User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Implementing Patient-Reported Outcome Measures in Your Practice: Pearls and Pitfalls
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
Superior Capsular Reconstruction: Clinical Outcomes After Minimum 2-Year Follow-Up
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
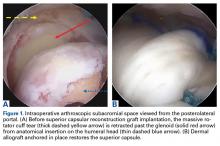
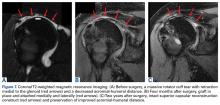
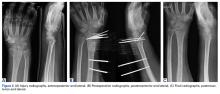
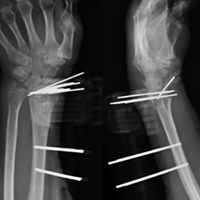
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
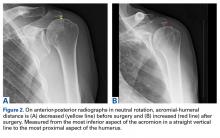
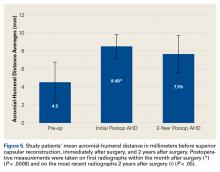
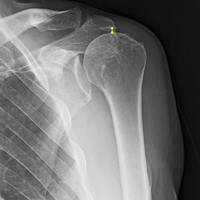
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Boldly Going (Where No Journal Has Gone Before)
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
Take-Home Points
- The optimal centrifugation protocol for production of rat PRP is 1300 rpm for 5 minutes.
- PRP administration in RCR improves tendon biomechanics in a rat model.
- Administration of NSAIDs following RCR has no significant effect on tendon biomechanical properties.
- NSAIDs may be co-administered with PRP without reducing efficacy of PRP.
- The role of PRP and NSAIDs in human RCR remains unclear.
Rotator cuff tears are a common source of shoulder pain and disability among older adults and athletes. Full-thickness tears alone occur in up to 30% of adults older than 60 years.1 Surgical repair is plagued by an unpredictable rate of recurrence (range, 11%-94%).1-10 As a result of improved suture materials, knotting patterns, and anchor designs, hardware issues are no longer the primary cause of rotator cuff repair (RCR) failures; now the principal mode of failure is biologic.2 Animal model studies have found that, after injury and subsequent healing, the tendon–bone interface remains abnormal.11 Rotator cuff research therefore has focused largely on biological enhancement of tendon-to-bone healing.
One means of biological augmentation is autologous platelet-rich plasma (PRP), which has supraphysiologic concentrations of platelets and their secreted growth factors. Although there is no consensus on the long-term efficacy of PRP, some studies suggest PRP accelerates healing over short and intermediate terms, which may contribute to a more rapid decrease in pain and more rapid return to normal activities.12-18 Similarly, systemic nonsteroidal anti-inflammatory drugs (NSAIDs) have long been used to treat musculoskeletal injuries, including rotator cuff pathology. However, NSAIDs inhibit cyclooxygenase activity, and clinical and experimental data have shown that cyclooxygenase 2 function is crucial in normal tendon-to-bone healing.19-21
Comprehensive studies have been conducted on the efficacy of both PRP and NSAIDs, but the interaction of concurrently used PRP and NSAIDs has not been determined. As many physicians use both modalities in the treatment of soft-tissue injuries, it is important to study the potential interactions when coadministered. Prior studies in small animal models suggest NSAIDs may impair tendon-to-bone healing in RCR, but there is no evidence regarding the effect of NSAIDs on the efficacy of PRP treatment.21
We conducted a study to determine the interaction of PRP and NSAIDs when used as adjuncts to RCR in a rat model. We hypothesized that PRP would increase the strength of RCR and that NSAIDs would interfere with the effects of PRP. A preliminary study objective was to determine an appropriate centrifugation protocol for producing PRP from rat blood, for use in this study and in future rat-based studies of PRP.
Materials and Methods
Part A: Pretesting Determination of PRP Centrifugation Protocol
Fourteen adult male Fischer rats were used in part A of this study, which was conducted to determine an appropriate PRP centrifugation protocol. Traditional PRP centrifugation protocols are established for human blood, but rat red blood cells (RBCs) and human RBCs differ in size.22 In our preliminary study, we wanted to determine the adjusted centrifuge speed and duration for producing clinically optimal PRP from rats. Clinically optimal PRP has reduced levels of RBCs, which decrease platelet affinity. Although the role of leukocytes in PRP preparations is debated, reducing the number of white blood cells (WBCs) decreases the number of matrix metalloproteinases and reactive oxygen species that may lead to inflammation. We used the platelet index (ratio of platelets to WBCs) and the RBC count to quantify the quality of our PRP sample.
Each rat in part A was anesthetized while supine. We used the Autologous Conditioned Plasma (ACP) system (Arthrex), which requires only 1 centrifugation cycle to create PRP. About 9 mL or 10 mL of blood was obtained by cardiac aspiration using an ACP Double Syringe (Arthrex). After blood retrieval, a thoracotomy was performed to confirm each rat’s death.
Part B: Determining the Effects of PRP and NSAIDs on RCR in a Rat Model
Operative Cohort. Of the 34 Fischer rats used in part B of this study, 6 were used as blood donors for PRP production, and the other 28 underwent bilateral rotator cuff surgeries. We used donor rats to maximize the amount of PRP retrieval, allocating about 1 donor rat per 5 operative rats. Fischer rats are an inbred strain, so the PRP from a donor Fischer rat simulates autologous blood in other Fischer rats. Use of allogenic blood is consistent with prior rat PRP studies.23,24
Operative Technique. Each bilateral surgery was performed by a single board-certified shoulder surgeon, and the anesthetic and surgical protocols were followed as approved by the home institution’s Institutional Animal Care and Use Committee. Before surgery, blood was harvested for PRP production from donor rats, as described earlier, and centrifuged for 5 minutes × 1300 rpm. After anesthetic induction and skin incision, the deltoid muscle was cut to expose the acromion and underlying rotator cuff. The distal supraspinatus tendon was sharply detached from the greater tuberosity. A bone-tunnel RCR was performed by drilling a transverse tunnel across the greater tuberosity and affixing the tendon to its footprint with a 5-0 polypropylene suture (Prolene; Ethicon). Each rat was then randomly assigned to receive 50 µL of donor PRP injected in 1 operative shoulder and saline in the contralateral shoulder. Injections were made in the supraspinatus tendon at its attachment to the humerus. Deltoid and skin were closed with 4-0 polyglactin (Vicryl) suture (Ethicon) and staples, respectively.
Tendon Preparation. Immediately post mortem, each shoulder was grossly dissected to isolate the supraspinatus muscle attached to the humerus. Shoulders were then frozen in 0.15-M saline solution until specified biomechanical testing dates.
On day of dimensional/biomechanical testing, each specimen was thawed at room temperature and finely dissected under a microscope (Stemi 200-C; Car Zeiss). After dissection, the humeral shaft was embedded in polymethylmethacrylate within a test tube. The free end of the supraspinatus tendon was glued within a “tab” of waterproofed emery cloth, leaving about 2 mm of tendon between the tab and the greater tuberosity.
Biomechanical Analysis. A 5848 MicroTester (Instron) was used for biomechanical testing. Each tabbed tendon, held by a pneumatic clamp attached to the MicroTester, was tested in a preconditioning phase and then a ramp-to-failure phase. A constant drip of 0.15-M saline was run through the apparatus to simulate physiologic hydration of tissue. After the embedded specimen was secure within the loading apparatus, an initial tensile preload of 0.2 N was applied. After preloading, the tendon was run through a preconditioning phase to account for viscoelastic relaxation. Immediately after preconditioning, each tendon was subjected to failure testing at a ramp rate of 0.1 mm/s. Force data were collected as a function of displacement, allowing for the calculation of 4 biomechanical parameters: failure force, tendon stiffness and normalized stiffness, energy to failure, and total energy. Tendon stiffness is the slope of a curve-fit line of the initial peak; failure force is the force of the highest peak; energy to failure is the area under the curve (AUC) to the highest peak; and total energy is the AUC from the start of failure ramping to the point at which the tendon is torn off completely. Two-way ANOVA was used to assess the differences between treatment groups and diet groups for all parameters. Statistical significance was set at P < .05.
A power analysis was performed to determine ability to detect differences between cohorts. For power of 80% and P = .05, a difference of 16% of the mean could be detected for failure force, 30% for energy to failure, 14% for total energy to failure, and 24% for stiffness. In addition, a difference of 4% of the mean could be detected for tendon length, 6% for width, and 10% for thickness.
Results
Across all collective treatment-diet groups and biomechanical parameters, there was only 1 statistically significant difference. Mean (SD) energy to failure was significantly higher (P = .03) in shoulders treated with PRP, 11.7 (7.3) N-mm, than in those treated without PRP, 8.7 (4.6) N-mm (Figure 4). There were no statistically significant differences between shoulders treated with indomethacin and those treated without indomethacin (Table 3), and no statistically significant relationships between treatment and drug for any other biomechanical parameter (Figures 5-7).
Discussion
Our preliminary objective in this study was to determine the optimal centrifugation protocol for producing rat-based PRP. Optimal PRP requires a dense concentration of platelets as well as reduced levels of RBCs and WBCs.25 We used the platelet index to quantify the quality of our PRP samples, and we obtained the highest platelet index for the protocol of 5 minutes × 1300 rpm. This finding may be useful in later rat studies involving PRP.
The primary objective of this study was to assess the effect of the interaction of PRP and NSAIDs on RCR. PRP has been found to augment RCR,12,26,27 but indomethacin may impair healing.21,25 We hypothesized that shoulders treated with PRP would have more biomechanical strength than control shoulders and that indomethacin would decrease biomechanical strength.
Our data showed increased energy to failure of the rotator cuff with PRP injections (P = .03). All other biomechanical parameters showed no significant differences with PRP treatment, though there were statistically insignificant trends of increased total energy, failure force, and stiffness in the PRP cohorts. There were no statistically significant differences between the indomethacin and no-indomethacin groups, and indomethacin had no effect on the efficacy of PRP treatment. It should be noted that the measurements of total energy, energy to failure, and failure force best reflect the strength of the tendon–bone interface. Other biomechanical measures, such as stiffness and normalized stiffness, are physical properties of the tendon itself and apply less to enthesis strength, which was the primary focus of this study.
Beck and colleagues23 studied the effect of allogeneic PRP on RCR in a rat model. They tested biomechanical and histologic outcomes 7, 14, and 21 days after surgery. There was no significant difference in failure load between the 2 groups at any time point. Compared with failure strain in the control group, failure strain in the PRP group was decreased at 7 days, normalized at 14 days, and increased at 21 days. The authors hypothesized that increased tendon failure strain at 21 days may have reduced forces being transmitted to the suture fixation site, which may be clinically significant and warrants further investigation. In a similar study, by Dolkart and colleagues,28 intraoperative PRP administration enhanced the maximal load-to-failure and stiffness of rats’ repaired rotator cuffs. On histologic examination, tendons treated with PRP (vs control tendons) had more organized collagen. Although these studies have limitations similar to our study, these results further support improved tendon-to-bone healing with PRP.
In clinical application, Barber and colleagues26 found that, compared with controls, suturing PRP fibrin matrix into the rotator cuff during repair decreased the incidence of magnetic resonance imaging–detected retears. However, in 2 prospective, randomized trials, Castricini and colleagues29 and Weber and colleagues30 found that use of PRP in RCR did not improve outcomes. All 3 studies differ from ours in that they used fibrin matrix. However, Ersen and colleagues31 found no difference in the effects of PRP on rotator cuff healing between injection and fibrin matrix; PRP improved biomechanical properties of repaired rotator cuff independent of administration method. In a meta-analysis of PRP supplementation in RCR, Warth and colleagues32 found a statistically significant improvement in retear rates for tears >3 cm repaired with a double-row technique, but otherwise no overall improvement in retear rates or outcome scores with PRP. The authors acknowledged that the significant heterogeneity of the studies in their meta-analysis may have affected the quality of their data.
Although our study provides some insight into the effectiveness of PRP in tendon repair, the lack of standardization in PRP preparation and time points tested makes comparisons with similar studies difficult.33 Recent reports have emphasized that not all PRP separation systems yield similar products.33 Platelet concentrations, and therefore platelet-derived growth factor concentrations, differ between systems and may yield different clinical outcomes. Our decision to use leukocyte-reduced PRP is supported by a meta-analysis by Riboh and colleagues,34 who reviewed the literature on the effect of leukocyte concentration on the efficacy of PRP products. They found that, in the treatment of knee osteoarthritis, use of leukocyte-poor PRP resulted in improved functional outcomes scores in comparison with placebo, but this improvement did not occur with leukocyte-rich PRP. However, there is still no consensus on optimal preparation, dosing, and route of administration of PRP, and preparations described in the literature vary.
This study also assessed the interaction of PRP and NSAIDs. Although there were no statistically significant differences between treatment and diet, shoulders treated with indomethacin alone showed a trend toward weaker biomechanical parameters in comparison with shoulders treated with saline alone, with PRP alone, or with both PRP and indomethacin. A larger sample would be needed to establish statistical significance. These trends are not surprising, as Cohen and colleagues21 found that NSAIDs, specifically indomethacin and celecoxib, significantly inhibited rotator cuff tendon-to-bone healing. The authors also found that a 2-week course of indomethacin was sufficient to significantly inhibit tendon-to-bone healing. In fact, although the drugs were discontinued after 14 days, biomechanical properties were negatively affected up to 8 weeks after repair. Our results differ from theirs even though the 2 studies used similar doses and administration protocols.
One strength of this study was that all surgeries were performed by a single board-certified surgeon using a standardized technique. In addition, a control group was established, and personnel and techniques for all fine dissections and biomechanical tests were consistent throughout. Blinded randomization and diet normalization, as well as adequate power for detecting significant effects, strengthened the study as well.
The study had several limitations. First, whereas most human rotator cuff tears are chronic, we used a model of acute injury and repair. As acute tears that are immediately repaired are more likely to heal, detection of differences between cohorts is less likely. However, using an acute model is still the most reliable strategy for inducing a controlled injury with reproducible severity. Second, we analyzed data at only 1 time point, which may not provide an accurate representation of long-term effects. Third, systemic administration of indomethacin did not allow for intra-rat shoulder comparisons of the different drug groups. Fourth, although it is possible that the dosage of NSAID was insufficient to produce significant differences in biomechanics, our dosage was consistent with that used in a study that found a significant effect on tendon healing.21
Conclusion
Our study found that the strength of the supraspinatus tendon enthesis as defined by energy to failure was increased with intratendinous PRP injection. Indomethacin showed no statistical effect, but there was a trend toward reduced strength after repair. However, the extent to which coadministration of indomethacin affects PRP remains unclear, and these data cannot necessarily be extrapolated to the typical human rotator cuff tear caused by chronic repetitive stress.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
1. Kinsella KG, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. https://www.census.gov/prod/2001pubs/p95-01-1.pdf. Published November 2001. Accessed September 24, 2017.
2. Gamradt SC, Rodeo SA, Warren RF. Platelet rich plasma in rotator cuff repair. Tech Orthop. 2007;22(1):26-33.
3. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
4. Harryman DT, Mack LA, Wang KY. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982-989.
5. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290-299.
6. Boileau P, Brassart N, Watkinson DJ, Carles M. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
7. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82(4):505-515.
8. Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2007;89(7):1533-1541.
9. Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90(10):1341-1347.
10. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423-2431.
11. Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81(9):1281-1290.
12. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
13. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14(12):1272-1280.
14. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171-1178.
15. Harmon KG. Muscle injuries and PRP: what does the science say? Br J Sports Med. 2010;44(9):616-617.
16. Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29(29):3983-3992.
17. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010;44(9):618-619.
18. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007-1017.
19. Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743-1747.
20. Aspenberg P. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2004;22(3):684.
21. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34(3):362-369.
22. Balazs T, Grice HC, Airth JM. On counting the blood cells of the rat with an electronic counter. Can J Comp Med Vet Sci. 1960;24(9):273-275.
23. Beck J, Evans D, Tonino PM, Yong S, Callaci JJ. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40(9):2037-2044.
24. Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93-99.
25. Chechik O, Dolkart O, Mozes G, Rak O, Alhajajra F, Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134(4):515-520.
26. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
27. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008;30(20-22):1584-1589.
28. Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E. A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg. 2014;134(9):1271-1277.
29. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258-265.
30. Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. Am J Sports Med. 2013;41(2):263-270.
31. Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg. 2014;134(3):405-411.
32. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306-320.
33. Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. Am J Sports Med. 2012;40(2):286-293.
34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792-800.
Acute Shortening Versus Bridging Plate for Highly Comminuted Olecranon Fractures
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
Take-Home Points
- The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability.
- Consider BP as an alternative to AS in unreconstructable olecranon fractures.
- Both BP and AS of olecranon fractures maintain elbow stability.
- BP has the advantage of maintaining elbow range of motion.
Olecranon fractures constitute about 10% of all forearm fractures.1 Many are low-energy fractures in osteoporotic bone in the elderly.1,2 Unstable fractures require operative fixation in which the goal is restoration of articular congruity and stability.3 Various fixation methods are used to treat unstable olecranon fractures, and outcomes are good overall.3-21 However, severely comminuted olecranon fractures, especially in osteoporotic bone, pose a unique challenge, where reconstruction may not be feasible.9 Although the articular surface can be reconstructed in most cases, reconstruction is not feasible with severe comminution or low bone mineral density. When articular congruity is no longer possible, the primary goal of fixation becomes elbow stability. Postoperative stability is linked to favorable outcomes, as it allows patients to engage in early range-of-motion (ROM) exercises, which improves joint function.5,21,22
When treating these severely comminuted olecranon fractures, surgeons have 2 options: bridge plating (BP) and acute shortening (AS). In BP, a plate is used to restore the length of the olecranon. The plate is spanned over the comminuted segment with fixation at proximal and distal pieces but without open reduction of the comminuted pieces.8 This process may be performed with or without bone grafting.21 Although any bony defect between the proximal and distal pieces may be filled, there is now a gap in articular congruity within the sigmoid notch. One concern with this fixation method is that joint stability is lost when this gap becomes too large. Surgeons therefore may decide to forgo BP and perform AS instead, as long as the coronoid is intact.21 In AS, often referred to as olecranon excision, comminuted fragments are removed and the triceps muscle advanced distally. AS constructs, often reserved for older, less active patients, yield acceptable results in this population.5 However, the long-term effects of AS in young, active patients are unclear, and biomechanical studies suggest reduced triceps muscle strength.23
Surgeons have had no studies guiding them in deciding which construct to use, BP or AS, in severely comminuted olecranon fractures in which the articular surface cannot be reconstructed.
We conducted a biomechanical study to determine the percentage loss of articular surface at which a BP construct becomes significantly clinically unstable. We also compared BP stability and AS stability for each percentage loss of articular surface and compared initial elbow ROM with the 2 methods. We hypothesized that, at a certain percentage loss of articular congruity, the BP construct would become too unstable and would require conversion to the AS construct.
Materials and Methods
Specimen Preparation
Eight fresh-frozen paired cadaveric upper limbs (2 male, 2 female; mean age, 61.8 years; age range, 56-74 years) were obtained from donors with no history of elbow trauma or prior surgery. Specimens were stored at –20°C, thawed to room temperature before testing, and, using clinical and radiographic evaluation, screened for abnormalities.
Each specimen was positioned with the arm draped in the lateral decubitus position, as in typical olecranon fracture surgery. A standard posterior approach to the olecranon was made with a midline posterior longitudinal skin incision. Subcutaneous flaps were developed, and the subcutaneous border of the proximal olecranon was exposed, preserving the medial and lateral collateral ligaments as well as the extensor mechanism. Baseline maximum flexion and extension of the elbow as well as olecranon length were measured with fluoroscopy (BV Pulsera, Philips) and ImageJ software (National Institutes of Health).
To ensure reproducible anatomical reduction during plating, a 3.5-mm 4-hole nonlocking periarticular anatomically contoured plate (Zimmer Biomet) was applied posteriorly to the intact olecranon through a longitudinal slit in the distal triceps tendon. The plate was predrilled to house 4 nonlocking screws, 2 proximal and 2 distal.
Fracture Generation and Testing of Fixation Constructs
Analysis
ImageJ software was used to analyze the C-arm radiographs. Measurements were divided into 4 groups of joint surface loss caused by the resections: 0% to 20%, 20% to 40%, 40% to 60%, and >60%. Differences in ROM between the BP and AS constructs were analyzed with a Wilcoxon signed rank test with statistical significance set at P < .05 (Prism 6; GraphPad Software).
Results
As many as 6 serial resections were made before the proximal fragment of the olecranon was judged too small to be secured to a plate with at least 2 screws. Only 7 specimens were large enough for the fifth cut, and only 4 were large enough for the sixth cut. After the final resection, mean loss of olecranon length was 77.3% (range, 63.7%-88%; median, 80.6%). All elbow specimens remained stable to manual valgus and varus testing in full extension, 30° of flexion, and full flexion in both supination and pronation. There was no medial or lateral opening of the ulnohumeral joint on fluoroscopy throughout testing, for either the BP or the AS constructs. There was no anterior or posterior subluxation throughout the entire ROM.
Discussion
Our goal in this study was to determine the maximum articular surface loss that can be tolerated before a BP construct becomes unstable. This finding applies to situations in which the degree of comminution makes reconstruction of the articular surface impossible. Contrary to our hypothesis, the ulnohumeral joint remained stable despite extensive loss of congruity within the sigmoid notch. In 1 specimen, the joint remained stable at 88% loss of olecranon. However, the 2 constructs had different ROM results: ROM was significantly lower at more resections with AS but remained unchanged from baseline with BP.
Dorsal plating has become standard treatment for comminuted olecranon fractures, and many studies, both clinical and biomechanical, have reported favorable results, good functional outcomes, and acceptable ROM.3,7,10,13,18-20,25 However, the multiple studies on the use of various plates in comminuted olecranon fractures did not address whether articular congruity was maintained during reductions or how much articular surface was reconstructed. Although we may reasonably assume larger studies included cases with some unmeasured loss of articular congruity, it is difficult to directly compare our findings with those of other studies. In addition, it is possible those studies did not include fractures that were deemed unfit for BP (because of very severe comminution) and underwent AS instead. Only 1 case series has focused on BP without complete articular reconstruction.8 The cases in that series had good outcomes with good stability—consistent with our finding of extreme comminution in a worst-case scenario.
Complete elbow stability after AS is consistent with findings in the literature.4,6,12,14,16 As AS is reserved for severely comminuted fractures and bone resections,21,23,26 our findings can be compared with the earlier findings. In AS, either the proximal pieces or the intermediate pieces are removed to create a smaller but congruent articular surface, with less concern for nonunion.21 When the proximal piece is removed, the triceps muscle is advanced to the ulnar shaft, creating a slinglike structure for the trochlea.4,11,16,23 When the intermediate piece is removed, the proximal piece is advanced to the shaft along with the triceps.12,14,27 In either technique, the triceps muscle is advanced distally, potentially affecting its extensibility and moment arm.23
Although small in numbers, case series and retrospective reviews have found that AS has good outcomes,4,14,16 whereas our study found significantly decreased ROM. A few patients in these studies lost ROM or triceps strength,12,14,16 but the cause, AS or fracture severity, is unclear. It is possible only 0% to 20% of the olecranon was resected in those cases, whereas our study found no significant change in ROM. It is also possible that cadaveric muscles do not stretch as well as muscles in vivo. Biomechanical studies have demonstrated changes in triceps stretch and strength,23,26 but perhaps these changes are subclinical or overcome with therapy and time.12,14 There are no data regarding whether patients who undergo AS (vs another fixation method) need more physical therapy. In extreme resection, some reduction in ROM is expected.13
The ulnohumeral joint is a primary static stabilizer of the elbow joint.28-30 Recent studies on the role of the ulnohumeral joint in elbow stability have focused mainly on the coronoid process in the setting of dislocation.28,29,31,32 According to these studies, 50% of the coronoid must remain intact for the elbow to be stable when all other stabilizers are intact.32 In our study, resections preserved the coronoid and the ligamentous stabilizers of the elbow. It is therefore possible that the elbow joint remained stable despite the considerable articular surface loss. Although the term ulnohumeral joint refers to both the coronoid and the remaining articular surface, our findings support the coronoid as a primary stabilizer and the remaining articular surface as a secondary static stabilizer.
This study had several limitations. First, its fractures were simulated by serial resection of only the middle portion of the olecranon. In reality, comminution could extend farther proximally or distally and involve the surrounding tissues, which help stabilize the elbow. However, our focus was on loss of articular surface and stability, so keeping surrounding structures intact avoided confounding factors that could contribute to stability. A second possible limitation is that the implant used here may be different from the implant used in a clinical setting. However, our focus was not on fixation quality, and stability alone should not be affected by plate type. Third, stability was measured not quantitatively but instead subjectively under manual stress and fluoroscopy. We chose this method because it mimics what happens during surgery and is the clinical standard for stability assessment.24 Fourth, soft-tissue properties of the cadaver models used in this biomechanical study may differ from soft-tissue properties in vivo. This study could not evaluate possible long-term complications, such as posttraumatic arthritis and heterotopic ossification.5,10 There are no long-term studies comparing BP and other olecranon fixation methods in terms of postoperative elbow arthritis.
Conclusion
The ulnohumeral joint can tolerate substantial articular surface loss without compromising stability. As a result, in the management of highly comminuted olecranon fractures, BP may be considered before AS is performed. Quality and amount of intact proximal bone, rather than degree of comminution, may be more important factors in deciding which fixation method to use.
This biomechanical study is the first to focus on olecranon fracture BP without complete reconstruction of the articular surface. When treating a highly comminuted olecranon fracture that has an unreconstructible articular surface, surgeons may consider BP with or without bone graft, as well as AS. Our study findings suggest that, though both constructs maintain elbow stability, BP may have the advantage of maintaining ROM too. BP can avoid effects on triceps and elbow ROM, which may be more important in younger, more active patients. Clinical correlates are needed to validate these findings, as overall outcomes may be affected by concurrent fractures and injuries to surrounding structures.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
1. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37(8):691-697.
2. Duckworth AD, Clement ND, Aitken SA, Court-Brown CM, McQueen MM. The epidemiology of fractures of the proximal ulna. Injury. 2012;43(3):343-346.
3. Bailey CS, MacDermid J, Patterson SD, King GJ. Outcome of plate fixation of olecranon fractures. J Orthop Trauma. 2001;15(8):542-548.
4. Adler S, Fay GF, Macausland WR Jr. Treatment of olecranon fractures. Indications for excision of the olecranon fragment and repair of the triceps tendon. J Trauma. 1962;2:597-602.
5. Baecher N, Edwards S. Olecranon fractures. J Hand Surg Am. 2013;38(3):593-604.
6. Bell TH, Ferreira LM, McDonald CP, Johnson JA, King GJW. Contribution of the olecranon to elbow stability: an in vitro biomechanical study. J Bone Joint Surg Am. 2010;92(4):949-957.
7. Buijze G, Kloen P. Clinical evaluation of locking compression plate fixation for comminuted olecranon fractures. J Bone Joint Surg Am. 2009;91(10):2416-2420.
8. Cervera-Irimia J, Tomé-Bermejo F, Gómez-Bermejo MA, Holgado-Moreno E, Stratenwerth EG. Treatment of comminuted olecranon fractures with olecranon plate and structural iliac crest graft. Acta Orthop Belg. 2012;78(6):703-707.
9. Edwards SG, Martin BD, Fu RH, et al. Comparison of olecranon plate fixation in osteoporotic bone: do current technologies and designs make a difference? J Orthop Trauma. 2011;25(5):306-311.
10. Erturer RE, Sever C, Sonmez MM, Ozcelik IB, Akman S, Ozturk I. Results of open reduction and plate osteosynthesis in comminuted fracture of the olecranon. J Shoulder Elbow Surg. 2011;20(3):449-454.
11. Estourgie RJ, Tinnemans JG. Treatment of grossly comminuted fractures of the olecranon by excision. Neth J Surg. 1982;34(3):127-129.
12. Fern ED, Brown JN. Olecranon advancement osteotomy in the management of severely comminuted olecranon fractures. Injury. 1993;24(4):267-269.
13. Gordon MJ, Budoff JE, Yeh ML, Luo ZP, Noble PC. Comminuted olecranon fractures: a comparison of plating methods. J Shoulder Elbow Surg. 2006;15(1):94-99.
14. Iannuzzi N, Dahners L. Excision and advancement in the treatment of comminuted olecranon fractures. J Orthop Trauma. 2009;23(3):226-228.
15. Ikeda M, Fukushima Y, Kobayashi Y, Oka Y. Comminuted fractures of the olecranon. Management by bone graft from the iliac crest and multiple tension-band wiring. J Bone Joint Surg Br. 2001;83(6):805-808.
16. McKeever FM, Buck RM. Fracture of the olecranon process of the ulna; treatment by excision of fragment and repair of triceps tendon. JAMA. 1947;135(1):1-5.
17. Rommens PM, Küchle R, Schneider RU, Reuter M. Olecranon fractures in adults: factors influencing outcome. Injury. 2004;35(11):1149-1157.
18. Siebenlist S, Torsiglieri T, Kraus T, Burghardt RD, Stöckle U, Lucke M. Comminuted fractures of the proximal ulna—preliminary results with an anatomically preshaped locking compression plate (LCP) system. Injury. 2010;41(12):1306-1311.
19. Tarallo L, Mugnai R, Adani R, Capra F, Zambianchi F, Catani F. Simple and comminuted displaced olecranon fractures: a clinical comparison between tension band wiring and plate fixation techniques. Arch Orthop Trauma Surg. 2014;134(8):1107-1114.
20. Wang Y, Tao R, Xu H, Cao Y, Zhou Z, Xu S. Mid-term outcomes of contoured plating for comminuted fractures of the olecranon. Orthop Surg. 2011;3(3):176-180.
21. Newman SD, Mauffrey C, Krikler S. Olecranon fractures. Injury. 2009;40(6):575-581.
22. Boyer MI, Galatz LM, Borrelli J, Axelrod TS, Ricci WM. Intra-articular fractures of the upper extremity: new concepts in surgical treatment. Instr Course Lect. 2003;52:591-605.
23. Didonna ML, Fernandez JJ, Lim TH, Hastings H, Cohen MS. Partial olecranon excision: the relationship between triceps insertion site and extension strength of the elbow. J Hand Surg Am. 2003;28(1):117-122.
24. Trumble T, Cornwall R, Budoff J. Core Knowledge in Orthopaedics: Hand, Elbow, and Shoulder. Philadelphia, PA: Mosby; 2006.
25. Simpson NS, Goodman LA, Jupiter JB. Contoured LCDC plating of the proximal ulna. Injury. 1996;27(6):411-417.
26. Ferreira LM, Bell TH, Johnson JA, King GJ. The effect of triceps repair techniques following olecranon excision on elbow stability and extension strength: an in vitro biomechanical study. J Orthop Trauma. 2011;25(7):420-424.
27. Colton CL. Fractures of the olecranon in adults: classification and management. Injury. 1973;5(2):121-129.
28. Hull JR, Owen JR, Fern SE, Wayne JS, Boardman ND 3rd. Role of the coronoid process in varus osteoarticular stability of the elbow. J Shoulder Elbow Surg. 2005;14(4):441-446.
29. Morrey BF, An KN. Stability of the elbow: osseous constraints. J Shoulder Elbow Surg. 2005;14(1 suppl S):174S-178S.
30. Williams G, Ramsey M, Wiesel S. Operative Techniques in Shoulder and Elbow Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
31. Schneeberger AG, Sadowski MM, Jacob HA. Coronoid process and radial head as posterolateral rotatory stabilizers of the elbow. J Bone Joint Surg Am. 2004;86(5):975-982.
32. Closkey RF, Goode JR, Kirschenbaum D, Cody RP. The role of the coronoid process in elbow stability. A biomechanical analysis of axial loading. J Bone Joint Surg Am. 2000;82(12):1749-1753.
Treating Unstable Distal Radius Fractures With a Nonspanning External Fixation Device: Comparison With Volar Locking Plates in Historical Control Group
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
Take-Home Points
- Clinical and radiographic outcomes of patients treated with non-spanning external fixation are comparable to those treated with open reduction and internal volar locked plate fixation.
- Non-spanning external fixation can lead to satisfactory outcomes based on the following features: fragment specific fixation, subchondral support, fixed angle strength, limited dissection, distraction/length adjustment, joint distraction avoidance, and ability to perform early rehabilitation.
- Non-spanning external fixation should be considered as a treatment option for complicated unstable comminuted intra-articular distal radius fractures, specifically in the elderly.
In the United States, distal radius fractures (DRFs) are among the most common fractures, comprising about 15% of all extremity fractures.1 With a DRF, the primary treatment goal is anatomical reduction with restoration of radiographic parameters and stable fixation of the fracture to restore wrist function.
This fracture type has a variety of treatment alternatives, including nonoperative closed reduction and casting of stable fractures, open reduction and internal fixation (ORIF) with dorsal or volar locking plates, and external fixation. Optimal surgical management of unstable DRFs remains controversial.2 Closed reduction with percutaneous pinning or external fixation has become less common with a trend toward using volar locking plates for internal fixation.3
External fixation of DRFs traditionally has involved either spanning or simple nonspanning devices. Spanning fixation is particularly useful in open or highly comminuted fractures with an unstable soft-tissue envelope. In the past, nonspanning external fixation typically was reserved for fractures with a noncomminuted extra-articular distal fragment to which several large pins or Kirschner wires (K-wires) could be secured. The Non-Bridging External Fixator (NBX; Nutek Orthopaedics) may be used in cases that traditionally might be treated with locked plating or fragment-specific fixation. Specifically, this device is indicated for comminuted intra-articular DRFs in which bone quality may be less than ideal. The NBX, also suitable in open fractures with a stable soft-tissue envelope, can restore and maintain articular alignment by providing subchondral support and stability with fragment-specific fixation. A key advantage of this type of external fixation is that it involves percutaneous fixation and allows for early postoperative range of motion (ROM).
Numerous studies have found excellent outcomes of treating unstable DRFs with ORIF with volar locking plates.4-6 However, few studies have compared the clinical and radiographic outcomes of ORIF with those of nonspanning external fixation in the treatment of unstable comminuted intra-articular DRFs. Windolf and colleagues7 found that, in cadaveric unstable intra-articular DRFs, nonspanning external fixation with multiplanar K-wires had biomechanical characteristics comparable to those of volar locking plates. Other suitable DRF treatment options have been found: an alternative nonbridging external fixator with multiplanar K-wires (Gradl and colleagues8) and the Cross-Pin Fixation system (A.M. Surgical) (Mirza and colleagues9).
We conducted a study to compare functional and radiographic outcomes of unstable comminuted intra-articular DRFs treated with a nonspanning external fixation device (NBX) with outcomes achieved with volar locking plates in a historical control group.
Materials and Methods
This retrospective case-control study was approved by our Institutional Review Board and conducted at 2 institutions. Included in the study were 25 consecutive patients (2 institutions) who underwent closed reduction and external fixation (CREF) with NBX as treatment for unstable DRFs (diagnosis based on radiographic parameters or inability to maintain acceptable alignment after closed reduction and casting). Of these 25 patients, 11 were available for clinical follow-up and medical records review; the other 14 were not available for followup but had their charts reviewed for radiographic data and treatment details. Six of the 14 patients declined to participate in the study, and the other 8 were lost to follow-up because of nonstandardized follow-up protocols. Patients were excluded from the study if their final follow-up had not occurred, or if it occurred before 6 months. For their participation in clinical follow-up, patients received nominal time compensation and mileage reimbursement through a grant from the NBX manufacturer.
The 25 patients underwent CREF with NBX between November 2008 and March 2013. Indications for external fixation consideration were intra-articular extension or significant comminution in patients with poor soft tissue or in patients who wanted to avoid invasive surgery or a permanent implant. Of the 11 patients who agreed to participate in the study, 7 were women and 4 were men; mean age was 64 years (range, 15-81 years). Of the 14 patients unable to follow up, 11 were women and 3 were men; mean age was 63 years (range, 26-89 years). At the last available follow-up, each of the 25 patients was doing well, was satisfied with treatment received and function regained, and had a healed DRF. In almost every case, the mechanism of injury was a fall onto an outstretched hand; most fractures were type C per AO (Arbeitsgemeinschaft für Osteosynthesefragen) classification (Table 1).
The surgical technique for this nonspanning external fixator involves closed reduction with longitudinal traction using ligamentotaxis to grossly align the fracture fragments, with small adjustments made throughout the procedure. A dorsally placed radiolucent fixator is used with fluoroscopic guidance to percutaneously affix a subchondral raft of smooth bicortical .062-inch K-wires. The fixator’s abundant pin holes allow for each specific distal fragment to be captured by pins that are a part of the external fixation construct. Furthermore, radially based pins that use a side bar allow for a “weave” of fixation. Radial length is then obtained and maintained by attaching the distal complex to proximal pins in the radial diaphysis. After pins are cut and wrist and digits are taken through full ROM to ensure smooth tracking, fluoroscopy is used to confirm final fracture fixation and alignment (Figure 1).
In ideal scenarios with good fixation, patients can begin gentle ROM exercises within 1 week after surgery. This regimen can progress to more aggressive motion exercises and even light strengthening (Figure 2).
The 11 clinical follow-up patients underwent directed clinical examination, including ROM and strength evaluation, by Dr. Dwyer and Dr. Crosby. Follow-up also included completion of questionnaires and review of radiographs.
During the clinical follow-up, a standard goniometer was used to evaluate active ROM (wrist flexion and extension and wrist radial and ulnar deviation, measured down the long axis of the forearm and the index ray), and forearm pronation and supination were measured from the 90° elbow flexion position using the humerus as the reference point with the shoulders in 0° of flexion, abduction, and external rotation. In addition, a calibrated dynamometer (Sammons Preston) was used to measure grip strength (position 3) and key pinch strength, and the average of 3 trials of each strength test was calculated. ROM and strength values were calculated as percentages of the contralateral (uninjured) side, as these ratios are more sensitive in detecting clinical changes.10 A 10% adjustment for dominant hand grip strength in right-handed patients was used for this comparison.11
Union (osseous bridging across fracture site on 2 of 3 views), radial height, radial inclination, and volar tilt were measured on standard posteroanterior and lateral radiographs taken at several points: time of injury, postreduction and/or preoperative, initial postoperative, and final follow-up. All radiographic measurements were independently taken by Dr. Dwyer and Dr. Crosby, who used a digital goniometer and ruler (Siemens Medical Solutions) or, when necessary, manual instruments. Means of the original and independent measurements were used for calculations.
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire, the Mayo wrist score, and the patient-rated wrist evaluation were used to assess activities of daily living, pain, and quality of life after surgery. Mayo wrist scores were adjusted for unemployed patients; work status was replaced with return to normal activities.
Complications of surgical treatment were evaluated. Major complications evaluated were loss of reduction, malunion, nonunion, deep infection, neuropathy, and tendon rupture. Minor complication possibilities were transient extensor tendon irritation, superficial infection, and finger stiffness. Also noted were 1 patient who subsequently required another procedure and 7 patients who were immobilized after external fixation removal.
We compared our study group’s outcomes with those of historical control patients who underwent fixation with internal volar locking plates. The 2 groups had similar demographic characteristics. To obtain the historical controls, we used the key words distal, radi*, volar, and plat* in a PubMed search. From the 169 citations found, we removed biomechanical cadaver studies, studies that focused on patients with demographics and fracture types dissimilar from our patient population’s, and studies that focused on special circumstances, such as complications or patient characteristics. Eight studies remained for historical comparison.
Results
Radiographic Outcomes
On the injury radiographs, mean volar tilt was –16.7° (range, 2° to –42°), mean radial inclination was 14.1° (range, –1° to 44°), and mean radial height was 5.3 mm (range, –2 mm to 11 mm). Minor improvement after reduction was noted. All patients had intraoperative or postoperative radiographs with external fixation in place (Figure 3).
On the final (post-fixation removal) radiographs, mean volar tilt was 3.3° (range, –16° to 21°), mean radial inclination was 20.7° (range, 0° to 31°), and mean radial height was 7.5 mm (range, 0 mm to 13 mm). Comparison of the injury and final means revealed correction of ~20° for volar tilt, 6° for radial inclination, and 2 mm for radial height. All but 5 patients had type C fractures (AO classification).
Clinical Outcomes
Eleven patients underwent clinical evaluation (functional assessment, physical examination). Mean DASH score was 11.4 (SD, 10.5; range, 0-27.3), mean Mayo wrist score was 79.0 (SD, 12.2; range, 65-100), and mean patient-rated wrist evaluation was 12.2 (SD, 11.9; range, 0-25.5). There was no statistical difference in DASH scores between this group and the historical control group (Table 3). ROM was measured under active effort. In our group, mean wrist flexion was 69.3° (86% of contralateral side), and mean extension was 64.0° (94%). Mean radial deviation of the wrist was 47.4° (135% of relative normal for patient), and mean ulnar deviation was 29.2° (101%). Mean (SD) pronation was 84.6° (4.7°), and mean (SD) supination was 82.3° (8.5°), or about 100% of contralateral pronosupination.
For each hand, 3 grip strength values and 3 key pinch strength values were obtained. These values were averaged, and the injury and contralateral sides were compared. Mean grip strength was 49.6 pounds (85% of contralateral), and mean key pinch strength was 14.0 pounds (97%).
Complications
Of the 25 patients, 6 (24%) had a pin-tract infection treated with oral antibiotics. One of these infections resulted in the removal of the entire fixator. One (4%) of the 25 patients reported transient hypoesthesia of the dorsal first webspace, and 3 (12%) reported pain at the pin sites.
Although all fractures achieved complete bony union, 1 patient (4%) had a refracture on the same fracture line after a fall within 6 weeks after fixator removal; this refracture was successfully treated with a cast worn for 6 weeks. Of the 3 patients with complete follow-up (27%) who lost reduction with external fixation in place, 2 had radiographic parameters maintained within acceptable limits, and 1 (9%) had a malunion with –16° volar tilt.
Our study patients had no tendon rupture, tendon irritation, or stiffness. By contrast, fixation with volar locking plates has been associated with extensor tendon and flexor tendon injury, flexor pollicis rupture, carpal tunnel syndrome, complex regional pain syndrome, loss of reduction, and hardware failure.19 Flexor pollicis longus ruptures that occur after volar plate fixation of DRFs are often attributed to plate positioning.20-22
Discussion
With volar locking plate internal fixation on the rise, CREF has become less widely used.3 This is especially true for comminuted and intra-articular fractures—most earlier external fixators required either spanning of the wrist or limited fixation in the distal articular fragment. Although many studies have found excellent outcomes of ORIF with volar locking plates in the treatment of unstable DRFs,4,6 few studies have compared volar locking plate ORIF with nonspanning external fixation for unstable comminuted intra-articular DRFs. Both Gradl and colleagues,8 using a nonbridging external fixator with multiplanar K-wires, and Mirza and colleagues,9 using the Cross-Pin Fixation system, found wrist function, quality-of-life, and radiographic outcomes similar to those of volar plate fixation in the treatment of DRFs. A comparative meta-analysis by Margaliot and colleagues17 revealed no superiority of internal fixation over external fixation for unstable DRFs, given the similarity in wrist function, radiographic, and subjective outcomes.
At a mean follow-up of 12.8 months (range, 6-23 months), our retrospective study found that the functional and radiographic outcomes of treating unstable comminuted DRFs with a nonspanning external fixator were similar to those reported in similarly matched control studies. Although followup of >2 years has been shown to be unnecessary,23-25 small differences may have been detected with interval results over these 2 years. The effect of selection bias on our study results should be considered in light of patients’ involvement in selecting fixation type. Our results parallel those of the temporal studies of Rozental and colleagues5 and Wei and colleagues12 (Table 2) while allowing for patients to return to function with limited morbidity and complications, similar to Orbay and Fernandez15 though with a less invasive procedure.
Although we found patient-rated outcome measure values analogous to those of the volar plate fixation group and bridging external fixator group in the study by Wright and colleagues,6 we did not measure intra-articular step-off. Another variable not addressed here was operative time. The nonspanning external fixator treatment that we investigated should undergo further study. A randomized prospective study that includes the additional outcome measures of intra-articular step-off and operative time is warranted.
We found that our study patients, who had their comminuted intra-articular DRFs treated with a nonspanning external fixator, and similar historical control patients, treated with volar locking plate internal fixation, had similar clinical and radiographic outcomes at final follow-up. There was no statistically significant difference in measured outcomes—wrist flexion and extension, radial deviation, pronation and supination, volar tilt, radial height, radial inclination, DASH scores—between the 2 groups. Compared with the historical control group, the external fixator group had significantly more postoperative ulnar deviation.
Given the functional and radiographic outcomes found at final follow-up in this study, we recommend considering a nonspanning external fixator in the treatment of unstable complex comminuted intra-articular DRFs, particularly those that occur in the elderly.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
1. Sanders WE. Distal radius fractures. In: Manske PR, ed. Hand Surgery Update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:117-123.
2. Shin EK, Jupiter JB. Current concepts in the management of distal radius fractures. Acta Chir Orthop Traumatol Cech. 2007;74(4):233-246.
3. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861.
4. Sammer DM, Kawamura K, Chung KC. Outcomes using an internal osteotomy and distraction device for corrective osteotomy of distal radius malunions requiring correction in multiple planes. J Hand Surg Am. 2006;31(10):1567-1577.
5. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837-1846.
6. Wright TW, Horodyski M, Smith DW. Functional outcome of unstable distal radius fractures: ORIF with a volar fixed-angle tine plate versus external fixation. J Hand Surg Am. 2005;30(2):289-299.
7. Windolf M, Schwieger K, Ockert B, Jupiter JB, Gradl G. A novel non-bridging external fixator construct versus volar angular stable plating for the fixation of intra-articular fractures of the distal radius—a biomechanical study. Injury. 2010;41(2):204-209.
8. Gradl G, Gradl G, Wendt M, Mittlmeier T, Kundt G, Jupiter JB. Non-bridging external fixation employing multiplanar K-wires versus volar locked plating for dorsally displaced fractures of the distal radius. Arch Orthop Trauma Surg. 2013;133(5):595-602.
9. Mirza A, Jupiter JB, Reinhart MK, Meyer P. Fractures of the distal radius treated with cross-pin fixation and a nonbridging external fixator, the CPX system: a preliminary report. J Hand Surg Am. 2009;34(4):603-616.
10. MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the Short Form-36, Disability of the Arm, Shoulder, and Hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am. 2000;25(2):330-340.
11. Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447.
12. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
13. Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J Hand Surg Am. 2006;31(3):359-365.
14. Osada D, Kamei S, Masuzaki K, Takai M, Kameda M, Tamai K. Prospective study of distal radius fractures treated with a volar locking plate system. J Hand Surg Am. 2008;33(5):691-700.
15. Orbay JL, Fernandez DL. Volar fixed-angle plate fixation for unstable distal radius fractures in the elderly patient. J Hand Surg Am. 2004;29(1):96-102.
16. Rein S, Schikore H, Schneiders W, Amlang M, Zwipp H. Results of dorsal or volar plate fixation of AO type C3 distal radius fractures: a retrospective study. J Hand Surg Am. 2007;32(7):954-961.
17. Margaliot Z, Haase SC, Kotsis SV, Kim HM, Chung KC. A meta-analysis of outcomes of external fixation versus plate osteosynthesis for unstable distal radius fractures. J Hand Surg Am. 2005;30(6):1185-1199.
18. Anderson RL. Practical Statistics for Analytical Chemists. New York, NY: Van Nostrand Reinhold; 1987.
19. Berglund LM, Messer TM. Complications of volar plate fixation for managing distal radius fractures. J Am Acad Orthop Surg. 2009;17(6):369-377.
20. Cross AW, Schmidt CC. Flexor tendon injuries following locked volar plating of distal radius fractures. J Hand Surg Am. 2008;33(2):164-167.
21. Bell JS, Wollstein R, Citron ND. Rupture of flexor pollicis longus tendon: a complication of volar plating of the distal radius. J Bone Joint Surg Br. 1998;80(2):225-226.
22. Klug RA, Press CM, Gonzalez MH. Rupture of the flexor pollicis longus tendon after volar fixed-angle plating of a distal radius fracture: a case report. J Hand Surg Am. 2007;32(7):984-988.
23. Kreder HJ, Hanel DP, Agel J, et al. Indirect reduction and percutaneous fixation versus open reduction and internal fixation for displaced intra-articular fractures of the distal radius: a randomised, controlled trial. J Bone Joint Surg Br. 2005;87(6):829-836.
24. Catalano LW 3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am. 1997;79(9):1290-1302.
25. Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J Jr. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633-639.
Radial Shaft Stress Fracture in a Major League Pitcher
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
Take-Home Points
- Stress fractures should always be considered when dealing with overuse injuries.
- Radial shaft stress fractures in overhead throwing athletes are rare.
- Stress fractures can occur anywhere increased muscular forces exceed the bone’s ability to remodel.
- Proper imaging is necessary to make the diagnosis of a stress fracture.
- Nonoperative management of radial shaft stress fractures is an effective treatment.
In athletes, the incidence of stress fractures has been reported to be 1.4% to 4.4%.1 Stress fractures of the upper extremity are less common and not as well described as lower extremity stress fractures. Although data is lacking, stress fractures involving the upper extremity appear to account for <6% of all stress fractures.2 Stress fractures of the upper extremity, though rare, are being recognized more often in overhead athletes.3-6 In baseball pitchers, stress fractures most commonly occur in the olecranon but have also been found in the ribs, clavicle, humerus, and ulnar shaft.2,4,7-10 Stress fractures of the radius are a rare cause of forearm pain in athletes, and there are only a few case reports involving overhead athletes.4,11-15 To our knowledge, a stress fracture of the radial shaft has not been reported in a throwing athlete. Currently, there are no reports on stress fractures of the proximal radial shaft.16-18
In this article, we report the case of a radial shaft stress fracture that was causing forearm pain in a Major League Baseball (MLB) pitcher. We also discuss the etiology, diagnosis, and management of stress fractures of the upper extremity of overhead throwing athletes. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 28-year-old right-hand-dominant MLB pitcher presented to the clinic with a 4-week history of right dorsal forearm pain that was refractory to a period of rest and physical therapy modalities. The pain radiated to the wrist and along the dorsal forearm. The pain started after the man attempted to develop a new pitch that required a significant amount of supination. The pain prevented him from pitching competitively. Indomethacin, diclofenac sodium topical gel, and methylprednisolone (Medrol Dosepak) reduced his symptoms only slightly.
Physical examination of the right elbow showed mild range of motion deficits; about 5° of extension and 5° of flexion were lacking. The patient had full pronation and supination. Palpation of the dorsal aspect of the forearm revealed marked tenderness in the area of the proximal radius. There was no tenderness over the posterior olecranon or the ulnar collateral ligament, and a moving valgus stress test was negative. No pain was elicited by resisted extension of the wrist or fingers. Motor innervation from the posterior interosseous nerve, anterior interosseous nerve, and ulnar nerve was intact with 5/5 strength, and there were no sensory deficits in the distribution of the radial, median, or ulnar nerves.
Discussion
Stress fractures account for 0.7% to 20% of sports medicine clinic injuries; <10% of all stress fractures involve the rib or upper extremity.4,6 When the intensity or frequency of physical activity is increased, as with overuse, bone resorption surpasses bone production, locally weakening the bone and making it prone to mechanical failure. Failure is thought to be induced by a combination of contractile muscular forces across damaged bone and increased mechanical loading caused by fatigue of supporting structures.5,6,19 These forces may have contributed to our baseball pitcher’s development of a stress fracture near the insertion of the supinator muscle in his throwing arm.
Given the insidious nature of stress fractures, the evaluating physician must have a high index of suspicion. Early recognition of a stress fracture is important in preventing further injury and allowing for early intervention, which is associated with faster healing.6,20 The clinical history often involves a change in training regimen within the weeks before pain onset. Furthermore, understanding the type of pitches used and the mechanics of each pitch can help with diagnosis. Often, pain increases as the inciting activity continues, and relief comes with rest. In an upper extremity examination, it is important to recall the usual stress fracture locations in throwers—the ribs, clavicle, humerus, ulnar shaft, and most often the olecranon—though the patient’s history often narrows the anatomical region of suspicion.2,4,7-10 Examination begins with inspection of the skin and soft tissues. Range of motion and strength testing results likely are normal throughout the upper extremity.3 Palpation over the suspected injury location often elicits pain and indicates further imaging is needed.6 The tuning fork test or the 3-point fulcrum test may elicit symptoms in occult fractures.3 Completing the assessment is a thorough neurovascular examination.
Insidious forearm pain requires a broad differential, including flexor-pronator mass or distal biceps injury, chronic exertional compartment syndrome, radial tunnel syndrome, intersection syndrome, pronator teres syndrome, anterior interosseous syndrome, thoracic outlet syndrome, musculocutaneous nerve compression, deep vein thrombosis of ulnar vein, and periostitis. Stress fractures distal to the elbow more commonly occur in weight-bearing athletes, though as this case shows it is important to consider stress fractures of the radius and ulna when evaluating forearm pain in a throwing athlete.21
The first imaging examination for a suspected stress fracture is a radiograph, which can be normal in up to 90% of patients, as it initially was in our athlete’s case.22 Often, radiographic evidence takes 2 to 12 weeks to appear.5 Even then, radiographs may be positive in only 50% of cases.19 CT, often regarded as insensitive during the early stages, is useful in visualizing fracture lines in a suspicious location.19,22 Radionuclide uptake scanning is highly sensitive during the early stages of stress injury but is nonspecific and may indicate neoplasm or infection; in addition, up to 46% of abnormal foci are asymptomatic.19 MRI has sensitivity comparable to that of radionuclide scanning but also many advantages, including lack of ionizing radiation, improved spatial resolution, and ability to image bone and soft tissue simultaneously.19 In our patient’s case, the unusual stress fracture location potentially could have hindered identification of the cause of injury. The lesion was just distal to the field of view of a normal elbow MRI and was not detected until a dedicated forearm MRI was examined. Both MRI and CT helped in identifying the stress fracture, and CT was used to follow interval healing.
In baseball players, upper extremity stress fractures are often nonoperatively treated with throwing cessation for 4 to 6 weeks followed by participation in a structured rehabilitation program.4,5 The throwing program that we suggest, and that was used in this case, has 21 stages of progression in duration, distance, and velocity of throwing. The athlete advances from each stage on the basis of symptoms.23 Other issues that may be addressed are vitamin D and calcium status and any flawed throwing mechanics that may have predisposed the athlete to injury. Such mechanics are gradually corrected.
The literature suggests that appropriate nonoperative management of stress fractures allows for return to sport in 8 to 10 weeks. It is important to note that most of the literature on stress fractures involves the lower extremity, and that treatment and time to return to play are therefore better described for such fractures.6 More study and evaluation of upper extremity stress fractures are needed to make return-to-sport predictions more reliable and successful treatment modalities more unified for this patient population. Last, it is imperative that clinical examination and symptoms be correlated with serial imaging when deciding on return to play. Our patient took 12 weeks to return to high-level sport. He progressed pain-free through the throwing program and showed radiographic evidence of healing on follow-up CT.
Conclusion
Radial shaft stress fractures are rare in throwing athletes. However, with a thorough history, a physical examination, and appropriate imaging, the correct diagnosis can be made early on, and proper treatment can be started to facilitate return to sport. To our knowledge, this is the first report of a stress fracture in the radial shaft of a MLB pitcher. Although the radial shaft is an uncommon location for stress fractures, we should keep in mind that they can occur wherever increased muscular forces exceed the ability of native bone to remodel. After diagnosis, the fracture usually heals with nonoperative treatment, and healing is confirmed with follow-up imaging, as was done in our patient’s case. Improved prediction of time to return to play for upper extremity fractures, such as the radial stress fracture described in this article, requires more study.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
1. Monteleone GP Jr. Stress fractures in the athlete. Orthop Clin North Am. 1995;26(3):423-432.
2. Iwamoto J, Takeda T. Stress fractures in athletes: review of 196 cases. J Orthop Sci. 2003;8(3):273-278.
3. Miller TL, Kaeding CC. Upper-extremity stress fractures: distribution and causative activities in 70 patients. Orthopedics. 2012;35(9):789-793.
4. Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174.
5. Brooks AA. Stress fractures of the upper extremity. Clin Sports Med. 2001;20(3):613-620.
6. Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309-325.
7. Gurtler R, Pavlov H, Torg JS. Stress fracture of the ipsilateral first rib in a pitcher. Am J Sports Med. 1985;13(4):277-279.
8. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
9. Wu C, Chen Y. Stress fracture of the clavicle in a professional baseball player. J Shoulder Elbow Surg. 1998;7(2):164-167.
10. Schickendantz MS, Ho CP, Koh J. Stress injury of the proximal ulna in professional baseball players. Am J Sports Med. 2002;30(5):737-741.
11. Loosli AR, Leslie M. Stress fractures of the distal radius. A case report. Am J Sports Med. 1991;19(5):523-524.
12. Inagaki H, Inoue G. Stress fracture of the scaphoid combined with the distal radial epiphysiolysis. Br J Sports Med. 1997;31(3):256-257.
13. Read MT. Stress fractures of the distal radius in adolescent gymnasts. Br J Sports Med. 1981;15(4):272-276.
14. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
15. Eisenberg D, Kirchner SG, Green NE. Stress fracture of the distal radius caused by “wheelies.” South Med J. 1986;79(7):918-919.
16. Brukner P. Stress fractures of the upper limb. Sports Med. 1998;26(6):415-424.
17. Farquharson-Roberts MA, Fulford PC. Stress fracture of the radius. J Bone Joint Surg Br. 1980;62(2):194-195.
18. Orloff AS, Resnick D. Fatigue fracture of the distal part of the radius in a pool player. Injury. 1986;17(6):418-419.
19. Anderson MW. Imaging of upper extremity stress fractures in the athlete. Clin Sports Med. 2006;25(3):489-504.
20. Bennell K, Brukner P. Preventing and managing stress fractures in athletes. Phys Ther Sport. 2005;6(4):171-180.
21. Sinha AK, Kaeding CC, Wadley GM. Upper extremity stress fractures in athletes: clinical features of 44 cases. Clin J Sport Med. 1999;9(4):199-202.
22. Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46-58.
23. Kaplan L, Lesniak B, Baraga M, et al. Throwing program for baseball players. 2009. http://uhealthsportsmedicine.com/documents/UHealth_Throwing_Program.pdf. Accessed May 24, 2016.
Information on Orthopedic Trauma Fellowships: Online Accessibility and Content
Take-Home Points
- The Internet is a popular resource for orthopedic fellowship applicants.
- 86% of OTF websites are accessible from Google and FREIDA.
- Accessible websites feature only 40% of fellowship applicant content.
- Accessibility and content of OTF websites are highly variable and largely deficient.
- Improvement of the accessibility and content of website information should be a future focus of OTF programs.
The Orthopaedic Trauma Fellowship Match facilitates the matching process for orthopedic residency graduates pursuing a career as orthopedic traumatologists. This match is supported by the Orthopaedic Trauma Association (OTA) and the San Francisco Matching Program (SFMP). Orthopedic trauma fellowship (OTF) programs are accredited by the OTA and may receive oversight by the American Council for Graduate Medical Education (ACGME), which defines uniform standards for fellowship training.1
Studies have found that the internet is an important and popular resource for applicants researching residency and fellowship programs.2-5 For many applicants, the internet is their initial and main source of information.5 Unfortunately, training programs do not have standardized website accessibility and content.
Few studies have addressed online content on orthopedic fellowship programs,4,6,7 and to our knowledge no one has studied online content on OTF programs. We conducted a study to assess the accessibility and ease of navigation of OTF websites and to evaluate the content on these sites. We wanted to identify content that applicants may reliably expect on OTF sites. Any deficits identified may be useful to fellowship programs and program directors interested in improving website quality. We hypothesized that the accessibility and content of online OTF content would be highly variable and largely deficient.
Methods
This study was conducted at New York University Hospital for Joint Diseases. On February 5, 2015, both the OTA database8 and the Fellowship and Residency Electronic Interactive Database (FREIDA)9 were accessed in order to create a comprehensive list of OTF programs. FREIDA, a catalog of all ACGME-accredited graduate medical education programs in the United States, is supported by the American Medical Association and provides cursory program information, including training program duration and number of positions per year.
The databases were reviewed for links to OTF program websites. An independent Google search for program websites was also initiated on February 5, 2015. The Google search was performed in the format “program name + orthopaedic trauma fellowship” to assess how accessible the program sites are from outside the 2 databases (OTA, FREIDA). Google was used because it is the most commonly used search engine.10 The first 25 search results were reviewed for links to OTF websites. Programs without accessible links to OTF websites—from the OTA database, from FREIDA, or from the Google search—were excluded from content assessment.
Accessible websites were electronically captured to ensure consistency of content during assessment. OTF site content was evaluated using methods described in similar investigations.4,5,11,12 In our dichotomous assessment of fellow education content, we awarded 1 point per content item on the website. The 10 education content items evaluated were call responsibilities, didactic instruction, journal club, research requirements, evaluation criteria, rotation schedule, operative experience, office/clinic experience, meetings attended, and courses attended. We also performed a dichotomous assessment of fellow recruitment content. The 10 recruitment content items evaluated were program description, application requirements, selection criteria, OTA link, SFMP link, location description, program contact information, fellow listing, faculty listing, and salary. Content items were chosen for evaluation on the basis of published OTF applicant experience.13 Percentages of education content, recruitment content, and total content were compared by program location, number of fellows, ACGME accreditation status,14 affiliation with a top 20 orthopedic hospital,15 and affiliation with a top 20 medical school,16 as in similar studies.7,17
Chi-square tests were used to compare content by fellowship location, number of fellows, ACGME accreditation status, affiliation with a top 20 orthopedic hospital, and affiliation with a top 20 medical school. For all tests, the significance level was set at P < .05.
Results
Of the 49 OTF programs identified with database queries, 9 appeared in both the OTA database and FREIDA, 39 appeared only in the OTA database, and 1 appeared only in FREIDA. There were 48 programs total in the OTA database and 10 total in FREIDA.
The OTA database had no OTF website links. Of the 10 OTF links in FREIDA, 3 (6%) were nonfunctioning, 6 (12%) had multiple steps for accessing program information, and 1 (2%) connected directly to program information. Therefore, FREIDA had a total of 7 accessible OTF links (14%). The independent Google search yielded website links for 42 (86%) of the 49 OTF programs. Five links (10%, 5/49) had multiple steps for accessing program information, and 37 links (76%, 37/49) connected directly to program information. The 7 OTF links accessible through FREIDA were accessible through Google as well. Table 1 summarizes the accessibility data.
All 42 accessible OTF websites were assessed for content. On average, these sites had 40% (range, 0%-75%) of the total assessed content. Mean (SD) education content score was 3.6 (2.2) out of 10. Operative experience (88%) and research requirements (81%) were the most consistently presented education items. Didactic learning (45%) and description of common office/clinic cases (43%) were next. Less than 5% of the sites had content on the training courses (eg, sponsored fracture courses) attended by fellows. Figure 1 summarizes the education items on the OTF websites.
Mean (SD) recruitment content score was 4.4 (2.2) out of 10. Program description (93%) and program contact information (88%) were the most consistently presented recruitment items. Clinical faculty (52%) and current and/or prior fellows (36%) were next. Fellow selection criteria appeared least often (12%). Figure 2 summarizes the recruitment items on the OTF websites.
Thirty-six percent of OTF programs with accessible websites were in the southern United States. However, there were no significant differences in online content between OTF program locations. Websites of programs with >1 fellow had significantly more education content (48% vs 33%; P = .043) and total content (46% vs 37%; P = .01) than websites of programs with 1 fellow. ACGME accreditation status, affiliation with a top 20 orthopedic hospital, and affiliation with a top 20 medical school did not have a significant effect on OTF website content. Table 2 summarizes OTF website content by location, number of fellows, top 20 orthopedic hospital affiliation, and top 20 medical school affiliation.
Discussion
We conducted this study to assess the accessibility of OTF program websites and to evaluate the content of the sites. Our hypothesis, that the accessibility and content of online OTF content would be highly variable and largely deficient, was supported by our findings. We found that the OTA database had no OTF website links and that FREIDA links connected directly to only 2% of OTF sites. The majority of OTF sites were accessed from the Google search, which had direct links to 76% of the OTF programs.
Other studies have had similar findings regarding the accessibility of fellowship websites. Mulcahey and colleagues6 evaluated sports medicine fellowship websites for accessibility and content, and found that the website of the American Orthopaedic Society for Sports Medicine directly linked to fellowship information for only 3% of programs; a Google search yielded direct links to 71% of program websites. Davidson and colleagues4 examined the quality and accessibility of online information on pediatric orthopedic fellowships and found no program links on the website of the Pediatric Orthopaedic Society of North America; a Google search yielded direct links to 68% of programs. Silvestre and colleagues7 assessed spine fellowship information on the Internet. The North American Spine Society website had working links to only 3% of fellowship sites, and FREIDA connected to only 6% of sites.
Content scores in our study were highly variable. Mean education and recruitment content scores were 3.6 (range, 0-9) and 4.4 (range, 0-10), respectively. Operative experience (88%) and program description (93%) were the most frequently presented education and recruitment items, respectively. Consistency in presenting program descriptions on OTF websites was slightly poorer than that in other orthopedic specialties. Sports medicine, pediatric orthopedic, and spine fellowship websites provided program descriptions for fellowship recruitment.4,6,7 Nevertheless, overall content scores in our study and in the aforementioned studies were similarly poor.
In our study, OTF websites showed no significant differences in content scores for program location, ACGME accreditation status, affiliation with a top 20 orthopedic hospital, or affiliation with a top 20 medical school. Lack of a significant effect of medical school or orthopedic hospital affiliation suggests academic prestige does not play a large role in attempts by OTF websites to attract applicants. However, programs with >1 fellow had significantly more education and total content than programs with 1 fellow. Results from a comparable study support this finding. Silvestre and colleagues18 assessed the accessibility of online plastic surgery residency content. Programs with 3 or 4 residents had significantly more online education content than programs with 1 resident. This finding may relate to the cost efficiency of developing low-cost websites to attract applicants to multiple positions.7
Despite lacking links to OTF websites, the OTA database had a large amount of content on 98% (48/49) of OTFs. In addition to presenting the content that we assessed in this study, the OTA database provided the number of inpatient beds at the primary teaching hospital, the annual number of emergency department visits, the annual number of trauma admissions, and the annual number of orthopedic trauma procedures. This standardized information may be very helpful to fellowship applicants and may be an important adjunct to fellowship websites.
FREIDA provided similar content, but accessible links were found for only 14% of the assessed programs. Although the deficiency in accessible OTF links in the OTA database and FREIDA is not well understood, it is important. The results of our study and of similar studies suggest that the listing of active fellowship program links on society websites would benefit orthopedic fellowship applicants, likely fostering a better understanding and a more efficient review of available programs. In addition, links on society websites afford fellowship directors the means to efficiently publicize their programs to large numbers of potential applicants, who likely use society websites as an initial informational resource.
Our study had limitations. First, its findings are subject to the dynamism of the internet, and OTF information may have been updated after this investigation was conducted. Second, our study did not rank-order accessible links, which may have provided more information on the efficiency of using Internet search engines in a review of OTF programs. In addition, our study involved dichotomous assessment of OTF content. Multichotomous evaluation may have further elucidated the quality of website information. Last, our study evaluated websites only for US-based OTF programs. Inclusion of international OTF programs, though outside the scope of this study, may have yielded different findings.
Conclusion
Our results highlight the difficulties that OTF applicants may experience in gathering fellowship information online. OTF website accessibility and content were found to be highly variable and largely deficient. Comparing our findings with those of similar studies revealed that fellowship websites generally provided little information that orthopedic specialty applicants could use. OTF programs should focus on improving their website accessibility and content.
1. Daniels AH, Grabel Z, DiGiovanni CW. ACGME accreditation of orthopaedic surgery subspecialty fellowship training programs. J Bone Joint Surg Am. 2014;96(11):e94.
2. Reilly EF, Leibrandt TJ, Zonno AJ, Simpson MC, Morris JB. General surgery residency program websites: usefulness and usability for resident applicants. Curr Surg. 2004;61(2):236-240.
3. Perron AD, Brady WJ. Sources of information on emergency medicine residency programs. Acad Emerg Med. 2002;9(12):1462-1463.
4. Davidson AR, Murphy RF, Spence DD, Kelly DM, Warner WC Jr, Sawyer JR. Accessibility and quality of online information for pediatric orthopaedic surgery fellowships. J Pediatr Orthop. 2014;34(8):831-834.
5. Rozental TD, Lonner JH, Parekh SG. The internet as a communication tool for academic orthopaedic surgery departments in the United States. J Bone Joint Surg Am. 2001;83(7):987-991.
6. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
7. Silvestre J, Guzman JZ, Skovrlj B, et al. The internet as a communication tool for orthopedic spine fellowships in the United States. Spine J. 2015;15(4):655-661.
8. Orthopaedic Trauma Association. Orthopaedic trauma fellowship directory. http://spec.ota.org/education/fellowshipcenter/fellowship_dir/dir_summary.cfm. Accessed February 5, 2015.
9. Fellowship and Residency Electronic Interactive Database. Orthopaedic trauma fellowship programs. https://freida.ama-assn.org/Freida/user/search/programSearch.do. Accessed February 5, 2015.
10. Experian Hitwise. Search engine analysis. http://www.experian.com/marketing-services/online-trends-search-engine.html. Accessed February 5, 2015.
11. Hinds RM, Klifto CS, Naik AA, Sapienza A, Capo JT. Hand society and matching program web sites provide poor access to information regarding hand surgery fellowship. J Hand Microsurg. 2016;8(2):91-95.
12. Hinds RM, Danna NR, Capo JT, Mroczek KJ. Foot and ankle fellowship websites: An assessment of accessibility and quality. Foot Ankle Spec. 2017;10(4):302-307.
13. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
14. American Council for Graduate Medical Education. Accredited orthopaedic trauma fellowship programs. https://www.acgme.org/ads/Public/Programs/Search?specialtyId=49&orgCode=&city=. Accessed February 5, 2015.
15. US News & World Report. Best hospitals for orthopedics. http://health.usnews.com/best-hospitals/rankings/orthopedics. Accessed February 5, 2015.
16. US News & World Report. Best medical schools: research. http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings?int=98fd08. Accessed February 5, 2015.
17. Silvestre J, Guzman JZ, Abbatematteo JM, Chang B, Levin LS. Evaluation of content and accessibility of hand fellowship websites. Hand (NY). 2015;10(3):516-521.
18. Silvestre J, Tomlinson-Hansen S, Fosnot J, Taylor JA. Plastic surgery residency websites: a critical analysis of accessibility and content. Ann Plast Surg. 2014;72(3):265-269.
Take-Home Points
- The Internet is a popular resource for orthopedic fellowship applicants.
- 86% of OTF websites are accessible from Google and FREIDA.
- Accessible websites feature only 40% of fellowship applicant content.
- Accessibility and content of OTF websites are highly variable and largely deficient.
- Improvement of the accessibility and content of website information should be a future focus of OTF programs.
The Orthopaedic Trauma Fellowship Match facilitates the matching process for orthopedic residency graduates pursuing a career as orthopedic traumatologists. This match is supported by the Orthopaedic Trauma Association (OTA) and the San Francisco Matching Program (SFMP). Orthopedic trauma fellowship (OTF) programs are accredited by the OTA and may receive oversight by the American Council for Graduate Medical Education (ACGME), which defines uniform standards for fellowship training.1
Studies have found that the internet is an important and popular resource for applicants researching residency and fellowship programs.2-5 For many applicants, the internet is their initial and main source of information.5 Unfortunately, training programs do not have standardized website accessibility and content.
Few studies have addressed online content on orthopedic fellowship programs,4,6,7 and to our knowledge no one has studied online content on OTF programs. We conducted a study to assess the accessibility and ease of navigation of OTF websites and to evaluate the content on these sites. We wanted to identify content that applicants may reliably expect on OTF sites. Any deficits identified may be useful to fellowship programs and program directors interested in improving website quality. We hypothesized that the accessibility and content of online OTF content would be highly variable and largely deficient.
Methods
This study was conducted at New York University Hospital for Joint Diseases. On February 5, 2015, both the OTA database8 and the Fellowship and Residency Electronic Interactive Database (FREIDA)9 were accessed in order to create a comprehensive list of OTF programs. FREIDA, a catalog of all ACGME-accredited graduate medical education programs in the United States, is supported by the American Medical Association and provides cursory program information, including training program duration and number of positions per year.
The databases were reviewed for links to OTF program websites. An independent Google search for program websites was also initiated on February 5, 2015. The Google search was performed in the format “program name + orthopaedic trauma fellowship” to assess how accessible the program sites are from outside the 2 databases (OTA, FREIDA). Google was used because it is the most commonly used search engine.10 The first 25 search results were reviewed for links to OTF websites. Programs without accessible links to OTF websites—from the OTA database, from FREIDA, or from the Google search—were excluded from content assessment.
Accessible websites were electronically captured to ensure consistency of content during assessment. OTF site content was evaluated using methods described in similar investigations.4,5,11,12 In our dichotomous assessment of fellow education content, we awarded 1 point per content item on the website. The 10 education content items evaluated were call responsibilities, didactic instruction, journal club, research requirements, evaluation criteria, rotation schedule, operative experience, office/clinic experience, meetings attended, and courses attended. We also performed a dichotomous assessment of fellow recruitment content. The 10 recruitment content items evaluated were program description, application requirements, selection criteria, OTA link, SFMP link, location description, program contact information, fellow listing, faculty listing, and salary. Content items were chosen for evaluation on the basis of published OTF applicant experience.13 Percentages of education content, recruitment content, and total content were compared by program location, number of fellows, ACGME accreditation status,14 affiliation with a top 20 orthopedic hospital,15 and affiliation with a top 20 medical school,16 as in similar studies.7,17
Chi-square tests were used to compare content by fellowship location, number of fellows, ACGME accreditation status, affiliation with a top 20 orthopedic hospital, and affiliation with a top 20 medical school. For all tests, the significance level was set at P < .05.
Results
Of the 49 OTF programs identified with database queries, 9 appeared in both the OTA database and FREIDA, 39 appeared only in the OTA database, and 1 appeared only in FREIDA. There were 48 programs total in the OTA database and 10 total in FREIDA.
The OTA database had no OTF website links. Of the 10 OTF links in FREIDA, 3 (6%) were nonfunctioning, 6 (12%) had multiple steps for accessing program information, and 1 (2%) connected directly to program information. Therefore, FREIDA had a total of 7 accessible OTF links (14%). The independent Google search yielded website links for 42 (86%) of the 49 OTF programs. Five links (10%, 5/49) had multiple steps for accessing program information, and 37 links (76%, 37/49) connected directly to program information. The 7 OTF links accessible through FREIDA were accessible through Google as well. Table 1 summarizes the accessibility data.
All 42 accessible OTF websites were assessed for content. On average, these sites had 40% (range, 0%-75%) of the total assessed content. Mean (SD) education content score was 3.6 (2.2) out of 10. Operative experience (88%) and research requirements (81%) were the most consistently presented education items. Didactic learning (45%) and description of common office/clinic cases (43%) were next. Less than 5% of the sites had content on the training courses (eg, sponsored fracture courses) attended by fellows. Figure 1 summarizes the education items on the OTF websites.
Mean (SD) recruitment content score was 4.4 (2.2) out of 10. Program description (93%) and program contact information (88%) were the most consistently presented recruitment items. Clinical faculty (52%) and current and/or prior fellows (36%) were next. Fellow selection criteria appeared least often (12%). Figure 2 summarizes the recruitment items on the OTF websites.
Thirty-six percent of OTF programs with accessible websites were in the southern United States. However, there were no significant differences in online content between OTF program locations. Websites of programs with >1 fellow had significantly more education content (48% vs 33%; P = .043) and total content (46% vs 37%; P = .01) than websites of programs with 1 fellow. ACGME accreditation status, affiliation with a top 20 orthopedic hospital, and affiliation with a top 20 medical school did not have a significant effect on OTF website content. Table 2 summarizes OTF website content by location, number of fellows, top 20 orthopedic hospital affiliation, and top 20 medical school affiliation.
Discussion
We conducted this study to assess the accessibility of OTF program websites and to evaluate the content of the sites. Our hypothesis, that the accessibility and content of online OTF content would be highly variable and largely deficient, was supported by our findings. We found that the OTA database had no OTF website links and that FREIDA links connected directly to only 2% of OTF sites. The majority of OTF sites were accessed from the Google search, which had direct links to 76% of the OTF programs.
Other studies have had similar findings regarding the accessibility of fellowship websites. Mulcahey and colleagues6 evaluated sports medicine fellowship websites for accessibility and content, and found that the website of the American Orthopaedic Society for Sports Medicine directly linked to fellowship information for only 3% of programs; a Google search yielded direct links to 71% of program websites. Davidson and colleagues4 examined the quality and accessibility of online information on pediatric orthopedic fellowships and found no program links on the website of the Pediatric Orthopaedic Society of North America; a Google search yielded direct links to 68% of programs. Silvestre and colleagues7 assessed spine fellowship information on the Internet. The North American Spine Society website had working links to only 3% of fellowship sites, and FREIDA connected to only 6% of sites.
Content scores in our study were highly variable. Mean education and recruitment content scores were 3.6 (range, 0-9) and 4.4 (range, 0-10), respectively. Operative experience (88%) and program description (93%) were the most frequently presented education and recruitment items, respectively. Consistency in presenting program descriptions on OTF websites was slightly poorer than that in other orthopedic specialties. Sports medicine, pediatric orthopedic, and spine fellowship websites provided program descriptions for fellowship recruitment.4,6,7 Nevertheless, overall content scores in our study and in the aforementioned studies were similarly poor.
In our study, OTF websites showed no significant differences in content scores for program location, ACGME accreditation status, affiliation with a top 20 orthopedic hospital, or affiliation with a top 20 medical school. Lack of a significant effect of medical school or orthopedic hospital affiliation suggests academic prestige does not play a large role in attempts by OTF websites to attract applicants. However, programs with >1 fellow had significantly more education and total content than programs with 1 fellow. Results from a comparable study support this finding. Silvestre and colleagues18 assessed the accessibility of online plastic surgery residency content. Programs with 3 or 4 residents had significantly more online education content than programs with 1 resident. This finding may relate to the cost efficiency of developing low-cost websites to attract applicants to multiple positions.7
Despite lacking links to OTF websites, the OTA database had a large amount of content on 98% (48/49) of OTFs. In addition to presenting the content that we assessed in this study, the OTA database provided the number of inpatient beds at the primary teaching hospital, the annual number of emergency department visits, the annual number of trauma admissions, and the annual number of orthopedic trauma procedures. This standardized information may be very helpful to fellowship applicants and may be an important adjunct to fellowship websites.
FREIDA provided similar content, but accessible links were found for only 14% of the assessed programs. Although the deficiency in accessible OTF links in the OTA database and FREIDA is not well understood, it is important. The results of our study and of similar studies suggest that the listing of active fellowship program links on society websites would benefit orthopedic fellowship applicants, likely fostering a better understanding and a more efficient review of available programs. In addition, links on society websites afford fellowship directors the means to efficiently publicize their programs to large numbers of potential applicants, who likely use society websites as an initial informational resource.
Our study had limitations. First, its findings are subject to the dynamism of the internet, and OTF information may have been updated after this investigation was conducted. Second, our study did not rank-order accessible links, which may have provided more information on the efficiency of using Internet search engines in a review of OTF programs. In addition, our study involved dichotomous assessment of OTF content. Multichotomous evaluation may have further elucidated the quality of website information. Last, our study evaluated websites only for US-based OTF programs. Inclusion of international OTF programs, though outside the scope of this study, may have yielded different findings.
Conclusion
Our results highlight the difficulties that OTF applicants may experience in gathering fellowship information online. OTF website accessibility and content were found to be highly variable and largely deficient. Comparing our findings with those of similar studies revealed that fellowship websites generally provided little information that orthopedic specialty applicants could use. OTF programs should focus on improving their website accessibility and content.
Take-Home Points
- The Internet is a popular resource for orthopedic fellowship applicants.
- 86% of OTF websites are accessible from Google and FREIDA.
- Accessible websites feature only 40% of fellowship applicant content.
- Accessibility and content of OTF websites are highly variable and largely deficient.
- Improvement of the accessibility and content of website information should be a future focus of OTF programs.
The Orthopaedic Trauma Fellowship Match facilitates the matching process for orthopedic residency graduates pursuing a career as orthopedic traumatologists. This match is supported by the Orthopaedic Trauma Association (OTA) and the San Francisco Matching Program (SFMP). Orthopedic trauma fellowship (OTF) programs are accredited by the OTA and may receive oversight by the American Council for Graduate Medical Education (ACGME), which defines uniform standards for fellowship training.1
Studies have found that the internet is an important and popular resource for applicants researching residency and fellowship programs.2-5 For many applicants, the internet is their initial and main source of information.5 Unfortunately, training programs do not have standardized website accessibility and content.
Few studies have addressed online content on orthopedic fellowship programs,4,6,7 and to our knowledge no one has studied online content on OTF programs. We conducted a study to assess the accessibility and ease of navigation of OTF websites and to evaluate the content on these sites. We wanted to identify content that applicants may reliably expect on OTF sites. Any deficits identified may be useful to fellowship programs and program directors interested in improving website quality. We hypothesized that the accessibility and content of online OTF content would be highly variable and largely deficient.
Methods
This study was conducted at New York University Hospital for Joint Diseases. On February 5, 2015, both the OTA database8 and the Fellowship and Residency Electronic Interactive Database (FREIDA)9 were accessed in order to create a comprehensive list of OTF programs. FREIDA, a catalog of all ACGME-accredited graduate medical education programs in the United States, is supported by the American Medical Association and provides cursory program information, including training program duration and number of positions per year.
The databases were reviewed for links to OTF program websites. An independent Google search for program websites was also initiated on February 5, 2015. The Google search was performed in the format “program name + orthopaedic trauma fellowship” to assess how accessible the program sites are from outside the 2 databases (OTA, FREIDA). Google was used because it is the most commonly used search engine.10 The first 25 search results were reviewed for links to OTF websites. Programs without accessible links to OTF websites—from the OTA database, from FREIDA, or from the Google search—were excluded from content assessment.
Accessible websites were electronically captured to ensure consistency of content during assessment. OTF site content was evaluated using methods described in similar investigations.4,5,11,12 In our dichotomous assessment of fellow education content, we awarded 1 point per content item on the website. The 10 education content items evaluated were call responsibilities, didactic instruction, journal club, research requirements, evaluation criteria, rotation schedule, operative experience, office/clinic experience, meetings attended, and courses attended. We also performed a dichotomous assessment of fellow recruitment content. The 10 recruitment content items evaluated were program description, application requirements, selection criteria, OTA link, SFMP link, location description, program contact information, fellow listing, faculty listing, and salary. Content items were chosen for evaluation on the basis of published OTF applicant experience.13 Percentages of education content, recruitment content, and total content were compared by program location, number of fellows, ACGME accreditation status,14 affiliation with a top 20 orthopedic hospital,15 and affiliation with a top 20 medical school,16 as in similar studies.7,17
Chi-square tests were used to compare content by fellowship location, number of fellows, ACGME accreditation status, affiliation with a top 20 orthopedic hospital, and affiliation with a top 20 medical school. For all tests, the significance level was set at P < .05.
Results
Of the 49 OTF programs identified with database queries, 9 appeared in both the OTA database and FREIDA, 39 appeared only in the OTA database, and 1 appeared only in FREIDA. There were 48 programs total in the OTA database and 10 total in FREIDA.
The OTA database had no OTF website links. Of the 10 OTF links in FREIDA, 3 (6%) were nonfunctioning, 6 (12%) had multiple steps for accessing program information, and 1 (2%) connected directly to program information. Therefore, FREIDA had a total of 7 accessible OTF links (14%). The independent Google search yielded website links for 42 (86%) of the 49 OTF programs. Five links (10%, 5/49) had multiple steps for accessing program information, and 37 links (76%, 37/49) connected directly to program information. The 7 OTF links accessible through FREIDA were accessible through Google as well. Table 1 summarizes the accessibility data.
All 42 accessible OTF websites were assessed for content. On average, these sites had 40% (range, 0%-75%) of the total assessed content. Mean (SD) education content score was 3.6 (2.2) out of 10. Operative experience (88%) and research requirements (81%) were the most consistently presented education items. Didactic learning (45%) and description of common office/clinic cases (43%) were next. Less than 5% of the sites had content on the training courses (eg, sponsored fracture courses) attended by fellows. Figure 1 summarizes the education items on the OTF websites.
Mean (SD) recruitment content score was 4.4 (2.2) out of 10. Program description (93%) and program contact information (88%) were the most consistently presented recruitment items. Clinical faculty (52%) and current and/or prior fellows (36%) were next. Fellow selection criteria appeared least often (12%). Figure 2 summarizes the recruitment items on the OTF websites.
Thirty-six percent of OTF programs with accessible websites were in the southern United States. However, there were no significant differences in online content between OTF program locations. Websites of programs with >1 fellow had significantly more education content (48% vs 33%; P = .043) and total content (46% vs 37%; P = .01) than websites of programs with 1 fellow. ACGME accreditation status, affiliation with a top 20 orthopedic hospital, and affiliation with a top 20 medical school did not have a significant effect on OTF website content. Table 2 summarizes OTF website content by location, number of fellows, top 20 orthopedic hospital affiliation, and top 20 medical school affiliation.
Discussion
We conducted this study to assess the accessibility of OTF program websites and to evaluate the content of the sites. Our hypothesis, that the accessibility and content of online OTF content would be highly variable and largely deficient, was supported by our findings. We found that the OTA database had no OTF website links and that FREIDA links connected directly to only 2% of OTF sites. The majority of OTF sites were accessed from the Google search, which had direct links to 76% of the OTF programs.
Other studies have had similar findings regarding the accessibility of fellowship websites. Mulcahey and colleagues6 evaluated sports medicine fellowship websites for accessibility and content, and found that the website of the American Orthopaedic Society for Sports Medicine directly linked to fellowship information for only 3% of programs; a Google search yielded direct links to 71% of program websites. Davidson and colleagues4 examined the quality and accessibility of online information on pediatric orthopedic fellowships and found no program links on the website of the Pediatric Orthopaedic Society of North America; a Google search yielded direct links to 68% of programs. Silvestre and colleagues7 assessed spine fellowship information on the Internet. The North American Spine Society website had working links to only 3% of fellowship sites, and FREIDA connected to only 6% of sites.
Content scores in our study were highly variable. Mean education and recruitment content scores were 3.6 (range, 0-9) and 4.4 (range, 0-10), respectively. Operative experience (88%) and program description (93%) were the most frequently presented education and recruitment items, respectively. Consistency in presenting program descriptions on OTF websites was slightly poorer than that in other orthopedic specialties. Sports medicine, pediatric orthopedic, and spine fellowship websites provided program descriptions for fellowship recruitment.4,6,7 Nevertheless, overall content scores in our study and in the aforementioned studies were similarly poor.
In our study, OTF websites showed no significant differences in content scores for program location, ACGME accreditation status, affiliation with a top 20 orthopedic hospital, or affiliation with a top 20 medical school. Lack of a significant effect of medical school or orthopedic hospital affiliation suggests academic prestige does not play a large role in attempts by OTF websites to attract applicants. However, programs with >1 fellow had significantly more education and total content than programs with 1 fellow. Results from a comparable study support this finding. Silvestre and colleagues18 assessed the accessibility of online plastic surgery residency content. Programs with 3 or 4 residents had significantly more online education content than programs with 1 resident. This finding may relate to the cost efficiency of developing low-cost websites to attract applicants to multiple positions.7
Despite lacking links to OTF websites, the OTA database had a large amount of content on 98% (48/49) of OTFs. In addition to presenting the content that we assessed in this study, the OTA database provided the number of inpatient beds at the primary teaching hospital, the annual number of emergency department visits, the annual number of trauma admissions, and the annual number of orthopedic trauma procedures. This standardized information may be very helpful to fellowship applicants and may be an important adjunct to fellowship websites.
FREIDA provided similar content, but accessible links were found for only 14% of the assessed programs. Although the deficiency in accessible OTF links in the OTA database and FREIDA is not well understood, it is important. The results of our study and of similar studies suggest that the listing of active fellowship program links on society websites would benefit orthopedic fellowship applicants, likely fostering a better understanding and a more efficient review of available programs. In addition, links on society websites afford fellowship directors the means to efficiently publicize their programs to large numbers of potential applicants, who likely use society websites as an initial informational resource.
Our study had limitations. First, its findings are subject to the dynamism of the internet, and OTF information may have been updated after this investigation was conducted. Second, our study did not rank-order accessible links, which may have provided more information on the efficiency of using Internet search engines in a review of OTF programs. In addition, our study involved dichotomous assessment of OTF content. Multichotomous evaluation may have further elucidated the quality of website information. Last, our study evaluated websites only for US-based OTF programs. Inclusion of international OTF programs, though outside the scope of this study, may have yielded different findings.
Conclusion
Our results highlight the difficulties that OTF applicants may experience in gathering fellowship information online. OTF website accessibility and content were found to be highly variable and largely deficient. Comparing our findings with those of similar studies revealed that fellowship websites generally provided little information that orthopedic specialty applicants could use. OTF programs should focus on improving their website accessibility and content.
1. Daniels AH, Grabel Z, DiGiovanni CW. ACGME accreditation of orthopaedic surgery subspecialty fellowship training programs. J Bone Joint Surg Am. 2014;96(11):e94.
2. Reilly EF, Leibrandt TJ, Zonno AJ, Simpson MC, Morris JB. General surgery residency program websites: usefulness and usability for resident applicants. Curr Surg. 2004;61(2):236-240.
3. Perron AD, Brady WJ. Sources of information on emergency medicine residency programs. Acad Emerg Med. 2002;9(12):1462-1463.
4. Davidson AR, Murphy RF, Spence DD, Kelly DM, Warner WC Jr, Sawyer JR. Accessibility and quality of online information for pediatric orthopaedic surgery fellowships. J Pediatr Orthop. 2014;34(8):831-834.
5. Rozental TD, Lonner JH, Parekh SG. The internet as a communication tool for academic orthopaedic surgery departments in the United States. J Bone Joint Surg Am. 2001;83(7):987-991.
6. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
7. Silvestre J, Guzman JZ, Skovrlj B, et al. The internet as a communication tool for orthopedic spine fellowships in the United States. Spine J. 2015;15(4):655-661.
8. Orthopaedic Trauma Association. Orthopaedic trauma fellowship directory. http://spec.ota.org/education/fellowshipcenter/fellowship_dir/dir_summary.cfm. Accessed February 5, 2015.
9. Fellowship and Residency Electronic Interactive Database. Orthopaedic trauma fellowship programs. https://freida.ama-assn.org/Freida/user/search/programSearch.do. Accessed February 5, 2015.
10. Experian Hitwise. Search engine analysis. http://www.experian.com/marketing-services/online-trends-search-engine.html. Accessed February 5, 2015.
11. Hinds RM, Klifto CS, Naik AA, Sapienza A, Capo JT. Hand society and matching program web sites provide poor access to information regarding hand surgery fellowship. J Hand Microsurg. 2016;8(2):91-95.
12. Hinds RM, Danna NR, Capo JT, Mroczek KJ. Foot and ankle fellowship websites: An assessment of accessibility and quality. Foot Ankle Spec. 2017;10(4):302-307.
13. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
14. American Council for Graduate Medical Education. Accredited orthopaedic trauma fellowship programs. https://www.acgme.org/ads/Public/Programs/Search?specialtyId=49&orgCode=&city=. Accessed February 5, 2015.
15. US News & World Report. Best hospitals for orthopedics. http://health.usnews.com/best-hospitals/rankings/orthopedics. Accessed February 5, 2015.
16. US News & World Report. Best medical schools: research. http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings?int=98fd08. Accessed February 5, 2015.
17. Silvestre J, Guzman JZ, Abbatematteo JM, Chang B, Levin LS. Evaluation of content and accessibility of hand fellowship websites. Hand (NY). 2015;10(3):516-521.
18. Silvestre J, Tomlinson-Hansen S, Fosnot J, Taylor JA. Plastic surgery residency websites: a critical analysis of accessibility and content. Ann Plast Surg. 2014;72(3):265-269.
1. Daniels AH, Grabel Z, DiGiovanni CW. ACGME accreditation of orthopaedic surgery subspecialty fellowship training programs. J Bone Joint Surg Am. 2014;96(11):e94.
2. Reilly EF, Leibrandt TJ, Zonno AJ, Simpson MC, Morris JB. General surgery residency program websites: usefulness and usability for resident applicants. Curr Surg. 2004;61(2):236-240.
3. Perron AD, Brady WJ. Sources of information on emergency medicine residency programs. Acad Emerg Med. 2002;9(12):1462-1463.
4. Davidson AR, Murphy RF, Spence DD, Kelly DM, Warner WC Jr, Sawyer JR. Accessibility and quality of online information for pediatric orthopaedic surgery fellowships. J Pediatr Orthop. 2014;34(8):831-834.
5. Rozental TD, Lonner JH, Parekh SG. The internet as a communication tool for academic orthopaedic surgery departments in the United States. J Bone Joint Surg Am. 2001;83(7):987-991.
6. Mulcahey MK, Gosselin MM, Fadale PD. Evaluation of the content and accessibility of web sites for accredited orthopaedic sports medicine fellowships. J Bone Joint Surg Am. 2013;95(12):e85.
7. Silvestre J, Guzman JZ, Skovrlj B, et al. The internet as a communication tool for orthopedic spine fellowships in the United States. Spine J. 2015;15(4):655-661.
8. Orthopaedic Trauma Association. Orthopaedic trauma fellowship directory. http://spec.ota.org/education/fellowshipcenter/fellowship_dir/dir_summary.cfm. Accessed February 5, 2015.
9. Fellowship and Residency Electronic Interactive Database. Orthopaedic trauma fellowship programs. https://freida.ama-assn.org/Freida/user/search/programSearch.do. Accessed February 5, 2015.
10. Experian Hitwise. Search engine analysis. http://www.experian.com/marketing-services/online-trends-search-engine.html. Accessed February 5, 2015.
11. Hinds RM, Klifto CS, Naik AA, Sapienza A, Capo JT. Hand society and matching program web sites provide poor access to information regarding hand surgery fellowship. J Hand Microsurg. 2016;8(2):91-95.
12. Hinds RM, Danna NR, Capo JT, Mroczek KJ. Foot and ankle fellowship websites: An assessment of accessibility and quality. Foot Ankle Spec. 2017;10(4):302-307.
13. Griffin SM, Stoneback JW. Navigating the Orthopaedic Trauma Fellowship Match from a candidate’s perspective. J Orthop Trauma. 2011;25(suppl 3):S101-S103.
14. American Council for Graduate Medical Education. Accredited orthopaedic trauma fellowship programs. https://www.acgme.org/ads/Public/Programs/Search?specialtyId=49&orgCode=&city=. Accessed February 5, 2015.
15. US News & World Report. Best hospitals for orthopedics. http://health.usnews.com/best-hospitals/rankings/orthopedics. Accessed February 5, 2015.
16. US News & World Report. Best medical schools: research. http://grad-schools.usnews.rankingsandreviews.com/best-graduate-schools/top-medical-schools/research-rankings?int=98fd08. Accessed February 5, 2015.
17. Silvestre J, Guzman JZ, Abbatematteo JM, Chang B, Levin LS. Evaluation of content and accessibility of hand fellowship websites. Hand (NY). 2015;10(3):516-521.
18. Silvestre J, Tomlinson-Hansen S, Fosnot J, Taylor JA. Plastic surgery residency websites: a critical analysis of accessibility and content. Ann Plast Surg. 2014;72(3):265-269.
Use of Intravenous Tranexamic Acid Improves Early Ambulation After Total Knee Arthroplasty and Anterior and Posterior Total Hip Arthroplasty
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
Take-Home Points
- IV-TXA significantly reduces intraoperative blood loss following TJA.
- Early mobilization correlates with reduced incidence of postoperative complications.
- IV-TXA minimizes postoperative anemia, facilitating improved early ambulation following TJA.
- IV-TXA significantly reduces the need for postoperative transfusions.
- IV-TXA is safe to use with no adverse events noted.
By the year 2020, use of primary total knee arthroplasty (TKA) in the United States will increase an estimated 110%, to 1.375 million procedures annually, and use of primary total hip arthroplasty (THA) will increase an estimated 75%, to more than 500,000 procedures.1 Minimizing perioperative blood loss and improving early postoperative ambulation both correlate with reduced postoperative morbidity, allowing patients to return to their daily lives expeditiously.
Tranexamic acid (TXA), a fibrinolytic inhibitor, competitively blocks lysine receptor binding sites of plasminogen, sustaining and stabilizing the fibrin architecture.2 TXA must be present to occupy binding sites before plasminogen binds to fibrin, validating the need for preoperative administration so the drug is available early in the fibrinolytic cascade.3 Intravenous (IV) TXA diffuses rapidly into joint fluid and the synovial membrane.4 Drug concentration and elimination half-life in joint fluid are equivalent to those in serum. Elimination of TXA occurs by glomerular filtration, with about 30% of a 10-mg/kg dose removed in 1 hour, 55% over the first 3 hours, and 90% within 24 hours of IV administration.5
The efficacy of IV-TXA in minimizing total joint arthroplasty (TJA) perioperative blood loss has been proved in small studies and meta-analyses.6-9 TXA-induced blood conservation decreases or eliminates the need for postoperative transfusion, which can impede valuable, early ambulation.10 In addition, the positive clinical safety profile of TXA supports routine use of TXA in TJA.6,11-15
The benefits of early ambulation after TJA are well established. Getting patients to walk on the day of surgery is a key part of effective and rapid postoperative rehabilitation. Early mobilization correlates with reduced incidence of venous thrombosis and postoperative complications.16 In contrast to bed rest, sitting and standing promotes oxygen saturation, which improves tissue healing and minimizes adverse pulmonary events. Oxygen saturation also preserves muscle strength and blood flow, reducing the risk of venous thromboembolism and ulcers. Muscle strength must be maintained so normal gait can be regained.17 Compared with rehabilitation initiated 48 to 72 hours after TKA, rehabilitation initiated within 24 hours reduced the number of sessions needed to achieve independence and normal gait; in addition, early mobilization improved patient reports of pain after surgery.18 An evaluation of Denmark registry data revealed that mobilization to walking and use of crutches or canes was achieved earlier when ambulation was initiated on day of surgery.19 Finally, mobilization on day of surgery and during the immediate postoperative period improved long-term quality of life after TJA.20
We conducted a retrospective cohort study to determine if use of IV-TXA improves early ambulation and reduces blood loss after TKA and anterior and posterior THA. We hypothesized that IV-TXA use would reduce postoperative anemia and improve early ambulation and outcomes without producing adverse events during the immediate postoperative period. TXA reduces bleeding, and reduced incidence of hemarthrosis, wound swelling, and anemia could facilitate ambulation, reduce complications, and shorten recovery in patients who undergo TJA.
Patients and Methods
In February 2014, this retrospective cohort study received Institutional Review Board approval to compare the safety and efficacy of IV-TXA (vs no TXA) in patients who underwent TKA, anterior THA, and posterior THA.
In March 2012, multidisciplinary protocols were standardized to ensure a uniform hospital course for patients at our institution. All patients underwent preoperative testing and evaluation by a nurse practitioner and an anesthesiologist. In March 2013, IV-TXA became our standard of care. TXA use was contraindicated in patients with thromboembolic disease or with hypersensitivity to TXA. Patients without a contraindication were given two 10-mg/kg IV-TXA doses, each administered over 15 to 30 minutes; the first dose was administered before incision, and the second was infused at case close and/or at least 60 minutes after the first dose. Most TKA patients received regional (femoral) anesthesia and analgesia, and most THA patients received spinal or epidural anesthesia and analgesia. In a small percentage of cases, IV analgesia was patient-controlled, as determined by the pain service. There were no significant differences in anesthesia/analgesia modality between the 2 study groups—patients who received TXA and those who did not. Patients were then transitioned to oral opioids for pain management, unless otherwise contraindicated, and were ambulated 4 hours after end of surgery, unless medically unstable. Hematology and chemistry laboratory values were monitored daily during admission.
Patients underwent physical therapy (PT) after surgery and until hospital discharge. Physical therapists blinded to patients’ intraoperative use or no use of TXA measured ambulation. After initial evaluation on postoperative day 0 (POD-0), patients were ambulated twice daily. The daily ambulation distance used for the study was the larger of the 2 daily PT distances (occasionally, patients were unable to participate fully in both sessions). Patients received either enoxaparin or rivaroxaban for postoperative thromboprophylaxis (the anticoagulant used was based on surgeon preference). Enoxaparin was subcutaneously administered at 30 mg every 12 hours for TKA, 40 mg once daily for THA, 30 mg once daily for calculated creatinine clearance under 30 mL/min, or 40 mg every 12 hours for body mass index (BMI) 40 or above. With enoxaparin, therapy duration was 14 days. Oral rivaroxaban was administered at 10 mg once daily for 12 days for TKA and 35 days for THA unless contraindicated.
The primary outcome variables were ambulation measured on POD-1 and POD-2 and intraoperative blood loss. In addition, hemoglobin and hematocrit were measured on POD-0, POD-1, and POD-2. Ambulation was defined as number of feet walked during postoperative hospitalization. To calculate intraoperative blood loss, the anesthesiologist subtracted any saline irrigation volume from the total volume in the suction canister. Also noted were postoperative transfusions and any diagnosis of postoperative venous thromboembolism—specifically, deep vein thrombosis (DVT) or pulmonary embolism (PE).
Demographic and clinical characteristics of the TXA and no-TXA groups were compared using either 2-sample t test (for continuous variables) or χ2 test (for categorical variables).
The ambulation outcome was log-transformed to meet standard assumptions of Gaussian residuals and equality of variance. Means and 95% confidence intervals (CIs) were calculated on the log scale and were anti-logged so the results could be presented in their original units.
A linear mixed model was used to model intraoperative blood loss as a function of group (TXA, no TXA), procedure (TKA, anterior THA, posterior THA), and potential confounders (age, sex, BMI, operative time).
Linear mixed models for repeated measures were used to compare outcomes (hemoglobin, hematocrit) between groups (TXA, no TXA) and procedures (TKA, anterior THA, posterior THA) and to compare changes in outcomes over time. Group, procedure, and operative time interactions were explored. Potential confounders (age, sex, BMI, operative time) were included in the model as well.
A χ2 test was used to compare the groups (TXA, no TXA) on postoperative blood transfusion (yes, no). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used. Need for transfusion was clinically assessed case by case. Symptomatic anemia (dyspnea on exertion, headaches, tachycardia) was used as the primary indication for transfusion once hemoglobin fell below 8 g/dL or hematocrit below 24%. Number of patients with a postoperative thrombus formation was minimal. Therefore, this outcome was described with summary statistics and was not formally analyzed.
Results
Of the 477 patients who underwent TJAs (275 TKAs, 98 anterior THAs, 104 posterior THAs; all unilateral), 111 did not receive TXA (June 2012-February 2013), and 366 received TXA (March 2013-January 2014). Other than for the addition of IV-TXA, the same standardized protocols instituted in March 2012 continued throughout the study period. The difference in sample size between the TXA and no-TXA groups was not statistically significant and did not influence the outcome measures.
Ambulation
There was a significant (P = .0066) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P < .0001), sex (P < .0001), BMI (P < .0001), and operative time (P = .8308). Regarding TKA, mean ambulation was higher for the TXA group than for the no-TXA group at POD-1 (8.36 vs 3.40 feet; P < .0001) and POD-2 (25.81 vs 18.75 feet; P = .0054). The same was true for anterior THA at POD-1 (10.86 vs 3.33 feet; P < .0001) and POD-2 (27.24 vs 13.19 feet; P < .0001) and posterior THA at POD-1 (10.64 vs 3.37 feet; P < .0001) and POD-2 (24.68 vs 12.93 feet; P = .0002). See Table 3.
Intraoperative Blood Loss
There was a significant 3-way interaction of TXA, procedure (P < .0053), and operative time (P < .0001) after adjusting for age (P < .6136), sex (P = .1147), and BMI (P = .6180). Regarding TKA, mean intraoperative blood loss was significantly lower for the TXA group than for the no-TXA group (241.58 vs 287.81 mL; P = .0004). The same was true for anterior THA (352.91 vs 533.79 mL; P < .0001). Regarding posterior THA, there was no significant difference between the TXA and no-TXA groups (326.00 vs 350.16 mL; P = .3246). See Table 4.
Hemoglobin
There was a significant (P = .0008) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .0174), sex (P < .0001), BMI (P = .0007), and operative time (P = .0002). Regarding TKA, postoperative hemoglobin levels were higher for the TXA group than for the no-TXA group at POD-0 (12.10 vs 11.68 g/dL; P = .0135), POD-1 (11.62 vs 10.67 g/dL; P < .0001), and POD-2 (11.02 vs 10.11 g/dL; P < .0001). The same was true for anterior THA at POD-1 (11.03 vs 10.19 g/dL; P = .0034) and POD-2 (10.57 vs 9.64 g/dL; P = .0009) and posterior THA at POD-2 (11.04 vs 10.16 g/dL; P = .0003). See Table 5.
Hematocrit
There was a significant (P < .0006) 3-way interaction of TXA, procedure, and operative time after adjusting for age (P = .1597), sex (P < .0001), BMI (P < .0001), and operative time (P = .0003). Regarding TKA, postoperative hematocrit levels were higher for the TXA group than for the no-TXA group at POD-0 (36.52% vs 34.65%; P < .0001), POD-1 (34.62% vs 31.83%; P < .0001), and POD-2 (33.01% vs 30.20%; P < .0001). The same was true for anterior THA at POD-1 (32.82% vs 30.59%; P = .0037) and POD-2 (31.58% vs 28.61%; P = .0004) and posterior THA at POD-2 (32.93% vs 30.17%; P < .0001). See Table 6.
Postoperative Transfusions
Of the 477 patients, 25 (5.24%) required a postoperative transfusion. Postoperative transfusions were less likely (P < .0001) required in the TXA group (1.64%, 6/366) than in the no-TXA group (17.12%, 19/111). Given the smaller number of events, a more complex model accounting for clustered data and potential confounders was not used, and the different procedures were not evaluated separately.
Deep Vein Thrombosis and Pulmonary Embolism
Of the 477 patients, 2 developed a DVT, and 5 developed a PE. Both DVTs occurred in the TXA group (2/366, 0.55%; 95% CI, 0.07%-1.96%). Of the 5 PEs, 4 occurred in the TXA group (4/366, 1.09%; 95% CI, 0.30%-2.77%), and 1 occurred in the no-TXA group (1/111, 0.90%; 95% CI, 0.02%-4.92%). Given the exceedingly small number of events, no statistical significance was noted between groups.
Discussion
Orthopedic surgeons carefully balance patient expectations, societal needs, and regulatory mandates while providing excellent care and working under payers’ financial restrictions. The Centers for Medicare & Medicaid Services announced that, starting in 2016, TJAs will be reimbursed in total as a single bundled payment, adding to the need to provide optimal care in a fiscally responsible manner.21 Standardized protocols implementing multimodal therapies are pivotal in achieving favorable postoperative outcomes.
Our study results showed that IV-TXA use minimized hemoglobin and hematocrit reductions after TKA, anterior THA, and posterior THA. Postoperative anemia correlates with decreased ambulation ability and performance during the early postoperative period. In general, higher postoperative hemoglobin and hematocrit levels result in improved motor performance and shorter recovery.22 In addition, early ambulation is a validated predictor of favorable TJA outcomes. In our study, for TKA, anterior THA, and posterior THA, ambulation on POD-1 and POD-2 was significantly better for patients who received TXA than for patients who did not.
Transfusion rates were markedly lower for our TXA group than for our no-TXA group (1.64% vs 17.12%), confirming the findings of numerous other studies on outcomes of TJA with TXA.2,3,6-12,14,15 Transfusions impede physical therapy and affect hospitalization costs.
Although potential thrombosis-related adverse events remain an endpoint in studies involving TXA, we found a comparably low incidence of postoperative venous thrombosis in our TXA and no-TXA groups (1.09% and 0.90%, respectively). In addition, no patient in either group developed a postoperative arterial thrombosis.
This is the largest single-center study of TXA use in TKA, anterior THA, and posterior THA. The effect of TXA use on postoperative ambulation was not previously found with TJA.
This study had its limitations. First, it was not prospective, randomized, or double-blinded. However, the physical therapists who mobilized patients and recorded ambulation data were blinded to the study and its hypothesis and followed a standardized protocol for all patients. In addition, intraoperative blood loss was recorded by an anesthesiologist using a standardized protocol, and patients received TXA per orthopedic protocol and surgeon preference, without selection bias. Another limitation was that ambulation data were captured only for POD-1 and POD-2 (most patients were discharged by POD-3). However, a goal of the study was to capture immediate postoperative data in order to determine the efficacy of intraoperative TXA. Subsequent studies can determine if this early benefit leads to long-term clinical outcome improvements.
In reducing blood loss and transfusion rates, intra-articular TXA is as efficacious as IV-TXA.23-25 We anticipate that the improved clinical outcomes found with IV-TXA in our study will be similar with intra-articular TXA, but more study is needed to confirm this hypothesis.
Conclusion
This retrospective cohort study found that use of IV-TXA in TJA improved early ambulation and clinical outcomes (reduced anemia, fewer transfusions) in the initial postoperative period, without producing adverse events.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83(4):596-601.
3. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty. Acta Orthop Scand. 2001;72(5):442-448.
4. Tanaka N, Sakahashi, H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83(5):702-705.
5. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47.
6. George DA, Sarraf KM, Nwaboku H. Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25(1):129-133.
7. Vigna-Taglianti F, Basso L, Rolfo P, et al. Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol. 2014;24(4):545-551.
8. Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care. 2003;31(5):529-537.
9. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829.
10. Sculco PK, Pagnano MW. Perioperative solutions for rapid recovery joint arthroplasty: get ahead and stay ahead. J Arthroplasty. 2015;30(4):518-520.
11. Lozano M, Basora M, Peidro L, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39-44.
12. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783.
13. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement. A systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
14. Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA. Clin Orthop Relat Res. 2011;469(10):2874-2880.
15. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
16. Stowers M, Lemanu DP, Coleman B, Hill AG, Munro JT. Review article: perioperative care in enhanced recovery for total hip and knee arthroplasty. J Orthop Surg (Hong Kong). 2014;22(3):383-392.
17. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation. Acta Orthop. 2008;79(5):624-630.
18. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
19. Husted H, Hansen HC, Holm G, et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Arch Orthop Trauma Surg. 2010;130(2):263-268.
20. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012;83(346):1-39.
21. Comprehensive Care for Joint Replacement Model. CMS.gov. https://innovation.cms.gov/initiatives/cjr. Updated October 5, 2017.
22. Wang X, Rintala DH, Garber SL, Henson H. Association of hemoglobin levels, acute hemoglobin decrease, age, and co-morbidities with rehabilitation outcomes after total knee replacement. Am J Phys Med Rehabil. 2005;84(6):451-456.
23. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.