User login
How should you treat Candida vaginitis in patients on antibiotics?
Oral and intravaginal antifungals for the treatment of uncomplicated vulvovaginal candidiasis (VVC) have similar effectiveness (strength of recommendation [SOR]: A, systematic review). However, no randomized controlled trials (RCTs) have addressed treatment options for patients taking antibiotics. Oral antifungals are contraindicated in pregnancy. While shorter courses of intravaginal therapy can be used by nonpregnant women, 7-day treatment may be necessary during pregnancy (SOR: A, systematic review). Products containing Lactobacillus species do not prevent postantibiotic vulvovaginitis (SOR: A, systematic review and RCT).
Most women would rather prevent than treat
Laura Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University
Many women complain about getting yeast infections after receiving antibiotics. Usually the patient will inform me of this while I’m writing the prescription for the antibiotic, asking for Diflucan “just in case.” Women prefer the convenience of the oral medicine over the hassle with topical applications. Some state that 1 dose of Diflucan does not cut it, and that they usually need 2. As a result, I find myself writing a prescription for Diflucan to be started along with the antibiotic, and then to be repeated as a second dose in 3 days. I have not heard any complaints from these patients about postantibiotic yeast infections.
Evidence summary
VVC is a common cause of vaginitis; Candida albicans accounts for 85% to 90% of cases. Risk factors include pregnancy, diabetes mellitus, and systemic antibiotics.1 Incidence increases with onset of sexual activity, but there is no direct evidence it is sexually transmitted.1 About 75% of women experience 1 VVC episode during their lifetime, 40% to 45% have 2 or more, and 5% to 8% have recurrent VVC (defined as 4 or more annually).1,2
The candidiasis/antibiotics link
A 2003 systematic review found the evidence supporting the association between antibiotics and VVC limited and contradictory.3 Most were case-control or cohort studies with small sample sizes. No RCTs compare the incidence of culture-confirmed VVC among women receiving antibiotics or placebo.
Nineteen reports of 18 original studies had sufficient data to calculate a relative risk or odds ratio for antibiotic-associated VVC. Thirteen of the 19 reports showed an increase (around twofold; range, 0.43–5) in vaginal Candida prevalence; however, 3 of the 13 reports had no mycological culture data. Six studies did not show significant association between antibiotics and vaginal yeast.3
Antibiotics are thought to increase risk of VVC by killing endogenous vaginal flora (particularly Lactobacillus), allowing microorganisms resistant to the antibiotics, like Candida, to flourish.1 Yet there is evidence that numbers of genital Lactobacillus are similar for women with and without symptomatic VVC.4 Further, decreasing Lactobacillus does not increase the risk of VVC.5
Topical and oral antifungals—both do the job
For the treatment of uncomplicated VVC, both topical and oral antifungals are clinically and mycologically effective, with comparable clinical cure rates >80%.6 No difference in persistent symptoms between single and multiple doses, or different durations of multiple dose regimens have been found, but samples may have been too small to detect clinically significant effects. An RCT found less nausea, headache, and abdominal pain with intravaginal imidazoles, but more vulvar irritation and vaginal discharge than oral fluconazole.6
For treatment of recurrent VVC, RCTs have shown the effectiveness of oral fluconazole and itraconazole maintenance therapy taken for 6 months after an initial regimen.7,8 Treating male sexual partners did not significantly improve resolution of the woman’s symptoms or reduce the rate of symptomatic relapse.9
Yogurt may not live up to its rep
Two poor-quality crossover RCTs provided insufficient evidence regarding effectiveness of a diet containing oral Lactobacillus yogurt to prevent recurrent VVC.9 A recent RCT of 278 women on short courses of antibiotics were randomized to oral lactobacilli or placebo and vaginal lactobacilli or placebo.10 The study was stopped early because there was no effect seen. Overall, 23% developed symptomatic vulvovaginitis.
Recommendations from others
The Infectious Diseases Society of America11 recommends treating uncomplicated VVC with short-course of oral or topical antifungals; treating complicated VVC with antimycotic therapy for 7 days, either daily as topical therapy or as two 150-mg doses of fluconazole 72 hours apart; treating non-albicans species of Candida with topical boric acid (600 mg/day for 14 days) or topical flucytosine; and treating recurrent VVC with induction therapy with 2 weeks of a topical or oral azole followed by a maintenance regimen for 6 months (fluconazole once a week or itraconazole twice a week).11
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
2. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
3. Xu J, Sobel JD. Antibiotic-associated vulvovaginal candidiasis. Curr Infect Dis Rep 2003;5:481-487.
4. Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol 1996;34:2497-2499.
5. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
6. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole antifungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2001;(1):CD002845.-
7. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004;351:876-883.
8. Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med 1997;42:83-87.
9. Spence D. Candidiasis (vulvovaginal). Clin Evid 2006;15:1-8.
10. Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing postantibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 2004;329:548.-
11. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
Oral and intravaginal antifungals for the treatment of uncomplicated vulvovaginal candidiasis (VVC) have similar effectiveness (strength of recommendation [SOR]: A, systematic review). However, no randomized controlled trials (RCTs) have addressed treatment options for patients taking antibiotics. Oral antifungals are contraindicated in pregnancy. While shorter courses of intravaginal therapy can be used by nonpregnant women, 7-day treatment may be necessary during pregnancy (SOR: A, systematic review). Products containing Lactobacillus species do not prevent postantibiotic vulvovaginitis (SOR: A, systematic review and RCT).
Most women would rather prevent than treat
Laura Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University
Many women complain about getting yeast infections after receiving antibiotics. Usually the patient will inform me of this while I’m writing the prescription for the antibiotic, asking for Diflucan “just in case.” Women prefer the convenience of the oral medicine over the hassle with topical applications. Some state that 1 dose of Diflucan does not cut it, and that they usually need 2. As a result, I find myself writing a prescription for Diflucan to be started along with the antibiotic, and then to be repeated as a second dose in 3 days. I have not heard any complaints from these patients about postantibiotic yeast infections.
Evidence summary
VVC is a common cause of vaginitis; Candida albicans accounts for 85% to 90% of cases. Risk factors include pregnancy, diabetes mellitus, and systemic antibiotics.1 Incidence increases with onset of sexual activity, but there is no direct evidence it is sexually transmitted.1 About 75% of women experience 1 VVC episode during their lifetime, 40% to 45% have 2 or more, and 5% to 8% have recurrent VVC (defined as 4 or more annually).1,2
The candidiasis/antibiotics link
A 2003 systematic review found the evidence supporting the association between antibiotics and VVC limited and contradictory.3 Most were case-control or cohort studies with small sample sizes. No RCTs compare the incidence of culture-confirmed VVC among women receiving antibiotics or placebo.
Nineteen reports of 18 original studies had sufficient data to calculate a relative risk or odds ratio for antibiotic-associated VVC. Thirteen of the 19 reports showed an increase (around twofold; range, 0.43–5) in vaginal Candida prevalence; however, 3 of the 13 reports had no mycological culture data. Six studies did not show significant association between antibiotics and vaginal yeast.3
Antibiotics are thought to increase risk of VVC by killing endogenous vaginal flora (particularly Lactobacillus), allowing microorganisms resistant to the antibiotics, like Candida, to flourish.1 Yet there is evidence that numbers of genital Lactobacillus are similar for women with and without symptomatic VVC.4 Further, decreasing Lactobacillus does not increase the risk of VVC.5
Topical and oral antifungals—both do the job
For the treatment of uncomplicated VVC, both topical and oral antifungals are clinically and mycologically effective, with comparable clinical cure rates >80%.6 No difference in persistent symptoms between single and multiple doses, or different durations of multiple dose regimens have been found, but samples may have been too small to detect clinically significant effects. An RCT found less nausea, headache, and abdominal pain with intravaginal imidazoles, but more vulvar irritation and vaginal discharge than oral fluconazole.6
For treatment of recurrent VVC, RCTs have shown the effectiveness of oral fluconazole and itraconazole maintenance therapy taken for 6 months after an initial regimen.7,8 Treating male sexual partners did not significantly improve resolution of the woman’s symptoms or reduce the rate of symptomatic relapse.9
Yogurt may not live up to its rep
Two poor-quality crossover RCTs provided insufficient evidence regarding effectiveness of a diet containing oral Lactobacillus yogurt to prevent recurrent VVC.9 A recent RCT of 278 women on short courses of antibiotics were randomized to oral lactobacilli or placebo and vaginal lactobacilli or placebo.10 The study was stopped early because there was no effect seen. Overall, 23% developed symptomatic vulvovaginitis.
Recommendations from others
The Infectious Diseases Society of America11 recommends treating uncomplicated VVC with short-course of oral or topical antifungals; treating complicated VVC with antimycotic therapy for 7 days, either daily as topical therapy or as two 150-mg doses of fluconazole 72 hours apart; treating non-albicans species of Candida with topical boric acid (600 mg/day for 14 days) or topical flucytosine; and treating recurrent VVC with induction therapy with 2 weeks of a topical or oral azole followed by a maintenance regimen for 6 months (fluconazole once a week or itraconazole twice a week).11
Oral and intravaginal antifungals for the treatment of uncomplicated vulvovaginal candidiasis (VVC) have similar effectiveness (strength of recommendation [SOR]: A, systematic review). However, no randomized controlled trials (RCTs) have addressed treatment options for patients taking antibiotics. Oral antifungals are contraindicated in pregnancy. While shorter courses of intravaginal therapy can be used by nonpregnant women, 7-day treatment may be necessary during pregnancy (SOR: A, systematic review). Products containing Lactobacillus species do not prevent postantibiotic vulvovaginitis (SOR: A, systematic review and RCT).
Most women would rather prevent than treat
Laura Kittinger-Aisenberg, MD
Chesterfield Family Medicine Residency Program, Virginia Commonwealth University
Many women complain about getting yeast infections after receiving antibiotics. Usually the patient will inform me of this while I’m writing the prescription for the antibiotic, asking for Diflucan “just in case.” Women prefer the convenience of the oral medicine over the hassle with topical applications. Some state that 1 dose of Diflucan does not cut it, and that they usually need 2. As a result, I find myself writing a prescription for Diflucan to be started along with the antibiotic, and then to be repeated as a second dose in 3 days. I have not heard any complaints from these patients about postantibiotic yeast infections.
Evidence summary
VVC is a common cause of vaginitis; Candida albicans accounts for 85% to 90% of cases. Risk factors include pregnancy, diabetes mellitus, and systemic antibiotics.1 Incidence increases with onset of sexual activity, but there is no direct evidence it is sexually transmitted.1 About 75% of women experience 1 VVC episode during their lifetime, 40% to 45% have 2 or more, and 5% to 8% have recurrent VVC (defined as 4 or more annually).1,2
The candidiasis/antibiotics link
A 2003 systematic review found the evidence supporting the association between antibiotics and VVC limited and contradictory.3 Most were case-control or cohort studies with small sample sizes. No RCTs compare the incidence of culture-confirmed VVC among women receiving antibiotics or placebo.
Nineteen reports of 18 original studies had sufficient data to calculate a relative risk or odds ratio for antibiotic-associated VVC. Thirteen of the 19 reports showed an increase (around twofold; range, 0.43–5) in vaginal Candida prevalence; however, 3 of the 13 reports had no mycological culture data. Six studies did not show significant association between antibiotics and vaginal yeast.3
Antibiotics are thought to increase risk of VVC by killing endogenous vaginal flora (particularly Lactobacillus), allowing microorganisms resistant to the antibiotics, like Candida, to flourish.1 Yet there is evidence that numbers of genital Lactobacillus are similar for women with and without symptomatic VVC.4 Further, decreasing Lactobacillus does not increase the risk of VVC.5
Topical and oral antifungals—both do the job
For the treatment of uncomplicated VVC, both topical and oral antifungals are clinically and mycologically effective, with comparable clinical cure rates >80%.6 No difference in persistent symptoms between single and multiple doses, or different durations of multiple dose regimens have been found, but samples may have been too small to detect clinically significant effects. An RCT found less nausea, headache, and abdominal pain with intravaginal imidazoles, but more vulvar irritation and vaginal discharge than oral fluconazole.6
For treatment of recurrent VVC, RCTs have shown the effectiveness of oral fluconazole and itraconazole maintenance therapy taken for 6 months after an initial regimen.7,8 Treating male sexual partners did not significantly improve resolution of the woman’s symptoms or reduce the rate of symptomatic relapse.9
Yogurt may not live up to its rep
Two poor-quality crossover RCTs provided insufficient evidence regarding effectiveness of a diet containing oral Lactobacillus yogurt to prevent recurrent VVC.9 A recent RCT of 278 women on short courses of antibiotics were randomized to oral lactobacilli or placebo and vaginal lactobacilli or placebo.10 The study was stopped early because there was no effect seen. Overall, 23% developed symptomatic vulvovaginitis.
Recommendations from others
The Infectious Diseases Society of America11 recommends treating uncomplicated VVC with short-course of oral or topical antifungals; treating complicated VVC with antimycotic therapy for 7 days, either daily as topical therapy or as two 150-mg doses of fluconazole 72 hours apart; treating non-albicans species of Candida with topical boric acid (600 mg/day for 14 days) or topical flucytosine; and treating recurrent VVC with induction therapy with 2 weeks of a topical or oral azole followed by a maintenance regimen for 6 months (fluconazole once a week or itraconazole twice a week).11
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
2. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
3. Xu J, Sobel JD. Antibiotic-associated vulvovaginal candidiasis. Curr Infect Dis Rep 2003;5:481-487.
4. Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol 1996;34:2497-2499.
5. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
6. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole antifungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2001;(1):CD002845.-
7. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004;351:876-883.
8. Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med 1997;42:83-87.
9. Spence D. Candidiasis (vulvovaginal). Clin Evid 2006;15:1-8.
10. Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing postantibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 2004;329:548.-
11. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
1. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
2. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
3. Xu J, Sobel JD. Antibiotic-associated vulvovaginal candidiasis. Curr Infect Dis Rep 2003;5:481-487.
4. Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol 1996;34:2497-2499.
5. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
6. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intra-vaginal imidazole and triazole antifungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev 2001;(1):CD002845.-
7. Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004;351:876-883.
8. Spinillo A, Colonna L, Piazzi G, Baltaro F, Monaco A, Ferrari A. Managing recurrent vulvovaginal candidiasis. Intermittent prevention with itraconazole. J Reprod Med 1997;42:83-87.
9. Spence D. Candidiasis (vulvovaginal). Clin Evid 2006;15:1-8.
10. Pirotta M, Gunn J, Chondros P, et al. Effect of lactobacillus in preventing postantibiotic vulvovaginal candidiasis: a randomised controlled trial. BMJ 2004;329:548.-
11. Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis 2004;38:161-189.
Evidence-based answers from the Family Physicians Inquiries Network
Screening for prostate cancer: Who and how often?
- Engage patients in shared decision making by discussing the benefits and risks of prostate cancer screening. Patients who review educational pamphlets before an office visit engage more fully in the decision-making process. (B)
- If performing prostate cancer screening, limit to men with greater than 10 years life expectancy. (B)
- Because the lead time of a diagnosis based on PSA screening is estimated to be 5 to 7 years, PSA screening every other year is unlikely to cause a loss of sensitivity. (B)
- Men with tumors with a Gleason score less than 5 are the best candidates for “watchful waiting,” having a favorable 20-year survival. (B)
Prostate cancer screening in asymptomatic men remains controversial, and it is difficult to present its benefits and risks quickly in a way that is understandable to patients. Yet many expert groups agree that physicians should enter into a mutual decision-making process with patients.1-3
This article reviews the latest information relevant to the controversy, offers “talking points” for family physicians to use when discussing screening with patients, and lists websites that patients may find helpful when making a decision about prostate cancer screening.
For this review, we searched for recent articles that are generalizable to a primary care population and of the highest evidence level available. We preferentially discuss population-based studies, studies from randomized trials of screening, and meta-analyses, rather than results that are hospital- or clinic-based. For a complete systematic review of this topic (from 2002), readers are referred to one conducted for the Agency for Healthcare Research and Quality.4 (See Scope of the problem).
Adenocarcinoma of the prostate is a significant public health burden. Age, family history, and race are the only known risk factors. Most cancers (86%) are diagnosed while still confined to the prostate; however, invasion beyond the capsule is sometimes not apparent until surgery.
Incidence. In 2005, there will be approximately 232,090 new cases of prostate cancer.5 American men have a 17% chance of being diagnosed with prostate cancer; African Americans have a 65% greater risk of developing prostate cancer than Caucasians.6 In fact, African Americans have the highest prostate cancer annual age-adjusted incidence rates in the world: 272/100,000 compared with 164/100,000 for Caucasian Americans.6 The rate for US Asian/Pacific Islander and Hispanic men is less than that for Caucasian Americans.
Mortality. There will be approximately 30,350 prostate cancer deaths in 2004.5 There is 3% chance of dying from prostate cancer; however, the risk of death is about 55% higher for African American men than Caucasian American men.6
Risk factors other than race. Risk of prostate cancer diagnosis increases with age: 1 in 48 men aged 40 to 59 years will be diagnosed with prostate cancer, while 1 in 8 men aged 60 to 79 years are at risk.7 A man who has a first-degree relative with prostate cancer is 2.4 times as likely to be diagnosed with prostate cancer as a man with no affected relatives.8
Key components of the controversy
How effective is screening?
A good screening test does 2 things. First, it detects a disease earlier than it would be detected with no screening at all, and it does so with sufficient accuracy to avoid a large number of false-positive and false-negative results.
Second, it leads to treatment of early disease that will likely produce a more favorable health outcome than waiting to treat patients who have signs and symptoms of disease.9 Unfortunately it is still unclear whether screening tests for prostate cancer meet these 2 criteria.
Skewed numbers. Yes, estimates of false-positive and false-negative results are available from numerous studies of different populations. However, in most studies, only men with abnormal test results receive a biopsy. Men with normal screening test results are not biopsied. Therefore the number of false negatives (and true negatives) is unknown. Furthermore, these estimates are often based on patients from urology clinics, a group more likely to have disease, thereby increasing the positive predictive value of a given screening test.
Whether current screening methods—in particular, prostate-specific antigen (PSA)—identify prostate cancers destined to become clinically relevant is also unknown. If screening does identify such cancers, a decrease in prostate cancer mortality among men who were screened is expected. If it does not, overdiagnosis and treatment of clinically insignificant cancers will negatively impact the quality of men’s lives without extending their life spans.
Clinical variability of the disease
The natural history of prostate cancer is uncertain. If cancer is left unidentified or untreated, more men will die with prostate cancer than of prostate cancer.
Clinically, prostate cancer ranges from an asymptomatic slow-growing tumor to an aggressive cancer with painful metastases. Treatment may be unnecessary at one end of the spectrum and palliative at the other.
The goal of screening is to identify slow-growing tumors destined to extend beyond the prostate while they are confined to the prostate and amenable to treatment, thereby decreasing the risk of prostate cancer morbidity and death.
Usually low morbidity. For a 50-year-old man, the risk of being diagnosed with prostate cancer by age 80 years is 15%; however, the same man has a 1.4% chance of dying of prostate cancer over that 30-year period.7 This 10-fold difference shows that prostate cancer usually is not a fatal illness. Another indication of the often benign nature of the disease is the high percentage of prostate cancers identified at surgery for bladder cancer; up to 40% of specimens contain unsuspected prostate cancer (level of evidence [LOE]: 2c).10,11
Most clinical diagnoses (80%) and prostate cancer deaths (90%) occur among men older than 65 years;6 the median age at diagnosis is 72 years.12 More than 75% of men older than 85 years will have histological prostate cancer (LOE: 2c).13 Many men live with their disease for more than 10 years, but do not die of it (LOE: 2c).12 Additionally, a review of several decision analyses indicates that men 75 years of age and older are not likely to benefit from screening and aggressive treatment (LOE: 2a).14
Hence the recommendation: if performing prostate cancer screening, limit to men with greater than 10 years life expectancy.15
Details of screening tests
Digital rectal examination insufficient
Digital rectal examination (DRE)—palpating the prostate gland to determine size and consistency—is one screening tool for prostate cancer, usually performed in conjunction with PSA testing.15 It is not a difficult or expensive test, but its reproducibility is only fair, even among experienced urologists (LOE: 2b).16
The sensitivity and specificity of DRE can only be estimated because men with a normal finding on DRE are not routinely biopsied in any studies (TABLE 1).17,18 One of two pertinent meta-analyses18 included studies that followed men with a negative DRE finding for development of prostate cancer (LOE: 2a). Both meta-analyses of DRE as a screening tool found that the included studies were heterogeneous in their study populations and definition of abnormal DRE test result (LOE: 2a). The positive predictive value (PPV) of DRE was 28% in one meta-analysis and 18% in the other.
TABLE 1
Characteristics of screening tests for prostate cancer
| TEST/SOURCE | DESCRIPTION (LEVEL OF EVIDENCE) | SN ESTIMATE (%) | SP ESTIMATE (%) | LIKELIHOOD RATIO | PPV (%) |
|---|---|---|---|---|---|
| Abnormal DRE18 | Meta-analysis of studies that performed biopsies for abnormal DRE (2a) | 59 (51%–67%) | 94 (91%–96%) | + 9.8 – 0.4 | 28 (20–36) |
| Abnormal DRE17 | Meta-analysis of studies that performed biopsies for abnormal DRE or PSA (2a) | 53.2 | 83.6 | + 3.24 – 0.56 | 17.8 |
| PSA >4 ng/mL12 | Review of multiple cohort studies with diverse populations, not systematic | Mean = 71 | Mean = 75 | + 2.8 – 0.30 | 37 |
| PSA >4 ng/mL19 | Longitudinal retrospective study of prostate cancers that developed within 2 years of PSA test (3b) | 73.2 | 85.4 | + 5.0 – 0.2 | |
| PSA >4 ng/mL20 | Nested case-control study of prostate cancers that developed within 5 years of PSA test in a cohort study (3b) | 86 | 94 | + 14.3 – 0.15 | |
| PSA > 4 ng/mL17 | Meta-analysis of studies that performed biopsies for abnormal PSA or DRE (2a) | 72.1 | 93.2 | + 10.2 – 0.30 | 25.1 |
| Sn, sensitivity; Sp, specificity; PPV, positive predictive value; | |||||
| PSA, prostate-specific antigen; DRE, digital rectal examination. | |||||
Prostate-specific antigen: Improving its clinical usefulness
Prostate-specific antigen (PSA), first detected in serum in 1979, is a protein produced by prostate epithelial cells. It was originally used to follow men treated for prostate cancer for evidence of recurrence. In the late 1980s, it became widely used in the US to screen for prostate cancer.
An elevated PSA level is suggestive but not diagnostic of prostate cancer. Elevated levels also occur with advancing age, increased prostate size, and prostatitis, and following ejaculation. Prostate manipulation such as biopsy and surgery (but not digital examination) also elevates PSA.
Customary cutpoint. An abnormal serum PSA level is commonly regarded as 4 ng/mL or greater. At this cutpoint, most studies report sensitivity for cancer of around 70%, with more variability in specificity (TABLE 1). Again, in most studies that report sensitivity and specificity, men with a PSA level less than 4 ng/mL did not undergo prostate biopsy; therefore, the number of false negatives and true negatives are only estimates.
In nested case-control studies of longitudinal cohorts, eligible cases were defined by the length of time between an abnormal PSA result and prostate cancer diagnosis, while controls were men who were not diagnosed with prostate cancer during the same time period (however, a biopsy was not usually performed). The PPV of an abnormal PSA level is estimated at 25% to 37%. As a comparison, the PPV of a positive mammogram finding for women 50 to 59 years is 4% to 9%, and 10% to 19% for women age 60 to 69.21
Suggested strategies to improve PSA accuracy. Approximately 70% of men with an elevated serum PSA level do not have cancer. To decrease the number of unnecessary biopsies, experts have suggested several strategies:
- using DRE with PSA
- calculating a ratio of free PSA (unbound to protein) or complexed PSA (bound to protein) to total PSA
- measuring PSA density, which incorporates the volume of the prostate gland and is subject to observer variability in prostate volume measurement
- recording PSA velocity, which is the annual rate of change in PSA level and requires 3 or more measurements.
Revise the cutpoint? The cutpoint of 4 ng/mL has been deemed too high by some clinicians. A recent report of men enrolled in a large prostate cancer prevention trial found that among the 9459 men receiving placebo, 2950 had a PSA level of 4 ng/mL or less and had normal prostates on DRE (LOE: 1b).22 All 2950 underwent a prostate biopsy; 449 (15.2%) were positive for cancer. Nearly 30% of those had a PSA level of 2 ng/mL or less.
Approximately 25% of tumors in men referred for biopsy because of abnormal PSA or DRE findings are thought to be discovered by chance due to the biopsy procedure and the high prevalence of disease (LOE: 2c).23 Furthermore, a recent study indicates that annual PSA fluctuation is substantial; 45% of men who initially had a value of 4 ng/mL or greater, subsequently had a normal level (LOE: 2b).24 Isolated abnormal levels should be confirmed before referral for biopsy.
Identifying clinically relevant cancers
The continued controversy around PSA screening relates to the potential over-diagnosis of localized prostate cancers that are not likely to become clinically significant. The ideal screening test would predictably identify cancers likely to progress.
Screening over-detects
Several computer simulations have estimated the amount of over-detection of prostate cancer. The National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry data demonstrated that the proportion of prostate cancer found through PSA testing that otherwise would not have been diagnosed in the patient’s lifetime was 29% for white men and 44% for black men (LOE: 2c).25
An Italian study found that the proportional excess of cancers detected by screening over those that would have been expected in the absence of screening is greater than 50% (LOE: 2c).26
Finally, a model based on results from the European randomized study of prostate cancer screening estimated that a screening program with a 4-year interval from age 55 to 67 has an over-detection rate of 48% (cancers that would not have been diagnosed in the absence of screening) (LOE: 2c).27 Additionally, the authors determined that such screening might advance prostate cancer diagnosis by at least 10 years. Previous estimates of diagnosis lead time have been in the 5- to 7-year range (LOE: 2b).28 These findings indicate that screening intervals of 2 to 4 years are unlikely to cause a loss of sensitivity.
Spread of tumor
Spread of the tumor beyond the prostate capsule is a poor prognostic sign.29 Unfortunately, this occurrence is often not known until surgery, and the result usually is an “up-staging” between clinical diagnosis and pathological diagnosis. Nevertheless, most cancers (86%) diagnosed between 1992 and 1999 were localized to the prostate.6 Because these cancers have not spread beyond the prostate at the time of diagnosis, they are more likely to be curable. They are also more likely to represent tumors that may grow so slowly that the host will die of something other than prostate cancer.
Gleason score
Gleason score is another predictor of cancers destined to become clinically relevant (FIGURE 1). A Gleason score of 7 or greater denotes moderate to poor cellular differentiation and indicates a greater potential for progression than lower Gleason values.29 A recent long-term follow-up report on a cohort of men with localized cancer treated conservatively demonstrated that men with low-grade tumors (Gleason score 2–4) have a minimal risk of dying from prostate cancer after 20 years, while men with high-grade tumors (Gleason score of 8–10) have high probability of prostate cancer death within 10 years of diagnosis (LOE: 2b).30 Ecological31 and clinical studies32 indicate that a substantial proportion of PSA-detected cancers are moderately differentiated. This is especially true in a first round of screening; as in the European randomized study of screening, where 36% of cancers were Gleason score 7 or higher (LOE: 1b).33
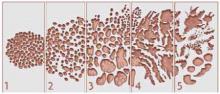
FIGURE 1
Calculating the Gleason score
The Gleason score is based on the level of differentiation and growth pattern of prostate cancer cells. Cancer cells that closely resemble the normal prostate cells when viewed under low-power magnification are well differentiated. Cancer cells that do not retain the structure of the surrounding normal cells are poorly differentiated. Scores range from 1 to 5.
In examining histologic samples of a patient’s prostate tissue, the pathologist will identify the 2 most commonly occurring patterns (types of differentiation) among the cancer cells and assign a numerical value to each pattern. The 2 numbers are then added to yield the final Gleason score. If a single pattern dominates, the pathologist will simply double the corresponding value.
Total scores range from 2 to 10. Scores in the range of 2–4 are considered well-differentiated, 5–7 are moderately differentiated, and 8–10 are poorly differentiated. In general, the higher the score, the worse the prognosis. Men with well-differentiated tumors that are treated conservatively have minimal risk of dying from prostate cancer.
Is declining mortality a sign of screening success?
Prostate cancer mortality has been declining since the mid-1990s in numerous parts of the world; the US,6,34 Canada,35 Australia,36 and the United Kingdom37 have all reported a reduction in the rate of prostate cancer deaths. Advocates of PSA screening point to this trend as evidence of the effectiveness of screening. But such ecological data are difficult to interpret. For instance, although much less PSA screening is performed in the UK, mortality trends are similar to those in the US where PSA testing has been used more widely.38
Aggressive screening not necessarily the reason. In the US, 2 geographic areas—Seattle, Washington and Connecticut—provided a natural experiment to compare the effect of aggressive screening on prostate cancer mortality (LOE: 2c).39 Although more aggressive screening and treatment took place in the Seattle area, prostate cancer mortality rates were similar to those in Connecticut over 11 years of follow-up. Similarly, in a study in British Columbia, prostate cancer mortality from 1985 to 1999 was not associated with the intensity of PSA screening (LOE: 2c).40
Other possible explanations. If the mortality decrease is not related to PSA screening, what could cause it? One explanation is “attribution bias.” Death certificate misattribution of cause of death from prostate cancer may partially explain the pattern of increasing, then decreasing mortality rates (LOE: 2c).41 Improvement in prostate cancer treatment, especially for advanced stage, and in particular hormone therapy, is another possible explanation for the decreasing prostate cancer mortality (LOE: 2c).14,42
Benefits of screening
The benefit of any effective screening test is a decrease in the risk of the screened-disease mortality. The best way to demonstrate decreased risk is through a randomized controlled study of the screening test, and 2 such trials are underway for prostate cancer. In the meantime, a decision model estimates that aggressive treatment of organ-confined disease potentially adds 3 years of life for men in their fifties, 1.5 years for men in their sixties, and 0.4 years for men in their seventies (LOE: 2c).3
Others have concluded that 25 men with clinically detected prostate cancer would need to be treated with surgery to prevent 1 prostate cancer death during a 6-year period, without evidence that quality of life is improved (LOE: 2c).43
Consider quality of life. With uncertainty surrounding improvement in the quantity of life as a result of prostate cancer screening, improved quality of life may be an issue for patients. Focus group research has demonstrated that some patients believe it is better to know if a cancer is present than to wonder if it will be diagnosed when it is too late for cure.44
General quality of life has been found to be similar among men treated for prostate cancer and age-matched controls without prostate cancer; however, urinary, sexual, and bowel function vary substantially between treated and untreated men and by treatment type (LOE: 3b) (TABLE 2).45,46 In general, men treated with radical prostatectomy and brachytherapy often report better general quality of life than men who undergo radiation treatment, despite having more urinary and sexual problems (LOE: 2b).47,48
TABLE 2
Estimates of risk associated with specific prostate cancer treatments 12 months or more after treatment
| TREATMENT OUTCOMES | RADICAL PROSTATECTOMY (%) | EXTERNAL BEAM RADIATION (%) | BRACHY-THERAPY* (%) | ANDROGEN DEPRIVATION THERAPY (%) | UNTREATED (%) |
|---|---|---|---|---|---|
| Death within 2 months of treatment | 0.5–0.7 | 0.2–0.5 | 0.2–0.5 | ||
| Urinary problems: | |||||
| Incontinence | 10–50 | 2–16 | 6–16 | ||
| Wearing pads | 5–32 | 2–12 | 2–16 | ||
| Urinary bother† | 4–20 | 3–15 | 3–16 | ||
| Sexual problems: | |||||
| Impotence | 50–80‡ | 30–60 | 20–60** | 70–92 | 20–50 |
| Sexual bother | 10–40 | 10–30 | 10–18 | 25–38 | 10–32 |
| Bowel problems: | |||||
| Bowel problems§ | 9–15 | 6–35 | 4–20 | ||
| Loose stools/diarrhea | 15–21 | 6–37 | 4–10 | ||
| Bowel bother | 1–3 | 4–12 | 2–10 | ||
| Other symptoms | Breast swelling: 5–25 | ||||
| Hot flashes: 50–60 | |||||
| * Fewer studies on brachytherapy are available, especially those with long-term follow-up; therefore, these findings are less certain than other entries. | |||||
| ‡ Includes nerve-sparing prostatectomy. | |||||
| † EBRT and brachytherapy patients are more likely to experience irritative voiding symptoms (i.e. dysuria, urgency and hesitancy and noctoria), while RP patients are more likely to experience incontinence. | |||||
| ** Impotence risk gradually increases with time after treatment. | |||||
| § Includes symptoms such as painful bowel movement and urgency | |||||
| Sources:references 14, 50–53, 65–71. | |||||
Harms of screening
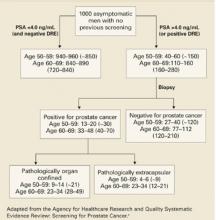
The chances of undergoing a biopsy based on an abnormal screening PSA are estimated at 15% to 40% depending on the patient’s age (FIGURE 2).3 There are adverse effects associated with transrectal biopsy of the prostate. In 2 large population-basedstudies of screening, the most frequent complications were hematuria and hematospermia (LOE: 1b, 2b) (TABLE 3), with more serious consequences such as sepsis and hospitalization occurring in fewer than 1% of patients. A study of 100 screened men with an abnormal PSA who underwent prostate biopsy found that although 69% felt moderate to severe pain with the biopsy, 80% would be willing to undergo a repeat biopsy (LOE: 1b).49
Treatment options. If the biopsy result is positive, the most common treatment options for localized cancer—which represents over 80% of all prostate cancers diagnosed6—include radical prostatectomy, external beam radiation therapy, brachytherapy (internal radiation therapy) or expectant management (watchful waiting). Population-based studies have reported outcomes for these treatment options (TABLE 2). Outcomes derived from hospital-based series of other prostate cancer treatments, such as cryotherapy and 3-dimensional radiation, are available, but the estimates often reflect the experience of only a few hospitals and are not representative of other facilities. Androgen ablation is the standard treatment for metastatic prostate cancer.
Untoward effects of treatment. Approximately 60% of radical prostatectomy patients report some incontinence 12 months or more after surgery (LOE: 2b),50,51 and about 30% of patients need to wear pads for urine leakage (LOE: 2b).50-53 Men undergoing radiation therapy have less urinary incontinence, but about 30% complain of diarrhea and loose stools (LOE: 2b).51,52 Both therapies are associated with a high percentage of erectile dysfunction: approximately 60% of radiation therapy patients and 75% of surgery patients report their erections are not firm enough for intercourse (LOE: 2b).51,52
Expectant management (following the cancer with regular PSA and ultrasound testing) is sometimes difficult to “sell” to patients whose fear of cancer dictates that the only logical response is to “cut it out.”44 A recent randomized trial indicated that radical prostatectomy lowers prostate cancer mortality, local progression, distant metastasis, and overall survival as compared with watchful waiting over a median of 8.2 years of follow-up (LOE: 1b).54 However, these results may have little relevance to prostate cancer screening since only 5% of the cancers were screen-detected and 76% were palpable.
FIGURE 2
Yield of screening 1000 men for prostate cancer
TABLE 3
Percentage of patients with specific complication of transrectal prostate biopsy
| CONDITION | TYROL STUDY63 | EUROPEAN RANDOMIZED STUDY OF SCREENING 64 |
|---|---|---|
| Gross hematuria >1 day | 12.5% | 22.6% |
| Hematospermia | 29.8% | 50.4% |
| Significant pain | 4.0% | 7.5% |
| Rectal bleeding | 0.6% | 1.3% |
| Nausea | 0.8% | 0.3% |
| Fever >38.5°C | 0.8% | 3.5% |
| Epididymitis | 0.7% | 0.07% |
| Sepsis | 0.3% | Not available |
| Hospitalization | Not available | 0.5% |
| Tyrol study63: LOE: 2b, N=6024 biopsies; ERSS study64: LOE: 1b, N=5802 biopsies. | ||
Recommendations from expert groups
Different expert groups have conflicting recommendations. Both the American Urological Association and the American Cancer Society recommend annual PSA screening starting at age 50 for most men; younger if risk factors are present. Groups that are evidence based tend to recommend a shared decision making process with patients. The AAFP and American College of Physicians advise physicians to counsel men on the known risks and uncertain benefits of screening for prostate cancer. The US Preventive Services Task Force 2002 update concluded that evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA or DRE. The National Cancer Institute cites a lack of evidence to determine a net benefit for PSA or DRE screening.
When will we know more?
Only 1 randomized controlled trial of prostate cancer screening has been completed55: 46,193 men were randomized to either PSA and DRE or no screening from 1989 to 1996. The study had methodological problems; for instance, only 23% of the group randomized to screening was screened. The investigators in the trial have interpreted its results as demonstrating a decrease in prostate cancer deaths in the screened group compared with the unscreened group (15 vs 48.7 per 100,000 man-years).55 Others have criticized the statistical analysis and calculated the results using an “intent to screen” analysis, finding no difference in prostate cancer deaths between the 2 groups.3,56
Two randomized controlled trials of screening are ongoing: the National Cancer Institute’s Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial57 and the European Randomized Study of Screening for Prostate Cancer.58 Both were started in the mid-1990s and will not have results available for a few more years. Also underway is a randomized trial of intervention (radical prostatectomy) versus expectant management, called the Prostate Cancer Intervention Versus Observation Trial (PIVOT).59
Counseling recommendations
However, providing men with information on prostate cancer screening before they discussed it with their family physician, rather than after the visit, resulted in patients having a significantly more active role in making a screening decision, and lower levels of decisional conflict (LOE: 2b).61 Informational pamphlets are available through the AAFP and CDC websites listed in TABLE 4. Additional websites containing prostate cancer screening information are found in TABLE 4. We also provide a bullet item list of key points for discussion with patients (TABLE 5), which can be used along with the balance sheet provided here (TABLE 2).
Shared decision-making is not an easy or quick process. Yet, the majority of patients will benefit from the discussion, regardless of the final decision. Of course, there are instances when a shared decision-making process is well-documented, and still results in an undesirable outcome;62 however, while the evidence for screening remains controversial, patients have the right to know that those controversies exist and why they exist.
TABLE 4
Useful websites for patients to find prostate cancer screening information
| CENTERS FOR DISEASE CONTROL AND PREVENTION |
| www.cdc.gov/cancer/prostate/decisionguide/index.htm |
| 10th grade reading level* |
| Good coverage of screening and treatment controversies |
| Offers downloadable PDF version |
| NATIONAL CANCER INSTITUTE |
| cis.nci.nih.gov/asp/FactSheetPub/AlphaSubList.asp?alpha=47 |
| 10th grade reading level |
| FAQ format |
| Offers Spanish version |
| AMERICAN CANCER SOCIETY |
| www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_ cancer_be_found_early_36.asp |
| 12th grade reading level |
| Lacks discussion of treatment options and their side effects |
| Biased in favor of screening but acknowledges that other distinguished organizations are not |
| AMERICAN UROLOGICAL ASSOCIATION |
| www.urologyhealth.org/adult/index.cfm?cat=09 |
| 12th grade reading level |
| Easy to navigate among screening and specific treatment pages |
| Biased in favor of PSA screening |
| AMERICAN ACADEMY OF FAMILY PHYSICIANS |
| familydoctor.org/healthfacts/361/ |
| 11th grade reading level |
| Question/answer format |
| Very straightforward, lacks depth |
| www.aafp.org/x19519.xml |
| 7th grade reading level |
| Separate information sheet for patients and physicians |
| Presents possible outcomes of PSA test and prostate cancer treatment in easy-to-follow format |
| WEBMD |
| my.webmd.com/medical_information/condition_centers/prostate_cancer/default.htm |
| 9th grade reading level |
| Question/answer format |
| Specifically addresses false negative and positives with current estimates |
| DARTMOUTH CENTER FOR SHARED DECISION MAKING |
| www.dhmc.org/dhmc-internet-upload/file_collection/PSA.pdf |
| 6th grade reading level |
| Well-designed, simple presentation of pros and cons of PSA testing |
| *Fleish-Kincaid grade level score based on average sentence length and average number of syllables per word. |
TABLE 5
Talking points for patients and physicians
| Prostate cancer is an important men’s health problem |
| Screening may prevent early prostate cancer death |
| DRE alone has little value as a screening test |
| Age, prostate size, prostatitis, ejaculation, prostate biopsy, and prostate surgery can cause a falsely elevated PSA test |
| Approximately 70% of men with an elevated serum PSA do not have cancer |
| The percentage of PSA screening false negatives ranges from 10%–22% in large studies |
| If the test is abnormal, a biopsy will be recommended |
| If the biopsy is positive, treatment options will be given |
| Many men experience long-term urinary incontinence and impotence related to their treatment |
CORRESPONDING AUTHOR
Kendra Schwartz, MD, MSPH, 101 E. Alexandrine, Detroit, MI 48201, E-mail: kensch@med.wayne.edu
1. American Academy of Family Physicians. Summary of recommendation for periodic health examinations. August 2002. Available at: www.aafp.org/PreBuilt/PHERev5.30802.pdf. Accessed on June 10, 2005.
2. US Preventive Services Task Force. Screening for Prostate Cancer. 2002. Available at: www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed on June 10, 2005.
3. American College of Physicians. Screening for prostate cancer. Position paper. Clinical Guideline: Part III. Ann Intern Med 1997;126:480-484.
4. Harris R, Lohr K, Beck R, Fink K, Godley P, Bunton A. Screening for prostate cancer. Systematic Evidence Review for AHRQ. Available at: www.ahrq.gov/uspstfix.htm. Accessed on June 10, 2005.
5. American Cancer Society. Cancer statistics 2005. CA Cancer J Clin 2005;55:10-30.
6. Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. Available at seer.cancer.gov/csr/1975_2002/. Accessed on June 10, 2005.
7. DevCan: Probability of dying of cancer [computer program]. Version 5.1. Bethesda, Md: National Cancer Instititute; 2003. Available at: srab.cancer.gov/devcan/. Accessed on June 10, 2005.
8. Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316-1321.
9. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996.
10. Kabalin J, McNeal J, Price H, Freiha F, Stamey T. Unsuspected adenocarcinoma of the prostate in patients undergooing cystoprostatectomy for other causes: incidence, histology and morphometric observations. J Urol 1989;141:1091-1094.
11. Montie J, Wood DJ, Pontes J, Boyett J, Levin H. Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 1989;63:381-385.
12. Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta 2002;315:71-97.
13. Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864.
14. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-929.
15. American Cancer Society. ACS Cancer Detection Guidelines. Available at: www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines _36.asp. Accessed June 10, 2005.
16. Smith D, Catalona W. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 1995;45:70-74.
17. Kishor M, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract 2003;16:95-101.
18. Hoogendam A, Buntix F, deVet HCW. The diagnostic value of digital rectal examination in primary care screening for prostate cancer: a meta-analysis. Fam Pract 1999;16:621-626.
19. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294.
20. Hakama M, Stenman UH, Aromaa A, Leinonen J, Hakulinen T, Knekt I. Validity of the prostate specific antigen test for prostate cancer screening: followup study with a bank of 21,000 sera in Finland. J Urol 2001;166:2189-2191.
21. Humphrey L, Helfand M, Chan B, Woolf S. Breast cancer screening: summary of the evidence. Ann Intern Med 2002;137:344-346.
22. Thompson I, Pauler D, Goodman P, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4 ng per milliliter. N Engl J Med 2004;350:2239-2246.
23. Collins MM, Ransohoff D, MJ Barry. Early detection of prostate cancer-serendipity strikes again. JAMA 1997;278:1516-1519.
24. Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA 2003;289:2695-2700.
25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-990.
26. Zappa M, Ciatto S, Bonardi R, Mazzotta A. Overdiagnosis of prostate carcinoma by screening: an estimate based on the results of the Florence Screening Pilot Study. Ann Oncol 1998;9:1297-1300.
27. Draisma G, Boer R, Otto S, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst 2003;95:868-878.
28. Auvin A, Maattanen L, Stenman UH, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279-285.
29. Gleason D, Mellinger G. Group at VACUR. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
30. Albertson PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-2101.
31. Schwartz K, Grignon D, Sakr W, Wood DJ. Prostate cancer histologic trends in the metropolitan Detroit are, 1982 to 1996. Urology 1999;53:769-774.
32. Smith D, Catalona W. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol 1994;152:1732-1736.
33. Hoedemaeker RF, van der Kwast T, Boer R, et al. Pathological features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001;93:1153-1158.
34. Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 2003;97:1507-1516.
35. National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: National Cancer Institute of Canada; 2001. Available at: www.ncic.cancer.ca. Accessed June 10, 2005.
36. Coory M, Baade P. Mortality from prostate cancer is decreasing. Med J Aust 2002;176:345-345.
37. Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales, 1971–1998. BJU Int 2000;85:1058-1062.
38. Oliver S, Gunnell D, Donovan J. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-1789.
39. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002;325:740.-
40. Coldman A, Phillips N, Pickles T. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003;168:31-35.
41. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-1032.
42. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003;361:1122-1128.
43. Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-693.
44. McFall SL, Hamm RM. Interpretation of prostate cancer screening events and outcomes: a focus group study. Patient Educ Couns 2003;49:207-218.
45. Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-135.
46. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep 2003;4:185-195.
47. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in the health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology 1999;53:180-186.
48. Bacon C, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804-1810.
49. Makinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology 2002;60:846-850.
50. Fowler FJ, Jr, Barry MJ, Lu-Yao G, Roman A, Wasson J, Wennberg JE. Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 1993;42:622-629.
51. Schwartz K, Bunner S, Bearer R, Severson RK. Complications from treatment for prostate carcinoma among men in the Detroit area. Cancer 2002;95:82-89.
52. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582-1592.
53. Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology 2002;60:1055-1058.
54. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-1184.
55. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38:83-91.
56. Alexander FE, Prescott RJ. Reply to Labrie et al. Results of the mortality analysis of the Quebec randomized controlled trial (RCT). Prostate 1999;40:135-137.
57. Prorok P, Andriole G, Bresalier R, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S.
58. Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80:1830-1834.
59. Wilt TJ, Brawer M. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol 1994;152:1910-1914.
60. Schapira M, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract 2000;49:418-424.
61. Davison B, Kirk P, Degner L, Hassard T. Information and patient participation in screening for prostate cancer. Patient Educ Couns 1999;37:255-263.
62. Merenstein D. Winners and losers. JAMA 2004;291:15-16.
63. Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Current Urol Reports 2004;5:220-225.
64. Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-830.
65. Fowler F, Barry MJ, Lu-Yao G, Wasson J, Bin L. Outcomes of external beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology, and End Results areas. J Clin Oncol 1996;14:2258-2265.
66. Hollenbeck BK, Dunn RL, Wei JT, Sandler HM, Sanda MG. Sexual health recovery after prostatectomy, external radiation, or brachytherapy for early stage prostate cancer. Curr Urol Rep 2004;5:212-219.
67. Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol 2001;19:3750-3757.
68. Lee R, Penson DF. Treatment outcomes in localized prostate cancer: a patient-oriented approach. Semin Urol Oncol 2002;20:63-73.
69. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-360.
70. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063-1068.
71. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med 1997;126:468-479.
- Engage patients in shared decision making by discussing the benefits and risks of prostate cancer screening. Patients who review educational pamphlets before an office visit engage more fully in the decision-making process. (B)
- If performing prostate cancer screening, limit to men with greater than 10 years life expectancy. (B)
- Because the lead time of a diagnosis based on PSA screening is estimated to be 5 to 7 years, PSA screening every other year is unlikely to cause a loss of sensitivity. (B)
- Men with tumors with a Gleason score less than 5 are the best candidates for “watchful waiting,” having a favorable 20-year survival. (B)
Prostate cancer screening in asymptomatic men remains controversial, and it is difficult to present its benefits and risks quickly in a way that is understandable to patients. Yet many expert groups agree that physicians should enter into a mutual decision-making process with patients.1-3
This article reviews the latest information relevant to the controversy, offers “talking points” for family physicians to use when discussing screening with patients, and lists websites that patients may find helpful when making a decision about prostate cancer screening.
For this review, we searched for recent articles that are generalizable to a primary care population and of the highest evidence level available. We preferentially discuss population-based studies, studies from randomized trials of screening, and meta-analyses, rather than results that are hospital- or clinic-based. For a complete systematic review of this topic (from 2002), readers are referred to one conducted for the Agency for Healthcare Research and Quality.4 (See Scope of the problem).
Adenocarcinoma of the prostate is a significant public health burden. Age, family history, and race are the only known risk factors. Most cancers (86%) are diagnosed while still confined to the prostate; however, invasion beyond the capsule is sometimes not apparent until surgery.
Incidence. In 2005, there will be approximately 232,090 new cases of prostate cancer.5 American men have a 17% chance of being diagnosed with prostate cancer; African Americans have a 65% greater risk of developing prostate cancer than Caucasians.6 In fact, African Americans have the highest prostate cancer annual age-adjusted incidence rates in the world: 272/100,000 compared with 164/100,000 for Caucasian Americans.6 The rate for US Asian/Pacific Islander and Hispanic men is less than that for Caucasian Americans.
Mortality. There will be approximately 30,350 prostate cancer deaths in 2004.5 There is 3% chance of dying from prostate cancer; however, the risk of death is about 55% higher for African American men than Caucasian American men.6
Risk factors other than race. Risk of prostate cancer diagnosis increases with age: 1 in 48 men aged 40 to 59 years will be diagnosed with prostate cancer, while 1 in 8 men aged 60 to 79 years are at risk.7 A man who has a first-degree relative with prostate cancer is 2.4 times as likely to be diagnosed with prostate cancer as a man with no affected relatives.8
Key components of the controversy
How effective is screening?
A good screening test does 2 things. First, it detects a disease earlier than it would be detected with no screening at all, and it does so with sufficient accuracy to avoid a large number of false-positive and false-negative results.
Second, it leads to treatment of early disease that will likely produce a more favorable health outcome than waiting to treat patients who have signs and symptoms of disease.9 Unfortunately it is still unclear whether screening tests for prostate cancer meet these 2 criteria.
Skewed numbers. Yes, estimates of false-positive and false-negative results are available from numerous studies of different populations. However, in most studies, only men with abnormal test results receive a biopsy. Men with normal screening test results are not biopsied. Therefore the number of false negatives (and true negatives) is unknown. Furthermore, these estimates are often based on patients from urology clinics, a group more likely to have disease, thereby increasing the positive predictive value of a given screening test.
Whether current screening methods—in particular, prostate-specific antigen (PSA)—identify prostate cancers destined to become clinically relevant is also unknown. If screening does identify such cancers, a decrease in prostate cancer mortality among men who were screened is expected. If it does not, overdiagnosis and treatment of clinically insignificant cancers will negatively impact the quality of men’s lives without extending their life spans.
Clinical variability of the disease
The natural history of prostate cancer is uncertain. If cancer is left unidentified or untreated, more men will die with prostate cancer than of prostate cancer.
Clinically, prostate cancer ranges from an asymptomatic slow-growing tumor to an aggressive cancer with painful metastases. Treatment may be unnecessary at one end of the spectrum and palliative at the other.
The goal of screening is to identify slow-growing tumors destined to extend beyond the prostate while they are confined to the prostate and amenable to treatment, thereby decreasing the risk of prostate cancer morbidity and death.
Usually low morbidity. For a 50-year-old man, the risk of being diagnosed with prostate cancer by age 80 years is 15%; however, the same man has a 1.4% chance of dying of prostate cancer over that 30-year period.7 This 10-fold difference shows that prostate cancer usually is not a fatal illness. Another indication of the often benign nature of the disease is the high percentage of prostate cancers identified at surgery for bladder cancer; up to 40% of specimens contain unsuspected prostate cancer (level of evidence [LOE]: 2c).10,11
Most clinical diagnoses (80%) and prostate cancer deaths (90%) occur among men older than 65 years;6 the median age at diagnosis is 72 years.12 More than 75% of men older than 85 years will have histological prostate cancer (LOE: 2c).13 Many men live with their disease for more than 10 years, but do not die of it (LOE: 2c).12 Additionally, a review of several decision analyses indicates that men 75 years of age and older are not likely to benefit from screening and aggressive treatment (LOE: 2a).14
Hence the recommendation: if performing prostate cancer screening, limit to men with greater than 10 years life expectancy.15
Details of screening tests
Digital rectal examination insufficient
Digital rectal examination (DRE)—palpating the prostate gland to determine size and consistency—is one screening tool for prostate cancer, usually performed in conjunction with PSA testing.15 It is not a difficult or expensive test, but its reproducibility is only fair, even among experienced urologists (LOE: 2b).16
The sensitivity and specificity of DRE can only be estimated because men with a normal finding on DRE are not routinely biopsied in any studies (TABLE 1).17,18 One of two pertinent meta-analyses18 included studies that followed men with a negative DRE finding for development of prostate cancer (LOE: 2a). Both meta-analyses of DRE as a screening tool found that the included studies were heterogeneous in their study populations and definition of abnormal DRE test result (LOE: 2a). The positive predictive value (PPV) of DRE was 28% in one meta-analysis and 18% in the other.
TABLE 1
Characteristics of screening tests for prostate cancer
| TEST/SOURCE | DESCRIPTION (LEVEL OF EVIDENCE) | SN ESTIMATE (%) | SP ESTIMATE (%) | LIKELIHOOD RATIO | PPV (%) |
|---|---|---|---|---|---|
| Abnormal DRE18 | Meta-analysis of studies that performed biopsies for abnormal DRE (2a) | 59 (51%–67%) | 94 (91%–96%) | + 9.8 – 0.4 | 28 (20–36) |
| Abnormal DRE17 | Meta-analysis of studies that performed biopsies for abnormal DRE or PSA (2a) | 53.2 | 83.6 | + 3.24 – 0.56 | 17.8 |
| PSA >4 ng/mL12 | Review of multiple cohort studies with diverse populations, not systematic | Mean = 71 | Mean = 75 | + 2.8 – 0.30 | 37 |
| PSA >4 ng/mL19 | Longitudinal retrospective study of prostate cancers that developed within 2 years of PSA test (3b) | 73.2 | 85.4 | + 5.0 – 0.2 | |
| PSA >4 ng/mL20 | Nested case-control study of prostate cancers that developed within 5 years of PSA test in a cohort study (3b) | 86 | 94 | + 14.3 – 0.15 | |
| PSA > 4 ng/mL17 | Meta-analysis of studies that performed biopsies for abnormal PSA or DRE (2a) | 72.1 | 93.2 | + 10.2 – 0.30 | 25.1 |
| Sn, sensitivity; Sp, specificity; PPV, positive predictive value; | |||||
| PSA, prostate-specific antigen; DRE, digital rectal examination. | |||||
Prostate-specific antigen: Improving its clinical usefulness
Prostate-specific antigen (PSA), first detected in serum in 1979, is a protein produced by prostate epithelial cells. It was originally used to follow men treated for prostate cancer for evidence of recurrence. In the late 1980s, it became widely used in the US to screen for prostate cancer.
An elevated PSA level is suggestive but not diagnostic of prostate cancer. Elevated levels also occur with advancing age, increased prostate size, and prostatitis, and following ejaculation. Prostate manipulation such as biopsy and surgery (but not digital examination) also elevates PSA.
Customary cutpoint. An abnormal serum PSA level is commonly regarded as 4 ng/mL or greater. At this cutpoint, most studies report sensitivity for cancer of around 70%, with more variability in specificity (TABLE 1). Again, in most studies that report sensitivity and specificity, men with a PSA level less than 4 ng/mL did not undergo prostate biopsy; therefore, the number of false negatives and true negatives are only estimates.
In nested case-control studies of longitudinal cohorts, eligible cases were defined by the length of time between an abnormal PSA result and prostate cancer diagnosis, while controls were men who were not diagnosed with prostate cancer during the same time period (however, a biopsy was not usually performed). The PPV of an abnormal PSA level is estimated at 25% to 37%. As a comparison, the PPV of a positive mammogram finding for women 50 to 59 years is 4% to 9%, and 10% to 19% for women age 60 to 69.21
Suggested strategies to improve PSA accuracy. Approximately 70% of men with an elevated serum PSA level do not have cancer. To decrease the number of unnecessary biopsies, experts have suggested several strategies:
- using DRE with PSA
- calculating a ratio of free PSA (unbound to protein) or complexed PSA (bound to protein) to total PSA
- measuring PSA density, which incorporates the volume of the prostate gland and is subject to observer variability in prostate volume measurement
- recording PSA velocity, which is the annual rate of change in PSA level and requires 3 or more measurements.
Revise the cutpoint? The cutpoint of 4 ng/mL has been deemed too high by some clinicians. A recent report of men enrolled in a large prostate cancer prevention trial found that among the 9459 men receiving placebo, 2950 had a PSA level of 4 ng/mL or less and had normal prostates on DRE (LOE: 1b).22 All 2950 underwent a prostate biopsy; 449 (15.2%) were positive for cancer. Nearly 30% of those had a PSA level of 2 ng/mL or less.
Approximately 25% of tumors in men referred for biopsy because of abnormal PSA or DRE findings are thought to be discovered by chance due to the biopsy procedure and the high prevalence of disease (LOE: 2c).23 Furthermore, a recent study indicates that annual PSA fluctuation is substantial; 45% of men who initially had a value of 4 ng/mL or greater, subsequently had a normal level (LOE: 2b).24 Isolated abnormal levels should be confirmed before referral for biopsy.
Identifying clinically relevant cancers
The continued controversy around PSA screening relates to the potential over-diagnosis of localized prostate cancers that are not likely to become clinically significant. The ideal screening test would predictably identify cancers likely to progress.
Screening over-detects
Several computer simulations have estimated the amount of over-detection of prostate cancer. The National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry data demonstrated that the proportion of prostate cancer found through PSA testing that otherwise would not have been diagnosed in the patient’s lifetime was 29% for white men and 44% for black men (LOE: 2c).25
An Italian study found that the proportional excess of cancers detected by screening over those that would have been expected in the absence of screening is greater than 50% (LOE: 2c).26
Finally, a model based on results from the European randomized study of prostate cancer screening estimated that a screening program with a 4-year interval from age 55 to 67 has an over-detection rate of 48% (cancers that would not have been diagnosed in the absence of screening) (LOE: 2c).27 Additionally, the authors determined that such screening might advance prostate cancer diagnosis by at least 10 years. Previous estimates of diagnosis lead time have been in the 5- to 7-year range (LOE: 2b).28 These findings indicate that screening intervals of 2 to 4 years are unlikely to cause a loss of sensitivity.
Spread of tumor
Spread of the tumor beyond the prostate capsule is a poor prognostic sign.29 Unfortunately, this occurrence is often not known until surgery, and the result usually is an “up-staging” between clinical diagnosis and pathological diagnosis. Nevertheless, most cancers (86%) diagnosed between 1992 and 1999 were localized to the prostate.6 Because these cancers have not spread beyond the prostate at the time of diagnosis, they are more likely to be curable. They are also more likely to represent tumors that may grow so slowly that the host will die of something other than prostate cancer.
Gleason score
Gleason score is another predictor of cancers destined to become clinically relevant (FIGURE 1). A Gleason score of 7 or greater denotes moderate to poor cellular differentiation and indicates a greater potential for progression than lower Gleason values.29 A recent long-term follow-up report on a cohort of men with localized cancer treated conservatively demonstrated that men with low-grade tumors (Gleason score 2–4) have a minimal risk of dying from prostate cancer after 20 years, while men with high-grade tumors (Gleason score of 8–10) have high probability of prostate cancer death within 10 years of diagnosis (LOE: 2b).30 Ecological31 and clinical studies32 indicate that a substantial proportion of PSA-detected cancers are moderately differentiated. This is especially true in a first round of screening; as in the European randomized study of screening, where 36% of cancers were Gleason score 7 or higher (LOE: 1b).33
FIGURE 1
Calculating the Gleason score
The Gleason score is based on the level of differentiation and growth pattern of prostate cancer cells. Cancer cells that closely resemble the normal prostate cells when viewed under low-power magnification are well differentiated. Cancer cells that do not retain the structure of the surrounding normal cells are poorly differentiated. Scores range from 1 to 5.
In examining histologic samples of a patient’s prostate tissue, the pathologist will identify the 2 most commonly occurring patterns (types of differentiation) among the cancer cells and assign a numerical value to each pattern. The 2 numbers are then added to yield the final Gleason score. If a single pattern dominates, the pathologist will simply double the corresponding value.
Total scores range from 2 to 10. Scores in the range of 2–4 are considered well-differentiated, 5–7 are moderately differentiated, and 8–10 are poorly differentiated. In general, the higher the score, the worse the prognosis. Men with well-differentiated tumors that are treated conservatively have minimal risk of dying from prostate cancer.
Is declining mortality a sign of screening success?
Prostate cancer mortality has been declining since the mid-1990s in numerous parts of the world; the US,6,34 Canada,35 Australia,36 and the United Kingdom37 have all reported a reduction in the rate of prostate cancer deaths. Advocates of PSA screening point to this trend as evidence of the effectiveness of screening. But such ecological data are difficult to interpret. For instance, although much less PSA screening is performed in the UK, mortality trends are similar to those in the US where PSA testing has been used more widely.38
Aggressive screening not necessarily the reason. In the US, 2 geographic areas—Seattle, Washington and Connecticut—provided a natural experiment to compare the effect of aggressive screening on prostate cancer mortality (LOE: 2c).39 Although more aggressive screening and treatment took place in the Seattle area, prostate cancer mortality rates were similar to those in Connecticut over 11 years of follow-up. Similarly, in a study in British Columbia, prostate cancer mortality from 1985 to 1999 was not associated with the intensity of PSA screening (LOE: 2c).40
Other possible explanations. If the mortality decrease is not related to PSA screening, what could cause it? One explanation is “attribution bias.” Death certificate misattribution of cause of death from prostate cancer may partially explain the pattern of increasing, then decreasing mortality rates (LOE: 2c).41 Improvement in prostate cancer treatment, especially for advanced stage, and in particular hormone therapy, is another possible explanation for the decreasing prostate cancer mortality (LOE: 2c).14,42
Benefits of screening
The benefit of any effective screening test is a decrease in the risk of the screened-disease mortality. The best way to demonstrate decreased risk is through a randomized controlled study of the screening test, and 2 such trials are underway for prostate cancer. In the meantime, a decision model estimates that aggressive treatment of organ-confined disease potentially adds 3 years of life for men in their fifties, 1.5 years for men in their sixties, and 0.4 years for men in their seventies (LOE: 2c).3
Others have concluded that 25 men with clinically detected prostate cancer would need to be treated with surgery to prevent 1 prostate cancer death during a 6-year period, without evidence that quality of life is improved (LOE: 2c).43
Consider quality of life. With uncertainty surrounding improvement in the quantity of life as a result of prostate cancer screening, improved quality of life may be an issue for patients. Focus group research has demonstrated that some patients believe it is better to know if a cancer is present than to wonder if it will be diagnosed when it is too late for cure.44
General quality of life has been found to be similar among men treated for prostate cancer and age-matched controls without prostate cancer; however, urinary, sexual, and bowel function vary substantially between treated and untreated men and by treatment type (LOE: 3b) (TABLE 2).45,46 In general, men treated with radical prostatectomy and brachytherapy often report better general quality of life than men who undergo radiation treatment, despite having more urinary and sexual problems (LOE: 2b).47,48
TABLE 2
Estimates of risk associated with specific prostate cancer treatments 12 months or more after treatment
| TREATMENT OUTCOMES | RADICAL PROSTATECTOMY (%) | EXTERNAL BEAM RADIATION (%) | BRACHY-THERAPY* (%) | ANDROGEN DEPRIVATION THERAPY (%) | UNTREATED (%) |
|---|---|---|---|---|---|
| Death within 2 months of treatment | 0.5–0.7 | 0.2–0.5 | 0.2–0.5 | ||
| Urinary problems: | |||||
| Incontinence | 10–50 | 2–16 | 6–16 | ||
| Wearing pads | 5–32 | 2–12 | 2–16 | ||
| Urinary bother† | 4–20 | 3–15 | 3–16 | ||
| Sexual problems: | |||||
| Impotence | 50–80‡ | 30–60 | 20–60** | 70–92 | 20–50 |
| Sexual bother | 10–40 | 10–30 | 10–18 | 25–38 | 10–32 |
| Bowel problems: | |||||
| Bowel problems§ | 9–15 | 6–35 | 4–20 | ||
| Loose stools/diarrhea | 15–21 | 6–37 | 4–10 | ||
| Bowel bother | 1–3 | 4–12 | 2–10 | ||
| Other symptoms | Breast swelling: 5–25 | ||||
| Hot flashes: 50–60 | |||||
| * Fewer studies on brachytherapy are available, especially those with long-term follow-up; therefore, these findings are less certain than other entries. | |||||
| ‡ Includes nerve-sparing prostatectomy. | |||||
| † EBRT and brachytherapy patients are more likely to experience irritative voiding symptoms (i.e. dysuria, urgency and hesitancy and noctoria), while RP patients are more likely to experience incontinence. | |||||
| ** Impotence risk gradually increases with time after treatment. | |||||
| § Includes symptoms such as painful bowel movement and urgency | |||||
| Sources:references 14, 50–53, 65–71. | |||||
Harms of screening
The chances of undergoing a biopsy based on an abnormal screening PSA are estimated at 15% to 40% depending on the patient’s age (FIGURE 2).3 There are adverse effects associated with transrectal biopsy of the prostate. In 2 large population-basedstudies of screening, the most frequent complications were hematuria and hematospermia (LOE: 1b, 2b) (TABLE 3), with more serious consequences such as sepsis and hospitalization occurring in fewer than 1% of patients. A study of 100 screened men with an abnormal PSA who underwent prostate biopsy found that although 69% felt moderate to severe pain with the biopsy, 80% would be willing to undergo a repeat biopsy (LOE: 1b).49
Treatment options. If the biopsy result is positive, the most common treatment options for localized cancer—which represents over 80% of all prostate cancers diagnosed6—include radical prostatectomy, external beam radiation therapy, brachytherapy (internal radiation therapy) or expectant management (watchful waiting). Population-based studies have reported outcomes for these treatment options (TABLE 2). Outcomes derived from hospital-based series of other prostate cancer treatments, such as cryotherapy and 3-dimensional radiation, are available, but the estimates often reflect the experience of only a few hospitals and are not representative of other facilities. Androgen ablation is the standard treatment for metastatic prostate cancer.
Untoward effects of treatment. Approximately 60% of radical prostatectomy patients report some incontinence 12 months or more after surgery (LOE: 2b),50,51 and about 30% of patients need to wear pads for urine leakage (LOE: 2b).50-53 Men undergoing radiation therapy have less urinary incontinence, but about 30% complain of diarrhea and loose stools (LOE: 2b).51,52 Both therapies are associated with a high percentage of erectile dysfunction: approximately 60% of radiation therapy patients and 75% of surgery patients report their erections are not firm enough for intercourse (LOE: 2b).51,52
Expectant management (following the cancer with regular PSA and ultrasound testing) is sometimes difficult to “sell” to patients whose fear of cancer dictates that the only logical response is to “cut it out.”44 A recent randomized trial indicated that radical prostatectomy lowers prostate cancer mortality, local progression, distant metastasis, and overall survival as compared with watchful waiting over a median of 8.2 years of follow-up (LOE: 1b).54 However, these results may have little relevance to prostate cancer screening since only 5% of the cancers were screen-detected and 76% were palpable.
FIGURE 2
Yield of screening 1000 men for prostate cancer
TABLE 3
Percentage of patients with specific complication of transrectal prostate biopsy
| CONDITION | TYROL STUDY63 | EUROPEAN RANDOMIZED STUDY OF SCREENING 64 |
|---|---|---|
| Gross hematuria >1 day | 12.5% | 22.6% |
| Hematospermia | 29.8% | 50.4% |
| Significant pain | 4.0% | 7.5% |
| Rectal bleeding | 0.6% | 1.3% |
| Nausea | 0.8% | 0.3% |
| Fever >38.5°C | 0.8% | 3.5% |
| Epididymitis | 0.7% | 0.07% |
| Sepsis | 0.3% | Not available |
| Hospitalization | Not available | 0.5% |
| Tyrol study63: LOE: 2b, N=6024 biopsies; ERSS study64: LOE: 1b, N=5802 biopsies. | ||
Recommendations from expert groups
Different expert groups have conflicting recommendations. Both the American Urological Association and the American Cancer Society recommend annual PSA screening starting at age 50 for most men; younger if risk factors are present. Groups that are evidence based tend to recommend a shared decision making process with patients. The AAFP and American College of Physicians advise physicians to counsel men on the known risks and uncertain benefits of screening for prostate cancer. The US Preventive Services Task Force 2002 update concluded that evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA or DRE. The National Cancer Institute cites a lack of evidence to determine a net benefit for PSA or DRE screening.
When will we know more?
Only 1 randomized controlled trial of prostate cancer screening has been completed55: 46,193 men were randomized to either PSA and DRE or no screening from 1989 to 1996. The study had methodological problems; for instance, only 23% of the group randomized to screening was screened. The investigators in the trial have interpreted its results as demonstrating a decrease in prostate cancer deaths in the screened group compared with the unscreened group (15 vs 48.7 per 100,000 man-years).55 Others have criticized the statistical analysis and calculated the results using an “intent to screen” analysis, finding no difference in prostate cancer deaths between the 2 groups.3,56
Two randomized controlled trials of screening are ongoing: the National Cancer Institute’s Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial57 and the European Randomized Study of Screening for Prostate Cancer.58 Both were started in the mid-1990s and will not have results available for a few more years. Also underway is a randomized trial of intervention (radical prostatectomy) versus expectant management, called the Prostate Cancer Intervention Versus Observation Trial (PIVOT).59
Counseling recommendations
However, providing men with information on prostate cancer screening before they discussed it with their family physician, rather than after the visit, resulted in patients having a significantly more active role in making a screening decision, and lower levels of decisional conflict (LOE: 2b).61 Informational pamphlets are available through the AAFP and CDC websites listed in TABLE 4. Additional websites containing prostate cancer screening information are found in TABLE 4. We also provide a bullet item list of key points for discussion with patients (TABLE 5), which can be used along with the balance sheet provided here (TABLE 2).
Shared decision-making is not an easy or quick process. Yet, the majority of patients will benefit from the discussion, regardless of the final decision. Of course, there are instances when a shared decision-making process is well-documented, and still results in an undesirable outcome;62 however, while the evidence for screening remains controversial, patients have the right to know that those controversies exist and why they exist.
TABLE 4
Useful websites for patients to find prostate cancer screening information
| CENTERS FOR DISEASE CONTROL AND PREVENTION |
| www.cdc.gov/cancer/prostate/decisionguide/index.htm |
| 10th grade reading level* |
| Good coverage of screening and treatment controversies |
| Offers downloadable PDF version |
| NATIONAL CANCER INSTITUTE |
| cis.nci.nih.gov/asp/FactSheetPub/AlphaSubList.asp?alpha=47 |
| 10th grade reading level |
| FAQ format |
| Offers Spanish version |
| AMERICAN CANCER SOCIETY |
| www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_ cancer_be_found_early_36.asp |
| 12th grade reading level |
| Lacks discussion of treatment options and their side effects |
| Biased in favor of screening but acknowledges that other distinguished organizations are not |
| AMERICAN UROLOGICAL ASSOCIATION |
| www.urologyhealth.org/adult/index.cfm?cat=09 |
| 12th grade reading level |
| Easy to navigate among screening and specific treatment pages |
| Biased in favor of PSA screening |
| AMERICAN ACADEMY OF FAMILY PHYSICIANS |
| familydoctor.org/healthfacts/361/ |
| 11th grade reading level |
| Question/answer format |
| Very straightforward, lacks depth |
| www.aafp.org/x19519.xml |
| 7th grade reading level |
| Separate information sheet for patients and physicians |
| Presents possible outcomes of PSA test and prostate cancer treatment in easy-to-follow format |
| WEBMD |
| my.webmd.com/medical_information/condition_centers/prostate_cancer/default.htm |
| 9th grade reading level |
| Question/answer format |
| Specifically addresses false negative and positives with current estimates |
| DARTMOUTH CENTER FOR SHARED DECISION MAKING |
| www.dhmc.org/dhmc-internet-upload/file_collection/PSA.pdf |
| 6th grade reading level |
| Well-designed, simple presentation of pros and cons of PSA testing |
| *Fleish-Kincaid grade level score based on average sentence length and average number of syllables per word. |
TABLE 5
Talking points for patients and physicians
| Prostate cancer is an important men’s health problem |
| Screening may prevent early prostate cancer death |
| DRE alone has little value as a screening test |
| Age, prostate size, prostatitis, ejaculation, prostate biopsy, and prostate surgery can cause a falsely elevated PSA test |
| Approximately 70% of men with an elevated serum PSA do not have cancer |
| The percentage of PSA screening false negatives ranges from 10%–22% in large studies |
| If the test is abnormal, a biopsy will be recommended |
| If the biopsy is positive, treatment options will be given |
| Many men experience long-term urinary incontinence and impotence related to their treatment |
CORRESPONDING AUTHOR
Kendra Schwartz, MD, MSPH, 101 E. Alexandrine, Detroit, MI 48201, E-mail: kensch@med.wayne.edu
- Engage patients in shared decision making by discussing the benefits and risks of prostate cancer screening. Patients who review educational pamphlets before an office visit engage more fully in the decision-making process. (B)
- If performing prostate cancer screening, limit to men with greater than 10 years life expectancy. (B)
- Because the lead time of a diagnosis based on PSA screening is estimated to be 5 to 7 years, PSA screening every other year is unlikely to cause a loss of sensitivity. (B)
- Men with tumors with a Gleason score less than 5 are the best candidates for “watchful waiting,” having a favorable 20-year survival. (B)
Prostate cancer screening in asymptomatic men remains controversial, and it is difficult to present its benefits and risks quickly in a way that is understandable to patients. Yet many expert groups agree that physicians should enter into a mutual decision-making process with patients.1-3
This article reviews the latest information relevant to the controversy, offers “talking points” for family physicians to use when discussing screening with patients, and lists websites that patients may find helpful when making a decision about prostate cancer screening.
For this review, we searched for recent articles that are generalizable to a primary care population and of the highest evidence level available. We preferentially discuss population-based studies, studies from randomized trials of screening, and meta-analyses, rather than results that are hospital- or clinic-based. For a complete systematic review of this topic (from 2002), readers are referred to one conducted for the Agency for Healthcare Research and Quality.4 (See Scope of the problem).
Adenocarcinoma of the prostate is a significant public health burden. Age, family history, and race are the only known risk factors. Most cancers (86%) are diagnosed while still confined to the prostate; however, invasion beyond the capsule is sometimes not apparent until surgery.
Incidence. In 2005, there will be approximately 232,090 new cases of prostate cancer.5 American men have a 17% chance of being diagnosed with prostate cancer; African Americans have a 65% greater risk of developing prostate cancer than Caucasians.6 In fact, African Americans have the highest prostate cancer annual age-adjusted incidence rates in the world: 272/100,000 compared with 164/100,000 for Caucasian Americans.6 The rate for US Asian/Pacific Islander and Hispanic men is less than that for Caucasian Americans.
Mortality. There will be approximately 30,350 prostate cancer deaths in 2004.5 There is 3% chance of dying from prostate cancer; however, the risk of death is about 55% higher for African American men than Caucasian American men.6
Risk factors other than race. Risk of prostate cancer diagnosis increases with age: 1 in 48 men aged 40 to 59 years will be diagnosed with prostate cancer, while 1 in 8 men aged 60 to 79 years are at risk.7 A man who has a first-degree relative with prostate cancer is 2.4 times as likely to be diagnosed with prostate cancer as a man with no affected relatives.8
Key components of the controversy
How effective is screening?
A good screening test does 2 things. First, it detects a disease earlier than it would be detected with no screening at all, and it does so with sufficient accuracy to avoid a large number of false-positive and false-negative results.
Second, it leads to treatment of early disease that will likely produce a more favorable health outcome than waiting to treat patients who have signs and symptoms of disease.9 Unfortunately it is still unclear whether screening tests for prostate cancer meet these 2 criteria.
Skewed numbers. Yes, estimates of false-positive and false-negative results are available from numerous studies of different populations. However, in most studies, only men with abnormal test results receive a biopsy. Men with normal screening test results are not biopsied. Therefore the number of false negatives (and true negatives) is unknown. Furthermore, these estimates are often based on patients from urology clinics, a group more likely to have disease, thereby increasing the positive predictive value of a given screening test.
Whether current screening methods—in particular, prostate-specific antigen (PSA)—identify prostate cancers destined to become clinically relevant is also unknown. If screening does identify such cancers, a decrease in prostate cancer mortality among men who were screened is expected. If it does not, overdiagnosis and treatment of clinically insignificant cancers will negatively impact the quality of men’s lives without extending their life spans.
Clinical variability of the disease
The natural history of prostate cancer is uncertain. If cancer is left unidentified or untreated, more men will die with prostate cancer than of prostate cancer.
Clinically, prostate cancer ranges from an asymptomatic slow-growing tumor to an aggressive cancer with painful metastases. Treatment may be unnecessary at one end of the spectrum and palliative at the other.
The goal of screening is to identify slow-growing tumors destined to extend beyond the prostate while they are confined to the prostate and amenable to treatment, thereby decreasing the risk of prostate cancer morbidity and death.
Usually low morbidity. For a 50-year-old man, the risk of being diagnosed with prostate cancer by age 80 years is 15%; however, the same man has a 1.4% chance of dying of prostate cancer over that 30-year period.7 This 10-fold difference shows that prostate cancer usually is not a fatal illness. Another indication of the often benign nature of the disease is the high percentage of prostate cancers identified at surgery for bladder cancer; up to 40% of specimens contain unsuspected prostate cancer (level of evidence [LOE]: 2c).10,11
Most clinical diagnoses (80%) and prostate cancer deaths (90%) occur among men older than 65 years;6 the median age at diagnosis is 72 years.12 More than 75% of men older than 85 years will have histological prostate cancer (LOE: 2c).13 Many men live with their disease for more than 10 years, but do not die of it (LOE: 2c).12 Additionally, a review of several decision analyses indicates that men 75 years of age and older are not likely to benefit from screening and aggressive treatment (LOE: 2a).14
Hence the recommendation: if performing prostate cancer screening, limit to men with greater than 10 years life expectancy.15
Details of screening tests
Digital rectal examination insufficient
Digital rectal examination (DRE)—palpating the prostate gland to determine size and consistency—is one screening tool for prostate cancer, usually performed in conjunction with PSA testing.15 It is not a difficult or expensive test, but its reproducibility is only fair, even among experienced urologists (LOE: 2b).16
The sensitivity and specificity of DRE can only be estimated because men with a normal finding on DRE are not routinely biopsied in any studies (TABLE 1).17,18 One of two pertinent meta-analyses18 included studies that followed men with a negative DRE finding for development of prostate cancer (LOE: 2a). Both meta-analyses of DRE as a screening tool found that the included studies were heterogeneous in their study populations and definition of abnormal DRE test result (LOE: 2a). The positive predictive value (PPV) of DRE was 28% in one meta-analysis and 18% in the other.
TABLE 1
Characteristics of screening tests for prostate cancer
| TEST/SOURCE | DESCRIPTION (LEVEL OF EVIDENCE) | SN ESTIMATE (%) | SP ESTIMATE (%) | LIKELIHOOD RATIO | PPV (%) |
|---|---|---|---|---|---|
| Abnormal DRE18 | Meta-analysis of studies that performed biopsies for abnormal DRE (2a) | 59 (51%–67%) | 94 (91%–96%) | + 9.8 – 0.4 | 28 (20–36) |
| Abnormal DRE17 | Meta-analysis of studies that performed biopsies for abnormal DRE or PSA (2a) | 53.2 | 83.6 | + 3.24 – 0.56 | 17.8 |
| PSA >4 ng/mL12 | Review of multiple cohort studies with diverse populations, not systematic | Mean = 71 | Mean = 75 | + 2.8 – 0.30 | 37 |
| PSA >4 ng/mL19 | Longitudinal retrospective study of prostate cancers that developed within 2 years of PSA test (3b) | 73.2 | 85.4 | + 5.0 – 0.2 | |
| PSA >4 ng/mL20 | Nested case-control study of prostate cancers that developed within 5 years of PSA test in a cohort study (3b) | 86 | 94 | + 14.3 – 0.15 | |
| PSA > 4 ng/mL17 | Meta-analysis of studies that performed biopsies for abnormal PSA or DRE (2a) | 72.1 | 93.2 | + 10.2 – 0.30 | 25.1 |
| Sn, sensitivity; Sp, specificity; PPV, positive predictive value; | |||||
| PSA, prostate-specific antigen; DRE, digital rectal examination. | |||||
Prostate-specific antigen: Improving its clinical usefulness
Prostate-specific antigen (PSA), first detected in serum in 1979, is a protein produced by prostate epithelial cells. It was originally used to follow men treated for prostate cancer for evidence of recurrence. In the late 1980s, it became widely used in the US to screen for prostate cancer.
An elevated PSA level is suggestive but not diagnostic of prostate cancer. Elevated levels also occur with advancing age, increased prostate size, and prostatitis, and following ejaculation. Prostate manipulation such as biopsy and surgery (but not digital examination) also elevates PSA.
Customary cutpoint. An abnormal serum PSA level is commonly regarded as 4 ng/mL or greater. At this cutpoint, most studies report sensitivity for cancer of around 70%, with more variability in specificity (TABLE 1). Again, in most studies that report sensitivity and specificity, men with a PSA level less than 4 ng/mL did not undergo prostate biopsy; therefore, the number of false negatives and true negatives are only estimates.
In nested case-control studies of longitudinal cohorts, eligible cases were defined by the length of time between an abnormal PSA result and prostate cancer diagnosis, while controls were men who were not diagnosed with prostate cancer during the same time period (however, a biopsy was not usually performed). The PPV of an abnormal PSA level is estimated at 25% to 37%. As a comparison, the PPV of a positive mammogram finding for women 50 to 59 years is 4% to 9%, and 10% to 19% for women age 60 to 69.21
Suggested strategies to improve PSA accuracy. Approximately 70% of men with an elevated serum PSA level do not have cancer. To decrease the number of unnecessary biopsies, experts have suggested several strategies:
- using DRE with PSA
- calculating a ratio of free PSA (unbound to protein) or complexed PSA (bound to protein) to total PSA
- measuring PSA density, which incorporates the volume of the prostate gland and is subject to observer variability in prostate volume measurement
- recording PSA velocity, which is the annual rate of change in PSA level and requires 3 or more measurements.
Revise the cutpoint? The cutpoint of 4 ng/mL has been deemed too high by some clinicians. A recent report of men enrolled in a large prostate cancer prevention trial found that among the 9459 men receiving placebo, 2950 had a PSA level of 4 ng/mL or less and had normal prostates on DRE (LOE: 1b).22 All 2950 underwent a prostate biopsy; 449 (15.2%) were positive for cancer. Nearly 30% of those had a PSA level of 2 ng/mL or less.
Approximately 25% of tumors in men referred for biopsy because of abnormal PSA or DRE findings are thought to be discovered by chance due to the biopsy procedure and the high prevalence of disease (LOE: 2c).23 Furthermore, a recent study indicates that annual PSA fluctuation is substantial; 45% of men who initially had a value of 4 ng/mL or greater, subsequently had a normal level (LOE: 2b).24 Isolated abnormal levels should be confirmed before referral for biopsy.
Identifying clinically relevant cancers
The continued controversy around PSA screening relates to the potential over-diagnosis of localized prostate cancers that are not likely to become clinically significant. The ideal screening test would predictably identify cancers likely to progress.
Screening over-detects
Several computer simulations have estimated the amount of over-detection of prostate cancer. The National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry data demonstrated that the proportion of prostate cancer found through PSA testing that otherwise would not have been diagnosed in the patient’s lifetime was 29% for white men and 44% for black men (LOE: 2c).25
An Italian study found that the proportional excess of cancers detected by screening over those that would have been expected in the absence of screening is greater than 50% (LOE: 2c).26
Finally, a model based on results from the European randomized study of prostate cancer screening estimated that a screening program with a 4-year interval from age 55 to 67 has an over-detection rate of 48% (cancers that would not have been diagnosed in the absence of screening) (LOE: 2c).27 Additionally, the authors determined that such screening might advance prostate cancer diagnosis by at least 10 years. Previous estimates of diagnosis lead time have been in the 5- to 7-year range (LOE: 2b).28 These findings indicate that screening intervals of 2 to 4 years are unlikely to cause a loss of sensitivity.
Spread of tumor
Spread of the tumor beyond the prostate capsule is a poor prognostic sign.29 Unfortunately, this occurrence is often not known until surgery, and the result usually is an “up-staging” between clinical diagnosis and pathological diagnosis. Nevertheless, most cancers (86%) diagnosed between 1992 and 1999 were localized to the prostate.6 Because these cancers have not spread beyond the prostate at the time of diagnosis, they are more likely to be curable. They are also more likely to represent tumors that may grow so slowly that the host will die of something other than prostate cancer.
Gleason score
Gleason score is another predictor of cancers destined to become clinically relevant (FIGURE 1). A Gleason score of 7 or greater denotes moderate to poor cellular differentiation and indicates a greater potential for progression than lower Gleason values.29 A recent long-term follow-up report on a cohort of men with localized cancer treated conservatively demonstrated that men with low-grade tumors (Gleason score 2–4) have a minimal risk of dying from prostate cancer after 20 years, while men with high-grade tumors (Gleason score of 8–10) have high probability of prostate cancer death within 10 years of diagnosis (LOE: 2b).30 Ecological31 and clinical studies32 indicate that a substantial proportion of PSA-detected cancers are moderately differentiated. This is especially true in a first round of screening; as in the European randomized study of screening, where 36% of cancers were Gleason score 7 or higher (LOE: 1b).33
FIGURE 1
Calculating the Gleason score
The Gleason score is based on the level of differentiation and growth pattern of prostate cancer cells. Cancer cells that closely resemble the normal prostate cells when viewed under low-power magnification are well differentiated. Cancer cells that do not retain the structure of the surrounding normal cells are poorly differentiated. Scores range from 1 to 5.
In examining histologic samples of a patient’s prostate tissue, the pathologist will identify the 2 most commonly occurring patterns (types of differentiation) among the cancer cells and assign a numerical value to each pattern. The 2 numbers are then added to yield the final Gleason score. If a single pattern dominates, the pathologist will simply double the corresponding value.
Total scores range from 2 to 10. Scores in the range of 2–4 are considered well-differentiated, 5–7 are moderately differentiated, and 8–10 are poorly differentiated. In general, the higher the score, the worse the prognosis. Men with well-differentiated tumors that are treated conservatively have minimal risk of dying from prostate cancer.
Is declining mortality a sign of screening success?
Prostate cancer mortality has been declining since the mid-1990s in numerous parts of the world; the US,6,34 Canada,35 Australia,36 and the United Kingdom37 have all reported a reduction in the rate of prostate cancer deaths. Advocates of PSA screening point to this trend as evidence of the effectiveness of screening. But such ecological data are difficult to interpret. For instance, although much less PSA screening is performed in the UK, mortality trends are similar to those in the US where PSA testing has been used more widely.38
Aggressive screening not necessarily the reason. In the US, 2 geographic areas—Seattle, Washington and Connecticut—provided a natural experiment to compare the effect of aggressive screening on prostate cancer mortality (LOE: 2c).39 Although more aggressive screening and treatment took place in the Seattle area, prostate cancer mortality rates were similar to those in Connecticut over 11 years of follow-up. Similarly, in a study in British Columbia, prostate cancer mortality from 1985 to 1999 was not associated with the intensity of PSA screening (LOE: 2c).40
Other possible explanations. If the mortality decrease is not related to PSA screening, what could cause it? One explanation is “attribution bias.” Death certificate misattribution of cause of death from prostate cancer may partially explain the pattern of increasing, then decreasing mortality rates (LOE: 2c).41 Improvement in prostate cancer treatment, especially for advanced stage, and in particular hormone therapy, is another possible explanation for the decreasing prostate cancer mortality (LOE: 2c).14,42
Benefits of screening
The benefit of any effective screening test is a decrease in the risk of the screened-disease mortality. The best way to demonstrate decreased risk is through a randomized controlled study of the screening test, and 2 such trials are underway for prostate cancer. In the meantime, a decision model estimates that aggressive treatment of organ-confined disease potentially adds 3 years of life for men in their fifties, 1.5 years for men in their sixties, and 0.4 years for men in their seventies (LOE: 2c).3
Others have concluded that 25 men with clinically detected prostate cancer would need to be treated with surgery to prevent 1 prostate cancer death during a 6-year period, without evidence that quality of life is improved (LOE: 2c).43
Consider quality of life. With uncertainty surrounding improvement in the quantity of life as a result of prostate cancer screening, improved quality of life may be an issue for patients. Focus group research has demonstrated that some patients believe it is better to know if a cancer is present than to wonder if it will be diagnosed when it is too late for cure.44
General quality of life has been found to be similar among men treated for prostate cancer and age-matched controls without prostate cancer; however, urinary, sexual, and bowel function vary substantially between treated and untreated men and by treatment type (LOE: 3b) (TABLE 2).45,46 In general, men treated with radical prostatectomy and brachytherapy often report better general quality of life than men who undergo radiation treatment, despite having more urinary and sexual problems (LOE: 2b).47,48
TABLE 2
Estimates of risk associated with specific prostate cancer treatments 12 months or more after treatment
| TREATMENT OUTCOMES | RADICAL PROSTATECTOMY (%) | EXTERNAL BEAM RADIATION (%) | BRACHY-THERAPY* (%) | ANDROGEN DEPRIVATION THERAPY (%) | UNTREATED (%) |
|---|---|---|---|---|---|
| Death within 2 months of treatment | 0.5–0.7 | 0.2–0.5 | 0.2–0.5 | ||
| Urinary problems: | |||||
| Incontinence | 10–50 | 2–16 | 6–16 | ||
| Wearing pads | 5–32 | 2–12 | 2–16 | ||
| Urinary bother† | 4–20 | 3–15 | 3–16 | ||
| Sexual problems: | |||||
| Impotence | 50–80‡ | 30–60 | 20–60** | 70–92 | 20–50 |
| Sexual bother | 10–40 | 10–30 | 10–18 | 25–38 | 10–32 |
| Bowel problems: | |||||
| Bowel problems§ | 9–15 | 6–35 | 4–20 | ||
| Loose stools/diarrhea | 15–21 | 6–37 | 4–10 | ||
| Bowel bother | 1–3 | 4–12 | 2–10 | ||
| Other symptoms | Breast swelling: 5–25 | ||||
| Hot flashes: 50–60 | |||||
| * Fewer studies on brachytherapy are available, especially those with long-term follow-up; therefore, these findings are less certain than other entries. | |||||
| ‡ Includes nerve-sparing prostatectomy. | |||||
| † EBRT and brachytherapy patients are more likely to experience irritative voiding symptoms (i.e. dysuria, urgency and hesitancy and noctoria), while RP patients are more likely to experience incontinence. | |||||
| ** Impotence risk gradually increases with time after treatment. | |||||
| § Includes symptoms such as painful bowel movement and urgency | |||||
| Sources:references 14, 50–53, 65–71. | |||||
Harms of screening
The chances of undergoing a biopsy based on an abnormal screening PSA are estimated at 15% to 40% depending on the patient’s age (FIGURE 2).3 There are adverse effects associated with transrectal biopsy of the prostate. In 2 large population-basedstudies of screening, the most frequent complications were hematuria and hematospermia (LOE: 1b, 2b) (TABLE 3), with more serious consequences such as sepsis and hospitalization occurring in fewer than 1% of patients. A study of 100 screened men with an abnormal PSA who underwent prostate biopsy found that although 69% felt moderate to severe pain with the biopsy, 80% would be willing to undergo a repeat biopsy (LOE: 1b).49
Treatment options. If the biopsy result is positive, the most common treatment options for localized cancer—which represents over 80% of all prostate cancers diagnosed6—include radical prostatectomy, external beam radiation therapy, brachytherapy (internal radiation therapy) or expectant management (watchful waiting). Population-based studies have reported outcomes for these treatment options (TABLE 2). Outcomes derived from hospital-based series of other prostate cancer treatments, such as cryotherapy and 3-dimensional radiation, are available, but the estimates often reflect the experience of only a few hospitals and are not representative of other facilities. Androgen ablation is the standard treatment for metastatic prostate cancer.
Untoward effects of treatment. Approximately 60% of radical prostatectomy patients report some incontinence 12 months or more after surgery (LOE: 2b),50,51 and about 30% of patients need to wear pads for urine leakage (LOE: 2b).50-53 Men undergoing radiation therapy have less urinary incontinence, but about 30% complain of diarrhea and loose stools (LOE: 2b).51,52 Both therapies are associated with a high percentage of erectile dysfunction: approximately 60% of radiation therapy patients and 75% of surgery patients report their erections are not firm enough for intercourse (LOE: 2b).51,52
Expectant management (following the cancer with regular PSA and ultrasound testing) is sometimes difficult to “sell” to patients whose fear of cancer dictates that the only logical response is to “cut it out.”44 A recent randomized trial indicated that radical prostatectomy lowers prostate cancer mortality, local progression, distant metastasis, and overall survival as compared with watchful waiting over a median of 8.2 years of follow-up (LOE: 1b).54 However, these results may have little relevance to prostate cancer screening since only 5% of the cancers were screen-detected and 76% were palpable.
FIGURE 2
Yield of screening 1000 men for prostate cancer
TABLE 3
Percentage of patients with specific complication of transrectal prostate biopsy
| CONDITION | TYROL STUDY63 | EUROPEAN RANDOMIZED STUDY OF SCREENING 64 |
|---|---|---|
| Gross hematuria >1 day | 12.5% | 22.6% |
| Hematospermia | 29.8% | 50.4% |
| Significant pain | 4.0% | 7.5% |
| Rectal bleeding | 0.6% | 1.3% |
| Nausea | 0.8% | 0.3% |
| Fever >38.5°C | 0.8% | 3.5% |
| Epididymitis | 0.7% | 0.07% |
| Sepsis | 0.3% | Not available |
| Hospitalization | Not available | 0.5% |
| Tyrol study63: LOE: 2b, N=6024 biopsies; ERSS study64: LOE: 1b, N=5802 biopsies. | ||
Recommendations from expert groups
Different expert groups have conflicting recommendations. Both the American Urological Association and the American Cancer Society recommend annual PSA screening starting at age 50 for most men; younger if risk factors are present. Groups that are evidence based tend to recommend a shared decision making process with patients. The AAFP and American College of Physicians advise physicians to counsel men on the known risks and uncertain benefits of screening for prostate cancer. The US Preventive Services Task Force 2002 update concluded that evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA or DRE. The National Cancer Institute cites a lack of evidence to determine a net benefit for PSA or DRE screening.
When will we know more?
Only 1 randomized controlled trial of prostate cancer screening has been completed55: 46,193 men were randomized to either PSA and DRE or no screening from 1989 to 1996. The study had methodological problems; for instance, only 23% of the group randomized to screening was screened. The investigators in the trial have interpreted its results as demonstrating a decrease in prostate cancer deaths in the screened group compared with the unscreened group (15 vs 48.7 per 100,000 man-years).55 Others have criticized the statistical analysis and calculated the results using an “intent to screen” analysis, finding no difference in prostate cancer deaths between the 2 groups.3,56
Two randomized controlled trials of screening are ongoing: the National Cancer Institute’s Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial57 and the European Randomized Study of Screening for Prostate Cancer.58 Both were started in the mid-1990s and will not have results available for a few more years. Also underway is a randomized trial of intervention (radical prostatectomy) versus expectant management, called the Prostate Cancer Intervention Versus Observation Trial (PIVOT).59
Counseling recommendations
However, providing men with information on prostate cancer screening before they discussed it with their family physician, rather than after the visit, resulted in patients having a significantly more active role in making a screening decision, and lower levels of decisional conflict (LOE: 2b).61 Informational pamphlets are available through the AAFP and CDC websites listed in TABLE 4. Additional websites containing prostate cancer screening information are found in TABLE 4. We also provide a bullet item list of key points for discussion with patients (TABLE 5), which can be used along with the balance sheet provided here (TABLE 2).
Shared decision-making is not an easy or quick process. Yet, the majority of patients will benefit from the discussion, regardless of the final decision. Of course, there are instances when a shared decision-making process is well-documented, and still results in an undesirable outcome;62 however, while the evidence for screening remains controversial, patients have the right to know that those controversies exist and why they exist.
TABLE 4
Useful websites for patients to find prostate cancer screening information
| CENTERS FOR DISEASE CONTROL AND PREVENTION |
| www.cdc.gov/cancer/prostate/decisionguide/index.htm |
| 10th grade reading level* |
| Good coverage of screening and treatment controversies |
| Offers downloadable PDF version |
| NATIONAL CANCER INSTITUTE |
| cis.nci.nih.gov/asp/FactSheetPub/AlphaSubList.asp?alpha=47 |
| 10th grade reading level |
| FAQ format |
| Offers Spanish version |
| AMERICAN CANCER SOCIETY |
| www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_ cancer_be_found_early_36.asp |
| 12th grade reading level |
| Lacks discussion of treatment options and their side effects |
| Biased in favor of screening but acknowledges that other distinguished organizations are not |
| AMERICAN UROLOGICAL ASSOCIATION |
| www.urologyhealth.org/adult/index.cfm?cat=09 |
| 12th grade reading level |
| Easy to navigate among screening and specific treatment pages |
| Biased in favor of PSA screening |
| AMERICAN ACADEMY OF FAMILY PHYSICIANS |
| familydoctor.org/healthfacts/361/ |
| 11th grade reading level |
| Question/answer format |
| Very straightforward, lacks depth |
| www.aafp.org/x19519.xml |
| 7th grade reading level |
| Separate information sheet for patients and physicians |
| Presents possible outcomes of PSA test and prostate cancer treatment in easy-to-follow format |
| WEBMD |
| my.webmd.com/medical_information/condition_centers/prostate_cancer/default.htm |
| 9th grade reading level |
| Question/answer format |
| Specifically addresses false negative and positives with current estimates |
| DARTMOUTH CENTER FOR SHARED DECISION MAKING |
| www.dhmc.org/dhmc-internet-upload/file_collection/PSA.pdf |
| 6th grade reading level |
| Well-designed, simple presentation of pros and cons of PSA testing |
| *Fleish-Kincaid grade level score based on average sentence length and average number of syllables per word. |
TABLE 5
Talking points for patients and physicians
| Prostate cancer is an important men’s health problem |
| Screening may prevent early prostate cancer death |
| DRE alone has little value as a screening test |
| Age, prostate size, prostatitis, ejaculation, prostate biopsy, and prostate surgery can cause a falsely elevated PSA test |
| Approximately 70% of men with an elevated serum PSA do not have cancer |
| The percentage of PSA screening false negatives ranges from 10%–22% in large studies |
| If the test is abnormal, a biopsy will be recommended |
| If the biopsy is positive, treatment options will be given |
| Many men experience long-term urinary incontinence and impotence related to their treatment |
CORRESPONDING AUTHOR
Kendra Schwartz, MD, MSPH, 101 E. Alexandrine, Detroit, MI 48201, E-mail: kensch@med.wayne.edu
1. American Academy of Family Physicians. Summary of recommendation for periodic health examinations. August 2002. Available at: www.aafp.org/PreBuilt/PHERev5.30802.pdf. Accessed on June 10, 2005.
2. US Preventive Services Task Force. Screening for Prostate Cancer. 2002. Available at: www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed on June 10, 2005.
3. American College of Physicians. Screening for prostate cancer. Position paper. Clinical Guideline: Part III. Ann Intern Med 1997;126:480-484.
4. Harris R, Lohr K, Beck R, Fink K, Godley P, Bunton A. Screening for prostate cancer. Systematic Evidence Review for AHRQ. Available at: www.ahrq.gov/uspstfix.htm. Accessed on June 10, 2005.
5. American Cancer Society. Cancer statistics 2005. CA Cancer J Clin 2005;55:10-30.
6. Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. Available at seer.cancer.gov/csr/1975_2002/. Accessed on June 10, 2005.
7. DevCan: Probability of dying of cancer [computer program]. Version 5.1. Bethesda, Md: National Cancer Instititute; 2003. Available at: srab.cancer.gov/devcan/. Accessed on June 10, 2005.
8. Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316-1321.
9. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996.
10. Kabalin J, McNeal J, Price H, Freiha F, Stamey T. Unsuspected adenocarcinoma of the prostate in patients undergooing cystoprostatectomy for other causes: incidence, histology and morphometric observations. J Urol 1989;141:1091-1094.
11. Montie J, Wood DJ, Pontes J, Boyett J, Levin H. Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 1989;63:381-385.
12. Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta 2002;315:71-97.
13. Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864.
14. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-929.
15. American Cancer Society. ACS Cancer Detection Guidelines. Available at: www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines _36.asp. Accessed June 10, 2005.
16. Smith D, Catalona W. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 1995;45:70-74.
17. Kishor M, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract 2003;16:95-101.
18. Hoogendam A, Buntix F, deVet HCW. The diagnostic value of digital rectal examination in primary care screening for prostate cancer: a meta-analysis. Fam Pract 1999;16:621-626.
19. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294.
20. Hakama M, Stenman UH, Aromaa A, Leinonen J, Hakulinen T, Knekt I. Validity of the prostate specific antigen test for prostate cancer screening: followup study with a bank of 21,000 sera in Finland. J Urol 2001;166:2189-2191.
21. Humphrey L, Helfand M, Chan B, Woolf S. Breast cancer screening: summary of the evidence. Ann Intern Med 2002;137:344-346.
22. Thompson I, Pauler D, Goodman P, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4 ng per milliliter. N Engl J Med 2004;350:2239-2246.
23. Collins MM, Ransohoff D, MJ Barry. Early detection of prostate cancer-serendipity strikes again. JAMA 1997;278:1516-1519.
24. Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA 2003;289:2695-2700.
25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-990.
26. Zappa M, Ciatto S, Bonardi R, Mazzotta A. Overdiagnosis of prostate carcinoma by screening: an estimate based on the results of the Florence Screening Pilot Study. Ann Oncol 1998;9:1297-1300.
27. Draisma G, Boer R, Otto S, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst 2003;95:868-878.
28. Auvin A, Maattanen L, Stenman UH, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279-285.
29. Gleason D, Mellinger G. Group at VACUR. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
30. Albertson PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-2101.
31. Schwartz K, Grignon D, Sakr W, Wood DJ. Prostate cancer histologic trends in the metropolitan Detroit are, 1982 to 1996. Urology 1999;53:769-774.
32. Smith D, Catalona W. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol 1994;152:1732-1736.
33. Hoedemaeker RF, van der Kwast T, Boer R, et al. Pathological features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001;93:1153-1158.
34. Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 2003;97:1507-1516.
35. National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: National Cancer Institute of Canada; 2001. Available at: www.ncic.cancer.ca. Accessed June 10, 2005.
36. Coory M, Baade P. Mortality from prostate cancer is decreasing. Med J Aust 2002;176:345-345.
37. Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales, 1971–1998. BJU Int 2000;85:1058-1062.
38. Oliver S, Gunnell D, Donovan J. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-1789.
39. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002;325:740.-
40. Coldman A, Phillips N, Pickles T. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003;168:31-35.
41. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-1032.
42. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003;361:1122-1128.
43. Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-693.
44. McFall SL, Hamm RM. Interpretation of prostate cancer screening events and outcomes: a focus group study. Patient Educ Couns 2003;49:207-218.
45. Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-135.
46. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep 2003;4:185-195.
47. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in the health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology 1999;53:180-186.
48. Bacon C, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804-1810.
49. Makinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology 2002;60:846-850.
50. Fowler FJ, Jr, Barry MJ, Lu-Yao G, Roman A, Wasson J, Wennberg JE. Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 1993;42:622-629.
51. Schwartz K, Bunner S, Bearer R, Severson RK. Complications from treatment for prostate carcinoma among men in the Detroit area. Cancer 2002;95:82-89.
52. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582-1592.
53. Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology 2002;60:1055-1058.
54. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-1184.
55. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38:83-91.
56. Alexander FE, Prescott RJ. Reply to Labrie et al. Results of the mortality analysis of the Quebec randomized controlled trial (RCT). Prostate 1999;40:135-137.
57. Prorok P, Andriole G, Bresalier R, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S.
58. Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80:1830-1834.
59. Wilt TJ, Brawer M. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol 1994;152:1910-1914.
60. Schapira M, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract 2000;49:418-424.
61. Davison B, Kirk P, Degner L, Hassard T. Information and patient participation in screening for prostate cancer. Patient Educ Couns 1999;37:255-263.
62. Merenstein D. Winners and losers. JAMA 2004;291:15-16.
63. Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Current Urol Reports 2004;5:220-225.
64. Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-830.
65. Fowler F, Barry MJ, Lu-Yao G, Wasson J, Bin L. Outcomes of external beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology, and End Results areas. J Clin Oncol 1996;14:2258-2265.
66. Hollenbeck BK, Dunn RL, Wei JT, Sandler HM, Sanda MG. Sexual health recovery after prostatectomy, external radiation, or brachytherapy for early stage prostate cancer. Curr Urol Rep 2004;5:212-219.
67. Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol 2001;19:3750-3757.
68. Lee R, Penson DF. Treatment outcomes in localized prostate cancer: a patient-oriented approach. Semin Urol Oncol 2002;20:63-73.
69. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-360.
70. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063-1068.
71. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med 1997;126:468-479.
1. American Academy of Family Physicians. Summary of recommendation for periodic health examinations. August 2002. Available at: www.aafp.org/PreBuilt/PHERev5.30802.pdf. Accessed on June 10, 2005.
2. US Preventive Services Task Force. Screening for Prostate Cancer. 2002. Available at: www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed on June 10, 2005.
3. American College of Physicians. Screening for prostate cancer. Position paper. Clinical Guideline: Part III. Ann Intern Med 1997;126:480-484.
4. Harris R, Lohr K, Beck R, Fink K, Godley P, Bunton A. Screening for prostate cancer. Systematic Evidence Review for AHRQ. Available at: www.ahrq.gov/uspstfix.htm. Accessed on June 10, 2005.
5. American Cancer Society. Cancer statistics 2005. CA Cancer J Clin 2005;55:10-30.
6. Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. Available at seer.cancer.gov/csr/1975_2002/. Accessed on June 10, 2005.
7. DevCan: Probability of dying of cancer [computer program]. Version 5.1. Bethesda, Md: National Cancer Instititute; 2003. Available at: srab.cancer.gov/devcan/. Accessed on June 10, 2005.
8. Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316-1321.
9. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996.
10. Kabalin J, McNeal J, Price H, Freiha F, Stamey T. Unsuspected adenocarcinoma of the prostate in patients undergooing cystoprostatectomy for other causes: incidence, histology and morphometric observations. J Urol 1989;141:1091-1094.
11. Montie J, Wood DJ, Pontes J, Boyett J, Levin H. Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 1989;63:381-385.
12. Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta 2002;315:71-97.
13. Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864.
14. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-929.
15. American Cancer Society. ACS Cancer Detection Guidelines. Available at: www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines _36.asp. Accessed June 10, 2005.
16. Smith D, Catalona W. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 1995;45:70-74.
17. Kishor M, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract 2003;16:95-101.
18. Hoogendam A, Buntix F, deVet HCW. The diagnostic value of digital rectal examination in primary care screening for prostate cancer: a meta-analysis. Fam Pract 1999;16:621-626.
19. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294.
20. Hakama M, Stenman UH, Aromaa A, Leinonen J, Hakulinen T, Knekt I. Validity of the prostate specific antigen test for prostate cancer screening: followup study with a bank of 21,000 sera in Finland. J Urol 2001;166:2189-2191.
21. Humphrey L, Helfand M, Chan B, Woolf S. Breast cancer screening: summary of the evidence. Ann Intern Med 2002;137:344-346.
22. Thompson I, Pauler D, Goodman P, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4 ng per milliliter. N Engl J Med 2004;350:2239-2246.
23. Collins MM, Ransohoff D, MJ Barry. Early detection of prostate cancer-serendipity strikes again. JAMA 1997;278:1516-1519.
24. Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA 2003;289:2695-2700.
25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-990.
26. Zappa M, Ciatto S, Bonardi R, Mazzotta A. Overdiagnosis of prostate carcinoma by screening: an estimate based on the results of the Florence Screening Pilot Study. Ann Oncol 1998;9:1297-1300.
27. Draisma G, Boer R, Otto S, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst 2003;95:868-878.
28. Auvin A, Maattanen L, Stenman UH, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279-285.
29. Gleason D, Mellinger G. Group at VACUR. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
30. Albertson PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-2101.
31. Schwartz K, Grignon D, Sakr W, Wood DJ. Prostate cancer histologic trends in the metropolitan Detroit are, 1982 to 1996. Urology 1999;53:769-774.
32. Smith D, Catalona W. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol 1994;152:1732-1736.
33. Hoedemaeker RF, van der Kwast T, Boer R, et al. Pathological features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001;93:1153-1158.
34. Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 2003;97:1507-1516.
35. National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: National Cancer Institute of Canada; 2001. Available at: www.ncic.cancer.ca. Accessed June 10, 2005.
36. Coory M, Baade P. Mortality from prostate cancer is decreasing. Med J Aust 2002;176:345-345.
37. Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales, 1971–1998. BJU Int 2000;85:1058-1062.
38. Oliver S, Gunnell D, Donovan J. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-1789.
39. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002;325:740.-
40. Coldman A, Phillips N, Pickles T. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003;168:31-35.
41. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-1032.
42. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003;361:1122-1128.
43. Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-693.
44. McFall SL, Hamm RM. Interpretation of prostate cancer screening events and outcomes: a focus group study. Patient Educ Couns 2003;49:207-218.
45. Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-135.
46. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep 2003;4:185-195.
47. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in the health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology 1999;53:180-186.
48. Bacon C, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804-1810.
49. Makinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology 2002;60:846-850.
50. Fowler FJ, Jr, Barry MJ, Lu-Yao G, Roman A, Wasson J, Wennberg JE. Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 1993;42:622-629.
51. Schwartz K, Bunner S, Bearer R, Severson RK. Complications from treatment for prostate carcinoma among men in the Detroit area. Cancer 2002;95:82-89.
52. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582-1592.
53. Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology 2002;60:1055-1058.
54. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-1184.
55. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38:83-91.
56. Alexander FE, Prescott RJ. Reply to Labrie et al. Results of the mortality analysis of the Quebec randomized controlled trial (RCT). Prostate 1999;40:135-137.
57. Prorok P, Andriole G, Bresalier R, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S.
58. Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80:1830-1834.
59. Wilt TJ, Brawer M. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol 1994;152:1910-1914.
60. Schapira M, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract 2000;49:418-424.
61. Davison B, Kirk P, Degner L, Hassard T. Information and patient participation in screening for prostate cancer. Patient Educ Couns 1999;37:255-263.
62. Merenstein D. Winners and losers. JAMA 2004;291:15-16.
63. Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Current Urol Reports 2004;5:220-225.
64. Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-830.
65. Fowler F, Barry MJ, Lu-Yao G, Wasson J, Bin L. Outcomes of external beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology, and End Results areas. J Clin Oncol 1996;14:2258-2265.
66. Hollenbeck BK, Dunn RL, Wei JT, Sandler HM, Sanda MG. Sexual health recovery after prostatectomy, external radiation, or brachytherapy for early stage prostate cancer. Curr Urol Rep 2004;5:212-219.
67. Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol 2001;19:3750-3757.
68. Lee R, Penson DF. Treatment outcomes in localized prostate cancer: a patient-oriented approach. Semin Urol Oncol 2002;20:63-73.
69. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-360.
70. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063-1068.
71. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med 1997;126:468-479.
Influenza vaccine does not prevent acute otitis media in young children
Administration of influenza vaccine to children aged 6 to 24 months to prevent acute otitis media is not recommended.
Administration of influenza vaccine to children aged 6 to 24 months to prevent acute otitis media is not recommended.
Administration of influenza vaccine to children aged 6 to 24 months to prevent acute otitis media is not recommended.
Antioxidant vitamins do not prevent cardiovascular disease
This meta-analysis of randomized controlled trials showed that neither beta-carotene nor vitamin E appears to prevent all-cause or cardiovascular mortality in patients with known heart disease or those at risk for heart disease. Similarly, use of these antioxidant vitamins did not affect number of stroke events. The use of beta-carotene and vitamin E should not be recommended for primary and secondary prevention of cardiovascular disease.
This meta-analysis of randomized controlled trials showed that neither beta-carotene nor vitamin E appears to prevent all-cause or cardiovascular mortality in patients with known heart disease or those at risk for heart disease. Similarly, use of these antioxidant vitamins did not affect number of stroke events. The use of beta-carotene and vitamin E should not be recommended for primary and secondary prevention of cardiovascular disease.
This meta-analysis of randomized controlled trials showed that neither beta-carotene nor vitamin E appears to prevent all-cause or cardiovascular mortality in patients with known heart disease or those at risk for heart disease. Similarly, use of these antioxidant vitamins did not affect number of stroke events. The use of beta-carotene and vitamin E should not be recommended for primary and secondary prevention of cardiovascular disease.
Topical ophthalmic NSAIDs reduce pain faster than placebo
Topical ophthalmic nonsteroidal anti-inflammatory drugs (NSAIDs) relieve the pain of uncomplicated acute corneal abrasions faster than placebo eyedrops.
The pain relief is small; whether the pain relief difference would be noticed by patients and how it compares with oral analgesics is unknown. Given the cost of topical NSAIDs, they are most useful for a select patient population: those who must return to work immediately, and those for whom opioid analgesia-induced sedation is intolerable.
Topical ophthalmic nonsteroidal anti-inflammatory drugs (NSAIDs) relieve the pain of uncomplicated acute corneal abrasions faster than placebo eyedrops.
The pain relief is small; whether the pain relief difference would be noticed by patients and how it compares with oral analgesics is unknown. Given the cost of topical NSAIDs, they are most useful for a select patient population: those who must return to work immediately, and those for whom opioid analgesia-induced sedation is intolerable.
Topical ophthalmic nonsteroidal anti-inflammatory drugs (NSAIDs) relieve the pain of uncomplicated acute corneal abrasions faster than placebo eyedrops.
The pain relief is small; whether the pain relief difference would be noticed by patients and how it compares with oral analgesics is unknown. Given the cost of topical NSAIDs, they are most useful for a select patient population: those who must return to work immediately, and those for whom opioid analgesia-induced sedation is intolerable.
Are ionized wrist bracelets better than placebo for musculoskeletal pain?
As a result of a profound placebo effect, this study showed that Q-Ray ionized wrist bracelets were not superior to placebo bracelets in self-reported pain improvement among patients with musculoskeletal pain.
Like many other studies involving the treatment of pain, the perception that the treatment would work profoundly improved its effectiveness. While the bracelet did not work better than placebo, many patients may experience less pain if they purchase and use it.
As a result of a profound placebo effect, this study showed that Q-Ray ionized wrist bracelets were not superior to placebo bracelets in self-reported pain improvement among patients with musculoskeletal pain.
Like many other studies involving the treatment of pain, the perception that the treatment would work profoundly improved its effectiveness. While the bracelet did not work better than placebo, many patients may experience less pain if they purchase and use it.
As a result of a profound placebo effect, this study showed that Q-Ray ionized wrist bracelets were not superior to placebo bracelets in self-reported pain improvement among patients with musculoskeletal pain.
Like many other studies involving the treatment of pain, the perception that the treatment would work profoundly improved its effectiveness. While the bracelet did not work better than placebo, many patients may experience less pain if they purchase and use it.
Factors associated with weaning in the first 3 months postpartum
OBJECTIVE: To determine the demographic, behavioral, and clinical factors associated with breastfeeding termination in the first 12 weeks postpartum.
STUDY DESIGN: This was a prospective cohort study.
POPULATION: Breastfeeding women in Michigan and Nebraska were interviewed by telephone at 3, 6, 9, and 12 weeks postpartum or until breastfeeding termination.
OUTCOMES MEASURED: We measured associations of demographic, clinical, and breastfeeding variables with weaning during the first 12 weeks postpartum.
RESULTS: A total of 946 women participated; 75% breastfed until 12 weeks. Women older than 30 years and women with at least a bachelor’s degree were more likely to continue breastfeeding in any given week. Mastitis, breast or nipple pain, bottle use, and milk expression in the first 3 weeks were all associated with termination. Beyond 3 weeks, women who expressed breast milk were 75% less likely to discontinue breastfeeding than women who did not. Women who used a bottle for some feedings during weeks 4 to 12 were 98% less likely to discontinue breastfeeding than women who did not use a bottle. "Not enough milk" was the most common reason given for termination in weeks 1 through 3 (37%) and weeks 4 through 6 (35%); “return to work” was the most common reason given in weeks 7 through 9 (53%) and weeks 10 through 12 (58%).
CONCLUSIONS: Younger women and less educated women need additional support in their breastfeeding efforts. Counseling and assistance should be provided to women with pain and mastitis. Exclusive breastfeeding for the first 3 weeks should be recommended. After the first 3 weeks, bottles and manual expression are not associated with weaning and may improve the likelihood of continuing breastfeeding, at least until 12 weeks.
- Younger and less educated women may need extra support for long-term breastfeeding success.
- Exclusive breastfeeding for the first 3 weeks decreases the risk of early weaning. At least 7 daily feedings of 10 or more minutes per feeding are recommended.
- The use of bottles and manual expression of milk after 3 weeks does not increase the risk of early weaning.
Family physicians are strongly encouraged to support and promote breastfeeding, the optimal form of infant nutrition.1 Despite its known benefits (fewer infant infections2-6 and decreased maternal risks of premenopausal breast cancer7 and post-menopausal hip fractures8), only 64% of mothers initiated breastfeeding in 19989 and only 29% of mothers fed their 6-month-old infant by breast, well below the Healthy People 2010 goal of 50% breastfeeding at 6 months.10 Clearly, determining the factors that influence breastfeeding beyond the early postpartum period would be beneficial.
Returning to work is a consistent risk factor for weaning.11-14 The impact of early bottle-feeding on the duration of breastfeeding has been studied with less consistent results.15,20 Insufficient milk supply is a common subjective reason given for termination.15,19,21,22 Older women and those with a higher level of education are at less risk of early breastfeeding termination.9,11,15,16,21,23,24
Few investigators have described how breastfeeding patterns may affect breastfeeding duration. Little is known about the effects of timing, frequency, and duration of individual breastfeedings, or the roles of breast pain and infection, sleep, and manual expression on early weaning. We studied women who indicated their intent to breastfeed prenatally to identify demographic factors and breastfeeding patterns associated with weaning in the first 12 weeks postpartum.
Methods
Population
We interviewed breastfeeding women by telephone at 3, 6, 9, and 12 weeks postpartum to investigate lactation mastitis risk factors and predictors of weaning. Pregnant women intending to breastfeed were recruited from 2 geographic sites between June 1994 and January 1998. In suburban Detroit, Michigan, women attending orientation at a freestanding birthing center were asked to participate. In Omaha, Nebraska, women at a single large company were recruited when applying for maternity leave.
Data collection
During the computer-assisted interview, subjects were asked to recall each of the previous 3 weeks. The initial interview, which collected demographic information, typically lasted 15 to 20 minutes; subsequent interviews were shorter. The survey addressed breastfeeding practices and recent health events. Exclusive breastfeeders were women who fed their infants only by breast. We did not collect information on pacifiers; therefore, exclusively breastfed infants may have also received pacifiers. Women who manually expressed or used a device to assist in expression were classified as “pumping” their breasts. Respondents were asked if they had bottle-fed the infant; they were not asked about bottle contents or volume.
Subjects were queried on potential difficulties including breast or nipple pain while nursing, nipple cracks, and mastitis (diagnosed by a health care provider), as well as other health problems and behaviors. Subjects who had stopped breastfeeding in the previous 3 weeks were asked when and why, given a list of possible explanations and an open-ended opportunity. Respondents could provide multiple reasons for termination.
Data analysis
Kaplan-Meier estimates describe the distribution of weaning times for the 2 sites. A log-rank test was used to assess group differences. Relationships between demographic factors and time of weaning were assessed by Cox regression analysis. Discrete survival analysis was used to determine whether variables measured on a weekly basis were related to breastfeeding cessation. Hazard ratios describe the association of the exposures between women who stopped breastfeeding at a given time and those who continued. Because breastfeeding cessation was a rare event in later weeks of the study, as were certain clinical or behavioral breastfeeding factors, weeks 4-12 were collapsed into a single interval. Two variables, number of daily feedings and duration of each feeding, were examined only in the first 3 weeks because the information was often missing beyond 3 weeks. All analyses were performed using the Statistical Package for the Social Sciences.25
Results
Description of subjects
A total of 1057 women agreed to be contacted. Of those, 946 (89.5%) participated in at least 1 interview. Of the 111 women who did not participate, 11 refused and 100 could not be located. Six hundred fifty-eight (69.6%) women completed all 4 interviews. The 56 women who entered the study at week 6 because they could not be reached for the first interview were similar in all factors to women who entered earlier. Of the 946, 711 (75.2%) were from Michigan and 235 (24.8%) were from Nebraska.
Subjects from Michigan were significantly more likely than those from Nebraska to be older than 30 years (52.0% vs 38.3%), have at least a bachelor’s degree (62.9% vs 48.5%), have 3 or more children (38.5% vs 19.6%), and have had a vaginal delivery (99.6% vs 77.0%) (Table W1).* The groups were similar in race, household income, and marital status.
Demographic factors
A total of 673 women (71.1%) continued breastfeeding until 12 weeks; 28% were exclusive breastfeeders. Michigan women were more likely to breastfeed at weeks 2 through 12 than their Nebraskan counterparts (P < .0001, Figure). A college degree was associated with 40% less weaning (Table 1). Age and annual household income were directly related to continued breastfeeding at both sites. Number of children in the household was not associated with termination. Previous breastfeeding experience showed a nonsignificant but consistent trend toward lower weaning risk.
TABLE 1
Relationships of demographics and other characteristics with time to weaning, by site
| Characteristic | Michigan women HR* (95% CI) | Nebraska women HR* (95% CI) |
|---|---|---|
| Older than 30 years | 0.5 (0.3,0.8) | 0.7 (0.5, 1.1) |
| BA/BS or higher | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.8) |
| Number of children in household | ||
| 1 | 1.0 | 1.0 |
| 2 | 1.0 (0.6, 1.6) | 0.7 (0.5, 1.2) |
| 3 or more | 0.6 (0.4, 1.0) | 0.9 (0.6, 1.5) |
| Household income ≥ $50,000 | 0.8 (0.5, 1.3) | 0.7 (0.5, 1.0) |
| Breastfed previously | 0.7 (0.5, 1.1) | 0.7 (0.5, 1.1) |
| Nonvaginal birth | † | 0.9 (0.6, 1.4) |
| NOTE: Bold numbers are significant at P < .05. | ||
| HR denotes hazard ratio; CI, confidence interval; BA, bachelor of arts degree; BS, bachelor of science degree. | ||
| *A hazard ratio of <1 indicates that subjects with this characteristic were less likely to wean during the 12 weeks. Unless otherwise noted, the referent group is the converse (eg, age < 30 years is the referent group for those older than 30 years). | ||
| †Too few observations to provide meaningful results. | ||
FIGURE
Probability of breastfeeding, by site, by postpartum week
Clinical and behavioral factors
Because time to weaning differed significantly by site, the survival analyses of clinical and behavioral factors were performed separately for Michigan and Nebraska and controlled for education, age, and previous breastfeeding experience.
During the first 3 weeks, Michigan women with mastitis were nearly 6 times more likely than Michigan women without mastitis to stop breast-feeding in the week of diagnosis (Table 2). Women from Nebraska showed nonsignificant results in the same direction in weeks 4 to 12. (No women from Nebraska with mastitis terminated during weeks 1 through 3.) Although nipple sores and cracks were not associated with weaning, breast pain was associated with weaning. For each day of pain in the first 3 weeks, there was a 10% increase in risk of cessation among Michigan women and a 26% increase among Nebraska women. The association between pain and weaning in weeks 4 through 12 is less clear. In these later weeks, women who reported pain were unexpectedly 75% to 80% more likely to continue breastfeeding than women who did not report pain, yet for Nebraska women the number of days with pain remained significantly associated with breastfeeding cessation.
Subjective depression and breastfeeding cessation were not related. The association between daily sleep and weaning varied by site. During weeks 4 through 12, Michigan women with more daily sleep were less likely to terminate. An opposite, but marginally significant trend, was observed for Nebraska women. Weaning was not associated with outside household help. Nonvaginal birth was not associated with weaning for Nebraska women. (There were only 2 cesarean sections in the Michigan group.)
Michigan women who expressed breast milk during the first 3 weeks were twice as likely to stop breastfeeding as those who did not pump. During the same period, Michigan women who used a bottle for some feedings were 9 times more likely to wean than nonbottle users. Respondents in Nebraska showed similar nonsignificant trends in the first 3 weeks. By contrast, during weeks 4 through 12, both Nebraska and Michigan women who pumped were about 75% less likely to wean, while women who used a bottle for some feedings were 98% less likely to stop breastfeeding.
Breast milk expression increased gradually over time, from 30% of women pumping an average of 3 times per day in the first 3 weeks to 45% of women pumping 5 times per day in the last 3 weeks. To determine if pumping and bottle-feeding had an effect independent of pain or mastitis on weaning in the first 3 weeks, we performed additional analyses controlling for pain, cracks and sores, and mastitis in the same week. The results were similar to those presented in Table 2. Michigan women who pumped were 3 times more likely to wean than those who did not pump (hazard ratio [HR] = 3.0, 95% confidence interval [CI], 1.3 - 6.7), while for Nebraska women there was no association between pumping and weaning (HR = 0.6, 95% CI, 0.3 - 1.5). Bottle-feeding was again significantly associated with weaning in weeks 1 through 3 for Michigan women (HR = 10.9, 95% CI, 4.5 - 26.7) and not associated in Nebraskans (HR = 0.8, 95% CI, 0.4 - 2.0).
Duration and frequency of feedings were investigated as weaning risk factors. There appeared to be a threshold for both variables during the first 3 weeks in Michigan women. Michigan women who breastfed less than 10 minutes per feeding were nearly 5 times more likely to stop breastfeeding than women who breastfed longer. Michigan women who breastfed 6 or fewer times per day were 8 times more likely to stop than those who breastfed more often. Results for Nebraska women fell in the same direction but were not statistically significant.
TABLE 2
Relationships of clinical and behavioral factors to breastfeeding cessation in the same week, adjusted for mother’s age, education, and previous breastfeeding experience
| Variable | Week | Michigan women HR (95% CI) | Nebraska women HR (95% CI) |
|---|---|---|---|
| Mastitis | 1 - 3 | 5.7 (1.3 - 25.9) | ‡ |
| 4 - 12 | ‡ | 2.1 (0.3 - 17.4) | |
| Engorgement | 1 - 3 | 0.6 (0.2 - 1.5) | 0.8 (0.3 - 2.1) |
| 4 - 12 | ‡ | 3.2 (0.6 - 15.8) | |
| Nipple sores/cracks | 1 - 3 | 1.1 (0.4 - 2.6) | 0.9 (0.4 - 2.3) |
| 4 - 12 | 2.6 (0.8 - 8.5) | 2.9 (0.8 - 10.7) | |
| Any pain † | 1 - 3 | 14.7 (6.8 - 32.0)§ | 9.1 (3.9 - 21.2)† |
| 4 - 12 | 0.3 (0.1 - 0.7) | 0.2 (0.1 - 0.5)† | |
| Days with pain* | 1 - 3 | 1.1 (1.0 - 1.2) | 1.3 (1.0 - 1.5) |
| 4 - 12 | 1.1 (1.0 - 1.2) | 1.1 (1.0 - 1.2) | |
| Returned to work | 1 - 3 | 0.4 (0.1 - 3.0) | ‡ |
| 4 - 12 | 2.1 (1.1 - 4.0) | 0.8 (0.4 - 1.7) | |
| Depressed | 1 - 3 | 0.9 (0.3 - 3.0) | 1.0 (0.4 - 2.6) |
| 4 - 12 | 0.9 (0.4 - 2.2) | 1.3 (0.6 - 2.7) | |
| Daily sleep hours | 1 - 3 | 0.9 (0.7 - 1.1) | 0.9 (0.8 - 1.2) |
| 4 - 12 | 0.7 (0.5 - 0.9) | 1.2 (1.0 - 1.5) | |
| Outside household help | 1 - 3 | 2.0 (0.8 - 4.8) | 0.9 (0.4 - 2.1) |
| 4 - 12 | 0.7 (0.3 - 2.6) | 0.7 (0.2 - 2.1) | |
| Pumping | 1 - 3 | 2.2 (1.1 - 4.6) | 1.3 (0.6 - 2.5) |
| 4 - 12 | 0.2 (0.1 - 0.5)§ | 0.3 (0.1 - 0.5) § | |
| Bottle feeding | 1 - 3 | 9.5 (4.3 - 21.0) § | 1.8 (0.9 - 3.5) |
| 4 - 12 | 0.03 (0.003 - 0.2) § | 0.02 (0.004 - 0.1) § | |
| Minutes per feeding | 1 - 3 | 1.0 (0.9, 1.0) | 1.1 (1.0, 1.1) |
| Less than 10 minutes per feeding | 1 - 3 | 4.8 (1.7, 13.4) | 2.2 (0.6, 8.1) |
| Feedings per day | 1 - 3 | 0.7 (0.6, 0.8) § | 0.9 (0.8, 1.1) |
| Less than 7 feedings/day | 1 - 3 | 8.1 (3.4, 19.2) § | 1.8 (0.7, 4.6) |
| NOTE: Bold numbers significant at P = .05 or less; those marked with § are significant at P = .001 or less. | |||
| HR denotes hazard ratio; CI, confidence interval. | |||
| *Subjects answered affirmatively to any of the following types of pain: pain when latching on, pain while nursing, pain when not nursing. | |||
| † Measured in 3-week periods. | |||
| ‡ Indicates there were too few observations to provide meaningful results; for example, there were no Nebraska women who had mastitis and stopped breastfeeding in the same week during weeks 1-3. | |||
Subjective factors
At each interview, women who had stopped breastfeeding in the previous 3 weeks were asked why they had made that decision. Most women (75%) provided only one reason. At the first interview, insufficient milk supply (37.3%) and breast pain or mastitis (32.9%) were the most common reasons for termination (Table 3). Insufficient milk supply was the reason most often given (35.0%) during weeks 4 through 6. At both weeks 9 and 12, return to work was the reason given most often (53.1% and 58.3%, respectively).
TABLE 3
Percentage of women citing given reason for termination of breastfeeding
| Week 3 | Week 6 | Week 9 | Week 12 | |
|---|---|---|---|---|
| Reason | (n = 67) | (n = 60) | (n = 32) | (n = 36) |
| Insufficient milk supply | 37.3 | 35.0 | 25.0 | 13.9 |
| Inconvenient | 17.9 | 25.0 | 21.9 | 33.3 |
| Returned to work | 4.5 | 31.7 | 53.1 | 58.3 |
| Breast pain or infection | 32.9 | 23.3 | 0 | 5.6 |
| Baby stopped nursing | 7.5 | 5.0 | 3.1 | 11.1 |
| Other | 22.4 | 18.3 | 3.1 | 5.6 |
| NOTE: Percentages total more than 100% because respondents could cite multiple reasons. | ||||
Discussion
Mastitis, pain, and days with pain in the first 3 weeks were important clinical factors associated with breastfeeding cessation in this cohort of women who prenatally self-identified as intending to breastfeed. Women who intend to breastfeed should be counseled regarding these possible complications, their temporary nature, prevention, and treatment. Mastitis is not an indication for breastfeeding termination; in fact, increased feedings and milk expression are considered treatment.26,27 Women who reported pain the first 3 weeks were more likely to stop breastfeeding than women who reported pain after the first 3 weeks. It is difficult to explain this finding; perhaps there are women who have pain during their entire breastfeeding career and yet continue to breastfeed because they are more pain-tolerant, have less severe or frequent pain than those who wean, or are more committed to breastfeeding.
Other clinical factors investigated were depression and daily sleep hours. Weaning was not associated with subjective depression. However, subjects did not undergo formal psychological testing as in the study that reported an association.24 The relationship between daily sleep hours and termination was not consistent, and likely not clinically significant.
The demographic risk factors related to breast-feeding termination in our study are similar to those previously reported,14,15,20,21,23,24 namely, younger maternal age and lower educational level. Investigations of parity have been inconsistent.16,28 We found no association of weaning with parity. Prior breastfeeding experience has been reported as improving breastfeeding rates15,28; our results are consistent with those findings, but not significantly so. All subjects had access to prenatal breastfeeding education and postnatal breastfeeding support, which may have diminished the differences between women with breastfeeding experience and those without experience.20
Michigan and Nebraska women who pumped or bottle-fed during weeks 4 through 12 were significantly less likely to terminate breastfeeding. In contrast, Michigan women who pumped or bottle-fed during the first 3 weeks postpartum were more likely to terminate even after controlling for pain and mastitis. A commitment to exclusive breastfeeding may be necessary in the early postpartum period for long-term success.15,19 To our knowledge, the seemingly protective effect associated with pumping and bottle-feeding after the first 3 weeks has not been previously reported.
Breastfeeding 6 or fewer times per day and feedings of 10 minutes or less were associated with termination during the first 3 weeks. Other studies also indicate that the ratio of breast to bottle feedings is important for long-term success. Feinstein and colleagues15 found that more than one daily bottle of formula supplementation was associated with shorter breastfeeding duration, which was minimized if there were 7 or more breastfeedings per day. Another study found no weaning difference between women who offered their infant only one bottle daily during weeks 2 through 6 and a bottle-avoiding group.17
The most frequent reasons given for termination were similar to those reported by others, namely, insufficient milk supply and return to work.11-15,21,22 Insufficient milk supply was a more common reason in the first few weeks after birth; return to work became an increasingly common reason after week 6.
We were unable to examine the role of pacifiers or smoking in breastfeedng termination because pacifier information was not collected and there were too few smokers for meaningful analysis. Smoking has been consistently reported as associated with early cessation.15,20,29,30 Although pacifier use does not appear to be directly related,31,32 it has been proposed as a marker for breastfeeding problems. The homogeneity of the sample limits our ability to make generalizations regarding other populations, such as women of color. However, the large sample size and the similarity of termination risk factors between 2 different populations of women lend confidence to our conclusions. As we did not assess mothers’ intentions, some of the variables found associated with termination might be intentional activities of weaning rather than risk factors for termination. The significant difference in termination risk between the sites also may be related to mothers’ intentions or level of commitment. The Michigan women may have intended to breastfeed longer from the outset. The Michigan recruitment site was an alternative birthing center. Women being delivered there may be more persistent in their breast-feeding efforts. Both sites provided access to breast-feeding support personnel, but the Michigan women, as a group, may have been more motivated to continue.
Our results provide clinically useful information. Additional support may be needed for younger and less educated women. Special efforts should be made for early diagnosis and treatment of mastitis and breast pain, particularly during the first 3 weeks. Exclusive breastfeeding without bottle supplementation should be recommended for the first 3 weeks, with at least 7 feedings per day. Each feeding should preferably last more than 10 minutes.
These results should also reassure breastfeeding women and their providers regarding the use of bottles. Bottle-feeding after 3 weeks does not appear to jeopardize breastfeeding success up to 12 weeks and may even improve it.
* Table W1 appears on the JFP Web site at www.jfponline.com.
Acknowledgments
This study was supported by National Institutes of Health grant #30866.
1. American Academy of Family Physicians. Policies on Health Issues: Infant Health. URL: http://aafp.org/policy/issues/i3.html
2. Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr 1995;126:696-702.
3. Dewey K, Heinig M, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr 1995;126:191-7.
4. Duncan B, Ey J, Holberg CJ, Wright AL, Martinez FD, Taussig LM. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics 1993;91:867-72.
5. Raisler J, Alexander C, O’Campo P. Breast-feeding and infant illness: a dose-reponse relationship? Am J Public Health 2000;90:1478-9.
6. Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol 1998;81:523-33.
7. Newcomb P, Storer B, Longnecker M, et al. Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med 1994;330:81-7.
8. Cumming RG, Klinieberg RJ. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int J Epidemiol 1993;22:884-91.
9. Mother’s Survey, Ross Products Division, Abbot Laboratories, Inc. Columbus OH, 1998.
10. U.S. Department of Health and Human Services. Healthy People 2010. (Conference edition in 2 volumes.) Washington, DC: January 2000.
11. Gielen AC, Faden RR, O’Campo P, Brown CH, Paige DM. Maternal employment during the early postpartum period: effects on initiation and continuation of breastfeeding. Pediatrics 1991;87:298-305.
12. Fein SB, Roe B. The effect of work status on initiation and duration of breast-feeding. Am J Public Health 1998;88:1042-6.
13. Kurinij N, Shiono PH, Ezrine SF, Rhoads GG. Does maternal employment affect breast-feeding? Am J Public Health 1989;79:1247-50.
14. Kearney MH, Cronenwett L. Breastfeeding and employment. J Obstet Gynecol Neonatal Nurs 1991;20:471-80.
15. Feinstein JM, Berkelhamer JE, Gruszka ME, Wong CA, Carey AE. Factors related to early termination of breast-feeding in an urban population. Pediatrics 1986;78:210-5.
16. Ryan AS, Wysong JL, Martinez GA, Simon SD. Duration of breast-feeding patterns established in the hospital. Clin Pediatr 1990;29:99-107.
17. Cronenwett L, Strukel T, Kearney M, et al. Single daily bottle use in the early weeks postpartum and breast-feeding outcomes. Pediatrics 1992;90:760-6.
18. Gray-Donald K, Kramer MS, Munday S, Leduc DG. Effect of formula supplementation in the hospital on the duration of breast-feeding; a controlled clinical trial. Pediatrics 1985;75:514-8.
19. Hill PD, Humenick SS, Brennan ML, Woolley D. Does early supplementation affect long-term breastfeeding? Clin Pediatr 1997;June:345-350.
20. Wright HJ, Walker PC. Prediction of duration of breast feeding in primiparas. J Epidemiol Comm Health 1983;37:89-94.
21. Hawkins LM, Nichols FH, Tanner JL. Predictors of the duration of breastfeeding in low-income women. Birth 1987;14:204-9.
22. Hill PD, Aldag JC. Insufficient milk supply among black and white breast-feeding mothers. Res Nurs Health 1993;16:203-11.
23. Kurinij N, Shiono PH, Rhoads GG. Breast-feeding incidence and duration in black and white women. Pediatrics 1988;81:365-71.
24. Cooper PJ, Murray L, Stein A. Psychosocial factors associated with the early termination of breast-feeding. J Psychosom Res 1993;37:171-6.
25. Statistical Package for the Social Sciences. Chicago, IL: SPSS Inc; 1998.
26. Marshall B, Hepper J. Zirbel. Sporadic mastitis: an infection that need not interrupt lactation. JAMA 1975;233:1377-9.
27. Lawrence R. Mastitis. In: Breastfeeding: a guide for the medical profession. 4th ed. St. Louis: Mosby; 1994.
28. Hill PD, Humenick SS, Argubright T, Aldag JC. Effects of parity and weaning practices on breastfeeding duration. Public Health Nurs 1997;14:227-34.
29. Hill PD, Aldag JC. Smoking and breastfeeding status. Res Nurs Health 1996;19:125-32.
30. Woodward A, Hand K. Smoking and reduced duration of breast-feeding. Med J Australia 1988;148:477-8.
31. Victora CG, Behague DP, Barros FC, Olinto MT, Weiderpass E. Pacifier use and short breastfeeding duration: cause, consequence, or coincidence. Pediatrics 1997;99:445-3.
32. Howard CR, Howard FM, Lanphear B, deBlieck EA, Eberly S, Lawrence RA. The effects of early pacifier use on breastfeeding duration? Pediatrics 1999;103:E33.-
OBJECTIVE: To determine the demographic, behavioral, and clinical factors associated with breastfeeding termination in the first 12 weeks postpartum.
STUDY DESIGN: This was a prospective cohort study.
POPULATION: Breastfeeding women in Michigan and Nebraska were interviewed by telephone at 3, 6, 9, and 12 weeks postpartum or until breastfeeding termination.
OUTCOMES MEASURED: We measured associations of demographic, clinical, and breastfeeding variables with weaning during the first 12 weeks postpartum.
RESULTS: A total of 946 women participated; 75% breastfed until 12 weeks. Women older than 30 years and women with at least a bachelor’s degree were more likely to continue breastfeeding in any given week. Mastitis, breast or nipple pain, bottle use, and milk expression in the first 3 weeks were all associated with termination. Beyond 3 weeks, women who expressed breast milk were 75% less likely to discontinue breastfeeding than women who did not. Women who used a bottle for some feedings during weeks 4 to 12 were 98% less likely to discontinue breastfeeding than women who did not use a bottle. "Not enough milk" was the most common reason given for termination in weeks 1 through 3 (37%) and weeks 4 through 6 (35%); “return to work” was the most common reason given in weeks 7 through 9 (53%) and weeks 10 through 12 (58%).
CONCLUSIONS: Younger women and less educated women need additional support in their breastfeeding efforts. Counseling and assistance should be provided to women with pain and mastitis. Exclusive breastfeeding for the first 3 weeks should be recommended. After the first 3 weeks, bottles and manual expression are not associated with weaning and may improve the likelihood of continuing breastfeeding, at least until 12 weeks.
- Younger and less educated women may need extra support for long-term breastfeeding success.
- Exclusive breastfeeding for the first 3 weeks decreases the risk of early weaning. At least 7 daily feedings of 10 or more minutes per feeding are recommended.
- The use of bottles and manual expression of milk after 3 weeks does not increase the risk of early weaning.
Family physicians are strongly encouraged to support and promote breastfeeding, the optimal form of infant nutrition.1 Despite its known benefits (fewer infant infections2-6 and decreased maternal risks of premenopausal breast cancer7 and post-menopausal hip fractures8), only 64% of mothers initiated breastfeeding in 19989 and only 29% of mothers fed their 6-month-old infant by breast, well below the Healthy People 2010 goal of 50% breastfeeding at 6 months.10 Clearly, determining the factors that influence breastfeeding beyond the early postpartum period would be beneficial.
Returning to work is a consistent risk factor for weaning.11-14 The impact of early bottle-feeding on the duration of breastfeeding has been studied with less consistent results.15,20 Insufficient milk supply is a common subjective reason given for termination.15,19,21,22 Older women and those with a higher level of education are at less risk of early breastfeeding termination.9,11,15,16,21,23,24
Few investigators have described how breastfeeding patterns may affect breastfeeding duration. Little is known about the effects of timing, frequency, and duration of individual breastfeedings, or the roles of breast pain and infection, sleep, and manual expression on early weaning. We studied women who indicated their intent to breastfeed prenatally to identify demographic factors and breastfeeding patterns associated with weaning in the first 12 weeks postpartum.
Methods
Population
We interviewed breastfeeding women by telephone at 3, 6, 9, and 12 weeks postpartum to investigate lactation mastitis risk factors and predictors of weaning. Pregnant women intending to breastfeed were recruited from 2 geographic sites between June 1994 and January 1998. In suburban Detroit, Michigan, women attending orientation at a freestanding birthing center were asked to participate. In Omaha, Nebraska, women at a single large company were recruited when applying for maternity leave.
Data collection
During the computer-assisted interview, subjects were asked to recall each of the previous 3 weeks. The initial interview, which collected demographic information, typically lasted 15 to 20 minutes; subsequent interviews were shorter. The survey addressed breastfeeding practices and recent health events. Exclusive breastfeeders were women who fed their infants only by breast. We did not collect information on pacifiers; therefore, exclusively breastfed infants may have also received pacifiers. Women who manually expressed or used a device to assist in expression were classified as “pumping” their breasts. Respondents were asked if they had bottle-fed the infant; they were not asked about bottle contents or volume.
Subjects were queried on potential difficulties including breast or nipple pain while nursing, nipple cracks, and mastitis (diagnosed by a health care provider), as well as other health problems and behaviors. Subjects who had stopped breastfeeding in the previous 3 weeks were asked when and why, given a list of possible explanations and an open-ended opportunity. Respondents could provide multiple reasons for termination.
Data analysis
Kaplan-Meier estimates describe the distribution of weaning times for the 2 sites. A log-rank test was used to assess group differences. Relationships between demographic factors and time of weaning were assessed by Cox regression analysis. Discrete survival analysis was used to determine whether variables measured on a weekly basis were related to breastfeeding cessation. Hazard ratios describe the association of the exposures between women who stopped breastfeeding at a given time and those who continued. Because breastfeeding cessation was a rare event in later weeks of the study, as were certain clinical or behavioral breastfeeding factors, weeks 4-12 were collapsed into a single interval. Two variables, number of daily feedings and duration of each feeding, were examined only in the first 3 weeks because the information was often missing beyond 3 weeks. All analyses were performed using the Statistical Package for the Social Sciences.25
Results
Description of subjects
A total of 1057 women agreed to be contacted. Of those, 946 (89.5%) participated in at least 1 interview. Of the 111 women who did not participate, 11 refused and 100 could not be located. Six hundred fifty-eight (69.6%) women completed all 4 interviews. The 56 women who entered the study at week 6 because they could not be reached for the first interview were similar in all factors to women who entered earlier. Of the 946, 711 (75.2%) were from Michigan and 235 (24.8%) were from Nebraska.
Subjects from Michigan were significantly more likely than those from Nebraska to be older than 30 years (52.0% vs 38.3%), have at least a bachelor’s degree (62.9% vs 48.5%), have 3 or more children (38.5% vs 19.6%), and have had a vaginal delivery (99.6% vs 77.0%) (Table W1).* The groups were similar in race, household income, and marital status.
Demographic factors
A total of 673 women (71.1%) continued breastfeeding until 12 weeks; 28% were exclusive breastfeeders. Michigan women were more likely to breastfeed at weeks 2 through 12 than their Nebraskan counterparts (P < .0001, Figure). A college degree was associated with 40% less weaning (Table 1). Age and annual household income were directly related to continued breastfeeding at both sites. Number of children in the household was not associated with termination. Previous breastfeeding experience showed a nonsignificant but consistent trend toward lower weaning risk.
TABLE 1
Relationships of demographics and other characteristics with time to weaning, by site
| Characteristic | Michigan women HR* (95% CI) | Nebraska women HR* (95% CI) |
|---|---|---|
| Older than 30 years | 0.5 (0.3,0.8) | 0.7 (0.5, 1.1) |
| BA/BS or higher | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.8) |
| Number of children in household | ||
| 1 | 1.0 | 1.0 |
| 2 | 1.0 (0.6, 1.6) | 0.7 (0.5, 1.2) |
| 3 or more | 0.6 (0.4, 1.0) | 0.9 (0.6, 1.5) |
| Household income ≥ $50,000 | 0.8 (0.5, 1.3) | 0.7 (0.5, 1.0) |
| Breastfed previously | 0.7 (0.5, 1.1) | 0.7 (0.5, 1.1) |
| Nonvaginal birth | † | 0.9 (0.6, 1.4) |
| NOTE: Bold numbers are significant at P < .05. | ||
| HR denotes hazard ratio; CI, confidence interval; BA, bachelor of arts degree; BS, bachelor of science degree. | ||
| *A hazard ratio of <1 indicates that subjects with this characteristic were less likely to wean during the 12 weeks. Unless otherwise noted, the referent group is the converse (eg, age < 30 years is the referent group for those older than 30 years). | ||
| †Too few observations to provide meaningful results. | ||
FIGURE
Probability of breastfeeding, by site, by postpartum week
Clinical and behavioral factors
Because time to weaning differed significantly by site, the survival analyses of clinical and behavioral factors were performed separately for Michigan and Nebraska and controlled for education, age, and previous breastfeeding experience.
During the first 3 weeks, Michigan women with mastitis were nearly 6 times more likely than Michigan women without mastitis to stop breast-feeding in the week of diagnosis (Table 2). Women from Nebraska showed nonsignificant results in the same direction in weeks 4 to 12. (No women from Nebraska with mastitis terminated during weeks 1 through 3.) Although nipple sores and cracks were not associated with weaning, breast pain was associated with weaning. For each day of pain in the first 3 weeks, there was a 10% increase in risk of cessation among Michigan women and a 26% increase among Nebraska women. The association between pain and weaning in weeks 4 through 12 is less clear. In these later weeks, women who reported pain were unexpectedly 75% to 80% more likely to continue breastfeeding than women who did not report pain, yet for Nebraska women the number of days with pain remained significantly associated with breastfeeding cessation.
Subjective depression and breastfeeding cessation were not related. The association between daily sleep and weaning varied by site. During weeks 4 through 12, Michigan women with more daily sleep were less likely to terminate. An opposite, but marginally significant trend, was observed for Nebraska women. Weaning was not associated with outside household help. Nonvaginal birth was not associated with weaning for Nebraska women. (There were only 2 cesarean sections in the Michigan group.)
Michigan women who expressed breast milk during the first 3 weeks were twice as likely to stop breastfeeding as those who did not pump. During the same period, Michigan women who used a bottle for some feedings were 9 times more likely to wean than nonbottle users. Respondents in Nebraska showed similar nonsignificant trends in the first 3 weeks. By contrast, during weeks 4 through 12, both Nebraska and Michigan women who pumped were about 75% less likely to wean, while women who used a bottle for some feedings were 98% less likely to stop breastfeeding.
Breast milk expression increased gradually over time, from 30% of women pumping an average of 3 times per day in the first 3 weeks to 45% of women pumping 5 times per day in the last 3 weeks. To determine if pumping and bottle-feeding had an effect independent of pain or mastitis on weaning in the first 3 weeks, we performed additional analyses controlling for pain, cracks and sores, and mastitis in the same week. The results were similar to those presented in Table 2. Michigan women who pumped were 3 times more likely to wean than those who did not pump (hazard ratio [HR] = 3.0, 95% confidence interval [CI], 1.3 - 6.7), while for Nebraska women there was no association between pumping and weaning (HR = 0.6, 95% CI, 0.3 - 1.5). Bottle-feeding was again significantly associated with weaning in weeks 1 through 3 for Michigan women (HR = 10.9, 95% CI, 4.5 - 26.7) and not associated in Nebraskans (HR = 0.8, 95% CI, 0.4 - 2.0).
Duration and frequency of feedings were investigated as weaning risk factors. There appeared to be a threshold for both variables during the first 3 weeks in Michigan women. Michigan women who breastfed less than 10 minutes per feeding were nearly 5 times more likely to stop breastfeeding than women who breastfed longer. Michigan women who breastfed 6 or fewer times per day were 8 times more likely to stop than those who breastfed more often. Results for Nebraska women fell in the same direction but were not statistically significant.
TABLE 2
Relationships of clinical and behavioral factors to breastfeeding cessation in the same week, adjusted for mother’s age, education, and previous breastfeeding experience
| Variable | Week | Michigan women HR (95% CI) | Nebraska women HR (95% CI) |
|---|---|---|---|
| Mastitis | 1 - 3 | 5.7 (1.3 - 25.9) | ‡ |
| 4 - 12 | ‡ | 2.1 (0.3 - 17.4) | |
| Engorgement | 1 - 3 | 0.6 (0.2 - 1.5) | 0.8 (0.3 - 2.1) |
| 4 - 12 | ‡ | 3.2 (0.6 - 15.8) | |
| Nipple sores/cracks | 1 - 3 | 1.1 (0.4 - 2.6) | 0.9 (0.4 - 2.3) |
| 4 - 12 | 2.6 (0.8 - 8.5) | 2.9 (0.8 - 10.7) | |
| Any pain † | 1 - 3 | 14.7 (6.8 - 32.0)§ | 9.1 (3.9 - 21.2)† |
| 4 - 12 | 0.3 (0.1 - 0.7) | 0.2 (0.1 - 0.5)† | |
| Days with pain* | 1 - 3 | 1.1 (1.0 - 1.2) | 1.3 (1.0 - 1.5) |
| 4 - 12 | 1.1 (1.0 - 1.2) | 1.1 (1.0 - 1.2) | |
| Returned to work | 1 - 3 | 0.4 (0.1 - 3.0) | ‡ |
| 4 - 12 | 2.1 (1.1 - 4.0) | 0.8 (0.4 - 1.7) | |
| Depressed | 1 - 3 | 0.9 (0.3 - 3.0) | 1.0 (0.4 - 2.6) |
| 4 - 12 | 0.9 (0.4 - 2.2) | 1.3 (0.6 - 2.7) | |
| Daily sleep hours | 1 - 3 | 0.9 (0.7 - 1.1) | 0.9 (0.8 - 1.2) |
| 4 - 12 | 0.7 (0.5 - 0.9) | 1.2 (1.0 - 1.5) | |
| Outside household help | 1 - 3 | 2.0 (0.8 - 4.8) | 0.9 (0.4 - 2.1) |
| 4 - 12 | 0.7 (0.3 - 2.6) | 0.7 (0.2 - 2.1) | |
| Pumping | 1 - 3 | 2.2 (1.1 - 4.6) | 1.3 (0.6 - 2.5) |
| 4 - 12 | 0.2 (0.1 - 0.5)§ | 0.3 (0.1 - 0.5) § | |
| Bottle feeding | 1 - 3 | 9.5 (4.3 - 21.0) § | 1.8 (0.9 - 3.5) |
| 4 - 12 | 0.03 (0.003 - 0.2) § | 0.02 (0.004 - 0.1) § | |
| Minutes per feeding | 1 - 3 | 1.0 (0.9, 1.0) | 1.1 (1.0, 1.1) |
| Less than 10 minutes per feeding | 1 - 3 | 4.8 (1.7, 13.4) | 2.2 (0.6, 8.1) |
| Feedings per day | 1 - 3 | 0.7 (0.6, 0.8) § | 0.9 (0.8, 1.1) |
| Less than 7 feedings/day | 1 - 3 | 8.1 (3.4, 19.2) § | 1.8 (0.7, 4.6) |
| NOTE: Bold numbers significant at P = .05 or less; those marked with § are significant at P = .001 or less. | |||
| HR denotes hazard ratio; CI, confidence interval. | |||
| *Subjects answered affirmatively to any of the following types of pain: pain when latching on, pain while nursing, pain when not nursing. | |||
| † Measured in 3-week periods. | |||
| ‡ Indicates there were too few observations to provide meaningful results; for example, there were no Nebraska women who had mastitis and stopped breastfeeding in the same week during weeks 1-3. | |||
Subjective factors
At each interview, women who had stopped breastfeeding in the previous 3 weeks were asked why they had made that decision. Most women (75%) provided only one reason. At the first interview, insufficient milk supply (37.3%) and breast pain or mastitis (32.9%) were the most common reasons for termination (Table 3). Insufficient milk supply was the reason most often given (35.0%) during weeks 4 through 6. At both weeks 9 and 12, return to work was the reason given most often (53.1% and 58.3%, respectively).
TABLE 3
Percentage of women citing given reason for termination of breastfeeding
| Week 3 | Week 6 | Week 9 | Week 12 | |
|---|---|---|---|---|
| Reason | (n = 67) | (n = 60) | (n = 32) | (n = 36) |
| Insufficient milk supply | 37.3 | 35.0 | 25.0 | 13.9 |
| Inconvenient | 17.9 | 25.0 | 21.9 | 33.3 |
| Returned to work | 4.5 | 31.7 | 53.1 | 58.3 |
| Breast pain or infection | 32.9 | 23.3 | 0 | 5.6 |
| Baby stopped nursing | 7.5 | 5.0 | 3.1 | 11.1 |
| Other | 22.4 | 18.3 | 3.1 | 5.6 |
| NOTE: Percentages total more than 100% because respondents could cite multiple reasons. | ||||
Discussion
Mastitis, pain, and days with pain in the first 3 weeks were important clinical factors associated with breastfeeding cessation in this cohort of women who prenatally self-identified as intending to breastfeed. Women who intend to breastfeed should be counseled regarding these possible complications, their temporary nature, prevention, and treatment. Mastitis is not an indication for breastfeeding termination; in fact, increased feedings and milk expression are considered treatment.26,27 Women who reported pain the first 3 weeks were more likely to stop breastfeeding than women who reported pain after the first 3 weeks. It is difficult to explain this finding; perhaps there are women who have pain during their entire breastfeeding career and yet continue to breastfeed because they are more pain-tolerant, have less severe or frequent pain than those who wean, or are more committed to breastfeeding.
Other clinical factors investigated were depression and daily sleep hours. Weaning was not associated with subjective depression. However, subjects did not undergo formal psychological testing as in the study that reported an association.24 The relationship between daily sleep hours and termination was not consistent, and likely not clinically significant.
The demographic risk factors related to breast-feeding termination in our study are similar to those previously reported,14,15,20,21,23,24 namely, younger maternal age and lower educational level. Investigations of parity have been inconsistent.16,28 We found no association of weaning with parity. Prior breastfeeding experience has been reported as improving breastfeeding rates15,28; our results are consistent with those findings, but not significantly so. All subjects had access to prenatal breastfeeding education and postnatal breastfeeding support, which may have diminished the differences between women with breastfeeding experience and those without experience.20
Michigan and Nebraska women who pumped or bottle-fed during weeks 4 through 12 were significantly less likely to terminate breastfeeding. In contrast, Michigan women who pumped or bottle-fed during the first 3 weeks postpartum were more likely to terminate even after controlling for pain and mastitis. A commitment to exclusive breastfeeding may be necessary in the early postpartum period for long-term success.15,19 To our knowledge, the seemingly protective effect associated with pumping and bottle-feeding after the first 3 weeks has not been previously reported.
Breastfeeding 6 or fewer times per day and feedings of 10 minutes or less were associated with termination during the first 3 weeks. Other studies also indicate that the ratio of breast to bottle feedings is important for long-term success. Feinstein and colleagues15 found that more than one daily bottle of formula supplementation was associated with shorter breastfeeding duration, which was minimized if there were 7 or more breastfeedings per day. Another study found no weaning difference between women who offered their infant only one bottle daily during weeks 2 through 6 and a bottle-avoiding group.17
The most frequent reasons given for termination were similar to those reported by others, namely, insufficient milk supply and return to work.11-15,21,22 Insufficient milk supply was a more common reason in the first few weeks after birth; return to work became an increasingly common reason after week 6.
We were unable to examine the role of pacifiers or smoking in breastfeedng termination because pacifier information was not collected and there were too few smokers for meaningful analysis. Smoking has been consistently reported as associated with early cessation.15,20,29,30 Although pacifier use does not appear to be directly related,31,32 it has been proposed as a marker for breastfeeding problems. The homogeneity of the sample limits our ability to make generalizations regarding other populations, such as women of color. However, the large sample size and the similarity of termination risk factors between 2 different populations of women lend confidence to our conclusions. As we did not assess mothers’ intentions, some of the variables found associated with termination might be intentional activities of weaning rather than risk factors for termination. The significant difference in termination risk between the sites also may be related to mothers’ intentions or level of commitment. The Michigan women may have intended to breastfeed longer from the outset. The Michigan recruitment site was an alternative birthing center. Women being delivered there may be more persistent in their breast-feeding efforts. Both sites provided access to breast-feeding support personnel, but the Michigan women, as a group, may have been more motivated to continue.
Our results provide clinically useful information. Additional support may be needed for younger and less educated women. Special efforts should be made for early diagnosis and treatment of mastitis and breast pain, particularly during the first 3 weeks. Exclusive breastfeeding without bottle supplementation should be recommended for the first 3 weeks, with at least 7 feedings per day. Each feeding should preferably last more than 10 minutes.
These results should also reassure breastfeeding women and their providers regarding the use of bottles. Bottle-feeding after 3 weeks does not appear to jeopardize breastfeeding success up to 12 weeks and may even improve it.
* Table W1 appears on the JFP Web site at www.jfponline.com.
Acknowledgments
This study was supported by National Institutes of Health grant #30866.
OBJECTIVE: To determine the demographic, behavioral, and clinical factors associated with breastfeeding termination in the first 12 weeks postpartum.
STUDY DESIGN: This was a prospective cohort study.
POPULATION: Breastfeeding women in Michigan and Nebraska were interviewed by telephone at 3, 6, 9, and 12 weeks postpartum or until breastfeeding termination.
OUTCOMES MEASURED: We measured associations of demographic, clinical, and breastfeeding variables with weaning during the first 12 weeks postpartum.
RESULTS: A total of 946 women participated; 75% breastfed until 12 weeks. Women older than 30 years and women with at least a bachelor’s degree were more likely to continue breastfeeding in any given week. Mastitis, breast or nipple pain, bottle use, and milk expression in the first 3 weeks were all associated with termination. Beyond 3 weeks, women who expressed breast milk were 75% less likely to discontinue breastfeeding than women who did not. Women who used a bottle for some feedings during weeks 4 to 12 were 98% less likely to discontinue breastfeeding than women who did not use a bottle. "Not enough milk" was the most common reason given for termination in weeks 1 through 3 (37%) and weeks 4 through 6 (35%); “return to work” was the most common reason given in weeks 7 through 9 (53%) and weeks 10 through 12 (58%).
CONCLUSIONS: Younger women and less educated women need additional support in their breastfeeding efforts. Counseling and assistance should be provided to women with pain and mastitis. Exclusive breastfeeding for the first 3 weeks should be recommended. After the first 3 weeks, bottles and manual expression are not associated with weaning and may improve the likelihood of continuing breastfeeding, at least until 12 weeks.
- Younger and less educated women may need extra support for long-term breastfeeding success.
- Exclusive breastfeeding for the first 3 weeks decreases the risk of early weaning. At least 7 daily feedings of 10 or more minutes per feeding are recommended.
- The use of bottles and manual expression of milk after 3 weeks does not increase the risk of early weaning.
Family physicians are strongly encouraged to support and promote breastfeeding, the optimal form of infant nutrition.1 Despite its known benefits (fewer infant infections2-6 and decreased maternal risks of premenopausal breast cancer7 and post-menopausal hip fractures8), only 64% of mothers initiated breastfeeding in 19989 and only 29% of mothers fed their 6-month-old infant by breast, well below the Healthy People 2010 goal of 50% breastfeeding at 6 months.10 Clearly, determining the factors that influence breastfeeding beyond the early postpartum period would be beneficial.
Returning to work is a consistent risk factor for weaning.11-14 The impact of early bottle-feeding on the duration of breastfeeding has been studied with less consistent results.15,20 Insufficient milk supply is a common subjective reason given for termination.15,19,21,22 Older women and those with a higher level of education are at less risk of early breastfeeding termination.9,11,15,16,21,23,24
Few investigators have described how breastfeeding patterns may affect breastfeeding duration. Little is known about the effects of timing, frequency, and duration of individual breastfeedings, or the roles of breast pain and infection, sleep, and manual expression on early weaning. We studied women who indicated their intent to breastfeed prenatally to identify demographic factors and breastfeeding patterns associated with weaning in the first 12 weeks postpartum.
Methods
Population
We interviewed breastfeeding women by telephone at 3, 6, 9, and 12 weeks postpartum to investigate lactation mastitis risk factors and predictors of weaning. Pregnant women intending to breastfeed were recruited from 2 geographic sites between June 1994 and January 1998. In suburban Detroit, Michigan, women attending orientation at a freestanding birthing center were asked to participate. In Omaha, Nebraska, women at a single large company were recruited when applying for maternity leave.
Data collection
During the computer-assisted interview, subjects were asked to recall each of the previous 3 weeks. The initial interview, which collected demographic information, typically lasted 15 to 20 minutes; subsequent interviews were shorter. The survey addressed breastfeeding practices and recent health events. Exclusive breastfeeders were women who fed their infants only by breast. We did not collect information on pacifiers; therefore, exclusively breastfed infants may have also received pacifiers. Women who manually expressed or used a device to assist in expression were classified as “pumping” their breasts. Respondents were asked if they had bottle-fed the infant; they were not asked about bottle contents or volume.
Subjects were queried on potential difficulties including breast or nipple pain while nursing, nipple cracks, and mastitis (diagnosed by a health care provider), as well as other health problems and behaviors. Subjects who had stopped breastfeeding in the previous 3 weeks were asked when and why, given a list of possible explanations and an open-ended opportunity. Respondents could provide multiple reasons for termination.
Data analysis
Kaplan-Meier estimates describe the distribution of weaning times for the 2 sites. A log-rank test was used to assess group differences. Relationships between demographic factors and time of weaning were assessed by Cox regression analysis. Discrete survival analysis was used to determine whether variables measured on a weekly basis were related to breastfeeding cessation. Hazard ratios describe the association of the exposures between women who stopped breastfeeding at a given time and those who continued. Because breastfeeding cessation was a rare event in later weeks of the study, as were certain clinical or behavioral breastfeeding factors, weeks 4-12 were collapsed into a single interval. Two variables, number of daily feedings and duration of each feeding, were examined only in the first 3 weeks because the information was often missing beyond 3 weeks. All analyses were performed using the Statistical Package for the Social Sciences.25
Results
Description of subjects
A total of 1057 women agreed to be contacted. Of those, 946 (89.5%) participated in at least 1 interview. Of the 111 women who did not participate, 11 refused and 100 could not be located. Six hundred fifty-eight (69.6%) women completed all 4 interviews. The 56 women who entered the study at week 6 because they could not be reached for the first interview were similar in all factors to women who entered earlier. Of the 946, 711 (75.2%) were from Michigan and 235 (24.8%) were from Nebraska.
Subjects from Michigan were significantly more likely than those from Nebraska to be older than 30 years (52.0% vs 38.3%), have at least a bachelor’s degree (62.9% vs 48.5%), have 3 or more children (38.5% vs 19.6%), and have had a vaginal delivery (99.6% vs 77.0%) (Table W1).* The groups were similar in race, household income, and marital status.
Demographic factors
A total of 673 women (71.1%) continued breastfeeding until 12 weeks; 28% were exclusive breastfeeders. Michigan women were more likely to breastfeed at weeks 2 through 12 than their Nebraskan counterparts (P < .0001, Figure). A college degree was associated with 40% less weaning (Table 1). Age and annual household income were directly related to continued breastfeeding at both sites. Number of children in the household was not associated with termination. Previous breastfeeding experience showed a nonsignificant but consistent trend toward lower weaning risk.
TABLE 1
Relationships of demographics and other characteristics with time to weaning, by site
| Characteristic | Michigan women HR* (95% CI) | Nebraska women HR* (95% CI) |
|---|---|---|
| Older than 30 years | 0.5 (0.3,0.8) | 0.7 (0.5, 1.1) |
| BA/BS or higher | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.8) |
| Number of children in household | ||
| 1 | 1.0 | 1.0 |
| 2 | 1.0 (0.6, 1.6) | 0.7 (0.5, 1.2) |
| 3 or more | 0.6 (0.4, 1.0) | 0.9 (0.6, 1.5) |
| Household income ≥ $50,000 | 0.8 (0.5, 1.3) | 0.7 (0.5, 1.0) |
| Breastfed previously | 0.7 (0.5, 1.1) | 0.7 (0.5, 1.1) |
| Nonvaginal birth | † | 0.9 (0.6, 1.4) |
| NOTE: Bold numbers are significant at P < .05. | ||
| HR denotes hazard ratio; CI, confidence interval; BA, bachelor of arts degree; BS, bachelor of science degree. | ||
| *A hazard ratio of <1 indicates that subjects with this characteristic were less likely to wean during the 12 weeks. Unless otherwise noted, the referent group is the converse (eg, age < 30 years is the referent group for those older than 30 years). | ||
| †Too few observations to provide meaningful results. | ||
FIGURE
Probability of breastfeeding, by site, by postpartum week
Clinical and behavioral factors
Because time to weaning differed significantly by site, the survival analyses of clinical and behavioral factors were performed separately for Michigan and Nebraska and controlled for education, age, and previous breastfeeding experience.
During the first 3 weeks, Michigan women with mastitis were nearly 6 times more likely than Michigan women without mastitis to stop breast-feeding in the week of diagnosis (Table 2). Women from Nebraska showed nonsignificant results in the same direction in weeks 4 to 12. (No women from Nebraska with mastitis terminated during weeks 1 through 3.) Although nipple sores and cracks were not associated with weaning, breast pain was associated with weaning. For each day of pain in the first 3 weeks, there was a 10% increase in risk of cessation among Michigan women and a 26% increase among Nebraska women. The association between pain and weaning in weeks 4 through 12 is less clear. In these later weeks, women who reported pain were unexpectedly 75% to 80% more likely to continue breastfeeding than women who did not report pain, yet for Nebraska women the number of days with pain remained significantly associated with breastfeeding cessation.
Subjective depression and breastfeeding cessation were not related. The association between daily sleep and weaning varied by site. During weeks 4 through 12, Michigan women with more daily sleep were less likely to terminate. An opposite, but marginally significant trend, was observed for Nebraska women. Weaning was not associated with outside household help. Nonvaginal birth was not associated with weaning for Nebraska women. (There were only 2 cesarean sections in the Michigan group.)
Michigan women who expressed breast milk during the first 3 weeks were twice as likely to stop breastfeeding as those who did not pump. During the same period, Michigan women who used a bottle for some feedings were 9 times more likely to wean than nonbottle users. Respondents in Nebraska showed similar nonsignificant trends in the first 3 weeks. By contrast, during weeks 4 through 12, both Nebraska and Michigan women who pumped were about 75% less likely to wean, while women who used a bottle for some feedings were 98% less likely to stop breastfeeding.
Breast milk expression increased gradually over time, from 30% of women pumping an average of 3 times per day in the first 3 weeks to 45% of women pumping 5 times per day in the last 3 weeks. To determine if pumping and bottle-feeding had an effect independent of pain or mastitis on weaning in the first 3 weeks, we performed additional analyses controlling for pain, cracks and sores, and mastitis in the same week. The results were similar to those presented in Table 2. Michigan women who pumped were 3 times more likely to wean than those who did not pump (hazard ratio [HR] = 3.0, 95% confidence interval [CI], 1.3 - 6.7), while for Nebraska women there was no association between pumping and weaning (HR = 0.6, 95% CI, 0.3 - 1.5). Bottle-feeding was again significantly associated with weaning in weeks 1 through 3 for Michigan women (HR = 10.9, 95% CI, 4.5 - 26.7) and not associated in Nebraskans (HR = 0.8, 95% CI, 0.4 - 2.0).
Duration and frequency of feedings were investigated as weaning risk factors. There appeared to be a threshold for both variables during the first 3 weeks in Michigan women. Michigan women who breastfed less than 10 minutes per feeding were nearly 5 times more likely to stop breastfeeding than women who breastfed longer. Michigan women who breastfed 6 or fewer times per day were 8 times more likely to stop than those who breastfed more often. Results for Nebraska women fell in the same direction but were not statistically significant.
TABLE 2
Relationships of clinical and behavioral factors to breastfeeding cessation in the same week, adjusted for mother’s age, education, and previous breastfeeding experience
| Variable | Week | Michigan women HR (95% CI) | Nebraska women HR (95% CI) |
|---|---|---|---|
| Mastitis | 1 - 3 | 5.7 (1.3 - 25.9) | ‡ |
| 4 - 12 | ‡ | 2.1 (0.3 - 17.4) | |
| Engorgement | 1 - 3 | 0.6 (0.2 - 1.5) | 0.8 (0.3 - 2.1) |
| 4 - 12 | ‡ | 3.2 (0.6 - 15.8) | |
| Nipple sores/cracks | 1 - 3 | 1.1 (0.4 - 2.6) | 0.9 (0.4 - 2.3) |
| 4 - 12 | 2.6 (0.8 - 8.5) | 2.9 (0.8 - 10.7) | |
| Any pain † | 1 - 3 | 14.7 (6.8 - 32.0)§ | 9.1 (3.9 - 21.2)† |
| 4 - 12 | 0.3 (0.1 - 0.7) | 0.2 (0.1 - 0.5)† | |
| Days with pain* | 1 - 3 | 1.1 (1.0 - 1.2) | 1.3 (1.0 - 1.5) |
| 4 - 12 | 1.1 (1.0 - 1.2) | 1.1 (1.0 - 1.2) | |
| Returned to work | 1 - 3 | 0.4 (0.1 - 3.0) | ‡ |
| 4 - 12 | 2.1 (1.1 - 4.0) | 0.8 (0.4 - 1.7) | |
| Depressed | 1 - 3 | 0.9 (0.3 - 3.0) | 1.0 (0.4 - 2.6) |
| 4 - 12 | 0.9 (0.4 - 2.2) | 1.3 (0.6 - 2.7) | |
| Daily sleep hours | 1 - 3 | 0.9 (0.7 - 1.1) | 0.9 (0.8 - 1.2) |
| 4 - 12 | 0.7 (0.5 - 0.9) | 1.2 (1.0 - 1.5) | |
| Outside household help | 1 - 3 | 2.0 (0.8 - 4.8) | 0.9 (0.4 - 2.1) |
| 4 - 12 | 0.7 (0.3 - 2.6) | 0.7 (0.2 - 2.1) | |
| Pumping | 1 - 3 | 2.2 (1.1 - 4.6) | 1.3 (0.6 - 2.5) |
| 4 - 12 | 0.2 (0.1 - 0.5)§ | 0.3 (0.1 - 0.5) § | |
| Bottle feeding | 1 - 3 | 9.5 (4.3 - 21.0) § | 1.8 (0.9 - 3.5) |
| 4 - 12 | 0.03 (0.003 - 0.2) § | 0.02 (0.004 - 0.1) § | |
| Minutes per feeding | 1 - 3 | 1.0 (0.9, 1.0) | 1.1 (1.0, 1.1) |
| Less than 10 minutes per feeding | 1 - 3 | 4.8 (1.7, 13.4) | 2.2 (0.6, 8.1) |
| Feedings per day | 1 - 3 | 0.7 (0.6, 0.8) § | 0.9 (0.8, 1.1) |
| Less than 7 feedings/day | 1 - 3 | 8.1 (3.4, 19.2) § | 1.8 (0.7, 4.6) |
| NOTE: Bold numbers significant at P = .05 or less; those marked with § are significant at P = .001 or less. | |||
| HR denotes hazard ratio; CI, confidence interval. | |||
| *Subjects answered affirmatively to any of the following types of pain: pain when latching on, pain while nursing, pain when not nursing. | |||
| † Measured in 3-week periods. | |||
| ‡ Indicates there were too few observations to provide meaningful results; for example, there were no Nebraska women who had mastitis and stopped breastfeeding in the same week during weeks 1-3. | |||
Subjective factors
At each interview, women who had stopped breastfeeding in the previous 3 weeks were asked why they had made that decision. Most women (75%) provided only one reason. At the first interview, insufficient milk supply (37.3%) and breast pain or mastitis (32.9%) were the most common reasons for termination (Table 3). Insufficient milk supply was the reason most often given (35.0%) during weeks 4 through 6. At both weeks 9 and 12, return to work was the reason given most often (53.1% and 58.3%, respectively).
TABLE 3
Percentage of women citing given reason for termination of breastfeeding
| Week 3 | Week 6 | Week 9 | Week 12 | |
|---|---|---|---|---|
| Reason | (n = 67) | (n = 60) | (n = 32) | (n = 36) |
| Insufficient milk supply | 37.3 | 35.0 | 25.0 | 13.9 |
| Inconvenient | 17.9 | 25.0 | 21.9 | 33.3 |
| Returned to work | 4.5 | 31.7 | 53.1 | 58.3 |
| Breast pain or infection | 32.9 | 23.3 | 0 | 5.6 |
| Baby stopped nursing | 7.5 | 5.0 | 3.1 | 11.1 |
| Other | 22.4 | 18.3 | 3.1 | 5.6 |
| NOTE: Percentages total more than 100% because respondents could cite multiple reasons. | ||||
Discussion
Mastitis, pain, and days with pain in the first 3 weeks were important clinical factors associated with breastfeeding cessation in this cohort of women who prenatally self-identified as intending to breastfeed. Women who intend to breastfeed should be counseled regarding these possible complications, their temporary nature, prevention, and treatment. Mastitis is not an indication for breastfeeding termination; in fact, increased feedings and milk expression are considered treatment.26,27 Women who reported pain the first 3 weeks were more likely to stop breastfeeding than women who reported pain after the first 3 weeks. It is difficult to explain this finding; perhaps there are women who have pain during their entire breastfeeding career and yet continue to breastfeed because they are more pain-tolerant, have less severe or frequent pain than those who wean, or are more committed to breastfeeding.
Other clinical factors investigated were depression and daily sleep hours. Weaning was not associated with subjective depression. However, subjects did not undergo formal psychological testing as in the study that reported an association.24 The relationship between daily sleep hours and termination was not consistent, and likely not clinically significant.
The demographic risk factors related to breast-feeding termination in our study are similar to those previously reported,14,15,20,21,23,24 namely, younger maternal age and lower educational level. Investigations of parity have been inconsistent.16,28 We found no association of weaning with parity. Prior breastfeeding experience has been reported as improving breastfeeding rates15,28; our results are consistent with those findings, but not significantly so. All subjects had access to prenatal breastfeeding education and postnatal breastfeeding support, which may have diminished the differences between women with breastfeeding experience and those without experience.20
Michigan and Nebraska women who pumped or bottle-fed during weeks 4 through 12 were significantly less likely to terminate breastfeeding. In contrast, Michigan women who pumped or bottle-fed during the first 3 weeks postpartum were more likely to terminate even after controlling for pain and mastitis. A commitment to exclusive breastfeeding may be necessary in the early postpartum period for long-term success.15,19 To our knowledge, the seemingly protective effect associated with pumping and bottle-feeding after the first 3 weeks has not been previously reported.
Breastfeeding 6 or fewer times per day and feedings of 10 minutes or less were associated with termination during the first 3 weeks. Other studies also indicate that the ratio of breast to bottle feedings is important for long-term success. Feinstein and colleagues15 found that more than one daily bottle of formula supplementation was associated with shorter breastfeeding duration, which was minimized if there were 7 or more breastfeedings per day. Another study found no weaning difference between women who offered their infant only one bottle daily during weeks 2 through 6 and a bottle-avoiding group.17
The most frequent reasons given for termination were similar to those reported by others, namely, insufficient milk supply and return to work.11-15,21,22 Insufficient milk supply was a more common reason in the first few weeks after birth; return to work became an increasingly common reason after week 6.
We were unable to examine the role of pacifiers or smoking in breastfeedng termination because pacifier information was not collected and there were too few smokers for meaningful analysis. Smoking has been consistently reported as associated with early cessation.15,20,29,30 Although pacifier use does not appear to be directly related,31,32 it has been proposed as a marker for breastfeeding problems. The homogeneity of the sample limits our ability to make generalizations regarding other populations, such as women of color. However, the large sample size and the similarity of termination risk factors between 2 different populations of women lend confidence to our conclusions. As we did not assess mothers’ intentions, some of the variables found associated with termination might be intentional activities of weaning rather than risk factors for termination. The significant difference in termination risk between the sites also may be related to mothers’ intentions or level of commitment. The Michigan women may have intended to breastfeed longer from the outset. The Michigan recruitment site was an alternative birthing center. Women being delivered there may be more persistent in their breast-feeding efforts. Both sites provided access to breast-feeding support personnel, but the Michigan women, as a group, may have been more motivated to continue.
Our results provide clinically useful information. Additional support may be needed for younger and less educated women. Special efforts should be made for early diagnosis and treatment of mastitis and breast pain, particularly during the first 3 weeks. Exclusive breastfeeding without bottle supplementation should be recommended for the first 3 weeks, with at least 7 feedings per day. Each feeding should preferably last more than 10 minutes.
These results should also reassure breastfeeding women and their providers regarding the use of bottles. Bottle-feeding after 3 weeks does not appear to jeopardize breastfeeding success up to 12 weeks and may even improve it.
* Table W1 appears on the JFP Web site at www.jfponline.com.
Acknowledgments
This study was supported by National Institutes of Health grant #30866.
1. American Academy of Family Physicians. Policies on Health Issues: Infant Health. URL: http://aafp.org/policy/issues/i3.html
2. Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr 1995;126:696-702.
3. Dewey K, Heinig M, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr 1995;126:191-7.
4. Duncan B, Ey J, Holberg CJ, Wright AL, Martinez FD, Taussig LM. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics 1993;91:867-72.
5. Raisler J, Alexander C, O’Campo P. Breast-feeding and infant illness: a dose-reponse relationship? Am J Public Health 2000;90:1478-9.
6. Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol 1998;81:523-33.
7. Newcomb P, Storer B, Longnecker M, et al. Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med 1994;330:81-7.
8. Cumming RG, Klinieberg RJ. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int J Epidemiol 1993;22:884-91.
9. Mother’s Survey, Ross Products Division, Abbot Laboratories, Inc. Columbus OH, 1998.
10. U.S. Department of Health and Human Services. Healthy People 2010. (Conference edition in 2 volumes.) Washington, DC: January 2000.
11. Gielen AC, Faden RR, O’Campo P, Brown CH, Paige DM. Maternal employment during the early postpartum period: effects on initiation and continuation of breastfeeding. Pediatrics 1991;87:298-305.
12. Fein SB, Roe B. The effect of work status on initiation and duration of breast-feeding. Am J Public Health 1998;88:1042-6.
13. Kurinij N, Shiono PH, Ezrine SF, Rhoads GG. Does maternal employment affect breast-feeding? Am J Public Health 1989;79:1247-50.
14. Kearney MH, Cronenwett L. Breastfeeding and employment. J Obstet Gynecol Neonatal Nurs 1991;20:471-80.
15. Feinstein JM, Berkelhamer JE, Gruszka ME, Wong CA, Carey AE. Factors related to early termination of breast-feeding in an urban population. Pediatrics 1986;78:210-5.
16. Ryan AS, Wysong JL, Martinez GA, Simon SD. Duration of breast-feeding patterns established in the hospital. Clin Pediatr 1990;29:99-107.
17. Cronenwett L, Strukel T, Kearney M, et al. Single daily bottle use in the early weeks postpartum and breast-feeding outcomes. Pediatrics 1992;90:760-6.
18. Gray-Donald K, Kramer MS, Munday S, Leduc DG. Effect of formula supplementation in the hospital on the duration of breast-feeding; a controlled clinical trial. Pediatrics 1985;75:514-8.
19. Hill PD, Humenick SS, Brennan ML, Woolley D. Does early supplementation affect long-term breastfeeding? Clin Pediatr 1997;June:345-350.
20. Wright HJ, Walker PC. Prediction of duration of breast feeding in primiparas. J Epidemiol Comm Health 1983;37:89-94.
21. Hawkins LM, Nichols FH, Tanner JL. Predictors of the duration of breastfeeding in low-income women. Birth 1987;14:204-9.
22. Hill PD, Aldag JC. Insufficient milk supply among black and white breast-feeding mothers. Res Nurs Health 1993;16:203-11.
23. Kurinij N, Shiono PH, Rhoads GG. Breast-feeding incidence and duration in black and white women. Pediatrics 1988;81:365-71.
24. Cooper PJ, Murray L, Stein A. Psychosocial factors associated with the early termination of breast-feeding. J Psychosom Res 1993;37:171-6.
25. Statistical Package for the Social Sciences. Chicago, IL: SPSS Inc; 1998.
26. Marshall B, Hepper J. Zirbel. Sporadic mastitis: an infection that need not interrupt lactation. JAMA 1975;233:1377-9.
27. Lawrence R. Mastitis. In: Breastfeeding: a guide for the medical profession. 4th ed. St. Louis: Mosby; 1994.
28. Hill PD, Humenick SS, Argubright T, Aldag JC. Effects of parity and weaning practices on breastfeeding duration. Public Health Nurs 1997;14:227-34.
29. Hill PD, Aldag JC. Smoking and breastfeeding status. Res Nurs Health 1996;19:125-32.
30. Woodward A, Hand K. Smoking and reduced duration of breast-feeding. Med J Australia 1988;148:477-8.
31. Victora CG, Behague DP, Barros FC, Olinto MT, Weiderpass E. Pacifier use and short breastfeeding duration: cause, consequence, or coincidence. Pediatrics 1997;99:445-3.
32. Howard CR, Howard FM, Lanphear B, deBlieck EA, Eberly S, Lawrence RA. The effects of early pacifier use on breastfeeding duration? Pediatrics 1999;103:E33.-
1. American Academy of Family Physicians. Policies on Health Issues: Infant Health. URL: http://aafp.org/policy/issues/i3.html
2. Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr 1995;126:696-702.
3. Dewey K, Heinig M, Nommsen-Rivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr 1995;126:191-7.
4. Duncan B, Ey J, Holberg CJ, Wright AL, Martinez FD, Taussig LM. Exclusive breast-feeding for at least 4 months protects against otitis media. Pediatrics 1993;91:867-72.
5. Raisler J, Alexander C, O’Campo P. Breast-feeding and infant illness: a dose-reponse relationship? Am J Public Health 2000;90:1478-9.
6. Hanson LA. Breastfeeding provides passive and likely long-lasting active immunity. Ann Allergy Asthma Immunol 1998;81:523-33.
7. Newcomb P, Storer B, Longnecker M, et al. Lactation and a reduced risk of premenopausal breast cancer. N Engl J Med 1994;330:81-7.
8. Cumming RG, Klinieberg RJ. Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. Int J Epidemiol 1993;22:884-91.
9. Mother’s Survey, Ross Products Division, Abbot Laboratories, Inc. Columbus OH, 1998.
10. U.S. Department of Health and Human Services. Healthy People 2010. (Conference edition in 2 volumes.) Washington, DC: January 2000.
11. Gielen AC, Faden RR, O’Campo P, Brown CH, Paige DM. Maternal employment during the early postpartum period: effects on initiation and continuation of breastfeeding. Pediatrics 1991;87:298-305.
12. Fein SB, Roe B. The effect of work status on initiation and duration of breast-feeding. Am J Public Health 1998;88:1042-6.
13. Kurinij N, Shiono PH, Ezrine SF, Rhoads GG. Does maternal employment affect breast-feeding? Am J Public Health 1989;79:1247-50.
14. Kearney MH, Cronenwett L. Breastfeeding and employment. J Obstet Gynecol Neonatal Nurs 1991;20:471-80.
15. Feinstein JM, Berkelhamer JE, Gruszka ME, Wong CA, Carey AE. Factors related to early termination of breast-feeding in an urban population. Pediatrics 1986;78:210-5.
16. Ryan AS, Wysong JL, Martinez GA, Simon SD. Duration of breast-feeding patterns established in the hospital. Clin Pediatr 1990;29:99-107.
17. Cronenwett L, Strukel T, Kearney M, et al. Single daily bottle use in the early weeks postpartum and breast-feeding outcomes. Pediatrics 1992;90:760-6.
18. Gray-Donald K, Kramer MS, Munday S, Leduc DG. Effect of formula supplementation in the hospital on the duration of breast-feeding; a controlled clinical trial. Pediatrics 1985;75:514-8.
19. Hill PD, Humenick SS, Brennan ML, Woolley D. Does early supplementation affect long-term breastfeeding? Clin Pediatr 1997;June:345-350.
20. Wright HJ, Walker PC. Prediction of duration of breast feeding in primiparas. J Epidemiol Comm Health 1983;37:89-94.
21. Hawkins LM, Nichols FH, Tanner JL. Predictors of the duration of breastfeeding in low-income women. Birth 1987;14:204-9.
22. Hill PD, Aldag JC. Insufficient milk supply among black and white breast-feeding mothers. Res Nurs Health 1993;16:203-11.
23. Kurinij N, Shiono PH, Rhoads GG. Breast-feeding incidence and duration in black and white women. Pediatrics 1988;81:365-71.
24. Cooper PJ, Murray L, Stein A. Psychosocial factors associated with the early termination of breast-feeding. J Psychosom Res 1993;37:171-6.
25. Statistical Package for the Social Sciences. Chicago, IL: SPSS Inc; 1998.
26. Marshall B, Hepper J. Zirbel. Sporadic mastitis: an infection that need not interrupt lactation. JAMA 1975;233:1377-9.
27. Lawrence R. Mastitis. In: Breastfeeding: a guide for the medical profession. 4th ed. St. Louis: Mosby; 1994.
28. Hill PD, Humenick SS, Argubright T, Aldag JC. Effects of parity and weaning practices on breastfeeding duration. Public Health Nurs 1997;14:227-34.
29. Hill PD, Aldag JC. Smoking and breastfeeding status. Res Nurs Health 1996;19:125-32.
30. Woodward A, Hand K. Smoking and reduced duration of breast-feeding. Med J Australia 1988;148:477-8.
31. Victora CG, Behague DP, Barros FC, Olinto MT, Weiderpass E. Pacifier use and short breastfeeding duration: cause, consequence, or coincidence. Pediatrics 1997;99:445-3.
32. Howard CR, Howard FM, Lanphear B, deBlieck EA, Eberly S, Lawrence RA. The effects of early pacifier use on breastfeeding duration? Pediatrics 1999;103:E33.-
What is the most effective regimen for eradication of Helicobacter pylori in patients who have failed a first eradication attempt?
ABSTRACT
BACKGROUND: Based on randomized clinical trials (RCTs), the most effective first-line eradication therapy for Helicobacter pylori is a combination of proton pump inhibitor (PPI) and 2 antimicrobial agents.1 Yet there remains a significant treatment failure rate of 5% to 25%. Antibiotic resistance is the major impediment of cure.2 Using a pooled analysis approach, the authors determined the second-line treatment strategy resulting in the greatest percentage of H pylorieradication.
POPULATION STUDIED: Studies of H pylori re-eradication in adults were retrieved from MEDLINE database, reference lists of retrieved research papers, and major congress abstract lists. All studies were performed between 1994 and 1999, were conducted prospectively (or without information on study design), contained detailed information on eradication agents, and included subjects with only one treatment failure. Eighteen articles and 47 abstracts were identified; 16 articles and 24 abstracts met the inclusion criteria.
STUDY DESIGN AND VALIDITY: The authors performed a pooled efficacy analysis of re-treatment regimens for H pylori eradication in adults. They included all prospective studies—randomized and nonrandomized—that reported eradication rates in patients previously treated with antibiotic therapy. The inclusion criteria were appropriate, and the search for relevant articles was complete in that the authors included abstracts from international gastroenterology meetings. An analysis strategy of simple pooling was used (total number of patients successfully treated divided by all those enrolled in given treatment category), which is appropriate since the primary outcome was the eradication rate.
OUTCOMES MEASURED: The authors calculated a pooled eradication rate with 95% confidence intervals for each of the 6 general treatment categories. They also determined the eradication rate of each second treatment, accounting for differences in initial treatment therapy regimen. Finally, they ascertained if the effectiveness of second-line therapy regimens improved when distinct antimicrobials not used in the first attempt at treatment were used.
RESULTS: The most effective second-line therapies for eradication of H pylori were quadruple therapy, either ranitidine-bismuth-based triple therapy (ranitidine-bismuth product plus 2 antimicrobials) at 80.2% (95% confidence interval [CI], 75%-85%) or H2-blocker or PPI, bismuth compound, and 2 antimicrobials) at 75.8% (95% CI, 73%-79%). The second-line treatment eradication rate was lower when the initial therapy was a PPI with 2 antimicrobials versus a PPI with one antimicrobial. Re-treatment was more difficult with an increased number of antimicrobials used in initial therapy. The re-treatment eradication rate was greater when 2 new antimicrobials were included in the regimen than when a single new antimicrobial was added (P=.0064).
If initial attempts fail at eradication of H pylori then quadruple therapy (an antisecretory agent, a bismuth compound, and 2 antimicrobials) or ranitidine-bismuth (Tritec) plus 2 antimicrobials is the most effective follow-up treatment. The latter approach is fairly expensive ($150). For patients whose first course included only one antimicrobial, using 2 new antimicrobials is just as effective as quadruple or ranitidine bismuth-based therapy. These approaches will achieve eradication in 75% to 80% of resistant cases.
ABSTRACT
BACKGROUND: Based on randomized clinical trials (RCTs), the most effective first-line eradication therapy for Helicobacter pylori is a combination of proton pump inhibitor (PPI) and 2 antimicrobial agents.1 Yet there remains a significant treatment failure rate of 5% to 25%. Antibiotic resistance is the major impediment of cure.2 Using a pooled analysis approach, the authors determined the second-line treatment strategy resulting in the greatest percentage of H pylorieradication.
POPULATION STUDIED: Studies of H pylori re-eradication in adults were retrieved from MEDLINE database, reference lists of retrieved research papers, and major congress abstract lists. All studies were performed between 1994 and 1999, were conducted prospectively (or without information on study design), contained detailed information on eradication agents, and included subjects with only one treatment failure. Eighteen articles and 47 abstracts were identified; 16 articles and 24 abstracts met the inclusion criteria.
STUDY DESIGN AND VALIDITY: The authors performed a pooled efficacy analysis of re-treatment regimens for H pylori eradication in adults. They included all prospective studies—randomized and nonrandomized—that reported eradication rates in patients previously treated with antibiotic therapy. The inclusion criteria were appropriate, and the search for relevant articles was complete in that the authors included abstracts from international gastroenterology meetings. An analysis strategy of simple pooling was used (total number of patients successfully treated divided by all those enrolled in given treatment category), which is appropriate since the primary outcome was the eradication rate.
OUTCOMES MEASURED: The authors calculated a pooled eradication rate with 95% confidence intervals for each of the 6 general treatment categories. They also determined the eradication rate of each second treatment, accounting for differences in initial treatment therapy regimen. Finally, they ascertained if the effectiveness of second-line therapy regimens improved when distinct antimicrobials not used in the first attempt at treatment were used.
RESULTS: The most effective second-line therapies for eradication of H pylori were quadruple therapy, either ranitidine-bismuth-based triple therapy (ranitidine-bismuth product plus 2 antimicrobials) at 80.2% (95% confidence interval [CI], 75%-85%) or H2-blocker or PPI, bismuth compound, and 2 antimicrobials) at 75.8% (95% CI, 73%-79%). The second-line treatment eradication rate was lower when the initial therapy was a PPI with 2 antimicrobials versus a PPI with one antimicrobial. Re-treatment was more difficult with an increased number of antimicrobials used in initial therapy. The re-treatment eradication rate was greater when 2 new antimicrobials were included in the regimen than when a single new antimicrobial was added (P=.0064).
If initial attempts fail at eradication of H pylori then quadruple therapy (an antisecretory agent, a bismuth compound, and 2 antimicrobials) or ranitidine-bismuth (Tritec) plus 2 antimicrobials is the most effective follow-up treatment. The latter approach is fairly expensive ($150). For patients whose first course included only one antimicrobial, using 2 new antimicrobials is just as effective as quadruple or ranitidine bismuth-based therapy. These approaches will achieve eradication in 75% to 80% of resistant cases.
ABSTRACT
BACKGROUND: Based on randomized clinical trials (RCTs), the most effective first-line eradication therapy for Helicobacter pylori is a combination of proton pump inhibitor (PPI) and 2 antimicrobial agents.1 Yet there remains a significant treatment failure rate of 5% to 25%. Antibiotic resistance is the major impediment of cure.2 Using a pooled analysis approach, the authors determined the second-line treatment strategy resulting in the greatest percentage of H pylorieradication.
POPULATION STUDIED: Studies of H pylori re-eradication in adults were retrieved from MEDLINE database, reference lists of retrieved research papers, and major congress abstract lists. All studies were performed between 1994 and 1999, were conducted prospectively (or without information on study design), contained detailed information on eradication agents, and included subjects with only one treatment failure. Eighteen articles and 47 abstracts were identified; 16 articles and 24 abstracts met the inclusion criteria.
STUDY DESIGN AND VALIDITY: The authors performed a pooled efficacy analysis of re-treatment regimens for H pylori eradication in adults. They included all prospective studies—randomized and nonrandomized—that reported eradication rates in patients previously treated with antibiotic therapy. The inclusion criteria were appropriate, and the search for relevant articles was complete in that the authors included abstracts from international gastroenterology meetings. An analysis strategy of simple pooling was used (total number of patients successfully treated divided by all those enrolled in given treatment category), which is appropriate since the primary outcome was the eradication rate.
OUTCOMES MEASURED: The authors calculated a pooled eradication rate with 95% confidence intervals for each of the 6 general treatment categories. They also determined the eradication rate of each second treatment, accounting for differences in initial treatment therapy regimen. Finally, they ascertained if the effectiveness of second-line therapy regimens improved when distinct antimicrobials not used in the first attempt at treatment were used.
RESULTS: The most effective second-line therapies for eradication of H pylori were quadruple therapy, either ranitidine-bismuth-based triple therapy (ranitidine-bismuth product plus 2 antimicrobials) at 80.2% (95% confidence interval [CI], 75%-85%) or H2-blocker or PPI, bismuth compound, and 2 antimicrobials) at 75.8% (95% CI, 73%-79%). The second-line treatment eradication rate was lower when the initial therapy was a PPI with 2 antimicrobials versus a PPI with one antimicrobial. Re-treatment was more difficult with an increased number of antimicrobials used in initial therapy. The re-treatment eradication rate was greater when 2 new antimicrobials were included in the regimen than when a single new antimicrobial was added (P=.0064).
If initial attempts fail at eradication of H pylori then quadruple therapy (an antisecretory agent, a bismuth compound, and 2 antimicrobials) or ranitidine-bismuth (Tritec) plus 2 antimicrobials is the most effective follow-up treatment. The latter approach is fairly expensive ($150). For patients whose first course included only one antimicrobial, using 2 new antimicrobials is just as effective as quadruple or ranitidine bismuth-based therapy. These approaches will achieve eradication in 75% to 80% of resistant cases.
What is the best approach to prevention and treatment of osteoporosis?
Randomized controlled trials (RCTs) have demonstrated decreased vertebral and nonvertebral fracture rates in postmenopausal osteoporotic women taking a bisphosphonate (alendronate or risedronate). Hormone replacement therapy (HRT) also has some evidence for osteoporotic fracture prevention. Concurrent calcium and vitamin D may also prevent fractures. Physical exercise and smoking cessation have been associated with increased bone density, but fracture prevention has not been reported (Grade of Recommendation: B, some extrapolation from individual trials needed).
Evidence Summary
Many trials report bone mineral density (BMD) as their primary outcome, which may or may not be associated with fracture rates. Relatively few studies have reported patient-oriented outcomes, such as vertebral or nonvertebral fracture.
Alendronate and risedronate have both reduced vertebral and nonvertebral fractures and increased BMD in clinical trials. The pooled risk estimates for alendronate’s antifracture effect are 48% to 53% vertebral and hip fracture reduction and 30% reduction of all clinical fractures (relative risk [RR] = 0.70; 95% confidence interval [CI], 0.59-0.82).1 Risedronate reduced hip fractures in osteoporotic women aged 70 to 79 years (RR = 0.6; 95% CI, 0.4-0.9), but women 80 years and older with nonskeletal risk factors did not benefit.2 One alendronate trial in 241 osteoporotic men demonstrated increased spine and hip BMD and a decreased incidence of vertebral fractures.3
HRT has been associated with increased BMD in RCTs, fewer fractures in observational studies, and fewer fractures in a recent meta-analysis of RCTs. HRT had the greatest benefit among women younger than 60 years (RR = 0.67; 95% CI, 0.56-0.94).4 For women older than 60 years, a nonsignificant trend toward benefit was found (RR = 0.88, 95% CI, 0.71-1.08). However, in a large RCT of postmenopausal women, 85% without osteoporotic BMD, 4 years of HRT did not reduce fracture incidence.5 Testosterone replacement in men is less studied; some small studies demonstrate increased BMD, but long-term benefits are unknown.6
Raloxifene and calcitonin have some supporting evidence as well. In a large 4-year placebo-controlled trial, raloxifene decreased vertebral fracture risk (RR = 0.7; 95% CI, 0.5-0.8) but had no effect on hip fracture and a threefold increase in thromboembolic risk (RR = 3.1; 95% CI, 1.5-6.2).7 Regarding calcitonin, one RCT compared 3 doses of intranasal calcitonin and placebo in osteoporotic women and showed an increase in lumbar spine BMD in treated groups.8 Over the 5-year study (59% of participants were lost to follow-up), there was a 39% reduction in risk of radiologic deformities (P=.03) only for women taking 200 IU.
Many subjects also used vitamin D and calcium, but effective doses of both agents are still in question. Combined calcium and vitamin D supplements have decreased fracture risk using 700 to 800 IU of vitamin D and 500 to 1200 mg of calcium daily,9 but a systematic review of vitamin D without calcium found no clear benefit.10 A meta-analysis showed that exercise training programs may play a preventive role, potentially preventing or reversing 1% of bone loss annually among women.11 Smoking cessation may also increase bone density.12
Recommendations From Others
The Royal College of Physician Guidelines on Osteoporosis Prevention and Treatment gives HRT an A grade of recommendation for preventing spinal fractures and a B grade for nonvertebral fracture prevention, while alendronate and risedronate received an A grade for both vertebral and nonvertebral fracture prevention. The American Association of Clinical Endocrinologists (AACE) states that HRT is the standard of care for preventing and treating postmenopausal bone loss but supports the use of bisphosphonates as an alternative. The US Preventive Services Task Force emphasizes smoking cessation, regular exercise, and adequate calcium intake, and recommends discussion with patients of the risks and benefits of HRT.
Sang-Ick Chang, MD
University of California San Francisco
It is very helpful to have bisphosphonates as a proven alternative to HRT, in cases where HRT is declined or not indicated. This review confirms what most of us have been doing in clinical practice: relying on HRT and bisphosphonates as the 2 preferred prescription medications for osteoporosis, relegating calcitonin to rare situations. Two difficult issues remain in the approach to osteoporosis, which are the dilemmas of who to screen and when to add a second agent.
1. Black DM, Thompson DE, Bauer DC, et al. J Clin Endocrinol Metab 2000;85:4118-24.
2. McClung MR, Geusens P, Miller PD, et al. N Engl J Med 2001;344:333-40.
3. Orwoll E, Ettinger M, Weiss S, et al. N Eng J Med 2000;343:604-10.
4. Torgerson DJ, Bell-Sayer, SEM. JAMA 2001;285:2891-97.
5. Cauley JA, Black DM, Barret-Connor E, et al. Am J Med 2001;110:442-50.
6. Kamel HK, Perry HM, Morley JE. J Am Geriatr Soc 2001;49:179-87.
7. Ettinger B, Black DM, Mitlak BH, et al. JAMA 1999;282:637-645.
8. Chesnut CH, Silverman S, Andirano K, et al. Am J Med 2000;109:267-76.
9. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. N Engl J Med 1997;670-76.
10. Gillespie WJ, Avenell A, Henry DA, O’Connell DL, Robertson J. Cochrane Database of Systematic Reviews, 2001; issue 1.
11. Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. Osteoporos Int 1999;9:1-12.
12. Hopper JL, Seeman E. N Eng J Med 1994;330:387-92.
Randomized controlled trials (RCTs) have demonstrated decreased vertebral and nonvertebral fracture rates in postmenopausal osteoporotic women taking a bisphosphonate (alendronate or risedronate). Hormone replacement therapy (HRT) also has some evidence for osteoporotic fracture prevention. Concurrent calcium and vitamin D may also prevent fractures. Physical exercise and smoking cessation have been associated with increased bone density, but fracture prevention has not been reported (Grade of Recommendation: B, some extrapolation from individual trials needed).
Evidence Summary
Many trials report bone mineral density (BMD) as their primary outcome, which may or may not be associated with fracture rates. Relatively few studies have reported patient-oriented outcomes, such as vertebral or nonvertebral fracture.
Alendronate and risedronate have both reduced vertebral and nonvertebral fractures and increased BMD in clinical trials. The pooled risk estimates for alendronate’s antifracture effect are 48% to 53% vertebral and hip fracture reduction and 30% reduction of all clinical fractures (relative risk [RR] = 0.70; 95% confidence interval [CI], 0.59-0.82).1 Risedronate reduced hip fractures in osteoporotic women aged 70 to 79 years (RR = 0.6; 95% CI, 0.4-0.9), but women 80 years and older with nonskeletal risk factors did not benefit.2 One alendronate trial in 241 osteoporotic men demonstrated increased spine and hip BMD and a decreased incidence of vertebral fractures.3
HRT has been associated with increased BMD in RCTs, fewer fractures in observational studies, and fewer fractures in a recent meta-analysis of RCTs. HRT had the greatest benefit among women younger than 60 years (RR = 0.67; 95% CI, 0.56-0.94).4 For women older than 60 years, a nonsignificant trend toward benefit was found (RR = 0.88, 95% CI, 0.71-1.08). However, in a large RCT of postmenopausal women, 85% without osteoporotic BMD, 4 years of HRT did not reduce fracture incidence.5 Testosterone replacement in men is less studied; some small studies demonstrate increased BMD, but long-term benefits are unknown.6
Raloxifene and calcitonin have some supporting evidence as well. In a large 4-year placebo-controlled trial, raloxifene decreased vertebral fracture risk (RR = 0.7; 95% CI, 0.5-0.8) but had no effect on hip fracture and a threefold increase in thromboembolic risk (RR = 3.1; 95% CI, 1.5-6.2).7 Regarding calcitonin, one RCT compared 3 doses of intranasal calcitonin and placebo in osteoporotic women and showed an increase in lumbar spine BMD in treated groups.8 Over the 5-year study (59% of participants were lost to follow-up), there was a 39% reduction in risk of radiologic deformities (P=.03) only for women taking 200 IU.
Many subjects also used vitamin D and calcium, but effective doses of both agents are still in question. Combined calcium and vitamin D supplements have decreased fracture risk using 700 to 800 IU of vitamin D and 500 to 1200 mg of calcium daily,9 but a systematic review of vitamin D without calcium found no clear benefit.10 A meta-analysis showed that exercise training programs may play a preventive role, potentially preventing or reversing 1% of bone loss annually among women.11 Smoking cessation may also increase bone density.12
Recommendations From Others
The Royal College of Physician Guidelines on Osteoporosis Prevention and Treatment gives HRT an A grade of recommendation for preventing spinal fractures and a B grade for nonvertebral fracture prevention, while alendronate and risedronate received an A grade for both vertebral and nonvertebral fracture prevention. The American Association of Clinical Endocrinologists (AACE) states that HRT is the standard of care for preventing and treating postmenopausal bone loss but supports the use of bisphosphonates as an alternative. The US Preventive Services Task Force emphasizes smoking cessation, regular exercise, and adequate calcium intake, and recommends discussion with patients of the risks and benefits of HRT.
Sang-Ick Chang, MD
University of California San Francisco
It is very helpful to have bisphosphonates as a proven alternative to HRT, in cases where HRT is declined or not indicated. This review confirms what most of us have been doing in clinical practice: relying on HRT and bisphosphonates as the 2 preferred prescription medications for osteoporosis, relegating calcitonin to rare situations. Two difficult issues remain in the approach to osteoporosis, which are the dilemmas of who to screen and when to add a second agent.
Randomized controlled trials (RCTs) have demonstrated decreased vertebral and nonvertebral fracture rates in postmenopausal osteoporotic women taking a bisphosphonate (alendronate or risedronate). Hormone replacement therapy (HRT) also has some evidence for osteoporotic fracture prevention. Concurrent calcium and vitamin D may also prevent fractures. Physical exercise and smoking cessation have been associated with increased bone density, but fracture prevention has not been reported (Grade of Recommendation: B, some extrapolation from individual trials needed).
Evidence Summary
Many trials report bone mineral density (BMD) as their primary outcome, which may or may not be associated with fracture rates. Relatively few studies have reported patient-oriented outcomes, such as vertebral or nonvertebral fracture.
Alendronate and risedronate have both reduced vertebral and nonvertebral fractures and increased BMD in clinical trials. The pooled risk estimates for alendronate’s antifracture effect are 48% to 53% vertebral and hip fracture reduction and 30% reduction of all clinical fractures (relative risk [RR] = 0.70; 95% confidence interval [CI], 0.59-0.82).1 Risedronate reduced hip fractures in osteoporotic women aged 70 to 79 years (RR = 0.6; 95% CI, 0.4-0.9), but women 80 years and older with nonskeletal risk factors did not benefit.2 One alendronate trial in 241 osteoporotic men demonstrated increased spine and hip BMD and a decreased incidence of vertebral fractures.3
HRT has been associated with increased BMD in RCTs, fewer fractures in observational studies, and fewer fractures in a recent meta-analysis of RCTs. HRT had the greatest benefit among women younger than 60 years (RR = 0.67; 95% CI, 0.56-0.94).4 For women older than 60 years, a nonsignificant trend toward benefit was found (RR = 0.88, 95% CI, 0.71-1.08). However, in a large RCT of postmenopausal women, 85% without osteoporotic BMD, 4 years of HRT did not reduce fracture incidence.5 Testosterone replacement in men is less studied; some small studies demonstrate increased BMD, but long-term benefits are unknown.6
Raloxifene and calcitonin have some supporting evidence as well. In a large 4-year placebo-controlled trial, raloxifene decreased vertebral fracture risk (RR = 0.7; 95% CI, 0.5-0.8) but had no effect on hip fracture and a threefold increase in thromboembolic risk (RR = 3.1; 95% CI, 1.5-6.2).7 Regarding calcitonin, one RCT compared 3 doses of intranasal calcitonin and placebo in osteoporotic women and showed an increase in lumbar spine BMD in treated groups.8 Over the 5-year study (59% of participants were lost to follow-up), there was a 39% reduction in risk of radiologic deformities (P=.03) only for women taking 200 IU.
Many subjects also used vitamin D and calcium, but effective doses of both agents are still in question. Combined calcium and vitamin D supplements have decreased fracture risk using 700 to 800 IU of vitamin D and 500 to 1200 mg of calcium daily,9 but a systematic review of vitamin D without calcium found no clear benefit.10 A meta-analysis showed that exercise training programs may play a preventive role, potentially preventing or reversing 1% of bone loss annually among women.11 Smoking cessation may also increase bone density.12
Recommendations From Others
The Royal College of Physician Guidelines on Osteoporosis Prevention and Treatment gives HRT an A grade of recommendation for preventing spinal fractures and a B grade for nonvertebral fracture prevention, while alendronate and risedronate received an A grade for both vertebral and nonvertebral fracture prevention. The American Association of Clinical Endocrinologists (AACE) states that HRT is the standard of care for preventing and treating postmenopausal bone loss but supports the use of bisphosphonates as an alternative. The US Preventive Services Task Force emphasizes smoking cessation, regular exercise, and adequate calcium intake, and recommends discussion with patients of the risks and benefits of HRT.
Sang-Ick Chang, MD
University of California San Francisco
It is very helpful to have bisphosphonates as a proven alternative to HRT, in cases where HRT is declined or not indicated. This review confirms what most of us have been doing in clinical practice: relying on HRT and bisphosphonates as the 2 preferred prescription medications for osteoporosis, relegating calcitonin to rare situations. Two difficult issues remain in the approach to osteoporosis, which are the dilemmas of who to screen and when to add a second agent.
1. Black DM, Thompson DE, Bauer DC, et al. J Clin Endocrinol Metab 2000;85:4118-24.
2. McClung MR, Geusens P, Miller PD, et al. N Engl J Med 2001;344:333-40.
3. Orwoll E, Ettinger M, Weiss S, et al. N Eng J Med 2000;343:604-10.
4. Torgerson DJ, Bell-Sayer, SEM. JAMA 2001;285:2891-97.
5. Cauley JA, Black DM, Barret-Connor E, et al. Am J Med 2001;110:442-50.
6. Kamel HK, Perry HM, Morley JE. J Am Geriatr Soc 2001;49:179-87.
7. Ettinger B, Black DM, Mitlak BH, et al. JAMA 1999;282:637-645.
8. Chesnut CH, Silverman S, Andirano K, et al. Am J Med 2000;109:267-76.
9. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. N Engl J Med 1997;670-76.
10. Gillespie WJ, Avenell A, Henry DA, O’Connell DL, Robertson J. Cochrane Database of Systematic Reviews, 2001; issue 1.
11. Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. Osteoporos Int 1999;9:1-12.
12. Hopper JL, Seeman E. N Eng J Med 1994;330:387-92.
1. Black DM, Thompson DE, Bauer DC, et al. J Clin Endocrinol Metab 2000;85:4118-24.
2. McClung MR, Geusens P, Miller PD, et al. N Engl J Med 2001;344:333-40.
3. Orwoll E, Ettinger M, Weiss S, et al. N Eng J Med 2000;343:604-10.
4. Torgerson DJ, Bell-Sayer, SEM. JAMA 2001;285:2891-97.
5. Cauley JA, Black DM, Barret-Connor E, et al. Am J Med 2001;110:442-50.
6. Kamel HK, Perry HM, Morley JE. J Am Geriatr Soc 2001;49:179-87.
7. Ettinger B, Black DM, Mitlak BH, et al. JAMA 1999;282:637-645.
8. Chesnut CH, Silverman S, Andirano K, et al. Am J Med 2000;109:267-76.
9. Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. N Engl J Med 1997;670-76.
10. Gillespie WJ, Avenell A, Henry DA, O’Connell DL, Robertson J. Cochrane Database of Systematic Reviews, 2001; issue 1.
11. Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. Osteoporos Int 1999;9:1-12.
12. Hopper JL, Seeman E. N Eng J Med 1994;330:387-92.
Evidence-based answers from the Family Physicians Inquiries Network
Is a 2-day course of oral dexamethasone more effective than 5 days of oral prednisone in improving symptoms and preventing relapse in children with acute asthma?
BACKGROUND: Dexamethasone, a long-acting corticosteroid successfully used in acute treatment of croup, may prevent more relapses than prednisone in asthmatic children.
POPULATION STUDIED: The authors studied known asthmatic persons (defined by 2 or more episodes of wheezing treated with b-agonists with or without steroids) aged 2 to 18 years presenting to a children’s health hospital emergency department (ED) with an acute asthma exacerbation requiring more than 1 albuterol nebulizer treatment. Nursing staff assessed asthma severity based on either peak expired flow rates or a validated asthma severity scoring system. Children were excluded for recent oral corticosteroid treatment, history of intubation, recent varicella exposure, stridor, possible foreign body, and certain chronic diseases. During an 11-month period, 628 subjects enrolled, of whom 533 (85%) completed the study. Two thirds were men, 84% were black, and the average age was between 6 and 7 years. Fifty-six percent of the children were classified as moderate asthma severity at presentation; the remainder was evenly distributed between mild and severe.
STUDY DESIGN AND VALIDITY: This controlled trial assigned children to receive oral prednisone (2 mg/kg, maximum 60 mg, n= 261) on odd days and dexamethasone (0.6 mg/kg, maximum 16 mg, n=272) on even days. The first dose was given in the ED; the prednisone group was sent home with a prescription for 4 daily doses, the dexamethasone group was given a prepackaged dose for the following day. Children who vomited 2 doses of steroid or were directly admitted to the hospital from the ED were dropped from the study.This was a quasirandomized study, in that children were placed on one drug on even days and the other steroid on odd days. As a result, the allocation to the specific treatment groups was not concealed. Although patient severity is unlikely to have varied systematically on even and odd days, a large potential exists for a bias to be introduced into this study. Nurses who believed one treatment was superior to another could have systematically altered enrollment of children into the study based on the treatment of that day. These 2 issues—lack of randomization and concealed allocation—could invalidate the results of the study. The majority of subjects were black. Asthma prevalence, morbidity, and mortality are higher among black children, especially those in urban settings.1 There is also some evidence of physiologic predisposition in this population, namely, higher serum immunoglobulin E levels and increased airway responsiveness.2 However, no literature suggests that there is a difference in asthma treatment response between black children and children of other races or ethnicities.
OUTCOMES MEASURED: The primary outcome was rate of relapse within 10 days of discharge from the ED. Secondary outcomes were rate of hospitalization, frequency of vomiting, medication compliance, persistence of symptoms, and work or school days missed.
RESULTS: By evaluating the children who completed the study, the authors determined that the relapse rates were similar between the 2 groups, 7.4% in the dexamethasone group and 6.9% in the prednisone group (P = NS). Intention-to-treat analysis also found no difference between treatments. The number of admissions after relapse and the prevalence of persistent symptoms was also similar between the 2 groups. More children in the prednisone group missed 2 or more school days (P =.05), and more parents in this group reported not giving the medication at home (P =.004).
For acute pediatric asthma, symptom improvement and relapse rate are similar whether our patients receive 2 doses of dexamethasone or 5 doses of oral prednisone. Given equal effectiveness, fewer school days missed, less vomiting, and fewer doses, dexamethasone may be preferable. However, we hesitate to make any recommendations for changes in practice based on this study, given the severe limitations in study design.
BACKGROUND: Dexamethasone, a long-acting corticosteroid successfully used in acute treatment of croup, may prevent more relapses than prednisone in asthmatic children.
POPULATION STUDIED: The authors studied known asthmatic persons (defined by 2 or more episodes of wheezing treated with b-agonists with or without steroids) aged 2 to 18 years presenting to a children’s health hospital emergency department (ED) with an acute asthma exacerbation requiring more than 1 albuterol nebulizer treatment. Nursing staff assessed asthma severity based on either peak expired flow rates or a validated asthma severity scoring system. Children were excluded for recent oral corticosteroid treatment, history of intubation, recent varicella exposure, stridor, possible foreign body, and certain chronic diseases. During an 11-month period, 628 subjects enrolled, of whom 533 (85%) completed the study. Two thirds were men, 84% were black, and the average age was between 6 and 7 years. Fifty-six percent of the children were classified as moderate asthma severity at presentation; the remainder was evenly distributed between mild and severe.
STUDY DESIGN AND VALIDITY: This controlled trial assigned children to receive oral prednisone (2 mg/kg, maximum 60 mg, n= 261) on odd days and dexamethasone (0.6 mg/kg, maximum 16 mg, n=272) on even days. The first dose was given in the ED; the prednisone group was sent home with a prescription for 4 daily doses, the dexamethasone group was given a prepackaged dose for the following day. Children who vomited 2 doses of steroid or were directly admitted to the hospital from the ED were dropped from the study.This was a quasirandomized study, in that children were placed on one drug on even days and the other steroid on odd days. As a result, the allocation to the specific treatment groups was not concealed. Although patient severity is unlikely to have varied systematically on even and odd days, a large potential exists for a bias to be introduced into this study. Nurses who believed one treatment was superior to another could have systematically altered enrollment of children into the study based on the treatment of that day. These 2 issues—lack of randomization and concealed allocation—could invalidate the results of the study. The majority of subjects were black. Asthma prevalence, morbidity, and mortality are higher among black children, especially those in urban settings.1 There is also some evidence of physiologic predisposition in this population, namely, higher serum immunoglobulin E levels and increased airway responsiveness.2 However, no literature suggests that there is a difference in asthma treatment response between black children and children of other races or ethnicities.
OUTCOMES MEASURED: The primary outcome was rate of relapse within 10 days of discharge from the ED. Secondary outcomes were rate of hospitalization, frequency of vomiting, medication compliance, persistence of symptoms, and work or school days missed.
RESULTS: By evaluating the children who completed the study, the authors determined that the relapse rates were similar between the 2 groups, 7.4% in the dexamethasone group and 6.9% in the prednisone group (P = NS). Intention-to-treat analysis also found no difference between treatments. The number of admissions after relapse and the prevalence of persistent symptoms was also similar between the 2 groups. More children in the prednisone group missed 2 or more school days (P =.05), and more parents in this group reported not giving the medication at home (P =.004).
For acute pediatric asthma, symptom improvement and relapse rate are similar whether our patients receive 2 doses of dexamethasone or 5 doses of oral prednisone. Given equal effectiveness, fewer school days missed, less vomiting, and fewer doses, dexamethasone may be preferable. However, we hesitate to make any recommendations for changes in practice based on this study, given the severe limitations in study design.
BACKGROUND: Dexamethasone, a long-acting corticosteroid successfully used in acute treatment of croup, may prevent more relapses than prednisone in asthmatic children.
POPULATION STUDIED: The authors studied known asthmatic persons (defined by 2 or more episodes of wheezing treated with b-agonists with or without steroids) aged 2 to 18 years presenting to a children’s health hospital emergency department (ED) with an acute asthma exacerbation requiring more than 1 albuterol nebulizer treatment. Nursing staff assessed asthma severity based on either peak expired flow rates or a validated asthma severity scoring system. Children were excluded for recent oral corticosteroid treatment, history of intubation, recent varicella exposure, stridor, possible foreign body, and certain chronic diseases. During an 11-month period, 628 subjects enrolled, of whom 533 (85%) completed the study. Two thirds were men, 84% were black, and the average age was between 6 and 7 years. Fifty-six percent of the children were classified as moderate asthma severity at presentation; the remainder was evenly distributed between mild and severe.
STUDY DESIGN AND VALIDITY: This controlled trial assigned children to receive oral prednisone (2 mg/kg, maximum 60 mg, n= 261) on odd days and dexamethasone (0.6 mg/kg, maximum 16 mg, n=272) on even days. The first dose was given in the ED; the prednisone group was sent home with a prescription for 4 daily doses, the dexamethasone group was given a prepackaged dose for the following day. Children who vomited 2 doses of steroid or were directly admitted to the hospital from the ED were dropped from the study.This was a quasirandomized study, in that children were placed on one drug on even days and the other steroid on odd days. As a result, the allocation to the specific treatment groups was not concealed. Although patient severity is unlikely to have varied systematically on even and odd days, a large potential exists for a bias to be introduced into this study. Nurses who believed one treatment was superior to another could have systematically altered enrollment of children into the study based on the treatment of that day. These 2 issues—lack of randomization and concealed allocation—could invalidate the results of the study. The majority of subjects were black. Asthma prevalence, morbidity, and mortality are higher among black children, especially those in urban settings.1 There is also some evidence of physiologic predisposition in this population, namely, higher serum immunoglobulin E levels and increased airway responsiveness.2 However, no literature suggests that there is a difference in asthma treatment response between black children and children of other races or ethnicities.
OUTCOMES MEASURED: The primary outcome was rate of relapse within 10 days of discharge from the ED. Secondary outcomes were rate of hospitalization, frequency of vomiting, medication compliance, persistence of symptoms, and work or school days missed.
RESULTS: By evaluating the children who completed the study, the authors determined that the relapse rates were similar between the 2 groups, 7.4% in the dexamethasone group and 6.9% in the prednisone group (P = NS). Intention-to-treat analysis also found no difference between treatments. The number of admissions after relapse and the prevalence of persistent symptoms was also similar between the 2 groups. More children in the prednisone group missed 2 or more school days (P =.05), and more parents in this group reported not giving the medication at home (P =.004).
For acute pediatric asthma, symptom improvement and relapse rate are similar whether our patients receive 2 doses of dexamethasone or 5 doses of oral prednisone. Given equal effectiveness, fewer school days missed, less vomiting, and fewer doses, dexamethasone may be preferable. However, we hesitate to make any recommendations for changes in practice based on this study, given the severe limitations in study design.