User login
Clinical Guideline Highlights for the Hospitalist: Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old
Invasive bacterial infections (IBI; ie, bacterial meningitis, bacteremia) are an uncommon but potentially devastating occurrence in young febrile infants. The challenge for clinicians is that physical examination cannot reliably exclude such infections. Thus, these infants have historically received comprehensive emergency department evaluation, including routine cerebrospinal fluid (CSF) assessment, and, often, required hospitalization for parenteral antibiotic administration while awaiting CSF culture results. The new American Academy of Pediatrics (AAP) guidelines were necessary given changing bacteriology, advances in diagnostic testing, greater insight into the differential risk of poor outcomes by site of infection, and better appreciation of the potential harms of unnecessary care and interventions.1 The 21 recommendations apply to well-appearing febrile infants 8 to 60 days of age, with recommendations stratified by age group, and exclude infants with certain conditions, including prematurity, focal bacterial infection, congenital or chromosomal abnormalities, and bronchiolitis. Four key recommendations are highlighted.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1: Diagnostic evaluation. For all age groups, blood culture and urinalysis (UA) are routinely recommended. For infants 8 to 21 days old, urine culture is routinely recommended. For older infants, urine culture is recommended if the UA is positive. All specimens for culture should be obtained via catheterization or suprapubic aspiration.
Infants 8 to 21 days old
- May assess inflammatory markers (grade B, weak).
- Should obtain CSF for analysis and culture (grade A, strong).
Infants 22 to 28 days old
- Should assess inflammatory markers (grade B, strong).
- May obtain CSF for analysis and culture even if no inflammatory marker obtained is abnormal (grade B, moderate).
- Should obtain CSF for analysis and culture if any inflammatory marker obtained is abnormal (procalcitonin >0.5 ng/mL [preferred]; C-reactive protein >20 mg/L; absolute neutrophil count >4000-5200/mm3; or temperature >38.5 °C) (grade B, moderate).
Infants 29 to 60 days old
- Should assess inflammatory markers (grade B, moderate).
- May obtain CSF for analysis and culture if any inflammatory marker is abnormal, (grade C, weak).
- Need not obtain CSF for analysis if all inflammatory markers obtained are normal (grade B, moderate).
Recommendation 2: Initial disposition decision
Infants 8 to 21 days old
- Admit (grade B, moderate).
Infants 22 to 28 days old
- Admit if CSF analysis is abnormal, UA is positive (A, strong), or if CSF is not obtained or is uninterpretable (grade B, weak).
- May manage at home if UA is normal, inflammatory markers are normal, CSF is normal or enterovirus positive, family has received verbal and written home monitoring instructions for concerning signs that should prompt immediate return for care, follow-up plan for reevaluation in 24 hours is in place, and means of communication for change in clinical status has been established (grade B, moderate).
Infants 29 to 60 days old
- Admit if CSF analysis is abnormal (grade A strong).
- May hospitalize if any inflammatory marker obtained is abnormal (grade B, moderate).
- Should manage at home if all the following are present: CSF is normal, if obtained; UA is negative; all inflammatory markers obtained are normal; teaching is complete; follow-up plan for reevaluation in 24 hours is in place; and means of communication for change in clinical status has been established (grade B, moderate).
Recommendation 3: Empiric antimicrobial treatment
Infants 8 to 21 days old
- Should initiate parenteral antimicrobial therapy (grade A, strong).
- This recommendation is based on the high prevalence of IBIs in this age category, and IBI may be present despite a negative UA and/or normal inflammatory markers.
Infants 22 to 28 days old
- Should initiate parenteral antimicrobial therapy if either CSF analysis suggests bacterial meningitis or UA is positive (grade A, strong).
- May administer parenteral antimicrobial therapy if any inflammatory marker is abnormal (grade B, moderate).
- May administer parenteral antimicrobial therapy even if everything is reassuring (grade B, weak).
- Should administer parenteral antimicrobial therapy to infant who will be managed at home even if all evaluation is reassuring (grade C, moderate).
Infants 29 to 60 days old
- Should start parenteral antimicrobials if CSF analysis suggests bacterial meningitis (grade A, strong).
- May use parenteral antimicrobials if any inflammatory marker is abnormal (grade B, moderate).
- Should initiate oral antimicrobial therapy if CSF is normal (if obtained), UA is positive, and no inflammatory markers obtained are abnormal (grade B, strong).
- Need not start antimicrobials if CSF is normal or enterovirus positive, UA is negative, and no inflammatory marker obtained is abnormal (grade B, moderate).
Recommendation 4: Hospital discharge decision
Infants 8 to 21 days old AND Infants 22 to 28 days ol
- Discontinue antibiotics and discharge infant when culture results are negative for 24 to 36 hours (or positive only for contaminants), the infant is well or improving, and there are no other reasons for hospitalization (grade B, strong).
Infants 29 to 60 days old
- Although no specific parameters are given for infants without UTI, presumably the discharge criteria for younger infants would also apply for this group.
- For infants with UTI, discharge if blood and CSF cultures are negative, infant is well or improving, and no other reasons for hospitalization remain (grade B, strong).
CRITIQUE
The guideline provides opportunities for safely doing less in a vulnerable population. For example, infants with UTIs may be managed differently (eg, often with oral antibiotics) from those with IBIs, which represents an important change from conventional practice.2 Additional strengths are the incorporation of procalcitonin, which has emerged as the most accurate marker for risk stratification;3 and deemphasis of complete blood count results.
Multiple exclusions for relatively common scenarios represent missed opportunities for a more complete set of recommendations for the febrile infant population. The decision to exclude infants in the first week of life is perplexing since infants 0 to 7 days old will receive CSF analysis, require admission, and generally be managed comparably to infants 8 to 21 days old. Infants with bronchiolitis are excluded; the absence of uniform guidance may perpetuate variability in management within and across institutions. Finally, exclusion of infants in whom perinatal or congenital herpes simplex virus is a consideration is not ideal. The requirement to consult separate guidance for herpes simplex virus evaluation fragments decision-making and may lead to inadvertent omissions of critical tests or treatment in at-risk infants.
Methods in Preparing the Guideline
The guideline working group included stakeholders from multiple specialties including general pediatrics, emergency medicine, hospital medicine, infectious diseases, and family medicine. In addition to published studies, the committee considered an Agency for Healthcare Research and Quality commissioned systematic review, as well as analyses of additional data solicited from previously published peer-reviewed studies. Once recommendations were formulated, additional input from physician focus groups and parents was solicited. Recommendations were rated based on strength of available evidence (A, B, C, D, X) as well as assessment of the benefit/harm profile (strong, moderate, weak).
Sources of Potential Conflicts of Interest or Bias
The guideline writing group was predominantly male, though we note that the broader working group was diverse in gender and specialty. No significant conflicts of interest were noted.
Generalizability
The complexity of this guideline, including age stratification, multiple exclusions, and multistep processes could lead to challenges in implementation; a health information technology application (app) could substantially ease the difficulty of implementation at the point of care.
AREAS IN NEED OF FUTURE STUDY
Additional areas in need of guidance include neonates with bronchiolitis and fever and neonates with focal infection. For the former, there is an abundance of evidence;4 what is needed is consensus. For the latter, additional study is needed such as the role of inflammatory markers in stratifying infants with focal infection who need additional evaluation prior to treatment.
1. Pantell RH, Roberts KB, Adams WG, et al; Subcommittee on Febrile Infants. Evaluation and management of well-appearing febrile infants 8-60 days old. Pediatrics. 2021; 148(2):e2021052228. https://doi.org/10.1542/peds.2021-052228
2. Chang PW, Wang ME, Schroeder AR. Diagnosis and management of UTI in febrile infants age 0-2 months: applicability of the AAP guideline. J Hosp Med. 2020;15(3): 176-180. https://doi.org/10.12788/jhm.3349
3. Wang ME, Srinivas N, McCulloh RJ. Clinical progress note: procalcitonin in the identification of invasive bacterial infections in febrile young infants. J Hosp Med. 2021; 16(3): 165-167. https://doi.org/10.12788/jhm.3451
4. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/1 0.1001/archpediatrics.2011.155
Invasive bacterial infections (IBI; ie, bacterial meningitis, bacteremia) are an uncommon but potentially devastating occurrence in young febrile infants. The challenge for clinicians is that physical examination cannot reliably exclude such infections. Thus, these infants have historically received comprehensive emergency department evaluation, including routine cerebrospinal fluid (CSF) assessment, and, often, required hospitalization for parenteral antibiotic administration while awaiting CSF culture results. The new American Academy of Pediatrics (AAP) guidelines were necessary given changing bacteriology, advances in diagnostic testing, greater insight into the differential risk of poor outcomes by site of infection, and better appreciation of the potential harms of unnecessary care and interventions.1 The 21 recommendations apply to well-appearing febrile infants 8 to 60 days of age, with recommendations stratified by age group, and exclude infants with certain conditions, including prematurity, focal bacterial infection, congenital or chromosomal abnormalities, and bronchiolitis. Four key recommendations are highlighted.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1: Diagnostic evaluation. For all age groups, blood culture and urinalysis (UA) are routinely recommended. For infants 8 to 21 days old, urine culture is routinely recommended. For older infants, urine culture is recommended if the UA is positive. All specimens for culture should be obtained via catheterization or suprapubic aspiration.
Infants 8 to 21 days old
- May assess inflammatory markers (grade B, weak).
- Should obtain CSF for analysis and culture (grade A, strong).
Infants 22 to 28 days old
- Should assess inflammatory markers (grade B, strong).
- May obtain CSF for analysis and culture even if no inflammatory marker obtained is abnormal (grade B, moderate).
- Should obtain CSF for analysis and culture if any inflammatory marker obtained is abnormal (procalcitonin >0.5 ng/mL [preferred]; C-reactive protein >20 mg/L; absolute neutrophil count >4000-5200/mm3; or temperature >38.5 °C) (grade B, moderate).
Infants 29 to 60 days old
- Should assess inflammatory markers (grade B, moderate).
- May obtain CSF for analysis and culture if any inflammatory marker is abnormal, (grade C, weak).
- Need not obtain CSF for analysis if all inflammatory markers obtained are normal (grade B, moderate).
Recommendation 2: Initial disposition decision
Infants 8 to 21 days old
- Admit (grade B, moderate).
Infants 22 to 28 days old
- Admit if CSF analysis is abnormal, UA is positive (A, strong), or if CSF is not obtained or is uninterpretable (grade B, weak).
- May manage at home if UA is normal, inflammatory markers are normal, CSF is normal or enterovirus positive, family has received verbal and written home monitoring instructions for concerning signs that should prompt immediate return for care, follow-up plan for reevaluation in 24 hours is in place, and means of communication for change in clinical status has been established (grade B, moderate).
Infants 29 to 60 days old
- Admit if CSF analysis is abnormal (grade A strong).
- May hospitalize if any inflammatory marker obtained is abnormal (grade B, moderate).
- Should manage at home if all the following are present: CSF is normal, if obtained; UA is negative; all inflammatory markers obtained are normal; teaching is complete; follow-up plan for reevaluation in 24 hours is in place; and means of communication for change in clinical status has been established (grade B, moderate).
Recommendation 3: Empiric antimicrobial treatment
Infants 8 to 21 days old
- Should initiate parenteral antimicrobial therapy (grade A, strong).
- This recommendation is based on the high prevalence of IBIs in this age category, and IBI may be present despite a negative UA and/or normal inflammatory markers.
Infants 22 to 28 days old
- Should initiate parenteral antimicrobial therapy if either CSF analysis suggests bacterial meningitis or UA is positive (grade A, strong).
- May administer parenteral antimicrobial therapy if any inflammatory marker is abnormal (grade B, moderate).
- May administer parenteral antimicrobial therapy even if everything is reassuring (grade B, weak).
- Should administer parenteral antimicrobial therapy to infant who will be managed at home even if all evaluation is reassuring (grade C, moderate).
Infants 29 to 60 days old
- Should start parenteral antimicrobials if CSF analysis suggests bacterial meningitis (grade A, strong).
- May use parenteral antimicrobials if any inflammatory marker is abnormal (grade B, moderate).
- Should initiate oral antimicrobial therapy if CSF is normal (if obtained), UA is positive, and no inflammatory markers obtained are abnormal (grade B, strong).
- Need not start antimicrobials if CSF is normal or enterovirus positive, UA is negative, and no inflammatory marker obtained is abnormal (grade B, moderate).
Recommendation 4: Hospital discharge decision
Infants 8 to 21 days old AND Infants 22 to 28 days ol
- Discontinue antibiotics and discharge infant when culture results are negative for 24 to 36 hours (or positive only for contaminants), the infant is well or improving, and there are no other reasons for hospitalization (grade B, strong).
Infants 29 to 60 days old
- Although no specific parameters are given for infants without UTI, presumably the discharge criteria for younger infants would also apply for this group.
- For infants with UTI, discharge if blood and CSF cultures are negative, infant is well or improving, and no other reasons for hospitalization remain (grade B, strong).
CRITIQUE
The guideline provides opportunities for safely doing less in a vulnerable population. For example, infants with UTIs may be managed differently (eg, often with oral antibiotics) from those with IBIs, which represents an important change from conventional practice.2 Additional strengths are the incorporation of procalcitonin, which has emerged as the most accurate marker for risk stratification;3 and deemphasis of complete blood count results.
Multiple exclusions for relatively common scenarios represent missed opportunities for a more complete set of recommendations for the febrile infant population. The decision to exclude infants in the first week of life is perplexing since infants 0 to 7 days old will receive CSF analysis, require admission, and generally be managed comparably to infants 8 to 21 days old. Infants with bronchiolitis are excluded; the absence of uniform guidance may perpetuate variability in management within and across institutions. Finally, exclusion of infants in whom perinatal or congenital herpes simplex virus is a consideration is not ideal. The requirement to consult separate guidance for herpes simplex virus evaluation fragments decision-making and may lead to inadvertent omissions of critical tests or treatment in at-risk infants.
Methods in Preparing the Guideline
The guideline working group included stakeholders from multiple specialties including general pediatrics, emergency medicine, hospital medicine, infectious diseases, and family medicine. In addition to published studies, the committee considered an Agency for Healthcare Research and Quality commissioned systematic review, as well as analyses of additional data solicited from previously published peer-reviewed studies. Once recommendations were formulated, additional input from physician focus groups and parents was solicited. Recommendations were rated based on strength of available evidence (A, B, C, D, X) as well as assessment of the benefit/harm profile (strong, moderate, weak).
Sources of Potential Conflicts of Interest or Bias
The guideline writing group was predominantly male, though we note that the broader working group was diverse in gender and specialty. No significant conflicts of interest were noted.
Generalizability
The complexity of this guideline, including age stratification, multiple exclusions, and multistep processes could lead to challenges in implementation; a health information technology application (app) could substantially ease the difficulty of implementation at the point of care.
AREAS IN NEED OF FUTURE STUDY
Additional areas in need of guidance include neonates with bronchiolitis and fever and neonates with focal infection. For the former, there is an abundance of evidence;4 what is needed is consensus. For the latter, additional study is needed such as the role of inflammatory markers in stratifying infants with focal infection who need additional evaluation prior to treatment.
Invasive bacterial infections (IBI; ie, bacterial meningitis, bacteremia) are an uncommon but potentially devastating occurrence in young febrile infants. The challenge for clinicians is that physical examination cannot reliably exclude such infections. Thus, these infants have historically received comprehensive emergency department evaluation, including routine cerebrospinal fluid (CSF) assessment, and, often, required hospitalization for parenteral antibiotic administration while awaiting CSF culture results. The new American Academy of Pediatrics (AAP) guidelines were necessary given changing bacteriology, advances in diagnostic testing, greater insight into the differential risk of poor outcomes by site of infection, and better appreciation of the potential harms of unnecessary care and interventions.1 The 21 recommendations apply to well-appearing febrile infants 8 to 60 days of age, with recommendations stratified by age group, and exclude infants with certain conditions, including prematurity, focal bacterial infection, congenital or chromosomal abnormalities, and bronchiolitis. Four key recommendations are highlighted.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1: Diagnostic evaluation. For all age groups, blood culture and urinalysis (UA) are routinely recommended. For infants 8 to 21 days old, urine culture is routinely recommended. For older infants, urine culture is recommended if the UA is positive. All specimens for culture should be obtained via catheterization or suprapubic aspiration.
Infants 8 to 21 days old
- May assess inflammatory markers (grade B, weak).
- Should obtain CSF for analysis and culture (grade A, strong).
Infants 22 to 28 days old
- Should assess inflammatory markers (grade B, strong).
- May obtain CSF for analysis and culture even if no inflammatory marker obtained is abnormal (grade B, moderate).
- Should obtain CSF for analysis and culture if any inflammatory marker obtained is abnormal (procalcitonin >0.5 ng/mL [preferred]; C-reactive protein >20 mg/L; absolute neutrophil count >4000-5200/mm3; or temperature >38.5 °C) (grade B, moderate).
Infants 29 to 60 days old
- Should assess inflammatory markers (grade B, moderate).
- May obtain CSF for analysis and culture if any inflammatory marker is abnormal, (grade C, weak).
- Need not obtain CSF for analysis if all inflammatory markers obtained are normal (grade B, moderate).
Recommendation 2: Initial disposition decision
Infants 8 to 21 days old
- Admit (grade B, moderate).
Infants 22 to 28 days old
- Admit if CSF analysis is abnormal, UA is positive (A, strong), or if CSF is not obtained or is uninterpretable (grade B, weak).
- May manage at home if UA is normal, inflammatory markers are normal, CSF is normal or enterovirus positive, family has received verbal and written home monitoring instructions for concerning signs that should prompt immediate return for care, follow-up plan for reevaluation in 24 hours is in place, and means of communication for change in clinical status has been established (grade B, moderate).
Infants 29 to 60 days old
- Admit if CSF analysis is abnormal (grade A strong).
- May hospitalize if any inflammatory marker obtained is abnormal (grade B, moderate).
- Should manage at home if all the following are present: CSF is normal, if obtained; UA is negative; all inflammatory markers obtained are normal; teaching is complete; follow-up plan for reevaluation in 24 hours is in place; and means of communication for change in clinical status has been established (grade B, moderate).
Recommendation 3: Empiric antimicrobial treatment
Infants 8 to 21 days old
- Should initiate parenteral antimicrobial therapy (grade A, strong).
- This recommendation is based on the high prevalence of IBIs in this age category, and IBI may be present despite a negative UA and/or normal inflammatory markers.
Infants 22 to 28 days old
- Should initiate parenteral antimicrobial therapy if either CSF analysis suggests bacterial meningitis or UA is positive (grade A, strong).
- May administer parenteral antimicrobial therapy if any inflammatory marker is abnormal (grade B, moderate).
- May administer parenteral antimicrobial therapy even if everything is reassuring (grade B, weak).
- Should administer parenteral antimicrobial therapy to infant who will be managed at home even if all evaluation is reassuring (grade C, moderate).
Infants 29 to 60 days old
- Should start parenteral antimicrobials if CSF analysis suggests bacterial meningitis (grade A, strong).
- May use parenteral antimicrobials if any inflammatory marker is abnormal (grade B, moderate).
- Should initiate oral antimicrobial therapy if CSF is normal (if obtained), UA is positive, and no inflammatory markers obtained are abnormal (grade B, strong).
- Need not start antimicrobials if CSF is normal or enterovirus positive, UA is negative, and no inflammatory marker obtained is abnormal (grade B, moderate).
Recommendation 4: Hospital discharge decision
Infants 8 to 21 days old AND Infants 22 to 28 days ol
- Discontinue antibiotics and discharge infant when culture results are negative for 24 to 36 hours (or positive only for contaminants), the infant is well or improving, and there are no other reasons for hospitalization (grade B, strong).
Infants 29 to 60 days old
- Although no specific parameters are given for infants without UTI, presumably the discharge criteria for younger infants would also apply for this group.
- For infants with UTI, discharge if blood and CSF cultures are negative, infant is well or improving, and no other reasons for hospitalization remain (grade B, strong).
CRITIQUE
The guideline provides opportunities for safely doing less in a vulnerable population. For example, infants with UTIs may be managed differently (eg, often with oral antibiotics) from those with IBIs, which represents an important change from conventional practice.2 Additional strengths are the incorporation of procalcitonin, which has emerged as the most accurate marker for risk stratification;3 and deemphasis of complete blood count results.
Multiple exclusions for relatively common scenarios represent missed opportunities for a more complete set of recommendations for the febrile infant population. The decision to exclude infants in the first week of life is perplexing since infants 0 to 7 days old will receive CSF analysis, require admission, and generally be managed comparably to infants 8 to 21 days old. Infants with bronchiolitis are excluded; the absence of uniform guidance may perpetuate variability in management within and across institutions. Finally, exclusion of infants in whom perinatal or congenital herpes simplex virus is a consideration is not ideal. The requirement to consult separate guidance for herpes simplex virus evaluation fragments decision-making and may lead to inadvertent omissions of critical tests or treatment in at-risk infants.
Methods in Preparing the Guideline
The guideline working group included stakeholders from multiple specialties including general pediatrics, emergency medicine, hospital medicine, infectious diseases, and family medicine. In addition to published studies, the committee considered an Agency for Healthcare Research and Quality commissioned systematic review, as well as analyses of additional data solicited from previously published peer-reviewed studies. Once recommendations were formulated, additional input from physician focus groups and parents was solicited. Recommendations were rated based on strength of available evidence (A, B, C, D, X) as well as assessment of the benefit/harm profile (strong, moderate, weak).
Sources of Potential Conflicts of Interest or Bias
The guideline writing group was predominantly male, though we note that the broader working group was diverse in gender and specialty. No significant conflicts of interest were noted.
Generalizability
The complexity of this guideline, including age stratification, multiple exclusions, and multistep processes could lead to challenges in implementation; a health information technology application (app) could substantially ease the difficulty of implementation at the point of care.
AREAS IN NEED OF FUTURE STUDY
Additional areas in need of guidance include neonates with bronchiolitis and fever and neonates with focal infection. For the former, there is an abundance of evidence;4 what is needed is consensus. For the latter, additional study is needed such as the role of inflammatory markers in stratifying infants with focal infection who need additional evaluation prior to treatment.
1. Pantell RH, Roberts KB, Adams WG, et al; Subcommittee on Febrile Infants. Evaluation and management of well-appearing febrile infants 8-60 days old. Pediatrics. 2021; 148(2):e2021052228. https://doi.org/10.1542/peds.2021-052228
2. Chang PW, Wang ME, Schroeder AR. Diagnosis and management of UTI in febrile infants age 0-2 months: applicability of the AAP guideline. J Hosp Med. 2020;15(3): 176-180. https://doi.org/10.12788/jhm.3349
3. Wang ME, Srinivas N, McCulloh RJ. Clinical progress note: procalcitonin in the identification of invasive bacterial infections in febrile young infants. J Hosp Med. 2021; 16(3): 165-167. https://doi.org/10.12788/jhm.3451
4. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/1 0.1001/archpediatrics.2011.155
1. Pantell RH, Roberts KB, Adams WG, et al; Subcommittee on Febrile Infants. Evaluation and management of well-appearing febrile infants 8-60 days old. Pediatrics. 2021; 148(2):e2021052228. https://doi.org/10.1542/peds.2021-052228
2. Chang PW, Wang ME, Schroeder AR. Diagnosis and management of UTI in febrile infants age 0-2 months: applicability of the AAP guideline. J Hosp Med. 2020;15(3): 176-180. https://doi.org/10.12788/jhm.3349
3. Wang ME, Srinivas N, McCulloh RJ. Clinical progress note: procalcitonin in the identification of invasive bacterial infections in febrile young infants. J Hosp Med. 2021; 16(3): 165-167. https://doi.org/10.12788/jhm.3451
4. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/1 0.1001/archpediatrics.2011.155
© 2021 Society of Hospital Medicine
Gender Distribution in Pediatric Hospital Medicine Leadership
There is a growing appreciation of gender disparities in career advancement in medicine. By 2004, approximately 50% of medical school graduates were women, yet considerable differences persist between genders in compensation, faculty rank, and leadership positions.1-3 According to the Association of American Medical Colleges (AAMC), women account for only 25% of full professors, 18% of department chairs, and 18% of medical school deans.1 Women are also underrepresented in other areas of leadership such as division directors, professional society leadership, and hospital executives.4-6
Specialties that are predominantly women, including pediatrics, are not immune to gender disparities. Women represent 71% of pediatric residents1 and currently constitute two-thirds of active pediatricians in the United States.7 However, there is a disproportionately low number of women ascending the pediatric academic ladder, with only 35% of full professors2 and 28% of department chairs being women.1 Pediatrics also was noted to have the fifth-largest gender pay gap across 40 specialties.3 These disparities can contribute to burnout, poorer patient outcomes, and decreased advancement of women known as the “leaky pipeline.”1,8,9
There is some evidence that gender disparities may be improving among younger professionals with increasing percentages of women as leaders and decreasing pay gaps.10,11 These potential positive trends provide hope that fields in medicine early in their development may demonstrate fewer gender disparities. One of the youngest fields of medicine is pediatric hospital medicine (PHM), which officially became a recognized pediatric subspecialty in 2017.12 There is no literature to date describing gender disparities in PHM. We aimed to explore the gender distribution of university-based PHM program leadership and to compare this gender distribution with that seen in the broader field of PHM.
METHODS
This study was Institutional Review Board–approved as non–human subjects research through University of Chicago, Chicago, Illinois. From January to March 2020, the authors performed web-based searches for PHM division directors or program leaders in the United States. Because there is no single database of PHM programs in the United States, we used the AAMC list of Liaison Committee on Medical Education (LCME)–accredited US medical schools; medical schools in Puerto Rico were not included, nor were pending and provisional institutions. If an institution had multiple practice sites for its students, the primary site for third-year medical student clerkship rotations was included. If a medical school had multiple branches, each with its own primary inpatient pediatrics site, these sites were included. If there was no PHM division director, a program leader (lead hospitalist) was substituted and counted as long as the role was formally designated. This leadership role is herein referred to under the umbrella term of “division director.”
We searched medical school web pages, affiliated hospital web pages, and Google. All program leadership information (divisional and fellowship, if present) was confirmed through direct communication with the program, most commonly with division directors, and included name, gender, title, and presence of associate/assistant leader, gender, and title. Associate division directors were only included if it was a formal leadership position. Associate directors of research, quality, etc, were not included due to the limited number of formal positions noted on further review. Of note, the terms “associate” and “assistant” are referring to leadership positions and not academic ranks.
Fellowship leadership was included if affiliated with a US medical school in the primary list. Medical schools with multiple PHM fellowships were included as separate observations. The leadership was confirmed using the methods described above and cross-referenced through the PHM Fellowship Program website. PHM fellowship programs starting in 2020 were included if leadership was determined.
All leadership positions were verified by two authors, and all authors reviewed the master list to identify errors.
To determine the overall gender breakdown in the specialty, we used three estimates: 2019 American Board of Pediatrics (ABP) PHM Board Certification Exam applicants, the 2019 American Academy of Pediatrics Section on Hospital Medicine membership, and a random sample of all PHM faculty in 25% of the programs included in this study.4
Descriptive statistics using 95% confidence intervals for proportions were used. Differences between proportions were evaluated using a two-proportion z test with the null hypothesis that the two proportions are the same and significance set at P < .05.
RESULTS
Of the 150 AAMC LCME–accredited medical school departments of pediatrics evaluated, a total of 142 programs were included; eight programs were excluded due to not providing inpatient pediatric services.
Division Leadership
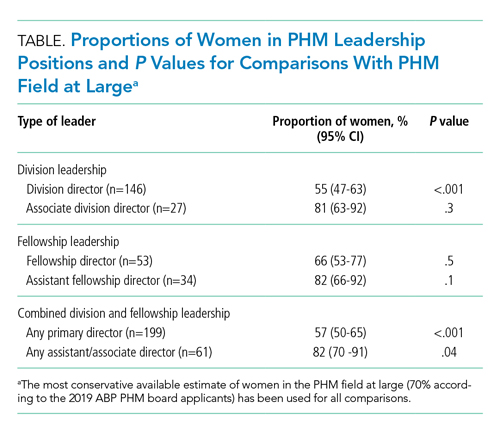
The proportion of women PHM division directors was 55% (95% CI, 47%-63%) in this sample of 146 leaders from 142 programs (4 programs had coleaders). In the 113 programs with standalone PHM divisions or sections, the proportion of women division directors was 56% (95% CI, 47%-64%). In the 29 hospitalist groups that were not standalone (ie, embedded in another division), the proportion of women leaders was similar at 52% (95% CI, 34%-69%). In 24 programs with 27 formally designated associate directors (1 program had 3 associate directors and 1 program had 2), 81% of associate directors were women (95% CI, 63%-92%).
Fellowship Leadership
A total of 51 PHM fellowship programs had 53 directors (2 had codirectors), and 66% of the fellowship directors were women (95% CI, 53%-77%). A total of 31 programs had 34 assistant directors (3 programs had 2 assistants), and 82% of the assistant fellowship directors were women (95% CI, 66%-92%).
Comparison With the Field at Large
The inaugural ABP PHM board certification exam in 2019 had 1,627 applicants with 70% women (95% CI, 68%-73%) (Suzanne Woods, MD, email communication, December 4, 2019). The American Academy of Pediatrics Section on Hospital Medicine, the largest PHM-specific organization, has 2,299 practicing physician members with 71% women (95% CI, 69%-73%) (Niccole Alexander, email communication, November 25, 2019). Our random sample of 25% of university-based PHM programs contained 1,063 faculty members with 72% women (95% CI, 69%-75%).
The Table provides P values for comparisons of the proportion of women in each of the above-described leadership roles compared to the most conservative estimate of women in the field from the estimates given above (ie, 70%). Compared with the field at large, women appear to be underrepresented as division directors (70% vs 55%; P < .001) but not as fellowship directors (70% vs 66%; P = .5). There is a higher proportion of women in all associate/assistant director roles, compared with the population (82% vs 70%; P = .04).
DISCUSSION
We found a significant difference between the proportion of women as PHM division directors (55%) when compared with the proportion of women physicians in PHM (70%), which suggests that women are underrepresented in clinical leadership at university-based pediatric hospitalist programs. Similar findings are described in other specialties, including notably adult hospital medicine.4 Burden et al found that only 16% of hospital medicine program leaders were women despite an equal number of women and men in the field. PHM has a much larger proportion of women, compared with that of hospital medicine, and yet women are still underrepresented as program leaders.
We found no disparities between the proportion of women as PHM fellowship directors and the field at large. These results are similar to those of other studies, which showed a higher number of women in educational leadership roles and lower representation in roles with influence over policy and allocation of resources.13,14 Although the proportion of women in educational roles itself is not a concern, there is evidence that these positions may be undervalued by some institutions, which provide these positions with lower salaries and fewer opportunities for career advancement.13,14
Interestingly, women are well-represented in associate/assistant director roles at both the division and fellowship leader level when comparing the distribution in those roles with that of the PHM field at large. This finding suggests that the pipeline of women is robust and potentially may indicate positive change. Alternatively, this finding may reflect a previously described phenomenon of the “sticky floor” in which women are “stuck” in these supportive roles and do not necessarily advance to higher-impact positions.15 We found a statistically significant higher proportion of women in the combined group of all associate/assistant directors compared with the overall population, which raises the concern that supportive leadership roles may represent “women’s work.”16 Future studies are needed to track whether these women truly advance or whether women are overrepresented in supportive leadership positions at the expense of primary leadership positions.
Adequate representation of women alone is not sufficient to achieve gender equity in medicine. We need to understand why there is a lower representation of women in leadership positions. Some barriers have already been described, including gender bias in promotions,17 higher demands outside of work,18 and lower pay,3 though none are specific to PHM. A further qualitative exploration of PHM leadership would help describe any barriers women in PHM specifically may be facing in their career trajectory. In addition, more information is needed to explore the experience of women with intersectional identities in PHM, especially since they may experience increased bias and discrimination.19
Limitations of this study include the lack of a centralized list of PHM programs and data on PHM workforce. Our three estimates for the proportion of women in PHM were similar at 70%-71%; however, these are only proxies for the true gender distribution of PHM physicians, which is unknown. PHM leadership targets of close to 70% women would be reflective of the field at large; however, institutional variation may exist, and ideally leadership should be diverse and reflective of its faculty members. Our study only describes university-based PHM programs and, therefore, is not necessarily generalizable to nonuniversity programs. Further studies are needed to evaluate any potential differences based on program type. In our study, gender was used in binary terms; however, we acknowledge that gender exists on a spectrum.
CONCLUSION
As a specialty early in development with a robust pipeline of women, PHM is in a unique position to lead the way in gender equity. However, women appear to be underrepresented as division directors at university-based PHM programs. Achieving proportional representation of women leaders is imperative for tapping into the full potential of the community and ensuring that the goals of the field are representative of the population.
Acknowledgment
Special thanks to Lucille Lester, MD, who asked the question that started this road to discovery.
1. Lautenberger DM, Dandar VM. State of Women in Academic Medicine 2018-2019 Exploring Pathways to Equity. AAMC; 2020. Accessed April 10, 2020. https://www.aamc.org/data-reports/data/2018-2019-state-women-academic-medicine-exploring-pathways-equity
2. Table 13: U.S. Medical School Faculty by Sex, Rank, and Department, 2017. AAMC; 2019. Accessed June 25, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
3. 2019 Physician Compensation Report. Doximity; March 2019. Accessed April 11, 2020. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round3.pdf
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Silver J, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019:179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303
6. Thomas R, Cooper M, Konar E, et al. Lean In: Women in the Workplace 2019. McKinsey & Company; 2019. Accessed July 1, 2020. https://wiw-report.s3.amazonaws.com/Women_in_the_Workplace_2019.pdf
7. Table 1.3: Number and Percentage of Active Physicians by Sex and Specialty, 2017. AAMC; 2017. Accessed April 12, 2020. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2017
8. Taka F, Nomura K, Horie S, et al. Organizational climate with gender equity and burnout among university academics in Japan. Ind Health. 2016;54(6):480-487. https://doi.org/10.2486/indhealth.2016-0126
9. Tsugawa Y, Jena A, Figueroa J, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. https://doi.org/10.1001/jamainternmed.2016.7875
10. Bissing MA, Lange EMS, Davila WF, et al. Status of women in academic anesthesiology: a 10-year update. Anesth Analg. 2019;128(1):137-143. https://doi.org/10.1213/ane.0000000000003691
11. Graf N, Brown A, Patten E. The narrowing, but persistent, gender gap in pay. Pew Research Center; March 22, 2019. Accessed April 20, 2020. https://www.pewresearch.org/fact-tank/2019/03/22/gender-pay-gap-facts/
12. American Board of Medical Specialties Officially Recognizes Pediatric Hospital Medicine Subspecialty Certification. News release. American Board of Medical Specialties; November 9, 2016. Accessed June 25, 2020. https://www.abms.org/media/120095/abms-recognizes-pediatric-hospital-medicine-as-a-subspecialty.pdf
13. Hofler LG, Hacker MR, Dodge LE, Schutzberg R, Ricciotti HA. Comparison of women in department leadership in obstetrics and gynecology with other specialties. Obstet Gynecol. 2016;127(3):442-447. https://doi.org/10.1097/aog.0000000000001290
14. Weiss A, Lee KC, Tapia V, et al. Equity in surgical leadership for women: more work to do. Am J Surg. 2014;208:494-498. https://doi.org/10.1016/j.amjsurg.2013.11.005
15. Tesch BJ, Wood HM, Helwig AL, Nattinger AB. Promotion of women physicians in academic medicine. Glass ceiling or sticky floor? JAMA. 1995;273(13):1022-1025.
16. Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95(10):1499-1506. https://doi.org/10.1097/acm.0000000000003555
17. Steinpreis RE, Anders KA, Ritzke D. The impact of gender on the review of the curricula vitae of job applicants and tenure candidates: a national empirical study. Sex Roles. 1999;41(7):509-528. https://doi.org/10.1023/A:1018839203698
18. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. https://doi.org/10.7326/m13-0974
19. Ginther DK, Kahn S, Schaffer WT. Gender, race/ethnicity, and National Institutes of Health R01 research awards: is there evidence of a double bind for women of color? Acad Med. 2016;91(8):1098-1107. https://doi.org/10.1097/acm.0000000000001278
There is a growing appreciation of gender disparities in career advancement in medicine. By 2004, approximately 50% of medical school graduates were women, yet considerable differences persist between genders in compensation, faculty rank, and leadership positions.1-3 According to the Association of American Medical Colleges (AAMC), women account for only 25% of full professors, 18% of department chairs, and 18% of medical school deans.1 Women are also underrepresented in other areas of leadership such as division directors, professional society leadership, and hospital executives.4-6
Specialties that are predominantly women, including pediatrics, are not immune to gender disparities. Women represent 71% of pediatric residents1 and currently constitute two-thirds of active pediatricians in the United States.7 However, there is a disproportionately low number of women ascending the pediatric academic ladder, with only 35% of full professors2 and 28% of department chairs being women.1 Pediatrics also was noted to have the fifth-largest gender pay gap across 40 specialties.3 These disparities can contribute to burnout, poorer patient outcomes, and decreased advancement of women known as the “leaky pipeline.”1,8,9
There is some evidence that gender disparities may be improving among younger professionals with increasing percentages of women as leaders and decreasing pay gaps.10,11 These potential positive trends provide hope that fields in medicine early in their development may demonstrate fewer gender disparities. One of the youngest fields of medicine is pediatric hospital medicine (PHM), which officially became a recognized pediatric subspecialty in 2017.12 There is no literature to date describing gender disparities in PHM. We aimed to explore the gender distribution of university-based PHM program leadership and to compare this gender distribution with that seen in the broader field of PHM.
METHODS
This study was Institutional Review Board–approved as non–human subjects research through University of Chicago, Chicago, Illinois. From January to March 2020, the authors performed web-based searches for PHM division directors or program leaders in the United States. Because there is no single database of PHM programs in the United States, we used the AAMC list of Liaison Committee on Medical Education (LCME)–accredited US medical schools; medical schools in Puerto Rico were not included, nor were pending and provisional institutions. If an institution had multiple practice sites for its students, the primary site for third-year medical student clerkship rotations was included. If a medical school had multiple branches, each with its own primary inpatient pediatrics site, these sites were included. If there was no PHM division director, a program leader (lead hospitalist) was substituted and counted as long as the role was formally designated. This leadership role is herein referred to under the umbrella term of “division director.”
We searched medical school web pages, affiliated hospital web pages, and Google. All program leadership information (divisional and fellowship, if present) was confirmed through direct communication with the program, most commonly with division directors, and included name, gender, title, and presence of associate/assistant leader, gender, and title. Associate division directors were only included if it was a formal leadership position. Associate directors of research, quality, etc, were not included due to the limited number of formal positions noted on further review. Of note, the terms “associate” and “assistant” are referring to leadership positions and not academic ranks.
Fellowship leadership was included if affiliated with a US medical school in the primary list. Medical schools with multiple PHM fellowships were included as separate observations. The leadership was confirmed using the methods described above and cross-referenced through the PHM Fellowship Program website. PHM fellowship programs starting in 2020 were included if leadership was determined.
All leadership positions were verified by two authors, and all authors reviewed the master list to identify errors.
To determine the overall gender breakdown in the specialty, we used three estimates: 2019 American Board of Pediatrics (ABP) PHM Board Certification Exam applicants, the 2019 American Academy of Pediatrics Section on Hospital Medicine membership, and a random sample of all PHM faculty in 25% of the programs included in this study.4
Descriptive statistics using 95% confidence intervals for proportions were used. Differences between proportions were evaluated using a two-proportion z test with the null hypothesis that the two proportions are the same and significance set at P < .05.
RESULTS
Of the 150 AAMC LCME–accredited medical school departments of pediatrics evaluated, a total of 142 programs were included; eight programs were excluded due to not providing inpatient pediatric services.
Division Leadership
The proportion of women PHM division directors was 55% (95% CI, 47%-63%) in this sample of 146 leaders from 142 programs (4 programs had coleaders). In the 113 programs with standalone PHM divisions or sections, the proportion of women division directors was 56% (95% CI, 47%-64%). In the 29 hospitalist groups that were not standalone (ie, embedded in another division), the proportion of women leaders was similar at 52% (95% CI, 34%-69%). In 24 programs with 27 formally designated associate directors (1 program had 3 associate directors and 1 program had 2), 81% of associate directors were women (95% CI, 63%-92%).
Fellowship Leadership
A total of 51 PHM fellowship programs had 53 directors (2 had codirectors), and 66% of the fellowship directors were women (95% CI, 53%-77%). A total of 31 programs had 34 assistant directors (3 programs had 2 assistants), and 82% of the assistant fellowship directors were women (95% CI, 66%-92%).
Comparison With the Field at Large
The inaugural ABP PHM board certification exam in 2019 had 1,627 applicants with 70% women (95% CI, 68%-73%) (Suzanne Woods, MD, email communication, December 4, 2019). The American Academy of Pediatrics Section on Hospital Medicine, the largest PHM-specific organization, has 2,299 practicing physician members with 71% women (95% CI, 69%-73%) (Niccole Alexander, email communication, November 25, 2019). Our random sample of 25% of university-based PHM programs contained 1,063 faculty members with 72% women (95% CI, 69%-75%).
The Table provides P values for comparisons of the proportion of women in each of the above-described leadership roles compared to the most conservative estimate of women in the field from the estimates given above (ie, 70%). Compared with the field at large, women appear to be underrepresented as division directors (70% vs 55%; P < .001) but not as fellowship directors (70% vs 66%; P = .5). There is a higher proportion of women in all associate/assistant director roles, compared with the population (82% vs 70%; P = .04).

DISCUSSION
We found a significant difference between the proportion of women as PHM division directors (55%) when compared with the proportion of women physicians in PHM (70%), which suggests that women are underrepresented in clinical leadership at university-based pediatric hospitalist programs. Similar findings are described in other specialties, including notably adult hospital medicine.4 Burden et al found that only 16% of hospital medicine program leaders were women despite an equal number of women and men in the field. PHM has a much larger proportion of women, compared with that of hospital medicine, and yet women are still underrepresented as program leaders.
We found no disparities between the proportion of women as PHM fellowship directors and the field at large. These results are similar to those of other studies, which showed a higher number of women in educational leadership roles and lower representation in roles with influence over policy and allocation of resources.13,14 Although the proportion of women in educational roles itself is not a concern, there is evidence that these positions may be undervalued by some institutions, which provide these positions with lower salaries and fewer opportunities for career advancement.13,14
Interestingly, women are well-represented in associate/assistant director roles at both the division and fellowship leader level when comparing the distribution in those roles with that of the PHM field at large. This finding suggests that the pipeline of women is robust and potentially may indicate positive change. Alternatively, this finding may reflect a previously described phenomenon of the “sticky floor” in which women are “stuck” in these supportive roles and do not necessarily advance to higher-impact positions.15 We found a statistically significant higher proportion of women in the combined group of all associate/assistant directors compared with the overall population, which raises the concern that supportive leadership roles may represent “women’s work.”16 Future studies are needed to track whether these women truly advance or whether women are overrepresented in supportive leadership positions at the expense of primary leadership positions.
Adequate representation of women alone is not sufficient to achieve gender equity in medicine. We need to understand why there is a lower representation of women in leadership positions. Some barriers have already been described, including gender bias in promotions,17 higher demands outside of work,18 and lower pay,3 though none are specific to PHM. A further qualitative exploration of PHM leadership would help describe any barriers women in PHM specifically may be facing in their career trajectory. In addition, more information is needed to explore the experience of women with intersectional identities in PHM, especially since they may experience increased bias and discrimination.19
Limitations of this study include the lack of a centralized list of PHM programs and data on PHM workforce. Our three estimates for the proportion of women in PHM were similar at 70%-71%; however, these are only proxies for the true gender distribution of PHM physicians, which is unknown. PHM leadership targets of close to 70% women would be reflective of the field at large; however, institutional variation may exist, and ideally leadership should be diverse and reflective of its faculty members. Our study only describes university-based PHM programs and, therefore, is not necessarily generalizable to nonuniversity programs. Further studies are needed to evaluate any potential differences based on program type. In our study, gender was used in binary terms; however, we acknowledge that gender exists on a spectrum.
CONCLUSION
As a specialty early in development with a robust pipeline of women, PHM is in a unique position to lead the way in gender equity. However, women appear to be underrepresented as division directors at university-based PHM programs. Achieving proportional representation of women leaders is imperative for tapping into the full potential of the community and ensuring that the goals of the field are representative of the population.
Acknowledgment
Special thanks to Lucille Lester, MD, who asked the question that started this road to discovery.
There is a growing appreciation of gender disparities in career advancement in medicine. By 2004, approximately 50% of medical school graduates were women, yet considerable differences persist between genders in compensation, faculty rank, and leadership positions.1-3 According to the Association of American Medical Colleges (AAMC), women account for only 25% of full professors, 18% of department chairs, and 18% of medical school deans.1 Women are also underrepresented in other areas of leadership such as division directors, professional society leadership, and hospital executives.4-6
Specialties that are predominantly women, including pediatrics, are not immune to gender disparities. Women represent 71% of pediatric residents1 and currently constitute two-thirds of active pediatricians in the United States.7 However, there is a disproportionately low number of women ascending the pediatric academic ladder, with only 35% of full professors2 and 28% of department chairs being women.1 Pediatrics also was noted to have the fifth-largest gender pay gap across 40 specialties.3 These disparities can contribute to burnout, poorer patient outcomes, and decreased advancement of women known as the “leaky pipeline.”1,8,9
There is some evidence that gender disparities may be improving among younger professionals with increasing percentages of women as leaders and decreasing pay gaps.10,11 These potential positive trends provide hope that fields in medicine early in their development may demonstrate fewer gender disparities. One of the youngest fields of medicine is pediatric hospital medicine (PHM), which officially became a recognized pediatric subspecialty in 2017.12 There is no literature to date describing gender disparities in PHM. We aimed to explore the gender distribution of university-based PHM program leadership and to compare this gender distribution with that seen in the broader field of PHM.
METHODS
This study was Institutional Review Board–approved as non–human subjects research through University of Chicago, Chicago, Illinois. From January to March 2020, the authors performed web-based searches for PHM division directors or program leaders in the United States. Because there is no single database of PHM programs in the United States, we used the AAMC list of Liaison Committee on Medical Education (LCME)–accredited US medical schools; medical schools in Puerto Rico were not included, nor were pending and provisional institutions. If an institution had multiple practice sites for its students, the primary site for third-year medical student clerkship rotations was included. If a medical school had multiple branches, each with its own primary inpatient pediatrics site, these sites were included. If there was no PHM division director, a program leader (lead hospitalist) was substituted and counted as long as the role was formally designated. This leadership role is herein referred to under the umbrella term of “division director.”
We searched medical school web pages, affiliated hospital web pages, and Google. All program leadership information (divisional and fellowship, if present) was confirmed through direct communication with the program, most commonly with division directors, and included name, gender, title, and presence of associate/assistant leader, gender, and title. Associate division directors were only included if it was a formal leadership position. Associate directors of research, quality, etc, were not included due to the limited number of formal positions noted on further review. Of note, the terms “associate” and “assistant” are referring to leadership positions and not academic ranks.
Fellowship leadership was included if affiliated with a US medical school in the primary list. Medical schools with multiple PHM fellowships were included as separate observations. The leadership was confirmed using the methods described above and cross-referenced through the PHM Fellowship Program website. PHM fellowship programs starting in 2020 were included if leadership was determined.
All leadership positions were verified by two authors, and all authors reviewed the master list to identify errors.
To determine the overall gender breakdown in the specialty, we used three estimates: 2019 American Board of Pediatrics (ABP) PHM Board Certification Exam applicants, the 2019 American Academy of Pediatrics Section on Hospital Medicine membership, and a random sample of all PHM faculty in 25% of the programs included in this study.4
Descriptive statistics using 95% confidence intervals for proportions were used. Differences between proportions were evaluated using a two-proportion z test with the null hypothesis that the two proportions are the same and significance set at P < .05.
RESULTS
Of the 150 AAMC LCME–accredited medical school departments of pediatrics evaluated, a total of 142 programs were included; eight programs were excluded due to not providing inpatient pediatric services.
Division Leadership
The proportion of women PHM division directors was 55% (95% CI, 47%-63%) in this sample of 146 leaders from 142 programs (4 programs had coleaders). In the 113 programs with standalone PHM divisions or sections, the proportion of women division directors was 56% (95% CI, 47%-64%). In the 29 hospitalist groups that were not standalone (ie, embedded in another division), the proportion of women leaders was similar at 52% (95% CI, 34%-69%). In 24 programs with 27 formally designated associate directors (1 program had 3 associate directors and 1 program had 2), 81% of associate directors were women (95% CI, 63%-92%).
Fellowship Leadership
A total of 51 PHM fellowship programs had 53 directors (2 had codirectors), and 66% of the fellowship directors were women (95% CI, 53%-77%). A total of 31 programs had 34 assistant directors (3 programs had 2 assistants), and 82% of the assistant fellowship directors were women (95% CI, 66%-92%).
Comparison With the Field at Large
The inaugural ABP PHM board certification exam in 2019 had 1,627 applicants with 70% women (95% CI, 68%-73%) (Suzanne Woods, MD, email communication, December 4, 2019). The American Academy of Pediatrics Section on Hospital Medicine, the largest PHM-specific organization, has 2,299 practicing physician members with 71% women (95% CI, 69%-73%) (Niccole Alexander, email communication, November 25, 2019). Our random sample of 25% of university-based PHM programs contained 1,063 faculty members with 72% women (95% CI, 69%-75%).
The Table provides P values for comparisons of the proportion of women in each of the above-described leadership roles compared to the most conservative estimate of women in the field from the estimates given above (ie, 70%). Compared with the field at large, women appear to be underrepresented as division directors (70% vs 55%; P < .001) but not as fellowship directors (70% vs 66%; P = .5). There is a higher proportion of women in all associate/assistant director roles, compared with the population (82% vs 70%; P = .04).

DISCUSSION
We found a significant difference between the proportion of women as PHM division directors (55%) when compared with the proportion of women physicians in PHM (70%), which suggests that women are underrepresented in clinical leadership at university-based pediatric hospitalist programs. Similar findings are described in other specialties, including notably adult hospital medicine.4 Burden et al found that only 16% of hospital medicine program leaders were women despite an equal number of women and men in the field. PHM has a much larger proportion of women, compared with that of hospital medicine, and yet women are still underrepresented as program leaders.
We found no disparities between the proportion of women as PHM fellowship directors and the field at large. These results are similar to those of other studies, which showed a higher number of women in educational leadership roles and lower representation in roles with influence over policy and allocation of resources.13,14 Although the proportion of women in educational roles itself is not a concern, there is evidence that these positions may be undervalued by some institutions, which provide these positions with lower salaries and fewer opportunities for career advancement.13,14
Interestingly, women are well-represented in associate/assistant director roles at both the division and fellowship leader level when comparing the distribution in those roles with that of the PHM field at large. This finding suggests that the pipeline of women is robust and potentially may indicate positive change. Alternatively, this finding may reflect a previously described phenomenon of the “sticky floor” in which women are “stuck” in these supportive roles and do not necessarily advance to higher-impact positions.15 We found a statistically significant higher proportion of women in the combined group of all associate/assistant directors compared with the overall population, which raises the concern that supportive leadership roles may represent “women’s work.”16 Future studies are needed to track whether these women truly advance or whether women are overrepresented in supportive leadership positions at the expense of primary leadership positions.
Adequate representation of women alone is not sufficient to achieve gender equity in medicine. We need to understand why there is a lower representation of women in leadership positions. Some barriers have already been described, including gender bias in promotions,17 higher demands outside of work,18 and lower pay,3 though none are specific to PHM. A further qualitative exploration of PHM leadership would help describe any barriers women in PHM specifically may be facing in their career trajectory. In addition, more information is needed to explore the experience of women with intersectional identities in PHM, especially since they may experience increased bias and discrimination.19
Limitations of this study include the lack of a centralized list of PHM programs and data on PHM workforce. Our three estimates for the proportion of women in PHM were similar at 70%-71%; however, these are only proxies for the true gender distribution of PHM physicians, which is unknown. PHM leadership targets of close to 70% women would be reflective of the field at large; however, institutional variation may exist, and ideally leadership should be diverse and reflective of its faculty members. Our study only describes university-based PHM programs and, therefore, is not necessarily generalizable to nonuniversity programs. Further studies are needed to evaluate any potential differences based on program type. In our study, gender was used in binary terms; however, we acknowledge that gender exists on a spectrum.
CONCLUSION
As a specialty early in development with a robust pipeline of women, PHM is in a unique position to lead the way in gender equity. However, women appear to be underrepresented as division directors at university-based PHM programs. Achieving proportional representation of women leaders is imperative for tapping into the full potential of the community and ensuring that the goals of the field are representative of the population.
Acknowledgment
Special thanks to Lucille Lester, MD, who asked the question that started this road to discovery.
1. Lautenberger DM, Dandar VM. State of Women in Academic Medicine 2018-2019 Exploring Pathways to Equity. AAMC; 2020. Accessed April 10, 2020. https://www.aamc.org/data-reports/data/2018-2019-state-women-academic-medicine-exploring-pathways-equity
2. Table 13: U.S. Medical School Faculty by Sex, Rank, and Department, 2017. AAMC; 2019. Accessed June 25, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
3. 2019 Physician Compensation Report. Doximity; March 2019. Accessed April 11, 2020. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round3.pdf
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Silver J, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019:179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303
6. Thomas R, Cooper M, Konar E, et al. Lean In: Women in the Workplace 2019. McKinsey & Company; 2019. Accessed July 1, 2020. https://wiw-report.s3.amazonaws.com/Women_in_the_Workplace_2019.pdf
7. Table 1.3: Number and Percentage of Active Physicians by Sex and Specialty, 2017. AAMC; 2017. Accessed April 12, 2020. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2017
8. Taka F, Nomura K, Horie S, et al. Organizational climate with gender equity and burnout among university academics in Japan. Ind Health. 2016;54(6):480-487. https://doi.org/10.2486/indhealth.2016-0126
9. Tsugawa Y, Jena A, Figueroa J, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. https://doi.org/10.1001/jamainternmed.2016.7875
10. Bissing MA, Lange EMS, Davila WF, et al. Status of women in academic anesthesiology: a 10-year update. Anesth Analg. 2019;128(1):137-143. https://doi.org/10.1213/ane.0000000000003691
11. Graf N, Brown A, Patten E. The narrowing, but persistent, gender gap in pay. Pew Research Center; March 22, 2019. Accessed April 20, 2020. https://www.pewresearch.org/fact-tank/2019/03/22/gender-pay-gap-facts/
12. American Board of Medical Specialties Officially Recognizes Pediatric Hospital Medicine Subspecialty Certification. News release. American Board of Medical Specialties; November 9, 2016. Accessed June 25, 2020. https://www.abms.org/media/120095/abms-recognizes-pediatric-hospital-medicine-as-a-subspecialty.pdf
13. Hofler LG, Hacker MR, Dodge LE, Schutzberg R, Ricciotti HA. Comparison of women in department leadership in obstetrics and gynecology with other specialties. Obstet Gynecol. 2016;127(3):442-447. https://doi.org/10.1097/aog.0000000000001290
14. Weiss A, Lee KC, Tapia V, et al. Equity in surgical leadership for women: more work to do. Am J Surg. 2014;208:494-498. https://doi.org/10.1016/j.amjsurg.2013.11.005
15. Tesch BJ, Wood HM, Helwig AL, Nattinger AB. Promotion of women physicians in academic medicine. Glass ceiling or sticky floor? JAMA. 1995;273(13):1022-1025.
16. Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95(10):1499-1506. https://doi.org/10.1097/acm.0000000000003555
17. Steinpreis RE, Anders KA, Ritzke D. The impact of gender on the review of the curricula vitae of job applicants and tenure candidates: a national empirical study. Sex Roles. 1999;41(7):509-528. https://doi.org/10.1023/A:1018839203698
18. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. https://doi.org/10.7326/m13-0974
19. Ginther DK, Kahn S, Schaffer WT. Gender, race/ethnicity, and National Institutes of Health R01 research awards: is there evidence of a double bind for women of color? Acad Med. 2016;91(8):1098-1107. https://doi.org/10.1097/acm.0000000000001278
1. Lautenberger DM, Dandar VM. State of Women in Academic Medicine 2018-2019 Exploring Pathways to Equity. AAMC; 2020. Accessed April 10, 2020. https://www.aamc.org/data-reports/data/2018-2019-state-women-academic-medicine-exploring-pathways-equity
2. Table 13: U.S. Medical School Faculty by Sex, Rank, and Department, 2017. AAMC; 2019. Accessed June 25, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
3. 2019 Physician Compensation Report. Doximity; March 2019. Accessed April 11, 2020. https://s3.amazonaws.com/s3.doximity.com/press/doximity_third_annual_physician_compensation_report_round3.pdf
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Silver J, Ghalib R, Poorman JA, et al. Analysis of gender equity in leadership of physician-focused medical specialty societies, 2008-2017. JAMA Intern Med. 2019:179(3):433-435. https://doi.org/10.1001/jamainternmed.2018.5303
6. Thomas R, Cooper M, Konar E, et al. Lean In: Women in the Workplace 2019. McKinsey & Company; 2019. Accessed July 1, 2020. https://wiw-report.s3.amazonaws.com/Women_in_the_Workplace_2019.pdf
7. Table 1.3: Number and Percentage of Active Physicians by Sex and Specialty, 2017. AAMC; 2017. Accessed April 12, 2020. https://www.aamc.org/data-reports/workforce/interactive-data/active-physicians-sex-and-specialty-2017
8. Taka F, Nomura K, Horie S, et al. Organizational climate with gender equity and burnout among university academics in Japan. Ind Health. 2016;54(6):480-487. https://doi.org/10.2486/indhealth.2016-0126
9. Tsugawa Y, Jena A, Figueroa J, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. https://doi.org/10.1001/jamainternmed.2016.7875
10. Bissing MA, Lange EMS, Davila WF, et al. Status of women in academic anesthesiology: a 10-year update. Anesth Analg. 2019;128(1):137-143. https://doi.org/10.1213/ane.0000000000003691
11. Graf N, Brown A, Patten E. The narrowing, but persistent, gender gap in pay. Pew Research Center; March 22, 2019. Accessed April 20, 2020. https://www.pewresearch.org/fact-tank/2019/03/22/gender-pay-gap-facts/
12. American Board of Medical Specialties Officially Recognizes Pediatric Hospital Medicine Subspecialty Certification. News release. American Board of Medical Specialties; November 9, 2016. Accessed June 25, 2020. https://www.abms.org/media/120095/abms-recognizes-pediatric-hospital-medicine-as-a-subspecialty.pdf
13. Hofler LG, Hacker MR, Dodge LE, Schutzberg R, Ricciotti HA. Comparison of women in department leadership in obstetrics and gynecology with other specialties. Obstet Gynecol. 2016;127(3):442-447. https://doi.org/10.1097/aog.0000000000001290
14. Weiss A, Lee KC, Tapia V, et al. Equity in surgical leadership for women: more work to do. Am J Surg. 2014;208:494-498. https://doi.org/10.1016/j.amjsurg.2013.11.005
15. Tesch BJ, Wood HM, Helwig AL, Nattinger AB. Promotion of women physicians in academic medicine. Glass ceiling or sticky floor? JAMA. 1995;273(13):1022-1025.
16. Pelley E, Carnes M. When a specialty becomes “women’s work”: trends in and implications of specialty gender segregation in medicine. Acad Med. 2020;95(10):1499-1506. https://doi.org/10.1097/acm.0000000000003555
17. Steinpreis RE, Anders KA, Ritzke D. The impact of gender on the review of the curricula vitae of job applicants and tenure candidates: a national empirical study. Sex Roles. 1999;41(7):509-528. https://doi.org/10.1023/A:1018839203698
18. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. https://doi.org/10.7326/m13-0974
19. Ginther DK, Kahn S, Schaffer WT. Gender, race/ethnicity, and National Institutes of Health R01 research awards: is there evidence of a double bind for women of color? Acad Med. 2016;91(8):1098-1107. https://doi.org/10.1097/acm.0000000000001278
© 2021 Society of Hospital Medicine
Promoting Gender Equity at the Journal of Hospital Medicine
Last year we pledged to lead by example and improve representation within the Journal of Hospital Medicine community.1 By emphasizing diversity, we expand the pool of faculty to whom leadership opportunities are available. A diverse team will put forth a broader range of ideas for consideration, spur greater innovation, and promote diversity in both published content and authorship, ensuring that the spectrum of content we publish reflects and benefits all patients to whom we provide care.
We write to share our progress, first reporting on gender equity. Currently, 45% of the journal leadership team are women, increased from 30% in 2018. In the past year, we also developed processes to collect peer reviewer and author demographic information through our manuscript management system. These processes helped us understand our baseline state.
Prior to developing these processes, we discussed our goals and potential approaches with Society of Hospital Medicine leaders; medical school deans of diversity, equity, and inclusion; department chairs in pediatrics and internal medicine; women, underrepresented minorities, and LGBTQ+ faculty; and trainees. We achieved consensus as a journal leadership team and implemented a new data collection system in July 2019. We focused on first and last authors given the importance of these positions for promotion and tenure. We requested that peer reviewers and authors provide demographic data, including gender (with nonbinary as an option), race, and ethnicity; “prefer not to answer” was a response option for each question. These data were not available during the manuscript decision process. Authors who did not submit information received up to three reminder emails from the Editor-in-Chief encouraging them to provide demographic information and stating the rationale for the request. We did not use gender identifying algorithms (eg, assignment of gender probability based on name) or visit professional websites; our intent was author self-identification.
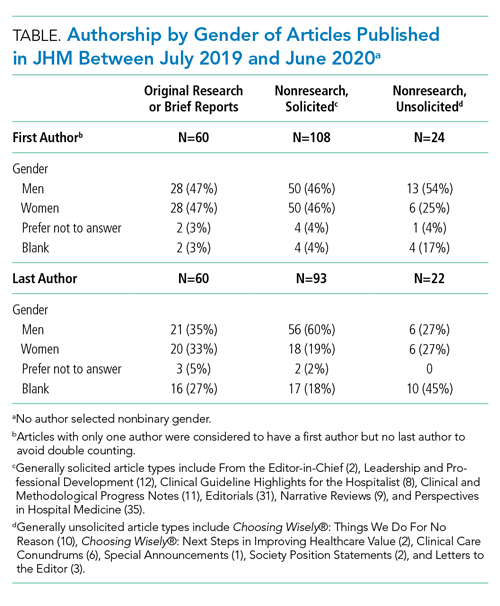
We categorized Journal of Hospital Medicine article types as research, generally solicited, and generally unsolicited (Table). Among research articles, the proportion of women and men were similar with women accounting for 47% of first authors (vs 47% men) and 33% of last authors (vs 35% men) (Table). However, 27% of last authors left this field blank. Among solicited article types, there was an equal proportion of women and men for first but not for last authors. Among unsolicited article types, a smaller proportion of women accounted for first authors. While the proportion of women and men was equal among last authors, 45% left this field blank.
Collecting author demographics and reporting our data on gender represent an important first step for the journal. In the upcoming year, we will develop strategies to obtain more complete data and report our performance on race, ethnicity, and intersectionality, and continue deliberate efforts to improve equity within all areas of the journal, including reviewer, author, and editorial roles. We are committed to continue sharing our progress.
1. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14:393. https://doi.org/10.12788/jhm.3247
Last year we pledged to lead by example and improve representation within the Journal of Hospital Medicine community.1 By emphasizing diversity, we expand the pool of faculty to whom leadership opportunities are available. A diverse team will put forth a broader range of ideas for consideration, spur greater innovation, and promote diversity in both published content and authorship, ensuring that the spectrum of content we publish reflects and benefits all patients to whom we provide care.
We write to share our progress, first reporting on gender equity. Currently, 45% of the journal leadership team are women, increased from 30% in 2018. In the past year, we also developed processes to collect peer reviewer and author demographic information through our manuscript management system. These processes helped us understand our baseline state.
Prior to developing these processes, we discussed our goals and potential approaches with Society of Hospital Medicine leaders; medical school deans of diversity, equity, and inclusion; department chairs in pediatrics and internal medicine; women, underrepresented minorities, and LGBTQ+ faculty; and trainees. We achieved consensus as a journal leadership team and implemented a new data collection system in July 2019. We focused on first and last authors given the importance of these positions for promotion and tenure. We requested that peer reviewers and authors provide demographic data, including gender (with nonbinary as an option), race, and ethnicity; “prefer not to answer” was a response option for each question. These data were not available during the manuscript decision process. Authors who did not submit information received up to three reminder emails from the Editor-in-Chief encouraging them to provide demographic information and stating the rationale for the request. We did not use gender identifying algorithms (eg, assignment of gender probability based on name) or visit professional websites; our intent was author self-identification.
We categorized Journal of Hospital Medicine article types as research, generally solicited, and generally unsolicited (Table). Among research articles, the proportion of women and men were similar with women accounting for 47% of first authors (vs 47% men) and 33% of last authors (vs 35% men) (Table). However, 27% of last authors left this field blank. Among solicited article types, there was an equal proportion of women and men for first but not for last authors. Among unsolicited article types, a smaller proportion of women accounted for first authors. While the proportion of women and men was equal among last authors, 45% left this field blank.
Collecting author demographics and reporting our data on gender represent an important first step for the journal. In the upcoming year, we will develop strategies to obtain more complete data and report our performance on race, ethnicity, and intersectionality, and continue deliberate efforts to improve equity within all areas of the journal, including reviewer, author, and editorial roles. We are committed to continue sharing our progress.
Last year we pledged to lead by example and improve representation within the Journal of Hospital Medicine community.1 By emphasizing diversity, we expand the pool of faculty to whom leadership opportunities are available. A diverse team will put forth a broader range of ideas for consideration, spur greater innovation, and promote diversity in both published content and authorship, ensuring that the spectrum of content we publish reflects and benefits all patients to whom we provide care.
We write to share our progress, first reporting on gender equity. Currently, 45% of the journal leadership team are women, increased from 30% in 2018. In the past year, we also developed processes to collect peer reviewer and author demographic information through our manuscript management system. These processes helped us understand our baseline state.
Prior to developing these processes, we discussed our goals and potential approaches with Society of Hospital Medicine leaders; medical school deans of diversity, equity, and inclusion; department chairs in pediatrics and internal medicine; women, underrepresented minorities, and LGBTQ+ faculty; and trainees. We achieved consensus as a journal leadership team and implemented a new data collection system in July 2019. We focused on first and last authors given the importance of these positions for promotion and tenure. We requested that peer reviewers and authors provide demographic data, including gender (with nonbinary as an option), race, and ethnicity; “prefer not to answer” was a response option for each question. These data were not available during the manuscript decision process. Authors who did not submit information received up to three reminder emails from the Editor-in-Chief encouraging them to provide demographic information and stating the rationale for the request. We did not use gender identifying algorithms (eg, assignment of gender probability based on name) or visit professional websites; our intent was author self-identification.
We categorized Journal of Hospital Medicine article types as research, generally solicited, and generally unsolicited (Table). Among research articles, the proportion of women and men were similar with women accounting for 47% of first authors (vs 47% men) and 33% of last authors (vs 35% men) (Table). However, 27% of last authors left this field blank. Among solicited article types, there was an equal proportion of women and men for first but not for last authors. Among unsolicited article types, a smaller proportion of women accounted for first authors. While the proportion of women and men was equal among last authors, 45% left this field blank.
Collecting author demographics and reporting our data on gender represent an important first step for the journal. In the upcoming year, we will develop strategies to obtain more complete data and report our performance on race, ethnicity, and intersectionality, and continue deliberate efforts to improve equity within all areas of the journal, including reviewer, author, and editorial roles. We are committed to continue sharing our progress.
1. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14:393. https://doi.org/10.12788/jhm.3247
1. Shah SS, Shaughnessy EE, Spector ND. Leading by example: how medical journals can improve representation in academic medicine. J Hosp Med. 2019;14:393. https://doi.org/10.12788/jhm.3247
© 2020 Society of Hospital Medicine
All Hands on Deck: Learning to “Un-specialize” in the COVID-19 Pandemic
Specialization, as detailed in Adam Smith’s 1776 landmark treatise, Wealth of Nations,1 has been an enduring trend in labor and economics for centuries. Mirroring evolution in other sectors of the economy, the healthcare workforce has become ever more specialized.2 General practitioners and family doctors have ceded ground to a bevy of specialists and subspecialists ranging from pediatric endocrinologists to otolaryngology-neurotologists. Given the growth in medical knowledge over the past century, this specialization seems both necessary and good. This same specialization that serves us in good times, though, leaves us woefully underprepared for an epidemic that will require large numbers of hospitalists/generalists and intensivists, such as the current coronavirus disease 2019 (COVID-19) pandemic.
A bit on terminology before we proceed. For purposes of this paper we define generalists as physicians trained in Internal Medicine, Family Medicine, Pediatrics, or Med/Peds who provide primary hospital care to adults and children. While some may argue that hospitalists are specialists in inpatient care, we would like to focus on hospitalists as generalists who focus on inpatient care and what we have in common with the broader community of generalists. We include as generalists anyone, irrespective of clinical training, who chooses broad primary patient responsibility over the narrower consultative role. There is always a specialist in our midst who knows more about a particular disease or condition; as generalists, most of us appreciate and welcome that expertise.
Sometimes it takes a pandemic like COVID-19 to highlight a tremendous blind spot in our healthcare system that, in retrospect, seems hard to have missed. What do we do when we need more generalists and have only a surplus of specialists, many of whom were involuntarily “furloughed” by canceled elective procedures and postponed clinics? How do we “un-specialize” our specialist workforce?
We will discuss some of the most pressing problems facing hospitals working to ensure adequate staffing for general inpatient units caused by the simultaneous reductions in physician availability (because of illness and/or quarantine) and markedly increased admissions of undifferentiated COVID-19–related illnesses. We will assume that hospitals have already activated all providers practicing in areas most similar to hospital medicine, including generalists who have mixed inpatient/outpatient practices, subspecialists with significant inpatient clinical roles, fellows, and advanced practice providers (APPs) with inpatient experience. The Accreditation Council for Graduate Medical Education released guidance around the roles of physician trainees during the pandemic.3 Despite these measures, though, further workforce augmentation will be vital. To that end, several challenges to clinical staffing are enumerated below, accompanied by strategies to address them.
CLINICAL STAFFING CHALLENGES
1. Clinicians eager to help, but out of practice in the inpatient setting: As hospitals across the country work to develop physician staffing contingency plans for scenarios in which general inpatient volumes increase by 50%-300% while 33%-50% of hospitalists either become infected or require quarantine, many hospitals are looking to bolster their physician depth. We have been extremely gratified by the tremendous response from the broader physician communities in which we work. We have encountered retired physicians who have volunteered to come back to work despite being at higher risk of severe COVID-19 complications and physician-scientists offering to step back into clinical roles. We have found outstanding subspecialists asking to work under the tutelage of experienced hospitalists; these specialists recognize how, despite years of clinical experience, they would need significant supervision to function in the inpatient setting. The humility and self-awareness of these volunteers has been phenomenal.
Retraining researchers, subspecialists, and retirees as hospitalists requires purposeful onboarding to target key educational goals. This onboarding should stress COVID-19–specific medical management, training in infection prevention and control, and hospital-specific workflow processes (eg, shift length, sign-over). Onboarding must also include access and orientation to electronic health records, training around inpatient documentation requirements, and billing practices. Non–COVID-19 healthcare will continue; hospitals and clinical leaders will need to determine whether certain specialists should focus on COVID-19 care alone and leave others to continue with speciality practice still needed. Ready access to hospital medicine and medical subspecialty consultation will be pivotal in supervising providers asked to step into hospitalist roles.
The onboarding process we describe might best be viewed through the lens of focused professional practice evaluation (FPPE). Required by the Joint Commission, FPPE is a process for the medical staff of a facility to evaluate privilege-specific competence by clinicians and is used for any new clinical privileges and when there may be question as to a current practitioner’s capabilities. The usual FPPE process includes reassessment of provider practice, typically at 3 to 6 months. Doing so may be challenging given overall workforce stress and the timing of clinical demand—eg, time for medical record review will be limited. Consideration of a “preceptorship” with an experienced hospitalist providing verbal oversight for providers with emergency privileges may be very appropriate. Indeed the Joint Commission recently published guidance around FPPE during the COVID-19 epidemic with the suggestion that mentorship and direct observation are reasonable ways to ensure quality.4
Concerns around scope of practice and medicolegal liability must be rapidly addressed by professional practice organizations, state medical boards, and medical malpractice insurers to protect frontline providers, nurses, and pharmacists. In particular, Joint Commission FPPE process requirements may need to be relaxed to respond to a surge in clinical demand. Contingency and crisis standards of care permit doing so. We welcome the introduction of processes to expedite provider licensure in many hard-hit states.
2. Clinicians who should not help because of medical comorbidities or age: Individuals with certain significant comorbidities (eg, inflammatory conditions treated with immunosuppressants, pulmonary disease, cancer with active treatment) or meeting certain age criteria should be discouraged from clinical work because the dangers of illness for them and of transmission of illness are high. Judgment and a version of mutual informed consent will be needed to address fewer clear scenarios, such as whether a 35-year-old physician who requires a steroid inhaler for asthma or a 64-year-old physician who is otherwise healthy have higher risk. It is our opinion that all physicians should contribute to the care of patients with documented or suspected COVID-19 unless they meet institutionally defined exclusion criteria. We should recognize that physicians who are unable to provide direct care to patients with COVID-19 infection may have significant remorse and feelings that they are letting down their colleagues and the oath they have taken. As the COVID -19 pandemic continues, we are quickly learning that physicians who have contraindications to providing care to patients with active COVID-19 infection can still contribute in numerous mission-critical ways. This may include virtual (telehealth) visits, preceptorship via telehealth of providers completing FPPE in hospital medicine practice, postdischarge follow-up of patients who are no longer infectious, and other care-coordination activities, such as triaging direct admission calls.
3. Clinicians who should be able to help but are fearful: All efforts must be undertaken to protect healthcare workers from acquiring COVID-19. Nevertheless, there are models predicting that ultimately the vast majority of the world’s population will be exposed, including healthcare workers.5,6 In our personal experience as hospitalists and leaders, the vast majority (95%-plus) of our hospitalists have not only continued to do their job but taken on additional responsibilities and clinical work despite the risk. We are hesitant to co-opt words like courage and bravery that we typically would reserve for people in far more hazardous lines of work than physicians, but in the current setting perhaps courage is the correct term. In quiet conversation, many are vaguely unnerved and some significantly so, but they set their angst aside and get to work. The same can be said for the numerous subspecialists, surgeons, nurses, and others who have volunteered to help.
Alternatively, as leaders, we must manage an extremely small minority of faculty who request to not care for patients with COVID-19 despite no clear contraindication. These situations are nuanced and fraught with difficulty for leaders. As physicians we have moral and ethical obligations to society.7 We also have contractual obligations to our employers. Finally, we have a professional duty to our colleagues. When such cases arise, as leaders we should try to understand the perspective of the physician making the request. It is also important to remember that as leaders we are obliged to be fair and equitable to all faculty; granting exceptions to some who ask to avoid COVID-19-related work, but not to others, is difficult to justify. Moreover, granting exceptions can undermine faith in leadership and inevitably sow discord. We suggest setting clear mutual expectations of engagement and not granting unwarranted exceptions.
CONCLUSION
In this time of a global pandemic, we face a looming shortage of hospital generalists, which calls for immediate and purposeful workforce expansion facilitated by learning to “un-specialize” certain providers. We propose utilizing the framework of FPPE to educate and support those joining hospital medicine teams. Hospitalists are innovators and health systems science leaders. Let’s draw on that strength now to rise to the challenge of COVID-19.
1. Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, Illinois: University of Chicago Press; 1976.
2. Cram P, Ettinger WH, Jr. Generalists or specialists--who does it better? Physician Exec. 1998;24(1):40-45.
3. Accreditation Council for Graduate Medical Education. ACGME Response to Pandemic Crisis. https://acgme.org/COVID-19. Accessed April 1, 2020.
4. The Joint Commission. Emergency Management—Meeting FPPE and OPPE Requirements During the COVID-19 Emergency. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medical-staff-ms/000002291/. Accessed April 1, 2020.
5. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. https://doi.org/10.1371/journal.pone.0231236.eCollection 2020.
6. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13222. https://doi.org/10.1111/eci.13222.
7. Antommaria M. Conflicting duties and reciprocal obligations during a pandemic. J Hosp Med. 2020;15(5):xx-xx. https://doi.org/10.12788/jhm.3425.
Specialization, as detailed in Adam Smith’s 1776 landmark treatise, Wealth of Nations,1 has been an enduring trend in labor and economics for centuries. Mirroring evolution in other sectors of the economy, the healthcare workforce has become ever more specialized.2 General practitioners and family doctors have ceded ground to a bevy of specialists and subspecialists ranging from pediatric endocrinologists to otolaryngology-neurotologists. Given the growth in medical knowledge over the past century, this specialization seems both necessary and good. This same specialization that serves us in good times, though, leaves us woefully underprepared for an epidemic that will require large numbers of hospitalists/generalists and intensivists, such as the current coronavirus disease 2019 (COVID-19) pandemic.
A bit on terminology before we proceed. For purposes of this paper we define generalists as physicians trained in Internal Medicine, Family Medicine, Pediatrics, or Med/Peds who provide primary hospital care to adults and children. While some may argue that hospitalists are specialists in inpatient care, we would like to focus on hospitalists as generalists who focus on inpatient care and what we have in common with the broader community of generalists. We include as generalists anyone, irrespective of clinical training, who chooses broad primary patient responsibility over the narrower consultative role. There is always a specialist in our midst who knows more about a particular disease or condition; as generalists, most of us appreciate and welcome that expertise.
Sometimes it takes a pandemic like COVID-19 to highlight a tremendous blind spot in our healthcare system that, in retrospect, seems hard to have missed. What do we do when we need more generalists and have only a surplus of specialists, many of whom were involuntarily “furloughed” by canceled elective procedures and postponed clinics? How do we “un-specialize” our specialist workforce?
We will discuss some of the most pressing problems facing hospitals working to ensure adequate staffing for general inpatient units caused by the simultaneous reductions in physician availability (because of illness and/or quarantine) and markedly increased admissions of undifferentiated COVID-19–related illnesses. We will assume that hospitals have already activated all providers practicing in areas most similar to hospital medicine, including generalists who have mixed inpatient/outpatient practices, subspecialists with significant inpatient clinical roles, fellows, and advanced practice providers (APPs) with inpatient experience. The Accreditation Council for Graduate Medical Education released guidance around the roles of physician trainees during the pandemic.3 Despite these measures, though, further workforce augmentation will be vital. To that end, several challenges to clinical staffing are enumerated below, accompanied by strategies to address them.
CLINICAL STAFFING CHALLENGES
1. Clinicians eager to help, but out of practice in the inpatient setting: As hospitals across the country work to develop physician staffing contingency plans for scenarios in which general inpatient volumes increase by 50%-300% while 33%-50% of hospitalists either become infected or require quarantine, many hospitals are looking to bolster their physician depth. We have been extremely gratified by the tremendous response from the broader physician communities in which we work. We have encountered retired physicians who have volunteered to come back to work despite being at higher risk of severe COVID-19 complications and physician-scientists offering to step back into clinical roles. We have found outstanding subspecialists asking to work under the tutelage of experienced hospitalists; these specialists recognize how, despite years of clinical experience, they would need significant supervision to function in the inpatient setting. The humility and self-awareness of these volunteers has been phenomenal.
Retraining researchers, subspecialists, and retirees as hospitalists requires purposeful onboarding to target key educational goals. This onboarding should stress COVID-19–specific medical management, training in infection prevention and control, and hospital-specific workflow processes (eg, shift length, sign-over). Onboarding must also include access and orientation to electronic health records, training around inpatient documentation requirements, and billing practices. Non–COVID-19 healthcare will continue; hospitals and clinical leaders will need to determine whether certain specialists should focus on COVID-19 care alone and leave others to continue with speciality practice still needed. Ready access to hospital medicine and medical subspecialty consultation will be pivotal in supervising providers asked to step into hospitalist roles.
The onboarding process we describe might best be viewed through the lens of focused professional practice evaluation (FPPE). Required by the Joint Commission, FPPE is a process for the medical staff of a facility to evaluate privilege-specific competence by clinicians and is used for any new clinical privileges and when there may be question as to a current practitioner’s capabilities. The usual FPPE process includes reassessment of provider practice, typically at 3 to 6 months. Doing so may be challenging given overall workforce stress and the timing of clinical demand—eg, time for medical record review will be limited. Consideration of a “preceptorship” with an experienced hospitalist providing verbal oversight for providers with emergency privileges may be very appropriate. Indeed the Joint Commission recently published guidance around FPPE during the COVID-19 epidemic with the suggestion that mentorship and direct observation are reasonable ways to ensure quality.4
Concerns around scope of practice and medicolegal liability must be rapidly addressed by professional practice organizations, state medical boards, and medical malpractice insurers to protect frontline providers, nurses, and pharmacists. In particular, Joint Commission FPPE process requirements may need to be relaxed to respond to a surge in clinical demand. Contingency and crisis standards of care permit doing so. We welcome the introduction of processes to expedite provider licensure in many hard-hit states.
2. Clinicians who should not help because of medical comorbidities or age: Individuals with certain significant comorbidities (eg, inflammatory conditions treated with immunosuppressants, pulmonary disease, cancer with active treatment) or meeting certain age criteria should be discouraged from clinical work because the dangers of illness for them and of transmission of illness are high. Judgment and a version of mutual informed consent will be needed to address fewer clear scenarios, such as whether a 35-year-old physician who requires a steroid inhaler for asthma or a 64-year-old physician who is otherwise healthy have higher risk. It is our opinion that all physicians should contribute to the care of patients with documented or suspected COVID-19 unless they meet institutionally defined exclusion criteria. We should recognize that physicians who are unable to provide direct care to patients with COVID-19 infection may have significant remorse and feelings that they are letting down their colleagues and the oath they have taken. As the COVID -19 pandemic continues, we are quickly learning that physicians who have contraindications to providing care to patients with active COVID-19 infection can still contribute in numerous mission-critical ways. This may include virtual (telehealth) visits, preceptorship via telehealth of providers completing FPPE in hospital medicine practice, postdischarge follow-up of patients who are no longer infectious, and other care-coordination activities, such as triaging direct admission calls.
3. Clinicians who should be able to help but are fearful: All efforts must be undertaken to protect healthcare workers from acquiring COVID-19. Nevertheless, there are models predicting that ultimately the vast majority of the world’s population will be exposed, including healthcare workers.5,6 In our personal experience as hospitalists and leaders, the vast majority (95%-plus) of our hospitalists have not only continued to do their job but taken on additional responsibilities and clinical work despite the risk. We are hesitant to co-opt words like courage and bravery that we typically would reserve for people in far more hazardous lines of work than physicians, but in the current setting perhaps courage is the correct term. In quiet conversation, many are vaguely unnerved and some significantly so, but they set their angst aside and get to work. The same can be said for the numerous subspecialists, surgeons, nurses, and others who have volunteered to help.
Alternatively, as leaders, we must manage an extremely small minority of faculty who request to not care for patients with COVID-19 despite no clear contraindication. These situations are nuanced and fraught with difficulty for leaders. As physicians we have moral and ethical obligations to society.7 We also have contractual obligations to our employers. Finally, we have a professional duty to our colleagues. When such cases arise, as leaders we should try to understand the perspective of the physician making the request. It is also important to remember that as leaders we are obliged to be fair and equitable to all faculty; granting exceptions to some who ask to avoid COVID-19-related work, but not to others, is difficult to justify. Moreover, granting exceptions can undermine faith in leadership and inevitably sow discord. We suggest setting clear mutual expectations of engagement and not granting unwarranted exceptions.
CONCLUSION
In this time of a global pandemic, we face a looming shortage of hospital generalists, which calls for immediate and purposeful workforce expansion facilitated by learning to “un-specialize” certain providers. We propose utilizing the framework of FPPE to educate and support those joining hospital medicine teams. Hospitalists are innovators and health systems science leaders. Let’s draw on that strength now to rise to the challenge of COVID-19.
Specialization, as detailed in Adam Smith’s 1776 landmark treatise, Wealth of Nations,1 has been an enduring trend in labor and economics for centuries. Mirroring evolution in other sectors of the economy, the healthcare workforce has become ever more specialized.2 General practitioners and family doctors have ceded ground to a bevy of specialists and subspecialists ranging from pediatric endocrinologists to otolaryngology-neurotologists. Given the growth in medical knowledge over the past century, this specialization seems both necessary and good. This same specialization that serves us in good times, though, leaves us woefully underprepared for an epidemic that will require large numbers of hospitalists/generalists and intensivists, such as the current coronavirus disease 2019 (COVID-19) pandemic.
A bit on terminology before we proceed. For purposes of this paper we define generalists as physicians trained in Internal Medicine, Family Medicine, Pediatrics, or Med/Peds who provide primary hospital care to adults and children. While some may argue that hospitalists are specialists in inpatient care, we would like to focus on hospitalists as generalists who focus on inpatient care and what we have in common with the broader community of generalists. We include as generalists anyone, irrespective of clinical training, who chooses broad primary patient responsibility over the narrower consultative role. There is always a specialist in our midst who knows more about a particular disease or condition; as generalists, most of us appreciate and welcome that expertise.
Sometimes it takes a pandemic like COVID-19 to highlight a tremendous blind spot in our healthcare system that, in retrospect, seems hard to have missed. What do we do when we need more generalists and have only a surplus of specialists, many of whom were involuntarily “furloughed” by canceled elective procedures and postponed clinics? How do we “un-specialize” our specialist workforce?
We will discuss some of the most pressing problems facing hospitals working to ensure adequate staffing for general inpatient units caused by the simultaneous reductions in physician availability (because of illness and/or quarantine) and markedly increased admissions of undifferentiated COVID-19–related illnesses. We will assume that hospitals have already activated all providers practicing in areas most similar to hospital medicine, including generalists who have mixed inpatient/outpatient practices, subspecialists with significant inpatient clinical roles, fellows, and advanced practice providers (APPs) with inpatient experience. The Accreditation Council for Graduate Medical Education released guidance around the roles of physician trainees during the pandemic.3 Despite these measures, though, further workforce augmentation will be vital. To that end, several challenges to clinical staffing are enumerated below, accompanied by strategies to address them.
CLINICAL STAFFING CHALLENGES
1. Clinicians eager to help, but out of practice in the inpatient setting: As hospitals across the country work to develop physician staffing contingency plans for scenarios in which general inpatient volumes increase by 50%-300% while 33%-50% of hospitalists either become infected or require quarantine, many hospitals are looking to bolster their physician depth. We have been extremely gratified by the tremendous response from the broader physician communities in which we work. We have encountered retired physicians who have volunteered to come back to work despite being at higher risk of severe COVID-19 complications and physician-scientists offering to step back into clinical roles. We have found outstanding subspecialists asking to work under the tutelage of experienced hospitalists; these specialists recognize how, despite years of clinical experience, they would need significant supervision to function in the inpatient setting. The humility and self-awareness of these volunteers has been phenomenal.
Retraining researchers, subspecialists, and retirees as hospitalists requires purposeful onboarding to target key educational goals. This onboarding should stress COVID-19–specific medical management, training in infection prevention and control, and hospital-specific workflow processes (eg, shift length, sign-over). Onboarding must also include access and orientation to electronic health records, training around inpatient documentation requirements, and billing practices. Non–COVID-19 healthcare will continue; hospitals and clinical leaders will need to determine whether certain specialists should focus on COVID-19 care alone and leave others to continue with speciality practice still needed. Ready access to hospital medicine and medical subspecialty consultation will be pivotal in supervising providers asked to step into hospitalist roles.
The onboarding process we describe might best be viewed through the lens of focused professional practice evaluation (FPPE). Required by the Joint Commission, FPPE is a process for the medical staff of a facility to evaluate privilege-specific competence by clinicians and is used for any new clinical privileges and when there may be question as to a current practitioner’s capabilities. The usual FPPE process includes reassessment of provider practice, typically at 3 to 6 months. Doing so may be challenging given overall workforce stress and the timing of clinical demand—eg, time for medical record review will be limited. Consideration of a “preceptorship” with an experienced hospitalist providing verbal oversight for providers with emergency privileges may be very appropriate. Indeed the Joint Commission recently published guidance around FPPE during the COVID-19 epidemic with the suggestion that mentorship and direct observation are reasonable ways to ensure quality.4
Concerns around scope of practice and medicolegal liability must be rapidly addressed by professional practice organizations, state medical boards, and medical malpractice insurers to protect frontline providers, nurses, and pharmacists. In particular, Joint Commission FPPE process requirements may need to be relaxed to respond to a surge in clinical demand. Contingency and crisis standards of care permit doing so. We welcome the introduction of processes to expedite provider licensure in many hard-hit states.
2. Clinicians who should not help because of medical comorbidities or age: Individuals with certain significant comorbidities (eg, inflammatory conditions treated with immunosuppressants, pulmonary disease, cancer with active treatment) or meeting certain age criteria should be discouraged from clinical work because the dangers of illness for them and of transmission of illness are high. Judgment and a version of mutual informed consent will be needed to address fewer clear scenarios, such as whether a 35-year-old physician who requires a steroid inhaler for asthma or a 64-year-old physician who is otherwise healthy have higher risk. It is our opinion that all physicians should contribute to the care of patients with documented or suspected COVID-19 unless they meet institutionally defined exclusion criteria. We should recognize that physicians who are unable to provide direct care to patients with COVID-19 infection may have significant remorse and feelings that they are letting down their colleagues and the oath they have taken. As the COVID -19 pandemic continues, we are quickly learning that physicians who have contraindications to providing care to patients with active COVID-19 infection can still contribute in numerous mission-critical ways. This may include virtual (telehealth) visits, preceptorship via telehealth of providers completing FPPE in hospital medicine practice, postdischarge follow-up of patients who are no longer infectious, and other care-coordination activities, such as triaging direct admission calls.
3. Clinicians who should be able to help but are fearful: All efforts must be undertaken to protect healthcare workers from acquiring COVID-19. Nevertheless, there are models predicting that ultimately the vast majority of the world’s population will be exposed, including healthcare workers.5,6 In our personal experience as hospitalists and leaders, the vast majority (95%-plus) of our hospitalists have not only continued to do their job but taken on additional responsibilities and clinical work despite the risk. We are hesitant to co-opt words like courage and bravery that we typically would reserve for people in far more hazardous lines of work than physicians, but in the current setting perhaps courage is the correct term. In quiet conversation, many are vaguely unnerved and some significantly so, but they set their angst aside and get to work. The same can be said for the numerous subspecialists, surgeons, nurses, and others who have volunteered to help.
Alternatively, as leaders, we must manage an extremely small minority of faculty who request to not care for patients with COVID-19 despite no clear contraindication. These situations are nuanced and fraught with difficulty for leaders. As physicians we have moral and ethical obligations to society.7 We also have contractual obligations to our employers. Finally, we have a professional duty to our colleagues. When such cases arise, as leaders we should try to understand the perspective of the physician making the request. It is also important to remember that as leaders we are obliged to be fair and equitable to all faculty; granting exceptions to some who ask to avoid COVID-19-related work, but not to others, is difficult to justify. Moreover, granting exceptions can undermine faith in leadership and inevitably sow discord. We suggest setting clear mutual expectations of engagement and not granting unwarranted exceptions.
CONCLUSION
In this time of a global pandemic, we face a looming shortage of hospital generalists, which calls for immediate and purposeful workforce expansion facilitated by learning to “un-specialize” certain providers. We propose utilizing the framework of FPPE to educate and support those joining hospital medicine teams. Hospitalists are innovators and health systems science leaders. Let’s draw on that strength now to rise to the challenge of COVID-19.
1. Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, Illinois: University of Chicago Press; 1976.
2. Cram P, Ettinger WH, Jr. Generalists or specialists--who does it better? Physician Exec. 1998;24(1):40-45.
3. Accreditation Council for Graduate Medical Education. ACGME Response to Pandemic Crisis. https://acgme.org/COVID-19. Accessed April 1, 2020.
4. The Joint Commission. Emergency Management—Meeting FPPE and OPPE Requirements During the COVID-19 Emergency. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medical-staff-ms/000002291/. Accessed April 1, 2020.
5. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. https://doi.org/10.1371/journal.pone.0231236.eCollection 2020.
6. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13222. https://doi.org/10.1111/eci.13222.
7. Antommaria M. Conflicting duties and reciprocal obligations during a pandemic. J Hosp Med. 2020;15(5):xx-xx. https://doi.org/10.12788/jhm.3425.
1. Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, Illinois: University of Chicago Press; 1976.
2. Cram P, Ettinger WH, Jr. Generalists or specialists--who does it better? Physician Exec. 1998;24(1):40-45.
3. Accreditation Council for Graduate Medical Education. ACGME Response to Pandemic Crisis. https://acgme.org/COVID-19. Accessed April 1, 2020.
4. The Joint Commission. Emergency Management—Meeting FPPE and OPPE Requirements During the COVID-19 Emergency. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medical-staff-ms/000002291/. Accessed April 1, 2020.
5. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. https://doi.org/10.1371/journal.pone.0231236.eCollection 2020.
6. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13222. https://doi.org/10.1111/eci.13222.
7. Antommaria M. Conflicting duties and reciprocal obligations during a pandemic. J Hosp Med. 2020;15(5):xx-xx. https://doi.org/10.12788/jhm.3425.
© 2020 Society of Hospital Medicine
The Future of Pediatric Hospital Medicine: Challenges and Opportunities
Pediatric hospital medicine (PHM) is in the midst of an exciting period of growth. In 2016, the American Board of Medical Specialties approved the petition for PHM to become the newest pediatric subspecialty, taking PHM on a divergent path from the Focused Practice in Hospital Medicine designation established for adult hospitalists. Establishment as a subspecialty has allowed PHM to define the unique skills and qualifications that hospitalists bring to patients and the healthcare system. These skills and qualifications are delineated in the PHM core competencies and national fellowship curriculum.1,2 In order to realize the vision of PHM to improve care for hospitalized children described by Roberts et al.,3 concerted efforts are needed to train and retain a workforce that is equipped with the skills to catalyze improvements in inpatient pediatric care. We discuss challenges and opportunities facing PHM in workforce development, sustainability of clinical work models, and interhospital collaboration.
FELLOWSHIP TRAINING AND THE PHM PIPELINE
The development of PHM as a subspecialty was driven by a number of factors.4 The acuity of hospitalized children has increased significantly, with a population comprised of more children with complex chronic conditions and/or technology dependence, serious complications of acute conditions, and acute mental health problems. At the same time, the medical and behavioral conditions seen by outpatient general pediatricians have become more complex and time intensive, with these practitioners less likely to work in inpatient settings. Hospitalist care has positive impacts on healthcare efficiency and value, and both parents and primary care pediatricians report high levels of satisfaction with the healthcare delivered by PHM services.4
A national count of the number of pediatric hospitalists is currently lacking. Conservative estimates suggest that at least 3,000 pediatric hospitalists currently practice in the United States.5 These hospitalists have highly varied scopes of practice and work across diverse settings—more diverse, perhaps, than any other pediatric subspecialty. Although difficult to quantify, we estimate that approximately one-third of pediatric hospitalists in the US work in community hospitals and the remainder practice at children’s hospitals.6 Many of the needs of hospitalized children differ across these settings, and the roles and challenges faced by hospitalists in these settings correspondingly differ. Community hospitalists frequently take active roles in newborn care and emergency department consultation, often without the support of other pediatric subspecialties.7 In contrast, hospitalists working at children’s hospitals more frequently care for highly complex patients, often collaborate across multiple specialties and assume nonclinical roles in quality improvement (QI), research, and medical education.
Residents graduating in July 2019 were the last cohort of residents eligible to pursue PHM subspecialty certification via the practice pathway. Accordingly, future residency graduates interested in PHM subspecialty certification will need to complete a PHM fellowship at an accredited program in the US or Canada. Since 2008, PHM fellowship directors have met yearly to collaborate and share best practices,8 developing the two-year fellowship curriculum that forms the basis for the American Board of Pediatrics training pathway.2 The curriculum allows significant flexibility to meet diverse needs, including tailored content for fellows planning to practice in community settings, fellows planning research careers, medicine-pediatrics hospitalist careers, and those desiring increased training in QI, medical education, or leadership/administration.2 In the spring of 2019, Pediatric Research in Inpatient Settings (PRIS) leadership, directors of existing PHM fellowship programs, and national academic society representatives met to develop a fellows’ research curriculum, training resources, and guidelines around scholarship expectations.9 This collaboration aims to accelerate the growth of high-quality clinical training and scholarship to benefit hospitalized children across many different settings.
Such collaboration is essential to address an emerging workforce challenge in PHM. Although the number of PHM fellowship positions is expected to grow in the coming years, there is currently a shortage relative to the anticipated demand. With approximately 2,800 US pediatric residents graduating annually and data indicating that 7% of graduating residents enter and remain in PHM for at least five years,10,11 almost 200 fellowship spots may be needed each year. As of November 2019, 77 fellowship positions were available for residents graduating in 2020,12 which is less than half of the potential demand. To address this mismatch, the PHM Fellowship Directors’ Council has led an annual training for new and potential fellowship directors, and 18 new programs are under development.13 However, this growth may be inadequate to meet the needs of the field. The extent to which limited PHM fellowship positions will adversely affect the pipeline of pediatricians pursuing PHM is unknown.
Efforts to support institutions in creating and expanding fellowship programs will be needed to address the potential shortage of fellowship positions. Continued guidance from the PHM Fellowship Directors’ Council in the many aspects of fellowship program development (eg, curriculum design, assessment) will be crucial in this endeavor. Furthermore, given that fellowships must support fellows to conduct scholarly work and demonstrate evidence of robust faculty scholarly activities to attain accreditation, an essential area of focus is faculty development. Considering barriers such as lack of time, mentorship, and resources, some divisions interested in starting a fellowship may find it challenging to achieve these standards.14 However, hospitalists are often engaged in areas such as QI and medical education, and there is potential to turn ongoing work into meaningful scholarship with appropriate guidance. Many of our supporting organizations (eg, Academic Pediatric Association, American Academy of Pediatrics, and Society of Hospital Medicine) provide training programs for faculty in areas such as educational scholarship, research, and QI; however, more may be needed. Leaders of PHM programs will need to be mindful and creative in accessing local, regional, and national resources to invest in faculty development.
CLINICAL WORK MODELS AND SUSTAINABILITY
As a group, pediatric hospitalists report high levels of satisfaction with their jobs.11 Despite this finding, there are a number of threats to the sustainability of current work models, some of which are unique to pediatrics given the overall lower patient volumes and greater seasonal variation compared with adult hospital medicine. Both university and community-based hospitalist programs report high weekend, overnight, and in-house clinical effort.7,15 Recent studies reported that a significant proportion of PHM program leaders (50% of division directors at university-affiliated programs and 37% of community program leaders) perceive their program to be unsustainable.7,15 Among university-affiliated programs, a higher burden of weekend work as well as university employment were associated with perceived unsustainability, while no specific program or employer characteristic was associated with this perception in community programs.
These findings indicate that efforts are needed to address PHM program sustainability and that different work models and interventions may be needed for university-based and community PHM programs. Wide variability exists in the ways that programs address overall clinical burden, with strategies including census caps, seasonal expansion of coverage, and formal back-up systems.7,15 Additional potential solutions may include differential weighting or financial incentives for nights and weekends, support for nonclinical work, loan repayment programs, and competitive salaries.11 In addition, structuring clinical and nonclinical roles to facilitate career development and advancement may enhance career longevity.15 Lessons learned from pediatric emergency medicine (PEM), which developed as a field a few decades ahead of PHM, may predict future challenges. A 2015 survey of PEM faculty found that despite a 15% decrease in weekly work hours over a 15-year period, a substantial number of PEM faculty report concerns about burnout, with 40% reporting a plan to decrease their clinical workload and 13% planning to leave the field within five years.16 Like PEM, the field of PHM may benefit from the development of best practice guidelines to improve well-being and career longevity.17
INTERHOSPITAL COLLABORATION
The culture of collaboration within PHM places the field in a solid position to address both workforce challenges and barriers to high-quality care for hospitalized children. There are several hospital-based learning networks actively working to strengthen our knowledge base and improve healthcare quality. The PRIS network (www.prisnetwork.org) aims to improve healthcare for children through multihospital studies, boasting 114 sites in the US and Canada. Numerous collaborative projects have linked hospitalists across programs to tackle problems ranging from handoff communication18 to eliminating monitor overuse.19 The Value in Inpatient Pediatrics network has similarly leveraged collaborations across multiple children’s and community hospitals to improve transitions of care20 and care for common conditions such as bronchiolitis, febrile infants, and asthma.21 These networks serve as models of effective collaboration between children’s hospitals and community hospitals, more of which is needed to increase research and QI initiatives in community hospitals, where the majority of US children receive their hospital-based care.6,22
With the rapid growth of scholarly networks in research, QI, and education, PHM has a solid infrastructure on which to base continued development as a subspeciality. Building on this infrastructure will be essential in order to address current challenges in workforce development, fellowship training, and program sustainability. Ultimately, achieving a strong, stable, and skilled workforce will enable PHM to fulfill its promise of improving the care of children across the diversity of settings where they receive their hospital-based care.
Disclosures
Dr. Leyenaar provides consultative services to the American Board of Pediatrics Foundation, which is not associated with this manuscript. Drs. Wang and Shaughnessy have no disclosures
1. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(S2):1-114. https://doi.org/10.1002/jhm.776.
2. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1). https://doi.org/10.1542/peds.2017-0698.
3. Roberts KB, Fisher ER, Rauch DA. A history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2019.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
5. American Board of Medical Specialities. American Board of Medical Specialities application for a new subspecialty certificate: Pediatric hospital medicine. http://www.abms.org/media/114649/abpeds-application-for-pediatric-hospital-medicine.pdf. Accessed November 6, 2019.
6. Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(10):682-685. https://doi.org/10.12788/jhm.3263.
8. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571.
9. Pediatric Hospital Medicine Fellowship Research Training Development. https://projectreporter.nih.gov/project_info_description.cfm?aid=9593276&icde=47889643. Accessed December 10, 2019.
10. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
11. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151.
12. PHM Fellowship Programs. http://phmfellows.org/phm-programs/. Accessed November 6, 2019.
13. Rassbach C [Personal communication]; 2019.
14. Bekmezian A, Teufel RJ, 2nd, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38-44. https://doi.org/10.1542/hpeds.2011-0006.
15. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977.
16. Gorelick MH, Schremmer R, Ruch-Ross H, Radabaugh C, Selbst S. Current workforce characteristics and burnout in pediatric emergency medicine. Acad Emerg Med. 2016;23(1):48-54. https://doi.org/10.1111/acem.12845.
17. American College of Emergency Physicians. Policy Statement: Emergency Physician Shift Work; June 2017.
18. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
19. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: Study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2.
20. Coghlin DT, Leyenaar JK, Shen M, et al. Pediatric discharge content: a multisite assessment of physician preferences and experiences. Hosp Pediatr. 2014;4(1):9-15. https://doi.org/10.1542/hpeds.2013-0022.
21. Value in inpatient pediatrics (VIP) Network. 2019. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed October 13, 2019.
22. McDaniel CE, Jennings R, Schroeder AR, et al. Aligning inpatient pediatric research with settings of care: A call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
Pediatric hospital medicine (PHM) is in the midst of an exciting period of growth. In 2016, the American Board of Medical Specialties approved the petition for PHM to become the newest pediatric subspecialty, taking PHM on a divergent path from the Focused Practice in Hospital Medicine designation established for adult hospitalists. Establishment as a subspecialty has allowed PHM to define the unique skills and qualifications that hospitalists bring to patients and the healthcare system. These skills and qualifications are delineated in the PHM core competencies and national fellowship curriculum.1,2 In order to realize the vision of PHM to improve care for hospitalized children described by Roberts et al.,3 concerted efforts are needed to train and retain a workforce that is equipped with the skills to catalyze improvements in inpatient pediatric care. We discuss challenges and opportunities facing PHM in workforce development, sustainability of clinical work models, and interhospital collaboration.
FELLOWSHIP TRAINING AND THE PHM PIPELINE
The development of PHM as a subspecialty was driven by a number of factors.4 The acuity of hospitalized children has increased significantly, with a population comprised of more children with complex chronic conditions and/or technology dependence, serious complications of acute conditions, and acute mental health problems. At the same time, the medical and behavioral conditions seen by outpatient general pediatricians have become more complex and time intensive, with these practitioners less likely to work in inpatient settings. Hospitalist care has positive impacts on healthcare efficiency and value, and both parents and primary care pediatricians report high levels of satisfaction with the healthcare delivered by PHM services.4
A national count of the number of pediatric hospitalists is currently lacking. Conservative estimates suggest that at least 3,000 pediatric hospitalists currently practice in the United States.5 These hospitalists have highly varied scopes of practice and work across diverse settings—more diverse, perhaps, than any other pediatric subspecialty. Although difficult to quantify, we estimate that approximately one-third of pediatric hospitalists in the US work in community hospitals and the remainder practice at children’s hospitals.6 Many of the needs of hospitalized children differ across these settings, and the roles and challenges faced by hospitalists in these settings correspondingly differ. Community hospitalists frequently take active roles in newborn care and emergency department consultation, often without the support of other pediatric subspecialties.7 In contrast, hospitalists working at children’s hospitals more frequently care for highly complex patients, often collaborate across multiple specialties and assume nonclinical roles in quality improvement (QI), research, and medical education.
Residents graduating in July 2019 were the last cohort of residents eligible to pursue PHM subspecialty certification via the practice pathway. Accordingly, future residency graduates interested in PHM subspecialty certification will need to complete a PHM fellowship at an accredited program in the US or Canada. Since 2008, PHM fellowship directors have met yearly to collaborate and share best practices,8 developing the two-year fellowship curriculum that forms the basis for the American Board of Pediatrics training pathway.2 The curriculum allows significant flexibility to meet diverse needs, including tailored content for fellows planning to practice in community settings, fellows planning research careers, medicine-pediatrics hospitalist careers, and those desiring increased training in QI, medical education, or leadership/administration.2 In the spring of 2019, Pediatric Research in Inpatient Settings (PRIS) leadership, directors of existing PHM fellowship programs, and national academic society representatives met to develop a fellows’ research curriculum, training resources, and guidelines around scholarship expectations.9 This collaboration aims to accelerate the growth of high-quality clinical training and scholarship to benefit hospitalized children across many different settings.
Such collaboration is essential to address an emerging workforce challenge in PHM. Although the number of PHM fellowship positions is expected to grow in the coming years, there is currently a shortage relative to the anticipated demand. With approximately 2,800 US pediatric residents graduating annually and data indicating that 7% of graduating residents enter and remain in PHM for at least five years,10,11 almost 200 fellowship spots may be needed each year. As of November 2019, 77 fellowship positions were available for residents graduating in 2020,12 which is less than half of the potential demand. To address this mismatch, the PHM Fellowship Directors’ Council has led an annual training for new and potential fellowship directors, and 18 new programs are under development.13 However, this growth may be inadequate to meet the needs of the field. The extent to which limited PHM fellowship positions will adversely affect the pipeline of pediatricians pursuing PHM is unknown.
Efforts to support institutions in creating and expanding fellowship programs will be needed to address the potential shortage of fellowship positions. Continued guidance from the PHM Fellowship Directors’ Council in the many aspects of fellowship program development (eg, curriculum design, assessment) will be crucial in this endeavor. Furthermore, given that fellowships must support fellows to conduct scholarly work and demonstrate evidence of robust faculty scholarly activities to attain accreditation, an essential area of focus is faculty development. Considering barriers such as lack of time, mentorship, and resources, some divisions interested in starting a fellowship may find it challenging to achieve these standards.14 However, hospitalists are often engaged in areas such as QI and medical education, and there is potential to turn ongoing work into meaningful scholarship with appropriate guidance. Many of our supporting organizations (eg, Academic Pediatric Association, American Academy of Pediatrics, and Society of Hospital Medicine) provide training programs for faculty in areas such as educational scholarship, research, and QI; however, more may be needed. Leaders of PHM programs will need to be mindful and creative in accessing local, regional, and national resources to invest in faculty development.
CLINICAL WORK MODELS AND SUSTAINABILITY
As a group, pediatric hospitalists report high levels of satisfaction with their jobs.11 Despite this finding, there are a number of threats to the sustainability of current work models, some of which are unique to pediatrics given the overall lower patient volumes and greater seasonal variation compared with adult hospital medicine. Both university and community-based hospitalist programs report high weekend, overnight, and in-house clinical effort.7,15 Recent studies reported that a significant proportion of PHM program leaders (50% of division directors at university-affiliated programs and 37% of community program leaders) perceive their program to be unsustainable.7,15 Among university-affiliated programs, a higher burden of weekend work as well as university employment were associated with perceived unsustainability, while no specific program or employer characteristic was associated with this perception in community programs.
These findings indicate that efforts are needed to address PHM program sustainability and that different work models and interventions may be needed for university-based and community PHM programs. Wide variability exists in the ways that programs address overall clinical burden, with strategies including census caps, seasonal expansion of coverage, and formal back-up systems.7,15 Additional potential solutions may include differential weighting or financial incentives for nights and weekends, support for nonclinical work, loan repayment programs, and competitive salaries.11 In addition, structuring clinical and nonclinical roles to facilitate career development and advancement may enhance career longevity.15 Lessons learned from pediatric emergency medicine (PEM), which developed as a field a few decades ahead of PHM, may predict future challenges. A 2015 survey of PEM faculty found that despite a 15% decrease in weekly work hours over a 15-year period, a substantial number of PEM faculty report concerns about burnout, with 40% reporting a plan to decrease their clinical workload and 13% planning to leave the field within five years.16 Like PEM, the field of PHM may benefit from the development of best practice guidelines to improve well-being and career longevity.17
INTERHOSPITAL COLLABORATION
The culture of collaboration within PHM places the field in a solid position to address both workforce challenges and barriers to high-quality care for hospitalized children. There are several hospital-based learning networks actively working to strengthen our knowledge base and improve healthcare quality. The PRIS network (www.prisnetwork.org) aims to improve healthcare for children through multihospital studies, boasting 114 sites in the US and Canada. Numerous collaborative projects have linked hospitalists across programs to tackle problems ranging from handoff communication18 to eliminating monitor overuse.19 The Value in Inpatient Pediatrics network has similarly leveraged collaborations across multiple children’s and community hospitals to improve transitions of care20 and care for common conditions such as bronchiolitis, febrile infants, and asthma.21 These networks serve as models of effective collaboration between children’s hospitals and community hospitals, more of which is needed to increase research and QI initiatives in community hospitals, where the majority of US children receive their hospital-based care.6,22
With the rapid growth of scholarly networks in research, QI, and education, PHM has a solid infrastructure on which to base continued development as a subspeciality. Building on this infrastructure will be essential in order to address current challenges in workforce development, fellowship training, and program sustainability. Ultimately, achieving a strong, stable, and skilled workforce will enable PHM to fulfill its promise of improving the care of children across the diversity of settings where they receive their hospital-based care.
Disclosures
Dr. Leyenaar provides consultative services to the American Board of Pediatrics Foundation, which is not associated with this manuscript. Drs. Wang and Shaughnessy have no disclosures
Pediatric hospital medicine (PHM) is in the midst of an exciting period of growth. In 2016, the American Board of Medical Specialties approved the petition for PHM to become the newest pediatric subspecialty, taking PHM on a divergent path from the Focused Practice in Hospital Medicine designation established for adult hospitalists. Establishment as a subspecialty has allowed PHM to define the unique skills and qualifications that hospitalists bring to patients and the healthcare system. These skills and qualifications are delineated in the PHM core competencies and national fellowship curriculum.1,2 In order to realize the vision of PHM to improve care for hospitalized children described by Roberts et al.,3 concerted efforts are needed to train and retain a workforce that is equipped with the skills to catalyze improvements in inpatient pediatric care. We discuss challenges and opportunities facing PHM in workforce development, sustainability of clinical work models, and interhospital collaboration.
FELLOWSHIP TRAINING AND THE PHM PIPELINE
The development of PHM as a subspecialty was driven by a number of factors.4 The acuity of hospitalized children has increased significantly, with a population comprised of more children with complex chronic conditions and/or technology dependence, serious complications of acute conditions, and acute mental health problems. At the same time, the medical and behavioral conditions seen by outpatient general pediatricians have become more complex and time intensive, with these practitioners less likely to work in inpatient settings. Hospitalist care has positive impacts on healthcare efficiency and value, and both parents and primary care pediatricians report high levels of satisfaction with the healthcare delivered by PHM services.4
A national count of the number of pediatric hospitalists is currently lacking. Conservative estimates suggest that at least 3,000 pediatric hospitalists currently practice in the United States.5 These hospitalists have highly varied scopes of practice and work across diverse settings—more diverse, perhaps, than any other pediatric subspecialty. Although difficult to quantify, we estimate that approximately one-third of pediatric hospitalists in the US work in community hospitals and the remainder practice at children’s hospitals.6 Many of the needs of hospitalized children differ across these settings, and the roles and challenges faced by hospitalists in these settings correspondingly differ. Community hospitalists frequently take active roles in newborn care and emergency department consultation, often without the support of other pediatric subspecialties.7 In contrast, hospitalists working at children’s hospitals more frequently care for highly complex patients, often collaborate across multiple specialties and assume nonclinical roles in quality improvement (QI), research, and medical education.
Residents graduating in July 2019 were the last cohort of residents eligible to pursue PHM subspecialty certification via the practice pathway. Accordingly, future residency graduates interested in PHM subspecialty certification will need to complete a PHM fellowship at an accredited program in the US or Canada. Since 2008, PHM fellowship directors have met yearly to collaborate and share best practices,8 developing the two-year fellowship curriculum that forms the basis for the American Board of Pediatrics training pathway.2 The curriculum allows significant flexibility to meet diverse needs, including tailored content for fellows planning to practice in community settings, fellows planning research careers, medicine-pediatrics hospitalist careers, and those desiring increased training in QI, medical education, or leadership/administration.2 In the spring of 2019, Pediatric Research in Inpatient Settings (PRIS) leadership, directors of existing PHM fellowship programs, and national academic society representatives met to develop a fellows’ research curriculum, training resources, and guidelines around scholarship expectations.9 This collaboration aims to accelerate the growth of high-quality clinical training and scholarship to benefit hospitalized children across many different settings.
Such collaboration is essential to address an emerging workforce challenge in PHM. Although the number of PHM fellowship positions is expected to grow in the coming years, there is currently a shortage relative to the anticipated demand. With approximately 2,800 US pediatric residents graduating annually and data indicating that 7% of graduating residents enter and remain in PHM for at least five years,10,11 almost 200 fellowship spots may be needed each year. As of November 2019, 77 fellowship positions were available for residents graduating in 2020,12 which is less than half of the potential demand. To address this mismatch, the PHM Fellowship Directors’ Council has led an annual training for new and potential fellowship directors, and 18 new programs are under development.13 However, this growth may be inadequate to meet the needs of the field. The extent to which limited PHM fellowship positions will adversely affect the pipeline of pediatricians pursuing PHM is unknown.
Efforts to support institutions in creating and expanding fellowship programs will be needed to address the potential shortage of fellowship positions. Continued guidance from the PHM Fellowship Directors’ Council in the many aspects of fellowship program development (eg, curriculum design, assessment) will be crucial in this endeavor. Furthermore, given that fellowships must support fellows to conduct scholarly work and demonstrate evidence of robust faculty scholarly activities to attain accreditation, an essential area of focus is faculty development. Considering barriers such as lack of time, mentorship, and resources, some divisions interested in starting a fellowship may find it challenging to achieve these standards.14 However, hospitalists are often engaged in areas such as QI and medical education, and there is potential to turn ongoing work into meaningful scholarship with appropriate guidance. Many of our supporting organizations (eg, Academic Pediatric Association, American Academy of Pediatrics, and Society of Hospital Medicine) provide training programs for faculty in areas such as educational scholarship, research, and QI; however, more may be needed. Leaders of PHM programs will need to be mindful and creative in accessing local, regional, and national resources to invest in faculty development.
CLINICAL WORK MODELS AND SUSTAINABILITY
As a group, pediatric hospitalists report high levels of satisfaction with their jobs.11 Despite this finding, there are a number of threats to the sustainability of current work models, some of which are unique to pediatrics given the overall lower patient volumes and greater seasonal variation compared with adult hospital medicine. Both university and community-based hospitalist programs report high weekend, overnight, and in-house clinical effort.7,15 Recent studies reported that a significant proportion of PHM program leaders (50% of division directors at university-affiliated programs and 37% of community program leaders) perceive their program to be unsustainable.7,15 Among university-affiliated programs, a higher burden of weekend work as well as university employment were associated with perceived unsustainability, while no specific program or employer characteristic was associated with this perception in community programs.
These findings indicate that efforts are needed to address PHM program sustainability and that different work models and interventions may be needed for university-based and community PHM programs. Wide variability exists in the ways that programs address overall clinical burden, with strategies including census caps, seasonal expansion of coverage, and formal back-up systems.7,15 Additional potential solutions may include differential weighting or financial incentives for nights and weekends, support for nonclinical work, loan repayment programs, and competitive salaries.11 In addition, structuring clinical and nonclinical roles to facilitate career development and advancement may enhance career longevity.15 Lessons learned from pediatric emergency medicine (PEM), which developed as a field a few decades ahead of PHM, may predict future challenges. A 2015 survey of PEM faculty found that despite a 15% decrease in weekly work hours over a 15-year period, a substantial number of PEM faculty report concerns about burnout, with 40% reporting a plan to decrease their clinical workload and 13% planning to leave the field within five years.16 Like PEM, the field of PHM may benefit from the development of best practice guidelines to improve well-being and career longevity.17
INTERHOSPITAL COLLABORATION
The culture of collaboration within PHM places the field in a solid position to address both workforce challenges and barriers to high-quality care for hospitalized children. There are several hospital-based learning networks actively working to strengthen our knowledge base and improve healthcare quality. The PRIS network (www.prisnetwork.org) aims to improve healthcare for children through multihospital studies, boasting 114 sites in the US and Canada. Numerous collaborative projects have linked hospitalists across programs to tackle problems ranging from handoff communication18 to eliminating monitor overuse.19 The Value in Inpatient Pediatrics network has similarly leveraged collaborations across multiple children’s and community hospitals to improve transitions of care20 and care for common conditions such as bronchiolitis, febrile infants, and asthma.21 These networks serve as models of effective collaboration between children’s hospitals and community hospitals, more of which is needed to increase research and QI initiatives in community hospitals, where the majority of US children receive their hospital-based care.6,22
With the rapid growth of scholarly networks in research, QI, and education, PHM has a solid infrastructure on which to base continued development as a subspeciality. Building on this infrastructure will be essential in order to address current challenges in workforce development, fellowship training, and program sustainability. Ultimately, achieving a strong, stable, and skilled workforce will enable PHM to fulfill its promise of improving the care of children across the diversity of settings where they receive their hospital-based care.
Disclosures
Dr. Leyenaar provides consultative services to the American Board of Pediatrics Foundation, which is not associated with this manuscript. Drs. Wang and Shaughnessy have no disclosures
1. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(S2):1-114. https://doi.org/10.1002/jhm.776.
2. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1). https://doi.org/10.1542/peds.2017-0698.
3. Roberts KB, Fisher ER, Rauch DA. A history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2019.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
5. American Board of Medical Specialities. American Board of Medical Specialities application for a new subspecialty certificate: Pediatric hospital medicine. http://www.abms.org/media/114649/abpeds-application-for-pediatric-hospital-medicine.pdf. Accessed November 6, 2019.
6. Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(10):682-685. https://doi.org/10.12788/jhm.3263.
8. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571.
9. Pediatric Hospital Medicine Fellowship Research Training Development. https://projectreporter.nih.gov/project_info_description.cfm?aid=9593276&icde=47889643. Accessed December 10, 2019.
10. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
11. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151.
12. PHM Fellowship Programs. http://phmfellows.org/phm-programs/. Accessed November 6, 2019.
13. Rassbach C [Personal communication]; 2019.
14. Bekmezian A, Teufel RJ, 2nd, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38-44. https://doi.org/10.1542/hpeds.2011-0006.
15. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977.
16. Gorelick MH, Schremmer R, Ruch-Ross H, Radabaugh C, Selbst S. Current workforce characteristics and burnout in pediatric emergency medicine. Acad Emerg Med. 2016;23(1):48-54. https://doi.org/10.1111/acem.12845.
17. American College of Emergency Physicians. Policy Statement: Emergency Physician Shift Work; June 2017.
18. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
19. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: Study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2.
20. Coghlin DT, Leyenaar JK, Shen M, et al. Pediatric discharge content: a multisite assessment of physician preferences and experiences. Hosp Pediatr. 2014;4(1):9-15. https://doi.org/10.1542/hpeds.2013-0022.
21. Value in inpatient pediatrics (VIP) Network. 2019. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed October 13, 2019.
22. McDaniel CE, Jennings R, Schroeder AR, et al. Aligning inpatient pediatric research with settings of care: A call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
1. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a framework for curriculum development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(S2):1-114. https://doi.org/10.1002/jhm.776.
2. Jerardi KE, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatrics. 2017;140(1). https://doi.org/10.1542/peds.2017-0698.
3. Roberts KB, Fisher ER, Rauch DA. A history of pediatric hospital medicine in the United States, 1996-2019. J Hosp Med. 2019.
4. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
5. American Board of Medical Specialities. American Board of Medical Specialities application for a new subspecialty certificate: Pediatric hospital medicine. http://www.abms.org/media/114649/abpeds-application-for-pediatric-hospital-medicine.pdf. Accessed November 6, 2019.
6. Leyenaar JK, Ralston SL, Shieh MS, et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624.
7. Alvarez F, McDaniel CE, Birnie K, et al. Community pediatric hospitalist workload: results from a national survey. J Hosp Med. 2019;14(10):682-685. https://doi.org/10.12788/jhm.3263.
8. Shah NH, Rhim HJ, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. https://doi.org/10.1002/jhm.2571.
9. Pediatric Hospital Medicine Fellowship Research Training Development. https://projectreporter.nih.gov/project_info_description.cfm?aid=9593276&icde=47889643. Accessed December 10, 2019.
10. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
11. House S, Frintner MP, Leyenaar JK. Factors influencing career longevity in pediatric hospital medicine. Hosp Pediatr. 2019;9(12):983-988. https://doi.org/10.1542/hpeds.2019-0151.
12. PHM Fellowship Programs. http://phmfellows.org/phm-programs/. Accessed November 6, 2019.
13. Rassbach C [Personal communication]; 2019.
14. Bekmezian A, Teufel RJ, 2nd, Wilson KM. Research needs of pediatric hospitalists. Hosp Pediatr. 2011;1(1):38-44. https://doi.org/10.1542/hpeds.2011-0006.
15. Fromme HB, Chen CO, Fine BR, Gosdin C, Shaughnessy EE. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. https://doi.org/10.12788/jhm.2977.
16. Gorelick MH, Schremmer R, Ruch-Ross H, Radabaugh C, Selbst S. Current workforce characteristics and burnout in pediatric emergency medicine. Acad Emerg Med. 2016;23(1):48-54. https://doi.org/10.1111/acem.12845.
17. American College of Emergency Physicians. Policy Statement: Emergency Physician Shift Work; June 2017.
18. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
19. Rasooly IR, Beidas RS, Wolk CB, et al. Measuring overuse of continuous pulse oximetry in bronchiolitis and developing strategies for large-scale deimplementation: Study protocol for a feasibility trial. Pilot Feasibility Stud. 2019;5:68. https://doi.org/10.1186/s40814-019-0453-2.
20. Coghlin DT, Leyenaar JK, Shen M, et al. Pediatric discharge content: a multisite assessment of physician preferences and experiences. Hosp Pediatr. 2014;4(1):9-15. https://doi.org/10.1542/hpeds.2013-0022.
21. Value in inpatient pediatrics (VIP) Network. 2019. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed October 13, 2019.
22. McDaniel CE, Jennings R, Schroeder AR, et al. Aligning inpatient pediatric research with settings of care: A call to action. Pediatrics. 2019;143(5). https://doi.org/10.1542/peds.2018-2648.
© 2020 Society of Hospital Medicine
Leading By Example: How Medical Journals Can Improve Representation in Academic Medicine
Women and racial and ethnic minorities remain underrepresented in senior faculty roles and academic leadership positions.1 Participation in peer review and publication in medical journals are important components of academic advancement that are emphasized in the promotion process. These efforts offer recognition of expertise and increase visibility in the scientific community, which may enhance opportunities for networking and collaboration, and provide other opportunities for career advancement. In addition, abundant evidence shows that organizations benefit from diverse teams, with better quality decisions and increased productivity resulting from diverse ideas and perspectives.2
Numerous studies have highlighted the prevalence and persistence of disparities in peer review and authorship.3,4 Much of this work has focused on gender though gaps in these measures likely exist for racial and ethnic minorities. Yet, there are few examples of journals implementing strategies to address disparities and track results of such efforts.5 While institutional barriers to advancement must be addressed, we believe that medical journals have an obligation to address unequal opportunities.
At the Journal of Hospital Medicine, we are committed to leading by example and developing approaches to create equity in all facets of journal leadership and authorship.6 The first step towards progress is to assess the current representation of women and racial and ethnic minorities in our journal community, including first and senior authors, invited expert contributors, reviewers, and editorial team members. Like most journals, we have not collected demographic information from authors or reviewers. But now, as part of the journal’s commitment to this cause, we request that everyone in the journal community (author, reviewer, editor) update their journal account (accessible at https://mc.manuscriptcentral.com/jhm) with demographic data, including gender, race, and ethnicity.
Inclusion of these data is voluntary. While each individual will be able to access and edit their personal demographic data, the individual data will remain private and unviewable to others. As such, it will not be available for nor will it be used in the manuscript review or decision process but rather for assessing our own inclusiveness. We will review these data in aggregate to broadly inform outreach efforts to promote diversity and inclusion in our author, invited expert contributor, reviewer, and journal leadership pools. We will report on the progress of these efforts in upcoming years.
We are committed to equity in providing opportunities for academic advancement across the journal community. Diversity and inclusion are important in raising the quality of the work that we publish. Different perspectives strengthen our journal and will help us continue to advance the field of Hospital Medicine.
Disclosures
The authors have nothing to disclose.
1. American Association of Medical Colleges. U.S. Medical School Faculty, 2018. https://www.aamc.org/data/facultyroster/reports/494946/usmsf18.html. Accessed May 6, 2019.
2. Turban S, Wu D, Zhang L. “When Gender Diversity Makes Firms More Productive” Harvard Business Review Feb 2019. https://hbr.org/2019/02/research-when-gender-diversity-makes-firms-more-productive. Accessed May 6, 2019.
3. Silver JK, Poorman JA, Reilly JM, Spector ND, Goldstein R, Zafonte RD. Assessment of women physicians among authors of perspective-type articles published in high-impact pediatric journals. JAMA Netw Open. 2018;1(3):e180802. doi: 10.1001/jamanetworkopen.2018.0802. PubMed
4. Jagsi R, Guancial EA, Worobey CC, Henault LE, Chang Y, Starr R, Tarbell NJ, Hylek EM. The “gender gap” in authorship of academic medical literature- a 35-year perspective. N Engl J Med. 2006;355(3):281-287. doi: 10.1056/NEJMsa053910. PubMed
5. Nature’s under-representation of women. Nature. 2018;558:344. doi: 10.1038/d41586-018-05465-7. PubMed
6. Shah SS. The Journal of Hospital Medicine in 2019 and beyond. J Hosp Med. 2019;14(1):7. doi: 10.12788/jhm.3143. PubMed
Women and racial and ethnic minorities remain underrepresented in senior faculty roles and academic leadership positions.1 Participation in peer review and publication in medical journals are important components of academic advancement that are emphasized in the promotion process. These efforts offer recognition of expertise and increase visibility in the scientific community, which may enhance opportunities for networking and collaboration, and provide other opportunities for career advancement. In addition, abundant evidence shows that organizations benefit from diverse teams, with better quality decisions and increased productivity resulting from diverse ideas and perspectives.2
Numerous studies have highlighted the prevalence and persistence of disparities in peer review and authorship.3,4 Much of this work has focused on gender though gaps in these measures likely exist for racial and ethnic minorities. Yet, there are few examples of journals implementing strategies to address disparities and track results of such efforts.5 While institutional barriers to advancement must be addressed, we believe that medical journals have an obligation to address unequal opportunities.
At the Journal of Hospital Medicine, we are committed to leading by example and developing approaches to create equity in all facets of journal leadership and authorship.6 The first step towards progress is to assess the current representation of women and racial and ethnic minorities in our journal community, including first and senior authors, invited expert contributors, reviewers, and editorial team members. Like most journals, we have not collected demographic information from authors or reviewers. But now, as part of the journal’s commitment to this cause, we request that everyone in the journal community (author, reviewer, editor) update their journal account (accessible at https://mc.manuscriptcentral.com/jhm) with demographic data, including gender, race, and ethnicity.
Inclusion of these data is voluntary. While each individual will be able to access and edit their personal demographic data, the individual data will remain private and unviewable to others. As such, it will not be available for nor will it be used in the manuscript review or decision process but rather for assessing our own inclusiveness. We will review these data in aggregate to broadly inform outreach efforts to promote diversity and inclusion in our author, invited expert contributor, reviewer, and journal leadership pools. We will report on the progress of these efforts in upcoming years.
We are committed to equity in providing opportunities for academic advancement across the journal community. Diversity and inclusion are important in raising the quality of the work that we publish. Different perspectives strengthen our journal and will help us continue to advance the field of Hospital Medicine.
Disclosures
The authors have nothing to disclose.
Women and racial and ethnic minorities remain underrepresented in senior faculty roles and academic leadership positions.1 Participation in peer review and publication in medical journals are important components of academic advancement that are emphasized in the promotion process. These efforts offer recognition of expertise and increase visibility in the scientific community, which may enhance opportunities for networking and collaboration, and provide other opportunities for career advancement. In addition, abundant evidence shows that organizations benefit from diverse teams, with better quality decisions and increased productivity resulting from diverse ideas and perspectives.2
Numerous studies have highlighted the prevalence and persistence of disparities in peer review and authorship.3,4 Much of this work has focused on gender though gaps in these measures likely exist for racial and ethnic minorities. Yet, there are few examples of journals implementing strategies to address disparities and track results of such efforts.5 While institutional barriers to advancement must be addressed, we believe that medical journals have an obligation to address unequal opportunities.
At the Journal of Hospital Medicine, we are committed to leading by example and developing approaches to create equity in all facets of journal leadership and authorship.6 The first step towards progress is to assess the current representation of women and racial and ethnic minorities in our journal community, including first and senior authors, invited expert contributors, reviewers, and editorial team members. Like most journals, we have not collected demographic information from authors or reviewers. But now, as part of the journal’s commitment to this cause, we request that everyone in the journal community (author, reviewer, editor) update their journal account (accessible at https://mc.manuscriptcentral.com/jhm) with demographic data, including gender, race, and ethnicity.
Inclusion of these data is voluntary. While each individual will be able to access and edit their personal demographic data, the individual data will remain private and unviewable to others. As such, it will not be available for nor will it be used in the manuscript review or decision process but rather for assessing our own inclusiveness. We will review these data in aggregate to broadly inform outreach efforts to promote diversity and inclusion in our author, invited expert contributor, reviewer, and journal leadership pools. We will report on the progress of these efforts in upcoming years.
We are committed to equity in providing opportunities for academic advancement across the journal community. Diversity and inclusion are important in raising the quality of the work that we publish. Different perspectives strengthen our journal and will help us continue to advance the field of Hospital Medicine.
Disclosures
The authors have nothing to disclose.
1. American Association of Medical Colleges. U.S. Medical School Faculty, 2018. https://www.aamc.org/data/facultyroster/reports/494946/usmsf18.html. Accessed May 6, 2019.
2. Turban S, Wu D, Zhang L. “When Gender Diversity Makes Firms More Productive” Harvard Business Review Feb 2019. https://hbr.org/2019/02/research-when-gender-diversity-makes-firms-more-productive. Accessed May 6, 2019.
3. Silver JK, Poorman JA, Reilly JM, Spector ND, Goldstein R, Zafonte RD. Assessment of women physicians among authors of perspective-type articles published in high-impact pediatric journals. JAMA Netw Open. 2018;1(3):e180802. doi: 10.1001/jamanetworkopen.2018.0802. PubMed
4. Jagsi R, Guancial EA, Worobey CC, Henault LE, Chang Y, Starr R, Tarbell NJ, Hylek EM. The “gender gap” in authorship of academic medical literature- a 35-year perspective. N Engl J Med. 2006;355(3):281-287. doi: 10.1056/NEJMsa053910. PubMed
5. Nature’s under-representation of women. Nature. 2018;558:344. doi: 10.1038/d41586-018-05465-7. PubMed
6. Shah SS. The Journal of Hospital Medicine in 2019 and beyond. J Hosp Med. 2019;14(1):7. doi: 10.12788/jhm.3143. PubMed
1. American Association of Medical Colleges. U.S. Medical School Faculty, 2018. https://www.aamc.org/data/facultyroster/reports/494946/usmsf18.html. Accessed May 6, 2019.
2. Turban S, Wu D, Zhang L. “When Gender Diversity Makes Firms More Productive” Harvard Business Review Feb 2019. https://hbr.org/2019/02/research-when-gender-diversity-makes-firms-more-productive. Accessed May 6, 2019.
3. Silver JK, Poorman JA, Reilly JM, Spector ND, Goldstein R, Zafonte RD. Assessment of women physicians among authors of perspective-type articles published in high-impact pediatric journals. JAMA Netw Open. 2018;1(3):e180802. doi: 10.1001/jamanetworkopen.2018.0802. PubMed
4. Jagsi R, Guancial EA, Worobey CC, Henault LE, Chang Y, Starr R, Tarbell NJ, Hylek EM. The “gender gap” in authorship of academic medical literature- a 35-year perspective. N Engl J Med. 2006;355(3):281-287. doi: 10.1056/NEJMsa053910. PubMed
5. Nature’s under-representation of women. Nature. 2018;558:344. doi: 10.1038/d41586-018-05465-7. PubMed
6. Shah SS. The Journal of Hospital Medicine in 2019 and beyond. J Hosp Med. 2019;14(1):7. doi: 10.12788/jhm.3143. PubMed
© 2019 Society of Hospital Medicine
In Reply to: “Practical Application of Pediatric Hospital Medicine Workforce Data: In Reference to ‘Pediatric Hospitalist Workload and Sustainability in University-Based Programs: Results from a National Interview-Based Survey’”
We appreciate the query by Drs. Douglas and Wilson. We hereby supply additional information that is critical for creating and administering sustainable staffing models.
For programs with a census cap, the majority cited 16 or fewer patients as the trigger for that cap. Nearly all programs with back-up used a census of 16 or fewer. Over 80% of programs cited a “safe 7
Regarding clinical weighting of nights, nighttime shifts were often more heavily weighted than day shifts, but approaches to weighting varied and have not been validated. Alternate staffing models for overnight pager calls varied greatly by individual program.
This is a time of significant growth for pediatric hospital medicine, and national workforce data are essential to hospitalists, administrators, and most importantly, patients. Our study1 provides pediatric hospital medicine leaders with data for discussions regarding appropriate FTE and staffing model considerations. The insights generated by our study are particularly relevant in expanding programs and solving problems related to recruitment and retention.
Disclosures
The authors have nothing to disclose.
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. PubMed
We appreciate the query by Drs. Douglas and Wilson. We hereby supply additional information that is critical for creating and administering sustainable staffing models.
For programs with a census cap, the majority cited 16 or fewer patients as the trigger for that cap. Nearly all programs with back-up used a census of 16 or fewer. Over 80% of programs cited a “safe 7
Regarding clinical weighting of nights, nighttime shifts were often more heavily weighted than day shifts, but approaches to weighting varied and have not been validated. Alternate staffing models for overnight pager calls varied greatly by individual program.
This is a time of significant growth for pediatric hospital medicine, and national workforce data are essential to hospitalists, administrators, and most importantly, patients. Our study1 provides pediatric hospital medicine leaders with data for discussions regarding appropriate FTE and staffing model considerations. The insights generated by our study are particularly relevant in expanding programs and solving problems related to recruitment and retention.
Disclosures
The authors have nothing to disclose.
We appreciate the query by Drs. Douglas and Wilson. We hereby supply additional information that is critical for creating and administering sustainable staffing models.
For programs with a census cap, the majority cited 16 or fewer patients as the trigger for that cap. Nearly all programs with back-up used a census of 16 or fewer. Over 80% of programs cited a “safe 7
Regarding clinical weighting of nights, nighttime shifts were often more heavily weighted than day shifts, but approaches to weighting varied and have not been validated. Alternate staffing models for overnight pager calls varied greatly by individual program.
This is a time of significant growth for pediatric hospital medicine, and national workforce data are essential to hospitalists, administrators, and most importantly, patients. Our study1 provides pediatric hospital medicine leaders with data for discussions regarding appropriate FTE and staffing model considerations. The insights generated by our study are particularly relevant in expanding programs and solving problems related to recruitment and retention.
Disclosures
The authors have nothing to disclose.
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. PubMed
1. Fromme HB, Chen C, Fine B, Gosdin C, Shaughnessy E. Pediatric hospitalist workload and sustainability in university-based programs: Results from a national interview-based survey. J Hosp Med. 2018;13(10):702-705. PubMed
© 2019 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
© 2019 Society of Hospital Medicine