User login
Systematic Review of Rapid Response Systems
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
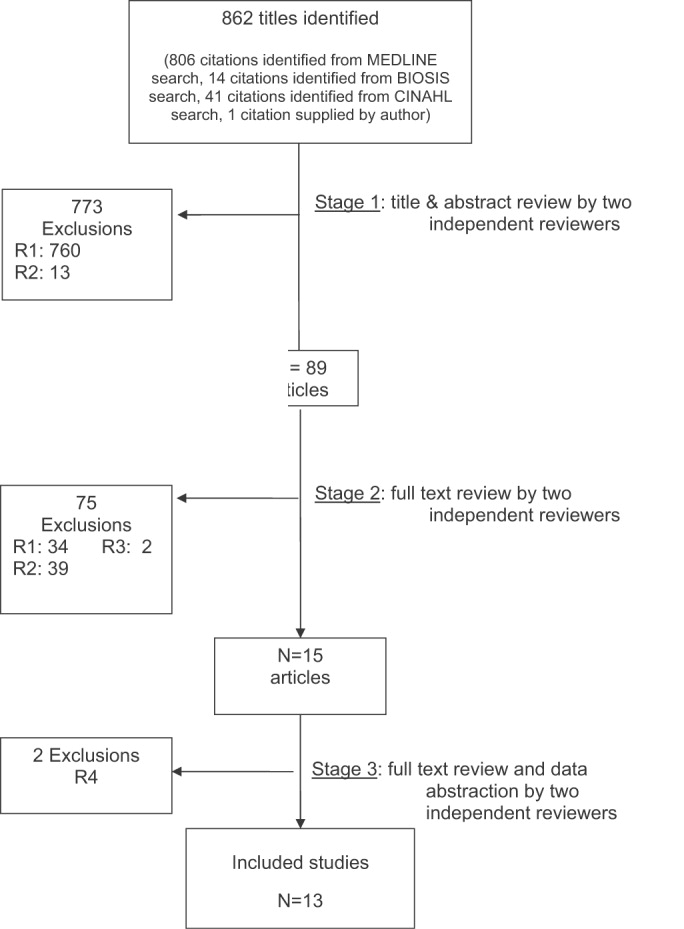
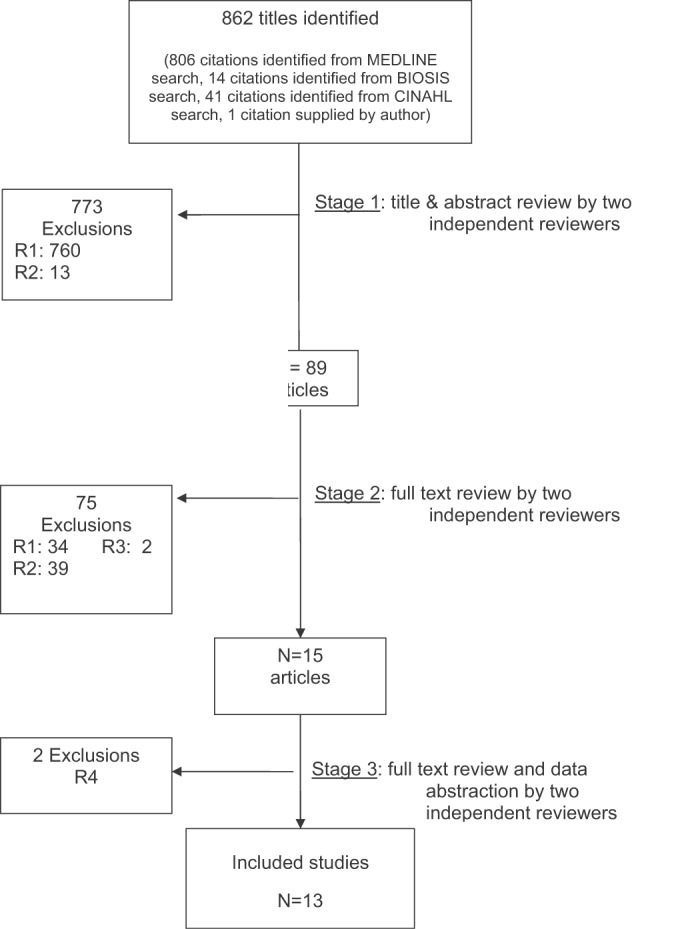
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.
Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
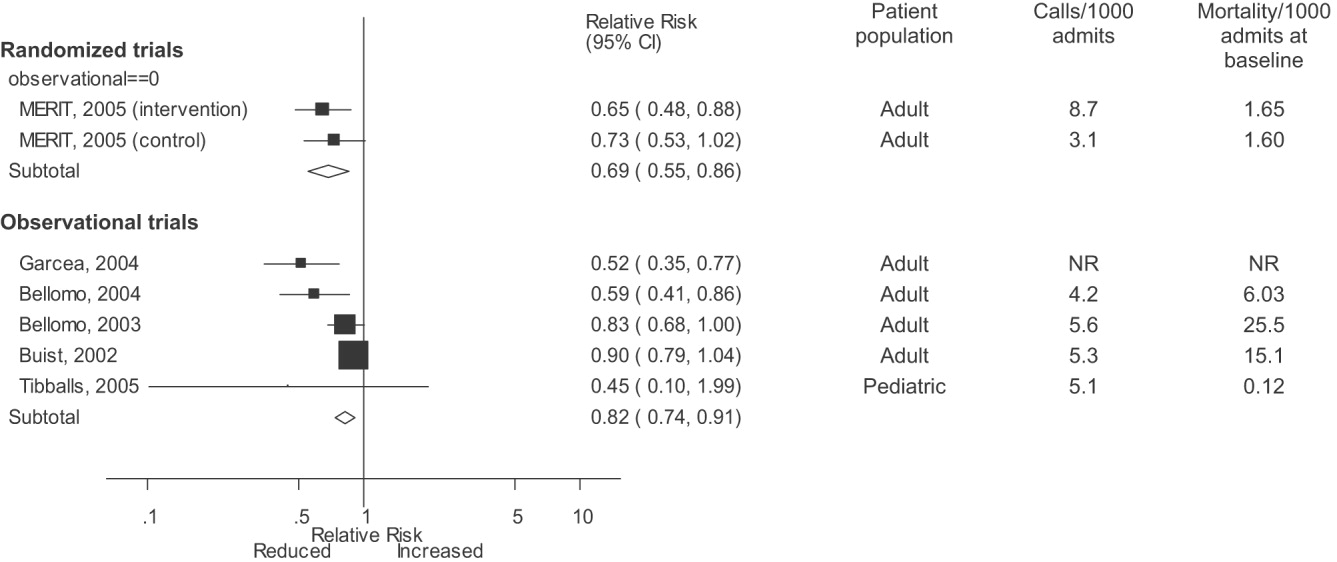
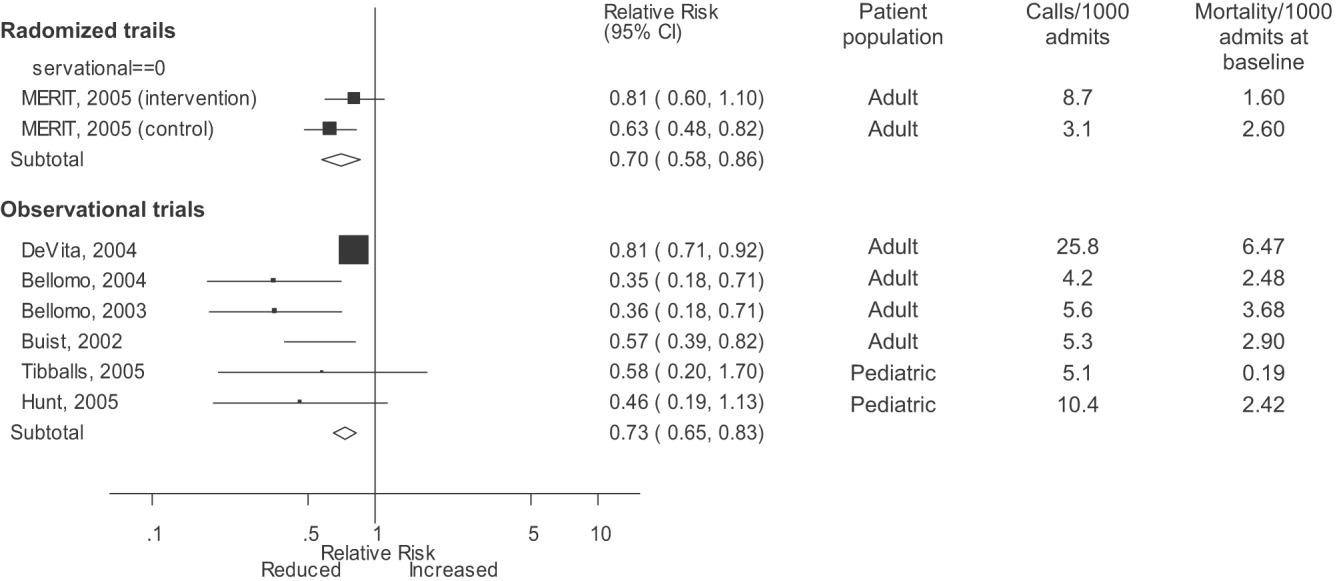
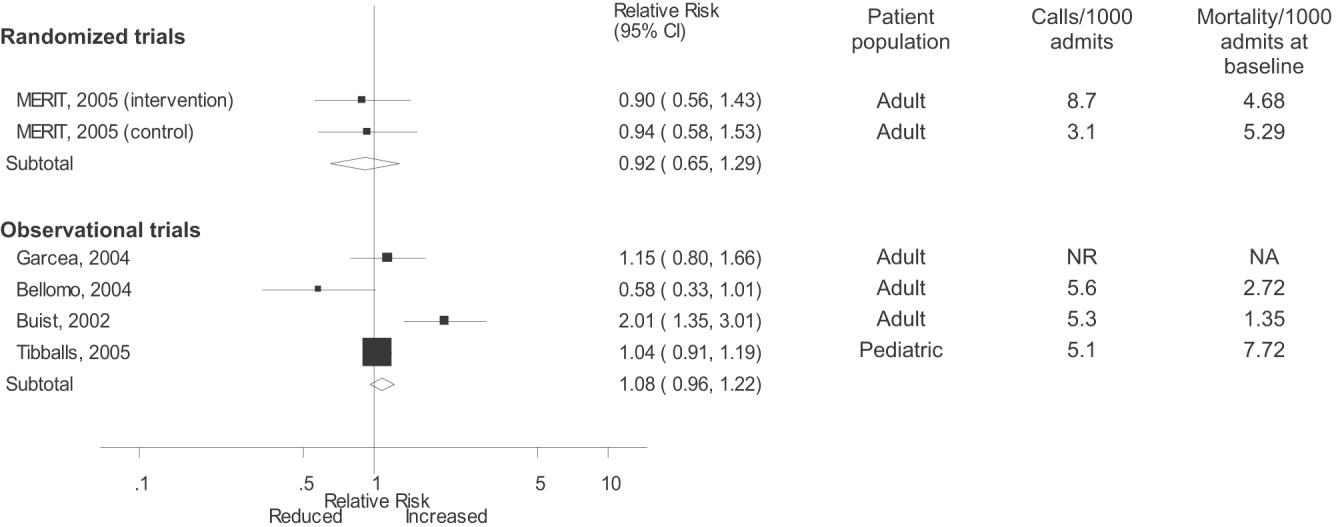
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).
DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.

Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).



DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.

Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).



DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
Preoperative Cardiac Risk Stratification 2007
More than 33 million patients undergo surgery annually in the United States. Approximately 8 million of these patients either have known coronary artery disease or risk factors for it, and an estimated 50,000 patients sustain a perioperative myocardial infarction, with an additional 1 million developing another medical complication. An integrated comprehensive approach is necessary to risk‐stratify these patients in an attempt to reduce these complications.
The basic role of risk stratification is to identify those patients at increased risk for complications; however, we are looking for a small number of patients at high risk in a population of relatively low‐risk patients. Most surgical patients do well, and further diagnostic testing has a low yield in predicting those likely to have a complication (poor positive predictive value [PPV]). Our goal should be to determine the underlying potential triggers of cardiac complications and institute measures to prevent them. After briefly reviewing pathophysiology, risk indices, and guidelines for preoperative cardiac risk assessment and diagnostic testing, we will focus on risk reduction strategies including prophylactic revascularization (CABG/PCI) and medical therapy.
PATHOPHYSIOLOGY OF PERIOPERATIVE MYOCARDIAL INFARCTION
Perioperative myocardial infarctions result from myocardial ischemia or plaque rupture and coronary thrombosis.1 Myocardial ischemia may be caused by increased oxygen demand or decreased oxygen delivery. Surgical trauma, anesthesia, pain, hypothermia, and bleeding trigger a stress state. This in turn increases catecholamine release, leading to tachycardia, hypertension, and increased oxygen demand. Anesthesia, hypotension, bleeding, and anemia may produce hypoxia, with subsequently decreased delivery of oxygen. Surgical trauma initiates an inflammatory response, leading to plaque fissuring, and a hypercoagulable state, which can result in acute coronary thrombosis. Perioperative prophylaxis should target these potential triggers.
CARDIAC RISK INDICES AND GUIDELINES
Over the past 3 decades, a number of cardiac risk indices have been published. The older group of indices was most notable for Goldman's original cardiac risk index2 and Detsky's modification.3 The newer group consists of the American College of Physicians (ACP) guidelines4 (now considered outdated), the American College of Cardiology/American Heart Association (ACC/AHA) guidelines (to be updated again in early 2007),5 and the Lee revised cardiac risk index (RCRI).6
The 2002 ACC guidelines5 outline how to determine the need for additional cardiac (usually noninvasive) testing (NIT): after ascertaining the urgency of surgery, history of revascularization procedures, and previous stress test results (if any), a combination of clinical risk predictors, surgery‐specific risk, and patient self‐reported exercise capacity should be entered into an algorithm. The guidelines state a shortcut can be used: noninvasive testing should be considered if a patient has any 2 of the following: (1) intermediate clinical risk (stable angina or old MI, compensated heart failure, diabetes mellitus, renal insufficiency), (2) high‐risk surgery (aortic or major vascular procedures, prolonged surgery with significant expected blood loss or fluid shifts), or (3) poor exercise capacity (<4 METs). Patients with major clinical predictors (unstable coronary syndromes, decompensated heart failure, severe valvular heart disease, or hemodynamically significant arrhythmias) should not undergo elective surgery without further workup or treatment. The ACP guidelines use the Detsky3 modified CRI and low‐risk variables to suggest any need for further testing depending on type of surgery (vascular or nonvascular). At times these 2 guidelines offer conflicting recommendations, with the ACC more likely than the ACP to recommend NIT. The RCRI, which was developed prospectively and has been validated, uses 6 predictors of major cardiac complicationshigh‐risk surgery, coronary artery disease, stroke, congestive heart failure, diabetes mellitus requiring insulin, and serum creatinine > 2 mg/dL. Patients with 0 or 1 risk factors are considered at low risk, those with 2 risk factors at moderate risk, and those with 3 or more risk factors at high risk (10% complication rate). Although the RCRI does not make recommendations about whether to test, it has been incorporated into a number of algorithms combining risk stratification with recommendations about noninvasive testing as well as use of perioperative beta‐blockers.710 0
|
DIAGNOSTIC CARDIAC TESTS
Tests should not be done if the results will not alter patient management. If further assessment is indicated based on the ACC/AHA algorithm, other risk indices,10 or criteria independent of the need for surgery, the physician must decide whether to do a noninvasive (eg, echocardiogram or stress test) or an invasive test (coronary angiography). Unless a patient has independent criteria for angiography or, occasionally, a very high prior probability of significant CAD based on multiple risk factors, noninvasive testing is usually the preferred first step. A resting echocardiogram is potentially useful for providing information about suspected valvular heart disease but is not a consistent predictor of ischemic events.
For ambulatory patients, exercise stress testing is usually preferred over pharmacologic testing; in the perioperative setting, the usefulness of exercise testing is limited by the indications for obtaining stress testing (namely, poor functional status) as well as its main limitation, patient inability to reach 85% of the target heart rate. As a result, pharmacologic stress testing should be the primary modality for patients requiring preoperative risk stratification. Pharmacologic stress testing can be done with nuclear imaging (dipyridamole or adenosine thallium) or echocardiography (dobutamine echocardiography). For the most part, the results are comparable,11, 12 with both having excellent negative predictive values (NPV > 95%) but poor positive predictive values (PPV < 20%); however, dobutamine echocardiography tends to have fewer false positives. Dipyridamole or adenosine testing is relatively contraindicated with bronchospasm and COPD but is preferred over exercise or dobutamine for patients with a left bundle‐branch block. Suspected critical aortic stenosis is a contraindication to stress testing. Positive noninvasive findings should result in prophylactic measures, either medical therapy or an invasive procedure.
CORONARY REVASCULARIZATION
Coronary Artery Bypass Grafting
Observational studies have shown that patients with CAD (in the CASS study) treated by coronary artery bypass grafting (CABG) surgery versus had a lower mortality (0.9% vs. 2.4%) and fewer nonfatal myocardial infarctions (0.7% vs. 1.1%) than patients treated with medical therapy who underwent noncardiac surgery months or years later.13 This protective effect of CABG lasted approximately 46 years; however, there was no benefit for low‐risk noncardiac procedures. Furthermore, the risk of perioperative mortality (3%) and morbidity associated with the CABG itself was not taken into account, which would have negated its potential benefit.
Percutaneous Coronary Intervention
Several reports suggested that a previous percutaneous coronary intervention (PCI) was also associated with a lower risk of perioperative mortality and nonfatal myocardial infarction (MI) compared to historical controls. Early studies suggested that noncardiac surgery could be performed as early as 710 days after balloon angioplasty (BA). As bare‐metal stents gradually replaced BA, subsequent reports highlighted the increased risk of noncardiac surgery within 2 weeks14 and then within 46 weeks15 after stenting. This was primarily because of in‐stent thrombosis associated with premature discontinuation of dual antiplatelet therapy or increased major bleeding if this therapy was continued. The current recommendation is to wait at least 46 weeks after inserting a bare‐metal stent and to discontinue clopidogrel aspirin at least 5 days before surgery. A recent review from the Mayo Clinic16 found BA to be reasonably safe if patients require surgery soon after cardiac intervention (after 2 weeks).
More recently drug‐eluting stents (DESs) have become the standard; however, the recommendations for antiplatelet therapy (in the absence of surgery) are for a minimum of 23 months after sirolimus‐coated stents and at least 6 months after stents with paclitaxel. There has been very little in the published literature on patients undergoing noncardiac surgery after drug‐eluting stents. A small retrospective review suggested that patients whose DES had been placed a median of 260 days before surgery had few cardiac events in the perioperative period.17 The recommendations of a French task force did not provide strong guidance, probably because of a lack of evidence.18 The only prospective study of stenting and noncardiac surgery involved continuing antiplatelet therapy (or stopping it less than 3 days before surgery) and using unfractionated heparin or enoxaparin in 103 patients. Despite this therapy, 5 patients died, 12 had myocardial infarctions, 22 had elevation of troponin, but only 4 had major bleeding. Patients with stenting less than 35 days before surgery were at the greatest risk.19 In view of these findings, if noncardiac surgery must be performed within 2 months and the patient is appropriate for PCI, balloon angioplasty or a bare‐metal stent is preferred over DES implantation. If a patient has a DES in place (particularly if it has been fewer than 6 months since implantation) and requires noncardiac surgery, the optimal approach would be to continue at least one if not both antiplatelet agents through surgery; if this is not possible, bridging therapy with intravenous IIB/IIIA receptor blockers has been a suggested approach.10
Revascularization Versus No Revascularization: the CARP Trial
The only randomized controlled study to compare invasive and noninvasive strategies was the Coronary Artery Revascularization Prophylaxis (CARP) trial.20 More than 5800 patients with stable cardiac symptoms scheduled for elective nonvascular surgery in VA hospitals were screened, approximately 20% underwent coronary angiography, and 510 patients (9% of the original group) were randomized to PCI/CABG or no revascularization. Revascularization was associated with 1.7% mortality and a 5.8% nonfatal MI rate, and an additional 4% died after successful revascularization while awaiting vascular surgery. Short‐term outcomes were similar in both the revascularization and no revascularization groups (3% 30‐day mortality and 8%12% perioperative nonfatal MI). The primary outcome, long‐term mortality, also did not differ between the groups (22% vs. 23%) after an average follow‐up of 2.7 years. The investigators concluded on the basis of this data that prophylactic revascularization could not be recommended for patients with stable CAD undergoing elective vascular surgery. Of note is that both groups of patients in the CARP trial were given intensive medical therapy, with 84% on beta‐blockers, 54% on statins, 51% on ACE inhibitors, and 73% on aspirin, which may have made it difficult to show any significant benefit of revascularization. Other limitations of that study are that it was underpowered to detect a short‐term benefit and excluded patients with unstable or more severe cardiac symptoms or disease (left main disease, aortic stenosis, and severe left ventricular dysfunction). In any case, the results of this support the ACC guidelines, which state that prophylactic revascularization is rarely necessary just to get the patient through surgery.
If the goal of risk stratification is to determine which patients are at increased risk and if revascularization fails to lower that risk, various medical therapies, including beta‐blockers, alpha‐agonists, and statins, should be considered as risk‐reduction strategies.
PHARMACOLOGIC STRATEGIES
Cardioprotection with Adrenergic Modulation and Statin Therapy
Support for adrenergic modulation (with beta‐blockers and alpha‐agonists) to prevent postoperative cardiac complications has been the subject of a number of reviews, including our own.7, 8, 21 Initial enthusiasm22, 23 has been tempered, however, as evidence has evolved.
The results of a randomized trial published in abstract form24 showed no significant difference in rates of a combined end point of mortality, myocardial infarction, heart failure, and ventricular arrhythmia 30 days after vascular surgery of 500 patients randomized to metoprolol or placebo. Furthermore, in a randomized trial of 107 aortic surgery patients with no history of coronary disease, metoprolol started on admission and continued for 7 days did not significantly reduce cardiac events.25 In addition, a well‐designed meta‐analysis suggested that there are too few data to definitively determine whether perioperative beta‐blockade is efficacious.26 Finally, the results of a rigorously analyzed observational trial using administrative data from nearly 700,000 patients suggested that perioperative beta‐blockade was protective (reduced mortality) only in higher‐risk patients (eg, RCRI 2 points). In those at lower risk, beta blockade was associated with a higher risk of complications, even if the lower‐risk patients had only 1 risk factor of either diabetes or coronary disease.27
Trials of alpha adrenergic agonists have also been summarized in at least 2 meta‐analyses. One of these meta‐analyses reported alpha‐2 agonists reduced mortality by nearly half and reduced postoperative myocardial infarction by a third in vascular patients, but had no benefit in others.28 Another meta‐analysis calculated that 83 patients needed to be treated with alpha‐agonists to prevent one cardiac event,29 a number higher than that for beta‐blockers.
Data on the effectiveness of statins is accumulating. The results of 5 observational trials3034 and 1 randomized study35 suggest that patients receiving statin therapy at the time of surgery (and afterward) have a lower risk of having a cardiac event and lower mortality, with relative reductions in risk between 80%30 and 30%.32 In the 1 randomized trial, of 100 vascular surgery patients, 20 mg/day of atorvastatin was begun 1 month before surgery and continued for 45 days,35 with beta‐blockers included per protocol. This protocol reduced the combined outcome of cardiac mortality, myocardial infarction, stroke, or unstable angina, but the overall number of events was very small (4 patients vs. 13 patients, P = .03). However, no patient required discontinuation of the drug because of side effects.
HOW SHOULD I INCORPORATE EVIDENCE INTO PRACTICE?
Target Patients Most Likely to Benefit
Recent trends in evidence increasingly support the idea that lower‐risk subgroups (such as those with the minor criteria employed by Mangano) may not benefit from perioperative beta‐blockers and that only higher‐risk subgroups should be targeted. This general approach was recommended in recent guidelines from the AHA‐ACC,36 as well as in an extensive review of perioperative cardiac risk management.10 The strongest recommendations were to continue beta‐blockers in patients already on them and to give them to patients scheduled for vascular surgery who had ischemia on a stress test. The ACC also stated that beta‐blockers were probably recommended for patients with known CAD or high cardiac risk scheduled for intermediate‐ to high‐risk surgery. Recommendations for other groups were weaker or lacked sufficient evidence.36 At this point, it seems prudent to target high‐risk patients (RCRI 2), as well as those who would require beta‐blockers or statin therapy regardless (eg, patients with known coronary artery disease). There are no data to suggest that dose titration of statins is required before surgery.
Be Aware of How Harm Might Be Produced
Notwithstanding its limitations, results from the recent observational trial from Lindenauer raise important questions about the effectiveness of beta‐blockers in practice. That is, are beta‐blockers safe and effective when used in surgical patients outside the tightly controlled setting of a randomized trial? It is apparent how titrating beta‐blockers to a target heart rate without careful clinical assessment (as occurred in most RCTs) might lead to beta‐blockers being used to treat tachycardia related to hypovolemia, pain, anemia, bleeding, or early sepsis. Interestingly, beta‐blockers may be associated with higher risk in other settings as well,37 so potential harm in the perioperative period are not completely surprising.
Use a Protocol That Sticks as Close to the Evidence as Possible
To stay as close as possible to what the evidence shows for the use of beta‐blockers, this drug should be started early enough to allow dose titration and continued for at least 7 days and optimally 30 days after surgery (indefinitely, if a patient requires it long term), working to ensure that patients are physiologically beta‐blocked (eg, heart rate 5565) for as much of the time that they are being treated as possible. Two recent studies demonstrated the importance of tight heart rate control38, 39higher doses of beta‐blockers and tight heart rate control were associated with reduced perioperative myocardial ischemia and troponin T release, which might obviate the need for preoperative cardiac testing in intermediate‐risk patients undergoing vascular surgery. A recent placebo‐controlled, randomized trial40 suggested that a simple strategy of 4 days of transdermal and oral clonidine reduced perioperative ischemia and mortality. Although this approach is very useful for patients who cannot take pills by mouth, it would necessitate a switch to beta‐blockers for patients who need them long term. In addition, use of clonidine may be associated with a higher risk of withdrawal than cardioselective beta‐blockers. No prospective trials have compared beta‐blockers and alpha‐2 agonists. Both produce hypotension and bradycardia, improve pain control, and rarely produce adverse pulmonary effects.41 At the least, consultants should be clear in their recommendations about the start and stop dates for beta‐blockers and should ensure a smooth outpatient transition of patients for whom long‐term statin or beta‐blocker therapy is needed.
Be Ready to Adjust Your Practice as the Evidence Continues to Evolve
Far too few patients have been randomized to beta‐blockers, adrenergic modulation, or statin therapy to date to provide a reasonable estimate of their effects on mortality. As a result, although it seems likely that some subgroups benefit from one or more of these therapies, the degree of risk requiredand an optimal dosing scheduleremains a subject of intense debate. The results of perioperative trials of adrenergic modulators have consistently provided evidence supporting their use in other patient populations, but larger studies may not confirm a beneficial effect. Ongoing Canadian (POISE) and European trials (DECREASE IV) should address sample size limitations and provide information critical for clinicians caring for patients in this era of rapidly evolving evidence.
- ,,,,,.Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: A review.CMAJ.2005;173:779–788.
- ,,, et al.Multifactorial index of cardiac risk in noncardiac surgical procedures.N Engl J Med.1977;297:845–850.
- ,,,,.Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index.Arch Intern Med.1986;146:2131–2134.
- ,.Perioperative assessment and management of risk from coronary artery disease.Ann Intern Med.1997;127:313–328.
- ,,, et al.ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery).Circulation.2002;105:1257–1267.
- .Reducing cardiac risk in noncardiac surgery.N Engl J Med.1999;341:1838–1840.
- ,.beta‐Blockers and reduction of cardiac events in noncardiac surgery: scientific review.JAMA.2002;287:1435–1444.
- ,.Clinical practice. Lowering cardiac risk in noncardiac surgery.N Engl J Med.2001;345:1677–1682.
- ,,, et al.Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy.JAMA.2001;285:1865–1873.
- ,.Assessing and reducing the cardiac risk of noncardiac surgery.Circulation.2006;113:1361–1376.
- ,,, et al.A meta‐analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery.Heart.2003;89:1327–1334.
- ,,,.Exercise echocardiography or exercise SPECT imaging? A meta‐analysis of diagnostic test performance.JAMA.1998;280:913–920.
- ,,,,,.Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study.Circulation.1997;96:1882–1887.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,, et al.Outcome of patients undergoing balloon angioplasty in the two months prior to noncardiac surgery.Am J Cardiol.2005;96:512–514.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,.Perioperative management of antiplatelet agents in patients with coronary stents: recommendations of a French Task Force.Br J Anaesth.2006;97:580–582.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,.Perioperative cardiac assessment for noncardiac surgery: eight steps to the best possible outcome.Circulation.2003;107:2771–2774.
- .Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.N Engl J Med.1997;336:1452; discussion1453–1454.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Metoprolol after vascular surgery (MAVS).Can J Anesth.2004;51:A7.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,.Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review.Anesth Analg.2003;97:623–633.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA.2004;291:2092–2099.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery The Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,, et al.Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial.JAMA.2001;285:1711–1718.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion975–976.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,.Atenolol in hypertension: is it a wise choice?Lancet.2004;364:1684–1689.
- ,,, et al.Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate‐risk patients receiving beta‐blocker therapy with tight heart rate control?J Am Coll Cardiol.2006;48:964–969.
- ,,, et al.High‐dose beta‐blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients.Circulation.2006;114:1344–1349.
- ,,, et al.Effect of clonidine on cardiovascular morbidity and mortality after noncardiac surgery.Anesthesiology.2004;101:284–293.
- ,,.Cardioselective beta‐blockers in patients with reactive airway disease: a meta‐analysis.Ann Intern Med.2002;137:715–725.
- ,,.Does this patient have an abnormal systolic murmur?JAMA.1997;277:564–571.
More than 33 million patients undergo surgery annually in the United States. Approximately 8 million of these patients either have known coronary artery disease or risk factors for it, and an estimated 50,000 patients sustain a perioperative myocardial infarction, with an additional 1 million developing another medical complication. An integrated comprehensive approach is necessary to risk‐stratify these patients in an attempt to reduce these complications.
The basic role of risk stratification is to identify those patients at increased risk for complications; however, we are looking for a small number of patients at high risk in a population of relatively low‐risk patients. Most surgical patients do well, and further diagnostic testing has a low yield in predicting those likely to have a complication (poor positive predictive value [PPV]). Our goal should be to determine the underlying potential triggers of cardiac complications and institute measures to prevent them. After briefly reviewing pathophysiology, risk indices, and guidelines for preoperative cardiac risk assessment and diagnostic testing, we will focus on risk reduction strategies including prophylactic revascularization (CABG/PCI) and medical therapy.
PATHOPHYSIOLOGY OF PERIOPERATIVE MYOCARDIAL INFARCTION
Perioperative myocardial infarctions result from myocardial ischemia or plaque rupture and coronary thrombosis.1 Myocardial ischemia may be caused by increased oxygen demand or decreased oxygen delivery. Surgical trauma, anesthesia, pain, hypothermia, and bleeding trigger a stress state. This in turn increases catecholamine release, leading to tachycardia, hypertension, and increased oxygen demand. Anesthesia, hypotension, bleeding, and anemia may produce hypoxia, with subsequently decreased delivery of oxygen. Surgical trauma initiates an inflammatory response, leading to plaque fissuring, and a hypercoagulable state, which can result in acute coronary thrombosis. Perioperative prophylaxis should target these potential triggers.
CARDIAC RISK INDICES AND GUIDELINES
Over the past 3 decades, a number of cardiac risk indices have been published. The older group of indices was most notable for Goldman's original cardiac risk index2 and Detsky's modification.3 The newer group consists of the American College of Physicians (ACP) guidelines4 (now considered outdated), the American College of Cardiology/American Heart Association (ACC/AHA) guidelines (to be updated again in early 2007),5 and the Lee revised cardiac risk index (RCRI).6
The 2002 ACC guidelines5 outline how to determine the need for additional cardiac (usually noninvasive) testing (NIT): after ascertaining the urgency of surgery, history of revascularization procedures, and previous stress test results (if any), a combination of clinical risk predictors, surgery‐specific risk, and patient self‐reported exercise capacity should be entered into an algorithm. The guidelines state a shortcut can be used: noninvasive testing should be considered if a patient has any 2 of the following: (1) intermediate clinical risk (stable angina or old MI, compensated heart failure, diabetes mellitus, renal insufficiency), (2) high‐risk surgery (aortic or major vascular procedures, prolonged surgery with significant expected blood loss or fluid shifts), or (3) poor exercise capacity (<4 METs). Patients with major clinical predictors (unstable coronary syndromes, decompensated heart failure, severe valvular heart disease, or hemodynamically significant arrhythmias) should not undergo elective surgery without further workup or treatment. The ACP guidelines use the Detsky3 modified CRI and low‐risk variables to suggest any need for further testing depending on type of surgery (vascular or nonvascular). At times these 2 guidelines offer conflicting recommendations, with the ACC more likely than the ACP to recommend NIT. The RCRI, which was developed prospectively and has been validated, uses 6 predictors of major cardiac complicationshigh‐risk surgery, coronary artery disease, stroke, congestive heart failure, diabetes mellitus requiring insulin, and serum creatinine > 2 mg/dL. Patients with 0 or 1 risk factors are considered at low risk, those with 2 risk factors at moderate risk, and those with 3 or more risk factors at high risk (10% complication rate). Although the RCRI does not make recommendations about whether to test, it has been incorporated into a number of algorithms combining risk stratification with recommendations about noninvasive testing as well as use of perioperative beta‐blockers.710 0
|
DIAGNOSTIC CARDIAC TESTS
Tests should not be done if the results will not alter patient management. If further assessment is indicated based on the ACC/AHA algorithm, other risk indices,10 or criteria independent of the need for surgery, the physician must decide whether to do a noninvasive (eg, echocardiogram or stress test) or an invasive test (coronary angiography). Unless a patient has independent criteria for angiography or, occasionally, a very high prior probability of significant CAD based on multiple risk factors, noninvasive testing is usually the preferred first step. A resting echocardiogram is potentially useful for providing information about suspected valvular heart disease but is not a consistent predictor of ischemic events.
For ambulatory patients, exercise stress testing is usually preferred over pharmacologic testing; in the perioperative setting, the usefulness of exercise testing is limited by the indications for obtaining stress testing (namely, poor functional status) as well as its main limitation, patient inability to reach 85% of the target heart rate. As a result, pharmacologic stress testing should be the primary modality for patients requiring preoperative risk stratification. Pharmacologic stress testing can be done with nuclear imaging (dipyridamole or adenosine thallium) or echocardiography (dobutamine echocardiography). For the most part, the results are comparable,11, 12 with both having excellent negative predictive values (NPV > 95%) but poor positive predictive values (PPV < 20%); however, dobutamine echocardiography tends to have fewer false positives. Dipyridamole or adenosine testing is relatively contraindicated with bronchospasm and COPD but is preferred over exercise or dobutamine for patients with a left bundle‐branch block. Suspected critical aortic stenosis is a contraindication to stress testing. Positive noninvasive findings should result in prophylactic measures, either medical therapy or an invasive procedure.
CORONARY REVASCULARIZATION
Coronary Artery Bypass Grafting
Observational studies have shown that patients with CAD (in the CASS study) treated by coronary artery bypass grafting (CABG) surgery versus had a lower mortality (0.9% vs. 2.4%) and fewer nonfatal myocardial infarctions (0.7% vs. 1.1%) than patients treated with medical therapy who underwent noncardiac surgery months or years later.13 This protective effect of CABG lasted approximately 46 years; however, there was no benefit for low‐risk noncardiac procedures. Furthermore, the risk of perioperative mortality (3%) and morbidity associated with the CABG itself was not taken into account, which would have negated its potential benefit.
Percutaneous Coronary Intervention
Several reports suggested that a previous percutaneous coronary intervention (PCI) was also associated with a lower risk of perioperative mortality and nonfatal myocardial infarction (MI) compared to historical controls. Early studies suggested that noncardiac surgery could be performed as early as 710 days after balloon angioplasty (BA). As bare‐metal stents gradually replaced BA, subsequent reports highlighted the increased risk of noncardiac surgery within 2 weeks14 and then within 46 weeks15 after stenting. This was primarily because of in‐stent thrombosis associated with premature discontinuation of dual antiplatelet therapy or increased major bleeding if this therapy was continued. The current recommendation is to wait at least 46 weeks after inserting a bare‐metal stent and to discontinue clopidogrel aspirin at least 5 days before surgery. A recent review from the Mayo Clinic16 found BA to be reasonably safe if patients require surgery soon after cardiac intervention (after 2 weeks).
More recently drug‐eluting stents (DESs) have become the standard; however, the recommendations for antiplatelet therapy (in the absence of surgery) are for a minimum of 23 months after sirolimus‐coated stents and at least 6 months after stents with paclitaxel. There has been very little in the published literature on patients undergoing noncardiac surgery after drug‐eluting stents. A small retrospective review suggested that patients whose DES had been placed a median of 260 days before surgery had few cardiac events in the perioperative period.17 The recommendations of a French task force did not provide strong guidance, probably because of a lack of evidence.18 The only prospective study of stenting and noncardiac surgery involved continuing antiplatelet therapy (or stopping it less than 3 days before surgery) and using unfractionated heparin or enoxaparin in 103 patients. Despite this therapy, 5 patients died, 12 had myocardial infarctions, 22 had elevation of troponin, but only 4 had major bleeding. Patients with stenting less than 35 days before surgery were at the greatest risk.19 In view of these findings, if noncardiac surgery must be performed within 2 months and the patient is appropriate for PCI, balloon angioplasty or a bare‐metal stent is preferred over DES implantation. If a patient has a DES in place (particularly if it has been fewer than 6 months since implantation) and requires noncardiac surgery, the optimal approach would be to continue at least one if not both antiplatelet agents through surgery; if this is not possible, bridging therapy with intravenous IIB/IIIA receptor blockers has been a suggested approach.10
Revascularization Versus No Revascularization: the CARP Trial
The only randomized controlled study to compare invasive and noninvasive strategies was the Coronary Artery Revascularization Prophylaxis (CARP) trial.20 More than 5800 patients with stable cardiac symptoms scheduled for elective nonvascular surgery in VA hospitals were screened, approximately 20% underwent coronary angiography, and 510 patients (9% of the original group) were randomized to PCI/CABG or no revascularization. Revascularization was associated with 1.7% mortality and a 5.8% nonfatal MI rate, and an additional 4% died after successful revascularization while awaiting vascular surgery. Short‐term outcomes were similar in both the revascularization and no revascularization groups (3% 30‐day mortality and 8%12% perioperative nonfatal MI). The primary outcome, long‐term mortality, also did not differ between the groups (22% vs. 23%) after an average follow‐up of 2.7 years. The investigators concluded on the basis of this data that prophylactic revascularization could not be recommended for patients with stable CAD undergoing elective vascular surgery. Of note is that both groups of patients in the CARP trial were given intensive medical therapy, with 84% on beta‐blockers, 54% on statins, 51% on ACE inhibitors, and 73% on aspirin, which may have made it difficult to show any significant benefit of revascularization. Other limitations of that study are that it was underpowered to detect a short‐term benefit and excluded patients with unstable or more severe cardiac symptoms or disease (left main disease, aortic stenosis, and severe left ventricular dysfunction). In any case, the results of this support the ACC guidelines, which state that prophylactic revascularization is rarely necessary just to get the patient through surgery.
If the goal of risk stratification is to determine which patients are at increased risk and if revascularization fails to lower that risk, various medical therapies, including beta‐blockers, alpha‐agonists, and statins, should be considered as risk‐reduction strategies.
PHARMACOLOGIC STRATEGIES
Cardioprotection with Adrenergic Modulation and Statin Therapy
Support for adrenergic modulation (with beta‐blockers and alpha‐agonists) to prevent postoperative cardiac complications has been the subject of a number of reviews, including our own.7, 8, 21 Initial enthusiasm22, 23 has been tempered, however, as evidence has evolved.
The results of a randomized trial published in abstract form24 showed no significant difference in rates of a combined end point of mortality, myocardial infarction, heart failure, and ventricular arrhythmia 30 days after vascular surgery of 500 patients randomized to metoprolol or placebo. Furthermore, in a randomized trial of 107 aortic surgery patients with no history of coronary disease, metoprolol started on admission and continued for 7 days did not significantly reduce cardiac events.25 In addition, a well‐designed meta‐analysis suggested that there are too few data to definitively determine whether perioperative beta‐blockade is efficacious.26 Finally, the results of a rigorously analyzed observational trial using administrative data from nearly 700,000 patients suggested that perioperative beta‐blockade was protective (reduced mortality) only in higher‐risk patients (eg, RCRI 2 points). In those at lower risk, beta blockade was associated with a higher risk of complications, even if the lower‐risk patients had only 1 risk factor of either diabetes or coronary disease.27
Trials of alpha adrenergic agonists have also been summarized in at least 2 meta‐analyses. One of these meta‐analyses reported alpha‐2 agonists reduced mortality by nearly half and reduced postoperative myocardial infarction by a third in vascular patients, but had no benefit in others.28 Another meta‐analysis calculated that 83 patients needed to be treated with alpha‐agonists to prevent one cardiac event,29 a number higher than that for beta‐blockers.
Data on the effectiveness of statins is accumulating. The results of 5 observational trials3034 and 1 randomized study35 suggest that patients receiving statin therapy at the time of surgery (and afterward) have a lower risk of having a cardiac event and lower mortality, with relative reductions in risk between 80%30 and 30%.32 In the 1 randomized trial, of 100 vascular surgery patients, 20 mg/day of atorvastatin was begun 1 month before surgery and continued for 45 days,35 with beta‐blockers included per protocol. This protocol reduced the combined outcome of cardiac mortality, myocardial infarction, stroke, or unstable angina, but the overall number of events was very small (4 patients vs. 13 patients, P = .03). However, no patient required discontinuation of the drug because of side effects.
HOW SHOULD I INCORPORATE EVIDENCE INTO PRACTICE?
Target Patients Most Likely to Benefit
Recent trends in evidence increasingly support the idea that lower‐risk subgroups (such as those with the minor criteria employed by Mangano) may not benefit from perioperative beta‐blockers and that only higher‐risk subgroups should be targeted. This general approach was recommended in recent guidelines from the AHA‐ACC,36 as well as in an extensive review of perioperative cardiac risk management.10 The strongest recommendations were to continue beta‐blockers in patients already on them and to give them to patients scheduled for vascular surgery who had ischemia on a stress test. The ACC also stated that beta‐blockers were probably recommended for patients with known CAD or high cardiac risk scheduled for intermediate‐ to high‐risk surgery. Recommendations for other groups were weaker or lacked sufficient evidence.36 At this point, it seems prudent to target high‐risk patients (RCRI 2), as well as those who would require beta‐blockers or statin therapy regardless (eg, patients with known coronary artery disease). There are no data to suggest that dose titration of statins is required before surgery.
Be Aware of How Harm Might Be Produced
Notwithstanding its limitations, results from the recent observational trial from Lindenauer raise important questions about the effectiveness of beta‐blockers in practice. That is, are beta‐blockers safe and effective when used in surgical patients outside the tightly controlled setting of a randomized trial? It is apparent how titrating beta‐blockers to a target heart rate without careful clinical assessment (as occurred in most RCTs) might lead to beta‐blockers being used to treat tachycardia related to hypovolemia, pain, anemia, bleeding, or early sepsis. Interestingly, beta‐blockers may be associated with higher risk in other settings as well,37 so potential harm in the perioperative period are not completely surprising.
Use a Protocol That Sticks as Close to the Evidence as Possible
To stay as close as possible to what the evidence shows for the use of beta‐blockers, this drug should be started early enough to allow dose titration and continued for at least 7 days and optimally 30 days after surgery (indefinitely, if a patient requires it long term), working to ensure that patients are physiologically beta‐blocked (eg, heart rate 5565) for as much of the time that they are being treated as possible. Two recent studies demonstrated the importance of tight heart rate control38, 39higher doses of beta‐blockers and tight heart rate control were associated with reduced perioperative myocardial ischemia and troponin T release, which might obviate the need for preoperative cardiac testing in intermediate‐risk patients undergoing vascular surgery. A recent placebo‐controlled, randomized trial40 suggested that a simple strategy of 4 days of transdermal and oral clonidine reduced perioperative ischemia and mortality. Although this approach is very useful for patients who cannot take pills by mouth, it would necessitate a switch to beta‐blockers for patients who need them long term. In addition, use of clonidine may be associated with a higher risk of withdrawal than cardioselective beta‐blockers. No prospective trials have compared beta‐blockers and alpha‐2 agonists. Both produce hypotension and bradycardia, improve pain control, and rarely produce adverse pulmonary effects.41 At the least, consultants should be clear in their recommendations about the start and stop dates for beta‐blockers and should ensure a smooth outpatient transition of patients for whom long‐term statin or beta‐blocker therapy is needed.
Be Ready to Adjust Your Practice as the Evidence Continues to Evolve
Far too few patients have been randomized to beta‐blockers, adrenergic modulation, or statin therapy to date to provide a reasonable estimate of their effects on mortality. As a result, although it seems likely that some subgroups benefit from one or more of these therapies, the degree of risk requiredand an optimal dosing scheduleremains a subject of intense debate. The results of perioperative trials of adrenergic modulators have consistently provided evidence supporting their use in other patient populations, but larger studies may not confirm a beneficial effect. Ongoing Canadian (POISE) and European trials (DECREASE IV) should address sample size limitations and provide information critical for clinicians caring for patients in this era of rapidly evolving evidence.
More than 33 million patients undergo surgery annually in the United States. Approximately 8 million of these patients either have known coronary artery disease or risk factors for it, and an estimated 50,000 patients sustain a perioperative myocardial infarction, with an additional 1 million developing another medical complication. An integrated comprehensive approach is necessary to risk‐stratify these patients in an attempt to reduce these complications.
The basic role of risk stratification is to identify those patients at increased risk for complications; however, we are looking for a small number of patients at high risk in a population of relatively low‐risk patients. Most surgical patients do well, and further diagnostic testing has a low yield in predicting those likely to have a complication (poor positive predictive value [PPV]). Our goal should be to determine the underlying potential triggers of cardiac complications and institute measures to prevent them. After briefly reviewing pathophysiology, risk indices, and guidelines for preoperative cardiac risk assessment and diagnostic testing, we will focus on risk reduction strategies including prophylactic revascularization (CABG/PCI) and medical therapy.
PATHOPHYSIOLOGY OF PERIOPERATIVE MYOCARDIAL INFARCTION
Perioperative myocardial infarctions result from myocardial ischemia or plaque rupture and coronary thrombosis.1 Myocardial ischemia may be caused by increased oxygen demand or decreased oxygen delivery. Surgical trauma, anesthesia, pain, hypothermia, and bleeding trigger a stress state. This in turn increases catecholamine release, leading to tachycardia, hypertension, and increased oxygen demand. Anesthesia, hypotension, bleeding, and anemia may produce hypoxia, with subsequently decreased delivery of oxygen. Surgical trauma initiates an inflammatory response, leading to plaque fissuring, and a hypercoagulable state, which can result in acute coronary thrombosis. Perioperative prophylaxis should target these potential triggers.
CARDIAC RISK INDICES AND GUIDELINES
Over the past 3 decades, a number of cardiac risk indices have been published. The older group of indices was most notable for Goldman's original cardiac risk index2 and Detsky's modification.3 The newer group consists of the American College of Physicians (ACP) guidelines4 (now considered outdated), the American College of Cardiology/American Heart Association (ACC/AHA) guidelines (to be updated again in early 2007),5 and the Lee revised cardiac risk index (RCRI).6
The 2002 ACC guidelines5 outline how to determine the need for additional cardiac (usually noninvasive) testing (NIT): after ascertaining the urgency of surgery, history of revascularization procedures, and previous stress test results (if any), a combination of clinical risk predictors, surgery‐specific risk, and patient self‐reported exercise capacity should be entered into an algorithm. The guidelines state a shortcut can be used: noninvasive testing should be considered if a patient has any 2 of the following: (1) intermediate clinical risk (stable angina or old MI, compensated heart failure, diabetes mellitus, renal insufficiency), (2) high‐risk surgery (aortic or major vascular procedures, prolonged surgery with significant expected blood loss or fluid shifts), or (3) poor exercise capacity (<4 METs). Patients with major clinical predictors (unstable coronary syndromes, decompensated heart failure, severe valvular heart disease, or hemodynamically significant arrhythmias) should not undergo elective surgery without further workup or treatment. The ACP guidelines use the Detsky3 modified CRI and low‐risk variables to suggest any need for further testing depending on type of surgery (vascular or nonvascular). At times these 2 guidelines offer conflicting recommendations, with the ACC more likely than the ACP to recommend NIT. The RCRI, which was developed prospectively and has been validated, uses 6 predictors of major cardiac complicationshigh‐risk surgery, coronary artery disease, stroke, congestive heart failure, diabetes mellitus requiring insulin, and serum creatinine > 2 mg/dL. Patients with 0 or 1 risk factors are considered at low risk, those with 2 risk factors at moderate risk, and those with 3 or more risk factors at high risk (10% complication rate). Although the RCRI does not make recommendations about whether to test, it has been incorporated into a number of algorithms combining risk stratification with recommendations about noninvasive testing as well as use of perioperative beta‐blockers.710 0
|
DIAGNOSTIC CARDIAC TESTS
Tests should not be done if the results will not alter patient management. If further assessment is indicated based on the ACC/AHA algorithm, other risk indices,10 or criteria independent of the need for surgery, the physician must decide whether to do a noninvasive (eg, echocardiogram or stress test) or an invasive test (coronary angiography). Unless a patient has independent criteria for angiography or, occasionally, a very high prior probability of significant CAD based on multiple risk factors, noninvasive testing is usually the preferred first step. A resting echocardiogram is potentially useful for providing information about suspected valvular heart disease but is not a consistent predictor of ischemic events.
For ambulatory patients, exercise stress testing is usually preferred over pharmacologic testing; in the perioperative setting, the usefulness of exercise testing is limited by the indications for obtaining stress testing (namely, poor functional status) as well as its main limitation, patient inability to reach 85% of the target heart rate. As a result, pharmacologic stress testing should be the primary modality for patients requiring preoperative risk stratification. Pharmacologic stress testing can be done with nuclear imaging (dipyridamole or adenosine thallium) or echocardiography (dobutamine echocardiography). For the most part, the results are comparable,11, 12 with both having excellent negative predictive values (NPV > 95%) but poor positive predictive values (PPV < 20%); however, dobutamine echocardiography tends to have fewer false positives. Dipyridamole or adenosine testing is relatively contraindicated with bronchospasm and COPD but is preferred over exercise or dobutamine for patients with a left bundle‐branch block. Suspected critical aortic stenosis is a contraindication to stress testing. Positive noninvasive findings should result in prophylactic measures, either medical therapy or an invasive procedure.
CORONARY REVASCULARIZATION
Coronary Artery Bypass Grafting
Observational studies have shown that patients with CAD (in the CASS study) treated by coronary artery bypass grafting (CABG) surgery versus had a lower mortality (0.9% vs. 2.4%) and fewer nonfatal myocardial infarctions (0.7% vs. 1.1%) than patients treated with medical therapy who underwent noncardiac surgery months or years later.13 This protective effect of CABG lasted approximately 46 years; however, there was no benefit for low‐risk noncardiac procedures. Furthermore, the risk of perioperative mortality (3%) and morbidity associated with the CABG itself was not taken into account, which would have negated its potential benefit.
Percutaneous Coronary Intervention
Several reports suggested that a previous percutaneous coronary intervention (PCI) was also associated with a lower risk of perioperative mortality and nonfatal myocardial infarction (MI) compared to historical controls. Early studies suggested that noncardiac surgery could be performed as early as 710 days after balloon angioplasty (BA). As bare‐metal stents gradually replaced BA, subsequent reports highlighted the increased risk of noncardiac surgery within 2 weeks14 and then within 46 weeks15 after stenting. This was primarily because of in‐stent thrombosis associated with premature discontinuation of dual antiplatelet therapy or increased major bleeding if this therapy was continued. The current recommendation is to wait at least 46 weeks after inserting a bare‐metal stent and to discontinue clopidogrel aspirin at least 5 days before surgery. A recent review from the Mayo Clinic16 found BA to be reasonably safe if patients require surgery soon after cardiac intervention (after 2 weeks).
More recently drug‐eluting stents (DESs) have become the standard; however, the recommendations for antiplatelet therapy (in the absence of surgery) are for a minimum of 23 months after sirolimus‐coated stents and at least 6 months after stents with paclitaxel. There has been very little in the published literature on patients undergoing noncardiac surgery after drug‐eluting stents. A small retrospective review suggested that patients whose DES had been placed a median of 260 days before surgery had few cardiac events in the perioperative period.17 The recommendations of a French task force did not provide strong guidance, probably because of a lack of evidence.18 The only prospective study of stenting and noncardiac surgery involved continuing antiplatelet therapy (or stopping it less than 3 days before surgery) and using unfractionated heparin or enoxaparin in 103 patients. Despite this therapy, 5 patients died, 12 had myocardial infarctions, 22 had elevation of troponin, but only 4 had major bleeding. Patients with stenting less than 35 days before surgery were at the greatest risk.19 In view of these findings, if noncardiac surgery must be performed within 2 months and the patient is appropriate for PCI, balloon angioplasty or a bare‐metal stent is preferred over DES implantation. If a patient has a DES in place (particularly if it has been fewer than 6 months since implantation) and requires noncardiac surgery, the optimal approach would be to continue at least one if not both antiplatelet agents through surgery; if this is not possible, bridging therapy with intravenous IIB/IIIA receptor blockers has been a suggested approach.10
Revascularization Versus No Revascularization: the CARP Trial
The only randomized controlled study to compare invasive and noninvasive strategies was the Coronary Artery Revascularization Prophylaxis (CARP) trial.20 More than 5800 patients with stable cardiac symptoms scheduled for elective nonvascular surgery in VA hospitals were screened, approximately 20% underwent coronary angiography, and 510 patients (9% of the original group) were randomized to PCI/CABG or no revascularization. Revascularization was associated with 1.7% mortality and a 5.8% nonfatal MI rate, and an additional 4% died after successful revascularization while awaiting vascular surgery. Short‐term outcomes were similar in both the revascularization and no revascularization groups (3% 30‐day mortality and 8%12% perioperative nonfatal MI). The primary outcome, long‐term mortality, also did not differ between the groups (22% vs. 23%) after an average follow‐up of 2.7 years. The investigators concluded on the basis of this data that prophylactic revascularization could not be recommended for patients with stable CAD undergoing elective vascular surgery. Of note is that both groups of patients in the CARP trial were given intensive medical therapy, with 84% on beta‐blockers, 54% on statins, 51% on ACE inhibitors, and 73% on aspirin, which may have made it difficult to show any significant benefit of revascularization. Other limitations of that study are that it was underpowered to detect a short‐term benefit and excluded patients with unstable or more severe cardiac symptoms or disease (left main disease, aortic stenosis, and severe left ventricular dysfunction). In any case, the results of this support the ACC guidelines, which state that prophylactic revascularization is rarely necessary just to get the patient through surgery.
If the goal of risk stratification is to determine which patients are at increased risk and if revascularization fails to lower that risk, various medical therapies, including beta‐blockers, alpha‐agonists, and statins, should be considered as risk‐reduction strategies.
PHARMACOLOGIC STRATEGIES
Cardioprotection with Adrenergic Modulation and Statin Therapy
Support for adrenergic modulation (with beta‐blockers and alpha‐agonists) to prevent postoperative cardiac complications has been the subject of a number of reviews, including our own.7, 8, 21 Initial enthusiasm22, 23 has been tempered, however, as evidence has evolved.
The results of a randomized trial published in abstract form24 showed no significant difference in rates of a combined end point of mortality, myocardial infarction, heart failure, and ventricular arrhythmia 30 days after vascular surgery of 500 patients randomized to metoprolol or placebo. Furthermore, in a randomized trial of 107 aortic surgery patients with no history of coronary disease, metoprolol started on admission and continued for 7 days did not significantly reduce cardiac events.25 In addition, a well‐designed meta‐analysis suggested that there are too few data to definitively determine whether perioperative beta‐blockade is efficacious.26 Finally, the results of a rigorously analyzed observational trial using administrative data from nearly 700,000 patients suggested that perioperative beta‐blockade was protective (reduced mortality) only in higher‐risk patients (eg, RCRI 2 points). In those at lower risk, beta blockade was associated with a higher risk of complications, even if the lower‐risk patients had only 1 risk factor of either diabetes or coronary disease.27
Trials of alpha adrenergic agonists have also been summarized in at least 2 meta‐analyses. One of these meta‐analyses reported alpha‐2 agonists reduced mortality by nearly half and reduced postoperative myocardial infarction by a third in vascular patients, but had no benefit in others.28 Another meta‐analysis calculated that 83 patients needed to be treated with alpha‐agonists to prevent one cardiac event,29 a number higher than that for beta‐blockers.
Data on the effectiveness of statins is accumulating. The results of 5 observational trials3034 and 1 randomized study35 suggest that patients receiving statin therapy at the time of surgery (and afterward) have a lower risk of having a cardiac event and lower mortality, with relative reductions in risk between 80%30 and 30%.32 In the 1 randomized trial, of 100 vascular surgery patients, 20 mg/day of atorvastatin was begun 1 month before surgery and continued for 45 days,35 with beta‐blockers included per protocol. This protocol reduced the combined outcome of cardiac mortality, myocardial infarction, stroke, or unstable angina, but the overall number of events was very small (4 patients vs. 13 patients, P = .03). However, no patient required discontinuation of the drug because of side effects.
HOW SHOULD I INCORPORATE EVIDENCE INTO PRACTICE?
Target Patients Most Likely to Benefit
Recent trends in evidence increasingly support the idea that lower‐risk subgroups (such as those with the minor criteria employed by Mangano) may not benefit from perioperative beta‐blockers and that only higher‐risk subgroups should be targeted. This general approach was recommended in recent guidelines from the AHA‐ACC,36 as well as in an extensive review of perioperative cardiac risk management.10 The strongest recommendations were to continue beta‐blockers in patients already on them and to give them to patients scheduled for vascular surgery who had ischemia on a stress test. The ACC also stated that beta‐blockers were probably recommended for patients with known CAD or high cardiac risk scheduled for intermediate‐ to high‐risk surgery. Recommendations for other groups were weaker or lacked sufficient evidence.36 At this point, it seems prudent to target high‐risk patients (RCRI 2), as well as those who would require beta‐blockers or statin therapy regardless (eg, patients with known coronary artery disease). There are no data to suggest that dose titration of statins is required before surgery.
Be Aware of How Harm Might Be Produced
Notwithstanding its limitations, results from the recent observational trial from Lindenauer raise important questions about the effectiveness of beta‐blockers in practice. That is, are beta‐blockers safe and effective when used in surgical patients outside the tightly controlled setting of a randomized trial? It is apparent how titrating beta‐blockers to a target heart rate without careful clinical assessment (as occurred in most RCTs) might lead to beta‐blockers being used to treat tachycardia related to hypovolemia, pain, anemia, bleeding, or early sepsis. Interestingly, beta‐blockers may be associated with higher risk in other settings as well,37 so potential harm in the perioperative period are not completely surprising.
Use a Protocol That Sticks as Close to the Evidence as Possible
To stay as close as possible to what the evidence shows for the use of beta‐blockers, this drug should be started early enough to allow dose titration and continued for at least 7 days and optimally 30 days after surgery (indefinitely, if a patient requires it long term), working to ensure that patients are physiologically beta‐blocked (eg, heart rate 5565) for as much of the time that they are being treated as possible. Two recent studies demonstrated the importance of tight heart rate control38, 39higher doses of beta‐blockers and tight heart rate control were associated with reduced perioperative myocardial ischemia and troponin T release, which might obviate the need for preoperative cardiac testing in intermediate‐risk patients undergoing vascular surgery. A recent placebo‐controlled, randomized trial40 suggested that a simple strategy of 4 days of transdermal and oral clonidine reduced perioperative ischemia and mortality. Although this approach is very useful for patients who cannot take pills by mouth, it would necessitate a switch to beta‐blockers for patients who need them long term. In addition, use of clonidine may be associated with a higher risk of withdrawal than cardioselective beta‐blockers. No prospective trials have compared beta‐blockers and alpha‐2 agonists. Both produce hypotension and bradycardia, improve pain control, and rarely produce adverse pulmonary effects.41 At the least, consultants should be clear in their recommendations about the start and stop dates for beta‐blockers and should ensure a smooth outpatient transition of patients for whom long‐term statin or beta‐blocker therapy is needed.
Be Ready to Adjust Your Practice as the Evidence Continues to Evolve
Far too few patients have been randomized to beta‐blockers, adrenergic modulation, or statin therapy to date to provide a reasonable estimate of their effects on mortality. As a result, although it seems likely that some subgroups benefit from one or more of these therapies, the degree of risk requiredand an optimal dosing scheduleremains a subject of intense debate. The results of perioperative trials of adrenergic modulators have consistently provided evidence supporting their use in other patient populations, but larger studies may not confirm a beneficial effect. Ongoing Canadian (POISE) and European trials (DECREASE IV) should address sample size limitations and provide information critical for clinicians caring for patients in this era of rapidly evolving evidence.
- ,,,,,.Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: A review.CMAJ.2005;173:779–788.
- ,,, et al.Multifactorial index of cardiac risk in noncardiac surgical procedures.N Engl J Med.1977;297:845–850.
- ,,,,.Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index.Arch Intern Med.1986;146:2131–2134.
- ,.Perioperative assessment and management of risk from coronary artery disease.Ann Intern Med.1997;127:313–328.
- ,,, et al.ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery).Circulation.2002;105:1257–1267.
- .Reducing cardiac risk in noncardiac surgery.N Engl J Med.1999;341:1838–1840.
- ,.beta‐Blockers and reduction of cardiac events in noncardiac surgery: scientific review.JAMA.2002;287:1435–1444.
- ,.Clinical practice. Lowering cardiac risk in noncardiac surgery.N Engl J Med.2001;345:1677–1682.
- ,,, et al.Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy.JAMA.2001;285:1865–1873.
- ,.Assessing and reducing the cardiac risk of noncardiac surgery.Circulation.2006;113:1361–1376.
- ,,, et al.A meta‐analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery.Heart.2003;89:1327–1334.
- ,,,.Exercise echocardiography or exercise SPECT imaging? A meta‐analysis of diagnostic test performance.JAMA.1998;280:913–920.
- ,,,,,.Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study.Circulation.1997;96:1882–1887.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,, et al.Outcome of patients undergoing balloon angioplasty in the two months prior to noncardiac surgery.Am J Cardiol.2005;96:512–514.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,.Perioperative management of antiplatelet agents in patients with coronary stents: recommendations of a French Task Force.Br J Anaesth.2006;97:580–582.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,.Perioperative cardiac assessment for noncardiac surgery: eight steps to the best possible outcome.Circulation.2003;107:2771–2774.
- .Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.N Engl J Med.1997;336:1452; discussion1453–1454.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Metoprolol after vascular surgery (MAVS).Can J Anesth.2004;51:A7.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,.Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review.Anesth Analg.2003;97:623–633.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA.2004;291:2092–2099.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery The Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,, et al.Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial.JAMA.2001;285:1711–1718.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion975–976.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,.Atenolol in hypertension: is it a wise choice?Lancet.2004;364:1684–1689.
- ,,, et al.Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate‐risk patients receiving beta‐blocker therapy with tight heart rate control?J Am Coll Cardiol.2006;48:964–969.
- ,,, et al.High‐dose beta‐blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients.Circulation.2006;114:1344–1349.
- ,,, et al.Effect of clonidine on cardiovascular morbidity and mortality after noncardiac surgery.Anesthesiology.2004;101:284–293.
- ,,.Cardioselective beta‐blockers in patients with reactive airway disease: a meta‐analysis.Ann Intern Med.2002;137:715–725.
- ,,.Does this patient have an abnormal systolic murmur?JAMA.1997;277:564–571.
- ,,,,,.Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: A review.CMAJ.2005;173:779–788.
- ,,, et al.Multifactorial index of cardiac risk in noncardiac surgical procedures.N Engl J Med.1977;297:845–850.
- ,,,,.Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index.Arch Intern Med.1986;146:2131–2134.
- ,.Perioperative assessment and management of risk from coronary artery disease.Ann Intern Med.1997;127:313–328.
- ,,, et al.ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery).Circulation.2002;105:1257–1267.
- .Reducing cardiac risk in noncardiac surgery.N Engl J Med.1999;341:1838–1840.
- ,.beta‐Blockers and reduction of cardiac events in noncardiac surgery: scientific review.JAMA.2002;287:1435–1444.
- ,.Clinical practice. Lowering cardiac risk in noncardiac surgery.N Engl J Med.2001;345:1677–1682.
- ,,, et al.Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta‐blocker therapy.JAMA.2001;285:1865–1873.
- ,.Assessing and reducing the cardiac risk of noncardiac surgery.Circulation.2006;113:1361–1376.
- ,,, et al.A meta‐analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery.Heart.2003;89:1327–1334.
- ,,,.Exercise echocardiography or exercise SPECT imaging? A meta‐analysis of diagnostic test performance.JAMA.1998;280:913–920.
- ,,,,,.Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study.Circulation.1997;96:1882–1887.
- ,,,,.Catastrophic outcomes of noncardiac surgery soon after coronary stenting.J Am Coll Cardiol.2000;35:1288–1294.
- ,,, et al.Clinical outcome of patients undergoing non‐cardiac surgery in the two months following coronary stenting.J Am Coll Cardiol.2003;42:234–240.
- ,,, et al.Outcome of patients undergoing balloon angioplasty in the two months prior to noncardiac surgery.Am J Cardiol.2005;96:512–514.
- ,,,,.Risk of noncardiac surgery after coronary drug‐eluting stent implantation.Am J Cardiol.2006;98:1212–1213.
- ,,,.Perioperative management of antiplatelet agents in patients with coronary stents: recommendations of a French Task Force.Br J Anaesth.2006;97:580–582.
- ,,,,,.Coronary artery stenting and non‐cardiac surgery—a prospective outcome study.Br J Anaesth.2006;96:686–693.
- ,,, et al.Coronary‐artery revascularization before elective major vascular surgery.N Engl J Med.2004;351:2795–2804.
- ,.Perioperative cardiac assessment for noncardiac surgery: eight steps to the best possible outcome.Circulation.2003;107:2771–2774.
- .Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery.N Engl J Med.1997;336:1452; discussion1453–1454.
- ,,, et al.The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery.Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group.N Engl J Med.1999;341:1789–1794.
- ,,,,.Metoprolol after vascular surgery (MAVS).Can J Anesth.2004;51:A7.
- ,,,,.Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial.J Vasc Surg.2005;41:602–609.
- ,,, et al.How strong is the evidence for the use of perioperative beta blockers in non‐cardiac surgery? Systematic review and meta‐analysis of randomised controlled trials.BMJ.2005;331:313–321.
- ,,,,,.Perioperative beta‐blocker therapy and mortality after major noncardiac surgery.N Engl J Med.2005;353:349–361.
- ,,.Alpha‐2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta‐analysis.Am J Med.2003;114:742–752.
- ,,.Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review.Anesth Analg.2003;97:623–633.
- ,,, et al.A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery.Eur J Vasc Endovasc Surg.2004;28:343–352.
- ,,,,.Lipid‐lowering therapy and in‐hospital mortality following major noncardiac surgery.JAMA.2004;291:2092–2099.
- ,,, et al.Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery The Statins for Risk Reduction in Surgery (StaRRS) study.J Am Coll Cardiol.2005;45:336–342.
- ,,, et al.Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery.Circulation.2003;107:1848–1851.
- ,,, et al.Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial.JAMA.2001;285:1711–1718.
- ,,, et al.Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial.J Vasc Surg.2004;39:967–975; discussion975–976.
- ,,, et al.ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology.Circulation.2006;113:2662–2674.
- ,,.Atenolol in hypertension: is it a wise choice?Lancet.2004;364:1684–1689.
- ,,, et al.Should major vascular surgery be delayed because of preoperative cardiac testing in intermediate‐risk patients receiving beta‐blocker therapy with tight heart rate control?J Am Coll Cardiol.2006;48:964–969.
- ,,, et al.High‐dose beta‐blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients.Circulation.2006;114:1344–1349.
- ,,, et al.Effect of clonidine on cardiovascular morbidity and mortality after noncardiac surgery.Anesthesiology.2004;101:284–293.
- ,,.Cardioselective beta‐blockers in patients with reactive airway disease: a meta‐analysis.Ann Intern Med.2002;137:715–725.
- ,,.Does this patient have an abnormal systolic murmur?JAMA.1997;277:564–571.
Impact of CT on PE Diagnosis
Spiral computed tomographic pulmonary angiography (CTPA) is a common first‐line test for the evaluation of suspected pulmonary embolism (PE). At our institution CTPA became the initial diagnostic study in 83% of patients with suspected PE within 3 years of the introduction of CT,1 and by 2001 CTPA had become the most common diagnostic test performed nationwide in patients diagnosed with PE.2 Most scans are interpreted as either positive or negative for pulmonary embolism, providing clinicians with a greater sense of diagnostic certainty than with the probabilistic results of lung scintigraphy. Initial studies of CTPA supported this appearance of diagnostic certainty, reporting sensitivity and specificity of greater than 90%,3, 4 but several subsequent studies have failed to reproduce these results.57 Newer multidetector CT scans are believed to be more accurate than earlier single‐detector CT,8 but true estimates of CTPA test characteristics will not be known until publication of the forthcoming PIOPED II study.9
Even without these data, CT‐based diagnostic algorithms have already appeared.1014 These algorithms generally focus on minimizing the false‐negative rate through use of serial testing (involving combinations of serum D‐dimer, lower‐extremity ultrasound, and CTPA). A recent meta‐analysis demonstrated that negative CTPA is highly accurate at ruling out PE, with test characteristics similar to conventional pulmonary angiography.15 Another meta‐analysis found that the 3‐month rate of subsequent venous thromboembolism after negative CTPA was 1.4% (95% CI 1.1%‐1.8%),16 supporting the strategy of withholding anticoagulants after negative CTPA in combination with other tests. However, use of serial testing to establish the diagnosis of PE and initiate anticoagulation has not been systematically evaluated or recommended, even for patients with a low pretest probability of PE.17
To assess the potential impact of these algorithms on the diagnosis of PE in clinical practice, we analyzed the clinical presentation and treatment of a cohort of patients at our institution who underwent CTPA for suspected PE.1 We calculated a range of posttest probabilities for pulmonary embolism for these patients, given the pretest probabilities, test results, and estimates of CTPA test characteristics. We then compared the treatment decisions of clinicians to the posttest probabilities of PE in order to establish the potential frequency of false‐positive and false‐negative diagnoses and to determine if patients were treated appropriately based on these estimates.
METHODS
Sites and Subjects
Details of the sites, subjects, and methods used to collect patient‐level data in this analysis have been previously published.1 The study was performed at Moffitt‐Long Hospital and San Francisco General Hospital, teaching hospitals affiliated with the University of California San Francisco School of Medicine. At both sites, single‐detector CT scans were available 24 hours a day throughout the study period and were read by attending radiologists who specialized in thoracic imaging. We excluded patients whose CTPA was not completed as the initial test in the evaluation of suspected PE, those who underwent testing for any indication other than suspected acute PE, and those with incomplete medical records or technically inadequate CTPA.
We randomly selected 345 patients who underwent CTPA between January 1, 1998, and December 31, 2000, from the Radiology Department databases. One investigator (R.L.T.) then abstracted charts of all patients. For each subject, we collected data about history and clinical presentation, diagnostic impressions of the treating clinicians, treatments administered both before and after diagnostic testing, CTPA result, results of other diagnostic tests for PE, and final clinical diagnosis. During the study period, there were no institution‐ or department‐specific guidelines or decision aids available for the diagnosis of PE. Ventilation‐perfusion scan, lower extremity ultrasound, and pulmonary angiography were available, but highly sensitive D‐dimer assays were not in use. The study was approved by the Institutional Review Boards of both sites, and requirement for written informed consent from patients was waived.
Estimates of Pretest Probabilities of Pulmonary Embolism and CTPA Test Characteristics
Several prediction rules1820 generate clinical pretest probabilities for patients with suspected PE. We used the Wells score18 to assign a pretest probability of low, moderate, or high to each patient on the basis of the following clinical variables: leg swelling, hemoptysis, tachycardia, history of recent immobilization, history of prior DVT or PE, active malignancy, and lack of a more likely alternative diagnosis. We chose this rule as (unlike other prediction rules such as the Geneva rule20) the Wells score has been validated for hospitalized patients with suspected PE and does not require arterial blood gas measurements. The prevalence of PE reported in the evaluation of the Wells score was 3.4%, 27.8%, and 78.3% for low, moderate, and high pretest probabilities, respectively.18
As in our previous study,1 we assumed CTPA to be 90% sensitive and 95% specific based on published estimates.3, 17 These values correspond to a positive likelihood ratio of 18 and a negative likelihood ratio of 0.1.21 We chose these values as a best‐case estimate of the test characteristics of CTPA, although other studies have found less impressive results.7 Using these pretest probabilities and likelihood ratios, we then used Bayes' theorem (Figure 1) to calculate the range of expected posttest probabilities of pulmonary embolism.
Calculation of Posttest Probabilities and Comparison to Treatment Outcomes
For each pretest probability category, we used the posttest probabilities calculated above to determine the number of true‐positive pulmonary emboli, as follows:
RESULTS
Patient Characteristics
After excluding 23 patients receiving anticoagulants for other indications prior to CTPA, the study cohort included 322 patients (57.7% female), with an average age of 58.6 years, of whom 20.5% had cancer and 4.5% had a prior history of thromboembolic disease. Scans were primarily ordered by the medicine service (47.7% of cases) and emergency department (22.9%). CTPA was the initial test for 9% of patients evaluated for suspected acute PE during the first 6 months of the study period, increasing to 83% by the end of 2000.1 The overall pretest probability distribution remained the same throughout the entire study period.1
Test Results and Treatment Decisions
Most patients in our cohort had a low (n = 184, 57.1%) or a moderate (n = 101, 31.4%) pretest probability of PE (Table 1). The likelihood of a positive CTPA increased as the pretest probability increased, but even among patients with high clinical risk, only 35.1% had positive CT scans. In total, scans were positive in 57 patients and negative in 265 patients. Clinicians treated 55 patients with a positive CTPA (96.5%); none of these patients underwent additional testing for DVT or PE after the imaging study. Among patients with a negative CTPA, 254 (95.8%) were not treated; none of the patients in whom anticoagulation was withheld underwent further testing, whereas the other 11 patients were treated on the basis of other tests (5 high‐probability ventilation‐perfusion scans, 3 positive leg ultrasounds, and 3 for unclear reasons). Overall, 66 patients (20.5%) were treated for pulmonary embolism.
| Pretest probability of PE (number of CTPA performed) | Low (N = 184) | Moderate (N = 101) | High (N = 37) | Total (N = 322) |
|---|---|---|---|---|
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| CTPA negative for PE (% of pretest probability group) | 162 (88.0%) | 79 (78.2%) | 24 (64.9%) | 265 (82.3%) |
| Patients with positive CT subsequently treated for PE (% of pretest probability group) | 21 (11.4%) | 21 (20.8%) | 13 (35.1%) | 55 (17.1%) |
| Patients treated for PE despite negative CT (% of pretest probability group) | 5 (2.7%) | 3 (3.0%) | 3 (8.1%) | 11 (3.4%) |
| Total patients treated for PE (% of pretest probability group) | 26 (14.1%) | 24 (23.8%) | 16 (43.2%) | 66 (20.5%) |
Literature‐Derived Estimates of Posttest Probabilities of Pulmonary Embolism
Patients who have a low pretest probability of PE and a positive CTPA have a posttest probability of 41.6% under our estimate of CTPA test characteristics. Patients with moderate pretest probability have a posttest probability of 87.4% and patients with a high pretest probability will have a 98.5% probability of embolism with a positive scan. The traditional treatment threshold for PE is a posttest probability of 90%.22
Observed Versus Expected PE Rates and Subsequent Treatment
Only 9 of the 22 patients (41%) with a low pretest probability and a positive CTPA likely represent true‐positive emboli. However, clinicians chose to treat 21 of the 22 patients with this combination of pretest probability and imaging findings. Thus, 12 emboli would be considered possible false‐positive diagnoses. Similarly, in the moderate pretest probability group, 2 of 21 patients with moderate pretest probability and 0 of 13 patients with high pretest probability treated for PE had a possibly false‐positive diagnosis. Thus, in total, 25.4% (14 of 55) patients treated for PE had a possible false‐positive diagnosis of pulmonary embolism and may have been unnecessarily administered anticoagulants (Table 2). All patients who potentially had a false‐positive PE had either a low or moderate pretest probability of PE; in fact, the majority (57.1%) of patients with a low pretest probability of PE who were subsequently treated for PE likely had a false‐positive diagnosis.
| Pretest probability | ||||
|---|---|---|---|---|
| Low (n = 184) | Moderate (n = 101) | High (n = 37) | Total (n = 322) | |
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| Patients with positive CTPA treated for pulmonary embolism (n, % treated in risk group) | 21 (95.4%) | 21 (95.4%) | 13 (100%) | 55 (96.5%) |
| Calculated number and rate of probable true‐positive evaluations | ||||
| Number of true‐positive PE (n, % treated in risk group) | 9 (42.9%) | 19 (90.5%) | 13 (100%) | 41 (74.6%) |
| Calculated number and rate of possible false‐positive evaluations | ||||
| Number of possible false‐positive PE (n, % in risk group with unexpected PE) | 12 (58.1%) | 2 (9.5%) | 0 | 14 (25.4%) |
Clinicians were more likely to overtreat a patient with a possible false‐positive CT scan than to withhold treatment from a patient with a possible false‐negative diagnosis. Using the same estimates of CTPA test characteristics, the incidence of possible false‐negative diagnosis of PE was 1.6% (4 possible false‐negative diagnoses among 254 patients with negative CTPA results who were not treated for PE.) All these patients had a high pretest probability of PE.
DISCUSSION
Physicians at our institution regarded CTPA results as definitive, anticoagulating 96.5% of patients with a positive CT and withholding treatment in 95.8% of patients with a negative scan. This practice pattern may result in unnecessary anticoagulation of many patients with a low pretest probability of PE who may have had false‐positive CTPA findings. In contrast, the rate of possible false‐negative diagnosis of PE was low, consistent with the results of several other studies.16
The use of CTPA is likely to increase because of the publication of multiple algorithms advocating that CTPA be the chief imaging study used in the diagnosis of PE.1014 These algorithms recommend serial testing on patients with a negative CTPA in order to minimize the false‐negative rate, but they do not require systematic follow‐up in patients with a positive scan, even if the pretest probability was low. In management trials, this approach resulted in a low false‐negative rate (1.0%‐1.8% at 3‐month follow‐up).1114 However, the rate of major bleeding in patients treated for PE was 3.2%‐6.0% at 3 months,1214 illustrating the potential risk of anticoagulating patients who may have false‐positive diagnoses. Furthermore, premature diagnostic closure after a CTPA positive for PE may result in additional morbidity as a result of missing the true diagnosis.
One potential explanation for the large number of potential false‐positive emboli seen in low‐risk patients is that it is difficult to accurately diagnose distal pulmonary emboli with CTPA. The interrater reliability of CTPA for diagnosis of subsegmental PE is suboptimal,23 and the clinical significance of these emboli remains uncertain.24 Thus, many emboli found in patients with low pretest probability actually may have been subsegmental PE that would not have been diagnosed by another radiologist. As CTPA is more accurate for diagnosing central PE,25 clinicians should consider reviewing positive scans with the interpreting radiologist, especially when the pretest probability was low and the filling defects identified are in distal vessels.
Our results may also illustrate that clinicians have a lower treatment threshold when presented with apparently definitive evidence of pulmonary embolism. Previous proposals on the appropriate treatment threshold for PE, which used Bayesian decision‐making methods similar to ours,22 incorporated PIOPED26 data on the pretest probability of pulmonary embolism, the test characteristics of ventilation‐perfusion scans, and the clinical outcomes of patients in each test result/pretest probability category. However, there is no corresponding data for CTPA, as its test characteristics are still uncertain, and long‐term clinical outcomes have not been documented for patients treated (or not treated) on the basis of CT results.
Our study had several limitations. First, charting bias potentially was introduced by our using a retrospective method of collecting data for calculating pretest probabilities. To address this potential bias, we collected data from the entire medical record, including information available at and preceding the time of the CT scan. We believe this method was effective, as the range of pretest probabilities and the prevalence of PE in our study were very similar to those seen in a number of prospective studies.1820, 26, 27 Although other risk indices exist, the Wells score has been shown to have predictive powers equal to other algorithms and to clinicians; implicit assessments.28, 29 In our cohort, 35.1% of patients with a high pretest probability were diagnosed with PE; although this was lower than that in the initial Wells cohort,18 it was very similar to a subsequent validation study using the Wells algorithm, in which the prevalence of PE in patients with high pretest probability was 37.5%.27 Plasma D‐dimer testing is not routinely used at our hospitals, but it is a component of some CTPA‐based diagnostic algorithms.1114 Although use of D‐dimer testing may have led to fewer scans in patients with negative D‐dimer test results and low pretest probability,30 the high false‐positive rate for D‐dimer assays31 makes it difficult to predict the effect of widespread D‐dimer use on the overall pretest probability distribution. Using our assumptions about CT test characteristics, a pretest probability of more than 30% is required to generate a posttest probability of PE of at least 90% (the traditional treatment threshold for anticoagulant therapy22) with a positive scan. Extensive D‐dimer use would be unlikely to cause such a shift in the distribution of pretest probabilities.
Finally, CT technology has continued to advance, and many institutions now use 64‐slice scanners32 in contrast to the single‐slice scanners in use at the time our data were collected. Our assumptions were that CTPA has a positive likelihood ratio of 18.0 and a negative likelihood ratio of 0.1 (corresponding to a sensitivity of 90% and a specificity of 95%), although many studies of single‐detector CTPA found less impressive values.5, 7 Multidetector CT is thought to be more accurate than was earlier technology, but the true diagnostic performance of multidetector CT is not yet known. However, our findings pertain primarily to clinicians' responses to test results, so even if newer scanners are more accurate, Bayesian analysis will still be required in order to appropriately treat patients. A recent meta‐analysis of diagnostic strategies for PE found CTPA to have a positive likelihood ratio of 24.1, but even using this higher value, patients with a low pretest probability and positive CTPA still have a posttest probability of PE below the traditional treatment threshold.33 As most patients undergoing evaluation for suspected PE have a low pretest probability,17 a substantial number of false‐positive diagnoses of PE may still occur, even with a more accurate diagnostic test.
CT pulmonary angiography has become the first‐line test for pulmonary embolism at our institution, a situation likely mirrored elsewhere. CTPA is safe and rapid and offers the advantage of revealing ancillary lung findings that may be clinically significant.12 Although the test is an important addition to a clinician's diagnostic armamentarium, Bayesian analysis must be used to interpret its results, especially when CTPA is used as the first‐line diagnostic test. Our data raise the troubling concern that reliance on CTPA as the sole diagnostic test for suspected pulmonary embolism may result in a large number of patients with false‐positive CT scans receiving anticoagulation treatment.
- ,,,,.The impact of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- ,,.Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism.Am J Cardiol.2004;93:1316–1317.
- ,,,.Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐old technique—comparison with pulmonary angiography.Radiology.1992;185:381–387.
- ,,, et al.Pulmonary embolism: validation of spiral CT angiography in 149 patients.Radiology.1996;201:467–470.
- ,,,,.Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta‐analysis.Clin Appl Thromb Hemost.2001;7(2):87–92.
- ,,,.The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism.Arch Intern Med.2000;160(3):293–298.
- ,,.Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review.Ann Intern Med.2000;132(3):227–232.
- ,,, et al.Suspected acute pulmonary embolism: evaluation with multi‐detector row CT versus digital subtraction pulmonary arteriography.Radiology.2004;233:806–815.
- ,,,.Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II.Semin Nucl Med.2002;32(3):173–182.
- ,,, et al.Management of suspected pulmonary embolism (PE) by D‐dimer and multi‐slice computed tomography in outpatients: an outcome study.J Thromb Haemost.2005;3:1926–1932.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,, et al.Single‐detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients.Ann Intern Med.2003;138:307–314.
- ,,, et al.Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study.Lancet.2002;260:1914–1920.
- ,,, et al.Diagnosing pulmonary embolism in outpatients with clinical assessment, D‐dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study.Am J Med.2004;116:291–299.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review.JAMA.2005;293:2012–2017.
- ,,,.Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,.Clinical Practice: The evaluation of suspected pulmonary embolism.N Engl J Med.2003;349:1247–1256.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,.A structured clinical model for predicting the probability of pulmonary embolism.Am J Med.2003;114(3):173–179.
- ,,,,.Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161(1):92–97.
- ,,,.Interpretation of diagnostic tests and strategies for their use in quantitative decision making. In:Diagnostic strategies for common medical problems.Philadelphia, PA:American College of Physicians,1999.
- ,,,.Strategy for diagnosis of patients with suspected acute pulmonary embolism.Chest.1993;103:1553–1559.
- ,,, et al.Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement.Eur Radiol.2003;13:823–829.
- ,.Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy.Chest.1995;108:978–981.
- ,,, et al.Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism.Ann Intern Med.2001;135(2):88–97.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED).The PIOPED Investigators.JAMA.1990;263:2753–2759.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer.Ann Intern Med.2001;135(2):98–107.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,, et al.Does this patient have pulmonary embolism?JAMA.2003;290:2849–2858.
- ,,,,.Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review.Ann Intern Med.2003;138:941–951.
- ,,, et al.D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review.Ann Intern Med.2004;140:589–602.
- .Multislice computed tomography for pulmonary embolism—a technological marvel.N Engl J Med2005;352(17):1812–4.
- ,,,,,.Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism.Br Med J.2005;331:259.
Spiral computed tomographic pulmonary angiography (CTPA) is a common first‐line test for the evaluation of suspected pulmonary embolism (PE). At our institution CTPA became the initial diagnostic study in 83% of patients with suspected PE within 3 years of the introduction of CT,1 and by 2001 CTPA had become the most common diagnostic test performed nationwide in patients diagnosed with PE.2 Most scans are interpreted as either positive or negative for pulmonary embolism, providing clinicians with a greater sense of diagnostic certainty than with the probabilistic results of lung scintigraphy. Initial studies of CTPA supported this appearance of diagnostic certainty, reporting sensitivity and specificity of greater than 90%,3, 4 but several subsequent studies have failed to reproduce these results.57 Newer multidetector CT scans are believed to be more accurate than earlier single‐detector CT,8 but true estimates of CTPA test characteristics will not be known until publication of the forthcoming PIOPED II study.9
Even without these data, CT‐based diagnostic algorithms have already appeared.1014 These algorithms generally focus on minimizing the false‐negative rate through use of serial testing (involving combinations of serum D‐dimer, lower‐extremity ultrasound, and CTPA). A recent meta‐analysis demonstrated that negative CTPA is highly accurate at ruling out PE, with test characteristics similar to conventional pulmonary angiography.15 Another meta‐analysis found that the 3‐month rate of subsequent venous thromboembolism after negative CTPA was 1.4% (95% CI 1.1%‐1.8%),16 supporting the strategy of withholding anticoagulants after negative CTPA in combination with other tests. However, use of serial testing to establish the diagnosis of PE and initiate anticoagulation has not been systematically evaluated or recommended, even for patients with a low pretest probability of PE.17
To assess the potential impact of these algorithms on the diagnosis of PE in clinical practice, we analyzed the clinical presentation and treatment of a cohort of patients at our institution who underwent CTPA for suspected PE.1 We calculated a range of posttest probabilities for pulmonary embolism for these patients, given the pretest probabilities, test results, and estimates of CTPA test characteristics. We then compared the treatment decisions of clinicians to the posttest probabilities of PE in order to establish the potential frequency of false‐positive and false‐negative diagnoses and to determine if patients were treated appropriately based on these estimates.
METHODS
Sites and Subjects
Details of the sites, subjects, and methods used to collect patient‐level data in this analysis have been previously published.1 The study was performed at Moffitt‐Long Hospital and San Francisco General Hospital, teaching hospitals affiliated with the University of California San Francisco School of Medicine. At both sites, single‐detector CT scans were available 24 hours a day throughout the study period and were read by attending radiologists who specialized in thoracic imaging. We excluded patients whose CTPA was not completed as the initial test in the evaluation of suspected PE, those who underwent testing for any indication other than suspected acute PE, and those with incomplete medical records or technically inadequate CTPA.
We randomly selected 345 patients who underwent CTPA between January 1, 1998, and December 31, 2000, from the Radiology Department databases. One investigator (R.L.T.) then abstracted charts of all patients. For each subject, we collected data about history and clinical presentation, diagnostic impressions of the treating clinicians, treatments administered both before and after diagnostic testing, CTPA result, results of other diagnostic tests for PE, and final clinical diagnosis. During the study period, there were no institution‐ or department‐specific guidelines or decision aids available for the diagnosis of PE. Ventilation‐perfusion scan, lower extremity ultrasound, and pulmonary angiography were available, but highly sensitive D‐dimer assays were not in use. The study was approved by the Institutional Review Boards of both sites, and requirement for written informed consent from patients was waived.
Estimates of Pretest Probabilities of Pulmonary Embolism and CTPA Test Characteristics
Several prediction rules1820 generate clinical pretest probabilities for patients with suspected PE. We used the Wells score18 to assign a pretest probability of low, moderate, or high to each patient on the basis of the following clinical variables: leg swelling, hemoptysis, tachycardia, history of recent immobilization, history of prior DVT or PE, active malignancy, and lack of a more likely alternative diagnosis. We chose this rule as (unlike other prediction rules such as the Geneva rule20) the Wells score has been validated for hospitalized patients with suspected PE and does not require arterial blood gas measurements. The prevalence of PE reported in the evaluation of the Wells score was 3.4%, 27.8%, and 78.3% for low, moderate, and high pretest probabilities, respectively.18
As in our previous study,1 we assumed CTPA to be 90% sensitive and 95% specific based on published estimates.3, 17 These values correspond to a positive likelihood ratio of 18 and a negative likelihood ratio of 0.1.21 We chose these values as a best‐case estimate of the test characteristics of CTPA, although other studies have found less impressive results.7 Using these pretest probabilities and likelihood ratios, we then used Bayes' theorem (Figure 1) to calculate the range of expected posttest probabilities of pulmonary embolism.

Calculation of Posttest Probabilities and Comparison to Treatment Outcomes
For each pretest probability category, we used the posttest probabilities calculated above to determine the number of true‐positive pulmonary emboli, as follows:
RESULTS
Patient Characteristics
After excluding 23 patients receiving anticoagulants for other indications prior to CTPA, the study cohort included 322 patients (57.7% female), with an average age of 58.6 years, of whom 20.5% had cancer and 4.5% had a prior history of thromboembolic disease. Scans were primarily ordered by the medicine service (47.7% of cases) and emergency department (22.9%). CTPA was the initial test for 9% of patients evaluated for suspected acute PE during the first 6 months of the study period, increasing to 83% by the end of 2000.1 The overall pretest probability distribution remained the same throughout the entire study period.1
Test Results and Treatment Decisions
Most patients in our cohort had a low (n = 184, 57.1%) or a moderate (n = 101, 31.4%) pretest probability of PE (Table 1). The likelihood of a positive CTPA increased as the pretest probability increased, but even among patients with high clinical risk, only 35.1% had positive CT scans. In total, scans were positive in 57 patients and negative in 265 patients. Clinicians treated 55 patients with a positive CTPA (96.5%); none of these patients underwent additional testing for DVT or PE after the imaging study. Among patients with a negative CTPA, 254 (95.8%) were not treated; none of the patients in whom anticoagulation was withheld underwent further testing, whereas the other 11 patients were treated on the basis of other tests (5 high‐probability ventilation‐perfusion scans, 3 positive leg ultrasounds, and 3 for unclear reasons). Overall, 66 patients (20.5%) were treated for pulmonary embolism.
| Pretest probability of PE (number of CTPA performed) | Low (N = 184) | Moderate (N = 101) | High (N = 37) | Total (N = 322) |
|---|---|---|---|---|
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| CTPA negative for PE (% of pretest probability group) | 162 (88.0%) | 79 (78.2%) | 24 (64.9%) | 265 (82.3%) |
| Patients with positive CT subsequently treated for PE (% of pretest probability group) | 21 (11.4%) | 21 (20.8%) | 13 (35.1%) | 55 (17.1%) |
| Patients treated for PE despite negative CT (% of pretest probability group) | 5 (2.7%) | 3 (3.0%) | 3 (8.1%) | 11 (3.4%) |
| Total patients treated for PE (% of pretest probability group) | 26 (14.1%) | 24 (23.8%) | 16 (43.2%) | 66 (20.5%) |
Literature‐Derived Estimates of Posttest Probabilities of Pulmonary Embolism
Patients who have a low pretest probability of PE and a positive CTPA have a posttest probability of 41.6% under our estimate of CTPA test characteristics. Patients with moderate pretest probability have a posttest probability of 87.4% and patients with a high pretest probability will have a 98.5% probability of embolism with a positive scan. The traditional treatment threshold for PE is a posttest probability of 90%.22
Observed Versus Expected PE Rates and Subsequent Treatment
Only 9 of the 22 patients (41%) with a low pretest probability and a positive CTPA likely represent true‐positive emboli. However, clinicians chose to treat 21 of the 22 patients with this combination of pretest probability and imaging findings. Thus, 12 emboli would be considered possible false‐positive diagnoses. Similarly, in the moderate pretest probability group, 2 of 21 patients with moderate pretest probability and 0 of 13 patients with high pretest probability treated for PE had a possibly false‐positive diagnosis. Thus, in total, 25.4% (14 of 55) patients treated for PE had a possible false‐positive diagnosis of pulmonary embolism and may have been unnecessarily administered anticoagulants (Table 2). All patients who potentially had a false‐positive PE had either a low or moderate pretest probability of PE; in fact, the majority (57.1%) of patients with a low pretest probability of PE who were subsequently treated for PE likely had a false‐positive diagnosis.
| Pretest probability | ||||
|---|---|---|---|---|
| Low (n = 184) | Moderate (n = 101) | High (n = 37) | Total (n = 322) | |
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| Patients with positive CTPA treated for pulmonary embolism (n, % treated in risk group) | 21 (95.4%) | 21 (95.4%) | 13 (100%) | 55 (96.5%) |
| Calculated number and rate of probable true‐positive evaluations | ||||
| Number of true‐positive PE (n, % treated in risk group) | 9 (42.9%) | 19 (90.5%) | 13 (100%) | 41 (74.6%) |
| Calculated number and rate of possible false‐positive evaluations | ||||
| Number of possible false‐positive PE (n, % in risk group with unexpected PE) | 12 (58.1%) | 2 (9.5%) | 0 | 14 (25.4%) |
Clinicians were more likely to overtreat a patient with a possible false‐positive CT scan than to withhold treatment from a patient with a possible false‐negative diagnosis. Using the same estimates of CTPA test characteristics, the incidence of possible false‐negative diagnosis of PE was 1.6% (4 possible false‐negative diagnoses among 254 patients with negative CTPA results who were not treated for PE.) All these patients had a high pretest probability of PE.
DISCUSSION
Physicians at our institution regarded CTPA results as definitive, anticoagulating 96.5% of patients with a positive CT and withholding treatment in 95.8% of patients with a negative scan. This practice pattern may result in unnecessary anticoagulation of many patients with a low pretest probability of PE who may have had false‐positive CTPA findings. In contrast, the rate of possible false‐negative diagnosis of PE was low, consistent with the results of several other studies.16
The use of CTPA is likely to increase because of the publication of multiple algorithms advocating that CTPA be the chief imaging study used in the diagnosis of PE.1014 These algorithms recommend serial testing on patients with a negative CTPA in order to minimize the false‐negative rate, but they do not require systematic follow‐up in patients with a positive scan, even if the pretest probability was low. In management trials, this approach resulted in a low false‐negative rate (1.0%‐1.8% at 3‐month follow‐up).1114 However, the rate of major bleeding in patients treated for PE was 3.2%‐6.0% at 3 months,1214 illustrating the potential risk of anticoagulating patients who may have false‐positive diagnoses. Furthermore, premature diagnostic closure after a CTPA positive for PE may result in additional morbidity as a result of missing the true diagnosis.
One potential explanation for the large number of potential false‐positive emboli seen in low‐risk patients is that it is difficult to accurately diagnose distal pulmonary emboli with CTPA. The interrater reliability of CTPA for diagnosis of subsegmental PE is suboptimal,23 and the clinical significance of these emboli remains uncertain.24 Thus, many emboli found in patients with low pretest probability actually may have been subsegmental PE that would not have been diagnosed by another radiologist. As CTPA is more accurate for diagnosing central PE,25 clinicians should consider reviewing positive scans with the interpreting radiologist, especially when the pretest probability was low and the filling defects identified are in distal vessels.
Our results may also illustrate that clinicians have a lower treatment threshold when presented with apparently definitive evidence of pulmonary embolism. Previous proposals on the appropriate treatment threshold for PE, which used Bayesian decision‐making methods similar to ours,22 incorporated PIOPED26 data on the pretest probability of pulmonary embolism, the test characteristics of ventilation‐perfusion scans, and the clinical outcomes of patients in each test result/pretest probability category. However, there is no corresponding data for CTPA, as its test characteristics are still uncertain, and long‐term clinical outcomes have not been documented for patients treated (or not treated) on the basis of CT results.
Our study had several limitations. First, charting bias potentially was introduced by our using a retrospective method of collecting data for calculating pretest probabilities. To address this potential bias, we collected data from the entire medical record, including information available at and preceding the time of the CT scan. We believe this method was effective, as the range of pretest probabilities and the prevalence of PE in our study were very similar to those seen in a number of prospective studies.1820, 26, 27 Although other risk indices exist, the Wells score has been shown to have predictive powers equal to other algorithms and to clinicians; implicit assessments.28, 29 In our cohort, 35.1% of patients with a high pretest probability were diagnosed with PE; although this was lower than that in the initial Wells cohort,18 it was very similar to a subsequent validation study using the Wells algorithm, in which the prevalence of PE in patients with high pretest probability was 37.5%.27 Plasma D‐dimer testing is not routinely used at our hospitals, but it is a component of some CTPA‐based diagnostic algorithms.1114 Although use of D‐dimer testing may have led to fewer scans in patients with negative D‐dimer test results and low pretest probability,30 the high false‐positive rate for D‐dimer assays31 makes it difficult to predict the effect of widespread D‐dimer use on the overall pretest probability distribution. Using our assumptions about CT test characteristics, a pretest probability of more than 30% is required to generate a posttest probability of PE of at least 90% (the traditional treatment threshold for anticoagulant therapy22) with a positive scan. Extensive D‐dimer use would be unlikely to cause such a shift in the distribution of pretest probabilities.
Finally, CT technology has continued to advance, and many institutions now use 64‐slice scanners32 in contrast to the single‐slice scanners in use at the time our data were collected. Our assumptions were that CTPA has a positive likelihood ratio of 18.0 and a negative likelihood ratio of 0.1 (corresponding to a sensitivity of 90% and a specificity of 95%), although many studies of single‐detector CTPA found less impressive values.5, 7 Multidetector CT is thought to be more accurate than was earlier technology, but the true diagnostic performance of multidetector CT is not yet known. However, our findings pertain primarily to clinicians' responses to test results, so even if newer scanners are more accurate, Bayesian analysis will still be required in order to appropriately treat patients. A recent meta‐analysis of diagnostic strategies for PE found CTPA to have a positive likelihood ratio of 24.1, but even using this higher value, patients with a low pretest probability and positive CTPA still have a posttest probability of PE below the traditional treatment threshold.33 As most patients undergoing evaluation for suspected PE have a low pretest probability,17 a substantial number of false‐positive diagnoses of PE may still occur, even with a more accurate diagnostic test.
CT pulmonary angiography has become the first‐line test for pulmonary embolism at our institution, a situation likely mirrored elsewhere. CTPA is safe and rapid and offers the advantage of revealing ancillary lung findings that may be clinically significant.12 Although the test is an important addition to a clinician's diagnostic armamentarium, Bayesian analysis must be used to interpret its results, especially when CTPA is used as the first‐line diagnostic test. Our data raise the troubling concern that reliance on CTPA as the sole diagnostic test for suspected pulmonary embolism may result in a large number of patients with false‐positive CT scans receiving anticoagulation treatment.
Spiral computed tomographic pulmonary angiography (CTPA) is a common first‐line test for the evaluation of suspected pulmonary embolism (PE). At our institution CTPA became the initial diagnostic study in 83% of patients with suspected PE within 3 years of the introduction of CT,1 and by 2001 CTPA had become the most common diagnostic test performed nationwide in patients diagnosed with PE.2 Most scans are interpreted as either positive or negative for pulmonary embolism, providing clinicians with a greater sense of diagnostic certainty than with the probabilistic results of lung scintigraphy. Initial studies of CTPA supported this appearance of diagnostic certainty, reporting sensitivity and specificity of greater than 90%,3, 4 but several subsequent studies have failed to reproduce these results.57 Newer multidetector CT scans are believed to be more accurate than earlier single‐detector CT,8 but true estimates of CTPA test characteristics will not be known until publication of the forthcoming PIOPED II study.9
Even without these data, CT‐based diagnostic algorithms have already appeared.1014 These algorithms generally focus on minimizing the false‐negative rate through use of serial testing (involving combinations of serum D‐dimer, lower‐extremity ultrasound, and CTPA). A recent meta‐analysis demonstrated that negative CTPA is highly accurate at ruling out PE, with test characteristics similar to conventional pulmonary angiography.15 Another meta‐analysis found that the 3‐month rate of subsequent venous thromboembolism after negative CTPA was 1.4% (95% CI 1.1%‐1.8%),16 supporting the strategy of withholding anticoagulants after negative CTPA in combination with other tests. However, use of serial testing to establish the diagnosis of PE and initiate anticoagulation has not been systematically evaluated or recommended, even for patients with a low pretest probability of PE.17
To assess the potential impact of these algorithms on the diagnosis of PE in clinical practice, we analyzed the clinical presentation and treatment of a cohort of patients at our institution who underwent CTPA for suspected PE.1 We calculated a range of posttest probabilities for pulmonary embolism for these patients, given the pretest probabilities, test results, and estimates of CTPA test characteristics. We then compared the treatment decisions of clinicians to the posttest probabilities of PE in order to establish the potential frequency of false‐positive and false‐negative diagnoses and to determine if patients were treated appropriately based on these estimates.
METHODS
Sites and Subjects
Details of the sites, subjects, and methods used to collect patient‐level data in this analysis have been previously published.1 The study was performed at Moffitt‐Long Hospital and San Francisco General Hospital, teaching hospitals affiliated with the University of California San Francisco School of Medicine. At both sites, single‐detector CT scans were available 24 hours a day throughout the study period and were read by attending radiologists who specialized in thoracic imaging. We excluded patients whose CTPA was not completed as the initial test in the evaluation of suspected PE, those who underwent testing for any indication other than suspected acute PE, and those with incomplete medical records or technically inadequate CTPA.
We randomly selected 345 patients who underwent CTPA between January 1, 1998, and December 31, 2000, from the Radiology Department databases. One investigator (R.L.T.) then abstracted charts of all patients. For each subject, we collected data about history and clinical presentation, diagnostic impressions of the treating clinicians, treatments administered both before and after diagnostic testing, CTPA result, results of other diagnostic tests for PE, and final clinical diagnosis. During the study period, there were no institution‐ or department‐specific guidelines or decision aids available for the diagnosis of PE. Ventilation‐perfusion scan, lower extremity ultrasound, and pulmonary angiography were available, but highly sensitive D‐dimer assays were not in use. The study was approved by the Institutional Review Boards of both sites, and requirement for written informed consent from patients was waived.
Estimates of Pretest Probabilities of Pulmonary Embolism and CTPA Test Characteristics
Several prediction rules1820 generate clinical pretest probabilities for patients with suspected PE. We used the Wells score18 to assign a pretest probability of low, moderate, or high to each patient on the basis of the following clinical variables: leg swelling, hemoptysis, tachycardia, history of recent immobilization, history of prior DVT or PE, active malignancy, and lack of a more likely alternative diagnosis. We chose this rule as (unlike other prediction rules such as the Geneva rule20) the Wells score has been validated for hospitalized patients with suspected PE and does not require arterial blood gas measurements. The prevalence of PE reported in the evaluation of the Wells score was 3.4%, 27.8%, and 78.3% for low, moderate, and high pretest probabilities, respectively.18
As in our previous study,1 we assumed CTPA to be 90% sensitive and 95% specific based on published estimates.3, 17 These values correspond to a positive likelihood ratio of 18 and a negative likelihood ratio of 0.1.21 We chose these values as a best‐case estimate of the test characteristics of CTPA, although other studies have found less impressive results.7 Using these pretest probabilities and likelihood ratios, we then used Bayes' theorem (Figure 1) to calculate the range of expected posttest probabilities of pulmonary embolism.

Calculation of Posttest Probabilities and Comparison to Treatment Outcomes
For each pretest probability category, we used the posttest probabilities calculated above to determine the number of true‐positive pulmonary emboli, as follows:
RESULTS
Patient Characteristics
After excluding 23 patients receiving anticoagulants for other indications prior to CTPA, the study cohort included 322 patients (57.7% female), with an average age of 58.6 years, of whom 20.5% had cancer and 4.5% had a prior history of thromboembolic disease. Scans were primarily ordered by the medicine service (47.7% of cases) and emergency department (22.9%). CTPA was the initial test for 9% of patients evaluated for suspected acute PE during the first 6 months of the study period, increasing to 83% by the end of 2000.1 The overall pretest probability distribution remained the same throughout the entire study period.1
Test Results and Treatment Decisions
Most patients in our cohort had a low (n = 184, 57.1%) or a moderate (n = 101, 31.4%) pretest probability of PE (Table 1). The likelihood of a positive CTPA increased as the pretest probability increased, but even among patients with high clinical risk, only 35.1% had positive CT scans. In total, scans were positive in 57 patients and negative in 265 patients. Clinicians treated 55 patients with a positive CTPA (96.5%); none of these patients underwent additional testing for DVT or PE after the imaging study. Among patients with a negative CTPA, 254 (95.8%) were not treated; none of the patients in whom anticoagulation was withheld underwent further testing, whereas the other 11 patients were treated on the basis of other tests (5 high‐probability ventilation‐perfusion scans, 3 positive leg ultrasounds, and 3 for unclear reasons). Overall, 66 patients (20.5%) were treated for pulmonary embolism.
| Pretest probability of PE (number of CTPA performed) | Low (N = 184) | Moderate (N = 101) | High (N = 37) | Total (N = 322) |
|---|---|---|---|---|
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| CTPA negative for PE (% of pretest probability group) | 162 (88.0%) | 79 (78.2%) | 24 (64.9%) | 265 (82.3%) |
| Patients with positive CT subsequently treated for PE (% of pretest probability group) | 21 (11.4%) | 21 (20.8%) | 13 (35.1%) | 55 (17.1%) |
| Patients treated for PE despite negative CT (% of pretest probability group) | 5 (2.7%) | 3 (3.0%) | 3 (8.1%) | 11 (3.4%) |
| Total patients treated for PE (% of pretest probability group) | 26 (14.1%) | 24 (23.8%) | 16 (43.2%) | 66 (20.5%) |
Literature‐Derived Estimates of Posttest Probabilities of Pulmonary Embolism
Patients who have a low pretest probability of PE and a positive CTPA have a posttest probability of 41.6% under our estimate of CTPA test characteristics. Patients with moderate pretest probability have a posttest probability of 87.4% and patients with a high pretest probability will have a 98.5% probability of embolism with a positive scan. The traditional treatment threshold for PE is a posttest probability of 90%.22
Observed Versus Expected PE Rates and Subsequent Treatment
Only 9 of the 22 patients (41%) with a low pretest probability and a positive CTPA likely represent true‐positive emboli. However, clinicians chose to treat 21 of the 22 patients with this combination of pretest probability and imaging findings. Thus, 12 emboli would be considered possible false‐positive diagnoses. Similarly, in the moderate pretest probability group, 2 of 21 patients with moderate pretest probability and 0 of 13 patients with high pretest probability treated for PE had a possibly false‐positive diagnosis. Thus, in total, 25.4% (14 of 55) patients treated for PE had a possible false‐positive diagnosis of pulmonary embolism and may have been unnecessarily administered anticoagulants (Table 2). All patients who potentially had a false‐positive PE had either a low or moderate pretest probability of PE; in fact, the majority (57.1%) of patients with a low pretest probability of PE who were subsequently treated for PE likely had a false‐positive diagnosis.
| Pretest probability | ||||
|---|---|---|---|---|
| Low (n = 184) | Moderate (n = 101) | High (n = 37) | Total (n = 322) | |
| ||||
| CTPA positive for PE (% of pretest probability group) | 22 (12.0%) | 22 (21.8%) | 13 (35.1%) | 57 (17.7%) |
| Patients with positive CTPA treated for pulmonary embolism (n, % treated in risk group) | 21 (95.4%) | 21 (95.4%) | 13 (100%) | 55 (96.5%) |
| Calculated number and rate of probable true‐positive evaluations | ||||
| Number of true‐positive PE (n, % treated in risk group) | 9 (42.9%) | 19 (90.5%) | 13 (100%) | 41 (74.6%) |
| Calculated number and rate of possible false‐positive evaluations | ||||
| Number of possible false‐positive PE (n, % in risk group with unexpected PE) | 12 (58.1%) | 2 (9.5%) | 0 | 14 (25.4%) |
Clinicians were more likely to overtreat a patient with a possible false‐positive CT scan than to withhold treatment from a patient with a possible false‐negative diagnosis. Using the same estimates of CTPA test characteristics, the incidence of possible false‐negative diagnosis of PE was 1.6% (4 possible false‐negative diagnoses among 254 patients with negative CTPA results who were not treated for PE.) All these patients had a high pretest probability of PE.
DISCUSSION
Physicians at our institution regarded CTPA results as definitive, anticoagulating 96.5% of patients with a positive CT and withholding treatment in 95.8% of patients with a negative scan. This practice pattern may result in unnecessary anticoagulation of many patients with a low pretest probability of PE who may have had false‐positive CTPA findings. In contrast, the rate of possible false‐negative diagnosis of PE was low, consistent with the results of several other studies.16
The use of CTPA is likely to increase because of the publication of multiple algorithms advocating that CTPA be the chief imaging study used in the diagnosis of PE.1014 These algorithms recommend serial testing on patients with a negative CTPA in order to minimize the false‐negative rate, but they do not require systematic follow‐up in patients with a positive scan, even if the pretest probability was low. In management trials, this approach resulted in a low false‐negative rate (1.0%‐1.8% at 3‐month follow‐up).1114 However, the rate of major bleeding in patients treated for PE was 3.2%‐6.0% at 3 months,1214 illustrating the potential risk of anticoagulating patients who may have false‐positive diagnoses. Furthermore, premature diagnostic closure after a CTPA positive for PE may result in additional morbidity as a result of missing the true diagnosis.
One potential explanation for the large number of potential false‐positive emboli seen in low‐risk patients is that it is difficult to accurately diagnose distal pulmonary emboli with CTPA. The interrater reliability of CTPA for diagnosis of subsegmental PE is suboptimal,23 and the clinical significance of these emboli remains uncertain.24 Thus, many emboli found in patients with low pretest probability actually may have been subsegmental PE that would not have been diagnosed by another radiologist. As CTPA is more accurate for diagnosing central PE,25 clinicians should consider reviewing positive scans with the interpreting radiologist, especially when the pretest probability was low and the filling defects identified are in distal vessels.
Our results may also illustrate that clinicians have a lower treatment threshold when presented with apparently definitive evidence of pulmonary embolism. Previous proposals on the appropriate treatment threshold for PE, which used Bayesian decision‐making methods similar to ours,22 incorporated PIOPED26 data on the pretest probability of pulmonary embolism, the test characteristics of ventilation‐perfusion scans, and the clinical outcomes of patients in each test result/pretest probability category. However, there is no corresponding data for CTPA, as its test characteristics are still uncertain, and long‐term clinical outcomes have not been documented for patients treated (or not treated) on the basis of CT results.
Our study had several limitations. First, charting bias potentially was introduced by our using a retrospective method of collecting data for calculating pretest probabilities. To address this potential bias, we collected data from the entire medical record, including information available at and preceding the time of the CT scan. We believe this method was effective, as the range of pretest probabilities and the prevalence of PE in our study were very similar to those seen in a number of prospective studies.1820, 26, 27 Although other risk indices exist, the Wells score has been shown to have predictive powers equal to other algorithms and to clinicians; implicit assessments.28, 29 In our cohort, 35.1% of patients with a high pretest probability were diagnosed with PE; although this was lower than that in the initial Wells cohort,18 it was very similar to a subsequent validation study using the Wells algorithm, in which the prevalence of PE in patients with high pretest probability was 37.5%.27 Plasma D‐dimer testing is not routinely used at our hospitals, but it is a component of some CTPA‐based diagnostic algorithms.1114 Although use of D‐dimer testing may have led to fewer scans in patients with negative D‐dimer test results and low pretest probability,30 the high false‐positive rate for D‐dimer assays31 makes it difficult to predict the effect of widespread D‐dimer use on the overall pretest probability distribution. Using our assumptions about CT test characteristics, a pretest probability of more than 30% is required to generate a posttest probability of PE of at least 90% (the traditional treatment threshold for anticoagulant therapy22) with a positive scan. Extensive D‐dimer use would be unlikely to cause such a shift in the distribution of pretest probabilities.
Finally, CT technology has continued to advance, and many institutions now use 64‐slice scanners32 in contrast to the single‐slice scanners in use at the time our data were collected. Our assumptions were that CTPA has a positive likelihood ratio of 18.0 and a negative likelihood ratio of 0.1 (corresponding to a sensitivity of 90% and a specificity of 95%), although many studies of single‐detector CTPA found less impressive values.5, 7 Multidetector CT is thought to be more accurate than was earlier technology, but the true diagnostic performance of multidetector CT is not yet known. However, our findings pertain primarily to clinicians' responses to test results, so even if newer scanners are more accurate, Bayesian analysis will still be required in order to appropriately treat patients. A recent meta‐analysis of diagnostic strategies for PE found CTPA to have a positive likelihood ratio of 24.1, but even using this higher value, patients with a low pretest probability and positive CTPA still have a posttest probability of PE below the traditional treatment threshold.33 As most patients undergoing evaluation for suspected PE have a low pretest probability,17 a substantial number of false‐positive diagnoses of PE may still occur, even with a more accurate diagnostic test.
CT pulmonary angiography has become the first‐line test for pulmonary embolism at our institution, a situation likely mirrored elsewhere. CTPA is safe and rapid and offers the advantage of revealing ancillary lung findings that may be clinically significant.12 Although the test is an important addition to a clinician's diagnostic armamentarium, Bayesian analysis must be used to interpret its results, especially when CTPA is used as the first‐line diagnostic test. Our data raise the troubling concern that reliance on CTPA as the sole diagnostic test for suspected pulmonary embolism may result in a large number of patients with false‐positive CT scans receiving anticoagulation treatment.
- ,,,,.The impact of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- ,,.Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism.Am J Cardiol.2004;93:1316–1317.
- ,,,.Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐old technique—comparison with pulmonary angiography.Radiology.1992;185:381–387.
- ,,, et al.Pulmonary embolism: validation of spiral CT angiography in 149 patients.Radiology.1996;201:467–470.
- ,,,,.Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta‐analysis.Clin Appl Thromb Hemost.2001;7(2):87–92.
- ,,,.The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism.Arch Intern Med.2000;160(3):293–298.
- ,,.Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review.Ann Intern Med.2000;132(3):227–232.
- ,,, et al.Suspected acute pulmonary embolism: evaluation with multi‐detector row CT versus digital subtraction pulmonary arteriography.Radiology.2004;233:806–815.
- ,,,.Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II.Semin Nucl Med.2002;32(3):173–182.
- ,,, et al.Management of suspected pulmonary embolism (PE) by D‐dimer and multi‐slice computed tomography in outpatients: an outcome study.J Thromb Haemost.2005;3:1926–1932.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,, et al.Single‐detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients.Ann Intern Med.2003;138:307–314.
- ,,, et al.Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study.Lancet.2002;260:1914–1920.
- ,,, et al.Diagnosing pulmonary embolism in outpatients with clinical assessment, D‐dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study.Am J Med.2004;116:291–299.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review.JAMA.2005;293:2012–2017.
- ,,,.Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,.Clinical Practice: The evaluation of suspected pulmonary embolism.N Engl J Med.2003;349:1247–1256.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,.A structured clinical model for predicting the probability of pulmonary embolism.Am J Med.2003;114(3):173–179.
- ,,,,.Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161(1):92–97.
- ,,,.Interpretation of diagnostic tests and strategies for their use in quantitative decision making. In:Diagnostic strategies for common medical problems.Philadelphia, PA:American College of Physicians,1999.
- ,,,.Strategy for diagnosis of patients with suspected acute pulmonary embolism.Chest.1993;103:1553–1559.
- ,,, et al.Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement.Eur Radiol.2003;13:823–829.
- ,.Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy.Chest.1995;108:978–981.
- ,,, et al.Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism.Ann Intern Med.2001;135(2):88–97.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED).The PIOPED Investigators.JAMA.1990;263:2753–2759.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer.Ann Intern Med.2001;135(2):98–107.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,, et al.Does this patient have pulmonary embolism?JAMA.2003;290:2849–2858.
- ,,,,.Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review.Ann Intern Med.2003;138:941–951.
- ,,, et al.D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review.Ann Intern Med.2004;140:589–602.
- .Multislice computed tomography for pulmonary embolism—a technological marvel.N Engl J Med2005;352(17):1812–4.
- ,,,,,.Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism.Br Med J.2005;331:259.
- ,,,,.The impact of helical computed tomography on diagnostic and treatment strategies in patients with suspected pulmonary embolism.Am J Med.2004;116:84–90.
- ,,.Trends in the use of diagnostic imaging in patients hospitalized with acute pulmonary embolism.Am J Cardiol.2004;93:1316–1317.
- ,,,.Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single‐breath‐old technique—comparison with pulmonary angiography.Radiology.1992;185:381–387.
- ,,, et al.Pulmonary embolism: validation of spiral CT angiography in 149 patients.Radiology.1996;201:467–470.
- ,,,,.Lung scintigraphy and helical computed tomography for the diagnosis of pulmonary embolism: a meta‐analysis.Clin Appl Thromb Hemost.2001;7(2):87–92.
- ,,,.The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism.Arch Intern Med.2000;160(3):293–298.
- ,,.Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review.Ann Intern Med.2000;132(3):227–232.
- ,,, et al.Suspected acute pulmonary embolism: evaluation with multi‐detector row CT versus digital subtraction pulmonary arteriography.Radiology.2004;233:806–815.
- ,,,.Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II.Semin Nucl Med.2002;32(3):173–182.
- ,,, et al.Management of suspected pulmonary embolism (PE) by D‐dimer and multi‐slice computed tomography in outpatients: an outcome study.J Thromb Haemost.2005;3:1926–1932.
- ,,, et al.Multidetector‐row computed tomography in suspected pulmonary embolism.N Engl J Med.2005;352:1760–1768.
- ,,, et al.Single‐detector helical computed tomography as the primary diagnostic test in suspected pulmonary embolism: a multicenter clinical management study of 510 patients.Ann Intern Med.2003;138:307–314.
- ,,, et al.Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study.Lancet.2002;260:1914–1920.
- ,,, et al.Diagnosing pulmonary embolism in outpatients with clinical assessment, D‐dimer measurement, venous ultrasound, and helical computed tomography: a multicenter management study.Am J Med.2004;116:291–299.
- ,,, et al.Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review.JAMA.2005;293:2012–2017.
- ,,,.Meta‐analysis: outcomes in patients with suspected pulmonary embolism managed with computed tomographic pulmonary angiography.Ann Intern Med.2004;141:866–874.
- ,.Clinical Practice: The evaluation of suspected pulmonary embolism.N Engl J Med.2003;349:1247–1256.
- ,,, et al.Use of a clinical model for safe management of patients with suspected pulmonary embolism.Ann Intern Med.1998;129:997–1005.
- ,,.A structured clinical model for predicting the probability of pulmonary embolism.Am J Med.2003;114(3):173–179.
- ,,,,.Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score.Arch Intern Med.2001;161(1):92–97.
- ,,,.Interpretation of diagnostic tests and strategies for their use in quantitative decision making. In:Diagnostic strategies for common medical problems.Philadelphia, PA:American College of Physicians,1999.
- ,,,.Strategy for diagnosis of patients with suspected acute pulmonary embolism.Chest.1993;103:1553–1559.
- ,,, et al.Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement.Eur Radiol.2003;13:823–829.
- ,.Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy.Chest.1995;108:978–981.
- ,,, et al.Performance of helical computed tomography in unselected outpatients with suspected pulmonary embolism.Ann Intern Med.2001;135(2):88–97.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED).The PIOPED Investigators.JAMA.1990;263:2753–2759.
- ,,, et al.Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d‐dimer.Ann Intern Med.2001;135(2):98–107.
- ,,, et al.Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism.Am J Med.2002;113(4):269–275.
- ,,, et al.Does this patient have pulmonary embolism?JAMA.2003;290:2849–2858.
- ,,,,.Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review.Ann Intern Med.2003;138:941–951.
- ,,, et al.D‐dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review.Ann Intern Med.2004;140:589–602.
- .Multislice computed tomography for pulmonary embolism—a technological marvel.N Engl J Med2005;352(17):1812–4.
- ,,,,,.Systematic review and meta‐analysis of strategies for the diagnosis of suspected pulmonary embolism.Br Med J.2005;331:259.
Copyright © 2006 Society of Hospital Medicine