs may also lead to osteoporosis and then a pathologic fracture of the vertebra. Several studies have found no association between bone mineral density and cumulative steroid dose,9,10 mean number of ESIs, or duration of ESIs,10 though other studies have found lower bone mineral density in postmenopausal women treated with ESIs.11-13
In a study of 3000 ESI patients propensity-matched to a non-ESI cohort, Mandel and colleagues14 found that each successive ESI increased the risk of osteoporotic spine fracture by 21%. This clinically relevant 21% increased risk might lead physicians to stop prescribing or using this intervention. However, the association between osteoporotic fractures and other types of steroid injections remains poorly understood and underinvestigated.
To further evaluate the relationship between steroid injections and osteoporotic fracture risk, we analyzed Medicare administrative claims data on both large-joint steroid injections (LJSIs) into knee and hip and transforaminal steroid injections (TSIs), as well as osteoporotic hip and wrist fractures. Our hypothesis was that a systemic effect of steroid injections would increase fracture risk in all skeletal locations regardless of injection site, whereas a local effect would produce a disproportionate increased risk of spine fracture with spine injection.
Materials and Methods
Medicare is a publicly funded US health insurance program for people 65 years old or older, people under age 65 years with certain disabilities, and people (any age) with end-stage renal disease or amyotrophic lateral sclerosis. The 5% Medicare Part B (physician, carrier) dataset contains individual claims records for a random sample of Medicare beneficiaries (~2.4 million enrollees). Patients who received steroid injections were identified from 5% Medicare claims made between January 1, 2004 and December 31, 2011. LJSIs were identified by Current Procedural Terminology (CPT) code 20610 and any of 16 other CPT codes: J0702, J1020, J1030, J1040, J1094, J1100, J1700, J1710, J1720, J2650, J2920, J2930, J3300, J3301, J3302, and J3303. ESIs were identified by CPT code 62310, 62311, 62318, or 62319, and TSIs by CPT code 64479, 64480, 64483, or 64484. Patients were followed in their initial injection cohort. For example, a patient who received an ESI initially and later received an LJSI remained in the ESI cohort.
Several groups of patients were excluded from the study: those who received Medicare coverage because of their age (under 65 years) and disabilities; those who received Medicare health benefits through health maintenance organizations (healthcare expenses were not submitted to the Centers for Medicare & Medicaid Services for payment, and therefore claims were not in the database or were incomplete); those with a prior claim history of <12 months (incomplete comorbidity history); and those who received a diagnosis of osteoporotic fracture (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 733.1x) before the initial steroid injection.
We determined the incidence of osteoporotic wrist, hip, and spine fractures within 1, 2, and 8 years after LJSI, ESI, and TSI. Wrist, hip, and spine fractures were identified by ICD-9-CM diagnosis codes 733.12, 733.13, and 733.14, respectively. We also determined the number of steroid injections given before wrist, hip, or spine fracture or, if no fracture occurred, before death or the end of the data period.
Statistical Analysis
Multivariate Cox regression analysis was performed to evaluate the risk factors for wrist, spine, and hip fractures. The covariates in this model included age, sex, race, census region, Medicare buy-in status, Charlson Comorbidity Index (CCI),15 year, and number of steroid injections before fracture, death, or end of data period. Medicare buy-in status, which indicates whether the beneficiary received financial assistance in paying insurance premiums, was used as a proxy for socioeconomic status. CCI is used as a composite score of a patient’s general health status in terms of comorbidities.15,16 Four previously established categories17 were used to group CCIs in this study: 0 (none), 1 to 2 (low), 3 to 4 (moderate), and 5 or more (high). In addition, several diagnoses made within the 12 months before initial steroid injection were considered: osteoporosis (ICD-9-CM codes 733.0x, V82.81), Cushing syndrome (ICD-9-CM code 255.0), long-term (current) use of bisphosphonates (ICD-9-CM code V58.68), asymptomatic postmenopausal status (ICD-9-CM code V49.81), postmenopausal hormone replacement therapy (ICD-9-CM code V07.4), and long-term (current) use of steroids (ICD-9-CM code V58.65). The comparison of relative risk between any groups was reported as the adjusted hazard ratio (AHR), which is the ratio of the hazard rates of that particular outcome, taking into account inherent patient characteristics such as age, sex, and race as covariates. AHR of 1 corresponds to equivalent risk, AHR of >1 to elevated risk, and AHR of <1 to reduced risk.
Results
Using the 5% Medicare data for 2004 to 2011, we identified 275,999 Medicare beneficiaries who underwent LJSI, 93,943 who underwent ESI, and 32,311 who underwent TSI. During this period, TSI use increased, ESI use decreased, and LJSI use was relatively stable (Figure).
The risk for osteoporotic spine fracture 1, 2, and 8 years after ESI, TSI, or LJSI was affected by age, race, sex, and CCI (P < .001 for all; Tables 2-4).
The risk for osteoporotic hip fracture after 1 and 2 years was affected by age and number of LJSIs and TSIs but not by number of ESIs. Sex and CCI were also risk factors for hip fracture at 1 and 2 years for ESI and LJSI patients, as was race for LJSI patients. Risk for osteoporotic wrist fracture at 1 and 2 years was affected by sex and race for ESI and LJSI patients; age, race, CCI, and long-term steroid use were risk factors for TSI patients at all time points. Higher number of LJSIs, but not ESIs or TSIs, was associated with lower wrist fracture risk.
Discussion
ESIs continue to be used in the nonoperative treatment of low back pain, radicular leg pain, and spinal stenosis. Although the present study found ESI use increased in the Medicare population between 1994 and 2001,18 the trend is reversing, decreasing by 25%, with rates of 264 per 10,000 Medicare enrollees in 2004 and 194 per 10,000 enrollees in 2011. ESI use may have changed after systematic reviews revealed there was no clear evidence of the efficacy of ESIs in managing low back pain and radicular leg pain3,5,6 or spinal stenosis.4
Nevertheless, ESIs are widely used because of the perceived benefit balanced against the perceived rarity of adverse events.6 Even if patients recognize a low likelihood of significant benefit, they may accept ESI as preferable to surgery. In addition, most private payers require extensive nonoperative treatment before they will approve surgery as a treatment option.
In a study by Mandel and colleagues,14 ESI increased the risk of vertebral compression fractures by 21%, which in turn increased the risk of death.19 If accurate, these findings obviously would challenge the perception that ESI is a low-risk intervention. In contrast to the Mandel study,14 the present analysis of the Medicare population revealed no clinically relevant change in risk of osteoporotic spine fracture with each successive ESI after the initial injection. After the initial injection, each successive ESI decreased the relative risk of osteoporotic spine fracture by 2%, and each successive LJSI decreased it by 4%. Although statistically significant, the small change in relative risk may not be clinically relevant. However, taken cumulatively over a number of successive injections, these effects may be clinically relevant.
The data also showed that, after the initial injection, each successive ESI had no effect on risk of osteoporotic hip or wrist fracture, and each successive LJSI reduced the risk. Similar to earlier findings,20,21 long-term steroid use increased the risk of spine fracture in ESI and LJSI patients. Prolonged exposure to steroids may be necessary to reduce bone formation and increase bone breakdown.12
Although the study by Mandel and colleagues14 and our study both used administrative databases and survival analysis methods, conclusions differed. First, Mandel and colleagues14 used a study inclusion criterion of spine-related steroid injections, whereas we used a criterion of any steroid injection. Second, they used 50 years as the lower age for study inclusion, and we used 65 years. Third, to control for patients who had osteoporosis before study entry, they excluded those who had a fracture in an adjacent vertebra after kyphoplasty and vertebroplasty. It is unclear if patients who had osteoporotic fractures at other sites were excluded as well. Thus, the 2 cohorts may not be directly comparable.
Whereas Mandel and colleagues14 based their definition of osteoporotic spine fracture on a keyword search of a radiology database, we used a specific reportable ICD-9-CM diagnosis code. As a result, they may have overreported osteoporotic spine fractures, and we may have underreported. Finally, our sample was much larger than theirs. Given the relative rarity of osteoporotic fractures, a study with a larger sample may have more power to detect differences. In addition, unlike Mandel and colleagues,14 we focused on an injection cohort. We did not include or make comparisons with a no-injection cohort because our study hypothesis involved the potential systemic effects of steroid injections based on injection site. Although chronic steroid use was found to have a significant effect in our study, it is unclear to what extent the diagnosis code was used, during the comorbidity assessment or only in the event of steroid-related complications.
Our study also found that, after the initial injection, each successive LJSI decreased the risk of osteoporotic wrist fracture by 10%, and each successive TSI decreased the risk of osteoporotic hip fracture by 5%. It is plausible these injections allowed improved mobility, mitigating the effects of osteoporosis induced by inactivity and lack of resistance training. It is also possible that improved mobility limited falls.
In summary, this analysis of the Medicare claims database revealed that ESI, TSI, and LJSI decreased osteoporotic spine fracture risk. However, the effect was small and may not be clinically meaningful. After the initial injection, successive ESIs had no effect on the risk of osteoporotic hip or wrist fracture, and successive LJSIs reduced the risk of osteoporotic wrist fracture, perhaps because of improved mobility. Prolonged oral steroid use increased spine fracture risk in ESI and LJSI patients. More studies are needed to evaluate the risk-benefit profile of steroid injections.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
1. Pethö G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92(4):1699-1775.
2. Saal J. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
3. Choi HJ, Hahn S, Kim CH, et al. Epidural steroid injection therapy for low back pain: a meta-analysis. Int J Technol Assess Health Care. 2013;29(3):244-253.
4. Chou R, Loeser JD, Owens DK, et al; American Pain Society Low Back Pain Guideline Panel. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine. 2009;34(10):1066-1077.
5. Savigny P, Watson P, Underwood M; Guideline Development Group. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:b1805.
6. Staal JB, de Bie RA, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low back pain: an updated Cochrane review. Spine. 2009;34(1):49-59.
7. Angeli A, Guglielmi G, Dovio A, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253-259.
8. Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177-186.
9. Dubois EF, Wagemans MF, Verdouw BC, et al. Lack of relationships between cumulative methylprednisolone dose and bone mineral density in healthy men and postmenopausal women with chronic low back pain. Clin Rheumatol. 2003;22(1):12-17.
10. Yi Y, Hwang B, Son H, Cheong I. Low bone mineral density, but not epidural steroid injection, is associated with fracture in postmenopausal women with low back pain. Pain Physician. 2012;15(6):441-449.
11. Al-Shoha A, Rao DS, Schilling J, Peterson E, Mandel S. Effect of epidural steroid injection on bone mineral density and markers of bone turnover in postmenopausal women. Spine. 2012;37(25):E1567-E1571.
12. Kang SS, Hwang BM, Son H, Cheong IY, Lee SJ, Chung TY. Changes in bone mineral density in postmenopausal women treated with epidural steroid injections for lower back pain. Pain Physician. 2012;15(3):229-236.
13. Kim S, Hwang B. Relationship between bone mineral density and the frequent administration of epidural steroid injections in postmenopausal women with low back pain. Pain Res Manag. 2014;19(1):30-34.
14. Mandel S, Schilling J, Peterson E, Rao DS, Sanders W. A retrospective analysis of vertebral body fractures following epidural steroid injections. J Bone Joint Surg Am. 2013;95(11):961-964.
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
16. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
17. Murray SB, Bates DW, Ngo L, Ufberg JW, Shapiro NI. Charlson index is associated with one-year mortality in emergency department patients with suspected infection. Acad Emerg Med. 2006;13(5):530-536.
18. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine. 2007;32(16):1754-1760.
19. Puisto V, Rissanen H, Heliövaara M, et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur Spine J. 2011;20(12):2181-2186.
20. Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Effects of low-dose corticosteroids on the bone mineral density of patients with rheumatoid arthritis: a meta-analysis. J Investig Med. 2008;56(8):1011-1018.
21. Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 1994;20(3):629-650.
Reverse Shoulder Arthroplasty and Latissimus Dorsi Tendon Transfer
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
Take-Home Points
- CTA with loss of teres minor has been associated with worse clinical outcomes.
- Combined RSA and LDTT has been proposed and studied as a solution to this problem.
- LD tendon can be transferred to native teres minor insertion or lateral bicipital groove.
- Published studies have shown significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength.
- Overall complication rates appear similar to RSA alone, however rates of neuropraxia may be higher.
Reverse shoulder arthroplasty (RSA) is a proven procedure that typically improves pain and function in patients with rotator cuff tear arthropathy.1 Worse clinical outcomes are seen in patients with loss of teres minor function.2,3 The teres minor is often the last important external rotator of the shoulder left in cuff tear arthropathy. When its function is lost, the ability to achieve active external rotation may become diminished. This phenomenon was termed combined loss of active elevation and external rotation (CLEER) by Boileau and colleagues.4 Patients with CLEER typically exhibit weakness with external rotation of the shoulder—most pronounced with the arm in an abducted position. Clinical examination may reveal a positive Hornblower test, and magnetic resonance imaging (MRI) of the shoulder often shows atrophy in the teres minor muscle.5
Patients with CLEER often do not exhibit the same degree of clinical improvement after RSA, largely because the external rotation strength deficit remains unchanged, causing persistent difficulty in completing activities of daily living (eg, combing hair, brushing teeth, eating).6 One option for treating patients with CLEER is to combine RSA with latissimus dorsi tendon transfer (LDTT) with or without teres major (TM)tendon transfer. In 1934, L’Episcopo7 was the first to describe performing LDTT with TM tendon transfer in an attempt to restore external rotation in patients with brachial plexus palsy. This procedure typically is used for irreparable posterior-superior rotator cuff tears in younger patients.8 Although the transfer was originally popularized with use of 2 incisions,9 Boileau and colleagues4 described a modified technique that allows the transfer to be performed through a single deltopectoral approach during RSA.
Although several authors have described the outcomes of RSA with LDTT, the expected clinical outcomes and complication rates remain elusive because of the relatively small number of patients in each case series. In a systematic review, we critically examined and synthesized the results of individual studies on RSA with LDTT. We had 3 questions: What are the demographics of patients treated with RSA-LDTT? What outcomes are associated with this combined procedure? What are the associated complications, and how often do they occur?
Methods
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed. PubMed and Scopus computerized literature databases were searched through July 2015. Articles were identified with keyword searches (Figure). In our review, we included only studies that were reported in English, that included a minimum of 10 patients at baseline, and that had follow-up of at least 12 months; we excluded review papers, case reports, and technique papers without patient data. Mr. Sheth performed the initial search, and he and Dr. Namdari reviewed the qualifying abstracts. If one of the authors selected a paper, it was moved to the next phase of the review process. At the final phase (full-text review), there were no disagreements about which articles ultimately would be included (Figure).
We obtained 36 articles from PubMed and 12 from Scopus (Figure). Of these 48 articles, 15 were removed on the basis of their titles (reviews or editorials), and 8 for being duplicates. The remaining 25 articles underwent abstract review, which eliminated 17: reviews, case reports, technique articles, instructional articles, and reports on small case series (<10 patients) or studies lacking the minimum 12-month follow-up. The remaining 8 articles underwent full-text review. Inclusion/exclusion criteria removed 1 article, leaving 7 qualifying articles for analysis.
None of the studies compared outcomes with those of a control (nonoperative) group or an alternative surgical treatment. One study reported outcomes of RSA with and without LDTT; in this instance, we included only the data specific to the RSA-with-LDTT cases. Data from the individual studies were compiled to obtain demographic statistics. In cases in which outcomes data were consistently reported between studies, results were pooled for calculation of percentages and frequency-weighted (FW) means. FW means and grouped standard deviations were used to generate P values, using the number of “subjects” as the number of studies. As a result, comparative statistics for each variable were reported as means that 95% of the studies would report.
Results
Seven studies met the inclusion/exclusion criteria and were included in this systematic review. Five were retrospective,10-14 and 2 were prospective.5,6 All were published between 2007 and 2015. Table 1 lists the full study characteristics between groups.
Demographics
All 7 studies reported number of patients at baseline (Table 1); 133 patients (study range, 11-40) underwent RSA with LDTT.5,6,10-14 All 7 studies reported patient ages; FW mean age was 69.5 years (range, 66-73 years).5,6,10-14 Six studies reported sex at follow-up; there were 36 men (33.6%) and 71 women (66.4%).5,6,10,12-14
Surgical Indications and Technique
All patients underwent RSA with LDTT with or without TM tendon transfer for the indications of cuff tear arthropathy and CLEER. All 7 studies assessed loss of elevation as active forward elevation of <80° or <90° and loss of external rotation as active external rotation of <0°, inability to maintain abducted arm at 0°, or external rotation lag sign of >30°. All surgeries were performed with the deltopectoral approach. Combined LD/TM tendons were transferred in 6 studies5,6,10,12-14 and only the LD tendon in the seventh.11 Of the 6 studies that indicated tendon transfer location, 4 reported attaching to the posterolateral aspect of the greater tuberosity at the level of the original teres minor insertion5,6,11,12 and 2 reported attaching to the lateral aspect of the bicipital groove at the level of the LD insertion,10,14 . Six studies reported use of a sling or brace for 6 weeks after surgery.5,6,10-12,14
Outcomes
The 7 studies reported outcomes data for 116 (87%) of their 133 baseline patients (Table 2). Patients were followed up an FW mean of 39.9 months (range, 18-65 months). Six studies reported postoperative Constant scores; FW mean Constant score was 28.7 before surgery and 64.4 afterward (P = .0001).5,6,10-13
With regard to functional evaluation on physical examination, all 7 studies reported preoperative and postoperative active forward elevation and external rotation.5,6,10-14 Active forward elevation improved to an FW mean of 136°, from 71° (P < .0001), and external rotation improved to an FW mean of 25°, from –4° (P < .0001). Three studies reported preoperative and postoperative abduction; abduction improved to an FW mean of 137°, from 72° (P = .003).6,10,13
Complications and Reoperations
The 7 studies reported 31 complications, for an overall complication rate of 22.8% (31/126).5,6,10-14 There were 9 cases of neuropraxia (7.1%), 7 infections (6.0%), 4 dislocations or subluxations (3.4%), 2 cases of aseptic loosening (1.7%), 2 deltoid separations (1.7%), 2 periprosthetic fractures (1.7%), 1 acromion fracture (0.9%), 1 hematoma (0.9%), 1 LD/TM tendon rupture (0.9%), 1 intraoperative metaphyseal fracture (0.9%), and 1 painful baseplate screw (prominent where it penetrated the scapular spine)7 (0.9%).
The 7 studies also reported 19 reoperations, for an overall reoperation rate of 15.1% (19/126).5,6,10-14 There were 4 wound revisions, 3 revision RSAs, 3 open reduction and internal fixations, 2 deltoid repairs, 2 irrigation and débridements, 1 revision to hemiarthroplasty, 1 acromioclavicular resection, 1 procedure for a shoulder dislocation, 1 cerclage wire fixation to correct an intraoperative metaphyseal fracture, and 1 procedure to burr down a protruding baseplate screw.
Discussion
RSA with LDTT improves postoperative function in patients with cuff tear arthropathy associated with profound external rotation weakness caused by loss of a functional teres minor muscle. That statement is consistent with the findings of our systematic review, as all 7 reviewed studies found functional improvements, particularly in active external rotation (~30° improvement). In addition, there were consistent reductions in pain and improvements in forward elevation.
Our review found a mean patient age of 69.5 years, similar to the 72.7 years reported in a recent population-based study on RSA utilization.15 Likewise, our percentage of women who underwent RSA with LDTT, 66.4%, is similar to the overall rate of 63.6%.15 It appears that the RSA-with-LDTT population and the traditional RSA population are not dramatically different.
The improvements we found in subjective outcome scores and range of motion can be compared with those found in RSA-only treatment of rotator cuff tear arthropathy. Wall and colleagues16 found an approximate 44-point Constant score improvement, to 65.1 from 21.7, which is similar to our 36-point improvement for RSA with LDTT. They also found an approximate 10-point increase in pain relief; ours was about 6 points. Regarding range of motion, they found 66° improvement in active forward elevation and 2° in active external rotation, and we found 65° and 29° improvement, respectively. Thus, the outcomes of RSA with LDTT and RSA alone appear to be comparable. Simovitch and colleagues17 evaluated RSA outcomes as a function of teres minor muscle atrophy and found that, compared with patients with stage 3 or 4 fatty infiltration, patients with stage 0, 1, or 2 infiltration had significantly better ultimate Constant scores, significantly better SSVs, and significantly more preoperative-to-postoperative improvement. On average, Constant scores and SSVs increased 32% and 25%, respectively, in patients with more extensive fatty atrophy, and these patients experienced an average net loss of 7° in external rotation. It appears that, whereas RSA-with-LDTT outcomes are similar to outcomes in a nonspecific group of cuff tear arthropathy patients treated with RSA alone, adding LDTT to RSA may substantially improve outcomes in cases in which the teres minor is of poor quality.
We found no differences in implant types. However, with the exception of the Arrow prosthesis, which had 8.5 mm of lateralization, all implants had a traditional Grammont design. Greiner and colleagues2 recently found a trend toward improved external rotation in lateralized RSA designs, and a statistically significant improvement in external rotation in patients with an intact teres minor. The impact of LDTT with use of a lateralized design is unknown.
Our review found a relatively high rate of complications, 22.8%, and a reoperation rate of 15.1%. These are not dramatically different from the historical rates of complications (21%) and reoperations (13.4%).18 Although RSA with LDTT appears to have a higher rate of a specific complication, nerve-related injury, this is not necessarily surprising given the proximity of the axillary and radial nerves, the operative field, and the tendons transferred. This review’s rate of neuropraxia, 7.1%, is higher than the historical rate of 1.2% reported for RSA alone.18
This systematic review was limited by the quality of the studies available for inclusion. Although we followed PRISMA guidelines, none of the reviewed studies reported methods for controlling bias, confounding, and chance. In addition, the number of patients included and the relatively short follow-up period limit the impact of our findings. Finally, the individual studies used different outcome measures and did not report raw patient data, which limited our ability to perform more advanced statistical analysis.
Conclusion
This systematic review describes the demographics and outcomes of patients who underwent RSA with LDTT. Compiled data and FW means showed significant improvements in various subjective values, active forward elevation, external rotation, and abduction strength. For RSA with LDTT and RSA alone, complication rates appear comparable, but the rate of neuropraxia may be higher for the combined procedure. Although this review provides valuable information on RSA with LDTT, its lack of a control comparison group and its relatively short follow-up period limited our ability to draw meaningful conclusions about the efficacy of the combined procedure in treating rotator cuff tear arthropathy in the absence of a functional teres minor.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
1. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
2. Greiner S, Schmidt C, Herrmann S, Pauly S, Perka C. Clinical performance of lateralized versus non-lateralized reverse shoulder arthroplasty: a prospective randomized study. J Shoulder Elbow Surg. 2015;24(9):1397-1404.
3. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
4. Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671-682.
5. Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466(3):584-593.
6. Boileau P, Rumian AP, Zumstein MA. Reversed shoulder arthroplasty with modified L’Episcopo for combined loss of active elevation and external rotation. J Shoulder Elbow Surg. 2010;19(2 suppl):20-30.
7. L’Episcopo JB. Tendon transplantation in obstetrical paralysis. Am J Surg. 1934;25:122-125.
8. Namdari S, Voleti P, Baldwin K, Glaser D, Huffman GR. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a systematic review. J Bone Joint Surg Am. 2012;94(10):891-898.
9. Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;(232):51-61.
10. Boughebri O, Kilinc A, Valenti P. Reverse shoulder arthroplasty combined with a latissimus dorsi and teres major transfer for a deficit of both active elevation and external rotation. Results of 15 cases with a minimum of 2-year follow-up. Orthop Traumatol Surg Res. 2013;99(2):131-137.
11. Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89(5):940-947.
12. Ortmaier R, Resch H, Hitzl W, et al. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2014;38(3):553-559.
13. Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elbow Surg. 2014;23(1):49-57.
14. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res. 2015;473(10):3212-3217.
15. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91-97.
16. Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
17. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89(5):934-939.
18. Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146-157.
Medial Oblique Meniscomeniscal Ligament of Knee
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
Take-Home Points
- Prevalence of the medial oblique meniscomeniscal ligament is 1% to 4%.
- It is important to distinguish this ligament from a meniscus tear on MRI.
- The functional characteristics of this ligament are not well understood.
- What may appear to be a meniscal tear in a younger patient could be a medial oblique meniscomeniscal ligament.
- Dr. Flanigan recommends leaving the ligament intact unless resection is needed to provide better visualization.
We report a case of aberrant meniscus attachment in the setting of anterior cruciate ligament (ACL) injury. An anomalous cordlike attachment ran from the anterior horn of the medial meniscus to the posterior horn of the lateral meniscus through the intercondylar notch. This attachment was previously named the medial oblique meniscomeniscal ligament1 but has seldom been reported in the literature. Prevalence is 1% to 4%.1,2 This case was treated at Ohio State University Wexner Medical Center in Columbus. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented with left knee pain after sustaining 2 injuries to the knee. The first injury occurred during a dodgeball game—when the knee buckled on landing from a jump. A “pop” was felt, and the knee swelled immediately. The second injury occurred about 3 months later, during soccer play. The patient was running when his foot slipped and caused the knee to buckle. Again, a “pop” was felt, and there was swelling. Mechanical symptoms of clicking then started. The patient reported no instability episodes. His medical history and family history were otherwise unremarkable. The patient was healthy and had a body mass index of 23.05 kg/m2.
Physical examination revealed no effusion, erythema, or warmth in the left knee. Range of motion was 0° to 135° in the left knee and 0° to 140° in the right knee. There was no pain on hyperextension of the knee or medial or lateral joint-line tenderness, but there was pain on hyperflexion, and the McMurray test was positive. Ligament examination was negative except for positive anterior drawer, Lachman, and pivot-shift tests.
Radiographs taken the day of the first clinic visit showed no acute osseous abnormality. Magnetic resonance imaging (MRI) showed complete disruption of the proximal fibers of the ACL (Figures 1, 2).
Also observed was a small oblique tear of the body of the lateral meniscus with slight blunting of the anterior horn of the medial meniscus, which may have been related to a small tear. A pivot-shift contusion pattern with impaction fracture of the lateral femoral condyle was also appreciated. There were no definite cartilage defects identified.
Discussion
The medial and lateral menisci typically are separate fibrocartilaginous structures acting as a cushion for the knee, but normal variant connections between the structures have been described. These connections include the anterior transverse meniscal ligament, the posterior transverse meniscal ligament, and the medial and lateral oblique meniscomeniscal ligaments.3 In the present case, a medial oblique meniscomeniscal ligament was identified. Its path between menisci was traceable on coronal and axial views. Video taken during arthroscopy also clearly showed its path and its relationship to other structures in the knee. To Dr. Flanigan’s knowledge, this ligament was not previously described with video. It is important to distinguish this ligament from a horizontal tear of the meniscus, given the potential for misinterpretation on MRI. A horizontal tear is a degenerative change that often occurs in older patients. Our patient was 18 years old at time of injury. In addition, the surface of his lower meniscus was smooth, whereas in a tear the edge is irregular and discontinuous. Dr. Flanigan prefers to leave this ligament intact unless resection would provide better visualization during arthroscopy. His reasoning is that the functional characteristics of the ligament are not well understood.
There are few reports on the medial oblique meniscomeniscal ligament.1 Sanders and colleagues1 found 3 cases of this normal variant. In the first, the ligament was interpreted as a flap tear on MRI; in the other 2 cases, the ligament was correctly identified. Kim and Laor2 and Dervin and Paterson4 also described this variant in case reports.
There are many abnormalities of the meniscus. In our literature review, we found reports on various anomalies, including discoid meniscus,5 ring-shape meniscus,6,7 accessory meniscus,8 double-layer meniscus,9-12 abnormal band formation,13,14 hypoplasia,15 Wrisberg meniscus,6 and congenital absence of meniscus.16 These variations have multifactorial causes, including congenital and developmental influences.
In a recent case report, Giordano and Goldblatt14 described an abnormal band of lateral meniscus extending from the posterior horn to the anterior-mid portion of the same meniscus. Lee and Min13 described the same band earlier, in a 2-patient case report.13 One patient presented symptomatically, nontraumatically, and the other with a posterior cruciate ligament tear. Each case was deemed congenital given the characteristic appearance and bilaterality of the anomaly.
In an 11-patient case series in Finland, Rainio and colleagues17 described an attachment from the anterior horn of the medial meniscus inserting into the ACL—a crescent band from the upper surface of the anterior horn that attached along the upper two thirds of the ACL.
At 2-year follow-up, our patient was doing well with rehabilitation and experienced only minimal symptoms. Radiologists and surgeons should be able to identify such variants. Knowing the common and rare variants, radiologists can help surgeons by identifying normal anatomy from pathology and providing a more clinically relevant report. Surgeons should be aware of the anatomical variability in the knee in order to provide the best care for their patients.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
1. Sanders TG, Linares RC, Lawhorn KW, Tirman PF, Houser C. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213(1):213-216.
2. Kim HK, Laor T. Oblique meniscomeniscal ligament: a normal variant. Pediatr Radiol. 2009;39(6):634.
3. Chan CM, Goldblatt JP. Unilateral meniscomeniscal ligament. Orthopedics. 2012;35(12):e1815-e1817.
4. Dervin GF, Paterson RS. Oblique menisco-meniscal ligament of the knee. Arthroscopy. 1997;13(3):363-365.
5. Sun Y, Jiang Q. Review of discoid meniscus. Orthop Surg. 2011;3(4):219-223.
6. Kim YG, Ihn JC, Park SK, Kyung HS. An arthroscopic analysis of lateral meniscal variants and a comparison with MRI findings. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):20-26.
7. Kim SJ, Jeon CH, Koh CH. A ring-shaped lateral meniscus. Arthroscopy. 1995;11(6):738-739.
8. Karahan M, Erol B. Accessory lateral meniscus: a case report. Am J Sports Med. 2004;32(8):1973-1976.
9. Okahashi K, Sugimoto K, Iwai M, Oshima M, Fujisawa Y, Takakura Y. Double-layered lateral meniscus. J Orthop Sci. 2005;10(6):661-664.
10. Karataglis D, Dramis A, Learmonth DJ. Double-layered lateral meniscus. A rare anatomical aberration. Knee. 2006;13(5):415-416.
11. Takayama K, Kuroda R, Matsumoto T, et al. Bilateral double-layered lateral meniscus: a report of two cases. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1336-1339.
12. Wang Q, Liu XM, Liu SB, Bai Y. Double-layered lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2050-2051.
13. Lee BI, Min KD. Abnormal band of the lateral meniscus of the knee. Arthroscopy. 2000;16(6):11.
14. Giordano B, Goldblatt J. Abnormal band of lateral meniscus. Orthopedics. 2009;32(1):51.
15. Ohana N, Plotquin D, Atar D. Bilateral hypoplastic lateral meniscus. Arthroscopy. 1995;11(6):740-742.
16. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
17. Rainio P, Sarimo J, Rantanen J, Alanen J, Orava S. Observation of anomalous insertion of the medial meniscus on the anterior cruciate ligament. Arthroscopy. 2002;18(2):E9.
Reliability of 3-Dimensional Glenoid Component Templating and Correlation to Intraoperative Component Selection
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
Take-Home Points
- Guidelines regarding glenoid component size selection for primary TSA are lacking.
- Intraoperative in situ glenoid sizing may not be ideal.
- 3-D digital models may be utilized for preoperative templating of glenoid component size in primary TSA.
- 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation can lead to consistent and reproducible templating of glenoid component size.
- 3-D templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio.
In 1974, Neer1 introduced the shoulder prosthesis. In 1982, Neer and colleagues2 found significant improvement in shoulder pain and function in patients with glenohumeral osteoarthritis treated with the Neer prosthesis. Since then, use of total shoulder arthroplasty (TSA) has increased. Between 1993 and 2007, TSA use increased 319% in the United States.3 Long-term outcomes studies have found implant survivorship ranging from 87% to 93% at 10 to 15 years.4
Although TSA is a successful procedure, glenoid component failure is the most common complication.5-10 Outcomes of revision surgery for glenoid instability are inferior to those of primary TSA.11 Recent research findings highlight the effect of glenoid size on TSA complications.12 A larger glenoid component increases the stability ratio (peak subluxation force divided by compression load).12 However, insufficient glenoid bone stock, small glenoid diameter, and inability to fit a properly sized reamer owing to soft-tissue constraints may lead surgeons to choose a smaller glenoid component in order to avoid peg penetration, overhang, and soft-tissue damage, respectively. Therefore, preoperative templating of glenoid size is a potential strategy for minimizing complications.
Templating is performed for proximal humeral components, but glenoid sizing typically is deferred to intraoperative in situ sizing with implant-specific targeting guides. This glenoid sizing practice arose out of a lack of standard digital glenoid templates and difficulty in selecting glenoid size based on plain radiographs and/or 2-dimensional (2-D) computed tomography (CT) scans. However, targeting devices are sporadically used during surgery, and intraoperative glenoid vault dimension estimates derived from visualization and palpation are often inaccurate. Often, rather than directly assess glenoid morphology, surgeons infer glenoid size from the size and sex of patients.13
Three-dimensional (3-D) CT can be used to accurately assess glenoid version, bone loss, and implant fit.14-19 We conducted a study to determine if 3-D digital imaging can be consistently and reproducibly used for preoperative templating of glenoid component size and to determine if glenoid sizes derived from templating correlate with the sizes of subsequently implanted glenoids.
Materials and Methods
This retrospective study was conducted at the Center for Shoulder, Elbow, and Sports Medicine at Columbia University Medical Center in New York City and was approved by our Institutional Review Board. Included in the study were all patients who underwent primary TSA for primary glenohumeral osteoarthritis over a 12-month period. Patients were required to have preoperative CT performed according to our study protocol. The CT protocol consisted of 0.5-mm axial cuts of the entire scapula and 3-D reconstruction of the scapula, glenoid, glenohumeral articulation, and proximal humerus. Patients were excluded from the study for primary TSA for a secondary cause of glenohumeral osteoarthritis, inflammatory arthritis, connective tissue disease, prior contralateral TSA, and prior ipsilateral scapula, glenoid, and proximal humerus surgery. Ultimately, 24 patients were included in the study.
CT data were formatted for preoperative templating. The CT images of each patient’s scapula were uploaded into Materialise Interactive Medical Image Control System (Mimics) software. Mimics allows 3-D image rendering and editing from various imaging modalities and formats. The software was used to create the 3-D scapula models for templating. Prior studies have validated the anatomical precision of 3-D models created with Mimics.20
Mimics was also used to digitize in 3-D the glenoid components from the Bigliani-Flatow Shoulder System (Zimmer Biomet). Glenoid components of 3 different sizes (40 mm, 46 mm, 52 mm) were used. (The Bigliani glenoid component was digitized, as this implant system was used for primary TSA in all 24 patients.) Each glenoid component was traced in 3-D with a Gage 2000 coordinate-measuring machine (Brown & Sharpe) and was processed with custom software. The custom software, cited in previous work by our group,17 created the same coordinate system for each scapula based on anatomical reference points. These digitized 3-D images of glenoid components were uploaded with the digitized 3-D scapulae derived from patients’ CT scans to the Magics software. Magics allows for manipulation and interaction of multiple 3-D models by creating electronic stereolithography files that provide 3-D surface geometry.
Three fellowship-trained shoulder surgeons and 4 shoulder fellows templated the most appropriately sized glenoid component for each of the 24 patients. At the time of templating, the surgeon was blinded to the size of the glenoid implant used in the surgery. In Magics, each scapula was positioned in 3-D similar to how it would appear with the patient in the beach-chair position during surgery. In both study arms, surgeons selected the largest component that maximized the area of contact while avoiding peg penetration of the glenoid vault or component overhang. In addition, surgeons were instructed to correct glenoid version to as near neutral as possible with component positioning but were not permitted to remove glenoid bone stock to correct deformity. All surgeons based placement of the glenoid component on the patient’s actual bone stock and not on osteophytes, which are readily appreciable on 3-D CT.
In study arm 1, the 3-D view of the glenoid was restricted to the initial view in the beach-chair position. The surgeon then manipulated the 3-D glenoid component template across a single 2-D plane, either the superior-inferior plane or the anterior-posterior plane, over the surface of the 3-D glenoid (Figure 1).
In study arm 2, surgeons were permitted to rotate the 3-D glenoid template and scapula in any manner (Figure 2).
Interobserver agreement was determined by comparing prosthetic glenoid component size selection among all study surgeons, and intraobserver agreement was determined by comparing glenoid size selection during 2 sessions separated by at least 3 weeks.
After each trial, the order of patients’ scapula images was randomly rearranged to reduce recall bias. Kappa (κ) coefficients were calculated for interobserver and intraobserver agreement. Kappas ranged from −1.0 (least agreement) to +1.0 (complete agreement). A κ of 0 indicated an observer selection was equivalent to random chance. The level of agreement was categorized according to κ using a system described by Landis and Koch21 (Table 1).
Results
The group of 24 patients consisted of 15 men and 9 women. Mean age was 70.3 years (range, 56-88 years). Primary TSA was performed in 14 right shoulders and 10 left shoulders. Of the 24 patients, 20 (83%) had a 46-mm glenoid component implanted, 3 male patients had a 52-mm glenoid component implanted, and 1 female patient had a 40-mm glenoid component implanted.
Study Arm 1: Glenoid Templating Based on 2 df
In study arm 1, overall intraobserver agreement was substantial, as defined in the statistical literature.21 Among all surgeons who participated, intraobserver agreeement was 0.76 (substantial), 0.60 (substantial), and 0.58 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.67, substantial agreement). Trial 1 interobserver agreement was 0.56 (moderate) (P < .001), 0.25 (fair) (P < .001), and 0.21 (fair) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.36, fair agreement) (P < .001), and trial 2 interobserver agreement was 0.58 (moderate) (P < .001), 0.18 (poor) (P = .003), and 0.24 (fair) (P <.001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.32, fair agreement) (P < .001). In study arm 1, therefore, trials 1 and 2 both showed fair interobserver agreement.
Study Arm 2: Glenoid Templating Based on 6 df
In study arm 2, a mean correlation of 0.42 (moderate agreement) was found between glenoid component size in 3-D templating and the glenoid component size ultimately selected during surgery (Table 3).
In study arm 2, overall intraobserver agreement was moderate. Among all surgeons who participated, intraobserver agreement was 0.80 (excellent), 0.43 (moderate), and 0.47 (moderate) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.58, moderate agreement). Trial 1 interobserver agreement was 0.75 (substantial) (P < .001), 0.39 (fair) (P < .001), and 0.50 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.54, moderate agreement) (P < .001), and trial 2 interobserver agreement was 0.66 (substantial) (P < .001), 0.28 (fair) (P = .003), and 0.40 (moderate) (P < .001) for the 40-mm, 46-mm, and 52-mm glenoid components, respectively (overall κ = 0.43, moderate agreement) (P < .001).
Discussion
Our results showed that 3-D glenoid templating had reproducible intraobserver and interobserver agreement. Overall intraobserver agreement was substantial (κ = 0.67) for study arm 1 and moderate (κ = 0.58) for study arm 2. Interobserver agreement was fair for trials 1 and 2 (κ = 0.36 and 0.32) in arm 1 and moderate for trials 1 and 2 (κ = 0.54 and 0.43) in arm 2.
Intraobserver and interobserver agreement values, particularly in study arm 2, which incorporated rotation (6 df), are consistent with values in commonly used classification systems, such as the Neer system for proximal humerus fractures, the Frykman system for distal radius fractures, and the King system for adolescent idiopathic scoliosis.22-30 Sidor and colleagues27 found overall interobserver agreement of 0.50 and overall intraobserver agreement of 0.66 for the Neer system, and Illarramendi and colleagues24 found overall interobserver agreement of 0.43 and overall intraobserver agreement of 0.61 for the Frykman system.
In study arm 2, overall interobserver and intraobserver agreement was moderate. A higher level of surgeon agreement is unlikely given the lack of well-defined parameters for determining glenoid component size. Therefore, glenoid size selection is largely a matter of surgeon preference. More research is needed to establish concrete guidelines for glenoid component size selection. Once guidelines are adopted, interobserver agreement in templating may increase.
In both study arms, the component that surgeons selected during templating tended to be smaller than the component they selected during surgery. In study arm 1, 32% of patients had a smaller component selected based on computer modeling, and 7% had a larger component selected. In study arm 2, the difference was narrower: 27% of patients had a smaller component selected during templating, and 16% had a larger component selected. A statistically significant difference (P < .001) in templated and implanted component sizes was found between men and women: Templated glenoid components were smaller than implanted components in 53% of women and larger than implanted components in 33% of men. Differences between templated and implanted components may be attributable to visualization differences. During templating, the entire glenoid can be visualized and the slightest peg penetration or component overhang detected; in contrast, during surgery, anatomical constraints preclude such a comprehensive assessment.
Differences in agreement between templated and implanted glenoid components suggest that the size of implanted components may not be ideal. In this study, the distribution of the templated glenoid sizes was much wider than that of the implanted glenoid sizes. During templating, each glenoid component can be definitively visualized and assessed for possible peg penetration and overhang. Visualization allows surgeons to base glenoid size selection solely on glenoid morphology, as opposed to factors such as patient sex and height. In addition, interobserver and intraobserver agreement values for the 40-mm glenoid component were considerably higher than those for components of other sizes, indicating that the 40-mm component was consistently and reproducibly selected for the same patients. Hence, templating may particularly help prevent peg penetration and component overhang for patients with a smaller diameter glenoid.
More research on 3-D templating is warranted given the results of this study and other studies.12,17,31 Scalise and colleagues31 found that, in TSA planning, surgeons’ use of 2-D (vs 3-D) imaging led them to overestimate glenoid component sizes (P = .006). In our study, the glenoid size selected during 3-D templating was, in many cases, smaller than the size selected during surgery. In order to avoid peg penetration and glenoid overhang, anecdotal guidelines commonly used in glenoid size selection, likely was the driving force in selecting smaller glenoid components during templating. Although anterior, superior, and inferior glenoid overhang typically can be assessed during surgery, posterior overhang is more difficult to evaluate. Three-dimensional modeling allows surgeons to determine optimal glenoid component size and position. In addition, intraoperative evaluation of glenoid component peg penetration is challenging, and peg penetration becomes evident only after it has occurred. During templating, however, surgeons were able to easily assess for peg penetration, and smaller glenoid components were selected.
A limitation of this study is that intraoperative glenoid version correction or peg containment was not quantified. More research is needed on the relationship between glenoid size selection and component overhang and peg penetration. Another limitation was use of only 1 TSA system (with 3 glenoid sizes, all with inline pegs); reliability of 3-D templating was not evaluated across different component designs. Last, given the absence of guidelines for glenoid component size selection, there was surgeon bias in preoperative templating and in intraoperative selection of glenoid size. Surgeons had differing opinions on the importance of maximizing the contact area of the component and correcting glenoid deformity and version.
Our study results showed that preoperative 3-D templating that allows for superior-inferior, anterior-posterior, and rotational translation was consistent and reproducible in determining glenoid component size, and use of this templating may reduce the risks of implant overhang, peg penetration, and decreased stability ratio. These results highlight the possibility that glenoid component sizes selected during surgery may not be ideal. More research is needed to determine if intraoperative glenoid size selection leads to adequate version correction and peg containment. The present study supports use of 3-D templating in primary TSA planning.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
1. Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1-13.
2. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
3. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
4. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
5. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
7. Matsen FA 3rd, Bicknell RT, Lippitt SB. Shoulder arthroplasty: the socket perspective. J Shoulder Elbow Surg. 2007;16(5 suppl):S241-S247.
8. Matsen FA 3rd, Clinton J, Lynch J, Bertelsen A, Richardson ML. Glenoid component failure in total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(4):885-896.
9. Pearl ML, Romeo AA, Wirth MA, Yamaguchi K, Nicholson GP, Creighton RA. Decision making in contemporary shoulder arthroplasty. Instr Course Lect. 2005;54:69-85.
10. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
11. Sanchez-Sotelo J, Sperling JW, Rowland CM, Cofield RH. Instability after shoulder arthroplasty: results of surgical treatment. J Bone Joint Surg Am. 2003;85(4):622-631.
12. Tammachote N, Sperling JW, Berglund LJ, Steinmann SP, Cofield RH, An KN. The effect of glenoid component size on the stability of total shoulder arthroplasty. J Shoulder Elbow Surg. 2007;16(3 suppl):S102-S106.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Briem D, Ruecker AH, Neumann J, et al. 3D fluoroscopic navigated reaming of the glenoid for total shoulder arthroplasty (TSA). Comput Aided Surg. 2011;16(2):93-99.
15. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
16. Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy. 2008;24(4):376-382.
17. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
18. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328-335.
19. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
20. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174.
22. Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM. Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1107-1111.
23. Humphrey CA, Dirschl DR, Ellis TJ. Interobserver reliability of a CT-based fracture classification system. J Orthop Trauma. 2005;19(9):616-622.
24. Illarramendi A, González Della Valle A, Segal E, De Carli P, Maignon G, Gallucci G. Evaluation of simplified Frykman and AO classifications of fractures of the distal radius. Assessment of interobserver and intraobserver agreement. Int Orthop. 1998;22(2):111-115.
25. Lenke LG, Betz RR, Bridwell KH, et al. Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1998;80(8):1097-1106.
26. Ploegmakers JJ, Mader K, Pennig D, Verheyen CC. Four distal radial fracture classification systems tested amongst a large panel of Dutch trauma surgeons. Injury. 2007;38(11):1268-1272.
27. Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. J Bone Joint Surg Am. 1993;75(12):1745-1750.
28. Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Joint Surg Am. 1993;75(12):1751-1755.
29. Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Br. 1991;73(4):676-678.
30. Ward WT, Vogt M, Grudziak JS, Tümer Y, Cook PC, Fitch RD. Severin classification system for evaluation of the results of operative treatment of congenital dislocation of the hip. A study of intraobserver and interobserver reliability. J Bone Joint Surg Am. 1997;79(5):656-663.
31. Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438-2445.
Medial Oblique Meniscomeniscal Ligament
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Arthroscopic identification and evaluation of the meniscomeniscal ligament.
Making Practice Perfect Download
Please click either of the links below to download your free eBook
Onecount Call To Arms
In-Office Diagnostic Needle Arthroscopy: Understanding the Potential Value for the US Healthcare System
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
Take-Home Points
- In-office diagnostic needle arthroscopy is a minimally invasive, rapid method for identification of intra-articular joint pathology.
- Cost savings of a significant value can be realized to both the patient and healthcare system via small-bore needle arthroscopy as opposed to MRI.
- Diagnostic needle arthroscopy can lead to quicker identification of pathology than MRI.
- Diagnostic needle arthroscopy can reduce the number of undue "formal" surgical diagnostic arthroscopies.
- Standardization of image quality of small bore arthroscopy may pose benefits to the variable quality of MRI.
Patient satisfaction and healthcare costs have taken a leading role in today’s health care market. Patient satisfaction, often categorized as the "patient experience," can be measured on numerous levels, such as access to healthcare professionals and diagnostic testing, wait time for appointments, and timely test results. Furthermore, patients’ having a full understanding of their pathology and treatment options may correlate with their overall satisfaction. Some metrics are subjective, but procedure costs are objective.
The algorithm for treating patients who present with knee or shoulder pathology to an orthopedic office involves taking a thorough history, performing a physical examination, and, in many cases, obtaining diagnostic imaging. After arriving at a diagnosis, the physician plans the patient’s treatment. In most cases in which magnetic resonance imaging (MRI) is required, the process can take 2 to 3 weeks.1
Surgical knee arthroscopy is one of the most common procedures in the United States.2,3 Worldwide, more than 2 million knee arthroscopies are performed yearly.4 For most procedures, the decision to treat is based on physical examination findings, and the diagnosis is confirmed with MRI. MRI has 86% sensitivity and 91% specificity for diagnosing ligamentous and meniscal tears.5 However, regular use of MRI has led to increased healthcare expenditures and a larger financial burden for patients, which can delay diagnosis.6
Since 2000, MRI use in the United States has risen significantly—by 10% over a 10-year period.7 According to a 2013 population analysis, 107 in 1000 US inhabitants had an MRI yearly.8
MRI costs vary widely because of several factors, including state/regional consideration, scanning in a hospital or an independent facility, and use of contrast and arthrography. In a 2017 study of the variation in noncontrast MRI costs at 71 hospitals and 26 independent facilities in Iowa, Westermann and colleagues9 found that, excluding radiologist interpretation fees, the mean MRI technical component cost to consumers was US $1874 (SD, $694; range, $500-$4000).
Patient factors may preclude use of MRI (Table).
Small-bore needle arthroscopy is a cost-effective alternative diagnostic tool with efficacy and accuracy similar to those of MRI and standard arthroscopy for intra-articular pathologies.6,11 The procedure is performed with a disposable handpiece equipped with an internal light source and optics; this handpiece attaches to a reusable tablet for ease of transportation and visualization (Figure 1).
In 2014, Voigt and colleagues6 reported a significant net healthcare system cost saving with use of a small-needle arthroscope for diagnostic testing. The saving was estimated at $115 million to $177 million for simple isolation of medial meniscus pathology—or, more specifically, for appropriate care after more accurate visualization with the diagnostic needle arthroscope coupled with a decrease in false positives compared with MRI use. Other factors include the economic impact of the patient’s lost work hours, often associated with the time off needed for the MRI and for the follow-up visit for review of results.
Methods
We retrospectively reviewed the patient charts for 200 in-office knee and shoulder diagnostic needle arthroscopies performed by 5 surgeons over a 12-month period and examined the costs. Medicare, Medicaid, worker’s compensation, self-pay, and motor vehicle cases were excluded to provide uniformity across commercial insurance payers. Only the reimbursement amounts for Current Procedural Terminology codes 29870 (diagnostic knee arthroscopy) and 29805 (diagnostic shoulder arthroscopy) were examined. Geographical differences in commercial payer reimbursements were considered. The 5 surgeons who submitted data for this study practice in different parts of the United States—the Northeast, the Mid-Atlantic, the Southeast, the Midwest, and the West Coast. Similarly, the costs of outpatient and inpatient MRI and MRA were reported by each physician based on regional rates. MRI reimbursement was considered only if the MRI magnet was 1.5 Tesla or stronger.
Results
We reviewed 200 (175 knee, 25 shoulder) in-office diagnostic needle arthroscopies of patients with commercial insurances. Average reimbursement was calculated across all commercial payers for both knee and shoulder arthroscopies (Figure 2).
For in-office diagnostic needle arthroscopy of the knee, average reimbursement was $628.92 (range, $340-$1391). For in-office diagnostic needle arthroscopy of the shoulder, average reimbursement was $492.38 (range, $471-$593). Outpatient MRI without contrast of the knee or shoulder averaged $1047 (range, $565-$2100) (Figure 3).
Discussion
Over the past decade, the combination of health and economics has often driven patient care and consumer demand. With rising deductibles and variations in secondary insurance carriers, patients often base healthcare decisions on their financial impact. Conversely, physicians are often in the difficult position of treating patients who are hesitant to obtain medical imaging out of financial concern. In addition, physicians and patients routinely are concerned about delays in care and timely reporting of test results. A patient’s ability to quickly obtain test results and start a course of definitive treatment may affect the patient’s perception of the overall healthcare experience with the physician, as has been noted in popular healthcare polls, such as Press-Ganey.13
Diagnostic needle arthroscopy performed in an office can yield a cost saving over MRI. Our review revealed in-office needle arthroscopy of the knee provided an average cost saving of $418.08 over standard MRI performed in an outpatient facility (Figure 3). That saving more than doubled, to $961.08, when MRI was performed in a hospital. Similarly, in-office needle arthroscopy of the shoulder provided an average cost saving of $554.62 over standard MRI. This saving also increased substantially, to $1097.62, over hospital MRI. An additional cost saving of $100 to $350 was found for knee or shoulder diagnostic needle arthroscopy over MRA.
Other factors affect the economic benefit of diagnostic needle arthroscopy over standard MRI. Having the procedure performed the same day as the presenting office visit can save the patient time and allow the physician to create a medical treatment plan sooner. In addition, the patient (and the insurance company) can save costs by avoiding a later office visit for review of MRI findings. Time spent going to MRI follow-up visits potentially can be analyzed as lost wages or as time lost from other segments of life. For the patient, this time can be defined as value hours. Last, there is a cost saving in avoiding nonoperative treatments in cases in which the initial definitive diagnosis would have called for surgical intervention. Accordingly, for patients who cannot undergo MRI, obtaining information on intra-articular pathology in the office may also decrease unnecessary "traditional" diagnostic arthroscopy in the operating room. Therefore, patients who do not require true formal arthroscopy to determine lack of pertinent intra-articular pathology can avoid unnecessary anesthesia, time off work, and associated healthcare expenses.
This study had several limitations. First, evaluating more cases would have increased the strength of the findings. Second, the large number of knee cases relative to shoulder cases may have been a by-product of the practice makeup of the surgeons rather than a matter of preference with this relatively new technology. However, the significant gap in cost savings between needle arthroscope and MRI cannot be discounted, and it provides a window on the potential cost savings the healthcare system can realize. Furthermore, analysis of payments made by the commercial payers in each state may have revealed a reimbursement fluctuation. The largest challenge in this study was the extreme variation in MRI costs. According to the literature, MRI of the upper or lower extremity ranges in cost from $500 to $4000.4 In addition, this cost is often negotiated between the patient and the MRI facility if the patient is willing to work outside insurance, which potentially can alter the overall average MRI cost.
The last points to consider are the reliability of users and the reproducibility of in-office diagnostic needle arthroscopy. Much as with true surgical arthroscopy and other diagnostic imaging practices, this procedure has a learning curve. We know that the number of successful diagnoses will increase with training and repetition, but so far there are no data on the number of procedures needed for proficiency. However, diagnostic needle arthroscopy images are of high quality and are static across users (Figures 5A, 5B). By contrast, the quality of MRI in the United States varies with the quality of the magnets used in individual facilities.
Conclusion
In-office diagnostic needle arthroscopy is a cost-effective and reproducible procedure with potential cost and quality-of-life benefits for commercial payers and patients. Although further study of long-term cost savings for the health care system is needed, significant value was realized in this 200-patient retrospective review. Minimum savings of $418 and $554.62 were realized for noncontrast knee and shoulder MRIs, respectively, in independent facilities. Those cost savings more than doubled in hospital-based facilities: $961.08 and $1097.62, respectively, for knee and shoulder noncontrast MRIs.
For More on In-office Arthroscopy...
Don’t miss Dr. Sean McMillan’s “Innovative Technique Update: In-Office Arthroscopy: My Technique and Results” at the upcoming Innovative Techniques® Knee, Hip, and Shoulder Course in Las Vegas. 29.5 CME/MOC available. Learn more
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.
1. O’Donnell J. Trice Medical literature. #4-10-0032 Rev A.
2. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994-1000.
3. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;(11):1-25.
4. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;(357):j1982.
5. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;(84):5-23.
6. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
7. Sharpe RE Jr, Levin DC, Parker L, Rao VM. The recent reversal of the growth trend in MRI: a harbinger of the future? J Am Coll Radiol. 2013;10(8):599-602.
8. Organisation for Economic Cooperation and Development (OECD). 46. Magnetic resonance imaging (MRI) exams, total per 1 000 population. OECD website. http://dx.doi.org/10.1787/mri-exam-total-table-2014-1-en. Published June 30, 2014. Accessed August 14, 2017.
9. Westermann RW, Schick C, Graves CM, Duchman KR, Weinstein SL. What does a shoulder MRI cost the consumer? Clin Orthop Relat Res. 2017;475(3):580-584.
10. Thakkar RS, Thakkar SC, Srikumaran U, McFarland EG, Fayad LM. Complications of rotator cuff surgery—the role of post-operative imaging in patient care. Br J Radiol. 2014;87(1039):20130630.
11. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
12. McMillan S, Saini S, Alyea E, Ford EA. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017.
13. Keeping me waiting: medical practice wait times and patient satisfaction [white paper]. South Bend, IN: Press Ganey; 2010. https://helpandtraining.pressganey.com/Documents_secure/Medical%20Practices/White%20Papers/Keep_Me_Waiting.pdf. Published 2010. Accessed August 14, 2017.