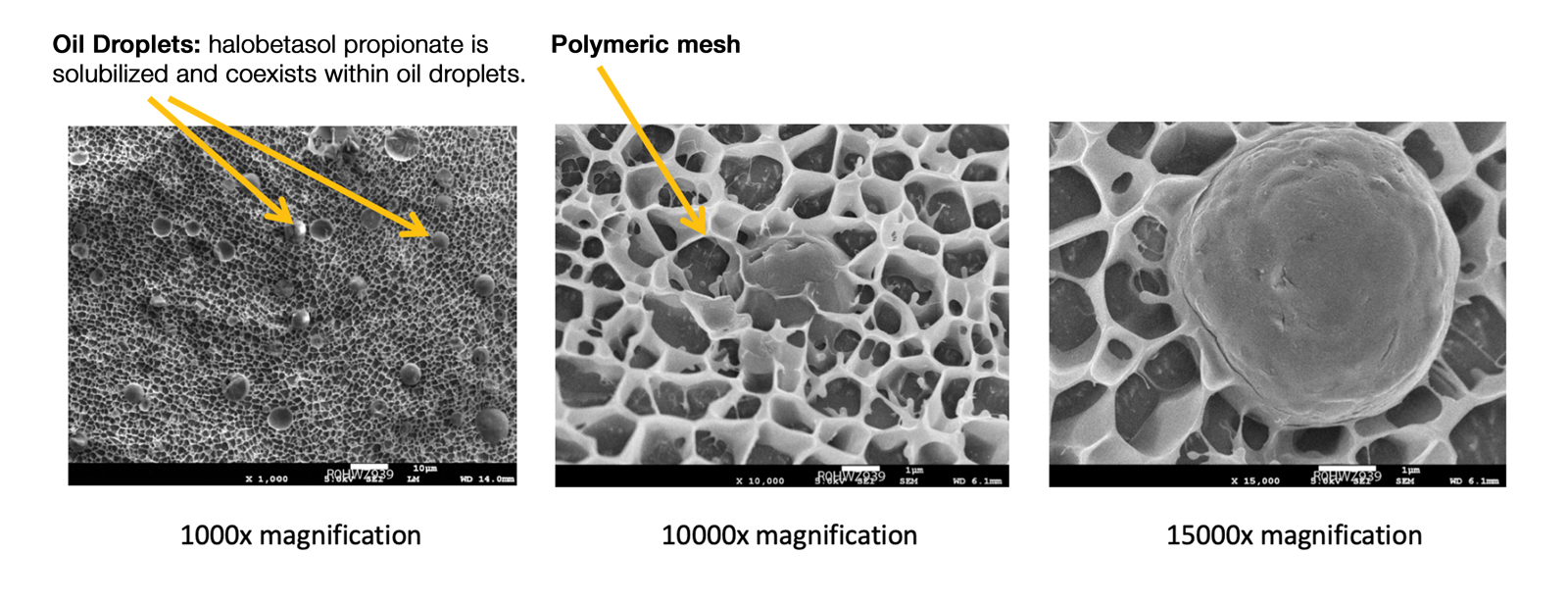
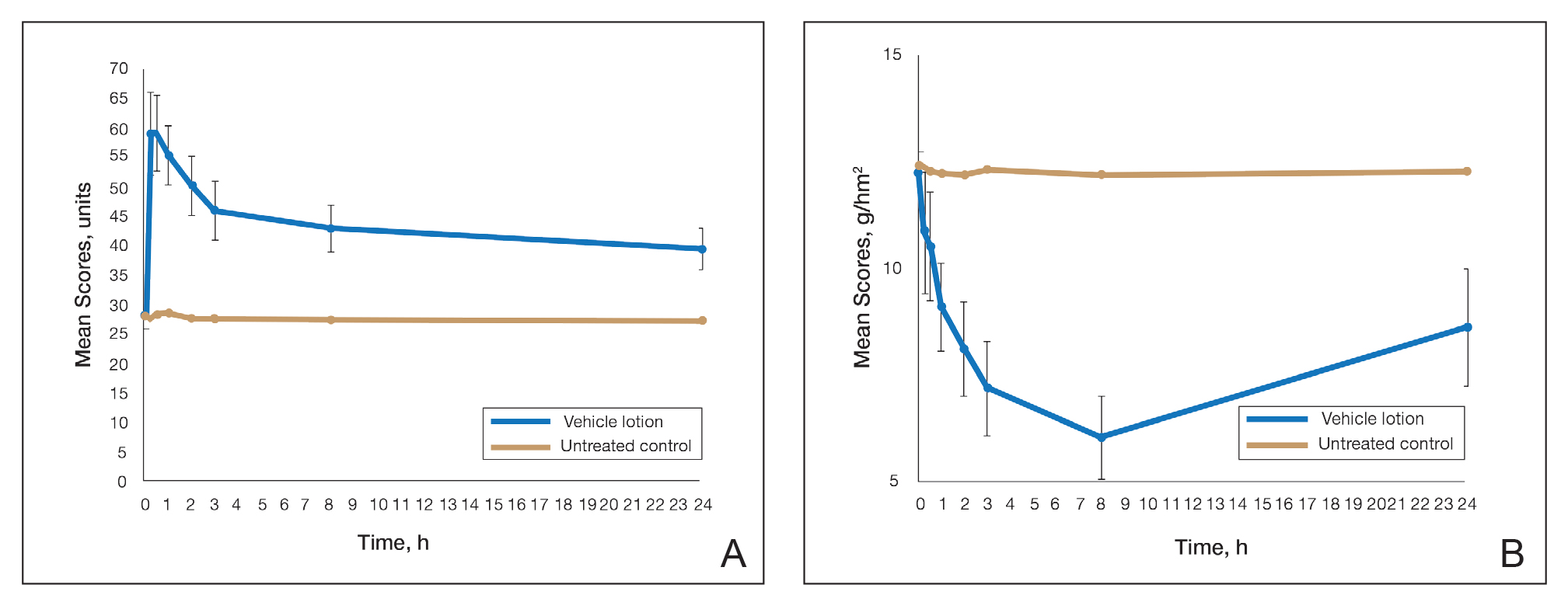
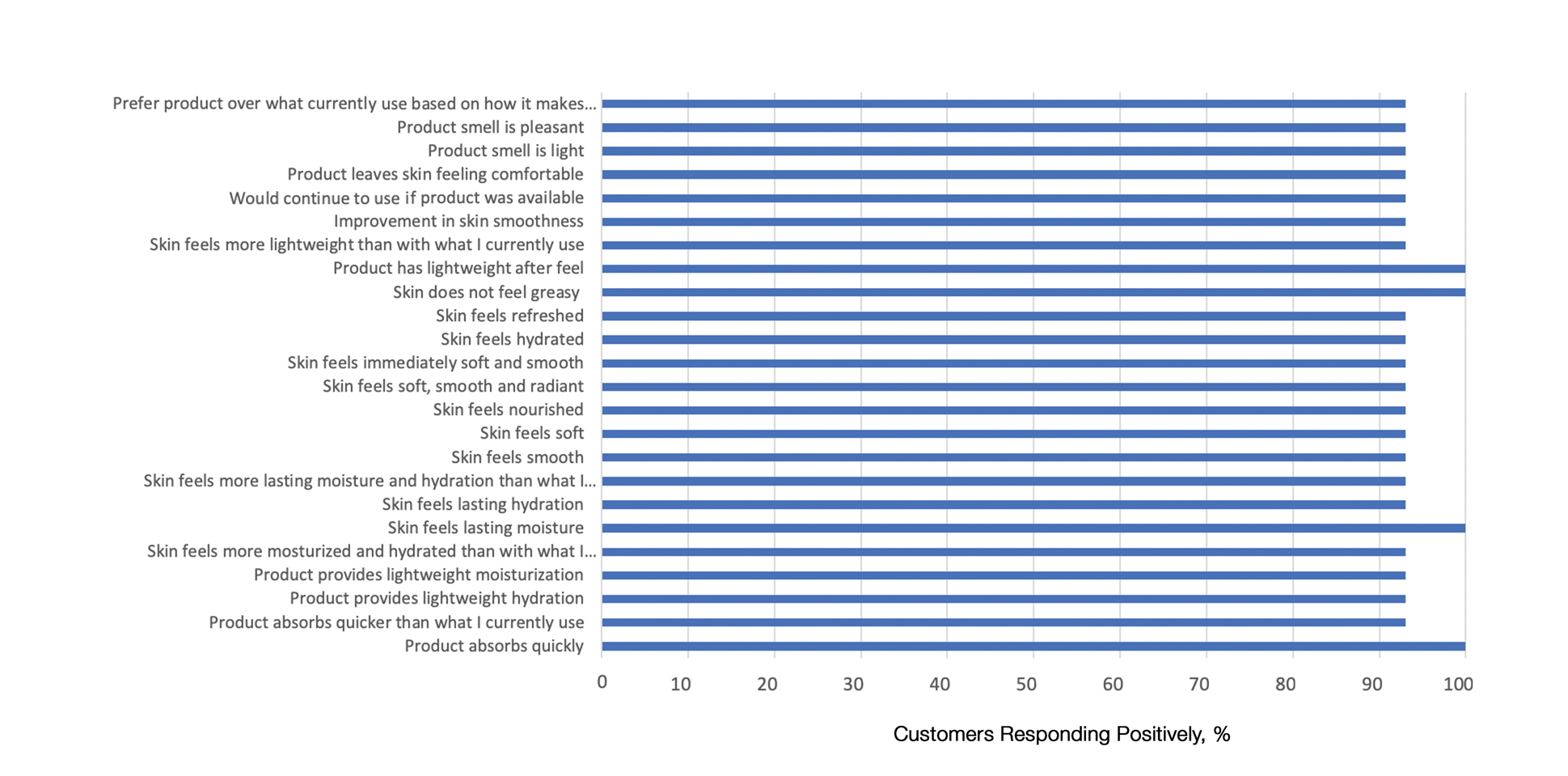
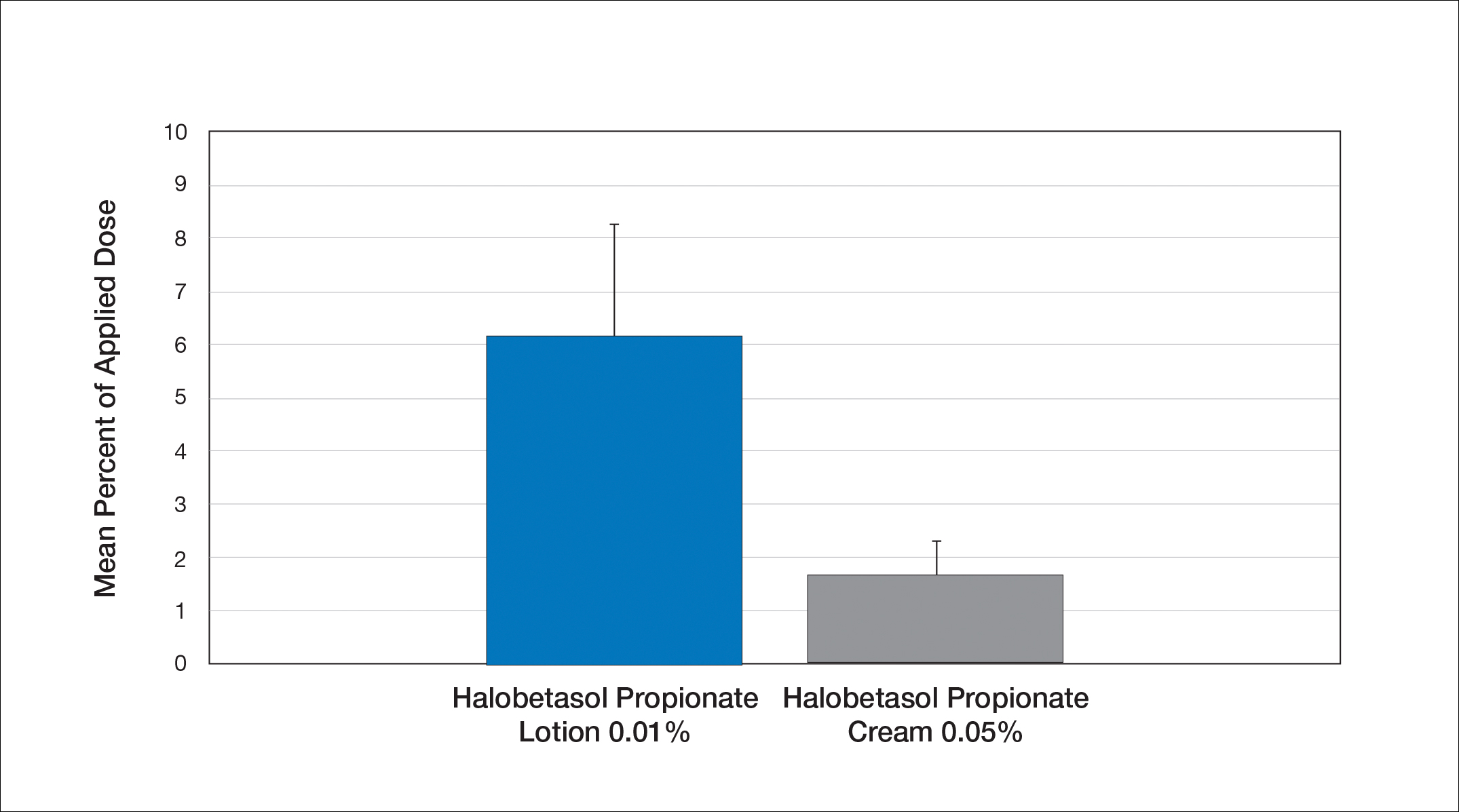
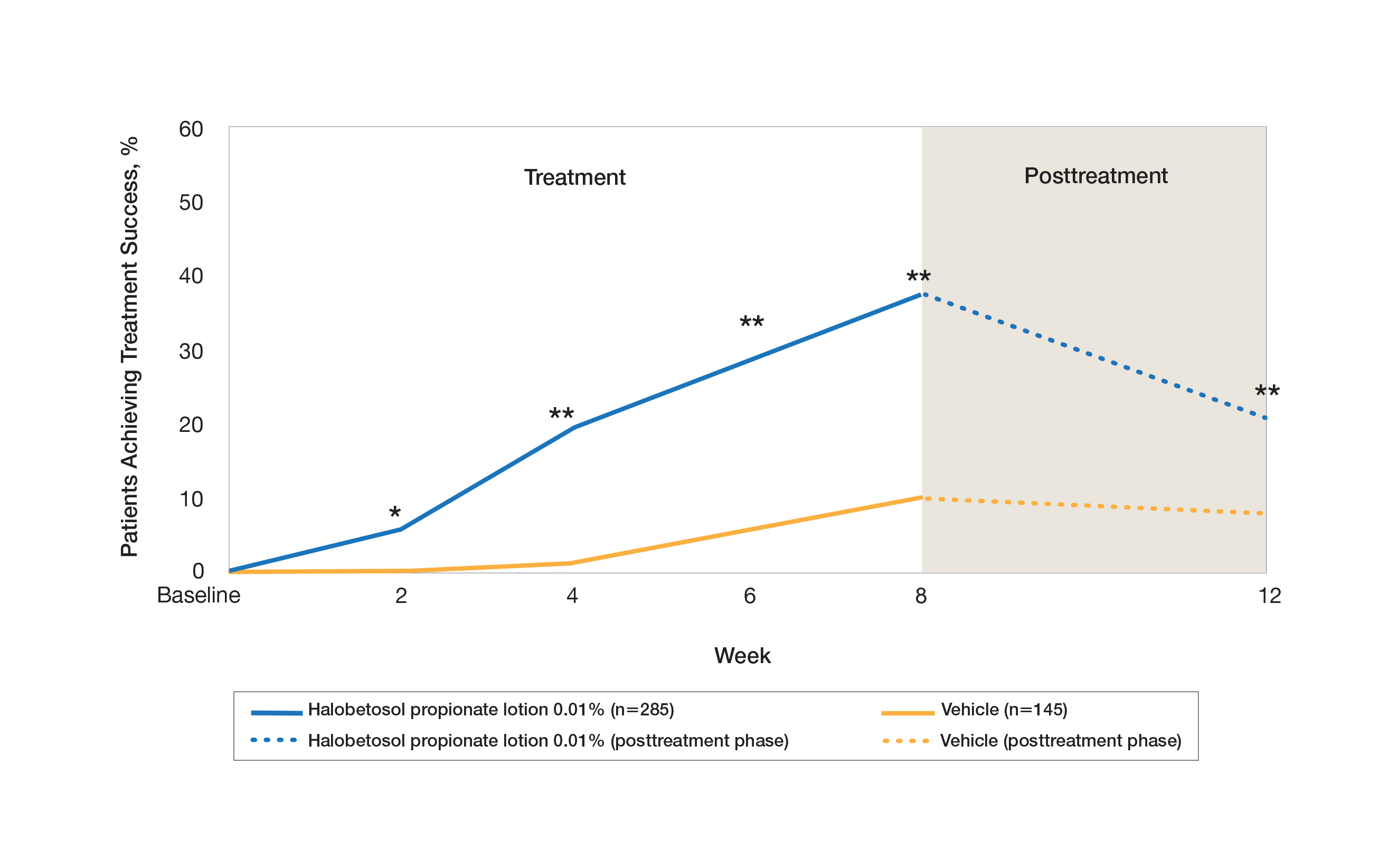
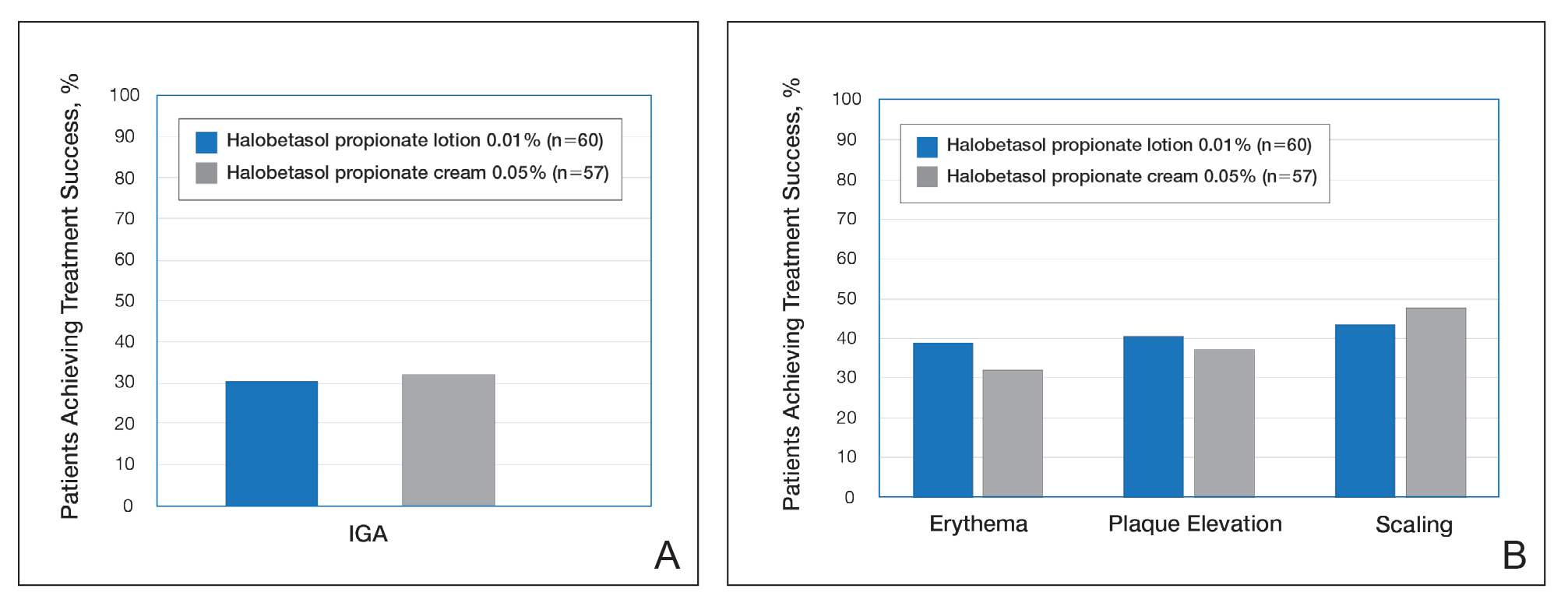
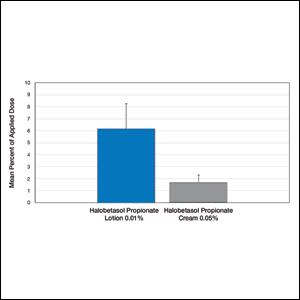
User login
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Phototherapy
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and
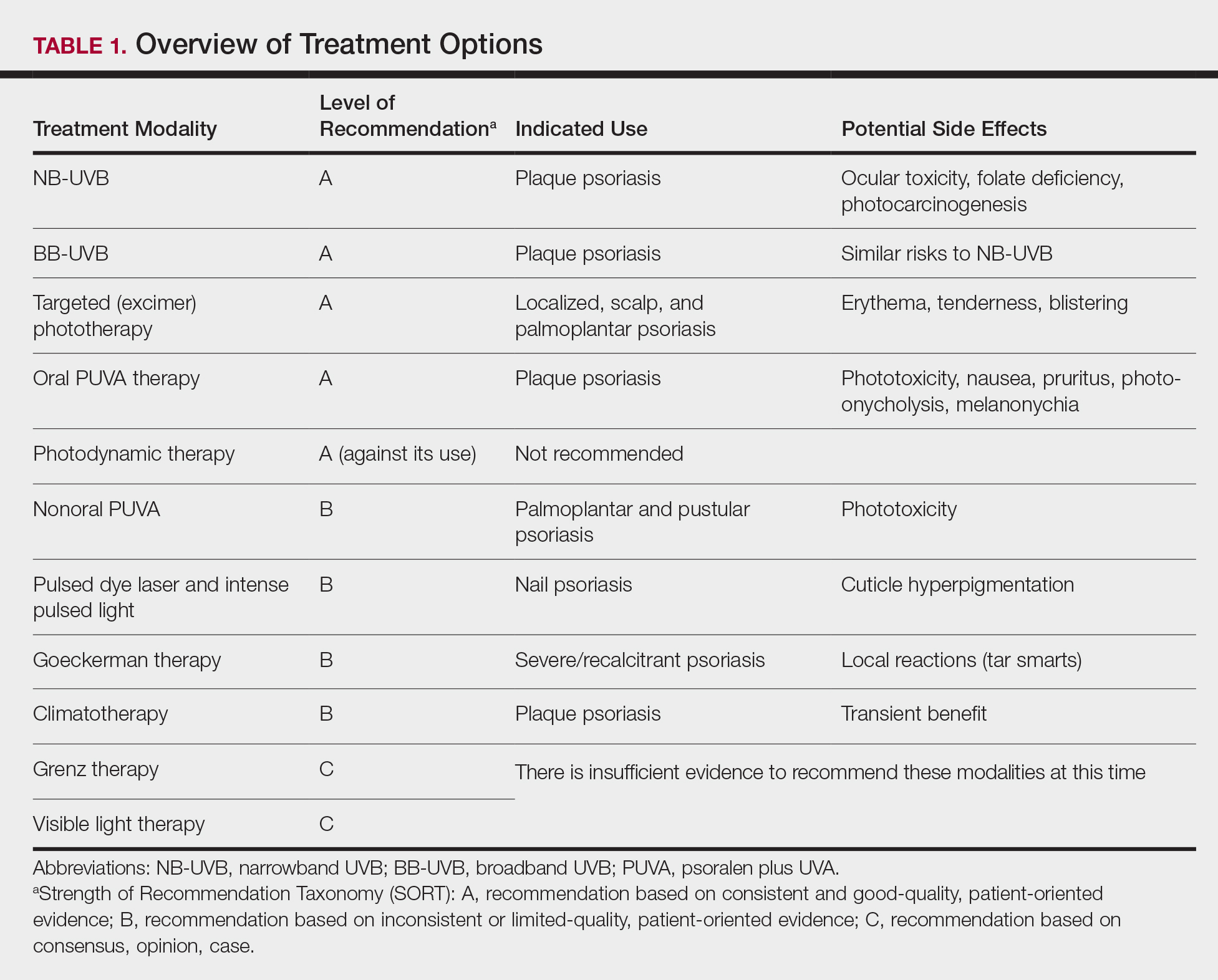
Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
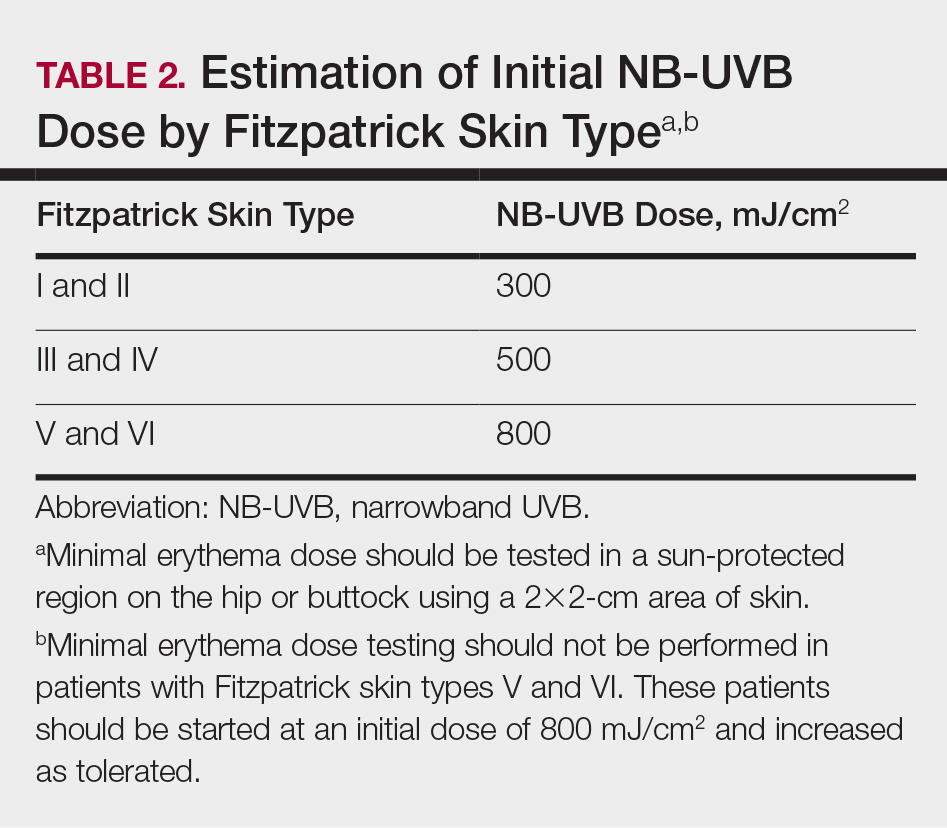
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.
For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
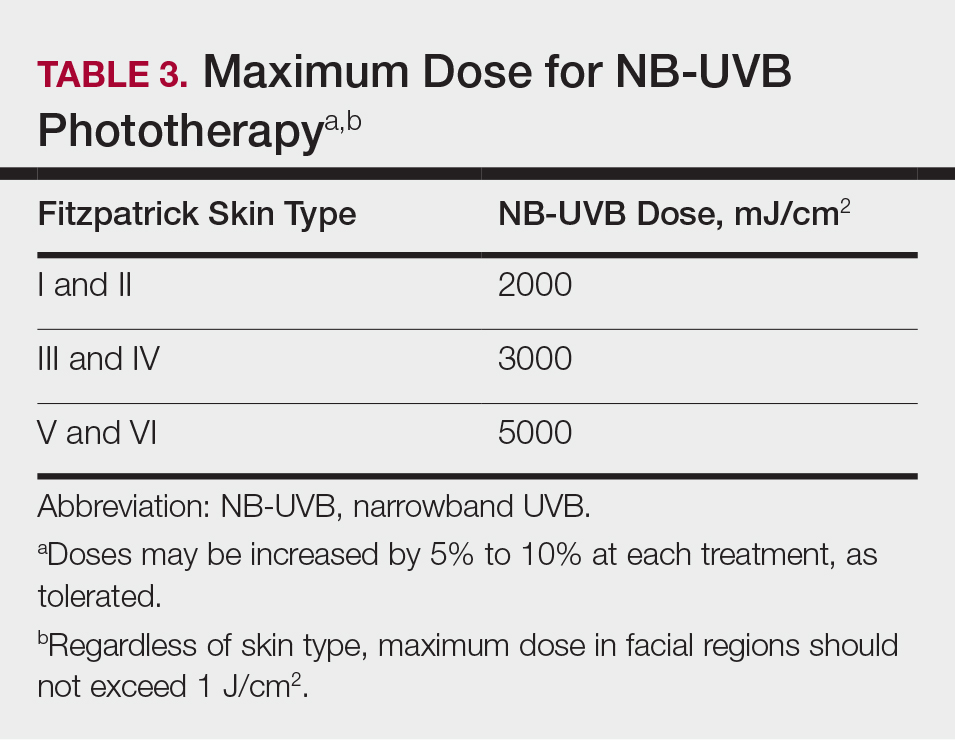
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).
Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Practice Points
- Phototherapy should be considered as an effective and low-risk treatment of psoriasis.
- Narrowband UVB, broadband UVB, targeted phototherapy (excimer laser and excimer lamp), and oral psoralen plus UVA have all received a grade A level of recommendation for the treatment of psoriasis in adults.
Tip Sheet: Teledermatology 101
Teledermatology Fast Facts
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
Halobetasol Propionate for the Management of Psoriasis
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
In clinical practice, for the majority of patients with psoriasis superpotent topical corticosteroids (TCSs) are used as initial therapy as well as ongoing breakthrough therapy to achieve quick resolution of target lesions. However, safe and effective long-term treatment and maintenance options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life, especially given that package inserts advise no more than 2 to 4 weeks of continuous use to limit side effects. The long-term use of superpotent TCSs can have a multitude of unwanted cutaneous side effects, such as skin atrophy, telangiectases, striae, and allergic vehicle responses.1,2 Tachyphylaxis, a decreased response to treatment over time, has been more controversial and may not occur with halobetasol propionate (HP) ointment 0.05%.3 In addition, TCSs are associated with relapse or rebound on withdrawal, which can be problematic but are poorly characterized.
We review the clinical data on HP, a superpotent TCS, in the treatment of psoriasis. We also explore both recent formulation developments and fixed-combination approaches to providing optimal treatment.
Clinical Experience With HP 0.05% in Various Formulations
Halobetasol propionate is a superpotent TCS with extensive clinical experience in treating psoriasis spanning nearly 30 years.1,2,3-7 Most recently, a twice-daily HP lotion 0.05% formulation was evaluated in patients with moderate to severe disease.8 Halobetasol propionate lotion 0.05% applied morning and night was shown to be significantly more effective than vehicle after 2 weeks of treatment (P<.001) in 2 parallel-group studies of 443 patients.9 Treatment success (ie, at least a 2-grade improvement in investigator global assessment [IGA] and IGA score of clear or almost clear) was achieved in 44.5% of patients treated with HP lotion 0.05% compared to 6.3% and 7.1% in the 2 vehicle arms. Treatment-related adverse events (AEs) were uncommon, with application-site pain reported in 2 patients treated with HP lotion 0.05% compared to 5 patients treated with vehicle.9
Several earlier studies have evaluated the short-term efficacy of twice-daily HP cream 0.05% and HP ointment 0.05% in the treatment of plaque psoriasis, but only 2 placebo-controlled trials have been reported, and data are limited.
Two 2-week studies of twice-daily HP ointment 0.05% (paired-comparison and parallel-group designs) in 204 patients with moderate plaque psoriasis reported improvement in plaque elevation, erythema, and scaling compared to vehicle. Patient global responses and physician global evaluation favored HP ointment 0.05%, and reports of stinging and burning were similar with active treatment and vehicle.4
Similarly, HP cream 0.05% applied twice daily was shown to be significantly superior to vehicle in reducing overall disease severity, erythema, plaque elevation, and scaling after 1 and 2 weeks of treatment in a paired-comparison study of 110 patients (P=.0001).5 A clinically significant reduction (at least a 1-grade improvement) in erythema, plaque elevation, pruritus, and scaling was noted in 81% to 92% of patients (P=.0001). Patients’ self-assessment of effectiveness rated HP cream 0.05% as excellent, very good, or good in 69% of patients compared to 20% for vehicle. Treatment-related AEs were reported by 4 patients.5
A small, noncontrolled, 2-week pediatric study (N=11) demonstrated the efficacy of combined therapy with HP cream 0.05% every morning and HP ointment 0.05% every night due to the then-perceived preference for creams as being more pleasant to apply during the day and ointments being more efficacious. Reported side effects were relatively mild, with application-site burning being the most common.10
Potential local AEs associated with HP are similar to those seen with other superpotent TCSs. Overall, they were reported in 0% to 13% of patients. The most common AEs were burning, pruritus, erythema, hypopigmentation, dryness, and folliculitis.5-8,10-14 Isolated cases of moderate telangiectasia and mild atrophy also have been reported.8,10
Comparative Studies With Other TCSs
In comparative studies of patients with severe localized plaque psoriasis, HP ointment 0.05% applied twice daily for up to 4 weeks was significantly superior compared to clobetasol propionate ointment 0.05% for the number of patients with none or mild disease (P=.0237) or comparisons of global evaluation scores (P=.01315) at week 2, or compared to betamethasone valerate ointment 0.1% (P=.02).6 It also was more effective than betamethasone dipropionate ointment 0.05% with healing seen in 40% of patients treated with HP ointment 0.05% within 24 days compared to 25% of patients treated with betamethasone dipropionate ointment 0.05%.8 Patient acceptance of HP ointment 0.05% based on cosmetic acceptability and ease of application was better (very good in 90% vs 80% of patients7) or significantly better compared to clobetasol propionate ointment 0.05% (P=.042 and P=.01915) and betamethasone dipropionate ointment 0.05% (P=.02).8
Evolving Management Strategies
A number of management strategies have been proposed to improve the safety and efficacy of long-term therapy with TCSs, including weekend-only or pulse therapy, dose reduction, rotating to another therapy, or combining with other topical therapies. Maintenance efficacy data are sparse. A small double-blind study in 44 patients with mild to moderate psoriasis was conducted wherein patients were treated with calcipotriene ointment in the morning and HP ointment in the evening for 2 weeks.16 Those patients who achieved at least a 50% improvement in disease severity (N=40) were randomized to receive HP ointment twice daily on weekends and calcipotriene ointment or placebo twice daily on weekdays for 6 months. Seventy-six percent of those patients treated with a HP/calcipotriene pulsed therapy maintained remission (achieving and maintaining a 75% improvement in physician global assessment) compared to 40% of those patients treated with HP only (P=.045). Mild AEs were reported in 4 patients treated with the combination regimen and 1 patient treated with HP only. No AE-related discontinuations occurred.16
In a real-world setting, a maintenance regimen that is less complicated enhances the potential for increased patient adherence and successful outcomes.17 After an initial 2-week regimen of twice-daily HP ointment 0.05% in combination with ammonium lactate lotion in patients with mild to moderate psoriasis (N=55), those rated clear or almost clear (41/55 [74.6%]) entered a maintenance phase, applying ammonium lactate lotion twice daily and either HP or placebo ointment twice daily on weekends. The probability of disease worsening by week 14 was 29% in the HP-treated group compared to 100% in the placebo group (P<.0001). By week 24, 12 patients (29.2%) remained clear or almost clear.17
Development of HP Lotion 0.01%
There are numerous examples in dermatology where advances in formulation development have made it possible to reduce the strength of active ingredients without compromising efficacy. Formulation advances also afford improved safety profiles that can extend a product’s utility. The vehicle affects not only the potency of an agent but also patient compliance, which is crucial for adequate response. Patients prefer lighter vehicles, such as lotions, over heavy ointments and creams.18,19
Recently, a polymeric honeycomb matrix (carbomer cross-linked polymers), which helps structure the oil emulsion and provide a uniform distribution of both active and moisturizing/hydrating ingredients (ie, sorbitol, light mineral oil, diethyl sebacate) at the surface of the skin, has been deployed for topical delivery of HP (eFigure 1). Ninety percent of the oil droplets containing solubilized halobetasol are 13 µm or smaller, an ideal size for penetration through follicular openings (unpublished data, Bausch Health, 2018).
This polymerized emulsion also forms a barrier by reducing epidermal water loss and improving skin hydration. Skin hydration and barrier protection of the lotion were assessed through corneometry and transepidermal water loss (TEWL) in 30 healthy female volunteers (aged 35–65 years) over 24 hours. The test material was applied to the volar forearm, with an untreated site serving as a control. Measurements using Tewameter and Corneometer were taken at baseline; 15 and 30 minutes; and 1, 2, 3, 8, and 24 hours postapplication. In addition, for the 8-hour study period, 15 patients applied the test material to the right side of the face and completed a customer-perception evaluation. Adverse events were noted throughout and irritation was assessed preapplication and postapplication. There were no AEs or skin irritation reported throughout the study. At baseline, mean (standard deviation [SD]) corneometry scores were 28.9 (2.9) and 28.1 (2.7) units for the test material and untreated control, respectively. There was an immediate improvement in water content that was maintained throughout the study. After 15 minutes, the mean (SD) score had increased to 59.1 (7.1) units in the vehicle lotion group (eFigure 2A). There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). At baseline, mean (SD) TEWL scores were 12.26 (0.48) and 12.42 (0.44) g/hm2, respectively (eFigure 2B). There was an immediate improvement in TEWL with a mean (SD) score of 6.04 (0.99) after 8 hours in the vehicle lotion group, a 50.7% change over baseline. There was no improvement at the control site, and differences were significant at all postapplication assessments (P<.001). Customer perception of the novel lotion formulation was positive, with the majority of patients (93%–100%) responding favorably to all questions about the various attributes of the test material (eFigure 3)(unpublished data, Bausch Health, 2018).
Comparison of Skin Penetration of HP Lotion 0.01% vs HP Cream 0.05%
Comparative percutaneous absorption of 2 HP formulations—0.01% lotion and 0.05% cream—was evaluated in vitro using human tissue from a single donor mounted on Bronaugh flow-through diffusion cells. Receptor phase samples were collected over the 24-hour study period and HP content assessed using liquid chromatography–mass spectrometry analysis. Halobetasol propionate lotion 0.01% demonstrated faster tissue permeation, with receptor phase levels of 0.91% of the applied dose at 24 hours compared to 0.28% of the applied dose with HP cream 0.05%. Although there was little differentiation of cumulative receptor fluid levels of HP at 6 hours, there was significant differentiation at 12 hours. Levels of HP were lowest in the receptor phase and highest in the epidermal layers of the skin, indicating limited permeation through the epidermis to the dermis. The mean (SD) for epidermal deposition of HP following the 24-hour duration of exposure was 6.17% (2.07%) and 1.72% (0.76%) for the 0.01% lotion and 0.05% cream, respectively (Figure 1)(unpublished data, Bausch Health, 2018).
Efficacy and Safety of HP Lotion 0.01% in Moderate to Severe Plaque Psoriasis
Two articles have been published on the use of HP lotion 0.01% in moderate to severe psoriasis: 2 pivotal studies comparing once-daily application with vehicle lotion over 8 weeks (N=430),20 and a comparative “label-restricted” 2-week study with HP lotion 0.01% and HP cream 0.05% (N=150).21
HP Lotion 0.01% Compared to Vehicle
Two multicenter, randomized, double-blind, vehicle-controlled phase 3 studies investigated the safety and efficacy of once-daily HP lotion 0.01% in moderate to severe plaque psoriasis (N=430).20 Patients were treated with HP lotion 0.01% or vehicle (randomized in a 2:1 ratio) for 8 weeks, with a 4-week posttreatment follow-up. Treatment success (defined as at least a 2-grade improvement in baseline IGA score and a score equating to clear or almost clear) was significantly greater with HP lotion 0.01% at all assessment points (Figure 2)(P=.003 for week 2; P<.001 for other time points). At week 8, 37.4% of patients receiving HP lotion 0.01% were treatment successes compared to 10.0% of patients receiving vehicle (P<.001). Additionally, a 2-grade improvement from baseline for each psoriasis sign—erythema, plaque elevation, and scaling—was achieved by 42.2% of patients receiving HP lotion 0.01% at week 8 compared to 11.4% of patients receiving vehicle (P<.001). Good efficacy was maintained posttreatment that was significant compared to vehicle (P<.001).20
There were corresponding reductions in body surface area (BSA) affected following treatment with HP lotion 0.01%.20 At baseline, the mean BSA was 6.1 (range, 3–12). By week 8, there was a 35.2% reduction in BSA compared to 5.9% with vehicle. Again, a significant reduction in BSA was maintained posttreatment compared to vehicle (P<.001).20
Halobetasol propionate lotion 0.01% was well tolerated with few treatment-related AEs.20 Most AEs were application-site reactions such as dermatitis (0.7%), infection, pruritus, and discoloration (0.4% each). Mild to moderate itching, dryness, burning, and stinging present at baseline all improved with treatment, and severity of local skin reactions was significantly lower than with vehicle at week 8 (P<.001). Quality-of-life data also highlighted the benefits of active treatment compared to vehicle for cutaneous tolerability. The Dermatology Life Quality Index (DLQI) is a 10-item patient-reported questionnaire consisting of questions concerning symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.22 Change from baseline for DLQI (how itchy, sore, painful, stinging) was significantly greater with HP lotion 0.01% at weeks 4 and 8 (P<.001). Changes in the overall DLQI score also were significantly greater with HP lotion 0.01% at both study visits (P=.006 and P=.014 at week 4 and P=.001 and P=.004 at week 8 for study 1 and study 2, respectively).20
HP Lotion 0.01% Compared to HP Cream 0.05%
Treatment success with HP lotion 0.01% also was shown to be comparable to the higher-concentration HP cream 0.05% in patients with moderate to severe psoriasis over a 2-week “label-restricted” treatment period (Figure 3). Both products were well tolerated over the 2-week treatment period. One patient reported application-site dermatitis (1.7%) with HP lotion 0.01%.21
Conclusion
Halobetasol propionate 0.05%—cream, ointment, and lotion—has been shown to be a highly effective short-term topical treatment for psoriasis. Longer-term treatment strategies using HP, which are important when considering management of a chronic condition, have been limited by safety concerns and labelling. However, there are data to suggest weekend or pulsed therapy may be an option.
A novel formulation of HP lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets. The polymerized honeycomb matrix and vehicle formulation form a barrier by reducing epidermal water loss and improving skin hydration. The oil droplets deliver uniform amounts of active ingredient in an optimal size for follicular penetration. Skin penetration has been shown to be quicker with greater retention in the epidermis with HP lotion 0.01% compared to HP cream 0.05%, with corresponding considerably lower penetration into the dermis.
Although there have been a number of clinical studies of HP for psoriasis, until recently there have been no comparative trials, with studies label restricted to a 2- to 4-week duration. Three clinical studies with HP lotion 0.01% have now been reported.Not only has HP lotion 0.01% been shown to be as effective as HP cream 0.05% in a 2-week comparative study (despite having one-fifth the concentration of HP), it also has been shown to be very effective and well tolerated following 8 weeks of daily use.20,21 Further studies involving longer treatment durations are required to better elucidate AEs, but HP lotion 0.01% may provide the first longer-term TCS treatment solution for moderate to severe psoriasis.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
- Kamili QU, Menter A. Topical treatment of psoriasis. Curr Probl Dermatol. 2009;38:37-58.
- Bailey J, Whitehair B. Topical treatments for chronic plaque psoriasis. Am Fam Physician. 2010;81:596.
- Czarnowicki T, Linkner RV, Suarez-Farinas M, et al. An investigator-initiated, double-blind, vehicle-controlled pilot study: assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol. 2014;71:954-959.
- Bernhard J, Whitmore C, Guzzo C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25:1170-1174.
- Katz HI, Gross E, Buxman M, et al. A double-blind, vehicle-controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. J Am Acad Dermatol. 1991;25:1175-1178.
- Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1153-1156.
- Goldberg B, Hartdegen R, Presbury D, et al. A double-blind, multicenter comparison of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25:1145-1148.
- Mensing H, Korsukewitz G, Yawalkar S. A double-blind, multicenter comparison between 0.05% halobetasol propionate ointment and 0.05% betamethasone dipropionate ointment in chronic plaque psoriasis. J Am Acad Dermatol. 1991;25:1149-1152.
- Pariser D, Bukhalo M, Guenthner S, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel enhanced lotion formulation of halobetasol propionate, 0.05% versus its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2017;16:234-240.
- Herz G, Blum G, Yawalkar S. Halobetasol propionate cream by day and halobetasol propionate ointment at night for the treatment of pediatric patients with chronic, localized psoriasis and atopic dermatitis. J Am Acad Dermatol. 1991;25:1166-1169.
- Datz B, Yawalkar S. A double-blind, multicenter trial of 0.05% halobetasol propionate ointment and 0.05% clobetasol 17-propionate ointment in the treatment of patients with chronic, localized atopic dermatitis or lichen simplex chronicus. J Am Acad Dermatol. 1991;25:1157-1160.
- Kantor I, Cook PR, Cullen SI, et al. Double-blind bilateral paired comparison of 0.05% halobetasol propionate cream and its vehicle in patients with chronic atopic dermatitis and other eczematous dermatoses. J Am Acad Dermatol. 1991;25:1184-1186.
- Yawalkar SJ, Schwerzmann L. Double-blind, comparative clinical trials with halobetasol propionate cream in patients with atopic dermatitis. J Am Acad Dermatol. 1991;25:1163-1166.
- Watson WA, Kalb RE, Siskin SB, et al. The safety of halobetasol 0.05% ointment in the treatment of psoriasis. Pharmacotherapy. 1990;10:107-111.
- Dhurat R, Aj K, Vishwanath V, et al. Evaluation of the efficacy and safety of 0.05% halobetasol propionate ointment and 0.05% clobetasol propionate ointment in chronic, localized plaque psoriasis. Asian J Pharm Clin Res. 2016;9:288-291.
- Lebwohl M, Yoles A, Lombardi K, et al. Calcipotriene ointment and halobetasol ointment in the long-term treatment of psoriasis: effects on the duration of improvement. J Am Acad Dermatol. 1998;39:447-450.
- Feldman SR, Horn EJ, Balkrishnan R, et al. Psoriasis: improvingadherence to topical therapy. J Am Acad Dermatol. 2008;59:1009-1016.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Eastman WJ, Malahias S, Delconte J, et al. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94:46-53.
- Green LJ, Kerdel FA, Cook-Bolden FE, et al. Safety and efficacy of halobetasol propionate 0.01% lotion in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase III randomized controlled trials. J Drugs Dermatol. 2018;17:1062-1069.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to compare the safety and efficacy of halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018].J Dermatolog Treat. 2019;30:333-339.
- Lewis V, Finlay AY. 10 years’ experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9:169-180.
Practice Points
- The widespread use of superpotent topical corticosteroids in treating psoriasis is limited by labelling that restricts short-term use, concerns about side effects, and a paucity of clinical data with longer-term use.
- Long-term management and treatment options are required for managing the chronic nature of psoriasis to improve patient satisfaction, adherence, and quality of life.
- A novel formulation of halobetasol propionate lotion 0.01% has been developed using a polymerized matrix with active ingredients and moisturizing excipients suspended in oil droplets.
Systemic Therapies in Psoriasis: An Update on Newly Approved and Pipeline Biologics and Oral Treatments
Recent advances in our understanding of psoriatic immune pathways have led to new generations of targeted therapies developed over the last 5 years. Although the pathogenesis of psoriasis remains to be fully elucidated, the success of these targeted therapies has confirmed a critical role of the IL-23/helper T cell (TH17) axis in maintaining the psoriatic immune cascade, a positive feedback loop in which IL-17, IL-12, and IL-23 released from myeloid dendritic cells lead to activation of helperT cells. Activated helper T cells—namely TH1, TH17, and TH22—release IL-17, IL-22, and other proinflammatory cytokines, amplifying the immune response and leading to keratinocyte proliferation and immune cell migration to psoriatic lesions. Inhibition of IL-17 and IL-23 by several biologics disrupts this aberrant inflammatory cascade and has led to dramatic improvements in outcomes, particularly among patients with moderate to severe disease.
Numerous biologics targeting these pathways and several oral treatments have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis; in addition, a number of promising therapies are on the horizon, and knowledge of these medications might help guide our treatment approach to the patient with psoriasis. This article provides an update on the most recent (as of 2019) approved therapies and medications in the pipeline for moderate to severe plaque psoriasis, with a focus on systemic agents in phase 3 clinical trials. (Medications targeting psoriatic arthritis, biosimilars, and existing medications approved by the FDA prior to 2019 will not be discussed.)
Risankizumab
Risankizumab-rzaa (formerly BI 655066) is a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, selectively inhibiting the role of this critical cytokine in psoriatic inflammation.
Phase 1 Trial
In a phase 1 proof-of-concept study, 39 patients with moderate to severe plaque psoriasis received varying dosages of intravenous or subcutaneous risankizumab or placebo.1 At week 12, the percentage of risankizumab-treated patients achieving reduction in the psoriasis area and severity index (PASI) score by 75% (PASI 75), 90% (PASI 90), and 100% (PASI 100) was 87% (27/31; P<.001 vs placebo), 58% (18/31; P=.007 vs placebo), and 16% (5/31; P=.590 vs placebo), respectively. Improvements in PASI scores were observed as early as week 2. Adverse events (AEs) were reported by 65% of the risankizumab group and 88% of the placebo group. Serious AEs were reported in 4 patients receiving risankizumab, none of which were considered related to the study medication.1
Phase 2 Trial
A phase 2 comparator trial demonstrated noninferiority at higher dosages of risankizumab in comparison to the IL-12/IL-23 inhibitor ustekinumab.2 Among 166 participants with moderate to severe plaque psoriasis, PASI 90 at week 12 was met by 77% of participants receiving 90 or 180 mg of risankizumab compared to 40% receiving ustekinumab (P<.001). Onset of activity with risankizumab was faster and the duration of effect longer vs ustekinumab; by week 8, at least PASI 75 was achieved by approximately 80% of participants in the 90-mg and 180-mg risankizumab groups compared to 60% in the ustekinumab group; PASI score reductions generally were maintained for as long as 20 weeks after the final dose of risankizumab was administered.2
Phase 3 Trials
The 52-week UltIMMa-1 and UltIMMa-2 phase 3 trials compared subcutaneous risankizumab (150 mg) to ustekinumab (45 or 90 mg [weight-based dosing]) or placebo administered at weeks 0, 4, 16, 28, and 40 in approximately 1000 patients with moderate to severe plaque psoriasis.3 Patients initially assigned to placebo switched to risankizumab 150 mg at week 16. At week 16, PASI 90 was achieved by 75.3% of risankizumab-treated patients, 42.0% of ustekinumab-treated patients, and 4.9% of placebo-treated patients in UltIMMa-1, and by 74.8% of risankizumab-treated patients, 47.5% of ustekinumab-treated patients, and 2.0% of placebo-treated patients in UltIMMa-2 (P<.0001 vs placebo and ustekinumab for both studies). Achievement of a static physician’s global assessment (sPGA) score of 0 or 1 at week 16 similarly favored risankizumab, with 87.8%, 63.0%, and 7.8% of patients in UltIMMa-1 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively, and 83.7%, 61.6%, and 5.1% in UltIMMa-2 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively (P<.0001 vs placebo and ustekinumab for both studies). Among patients initially assigned to risankizumab, improvements in PASI and sPGA continued to increase until week 52, with 81.9% achieving PASI 90 at week 52 compared to 44.0% on ustekinumab in UltIMMa-1, and 80.6% achieving PASI 90 at week 52 compared to 50.5% on ustekinumab in UltIMMa-2 (P<.0001 vs ustekinumab for both studies). Treatment-emergent AE profiles were similar for risankizumab and ustekinumab in both studies, and there were no unexpected safety findings.3
Risankizumab received FDA approval for the treatment of moderate to severe plaque psoriasis in April 2019.
Bimekizumab
Bimekizumab (UCB4940), a humanized IgG1 monoclonal antibody, selectively neutralizes the biologic functions of IL-17A and IL-17F, the latter of which has only recently been implicated in contributing to the psoriatic immune cascade.4
First-in-Human Study
Thirty-nine participants with mild psoriasis demonstrated efficacy after single-dose intravenous bimekizumab, with maximal improvements in all measures of disease activity observed between weeks 8 and 12 in participants receiving 160 to 640 mg.5
Proof-of-Concept Phase 1b Study
A subsequent trial of 53 participants with psoriatic arthritis demonstrated sustained efficacy to week 20 with varying dosages of intravenous bimekizumab.6 At week 8, PASI 100 was met by 86.7% of participants receiving the top 3 dosages of bimekizumab compared to none of the placebo-treated participants. Treatment-emergent AEs, including neutropenia and elevation of liver transaminases, were mostly mild to moderate and resolved spontaneously. There were 3 severe AEs and 3 serious AEs, none of which were related to treatment.6
Importantly, bimekizumab was shown in this small study to have the potential to be highly effective at treating psoriatic arthritis. American College of Rheumatology ACR20, ACR50, and ACR70 response criteria were very high, with an ACR20 of 80% and an ACR50 of 40%.6 Further trials are necessary to gather more data and confirm these findings; however, these levels of response are higher than those of any other biologic on the market.
Phase 2b Dose-Ranging Study
In this trial, 250 participants with moderate to severe plaque psoriasis received either 64 mg, 160 mg with a 320-mg loading dose, 320 mg, or 480 mg of subcutaneous bimekizumab or placebo at weeks 0, 4, and 8.7 At week 12, PASI 90 was achieved by significantly more patients in all bimekizumab-treated groups compared to the placebo group (46.2%–79.1% vs 0%; P<.0001 for all dosages); PASI 100 also was achieved by significantly more bimekizumab-treated patients (27.9%–60.0% vs 0%; P<.0002). Improvement began as early as week 4, with clinically meaningful responses observed in all bimekizumab groups across all measures of disease activity. Treatment-emergent AEs occurred more frequently in bimekizumab-treated participants (61%) than in placebo-treated participants (36%); the most common AEs were nasopharyngitis and upper respiratory tract infection. Of note, fungal infections were reported by 4.3% of participants receiving bimekizumab; all cases were localized superficial infection, and none led to discontinuation. Three serious AEs were reported, none of which were considered related to the study treatment.7
Mirikizumab
Mirikizumab (LY3074828) is a humanized IgG4 monoclonal antibody that selectively binds and inhibits the p19 subunit of IL-23, with no action on IL-12.
Phase 1 Trial
Mirikizumab was shown to improve PASI scores in patients with plaque psoriasis.8
Phase 2 Trial
Subsequently, a trial of 205 participants with moderate to severe plaque psoriasis compared 3 dosing regimens of subcutaneous mirikizumab—30, 100, or 300 mg—at weeks 0 and 8 compared to placebo.9 Primary end point results at week 16 demonstrated PASI 90 response rates of 0%, 29% (P=.009), 59% (P<.001), and 67% (P<.001) in the placebo, 30-mg, 100-mg, and 300-mg mirikizumab groups, respectively. Complete clearance of psoriasis, measured by PASI 100 and sPGA 0, was achieved by 0%, 16%, 31%, and 31%, respectively (P=.039 for 30 mg vs placebo; P=.007 for the higher dosage groups vs placebo). Response rates for all efficacy outcomes were statistically significantly higher for all mirikizumab treatment groups compared to placebo and were highest in the 100-mg and 300-mg treatment groups. Frequencies of participants reporting AEs were similar across treatment and placebo groups.9
Oral Medications
Only a few small-molecule, orally bioavailable therapies are on the market for the treatment of psoriasis, some of which are associated with unfavorable side-effect profiles that preclude long-term therapy.
BMS-986165
The intracellular signaling enzyme tyrosine kinase 2 is involved in functional responses of IL-12 and IL-23. BMS-986165, a potent oral inhibitor of tyrosine kinase 2 with greater selectivity than other tyrosine kinase inhibitors, demonstrated efficacy in a phase 2 trial of 267 participants with moderate to severe plaque psoriasis receiving any of 5 dosing regimens—3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily—compared to placebo.10 At week 12, the percentage of patients with a 75% or greater reduction in PASI was 7% with placebo, 9% with 3 mg every other day (P=.49 vs placebo), 39% with 3 mg daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). Adverse events occurred in 51% of patients in the placebo group and in 55% to 80% of BMS-986165–treated patients; the most common AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection.10
A phase 3 trial comparing BMS-986165 with placebo and apremilast is underway (ClinicalTrials.gov Identifier NCT03611751).
Piclidenoson (CF101)
A novel small molecule that binds the Gi protein–associated A3 adenosine receptor piclidenoson induces an anti-inflammatory response via deregulation of the Wnt and nuclear factor κB signal transduction pathways, leading to downregulation of proinflammatory cytokines, including IL-17 and IL-23.11
In a phase 2 dose-ranging study, 75 patients with moderate to severe plaque psoriasis received varying dosages—1, 2, or 4 mg—of oral piclidenoson or placebo twice daily for 12 weeks.12 Progressive improvement in the mean change from baseline PASI score was observed in the 2-mg group, with statistically significant differences at weeks 8 and 12 compared to placebo (P=.047 and P=.031, respectively). At week 12, 35.3% of the 2-mg group achieved at least PASI 50. Improvements in PASI were less pronounced in the 4-mg group, and no therapeutic benefit was observed in the 1-mg group. Of the 20 AEs reported, 15 possibly were related to the study drug; 1 AE was severe.12
In a subsequent phase 2/3 trial, patients with moderate to severe plaque psoriasis received piclidenoson—1 or 2 mg—or placebo twice daily.13 At week 12, PASI 75 was achieved by 8.5% of patients in the 2-mg group and by 6.9% of patients receiving placebo (P=.621), thereby not meeting the primary study end point. Results at week 32 were more encouraging. In the 2-mg group, PASI mean percentage improvement was 57% (P<.002) compared to baseline, with linear improvements observed in PASI 50 (63.5%), PASI 75 (35.5%), PASI 90 (24.7%), and PASI 100 (10.6%).13
A phase 3 trial comparing piclidenoson 2 and 3 mg to apremilast and placebo is in progress (ClinicalTrials.gov Identifier NCT03168256).
Future Directions
Despite abundant options for treating moderate to severe plaque psoriasis and psoriatic arthritis, the pipeline remains rich. Novel treatments might have improved efficacy, favorable safety profiles, and different modes of administration compared to current medications. In addition to the novel therapeutics covered here, several treatments are in development further down the pipeline, with only phase 1 or 2 data available. Remtolumab (ABT-122), a tumor necrosis factor α– and IL-17A–targeted immunoglobulin, is unique among biologics, given its dual inhibition of tumor necrosis factor α and IL-17A.14 M1095 (ALX-0761), a novel trivalent bispecific nanobody, is another intriguing candidate. This dual inhibitor of IL-17A/F might exhibit a number of advantages over conventional antibodies, including better tissue penetration, reduced immunogenicity, and a longer half-life (ClinicalTrials.gov Identifier NCT03384745).15,16
As always with drug development, numerous medications that were under development failed to meet primary end points in phase 2 trials and have therefore been discontinued, including namilumab and prurisol. It is reassuring that the pace of drug discovery and development in psoriasis does not seem to be slowing; to our patients’ benefit, we will have an array of treatments available to tailor therapy to the individual.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Maroof A, Baeten D, Archer S, et al. 02.13 Il-17f contributes to human chronic inflammation in synovial tissue: preclinical evidence with dual IL-17a and IL-17f inhibition with bimekizumab in psoriatic arthritis. Ann Rheum Dis. 2017;76(Suppl 1):A13.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of bothinterleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-286.e10.
- Maari C. Safety, efficacy, and pharmacokinetics of a p19-directed IL-23 antibody in patients with plaque psoriasis and healthy subjects. Presented at: 25th European Academy of Dermatology and Venereology Congress; Vienna, Austria; September 28-October 2, 2016.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88-95.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Cohen S, Barer F, Itzhak I, et al. Inhibition of IL-17 and IL-23 in human keratinocytes by the A3 adenosine receptor agonist piclidenoson. J Immunol Res. 2018;2018:2310970.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
- 13. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol. 2016;15:931-938.
- Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70:1778-1789.
- Nanobodies’ competitive features. Ablynx website. http://www.ablynx.com/technology-innovation/nanobodies-competitive-features. Accessed July 4, 2019.
- Svecova D, Lubell MW, Casset-Semanaz F, et al. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196-203.
Recent advances in our understanding of psoriatic immune pathways have led to new generations of targeted therapies developed over the last 5 years. Although the pathogenesis of psoriasis remains to be fully elucidated, the success of these targeted therapies has confirmed a critical role of the IL-23/helper T cell (TH17) axis in maintaining the psoriatic immune cascade, a positive feedback loop in which IL-17, IL-12, and IL-23 released from myeloid dendritic cells lead to activation of helperT cells. Activated helper T cells—namely TH1, TH17, and TH22—release IL-17, IL-22, and other proinflammatory cytokines, amplifying the immune response and leading to keratinocyte proliferation and immune cell migration to psoriatic lesions. Inhibition of IL-17 and IL-23 by several biologics disrupts this aberrant inflammatory cascade and has led to dramatic improvements in outcomes, particularly among patients with moderate to severe disease.
Numerous biologics targeting these pathways and several oral treatments have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis; in addition, a number of promising therapies are on the horizon, and knowledge of these medications might help guide our treatment approach to the patient with psoriasis. This article provides an update on the most recent (as of 2019) approved therapies and medications in the pipeline for moderate to severe plaque psoriasis, with a focus on systemic agents in phase 3 clinical trials. (Medications targeting psoriatic arthritis, biosimilars, and existing medications approved by the FDA prior to 2019 will not be discussed.)
Risankizumab
Risankizumab-rzaa (formerly BI 655066) is a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, selectively inhibiting the role of this critical cytokine in psoriatic inflammation.
Phase 1 Trial
In a phase 1 proof-of-concept study, 39 patients with moderate to severe plaque psoriasis received varying dosages of intravenous or subcutaneous risankizumab or placebo.1 At week 12, the percentage of risankizumab-treated patients achieving reduction in the psoriasis area and severity index (PASI) score by 75% (PASI 75), 90% (PASI 90), and 100% (PASI 100) was 87% (27/31; P<.001 vs placebo), 58% (18/31; P=.007 vs placebo), and 16% (5/31; P=.590 vs placebo), respectively. Improvements in PASI scores were observed as early as week 2. Adverse events (AEs) were reported by 65% of the risankizumab group and 88% of the placebo group. Serious AEs were reported in 4 patients receiving risankizumab, none of which were considered related to the study medication.1
Phase 2 Trial
A phase 2 comparator trial demonstrated noninferiority at higher dosages of risankizumab in comparison to the IL-12/IL-23 inhibitor ustekinumab.2 Among 166 participants with moderate to severe plaque psoriasis, PASI 90 at week 12 was met by 77% of participants receiving 90 or 180 mg of risankizumab compared to 40% receiving ustekinumab (P<.001). Onset of activity with risankizumab was faster and the duration of effect longer vs ustekinumab; by week 8, at least PASI 75 was achieved by approximately 80% of participants in the 90-mg and 180-mg risankizumab groups compared to 60% in the ustekinumab group; PASI score reductions generally were maintained for as long as 20 weeks after the final dose of risankizumab was administered.2
Phase 3 Trials
The 52-week UltIMMa-1 and UltIMMa-2 phase 3 trials compared subcutaneous risankizumab (150 mg) to ustekinumab (45 or 90 mg [weight-based dosing]) or placebo administered at weeks 0, 4, 16, 28, and 40 in approximately 1000 patients with moderate to severe plaque psoriasis.3 Patients initially assigned to placebo switched to risankizumab 150 mg at week 16. At week 16, PASI 90 was achieved by 75.3% of risankizumab-treated patients, 42.0% of ustekinumab-treated patients, and 4.9% of placebo-treated patients in UltIMMa-1, and by 74.8% of risankizumab-treated patients, 47.5% of ustekinumab-treated patients, and 2.0% of placebo-treated patients in UltIMMa-2 (P<.0001 vs placebo and ustekinumab for both studies). Achievement of a static physician’s global assessment (sPGA) score of 0 or 1 at week 16 similarly favored risankizumab, with 87.8%, 63.0%, and 7.8% of patients in UltIMMa-1 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively, and 83.7%, 61.6%, and 5.1% in UltIMMa-2 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively (P<.0001 vs placebo and ustekinumab for both studies). Among patients initially assigned to risankizumab, improvements in PASI and sPGA continued to increase until week 52, with 81.9% achieving PASI 90 at week 52 compared to 44.0% on ustekinumab in UltIMMa-1, and 80.6% achieving PASI 90 at week 52 compared to 50.5% on ustekinumab in UltIMMa-2 (P<.0001 vs ustekinumab for both studies). Treatment-emergent AE profiles were similar for risankizumab and ustekinumab in both studies, and there were no unexpected safety findings.3
Risankizumab received FDA approval for the treatment of moderate to severe plaque psoriasis in April 2019.
Bimekizumab
Bimekizumab (UCB4940), a humanized IgG1 monoclonal antibody, selectively neutralizes the biologic functions of IL-17A and IL-17F, the latter of which has only recently been implicated in contributing to the psoriatic immune cascade.4
First-in-Human Study
Thirty-nine participants with mild psoriasis demonstrated efficacy after single-dose intravenous bimekizumab, with maximal improvements in all measures of disease activity observed between weeks 8 and 12 in participants receiving 160 to 640 mg.5
Proof-of-Concept Phase 1b Study
A subsequent trial of 53 participants with psoriatic arthritis demonstrated sustained efficacy to week 20 with varying dosages of intravenous bimekizumab.6 At week 8, PASI 100 was met by 86.7% of participants receiving the top 3 dosages of bimekizumab compared to none of the placebo-treated participants. Treatment-emergent AEs, including neutropenia and elevation of liver transaminases, were mostly mild to moderate and resolved spontaneously. There were 3 severe AEs and 3 serious AEs, none of which were related to treatment.6
Importantly, bimekizumab was shown in this small study to have the potential to be highly effective at treating psoriatic arthritis. American College of Rheumatology ACR20, ACR50, and ACR70 response criteria were very high, with an ACR20 of 80% and an ACR50 of 40%.6 Further trials are necessary to gather more data and confirm these findings; however, these levels of response are higher than those of any other biologic on the market.
Phase 2b Dose-Ranging Study
In this trial, 250 participants with moderate to severe plaque psoriasis received either 64 mg, 160 mg with a 320-mg loading dose, 320 mg, or 480 mg of subcutaneous bimekizumab or placebo at weeks 0, 4, and 8.7 At week 12, PASI 90 was achieved by significantly more patients in all bimekizumab-treated groups compared to the placebo group (46.2%–79.1% vs 0%; P<.0001 for all dosages); PASI 100 also was achieved by significantly more bimekizumab-treated patients (27.9%–60.0% vs 0%; P<.0002). Improvement began as early as week 4, with clinically meaningful responses observed in all bimekizumab groups across all measures of disease activity. Treatment-emergent AEs occurred more frequently in bimekizumab-treated participants (61%) than in placebo-treated participants (36%); the most common AEs were nasopharyngitis and upper respiratory tract infection. Of note, fungal infections were reported by 4.3% of participants receiving bimekizumab; all cases were localized superficial infection, and none led to discontinuation. Three serious AEs were reported, none of which were considered related to the study treatment.7
Mirikizumab
Mirikizumab (LY3074828) is a humanized IgG4 monoclonal antibody that selectively binds and inhibits the p19 subunit of IL-23, with no action on IL-12.
Phase 1 Trial
Mirikizumab was shown to improve PASI scores in patients with plaque psoriasis.8
Phase 2 Trial
Subsequently, a trial of 205 participants with moderate to severe plaque psoriasis compared 3 dosing regimens of subcutaneous mirikizumab—30, 100, or 300 mg—at weeks 0 and 8 compared to placebo.9 Primary end point results at week 16 demonstrated PASI 90 response rates of 0%, 29% (P=.009), 59% (P<.001), and 67% (P<.001) in the placebo, 30-mg, 100-mg, and 300-mg mirikizumab groups, respectively. Complete clearance of psoriasis, measured by PASI 100 and sPGA 0, was achieved by 0%, 16%, 31%, and 31%, respectively (P=.039 for 30 mg vs placebo; P=.007 for the higher dosage groups vs placebo). Response rates for all efficacy outcomes were statistically significantly higher for all mirikizumab treatment groups compared to placebo and were highest in the 100-mg and 300-mg treatment groups. Frequencies of participants reporting AEs were similar across treatment and placebo groups.9
Oral Medications
Only a few small-molecule, orally bioavailable therapies are on the market for the treatment of psoriasis, some of which are associated with unfavorable side-effect profiles that preclude long-term therapy.
BMS-986165
The intracellular signaling enzyme tyrosine kinase 2 is involved in functional responses of IL-12 and IL-23. BMS-986165, a potent oral inhibitor of tyrosine kinase 2 with greater selectivity than other tyrosine kinase inhibitors, demonstrated efficacy in a phase 2 trial of 267 participants with moderate to severe plaque psoriasis receiving any of 5 dosing regimens—3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily—compared to placebo.10 At week 12, the percentage of patients with a 75% or greater reduction in PASI was 7% with placebo, 9% with 3 mg every other day (P=.49 vs placebo), 39% with 3 mg daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). Adverse events occurred in 51% of patients in the placebo group and in 55% to 80% of BMS-986165–treated patients; the most common AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection.10
A phase 3 trial comparing BMS-986165 with placebo and apremilast is underway (ClinicalTrials.gov Identifier NCT03611751).
Piclidenoson (CF101)
A novel small molecule that binds the Gi protein–associated A3 adenosine receptor piclidenoson induces an anti-inflammatory response via deregulation of the Wnt and nuclear factor κB signal transduction pathways, leading to downregulation of proinflammatory cytokines, including IL-17 and IL-23.11
In a phase 2 dose-ranging study, 75 patients with moderate to severe plaque psoriasis received varying dosages—1, 2, or 4 mg—of oral piclidenoson or placebo twice daily for 12 weeks.12 Progressive improvement in the mean change from baseline PASI score was observed in the 2-mg group, with statistically significant differences at weeks 8 and 12 compared to placebo (P=.047 and P=.031, respectively). At week 12, 35.3% of the 2-mg group achieved at least PASI 50. Improvements in PASI were less pronounced in the 4-mg group, and no therapeutic benefit was observed in the 1-mg group. Of the 20 AEs reported, 15 possibly were related to the study drug; 1 AE was severe.12
In a subsequent phase 2/3 trial, patients with moderate to severe plaque psoriasis received piclidenoson—1 or 2 mg—or placebo twice daily.13 At week 12, PASI 75 was achieved by 8.5% of patients in the 2-mg group and by 6.9% of patients receiving placebo (P=.621), thereby not meeting the primary study end point. Results at week 32 were more encouraging. In the 2-mg group, PASI mean percentage improvement was 57% (P<.002) compared to baseline, with linear improvements observed in PASI 50 (63.5%), PASI 75 (35.5%), PASI 90 (24.7%), and PASI 100 (10.6%).13
A phase 3 trial comparing piclidenoson 2 and 3 mg to apremilast and placebo is in progress (ClinicalTrials.gov Identifier NCT03168256).
Future Directions
Despite abundant options for treating moderate to severe plaque psoriasis and psoriatic arthritis, the pipeline remains rich. Novel treatments might have improved efficacy, favorable safety profiles, and different modes of administration compared to current medications. In addition to the novel therapeutics covered here, several treatments are in development further down the pipeline, with only phase 1 or 2 data available. Remtolumab (ABT-122), a tumor necrosis factor α– and IL-17A–targeted immunoglobulin, is unique among biologics, given its dual inhibition of tumor necrosis factor α and IL-17A.14 M1095 (ALX-0761), a novel trivalent bispecific nanobody, is another intriguing candidate. This dual inhibitor of IL-17A/F might exhibit a number of advantages over conventional antibodies, including better tissue penetration, reduced immunogenicity, and a longer half-life (ClinicalTrials.gov Identifier NCT03384745).15,16
As always with drug development, numerous medications that were under development failed to meet primary end points in phase 2 trials and have therefore been discontinued, including namilumab and prurisol. It is reassuring that the pace of drug discovery and development in psoriasis does not seem to be slowing; to our patients’ benefit, we will have an array of treatments available to tailor therapy to the individual.
Recent advances in our understanding of psoriatic immune pathways have led to new generations of targeted therapies developed over the last 5 years. Although the pathogenesis of psoriasis remains to be fully elucidated, the success of these targeted therapies has confirmed a critical role of the IL-23/helper T cell (TH17) axis in maintaining the psoriatic immune cascade, a positive feedback loop in which IL-17, IL-12, and IL-23 released from myeloid dendritic cells lead to activation of helperT cells. Activated helper T cells—namely TH1, TH17, and TH22—release IL-17, IL-22, and other proinflammatory cytokines, amplifying the immune response and leading to keratinocyte proliferation and immune cell migration to psoriatic lesions. Inhibition of IL-17 and IL-23 by several biologics disrupts this aberrant inflammatory cascade and has led to dramatic improvements in outcomes, particularly among patients with moderate to severe disease.
Numerous biologics targeting these pathways and several oral treatments have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis; in addition, a number of promising therapies are on the horizon, and knowledge of these medications might help guide our treatment approach to the patient with psoriasis. This article provides an update on the most recent (as of 2019) approved therapies and medications in the pipeline for moderate to severe plaque psoriasis, with a focus on systemic agents in phase 3 clinical trials. (Medications targeting psoriatic arthritis, biosimilars, and existing medications approved by the FDA prior to 2019 will not be discussed.)
Risankizumab
Risankizumab-rzaa (formerly BI 655066) is a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, selectively inhibiting the role of this critical cytokine in psoriatic inflammation.
Phase 1 Trial
In a phase 1 proof-of-concept study, 39 patients with moderate to severe plaque psoriasis received varying dosages of intravenous or subcutaneous risankizumab or placebo.1 At week 12, the percentage of risankizumab-treated patients achieving reduction in the psoriasis area and severity index (PASI) score by 75% (PASI 75), 90% (PASI 90), and 100% (PASI 100) was 87% (27/31; P<.001 vs placebo), 58% (18/31; P=.007 vs placebo), and 16% (5/31; P=.590 vs placebo), respectively. Improvements in PASI scores were observed as early as week 2. Adverse events (AEs) were reported by 65% of the risankizumab group and 88% of the placebo group. Serious AEs were reported in 4 patients receiving risankizumab, none of which were considered related to the study medication.1
Phase 2 Trial
A phase 2 comparator trial demonstrated noninferiority at higher dosages of risankizumab in comparison to the IL-12/IL-23 inhibitor ustekinumab.2 Among 166 participants with moderate to severe plaque psoriasis, PASI 90 at week 12 was met by 77% of participants receiving 90 or 180 mg of risankizumab compared to 40% receiving ustekinumab (P<.001). Onset of activity with risankizumab was faster and the duration of effect longer vs ustekinumab; by week 8, at least PASI 75 was achieved by approximately 80% of participants in the 90-mg and 180-mg risankizumab groups compared to 60% in the ustekinumab group; PASI score reductions generally were maintained for as long as 20 weeks after the final dose of risankizumab was administered.2
Phase 3 Trials
The 52-week UltIMMa-1 and UltIMMa-2 phase 3 trials compared subcutaneous risankizumab (150 mg) to ustekinumab (45 or 90 mg [weight-based dosing]) or placebo administered at weeks 0, 4, 16, 28, and 40 in approximately 1000 patients with moderate to severe plaque psoriasis.3 Patients initially assigned to placebo switched to risankizumab 150 mg at week 16. At week 16, PASI 90 was achieved by 75.3% of risankizumab-treated patients, 42.0% of ustekinumab-treated patients, and 4.9% of placebo-treated patients in UltIMMa-1, and by 74.8% of risankizumab-treated patients, 47.5% of ustekinumab-treated patients, and 2.0% of placebo-treated patients in UltIMMa-2 (P<.0001 vs placebo and ustekinumab for both studies). Achievement of a static physician’s global assessment (sPGA) score of 0 or 1 at week 16 similarly favored risankizumab, with 87.8%, 63.0%, and 7.8% of patients in UltIMMa-1 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively, and 83.7%, 61.6%, and 5.1% in UltIMMa-2 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively (P<.0001 vs placebo and ustekinumab for both studies). Among patients initially assigned to risankizumab, improvements in PASI and sPGA continued to increase until week 52, with 81.9% achieving PASI 90 at week 52 compared to 44.0% on ustekinumab in UltIMMa-1, and 80.6% achieving PASI 90 at week 52 compared to 50.5% on ustekinumab in UltIMMa-2 (P<.0001 vs ustekinumab for both studies). Treatment-emergent AE profiles were similar for risankizumab and ustekinumab in both studies, and there were no unexpected safety findings.3
Risankizumab received FDA approval for the treatment of moderate to severe plaque psoriasis in April 2019.
Bimekizumab
Bimekizumab (UCB4940), a humanized IgG1 monoclonal antibody, selectively neutralizes the biologic functions of IL-17A and IL-17F, the latter of which has only recently been implicated in contributing to the psoriatic immune cascade.4
First-in-Human Study
Thirty-nine participants with mild psoriasis demonstrated efficacy after single-dose intravenous bimekizumab, with maximal improvements in all measures of disease activity observed between weeks 8 and 12 in participants receiving 160 to 640 mg.5
Proof-of-Concept Phase 1b Study
A subsequent trial of 53 participants with psoriatic arthritis demonstrated sustained efficacy to week 20 with varying dosages of intravenous bimekizumab.6 At week 8, PASI 100 was met by 86.7% of participants receiving the top 3 dosages of bimekizumab compared to none of the placebo-treated participants. Treatment-emergent AEs, including neutropenia and elevation of liver transaminases, were mostly mild to moderate and resolved spontaneously. There were 3 severe AEs and 3 serious AEs, none of which were related to treatment.6
Importantly, bimekizumab was shown in this small study to have the potential to be highly effective at treating psoriatic arthritis. American College of Rheumatology ACR20, ACR50, and ACR70 response criteria were very high, with an ACR20 of 80% and an ACR50 of 40%.6 Further trials are necessary to gather more data and confirm these findings; however, these levels of response are higher than those of any other biologic on the market.
Phase 2b Dose-Ranging Study
In this trial, 250 participants with moderate to severe plaque psoriasis received either 64 mg, 160 mg with a 320-mg loading dose, 320 mg, or 480 mg of subcutaneous bimekizumab or placebo at weeks 0, 4, and 8.7 At week 12, PASI 90 was achieved by significantly more patients in all bimekizumab-treated groups compared to the placebo group (46.2%–79.1% vs 0%; P<.0001 for all dosages); PASI 100 also was achieved by significantly more bimekizumab-treated patients (27.9%–60.0% vs 0%; P<.0002). Improvement began as early as week 4, with clinically meaningful responses observed in all bimekizumab groups across all measures of disease activity. Treatment-emergent AEs occurred more frequently in bimekizumab-treated participants (61%) than in placebo-treated participants (36%); the most common AEs were nasopharyngitis and upper respiratory tract infection. Of note, fungal infections were reported by 4.3% of participants receiving bimekizumab; all cases were localized superficial infection, and none led to discontinuation. Three serious AEs were reported, none of which were considered related to the study treatment.7
Mirikizumab
Mirikizumab (LY3074828) is a humanized IgG4 monoclonal antibody that selectively binds and inhibits the p19 subunit of IL-23, with no action on IL-12.
Phase 1 Trial
Mirikizumab was shown to improve PASI scores in patients with plaque psoriasis.8
Phase 2 Trial
Subsequently, a trial of 205 participants with moderate to severe plaque psoriasis compared 3 dosing regimens of subcutaneous mirikizumab—30, 100, or 300 mg—at weeks 0 and 8 compared to placebo.9 Primary end point results at week 16 demonstrated PASI 90 response rates of 0%, 29% (P=.009), 59% (P<.001), and 67% (P<.001) in the placebo, 30-mg, 100-mg, and 300-mg mirikizumab groups, respectively. Complete clearance of psoriasis, measured by PASI 100 and sPGA 0, was achieved by 0%, 16%, 31%, and 31%, respectively (P=.039 for 30 mg vs placebo; P=.007 for the higher dosage groups vs placebo). Response rates for all efficacy outcomes were statistically significantly higher for all mirikizumab treatment groups compared to placebo and were highest in the 100-mg and 300-mg treatment groups. Frequencies of participants reporting AEs were similar across treatment and placebo groups.9
Oral Medications
Only a few small-molecule, orally bioavailable therapies are on the market for the treatment of psoriasis, some of which are associated with unfavorable side-effect profiles that preclude long-term therapy.
BMS-986165
The intracellular signaling enzyme tyrosine kinase 2 is involved in functional responses of IL-12 and IL-23. BMS-986165, a potent oral inhibitor of tyrosine kinase 2 with greater selectivity than other tyrosine kinase inhibitors, demonstrated efficacy in a phase 2 trial of 267 participants with moderate to severe plaque psoriasis receiving any of 5 dosing regimens—3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily—compared to placebo.10 At week 12, the percentage of patients with a 75% or greater reduction in PASI was 7% with placebo, 9% with 3 mg every other day (P=.49 vs placebo), 39% with 3 mg daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). Adverse events occurred in 51% of patients in the placebo group and in 55% to 80% of BMS-986165–treated patients; the most common AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection.10
A phase 3 trial comparing BMS-986165 with placebo and apremilast is underway (ClinicalTrials.gov Identifier NCT03611751).
Piclidenoson (CF101)
A novel small molecule that binds the Gi protein–associated A3 adenosine receptor piclidenoson induces an anti-inflammatory response via deregulation of the Wnt and nuclear factor κB signal transduction pathways, leading to downregulation of proinflammatory cytokines, including IL-17 and IL-23.11
In a phase 2 dose-ranging study, 75 patients with moderate to severe plaque psoriasis received varying dosages—1, 2, or 4 mg—of oral piclidenoson or placebo twice daily for 12 weeks.12 Progressive improvement in the mean change from baseline PASI score was observed in the 2-mg group, with statistically significant differences at weeks 8 and 12 compared to placebo (P=.047 and P=.031, respectively). At week 12, 35.3% of the 2-mg group achieved at least PASI 50. Improvements in PASI were less pronounced in the 4-mg group, and no therapeutic benefit was observed in the 1-mg group. Of the 20 AEs reported, 15 possibly were related to the study drug; 1 AE was severe.12
In a subsequent phase 2/3 trial, patients with moderate to severe plaque psoriasis received piclidenoson—1 or 2 mg—or placebo twice daily.13 At week 12, PASI 75 was achieved by 8.5% of patients in the 2-mg group and by 6.9% of patients receiving placebo (P=.621), thereby not meeting the primary study end point. Results at week 32 were more encouraging. In the 2-mg group, PASI mean percentage improvement was 57% (P<.002) compared to baseline, with linear improvements observed in PASI 50 (63.5%), PASI 75 (35.5%), PASI 90 (24.7%), and PASI 100 (10.6%).13
A phase 3 trial comparing piclidenoson 2 and 3 mg to apremilast and placebo is in progress (ClinicalTrials.gov Identifier NCT03168256).
Future Directions
Despite abundant options for treating moderate to severe plaque psoriasis and psoriatic arthritis, the pipeline remains rich. Novel treatments might have improved efficacy, favorable safety profiles, and different modes of administration compared to current medications. In addition to the novel therapeutics covered here, several treatments are in development further down the pipeline, with only phase 1 or 2 data available. Remtolumab (ABT-122), a tumor necrosis factor α– and IL-17A–targeted immunoglobulin, is unique among biologics, given its dual inhibition of tumor necrosis factor α and IL-17A.14 M1095 (ALX-0761), a novel trivalent bispecific nanobody, is another intriguing candidate. This dual inhibitor of IL-17A/F might exhibit a number of advantages over conventional antibodies, including better tissue penetration, reduced immunogenicity, and a longer half-life (ClinicalTrials.gov Identifier NCT03384745).15,16
As always with drug development, numerous medications that were under development failed to meet primary end points in phase 2 trials and have therefore been discontinued, including namilumab and prurisol. It is reassuring that the pace of drug discovery and development in psoriasis does not seem to be slowing; to our patients’ benefit, we will have an array of treatments available to tailor therapy to the individual.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Maroof A, Baeten D, Archer S, et al. 02.13 Il-17f contributes to human chronic inflammation in synovial tissue: preclinical evidence with dual IL-17a and IL-17f inhibition with bimekizumab in psoriatic arthritis. Ann Rheum Dis. 2017;76(Suppl 1):A13.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of bothinterleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-286.e10.
- Maari C. Safety, efficacy, and pharmacokinetics of a p19-directed IL-23 antibody in patients with plaque psoriasis and healthy subjects. Presented at: 25th European Academy of Dermatology and Venereology Congress; Vienna, Austria; September 28-October 2, 2016.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88-95.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Cohen S, Barer F, Itzhak I, et al. Inhibition of IL-17 and IL-23 in human keratinocytes by the A3 adenosine receptor agonist piclidenoson. J Immunol Res. 2018;2018:2310970.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
- 13. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol. 2016;15:931-938.
- Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70:1778-1789.
- Nanobodies’ competitive features. Ablynx website. http://www.ablynx.com/technology-innovation/nanobodies-competitive-features. Accessed July 4, 2019.
- Svecova D, Lubell MW, Casset-Semanaz F, et al. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196-203.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Maroof A, Baeten D, Archer S, et al. 02.13 Il-17f contributes to human chronic inflammation in synovial tissue: preclinical evidence with dual IL-17a and IL-17f inhibition with bimekizumab in psoriatic arthritis. Ann Rheum Dis. 2017;76(Suppl 1):A13.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of bothinterleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-286.e10.
- Maari C. Safety, efficacy, and pharmacokinetics of a p19-directed IL-23 antibody in patients with plaque psoriasis and healthy subjects. Presented at: 25th European Academy of Dermatology and Venereology Congress; Vienna, Austria; September 28-October 2, 2016.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88-95.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Cohen S, Barer F, Itzhak I, et al. Inhibition of IL-17 and IL-23 in human keratinocytes by the A3 adenosine receptor agonist piclidenoson. J Immunol Res. 2018;2018:2310970.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
- 13. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol. 2016;15:931-938.
- Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70:1778-1789.
- Nanobodies’ competitive features. Ablynx website. http://www.ablynx.com/technology-innovation/nanobodies-competitive-features. Accessed July 4, 2019.
- Svecova D, Lubell MW, Casset-Semanaz F, et al. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196-203.
Practice Points
- New systemic options for the treatment of psoriasis continue to emerge.
- With more choices, we can now tailor therapeutic approaches to the patient rather than base treatment choices purely on efficacy.
- New and upcoming biologics may offer improved skin clearance in line with the National Psoriasis Foundation’s treat-to-target approach, while others may offer increased efficacy in treating psoriatic arthritis.
- Novel small-molecule oral medications are in development and may have improved efficacy over current options.
What’s New in Topical Treatments for Psoriasis
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
In an era when we have access to a dizzying array of biologics for psoriasis treatment, it is easy to forget that topical therapies are still the bread and butter of treatment. For the majority of patients living with psoriasis, topical treatment is the only therapy they receive; indeed, a recent study examining a large national payer database found that 86% of psoriasis patients were managed with topical medications only.1 Thus, it is extremely important to understand how to optimize topical treatments, recognize pitfalls in management, and utilize newer agents that can been added to our treatment armamentarium for psoriasis.
In general, steroids have been the mainstay of topical treatment of psoriasis. Their broad anti-inflammatory activity works well against both the visible signs and symptoms of psoriasis as well as the underlying inflammatory milieu of the disease; however, these treatments are not without their downsides. Hypothalamic-pituitary-adrenal (HPA) axis suppression, especially in higher-potency topical steroids, is a serious concern that limits their use. In one study comparing lotion and cream formulations of clobetasol propionate, HPA axis suppression was seen in 80% (8/10) of adults in the lotion group and 30% (3/10) in the cream group after 4 weeks of treatment.2 These findings are not new; a 1987 study found that patients using less than 50 g of topical clobetasol per week, which is considered a low dose, could still exhibit HPA axis suppression.3 Severe HPA axis suppression may occur; one study of various topical steroids found some degree of HPA axis suppression in 38% (19/50) of patients, with a direct correlation with topical steroid potency.4 Additionally, cutaneous side effects such as striae formation, atrophy, and the possibility of tachyphylaxis must be considered. Various treatment regimens have been developed to limit topical steroid use, including steroid-sparing medications (eg, calcipotriene) used in conjunction with topical steroids, systemic treatments (eg, phototherapy) added on, or higher-potency topical steroids rotated with lower-potency steroids. Implementing other agents, such as topical retinoids or keratolytics, into the treatment regimen also is an important consideration in the overall approach to topical psoriasis therapy.
Notably, a number of newly approved topical treatments for psoriasis have emerged, and more are in the pipeline. When evaluating these agents, important considerations include safety, length of treatment course, and efficacy. Several of these agents hold promise for patients with psoriasis.
An alcohol-free, fixed-combination aerosol foam formulation of calcipotriene 0.005% and betamethasone dipropionate 0.064% was approved by the US Food and Drug Administration for plaque psoriasis in 2015. This agent was shown to be more efficacious than the same combination of active ingredients in an ointment formulation as well as either agent alone, with psoriasis area and severity index 75 response achieved in more than 50% of patients at week 4 of treatment.5 Notably, this product offers once-daily application with positive patient satisfaction scores.6 The novelty of this foam is in its ability to supersaturate the active ingredients on the surface of the skin with improved penetration and drug delivery.
A novel spray formulation of betamethasone dipropionate 0.05% also has been developed and has been compared to augmented betamethasone dipropionate lotion. One benefit of this spray is that, based on the vasoconstriction test, the potency is similar to a mid-potency steroid while the efficacy is not significantly different from betamethasone dipropionate lotion, a class I steroid.7 Hypothalamic-pituitary-adrenal axis suppression was similar following a 4-week treatment course compared to a 2-week course of the lotion formulation.8
The newest agent, halobetasol propionate lotion 0.01%, was approved for treatment of psoriasis in October 2018. Compared to halobetasol 0.05% cream or ointment, halobetasol propionate lotion 0.01% has one-fifth the concentration of the active ingredient with the same degree of success in efficacy scores.9 This reduction in drug concentration is possible because the proprietary lotion base allows for better drug delivery of the active ingredient. Importantly, HPA axis suppression was assessed over an 8-week period of use and no suppression was noted.9 Generic class I steroids should only be used for 2 weeks, which is the standard treatment period used in comparator trials; however, many patients will still have active lesions on their body after 2 weeks of treatment, and if using generic clobetasol or betamethasone dipropionate, the choice becomes whether to keep applying the medication and risk HPA axis suppression and cutaneous side effects or switch to a less effective treatment. However, some of the newer agents are indicated for 4 to 8 weeks of treatment.
Utilizing other classes of agents such as retinoids and keratolytics in our treatment armamentarium for psoriasis often is helpful. It has long been known that tazarotene can be combined with topical steroids for increased efficacy and limitation of the irritating effects of the retinoid.10 Similarly, keratolytics play a role in allowing a topically applied medication to penetrate deep enough to affect the underlying inflammation of psoriasis. Medications that include salicylic acid or urea may help to remove ostraceous scales from thick psoriasis lesions that would otherwise prevent delivery of topical steroids to achieve clinically meaningful results. For scalp psoriasis, there are salicylic acid solutions as well as newer agents such as a dimethicone-based topical product.11
Nonsteroidal topical anti-inflammatories also have been used off label for psoriasis treatment. These agents are especially useful in patients who were not successfully treated with calcipotriene or need adjunctive therapy. Although not extremely effective against plaque psoriasis, topical tacrolimus in particular seems to have a place in the treatment of inverse psoriasis where it can be utilized without concern for long-term side effects.12 Crisaborole ointment, a topical medication approved for treatment of atopic dermatitis, was studied in phase 2 trials, but development has not progressed for a psoriasis indication.13 It is reasonable to consider this medication in the same way that tacrolimus has been used, however, considering that the mechanism of action—phosphodiesterase type 4 inhibition—has successfully been implemented in an oral medication to treat psoriasis, apremilast.
There are numerous topical medications in the pipeline that are being developed to treat psoriasis. Of them, the most relevant is a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% in a proprietary lotion vehicle. A decision from the US Food and Drug Administration is expected in the first quarter of 2019. This medication capitalizes on the aforementioned synergistic effects of tazarotene and a superpotent topical steroid to achieve improved efficacy. Similar to halobetasol lotion 0.01%, this product was evaluated over an 8-week period, and no HPA axis suppression was observed. Efficacy was significantly improved versus both placebo and either halobetasol or tazarotene alone.14
Overall, it is promising that after a long period of relative stagnancy, we have numerous new agents available and upcoming for the topical treatment of psoriasis. For the vast majority of patients, topical medications still represent the mainstay of treatment, and it is important that we have access to better, safer medications in this category.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study [published online October 25, 2018]. J Med Econ. doi:10.1080/13696998.2018.1540424.
- Clobex [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2005.
- Ohman EM, Rogers S, Meenan FO, et al. Adrenal suppression following low-dose topical clobetasol propionate. J R Soc Med. 1987;80:422-424.
- Kerner M, Ishay A, Ziv M, et al. Evaluation of the pituitary-adrenal axis function in patients on topical steroid therapy. J Am Acad Dermatol. 2011;65:215-216.
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Paul C, Bang B, Lebwohl M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: rationale for development and clinical profile. Expert Opin Pharmacother. 2017;18:115-121.
- Fowler JF Jr, Hebert AA, Sugarman J. DFD-01, a novel medium potency betamethasone dipropionate 0.05% emollient spray, demonstrates similar efficacy to augmented betamethasone dipropionate 0.05% lotion for the treatment of moderate plaque psoriasis. J Drugs Dermatol. 2016;15:154-162.
- Sidgiddi S, Pakunlu RI, Allenby K. Efficacy, safety, and potency of betamethasone dipropionate spray 0.05%: a treatment for adults with mildto-moderate plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:14-22.
- Kerdel FA, Draelos ZD, Tyring SK, et al. A phase 2, multicenter, doubleblind, randomized, vehicle-controlled clinical study to compare the safety and efficacy of a halobetasol propionate 0.01% lotion and halobetasol propionate 0.05% cream in the treatment of plaque psoriasis [published online November 5, 2018]. J Dermatolog Treat. doi:10.1080/09 546634.2018.1523362.
- Lebwohl M, Poulin Y. Tazarotene in combination with topical corticosteroids. J Am Acad Dermatol. 1998;39(4 pt 2):S139-S143.
- Hengge UR, Roschmann K, Candler H. Single-center, noninterventional clinical trial to assess the safety, efficacy, and tolerability of a dimeticone-based medical device in facilitating the removal of scales after topical application in patients with psoriasis corporis or psoriasis capitis. Psoriasis (Auckl). 2017;7:41-49.
- Malecic N, Young H. Tacrolimus for the management of psoriasis: clinical utility and place in therapy. Psoriasis (Auckl). 2016;6:153-163.
- Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
Biologics and Systemic Therapies for Psoriasis: Treat the Patient, Not the Disease
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
What do patients need to know initially about psoriasis treatment?
It is important to set expectations with the patient based on the treatment selected, not only for patient satisfaction but to forge an enduring bond with the patient so he/she will trust you to guide the treatment plan if the first therapy does not work as well as anticipated. Because psoriasis is a longitudinal disease process, the patient-physician relationship should be, too. Certainly, these principles generally apply among all patient groups and demographics; however, one may take into account a few special circumstances when dealing with psoriasis. In a pediatric patient, I may try to see if topical therapy including calcipotriene can adequately treat the skin disease before pursuing systemic treatment. The rationale is 2-fold: (1) this patient would be committed to an extended period on immunomodulatory therapy if he/she truly requires it, and (2) some of the forms of psoriasis in children, such as guttate psoriasis, may be self-limited, so it is reasonable to see if it will persist before forging ahead with a long-term systemic medication. In patients with a recent history of cancer, I would likely choose an oral medication such as apremilast before a biologic; even though there are no real data to suggest biologics are associated with higher rates of solid-organ malignancy, most practitioners would err on the side of being more conservative. For patients with human immunodeficiency virus, the tendency is to use the agents with more data (eg, tumor necrosis factor α inhibitors) due to safety concerns with an immunomodulatory medication.
What are your go-to treatments?
I tend to be as aggressive as the patient wants to be with therapy. I regularly see patients in whom multiple systemic treatments have failed and a more creative regimen is needed, such as combining a biologic medication with an oral antipsoriatic treatment (eg, apremilast, acitretin). However, I do have patients with moderate to severe psoriasis who have not seen a dermatologist before. I do not find it necessary to have topical treatments fail before starting a biologic; after all, the sequelae of long-term topical steroid use are notable.
With the newer biologics on the market, such as the IL-17 and IL-23 inhibitors, the sky's the limit for psoriasis area and severity index clearance, but the true benefit is that these medications are much more targeted toward the pathogenesis of psoriasis. Unfortunately, we have to be mindful of insurance and formulary restrictions, but when faced with choosing a broad-acting immunomodulatory agent or a more specific/targeted immunomodulatory agent for an inflammatory disease, most dermatologists would choose the more targeted medication. The data support that the newer agents have better psoriasis area and severity index responses and a much greater proportion of clearance, but there is something to be said about biologics such as etanercept, adalimumab, and ustekinumab, which have been on the market for much longer and have shown durable response with a longer track record of safety and efficacy. Recent head-to-head comparisons can help guide treatment. For instance, patients who achieved suboptimal clearance on ustekinumab can safely and reasonably be switched to guselkumab based on the findings of the NAVIGATE study, which looked at this exact situation. More of these studies looking at specific prior treatment failures and improvement upon switching to a newer agent are needed to underscore the efficacy of these drugs and also to help argue for their placement on insurance formularies.
For a new patient with psoriasis, I will screen for psoriatic arthritis, look at involvement (eg, body surface area, individual plaque severity/thickness, locations such as scalp and extremities), and assess patient attitudes toward different treatments. Two patients with the exact same clinical appearance might have completely different strategies, one wanting to be as aggressive as possible to get rid of the psoriasis and the other not believing in systemic treatments and wanting to be as "natural" as possible.
For patients with only cutaneous involvement, the dosing frequency and efficacy of the newer IL-17 and IL-23 classes of medications are hard to beat. If a patient has notable psoriatic arthritis, I still tend to reach for a tumor necrosis factor α inhibitor first. For patients with limited involvement, especially those with scalp and/or palmoplantar psoriasis, I have found that apremilast works quite well. Apremilast, in general, would be a good first-step medication for patients wary of systemic therapy, and with its relatively benign side-effect profile, it has almost completely supplanted methotrexate in my practice. We also have a few newer topical medicines such as a calcipotriene 0.005%-betamethasone dipropionate 0.064% foam and a betamethasone dipropionate spray 0.05% that have proven useful, with more products in the pipeline.
How do you keep patients compliant with treatment?
Setting expectations is most important, and letting patients know what to expect from their first visit really helps to keep them satisfied with the plan and progress. Giving the patient a say in guiding the treatment and perhaps coming up with a rough treatment plan with a defined timeline also helps, such as starting with a topical regimen but moving on to an oral medicine if the topical does not work within 2 to 3 months, and then a biologic if oral therapy does not work well within 3 to 6 months. It is important not to push the patient to pursue a more aggressive therapy unless he/she wants to, otherwise the patient might not be compliant or may stop altogether.
What do you do if they refuse treatment?
If the patient is in your office, clearly he/she does want some help. Try to figure out what is at the root of the treatment refusal. Is the patient refusing topical steroids because he/she is afraid of them? Is the patient unable to stomach having to inject himself/herself? Finding the basis of their reticence may take more time, but we usually can find a mutually agreeable plan of action. Even if the first step is to watch and wait, you want the patient leaving your office knowing that if things do not progress as expected or get worse, they can have faith in you to come back and get more help.
What resources do you recommend to patients for more information?
The National Psoriasis Foundation is a great resource for patients. They have numerous outreach programs and a wealth of patient information. Also, the American Academy of Dermatology is a good resource, not just for patients but for providers; for example, the academy offers appeals letters that can be sent to insurance companies to try to advocate for a specific medication for patients.
Suggested Readings
Help patients appeal denial of psoriasis drugs. American Academy of Dermatology website. https://www.aad.org/members/publications/member-to-member/2017/jan-27-2017/help-patients-appeal-denial-of-psoriasis-drugs. Accessed February 9, 2018.
Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial [published online October 10, 2017]. Br J Dermatol. 2018;178:114-123.
Psoriatic Arthritis Treatment: The Dermatologist’s Role
Spot Psoriatic Arthritis Early in Psoriasis Patients
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
Applications of Lasers in Medical Dermatology
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.