User login
AP-1 plays key role in various AML subtypes, team says
The AP-1 transcription factor family is of “major importance” in acute myeloid leukemia (AML), according to researchers.
The team said they identified transcription factor networks specific to AML subtypes, which showed that leukemic growth is dependent upon certain transcription factors, and “the global activation of signaling pathways parallels a growth dependence on AP-1 activity in multiple types of AML.”
Constanze Bonifer, PhD, of the University of Birmingham in the U.K., and her colleagues conducted this research and detailed their findings in Nature Genetics.
The researchers noted that previous work revealed the existence of gene regulatory networks in different types of AML classified by gene expression and DNA methylation patterns.
“Our work now defines these networks in detail and shows that leukemic drivers determine the regulatory phenotype by establishing and maintaining specific gene regulatory and signaling networks that are distinct from those in normal cells,” Dr. Bonifer and her colleagues wrote.
The researchers combined data obtained via several analytic techniques to construct transcription factor networks in normal CD34+ cells and cells from AML patients with defined mutations, including RUNX1 mutations, t(8;21) translocations, mutations of both alleles of the CEBPA gene, and FLT3-ITD with or without NPM1 mutation.
The AP-1 family network was of “high regulatory relevance” for all AML subtypes evaluated, the team reported.
Follow-up in vitro and in vivo studies confirmed the importance of AP-1 for different AML subtypes.
In the in vitro study, the researchers transduced AML cells with a doxycycline-inducible version of a dominant-negative (dn) FOS protein.
“AP-1 is a heterodimer formed by members of the FOS, JUN, ATF, CREB, and JDP families of transcription factors,” the researchers wrote. “[T]hus, it is challenging to target by defined RNA interference approaches.”
Results of the in vitro study showed that induction of dnFOS, mediated by doxycycline, inhibited proliferation of t(8;21)+ Kasumi-1 cells and FLT3-ITD-expressing MV4-11 cells.
Induction of dnFOS also inhibited the colony-forming ability of primary CD34+ FLT3-ITD cells but not CD34+ hematopoietic stem and progenitor cells.
To evaluate the relevance of AP-1 for leukemia propagation in vivo, the researchers transplanted either of two cell lines—Kasumi-1 or MV4-11—expressing inducible dnFOS in immunodeficient mice.
With Kasumi-1, granulosarcomas developed in six of seven untreated control mice and two mice treated with doxycycline, neither of which expressed the inducible protein.
With MV4-11, doxycycline inhibited leukemia development, and untreated mice rapidly developed tumors.
The researchers declared no competing interests related to this work, which was funded by Bloodwise, Cancer Research UK, a Kay Kendall Clinical Training Fellowship, and an MRC/Leuka Clinical Training Fellowship.
The AP-1 transcription factor family is of “major importance” in acute myeloid leukemia (AML), according to researchers.
The team said they identified transcription factor networks specific to AML subtypes, which showed that leukemic growth is dependent upon certain transcription factors, and “the global activation of signaling pathways parallels a growth dependence on AP-1 activity in multiple types of AML.”
Constanze Bonifer, PhD, of the University of Birmingham in the U.K., and her colleagues conducted this research and detailed their findings in Nature Genetics.
The researchers noted that previous work revealed the existence of gene regulatory networks in different types of AML classified by gene expression and DNA methylation patterns.
“Our work now defines these networks in detail and shows that leukemic drivers determine the regulatory phenotype by establishing and maintaining specific gene regulatory and signaling networks that are distinct from those in normal cells,” Dr. Bonifer and her colleagues wrote.
The researchers combined data obtained via several analytic techniques to construct transcription factor networks in normal CD34+ cells and cells from AML patients with defined mutations, including RUNX1 mutations, t(8;21) translocations, mutations of both alleles of the CEBPA gene, and FLT3-ITD with or without NPM1 mutation.
The AP-1 family network was of “high regulatory relevance” for all AML subtypes evaluated, the team reported.
Follow-up in vitro and in vivo studies confirmed the importance of AP-1 for different AML subtypes.
In the in vitro study, the researchers transduced AML cells with a doxycycline-inducible version of a dominant-negative (dn) FOS protein.
“AP-1 is a heterodimer formed by members of the FOS, JUN, ATF, CREB, and JDP families of transcription factors,” the researchers wrote. “[T]hus, it is challenging to target by defined RNA interference approaches.”
Results of the in vitro study showed that induction of dnFOS, mediated by doxycycline, inhibited proliferation of t(8;21)+ Kasumi-1 cells and FLT3-ITD-expressing MV4-11 cells.
Induction of dnFOS also inhibited the colony-forming ability of primary CD34+ FLT3-ITD cells but not CD34+ hematopoietic stem and progenitor cells.
To evaluate the relevance of AP-1 for leukemia propagation in vivo, the researchers transplanted either of two cell lines—Kasumi-1 or MV4-11—expressing inducible dnFOS in immunodeficient mice.
With Kasumi-1, granulosarcomas developed in six of seven untreated control mice and two mice treated with doxycycline, neither of which expressed the inducible protein.
With MV4-11, doxycycline inhibited leukemia development, and untreated mice rapidly developed tumors.
The researchers declared no competing interests related to this work, which was funded by Bloodwise, Cancer Research UK, a Kay Kendall Clinical Training Fellowship, and an MRC/Leuka Clinical Training Fellowship.
The AP-1 transcription factor family is of “major importance” in acute myeloid leukemia (AML), according to researchers.
The team said they identified transcription factor networks specific to AML subtypes, which showed that leukemic growth is dependent upon certain transcription factors, and “the global activation of signaling pathways parallels a growth dependence on AP-1 activity in multiple types of AML.”
Constanze Bonifer, PhD, of the University of Birmingham in the U.K., and her colleagues conducted this research and detailed their findings in Nature Genetics.
The researchers noted that previous work revealed the existence of gene regulatory networks in different types of AML classified by gene expression and DNA methylation patterns.
“Our work now defines these networks in detail and shows that leukemic drivers determine the regulatory phenotype by establishing and maintaining specific gene regulatory and signaling networks that are distinct from those in normal cells,” Dr. Bonifer and her colleagues wrote.
The researchers combined data obtained via several analytic techniques to construct transcription factor networks in normal CD34+ cells and cells from AML patients with defined mutations, including RUNX1 mutations, t(8;21) translocations, mutations of both alleles of the CEBPA gene, and FLT3-ITD with or without NPM1 mutation.
The AP-1 family network was of “high regulatory relevance” for all AML subtypes evaluated, the team reported.
Follow-up in vitro and in vivo studies confirmed the importance of AP-1 for different AML subtypes.
In the in vitro study, the researchers transduced AML cells with a doxycycline-inducible version of a dominant-negative (dn) FOS protein.
“AP-1 is a heterodimer formed by members of the FOS, JUN, ATF, CREB, and JDP families of transcription factors,” the researchers wrote. “[T]hus, it is challenging to target by defined RNA interference approaches.”
Results of the in vitro study showed that induction of dnFOS, mediated by doxycycline, inhibited proliferation of t(8;21)+ Kasumi-1 cells and FLT3-ITD-expressing MV4-11 cells.
Induction of dnFOS also inhibited the colony-forming ability of primary CD34+ FLT3-ITD cells but not CD34+ hematopoietic stem and progenitor cells.
To evaluate the relevance of AP-1 for leukemia propagation in vivo, the researchers transplanted either of two cell lines—Kasumi-1 or MV4-11—expressing inducible dnFOS in immunodeficient mice.
With Kasumi-1, granulosarcomas developed in six of seven untreated control mice and two mice treated with doxycycline, neither of which expressed the inducible protein.
With MV4-11, doxycycline inhibited leukemia development, and untreated mice rapidly developed tumors.
The researchers declared no competing interests related to this work, which was funded by Bloodwise, Cancer Research UK, a Kay Kendall Clinical Training Fellowship, and an MRC/Leuka Clinical Training Fellowship.
Reprocessed bronchoscopes found to harbor microbial growth
even when done in accordance with endoscope reprocessing standards, according to results of a prospective, multisite investigation.
All clinically used bronchoscopes evaluated in the study had residual contamination after reprocessing, and more than half showed microbial growth, the researchers reported in the journal CHEST.
These findings suggest that systematic changes are needed to improve cleaning and disinfection and to avoid the retention of bioburden, said researcher Cori L. Ofstead, MSPH, and her coinvestigators (Chest 2018 Nov;154[5]:1024-34).
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Ms. Ofstead and her colleagues said in their report.
Institutions also should consider shifting from high-level disinfection (HLD) to sterilization to reduce patient exposure to contaminated bronchoscopes, they added.
The study was conducted in three large, tertiary hospitals that contributed a total of 24 clinically used devices. That total comprised nine therapeutic, nine pediatric, and six endobronchial ultrasound (EBUS) bronchoscopes that were all reprocessed in accordance with each institution’s standard practices.
Proteins were detected in 100% of the bronchoscopes after HLD, according to researchers.
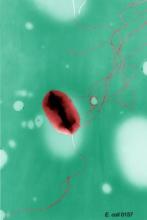
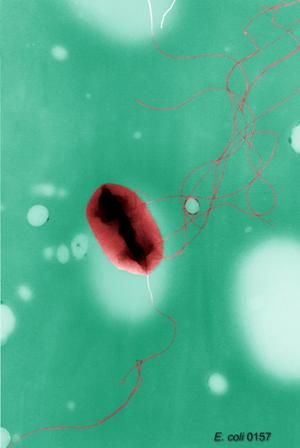
Looking at 20 paired postcleaning and post-HLD samples, the researchers found microbial growth in 11 of 20 (55%) manually-cleaned bronchoscopes and 14 of 24 (58%) bronchoscopes after HLD. The post-HLD samples included mold and recognized pathogens such as Escherichia coli, as well as normal flora and environmental bacteria, they said.
All 24 of the bronchoscopes had visible irregularities, including brown, red, or oily residue, retained fluid, debris in channels, scratches, or damage at insertion tubes and distal ends, they added.
Substandard reprocessing practices were found at two of the three participating institutions, according to the investigators. At one site, technicians reused syringes to flush channels with alcohol stored in an uncovered bowl during the day, according to the report, and bronchoscopes at that site were dried with reused towels and stored in a cabinet without active ventilation.
“Nursing staff were observed handling patient-ready bronchoscopes with bare hands,” the investigators reported.
Although clinical outcomes were not measured, the contamination, microbial growth, and defects observed in this study are “worrisome,” according to authors, because of the high infection risk in many patients undergoing bronchoscopy, and because of the infectious outbreaks and patient deaths linked to contaminated bronchoscopes in previous investigations.
Research funding for the study was provided by 3M Company. Study materials were provided by 3M Company and Healthmark Industries. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received research funding and speaking fees related to infection prevention from 3M Company, Healthmark Industries, Advanced Sterilization Products (Johnson & Johnson), and others.
The senior author of the study was J. Scott Ferguson, MD, of the division of pulmonary and critical care medicine at the University of Wisconsin School of Medicine and Public Health, Madison. Dr. Ferguson provided disclosures related to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
SOURCE: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
Results of this study, which document biological contamination of inadequately reprocessed bronchoscopes, are provocative and “alarming,” warranting attention from physicians, paramedical staff, administrators, and manufacturers, Atul C. Mehta, MBBS, and Thomas Gildea, MD, wrote in an editorial (Chest 2018 Nov;154[5]:1001-3).
A breach in the disinfection protocol is the “most common culprit” behind the spread of infection during bronchoscopy, they noted.
“In our opinion, the interventional pulmonology community has buried its head in the sand regarding this issue,” they wrote.
While cases of true infection related to bronchoscopy “can seldom be differentiated” in the literature, it is nevertheless “mandatory” that biologic contamination from one patient to another be avoided, they wrote.
One dismaying observation in the present study is that human proteins were detected in the working channels of all of bronchoscopes after high-level disinfection (HLD), Dr. Mehta and Dr. Gildea stated.
“It is critical that if HLD remains the standard of care and is sufficient, it must be done properly,” they wrote. “This study raises concerns that HLD itself may not be sufficient, and we have no options for disposable specialty bronchoscopes.”
Unfortunately, requiring sterilized bronchoscopes is time-consuming and impractical for a busy bronchoscopy practice, according to the authors, while disposable bronchoscopes need to be established as clinically effective and cost effective.
In the meantime, the authors recommended that clinicians proactively consider initiatives to ensure patient safety, including formally assessing HLD processes, examining all bronchoscopes for visible damage, and ensuring that HLD guidelines are met or exceeded.
Dr. Mehta and Dr. Gildea are with the department of pulmonary medicine at Cleveland Clinic. Neither author reported conflicts of interest related to the editorial, which was published in Chest.
Results of this study, which document biological contamination of inadequately reprocessed bronchoscopes, are provocative and “alarming,” warranting attention from physicians, paramedical staff, administrators, and manufacturers, Atul C. Mehta, MBBS, and Thomas Gildea, MD, wrote in an editorial (Chest 2018 Nov;154[5]:1001-3).
A breach in the disinfection protocol is the “most common culprit” behind the spread of infection during bronchoscopy, they noted.
“In our opinion, the interventional pulmonology community has buried its head in the sand regarding this issue,” they wrote.
While cases of true infection related to bronchoscopy “can seldom be differentiated” in the literature, it is nevertheless “mandatory” that biologic contamination from one patient to another be avoided, they wrote.
One dismaying observation in the present study is that human proteins were detected in the working channels of all of bronchoscopes after high-level disinfection (HLD), Dr. Mehta and Dr. Gildea stated.
“It is critical that if HLD remains the standard of care and is sufficient, it must be done properly,” they wrote. “This study raises concerns that HLD itself may not be sufficient, and we have no options for disposable specialty bronchoscopes.”
Unfortunately, requiring sterilized bronchoscopes is time-consuming and impractical for a busy bronchoscopy practice, according to the authors, while disposable bronchoscopes need to be established as clinically effective and cost effective.
In the meantime, the authors recommended that clinicians proactively consider initiatives to ensure patient safety, including formally assessing HLD processes, examining all bronchoscopes for visible damage, and ensuring that HLD guidelines are met or exceeded.
Dr. Mehta and Dr. Gildea are with the department of pulmonary medicine at Cleveland Clinic. Neither author reported conflicts of interest related to the editorial, which was published in Chest.
Results of this study, which document biological contamination of inadequately reprocessed bronchoscopes, are provocative and “alarming,” warranting attention from physicians, paramedical staff, administrators, and manufacturers, Atul C. Mehta, MBBS, and Thomas Gildea, MD, wrote in an editorial (Chest 2018 Nov;154[5]:1001-3).
A breach in the disinfection protocol is the “most common culprit” behind the spread of infection during bronchoscopy, they noted.
“In our opinion, the interventional pulmonology community has buried its head in the sand regarding this issue,” they wrote.
While cases of true infection related to bronchoscopy “can seldom be differentiated” in the literature, it is nevertheless “mandatory” that biologic contamination from one patient to another be avoided, they wrote.
One dismaying observation in the present study is that human proteins were detected in the working channels of all of bronchoscopes after high-level disinfection (HLD), Dr. Mehta and Dr. Gildea stated.
“It is critical that if HLD remains the standard of care and is sufficient, it must be done properly,” they wrote. “This study raises concerns that HLD itself may not be sufficient, and we have no options for disposable specialty bronchoscopes.”
Unfortunately, requiring sterilized bronchoscopes is time-consuming and impractical for a busy bronchoscopy practice, according to the authors, while disposable bronchoscopes need to be established as clinically effective and cost effective.
In the meantime, the authors recommended that clinicians proactively consider initiatives to ensure patient safety, including formally assessing HLD processes, examining all bronchoscopes for visible damage, and ensuring that HLD guidelines are met or exceeded.
Dr. Mehta and Dr. Gildea are with the department of pulmonary medicine at Cleveland Clinic. Neither author reported conflicts of interest related to the editorial, which was published in Chest.
even when done in accordance with endoscope reprocessing standards, according to results of a prospective, multisite investigation.
All clinically used bronchoscopes evaluated in the study had residual contamination after reprocessing, and more than half showed microbial growth, the researchers reported in the journal CHEST.
These findings suggest that systematic changes are needed to improve cleaning and disinfection and to avoid the retention of bioburden, said researcher Cori L. Ofstead, MSPH, and her coinvestigators (Chest 2018 Nov;154[5]:1024-34).
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Ms. Ofstead and her colleagues said in their report.
Institutions also should consider shifting from high-level disinfection (HLD) to sterilization to reduce patient exposure to contaminated bronchoscopes, they added.
The study was conducted in three large, tertiary hospitals that contributed a total of 24 clinically used devices. That total comprised nine therapeutic, nine pediatric, and six endobronchial ultrasound (EBUS) bronchoscopes that were all reprocessed in accordance with each institution’s standard practices.
Proteins were detected in 100% of the bronchoscopes after HLD, according to researchers.
Looking at 20 paired postcleaning and post-HLD samples, the researchers found microbial growth in 11 of 20 (55%) manually-cleaned bronchoscopes and 14 of 24 (58%) bronchoscopes after HLD. The post-HLD samples included mold and recognized pathogens such as Escherichia coli, as well as normal flora and environmental bacteria, they said.
All 24 of the bronchoscopes had visible irregularities, including brown, red, or oily residue, retained fluid, debris in channels, scratches, or damage at insertion tubes and distal ends, they added.
Substandard reprocessing practices were found at two of the three participating institutions, according to the investigators. At one site, technicians reused syringes to flush channels with alcohol stored in an uncovered bowl during the day, according to the report, and bronchoscopes at that site were dried with reused towels and stored in a cabinet without active ventilation.
“Nursing staff were observed handling patient-ready bronchoscopes with bare hands,” the investigators reported.
Although clinical outcomes were not measured, the contamination, microbial growth, and defects observed in this study are “worrisome,” according to authors, because of the high infection risk in many patients undergoing bronchoscopy, and because of the infectious outbreaks and patient deaths linked to contaminated bronchoscopes in previous investigations.
Research funding for the study was provided by 3M Company. Study materials were provided by 3M Company and Healthmark Industries. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received research funding and speaking fees related to infection prevention from 3M Company, Healthmark Industries, Advanced Sterilization Products (Johnson & Johnson), and others.
The senior author of the study was J. Scott Ferguson, MD, of the division of pulmonary and critical care medicine at the University of Wisconsin School of Medicine and Public Health, Madison. Dr. Ferguson provided disclosures related to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
SOURCE: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
even when done in accordance with endoscope reprocessing standards, according to results of a prospective, multisite investigation.
All clinically used bronchoscopes evaluated in the study had residual contamination after reprocessing, and more than half showed microbial growth, the researchers reported in the journal CHEST.
These findings suggest that systematic changes are needed to improve cleaning and disinfection and to avoid the retention of bioburden, said researcher Cori L. Ofstead, MSPH, and her coinvestigators (Chest 2018 Nov;154[5]:1024-34).
“Evidence-based, bronchoscope-specific reprocessing and maintenance guidelines are needed, along with quality management programs to ensure that these complex processes are carried out effectively,” Ms. Ofstead and her colleagues said in their report.
Institutions also should consider shifting from high-level disinfection (HLD) to sterilization to reduce patient exposure to contaminated bronchoscopes, they added.
The study was conducted in three large, tertiary hospitals that contributed a total of 24 clinically used devices. That total comprised nine therapeutic, nine pediatric, and six endobronchial ultrasound (EBUS) bronchoscopes that were all reprocessed in accordance with each institution’s standard practices.
Proteins were detected in 100% of the bronchoscopes after HLD, according to researchers.
Looking at 20 paired postcleaning and post-HLD samples, the researchers found microbial growth in 11 of 20 (55%) manually-cleaned bronchoscopes and 14 of 24 (58%) bronchoscopes after HLD. The post-HLD samples included mold and recognized pathogens such as Escherichia coli, as well as normal flora and environmental bacteria, they said.
All 24 of the bronchoscopes had visible irregularities, including brown, red, or oily residue, retained fluid, debris in channels, scratches, or damage at insertion tubes and distal ends, they added.
Substandard reprocessing practices were found at two of the three participating institutions, according to the investigators. At one site, technicians reused syringes to flush channels with alcohol stored in an uncovered bowl during the day, according to the report, and bronchoscopes at that site were dried with reused towels and stored in a cabinet without active ventilation.
“Nursing staff were observed handling patient-ready bronchoscopes with bare hands,” the investigators reported.
Although clinical outcomes were not measured, the contamination, microbial growth, and defects observed in this study are “worrisome,” according to authors, because of the high infection risk in many patients undergoing bronchoscopy, and because of the infectious outbreaks and patient deaths linked to contaminated bronchoscopes in previous investigations.
Research funding for the study was provided by 3M Company. Study materials were provided by 3M Company and Healthmark Industries. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received research funding and speaking fees related to infection prevention from 3M Company, Healthmark Industries, Advanced Sterilization Products (Johnson & Johnson), and others.
The senior author of the study was J. Scott Ferguson, MD, of the division of pulmonary and critical care medicine at the University of Wisconsin School of Medicine and Public Health, Madison. Dr. Ferguson provided disclosures related to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
SOURCE: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
FROM CHEST
Key clinical point: Bronchoscope reprocessing was ineffective, even when performed in accordance with endoscope reprocessing standards.
Major finding: After high-level disinfection, residual contamination was found in 100% of bronchoscopes, while microbial growth was seen in 58%.
Study details: Observation and testing of 24 bronchoscopes contributed by three tertiary care hospitals.
Disclosures: 3M Company and Healthmark Industries provided study materials. Ms. Ofstead and several coauthors reported employment with Ofstead & Associates, which has received funding related to infection prevention from these two companies, among others. The senior study author disclosed ties to NewWave Medical, Pharmaceutical Product Development, Oncocyte, Concordia, and PneumRx.
Source: Ofstead C et al. Chest. 2018 Nov;154(5):1024-34.
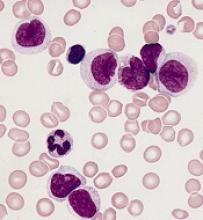
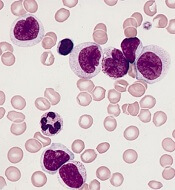
Transcription factor plays key role in AML gene regulatory networks
The AP-1 transcription factor family, important in many tumor types, plays a major role in acute myeloid leukemia, according to researchers who conducted a comprehensive global analysis of gene regulatory networks involved in this disease.
This observation suggests new opportunities for targeted treatment of AML, according to the researchers, led by Peter N. Cockerill, PhD, and Constanze Bonifer, PhD, with the Institute of Cancer and Genomic Sciences, University of Birmingham, England.
“Induced and aberrantly expressed transcription factors are not bystanders, but are important for network maintenance and leukemic growth,” the investigators wrote in Nature Genetics.
Investigators combined data obtained via several different analytic techniques to construct transcription factor networks in normal CD34+ cells and cells from specific subgroups of subjects with defined mutations, including RUNX1 mutations, t(8;21) translocations, mutations of both alleles of the CEBPA gene, and FLT3-ITD with or without NPM1 mutation.
The AP-1 family network was of “high regulatory relevance” for all AML subtypes evaluated, the investigators reported.
Previous work revealed the existence of gene regulatory networks in different types of AML classified by gene expression and DNA-methylation patterns.
“Our work now defines these networks in detail, and shows that leukemic drivers determine the regulatory phenotype by establishing and maintaining specific gene regulatory and signaling networks that are distinct from those in normal cells,” the authors said in their report.
Follow-up in vitro and in vivo studies confirmed the importance of AP-1 for different AML subtypes.
In the in vitro study, investigators transduced AML cells with a doxycycline-inducible version of a dominant negative FOS protein.
“AP-1 is a heterodimer formed by members of the FOS, JUN, ATF, CREB and JDP families of transcription factors, thus it is challenging to target by defined RNA interference approaches,” the investigators explained.
Results of the in vitro study showed that induction of that protein, mediated by doxycycline, inhibited proliferation and colony-forming ability in AML cell lines.
To evaluate the relevance of AP-1 for leukemia propagation in vivo, they transplanted two different types of cells expressing inducible dominant negative FOS protein in immunodeficient mice.
For the first cell type, granulosarcomas developed in six out of seven mice in a control group, but in only two mice treated with doxycycline, neither of which expressed the inducible protein, suggesting that the transgene was silenced, according to the investigators. For the second cell type, doxycycline inhibited leukemia development, while untreated mice rapidly developed tumors.
“Taken together, these findings demonstrate the importance of AP-1 for several AML subtypes and emphasize the potential of transcriptional network analyses to predict transcription factors crucial for malignant propagation,” the investigators wrote.
They declared no competing interests related to their research, which was funded by Bloodwise, Cancer Research UK, a Kay Kendall Clinical Training Fellowship and a MRC/Leuka Clinical Training Fellowship.
SOURCE: Assi SA et al. Nat Genet. 2018 Nov 12. doi: 10.1038/s41588-018-0270-1.
The AP-1 transcription factor family, important in many tumor types, plays a major role in acute myeloid leukemia, according to researchers who conducted a comprehensive global analysis of gene regulatory networks involved in this disease.
This observation suggests new opportunities for targeted treatment of AML, according to the researchers, led by Peter N. Cockerill, PhD, and Constanze Bonifer, PhD, with the Institute of Cancer and Genomic Sciences, University of Birmingham, England.
“Induced and aberrantly expressed transcription factors are not bystanders, but are important for network maintenance and leukemic growth,” the investigators wrote in Nature Genetics.
Investigators combined data obtained via several different analytic techniques to construct transcription factor networks in normal CD34+ cells and cells from specific subgroups of subjects with defined mutations, including RUNX1 mutations, t(8;21) translocations, mutations of both alleles of the CEBPA gene, and FLT3-ITD with or without NPM1 mutation.
The AP-1 family network was of “high regulatory relevance” for all AML subtypes evaluated, the investigators reported.
Previous work revealed the existence of gene regulatory networks in different types of AML classified by gene expression and DNA-methylation patterns.
“Our work now defines these networks in detail, and shows that leukemic drivers determine the regulatory phenotype by establishing and maintaining specific gene regulatory and signaling networks that are distinct from those in normal cells,” the authors said in their report.
Follow-up in vitro and in vivo studies confirmed the importance of AP-1 for different AML subtypes.
In the in vitro study, investigators transduced AML cells with a doxycycline-inducible version of a dominant negative FOS protein.
“AP-1 is a heterodimer formed by members of the FOS, JUN, ATF, CREB and JDP families of transcription factors, thus it is challenging to target by defined RNA interference approaches,” the investigators explained.
Results of the in vitro study showed that induction of that protein, mediated by doxycycline, inhibited proliferation and colony-forming ability in AML cell lines.
To evaluate the relevance of AP-1 for leukemia propagation in vivo, they transplanted two different types of cells expressing inducible dominant negative FOS protein in immunodeficient mice.
For the first cell type, granulosarcomas developed in six out of seven mice in a control group, but in only two mice treated with doxycycline, neither of which expressed the inducible protein, suggesting that the transgene was silenced, according to the investigators. For the second cell type, doxycycline inhibited leukemia development, while untreated mice rapidly developed tumors.
“Taken together, these findings demonstrate the importance of AP-1 for several AML subtypes and emphasize the potential of transcriptional network analyses to predict transcription factors crucial for malignant propagation,” the investigators wrote.
They declared no competing interests related to their research, which was funded by Bloodwise, Cancer Research UK, a Kay Kendall Clinical Training Fellowship and a MRC/Leuka Clinical Training Fellowship.
SOURCE: Assi SA et al. Nat Genet. 2018 Nov 12. doi: 10.1038/s41588-018-0270-1.
The AP-1 transcription factor family, important in many tumor types, plays a major role in acute myeloid leukemia, according to researchers who conducted a comprehensive global analysis of gene regulatory networks involved in this disease.
This observation suggests new opportunities for targeted treatment of AML, according to the researchers, led by Peter N. Cockerill, PhD, and Constanze Bonifer, PhD, with the Institute of Cancer and Genomic Sciences, University of Birmingham, England.
“Induced and aberrantly expressed transcription factors are not bystanders, but are important for network maintenance and leukemic growth,” the investigators wrote in Nature Genetics.
Investigators combined data obtained via several different analytic techniques to construct transcription factor networks in normal CD34+ cells and cells from specific subgroups of subjects with defined mutations, including RUNX1 mutations, t(8;21) translocations, mutations of both alleles of the CEBPA gene, and FLT3-ITD with or without NPM1 mutation.
The AP-1 family network was of “high regulatory relevance” for all AML subtypes evaluated, the investigators reported.
Previous work revealed the existence of gene regulatory networks in different types of AML classified by gene expression and DNA-methylation patterns.
“Our work now defines these networks in detail, and shows that leukemic drivers determine the regulatory phenotype by establishing and maintaining specific gene regulatory and signaling networks that are distinct from those in normal cells,” the authors said in their report.
Follow-up in vitro and in vivo studies confirmed the importance of AP-1 for different AML subtypes.
In the in vitro study, investigators transduced AML cells with a doxycycline-inducible version of a dominant negative FOS protein.
“AP-1 is a heterodimer formed by members of the FOS, JUN, ATF, CREB and JDP families of transcription factors, thus it is challenging to target by defined RNA interference approaches,” the investigators explained.
Results of the in vitro study showed that induction of that protein, mediated by doxycycline, inhibited proliferation and colony-forming ability in AML cell lines.
To evaluate the relevance of AP-1 for leukemia propagation in vivo, they transplanted two different types of cells expressing inducible dominant negative FOS protein in immunodeficient mice.
For the first cell type, granulosarcomas developed in six out of seven mice in a control group, but in only two mice treated with doxycycline, neither of which expressed the inducible protein, suggesting that the transgene was silenced, according to the investigators. For the second cell type, doxycycline inhibited leukemia development, while untreated mice rapidly developed tumors.
“Taken together, these findings demonstrate the importance of AP-1 for several AML subtypes and emphasize the potential of transcriptional network analyses to predict transcription factors crucial for malignant propagation,” the investigators wrote.
They declared no competing interests related to their research, which was funded by Bloodwise, Cancer Research UK, a Kay Kendall Clinical Training Fellowship and a MRC/Leuka Clinical Training Fellowship.
SOURCE: Assi SA et al. Nat Genet. 2018 Nov 12. doi: 10.1038/s41588-018-0270-1.
FROM NATURE GENETICS
Key clinical point:
Major finding: The AP-1 factor family gene regulatory network was of high regulatory relevance in multiple subtypes of AML with defined mutations.
Study details: Analysis of normal CD34+ cells and cells from AML subjects.
Disclosures: Funding came from Bloodwise and Cancer Research UK, among other sources. The researchers reported having no competing financial interests.
Source: Assi SA et al. Nat Genet. 2018 Nov 12. doi: 10.1038/s41588-018-0270-1.
Concussion/TBI linked to suicide risk, meta-analysis suggests
Risk of suicide was doubled in persons who experienced a concussion or mild traumatic brain injury (TBI) earlier in life, according to results of a meta-analysis of 17 studies representing nearly 7 million patients.
However, the absolute risk of suicide remained quite low, according to Michael Fralick, MD, of the University of Toronto, and co-investigators.
“Nearly all patients diagnosed with concussion and/or mild TBI did not die by suicide,” Dr. Fralick and colleagues said in their report on the study, which appears in JAMA Neurology.
Nevertheless, the meta-analysis illustrates evidence for an increased risk of suicide, suicide attempts, and suicidal ideation for persons with a history of these injuries, they said in the report.
The meta-analysis included 10 cohort studies, 5 cross-sectional studies, and 2 case-control studies looking at the risk of suicide, suicide attempts, or suicidal ideation after a concussion or mild TBI. Those studies included a roughly 714,000 individuals with a concussion and/or TBI diagnosis, and 6,236,000 without a diagnosis.
For people diagnosed with at least one concussion and/or mild TBI, the risk of suicide was 2-fold higher (relative risk, 2.03; 95% CI, 1.47-2.80; P less than 0.001), according to the report.
The risk was “slightly stronger,” investigators said, when the analysis was limited to studies adjusting for factors associated with those brain injuries and with suicide (RR, 2.10; 95% CI, 1.40-3.13; P less than 0.01).
Four of the 5 cohort studies reported absolute risk of suicide, according to Dr. Fralick and coauthors. In one study with a median follow-up of 3.6 years, 0.50% of individuals with a concussion and/or TBI subsequently died of suicide, while similarly, 0.59% died in a study with 4.0 years of follow-up, 0.28% in a study with 9.3 years follow-up, and 0.49% in one with a 12.3 year median follow-up.
Most of the studies in the meta-analysis reported an increased risk of suicide attempt after concussion and/or mild TBI, according to Dr. Fralick and his collaborators, while the eight studies looking at suicidal ideation all reported heightened risk after those brain injuries.
The researchers acknowledged some limitations of their analysis. Recall bias could have led to an overestimation of the association between concussion and suicide risk, since suicide attempts may affect reporting of concussion history, they said.
Furthermore, most of the studies were retrospective, and did not include an active comparator group, such as individuals with non-neurologic injuries, they added.
“Until large prospective studies with sufficiently large durations of follow-up are available, we have to rely on the currently available data,” they said in the report.
Dr. Fralick and co-authors reported no conflict of interest disclosures related to the study.
SOURCE: Fralick M, et al. JAMA Neurol. 2018 Nov 12.
This meta-analysis provides a comprehensive review of medical science that suggests a significant association between concussions and later suicide, according to Donald A Redelmeier, MD, and Junaid A. Bhatti, MBBS.
In an editorial, Dr. Redelmeier and Dr. Bhatti noted “media speculation” on the link between concussion and suicide, and commented that medical science progresses more slowly than the news cycle.
“A meta-analysis always has limitations and these authors maintained a thoughtful approach to avoid overstatements,” they said in their editorial.
Although the absolute risks of suicide are modest, this meta-analysis highlights that a concussion could contribute to long-term neuropsychiatric illness, they added.
Health care should aim to prevent concussions, while clinicians need to avoid language such as “dinged” that trivializes the effects of concussion, according to the authors.
In particular, they said neurologists should be aware of the suicide risks highlighted in this meta-analysis, and may want to screen concussion patients for other factors such as mood disorders, substance use, or past suicide attempts, since there is some evidence that concussions may amplify latent psychiatric illnesses.
Likewise, they said, psychiatrists should look for a concussion history when evaluating a particular patient’s risk of suicide.
“We should all recognize that a concussion, in its own way, can be lethal,” the authors concluded.
Dr. Redelmeier and Dr. Bhatti are with the Departments of Medicine and of Surgery, University of Toronto. Their editorial was published in JAMA Neurology. Dr. Redelmeier reported support from the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, and the BrightFocus Foundation, while Dr. Bhatti reported support from the Sunnybrook Research Institute.
This meta-analysis provides a comprehensive review of medical science that suggests a significant association between concussions and later suicide, according to Donald A Redelmeier, MD, and Junaid A. Bhatti, MBBS.
In an editorial, Dr. Redelmeier and Dr. Bhatti noted “media speculation” on the link between concussion and suicide, and commented that medical science progresses more slowly than the news cycle.
“A meta-analysis always has limitations and these authors maintained a thoughtful approach to avoid overstatements,” they said in their editorial.
Although the absolute risks of suicide are modest, this meta-analysis highlights that a concussion could contribute to long-term neuropsychiatric illness, they added.
Health care should aim to prevent concussions, while clinicians need to avoid language such as “dinged” that trivializes the effects of concussion, according to the authors.
In particular, they said neurologists should be aware of the suicide risks highlighted in this meta-analysis, and may want to screen concussion patients for other factors such as mood disorders, substance use, or past suicide attempts, since there is some evidence that concussions may amplify latent psychiatric illnesses.
Likewise, they said, psychiatrists should look for a concussion history when evaluating a particular patient’s risk of suicide.
“We should all recognize that a concussion, in its own way, can be lethal,” the authors concluded.
Dr. Redelmeier and Dr. Bhatti are with the Departments of Medicine and of Surgery, University of Toronto. Their editorial was published in JAMA Neurology. Dr. Redelmeier reported support from the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, and the BrightFocus Foundation, while Dr. Bhatti reported support from the Sunnybrook Research Institute.
This meta-analysis provides a comprehensive review of medical science that suggests a significant association between concussions and later suicide, according to Donald A Redelmeier, MD, and Junaid A. Bhatti, MBBS.
In an editorial, Dr. Redelmeier and Dr. Bhatti noted “media speculation” on the link between concussion and suicide, and commented that medical science progresses more slowly than the news cycle.
“A meta-analysis always has limitations and these authors maintained a thoughtful approach to avoid overstatements,” they said in their editorial.
Although the absolute risks of suicide are modest, this meta-analysis highlights that a concussion could contribute to long-term neuropsychiatric illness, they added.
Health care should aim to prevent concussions, while clinicians need to avoid language such as “dinged” that trivializes the effects of concussion, according to the authors.
In particular, they said neurologists should be aware of the suicide risks highlighted in this meta-analysis, and may want to screen concussion patients for other factors such as mood disorders, substance use, or past suicide attempts, since there is some evidence that concussions may amplify latent psychiatric illnesses.
Likewise, they said, psychiatrists should look for a concussion history when evaluating a particular patient’s risk of suicide.
“We should all recognize that a concussion, in its own way, can be lethal,” the authors concluded.
Dr. Redelmeier and Dr. Bhatti are with the Departments of Medicine and of Surgery, University of Toronto. Their editorial was published in JAMA Neurology. Dr. Redelmeier reported support from the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, and the BrightFocus Foundation, while Dr. Bhatti reported support from the Sunnybrook Research Institute.
Risk of suicide was doubled in persons who experienced a concussion or mild traumatic brain injury (TBI) earlier in life, according to results of a meta-analysis of 17 studies representing nearly 7 million patients.
However, the absolute risk of suicide remained quite low, according to Michael Fralick, MD, of the University of Toronto, and co-investigators.
“Nearly all patients diagnosed with concussion and/or mild TBI did not die by suicide,” Dr. Fralick and colleagues said in their report on the study, which appears in JAMA Neurology.
Nevertheless, the meta-analysis illustrates evidence for an increased risk of suicide, suicide attempts, and suicidal ideation for persons with a history of these injuries, they said in the report.
The meta-analysis included 10 cohort studies, 5 cross-sectional studies, and 2 case-control studies looking at the risk of suicide, suicide attempts, or suicidal ideation after a concussion or mild TBI. Those studies included a roughly 714,000 individuals with a concussion and/or TBI diagnosis, and 6,236,000 without a diagnosis.
For people diagnosed with at least one concussion and/or mild TBI, the risk of suicide was 2-fold higher (relative risk, 2.03; 95% CI, 1.47-2.80; P less than 0.001), according to the report.
The risk was “slightly stronger,” investigators said, when the analysis was limited to studies adjusting for factors associated with those brain injuries and with suicide (RR, 2.10; 95% CI, 1.40-3.13; P less than 0.01).
Four of the 5 cohort studies reported absolute risk of suicide, according to Dr. Fralick and coauthors. In one study with a median follow-up of 3.6 years, 0.50% of individuals with a concussion and/or TBI subsequently died of suicide, while similarly, 0.59% died in a study with 4.0 years of follow-up, 0.28% in a study with 9.3 years follow-up, and 0.49% in one with a 12.3 year median follow-up.
Most of the studies in the meta-analysis reported an increased risk of suicide attempt after concussion and/or mild TBI, according to Dr. Fralick and his collaborators, while the eight studies looking at suicidal ideation all reported heightened risk after those brain injuries.
The researchers acknowledged some limitations of their analysis. Recall bias could have led to an overestimation of the association between concussion and suicide risk, since suicide attempts may affect reporting of concussion history, they said.
Furthermore, most of the studies were retrospective, and did not include an active comparator group, such as individuals with non-neurologic injuries, they added.
“Until large prospective studies with sufficiently large durations of follow-up are available, we have to rely on the currently available data,” they said in the report.
Dr. Fralick and co-authors reported no conflict of interest disclosures related to the study.
SOURCE: Fralick M, et al. JAMA Neurol. 2018 Nov 12.
Risk of suicide was doubled in persons who experienced a concussion or mild traumatic brain injury (TBI) earlier in life, according to results of a meta-analysis of 17 studies representing nearly 7 million patients.
However, the absolute risk of suicide remained quite low, according to Michael Fralick, MD, of the University of Toronto, and co-investigators.
“Nearly all patients diagnosed with concussion and/or mild TBI did not die by suicide,” Dr. Fralick and colleagues said in their report on the study, which appears in JAMA Neurology.
Nevertheless, the meta-analysis illustrates evidence for an increased risk of suicide, suicide attempts, and suicidal ideation for persons with a history of these injuries, they said in the report.
The meta-analysis included 10 cohort studies, 5 cross-sectional studies, and 2 case-control studies looking at the risk of suicide, suicide attempts, or suicidal ideation after a concussion or mild TBI. Those studies included a roughly 714,000 individuals with a concussion and/or TBI diagnosis, and 6,236,000 without a diagnosis.
For people diagnosed with at least one concussion and/or mild TBI, the risk of suicide was 2-fold higher (relative risk, 2.03; 95% CI, 1.47-2.80; P less than 0.001), according to the report.
The risk was “slightly stronger,” investigators said, when the analysis was limited to studies adjusting for factors associated with those brain injuries and with suicide (RR, 2.10; 95% CI, 1.40-3.13; P less than 0.01).
Four of the 5 cohort studies reported absolute risk of suicide, according to Dr. Fralick and coauthors. In one study with a median follow-up of 3.6 years, 0.50% of individuals with a concussion and/or TBI subsequently died of suicide, while similarly, 0.59% died in a study with 4.0 years of follow-up, 0.28% in a study with 9.3 years follow-up, and 0.49% in one with a 12.3 year median follow-up.
Most of the studies in the meta-analysis reported an increased risk of suicide attempt after concussion and/or mild TBI, according to Dr. Fralick and his collaborators, while the eight studies looking at suicidal ideation all reported heightened risk after those brain injuries.
The researchers acknowledged some limitations of their analysis. Recall bias could have led to an overestimation of the association between concussion and suicide risk, since suicide attempts may affect reporting of concussion history, they said.
Furthermore, most of the studies were retrospective, and did not include an active comparator group, such as individuals with non-neurologic injuries, they added.
“Until large prospective studies with sufficiently large durations of follow-up are available, we have to rely on the currently available data,” they said in the report.
Dr. Fralick and co-authors reported no conflict of interest disclosures related to the study.
SOURCE: Fralick M, et al. JAMA Neurol. 2018 Nov 12.
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: For people diagnosed with at least one concussion and/or mild TBI, the risk of suicide was 2-fold higher.
Study details: A meta-analysis of 17 studies representing nearly 7 million individuals with or without a concussion diagnosis.
Disclosures: The authors reported no conflicts of interest.
Source: Fralick M, et al. JAMA Neurol. 2018 Nov 12.
Nearly half of infants don’t sleep through the night at 1 year
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Multiple studies looking at whether sleep matters in infants and no clear consensus, the answer going forward may depend on the primary outcome evaluated, Jodi A. Mindell, PhD, and Melisa Moore, PhD
“The jury is still out,” Dr. Mindell and Dr. Moore wrote in an editorial discussing the present study, which like others before it have found no relationship or limited relationships between infant sleep and later development.
On the other hand, several studies have found that fragmented sleep is associated with negative outcomes with respect to development, the editorial authors said.
One reason for the lack of agreement between studies may be differences in measurement, as the studies to date have used a variety of different measures for both sleep and development, they said. Moreover, the age of infants varies across studies, as does their location, raising the possibility that cultural differences may account for the disparate results.
Beyond that, they added, there is no single primary sleep outcome that has been applied, with some studies looking at sleep duration, and others looking at sleep consolidation, longest stretch of sleep, or duration of night wakings.
What some of these studies may miss is that many other factors may influence development, including genetics, nutrition, parental education, and interaction between child and parent.
“Sleep may be a drop in the bucket for broad development but, instead, have a more significant impact on next-day functioning,” they said.
Thus, the editorialists propose that future studies evaluate function instead of development to assess the importance of infant sleep, as some studies to date have shown that sleep in infants is important for language learning and memory consolidation.
“Rather than investigate gross development, we propose that day-to-day functioning and skill development may be better indicators of the impact of sleep on development in early childhood,” they concluded.
Dr. Mindell, and Dr. Moore are with the Sleep Center, Children’s Hospital of Philadelphia. Their editorial appears in Pediatrics. Dr. Mindell reported she is a consultant for Johnson & Johnson Consumer. Dr. Moore reported no financial relationships relevant to the article.
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Just over half of infants get 8 hours of uninterrupted sleep at 12 months of age, an analysis of findings from a longitudinal birth cohort study showed.
It also found that whether an infant sleeps through night has no significant associated with any variations in mental or psychomotor development.
However, the rate of breastfeeding was significantly higher among infants who did not sleep through the night, investigators said in their report on the analysis, published in Pediatrics.
Being informed about the normal development of the sleep-wake cycle could be reassuring for parents, according to the authors, who said that new mothers tend to be “greatly surprised” by the sleep disturbance and exhaustion they experience.
“Keeping in mind the wide variability in the age when an infant starts to sleep through the night, expectations for early sleep consolidation could be moderated,” said Marie-Hélène Pennestri, PhD, of the Department of Educational and Counselling Psychology at McGill University, Montreal, and her coauthors.
Dr. Pennestri and colleagues reported on 388 mother-infant dyads in a longitudinal birth cohort study called Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN). Pregnant mothers were recruited from obstetric clinics in Canada. When their infants reached the age of 6 and 12 months, the mothers responded to questionnaires about sleep habits.
At 6 months, 62.4% of infants attained at least 6 hours of uninterrupted sleep, mothers reported, while 43.0% had reached 8 hours, the mothers reported. By 12 months of age, 72.1% of the infants attained 6 hours, and 56.6% attained 8 hours.
There were no associations between sleeping through the night and concurrent mental or psychomotor development, as measured by the Bayley Scales of Infant Development II at both 6 or 12 months of age, with P values greater than 0.05, investigators reported.
A similar lack of association between uninterrupted sleep and development or maternal mood was seen in a follow-up measurement at 36 months of age.
Sleeping through the night was likewise not associated with maternal mood, assessed using a depression scale with items that reflected symptom frequency in the previous week. “This is noteworthy because maternal sleep deprivation is often invoked to support the introduction of early behavioral interventions,” investigators said in a discussion of the results.
By contrast, sleeping through the night was linked to lower rates of breastfeeding as reported by mothers on retrospective questionnaires administered at both 6 and 12 months. At 12 months of age, 22.1% of infants sleeping through the night were breastfed, compared to 47.1% of infants not sleeping through the night (P less than 0.0001), Dr. Pennestri and colleagues reported.
However, that breastfeeding observation needs to be further investigated, according to the authors.
“The results of our study do not allow for the drawing of any causality between not sleeping through the night and breastfeeding,” they wrote.
Dr. Pennestri and coauthors said they had no financial relationships or potential conflicts of interest to disclose relevant to their report. They reported funding from the Ludmer Center for Neuroinformatics and Mental Health, Canadian Institutes of Health Research, and several other research institutions.
SOURCE: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
FROM PEDIATRICS
Key clinical point: Sleeping through the night in infancy was not significantly associated with any variations in development or maternal mood.
Major finding: There were no associations between 6 or 8 hours of uninterrupted sleep and concurrent mental or psychomotor development or reported depressive symptoms.
Study details: An analysis of 388 mother-infant dyads in a longitudinal birth cohort study.
Disclosures: Authors said they had no financial relationships or potential conflicts of interest to disclose.
Source: Pennestri MH, et al. Pediatrics. 2018;142(6):e20174330.
Apixaban is safest effective DOAC for stroke prevention in Afib, per AHRQ report
, according to results of an updated comparative effectiveness review.
Dabigatran (Pradaxa), by contrast, has shown reductions in stroke events but a similar rate of bleeding events compared to warfarin, according to the report from the Duke Evidence-based Practice Center, Durham, N.C.
Rivaroxaban (Xarelto), meanwhile, is “similar in both benefits and harms with warfarin” in evidence to date, investigators wrote in the report, which was prepared for the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI).
Finally, edoxaban (Savaysa) is “most likely similar” to warfarin with respect to preventing stroke or systemic embolism, with less risk for major bleeding and hemorrhagic stroke, investigators wrote in a summary of their findings on the AHRQ website.
“Effectiveness of these direct oral anticoagulants as compared to one another however is limited by the lack of randomized studies directly comparing their safety and effectiveness,” concluded investigators, led by Gillian D. Sanders, PhD, of Duke University.
The 612-page report details a systematic review based on 320 articles representing 185 unique studies. The review was designed to update a 2013 AHRQ report that evaluated evidence not only for treatment options to prevent stroke in patients with atrial fibrillation, but also for tools used to predict risk of stroke or bleeding.
In the 2013 report, investigators concluded that the newer anticoagulants showed “early promise” in reducing stroke and bleeding events compared with warfarin.
That earlier report said that CHA2 and CHA2DS2-VASc had the best evidence to support prediction of stroke events, while HAS-BLED provided the best discrimination of bleeding risk.
The updated report adds the ABC stroke risk score as a tool that, along with CHADS2 and CHA2DS2-VASc, has the “best evidence” predicting thromboembolic risk, authors said.
Imaging tools, on the other hand, still need more evidence supporting their use to predict thromboembolic risk, Dr. Sanders and colleagues said in their report.
The literature review, which covered the January 2000 through February 2018, turned up 61 studies relevant to predicting thromboembolic risk, 38 on bleeding risk, and 117 on preventing thromboembolic events with anticoagulation therapies, antiplatelet therapies, or procedures.
Direct oral anticoagulants were evaluated in randomized clinical trials that were “often very large, of good quality, and considered definitive in the field,” Dr. Sanders and colleagues wrote in their report.
However, these trials were constrained to comparing direct oral anticoagulants with warfarin or aspirin, and have not involved head-to-head comparison among the newer agents, they added.
“Based on these trials though, clinical leaders and professional societies have determined that these newer agents are better than the prior lone treatment of warfarin in terms of stroke prevention, side effects, and risk of bleeding,” they said in the published report.
SOURCE: Sanders GD, et al. 2018 Oct 30. AHRQ Publication No. 18(19)-EHC018-EF.
, according to results of an updated comparative effectiveness review.
Dabigatran (Pradaxa), by contrast, has shown reductions in stroke events but a similar rate of bleeding events compared to warfarin, according to the report from the Duke Evidence-based Practice Center, Durham, N.C.
Rivaroxaban (Xarelto), meanwhile, is “similar in both benefits and harms with warfarin” in evidence to date, investigators wrote in the report, which was prepared for the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI).
Finally, edoxaban (Savaysa) is “most likely similar” to warfarin with respect to preventing stroke or systemic embolism, with less risk for major bleeding and hemorrhagic stroke, investigators wrote in a summary of their findings on the AHRQ website.
“Effectiveness of these direct oral anticoagulants as compared to one another however is limited by the lack of randomized studies directly comparing their safety and effectiveness,” concluded investigators, led by Gillian D. Sanders, PhD, of Duke University.
The 612-page report details a systematic review based on 320 articles representing 185 unique studies. The review was designed to update a 2013 AHRQ report that evaluated evidence not only for treatment options to prevent stroke in patients with atrial fibrillation, but also for tools used to predict risk of stroke or bleeding.
In the 2013 report, investigators concluded that the newer anticoagulants showed “early promise” in reducing stroke and bleeding events compared with warfarin.
That earlier report said that CHA2 and CHA2DS2-VASc had the best evidence to support prediction of stroke events, while HAS-BLED provided the best discrimination of bleeding risk.
The updated report adds the ABC stroke risk score as a tool that, along with CHADS2 and CHA2DS2-VASc, has the “best evidence” predicting thromboembolic risk, authors said.
Imaging tools, on the other hand, still need more evidence supporting their use to predict thromboembolic risk, Dr. Sanders and colleagues said in their report.
The literature review, which covered the January 2000 through February 2018, turned up 61 studies relevant to predicting thromboembolic risk, 38 on bleeding risk, and 117 on preventing thromboembolic events with anticoagulation therapies, antiplatelet therapies, or procedures.
Direct oral anticoagulants were evaluated in randomized clinical trials that were “often very large, of good quality, and considered definitive in the field,” Dr. Sanders and colleagues wrote in their report.
However, these trials were constrained to comparing direct oral anticoagulants with warfarin or aspirin, and have not involved head-to-head comparison among the newer agents, they added.
“Based on these trials though, clinical leaders and professional societies have determined that these newer agents are better than the prior lone treatment of warfarin in terms of stroke prevention, side effects, and risk of bleeding,” they said in the published report.
SOURCE: Sanders GD, et al. 2018 Oct 30. AHRQ Publication No. 18(19)-EHC018-EF.
, according to results of an updated comparative effectiveness review.
Dabigatran (Pradaxa), by contrast, has shown reductions in stroke events but a similar rate of bleeding events compared to warfarin, according to the report from the Duke Evidence-based Practice Center, Durham, N.C.
Rivaroxaban (Xarelto), meanwhile, is “similar in both benefits and harms with warfarin” in evidence to date, investigators wrote in the report, which was prepared for the Agency for Healthcare Research and Quality (AHRQ) and the Patient-Centered Outcomes Research Institute (PCORI).
Finally, edoxaban (Savaysa) is “most likely similar” to warfarin with respect to preventing stroke or systemic embolism, with less risk for major bleeding and hemorrhagic stroke, investigators wrote in a summary of their findings on the AHRQ website.
“Effectiveness of these direct oral anticoagulants as compared to one another however is limited by the lack of randomized studies directly comparing their safety and effectiveness,” concluded investigators, led by Gillian D. Sanders, PhD, of Duke University.
The 612-page report details a systematic review based on 320 articles representing 185 unique studies. The review was designed to update a 2013 AHRQ report that evaluated evidence not only for treatment options to prevent stroke in patients with atrial fibrillation, but also for tools used to predict risk of stroke or bleeding.
In the 2013 report, investigators concluded that the newer anticoagulants showed “early promise” in reducing stroke and bleeding events compared with warfarin.
That earlier report said that CHA2 and CHA2DS2-VASc had the best evidence to support prediction of stroke events, while HAS-BLED provided the best discrimination of bleeding risk.
The updated report adds the ABC stroke risk score as a tool that, along with CHADS2 and CHA2DS2-VASc, has the “best evidence” predicting thromboembolic risk, authors said.
Imaging tools, on the other hand, still need more evidence supporting their use to predict thromboembolic risk, Dr. Sanders and colleagues said in their report.
The literature review, which covered the January 2000 through February 2018, turned up 61 studies relevant to predicting thromboembolic risk, 38 on bleeding risk, and 117 on preventing thromboembolic events with anticoagulation therapies, antiplatelet therapies, or procedures.
Direct oral anticoagulants were evaluated in randomized clinical trials that were “often very large, of good quality, and considered definitive in the field,” Dr. Sanders and colleagues wrote in their report.
However, these trials were constrained to comparing direct oral anticoagulants with warfarin or aspirin, and have not involved head-to-head comparison among the newer agents, they added.
“Based on these trials though, clinical leaders and professional societies have determined that these newer agents are better than the prior lone treatment of warfarin in terms of stroke prevention, side effects, and risk of bleeding,” they said in the published report.
SOURCE: Sanders GD, et al. 2018 Oct 30. AHRQ Publication No. 18(19)-EHC018-EF.
No difference for blacks vs. whites in precancerous colorectal neoplasm prevalence: A meta-analysis
, prompting investigators to suggest that the age at which screening starts need not differ based on race.
There was also no difference in advanced neoplasia in the proximal colon between black and white screen-eligible individuals in the most rigorous of the studies included in the meta-analysis, investigators reported.
Those findings support eliminating the age difference at which to begin screening of average-risk individuals, as is currently recommended in some guidelines, said Thomas F. Imperiale, MD, the Lawrence Lumeng Professor of Gastroenterology and Hepatology at Indiana University, Indianapolis, and his coinvestigators.
In areas with no disparities in screening access, average-risk screening could begin at age 50 years, regardless of race, at least based on results of this meta-analysis, Dr. Imperiale and his colleagues said in their report.
“To the extent that advanced adenoma is the precursor lesion for colorectal cancer, tailoring the age at which to begin screening and how to screen based on race is not supported by our findings,” they said in the report, which appears in the journal Gastroenterology.
Dr. Imperiale and his coinvestigators scanned the medical literature and identified nine studies looking at the prevalence of advanced adenomas or advanced precancerous colorectal neoplasms in both black and white individuals of average risk who had undergone screening colonoscopy.
Those nine cross-sectional studies, all published during 2010-2017, represented a total of 302,128 participants. Six studies were of high methodologic quality and had a low risk of bias, while the remaining three failed to adjust for age and sex, authors of the meta-analysis said in their report.
Prevalence of advanced adenomas or advanced precancerous colorectal neoplasms ranged from 2% to 10% for whites and from 5% to 12% for blacks in the nine studies, with only one study, which had no histology results available, showing a higher prevalence in blacks, investigators found.
Taken together, there was no difference between racial groups, with a point prevalence of 6.57% for blacks and 6.20% for whites (odds ratio, 1.03; 95% confidence interval, 0.81-1.30) and an absolute risk difference of zero, according to the statistical analysis.
Of five studies that included data on proximal advanced adenomas or advanced precancerous colorectal neoplasms, two showed a greater prevalence in blacks versus whites, with point prevalences of 3.30% and 2.42%, respectively. However, there was no difference in prevalence for the “best subset” of three studies with a moderate degree of heterogeneity, investigators said.
Given these findings, the higher colorectal cancer incidence and mortality seen in black adults is less likely because of biology, and more likely from differences in symptom recognition, diagnostic evaluation, or acceptance of preventive services, Dr. Imperiale and his coauthors said in a discussion of the results.
Some current guidelines suggest starting colorectal cancer screening at age 40 years for average-risk blacks, which is 5-10 years earlier than for nonblacks, investigators said, though of note, the most recent American Cancer Society recommendations recommend screening starting at age 45 years for all average-risk individuals.
“If this recommendation is followed broadly, it would lessen the clinical and policy implications of our findings,” they wrote. “However, the uptake of this recommendation is yet to be determined, as it differs from those of all other professional organizations.”
The study was supported by Indiana CTSI Collaboration in Translational Research Grants. Dr. Imperiale and hiscoauthors reported no conflicts of interest.
SOURCE: Imperiale TF et al. Gastroenterology. 2018 Aug 21. doi: 10.1053/j.gastro.2018.08.020.
, prompting investigators to suggest that the age at which screening starts need not differ based on race.
There was also no difference in advanced neoplasia in the proximal colon between black and white screen-eligible individuals in the most rigorous of the studies included in the meta-analysis, investigators reported.
Those findings support eliminating the age difference at which to begin screening of average-risk individuals, as is currently recommended in some guidelines, said Thomas F. Imperiale, MD, the Lawrence Lumeng Professor of Gastroenterology and Hepatology at Indiana University, Indianapolis, and his coinvestigators.
In areas with no disparities in screening access, average-risk screening could begin at age 50 years, regardless of race, at least based on results of this meta-analysis, Dr. Imperiale and his colleagues said in their report.
“To the extent that advanced adenoma is the precursor lesion for colorectal cancer, tailoring the age at which to begin screening and how to screen based on race is not supported by our findings,” they said in the report, which appears in the journal Gastroenterology.
Dr. Imperiale and his coinvestigators scanned the medical literature and identified nine studies looking at the prevalence of advanced adenomas or advanced precancerous colorectal neoplasms in both black and white individuals of average risk who had undergone screening colonoscopy.
Those nine cross-sectional studies, all published during 2010-2017, represented a total of 302,128 participants. Six studies were of high methodologic quality and had a low risk of bias, while the remaining three failed to adjust for age and sex, authors of the meta-analysis said in their report.
Prevalence of advanced adenomas or advanced precancerous colorectal neoplasms ranged from 2% to 10% for whites and from 5% to 12% for blacks in the nine studies, with only one study, which had no histology results available, showing a higher prevalence in blacks, investigators found.
Taken together, there was no difference between racial groups, with a point prevalence of 6.57% for blacks and 6.20% for whites (odds ratio, 1.03; 95% confidence interval, 0.81-1.30) and an absolute risk difference of zero, according to the statistical analysis.
Of five studies that included data on proximal advanced adenomas or advanced precancerous colorectal neoplasms, two showed a greater prevalence in blacks versus whites, with point prevalences of 3.30% and 2.42%, respectively. However, there was no difference in prevalence for the “best subset” of three studies with a moderate degree of heterogeneity, investigators said.
Given these findings, the higher colorectal cancer incidence and mortality seen in black adults is less likely because of biology, and more likely from differences in symptom recognition, diagnostic evaluation, or acceptance of preventive services, Dr. Imperiale and his coauthors said in a discussion of the results.
Some current guidelines suggest starting colorectal cancer screening at age 40 years for average-risk blacks, which is 5-10 years earlier than for nonblacks, investigators said, though of note, the most recent American Cancer Society recommendations recommend screening starting at age 45 years for all average-risk individuals.
“If this recommendation is followed broadly, it would lessen the clinical and policy implications of our findings,” they wrote. “However, the uptake of this recommendation is yet to be determined, as it differs from those of all other professional organizations.”
The study was supported by Indiana CTSI Collaboration in Translational Research Grants. Dr. Imperiale and hiscoauthors reported no conflicts of interest.
SOURCE: Imperiale TF et al. Gastroenterology. 2018 Aug 21. doi: 10.1053/j.gastro.2018.08.020.
, prompting investigators to suggest that the age at which screening starts need not differ based on race.
There was also no difference in advanced neoplasia in the proximal colon between black and white screen-eligible individuals in the most rigorous of the studies included in the meta-analysis, investigators reported.
Those findings support eliminating the age difference at which to begin screening of average-risk individuals, as is currently recommended in some guidelines, said Thomas F. Imperiale, MD, the Lawrence Lumeng Professor of Gastroenterology and Hepatology at Indiana University, Indianapolis, and his coinvestigators.
In areas with no disparities in screening access, average-risk screening could begin at age 50 years, regardless of race, at least based on results of this meta-analysis, Dr. Imperiale and his colleagues said in their report.
“To the extent that advanced adenoma is the precursor lesion for colorectal cancer, tailoring the age at which to begin screening and how to screen based on race is not supported by our findings,” they said in the report, which appears in the journal Gastroenterology.
Dr. Imperiale and his coinvestigators scanned the medical literature and identified nine studies looking at the prevalence of advanced adenomas or advanced precancerous colorectal neoplasms in both black and white individuals of average risk who had undergone screening colonoscopy.
Those nine cross-sectional studies, all published during 2010-2017, represented a total of 302,128 participants. Six studies were of high methodologic quality and had a low risk of bias, while the remaining three failed to adjust for age and sex, authors of the meta-analysis said in their report.
Prevalence of advanced adenomas or advanced precancerous colorectal neoplasms ranged from 2% to 10% for whites and from 5% to 12% for blacks in the nine studies, with only one study, which had no histology results available, showing a higher prevalence in blacks, investigators found.
Taken together, there was no difference between racial groups, with a point prevalence of 6.57% for blacks and 6.20% for whites (odds ratio, 1.03; 95% confidence interval, 0.81-1.30) and an absolute risk difference of zero, according to the statistical analysis.
Of five studies that included data on proximal advanced adenomas or advanced precancerous colorectal neoplasms, two showed a greater prevalence in blacks versus whites, with point prevalences of 3.30% and 2.42%, respectively. However, there was no difference in prevalence for the “best subset” of three studies with a moderate degree of heterogeneity, investigators said.
Given these findings, the higher colorectal cancer incidence and mortality seen in black adults is less likely because of biology, and more likely from differences in symptom recognition, diagnostic evaluation, or acceptance of preventive services, Dr. Imperiale and his coauthors said in a discussion of the results.
Some current guidelines suggest starting colorectal cancer screening at age 40 years for average-risk blacks, which is 5-10 years earlier than for nonblacks, investigators said, though of note, the most recent American Cancer Society recommendations recommend screening starting at age 45 years for all average-risk individuals.
“If this recommendation is followed broadly, it would lessen the clinical and policy implications of our findings,” they wrote. “However, the uptake of this recommendation is yet to be determined, as it differs from those of all other professional organizations.”
The study was supported by Indiana CTSI Collaboration in Translational Research Grants. Dr. Imperiale and hiscoauthors reported no conflicts of interest.
SOURCE: Imperiale TF et al. Gastroenterology. 2018 Aug 21. doi: 10.1053/j.gastro.2018.08.020.
FROM GASTROENTEROLOGY
Key clinical point: Rates of advanced precancerous neoplasia did not differ between average-risk black and white individuals who underwent screening colonoscopy; starting age for screening may need not differ based on race.
Major finding: There was no difference between racial groups, with a point prevalence of 6.57% for blacks and 6.20% for whites (odds ratio, 1.03; 95% confidence interval, 0.81-1.30).
Study details: A meta-analysis of nine cross-sectional studies published during 2010-2017 and representing 302,128 individuals.
Disclosures: The study was supported by Indiana CTSI Collaboration in Translational Research Grants. Dr. Imperiale and coauthors reported no conflicts of interest.
Source: Imperiale TF et al. Gastroenterology. 2018 Aug 21. doi: 10.1053/j.gastro.2018.08.020.
Single-dose zoliflodacin successfully treats uncomplicated urogenital gonorrhea
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
This study represents a “step forward” in identifying new antimicrobial treatment options for patients with gonorrhea, according to Susan Blank, MD, and Demetre C. Daskalakis, MD.
“Given the challenges in clinical follow-up in this patient population, the single-dose regimen is promising,” Dr. Blank and Dr. Daskalakis wrote in a editorial.
While zoliflodacin has the potential to be an effective treatment for gonorrhea, its activity needs to be better defined, particularly in key anatomical sites of infection such as the pharynx, where limited activity was observed.
Progression of resistance of Neisseria gonorrhoeae is an “ever-present concern” given the history of the organism, the authors wrote.
“We are facing the real danger of multidrug-resistant, nearly untreatable gonorrhea,” they wrote. “To avoid untreatable cases of this high-incidence infection, we need to advance diagnostic technology and develop treatments with different mechanisms of action.”
Dr. Blank and Dr. Daskalakis are with the division of disease control in the New York City Department of Health and Mental Hygiene. Their editorial appears in the New England Journal of Medicine . Both reported having no conflicts of interest.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
A new antibiotic successfully treated uncomplicated urogenital and rectal gonococcal infections, but was not as effective as ceftriaxone in treating pharyngeal infections, according to the results of a randomized, phase 2 study.
About 96% of patients with infection at urogenital sites had microbiologic cure with zoliflodacin, a novel antimicrobial agent that inhibits DNA biosynthesis. The cure rate was 100% for rectal infections, but was just 50%-82% for pharyngeal infections, though few participants in this study had infection at either of those sites.
The study investigators, led by Stephanie N. Taylor, MD, professor of medicine and microbiology at Louisiana State University, New Orleans, wrote that the need for new antimicrobial agents has been underscored by reports of multidrug-resistant Neisseria gonorrhoeae and the possibility of untreatable gonorrhea.
“This phase 2 trial creates equipoise for larger, more definitive studies of zoliflodacin,” Dr. Taylor and her coauthors wrote in the New England Journal of Medicine.
At this point, N. gonorrhoeae has developed resistance to every recommended antibiotic class, including cephalosporins and macrolides, they added.
Zoliflodacin (ETX0914) is an antimicrobial that has received fast-track designation from the Food and Drug Administration specifically for development as an oral treatment for gonococcal infections, the authors noted.
“The mechanism of action of zoliflodacin differs from currently available therapies in that it inhibits microbial biosynthesis by arresting the cleaved covalent gyrase complex and the formation of fused circular DNA required for biosynthesis,” they wrote.
Dr. Taylor and her colleagues studied single 2- and 3-gram doses of zoliflodacin in comparison with 500 mg of intramuscular ceftriaxone in 181 patients with uncomplicated urogenital gonorrhea enrolled in the open-label, randomized, phase 2 study between November 2014 and December 2015.
A total of 141 patients included in the microbiologic intention-to-treat analysis had confirmed positive urethral or cervical cultures. Cures were seen in 55 of 57 infections treated with 2 grams (96%) and 54 of 56 treated with 3 grams (96%) of zoliflodacin, and in 28 of 28 infections (100%) treated with ceftriaxone.
Of 15 confirmed rectal infections, 100% were cured, including 12 treated with zoliflodacin at 2 or 3 grams and 3 treated with ceftriaxone. Of 23 confirmed pharyngeal infections, cures were seen in 4 of 8 (50%) treated with 2 grams of zoliflodacin and 9 of 11 (82%) treated with 3 grams, compared with 4 of 4 cured (100%) with ceftriaxone.
That suggests zoliflodacin was not as effective as ceftriaxone in treating pharyngeal gonorrhea, which is generally considered more difficult to treat than infections at other sites, according to Dr. Taylor and her coauthors.
“Currently, this limitation has not curtailed recommendations for the use of drugs such as spectinomycin or fluoroquinolones for the treatment of gonorrhea,” they wrote.
The study was funded by the National Institutes of Health and Entasis Therapeutics. Dr. Taylor reported grants from the NIH during the study, and other disclosures related to a variety of pharma companies. Her coauthors reported disclosures related to AstraZeneca (parent company of Entasis, which is developing zoliflodacin) and other pharmaceutical companies.
SOURCE: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Cure rates of 96% and 100% were reported for urethral/cervical and rectal infections, respectively, and ranged from 50% to 82% in pharyngeal infections.
Study details: A randomized, open-label, phase 2 study including 181 patients with uncomplicated urogenital gonorrhea who received zoliflodacin or ceftriaxone.
Disclosures: The study authors reported disclosures related to AstraZeneca (parent company of Entasis Therapeutics, which is developing zoliflodacin) and other pharmaceutical companies.
Source: Taylor SN et al. N Engl J Med. 2018 Nov 7; 379:1835-45.
Caffeinated coffee intake linked to lower rosacea risk
Caffeinated coffee intake is linked to a decreased incidence of rosacea, results of a large, observational study suggest.
Increased levels of caffeinated coffee consumption were associated with progressively lower levels of incident rosacea in a study of more than 82,000 participants representing more than 1.1 million person-years of follow-up.
By contrast, caffeine from other foods was not associated with rosacea incidence, reported Wen-Qing Li, PhD, of the department of dermatology at Brown University, Providence, R.I., and his coinvestigators. Those findings may have implications for the “causes and clinical approach” to rosacea.
“Our findings do not support limiting caffeine intake as a preventive strategy for rosacea,” they concluded in the study, published in JAMA Dermatology.
This is not the first study looking for potential links between rosacea and caffeine or coffee intake. However, previous studies didn’t distinguish between caffeinated coffee versus other beverages, and only one previous study made a distinction between the amounts of caffeine and coffee consumed, according to the authors.
Their research was based on data from the Nurses’ Health Study II, a prospective cohort study started in 1989. They looked specifically at 82,737 women who, in 2005, responded to the question about whether they had been diagnosed with rosacea. They identified 4,945 incident rosacea cases over the 1,120,051 person-years of follow-up.
A significant inverse association was found between caffeinated coffee intake and rosacea: Individuals who consumed four or more servings a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001). They also found a dose-dependent effect, with the absolute risk of rosacea decreased by 131 per 100,000 person-years with at least four daily servings of caffeinated coffee, compared with under one serving a month.
By contrast, decaffeinated coffee was not associated with a reduced risk of rosacea, and in further analysis, the investigators found that there was no significant inverse association when they looked just at caffeine intake from sources other than coffee, such as chocolate, tea, and soda.
Caffeine could influence rosacea incidence by one of several mechanisms, including its effect on vascular contractility, the investigators hypothesized. “Increased caffeine intake may decrease vasodilation and consequently lead to diminution of rosacea symptoms.”
However, caffeine also has documented immunosuppressant effects that could possibly decrease rosacea-associated inflammation and has been shown to modulate hormone levels. “Hormonal factors have been implicated in the development of rosacea, and caffeine can modulate hormone levels,” they wrote.
Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from several sources, including National Institutes of Health grants for the Nurses’ Health Study II.
SOURCE: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
This study shows an inverse association between caffeine intake and incidence rosacea, which suggests that patients with rosacea need not avoid coffee, according to Mackenzie R. Wehner, MD, and Eleni Linos, MD, MPH.
For everyone else, the findings offer yet another reason to keep indulging in one of “life’s habitual pleasures,” they wrote. “We will raise an insulated travel mug to that.”
This latest study fits in with numerous studies suggesting coffee may be protective against a number of maladies, including cancer, cardiovascular disease, type 2 diabetes, and Parkinson’s disease, they wrote in their editorial published in JAMA Dermatology.
However, this is an observational study, not a rigorous, randomized trial that could more conclusively prove coffee actually provides an antirosacea benefit that cannot be explained by other factors, such as systematic differences between people who do and do not drink coffee. Enrollment of all women, mostly white, in the Nurses’ Health Study II is another limitation, they added.
Nevertheless, studies like this are the “next-best option” in lieu of randomized, controlled trials to evaluate these relationships, they wrote. “Importantly, the strength of the protective effect noted and the dose-response relationship with increasing coffee and caffeine intake are convincing.”
Dr. Wehner , is with the department of dermatology at the University of Pennsylvania, Philadelphia. Dr. Linos is with the department of dermatology at the University of California, San Francisco. Dr. Wehner reported support from a National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Dermatology Research Training grant. Dr. Linos reported support from the National Cancer Institute and the National Institute of Aging.
This study shows an inverse association between caffeine intake and incidence rosacea, which suggests that patients with rosacea need not avoid coffee, according to Mackenzie R. Wehner, MD, and Eleni Linos, MD, MPH.
For everyone else, the findings offer yet another reason to keep indulging in one of “life’s habitual pleasures,” they wrote. “We will raise an insulated travel mug to that.”
This latest study fits in with numerous studies suggesting coffee may be protective against a number of maladies, including cancer, cardiovascular disease, type 2 diabetes, and Parkinson’s disease, they wrote in their editorial published in JAMA Dermatology.
However, this is an observational study, not a rigorous, randomized trial that could more conclusively prove coffee actually provides an antirosacea benefit that cannot be explained by other factors, such as systematic differences between people who do and do not drink coffee. Enrollment of all women, mostly white, in the Nurses’ Health Study II is another limitation, they added.
Nevertheless, studies like this are the “next-best option” in lieu of randomized, controlled trials to evaluate these relationships, they wrote. “Importantly, the strength of the protective effect noted and the dose-response relationship with increasing coffee and caffeine intake are convincing.”
Dr. Wehner , is with the department of dermatology at the University of Pennsylvania, Philadelphia. Dr. Linos is with the department of dermatology at the University of California, San Francisco. Dr. Wehner reported support from a National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Dermatology Research Training grant. Dr. Linos reported support from the National Cancer Institute and the National Institute of Aging.
This study shows an inverse association between caffeine intake and incidence rosacea, which suggests that patients with rosacea need not avoid coffee, according to Mackenzie R. Wehner, MD, and Eleni Linos, MD, MPH.
For everyone else, the findings offer yet another reason to keep indulging in one of “life’s habitual pleasures,” they wrote. “We will raise an insulated travel mug to that.”
This latest study fits in with numerous studies suggesting coffee may be protective against a number of maladies, including cancer, cardiovascular disease, type 2 diabetes, and Parkinson’s disease, they wrote in their editorial published in JAMA Dermatology.
However, this is an observational study, not a rigorous, randomized trial that could more conclusively prove coffee actually provides an antirosacea benefit that cannot be explained by other factors, such as systematic differences between people who do and do not drink coffee. Enrollment of all women, mostly white, in the Nurses’ Health Study II is another limitation, they added.
Nevertheless, studies like this are the “next-best option” in lieu of randomized, controlled trials to evaluate these relationships, they wrote. “Importantly, the strength of the protective effect noted and the dose-response relationship with increasing coffee and caffeine intake are convincing.”
Dr. Wehner , is with the department of dermatology at the University of Pennsylvania, Philadelphia. Dr. Linos is with the department of dermatology at the University of California, San Francisco. Dr. Wehner reported support from a National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Dermatology Research Training grant. Dr. Linos reported support from the National Cancer Institute and the National Institute of Aging.
Caffeinated coffee intake is linked to a decreased incidence of rosacea, results of a large, observational study suggest.
Increased levels of caffeinated coffee consumption were associated with progressively lower levels of incident rosacea in a study of more than 82,000 participants representing more than 1.1 million person-years of follow-up.
By contrast, caffeine from other foods was not associated with rosacea incidence, reported Wen-Qing Li, PhD, of the department of dermatology at Brown University, Providence, R.I., and his coinvestigators. Those findings may have implications for the “causes and clinical approach” to rosacea.
“Our findings do not support limiting caffeine intake as a preventive strategy for rosacea,” they concluded in the study, published in JAMA Dermatology.
This is not the first study looking for potential links between rosacea and caffeine or coffee intake. However, previous studies didn’t distinguish between caffeinated coffee versus other beverages, and only one previous study made a distinction between the amounts of caffeine and coffee consumed, according to the authors.
Their research was based on data from the Nurses’ Health Study II, a prospective cohort study started in 1989. They looked specifically at 82,737 women who, in 2005, responded to the question about whether they had been diagnosed with rosacea. They identified 4,945 incident rosacea cases over the 1,120,051 person-years of follow-up.
A significant inverse association was found between caffeinated coffee intake and rosacea: Individuals who consumed four or more servings a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001). They also found a dose-dependent effect, with the absolute risk of rosacea decreased by 131 per 100,000 person-years with at least four daily servings of caffeinated coffee, compared with under one serving a month.
By contrast, decaffeinated coffee was not associated with a reduced risk of rosacea, and in further analysis, the investigators found that there was no significant inverse association when they looked just at caffeine intake from sources other than coffee, such as chocolate, tea, and soda.
Caffeine could influence rosacea incidence by one of several mechanisms, including its effect on vascular contractility, the investigators hypothesized. “Increased caffeine intake may decrease vasodilation and consequently lead to diminution of rosacea symptoms.”
However, caffeine also has documented immunosuppressant effects that could possibly decrease rosacea-associated inflammation and has been shown to modulate hormone levels. “Hormonal factors have been implicated in the development of rosacea, and caffeine can modulate hormone levels,” they wrote.
Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from several sources, including National Institutes of Health grants for the Nurses’ Health Study II.
SOURCE: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
Caffeinated coffee intake is linked to a decreased incidence of rosacea, results of a large, observational study suggest.
Increased levels of caffeinated coffee consumption were associated with progressively lower levels of incident rosacea in a study of more than 82,000 participants representing more than 1.1 million person-years of follow-up.
By contrast, caffeine from other foods was not associated with rosacea incidence, reported Wen-Qing Li, PhD, of the department of dermatology at Brown University, Providence, R.I., and his coinvestigators. Those findings may have implications for the “causes and clinical approach” to rosacea.
“Our findings do not support limiting caffeine intake as a preventive strategy for rosacea,” they concluded in the study, published in JAMA Dermatology.
This is not the first study looking for potential links between rosacea and caffeine or coffee intake. However, previous studies didn’t distinguish between caffeinated coffee versus other beverages, and only one previous study made a distinction between the amounts of caffeine and coffee consumed, according to the authors.
Their research was based on data from the Nurses’ Health Study II, a prospective cohort study started in 1989. They looked specifically at 82,737 women who, in 2005, responded to the question about whether they had been diagnosed with rosacea. They identified 4,945 incident rosacea cases over the 1,120,051 person-years of follow-up.
A significant inverse association was found between caffeinated coffee intake and rosacea: Individuals who consumed four or more servings a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001). They also found a dose-dependent effect, with the absolute risk of rosacea decreased by 131 per 100,000 person-years with at least four daily servings of caffeinated coffee, compared with under one serving a month.
By contrast, decaffeinated coffee was not associated with a reduced risk of rosacea, and in further analysis, the investigators found that there was no significant inverse association when they looked just at caffeine intake from sources other than coffee, such as chocolate, tea, and soda.
Caffeine could influence rosacea incidence by one of several mechanisms, including its effect on vascular contractility, the investigators hypothesized. “Increased caffeine intake may decrease vasodilation and consequently lead to diminution of rosacea symptoms.”
However, caffeine also has documented immunosuppressant effects that could possibly decrease rosacea-associated inflammation and has been shown to modulate hormone levels. “Hormonal factors have been implicated in the development of rosacea, and caffeine can modulate hormone levels,” they wrote.
Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from several sources, including National Institutes of Health grants for the Nurses’ Health Study II.
SOURCE: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
FROM JAMA DERMATOLOGY
Key clinical point: Caffeinated coffee intake was linked to decreased incidence of rosacea, while decaffeinated coffee and noncoffee sources of caffeine had no such effect.
Major finding: Consuming four or more servings of caffeinated coffee was associated with lower risk of rosacea versus one or fewer servings per month (hazard ratio, 0.77; P less than .001).
Study details: An analysis based on 82,737 participants in the Nurses’ Health Study II who responded to a question about rosacea.
Disclosures: Two study authors reported disclosures related to AbbVie, Amgen, Astellas Pharma, Janssen, Merck, Novartis, and Pfizer, among others. Funding for the study came from National Institutes of Health grants for the Nurses’ Health Study II and other sources.
Source: Li W-Q et al. JAMA Dermatol. 2018 Oct 17. doi: 10.1001/jamadermatol.2018.3301.
Bradycardia guideline sets new bar for shared decision-making in pacemaker placement
A new clinical practice guideline on the management of bradycardia and cardiac conduction system disorders in adults emphasizes the importance of patient-centered care and “shared decision-making” between patient and clinician, particularly with regard to patients who have indications for pacemaker implantation.
Shared decision-making extends to the end-of-life setting where “complex” informed consent and refusal of care decisions need to be patient-specific, and must involve all stakeholders, according to the new 2018 guidelines from the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Rhythm Society (HRS).
“Patients with decision-making capacity or his/her legally defined surrogate has the right to refuse or request withdrawal of pacemaker therapy, even if the patient is pacemaker dependent, which should be considered palliative, end-of-life care, and not physician-assisted suicide,” the guidelines read.
The guidelines additionally update the evaluation and treatment of sinus node dysfunction, atrioventricular block, and conduction disorders, based in part on a comprehensive evidence review conducted from January to September 2017. They supersede a 2008 guideline from the three societies on device-based therapy of cardiac rhythm abnormalities, and the focused update to that guideline published in 2012.
These guidelines will be useful not only to arrhythmia specialists, but also to internists and family physicians, cardiologists, surgeons, emergency physicians, and anesthesiologists, according to the guideline writing committee, which included representativens of ACC, AHA, HRS, and several other national organizations. The committee included cardiac electrophysiologists, cardiologists, surgeons, an anesthesiologist, and other clinicians, as well as a patient/lay representative, and was chaired by Fred M. Kusumoto, MD, of Mayo Clinic Florida in Jacksonville.
For sinus node dysfunction, no minimum heart rate or pause duration has been determined for which permanent pacing would be recommended, the guidelines state. To determine whether permanent pacing is necessary in those patients, clinicians should work to establish a temporal correlation bewteen bradycardia and symptoms, according to the guideline authors.
Left bundle branch block, when found on echocardiogram, greatly increases the chances of underlying structural heart disease and of a left ventricular systolic dysfunction diagnosis, according to the guidelines, which state that echocardiography is the most appropriate initial screening test for left ventricular systolic dysfunction and other structural heart disease.
Permanent pacing is recommended for certain types of atrioventricular (AV) block, according to the guidelines, which include high-grade AV block, acquired second-degree Mobitz type II AV block, and third-degree AV block not related to reversible or physiologic causes.
Treatment of sleep apnea can reduce frequency of nocturnal bradycardia and may provide a cardiovascular benefit, the guideline authors state. Patients with nocturnal bradycardias should be screened for sleep apnea, though authors cautioned that these arrhythmias are not, in and of themselves, an indication for permanent pacing.
“Treatment decisions are based not only on the best available evidence, but also on the patient’s goals of care and preferences,” Dr. Kusumoto said in a press release jointly issued by the ACC, AHA, and HRS. Toward that end, patients should receive “trusted material” to help them understand the consequences and risks of any proposed management decision.
Emerging pacing technologies such as His bundle pacing and transcatheter leadless pacing systems need more study to determine which patient populations will benefit most from them, the guidelines state.
“Regardless of technology, for the foreseeable future, pacing therapy requires implantation of a medical device,” Dr. Kusumoto said in the release. “Future studies are warranted to focus on the long-term implications associated with lifelong therapy.”
The 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay is now published in the Journal of the American College of Cardiology, and simultaneously in the journals Circulation and HeartRhythm.
Dr. Kusumoto reported no relationships with industry or other entities. Guideline co-authors provided disclosures related to Boston Scientific, Janssen Pharmaceuticals, Medtronic, Daiichi-Sankyo, Sanofi-Aventis, St. Jude Medical, and Abbott, among others.
SOURCE: Kusumoto FM, et al. J Am Coll Cardiol. 2018 Nov 6.
A new clinical practice guideline on the management of bradycardia and cardiac conduction system disorders in adults emphasizes the importance of patient-centered care and “shared decision-making” between patient and clinician, particularly with regard to patients who have indications for pacemaker implantation.
Shared decision-making extends to the end-of-life setting where “complex” informed consent and refusal of care decisions need to be patient-specific, and must involve all stakeholders, according to the new 2018 guidelines from the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Rhythm Society (HRS).
“Patients with decision-making capacity or his/her legally defined surrogate has the right to refuse or request withdrawal of pacemaker therapy, even if the patient is pacemaker dependent, which should be considered palliative, end-of-life care, and not physician-assisted suicide,” the guidelines read.
The guidelines additionally update the evaluation and treatment of sinus node dysfunction, atrioventricular block, and conduction disorders, based in part on a comprehensive evidence review conducted from January to September 2017. They supersede a 2008 guideline from the three societies on device-based therapy of cardiac rhythm abnormalities, and the focused update to that guideline published in 2012.
These guidelines will be useful not only to arrhythmia specialists, but also to internists and family physicians, cardiologists, surgeons, emergency physicians, and anesthesiologists, according to the guideline writing committee, which included representativens of ACC, AHA, HRS, and several other national organizations. The committee included cardiac electrophysiologists, cardiologists, surgeons, an anesthesiologist, and other clinicians, as well as a patient/lay representative, and was chaired by Fred M. Kusumoto, MD, of Mayo Clinic Florida in Jacksonville.
For sinus node dysfunction, no minimum heart rate or pause duration has been determined for which permanent pacing would be recommended, the guidelines state. To determine whether permanent pacing is necessary in those patients, clinicians should work to establish a temporal correlation bewteen bradycardia and symptoms, according to the guideline authors.
Left bundle branch block, when found on echocardiogram, greatly increases the chances of underlying structural heart disease and of a left ventricular systolic dysfunction diagnosis, according to the guidelines, which state that echocardiography is the most appropriate initial screening test for left ventricular systolic dysfunction and other structural heart disease.
Permanent pacing is recommended for certain types of atrioventricular (AV) block, according to the guidelines, which include high-grade AV block, acquired second-degree Mobitz type II AV block, and third-degree AV block not related to reversible or physiologic causes.
Treatment of sleep apnea can reduce frequency of nocturnal bradycardia and may provide a cardiovascular benefit, the guideline authors state. Patients with nocturnal bradycardias should be screened for sleep apnea, though authors cautioned that these arrhythmias are not, in and of themselves, an indication for permanent pacing.
“Treatment decisions are based not only on the best available evidence, but also on the patient’s goals of care and preferences,” Dr. Kusumoto said in a press release jointly issued by the ACC, AHA, and HRS. Toward that end, patients should receive “trusted material” to help them understand the consequences and risks of any proposed management decision.
Emerging pacing technologies such as His bundle pacing and transcatheter leadless pacing systems need more study to determine which patient populations will benefit most from them, the guidelines state.
“Regardless of technology, for the foreseeable future, pacing therapy requires implantation of a medical device,” Dr. Kusumoto said in the release. “Future studies are warranted to focus on the long-term implications associated with lifelong therapy.”
The 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay is now published in the Journal of the American College of Cardiology, and simultaneously in the journals Circulation and HeartRhythm.
Dr. Kusumoto reported no relationships with industry or other entities. Guideline co-authors provided disclosures related to Boston Scientific, Janssen Pharmaceuticals, Medtronic, Daiichi-Sankyo, Sanofi-Aventis, St. Jude Medical, and Abbott, among others.
SOURCE: Kusumoto FM, et al. J Am Coll Cardiol. 2018 Nov 6.
A new clinical practice guideline on the management of bradycardia and cardiac conduction system disorders in adults emphasizes the importance of patient-centered care and “shared decision-making” between patient and clinician, particularly with regard to patients who have indications for pacemaker implantation.
Shared decision-making extends to the end-of-life setting where “complex” informed consent and refusal of care decisions need to be patient-specific, and must involve all stakeholders, according to the new 2018 guidelines from the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Rhythm Society (HRS).
“Patients with decision-making capacity or his/her legally defined surrogate has the right to refuse or request withdrawal of pacemaker therapy, even if the patient is pacemaker dependent, which should be considered palliative, end-of-life care, and not physician-assisted suicide,” the guidelines read.
The guidelines additionally update the evaluation and treatment of sinus node dysfunction, atrioventricular block, and conduction disorders, based in part on a comprehensive evidence review conducted from January to September 2017. They supersede a 2008 guideline from the three societies on device-based therapy of cardiac rhythm abnormalities, and the focused update to that guideline published in 2012.
These guidelines will be useful not only to arrhythmia specialists, but also to internists and family physicians, cardiologists, surgeons, emergency physicians, and anesthesiologists, according to the guideline writing committee, which included representativens of ACC, AHA, HRS, and several other national organizations. The committee included cardiac electrophysiologists, cardiologists, surgeons, an anesthesiologist, and other clinicians, as well as a patient/lay representative, and was chaired by Fred M. Kusumoto, MD, of Mayo Clinic Florida in Jacksonville.
For sinus node dysfunction, no minimum heart rate or pause duration has been determined for which permanent pacing would be recommended, the guidelines state. To determine whether permanent pacing is necessary in those patients, clinicians should work to establish a temporal correlation bewteen bradycardia and symptoms, according to the guideline authors.
Left bundle branch block, when found on echocardiogram, greatly increases the chances of underlying structural heart disease and of a left ventricular systolic dysfunction diagnosis, according to the guidelines, which state that echocardiography is the most appropriate initial screening test for left ventricular systolic dysfunction and other structural heart disease.
Permanent pacing is recommended for certain types of atrioventricular (AV) block, according to the guidelines, which include high-grade AV block, acquired second-degree Mobitz type II AV block, and third-degree AV block not related to reversible or physiologic causes.
Treatment of sleep apnea can reduce frequency of nocturnal bradycardia and may provide a cardiovascular benefit, the guideline authors state. Patients with nocturnal bradycardias should be screened for sleep apnea, though authors cautioned that these arrhythmias are not, in and of themselves, an indication for permanent pacing.
“Treatment decisions are based not only on the best available evidence, but also on the patient’s goals of care and preferences,” Dr. Kusumoto said in a press release jointly issued by the ACC, AHA, and HRS. Toward that end, patients should receive “trusted material” to help them understand the consequences and risks of any proposed management decision.
Emerging pacing technologies such as His bundle pacing and transcatheter leadless pacing systems need more study to determine which patient populations will benefit most from them, the guidelines state.
“Regardless of technology, for the foreseeable future, pacing therapy requires implantation of a medical device,” Dr. Kusumoto said in the release. “Future studies are warranted to focus on the long-term implications associated with lifelong therapy.”
The 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay is now published in the Journal of the American College of Cardiology, and simultaneously in the journals Circulation and HeartRhythm.
Dr. Kusumoto reported no relationships with industry or other entities. Guideline co-authors provided disclosures related to Boston Scientific, Janssen Pharmaceuticals, Medtronic, Daiichi-Sankyo, Sanofi-Aventis, St. Jude Medical, and Abbott, among others.
SOURCE: Kusumoto FM, et al. J Am Coll Cardiol. 2018 Nov 6.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY